Abstract
Background
Intradermal administration of fractional inactivated poliovirus vaccine (fIPV) is a dose-sparing alternative to intramuscular full dose. We assessed the recommendation of two fIPV doses or one IPV dose for routine immunization, and a fIPV booster dose for outbreak response.
Methods
We conducted an open-label, randomized, controlled, inequality, non-inferiority trial in two Dhaka clinics, Bangladesh. Healthy infants were randomized at 6 weeks to one of four arms: (A) IPV at 14 weeks and IPV at 22 weeks (booster); (B) IPV at 14 weeks and fIPV booster; (C) IPV at 6 weeks and fIPV booster; or (D) fIPV at 6+14 weeks and fIPV booster. Vaccines were administered by needle-syringe, with intradermal adapter for fIPV. Vaccine response (seroconversion from seronegative (<1:8) at baseline to seropositive (≥1:8) or four-fold increase in reciprocal antibody titers adjusted for maternal antibody decay) to types 1, 2, and 3 at 22 weeks (routine immunization) and 26 weeks (outbreak response) was assessed in the intention-to-treat population. Non-inferiority margin was 12·5%. (Registered at ClinicalTrials.gov, number NCT02847026).
Findings
From September 1, 2016 to May 2, 2017, 1,076 participants were assigned to Arms A (n=271), B (n=267), C (n=268), and D (n=270). Vaccine response at 22 weeks to two doses of fIPV was significantly higher (p<0.0001) than one dose of IPV (Arm D versus A/B) for type 1 [212 (79%, 95%CI: 73%−83%) versus 305 (57%, 95%CI: 53%−61%)], type 2 [173 (64%, 95%CI: 58%−70%) versus 249 (46%, 95%CI: 42%−51%)], and type 3 [196 (73%, 95%CI: 67%−78%) versus 196 (36%, 95%CI: 33%−41%)]. At 26 weeks, fIPV booster was non-inferior to IPV (Arm B versus A) to types 1 (−1·1%, 90%CI: −2·2% - −0·1%), type 2 (0·4%, 90%CI: −2·2% - 1·4%), and type 3 (−1·5%, 90%CI: −3·2% - −0·2%). Of 129 adverse events, 21 were serious including one death; none were attributed to IPV/fIPV.
Interpretation
fIPV is an effective dose-sparing strategy for routine immunization and outbreak response.
Funding
U.S. Centers for Disease Control and Prevention
Keywords: Fractional inactivated poliovirus vaccine, inactivated poliovirus vaccine, immunogenicity, Bangladesh
Introduction
After type 2 wild poliovirus was certified eradicated in 2015, the Global Polio Eradication Initiative (GPEI) conducted a globally synchronized withdrawal of oral poliovirus vaccine (OPV) type 2 in April 2016 by replacing trivalent OPV (tOPV; types 1, 2, and 3) with bivalent OPV (bOPV; types 1 and 3).1 Cessation of routine use of OPV2 was essential to mitigate risk that the live, attenuated type 2 vaccine virus would continue circulation in under-immunized populations and genetically revert and reacquire neurovirulence thereby causing paralysis.2 To offset the gap in type 2 immunity, the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization (SAGE) recommended all OPV-using countries to introduce one dose of inactivated poliovirus vaccine (IPV; types 1, 2, and 3) at age 14 weeks or later prior to OPV2 cessation.3 Depending on the age of administration, vaccine response to one IPV dose was between 34–77% for type 2; evidence of priming among seronegative children was such that the cumulative vaccine response (vaccine response plus priming) to one dose of IPV was ≥90% [Anand, personal communication].4–6 In the event of a type 2 outbreak, a dose of type 2 containing vaccine in a population that had received at least one dose of IPV would rapidly induce protective levels of immunity against paralysis. However, IPV manufacturers were unable to meet the global supply demand and 49 countries either delayed IPV introduction or experienced a stock-out after introduction.7
Intradermal administration of fractional dose of IPV (fIPV) has been investigated since 1953 and studies in the 1990’s demonstrated that a one-fifth fIPV dose (0.1ml) of the enhanced-potency IPV (i.e., current IPV formulation, 0.5ml) was immunogenic.8–12 Since the 2008 World Health Assembly, fIPV has been further explored as a cost-saving option for countries due to the substantially higher cost of IPV compared with OPV;13 however, fIPV is also being pursued as a dose-sparing option in light of the limited IPV supply.
In 2016, SAGE encouraged countries to evaluate the cost-benefits, trade-offs, and programmatic feasibility of providing two fIPV doses at ages 6 and 14 weeks as an alternative to one IPV dose.14 It was inferred that this schedule would provide a higher vaccine response based on comparisons of study arm(s) from multiple clinical trials4–6,15–20 but no clinical trial has directly compared these two options. As of September 2018, this schedule has been introduced in Bangladesh, Cuba, Ecuador, India, Nepal, and Sri Lanka.
The global IPV shortage also has implications for outbreak response activities. GPEI had proposed that response activities include IPV as a booster to quickly improve immunity, especially to type 2. IPV supply shortages have led countries to stretch supplies by using fIPV when responding to type 2 poliovirus events.21,22 Based on studies in adults and older children previously vaccinated with OPV,12,14 SAGE recommended in October 2016 that fIPV be used in response campaigns if IPV was deemed necessary. No studies have compared type 2 immunogenicity of a fIPV booster instead of IPV among OPV-naïve children vaccinated with a single IPV dose.
We conducted a clinical trial among OPV-naïve infants to compare the immunogenicity of two fIPV doses at ages 6 and 14 weeks to one dose of IPV at age 14 weeks. The use of IPV in OPV-naïve infants also allowed us to investigate the immunogenicity of two doses of IPV in comparison with three doses of fIPV to inform deliberations of vaccination schedules after OPV use is discontinued globally. To inform policies related to fIPV use in outbreak settings, we compared the immunogenicity of an IPV booster with a fIPV booster, both given to infants who received IPV at 14 weeks of age. We also assessed immunogenicity of a fIPV booster administered to infants who received IPV at 14 weeks of age in comparison with IPV given at 6 weeks, or fIPV given at 6 and 14 weeks.
Methods
Study design and participants
We conducted a randomized, controlled, parallel, open-label, inequality, non-inferiority trial in Mirpur in urban Dhaka, Bangladesh. A second site was established shortly thereafter in the Mohakahli area of Dhaka to increase participant enrollment. Bangladesh withdrew OPV2 use in April 2016. The study included an independent evaluation of immunogenicity to oral rotavirus vaccines; for simplicity, details and results presented in this paper focus only on the poliovirus component as there is no reported interference between IPV and oral rotavirus vaccines. The study and amendments were approved by icddr,b’s Institutional Review Board. Study and amendments were shared with CDC but deferred to icddr,b’s IRB; CDC staff had no interaction with human subjects nor access to personally identifiable information.
Field workers identified expectant mothers within assigned communities and interested parents were invited to participate. Infants aged 6 weeks (42–48 days) were eligible if they were full-term (>37 weeks) singleton births and would remain in the area for the study duration. Written informed consent was obtained from parents. Infants were excluded if they had evidence or indication of a medical condition that contraindicated venipuncture or parenteral administration of IPV; chronic medical condition identified by a study medical officer (not including stunting or wasting); severe illness that required hospital admission; vomiting or intolerance to liquids in the 24 hours prior to enrollment; receipt of any poliovirus or rotavirus vaccine prior to enrollment; any known allergies or sensitivity to polio or rotavirus vaccines or contents; or history of intussusception, intestinal malformation, or abdominal surgery. Parents could withdraw consent for participation at any time. Study staff withdrew participants if poliovirus or rotavirus vaccine was received outside the study; identified a medical condition in which continued participation posed a risk; used immunosuppressive medications; or were unable to obtain blood during the first visit.
Randomization and masking
Participants were randomly allocated (1:1:1:1) to one of four study arms, each arm representing a potential routine immunization schedule (i.e., primary series) and a booster dose. The four arms were: A) IPV at age 14 weeks and IPV booster at age 22 weeks (IPV14+IPV); B) IPV at age 14 weeks and fIPV booster at age 22 weeks (IPV14+fIPV); C) IPV at age 6 weeks and fIPV booster at age 22 weeks (IPV6+fIPV); and D) fIPV at age 6 and 14 weeks and fIPV booster at age 22 weeks (fIPV6/14+fIPV). At each study clinic, block randomization was used with block sizes of 8, 16, 24, and 32. Investigators with no participant engagement generated the randomization sequence using R (R foundation, version 3.2.1) and concealed randomization assignment in sequentially numbered, sealed, opaque envelopes. Study clinic staff had no a-priori knowledge of the randomization scheme; arm assignment was unmasked to parents and study clinic staff when envelopes were opened. Only laboratory staff remained blinded to assignment during and after the trial.
Procedures
Upon enrollment, staff obtained the infant’s clinical history (i.e., breastfeeding, previous vaccination, and health status), conducted a physical examination (including temperature, weight, and length), collected a sample of blood, administered IPV or fIPV (IPV6+fIPV and fIPV6/14+fIPV arms only), and monitored infants for 30 minutes for any systemic or site of injection adverse events. Weight and length were measured twice using an electronic scale with precision to 100 grams and a measuring board with precision to 1 mm, respectively. The mean of the two measurements were used to assess whether participants had evidence of wasting (reduced weight for age) or stunting (reduced length for age) using the child-growth standard curves from WHO’s Multicenter Growth Reference Study.23 Wasting or stunting was present if participant measurements were more than two standard deviations below the mean of the reference population. Participants returned for study clinic visits at ages 14 weeks, 22 weeks, 23 weeks, and 26 weeks to complete polio-related activities; visits at age 10 and 18 weeks were also conducted for rotavirus vaccine-related activities. At each visit, staff again collected information on the participant’s clinical history, conducted physical examinations, collected a sample of blood (22, 23, and 26 weeks), administered IPV or fIPV (14 and 22 weeks), and monitored for adverse events. All blood samples were collected prior to study vaccine administration.
The IPV/fIPV used in this trial was manufactured by Sanofi Pasteur (Lyon, France) and each full dose contained type 1 (40 D-antigen unit of the Mahoney strain), type 2 (8 D-antigen unit of the MEF-1 strain), and type 3 (32 D-antigen unit of the Saukett strain). IPV was only available for procurement as a pre-filled syringe and was used in accordance with the manufacturer’s instructions when administered as an intramuscular full dose (0·5ml). For fIPV administration, the contents of the pre-filled IPV syringe were transferred into a sterile vial and 0·1ml of vaccine was withdrawn using the HelmJect auto-disable syringe 0·1ml 27Gx1/2”. The Helms intradermal adapter was then affixed to the needle-syringe for intradermal administration. Sanavita (formerly Helm Medical GmbH) donated the needle-syringe and intradermal adapter as a blister pack. IPV and fIPV were administered on the outer, upper right thigh of participants. Participants also received all routine immunization vaccines (except polio) according to the schedule of the Expanded Programme on Immunization of the Bangladesh Ministry of Health and Family Welfare. This included pentavalent (diphtheria, pertussis, tetanus, Hepatitis B, Haemophilus influenzae type B) and the pneumococcal conjugate vaccine (PCV, Streptococcus pneumoniae). Upon participation completion, infants in Arms C and D received one dose of IPV and all infants received three doses of bOPV at four-week intervals beginning at 26 weeks of age to ensure compliance with national guidelines for polio vaccination. All vaccines remained in cold chain per manufacturer’s recommendations.
Blood samples (1 or 1.5 ml, depending on poliovirus and rotavirus testing needs) were collected prior to study vaccination by venipuncture and transported to icddr,b’s laboratory by the end of the day; samples were stored and transported at 2–8°C. Samples were centrifuged within 24 hours of collection and serum were aliquoted for testing (stored at −20°C) and storage (stored at −70°C). Upon completion of all study activities, sera were sent to the Centers for Disease Control and Prevention laboratory in Atlanta, GA, USA, for testing. The polio microneutralization assay was used to measure antibody titers to poliovirus types 1, 2, and 3 and the upper limit of detection was ≥1448.24
Outcomes
The primary outcome was vaccine response measured at two time points: 22 weeks of age (8–16 weeks after the last primary series vaccination); and 26 weeks of age (four weeks after the booster dose at 22 weeks of age). Vaccine response was defined as seroconversion from seronegative (<1:8) at baseline (i.e., 6 weeks of age) to seropositive (≥1:8) after vaccination, or a four-fold rise in antibody titers between baseline and post-vaccination adjusted for the exponential decay of maternal antibodies assuming a half-life of 28 days. Vaccine response at 22 weeks of age (i.e., vaccine response to primary series) was used to assess any differences in immunogenicity of two doses of fIPV in comparison with one dose of IPV (Arm D vs A/B). Vaccine response at 26 weeks of age (i.e., vaccine response to booster) was used to assess: 1) non-inferiority of a fIPV booster in comparison with an IPV booster (Arm B vs A); 2) differences in immunogenicity of fIPV booster when given after two doses of fIPV in comparison with one dose IPV (Arm D vs B); 3) non-inferiority of a fIPV booster when given after one dose of IPV at 6 weeks in comparison with IPV at 14 weeks (Arm C vs B); and 4) differences in immunogenicity between three fIPV doses in comparison with two doses of IPV (Arm D vs A).
The secondary outcomes were priming, cumulative vaccine response, and median reciprocal antibody titers. Priming was defined as the absence of type-specific vaccine response at 22 weeks with subsequent evidence of response at 23 weeks. Cumulative vaccine response to one dose of IPV was defined as vaccine response at 22 weeks or priming response at 23 weeks. Assessments of priming and cumulative vaccine response were the same as previously noted but restricted to arms in which one dose of IPV was given in the primary series (i.e., no comparisons with Arm D). Median reciprocal antibody titers were calculated at the same time points of interest as vaccine response (22 and 26 weeks of age), priming (23 weeks of age), and cumulative vaccine response (22 and 23 weeks of age).
Systemic and site of injection adverse events were monitored during the course of the study. Adverse events were defined as any illness experienced by the participant during the study period. Serious adverse events were defined as death, hospitalization, paralysis or severe disability, and anaphylaxis reaction after vaccine administration. During clinic visits, parents were asked about any illness since the last clinic visit and participants were monitored for adverse events for 30 minutes immediately after vaccination. Parents were instructed to seek care immediately and contact the study clinic if their infant became ill between study clinic visits. All adverse event reports were reviewed by the principal investigator and all serious adverse event reports were shared within 24 hours to icddr,b’s institutional review board, the Data Safety and Monitoring Board, Sanavita, and CDC.
Sample Size and Statistical Analysis
The sample size for the study was calculated to address the primary objectives. A sample size of 888 was calculated (222 per arm). To account for 10% of participants who would be seropositive with antibody titers close to the upper limit of detection at baseline and 10% attrition, we calculated an enrollment target of 1,144 infants (286 per arm). To assess non-inferiority of a fIPV booster in comparison with an IPV booster among participants who have received one IPV, we used a conservative vaccine response estimate of 60% [Anand, personal communication].6 The enrollment target of 286 per arm was sufficient for non-inferiority defined as at least a 12·5% difference in vaccine response, power of 85% with a one-sided α of 0·05, and evaluated at no difference between arms under the alternative hypothesis of non-inferiority. To evaluate differences in vaccine response among infants given two fIPV in comparison with one IPV, we estimated that 80% of infants would respond after two fIPV at 6 and 14 weeks of age and 70% of infants would respond to one IPV at 14 weeks of age [Anand, personal communication].4,6 An enrollment target of 286 in Arm D (two doses fIPV) and 572 in Arms A and B combined (after one dose IPV) was sufficient to obtain 80% power with two-sided α of 0·05 to detect a statistically significant difference in immune response of at least 12·5%.
Inequality tests were done by Fisher’s exact to evaluate differences in vaccine response, and differences in priming response. Non-inferiority was assessed by comparing the lower bound of a 90% Wald Confidence Interval (CI) to the non-inferiority margin. The Kruskal-Wallis test was used to assess differences in measured antibody titer distributions among responders between study arms. Multiple comparison correction was not applied to the analyses because a-priori hypotheses were investigated at different outcome endpoints. Post-hoc analyses were performed to assess the influence of maternal antibodies. Reverse cumulative distribution function curves were used to visualize differences in antibody titers among responders with the y-axis denoting the proportion of infants with antibody titers at the corresponding x-axis and greater. Descriptive analyses (percentages and medians) were performed for baseline characteristics, adverse events, characteristics of fIPV injections, and influence of maternal antibodies. The presence of poliovirus antibody titers at baseline was assumed to represent maternal antibodies; titers ≥64 were categorized as “high” while titers <64 were categorized as “low/undetectable”. Results and conclusions by intention-to-treat and per protocol analyses did not differ; we present results from the intention-to-treat analysis and include per protocol results in the appendix. Data were analyzed in SAS (Cary NC, Version 9.4) and R (R Foundation, Version 3.3.3). This trial is registered at ClinicalTrials.gov (number NCT02847026).
Role of the funding source
The sponsor of the study participated in study design, protocol development, data analysis, data interpretation, and manuscript development. The sponsor did not participate in data collection. The corresponding author had full access to all study data, except personally identifiable information, and had final responsibility for the decision to submit for publication.
Results
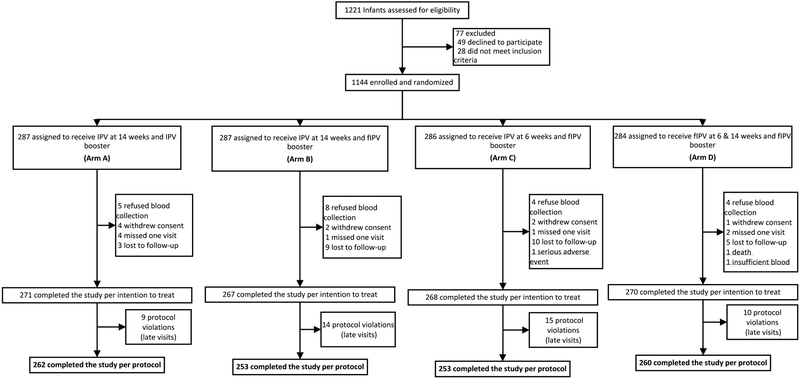
Of 1,221 parents approached for the study, 1,144 infants were enrolled from September 1, 2016 to May 2, 2017 (Figure 1). The intention-to-treat analysis included 1,076 (94%) participants. Baseline characteristics by study arm are summarized in Table 1.
Figure 1. Trial profile, Bangladesh 2016–2017.
IPV=inactivated poliovirus vaccine. fIPV=fractional inactivated poliovirus vaccine.
Table 1.
Baseline characteristics of the intention-to-treat population
| Baseline Characteristics | Arm A | Arm B | Arm C | Arm D | ||||
|---|---|---|---|---|---|---|---|---|
| IPV14+IPV booster | IPV14+fIPV booster | IPV6+fIPV booster | fIPV6/14+fIPV booster | |||||
| (n=271) | (n=267) | (n=268) | (n=270) | |||||
| Age (days) | 44 (43–47) | 44 (42–47) | 44 (42–47) | 44 (43–47) | ||||
| Male | 136 | 50% | 128 | 48% | 136 | 51% | 127 | 47% |
| Mother’s education | ||||||||
| No formal school | 48 | 18% | 48 | 18% | 45 | 17% | 45 | 17% |
| Primary | 98 | 36% | 109 | 41% | 110 | 41% | 92 | 34% |
| Middle | 62 | 23% | 66 | 25% | 63 | 24% | 73 | 27% |
| High | 46 | 17% | 34 | 13% | 40 | 15% | 45 | 17% |
| Graduate | 17 | 6% | 10 | 4% | 10 | 4% | 15 | 6% |
| Exclusive breastfeeding | 66 | 24% | 65 | 24% | 67 | 25% | 87 | 32% |
| Wasting present | 16 | 6% | 19 | 7% | 16 | 6% | 18 | 7% |
| Stunting present | 27 | 10% | 43 | 16% | 38 | 14% | 31 | 11% |
| Type 1 poliovirus | ||||||||
| Seropositive | 149 | 55% | 132 | 49% | 146 | 54% | 140 | 52% |
| Reciprocal titers | 28 | (14–91) | 28 | (14–114) | 28 | (11–114) | 36 | (14–144) |
| Type 2 poliovirus | ||||||||
| Seropositive | 165 | 61% | 139 | 52% | 139 | 52% | 149 | 55% |
| Reciprocal titers | 18 | (11–36) | 18 | (11–36) | 18 | (11–45) | 18 | (11–36) |
| Type 3 poliovirus | ||||||||
| Seropositive | 87 | 32% | 91 | 34% | 98 | 37% | 81 | 30% |
| Reciprocal titers | 18 | (11–45) | 23 | (11–57) | 23 | (11–57) | 18 | (11–45) |
Data are n (%), median (range) for age in days, or median (interquartile range) for reciprocal antibody titers among seropositive participants. Baseline measurements for participants were obtained at 6 weeks of age.
At 22 weeks of age, participants who received two fIPV (Arm D) had significantly higher (p<0.0001) vaccine response (Table 2A) and median antibody titers (Table 2B) for all serotypes than participants who received one IPV (Arms A/B). For type 1 this was 212 participants (79%, 95%CI: 73%−83%) compared with 305 (57%, 95%CI: 53%−61%)], type 2 was 173 participants (64%, 95%CI: 58%−70%) compared with 249 participants (46%, 95%CI: 42%−51%)], and type 3 was 196 participants (73%, 95%CI: 67%−78%) compared with 196 participants (36%, 95%CI: 33%−41). Median reciprocal antibody titers among those with vaccine response who received two doses of fIPV was 144 (interquartile range (IQR): 51–455) in comparison with one dose IPV which was 23 (IQR): 14–57) for type 1, 45 (IQR: 18–144) compared with 14 (IQR: 11–23) for type 2, and 91 (IQR: 36–455) in comparison with 18 (IQR: 11–57) for type 3. In assessing a potential vaccine schedule of two IPV (Arm A) in comparison with three fIPV (Arm D) at 26 weeks of age, there was a significant difference for type 1 (100% vs 98%, Table 2A). Median antibody titers among those with vaccine response were 3–6 fold higher among participants who received two IPV when compared to three fIPV (Table 2B).
Table 2A.
Summary of vaccine response for poliovirus types 1, 2, and 3 by study arms
| Vaccine response | Arm A | Arm B | Arm C | Arm D | Fisher’s Exact Test | ||||
|---|---|---|---|---|---|---|---|---|---|
| IPV14+IPV booster | IPV14+fIPV booster | IPV6+fIPV booster | fIPV6/14+fIPV booster | ||||||
| (n=271) | (n=267) | (n=268) | (n=270) | ||||||
| Type 1 | |||||||||
| Vaccine response to primary series | 164/271 | 61% (55–66%) | 141/267 | 53% (47–59%) | 101/268 | 38% (32–44%) | 212/270 | 79% (73–83%) | B v A: p = 0.08 D v A, D v B: p < 0.0001 C v B: p = 0.0005 |
| Priming response | 107/107 | 100% (97–100%) | 124/126 | 98% (94–100%) | 155/167 | 93% (88–96%) | - | - | B v A: p = 0.50 C v B: p = 0.0281 |
| Cumulative vaccine response | 271/271 | 100% (99–100%) | 265/267 | 99% (97–100%) | 256/268 | 96% (92–97%) | - | - | B v A: p = 0.25 C v B: p = 0.0118 |
| Vaccine response to booster | 271/271 | 100% (97–100%) | 264/267 | 99% (97–100%) | 255/268 | 95% (92–97%) | 264/270 | 98% (95–99%) | D v A: p = 0.0150 D v B: p = 0.50 |
| Type 2 | |||||||||
| Vaccine response to primary series | 126/271 | 47% (41–52%) | 123/267 | 46% (40–52%) | 70/268 | 26% (21–32%) | 173/270 | 64% (58–70%) | B v A: p = 0.93 D v A, C v B, D v B: p < 0.0001 |
| Priming response | 143/145 | 99% (95–100%) | 139/144 | 97% (92–99%) | 167/198 | 84% (79–89%) | - | - | B v A: p = 0.28 C v B: p = 0.0003 |
| Cumulative vaccine response | 269/271 | 99% (97–100%) | 262/267 | 98% (96–99%) | 237/268 | 88% (84–92%) | - | - | B v A: p = 0.28 C v B: p < 0.0001 |
| Vaccine response to booster | 267/271 | 99% (96–99%) | 262/267 | 98% (96–99%) | 239/268 | 89% (85–92%) | 260/270 | 96% (93–98%) | D v A: p = 0.11 D v B: p = 0.30 |
| Type 3 | |||||||||
| Vaccine response to primary series | 99/271 | 37% (31–42%) | 97/267 | 36% (31–42%) | 92/268 | 34% (29–40%) | 196/270 | 73% (67–78%) | B v A: p = 1.0 D v A, D v B: p < 0.0001 C v B: p = 0.65 |
| Priming response | 171/172 | 99% (97–100%) | 166/170 | 98% (94–99%) | 170/176 | 97% (93–98%) | - | - | B v A: p = 0.21 C v B: p = 0.75 |
| Cumulative vaccine response | 270/271 | 100% (98–100%) | 263/267 | 99% (96–99%) | 262/268 | 98% (95–99%) | - | - | B v A: p = 0.21 C v B: p= 0.75 |
| Vaccine response to booster | 269/271 | 99% (97–100%) | 261/267 | 98% (95–99%) | 257/268 | 96% (93–98%) | 266/270 | 99% (96–99%) | D v A: p = 0.45 D v B: p = 0.54 |
Data are the percentage of participants with vaccine response expressed as n/N including 95% confidence interval (CI). Vaccine response defined as seroconversion from seronegative (<1:8) to seropositive (≥1:8) after vaccination, or a four-fold rise in antibody titers after vaccination adjusted for maternal antibody decay. Priming defined as absence of vaccine response at 22 weeks with subsequent evidence of response at 23 weeks. Cumulative vaccine response defined as vaccine response at 22 weeks or priming response at 23 weeks. IPV=inactivated poliovirus vaccines. fIPV=fractional inactivated poliovirus vaccine. Fisher’s Exact test was used to test for inequality of proportions between study arms.
Table 2B.
Summary of reciprocal antibody titers to vaccination for poliovirus types 1, 2, and 3 by study arms
| Vaccine Response | Arm A | Arm B | Arm C | Arm D | Kruskal-Wallis Test | ||||
|---|---|---|---|---|---|---|---|---|---|
| IPV14+IPV booster | IPV14+fIPV booster | IPV6+fIPV booster | fIPV6/14+fIPV booster | ||||||
| (n=271) | (n=267) | (n=268) | (n=270) | ||||||
| Type 1 | |||||||||
| Vaccine response to primary series | 164 | 23 (13–57) | 141 | 23 (14–57) | 101 | 28 (14–144) | 212 | 144 (51–455) | B v A: p = 0.51 D v A, D v B: p < 0.0001 C v B: p = 0.25 |
| Priming response | 107 | ≥1448 (1152-≥1448) | 124 | 576 (288–1152) | 155 | 1152 (362-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.0057 |
| Cumulative vaccine response | 271 | ≥1448 (1152-≥1448) | 265 | 910 (455-≥1448) | 256 | ≥1448 (576-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.0026 |
| Vaccine response to booster | 271 | ≥1448 (724-≥1448) | 264 | 576 (228–1152) | 255 | ≥1448 (576-≥1448) | 264 | 288 (114–910) | B v A, D v A, D v B, C v B: p < 0.0001 |
| Type 2 | |||||||||
| Vaccine response to primary series | 126 | 14 (9–23) | 123 | 14 (11–23) | 70 | 14 (11–28) | 173 | 45 (18–144) | B v A: p = 0.17 D v A, D v B: p < 0.0001 C v B: p = 0.58 |
| Priming response | 143 | ≥1448 (910-≥1448) | 139 | 576 (228–910) | 167 | 724 (114-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.62 |
| Cumulative vaccine response | 269 | ≥1448 (1152-≥1448) | 262 | 910 (362–1152) | 237 | 910 (228-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.34 |
| Vaccine response to booster | 267 | 1152 (576-≥1448) | 262 | 362 (144–724) | 239 | 724 (228-≥1448) | 260 | 181 (72–576) | B v A, D v A, D v B, C v B: p < 0.0001 |
| Type 3 | |||||||||
| Vaccine response to primary series | 99 | 28 (11–91) | 97 | 14 (11–36) | 92 | 20 (11–144) | 196 | 91 (36–455) | B v A: p = 0.0136 D v A, D v B: p < 0.0001 C v B: p = 0.33 |
| Priming response | 171 | ≥1448 (≥1448 -≥1448) | 166 | ≥1448 (910-≥1448) | 170 | ≥1448 (724-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.70 |
| Cumulative vaccine response | 270 | ≥1448 (≥1448-≥1448) | 263 | ≥1448 (910-≥1448) | 262 | ≥1448 (910-≥1448) | - | - | B v A: p < 0.0001 C v B: p = 0.92 |
| Vaccine response to booster | 269 | ≥1448 (1152-≥1448) | 261 | 910 (455-≥1448) | 257 | ≥1448 (576-≥1448) | 266 | 455 (144–1152) | B v A, D v A, D v B: p < 0.0001 C v B: p = 0.0010 |
Data are the number of vaccine responders (n) and the median (interquartile range) of reciprocal antibody titers among vaccine responders. Vaccine response defined as seroconversion from seronegative (<1:8) to seropositive (≥1:8) after vaccination, or a four-fold rise in antibody titers after vaccination adjusted for maternal antibody decay. Priming defined as absence of vaccine response at 22 weeks with subsequent evidence of response at 23 weeks. Cumulative vaccine response defined as vaccine response at 22 weeks or priming response at 23 weeks. IPV=inactivated poliovirus vaccines. fIPV=fractional inactivated poliovirus vaccine. Kruskal-Wallis Test was used to test for inequality of antibody titer distributions between study arms.
Findings from the priming assessment show that participants who received IPV at 14 weeks of age had similar priming response (97%−100%) and cumulative vaccine response (98%−100%) to all types irrespective of IPV (Arm A) or fIPV booster (Arm B, Table 2A). However, median titers were significantly higher among those who received an IPV booster compared with fIPV booster (Table 2B) despite high overall median titers (≥256) in both groups. Participants who received IPV6+fIPV booster (Arm C) had significantly lower priming and cumulative vaccine response to types 1 and 2 than those who received IPV14+fIPV booster (Arm B). Median antibody titers of priming and cumulative vaccine response in the IPV6+fIPV arm was high (>724) for all types and was significantly higher than IPV14+fIPV (Arm B) for type 1.
Results from the booster dose analyses indicate that fIPV was non-inferior to IPV for all types following IPV at 14 weeks of age (Figure 2A). Vaccine response to IPV6+fIPV booster (Arm C) was non-inferior to IPV14+fIPV booster (Arm B) for types 1 and 3 (Figure 2B). The lower limit of the confidence interval for the type 2 difference was 12·4%. Vaccine response to IPV14+fIPV booster (Arm B) and fIPV6/14+fIPV booster (Arm D) was similar for all serotypes (>96%). Overall, antibody titers were highest among participants in IPV14+IPV booster (Arm A) and lowest among participants in fIPV6/14+fIPV booster (Arm D, Figure 3).
Figure 2. Non-inferiority assessment of vaccine response to poliovirus types 1, 2, and 3, four weeks after booster vaccination.
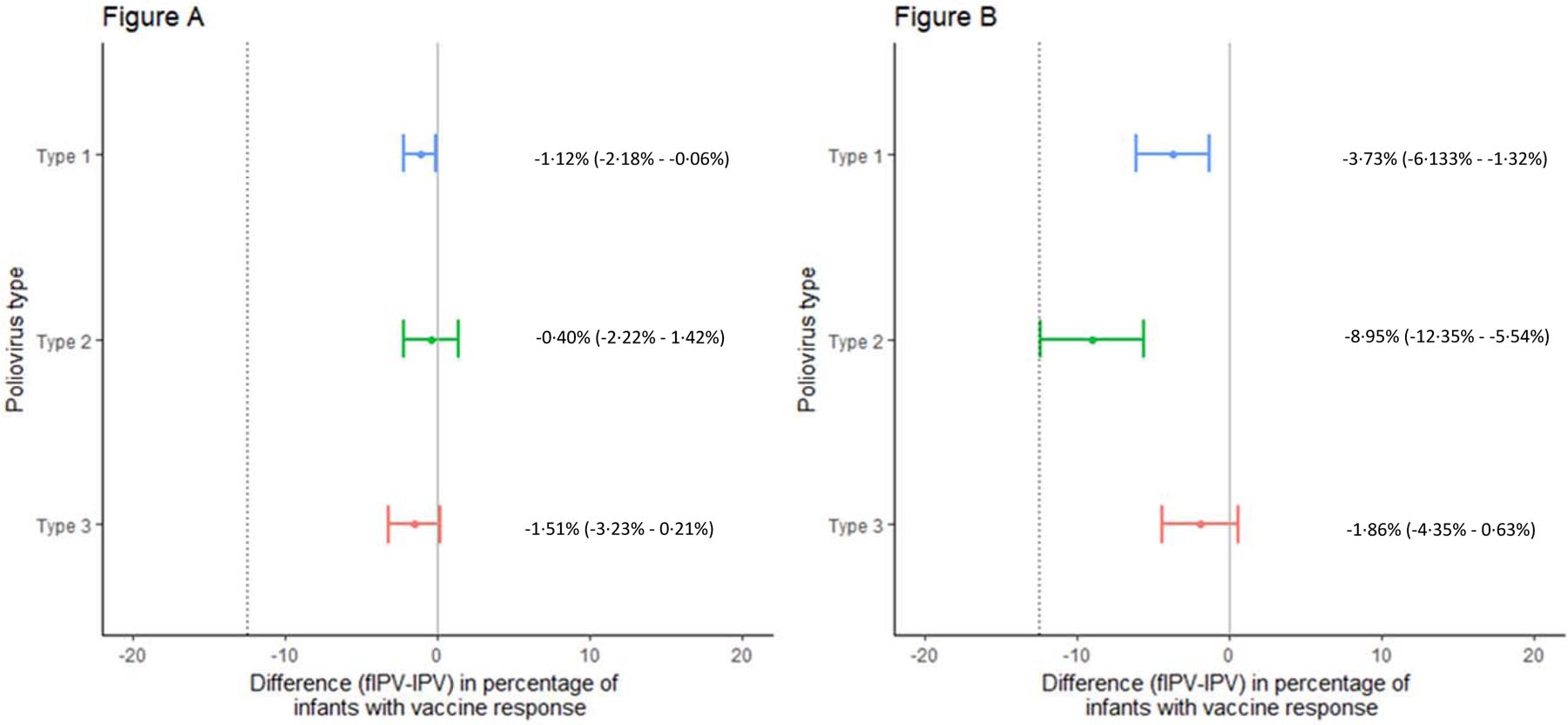
Differences in vaccine response are presented along with 90% confidence intervals around the estimated difference. The hashed line represents the non-inferiority margin defined at −12.5%. Non-inferiority is concluded if the lower bound of the 90% confidence interval falls to the right of the non-inferiority margin. (2A) IPV at 14 weeks and fIPV booster (Arm B) in comparison with IPV at 14 weeks and IPV booster (Arm A). (2B) IPV at 6 weeks and fIPV booster (Arm C) in comparison with IPV at 14 weeks and fIPV booster (Arm B).
Figure 3. Reverse cumulative distribution function curves of reciprocal antibody titers to poliovirus types 1, 2, and 3 by study arm.
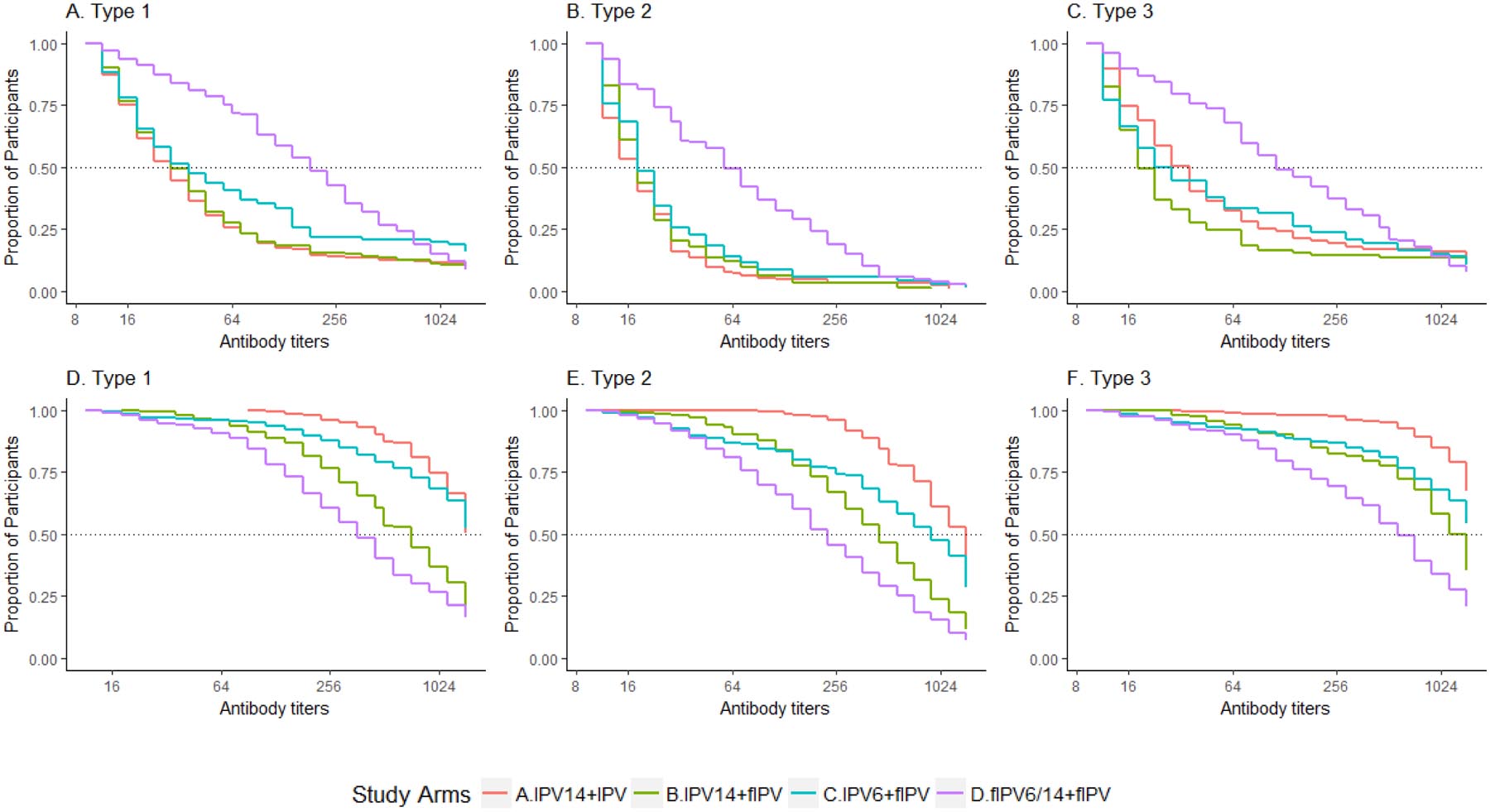
(3A-3C) Proportion of participants (y-axis) with measured reciprocal antibody titers and all greater titers (x-axis) among vaccine responders at 22 weeks of age by poliovirus type (prior to booster vaccination). (3D-3F) Proportion of participants with measured reciprocal antibody titers and all greater titers among vaccine responders at 26 weeks of age by poliovirus type (four weeks after booster vaccination).
A total of 129 adverse events were reported of which 21 (16%) were classified as serious including one death due to aspiration; none of the adverse events were attributed to use of the polio vaccines. The most commonly reported illnesses were respiratory infections (n=68), diarrhea (n=29), and dermatological conditions (i.e., cellulitis, chicken pox, scabies, and tinea capitis) (n=17). Other reported illnesses included conjunctivitis (n=5), oral thrush (n=4), respiratory and gastrointestinal co-infection (n=3), and single reports of measles and meningitis. All participants reported one adverse event except one participant who reported two.
There were 1,296 fIPV administrations during the study. The median bleb size was 8 mm (range: 4–11 mm) and 15 (1·2%) were ≤5 mm. There was no evidence of wetness (i.e., vaccine visible on the skin) among 1,267 (97·8%) injections. Of the 29 administrations with evidence of a small drop (28) or partial wetness (1), five (17·2%) were observed with bleb sizes that were small (≤5 mm).
High maternal antibody titers at baseline were associated with lower type 2 vaccine response at 22 weeks of age. Among participants who received IPV at age 14 weeks, 243 of 491 (49·5%) with low/undetectable maternal antibody titers responded compared to 6 of 47 (12·8%) with high titers. Likewise, participants who received IPV at age 6 weeks and had low/undetectable maternal antibody titers had higher vaccine response [70 of 245 (28·6%)] than those with high titers [0 of 23 (0%)]. Finally, 173 of 251 (68·9%) of participants who received fIPV at 6 and 14 weeks and low/undetectable maternal antibodies had vaccine response compared to 0 of 19 (0%) with high titers. A similar trend was not observed for types 1 and 3 (data not shown).
Discussion
Several important findings from our study can be used to inform the use of fIPV in routine immunization schedules and outbreak response. First, our study is the first to demonstrate in a direct comparison that two intradermal doses of fIPV at 6 and 14 weeks of age is more immunogenic for all types than one intramuscular dose of IPV at 14 weeks of age. Type-specific vaccine response to two fIPV was 16–36% higher when compared to one IPV and antibody titers were 5–6 times as high. Type-specific response to two fIPV in our study (64–79%) was similar to a study in Oman (69%−72%)18 but lower than a previous study in the same Dhaka community of similarly aged children (81%−89%)4 and slightly older children in Cuba (93%−98%)5. Higher type 2 response to one dose of IPV at 14 weeks was reported in studies from Bangladesh (73%) [Anand, personal communication], India (69%),6 Panama (75%),20 and Latin American countries (80%)17 compared with our study (46%). The observed differences for both fIPV and IPV groups may be due to absence of secondary exposure to type 2 because our study was conducted after OPV2 withdrawal. Other possible reasons include IPV from different manufacturers, different interval between fIPV vaccinations,5,18 baseline maternal antibodies, and different devices used for fIPV administration.
Second, participants who received IPV at 14 weeks of age had ≥98% cumulative vaccine response to all types irrespective of receiving IPV or a fIPV booster. This high cumulative vaccine response is consistent with a previous study in the same community [Anand, personal communication] and another in India.6 Participants who received IPV at 6 weeks and fIPV booster had relatively high cumulative vaccine response to types 1 and 3 (>96%) but type 2 (88%) response was ~10% lower than those who received IPV at 14 weeks and fIPV booster. This finding differs from another study in this community where type 2 cumulative response was 99% among those who received IPV at 6 weeks. However, secondary transmission from tOPV use during the latter study may have led to higher cumulative response. Another previous study in the same Dhaka community detected 14% vaccine response to type 2 among participants who only received bOPV.4 Moreover, an IPV booster was administered in that study instead of fIPV, which may have led to higher priming and cumulative vaccine response. Our finding suggests that a full dose IPV may be necessary to obtain high cumulative vaccine response if the first IPV dose is given before 14 weeks of age.
Third, a fIPV booster is non-inferior to an IPV booster for all serotypes (<2% difference) when given to individuals who have received IPV at 14 weeks of age. While type 1 and 3 responses to a fIPV booster among infants who received IPV at 6 weeks in comparison with IPV at 14 weeks were non-inferior, the type 2 response was interpreted as inferior because the lower limit of the difference (12·4%) almost reached the 12·5% non-inferiority margin. Due to the large programmatic implications of misinterpreting the result, we erred on the side of caution in our interpretation. There was no difference in vaccine response for all serotypes (>96%) when a fIPV booster was given to participants who received two fIPV compared to IPV at 14 weeks. This is the first study to assess immunogenicity of fIPV booster in OPV-naïve children who previously received a single IPV at 14 weeks of age or two fIPV doses at 6 and 14 weeks of age. The feasibility of fIPV use in campaigns was recently demonstrated in India and Pakistan using needle-syringe21,22 and a needle-free jet injector.25 All reported high vaccination coverage although issues with identifying skilled and well-trained vaccinators to administer intradermal injections with needle-syringe were noted. Devices such as the intradermal adapter used in this study and needle-free jet injectors may overcome these issues but immunogenicity of needle-free devices should be assessed if any changes to the device are made since previous immunogenicity trials as changes may unintentionally affect immunogenicity.
Finally, our evaluation of a potential future schedule of two IPV doses versus three fIPV doses found high vaccine response (>96%) to all serotypes for both schedules. While there was a significant difference observed for type 1 (p=0.02), programmatically this difference of 98% versus 100% is not meaningful. Discussions are ongoing about the introduction of a second dose of IPV in preparation for OPV cessation from routine immunization programs. For countries that have already introduced two fIPV doses, our finding provides clinical evidence indicating a schedule with three fIPV doses (6, 14, and 22 weeks) would be as immunogenic as a schedule with two IPV doses (14 and 22 weeks). Vaccine response may differ depending on the age at first dose and the time interval between vaccinations.
Interference from maternal antibodies has been reported in other studies that evaluated immunogenicity of fIPV and IPV.18,19 We observed maternal antibody interference to type 2 in all study arms when vaccine response was assessed after the primary series at 22 weeks; a similar type 2 only affect was observed in Cuba.26However, a robust type 1 response was observed despite similar prevalence and higher titers at baseline than type 2. Interference of maternal antibodies to type 1 may not have been observed due to secondary exposure to bOPV or another undetermined factor; the study in Cuba was conducted at a time of no secondary exposure to OPV.
Participants who received IPV at 6 weeks of age followed by a fIPV booster had lower levels of type 2 priming, cumulative vaccine response, and vaccine response to the booster compared to those who received IPV at 14 weeks of age. Further research on whether an IPV booster could close the immunity gap compared with fIPV booster would be informative, especially if countries are considering a schedule in which IPV is given at 6 weeks to increase overall polio vaccination coverage or to protect against VAPP when followed by bOPV.
The IPV booster led to consistently higher antibody titers when compared with the fIPV booster; other studies reported similar findings.27–29 This finding may be explained by the lower antigen content in fIPV than in IPV, which did not appear to be compensated by the enhanced immune response expected from the introduction of the antigen in the dendritic cell-rich environment of the dermis. Poor intradermal administration technique is unlikely to explain the low response given the relatively large bleb size and minimal wetness observed in our study. The implication of the lower antibody titers on individual protection is unclear. Detectable antibody titers (>1:8) are globally recognized as protective against paralysis. Antibody titers decay over time; high antibody titers after primary vaccination remain above detectable levels for a longer period than low antibody titers suggesting a longer duration of protection against paralysis.30 However, evidence suggests that immunological memory may persist even in the absence of detectable antibody titers,31 yet whether secondary response could be induced fast enough to prevent paralysis is unknown.
Our study had several strengths and limitations. It was conducted among OPV-naïve infants that allowed us to examine the effect of a fIPV booster in those who had received IPV or fIPV only. Furthermore, it was conducted after OPV2 withdrawal and provides an estimate of type 2 response that is unaffected by secondary type 2 transmission. But bOPV is still in use and secondary transmission of types 1 and 3 may have led to higher observed vaccine response. This is thought to be minimal for types 1 (5%) and 3 (3%) based on a recent study in the same population [Zaman, personal communication]. Furthermore, we believe our interpretations remain accurate because all study arms would have been equally affected. We used intradermal adapters to minimize the effect of poor injection quality on our interpretation of findings related to fIPV, which differs from use of needle-syringe only in routine immunization programs. The high percentage of good injection quality (e.g., larger bleb sizes and wetness in <3% of injections) may have led to higher percentages of vaccine response than what would be routinely observed in the field.
Our study supports SAGE’s recommendation to introduce two doses of fIPV at 6 and 14 weeks of age in routine immunization schedules. Furthermore, it supports SAGE’s recommendation to use a fIPV booster as part of outbreak response efforts to rapidly increase immunity in children previously vaccinated with IPV or fIPV, especially for type 2 outbreaks affecting cohorts with no exposure to OPV2. Use of fIPV in both situations serves as an effective dose-sparing strategy without compromising individual and population immunity.
Supplementary Material
Table 0.
Key time points for study activities by study arm
| Arm | Age of participant | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 weeks | 10 weeks | 14 weeks | 18 weeks | 22 weeks | 23 weeks | 26 weeks | ||
| A | ● | IPV | ● IPV | ● | ● | |||
| B | ● | IPV | ● fIPV | ● | ● | |||
| C | ● IPV | ● fIPV | ● | ● | ||||
| D | ● fIPV | fIPV | ● fIPV | ● | ● | |||
indicates blood collection. IPV=inactivated poliovirus vaccines. fIPV=fractional inactivated poliovirus vaccine.
Panel: Research in context.
Evidence before this study
In 2013, the Strategic Advisory Group of Experts on Immunization recommended countries introduce one dose of intramuscular inactivated poliovirus vaccine (IPV) as a risk mitigation strategy against the decline in type 2 immunity that would occur with the planned removal of type 2 in the oral poliovirus vaccine (OPV) in 2016. A global IPV supply shortage ensued as vaccine manufacturers were unable to meet the increased demand. The Strategic Advisory Group of Experts on Immunization in 2016 encouraged countries to assess the feasibility of introducing two intradermal doses of fractional IPV (fIPV) at 6 and 14 weeks of age in lieu of one dose IPV at 14 weeks of age as a means to stretch the limited global supply. This recommendation was based on evidence from indirect comparisons that suggested two fIPV doses would be more immunogenic than one IPV dose. They also recommended in October 2016 that if IPV was to be used in response activities, fIPV be used instead to rapidly protect individuals and populations based on studies in adults and older children who had been vaccinated with OPV.
We conducted a PubMed search to confirm the absence of data that would further inform the recommendations. We identified English language publications between 1 January 1990 to 15 July 2018 using the terms “fractional inactivated poliovirus vaccine”, “inactivated poliovirus vaccine”, and “intradermal”. We restricted our search to clinical trials after 1990 when enhanced IPV (current formulation) was more commonly used. We selected studies that assessed vaccine response to IPV or fIPV in the absence of OPV use, regardless of use as part of primary vaccination series or as a booster. Trials of IPV and OPV that included an IPV or fIPV only arm(s) were included in our search. Recent review articles on fIPV were also used to confirm our search strategy. We did not identify any studies that directly compared two doses of fIPV at 6 and 14 weeks of age with one dose of IPV at 14 weeks of age.
There were no data on type 2 vaccine response to a fIPV booster in OPV-naïve children who previously received IPV or fIPV.
Added value of this study
This is the first study to directly compare the recommended two dose fIPV schedule at 6 and 14 weeks of age with one dose of IPV at 14 weeks. Results indicate that two doses of fIPV led to higher vaccine response and antibody titers than one dose of IPV. This is also the first study to assess fIPV as a booster amongst OPV-naïve infants who had received IPV or fIPV only. Vaccine response to a fIPV booster was non-inferior to an IPV booster when given to infants who had received IPV at 14 weeks of age. Furthermore, a fIPV booster was able to elicit similar vaccine response among infants who previously received IPV at 14 weeks or two fIPV at 6 and 14 weeks. However, antibody titers were consistently higher among those who had received IPV as part of a primary series or as a booster.
Implication of all the available evidence
Findings from this study directly support the Strategic Advisory Group of Experts on Immunization’s recommendations to use fIPV as a dose-sparing strategy for routine immunization schedules and outbreak response. It is vital to balance IPV use between these two competing priorities. Results from this study should be used to further encourage countries that have yet to introduce IPV in routine immunization schedules, or have not received additional IPV supplies since initial introduction, to introduce fIPV to protect their population against type 2. Furthermore, fIPV should be considered for use in outbreak response campaigns if IPV is to be used, permitting vaccination of five-times more children per IPV vial than a full dose.
ACKNOWLEDGEMENTS
icddr,b acknowledges with gratitude the commitment of CDC to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden and UK for providing core/unrestricted support. We thank the study staff at the Mirpur and Mohakahli sites in Dhaka, Bangladesh; Deborah Moore, Yiting Zhang, Sharla McDonald, Will Hendley, Larin McDuffie, Mario Nicolas, Kathryn Ann Vetter Manly, and Heather Jost for processing and testing the samples at the Polio and Picornavirus Laboratory Branch in the Centers for Disease Control and Prevention; Howard E Gary Jr for design and statistical support during protocol development; and all parents and infants who participated in this study. We also thank Sanavita (formerly Helm Medical GmbH) for donating the HelmJect needle-syringe and Helm intradermal adapter used in this study.
FUNDING STATEMENT
The study was funded by the Global Immunization Division of the U.S. Centers for Disease Control and Prevention. Sanavita (formerly Helm Medical GmbH) donated the needle-syringe and intradermal adapter used in this study.
Footnotes
DECLARATION OF INTERESTS
CS’s family member previously owned stock in SanofiPasteur. All other authors declare that they have no conflict of interest.
DATA SHARING
The study is registered on the clinicaltrials.gov website (number NCT02847026) and aggregated data from Tables 1 - 3 will added to the registration with publication. In accordance with the protocol, icddr,b investigators will have access to participant data with identifiers; external investigators will have access to de-identified participant data; de-identified data may be shared with national and international vaccine manufacturers and regulatory authorities upon request; and no participant-level data will be shared further.
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
References
- 1.Adams A, Salisbury DM. Eradicating polio. Science 2015; 350(6261): 609. [DOI] [PubMed] [Google Scholar]
- 2.Aylward RB, Sutter RW, Heymann DL. Policy. OPV cessation--the final step to a “polio-free” world. Science 2005; 310(5748): 625–6. [DOI] [PubMed] [Google Scholar]
- 3.Meeting of the Strategic Advisory Group of Experts on immunization, April 2013 - conclusions and recommendations. Wkly Epidemiol Rec 2013; 88(20): 201–6. [PubMed] [Google Scholar]
- 4.Anand A, Zaman K, Estivariz CF, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine 2015; 33(48): 6816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resik S, Tejeda A, Sutter RW, et al. Priming after a fractional dose of inactivated poliovirus vaccine. N Engl J Med 2013; 368(5): 416–24. [DOI] [PubMed] [Google Scholar]
- 6.Sutter RW, Bahl S, Deshpande JM, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 2015; 386(10011): 2413–21. [DOI] [PubMed] [Google Scholar]
- 7.Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of Trivalent Oral Poliovirus Vaccine and Introduction of Inactivated Poliovirus Vaccine - Worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016; 65(35): 934–8. [DOI] [PubMed] [Google Scholar]
- 8.Nirmal S, Cherian T, Samuel BU, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to fractional doses of intradermally administered inactivated poliovirus vaccine. Vaccine 1998; 16(9–10): 928–31. [DOI] [PubMed] [Google Scholar]
- 9.Samuel BU, Cherian T, Rajasingh J, Raghupathy P, John TJ. Immune response of infants to inactivated poliovirus vaccine injected intradermally. Vaccine 1992; 10(2): 135. [DOI] [PubMed] [Google Scholar]
- 10.Samuel BU, Cherian T, Sridharan G, Mukundan P, John TJ. Immune response to intradermally injected inactivated poliovirus vaccine. Lancet 1991; 338(8763): 343–4. [DOI] [PubMed] [Google Scholar]
- 11.Salk JE. Recent studies on immunization against poliomyelitis. Pediatrics 1953; 12(5): 471–82. [PubMed] [Google Scholar]
- 12.Okayasu H, Sein C, Chang Blanc D, et al. Intradermal Administration of Fractional Doses of Inactivated Poliovirus Vaccine: A Dose-Sparing Option for Polio Immunization. J Infect Dis 2017; 216(suppl_1): S161–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH. Sixty-first World Health Assembly - Resolution and Decisions World Health Assembly; 2008 19–24 May 2008; Geneva, Switzerland: WHO; 2008. p. 1–3. [Google Scholar]
- 14.Meeting of the Strategic Advisory Group of Experts on immunization, April 2016 - conclusions and recommendations. Wkly Epidemiol Rec 2016; 91(21): 266–84. [PubMed] [Google Scholar]
- 15.Meeting of the Strategic Advisory Group of Experts on immunization, April 2017 - conclusions and recommendations. Wkly Epidemiol Rec 2017; 92(22): 301–20. [PubMed] [Google Scholar]
- 16.Anand A, Molodecky NA, Pallansch MA, Sutter RW. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule. Vaccine 2017; 35(22): 2993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asturias EJ, Bandyopadhyay AS, Self S, et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial. Lancet 2016; 388(10040): 158–69. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed AJ, AlAwaidy S, Bawikar S, et al. Fractional doses of inactivated poliovirus vaccine in Oman. N Engl J Med 2010; 362(25): 2351–9. [DOI] [PubMed] [Google Scholar]
- 19.Resik S, Tejeda A, Lago PM, et al. Randomized controlled clinical trial of fractional doses of inactivated poliovirus vaccine administered intradermally by needle-free device in Cuba. J Infect Dis 2010; 201(9): 1344–52. [DOI] [PubMed] [Google Scholar]
- 20.Saez-Llorens X, Clemens R, Leroux-Roels G, et al. Immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine in infants: a comparative, observer-blind, randomised, controlled trial. Lancet Infect Dis 2016; 16(3): 321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahl S, Verma H, Bhatnagar P, et al. Fractional-Dose Inactivated Poliovirus Vaccine Immunization Campaign - Telangana State, India, June 2016. MMWR Morb Mortal Wkly Rep 2016; 65(33): 859–63. [DOI] [PubMed] [Google Scholar]
- 22.Pervaiz A, Mbaeyi C, Baig MA, et al. Fractional-Dose Inactivated Poliovirus Vaccine Campaign - Sindh Province, Pakistan, 2016. MMWR Morb Mortal Wkly Rep 2017; 66(47): 1295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Group WMGRS. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. p. 312. [Google Scholar]
- 24.Weldon WC, Oberste MS, Pallansch MA. Standardized Methods for Detection of Poliovirus Antibodies. Methods Mol Biol 2016; 1387: 145–76. [DOI] [PubMed] [Google Scholar]
- 25.Yousafzai MT, Saleem AF, Mach O, Baig A, Sutter RW, Zaidi AKM. Feasibility of conducting intradermal vaccination campaign with inactivated poliovirus vaccine using Tropis intradermal needle free injection system, Karachi, Pakistan. Heliyon 2017; 3(8): e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuba IPVSCG. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. N Engl J Med 2007; 356(15): 1536–44. [DOI] [PubMed] [Google Scholar]
- 27.Clarke E, Saidu Y, Adetifa JU, et al. Safety and immunogenicity of inactivated poliovirus vaccine when given with measles-rubella combined vaccine and yellow fever vaccine and when given via different administration routes: a phase 4, randomised, non-inferiority trial in The Gambia. Lancet Glob Health 2016; 4(8): e534–47. [DOI] [PubMed] [Google Scholar]
- 28.Estivariz CF, Jafari H, Sutter RW, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis 2012; 12(2): 128–35. [DOI] [PubMed] [Google Scholar]
- 29.Soonawala D, Verdijk P, Wijmenga-Monsuur AJ, et al. Intradermal fractional booster dose of inactivated poliomyelitis vaccine with a jet injector in healthy adults. Vaccine 2013; 31(36): 3688–94. [DOI] [PubMed] [Google Scholar]
- 30.Vidor EPS. Poliovirus vaccine - inactivated In: Plotkin SA OW, Offit PA, ed. Vaccines. 6th ed: W.B. Saunders Co; 2013: 573–97. [Google Scholar]
- 31.Abbink F, Buisman AM, Doornbos G, Woldman J, Kimman TG, Conyn-van Spaendonck MA. Poliovirus-specific memory immunity in seronegative elderly people does not protect against virus excretion. J Infect Dis 2005; 191(6): 990–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.