Abstract
Hydrogen sulfide (H2S) is one of the important biological mediators involved in physiological and pathological processes in mammals. Recently developed H2S donors show promising effects against several pathological processes in preclinical and early clinical studies. For example, H2S donors have been found to be effective in the prevention of gastrointestinal ulcers during anti-inflammatory treatment. Notably, there are well-established medicines used for the treatment of a variety of diseases, whose chemical structure contains sulfur moieties and may release H2S. Hence, the therapeutic effect of these drugs may be partly the result of the release of H2S occurring during drug metabolism and/or the effect of these drugs on the production of endogenous hydrogen sulfide. In this work, we review data regarding sulfur drugs commonly used in clinical practice that can support the hypothesis about H2S-dependent pharmacotherapeutic effects of these drugs.
Keywords: hydrogen sulfide, H2S donors, H2S pro-drugs, sulfur-containing drugs, cardiovascular, neuromodulation, anticancer drug, anti-inflammatory agents
1. Hydrogen Sulfide in Physiology and Pharmacology
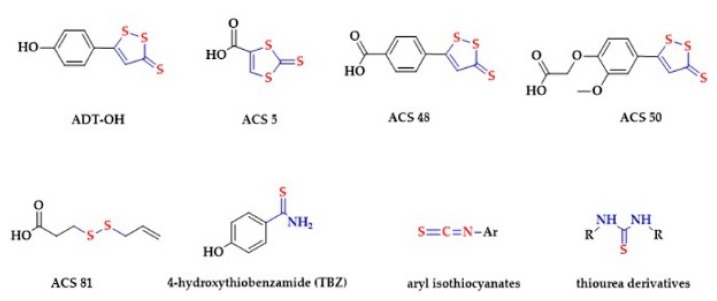
Hydrogen sulfide (H2S) is produced by a variety of organisms, e.g., bacteria, fungi, plants and animals. First reports linking H2S with the smell of rotten eggs can be traced back in the 18th century (reviewed in [1]). Similarly, the toxic effects of H2S on mammals have been known over the centuries. The 1996 report from Abe and Kimura, suggesting the role of endogenously produced H2S in neuromodulation, started a new era in H2S research, and its role in biology and medicine [2]. Later, a number of important biological actions of H2S were described, including vasorelaxation [3], changes in brain neurotransmission [4,5,6], and the effect on neuronal K+ channel activity [7]. These effects are believed to be mediated by physiological concentrations of H2S. Therefore, H2S is now regarded as a third gaseous signaling molecule, next to nitric oxide (NO) and carbon monoxide (CO). In order to develop H2S-releasing donors, researchers started to modify chemical structures of well-described sulfide releasing agents, obtaining several H2S donors including Lawesson’s reagent and analogues [8], DTT (1,2-dithiole-3-thiones) derivatives like ADT-OH, ACS 5, ACS 48 and ACS 50 [9,10,11], diallyl disulfide (DADS) derivatives like ACS 81 [12], arylthioamides (TBZ) [13], aryl isothiocyanates [14] and thiourea derivatives [15] (Figure 1).
Figure 1.
Examples of hydrogen sulfide (H2S)-releasing groups that can be coupled to existing pharmacologically active compounds.
Interestingly, there are numerous well-established medicines which contain sulfur moieties. It can be assumed that drugs containing sulfur in their structure may release H2S or affect its endogenous production. The possibility of releasing H2S from drugs can enhance their biological activity and provide additional therapeutic benefits, but also generate some adverse effects. This paper reviews experimental and clinical data that may suggest that the pharmacological effects of several commonly used drugs may in part depend on the presence of sulfur groups and/or on the release of H2S from the active molecule.
1.1. H2S Production
The H2S gas is colorless and flammable. Free sulfide is a weak acid that dissociates in the body fluids with pH 7.4, the pKa1 = 6.8 and pKa2 > 12 at 37 °C to yield ~20% of H2S and ~80% of HS– and negligible amounts of S2- [16]. In cellular compartments the pH affects the relative proportion to total sulfide, from 90% of HS– in the mitochondrial matrix (pH = 8) to over 90% of H2S in lysosomes (pH = 5). The lipophilic property enables a rapid diffusion of H2S through the lipid bilayer of cell membranes [17]. On the other hand, HS– is not permeable and requires transporters in order to enter the cell [18,19].
H2S is produced in mammalian organisms by non-enzymatic and enzymatic pathways. Sulfate-reducing bacteria (SRB) colonize the gut and in the presence of an electron donor reduce sulfate to produce H2S [20]. In addition, erythrocytes are able to convert elemental sulfur to HS- by non-enzymatic reduction [21]. H2S is generated in the tissues by cysteine metabolizing enzymes, cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfurtransferase (3-MST) in conjunction with cysteine aminotransferase (CAT). CBS and CSE are a part of the reverse transsulfuration pathway. The β-replacement reaction of homocysteine with serine is catalyzed by the CBS and generates cystathionine. CSE catalyzes the α,γ-elimination of cystathionine to cysteine, α-ketobutyrate and NH3. H2S is generated subsequently by the β-elimination reaction of cysteine catalyzed by either CBS or CSE. Alternatively, CSE catalyzes the conversion of cystine to thiocysteine, pyruvate and NH3, subsequently liberating H2S [22]. CAT catalyzes the conversion of cysteine to α-ketoglutarate, producing 3-mercaptopyruvate. 3-MST subsequently forms a persulfide on the enzyme, liberating H2S under reducing conditions [23]. CBS represents the main H2S-generating enzyme in the brain, whereas CSE dominates in the cardiovascular system [24,25]. The activity of 3-MST seems to be highest in the adrenal cortex [26]. The expression of the enzyme was also reported in erythrocytes [27]. All enzymes can be found in the lungs, liver, kidneys and gastrointestinal tract [28,29,30,31]. Regarding the sub-cellular distribution, CBS and CSE are cytosolic enzymes [32], whereas 3-MST is present mostly in the mitochondria [33]. However, translocation of these enzymes between compartments is possible under specific conditions [33,34]. In addition to cysteine metabolism, several other pathways of H2S biosynthesis were proposed, including the reduction of thioredoxin by catalase or thiosulfate by thiosulfate reductase [35,36]. Finally, gut bacteria express specific H2S-producing enzymes, namely cysteine desulfhydrase [37,38] and sulfite reductase [39].
1.2. H2S Excretion
The main route of elimination is the oxidation of H2S in the mitochondria. H2S is converted to thiosulfate and further oxidized to sulfate and excreted by the kidneys. The main enzymes involved in the elimination pathway are sulfide quinone oxidoreductase (SQR), persulfide dioxygenase (ETHE1), thiosulfate sulfurtransferase (TST) and sulfite oxidase (SO). Firstly, a SQR cysteine persulfide is formed. The sulfane can be further transferred to glutathione to form glutathione persulfide or to sulfite and form thiosulfate. The glutathione persulfide may be oxidized by ETHE1 and thiosulfate by TST to regenerate sulfite, which is oxidized by SO to sulfate [36,40,41]. Additionally, a part of H2S is exhaled or scavenged in the blood by methemoglobin to form sulfhemoglobin [42,43,44].
1.3. H2S Concentrations in Plasma and Tissues
The concentration of free H2S in plasma and tissues is in nanomolar range [45]. In contrast, very high concentrations of H2S are found in the large intestine [46,47,48]. This is because of enzymatic production by the intestinal tissue, and non-enzymatic and enzymatic production by gut microbiota. It has been found that colonic epithelial cells convert sulfide into thiosulfate more efficiently than other tissues [49,50]. Shen et al. reported that germ-free mice have lower systemic levels of H2S in plasma and various tissues, suggesting that gut microbiota regulates the systemic bioavailability and metabolism of H2S [51].
Free H2S may exist in bound form as sulfane sulfur or acid labile sulfur. Inorganic (H2Sn) or organic (RSnH or RSnR) persulfides (n = 2) and polysulfides (n = 3–8) represent the sulfane sulfur store [52]. These species are formed either by direct interaction between H2S and oxidants (GSSG, NO) or by enzymatic oxidation. For instance, the persulfidation of 3-MST (3-MST-SSH) or SQR (SQR-SSH) can represent a source of organic persulfides [32,35]. In addition, 3-MST, super oxide dismutase (SOD) and catalase may oxidize H2S and form inorganic and organic per-/poly-sulfides [36,53,54,55,56]. Interestingly, catalase acts as sulfide-sulfur oxido-reductase, catalyzing both the H2S oxidation or the thiols reduction and H2S production [34]. Endogenous reductants subsequently liberate H2S from sulfane sulfur stores or the sulfane may be transported and transferred to other molecules to mediate sulfur signaling [52,56]. The acid labile sulfur is formed by the interaction between H2S and iron centers of proteins. However, the H2S release from the acid labile store requires low pH < 5.4 [57].
1.4. H2S Signaling
A number of cellular and molecular mechanisms of H2S actions have been proposed, including the interaction of H2S with several ion channels, enzymes regulating redox balance, the persulfidation or a direct interaction with heme proteins.
Increasing evidence suggests that physiological effects of H2S are linked with the persulfidation of the target protein residues [58,59]. The persulfidation is a crucial post-translational modification that regulates the function of the proteins. In order to form a cysteine persulfide, the oxidation of H2S to per-/poly-sulfide or the oxidation of the target cysteine to sulfenic acid or disulfide is needed [60]. Recently, the endogenous source of persulfides was identified in the mitochondria, namely the cysteinyl–tRNA synthetases, which incorporate cysteine persulfides into the proteins during translation. It was hypothesized that the cysteine persulfides may be released to cytosol in order to mediate further post-translational persulfidation of target proteins [61].
In addition, the interaction of H2S with metal centers of target proteins, particularly the interaction with heme proteins, was investigated thoroughly [62]. H2S may induce a covalent modification of heme, resulting in sulfheme formation [63]. Secondly, the oxidative detoxification of H2S by heme proteins results in the formation of polysulfides and thiosulfate [27]. For instance, the toxic effect of H2S is based on the inhibition of mitochondrial electron transport at cytochrome C oxidase [64,65,66]. H2S reversibly binds to the heme center of cytochrome C oxidase, thereby inhibiting the binding of oxygen, resulting in the shutdown of ATP generation [66,67]. On the other hand, low concentrations of H2S (≤1 µM) stimulate cellular energetic. The persulfidation of SQR is coupled with the transfer of electrons to coenzyme Q, thereby enhancing mitochondrial electron transport, resulting in higher ATP production [68,69].
H2S may also modulate the production and activity of other gasotransmitters. The persulfidation of endothelial NO-synthase (eNOS) Cys433 residue promotes the production of NO [70]. The persulfidation of Keap 1 Cys151 leads to the dissociation of the protein from Nrf2, subsequent translocation of Nrf2 into the nucleus, thereby promoting the heme oxygenase 1 (HO-1) induced CO production [71]. Similar to persulfidation, NO may modulate protein function via S-nitrosation. However, Wolhuter et al. reported that S-nitrosation is not a stable regulatory modification in the cells. They proposed that S-nitrosothiols are transient intermediates that react with thiols to form stable persulfides [72]. The direct interaction between H2S and NO results in the formation of biologically active nitrosopersulfide and polysulfides [73,74,75,76]. In addition, H2S may interact with other reactive species, e.g., oxygen, nitrogen, sulfur and selenium species. These species are produced by various cellular enzymes (NADPH oxidase, xanthine oxidase, uncoupled NOS) and their mutual interaction leads to the formation of numerous products, contributing mostly to the redox biology of the cell [77,78,79,80,81].
1.5. H2S in the Cardiovascular System
Vasodilation and blood pressure lowering induced by exogenous H2S salts and H2S donors have been reported by several groups [82,83,84,85,86,87,88,89,90,91,92]. The endogenous production of H2S by CSE was decreased in various types of hypertension, e.g., in spontaneously hypertensive rats, in rats with pulmonary hypertension and in women with pre-eclampsia, compared to healthy controls [24,93,94,95]. Moreover, the deletion of CSE in mice resulted in the development of hypertension and impaired endothelium-dependent vasorelaxation [24]. We have recently shown that, besides tissue enzymes, the gut microbiota-derived H2S may be involved in the development of hypertension [83,89,96]. In addition, H2S donors were found to relax corpus cavernosum and were tested for the treatment of erectile dysfunction [97,98,99,100].
The opening of ATP-sensitive potassium channels (KATP) is believed to mediate the vasodilation induced by H2S donors [83,86,101]. Namely, the activation of the channel by persulfidation of the sulfonylurea receptor 1 (SUR1) Cys6 and Cys26 subunit [101,102]. Several studies confirm that the H2S-related vasodilation is associated with the promotion of NO synthesis (Cys433 eNOS persulfidation) and/or NO signalling (reviewed in [103]). For instance, the H2S/NO interaction product nitrosopersulfide mediates vasodilation and increases levels of cyclic guanosine monophosphate (cGMP) [73]. In addition, Stubbert et al. proposed a NO-independent mechanism of direct activation of protein kinase G (PKG) 1α by Cys42 persulfidation. They showed that transgenic knock-in mice, where Cys42 within PKG1α is replaced with redox-dead Ser, do not respond to H2S salt by blood pressure lowering [104].
The administration of H2S donors stimulates vascularization (reviewed in [105]) and silencing of 3-MST reduces cell growth, migration and network formation [106]. The activation of vascular endothelial growth factor (VEGF) or the inhibition of phosphatase and tensin homolog (PTEN) were proposed to mediate the pro-angiogenic actions of H2S. In detail, the direct reduction of Cys1024-Cys1045 within VEGF2 by H2S was reported [107]. In addition, the persulfidation of Cys68 and Cys755 specificity protein 1 (Sp 1) promoted the transcription of VEGF2 [108]. Greiner et al. observed the formation of PTEN Cys124 and Cys71 disulfide bond as a response to H2S salts [60].
1.6. H2S and the Immune System
Two major pathways regulate the inflammatory signaling in cells, namely the nuclear factor-κB (NF-κB) pathway and nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway. Accumulating evidence suggests that H2S signaling promotes the Nrf2 signaling, thereby activating the antioxidant defense of the cell [71,109,110,111,112,113,114,115]. Nrf2 is sequestered by Kelch-like ECH-associated protein (Keap) 1 in the cytosol. The persulfidation of Keap 1 Cys151 leads to the dissociation of the protein from Nrf2 and subsequent translocation of Nrf2 to the nucleus, thus promoting the transcription of antioxidant response elements [71]. In addition, the persulfidation of Cys38 p65 subunit of NF-κB augments the binding to the ribosomal protein S3 (RPS3), thereby promoting the transcription of anti-apoptotic genes [116].
1.7. H2S in the Nervous System
Memory loss was reported in individuals exposed to toxic concentration of H2S [117]. In contrast, the breakthrough report from Abe and Kimura showed that micromolar H2S concentrations facilitate the induction of hippocampal long-term potentiation (LTP) [2]. The activation of N-methyl-D-aspartate (NMDA) receptor and the induction of Ca2+ influx by transient receptor potential ankyrin1 (TRPA1) channel opening were proposed to mediate the LTP induction by H2S donors [118]. In detail, the administration of sulfide salts and inorganic polysulfides led to the persulfidation of TRPA1 N-terminal cysteine residues and Ca2+ influx in astrocytes [118]. In addition, Kimura proposed that these sulfide species activate the NMDA receptor indirectly via the downstream TRPA1 signaling [119,120].
Decreased levels of H2S were reported in neurodegenerative disorders including Alzheimer disease and Parkinson disease in comparison to healthy controls [121,122,123,124]. Besides, lower expression of CSE was found in patients with Huntington disease [123]. On the other hand, a mutation of ETHE1 gene was found in ethylmalonyl encephalopathy patients resulting in the accumulation of H2S in the brain [125]. Several studies support the neuroprotective effects of H2S (reviewed in [118]). H2S may promote the glutathione (GSH) production via the activation of cystine/glutamate antiporter, cysteine transporter or glutamate cysteine lyase, and thus promote the antioxidative defense [126,127]. In addition, the opening of KATP and cystic fibrosis transmembrane conductance regulator Cl- channels by H2S results in stabilizing of the neuronal plasma membrane [127]. The inactivation of neuroprotective ubiquitin E3 ligase of parkin plays a crucial role in the development of Parkinson disease. Vandiver et al. showed that persulfidation of parkin promotes the ubiquitin E3 ligase activity and thus mediates cytoprotection. Furthermore, they found that Parkinson’s patients have depleted persulfidated parkin in the brain [128].
1.8. Other Effects of H2S
A bell-shaped model characterizes the cellular effects of H2S. At lower concentrations, H2S promotes cell survival, whereas higher H2S concentrations can lead to cell death. The cytoprotective and anti-inflammatory properties of H2S are associated with faster dermal wound healing, mucosal defense and ulcer healing in the gastrointestinal system (reviewed in [129,130]). An improved clinical severity index of psoriasis was shown after the topical administration of H2S donor [131]. Furthermore, H2S-releasing derivatives of nonsteroidal anti-inflammatory drugs (HS-NSAIDs) reduced the gastric damage induced by the corresponding parent drugs (reviewed in [132]). H2S donors were also shown to relieve visceral pain [133,134,135] and an HS-trimebutine is now in Phase II clinical trials as an abdominal analgesic (NCT01926444). The role of H2S has also been investigated in the etiology of cancer and diabetes, however, the studies show contradictory results [136,137,138,139,140,141,142,143,144,145,146,147]
2. Sulfur-Drugs and Their Therapeutic Potential
Sulfur is essential to the life and growth of all organisms and plays a crucial role in the regulation of various biological processes in the human body. Sulfur can obtain oxidation states anywhere between −2 to +6 and represents one of the most chemically versatile elements. Generally, organo-sulfur compounds are organic compounds containing a carbon–sulfur bond. Many organo-sulfur compounds are sulfur equivalents of oxygen-containing organic compounds, for example, thioethers, thiols or thioesters. Therefore, sulfur-containing products can form a variety of molecular arrangements and exhibit diverse biological activities. The organo-sulfur compounds were already used as ointments with mild antiseptic effects in ancient times. The colloidal sulfur was regularly administered to patients suffering from rheumatoid arthritis. At present, the diversity of elements among approved pharmaceuticals reveal that sulfur is the fifth most used element after carbon, hydrogen, oxygen and nitrogen [148]. Sulfur-derived functional groups possess a variety of pharmacological properties and represent a useful tool for the development of new therapeutic agents. Sulfur moieties can be found in pharmaceuticals with various therapeutic applications, particularly in antihypertensive drugs, analgesics, antibacterial, anti-inflammatories, anticancer agents and many others.
2.1. Natural Products Containing Hydrogen Sulfide-Releasing Moieties
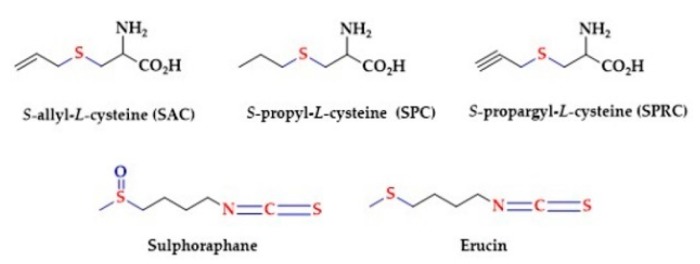
Natural products capable of releasing H2S have drawn a lot of attention [149]. Commonly isolated compounds from sulfur natural products are allyl-substituted polysulfides (mainly in form di-, trisulfides and/or tetrasulfides) [79,150,151,152,153]. The garlic-derived sulfur compounds like the diallyl disulfide (DADS) require the presence of reduced glutathione to release H2S. H2S generation relies on nucleophilic substitution of GSH at the a-carbon of the allyl substituent to form an allyl perthiol, which further undergoes a thiol/disulfide exchange to release H2S. Similarly, red blood cells released H2S rapidly from DADS under anoxic conditions and in the presence of glutathione [154]. The health benefits of garlic have been postulated for thousands of years and several studies demonstrated the positive impact of garlic on the cardio-vascular system. This includes lowering of arterial blood pressure, the reduction of blood cholesterol and platelet aggregation, and the reduction of oxidative stress. It was suggested that S-allyl-l-cysteine (SAC) is a potential source of H2S and is responsible for the cardioprotective effects of garlic. Other garlic-derived compounds are S-propyl-L-cysteine (SPC) and S-propargyl-l-cysteine (SPRC) [155,156,157]. In addition to garlic, there are many other natural products containing functional groups that can be considered as potential H2S donors, for example Sulphoraphane and Erucin (Figure 2) [158,159].
Figure 2.
Naturally occurring H2S-donating compounds.
Sulforaphane is sulfur-organic molecule from the group of isothiocyanates. Sulphoraphane occurs in cruciferous vegetables and its highest concentrations are found in broccoli sprouts. Sulphoraphane has been postulated to exert anticancer property, to suppress the proliferation of prostate cancer cells and to enhance the expression of CBS and CSE [160]. It has also been postulated that the consumption of Broccoli sprouts, containing Sulforaphane, reduces nephropathy and vascular complications [158].
2.2. Sulfur Amino Acids
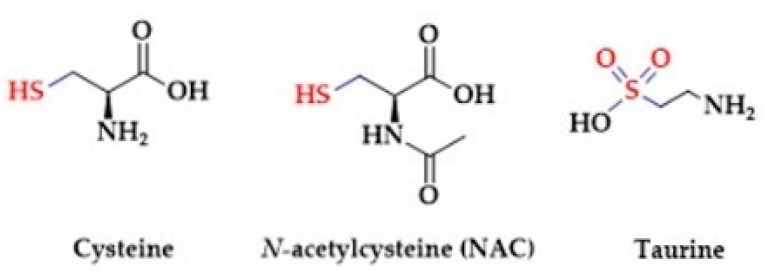
Several studies confirm that dietary sulfur amino acids, cysteine and taurine (Figure 3), have beneficial effects on human health [161].
Figure 3.
The sulfur-containing amino acids.
Sulfur amino acids participate in the synthesis of essential bio-molecules like antioxidants, vitamins and co-factors (thiamine, lipoic acid, biotin, coenzyme A). Giannis et al. showed that thiol amino acids are potential H2S donors [162]. They observed the release of H2S from thioglycine and thiovaline (Figure 4) in the presence of bicarbonate. In addition, both sulfur amino acids promoted the cGMP formation and relaxation of mouse aortic rings [163].
Figure 4.
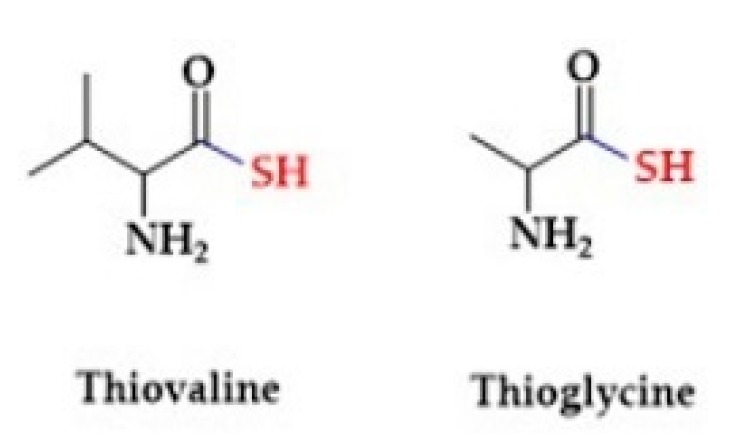
The structures of selected thiol amino acids.
2.2.1. Cysteine
The chemical structure of cysteine contains a nucleophilic thiol (-SH) (Figure 3) that may be readily oxidized, thus mediating biological activity of the cells. The thiol group enables direct scavenging of free radicals or the regeneration of oxidized molecules to their reduced states. Furthermore, cysteine serves as a substrate for the production of glutathione and H2S. Cysteine residues incorporated within proteins play a key role in the regulation of structural and functional properties of proteins. Particularly, the formation of cysteine disulfides and persulfidation of cysteine residues (described in section Signaling) are crucial post-translational modifications. Cysteine is endogenously produced from an essential amino acid methionine. In detail, the demethylation of methionine results in the formation of S-Adenosyl-l-homocysteine (SAH), which is subsequently hydrolyzed to homocysteine. Homocysteine enters the transulfuration pathway to produce cysteine by CBS and CSE. Accumulating evidence suggests that cysteine plays a key role in the maintenance of mammalian homeostasis [164,165,166,167,168]. However, due to its unstable nature cysteine is not suitable for clinical use. N-acetylcysteine (NAC) has been used instead as a nutritional supplement over the years. Several reports confirm that administration of NAC prevented the development of hypertension in rodents and humans. In addition, NAC attenuated the hypertensive-related complications, namely increased nitric oxide bioavailability, improved renal function and attenuated the development of insulin resistance. In addition, the antioxidant properties of NAC are used to prevent the development of neurodegenerative disorders, inflammatory bowel disease or to treat paracetamol-induced poisoning [169,170].
2.2.2. Taurine
Taurine (2-aminoethanesulfonic acid) is one of the few naturally occurring sulfonic acids -SO3H (Figure 3). It is endogenously produced via cysteine sulfinic acid pathway or acquired by diet [171,172]. In detail, the thiol moiety of cysteine is oxidized by cysteine dioxygenase to sulfinic acid. Further decarboxylation by sulfinoalanine decarboxylase forms hypotaurine, which is subsequently oxidized to taurine by hypotaurine dehydrogenase. Taurine is abundant in the brain, retina, skeletal muscle and liver of mammals. The transport of taurine through plasma membranes is mediated via transporters: SLC6A6 (TauT) and SLC36A1 (PAT1) [173]. Taurine is an important substrate for microbial production of H2S. Taurine is used by known intestinal microbe Bilophila wadsworthia as an electron acceptor for anaerobic respiration. This pathway results in sulfite production, which is subsequently converted to H2S [174]. Similar to cysteine, blood pressure lowering and antioxidative effects were reported after taurine supplementation [175,176,177,178,179,180]. Moreover, the development of hypertension was accelerated in taurine-deficient rats [181]. Taurine does not incorporate into proteins and the biochemical nature of its actions is not clear. Interestingly, the antihypertensive effect of taurine was associated with increased levels of H2S in the plasma of prehypertensive patients. Taurine upregulates the expression of H2S-synthesizing enzymes CBS and CSE, and thereby contributes to increasing the level of endogenous H2S [182].
2.3. Antihypertensive Drugs
Hypertension is a leading cause of morbidity and mortality worldwide. Numerous antihypertensive drug classes were developed, e.g., renin–angiotensin–aldosterone system (RAAS) inhibitors, calcium channel blockers, beta-blockers and diuretics. The RAAS is a key regulator of blood volume and systemic vascular resistance. The decrease of systemic blood pressure leads to the release of renin by the kidneys, thus stimulating the formation of angiotensin, which in turn promotes the release of aldosterone from the adrenal cortex, resulting in sodium and water retention in the kidney [183,184]. To date, over 20 compounds targeting the RAAS have been introduced, and some of them possess a sulfur moiety.
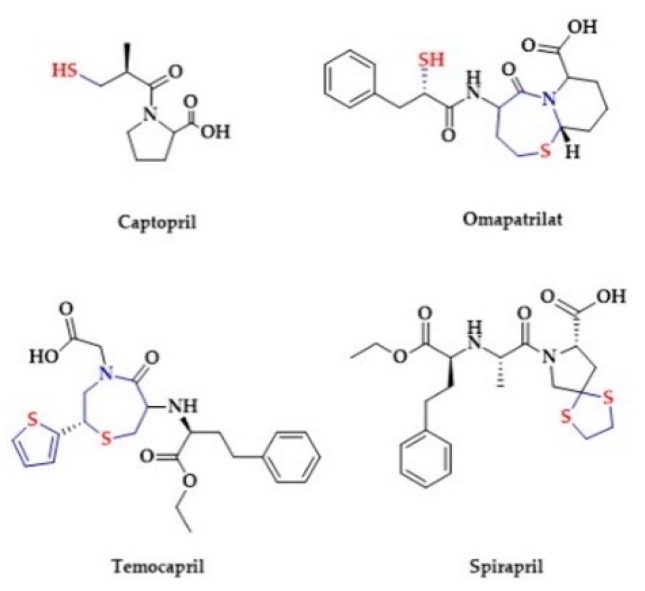
The group of angiotensin-converting enzyme inhibitors (ACE-I) is a cornerstone of antihypertensive treatment [185,186]. The first ACE-I, i.e., Captopril, was patented and approved for clinical use in the 1980 [187]. The chemical structure of Captopril contains a thiol. In the plasma Captopril forms its disulfide or reacts with cysteine and glutathione to form mixed disulfides, thus representing a sulfane sulfur source (Figure 5).
Figure 5.
The structures of selected ACE inhibitors.
However, the possible involvement of sulfide signaling in the Captopril-dependent effects remains unclear. Besides Captopril, Lisinopril is also administered as an active drug. Other ACE-I inhibitors are pro-drugs, undergoing hydrolysis in the liver to active forms containing a hydroxyl group [188]. Zofenopril, an ACE-I inhibitor approved for medical use in 2000, undergoes hydrolysis and forms an active metabolite Zofenoprilat containing a thiol group (Figure 6). Several studies confirmed that Zofenopril administration increases the levels of H2S-metabolites in the plasma of mice and pigs [188]. Pro-angiogenic, anti-inflammatory and anti-apoptotic actions of Zofenopril were reported in association with H2S release [189,190,191,192,193].
Figure 6.
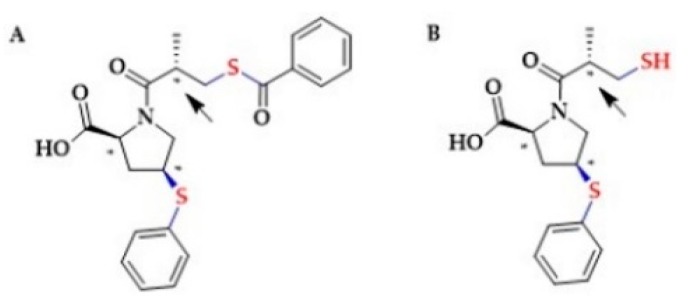
The structures of (A) Zofenopril and (B) Zofenoprilat. The asterisks denote the S configurations at the chiral centers. The arrow points to the only carbon atom whose configuration leads to R-Zofenoprilat.
In addition, Bucci et al. reported that Zofenopril improved vascular function in a model of spontaneous hypertension, which was associated with H2S release and was dependent on the inhibition of ACE. Namely, S-Zofenoprilat, the active diasteroisomer, as well as the inactive R-Zofenoprilat, restored vascular response of hypertensive rats (Figure 6). On the other hand, Enalapril, a non-thiol ACE inhibitor, failed to improve the vascular function (Figure 7) [192].
Figure 7.
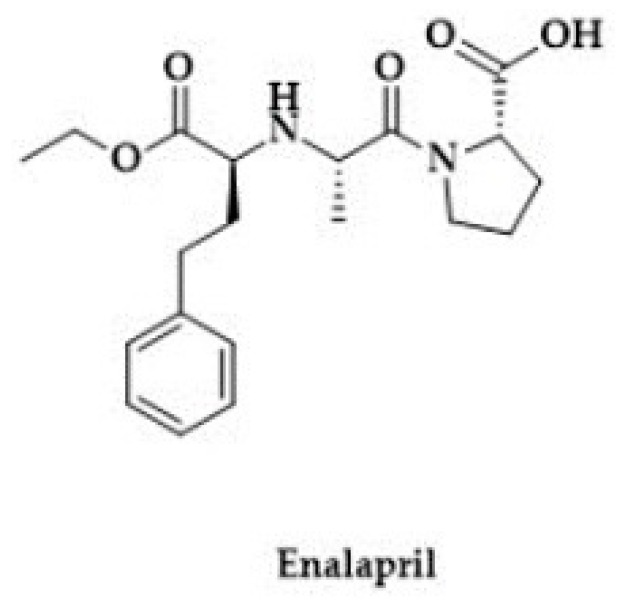
The structure of Enalapril.
Spirapril and Temocapril are hydroxyl-based ACE inhibitors, administered in the pro-drug esterified form. The chemical structure of these drugs contains cyclic sulfur moieties (Figure 5). Spirapril contains a sulfur atom in a dithioketal ring. Temocapril contains two sulfur atoms, one in the thiophene ring and the other in thiazepine ring [193]. In 2003, an experimental ACE inhibitor Omapatrilat was introduced. Omapatrilat contains a hydroxyl group as well as a thiol group and another sulfur atom in a thiazepine ring (Figure 5). It can simultaneously inhibit ACE and neutral endopeptidase (NEP). Interestingly, the ACE inhibition by Omapatrilat is longer in comparison to Enalapril [194]. Another sulfur-based drug is Remikiren, a direct renin inhibitor containing a sulfonyl moiety in its structure (Figure 8) [195].
Figure 8.
The structures of renin inhibitors Remikiren and Enalkiren.
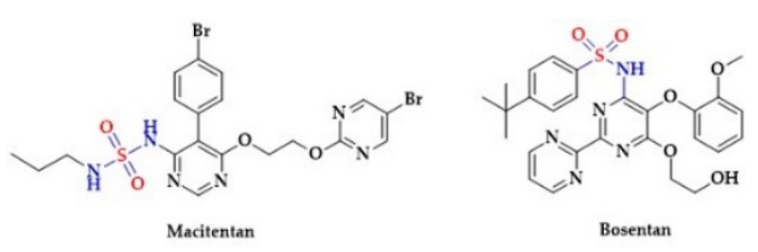
Notably, a clinical study showed a greater potency of Remikiren to lower blood pressure in comparison to a non-sulfur renin inhibitor Enalkiren [196]. Several other antihypertensive drugs possess a sulfonic group in their structure. For instance, endothelin receptor antagonists Macitentan and Bosentan contain sulfones in their structure (Figure 9) [197,198].
Figure 9.
The structures of selected endothelin receptor antagonists.
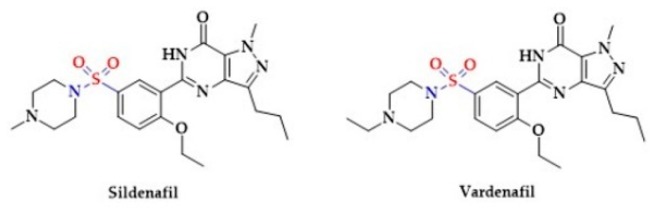
Moreover, the phosphodiesterase inhibitors, Vardenafil [199,200,201,202] and Sildenafil [199,203,204], used for the treatment of erectile dysfunction, are sulfonic acids (Figure 10).
Figure 10.
The structures of selected inhibitors of phosphodiesterase activity.
In addition, the calcium-channel blocker Diltiazem contains a thiazepine ring (Figure 11) [205].
Figure 11.
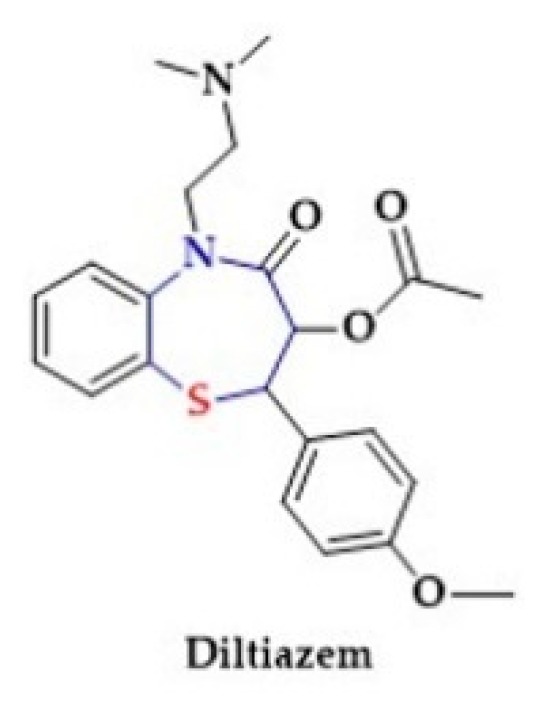
The structure of calcium-channel blocker (Diltiazem).
2.4. Central Nervous System Agents
Numerous studies show that H2S exerts a number of biological actions in the Central Nervous System (CNS), including anti-inflammatory, anti-oxidant, anti-apoptotic, and neuroprotective effects [206].
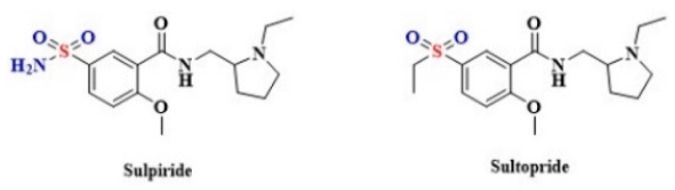
Despite the potentially beneficial effect of H2S on cellular functions, an excessive amount of H2S and polysulfides may impair brain functions in what is referred to as the so-called “sulfide stress” [207]. Sulfide stress is characterized by an increase in H2S/polysulfide production as a result of elevated levels of 3-MST enzyme. This may result from an inflammatory/oxidative insult to the brain. There is some evidence that the H2S/polysulfide production system is upregulated in schizophrenia. A more detailed explanation of the role of sulfide stress in the development of schizophrenia may give a new direction to develop a more effective treatment for this disorder [208]. However, it is worth stressing that several sulfur-based drugs are used in the treatment of schizophrenia, including Sulpiride and Sultopride. These medicines contain a sulfonamide group that is S-linked to a benzene ring (Figure 12) [209,210,211]. Whether the use of sulfuric drugs may affect the course of schizophrenia by modulating the endogenous H2S levels is unknown.
Figure 12.
The structures of Sulpiride and Sultopride.
Parkinson’s disease is a neurodegenerative disorder caused by progressive loss of dopaminergic neurons in the substantia nigra. The most widely used therapy is Levodopa (L-DOPA), but it does not stop disease progression [212]. Numerous studies indicate that the endogenous H2S levels are markedly reduced in various Parkinson’s disease models. Xue et al. showed that NaHS treatment reduces the loss of substantia nigra neurons and slows the development of motor dysfunction in animal models [213]. Other groups also found that intraperitoneal injection of NaHS (as H2S donor) and the inhalation of H2S exerted protective effects in animal models of Parkinson’s disease [214]. Based on these reports, it was stated that the combination of L-DOPA and H2S may have a potential therapeutic value. Lee at al. have developed four L-DOPA hybrids based on coupling L-DOPA to different hydrogen sulfide-donating compounds: ACS 48, ACS 50, ACS 5 and ACS 8 (Figure 13). H2S donor structures present in L-DOPA hybrids release hydrogen sulfide by hydrolysis.
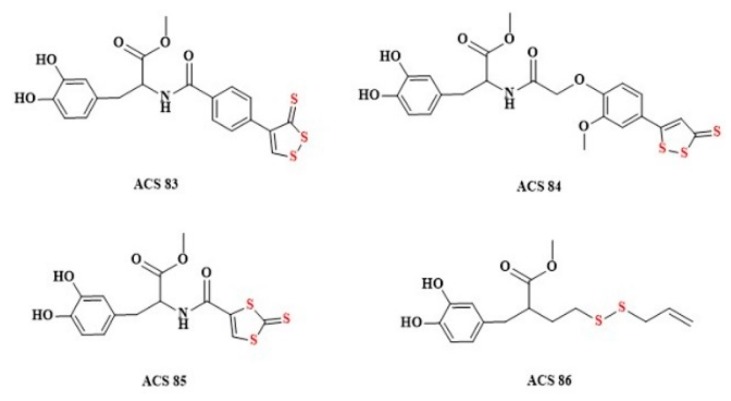
Figure 13.
The structures of the H2S-releasing L-DOPA derivatives (ACS83, ACS84, ACS85, and ACS86).
After intravenous administration of H2S-releasing L-DOPA derivatives (Figure 13) a large increase in dopamine and glutathione has been observed in intracerebral fluid [215].
2.5. Dithiolethiones and Their NSAID Hybrids
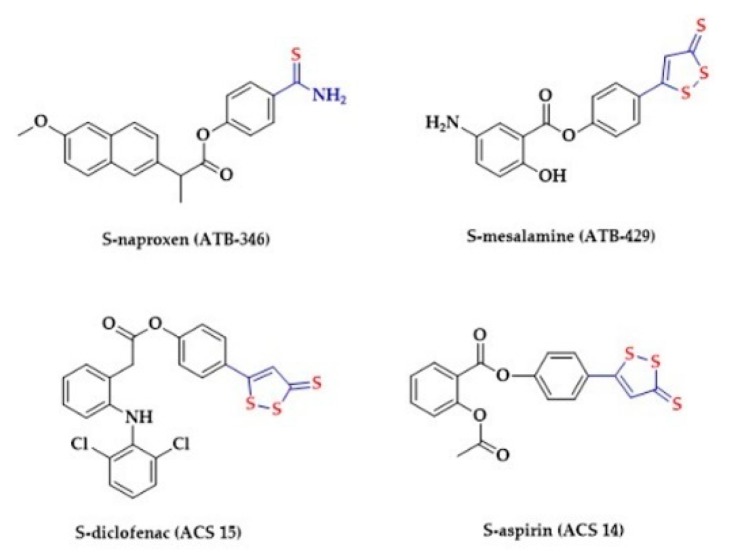
NSAIDs have high efficacy in reducing pain and inflammation. The NSAIDs act by the inhibition of cyclooxygenases (COXs). Traditional NSAIDs are non-specific inhibitors of both COX-1 and COX-2. Adverse effects of NSAIDs on the gastrointestinal tract are associated with the reduction of prostaglandin synthesis due to the inhibition of COX-1. Numerous studies showed that H2S may reduce adverse effects of NSAIDs in the gastrointestinal tract [216,217]. 1,2-Dithiole-3-thiones (DTTs), anethole trithione (ADT) and the phenol derivative of ADT (ADT-OH) belong to the family of hydrolysis-triggered H2S donors (Figure 14). A rapid generation of H2S from DTT derivates was observed in the presence of mitochondria [218]. They are commonly used in the design of HS-NSAIDs (hydrogen sulfide-releasing non-steroidal anti-inflammatory drugs) [217,218]. Sparatore et al. synthesized a S-aspirin (ACS 14) and compared the gastric damages caused by ACS 14 and aspirin in rats (Figure 14) [219]. ACS 14 protected the gastric mucosa through increased H2S/glutathione production, HO-1 activation and isoprostane suppression. S-diclofenac (ACS 15) has also been studied. This drug showed increased anti-inflammatory activity compared to diclofenac in several models [220,221]. Another hybrid drug, S-mesalamine (ATB-429), has been well characterized in animal models of Crohn’s disease and ulcerative colitis and has turned out to be more effective than mesalamine [222]. Similarly, S-naproxen (ATB-346) has been found to cause less gastric damage than its parent drug [223]. Chattopadhyay et al. evaluated the effects of four different HS-NSAIDs on the growth of different human cancer cell lines. All tested HS-NSAIDs effectively inhibited the growth of cancer cells [224].
Figure 14.
The structures of selected HS-NSAIDs.
2.6. The Coxibs, Selective Inhibitors of Cyclooxygenase-2 (COX-2)
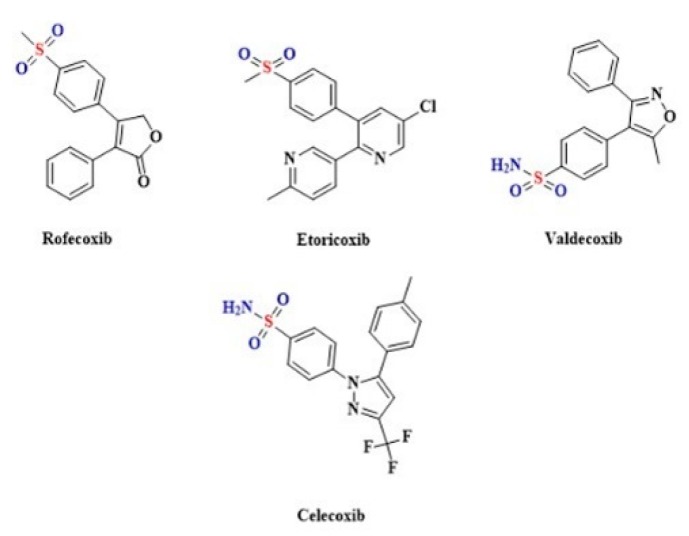
The Coxibs belong to the group of anti-inflammatory drugs that are selective inhibitors of COX-2 [225,226]. Celecoxib, Rofecoxib [227], Etoricoxib [228] and Valdecoxib [229] contain a sulfonamide group that is S-linked to a benzene ring (Figure 15).
Figure 15.
The structures of selective COX-2 inhibitors.
Treatment with selective COX-2 inhibitors such as Celecoxib seems to produce fewer side effects in comparison with non-selective NSAIDs [230,231]. Szabó et al. synthesized a series of Celecoxib derivatives with various substituents on the benzenesulfonamide moiety. The gastrointestinal adverse reaction profile was more favorable compared to the parent drug [232]. Celecoxib is often used to counteract the multiple side effects of Cyclosporin A (CsA), an immunosuppressant drug used in the treatment of inflammatory diseases of autoimmune origin [233,234,235]. H2S was shown to prevent the CsA-induced vasomotor alteration and nephrotoxicity [236,237]. In addition, Helmy et al. confirmed that upregulation of CSE/H2S pathway underlies the capacity of Celecoxib to compromise the hypertensive and renal insult caused by CsA in rats [238].
2.7. Thiourea Derivatives As Antithyroid and Anesthetics Drugs
The antithyroid activity of thiourea and its derivatives has been confirmed in numerous studies. Thyreostatics containing thiourea in a cyclic form are Propylthiouracil, Thiamazole and Carbimazole (Figure 16).
Figure 16.
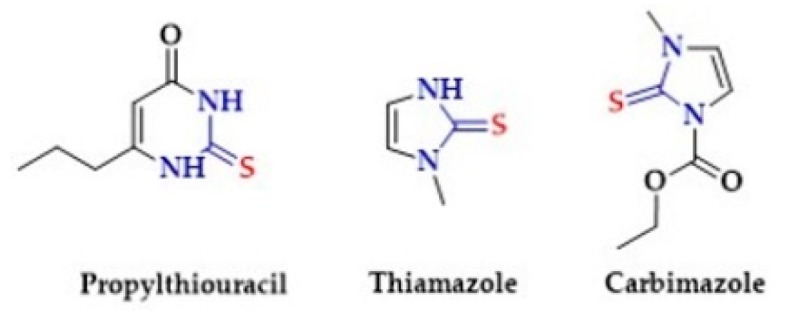
The structures of Propylthiouracil, Thiamazole and Carbimazole.
Propylthiouracil inhibits the synthesis of thyroxine and inhibits conversion of thyroxine to triiodothyronine. Thiamazole (other name Methimazole) may directly inhibit thyroid peroxidase or directly inhibit thyroglobulin, hence reducing the production of the thyroid hormones T3 and T4 (thyroxine) [239,240]. Carbimazole is a pro-drug which is converted to the active form, methimazole [241]. In our work from 2018, we proved that compounds based on thiourea can act as controlled hydrolysis-based H2S donors [15]. In turn, numerous studies indicate an association between thyroid hormone (TH) level and H2S level [242,243].
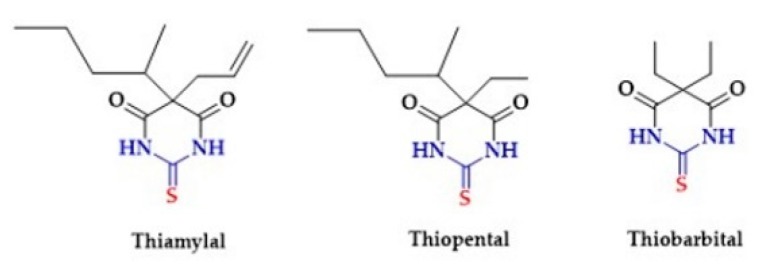
Another group of drugs containing thiourea moiety in the structure are barbiturates. Barbiturates act as CNS depressants. They are also used as anxiolytics, hypnotics, and anticonvulsants. The examples of sulfur-containing barbiturates are Thiamylal, Thiopental and Thiobarbital (Figure 17). Both Thiamylal, and Thiopental are used for short-term anesthesia and short surgical procedures associated with minimal painful stimuli [244,245]. Thiobarbital has sedative effects [246].
Figure 17.
The structures of Thiamylal, Thiopental and Thiobarbital.
2.8. Other Drugs
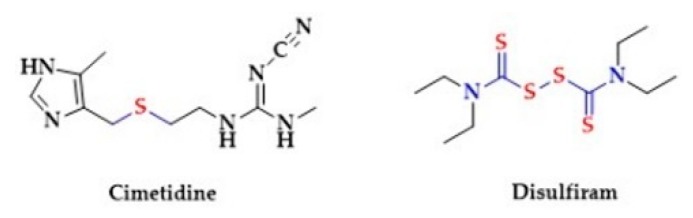
Disulfiram in chemical terms is tetraethylthiuram disulfide. Thiuram disulfides are a class of organo-sulfur compounds with the formula (R2NCSS)2 (Figure 18).
Figure 18.
The structures of Cimetidine and Disulfiram.
There are two dithiocarbamate subunits which are linked by an S−S bond in the chemical structure of Disulfiram. This drug is used for the treatment of alcohol dependence [247]. It belongs to a group of aldehyde dehydrogenase inhibitors that increase the blood level of acetaldehyde after the ingestion of ethanol. The disulfiram–ethanol reaction (DER) is the cause of highly unpleasant symptoms referred to as “acetaldehyde syndrome,” including flushing, systemic vasodilation, respiratory difficulties, nausea and hypotension. The latter is one of the most common and potentially life-threatening side effects of the drug. The observed blood pressure-lowering effect has been attributed to the vasodilatory action of acetaldehyde [248].
Cimetidine, a histamine receptor blocker, contains a sulfur moiety in the form of thioether (Figure 18). Thioethers are sulfuric ether analogues with the general formula R−S−R. The effects of Cimetidine include reduction of gastric acid secretion and reduction in gastric volume and acidity. Interestingly, a common side-effect of Cimetidine is hypotension. It has been reported, that intravenous administration of Cimetidine induces a short-lasting (5–15 min) hypotension in anaesthetized rats due to arterial vasodilatation. Notably, the pretreatment with diphenhydramine, an antihistamine agent, did not reduce the hypotensive effect. This suggests no involvement of histamine receptors in the hypotensive action of cimetidine. It may be speculated that the release of H2S from the thioether moiety may be responsible for the cimetidine-induced hypotension [249].
Several studies suggest that H2S may regulate cancer cell growth and tumor progression and that the expression of CSE and CBS is reduced in antiandrogen-resistant prostate cancer cells. Additionally, in antiandrogen-resistant prostate cancer cells, lower levels of endogenous H2S were found [250,251]. Interestingly, Enzalutamide [252,253] and Apalutamide [254], the androgen receptor antagonists that are used in the prostate cancer treatment, are N, N-disubstituted thiourea derivatives (Figure 19). The thiourea moiety presence in the structure of Enzalutamide and Apalutamide may release H2S and strengthen their androgen receptor antagonist properties. Hydrolysis is the mechanism of H2S generation from thiourea derivatives. [15].
Figure 19.
The structures of selected androgen receptor (AR) antagonists.
3. Perspectives and Limitations
Accumulating evidence suggests that H2S contributes to the regulation of essential biological processes in mammals. In spite of the significant progress in the field of developing H2S donors, there is still a lack of compounds that would meet all requirements for the ideal H2S donor in clinical studies. Notably, there are a number of commonly used drugs containing sulfur moieties, which have been found to release H2S ex vivo, and some of them in vivo. This may significantly contribute to pharmacokinetics and pharmacodynamics of those drugs. Nevertheless, there are significant gaps in our knowledge that hinder clinical use of H2S donors. A list of questions to be answered includes, but is not limited to, the following: (i) What are therapeutic vs. toxic concentrations of H2S and its products? (ii) What are the mechanisms of H2S release from the drug? (iii) How to deliver H2S chronically in vivo at a constant rate? (iv) How to monitor plasma concentration of H2S and its products? (v) What are the mechanisms of H2S action?
4. Conclusions
There are numerous well-established medicines containing sulfur moieties that release H2S ex vivo and may release H2S in vivo. Thus, the sulfur moieties present in the drug structure may function as an H2S donor and/or affect endogenous H2S metabolism. Further research is needed to clarify whether the released H2S may contribute to the therapeutic effect of these drugs, and, if so, which of the mechanisms is dominant. If it is true, the addition of sulfur moieties may significantly affect the pharmacotherapeutic profile of parent drugs.
Author Contributions
All authors have contributed to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Centre, Poland, grant no. UMO-2016/22/E/NZ5/00647. L.T. was supported by the VEGA Grant Agency of the Slovak Republic, grant no. 2/0079/19 and the University Science Park for Biomedicine (ITMS 26240220087).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wang R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 2.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruszyna H., Kruszyna R., Smith R.P. Cyanide and sulfide interact with nitrogenous compounds to influence the relaxation of various smooth muscles. Proc. Soc. Exp. Biol. Med. 1985;179:44–49. doi: 10.3181/00379727-179-42062. [DOI] [PubMed] [Google Scholar]
- 4.Kombian S.B., Warenycia M.W., Mele F.G., Reiffenstein R.J. Effects of acute intoxication with hydrogen sulfide on central amino acid transmitter systems. Neurotoxicology. 1988;9:587–595. [PubMed] [Google Scholar]
- 5.Warenycia M.W., Goodwin L.R., Benishin C.G., Reiffenstein R.J., Francom D.M., Taylor J.D., Dieken F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharm. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 6.Warenycia M.W., Kombian S.B., Reiffenstein R.J. Stress-induced increases in brainstem amino acid levels are prevented by chronic sodium hydrosulfide treatment. Neurotoxicology. 1990;11:93–98. [PubMed] [Google Scholar]
- 7.Kombian S.B., Reiffenstein R.J., Colmers W.F. The actions of hydrogen sulfide on dorsal raphe serotonergic neurons in vitro. J. Neurophysiol. 1993;70:81–96. doi: 10.1152/jn.1993.70.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Ozturk T., Ertas E., Mert O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007;107:5210–5278. doi: 10.1021/cr040650b. [DOI] [PubMed] [Google Scholar]
- 9.Zanatta S.D., Jarrott B., Williams S.J. Synthesis and Preliminary Pharmacological Evaluation of Aryl Dithiolethiones with Cyclooxygenase-2-Selective Inhibitory Activity and Hydrogen Sulfide-Releasing Properties. Aust. J. Chem. 2010;63:946–957. doi: 10.1071/CH09517. [DOI] [Google Scholar]
- 10.Perrino E., Cappelletti G., Tazzari V., Giavini E., Soldato P.D., Sparatore A. New sulfurated derivatives of. valproic acid with enhanced histone deacetylase inhibitory activity. Bioorg. Med. Chem. Lett. 2008;18:1893–1897. doi: 10.1016/j.bmcl.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Tazzari V., Cappelletti G., Casagrande M., Perrino E., Renzi L., Del Soldato P. New aryldithiolethione derivatives as potent histone deacetylase inhibitors. Bioorg. Med. Chem. 2010;18:4187–4194. doi: 10.1016/j.bmc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y.R., Hu C.H. Computational Study of H2S Release in Reactions of Diallyl Polysulfides with Thiols. J. Phys. Chem. B. 2017;121:6359–6366. doi: 10.1021/acs.jpcb.7b03683. [DOI] [PubMed] [Google Scholar]
- 13.Martelli A., Testai L., Citi V., Marino A., Pugliesi I., Barresi E., Nesi G., Rapposelli S., Taliani S., Da Settimo F., et al. Arylthioamides as H2S donors: L-cysteineactivated releasing properties and vascular effects in vitro and in vivo. ACS Med. Chem. Lett. 2013;4:904–908. doi: 10.1021/ml400239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martelli A., Testai L., Citi V., Marino A., Bellagambi F.G., Ghimenti S., Breschi M.C., Calderone V. Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vascul. Pharmacol. 2014;60:32–41. doi: 10.1016/j.vph.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Zaorska E., Hutsch T., Gawryś-Kopczyńska M., Ostaszewski R., Ufnal M., Koszelewski D. Evaluation of thioamides, thiolactams and thioureas as hydrogen sulfide (H2S) donors for lowering blood pressure. Bioorg. Chem. 2019;88:10294. doi: 10.1016/j.bioorg.2019.102941. [DOI] [PubMed] [Google Scholar]
- 16.Hughes M.N., Centelles M.N., Moore K.P. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: A review. Free Radic. Biol. Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Mathai J.C., Missner A., Kügler P., Saparov S.M., Zeidel M.L., Lee J.K., Pohl P. No facilitator required for membrane transport of hydrogen sulfide. Proc. Natl. Acad. Sci. USA. 2009;106:16633–16638. doi: 10.1073/pnas.0902952106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings M.L. Transport of H2S and HS− across the human red blood cell membrane: Rapid H2S diffusion and AE1-mediated Cl−/HS− exchange. Am. J. Physiol. Cell Physiol. 2013;305:C941–C950. doi: 10.1152/ajpcell.00178.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czyzewski B.K., Wang D.N. Identification and characterization of a bacterial hydrosulphide ion channel. Nature. 2012;483:494–497. doi: 10.1038/nature10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbonero F., Benefiel A.C., Gaskins H.R. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2012;9:504–518. doi: 10.1038/nrgastro.2012.85. [DOI] [PubMed] [Google Scholar]
- 21.Searcy D.G., Lee S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998;282:310–322. doi: 10.1002/(SICI)1097-010X(19981015)282:3<310::AID-JEZ4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Singh S., Padovani D., Leslie R.A., Chiku T., Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav P.K., Yamada K., Chiku T., Koutmos M., Banerjee R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013;288:20002–20013. doi: 10.1074/jbc.M113.466177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S., et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bao L., Vlcek C., Paces V., Kraus J.P. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch. Biochem. Biophys. 1998;350:95–103. doi: 10.1006/abbi.1997.0486. [DOI] [PubMed] [Google Scholar]
- 26.Taniguchi T., Kimura T. Role of 3-mercaptopyruvate sulfurtransferase in the formation of the iron-sulfur chromophore of adrenal ferredoxin. Biochim. Biophys. Acta. 1974;364:284–295. doi: 10.1016/0005-2744(74)90014-X. [DOI] [PubMed] [Google Scholar]
- 27.Vitvitsky V., Yadav P.K., Kurthen A., Banerjee R. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J. Biol. Chem. 2015;290:8310–8320. doi: 10.1074/jbc.M115.639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabil O., Vitvitsky V., Xie P., Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linden D.R., Sha L., Mazzone A., Stoltz G.J., Bernard C.E., Furne J.K., Levitt M.D., Farrugia G., Szurszewski J.H. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J. Neurochem. 2008;106:577–585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramasamy S., Singh S., Taniere P., Langman M.J., Eggo M.C. Sulfide-detoxifying enzymes in the human colon are decreased in cancer and upregulated in differentiation. Am. J. Physiol. Gastrointest Liver Physiol. 2006;291:G288–G296. doi: 10.1152/ajpgi.00324.2005. [DOI] [PubMed] [Google Scholar]
- 31.Mudd S.H., Finkelstein J.D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J. Biol. Chem. 1965;240:4382–4392. [PubMed] [Google Scholar]
- 32.Teng H., Wu B., Zhao K., Yang G., Wu L., Wang R. Oxygen-sensitive mitochondrial accumulation of cystathionine beta-synthase mediated by Lon protease. Proc. Natl. Acad. Sci. USA. 2013;110:12679–12684. doi: 10.1073/pnas.1308487110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson M.R., Melideo S.L., Jorns M.S. Human sulfide: Quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 34.Shibuya N., Tanaka M., Yoshida M., Ogasawara Y., Togawa T., Ishii K., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal N., Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine beta-synthase sumoylation. PLoS ONE. 2008;3:e4032. doi: 10.1371/journal.pone.0004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson K.R., Gao Y., DeLeon E.R., Arif M., Arif F., Arora N., Straub K.D. Catalase as a sulfide-sulfur oxido-reductase: An ancient (and modern?) regulator of reactive sulfur species (RSS) Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awano N., Wada M., Mori H., Nakamori S., Takagi H. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl. Environ. Microbiol. 2005;71:4149–4152. doi: 10.1128/AEM.71.7.4149-4152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumagai H., Sejima S., Choi Y.J., Tanaka H., Yamada H. Crystallization and properties of cysteine desulfhydrase from Aerobacter aerogenes. FEBS Lett. 1975;52:304–307. doi: 10.1016/0014-5793(75)80831-3. [DOI] [PubMed] [Google Scholar]
- 39.Murphy M.J., Siegel L.M., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. VI. The reaction of carbon monoxide with the Escherichia coli holoenzyme, the hemoprotein, and free siroheme. J. Biol. Chem. 1974;249:1610–1614. [PubMed] [Google Scholar]
- 40.Augustyn K.D., Jackson M.R., Jorns M.S. Use of Tissue Metabolite Analysis and Enzyme Kinetics To Discriminate between Alternate Pathways for Hydrogen Sulfide Metabolism. Biochemistry. 2017;56:986–996. doi: 10.1021/acs.biochem.6b01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung M., Kasamatsu S., Matsunaga T., Akashi S., Ono K., Nishimura A., Morita M., Abdul Hamid H., Fujii S., Kitamura H., et al. Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 2016;480:180–186. doi: 10.1016/j.bbrc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 42.Nichol A.W., Hendry I., Morell D.B. Mechanism of formation of sulphhaemoglobin. Biochim. Biophys. Acta. 1968;156:97–108. doi: 10.1016/0304-4165(68)90108-6. [DOI] [PubMed] [Google Scholar]
- 43.Saeedi A., Najibi A., Mohammadi-Bardbori A. Effects of long-term exposure to hydrogen sulfide on human red blood cells. Int. J. Occup. Environ. Med. 2015;6:20–25. doi: 10.15171/ijoem.2015.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costello B.P., Ewen R.J., Ratcliffe N.M. A sensor system for monitoring the simple gases hydrogen, carbon monoxide, hydrogen sulfide, ammonia and ethanol in exhaled breath. J. Breath Res. 2008;2:037011. doi: 10.1088/1752-7155/2/3/037011. [DOI] [PubMed] [Google Scholar]
- 45.Furne J., Saeed A., Levitt M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1479–R1485. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 46.Levitt M.D., Springfield J., Furne J., Koenig T., Suarez F.L. Physiology of sulfide in the rat colon: Use of bismuth to assess colonic sulfide production. J. Appl. Physiol. 2002;92:1655–1660. doi: 10.1152/japplphysiol.00907.2001. [DOI] [PubMed] [Google Scholar]
- 47.Magee E.A., Richardson C.J., Hughes R., Cummings J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000;72:488–1494. doi: 10.1093/ajcn/72.6.1488. [DOI] [PubMed] [Google Scholar]
- 48.Deplancke B., Finster K., Graham W.V., Collier C.T., Thurmond J.E., Gaskins H.R. Gastrointestinal and microbial responses to sulfate-supplemented drinking water in mice. Exp. Biol. Med. 2003;228:424–433. doi: 10.1177/153537020322800413. [DOI] [PubMed] [Google Scholar]
- 49.Furne J., Springfield J., Koenig T., DeMaster E., Levitt M.D. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: A specialized function of the colonic mucosa. Biochem. Pharmacol. 2001;62:255–259. doi: 10.1016/S0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 50.Levitt M.D., Furne J., Springfield J., Suarez F., DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. J. Clin. Investig. 1999;104:1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen X., Carlström M., Borniquel S., Jädert C., Kevil C.G., Lundberg J.O. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic. Biol. Med. 2013;60:195–200. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuto J.M., Ignarro L.J., Nagy P., Wink D.A., Kevil C.G., Feelisch M., Cortese-Krott M.M., Bianco C.L., Kumagai Y., Hobbs A.J., et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018;592:2140–2152. doi: 10.1002/1873-3468.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H2S) and Production of Reactive Sulfur Species (RSS) by superoxide dismutase. Redox Biol. 2018;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura Y., Toyofuku Y., Koike S., Shibuya N., Nagahara N., Lefer D., Ogasawara Y., Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015;5:14774. doi: 10.1038/srep14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kimura Y., Koike S., Shibuya N., Lefer D., Ogasawara Y., Kimura H. 3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S. Sci. Rep. 2017;7:10459. doi: 10.1038/s41598-017-11004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikami Y., Shibuya N., Kimura Y., Nagahara N., Ogasawara Y., Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 57.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 58.Mustafa A.K., Gadalla M.M., Sen N., Kim S., Mu W., Gazi S.K., Barrow R.K., Yang G., Wang R., Snyder S.H. HS signals through protein S-Sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang D., Du J., Tang C., Huang Y., Jin H. H2S-Induced Sulfhydration: Biological Function and Detection Methodology. Front. Pharmacol. 2017;8:608. doi: 10.3389/fphar.2017.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greiner R., Palinkas Z., Basell K., Becher D., Antelmann H., Nagy P., Dick T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013;19:1749–1765. doi: 10.1089/ars.2012.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akaike T., Ida T., Wei F.Y., Nishida M., Kumagai Y., Alam M.M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., et al. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 2017;8:1177. doi: 10.1038/s41467-017-01311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boubeta F.M., Bieza S.A., Bringas M., Palermo J.C., Boechi L., Estrin D.A., Bari S.E. Hemeproteins as Targets for Sulfide Species. Antioxid. Redox Signal. 2020;32:247–257. doi: 10.1089/ars.2019.7878. [DOI] [PubMed] [Google Scholar]
- 63.Rios-Gonzalez B.B., Roman-Morales E.M., Pietri R., Lopez-Garriga J. Hydrogen sulfide activation in hemeproteins: The sulfheme scenario. J. Inorg. Biochem. 2014;133:78–86. doi: 10.1016/j.jinorgbio.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petersen L.C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta. 1977;460:299–307. doi: 10.1016/0005-2728(77)90216-X. [DOI] [PubMed] [Google Scholar]
- 65.Hill B.C., Woon T.C., Nicholls P., Peterson J., Greenwood C., Thomson A.J. Interactions of sulphide and other ligands with cytochrome c oxidase. An electron-paramagnetic-resonance study. Biochem. J. 1984;224:591–600. doi: 10.1042/bj2240591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nicholls P. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts of the reduced alpha-peak. Biochim. Biophys. Acta. 1975;396:24–35. doi: 10.1016/0005-2728(75)90186-3. [DOI] [PubMed] [Google Scholar]
- 67.Nicholls P., Petersen L.C., Miller M., Hansen F.B. Ligand-induced spectral changes in cytochrome c oxidase and their possible significance. Biochim. Biophys. Acta. 1976;449:188–196. doi: 10.1016/0005-2728(76)90132-8. [DOI] [PubMed] [Google Scholar]
- 68.Goubern M., Andriamihaja M., Nubel T., Blachier F., Bouillaud F. Sulfide, the first inorganic substrate for human cells. FASEB J. 2007;21:1699–1706. doi: 10.1096/fj.06-7407com. [DOI] [PubMed] [Google Scholar]
- 69.Modis K., Coletta C., Erdelyi K., Papapetropoulos A., Szabo C. Intramitochondrial hydrogen sulfide production by 3-mercaptopyruvate sulfurtransferase maintains mitochondrial electron flow and supports cellular bioenergetics. FASEB J. 2013;27:601–611. doi: 10.1096/fj.12-216507. [DOI] [PubMed] [Google Scholar]
- 70.Altaany Z., Ju Y., Yang G., Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 2014;7:ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 71.Xie L., Gu Y., Wen M., Zhao S., Wang W., Ma Y., Meng G., Han Y., Wang Y., Liu G., et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes. 2016;65:3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolhuter K., Whitwell H.J., Switzer C.H., Burgoyne J.R., Timms J.F., Eaton P. Evidence against Stable Protein S-Nitrosylation as a Widespread Mechanism of Post-translational Regulation. Mol. Cell. 2018;69:438–450. doi: 10.1016/j.molcel.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortese-Krott M.M., Kuhnle G.G.C., Dyson A., Fernandez B.O., Grman M., DuMond J.F., Barrow M.P., McLeod G., Nakagawa H., Ondrias K., et al. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA. 2015;112:E4651–E4660. doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kristek F., Grman M., Ondrias K. In Vivo Measurement of H2S, Polysulfides, and “SSNO(-) Mix”-Mediated Vasoactive Responses and Evaluation of Ten Hemodynamic Parameters from Rat Arterial Pulse Waveform. Methods Mol. Biol. 2019;2007:109–124. doi: 10.1007/978-1-4939-9528-8_8. [DOI] [PubMed] [Google Scholar]
- 75.Cacanyiova S., Berenyiova A., Balis P., Kristek F., Grman M., Ondrias K., Breza J., Breza J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017;68:527–538. [PubMed] [Google Scholar]
- 76.Berenyiova A., Grman M., Mijuskovic A., Stasko A., Misak A., Nagy P., Ondriasova E., Cacanyiova S., Brezova V., Feelisch M., et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide. 2015;46:123–130. doi: 10.1016/j.niox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., van Goor H., et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kharma A., Grman M., Misak A., Dominguez-Alvarez E., Nasim M.J., Ondrias K., Chovanec M., Jacob C. Inorganic Polysulfides and Related Reactive Sulfur-Selenium Species from the Perspective of Chemistry. Molecules. 2019;24:1359. doi: 10.3390/molecules24071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grman M., Nasim M.J., Leontiev R., Misak A., Jakusova V., Ondrias K., Jacob C. Inorganic Reactive Sulfur-Nitrogen Species: Intricate Release Mechanisms or Cacophony in Yellow, Blue and Red? Antioxidants. 2017;6:14. doi: 10.3390/antiox6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Misak A., Kurakova L., Goffa E., Brezova V., Grman M., Ondriasova E., Chovanec M., Ondrias K. Sulfide (Na2S) and Polysulfide (Na2S2) Interacting with Doxycycline Produce/Scavenge Superoxide and Hydroxyl Radicals and Induce/Inhibit DNA Cleavage. Molecules. 2019;24:1148. doi: 10.3390/molecules24061148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Misak A., Grman M., Bacova Z., Rezuchova I., Hudecova S., Ondriasova E., Krizanova O., Brezova V., Chovanec M., Ondrias K. Polysulfides and products of H2S/S-nitrosoglutathione in comparison to H2S, glutathione and antioxidant Trolox are potent scavengers of superoxide anion radical and produce hydroxyl radical by decomposition of H2O2. Nitric Oxide. 2018;76:136–151. doi: 10.1016/j.niox.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Drobna M., Misak A., Holland T., Kristek F., Grman M., Tomasova L., Berenyiova A., Cacanyiova S., Ondrias K. Captopril partially decreases the effect of H2S on rat blood pressure and inhibits H2S-induced nitric oxide release from S-nitrosoglutathione. Physiol. Res. 2015;64:479–486. doi: 10.33549/physiolres.932772. [DOI] [PubMed] [Google Scholar]
- 83.Tomasova L., Jurkowska H., Wrobel M., Huc T., Ondrias K., Ostaszewski R., Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016;60:50–58. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 84.Tomasova L., Pavlovicova M., Malekova L., Misak A., Kristek F., Grman M., Cacanyiova S., Tomasek M., Tomaskova Z., Perry A., et al. Effects of AP39, a novel triphenylphosphonium derivatised anethole dithiolethione hydrogen sulfide donor, on rat haemodynamic parameters and chloride and calcium Cav3 and RyR2 channels. Nitric Oxide. 2015;46:131–144. doi: 10.1016/j.niox.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 85.Zhao W., Zhang J., Lu Y., Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hosoki R., Matsuki N., Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 87.Ali M.Y., Ping C.Y., Mok Y.Y., Ling L., Whiteman M., Bhatia M., Moore P.K. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulphide? Br. J. Pharmacol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sikora M., Drapala A., Ufnal M. Exogenous hydrogen sulfide causes different hemodynamic effects in normotensive and hypertensive rats via neurogenic mechanisms. Pharm. Rep. 2014;66:751–758. doi: 10.1016/j.pharep.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 89.Tomasova L., Drapala A., Jurkowska H., Wrobel M., Ufnal M. Na2S, a fast-releasing H2S donor, given as suppository lowers blood pressure in rats. Pharm. Rep. 2017;69:971–977. doi: 10.1016/j.pharep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Drapala A., Koszelewski D., Tomasova L., Ostaszewski R., Grman M., Ondrias K., Ufnal M. Parenteral Na2S, a fast-releasing H2S donor, but not GYY4137, a slow-releasing H2S donor, lowers blood pressure in rats. Acta Biochim. Pol. 2017;64:561–566. doi: 10.18388/abp.2017_1569. [DOI] [PubMed] [Google Scholar]
- 91.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., Zhao Y., Baskar R., Tan C.H., Moore P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 92.Cacanyiova S., Berenyiova A., Kristek F., Drobna M., Ondrias K., Grman M. The adaptive role of nitric oxide and hydrogen sulphide in vasoactive responses of thoracic aorta is triggered already in young spontaneously hypertensive rats. J. Physiol. Pharmacol. 2016;67:501–512. [PubMed] [Google Scholar]
- 93.Du J., Yan H., Tang C. Endogenous H2S is involved in the development of spontaneous hypertension. Beijing Da Xue Xue Bao Yi Xue Ban. 2003;35:102. [PubMed] [Google Scholar]
- 94.Wei H.L., Zhang C.Y., Jin H.F., Tang C.S., Du J.B. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol. Sin. 2008;29:670–679. doi: 10.1111/j.1745-7254.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed A. Molecular mechanisms and therapeutic implications of the carbon monoxide/hmox1 and the hydrogen sulfide/CSE pathways in the prevention of pre-eclampsia and fetal growth restriction. Pregnancy Hypertens. 2014;4:243–244. doi: 10.1016/j.preghy.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Tomasova L., Konopelski P., Ufnal M. Gut bacteria and hydrogen sulfide: The new old players in circulatory system homeostasis. Molecules. 2016;21:1558. doi: 10.3390/molecules21111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.La Fuente J.M., Fernandez A., Pepe-Cardoso A.J., Martinez-Salamanca J.I., Louro N., Angulo J. L-cysteine/hydrogen sulfide pathway induces cGMP-dependent relaxation of corpus cavernosum and penile arteries from patients with erectile dysfunction and improves arterial vasodilation induced by PDE5 inhibition. Eur. J. Pharm. 2019;863:172675. doi: 10.1016/j.ejphar.2019.172675. [DOI] [PubMed] [Google Scholar]
- 98.Dayar E., Kara E., Yetik-Anacak G., Hocaoglu N., Bozkurt O., Gidener S., Durmus N. Do penile haemodynamics change in the presence of hydrogen sulphide (H2S) donor in metabolic syndrome-induced erectile dysfunction? Andrologia. 2018;50:e12885. doi: 10.1111/and.12885. [DOI] [PubMed] [Google Scholar]
- 99.Yetik-Anacak G., Dikmen A., Coletta C., Mitidieri E., Dereli M., Donnarumma E., d’Emmanuele di Villa Bianca R., Sorrentino R. Hydrogen sulfide compensates nitric oxide deficiency in murine corpus cavernosum. Pharmacol. Res. 2016;113:38–43. doi: 10.1016/j.phrs.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 100.Shukla N., Rossoni G., Hotston M., Sparatore A., Del Soldato P., Tazzari V., Persad R., Angelini G.D., Jeremy J.Y. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang G.H., Adebiyi A., Leo M.D., McNally E.M., Leffler C.W., Jaggar J.H. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H2088–H2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang B., Tang G., Cao K., Wu L., Wang R. Molecular mechanism for H2S-induced activation of K ATP channels. Antioxid. Redox Signal. 2010;12:1167–1178. doi: 10.1089/ars.2009.2894. [DOI] [PubMed] [Google Scholar]
- 103.Wu D., Hu Q., Zhu D. An Update on Hydrogen Sulfide and Nitric Oxide Interactions in the Cardiovascular System. Oxid. Med. Cell. Longev. 2018;2018:4579140. doi: 10.1155/2018/4579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stubbert D., Prysyazhna O., Rudyk O., Scotcher J., Burgoyne J.R., Eaton P. Protein kinase G Iα oxidation paradoxically underlies blood pressure lowering by the reductant hydrogen sulfde. Hypertenstion. 2014;64:1344–1351. doi: 10.1161/HYPERTENSIONAHA.114.04281. [DOI] [PubMed] [Google Scholar]
- 105.Kanagy N.L., Szabo C., Papapetropoulos A. Vascular biology of hydrogen sulfide. American journal of physiology. Cell Physiol. 2017;312:C537–C549. doi: 10.1152/ajpcell.00329.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Coletta C., Modis K., Szczesny B., Brunyanszki A., Olah G., Rios E.C., Yanagi K., Ahmad A., Papapetropoulos A., Szabo C. Regulation of Vascular Tone, Angiogenesis and Cellular Bioenergetics by the 3-Mercaptopyruvate Sulfurtransferase/H2S Pathway: Functional Impairment by Hyperglycemia and Restoration by DL-alpha-Lipoic Acid. Mol. Med. 2015;21:1–14. doi: 10.2119/molmed.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tao B.B., Liu S.Y., Zhang C.C., Fu W., Cai W.J., Wang Y., Shen Q., Wang M.J., Chen Y., Zhang L.J., et al. VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid. Redox Signal. 2013;19:448–464. doi: 10.1089/ars.2012.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saha S., Chakraborty P.K., Xiong X., Dwivedi S.K., Mustafi S.B., Leigh N.R., Ramchandran R., Mukherjee P., Bhattacharya R. Cystathionine beta-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016;30:441–456. doi: 10.1096/fj.15-278648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lohninger L., Tomasova L., Praschberger M., Hintersteininger M., Erker T., Gmeiner B.M., Laggner H. Hydrogen sulphide induces HIF-1alpha and Nrf2 in THP-1 macrophages. Biochimie. 2015;112:187–195. doi: 10.1016/j.biochi.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 110.Aghagolzadeh P., Radpour R., Bachtler M., van Goor H., Smith E.R., Lister A., Odermatt A., Feelisch M., Pasch A. Hydrogen sulfide attenuates calcification of vascular smooth muscle cells via KEAP1/NRF2/NQO1 activation. Atherosclerosis. 2017;265:78–86. doi: 10.1016/j.atherosclerosis.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 111.Corsello T., Komaravelli N., Casola A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-Dependent Maintenance of Cellular Redox Balance. Antioxidants. 2018;7:129. doi: 10.3390/antiox7100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J., Shi C., Wang H., Gao C., Chang P., Chen X., Shan H., Zhang M., Tao L. Protective Effects of Hydrogen Sulfide on a Cell Culture Model of Traumatic Scratch Injury involving Suppression of Oxidative Stress and Upregulation of Nrf-2. Int. J. Biochem. Cell Biol. 2019;117:105636. doi: 10.1016/j.biocel.2019.105636. [DOI] [PubMed] [Google Scholar]
- 113.Chen H., Xie K., Chen Y., Wang Y., Wang Y., Lian N., Zhang K., Yu Y. Nrf2/HO-1 signaling pathway participated in the protection of hydrogen sulfide on neuropathic pain in rats. Int. Immunopharmacol. 2019;75:105746. doi: 10.1016/j.intimp.2019.105746. [DOI] [PubMed] [Google Scholar]
- 114.Kumar M., Sandhir R. Neuroprotective Effect of Hydrogen Sulfide in Hyperhomocysteinemia Is Mediated Through Antioxidant Action Involving Nrf2. Neuromol. Med. 2018;20:475–490. doi: 10.1007/s12017-018-8505-y. [DOI] [PubMed] [Google Scholar]
- 115.Ling K., Xu A., Chen Y., Chen X., Li Y., Wang W. Protective effect of a hydrogen sulfide donor on balloon injury-induced restenosis via the Nrf2/HIF-1alpha signaling pathway. Int. J. Mol. Med. 2019;43:1299–1310. doi: 10.3892/ijmm.2019.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen Sulfide-Linked Sulfhydration of NF-κB Mediates Its Antiapoptotic Actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reiffenstein R.J., Hulbert W.C., Roth S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 118.Kimura H. Signaling by hydrogen sulfide (H2S) and polysulfides (H2Sn) in the central nervous system. Neurochem. Int. 2019;126:118–125. doi: 10.1016/j.neuint.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 119.Hatakeyama Y., Takahashi K., Tominaga M., Kimura H., Ohta T. Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol. Pain. 2015;11:24. doi: 10.1186/s12990-015-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shigetomi E., Jackson-Weaver O., Huckstepp R.T., O’Dell T.J., Khakh B.S. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J. Neurosci. 2013;33:10143–10153. doi: 10.1523/JNEUROSCI.5779-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eto K., Asada T., Arima K., Makifuchi T., Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L.M., Jiang C.X., Liu D.W. Hydrogen sulfide attenuates neuronal injury induced by vascular dementia via inhibiting apoptosis in rats. Neurochem. Res. 2009;34:1984–1992. doi: 10.1007/s11064-009-0006-9. [DOI] [PubMed] [Google Scholar]
- 123.Hu L.F., Lu M., Tiong C.X., Dawe G.S., Hu G., Bian J.S. Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell. 2010;9:135–146. doi: 10.1111/j.1474-9726.2009.00543.x. [DOI] [PubMed] [Google Scholar]
- 124.Paul B.D., Sbodio J.I., Xu R., Vandiver M.S., Cha J.Y., Snowman A.M., Snyder S.H. Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature. 2014;509:96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tiranti V., Viscomi C., Hildebrandt T., Di Meo I., Mineri R., Tiveron C., Levitt M.D., Prelle A., Fagiolari G., Rimoldi M., et al. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat. Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 126.Kimura Y., Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 127.Kimura Y., Goto Y., Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010;12:1–13. doi: 10.1089/ars.2008.2282. [DOI] [PubMed] [Google Scholar]
- 128.Vandiver M.S., Paul B.D., Xu R., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Lee Y.I., Dawson V.L., Dawson T.M., et al. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Coavoy-Sanchez S.A., Costa S.K.P., Muscara M.N. Hydrogen sulfide and dermatological diseases. Br. J. Pharmacol. 2019:1–9. doi: 10.1111/bph.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wallace J.L., Ianaro A., de Nucci G. Gaseous Mediators in Gastrointestinal Mucosal Defense and Injury. Dig. Dis. Sci. 2017;62:2223–2230. doi: 10.1007/s10620-017-4681-0. [DOI] [PubMed] [Google Scholar]
- 131.Schmidt T.P., Lopes L.B., Rodrigues L., Wood M., Whiteman M., Muscará M.N., Costa S.K.P. PP32—Antipsoriatic activity of GYY4137 (a slow-releasing hydrogen sulphide donor) microemulsion system using a mouse skin model of psoriasis. Nitric Oxide. 2015;47:S26. doi: 10.1016/j.niox.2015.02.062. [DOI] [Google Scholar]
- 132.Sulaieva O., Wallace J.L. Gaseous mediator-based anti-inflammatory drugs. Curr. Opin. Pharm. 2015;25:1–6. doi: 10.1016/j.coph.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 133.Distrutti E., Sediari L., Mencarelli A., Renga B., Orlandi S., Russo G., Caliendo G., Santagada V., Cirino G., Wallace J.L., et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J. Pharm. Exp. 2006;319:447–458. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- 134.Ekundi-Valentim E., Santos K.T., Camargo E.A., Denadai-Souza A., Teixeira S.A., Zanoni C.I., Grant A.D., Wallace J., Muscara M.N., Costa S.K. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br. J. Pharmacol. 2010;159:1463–1474. doi: 10.1111/j.1476-5381.2010.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Distrutti E., Sediari L., Mencarelli A., Renga B., Orlandi S., Antonelli E., Roviezzo F., Morelli A., Cirino G., Wallace J.L., et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 136.Szabo C., Coletta C., Chao C., Modis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-beta-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lagoutte E., Mimoun S., Andriamihaja M., Chaumontet C., Blachier F., Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim. Biophys. Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 138.Modis K., Coletta C., Asimakopoulou A., Szczesny B., Chao C., Papapetropoulos A., Hellmich M.R., Szabo C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-beta-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide. 2014;41:146–156. doi: 10.1016/j.niox.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhattacharyya S., Saha S., Giri K., Lanza I.R., Nair K.S., Jennings N.B., Rodriguez-Aguayo C., Lopez-Berestein G., Basal E., Weaver A.L., et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE. 2013;8:e79167. doi: 10.1371/journal.pone.0079167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hellmich M.R., Szabo C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Veldman B.A., Vervoort G., Blom H., Smits P. Reduced plasma total homocysteine concentrations in Type 1 diabetes mellitus is determined by increased renal clearance. Diabet. Med. 2005;22:301–305. doi: 10.1111/j.1464-5491.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 142.Yang W., Yang G., Jia X., Wu L., Wang R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005;569:519–531. doi: 10.1113/jphysiol.2005.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu L., Yang W., Jia X., Yang G., Duridanova D., Cao K., Wang R. Pancreatic islet overproduction of H2S and suppressed insulin release in Zucker diabetic rats. Lab. Investig. 2009;89:59–67. doi: 10.1038/labinvest.2008.109. [DOI] [PubMed] [Google Scholar]
- 144.Szadvari I., Hudecova S., Chovancova B., Matuskova M., Cholujova D., Lencesova L., Valerian D., Ondrias K., Babula P., Krizanova O. Sodium/calcium exchanger is involved in apoptosis induced by H2S in tumor cells through decreased levels of intracellular pH. Nitric Oxide. 2019;87:1–9. doi: 10.1016/j.niox.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 145.Markova J., Hudecova S., Soltysova A., Sirova M., Csaderova L., Lencesova L., Ondrias K., Krizanova O. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the beta1 and beta3 but not beta2 adrenergic receptors, and induces apoptosis. Pflug. Arch. 2014;466:1329–1342. doi: 10.1007/s00424-013-1366-1. [DOI] [PubMed] [Google Scholar]
- 146.Lencesova L., Hudecova S., Csaderova L., Markova J., Soltysova A., Pastorek M., Sedlak J., Wood M.E., Whiteman M., Ondrias K., et al. Sulphide signalling potentiates apoptosis through the up-regulation of IP3 receptor types 1 and 2. Acta Physiol. 2013;208:350–361. doi: 10.1111/apha.12105. [DOI] [PubMed] [Google Scholar]
- 147.Cao X., Ding L., Xie Z.Z., Yang Y., Whiteman M., Moore P., Bian J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019;31:1–38. doi: 10.1089/ars.2017.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Smith B.R., Eastman C.M., Njardarson J.T. Beyond C, H, O, and N! Analysis of the elemental composition of U.S. FDA approved drug architectures. J. Med. Chem. 2014;57:9764–9773. doi: 10.1021/jm501105n. [DOI] [PubMed] [Google Scholar]
- 149.Pluth M., Bailey T., Hammers M., Hartle M., Henthorn H., Steiger A. Natural Products Containing Hydrogen Sulfide Releasing Moieties. Synlett. 2015;26:2633–2643. doi: 10.1055/s-0035-1560638. [DOI] [Google Scholar]
- 150.Kamyshny A., Goifman A., Gun J., Rizkov D., Lev O. Equilibrium Distribution of Polysulfide Ions in Aqueous Solutions at 25 °C: A New Approach for the Study of Polysulfides’ Equilibria. Environ. Sci. Technol. 2014;38:6633–6644. doi: 10.1021/es049514e. [DOI] [PubMed] [Google Scholar]
- 151.Munday R., Munday J.S., Munday C.M. Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the Allium family: Redox cycling in vitro and hemolytic activity and Phase 2 enzyme induction in vivo. Free Radic. Biol. Med. 2003;34:1200–1211. doi: 10.1016/S0891-5849(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 152.Steudel R. The Chemistry of Organic Polysulfanes R−Sn−R (n> 2) Chem. Rev. 2002;102:3905–3946. doi: 10.1021/cr010127m. [DOI] [PubMed] [Google Scholar]
- 153.Münchberg U., Anwar A., Mecklenburg S., Jacob C. Polysulfides as biologically active ingredients of garlic. Org. Biomol. Chem. 2007;5:1505–1518. doi: 10.1039/B703832A. [DOI] [PubMed] [Google Scholar]
- 154.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rahman M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007;10:245–268. doi: 10.1080/10942910601113327. [DOI] [Google Scholar]
- 156.Rana S.V., Pal R., Vaiphei K., Sharma S.K., Ola R.P. Garlic in health and disease. Nutr. Res. Rev. 2011;24:60–71. doi: 10.1017/S0954422410000338. [DOI] [PubMed] [Google Scholar]
- 157.Butt M.S., Sultan M.T., Butt M.S., Iqbal J. Garlic: Nature’s protection against physiological threats. Crit. Rev. Food Sci. Nutr. 2009;49:538–551. doi: 10.1080/10408390802145344. [DOI] [PubMed] [Google Scholar]
- 158.Guo W., Cheng Z., Zhu Y. Hydrogen sulfide and translational medicine. Acta Pharm. Sin. 2013;34:1284–1291. doi: 10.1038/aps.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Citi V., Martelli A., Testai L., Marino A., Breschi M., Calderone V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effects of Brassicaceae? Planta Med. 2014;80:610–613. doi: 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- 160.Pei Y., Wu B., Cao Q., Wu L., Yang G. Hydrogen sulfide mediates the antisurvival effect of sulforaphane on human prostate cancer cells. Toxicol. Appl. Pharm. 2011;257:420–428. doi: 10.1016/j.taap.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 161.Vasdev S., Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int. J. Angiol. 2010;19:7–20. doi: 10.1055/s-0031-1278362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brosnan J.T., Brosnan M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 163.Zhou Z., von Wantoch Rekowski M., Coletta C., Szabo C., Bucci M., Cirino G., Topouzis S., Papapetropoulos A., Giannis A. Thioglycine and l-thiovaline: Biologically active H2S-donors. Bioorg. Med. Chem. 2012;20:2675–2678. doi: 10.1016/j.bmc.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 164.Vasdev S., Singal P., Gill V. The antihypertensive effect of cysteine. Int. J. Angiol. 2009;18:7–21. doi: 10.1055/s-0031-1278316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mills J.L., McPartlin J.M., Kirke P.N., Lee Y.J., Conley M.R., Weir D.G., Scott J.M. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345:149–151. doi: 10.1016/S0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 166.Refsum H., Ueland P.M., Nygard O., Vollset S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- 167.Arner E.S. Selenoproteins-What unique properties can arise with selenocysteine in place of cysteine? Exp. Cell Res. 2010;316:1296–1303. doi: 10.1016/j.yexcr.2010.02.032. [DOI] [PubMed] [Google Scholar]
- 168.Nagy P., Winterbourn C.C. Redox Chemistry of Biological Thiols. Adv. Mol. Toxicol. 2010;4:183–222. [Google Scholar]
- 169.Turell L., Radi R., Alvarez B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013;65:244–253. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mokhtari V., Afsharian P., Maryam Shahhoseini M., Kalantar S.M., Moini A.A. Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017;19:11–17. doi: 10.22074/cellj.2016.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schaffer S., Kim H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018;26:225–241. doi: 10.4062/biomolther.2017.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tiruppathi C., Brandsch M., Miyamoto Y., Ganapathy V., Leibach F.H. Constitutive expression of the taurine transporter in a human colon carcinoma cell line. Am. J. Physiol. 1992;263:625–631. doi: 10.1152/ajpgi.1992.263.5.G625. [DOI] [PubMed] [Google Scholar]
- 173.Chen Z., Fei Y.J., Anderson C.M., Wake K.A., Miyauchi S., Huang W. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J. Physiol. 2003;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carbonero F., Benefiel A.C., Alizadeh-Ghamsari A.H., Gaskins H.R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 2012;3:448. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ideishi M., Miura S., Sakai T., Sasaguri M., Misumi Y., Arakawa K. Taurine amplifies renal kallikrein and prevents salt-induced hypertension in Dahl rats. J. Hypertens. 1994;12:653–661. doi: 10.1097/00004872-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 176.Dawson R., Jr., Liu S., Jung B., Messina S., Eppler B. Effects of high salt diets and taurine on the development of hypertension in the stroke-prone spontaneously hypertensive rat. Amino Acids. 2000;19:643–665. doi: 10.1007/s007260070014. [DOI] [PubMed] [Google Scholar]
- 177.Harada H., Isujino T., Watari Y., Nonaka H., Emoto N., Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–136. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- 178.Hagar H.H., El Etter E., Arafa M. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clin. Exp. Pharm. Physiol. 2006;33:189–196. doi: 10.1111/j.1440-1681.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- 179.Hu J., Xu X., Yang J., Wu G., Sun C., Lv Q. Antihypertensive effect of taurine in rat. Adv. Exp. Med. Biol. 2009;643:75–84. doi: 10.1007/978-0-387-75681-3_8. [DOI] [PubMed] [Google Scholar]
- 180.Shimada K., Jong C.J., Takahashi K., Schaffer S.W. Role of ROS Production and Turnover in the Antioxidant Activity of Taurine. Adv. Exp. Med. Biol. 2015;803:581–596. doi: 10.1007/978-3-319-15126-7_47. [DOI] [PubMed] [Google Scholar]
- 181.Mozaffari M.S., Patel C., Abdelsayed R., Schaffer S.W. Accelerated NaCl-induced hypertension in taurine-deficient rat: Role of renal function. Kidney Int. 2006;70:329–337. doi: 10.1038/sj.ki.5001503. [DOI] [PubMed] [Google Scholar]
- 182.Sun Q., Wang B., Li Y., Sun F., Li P., Xia W., Zhou X.Q., Wang X., Chen J., Zeng X., et al. Taurine supplementation lowers blood pressure and improves vascular function in prehypertension: Randomized, double-blind, placebo controlled study. Hypertension. 2016;67:541–549. doi: 10.1161/HYPERTENSIONAHA.115.06624. [DOI] [PubMed] [Google Scholar]
- 183.Dzau V. The cardiovscular continuum and renin-angiotensin-aldosterone system blockade. J. Hypertens. 2005;13:S9–S17. doi: 10.1097/01.hjh.0000165623.72310.dd. [DOI] [PubMed] [Google Scholar]
- 184.Savoia C., Schiffrin E.L. Inhibition of the renin angiotensin system: Implications for the endothelium. Curr. Diab. Rep. 2006;6:274–278. doi: 10.1007/s11892-006-0060-5. [DOI] [PubMed] [Google Scholar]
- 185.Ondetti M.A., Rubin B., Cushman D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science. 1977;196:441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- 186.Liu X., Engelman R.M., Ronson J.A., Cordis G.A., Das D.K. Attenuation of myocardial reperfusion injury by sulphydryl-containing angiotensin converting enzyme inhibitors. Cardiovasc. Drugs. 1992;6:437–443. doi: 10.1007/BF00054194. [DOI] [PubMed] [Google Scholar]
- 187.Atkinson A.B., Robertson J.I. Captopril in the treatment of clinical hypertension and cardiac failure. Lancet. 1979;2:836–839. doi: 10.1016/S0140-6736(79)92186-X. [DOI] [PubMed] [Google Scholar]
- 188.Donnarumma E., Ali M.J., Rushing A.M., Scarborough A.L., Bradley J.M., Organ C.L., Islam K.N., Polhemus D.J., Evangelista S., Cirino G., et al. Zofenopril Protects against Myocardial Ischemia-Reperfusion Injury by Increasing Nitric Oxide and Hydrogen Sulfide Bioavailability. J. Am. Heart Assoc. 2016;5:1–17. doi: 10.1161/JAHA.116.003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Terzuoli E., Monti M., Vellecco V., Bucci M.G., Cirino G., Ziche M., Morbidelli L. Characterization of zofenoprilat as an inducer of functional angiogenesis through increased H2S availability. Br. J. Pharmacol. 2015;172:2961–2973. doi: 10.1111/bph.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Monti M., Terzuoli E., Ziche M., Morbidelli L. The sulphydryl containing ACE inhibitor Zofenoprilat protects coronary endothelium from Doxorubicin-induced apoptosis. Pharmacol. Res. 2013;76:171–181. doi: 10.1016/j.phrs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 191.Monti M., Terzuoli E., Ziche M., Morbidelli L. H2S dependent and independent anti-inflammatory activity of zofenoprilat in cells of the vascular wall. Pharmacol. Res. 2016;113:426–437. doi: 10.1016/j.phrs.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 192.Bucci M., Vellecco V., Cantalupo A., Brancaleone V., Zhou Z., Evangelista S., Calderone V., Papapetropoulos A., Cirino G. Hydrogen sulfide accounts for the peripheral vascular effects of zofenopril independently of ACE inhibition. Cardiovasc. Res. 2014;102:138–147. doi: 10.1093/cvr/cvu026. [DOI] [PubMed] [Google Scholar]
- 193.Furuta S., Kiyosawa K., Higuchi M., Kasahara H., Saito H., Shioya H., Oguchi H. Pharmacokinetics of temocapril, an ACE inhibitor with preferential biliary excretion, in patients with impaired liver function. Eur. J. Clin. Pharm. 1993;44:383–385. doi: 10.1007/BF00316478. [DOI] [PubMed] [Google Scholar]
- 194.Packer M., Califf R.M., Konstam M.A., Krum H., McMurray J.J., Rouleau J.L. Comparison of Omapatrilat and Enalapril in Patients With Chronic Heart Failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.CIR.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 195.MacFadyen R.J., Jones C.R., Doig J.K., Birnbock H., Reid J.L. Responses to an Orally Active Renin Inhibitor, Remikiren (Ro 42–5892), After Controlled Salt Depletion in Humans. J. Cardiovasc. Pharmacol. 1995;25:347–353. doi: 10.1097/00005344-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 196.Clozel J.P., Fischli W. Comparative effects of three different potent renin inhibitors in primates. Hypertension. 1993;22:9–17. doi: 10.1161/01.HYP.22.1.9. [DOI] [PubMed] [Google Scholar]
- 197.Iglarz M., Landskroner K., Rey M., Wanner D., Hess P., Clozel M. Optimization of tissue targeting properties of macitentan, a new dual endothelin receptor antagonist, improves its efficacy in a rat model of pulmonary fibrosis associated with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2011;183:A6445. [Google Scholar]
- 198.Kiowski W., Sutsch G., Oechslin E., Bertel O. Hemodynamic effects of bosentan in patients with chronic heart failure. Heart Fail. Rev. 2001;6:325–334. doi: 10.1023/A:1011460426786. [DOI] [PubMed] [Google Scholar]
- 199.Teixeira C., Priviero F., Webb R. Differential effects of the phosphodiesterase type 5 inhibitors sildenafil, vardenafil, and tadalafil in rat aorta. J. Pharmacol. Exp. Ther. 2006;316:654–661. doi: 10.1124/jpet.105.092544. [DOI] [PubMed] [Google Scholar]
- 200.Auerbach S.M., Gittelman M., Mazzu A., Cihon F., Sundaresan P., White W.B. Simultaneous administration of vardenafil and tamsulosin does not induce clinically significant hypotension in patients with benign prostatic hyperplasia. Urology. 2004;64:998–1003. doi: 10.1016/j.urology.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 201.Kim N.N., Huang Y.H., Goldstein I., Bischoff E., Traish A.M. Inhibition of cyclic GMP hydrolysis in human corpus cavernosum smooth muscle cells by vardenafil, a novel, selective phosphodiesterase type 5 inhibitor. Life Sci. 2001;69:2249–2256. doi: 10.1016/S0024-3205(01)01308-X. [DOI] [PubMed] [Google Scholar]
- 202.Saenz de Tejada I., Angulo J., Cuevas P., Fernandez A., Moncada I., Allona A., Lledo E., Korschen H.G., Niewohner U., Haning H., et al. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int. J. Impot. Res. 2001;13:282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- 203.Boolell M., Allen M.J., Ballard S.A., Gepi-Atlee S., Murihead G.J., Naylor A.M., Osterloh J.H., Gingell C. Sildenafil: An orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 204.Cheitlin M.D., Hutter A.M., Jr., Brindis R.G., Ganz P., Kaul S., Russell R.O., Jr., Zusman R.M. ACC/AHA expert consensus document. Use of sildenafil (Viagra) in patients with cardiovascular disease. J. Am. Coll. Cardiol. 1999;1:273–282. doi: 10.1016/S0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- 205.O’Connor S.E., Grosset A., Janiak P. The pharmacological basis and pathophysiological significance of the heart rate-lowering property of diltiazem. Fundam. Clin. Pharmacol. 1999;13:145–153. doi: 10.1111/j.1472-8206.1999.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 206.Hu L.F., Lu M., Hon Wong P.T., Bian J.S. Hydrogen sulfide: Neurophysiology and neuropathology. Antioxid. Redox Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 207.Perez-Torres I., Guarner-Lans V., Rubio-Ruiz M.E. Reductive stress in inflammation-associated diseases and the pro-oxidant effect of antioxidant agents. Int. J. Mol. Sci. 2017;18:2098. doi: 10.3390/ijms18102098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ide M., Ohnishi T., Toyoshima M., Balan S., Maekawa M., Shimamoto-Mitsuyama C., Yoshikawa T. Excess hydrogen sulfide and polysulfides production underlies a schizophrenia pathophysiology. EMBO Mol. Med. 2019;e10695:1–24. doi: 10.15252/emmm.201910695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cavallotti C., Nuti F., Bruzzone P., Mancone M. Age-related changes in dopamine D2 receptors in rat heart and coronary vessels. Clin. Exp. Pharmacol. Physiol. 2002;29:412–418. doi: 10.1046/j.1440-1681.2002.03677.x. [DOI] [PubMed] [Google Scholar]
- 210.Jaber M., Tison F., Fournier M.C., Bloch B. Differential influence of haloperidol and sulpiride on dopamine receptors and peptide mRNA levels in the rat striatum and pituitary. Brain Res. Mol. Brain Res. 1994;23:14–20. doi: 10.1016/0169-328X(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 211.Chen X., Ji Z.L., Chen Y.Z. TTD: Therapeutic Target Database. Nucleic Acids Res. 2002;30:412–415. doi: 10.1093/nar/30.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zoccolella S., Lamberti P., Armenise E. Plasma homocysteine levels in Parkinson’s disease: Role of antiparkinsonian medications. Parkinsonism Relat. Disord. 2005;11:131–133. doi: 10.1016/j.parkreldis.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 213.Xue X., Bian J.S. Neuroprotective effects of hydrogen sulfide in Parkinson’s disease animal models: Methods and protocols. Methods Enzym. 2015;554:169–186. doi: 10.1016/bs.mie.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 214.Kida K., Yamada M., Tokuda K., Marutani E., Kakinohana M., Kaneki M., Ichinose F. Inhaled Hydrogen Sulfide Prevents Neurodegeneration and Movement Disorder in a Mouse Model of Parkinson’s Disease. Antioxid. Redox Signal. 2011;15:343–352. doi: 10.1089/ars.2010.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee M., Tazzari V., Giustarini D., Rossi R., Sparatore A., Del Soldato P., McGeer P.L. Effects of Hydrogen Sulfide-releasing l-DOPA Derivatives on Glial Activation. J. Biol. Chem. 2010;285:17318–17328. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wolfe M.M., Lichtenstein D.R., Singh G. Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N. Engl. J. Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 217.Sparatore A., Santus G., Giustarini D., Rossi R., Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev. Clin. Pharm. 2011;4:109–121. doi: 10.1586/ecp.10.122. [DOI] [PubMed] [Google Scholar]
- 218.Chan M.V., Wallace J.L. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: Translating physiology to treatments. Am. J. Physiol. Gastrointest Liver. Physiol. 2013;305:G467–G473. doi: 10.1152/ajpgi.00169.2013. [DOI] [PubMed] [Google Scholar]
- 219.Sparatore. A., Perrino E., Tazzari V., Giustarini D., Rossi R., Rossoni G., Erdman K., Schreoder H., Del Soldato P. Pharmacological profile of a novel H2S-releasing aspirin. Free Radic. Biol. Med. 2009;46:586–592. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 220.Li L., Rossoni G., Sparatore A., Lee L.C., Del Soldato P., Moore P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007;42:706–719. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 221.Wallace J.L., Caliendo G., Santagata V., Cirino G., Fiorucci S. Gastrointestinal safety and antiinflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 222.Fiorucci S., Orlandi S., Mencarelli A., Caliendo G., Santagada V., Distrutti E., Santucci L., Cirino G., Wallace J.L. Enhanced activity of a hydrogen sulphide releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br. J. Pharm. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wallace J.L., Caliendo G., Santagada V., Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br. J. Pharm. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chattopadhyay M., Kodela R., Nath N., Dastagirzada Y.M., Velazquez-Martinez C.A., Boring D., Kashfi K. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: A general property and evidence of a tissue type-independent effect. Biochem. Pharm. 2012;83:715–722. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 225.FitzGerald G.A. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2003;2:879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 226.FitzGerald G.A., Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N. Engl. J. Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 227.Prasit P., Wang Z., Brideau C., Chan C.C., Charleson S., Cromlish W., Ethier D., Evans J.F., Ford-Hutchinson A.W., Gauthier J.Y., et al. The discovery of rofecoxib, [MK 966, VIOXX, 4-(4′-methylsulfonylphenyl)-3-phenyl- 2(5H)-furanone], an orally active cyclooxygenase-2 inhibitor. Bioorg. Med. Chem. Lett. 1999;9:1773–1778. doi: 10.1016/S0960-894X(99)00288-7. [DOI] [PubMed] [Google Scholar]
- 228.Sorbera L.A., Castaner R.M., Silvestre J., Castaner J. Etoricoxib. Analgesic drug, antiarthritic, cyclooxygenase-2 inhibitor. Drugs Future. 2001;26:346–353. doi: 10.1358/dof.2001.026.04.616043. [DOI] [Google Scholar]
- 229.Talley J.J., Brown D.L., Carter J.S., Graneto M.J., Kobolt C.M., Masferrer J.L., Perkins W.E., Rogers R.S., Shaffer A.F., Zhang Y.Y., et al. 4-[5-Methyl-3-phenylisoxazol-4- yl]- benzenesulfonamide, Valdecoxib: A potent and selective inhibitor of COX-2. J. Med. Chem. 2000;43:775–777. doi: 10.1021/jm990577v. [DOI] [PubMed] [Google Scholar]
- 230.Singh G., Fort J.G., Goldstein J.L., Levy R.A., Hanrahan P.S., Bello A.E., Andrade-Ortega L., Wallemark C., Agrawal N.M., Eisen G.M., et al. Celecoxib Versus Naproxen and Diclofenac in Osteoarthritis Patients: SUCCESS-I Study. Am. J. Med. 2006;119:255–266. doi: 10.1016/j.amjmed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 231.Gordo A.C., Walker C., Armada B., Zhou D. Efficacy of celecoxib versus ibuprofen for the treatment of patients with osteoarthritis of the knee: A randomized double-blind, non-inferiority trial. J. Int. Med. Res. 2017;45:59–74. doi: 10.1177/0300060516673707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Szabó G., Fischer J., Kis-Varga Á., Gyires K. New Celecoxib Derivatives as Anti-Inflammatory Agents. J. Med. Chem. 2008;51:142–147. doi: 10.1021/jm070821f. [DOI] [PubMed] [Google Scholar]
- 233.Faulds D., Goa K.L., Benfield P. Cyclosporin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45:953–1040. doi: 10.2165/00003495-199345060-00007. [DOI] [PubMed] [Google Scholar]
- 234.El-Bassossy H.M., Awan Z., El-Mas M.M. Perinatal ciclosporin A exposure elicits sex-related cardiac dysfunction and inflammation in the rat progeny. Toxicol. Lett. 2017;281:35–43. doi: 10.1016/j.toxlet.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 235.Hammoud S.H., Alkhansa S., Mahjoub N., Omar A.G., El-Mas M.M., Eid A.A. Molecular basis of the counteraction by calcium channel blockers of Cyclosporine nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2018;315:F572–F582. doi: 10.1152/ajprenal.00275.2017. [DOI] [PubMed] [Google Scholar]
- 236.Ping N.N., Mi Y.N., Liu D.Z., Zhang S., Chen J.G., Cao Y.X. H2S prevents cyclosporine A-induced vasomotor alteration in rats. Cardiovasc. Toxicol. 2017;17:287–296. doi: 10.1007/s12012-016-9383-x. [DOI] [PubMed] [Google Scholar]
- 237.Lee G., Hosgood S.A., Patel M.S., Nicholson M.L. Hydrogen sulphide as a novel therapy to ameliorate cyclosporine nephrotoxicity. J. Surg. Res. 2015;197:419–426. doi: 10.1016/j.jss.2015.02.061. [DOI] [PubMed] [Google Scholar]
- 238.Helmy M.M., Helmy M.W., El-Mas M.M. Upregulation of cystathionine-γ-lyase/hydrogen sulfide pathway underlies the celecoxib counteraction of cyclosporine-induced hypertension and renal insult in rats. Prostaglandins Lipid Mediat. 2019;141:1–10. doi: 10.1016/j.prostaglandins.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 239.Sugawara M., Sugawara Y., Wen K. Methimazole and Propylthiouracil Increase Cellular Thyroid Peroxidase Activity and Thyroid Peroxidase mRNA in Cultured Porcine Thyroid Follicles. Thyroid. 1999;9:513–518. doi: 10.1089/thy.1999.9.513. [DOI] [PubMed] [Google Scholar]
- 240.Guilhem I., Massart C., Poirier J.Y., Maugendre D. Differential evolution of thyroid peroxidase and thyrotropin receptor antibodies in Graves’ disease: Thyroid peroxidase antibody activity reverts to pretreatment level after carbimazole withdrawal. Thyroid. 2006;16:1041–1045. doi: 10.1089/thy.2006.16.1041. [DOI] [PubMed] [Google Scholar]
- 241.Manna D., Roy G., Mugesh G. Antithyroid Drugs and Their Analogues: Synthesis, Structure, and Mechanism of Action. Acc. Chem. Res. 2013;46:2706–2715. doi: 10.1021/ar4001229. [DOI] [PubMed] [Google Scholar]
- 242.Hine C., Kim H.J., Zhu Y., Harputlugil E., Longchamp A., Matos M.S., Ramadoss P., Bauerle K., Brace L., Asara J.M., et al. Hypothalamic-pituitary axis regulates hydrogen sulfide production. Cell Metab. 2017;25:1320–1333. doi: 10.1016/j.cmet.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tahiliani P., Kar A. The combined effects of Trigonella and Allium extracts in the regulation of hyperthyroidism in rats. Phytomedicine. 2003;10:665–668. doi: 10.1078/0944-7113-00277. [DOI] [PubMed] [Google Scholar]
- 244.Perez-Barcena J., Barcelo B., Homar J., Abadal J.M., Molina F.J., de la Pena A., Sahuquillo J., Ibanez J. Comparison of the effectiveness of pentobarbital and thiopental in patients with refractory intracranial hypertension. Preliminary report of 20 patients. Neurocirugia. 2005;16:5–12. [PubMed] [Google Scholar]
- 245.Morgan D.J., Blackman G.L., Paull J.D., Wolf L.J. Pharmacokinetics and plasma binding of thiopental. II: Studies at cesarean section. Anesthesiology. 1981;54:474–480. doi: 10.1097/00000542-198106000-00006. [DOI] [PubMed] [Google Scholar]
- 246.Bercovitz A.B., Godke R.A., Biellier H.V., Short C.E. Surgical anesthesia in turkeys with thialbarbital sodium. Am. J. Vet. Res. 1975;36:301–302. [PubMed] [Google Scholar]
- 247.Moreels S.E., Neyrinck A., Desmet W. Intractable hypotension and myocardial ischaemia induced by co-ingestion of ethanol and disulfiram. Acta Cardiol. 2012;67:491–493. doi: 10.1080/AC.67.4.2170696. [DOI] [PubMed] [Google Scholar]
- 248.Ho M.P., Liu C.M., Yo C.H., Lee C.C., Chen C.L. Refractive Hypotension in a Patient with Disulfiram-Ethanol Reaction. Am. J. Med. Sci. 2007;333:53–55. doi: 10.1097/00000441-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 249.Paakkari I., Tötterman K.J., Kupari M., Karppanen H., Paakkari P. Peripheral hypotensive and central hypertensive effects of cimetidine. Agents Actions. 1982;12:152–155. doi: 10.1007/BF01965129. [DOI] [PubMed] [Google Scholar]
- 250.Zhao K., Li S., Wu L., Lai C., Yang G. Hydrogen Sulfide Represses Androgen Receptor Transactivation by Targeting at the Second Zinc Finger Module. J. Biol. Chem. 2014;289:20824–20835. doi: 10.1074/jbc.M114.559518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Duan F., Li Y., Chen L., Zhou X., Chen J., Chen H., Li R. Sulfur inhibits the growth of androgen-independent prostate cancer in vivo. Oncol. Lett. 2014;9:437–441. doi: 10.3892/ol.2014.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nadiminty N., Tummala R., Liu C., Yang J., Lou W., Evans C.P., Gao A.C. NF- B2/p52 Induces Resistance to Enzalutamide in Prostate Cancer: Role of Androgen Receptor and Its Variants. Mol. Cancer. 2013;12:1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gibbons J.A., de Vries M., Krauwinkel W., Ohtsu Y., Noukens J., van der Walt J.S., Ouatas T. Pharmacokinetic Drug Interaction Studies with Enzalutamide. Clin. Pharm. 2015;54:1057–1069. doi: 10.1007/s40262-015-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rathkopf D.E., Scher H.I. Apalutamide for the treatment of prostate cancer. Expert Rev. Anticancer. 2018;18:823–836. doi: 10.1080/14737140.2018.1503954. [DOI] [PMC free article] [PubMed] [Google Scholar]