Abstract
Various ferulic acid (FA) dimers occurring in plant cell walls, such as 8-5-, 8-O-4-, 5-5-, and 8-8-coupled dimers, are effective antioxidants and potential antimicrobials. It is necessary to access these diferulates as reference compounds to validate those isolated from plants. 3,6-bis(4-hydroxy-3-methoxyphenyl)-tetrahydrofuro-[3,4-c]furan-1,4-dione, a 8-8-coupled FA dilactone generated from ferulic acid via radical coupling, has been used to synthesize 8-8-coupled FA dimers although few reports investigated the distribution of products and mechanisms involved in the transformation of FA dilactone. In this work, the FA dilactone, obtained from FA by a peroxidase-catalyzed radical coupling, was reacted under various base/acid conditions. Effects of reaction conditions and workup procedures on the distribution of products were investigated by GC-MS. The isolated products from such treatments of FA dilactone were characterized by NMR. New derivatives of FA dimer including 2-(4-hydroxy-3-methoxybenzylidene)-3-(hydroxyl-(4-hydroxy-3-methoxyphenyl)methyl)succinic acid and 2-(bis(4-hydroxy-3-methoxyphenyl)-methyl)-succinic acid were produced from NaOH treatment. Another novel 8-8-coupled cyclic FA dimer, diethyl 6-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-methoxy-1,2-dihydronaphthalene-2,3-dicarboxylate was identified in products from FA dilactone treated by dry HCl in absolute ethanol. Mechanisms involved in such transformations were proposed.
Keywords: dehydrodimers, alkali/acid treatment, GC-MS, NMR, radical coupling
1. Introduction
Natural phenolics, the secondary metabolites extracted from plants, such as gallic acid [1] and ellagic acid [2], have been shown to have various biological activities including antioxidant, antimicrobial, and anticancer capacities [3,4]. Ferulic acid (FA), 4-hydroxy-3-methoxycinnamic acid, one of the large families of biologically active phenolics in plants, is an excellent representative having various applications [5]. In addition to monomeric FA, various FA dimers occurring in plant cell walls, including 5-5-, 8-8-, 8-5-, and 8-O-4-coupled dehydrodimers [6] were demonstrated to have potential antimicrobial properties. FA dimers found in hydrolysate of corn Stover were showed to inhibit the growth of S. cerevisiae revealing that poacic acid, an 8-5-coupled decarboxylated ferulic acid dimer (8-5 DC), had the greatest antifungal activity with an IC50 of 111 μg/mL (324 μM) against S. cerevisiae. This inhibition is comparable to that of the widely used fungicides picoxystrobin (IC50 of 308 μM) or polyoxin D (IC50 of 340 μM) and substantially lower than that of the primary fungicide used in organic agriculture, copper sulfate (IC50 of 2.4 mM) [7]. Some dimeric ferulates showed higher radical-scavenging efficacy than the monomers [8].
The rich structural diversity of 8-8-coupled FA dimers have attracted considerable attention in recent decades because of their unique architectures and diverse biological activities [9,10]. However, extracting and isolating such dehydrodimers on a large scale from plants is challenging due to their low abundance [11]. It is desirable and needed to devise synthetic strategies to make FA dimers with potential antimicrobial capabilities. 3,6-bis(4-hydroxy-3-methoxyphenyl)-tetrahydrofuro-[3,4-c]furan-1,4-dione, a FA dilactone, was first synthesized by Cartwright [12] using FeCl3. It also can be conveniently prepared from an enzymatic oxidative coupling of ferulic acid [12,13].
FA dilactone has been used to prepare 4,8-bis(4-hydroxy-3-methoxyphenyl)-3,7-dioxabicyclo[3.3.0]octan-2-one, a 8-β-coupled dimer formed by radical coupling of ferulic acid or its ester and coniferyl alcohol [14]. Alkali or acid treatment of FA dilactone produced 8-8-coupled diferulic acids (DFA), 8-8-o DFA (compound 2) and/or 8-8-c DFA (compound 3) (Figure 1). The FA dilactone has been treated with 2 M NaOH to afford trans-lactone acid 4 as amorphous solid in very high yield following a silica column separation [15]. Acid-catalyzed transformation of the 5-substituted ferulate or their corresponding dilactones produced tetrahydrofuran or dibenzylidenesuccinate types of products [16]. The trans- and cis-isomers of 1,2-dihydronaphthylene diethyl esters can be produced in such a treatment [17]. Acid-catalyzed transformation of FA dilactone 1 in dry methanol was reported to produce trans-1,2-dihydronaphthylene diethyl esters (8-8-c dimethyl diferulic acid ester) [18]. However, no detailed product distributions from such transformations of FA dilactone were reported.
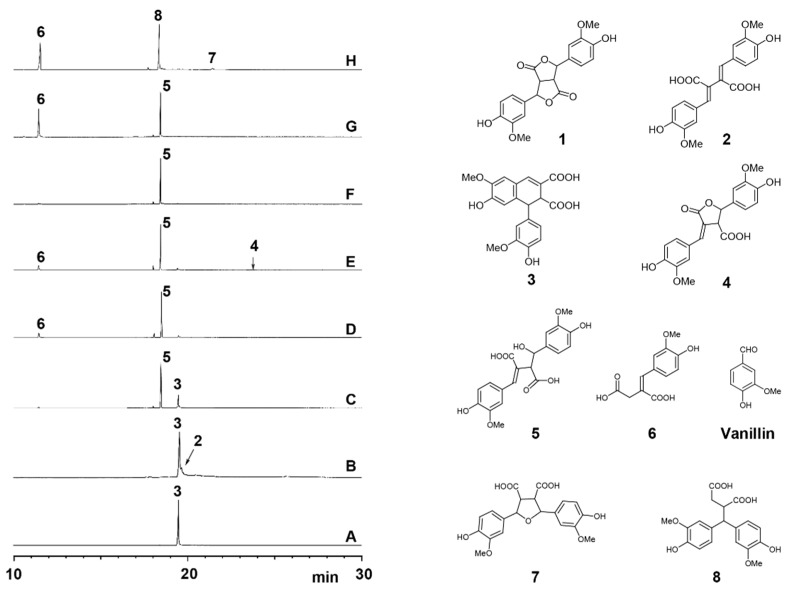
Figure 1.
Left: Total ion chromatograms of crude products obtained from transformation of FA dilactone by 12-h alkali treatments: (A) 4 eq. 0.1M NaOH; (B) 15 eq. 0.1M NaOH; (C) 15 eq. 0.5 M NaOH; and (D–H) 120 eq. NaOH (D) 1.0 M; (E) 2.0 M; (F) 4.0 M; (G) 8.0 M; and (H) 16.0 M). Right: The structures of compounds 1–8 described in this work.
In this work, FA dilactone was reacted under various conditions. Effects of base/acid types, reagent concentrations, and reaction media on the distribution of the resultant products from FA dilactone were investigated by GC-MS and NMR analysis. Products derived from FA dilactone under various treatment conditions were isolated by using Thin Layer Chromatography (TLC) and flash chromatography and were characterized by NMR. Reported here were several new compounds obtained from FA lactone treated under various conditions. Hypothetic mechanisms or pathways leading to those new products were proposed.
2. Experimental Section
2.1. Materials and Methods
Ferulic acid was a commercial product from Wuhan Yuan-Cheng Gong-Chuang Technology, Co. Ltd. (Wuhan, China). The FA dilactone dimer was synthesized according to an enzyme-catalyzed process (details are included in the Supplemental Materials). NH4OH (25%–28% wt), absolute ethanol, 1,4-dioxane, and acetyl chloride were analytical reagent (AR) grade and obtained from Macklin (Shanghai, China). NaOH and Na2CO3 were also purchased from Macklin (Shanghai, China).
The crude products from preparative-scale experiments using various treatment of FA dilactone were separated by flash chromatography (Isolera One, Biotage, Shanghai) with Snap silica columns or TLC (Silica gel 60 F254, 1 mm plate). The isolated compounds were characterized by NMR (Bruker 600 MHz, in acetone-d6). NMR spectrum assignments were made according to published data for known compounds or by 2D NMR experiments including COSY, HSQC, and HMBC for new compounds. The NMR data are included in the Supplementary Materials.
High-resolution mass spectra were acquired on a high-resolution mass spectrometer (HRMS, Agilent 1290/maxis impact, Karlsruhe, Germany) with Electron Spray Ionization (ESI) resource. Trimethylsilyl (TMS-) derivatives of products were analyzed by GC-MS (GCMS-TQ8040, Schimadzu, Shanghai). He (1mL·min−1) was used as the carrier gas. GC conditions were as follows: SH-Rxi-5Sil MS column (30 m × 25 mm × 25 µm); initial temperature at 200 °C and held for 1 min, and ramped at 10 °C min−1 to 280 °C and held for 30 min; injector temperature 250 °C; ionization energy 200 eV; and scan range: m/z 50–700.
2.2. Transformations of FA Dilactone under Various Conditions
2.2.1. Alkali Treatments
The FA dilactone (20–60 mg, 0.05–0.15 mmol) was added separately in aqueous base solutions containing given equivalents of bases (Na2CO3, NH4OH, and NaOH) in various concentrations. The reactions were terminated by adding 1 M HCl to acidify the reaction media to pH value around 3. A given amounts of internal standard dissolved in ethyl acetate was added, and the products were recovered by ethyl acetate (EtOAc) extraction. The organic phase was dried over anhydrous MgSO4. About 0.2 mL EtOAc containing 3 mg of the crude product were taken for TMS-derivatization and subjected to GC-MS analysis.
For another set of NaOH treatments, the recovered products in ethyl acetate solution (wet, without drying) were evaporated to generate an oil-like product. The products were kept at 25 °C overnight prior to GC-MS analysis (their total ion chromatograms showed significant amounts of product 4; Figure S1 in Supplementary Materials).
2.2.2. Acid Treatments
In aqueous solution: The FA dilactone (50 mg, 0.13 mmol) was dissolved in aqueous dioxane (50% or 99%, v/v) solutions containing HCl in various concentrations. The mixture was stirred at 70 °C for 12 h and was monitored by TLC (n-hexanes/EtOAc, 75:25 v/v). The reaction mixtures were evaporated under reduced pressure to remove all water or solvents. The residues were dissolved in 10 mL absolute ethanol, from which a small amount (0.1 mL) of products was allocated for GC-MS analysis. The ethanol solution was evaporated under reduced pressure to dryness. The residues were redissolved in 10 mL absolute ethanol and esterified by slowly adding 0.5 mL acetyl chloride. The mixture was kept at 25 °C for 24 h. The resultant solution was evaporated to dryness under reduced pressure. The residue was dissolved in acetone and evaporated again (this operation was repeated 2 times). The products were analyzed by NMR.
In absolute ethanol: 0.75 mL acetyl chloride was added dropwise into 10 mL absolute ethanol containing 50 mg FA dilactone (0.13 mmol). The reaction mixture was stirred at 25 °C for 12 h when the resultant yellow solution was evaporated under reduced pressure to dryness. The residues were dissolved in 20 mL EtOAc and washed with saturated NH4Cl. The EtOAc solution was dried over anhydrous MgSO4 and filtered off to remove the solids. The products were obtained after evaporating off the EtOAc solvent and were analyzed by NMR.
3. Results and Discussion
3.1. Transformation of Dilactone in Alkali Solutions
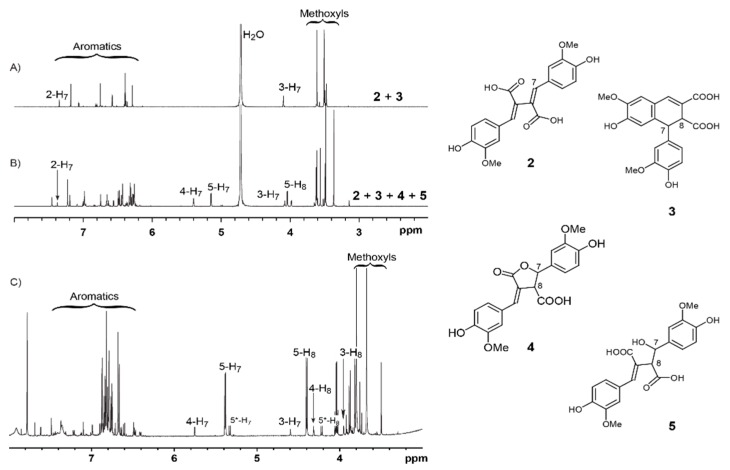
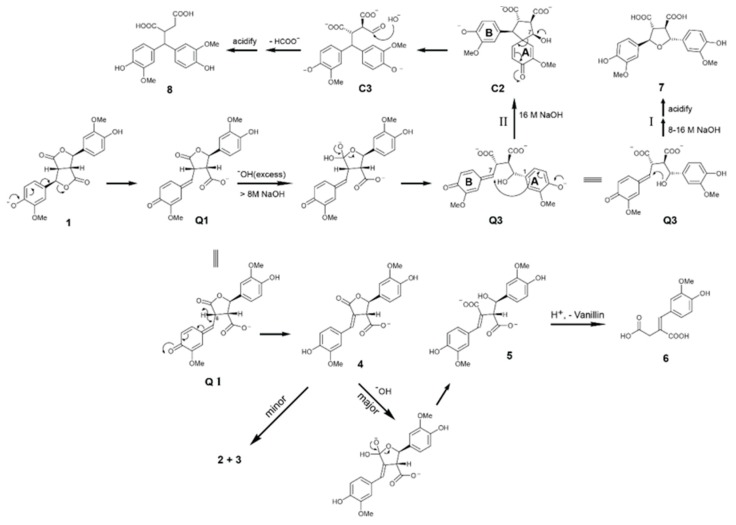
It was reported that lactone product 4 (Figure 1) was obtained in high yield by a 2 M NaOH treatment of FA dilactone at 25 °C followed by a silica column separation [15]. FA dilactone was reacted with 1 M or 2 M aqueous Na2CO3 at 25 °C overnight. producing compounds 2 and 3 completely (Figure S2). We were wondering why the lactone 4 seems pretty stable in 2 M NaOH solution at 25 °C whereas, in aqueous 1 M Na2CO3, no lactone product 4 was obtained from the FA dilactone. To solve this puzzle, the 2 M NaOH treatment of FA lactone was revisited. The crude products from such a treatment (with 2 M NaOH for 16 h) were recovered by ethyl acetate solvent extraction after acidification and directly analyzed by GC-MS. It was surprising that the major product was compound 5 along with small amounts of compound 6 and trace amounts of 3 (Figure 1). NMR analysis of the crude products also confirmed the GC-MS result (Figure 2). The NMR analysis suggested that compound 5 is a hydration product of compound 2. The mass spectrum of TMS-derived 5 has a base ion fragment (m/z) of 297, suggesting that a benzylic hydroxyl (C7-OH) group is present in this compound. Its high-resolution mass data also supported the formula given in the suggested structure of 5.
Figure 2.
In situ 1H NMR (in D2O) of products from ferulic acid (FA) dilactone treated by (A) 1 M Na2CO3 and (B) 1 M NaOH. (C) 1H NMR (in Acetone-d6) of crude products recovered from 2 M NaOH treatment of FA dilactone.
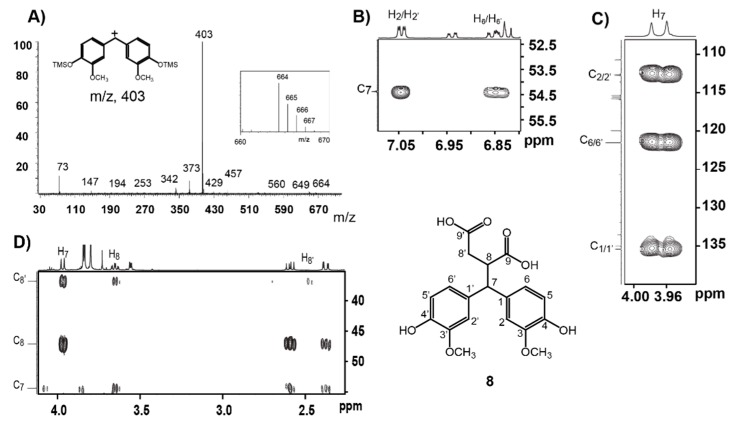
More surprising was that almost no lactone product 4 was detected (Figure 1 left panel, E) although NMR of the crude products proved the presence of compound 4 (Figure 2). This interesting finding promoted further study on transformation of the FA dilactone under various alkali conditions. Thus, effects of NaOH concentrations and its stoichiometry on the transformations of FA dilactone were investigated. The total crude products recovered by ethyl acetate solvent extraction after acidification were directly silylated and analyzed by GC-MS (Figure 1). In 0.1 M NaOH solution, using less than 15 eq. of NaOH, the major product was compound 3, 8-8-c diferulic acid, whereas compound 5, a hydrolysis product, was the main product when in 0.5 M NaOH using 15 eq. of NaOH. When large excess amounts of NaOH (120 eq.) were used, the dominate product was still compound 5 even though the NaOH concentrations used varied from 1 M to 4 M. Higher concentrations resulted in large amount of compound 6, a retro-claisen condensation product. Methyl ether of 6, veratrylidenesuccinic acid, has been obtained from NaOH treatment of dimethyl ether of dilactone 1 before [12]. However, at the concentration (16 M) close to saturation point, a new major product, compound 8, was generated from FA dilactone 1 although compounds 6 and 7 were also detected. In total, seven products, compounds 2–8, were isolated by silica column separation from preparative scale experiments and characterized by NMR (see Supplementary Materials for detailed NMR data). Compounds 2 and 3 were isolated from 0.1 M NaOH treatment. Compound 4 was hardly detected by GC-MS in products generated from any NaOH treatment of dilactone 1 (Figure 1). This contrasted the previous report that compound 4 was obtained in high yield from a 2 M NaOH treatment [15]. However, in an experiment where oil-like products obtained by evaporating the wet (no drying) ethyl acetate solution of products were kept at 25 °C overnight, compound 4 was found to be one of the major products while 5 decreased significantly, as shown by GC-MS analysis (Figure S1, Supplementary Material). This suggested that compound 5 was not stable and converted to compound 4 and/or compound 6 under the storage conditions. It was also found that compound 4 can be formed, apparently from compound 5, during column separation or by mixing with silica gel in ethyl acetate (Figure S3). All these results above suggest that the majority of compound 4 reported might not be formed directly from the NaOH treatment; instead, it was actually generated during posttreatments including silica column separation. In situ NMR experiment also indicated that major species derived from FA dilactone 1 in 1 M NaOH D2O solution (excess amount) is compound 5 while the rest of the minor ones are compounds 2–4 (Figure 2B). Compound 7, a ferulic acid dimer with a symmetry tetrahydrofuran (THF) ring, was identified here for the first time from 16 M NaOH treatment of 1. It was verified by comparing its GC retention time, mass spectrum, and NMR data with those of the reference compound synthesized according to a published method [19]. Compound 8, a rearrangement product, is also a new compound derived from FA dilactone 1. The TMS-derivative of compound 8 (Figure 3A) has a molecular ion (m/z) of 664, which is not typical of any 8-8-coupled FA dimers reported before. The base ion fragment (m/z) of compound 8 (TMS-derived) was 403, implying that product 8 has a bis-guaiacyl methylene structure. Although being mixed with minor compound 7, it was successful to figure out the structure of compound 8 by 2D NMR experiments (Figure 3). From the HMBC spectrum of compound 8 (Figure 3B), it can be seen that the C7 signal (54.38 ppm) correlates to two sets of aromatic proton signals (6.85 ppm and 7.05 ppm, assigned to protons at C6 and C2 of guaiacyl units. In addition, what can be seen (Figure 3C) is that 1H signal at 3.97 ppm (assigned to H7) correlates to three pairs of 13C signals (134.98/135.40 ppm, 112.60/112.75 ppm, and 121.51/121.51 ppm) corresponding to C1, C2, and C6 of guaiacyl units. Further examination of the NMR (Figure 3D) suggested that 1H signals at 3.97 ppm, 3.65 ppm, and 2.59–2.38 ppm are correlated with each other and can be assigned to aliphatic protons at C7, C8, and C8′, respectively. High-resolution mass spectrum also supported the formula of the structure of compound 8 as shown in Figure 3.
Figure 3.
HMBC NMR (B–D) and mass spectra (A) of compound 8, showing characteristic features to establish its chemical structure shown.
All these products were quantified by GC-TIC with their GC-MS response factors against an internal standard, (E)-4-(4-(4-hydroxy-3-methoxyphenyl)but-3-en-2-yl)-2-methoxyphenol (vinlyguaiacol dimer) (Table 1). Na2CO3 and NH4OH with various concentrations were also used for treatment of dilactone 1, and their results are listed in Table 1. Apparently, the ratios of the products from FA dilactone 1 treated by various concentrations (1 M to 5 M) of Na2CO3 at 25 °C did not change that much. This could be explained by the fact that the basicity of aqueous Na2CO3 does not change much in concentrations ranging from 1 M to 5 M due to its buffer capacity (pHs of 1 M, 2 M, and 5 M Na2CO3 solutions are 11.38, 11.55, and 11.72, respectively). However, ammonia concentrations used for treating dilactone 1 produced various ratios of the products 2 and 3, showing that higher ammonia concentration favored the formation of 2. This is because higher concentrations of ammonia (hydroxide ion) would favor the elimination of proton H7 from Q2 (Figure 4), leading to the formation of compound 2.
Table 1.
The products and yields (%) obtained under various base concentrations.
| Base Concentrations | Eq. | Temp. (°C) | Time (h) | Products (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 5 | 6 | 7 | 8 | ||||
| 0.1 M NaOH | 3 | 25 | 16 | 5.8 | 91.4 | ||||
| 0.1 M NaOH | 4 | 5.6 | 92.1 | ||||||
| 0.1 M NaOH | 15 | 6.0 | 89.3 | ||||||
| 0.5 M NaOH | 15 | 2.8 | 65.4 | 21.2 | 4.0 | ||||
| 1 M NaOH | 120 | 3.0 | 7.6 | 79.5 | 3.3 | ||||
| 2 M NaOH | 2.4 | 8.7 | 78.9 | 3.7 | |||||
| 4 M NaOH | 87.2 | 0.8 | |||||||
| 8 M NaOH | 62.5 | 23.4 | 3.7 | ||||||
| 16 M NaOH | 21.8 | 15.5 | 60.2 | ||||||
| 1 M Na2CO3 | 120 | 25 | 16 | 8.7 | 88.7 | ||||
| 2 M Na2CO3 | 9.2 | 89.5 | |||||||
| 5 M Na2CO3 | 8.7 | 89.2 | |||||||
| 0.5 M NH4·OH | 120 | 25 | 16 | 31.5 | 67.7 | ||||
| 5 M NH4·OH | 42.4 | 56.8 | |||||||
| 14 M NH4·OH | 50.9 | 46.5 | |||||||
Figure 4.
The proposed mechanisms leading to the formation of compounds 2 and 3.
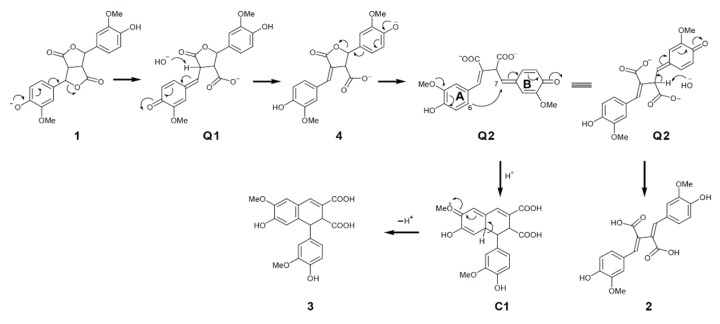
As for how these products were formed from FA dilactone under aqueous alkali treatment conditions, a plausible mechanism or pathways were proposed and illustrated in Figure 4 and Figure 5. In Na2CO3, NH4OH, or diluted NaOH solution, the FA dilactone was converted into quinomethide Q1 that loses a proton from 8-position readily to produce lactone 4, from which quinomethide Q2 was produced through opening the lactone by electron pushing. Two possible options for stabling Q2. One is a nucleophilic attack from carbon-A6 to the 7-position of B-ring to form compound 3 eventually through aromatization of intermediate C1. The other is an elimination of a proton from the 8-position producing compound 2 (Figure 4).
Figure 5.
The proposed mechanisms leading to the formation of products from FA dilactone in aqueous NaOH solution followed by acidification. Note: all compounds are racemic although one isomer is drawn.
As Figure 5 shows, in excess NaOH solutions with molar concentrations beyond 0.5 M, dilactone 1 was prone to quickly forming quinone methide Q1. As an intermediate produced from Q1 by elimination of one H8, compound 4 was hydrolyzed quickly, giving rise to a major product, compound 5, which is suggested or supported by the fact that one dominate diastereomer was detected by GC-MS (Figure 1) or by in situ NMR (Figure 2). In higher (8 M and beyond) concentrations of NaOH, however, direct hydrolysis of Q1 is a fast and dominate reaction generating Q3 (Figure 5). From Q3, two possible pathways lead to the formation of products 7 or 8. In one direction, the newly formed hydroxyl group attacks the C7 of B-ring forming product 7 (pathway I). In another direction (pathway II), the C1 of A-ring competitively attacks the 7-position of the B ring driven by electron pushing from phenoxide of A-ring, forming a spiro-dienone intermediate B, which was then rearranged through cleavage of C1–C7 bonds by the electron pushing from 7-OH of A-ring. The resultant aldehyde C3 is attacked by a hydroxide ion, eliminating a formate to produce compound 8 eventually (Figure 5). Meanwhile, vanillin and compound 6 were also produced from compound 5 through retro-claisen condensation during acidification.
3.2. Transformation of Dilactone in Acidic Solutions
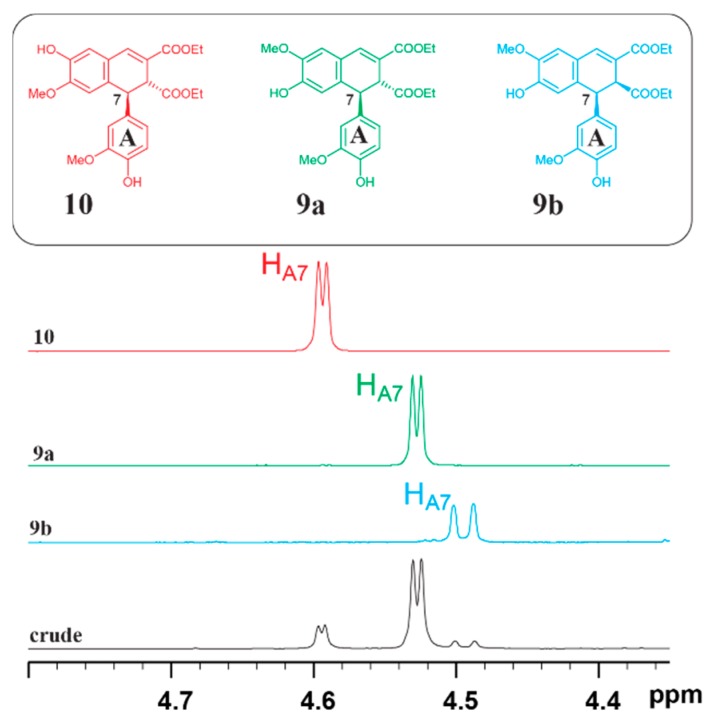
Compound 3 (8-8-c diferulic acid) belongs to an important group of arylnaphthalene lignans with diverse architectures and biological activities [20,21]. Acid-catalyzed transformation of FA dilactone 1 in absolute alcohol (methanol) produced 8-8-c diferulic acid ester, as reported by Takei et al [18]. However, no other products have ever been reported from such acid catalyzed transformations. In the current study, FA dilactone 1 was treated with dry HCl (generated from acetyl chloride and absolute ethanol) in ethanol at 70 °C for 12 h; the crude products were characterized by GC-MS and NMR after completely removing all volatile chemicals. Although GC-MS chromatogram of the crude products showed one major peak accompanied with a minor one (Figure S4, Supplementary Materials), NMR spectrum of the crude products indicated that there was an unknown compound of which the 1H chemical shift at around 4.6 ppm, tentatively assigned to A7-proton, was different from those of the well-documented trans- and cis-isomers of 8-8-c diferulic acid ethyl esters 9a and 9b (Figure 6). Our curiosity led to an effort to reveal the identity of the unknown product. Thus, the crude product of a preparative scale reaction was subjected to a flash silica column separation with n-hexanes/EtOAc (50:50, v/v) as eluent resulting in two fractions. The major fraction contained compound 9, from which the two isomers (9a and 9b) were separated by TLC. The minor fraction was identified by 2D NMR analysis to be compound 10 of which the structure is shown in Figure 6.
Figure 6.
Partial 1H NMR spectra showing the characteristic chemical shifts of A-7 protons in crude products from acid catalyzed transformation of FA dilactone and compounds 9 and 10.
The detailed NMR assignment of compound 10 can be found in the Supplementary Materials (Figures S5 and S6). To the best of our knowledge, it was the first time that compound 10 was discovered in products obtained by such a treatment.
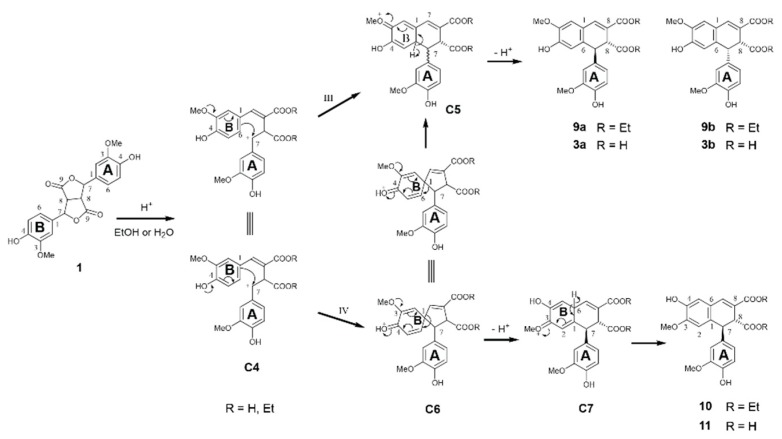
Figure 7 shows the plausible pathway leading to the formation of compounds 9 and 10 from FA dilactone 1. In acidic solutions, the intermediate cation C4 is formed from FA dilactone 1. Nucleophilic attacking from B-ring at its 6-position to the 7-position of A-ring forms cation C5 (pathway III). The elimination of a proton from C5 leads to the formation of compound 9 (9a and 9b). Meanwhile, a competing reaction (pathway IV) is the nucleophilic attacking from B-ring at its 1-position to the 7-position of A-ring, driven by electron pushing from phenolic hydroxyl on B-ring, generating a spiro-dienone C6. Electron pushing from the methoxyl group on the B-ring of C3 would rearrange bonding on the 1-position of the B-ring so that two cations (C5 and C7) are possibly formed, as shown in Figure 7. Re-aromatization of C7 produces compound 10. The molar ratios of these three isomeric products generated in dry 1 M HCl absolute ethanol of FA dilactone was determined by 1H NMR to be 73.4% (9a):8.2% (9b):18.3% (10).
Figure 7.
Proposed mechanisms involved in acid treatment of FA dilactone leading to the formation of compounds 10 and 11.
In acidic (0.5 M HCl) aqueous 1,4-dioxane (50%) solution, dilactone 1 was dominantly transformed to compound 3 although there were small amounts of other products which were difficult to identify by either GC-MS or NMR. Therefore, the crude product was esterified by dry HCl in absolute ethanol and characterized by 1H NMR. Comparing the NMR spectrum of the esterified crude product to that of compounds 9 and 10 confirmed that three compounds, 3a, 3b, and 11, were indeed produced (Figure S7, Supplementary Materials). The molar ratios of these dimers were determined by integrating their characteristic signals (assigned to H7 of A-ring) at around 4.5–4.6 ppm and are listed in Table 2.
Table 2.
The molar ratios of products from FA dilactone under various acidic conditions *.
| Acid Concentrations | Reaction Media | Ratios of Products (%) |
|---|---|---|
| 1.0 M HCl | Dry HCl in ethanol | 10 (18.3):9a (73.4):9b (8.2) |
| 0.5 M HCl | 50% dioxane | 11 (4.1):3a (89.2):3b (6.6) |
| 1.2 M HCl | 50% dioxane | 11 (22.7):3a (70.5):3b (6.8) |
| 1.2 M HCl | 99% dioxane | 11 (27.8):3a (63.8):3b (8.3) |
* Note: reactions were performed at 70 °C for 12 h. The ratios were determined by 1H NMR. The ratios among 11, 3a, and 3b were determined according to the corresponding ratios among 10, 9a, and 9b after esterification.
When acid concentration in reaction media was increased from 0.5 to 1.2 M, the percentage of dimer 11 was increased from 4.1% to 22.7%, suggesting that higher acid concentration favors the formation of compound 11. At the same acid concentration (1.2 M), decreasing water content of the reaction media produced more compound 11. The reaction mechanisms involved for the transformation of FA dilactone in aqueous acid treatment can also be described similarly in Figure 7.
In summary, the identified new compounds in the current study implicate that the easily available FA dilactone 1 poses as an excellent starting material to access diverse diferulic acids. More importantly, compounds produced in the transformation could be considered as “plant-derived” chemicals potentially with good biological activities (studies in this regard are undergoing promising progress), such as antibacterial, antioxidant, and anti-inflammatory abilities.
4. Conclusions
In this study, FA dilactone was reacted under various alkali/acidic conditions to produce degradation products or diferulates with diverse structures. Chemicals (bases or acid), their stoichiometry and concentrations, and reaction media were factors influencing the transformation outcome and reaction pathways leading to various products. New derivatives including 2-(4-hydroxy-3-methoxybenzylidene)-3-(hydroxy(4-hydroxy-3-methoxyphenyl)methyl)succinic acid (compound 5), (trans, trans, trans)-2,5-bis(4-hydroxy-3-methoxyphenyl)-tetrahydrofuran-3,4-dicarboxylic acid (compound 7), 2-(bis(4-hydroxy-3-methoxyphenyl)methyl)succinic acid (compound 8), and diethyl 6-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-methoxy-1,2-dihydronaphthalene-2,3-dicarboxylate (compound 10) from FA dilactone were discovered and identified. Reaction pathways involving spiro-dienone intermediates were proposed to be responsible for the formation of the newly discovered products from the transformation of FA dilactone under various alkali/acidic conditions.
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/2218-273X/10/2/175/s1.
Author Contributions
Conceptualization, F.L.; Funding acquisition, F.L.; Investigation, Y.H. and Y.J.; Methodology, Y.H.; Writing–original draft, Y.H.; Writing–review and editing, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31770621) and by Guangdong Province Science Foundation for Cultivating National Engineering Research Center for Efficient Utilization of Plant Fibers (2017B090903003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.He Z., Chen A.Y., Rojanaskul Y., Rankin G.O., Chen Y.C. Gallic acid, a phenolic compound, exerts anti-angiogenic effects via the PTEN/AKT/HIF-1alpha/VEGF signaling pathway in ovarian cancer cells. Oncol. Rep. 2016;35:291–297. doi: 10.3892/or.2015.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassim M., Achoui M., Mustafa M.R., Moh M.A., Yusoff K.M. Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity. Nutr. Res. 2010;30:650–659. doi: 10.1016/j.nutres.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Vaquero M.J.R., Serravalle L.R.T., Nadra M.C.M.d., Saad A.M.S.d. Antioxidant capacity and antibacterial activity of phenolic compounds from argentinean herbs infusions. Food Control. 2010;21:779–785. doi: 10.1016/j.foodcont.2009.10.017. [DOI] [Google Scholar]
- 4.Boz H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015;50:2323–2328. doi: 10.1111/ijfs.12898. [DOI] [Google Scholar]
- 5.Yue F., Gao R., Piotrowski J.S., Kabbage M., Lu F., Ralph J. Scaled-up production of poacic acid, a plant-derived antifungal agent. Ind. Crops Prod. 2017;103:240–243. doi: 10.1016/j.indcrop.2017.03.045. [DOI] [Google Scholar]
- 6.Zhang A., Lu F., Sun R., Ralph J. Ferulate-coniferyl alcohol cross-coupled products formed by radical coupling reactions. Planta. 2009;229:1099–1108. doi: 10.1007/s00425-009-0894-6. [DOI] [PubMed] [Google Scholar]
- 7.Piotrowski J.S., Okada H., Lu F., Li S.C., Hinchman L., Ranjan A., Smith D.L., Higbee A.J., Ulbrich A., Coon J.J., et al. Plant-derived antifungal agent poacic acid targets beta-1,3-glucan. Proc. Natl. Acad. Sci. USA. 2015;112:E1490–E1497. doi: 10.1073/pnas.1410400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia Y., He Y., Lu F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018;269:480–485. doi: 10.1016/j.foodchem.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Neudorffer A., Desvergne J.-P., Bonnefont-Rousselot D., Legrand A., Fleury M.-B., Largeron M. Protective Effects of 4-Hydroxycinnamic Ethyl Ester Derivatives and Related Dehydrodimers against Oxidation of LDL: Radical Scavengers or Metal Chelators? J. Agric. Food Chem. 2006;54:1898–1905. doi: 10.1021/jf052923p. [DOI] [PubMed] [Google Scholar]
- 10.Neudorffer A., Bonnefont-Rousselot D., Legrand A., Fleury M.-B., Largeron M. 4-Hydroxycinnamic Ethyl Ester Derivatives and Related Dehydrodimers: Relationship between Oxidation Potential and Protective Effects against Oxidation of Low-Density Lipoproteins. J. Agric. Food Chem. 2004;52:2084–2091. doi: 10.1021/jf035068n. [DOI] [PubMed] [Google Scholar]
- 11.Bunzel M., Funk C., Steinhart H. Semipreparative isolation of dehydrodiferulic and dehydrotriferulic acids as standard substances from maize bran. J. Sep. Sci. 2004;27:1080–1086. doi: 10.1002/jssc.200301703. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright N.J., Haworth R.D. The constituents of natural phenolic resins. Part XIX. The oxidation of ferulic acid. J. Chem. Soc. 1944:535–538. doi: 10.1039/jr9440000535. [DOI] [PubMed] [Google Scholar]
- 13.Adelakun O.E., Kudanga T., Parker A., Green I.R., Roes-Hill M.L., Burton S.G. Laccase-catalyzed dimerization of ferulic acid amplifies antioxidant activity. J. Mol. Catal. B Enzym. 2012;74:29–35. doi: 10.1016/j.molcatb.2011.08.010. [DOI] [Google Scholar]
- 14.Quideau S., Ralph J. Synthesis of 4,8-bis(4-hydroxy-3-methoxyphenyl)-3,7-dioxabicyclo[3.3.0]-octan- 2-ones and determination of their relative configuration via long-range proton couplings. J. Chem. Soc. Perkin Trans. 1. 1993:653–659. doi: 10.1039/p19930000653. [DOI] [Google Scholar]
- 15.Ralph J., Quideau S., Grabber J.H., Hatfield R.D. Identification and synthesis of new ferulic acid dehydrodimers present in grass cell walls. J. Chem. Soc. Perkin Trans. 1. 1994:3485–3495. doi: 10.1039/p19940003485. [DOI] [Google Scholar]
- 16.Li W., Liu H., Xu J., Zang P., Liu Q., Li W. Regioselective biomimetic oxidative cyclization strategy for the synthesis of aryldihydronaphthalene lignans. Eur. J. Org. Chem. 2014;16:3475–3482. doi: 10.1002/ejoc.201301825. [DOI] [Google Scholar]
- 17.Datta P.K., Yau C., Hooper T.S., Brigitte L.Y., Charlton J.L. Acid-catalyzed cyclization of 2,3-dibenzylidenesuccinates: Synthesis of lignans (±)-cagayanin and (±)-galbulin. J. Org. Chem. 2001;66:8606–8611. doi: 10.1021/jo0161025. [DOI] [PubMed] [Google Scholar]
- 18.Takei Y., Mori K., Matsui M. Synthesis of dl-matairesinol dimethyl Ether, dehydrodimethyl-conidendrin and dehydrodimethylretrodendrin from ferulic acid. Agric. Biol. Chem. 1973;37:637–641. [Google Scholar]
- 19.Stevenson R., Williams J.R. Synthesis of tetrahydrofuran lignans, (±)-galbelgin and (±)-grandisin. Tetrahedron. 1977;33:285–288. doi: 10.1016/0040-4020(77)80107-5. [DOI] [Google Scholar]
- 20.Luo J., Hu Y., Kong W., Wang M. Evaluation and Structure-Activity Relationship Analysis of a New Series of Arylnaphthalene lignans as Potential Anti-Tumor Agents. PLoS ONE. 2014;9:1–16. doi: 10.1371/journal.pone.0093516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y., Hui J., Zhu L. Synthesis and bioevaluation of novel arylnaphthalene lignans as anticancer agents. J. Med. Chem. 2013;22:2505–2510. doi: 10.1007/s00044-012-0245-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.