Abstract
We genetically engineered expression of an activated form of P110 alpha, the catalytic subunit of PI3K in mouse prostate epithelium, to create a mouse model of direct PI3K activation (Pbsn-cre4Prb;PI3KGOF/+). We hypothesized that direct activation would cause rapid neoplasia and cancer progression. Pbsn-cre4Prb;PI3KGOF/+ mice develop widespread prostate intraepithelial hyperplasia, but stromal invasion is limited and overall progression is slower than anticipated. However, the model produced profound and progressive stromal remodeling prior to explicit epithelial neoplasia. Increased stromal cellularity and inflammatory infiltrate are evident as early as 4 months of age and progressively increase through 12 months of age, the terminal endpoint of this study. Prostatic collagen density and phosphorylated SMAD2-positive prostatic stromal cells are expansive and accumulate with age, consistent with pro-fibrotic TGFβ pathway activation. Few reported mouse models accumulate prostate-specific collagen to the degree observed in Pbsn-cre4Prb;PI3KGOF/+. Our results indicate a signaling process beginning with prostatic epithelial PI3K and TGFβ signaling driving prostatic stromal hypertrophy and collagen accumulation. These mice afford a unique opportunity to explore molecular mechanisms of prostatic collagen accumulation which is relevant to cancer progression, metastasis, inflammation, and urinary dysfunction.
Keywords: prostate, collagen, fibrosis, PI3KCA, cancer, stroma, mouse model
INTRODUCTION
The phosphoinositide 3-kinase (PI3K) pathway is activated in 40% of early-stage human tumors[1,2] and pathway activation is sufficient for tumorigenesis[3–6]. Consequentially, mouse models of human cancers often involve mutations in PI3K pathway constituents[7]. One of the most widely used prostate cancer models harbors an inactivating mutation in Phosphatase and tensin homolog (Pten), a negative regulator of the PI3K pathway[8]. Prostate epithelial specific deletion of Pten is a commonly used mouse model for prostate cancer although the degree of cancer progression and timing of progression has varied significantly in different reports, possibly due to differences in strain background[8,9]. However, a recent report of a mouse model of colon cancer found that expression of a mutated/activated form of the PI3K catalytic subunit alpha (also known as P110 and PIK3CA) in colonic epithelium activated PI3K pathway targets and resulted in aggressive and invasive adenocarcinoma[10]. Therefore, we hypothesized that constitutive expression of the mutated form of PI3KCA in mouse prostatic epithelium (Pbsn-cre4Prb/+;PI3KGOF/+) would accelerate prostatic tumor formation and aggression relative to Pten inactivating mutations.
We previously characterized the prostatic cell composition of 4-month-old Pbsn-cre4Prb/+;PI3KGOF/+ mice[11]. In our initial characterization, we observed several reactive stroma characteristics including hematolymphoid-derived inflammatory infiltrate, expanded populations of putative fibroblasts, and loss of smooth muscle. Similar stromal phenotypes often accompany solid tumors and are correlated with poor disease outcomes[12–16]. It is known that the extracellular matrix (ECM) of tumor-adjacent stroma is often more dense compared to areas without associated tumors[17–21]. Of all known ECM components, collagen is the most prevalent in both benign and malignant tissue. In organs with highly collagenous reactive stroma, the structural properties of collagen (density, alignment, orientation, etc.) contribute to tumor invasion and metastasis[19,21–25]. Collagen has also been implicated in pro-tumorigenic signaling, serving as the ligand for tumor cell integrins[16–19] and can compromise drug delivery to tumors[20]. In urologic research, there is increasing evidence that collagen, especially in the prostate gland, may cause benign urinary dysfunction in both humans and rodents[26–29]. In fact, pathological roles for collagen are so prevalent that benign and malignant prostatic disease research have recently converged on the goal of identifying mechanisms responsible for collagen accumulation.
Pbsn-cre4Prb/+;PI3KGOF/+ mice provide a unique opportunity to understand the process of prostatic collagen deposition. Here, we report the histological and molecular changes occurring in Pbsn-cre4Prb/+;PI3KGOF/+ mouse prostates at 4, 7, and 12 months of age. We confirmed PI3K pathway activation via immunohistochemical identification of a known target downstream of PI3K: phosphorylated ribosomal protein S6 kinase beta-1 (p-p70S6K). Epithelial neoplasia progresses slowly and fails to develop aggressive stromal invasion by 12 months, but the prostatic stroma is significantly transformed. The most notable stromal transformation is pervasive prostatic collagen accumulation. We explored potential mechanisms for collagen accumulation in Pbsn-cre4Prb/+;PI3KGOF/+ mice and identified transforming growth factor β (TGFβ) pathway activation. Overall, our data indicate that prostate epithelial-specific activating mutations in the Pik3ca gene result in a robust stromal response featuring extensive collagen deposition. Further resolving the mechanisms behind this fibrotic phenotype will be valuable for future cancer research as well as benign disease research.
MATERIALS AND METHODS
Husbandry
Mice were housed in polysulfone cages containing corn cob bedding and maintained on a 12 hr light and dark cycle at 20.5 ± 5°C and 30–70% relative humidity. Mice were fed a 5015 Diet (PMI Nutrition International, Brentwood MO) from conception through weaning (PND 21) and an 8604 Teklad Rodent Diet thereafter (Harlan Laboratories, Madison WI). Feed and water were available ad libitum. All procedures were approved by the University of Wisconsin Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Mouse Genetics
Mice were purchased from Jackson Laboratory (Bar Harbor, ME) and used to establish a breeding colony in our laboratory. Mice were maintained on a mixed background consisting of Tg(Pbsn-cre)4Prb/J (PB-Cre4, stock number 026662), 129S1/Svlmj (stock number 002448), and C57BL/6-Gt(ROSA)26Sortm7(Pik3ca*,EGFP)Rsky/J (R26StopFLP110*, stock number 012343) [30,31]. Mutant mice harbor a single copy of cre and a single copy of the PI3K GOF allele (Pbsn-cre4Prb/+;PI3KGOF/+). Control mice have no copies of cre (Pbsn-cre4Prb+/+;PI3KGOF/+) and a single copy of the PI3K GOF allele.
Immunohistochemistry (IHC)
Tissues were fixed in 4% paraformaldehyde, dehydrated in alcohol, cleared in xylene, and infiltrated with paraffin as described previously[32]. 5 μm sections were generated, mounted on Superfrost™ Plus Gold Slides (Thermo Fisher Scientific; Waltham, MA) and immunolabeled using antibodies against phosphorylated ribosomal protein S6 kinase beta-1 (p-p70S6K) (1:50, Cell Signaling Technology; Danvers, MA, Catalog #9206L, Lot 22; RRID:AB_2285392), smooth muscle actin alpha (SMA) (1:250, Leica; Wetzlar, Germany, Catalog #NCL-L-SMA; RRID:AB_442134, [33]), elastin (1:50, Abcam, Cambridge, United Kingdom, Catalog #ab21610, RRID:AB_446423), collagen IV (1:250, Abcam, Cambridge, United Kingdom, Catalog #ab6586, RRID:AB_305584), and phosphorylated Mothers Against Decapentaplegic Homolog 2 (p-SMAD 2) (1:500, Thermo Fisher; Waltham, MA, Catalog #44–244G; RRID:AB_1502057). For colorimetric IHC, endogenous peroxidase was quenched via incubation in 3% H2O2 for 10 minutes. Non-specific binding was blocked using a Mouse on Mouse Kit (BMK-2202; Vector Laboratories, Burlingame, CA). After primary antibody incubation, the slides were treated with anti-mouse IgG secondary antibody for one hour then Vectastain ABC Kit (PK-6100; Vector Laboratories, Burlingame, CA) after several rinses in PBS. The slides were then incubated with DAB Peroxidase Substrate Kit (SK-4100; Vector Laboratories, Burlingame, CA) for 5–10 minutes. The DAB oxidation was halted by submerging slides in water. Slides were counterstained in hematoxylin, dehydrated in graded ethanol and CitriSolv, (1601, Deacon Labs, King of Prussia, PA) then mounted using Permount mounting medium (SP15–100; Fisher Scientific, Hampton, NH). For fluorescent IHC, non-specific binding sites were blocked for 1 hr in TBSTw containing 1% Blocking Reagent (11096176001, Roche Diagnostics, Indianapolis, IN), 5% normal goat sera, and 1% bovine serum albumin fraction 5 (RGBTw). Tissues were incubated overnight at 4°C with primary antibodies. After several washes with TBSTw, tissues were incubated for 1 hour at room temperature with RGBTw containing 1:250 diluted fluorescent secondary antibodies. 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI) (1:1000) was used to visualize nuclei and slides were mounted in anti-fade media (phosphate-buffered saline containing 80% glycerol and 0.2% n-propyl gallate). Antibodies were selected for either colorimetric or fluorescent IHC based on whether co-stains were required and to facilitate best visualization of the protein of interest. Staining was imaged at 40X brightfield using a Leica DM LB Microscope (Leica, Wetzlar, Germany) and QImaging Micropublisher 5.0 RTV camera and software (01-MP5.0-RTV-R-CLR-10; QImaging, Surrey, BC, Canada) or at 20x (PlanFluor, NA 0.45) using a BZ-X710 digital microscope (Keyence, Itasca, IL). Tiled images were captured using Keyence image acquisition software (Keyence, Itasca, IL) and stitched to generate an image across the entire prostate section.
Cell Counting
For p-p70S6K staining, three 20x randomly selected fields per mouse containing at least 50 hematoxylin-stained cells were chosen for cell counting. Data is presented as the percentage of positively DAB-stained epithelial or stromal cells to total number of epithelial or stromal cells.
For p-SMAD2 staining, whole prostate lobe scans were taken. Each individual 20x field was then used as a region of interest (ROI) for counting. ≥ 10% of the total ROIs were used for counting with a minimum of 5 ROIs counted for each group. ROI’s were counted if the image was ≥ 50% tissue. Stromal cells were designated as either p-SMAD2+ or p-SMAD2- with 720–6,628 total cells counted per animal.
Hematoxylin and Eosin (H&E)
Tissues were fixed in 4% paraformaldehyde, dehydrated in ethanol, cleared in xylene, infiltrated with paraffin, and 5 μm sections were stained with hematoxylin and eosin. Brightfield imaging was performed using a BZ-X710 digital microscope (Keyence, Itasca, IL) using a 10x dry objective (PlanFluor, NA 0.45). Tiled images were captured using Keyence image acquisition software (Keyence, Itasca, IL) and stitched to generate an image of the complete prostate section as represented on the microscope slide. All lesion scoring and tumor staging was performed by two American College of Veterinary Pathologists certified pathologists.
Picrosirius Red (PSR)
Tissues were fixed in 4% paraformaldehyde, dehydrated in alcohol, cleared in xylene, infiltrated with paraffin, and 5 μm sections were stained with PSR as described previously [34]. Fluorescent imaging was performed using a BZ-X710 digital microscope (Keyence, Itasca, IL) using a 20x dry objective (PlanFluor, NA 0.45). Tiled images were captured using Keyence image acquisition software (Keyence, Itasca, IL) and stitched to generate an image across the entire prostate section. Specific collagen staining was isolated by subtracting the tissue autofluorescence from the acquired images. The region of interest was then isolated further by manually removing non-prostatic tissue (primarily epithelial secretions). Total tissue area was measured by creating a freehand selection boundary around the perimeter of the prostate lobe and measuring the pixel area of the selected area. Stromal area was measured by drawing selection boundaries around the perimeter of each prostate duct and then subtracting this area from the total tissue area. Fiber counts were obtained via CT-FIRE fiber analysis software (LOCI, Madison, WI). Collagen density was measured by quantifying the area of PSR staining to total tissue area. Stromal collagen fiber density was measured by normalizing CT-FIRE fiber counts to the calculated stromal area of the tissue.
Smooth Muscle Quantification
Fluorescent IHC was performed for SMA as described above. On resulting images, periductal smooth muscle thickness was quantified by measuring the distance between the basal epithelial surface and outer boundary of the periductal smooth muscle layer. At least three ducts were quantified per 20x field, with at least three fields quantified per animal. All quantification was performed using the measure tool of FIJI[35].
RNAscope (in situ hybridization)
Tissues were fixed in 4% paraformaldehyde, dehydrated in alcohol, cleared in xylene, and infiltrated with paraffin as described previously[32]. 5 μm sections were generated, mounted on Superfrost™ Plus Gold Slides (Thermo Fisher Scientific; Waltham, MA). A probe (Catalog# 407751) synthesized to recognize TGFβ1 (accession no. NM_011577.1) was acquired from Advanced Cell Diagnostics, Inc., [(ACD), Hayward, CA)] for use in the RNAscope assay. Sections were deparaffinized in xylene, rehydrated in 100% ethanol, and air-dried for 5 minutes at room temperature. Sections were then treated serially with: 1) endogenous hydrogen peroxidase block solution for 10 minutes at room temperature, 2) 25 minutes of immersion in pretreatment 2 solution at 100–104°C, and 3) digested with protease solution at 40°C for 28 minutes. Slides were rinsed with distilled water twice after each pretreatment step. Sections were then hybridized in TGFβ1 probe at 40°C for 2 hours in a humidified chamber. After wash buffer steps, signal amplification from the hybridized probes was performed by the serial application of amplification solutions per the RNAscope instructions. Horseradish peroxidase (HRP) activity was then observed by the application of 3,3′-diaminobenzidine (DAB) for 10 minutes at ambient temperature. Sections were then counterstained with Harris hematoxylin for 30 seconds, dehydrated through graded ethanol and xylene and then mounted in Richard-Allan mounting medium (Thermo Fisher Scientific, Waltham MA).
Statistics
Experimental groups consisted of 2–3 animals for IHC analysis and 2–4 animals for PSR analysis; figure images are representative of each treatment group. For all Pbsn-cre4Prb/+;PI3KGOF/+ time course experiments, a Shapiro-Wilk test was used to test for normality and a two-way ANOVA was conducted to identify differences among means. A Sidaks test for multiple comparisons was used as a post-hoc test. Results are reported as mean ± standard error of the mean (SE). A difference of p < 0.05 was considered significant. Statistical power was determined using calculations for normal distributions [36]. A power level of 0.80 was considered appropriate for histological quantifications (Fig 1. = 0.889, Fig 3. = 0.997, Fig 4. ≥ 0.999, Fig 5. = 0.999). All statistics were performed using GraphPad statistical software (GraphPad Software, San Diego, CA).
Figure 1. The PI3K pathway is activated in Pbsn-cre4Prb/+;PI3KGOF/+ prostates.
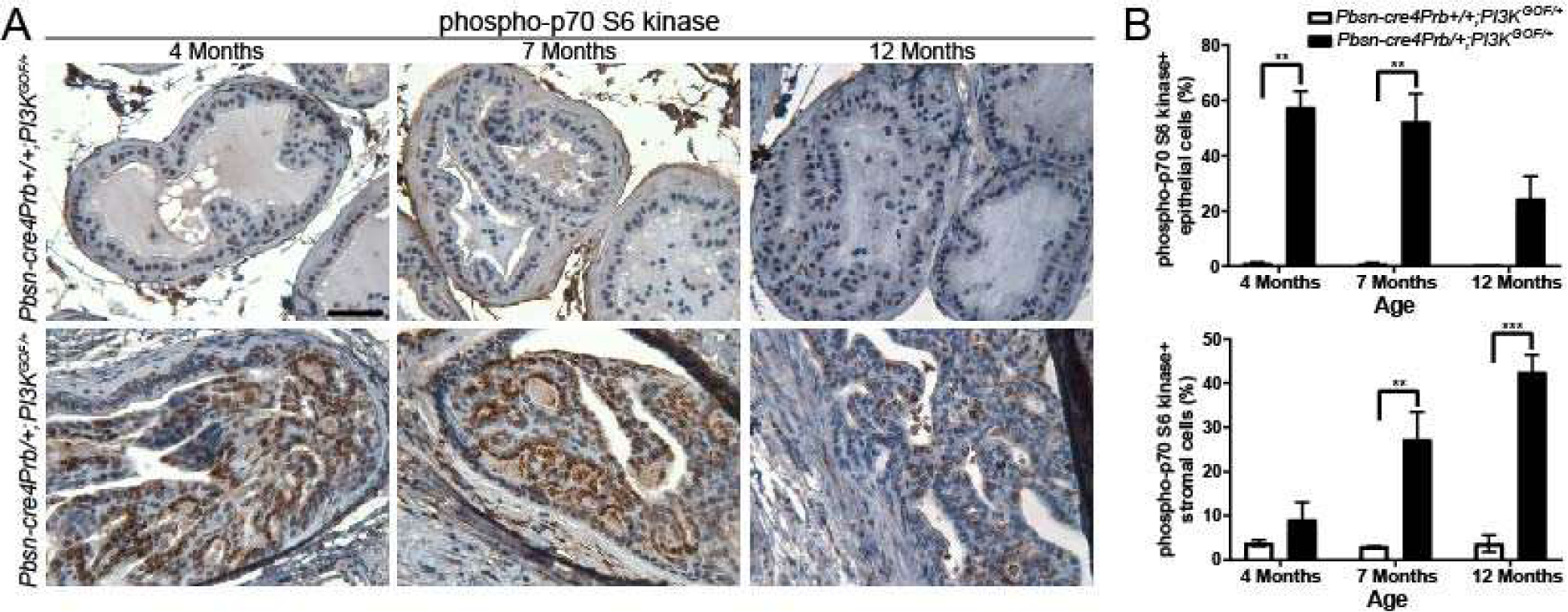
(A) Dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained with an antibody recognizing the phosphorylated form of p70 S6 kinase. (B) There is a greater frequency of phospho-p70 S6 kinase immunopositive epithelial cells in Pbsn-cre4Prb/+;PI3KGOF/+ mice than in controls at 4, 7, and 12 months of age, and a greater frequency of phospho-p70 S6 kinase immunopositive stromal cells in Pbsn-cre4Prb/+;PI3KGOF/+ mice than in controls at 7 and 12 months of age Results are mean ± SE of 2–4 mice. Significant differences among groups: * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001. Scale bar = 50 μm.
Figure 3. Characterization of Pbsn-cre4Prb/+;PI3KGOF/+ prostate epithelial and stromal changes.
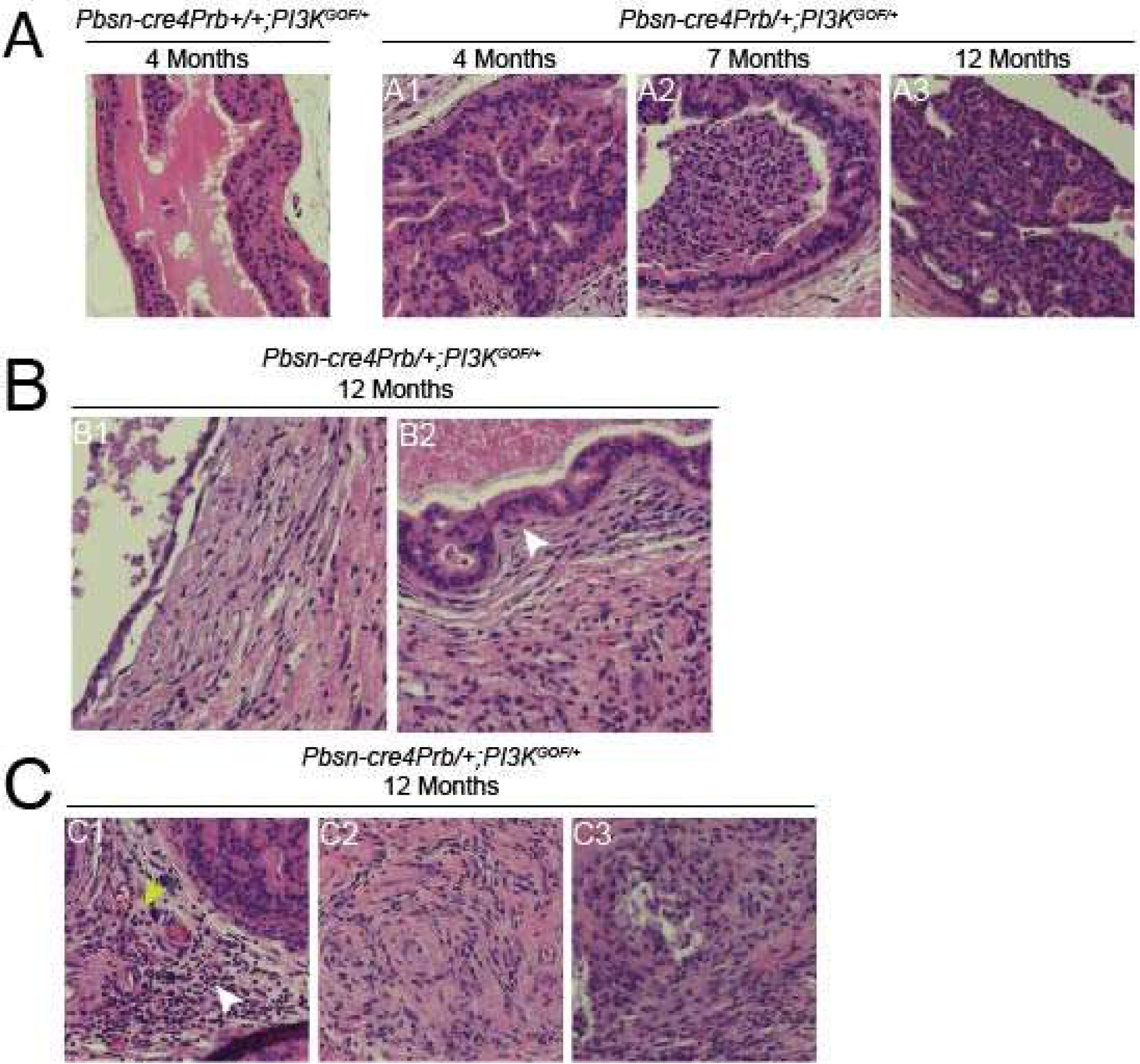
Hematoxylin and eosin stained dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice imaged at 20x. (A1) 4 month old Pbsn-cre4Prb/+;PI3KGOF/+ mice exhibit epithelial hyperplasia. (A2, A3) Grade 3 mouse prostatic intraepithelial neoplasia (mPIN3) is present in Pbsn-cre4Prb/+;PI3KGOF/+ at 7 and 12 months of age. (B1) Large cyst-like structures are apparent beginning at 7 and 12 months of age. (B2) Discreet instances of epithelial invasion into the stroma are present at 12 months of age (white arrow), yet no tumor formation is observed. (C1) At 7 and 12 months of age the stroma contains a mixed population of immune infiltrate consisting of lymphocytes (white arrow) and neutrophils (yellow arrow). (C2) The stroma is filled with pockets of spindle-shaped cells and (C3) necrosis. Images are representative of 2–4 mice per genotype.
Figure 4. Collagen accumulates in Pbsn-cre4Prb/+;PI3KGOF/+ mouse prostates.
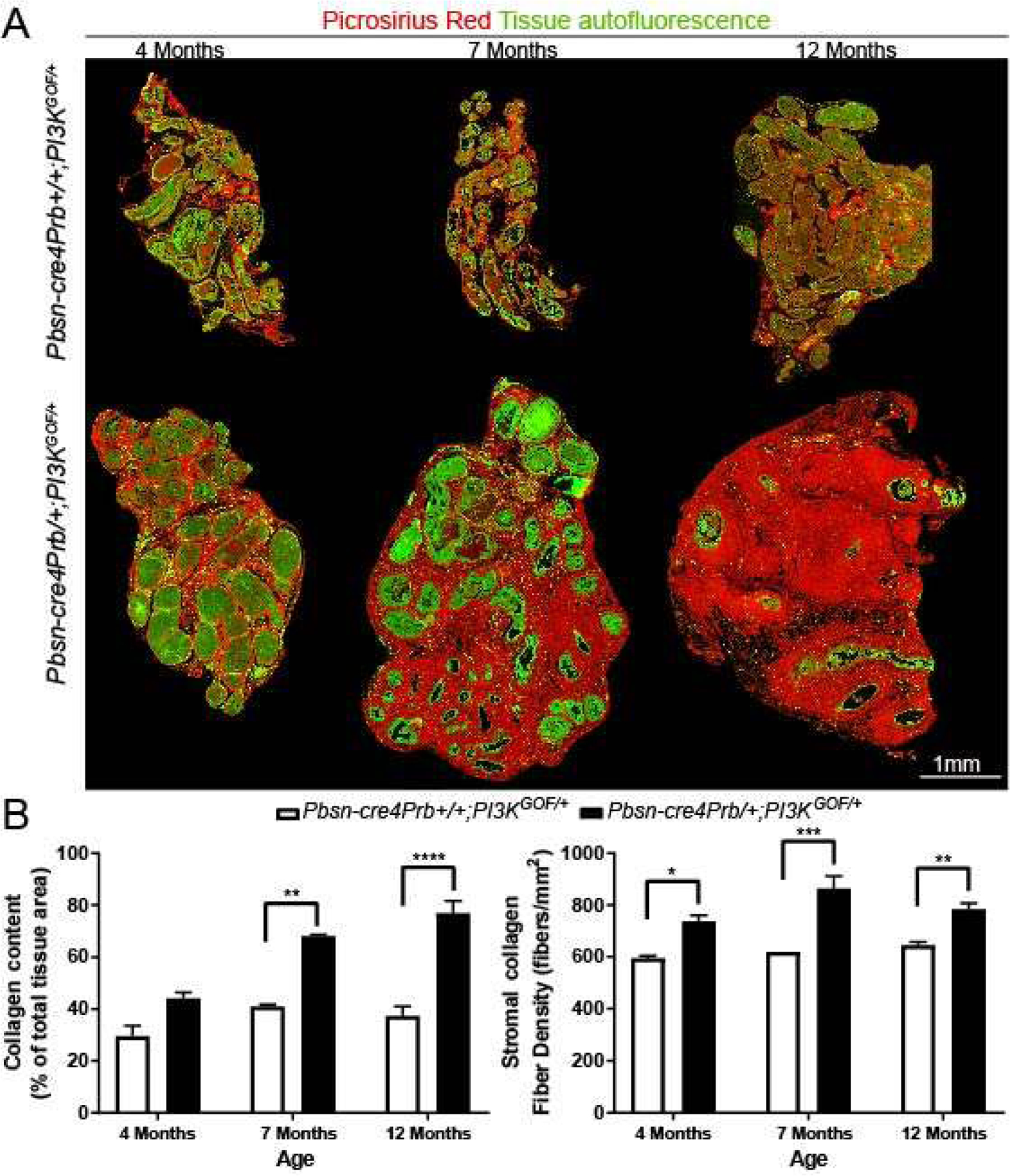
(A) Dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained with picrosirius red and imaged using fluorescent microscopy. Following whole tissue imaging, images were assembled into a montage. (B) Collagen abundance and density was quantified across the entire prostate lobe and begins to differ between groups starting at 7 months and continuing until at least 12 months of age. Results are mean ± SE of 2–4 mice. Significant differences among groups: * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001; **** = p ≤ 0.0001. Scale bar = 1 mm.
Figure 5. Characterization of stromal fibrous elements in 12 month old Pbsn-cre4Prb/+;PI3KGOF/+mice.
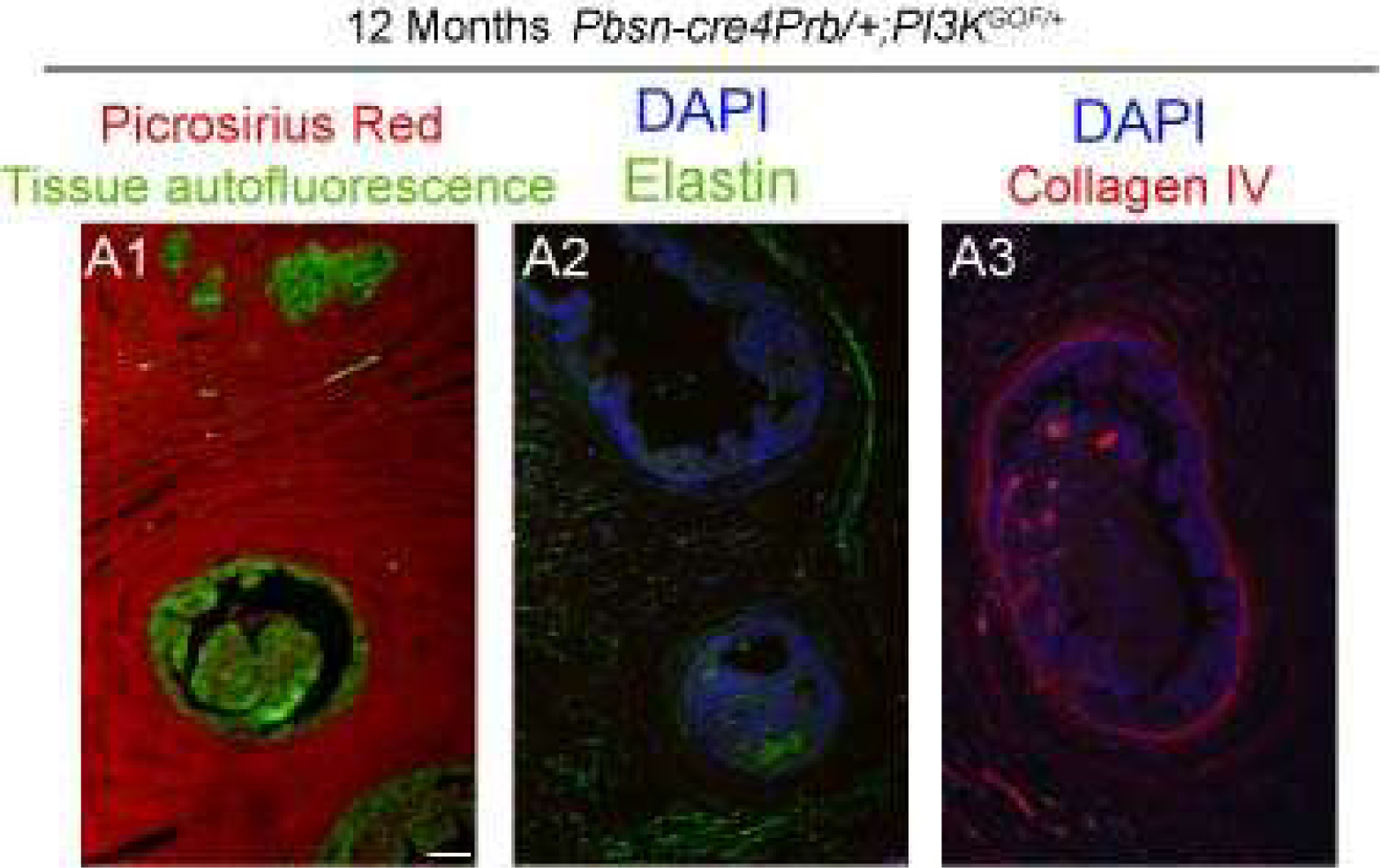
Dorsal prostate sections from 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained with picrosirius red or antibodies recognizing the elastin and collagen subtype IV. Tissues were imaged using fluorescent microscopy at 10x. (A1) Dense, fibrous collagen is prevalent in the stroma of Pbsn-cre4Prb/+;PI3KGOF/+ mice. (A2) Elastin often associates with the presence of collagen fibers, but is not the dominant fiber present. (A3) Non-fibrillar collagen subtype IV is present in the prostate but is not the dominant collagen present. Images are representative of 2–4 mice. Scale bars = 50 μm.
RESULTS
Pbsn-cre4Prb/+;PI3KGOF/+ mice have activated prostatic PI3K/AKT/mTOR signaling.
Pten is the fifth most mutated gene in human prostate cancer and is a crucial component of the PI3K pathway[37]. This observation has led to numerous studies using Pten mutant mice to drive prostate cancer. However, a more direct method to activate the PI3K pathway is overexpression of the eighth-most mutated gene in prostate cancer[37], Pik3ca. Based on previous results in the colon, we hypothesized that overexpression of an active mutant form of Pik3ca (Pbsn-cre4Prb/+;PI3KGOF/+) would drive aggressive, invasive prostate carcinoma[10]. We assessed cellular and histological composition of the prostate from Pbsn-cre4Prb/+;PI3KGOF/+ mice at 4, 7, and 12 months of age. Mice remain active and healthy throughout this period and no mortality was observed. Previous studies have confirmed PI3K pathway activation by immunostaining for a downstream target, phosphorylated ribosomal protein S6 kinase beta-1 (p-p70S6K)[38–40]. P-p70S6K-positive epithelial cell frequency in 4, 7, and 12-month-old Pbsn-cre4Prb/+;PI3KGOF/+ mice is significantly greater than that of age matched control mice (Figure 1). There is an overall decrease in epithelial PI3K signaling with age that is likely due to the dramatic decrease in the epithelial:stromal tissue ratio in Pbsn-cre4Prb/+;PI3KGOF/+ mouse prostates. Interestingly, there is also an increase in the stromal frequency of p-p70S6K-positive cells at 7 and 12 months of age. This is not unexpected given the role of PI3K signaling in inflammation and immune response[41]. These data provide compelling evidence that a genetically-engineered activating mutation in the p110 catalytic subunit of PI3K successfully activates prostatic PI3K/AKT/mTOR signaling in vivo.
We hypothesized that Pbsn-cre4Prb/+;PI3KGOF/+ mice, in which there is direct activation of the PI3K pathway, would have increased pathway activation compared to the commonly used mouse model, Pbsn-cre4Prb/+;PtenLOF/LOF, in which pathway activation is indirect. We performed a brief histological comparison to identify phenotypic differences between these distinct and relevant models of PI3K pathway activation. Using control samples from a previous study[42], we compared p-p70S6K expression between 4 month old Pbsn-cre4Prb/+;PI3KGOF/+ and Pbsn-cre4Prb/+;PtenLOF/LOF mice. Although these experimental groups differ in genetic background, we observed that the relative abundance of p-p70S6K-positive cells is significantly greater in Pbsn-cre4Prb/+;PI3KGOF/+ mice than Pbsn-cre4Prb/+;PtenLOF/LOF mice (Supplemental Figure 1A).
Pbsn-cre4Prb/+;PI3KGOF/+ mice undergo marked stromal cell expansion with age.
We assessed prostate gland histomorphology at 4, 7, and 12 months of age to determine the timeline of cancer progression in Pbsn-cre4Prb/+;PI3KGOF/+ mice. Prostate cross-sectional area does not appear to differ between 4-month-old PI3K mutant and control mice but appears to increase in 7 and 12-month-old PI3K mutant mice (Figure 2A). Epithelial hyperplasia is exhibited as early as 4 months of age, characterized by increased epithelial cells in either a single epithelial lining or accompanied by tufting, papillary, or even cribriform changes[43]. Grade 3 mouse prostatic intraepithelial neoplasia (mPIN3) is evident in Pbsn-cre4Prb/+;PI3KGOF/+ mice at 7 and 12 months, characterized by obvious nuclear atypia and cells filling or almost filling the duct lumens [43,44] (Figure 3A). Prostatic inflammation characterized by expanded stroma and leukocyte infiltration is evident in Pbsn-cre4Prb/+;PI3KGOF/+ mice prostate beginning as early as 4 months of age and continues through 12 months of age. At 4 months there are abundant leukocytes (predominantly lymphocytes) infiltrating an expanded stromal compartment containing abundant spindle shaped cells and scant extracellular matrix proteins (Figure 3C). At 7 and 12 months any normal stromal tissue has been effaced and replaced by a dense extracellular matrix containing leukocytes and spindle shaped cells. The matrix contains pockets of necrosis and occasionally is accompanied by large cyst-like structures. No tumor formation exists up to 12 months of age. Although a few cells were observed to extend into the stroma, they were few in number, in close proximity to the ducts, static in their progression across the time points, and the lesion remained minimal in even the latest time points assessed (Figure 3B).
Figure 2. Pbsn-cre4Prb/+;PI3KGOF/+ prostates exhibit dense, fibrous stroma.
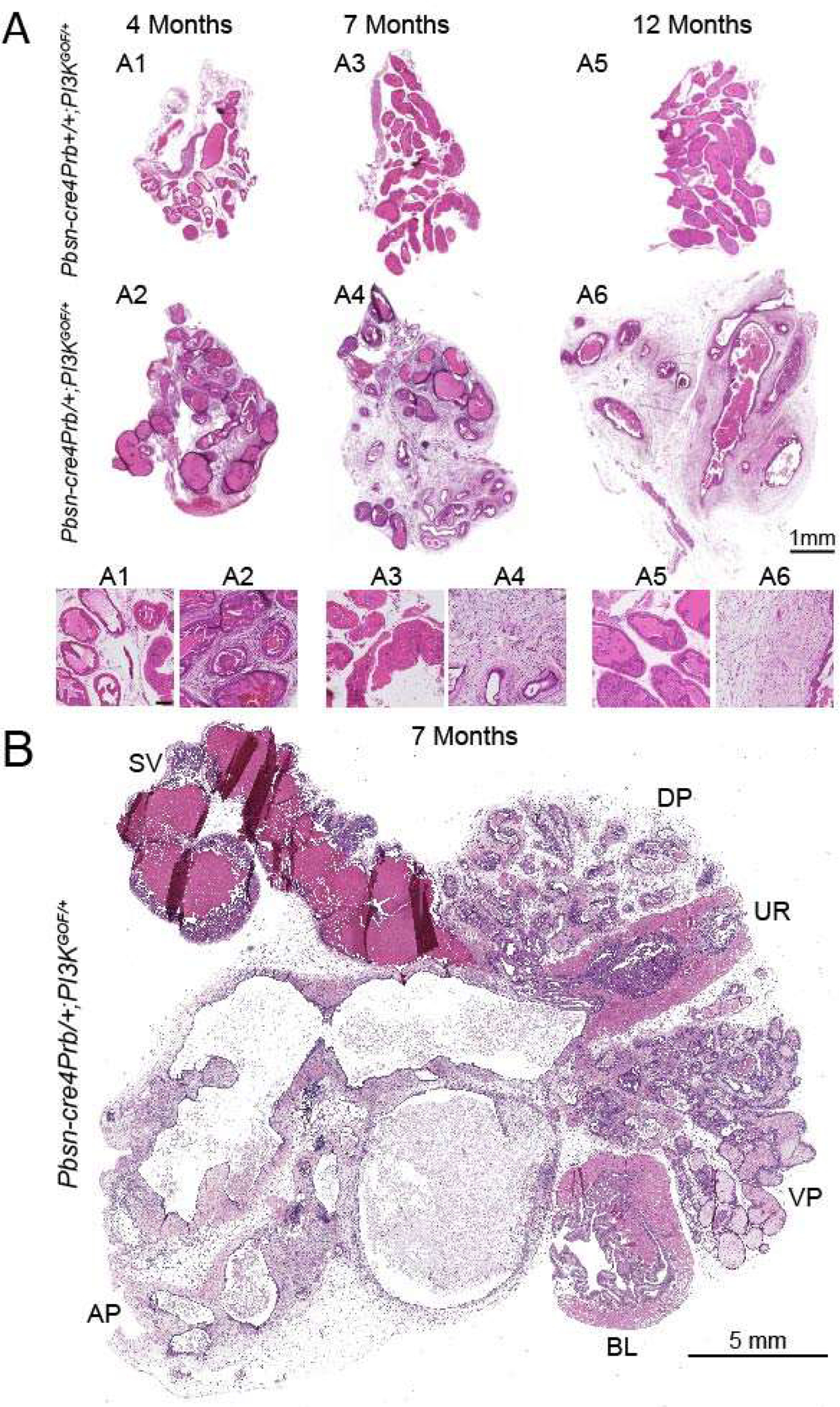
(A) Hematoxylin and eosin stained dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice. Whole prostate sections were imaged and assembled into a montage. Magnified insets show that the prostatic stroma of Pbsn-cre4Prb/+;PI3KGOF/+ mice is more dense and eosinophilic than that of control mice. (B) Hematoxylin and eosin stained complete lower urinary tract section of a 7 month old Pbsn-cre4Prb/+;PI3KGOF/+ mouse. The prostate is disproportionally larger than the urethra and bladder and harbors large cystic structures. Images are representative of 2–4 mice per genotype. Abbreviations: SV = seminal vesicle, BL = Bladder, UR = Urethra, DP = Dorsal Prostate, VP = Ventral Prostate, AP = Anterior Prostate. Scale bar = 1 mm (A), 100 μm (A insets), 5 mm (B).
Pbsn-cre4Prb/+;PI3KGOF/+ mice accumulate prostatic collagen
Given the marked expansion of stroma and the known roles of the PI3K pathway in fibrosis and collagen deposition, we next assessed stromal collagen content to test whether it is elevated in Pbsn-cre4Prb/+;PI3KGOF/+ mice[45–49]. We used picrosirius red (PSR), a ubiquitous collagen stain that has been shown previously to bind both fibrillar and non-fibrillar collagen subtypes[34]. Pbsn-cre4Prb/+;PI3KGOF/+ mice have significantly more prostatic collagen than controls at 7 and 12 months of age, but no difference was noted at 4 months (Figure 4B). We applied image masks to assess collagen density exclusively within the stromal compartment in order to control for bias due to loss of prostatic epithelial luminal space in mutant mice. Using CT-FIRE fiber detection software[50], we quantified the number of collagen fibers per unit area of stromal tissue and examined spatial distribution of collagens across the entire prostate gland (Figure 4A). At 4, 7, and 12 months of age the stromal collagen density of Pbsn-cre4Prb/+;PI3KGOF/+ mice exceeds that of control mice. As an additional step in characterizing the stromal elements present in Pbsn-cre4Prb/+;PI3KGOF/+ mice, we performed immunohistochemistry for additional extracellular fiber types in 12 month old prostate samples (Figure 5). We observed a clear abundance of PSR-stained collagen throughout the gland. To separate the contributions of non-fibrillar collagen, we used an antibody recognizing collagen subtype IV. Collagen IV is abundant in the stroma, particularly in periductal and vascular-associated regions, however it is only a small component of the dense, PSR-stained extracellular matrix. Given that the presence of collagen often associates elastin[51], we also stained the 12 month samples to assess density of elastin fibers in the stroma. Again, elastin fibers are present, but do not comprise a majority of the stromal fibers observed. Most of these fibers are likely fibrous collagen subtype I.
To contextualize the collagen build-up in Pbsn-cre4Prb/+;PI3KGOF/+ mice, we compared collagen density between Pbsn-cre4Prb/+;PI3KGOF/+ and Pbsn-cre4Prb/+;PtenLOF/LOF mice. At the 4 month time point, Pbsn-cre4Prb/+;PI3KGOF/+ and Pbsn-cre4Prb/+;PtenLOF/LOF mice have similar prostatic collagen density (Supplemental Figure 1B).
Pbsn-cre4Prb/+;PI3KGOF/+ mice lose periductal smooth muscle in prostate.
A myofibroblast / fibroblast-dominated stroma replaces periductal smooth muscle in human prostate cancer[18]. We performed immunohistochemistry for smooth muscle actin alpha (SMA) to determine whether Pbsn-cre4Prb/+;PI3KGOF/+ mice recapitulate this process. SMA+ periductal stroma is thinner in Pbsn-cre4Prb/+;PI3KGOF/+ mice at 4, 7, and 12 months of age (Figure 6). To quantify the integrity of the periductal smooth muscle layer, SMA+ area was measured in a 10 μm band around prostate ducts. Pbsn-cre4Prb/+;PI3KGOF/+ mice have significantly lower SMA+ area in this periductal region compared to controls at 4, 7, and 12 months of age. Infrequent, individual SMA+ cells are detectable in Pbsn-cre4Prb/+;PI3KGOF/+ mice, but are not clustered in the periductal space and likely represent activated myofibroblasts. Although indicative of advanced prostate cancer in humans, loss of periductal smooth muscle in Pbsn-cre4Prb/+;PI3KGOF/+ mice does not appear to drive large scale epithelial invasion.
Figure 6. Pbsn-cre4Prb/+;PI3KGOF/+ mice lose integrity of dorsal prostate periductal smooth muscle.
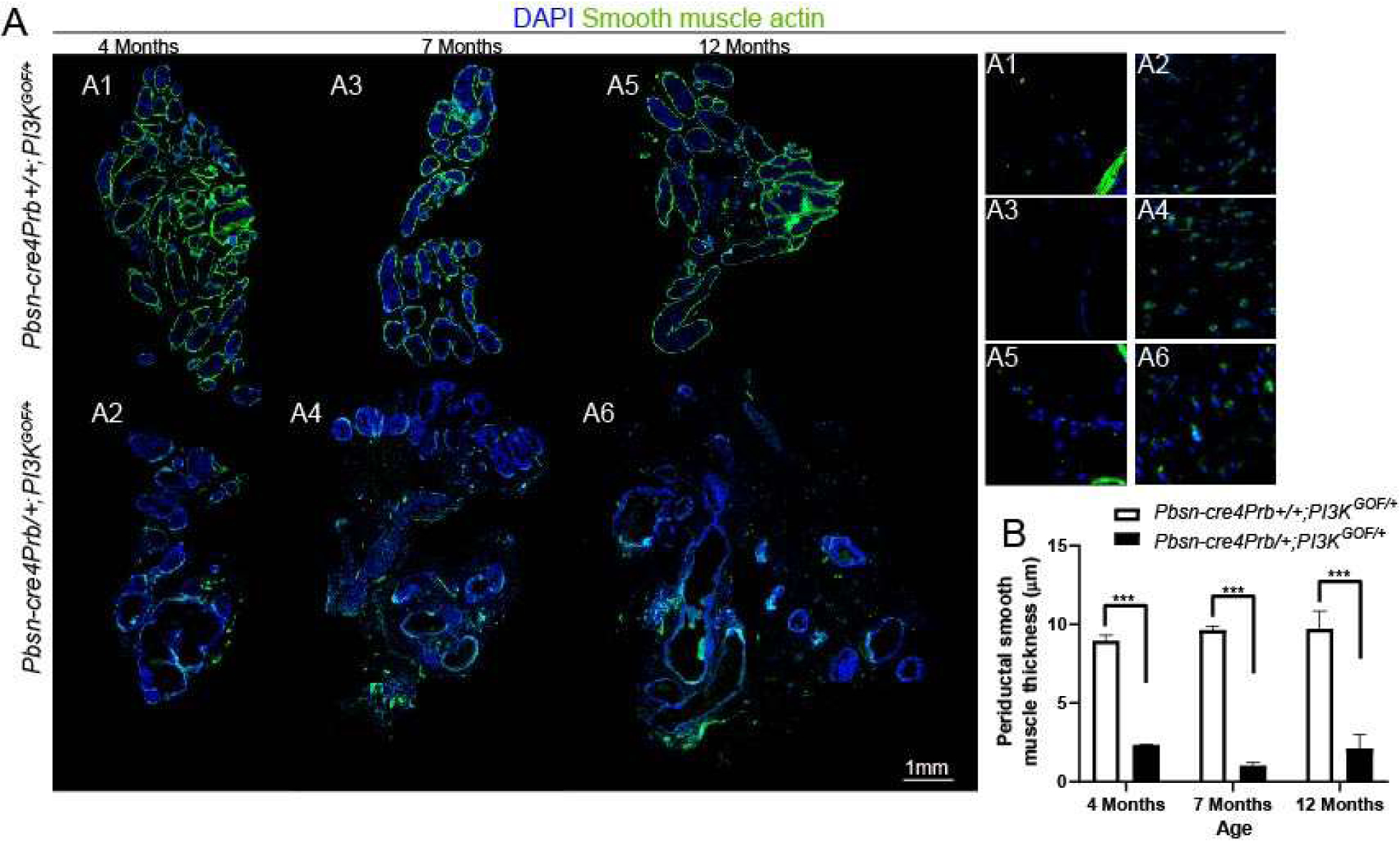
(A) Dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained with an antibody recognizing smooth muscle actin alpha (SMA) and DAPI to mark nuclei. Slides were imaged using fluorescent microscopy and assembled into a montage. Smooth muscle is significantly diminished in Pbsn-cre4Prb/+;PI3KGOF/+ mice compared to controls at all time points. Magnified insets show the increase in scattered, putative myofibroblasts (SMA+) cells throughout the stroma of of Pbsn-cre4Prb/+;PI3KGOF/+ mice. (B) The periductal SMA+ layer is significantly thinner in Pbsn-cre4Prb/+;PI3KGOF/+ mice than controls. Results are mean ± SE of 2–4 mice. Significant differences among groups: * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001; **** = p ≤ 0.0001. Scale bar = 1 mm.
Pbsn-cre4Prb/+;PI3KGOF/+ mice accumulate prostatic stromal p-SMAD2, evidence of TGFβ activation.
We next investigated the signaling pathway responsible for the marked expansion of the collagen matrix and of SMA- fibroblasts in Pbsn-cre4Prb/+;PI3KGOF/+ mouse prostates. TGFβ signaling is intimately involved in the regulation of many wound repair and angiogenic processes in the reactive stroma of premalignant prostate cancer[14,52–57]. Previous studies used p-SMAD2 as a histological index of TGFβ pathway activity[58]. We used immunohistochemistry to stain and quantify the frequency of p-SMAD2+ stromal cells and found no difference between 4 month old Pbsn-cre4Prb/+;PI3KGOF/+ and control mice, but significantly more p-SMAD2+ stromal cells in 7 and 12 month Pbsn-cre4Prb/+;PI3KGOF/+ compared to age-matched controls (Figure 7B). The limited staining present in the prostate epithelium of Pbsn-cre4Prb/+;PI3KGOF/+ mice at all time points supports that phosphorylation of SMAD2 is a paracrine response to activation of PI3K and not caused by off-target Cre-induced recombination. To further link transgene expression to stromal activation of the TGFβ pathway, we performed RNAscope to identify expression of Tgfβ mRNA. Tgfβ mRNA is noticeably more abundant in the epithelium of 4 month old Pbsn-cre4Prb/+;PI3KGOF/+ mice compared to that of controls (Figure 5C).
Figure 7. Pbsn-cre4Prb/+;PI3KGOF/+ mouse prostatic stroma has increased levels of phospho-SMAD2, a biomarker of transforming growth factor β activity.
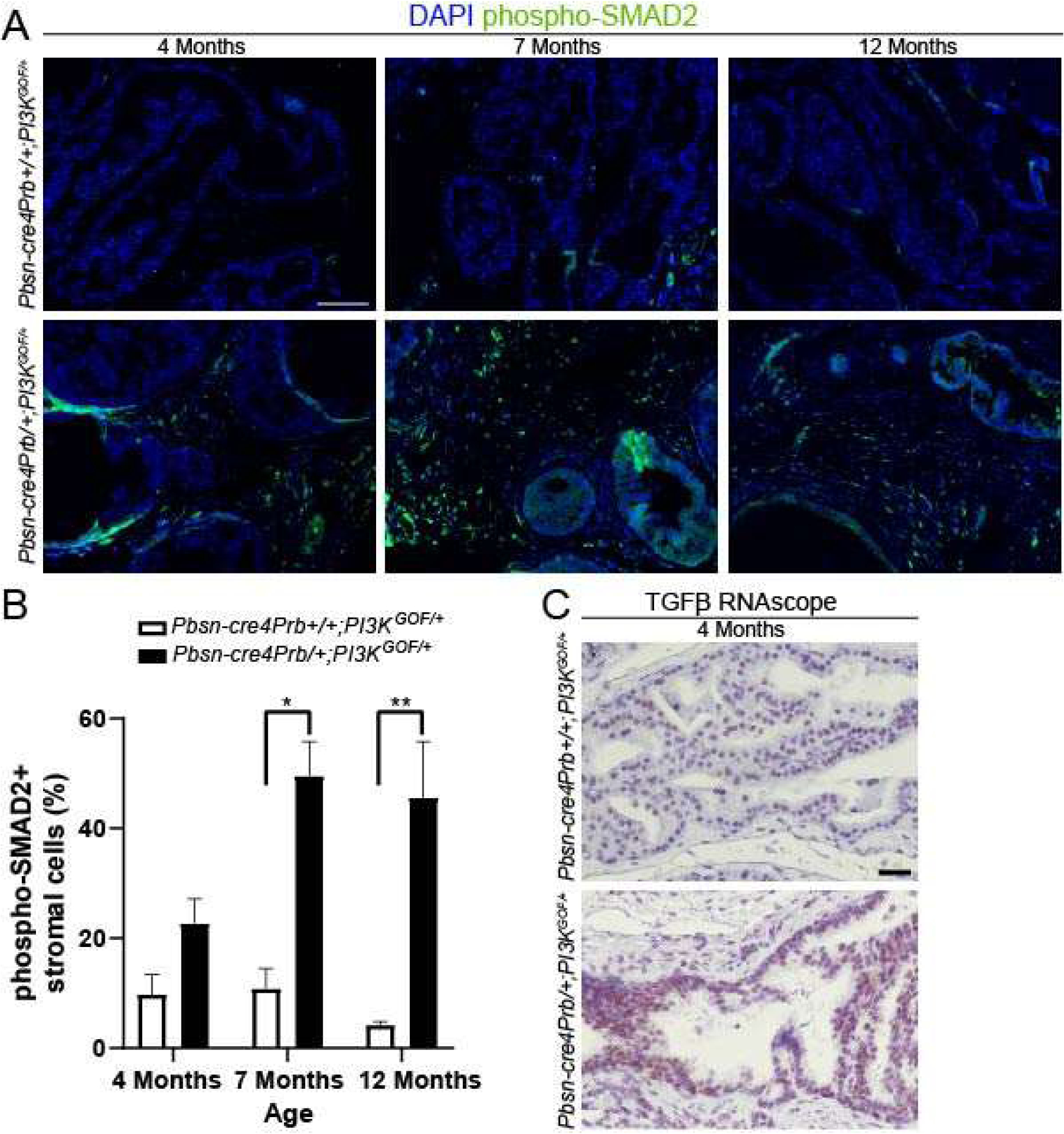
(A) Dorsal prostate sections from 4, 7 and 12 month old Pbsn-cre4Prb+/+;PI3KGOF/+ (control) and Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained with an antibody recognizing the phosphorylated form of SMAD2 (p-SMAD2) and DAPI to mark nuclei. Slides were imaged using fluorescent microscopy. (B) The frequency of p-SMAD2+ stromal cells begins to differ between groups starting at 7 months and continuing until at least 12 months of age. (C) Pbsn-cre4Prb/+;PI3KGOF/+ male mice were stained using RNAscope to visualize TGFβ mRNA. Representative image of greater TGFβ mRNA abundance in the epithelium of Pbsn-cre4Prb/+;PI3KGOF/+ mice. Results are mean ± SE of n = 2–4 mice. Significant differences among groups: * = p ≤ 0.05; ** = p ≤ 0.01. Scale bars = 50 μm.
DISCUSSION
Numerous mouse models of prostate cancer are currently used for basic research and intervention validation[59]. One of the most commonly used models is the Pbsn-cre4Prb/+;PtenLOF/LOF model which recapitulates the frequent loss of PTEN observed in human prostate cancers, results in activation of the PI3K pathway, and causes prostate cancers. Activation of the PI3K pathway by mutations in the PI3KCA gene are also common in human prostate cancers and are modeled in this study.
Pbsn-cre4Prb/+;PI3KGOF/+ do not become moribund and have 100% survival until 12 months of age. High grade PIN and hyperplasia is observed in all the prostate lobes; however, instances of stromal invasion were limited and when present were localized. Prostate lobes larger than controls and contain large cystic structures, which appear to be dilated ducts resulting from impeded flow of prostatic secretions. A diagnosis of carcinoma was not favored given the minimal and regionally restricted extent of epithelial invasion even at late time-points; a diagnosis of carcinoma in the context of an animal model, in which it is more conservative to avoid over-interpretation of lesions, demands clear evidence of malignant behavior. Overall, cancer progression in Pbsn-cre4Prb/+;PI3KGOF/+ is relatively mild compared to published reports for Pbsn-cre4Prb/+;PtenLOF/LOF mice[8,9].
Despite lacking a potent cancerous phenotype, Pbsn-cre4Prb/+;PI3KGOF/+ undergo extreme stromal ECM remodeling. We documented a clear progression of stromal desmoplasia that is consistent with previous findings that PI3K can initiate collagen production and fibrosis[45,47–49]. However, our study is the first to apply an innovative method to evaluate and quantify collagen across complete cross-sections of mouse prostate. Our process of evaluating collagen content across the entire tissue is rigorous and accounts for naturally occurring variations in collagen density across the mouse prostate gland as seen in other species[60]. We observed a dramatic increase in interductal collagen in Pbsn-cre4Prb/+;PI3KGOF/+ mice, however this was not accompanied by a concurrent increase in elastin fibers. This reliable, targeted method to specifically drive collagen accumulation will have broad utility to the research community given the role of collagen in inflammation, benign disease, and cancer progression/metastasis, [22–24,26,29,61–66].
The Pbsn-cre4Prb/+;PI3KGOF/+ mouse model also provides a unique opportunity to identify pro-fibrotic pathways specific to the prostate gland. We showed that collagens do not begin to accumulate until after 4 months of age in Pbsn-cre4Prb/+;PI3KGOF/+ mice. Collagen density then sharply increases between 4 and 7 months of age. We have therefore defined a critical window in which collagen accumulation occurs. We probed a potential mechanism in which TGFβ signaling drives the stromal transformation of Pbsn-cre4Prb/+;PI3KGOF/+ mice. We observed progressively increasing TGFβ pathway activation in the stroma of Pbsn-cre4Prb/+;PI3KGOF/+ mice at 7 and 12 months of age – the same time points at which collagen density is significantly increased. Since TGFβ signaling is pro-fibrotic[67,68] and has been shown to associate with PIN lesions in humans[52], it is a plausible trigger for collagen accumulation in the Pbsn-cre4Prb/+;PI3KGOF/+ model. We observed an apparent increase in epithelial Tgfβ mRNA production at 4 months that precedes the stromal response at 7 and 12 months. This lends itself to the hypothesis that PI3K activation triggers an autonomous signaling cascade ultimately leading to production and secretion of TGFβ by prostatic epithelium, which activates stromal SMAD phosphorylation and collagen transcription in prostatic stroma. Further studies should isolate the specific cell types producing collagen and, by comparing to existing models of prostate collagen accumulation, determine whether the identity of the collagen-producing cell, or the cellular signals responsible for its activation, are insult specific.
The time course performed in this study highlights the potential utility of the Pbsn-cre4Prb/+;PI3KGOF/+ as a model of organ-specific collagen accumulation. The highly localized stromal response is useful for testing anti-fibrotic agents or biomarker discovery. Perhaps the most promising feature of the model is that the stromal responses to epithelial-derived PI3K pathway activation appear to precede any neoplastic epithelial changes, and continues afterward as well. This model provides the opportunity to study collagen production in both the presence and absence of precancerous neoplasia, making the model well suited for both benign and cancer studies. Further unraveling the nuances of the epithelial-stromal communication in Pbsn-cre4Prb/+;PI3KGOF/+ mice will undoubtedly yield the data necessary to prevent formation and deposition of dense, reactive stroma and thus enhance therapies for both cancer and fibrotic diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to recognize Dr. Ruth Sullivan and Dr. LaTasha Crawford for histological analysis and input on experimental design.
This work was supported by National Institutes of Health [grant numbers U54DK104310S1, U54DK104310, F31ES028594, T32ES007015, CA195313, TL1TR002375, and F30DK122686] and the American Motorcycle Association Ride for Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: The authors have no conflict of interest.
REFERENCES
- 1.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19: 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch E, Ciraolo E, Franco I, et al. PI3K in cancer–stroma interactions: bad in seed and ugly in soil. Oncogene 2014; 33: 3083–3090 [DOI] [PubMed] [Google Scholar]
- 4.Park S, Kim YS, Kim DY, et al. PI3K pathway in prostate cancer: All resistant roads lead to PI3K. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer 2018; 1870: 198–206 [DOI] [PubMed] [Google Scholar]
- 5.Chalhoub N, Baker SJ. PTEN and the PI3-Kinase Pathway in Cancer. Annual review of pathology 2009; 4: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMenamin ME, Soung P, Perera S, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 1999; 59: 4291–4296 [PubMed] [Google Scholar]
- 7.Carnero A, Paramio JM. The PTEN/PI3K/AKT Pathway in vivo, Cancer Mouse Models. Frontiers in Oncology 2014; 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003; 4: 209–221 [DOI] [PubMed] [Google Scholar]
- 9.Svensson RU, Haverkamp JM, Thedens DR, et al. Slow disease progression in a C57BL/6 pten-deficient mouse model of prostate cancer. Am J Pathol 2011; 179: 502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leystra AA, Deming DA, Zahm CD, et al. Mice Expressing Activated PI3K Rapidly Develop Advanced Colon Cancer. Cancer Res 2012; 72: 2931–2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegner KA, Cadena MT, Trevena R, et al. An immunohistochemical identification key for cell types in adult mouse prostatic and urethral tissue sections. PLOS ONE 2017; 12: e0188413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocrine Related Cancer 2012; 19: R187–R204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev 1998; 17: 411–419 [DOI] [PubMed] [Google Scholar]
- 14.TUXHORN JA, AYALA GE, ROWLEY DR. REACTIVE STROMA IN PROSTATE CANCER PROGRESSION. The Journal of Urology 2001; 166: 2472–2483 [PubMed] [Google Scholar]
- 15.Levesque C, Nelson PS. Cellular Constituents of the Prostate Stroma: Key Contributors to Prostate Cancer Progression and Therapy Resistance. Cold Spring Harb Perspect Med 2018; 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation 2002; 70: 473–485 [DOI] [PubMed] [Google Scholar]
- 17.da Silva MM, Matheus WE, Garcia PV, et al. Characterization of reactive stroma in prostate cancer: involvement of growth factors, metalloproteinase matrix, sexual hormones receptors and prostatic stem cells. Int Braz J Urol 2015; 41: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuxhorn JA, Ayala GE, Smith MJ, et al. Reactive stroma in human prostate cancer induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical Cancer Research 2002; 8: 2912–2923 [PubMed] [Google Scholar]
- 19.Provenzano PP, Inman DR, Eliceiri KW, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med 2008; 6: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paszek MJ, Weaver VM. The Tension Mounts: Mechanics Meets Morphogenesis and Malignancy. J Mammary Gland Biol Neoplasia 9: 325–342 DOI: 10.1007/s10911-004-1404-x. [DOI] [PubMed] [Google Scholar]
- 21.Provenzano PP, Eliceiri KW, Campbell JM, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Medicine 2006; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcus CE, Keely PJ, Eliceiri KW, et al. Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells. J Biol Chem 2013; 288: 12722–12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conklin MW, Eickhoff JC, Riching KM, et al. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am J Pathol 2011; 178: 1221–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shea MP, O’Leary KA, Wegner KA, et al. High collagen density augments mTOR-dependent cancer stem cells in ERα+ mammary carcinomas, and increases mTOR-independent lung metastases. Cancer Lett 2018; 433: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcus CE, Holt EC, Keely PJ, et al. Dense collagen-I matrices enhance pro-tumorigenic estrogen-prolactin crosstalk in MCF-7 and T47D breast cancer cells. PLoS ONE 2015; 10: e0116891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R, et al. Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. The Prostate 2013; 73: 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauman TM, Nicholson TM, Abler LL, et al. Characterization of Fibrillar Collagens and Extracellular Matrix of Glandular Benign Prostatic Hyperplasia Nodules Emmert-Buck MR, ed. PLoS ONE 2014; 9: e109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Gharaee-Kermani M, Kunju L, et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol 2012; 188: 1375–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong L, Hutson PR, Bushman W. Prostatic Inflammation Induces Fibrosis in a Mouse Model of Chonic Bacterial Infection Thumbikat P, ed. PLoS ONE 2014; 9: e100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 2001; 101: 61–69 [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell 2009; 139: 573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta V, Abler LL, Keil KP, et al. Atlas of Wnt and R-Spondin Gene Expression in the Developing Male Mouse Lower Urogenital Tract. Dev Dyn 2011; 240: 2548–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vezina Chad. GUDMAP Consortium. Available from: https://www.gudmap.org/id/16-QP8M.
- 34.Wegner KA, Keikhosravi A, Eliceiri KW, et al. Fluorescence of Picrosirius Red Multiplexed With Immunohistochemistry for the Quantitative Assessment of Collagen in Tissue Sections. J Histochem Cytochem July 2017: 22155417718541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nature Methods 2012; 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosner B Fundamentals of Biostatistics. Cengage Learning; 2010. [Google Scholar]
- 37.Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nature Genetics 2018; 50: 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin NE, Gerke T, Sinnott JA, et al. Measuring PI3K Activation: Clinicopathologic, Immunohistochemical, and RNA Expression Analysis in Prostate Cancer. Mol Cancer Res 2015; 13: 1431–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zagorac I, Fernandez-Gaitero S, Penning R, et al. In vivo phosphoproteomics reveals kinase activity profiles that predict treatment outcome in triple-negative breast cancer. Nat Commun 2018; 9: 3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Li L, Duan Q, et al. Krüppel-like factor 2 suppresses human gastric tumorigenesis through inhibiting PTEN/AKT signaling. Oncotarget 2017; 8: 100358–100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins PT, Stephens LR. PI3K signalling in inflammation. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2015; 1851: 882–897 [DOI] [PubMed] [Google Scholar]
- 42.Le B, Powers GL, Tam YT, et al. Multi-drug loaded micelles delivering chemotherapy and targeted therapies directed against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer. PLoS One 2017; 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ittmann M, Huang J, Radaelli E, et al. Animal Models of Human Prostate Cancer: The Consensus Report of the New York Meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013; 73: 2718–2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park J-H, Walls JE, Galvez JJ, et al. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol 2002; 161: 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilly R, Mroz MS, Dempsey E, et al. Targeting the PI3K/Akt/mTOR signalling pathway in Cystic Fibrosis. Sci Rep 2017; 7: 7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li H-Y, Zhang Q-G, Chen J-W, et al. The fibrotic role of phosphatidylinositol-3-kinase/Akt pathway in injured skeletal muscle after acute contusion. Int J Sports Med 2013; 34: 789–794 [DOI] [PubMed] [Google Scholar]
- 47.Campa CC, Silva RL, Margaria JP, et al. Inhalation of the prodrug PI3K inhibitor CL27c improves lung function in asthma and fibrosis. Nature Communications 2018; 9: 5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C, Li J, Tiantian M. PI3K/Akt signaling pathway and liver fibrosis. Chinese Pharmacological Bulletin 2011; 27: 1037–1041 [Google Scholar]
- 49.Yokoyama K, Kimoto K, Itoh Y, et al. The PI3K/Akt pathway mediates the expression of type I collagen induced by TGF-β2 in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 2012; 250: 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bredfeldt JS, Liu Y, Pehlke CA, et al. Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J Biomed Opt 2014; 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123: 4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cardillo MR, Petrangeli E, Perracchio L, et al. Transforming growth factor-beta expression in prostate neoplasia. Anal Quant Cytol Histol 2000; 22: 1–10 [PubMed] [Google Scholar]
- 53.Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986; 83: 4167–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strand DW, Liang Y-Y, Yang F, et al. TGF-β induction of FGF-2 expression in stromal cells requires integrated smad3 and MAPK pathways. Am J Clin Exp Urol 2014; 2: 239–248 [PMC free article] [PubMed] [Google Scholar]
- 55.Yang F, Chen Y, Shen T, et al. Stromal TGF-β signaling induces AR activation in prostate cancer. Oncotarget 2014; 5: 10854–10869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eastham JA, Truong LD, Rogers E, et al. Transforming growth factor-beta 1: comparative immunohistochemical localization in human primary and metastatic prostate cancer. Lab Invest 1995; 73: 628–635 [PubMed] [Google Scholar]
- 57.Ihn H Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol 2002; 14: 681–685 [DOI] [PubMed] [Google Scholar]
- 58.Franco OE, Jiang M, Strand DW, et al. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res 2011; 71: 1272–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parisotto M, Metzger D. Genetically engineered mouse models of prostate cancer. Molecular Oncology 2013; 7: 190–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruetten H, Wegner KA, Romero MF, et al. Prostatic collagen architecture in neutered and intact canines. The Prostate 2018; 78: 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.BURNS-COX N, AVERY NC, GINGELL JC, et al. CHANGES IN COLLAGEN METABOLISM IN PROSTATE CANCER: A HOST RESPONSE THAT MAY ALTER PROGRESSION. The Journal of Urology 2001; 166: 1698–1701 [DOI] [PubMed] [Google Scholar]
- 62.Hall CL, Dubyk CW, Riesenberger TA, et al. Type I Collagen Receptor (α2β1) Signaling Promotes Prostate Cancer Invasion through RhoC GTPase. Neoplasia 2008; 10: 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiefer J, Alexander A, Farach-Carson MC. Type I Collagen-Mediated Changes in Gene Expression and Function of Prostate Cancer Cells. In Keller ET, Chung LWK, eds. The Biology of Skeletal Metastases. Cancer Treatment and Research. Boston, MA: Springer US, 2004; 101–124. [DOI] [PubMed] [Google Scholar]
- 64.Bell-Cohn A, Mazur DJ, Hall C, et al. Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type 2 cytokine signaling. Am J Physiol Renal Physiol 2019; 316: F682–F692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ricke WA, Lee CW, Clapper TR, et al. In Utero and Lactational TCDD Exposure Increases Susceptibility to Lower Urinary Tract Dysfunction in Adulthood. Toxicological Sciences 2016: kfw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholson TM, Ricke EA, Marker PC, et al. Testosterone and 17β-Estradiol Induce Glandular Prostatic Growth, Bladder Outlet Obstruction, and Voiding Dysfunction in Male Mice. Endocrinology 2012; 153: 5556–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Branton MH, Kopp JB. TGF-β and fibrosis. Microbes and Infection 1999; 1: 1349–1365 [DOI] [PubMed] [Google Scholar]
- 68.Pohlers D, Brenmoehl J, Löffler I, et al. TGF-β and fibrosis in different organs — molecular pathway imprints. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2009; 1792: 746–756 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


