Abstract
Heat stress (HS) is a financial and physiological burden on the poultry industry and the mitigation of the adverse effects of HS is vital to poultry production sustainability. The purpose of this study was, therefore, to determine the effects of an amino acid-chelated trace mineral supplement on growth performance, stress and inflammatory markers, and meat quality in heat-stressed broilers. One day-old Cobb 500 male broilers (n = 480) were allocated into 12 environmental chambers (24 floor pens) and divided into two groups: one group supplemented with amino acid-chelated trace mineral in drinking water and one control group. On day 28, birds were subjected to chronic heat stress (HS, 2 wk, 35 °C and 20% to 30% RH) or maintained at thermoneutral condition (TN, 24 °C) in a 2 × 2 factorial design. Feed intake (FI), water consumption, and body weight were recorded. At day 42, serum fluorescein isothiocyanate dextran (FITC-D) levels, blood gas, electrolyte, and stress markers were measured. Jejunum samples were collected to measure gene expression of stress, inflammation, and tight junction proteins. The rest of the birds were processed to evaluate carcass traits. HS resulted in an increase in core body temperature, which increased water intake and decreased FI, body weight, and feed efficiency (P < 0.05). HS reduced carcass yield and the weight of all parts (P < 0.05). HS significantly increased levels of circulating corticosterone (CORT), heat shock protein 70 (HSP70), interleukin 18 (IL-18), tumor necrosis factor alpha, C-reactive protein, and nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain-containing 3 expression. HS significantly increased serum FITC-D levels and the expression of HSP70 and IL-18 in the jejunum. Although it did not affect the growth performance, amino acid-chelated trace mineral supplementation reversed the effect of HS by reducing CORT and FITC-D levels and the expression of stress and proinflammatory cytokines in the circulation and the jejunum. However, it upregulated these parameters in birds maintained under TN conditions. Together, these data indicate that the amino acid-chelated trace mineral might alleviate stress and inflammation and improve gut integrity in heat-stressed but not thermoneutral broilers.
Keywords: Avalar, broilers, cytokines, heat stress, tight junction
Introduction
Heat stress (HS) is one of the most significant environmental stressors challenging poultry production worldwide (Lara and Rostagno, 2013; Greene et al., 2019b). Heat stress has adverse effects across all agricultural systems; however, poultry are particularly susceptible due to their high metabolic activity and heat production and decreased thermo-tolerance associated with their high growth rate (Deeb and Cahaner, 2002). Heat stress negatively impacts feed intake (FI), growth performance, meat yield, welfare, and mortality in the modern broilers. Globally, widespread extreme heat waves have repeatedly occurred and have caused great losses in the past. Based on a 2003 analysis, American animal agriculture loses an estimated US$1.69 to US$2.36 billion dollars annually due to HS, with poultry-specific losses ranging from US$128 to US$165 million (St-Pierre et al., 2003). As these values are over a decade old, they are likely considerably less than the current economic burden of HS. Additionally, as global temperatures are predicted to increase over the coming decades (Stillman, 2019), these negative events are projected to have an even greater impact on animal health and performance, economic losses, and food security for a growing world population.
Current methodologies for alleviating HS in poultry are only partially effective, as productivity still declines during warmer seasons. Currently, research efforts are focused toward management and nutritional strategies to help poultry better withstand HS challenges and maintain broiler health and productivity. Trace mineral supplementation, in particular, is a potential approach due to the known function of these minerals in growth, the immune response, and for their antioxidant characteristics (Richards et al., 2010; Światkiewicz et al., 2014). Birds are also likely mineral-deficient during HS, due to decreased intake and increased excretion (Belay and Teeter, 1996), as well as changes in metabolism affecting requirements (Coelho and McNaughton, 1995). Compared with inorganic, organic minerals, particularly amino acid-chelated minerals, are more bioavailable to the animal and prevent potential antagonism between other minerals and nutrients (Światkiewicz et al., 2014). Additionally, organic minerals have been reported to improve the antioxidant system and immune response and disease resistance, and reduce mortality (Kidd et al., 1996; Downs et al., 2000; Ferket et al., 2009). As HS is well known to induce oxidative stress and immunosuppression and reduce well-being and growth performances in broilers, we hypothesized that organic mineral supplementation may alleviate the adverse effect of HS. We, therefore, undertook the present study to determine the effect of a commercially available amino acid-chelated mineral (Avalar, Tracer Minerals, Cimmaron, KS) supplementation on growth performances and on the expression of heat shock proteins (HSPs) and cytokines in gut and blood of heat-stressed broilers.
Materials and Methods
The present study was conducted in accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health and the protocols were approved by the University of Arkansas Institutional Animal Care and Use Committee under protocol # 16084.
Animal procedure and environment
Four hundred eighty day-old Cobb500 broiler chicks were obtained from Cobb-Vantress hatchery (Siloam Springs, AR) and housed in environmentally controlled chambers in the Poultry Environmental Research Laboratory at the University of Arkansas. Each environmental chamber was divided into two pens with separate feeders and water containers (12 chambers, 24 pens, 20 birds/pen) where temperature, relative humidity (RH), and photoperiod can be managed accurately. On the day of hatch, chicks were individually weighed and tagged and kept at a density of approximately 0.15 m2/bird in all pens. Diets were formulated to meet Cobb-Vantress requirements (Table 1) and were fed ab libitum. An amino acid-chelated mineral supplement (Avalar, Tracer Minerals, Cimmaron, KS) was added in drinking water at the manufacturer’s recommended dose (Table 2). The ambient temperature was reduced gradually from 32 °C on day 1 to 24 °C on day 21, with RH at 55 ± 5%. On day 28, chambers were randomly divided into two environmental conditions (thermoneutral [TN], 24 °C vs. HS, 35 °C) and pens were assigned a treatment (Control, C vs. Avalar amino acid-chelated mineral treatment, M) in a 2 × 2 factorial design. The day prior to HS challenge, the chickens (12 birds/group) were equipped with a Thermochron temperature logger (iButton, DS1922L, Maxim, CA) for continuous monitoring of the core body temperature. Environmental temperature and RH were recorded daily in each chamber. Feed and water intake were recorded daily for each pen. Mortalities were recorded daily and FI (individual and cumulative) was adjusted for any losses. Bodyweight was recorded weekly, and body weight gain, feed conversion ratio (FCR), and feed efficiency were determined as previously described (Rajaei-Sharifabadi et al., 2017).
Table 1.
Ingredient and nutrient composition of the basal diet
| Starter 0 to 14 d | Grower 15 to 42 d | |
|---|---|---|
| Ingredient, % of diet | ||
| Corn | 60.099 | 65.070 |
| Soybean meal, 46% | 33.381 | 28.286 |
| Poultry fat | 2.473 | 2.821 |
| Dicalcium phosphate | 1.610 | 1.481 |
| Limestone | 1.015 | 0.981 |
| Salt | 0.355 | 0.359 |
| Sodium bicarbonate | 0.120 | 0.120 |
| DL-methionine | 0.330 | 0.285 |
| l-lysine HCl | 0.244 | 0.233 |
| l-threonine | 0.102 | 0.096 |
| Choline chloride, 60% | 0.031 | 0.029 |
| Vitamin premix1 | 0.100 | 0.100 |
| Trace mineral premix2 | 0.100 | 0.100 |
| Selenium premix3 | 0.020 | 0.020 |
| Santoquin | 0.020 | 0.020 |
| Calculated composition, % | ||
| Dry matter | 88.12 | 87.99 |
| ME, kcal/kg | 3,035 | 3,108 |
| CP | 21.20 | 19.10 |
| AID Lys | 1.18 | 1.05 |
| AID Met | 0.61 | 0.54 |
| AID TSAA | 0.89 | 0.80 |
| AID Thr | 0.77 | 0.69 |
| AID Trp | 0.22 | 0.19 |
| AID Arg | 1.27 | 1.12 |
| AID Ile | 0.79 | 0.71 |
| AID Val | 0.86 | 0.78 |
| Total ca | 0.90 | 0.84 |
| Total P | 0.71 | 0.66 |
| Available P | 0.45 | 0.42 |
| Analyzed nutrient | ||
| Crude protein, % | 21.5 | 21.1 |
| Energy, Kcal/kg | 4,061 | 4,046 |
| Fat, % | 5.51 | 5.26 |
1Supplied per kilogram of diet: manganese, 100 mg; magnesium, 27 mg; zinc, 100 mg; iron, 50 mg; copper, 10 mg; iodine, 1 mg.
2Supplied per kilogram of diet: vitamin A, 30,863 IU; vitamin D3, 22,045 ICU; vitamin E, 220 IU; vitamin B12, 0.05 mg; menadione, 6.0 mg; riboflavin, 26 mg; d-pantothenic acid, 40 mg; thiamine, 6.2 mg; niacin, 154 mg; pyridoxine, 11 mg; folic acid, 3.5 mg; biotin, 0.33 mg.
3Supplied 0.12 mg of selenium per kg of diet.
Table 2.
Composition and dosing schedule for the amino acid-chelated trace mineral
| Composition1 | Treatment2 | ||
|---|---|---|---|
| Mineral | Quantity | Day | Dose (mL/L) |
| Zn | 1,800 ppm | 1 to 6 | 9.8 |
| Mn | 530 ppm | 10 to 12 | 7.8 |
| Fe | 330 ppm | 17 to 19 | 7.8 |
| Cu | 130 ppm | 24 to 26 | 7.8 |
| Co | 18 ppm | 31 to 32 | 7.8 |
| Mg | 0.6% | 38 to 39 | 7.8 |
| K | 0.5% | ||
| Ca | 0.075% |
1The product is in accordance with AAFCO 57.142 Metal Amino Acid Chelate and AAFCO 57.150 Metal Amino Acid Complex (for the potassium).
2Dose (in drinking water) and timing based on the manufacturer’s recommendation. Control groups received un-supplemented water for the duration of the experiment. Ca, calcium; Co, cobalt; Cu, copper; Fe, iron; K, potassium; Mg, magnesium; Mn, manganese; Zn, zinc.
Sample collection
Selected birds were euthanized by cervical dislocation after chronic (2 wk) HS. For RNA analysis, blood samples (1 mL) were collected from wing vein into sterile tubes containing Trizol-LS reagent (Thermo Fisher Scientific, Waltham, MA). For plasma, blood samples (2.5 to 3.5 mL) were collected in vacutainer tubes with plasma separation tube gel and lithium heparin and after centrifugation (1,500 × g, 10 min, 4 °C), plasma was separated and stored at −20 °C for later analysis. Blood chemistry was analyzed using a portable analyzer (i-STAT Alinity, Abbott Laboratories, USA; cartridge Cg8+). Blood is introduced into the cartridge by using a syringe, and the cartridge is then inserted into the analyzer, and operator and animal identification were entered into the system. A detailed technical description and use of iSTAT has been described elsewhere (Erickson and Wilding, 1993). iSTAT analysis has been validated in chickens (Steinmetz et al., 2007; Martin et al., 2010; Wang et al., 2018; Greene et al., 2019a). The following parameters were measured: hematocrit (Hct, % packed cell volume), hemoglobin (Hb, g/dL), pH, partial pressure carbon dioxide (pCO2, mmHg), partial pressure oxygen (pO2, mmHg), base excess (BE, ecf, mmol/L), total carbon dioxide (TCO2, mmol/L), oxygen saturation (sO2, %), sodium (Na+, mmol/L), potassium (K+, mmol/L), ionized calcium (iCa, mmol/L), bicarbonate (HCO3−, mmol/L), and glucose (mg/dL). Blood samples were collected to assess the gene expression of cytokines (interleukin [IL]-18, IL-1β, nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 3 [NLRP3], and tumor necrosis factor alpha [TNFα], and C-reactive protein [CRP]) and stress markers (HSP60 and HSP70) and corticosterone (CORT). To assess the expression of HSPs, tight junction proteins, and cytokines, the upper jejunum (approximately 10 cm below bile duct entrance into distal duodenum) was collected, cleaned of digesta by gently pressing the tissue, and rinsed in phosphate-buffered saline solution. Once collected, blood and tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C until use for molecular and biochemical analysis.
Corticosterone radioimmunoassay
Plasma CORT levels were determined by radioimmunoassay as previously described (Madison et al., 2008). All samples were assayed in duplicate. The inter- and intra-assay coefficient of variation were lower than 5%.
RNA isolation, reverse transcription, and quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from blood and jejunal samples using Trizol reagent (Thermo Fisher Scientific, Rockford, IL) following manufacturer’s recommendations. RNA concentrations and purity were measured in duplicate for each sample using the Take 3 Micro-Volume Plate and the Synergy HT multimode microplate reader (BioTek, Winooski, VT). RNA integrity and quality were further verified using 1% agarose gel electrophoresis. qScript cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD) was used to transcribe 1 µg of RNA into cDNA. Real-time quantitative PCR (Applied Biosystems 7500 Real-Time PCR system) was performed by mixing 5 µL of 10× diluted cDNA, 0.5 µM of each forward and reverse specific primer, and SYBR Green Master Mix (Thermo Fisher Scientific, Rockford, IL) in a total volume of 20 µL per reaction. Oligonucleotides primers specific for chicken IL-1β, IL-18, NLRP3, CRP, TNFα, HSP60, HSP70, and the housekeeping gene, ribosomal 18S, are summarized in Table 3. The qPCR cycling conditions were the same as described previously (Lassiter et al., 2015). Relative expression of target genes was determined by the 2−ΔΔCt method (Schmittgen and Livak, 2008) and the control treatment under TN conditions was used as calibrator.
Table 3.
Oligonucleotide real-time qPCR primers
| Gene | Accession number1 | Primer sequence (5′ → 3′) | Orientation | Product size (bp) |
|---|---|---|---|---|
| IL-1β | NM_204524 | CGAGGAGCAGGGACTTTGC GAAGGTGACGGGCTCAAAAA | Forward Reverse | 71 |
| IL-18 | GU119895 | TGCAGCTCCAAGGCTTTTAAG CTCAAAGGCCAAGAACATTCCT | Forward Reverse | 63 |
| TNFα | NM_204267 | CGTTTGGGAGTGGGCTTTAA GCTGATGGCAGAGGCAGAA | Forward Reverse | 61 |
| NLRP3 | XM_001233261 | GTTGGGCAGTTTCACAGGAATAG GCCGCCTGGTCATACAGTGT | Forward Reverse | 63 |
| CRP | NM_001039564 | AAGCTCAGGACAACGAGATCCT TTTCCCCCCCACGTAGAAG | Forward Reverse | 71 |
| HSP60 | NM_001012916 | CGCAGACATGCTCCGTTTG TCTGGACACCGGCCTGAT | Forward Reverse | 55 |
| HSP70 | J02579 | GGGAGAGGGTTGGGCTAGAG TTGCCTCCTGCCCAATCA | Forward Reverse | 55 |
| OCLN | NM_205128 | CGCAGATGTCCAGCGGTTA GTAGGCCTGGCTGCACATG | Forward Reverse | 59 |
| CLDN1 | NM_001013611 | CCCACGTTTTCCCCTGAAA GCCAGCCTCACCAGTGTTG | Forward Reverse | 61 |
| 18S | AF173612 | TCCCCTCCCGTTACTTGGAT GCGCTCGTCGGCATGTA | Forward Reverse | 60 |
1Accession number refers to Genbank (NCBI). CLDN1, claudin 1; CRP, C-reactive protein; HSP, heat shock protein; IL, interleukin; NLRP3, nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 3; OCLN, occluding; TNFα, tumor necrosis factor alpha.
Intestinal permeability
Paracellular gut leakage was measured using the fluorescent marker flouresisothyiocynate-dextran (FITC-D) as previously described (Baxter et al., 2019). In brief, the dose of FITC-D was calculated based on the average pen body weight. Chickens were gavaged with FITC-D (8.32 mg/kg of body weight) and blood was collected l h post gavage. Fluorescence was measured at an excitation wavelength of 428 nm and an emission wavelength of 528 nm using the Synergy HT multimode microplate reader (BioTek, Winooski, VT).
Processing and woody breast scoring
At the end of the trial (day 42), the remaining birds were processed at the University of Arkansas Pilot Processing Plant (Fayetteville, AR) using a commercial inline system, and carcass quality traits including live weight, hot and chilled carcass weight, fat, breast, tender, wing, and leg quarter weights were determined as previously described (Orlowski et al., 2018). Whole breast fillets were evaluated for degree of hardness (woody breast [WB]) based on tactile evaluation using the scale developed by Tijare et al. (2016) with categories of normal (NORM), moderate (MOD), and severe (SEV).
Statistical analysis
All data are expressed as mean ± SEM. Data were analyzed by two-way ANOVA using general linear model (GLM) procedures of SAS (v9.4Cary, NC) or GraphPad Prism version 6.0 (La Jolla, CA). The main effects were mineral supplementation (Control vs. Avalar), ambient temperature (TN vs. HS), and their interaction. When ANOVA showed a significant effect, means were compared by Student Newman Keuls (SNK) multiple comparison test. P < 0.05 was considered significant. WB scores were considered an ordinal variable and means between groups were separated using Pearson’s Chi-square. Differences between the frequency of each score were also determined using Proc GLM in SAS, with Diet and Temp as fixed effects. Means were separated using the least square means (LSMEANS) procedure, and significance set at P < 0.05.
Results
Growth performance and carcass characteristics
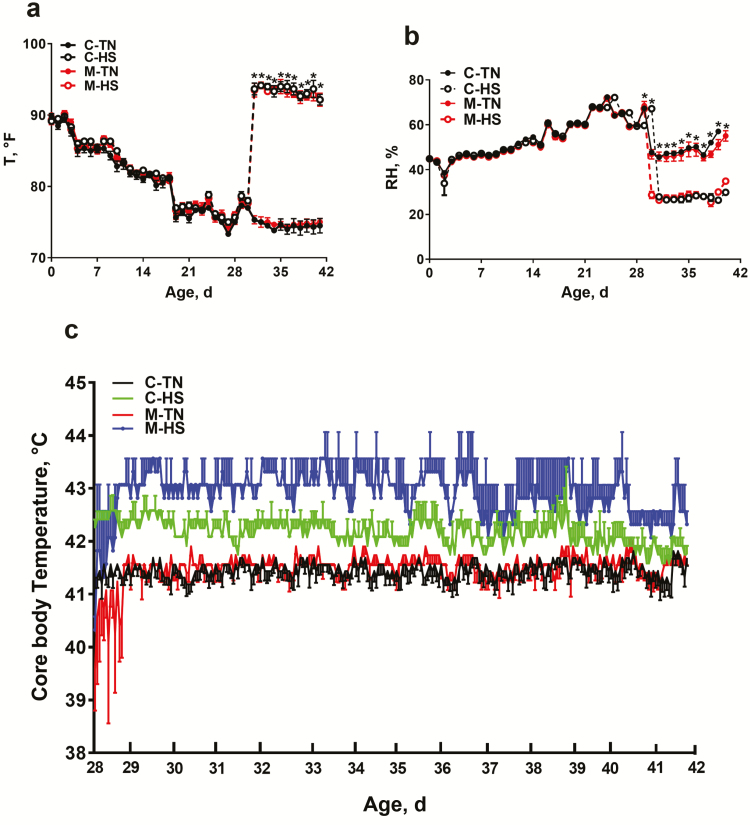
Before HS initiation, the environment temperature and RH did not differ among the environmental chambers. After the onset of HS, the environmental temperature was significantly higher and RH was significantly lower in the HS as compared with the TN chambers (Figure 1a and b). Core body temperature in the HS groups was ~1 to 1.5 °C higher than the control groups.
Figure 1.
Effect of amino acid-chelated trace mineral supplementation on core body temperature of heat-stressed broilers. The chamber temperatures (a), relative humidity (b), and the core body temperature (c) were monitored. Data are presented as mean ± SEM (n = 12 birds/group). *indicates significant difference at P < 0.05. C, control; HS, heat stress; M, mineral supplementation; RH, relative humidity; T, barn temperature; TN, thermoneutral.
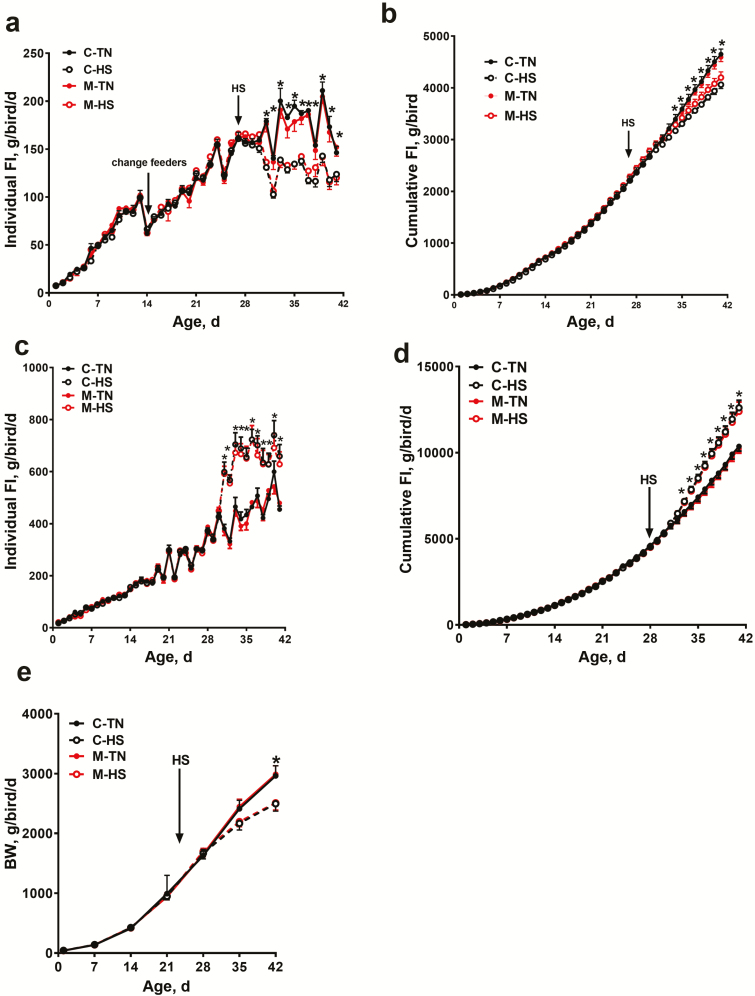
FI between control and amino acid-chelated trace mineral-supplemented group did not differ prior to the onset of HS. After HS initiation, however, individual FI was significantly lower in the HS pens as compared with TN. There were no significant differences in FI between the control and the amino acid-chelated trace mineral groups, regardless of environmental temperature (Figure 2a and b). However, under HS, the amino acid-chelated trace mineral group had higher FI compared with the control group (4,199.4 g ± 110 vs. 4,061.14 g ± 75.9, P = 0.2). Before HS initiation, there was no significant difference in water intake between any of the treatment groups. After HS, water intake was significantly higher in chickens in the HS chambers. Regardless of environmental conditions, there was no significant effect of the amino acid-chelated trace mineral supplementation on water intake (Figure 2c and d).
Figure 2.
Effect of amino acid-chelated trace mineral supplementation on growth performance in heat-stressed broilers. Individual and cumulative FI (a, b), individual and cumulative water intake (WI) (c, d), and body weight and body weight gain (e). Data are presented as mean ± SEM (n = 120 birds/group for body weight and n = 6 pens/group for FI and WI). *indicates significant difference at P < 0.05.
Before HS, all treatment groups had similar average body weight and initial body weight gains (Figure 2e). Chickens under TN conditions had a higher body weight and higher body weight gain from day 35 to day 42 than HS chickens (Figure 2e). Regardless of the environmental challenge, there was no significant difference in growth between the control and the amino acid-chelated trace mineral-supplemented chickens. HS increased FCR in both control and amino acid-chelated trace mineral groups, and amino acid-chelated trace mineral supplementation averaged 4 points better FCR compared with control diets under both environmental conditions (1.57 ± 0.01 vs. 1.53 ± 0.01 and 1.66 ± 0.01 vs. 1.62 ± 0.01 in control vs. amino acid-chelated trace mineral under TN and HS conditions, respectively; P < 0.0001 for the effects of diet and environmental; P > 0.99 for the interaction).
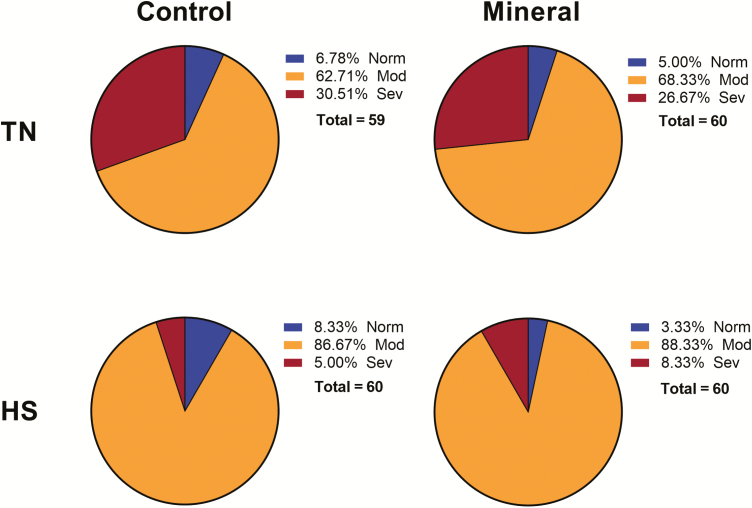
The effects of HS and amino acid-chelated trace mineral supplementation on processing data are shown in Table 4. HS caused a significant reduction in live weight, carcass weight (pre and post chill), wing, breast, tender, and leg quarter weight. Under TN conditions, control chickens had a WB incidence of 6.78% normal, 62.71% moderate, and 30.51% severe. Control chickens under HS conditions had an incidence of 8.33% normal breast, 86.67% moderate, and 5.00% severe. With amino acid-chelated trace mineral supplementation under TN conditions, 5.00% of breasts were scored as normal, 68.33% as moderate, and 26.67% as severe. With amino acid chelated trace mineral treatment under HS conditions, 3.33% of the fillets were normal, 88.33% were moderate, and 8.33% were scored as severe for WB (Figure 3, Table 5).
Table 4.
The effects of HS and amino acid-chelated mineral supplementation on carcass parameters of broilers1
| Diet | Control | Avalar | P-values | ||||
|---|---|---|---|---|---|---|---|
| Environment | TN | HS | TN | HS | Diet (D) | Environment (E) | Interaction (D × E) |
| LW, g | 2,974.7 ± 171.5 | 2,444.9 ± 136.5 | 3,010.8 ± 132.8 | 2,526.0 ± 104.5 | 0.677 | 0.002 | 0.872 |
| HCW, g | 2,304.1 ± 145.1 | 1,913.3 ± 109.5 | 2,318.1 ± 113.8 | 1,950.1 ± 84.6 | 0.828 | 0.004 | 0.922 |
| CCW, g | 2,368.6 ± 148.7 | 1,965.8 ± 109.9 | 2,383.8 ± 114.6 | 2,004.5 ± 85.4 | 0.820 | 0.003 | 0.921 |
| Fat, % | 1.38 ± 0.16 | 1.42 ± 0.14 | 1.48 ± 0.15 | 1.41 ± 0.16 | 0.573 | 0.934 | 0.418 |
| Breast, g | 599.8 ± 16.4 | 480.0 ± 11.4 | 608.4 ± 13.9 | 487.5 ± 9.8 | 0.848 | 0.009 | 0.989 |
| Breast, % | 25.13 ± 0.29 | 24.30 ± 0.23 | 25.39 ± 0.26 | 25.24 ± 0.24 | 0.432 | <0.0001 | 0.121 |
| Tender, g | 118.29 ± 7.54 | 100.82 ± 6.70 | 120.62 ± 6.95 | 101.60 ± 6.22 | 0.823 | 0.015 | 0.912 |
| Wing, g | 235.14 ± 13.02 | 204.67 ± 9.97 | 235.97 ± 10.74 | 207.40 ± 7.80 | 0.868 | 0.011 | 0.929 |
| Leg quarter, g | 702.98 ± 43.42 | 586.37 ± 34.53 | 706.28 ± 31.57 | 602.30 ± 26.48 | 0.857 | 0.005 | 0.784 |
1Data are means ± SEM. LW, live weight, HCW: Hot carcass weight, CCW: chilled carcass weight.
Figure 3.
Effect of amino acid-chelated trace mineral supplementation on WB incidence. At day 42, breast filets were macroscopically scored and classified to WB categories to normal (NORM, score 0), moderate (MOD, score 0.5 to 1.5), and severe (SEV, score 2 to 3). n = 59 to 60 breast fillets/group.
Table 5.
Effect of amino acid-chelated mineral supplement and heat stress on WB categories1
| WB category | Diet | Temp. | Diet × Temp. |
|---|---|---|---|
| Normal | 0.2377 | 1.0000 | 0.5497 |
| Moderate | 0.5497 | 0.0101 | 0.6893 |
| Severe | 1.0000 | 0.0110 | 0.6708 |
1Values represent P-values as determined using Proc GLM and LSMEANS procedure of SAS.
Circulating stress markers
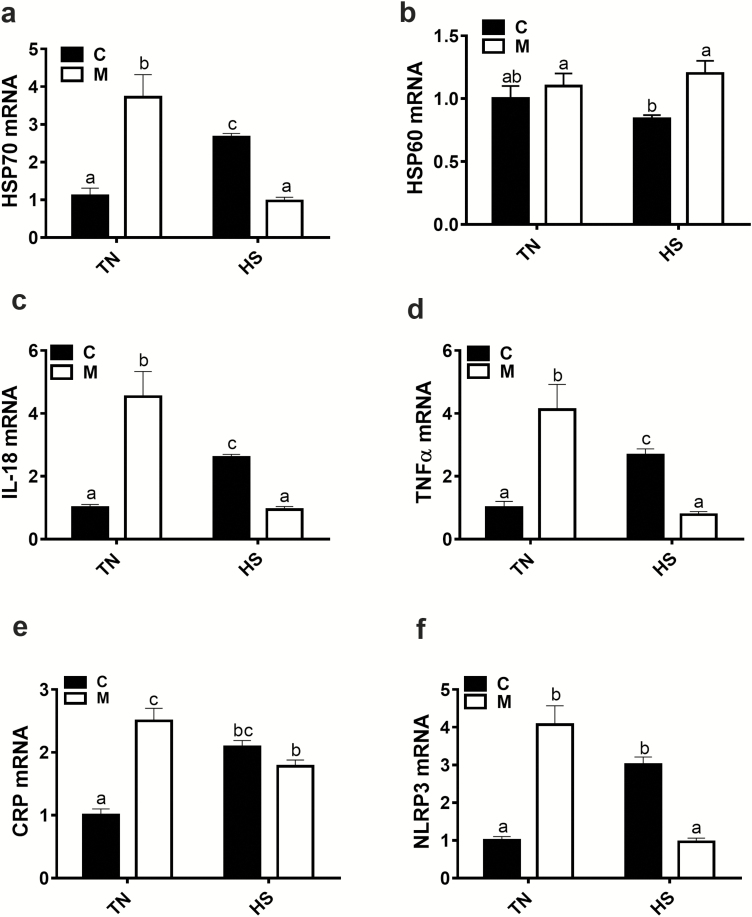
Amino acid-chelated trace mineral supplementation reduces circulating CORT levels by 34% and 12% compared with the control group under both TN and chronic HS conditions (186 ± 33 vs. 123.4 ± 10 pg/mL and 325.1 ± 39 vs. 286 ± 33 pg/mL in control vs. amino acid-chelated trace mineral-supplemented group under TN and HS conditions, respectively; P = 0.03, P = 0.43, and P = 0.85 for the effect of HS, amino acid-chelated trace mineral, and their interaction, respectively). Similarly, amino acid-chelated trace mineral supplementation significantly downregulates the expression of blood HSP70, IL-18, TNFα, and NLRP3 under chronic HS conditions (Figure 4a, c, d, and f). However, blood HSP60 mRNA levels were significantly increased, and CRP levels remained unchanged in amino acid-chelated trace mineral-supplemented and heat-stressed birds compared with control (Figure 4b and e). Under TN conditions, amino acid-chelated trace mineral administration significantly upregulates the expression of blood HSP70, IL-18, TNFα, CRP, and NLRP3 without affecting that of HSP60 compared with the untreated group (Figure 4a–f).
Figure 4.
Effect of amino acid-chelated trace mineral supplementation on circulating stress and inflammatory markers. The relative gene expression of HSP70 (a), HSP60 (b), IL-18 (c), TNFα (d), CRP (e), and NLRP3 (f) was determined by qPCR and analyzed by 2–ΔΔCt method using C-TN group as a calibrator. Data are presented as mean ± SEM (n = 6 to 10 birds per group). Different letters indicate significant difference at P < 0.05. C, control; CRP, C-reactive protein; HSP, heat shock protein; IL, interleukin; M, mineral (Avalar); NLRP3, nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 3; TNFα, tumor necrosis factor alpha.
Intestinal integrity and stress markers
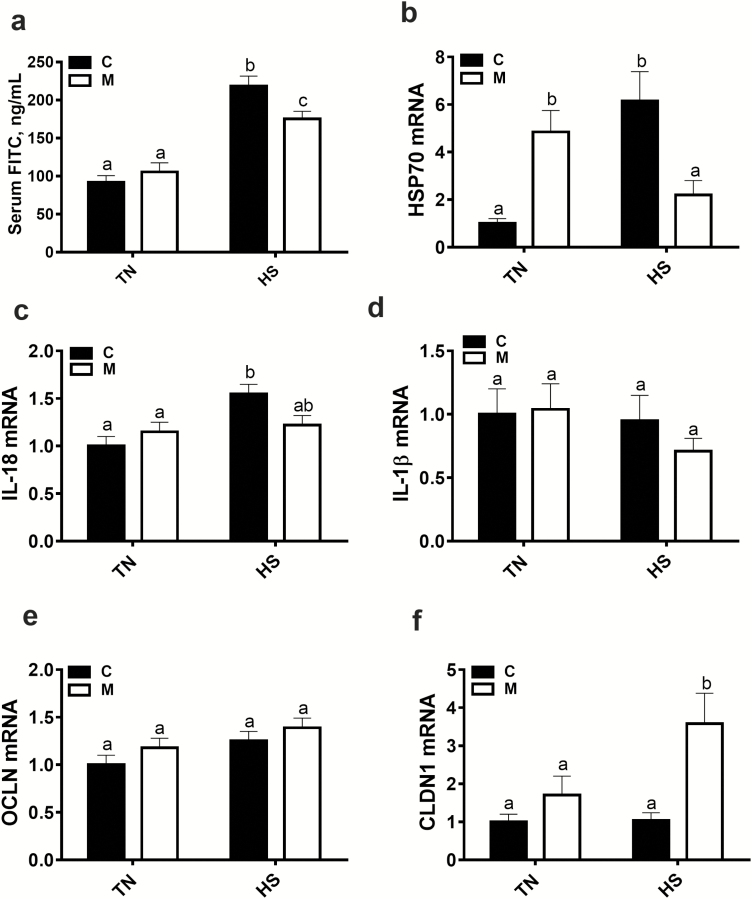
Chronic HS significantly increases serum FITC-D levels compared with TN conditions and amino acid-chelated trace mineral supplementation significantly reduces serum FITC-D concentrations compared with the control group under HS conditions (Figure 5a). As for the blood, amino acid-chelated trace mineral supplementation significantly downregulates the expression of HSP70 in the jejunum compared with the control group under chronic HS conditions (Figure 5b). Amino acid-chelated trace mineral supplementation significantly upregulates the expression of claudin 1 (CLDN1), but not that of occluding (OCLN), in the jejunum compared with the control group under HS conditions (Figure 5e and f).
Figure 5.
Effect of amino acid-chelated trace mineral supplementation on serum FITC-D concentrations and on intestinal stress and inflammatory markers. Intestinal permeability was assessed by measuring serum FITC-D levels (a). The relative gene expression of HSP70 (b), IL-18 (c), IL-1β (d), OCLN (e), and CLDN1 (f) was determined by qPCR and analyzed by 2–ΔΔCt method using C–TN group as a calibrator. Data are presented as mean ± SEM (n = 6 to 10 birds/group). Different letters indicate significant difference at P < 0.05. C, control; CLDN1, claudin 1; FITC, fluorescein isothiocyanate; HSP, heat shock protein; IL, interleukin; M, mineral (Avalar); OCLN, occludin.
Blood gasses and electrolytes
Hb levels were significantly increased by HS but were unaffected by amino acid-chelated trace mineral supplementation (Table 6). The levels of pCO2 were significantly increased by HS only in the amino acid-chelated trace mineral-supplemented group and not in the control birds (Table 6). There was a significant interaction between HS and mineral treatment on HCO3, BE, and total CO2.
Table 6.
Effect of chronic HS and amino acid-chelated trace mineral supplementation on blood parameters in chicken1
| Diet | Control | Avalar | P-values | ||||
|---|---|---|---|---|---|---|---|
| Environment | TN | HS | TN | HS | Diet (D) | Environment (E) | Interaction (D × E) |
| pH | 7.49 ± 0.053 | 7.47 ± 0.062 | 7.48 ± 0.056 | 7.44 ± 0.073 | 0.318 | 0.142 | 0.622 |
| pCO2, mmHg | 33.1 ± 6.2 | 33.2 ± 7.6 | 31.8 ± 5.3 | 40.5 ± 11.3 | 0.243 | 0.091 | 0.094 |
| pO2, mmHg | 71.4 ± 15.6 | 79.4 ± 13.9 | 77.0 ± 17.5 | 74.8 ± 17.8 | 0.925 | 0.580 | 0.326 |
| HCO3, mmol/L | 24.7 ± 3.3 | 23.2 ± 3.4 | 23.1 ± 2.1 | 26.3 ± 4.3 | 0.670 | 0.431 | 0.031 |
| BE, mmol/L | 2.5 ± 3.3 | 1.0 ± 3.1 | 0.8 ± 2.2 | 3.8 ± 4.3 | 0.599 | 0.474 | 0.037 |
| sO2, % | 89.9 ± 6.3 | 91.1 ± 4.8 | 91.6 ± 5.5 | 87.0 ± 8.6 | 0.560 | 0.410 | 0.163 |
| TCO2, mmol/L | 25.6 ± 3.5 | 23.9 ± 3.6 | 23.8 ± 2.2 | 27.4 ± 4.4 | 0.450 | 0.399 | 0.023 |
| Na, mmol/L | 146.1 ± 1.6 | 146.3 ± 2.1 | 146.7 ± 1.7 | 145.3 ± 1.8 | 0.729 | 0.302 | 0.171 |
| K, mmol/L | 4.7 ± 0.3 | 4.7 ± 0.3 | 4.6 ± 0.2 | 4.5 ± 0.2 | 0.122 | 0.629 | 0.189 |
| iCa, mmol/L | 1.19 ± 0.15 | 1.18 ± 0.16 | 1.23 ± 0.11 | 1.29 ± 0.11 | 0.088 | 0.496 | 0.373 |
| Glucose, mg/dL | 205.5 ± 28.4 | 199.9 ± 26.9 | 203.9 ± 19.7 | 223.9 ± 20.2 | 0.151 | 0.351 | 0.102 |
| Hct, %PCV | 18.7 ± 1.6 | 20.5 ± 3.4 | 19.1 ± 2.0 | 19.9 ± 2.0 | 0.916 | 0.105 | 0.505 |
| Hb, g/dL | 6.3 ± 0.6 | 7.1 ±1.0 | 6.5 ± 0.7 | 6.8 ± 0.7 | 0.689 | 0.046 | 0.351 |
1Data are means ± SEM. n = 10 per group. pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; HCO3, bicarbonate; BE, base excess; sO2, oxygen saturation; TCO2, total carbon dioxide; Na, sodium; K, potassium; iCa, ionized calcium; Hct, hematocrit; Hb, hemoglobin; PCV, packed cell volume.
Discussion
HS is a global issue affecting the performance and welfare of animals in the agricultural industry. Currently, there is no consensus or published guideline for poultry mineral requirements during HS, where birds consume less feed, have poorer digestibility, and greater excretion of dietary minerals (Hai et al., 2000) making these dietary components a hot spot and critical target for research. In this study, as expected, and in agreement with previously published research (Leenstra and Cahaner, 1992; Gonzalez-Esquerra and Leeson, 2005; Flees et al., 2017), exposure to HS decreased FI and body weight (BW) gains and increased water intake relative to TN conditions. The lack of a difference between water consumption in the control and amino acid-chelated trace mineral groups indicates that amino acid-chelated trace mineral supplementation did not affect palatability or birds’ ability to drink. These data support the feasibility of drinking water-mineral as an effective delivery method. Others have shown that birds may refuse mineral-supplemented feed, but only at excessive concentrations (Ferket and Gernat, 2006). The increase (~138 g/bird/42 d) in FI in the amino acid-chelated trace mineral group as compared with the control birds under HS conditions suggests that amino acid-chelated mineral supplementation may help stimulate appetite and FI. This stimulatory effect of specific trace minerals on FI has been observed previously. For instance, supplementation with organic iron or iron in combination with copper resulted in a significant increase in FI with no effect on body weight in broilers, whereas supplementation with zinc resulted in increases in both FI and body weight gains (Bao et al., 2010). Conversely, lower FI in broilers has been reported to be a consequence of trace mineral deficiencies (Bao et al., 2007). This suggests that the combination of minerals in amino acid-chelated trace mineral might stimulate appetite through orexigenic peptides coupled to the afferent vagus nerve (Marreiro et al., 2006; Akarsu et al., 2007; Suzuki et al., 2011; Nishiuchi et al., 2018). The slightly higher core body temperature (~0.5 °C) observed during HS in the amino acid-chelated trace mineral-supplemented birds may also be due to the diet-induced thermogenesis and/or higher metabolic function associated with the increase in FI. Regardless of environmental conditions, amino acid-chelated trace mineral treatment had no significant effect on BW, BW gain, or FCR. Overall, the reported effects of mineral supplementation during HS in the literature are inconsistent, with some showing no changes (Bartlett and Smith, 2003; Pacheco et al., 2017) and others increasing performance parameters (Kucuk et al., 2003; Sahin et al., 2005; Laganá et al., 2007; Kucuk 2008; Yang et al., 2012). These discrepancies may be due to the use of varying sources, doses, and forms of mineral, as well as differences in supplementation timing and differences in the mineral content of the basal diets.
At the cellular level, a small increase in temperature induces alterations such as protein misfolding and aggregation, transcription modulation, and cell cycle arrest (Richter et al., 2010). Many of the observed effects of HS can be attributed to the aggregation of intracellular proteins and an overall imbalance of protein homeostasis. To prevent these deleterious effects, the cell has a coordinated and highly conserved response system. Depending on the severity and duration of the stress, cells can utilize highly efficient stress response and protein quality control systems to ensure their survival or activate stress signaling cascades that result in cell-death pathways (Santoro, 2000). At the molecular level, a common rapid response to HS is the increased synthesis of HSPs. Here, in concurrence with other research, HSP70 gene expression was upregulated during HS in the circulation and in the jejunum of control birds (Varasteh et al., 2015; Rajkumar et al., 2018; Xu et al., 2018; Greene et al., 2019b), indicating a systemic and local (intestinal) stress status. Interestingly, amino acid-chelated trace mineral supplementation reverses this effect, suggesting a mitigation of stress induced by heat load. The anti-stress effects of the amino acid-chelated trace mineral are further supported by the reduction of plasma CORT (the gold standard stress marker) levels in heat-stressed broilers (Quinteiro-Filho et al., 2010; Xu et al., 2018). A similar effect on HSP70 expression has been shown with individual supplementation with specific minerals, including zinc (Kucuk et al., 2003; Sahin et al., 2005; Rajkumar et al., 2018), and manganese (Zhu et al., 2015), both of which are components of the amino acid-chelated trace mineral.
The circulatory system and the gastrointestinal tract are primarily responsive to heat stress and a variety of changes can be observed, including inflammation and impairment of intestinal barrier integrity (Lambert et al., 2002; Pockley, 2002; Song et al., 2014; Li et al., 2019b; Koch et al., 2019). This is evident here following the induction of proinflammatory cytokines (IL-18, TNFα, CRP, and NLRP3) in the circulation and IL-18 in the jejunum of heat-stressed birds, which corroborates previous studies (Welc et al., 2013; Ohtsu et al., 2015; Saleh and Al-Zghoul, 2019). The NLRP3 is an intracellular sensor that detects a broad range of endogenous danger signals and environmental irritants, resulting in the assembly and activation of the NLRP3 inflammasome and caspase 1-dependent release of the proinflammatory cytokines IL-1β and IL-18 (Martinon et al., 2002; Duncan et al., 2007; Mangan et al., 2018). Although the upstream mediators of NLRP3 inflammasome activation are not known in this study, it is possible that HS induces TNF-α which leads to NF-kB activation and NLRP3 transcription (Bauernfeind et al., 2009; Franchi et al., 2009). It is also plausible that HS induces NLRP3 activation via CRP-upregulating NF-kB activity (Bello et al., 2016; Bian et al., 2019). In addition to stress alleviation, the downregulation of proinflammatory cytokine expression indicates that the amino acid-chelated trace mineral may reduce inflammation in heat-stressed broilers. In fact, minerals are crucial components of enzymes necessary for antioxidant function, and dietary iron (Sun et al., 2015), zinc (Bun et al., 2011), magnesium (Yang et al., 2006, 2012), copper (Dameron and Harris, 1987; Ognik et al., 2018), and manganese (Lu et al., 2007; Li et al., 2011; Zhu et al., 2015) have all been shown to improve antioxidant function and reduce inflammation in poultry.
It is well known that heat stress and proinflammatory cytokines induce leaky gut syndrome via disruption of the intestinal barrier integrity (Lambert et al., 2002; Dann et al., 2008), which is obvious here due to the increase of serum FITC-D levels in heat-stressed birds. The upregulation of CLDN1 gene expression in the jejunum of heat-stressed broilers indicates a protective role of the amino acid-chelated trace mineral. CLDN1 is widely expressed in the intestinal epithelium and it is known by its barrier-forming ability (Günzel and Yu, 2013). It has been reported that the upregulation of CLDN1 increases transepithelial electrical resistance and maintain intestinal barrier integrity (Luissint et al., 2016; Wu et al., 2018; Li et al., 2019a; Nishii et al., 2019). Taken together, amino acid-chelated trace mineral supplementation seems to reduce systemic and local (intestinal) stress and inflammation, and, in turn, improves intestinal barrier integrity in heat-stressed broilers. However and unexpectedly, amino acid-chelated trace mineral also upregulates the expression of HSP70 and proinflammatory cytokines in chickens maintained under TN conditions. This may be due to trace mineral levels in excess of requirements from the combined diet and water supplementation, as diets and water were not adjusted for the mineral content of amino acid-chelated trace mineral. Therefore, perhaps excessive mineral intakes lead to the production of proinflammatory cytokines (Kogut, 2017).
As WB is associated with oxidative stress and because minerals are recommended as cofactors and external antioxidants in the management of oxidative stress (Willcox et al., 2004; Wolonciej et al., 2016), we sought, next, to determine the effects of the amino acid-chelated trace mineral on WB incidence. WB is a muscle myopathy, characterized by palpable stiffness of the breast muscle and a myodegeneration within the fillet (Petracci and Cavani, 2012). It can cause significant economic losses to the industry, due to changes in meat texture, protein content, and water-holding capacity, and ultimately, consumer acceptance (Kuttappan et al., 2012, 2017). The heavy selection for growth in broiler chickens has increased muscle fiber diameter, reducing vascularization in the muscles, which concurrently reduces nutrient supply to the breast muscle and increases oxidative stress (Velleman and Clark, 2015). Here, HS reduced the severity of WB and this is not surprising due to a decrease in FI and body weight. Amino acid-chelated trace mineral supplementation led to a ~3.8% reduction and ~3.8% increase in the incidence of severe WB in birds maintained under TN and HS conditions, respectively. This result is intriguing, and due to the complexity of WB myopathy and current lack of understanding of its etiology, other research on the effects of dietary trace minerals are needed. Sirri et al. (2016) used high and low doses of an organic trace mineral mix and found no effect on the incidence of WB at 51 d of age. Echeverry et al. (2016), on the other hand, have shown that supplementation with zinc resulted in increased zinc status in the breast muscle, improving oxidative stability; however meat quality was not assessed ().
When exposed to higher temperatures, chicken use multiple physiological mechanisms to thermoregulate, including decreased feeding and moving, as well as increased drinking behavior, laying, spreading of wings, and panting. Panting is considered the most obvious sign of HS and can lead to respiratory alkalosis (Fedde, 1998). Here, we show an interaction of mineral supplementation and HS on several parameters related to respiratory alkalosis. In particular, TCO2, BE, and HCO3− were lower under TN conditions, but higher with amino acid-chelated trace mineral supplementation during HS. Though not measured, this may indicate that the mineral supplement may mitigate the effects of HS through decreased panting, leading to a more stable acid–base balance in the blood. Indeed, Wang et al. (2018) have shown TCO2 and HCO3− to be higher under HS in thermo-resistant (Fayoumi) as compared to sensitive (Leghorn) chicken lines and suggest these measures as potential selection markers for thermo-tolerance.
In summary, the beneficial effects of amino acid-chelated trace mineral supplementation seem to be environment-dependent. It is protective as it reduces circulating and intestinal stress and inflammation in heat-stressed birds; however, it increases the expression of stress and proinflammatory cytokines in birds maintained under TN conditions. These data open a new vista for further in-depth investigations to delineate the mode of amino acid-chelated trace mineral action and to define the mineral requirement of broilers under both TN and HS conditions.
Acknowledgments
This work was supported by a grant from Tracer Minerals Cimmaron, KS (to S.D.). Tracer Minerals had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
Glossary
Abbreviations
- BE
base excess
- BW
body weight
- CLDN1
claudin 1
- CORT
corticosterone
- CRP
C-reactive protein
- FCR
feed conversion ratio
- FI
feed intake
- FITC
Fluorescein Isothiocyanate
- GLM
general linear model
- Hb
hemoglobin
- HS
heat stress
- HSP
heat shock protein
- IL
interleukin
- NLRP3
nucleotide-binding oligomerization domain leucine-rich repeat and pyrin domain containing 3
- OCLN
occluding
- PCR
polymerase chain reaction
- RH
relative humidity
- SNK
Student Newman Keuls
- TN
thermoneutral
- TNFα
tumor necrosis factor alpha
- WB
woody breast
- WI
water intake
Author contributions
S.D. conceptualize and design the research. E.S.G., M.B., G.T.I., S.O., and S.D. performed the live bird trial. S.D. purchased the reagents. S.O., E.S.G., and S.D. assisted in the processing of the trial, and collection of meat quality data. M.B. and S.D. conducted gene expression analysis and statistical analysis. S.D. wrote the manuscript. E.S.G., M.B., M.T.K., G.T.I., and S.O. provided input and revised manuscript drafts.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Literature Cited
- Akarsu S., Ustundag B., Gurgoze M. K., Sen Y., and Aygun A. D.. . 2007. Plasma ghrelin levels in various stages of development of iron deficiency anemia. J. Pediatr. Hematol. Oncol. 29:384–387. doi: 10.1097/MPH.0b013e3180645170 [DOI] [PubMed] [Google Scholar]
- Bao Y. M., Choct M., Iji P. A., and Bruerton K.. . 2007. Effect of organically complexed copper, iron, manganese, and zinc on broiler performance, mineral excretion, and accumulation in tissues. J Appl Poult Res. 16(3):448–455. doi: 10.1093/japr/16.3.448 [DOI] [Google Scholar]
- Bao Y. M., Choct M., Iji P. A., and Bruerton K.. . 2010. Trace mineral interactions in broiler chicken diets. Br. Poult. Sci. 51:109–117. doi: 10.1080/00071660903571904 [DOI] [PubMed] [Google Scholar]
- Bartlett J. R., and Smith M. O.. . 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult. Sci. 82:1580–1588. doi: 10.1093/ps/82.10.1580 [DOI] [PubMed] [Google Scholar]
- Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., . et al. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183:787–791. doi: 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M. F. A., Dridi S., Koltes D. A., Latorre J. D., Bottje W. G., Greene E. S., Bickler S. W., Kim J. H., Merino-Guzman R., Hernandez-Velasco X., . et al. 2019. Evaluation of intestinal permeability and liver bacterial translocation in two modern broilers and their jungle fowl ancestor. Front. Genet. 10:480. doi: 10.3389/fgene.2019.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belay T., and Teeter R. G.. . 1996. Effects of ambient temperature on broiler mineral balance partitioned into urinary and faecal loss. Br. Poult. Sci. 37:423–433. doi: 10.1080/00071669608417873 [DOI] [PubMed] [Google Scholar]
- Bello A. U., Sulaiman J. A., and Aliyu M. S.. . 2016. Acute phase protein mRNA expressions and enhancement of antioxidant defense system in Black-meated Silkie Fowls supplemented with clove (Eugenia caryophyllus) extracts under the influence of chronic heat stress. J. Anim. Sci. Technol. 58:39. doi: 10.1186/s40781-016-0122-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian F., Yang X.-Y., Xu G., Zheng T., and Jin S.. . 2019. CRP-induced NLRP3 inflammasome activation increases LDL transcytosis across endothelial cells. Front. Pharmacol. 10:40. doi: 10.3389/fphar.2019.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bun S. D., Guo Y. M., Guo F. C., Ji F. J., and Cao H.. . 2011. Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poult. Sci. 90:1220–1226. doi: 10.3382/ps.2010-01308 [DOI] [PubMed] [Google Scholar]
- Coelho M. B., and McNaughton J. L.. . 1995. Effect of composite vitamin supplementation on broilers. J. Appl. Poult. Res. 4(3):219–229. [Google Scholar]
- Dameron C. T., and Harris E. D.. . 1987. Regulation of aortic CuZn-superoxide dismutase with copper. Effects in vivo. Biochem. J. 248(3):663–668. doi: 10.1042/bj2480663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann S. M., Spehlmann M. E., Hammond D. C., Iimura M., Hase K., Choi L. J., Hanson E., and Eckmann L.. . 2008. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J. Immunol. 180:6816–6826. doi: 10.4049/jimmunol.180.10.6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb N., and Cahaner A.. . 2002. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 81(3):293–301. doi 10.1093/ps/81.3.293 [DOI] [PubMed] [Google Scholar]
- Downs K. M., Hess J. B., and Bilgili S. F.. . 2000. Selenium source effect on broiler carcass characteristics, meat quality and drip loss. J. Appl. Anim. Res. 18(1):61–71. doi: 10.1080/09712119.2000.9706324 [DOI] [Google Scholar]
- Duncan J. A., Bergstralh D. T., Wang Y., Willingham S. B., Ye Z., Zimmermann A. G., and Ting J. P.. . 2007. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 104:8041–8046. doi: 10.1073/pnas.0611496104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry H., Yitbarek A., Munyaka P., Alizadeh M., Cleaver A., Camelo-Jaimes G., Wang P., O K., and Rodriguez-Lecompte J. C.. . 2016. Organic trace mineral supplementation enhances local and systemic innate immune responses and modulates oxidative stress in broiler chickens. Poult. Sci. 95:518–527. doi: 10.3382/ps/pev374 [DOI] [PubMed] [Google Scholar]
- Erickson K. A., and Wilding P.. . 1993. Evaluation of a novel point-of-care system, the i-STAT portable clinical analyzer. Clin. Chem. 39:283–287. doi: 10.1093/clinchem/39.2.283 [DOI] [PubMed] [Google Scholar]
- Fedde M. R. 1998. Relationship of structure and function of the avian respiratory system to disease susceptibility. Poult. Sci. 77(8):1130–1138. doi: 10.1093/ps/77.8.1130 [DOI] [PubMed] [Google Scholar]
- Ferket P., and Gernat A.. . 2006. Factors that affect feed intake of meat birds: a review. Int. J. Poult. Sci. 5(10):905–911. doi: 10.3923/ijps.2006.905.911 [DOI] [Google Scholar]
- Ferket P. R., Oviedo-Rondón E. O., Mente P. L., Bohórquez D. V., Santos A. A. Jr, Grimes J. L., Richards J. D., Dibner J. J., and Felts V.. . 2009. Organic trace minerals and 25-hydroxycholecalciferol affect performance characteristics, leg abnormalities, and biomechanical properties of leg bones of turkeys. Poult. Sci. 88:118–131. doi: 10.3382/ps.2008-00200 [DOI] [PubMed] [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B. M., Ellestad L., Porter T., Donoghue A., Bottje W. G., and Dridi S.. . 2017. Effect of Morinda citrifolia (Noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 8:919. doi: 10.3389/fphys.2017.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L., Eigenbrod T., and Núñez G.. . 2009. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183:792–796. doi: 10.4049/jimmunol.0900173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Esquerra R., and Leeson S.. . 2005. Effects of acute versus chronic heat stress on broiler response to dietary protein. Poult. Sci. 84:1562–1569. doi: 10.1093/ps/84.10.1562 [DOI] [PubMed] [Google Scholar]
- Greene E., Flees J., Dadgar S., Mallmann B., Orlowski S., Dhamad A., Rochell S., Kidd M., Laurendon C., Whitfield H., . et al. 2019a. Quantum blue reduces the severity of woody breast myopathy via modulation of oxygen homeostasis-related genes in broiler chickens. Front. Physiol. 10:1251. doi: 10.3389/fphys.2019.01251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene E. S., Rajaei-Sharifabadi H., and Dridi S.. . 2019b. Feather HSP70: a novel non-invasive molecular marker for monitoring stress induced by heat exposure in broilers. Poult. Sci. 98:3400–3404. doi: 10.3382/ps/pez120 [DOI] [PubMed] [Google Scholar]
- Günzel D., and Yu A. S.. . 2013. Claudins and the modulation of tight junction permeability. Physiol. Rev. 93:525–569. doi: 10.1152/physrev.00019.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai L., Rong D., and Zhang Z. Y.. . 2000. The effect of thermal environment on the digestion of broilers. J. Anim. Physiol. Anim. Nutr. 83(2):57–64. doi: 10.1046/j.1439-0396.2000.00223.x [DOI] [Google Scholar]
- Kidd M. T., Ferket P. R., and Qureshi M. A.. . 1996. Zinc metabolism with special reference to its role in immunity. World Poult. Sci. J. 52(3):309–324. doi: 10.1079/WPS19960022 [DOI] [Google Scholar]
- Koch F., Thom U., Albrecht E., Weikard R., Nolte W., Kuhla B., and Kuehn C.. . 2019. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc. Natl. Acad. Sci. U. S. A. 116:10333–10338. doi: 10.1073/pnas.1820130116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M. H. 2017. Issues and consequences of using nutrition to modulate the avian immune response. J. Appl. Poult. Res. 26(4):605–612. doi: 10.3382/japr/pfx028 [DOI] [Google Scholar]
- Kucuk O. 2008. Zinc in a combination with magnesium helps reducing negative effects of heat stress in quails. Biol. Trace Elem. Res. 123:144–153. doi: 10.1007/s12011-007-8083-6 [DOI] [PubMed] [Google Scholar]
- Kucuk O., Sahin N., and Sahin K.. . 2003. Supplemental zinc and vitamin A can alleviate negative effects of heat stress in broiler chickens. Biol. Trace Elem. Res. 94:225–235. doi: 10.1385/BTER:94:3:225 [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Bottje W., Ramnathan R., Hartson S. D., Coon C. N., Kong B. W., Owens C. M., Vazquez-Añon M., and Hargis B. M.. . 2017. Proteomic analysis reveals changes in carbohydrate and protein metabolism associated with broiler breast myopathy. Poult. Sci. 96:2992–2999. doi: 10.3382/ps/pex069 [DOI] [PubMed] [Google Scholar]
- Kuttappan V. A., Lee Y. S., Erf G. F., Meullenet J. F., McKee S. R., and Owens C. M.. . 2012. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult. Sci. 91:1240–1247. doi: 10.3382/ps.2011-01947 [DOI] [PubMed] [Google Scholar]
- Laganá C., Ribeiro A. M. L., Kessler A. M., Kratz L. R., and Pinheiro C. C.. . 2007. Effect of the supplementation of vitamins and organic minerals on the performance of broilers under heat stress. Braz. J. Poult. Sci. 9:39–43. doi: 10.1590/S1516-635X2007000100006 [DOI] [Google Scholar]
- Lambert G. P., Gisolfi C. V., Berg D. J., Moseley P. L., Oberley L. W., and Kregel K. C.. . 2002. Selected contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J. Appl. Physiol. (1985). 92:1750–61; discussion 1749. doi: 10.1152/japplphysiol.00787.2001 [DOI] [PubMed] [Google Scholar]
- Lara L. J., and Rostagno M. H.. . 2013. Impact of heat stress on poultry production. Animals (Basel). 3:356–369. doi: 10.3390/ani3020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter K., Greene E., Piekarski A., Faulkner O. B., Hargis B. M., Bottje W., and Dridi S.. . 2015. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308:R173–R187. doi: 10.1152/ajpregu.00394.2014 [DOI] [PubMed] [Google Scholar]
- Leenstra F., and Cahaner A.. . 1992. Effects of low, normal, and high temperatures on slaughter yield of broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult. Sci. 71:1994–2006. doi: 10.3382/ps.0711994 [DOI] [PubMed] [Google Scholar]
- Li S., Lu L., Hao S., Wang Y., Zhang L., Liu S., Liu B., Li K., and Luo X.. . 2011. Dietary manganese modulates expression of the manganese-containing superoxide dismutase gene in chickens. J. Nutr. 141:189–194. doi: 10.3945/jn.110.126680 [DOI] [PubMed] [Google Scholar]
- Li X., Mao M., Zhang Y., Yu K., and Zhu W.. . 2019a. Succinate modulates intestinal barrier function and inflammation response in pigs. Biomolecules 9(9):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Wang G., Zhang X. L., He G. L., Luo X., Yang J., Luo Z., Shen T. T., and Yang X. S.. . 2019b. MicroRNA-155 promotes heat stress-induced inflammation via targeting liver X receptor α in microglia. Front. Cell. Neurosci. 13:12. doi: 10.3389/fncel.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Luo X. G., Ji C., Liu B., and Yu S. X.. . 2007. Effect of manganese supplementation and source on carcass traits, meat quality, and lipid oxidation in broilers. J. Anim. Sci. 85:812–822. doi: 10.2527/jas.2006-229 [DOI] [PubMed] [Google Scholar]
- Luissint A.-C., Parkos C. A., and Nusrat A.. . 2016. Inflammation and the intestinal barrier: leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterol. 151(4):616–632. doi: 10.1053/j.gastro.2016.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison F. N., Jurkevich A., and Kuenzel W. J.. . 2008. Sex differences in plasma corticosterone release in undisturbed chickens (Gallus gallus) in response to arginine vasotocin and corticotropin releasing hormone. Gen. Comp. Endocrinol. 155:566–573. doi: 10.1016/j.ygcen.2007.08.014 [DOI] [PubMed] [Google Scholar]
- Mangan M. S. J., Olhava E. J., Roush W. R., Seidel H. M., Glick G. D., and Latz E.. . 2018. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 17:588–606. doi: 10.1038/nrd.2018.97 [DOI] [PubMed] [Google Scholar]
- Marreiro D. N., Geloneze B., Tambascia M. A., Lerário A. C., Halpern A., and Cozzolino S. M.. . 2006. Effect of zinc supplementation on serum leptin levels and insulin resistance of obese women. Biol. Trace Elem. Res. 112:109–118. doi: 10.1385/bter:112:2:109 [DOI] [PubMed] [Google Scholar]
- Martin M. P., Wineland M., and Barnes H. J.. . 2010. Selected blood chemistry and gas reference ranges for broiler breeders using the i-STAT handheld clinical analyzer. Avian Dis. 54:1016–1020. doi: 10.1637/9223-122209-Reg.1 [DOI] [PubMed] [Google Scholar]
- Martinon F., Burns K., and Tschopp J.. . 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10:417–426. doi: 10.1016/s1097-2765(02)00599-3 [DOI] [PubMed] [Google Scholar]
- Nishii N., Oshima T., Li M., Eda H., Nakamura K., Tamura A., Ogawa T., Yamasaki T., Kondo T., and Kono T.. . 2020. Lubiprostone induces claudin-1 and protects intestinal barrier function. Pharmacol. 105:102–108. doi: 10.1159/000503054 [DOI] [PubMed] [Google Scholar]
- Nishiuchi M., Sakai K., Tajima H., Katayama K., Kimura F., Hoshi S., Goto T., Shirakawa H., and Komai M.. . 2018. Orexigenic action of oral zinc: metabolomic analysis in the rat hypothalamus. Biosci. Biotechnol. Biochem. 82:2168–2175. doi: 10.1080/09168451.2018.1516543 [DOI] [PubMed] [Google Scholar]
- Ognik K., Sembratowicz I., Cholewińska E., Jankowski J., Kozłowski K., Juśkiewicz J., and Zduńczyk Z.. . 2018. The effect of administration of copper nanoparticles to chickens in their drinking water on the immune and antioxidant status of the blood. Anim. Sci. J. 89:579–588. doi: 10.1111/asj.12956 [DOI] [PubMed] [Google Scholar]
- Ohtsu H., Yamazaki M., Abe H., Murakami H., and Toyomizu M.. . 2015. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 52:282–287. doi: 10.2141/jpsa.0150062 [DOI] [Google Scholar]
- Orlowski S., Flees J., Greene E. S., Ashley D., Lee S. O., Yang F. L., Owens C. M., Kidd M., Anthony N., and Dridi S.. . 2018. Effects of phytogenic additives on meat quality traits in broiler chickens1. J. Anim. Sci. 96:3757–3767. doi: 10.1093/jas/sky238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco B. H. C., Nakagi V. S., Kobashigawa E. H., Caniatto A. R. M., Faria D. E., and Faria Filho D. E.. . 2017. Dietary levels of zinc and manganese on the performance of broilers between 1 to 42 days of age. Braz. J. Poult. Sci. 19:171–178. doi: 10.1590/1806-9061-2016-0323 [DOI] [Google Scholar]
- Petracci M., and Cavani C.. . 2012. Muscle growth and poultry meat quality issues. Nutrients 4:1–12. doi: 10.3390/nu4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley A. G. 2002. Heat shock proteins, inflammation, and cardiovascular disease. Circulation 105:1012–1017. doi: 10.1161/hc0802.103729 [DOI] [PubMed] [Google Scholar]
- Quinteiro-Filho W. M., Ribeiro A., Ferraz-de-Paula V., Pinheiro M. L., Sakai M., Sá L. R., Ferreira A. J., and Palermo-Neto J.. . 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 89:1905–1914. doi: 10.3382/ps.2010-00812 [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W. G., and Dridi S.. . 2017. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress- and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 8:192. doi: 10.3389/fgene.2017.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar U., Vinoth A., Reddy E. P. K., Shanmugam M., and Rao S. V. R.. . 2018. Effect of supplemental trace minerals on Hsp-70 mRNA expression in commercial broiler chicken. Anim. Biotechnol. 29:20–25. doi: 10.1080/10495398.2017.1287712 [DOI] [PubMed] [Google Scholar]
- Richards J. D., Zhao J., Harrell R. J., Atwell C. A., and Dibner J. J.. . 2010. Trace mineral nutrition in poultry and swine. Asian-Australas. J. Anim. Sci. 23(11):1527–1534. doi: 10.5713/ajas.2010.r.07 [DOI] [Google Scholar]
- Richter K., Haslbeck M., and Buchner J.. . 2010. The heat shock response: life on the verge of death. Mol. Cell 40:253–266. doi: 10.1016/j.molcel.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Sahin K., Smith M. O., Onderci M., Sahin N., Gursu M. F., and Kucuk O.. . 2005. Supplementation of zinc from organic or inorganic source improves performance and antioxidant status of heat-distressed quail. Poult. Sci. 84:882–887. doi: 10.1093/ps/84.6.882 [DOI] [PubMed] [Google Scholar]
- Saleh K. M. M., and Al-Zghoul M. B.. . 2019. Effect of acute heat stress on the mRNA levels of cytokines in broiler chickens subjected to embryonic thermal manipulation. Animals 9(8):499. doi: 10.3390/ani9080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G. 2000. Heat shock factors and the control of the stress response. Biochem. Pharmacol. 59:55–63. doi: 10.1016/s0006-2952(99)00299-3 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D., and Livak K. J.. . 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108. doi: 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Sirri F., Maiorano G., Tavaniello S., Chen J., Petracci M., and Meluzzi A.. . 2016. Effect of different levels of dietary zinc, manganese, and copper from organic or inorganic sources on performance, bacterial chondronecrosis, intramuscular collagen characteristics, and occurrence of meat quality defects of broiler chickens. Poult. Sci. 95:1813–1824. doi: 10.3382/ps/pew064 [DOI] [PubMed] [Google Scholar]
- Song J., Xiao K., Ke Y. L., Jiao L. F., Hu C. H., Diao Q. Y., Shi B., and Zou X. T.. . 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 93:581–588. doi: 10.3382/ps.2013-03455 [DOI] [PubMed] [Google Scholar]
- Steinmetz H. W., Vogt R., Kästner S., Riond B., and Hatt J. M.. . 2007. Evaluation of the i-STAT portable clinical analyzer in chickens (Gallus gallus). J. Vet. Diagn. Invest. 19:382–388. doi: 10.1177/104063870701900407 [DOI] [PubMed] [Google Scholar]
- Stillman J. H. 2019. Heat waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology (Bethesda). 34:86–100. doi: 10.1152/physiol.00040.2018 [DOI] [PubMed] [Google Scholar]
- St-Pierre N., Cobanov B., and Schnitkey G.. . 2003. Economic losses from heat stress by US livestock industries. J Dairy Sci. 86(E.Suppl):E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Sun J., Liu D., and Shi R.. . 2015. Supplemental dietary iron glycine modifies growth, immune function, and antioxidant enzyme activities in broiler chickens. Livest. Sci. 176:129–134. doi 10.1016/j.livsci.2015.03.004 [DOI] [Google Scholar]
- Suzuki K., Jayasena C. N., and Bloom S. R.. . 2011. The gut hormones in appetite regulation. J. Obes. 2011:528401. doi: 10.1155/2011/528401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Światkiewicz S., Arczewska-Wlosek A., and Jozefiak D.. . 2014. The efficacy of organic minerals in poultry nutrition: review and implications of recent studies. World Poult. Sci. J. 70(3):475–486. doi 10.1017/S0043933914000531 [DOI] [Google Scholar]
- Tijare V. V., Yang F. L., Kuttappan V. A., Alvarado C. Z., Coon C. N., and Owens C. M.. . 2016. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult. Sci. 95:2167–2173. doi: 10.3382/ps/pew129 [DOI] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., and Fink-Gremmels J.. . 2015. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides. PLoS One 10:e0138975. doi: 10.1371/journal.pone.0138975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velleman S. G., and Clark D. L.. . 2015. Histopathologic and myogenic gene expression changes associated with wooden breast in broiler breast muscles. Avian Dis. 59:410–418. doi: 10.1637/11097-042015-Reg.1 [DOI] [PubMed] [Google Scholar]
- Wang Y., Saelao P., Chanthavixay K., Gallardo R., Bunn D., Lamont S. J., Dekkers J. M., Kelly T., and Zhou H.. . 2018. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 97:770–780. doi: 10.3382/ps/pex363 [DOI] [PubMed] [Google Scholar]
- Welc S. S., Clanton T. L., Dineen S. M., and Leon L. R.. . 2013. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J. Appl. Physiol. (1985). 115:1126–1137. doi: 10.1152/japplphysiol.00636.2013 [DOI] [PubMed] [Google Scholar]
- Willcox J. K., Ash S. L., and Catignani G. L.. . 2004. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 44:275–295. doi: 10.1080/10408690490468489 [DOI] [PubMed] [Google Scholar]
- Wołonciej M., Milewska E., and Roszkowska-Jakimiec W.. . 2016. Trace elements as an activator of antioxidant enzymes. Postepy Hig. Med. Dosw. (Online) 70:1483–1498. doi: 10.5604/17322693.1229074 [DOI] [PubMed] [Google Scholar]
- Wu T. K., Lim P. S., Jin J. S., Wu M. Y., and Chen C. H.. . 2018. Impaired gut epithelial tight junction expression in hemodialysis patients complicated with intradialytic hypotension. Biomed Res. Int. 2018:2670312. doi: 10.1155/2018/2670312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lai X., Li Z., Zhang X., and Luo Q.. . 2018. Effect of chronic heat stress on some physiological and immunological parameters in different breed of broilers. Poult. Sci. 97:4073–4082. doi: 10.3382/ps/pey256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gao M., Nie W., Yuan J., Zhang B., Wang Z., and Wu Z.. . 2012. Dietary magnesium sulfate supplementation protects heat stress-induced oxidative damage by restoring the activities of anti-oxidative enzymes in broilers. Biol. Trace Elem. Res. 146:53–58. doi: 10.1007/s12011-011-9210-y [DOI] [PubMed] [Google Scholar]
- Yang Y., Wu Z., Chen Y., Qiao J., Gao M., Yuan J., Nie W., and Guo Y.. . 2006. Magnesium deficiency enhances hydrogen peroxide production and oxidative damage in chick embryo hepatocyte in vitro. Biometals 19:71–81. doi: 10.1007/s10534-005-6898-1 [DOI] [PubMed] [Google Scholar]
- Zhu Y. W., Lu L., Li W. X., Zhang L. Y., Ji C., Lin X., Liu H. C., Odle J., and Luo X. G.. . 2015. Effect of dietary manganese on antioxidant status and expression levels of heat-shock proteins and factors in tissues of laying broiler breeders under normal and high environmental temperatures. Br. J. Nutr. 114:1965–1974. doi: 10.1017/S0007114515003803 [DOI] [PubMed] [Google Scholar]