Abstract
Objective.
Human Immunodeficiency Virus (HIV) is well-known to cause impairment of the human immune system, and until recently was a leading cause of death. It has been shown that T-lymphocytes are the main targets of HIV. The virus inactivates T-lymphocytes by interfering with a wide range of cellular and molecular targets leading to suppression of the immune system. The obljective of this review is to investigate to what extent microRNAs (miRNAs) are involved in HIV pathogenesis.
Methods.
The scientific literature (Pubmed and Google scholar) was searched between 1988 and 2019.
Results.
Mounting evidence has revealed that miRNAs are involved in viral replication and immune response, whether by direct targeting of viral transcripts, or through indirect modulation of virus-related host pathways. In addition, exosomes have been found to act as nano-scale carriers involved in HIV pathogenesis. These nanovehicles target their cargos (i.e., DNA, RNA, viral proteins, and miRNAs) leading to alteration of the behavior of recipient cells.
Conclusion.
miRNAs and exosomes are important players in HIV pathogenesis. Additionally, there are potential diagnostic applications of miRNAs as biomarkers in HIV infection.
Keywords: Human immunodeficiency virus, AIDS, microRNA, exosomes, pathogenesis mechanisms, diagnostic biomarkers
Introduction
Human immunodeficiency virus 1 (HIV-1) is a member of the lentiviral family of retroviruses which causes human infection, and drastically decreases the number of CD4 T-lymphocytes as the infection progresses. Consequently, the affected subjects become highly susceptible to acquired immunodeficiency syndrome (AIDS) (1–3). According to a World Health Organization report, since the discovery of the HIV/AIDS, it has been estimated that the number of patients who acquired HIV/AIDS was 70 million, of whom 35 million have died. At the end of 2017, around 36.9 million HIV-infected people were living throughout the world (4, 5).
Since inflammation and dysfunction of immune response are hallmarks of chronic untreated HIV disease, this could be a cause of serious non-AIDS events (SNAEs), and various clinical sequelae that afflict AIDS patients (6, 7). There are some reports have documented the effect of early anti-retroviral therapy (ART) on the development of inflammatory diseases in AIDS. The impact of early ART on markers of inflammation is less clear. Early ART has been related to a significant decrease in the frequency of latently infected cells, which is more pronounced if ART is initiated within days to weeks (rather than months) following infection. Although early ART can potentially decrease serious non-AIDS events (such as inflammatory end-stage organ disease) and related mortality, longer prospective studies with clinical endpoints are still required to determine the benefits of early ART (8–12).
Due to the success of ART, HIV has transitioned into a more long-term chronic disease in most countries, where the previous serious effects of AIDS are not now a major concern (13). Instead of addressing acute immune suppression that threatens patients’ lives, clinical professionals now manage persistent disease, which may continue for several years. HIV care now requires clinicians and health care organizations to change from focusing on acute care, to long-term management (13). Clinical professionals not only require to be experts in anti-retroviral control, but also require additional skills for the prevention and management of cardio-vascular disease, and other co-morbidities related to aging. Bio-medical studies should also provide new approaches in this regard (13). One of the high priorities for handling HIV in the long term, is to understand the reasons for the persistent inflammation arising during ART, and how it results in morbidity and additional health problems. Moreover, there should affordable methods for preventing non-transmissible diseases and tuberculosis (TB) in populations who live in regions lacking robust care health systems (13).
MicroRNAs (miRNAs) are small non-coding RNAs that have been implicated in the causation and progression of a wide range of different diseases, and have recently been proposed as therapeutic targets in cancer, infections, cardiovascular disease, and diabetes (14–19). miRNAs in serum (circulating miRNAs) or in PBMC of HIV-1 infected patients can play a role in the progress of HIV-1 infections, by modulating HIV-1 proteins or affecting HIV-1 replication-associated host parameters (20). Several different miRNAs may inhibit the replication HIV-1; however they are not expected to influence the integration of viral DNA. Although viral protein expression may be reduced or blocked, HIV-1 latency could still be observed, which is one of the big barriers for treatment of HIV-1 infections (20). Integrated pro-virus within the HIV-1 latency reservoir, is able to be reactivated under proper stimulation leading to HIV-1 recurrence. This situation suggests the possible involvement of cellular miRNAs in establishing HIV-1 latency. Promising strategies for clearing the viral reservoir could result from studies on the mechanism of how miRNAs influence viral protein expression (20).
Lipid membrane particles or vesicles with nanometer dimensions are widely released by mammalian cells. These particles contain different cargos related to their cells of origin, including nucleic acids and proteins (21). These cell-secreted vesicles have been shown to important in inter-cellular communication. There is still uncertainty concerning the terminology and precise dimensions of different vesicles secreted by cells. These vesicles have been called exosomes, extra-cellular vesicles, oncosomes, micro-vesicles, and so forth (22). In this review, they are called exosomes for clarity.
Numerous definitions that have been provided for cell-derived vesicle subtypes have led to significant overlap with some properties of viruses. In practice, cell-related vesicles and viruses can both play a role in inter-cellular communication by traveling in the circulation, attaching to, and being taken up by cells, and delivering their cargo to the target or recipient cells (23). There are similarities between viruses and exosomes in terms of function and composition, which is caused by their overlap in biogenesis and functions. In the infected cells, exosomes and viruses are generated at the same time, and result in the incorporation of viral-derived materials into exosomes. Such considerations lead to difficulties in differentiation and separation of these two types of particles. It is necessary to better distinguish between viruses and exosomes in biological specimens. Purification and isolation procedures should be designed to distinguish between viruses and exosomes, but even recent separation protocols have disadvantages, which can affect the conclusions (24). Due to varying specific features of exosomes, the absence of a global marker, and the possibility that exosomes can contain viral materials, validation of homogenous purification from a heterogenous population is still problematic.
In the current review, we summarize the role of microRNAs and exosomes in the pathogenesis of HIV infection. Moreover, we highlight the potential diagnostic roles of microRNAs and exosomes in HIV patients.
MicroRNAs and Exosomes: Insights into HIV pathogenesis
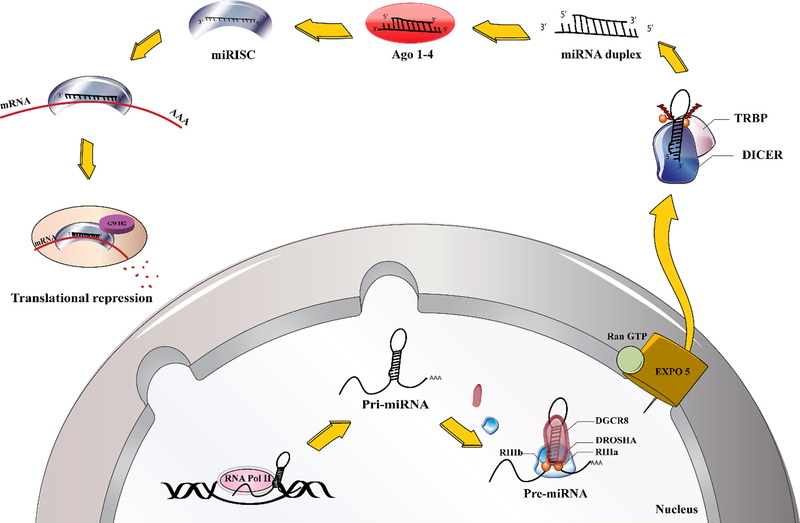
miRNAs belong to the class of non-coding RNAs, and are about 19–25 nucleotides in length. They are generated from endogenous primary miRNA precursors by RNA polymerase II acting on genomic DNA sequences. Primary miRNAs are catalyzed and processed into single-strand mature miRNAs by two ribonucleases (RNase) III enzymes, “Drosha and Dicer” (Figure 1) (25).
Figure 1.
microRNA biogenesis
One role of miRNAs may be to control virus propagation and replication. For instance, the regulatory effects of miRNAs on the propagation of viral infection have been recently demonstrated. Furthermore, some viral miRNAs encoded by viral genomes are expressed within host cells and play a role in the cell cycle and cellular outcomes of infection (26).
While several viruses have been shown to encode for viral miRNAs, controversy persists over whether a functional miRNA is encoded in the HIV-1 genome (27). However, it has been reported that HIV-1 infectivity is influenced by cellular miRNAs. Either through directly targeting the viral genome, or by targeting the host cellular proteins required for successful virus replication, multiple cellular miRNAs may modulate HIV-1 infection and replication. Perhaps as a survival strategy, HIV-1 may modulate proteins in the miRNA biogenesis pathway to subvert the miRNA-induced antiviral effects (27).
miRNAs regulation is anchored on genomic information processing on four scenarios that may possibly explain the confounded nature of their effects in virus-infected host systems. First, HIV-1 infection alters host miRNAs networks to initiate successful viral invasion and latency, thus, affecting global host microRNA regulome. Second, HIV-1 miRNAs are produced from both sense and antisense transcripts to target either its own viral transcript or host genes for immune compromise. Third, the host miRNAs systems may consequentially target the HIV-1 genomic elements or its genes to innate immune responses. Fourth, the interplay of miRNAs and target mRNA between host and HIV-1 can be organized into regulatory modules (cis-and trans- regulation) of essential biochemical pathways as critical determinants of host cell fate and survival.
It has also been reported that viral protein Tat, by targeting PTEN, up-regulates the expression of miR-21 and miR-222 contributing to apoptosis resistance in CD4+ T cells infected by HIV-1 (28). In addition, some miRNAs such as miR-29a, miR-128, miR-28, and miR-223 can lead to a decrease in HIV replication by targeting the HIV 3’-UTR region, PTEN, and the 3’-end of HIV mRNA, respectively (29–31). Thus, miRNAs can not only facilitate viral replication, but also promote disease progression. On the other hand some other miRNAs can decrease or inhibit the virus life cycle.
In general, HIV-1 infection may be affected by miRNAs via several mechanisms. Anti-HIV-1 miRNAs inhibits HIV-1 activity via CCR5 or CXCR4, auxiliary receptors for HIV-1, or target HIV-1 directly through env, pol, gag, vif, and tat genes of HIV-1 genome (32). Hariharan et al. applied a consensus scoring technique, and demonstrated that miR-29a and miR-29b targeted nef, miR-149 targeted vpr, miR-378 targeted env, and miR-324–5p targeted vif inside the HIV-1 genome (33). Huang et al. found that miR-28, miR-125b, miR-150, miR-223, and miR-382–5p targeted 3′UTR region of HIV-1 mRNA in the cells, which declines CD4+ T-cell actuation during the resting time (34). Monocytes have numerous anti-HIV-1 miRNAs, which may be down-regulated in macrophages after differentiation, and could render macrophages more vulnerable to HIV-1 infection. Inhibiting such miRNAs enhanced the susceptibility of mononuclear cells to HIV-1 infection (34). The 3′UTR region of the HIV-1 RNA genome has been identified as the target of miR-196b and miR-1290 (35). Suppressing these 2 miRNAs may result in the activation of latent HIV-1, which could lead to clearance of the latent viral reservoirs through virus-generated cytolysis and the host anti-viral immune response generated in the presence of ART (35, 36).
It can be concluded that the reason for the low number of validated HIV-1 encoded miRNAs in the miRBase database, reflects difficulties in their detection, and makes them among the least described virus-induced miRNAs (37). The reason for this low number may be due to the small genome size of HIV-1, or to low levels of expression that cannot easily be detected by common biochemical methods. Therefore, improved procedures for detection are needed (38). A previous study estimated that retroviral miRNAs comprise just 0.5% of the total miRNAs that can be detected in cells infected with HIV-1 (39). Additionally, the biogenesis of viral miRNAs could be limited due to the poor accessibility of the primiRNAs to nuclear miRNA processing machinery, and natural destabilization (37). Moreover, numerous studies have reported that Dicer or Drosha can carry out endonucleolytic destruction of viral RNA genomes, which will eventually reduce the production of viral miRNAs (40).
Nonetheless, the emergence of technologies with higher sensitivity such as next generation sequencing, RNAse protection assays (RPA), and improved computational power could play a role in the discovery of novel HIV-1-derived miRNAs. Recently a pyro-sequencing approach suggested that not less than 40% (or 125) of the candidate HIV-1 miRNAs originated from the TAR, RRE and nef regions, and constituted the majority of non-coding RNAs in cells infected with HIV-1 (41). Deep sequencing studies have further confirmed these findings, since it has been reported that HIV-1 miRNAs resulting from the structured regions of the genome, facilitate Drosha and Dicer mediated RNA processing (42).
It seems likely that interactions between HIV-1 miRNAs and the targeted mRNAs function as a regulator of the viral genome. More investigation is needed to confirm additional functionality of HIV-1 miRNAs, such as targeting host cellular transcripts for immune evasion (43).
Exosomes are biological nanovehicles, which are characteristic of many pathological and physiological processes (44, 45). Nearly all the different types of mammalian cells release exosomes into the extracellular environment (46) and they have been found to be abundant in several biological fluids, such as, blood, saliva, breast milk, semen and urine (47–53). Exosomes possess lipid-bilayer membranes, and have a roughly spherical shape (54). The contents of the exosomes depend on the cell type from which they were formed by a budding process, and on the condition of the host cells (e.g. virally infected or cancer cells) (55–57). Exosomes normally contain a range of different molecules, such as nucleic acids (DNA, RNA, mRNA, viral genome and microRNAs), annexins, tetraspanins (i.e., CD9, CD63, CD81, CD82), enzymes, cytoskeletal proteins, MHC molecules, signal transduction proteins and heat shock proteins (54, 58). It has been proposed that exosomes are involved in cell-cell communication. By regulation of cell signaling, as well as the ability to be taken up by targeted cells, they are thought to play significant roles in intercellular communications via interactions with membrane receptors (46, 59). Moreover, exosomes have the ability to transfer miRNAs into target cells, and may take part in intracellular communication by the repression of target mRNAs in recipient cells. Consequently, after merging with target cells, the viral and cellular miRNAs delivered by exosomes alter the levels of gene expression, by inhibition of mRNA translation (60). There are several studies that show that exosomes contain HIV proteins and fragments of the HIV genome (61, 62). Furthermore, as has been shown in HIV-infected patients, exosomes also contain HIV-derived transactivating responsive (TAR) RNA which can inhibit apoptosis by decreasing the expression of pro-apoptotic proteins, such as Cdk9 and Bim (61). Recently, several studies have suggested that exosomes isolated from the semen of healthy men, and from the breast milk of healthy women suppressed HIV infection (62).
HIV Long-Term Non-Progressors and microRNAs
To control viral replication, the majority of HIV-infected patients require long-term antiretroviral therapy (ART). However, approximately 1% to 5% of affected individuals (which are called long-term non-progressors (LTNPs) can control their HIV infection for more than 7 years without receiving ART (63, 64). Elite controllers (ECs) and viremic controllers (VCs) have been identified as small subsets of patients with HIV infection (65, 66). Without ART, the viral load in ECs can reach an undetectable level (<50 copy/ml) and the CD4-cell count remains high (200 to 1000/μl) (67–70). However, the viral load of VCs usually lies between 200 and 2000 copies/ml in the absence of ART. Viremic progressors (VPs) are HIV-positive patients who have high levels of viral load and progress towards AIDS if they do not receive ART (71, 72). The exact mechanism of this LTNP phenomenon has not yet been completely elucidated (73). The effects of miRNAs on HIV replication have been reported (74–76), but their roles in ECs have not been adequately studied. However, some studies have shown deregulated expression of some miRNAs in ECs compared to non-infected controls (77).
In 2012, Witwer and colleagues conducted an investigation to evaluate the expression profile of miRNAs, and their correlation with viral load versus number of CD4+ T-cell in three groups (uninfected controls, untreated viremic patients, and ECs). They found that in both ECs and viremic patients, miR-125b and miR-150 were significantly down-regulated. Also, there was a negative correlation between miR-181b and CD4 counts, while the correlation between miR-150, miR-31 and miR-29a was remarkably positive; however, no significant rcorrelation was observed between miRNAs expression and viral load (78). Reynoso and colleagues reported that, in plasma obtained from ECs, the expression of miR-29b-3p, miR-146a-5p and miR-33a-5p was up-regulated in comparison with chronically HIV-infected patients. In addition, up-regulation of miR-33a-5p and miR-29b-3p correlated with a notable decrease in viral production in primary CD4+ T cells and MT2 cells (69). In another study, Egana-Gorrono et al., screened 286 miRNAs in phyto-hemagglutinin-stimulated PBMCs from 29 individuals divided into four groups: ECs, VPs, patients receiving ART, and uninfected individuals, using TaqMan low-density arrays (TLDA). The results showed that the expression pattern of23 miRNAs was significantly different between ECs and VPs (Table 1). Next, all the subjects were divided into two groups and the alteration of mRNA expression levels were analyzed in two blocks (block 1: ECs and uninfected individuals; and block 2: viremic-progressors and patients receiving ART). The outcomes of their analysis confirmed that miR-27a, −27b, −29b and −221 were up-regulated in block 1, and the expression of the miRNAs was down-regulated in block 2. In addition, 19 other miRNAs were down-regulated in block 1, while they were up-regulated in block 2 (77). Overall, the assessment of the altered expression patterns of miRNA in ECs, which was similar to non-infected subjects, and different from chronically HIV-infected patients, could provide useful information to identify novel and prognostic biomarkers for predicting HIV disease progression towards AIDS, and could contribute to anti-HIV drug discovery efforts.
Table 1:
Expression of Long term non-progressor (LTNP) microRNAs and Viramic progressors microRNAs
| microRNA | EC/VC/VP | Expression | Method | Matrial (Plasma, PBMC, T cell) | Sample (n) | Note | Ref |
|---|---|---|---|---|---|---|---|
| miR-16 | EC and untreated viremic patients | Up | TaqMan low-density arrays (TLDA), qPCR | PBMC | 13 (EC+VC) | (78) | |
| miR-22 | EC and untreated viremic patients | Up | TLDA | PBMC | 13 (EC+VC) | (78) | |
| miR-155 | EC and untreated viremic patients | Up | qPCR | PBMC | 13 (EC+VC) | (78) | |
| miR-34a | Untreated viremic patients | Up | qPCR | PBMC | 6 | Significantly correlated with CD4 counts only in elite controllers | (78) |
| miR-21 | Untreated viremic patients | Up | qPCR | PBMC | 6 | (78) | |
| miR-27a | Untreated viremic patients | Up | qPCR | PBMC | 6 | (78) | |
| miR-181b | Untreated viremic patients | Up | Nanostring, qPCR | PBMC | 6 | Negatively correlated with CD4 counts | (78) |
| miR-181d | Untreated viremicpatients | Up | Nanostring | PBMC | 6 | (78) | |
| miR-155 | Untreated viremic patients | Up | Nanostring | PBMC | 6 | (78) | |
| miR-9 | Untreated viremic patients | Up | Nanostring | PBMC | 6 | (78) | |
| miR-29b-3p | EC | Up | Real-time qPCR | Plasma | 18 (9 EC+9 CH) | The expression level of miR-29b-3p was higher in plasma from ECs than chronic HIV progressors (CH). | (69) |
| miR-33a-5p | EC | Up | Real-time qPCR | Plasma | 18 (9 EC+9 CH) | The expression level of miR-33a-5p was higher in plasma from ECs than CHs | (69) |
| miR-146a-5p | EC | Up | Real-time qPCR | Plasma | 18 (9 EC+9 CH) | The expression level of miR-146a-5p was higher in plasma from ECs than CHs. | (69) |
| miR-221 | EC | Up | TLDA | PBMC | 8 | Upregulated in Ec and uninfected individuals while downregulated in VCs and patients under ART | (77) |
| miR-27b | EC | Up | TLDA | PBMC | 8 | Upregulated in Ec and uninfected individuals while downregulated in VCs and patients under ART | (77) |
| miR-29b | EC | Up | TLDA | PBMC | 8 | Upregulated in Ec and uninfected individuals while downregulated in VCs and patients under ART | (77) |
| miR106a | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| mir-155 | EC and VC | Up | Microarray | CD8+T- cells | 30 (EC+VC) | Upregulated mir-155 expression when resting CD8+ T-cells were stimulated | (66) |
| miR-140 | VP | Up | TLDA | CD8+ T-cells | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-146a | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-4484 | EC, VC and VP | Up | Microarray | CD8+T- cells | 43 (EC+VC+VP) | Upregulated mir-4484 expression when resting CD8+ T-cells were stimulated | (66) |
| miR-125a | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-484 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-590 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-17 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-146b | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-155 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-16 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-186 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-27a | EC | Up | TLDA | PBMC | 8 | Upregulated in Ec and uninfected individuals while downregulated in Vcs and patients under ART | (77) |
| miR-191 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-197 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-339 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-374 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-200b | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-200c | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-422 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-454 | VP | Up | TLDA | PBMC | 8 | Upregulated in VCs and patients under ART while Downregulated in ECs and uninfected individuals | (77) |
| miR-125b | Untreated viremic patients | Down | TLDA | PBMC | 6 | (78) | |
| Let-7g | Untreated viremic patients | Down | Nanostring, TLDA | PBMC | 6 | (78) | |
| miR-146b-5p | Untreated viremic patients | Down | Nanostring, TLDA | PBMC | 6 | (78) | |
| miR-150 | Untreated viremic patients | Down | Nanostring | PBMC | 6 | Considerably positively correlated with CD4 counts | (78) |
| miR-150 | EC and untreated viremic patients | Down | qPCR | PBMC | 13 (EC+VC) | Considerably positively correlated with CD4 counts | (78) |
| miR-29a | Untreated viremic patients | Down | Nanostring, TLDA, qPCR | PBMC | 6 | Considerably positively correlated with CD4 counts | (78) |
| miR-29b | Untreated viremic patients | Down | Nanostring | PBMC | 6 | (78) | |
| miR-29c | Untreated viremic patients | Down | Nanostring | PBMC | 6 | (78) | |
| miR-221 | VP | Down | TLDA | PBMC | 8 | Downregulated in VCs and patients under ART while upregulated in ECs and uninfected individuals | (77) |
| miR-27a | VP | Down | TLDA | PBMC | 8 | Downregulated in VCs and patients under ART while upregulated in ECs and uninfected individuals | (77) |
| miR-27b | VP | Down | TLDA | PBMC | 8 | Downregulated in VCs and patients under ART while upregulated in ECs and uninfected individuals | (77) |
| miR-29b | VP | Down | TLDA | PBMC | 8 | Downregulated in VCs and patients under ART while upregulated in ECs and uninfected individuals | (77) |
| miR106a | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-125a | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-140 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-146a | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-146b | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-155 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-16 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-17 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-186 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-191 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-197 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-200b | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-200c | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-339 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-374 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-422 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-454 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-484 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-590 | EC | Down | TLDA | PBMC | 8 | Downregulated in ECs and uninfected individuals while Upregulated in VCs and patients under ART | (77) |
| miR-4734 | VP | Down | Microarray | CD8+ T-cells | 13 | Downregulated miR-4734 in VPs Compared with uninfected individuals | (66) |
| miR-4505 | EC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-4505 in ECs Compared with uninfected individuals | (66) |
| miR-4505 | VC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-4505 in VCs Compared with uninfected individuals | (66) |
| mir-4508 | EC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-4508 in ECs Compared with VP | (66) |
| miR-3620 | EC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-3620 in ECs Compared with VP | (66) |
| miR-4492 | EC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-4492 in ECs Compared with VP | (66) |
| miR-4507 | EC | Down | Microarray | CD8+ T-cells | 15 | Downregulated miR-4507 in ECs Compared with VP | (66) |
| miR-181a | VP | Down | Microarray | CD8+ T-cells | 13 | Downregulatedmir-181a expression when resting CD8+ T-cells were stimulated | (66) |
| let-7b-5p | VP | Down | Microarray | CD8+ T-cells | 13 | Downregulated let-7b-5p expression when resting CD8+ T-cells were stimulated | (66) |
| miR-150 | VC | Down | Microarray | CD8+ T-cells | 15 | Downregulated mir-150 expression when resting CD8+ T-cells were stimulated | (66) |
| miR-1202 | VC | Down | Microarray | CD8+ T-cells | 15 | Downregulated mir-1202 expression when resting CD8+ T-cells were stimulated | (66) |
Egaña-Gorroño et al. evaluated the differential miRNA profile in CD8+ T cells between patients infected with HIV, who had differences with regard to control of viral replication and immune response (79). They reported down-regulation of miRNA when comparing samples from elite suppressors (ES), ART-treated, and viremic HIV-infected groups, and showed that hsa-miR-4492 had the highest down-regulation. Even though miRNA down-regulation was more pronounced when comparing stimulated CD8+ T cells with their resting counterparts, viremic patients (VP) still exhibited a differential miRNA expression pattern. Indeed, hsa-miR-155 and hsa-miR-181a were down-regulated in VP, while up-regulation or no difference was found after stimulation in the other groups. In general, functional enrichment analysis showed that the expected target genes contributed to activation of signal transduction pathways, metabolic modulation, apoptosis, and immune responses (79).
Examination of the miRNA profile in patients infected with HIV at various stages of the infection might show a dysregulated miRNA pattern with diagnostic and prognostic value for HIV-1 treatment (79). Moreover, the differential modulation of miRNAs in CD8+ T-lymphocytes might be helpful in further understanding the basic mechanisms of host anti-viral responses.
So far, researchers have not observed a relationship between level of miRNA expression and resistance of the resting memory CD4+ T cells to HIV-1 infection. In comparison with activated CD4+ T cells, resting memory CD4+ T cells exhibited up-regulation of five miRNAs: miR-28, miR-125b, miR-150, miR-223, and miR-382, which negatively target the 3′-ends of HIV-1 mRNAs (34). For this reason, in resting CD4+ T cells infected with HIV-1 (infected clones or isolated from cART-treated HIV-1 patients) knock-down of miRNAs using antisense suppressors increased the generation of viral proteins and virions. Moreover, over-expression and knockdown studies suggested there was a negative relationship between miR-125b expression and HIV-1 infection in a T cell line (80). Additionally, specific miRNAs apparently indirectly regulate HIV-1 infection in resting CD4+ T cells, via regulation of the expression of cellular co-factors. Levels of cyclin T1 protein, which is critically involved in the viral Tat-mediated trans-activation of HIV-1 LTR-driven gene expression, were up-regulated, regardless of transcript levels, when the resting CD4+ T cells were activated (81). It is interesting that up-regulation of cyclin T1 was followed by a considerable down-regulation of a group of miRNAs, including miR-27b, miR-29b, miR-150, and miR-223 in activated CD4+ T cells. Researchers confirmed this observation via over-expression or depletion of miRNAs, which decreased or enhanced the cyclin T1 protein levels, respectively. Nonetheless, it was found that only miR-27b directly modulated the expression of cyclin T1, while miR-29b, miR-150, and miR-223 had an indirect impact on the levels of cyclin T1.
Monocytes do not allow HIV-1 replication; however, they are susceptible to infection upon differentiation into monocyte-derived macrophages (MDM) or monocyte-derived dendritic cells (MDDC). Although multiple mechanisms have been proposed for the post-entry restriction of HIV-1 in monocytes, some reports suggested a relationship between levels of miRNA expression and the resistance of monocytes to HIV-1 infection. Wang et al. (82) observed that monocytes expressed higher levels of cellular miRNAs, miR-125b-5p, miR-28–5p, miR-150–5p, miR-223–3p, and miR-382–5p. Huang et al. (34) reported that these miRNAs inhibited HIV-1 replication in resting CD4+ T cells. Since knock-down of these miRNAs in monocytes increased HIV-1 infection, and miRNA over-expression in the MDMs suppressed HIV-1 replication, researchers suggested that levels of miRNA expression could define the susceptibility of monocytes or MDMs to HIV-1. Figure 2 illustrates the various miRNAs involved in HIV pathogenesis.
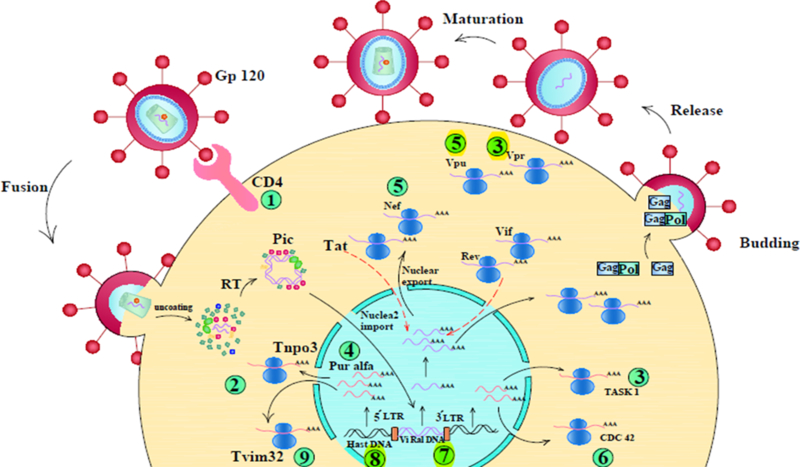
Figure 2. A schematic of microRNA networks affected by HIV virus.
(1) Up-regulation of miR-221 & 222 blocks CD4 receptor and inhibits entry of virus into the cell. (2) Up-regulation of miR-128 represses nuclear import of the virus. (3) Down regulation of miR-34a enhances release of virus and increases vpu activity. (4) Up-regulation of miR-20a decreases tat activity, inhibits binding of pur α to the LTR promoter, and represses virus replication in monocytes. (5) Up-regulation of miR-223 represses nef, vpr and blocks apoptosis in non-infected CD4 T-cells; miR-n367 decreases nef protein and virus virulence. (6) Down-regulation of miR-29 a/b increases cdc42 expression and promotes apoptosis in CD4 T-cells, (7) Up-regulation of miR-H3 interacts with the tata box in 5́ LTR, enhances promoter activity and increases RNA transcription and protein expression. (8) Up-regulation of miR-TaR3p decreases virus replication via targeting the TaR element in the 5́ LTR. (9) Up-regulation of miR-155 maintains the virus in the latent phase and reduces Nf-κβ signaling pathway
HIV-encoded miRNAs
Despite the efforts that have been made to find a permanent cure for HIV/AIDS, the HIV virus unfortunately uses a range of strategies to escape recognition and elimination by the host immune system (83, 84). These strategies depend on viral products which mimic some host cell-specific components to evade immune recognition (85). It has been revealed that the virus and viral proteins are able to encode small non-coding RNAs (miRNAs) leading to manipulation of cellular and viral transcripts contributing to the proliferation and infectivity of the virus, as well as actively curtailing the host immune responses against the virus (86). Recently, it has been demonstrated that both non-coding and coding region of the HIV genome can produce miRNAs, which regulate both host and viral gene expression (Table 2). The essential functions of viral mi RNAs (vmiRNAs) have not yet been addressed in depth (87, 88). Several studies have indicated that vmiRNAs originate from the trans-activation response (TAR) and negative regulatory factor (Nef) proteins of HIV(89–92). Klase et al. showed how to isolate vmiRNAs (TAR-miR-3p and −5p) from TAR, which protect the HIV-infected cells against apoptosis through decreasing ERCC1 and IER3 gene expression involved in apoptosis (91). The results suggested that TAR-miRNA shelters HIV-infected cells from programmed cell death by down-regulation of cellular genes implicated in apoptosis (91). You et al. reported that by targeting the PABPC4 in lymphokine cells, miR-N367 could suppress the expression of lymphokine mRNA, and also contribute to the maintenance of viral latency (93). Furthermore, Omoto and colleagues demonstrated that Nef-derived miRNAs were produced persistently in HIV infected cells. Their results showed that miR-N367 inhibited Nef expression, as well as transcription of the long terminal repeat (LTR), possibly reducing HIV transcription (92). The miR-H3 sequence is embedded in the HIV reverse transcriptase coding region, which is highly conserved between HIV subtypes. This provides possibilities for miR-H3 to target the HIV TATA box at 5′-LTR, leading to the activation of viral promoter transcription. Subsequently, up-regulation of miR-H3 can result in massive production of the virus; however, mutations within miR-H3 sequence significantly decrease the replication of wild-type HIV viruses (94). The production of the antagonizing transcription factor (AATF) has a role in DNA damage, cell cycle, transcriptional regulation, and in apoptosis. Thus, it is possible that, by silencing the AATF gene, apoptosis induction would increase (95). Kaul et al. reported that miR-H1 down-regulates AATF gene expression and induces apoptosis in infected cells, caused by vmiR-TAR. Consequently, miR-H1 decreases the expression of miR-149 which has been identified as a target-transcript of HIVVpr (96).
Table 2:
HIV-encoded miRNAs
| vmiRNA | Target | Function | Ref |
|---|---|---|---|
| miR-N367 | Nef | Block HIVNef expression in vitro (long term non-progressors) miR-N367 corresponding to suppression of Nef expression in vivo (Inhibition of Nef expression by siRNA/miR-N367 in mice) |
(92) |
| miR-N367 | PABPC4 (iPABP) | MiR-N367 might repress the activation of lymphokine mRNA expression through targeting PABPC4 to help maintain viral latency. | (93) |
| HIV-1-miR-H1 | AATF | Induced AATF gene knockdown within human mononuclear cells initiates their apoptosis. | (96) |
| miR-H3 | HIV 5′ LTR | Interacts with the TATA box in HIV 5′ LTR miR-H3 upregulates HIV RNA transcription and protein expression. |
(94) |
| vmiR99 | Stimulated human macrophage TNFα release | (109) | |
| vmiR88 | Stimulated human macrophage TNFα release | (109) | |
| vmiR-TAR | IER3, ERCC1, Caspase 8, Ikaros, Aiolos | Protects infected cells from apoptosis | (91, 109) |
| vmiRNA#1–5 | - | Proteins involved in, for example, signal transduction, protein synthesis, and degradation, DNA methylation | (87) |
| miR-TAR-3p | TAR (HIV) | Inhibitory effect on viral replication | (101) |
Studies have shown that specific families of viruses encode certain miRNAs that are commonly called viral miRNAs or vmiRNAs. These vmiRNAs affect viral replication (97), and are an important factor in the activity of DNA viruses (98, 99). Although most RNA viruses do not encode for vmiRNAs, the question of whether retro-viruses (and specifically HIV-1) do or do not encode for any functional miRNA(s) is still under dispute (100). Another member of the Retroviridae family, called bovine leukemia virus clearly does encode for a functional vmiRNA (101). Hence, researchers have argued that retroviruses that have access to both the nuclear and cytoplasmic miRNA processing machinery may indeed express vmiRNAs.
A recently conducted study used bio-informatic prediction algorithms, and suggested that the HIV-1 genome might encode for five candidate vmiRNAs (102). Reports showed that the HIV-1 Nef coding region contained specific vmiRNAs (Nef-U3-miR-N367) (103). Additionally, the 3′ end of HIV-1 RNA encodes for a vmiRNA (HIV-1-miR-H1) (104). HIV-1 trans-activation RNA (TAR) that attaches to the HIV-1 protein Tat, and modulates viral translation, encodes for a vmiRNA (known as TAR-miR-5p & 3p) (105, 106). Although the above studies demonstrated the presence of vmiRNAs inside the HIV-1 RNA genome, other studies reported contrary findings, and could not demonstrate vmiRNA expression post-HIV-1 infection. Another recent study used sensitive deep sequencing technology to show that HIV-1 did not express any functional vmiRNAs in infected cell lines, primary peripheral blood mono-nuclear cells (PBMCs), and in primary macrophages (107). Moreover, the researchers conducted photo-activatable, ribonucleoside-induced cross-linking, togther with immuno-precipitation (PAR-CLIP) assays, and provided evidence implying that HIV-1 genome would not be likely to be targeted by cellular miRNAs (107). Zhang et al. showed the presence of a new vmiRNA encoded by HIV-1 and known as miR-H3 (108). They found that miR-H3 was located in the region of the HIV-1 RNA genome, which encodes for reverse transcriptase, and attached to the TATA binding sites on HIV-1 5′LTR to increase viral transcription (108).
Contradictory findings regarding whether vmiRNAs are encoded by HIV-1 can be partly explained by the differences in the types of cells investigated, different technology employed, or different strategies employed for detecting and confirming vmiRNA expression. Further research is necessary to reconcile the differences, using standardized protocols, and improving collaboration and exchange of data between research groups who work in this field. The actual practical contributions of vmiRNAs encoded by HIV-1, should be followed by careful monitoring of their role in HIV-1 infection and replication.
MicroRNAs as diagnostic and prognostic biomarkers in HIV
MicroRNAs (miRNAs), 18–25 nucleotides in length, are non-coding RNAs that are able to target mRNAs and regulate their expression (110–112). In terms of functionality, miRNAs are important agents for gene silencing and post-transcription regulation of proteins. During viral infection, some of the viral genes that play essential roles in the pathogenesis of infection, also cause miRNA production through interacting with the virus or host cell mRNAs (113). Mounting data suggests that these biomarkers can be considered appropriate for screening and monitoring of HIV patients in various phases of infection. Some investigations have focused on the elaboration of HIV miRNAs and their expression levels. It has been found that a dysregulated profile of miRNA expression would be helpful to differentiate HIV infection, and the phase of infection in affected patients. Recently, it was concluded that miRNAs and their expression levels could be different in different phases. For instance, miR-3162–3p is down-regulated in the plasma of patients in the acute phase of HIV infection. Therefore, this biomarker could useful to detect new infections (114, 115).
In HIV patients, the expression levels of miRNAs have been evaluated by several investigations. Although some miRNAs have shown variability in expression levels between both plasma and infected cells, their expression levels can be useful to determine HIV infection progression. For example, miR-146b-5p and miR-150 are dysregulated in various phases of HIV infection in plasma and in PBMC. Additionally, it has been reported that miR-150 and miR-146b-5p are up-regulated in plasma, but down-regulated in PBMC of HIV patients in the AIDS phase (116). It has been reported that miR-34a up-regulation can increase trans-activation of the virus by affecting Tat expression and increasing its functionality (117). Several studies have reported the expression levels of miRNAs in different cells from AIDS patients in different phases of infection. In primary CD4+ T cells, the expression of miR-124a, miR-29a, miR-223, miR-27a, miR-19b, miR-151–3p, miR-28–5p, miR-766 and miR-30a-3p were up-regulated, while the expression of miR-125b which increases the virus entry to the cells, was down-regulated. In addition, miR-181b was up-regulated in monocyte-derived dendritic cells (MDDCs) in the AIDS phase (118–121).
During HIV infection, the virus recruits employs several mechanisms to induce the latency phase inside the host cells. The mechanisms that the virus uses for induction of the latency phase, are not yet fully understood. In the latent phase of HIV infection, the virus does not replicate. HIV latency in resting primary CD4+ T cells is one of the most important problems affecting treatment of this infection, even when using HAART (highly active antiretroviral therapy) (122–124). Investigations on HIV latency and its correlation with miRNA expression support this idea that miRNAs play a part in this process. Huang and colleges found that miR-28, miR-125b, miR-150, miR-223, and miR-382 were up-regulated in HIV infected cells in the latency phase. By targeting the 3′ ends of HIV messenger RNAs, these miRNAs inhibit virus production in infected resting CD4+ T cells (125). Other studies have strengthened the correlation between HIV latency and miRNAs, in which miRNAs expression could regulate virus replication in infected cells. It has been found that, during the latency phase of HIV infected cells, miR-17–5p, miR-20a and, miR-29a were up-regulated and also could suppress viral replication. It was shown that miR-17–5p and miR-20a could reduce virus replication, meanwhile, they affect the Tat cofactor PCAF (p300/CBP-associated factor) (74, 126). Another relevant issue for HIV replication concerns the simultaneous up-regulation of some cellular and viral miRNAs that promote HIV replication in infected cells. It has been found that miR-TAR reduced apoptosis and promoted viral replication. Also, miRNA-H3 (viral miRNA) and miRNA-132 can act as viral replication promoters and their expression levels increase in infected cells (91, 94, 127). Wang et al. showed that miR-196b and miR-1290 were up-regulated in infected cells, and also reported that, through targeting the 3’ untranslated region of HIV, these miRNAs reduced viral replication. This study suggests that the inhibition of some cellular miRNAs could affect HIV latency, leading to the eradication of a reservoir of infection (128).
HIV infected individuals do not solely suffer from immune suppression, but they may also suffer from other complications, such as HIV-associated neurocognitive disorders (HAND), further exacerbating the severity of the condition. The HAND syndrome is a result of excessive macrophage activation, and this condition even occurs in patients who successfully respond to ART. Investigations on miRNAs and HAND have found that there are some miRNAs that could be related to this condition. An in-vitro study showed that miR-196a induced apoptosis in neuronal cells. Moreover, miR-500a-5p, miR-381–3p, miR-93–3p and miR-34c-3p were up-regulated in macrophages in human brain tissue. By decreasing neuroprotective proteins, they play a role in neuronal pathogenesis and also affect the innate immune responses leading to neurocognitive dysfunction. In addition, there are other miRNAs playing a crucial role in HIV neuronal complications. For instance, up-regulation of miR-101 in human brain microvascular endothelial cells caused destruction of endothelial permeability, and miR-128a disrupted the neuronal activity in primary cortical neurons, and could contribute to HIV encephalopathy (HIVE) (129–132). Nowadays, it is well-accepted that miRNAs play a role in other HIV complications, such as HIV cardiomyopathy (HIVCM) caused by increased monocyte adhesion to the endothelium in HIV patients. This adhesion is caused by increased ICAM-1 expression induced in infected cells by HIV Tat. Also, Tat can recruit miR-221 and miR-222 to cause an increase in ICAM-1 leading to cardiomyopathy in the untreated infection animals (133). It has been demonstrated that miRNAs are important agents in the regulation of glomerular hemostasis in different conditions. The role of miRNAs in HIV-associated nephropathy (HIVAN) has not been fully documented, but in a study conducted by Cheng et al. on transgenic mice (Tg26) and in vitro human podocytes, they observed that miR-200 and miR-33 were down-regulated during HIV infection (untreated infection). They suggested that these miRNAs could play a role in the pathogenesis of the proliferative phenotype of HIVAN (134).
Regardless of the disease phase, different levels of miRNAs in HIV infected patients when compared to controls, show that expression patterns of miRNAs in different cells are more complicated than what could be first thought. For instance, several studies demonstrated that in monocytes and monocyte-derived macrophages (MDMs), miR-198, miR-155, miR-1236, miR-221, miR-15a, miR-15b, miR-16 and miR-93 were up-regulated. Furthermore, the expression of miR-21, miR-222, miR-29, miR-34a, miR-125b, miR-28–5p, miR-150, miR-223 and miR-290 were also up-regulated in lymphocytes, and vice versa for miR-34c-5p, miR-20a, miR-106, miR-155, miR-29 and miR-2 (Table 3).
Table 3:
Expression of microRNAs in different phases of HIV infection
| miRNA | Expression | Type of cell | HIV-associated phase/disease | Model | Method | Note | Ref |
|---|---|---|---|---|---|---|---|
| miR-28, 125b, 150, 223, 382 | Up | Resting primary CD4+ T cell | Latency | Human, in vitro | Microarray stem-loop RT-PCR | Targets the 3′ ends of HIV messenger RNAs | (125) |
| miR-17-5p, 20a | Up | CD8+ T cell | Latency | Human, in vitro | Northern blot | Suppression of HIV replication by reducing PCAF (Tat co factor) expression | (74) |
| miR-132 | Up | Active CD4+ T cell | Latency | Human, in vitro | Real-time RT-PCR | Promotes HIV Replication | (127) |
| miR-H3 | Up | Resting CD4+ T cell | Latency | In vitro | Deep sequencing | Enhances viral production and Replication activates HIV latency | (94) |
| miR-196b, 1290 | Up | - | Latency | In vitro, Human | Microarray Real-time RT-PCR | Keep the virus in the latent phase | (128) |
| miR-155 | Up | - | Latency | In vitro | Real-time RT-PCR | Keep the virus in the latent phase and decrease of NF-KB Signaling pathway | (138) |
| miR-29a | Up | - | Latency | Human, in vitro | Real time PCR | Repress active HIV replication in Latent phase | (126) |
| miR-128 | Up | CD4+ T cells blood-derived monocytes | Maybe in Latency | In vitro | - | Type I interferon induce expression of mir-128 and inhibition of viral nuclear entry and viral replication | (30) |
| miR-34a | Up | - | AIDS | In vitro | Real time PCR | Up regulation n of miR-34a enhanced Tat-induced LTR transactivation through repressing SIRT1 and increasing the transcriptional activity of NF-kB | (117) |
| miR-181b | Up | Monocyte-derived dendritic cells (MDDCs) | AIDS | in vitro | Real time PCR | Gp 120 increase level of miR-181b and induce of IL-6 expression, As a result, chronic inflammation occurs | (139) |
| miR-124a | Up | CD4+ T cells | AIDS | HUman | Real-time RT-PCR | Decrees level of SIRT1 protein and activating Th2 type CD4+ T cells leads to secretion of IL-10 and TGF-β cytokines | (119) |
| miR-29a, 223, 27a, 19b, 151-3p, 28-5p, 766, 30a-3p, | Up | CD4+ T cells | HIV/AIDS | Human | Real-time RT-PCR TaqMan Low-Density Array | (120) | |
| mir-146a | Up | Microglial cells | Neuro AIDS | Human | Real-time RT-PCR | Keeping HIV-mediated chronic inflammation of the brain, induce the latency of the virus in microglial cells | (140) |
| miR-101 | Up | human brain microvascular endothelial cells | HIV-associated neurological disorders | In vitro | Real-time RT-PCR | TAT C protein induces expression of miR-101 and block VE-Cadherin, Destruction of endothelial permeability | (130) |
| miR-34c-3p, 93-3p, 381-3p, 500a-5p | Up | Primary macrophages | HIV-associated neurocognitive disorders | Human, in vitro | Microarray | Decline levels of peroxisomal proteins and defense against anti-viral | (131) |
| mir-128a | Up | Primary cortical neurons | HIV Encephalopathy (HIVE) | Human, in vitro | Microarray Real-time RT-PCR | TAT induced expression of mir-128a and disrupt neuronal activity | (141) |
| miR-198 | Up | Monocytes (macrophage) | - | In vitro | Microarray | Decreased cyclin T1 protein and repress of HIV replication and gene expression in monocytic | (142) |
| miR-217 | Up | Galactosidase indicator (MAGI) cells | - | In vitro | Real time PCR | miR-217 promotes Tat-induced HIV LTR transactivation by inhibit of SIRT1 expression and decrease AMPK signaling pathway, promote NF-κB activation | (143) |
| miR-155 | - | Macrophages | - | In vitro | Microarray | TLR3 and TLR4 stimulation in Macrophage, promote expression of miRNA and inhibit Inhibition of the post-entry, pre-integration steps | (144) |
| miR-32 | Up | Microglia cell | - | In vitro | Real time PCR | The Tat C protein induces miRNA and down regulate the expression level of TRAF3, over expression IRF3 and IRF7 | (145) |
| miR-29a | Up | T lymphocytes | - | In vitro | Real Time RT-PCR | Represses HIV Replication | (29) |
| miR-223 | Up | CD4+CD8- | - | In vitro | microarray Real time PCR | Inhibit of Vif. Vpr and Nef proteins | (146) |
| miR-9 | Up | Macrophages | - | Human, in vitro | Real time PCR | Methamphetamine and HIV promote expression of miR-9. Induce neurotransmitter release in dopaminergic neuron | (147) |
| miR-34a | Up | vascular endothelial cells | - | Human, in vitro, in vivo | Real time PCR | TAT protein, p53, lopinavir/ritonavir induce miR-34a expression and increased EC senescence | (148) |
| miR-TAR-3p | Up | monocyte-derived macrophages(MDMs) | - | In vitro | Real time PCR | MiR-TAR-3p Decrease virus replication via targeting the TAR element in the 5´ LTR and reduce genome transcription | (149) |
| miR-1236 | Up | Monocytes | - | In vitro | Real-time RT-PCR | Repress HIV Infection in Monocytes | (150) |
| miR-222 | Up | CD4 T cell | - | In vitro | Real-time RT-PCR | Up regulation mir-222 by HIV tat and NFKB, decreasing rate of CD4 | (151) |
| miR-150, 28-5p, 125b | Up | CD4+ T cell | - | Human, in vitro | Real-time RT-PCR | Methamphetamine reduces HIV Replication via up regulation of mir-125b, miR-150 and miR-28-5p | (152) |
| miR-34a | Up | T-cells | - | In vitro | Real time PCR | Promotes HIV replication in t cell | (153) |
| miR-29 family | Up | CD4+ T lymphocytes CD14+ monocytes | - | Human | Real time PCR | - | (154) |
| miR-29 | - | primary lymphoid CD4 T cells | - | Human, in vitro, in vivo | Real time PCR | IL-21 increase level of mir-29 and inhibit HIV replication | (155) |
| miR-221, 222 | Up | monocyte-derived macrophages (MDMs) | - | In vitro | RNA-seq | TNFα induce expression of mir-222/221 and restrict HIV entry in Macrophages | (156) |
| hsa-miR-21, 222 | Up | CD4+ T cells | - | In vitro | Microarray | Inhibition of apoptosis through reduction of PTEN-AKT-FOXO3a signaling pathway, enhance hiv1 replication and promote energy and depletion of CD4+ T Cell, Increasing the expression of miRNA by tat protein | (28) |
| hsa-miR-29a | Up | - | - | In vitro | Real time PCR | Block Nef expression and decrease of virus levels | (157) |
| miR-326 | Up | - | - | In vitro | Real time PCR | Inhibit of 3´ LTR genome and decreased HIV replication | (158) |
| miR-182 | Up | - | - | In vitro | Real time PCR | Increase in Tat-induced HIV LTR transactivation and promoting the transcriptional activity of NF-kB | (159) |
| miR-19b, 146a, 615-3p, 382, 34a, 144, 155 | Up | - | - | Human, in vitro | Real-time RT-PCR | Useful biomarker for the determination of prognosis | (160) |
| let-7c | Up | - | - | In vitro | Real time PCR | Increase viral replication and spread, promote virion release and higher copy number of viral genome transcripts in infected cells | (161) |
| miR-34a, 124a | Up | - | - | In vitro | Real time PCR | Increase viral replication and spread, promote virion release and higher copy number of viral genome transcripts in infected cells | (161) |
| miR-15a/b, 16, 20a, 93, 106b | Up | Monocytes monocyte-derived DCs (MDDCs) | - | In vitro | Microarray Real-time RT-PCR | Decrease of tat and Pur-α proteins, Represses HIV replication in monocytes | (162) |
| hsa-miR541-3p, 518f-3p, 195-3p | Up | - | - | Human, in vitro | Real-time RT-PCR | Represses HIV replication and induce antiviral responses | (163) |
| miR-9 | Down | CD4+ cells | Latency | Human, in vitro | Real-time RT-PCR | Inhibit of IL2 transcription and development of T cell exhaustion during chronic HIV infection | (164) (165) |
| miR-31, 29b, 590-5p | Down | CD4+ T cell | Latency | Human | Real time PCR | Useful for prognostic and diagnostic, strategy to choose patients who would benefit from earlier ART | (166) |
| hmiR-che-1 | Down | CD4+ T cell | Latency AIDS | Human, in vitro | Real time PCR | Increase Vpr protein and over expression of hiv1-mir-H1 | (167) |
| miR-125b | Down | Primary CD4+ T cells | AIDS | Human, in vitro | Microarray Real Time RT-PCR | Cocaine promotes HIV replication and increase of virus entry | (121) |
| miR-146b-5p | Up Down | - | AIDS | Human | Real-time RT-PCR | (116) | |
| miR-150 | Up | - | AIDS | Human | Real-time RT-PCR | (116) | |
| miR-21, 155 | Down | Monocyte-derived dendritic cells (MDDCs) | Suggest in AIDS | In vitro | Real time PCR | Gp 120 decreases level of miR-21/155 and induces IL-6 expression, as a result, chronic inflammation occurs | (139) |
| miR-221, 222 | Down | Monocytes | HIV-Associated Cardiomyopathy | In vitro, in vivo | Real-time RT-PCR | TAT promotes Expression of ICAM-1 and increase NF-kB, MAPK signaling pathways | (133) |
| miR-200, 33 | Down | Podocytes | HIV-associated nephropathy (HIVAN) | In vitro, in vivo | microarray Real time PCR | Contributes to the progress of the proliferative phenotype of HIVAN | (134) |
| miR-196a | Down | Neuronal | HIV associated neurocognitive disorders (HAND) | In vitro, in vivo | Real time PCR | TAT induce of p73, p53 and inhibit miR-196a Increase apoptosis in neuronal cells | (132) |
| miR-3162-3p | Down | - | Acute | Human, in vitro | microarray Real time PCR | Potential biomarker to identify HIV new infections in HIV infected persons | (114) |
| miR-29a/b | Down | CD4+CD8- | - | In vitro | microarray Real time PCR | Increase Nef and cdc42 promotes apoptosis in cd4 cells | (146) |
| miR-155, 21 | Down | CD4+CD8- | - | In vitro | microarray Real time PCR | - | (146) |
| miR-155, 20a | Down | Monocyte-derived dendritic cells (MDCCs) | - | In vitro | Real-time RT-PCR | Cocaine reduces CD83 and enhances DC-SIGN, LTR and pu.1 levels in monocyte-derived dendritic cells (MDCCs), elevates HIV Infectivity | (168) |
| miR-106b | Down | CD4+ T cell | - | In vitro | Real time PCR | Increase of p21 expression, Useful to provide a new therapeutic approach | (169) |
| miR-20a | Down | CD4+ T cell | - | In vitro | Real time PCR | Increase of p21 expression, Useful to provide a new therapeutic approach | (169) |
| miR-34c-5p | Down | Naïve CD4 T cells | - | Human, in vitro | miR RT-qPCR assays | Promotes HIV replication | (170) |
| miR-21 | Down | Monocytes | - | Human, in vitro | Real time PCR | Increased levels of NFKB and IFN-stimulated gene 15 lead to promoting inflammation | (171) |
| miR-181a | Down | Astrocytes compared to microglia | - | In vitro | Real-time RT-PCR | Suppression of HIV replication | (172) |
| hsa-miR1225-5p, 18a, 335 | Down | - | - | Human, in vitro | Real-time RT-PCR | - | (163) |
| miR-29a-5p | Down | - | - | Human | Real time PCR | - | (173) |
| miR-27b | Up | Resting CD4+ T cell | - | In vitro In vivo | Microarray | In resting CD4+ T cells this miRNA directly binds the 3ʹ-UTR of cyclin T1 and inhibits expression, repressing HIV replication | (174) |
| miR-29b, 150, 223 | Up | Resting CD4+ T cell | - | In vitro In vivo | Microarray | In resting CD4+ T cells this miRNA directly binds the 3ʹ-UTR of cyclin T1 and inhibits expression, repressing HIV replication | (174) |
| miR-28, 150, 223, | Up | Monocytes/macrophages | - | In vitro | Real-time RT-PCR | Represses HIV replication in macrophages and enhances replication in monocytes | (175) |
| miR-382 | Up | Monocytes/macrophages | - | In vitro | Real-time RT-PCR | Represses HIV replication in macrophages and enhances replication in monocytes | (175) |
| miR-146a | Down | Resting primary CD4+ T lymphocytes | - | In vitro | Real time PCR | In resting primary CD4 T lymphocytes prevents HIV entry into T lymphocytes and myeloid cells In active CD4+ T lymphocytes enhances HIV entry | (176) |
| miR-N367 | - | T cell | Latency | In vitro, in vivo | Northern blot | Blocks Nef expression, decreases HIV virulence | (92) |
| miR-TAR-5p, TAR-3p | - | - | Latency | In vitro | Sequencing | Reducing apoptosis and promotes virus replication | (91) |
| miR-H1 | - | CD4+ T cell | Latency AIDS | Human, in vitro | Real time PCR | Promote CD4+ lymphopenia by decreasing AATF gene | (167) |
| vmiR88, 99 | - | Macrophage | AIDS | In vitro | Real-time RT-PCR | Stimulated human macrophage TNFα release | (109) |
| miR-N367 | - | T cell | - | In vitro | Northern blot | Decreases HIV transcription via the NRE of the 5´-LTR U3 region and Nef sequences at the 3´-UTR | (177) |
| miR-TAR-5p, TAR-3p | - | - | - | In vitro, in vivo | Northern blot | Effective on virus replication and Increased efficiency of host antiviral defense | (178) |
| hiv1-mir-H1 | - | - | - | In vitro | - | Suppression of mir149 expression and blocks AATF, Bcl-2, c-myc, Par-4 and Dicer genes, up-regulates Vpr protein, and increases apoptosis | (96) |
| hsa-miR-148 | - | - | - | Human | Real time PCR | (179) |
Approximately 10∼15% of the patients infected with HIV-1 exhibit a sharp decline in CD4+ T-cell counts in the initial phase of infection, followed by a rapid progression to full-blown AIDS. Researchers compared miRNA expression profiles in PBMCs isolated from rapid and slow progressors, and showed different levels of miR-31, miR-200c, miR-526a, miR-99a, and miR-503, which might be useful as bio-markers for fast progressors (135). Duskova et al. demonstrated miR-19b, miR-146a, miR-615–3p, miR-382, miR-34a, miR-144, and miR-155 that modulate innate immune-associated and inflammation-associated genes, were remarkably up-regulated in PBMCs with the increased viral loading in the people infected with HIV-1 (136).
Munshi et al. compared the expression levels of miR-16, miR-146b-5p, miR-150, miR-191, miR-223, and miR-146b-5p in plasma taken from normal individuals without AIDS, patients infected with HIV who had not been given ART, patients who had taken ART, and those who had been given ART but did not respond (116). The researchers found that levels of miR-146b-5p in plasma and PBMCs varied between groups, and predicted the response to ART. Additionally, it may be possible to use miR-150 as a biomarker to monitor HIV/AIDS prevalence and the impact of ART (116). Rocca et al. observed an inverse relationship between miR-29a levels and HIV viral load and the degrees of immunosuppression (numbers of CD4+ T cells and the CD4+ T/CD8+ T ratio) in 165 young patients chronically infected with HIV-1 (137). MiR-29a levels and response to treatment varied among patients. Low miR-29a levels were observed in the patients who failed treatment (CD4 < 350 cells/μL). Hence, it may be possible to use miR-29a as a biomarker of long-term survivors and response to ART. Moreover, miR-29a may predict disease prognosis and progression (137).
Longitudinal studies of miRNAs in non-treated patients who suffer HIV-1 progression may give insights into disease progression and potential therapeutic targets. However, guidelines to start ART as soon as possible after diagnosis of HIV-1 infection, have made this type of study more difficult to carry out.
MicroRNAs as therapeutic targets in HIV
The cellular RNAi machinery, such as miRNAs, plays a pivotal role in controlling several diseases, such as viral infection, cancer, and probably HIV. Some details of the interaction between host-cellular miRNAs and HIV have been reported. In fact, the expression profile of miRNAs changes during HIV infection supporting the idea that dysregulation of cellular miRNAs is associated with HIV disease progression (180–182). It has been observed that some miRNAs hinder HIV infection via several mechanisms. Some cellular miRNAs target HIV accessory genes and host protein/genes involved in the life cycle of HIV(183), affecting the interaction between cell surface glycoprotein CD4 and the HIV ligands, leading to HIV entry (184). Lodge et al. demonstrated that, through down-regulation of CD4 expression, miR-221 and miR-222 were up-regulated in macrophages infected with HIV, resulting in the inhibition of CD4-mediated HIV entry (156). Moreover, the co-receptor CXCR4 is another facilitator which helps HIV entry into T lymphocytes and myeloid cells (185, 186). Quaranta and colleagues performed an investigation to test the efficacy of treatment with AMD3100 and promyelocytic leukemia zinc finger (PLZF) on the CXCR4 and miR-146a expression levels in human primary CD4+ T lymphocytes infected by HIV. They showed that PLZF, an amiR-146a repressor, increased the expression levels of TRAF6 and CXCR4 proteins in CD4+ T lymphocytes. Therefore, using gene-silencing treatment of PLZF or AMD3100, it was observed that the up-regulation of miR-146a restricted HIV entry into leukemic monocytic cells and CD4+ T lymphocytes through suppression of CXCR4 (176). The mechanism of HIV replication is associated with host cellular factor cyclinT1, which binds to viral trans-activator protein (Tat) leading to the activation of transcription of the integrated virus (provirus) (187). A new anti-HIV property of miR-198 was recently shown by Sung and colleagues. They showed that up-regulation of miR-198 restricts HIV replication via targeting 3UTR of cyclinT1 mRNA in monocytes (142). Previous studies have indicated that TNPO3 regulates nuclear import and replication of HIV in host cells (188, 189). Through induction of type I interferon, miR-128 directly targets TNPO3 mRNA. Consequently, TNPO3 mRNA expression levels and protein are remarkably reduced, and miR-128 suppresses virus replication in HIV-infected cells (30). miRNAs can also target Nef, an important virulence factor of primate lentiviruses, such as HIV (190). Ahluwalia et al. suggested that repression of miR-29a notably promoted HIV infection. Furthermore, it was demonstrated that miR-29a decreased the expression of Nef protein, which causes interference with HIV replication (157). In addition, Waminathan and colleagues reported that miR-155 coulkd serve as an anti-HIV agent via affecting some HIV-dependent factors, such as LEDGF, Nup153, TNPO3 and ADAM10 involved in pre-integration and post-entry events (144). Triboulet et al observed that down-regulation of pri-miR-17/92 enhanced HIV production in Jurkat cells, and it is known that HIV actively down-regulates the expression of the miR-17/92 cluster. By affecting the PCAF (p300/CBP-associated factor) levels, these miRNAs could decrease HIV transcription, because PCAF enhances Tat binding to CDK9/P-TEFb as well as transcription of HIV genes (74, 191).
As mentioned earlier, studies have reported that several cellular miRNAs contribute to HIV replication by targeting both cellular and viral factors. As seen in table 4, some miRNAs can enhance HIV infection by repressing cellular inhibitors of viral replication. For instance, miR-217 and miR-34a down-regulate SIRT-1, a Tat and p65 deacetylase, leading to increased efficiency of HIV transcription (118, 143, 192). miR-132 is the first miRNA shown to elevate HIV replication. Chiang et al. found that miR-132 was over-expressed due to the activation of CD4+ T cells. They also reported that, by targeting of MeCP2, miR-132 may be potentially associated with enhanced HIV replication (127). The phosphatase 1 nuclear targeting subunit (PNUTS) is involved in regulation of HIV transcription. Kapoor et al. reported that miR-34a was up-regulated in HIV-infected T cells. They also indicated that miR-34a could overcome the suppressive effects of PNUTS and increase HIV replication (153). In one investigation, Farberov et al. showed that the up-regulation of miR-124a, miR-34a and let-7c increased virion release and the viral genome copy number in the infected Jurkat cell line. These miRNAs, by targeting TASK1 and p21, inhibited cellular proteins that restrict viral replication, and enhanced viral replication and release from HIV-infected cells (161). As well, Zhang and colleagues suggested that HIV-Tat protein induces the over-expression of miR-34a (long terminal repeat (LTR) trans-activation via the SIRT1/NF-kB pathway) in TZM-bl cells(117). These findings suggest that some miRNAs can be considered as novel agents to control HIV infection or in conjunction with antiviral drugs contributing to improve the viral disease.
Table 4:
MicroRNA as therapeutic targets in HIV
| microRNA | Expression | Target | Model | Method | Function (s) | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Reversal of latency | Apoptosis inhibition | Prevention of HIV entry | Other | ||||||
| miR-132 | Up | MeCP2 | Human | miR-132 enhances HIV replication | (127) | ||||
| mir-146a | Up | CCL8/MCP-2 | In vitro | Real-time PCR | the maintenance of HIV-mediated chronic inflammation of the brain | (140) | |||
| miR-34a | Up | PNUTS/PPP1R10 | In vitro | Real-time PCR | enhances HIV replication | (153) | |||
| hsa-miR-21, 222 | Up | PTEN | In vitro | Microarray | * | (28) | |||
| miR-29a | Up | HIV 3 UTR region | In vitro | Represses HIV Replication | (29) | ||||
| miR-222, 221 | Up | CD4 mRNA | In vitro | RNA-seq | * | (156) | |||
| miR-217 | Up | SIRT1 | In vitro | miR-217 promotes Tat-induced HIV LTR transactivation | (115) | ||||
| let-7c | Up | p21 | In vitro | Real-time PCR | Enhance viral replication and spread | (161) | |||
| miR-34a | Up | TASK1 | In vitro | Real-time PCR | Enhance viral replication and spread | (161) | |||
| miR-34a | Up | SIRT1 3ʹ-UTR | In vitro | Real-time PCR | Promotes Tat-induced HIV transactivation | (117) | |||
| miR-217 | Up | In vitro | Real-time PCR | miR-217 influenced promotes Tat-induced HIV transactivation via decreases of SIRT1 expression | (143) | ||||
| miR-124a | Up | TASK1 | In vitro | Real-time PCR | Enhance viral replication and spread | (161) | |||
| miR-29 family | Up | 63 | Real-time PCR | Influence the clinical progression of HIV infection, the HIVproviral load and the innate immune response against HIV | (154) | ||||
| miR-34a | Up | Sirt1 | In vivo, In vitro | Real-time PCR | (193) | ||||
| let-7, miR-124a, 34a | Up | p21 protein | In vitro | Real-time PCR | Enhances viral production and Replication | (161) | |||
| miR-146a | Down | CXCR4 TRAF-6 | In vitro | * | (176) | ||||
| miR-34c-5p | Down | Human | miR RT-qPCR assays | Promotes HIV replication | (170) | ||||
| miR-21 | Down | IP-10 | Human, In vitro | Real-time PCR | miR-21 has the ability to inhibiting the expression of IP-10 via monocytes, that is a significant source of inflammation in HIV positive patients. | (171) | |||
| miR-20a | Down | p21 | In vitro | Real-time PCR | HIV can exploit host cellular machinery through miR-20a to regulate the selective gene in target cells. | (169) | |||
| miR-106b | Down | p21 | In vitro | Real-time PCR | HIV can exploit host cellular machinery through miR-106b to regulate the selective gene in target cells. | (169) | |||
| miR-27b | Down | 3ʹ-UTR of cyclin T1 | In vitro | Microarray | Regulation of HIV Replication in Resting CD4+T Lymphocytes | (174) | |||
| miR-196b, 1290 | 3ʹ untranslated region of HIV | In vitro | * | Contribute to HIV latency suppressing effects on HIV infectivity | (128) | ||||
| miR-1236 | VprBP | In vitro | Inhibits HIV Infection of Monocytes | (194) | |||||
| hsa-miR29a | Nef (HIV protein) | In vitro | Downregulates the expression of Nef protein and interferes with HIV replication | (157) | |||||
| miR-128 | TNPO3 mRNA | In vitro | * | Inhibition of viral replication | (30) | ||||
| miR-182 | NAMPT Mrna | In vitro | Real-time PCR | Increase in Tat-induced HIV LTR transactivation | (159) | ||||
| miR-155 | 3ʹ-UTR of TRIM32 | In vitro | Real-time PCR | * | (138) | ||||
| miR-198 | Cyclin T1 mRNA | In vitro | Real-time PCR | Restrict HIV replication in monocytes | (142) | ||||
| miR-155 | LEDGF ADAM10 TNPO3 Nup153 | In vitro | Real-time PCR | Inhibits PIC nuclear import | (144) | ||||
In conclusion, most studies were carried out in vitro. Whether miRNAs can inhibit latency, boost immune function, prevent re-infection with HIV-1, or allow long-term proviral latency remains to be answered in humans. Therefore, future research studies need to confirm the functional relevance of miRNA in HIV-1 infection.
HIV and Exosomes
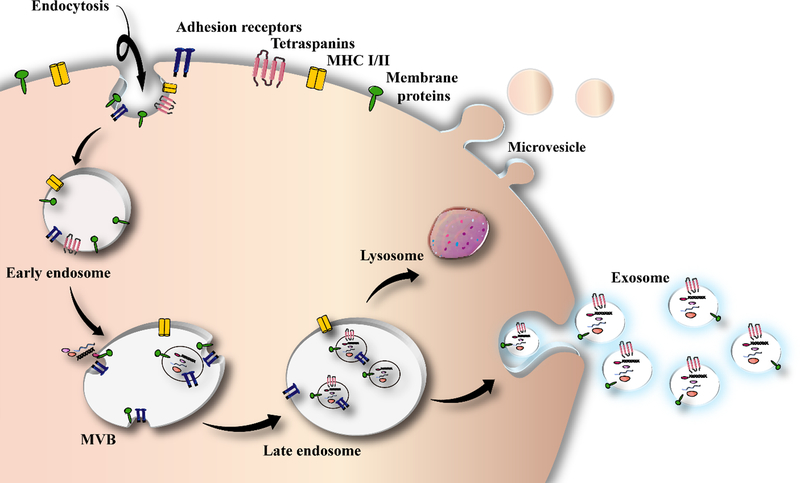
A majority of the cells are able to release membrane-surrounded vesicles, usually known as extra-cellular vesicles (EVs), into the extra-cellular spaces for inter-cellular communication at local and distant sites. They mediate molecular transfer between cells, and may carry out immune modulation (195). EVs have a high degree of heterogeneity and dynamic interconversion, and are commonly categorized into exosomes (200), macro-vesicles (196), and apoptotic bodies, on the basis of biogenesis and vesicle cellular origin (197). Exosomes are produced as intra-luminal vesicles, by budding away from the cytoplasm into an intermediate endocytic compartment, that is called a “multi-vesicular body” (MVB). Exosomes are shed from the cells when the MVB is combined with the plasma membranes (198). Exosomes include different molecular cargoes depending on their cells of origin, such as RNAs and proteins (198). Even though widely-employed exosome purification protocols in some past publications, have frequently co-isolated various kinds of EVs, differential ultra-centrifugation technique can separate EVs containing CD63, CD81, and CD9 tetraspanins, and endosome marker-enriched vesicles that are considered to be the characteristic markers of exosomes (199). Figure 3 illustates a schema of exosome biogenesis.
Figure 3.
Exosome biogenesis
It is possible to isolate exosomes from the medium of HIV-1-infected cells, and from sera of individuals with HIV infection (Table 5) (200). The exosomes from latent HIV-1-infected Jurkat cells (J1.1) do not contain intact HIV-1 viral particles, even though these exosomes include some viral proteins, including Gag and the precursor form of Env protein (p160) (201). The HIV transactivation response (TAR) element RNA, which is a precursor of several HIV-encoded miRNAs, establishes a stem–loop folded structure in the nascent transcript, that facilitates the attachment of viral transcriptional trans-activator (Tat) protein to enhance transcription and replication of HIV (202). Exosomes isolated from HIV-1-infected cell culture supernatants, or from HIV-infected patient sera contained TAR RNA in the total viral RNA (201). TAR RNA-bearing exosomes generated the pro-inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-β (TNF-β) when added to primary macrophages (200).
Table 5:
Exosomal cargoes released from HIV-infected cells
| Type of cargo | Expression | Exosome Source | Sample (n) | Ref | |
|---|---|---|---|---|---|
| MicroRNA | miR-155 | Up regulation | Plasma | 43 | (243) |
| miR-223 | Up regulation | Plasma | 43 | (243) | |
| miR-548a | Upregulation | HIV-infected macrophage | (221) | ||
| miR-30e | Upregulation | HIV-infected macrophage | (221) | ||
| miR-338 | Upregulation | HIV-infected macrophage | (221) | ||
| miR-454 | Upregulation | HIV-infected macrophage | (221) | ||
| miR-518f | Upregulation | HIV-infected macrophage | (221) | ||
| miR-1243 | Upregulation | HIV-infected macrophage | (221) | ||
| miR-1247a | Up regulation | HIV-infected macrophage | (221) | ||
| miR-150 | Up regulation | HIV-infected macrophage | (221) | ||
| miR-29a | Up regulation | HIV-infected macrophage | (221) | ||
| miR-302c | Up regulation | HIV-infected macrophage | (221) | ||
| miR-636 | Up regulation | HIV-infected macrophage | (221) | ||
| miR-872 | Up regulation | HIV-infected macrophage | (221) | ||
| miR-875 | Up regulation | HIV-infected macrophage | (221) | ||
| vmiR-88 | Up regulation | Serum | 14 | (109) | |
| vmiR-99 | Up regulation | Serum | 14 | (109) | |
| miR-29b | Up regulation | - | 16 | (244) | |
| TAR miRNA | Up regulation | PBMC | 6 | (61) | |
| miR-130a | Up regulation | IV-infected and cocaine-treated human monocyte derived macrophages | (245) | ||
| miR-17 | Up regulation | Human monocytic U937 cells | (222) | ||
| miR-382 | Down regulation | Human monocytic U937 cells | (222) | ||
| Protein | Tat | Up regulation | Engineered human cellular exosomes to express HIV Tat | 14 | (246) |
| Nef | Up regulation | Expresstion of HIVs Nef proteins in HEK-293 | (223) | ||
| Hck (hemopoietic cell kinase), ADAM17 | Up regulation | Plasma, Myeloid cells | 8 | (226) | |
| Nef and ADAM17 | Up regulation | Plasma | 8 | ||
| Nef | Up regulation | Induce pro-inflammatory vesicles | (226) | ||
| DAP-3 | Up regulation | Dendritic cells | (247) | ||
| Nef | Up regulation | A3.01 T cell line | (224) | ||
| Nef-ADAM17-TNFα | Up regulation | U937 cells | (248) | ||
| Nef | Up regulation | Astrocyte cultures | (249) | ||
| Fibronectin and Galectin-3 | Up regulation | HIV infected DC | - | (250) | |
| Nef | Up regulation | HEK 293 cells | (251) | ||
| Nef | Up regulation | Plasma, neuroblastoma cell line SH-SY5Y | 12 | (229) | |
| Nef domain (the62EEEE65acidic) | Up regulation | (252) | |||
| Nef | Up regulation | (253) | |||
| Nef | Up regulation | PBLs | (225) | ||
| Gag | Up regulation | Jurkat T cells | (254) | ||
| Nef | Up regulation | Microglial cells | (230) | ||
| Tat | Up regulation | Tat-expressing primary astrocytes | (228) | ||
| Cytokin/ chemokine | Nef and IL-1, IL-2, IL-2Ra, IL-4, IL-5, IL-7, IL-9, IL-12p70, IL-15, IL-16, TNF-α2/α, IFN-β, CXCL10, CCL2/3/4,CD-40L, G-CSF, sFasL, sICAM, | Up regulation | Plasma | 25 | (227) |
| RNA | Trans-activation response (TAR) RNA and TAR-gag | Up regulation | Jurkat E4 cells (HIV-infected T cells) and othere cell lines | In vitro, human PBMC | (255) |
| Nef messenger RNA | Up regulation | SH-SY5Y cells, HEK293 | (229) | ||
| AATK, SLC27A1, and CDKAL | Up regulation | Nef-expressing U937 monocytic cells | (232) | ||
| MECP2, HMOX1, AARSD1,ATF2 | Up regulation | Nef-expressing U937monocytic cells | (232) | ||
| nef messenger RNA | Up regulation | Neuroblastoma cell line SH-SY5Y | (229) | ||
| TAR RNA | Up regulation | (256) | |||
| Defective HIV | defective HIV (F12/Hut-78 cells) andNefF12 | E Up regulation | Hut-78, D10/Hut-78, F12/Hut-78, and Rev-CEM and HEK293 wich infected by virus | (231) | |
Currently, the usefulness of exosomes and other extracellular vesicles (EVs) is an evolving approach to diagnose or even treat HIV infection (203). Determining the possible correlation between HIV and release patterns of exosomes may be helpful to tackle HIV and its related complications, leading to production of new medications or therapeutic agents (203–206). Despite all the attempts that have been made during the prior 20 years, to discover an effective and potent vaccine to protect the immune system against HIV, there are still numerous puzzles to be solved (207, 208). These problems may originate from unknown mechanisms related to host-pathogen interactions and the ability of the virus to evade the immune ssytem. For instance, one of the ways to escape immune detection is to take advantage of intercellular communication processes. Cytoplasmic tunnels that resemble cell-to-cell channels or tunneling nano-tubes are other possibilities. In this way, macromolecules and cellular constituents can be physically transported from one cell to another cell. These channels can be used in microbial propagation, when two immune cells form synapses to present antigens in humoral immunity. This virus can then move from one T cell to infect all of them (209).
The complexity of performing studies on exosomes secreted from cells infected with HIV-1 is due to particular features of exosomes and HIV-1 viruses. Their biophysical molecular properties, biogenesis and uptake mechanisms are complex and different. The density of HIV-1 ranges from 1.16 to 1.18 g·mL−1, while the density of exosomes ranges from 1.13 to 1.21 g·mL−1 (210, 211). HIV-1 is slightly larger in size than exosomes, with the virus diameter ranging from 100–120 nm, in comparison with the diameter of exosomes being 40–100 nm (24). Moreover, it is believed that HIV-1 could be produced via similar pathways that apply to exosome biogenesis (212). HIV-1 budding is suggested to be involved with numerous cellular processes, such as Alix and TSG101 that are also involved in exosome biogenesis (213). The convergence of the biogenesis of HIV-1 and exosomes, suggests that HIV-1 products, such as proteins and RNAs might be encased within the exosomes, and the fact that exosomes can be isolated from fluids of indivuduals infected with HIV-1 raises the question of whether this could be contamination. However, rigorous purification methods, including immune-affinity methods and centrifugation on density gradients are able to separate exosomes from HIV-1 particles (24, 214). The remarkable similarity between the biogenesis of exosomes and enveloped viruses generally, and HIV-1 in particular, resulted in the “Trojan exosome” hypothesis of HIV-1 assembly and cell-cell transmission. This suggests that the HIV-1 virus evolved to use the biogenesis of exosomes and their cell uptake pathways, to allow envelope-independent entry of the virus (215). The constituents of the HIV virus may be packaged into the intraluminal vesicles (ILV) while there is uncertainty as to whether the cells are infected by mature progeny viral or not (216). It is possible that, exosomes that bind via their unique cell surface receptors, or by engagement of intact virus could be involved in viral infections (217). In other words HIV could employ exosomes to increase the infection rate and to escape from immune defense. This process, by which exosomes may facilitate the viral access into cells of the innate and adaptive immune system is very important, and could be a mechanism by which HIV develops resistance to some neutralizing antibodies (217).
The composition of exosomes derived from biological fluids is extremely variable, and it has been proposed that the composition may govern their biological effects. Accumulating evidence suggests that the effects of exosomes on HIV pathogenesis may depend on their cellular source (218). In most cases (but not all) exosomes derived from HIV-infected cells are more virulent and boost infection, while exosomes derived from uninfected cells have more protective properties (219). Regarding the source of exosomal release, It appears that the origin of the exosomes is the determining factor for their effects on HIV (218). For example, exosomes derived from human blood infected with HIV, may be derived from various types of cells, including both HIV-infected cells as well as uninfected cells. It has been postulated that HIV hijacks the exosome biogenesis pathway which transports various viral proteins and RNAs for the viral dissemination process(220).
In recent years, some studies have focused on the exosomal contents in HIV patients and HIV infected cells. Roth et al. used MDM cells (human monocytes differentiated to macrophages) to show that miR-548a, miR-30e, miR-338, miR-454, miR-518f, miR-1243, miR-1247a, miR-150, miR-29a, miR-302c, miR-636, miR-872, miR-875 were all up-regulated in HIV infected cells (221). Also, Aqil et al. used MicroRNA Array screening to measure miR-149, miR-382, miR-378, miR-324–5p, miR-223, miR-150, miR-125b, miR-29a, miR-29b, miR-29c, miR-92a, miR-28, miR-26a, miR-20a, miR-19a, miR-19b, miR-17. They demonstrated that these miRNAs were down-regulated in HIV Nef expressing human monocytic U937 cells. They also, found that some miRNAs could prevent replication of HIV (222). Regardless of the miRNAs, there are other factors which they could have an impact on HIV progression. McNamara and colleagues investigated expression of HIV Nef proteins in HEK-293 cells, and they showed that HIV Nef protein could be involved in extracellular communication and viral pathogenesis (223). It has also been suggested that exosomal Nef could have an effect on CD4+ T lymphocytes and could activate resting lymphocytes (109). Further results reported by Konadu et al. using a TUNEL assay and immune blot analysis suggested that the secretion modification region (SMR) motif of HIV Nef could play an important role in viral pathogenesis (224).
It has also been found that there are components in the exosomes of HIV infected cells, which can alter immune response and induce apoptosis. In this regard, Lenassi and et al. showed that exosomal Nef induced cell death in HIV-1 infected peripheral blood lymphocytes (PBLs) (225). Furthermore, it was found, that TAR miRNA could down-regulate apoptosis by targeting the Bim protein. These results led Narayanan et al. to investigate exosomes in PBM cells from uninfected controls, HAART-treated, and long term non-progressor patients (61). Lee et al. investigated exosomes obtained from myeloid cells isolated from HIV patients, and found that the exosomes contiained Nef and the myeloid tyrosine kinase, Hck leading to the release of pro-inflammatory cytokines which correlated with immune suppression in chronic HIV Infection (226). Analysis of the exosomal cargoes obtained from plasma, has been reported by Konadu et al, who showed that cytokines and chemokines such as IL-1, IL-2, IL-2Ra, IL-4, IL-5, IL-7, IL-9, IL-12p70, IL-15, IL-16, TNF- α, IFN-β and CXCL10, CCL2/3/4, CD-40L, G-CSF, sFasL, and sICAM could be found accompanying the Nef in exosomes. Researchers observed that the levels of each cytokine and chemokine, measured in the exosomal fraction, were higher in the infected individuals compared to non-affected cases. It should be noted that a relationship between the majority of cytokines/chemokines and exosomes or MVs has not yet been confirmed by mass spectrometry or other protein detection techniques. Nonetheless, higher amounts of these immune mediators in the EV fraction of patients infected with HIV, emphasize the potential significance of an exosome-based delivery mechanism for HIV infection, and other pathological conditions (Table 5 ) (227).
Nowadays the use antiretroviral therapy has lessened many of the complications of HIV infection, but HIV-associated neurocognitive disorder (HAND) is still troubling. Investigations into HIV infected exosomes have shown a connection between the exosomal cargoes and HAND. In one study, Rahimian and colleagues showed that Tat protein derived from primary astrocytes could induce death in neurons (228). Khan et al. using neuroblastoma cell lines showed that Nef could be important in HAND pathogenesis by increased secretion of beta-amyloid (Aβ) and Aβ peptides. They also showed that Nef mRNA could induce secretion of beta-amyloid (Aβ) (229). Exosomal Nef can also affect the blood-brain barrier and its integrity, and can induce microglial cells to secrete cytokines and chemokines (230). It has been shown that exosomes derived from HIV infected cells, were able to transport different mRNAs derived from HIV. Arenaccio and colleagues found that HIV derivatives in the exosomal cargo could increase the susceptibility of unstimulated T CD4+ cells (231). It was also sugested by Aqil et al. that, AATK, SLC27A1, and CDKAL mRNAs could be important for triggering apoptosis in Nef-expressing U937 monocytic cells (232).
It seems that EVs obtained from human semen may possess a significant anti-retroviral activity. Madison et al. demonstrated the ability of EVs isolated from semen taken from healthy males to inhibit viral replication when incubated with various strains of HIV (233). Researchers argued that EV-mediated disruption of the reverse transcriptase activity caused defective viral replication. Interestingly, anti-retroviral activity has been found in semen-derived EVs, but not in the EVs isolated from blood (233). Researchers showed that EVs isolated from the healthy semen were capable of inhibiting HIV spread into vaginal cells and of disrupting viral replication in the vaginal epithelium (234). These results strengthened the significance of the examination of anti-retroviral activity of exosomes (and other vesicles extracted from semen) for contributing to the discovery of novel treatments for HIV. Moreover, studies on the isolation of EVs from breast milk provided fascinating findings concerning HIV suppression. Reports showed that EVs isolated from breast milk provided regulatory activity on the immune system (235). Näslund et al. demonstrated that EVs extracted from human breast milk from normal donors suppressed HIV infection of MDDC and viral transmission to CD4+ T cells (236). A reason for this protection against infection, may be that the EVs attach to the DC-SIGN receptor, thus competing with the virus, and inhibit infection of CD4+ T cells (236). Analyzing exosomal proteins extracted from saliva might be used for monitoring the positive or negative impacts on the progress of HIV infection and AIDS. Dominy et al. studied heroin abusers, and provided evidence for the effect of HIV infection on the release of proteins contained in EVs from salivary glands and oral epithelium (237). They explained that EVs isolated from saliva contained cargo proteins modified by HIV infection (237). Researchers have examined EVs in the urine as effectors of innate immunity (innate immune proteins with anti-microbial activities). For instance, urinary EVs suppressed the growth of Escherichia coli (two pathogenic and commensal strains). This indicated a probable immune defense system of the host urinary tract (238). As far as we know, there have not yet been any reports examining the relationship between HIV infection and EVs isolated from urine. The use of urinary exosomes (and other EVs) may possibly provides a replacement for normal blood samples for monitoring spread of infection in HIV-positive individuals. Urinary EVs might also be applied for the investigation of any relationship between kidney diseases and HIV (239). Similarly, EVs derived from vaginal fluid have been suggested to be a protective agent against HIV infection (240). It is still necessary to do further work on the contribution of exosomes and EVs in various body fluids and tissues. Investigation of exosomes extracted from other biological fluids, particularly the cerebro-spinal fluid (which has a close relationship to the immuno-privileged state of the brain) might provide more information on the transport of immuno-modulatory molecules in the context of HIV-associated brain manifestations. Moreover, a greater understanding of the mechanistic basis of how exosome derived from different bio-fluids inhibit HIV-1 replication may be useful in future strategies to design HIV-1 vaccines.
The isolation and purification of nano-vesicles can constitute a methodological concern because of the variation of the phenotypic properties of exosomes. Isolation and purification may be carried out by centrifugation, which involves using filters and density gradients. Moreover, a growing variety of commercial kits now exist for facilitating the procedure. Assessment of protein markers is necessary for isolating and purifying exosomes so that the resulting material is well-characterized (241). It is possible to directly visualize exosomes using electron microscopy, where they frequently show a cup-shaped morphology, but this may be produced by the electron microscopy preparation process (242). In addition, the similar features of exosomes and retro-viral particles, adds another layer of complication to the characterization and isolation of vesicles from HIV infections. One popular approach is to use density gradient isolation to separate exosomes from viral particles, accompanied by an enzymatic assay for acetylcholinesterase (AChe), which is a marker of exosomes (203). However current thinking is to consider Ache to be a marker of ectosomes or micro-vesicles instead of exosomes, as demonstrated by numerous proteomic studies accessible on Exocarta (http://www.exocarta.org/) and Vesiclepedia (http://www.microvesicles.org/). It is clear that protocols for the separation of HIV from EVs (with similar dimensions) has no effect on separating various EV sub-populations. Thus, it is necessary for further progress in the existing separation techniques in order to further understand the contributions of exosomes (and other EVs) to the pathophysiology of HIV infection.
To summarize, the influence of exosomes on HIV is still poorly understood. In a general way, exosomes can modulate immune responses and may affect HIV pathogenesis, playing arelevant role in HIV pathophysiology. This seems to be mediated by the exosomal cargo,which comprises mainly membrane proteins and non-coding RNAs. The current state of the field can be summarized as follows: (I) A lack of distinction between exosomes and other EVs creates much confusion in the literature. The labeling of other types of vesicles as “exosomes” makes the comparison of results from different studies very difficult. Besides, a range of experiments were performed using a mixture of exosomes with other types of EVs;(II) Over the years, mounting evidence has left little room for the hypothesis of HIVexploiting the MVB/exosome export machinery for its release (thought to occur especially ininfected macrophages), although it is clear that the virus may explore components that areshared between exosome and vesicles budding at the plasma membrane;(III) An alternative hypothesis of the exploitation of exosome/EV release by HIV is acamouflage of the viral particles by surrounding themselves with exosomes, which couldenhance the infectivity of viral particles by facilitating their contact with target cells;(IV) Nef plays an important role in increasing import/export processes in infected cellsand regulating exosomal cargo. Nef is long known to be secreted, and a body of work sustainsthat most of it is secreted in EVs. Although most of the experimental evidence implicatesectosomes as the primary source of secreted Nef, exosomes could also play a role. Nefsecreted in EVs was suggested to have a range of effects on target cells, such as induction ofapoptosis and, at a neurological level, Beta-Amyloid induction and the disruption of theblood-brain barrier; (V) HIV induces the expression of several types of RNA, such as vRNA-TAR, that are incorporated in exosomes and have been reported to have important functions in target cells, suggesting an important role in HIV infection. Conversely, host miRNAs carried by host exosomes could exert a protective effect against HIV pathogenesis. The viral protein Nef was described to influence miRNA export in exosomes/retention in Nef-expressing cells, thusinfluencing gene expression; (VI) The identification of the biomolecules transported by exosomes and the elucidation of their immune regulatory effect in HIV-positive individuals will provide new insights into the role of these immunomodulatory nanovesicles in AIDS pathogenesis; (VII) Exosomes from different cell sources play different roles in HIV pathogenesis. Whereas exosomes/EVs from infected cells may promote viral replication and thedissemination of infection, exosomes/EVs from uninfected tissues or cells could protect theimmune system against the virus, as described in CD8 cells. The direction of the actiondepends on the cargo, the type of their cell of origin, and the interaction with viral proteins;(VIII) Exosomes from semen and breast milk have a potential anti-HIV activity. Thebiomonitoring of HIV/AIDS progression could be performed through the evaluation ofexosomes derived from different biological fluids; (IX) Engineered exosomes could be used as vectors for therapeutic molecules and nanosystems.
Conclusions
In recent decades, several studies have confirmed that microRNAs are major players in the pathogenesis of various diseases such as cancer, cardiovascular diseases, and also some infectious diseases. These molecules are able to inhibit, activatie, and modulate a wide range of different cellular processes, and can affect molecular targets mediating their pleiotropic effects. It has been shown that the HIV virus can affect the regulation and modulation of miRNA networks dictating their effects on the infected cells. Deregulation of miRNAs is associated with HIV progression in infected patients. Hence, identification of the miRNA sequences in patients infected with HIV, will facilitate ongoing research providing a better understanding of underlying cellular and molecular pathways involvied in HIV pathogenesis. Moreover, it is likely that miRNAs could be employed as diagnostic biomarkers for monitoring patients who are infected with HIV. Besides miRNAs, exosomes are another factor involved in HIV pathogenesis. Exosomes by targeting their cargos (i.e., DNAs, RNAs, miRNAs, viral proteins) to recipient cells could be involved in HIV pathogenesis. To understand the interaction between exosomes and/or EVs and their respective cargos, with HIV, and the modulation of the immune system occurring during viral infection, it is necessary to improve, standardize, and better describe techniques for isolation and characterization. A clear determination of the type(s) of vesicles contributing to Nef transfer might help researchers to find methods for specific blockage of the pathways involved in controlling viral transmission. Studies of exosome or EVs and viral infection is still an emerging area of HIV research. Nonetheless, our assumption is that researchers can overcome these problems during the coming years. so that more applications of exosomes will be used for disease management and drug discovery of HIV infection. Apparently, HIV controls cell export mechanisms in multiple ways. It is possible that there is a relationship between viral and host RNA export and exosomal pathways, whereas intact virus export and secretion of Nef are apparently a cell membrane-driven process. Nevertheless, there is insufficient information available on the pathways due to difficulties in distinguishing exosomes from ectosomes or micro-vesicles. Our belief is that a clear understanding of the relationship between HIV infection and progression, and micro-vesicles or exosomes, would make it possible to better understand HIV infection and may provide new opportunities for an effective cure.
In conclusion, identification of various miRNAs and exosomes involved in HIV pathogenesis could pave the way for the development of new and effective therapeutic approaches which offer new hope in patients infected with HIV. On the other hand, utilization of microRNAs and exosomes are subject to some limitations, such as the variation of microRNA expression patterns in different samples, and the high cost of isolation and characterization of exosomes, which are partly responsible for the fact that no microRNA and exosome applications have yet been introduced into clinical practice.
Acknowledgments
Funding
Michael R Hamblin was supported by US NIH Grants R01AI050875 and R21AI121700.
Abbreviations
- AARSD1
Alanyl-TRNA Synthetase Domain Containing 1
- AATF
Apoptosis Antagonizing Transcription Factor
- AATK
Apoptosis Associated Tyrosine Kinase
- AIDS
acquired immune deficiency syndrome
- AP-1
activating protein-1
- ART
Antiretroviral Therapy
- ATF2
Activating Transcription Factor 2
- Cdk9
cyclin-dependent kinase 9
- CXCR
C-X-C chemokine receptor
- DAP3
death-associated protein 3
- ECs
Elite controllers
- EVs
extracellular vesicles
- FOXO3
Forkhead box O3
- G-CSF
Granulocyte colony-stimulating factor
- HAART
highly active antiretroviral therapy
- HAND
Human immunodeficiency virus-associated neurological disorders
- Hck
hemopoietic cell kinase
- HEK 293
Human embryonic kidney 293 cells
- HIV
Human Immunodeficiency Virus
- HIVCM
Human Immunodeficiency Virus cardiomyopathy
- HIVE
Human Immunodeficiency Virus Encephalopathy
- HMOX1
hemeoxygenase (decycling) 1
- IER3
immediate early response 3
- ILV
intraluminal vesicle
- IRF
Interferon regulatory factor
- LTNP
Long term non-progressor
- LTR
Long terminal repeat
- MAPK
Mitogen-activated protein kinase
- MDCCs
monocyte-derived dendritic cells
- MDM
monocyte-derived macrophages
- MECP2
methyl CpG binding protein 2
- microRNA
microRNA
- NAMPT
Nicotinamidephosphoribosyltransferase
- Nef
Negative Regulatory Factor
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cell
- Nup153
Nucleoporin 153
- PABPC4
poly(A) binding protein cytoplasmic 4
- PBL
Peripheral blood lymphocyte
- PBMC
Peripheral blood mononuclear cell
- PCAF
P300/CBP-associated factor
- PLZF
promyelocytic leukemia zinc finger
- PNUTS
phosphatase 1 nuclear-targeting subunit
- PTEN
Phosphatase and tensin homolog
- RNase
ribonucleases
- SIRT1
Sirtuin 1
- SLC27A1
Solute Carrier Family 27 Member 1
- SMR
secretion modification region
- Sp1
specificity protein 1
- TAR
Trans-activation response element
- Tat
Trans-Activator of Transcription
- TLDA
TaqMan low-density array
- TLR
Toll-like receptor
- TNPO3
Transportin-3
- TRAF6
TNF receptor associated factor 6
- UTR
Untranslated region
- VCs
viremic controllers
- Vif
Viral infectivity factor
- VPs
viremic progressors
Footnotes
Conflicts of interest
Michael R Hamblin is on the following Scientific Advisory Boards
Transdermal Cap Inc, Cleveland, OH
BeWell Global Inc, Wan Chai, Hong Kong
Hologenix Inc. Santa Monica, CA
LumiThera Inc, Poulsbo, WA
Vielight, Toronto, Canada
Bright Photomedicine, Sao Paulo, Brazil
Quantum Dynamics LLC, Cambridge, MA
Global Photon Inc, Bee Cave, TX
Medical Coherence, Boston MA
NeuroThera, Newark DE
JOOVV Inc, Minneapolis-St. Paul MN
AIRx Medical, Pleasanton CA
FIR Industries, Inc. Ramsey, NJ
UVLRx Therapeutics, Oldsmar, FL
Ultralux UV Inc, Lansing MI
Illumiheal & Petthera, Shoreline, WA
MB Lasertherapy, Houston, TX
ARRC LED, San Clemente, CA
Varuna Biomedical Corp. Incline Village, NV
Niraxx Light Therapeutics, Inc, Boston, MA
Dr Hamblin has been a consultant for
Lexington Int, Boca Raton, FL
USHIO Corp, Japan
Merck KGaA, Darmstadt, Germany
Philips Electronics Nederland B.V.
Johnson & Johnson Inc, Philadelphia, PA
Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany
Dr Hamblin is a stockholder in
Global Photon Inc, Bee Cave, TX
Mitonix, Newark, DE.
References
- 1.Blattner W, Gallo RC and Temin H. Hiv causes AIDS. Science. 1988; 241:515–6. [DOI] [PubMed] [Google Scholar]
- 2.Lee C, Kernoff PA, Phillips A, et al. Serial CD4 lymphocyte counts and development of AIDS. The Lancet. 1991; 337:389–92. [DOI] [PubMed] [Google Scholar]
- 3.Maracy MR, Mostafaei S, Moghoofei M and Mansourian M. Impact of HIV risk factors on survival in Iranian HIV-infected patients: A Bayesian approach to retrospective cohort. HIV & AIDS Review International Journal of HIV-Related Problems. 2017; 16:100–6. [Google Scholar]
- 4.Zhang Q, Frange P, Blanche S and Casanova J-L. Pathogenesis of infections in HIV-infected individuals: insights from primary immunodeficiencies. Current opinion in immunology. 2017; 48:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio C. The global HIV epidemic: What the pathologist needs to know Seminars in diagnostic pathology. Vol 34, Elsevier, 2017: 314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non–AIDS-defining morbid events during suppressive antiretroviral treatment. The Journal of infectious diseases. 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PloS one. 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D: A: D): a multicohort collaboration. The Lancet. 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 9.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS pathogens. 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. The Lancet infectious diseases. 2014; 14:281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucero C, Torres B, León A, et al. Rate and predictors of non-AIDS events in a cohort of HIV-infected patients with a CD4 T cell count above 500 cells/mm3. AIDS research and human retroviruses. 2013; 29:1161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberg JA. Aging, inflammation, and HIV infection. Topics in antiviral medicine. 2012; 20:101. [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks SG, Lewin SR and Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet (London, England). 2013; 382:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavakolizadeh J, Roshanaei K, Salmaninejad A, et al. MicroRNAs and exosomes in depression: Potential diagnostic biomarkers. 2018; 119:3783–97. [DOI] [PubMed] [Google Scholar]
- 15.Rabieian R, Boshtam M, Zareei M, Kouhpayeh S, Masoudifar A and Mirzaei H. Plasminogen Activator Inhibitor Type-1 as a Regulator of Fibrosis. 2018; 119:17–27. [DOI] [PubMed] [Google Scholar]
- 16.Saeedi Borujeni MJ, Esfandiary E, Taheripak G, Codoner-Franch P, Alonso-Iglesias E and Mirzaei H. Molecular aspects of diabetes mellitus: Resistin, microRNA, and exosome. 2018; 119:1257–72. [DOI] [PubMed] [Google Scholar]
- 17.Keshavarzi M, Sorayayi S, Jafar Rezaei M, et al. MicroRNAs-Based Imaging Techniques in Cancer Diagnosis and Therapy. 2017; 118:4121–8. [DOI] [PubMed] [Google Scholar]
- 18.Mirzaei H, Ferns GA, Avan A and Mobarhan MG. Cytokines and MicroRNA in Coronary Artery Disease. Advances in clinical chemistry. 2017; 82:47–70. [DOI] [PubMed] [Google Scholar]
- 19.Gholamin S and Mirzaei H. GD2-targeted immunotherapy and potential value of circulating microRNAs in neuroblastoma. 2018; 233:866–79. [DOI] [PubMed] [Google Scholar]
- 20.Sun B, Yang R and Mallardo M. Roles of microRNAs in HIV-1 Replication and Latency. MicroRNA (Shariqah, United Arab Emirates). 2016; 5:120–3. [DOI] [PubMed] [Google Scholar]
- 21.Raposo G and Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013; 200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch JL, Stapleton JT and Okeoma CM. Vehicles of intercellular communication: exosomes and HIV-1. The Journal of general virology. 2019; 100:350–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abels ER and Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cellular and molecular neurobiology. 2016; 36:301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantin R, Diou J, Belanger D, Tremblay AM and Gilbert C. Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. Journal of immunological methods. 2008; 338:21–30. [DOI] [PubMed] [Google Scholar]
- 25.Wahid F, Shehzad A, Khan T and Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2010; 1803:1231–43. [DOI] [PubMed] [Google Scholar]
- 26.Bruscella P, Bottini S, Baudesson C, Pawlotsky J-M, Feray C and Trabucchi M. Viruses and miRNAs: More friends than foes. Frontiers in microbiology. 2017; 8:824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balasubramaniam M, Pandhare J and Dash C. Are microRNAs Important Players in HIV-1 Infection? An Update. Viruses. 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sánchez-Del Cojo M, López-Huertas MR, Díez-Fuertes F, et al. Changes in the cellular microRNA profile by the intracellular expression of HIV-1 Tat regulator: A potential mechanism for resistance to apoptosis and impaired proliferation in HIV-1 infected CD4+ T cells. PloS one. 2017; 12:e0185677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nathans R, Chu C-y, Serquina AK, Lu C-C, Cao H and Rana TM. Cellular microRNA and P bodies modulate host-HIV-1 interactions. Molecular cell. 2009; 34:696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen I, Bochnakiem A, Zisoulis D, et al. Interferon-inducible miR-128 modulates HIV-1 replication by targeting TNPO3 mRNA. bioRxiv. 2017:195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai LM and Yu D. MicroRNAs in common diseases and potential therapeutic applications. Clinical and Experimental Pharmacology and Physiology. 2010; 37:102–7. [DOI] [PubMed] [Google Scholar]
- 32.Su B, Fu Y, Liu Y, et al. Potential Application of MicroRNA Profiling to the Diagnosis and Prognosis of HIV-1 Infection. Frontiers in microbiology. 2018; 9:3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hariharan M, Scaria V, Pillai B and Brahmachari SK. Targets for human encoded microRNAs in HIV genes. Biochemical and biophysical research communications. 2005; 337:1214–8. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nature medicine. 2007; 13:1241–7. [DOI] [PubMed] [Google Scholar]
- 35.Wang P, Qu X, Zhou X, et al. Two cellular microRNAs, miR-196b and miR-1290, contribute to HIV-1 latency. Virology. 2015; 486:228–38. [DOI] [PubMed] [Google Scholar]
- 36.Wang FS, Zhang L, Douek D, McMichael A, Xu XN and Lewin SR. Strategies for an HIV cure: progress and challenges. Nature immunology. 2018; 19:1155–8. [DOI] [PubMed] [Google Scholar]
- 37.Grundhoff A and Sullivan CS. Virus-encoded microRNAs. Virology. 2011; 411:325–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Althaus CF, Vongrad V, Niederost B, et al. Tailored enrichment strategy detects low abundant small noncoding RNAs in HIV-1 infected cells. Retrovirology. 2012; 9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J and Cullen BR. Analysis of the interaction of primate retroviruses with the human RNA interference machinery. Journal of virology. 2007; 81:12218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouellet DL and Provost P. Current knowledge of MicroRNAs and noncoding RNAs in virus-infected cells. Methods in molecular biology (Clifton, NJ). 2010; 623:35–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeung ML, Bennasser Y, Watashi K, Le SY, Houzet L and Jeang KT. Pyrosequencing of small non-coding RNAs in HIV-1 infected cells: evidence for the processing of a viral-cellular double-stranded RNA hybrid. Nucleic acids research. 2009; 37:6575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schopman NC, Willemsen M, Liu YP, et al. Deep sequencing of virus-infected cells reveals HIV-encoded small RNAs. Nucleic acids research. 2012; 40:414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boss IW and Renne R. Viral miRNAs and immune evasion. Biochimica et biophysica acta. 2011; 1809:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urbanelli L, Magini A, Buratta S, et al. Signaling pathways in exosomes biogenesis, secretion and fate. Genes. 2013; 4:152–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abels ER and Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Springer, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lotvall J and Valadi H. Cell to cell signalling via exosomes through esRNA. Cell adhesion & migration. 2007; 1:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caby M-P, Lankar D, Vincendeau-Scherrer C, Raposo Gß and Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International immunology. 2005; 17:879–87. [DOI] [PubMed] [Google Scholar]
- 48.Pisitkun T, Shen R-F and Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America. 2004; 101:13368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J and Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PloS one. 2010; 5:e8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. The Journal of immunology. 2007; 179:1969–78. [DOI] [PubMed] [Google Scholar]
- 51.Admyre C, Grunewald J, Thyberg J, et al. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. European Respiratory Journal. 2003; 22:578–83. [DOI] [PubMed] [Google Scholar]
- 52.Sahl√©n GrE, Egevad L, Ahlander A, Norl√©n BJ, Ronquist G and Nilsson BO. Ultrastructure of the secretion of prostasomes from benign and malignant epithelial cells in the prostate. The Prostate. 2002; 53:192–9. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan R, Saez F, Girouard J and Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells, Molecules, and Diseases. 2005; 35:1–10. [DOI] [PubMed] [Google Scholar]
- 54.Chahar HS, Bao X and Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015; 7:3204–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schorey JS, Cheng Y, Singh PP and Smith VL. Exosomes and other extracellular vesicles in host‚Äìpathogen interactions. EMBO reports. 2015; 16:24–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janas AM, Sapo≈Ñ K, Janas T, Stowell MH and Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2016; 1858:1139–51. [DOI] [PubMed] [Google Scholar]
- 57.Meckes DG and Raab-Traub N. Microvesicles and viral infection. Journal of virology. 2011; 85:12844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Théry C, Boussac M, Véron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. The Journal of Immunology. 2001; 166:7309–18. [DOI] [PubMed] [Google Scholar]
- 59.Camussi G, Deregibus MC, Bruno S, Cantaluppi V and Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010; 78:838–48. [DOI] [PubMed] [Google Scholar]
- 60.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences. 2010; 107:6328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narayanan A, Iordanskiy S, Das R, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. Journal of Biological Chemistry. 2013; 288:20014–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ellwanger JH, Veit TD and Chies JAB. Exosomes in HIV infection: A review and critical look. Infection, Genetics and Evolution. 2017; 53:146–54. [DOI] [PubMed] [Google Scholar]
- 63.Zeller JM, McCain NL and Swanson B. Immunological and virological markers of HIV-disease progression. Journal of the Association of Nurses in AIDS Care. 1996; 7:15–27. [DOI] [PubMed] [Google Scholar]
- 64.Pantaleo G, Menzo S, Vaccarezza M, et al. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. New England Journal of Medicine. 1995; 332:209–16. [DOI] [PubMed] [Google Scholar]
- 65.Gonzalo-Gil E, Ikediobi U and Sutton RE. Focus: Infectious Diseases: Mechanisms of Virologic Control and Clinical Characteristics of HIV+ Elite/Viremic Controllers. The Yale journal of biology and medicine. 2017; 90:245. [PMC free article] [PubMed] [Google Scholar]
- 66.Egaña-Gorroño L, Guardo AC, Bargalló ME, et al. MicroRNA profile in CD8+ T-lymphocytes from HIV-infected individuals: relationship with antiviral immune response and disease progression. PloS one. 2016; 11:e0155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikhail M, Wang B and Saksena NK. Mechanisms involved in non-progressive HIV disease. AIDS Rev 2003; 5:230–44. [PubMed] [Google Scholar]
- 68.Walker BD. Elite control of HIV Infection: implications for vaccines and treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA 2007; 15:134–6. [PubMed] [Google Scholar]
- 69.Reynoso R, Laufer N, Hackl M, et al. MicroRNAs differentially present in the plasma of HIV elite controllers reduce HIV infection in vitro. Scientific reports. 2014; 4:5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okulicz JF and Lambotte O. Epidemiology and clinical characteristics of elite controllers. Current Opinion in HIV and AIDS. 2011; 6:163–8. [DOI] [PubMed] [Google Scholar]
- 71.Egaña-Gorroño L, Guardo AC, Bargalló ME, et al. MicroRNA profile in CD8+ T-lymphocytes from HIV-infected individuals: relationship with antiviral immune response and disease progression. PloS one. 2016; 11:e0155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV natural history study. The Journal of infectious diseases. 2009; 200:1714–23. [DOI] [PubMed] [Google Scholar]
- 73.Blankson J. Control of HIV-1 replication in elite suppressors. Discovery medicine. 2010; 9:261–6. [PubMed] [Google Scholar]
- 74.Triboulet R, Mari B, Lin Y-L, et al. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science. 2007; 315:1579–82. [DOI] [PubMed] [Google Scholar]
- 75.Klase Z, Houzet L and Jeang K-T. MicroRNAs and HIV-1: complex interactions. Journal of Biological Chemistry. 2012; 287:40884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernardi G. Cellular microRNAs in HIV replication and latency; 2017. [Google Scholar]
- 77.Egaña-Gorroño L, Escribà T, Boulanger N, et al. Differential microRNA expression profile between stimulated PBMCs from HIV-1 infected elite controllers and viremic progressors. PLoS One. 2014; 9:e106360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Witwer KW, Watson AK, Blankson JN and Clements JE. Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients. Retrovirology. 2012; 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Egana-Gorrono L, Guardo AC, Bargallo ME, et al. MicroRNA Profile in CD8+ T-Lymphocytes from HIV-Infected Individuals: Relationship with Antiviral Immune Response and Disease Progression. PloS one. 2016; 11:e0155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantri CK, Pandhare Dash J, Mantri JV and Dash CC. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PloS one. 2012; 7:e51387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chiang K, Sung TL and Rice AP. Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. Journal of virology. 2012; 86:3244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Ye L, Hou W, et al. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood. 2009; 113:671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Batorsky R, Sergeev RA and Rouzine IM. The route of HIV escape from immune response targeting multiple sites is determined by the cost-benefit tradeoff of escape mutations. PLoS computational biology. 2014; 10:e1003878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guha D and Ayyavoo V. Innate immune evasion strategies by human immunodeficiency virus type 1. Isrn Aids. 2013; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfeffer S, Zavolan M, Grässer FA, et al. Identification of virus-encoded microRNAs. Science. 2004; 304:734–6. [DOI] [PubMed] [Google Scholar]
- 86.Fruci D, Rota R and Gallo A. The role of HCMV and HIV-1 microRNAs: processing, and mechanisms of action during viral infection. Frontiers in microbiology. 2017; 8:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bennasser Y, Le S-Y, Yeung ML and Jeang K-T. HIV-1 encoded candidate micro-RNAs and their cellular targets. Retrovirology. 2004; 1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uğur AR and Özdemir M. Might miRNAs Be Related to Mother-to-Child Transmission of HIV-1? A Short Review on Putative Viral miRNAs Encoded by HIV-1. Journal of Pediatric Infectious Diseases. 2017. [Google Scholar]
- 89.Ouellet DL, Plante I, Landry P, et al. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic acids research. 2008; 36:2353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klase Z, Kale P, Winograd R, et al. HIV-1 TAR element is processed by Dicer to yield a viral micro-RNA involved in chromatin remodeling of the viral LTR. BMC molecular biology. 2007; 8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klase Z, Winograd R, Davis J, et al. HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology. 2009; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Omoto S, Ito M, Tsutsumi Y, et al. HIV-1 nef suppression by virally encoded microRNA. Retrovirology. 2004; 1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.You X, Zhang Z, Fan J, Cui Z and Zhang X-E. Functionally orthologous viral and cellular microRNAs studied by a novel dual-fluorescent reporter system. PLoS One. 2012; 7:e36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Fan M, Geng G, et al. A novel HIV-1-encoded microRNA enhances its viral replication by targeting the TATA box region. Retrovirology. 2014; 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma M. Apoptosis-antagonizing transcription factor (AATF) gene silencing: role in induction of apoptosis and down-regulation of estrogen receptor in breast cancer cells. Biotechnology letters. 2013; 35:1561–70. [DOI] [PubMed] [Google Scholar]
- 96.Kaul D, Ahlawat A and Gupta SD. HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Molecular and cellular biochemistry. 2009; 323:143–8. [DOI] [PubMed] [Google Scholar]
- 97.Nair V and Zavolan M. Virus-encoded microRNAs: novel regulators of gene expression. Trends in microbiology. 2006; 14:169–75. [DOI] [PubMed] [Google Scholar]
- 98.Sullivan CS, Sung CK, Pack CD, et al. Murine Polyomavirus encodes a microRNA that cleaves early RNA transcripts but is not essential for experimental infection. Virology. 2009; 387:157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boss IW, Plaisance KB and Renne R. Role of virus-encoded microRNAs in herpesvirus biology. Trends in microbiology. 2009; 17:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pfeffer S, Sewer A, Lagos-Quintana M, et al. Identification of microRNAs of the herpesvirus family. Nature methods. 2005; 2:269–76. [DOI] [PubMed] [Google Scholar]
- 101.Kincaid RP, Burke JM and Sullivan CS. RNA virus microRNA that mimics a B-cell oncomiR. Proceedings of the National Academy of Sciences of the United States of America. 2012; 109:3077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennasser Y, Le SY, Yeung ML and Jeang KT. HIV-1 encoded candidate micro-RNAs and their cellular targets. Retrovirology. 2004; 1:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Omoto S and Fujii YR. Regulation of human immunodeficiency virus 1 transcription by nef microRNA. The Journal of general virology. 2005; 86:751–5. [DOI] [PubMed] [Google Scholar]
- 104.Kaul D, Ahlawat A and Gupta SD. HIV-1 genome-encoded hiv1-mir-H1 impairs cellular responses to infection. Molecular and cellular biochemistry. 2009; 323:143–8. [DOI] [PubMed] [Google Scholar]
- 105.Ouellet DL, Plante I, Landry P, et al. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic acids research. 2008; 36:2353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klase Z, Winograd R, Davis J, et al. HIV-1 TAR miRNA protects against apoptosis by altering cellular gene expression. Retrovirology. 2009; 6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whisnant AW, Bogerd HP, Flores O, et al. In-depth analysis of the interaction of HIV-1 with cellular microRNA biogenesis and effector mechanisms. mBio. 2013; 4:e000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y, Fan M, Geng G, et al. A novel HIV-1-encoded microRNA enhances its viral replication by targeting the TATA box region. Retrovirology. 2014; 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bernard MA, Zhao H, Yue SC, Anandaiah A, Koziel H and Tachado SD. Novel HIV-1 miRNAs stimulate TNFα release in human macrophages via TLR8 signaling pathway. PLoS One. 2014; 9:e106006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saeedi Borujeni MJ, Esfandiary E, Baradaran A, et al. Molecular aspects of pancreatic beta-cell dysfunction: Oxidative stress, microRNA, and long noncoding RNA. 2018. [DOI] [PubMed] [Google Scholar]
- 111.Khani P, Nasri F, Khani Chamani F, et al. Genetic and epigenetic contribution to astrocytic gliomas pathogenesis. 2018. [DOI] [PubMed] [Google Scholar]
- 112.Jamali L, Tofigh R, Tutunchi S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. 2018; 233:8538–50. [DOI] [PubMed] [Google Scholar]
- 113.Keshavarz M and Mirzaei H. Influenza vaccine: Where are we and where do we go? 2018:e2014. [Google Scholar]
- 114.Huang Q, Huang C, Luo Y, He F and Zhang R. Circulating lncRNA NEAT1 correlates with increased risk, elevated severity and unfavorable prognosis in sepsis patients. The American journal of emergency medicine. 2018; 36:1659–63. [DOI] [PubMed] [Google Scholar]
- 115.Moghoofei M, Bokharaei-Salim F, Esghaei M, et al. microRNAs 29, 150, 155, 223 level and their relation to viral and immunological markers in HIV-1 infected naive patients. Future Virology. 2018; 13:637–45. [Google Scholar]
- 116.Munshi SU, Panda H, Holla P, Rewari BB and Jameel S. MicroRNA-150 is a potential biomarker of HIV/AIDS disease progression and therapy. PLoS One. 2014; 9:e95920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang H-S, Chen X-Y, Wu T-C, Sang W-W and Ruan Z. MiR-34a is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation through the SIRT1/NFκB pathway. FEBS letters. 2012; 586:4203–7. [DOI] [PubMed] [Google Scholar]
- 118.Zhang H-S, Chen X-Y, Wu T-C, Sang W-W and Ruan Z. MiR‚Äê34a is involved in Tat‚Äêinduced HIV‚Äê1 long terminal repeat (LTR) transactivation through the SIRT1/NFŒ∫B pathway. FEBS letters. 2012; 586:4203–7. [DOI] [PubMed] [Google Scholar]
- 119.Zhao W, Liu C, Shi C, Fan T, Chu K and Ma Y. Role of miR-124a in T cell activation and immunity in AIDS patients. Experimental and therapeutic medicine. 2017; 14:4807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qi Y, Hu H, Guo H, et al. MicroRNA profiling in plasma of HIV-1 infected patients: potential markers of infection and immune status. Journal of Public Health and Emergency. 2017; 1. [Google Scholar]
- 121.Mantri CK, Dash JP, Mantri JV and Dash CC. Cocaine enhances HIV-1 replication in CD4+ T cells by down-regulating MiR-125b. PloS one. 2012; 7:e51387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 123.Wong JK, Hezareh M, G√ºnthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997; 278:1291–5. [DOI] [PubMed] [Google Scholar]
- 124.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003; 9:727. [DOI] [PubMed] [Google Scholar]
- 125.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nature medicine. 2007; 13:1241. [DOI] [PubMed] [Google Scholar]
- 126.Patel P, Ansari MY, Bapat S, Thakar M, Gangakhedkar R and Jameel S. The microRNA miR-29a is associated with human immunodeficiency virus latency. Retrovirology. 2014; 11:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chiang K, Liu H and Rice AP. miR-132 enhances HIV-1 replication. Virology. 2013; 438:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang P, Qu X, Zhou X, et al. Two cellular microRNAs, miR-196b and miR-1290, contribute to HIV-1 latency. Virology. 2015; 486:228–38. [DOI] [PubMed] [Google Scholar]
- 129.Eletto D, Russo G, Passiatore G, et al. Inhibition of SNAP25 expression by HIV‚Äê1 Tat involves the activity of mir‚Äê128a. Journal of cellular physiology. 2008; 216:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mishra R and Singh SK. HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. Journal of Neuroscience. 2013; 33:5992–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu Z, Asahchop EL, Branton WG, Gelman BB, Power C and Hobman TC. MicroRNAs upregulated during HIV infection target peroxisome biogenesis factors: Implications for virus biology, disease mechanisms and neuropathology. PLoS pathogens. 2017; 13:e1006360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bagashev A, Mukerjee R, Santerre M, et al. Involvement of miR-196a in HIV-associated neurocognitive disorders. Apoptosis. 2014; 19:1202–14. [DOI] [PubMed] [Google Scholar]
- 133.Duan M, Yao H, Hu G, Chen X, Lund AK and Buch S. HIV Tat induces expression of ICAM-1 in HUVECs: implications for miR-221/−222 in HIV-associated cardiomyopathy. PloS one. 2013; 8:e60170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng K, Rai P, Plagov A, et al. MicroRNAs in HIV-associated nephropathy (HIVAN). Experimental and molecular pathology. 2013; 94:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sung TL and Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS pathogens. 2009; 5:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duskova K, Nagilla P, Le HS, et al. MicroRNA regulation and its effects on cellular transcriptome in human immunodeficiency virus-1 (HIV-1) infected individuals with distinct viral load and CD4 cell counts. BMC infectious diseases. 2013; 13:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosca A, Anton G, Botezatu A, et al. miR-29a associates with viro-immunological markers of HIV infection in treatment experienced patients. Journal of medical virology. 2016; 88:2132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruelas DS, Chan JK, Oh E, et al. MicroRNA-155 reinforces HIV latency. Journal of Biological Chemistry. 2015; 290:13736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masotti A, Donninelli G, Da Sacco L, Varano B, Del Cornò M and Gessani S. HIV-1 gp120 influences the expression of microRNAs in human monocyte-derived dendritic cells via STAT3 activation. BMC genomics. 2015; 16:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rom S, Rom I, Passiatore G, et al. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. The FASEB Journal. 2010; 24:2292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eletto D, Russo G, Passiatore G, et al. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. Journal of cellular physiology. 2008; 216:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sung T-L and Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS pathogens. 2009; 5:e1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang H-S, Wu T-C, Sang W-W and Ruan Z. MiR-217 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation by down-regulation of SIRT1. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2012; 1823:1017–23. [DOI] [PubMed] [Google Scholar]
- 144.Swaminathan G, Rossi F, Sierra L-J, Gupta A, Navas-Martín S and Martín-García J. A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages. PLoS pathogens. 2012; 8:e1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mishra R, Chhatbar C and Singh SK. HIV-1 Tat C-mediated regulation of tumor necrosis factor receptor-associated factor-3 by microRNA 32 in human microglia. Journal of neuroinflammation. 2012; 9:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun G, Li H, Wu X, et al. Interplay between HIV-1 infection and host microRNAs. Nucleic acids research. 2011; 40:2181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tatro ET, Hefler S, Shumaker-Armstrong S, et al. Modulation of BK channel by MicroRNA-9 in neurons after exposure to HIV and methamphetamine. Journal of neuroimmune pharmacology. 2013; 8:1210–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao JY, Shen XJ, Zhang BF, et al. [Surveillance for viral diarrhea in sentinel hospitals in Henan province, 2013–2015]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2016; 37:1392–6. [DOI] [PubMed] [Google Scholar]
- 149.Yu B, Shao H, Su C, et al. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep 2016; 6:34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pita I, Horta-Vale AM, Cardoso H and Macedo G. Hepatitis B inactive carriers: An overlooked population? GE Portuguese Journal of Gastroenterology. 2014; 21:241–9. [Google Scholar]
- 151.Orecchini E, Doria M, Michienzi A, et al. The HIV-1 Tat protein modulates CD4 expression in human T cells through the induction of miR-222. RNA biology. 2014; 11:334–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mantri CK, Mantri JV, Pandhare J and Dash C. Methamphetamine Inhibits HIV-1 Replication in CD4+ T Cells by Modulating Anti–HIV-1 miRNA Expression. The American journal of pathology. 2014; 184:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kapoor R, Arora S, Ponia SS, Kumar B, Maddika S and Banerjea AC. The miRNA miR-34a enhances HIV-1 replication by targeting PNUTS/PPP1R10, which negatively regulates HIV-1 transcriptional complex formation. Biochemical Journal. 2015; 470:293–302. [DOI] [PubMed] [Google Scholar]
- 154.Monteleone K, Selvaggi C, Cacciotti G, et al. MicroRNA-29 family expression and its relation to antiviral immune response and viro-immunological markers in HIV-1-infected patients. BMC infectious diseases. 2015; 15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Adoro S, Cubillos-Ruiz JR, Chen X, et al. IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nature communications. 2015; 6:7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lodge R, Barbosa JAF, Lombard-Vadnais F, et al. Host MicroRNAs-221 and-222 inhibit HIV-1 entry in macrophages by targeting the CD4 viral receptor. Cell reports. 2017; 21:141–53. [DOI] [PubMed] [Google Scholar]
- 157.Ahluwalia JK, Khan SZ, Soni K, et al. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology. 2008; 5:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Houzet L, Klase Z, Yeung ML, et al. The extent of sequence complementarity correlates with the potency of cellular miRNA-mediated restriction of HIV-1. Nucleic acids research. 2012; 40:11684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen X-Y, Zhang H-S, Wu T-C, Sang W-W and Ruan Z. Down-regulation of NAMPT expression by miR-182 is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation. The international journal of biochemistry & cell biology. 2013; 45:292–8. [DOI] [PubMed] [Google Scholar]
- 160.Duskova K, Nagilla P, Le H-S, et al. MicroRNA regulation and its effects on cellular transcriptome in human immunodeficiency virus-1 (HIV-1) infected individuals with distinct viral load and CD4 cell counts. BMC infectious diseases. 2013; 13:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Farberov L, Herzig E, Modai S, Isakov O, Hizi A and Shomron N. MicroRNA-mediated regulation of p21 and TASK1 cellular restriction factors enhances HIV-1 infection. J Cell Sci 2015; 128:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen C-J, Jia Y-H, Tian R-R, Ding M, Zhang C and Wang J-H. Translation of Pur-α is targeted by cellular miRNAs to modulate the differentiation-dependent susceptibility of monocytes to HIV-1 infection. The FASEB Journal. 2012; 26:4755–64. [DOI] [PubMed] [Google Scholar]
- 163.Devadas K, Biswas S, Haleyurgirisetty M, et al. Identification of host micro RNAs that differentiate HIV-1 and HIV-2 infection using genome expression profiling Techniques. Viruses. 2016; 8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Seddiki N, Phetsouphanh C, Swaminathan S, et al. The microRNA-9/B-lymphocyte-induced maturation protein-1/IL-2 axis is differentially regulated in progressive HIV infection. European journal of immunology. 2013; 43:510–20. [DOI] [PubMed] [Google Scholar]
- 165.Swaminathan S and Kelleher AD. MicroRNA modulation of key targets associated with T cell exhaustion in HIV-1 infection. Current Opinion in HIV and AIDS. 2014; 9:464–71. [DOI] [PubMed] [Google Scholar]
- 166.Zhu L, Qiu C, Ma C, Zhang X and Xu J. The prognostic and diagnostic use of microRNA expression in chronic HIV infection. Retrovirology. 2012; 9:P166. [Google Scholar]
- 167.Kaul D and Hussain A. Cellular AATF gene encodes a novel miRNA that can contribute to HIV-1 latency. 2009. [PubMed] [Google Scholar]
- 168.Napuri J, Pilakka-Kanthikeel S, Raymond A, et al. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PloS one. 2013; 8:e83682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Guha D, Mancini A, Sparks J and Ayyavoo V. HIV-1 Infection Dysregulates Cell Cycle Regulatory Protein p21 in CD4+ T Cells Through miR-20a and miR-106b Regulation. Journal of cellular biochemistry. 2016; 117:1902–12. [DOI] [PubMed] [Google Scholar]
- 170.Amaral AJ, Andrade J, Foxall RB, et al. miRNA profiling of human naive CD4 T cells links miR-34c-5p to cell activation and HIV replication. The EMBO journal. 2017; 36:346–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu X, Zhang L-L, Yin L-B, et al. Deregulated Microrna-21 expression in Monocytes from hiV-infected Patients contributes to elevated iP-10 secretion in hiV infection. Frontiers in immunology. 2017; 8:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pilakka-Kanthikeel S, Raymond A, Atluri VSR, et al. Sterile alpha motif and histidine/aspartic acid domain-containing protein 1 (SAMHD1)-facilitated HIV restriction in astrocytes is regulated by miRNA-181a. Journal of neuroinflammation. 2015; 12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Advay S, Ahmadi A, Abdi M and Arabzadeh AM. Study of miR-29a-5p expression in HIV positive and HIV/HCV co-infected patients in Sanandaj-Iran. Hepatitis Monthly. 2017; 17. [Google Scholar]
- 174.Chiang K, Sung T-L and Rice AP. Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. Journal of virology. 2012; 86:3244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang X, Ye L, Hou W, et al. Cellular microRNA expression correlates with susceptibility of monocytes/macrophages to HIV-1 infection. Blood. 2009; 113:671–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Quaranta MT, Olivetta E, Sanchez M, et al. miR-146a controls CXCR4 expression in a pathway that involves PLZF and can be used to inhibit HIV-1 infection of CD4+ T lymphocytes. Virology. 2015; 478:27–38. [DOI] [PubMed] [Google Scholar]
- 177.Omoto S and Fujii YR. Regulation of human immunodeficiency virus 1 transcription by nef microRNA. Journal of General Virology. 2005; 86:751–5. [DOI] [PubMed] [Google Scholar]
- 178.Ouellet DL, Plante I, Landry P, et al. Identification of functional microRNAs released through asymmetrical processing of HIV-1 TAR element. Nucleic acids research. 2008; 36:2353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kulkarni S, Savan R, Qi Y, et al. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature. 2011; 472:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Swaminathan G, Navas-Mart√≠n S and Mart√≠n-Garc√≠a J. MicroRNAs and HIV-1 infection: antiviral activities and beyond. Journal of molecular biology. 2014; 426:1178–97. [DOI] [PubMed] [Google Scholar]
- 181.Pilakka-Kanthikeel S and Nair MP. Interaction of drugs of abuse and microRNA with HIV: a brief review. Frontiers in Microbiology. 2015; 6:967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mirzaei H, Khataminfar S, Mohammadparast S, et al. Circulating microRNAs as potential diagnostic biomarkers and therapeutic targets in gastric cancer: current status and future perspectives. Current medicinal chemistry. 2016; 23:4135–50. [DOI] [PubMed] [Google Scholar]
- 183.Lecellier C-H, Dunoyer P, Arar K, et al. A cellular microRNA mediates antiviral defense in human cells. Science. 2005; 308:557–60. [DOI] [PubMed] [Google Scholar]
- 184.Silberman S, Goldman S, Mitchell D, et al. The interaction of CD4 with HIV-1 gp120. Seminars in immunology. Vol 3, 1991: 187–92. [PubMed] [Google Scholar]
- 185.Bleul CC, Wu L, Hoxie JA, Springer TA and Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proceedings of the National Academy of Sciences. 1997; 94:1925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Doranz BJ, Grovit-Ferbas K, Sharron MP, et al. A small-molecule inhibitor directed against the chemokine receptor CXCR4 prevents its use as an HIV-1 coreceptor. Journal of Experimental Medicine. 1997; 186:1395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kwak YT, Ivanov D, Guo J, Nee E and Gaynor RB. Role of the human and murine cyclin T proteins in regulating HIV-1 tat-activation. Journal of molecular biology. 1999; 288:57–69. [DOI] [PubMed] [Google Scholar]
- 188.De Iaco A, Santoni F, Vannier A, Guipponi M, Antonarakis S and Luban J. TNPO3 protects HIV-1 replication from CPSF6-mediated capsid stabilization in the host cell cytoplasm. Retrovirology. 2013; 10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fricke T, Valle-Casuso JC, White TE, et al. The ability of TNPO3-depleted cells to inhibit HIV-1 infection requires CPSF6. Retrovirology. 2013; 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wonderlich ER, Leonard JA and Collins KL. HIV immune evasion: disruption of antigen presentation by the HIV Nef protein. Advances in virus research. Vol 80, Elsevier, 2011: 103–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roof P, Ricci M, Genin P, et al. Differential regulation of HIV-1 clade-specific B, C, and E long terminal repeats by NF-κB and the Tat transactivator. Virology. 2002; 296:77–83. [DOI] [PubMed] [Google Scholar]
- 192.Frattari G, Aagaard L and Denton PW. The role of miR-29a in HIV-1 replication and latency. Journal of virus eradication. 2017; 3:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhan J, Qin S, Lu L, et al. miR-34a is a common link in both HIV-and antiretroviral therapy-induced vascular aging. Aging (Albany NY). 2016; 8:3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ma L, Shen C-J, Cohen EA, Xiong S-D and Wang J-H. miRNA-1236 inhibits HIV-1 infection of monocytes by repressing translation of cellular factor VprBP. PloS one. 2014; 9:e99535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Colombo M, Raposo G and Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014; 30:255–89. [DOI] [PubMed] [Google Scholar]
- 196.Antonyak MA and Cerione RA. Microvesicles as mediators of intercellular communication in cancer. Methods in molecular biology (Clifton, NJ). 2014; 1165:147–73. [DOI] [PubMed] [Google Scholar]
- 197.Yanez-Mo M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015; 4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tkach M and Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016; 164:1226–32. [DOI] [PubMed] [Google Scholar]
- 199.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. 2016; 113:E968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sampey GC, Saifuddin M, Schwab A, et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. 2016; 291:1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Narayanan A, Iordanskiy S, Das R, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. The Journal of biological chemistry. 2013; 288:20014–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Berkhout B, Silverman RH and Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989; 59:273–82. [DOI] [PubMed] [Google Scholar]
- 203.Ellwanger JH, Veit TD and Chies JAB. Exosomes in HIV infection: A review and critical look. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2017; 53:146–54. [DOI] [PubMed] [Google Scholar]
- 204.DeMarino C, Pleet ML, Cowen M, et al. Antiretroviral Drugs Alter the Content of Extracellular Vesicles from HIV-1-Infected Cells. Sci Rep 2018; 8:7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.DeMarino C and Kashanchi F. Presence of Viral microRNA in Extracellular Environments. EBioMedicine. 2017; 20:9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barclay RA, Schwab A, DeMarino C, et al. Exosomes from uninfected cells activate transcription of latent HIV-1. The Journal of biological chemistry. 2017; 292:11682–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Walker LM, Phogat SK, Chan-Hui P-Y, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009; 326:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cohen J. HIV/AIDS Researchers Reach for High-Hanging Fruit. American Association for the Advancement of Science, 2009. [DOI] [PubMed] [Google Scholar]
- 209.Kadiu I and Gendelman HE. Macrophage bridging conduit trafficking of HIV-1 through the endoplasmic reticulum and Golgi network. Journal of proteome research. 2011; 10:3225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang JJ, Horton R, Varthakavi V, Spearman P and Ratner L. Formation and release of virus-like particles by HIV-1 matrix protein. AIDS (London, England). 1999; 13:281–3. [DOI] [PubMed] [Google Scholar]
- 211.Thery C, Boussac M, Veron P, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. Journal of immunology (Baltimore, Md : 1950). 2001; 166:7309–18. [DOI] [PubMed] [Google Scholar]
- 212.Fang Y, Wu N, Gan X, Yan W, Morrell JC and Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS biology. 2007; 5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Usami Y, Popov S, Popova E, Inoue M, Weissenhorn W and Göttlinger HG. The ESCRT pathway and HIV-1 budding. Portland Press Limited, 2009. [DOI] [PubMed] [Google Scholar]
- 214.Chertova E, Chertov O, Coren LV, et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol 2006; 80:9039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gould SJ, Booth AM and Hildreth JE. The Trojan exosome hypothesis. Proceedings of the National Academy of Sciences. 2003; 100:10592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Amara A and Littman DR. After Hrs with HIV. The Journal of cell biology. 2003; 162:371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kadiu I, Narayanasamy P, Dash PK, Zhang W and Gendelman HE. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages. The Journal of Immunology. 2012; 189:744–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Madison MN and Okeoma CM. Exosomes: implications in HIV-1 pathogenesis. Viruses. 2015; 7:4093–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Teow S-Y, Nordin AC, Ali SA and Khoo AS-B. Exosomes in human immunodeficiency virus type I pathogenesis: threat or opportunity? Advances in Virology. 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Izquierdo-Useros N, Puertas MC, Borràs FE, Blanco J and Martinez-Picado J. Exosomes and retroviruses: the chicken or the egg? Cellular microbiology. 2011; 13:10–7. [DOI] [PubMed] [Google Scholar]
- 221.Roth WW, Huang MB, Addae Konadu K, Powell MD and Bond VC. Micro RNA in exosomes from HIV-infected macrophages. International journal of environmental research and public health. 2015; 13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Aqil M, Naqvi AR, Mallik S, Bandyopadhyay S, Maulik U and Jameel S. The HIV Nef protein modulates cellular and exosomal miRNA profiles in human monocytic cells. Journal of extracellular vesicles. 2014; 3:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McNamara RP, Costantini LM, Myers TA, et al. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. mBio. 2018; 9:e02344–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Konadu KA, Anderson JS, Huang M-B, et al. Hallmarks of HIV-1 pathogenesis are modulated by Nef’s Secretion Modification Region. Journal of AIDS & clinical research. 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lenassi M, Cagney G, Liao M, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010; 11:110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee J-H, Ostalecki C, Zhao Z, et al. HIV Activates the Tyrosine Kinase Hck to Secrete ADAM Protease-Containing Extracellular Vesicles. EBioMedicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Konadu KA, Chu J, Huang MB, et al. Association of Cytokines With Exosomes in the Plasma of HIV-1–Seropositive Individuals. The Journal of infectious diseases. 2014; 211:1712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rahimian P and He JJ. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. Journal of neurovirology. 2016; 22:774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Khan MB, Lang MJ, Huang M-B, et al. Nef exosomes isolated from the plasma of individuals with HIV-associated dementia (HAD) can induce Aβ 1–42 secretion in SH-SY5Y neural cells. Journal of neurovirology. 2016; 22:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Raymond A, Diaz P, Chevelon S, et al. Microglia-derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine-based delivery of Nef peptides. Journal of neurovirology. 2016; 22:129–39. [DOI] [PubMed] [Google Scholar]
- 231.Arenaccio C, Chiozzini C, Columba-Cabezas S, Manfredi F and Federico M. Cell activation and HIV-1 replication in unstimulated CD4+ T lymphocytes ingesting exosomes from cells expressing defective HIV-1. Retrovirology. 2014; 11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Aqil M, Mallik S, Bandyopadhyay S, Maulik U and Jameel S. Transcriptomic analysis of mRNAs in human monocytic cells expressing the HIV-1 Nef protein and their exosomes. BioMed research international. 2015; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Madison MN, Roller RJ and Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 2014; 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Madison MN, Jones PH and Okeoma CM. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology. 2015; 482:189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. Journal of immunology (Baltimore, Md : 1950). 2007; 179:1969–78. [DOI] [PubMed] [Google Scholar]
- 236.Naslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK and Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS (London, England). 2014; 28:171–80. [DOI] [PubMed] [Google Scholar]
- 237.Dominy SS, Brown JN, Ryder MI, Gritsenko M, Jacobs JM and Smith RD. Proteomic analysis of saliva in HIV-positive heroin addicts reveals proteins correlated with cognition. PloS one. 2014; 9:e89366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Hiemstra TF, Charles PD, Gracia T, et al. Human urinary exosomes as innate immune effectors. Journal of the American Society of Nephrology : JASN. 2014; 25:2017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dimov I, Jankovic Velickovic L and Stefanovic V. Urinary exosomes. TheScientificWorldJournal. 2009; 9:1107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Smith JA and Daniel R. Human vaginal fluid contains exosomes that have an inhibitory effect on an early step of the HIV-1 life cycle. AIDS (London, England). 2016; 30:2611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mathias RA, Lim JW, Ji H and Simpson RJ. Isolation of extracellular membranous vesicles for proteomic analysis. Methods in molecular biology (Clifton, NJ). 2009; 528:227–42. [DOI] [PubMed] [Google Scholar]
- 242.Genneback N, Hellman U, Malm L, et al. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. Journal of extracellular vesicles. 2013; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hubert A, Subra C, Jenabian M-A, et al. Elevated abundance, size, and MicroRNA content of plasma extracellular vesicles in viremic HIV-1+ patients: Correlations with known markers of disease progression. Journal of acquired immune deficiency syndromes (1999). 2015; 70:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hu G, Yao H, Chaudhuri A, et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell death & disease. 2012; 3:e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sharma H, Chinnappan M, Agarwal S, et al. Macrophage-derived extracellular vesicles mediates smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV-infection and substance abuse. The FASEB Journal. 2018:fj. 201701558R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Tang X, Lu H, Dooner M, Chapman S, Quesenberry PJ and Ramratnam B. Exosomal Tat protein activates latent HIV-1 in primary, resting CD4+ T lymphocytes. JCI insight. 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mfunyi CM, Vaillancourt M, Vitry J, et al. Exosome release following activation of the dendritic cell immunoreceptor: a potential role in HIV-1 pathogenesis. Virology. 2015; 484:103–12. [DOI] [PubMed] [Google Scholar]
- 248.Arenaccio C, Anticoli S, Manfredi F, Chiozzini C, Olivetta E and Federico M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology. 2015; 12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Saribas AS, Cicalese S, Ahooyi TM, Khalili K, Amini S and Sariyer IK. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell death & disease. 2017; 8:e2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kulkarni R and Prasad A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Scientific reports. 2017; 7:14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Balakrishnan KV, Mahdii BA, Mahdi SA, et al. Stable transfection study for cloning and expression of HIV-1 NEF protein in HEK 293 cells. Rasayan Journal of Chemistry. 2017; 10:176–89. [Google Scholar]
- 252.Arenaccio C, Chiozzini C, Columba-Cabezas S, et al. Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef-and ADAM17-dependent mechanism. Journal of virology. 2014; 88:11529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Aqil M, Naqvi AR, Bano AS and Jameel S. The HIV-1 Nef protein binds argonaute-2 and functions as a viral suppressor of RNA interference. PLoS One. 2013; 8:e74472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Fang Y, Wu N, Gan X, Yan W, Morrell JC and Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS biology. 2007; 5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Barclay RA, Schwab A, DeMarino C, et al. Exosomes from uninfected cells activate transcription of latent HIV-1. Journal of Biological Chemistry. 2017; 292:11682–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Sampey GC, Saifuddin M, Schwab A, et al. Exosomes from HIV-1-infected cells stimulate production of pro-inflammatory cytokines through trans-activating response (TAR) RNA. Journal of Biological Chemistry. 2016; 291:1251–66. [DOI] [PMC free article] [PubMed] [Google Scholar]