Abstract
Ribosomal proteins (RPs) are indispensable in ribosome biogenesis and protein synthesis, and play a crucial role in diverse developmental processes. In the present study, we carried out a comprehensive analysis of RPs in chordates and examined the expression profiles of the complete set of 92 cytoplasmic RP genes in Nile tilapia. The RP genes were randomly distributed throughout the tilapia genome. Phylogenetic and syntenic analyses revealed the existence of duplicated RP genes from 2R (RPL3, RPL7, RPL22 and RPS27) and 3R (RPL5, RPL19, RPL22, RPL41, RPLP2, RPS17, RPS19 and RPS27) in tilapia and even more from 4R in common carp and Atlantic salmon. The RP genes were found to be expressed in all tissues examined, but their expression levels differed among different tissues. Gonadal transcriptome analysis revealed that almost all RP genes were highly expressed, and their expression levels were highly variable between ovaries and testes at different developmental stages in tilapia. No sex- and stage-specific RP genes were found. Eleven RP genes displayed sexually dimorphic expression with nine higher in XY gonad and two higher in XX gonad at all stages examined, which were proved to be phenotypic sex dependent. Quantitative real-time PCR and immunohistochemistry ofRPL5b and RPL24 were performed to validate the transcriptome data. The genomic resources and expression data obtained in this study will contribute to a better understanding of RPs evolution and functions in chordates.
Keywords: cytoplasmic ribosomal protein gene, evolution, transcriptome analysis, Nile tilapia
1. Introduction
Ribosomes are absolutely essential for life, generating all proteins required for cell growth and maintenance. In eukaryotes, a mature ribosome consists of four different ribosomal RNAs (rRNAs; 18S in the small subunit and 28S, 5.8S, and 5S in the large subunit), as well as ~80 ribosomal proteins (RPs) [1]. RPs from the small and large subunits are named as RPS and RPL, respectively. Great progress has been made in recent years in elucidating the structure and function of the RPs [2,3]. The cellular levels of some RP transcripts change as a function of growth, development and certain tumors [4,5,6]. In addition, many RPs are believed to have important functions in various other cellular processes, the so-called extraribosomal functions [7,8]. Over the past years, mutations in ribosomal proteins or ribosomal biogenesis factors have been identified in patients with varying disease [9,10]. For example, haploinsufficiency of the RPS4 genes has been suggested to contribute to anatomic abnormalities associated with the Turner syndrome in humans [11]. In zebrafish, many ribosomal protein mutations are associated with growth impairment and tumor predisposition [12]. Knockdown of RPS7 causes developmental abnormalities via p53 dependent and independent pathways [13].
Genomic mapping of the RPs is of great interest because of its value in understanding genome evolution. The sequence information of these genes is required for mapping them onto chromosomes. The large number of RPs has complicated the quest for a complete understanding of their sequence information, gene structures and genomic organizations. The first complete identification of human ribosomal proteins was published in 2001 [14,15]. Although fish represent the largest group of vertebrates, a complete map of RP genes from any teleost species has not been available until recently. The complete set of RP genes has been identified from cDNA library in channel catfish, Senegalese sole and Atlantic halibut [16,17,18,19]. However, the exact number of RP genes in fish species is unclear due to lack of complete genome sequences. The rapid development of genomics, sequencing and disclosure of more and more animal genomes in recent years has made it feasible to elucidate the evolution of RP genes in vertebrates.
The expression pattern of RP genes in different tissues and developmental stages has been reported in several species. In Haemaphysalis longicornis, RPS-27 was expressed in all life stages, and it was more highly expressed in the salivary gland than in the midgut at the tissue level [20]. In channel catfish, Senegalese sole and Atlantic halibut, RP genes were expressed ubiquitously at a similar level among tissues, although they differed in the relative abundance of their transcripts [16,17,18,19]. Sexually dimorphic expression of RP genes was also observed in some species. For example, two ribosomal proteins, RPL17 and RPL37, were expressed higher in males compared to females in the zebra finch brain [21,22,23]. Later, RPS6 was also found to be enhanced in the male zebra finch brain as compared to the female’s throughout life [24]. In marine shrimp, RPL24 was differentially expressed in ovary and testis [25]. In zebrafish, RPL39 and RPL23A were found to be the female-biased genes [26]. Existing studies on global RP gene expression are mainly based on expressed sequence tag (EST) approach, the expression level of genes probably not represented properly. The advances of transcriptomics make it possible to carry out more accurate expression analyses of the complete set of RPs in different tissues and different developmental stages in vertebrates.
The Nile tilapia (Oreochromis niloticus) is a commercially important farmed fish species in aquaculture worldwide, with the growth rate of males substantially higher than that of females [27]. Because of the key role RPs play in cellular growth and proliferation, it is important to elucidate the expression pattern of RPs in different tissues in tilapia. As is known to all, during fish gonadal differentiation and development, the male and female gonads display different gene expression profiles. Thus, the gonadal model provides a unique opportunity to delineate the coordinated expression of RPs and other genes during gonadal differentiation and development. However, the complete set of Nile tilapia ribosomal protein genes has not been previously documented or characterized, and the expression of RP genes during gonadal differentiation and development in fish has not been reported to date. The availability of the whole genome sequences of tilapia and tissue transcriptomes [28,29], together with gonadal transcriptomes at different developmental stages [30,31], has made it an excellent model for genome-wide identification, tissue distribution and gonadal expression profile investigation of RP genes involved in gonadal differentiation and development.
RPs are highly conserved even between vertebrates and invertebrates. This sequence identity enables us to name RPs with significant confidence and also enables the use of antibodies raised against mouse RPs on tilapia proteins. In the present study, we carried out a comprehensive analysis of RP genes in tilapia and other chordates, including genome-wide search, chromosomal location, phylogeny, synteny and spatial and temporal expression profiles. Our results provided a new insight into evolution of RPs and revealed their potential role in teleosts.
2. Results
2.1. Identification of RP Genes in Tilapia
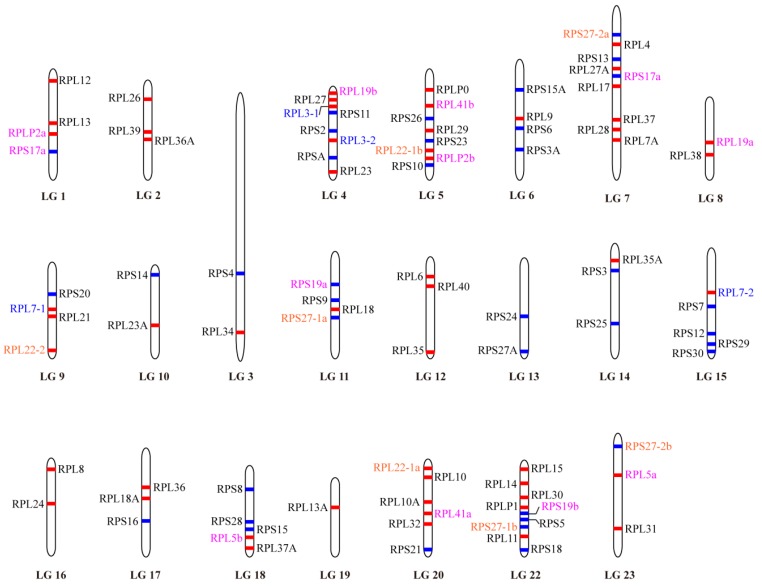
To obtain the complete set of RP genes, a blast search against tilapia genome sequences at NCBI was performed using zebrafish and human RP genes as reference. A total of 92 RP genes, including 55 encoding large-subunit proteins and 37 encoding small-subunit proteins (Table 1), were identified in tilapia. The GenBank accession number, Chromosome location, number of exons, gene length (bp) and protein length (aa) for the complete set of RP genes are shown in Table 1. All the 92 RP genes have complete open reading frames (ORFs) and gene sequences. The average size of the genes from the transcription start site is ~3.61 kb. RPS17a is the largest (~7.98 kb), whereas RPL41bis the smallest (only ~0.91 kb). Each gene has an average of 5.5 exons, ranging from 3 (RPL39 and RPS29) to 10 (RPL3-2 and RPL4). All of the 92 RP genes were assigned to the linkage maps of the tilapia genome (Figure 1). The RP genes were found to be randomly distributed throughout the tilapia genome covering all 22 linkage groups (LG). Each LG carried at least one RP gene. Both the LG7 and LG22 carried a total of nine genes. LG19 is the only example with only one RP gene (RPL13A) present.
Table 1.
Ribosomal protein (RP) genes identified in Nile tilapia genome.
| Gene | Accession Number |
Chromosome Location | Exon Count | Gene Length (bp) | Protein Length (aa) |
|---|---|---|---|---|---|
| RPSA | LOC100707344 | LG4 | 8 | 2836 | 307 |
| RPS2 | LOC100693457 | LG4 | 7 | 2113 | 279 |
| RPS3 | LOC100696630 | LG14 | 7 | 6031 | 245 |
| RPS3A | LOC100690034 | LG6 | 6 | 3940 | 266 |
| RPS4 | LOC100709648 | LG3 | 7 | 7165 | 263 |
| RPS5 | LOC100691205 | LG22 | 6 | 3187 | 203 |
| RPS6 | LOC100707655 | LG6 | 6 | 4440 | 249 |
| RPS7 | LOC100704343 | LG15 | 7 | 6283 | 194 |
| RPS8 | LOC100693356 | LG18 | 6 | 4026 | 208 |
| RPS9 | LOC100694704 | LG11 | 5 | 2450 | 194 |
| RPS10 | LOC100708197 | LG5 | 6 | 4577 | 166 |
| RPS11 | LOC100696602 | LG4 | 5 | 2026 | 161 |
| RPS12 | LOC100534443 | LG15 | 5 | 2914 | 132 |
| RPS13 | LOC100696829 | LG7 | 6 | 2934 | 151 |
| RPS14 | LOC100696222 | LG10 | 5 | 2925 | 151 |
| RPS15 | LOC100695708 | LG18 | 4 | 2627 | 145 |
| RPS15A | LOC100698782 | LG6 | 5 | 5166 | 130 |
| RPS16 | LOC100703910 | LG17 | 6 | 3101 | 146 |
| RPS17a | LOC100691912 | LG7 | 5 | 7981 | 134 |
| RPS17b | LOC100699764 | LG1 | 5 | 5281 | 134 |
| RPS18 | LOC100699803 | LG22 | 6 | 3445 | 152 |
| RPS19a | LOC100689943 | LG11 | 6 | 6770 | 146 |
| RPS19b | LOC100690398 | LG22 | 5 | 7095 | 152 |
| RPS20 | LOC100696588 | LG9 | 4 | 1505 | 119 |
| RPS21 | LOC100701551 | LG20 | 6 | 4172 | 83 |
| RPS23 | LOC100690746 | LG5 | 4 | 2080 | 143 |
| RPS24 | LOC100700493 | LG13 | 5 | 4576 | 131 |
| RPS25 | LOC100690179 | LG14 | 5 | 2542 | 123 |
| RPS26 | LOC100700449 | LG5 | 4 | 1749 | 115 |
| RPS27-1a | LOC100705775 | LG11 | 4 | 5189 | 84 |
| RPS27-1b | LOC100696027 | LG22 | 4 | 2376 | 84 |
| RPS27-2a | LOC100691333 | LG7 | 4 | 2298 | 84 |
| RPS27-2b | LOC100690174 | LG23 | 4 | 2477 | 84 |
| RPS27A | LOC100697017 | LG13 | 6 | 2770 | 156 |
| RPS28 | LOC100696499 | LG18 | 4 | 2909 | 69 |
| RPS29 | LOC100703241 | LG15 | 3 | 1056 | 56 |
| RPS30 | LOC100704053 | LG15 | 5 | 1783 | 133 |
| RPL3-1 | LOC100711099 | LG4 | 9 | 4294 | 403 |
| RPL3-2 | LOC100694819 | LG4 | 10 | 3972 | 408 |
| RPL4 | LOC100694481 | LG7 | 10 | 3520 | 369 |
| RPL5a | LOC100708520 | LG23 | 8 | 6819 | 297 |
| RPL5b | LOC100700425 | LG18 | 8 | 3792 | 298 |
| RPL6 | LOC100699139 | LG12 | 6 | 6639 | 227 |
| RPL7-1 | LOC100702526 | LG9 | 7 | 4215 | 245 |
| RPL7-2 | LOC100700318 | LG15 | 7 | 4406 | 246 |
| RPL7A | LOC100693537 | LG7 | 8 | 3256 | 266 |
| RPL8 | LOC100706674 | LG16 | 6 | 3269 | 257 |
| RPL9 | LOC100703191 | LG6 | 8 | 5781 | 192 |
| RPL10 | LOC100697190 | LG20 | 6 | 3989 | 215 |
| RPL10A | LOC100702190 | LG20 | 6 | 1982 | 216 |
| RPL11 | LOC100695881 | LG22 | 6 | 3847 | 178 |
| RPL12 | LOC100695084 | LG1 | 6 | 3525 | 165 |
| RPL13 | LOC100712089 | LG1 | 6 | 4141 | 211 |
| RPL13A | LOC100708460 | LG19 | 7 | 3290 | 205 |
| RPL14 | LOC100534449 | LG22 | 6 | 3561 | 137 |
| RPL15 | LOC100697988 | LG22 | 4 | 2672 | 204 |
| RPL17 | LOC100707041 | LG7 | 7 | 4249 | 184 |
| RPL18 | LOC100534549 | LG11 | 7 | 5614 | 188 |
| RPL18A | LOC100706131 | LG17 | 5 | 3492 | 176 |
| RPL19a | LOC100706003 | LG8 | 6 | 2335 | 195 |
| RPL19b | LOC100709589 | LG4 | 6 | 3080 | 194 |
| RPL21 | LOC100711190 | LG9 | 6 | 2393 | 160 |
| RPL22-1a | LOC100699599 | LG20 | 4 | 3986 | 129 |
| RPL22-1b | LOC100710673 | LG5 | 4 | 3264 | 129 |
| RPL22-2 | LOC100699889 | LG9 | 4 | 2079 | 129 |
| RPL23 | LOC100701640 | LG4 | 5 | 4091 | 140 |
| RPL23A | LOC100705596 | LG10 | 5 | 2677 | 155 |
| RPL24 | LOC100707648 | LG16 | 6 | 3744 | 157 |
| RPL26 | LOC100708525 | LG2 | 4 | 3651 | 145 |
| RPL27 | LOC100711912 | LG4 | 5 | 3838 | 136 |
| RPL27A | LOC100702758 | LG7 | 5 | 6769 | 148 |
| RPL28 | LOC100704095 | LG7 | 5 | 2011 | 137 |
| RPL29 | LOC106098935 | LG5 | 4 | 3933 | 64 |
| RPL30 | LOC100710306 | LG22 | 5 | 3112 | 116 |
| RPL31 | LOC100696251 | LG23 | 5 | 3056 | 124 |
| RPL32 | LOC100534436 | LG20 | 4 | 3255 | 135 |
| RPL34 | LOC109195715 | LG3 | 5 | 2953 | 117 |
| RPL35 | LOC100711334 | LG12 | 4 | 2608 | 123 |
| RPL35A | LOC100711077 | LG14 | 5 | 4038 | 110 |
| RPL36 | LOC 100691103 | LG17 | 4 | 3201 | 105 |
| RPL36A | LOC100690460 | LG2 | 5 | 4870 | 106 |
| RPL37 | LOC100705081 | LG7 | 4 | 4669 | 97 |
| RPL37A | LOC100692490 | LG18 | 4 | 2432 | 92 |
| RPL38 | LOC100711498 | LG8 | 5 | 3295 | 70 |
| RPL39 | LOC100692256 | LG2 | 3 | 3583 | 51 |
| RPL40 | LOC100699676 | LG12 | 5 | 3438 | 128 |
| RPL41a | LOC100711481 | LG20 | 4 | 911 | 25 |
| RPL41b | LOC100699101 | LG5 | 4 | 1905 | 25 |
| RPLP0 | LOC100534569 | LG5 | 8 | 2532 | 315 |
| RPLP1 | LOC100692069 | LG22 | 4 | 2095 | 114 |
| RPLP2a | LOC100691806 | LG1 | 5 | 2962 | 114 |
| RPLP2b | LOC100697185 | LG5 | 5 | 1987 | 114 |
Figure 1.
Chromosomal maps of the RP genes in Nile tilapia. RP genes are depicted on a linkage group (LG) of the genome (Release 2). LG21 is absent because it has been combined withLG16. Red and blue horizontal lines indicate the large subunit and small subunit RP genes, respectively. Gene name in blue indicates genes derived from 2R event. Gene name in magenta indicates genes derived from 3R event. Gene name in orange indicates genes derived from both 2R and 3R events.
2.2. Comparative Analysis of RP Genes in Chordates
To understand the conservation of RP genes in vertebrates, the putative amino acid sequences of tilapia RPs were compared with those from the zebrafish, medaka, fugu and human (Table S1). Overall identities of tilapia RPs were97%, 96.5%, 94.1%and 92.7% to those of medaka, fugu, zebrafish and human, respectively. The most conserved RPs in the five species (overall mean identity ≥98%) were RPL23, RPL38, RPL39, RPL40, RPS13, RPS14, RPS23 and RPS27A.
The numbers of amino acids in the RPs are highly conserved among species. Among the five species compared, 48 of the 92 RPs (52.2%) have the same number of amino acids. The other RPs display different sizes in either one species (RPL5a, RPL9, RPL18 and RPL28 in zebrafish and RPL10A, RPL13A, RPL23A, RPL29, RPS3, RPS10, RPS27-1a and RPS19a in human), or two to four species (21 RPs including RPL4, RPL6, RPL7-1, RPL10, RPL14, RPL19a, RPL19b, RPL22-1b, RPL22-2, RPL29, RPL30, RPL31, RPLP0, RPLP1, RPLP2a, RPSA, RPS2, RPS3A, RPS11, RPS24 and RPS25) or even five species (RPL4, RPL14 and RPL29). In all three cases, fish RPs have fewer amino acids than their mammalian counterparts.
Given that the whole genome duplication (WGD) can drive the expansion of gene families, we surveyed the number changes of the RP genes in 13 representative chordates with different rounds of WGD, from first round to fourth round (referred to as 1R, 2R, 3R and 4R) (Figure 2, Figure 3 and Table S2). After comprehensive analysis, we identified 79, 64, 86, 84, 83, 80, 85, 125, 90, 148, 88, 92 and 86 RP genes in sea squirt, lamprey, coelacanth, human, mouse, spotted gar, zebrafish, common carp, channel catfish, Atlantic salmon, medaka, tilapia and fugu genome, respectively. Compared with Atlantic salmon, the absence of certain RPs in common carp may be due to more secondary loss or incomplete genome sequencing and assembly.
Figure 2.
Phylogenetic relationship of 13 representative chordates analyzed and number variation of RP genes. 1R, 2R, 3R and 4R indicate the four rounds of WGD that occurred during vertebrate evolution. Slash indicates no homologous gene detected, probably due to secondary loss or incomplete genome sequences. * indicates two isoforms of RPS4 located on the human X and Y chromosomes. Cin, Ciona intestinalis; Pma, Petromyzon marinus; Lch, Latimeria chalumnae; Hsa, Homo sapiens; Mmu, Mus musculus; Loc, Lepisosteus oculatus; Dre, Danio rerio; Cca, Cyprinus carpio; Ipu, Ictalurus punctatus; Ssa, Salmo salar; Ola, Oryzias latipes; Oni, Oreochromis niloticus; Tru, Takifugu rubripes.
Figure 3.
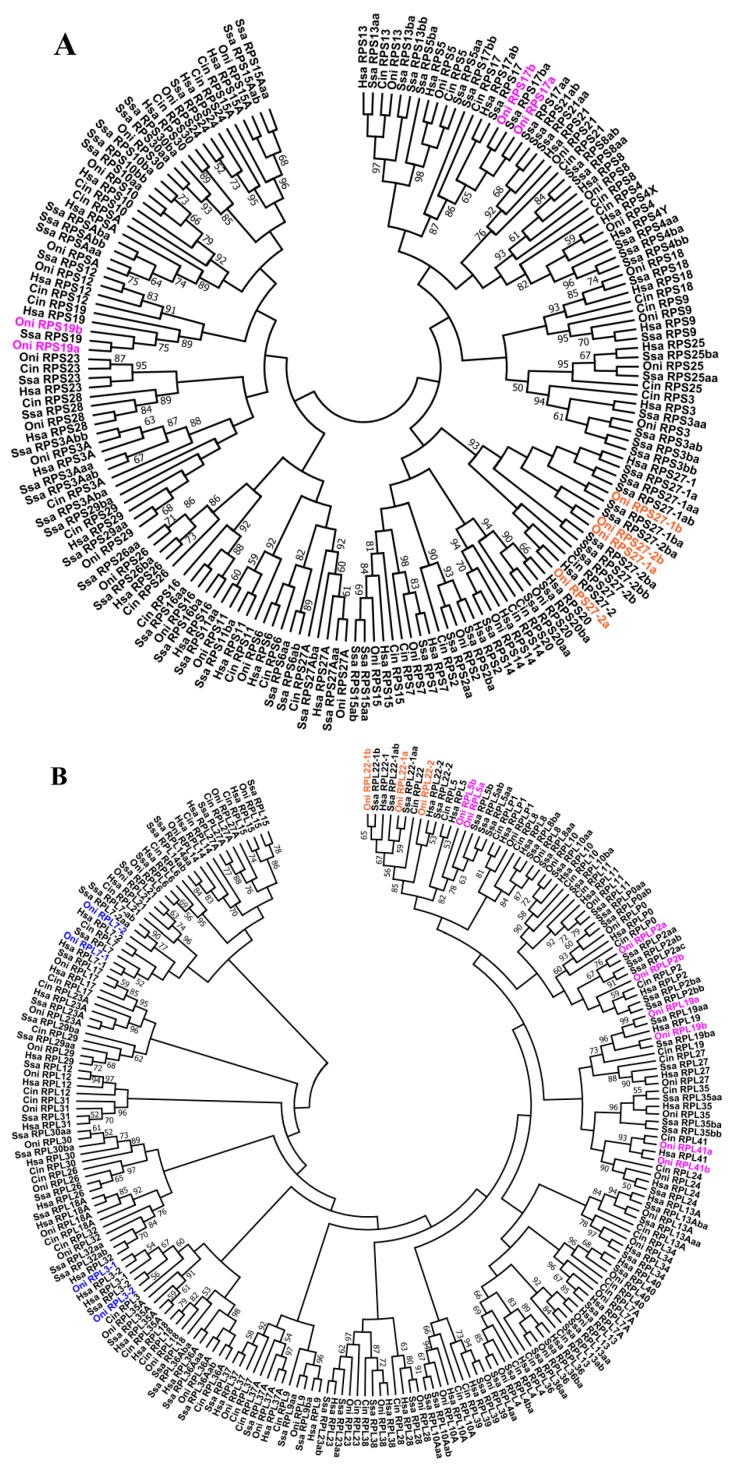
Phylogenetic tree of the small subunit (A) and large subunit (B) RP genes from sea squirt (1R), human (2R), tilapia (3R) and Atlantic salmon (4R). Gene name in blue indicates genes derived from 2R event in vertebrates. Gene name in magenta indicates genes derived from3R event in teleosts. Gene name in orange indicates genes derived from both 2R and 3R events. Cin, Cionaintestinalis; Hsa, Homo sapiens; Oni, Oreochromis niloticus; Ssa, Salmo salar.
2.3. Phylogenetic and Syntenic Analyses of RP Paralogous Genes in Tilapia
Phylogenetic and syntenic analyses were performed to understand the evolution of RP genes in vertebrates. Ten RP genes with different numbers of paralogous genes were identified in tilapia, including two for RPL3, RPL5, RPL7, RPL19, RPL41, RPLP2, RPS17 and RPS19, three for RPL22, and four for RPS27. All of these RP paralogous genes were unevenly distributed throughout the genome as shown in Figure 1. Interestingly, RPS19a and RPS27-1a, RPS19b and RPS27-1b, were located on LG11 and LG22, respectively.RPL41a and RPL22-1a, RPL41b and RPL22-1b, were located on LG20 and LG5, respectively. In contrast, both RPL3-1 and RPL3-2 were located on LG4.
A phylogenetic analysis was carried out for these duplicated genes, including sequences available in the GenBank and Ensembl for representative fish and tetrapod counterparts (Figure S1). Paralogs of RPL41 were not included in this analysis due to their small ORFlength and identical amino acid sequence (25 aa). In the tree, paralogs of RPL3-1 and RPL3-2 were grouped into two distinct well-supported clades that included the fish and tetrapod counterparts (Figure S1A). Similarly, the RPL7-1 and RPL7-2 paralogs also clustered into two separate sister clades (Figure S1B). The phylogenetic relationship of these genes suggests that they could have arisen from 2R and evolved independently. Paralogs of RPL22-1a/RPL22-1b and RPL22-2 were grouped into two paraphyletic groups, including fish and tetrapod counterparts as shown in Figure 3A. RPL22-1b appeared closely related to the other fish RPL22 sequences, although they did not form a well-resolved clade (Figure S1C). The four paralogous genes for RPS27 in tilapia clustered into two paraphyletic groups with their fish and tetrapod counterparts (Figure S1D). The tilapia RPS27-1a and RPS27-1b showed a monophyletic origin with tetrapod counterparts as a paraphyletic group, whereas tilapiaRPS27-2b grouped with other fish counterparts in a well-supported clade, and tilapiaRPS27-2awas closely related to its cichlid counterparts and RPS27-2 sequences from tetrapod. Both theRPL5 and RPL19 paralogs showed a monophyletic origin with tetrapod counterparts as a paraphyletic group (Figure S1E,F, respectively). RPLP2 paralogs clustered into two separate sister clades (Figure S1G). RPLP2b in tilapia grouped with other fish counterparts in a well-supported clade, whereas the RPLP2a in tilapia was grouped with counterparts from medaka, fugu, three-spined stickleback, Atlantic salmon, channel catfish, zebrafish, carp and RPLP2 from spotted gar, coelacanth and tetrapods. Paralogs of RPS17 also clustered into two separate sister clades (Figure S1H). RPS17b in tilapia grouped with other fish counterparts in a well-supported clade, whereas the RPS17a was closely related to counterparts from medaka, three-spined stickleback, fugu, zebrafish, carpand RPS17 sequences from spotted gar, coelacanth and tetrapod.Two RPS19paralogs were found in tilapia for the first time(Figure S1I), and they share 84.2% identity at the amino acid level. Similar to RPL5 and RPL19, the RPS19 paralogs also showed a monophyletic origin with tetrapod counterparts as a paraphyletic group, but the RPS19b was only detected in species from Percomorpha, including Perciformes, Cyprinodontiformes and Pleuronectiformes.
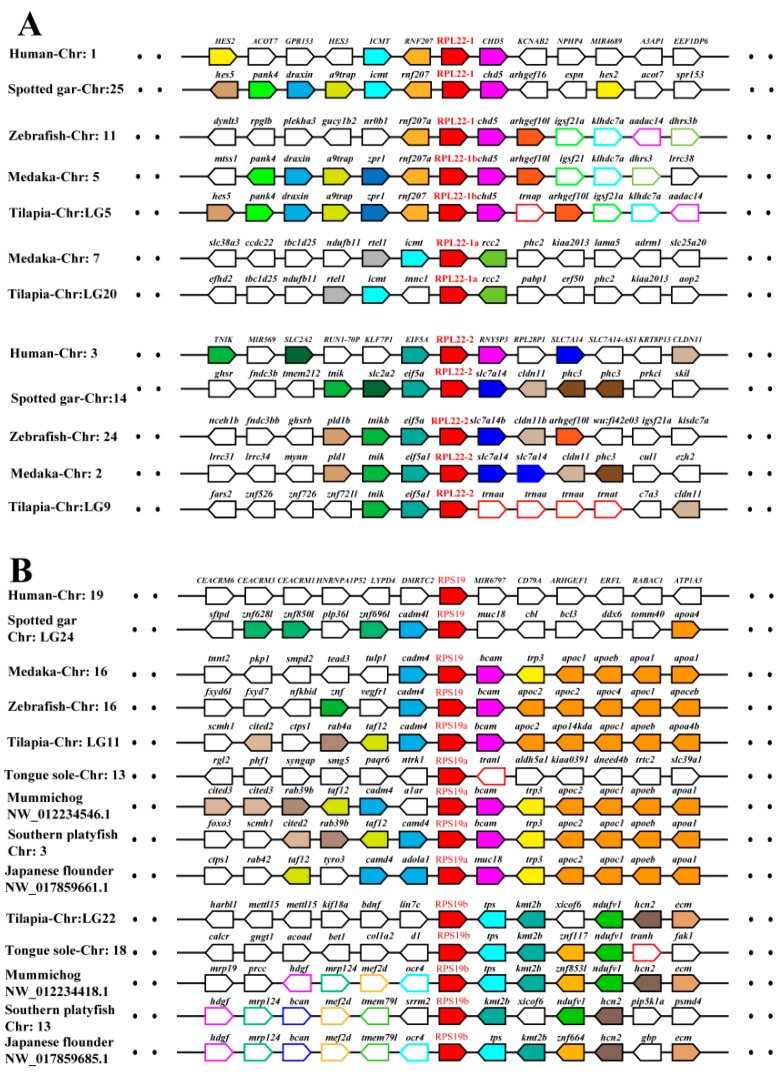
In addition, synteny analysis showed that all the duplicates of tilapia RP genes and their adjacent genes were in regions of conserved synteny in teleosts and other vertebrates (Figure 4 and Figure S2). Phylogenetic and syntenic analyses revealed the existence of paralogous genes from 2R (RPL3, RPL7, RPL22 and RPS27) and 3R (RPL5, RPL19, RPL22, RPL41, RPLP2, RPS17, RPS19 and RPS27). RPL3 and RPL7 only experienced 2R event, and RPL22 and RPS27 experienced both 2R and 3R events. In addition, duplication ofRPS19 was observed only in Percomorpha. Duplicates of RPL5, RPL19, RPL41, RPLP2, RPS17 and RPS19were clustered in two independent clades in the teleost lineage, whereas only one copy was found in one clade in tetrapods and spotted gar. These results indicated that those duplicates were derived from 3R event after teleost fish diverged from spotted gar.
Figure 4.
Synteny analyses of RPL22 (A) and RPS19 (B) and their adjacent genes in tilapia and other vertebrates. Rectangles represent genes in chromosome/scaffold. Dotted lines represent omitted genes of the chromosome/scaffold. The direction of the arrows indicates the gene orientation. The RP genes are shown in red, while the other genes are shown in different color.
2.4. Tissue Distribution and Ontogeny Expression of RP Genes in Gonads of Tilapia by Transcriptomic Analysis
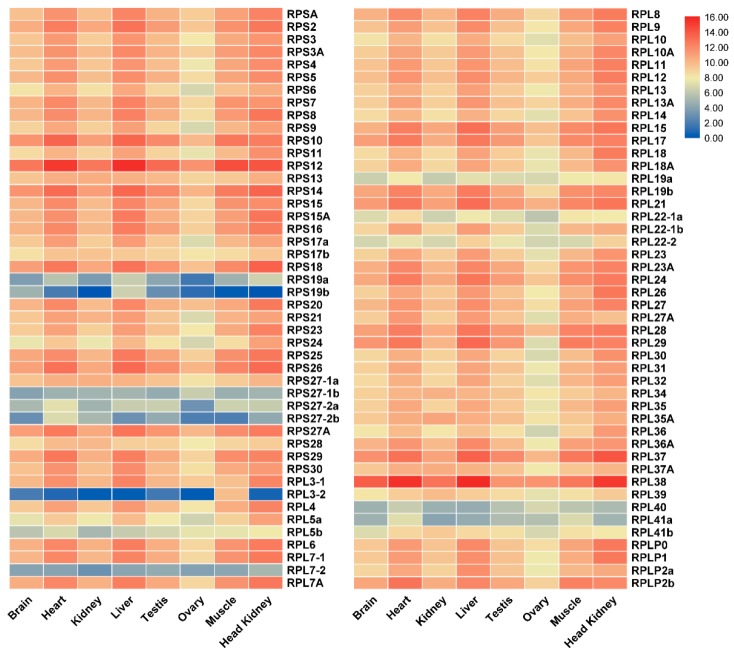
Transcriptome data from eight adult tissues and gonads from four developmental stages of tilapia were analyzed to understand the expression profile of RP genes. The RP genes were found to be expressed in all tissues examined (Figure 5). Interestingly, most RP genes exhibited tissue-biased expression patterns especially in heart, liver, muscle and head kidney. RPS10, RPS12, RPS14, RPS18, RPS26, RPL37 and RPL38 were highly expressed, while RPL3-2 showed background expression level in all eight tissues. In addition, RPL38 was the most highly expressed in all eight tissues.
Figure 5.
A heat map showing tissue distribution (RPKM: reads per kb per million reads) of RP genes in eight tissues based on transcriptome data in tilapia. Red and blue indicate high and low expression, respectively. Each row represents a different gene, and each column represents an independent tissue sample. The widespread complex expression patterns in all tissues were readily discernable. Most of the RP genes displayed high and ubiquitous expression in all tissues examined.
Most RP genes were generally highly expressed in tilapia gonads (Figure 6). The total and average reads per kb per million (RPKM) values were 804,840 and 8748 for XX gonads, and 866,548 and 9419 for XY gonads, respectively (Table 2). No sex- and stage-specific RP genes were observed. With regard to single RP genes in the four developmental stages, 57 and 69 RP genes were found to express at an average RPKM value above 1000 in XX and XY gonads, respectively. In contrast, some RP genes were rarely expressed both in XX and XY gonads at the four developmental stages, including RPL3-2, RPL7-2, RPS19b, RPS27-1b and RPS28, which were present at an average RPKM value lower than 100.
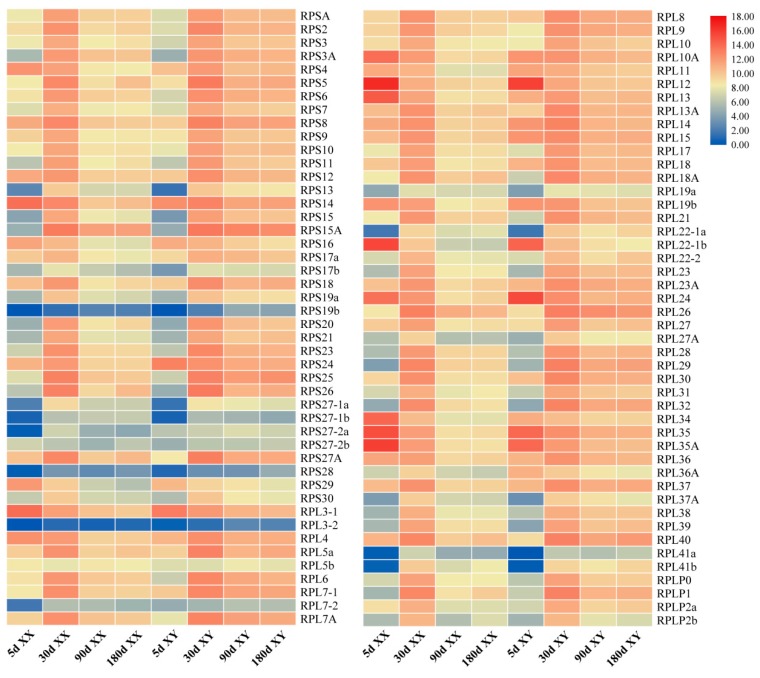
Figure 6.
The expression profiles of RP genes in XX and XY gonads based on transcriptome data from tilapia. RNA preparations from gonads of XX and XY fish at 5, 30, 90 and 180 dah (day after hatching) were sequenced previously [30]. RPKM (reads per kb per million reads) was used to normalize the expression profiles of RP genes.
Table 2.
Statistics of RP gene expressions in tilapia gonads at four developmental stages.
| 5 dah | 30 dah | 90 dah | 180 dah | |||||
|---|---|---|---|---|---|---|---|---|
| XX | XY | XX | XY | XX | XY | XX | XY | |
| Total | 409,026 | 229,345 | 299,234 | 372,252 | 45,903 | 133,854 | 50,678 | 131,097 |
| Average | 4446 | 2493 | 3253 | 4046 | 499 | 1455 | 551 | 1425 |
Total indicates the total RPKM of all RP genes; average indicates the average RPKM of all RP genes.
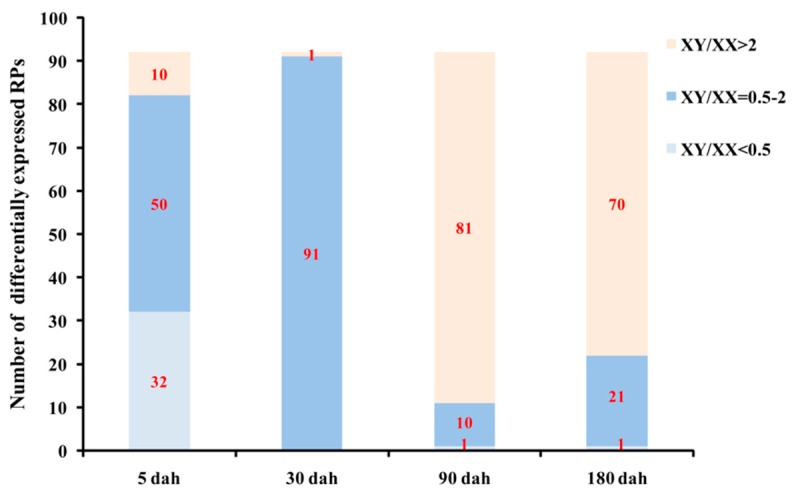
At 5 dah (day after hatching), the total and average RPKM valuesof the RP genes were 409,026 and 4446 for XX gonads, and 229,345 and 2493 for XY gonads, respectively (Table 2). Twenty-eight and 25 RP genes were expressed at RPKM values above 1000 in XX and XY gonads, respectively. Thirty-two RP genes were expressed at 2-fold higher levels in XX than XY gonads (XY/XX RPKM value < 0.5), and 10 genes showed an approximately 2-fold higher expression in XY than XX gonads (XY/XX RPKM value > 2), 50 genes displayed no significant differences in expression level between XX and XY gonads (XY/XX RPKM value = 0.5–2) (Figure 6 and Figure 7).
Figure 7.
Number of differentially expressed RPs in XX and XY gonads of tilapia at 5, 30, 90 and 180 dah.XY/XX ratio was measured by RPKM values.
At 30 dah, the expression levels of RP genes in XX gonads were down-regulated with the total and average RPKM values dropping to 299,234 and 3253, respectively, while in XY gonads they were up-regulated with the total and average RPKM values rising to 372,252 and 4046, respectively (Table 2). Seventy-one and 72 genes were expressed at RPKM values above 1000 in XX and XY gonads, respectively. At this stage, all RP genes except one (RPS19b with RPKM values of 1.47 and 3.76 in XX and XY gonads, respectively) displayed no significant differences in expression level between XX and XY gonads (XY/XX RPKM value= 0.5–2) (Figure 6 and Figure 7).
At 90 dah, the expression levels of RP genes were remarkably down-regulated in both XX and XY gonads, with the total and average RPKM values decreased to 45,903 and 499 for XX and 133,854 and 1455 for XY, respectively (Table 2). Six and 55 RP genes were expressed at RPKM values above 1000 in XX and XY gonads, respectively. Eighty-one out of 92 genes (88%) were expressed 2-fold higher in XY than XX gonads (XY/XX RPKM value > 2), and only 1 RP gene was expressed at 2-fold higher levels in XX than XY gonads (XY/XX RPKM value < 0.5), 10 genes displayed no significant differences in expression level between XX and XY gonads (XY/XX RPKM value = 0.5–2) (Figure 6 and Figure 7).
At 180 dah, the expression patterns of RP genes in both XX and XY gonads were similar to those at 90 dah, the total and average RPKM values of the RP genes were 50,678 and 551 for XX gonads, and 131,096 and 1425 for XY gonads, respectively (Table 2). Ten and 55 RP genes were expressed at RPKM values above 1000 in XX and XY gonads, respectively. Seventy out of 92 genes (76%) were expressed at 2-fold higher levels in XY than XX gonads (XY/XX RPKM value > 2), and only 1 RP gene was expressed at 2-fold higher levels in XX than XY gonads (XY/XX RPKM value < 0.5), 21 genes displayed no significant differences in expression level between XX and XY gonads (XY/XX RPKM value = 0.5–2) (Figure 6 and Figure 7).
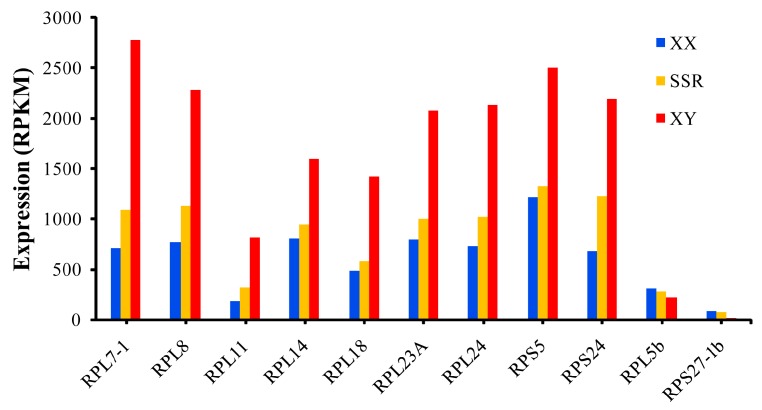
Overall, the number and expression levels of differentially expressed RP genes were higher in XX than in XY gonads at 5 dah stage, while contrasting expression patterns were observed at 90 and 180 dah stages. At 30 dah stage, all RP genes except one (RPS19b) displayed no significant differences in expression level between XX and XY gonads. In addition, 11 RP genes displayed sexually dimorphic expressionin the gonad with 9 expressed higher in XY gonad and 2 higher in XX gonad at all stages examined. Transcriptome analysis of gonads from the sex-reversed fishrevealeda reversed expression profile of these 11 RP genes, indicating the sexual dimorphism is phenotypicsex dependent (Figure 8). Taken together, our results revealed a strong sex- and stage-dependent expression pattern of RP genes in tilapia gonad.
Figure 8.
Sexually dimorphic expression of RP genes in XX and XY gonads based on gonadal transcriptome data from normal and secondary sex-reversal (SSR) tilapia [31]. SSR tilapia were obtained by feeding XX fish a diet sprayed with 95% ethanol containing Fadrozole at a concentration of 200 μg/g diet from 90 to 180 dah. The control XX and XY fish were fed a diet sprayed with 95% ethanol but without Fadrozole. RNA preparations from gonads of XX, XY and sex-reversed individuals at 180 dah were sequenced using Illumina 2000 HiSeq technology in our previous study. RPKM (reads per kb per million reads) was used to normalize the expression profiles of RP genes.
2.5. Validation by qRT-PCR and Immunohistochemistry
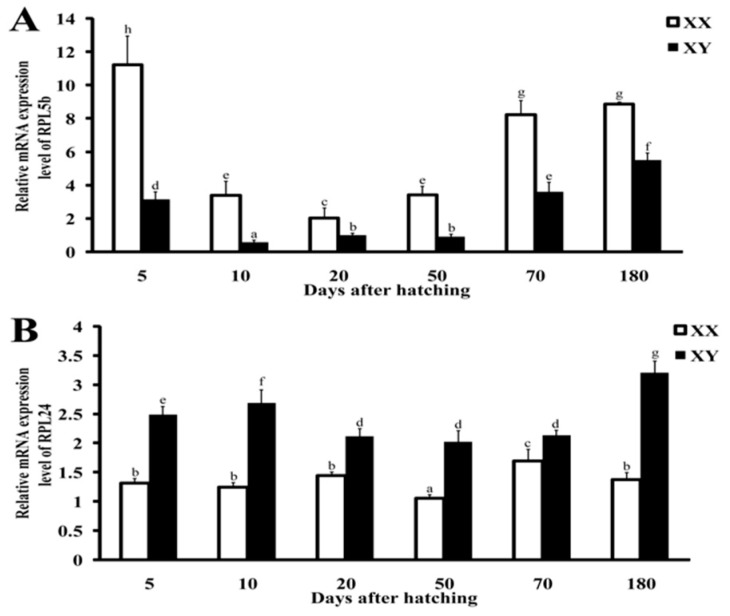
Immunohistochemistry (IHC) and qRT-PCR were performed to validate the transcriptome data. RPL5b and RPL24, the most significantly differentially expressed genes in XX and XY gonads and with commercial antibodies available, were selected for validation. By qRT-PCR, RPL5b was continuously expressed higher while RPL24 was continuously expressed lower in XX than XY gonads at 5, 10, 20, 50, 70 and 180 dah, even though their expression varied at different stages of gonad development (Figure 9).
Figure 9.
Ontogeny expression of RPL5b (A) and RPL24 (B) in tilapia gonads by qRT-PCR. Data are expressed as mean±SD for triplicates. Bars bearing different letters differ (p < 0.05) by one-way ANOVA followed by post-hoc test.
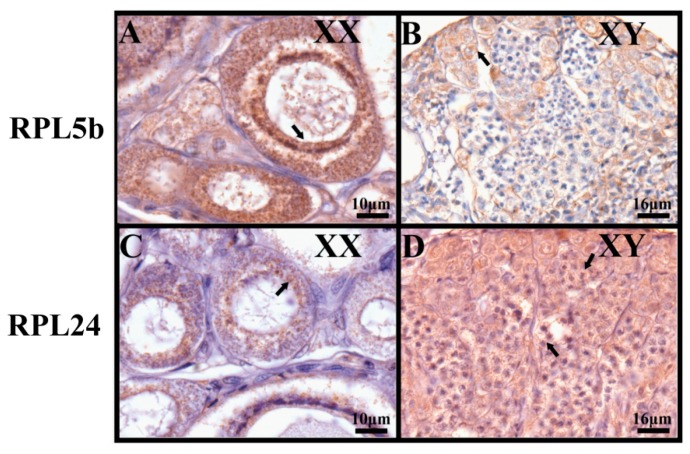
By immunohistochemistry, strong specific signals of RPL5b were observed mainly in the cytoplasm of oocytes in the ovary, while weak signals were detected in the spermatocytes in the testis. However, nearly no signals were detected in other spermatogenic cells (Figure 10A,B). In contrast, RPL24 was found to be expressed ubiquitously at high levels in different spermatogeniccells, while very weak signals were observed in the cytoplasm of oocytes in the ovary (Figure 10C,D).
Figure 10.
Sexually dimorphic expression of RPL5b and RPL24 in tilapia ovary and testis by immunohistochemistry. Samples were taken at 120 dah. Signals of RPL5b were observed mainly in the cytoplasm of oocytes in the ovary (A), while weak signals were detected in the spermatocytes in the testis (B). In contrast, very weak signals of RPL24 were observed in the cytoplasm of oocytes in the ovary (C), while strong signals were detected ubiquitously in different spermatogenic cells (D). Black arrows indicate positive signals. Scale bar, A and C, 10 μm; B and D, 16 μm.
3. Discussion
3.1. Evolution of RP Genes in Chordates
In prokaryotic genomes, RP genes were found to be clustered in operons [32]. In the Arabidopsis genome, RP genes were reported not to be uniformly distributed with much higher density in several regions [33]. In rice, the RPS genes were found to be distributed throughout the rice genome. Both arms of the chromosome randomly carried the RPS genes. Each chromosome carried at least one member of the RPS gene family [34]. In humans, RP genes are widely scattered across the genome, both sex chromosomes and 20 autosomes (all but chromosomes 7 and 21) were found to carry one or more RP genes [15]. In the present study, the complete set of 92RP genes were randomly distributed throughout the tilapia genome, with each LG carrying one or more genes, similar to the RP gene distribution pattern observed in rice and humans.
Ribosomal proteins are indispensable in ribosome biogenesis and protein synthesis, and play a crucial role in diverse developmental processes. The rapid development of genome sequencing and bioinformatics has increased the availability of complete sets of RPs for a wide range of species, which allowed their application in phylogenetic analysis [35,36,37,38]. The identification and characterization of the RPs in channel catfish, Senegalese sole and Atlantic halibut add more molecular markers for studying genome evolution and phylogenetic relationships in teleosts [16,17,18,19]. However, the exact number of RP genes in chordates, especially in teleosts, has not been fully understood yet. In this work, we identified 79, 64, 86, 84, 83, 80, 85, 125, 90, 148, 88, 92 and 86 RP genes in the sea squirt, lamprey, coelacanth, human, mouse, spotted gar, zebrafish, common carp, channel catfish, Atlantic salmon, medaka, tilapia and fugu genome, respectively. We also updated the number of the RP genes from 80 [15] to 84in humans because of the isolation of four more paralogs of RPS27, RPL3, RPL7 and RPL22 from the human genome. These results revealed that the number of RP genes does not change much in chordates following 2R and 3R events, and significant expansion of RP genes is only observed in teleost fishes with 4R. This work should serve as a basis to allow comparative analysis of genome evolution and function of RP genes inchordates, especially in teleosts.
Four rounds of large-scale genome duplications (referred to as 1R, 2R, 3R and 4R) shaped genome evolution in fish [39,40]. Whole genome duplication events, followed by deletion or decay of some of the RP genes, are the major contributors to the diversity of models of evolution of RP genes. RP genes, which are highly expressed, exist in many copies and are essential for ribosomal function [41,42]. Different gene copies have been described for some RPs in fish. For example, channel catfish have two paralogous genes for RPL5, RPS26 and RPS27 [16,17]. In Senegalese sole, two paralogous genes for RPL13A, RPL19 and RPS27, as well as three different RPL22 genes have been identified. In Atlantic halibut, two paralogous genes for RPL3, RPL7, RPL19, RPL22, RPL41and RPLP2, and four different RPS27 genes were identified [18,19]. In the present study, paralogous genes for10RP types were identified in tilapia genome, of which RPL3, RPL5, RPL7, RPL19, RPL22, RPL41, RPLP2, RPS17 and RPS27 paralogs could be found in most fish species, including channel catfish, Senegalese sole and Atlantic halibut. We newly identified RPS19 paralogs in tilapia, which were also detected in other species from Percomorpha but not in other teleosts, indicating that they are derived from lineage-specific duplication.
RPL3 and RPL7 could have originated from 2R duplications since a two-branch tree topology containing the fish and tetrapod counterparts was observed. The 3R duplication would explain the RPL22-1 paralogs (RPL22-1a and RPL22-1b) observed in tilapia and other teleosts, which is closely phylogenetically related to RPL22-1 found in species without 3R and to RPL22-2 derived from 2R. RPL5, RPL19 and RPS17 obtained a paralogfrom3R duplication in teleosts [17,19], and one more for RPL5 in Atlantic salmon and rainbow trout with 4R. Although the previous study indicated the RPS27 gene as a mammalian-specific isoform [43], the identification of four RPS27 genes in tilapia supports the hypothesis of two RPS27 paralogs in tetrapod and at least two in fish as a common feature. The two additional paralogousRPS27genes might have appeared in the 3R or fish-specific genome duplication [16,18]. Both of the two paralogs in tetrapods seem to have duplicated in teleosts, making four RPS27 genes more likely where a few species have lost some of these four. This was further supported by the isolation of eight RPS27 genes in species with 4R. In summary, most of the duplicated RP genes from 2R were lost, but four of them, including paralogs of RPL3, RPL7, RPL22 and RPS27, were retained in vertebrates including human. Two of them (RPL22 and RPS27) even experienced 3R and retained 3 to 4 copies in teleosts. Taken together, our data clearly support the birth-and-death model for the evolution of RP genes.
3.2. Possible Roles of RP Genes in Different Tissues, Especially in Gonads
Spatial and temporal gene expression patterns are important for understanding gene regulation and function [44,45]. RPs are key components of the translational machinery responsible for protein synthesis in all cells, and thereby participate in multiple cellular processes including growth and development [46,47]. In zebrafish, RP genes were found to be expressed in a model of continuous coordinate increase from the onset of mid-blastula transition to hatching [6,48]. In Atlantic halibut, expression levels of 40 and 41 RPs were increased from embryos to 1-day-old yolk sac larvae and in fast skeletal muscle in juveniles, respectively [49]. In channel catfish, Senegalese sole and Atlantic halibut, the expression profiles of RP genes in tissues have been shown to be associated with the protein biosynthetic requirements and cellular demands [16,17,18,19]. In the present study, transcriptomic analysis revealed that the RP genes expressed in all tissues examined, but their expression levels differed among different tissues in tilapia, indicating their essential roles in various physiological processes.
To date, there are a few researches focused on the roles of RPs involved in gonad differentiation and development. In Chinese mitten crab, RPS27 and RPL40 were found to play key roles in gametogenesis and reproductive success [50]. In the present study, RP gene expression profile was obtained from transcriptome analyses of the gonad samples from tilapia at 5, 30, 90 and 180 dah, which represent sex determination and differentiation, initiation of germ cell meiosis in ovary, initiation of germ cell meiosis in testis, and vitellogenesis in ovary and sperm maturation in testis, respectively [30]. In tilapia gonads, most RP genes were highly expressed, and some of them displayed sexually dimorphic expression at different stages of development. At 5 dah, the number and expression levels of differentially expressed RP genes were higher in XX than XY gonads. At 30 dah, all RP genes except one displayed no significant differences between XX and XY gonads. At 90 and 180 dah, the number and expression levels of differentially expressed RP genes were higher in XY than XX gonads. The expression profile of RP genes in XX and XY gonads is similar to that of the steroidogenic enzyme genes and clearly associated with the biosynthesis of different numbers and types of proteins needed for gonadal differentiation and development, as revealed by gonadal transcriptomic analyses [30]. In addition, 11 RP genes displayed sexually dimorphic expression (phenotype dependent as revealed by analysis of the sex-reversed fish) in gonads with 9 (RPL7-1, RPL8, RPL11, RPL14, RPL18, RPL23A, RPL24, RPS5 and RPS24) expressed higher in XY gonad and 2 (RPL5b and RPS27-1b) higher in XX gonad at all stages examined. qRT-PCR and IHC of RPL5b and RPL24 validated the transcriptome data. The sexually dimorphic expression of these 11 RP genes at 5 dah indicates that they may play a crucial role in sex differentiation in tilapia. Furthermore, RPL7-1, RPL8, RPL11, RPL14, RPL18, RPL23A, RPL24, RPS5 and RPS24 may play important roles in the development of testis and spermatogenesis, while RPL5b and RPS27-1b may play important roles in the development of ovary and oogenesis. Sex-specific expression of translation elongation factors eEF1α1b in the testis and 42Sp50 in the ovary have been reported in frogs and several fish species, including tilapia [51,52,53,54]. Taken together, these results indicate that the gonad tightly controls its biosynthesis machinery to meet the needs of proteins required for oogenesis and spermatogenesis.
4. Materials and Methods
4.1. Animal Rearing
Nile tilapia fishes used in this study were reared in recirculating freshwater tanks at 26°C and under natural photoperiod. Animal experiments were performed following the regulations of the Guide for Care and approved by the Institutional Animal Care and Use Committee of Southwest University (No. IACUC-20181015-12, 15 October 2018).
4.2. Identification of RP genes from different chordates
The genomes of 13 species (sea squirt, lamprey, coelacanth, human, mouse, spotted gar, zebrafish, common carp, channel catfish, Atlantic salmon, medaka, tilapia and fugu) were examined to identify RP genes in each species. The genomic sequences of all species are available at the NCBI(https://www.ncbi.nlm.nih.gov/) and Ensembl (http://asia.ensembl.org/index.html) database. All RP genes were identified by tblastn (E = 2e−5) against genome sequences, using zebrafish and human RP proteins as the query sequences. The identified RP genes were named according to the principle described in the previous study [55]. Genomic distribution of RP genes was performed using NCBI and UCSC (http://genome.ucsc.edu/) databases.
4.3. Phylogenetic and Syntenic Analyses
Sequences were analyzed using the EditSeq and Megalign program with the Laser gene sequence analysis software package (DNAStar, Madison, WI, USA). Both amino acid and nucleotide sequences were used, and both maximum-likelihood (ML) and neighbor-joining (NJ) analyses were carried out for RP genes using MEGA 6.0 software (Tempe, AZ, USA) [56]. Phylogenetic trees for the small and large subunit RPs were generated by ML method with the amino acid sequences. Phylogenetic trees of the paralogous RP genes were constructed by NJ method with DNA sequences from a wide range of species. The degree of confidence assigned to nodes in trees was achieved by bootstrapping with 1000 replicates.
For syntenic analysis, position and orientation of RP genes and their adjacent genes on the chromosome were determined using Genomicus (available online: http://www.genomicus.biologie.ens.fr/genomicus-89.01/cgi-bin/search.pl) [53].
4.4. Expression Analyses of Tilapia RP Genes in Adult Tissues and Gonads at Different Developmental Stages
The transcriptomes of eight tissues from adult tilapia, including brain, heart, liver, ovary, testis, kidney, muscle and head kidney (Accession codes: PRJNA78915 and SRR1916191) [28], four pairs of XX and XY gonads from tilapia at 5, 30, 90, and 180 dah (Accession codes: SRA055700) [30] were downloaded from the NCBI database. Gonadal transcriptomes of control and secondary sex reversal (SSR) tilapia (Normal XX fish at 90 dah were fed a diet sprayed with 95% ethanol containing Fadrozole at a concentration of 200 μg/g diet for 90 days, while the control XX and XY fish were fed a diet sprayed with 95% ethanol but without Fadrozole) were reported by our previous study (Accession codes: SRP014017) [31]. Genome-wide expression analysis of RNA-Seq data was performed by TopHat2 v2.1.1 and Cufflinks [57]. RPKM was used to normalize the expression profile of RP genes. Only those transcripts mapped to unique loci in the tilapia genome were used to calculate RPKM values. Identification of XX/XY-enriched RP genes was performed as described previously [58,59]. Detection of genes differentially enriched was analyzed with Tbtools [60].
4.5. Validation of Differentially Expressed Genesby qRT-PCR and IHC
Two differentially expressed RP genes between ovaries and testes, RPL5b and RPL24, were selected to perform qRT-PCR analyses. The results were compared with the transcriptome data. To perform qRT-PCR, gonads were dissected from XX and XY tilapia at 5, 10, 20, 50, 70 and 180 dah, and total RNA was isolated from each sample and reverse-transcribed using MMLV reverse transcriptase (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s protocol. Gene-specific primers, RPL5b-F 5′- CCTGGTGCCTTCACGTGTTA-3′, RPL5b-R 5′- GTAGCCGGGGAAACGTTTC-3′, RPL24-F 5′- CCGATACGCCAGGATAGACG-3′, RPL24-R 5′- CCTTCTTGTGCTTGCGTCTG-3′, were used for qRT-PCR. qRT-PCR examination was performed according to the manufacturer’s protocol using the SYBR Green I Master Mix (TaKaRa, Dalian, China). Tilapia eef1a1a was used as internal control to normalize the expression of these two genes. The relative abundance ofmRNA transcripts was evaluated using the formula R = 2−ΔΔCt, as described previously [61]. Data are expressed as mean ± SD for triplicates. The statistical package GraphPad Prism (GraphPad Software, Inc. San Diego, CA, USA) was used to analyze data from qRT-PCR experiments. One-way ANOVA followed by posthoc test was performed to determine the significance. p < 0.05 was considered to be significantly different.
To validate which population of cells in the developing gonads expressed RPL5b and RPL24, IHC was performed using ovaries and testes from tilapia at 120 dah. Gonads were dissected, fixed in Bouin’s solution at room temperature overnight, embedded in paraffin and sectioned at 5 μm thickness. Paraffin sections were de-paraffinized and hydrated. The primary antibodies anti-mouse RPL5b(ARP56126_P050) and RPL24(ARP65377_P050) were purchased from AVIVA (Beijing AVIVA Systems Biology, Beijing, China). After overnight incubation with freshly diluted primary antibody (1000 times dilution) at 4 °C, the slides were washed twice in 1×PBS (phosphate-buffered saline) for 10 min, and then, incubated with anti-mouse IgG at room temperature for 1 h. After washing, 3,3′-diaminobenzidine tetrahydrochloride was applied for the color reaction, the slides were then counterstained in hematoxylin, dehydrated and mounted [62].
5. Conclusions
Comparative analyses of the RP genes in tilapia and other chordates provide a clear perspective on the evolution of RP genes. The number of the RP genes does not change much in vertebrates following 2R and 3R events, and significant expansion of RP genes was only observed in teleost fishes with 4R. The RP genes were found to be expressed in all tissues examined, but their expression levels differed among different tissues in tilapia, indicating their essential roles in various physiological processes. In addition, we also found, for the first time, that some of them displayed sexually dimorphic expression in developing gonads in fish. Taken together, these results present a new perspective to understand the evolution and function of RP genes in chordates and even other organisms. Future functional characterization of these RP genes with sexually dimorphic expression in teleosts will help us to better understand their important roles in sex differentiation and gonad development.
Acknowledgments
This work was supported by grants 31861123001, 31630082, 31872556 and 31972778 from the National Natural Science Foundation of China; grant 2018IB019 from Yunnan Science and Technology project.
Abbreviations
| RP | ribosomal protein |
| WGD | whole genome duplication |
| 1R | first round of genome duplication |
| 2R | second round of genome duplication |
| 3R | third round of genome duplication |
| 4R | fourth round of genome duplication |
| qRT-PCR | quantitative real time polymerase chain reaction |
| IHC | Immunohistochemistry |
| ML | maximum-likelihood |
| NJ | neighbour-joining |
| dah | day after hatching |
| RPKM | reads per kb per million reads |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/4/1230/s1.
Author Contributions
Conceptualization, G.K. and D.W.; formal analysis, G.K., W.T., S.Z. and X.W.; methodology, G.K., W.T., S.Z. and X.W.; project administration, D.W.; software, W.T., S.Z. and X.W.; supervision, D.W.; validation, G.K.; writing—review and editing, G.K. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China: 31861123001, 31630082, 31872556 and 31972778; Yunnan Science and Technology project: 2018IB019.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.McCann K.L., Baserga S.J. Mysterious ribosome pathies. Sicence. 2013;341:849–850. doi: 10.1126/science.1244156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolze A., Mahlaoui N., Byun M., Turner B., Trede N., Ellis S.R., Abhyankar A., Itan Y., Patin E., Brebner S. Ribosomal protein SA haploinsufficiency in humans with isolated congenital asplenia. Science. 2013;340:976–978. doi: 10.1126/science.1234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das P., Basu A., Biswas A., Poddar D., Andrews J., Barik S., Komar A.A., Mazumder B. Insights into the mechanism of ribosomal incorporation of mammalian L13a protein during ribosome biogenesis. Mol. Cell Biol. 2013;33:2829–2842. doi: 10.1128/MCB.00250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand P., Gruppuso P.A. Rapamycin inhibits liver growth during refeeding in rats via control of ribosomal protein translation but not cap-dependent translation initiation. J. Nutr. 2006;136:27–33. doi: 10.1093/jn/136.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowalczyk P., Woszczynski M., Ostrowski J. Increased expression of ribosomal protein S2 in liver tumors, posthepactomized livers, and proliferating hepatocytes in vitro. Acta. Biochim. Pol. 2002;49:615–624. doi: 10.18388/abp.2002_3770. [DOI] [PubMed] [Google Scholar]
- 6.Mathavan S., Lee S.G.P., Mak A., Miller L.D., Murthy K.R.K., Govindarajan K.R., Tong Y., Wu Y.L., Lam S.H., Yang H., et al. Transcriptome analysis of zebrafish embryogenesis using microarrays. PLoS Genet. 2005;1:260–276. doi: 10.1371/journal.pgen.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutt S., Narla A., Lin K., Mullally A., Abayasekara N., Megerdichian C., Wilson F.H., Currie T., Khanna-Gupta A., Berliner N. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117:2567–2576. doi: 10.1182/blood-2010-07-295238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dionne K.L., Bergeron D., Landry-Voyer A.M., Bachand F. The 40S ribosomal protein uS5 (RPS2) assembles into an extraribosomal complex with human ZNF277 that competes with the PRMT3-uS5 interaction. J. Biol. Chem. 2019;294:1944–1955. doi: 10.1074/jbc.RA118.004928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burwick N., Coats S.A., Nakamura T., Shimamura A. Impaired ribosomal subunit association in Shwachman-Diamond syndrome. Blood. 2012;120:5143–5152. doi: 10.1182/blood-2012-04-420166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Gijn D.R., Tucker A.S., Cobourne M.T. Craniofacial development: Current concepts in the molecular basis of Treacher Collins syndrome. Br. J. Oral. Max. Surg. 2013;51:384–388. doi: 10.1016/j.bjoms.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy N., Kessel R., Bhagat T.D., Bhattacharyya S., Yu Y.T., Mcmahon C., Verma A. Alterations in the ribosomal machinery in cancer and hematologic disorders. J. Hematol. Oncol. 2012;5:32. doi: 10.1186/1756-8722-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai K., Amsterdam A., Farrington S., Bronson R.T., Hopkins N., Lees J.A. Many ribosomal protein mutations are associated with growth impairment and tumor predisposition in zebrafish. Dev. Dyn. 2009;238:76–85. doi: 10.1002/dvdy.21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duan J., Ba Q., Wang Z., Hao M., Li X., Hu P., Zhang D., Zhang R., Wang H. Knockdown of ribosomal protein S7 causes developmental abnormalities via p53 dependent and independent pathways in zebrafish. Int. J. Bioche. Cell Biol. 2011;43:1218–1227. doi: 10.1016/j.biocel.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Wool I.G., Chan Y.L., Gluck A. Structure and evolution of mammalian ribosomal proteins. Biochem. Cell Biol. 1995;73:933–947. doi: 10.1139/o95-101. [DOI] [PubMed] [Google Scholar]
- 15.Uechi T., Tanaka T., Kenmochi N. A complete map of the human ribosomal protein genes: Assignment of 80 genes to the cytogenetic map and implications for human disorders. Genomics. 2001;72:223–230. doi: 10.1006/geno.2000.6470. [DOI] [PubMed] [Google Scholar]
- 16.Karsi A., Patterson A., Feng J., Liu Z. Translational machinery of channel catfish: I. A transcriptomic approach to the analysis of 32 40S ribosomal protein genes and their expression. Gene. 2002;291:177–186. doi: 10.1016/S0378-1119(02)00595-4. [DOI] [PubMed] [Google Scholar]
- 17.Patterson A., Karsi A., Feng J.N., Liu Z.J. Translational machinery of channel catfish: II. Complementary DNA and expression of the complete set of 47 60S ribosomal proteins. Gene. 2003;305:151–160. doi: 10.1016/S0378-1119(02)01183-6. [DOI] [PubMed] [Google Scholar]
- 18.Manchado M., Infante C., Asensio E., Canavate J.P., Douglas S.E. Comparative sequence analysis of the complete set of 40S ribosomal proteins in the Senegalese sole (Soleasenegalensis Kaup) and Atlantic halibut (Hippoglossus hippoglossus L.) (Teleostei: Pleuronectiformes): Phylogeny and tissue- and development-specific expression. BMC Evol. Biol. 2007;7:107. doi: 10.1186/1471-2148-7-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuoka M.P., Infante C., Reith M., Canavate J.P., Douglas S.E., Manchado M. Translational machinery of Senegalese sole (Soleasenegalensis Kaup) and Atlantic halibut (Hippoglossus hippoglossus L.): Comparative sequence analysis of the complete set of 60s ribosomal proteins and their expression. Mar. Biotechnol. 2008;10:676–691. doi: 10.1007/s10126-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 20.Rahman M.K., Kim B., You M. Molecular cloning, expression and impact of ribosomal protein S-27 silencing in Haemaphysalis longicornis (Acari: Ixodidae) Exp. Parasitol. 2019;209:107829. doi: 10.1016/j.exppara.2019.107829. [DOI] [PubMed] [Google Scholar]
- 21.Wade J.L., Tang Y.P., Peabody C.P., Tempelma R.J. Enhanced gene expression in the forebrain of hatchling and juvenile male zebra finch. J. Neurobiol. 2005;64:224–248. doi: 10.1002/neu.20141. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y.P., Wade J. Sexually dimorphic expression of the genes encoding ribosomal proteins L17 and L37 in the song control nuclei of juvenile zebra finches. Brain Res. 2006;1126:102–108. doi: 10.1016/j.brainres.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Y.P., Wade J. Sex- and age-related differences in ribosomal proteins L17 and L37, as well as androgen receptor protein, in the song control system of zebra finches. Neuroscience. 2010;171:1131–1140. doi: 10.1016/j.neuroscience.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acharya K.D., Veney S.L. Sexually dimorphic expression and estradiol mediated up-regulation of a sex-linked ribosomal gene, RPS6, in the zebra finch brain. Dev. Neurobiol. 2013;73:599–608. doi: 10.1002/dneu.22085. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z., Wang Y., Jiang Y., Lin P., Jia X., Zou Z. Ribosomal protein L24 is differentially expressed in ovary and testis of the marine shrimp marsupenaeus japonicus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007;147:466–474. doi: 10.1016/j.cbpb.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Zheng W.L., Xu H.Y., Lam S.H., Luo H., Karuturi K.M., Gong Z.Y. Transcriptomic analyses of sexual dimorphism of the zebrafish liver and the effect of sex hormones. PLoS ONE. 2013;8:e53562. doi: 10.1371/journal.pone.0053562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafedh Y.S.A. Effects of dietary protein on growth and body composition of Nile tilapia, Oreochromisniloticus L. Aquac. Res. 1999;30:385–393. doi: 10.1046/j.1365-2109.1999.00343.x. [DOI] [Google Scholar]
- 28.Brawand D., Wagner C.E., Li Y.I., Malinsky M., Keller I., Fan S., Simakov O., Ng A.Y., Lim Z.W., Bezault E., et al. The genomic substrate for adaptive radiation in African cichlid fish. Nature. 2014;513:375–381. doi: 10.1038/nature13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conte M.A., Gammerdinger W.J., Bartie K.L., Penman D.J., Kocher T.D. A high quality assembly of the Nile tilapia (Oreochromis niloticus) genome reveals the structure of two sex determination regions. BMC Genomics. 2017;18:341. doi: 10.1186/s12864-017-3723-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao W.J., Yuan J., Zhou L.Y., Sun L.N., Sun Y.L., Yang S.J., Li M.H., Zeng S., Huang B.F., Wang D.S. Characterization of gonadal transcriptomes from Nile tilapia (Oreochromis niloticus) reveals differentially expressed genes. PLoS ONE. 2013;8:e63604. doi: 10.1371/journal.pone.0063604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L.N., Jiang X.L., Xie Q.P., Yuan J., Huang B.F., Tao W.J., Zhou L.Y., Nagahama Y., Wang D.S. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology. 2014;155:1476–1488. doi: 10.1210/en.2013-1959. [DOI] [PubMed] [Google Scholar]
- 32.Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu. Rev. Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- 33.Barakat A., Szick-Miranda K., Chang I.F., Guyot R., Blanc G., Cooke R., Delseny M., Bailey-Serres J. The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 2001;127:398–415. doi: 10.1104/pp.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha A., Das S., Moin M., Dutta M., Bakshi A., Madhav M.S., Kirti P.B. Genome-wide identification and comprehensive expression profiling of ribosomal protein small subunit (rps) genes and their comparative analysis with the large subunit (rpl) genes in rice. Front. Plant Sci. 2017;8:1553. doi: 10.3389/fpls.2017.01553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finn R.D., Tate J., Mistry J., Coggill P.C., Sammut S.J., Hotz H.R., Ceric G., Forslund K., Eddy S.R., Sonnhammer E.L.L., et al. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–D288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arragain S., Garcia-Serres R., Blondin G., Douki T., Clemancey M., Latour J.M., Forouhar F., Neely H., Montelione G.T., Hunt J.F., et al. Post-translational modification of ribosomal proteins structural and functional characterization of rimo from Thermotoga maritima, a radical s-adenosylmethionine methylthiotransferase. J. Biol. Chem. 2010;285:5792–5801. doi: 10.1074/jbc.M109.065516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barthelemy R.M., Chenuil A., Blanquart S., Casanova J.P., Faure E. Translational machinery of the chaetognath Spadella cephaloptera: A transcriptomic approach to the analysis of cytosolic ribosomal protein genes and their expression. BMC Evol. Biol. 2007;7:146–162. doi: 10.1186/1471-2148-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perina D., Cetkovic H., Harcet M., Premzl M., Lukic-Bilela L., Muller W.E., Gamulin V. The complete set of ribosomal proteins from the marine sponge Suberites domuncula. Gene. 2006;366:275–284. doi: 10.1016/j.gene.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Hoegg S., Brinkmann H., Taylor J.S., Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J. Mol. Evol. 2004;59:190–203. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- 40.Berthelot C., Brunet F., Chalopin D., Juanchich A., Bernard M., Noel B., Bento P., Da Silva C., Labadie K., Alberti A. The rainbow trout genome provides novel insights into evolution after whole-genome duplication in vertebrates. Nat. Commun. 2014;5:3657. doi: 10.1038/ncomms4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blomme T., Vandepoele K., De Bodt S., Simillion C., Maere S., Van de Peer Y. The gain and loss of genes during 600 million years of vertebrate evolution. Genome Biol. 2006;7:R43. doi: 10.1186/gb-2006-7-5-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Zheng C., Sankoff D. Evolutionary model for the statistical divergence of paralogous and orthologous gene pairs generated by whole genome duplication and speciation. IEEE/ACM Trans. Comput. Biol. Bioinform. 2017;15:1579–1584. doi: 10.1109/TCBB.2017.2712695. [DOI] [PubMed] [Google Scholar]
- 43.Thomas E.A., Alvarez C.E., Sutcliffe J.G. Evolutionarily distinct classes of S27 ribosomal proteins with differential mRNA expression in rat hypothalamus. J. Neurochem. 2000;74:2259–2267. doi: 10.1046/j.1471-4159.2000.0742259.x. [DOI] [PubMed] [Google Scholar]
- 44.Wei L., Yang C., Tao W.J., Wang D.S. Genome-wide identification and transcriptome-based expression profiling of the Sox gene family in the Nile tilapia (Oreochromis niloticus) Int. J. Mol. Sci. 2016;17:270. doi: 10.3390/ijms17030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thisse C., Degrave A., Kryukov G.V., Gladyshev V.N., Obrecht-Pflumio S., Krol A., Thisse B., Lescure A. Spatial and temporal expression patterns of selenoprotein genes during embryogenesis in zebrafish. Gene Expr. Patterns. 2003;3:525–532. doi: 10.1016/S1567-133X(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 46.Fortier S., MacRae T., Sargeant T., Sauvageau G. Regulation of mouse embryonic stem cell fate by ribosomal proteins. Exp. Hematol. 2013;41:S29. doi: 10.1016/j.exphem.2013.05.116. [DOI] [Google Scholar]
- 47.Zhou X., Liao W.J., Liao J.M., Liao P., Lu H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015;7:92–104. doi: 10.1093/jmcb/mjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small C.M., Carney G.E., Mo Q.X., Vannucci M., Jones A.G. A microarray analysis of sex- and gonad-biased gene expression in the zebrafish: Evidence for masculinization of the transcriptome. BMC Genom. 2019;10:579. doi: 10.1186/1471-2164-10-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai J.L., Solberg C., Fernandes J.M.O., Johnston I.A. Profiling of maternal and developmental-stage specific mRNA transcripts in Atlantic halibut Hippoglossus hippoglossus. Gene. 2006;386:202–210. doi: 10.1016/j.gene.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q., Chen L., Wang Y., Li W., He L., Jiang H. Expression characteristics of two ubiquitin/ribosomal fusion protein genes in the developing testis, accessory gonad and ovary of chinese mitten crab, eriocheir sinensis. Mol. Biol. Rep. 2012;39:6683–6692. doi: 10.1007/s11033-012-1474-6. [DOI] [PubMed] [Google Scholar]
- 51.Deschamps S., Morales J., Mazabraud A., Le M.M., Denis H., Brown D.D. Two forms of elongation factor 1 alpha (EF-1 alpha and 42Sp50), present in oocytes, but absent in somatic cells of Xenopus laevis. J. Cell Biol. 1991;114:1109–1111. doi: 10.1083/jcb.114.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chambers D.M., Peters J., Abbott C.M. The lethal mutation of the mouse wasted (wst) is a deletion that abolishes expression of a tissue-specific isoform of translation elongation factor 1α, encoded by the eEFla2 gene. Proc. Natl. Acad. Sci. USA. 1998;98:4463–4468. doi: 10.1073/pnas.95.8.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckhardt K., Troger J., Reissmann J., Katschinski D.M., Wagner K.F., Stengel P., Spielmann P., Stiehl D.P., Camenisch G., Wenger R.H. Male germ cell expression of the pas domain kinase paskin and its novel target eukaryotic translation elongation factor eEF1A1. Cell Physiol. Biochem. 2007;20:227–240. doi: 10.1159/000104169. [DOI] [PubMed] [Google Scholar]
- 54.Chen J., Jiang D., Tan D., Fan Z., Wei Y., Li M., Wang D. Heterozygous mutation of eEF1A1b resulted in spermatogenesis arrest and infertility in male tilapia, Oreochromis niloticus. Sci. Rep. 2017;7:43733. doi: 10.1038/srep43733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshihama M., Uechi T., Asakawa S., Kawasaki K., Kato S., Higa S., Maeda N., Minoshima S., Tanaka T., Shimizu N., et al. The human ribosomal protein genes: Sequencing and comparative analysis of 73 genes. Genome Res. 2002;12:379–390. doi: 10.1101/gr.214202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D.R., Pimentel H., Salzberg S.L., Rinn J.L., Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muffato M., Louis A., Poisnel C.E., RoestCrollius H. Genomicus: A database and a browser to study gene synteny in modern and ancestral genomes. Bioinformatics. 2010;26:1119–1121. doi: 10.1093/bioinformatics/btq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan J., Tao W.J., Cheng Y.Y., Huang B.F., Wang D.S. Genome-wide identification, phylogeny, and gonadal expression of fox genes in Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2014;40:1239–1252. doi: 10.1007/s10695-014-9919-6. [DOI] [PubMed] [Google Scholar]
- 60.Chen C.j., Chen H., He Y.H., Xia R. TBtools, a toolkit for biologists integrating various biological data handling tools with a user-friendly interface. BioRxiv. 2018 doi: 10.1101/289660. [DOI] [Google Scholar]
- 61.Shved N., Berishvili G., D’Cotta H., Baroiller J.F., Segner H., Eppler E., Reinecke M. Ethinylestradiol differentially interferes with IGF-I in liver and extrahepatic sites during development of male and female bony fish. J. Endocrinol. 2007;195:513–523. doi: 10.1677/JOE-07-0295. [DOI] [PubMed] [Google Scholar]
- 62.Li M.H., Sun Y.L., Zhao J., Shi H.J., Zeng S., Ye K., Jiang D.N., Zhou L.Y., Sun L.N., Tao W.J., et al. A tandem duplicate of anti-Mullerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genet. 2015;11:e1005678. doi: 10.1371/journal.pgen.1005678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.