Abstract
Tamoxifen efficacy in breast cancer is suspected to depend on adherence and intact drug metabolism. We evaluated the role of adherence behavior and pharmacogenetics on the formation rate of (Z)‐endoxifen. In 192 Brazilian patients, we assessed plasma levels of tamoxifen and its metabolites at 3, 6, and 12 months of treatment (liquid‐chromatography tandem mass spectrometry), adherence behavior (Morisky, Green, and Levine medication adherence scale), and cytochrome P450 2D6 (CYP2D6) and other pharmacogene polymorphisms (matrix‐assisted laser‐desorption‐ionization time of flight) mass spectrometry, real‐time polymerase chain reaction). Adherence explained 47% of the variability of tamoxifen plasma concentrations (P < 0.001). Although CYP2D6 alone explained 26.4%, the combination with adherence explained 40% of (Z)‐endoxifen variability at 12 months (P < 0.001). The influence of low adherence to not achieving relevant (Z)‐endoxifen levels was highest in patients with noncompromised CYP2D6 function (relative risk 3.65; 95% confidence interval 1.48–8.99). As a proof‐of‐concept, we demonstrated that (Z)‐endoxifen levels are influenced both by patient adherence to tamoxifen and CYP2D6, which is particularly relevant for patients with full CYP2D6 function.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Poor patient adherence to tamoxifen is associated with reduced clinical efficacy. cytochrome P450 2D6 (CYP2D6) is a key factor of tamoxifen metabolism, however, genetic variants only partially explain the variability of plasma concentrations of the active metabolite (Z)‐endoxifen.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We studied the influence of patient adherence behavior and CYP2D6 phenotype (and other pharmacogenes) on plasma metabolite concentrations. We investigated whether (Z)‐endoxifen concentrations depended on either factor alone or in combination.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Adherence and CYP2D6 status are independent determinants of tamoxifen and (Z)‐endoxifen plasma levels. Their combined influence is particularly relevant for patients with full CYP2D6 function.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The dual monitoring of tamoxifen (surrogate for adherence) and (Z)‐endoxifen (surrogate for clinical response) plasma levels could be a strategy to improve tamoxifen outcome in the future.
1.
One‐third of patients with early breast cancer with estrogen receptor (ER)‐positive tumors treated with the selective estrogen receptor modulator tamoxifen either relapse or die from the disease in the following decade.1, 2 Improvement of tamoxifen efficacy requires a better knowledge of factors determining outcome of which the medication‐taking behavior (i.e., adherence), is a strong suspect.3, 4 Discontinuation and nonadherence to tamoxifen are frequent and result in increased mortality.5, 6, 7, 8, 9 A large study using automated pharmacy records showed that 31% of patients discontinued therapy, and among those who continued, only 70% adhered at 4.5 years with only 50% being fully adherent.10 Nonadherence gradually evolves over time from 10% in the first year to > 50% in the fifth year6, 8, 10, 11 being most pronounced in young women being below 40 years of age and women older than 75 years.10 Ten‐year survival rates significantly differed between patients who continued treatment (82%) compared with patients who did not adhere (78%).11 Recently, the BIG I‐98 trial reported a considerably reduced disease‐free survival (hazard ratio 1.45; 95% confidence interval (CI) 1.09–1.93) for patients who ceased protocol‐assigned endocrine treatment.12
To assess drug adherence, commonly used prescription‐refill patterns infer that the medication is taken exactly as prescribed, yet, partial adherence or actual use of the medication cannot be controlled.13 In contrast, an objective surrogate of tamoxifen adherence is the monitoring of drug concentrations, including the parent drug and its active metabolite (Z)‐endoxifen. (Z)‐endoxifen can be easily measured in the patients' plasma, however, individual concentrations depend on pharmacogene polymorphisms, particularly those of the liver enzyme cytochrome P450 2D6 (CYP2D6).14 Polymorphisms are responsible for different phenotypes, which aside from individuals with normal CYP2D6 function (extensive metabolizer (EM)), comprise individuals with absent (poor metabolizer (PM)), reduced (intermediate metabolizer (IM)), and increased (ultrarapid metabolizer (UM)) CYP2D6 activity. Among Europeans, the frequency of PM and IM individuals is 9% and 40%, respectively. In Brazil, the observed PM frequency is 2.5% with an increased 4% frequency in patients with ER‐positive breast cancer.15, 16
For patients receiving tamoxifen therapy, CYP2D6 genotyping allows the prediction of (Z)‐endoxifen plasma concentrations.17, 18, 19 A putative threshold of 5.9 ng/mL has been proposed in the Women's Healthy Eating and Living (WHEL) study suggesting that a minimal concentration threshold is required above which (Z)‐endoxifen is more effective against the recurrence of breast cancer and below which patients are at higher risk for recurrence,20 likely due to incomplete inhibition of ER‐dependent growth signaling.21 Although several outcome studies demonstrated the importance of CYP2D6 genotyping for the prediction of the risk‐to‐relapse in adjuvant and metastatic settings,22, 23, 24, 25 other studies did not confirm this association,26, 27, 28, 29 the reason why current clinical guidelines do not support the use of CYP2D6 genotypes for predicting tamoxifen response.30 Therefore, it is important to identify confounders that may mask the tamoxifen CYP2D6 association, with tamoxifen adherence being a prime candidate.13 The combined analysis of adherence behavior and tamoxifen metabolism may shed new light on this important issue, particularly because first evidence has been reported of an increased effect of CYP2D6 genotype on patient outcome when adjusted for adherence to tamoxifen therapy.31 Here, we present a prospective study of mainly patients with early breast cancer from Brazil, in which interview‐informed tamoxifen adherence together with CYP2D6 metabolizer status have been measured in order to evaluate their combined contribution to the lowering of the patients' plasma (Z)‐endoxifen concentrations to potentially subtherapeutic levels. The study demonstrates how patients with breast cancer of underserved patient populations can contribute valuable pharmacokinetic and pharmacogenetic information in the field of breast cancer biomarker research.
2. Materials and Methods
2.1. Patients and study design
Patients were consecutively recruited at the Erasto Gaertner Hospital, Curitiba, Southern Brazil, a national reference center for oncology treatment. Between April 2014 and June 2017, 192 patients with ER‐positive breast cancer treated with tamoxifen were included to investigate the relevance of drug adherence on the plasma levels of active tamoxifen metabolites. According to recommendations for tumor marker studies (REMARK),32 inclusion criteria were defined as women aged 18 years or older who were diagnosed with any stage, histologic, or molecular subtype of ER‐positive breast cancer, and who started daily treatment with 20 mg tamoxifen for an intended 5 years of therapy. Exclusion criteria were age beyond 82 years and patients unable to complete the study schedule and questionnaire. Patients were followed during the first year of treatment at months 3, 6, and 12 for the assessment of adherence to tamoxifen intake based on interview, measurement of plasma levels of tamoxifen and its metabolites, and assessment of CYP2D6 metabolizer status (and other relevant drug metabolizing enzymes (DMEs)) based on genotypes. Ethical approval was obtained from the Brazilian National Commission of Ethical Research. All patients provided written informed consent. Study size calculation (99.9% power) revealed a minimum of 42 patients required to detect an association of (Z)‐endoxifen variability with CYP2D6 polymorphism, based on the prevalence of IM and PM patients of 40% in Brazil33 and an expected effect size of R 2 = 0.4.17
2.2. Assessment of adherence to tamoxifen therapy
Tamoxifen adherence was assessed using the Morisky, Green, and Levine Medication Adherence Scale questionnaire, a structured four‐item self‐reported adherence measure validated for a wide range of diseases, including cancer.34 This questionnaire has been successfully used in patients with low literacy and its feasibility was previously confirmed in our hospital for inpatients and outpatients with cancer.35 Four trained pharmacists performed the questionnaire‐based interviews at 3, 6, and 12 months after starting tamoxifen therapy and included the following questions: (i) Do you ever forget to take your medicines?; (ii) Are you careless at times about taking your medicines?; (iii) When you feel better do you sometimes stop taking your medicine?; and (iv) Sometimes, if you feel worse when you take the medicine, do you stop taking it? For each patient and visit “yes” (1 point) or “no” (0 point) answers were documented and the four‐item scores summed up to define three adherence levels: high (0), medium (1–2), and low adherence (3–4). Information on concomitant medication and self‐reported adverse events during tamoxifen treatment were recorded during all visits.
2.3. Genotyping and quantification of tamoxifen and its active metabolites from plasma
Genomic DNA obtained from peripheral blood mononuclear cells was genotyped for CYP2D6 polymorphisms as previously described.24 A CYP2D6 enzyme activity score (AS) was assigned to genotypes (diplotypes) based on allele scores of 0 (PM), 0.5 (IM), 1 (EM), and 2 (UM)36, 37 and CYP2D6 phenotypes were deduced from AS: PM (0), IM (0.5 to 1.0), EM (1.5 to 2.0), and UM (3.0). Other DME gene polymorphisms included CYP2C9*2 and *3, CYP2C19*2 and *17, and CYP3A4*22 and CYP3A5*3 (Supplemental Material).
Heparinized plasma samples were obtained at months 3, 6, and 12 after the start of tamoxifen therapy. Plasma levels of tamoxifen and the inactive major metabolite DM‐Tam as well as active metabolites (Z)‐endoxifen and (Z)‐4‐OH‐Tam) were measured by liquid chromatography tandem mass spectrometry as previously described.17
2.4. Statistical analysis
DME genotype frequencies were tested for Hardy–Weinberg Equilibrium. Parametric and nonparametric tests were applied to determine whether tamoxifen and its metabolite concentrations as well as metabolic ratio's (MRs) differ between DME genotypes and adherence behavior. Multiple linear regression modeling was applied to evaluate the contribution of factors to the variability of plasma concentrations of tamoxifen, (Z)‐endoxifen, DM‐Tam, and (Z)‐4‐OH‐Tam, as well as the respective MRs. Relative risk (RR) and 95% CIs were calculated at the 12‐month time point to evaluate the risk of patients not achieving a previously proposed clinical threshold of 5.9 ng/mL (15.8 nM) (Z)‐endoxifen.20 All P values were two‐sided, and values < 0.05 were considered statistically significant (details provided in Supplemental Material).
3. Results
3.1. Patient adherence to treatment
Demographic and clinical characteristics together with adherence assessment at specific time points are given in Table 1 and Table S1 . Adherence was assessed for 163 patients at month 3 (85%), 173 patients at month 6 (90%), and 170 patients at month 12 (89%). Median age at diagnosis was 51.5 years (range 24–82 years); 127 patients (66%) were premenopausal. At 3 and 6 months, 74–76% of patients showed high adherence rates to tamoxifen treatment, which dropped to 63% at 12 months (Figure 1 a). Low adherence was not observed during the first 3 months but increased to 10.6% at 12 months (Figure 1 a; P < 0.05). High adherence at 12 months was more prevalent in patients without reported adverse events compared with those who reported adverse events (Figure 1 b; P < 0.05). With the exception of age at diagnosis, menopausal status, and self‐reported adverse events at 12 months, patient and tumor characteristics did not differ between adherence subgroups across time points (Table 1). Adverse events at 3, 6, and 12 months were reported for 53%, 54%, and 66% of patients, respectively (Table 1), with hot flashes being most frequent (62%, 65%, and 68%, respectively). Others included edema of the inferior members (17%, 25%, and 20%), fatigue (14%, 25%, and 30%), and nausea/vomiting (15%, 12%, and 12%) at 3, 6, and 12 months, respectively.
Table 1.
Adherence behavior to tamoxifen treatment (20 mg daily) of Brazilian patients with breast cancer in relation to their demographic and clinical characteristics
| Characteristic | Overall distribution (n = 192) | Adherence at 3 months (n = 163) | OR | P value | Adherence at 6 months (n = 173) | OR | P value | Adherence at 12 months (n = 170) | OR | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High, n (%) | Medium/low, n (%) | 95% CI | High, n (%) | Medium/low, n (%) | 95% CI | High, n (%) | Medium/low, n (%) | 95% CI | |||||
| Ethnicity,a n (%) | |||||||||||||
| White | 139 (72.4) | 89 (74.8) | 30 (25.2) | 0.804 (0.37–1.73) | 0.689 | 98 (79.7) | 25 (20.3) | 2.019 (0.97–4.20) | 0.078 | 78 (62.9) | 46 (37.1) | 0.904 (0.45–1.84) | 0.859 |
| Black | 11 (5.7) | 31 (70.5) | 13 (29.5) | 33 (66.0) | 17 (34.0) | 30 (65.2) | 16 (34.8) | ||||||
| Pardo | 37 (19.3) | ||||||||||||
| Asian/Indian | 5 (2.6) | ||||||||||||
| Educational level,b n (%) | |||||||||||||
| None or incomplete basic | 68 (35.4) | 46 (76.7) | 14 (23.3) | 0.777 (0.37–1.62) | 0.582 | 48 (77.4) | 14 (22.6) | 1.157 (0.56–2.41) | 0.853 | 40 (67.8) | 19 (32.2) | 1.331 (0.68–2.59) | 0.503 |
| Basic | 50 (26.0) | 74 (71.8) | 29 (28.2) | 83 (74.8) | 28 (25.2) | 68 (61.3) | 43 (38.7) | ||||||
| Upper secondary | 53 (27.6) | ||||||||||||
| University (Bachelor/Master/Doctoral) | 21 (10.9) | ||||||||||||
| Age at diagnosis, median (range), years | 51.5 (24–82) | 54 (33–82) | 47 (28–76) | < 0.001 | 54 (24–82) | 49 (32–77) | 0.007 | 56 (24–82) | 48 (28–79) | < 0.001 | |||
| < 65 | 145 (75.5) | 87 (69.0) | 39 (31.0) | 0.270 (0.09–0.82) | 0.018 | 94 (71.8) | 37 (28.2) | 2.913 (1.03–7.98) | 0.038 | 71 (55.9) | 56 (44.1) | 4.86 (1.92–12.34) | < 0.001 |
| 65 | 47 (24.5) | 33 (89.2) | 4 (10.8) | 37 (88.1) | 5 (11.9) | 37 (86.0) | 6 (14.0) | ||||||
| Menopausal status, n (%) | |||||||||||||
| Premenopausal | 127 (66.1) | 74 (66.7) | 37 (33.3) | 0.261 (0.10–0.67) | 0.004 | 81 (70.4) | 34 (29.6) | 2.623 (1.13–6.12) | 0.025 | 60 (53.6) | 52 (46.4) | 4.160 (1.92–9.04) | < 0.001 |
| Postmenopausal | 65 (33.9) | 46 (88.5) | 6 (11.5) | 50 (86.2) | 8 (13.8) | 48 (82.8) | 10 (17.2) | ||||||
| ECOG performance status, n (%) | |||||||||||||
| 0–1 | 187 (97.4) | 116 (73.0) | 43 (27.0) | 1.371 (1.25–1.51) | 0.574 | 126 (75.0) | 42 (25.0) | 1.333 (1.22–1.46) | 0.337 | 103 (62.4) | 62 (37.6) | 1.602 (1.42–1.80) | 0.160 |
| 2–4 | 5 (2.6) | 4 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | ||||||
| Clinical stage at diagnosis,c n (%) | |||||||||||||
| 0 (in situ) | 2 (1.0) | 93 (72.1) | 36 (27.9) | 0.670 (0.27–1.67) | 0.513 | 104 (75.4) | 34 (24.6) | 1.103 (0.46–2.66) | 1.000 | 85 (61.6) | 53 (38.4) | 1.593 (0.69–3.70) | 0.314 |
| IA/IB | 56 (29.2) | ||||||||||||
| IIA/IIB | 92 (47.9) | ||||||||||||
| IIIA/IIIB/IIIC | 33 (17.2) | 27 (79.4) | 7 (20.6) | 27 (77.1) | 8 (22.9) | 23 (71.9) | 9 (28.1) | ||||||
| IV | 9 (4.7) | ||||||||||||
| Concomitant medications,d n (%) | |||||||||||||
| < 3 medications | 122 (63.6) | 92 (71.3) | 37 (28.7) | 1.877 (0.72–4.91) | 0.274 | 107 (77.0) | 32 (23.0) | 1.393 (0.60–3.22) | 0.504 | 92 (62.2) | 56 (37.8) | 0.616 (0.23–1.67) | 0.477 |
| 3 or more medication | 70 (36.5) | 28 (82.4) | 6 (17.6) | 24 (70.6) | 10 (29.4) | 16 (72.7) | 6 (27.3) | ||||||
| Self‐reported adverse events | |||||||||||||
| Yes | 155 (80.7) | 60 (69.8) | 26 (30.2) | 0.654 (0.32–1.33) | 0.287 | 66 (70.2) | 28 (29.8) | 1.970 (0.95–4.08) | 0.076 | 63 (55.8) | 50 (44.2) | 2.976 (1.42–6.22) | 0.004 |
| No | 37 (19.3) | 60 (77.9) | 17 (22.1) | 65 (82.3) | 14 (17.7) | 45 (78.9) | 12 (21.1) | ||||||
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OR, odds ratio.
P: significance value for Fisher's exact test for qualitative variables and Mann–Whitney test for quantitative variable (age).
Black, Pardo, and Asian/Indian were grouped to be tested against white. In Brazil, Pardo is an ethnic/skin color category used by the Brazilian Institute of Geography and Statistics (IBGE) in the Brazilian censuses. The term “pardo” is commonly used to refer to Brazilians of mixed ethnic ancestries. Pardo Brazilians represent a wide range of skin colors and backgrounds. They are typically a mixture of white Brazilian, Afro‐Brazilian, and Native Brazilian. Indian refers to Native Brazilian.
Basic, upper secondary and university levels were grouped to be tested against no instruction.
0 (in situ), IA/IB and IIA/IIB were grouped to be tested against IIIA/IIIB/IIIC and IV.
Concomitant medication in this analysis was assessed to evaluate the impact of polypharmacy in adherence behavior. The main prescribed pharmacologic classes were antihypertensive drugs (29.2%, 29.4%, and 26.3% for 3, 6, and 12 months, respectively) and nonopioid analgesics and nonsteroidal anti‐inflammatory drugs (13.2%, 16.0%, and 22.0% for 3, 6, and 12 months, respectively). Strong cytochrome P450 2D6 (CYP2D6) inhibitors were near 1.0% of all concomitant medications for all time points (0.5%, 0.9%, and 1.2% for 3, 6, and 12 months, respectively).
Figure 1.
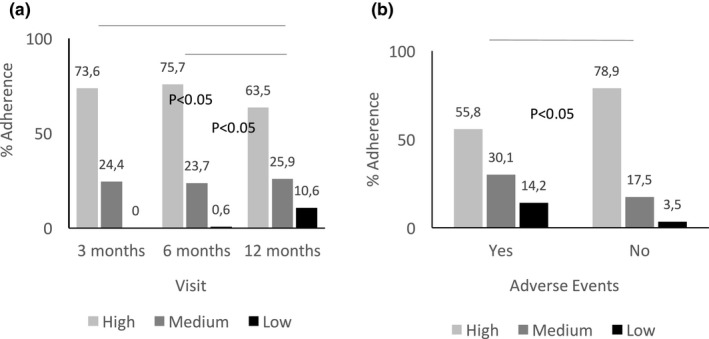
Adherence levels at consecutive time points after starting tamoxifen therapy of 20 mg daily. (a) 3 months (n = 163); 6 months (n = 173); and 12 months (n = 170). (b) 12 months, stratified by the occurrence of self‐reported adverse events: presence of adverse events (Yes; N = 113); absence of adverse events (No; N = 57). Categories high (light grey), medium (dark grey), and low (black) are given as % for each group; P values refer to contingency Chi‐square tests of a difference between visit a or grouping by adverse events b.
3.2. Genotype frequencies and enzyme AS
Genotypes met Hardy–Weinberg Equilibrium with the exception of CYP2D6*29 (Table S2 ). Although allele frequencies differed significantly between ethnic groups (P < 0.001; Table S2 ), ethnicity was not a prognostic factor for DME phenotypes or plasma concentration of tamoxifen and its major metabolites. DME phenotypes/AS predicted from 17 polymorphic loci tested in the 192 patients are summarized in Figure S1 .
3.3. Association between treatment adherence and plasma concentrations of tamoxifen and its metabolites
At 3 months of treatment, patients with good adherence had 26% higher tamoxifen concentrations than patients with medium adherence (318 ± 97 nM vs. 236 ± 115 nM; P < .001; Figure 2 a). Multiple linear regression analysis showed that 16–21% of the variability of tamoxifen levels at months 3 and 6 were explained by adherence to treatment and age at diagnosis (P < 0.001; Table 2). At 12 months, tamoxifen plasma levels strongly correlated with adherence (r = 0.70; P < 0.001) with tamoxifen levels being highest in patients with high adherence (389 ± 99 nM) compared with those with low or medium adherence (157 ± 67 nM and 258 ± 61 nM; P < 0.001; Figure 2 a). In multivariate analyses, adherence was confirmed as the sole determinant that explained 47% of tamoxifen plasma concentration variability (P < 0.001; Table 2).
Figure 2.
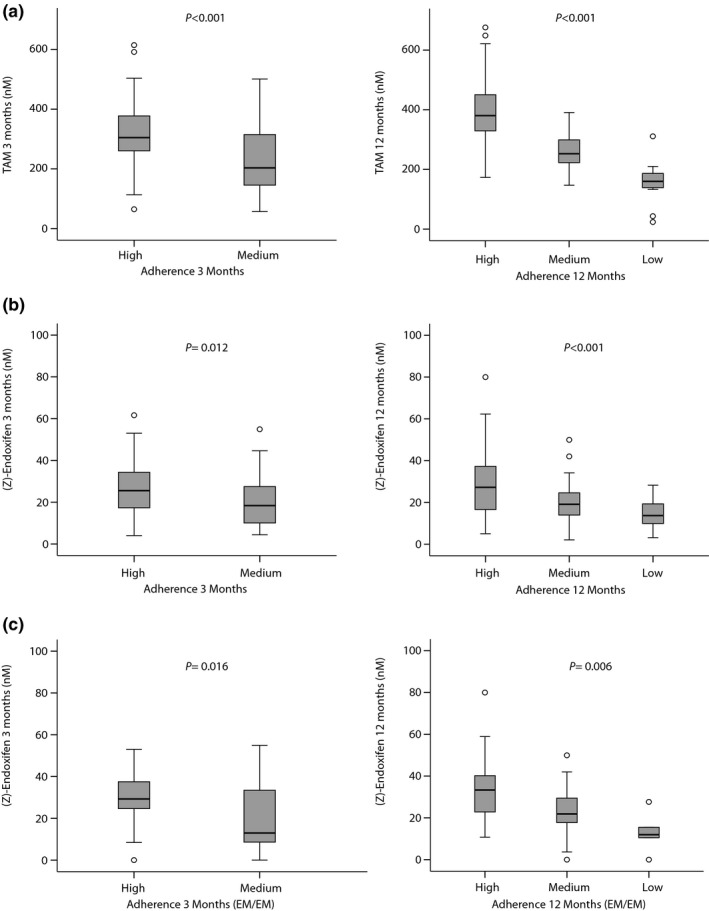
Influence of treatment adherence on tamoxifen and (Z)‐endoxifen plasma concentration at 3 months (n = 156 patients) and 12 months (n = 139 patients). Plasma concentrations are presented as boxplots with boxes representing medians, 25% and 75% percentiles, and whiskers defined by the 5th and 95th percentiles and extreme values outside the whiskers. (a) TAM: Tamoxifen (parent drug). (b) (Z)‐endoxifen. (c) (Z)‐endoxifen for the subgroup of cytochrome P450 2D6 (CYP2D6). Extensive metabolizer (EM/EM) patients at 3 months (n = 67) and 12 months (n = 55). P values refer to test for different plasma concentrations between adherence categories. Data at 6 months not shown.
Table 2.
Summary of multiple linear regression models for tamoxifen and selected metabolites
| Metabolite/time point | r2 | F | P valuea | Parameters in the model |
|---|---|---|---|---|
| Tam 3 months | 0.212 | 19.750 | < 0.001 | Medium adherence; age at diagnosis |
| Tam 6 months | 0.161 | 9.520 | < 0.001 | Low and medium adherence; age at diagnosis |
| Tam 12 months | 0.471 | 39.130 | < 0.001 | Low and medium adherence |
| DM‐Tam 3 months | 0.412 | 8.995 | < 0.001 | CYP2D6 PM/IM, IM/IM, EM/PM, EM/IM; medium adherence; age at diagnosis; Asian/Indian ethnicity |
| DM‐Tam 6 months | 0.217 | 4.384 | < 0.001 | CYP2D6 PM/PM, EM/PM; low and medium adherence; age at diagnosis |
| DM‐Tam 12 months | 0.427 | 10.451 | < 0.001 | CYP2D6 PM/IM, EM/PM, EM/IM; low and medium adherence; age at diagnosis |
| Z‐Endo 3 months | 0.345 | 7.370 | < 0.001 | CYP2D6 PM/PM, PM/IM, EM/PM; medium adherence; Asian/Indian ethnicity |
| Z‐Endo 6 months | 0.322 | 8.421 | < 0.001 | CYP2D6 PM/PM, PM/IM, EM/PM, IM/IM; medium adherence |
| Z‐Endo 12 months | 0.403 | 10.877 | < 0.001 | CYP2D6 PM/PM, PM/IM, EM/PM, IM/IM; low and medium adherence |
| Metabolic ratio | ||||
| DM‐Tam/Z‐Endo 3 months | 0.556 | 30.002 | < 0.001 | CYP2D6 PM/IM, IM/IM, EM/PM, EM/IM |
| DM‐Tam/Z‐Endo 6 months | 0.510 | 20.195 | < 0.001 | CYP2D6 PM/IM, IM/IM, EM/PM, EM/IM |
| DM‐Tam/Z‐Endo 12 months | 0.575 | 15.540 | < 0.001 | CYP2D6 PM/IM, EM/PM, EM/IM; black ethnicity |
Cytochrome P450 2D6 (CYP2D6) diplotypes PM/PM, two null alleles; DM‐Tam, N‐desmethyltamoxifen; EM/IM, one normal and one reduced activity allele; EM/PM, one normal and one null activity allele; EM/UM, patient with gene duplications of alleles with normal activity; F, F‐test (ANOVA) for the model; IM/IM, two reduced activity alleles; PM/IM, one null activity and one reduced activity allele; r 2, model's coefficient of determination; Tam, tamoxifen; Z‐Endo, (Z)‐endoxifen.
Significance value of F‐test (analysis of variance) for the proposed model.
Similar associations with adherence were obtained for tamoxifen metabolite (Z)‐endoxifen (Figure 2 b). In subgroup analysis of patients with functional CYP2D6 (EM/EM) treatment adherence was significantly correlated with tamoxifen metabolites at all three time points (Figure 2 c, data shown for months 3 and 12).
3.4. Association between genotypes of pharmacogenes and plasma concentrations of tamoxifen and its metabolites
Plasma concentrations of all tamoxifen metabolites were affected by CYP2D6 phenotype with a strongest effect for (Z)‐endoxifen (Table S3 ). CYP2D6 AS demonstrated gene‐dose effects on both (Z)‐endoxifen and MR of N‐desmethyltamoxifen (DM‐Tam)/(Z)‐endoxifen confirming the importance of CYP2D6 for the bioactivation of tamoxifen to (Z)‐endoxifen via DM‐Tam (Figure S2 ; P < 0.001). CYP2D6 deficient PM patients (PM/PM) had 4.5 to 5.5 times lower (Z)‐endoxifen concentrations than EM patients with functional CYP2D6 (EM/EM). The mean concentrations of PM compared with EM patients at 3, 6, and 12 months were 6.5 ± 2.7 nM vs. 29.6 ± 12.9 nM; 7.2 ± 3.3 nM vs. 30.3 ± 14.5 nM; and 5.4 ± 1.1 nM vs. 30.3 ± 14.5 nM. In multivariate analyses, CYP2D6 phenotype was a significant predictor of tamoxifen metabolites and MRs across all time points (Table 2).
Due to the local prescription practice there were few patients with concurrent comedication of strong CYP2D6 inhibitors. Although three of the four patients taking a strong CYP2D6 inhibitor (fluoxetine) showed low levels of (Z)‐endoxifen (6–15 nM), CYP2D6 inhibitor use was not significantly associated with tamoxifen metabolite concentrations. Among other covariates, age at diagnosis was positively associated with increased tamoxifen and DM‐TAM concentrations, whereas DMEs other than CYP2D6 had little influence (Table S3 ).
3.5. Combined analysis of factors influencing (Z)‐endoxifen concentrations
Adherence and CYP2D6 phenotype were jointly associated with (Z)‐endoxifen concentrations across all time points (Table 2). The additive effect of adherence and CYP2D6 phenotype was most evident at 12 months. Although CYP2D6 as a single factor explained 26.4% of (Z)‐endoxifen variability (P < 0.001), the explained variability increased to 40% when adherence was included in multivariate analysis (P < 0.001; Table 2). We next evaluated whether adherence or CYP2D6 polymorphisms had a stronger effect on (Z)‐endoxifen concentrations based on standardized beta coefficients (Table 3). Across all time points, severe CYP2D6 impairment (AS ≤ 0.5; PM/PM and PM/IM) was the strongest determinant of (Z)‐endoxifen variability. The second strongest effect resulted from low or medium treatment adherence at 6 and 12 months with an effect size equal or greater to that resulting from a CYP2D6 AS > 0.5 (IM and EM; Table 3). As a putative surrogate predictor of clinical response, we used plasma concentrations of (Z) endoxifen and evaluated the relevance of adherence vs. CYP2D6 phenotype based on the RR of not achieving the threshold plasma concentration of 5.9 ng/mL (Z)‐endoxifen (Figure 3). The risk to not achieve the threshold was highest in patients with CYP2D6 PM phenotype (RR 4.1; 95% CI 3.04–5.50), followed by patients with either CYP2D6 IM phenotype (RR 2.54; 95% CI 1.41–4.60) or low treatment adherence (RR 2.44; 95% CI 1.40–4.25). In subgroup analyses in patients with noncompromised CYP2D6 function (EM phenotype), low adherence showed to be a strong risk factor of not achieving clinical threshold concentrations (RR 3.65; 95% CI 1.48–8.99). This association was less pronounced in patients with reduced CYP2D6 activity (IM phenotype; RR 2.19; 95% CI 1.21–3.97).
Table 3.
Evaluation of contributing variables based on linear regression coefficients for the prediction of (Z)‐endoxifen plasma variability
| 3 Months | 6 Months | 12 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | St beta coef.a | P valueb | Variable | St beta coef.a | P valueb | Variable | St beta coef.a | P valueb |
| CYP2D6 PM/IM | −0.353 | < 0.001 | CYP2D6 PM/IM | −0.378 | < 0.001 | CYP2D6 PM/PM | −0.374 | < 0.001 |
| CYP2D6 PM/PM | −0.312 | < 0.001 | CYP2D6 PM/PM | −0.307 | < 0.001 | CYP2D6 PM/IM | −0.342 | < 0.001 |
| CYP2D6 EM/PM | −0.249 | 0.001 | Adherence‐medium | −0.266 | < 0.001 | Adherence‐low | −0.337 | < 0.001 |
| Adherence‐medium | −0.189 | 0.008 | CYP2D6 EM/PM | −0.203 | 0.010 | CYP2D6 EM/PM | −0.208 | 0.007 |
| CYP2D6 IM/IM | −0.119 | 0.103 | CYP2D6 IM/IM | −0.173 | 0.016 | Adherence‐medium | −0.206 | 0.004 |
| CYP2D6 EM/IM | −0.146 | 0.055 | CYP2D6 EM/IM | −0.134 | 0.083 | CYP2D6 IM/IM | −0.136 | 0.050 |
| Black | −0.092 | 0.206 | Adherence‐low | −0.110 | 0.114 | CYP2D6 EM/IM | −0.137 | 0.069 |
| Brown/mixed | −0.052 | 0.456 | CYP2D6 EM/UM | −0.010 | 0.887 | CYP2D6 EM/UM | 0.140 | 0.051 |
| Asian/Indian | 0.144 | 0.042 | ||||||
| CYP2D6 EM/UM | 0.102 | 0.156 | ||||||
Black, Brown/Mixed, Asian/Indian refers to the non‐European ethnicities included in the study cohort; cytochrome P450 2D6 (CYP2D6) diplotypes EM/IM, one normal and one reduced activity allele; EM/PM, one normal and one null activity allele; EM/UM, patient with gene duplications of alleles with normal activity; IM/IM, two reduced activity alleles; PM/IM, one null activity and one reduced activity allele; PM/PM, two null alleles; St, standard.
Variables are listed according to their relevance in the model according to standardized beta coefficients.
Only variables with P < 0.05 (t‐test) are relevant in the model.
Figure 3.
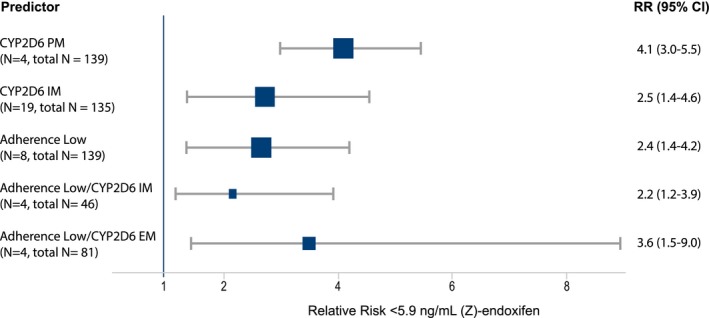
Forest plot for the relative risk of not achieving the clinical threshold of a (Z)‐endoxifen concentration ≥ 5.9 ng/mL (15.8 nM) at 12 months. RR estimates are given as squared symbols with size weighted by the number of included patients. 95% CI, 95% confidence interval; EM, extensive metabolizer; IM, intermediate metabolizer; N, number of patients positive for predictor variable and in total analyses; PM, poor metabolizer; RR, relative risk.
4. Discussion
We provide first evidence that the ability to achieve clinically relevant (Z)‐endoxifen plasma concentrations during breast cancer tamoxifen treatment is cooperatively influenced by treatment adherence behavior, CYP2D6 genomic background, and pharmacokinetic capacity. Conjointly, this could improve the prediction of active tamoxifen metabolite levels (Z‐endoxifen) and possibly clinical efficacy,17, 20, 38 a high priority goal in personalized endocrine treatment. Previous attempts to predict the variability of (Z)‐endoxifen levels relied on pharmacokinetic and pharmacogenomic knowledge,17, 38, 39 which has been implemented into therapeutic recommendations based on CYP2D6 genotyping.14 Although a recent drug monitoring study showed that tamoxifen metabolites may be predictive of side effects, such as nausea and vaginal dryness,40 clinical tamoxifen outcome studies as of yet provided controversial results possibly due to the influence of unidentified confounders.22, 23, 24, 26, 27, 28 Because it is well known that women stop taking their medication before completing the standard 5‐year regimen, we investigated patients' adherence behavior during the first year of tamoxifen treatment in relation to their metabolic capacity for achieving relevant (Z)‐endoxifen concentrations. Our observed 12‐month adherence rate is considerably lower than the 1‐year tamoxifen adherence reported by Dezentje et al.41 (93%) based on prescription refill data. The latter assumed that prescription‐refilling patterns correspond to patient medication‐taking behavior and that the medication is taken exactly as prescribed independent of patients' beliefs or concerns about treatment. A 90% 1‐year adherence rate was also reported by Makubate et al.42 in a retrospective cohort of endocrine‐treated breast cancers that declined to 51% in the fifth year, and that demonstrated an association of low adherence (< 80%) with poor survival. Our prospective study used the Morisky, Green, and Levine Medication Adherence Scale questionnaire in a pharmacist‐guided interview to assess adherence rates, a tool previously used in an oncology setting in Brazil35, 43 and which is considered reliable based on the validation of self‐report questionnaires in relation to medication‐monitoring devices.44 Notably, Morisky's selected adherence scale is an easy to perform and fast questionnaire suitable for our prospective setting with 35% of patients with low‐literacy.13, 45 The questionnaire provided us with the benefit to retrieve valuable tamoxifen adherence behavior and pharmacokinetic information from a health disparity cancer population that otherwise would not be accessible. The high observed tamoxifen nonadherence rate within the first year may reflect the socioeconomic condition of this population and the high percentage of young women (two‐thirds) known to be at increased risk to stop medication.10
We confirmed that CYP2D6 phenotype is a strong determinant of plasma (Z)‐endoxifen levels in that increasing CYP2D6 allele activity correlated with increasing plasma metabolite concentrations. Our data are sound as the prevalence of reduced‐function and null‐function alleles compares with those reported from Brazil33 and are in line with population admixture of Europeans, Native Americans, Africans, and Asians, with our institution serving as a national reference center for oncology treatment. (Z)‐endoxifen plasma levels were in the range of those reported by others,15, 17 and, as expected, CYP2D6 polymorphism only partially predicted its variability.19 Potential confounders, such as DME polymorphisms or strong CYP2D6 inhibitors, do not play a significant role as the former showed no effect and the latter were infrequently used by our patients given the low prescription rates at our hospital.
Of note, the observed effect of adherence behavior on active metabolite levels depended on CYP2D6 functionality. Although PM status was still the most important predictor of reduced (Z)‐endoxifen concentrations, and the risk to not achieve sufficient concentration due to low‐adherence was moderate across all patients (RR 2.44; 95% CI 1.40–4.25), low‐adherence was a strong predictor in EM patients (RR 3.65; 95% CI 1.48–8.99). The latter clearly indicates an independent influence of low‐adherence resulting in suboptimal concentrations of active tamoxifen metabolites. An immediate consequence from this finding is that EM and IM patients who are now in their ensuing year(s) of the 5‐year treatment must be encouraged to adhere to tamoxifen, as the expected benefit will depend on their own authority. Known barriers to prevent tamoxifen adherence are manifold and include low recurrence risk perception, side effects, age extremes, medication cost, ethnicity, educational level, lack of social support, and suboptimal patient physician communication.3, 4, 13
Importantly, we confirmed the relevance of age on tamoxifen adherence that patients at 65 years of age or older showed better adherence to tamoxifen than younger patients. Moreover, multivariate modeling identified age as an independent predictor of parent drug levels (i.e., tamoxifen). Previous findings by others showed that plasma concentrations of tamoxifen and its metabolites increase with age,46 which, according to our findings, may be explained at least in part by patient adherence behavior. Given the strong predictive value of adherence for the variability of tamoxifen plasma concentrations we propose that drug monitoring is a powerful surrogate to assess tamoxifen adherence for which we identified a threshold concentration of 157 nM (± 67 nM) to stratify patients into low vs. medium/high tamoxifen adherent. This is particularly relevant to young patients, as their risk to discontinue tamoxifen is among the highest4, 10 with tamoxifen currently being the sole standard‐of‐care treatment in this patient group.
Our study provides a first link between poor patient adherence to tamoxifen and the risk to not achieve relevant (Z)‐endoxifen plasma concentrations. However, the study is not without limitations. We are aware that the Morisky, Green, and Levine Medication Adherence Scale scoring system does not quantitatively capture adherence, yet we consider this approach appropriate as it allowed us to assess tamoxifen adherence in this patient group with a high proportion of illiteracy. Moreover, the putative threshold of 5.9 ng/mL (Z)‐endoxifen20 has not been prospectively validated and controversies on the CYP2D6—endoxifen relationship for the prediction of tamoxifen efficacy have not been finally resolved, which currently limits the clinical utility of our findings.29, 47, 48, 49, 50, 51, 52
In conclusion, our proof‐of‐concept study suggests that the dual monitoring of tamoxifen plasma levels as surrogate marker for adherence and (Z)‐endoxifen formation as surrogate marker for clinical response could be a strategy to improve patients' chances to avoid recurrence and premature death in the future.
5. Funding
This work was conducted during a scholarship supported by the International Cooperation Program CAPES/STICAMSUD at the Pontifical Catholic University of Parana, Curitiba, Brazil and financed by CAPES – Brazilian Federal Agency for Support and Evaluation of Graduate Education within the Ministry of Education of Brazil. The work was also funded in part by the Robert Bosch Foundation, Stuttgart, the HORIZON 2020‐PHC‐2015 grant U‐Px 668353, Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy ‐ EXC 2180 – 390900677, and DFG, SCHR 1323/2‐1 and MU 1727/2‐1), Interfaculty Center for Pharmacogenomics and Drug Research (ICEPHA) University of Tübingen, and The German Cancer Consortium (DKTK), partner site Tuebingen), Germany.
Conflict of Interest
The authors declared no competing interests for this work.
Authors Contributions
J.M.N., J.C.C.‐d.‐R., T.A.A., H.B., W.S., T.M., R.H., M.S., and R.F.P.‐F. wrote the manuscript. J.C.C.‐d.‐R., J.M.N., T.A.A., and H.B. designed the research. J.M.N., T.A.A., E.C.L.V., J.P.K., S.P., D.M., S.D.R.d.M., R.F.P.‐F., T.M., and W.S. performed the research. J.M.N., J.C.C.‐d.‐R., T.A.A., W.S., T.M., R.H., M.S., and H.B. analyzed the data.
Supporting information
Figure S1.
Figure S2.
Table S1.
Table S2.
Table S3.
Supplementary Material.
References
- 1. Early Breast Cancer Trialists' Collaborative Group . Aromatase inhibitors versus tamoxifen in early breast cancer: patient‐level meta‐analysis of the randomised trials. Lancet 386, 1341–1352 (2015). [DOI] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists' Collaborative Group . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient‐level meta‐analysis of randomised trials. Lancet 378, 771–784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Adherence to Long‐Term Therapies: Evidence for Action (World Health Organization, Geneva, Switzerland, 2003). [Google Scholar]
- 4. Chlebowski, R.T. , Kim, J. & Haque, R. Adherence to endocrine therapy in breast cancer adjuvant and prevention settings. Cancer Prev. Res. (Phila.) 7, 378–387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimmick, G. , Anderson, R. , Camacho, F. , Bhosle, M. , Hwang, W. & Balkrishnan, R. Adjuvant hormonal therapy use among insured, low‐income women with breast cancer. J. Clin. Oncol. 27, 3445–3451 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCowan, C. , Wang, S. , Thompson, A.M. , Makubate, B. & Petrie, D.J. The value of high adherence to tamoxifen in women with breast cancer: a community‐based cohort study. Br. J. Cancer 109, 1172–1180 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owusu, C. et al Predictors of tamoxifen discontinuation among older women with estrogen receptor‐positive breast cancer. J. Clin. Oncol. 26, 549–555 (2008). [DOI] [PubMed] [Google Scholar]
- 8. Partridge, A.H. , Wang, P.S. , Winer, E.P. & Avorn, J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J. Clin. Oncol. 21, 602–606 (2003). [DOI] [PubMed] [Google Scholar]
- 9. Silliman, R.A. et al Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J. Clin. Oncol. 20, 2680–2688 (2002). [DOI] [PubMed] [Google Scholar]
- 10. Hershman, D.L. et al Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early‐stage breast cancer patients. J. Clin. Oncol. 28, 4120–4128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hershman, D.L. et al Early discontinuation and non‐adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 126, 529–537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chirgwin, J.H. et al Treatment adherence and its impact on disease‐free survival in the Breast International Group 1–98 Trial of tamoxifen and letrozole, alone and in sequence. J. Clin. Oncol. 34, 2452–2459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lam, W.Y. & Fresco, P. Medication adherence measures: an overview. Biomed. Res. Int. 2015, 217047 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goetz, M.P. et al Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and tamoxifen therapy. Clin. Pharmacol. Ther. 103, 770–777 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antunes, M.V. et al Endoxifen levels and its association with CYP2D6 genotype and phenotype: evaluation of a southern Brazilian population under tamoxifen pharmacotherapy. Ther. Drug Monit. 34, 422–431 (2012). [DOI] [PubMed] [Google Scholar]
- 16. Friedrich, D.C. et al Distribution of CYP2D6 alleles and phenotypes in the Brazilian population. PLoS One 9, e110691 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murdter, T.E. et al Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin. Pharmacol. Ther. 89, 708–717 (2011). [DOI] [PubMed] [Google Scholar]
- 18. Stearns, V. et al Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 95, 1758–1764 (2003). [DOI] [PubMed] [Google Scholar]
- 19. Schroth, W. et al Improved prediction of endoxifen metabolism by CYP2D6 genotype in breast cancer patients treated with tamoxifen. Front. Pharmacol. 8, 582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madlensky, L. et al Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin. Pharmacol. Ther. 89, 718–725 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maximov, P.Y. et al Simulation with cells in vitro of tamoxifen treatment in premenopausal breast cancer patients with different CYP2D6 genotypes. Br. J. Pharmacol. 171, 5624–5635 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goetz, M.P. et al CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin. Cancer Res. 19, 500–507 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Province, M.A. et al CYP2D6 genotype and adjuvant tamoxifen: meta‐analysis of heterogeneous study populations. Clin. Pharmacol. Ther. 95, 216–227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schroth, W. et al Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302, 1429–1436 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karle, J. et al Influence of CYP2D6‐genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res. Treat. 139, 553–560 (2013). [DOI] [PubMed] [Google Scholar]
- 26. Rae, J.M. et al CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen‐treated breast cancer patients. J. Natl. Cancer Inst. 104, 452–460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Regan, M.M. et al CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine‐responsive breast cancer: the Breast International Group 1‐98 Trial. J. Natl. Cancer Inst. 104, 441–451 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wegman, P. , Elingarami, S. , Carstensen, J. , Stal, O. , Nordenskjold, B. & Wingren, S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 9, R7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez‐Spitman, A. et al Tamoxifen pharmacogenetics and metabolism: results from the prospective CYPTAM study. J. Clin. Oncol. 37, 636–646 (2019). [DOI] [PubMed] [Google Scholar]
- 30. Harris, L.N. et al Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 34, 1134–1150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson, A.M. et al Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res. Treat. 125, 279–287 (2011). [DOI] [PubMed] [Google Scholar]
- 32. McShane, L.M. et al Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 97, 1180–1184 (2005). [DOI] [PubMed] [Google Scholar]
- 33. Prado, C.M. Desenvolvimento de metodologia para determinação dos genótipos principais dos genes CYP2D6, CYP2C19 e CYP2C9: aplicação na Farmacogenética. Tese de Doutorado, Universidade de São Paulo; (2009). [Google Scholar]
- 34. Morisky, D.E. , Green, L.W. & Levine, D.M. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med. Care 24, 67–74 (1986). [DOI] [PubMed] [Google Scholar]
- 35. Oliveira, A. , Munhoz, E.C. , Nardin, J.M. & Carneiro, M.B. Assessment of imatinib mesylate adherence of patients with chronic myeloid leukemia. Rev. Bras. Farm. Hosp. Serv. Saúde 4, 6–12 (2013). [Google Scholar]
- 36. Zineh, I. et al Pharmacokinetics and CYP2D6 genotypes do not predict metoprolol adverse events or efficacy in hypertension. Clin. Pharmacol. Ther. 76, 536–544 (2004). [DOI] [PubMed] [Google Scholar]
- 37. Gaedigk, A. , Simon, S.D. , Pearce, R.E. , Bradford, L.D. , Kennedy, M.J. & Leeder, J.S. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 83, 234–242 (2008). [DOI] [PubMed] [Google Scholar]
- 38. Saladores, P. et al Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 15, 84–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Desta, Z. , Ward, B.A. , Soukhova, N.V. & Flockhart, D.A. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 310, 1062–1075 (2004). [DOI] [PubMed] [Google Scholar]
- 40. Helland, T. et al Drug monitoring of tamoxifen metabolites predicts vaginal dryness and verifies a low discontinuation rate from the Norwegian Prescription Database. Breast Cancer Res. Treat. 177, 185–195 (2019). [DOI] [PubMed] [Google Scholar]
- 41. Dezentje, V.O. et al Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early‐stage breast cancer. J. Clin. Oncol. 28, 2423–2429 (2010). [DOI] [PubMed] [Google Scholar]
- 42. Makubate, B. , Donnan, P.T. , Dewar, J.A. , Thompson, A.M. & McCowan, C. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br. J. Cancer 108, 1515–1524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliveira, A.T. & Queiroz, A.P.A. Profile use of oral antineoplastic therapy: the importance of pharmaceutical guidance. Rev. Bras. Farm. Hosp. Serv. Saúde 3, 24–29 (2012). [Google Scholar]
- 44. Shi, L. , Liu, J. , Koleva, Y. , Fonseca, V. , Kalsekar, A. & Pawaskar, M. Concordance of adherence measurement using self‐reported adherence questionnaires and medication monitoring devices. Pharmacoeconomics 28, 1097–1107 (2010). [DOI] [PubMed] [Google Scholar]
- 45. Culig, J. & Leppee, M. From Morisky to Hill‐bone; self‐reports scales for measuring adherence to medication. Coll. Antropol. 38, 55–62 (2014). [PubMed] [Google Scholar]
- 46. Lien, E.A. et al Serum concentrations of tamoxifen and its metabolites increase with age during steady‐state treatment. Breast Cancer Res. Treat. 141, 243–248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brauch, H. & Schwab, M. Prediction of tamoxifen outcome by genetic variation of CYP2D6 in postmenopausal women with early breast cancer. Br. J. Clin. Pharmacol. 77, 695–703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goetz, M.P. & Ingle, J.N. CYP2D6 genotype and tamoxifen: considerations for proper nonprospective studies. Clin. Pharmacol. Ther. 96, 141–144 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rae, J.M. CYP2D6 genotype should not be used to determine endocrine therapy in postmenopausal breast cancer patients. Clin. Pharmacol. Ther. 94, 183–185 (2013). [DOI] [PubMed] [Google Scholar]
- 50. Braal, C.L. et al Relevance of endoxifen concentrations: absence of evidence is not evidence of absence. J. Clin. Oncol. 37, 1980–1981 (2019). [DOI] [PubMed] [Google Scholar]
- 51. Brauch, H. , Schroth, W. , Murdter, T. & Schwab, M. Tamoxifen pharmacogenetics and metabolism: the same is not the same. J. Clin. Oncol. 37, 1981–1982 (2019). [DOI] [PubMed] [Google Scholar]
- 52. Goetz, M.P. , Suman, V.J. , Nakamura, Y. , Kiyotani, K. , Jordan, V.C. & Ingle, J.N. Tamoxifen metabolism and breast cancer recurrence: a question unanswered by CYPTAM. J. Clin. Oncol. 37, 1982–1983 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Table S1.
Table S2.
Table S3.
Supplementary Material.


