Abstract
Loading and testosterone may influence musculoskeletal recovery after spinal cord injury (SCI). Our objectives were to determine 1) the acute effects of bodyweight-supported treadmill training (TM) on hindlimb cancellous bone microstructure and muscle mass in adult rats after severe contusion SCI and 2) whether longer-term TM with adjuvant testosterone-enanthate (TE) delivers musculoskeletal benefit. In Study 1, TM (40-min/day, 5-days/week, beginning 1-week post-surgery) did not prevent SCI-induced hindlimb cancellous bone loss after 3-weeks. In Study 2, TM did not attenuate SCI-induced plantar flexor muscles atrophy nor improve locomotor recovery after 4-weeks. In our Main Study, SCI produced extensive distal femur and proximal tibia cancellous bone deficits, a deleterious slow-to-fast fiber-type transition in soleus, lower muscle fiber cross-sectional area (fCSA), impaired muscle force production, and levator ani/bulbocavernosus (LABC) muscle atrophy after 8-weeks. TE-alone (7.0-mg/week) suppressed bone resorption, attenuated cancellous bone loss, constrained the soleus fiber-type transition, and prevented LABC atrophy. In comparison, TE+TM concomitantly suppressed bone resorption and stimulated bone formation after SCI, produced near-complete cancellous bone preservation, prevented the soleus fiber-type transition, attenuated soleus fCSA atrophy, maintained soleus force production, and increased LABC mass. 75% of SCI+TE+TM animals recovered voluntary over-ground hindlimb stepping, while no SCI and only 20% of SCI+TE animals regained stepping ability. Positive associations between testosterone and locomotor function suggest that TE influenced locomotor recovery. In conclusion, short-term TM alone did not improve bone, muscle, or locomotor recovery in adult rats after severe SCI, while longer-term TE+TM provided more comprehensive musculoskeletal benefit than TE-alone.
Keywords: androgen, estradiol, exercise, physical rehabilitation, neuromuscular plasticity, osteoporosis, hypogonadism, regenerative rehabilitation
Resource Identifiers (RRID): AB_60395, AB_2556551, AB_1500896, AB_138404, AB_2235587, AB_2147165, AB_881987, AB_1157865, AB_1157897, AB_2099233, AB_330924
Graphical Abstract
In our rodent severe contusion SCI model, a regenerative rehabilitation strategy combining pharmacologic testosterone with bodyweight-supported treadmill training (TE+TM) produced more comprehensive muscle and bone recovery than TE alone. 75% of TE+TM animals also regained voluntary over-ground hindlimb stepping, while no SCI and only 20% of SCI+TE recovered this ability.
1. Introduction
Skeletal muscle atrophy and impaired muscle function are hallmarks of spinal cord injury (SCI) that are negatively associated with walking function (1) and that worsen risk for several metabolic co-morbidities (2). SCI also produces severe bone mineral density (BMD) deficits and deleterious alterations to skeletal microarchitecture in the unloaded limbs, which contribute to increased fracture risk (3). These musculoskeletal deficits are exacerbated by the unloading and/or paralysis that occurs secondary to the central nervous system (CNS) injury (4). Specialized activity-based physical rehabilitation regimens that focus on locomotor retraining [e.g., bodyweight-supported treadmill training (TM)] have shown promise in hastening muscular recovery in persons with incomplete SCI (5). Similarly, in animal models of mild or moderate severity incomplete SCI, quadrupedal bodyweight-supported TM training attenuates muscle atrophy, increases muscle force production (6–9), and promotes recovery of voluntary over-ground locomotion, in-part, by improving afferent neuromodulation of the central pattern generator, by preventing axonal degradation, and by normalizing the spinal reflex pathways that regulate spasticity and motoneuron excitability (10, 11). However, after more severe SCI, humans (12) and animal models (13) exhibit only minimal muscular improvement and negligible recovery of over-ground walking ability in response to bodyweight-supported TM training. Similarly, the effectiveness of TM training and of other reloading strategies in regenerating bone after SCI remains contentious (14), especially at the sites most prone to fracture (i.e., distal femur and proximal tibia) in this population (15). These results suggest that alternative regenerative rehabilitation strategies are needed to enhance musculoskeletal recovery after more severe SCI (16).
In addition to paralysis, several systemic hormonal irregularities occur secondary to SCI and may worsen the musculoskeletal deficits in this population (17). Low serum testosterone (i.e., hypogonadism) is one of these irregularities that is present in nearly all men with SCI during the first 12-months after injury (18), with hypogonadism persisting in roughly 50% of men with SCI throughout the life-span (19). In non-neurologically-impaired men, low serum testosterone is associated with reduced skeletal muscle mass, low muscle force output, and low BMD (20) and testosterone replacement therapy (TRT) has been shown to increase muscle mass and strength (21), and BMD (22). Similarly, serum testosterone has been positively associated with whole thigh muscle cross-sectional area (CSA) in persons with SCI (23), and a small clinical study and case series have reported that TRT increased lower-extremity fat-free mass (24) and knee extensor CSA (25), respectively, in men with motor-complete SCI. However, a recent open-label trial reported that low-dose TRT did not increase fat-free mass, lower-extremity muscle CSA, or neuromuscular electrical stimulation (NMES)-elicited muscle contractile properties in paralyzed men with motor-complete SCI, while these measures were improved in men undergoing lower-extremity NMES resistance training with TRT (26, 27). Given these findings, research is needed to determine the individual and combined effects of testosterone treatment and activity-based physical rehabilitation on bone and muscle preservation after SCI.
Animal models are commonly utilized to examine therapeutic strategies intended to improve bone and muscle recovery after SCI, with the musculoskeletal adaptations having been characterized in several preclinical models of varying injury severity (28). For example, after spinal cord transection, mammals exhibit continual sublesional paralysis due to the complete lack of descending spinal circuitry (29), which produces extensive soleus atrophy and a gradual slow-to-fast fiber transition that is characterized by fewer oxidative fibers and higher proportion of glycolytic fibers (28), and severe sublesional bone loss (30). However, spinal transection eliminates CNS input to the motor unit and to bone and does not reproduce the injury mechanism that occurs most frequently in persons with SCI, suggesting that pathophysiologic differences exist between the transection model and most humans with SCI (31). Alternatively, the rodent moderate contusion SCI model mimics the injury mechanism and pathophysiology occurring in persons with incomplete SCI. In this model, muscle atrophy peaks with 7–14 days of injury (8), but is transient, with muscle CSA, contractile properties, and fiber-type distribution reverting to control levels within several weeks of injury, apparently due to spontaneous recovery of voluntary hindlimb locomotion (8, 32). Furthermore, little to no detectable bone loss occurs in rodents in response to mild or moderate contusion SCI (33), likely because occasional voluntary hindlimb stepping is preserved in these models. In comparison, only a few studies have examined musculoskeletal adaptations occurring after severe contusion SCI (30, 33–36), a model that is indicative of the severely impaired incomplete SCI population and that is not typically associated with recovery of hindlimb bone or muscle parameters or the ability to perform hindlimb stepping (36). Our laboratory has developed a rodent severe contusion SCI model, that displays several characteristics of the severe incomplete SCI population, including the complete inability to support the hindlimbs in stance or to perform voluntary over-ground stepping for at least 3-months post-injury, extensive cancellous bone deficits at the distal femur and proximal tibia (36), and ~50% lower circulating testosterone than uninjured controls for upwards of 2-months post-injury (36–38). Using this model, we have reported a >50% reduction in soleus muscle fiber cross-sectional area (fCSA) occurs within 21-days of SCI, although, we did not observe definitive signs of the hallmark slow-oxidative to fast-glycolytic fiber-type transition at this relatively early post-injury time point (34). Furthermore, we have reported that testosterone-enanthate (TE) drug treatment attenuated cancellous bone loss and maintained mass of the sublesional androgen-sensitive non-weight bearing levator ani/bulbocavernosus (LABC) muscle complex in both young (38) and skeletally-mature rodents after severe SCI (34, 39), with supraphysiologic TE producing the most robust effects. However, in our previous studies, supraphysiologic TE did not prevent the soleus fCSA atrophy after severe SCI, perhaps because the soleus exhibits ~70% lower androgen receptor expression than the androgen-sensitive LABC (34) or because the TE treatment duration (21-days) was not sufficient to observe muscular improvements. Furthermore, we are unaware of any study that has examined the bone or muscle responses to TE treatment when administered adjuvant to bodyweight-supported TM training. Examining the skeletal response to this strategy remains important because some reports indicate that androgens constrain the osteogenic responsiveness to mechanical loading (40, 41), while others indicate androgens enhance osteoblast recruitment and matrix osteoblast production in bone explants undergoing cyclic strain (42, 43).
Our objectives were 1) to assess the acute effects of TM training on recovery of cancellous bone microstructure and muscle mass in rodents after severe contusion SCI, 2) to characterize the magnitude of muscle atrophy and impaired muscle force mechanics that persist after 8-weeks in our severe SCI model, and 3) to determine whether 8-week treatment with TE alone or TE adjuvant to TM improves bone and muscle recovery in our severe SCI model that exhibits both low testosterone and persistent disuse. We hypothesized that 1) TM would lessen cancellous bone loss and muscle loss after SCI, 2) TE would attenuate muscle atrophy and prevent SCI-induced cancellous bone deficits by suppressing bone resorption, and 3) TE+TM would produce more robust musculoskeletal benefits than TE alone.
2. Materials and Methods
2.1. Animal care
The experimental design involved three studies. For all, barrier-raised and specific pathogen-free 16-week-old Sprague-Dawley rats were obtained from Charles River (Wilmington, MA). Rats were housed individually in a temperature- and light-controlled room on a 12 h light:dark cycle, and were fed commercial rodent chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories Inc., Indianapolis, IN) and tap water ad libitum. All procedures conformed to the ILAR Guide to the Care and Use of Experimental Animals and were approved by the Institutional Care and Use Committee at Malcom Randall VAMC or the University of Florida.
2.2. Experimental Design
2.2.1. Preliminary Bone Experimental Design – Study 1
For our preliminary bone experiment, we acquired femurs and tibias from an experiment that evaluated recovery from severe (incomplete) SCI with and without TM training (35). Surgical and post-operative care details are published (35). Briefly, female rats were randomized to the following groups: 1) non-surgical Controls (n=8), 2) T9 laminectomy with severe contusion SCI (n=8), and 3) SCI+TM (n=4). Manually-assisted, bodyweight-supported TM training began 1-week post-SCI (40-min/day, 5-days/week) and was conducted in an identical manner to the protocol that we have previously shown improves hindlimb muscle fCSA and muscle force output in rats after moderate SCI (details below) (6, 7, 10, 11, 44). Animals were sacrificed at Day 21 and tissues were excised. The left femurs and tibias were weighed, measured, wrapped in salinated gauze, and stored at −20° C prior to microcomputed tomography (μCT).
2.2.2. Preliminary Muscle Experimental Design – Study 2
For our preliminary muscle study, we obtained plantar flexor muscles from a separate experiment that assessed the effects of bodyweight-supported TM training on recovery from severe (incomplete) SCI. Male rats were stratified based on body mass and randomized into the following groups: 1) T9 laminectomy (SHAM, n=11), 2) T9 laminectomy with severe contusion SCI (n=10), or 3) SCI+TM (n=11). TM training began 1-week post-SCI (40-min/day, 5-days/week) and was conducted in an identical manner to Preliminary Study 1. Open-field locomotion was assessed with the Basso-Beattie-Bresnahan (BBB) locomotor rating scale (45) at Day 7 and weekly thereafter by two blinded observers. Rats were euthanized at Day 28 via thoracotomy and exsanguination, while under isoflurane anesthesia. The soleus, gastrocnemius, and plantaris were excised and weighed in a blinded manner.
2.2.3. Main Experimental Design – Study 3
Our main experiment was designed to assess the effects of TE or TE+TM treatment on bone and muscle recovery after SCI. Male rats were stratified according to body mass and randomized into the following groups: 1) SHAM + vehicle (n=10), 2) T9 laminectomy with severe contusion SCI + vehicle (n=11), 3) SCI+TE (n=10), and 4) SCI+TE+TM (n=10). Animals received TE (7.0 mg/week, Savient Pharmaceutical, East Brunswick, NJ) or vehicle (0.1 ml/week sesame oil) weekly via i.m. injection, under brief isoflurane anesthesia. TM training began 1-week post-SCI (40-min/day, 5-days/week) and was performed identically to Preliminary Study 1 and 2 and our previous studies (6, 7, 10, 11, 44). Open-field locomotion was assessed at Days 7 and 14, and every other week thereafter, as described above. Blood was sampled monthly from the tail. Decloymycin and calcein were administered (15 mg/kg body mass, s.c.) at 10 and 3 days before euthanasia respectively, to fluorochrome label bone surfaces. Rats were euthanized at Day 56 as described above, blood was acquired by cardiac puncture, femurs and tibias were excised, weighed, and measured, and the left and right soleus and the LABC muscle complex were excised and weighed in a blinded manner. Femurs were wrapped in salinated gauze and stored at −20° C prior to μCT and bone mechanical testing. The left tibia was prepped for histomorphometry (described below). The left soleus was prepared for immediate assessment of isolated ex vivo force mechanics and subsequently preserved for histologic analysis. The remaining muscles were frozen in liquid nitrogen and stored at −80° C. A section of the spinal cord, including the lesion site, was excised and fixed as described below.
Bone outcomes were evaluated at the distal femur or proximal tibia because these skeletal sites display dramatic bone loss and are the most prone to fracture in persons with SCI (15). Muscle outcomes were evaluated in the soleus because it is mostly composed of type I fibers that exhibit significant atrophy (34) and a phenotypic slow-oxidative to fast-glycolytic fiber-type transition in response to disuse and because the rat soleus is recruited during locomotion and standing (6–9). The LABC was assessed because it is an androgen-sensitive sublesional non-weight bearing muscle (38).
We did not include female rodents in our main experiment because the purpose of this experiment was to assess the effects of TE+TM in an SCI model that displays both persistent disuse and a testosterone deficiency, which only occurs in males, and because testosterone is a male hormone that produces androgenizing effects in the doses provided in this study, indicating that this therapy is not clinically applicable to females. From a pragmatic standpoint, we also did not include a stand-alone TM group in our main study because findings from our two preliminary studies (discussed below) indicated that TM did not improve and, in some cases, worsened cancellous bone loss after severe SCI and because TM did not improve plantar flexor mass nor enhance locomotor recovery. However, it is important to note that our study design directly matched that of a recent clinical trial that evaluated whether TRT improved muscular outcomes in men with complete SCI, when administered alone or in combination with lower-extremity NMES resistance training (26, 27), providing rationale for our design.
2.3. Surgery and Post-surgical Care
Fully anesthetized rats received T9 laminectomy to expose the spinal cord. A contusion SCI was induced by applying 250-kilodyne force to the T9 segment of the spinal cord using the IH Impactor (Precision Systems and Instrumentation, Lexington, KY), per our methods (36–39). Rats received buprenorphine (0.05-mg/kg qd, sc) and ketoprofen (5.0-mg/kg qd, sc) to reduce inflammation and pain for 36–72 h, and ampicillin (100-mg/kg qd, sc) for 5-days. Throughout the experiment, rat cages were placed on heated water pads to ensure animal thermoneutrality. Post-operative care included daily examinations for signs of distress, weight loss, dehydration, fecal clearance, and bladder dysfunction. Bladders were manually expressed twice daily until spontaneous voiding returned. Ringer’s (sc) was provided post-surgery and when signs of dehydration were present. Apples, Jell-O with added protein/fat, and Froot Loops® were provided to assist with body mass recovery.
2.4. Verification of SCI Severity
Our lab has developed a standardized a priori approach to exclude animals that do not display functional characteristics consistent with 250-kilodyne severe SCI (36, 37). This approach is implemented at Day 7 post-surgery, to ensure that the injury severity is homogenous across all animals prior to initiating locomotor retraining. Specifically, we excluded all SCI animals that exhibited a BBB score >4 on Day 7, when averaging the hindlimbs, or a BBB score >7 for either the right or left hindlimbs, irrespective of the contralateral limb. With this approach, we exclude most animals that would regain the ability to perform over-ground weight-supported stepping (i.e., BBB ≥9) without any intervention because this degree of spontaneous locomotor recovery is not typically observed in adult male rodents receiving severe contusion SCI (36, 37).
2.5. Treadmill Training Protocol
Quadrupedal bodyweight-supported TM training was performed (40-min/day, 5-days/week), using our protocol (6–11, 44). Briefly, rats were suspended by a harness that provided 40% bodyweight support, while the trainer positioned the paralyzed hindlimbs into plantar stepping positions on a moving treadmill (3.5-m/min). On training day 1, rats were given 5-min to explore the treadmill and were then acclimated to TM training, which involved performing four 5-min bouts, with 5-min rest between bouts. On training day 2, rats performed two 10-min bouts per day, twice daily. On all remaining training days, rats completed two 20-min bouts/day, with 4–5 h rest between bouts, totaling 200-min/week of individualized training per animal. Treadmill speed progressively increased and bodyweight support was gradually reduced as rats acclimated to training (Table 1). Hindlimb manual assistance was provided during all sessions to ensure consistent plantar stepping.
Table 1.
Treadmill speed and percent bodyweight support for animals in our primary study that received spinal cord injury with testosterone-enanthate and bodyweight-supported treadmill training (SCI+TE+TM).
| Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | |
|---|---|---|---|---|---|---|---|---|
| Speed, m/min | N/A | 4.1 ± 0.4 | 4.8 ± 0.6 | 5.3 ± 0.2 | 5.8 ± 0.1 | 6.0 ± 0.2 | 6.1 ± 0.7 | 6.4 ± 0.7 |
| Bodyweight support, % | N/A | 39.2 ± 3.3 | 39.1 ± 2.3 | 36.1 ± 1.4 | 35.1 ± 0.8 | 33.9 ± 1.3 | 33.7 ± 1.6 | 31.9 ± 6.3 |
Values are means ± SD, n=8. N/A = Not applicable, training was not initiated until one-week post-surgery.
2.6. Drug Selection Rationale
The TE regimen we used is standard in our lab because 1) it elevates serum testosterone for approximately one-week post-injection (46), 2) it is sufficient to increase mass of the androgen-sensitive LABC muscle complex after orchiectomy (47) via actions involving the suppression of catabolic genes and the stimulation of anabolic gene expression (48), 3) it mimics findings from our clinical trial that confirmed higher-than-replacement TE treatment produced muscular improvements in ambulatory hypogonadal men (49), and 4) intramuscular TE administration is FDA-approved (22). Furthermore, we have previously reported that this TE regimen attenuated hindlimb cancellous bone loss and LABC muscle loss in both young and older male rodents after severe contusion SCI (34, 37, 39), while lower TE doses produced less robust effects (38).
2.7. Isolated Muscle Mechanics
The left soleus was excised, cleaned, and immersed in Ringer’s solution (25°C, pH=7.4, equilibrated 95% O2/5% CO2) for contractile experiments (n=8–11/group), which were performed by a single investigator blinded to the treatment groups. The soleus was then aligned vertically between a servomotor lever arm and a stainless steel fixed-post (Aurora Scientific, Inc., Ontario, CA). The muscle was stimulated by pulses transmitted between two platinum electrodes placed longitudinally on either side of the muscle. The muscle was set to the optimal length at which the twitch elicited from single stimuli, delivered once every 10-s, was maximal. Tetanic tension was the maximal tension elicited by a train of pulses at 120-Hz with a pulse duration of 800-ms. In isometric twitch, the time courses of tension development (i.e., the time from onset of stimulation to peak tension or TPT) and relaxation (i.e., the time of return to 50% of maximal twitch tension or ½RT) were measured. Typically, in culture, the TPT and ½RT of muscle that exhibits higher fast MHC expression are faster (i.e., of a shorter duration) than muscle that exhibits more slow MHC (50).
2.8. Muscle Histology
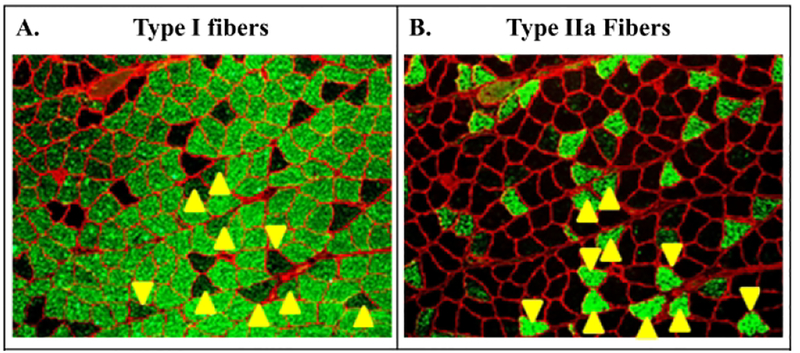
Following force mechanics, the left soleus was pinned at resting length on cork board, coated with Tissue-Tek® OCT (Sakura Finetek, Torrance, CA), frozen in liquid nitrogen-cooled 2-methylbutane, and stored at −80° C for histological analysis. Serial (10-μm) transverse sections of the soleus mid-belly were obtained on a CM1850 cryostat (Leica Biosystems, Buffalo Grove, IL) and mounted on gelatin-coated glass slides. Immunohistochemistry (n=6–8/group) was performed for determination of fCSA and fiber-type distribution, as we have described (34, 35), with the investigator blinded to the experimental groups. Antibodies used were: rabbit anti-laminin (1:200, #RB-082-A, RRID:AB_60395), rhodamine-conjugated goat anti-mouse IgG (1:500, #R-6394, RRID:AB_2556551), Alexa Fluor® 350 goat anti-mouse IgG (1:200, #A21140, RRID:AB_1500896), Alexa Fluor® 488 goat anti-mouse IgM (1:200, #A-11029, RRID:AB_138404) (Thermo Scientific, Waltham, MA); and anti-MHC I (#BA-D5, RRID:AB_2235587) and anti-MHC IIa (#SC-71, RRID:AB_2147165) [Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA]. Image acquisition was performed on a Nikon Eclipse TE2000-S microscope (Melville, NY) with fiber-type distribution and fCSA determined from >150 fibers per animal using Image J (NIH). Fibers staining exclusively with anti-MHC I or anti-MHC IIa were characterized as such. Serial fibers staining with both anti-MHC-I and anti-MHC IIa were considered dually-stained I/IIa hybrid fibers, as previously described (Figure 1) (34).
Figure 1.
Representative serial slices from soleus of an animal in our primary study that received spinal cord injury with testosterone-enanthate (SCI+TE). Samples were stained with (A) anti-MHC-I (green fibers) or (B) anti-MHC IIA (green fibers), as described in the methods. Yellow arrows indicate dually-stained I/IIa hybrid fibers that were assessed according to our methods (34).
2.9. Muscle Homogenization, SDS-PAGE, and Western Immunoblotting
Soleus muscle (25–30 mg) was homogenized (n=7–11/group) in a 1:10 w/v cocktail of RIPA buffer with protease and phosphatase inhibitors (Halt 100x, Pierce, Rockford, IL) using an automated bead homogenizer (FastPrep-24, MP Biomedicals, Santa Ana, CA). Homogenates were left on ice for 10-min and were then centrifuged at 4°C and 14,000 g for 15-min. Protein concentrations were determined from the supernatants using a microBCA kit (Pierce, Rockford, IL). 60-μg of protein was then mixed with 2x Laemmli buffer and 5% beta-mercaptoethanol, boiled for 5-min, and loaded into Criterion 4–15% gradient polyacrylamide gels (Bio-Rad, Hercules, CA). The gels were run for 60–90 min at 180-V, then transferred to a PVDF membrane using a semi-dry transfer of 20-V for 50-min to assess peroxisome proliferator activated receptor gamma co-activator 1 alpha (PGC-1α) and beta (PGC-1β) or were wet transferred for 2-h at 0.05-A on ice to assess myosin isoforms. Membranes were stained with Ponceau S, imaged using a CCD digital imager (AI600, Amersham, Madison, WI), de-stained with tris-buffered saline with 0.01%-Tween 20 (TBST), and blocked at room temperature for 60-min in 5% w/v bovine serum albumin (BSA)-TBST solution. Membranes were incubated overnight at 4°C with primary antibody in 1% BSA-TBST, as follows: 1:1000 for PGC-1α and PGC-1β (Abcam, #ab54481, RRID:AB_881987 and #ab176328) and 1:500 for the myosin isoforms (type I: #BA-D5, RRID:AB_2235587; type IIa: #2F7, RRID:AB_1157865; type IIx: #6H1, RRID:AB_1157897; DSHB, Iowa City, IA]. The next morning, membranes were washed 3×10-min in TBST and placed in anti-rabbit (#7074, RRID:AB_2099233) or anti-mouse (#7076, RRID:AB_330924) horseradish peroxidase (HRP)-linked secondary antibody (Cell Signaling) for 60-min at room temperature. After another 3×10-min TBST washes, membranes were incubated for 5-min at room temperature in HRP reactive chemiluminescence (ECL Prime, Amersham, Madison, WI), and imaged with an AI600 CCD imager. Densitometry was measured using ImageQuant 8.0 (GE Healthcare, Marlborough, MA) and blots were normalized to whole lane densitometry values using Ponceau S stain.
2.10. Bone Histomorphometry
The proximal tibial metaphysis was evaluated via histomorphometry, as previously described (36, 38). Briefly, tibiae were fixed in 10% phosphate-buffered formalin, dehydrated in ethanol, and embedded undecalcified in methyl methacrylate. 4-μm-thick proximal tibia sections underwent Von Kossa staining with a tetrachrome counterstain (Polysciences Inc., Warrington, PA) to assess cancellous bone structure. 8-μm-thick sections remained unstained to evaluate fluorochrome-based bone formation indices. The proximal tibia region of interest (ROI) began 0.5 mm distal to the growth plate and excluded the primary spongiosa and cancellous bone within 0.25 mm of the endocortical border. The Osteomeasure System (Osteometrics, Atlanta, GA) was used to measure: cancellous bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and the percentage of cancellous bone covered by osteoblasts (Ob.S/BS) and osteoclasts (Oc.S/BS). Fluorochrome-based bone formation indices were measured under ultraviolet illumination. Mineralizing surface (MS/BS), an index of active bone formation, was calculated as the percentage of cancellous bone surfaces with double fluorochrome labels. Mineral apposition rate (MAR), an index of osteoblast activity, was calculated by dividing the interlabel distance by the time interval between fluorochrome label administrations. Bone formation rate (BFR/BS) was calculated by multiplying MS/BS by MAR.
2.11. μCT Analysis of Bone Microstructure
The tibias and femurs were scanned with a Bruker Skyscan 1172 μCT (Kontich, Belgium) at: 80 kVP/120 μA, 0.5 mm Al filter, 2K camera resolution, 9.86 μm voxel, 180° tomographic rotation, and 0.7° rotation step. The cancellous ROIs encompassed 4.0 mm, starting 1.5 mm distal or proximal to the metaphyseal growth plate at the proximal tibia or distal femur, respectively. Cancellous outcomes were: BV/TV, Tb.N, Tb.Sp, Tb.Th, structure model index (SMI), and volumetric (v)BMD, which was determined after calibration with hydroxyapatite phantoms.
2.12. Bone Mechanical Testing
After μCT, the right femora underwent a medial-lateral 3-point bending test (47) and the left femora underwent an anterior-posterior cantilever test, as previously described (44, 51). Briefly, prior to testing, 10 cycles of sinusoidal preload (0 to 10 N) were applied to the distal anterior femur (cantilever test) or the medial femoral diaphysis (3-point bending test) with a flat steel fixture attached to a servohydraulic testing machine (MTS 858 Bionix Test System, MTX, Eden Prairie, MN). The bending loads were applied at 1.0 mm/s, until specimen failure. Maximal load (N), displacement at maximal load (mm), and energy to failure (N*mm) were determined from the load-deformation curves.
2.13. Serum Measurements
Blood was centrifuged at 3000 g, with serum aliquots stored at −80°C. Serum measurements (n=7–11/group) were performed in duplicate on a single plate. Testosterone and estradiol were determined by EIA with an intra-assay CVs <9% (ALPCO, Salem, NH). Free (unbound) brain derived neurotrophic factor (BDNF) was determined by ELISA with an intra-assay CV <6.2% (R&D Systems, Minneapolis, MN). Tartrate resistant acid phosphatase 5b (TRAP5b), a bone resorption marker, and procollagen type 1 N-terminal propeptide (P1NP), a bone formation marker, were determined by EIA with intra-assay CVs <7.4% (IDS, Fountain Hills, AZ).
2.14. Spinal Cord Histology
The T7-T11 segment of the spinal column encompassing the lesion site was excised from a representative subset of animals (n=3/group), post-fixed in 4% paraformaldehyde, trimmed to a length of ~5 mm, paraffin embedded, and the middle 3 mm of this segment that encompassed the lesion epicenter was microtome sectioned (10-μm thickness), and stained with hematoxylin and eosin for qualitative histological analysis by a blinded investigator. Light microscopic images were obtained under low- (5X) and high-magnification (40X) with a Zeiss Axio Imager Z2 microscope (Carl Zeiss, Gottingen, Germany) to assess injury severity by qualitatively evaluating the injured cavity, tissue debris, extent of myelination, characteristics of the remaining axons in the spared tissue, and the degree of collagen infiltration, as previously described (36, 38).
2.15. Statistical Analysis
Our main experiment was powered around the following variables: soleus muscle mass and peak tetanic force and distal femur cancellous BV/TV and Tb.N. A sample size of N=6–10/group was determined necessary to detect differences in these key variables at an α level of p<0.05 and 80% power. Other bone and muscle variables and hormone responses were evaluated to assist in proper data interpretation and to characterize our SCI model. All data points were independent measures, except in the case of variables that were assessed at multiple time points within the same animal (i.e., body mass and BBB). Mixed-Model Repeated Measures ANOVAs were used to analyze variables that were assessed at multiple time points. One-Way ANOVAs were used to analyze all other outcomes. Fisher’s LSD post hoc tests were performed for multiple comparisons among groups when appropriate. Pearson correlation coefficients were used to determine associations among select variables, with the Holm-Bonferonni correction used to control Type I error. Results are reported as means ± standard deviation (SD). Group main effects and interactions (when applicable) are reported, with exact p-values derived from post hoc analyses reported throughout. An α level of p<0.05 was defined as the threshold of significance. Statistical analyses were performed with SPSS v24.0.0 (IBM, Chicago, IL).
3. Results
3.1. Preliminary Bone Study
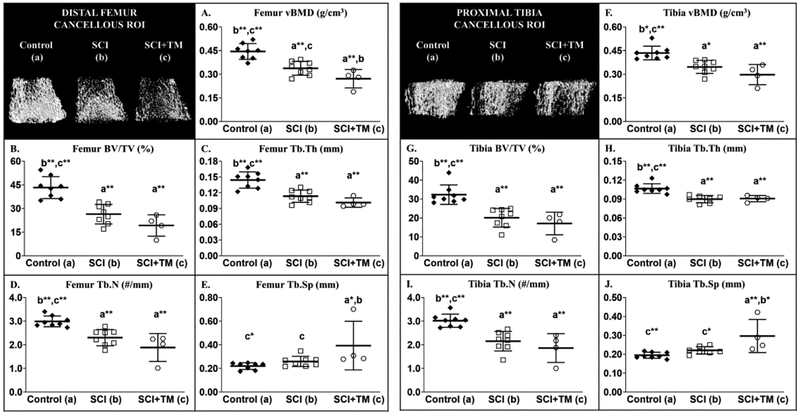
In our preliminary bone study, differences in distal femur and proximal tibia cancellous bone microstructural were present among groups for the following outcomes: vBMD [femur: F(2,19) = 18.819, p<0.001; tibia: F(2,19) = 13.423, p<0.001], BV/TV [femur: F(2,19) = 21.884, p<0.001; tibia: F(2,19) = 15.915, p<0.001], Tb.N [femur: F(2,19) = 14.317, p<0.001; tibia: F(2,19) = 13.933, p<0.001], Tb.Th [femur: F(2,19) = 18.392, p<0.001; tibia: F(2,19) = 14.727, p<0.001], and Tb.Sp [femur: F(2,19) = 4.759, p=0.023; tibia: F(2,19) = 8.208, p=0.004]. SCI displayed 20–30% lower cancellous vBMD at the distal femur (Figure 2 A–E) and proximal tibial metaphysis (Figure 2 F–J) vs Control, characterized by lower BV/TV, Tb.Th, and Tb.N (all p<0.001). SCI+TM exhibited 20% lower distal femur vBMD (p=0.042) and 50% higher Tb.Sp vs SCI (p=0.030), and the lowest cancellous values among groups at both skeletal sites (all outcomes: p=0.007 to p<0.001 vs Control). BBB scores were not available for the study, so we did not employ our a priori approach to exclude animals that did not display functional characteristics consistent with severe SCI.
Figure 2 A–J.
Microcomputed tomography (μCT)-derived cancellous bone outcomes from our preliminary bone study that involved non-surgical Control animals or those that received spinal cord injury (SCI) alone or in combination with bodyweight-supported treadmill training (SCI+TM). Images represent cancellous bone within each region of interest (ROI). Values are means ± SD, n=4–8/group, as described in methods. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs Control, b = vs SCI, c = vs SCI+TM). vBMD, volumetric bone mineral density; BV/TV, cancellous bone volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation.
3.2. Preliminary Muscle Study
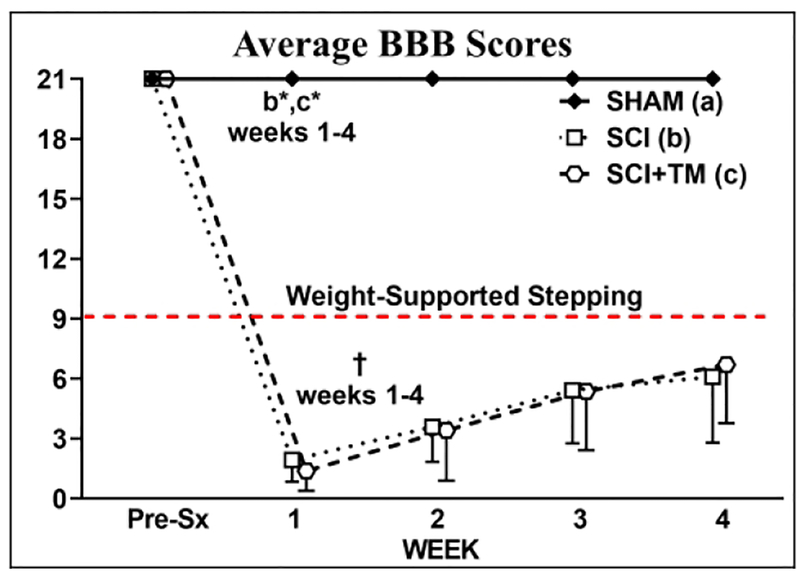
In our preliminary muscle study, four SCI and SCI+TM animals displayed BBB scores above our exclusionary values (average = 6.7 ± 1.1) at week 1 and were removed from the study before beginning TM training. All remaining animals displayed hindlimb locomotor deficits that were functionally consistent with severe SCI (Figure 3). A main effect for group [F(4,116) = 379.401, p<0.001] and a group × time interaction [F(8,116) = 96.751, p<0.001] were present for BBB scores. At week 1, all SCI animals displayed extensive hindlimb locomotor deficits (all SCI groups: p<0.01 vs baseline), while SHAM displayed normal locomotor behavior (Figure 3). From weeks 1–4, SCI and SCI+TM animals displayed a similar degree of spontaneous improvement in hindlimb motor function (weeks 3–4, p<0.01 vs week 1 for both), with most animals regaining slight-to-extensive movement in 2–3 hindlimb joints and demonstrating no ability to support the bodyweight in stance. BBB scores in SCI and SCI+TM both remained below SHAM at all time points (p<0.01) and no differences in BBB scores were present between SCI groups at any time point.
Figure 3.
BBB scores from animals in preliminary muscle study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with bodyweight-supported treadmill training (SCI+TM). Values are means ± SD, n=10–11 per group, as described in Table 2. Red dashed line indicates voluntary over-ground weight-supported plantar stepping. †Indicates different than pre-surgery (Pre-Sx). Letters indicate differences vs respectively labeled groups at p<0.05 or *p<0.01 (a = vs SHAM, b = vs SCI, c = vs SCI+TM). Note: No differences are present between SCI and SCI+TM groups at any timepoint.
For body mass, a main effect for group [F(4,116) = 57.070, p<0.001] and a group × time interaction [F(8,116) = 14.961, p<0.001] were present. Baseline body mass was similar among all groups (Table 2). SHAM body mass progressively increased throughout the experiment (data not shown), being 8% higher than baseline at week 4 (p<0.01, Table 2) and higher than both SCI groups at all post-surgical time points (p<0.01). In both SCI groups, body mass declined ~10% within the two-weeks post-surgery (p<0.01 vs pre-surgery) and increased thereafter.
Table 2.
Body mass and muscle mass from animals in preliminary muscle study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with bodyweight-supported treadmill training (SCI+TM).
| SHAM (a) N=11 | SCI (b) N=10 | SCI+TM (c) N=11 | |
|---|---|---|---|
| BODY MASS | |||
| Pre-surgery, g | 509 ± 24 | 523 ± 19 | 520 ± 25 |
| Δ at sacrifice, g | 43 ± 17 †,b*,c* | −29 ± 25 †,a* | −11 ± 31 a* |
| TRICEPS SURAE MASS AT SACRIFICE | |||
| Absolute, g | 3.43 ± 0.19 b**,c** | 2.63 ± 0.44 a** | 2.82 ± 0.34 a** |
| Relative, % body mass | 0.62 ± 0.04 b**,c* | 0.53 ± 0.07 a** | 0.55 ± 0.05 a* |
| SOLEUS MASS AT SACRIFICE | |||
| Absolute, g | 0.24 ± 0.03 b**,c** | 0.14 ± 0.03 a** | 0.15 ± 0.03 a** |
| Relative, % body mass | 0.044 ± 0.006 b**,c** | 0.028 ± 0.006 a** | 0.030 ± 0.006 a** |
| GASTROCNEMIUS MASS AT SACRIFICE | |||
| Absolute, g | 2.67 ± 0.16 b **,c** | 2.09 ± 0.34 a** | 2.23 ± 0.27 a** |
| Relative, % body mass | 0.485 ± 0.028 b**,c* | 0.422 ± 0.053 a** | 0.438 ± 0.042 a* |
| PLANTARIS MASS AT SACRIFICE | |||
| Absolute, g | 0.52 ± 0.03 b*,c* | 0.40 ± 0.08 a* | 0.44 ± 0.06 a* |
| Relative, % body mass | 0.094 ± 0.006 b* | 0.081 ± 0.013 a* | 0.086 ± 0.011 |
Values are means ± SD. †Indicates different than pre-surgery. Letters indicate differences vs respectively labeled groups at p<0.05, *p<0.01, or **p<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TM).
Differences among groups were present for absolute and relative muscle mass of the triceps surae [absolute: F(2,31) = 16.368, p<0.001; relative: F(2,31) = 8.349, p=0.001], soleus [absolute: F(2,31) = 36.822, p<0.001; relative: F(2,31) = 24.412, p<0.001], gastrocnemius [absolute: F(2,31) = 13.746, p<0.001; relative: F(2,31) = 6.372, p=0.005], and plantaris [F(2,31) = 10.709, p<0.001; F(2,31) = 4.459, p=0.020]. SCI and SCI+TM displayed 35–45% lower absolute soleus mass and 30–40% lower relative soleus mass vs SHAM (all p<0.001), along with deficits in triceps surae, gastrocnemius, and plantaris masses when compared with SHAM (Table 2). No differences in muscle mass was present between SCI groups.
3.3. Main Bone and Muscle Experiment
3.3.1. Post-Surgical Recovery
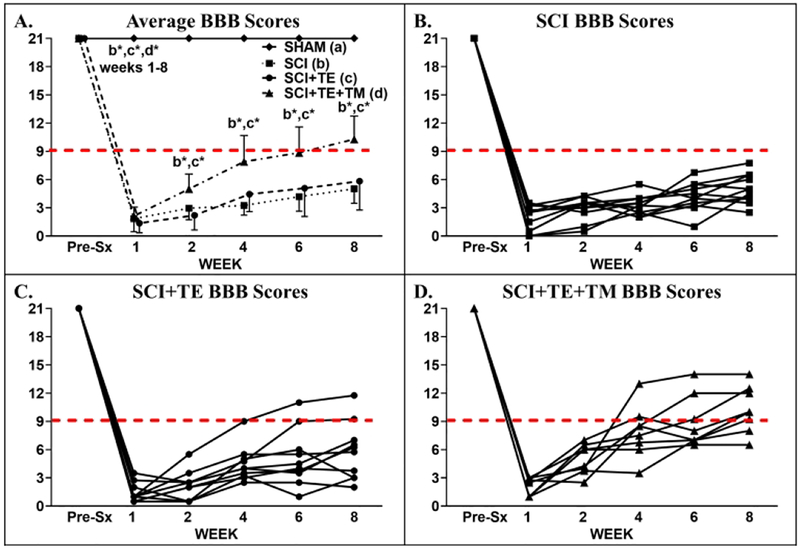
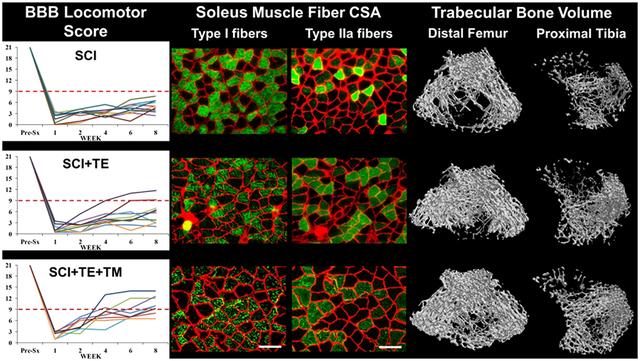
In our main study, no animals met our a priori criteria for exclusion. A main effect for time [F(5,175) = 677.885, p<0.001] and a group × time interaction [F(15,175) = 84.831, p<0.001] were present for BBB scores. At week 1, all SCI animals displayed extensive hindlimb locomotor deficits that were consistent with a severe T9 SCI (Figure 4 A–D), while SHAM displayed normal locomotor behavior. From weeks 1–8, SCI and SCI+TE animals exhibited an expected minimal degree of hindlimb locomotor improvement (weeks 2–8, p<0.01 vs week 1 for both). In comparison, SCI+TE+TM exhibited more pronounced recovery of voluntary locomotor function vs SCI and SCI+TE (p<0.01 vs both at weeks 2–8). Ultimately, 6 of 8 SCI+TE+TM animals regained the ability to perform voluntary weight-supported plantar placement and/or stepping (BBB = 9), while 0 of 11 SCI and only 2 of 10 SCI+TE animals achieved this level of locomotor recovery (Figure 4 B–D). BBB scores in all SCI groups remained below SHAM at all post-surgical time points (p<0.01).
Figure 4 A-D.
BBB scores from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=8–11 per group, as described in Table 3. Horizontal dashed line indicates voluntary over-ground weight-supported plantar stepping (i.e., BBB ≥9). †Indicates different than pre-surgery (Pre-Sx). Letters indicate differences vs respectively labeled groups at p<0.05 or *p<0.01 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM). Individual symbols and lines (B–D) represent individual animal values; SHAM values are not shown because all animals exhibited BBB=21 throughout. Note 0/11 SCI, 2/10 SCI+TE, and 6/8 SCI+TE+TM animals exhibited BBB ≥9 at 8-weeks post-surgery.
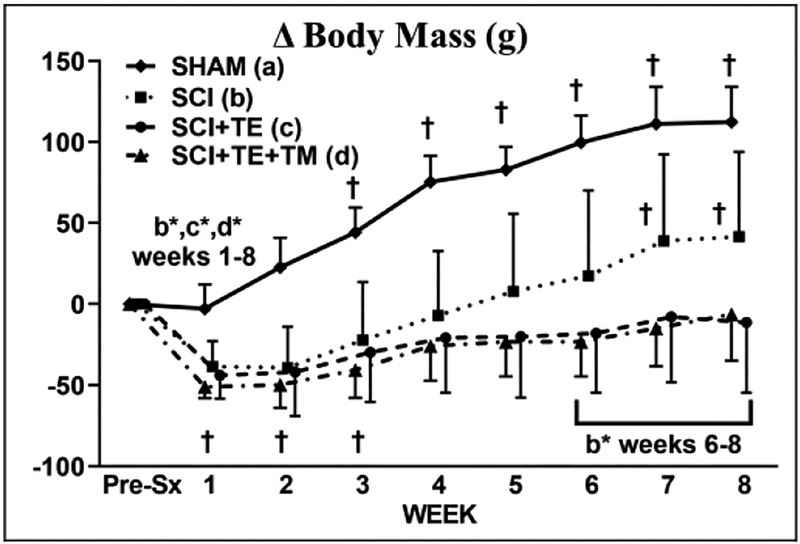
For body mass, a main effect for time [F(8,280) = 71.595, p<0.001] and group × time interaction [F(24,280) = 12.659, p<0.001] were present. Baseline body mass was similar among groups (Table 3). SHAM animals exhibited a progressive increase in body mass, with values being higher than baseline from post-surgery weeks 3–8 (p<0.01) and higher than all SCI groups throughout (p<0.01) (Figure 5). All SCI groups exhibited an initial ~10% body mass loss within two-weeks of surgery (weeks 1–2 p<0.01 vs baseline) and gradual body mass gain thereafter. At sacrifice, body mass in SCI animals was 9% higher than baseline (p<0.01), while SCI+TE and SCI+TE+TM did not exceed baseline (both p<0.01 vs SCI).
Table 3.
Body mass and soleus and levator ani/bulbocavernosus (LABC) muscle mass from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM).
| SHAM (a) N=10 | SCI (b) N=11 | SCI+TE (c) N=10 | SCI+TE+TM (d) N=8 | |
|---|---|---|---|---|
| BODY MASS | ||||
| Pre-surgery, g | 496 ± 30 | 486 ± 25 | 492 ± 28 | 472 ± 16 |
| Δ at sacrifice, g | 112 ± 22 †,b*,c*,d* | 42 ± 52 †,a*,c*,d* | −11 ± 43 a*,b* | −6 ± 29 a*,b* |
| SOLEUS MASS AT SACRIFICE | ||||
| Absolute, g | 0.24 ± 0.01 b**,c**,d | 0.14 ± 0.02 a**,d* | 0.15 ± 0.03 a**,d | 0.20 ± 0.03 a,b*,c |
| Relative, % body mass | 0.041 ± 0.002 b**,c** | 0.028 ± 0.003 a**,d** | 0.032 ± 0.005 a**,d** | 0.043 ± 0.007 b**,c** |
| LABC MASS AT SACRIFICE | ||||
| Absolute, g | 1.37 ± 0.06 b,d** | 1.19 ± 0.04 a,c*,d** | 1.43 ± 0.08 b*,d** | 1.79 ± 0.06 a**,b**,c** |
| Relative, % body mass | 0.227 ± 0.036 c**,d** | 0.223 ± 0.021 c**,d** | 0.311 ± 0.027 a**,b**,d** | 0.387 ± 0.030 a**,b**,c** |
Values are means ± SD. †Indicates different than pre-surgery. Letters indicate differences vs respectively labeled groups at p<0.05, *p<0.01, or **p<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
Figure 5.
Body mass change from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=8–11 per group, as described in Table 3. †Indicates different than pre-surgery (week 0). Letters indicate differences vs respectively labeled groups at p<0.05 or *p<0.01 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
3.3.2. Muscle Mass
Differences in absolute soleus mass [F(3,35) = 29.531, p<0.001] and relative soleus mass [F(3,35) = 22.016, p<0.001] were present among groups. Both absolute and relative soleus mass were 30–40% lower in SCI vs SHAM (p<0.001, Table 3). TE did not prevent these reductions (p<0.001 vs SHAM). In comparison, TE+TM attenuated SCI-induced soleus muscle loss, evidenced by 20–35% higher absolute mass and 35–55% higher relative mass vs SCI and SCI+TE (all p<0.001). Ultimately, SCI+TE+TM displayed no difference in relative soleus mass vs SHAM. Across all SCI groups, absolute and relative soleus mass were positively associated with serum testosterone (r=0.453 – 0.508, p=0.011 – 0.026) and with week 8 BBB score (r=0.747 – 0.837, both p<0.001).
Differences among groups were observed for absolute LABC mass [F(3,38) = 15.618, p<0.001] and relative LABC mass [F(3,38) = 64.260, p<0.001]. Absolute LABC mass was 13% lower in SCI vs SHAM (p=0.033, Table 3), with no difference in relative mass. TE completely prevented SCI-induced LABC muscle loss (p=0.006 vs SCI) and produced a higher relative mass vs SHAM and SCI (both p<0.001). In comparison, absolute and relative LABC mass were higher in SCI+TE+TM vs all other groups (p<0.001). Across all SCI groups, absolute and relative LABC mass were positively associated with serum testosterone (r=0.710 – 0.755, both p<0.001) and with week 8 BBB score (r=0.550 – 0.628, p=0.002 to p<0.001).
3.3.3. Soleus Muscle Histology
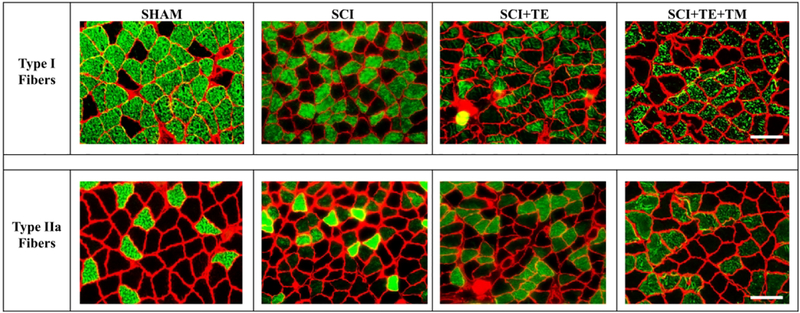
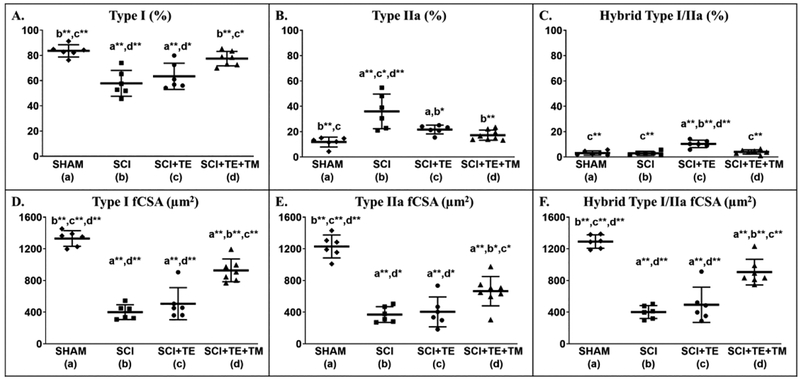
Representative histologic images of type I and IIa fibers from soleus muscle of each group are presented in Figure 6. Differences among groups were present for the percentages of type I fibers [F(3,24) = 13.312, p<0.001], type IIa fibers [F(3,24) = 12.242, p<0.001], and I/IIa hybrid fibers [F(3,24) = 18.271, p<0.001]. SCI produced an expected slow-to-fast fiber-type transition in the soleus, exemplified by a lower proportion of type I fibers (p<0.001) and higher proportion of type IIa fibers vs SHAM (p<0.001, Figure 7 A–B). TE partially prevented this fiber-type transition, as indicated by a lower proportion of type IIa fibers (p=0.003) and a higher proportion of dually-stained I/IIa hybrid fibers vs SCI (p<0.001, Figure 7 C). TE+TM completely prevented the slow-to-fast fiber transition and maintained all fiber proportions similar to SHAM, evidenced by a higher proportion of type I fibers vs SCI (p<0.001) and SCI+TE (p=0.005), by a lower proportion of type IIa fibers vs SCI (p<0.001), and by a lower proportion of type I/IIa hybrid fibers vs SCI+TE (p<0.001). A similar proportion of soleus fibers (1–5%) remained unstained in all groups, presumably representing type IIx or IIb fibers (data not shown). Across all SCI groups, week 8 BBB score was associated with the percentage of type I fibers (r=0.609, p=0.006), while testosterone (r= −0.536, p=0.022) and BBB (r= −0.533, p=0.016) were each negatively associated with the percentage of type IIa fibers.
Figure 6.
Histological images of soleus type I (top row) and type IIa (bottom row) fiber-type staining from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Samples were stained with anti-MHC-I (green fibers – top row) or anti-MHC IIA (green fibers – bottom row), as described in the methods and according to our published methods (34). Scale bar = 50 μm.
Figure 7 A–F.
Soleus type I, IIa, and hybrid I/IIa muscle fiber proportions and cross-sectional area (fCSA) from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=6–8/group. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
Differences among groups were noted in fCSA for type I [F(3,24) = 54.105, p<0.001], type II [F(3,24) = 36.300, p<0.001], and type I/IIa hybrid fibers [F(3,24) = 44.805, p<0.001]. SCI exhibited 70% lower soleus fCSA for all fiber types vs SHAM (p<0.001, Figure 7 D–F). TE did not prevent these reductions, with fCSA remaining 60–65% lower than SHAM (p<0.001). TE+TM attenuated fCSA loss, evidenced by higher type I (p<0.001 vs SCI and SCI+TE), IIa (p<0.001 vs SCI; p=0.003 vs SCI+TE), and hybrid I/IIa fCSA (p<0.001 vs SCI and SCI+TE). However, fCSA remained 30–45% lower in SCI+TE+TM vs SHAM for all fiber-types examined (p<0.001). Across all SCI groups, BBB score at week 8 was associated with type I, IIa, and hybrid I/IIa fCSA (r=0.749 – 0.840, all p<0.001), with no significant correlations present among testosterone and fCSA.
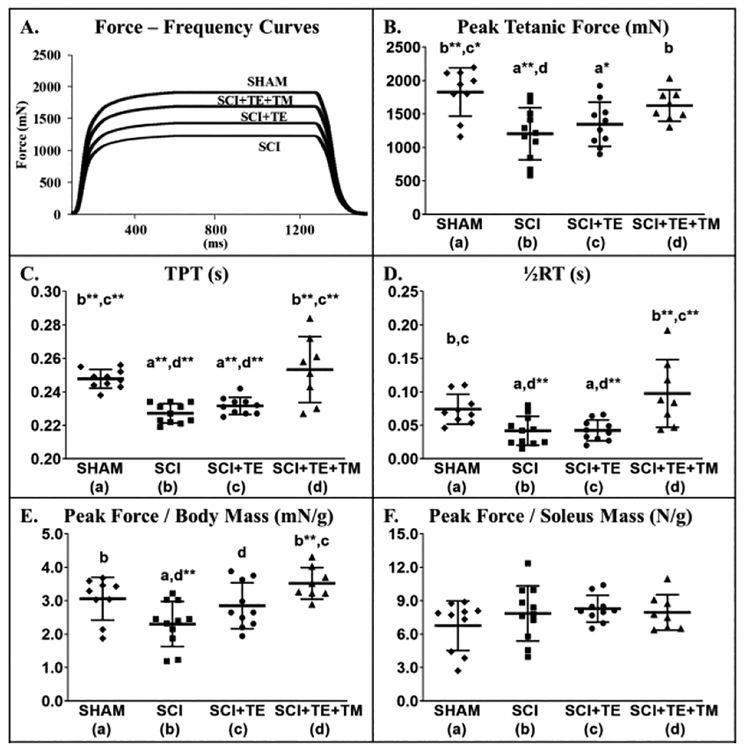
3.3.4. Isolated Soleus Force Mechanics
Representative soleus force mechanics tracings are presented in Figure 8 A. Differences in peak tetanic force [F(3,37) = 6.580, p=0.001], peak force:body mass ratio [F(3,37) = 6.012, p=0.002], TPT [F(3,37) = 14.628, p<0.001], and ½RT [F(3,37) = 7.810, p<0.001] were observed between groups. SCI resulted in 35% lower peak force vs SHAM (p<0.001, Figure 8 B) and produced changes consistent with a slow-to-fast fiber-type transition, including 8% faster TPT (p<0.001) and 45% faster ½RT (p=0.019) (Figure 8 C–D). TE alone did not prevent these changes. In comparison, TE+TM produced 35% higher peak force vs SCI (p=0.011) and preserved TPT and ½RT at SHAM levels (p<0.001 vs SCI and SCI+TE). Soleus peak force:body mass ratio was lower in SCI vs SHAM (p=0.012, Figure 8 E) and TE attenuated this reduction (trend, p = 0.057 vs SCI). TE+TM preserved the force:body mass ratio similar to SHAM values (p<0.001 vs SCI; p=0.032 vs SCI+TE). No differences in peak force:soleus mass were present among groups [F(3,37) = 1.162, p=0.338, Figure 8 F). Across all SCI groups, BBB score at week 8 was associated with soleus peak force, peak force:body mass, TPT, and ½RT (r=0.646 – 0.749, all p<0.001), with no significant associations present for testosterone.
Figure 8 A–F.
Isolated soleus force mechanics from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=8–11/group, as described in Table 3. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM). TPT, time to peak tension; ½RT, half relaxation time.
3.3.5. Soleus Muscle Protein Expression
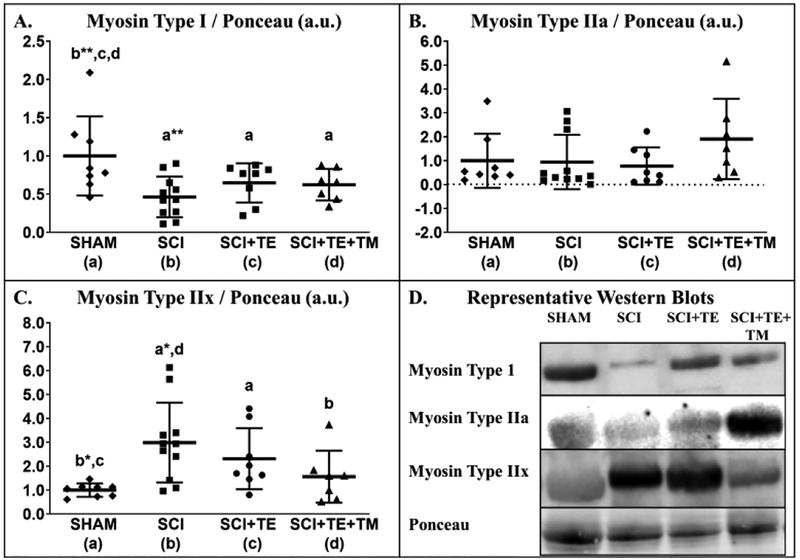
Differences in soleus myosin type I [F(3,33) = 4.148, p=0.014] and type IIx protein expression [F(3,33) = 4.423, p=0.011] were present among groups, while no difference was present for myosin type IIa expression [F(3,33) = 1.333, p=0.282]. Soleus myosin type I expression was 54% lower in SCI vs SHAM (p=0.001, Figure 9 A) and remained ~35% lower than SHAM in the TE (p=0.041) and TE+TM groups (p=0.037). Although, no differences in myosin IIa expression existed among groups, the relative value was ~100% higher in SCI+TE+TM vs all other groups (non-significant, Figure 9 B). SCI produced 3-fold higher soleus myosin IIx expression vs SHAM (p=0.002, Figure 9 C). TE partially prevented this effect, with myosin IIx remaining 130% higher than SHAM (p=0.044). In comparison, TE+TM maintained myosin IIx expression at SHAM levels (p=0.025 vs SCI).
Figure 9 A–D.
Relative protein expression of myosin (A) type I, (B) type IIa, and (C) type IIx, and (D) representative western blot images from soleus muscle of animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=7–11/group. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
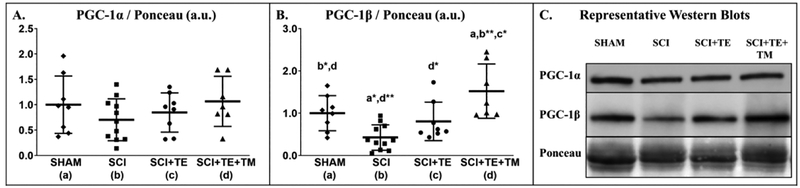
No differences in soleus PGC-1α protein expression were observed among groups [F(3,33) = 1.085, p=0.371], although, PGC-1α appeared 30% lower in SCI vs SHAM and near SHAM levels in SCI+TE+TM (both non-significant, Figure 10 A). In comparison, soleus PGC-1β expression was different among groups [F(3,33) = 8.786, p<0.001]. Specifically, PGC-1β expression was ~60% lower in SCI vs SHAM (p=0.010, Figure 10 B). TE attenuated this effect with PGC-1β expression remaining near SHAM levels, while, TE+TM increased PGC-1β expression vs all other groups, with relative values being 50–90% higher than SHAM (p=0.032) and SCI+TE (p=0.004) and >3-fold higher than SCI (p<0.001). Across all SCI groups, PGC-1β was associated with testosterone (r=0.654, p=0.001), BBB score at week 8 (r=0.585, p=0.005), and soleus TPT (r=0.528, p=0.006).
Figure 10 A–C.
Relative protein expression of (A) PGC-1α and (B) PGC-1β, and (C) representative western blot images from soleus muscle of animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=7–11/group. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
3.3.6. Histomorphometry-derived Analysis of Bone Turnover
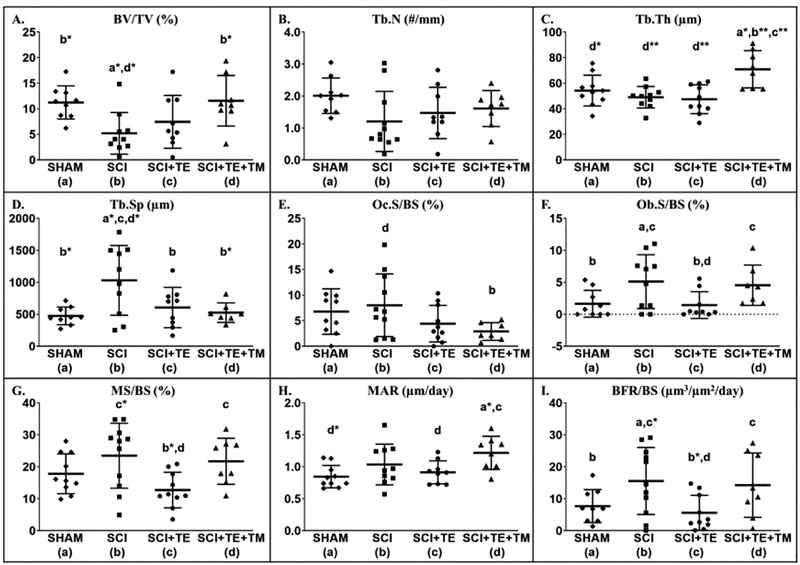
At the proximal tibial metaphysis, differences in histomorphometry-derived measurements of cancellous BV/TV [F(3,36) = 4.478, p=0.010], Tb.Th [F(3,36) = 7.272, p=0.001], Tb.Sp [F(3,36) = 5.014, p=0.006], Ob.S/BS [F(3, 36) = 3.527, p=0.026], MS/BS [F(3,36) = 3.973, p=0.015], MAR [F(3,36) = 3.997, p=0.016], and BFR/BS [F(3,36) = 3.466, p=0.027] were present among groups. BV/TV was 44% lower in SCI vs SHAM (p=0.005), characterized by 40% lower Tb.N (non-significant) and increased Tb.Sp (p=0.002, Figure 11 A–D). These bone deficits appeared to result from increased bone turnover, evidenced by 18% higher Oc.S/BS (non-significant), 3-fold higher Ob.S/BS (p=0.019), and 100% higher BFR/BS (p=0.040, Figure 11 E–I) vs SHAM. TE suppressed bone turnover, evidenced by 45% lower Oc.S/BS (non-significant) and by lower Ob.S/BS (p=0.014), MS/BS (p=0.003) and BFR/BS (p=0.009) vs SCI. In comparison, TE+TM produced concomitant antiresorptive and bone anabolic actions, exemplified by 64% lower Oc.S/BS vs SCI (non-significant) and by higher Ob.S/BS (p=0.05 vs SCI+TE; trend, p=0.069 vs SHAM), MS/BS (p=0.017 vs SCI+TE), MAR (p=0.003 vs SHAM; p=0.040 vs SCI+TE), and BFR/BS (p=0.033 vs SCI+TE), which preserved BV/TV and Tb.Sp at SHAM levels (both p=0.005 vs SCI) and produced 30–50% higher Tb.Th vs all other groups (p=0.005 vs SHAM; p<0.001 vs both SCI groups). Across all SCI groups, testosterone was negatively associated with Oc.S/BS (r= −0.472, p=0.017) and Tb.Sp (r= −0.559, p=0.006) and BBB score at week 8 was positively associated with Tb.Th (r=0.611, p=0.001).
Figure 11 A–I.
Cancellous bone histomorphometric outcomes at the proximal tibia from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=7–11/group. Symbols represent individual animal data points. Letters indicate differences vs labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM). BV/TV, cancellous bone volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; Oc.S/BS, osteoclast surface; Ob.S/BS, osteoblast surface; MS/BS, mineralizing surface; MAR, mineral apposition rate; BFR/BS, bone formation rate.
3.3.7. μCT Analysis and Bone Mechanical Characteristics
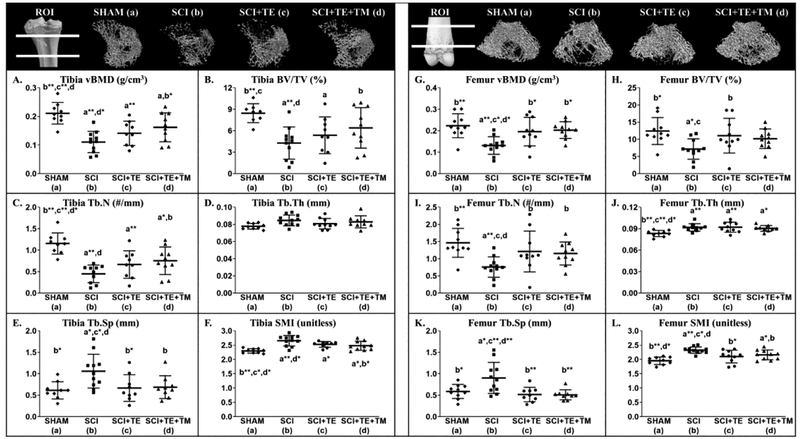
Representative three-dimensional (3D) μCT-derived images of cancellous bone at the proximal tibia and distal femur ROIs are presented in Figure 12. Differences were noted among groups for cancellous vBMD [tibia: F(3,38) = 9.741, p<0.001; femur: F(3,40) = 6.379, p=0.001], BV/TV [tibia: F(3,38) = 5.214, p=0.005; femur: F(3,40) = 3.664, p=0.021], Tb.N [tibia: F(3,38) = 10.699, p<0.001; femur: F(3,40) = 4.986, p=0.005], Tb.Th [femur: F(3,40) = 5.548, p=0.003], Tb.Sp [tibia: F(3,38) = 4.590, p=0.008; femur: F(3,40) = 6.683, p=0.001], and SMI [tibia: F(3,38) = 10.659, p<0.001; femur: F(3,40) = 8.733, p<0.001] (Figure 12 A–L). Cancellous vBMD was ~40–50% lower in SCI vs SHAM at the proximal tibia and distal femur (both p<0.001), characterized by lower BV/TV (tibia: p<0.001; femur: p=0.003) and Tb.N (both p=0.001), and by higher Tb.Sp (tibia: p=0.003; femur: p=0.004) and SMI (both p<0.001). TE partially prevented cancellous bone loss at these sites, evidenced by higher vBMD (femur: p=0.007), BV/TV (femur: p=0.024), and Tb.N (femur: p=0.019) and by lower Tb.Sp (tibia: p=0.007; femur: p=0.001) and SMI (tibia: trend, p=0.053; femur: p=0.004) vs SCI. In comparison, TE+TM more completely prevented proximal tibial cancellous bone loss and preserved distal femur cancellous bone in a roughly similar magnitude to TE, with SCI+TE+TM exhibiting ~50% higher vBMD vs SCI (tibia: p=0.008; femur: p=0.003), along with higher BV/TV (tibia: p=0.046) and Tb.N (tibia: p=0.019; femur: p=0.040), and lower Tb.Sp (tibia: p=0.011; femur: p=0.001) and SMI (tibia: p=0.008; femur: p=0.027). Across all SCI groups, testosterone was positively associated with vBMD (femur: r=0.519, p=0.007), BV/TV (femur: r=0.454, p=0.020), Tb.N (femur: r=0.501, p=0.009) and negatively associated with Tb.Sp. (tibia: r= −0.517, p=0.010; femur: r=−0.561, p=0.004) and SMI (tibia: r= −0.525, p=0.007; femur: r= −0.550,=0.004). BBB score at week 8 was not associated with any tibial or femoral μCT-derived bone microstructural outcome.
Figure 12 A–L.
Microcomputed tomography (μCT)-based cancellous bone characteristics at the proximal tibia and distal femur from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Images represent cancellous bone within each region of interest (ROI). Values are means ± SD, n=10–11/group. Symbols represent individual animal data points. Letters indicate differences vs labeled groups at p<0.05, *<0.01, or **<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM). vBMD, volumetric bone mineral density; BV/TV, cancellous bone volume; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; SMI, structure model index.
Despite the robust cancellous bone deficits after SCI, no differences in distal femur or femoral diaphysis whole bone maximal load [distal femur: F(3,35) = 0.656, p=0.585; femoral midshaft: F(3,38) = 2.653, p=0.064] or energy to failure [distal femur: F(3,35) = 1.994, p=0.135; femoral midshaft F(3,38) = 2.741, p=0.058] were present among groups (Table 4). In comparison, a difference among groups was present for displacement at maximal load at the distal femur [F(3,35) = 4.782, p=0.007], but not the femoral diaphysis [F(3,35) = 0.463, p=0.710].
Table 4.
Whole bone mechanical characteristics from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus treadmill training (SCI+TE+TM).
| SHAM (a) | SCI (b) | SCI+TE (c) | SCI+TE+TM (d) | |
|---|---|---|---|---|
| DISTAL FEMUR | ||||
| Maximum load, N | 97 ± 14 | 90 ± 19 | 96 ± 14 | 89 ± 14 |
| Displacement, mm | 1.2 ± 0.12d* | 1.1 ± 0.22 | 1.2 ± 0.14d* | 0.9 ± 0.17a*,c* |
| Energy to failure, N*mm | 65 ± 12 | 60 ± 18 | 67 ± 10 | 51 ± 17 |
| FEMORAL DIAPHYSIS | ||||
| Maximum load, N | 223 ± 29 | 192 ± 19 | 198 ± 21 | 192 ± 45 |
| Displacement, mm | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.3 |
| Energy to failure, N*mm | 123 ± 27 | 94 ± 19 | 101 ± 13 | 95 ± 42 |
Values are means ± SD, n=7–11/group. Letters indicate differences vs respectively labeled groups at p<0.05 or *p<0.01 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
3.3.8. Serum Hormones
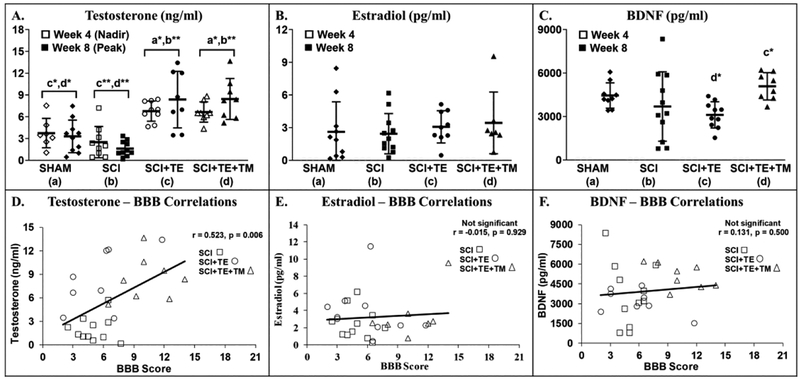
A difference among groups was noted for testosterone at week 4 [F(3,32) = 12.457, p<0.001] and week 8 [F(3,35) = 16.142, p<0.001, Figure 13 A]. No statistical differences were present between SCI and SHAM groups at either timepoint, although, mean testosterone was 33–50% lower in SCI vs SHAM at week 4 (non-significant, p=0.174) and week 8 (non-significant, p=0.159). In comparison, peak and nadir testosterone (occurring 1-day and 1-week after TE injection, respectively) were higher in both TE treated groups vs SHAM and SCI (all p<0.001). No differences in estradiol were present among groups at week 8 [F(3,36) = 0.341, p=0.796, Figure 13 B]. A difference in free BDNF was present among groups at week 8 [F(3,37) = 2.969, p=0.046], with BDNF being 40–60% higher in SCI+TE+TM vs SCI (trend, p=0.055) and SCI+TE (p=0.009, Figure 13 C). Across all SCI groups, week 8 BBB scores were positively associated with testosterone (r=0.523, p=0.006), but not with estradiol or BDNF (Figure 13 D–F). Estradiol and BDNF were not evaluated at week 4 due to insufficient serum.
Figure 13 A–F.
Serum hormones from animals in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Correlations (D–F) only involve animals receiving SCI. Values are means ± SD, n=7–11 per group. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at *p<0.01 or ** p<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM). Estradiol and BDNF could not be evaluated at week 4 due to insufficient serum.
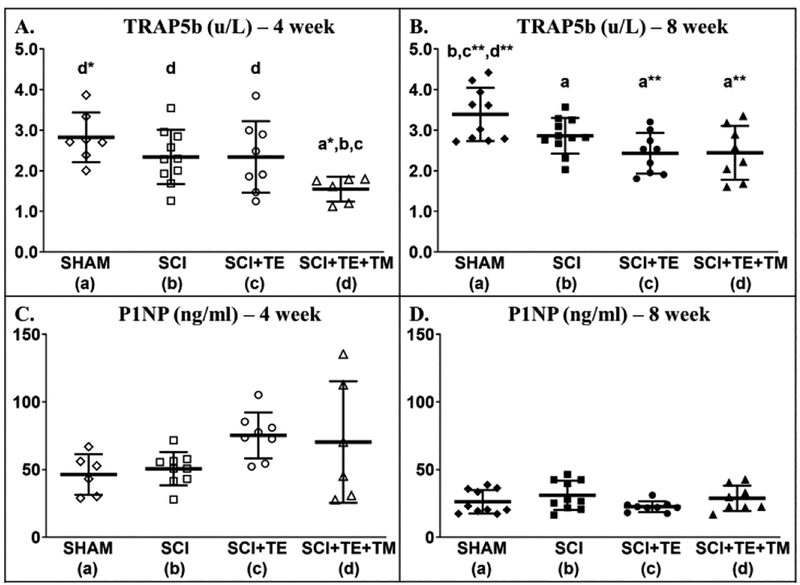
A difference among groups was present for circulating TRAP5b (bone resorption marker) at week 4 [F(3,30) = 3.975, p=0.018] and week 8 [F(3,37) = 5.995, p=0.002]. At week 4, TRAP5b was 33–45% lower in SCI+TE+TM vs SHAM (p=0.002), SCI (p=0.030), and SCI+TE (p=0.037, Figure 14 A). At week 8 all SCI groups displayed lower TRAP5b than SHAM (p=0.040 for SCI; p=0.001 for both TE groups, Figure 14 B). No differences in P1NP were observed at week 4 [F(3,28) = 2.557, p=0.078] or week 8 [F(3,36) = 1.610, p=0.206) (Figure 14 C–D).
Figure 14 A–D.
Serum tartrate-resistant acid phosphatase (TRAP5b), a bone resorption marker, and (P1NP), a bone formation marker, in our primary study after sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Values are means ± SD, n=6–10 per group. Symbols represent individual animal data points. Letters indicate differences vs respectively labeled groups at p<0.05, *p<0.01, or **p<0.001 (a = vs SHAM, b = vs SCI, c = vs SCI+TE, d = vs SCI+TE+TM).
3.3.9. Spinal Cord Histology
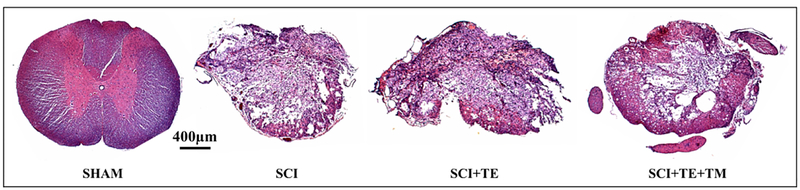
Qualitative assessment of a subset of spinal cords from SCI and SCI+TE groups revealed an injury severity consistent with a severe contusion SCI. The injury epicenter was mostly symmetrical with loss of white and gray matter in both sides of the cord (Figure 15). At the injury epicenter, a thin layer of white matter was spared mostly in the ventral part of the cord, which exhibited visible loss of axons, myelin, and collagen morphology under high-magnification, and often appeared thin and porous. Diffuse tissue debris was also present. Spinal cords from SCI+TE+TM animals exhibited a pronounced spared rim of white matter that often extended throughout the ventral, ventrolateral, and dorsal cord and some preservation of myelin, axons, and collagen morphology. Single to multiple cavities were present throughout the spinal gray and white matter, with less tissue debris evident at the epicenter.
Figure 15.
Representative histologic images of spinal cords (hematoxylin and eosin stained, 5X magnification) from animals in our primary study that received sham surgery (T9 laminectomy) or spinal cord injury (SCI) alone or in combination with testosterone-enanthate (SCI+TE) or TE plus bodyweight-supported treadmill training (SCI+TE+TM). Sections were qualitatively evaluated by examining the injured cavity, tissue debris, extent of myelination, characteristics of remaining axons in the spared tissue, and the degree of collagen infiltration under low- (5X) and high-magnification (40X). In SCI and SCI+TE groups, most injuries displayed symmetrical loss of white and gray matter in both sides of the cord and an injury severity graded as severe, with a thin layer of white matter spared mostly in the ventral part of the cord. The spared tissue exhibited pronounced loss of axons, myelin, and collagen morphology and often appeared thin and porous, with diffuse tissue debris throughout. The epicenter of the SCI+TE+TM group exhibited a pronounced spared rim of white matter that often extended throughout the ventral, ventrolateral, and dorsal parts of the cord and some preservation of myelin, axons, and collagen morphology. Single to multiple cavities were also present throughout the spinal gray and white matters, with less tissue debris evident at the epicenter. Scale bar = 400 μm.
4. Discussion
Bone loss, muscle loss, and impaired muscle contractile properties are hallmarks of SCI (28) that contribute to the high fracture risk in this population (15). Reduced weight bearing and low testosterone (23) are factors that influence these bone and muscle deficits, suggesting that combinatory therapies addressing both impairments may enhance musculoskeletal recovery after motor-incomplete SCI (4). To address this possibility, we conducted two preliminary studies to examine the acute effects of a standardized quadrupedal bodyweight-supported TM training regimen (two 20-min bouts/day, 5-days/week) on recovery of cancellous bone microstructural variables and muscle mass in skeletally-mature rodents after severe SCI. In our preliminary bone study, we observed that 2-weeks of TM worsened distal femur cancellous bone loss and did not prevent bone structural deficits at the proximal tibia. In our preliminary muscle study, we observed no differences in mass of the plantar flexors or BBB scores between SCI and SCI+TM groups after 3-weeks of TM, despite previous reports indicating that an identical TM regimen improved muscular recovery in rodents after moderate SCI with only 1-week of training (7–9). In our main study, we more thoroughly characterized the bone and muscle adaptations occurring in response to severe contusion SCI alone or in combination with TE or TE+TM. The primary findings of this study are described below. SCI produced soleus fCSA atrophy, a slow-oxidative to fast-glycolytic fiber-type transition in soleus, impairments in isolated soleus force mechanics, and LABC muscle loss at two-months post-injury, which was accompanied by severe cancellous bone loss at the distal femur and proximal tibia, likely a result of increased bone turnover. In our model, TE attenuated cancellous bone loss by normalizing bone turnover, constrained the slow-to-fast fiber transition in soleus after SCI, and completely prevented LABC muscle loss, while producing no measurable improvement in other soleus muscle characteristics. In comparison, a combinatory strategy involving TM training with adjuvant TE attenuated soleus fCSA atrophy by ~35–50%, prevented the soleus fiber-type shift, and preserved isolated force production at SHAM levels, increased LABC mass in comparison with all other groups. Furthermore, TE+TM suppressed bone resorption markers and simultaneously stimulated bone formation, resulting in robust cancellous bone preservation. Interestingly, 6 of 8 SCI+TE+TM animals regained the ability to perform voluntary hindlimb over-ground stepping, while no SCI animals and only two SCI+TE animals exhibited this degree of locomotor recovery. Across all SCI groups, serum testosterone was positively associated with BBB scores at week 8, suggesting that TE may have influenced locomotor recovery. Our results indicate that 2–3 weeks of quadrupedal TM training did not improve plantar flexor muscle mass, hindlimb locomotor function in skeletally-mature male rats after severe contusion SCI. In comparison, a multimodal regenerative rehabilitation approach involving 7-weeks of TE+TM hastened muscular recovery in our model, more so than what occurred in response to TE-alone, and produced concomitant antiresorptive and bone anabolic actions at the proximal tibia, resulting in more complete cancellous bone preservation than TE-alone. However, our findings are tempered with the understanding that our study design did not allow for determination of the independent effects of TM on the outcomes reported in our main study.
The muscular adaptations to SCI have been characterized in several preclinical models of varying injury severities (28), although, we are aware of only a few studies that examined muscular adaptations after severe contusion SCI, a model of severely impaired incomplete SCI. For example, Ye et al reported that triceps surae CSA was 25% lower within 7 days of severe (50-mm NYU weight-drop) SCI, remaining relatively stable thereafter, and that soleus in situ peak tetanic force was 25% lower in SCI versus non-surgical controls at 21-days post-injury (35). Similarly, we have previously reported that our male rodent severe contusion SCI model, displays a >50% reduction in soleus fCSA within 21-days of SCI, although, definitive evidence of a slow-to-fast fiber transition was not present at this time point (34). Herein, we expand upon these data by reporting that soleus atrophy persisted in male rodents for at least two-months after severe SCI, exemplified by 30–40% lower absolute and relative soleus mass and >70% smaller type I, type IIa, and hybrid type I/IIa fCSA in comparison with SHAM. These changes likely influenced the lower soleus peak tetanic force and peak force:body mass in SCI vs SHAM, considering that no differences in the peak force:soleus mass ratio were observed any group. In addition, our immunohistochemical and protein expression findings support a slow-to-fast fiber transition after SCI, given that 1) SCI soleus exhibited a lower proportion of type I fibers and higher proportions of type IIa and hybrid I/IIa fibers versus SHAM and 2) SCI soleus displayed >50% lower type I MHC protein expression and 3-fold higher type IIx MHC expression versus SHAM. Furthermore, the faster TPT and ½RT present in SCI soleus indicates a functionally faster muscle after severe SCI. Similarly, cultured skeletal muscle with higher fast MHC exhibits faster TPT and ½RT (50). In comparison, Lin et al recently reported that young female rodents receiving an identical 250-kilodye contusion SCI exhibited only 15% smaller soleus and tibialis anterior fCSA at 3-months post-injury, when compared with controls (30). However, SCI animals in the Lin et al study exhibited spontaneous recovery of hindlimb weight-supported stepping from weeks 4–13, indicating greater functional recovery occurred in young female rodents than in our skeletally-mature male rodent model. In this regard, 0 of 11 SCI animals in our study regained the ability to support the hindlimbs in stance or to perform hindlimb weight-supported stepping. Others have suggested that young female rodents exhibit more pronounced spontaneous locomotor recovery than males after SCI (13), perhaps because estradiol concentrations are greater in females versus males (52) which may reduce secondary apoptotic and inflammatory damage within the spinal cord (53). We did not detect differences in circulating estradiol among groups in our study. However, it remains possible that estradiol concentrations within the CNS differ from that of the circulation, given that 1) various cell types throughout the CNS, including lumbar spinal motoneurons, express aromatase and have been shown to synthesize estradiol in culture (54, 55), and 2) circulating estradiol concentrations do not necessarily reflect concentrations in peripheral tissues that express aromatase and other sex-steroid hormone metabolizing enzymes (52, 56).
Testosterone treatment is known to increase lean body mass and muscle strength in non-neurologically impaired persons (21). A small study and a separate case series also reported that TRT increased lower-extremity lean body mass (24) and muscle cross-sectional area (25), respectively, in men with motor-complete SCI. However, a recent open-label trial reported no improvements in lean mass or knee extensor CSA in men with low-normal testosterone who received low-dose TRT for 16-weeks after chronic motor-complete SCI (27). Similarly, our group has not detected significant improvement in soleus mass or soleus fCSA in skeletally-mature male rodents receiving TE for 21-days after SCI (34). In comparison, we have consistently demonstrated that TE (identical dose to our current study) increases mass of the non-weight bearing sublesional LABC muscle complex after SCI (34, 38, 39) and others have reported that androgen treatment increased quadriceps fCSA after moderate SCI (57, 58). The different responses among these muscle groups may have occurred because of differing androgen treatment durations and/or doses among studies or because androgen receptor (AR) expression differs among muscle groups. Indeed, we have previously reported that the soleus muscle exhibits 70% lower AR mRNA expression than the LABC (34). Herein, we expand upon our previous data by reporting that 8-weeks of TE treatment attenuated the slow-to-fast fiber-type transition, evidenced by a lower proportion of type IIa fibers and a higher proportion of hybrid I/IIa fibers versus SCI, which corroborates findings from others (59). As such, it was surprising that SCI+TE did not exhibit slower TPT or ½RT than SCI animals. Furthermore, TE did not improve soleus peak tetanic force, likely because TE did not attenuate soleus fCSA atrophy. Our results appear to corroborate those of Holman et al who recently reported that low-dose TRT alone did not improve NMES-elicited lower-extremity muscle contractile properties in paralyzed men (26). In contrast, TE completely prevented SCI-induced LABC mass loss in our study, with SCI+TE exhibiting higher relative LABC mass than SHAM. These findings support the contention that muscles with low AR expression exhibit reduced androgen-responsiveness, although, future research examining whether SCI or TE treatment alter AR expression across muscles would assist in elucidating the mechanisms underlying the differing muscular responses to androgen treatment.
In rodents with mild or moderate contusion SCI, quadrupedal bodyweight-supported TM training improves voluntary over-ground ambulation (13), which hastens recovery of soleus fCSA (6–8) and muscle force production (9), and reverses the slow-to-fast fiber transition (9). As such, we were surprised that 3-weeks of quadrupedal TM training did not improve BBB score or mass of the plantar flexors in our preliminary muscle experiment, especially considering previous reports indicating that female rodents undergoing an identical TM regimen after moderate SCI displayed improvements in voluntary hindlimb plantar stepping, soleus muscle function, and soleus fCSA within 1-week (7–9). These findings suggest that TM training is less effective in hastening muscular recovery as SCI severity increases, as suggested by others (13), and/or that sex may be a factor for the incongruities in our preliminary data compared to the literature. In comparison, the combinatory TE+TM strategy that we employed maintained relative soleus mass near SHAM levels, prevented the slow-to-fast fiber-type transition in soleus, attenuated soleus fCSA atrophy, and maintained soleus peak tetanic force and other contractile characteristics after severe contusion SCI. Given these results, it appears that TE and TM produced complimentary muscular effects in our severe contusion SCI model. Alternatively, it may be possible that TM training requires a longer period of time to produce muscle recovery after severe contusion SCI.
To gain insight into known regulators of type I fiber expression and mitochondrial status, we evaluated PGC-1α and PGC-1β protein expression, which regulate type I fiber gene expression and mitochondrial biogenesis (60, 61), and mitochondrial function (62), respectively. In spinalized rodents, PGC-1α protein levels are suppressed within two-weeks (63), with 50% lower total and nuclear PGC-1α protein expression (64) and 50% lower PGC-1β mRNA (65) persisting for at least 8-weeks. In our primary study, PGC-1α expression appeared relatively lower at 8-weeks after severe SCI vs SHAM and near identical in SCI+TE+TM and SHAM groups, although, these differences were not significant and were less pronounced than after spinal transection. In comparison, PGC-1β protein levels were ~60% lower in SCI vs SHAM, suggesting that impairments in mitochondrial function may have accompanied the slow-to-fast fiber-type shift in our model. In our study, TE partially prevented the decline in PGC-1β expression, with protein levels remaining near SHAM levels. Similarly, Wu et al also reported that spinalized animals treated with testosterone exhibited gastrocnemius PGC-1β mRNA near controls (i.e., >100% increase vs untreated spinal transection) (64). However, the most dramatic change we observed was that SCI+TE+TM displayed 50–300% higher soleus PGC-1β protein levels than SHAM and all other SCI groups. The functional significance of increased PGC-1β in SCI+TE+TM animals is unknown, although, it is interesting to note that across all SCI groups relative PGC-1β protein expression was associated with a slower TPT, likely because PGC-1β is predominantly expressed in type I fibers. Furthermore, PGC-1β protein expression was more highly correlated with serum testosterone than with BBB score at week 8, suggesting that these changes may have been influenced by TE treatment.
We also observed that the magnitude of improvement in voluntary over-ground hindlimb stepping that developed in SCI+TE+TM animals was comparatively greater than what occurred in response to TM-alone in our preliminary muscle study after 3-weeks of training and significantly greater than the SCI and SCI+TE groups in our main experiment. Given that TE+TM promoted recovery of voluntary over-ground stepping, it is likely that some tissue sparing occurred throughout the lesion epicenter because the ventral and lateral white matter tracts subserve most of the important hindlimb locomotor functions via several descending pathways, including the reticulospinal, vestibulospinal, and rubrospinal tracts. To assess the possibility, we histologically examined the spinal lesion in a subset of animals from each group and our qualitative assessment indicated a pronounced rim of white matter was preserved at the injury epicenter of SCI+TE+TM animals. Although, quantitative assessment of white matter sparing is necessary to validate this preliminary observation and to compare spared tissue volume among groups. Importantly, we do not believe that the pronounced white matter in SCI+TE+TM animals was indicative of a less severe injury in this group because all SCI animals received an identical injury and exhibited similar BBB scores at week 1, providing evidence of a functionally consistent injury among groups prior to beginning TM training. As such, it is interesting to note that 75% of SCI+TE+TM animals regained the ability to support the hindlimbs in stance and/or to perform voluntary over-ground weight-supported dorsal stepping (i.e., BBB ≥9) at week 8, >60% exhibited occasional weight-supported plantar stepping (i.e., BBB ≥10), and nearly 40% exhibited frequent to consistent weight-supported plantar stepping with occasional forelimb-hindlimb coordination (i.e., BBB ≥12). In comparison, one SCI+TE animal regained the ability to perform dorsal stepping and one exhibited plantar stepping by week 8, which is consistent with our previous reports (36, 38), while no SCI animals recovered the ability to support the hindlimbs in stance. Interestingly, Lin et al reported that young female rodents exhibited a degree of spontaneous locomotor improvement after severe SCI that was comparable to that observed in our male SCI+TE+TM animals. As discussed above, inter-sex comparison of functional recovery after SCI is confounded by the potential neuroprotective effects of higher estradiol in females (4). We observed no differences in circulating estradiol among groups in our main study and no associations among estradiol and BBB scores. However, circulating testosterone was 35–50% lower after SCI, suggesting potential differences in CNS estradiol concentrations, given that spinal motoneurons express aromatase and actively synthesize estradiol in culture (54, 55). In support of this contention, testosterone treatment has been shown to attenuate the regression of dendritic length in spinal motoneurons after SCI (58), with androgenic and estrogenic actions each producing approximately 50% of the total effect (57). As such, examining the role of aromatase in mediating the effects of administered TE on locomotor recovery remains an important area of investigation. BDNF, a known molecular mediator of activity-dependent plasticity (66), also interacts with androgens to maintain dendritic length in spinal motoneurons innervating the quadriceps and the non-weight-bearing bulbocavernosus muscles after SCI (57, 58), demonstrating neuroprotective effects of both molecules. Interestingly, SCI+TE+TM displayed the highest circulating BDNF among groups, which may have influenced the relatively profound degree of muscular plasticity occurring in this group. Future studies are needed to determine whether TE+TM increases BDNF expression within the CNS and whether this multimodal therapy preserves dendritic length more so than either individual component.
In the neurologically-intact state, episodic loading limits disuse-induced bone loss by producing antiresorptive and bone anabolic actions (67). Residual voluntary musculoskeletal strain also reduces bone loss after neurologic injury, as evidenced by reports indicating that 1) higher BMD persists in the less neurologically impaired limb after SCI (68) and 2) cast immobilization, which eliminates residual muscle contractility, worsened bone loss after SCI (44). However, no stand-alone physical rehabilitation regimen has demonstrated consistent success in preventing bone loss or regenerating bone after SCI (14). In support of this, bodyweight-supported TM training did not improve cancellous bone morphology in our preliminary bone study, with SCI+TM exhibiting the lowest distal femur and proximal tibia cancellous bone variables among all groups. While these findings may appear surprising, they corroborate reports that indicate after extended disuse, rodents exhibit reduced cancellous BMD in response to reambulation (69) or dynamic moderate-intensity physical rehabilitation (70), perhaps because the residual weakened trabecular network is insufficient to support dynamic loading. Similarly, several clinical trials and case-studies reported that locomotor-based rehabilitation strategies did not increase BMD nor prevent BMD loss in the most fracture-prone sites after SCI (71–74). The mechanisms underlying the diminished cancellous bone mechano-responsiveness after SCI require further elucidation, but may be influenced by impaired osteoblast (75) or osteocyte signaling (76). As evidence, preclinical (36, 77, 78) and clinical studies (79) report increased bone resorption and concomitantly reduced bone formation (i.e., uncoupled bone turnover) acutely after SCI, likely indicating the presence of a bone formation defect. Indeed, Jiang et al reported that cultured osteoblasts obtained from rats 3-weeks post spinal cord transection exhibit reduced bone formation indices and lower osteogenic gene expression in response to cyclic strain, when compared with osteoblasts obtained from normally ambulating animals or those undergoing hindlimb immobilization (80). Qin et al also reported that NMES reduced bone resorption and osteoclastogenesis in rats 16-weeks post spinal cord transection, demonstrating the expected antiresorptive response to reloading, while bone formation indices and osteoblast differentiation remained unaltered (81). In our main experiment, we did not detect increased TRAP5b at 1- or 2-months post-SCI and proximal tibia Oc.S/BS was only modestly elevated in SCI animals, despite noticeably lower cancellous bone mass, suggesting that the majority of bone loss occurred rapidly after experimental SCI, as previously reported (36–38, 44). In contrast, Ob.S/BS and cancellous BFR/BS were higher in SCI vs SHAM at 2-month post-surgery in our current study. We have previously reported a near-complete ablation of Ob.S/BS and BFR/BS occurs 2–3 weeks post-SCI (37, 38), with renormalization of these measures by 2-months post-surgery (36), which supports our current findings. These temporal and directional differences in cancellous bone formation between the acute and sub-chronic phases in our studies suggest that any uncoupling of bone turnover is transient in our SCI model.
Androgens produce direct antiresorptive influence on cancellous bone (82), in a manner that is independent from muscle contractility (83). Additionally, androgens influence the osteogenic responsiveness to mechanical strain, with some studies reporting additive skeletal benefit (42, 43). For example, bone explants incubated with dihydrotestosterone, a highly potent non-aromatizable androgen (56), exhibited synergistic increases in 3H-thymidine and 3H-proline incorporation in response to strain, indicative of improved osteoblast recruitment and matrix osteoblast production, respectively (42). Our findings expand on this by demonstrating that TE+TM increased Ob.S/BS, along with cancellous bone formation in comparison with TE-alone. We did not observe elevated estradiol in either TE group, suggesting that the skeletal responses to administered TE may have been androgen-mediated, as we have previously reported (82), or that testosterone was locally aromatized to estradiol in bone. Interestingly, TE+TM also produced antiresorptive actions, as demonstrated by SCI+TE+TM exhibiting the lowest circulating TRAP5b and Oc.S/BS among all groups. These combinatory antiresorptive and bone anabolic actions are striking given that 1) findings from our preliminary bone study demonstrated an identical, albeit shorter duration, TM protocol worsened cancellous bone loss after SCI, when initiated in females without TE, and 2) TM training was performed with 30–40% bodyweight support, suggesting only a minimal mechanical strain threshold may be necessary to stimulate bone anabolic and/or antiresorptive actions in cancellous bone in the presence of adjuvant TE.
Several limitations to our study design also merit mention. First, we did not quantify the musculoskeletal strain resulting from our TM protocol and we cannot quantify the amount of stepping necessary for bone and muscle improvement in our main study because most SCI+TE+TM animals regained the ability to perform some voluntary over-ground hindlimb stepping. However, we do not view this as a serious limitation because we utilized a physical rehabilitation regimen that improves corticospinal neuroplasticity in humans (84) and in animals with motor-incomplete SCI (10, 16), and because the presence of facilitated and voluntary stepping was the sole difference between the SCI+TE+TM and SCI+TE groups. Indeed, all SCI animals in our primary study presented with a uniform degree of post-surgical hindlimb locomotor impairment at 1-week post-injury (before initiation of TM training), while SCI+TE+TM exhibited more pronounced locomotor recovery thereafter, likely indicating some degree of corticospinal plasticity may have influenced the musculoskeletal outcomes in this group. Second, we did we did not evaluate the bone or muscle responses to TM-alone in our main study because our preliminary bone study indicated worsened cancellous bone loss in response to 2-weeks of TM-alone and our preliminary muscle study indicated no improvement in plantar flexor muscle mass or BBB scores in response to 3-weeks of TM-alone. Moreover, the logistics of performing 200-min/week of individualized one-on-one TM training for 7 continuous weeks would have made inclusion of another experimental group unfeasible in our main experiment. In this regard, it should be noted that our study design matched that of a recent clinical trial that evaluated whether TRT, when administered alone or in combination with lower-extremity NMES resistance training, improved muscular outcomes in men with complete SCI, demonstrating our study design is relevant to the clinical literature (26, 27). Nevertheless, future studies comparing bone and muscle adaptations to TM and TE when administered alone and in combination are necessary to determine the degree to which each individual therapy contributed to the musculoskeletal benefits of this multimodal strategy. Lastly, we did not include female rodents in our main experiment because the purpose of our experiment was to assess the effects of TE+TM in an SCI model that displays persistent disuse and a testosterone deficiency, which only occurs in males, and because testosterone is a male hormone that produces androgenizing effects in the doses provided in this study, so our therapy is not clinically applicable to females.
In summary, our findings demonstrate that significant deficits in soleus muscle fCSA and isolated muscle function persisted in the soleus of skeletally-mature male rats for at least 8-weeks after severe SCI and were accompanied by severe cancellous bone deficits at the distal femur and proximal tibia. Additionally, we provide immunohistochemistry, protein expression, and functional evidence supporting a slow-to-fast fiber-type transition in the soleus, a primary loading muscle in rodents, in our model. TE treatment prevented LABC atrophy and attenuated the slow-to-fast fiber transition in soleus but did not increase soleus fCSA or improve isolated muscle performance after SCI. Additionally, TE treatment normalized indices of bone turnover, resulting in partial prevention of cancellous bone loss after SCI. In comparison, TE+TM attenuated the SCI-induced reductions in soleus mass and soleus fCSA, prevented the slow-to-fast fiber-type shift, maintained soleus peak tetanic force, TPT, and ½RT near SHAM levels, and increased LABC mass. Furthermore, TE+TM produced concomitant antiresorptive and bone anabolic actions that resulted in robust cancellous bone preservation after SCI. It is likely that the improvement in hindlimb locomotor function occurring in SCI+TE+TM animals contributed to the magnitude of musculoskeletal improvements in this group, although, further research is needed to verify this assertion. In conclusion, our results suggest that a regenerative rehabilitation approach involving TM and adjuvant TE preserved cancellous bone and promoted muscle plasticity in the context of our rodent model that exhibits low testosterone and disuse after severe contusion SCI. These results provide rationale for future studies intended to determine 1) the degree to which TE and TM each contributed to the effects of the multimodal therapy, 2) whether the aromatization of testosterone within the CNS or bone influences neuromuscular plasticity or bone preservation, respectively, and 3) the molecular mechanisms underlying the improved musculoskeletal outcomes resulting from TE+TM.
Significance Statement.
The bone and muscle deficits that occur after spinal cord injury (SCI) are precipitated by the neural injury and subsequent unloading and exacerbated by a host of secondary factors, including suppressed testosterone. We present the first evidence indicating that a regenerative rehabilitation approach involving bodyweight-supported treadmill training (TM) with adjuvant testosterone (TE) produced comprehensive musculoskeletal preservation in rodents after severe contusion SCI. Specifically, TE+TM concomitantly suppressed bone resorption and stimulated bone formation, produced near-complete cancellous bone preservation, prevented the deleterious oxidative-to-glycolytic muscle fiber-type transition in soleus, attenuated soleus muscle atrophy, and maintained soleus force production and muscle contractile properties.
5. Acknowledgements
The antibodies SC.71, BA.D5, and BF.F3 developed by Professor Stefano Schiaffino and 2F7 and 6H1 developed by Christine A. Lewis were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242.
8 Funding
This work was supported by a Paralyzed Veterans of America (PVA) Fellowship (#2939 to FY), by the Office of Research and Development, Rehabilitation Research and Development (RR&D) Service, Department of Veterans Affairs 1I21RX001273-01 and Presidential Early Career Award for Scientists and Engineers (PECASE #B9280-O) to JFY and VA RR&D Center grant 1I50RX002020-1, by the National Institutes of Health (P01 HD059751-01A1 to KV), and, in-part, by resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Grant Funding: This work was supported by a Paralyzed Veterans of America (PVA) Fellowship (#2939 to FY), by the Office of Research and Development, Rehabilitation Research and Development (RR&D) Service, Department of Veterans Affairs [1I21RX001273-01 and Presidential Early Career Award for Scientists and Engineers (PECASE #B9280-O) to JFY and 1I50RX002020-1], by the National Institutes of Health (P01 HD059751-01A1 to KV), and, in-part, by resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
7 Conflict of Interest Statement
All authors declare no conflicts of interest and no competing financial interests exist.
9 Data Availability Statement
The data generated and/or analyzed in this study will be provided, upon written request, per policy of the US Department of Veterans Affairs and in accordance with the United States Freedom of Information Act.
10 References
- 1.DiPiro ND, Holthaus KD, Morgan PJ, Embry AE, Perry LA, Bowden MG, et al. Lower Extremity Strength Is Correlated with Walking Function After Incomplete SCI. Top Spinal Cord Inj Rehabil. (2015) 21(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DL Jr., Yarar-Fisher C. Contributors to Metabolic Disease Risk Following Spinal Cord Injury. Curr Phys Med Rehabil Rep. (2016) 4(3):190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCarthy ID, Bloomer Z, Gall A, Keen R, Ferguson-Pell M. Changes in the structural and material properties of the tibia in patients with spinal cord injury. Spinal Cord. (2012) 50(4):333–7. [DOI] [PubMed] [Google Scholar]
- 4.Otzel DM, Lee J, Ye F, Borst SE, Yarrow JF. Activity-Based Physical Rehabilitation with Adjuvant Testosterone to Promote Neuromuscular Recovery after Spinal Cord Injury. Int J Mol Sci. (2018) 19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. (2012) 93(9):1508–17. [DOI] [PubMed] [Google Scholar]
- 6.Jayaraman A, Liu M, Ye F, Walter GA, Vandenborne K. Regenerative responses in slow- and fast-twitch muscles following moderate contusion spinal cord injury and locomotor training. Eur J Appl Physiol. (2013) 113(1):191–200. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Stevens-Lapsley JE, Jayaraman A, Ye F, Conover C, Walter GA, et al. Impact of treadmill locomotor training on skeletal muscle IGF1 and myogenic regulatory factors in spinal cord injured rats. Eur J Appl Physiol. (2010) 109(4):709–20. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Bose P, Walter GA, Thompson FJ, Vandenborne K. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord. (2008) 46(7):488–93. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JE, Liu M, Bose P, O’Steen WA, Thompson FJ, Anderson DK, et al. Changes in soleus muscle function and fiber morphology with one week of locomotor training in spinal cord contusion injured rats. J Neurotrauma. (2006) 23(11):1671–81. [DOI] [PubMed] [Google Scholar]
- 10.Bose PK, Hou J, Parmer R, Reier PJ, Thompson FJ. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front Physiol. (2012) 3:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma. (2014) 31(12):1088–106. [DOI] [PubMed] [Google Scholar]
- 12.Behrman AL, Ardolino EM, Harkema SJ. Activity-Based Therapy: From Basic Science to Clinical Application for Recovery After Spinal Cord Injury. J Neurol Phys Ther. (2017) 41 Suppl 3:S39–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battistuzzo CR, Callister RJ, Callister R, Galea MP. A systematic review of exercise training to promote locomotor recovery in animal models of spinal cord injury. J Neurotrauma. (2012) 29(8):1600–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panisset MG, Galea MP, El-Ansary D. Does early exercise attenuate muscle atrophy or bone loss after spinal cord injury? Spinal Cord. (2016) 54(2):84–92. [DOI] [PubMed] [Google Scholar]
- 15.Cirnigliaro CM, Myslinski MJ, La Fountaine MF, Kirshblum SC, Forrest GF, Bauman WA. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int. (2017) 28(3):747–65. [DOI] [PubMed] [Google Scholar]
- 16.de Leon RD, Dy CJ. What Did We Learn from the Animal Studies of Body Weight-Supported Treadmill Training and Where Do We Go from Here? J Neurotrauma. (2017) 34(9):1744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. (2010) 1211:66–84. [DOI] [PubMed] [Google Scholar]
- 18.Clark MJ, Schopp LH, Mazurek MO, Zaniletti I, Lammy AB, Martin TA, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. (2008) 87(9):758–67. [DOI] [PubMed] [Google Scholar]
- 19.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. (2014) 37(1):32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia H, Sullivan CT, McCoy SC, Yarrow JF, Morrow M, Borst SE. Review of health risks of low testosterone and testosterone administration. World J Clin Cases. (2015) 3(4):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, et al. Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2018) 9(3):465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borst SE, Yarrow JF. Injection of testosterone may be safer and more effective than transdermal administration for combating loss of muscle and bone in older men. Am J Physiol Endocrinol Metab. (2015) 308(12):E1035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abilmona SM, Sumrell RM, Gill RS, Adler RA, Gorgey AS. Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. (2011) 43(8):574–9. [DOI] [PubMed] [Google Scholar]
- 25.Moore PD, Gorgey AS, Wade RC, Khalil RE, Lavis TD, Khan R, et al. Neuromuscular electrical stimulation and testosterone did not influence heterotopic ossification size after spinal cord injury: A case series. World J Clin Cases. (2016) 4(7):172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman ME, Gorgey AS. Testosterone and Resistance Training Improve Muscle Quality in Spinal Cord Injury. Med Sci Sports Exerc. (2019). [DOI] [PubMed] [Google Scholar]
- 27.Gorgey AS, Khalil R, Gill RS, Gater DR, Lavis TR, Cardozo C, et al. Low-Dose Testosterone and Evoked Resistance Exercise after Spinal Cord Injury [TEREX-SCI] on Cardio-metabolic Risk Factors: An open-label randomized clinical trial. J Neurotrauma. (2019). [DOI] [PubMed] [Google Scholar]
- 28.Biering-Sorensen B, Kristensen IB, Kjaer M, Biering-Sorensen F. Muscle after spinal cord injury. Muscle Nerve. (2009) 40(4):499–519. [DOI] [PubMed] [Google Scholar]
- 29.Baldwin KM, Haddad F, Pandorf CE, Roy RR, Edgerton VR. Alterations in muscle mass and contractile phenotype in response to unloading models: role of transcriptional/pretranslational mechanisms. Front Physiol. (2013) 4:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CY, Androjna C, Rozic R, Nguyen B, Parsons B, Midura RJ, et al. Differential Adaptations of the Musculoskeletal System after Spinal Cord Contusion and Transection in Rats. J Neurotrauma. (2018) 35(15):1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, et al. Animal models of spinal cord injury: a systematic review. Spinal Cord. (2017) 55(8):714–21. [DOI] [PubMed] [Google Scholar]
- 32.Hutchinson KJ, Linderman JK, Basso DM. Skeletal muscle adaptations following spinal cord contusion injury in rat and the relationship to locomotor function: a time course study. J Neurotrauma. (2001) 18(10):1075–89. [DOI] [PubMed] [Google Scholar]
- 33.Voor MJ, Brown EH, Xu Q, Waddell SW, Burden RL Jr., Burke DA, et al. Bone loss following spinal cord injury in a rat model. J Neurotrauma. (2012) 29(8):1676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, et al. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS One. (2018) 13(3):e0194440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye F, Baligand C, Keener JE, Vohra R, Lim W, Ruhella A, et al. Hindlimb muscle morphology and function in a new atrophy model combining spinal cord injury and cast immobilization. J Neurotrauma. (2013) 30(3):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otzel DM, Conover CF, Ye F, Phillips EG, Bassett T, Wnek RD, et al. Longitudinal Examination of Bone Loss in Male Rats After Moderate-Severe Contusion Spinal Cord Injury. Calcif Tissue Int. (2018) 104(1):79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, et al. Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res. (2015) 30(4):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, et al. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma. (2014) 31(9):834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarrow JF, Phillips EG, Conover CF, Bassett TE, Chen C, Teurlings T, et al. Testosterone Plus Finasteride Prevents Bone Loss without Prostate Growth in a Rodent Spinal Cord Injury Model. J Neurotrauma. (2017) 34(21):2972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinnesael M, Laurent MR, Jardi F, Dubois V, Deboel L, Delisser P, et al. Androgens inhibit the osteogenic response to mechanical loading in adult male mice. Endocrinology. (2015) 156(4):1343–53. [DOI] [PubMed] [Google Scholar]
- 41.Callewaert F, Bakker A, Schrooten J, Van Meerbeek B, Verhoeven G, Boonen S, et al. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J Bone Miner Res. (2010) 25(1):124–31. [DOI] [PubMed] [Google Scholar]
- 42.Cheng MZ, Zaman G, Rawlinson SC, Pitsillides AA, Suswillo RF, Lanyon LE. Enhancement by sex hormones of the osteoregulatory effects of mechanical loading and prostaglandins in explants of rat ulnae. J Bone Miner Res. (1997) 12(9):1424–30. [DOI] [PubMed] [Google Scholar]
- 43.Cheng MZ, Zaman G, Rawlinson SC, Suswillo RF, Lanyon LE. Mechanical loading and sex hormone interactions in organ cultures of rat ulna. J Bone Miner Res. (1996) 11(4):502–11. [DOI] [PubMed] [Google Scholar]
- 44.Yarrow JF, Ye F, Balaez A, Mantione JM, Otzel DM, Chen C, et al. Bone loss in a new rodent model combining spinal cord injury and cast immobilization. J Musculoskelet Neuronal Interact. (2014) 14(3):255–66. [PMC free article] [PubMed] [Google Scholar]
- 45.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. (1995) 12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 46.Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, et al. Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab. (2008) 295(5):E1213–22. [DOI] [PubMed] [Google Scholar]
- 47.McCoy SC, Yarrow JF, Conover CF, Borsa PA, Tillman MD, Conrad BP, et al. 17beta-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone. (2012) 51(4):667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye F, McCoy SC, Ross HH, Bernardo JA, Beharry AW, Senf SM, et al. Transcriptional regulation of myotrophic actions by testosterone and trenbolone on androgen-responsive muscle. Steroids. (2014) 87:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, et al. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab. (2014) 306(4):E433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang YC, Dennis RG, Baar K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation. Am J Physiol Cell Physiol. (2006) 291(1):C11–7. [DOI] [PubMed] [Google Scholar]
- 51.Yarrow JF, Phillips EG, Conover CF, Bassett TE, Chen C, Teurlings T, et al. Testosterone Plus Finasteride Prevents Bone Loss without Prostate Growth in a Rodent Spinal Cord Injury Model. J Neurotrauma. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarrow JF, Conover CF, Lipinska JA, Santillana CA, Wronski TJ, Borst SE. Methods to quantify sex steroid hormones in bone: applications to the study of androgen ablation and administration. Am J Physiol Endocrinol Metab. (2010) 299(5):E841–7. [DOI] [PubMed] [Google Scholar]
- 53.Elkabes S, Nicot AB. Sex steroids and neuroprotection in spinal cord injury: a review of preclinical investigations. Exp Neurol. (2014) 259:28–37. [DOI] [PubMed] [Google Scholar]
- 54.Ji YX, Zhao M, Liu YL, Chen LS, Hao PL, Sun C. Expression of aromatase and estrogen receptors in lumbar motoneurons of mice. Neurosci Lett. (2017) 653:7–11. [DOI] [PubMed] [Google Scholar]
- 55.Rakotoarivelo C, Petite D, Lambard S, Fabre C, Rouleau C, Lumbroso S, et al. Receptors to steroid hormones and aromatase are expressed by cultured motoneurons but not by glial cells derived from rat embryo spinal cord. Neuroendocrinology. (2004) 80(5):284–97. [DOI] [PubMed] [Google Scholar]
- 56.Yarrow JF, Wronski TJ, Borst SE. Testosterone and Adult Male Bone: Actions Independent of 5alpha-Reductase and Aromatase. Exerc Sport Sci Rev. (2015) 43(4):222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sengelaub DR, Han Q, Liu NK, Maczuga MA, Szalavari V, Valencia SA, et al. Protective Effects of Estradiol and Dihydrotestosterone following Spinal Cord Injury. J Neurotrauma. (2018) 35(6):825–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byers JS, Huguenard AL, Kuruppu D, Liu NK, Xu XM, Sengelaub DR. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. J Comp Neurol. (2012) 520(12):2683–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregory CM, Vandenborne K, Huang HF, Ottenweller JE, Dudley GA. Effects of testosterone replacement therapy on skeletal muscle after spinal cord injury. Spinal Cord. (2003) 41(1):23–8. [DOI] [PubMed] [Google Scholar]
- 60.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. (2002) 418(6899):797–801. [DOI] [PubMed] [Google Scholar]
- 61.Kang C, Goodman CA, Hornberger TA, Ji LL. PGC-1alpha overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J. (2015) 29(10):4092–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gali Ramamoorthy T, Laverny G, Schlagowski AI, Zoll J, Messaddeq N, Bornert JM, et al. The transcriptional coregulator PGC-1beta controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat Commun. (2015) 6:10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Higashino K, Matsuura T, Suganuma K, Yukata K, Nishisho T, Yasui N. Early changes in muscle atrophy and muscle fiber type conversion after spinal cord transection and peripheral nerve transection in rats. J Neuroeng Rehabil. (2013) 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Zhao J, Zhao W, Pan J, Bauman WA, Cardozo CP. Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma. (2012) 29(8):1663–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Collier L, Qin W, Creasey G, Bauman WA, Jarvis J, et al. Electrical stimulation modulates Wnt signaling and regulates genes for the motor endplate and calcium binding in muscle of rats with spinal cord transection. BMC Neurosci. (2013) 14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cote MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. (2011) 28(2):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price JS, Sugiyama T, Galea GL, Meakin LB, Sunters A, Lanyon LE. Role of endocrine and paracrine factors in the adaptation of bone to mechanical loading. Curr Osteoporos Rep. (2011) 9(2):76–82. [DOI] [PubMed] [Google Scholar]
- 68.Lichy AM, Groah S. Asymmetric lower-limb bone loss after spinal cord injury: case report. J Rehabil Res Dev. (2012) 49(2):221–6. [DOI] [PubMed] [Google Scholar]
- 69.Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J Musculoskelet Neuronal Interact. (2006) 6(3):217–25. [PubMed] [Google Scholar]
- 70.Yarrow JF, McCoy SC, Ferreira JA, Pingel JE, Conrad BP, Wronski TJ, et al. A rehabilitation exercise program induces severe bone mineral deficits in estrogen-deficient rats after extended disuse. Menopause. (2012) 19(11):1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AISB spinal cord injury. J Spinal Cord Med. (2008) 31(5):509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. (2006) 31(3):283–91. [DOI] [PubMed] [Google Scholar]
- 73.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, et al. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. (2005) 43(11):649–57. [DOI] [PubMed] [Google Scholar]
- 74.Coupaud S, Jack LP, Hunt KJ, Allan DB. Muscle and bone adaptations after treadmill training in incomplete Spinal Cord Injury: a case study using peripheral Quantitative Computed Tomography. J Musculoskelet Neuronal Interact. (2009) 9(4):288–97. [PubMed] [Google Scholar]
- 75.Jiang SD, Yan J, Jiang LS, Dai LY. Down-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in osteoblasts from rats with chronic spinal cord injury. Joint Bone Spine. (2011) 78(5):488–92. [DOI] [PubMed] [Google Scholar]
- 76.Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, et al. Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res. (2015) 30(11):1994–2004. [DOI] [PubMed] [Google Scholar]
- 77.Morse L, Teng YD, Pham L, Newton K, Yu D, Liao WL, et al. Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos Int. (2008) 19(5):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin T, Tong W, Chandra A, Hsu SY, Jia H, Zhu J, et al. A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats. Bone Res. (2015) 3:15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan CO, Battaglino RA, Morse LR. Spinal Cord Injury and Osteoporosis: Causes, Mechanisms, and Rehabilitation Strategies. Int J Phys Med Rehabil. (2013) 1. [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang SD, Yang YH, Chen JW, Jiang LS. Isolated osteoblasts from spinal cord-injured rats respond less to mechanical loading as compared with those from hindlimb-immobilized rats. J Spinal Cord Med. (2013) 36(3):220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin W, Sun L, Cao J, Peng Y, Collier L, Wu Y, et al. The central nervous system (CNS)-independent anti-bone-resorptive activity of muscle contraction and the underlying molecular and cellular signatures. J Biol Chem. (2013) 288(19):13511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beck DT, Yarrow JF, Beggs LA, Otzel DM, Ye F, Conover CF, et al. Influence of aromatase inhibition on the bone-protective effects of testosterone. J Bone Miner Res. (2014) 29(11):2405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laurent MR, Jardi F, Dubois V, Schollaert D, Khalil R, Gielen E, et al. Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone. (2016) 93:33–42. [DOI] [PubMed] [Google Scholar]
- 84.Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. (2013) 94(11):2297–308. [DOI] [PubMed] [Google Scholar]