Abstract
Objective:
To develop a predictive model of neurocognitive trajectories in children with perinatal HIV (pHIV).
Design:
Machine learning analysis of baseline and longitudinal predictors derived from clinical measures utilized in pediatric HIV.
Methods:
285 children (ages 2–14 years at baseline; Mage=6.4 years) with pHIV in Southeast Asia underwent neurocognitive assessment at study enrollment and twice annually thereafter for an average of 5.4 years. Neurocognitive slopes were modeled to establish two subgroups (above (n=145) and below average (n=140) trajectories). Gradient-boosted multivariate regressions (GBM) with five-fold cross validation were conducted to examine baseline (pre-ART) and longitudinal predictive features derived from demographic, HIV disease, immune, mental health, and physical health indices (i.e., complete blood count; CBC).
Results:
The baseline GBM established a classifier of neurocognitive group designation with an average AUC of 79% built from HIV disease severity and immune markers. GBM analysis of longitudinal predictors with and without interactions improved the average AUC to 87% and 90%, respectively. Mental health problems and hematocrit levels also emerged as salient features in the longitudinal models, with novel interactions observed between mental health problems and both CD4 count and hematocrit levels. Average AUCs derived from each GBM model were higher than results obtained using logistic regression.
Conclusions:
Our findings support the feasibility of machine learning to identify children with pHIV at risk for suboptimal neurocognitive development. Results also point towards interactions between HIV disease and mental health problems as early antecedents to neurocognitive difficulties in later childhood among individuals with pHIV.
Keywords: perinatal HIV, cognition, machine learning, mental health, development
Introduction
A subset of children with perinatal human immunodeficiency virus (pHIV) experience long-term neurocognitive difficulties [1–3]. While initial findings from a prospective investigation of newborns with pHIV [4] described neurocognitive gains associated with the initiation of anti-retroviral treatment (ART) within the first 30 days of life, a recent follow-up study of the same cohort identified neurocognitive difficulties in a subset of children after approximately 5 years of treatment [5]. Similar findings of persistent neurocognitive symptoms have been reported in separate cohorts of older children with pHIV who initiated ART within the first weeks after birth [6,7], and children with pHIV who initiated de novo ART after surviving the first year or longer without ART [8–13].
Predicting individual neurocognitive outcomes for young children with pHIV is challenging, especially for individuals residing in resource-limited environments where the majority of the global population of pHIV reside. Prior investigations of youth with pHIV reveal independent risk factors of suboptimal neurodevelopment, including HIV disease indices (e.g., low CD4 T-cell count, high viral load [14–16]) and numerous psychosocial factors (e.g., poverty, parental mental health problems) [17–22]. However, previous investigations have utilized traditional analytic methods that are generally insensitive to nonlinearities and multiple interactions among predictor variables [23].
Machine learning provides a complementary approach to traditional statistical models by leveraging methods capable of defining structure in complex, multi-dimensional data [23–30]. Machine learning methods have been shown to improve diagnostic accuracy and prediction of disease and treatment outcomes when compared to traditional analytics [26–33]. Additionally, machine learning provides a unique opportunity to discover linear and nonlinear (e.g., polynomial) mechanisms and multiple high-level interactions among risk factors that can then facilitate the development of tailored clinical interventions [23, 27]. Only a handful of studies have applied machine learning to investigate neurobehavioral features of HIV, and none have focused on pediatric samples [28–30]. For example, Ogishi et al. [30] utilized machine learning to discover three viral proteins that distinguished HIV-infected adults with dementia from those without dementia with approximately 90% accuracy. The identification of novel HIV disease mechanisms associated with severe cognitive impairment is an important finding, but the results do not readily translate to the larger population of individuals affected by HIV with less severe neurocognitive problems or to pediatric populations. To date, no studies have utilized machine learning to establish a data-driven predictive model of neurocognitive development in children with pHIV.
The current study was designed to address these gaps and contribute to the existing literature on pediatric HIV. Following precedence by Papini et al. [31], we built the predictive algorithms using risk factors (i.e., input features) from readily accessible clinical information. Second, we compared model performance between additive and interactive algorithms built from information obtained only at the first study visit (baseline, pre-ART) and information acquired over the course of structured follow-up visits (average of 5.4 years). These comparisons established an empirical benchmark to quantify the gain in accuracy using more complex models. Finally, we compared the machine learning algorithms to a standard analytic approach using the same number of predictive features in each method.
Methods
Study Design
Data from 285 children with pHIV were included in the current study. Participants included Thai (n=170) and Cambodian (n=115) children enrolled in the Pediatric Randomized to Early vs. Deferred Initiation in Cambodia and Thailand (PREDICT) clinical trial (clinicaltrials.gov identification: U19AI53741) [8]. The PREDICT study began enrollment in 2006, when HIV treatment guidelines in Thailand recommended ART in children with CD4<15%. Accordingly, the PREDICT study enrolled male and female children with pHIV who had survived the first year of life (PREDICT enrollment age=1–14 years) with a CD4 between 15–25% and no history of ART (including no exposure to ART in utero). Participants were then randomized to begin ART at CD4 <25% (immediate treatment arm) or CD4<15% (deferred treatment arm). The main outcomes of PREDICT included mortality and the frequency of AIDS-defining illnesses 3 years after enrollment, neither of which differed by treatment arm [8].
The exclusion criteria from PREDICT were applied for the current study. Specifically, individuals were excluded if they reported a history of brain infection, neurological disorder, congenital abnormalities, previous use of immune modulators within 4 weeks of enrollment, baseline absolute neutrophil count <750 cells/μL, hemoglobin <7.5 g/dL, baseline platelet counts <50,000/μL, or alanine aminotransferase >4 times the upper limit of normative values. The protocols were approved by the Institutional Review Boards in Thailand, Cambodia, and the US. Caregivers provided informed consent with assent obtained from children over 7 years of age.
First-line ART included zidovudine, lamivudine, and nevirapine. Prior to the last study visit for this analysis, 124 (43%) participants enrolled in the deferred arm experienced a decline in CD4 to <15%, at which point ART was initiated. The remaining 33 (11%) children in the deferred treatment arm maintained a CD4>15% throughout the observation period for this study. One participant was diagnosed with an opportunistic infection within weeks of enrollment (prompting initiation of ART). Otherwise, no cases of AIDS-defining illnesses were observed during the study.
Outcome measure
Neurocognitive development was assessed using the Beery-Buktenica Developmental Test of Visual-Motor Integration, Fourth Edition (Beery VMI) [34]. The Beery VMI is a test of graphomotor construction of 30 increasingly complex line drawings presented in sequential order. The test is suitable for individuals age 2 and older and provides a sensitive measure of neurocognitive development for individuals from diverse environmental, educational, and cultural backgrounds [34]. Performance is strongly correlated with chronological age [35] and is closely linked to performance on functional skills, academic achievement, learning difficulties, and neurodevelopmental delay [35–39]. The Beery VMI was administered to Thai and Cambodian children at baseline and again twice per year for an average of 5.4 years. Baseline scores were not available for 12 participants (6 in each treatment arm of PREDICT). For these cases, performance acquired at the next clinic visit (approximately 6 months after enrollment) was utilized as the first assessment for the slope analysis.
Neurocognitive trajectories on the Beery VMI were defined by the average percent change in raw score performance modeled across all follow-up visits. Individual trajectories (i.e., slopes) were designated as above average (positive slope; n=145) or below average (negative slope; n=140) in comparison to the within sample mean. This approach yielded a robust set of features (approximately 220,000 data elements) for analysis.
Predictors
Multi-dimensional predictor variables included: 1) demographics (e.g., age, sex, family income); 2) HIV disease (plasma viral load); 3) immune markers (e.g., CD4% and count, CD8% and count, total lymphocyte count, white blood cell count), 4) blood markers of physical health (e.g., red blood cell count (RBC), platelet count, hematocrit, hemoglobin, glucose); and 5) mental health indices (subscale and overall indices from the Child Behavior Checklist-Caregiver version; CBCL) [40]. The CBCL was translated into Thai [9, 41] and Khmer [9] in prior studies. Primary caregivers completed the preschool (age 2–6 years) and school-age (age 6–18 years) versions at the baseline visit and every six months thereafter (following the same sequence as the Beery VMI). Raw scores on the composite scales (Internalizing, Externalizing, and Total Problem scores) and subscales (Anxiousness, Aggressive Behavior, Rule-Breaking Behaviors, Thought Problems, Affective, Somatic Complaints, and Social Competence) were included as predictors.
Machine learning approach
Gradient boosted multivariate regression (GBM), a form of ensemble machine learning, was utilized to build the algorithms. GBM leverages a “wisdom of crowds” approach to combine accuracy and error information from multiple individual algorithms to establish a robust overall model that optimizes accuracy while controlling for overfitting of the data [23]. Recent studies have utilized GBM to identify novel features of psychiatric conditions (e.g., Post-Traumatic Stress Disorder) [31], neurodegenerative disease (e.g., probable Alzheimer’s disease) [42], and complex brain structure-function relationships (e.g., subcortical-cortical networks) [43].
Neurocognitive trajectories were assigned using probability scores based on the sigmoid function by a (1/(1+e^(-x)), using a 0.5 decision boundary, and gradient descent to minimize prediction error. Accuracy defined from Receiver Operator Curves (AUC) served as the main metric of model performance. Sensitivity, specificity, F1 score (i.e., harmonic balance between sensitivity and specificity), and AUC interpretation followed standard convention (poor = 0.6–0.7, fair = 0.7–0.8, good = 0.8–0.9; excellent ≥ 0.9) [44].
Feature selection
Feature selection was conducted using an in-house program based on SciKit [45] and PDPBox [46]. Consistent with prior studies [31], we examined several GBM models that differed by model complexity to determine relative gain in average AUC [31]. The first model focused on baseline features acquired before participants began ART. The second model included longitudinal features acquired during the follow-up study visits of the PREDICT trial. Measures of central tendency (e.g., average, max value) and dispersion were included in the longitudinal approach to facilitate discovery of novel mechanisms. A third GBM examined the relative gain in model performance by allowing for up to 2-way interactions among input features.
Model stability and validity
Several methods were employed to optimize stability and validity. First, as described above we utilized a form of ensemble machine learning that is robust to overfitting compared to other machine learning methods [23,31]. Second, we focused the final algorithms on the 6 risk features with the highest classification strengths (i.e., mutual information criterion scores). This approach provided additional control against overfitting the data and aided clinical interpretation of the final model. Finally, we conducted 5-fold cross validation with multiple repeats (total of 25 validation trials) using data that were not included in the development of the algorithms. To accomplish this task, we partitioned the data into four training sets as well as a fifth validation set. The algorithms were trained using each iteration of the folds, and then validated on the remaining data. As noted above, the accuracy from the ROC analyses, averaged across the validation trials, served as the final metric of model performance.
Additional analyses
Repeated measures univariate analyses of variance (RM-ANOVA) models examined change from the first to last assessment among key risk factors. These analyses compared change by neurocognitive group trajectory subclass (below vs above average) as well as the time by group interaction (subgroup x time). Classification accuracy (AUC) was also examined using logistic regression to serve as a benchmark comparison to the GBM models. Logistic regression is a traditional statistical method that utilizes the base principle of machine learning (i.e., iterative learning) to establish an algorithm to predict a binary outcome. Logistic regression is ideally suited for hypothesis confirmation (rather than discovery) because the algorithm is based on a limited number of pre-defined predictors [23]. To facilitate comparison to GBM, the logistic regression was conducted using a two-step procedure; the first step ranked the relative importance of the same input features as utilized in the GBM models, and the second step determined classification accuracy using 6 features with highest coefficient rankings from the first step.
Results
Baseline GBM analysis
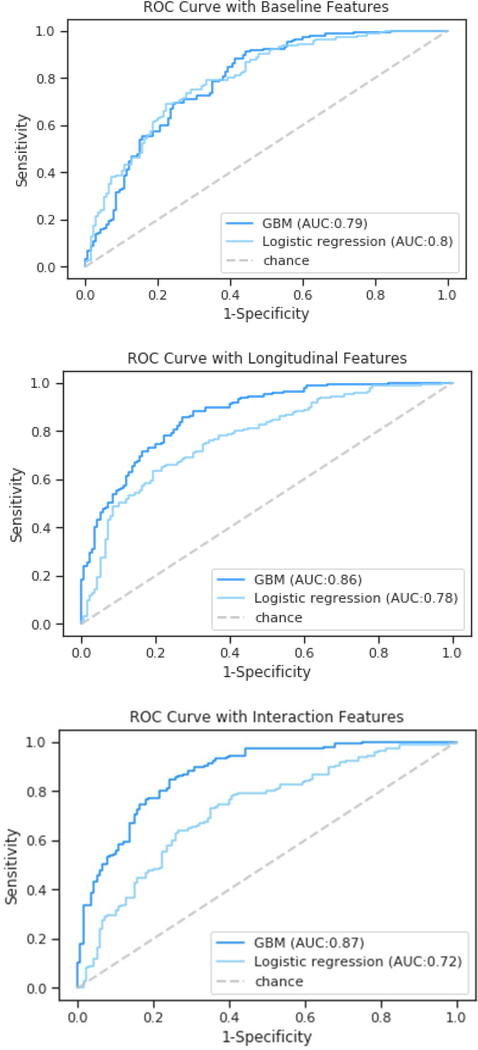
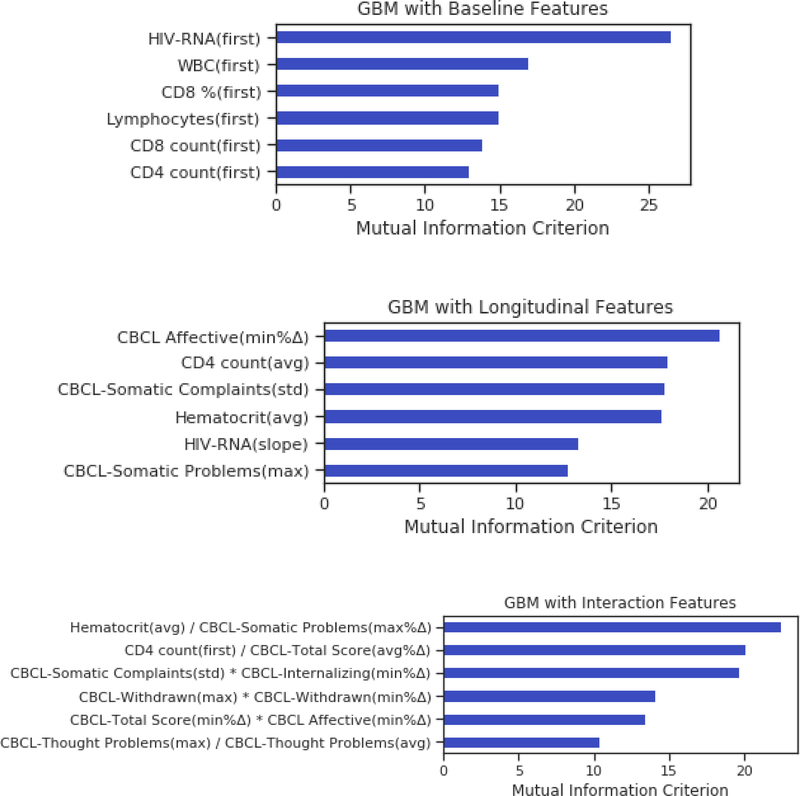
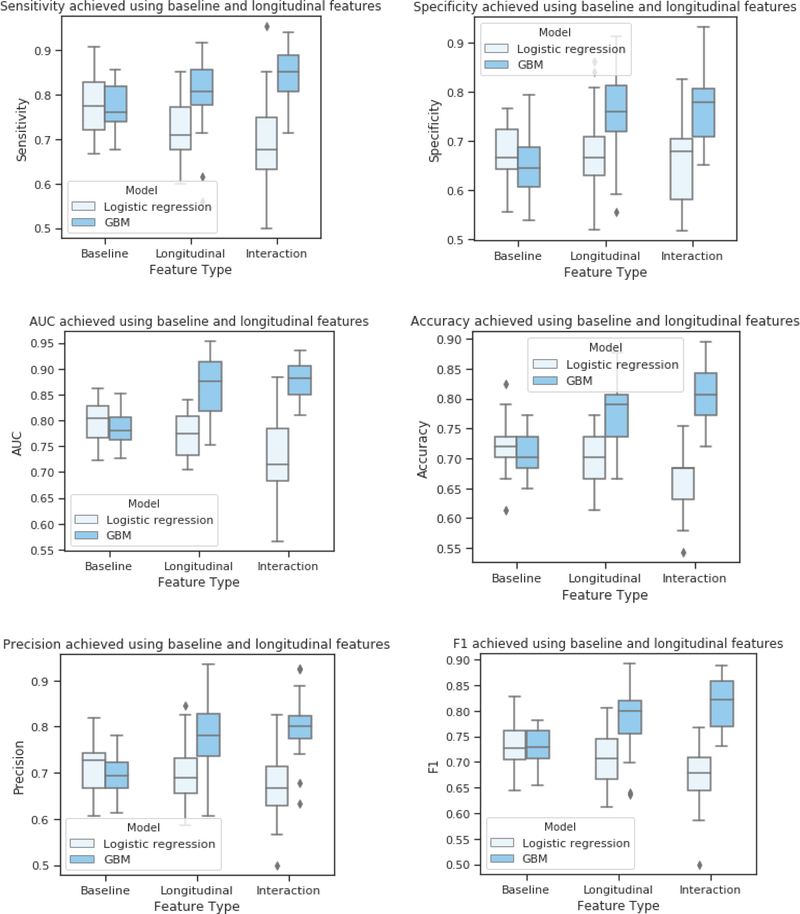
Results from the baseline GBM are depicted in Figure 1. The algorithm classified children with pHIV according to neurocognitive trajectory designation (above average/below average) with 71% accuracy (AUC of 79%, sensitivity of 76%, specificity of 66%, and F1 score of 72%). Top predictor variables of the baseline GBM included HIV disease (higher viral load) and immune markers (higher CD8% and CD8 count, and higher white blood cell and lymphocyte counts) (Fig. 2).
Fig. 1.
Receiver Operator Curves comparing average AUC for the baseline, longitudinal and interactive GBM and logistic regression analyses.
Fig. 2.
Feature importance ranking for the baseline, longitudinal, and interactive GBM models. Baseline model (top panel): HIV-RNA (copies/mL), white blood cell count (WBC) CD8 T cell %, Lymphocytes, CD8 T cell count, and CD4 T cell count. Longitudinal model (middle panel): CBCL Affective score minimum percent change (min % Δ); CD4 T cell count average value (avg), CBCL Somatic Complaints standard deviation (std), hematocrit (avg), HIV RNA slope, and CBCL Somatic Problems score maximum (max). Interaction model (bottom panel): hematocrit (avg) x CBCL Somatic Problems score (max % Δ), CD4 count at baseline (first) x CBCL Total Score (avg % Δ), CBCL Somatic Complaints score (std) x CBCL Internalizing score (min % Δ), CBCL Withdrawn score (max) x CBCL Withdrawn score (min % Δ), CBCL Total Score (min % Δ) x CBCL Affective score (min % Δ), and CBCL Thought Problems score (max) x CBCL Thought Problems score (avg).
Longitudinal GBM without interactions
The addition of predictor data from the follow-up visits improved accuracy to 77%, with an average AUC of 87%, sensitivity of 78%, specificity of 76%, and F1 score of 77%. Key predictor variables of the longitudinal GBM included HIV disease (higher viral load), immune indices (lower average CD4 cell count), blood markers of physical health (hematocrit levels), and mental health indices (raw scores on the Affective Problems, Withdrawn, and Somatic Complaints scales).
Longitudinal GBM with 2-way interactions
The longitudinal analysis allowing for up to 2-way interactions yielded the best model performance, with an average accuracy of 80% (AUC of 90%, sensitivity of 83%, specificity of 78%, and F1 score of 81%). Predictors (Fig. 3) included interactions between blood markers of physical health (average hematocrit level) with mental health (Somatic Complaints scale), immune status (baseline CD4 count) with mental health (CBCL Total Score), and interactions between mental health indices representing severity and chronicity of problems reported on the CBCL (i.e., Thought Problems, Affective, Withdrawn, Internalizing, and Somatic Complaints scales) (Supp. Figs. 1–2).
Fig. 3.
Classification performance comparing baseline, longitudinal and interactive GBM to logistic regression.
Subgroup comparisons of predictor variables
At baseline, participants in the above average trajectory group had lower plasma viral load (p < .001), CD8 (p < .001), total lymphocyte (p = .04), and white blood cell counts (p <. 001); CD4% did not differ at baseline (p = .77) (Table 2). Children in the below average trajectory group had lower hematocrit (p < .001) and hemoglobin (p < .001) at baseline. They also weighed less (p < .001) and they were shorter (p < .001) than individuals in the above average trajectory group. BMI did not differ by group (p =.11) at the baseline visit. On the CBCL, raw scores were higher on each subscale and composite scale among youth with pHIV in the below average trajectory group (p < .05).
Table 2. Predictive classifiers of each neurocognitive trajectory subgroup at first and last assessment and tested over time.
Child Behavior Checklist Values (CBCL) values represent raw scores. Caregivers completed age-appropriate versions of the CBCL (age 2 to 5 years or age 6 years and over). Green highlight identifies significant differences in unadjusted analyses.
| First Assessment | Last Assessment | Repeated Measures Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| Predictor Variable | Above Average (n=145) | Below Average (n=140) | P | Above Average (n=145) | Below Average (n=140) | P | Time Main Effect: Λ, p-value | Group x Time Effect: Λ, p-value |
| HIV Disease Markers | ||||||||
| Viral load (/mL) / % detectable | 33,100 / 100% | 100,000 / 100% | <.001 | 34,100 / 26% | 11,230 /17% | .07 | .76, p<.001 | .96, p=.001 |
| Immune Markers | ||||||||
| CD8 cell count (cells/mm3) | 1,311 (607.17) | 1,974.90 (113.31) | <.001 | 1,071.54 (429.10) | 1,152.92 (464.83) | .13 | .73, p<.001 | .90, p<.001 |
| CD4 % | 20.01 (2.93) | 20.12 (2.94) | 0.77 | 27.47 (8.33) | 30.37 (7.10) | .002 | .47, p<.001 | .97, p=.005 |
| Lymphocytes (10^3/ul) | 6.26 (10.85) | 9.35 (13.72) | .04 | 3.48 (5.98) | 5.64 (10.65) | .04 | .93, p<.001 | .99, p=.49 |
| White blood cell count | 7.75 (2.52) | 10.33 (4.14) | <.001 | 6.84 (2.15) | 7.27 (2.10) | .09 | .76, p<.001 | .92, p<.001 |
| Blood Indices of Physical Health | ||||||||
| Hematocrit | 34.71 (2.61) | 33.11 (3.21) | <.001 | 36.89 (3.38) | 34.97 (3.37) | <.001 | .76, p<.001 | .99, p=.47 |
| Hemoglobin | 11.45 (1.00) | 10.86 (1.14) | <.001 | 12.49 (1.29) | 11.97 (1.25) | .001 | .56, p<.001 | .99, p=.64 |
| Mental Health Indices (CBCL Scales) | ||||||||
| CBCL Affective | 2.95 (2.32) | 2.27 (2.08) | .01 | 2.14 (3.61) | 1.82 (2.45) | .39 | .96, p=.002 | .99, p=.37 |
| CBCL Internalizing | 9.65 (6.26) | 6.75 (5.36) | <.001 | 6.72 (7.83) | 5.41 (6.17) | .12 | .91, p<.001 | .98, p=.06 |
| CBCL Somatic Problems | 2.52 (2.26) | 1.50 (1.69) | <.001 | 1.42 (2.12) | 1.05 (1.89) | .12 | .90, p<.001 | .98, p=.02 |
| CBCL Withdrawn | 2.79 (2.22) | 1.99 (1.78) | .01 | 2.06 (2.28) | 1.66 (1.85) | .11 | .96, p=.001 | .99, p=.19 |
| CBCL Thought Problems | 1.98 (2.16) | 1.38(2.00) | .02 | 1.60 (3.27) | .99 (2.10) | .06 | .98, p<.02 | 1.00, p=.99 |
| CBCL Total Score | 31.86 (19.25) | 27.61 (18.29) | .06 | 23.86 (23.98) | 20.31 (19.36) | .17 | .88, p<.001 | 1.00, p=.77 |
| Physical Development | ||||||||
| Weight (Kg) | 23.97 (7.62) | 19.06 (6.11) | <.001 | 39.72 (11.18) | 28.39 (10.42) | <.001 | .26, p<.001 | .84, p<.001 |
| Height (cm) | 123.45 (12.74) | 111.55 (10.62) | <.001 | 146.78 (12.65) | 130.64 (13.81) | <.001 | .16, p<.001 | .95, p<.001 |
| BMI (weight/height2)*10000 | 15.38 (2.02) | 15.03 (1.63) | .11 | 18.10 (3.15) | 16.13 (2.51) | <.001 | .51, p<.001 | .85, p<.001 |
When considered longitudinally, plasma viral load, CD8 count, and white blood cell count declined for both groups between the first and last study visit, with a trend for higher viral load in the below average trajectory group at follow-up (p = .07). The two groups did not differ on CD8 cell count (p = .13) or white blood cell count (p = .09) at the final visit. Total lymphocytes (p = .04), and CD4%, (p = .002) were higher in the below average trajectory group at follow-up. Children in the low trajectory group continued to have lower body weight (p < .001), were shorter (p < .001) and had a lower BMI (p < .001) at the final follow-up visit. CBCL raw scores decreased for both groups (p < .05) overall, with no group differences at the final visit. The pattern of results was unchanged in adjusted analyses. The comparison to logistic regression revealed higher accuracy using the GBM algorithms (p < .05; Fisher’s Exact test).
Conclusions
Study findings provide several novel contributions to the current understanding of long-term neurocognitive outcomes in children with pHIV. First, we demonstrate that HIV disease indices acquired using standard clinical measures distinguish children who are at risk for suboptimal neurocognitive development over a 5-year period. Second, we demonstrate significant gains in model accuracy using information acquired during structured follow-up visits compared to a single cross-sectional assessment before ART. The longitudinal GBM analyses identified novel features of suboptimal neurocognitive trajectories (i.e., emergence of mental health problems and elevations in a blood marker of anemia) that were not observed in the analysis of predictors from the baseline visit. Third, inclusion of 2-way interactions revealed relationships between mental health problems and laboratory measures of HIV disease, immune dysregulation, and laboratory indices of physical health. These findings have potential to guide the development of intervention strategies to maximize neurodevelopmental outcomes in youth exposed to HIV during critical stages of brain development.
Our predictive models were built using clinical measures that can be acquired in resource-limited settings. This is important because regions of the world that shoulder the highest prevalence of pHIV typically have the fewest resources (e.g., neuroimaging) to guide precision medicine initiatives. We further enhanced the clinical relevance of the present study by identifying risk factors using information from the baseline assessment (pre-ART). This mirrors the initial point of contact in a clinical environment, when clinicians are limited to a “snapshot” of information to predict long-term outcomes and help guide treatment decisions. Risk variables identified in the baseline GBM underscore the importance of early HIV disease (plasma viral load) and immune (CD8%, CD8 count, white blood cells, lymphocytes) indices, which collectively yielded an average AUC of nearly 80% using five-fold cross validation with multiple repeats.
Interestingly, baseline HIV viral load was significantly elevated in children in the below average trajectory group. Total lymphocyte count and CD8 T-cell count were also higher in these children. In adults with HIV, increased CD8 activity precipitates T-cell exhaustion, which corresponds to worse disease outcomes [47,48]. Whether children and adolescents with pHIV exhibit T-cell exhaustion in the context of suppressive ART is unknown. Ongoing efforts by our team include studies focused on the interplay between viremia in early childhood, initial immune response (particularly CD8 T-cell activation and exhaustion), and chemokine co-receptor tropism (i.e., X4 and CCR5 chemokine co-receptors). These variables were not available for analysis in the present study, but there is strong precedence from recent work in adults with acute HIV that early viral-host dynamics set the stage for mental health complications in a subset of individuals [49]. Our previous study demonstrating neurocognitive difficulties in young children with pHIV in PREDICT who subsequently met criteria as long-term non-progressors [11] is consistent with the importance of early life immunological dynamics on cognitive and mental health outcomes.
Results from the longitudinal GBM models (with and without interactions) revealed hematocrit as an important predictor of neurocognitive trajectory subgroup. Hematocrit represents the percentage of oxygen-rich red blood cells in the total volume of blood. Low hematocrit is suggestive of anemia and is common in youth with pHIV residing in resource limited environments, including Southeast Asia [1,7,50–52]. Levels of hematocrit, hemoglobin, and red blood cell count were lower in children in the below average trajectory group. Hematocrit level was not a top predictor in the baseline (pre-ART) model, but this variable emerged as a strong risk factor in the longitudinal analyses. Participants enrolled in the PREDICT trial were prescribed zidovudine as first-line ART, and this drug is known to cause anemia [53–55]. However, abnormal hematocrit levels may also reflect disease duration and/or other environmental factors unrelated to HIV and ART per se. Hoare et al. [56] reported that blood markers of anemia were strong correlates of brain white matter abnormalities in children with pHIV who were not taking zidovudine, arguing against an iatrogenic effect from this drug. In the present study, children in the below average trajectory group weighed less and were shorter at baseline (before ART) than their counterparts in the above average subgroup which further implicates variables other than drug regimen.
Accuracy of the GBMs improved when information from follow-up visits was included as predictor variables. More importantly, the longitudinal GBMs (with and without interactions) discovered features that were not observed in the baseline analysis. Specifically, mental health problems became prominent predictors of neurocognitive development as children aged, involving interactions between caregiver ratings of anxiousness, withdrawn behavior, and thought problems with HIV disease (i.e., viral load), immune (e.g., CD4, CD8) and physical health (i.e., hematocrit level) indices. The Affective and Withdrawn subscales of the CBCL include symptoms of depression, and a robust literature from studies of adults with HIV demonstrate poor treatment compliance among individuals with symptoms of depression [57–60]. The association between developmental trajectories and Thought Disorder is more complicated because the items that comprise this scale on the CBLC are heterogeneous (e.g., sleep problems, hearing voices, mind wandering) and they are more likely to be misinterpreted and endorsed by caregivers of children living in lower socioeconomic settings [61]. Item level analyses are needed to more fully understand the symptom profiles that correspond to divergent neurocognitive trajectories.
Additionally, our results reinforce the need for psychosocial interventions to address the mental health needs in children with pHIV [62–65]. Prior work conducted in Thailand by members of our team demonstrate significant gains in mental health of youth and their caregivers following a brief, theoretically-informed psychosocial intervention [66]. Other studies using cognitive training strategies demonstrate improved cognitive performance with evidence of task transfer [67,68]. Additional studies are needed using data-driven models to continue the development of precision health initiatives capable of delivering the most effective therapeutic strategy, or combination of strategies, tailored to individual needs/strengths. Complimentary application of data science and traditional statistics methods are essential to achieve this goal.
It is important to note that participants enrolled in the PREDICT trial represent a unique group of children with pHIV who survived at least the first year of life without ART. As such, the results may not generalize to all children with pHIV (e.g., individuals exposed to ART in utero) or to uninfected children who do not share HIV disease risk factors. Information related to environmental stressors (e.g., early life trauma, food insecurity, pollution) that have potential to impact neurodevelopment was not available for this study [6,69,70]. Future investigations utilizing independent cohorts are needed to determine whether these factors and others (e.g., hospital admissions, neuropsychiatric diagnosis) represent key explanatory variables.
In summary, our study demonstrates that common clinical measures can identify youth with pHIV who are at risk for long-term neurodevelopmental problems. Synergies between mental health indices, hematocrit levels from blood, and HIV disease dynamics merit additional investigation to delineate causal pathways. Further, our finding that viral suppression was more common, albeit modestly, in children with worse neurocognitive trajectories suggests that traditional HIV clinical metrics of disease severity (i.e., viral detection) are not reliable markers of developmental risk in youth with pHIV. Our results add to a growing body of evidence that early HIV disease mechanisms and mental health problems represent key risk variables among youth with pHIV. Adjunctive interventions are needed to support neurodevelopment in this vulnerable population.
Supplementary Material
Table 1.
Baseline demographics and clinical variables for all participants.
| Demographic Variables | |
| Sex | 42% male; 58% female |
| Age at enrollment (years) | 6.21 (2.8) |
| Ethnicity: Percent Thai / Cambodian | 60% / 40% |
| HIV Disease | |
| Plasma HIV viral load (copies/mL); median | 63,300 |
| Immune Markers: Mean (Standard Deviation) | |
| Plasma CD4 T cell count (cells/mm3) | 725.80 (390.35) |
| Plasma CD4 T cell percent | 20.06 (24.0) |
| Plasma CD8 T cell count (cells/mm3) | 1,637.30 (964.59) |
| Plasma CD8 T cell percent | 44.30(9.05) |
| Plasma CD4/CD8 ratio | 0.48 (0.14) |
| Blood Indices of Physical Health: Mean (Standard Deviation) | |
| Hematocrit (%) | 33.92 (3.02) |
| Neutrophils (10^3/ul) | 8.59 (13.39) |
| Monocytes (10^3/ul) | 1.54 (2.64) |
| Lymphocytes (10^3/ul) | 7.78 (12.42) |
| White blood cell count | 9.02 (3.65) |
| Red blood cell count (10^6/ul) | 4.66 (0.49) |
| Hemoglobin (pg/cell) | 11.15 (1.11) |
| Triglycerides (ml/dL) | 118.20 (62.5) |
| Mental Health Indices (CBCL Scales): Mean (Standard Deviation) | |
| Anxious | 2.98 (3.40) |
| Withdrawn | 2.39 (2.05) |
| Somatic Complaints | 2.85 (2.88) |
| Social Problem | 2.02 (2.07) |
| Thought Problems | 1.68 (2.10) |
| Attention Problems | 3.74 (2.94) |
| Rule-Breaking Problems | 2.46 (2.59) |
| Aggressive Behavior | 6.41 (4.62) |
| Internalizing | 8.11 (6.01) |
| Externalizing | 8.86 (6.60) |
| Total Score | 29.77 (18.87) |
Acknowledgement
We are grateful to the children and caregivers for their participation in this study. The PREDICT study was sponsored by the National Institute of Allergy and Infectious Disease (NIAID), Grant number U19 AI053741, Clinicaltrial.gov identification number NCT00234091. Antiretroviral drugs for PREDICT were provided by ViiV Healthcare (AZT, 3TC), Boehringer Ingelheim (NVP), Merck (EFV), Abbott (RTV) and Roche (NFV). The neurodevelopment work was supported by initial funds by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, and later funded by R01MH089722 (Valcour) and R01MH102151 (Ananworanich and Puthanakit). Finally, we are grateful for the scientific insights related to data analysis, interpretation, and conclusions provided by Dr. Pim Brouwers, Deputy Director, Division of AIDS Research, NIH-NIMH.
Sources of Funding: The PREDICT study was sponsored by the National Institute of Allergy and Infectious Disease (NIAID), Grant number U19 AI053741, Clinicaltrial.gov identification number NCT00234091. Antiretroviral drugs for PREDICT were provided by ViiV Healthcare (AZT, 3TC), Boehringer Ingelheim (NVP), Merck (EFV), Abbott (RTV) and Roche (NFV). The neurodevelopment work was supported by initial funds by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, and later funded by R01MH089722 (Valcour) and R01MH102151(Ananworanich and Puthanakit).
Footnotes
The PREDICT Study Group
HIV Netherlands Australia Thailand (HIV-NAT) Research Collaboration, Thai Red Cross AIDS Research Center, Bangkok, Thailand; Kiat Ruxrungtham, Jintanat Ananworanich, Thanyawee Puthanakit, Chitsanu Pancharoen, Torsak Bunupuradah, Stephen Kerr, Theshinee Chuenyam, Sasiwimol Ubolyam, Apicha Mahanontharit, Tulathip Suwanlerk, Jintana Intasan, Thidarat Jupimai, Primwichaya Intakan, Tawan Hirunyanulux, Praneet Pinklow, Kanchana Pruksakaew, Oratai Butterworth, Nitiya Chomchey, Chulalak Sriheara, Anuntaya Uanithirat, Sunate Posyauattanakul, Thipsiri Prungsin, Pitch Boonrak, Waraporn Sakornjun, Tanakorn Apornpong, Jiratchaya Sophonphan, Ormrudee Ritim, Nuchapong Noumtong, Noppong Hirunwadee, Chowalit Phadungphon, Wanchai Thongsee, Orathai Chaiya, Augchara Suwannawat, Threepol Sattong, Niti Wongthai,Kesdao Nantapisan, Umpaporn Methanggool, Narumon Suebsri, Taksin Panpuy, Chayapa Phasomsap, Boonjit Deeaium, Pattiya Jootakarn.
Bamrasnaradura Infectious Diseases Institute, Nonthaburi, Thailand; Jurai Wongsawat, Rujanee Sunthornkachit, Visal Moolasart, Natawan Siripongpreeda, Supeda Thongyen, Piyawadee Chathaisong, Vilaiwan Prommool, Duangmanee Suwannamass, Simakan Waradejwinyoo, Nareopak Boonyarittipat, Thaniya Chiewcharn, Sirirat Likanonsakul, Chatiya Athichathana, Boonchuay Eampokalap, Wattana Sanchiem.
Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand; Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand; Pope Kosalaraksa, Pagakrong Lumbiganon, Chulapan Engchanil, Piangjit Tharnprisan, Chanasda Sopharak,Viraphong Lulitanond, Samrit Khahmahpahte, Ratthanant Kaewmart, Prajuab Chaimanee, Mathurot Sala, Thaniita Udompanit,Ratchadaporn Wisai,Somjai Rattanamanee, Yingrit Chantarasuk, Sompong Sarvok, Yotsombat Changtrakun, Soontorn Kunhasura, Sudthanom Kamollert.
Queen Savang Vadhana Memorial Hospital, Chonburi, Thailand; Wicharn Luesomboon, Dr. Pairuch Eiamapichart, Dr. Tanate Jadwattanakul, Isara Limpetngam, Daovadee Naraporn, Pornpen Mathajittiphun, Chatchadha Sirimaskul, Woranun Klaihong, Pipat Sittisak, Tippawan Wongwian, Kansiri Charoenthammachoke, Pornchai Yodpo.
Nakornping Hospital, Chiang Mai, Thailand; Suparat Kanjanavanit, Maneerat Ananthanavanich, Penpak Sornchai, Thida Namwong, Duangrat Chutima, Suchitra Tangmankhongworakun, Pacharaporn Yingyong, Juree Kasinrerk, Montanee Raksasang, Pimporn Kongdong, Siripim Khampangkome, Suphanphilat Thong-Ngao, Sangwan Paengta, Kasinee Junsom, Ruttana Khuankaew M, Parichat Moolsombat, Duanpen Khuttiwung, Chanannat Chanrin.
Chiangrai Regional Hospital, ChiangRai, Thailand; Rawiwan Hansudewechakul, Yaowalak Jariyapongpaiboon, Chulapong Chanta, Areerat Khonponoi, Chaniporn Yodsuwan, Warunee Srisuk, Pojjavitt Ussawawuthipong, Yupawan Thaweesombat, Polawat Tongsuk, Chaiporn Kumluang, Ruengrit Jinasen, Noodchanee Maneerat, Kajorndej Surapanichadul, Pornpinit Donkaew.
Prapokklao Hospital, Chantaburi,Thailand; Chaiwat Ngampiyaskul, Naowarat Srisawat, Wanna Chamjamrat, Sayamol Wattanayothin, Pornphan Prasertphan, Tanyamon Wongcheeree, Pisut Greetanukroh, Chataporn Imubumroong, Pathanee Teirsonsern.
Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; Virat Sirisanthana, Linda Aurpibul, Pannee Visrutaratna, Siriporn Taphey, Tawalchaya Cholecharoentanan, Nongyow Wongnum, Chintana Khamrong, Rassamee Kaewvichit, Kittipong Rungroengthanakit.
Chiang Mai University: Kulvadee Thongpibul
Disclaimer: The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above, the U.S. Department of the Army or the U.S. Department of Defense, the National Institutes of Health, the Department of Health and Human Services, or the United States government, nor does mention of trade names, commercial products, or organizations imply endorsement by the Thai Red Cross AIDS Research Centre.
Conflicts of Interest: Dr. Jintanat Ananworanich received honoraria for participating in advisory meetings for ViiV Healthcare, Gilead, Merck, Roche and AbbVie. Dr. Victor Valcour received honoraria from ViiV Healthcare. No conflicts reported for the remaining authors.
References
- 1.Ezeamama AE, Kizza FN, Zalwango SK, Nkwata AK, Zhang M, Rivera ML, et al. Perinatal HIV Status and Executive Function During School-Age and Adolescence. Medicine (Baltimore) 2016; 95. doi: 10.1097/MD.0000000000003438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garvie PA, Brummel SS, Allison SM, Malee K, Mellins CA, Wilkins ML, et al. Roles of Medication Responsibility, Executive and Adaptive Functioning in Adherence for Children and Adolescents with Perinatally Acquired HIV. Pediatr Infect Dis J 2017; 36:751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunupuradah T, Puthanakit T, Kosalaraksa P, Kerr SJ, Kariminia A, Hansudewechakul R, et al. Poor quality of life among untreated Thai and Cambodian children without severe HIV symptoms. AIDS Care 2012; 24:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughton B, Cornell M, Grove D, Kidd M, Springer PE, Dobbels E, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS Lond Engl 2012; 26:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughton B, Cornell M, Kidd M, Springer PE, Dobbels EFM-T, Rensburg AJV, et al. Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy. J Int AIDS Soc 2018; 21:e25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debeaudrap P, Bodeau-Livinec F, Pasquier E, Germanaud D, Ndiang ST, Nlend AN, et al. Neurodevelopmental outcomes in HIV-infected and uninfected African children. AIDS 2018; 32:2749. [DOI] [PubMed] [Google Scholar]
- 7.Boivin MJ, Barlow-Mosha L, Chernoff MC, Laughton B, Zimmer B, Joyce C, et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS Lond Engl 2018; 32:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puthanakit T, Saphonn V, Ananworanich J, Kosalaraksa P, Hansudewechakul R, Vibol U, et al. Early versus deferred antiretroviral therapy for children older than 1 year infected with HIV (PREDICT): a multicentre, randomised, open-label trial. Lancet Infect Dis 2012; 12:933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puthanakit T, Ananworanich J, Vonthanak S, Kosalaraksa P, Hansudewechakul R, van der Lugt J, et al. Cognitive Function and Neurodevelopmental Outcomes in HIV-Infected Children Older than 1 Year of Age Randomized to Early Versus Deferred Antiretroviral Therapy: The PREDICT Neurodevelopmental Study. Pediatr Infect Dis J 2013; 32:501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014; 26:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul R, Apornpong T, Prasitsuebsai W, Puthanakit T, Saphonn V, Aurpibul L, et al. Cognition, Emotional Health, and Immunological Markers in Children With Long-Term Nonprogressive HIV. JAIDS J Acquir Immune Defic Syndr 2018; 77:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul R, Prasitsuebsai W, Jahanshad N, Puthanakit T, Thompson P, Aurpibul L, et al. Structural Neuroimaging and Neuropsychologic Signatures in Children With Vertically Acquired HIV. Pediatr Infect Dis J 2018; 37:662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ananworanich J, Kerr SJ, Jaimulwong T, Vibol U, Hansudewechakul R, Kosalaraksa P, et al. Soluble CD163 and monocyte populations in response to antiretroviral therapy and in relationship with neuropsychological testing among HIV-infected children. J Virus Erad; 1:196–202. [PMC free article] [PubMed] [Google Scholar]
- 14.Smith R, Chernoff M, Williams PL, Malee KM, Sirois PA, Kammerer B, et al. Impact of Human Immunodeficiency Virus Severity on Cognitive and Adaptive Functioning during Childhood and Adolescence. Pediatr Infect Dis J 2012; 31. doi: 10.1097/INF.0b013e318253844b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol 2006; 30:633–657. [DOI] [PubMed] [Google Scholar]
- 16.Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, et al. Neurocognitive and Motor Deficits in HIV-Infected Ugandan Children With High CD4 Cell Counts. Clin Infect Dis Off Publ Infect Dis Soc Am 2012; 54:1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellins CA, Brackis‐Cott E, Leu C-S, Elkington KS, Dolezal C, Wiznia A, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry 2009; 50:1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hochhauser CJ, Gaur S, Marone R, Lewis M. The impact of environmental risk factors on HIV-associated cognitive decline in children. AIDS Care 2008; 20:692–699. [DOI] [PubMed] [Google Scholar]
- 19.Coscia JM, Christensen BK, Henry RR, Wallston K, Radcliffe J, Rutstein R. Effects of Home Environment, Socioeconomic Status, and Health Status on Cognitive Functioning in Children With HIV-1 Infection. J Pediatr Psychol 2001; 26:321–329. [DOI] [PubMed] [Google Scholar]
- 20.Kandawasvika GQ, Kuona P, Chandiwana P, Masanganise M, Gumbo FZ, Mapingure MP, et al. The burden and predictors of cognitive impairment among 6- to 8-year-old children infected and uninfected with HIV from Harare, Zimbabwe: A cross-sectional study. Child Neuropsychol 2015; 21:106–120. [DOI] [PubMed] [Google Scholar]
- 21.Crowell CS, Huo Y, Tassiopoulos K, Malee KM, Yogev R, Hazra R, et al. Early Viral Suppression Improves Neurocognitive Outcomes in HIV-infected Children. AIDS Lond Engl 2015; 29:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malee K, Kerr S, Paul R, Puthanakit T, Thongpibul K, Kosalaraksa P, et al. Emotional and behavioral resilience among children with perinatally acquired HIV in Thailand and Cambodia. Aids 2019; 33. doi: 10.1097/QAD.0000000000002182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller PJ, Lubke GH, McArtor DB, Bergeman CS. Finding structure in data using multivariate tree boosting. Psychol Methods 2016; 21:583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg A, Tai K. Comparison of statistical and machine learning methods in modelling of data with multicollinearity. Int J Model Identif Control 2013; 18:295–312. [Google Scholar]
- 25.Lakhani P, Prater AB, Hutson RK, Andriole KP, Dreyer KJ, Morey J, et al. Machine Learning in Radiology: Applications Beyond Image Interpretation. J Am Coll Radiol 2018; 15:350–359. [DOI] [PubMed] [Google Scholar]
- 26.Singal AG, Mukherjee A, Elmunzer BJ, Higgins PD, Lok AS, Zhu J, et al. Machine Learning Algorithms Outperform Conventional Regression Models in Predicting Development of Hepatocellular Carcinoma. Am J Gastroenterol 2013; 108:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deo RC. Machine Learning in Medicine. Circulation 2015; 132:1920–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade BSC, Valcour VG, Wendelken-Riegelhaupt L, Esmaeili-Firidouni P, Joshi SH, Gutman BA, et al. Mapping abnormal subcortical brain morphometry in an elderly HIV + cohort. NeuroImage Clin 2015; 9:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Kwon D, Esmaeili‐Firidouni P, Pfefferbaum A, Sullivan EV, Javitz H, et al. Extracting patterns of morphometry distinguishing HIV associated neurodegeneration from mild cognitive impairment via group cardinality constrained classification. Hum Brain Mapp 2016; 37:4523–4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogishi M, Yotsuyanagi H. Prediction of HIV-associated neurocognitive disorder (HAND) from three genetic features of envelope gp120 glycoprotein. Retrovirology 2018; 15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papini S, Pisner D, Shumake J, Powers MB, Beevers CG, Rainey EE, et al. Ensemble machine learning prediction of posttraumatic stress disorder screening status after emergency room hospitalization. J Anxiety Disord 2018; 60:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Tjortjis C, Zeng X, Qiao H, Buchan I, Keane J. Comparing data mining methods with logistic regression in childhood obesity prediction. Inf Syst Front 2009; 11:449–460. [Google Scholar]
- 33.Feng J-Z, Wang Y, Peng J, Sun M- W, Zeng J, Jiang H. Comparison between logistic regression and machine learning algorithms on survival prediction of traumatic brain injuries. J Crit Care 2019; 54:110–116. [DOI] [PubMed] [Google Scholar]
- 34.Beery K, Buktenica N. Developmental test of Visual-Motor Integration. 4th edition, revised. New Jersey: Modern Curriculum Press: Parsippany; 1997. [Google Scholar]
- 35.Crotty K, Baron IS (2011) Beery Developmental Test of Visual-Motor Integration (VMI) In: Kreutzer JS, DeLuca J, Caplan B (eds) Encyclopedia of Clinical Neuropsychology. Springer, New York, NY [Google Scholar]
- 36.Kurdek LA, Sinclair RJ. Psychological, family, and peer predictors of academic outcomes in first- through fifth-grade children. J Educ Psychol 2000; 92:449–457. [Google Scholar]
- 37.Green RR, Bigler ED, Froehlich A, Prigge MBD, Travers BG, Cariello AN, et al. Beery VMI Performance in Autism Spectrum Disorder. Child Neuropsychol J Norm Abnorm Dev Child Adolesc 2016; 22:795–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sortor JM, Kulp and MT. Are the Results of the Beery-Buktenica Developmental Test of Visual-Motor Integration and Its Subtests Related to Achievement Test Scores? Optom Vis Sci 2003; 80:758. [DOI] [PubMed] [Google Scholar]
- 39.Barnhardt C, Borsting E, Deland P, Pham N, Vu T. Relationship Between Visual-Motor Integration and Spatial Organization of Written Language and Math. Optom Vis Sci 2005; 82:138. [DOI] [PubMed] [Google Scholar]
- 40.Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Aseba Burlington, VT:; 2001. [Google Scholar]
- 41.Achenbach TM, Becker A, Döpfner M, Heiervang E, Roessner V, Steinhausen H-C, et al. Multicultural assessment of child and adolescent psychopathology with ASEBA and SDQ instruments: research findings, applications, and future directions. J Child Psychol Psychiatry 2008; 49:251–275. [DOI] [PubMed] [Google Scholar]
- 42.Riedel BC, Daianu M, Ver GS, Mezher A, Salminen LE, Galstyan A, et al. Uncovering Biologically Coherent Peripheral Signatures of Health and Risk for Alzheimer’s Disease in the Aging Brain. Front Aging Neurosci 2018; 10:390–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viejo G, Cortier T, Peyrache A. Brain-state invariant thalamo-cortical coordination revealed by non-linear encoders. PLOS Comput Biol 2018; 14:e1006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones CM, Athanasiou T. Summary Receiver Operating Characteristic Curve Analysis Techniques in the Evaluation of Diagnostic Tests. Ann Thorac Surg 2005; 79:16–20. [DOI] [PubMed] [Google Scholar]
- 45.scikit-learn: machine learning in Python — scikit-learn 0.21.2 documentation. https://scikit-learn.org/stable/ (accessed 12 Jul2019).
- 46.Jiangchun L SauceCat/PDPbox.; 2019. https://github.com/SauceCat/PDPbox (accessed 5 Nov2019).
- 47.Fujita T, Burwitz BJ, Chew GM, Reed JS, Pathak R, Seger E, et al. Expansion of Dysfunctional Tim-3–Expressing Effector Memory CD8 + T Cells during Simian Immunodeficiency Virus Infection in Rhesus Macaques. J Immunol 2014; 193:5576–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J, et al. Exhaustion of Activated CD8 T Cells Predicts Disease Progression in Primary HIV-1 Infection. PLoS Pathog 2016; 12. doi: 10.1371/journal.ppat.1005661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellmuth J, Colby D, Valcour V, Suttichom D, Spudich S, Ananworanich J, et al. Depression and Anxiety are Common in Acute HIV Infection and Associate with Plasma Immune Activation. AIDS Behav 2017; 21:3238–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmbhatt H, Boivin M, Ssempijja V, Kagaayi J, Kigozi G, Serwadda D, et al. Impact of HIV and Atiretroviral Therapy on Neurocognitive Outcomes Among School Aged Children. J Acquir Immune Defic Syndr 1999 2017; 75:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosalaraksa P, Bunupuradah T, Vonthanak S, Wiangnon S, Hansudewechakul R, Vibol U, et al. Prevalence of Anemia and Underlying Iron Status in Naive Antiretroviral Therapy HIV-Infected Children with Moderate Immune Suppression. AIDS Res Hum Retroviruses 2012; 28:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bunupuradah T, Kariminia A, Chan K-C, Ramautarsing R, Huy BV, Han N, et al. Incidence and predictors of severe anemia in Asian HIV-infected children using first-line antiretroviral therapy. Int J Infect Dis 2013; 17:e806–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikunaiye NY, Denue BA, Aina BA, Aderemi-Williams R, Rawizza HE. Incidence of Anemia among HIV-Infected patients treated with zidovudine-containing antiretroviral therapy in Northeastern Nigeria. Ann Ib Postgrad Med 2018; 16:115–124.31217768 [Google Scholar]
- 54.Dash KR, Meher LK, Hui PK, Behera SK, Nayak SN. High Incidence of Zidovudine Induced Anaemia in HIV Infected Patients in Southern Odisha. Indian J Hematol Blood Transfus 2015; 31:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renner LA, Dicko F, Kouéta F, Malateste K, Gueye RD, Aka E, et al. Anaemia and zidovudine‐containing antiretroviral therapy in paediatric antiretroviral programmes in the IeDEA Paediatric West African Database to evaluate AIDS. J Int AIDS Soc 2013; 16. doi: 10.7448/IAS.16.1.18024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoare J, Fouche J-P, Phillips N, Joska JA, Donald KA, Thomas K, et al. Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. J Neurovirol 2015; 21:120–128. [DOI] [PubMed] [Google Scholar]
- 57.Malee KM, Tassiopoulos K, Huo Y, Siberry G, Williams PL, Hazra R, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care 2011; 23:1533–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerr SJ, Puthanakit T, Malee KM, Thongpibul K, Ly PS, Sophonphan J, et al. Increased risk of executive function and emotional-behavioural problems among virologically well-controlled perinatally HIV-infected adolescents in Thailand and Cambodia. J Acquir Immune Defic Syndr 1999 Published Online First: 4 July 2019. doi: 10.1097/QAI.0000000000002132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermetet-Lindsay KD, Correia KF, Williams PL, Smith R, Malee KM, Mellins CA, et al. Contributions of Disease Severity, Psychosocial Factors, and Cognition to Behavioral Functioning in US Youth Perinatally Exposed to HIV. AIDS Behav 2017; 21:2703–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mellins CA, Elkington KS, Leu C-S, Santamaria EK, Dolezal C, Wiznia A, et al. Prevalence and Change in Psychiatric Disorders Among Perinatally HIV-Infected and HIV-Exposed Youth. AIDS Care 2012; 24:953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Longeway K, Johnson S, Garwood M, Davis L. Diagnosing Childhood Thought Disorder: Do Parent Checklists Yield False Positives? [Paper] 108th Annual Conference of the American Psychological Association (APA) 4–8 August 2000. [Google Scholar]
- 62.Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS 2019; 33:1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elkington KS, Robbins RN, Bauermeister JA, Abrams EJ, McKay M, Mellins CA. Mental Health in Youth Infected with and Affected by HIV: The Role of Caregiver HIV. J Pediatr Psychol 2011; 36:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mellins CA, Brackis-Cott E, Dolezal C, Leu CS, Valentin C, Meyer-Bahlburg HFL. Mental Health of Early Adolescents from High-risk Neighborhoods: The Role of Maternal HIV and Other Contextual, Self-Regulation, and Family Factors. J Pediatr Psychol 2008; 33:1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc 2013; 16:18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKay M, Block M, Mellins C, Traube DE, Brackis-Cott E, Minott D, et al. Adapting a Family-Based HIV Prevention Program for HIV-Infected Preadolescents and Their Families: Youth, Families and Health Care Providers Coming Together to Address Complex Needs. Soc Work Ment Health 2007; 5:355–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boivin MJ, Busman RA, Parikh SM, Bangirana P, Page CF, Opoka RO, et al. A pilot study of the neuropsychological benefits of computerized cognitive rehabilitation in Ugandan children with HIV. Neuropsychology 2010; 24:667–673. [DOI] [PubMed] [Google Scholar]
- 68.Boivin MJ, Nakasujja N, Sikorskii A, Opoka RO, Giordani B. A Randomized Controlled Trial to Evaluate if Computerized Cognitive Rehabilitation Improves Neurocognition in Ugandan Children with HIV. AIDS Res Hum Retroviruses 2016; 32:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suter MK, Karr CJ, John-Stewart GC, Gómez LA, Moraa H, Nyatika D, et al. Implications of Combined Exposure to Household Air Pollution and HIV on Neurocognition in Children. Int J Environ Res Public Health 2018; 15. doi: 10.3390/ijerph15010163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spies G, Ahmed-Leitao F, Fennema-Notestine C, Cherner M, Seedat S. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J Neurovirol 2016; 22:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.