Abstract
Prostate cancer (PCa) represents a major cause of cancer mortality among men in developed countries. Patients with recurrent disease initially respond to androgen-deprivation therapy, but the tumor eventually progresses into castration-resistant PCa; in this condition, tumor cells acquire the ability to escape cell death and develop resistance to current therapies. Thus, new therapeutic approaches for PCa management are urgently needed. In this setting, natural products have been extensively studied for their anti-PCa activities, such as tumor growth suppression, cell death induction, and inhibition of metastasis and angiogenesis. Additionally, numerous studies have shown that phytochemicals can specifically target the androgen receptor (AR) signaling, as well as the PCa stem cells (PCSCs). Interestingly, many clinical trials have been conducted to test the efficacy of nutraceuticals in human subjects, and they have partially confirmed the promising results obtained in vitro and in preclinical models. This article summarizes the anti-cancer mechanisms and therapeutic potentials of different natural compounds in the context of PCa prevention and treatment.
Keywords: prostate cancer, natural compounds, phytochemicals, chemoprevention, novel therapeutic strategies
1. Introduction
Globally, prostate cancer (PCa) is the most frequently diagnosed tumor in men, being particularly common in Western countries [1]. In about 90% of cases, PCa is still organ-confined or only locally advanced at diagnosis, which makes it effectively treatable with prostatectomy or local radiotherapy. However, 30–40% of patients usually experience progression of disease [2]; at this stage, where tumor growth depends on androgens, the most effective treatment is represented by androgen-deprivation therapy, aimed at blocking hormone secretion and/or activity. This therapy is based on pharmacological castration, obtained by administration of GnRH agonists, alone or in combination with antiandrogens [3,4]; more recently, two major clinical trials, CHAARTED and STAMPEDE, have also demonstrated benefits of early initiation of chemotherapy concomitantly with hormonal therapy [5,6]. However, despite a good initial response, relapse occurs in the majority of patients within 2–3 years, and the tumor progresses towards a condition of resistance to castration [7]. Improved therapeutic options for castration-resistant patients are needed, since taxane-based (i.e., docetaxel) treatment and immunotherapy, as well as the novel therapies with enzalutamide and abiraterone, generally offer a progression-free survival of a few months [8,9]. Parallelly, bone metastases, occurring in 80% of advanced PCas and usually treated with radiation therapy and chemotherapy, are associated with considerable morbidity, adversely affect quality of life and several skeletal-related events [4,10]. Therefore, in the last years natural compounds have gained a lot of interest, due to their various anti-cancer effects. In fact, accumulating evidence has highlighted that nutraceuticals can exert growth-suppressing, pro-death, anti-metastatic, and anti-angiogenic activity in PCa cell lines and xenografts, while sparing normal prostate epithelial cells [11]. In particular, several mechanisms are involved in the anti-PCa actions of these molecules, including inhibition of androgen receptor (AR) axis and targeting of cancer stemness [12,13]. This review is aimed at summarizing the recent evidence about the role of different nutraceuticals in PCa prevention and therapy.
2. Natural Compounds with Potential to Treat Prostate Cancer
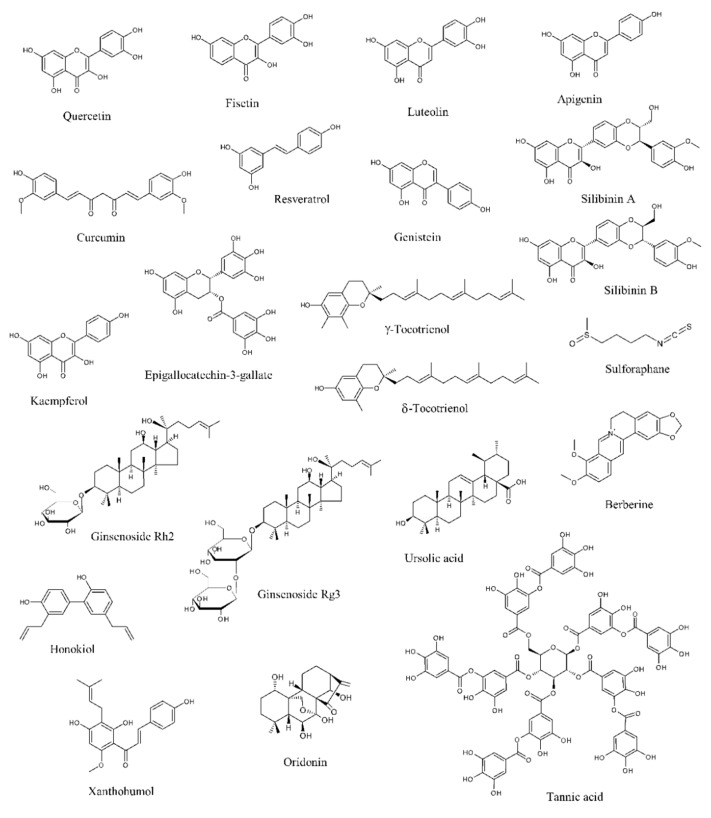
Data from literature have pointed out that several natural products can selectively target numerous molecules and signaling pathways implicated in tumor development and progression [11,12,13]. Many of them have been tested in in vitro and in vivo studies, while some clinical trials have been conducted or are currently ongoing [11,12,13]. Among these naturally occurring molecules, quercetin, fisetin, luteolin, apigenin, curcumin, resveratrol, genistein, silibinin, kaempferol, epigallocatechin-3-gallate (EGCG), tocotrienols, sulforaphane, ginsenosides, ursolic acid, berberine, honokiol, xanthoumol, oridonin, and tannic acid have shown outstanding potential as anti-PCa agents in in vitro and preclinical experiments (Figure 1).
Figure 1.
Chemical structures of the major anti-prostate cancer (PCa) phytochemicals.
2.1. Natural Compounds Modulating the Androgen Receptor Axis
A number of studies indicates that PCa growth and progression are driven by the AR, a ligand-dependent transcription factor and member of the nuclear receptor family [14]. The AR is encoded by the AR gene located on the X chromosome at Xq11-12 and displays a N-terminal regulatory domain, a DNA-binding domain (DBD), a ligand-binding domain (LBD), and a C-terminal domain. In the absence of androgens, particularly dihydrotestosterone (DHT) and testosterone, it is complexed with chaperone proteins, heat-shock protein 90 (Hsp90) and 70 (Hsp70), in the cell cytoplasm. Upon ligand binding, it is transferred to the nucleus, where it homodimerizes due to the interactions of dedicated motifs in the DBD and in the LBD. Then, the dimerized receptor recognizes cognate DNA response elements in regulatory regions located in proximal or more distal intra- and inter-genic regions of androgen target genes [15,16]. It then recruits different coregulator proteins and epigenetic factors to generate a transcriptionally active complex able to upregulate downstream pro-survival gene expression [14].
Given its fundamental role in PCa cell proliferation, the AR signaling represents a crucial target for PCa management. In this context, pharmacological castration obtained via androgen-deprivation therapy is currently the most effective strategy for PCa treatment. However, PCa often becomes castration resistant [8,9]. One of the mechanisms underlying this change is an enhanced AR expression in the tumor cell. In particular, it has been shown that 28% of cancers resistant to androgen-deprivation therapy display AR upregulation due to amplification of its gene [17]. Another mechanism responsible for PCa androgen-independent growth is ligand promiscuity, caused by mutations of the AR gene that lead to amino acid substitutions in the LBD and subsequent decrease in the specificity and selectivity for ligands: the most common of them are T877A, F876L, W741L, and L701H. These mutant AR proteins bind to other steroids, including progesterone, estrogens, and glucocorticoids, which can activate the AR signaling pathway and promote PCa progression [18]. AR activation via ligand-independent mechanisms represents the third mechanism of androgen-independent PCa development [19]. Indeed, it has been found that tyrosine kinase receptor-activating ligands, such as epidermal growth factor (EGF) and insulin-like growth-factor-1 (IGF-1), can activate the AR through the phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway [20,21,22,23,24]. Finally, various AR splice variants lacking the LBD have been recently reported: the AR N-terminal domain becomes constitutively active in the absence of the LBD, thereby promoting castration resistant proliferation [25,26].
Interestingly, various phytochemicals have been shown to modulate AR expression and activity.
Quercetin is a penta-hydroxylated flavonol, naturally occurring in tea, onions, apples, tomatoes, and capers and endowed with important chemopreventive and anti-cancer properties [27]. Yuan et al. demonstrated that in LNCaP PCa cells a protein complex containing the AR, specific protein 1 (Sp1) and c-Jun was generated in response to quercetin treatment and suppressed AR function. This resulted in the inhibition of the production of the prostate-specific, androgen-related tumor markers prostate-specific antigen (PSA) and human kallikrein-2 (hK2), as well as in the downregulation of androgen-related genes, such as ornithine decarboxylase (ODC) and NKX3.1 [28,29,30,31]. Interestingly, quercetin was also able to repress the expression of the AR splice variant 7 (AR-V7), which correlates to resistance to enzalutamide and poor prognosis, via Hsp70 inhibition [32].
Fisetin, a flavonol present in strawberries, apples, persimmons, onions, kiwi, and cucumbers, has been recently demonstrated to exert not only potent neuroprotective effects but also different anti-tumor activities [33,34]. In PCa, it was shown to specifically bind to the AR LBD. This interaction resulted in a decreased AR stability and amino-terminal/carboxyl-terminal (N-C) interaction, leading to a reduced transactivation of AR target genes. Moreover, fisetin treatment of LNCaP cells was followed by a downregulation of AR levels, due to a reduction in its promoter activity and to an increase of its degradation. In this cell line, the flavonol also synergized with bicalutamide in promoting apoptotic cell death. Finally, in AR-positive CWR22υ1 PCa cell-bearing mice, fisetin inhibited tumor growth and decreased PSA serum levels, suggesting that this compound is able to suppress AR activity also in vivo [35].
Luteolin, a flavone abundant in rosemary, thyme, parsley, broccoli, and celery, is characterized by anti-inflammatory, neuroprotective, and anti-cancer activity [36,37]. It was observed to induce a dose- and time-dependent decrease in AR mRNA and protein expression, as well as of intracellular and secreted PSA levels, in PCa cells. In particular, it appears to promote the AR-Hsp90 complex dissociation, causing AR degradation via the proteasome-ubiquitin pathway [38].
Curcumin is a polyphenol extracted from turmeric (Curcuma longa), which has shown great therapeutic potential [39,40,41]. This compound was demonstrated not only to decrease the expression of AR and AR-related cofactors, such as activator protein-1 (AP-1), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), CREB-binding protein (CBP), and NKX3.1, but also to reduce testosterone production in PCa cell lines and xenografts. This reduction in testosterone levels was associated with a downregulation of steroidogenic acute regulatory proteins, including cytochrome P450 11A1 (CYP11A1) and 3-beta-hydroxysteroid dehydrogenase 2 (HSD3B2), and in an enhanced expression of aldo-keto reductase 1C2 (AKR1C2), a 3-ketosteroid reductase responsible for the elimination of 5alpha-DHT and subsequent inactivation of AR [42,43,44,45].
Resveratrol is a grape-derived polyphenol that possesses numerous health benefits, including various chemopreventive effects [46]. It was found to target the AR axis in different in vitro and in vivo PCa models [47,48,49,50,51]. On one hand, in LNCaP cells it inhibited β-catenin nuclear translocation through hypoxia-inducible factor 1-α (HIF-1α) downregulation, thus suppressing β-catenin-mediated AR signaling [52]; similarly, it also repressed interleukin-6 (IL-6)-induced AR transcriptional activity [53]. On the other hand, in 22RV1 cells it promoted the AR splice variant ARV7 proteasomal degradation, by enhancing its polyubiquitination. These data indicate that resveratrol could be used not only for the treatment of androgen-responsive PCa but also for the management of the ARV7-positive castration-resistant tumor [54].
Genistein is a common phytoestrogen that can be obtained from soybeans [55]. Indeed, it was shown to inhibit the AR signaling via estrogen receptor-β (ER-β) and estrogen-related pathways, as well as through suppression of Akt/Forkhead box O3a (FOXO3a)/glycogen synthase kinase 3β (GSK-3β) and histone deacetylase 6 (HDAC6)-Hsp90 function, needed to stabilize the AR [56,57,58,59]. Notably, in a recent study by Mahmoud et al., genistein was also demonstrated to bind to both the wild and the T877A-mutant types of AR, specifically competing with androgens. In particular, while it suppressed proliferation of AR wild-type LAPC-4 cells, it exerted a dual role in T877A-mutated LNCaP and PC3 cell lines, by stimulating cell growth at lower doses and inducing cell death at higher concentrations [60]. Finally, in PCa cells genistein downregulated prostate androgen-regulated transcript-1 (PART-1) gene expression induced by DHT, thus affecting cell proliferation [61].
Other natural products that have been demonstrated to trigger similar inhibitory effects on the AR axis are sulforaphane [62,63,64,65], epigallocatechin-3-gallate (EGCG) [66,67], ginsenosides [68,69,70,71], silymarin [72], berberine [73], honokiol [74], and celastrol [75].
2.2. Natural Compounds Affecting Proliferation
Numerous natural compounds have been reported to exert growth-suppressive and anti-proliferative activities in PCa cells and xenografts.
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase whose activation is associated with an increase in cell growth and survival, which explains why EGFR is commonly overexpressed/overactivated in tumors of epithelial origin, including PCa. In particular, after binding to its specific ligands, such as EGF and transforming growth factor α (TGFα), it triggers several downstream signaling pathways, including PI3K/Akt/mTOR, mitogen-activated protein kinases (MAPKs), Hedgehog (Hh) signaling, and NF-κB [76]. Many phytochemicals, including quercetin, luteolin, resveratrol, genistein, and berberine, have been shown to reduce EGFR levels, as well as to suppress its intrinsic tyrosine kinase activity and its ligand-induced activation, in different PCa cell lines and in vivo models [77,78,79,80,81].
The IGF axis is a complex signaling network implicated in different tumorigenic processes, particularly in cancer proliferation, survival, and metabolism. It involves the interaction between the peptide-ligands IGF1 and IGF2 and the receptors IGF1R and IGF2R, and its activation elicits downstream signals, such as the PI3K/AKT and the MAPK pathways [82]. Interestingly, the IGF axis represents a major target for the anti-PCa action of silibinin, a flavonoid endowed with antioxidant properties commonly found in the milk thistle (Silybum marianum) [83,84]. Indeed, it decreased IGF1 expression and increased IGFBP-3 levels in transgenic adenocarcinoma of the mouse prostate (TRAMP) models, thus inhibiting tumor growth and progression [85,86,87]. Similar results were also obtained after treatment of PCa-bearing mice with luteolin [88].
Emerging evidence has highlighted the key role played by the PI3K/AKT pathway in the development of castration resistant PCa. This cascade, which is activated in most of advanced PCas, acts as a fundamental driver for tumor cell proliferation, thereby allowing cancer cells to survive to the androgen deprivation-related cytotoxicity. Moreover, preclinical studies have highlighted a strict correlation between the PI3K/AKT and AR axes, evidencing a dynamic cross-talk between these cascades in the acquisition of androgen-deprivation therapy resistance. Therefore, there is an evident rationale for the development of novel PI3K inhibitors, which may be able to block castration-resistant PCa growth and survival [89]. In this setting, the interest in natural products has recently increased, due to their ability to specifically target the PI3K/AKT cascade. In particular, quercetin, apigenin, curcumin, genistein, sulforaphane, and EGCG have been demonstrated to attenuate PCa cell growth by downregulating this signaling pathway [90,91,92,93,94,95,96,97,98].
During PCa progression, both tumor invasion and chemoresistance are promoted by NF-κB. Indeed, constitutive activation of this protein has been commonly found in primary PCas and it is associated with AR loss and castration-resistant features. Thus, NF-κB is an important target for PCa management, owing to its role in tumorigenesis and therapy resistance [99]. Notably, downregulation of this protein and of its target genes has been highlighted after resveratrol, genistein, sulforaphane, ursolic acid, tocotrienol, and celastrol treatment [100,101,102,103,104,105].
Hh pathway activation is implicated in the development of different types of tumors, including PCa. In particular, many studies have pointed out that this signaling plays a crucial role in the progression of PCa to more aggressive and chemoresistant states [106]. Slusarz et al. demonstrated that seven common nutraceuticals, (i.e., genistein, curcumin, EGCG, resveratrol, apigenin, baicalein, and quercetin) can suppress the Hh pathway both in vitro and in vivo, with four of them (i.e., genistein, curcumin, resveratrol, and EGCG) decreasing not only Hh effector Gli1 expression but also Gli1 reporter activity [107].
Genome sequencing and gene expression analyses have evidenced the importance of the Wnt pathway in the development of castration resistant PCa [108]. Wnt signaling is also implicated in the cross-talk with the PCa microenvironment, where this protein is secreted by the tumor stroma and promotes therapy resistance, as well as in PCa stem cell self-renewal or expansion [109]. Preclinical studies have illustrated the potential of Wnt inhibitors in preventing PCa progression. Some of them have already been tested in phase I trials, although they have not been administered to PCa patients yet [108,109]. Interestingly, treatment of PCa cells with quercetin, curcumin, genistein, and silibinin resulted in growth suppression through Wnt cascade modulation [110,111,112,113].
MicroRNAs (miRNAs) are endogenous, ≈22 nucleotides, non-coding RNAs able to induce both transcriptional and translational arrest, thus functioning as either oncogenes or oncosuppressors, depending on the specific tumor type [114]. Concerning PCa, genistein has shown promise in modulating the levels of different oncogenic (i.e., miR221, miR222, miR151, and miR1260b) and oncosuppressor (i.e., miR-574-3p and miR34a) miRNAs, thus affecting cancer cell proliferation [115,116,117,118,119,120]. Similar encouraging data were also obtained from in vitro studies with luteolin, curcumin, resveratrol, ginsenoside Rh2, and celastrol [121,122,123,124,125,126].
2.3. Natural Compounds Inducing Canonical and Non-Canonical Cell Deaths
Apoptosis is commonly induced in PCa cells and xenografts treated with phytochemicals. In particular, many natural products have been found to trigger both the extrinsic and intrinsic apoptotic pathways, by activating cell surface death receptors, altering Bax/Bcl-2 ratio, increasing p21 levels and triggering caspase-8, -9, -3, and poly (ADP-ribose) polymerase (PARP) cleavage [127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153]. In this setting, proteostasis disruption appears to play a key role in the modulation of the nutraceutical-related apoptotic cell death. Indeed, while apigenin, luteolin, genistein, and celastrol inhibited the proteasomal activity and caused ubiquitinated protein accumulation in different PCa cell lines [154,155,156], quercetin, curcumin, silibinin, and tannic acid induced endoplasmic reticulum (ER) stress [157,158,159,160], a condition where unfolded/misfolded proteins accumulate in the ER lumen and promote the activation of distinct pro-death cascades, including the double-stranded RNA-dependent protein kinase PKR-like ER kinase (PERK)/eukaryotic initiation factor 2α (eIF2α)/activating transcription factor 4 (ATF4)/C/EBP homologous protein (CHOP) pathway and the inositol-requiring enzyme 1α (IRE1)/c-Jun N-terminal kinase (JNK)/p38 MAPK cascade [161]. Notably, curcumin- and silibinin-mediated ER stress was associated with generation of reactive oxygen species (ROS) and redox homeostasis alteration [159,162], which were also observed in resveratrol- and sulforaphane-treated PCa cells [163,164,165,166].
Interestingly, apoptotic cell death is not the only death mode triggered by natural compounds.
Berberine is a benzylisoquinoline alkaloid commonly found in the plants of the genus Berberis [167,168]. In a recent study by Zhang et al., it was shown to induce programmed necrosis in LNCaP and PC-82 PCa cell lines. In particular, mitochondrial protein cyclophilin-D (Cyp-D) was observed to be crucially involved in the modulation of berberine-related necrotic cell death. Indeed, berberine treatment resulted in ROS production, which promoted p53 translocation to mitochondria and its interaction with Cyp-D to open the mitochondrial permeability transition pore (mPTP), ultimately leading to necrosis induction [169]. Pro-necrotic effects were also exerted by curcumin in DU145 cells [170].
Paraptosis is a programmed cell death mode characterized by cytoplasmic vacuolation, particularly by ER dilatation and mitochondrial swelling [171,172]. Recently, we have demonstrated that δ-tocotrienol, a vitamin E derivative particularly abundant in annatto seeds, rice bran, and palm oil [173,174], can trigger both apoptosis and paraptosis in PC3 and DU145 cell lines. The mechanisms underlying its pro-paraptotic effects were found to correlate with activation of JNK and p38, as well as with proteotoxicity, since not only the protein synthesis inhibitor cycloheximide but also the ER stress inhibitor salubrinal successfully prevented the cytoplasmic vacuolation evoked by the treatment with this natural compound [175]. Similarly, paraptosis-like cytoplasmic vacuolation was also observed in celastrol-treated PC3 cells [176].
Autophagy is an evolutionarily conserved catabolic process generally used by the cell to eliminate cytoplasmic material, including misfolded proteins and damaged organelles, via lysosomal degradation: it involves the formation of double-membrane vesicles, the autophagosomes, that promote cytoplasmic cargo recycling after fusion with lysosomes, and it is regulated by different proteins, particularly by microtubule-associated proteins 1A/1B light chain 3B, commonly called LC3 [177]. It is now well known that autophagy can act as both tumor promoter and suppressor. The dual role of this mechanism in cancer cells apparently depends on tumor type, stage, and genetic context. Indeed, while on one hand the autophagic flux clearly suppresses tumorigenesis, on the other hand it acts as a key survival mechanism in response to stress, thus promoting cancer cell proliferation. In the context of PCa, curcumin, sulforaphane, silibinin, ursolic acid, honokiol, and oridonin triggered cytoprotective autophagy [178,179,180,181,182,183,184]; on the contrary, fisetin, resveratrol, and celastrol treatment resulted in autophagic cell death [185,186,187]. In particular, the fisetin- and resveratrol-mediated autophagic flux was associated with Akt/mTOR signaling pathway downregulation and AMP-activated protein kinase (AMPK) activation [185,186]. The autophagy induced by celastrol, a pentacyclic triterpenoid extracted from Tripterygium Wilfordi roots [188], was instead correlated to suppression of AR/miR-101 cascade [187].
2.4. Natural Compounds Impairing Metabolism
Tumor metabolism is usually characterized by a high flux of glucose through glycolysis and the pentose phosphate pathway, thus representing an important pharmacological target. Indeed, treatments aimed at blocking these pathways and/or shifting lactic acid fermentation towards mitochondrial oxidative phosphorylation have shown promise in reducing tumor growth [189]. In recent studies by Fonseca J et al., resveratrol was found to promote a shift towards mitochondrial oxidation in PCa cells concomitantly with the suppression of proliferation, and when this change was prevented by culturing tumor cells in glucose-free medium or via prolyl hydroxylase (PHD) inhibition-mediated stabilization of HIF-1α, the phenol did not affect oxidative phosphorylation and cell growth, indicating that the metabolic shift from glucose fermentation to oxidation is fundamental for its anti-cancer effects [190,191].
As mentioned above, cancer cells need an increase in glucose uptake to satisfy their high demand for cell growth and proliferation. This is mediated by glucose transporters (GLUTs) by a mechanism of facilitated diffusion. Fourteen different GLUT receptors (GLUT1-12, GLUT14, and H/myo-inositol transporter) exist: the enhanced glucose consumption observed in tumor cells has been associated with overexpression of GLUT1, commonly found in brain and erythrocytes, but may also involve other GLUTs, including the heart-, skeletal muscle-, and adipose tissue-specific GLUT4 [192]. Gonzalez-Menendez et al. showed that GLUT1 and 4 proteins are expressed in LNCaP and PC3 cells and that apigenin and phloretin are able not only to reduce glucose uptake but also to modify GLUT levels in these cell lines [193].
Phosphoglucomutase 3 (PGM3) belongs to the hexose-phosphate mutase family, and it mediates the conversion of glucose-1-phosphate to glucose-6-phosphate, thus regulating glycolysis and pentose phosphate shunt [194]. Recently, it has been demonstrated to be a specific target for the anti-PCa activity of sulforaphane [195], an organic isothiocyanate derived from broccoli and other cruciferous plants [196,197].
In the last decade, the metabolic rewiring underlying tumor increased proliferation has been reported to not only involve glucose metabolism but also lipid synthesis. The crucial role played by lipids in tumor progression has been evidenced by different studies demonstrating that normal cells, except for adipocytes and hepatocytes, uptake the fatty acids necessary for their growth from the diet; however, in tumor cells lipids are mostly obtained via de novo lipogenesis. In the case of PCa, many studies have highlighted that its precursor lesions are characterized by elevated endogenous lipogenesis, regardless of the levels of extracellular/circulating lipids [198,199,200]. The increased de novo lipogenesis observed in PCa cells has been associated with their enhanced request for energy production, redox homeostasis, membrane formation, cell death escape, and modulation of many intracellular proliferative pathways [198,199,200,201]. Moreover, during androgen-deprivation therapy, cholesterol plays a key role in the de novo androgen synthesis, thus promoting self-sufficiency in AR signaling and hormone-refractory progression of the tumor [202,203]. Therefore, these unique metabolic features of PCa represent an optimal target for the management of this cancer. In this setting, silibinin treatment lead to the suppression of PCa aberrant lipid metabolism, both in vitro and in vivo. Mechanistically, this compound activated increased AMPK-mediated phosphorylation of sterol regulatory element-binding protein-1 (SREBP-1) and inhibited its nuclear translocation, thus reducing lipid and cholesterol accumulation and suppressing the development of androgen-independence. Moreover, the lipogenic phenotype promoted by hypoxia in PCa cells was abrogated by silibinin via inhibition of acetyl-Co A carboxylase (ACC) and fatty acid synthase (FASN) [204,205,206]. Notably, these two enzymes were also downregulated by other nutraceuticals, such as luteolin, quercetin, kaempferol, apigeninin, EGCG, and sulforaphane, in normoxic conditions [207,208,209,210].
Glutamine uptake and use is increased in various tumors, including PCa, primarily to support de novo lipogenesis. In fact, in the process of glutaminolysis, glutamine is first converted into glutamate and then into α-ketoglutarate, that can enter the Krebs cycle to drive citrate synthesis for lipogenesis [211]. Interestingly, inhibition of the glutamate-to-α-ketoglutarate conversion blocked resveratrol-related cytotoxicity in PCa cells. A similar effect was also obtained by reducing glutamine content in the culture medium, indicating that resveratrol-mediated anti-PCa effects are dependent on glutamine metabolism [212]. In addition, untargeted metabolomics and metabolic flux analysis using isotopically labeled glutamine pointed out that resveratrol in combination with ursolic acid and curcumin severely altered glutamine metabolism [213]; in particular, alanine serine cysteine transporter 2 (ASCT2) levels were found to be downregulated [213].
2.5. Natural Compounds Inhibiting Invasion
Metastasis, the spread of cancer cells from the primary tumor to new body tissues and organs, is a key step in PCa growth and progression [214].
During cancer development, tumor cells undergo dynamic changes leading to the acquisition of a highly invasive phenotype and to their detachment from the original tissue. Epithelial-to-mesenchymal transition (EMT) is the hallmark of this phenomenon, during which an important change in the expression of adhesion molecules regulating the interaction of tumor cells with the extracellular matrix and their microenvironment occurs. Indeed, a common characteristic of tumors of epithelial origin is an increase in the expression of N-cadherin and a parallel downregulation of E-cadherin, a major component of adherent junctions. This molecular switch is called cadherin switching, and it is generally accompanied by the upregulation of other invasion markers, such as Twist, Snail, and Slug, culminating in the enhanced metastatic potential of the tumor cell [215]. Numerous natural compounds have been shown to revert EMT in PCa cells and xenografts, particularly by modulating the PI3K/Akt and Wnt/β-catenin signaling cascades [216,217,218,219,220,221,222,223,224,225,226]. In addition, urokinase-type plasminogen activator (uPA)-, Y-box binding protein-1 (YB-1)- and SPARC/osteonectin, cwcv, and kazal-like domains proteoglycan 1 (SPOCK1)-mediated suppression of EMT contributed to the anti-invasive activity of quercetin, fisetin, and apigenin, respectively [227,228,229].
Extracellular matrix (ECM) proteolytic degradation is a key event in the metastatic process. Among more than 100 distinct proteinases, matrix metalloproteinases (MMPs) appear to be primarily responsible for most of the ECM degradation observed during metastasis [230]. In particular, MMP-2 and MMP-9 have been frequently associated with the invasiveness of tumors, including PCa. Reduction in MMP-2 and MMP-9 levels was observed after treatment with various nutraceuticals, and it generally correlates with MAPK inactivation [231,232,233,234,235,236,237,238,239,240,241,242,243,244].
One of the main events occurring during metastasis is the substitution of cell–cell interactions with integrin-based cell-matrix communication, in order to promote tumor cell invasiveness [215]. In PCa cells, silibinin treatment not only modulated the fibronectin-mediated expression of integrins (α5, αV, β1, and β3) but also induced actin remodeling and cytoskeleton disorganization via focal adhesion kinase (FAK)/Src signaling pathway inhibition [245]. Notably, disruption of microfilament-driven cell motility was also found after apigenin and curcumin treatment [246,247].
Like most tumors, PCa is characterized by CD44 dysregulation. CD44 standard (CD44s), which is present in normal epithelium, is lost in the tumor, whereas pro-invasive splice variant isoform CD44v7-10 is overexpressed [248]. CD44 inhibition is one of the mechanisms through which silibinin decreases PCa tumorigenicity. Indeed, in PC-3M cells silibinin dose-dependently reduced the mRNA and protein levels of CD44v7-10, also inhibiting early growth response protein 1 (EGR1), a regulator of CD44 promoter activity [249].
Different studies demonstrated a direct correlation between loss of metastasis suppressors/overexpression of invasion promoters and poor prognosis in human PCas. Interestingly, while genistein and EGCG induced the expression of the invasion suppressors kangai-1 (KAI1) and tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) [250,251], sulforaphane-cysteine, ginsenoside Rg3, and pterostilbene inhibited the metastasis promoters galectin 1, aquaporin 1, and metastasis-associated protein 1 (MTA1) [252,253,254], respectively.
As reported above, bone metastasis commonly occurs in advanced PCas, and it responsible for considerable morbidity, such as pathologic fractures, spinal cord compression, and pain [10]. Curcumin was reported to suppress PCa bone metastasis by upregulating the invasion inhibitor bone morphogenic protein-7 (BMP-7) in vivo [255]. Bone metastasis inhibition was also observed after genistein and celastrol treatment [256,257].
2.6. Natural Compounds Reducing Angiogenesis
Angiogenesis, the formation of new blood vessels from preexisting capillaries, is a fundamental step in cancer development, enabling the proliferating tumor to receive oxygen and nutrients [258]. In particular, angiogenesis is characterized by the activation and migration of endothelial cells towards specific stimuli secreted by the tumor. Among several cancer-derived angiogenic factors, the most important is vascular endothelial growth factor (VEGF). The specific mitogenic effects of VEGF on the endothelial cells are mainly regulated by VEGFR-1 and VEGFR-2, two receptor tyrosine kinases. Of the two receptors, VEGFR-2 plays a fundamental role in promoting proliferation, migration, and tube formation of endothelial cells by activating multiple downstream signals, such as PI3K/Akt and MAPKs [259,260]. Interestingly, quercetin, luteolin, and celastrol at non-toxic concentrations were shown to suppress endothelial cell growth and invasion and microvessel sprouting in vitro, as well as to inhibit ex vivo angiogenesis. Mechanistically, these compounds were demonstrated to block VEGF-induced activation of VEGFR-2 and of its downstream target PI3K/Akt [261,262,263].
Hypoxia and transforming growth factor-β (TGF-β) are the two main factors implicated in the increase of VEGF secretion [264,265]. Quercetin, apigenin, and genistein were found to reduce HIF-1α expression in PCa cells, successfully preventing VEGF release [266,267,268]. Parallelly, apigenin was also shown to decrease TGF-β-induced VEGF expression by blocking the phosphorylation and nuclear translocation of Smad2 and Smad3 and by downregulating the FAK/Src/Akt pathway [269].
Thrombospondin-1 (TSP-1) is a 450 kDa extracellular calcium binding glycoprotein and a potent endogenous anti-angiogenic factor [270]. Yang et al. have recently reported that quercetin can upregulate TSP-1 mRNA and protein expression in PCa xenografts [271].
Hyaluronan is a major component of the ECM. It is a non-sulfated, linear polymer formed by repeating disaccharides of glucuronic acid (GlcUA) and N-acetyl glucosamine units (GlcNAc), and it is synthesized at the cell surface by the membrane-bound enzyme hyaluronan synthase, while being degraded by hyaluronidases [272]. In particular, the native anti-angiogenic molecule of hyaluronan can be fragmented into a smaller pro-inflammatory and pro-tumor form. Indeed, high levels of hyaluronan low-molecular-weight fragments correlate with malignant progression and poor survival in different tumor types, including PCa [273,274]. In a recent study, hyaluronan has been identified as a specific target for fisetin anti-PCa activity in tumor xenografts and TRAMP mouse models, where increased levels of anti-angiogenic high-molecular-weight hyaluronan have been found [275].
2.7. Natural Compounds Targeting Cancer Stem Cells
PCa stem cells (PCSCs) represent a small subpopulation of stem-like cells endowed with self-renewal and differentiation abilities, as well as with tumor-initiating and propagating functions. Expression of cell surface markers, including CD44, CD133, and α2β1 integrin, is commonly used to identify and enrich PCSCs. Owing to their resistance to standard therapies, their role in metastasis and relapse and their contribution to the progression towards castration-resistant PCa, PCSCs are currently under extensive study, especially in the field of anti-cancer drug discovery [276].
Quercetin and luteolin successfully reduced the anchorage-independent spheroid formation and the expression of CD44, ABCG2, Sox2, and Nanog in highly invasive PCa cells [277]. Moreover, while quercetin was able to block the proliferation of LNCaP- and PC3-derived CD44+/CD133+ and CD44+ stem cells [278], luteolin suppressed PCa stemness via upregulation of frizzled class receptor 6 (FZD6), thus inhibiting Wnt signaling [279].
Apigenin dose-dependently suppressed PCSC growth, by increasing p21 and p27 levels. In these cells, this compound also triggered extrinsic apoptosis via upregulation of TNF-α, caspase-8 and -3, and it strongly reduced invasion through downregulation of MMP-2, -9, Snail, and Slug. Furthermore, apigenin treatment induced a PI3K/Akt/NF-κB-mediated decrease in pluripotency marker Oct3/4 protein expression [280,281]. Finally, it sensitized human CD44+ PCSCs to cisplatin [282].
Curcumin inhibited DU145 and 22RV1-derived CD44+/CD133+ PCSC proliferation and invasion by ceRNA effect of miR-145 and lncRNA-ROR, as well as through modulation of DLK1-DIO3 imprinted gene cluster miRNAs. In fact, bioinformatic analyses and luciferase activity assays demonstrated that both the lncRNA-ROR and Oct4 mRNA contain miR-145 binding sites, and that Oct4 and lncRNA-ROR directly compete for miRNA binding. Decreasing the lncRNA-ROR endogenous levels via curcumin treatment could effectively enhance the available concentration of miR-145 in PCSCs, where miR-145 prevented cell growth by reducing Oct4 expression. Parallelly, miR-770-5p and miR-1247 expression levels were found to be significantly higher in curcumin-treated than in control PCSCs [283,284].
PCSC-like traits, including aldehyde dehydrogenase 1 (ALDH1) accelerated activity, CD49f+ fraction enrichment, and sphere formation capability, were abrogated by sulforaphane treatment. Notably, sulforaphane-induced suppression of PCSC-like phenotype was counteracted when c-Myc was overexpressed in PCa cells, suggesting that sulforaphane may target c-Myc-regulated PCSC-like characteristics [285].
Sphere formation was markedly suppressed after treatment of PCa cells with genistein. Moreover, treatment of PCSC-enriched spheres with genistein inhibited their growth and tumorigenicity in vivo. Additionally, this compound not only downregulated CD44 expression, but also inhibited the Hh-Gli1 pathway, which presumably contributes to the anti-CSC effect of genistein in PCa [286].
It has been reported that γ-tocotrienol could reduce CD133 and CD44 markers in castration-resistant PCa cells, also suppressing their anchorage-independent growth and spheroidogenic ability. In addition, γ-tocotrienol pretreatment of PCa cells lead tumor initiation suppression after their inoculation in nude mice. Moreover, despite being highly resistant to docetaxel, CD133+ cells were as responsive to γ-tocotrienol as the CD133- population [287]. Similar experiments were performed by Lee et al., who confirmed the γ-tocotrienol capability to eliminate the CSC subpopulation in various PCa cell lines and mouse models, significantly inhibiting castration-resistant tumor proliferation [288]. Recent evidence indicates that also δ-tocotrienol can block PCSC growth under hypoxia via inactivation of the HIF-1α signaling [289].
3. Clinical Impact
To date, various clinical trials have been conducted to test the efficacy of natural compounds in PCa patients.
Two randomized, double-blind, placebo-controlled trials, aimed at evaluating the effects of curcumin on PCa patients undergoing radiotherapy, showed that this phenol could mitigate radiation-induced proctitis and oxidative stress [290,291], while six-month intake of the compound reduced the elevation of PSA in PCa men who received intermittent androgen deprivation (IAD), despite not significantly affecting the overall off-treatment duration of the therapy [292].
Accumulating epidemiological evidence has highlighted a geographical basis for PCa incidence, and isoflavone consumption may be related to this phenomenon. Indeed, PCa is more common in Western than Asian populations, and several trials have demonstrated that soy derivatives genistein and daidzein can prevent the development and progression of this tumor in Japanese and Chinese men [293,294,295,296]. On the contrary, the data collected in European patients are still contradictory. While the results obtained from two population-based case-control studies on diet, inherited susceptibility and PCa support the idea that a phytoestrogen-enriched diet may protect against the tumor in Scottish and Sicilian men [297,298], in a European Prospective Investigation into Cancer and Nutrition study genistein concentrations in the plasma samples of 1605 PCa cases and 1697 matched control participants were not correlated with cancer risk [299]. Globally, a recent meta-analysis of single patient data from seven prospective studies (two Japanese studies with 241 cases and 503 controls and five European studies with 2828 cases and 5593 controls) did not show any significant correlation between prediagnostic intake of isoflavones and PCa development, although further studies should be performed in populations where isoflavone intakes are high [300]. In this respect, it should be underlined that purified genistein have been demonstrated to be well tolerated in 20 PCa patients treated with 300 or 600 mg isoflavone/day for 84 days, showing no genotoxicity [301] and causing only minor estrogenic effects, such as hot flashes and breast changes [302].
Increased PSA serum levels are commonly observed in PCa after radical prostatectomy and are defined “biochemical recurrence” [303]. Oral administration of 60 mg/day of sulforaphane for six months, followed by two months with no treatment, led to a partial reduction of PSA levels in PCa patients who underwent prostate removal [304]. Similarly, treatment with 200 μmoles/day of sulforaphane resulted in a small (<50%) PSA decrease in patients with recurrent PCa, with a significant lengthening of the on-treatment PSA doubling time (PSADT) with respect to the pretreatment (9.6 months on-treatment vs. 6.1 months pretreatment) [305].
In a randomized placebo-controlled clinical study, PCa middle-aged men were given two doses of resveratrol (150 or 1000 mg/day) for four months: the levels of circulating androgen precursors were shown to be reduced, but no effect was observed on testosterone, DHT, and PSA levels, as well as on prostate volume [306]. In a phase I clinical trial, different doses of pulverized muscadine grape (Vitis rotundifolia) skin containing 4.4 μg resveratrol/500 mg extract were administered to 14 men with recurrent PCa for 2–31 months. The highest dose (4000 mg) was found to be safe and able to elongate PSADT of about 5.3 months [307]. The benefits of both the high and low (500 mg) doses were then explored in a 12-month, randomized, multicenter, placebo-controlled, phase II trial, where no changes in PSADT were evidenced in 125 patients with biochemically recurrent PCa; however, in a preplanned exploratory analysis, a significant PSADT pre-to-post increase was highlighted in patients with SOD2 Alanine/Alanine genotype (26% of total patients) treated with muscadine grape skin extract with respect to the control group, revealing the existence of a patient subpopulation which may be responsive to the treatment [308].
PCa patients scheduled for radical prostatectomy received daily doses of Polyphenon E, containing 800 mg of EGCG, until the day of surgery: serum levels of PSA, HGF, and VEGF were found to be decreased [309,310]. However, daily intake of this mixture for one year did not reduce the risk of PCa in men with high-grade prostatic intraepithelial neoplasia (HGPIN) and/or atypical small acinar proliferation (ASAP), despite being well tolerated [311]. On the contrary, positive results were obtained by treating 60 volunteers with HGPIN with 600 mg/day of EGCG: after one year, only one case of cancer was found among the 30 EGCG-treated men, while nine tumors were diagnosed among the 30 placebo-treated men. Moreover, EGCG-treated men showed lower PSA values compared to placebo-treated ones, although no significant difference was evidence between the two arms. Finally, a significant improvement of the International Prostate Symptom Score (IPSS) was observed in EGCG-treated men with benign prostatic hyperplasia [312]. In this regard, it should also be noted that PCA risk among Hong Kong and Japanese populations inversely correlates to green tea consumption and EGCG intake [313,314].
Silybin-phytosome is a commercially available formulation containing silibinin. In a phase I trial, it was orally administered to 13 patients with advanced PCa, starting from 2.5 g/day and gradually escalating to 20 g/day. No side effect was observed, except for nine cases of grade 1–2 hyperbilirubinemia. In particular, a daily dose of 13 g appeared to be well tolerated [315]. Therefore, in the subsequent study six patients with localized PCA and scheduled for prostatectomy were selected to receive three daily doses of the formulation (13 g tot), while six were chosen as controls. Silibinin blood concentrations reached a mean value of 19.7 μM after 1 h, while trough levels were 1.2 μM at the end of the 14–31 (with a mean of 20) days of treatment. On the contrary, the highest silibinin concentration observed in the prostate tissue was 496.6 pmol/g. Toxic effects were similar to those found in the previous trial. Notably, no objective PSA, IGF-I, and IGFBP-3 responses were observed in both the studies [316].
A randomized prospective double-blind study called Selenium and Vitamin E Cancer Prevention Trial (SELECT) was initiated in 2001 to determine whether vitamin E and selenium could reduce the risk of PCa in healthy men [317]. It involved more than 35,000 patients followed for up to 12 years [318]. Unfortunately, none of the tested agents, alone or in combination with each other, showed significant chemopreventive effects [318,319,320].
4. Conclusions
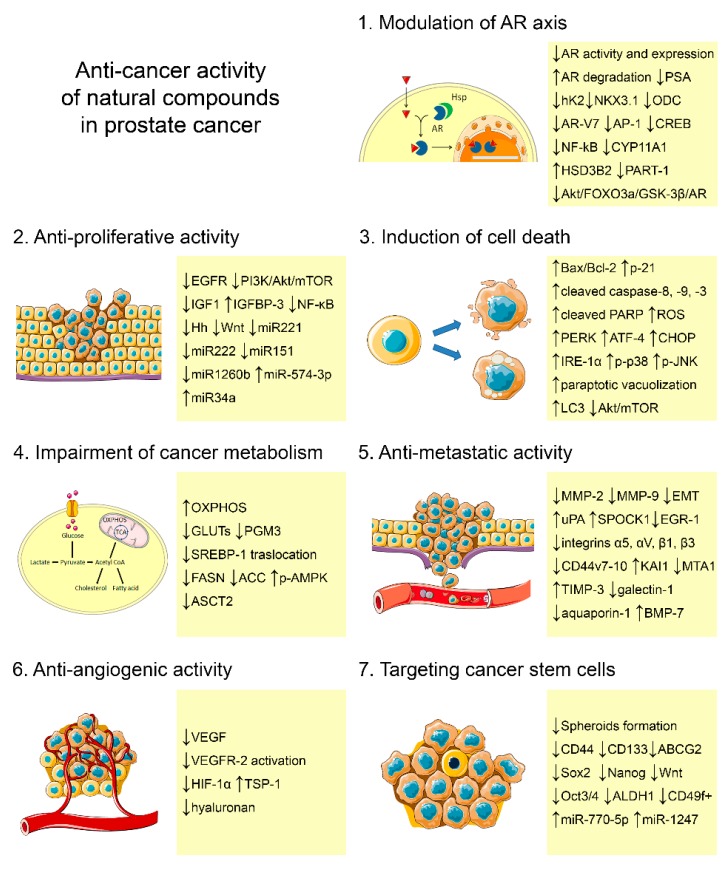
This article provides an overview of recent findings about the anti-PCa activity of different natural compounds (Figure 2, Table 1).
Figure 2.
Mechanisms of action and molecular targets of phytochemicals in PCa.
Table 1.
Main signaling pathways modulated by phytochemicals in PCa.
| Natural Compound. | Downregulated Pathways | Upregulated Pathways | Ref. |
|---|---|---|---|
| Apigenin | PI3K/Akt, Hh axis Proteosomal activity Glucose uptake Invasion/Motility Angiogenesis PCa cell stemness |
Extrinsic apoptotic cell death in PCa stem cells | [91,92,112,193,217,229,246,267,268,269,280,281,282] |
| Berberine | AR axis EGFR levels and activity Invasion/Motility |
Apoptotic cell death Programmed-necrotic cell death |
[73,148,149,168,169,225] |
| Celastrol | AR axis Proteosomal activity Oncogenic miRNAs Invasion/Motility Bone metastasis Angiogenesis |
ER stress Apoptotic cell death Paraptotic cell death Autophagic cell death |
[75,126,156,161,176,188,226,257,263] |
| Curcumin | AR signaling Testosterone levels PI3K/Akt, Hh, Wnt axis Oncogenic miRNAs Glutaminolysis Invasion/Motility Bone metastasis PCa cell stemness |
ER stress Apoptotic cell death ROS production Programmed-necrotic cell death Oncosuppressive miRNAs |
[42,43,44,45,93,94,107,111,122,132,133,134,158,161,162,170,213,218,233,234,235,247,255,283,284] |
| EGCG | AR signaling PI3K/Akt, Hh axis Lipogenesis Invasion/Motility |
Apoptotic cell death | [66,67,98,107,145,209,241,242,243,251] |
| Fisetin | AR stability and function Invasion/Motility Angiogenesis |
Autophagic cell death | [35,185,228,232,275] |
| Genistein | AR signaling EGFR levels and activity PI3K/Akt, NFκB, Hh, Wnt axis Oncogenic miRNAs Proteosomal activity Lipogenesis Invasion/Motility Bone metastasis PCa cell stemness |
Apoptotic cell death Oncosuppressive miRNAs | [56,57,58,59,60,61,81,95,96,101,102,107,112,115,116,117,118,119,120,140,154,155,208,219,220,236,237,238,239,240,250,256,286] |
| Ginsenosides | AR axis NFκB signaling Oncogenic miRNAs Metastasis promoters |
Oncosuppressive miRNAs | [68,69,70,71,125,253] |
| Honokiol | AR axis | Apoptotic cell death | [74,153] |
| Kaempferol | Apoptotic cell death | [131] | |
| Luteolin | AR signaling EGFR levels and activity IGFR signaling Oncogenic miRNAs Proteosomal activity Lipogenesis Endothelial cell growth Microvessel sprouting PCa cell stemness |
Apoptotic cell death Oncosuppressive miRNAs |
[38,79,88,121,130,207,262,277,279] |
| Quercetin | AR signaling AR-V7 activity EGFR levels and activity Pi3K/Akt, Hh, Wnt axis Lipogenesis Invasion/Motility Endothelial cell growth Microvessel sprouting PCa cell stemness |
ER stress Apoptotic cell death |
[28,29,30,31,32,77,78,90,110,127,128,129,157,161,216,227,231,261,266,271,277,278] |
| Resveratrol | AR signaling EGFR levels and activity NFκB, Hh signaling Oncogenic miRNAs Glucose fermentation Glutaminolysis |
ER stress Apoptotic cell death ROS production Autophagic cell death Mitochondrial oxidation Oncosuppressive miRNAs |
[47,48,49,50,51,52,53,54,80,100,107,123,124,135,136,137,138,139,161,163,164,186,190,191,212] |
| Sibilinin | IGF1 expression Wnt cascade Lipogenesis and lipid-dependent metabolism Invasion/Motility |
ER stress Apoptotic cell death ROS production |
[72,85,86,87,113,147,159,161,204,205,206,222,223,224,245,249] |
| Sulforaphane | AR function PI3K/Akt, NFκB axis Glycolysis Penthose Phosphate shunt Lipogenesis and lipid-dependent metabolism Invasion/Motility Metastasis promoters PCa cell stemness |
Apoptotic cell death ROS production |
[62,63,64,65,97,103,141,142,143,144,165,166,195,196,197,210,221,252,285] |
| Tannic acid | ER stress Apoptotic cell death |
[160,161] | |
| Tocotrienols | NFκB signaling PCa cell stemness |
ER stress Apoptotic cell death Paraptotic cell death Autophagic cell death |
[105,161,175,287,289] |
| Ursolic acid | NFκB signaling Glutaminolysis |
Apoptotic cell death | [104,150,152,213] |
The use of phytochemicals for PCa management offers several advantages. Firstly, natural products are safe and well tolerated, as well as usually economically affordable. Moreover, they are endowed with various in vitro and in vivo anti-tumor properties, including growth-suppressing, pro-death, anti-invasive, and anti-angiogenic activities. In particular, they appear to be able to selectively target the AR axis and the CSC subpopulation. However, these promising pleiotropic effects have been just partly confirmed in PCa patients, where nutraceutical intake has been associated with chemoprevention and PSA reduction rather than with tumor eradication. Thus, new clinical trials aimed at validating nutraceutical effectiveness in human subjects are urgently needed.
Funding
This research was funded by MIUR Progetto di Eccellenza (Department of Pharmacological and Biomolecular Sciences, Università degli Studi di Milano) and PRIN 2015, project n. 2015B7M39T_004 (Patrizia Limonta).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., Dandona L., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol. 2017;3:524. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson A.J., Scardino P.T., Eastham J.A., Bianco F.J., Jr., Dotan Z.A., Fearn P.A., Kattan M.W. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J. Natl. Cancer Inst. 2006;98:715–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlmutter M.A., Lepor H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007;9:S3–S8. [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas T.S., Pachynski R.K. Treatment of advanced prostate cancer. Mo. Med. 2018;115:156–161. [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney C.J., Chen Y.H., Carducci M., Liu G., Jarrard D.F., Eisenberger M., Wong Y.N., Hahn N., Kohli M., Cooney M.M., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl. J. Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W., Parker C.C., Russell J.M., Attard G., et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limonta P., Moretti R.M., Marzagalli M., Montagnani Marelli M. Castration Resistant Prostate Cancer: From emerging molecular pathways to targeted therapeutic approaches. Clin. Cancer Drugs. 2014;1:11–27. doi: 10.2174/2212697X0101131204151357. [DOI] [Google Scholar]
- 8.Nelson A.W., Shah N. Prostate cancer. Surg. (United Kingdom) 2019;37:500–507. doi: 10.1016/j.mpsur.2019.07.006. [DOI] [Google Scholar]
- 9.Teo M.Y., Rathkopf D.E., Kantoff P. Treatment of advanced prostate cancer. Annu. Rev. Med. 2019;70:479–499. doi: 10.1146/annurev-med-051517-011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cathomas R., Bajory Z., Bouzid M., El Ghoneimy A., Gillessen S., Goncalves F., Kacso G., Kramer G., Milecki P., Pacik D., et al. Management of bone metastases in patients with castration-resistant prostate cancer. Urol. Int. 2014;92:377–386. doi: 10.1159/000358258. [DOI] [PubMed] [Google Scholar]
- 11.Salehi B., Fokou P.V.T., Yamthe L.R.T., Tali B.T., Adetunji C.O., Rahavian A., Mudau F.N., Martorell M., Setzer W.N., Rodrigues C.F., et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients. 2019;11:1483. doi: 10.3390/nu11071483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallifatidis G., Hoy J.J., Lokeshwar B.L. Bioactive natural products for chemoprevention and treatment of castration-resistant prostate cancer. Semin. Cancer Biol. 2016;40–41:160–169. doi: 10.1016/j.semcancer.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor W.F., Jabbarzadeh E. The use of natural products to target cancer stem cells. Am. J. Cancer Res. 2017;7:1588–1605. [PMC free article] [PubMed] [Google Scholar]
- 14.Dai C., Heemers H., Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017;7:a030452. doi: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadal M., Prekovic S., Gallastegui N., Helsen C., Abella M., Zielinska K., Gay M., Vilaseca M., Taulès M., Houtsmuller A.B., et al. Structure of the homodimeric androgen receptor ligand-binding domain. Nat. Commun. 2017;8:14388. doi: 10.1038/ncomms14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Royen M.E., van Cappellen W.A., de Vos C., Houtsmuller A.B., Trapman J. Stepwise androgen receptor dimerization. J. Cell Sci. 2012;125:1970–1979. doi: 10.1242/jcs.096792. [DOI] [PubMed] [Google Scholar]
- 17.Koivisto P., Kononen J., Palmberg C., Tammela T., Hyytinen E., Isola J., Trapman J., Cleutjens K., Noordzij A., Visakorpi T., et al. Androgen receptor gene amplification: A possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 18.Buchanan G., Greenberg N.M., Scher H.I., Harris J.M., Marshall V.R., Tilley W.D. Collocation of androgen receptor gene mutations in prostate cancer. Clin. Cancer Res. 2001;7:1273–1281. [PubMed] [Google Scholar]
- 19.Jenster G. Ligand-independent activation of the androgen receptor in prostate cancer by growth factors and cytokines. J. Pathol. 2000;191:227–228. doi: 10.1002/1096-9896(200007)191:3<227::AID-PATH636>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Green S.M., Mostaghel E.A., Nelson P.S. Androgen action and metabolism in prostate cancer. Mol. Cell. Endocrinol. 2012;360:3–13. doi: 10.1016/j.mce.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mellinghoff I.K., Vivanco I., Kwon A., Tran C., Wongvipat J., Sawyers C.L. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan N.P., Liu Y., Majumder S., Warren M.R., Parker C.E., Mohler J.L., Earp H.S., Whang Y.E. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y., Karaca M., Zhang Z., Gioeli D., Earp H.S., Whang Y.E. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010;29:3208–3216. doi: 10.1038/onc.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraus S., Gioeli D., Vomastek T., Gordon V., Weber M.J. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–11054. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- 25.Sharifi N. Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology. 2013;154:4010–4017. doi: 10.1210/en.2013-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Z., Yang X., Sun F., Jiang R., Linn D.E., Chen H., Chen H., Kong X., Melamed J., Tepper C.G., et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauf A., Imran M., Khan I.A., Ur-Rehman M.-, Gilani S.A., Mehmood Z., Mubarak M.S. Anticancer potential of quercetin: A comprehensive review. Phyther. Res. 2018;32:2109–2130. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 28.Xing N. Quercetin inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:409–414. doi: 10.1093/carcin/22.3.409. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H., Pan Y., Young C.Y.F. Overexpression of c-Jun induced by quercetin and resverol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2004;213:155–163. doi: 10.1016/j.canlet.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Yuan H., Gong A., Young C.Y.F. Involvement of transcription factor Sp1 in quercetin-mediated inhibitory effect on the androgen receptor in human prostate cancer cells. Carcinogenesis. 2005;26:793–801. doi: 10.1093/carcin/bgi021. [DOI] [PubMed] [Google Scholar]
- 31.Yuan H., Young C.Y.F., Tian Y., Liu Z., Zhang M., Lou H. Suppression of the androgen receptor function by quercetin through protein-protein interactions of Sp1, c-Jun, and the androgen receptor in human prostate cancer cells. Mol. Cell. Biochem. 2010;339:253–262. doi: 10.1007/s11010-010-0388-7. [DOI] [PubMed] [Google Scholar]
- 32.Kita K., Shiota M., Tanaka M., Otsuka A., Matsumoto M., Kato M., Tamada S., Iwao H., Miura K., Nakatani T., et al. Heat shock protein 70 inhibitors suppress androgen receptor expression in LNCaP95 prostate cancer cells. Cancer Sci. 2017;108:1820–1827. doi: 10.1111/cas.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal H.C., Pearlman R.L., Afaq F. Fisetin and its role in chronic diseases. Adv. Exp. Med. Biol. 2016;928:213–244. doi: 10.1007/978-3-319-41334-1_10. [DOI] [PubMed] [Google Scholar]
- 34.Lall R.K., Adhami V.M., Mukhtar H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol. Nutr. Food Res. 2016;60:1396–1405. doi: 10.1002/mnfr.201600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan N., Asim M., Afaq F., Zaid M.A., Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–8563. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabavi S.F., Braidy N., Gortzi O., Sobarzo-Sanchez E., Daglia M., Skalicka-Woźniak K., Nabavi S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015;119:1–11. doi: 10.1016/j.brainresbull.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M., et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 38.Chiu F.L., Lin J.K. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate. 2008;68:61–71. doi: 10.1002/pros.20690. [DOI] [PubMed] [Google Scholar]
- 39.Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Ramirez-Tortosa M.C. Curcumin and health. Molecules. 2016;21:264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174:1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomeh M.A., Hadianamrei R., Zhao X. A review of curcumin and its derivatives as anticancer agents. Int. J. Mol. Sci. 2019;20:1033. doi: 10.3390/ijms20051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakamura K., Yasunaga Y., Segawa T., Ko D., Moul J., Srivastava S., Rhim J. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002;21:825–830. doi: 10.3892/ijo.21.4.825. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H.N., Yu C.X., Zhang P.J., Chen W.W., Jiang A.L., Kong F., Deng J.T., Zhang J.Y., Young C.Y.F. Curcumin downregulates homeobox gene NKX3.1 in prostate cancer cell LNCaP. Acta Pharmacol. Sin. 2007;28:423–430. doi: 10.1111/j.1745-7254.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 44.Guo H., Xu Y.-M., Ye Z.-Q., Yu J.-H., Hu X.-Y. Curcumin induces cell cycle arrest and apoptosis of prostate cancer cells by regulating the expression of IkappaBalpha, c-Jun and androgen receptor. Pharmazie. 2013;68:431–434. [PubMed] [Google Scholar]
- 45.Ide H., Lu Y., Noguchi T., Muto S., Okada H., Kawato S., Horie S. Modulation of AKR1C2 by curcumin decreases testosterone production in prostate cancer. Cancer Sci. 2018;109:1230–1238. doi: 10.1111/cas.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauf A., Imran M., Butt M.S., Nadeem M., Peters D.G., Mubarak M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018;58:1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 47.Gao S., Liu G.-Z., Wang Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate. 2004;59:214–225. doi: 10.1002/pros.10375. [DOI] [PubMed] [Google Scholar]
- 48.Jones S.B., DePrimo S.E., Whitfield M.L., Brooks J.D. Resveratrol-induced gene expression profiles in human prostate cancer cells. Cancer Epidemiol. Biomarkers Prev. 2005;14:596–604. doi: 10.1158/1055-9965.EPI-04-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benitez D.A., Pozo-Guisado E., Clementi M., Castellón E., Fernandez-Salguero P.M. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer. 2007;96:1595–1604. doi: 10.1038/sj.bjc.6603755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harada N., Murata Y., Yamaji R., Miura T., Inui H., Nakano Y. Resveratrol down-regulates the androgen receptor at the post-translational level in prostate cancer cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2007;53:556–560. doi: 10.3177/jnsv.53.556. [DOI] [PubMed] [Google Scholar]
- 51.Wang T.T.Y., Hudson T.S., Wang T.C., Remsberg C.M., Davies N.M., Takahashi Y., Kim Y.S., Seifried H., Vinyard B.T., Perkins S.N., et al. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis. 2008;29:2001–2010. doi: 10.1093/carcin/bgn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitani T., Harada N., Tanimori S., Nakano Y., Inui H., Yamaji R. Resveratrol inhibits hypoxia-inducible factor-1α-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J. Nutr. Sci. Vitaminol. 2014;60:276–282. doi: 10.3177/jnsv.60.276. [DOI] [PubMed] [Google Scholar]
- 53.Lee M.H., Kundu J.K., Keum Y.S., Cho Y.Y., Surh Y.J., Choi B.Y. Resveratrol inhibits IL-6-induced transcriptional activity of AR and STAT3 in human prostate cancer LNCaP-FGC cells. Biomol. Ther. 2014;22:426–430. doi: 10.4062/biomolther.2014.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson S., Cavero L., Tong D., Liu Q., Geary K., Talamonti N., Xu J., Fu J., Jiang J., Zhang D. Resveratrol enhances polyubiquitination-mediated ARV7 degradation in prostate cancer cells. Oncotarget. 2017;8:54683–54693. doi: 10.18632/oncotarget.18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuli H.S., Tuorkey M.J., Thakral F., Sak K., Kumar M., Sharma A.K., Sharma U., Jain A., Aggarwal V., Bishayee A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019;10:1336. doi: 10.3389/fphar.2019.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bektic J., Berger A.P., Pfeil K., Dobler G., Bartsch G., Klocker H. Androgen Receptor Regulation by Physiological Concentrations of the Isoflavonoid Genistein in Androgen-Dependent LNCaP Cells Is Mediated by Estrogen Receptor β. Eur. Urol. 2004;45:245–251. doi: 10.1016/j.eururo.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi Y., Hursting S.D., Perkins S.N., Wang T.C., Wang T.T.Y. Genistein affects androgen-responsive genes through both androgen- and estrogen-induced signaling pathways. Mol. Carcinog. 2006;45:18–25. doi: 10.1002/mc.20153. [DOI] [PubMed] [Google Scholar]
- 58.Li Y., Wang Z., Kong D., Li R., Sarkar S.H., Sarkar F.H. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J. Biol. Chem. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basak S., Pookot D., Noonan E.J., Dahiya R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol. Cancer Ther. 2008;7:3195–3202. doi: 10.1158/1535-7163.MCT-08-0617. [DOI] [PubMed] [Google Scholar]
- 60.Mahmoud A.M., Zhu T., Parray A., Siddique H.R., Yang W., Saleem M., Bosland M.C. Differential effects of genistein on prostate cancer cells depend on mutational status of the androgen receptor. PLoS ONE. 2013;8:e78479. doi: 10.1371/journal.pone.0078479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu L., Blackburn G.L., Zhou J.-R. Genistein and Daidzein Downregulate Prostate Androgen-Regulated Transcript-1 (PART-1) Gene expression induced by dihydrotestosterone in human prostate LNCaP cancer cells. J. Nutr. 2003;133:389–392. doi: 10.1093/jn/133.2.389. [DOI] [PubMed] [Google Scholar]
- 62.Kim S.-H., Singh S.V. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol. Cancer Ther. 2009;8:1946–1954. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gibbs A., Schwartzman J., Deng V., Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc. Natl. Acad. Sci. USA. 2009;106:16663–16668. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khurana N., Talwar S., Chandra P.K., Sharma P., Abdel-Mageed A.B., Mondal D., Sikka S.C. Sulforaphane increases the efficacy of anti-androgens by rapidly decreasing androgen receptor levels in prostate cancer cells. Int. J. Oncol. 2016;49:1609–1619. doi: 10.3892/ijo.2016.3641. [DOI] [PubMed] [Google Scholar]
- 65.Khurana N., Kim H., Chandra P.K., Talwar S., Sharma P., Abdel-Mageed A.B., Sikka S.C., Mondal D. Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer. Oncol. Rep. 2017;38:2774–2786. doi: 10.3892/or.2017.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren F., Zhang S., Mitchell S.H., Butler R., Young C.Y. Tea polyphenols down-regulate the expression of the androgen receptor in LNCaP prostate cancer cells. Oncogene. 2000;19:1924–1932. doi: 10.1038/sj.onc.1203511. [DOI] [PubMed] [Google Scholar]
- 67.Chuu C.-P., Chen R.-Y., Kokontis J.M., Hiipakka R.A., Liao S. Suppression of androgen receptor signaling and prostate specific antigen expression by (-)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009;275:86–92. doi: 10.1016/j.canlet.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bae J.-S., Park H.-S., Park J.-W., Li S.-H., Chun Y.-S. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J. Nat. Med. 2012;66:476–485. doi: 10.1007/s11418-011-0609-8. [DOI] [PubMed] [Google Scholar]
- 69.Nanao-Hamai M., Son B.K., Komuro A., Asari Y., Hashizume T., Takayama K., Ogawa S., Akishita M. Ginsenoside Rb1 inhibits vascular calcification as a selective androgen receptor modulator. Eur. J. Pharmacol. 2019;859:172546. doi: 10.1016/j.ejphar.2019.172546. [DOI] [PubMed] [Google Scholar]
- 70.Cao B., Liu X., Li J., Liu S., Qi Y., Xiong Z., Zhang A., Wiese T., Fu X., Gu J., et al. 20(S)-protopanaxadiol-aglycone downregulation of the full-length and splice variants of androgen receptor. Int. J. Cancer. 2013;132:1277–1287. doi: 10.1002/ijc.27754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao B., Qi Y., Yang Y., Liu X., Xu D., Guo W., Zhan Y., Xiong Z., Zhang A., Wang A.R., et al. 20(S)-protopanaxadiol inhibition of progression and growth of castration-resistant prostate cancer. PLoS ONE. 2014;9:e111201. doi: 10.1371/journal.pone.0111201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu W. Silymarin inhibits function of the androgen receptor by reducing nuclear localization of the receptor in the human prostate cancer cell line LNCaP. Carcinogenesis. 2001;22:1399–1403. doi: 10.1093/carcin/22.9.1399. [DOI] [PubMed] [Google Scholar]
- 73.Li J., Cao B., Liu X., Fu X., Xiong Z., Chen L., Sartor O., Dong Y., Zhang H. Berberine suppresses androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2011;10:1346–1356. doi: 10.1158/1535-7163.MCT-10-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hahm E.-R., Karlsson A.I., Bonner M.Y., Arbiser J.L., Singh S. V Honokiol inhibits androgen receptor activity in prostate cancer cells. Prostate. 2014;74:408–420. doi: 10.1002/pros.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao L., Zhou Z., Cai Y., Castro P., Dakhov O., Shi P., Bai Y., Ji H., Shen W., Wang J. Celastrol suppresses tumor cell growth through targeting an AR-ERG-NF-κB pathway in TMPRSS2/ERG fusion gene expressing prostate cancer. PLoS ONE. 2013;8:e58391. doi: 10.1371/journal.pone.0058391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Normanno N., De Luca A., Bianco C., Strizzi L., Mancino M., Maiello M.R., Carotenuto A., De Feo G., Caponigro F., Salomon D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 77.Huynh H., Nguyen T.T.T., Chan E., Tran E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-alpha)- and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int. J. Oncol. 2003;23:821–829. doi: 10.3892/ijo.23.3.821. [DOI] [PubMed] [Google Scholar]
- 78.Firdous A.B., Sharmila G., Balakrishnan S., Rajasingh P., Suganya S., Srinivasan N., Arunakaran J. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food Funct. 2014;5:2632–2645. doi: 10.1039/C4FO00255E. [DOI] [PubMed] [Google Scholar]
- 79.Markaverich B.M., Vijjeswarapu M., Shoulars K., Rodriguez M. Luteolin and gefitinib regulation of EGF signaling pathway and cell cycle pathway genes in PC-3 human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2010;122:219–231. doi: 10.1016/j.jsbmb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart J.R., O’Brian C.A. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest. New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- 81.Oh H.Y., Leem J., Yoon S.J., Yoon S., Hong S.J. Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and down-regulation of androgen receptor. Biochem. Biophys. Res. Commun. 2010;393:319–324. doi: 10.1016/j.bbrc.2010.01.133. [DOI] [PubMed] [Google Scholar]
- 82.Heidegger I., Kern J., Ofer P., Klocker H., Massoner P. Oncogenic functions of IGF1R and INSR in prostate cancer include enhanced tumor growth, cell migration and angiogenesis. Oncotarget. 2014;5:2723–2735. doi: 10.18632/oncotarget.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wing Ying Cheung C., Gibbons N., Wayne Johnson D., Lawrence Nicol D. Silibinin – A promising new treatment for cancer. Anticancer. Agents Med. Chem. 2010;10:186–195. doi: 10.2174/1871520611009030186. [DOI] [PubMed] [Google Scholar]
- 84.Zhu X.X., Ding Y.H., Wu Y., Qian L.Y., Zou H., He Q. Silibinin: A potential old drug for cancer therapy. Expert Rev. Clin. Pharmacol. 2016;9:1323–1330. doi: 10.1080/17512433.2016.1208563. [DOI] [PubMed] [Google Scholar]
- 85.Zi X., Zhang J., Agarwal R., Pollak M. Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res. 2000;60:5617–5620. [PubMed] [Google Scholar]
- 86.Singh R.P., Dhanalakshmi S., Tyagi A.K., Chan D.C.F., Agarwal C., Agarwal R. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–3069. [PubMed] [Google Scholar]
- 87.Raina K., Blouin M.-J., Singh R.P., Majeed N., Deep G., Varghese L., Glodé L.M., Greenberg N.M., Hwang D., Cohen P., et al. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007;67:11083–11091. doi: 10.1158/0008-5472.CAN-07-2222. [DOI] [PubMed] [Google Scholar]
- 88.Fang J., Zhou Q., Shi X.L., Jiang B.H. Luteolin inhibits insulin-like growth factor 1 receptor signaling in prostate cancer cells. Carcinogenesis. 2007;28:713–723. doi: 10.1093/carcin/bgl189. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh A., Edlind M. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J. Androl. 2014;16:378. doi: 10.4103/1008-682X.122876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ward A.B., Mir H., Kapur N., Gales D.N., Carriere P.P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018;16:108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shukla S., Gupta S. Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle. 2007;6:1102–1114. doi: 10.4161/cc.6.9.4146. [DOI] [PubMed] [Google Scholar]
- 92.Shukla S., Bhaskaran N., Babcook M.A., Fu P., MacLennan G.T., Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chaudhary L.R., Hruska K.A. Inhibition of cell survival signal protein kinase B/Akt by curcumin in human prostate cancer cells. J. Cell. Biochem. 2003;89:1–5. doi: 10.1002/jcb.10495. [DOI] [PubMed] [Google Scholar]
- 94.Yu S., Shen G., Khor T.O., Kim J.-H., Kong A.-N.T. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao F., Jin T.-Y., Zhou Y.-F. Inhibitory effect of isoflavones on prostate cancer cells and PTEN gene. Biomed. Environ. Sci. 2006;19:35–41. [PubMed] [Google Scholar]
- 96.El Touny L.H., Banerjee P.P. Akt–GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis. 2007;28:1710–1717. doi: 10.1093/carcin/bgm103. [DOI] [PubMed] [Google Scholar]
- 97.Keum Y.S., Oo Khor T., Lin W., Shen G., Han Kwon K., Barve A., Li W., Kong A.N. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) Mice: Implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase p. Pharm. Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 98.Siddiqui I.A., Adhami V.M., Afaq F., Ahmad N., Mukhtar H. Modulation of phosphatidylinositol-3-kinase/protein kinase B- and mitogen-activated protein kinase-pathways by tea polyphenols in human prostate cancer cells. J. Cell. Biochem. 2004;91:232–242. doi: 10.1002/jcb.10737. [DOI] [PubMed] [Google Scholar]
- 99.Verzella D., Fischietti M., Capece D., Vecchiotti D., Del Vecchio F., Cicciarelli G., Mastroiaco V., Tessitore A., Alesse E., Zazzeroni F. Targeting the NF-κB pathway in prostate cancer: A promising therapeutic approach? Curr. Drug Targets. 2016;17:311–320. doi: 10.2174/1389450116666150907100715. [DOI] [PubMed] [Google Scholar]
- 100.Benitez D.A., Hermoso M.A., Pozo-Guisado E., Fernández-Salguero P.M., Castellón E.A. Regulation of cell survival by resveratrol involves inhibition of NFκB-regulated gene expression in prostate cancer cells. Prostate. 2009;69:1045–1054. doi: 10.1002/pros.20953. [DOI] [PubMed] [Google Scholar]
- 101.Davis J.N., Kucuk O., Sarkar F.H. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr. Cancer. 1999;35:167–174. doi: 10.1207/S15327914NC352_11. [DOI] [PubMed] [Google Scholar]
- 102.Li Y., Sarkar F.H. Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 2002;8:2369–2377. [PubMed] [Google Scholar]
- 103.Xu C., Shen G., Chen C., Gélinas C., Kong A.N.T. Suppression of NF-κB and NF-κB-regulated gene expression by sulforaphane and PEITC through IκBα, IKK pathway in human prostate cancer PC-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 104.Shanmugam M.K., Rajendran P., Li F., Nema T., Vali S., Abbasi T., Kapoor S., Sharma A., Kumar A.P., Ho P.C., et al. Ursolic acid inhibits multiple cell survival pathways leading to suppression of growth of prostate cancer xenograft in nude mice. J. Mol. Med. 2011;89:713–727. doi: 10.1007/s00109-011-0746-2. [DOI] [PubMed] [Google Scholar]
- 105.Yap W.N., Chang P.N., Han H.Y., Lee D.T.W., Ling M.T., Wong Y.C., Yap Y.L. γ-Tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer. 2008;99:1832–1841. doi: 10.1038/sj.bjc.6604763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonnissen A., Isebaert S., Haustermans K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int. J. Mol. Sci. 2013;14:13979–14007. doi: 10.3390/ijms140713979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slusarz A., Shenouda N.S., Sakla M.S., Drenkhahn S.K., Narula A.S., MacDonald R.S., Besch-Williford C.L., Lubahn D.B. Common botanical compounds inhibit the Hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. doi: 10.1158/0008-5472.CAN-09-3012. [DOI] [PubMed] [Google Scholar]
- 108.Yardy G.W., Brewster S.F. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- 109.Murillo-Garzón V., Kypta R. WNT signalling in prostate cancer. Nat. Rev. Urol. 2017;14:683–696. doi: 10.1038/nrurol.2017.144. [DOI] [PubMed] [Google Scholar]
- 110.Baruah M.M., Khandwekar A.P., Sharma N. Quercetin modulates Wnt signaling components in prostate cancer cell line by inhibiting cell viability, migration, and metastases. Tumor Biol. 2016;37:14025–14034. doi: 10.1007/s13277-016-5277-6. [DOI] [PubMed] [Google Scholar]
- 111.Teiten M.H., Gaascht F., Cronauer M., Henry E., Dicato M., Diederich M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011;38:603–611. doi: 10.3892/ijo.2011.905. [DOI] [PubMed] [Google Scholar]
- 112.Liss M.A., Schlicht M., Kahler A., Fitzgerald R., Thomassi T., Degueme A., Hessner M., Datta M.W. Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics. 2010;7:245–252. [PubMed] [Google Scholar]
- 113.Lu W., Lin C., King T.D., Chen H., Reynolds R.C., Li Y. Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell. Signal. 2012;24:2291–2296. doi: 10.1016/j.cellsig.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal. Transduct. Target. Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y., Zaman M.S., Deng G., Majid S., Saini S., Liu J., Tanaka Y., Dahiya R. MicroRNAs 221/222 and genistein-mediated regulation of ARHI tumor suppressor gene in prostate cancer. Cancer Prev. Res. (Phila). 2011;4:76–86. doi: 10.1158/1940-6207.CAPR-10-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rabiau N., Trraf H.-K., Adjakly M., Bosviel R., Guy L., Fontana L., Bignon Y.-J., Bernard-Gallon D.J. miRNAs differentially expressed in prostate cancer cell lines after soy treatment. In Vivo. 2011;25:917–921. [PubMed] [Google Scholar]
- 117.Chiyomaru T., Yamamura S., Zaman M.S., Majid S., Deng G., Shahryari V., Saini S., Hirata H., Ueno K., Chang I., et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic MicroRNA-151. PLoS ONE. 2012;7:e43812. doi: 10.1371/journal.pone.0043812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiyomaru T., Yamamura S., Fukuhara S., Hidaka H., Majid S., Saini S., Arora S., Deng G., Shahryari V., Chang I., et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS ONE. 2013;8:e58929. doi: 10.1371/journal.pone.0058929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiyomaru T., Yamamura S., Fukuhara S., Yoshino H., Kinoshita T., Majid S., Saini S., Chang I., Tanaka Y., Enokida H., et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE. 2013;8:e70372. doi: 10.1371/journal.pone.0070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hirata H., Hinoda Y., Shahryari V., Deng G., Tanaka Y., Tabatabai Z.L., Dahiya R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer. 2014;110:1645–1654. doi: 10.1038/bjc.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han K., Meng W., Zhang J.-J., Zhou Y., Wang Y., Su Y., Lin S., Gan Z., Sun Y., Min D.-L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. Onco. Targets. Ther. 2016;26:3085–3094. doi: 10.2147/OTT.S102862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu M., Zheng Z., Huang J., Ma X., Huang C., Wu R., Li X., Liang Z., Deng F., Wu J., et al. Modulation of miR-34a in curcumin-induced antiproliferation of prostate cancer cells. J. Cell. Biochem. 2019;120:15616–15624. doi: 10.1002/jcb.28828. [DOI] [PubMed] [Google Scholar]
- 123.Sheth S., Jajoo S., Kaur T., Mukherjea D., Sheehan K., Rybak L.P., Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE. 2012;7:e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dhar S., Kumar A., Rimando A.M., Zhang X., Levenson A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget. 2015;6:27214–27226. doi: 10.18632/oncotarget.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao Q., Zheng J. Ginsenoside Rh2 inhibits prostate cancer cell growth through suppression of microRNA-4295 that activates CDKN1A. Cell Prolif. 2018;51:e12438. doi: 10.1111/cpr.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao L., Zhang X., Cao F., Wang Y., Shen Y., Yang C., Uzan G., Peng B., Zhang D. Inhibiting inducible miR-223 further reduces viable cells in human cancer cell lines MCF-7 and PC3 treated by celastrol. BMC Cancer. 2015;15:873. doi: 10.1186/s12885-015-1909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vijayababu M.R., Kanagaraj P., Arunkumar A., Ilangovan R., Dharmarajan A., Arunakaran J. Quercetin induces p53-independent apoptosis in human prostate cancer cells by modulating Bcl-2-related proteins: A possible mediation by IGFBP-3. Oncol. Res. 2006;16:67–74. doi: 10.3727/000000006783981224. [DOI] [PubMed] [Google Scholar]
- 128.Senthilkumar K., Elumalai P., Arunkumar R., Banudevi S., Gunadharini N.D., Sharmila G., Selvakumar K., Arunakaran J. Quercetin regulates insulin like growth factor signaling and induces intrinsic and extrinsic pathway mediated apoptosis in androgen independent prostate cancer cells (PC-3) Mol. Cell. Biochem. 2010;344:173–184. doi: 10.1007/s11010-010-0540-4. [DOI] [PubMed] [Google Scholar]
- 129.Lee D.H., Szczepanski M., Lee Y.J. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem. Pharmacol. 2008;75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Horinaka M., Yoshida T., Shiraishi T., Nakata S., Wakada M., Nakanishi R., Nishino H., Matsui H., Sakai T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene. 2005;24:7180–7189. doi: 10.1038/sj.onc.1208874. [DOI] [PubMed] [Google Scholar]
- 131.Pham H.N.T., Sakoff J.A., Van Vuong Q., Bowyer M.C., Scarlett C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Br. 2018;21:1033–1036. doi: 10.1016/j.dib.2018.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dorai T., Gehani N., Katz A. Therapeutic potential of curcumin in human prostate cancer - I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 133.Shankar S., Srivastava R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007;30:905–918. doi: 10.3892/ijo.30.4.905. [DOI] [PubMed] [Google Scholar]
- 134.Yang J., Ning J., Peng L., He D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int. J. Clin. Exp. Pathol. 2015;8:9272–9278. [PMC free article] [PubMed] [Google Scholar]
- 135.Lin H.Y., Shih A., Davis F.B., Tang H.Y., Martino L.J., Bennett J.A., Davis P.J. Resveratrol induced serine phosphorylation of p53 causes apoptosis in a mutant p53 prostate cancer cell line. J. Urol. 2002;168:748–755. doi: 10.1016/S0022-5347(05)64739-8. [DOI] [PubMed] [Google Scholar]
- 136.Morris G.Z., Williams R.L., Elliott M.S., Beebe S.J. Resveratrol induces apoptosis in LNCaP cells and requires hydroxyl groups to decrease viability in LNCaP and DU 145 cells. Prostate. 2002;52:319–329. doi: 10.1002/pros.10122. [DOI] [PubMed] [Google Scholar]
- 137.Aziz M.H., Nihal M., Fu V.X., Jarrard D.F., Ahmad N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3′-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 138.Benitez D.A., Pozo-Guisado E., Alvarez-Barrientos A., Fernandez-Salguero P.M., Castellon E.A. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J. Androl. 2006;28:282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 139.Kai L., Samuel S.K., Levenson A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. cancer. 2010;126:1538–1548. doi: 10.1002/ijc.24928. [DOI] [PubMed] [Google Scholar]
- 140.Kumi-Diaka J., Sanderson N.A., Hall A. The mediating role of caspase-3 protease in the intracellular mechanism of genistein-induced apoptosis in human prostatic carcinoma cell lines, DU145 and LNCaP. Biol. Cell. 2000;92:595–604. doi: 10.1016/S0248-4900(00)01109-6. [DOI] [PubMed] [Google Scholar]
- 141.Chiao J., Chung F.-L., Kancherla R., Ahmed T., Mittelman A., Conaway C. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int. J. Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 142.Singh A.V., Xiao D., Lew K.L., Dhir R., Singh S.V. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 143.Choi S., Lew K.L., Xiao H., Herman-Antosiewicz A., Xiao D., Brown C.K., Singh S.V. D,L-Sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and Apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 144.Clarke J.D., Hsu A., Yu Z., Dashwood R.H., Ho E. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol. Nutr. Food Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hagen R.M., Chedea V.S., Mintoff C.P., Bowler E., Morse H.R., Ladomery M.R. Epigallocatechin-3-gallate promotes apoptosis and expression of the caspase 9a splice variant in PC3 prostate cancer cells. Int. J. Oncol. 2013;43:194–200. doi: 10.3892/ijo.2013.1920. [DOI] [PubMed] [Google Scholar]
- 146.Ben-Eltriki M., Deb S., Adomat H., Tomlinson Guns E.S. Calcitriol and 20(S)-protopanaxadiol synergistically inhibit growth and induce apoptosis in human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2016;158:207–219. doi: 10.1016/j.jsbmb.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 147.Agarwal C., Tyagi A., Kaur M., Agarwal R. Silibinin inhibits constitutive activation of Stat3, and causes caspase activation and apoptotic death of human prostate carcinoma DU145 cells. Carcinogenesis. 2007;28:1463–1470. doi: 10.1093/carcin/bgm042. [DOI] [PubMed] [Google Scholar]
- 148.Mantena S.K., Sharma S.D., Katiyar S.K. Berberine, a natural product, induces G1-phase cell cycle arrest and caspase-3-dependent apoptosis in human prostate carcinoma cells. Mol. Cancer Ther. 2006;5:296–308. doi: 10.1158/1535-7163.MCT-05-0448. [DOI] [PubMed] [Google Scholar]
- 149.Choi M.S., Oh J.H., Kim S.M., Jung H.Y., Yoo H.S., Lee Y.M., Moon D.C., Han S.B., Hong J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009;34:1221–1230. [PubMed] [Google Scholar]
- 150.Zhang Y., Kong C., Wang H., Wang L., Xu C., Sun Y. Phosphorylation of Bcl-2 and activation of caspase-3 via the c-Jun N-terminal kinase pathway in ursolic acid-induced DU145 cells apoptosis. Biochimie. 2009;91:1173–1179. doi: 10.1016/j.biochi.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 151.Zhang Y., Kong C., Wang L., Li J., Liu X., Xu B., Xu C., Sun Y. Ursolic acid overcomes Bcl-2-mediated resistance to apoptosis in prostate cancer cells involving activation of JNK-induced Bcl-2 phosphorylation and degradation. J. Cell. Biochem. 2009;109:764–773. doi: 10.1002/jcb.22455. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Y., Kong C., Zeng Y., Wang L., Li Z., Wang H., Xu C., Sun Y. Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol. Carcinog. 2010;49:374–385. doi: 10.1002/mc.20610. [DOI] [PubMed] [Google Scholar]
- 153.Hahm E.R., Arlotti J.A., Marynowski S.W., Singh S.V. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008;14:1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 154.Chen D. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front. Biosci. 2007;12:1935. doi: 10.2741/2199. [DOI] [PubMed] [Google Scholar]
- 155.Kazi A., Daniel K.G., Smith D.M., Kumar N.B., Dou Q.P. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem. Pharmacol. 2003;66:965–976. doi: 10.1016/S0006-2952(03)00414-3. [DOI] [PubMed] [Google Scholar]
- 156.Dai Y., Desano J., Tang W., Meng X., Meng Y., Burstein E., Lawrence T.S., Xu L. Natural proteasome inhibitor celastrol suppresses androgen-independent prostate cancer progression by modulating apoptotic proteins and NF-kappaB. PLoS ONE. 2010;5:e14153. doi: 10.1371/journal.pone.0014153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu K.-C., Yen C.-Y., Wu R.S.-C., Yang J.-S., Lu H.-F., Lu K.-W., Lo C., Chen H.-Y., Tang N.-Y., Wu C.-C., et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environ. Toxicol. 2014;29:428–439. doi: 10.1002/tox.21769. [DOI] [PubMed] [Google Scholar]
- 158.Rivera M., Ramos Y., Rodríguez-Valentín M., López-Acevedo S., Cubano L.A., Zou J., Zhang Q., Wang G., Boukli N.M. Targeting multiple pro-apoptotic signaling pathways with curcumin in prostate cancer cells. PLoS ONE. 2017;12:e0179587. doi: 10.1371/journal.pone.0179587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim S.H., Kim K.Y., Yu S.N., Seo Y.K., Chun S.S., Yu H.S., Ahn S.C. Silibinin induces mitochondrial NOX4-mediated endoplasmic reticulum stress response and its subsequent apoptosis. BMC Cancer. 2016;16:452. doi: 10.1186/s12885-016-2516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nagesh P., Hatami E., Chowdhury P., Kashyap V., Khan S., Hafeez B., Chauhan S., Jaggi M., Yallapu M. Tannic acid induces endoplasmic reticulum stress-mediated apoptosis in prostate cancer. Cancers. 2018;10:68. doi: 10.3390/cancers10030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Limonta P., Moretti R., Marzagalli M., Fontana F., Raimondi M., Montagnani Marelli M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int. J. Mol. Sci. 2019;20:961. doi: 10.3390/ijms20040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee W.-J., Chien M.-H., Chow J.-M., Chang J.-L., Wen Y.-C., Lin Y.-W., Cheng C.-W., Lai G.-M., Hsiao M., Lee L.-M. Nonautophagic cytoplasmic vacuolation death induction in human PC-3M prostate cancer by curcumin through reactive oxygen species -mediated endoplasmic reticulum stress. Sci. Rep. 2015;5:10420. doi: 10.1038/srep10420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar S., Stokes J., Singh U.P., Scissum-Gunn K., Singh R., Manne U., Mishra M.K. Prolonged exposure of resveratrol induces reactive superoxide species–independent apoptosis in murine prostate cells. Tumor Biol. 2017;39:101042831771503. doi: 10.1177/1010428317715039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang D., Gao Z., Zhang X. Resveratrol induces apoptosis in murine prostate cancer cells via hypoxia-inducible factor 1-alpha (HIF-1α)/reactive oxygen species (ROS)/P53 signaling. Med. Sci. Monit. 2018;24:8970–8976. doi: 10.12659/MSM.913290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh S.V., Srivastava S.K., Choi S., Lew K.L., Antosiewicz J., Xiao D., Zeng Y., Watkins S.C., Johnson C.S., Trump D.L., et al. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 166.Xiao D., Powolny A.A., Antosiewicz J., Hahm E.R., Bommareddy A., Zeng Y., Desai D., Amin S., Herman-Antosiewicz A., Singh S.V. Cellular responses to cancer chemopreventive agent D,L-sulforaphane in human prostate cancer cells are initiated by mitochondrial reactive oxygen species. Pharm. Res. 2009;26:1729–1738. doi: 10.1007/s11095-009-9883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Imenshahidi M., Hosseinzadeh H. Berberis vulgaris and berberine: An update review. Phytother. Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 168.Zhang C., Sheng J., Li G., Zhao L., Wang Y., Yang W., Yao X., Sun L., Zhang Z., Cui R. Effects of berberine and its derivatives on cancer: A systems pharmacology review. Front. Pharmacol. 2019;10:1461. doi: 10.3389/fphar.2019.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang L.Y., Wu Y.L., Gao X.H., Guo F. Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells. Biochem. Biophys. Res. Commun. 2014;450:697–703. doi: 10.1016/j.bbrc.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 170.Kang D., Park W., Lee S., Kim J.H., Song J.J. Crosstalk from survival to necrotic death coexists in DU-145 cells by curcumin treatment. Cell. Signal. 2013;25:1288–1300. doi: 10.1016/j.cellsig.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 171.Lee D., Kim I.Y., Saha S., Choi K.S. Paraptosis in the anti-cancer arsenal of natural products. Pharmacol. Ther. 2016;162:120–133. doi: 10.1016/j.pharmthera.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 172.Fontana F., Raimondi M., Marzagalli M., Di Domizio A., Limonta P. The emerging role of paraptosis in tumor cell biology: Perspectives for cancer prevention and therapy with natural compounds. Biochim. Biophys. Acta Rev. Cancer. 2020;1873:188338. doi: 10.1016/j.bbcan.2020.188338. [DOI] [PubMed] [Google Scholar]
- 173.Montagnani Marelli M., Marzagalli M., Fontana F., Raimondi M., Moretti R.M., Limonta P. Anticancer properties of tocotrienols: A review of cellular mechanisms and molecular targets. J. Cell. Physiol. 2019;234:1147–1164. doi: 10.1002/jcp.27075. [DOI] [PubMed] [Google Scholar]
- 174.Fontana F., Raimondi M., Marzagalli M., Moretti R.M., Marelli M.M., Limonta P. Tocotrienols and cancer: From the state of the art to promising novel patents. Recent Pat. Anticancer. Drug Discov. 2019;14:5–18. doi: 10.2174/1574892814666190116111827. [DOI] [PubMed] [Google Scholar]
- 175.Fontana F., Moretti R.M., Raimondi M., Marzagalli M., Beretta G., Procacci P., Sartori P., Montagnani Marelli M., Limonta P. δ-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019;52:e12576. doi: 10.1111/cpr.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang W.B., Feng L.X., Yue Q.X., Wu W.Y., Guan S.H., Jiang B.H., Yang M., Liu X., Guo D.A. Paraptosis accompanied by autophagy and apoptosis was induced by celastrol, a natural compound with influence on proteasome, ER stress and Hsp90. J. Cell. Physiol. 2012;227:2196–2206. doi: 10.1002/jcp.22956. [DOI] [PubMed] [Google Scholar]
- 177.Glick D., Barth S., Macleod K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang C., Ma X., Wang Z., Zeng X., Hu Z., Ye Z., Shen G. Curcumin induces apoptosis and protective autophagy in castration-resistant prostate cancer cells through iron chelation. Drug Des. Devel. Ther. 2017;11:431–439. doi: 10.2147/DDDT.S126964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Herman-Antosiewicz A., Johnson D.E., Singh S.V. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 180.Watson G.W., Wickramasekara S., Fang Y., Palomera-Sanchez Z., Maier C.S., Williams D.E., Dashwood R.H., Perez V.I., Ho E. Analysis of autophagic flux in response to sulforaphane in metastatic prostate cancer cells. Mol. Nutr. Food Res. 2015;59:1954–1961. doi: 10.1002/mnfr.201500283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim S.-H., Kim K.-Y., Yu S.-N., Park S.-K., Choi H.-D., Ji J.-H., Ahn S.-C. Autophagy inhibition enhances silibinin-induced apoptosis by regulating reactive oxygen species production in human prostate cancer PC-3 cells. Biochem. Biophys. Res. Commun. 2015;468:151–156. doi: 10.1016/j.bbrc.2015.10.143. [DOI] [PubMed] [Google Scholar]
- 182.Shin S.W., Kim S.Y., Park J.-W. Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells. Biochim. Biophys. Acta Mol. Cell Res. 2012;1823:451–457. doi: 10.1016/j.bbamcr.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 183.Delmulle L., Vanden Berghe T., De Keukeleire D., Vandenabeele P. Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death. Phyther. Res. 2008;22:197–203. doi: 10.1002/ptr.2286. [DOI] [PubMed] [Google Scholar]
- 184.Hahm E.R., Sakao K., Singh S.V. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74:1209–1221. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suh Y., Afaq F., Khan N., Johnson J.J., Khusro F.H., Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–1433. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Selvaraj S., Sun Y., Sukumaran P., Singh B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2016;55:818–831. doi: 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo J., Huang X., Wang H., Yang H. Celastrol induces autophagy by targeting AR/miR-101 in prostate cancer cells. PLoS ONE. 2015;10:e0140745. doi: 10.1371/journal.pone.0140745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kashyap D., Sharma A., Tuli H.S., Sak K., Mukherjee T., Bishayee A. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018;128:70–81. doi: 10.1016/j.critrevonc.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 189.Kalyanaraman B. Teaching the basics of cancer metabolism: Developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi: 10.1016/j.redox.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fonseca J., Moradi F., Valente A.J.F., Stuart J.A. Oxygen and glucose levels in cell culture media determine resveratrol’s effects on growth, hydrogen peroxide production, and mitochondrial dynamics. Antioxidants. 2018;7:157. doi: 10.3390/antiox7110157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fonseca J., Moradi F., Maddalena L., Ferreira-Tollstadius B., Selim S., Stuart J.A. Resveratrol integrates metabolic and growth effects in PC3 prostate cancer cells-involvement of prolyl hydroxylase and hypoxia inducible factor-1. Oncol. Lett. 2018;17:697–705. doi: 10.3892/ol.2018.9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Szablewski L. Expression of glucose transporters in cancers. Biochim. Biophys. Acta - Rev. Cancer. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 193.Gonzalez-Menendez P., Hevia D., Rodriguez-Garcia A., Mayo J.C., Sainz R.M. Regulation of GLUT transporters by flavonoids in androgen-sensitive and -insensitive prostate cancer cells. Endocrinology. 2014;155:3238–3250. doi: 10.1210/en.2014-1260. [DOI] [PubMed] [Google Scholar]
- 194.Pang H., Koda Y., Soejima M., Kimura H. Identification of human phosphoglucomutase 3 (PGM3) as N-acetylglucosamine-phosphate mutase (AGM1) Ann. Hum. Genet. 2002;66:139–144. doi: 10.1046/j.1469-1809.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 195.Lee C.H., Jeong S.J., Yun S.M., Kim J.H., Lee H.J., Ahn K.S., Won S.H., Kim H.S., Lee H.J., Ahn K.S., et al. Down-regulation of phosphoglucomutase 3 mediates sulforaphane-induced cell death in LNCaP prostate cancer cells. Proteome Sci. 2010;8:67. doi: 10.1186/1477-5956-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Briones-Herrera A., Eugenio-Pérez D., Reyes-Ocampo J.G., Rivera-Mancía S., Pedraza-Chaverri J. New highlights on the health-improving effects of sulforaphane. Food Funct. 2018;9:2589–2606. doi: 10.1039/C8FO00018B. [DOI] [PubMed] [Google Scholar]
- 197.Bayat Mokhtari R., Baluch N., Homayouni T.S., Morgatskaya E., Kumar S., Kazemi P., Yeger H. The role of sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018;12:91–101. doi: 10.1007/s12079-017-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zadra G., Photopoulos C., Loda M. The fat side of prostate cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Suburu J., Chen Y.Q. Lipids and prostate cancer. Prostaglandins Other Lipid Mediat. 2012;98:1–10. doi: 10.1016/j.prostaglandins.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Flavin R., Zadra G., Loda M. Metabolic alterations and targeted therapies in prostate cancer. J. Pathol. 2011;223:283–294. doi: 10.1002/path.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rysman E., Brusselmans K., Scheys K., Timmermans L., Derua R., Munck S., Van Veldhoven P.P., Waltregny D., Daniëls V.W., Machiels J., et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- 202.Leon C.G., Locke J.A., Adomat H.H., Etinger S.L., Twiddy A.L., Neumann R.D., Nelson C.C., Guns E.S., Wasan K.M. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2009;70:390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 203.Cai C., Chen S., Ng P., Bubley G.J., Nelson P.S., Mostaghel E.A., Marck B., Matsumoto A.M., Simon N.I., Wang H., et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Raina K., Serkova N.J., Agarwal R. Silibinin feeding alters the metabolic profile in TRAMP prostatic tumors:1h-nmrs-based metabolomics study. Cancer Res. 2009;69:3731–3735. doi: 10.1158/0008-5472.CAN-09-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nambiar D.K., Deep G., Singh R.P., Agarwal C., Agarwal R. Silibinin inhibits aberrant lipid metabolism, proliferation and emergence of androgen-independence in prostate cancer cells via primarily targeting the sterol response element binding protein 1. Oncotarget. 2014;5:10017–10033. doi: 10.18632/oncotarget.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Deep G., Kumar R., Nambiar D.K., Jain A.K., Ramteke A.M., Serkova N.J., Agarwal C., Agarwal R. Silibinin inhibits hypoxia-induced HIF-1α-mediated signaling, angiogenesis and lipogenesis in prostate cancer cells: In vitro evidence and in vivo functional imaging and metabolomics. Mol. Carcinog. 2017;56:833–848. doi: 10.1002/mc.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Coleman D.T., Bigelow R., Cardelli J.A. Inhibition of fatty acid synthase by luteolin post-transcriptionally down-regulates c-Met expression independent of proteosomal/lysosomal degradation. Mol. Cancer Ther. 2009;8:214–224. doi: 10.1158/1535-7163.MCT-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Brusselmans K., Vrolix R., Verhoeven G., Swinnen J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005;280:5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 209.Brusselmans K., De Schrijver E., Heyns W., Verhoeven G., Swinnen J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- 210.Singh K.B., Kim S.H., Hahm E.R., Pore S.K., Jacobs B.L., Singh S.V. Prostate cancer chemoprevention by sulforaphane in a preclinical mouse model is associated with inhibition of fatty acid metabolism. Carcinogenesis. 2018;39:826–837. doi: 10.1093/carcin/bgy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eidelman E., Twum-Ampofo J., Ansari J., Siddiqui M.M. The metabolic phenotype of prostate cancer. Front. Oncol. 2017;7:131. doi: 10.3389/fonc.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Freeman M.R., Kim J., Lisanti M.P., Di Vizio D. A metabolic perturbation by U0126 identifies a role for glutamine in resveratrol-induced cell death. Cancer Biol. Ther. 2011;12:966–977. doi: 10.4161/cbt.12.11.18136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lodi A., Saha A., Lu X., Wang B., Sentandreu E., Collins M., Kolonin M.G., DiGiovanni J., Tiziani S. Combinatorial treatment with natural compounds in prostate cancer inhibits prostate tumor growth and leads to key modulations of cancer cell metabolism. NPJ Precis. Oncol. 2017:1. doi: 10.1038/s41698-017-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Seyfried T.N., Huysentruyt L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013;18:43–73. doi: 10.1615/CritRevOncog.v18.i1-2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dongre A., Weinberg R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019;20:69–84. doi: 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 216.Bhat F.A., Sharmila G., Balakrishnan S., Arunkumar R., Elumalai P., Suganya S., Raja Singh P., Srinivasan N., Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J. Nutr. Biochem. 2014;25:1132–1139. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 217.Zhu Y., Wu J., Li S., Wang X., Liang Z., Xu X., Xu X., Hu Z., Lin Y., Chen H., et al. Apigenin inhibits migration and invasion via modulation of epithelial mesenchymal transition in prostate cancer. Mol. Med. Rep. 2015;11:1004–1008. doi: 10.3892/mmr.2014.2801. [DOI] [PubMed] [Google Scholar]
- 218.Hu H.J., Lin X.L., Liu M.H., Fan X.J., Zou W.W. Curcumin mediates reversion of HGF-induced epithelial-mesenchymal transition via inhibition of c-Met expression in DU145 cells. Oncol. Lett. 2016;11:1499–1505. doi: 10.3892/ol.2015.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhang L., Li L., Wu D., Fan J., Li X., Wu K., Wang X., He D. A novel anti-cancer effect of genistein: Reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol. Sin. 2008;29:1060–1068. doi: 10.1111/j.1745-7254.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 220.Lee J., Ju J., Park S., Hong S.J., Yoon S. Inhibition of IGF-1 signaling by genistein: Modulation of E-cadherin expression and downregulation of β-catenin signaling in hormone refractory PC-3 prostate cancer cells. Nutr. Cancer. 2012;64:153–162. doi: 10.1080/01635581.2012.630161. [DOI] [PubMed] [Google Scholar]
- 221.Peng X., Zhou Y., Tian H., Yang G., Li C., Geng Y., Wu S., Wu W. Sulforaphane inhibits invasion by phosphorylating ERK1/2 to regulate E-cadherin and CD44v6 in human prostate cancer DU145 cells. Oncol. Rep. 2015;34:1565–1572. doi: 10.3892/or.2015.4098. [DOI] [PubMed] [Google Scholar]
- 222.Raina K., Rajamanickam S., Singh R.P., Deep G., Chittezhath M., Agarwal R. Stage-specific inhibitory effects and associated mechanisms of silibinin on tumor progression and metastasis in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2008;68:6822–6830. doi: 10.1158/0008-5472.CAN-08-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Singh R.P., Raina K., Sharma G., Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin. Cancer Res. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Deep G., Gangar S.C., Agarwal C., Agarwal R. Role of E-cadherin in antimigratory and antiinvasive efficacy of silibinin in prostate cancer cells. Cancer Prev. Res. 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu C.-H., Tang W.-C., Sia P., Huang C.-C., Yang P.-M., Wu M.-H., Lai I.-L., Lee K.-H. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int. J. Med. Sci. 2015;12:63–71. doi: 10.7150/ijms.9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kang H., Lee M., Jang S.-W. Celastrol inhibits TGF-β1-induced epithelial–mesenchymal transition by inhibiting Snail and regulating E-cadherin expression. Biochem. Biophys. Res. Commun. 2013;437:550–556. doi: 10.1016/j.bbrc.2013.06.113. [DOI] [PubMed] [Google Scholar]
- 227.Senthilkumar K., Arunkumar R., Elumalai P., Sharmila G., Gunadharini D.N., Banudevi S., Krishnamoorthy G., Benson C.S., Arunakaran J. Quercetin inhibits invasion, migration and signalling molecules involved in cell survival and proliferation of prostate cancer cell line (PC-3) Cell Biochem. Funct. 2011;29:87–95. doi: 10.1002/cbf.1725. [DOI] [PubMed] [Google Scholar]
- 228.Khan M.I., Adhami V.M., Lall R.K., Sechi M., Joshi D.C., Haidar O.M., Syed D.N., Siddiqui I.A., Chiu S.-Y., Mukhtar H. YB-1 expression promotes epithelial-to-mesenchymal transition in prostate cancer that is inhibited by a small molecule fisetin. Oncotarget. 2014;5:2462–2474. doi: 10.18632/oncotarget.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chien M.-H., Lin Y.-W., Wen Y.-C., Yang Y.-C., Hsiao M., Chang J.-L., Huang H.-C., Lee W.-J. Targeting the SPOCK1-snail/slug axis-mediated epithelial-to-mesenchymal transition by apigenin contributes to repression of prostate cancer metastasis. J. Exp. Clin. Cancer Res. 2019;38:246. doi: 10.1186/s13046-019-1247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gialeli C., Theocharis A.D., Karamanos N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 231.Vijayababu M.R., Arunkumar A., Kanagaraj P., Venkataraman P., Krishnamoorthy G., Arunakaran J. Quercetin downregulates matrix metalloproteinases 2 and 9 proteins expression in prostate cancer cells (PC-3) Mol. Cell. Biochem. 2006;287:109–116. doi: 10.1007/s11010-005-9085-3. [DOI] [PubMed] [Google Scholar]
- 232.Chien C.-S., Shen K.-H., Huang J.-S., Ko S.-C., Shih Y.-W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell. Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 233.Hong J.H., Ahn K.S., Bae E., Jeon S.S., Choi H.Y. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006;9:147–152. doi: 10.1038/sj.pcan.4500856. [DOI] [PubMed] [Google Scholar]
- 234.Cheng T.-S., Chen W.-C., Lin Y.-Y., Tsai C.-H., Liao C.-I., Shyu H.-Y., Ko C.-J., Tzeng S.-F., Huang C.-Y., Yang P.-C., et al. Curcumin-targeting pericellular serine protease matriptase role in suppression of prostate cancer cell invasion, tumor growth, and metastasis. Cancer Prev. Res. 2013;6:495–505. doi: 10.1158/1940-6207.CAPR-12-0293-T. [DOI] [PubMed] [Google Scholar]
- 235.Yang J., Wang C., Zhang Z., Chen X., Jia Y., Wang B., Kong T. Curcumin inhibits the survival and metastasis of prostate cancer cells via the Notch-1 signaling pathway. APMIS. 2017;125:134–140. doi: 10.1111/apm.12650. [DOI] [PubMed] [Google Scholar]
- 236.Huang X., Chen S., Xu L., Liu Y., Deb D.K., Platanias L.C., Bergan R.C. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65:3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 237.Xu L., Bergan R.C. Genistein inhibits matrix metalloproteinase type 2 activation and prostate cancer cell invasion by blocking the transforming growth factor β-mediated activation of mitogen-activated protein kinase-activated protein kinase 2-27-kDa heat shock protein pathway. Mol. Pharmacol. 2006;70:869–877. doi: 10.1124/mol.106.023861. [DOI] [PubMed] [Google Scholar]
- 238.Kumi-Diaka J.K., Hassanhi M., Merchant K., Horman V. Influence of genistein isoflavone on matrix metalloproteinase-2 expression in prostate cancer cells. J. Med. Food. 2006;9:491–497. doi: 10.1089/jmf.2006.9.491. [DOI] [PubMed] [Google Scholar]
- 239.Xu L., Ding Y., Catalona W.J., Yang X.J., Anderson W.F., Jovanovic B., Wellman K., Killmer J., Huang X., Scheidt K.A., et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J. Natl. Cancer Inst. 2009;101:1141–1155. doi: 10.1093/jnci/djp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhang H., Gordon R., Li W., Yang X., Pattanayak A., Fowler G., Zhang L., Catalona W.J., Ding Y., Xu L., et al. Genistein treatment duration effects biomarkers of cell motility in human prostate. PLoS ONE. 2019;14:e0214078. doi: 10.1371/journal.pone.0214078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vayalil P.K., Katiyar S.K. Treatment of epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and -9 via inhibition of activation of mitogen-activated protein kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145 cells. Prostate. 2004;59:33–42. doi: 10.1002/pros.10352. [DOI] [PubMed] [Google Scholar]
- 242.Pezzato E., Sartor L., Dell’Aica I., Dittadi R., Gion M., Belluco C., Lise M., Garbisa S. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (-)epigallocatechin-3-gallate. Int. J. cancer. 2004;112:787–792. doi: 10.1002/ijc.20460. [DOI] [PubMed] [Google Scholar]
- 243.Roomi M.W., Ivanov V., Kalinovsky T., Niedzwiecki A., Rath M. Anti-tumor effect of ascorbic acid, lysine, proline, arginine, and epigallocatechin gallate on prostate cancer cell lines PC-3, LNCaP, and DU145. Res. Commun. Mol. Pathol. Pharmacol. 2004;115–116:251–264. [PubMed] [Google Scholar]
- 244.Wu K.J., Zeng J., Zhu G.D., Zhang L.L., Zhang D., Li L., Fan J.H., Wang X.Y., He D.L. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol. Sin. 2009;30:1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Deep G., Kumar R., Jain A.K., Agarwal C., Agarwal R. Silibinin inhibits fibronectin induced motility, invasiveness and survival in human prostate carcinoma PC3 cells via targeting integrin signaling. Mutat. Res. Mol. Mech. Mutagen. 2014;768:35–46. doi: 10.1016/j.mrfmmm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Franzen C.A., Amargo E., Todorović V., Desai B.V., Huda S., Mirzoeva S., Chiu K., Grzybowski B.A., Chew T.-L., Green K.J., et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev. Res. 2009;2:830–841. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]
- 247.Holy J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell Motil. Cytoskeleton. 2004;58:253–268. doi: 10.1002/cm.20012. [DOI] [PubMed] [Google Scholar]
- 248.Chen C., Zhao S., Karnad A., Freeman J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018;11:64. doi: 10.1186/s13045-018-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Handorean A.M., Yang K., Robbins E.W., Flaig T.W., Iczkowski K.A. Silibinin suppresses CD44 expression in prostate cancer cells. Am. J. Transl. Res. 2009;1:80–86. [PMC free article] [PubMed] [Google Scholar]
- 250.El Touny L.H., Banerjee P.P. Genistein induces the metastasis suppressor kangai-1 which mediates its anti-invasive effects in TRAMP cancer cells. Biochem. Biophys. Res. Commun. 2007;361:169–175. doi: 10.1016/j.bbrc.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Deb G., Shankar E., Thakur V.S., Ponsky L.E., Bodner D.R., Fu P., Gupta S. Green tea–induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol. Carcinog. 2019;58:1194–1207. doi: 10.1002/mc.23003. [DOI] [PubMed] [Google Scholar]
- 252.Tian H., Zhou Y., Yang G., Geng Y., Wu S., Hu Y., Lin K., Wu W. Sulforaphane-cysteine suppresses invasion via downregulation of galectin-1 in human prostate cancer DU145 and PC3 cells. Oncol. Rep. 2016;36:1361–1368. doi: 10.3892/or.2016.4942. [DOI] [PubMed] [Google Scholar]
- 253.Pan X.Y., Guo H., Han J., Hao F., An Y., Xu Y., Xiaokaiti Y., Pan Y., Li X.J. Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur. J. Pharmacol. 2012;683:27–34. doi: 10.1016/j.ejphar.2012.02.040. [DOI] [PubMed] [Google Scholar]
- 254.Dhar S., Kumar A., Zhang L., Rimando A.M., Lage J.M., Lewin J.R., Atfi A., Zhang X., Levenson A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget. 2016;7:18469–18484. doi: 10.18632/oncotarget.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dorai T., Diouri J., O’Shea O., Doty – S.B. Curcumin inhibits prostate cancer bone metastasis by up-regulating bone morphogenic protein-7 in vivo. J. Cancer Ther. 2014;5:369–386. doi: 10.4236/jct.2014.54044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li Y., Che M., Bhagat S., Ellis K.L., Kucuk O., Doerge D.R., Abrams J., Cher M.L., Sarkar F.H. Regulation of gene expression and inhibition of experimental prostate cancer bone metastasis by dietary genistein. Neoplasia. 2004;6:354–363. doi: 10.1593/neo.03478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Kuchta K., Xiang Y., Huang S., Tang Y., Peng X., Wang X., Zhu Y., Li J., Xu J., Lin Z., et al. Celastrol, an active constituent of the TCM plant Tripterygium wilfordii Hook.f., inhibits prostate cancer bone metastasis. Prostate Cancer Prostatic Dis. 2017;20:156–164. doi: 10.1038/pcan.2016.61. [DOI] [PubMed] [Google Scholar]
- 258.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shibuya M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes and Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 261.Pratheeshkumar P., Budhraja A., Son Y.O., Wang X., Zhang Z., Ding S., Wang L., Hitron A., Lee J.C., Xu M., et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS ONE. 2012;7:e47516. doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pratheeshkumar P., Son Y.O., Budhraja A., Wang X., Ding S., Wang L., Hitron A., Lee J.C., Kim D., Divya S.P., et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE. 2012;7:e52279. doi: 10.1371/journal.pone.0052279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Pang X., Yi Z., Zhang J., Lu B., Sung B., Qu W., Aggarwal B.B., Liu M. Celastrol suppresses angiogenesis-mediated tumor growth through inhibition of AKT/Mammalian target of rapamycin pathway. Cancer Res. 2010;70:1951–1959. doi: 10.1158/0008-5472.CAN-09-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Krock B.L., Skuli N., Simon M.C. Hypoxia-Induced Angiogenesis: GOOD AND EVil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.van Meeteren L., Goumans M.-J., ten Dijke P. TGF-β receptor signaling pathways in angiogenesis; emerging targets for anti-angiogenesis therapy. Curr. Pharm. Biotechnol. 2011;12:2108–2120. doi: 10.2174/138920111798808338. [DOI] [PubMed] [Google Scholar]
- 266.Lee D.H., Lee Y.J. Quercetin suppresses hypoxia-induced accumulation of hypoxia-inducible factor-1α (HIF-1α) through inhibiting protein synthesis. J. Cell. Biochem. 2008;105:546–553. doi: 10.1002/jcb.21851. [DOI] [PubMed] [Google Scholar]
- 267.Mirzoeva S., Nam D.K., Chiu K., Franzen C.A., Bergan R.C., Pelling J.C. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol. Carcinog. 2008;47:686–700. doi: 10.1002/mc.20421. [DOI] [PubMed] [Google Scholar]
- 268.Guo Y., Wang S., Hoot D.R., Clinton S.K. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J. Nutr. Biochem. 2007;18:408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 269.Mirzoeva S., Franzen C.A., Pelling J.C. Apigenin inhibits TGF-β-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and Src-dependent mechanism. Mol. Carcinog. 2014;53:598–609. doi: 10.1002/mc.22005. [DOI] [PubMed] [Google Scholar]
- 270.Shi X., Deepak V., Wang L., Ba X., Komori T., Zeng X., Liu W. Thrombospondin-1 is a putative target gene of Runx2 and Runx3. Int. J. Mol. Sci. 2013;14:14321–14332. doi: 10.3390/ijms140714321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yang F., Jiang X., Song L., Wang H., Mei Z., Xu Z., Xing N. Quercetin inhibits angiogenesis through thrombospondin-1 upregulation to antagonize human prostate cancer PC-3 cell growth in vitro and in vivo. Oncol. Rep. 2016;35:1602–1610. doi: 10.3892/or.2015.4481. [DOI] [PubMed] [Google Scholar]
- 272.Toole B.P., Wight T.N., Tammi M.I. Hyaluronan-cell interactions in cancer and vascular disease. J. Biol. Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 273.Yang C., Cao M., Liu H., He Y., Xu J., Du Y., Liu Y., Wang W., Cui L., Hu J., et al. The high and low molecular weight forms of hyaluronan have distinct effects on CD44 clustering. J. Biol. Chem. 2012;287:43094–43107. doi: 10.1074/jbc.M112.349209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Lokeshwar V.B., Rubinowicz D., Schroeder G.L., Forgacs E., Minna J.D., Block N.L., Nadji M., Lokeshwar B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- 275.Lall R.K., Syed D.N., Khan M.I., Adhami V.M., Gong Y., Lucey J.A., Mukhtar H. Dietary flavonoid fisetin increases abundance of high-molecular-mass hyaluronan conferring resistance to prostate oncogenesis. Carcinogenesis. 2016;37:918–928. doi: 10.1093/carcin/bgw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Skvortsov S., Skvortsova I.-I., Tang D.G., Dubrovska A. Concise review: Prostate cancer stem cells: Current understanding. Stem Cells. 2018;36:1457–1474. doi: 10.1002/stem.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tsai P.H., Cheng C.H., Lin C.Y., Huang Y.T., Lee L.T., Kandaswami C.C., Lin Y.C., Lee K.P.H., Hung C.C., Hwang J.J., et al. Dietary flavonoids luteolin and quercetin suppressed cancer stem cell properties and metastatic potential of isolated prostate cancer cells. Anticancer Res. 2016;36:6367–6380. doi: 10.21873/anticanres.11234. [DOI] [PubMed] [Google Scholar]
- 278.Erdogan S., Turkekul K., Dibirdik I., Doganlar O., Doganlar Z.B., Bilir A., Oktem G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018;107:793–805. doi: 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 279.Han K., Lang T., Zhang Z., Zhang Y., Sun Y., Shen Z., Beuerman R.W., Zhou L., Min D. Luteolin attenuates Wnt signaling via upregulation of FZD6 to suppress prostate cancer stemness revealed by comparative proteomics. Sci. Rep. 2018;8:8537. doi: 10.1038/s41598-018-26761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Erdogan S., Doganlar O., Doganlar Z.B., Serttas R., Turkekul K., Dibirdik I., Bilir A. The flavonoid apigenin reduces prostate cancer CD44 + stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 281.Erdogan S., Turkekul K., Dibirdik I., Doganlar Z.B., Doganlar O., Bilir A. Midkine silencing enhances the anti–prostate cancer stem cell activity of the flavone apigenin: Cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF-κB. Invest. New Drugs. 2019 doi: 10.1007/s10637-019-00774-8. [DOI] [PubMed] [Google Scholar]
- 282.Erdogan S., Turkekul K., Serttas R., Erdogan Z. The natural flavonoid apigenin sensitizes human CD44+prostate cancer stem cells to cisplatin therapy. Biomed. Pharmacother. 2017;88:210–217. doi: 10.1016/j.biopha.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 283.Liu T., Chi H., Chen J., Chen C., Huang Y., Xi H., Xue J., Si Y. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29–38. doi: 10.1016/j.gene.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 284.Zhang H., Zheng J., Shen H., Huang Y., Liu T., Xi H., Chen C. Curcumin suppresses in vitro proliferation and invasion of human prostate cancer stem cells by modulating DLK1-DIO3 imprinted gene cluster microRNAs. Genet. Test. Mol. Biomarkers. 2018;22:43–50. doi: 10.1089/gtmb.2017.0179. [DOI] [PubMed] [Google Scholar]
- 285.Vyas A.R., Moura M.B., Hahm E.R., Singh K.B., Singh S.V. Sulforaphane inhibits c-Myc-mediated prostate cancer stem-like traits. J. Cell. Biochem. 2016;117:2482–2495. doi: 10.1002/jcb.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Zhang L., Li L., Jiao M., Wu D., Wu K., Li X., Zhu G., Yang L., Wang X., Hsieh J.T., et al. Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog-Gli1 pathway. Cancer Lett. 2012;323:48–57. doi: 10.1016/j.canlet.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 287.Luk S.U., Yap W.N., Chiu Y.-T., Lee D.T.W., Ma S., Lee T.K.W., Vasireddy R.S., Wong Y.-C., Ching Y.P., Nelson C., et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. cancer. 2011;128:2182–2191. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- 288.Lee S.O., Ma Z., Yeh C.-R., Luo J., Lin T.-H., Lai K.-P., Yamashita S., Liang L., Tian J., Li L., et al. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. J. Mol. Cell Biol. 2013;5:14–26. doi: 10.1093/jmcb/mjs042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kaneko S., Sato C., Shiozawa N., Sato A., Sato H., Virgona N., Yano T. Suppressive effect of delta-tocotrienol on hypoxia adaptation of prostate cancer stem-like cells. Anticancer Res. 2018;38:1391–1399. doi: 10.21873/anticanres.12362. [DOI] [PubMed] [Google Scholar]
- 290.Hejazi J., Rastmanesh R., Taleban F.A., Molana S.H., Hejazi E., Ehtejab G., Hara N. Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutr. Cancer. 2016;68:77–85. doi: 10.1080/01635581.2016.1115527. [DOI] [PubMed] [Google Scholar]
- 291.Saadipoor A., Razzaghdoust A., Simforoosh N., Mahdavi A., Bakhshandeh M., Moghadam M., Abdollahi H., Mofid B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phyther. Res. 2019;33:370–378. doi: 10.1002/ptr.6230. [DOI] [PubMed] [Google Scholar]
- 292.Choi Y.H., Han D.H., Kim S., Kim M., Sung H.H., Jeon H.G., Jeong B.C., Seo S.I., Jeon S.S., Lee H.M., et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate. 2019;79:614–621. doi: 10.1002/pros.23766. [DOI] [PubMed] [Google Scholar]
- 293.Nagata Y., Sonoda T., Mori M., Miyanaga N., Okumura K., Goto K., Naito S., Fujimoto K., Hirao Y., Takahashi A., et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J. Nutr. 2007;137:1974–1979. doi: 10.1093/jn/137.8.1974. [DOI] [PubMed] [Google Scholar]
- 294.Kurahashi N., Iwasaki M., Inoue M., Sasazuki S., Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: The Japan Public Health Center. J. Clin. Oncol. 2008;26:5923–5929. doi: 10.1200/JCO.2008.16.8807. [DOI] [PubMed] [Google Scholar]
- 295.Nagata Y., Sugiyama Y., Fukuta F., Takayanagi A., Masumori N., Tsukamoto T., Akasaka H., Ohnishi H., Saitoh S., Miura T., et al. Relationship of serum levels and dietary intake of isoflavone, and the novel bacterium Slackia sp. strain NATTS with the risk of prostate cancer: A case–control study among Japanese men. Int. Urol. Nephrol. 2016;48:1453–1460. doi: 10.1007/s11255-016-1335-7. [DOI] [PubMed] [Google Scholar]
- 296.Wu Y., Zhang L., Na R., Xu J., Xiong Z., Zhang N., Dai W., Jiang H., Ding Q. Plasma genistein and risk of prostate cancer in Chinese population. Int. Urol. Nephrol. 2015;47:965–970. doi: 10.1007/s11255-015-0981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Heald C.L., Ritchie M.R., Bolton-Smith C., Morton M.S., Alexander F.E. Phyto-oestrogens and risk of prostate cancer in Scottish men. Br. J. Nutr. 2007;98:388–396. doi: 10.1017/S0007114507700703. [DOI] [PubMed] [Google Scholar]
- 298.Russo G.I., Di Mauro M., Regis F., Reale G., Campisi D., Marranzano M., Lo Giudice A., Solinas T., Madonia M., Cimino S., et al. Association between dietary phytoestrogens intakes and prostate cancer risk in Sicily. Aging Male. 2018;21:48–54. doi: 10.1080/13685538.2017.1365834. [DOI] [PubMed] [Google Scholar]
- 299.Travis R.C., Allen N.E., Appleby P.N., Price A., Kaaks R., Chang-Claude J., Boeing H., Aleksandrova K., Tjønneland A., Johnsen N.F., et al. Prediagnostic concentrations of plasma genistein and prostate cancer risk in 1,605 men with prostate cancer and 1,697 matched control participants in EPIC. Cancer Causes Control. 2012;23:1163–1171. doi: 10.1007/s10552-012-9985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Perez-Cornago A., Appleby P.N., Boeing H., Gil L., Kyrø C., Ricceri F., Murphy N., Trichopoulou A., Tsilidis K.K., Khaw K.T., et al. Circulating isoflavone and lignan concentrations and prostate cancer risk: A meta-analysis of individual participant data from seven prospective studies including 2,828 cases and 5,593 controls. Int. J. Cancer. 2018;143:2677–2686. doi: 10.1002/ijc.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Miltyk W., Craciunescu C.N., Fischer L., Jeffcoat R.A., Koch M.A., Lopaczynski W., Mahoney C., Jeffcoat R.A., Crowell J., Paglieri J. Lack of significant genotoxicity of purified soy isoflavones (genistein, daidzein, and glycitein) in 20 patients with prostate cancer. Am. J. Clin. Nutr. 2003;77:875–882. doi: 10.1093/ajcn/77.4.875. [DOI] [PubMed] [Google Scholar]
- 302.Fischer L., Mahoney C., Jeffcoat A.R., Koch M.A., Thomas B.E., Valentine J.L., Stinchcombe T., Boan J., Crowell J.A., Zeisel S.H. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Multiple-dose administration to men with prostate neoplasia. Nutr. Cancer. 2004;48:160–170. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 303.Tourinho-Barbosa R., Srougi V., Nunes-Silva I., Baghdadi M., Rembeyo G., Eiffel S.S., Barret E., Rozet F., Galiano M., Cathelineau X., et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. braz j urol. 2018;44:14–21. doi: 10.1590/s1677-5538.ibju.2016.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Cipolla B.G., Mandron E., Lefort J.M., Coadou Y., Della Negra E., Corbel L., Le Scodan R., Azzouzi A.R., Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev. Res. 2015;8:712–719. doi: 10.1158/1940-6207.CAPR-14-0459. [DOI] [PubMed] [Google Scholar]
- 305.Alumkal J.J., Slottke R., Schwartzman J., Cherala G., Munar M., Graff J.N., Beer T.M., Ryan C.W., Koop D.R., Gibbs A., et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest. New Drugs. 2015;33:480–489. doi: 10.1007/s10637-014-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Kjaer T.N., Ornstrup M.J., Poulsen M.M., Jørgensen J.O., Hougaard D.M., Cohen A.S., Neghabat S., Richelsen B., Pedersen S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75:1255–1263. doi: 10.1002/pros.23006. [DOI] [PubMed] [Google Scholar]
- 307.Paller C.J., Rudek M.A., Zhou X.C., Wagner W.D., Hudson T.S., Anders N., Hammers H.J., Dowling D., King S., Antonarakis E.S., et al. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate. 2015;75:1518–1525. doi: 10.1002/pros.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Paller C.J., Zhou X.C., Heath E.I., Taplin M.E., Mayer T., Stein M.N., Bubley G.J., Pili R., Hudson T., Kakarla R., et al. Muscadine grape skin extract (MPX) in men with biochemically recurrent prostate cancer: A randomized, multicenter, placebo-controlled clinical trial. Clin. Cancer Res. 2018;24:306–315. doi: 10.1158/1078-0432.CCR-17-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.McLarty J., Bigelow R.L.H., Smith M., Elmajian D., Ankem M., Cardelli J.A. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev. Res. 2009;2:673–682. doi: 10.1158/1940-6207.CAPR-08-0167. [DOI] [PubMed] [Google Scholar]
- 310.Nguyen M.M., Ahmann F.R., Nagle R.B., Hsu C.-H., Tangrea J.A., Parnes H.L., Sokoloff M.H., Gretzer M.B., Chow H.-H.S. Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: Evaluation of potential chemopreventive activities. Cancer Prev. Res. 2012;5:290–298. doi: 10.1158/1940-6207.CAPR-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kumar N.B., Pow-Sang J., Egan K.M., Spiess P.E., Dickinson S., Salup R., Helal M., McLarty J., Williams C.R., Schreiber F., et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Cancer Prev. Res. 2015;8:879–887. doi: 10.1158/1940-6207.CAPR-14-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bettuzzi S., Brausi M., Rizzi F., Castagnetti G., Peracchia G., Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 313.Lee P.M.Y., Ng C.F., Liu Z.M., Ho W.M., Lee M.K., Wang F., Kan H.D., He Y.H., Ng S.S.M., Wong S.Y.S., et al. Reduced prostate cancer risk with green tea and epigallocatechin 3-gallate intake among Hong Kong Chinese men. Prostate Cancer Prostatic Dis. 2017;20:318–322. doi: 10.1038/pcan.2017.18. [DOI] [PubMed] [Google Scholar]
- 314.Kurahashi N., Sasazuki S., Iwasaki M., Inoue M., Tsugane S., JPHC Study Group Green tea consumption and prostate cancer risk in Japanese men: A prospective study. Am. J. Epidemiol. 2008;167:71–77. doi: 10.1093/aje/kwm249. [DOI] [PubMed] [Google Scholar]
- 315.Flaig T.W., Gustafson D.L., Su L.J., Zirrolli J.A., Crighton F., Harrison G.S., Pierson A.S., Agarwal R., Glodé L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 316.Flaig T.W., Glodé M., Gustafson D., van Bokhoven A., Tao Y., Wilson S., Su L.J., Li Y., Harrison G., Agarwal R., et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70:848–855. doi: 10.1002/pros.21118. [DOI] [PubMed] [Google Scholar]
- 317.Klein E.A., Thompson I.M., Lippman S.M., Goodman P.J., Albanes D., Taylor P.R., Coltman C. SELECT: The next prostate cancer prevention trial. Selenum and vitamin E cancer prevention trial. J. Urol. 2001;166:1311–1315. doi: 10.1016/S0022-5347(05)65759-X. [DOI] [PubMed] [Google Scholar]
- 318.Lippman S.M., Klein E.A., Goodman P.J., Lucia M.S., Thompson I.M., Ford L.G., Parnes H.L., Minasian L.M., Gaziano J.M., Hartline J.A., et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Dunn B.K., Richmond E.S., Minasian L.M., Ryan A.M., Ford L.G. A nutrient approach to prostate cancer prevention: The selenium and vitamin E cancer prevention trial (SELECT) Nutr. Cancer. 2010;62:896–918. doi: 10.1080/01635581.2010.509833. [DOI] [PubMed] [Google Scholar]
- 320.Ledesma M.C., Jung-Hynes B., Schmit T.L., Kumar R., Mukhtar H., Ahmad N. Selenium and vitamin E for prostate cancer: Post-SELECT (selenium and vitamin E cancer prevention trial) status. Mol. Med. 2011;17:134–143. doi: 10.2119/molmed.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]