Abstract
Adipose tissue is comprised of adipocytes and many other cell types which engage in dynamic crosstalk in a highly innervated and vascularized tissue matrix. Although studied for decades, it is only recently appreciated that extensive arbors of sensory and sympathetic nerve fibers play a dominant role in regulation of adipose functions. Here we summarize recent literature suggesting adipocytes signal to local sensory nerve fibers in response to perturbations in lipolysis and lipogenesis. Such adipocyte signaling to the central nervous system (CNS) causes sympathetic output to distant adipose depots and potentially other metabolic tissues to regulate systemic glucose homeostasis. Recently identified paracrine factors that mediate such adipocyte-neuron crosstalk are reviewed. Similarly, immune cells and endothelial cells within adipose tissue communicate with local nerve fibers to modulate neurotransmitter tone, blood flow, adipocyte differentiation and energy expenditure, including adipose “browning” to produce heat. This understudied field of “neurometabolism” related to adipose tissue biology has great potential to reveal new mechanistic insights and potential therapeutic strategies for obesity and type 2 diabetes.
How fat is stored in mammals sparked vigorous scientific debate in the mid to late 1800s, yielding conflicting concepts and false starts. Major schools of thought proposed that fat accumulates in unspecialized connective tissue cells in the form of multilocular “mulberry” cells, or that “wandering cells” in the plasma accumulate fat and gather in the connective tissue. Another view posited the current concept that adipocytes occupy a specialized “glandular” tissue, but as late as 1901, this hypothesis was the least appealing in a comprehensive review of the literature1. How surprising it would be to these early investigators that adipose tissue is indeed “glandular” in the true sense, functioning as an endocrine organ that can control systemic functions2–5, and that it secretes potent paracrine and autocrine factors that modify its “glandular” nature4,6,7. It would be even more startling for these investigators to learn that adipose tissue can be a major heat generating tissue, critical for many animals to sustain body temperature during extreme cold exposure8,9. Viewed in this context, the multiple roles that adipose tissue plays in mammalian physiology is truly remarkable and unexpected.
During the last couple of decades, it was also revealed that adipose tissue has a strong influence on whole-body glucose and lipid metabolism through its effects on major tissues such as skeletal muscle, liver and brain10. Figure 1A and 1B illustrate several well-studied pathways through which this tissue crosstalk has been shown to operate. First, storage of lipid in white adipocytes, mostly derived from hydrolysis and intracellular re-esterification of triglyceride in circulating lipoproteins11–13, serves not only as a reservoir of calories for future use but also as a means to sequester lipid away from peripheral tissues vulnerable to “lipotoxicity” that disrupts insulin’s actions to control metabolism10,14,15. Thus, in obesity, fatty acids (FA) derived from dietary intake and white adipocyte lipolysis can elevate hepatic gluconeogenesis through the generation of the allosteric effector acetyl CoA16,17 and attenuate skeletal muscle glucose utilization through inhibiting glucose transport and metabolism18–21. These actions, combined with the dampening of pancreatic islet production of insulin, promote glucose intolerance, leading to type 2 diabetes. On the other hand, high rates of FA oxidation observed in adipocytes of brown adipose tissue (BAT) and brown-like “beige” adipocytes in white adipose tissue (WAT) that express uncoupling protein 1 (UCP1) within the mitochondria can also decrease lipid accumulation in peripheral tissues and increase glucose tolerance8. Thus, these main functions of white adipocytes to store fat and brown adipocytes to oxidize fat allow adipose tissue to control systemic insulin sensitivity and susceptibility to metabolic disease. Many reviews have extensively investigated the endocrine regulation of adipocyte lipid metabolism in detail5,10,14,22.
Figure 1: Proposed mechanisms whereby adipose tissue controls systemic insulin sensitivity.
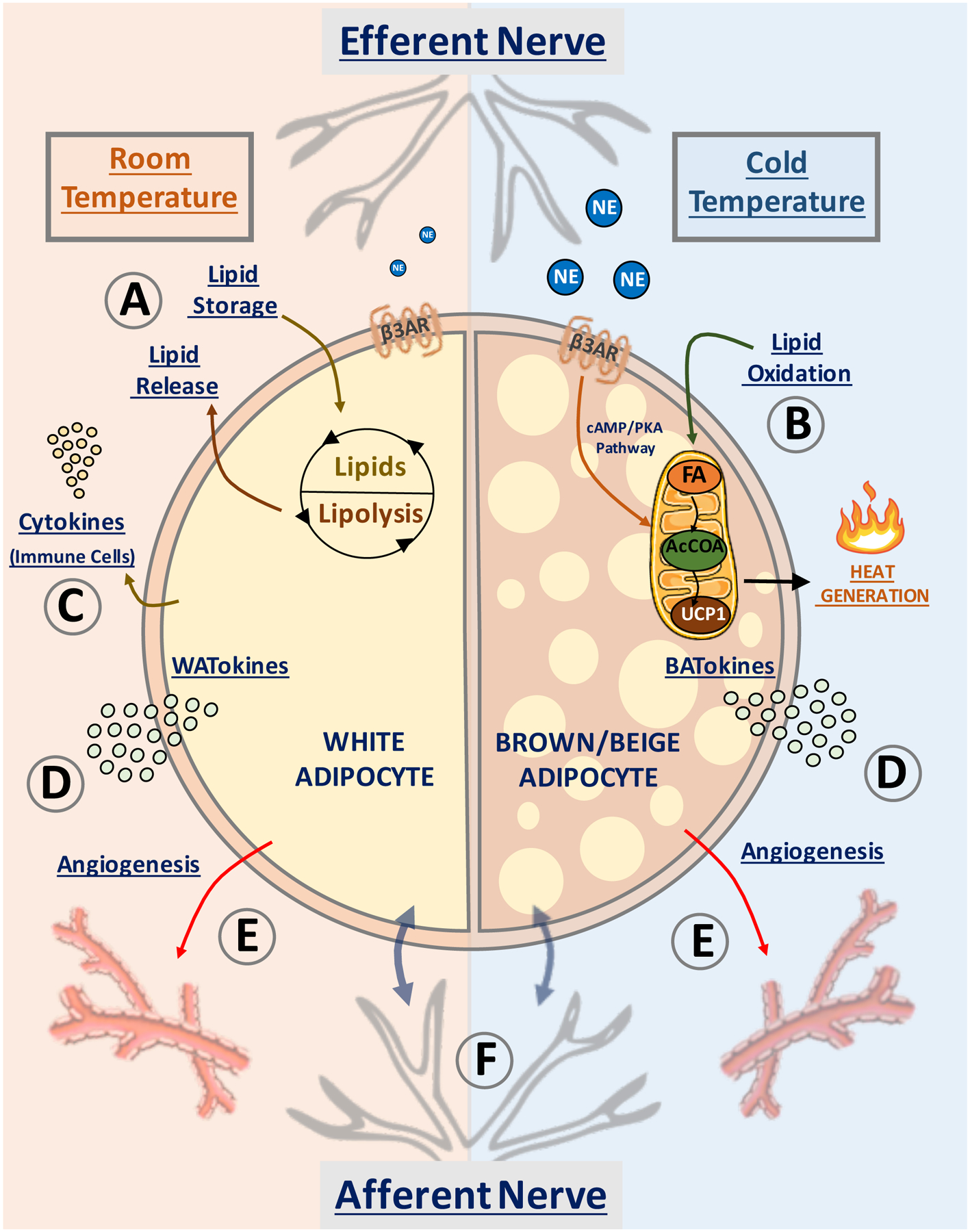
Depicted are pathways in white adipocytes (left semicircle) and beige/brown adipocytes (right semicircle) that have been proposed to affect whole body insulin sensitivity and systemic metabolism. At room temperature (22°C) and above, most of WAT is composed of white adipocytes (left), while at low temperature (6°C) brown/beige adipocytes appear in WAT (right). (A) White adipocytes promote fatty acid esterification into triglycerides for storage, sequestering fat away from liver and skeletal muscle to prevent “lipotoxicity”. (B) Sustained release of norepinephrine (NE) by adipose efferent nerves activates β3-adrenergic (β3AR) receptor and induces “beige” adipocyte formation within white adipose tissue. Beige adipocytes display increased mitochondrial density and high capacity for fatty acid (FA) oxidation into acetyl-CoA (AcCOA) which fuels heat production via mitochondrial uncoupling protein-1 (UCP1) within the electron transport chain. (C) White adipocytes upregulate resident immune cells in obesity, releasing cytokines into the circulation. (D) Secretion of white adipocyte-derived bioactive molecules (denoted WATokines) and beige or brown adipocyte-derived factors (denoted BATokines) may modulate (E) adipose vascularization and (F) activate local afferent nerve fibers. These factors can also be released into the circulation to affect distant tissues.
Figure 1C illustrates that white adipocytes modulate the accumulation and activities of immune cells within adipose tissues, for example, by attracting macrophages upon adipocyte death23 and by secreting paracrine factors such as monocyte chemoattractant protein 1 (MCP1) and tumor necrosis factor-α (TNFα) that attract or activate macrophages, T and B lymphocytes and other immune cells24,25, 26,27. Such cells, in turn, secrete cytokines which can locally disrupt adipose lipid sequestration10,14,28 and lipolysis29 or act systemically to inhibit insulin secretion or insulin action in other tissues30. Adipocytes also secrete factors (Figure 1D) denoted adipokines (“WATokines” from WAT and “BATokines” from BAT) that can directly act on the local vasculature31 (Figure 1E) or on distant tissues with leptin being the prototypic such circulating factor that acts on the brain to suppress appetite and increase energy expenditure32. Each of the above adipocyte-mediated endocrine and paracrine pathways can mediate systemic effects of adipose tissue on metabolic homeostasis.
A growing literature highlights an additional mode of adipose control over systemic metabolism: paracrine signaling from adipose cells to localized nerve fibers (Box 1 and Figure 1F). These adipocyte signals to local nerve fibers can be transmitted to the central nervous system (CNS), which can then initiate output to other tissues. Therefore, adipose-initiated CNS stimulation through local adipose afferent neuronal fibers could be a major modality of adipose control over systemic glucose tolerance and insulin resistance. While there have been some previous reviews on this topic33–36, abundant new relevant data has emerged over the last few years (Box 1), and major new insights have been gained. The aim of this article is to critically review the most recent literature on signaling between adipose cells and local sensory and sympathetic nerve fibers within the tissue, and on the potential to exploit these processes to develop strategies for future therapies targeting metabolic disease.
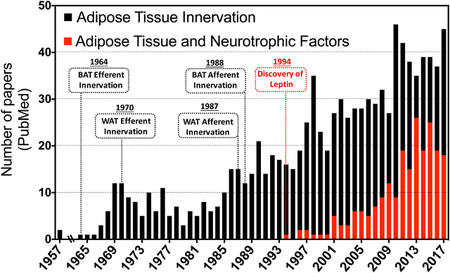
Box 1: Interest in neurotrophic factors in adipose tissue spikes at the turn of the century.
It has long been known that adipose tissue is innervated, but its major significance has only been more recently apparent. Sympathetic nerve fibers within adipose tissue were revealed by the mid-1960s70,234, but evidence of sensory innervation of adipose tissue was not reported until over twenty years later42,235. Beginning in the 1990s a growing number of publications appeared on the role of adipose tissue innervation in adipocyte function. The Bartness laboratory, in particular, generated a deep literature on this topic, and adipose tissue biology experienced a renaissance as well, generating the realization that it is a highly complex endocrine organ with extensive influence over systemic homeostasis. Key discoveries in this field have included the finding that fasting increases adipocyte lipolysis primarily through local release of catecholamines through enhanced adipose sympathetic activity rather than through increased circulating catecholamines132,235. Similarly, it was discovered that the antilipolytic effect of insulin is also in part mediated through inhibition of sympathetic activity at the level of the CNS121,133. The discovery of leptin in 1994 revealed the capacity of adipose tissue to communicate with the CNS and other distant organs through the circulation, and the discovery of many additional adipokines with various potential activities have continued to reinforce this paradigm32. The table, taken from PubMed searches of the terms listed, shows the rapidly expanding discovery of neurotrophic factors emitted by adipose tissue that could directly interact with local nerve fibers and other cell types within the tissue. Such adipocyte-nerve crosstalk has added to our knowledge of the complexity of adipose tissue and its relationship to whole body metabolism, and has created a new perspective on the basis of metabolic disorders such as diabetes, obesity, and cachexia. Targeting these disorders through the neuro-adipose connection offers new strategies for future therapeutic interventions. The number of publications were identified in PubMed and are expressed as total number of results for the specific keywords “Adipose Tissue Innervation” (black bars) and “Adipose Tissue and Neurotrophic Factors” (red bars).
Crosstalk between adipose tissue and local sensory nerve fibers
Sensory Innervation of adipose tissue.
Two modes of communication have been described whereby adipose tissue metabolic status is communicated to the brain. The first is exemplified by the WATokine leptin, which is released from adipose tissue into the circulation and acts on the hypothalamus to regulate appetite and systemic metabolism36,37. The discovery of leptin in 1994 definitively established a functional role of adipocytes as endocrine cells37,38. The second mechanism of adipose to brain communication is mediated through the sensory innervation of adipose tissue, comprising an adipose-neuronal track capable of dissemination of adipose tissue signals over long distances to the CNS39–41. While early studies revealed the presence of sympathetic innervation within adipose tissue (Box1), results demonstrating the presence of adipose sensory nerves and their function are more recent. Using the fluorescent retrograde True Blue, sensory fibers in adipose tissue were clearly observed42. Moreover, immunohistochemical analysis of adipose tissue labeled with antibodies against molecular markers selectively expressed in afferent neurons, such as calcitonin-related gene peptide (CGRP), strongly supported the existence of adipose sensory innervation43,44. Subsequently, a series of elegant studies conducted by Bartness and colleagues showed that adipose tissue is innervated by sensory nerve fibers that convey signals to the brain40,45–48. In those studies, the H129 strain of herpes simplex virus-1, an anterograde tract tracer, was used to track routes between adipose tissue and the brain through sensory nerves45,46.
Adipose signals to the CNS through the above afferent pathway can in turn trigger peripheral responses through sympathetic outflow, as has been reported for WAT signaling to BAT40,48,49. This adipose depot intercommunication may be critical for energy balance and proper control of systemic metabolism40,48,50. Accordingly, selective denervation of sensory fibers within WAT suppresses sympathetic outflow and thermogenesis in BAT triggered by lipolysis in distant WAT depots48. Collectively, these results provide evidence that adipose sensory nerves play a significant role in detecting metabolic cues within adipose tissue and in conveying signals to the brain from such cues, contributing to metabolic homeostasis (Figure 2).
Figure 2: Adipose Signaling to Local Nerve Fibers Regulates Systemic Metabolism.
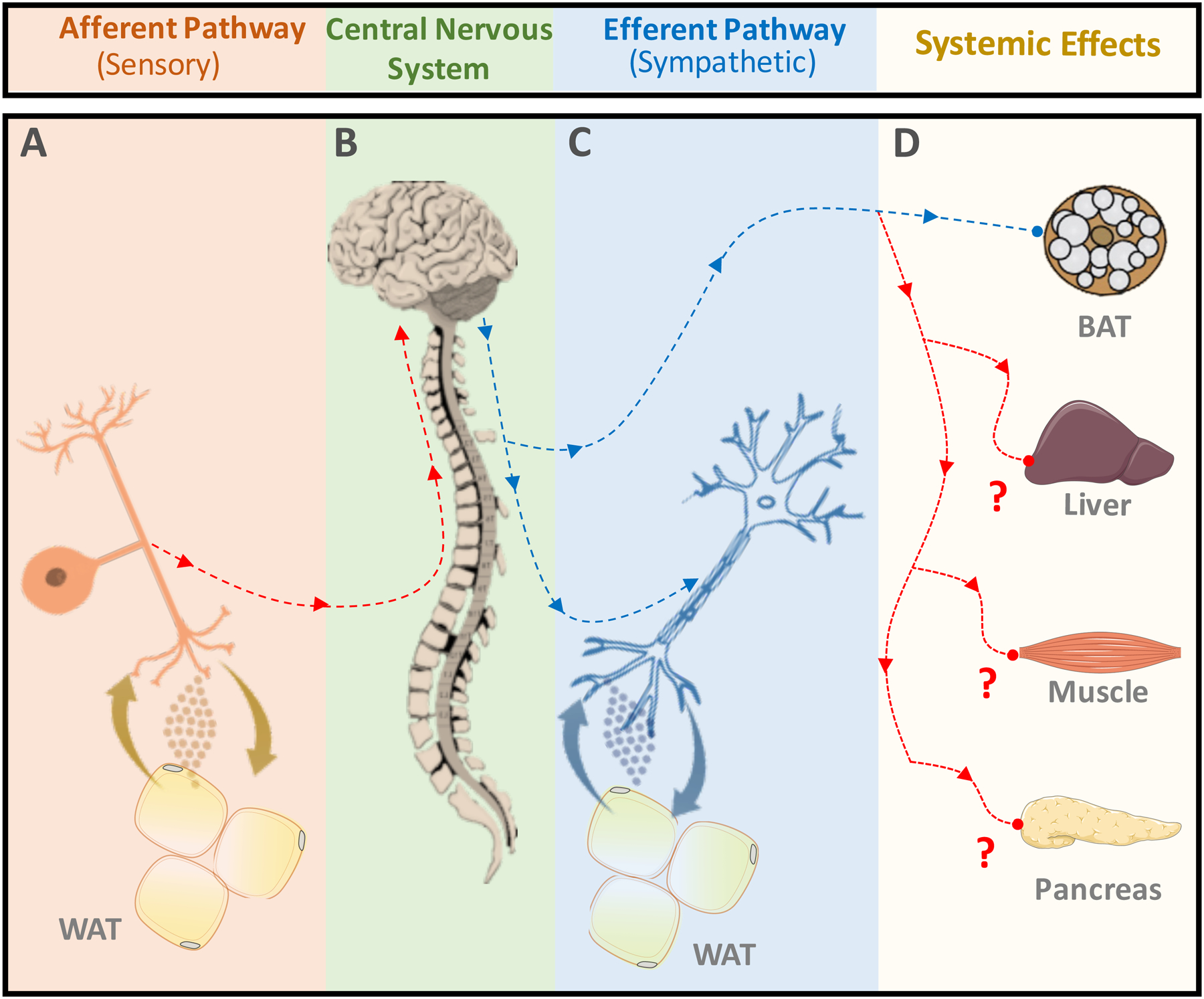
(A) Sensory nerves relay information from the white adipose tissue (WAT) microenvironment to the central nervous system. (B) The central nervous system (CNS) integrates adipose tissue signals to orchestrate a response to the adipose tissue microenvironment. The CNS conveys its response via sympathetic outflow back into the periphery. (C) The sympathetic nerve innervating WAT releases signaling factors that influence the adipose tissue microenvironment. (D) The autonomic nervous system also affects other metabolic organs in order to promote whole-body homeostasis. Whether adipose metabolic cues are conveyed to CNS to control sympathetic outflow into liver, muscle and pancreas (represented in red lines) is still unknown. Thus, the adipose tissue microenvironment may have a role in regulating systemic metabolism through signaling to local nerve fibers. The depicted cartoon illustrates a general concept. The scale in which the diagrams were drawn is not anatomically accurate.
Adipose Tissue Factors that Signal to Sensory Nerves
Leptin.
Recent progress in identifying the adipose-derived molecular signals that regulate local sensory nerve fibers and ultimately modulate adipose-brain communication has been encouraging (Figure 3). Remarkably, the endocrine factor leptin can function in this capacity as a local paracrine factor that modulates local afferent nerve fibers in adipose tissue51,52. Afferent fibers innervating WAT express leptin receptor and are markedly activated upon direct leptin injections into adipose tissue53. Additionally, selective deletion of leptin receptor in vagal sensory neurons disrupts hypothalamic control of food intake and increases the propensity for obesity in mice54. Leptin-stimulated afferent nerve fibers in WAT convey functional signals to the CNS since they elicit enhanced sympathetic drive into adipose tissue55. Central administration of leptin was shown to stimulate the SNS in skeletal muscle as well56. Altogether, these results suggest that leptin is one adipocyte factor that signals to local afferent nerve fibers, communicating the presence of increased fat stores to the brain. Thus, leptin acts both as a circulating factor to control whole body metabolism through hypothalamic regulation as well as a paracrine factor to directly activate adipose afferent fibers that signal to the CNS.
Figure 3: Adipose Tissue-Sensory Nerve Crosstalk.
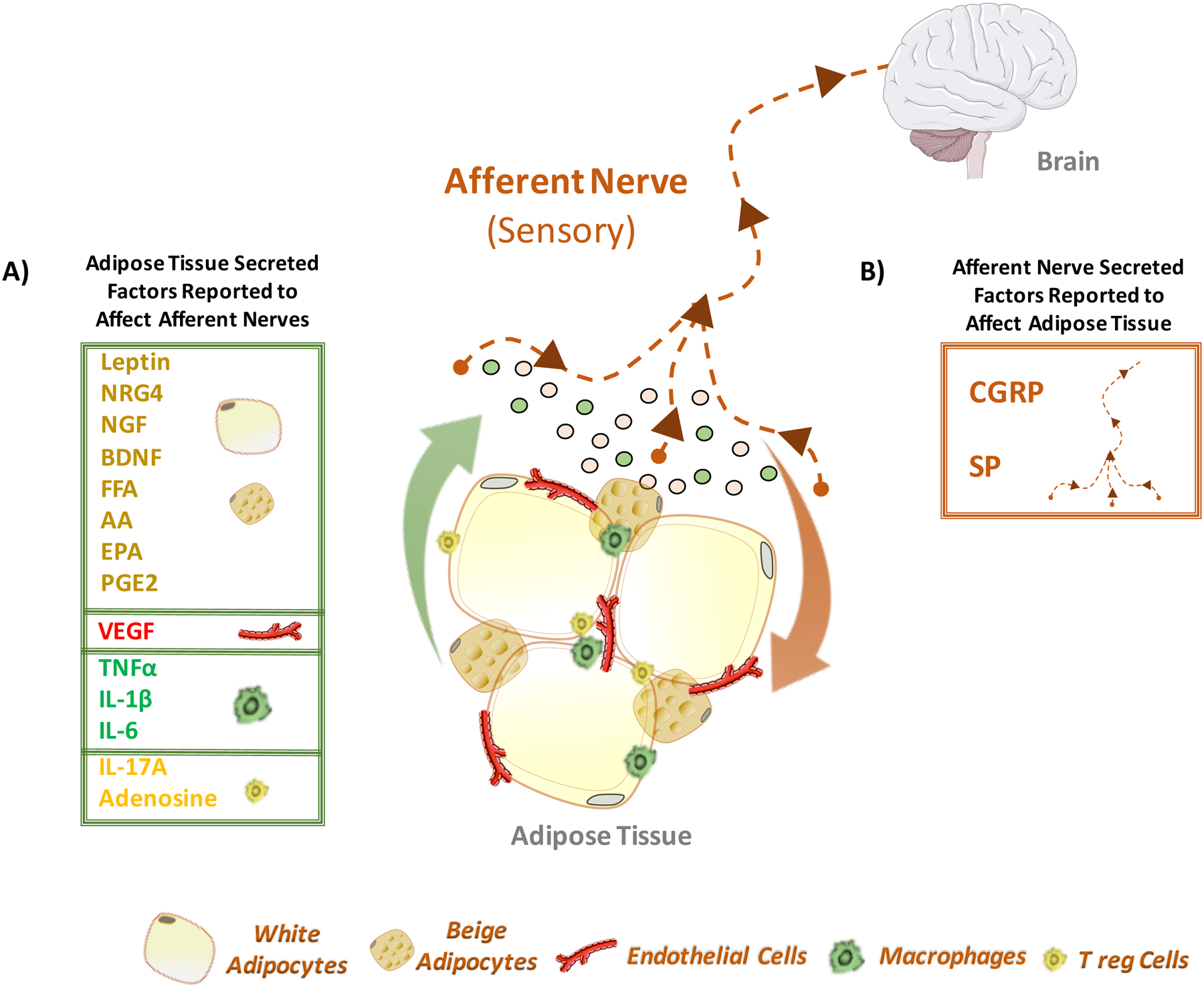
(A) Adipose tissue resident cells release molecular mediators that can act on the afferent neuronal pathway and invoke a central response to the tissue microenvironment. The principle component of adipose tissue, the adipocyte, can release various neuro-active and neurotrophic peptides, such as leptin, neuregulin-4 (NRG4), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), free fatty acids (FFA), arachidonic acid (AA) and eicosanoids, such as eicosapentaenoic acid (EPA) and prostaglandin-E2 (PGE2), that act on surrounding cells, including sensory nerve fibers. Endothelial cells, comprising the vasculature of adipose tissue, secrete vascular endothelial growth factor (VEGF) that can promote nerve sprouting and sensory hypersensitization upon interaction with the VEGF receptor family (VEGFR). Cytokines secreted from macrophages, such as tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6), may act directly on the sensory nerve itself, or indirectly through their pro-inflammatory effects within the adipose tissue microenvironment. Anti-inflammatory cytokines, such as interleukin-17A (IL-17A) and adenosine, originating from alternatively-activated macrophages and various lymphocytes, including regulatory T-cells (Tregs), can act similarly. (B) Conversely, signaling from the sensory nerve terminals to cells within adipose tissue has the potential to modulate adipocyte functions. Upon stimulation, the sensory nerve can release calcitonin gene-related peptide (CGRP) and substance P (SP) into the innervated tissue that modulates the microenvironment surrounding the sensory nerve.
Fatty Acids (FA).
Adipose tissue-derived FA have also been reported to be potent activators of sensory nerve fibers in adipose tissue40, suggesting that local afferent fibers sense adipose tissue lipolysis and communicate this metabolic flux to the CNS (Figure 3). Importantly, in response to local FA release from WAT, the brain increases sympathetic drive to BAT to enhance thermogenesis40. Other bioactive lipids such as arachidonic acid (AA), eicosanoids and their derived lipids can be produced by adipocytes as well as other adipose tissue-resident cells such as immune cells and endothelial cells, and may also act on local sensory nerves40. Nonetheless, further investigation is necessary to more clearly determine the physiological relevance of these bioactive lipids in the activation of adipose sensory nerves.
Adipose-derived Neurotrophic Factors.
Another class of agents released by adipose tissue resident cells that may regulate local afferent fibers are exemplified by several known neurotrophic factors (Figure 3A). These factors are bioactive molecules known to control many aspects of neuronal function in both the peripheral and central nervous system. The neurotrophic molecules are essential for neuronal development, regeneration, survival and maintenance57,58. Examples of adipose-derived neurotrophic factors include neuregulin-4 (Nrg4)59,60, brain-derived neurotrophic factor (BDNF)61 and nerve growth factor (NGF)62. These proteins are powerful regulators of proliferation, survival, migration and differentiation of neurons, and implicated in several systemic nervous system functions. These and other neurotrophic peptides produced by adipose tissue may act on local sensory nerves to either expand innervation or stimulate electrical activity or both61–64. Results demonstrating that sensory neurons express the receptors for NRG4, NGF and BDNF, such as ErbB3/4, TrkA and TrkB, respectively, support this notion65–67. Nonetheless, studies on selective genetic deletion of these receptors in sensory neurons will be necessary to determine whether they actually regulate adipose tissue sensory nerve functions in vivo. A major caveat of this approach, however, is the lack of tools to ablate receptors selectively in adipose tissue sensory nerve fibers without perturbing sensory nerves in other tissues.
VEGF.
A remarkably close anatomical and functional relationship between vascularization and innervation of adipose tissue has been long known68–71. Morphological analysis revealed that fibers innervating adipose tissue juxtapose the vasculature68,69. Moreover, endothelial cells and adipocytes secrete vascular endothelial growth factor (VEGF)31 (Figure 3A), a peptide known to stimulate angiogenesis and promote vascular sympathetic innervation and increase sensory nerve density72–74. Recent findings highlighted the role of adipose VEGF-A in the control of adipose tissue function and in improving systemic energy metabolism and glucose tolerance through the enhancement of adipose vascularization31,75,76. As sensory neurons express VEGF receptor (VEGFR)77, sensory-neuron-specific genetic deletion of VEGFR would be helpful to discover whether VEGF acts through a sensory nerve VEGFR signaling pathway to enhance adipose vascularization, sensory innervation and systemic metabolism.
Cytokines.
White adipocytes communicate with adipose tissue-resident immune cells, such as macrophages and lymphocytes, to modulate cytokine production and thus regulate the levels of adipose inflammation, as occurs in obese rodents and humans25,78,79. Cytokines secreted from macrophages, such as TNFα, interleukin-1β (IL-1β) and interleukin-6 (IL-6), may act directly on the sensory neuron itself80–82, or indirectly through their pro-inflammatory effects within the adipose tissue microenvironment (Figure 3A). On the other hand, anti-inflammatory cytokines, such as interleukin-17A (IL-17A) and adenosine, originating from alternatively-activated macrophages, regulatory T-cells (Tregs) and T-helper cells, can also interact directly with sensory nerve terminals or indirectly through modulation of the adipose microenvironment to elicit neuronal responses83,84. As such, the inflammatory status of adipose tissue is likely to be highly influential on afferent signaling to the CNS. Altogether, these observations suggest that many adipose tissue-derived bioactive molecules function as paracrine factors to regulate local sensory nerves, and that additional such factors await to be discovered.
Sensory Nerve Factors that Signal to Adipose Tissue
Calcitonin Related-Gene Peptide (CGRP).
While adipose resident cells secrete factors that act on local sensory nerve fibers, stimulated sensory nerves may also release factors that modulate adipose tissue function in a way that affects whole-body metabolism (Figure 3B). This phenomenon, known as the axon reflex, allows for afferent fibers to signal directly to the peripheral tissue that they innervate, circumventing central signal integration and response85. An example is CGRP, a member of the calcitonin family of peptides, which is synthesized and released from nerves in the CNS as well as in peripheral tissues86. CGRP expression appears to be concentrated in sensory afferents that innervate the vasculature86, suggesting the endothelium as a primary target for CGRP released by sensory nerves. Accordingly, CGRP acts as a potent vasodilator when it binds to its cognitive calcitonin receptor-like receptor (CLR) present in endothelial cells87. The activation of CLR signaling has also been reported to promote immune cell adhesion and migration through the endothelium and modulate tissue inflammation88. In this regard, it is conceivable that CGRP released by adipose sensory nerves plays a key role in adipose vascular homeostasis and inflammation and thus systemic metabolism. Therefore, the sensory nerve-derived CGRP targets adipose tissue vasculature, modulating tissue metabolism. Recent studies using genetic deletion of the Cgrp gene suggest a functional role of CGRP in adipose tissue metabolism, thermogenesis and energy balance89,90. Whether these phenotypes noted in mice lacking Cgrp gene are due to changes in adipose tissue vascular function and levels of adipose tissue inflammation has not been yet investigated.
Substance P (SP).
Substance P (SP) is a neuropeptide released by peripheral sensory nerves that, similar to CGRP protein, acts as a potent vasodilator and immune cell regulator91 (Figure 3B). SP exerts its biological activity via the high-affinity neurokinin-1 receptor (NK1R) present in endothelial and immune cells92. Interestingly, SP stimulates the expression of several pro-inflammatory cytokines by immune cells93, suggesting a role for this neuropeptide as a mediator of adipose tissue inflammation in obesity and metabolic dysfunctions. Selective genetic deletion of NKR1 receptors expressed in adipose tissue endothelial or immune cells will be necessary to definitively determine whether SP/NKR1 signaling modulates adipose tissue functions and whole-body metabolism.
Sensory Dysregulation in Obesity and Diabetes
A hallmark of adipose tissue dysfunction in obese animal models and humans is a low grade chronic inflammatory state10,78,94. Immune cell infiltration, macrophage proliferation and increased pro-inflammatory cytokine production are noted in adipose tissue in obesity25,78. As inflammatory mediators have been shown to trigger neuritis and disrupt sensory neuron activity95,96, it is conceivable that the increased production of cytokines in adipose tissue may impair the ability of local sensory nerves to convey information from the adipose tissue to the brain. Indeed, sensory nerve dysfunctions in the initial stages of glucose intolerance in obese subjects have been recently reported97.
Thus, obesity-associated adipose dysfunction and chronic inflammation that disrupts local afferent signaling to the CNS may have adverse consequences for adipose tissue functions and whole-body metabolism. Studies designed to test this hypothesis will be of high interest.
It is also possible that adipose tissue sensory nerve fibers contribute to the adipose tissue inflammation in obesity. The adipose tissue expansion and increased release of leptin and free fatty acids (FFA) in obese humans and rodents may cause hyperactivation of local sensory nerve fibers and consequent release of vasoactive and pro-inflammatory neurotransmitters, such as CGRP and SP. This process has been well-studied in other peripheral tissues and is known as neurogenic inflammation98,99. It occurs when activated afferent nerves release inflammatory mediators, triggering tissue inflammation98,99. Importantly, neurogenic inflammation also appears to play a role in neuritis and nerve damage, pathologies often seen in obese and diabetic subjects100,101. Therefore, an exciting hypothesis to test is whether sensory neurogenic inflammation may contribute to adipose inflammation and dysfunction in obesity.
Central Integration of Adipose Signals:
One key area of focus introduced by the findings that adipose tissue depots are innervated by sensory fibers is determining the destination and central function of these afferent fibers. Analyses conducted by the Bartness group applying anterograde tract tracing with HSV H129 demonstrated that sensory innervation of adipose tissue arises from dorsal root ganglion (DRG) neurons45,46. This neuronal tracing method also revealed extensive projections of the adipose afferent network as well as surprising results regarding the anatomy of afferent fibers innervating different adipose tissue depots. For instance, subcutaneous inguinal adipose tissue (iWAT), the WAT depot most susceptible to browning, appears to be entirely innervated by spinal afferents typically associated with somatosensory information. Moreover, no signal arising from the nodose ganglion, which is typically associated with visceral afferents, was detected in iWAT. Thus, this distinct sensory innervation in iWAT likely contributes to its increased ability to promptly respond to changes in physiological conditions such as cold exposure compared to other adipose depots. Importantly, these analyses also revealed that these afferent nerves traversed up the spinal cord, localizing in all areas of the brain - the hindbrain, midbrain, and forebrain. Of these areas, the forebrain is highly relevant to systemic metabolism, as it houses the hypothalamus.
Essential role of the hypothalamus –
The hypothalamus (Figure 4) is essential for regulation of thermogenesis102,103, energy balance and systemic metabolism104–106, integrating signals from peripheral organs and tissues to generate proper physiological responses. In addition to neuroendocrine signaling to the pituitary107, the hypothalamus relies on neuronal circuits and connections to communicate directly with peripheral organs and tissues. One example of such a neuronal circuit is the adipose-hypothalamus-adipose neuronal circuit40,41. As shown in Figure 4A, the pseudounipolar afferent sensory fibers innervating white adipose tissue depots arise from the DRG proximal to the spinal cord. The DRG, which also projects to the brain via the dorsal horn of the spinal cord, relays sensory information from the periphery to the CNS for integration (Figure 4A). Then, primarily through this hypothalamic integration, signals can be conveyed as sympathetic outflow to adipose tissue and peripheral organs via the intermediolateral nucleus of the spinal cord (IML) (Figure 4A).
Figure 4: Central Integration of Adipose Signals and Obesity-mediated Dysregulation.
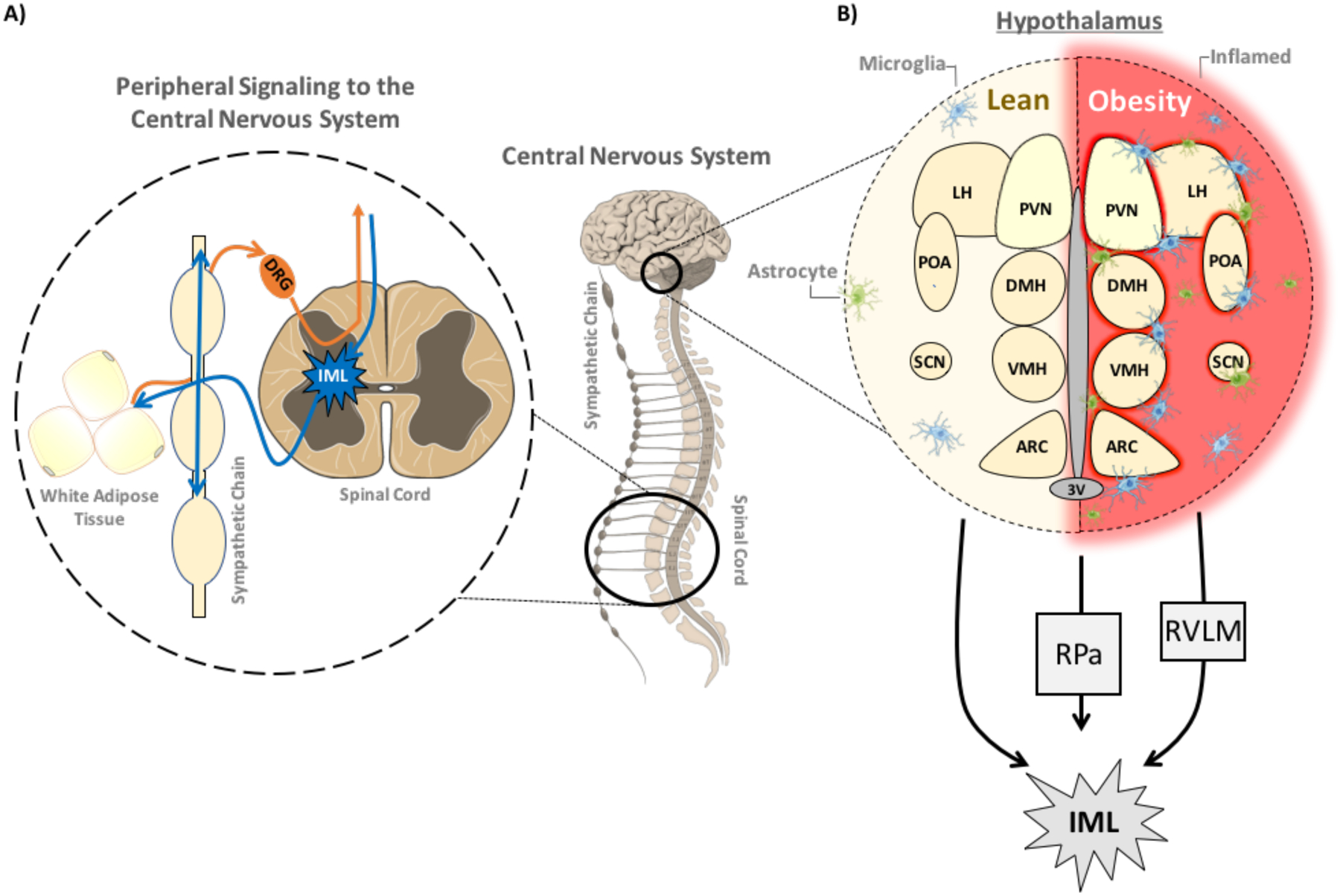
(A) Afferent sensory nerve fibers innervating white adipose tissue depots arise from dorsal root ganglia (DRG) proximal to the spinal cord. The DRG also projects to the brain via the dorsal horn of the spinal cord, relaying sensory information from the periphery to the central nervous system for integration. (B) The hypothalamus is a primary area for metabolic regulation in the central nervous system, influencing thermogenesis and food intake, as well as other critical homeostatic functions throughout the body. Projections into the preoptic area (POA), as well as resident temperature-sensitive neurons, relay critical thermoregulatory information to the dorsomedial hypothalamic nucleus (DMH), a core component of the orexinergic system and thermoregulatory function of the hypothalamus. The paraventricular hypothalamus (PVN), which is proximal to the third ventricle (3V), is involved food intake, thermoregulation and neuroendocrine functions through projections to the pituitary. The arcuate nucleus (ARC), along with the ventromedial nucleus of the hypothalamus (VMH) and lateral hypothalamus (LH), are also involved in appetitive behavior and food reward. The suprachiasmatic nucleus (SCN) is a critical area for regulating circadian rhythm. All of these centers play direct or indirect roles in influencing hypothalamic thermogenic regulation. Hypothalamic inflammation has been linked to metabolic dysregulation and obesity-related insulin resistance through excessive gliosis, leading to neuronal damage, particularly noted within arcuate nucleus. The hypothalamus sends sympathetic projections to periphery either directly through the intermediolateral nucleus of the spinal cord (IML), or via relay through the raphe pallidus nucleus (RPa) or the rostral ventrolateral medulla (RVLM) to the IML. The IML houses the preganglionic neurons responsible for synapsing onto the catecholaminergic postganglionic sympathetic fibers innervating the target tissues.
Subsequent studies utilizing both anterograde HSV H129 tracing and retrograde pseudorabies virus (PRV) tracing from white and brown adipose depots revealed areas of colocalization within the hypothalamus, suggesting the existence of a reflex circuit that converts adipose sensory cues into sympathetic outflow back into adipose tissue39,108. Corroborating this notion, in an extensive screening of central H129 and PRV colocalization from iWAT injections, Bartness and colleagues revealed a high degree of doubly-labeled neurons, upwards of 75%, suggesting an extensive sensory-sympathetic iWAT feedback system47. However, an important point to consider related to these findings is the caveats associated with the use of HSV-H129. In particular, there is the potential for double labeling of afferent and efferent fibers, making the interpretation of the above results less clear. Moreover, as the HSV-129 tracing experiments were conducted on hamsters, replication from these results in other animal species, such as mice will be necessary to extend these findings
An additional remarkable finding was the observation of differential sympathetic outflows to various depots of WAT. Dual PRV tracing of mesenteric WAT (mWAT) and iWAT revealing the sympathetic pathways innervating both tissue depots showed iWAT had considerably more single-labeled neurons within the brain when compared to mWAT109. However, areas more typically associated with sympathetic regulation of metabolism, such as the paraventricular hypothalamic nucleus, lacked such a striking difference in labeling. This finding suggests that the differences observed in metabolic phenotypes associated with subcutaneous versus visceral adiposity may have a neuronal component. If the neural origins of sympathetic innervation vary between adipose depots, there may exist a hardwired differential sympathetic drive to each tissue, further suggesting specialized, depot-specific roles. An earlier study also showed less PRV labeling within the suprachiasmatic, arcuate, and dorsomedial hypothalamic nuclei from epididymal WAT (eWAT) viral injections when compared to iWAT injections110. One should note that when dual tract tracing is performed with the same strain of the virus, there is a potential for one virus to interfere with the infection of the same neuron by the second, leading to an underestimation of dually-labeled neurons. These critical studies indicate that adipose sensory and sympathetic nerves are connected and have the potential for communication via hypothalamic relay, as well as via areas of the hind- and midbrain. Additionally, divergences in these pathways between WAT depots have emerged as provocative areas to probe when addressing the phenotypic disparity contributed by visceral and subcutaneous adiposity. While these adipose-hypothalamus-adipose neuronal signaling axes exist as potential significant networks in metabolic communication, traditional regulators of sympathetic drive, such as central leptin and insulin signaling, remain prominent modes of metabolic signaling within the hypothalamus. It is important to emphasize the essential role of central leptin signaling in the regulation of regional sympathetic nerve activity, energy expenditure and metabolic homeostasis. Excellent reviews discussing the contribution of hypothalamic leptin signaling on SNS activation and glucose systemic metabolism have been published in recent years36,106,111–114.
Hypothalamic insulin signaling –
The functional relevance of adipose-hypothalamic neuronal circuitry for adipose thermogenesis and therefore systemic metabolism was also demonstrated in experiments aimed to investigate the role of hypothalamic insulin signaling in peripheral tissues and systemic metabolism. Insulin was shown to engage with insulin receptors (IR) in two neuronal populations, the cocaine- and amphetamine-related transcript/proopiomelanocortin (CART/POMC) neurons and the NPY/agouti-related peptide (NPY/AgRP) neurons present within the arcuate nucleus (Arc) of the hypothalamus115,116. Insulin signaling in these neurons affects neuronal excitability, peptide expression and stimulates the appetite-suppressing population of POMC neurons, which in turn inhibits the appetite-promoting population of AgRP neurons115,117,118. Importantly, insulin receptor signaling in POMC and AgRP appears to be essential to regulate energy balance and systemic metabolism, as genetic disruption of components in the insulin receptor (IR) signaling pathway in those neurons alter systemic metabolism119,120. For instance, IR deletion in POMC neurons abolishes the ability of insulin to suppress adipose lipolysis, while preserving its ability to suppress hepatic glucose production, and led to the development of hepatic steatosis in mice fed a high-fat diet121. Furthermore, deletion of IR in AgRP neurons impaired insulin’s ability to suppress hepatic glucose production, while its action to inhibit lipolysis was preserved121.
The above observations raise an important question regarding the relevance of central integration for metabolic homeostasis. Results supporting an essential role of CNS and hypothalamus in controlling whole-body metabolism are well established. In one such study, fed, chronic decerebrated rats were shown to be hyperinsulinemic, hyperadiponectinemic and hyperleptinemic when compared to their control counterparts122. The presence of the forebrain was shown to be critical for maintaining normal circulating insulin levels, body temperature, energy expenditure, and a healthy balance of lean and fat mass122. Whether these findings result from a disrupted neuroendocrine axis, disrupted direct sympathetic outflow or a combination of the two should be further investigated. Altogether, these results demonstrate the importance of the central integration of peripheral signals and also the hypothalamic regulation of adipose sympathetic drive and systemic metabolism.
Central Dysregulation in Obesity and Diabetes
Obesity causes significant morphological changes within the hypothalamus in a mouse model. In particular, it enhances inflammation and microglial expansion123–127 (Figure 4B). These observations were extended to obese subjects who displayed increased inflammation and gliosis throughout the mediobasal hypothalamus (MBH), along with functional impairment123,128. Moreover, results describing MBH gliosis linked to obesity and insulin resistance in humans were recently published128,129. Thus, persistent hypothalamic inflammation positively correlates with metabolic dysfunctions in obese animal models and humans. To elucidate whether hypothalamic inflammation is implicated in metabolic dysfunction, recent studies have investigated whether targeting hypothalamic inflammation would improve systemic metabolism. In one study, evidence suggested that the NF-κB pathway is necessary for hypothalamic microgliosis and metabolic dysfunction in obesity130. Through either pharmacological depletion of microglia or inhibition of NF-κB pathway in these cells, hypothalamic inflammation was prevented and a normal systemic energy balance was preserved130. Overall, these results strongly support the notion that hypothalamic inflammation in obesity contributes to metabolic dysfunctions and energy imbalance. Moreover, persistent hypothalamic inflammation can affect central integration and systemic metabolism, disrupting the adipose afferent reflex through uncoupling the adipose microenvironment from the central relay through sympathetic outflow into the periphery.
Crosstalk between adipose tissue and local sympathetic nerve fibers
Sympathetic Innervation of Adipose
Immunohistochemical analysis of selective sympathetic neurons markers, such as the catecholamine biosynthetic enzyme tyrosine hydroxylase (TH), revealed sympathetic fibers in adipose tissue in close anatomical association with the vasculature, similar to adipose afferent nerves68,131. Sympathetic innervation of adipose tissue and release of NE from these nerve fibers are also essential for lipolysis of TAG in white adipocytes during fasting conditions132 (Figure 5). In contrast, the secretion of epinephrine by the adrenal gland does not seem to be required for adipocyte lipolysis in fasting132, supporting the notion that adipose sympathetic drive is indispensable for mobilization of FFA and its use as fuel in other tissues. The importance of adipose sympathetic drive for the proper control of adipose tissue lipolysis is also illustrated by the experiments from Buettner and colleagues in which central insulin administration suppresses lipolysis in peripheral adipocytes133. Thus, insulin acts not only cell autonomously in adipocytes, but also in the hypothalamus to attenuate lipolysis. The latter occurs through insulin-mediated inhibition of sympathetic activity in adipose tissue through unknown mechanisms133. Collectively, these observations provide strong evidence that major physiological pathways that mediate both stimulation and inhibition of adipocyte lipolysis are driven by NE and insulin regulation of sympathetic neurons. Thus, brain to adipocyte communication through sympathetic innervation of adipose tissue appears to be pivotal for homeostatic control of whole-body metabolism50.
Figure 5: Adipose Tissue-Sympathetic Nerve Crosstalk.
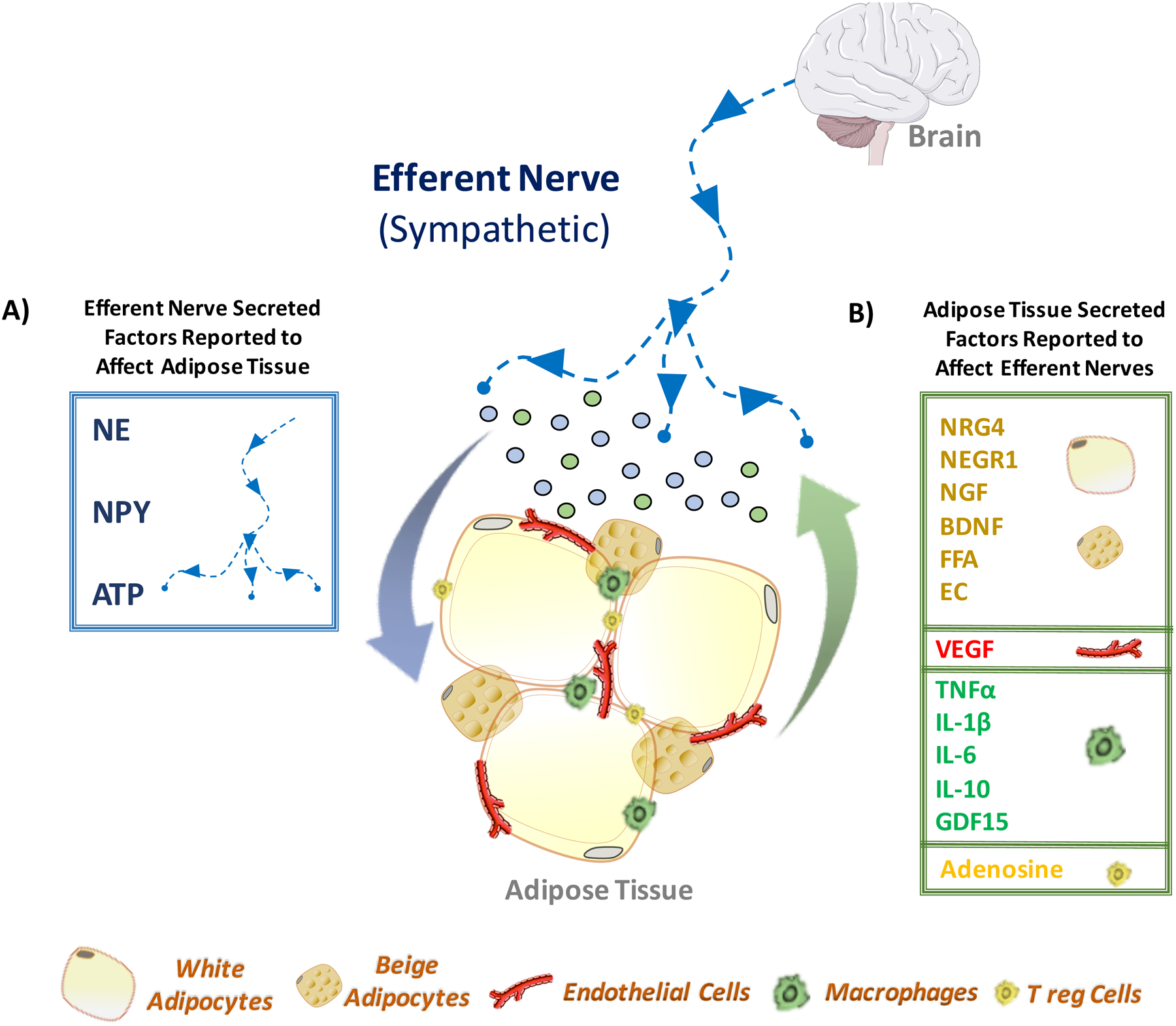
(A) Efferent nerve fibers are known to regulate adipose tissue functions through secretion of bioactive factors. Among them are catecholamine (norepinephrine), neuropeptide Y (NPY) and adenosine triphosphate (ATP). The central nervous system stimulates sympathetic outflow to adipose tissue, triggering the secretion of norepinephrine, NPY or ATP (represented by blue dots). These sympathetic-derived secreted factors, through the activation of their respective receptors, affect not only adipocytes, but also other adipose resident cells, such as endothelial cells, macrophages and lymphocytes. (B) In turn, the stimulated adipose cells also produce a number of secreted bioactive factors that communicate with adipose sympathetic fibers. For instance, multilocular beige adipocytes - induced via sympathetic norepinephrine and/or adenosine molecules - produce the neurotrophic factor neuregulin-4 (NRG4), which is known to promote neurite outgrowth. Additionally, white adipocytes have been shown to synthesize several factors with neurotrophic activity that may enhance sympathetic innervation of adipose tissue. Among these factors are the neuronal growth regulator 1 (NEGR1), nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), free fatty acids (FFA) and endocannabinoids (EC). The vascular endothelial growth factor (VEGF) secreted by endothelial cells and adipocytes elicits sympathetic innervation in adipose tissue. Adipose resident immune cells, such as macrophage and lymphocytes, secrete cytokines, tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL-1β), and factors demonstrated to affect neurite outgrowth and possibly adipose sympathetic innervation. Among them are interleukin-6 (IL6), interleukin-10 (IL10) and growth/differentiation factor 15 (GDF15).
Sympathetic nerve fibers are also detected within the adipose parenchyma, particularly in conditions such as cold stimulus that trigger sympathetic expansion and activity in adipose tissue134,135. The increased sympathetic innervation within WAT and BAT parenchyma is critical for the increased expression of thermogenic genes in adipose tissue in response to cold-exposure134–136. Accordingly, selective sympathetic denervation of WAT and BAT disrupts cold-induced thermogenesis135–137. The importance of adipose sympathetic drive for thermogenesis and systemic metabolism was also consolidated in a recent report showing regional sympathectomy compromises adipose thermogenesis and renders mice susceptible to obesity50.
Sympathetic Nerve Factors that Signal to Adipose Tissue
Efferent sympathetic nerve fibers regulate adipose tissue functions through the secretion of the neurotransmitters NE, NPY and ATP, as illustrated in Figure 5A. These sympathetic-derived factors are present within the nerve fibers and are released upon the generation of an action potential. Reports suggesting sympathetic co-transmission and coordinated actions of NE and ATP electrically released from nerve endings in vessels have been published over the least three decades138–140. Importantly, while results depicting sympathetic co-transmission by the adipose nerve endings are not abundant, recent evidence supports the existence of this process in adipose nerve terminals. Accordingly, synergistic modulation of brown adipocyte functions by sympathetic adrenergic and purinergic pathways has been proposed141. Moreover, it was recently revealed that ATP is a necessary sympathetic co-transmitter for the high efficacy and specificity of NE-induced thermogenesis in brown adipocytes142.
In response to sympathetic neurotransmitters, adipocytes and other adipose resident cells produce bioactive factors, which modulate the adipocytes’ functions, including the cellular responses to the neurotransmitters themselves. In this regard, the neurotransmitters secreted by adipose sympathetic nerve endings set a “tone” upon which other inputs originating from adipose resident cells would regulate adipocyte metabolism and fine tune the adipocyte response to the neurotransmitters (Figure 5).
Norepinephrine (NE).
The neurotransmitter NE and its role in adipose metabolic processes have been well studied for many years and its actions within adipose tissue have been recently reviewed143,144. Upon CNS stimulus, sympathetic nerve fibers release NE within adipose tissue that upon binding to adrenergic receptors (AR) modulates several metabolic processes, including lipolysis, thermogenesis and adipose tissue remodeling143,145 (Figure 5A). Adrenergic receptors activate the classical cAMP signaling pathway that activates protein kinase A (PKA), which activates lipolysis of TAG within adipocyte lipid droplets in WAT and BAT8,143. This pathway also induces the “browning” of WAT through the appearance of beige adipocytes and the BAT thermogenic gene program, enhancing the expression of mitochondrial and oxidative genes, including the mitochondrial uncoupling protein UCP18,143,146. The β-adrenergic receptors (β1, β2, and β3) are necessary mediators of NE’s actions on adipose tissue, as mouse models lacking these three receptor subtypes present dysregulation of energy metabolism and are prone to obesity147,148. Sustained release of NE and consequent receptor activation also exerts profound morphological changes in adipose cellularity and tissue remodeling145. Among those changes is the enhanced blood flow in BAT during cold acclimation. The secretion of local vasodilating factors, such as nitric oxide (NO) has been proposed to increase blood flow in BAT during cold-induced thermogenesis149,150. The sympathetic stimulus seems to participate in this process, as NE enhances the regional blood flow, along with the induction of the NO-biosynthetic enzyme (NOS) in BAT151,152. Altogether, these dramatic changes mediate the adaptive responses of adipose tissue to chronic exposure to low temperature or to metabolic stress, such as caloric restriction153.
Neuropeptide-Y (NPY).
Although neuropeptide-Y is largely produced by CNS neurons, this neuropeptide is also synthesized by peripheral sympathetic nerve fibers, acting as a neurotransmitter that appears to affect adipose cells154 (Figure 5A). NPY receptor sub-types 1 and 2 mediate the actions of NPY and belong to the seven-transmembrane Gi/o-protein coupled receptor family that inhibits adenylyl cyclase and thus the cAMP/PKA pathway, acting antagonistically to adrenergic signaling154. Activation of NPY receptor suppresses lipolysis in adipocytes155 while activating the extracellular-regulated kinase (ERK) pathway and adipogenesis156. Similar to NE, NPY is located in cytosolic granules in the terminals of sympathetic neurons. In mice, its release and actions can be triggered by stressful situations, such as exposure to cold or overfeeding157.
Importantly, signals emanating from NPY receptors promote adipose angiogenesis, along with differentiation of new adipocytes157. In addition, obese mice were found to have elevated levels of circulating NPY and increased expression of its receptors in WAT, suggesting a role for this neuropeptide in adipose tissue accretion during obesity. Indeed, this notion was further supported by adipose-targeted knockdown and pharmacological inhibition of the NPY2 receptor, reducing obesity and metabolic abnormalities in mice157. Altogether, it appears that NPY produced by adipose sympathetic nerves functions in a system of feedback responses that oppose the actions of NE. Further studies to identify the signals that trigger the sympathetic production, release and actions of NPY are needed to clarify the exact role of NPY in regulating systemic metabolism and metabolic syndrome.
Adenosine triphosphate (ATP).
Similar to NE and NPY, adenosine triphosphate is a co-transmitter released by sympathetic nerve endings158 (Figure 5A). When secreted from neurons, ATP exerts its effects via the ionotropic purinergic receptor subtypes (P2XRs) and metabotropic purinergic receptor subtypes (P2YRs)159. Evidence suggests that signaling through these purinergic receptors affect several different processes in adipose tissue, including lipid metabolism (enhanced lipogenesis and lipolysis), increased thermogenesis, inflammation and endocrine functions159,160. For example, P2RX5 expression is induced in BAT and WAT upon chronic cold exposure and correlates with UCP1 expression161. Nonetheless, the functional role of these receptor subtypes on adipose and systemic metabolism need to be further rigorously investigated, applying both pharmacological and genetic approaches in order to determine their full functional relevance.
Adipose Tissue Factors that Signal to Sympathetic Nerves
Neurotrophic Factors.
It seems probable that adipose cells affected by SNS secretions signal back to local sympathetic nerve fibers within coordinated feedback responses (Figure 5B). Accordingly, several WAT- and BAT-derived secreted factors regulated by NE stimulus have been reported to act on efferent nerves. Among these factors are the neurotrophic factors NRG459,60, neuronal growth factor 1 (NEGR1)162, NGF62 and BDNF61. Chronic β-adrenergic receptor activation by NE induces the formation of beige adipocytes in WAT, which also produce some of these neuropeptides7. Since these factors act as potent neuronal regulators, it is plausible they enhance sympathetic innervation in adipose tissue during cold-induced thermogenesis and browning. This positive loop of beige adipocytes promoting their own formation through enhanced sympathetic nerve density may be critical for adipose thermogenesis. In support of this possibility, inhibiting NGF and its receptor TrkA suppresses browning of adipose tissue62. This study suggests that NGF mediates sympathetic plasticity within adipose tissue through enhanced innervation during cold exposure62. Such neuronal plasticity resembles the active remodeling of adipose tissue depots during times of stress. As the TrkA protein is expressed in other tissues, it would be important to selectively inactivate NGF/TrkA signaling in adipose sympathetic nerve endings to confirm the requirement for this pathway in adipose tissue remodeling during cold exposure.
Fatty acids (FA) and bioactive lipid metabolites.
While the effects of FA and bioactive lipids on adipose sensory nerve fibers have been investigated, mechanistic information on how these molecules affect adipose sympathetic nerve is limited (Figure 5B). It is conceivable that lipolysis of TAG and de novo biosynthesis of FA (DNL) in adipocytes can generate FA and lipid metabolites which may act on adipose efferent fibers163. Indeed, in vivo studies shown that chronic infusion of FA reduces peripheral sympathetic activity in rodents164. However, this effect may be through CNS regulation by FA165 and whether physiologically relevant FA also controls peripheral sympathetic nerve directly remains to be determined.
Endocannabinoids.
Recent studies demonstrated that adipocytes produce significant levels of endocannabinoids (EC)166–168 (Figure 5B). Acting through their receptors (CB1 and CB2), these bioactive lipids stimulate energy intake and inhibit energy expenditure, playing an important role in energy balance169. Importantly, dysregulations on EC adipose production and CB1 activation may contribute to metabolic dysfunctions. Thus, in obese subjects high circulating levels of EC positively correlate with increased visceral fat170,171, suggesting that increased EC levels may contribute to metabolic dysregulation during obesity. Furthermore, adipose-specific inactivation of CB1 appears to enhance adipose thermogenesis172, attenuating obesity-induced metabolic dysfunctions in mouse models169.
Although some evidence indicates a direct action of EC on the adipocyte, other studies have suggested an inhibitory effect of EC on neural circuits that blunt adipose sympathetic activity and thermogenesis173,174. While these effects are likely mediated through central mechanisms, some recent reports suggest EC may also act directly on peripheral sympathetic nerves175,176. In the perfused mesenteric vascular bed of the rat, activation of CB1 receptor by the EC anandamide suppresses the release of noradrenaline and ATP from sympathetic nerve terminals175. Altogether, these observations suggest that adipose-derived FA and bioactive lipids such as the EC may act on local sympathetic fibers, suppressing their activity. Thus, the increased adipose biosynthesis of EC noted in obesity may inhibit adipose sympathetic outflow, contributing to metabolic dysfunction. Adipose EC may also signal through afferent nerve fibers, which should be studied in more detail.
Cytokines and GDF15.
Adipocytes are not the only adipose tissue resident cells that secrete neuronal modulators that may affect adipose sympathetic nerve fibers (Figure 5B). Adipose tissue macrophages (ATMs) produce a number of pro-inflammatory and anti-inflammatory cytokines, such as TNFα, IL-1β, IL-6 and IL-10, which are all reported to modulate sympathetic nerve activity177. When produced in peripheral tissues, cytokines can enter the circulation and gain access to the brain, enhancing the sympathetic outflow via CNS regulation177. However, in chronic inflammatory states as occurs in adipose tissue during obesity, increased local levels of inflammatory cytokines may cause repulsion of sympathetic nerve fibers177 from inflamed adipose tissue regions or even nerve damage, depending on the severity of inflammation. This sympathetic neuronal response during a persistent inflammatory state, as occurs in arthritis, has been investigated in depth and previously described177. However, evidence supporting that sympathetic nerve repulsion occurs in inflamed adipose tissue remains speculative and experiments to rigorously test this hypothesis are necessary. Thus, while adipose sympathetic outflow produces an anti-inflammatory response (see below), persistent cytokine production and chronic inflammation noted in obesity may disrupt adipose efferent nerve function.
Other reported neuronal modulatory factors potentially produced by adipose cells are the growth differentiation factor-15 (GDF15)178 and adenosine179. Although initially identified as a cytokine secreted by macrophages180, GDF15 appear to be expressed predominantly in liver, with lower amounts produced by other tissues181. However, under metabolic stress conditions such as obesity, the adipose expression levels of GDF15 is strongly upregulated178, which may be due to increased ATM contents. Although the effects of GDF15 on energy metabolism are known to be mediated through suppression of food intake mechanisms in the brain182,183, peripheral actions of GDF15, including sympathetic neuronal regulation may also occur. Consistent with this notion, sympathetic neurons express the GDF15 receptor GFRAL183 and signaling through this receptor induces a potent neurotropic effect both in vitro and in vivo184. Nonetheless, it remains to be tested whether adipose-derived GDF15 plays a direct role as a peripheral neuronal modulator.
Adenosine.
Recent studies indicate that adenosine, through its A2A receptors, induces BAT and WAT thermogenesis185. This metabolite is released from adipocytes or from other adipose resident cells (Figure 5B). Moreover, adenosine can also be produced from extracellular ATP by T-reg lymphocytes and possibly endothelial cells, which express the enzymes ecto-5’-nucleotidases CD33 and CD73, cell surface proteins necessary for extracellular adenosine generation from ATP179. Importantly, the presence of adenosine receptors was also detected in peripheral efferent fibers in the vasculature and signaling via these receptors appears to blunt the secretion of neurotransmitters in sympathetic nerve endings186,187, suggesting that adenosine within adipose tissue may also be involved in a negative feedback mechanism.
Sympathetic Dysregulation in Obesity and Diabetes
General SNS dysregulation in metabolic disease is well established and was reviewed recently188. Since sympathetic outflow into adipose tissue controls such important adipocyte metabolic processes34,132,133, it is therefore not surprising that obesity disrupts catecholamine-mediated adipocyte lipolysis189,190, mitochondrial biogenesis191 and adipose tissue remodeling192. Sympathetic signals emanating from the CNS to adipose tissue may be altered in obesity by the associated hyperinsulinemia193, since insulin receptor signaling in the brain is known to suppress adipose sympathetic outflow in lean mice133. Thus, chronic hyperinsulinemia may cause insufficient sympathetic activity within adipose tissue, attenuating catecholamine-mediated actions. Consistent with this notion, eliminating the hyperinsulinemia normally observed in high fat diet-fed mice by genetic deletion of 3 insulin alleles reprograms WAT to express UCP1 protein and increase energy expenditure193,194. Thus, pathways that are enhanced by catecholamine are promoted upon reduction of circulating insulin levels. Chronic hyperinsulinemia in obesity also inhibits β-adrenergic receptor signaling through marked downregulation of β3-adrenergic receptors, as noted in adipocytes from obese mice192,195. This decreased adipocyte β3-adrenergic receptor expression and signaling is reversed by treatment of mice with diazoxide to reduce insulin secretion from β-cells196. Moreover, suppression of β-adrenergic receptor signaling in adipocytes in obesity has been consistently observed in other experimental models94,192.
Although the inhibitory effect of central insulin action on sympathetic outflow into white adipose tissue has been previously demonstrated by different studies121,133,194, it is important to emphasize that in other studies insulin signaling in the brain has been claimed to increase sympathetic drive into peripheral tissues, such as kidney and brown fat197,198. Thus, insulin signaling in the brain appears to elicit distinct sympathetic effects, depending on the tissue. As central insulin signaling is disrupted during metabolic dysfunctions, it would be important to examine whether perturbation of brain insulin signaling impacts the increased sympathetic drive into some peripheral tissues.
Similar to adipose sensory innervation, local sympathetic innervation may also be affected by the persistent adipose inflammation observed in obesity199. Interestingly, moderate reduction of hyperinsulinemia in obese mice decreased adipose inflammation and improved insulin signaling, along with an improvement in systemic metabolism78. However, whether these metabolic enhancements were associated with elevated adipose sympathetic tone and/or activity was not investigated.
Signaling networks among diverse adipose cell types and local nerve fibers
The many types of resident cells within adipose tissue communicate with each other through complex signaling networks, as exemplified in Figure 6A. Sympathetic neurotransmitters control key functions in endothelial cells and multiple types of immune cells in addition to adipocytes, and each of these cell types communicate with adipocytes and each other, adjusting their metabolic pathways and shaping the tissue microenvironment. This adipose tissue complexity also includes external signals that are integrated within the tissue and then relayed to other tissues from the adipose tissue in the form of secreted factors that act through the circulation or on sensory nerve fibers. Thus, adipose depots act as both sensors and integrators of complex signaling networks, and adipose innervation is critical to this function.
Figure 6: Integrating peripheral signals in adipose tissue.
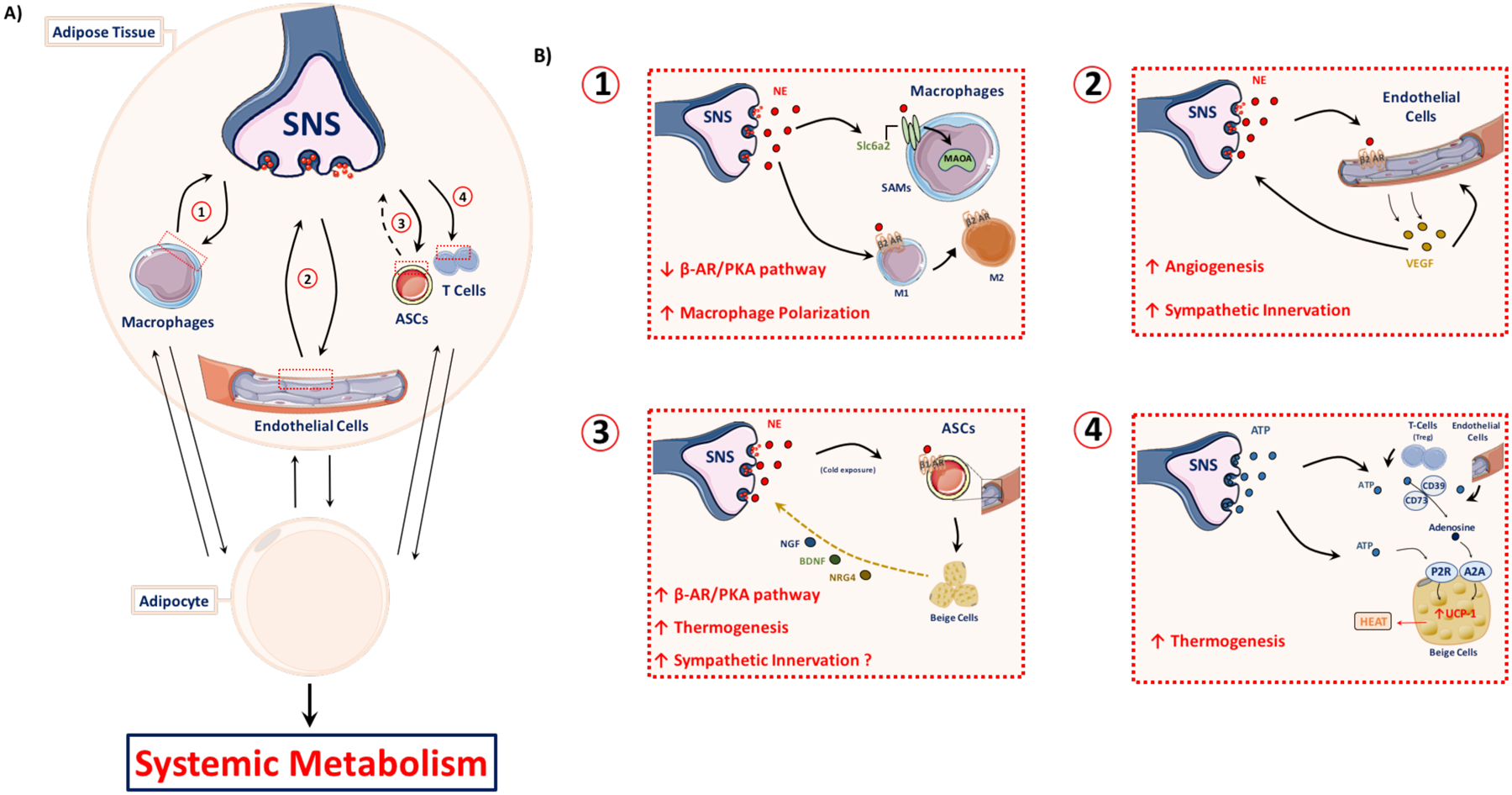
(A) Much like the integration of adipose signals within the brain, neuro-adipose signal integration also occurs within the periphery. Examples include modulation of the adipose tissue microenvironment via interaction with immune cell populations, endothelial cells, and adipose stem cells (ASCs). Responses of adipose tissue to sympathetic nervous system (SNS) cues allows for the rapid adaptation and remodeling that is required to maintain systemic metabolic homeostasis. (B) Sympathetic nerve fibers engage in unique interactions with adipose tissue cell populations. (1) Norepinephrine (NE) release from sympathetic terminals leads to adipose macrophage polarization from a proinflammatory (M1) to an anti-inflammatory, alternatively-activated (M2) profile. Sympathetic neuron-associated macrophages (SAMs) localize around sympathetic synapses and take up secreted NE through the solute carrier family 6 member 2 (Slc6a2) NE transporter. Monoamine oxidase A (MAOA) catalyzes the degradation of NE within the SAMs. (2) NE release from sympathetic nerve endings stimulates the β2 adrenergic receptor (β2AR) of the endothelial cells of the vasculature, leading to vascular endothelial growth factor (VEGF) secretion from the endothelium. VEGF stimulates angiogenesis and neurite outgrowth, driving increased irrigation and innervation of the adipose tissue. (3) Adipose-derived stem cell (ASCs) β1 adrenergic receptor (β1AR) activation by sympathetic NE drives beige adipocyte differentiation. These beige adipocytes have an enhanced thermogenic capacity relative to white adipocytes and a brown-like adipokine expression profile, which may include neurotrophic factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neuregulin-4 (NRG4). These factors can drive increased sympathetic innervation and arborization. (4) The sympathetic cotransmitter adenosine triphosphate (ATP) is cleaved by regulatory T-cells (Tregs) into adenosine via CD73 and CD39-mediated degradation to create the anti-inflammatory “purinergic halo” surrounding the Tregs. The adenosine interacts with the adenosine A2A receptor to drive beige adipocyte thermogenesis through mitochondrial uncoupling protein 1 (UCP1) upregulation. Sympathetic-derived ATP can also directly interact with the purinergic P2 receptor family (P2R) to drive beige adipocyte thermogenesis.
Neuron-Immune cell communication in adipose tissue.
A variety of adipose resident immune cells express adrenoreceptors that can be activated upon the release of NE by adipose sympathetic nerve endings. ATMs represent one such example that play a pivotal role in adipose inflammation and systemic metabolism25,78. ATMs can be sorted in two major subtypes, denoted M1 and M2, which exert either primarily pro-inflammatory (i.e. M1) or anti-inflammatory (i.e. M2) functions200,201. Although macrophages likely form a continuum of states between these two extreme types, several studies have shown that in healthy, functional adipose tissue the ratio of M1- to M2-defined macrophages is low, but is shifted towards an M1 profile in unhealthy adipose tissue, as in obesity202,203. Strategies to shift this ratio towards an M2-dominant population appear to improve adipose and whole body metabolism204,205. Interestingly, catecholamines exert a potent effect on macrophages through β2-adrenergic receptors that promote M1 macrophage differentiation into the M2 state206, indicating sympathetic signals favor an anti-inflammatory state (Figure 6B, panel 1). This concept is supported by a study showing sympathetic nerve activity maintains an anti-inflammatory state in adipose tissue by inhibiting TNF-α expression in macrophages through activation of the β2-adrenergic receptor/PKA pathway207. Conversely, adipose sympathetic denervation leads to a marked increase in tissue inflammation and dysfunction207.
Surprisingly, a newly identified macrophage subtype (M1-like) appears to participate in a mechanism to blunt sympathetic signaling in adipose and other tissues (Figure 6B, panel 1). These macrophages display a close anatomical and functional association with local sympathetic nerve fibers and are therefore named sympathetic neuron-associated macrophages (SAMs)199,208. Moreover, these macrophages possess high levels of a NE transporter (SLC6A2) in their plasma membranes and are active in taking up NE released by sympathetic neurons and degrading it using the monoamine oxidase A (MAOA) pathway199,208. Interestingly, genetic deletion of Slc6a2 in macrophages inhibits NE uptake by SAMs, enhancing sympathetic signaling to the adipose tissue and improving energy homeostasis199. Thus, the above pathways reveal tight communication between neurons and immune cells in adipose tissue: on the one hand, sympathetic nerve endings signal to reduce adipose inflammation by shifting the population of macrophages to an M2 phenotype, while on the other hand, SAMs decrease the availability of released catecholamine, and therefore, the sympathetic signal itself. Interestingly, obesity and age-related metabolic dysfunction are associated with increased SAM content which suppresses sympathetic drive and elevates adipose tissue inflammation199,208,209. These findings indicate that perturbations in neuron-immune cell interactions may be key contributors to metabolic disease.
Neuron-endothelial cell communication in adipose tissue.
The close association between adipose nerve fibers and the vasculature indicates interdependency between these two structures that may be essential for their functional role in regulating local and systemic metabolism210. Indeed, as shown in Figure 6B, panel 2, NE secreted by sympathetic nerve endings activate β-adrenergic receptors in endothelial cells to induce VEGF production211. In turn, endothelial-derived VEGF may promote angiogenesis through stimulation of endothelial cells and increase sympathetic innervation through activation of VEGF receptor (VEGFR) signaling in sympathetic neurons212,213. This physiological response of endothelial cells to sympathetic stimulus may be essential in situations where increased vascularization and innervation are needed, such as adipose tissue expansion during overfeeding31 or in cold-induced thermogenesis214. Accordingly, inhibition of VEGFR signaling in adipose cells disrupts thermogenesis and browning in WAT induced by cold temperature215–217. Conversely, overexpression of VEGF peptide elicits browning in WAT75,215,218,219, consistent with a key role for VEGF in promoting adipose pathways that benefit systemic metabolism.
Neuron-adipocyte progenitor communication.
Adipose-resident perivascular cells (illustrated in Figure 6B, Panel 3) represent a subset of progenitor cells that through activation of their β1-adrenergic receptors (ADRB1) differentiate into beige adipocytes220. This mechanism appears to be distinct from the well-known β3-adrenergic receptor-induced browning of WAT, as it relies on β1-adrenergic receptor rather than the β3-receptor isotype220. Interestingly, Adrb1-null mice are intolerant to cold exposure and cannot defend their body temperature, highlighting the importance of ADRB1 for adipose thermogenesis and euthermia221. Additionally, these perivascular-derived beige adipocytes not only display higher thermogenic capacity, but also may secrete neurotrophic factors to promote adipose sympathetic innervation (Figure 6B, panel 3). Thus, sympathetic stimulation and differentiation of perivascular progenitor cells into beige adipocytes may strongly contribute to browning of WAT during cold stimulus, especially in mice that have not been previously exposed to the cold137. The importance of this mechanism remains to be determined by inactivating ADRB1 receptors in perivascular cells and examining the possible consequences for energy metabolism.
Dysregulation of Peripheral Signal Integration in Obesity and Diabetes
Similar to how hypothalamic dysregulation negatively affect systemic metabolism, a failure in peripheral signal integration can occur in obesity and may lead to metabolic dysfunctions and diabetes. Dysregulation of a homeostatic mechanism may arise from and result in the overproduction or underproduction of potent signaling molecules. The overproduction of neuropeptide-Y (NPY) in obesity is one such case of signaling molecule dysregulation157. As detailed above, this neuropeptide favors adipose tissue expansion and has been implicated in obesity-linked metabolic dysfunctions. Strategies aimed to block signaling emanated from one of the NPY receptors subtype (NPY2) were successful in attenuating various metabolic abnormalities during obesity157. Moreover, NPY signaling in vasculature increased adhesion of leukocytes to endothelial cells222,223 and treatment with a global NPY receptor antagonist effectively improved chronic inflammation222,224. Thus, it is conceivable that persistent activation of adipose cells by enhanced NPY levels promote adipose tissue accretion, favor immune cell infiltration and exacerbate adipose inflammation. Imbalance due to persistent signals emanating from chronically activated NPY receptors in obesity may contribute to the disruption of adipose tissue functions, such as local signal integration. However, a recent study suggested that macrophage/hematopoietic cells-derived NPY initiates an anti-inflammatory response during early stage of obesity in mice225. Accordingly, deletion of NPY in macrophage/hematopoietic cells promotes inflammation in adipose tissue225. The reason for the discrepancy between this recent study and the previous results could be the differential effect of NPY at different stages of adipose tissue expansion in obesity, the origin of the NPY (nerve versus immune or other cells types) or the differential signaling by receptor 1 versus receptor 2. Additional studies will be necessary to better understand the how NPY originates from different adipose cells and how its signaling from receptor subtypes impacts adipose and whole body metabolism at different stages of obesity and metabolic dysfunction.
Adenosine triphosphate (ATP) is a sympathetic co-transmitter in which dysregulation in its production and/or degradation by adipose resident cells may be implicated in obesity-linked adipose dysfunction and disruption of adipose signaling integration. An increase in the extracellular levels of ATP is often associated with tissue inflammation and metabolic abnormalities as occur in adipose tissue during obesity226,227. Consistent with this notion, the adipose tissue from obese and metabolically unhealthy subjects releases elevated levels of ATP when compared to lean and metabolically healthy individuals226. Such an increase in the extracellular levels of ATP stimulates purinergic receptor P2RX7 signaling in M1 macrophages to enhance pro-inflammatory cytokine production and adipose inflammation228. Therefore, local mechanisms controlling the extracellular ATP levels are essential for tissue homeostasis. One such local mechanism that plays a role in regulating extracellular ATP concentrations is the cleavage of ATP into adenosine.
In contrast to the pro-inflammatory role of extracellular ATP, adenosine produced by ATP degradation is known to limit severe inflammation and tissue damage227. The secreted ATP can be metabolized into adenosine by two extracellular ecto-5’-nucleotidases CD39/CD73227. These two enzymes, expressed at the cellular surface of regulatory T lymphocytes (Tregs)83,84 and in the adipose vasculature229, (Figure 6, panel 4) may play a key role in controlling the extracellular concentrations of ATP and adenosine. Therefore, adipose Tregs and endothelial cells can modulate the tissue inflammation state by controlling the extracellular ATP conversion into adenosine (Figure 6, panel 4). Disruptions of adipose endothelial function and reduction in Tregs content, as noted in obesity, would diminish ATP hydrolysis, increasing the ATP:adenosine ratio in the extracellular compartment. Consequently, high ATP levels would activate M1 macrophages and favor the chronic inflammatory state typically seen in adipose tissue in obesity.
Hence, inability to control the extracellular levels of ATP, either due to overproduction by sympathetic endings or due to disruption in Tregs and endothelial cells (or both) may impact adipose functions and proper integration of peripheral signals to control systemic metabolism. Consistent with this notion, global genetic deletion of CD39 leads to an inability to control extracellular ATP levels in hepatocytes, enhanced inflammation and systemic insulin resistance229. It will be important to examine the role of the adipose CD39 and CD73 as modulators of ATP and adenosine signaling in adipose tissue metabolism and possible influences in whole-body metabolism.
In summary, the ability of adipose tissue to integrate central and peripheral signals relies on the accurate regulation of adipose-neuron crosstalk and maintenance of tissue homeostasis. In obesity, adipose expansion triggers tissue inflammation favoring pro-inflammatory M1 macrophages and diminishing the local sympathetic signaling (Figure 6, panel 1). The reduction in NE levels will not only favor a pro-inflammatory state, but also blunt VEGF-mediated angiogenesis and adipose innervation (Figure 6, panel 2). Lower levels of NE may also reduce differentiation of perivascular-derived beige cells, affecting adipose thermogenesis and potentially innervation (Figure 6, panel 3). Chronic adipose inflammation may also reduce vascular function and the presence of anti-inflammatory Treg lymphocytes, resulting in extracellular ATP accumulation and persistent tissue inflammation (Figure 6, panel 4). Fine-tuned regulation of these processes may be essential for adipose peripheral integration and ultimately whole-body metabolic homeostasis. A failure to appropriately regulate such processes may be linked to metabolic disorders in obesity and type-2 diabetes.
Conclusions
Recent findings showing dynamic crosstalk between adipose cells and local nerve fibers have opened a fertile area of adipose biology for more detailed study. The revelation that lipolysis of fat stores, requisite for survival in fasting, is stimulated by NE released from efferent, sympathetic nerve fibers within adipose tissue rather than by circulating catecholamines reinforces the importance of this topic132. Even the dramatic suppression of adipocyte lipolysis during feeding by insulin involves regulation of sympathetic tone in adipose tissue121,133. Similarly, it is plausible that afferent, sensory nerve fibers might also modulate adipose cells, providing an exciting area for further research to identify factors that mediate such putative effects. Conversely, it is now appreciated that communication moves in the other direction as well: adipocytes and other adipose cell types can signal to nearby sensory nerve fibers that transmit information to the CNS and to sympathetic neurons that control SNS tone. Some adipose cell factors that act on sensory neuron have been identified, including leptin and FA, but this area of investigation is still at an early stage and offers considerable opportunities for discovery. Importantly, much of the data available and cited in this review relates to mouse studies, and it is critical for the field to test these findings and ideas in human tissues.
Another fertile area for investigation relates to the key role of endothelial cells, adipose progenitor cells and immune cells as both targets of neuronal signals within adipose tissue and as generators of signals to local nerve fibers. NE and the neurotransmitters CGRP and SP are vasoactive factors and immune modulators that can regulate the endothelium and immune cells within adipose tissue when released by sympathetic and sensory nerve endings, respectively. Importantly, dysregulation of CGRP and SP may contribute to endothelial dysfunctions and persistent adipose inflammation. This immune reaction resembles the neurogenic inflammation noted in other tissues in diabetes and, likewise, may lead to “adipose neuropathy”. However, the extent to which adipose sensory nerve dysregulation and afferent nerve-induced neurogenic inflammation contribute to immune cell expansion in adipose tissue in obesity is unknown. Might targeting adipose neurogenic inflammation by blocking CGRP and SP receptors attenuate chronic adipose inflammation and thus improve systemic metabolism in obesity?
Adipose innervation and the regulation of adipose function by the CNS are likely important targets for disruption by obesity and type 2 diabetes. Imbalance associated with metabolic disease in the levels of sympathetic neurotransmitters NE, NPY, and ATP is linked to chronic adipose dysfunction and the failure of adipose tissue to regulate systemic metabolism. Obesity and pro-inflammatory cytokines also reduce the biosynthesis of adipocyte-derived neurotrophic factors, such as NRG463,230, which has also been shown to be a beneficial circulating agent controlling glucose homeostasis and liver fat metabolism. Similarly, there is high interest in identifying what triggers hypothalamic inflammation and to what extent it causes metabolic dysfunctions in obesity. Bioactive lipids, glycosylated proteins, pro-inflammatory cytokines and perhaps hyperinsulinemia are top candidates among the suspected factors that might elicit hypothalamic inflammation. As this research area is still relatively new, taking advantage of new techniques and developing more refined methods will be critical to optimize progress in understanding communication between adipose cells and local nerve fibers. Rather than relying on genetic deletion of neuronal receptors or ligands in all sensory or sympathetic nerve fibers, more localized perturbations and reporters will be required. Optogenetic55,231 and chemical genetic approaches232, e.g., designer receptors exclusively activated by designer drugs (DREADD), will be valuable approaches for future studies.
Finally, the discovery that WAT metabolic activity can modulate the function of distant BAT depots through sensory nerve signaling to the CNS to enhance sympathetic outflow represents an exciting future direction for research in this field39,40,48,49. Coupled with extensive data233 showing that the CNS can regulate metabolic tissues, including liver, skeletal muscle and pancreatic islets, to control systemic glucose homeostasis, these findings infer that adipose tissue may also signal to these other tissues through the brain. Much more work is required to test this idea and to define its full significance, but hopefully this hypothesis will be enticing to many scientists in the field.
Key Points.
Adipocytes modulate whole body metabolism through secretion of endocrine and paracrine factors that modulate local immune cell cytokine secretion, endothelium blood flow and neuronal signaling to the brain.
Adipocytes, immune cells and endothelial cells within adipose tissues secrete factors such as leptin, TNFα and VEGF that regulate local sensory nerve fibers.
Adipocyte lipid metabolism communicates with local sensory nerve fibers, sending signals to the CNS, and conversely, sensory nerve fibers secrete factors such as CGRP and SP that may regulate adipocytes and other adipose cells.
Increased lipolysis in white adipose tissue in response to sympathetic activation can cause sensory nerve fibers to regulate the metabolic activity of distant brown adipose tissue depots.
Extensive and dynamic signaling networks among the diverse cell types in adipose tissue integrate and mediate communication through bioactive lipids to local sensory nerve fibers and neurotrophic factors to sympathetic nerve fibers.
Identifying factors within adipose tissues that regulate sensory and sympathetic nerve fibers may reveal therapeutic strategies for obesity and type 2 diabetes.
Acknowledgements
Work from the Michael Czech laboratory discussed in this Review was supported by NIH grants 676 DK30898 and DK103047 to M.P.C.
Glossary
- Browning of WAT
The formation of beige adipocytes and or the conversion of white adipocytes into beige adipocytes within WAT occurring in response to cold or other stimulus. Through this process expression of UCP1 protein levels and thermogenic capacity of WAT are enhanced
- Beige Cells
UCP1-positive, thermogenic adipocytes that appear within white adipose tissue upon stimulus. Are often called “inducible brown”, “beige”, or “brite” (brown in white)
- Uncoupling protein 1 (UCP1)
Mitochondrial protein found in beige and brown adipocytes involved in thermogenesis. It uncouples mitochondrial respiration from ATP production, converting energy of membrane electrical potential into heat
- β-adrenergic receptor
The β-adrenergic receptors belong to a class of G protein-coupled receptors that are activated by catecholamines
- Macrophage Polarization
The shift in macrophage phenotypic profile between pro-inflammatory M1 subtypes and anti-inflammatory M2 subtypes depending on external cues
- Hyperinsulinemia
A state of supraphysiological levels of circulating insulin. It is closely associated with glucose intolerance, obesity, hypertension and dyslipidemia
- Afferent Nerve
Consists of sensory nerve fibers that can transmit information from the periphery to the central nervous system
- Efferent Nerve
Consists of sympathetic nerve fibers that travel away from the central nervous system to innervate adipose tissue and other peripheral tissues
- Neurogenic Inflammation
Local inflammation generated by signals originating from nerve fibers
- Neurotrophic Factors
Molecules that regulate growth, morphological plasticity and survival potential of neurons
- Neuropathy
The damage or destruction of nerves
- Crosstalk
In this review we refer to the intercommunication between adipose resident cells and the local neuronal fibers as crosstalk, which is determined by the interactions between secreted molecules from one cell type with the related receptor(s) in a distinct cell type
Footnotes
Competing interests
The authors declare no competing interests.
REFERENCES
- 1.Shaw HB A Contribution to the Study of the Morphology of Adipose Tissue. J Anat Physiol 36, 1–13 (1901). [PMC free article] [PubMed] [Google Scholar]
- 2.Kershaw EE & Flier JS Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89, 2548–2556, doi: 10.1210/jc.2004-0395 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Ouchi N, Parker JL, Lugus JJ & Walsh K Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11, 85–97, doi: 10.1038/nri2921 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villarroya F, Cereijo R, Villarroya J & Giralt M Brown adipose tissue as a secretory organ. Nat Rev Endocrinol 13, 26–35, doi: 10.1038/nrendo.2016.136 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Stern JH, Rutkowski JM & Scherer PE Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell metabolism 23, 770–784, doi: 10.1016/j.cmet.2016.04.011 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J et al. SDF-1 is an Autocrine Insulin-Desensitizing Factor in Adipocytes. Diabetes, doi: 10.2337/db17-0706 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Villarroya F, Gavalda-Navarro A, Peyrou M, Villarroya J & Giralt M The Lives and Times of Brown Adipokines. Trends Endocrinol Metab 28, 855–867, doi: 10.1016/j.tem.2017.10.005 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol Rev 84, 277–359, doi: 10.1152/physrev.00015.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Rosen ED & Spiegelman BM What we talk about when we talk about fat. Cell 156, 20–44, doi: 10.1016/j.cell.2013.12.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilherme A, Virbasius JV, Puri V & Czech MP Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9, 367–377, doi: 10.1038/nrm2391 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nye C, Kim J, Kalhan SC & Hanson RW Reassessing triglyceride synthesis in adipose tissue. Trends Endocrinol Metab 19, 356–361, doi: 10.1016/j.tem.2008.08.003 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Teusink B et al. Contribution of fatty acids released from lipolysis of plasma triglycerides to total plasma fatty acid flux and tissue-specific fatty acid uptake. Diabetes 52, 614–620 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Kersten S Physiological regulation of lipoprotein lipase. Biochim Biophys Acta 1841, 919–933, doi: 10.1016/j.bbalip.2014.03.013 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Czech MP, Tencerova M, Pedersen DJ & Aouadi M Insulin signalling mechanisms for triacylglycerol storage. Diabetologia 56, 949–964, doi: 10.1007/s00125-013-2869-1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unger RH, Clark GO, Scherer PE & Orci L Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801, 209–214, doi: 10.1016/j.bbalip.2009.10.006 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Perry RJ et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758, doi: 10.1016/j.cell.2015.01.012 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Titchenell PM et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell metabolism 23, 1154–1166, doi: 10.1016/j.cmet.2016.04.022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clerk LH, Rattigan S & Clark MG Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51, 1138–1145 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Dresner A et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. The Journal of clinical investigation 103, 253–259, doi: 10.1172/JCI5001 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF & Shulman GI Cellular mechanism of insulin resistance in skeletal muscle. J R Soc Med 95 Suppl 42, 8–13 (2002). [PMC free article] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Bonadonna RC & Ferrannini E Pathogenesis of NIDDM. A balanced overview. Diabetes Care 15, 318–368 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Walther TC, Chung J & Farese RV Jr. Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol 33, 491–510, doi: 10.1146/annurev-cellbio-100616-060608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cinti S et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research 46, 2347–2355, doi: 10.1194/jlr.M500294-JLR200 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Kanda H et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation 116, 1494–1505, doi: 10.1172/JCI26498 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amano SU et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism 19, 162–171, doi: 10.1016/j.cmet.2013.11.017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisberg SP et al. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation 112, 1796–1808, doi: 10.1172/JCI19246 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation 112, 1821–1830, doi: 10.1172/JCI19451 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafson B & Smith U Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. The Journal of biological chemistry 281, 9507–9516, doi: 10.1074/jbc.M512077200 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Grant RW & Stephens JM Fat in flames: influence of cytokines and pattern recognition receptors on adipocyte lipolysis. American journal of physiology. Endocrinology and metabolism 309, E205–213, doi: 10.1152/ajpendo.00053.2015 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Aouadi M et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci U S A 110, 8278–8283, doi: 10.1073/pnas.1300492110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung HK et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab 17, 61–72, doi: 10.1016/j.cmet.2012.12.010 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Halaas JL et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269, 543–546 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Bartness TJ, Kay Song C, Shi H, Bowers RR & Foster MT Brain-adipose tissue cross talk. The Proceedings of the Nutrition Society 64, 53–64 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Bartness T & Kay Song C Innervation of Brown Adipose Tissue and its Role in Thermogenesis. Vol. 29 (2005). [Google Scholar]
- 35.Bartness TJ & Ryu V Neural control of white, beige and brown adipocytes. International journal of obesity supplements 5, S35–39, doi: 10.1038/ijosup.2015.9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caron A, Lee S, Elmquist JK & Gautron L Leptin and brain-adipose crosstalks. Nature reviews. Neuroscience 19, 153–165, doi: 10.1038/nrn.2018.7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedman J 20 years of leptin: leptin at 20: an overview. The Journal of endocrinology 223, T1–8, doi: 10.1530/JOE-14-0405 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432, doi: 10.1038/372425a0 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Ryu V, Garretson JT, Liu Y, Vaughan CH & Bartness TJ Brown adipose tissue has sympathetic-sensory feedback circuits. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 2181–2190, doi: 10.1523/jneurosci.3306-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garretson JT et al. Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis. Molecular metabolism 5, 626–634, doi: 10.1016/j.molmet.2016.06.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris RBS Denervation as a tool for testing sympathetic control of white adipose tissue. Physiol Behav 190, 3–10, doi: 10.1016/j.physbeh.2017.07.008 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fishman RB & Dark J Sensory innervation of white adipose tissue. The American journal of physiology 253, R942–944, doi: 10.1152/ajpregu.1987.253.6.R942 (1987). [DOI] [PubMed] [Google Scholar]
- 43.Shi H, Song CK, Giordano A, Cinti S & Bartness TJ Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity. American journal of physiology. Regulatory, integrative and comparative physiology 288, R1028–1037, doi: 10.1152/ajpregu.00648.2004 (2005). [DOI] [PubMed] [Google Scholar]
- 44.De Matteis R, Ricquier D & Cinti S TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. Journal of neurocytology 27, 877–886 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Song CK, Schwartz GJ & Bartness TJ Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. American journal of physiology. Regulatory, integrative and comparative physiology 296, R501–511, doi: 10.1152/ajpregu.90786.2008 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughan CH & Bartness TJ Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. American journal of physiology. Regulatory, integrative and comparative physiology 302, R1049–1058, doi: 10.1152/ajpregu.00640.2011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryu V & Bartness TJ Short and long sympathetic-sensory feedback loops in white fat. American journal of physiology. Regulatory, integrative and comparative physiology 306, R886–900, doi: 10.1152/ajpregu.00060.2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen NLT, Xue B & Bartness TJ Sensory denervation of inguinal white fat modifies sympathetic outflow to white and brown fat in Siberian hamsters. Physiol Behav, doi: 10.1016/j.physbeh.2018.02.019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilherme A et al. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Molecular metabolism, doi: 10.1016/j.molmet.2018.06.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pereira MM et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat Commun 8, 14967, doi: 10.1038/ncomms14967 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niijima A Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. Journal of the autonomic nervous system 73, 19–25 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Niijima A Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neuroscience letters 262, 125–128 (1999). [DOI] [PubMed] [Google Scholar]
- 53.Murphy KT et al. Leptin-sensitive sensory nerves innervate white fat. American journal of physiology. Endocrinology and metabolism 304, E1338–1347, doi: 10.1152/ajpendo.00021.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lartigue G, Ronveaux CC & Raybould HE Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab 3, 595–607, doi: 10.1016/j.molmet.2014.06.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeng W et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94, doi: 10.1016/j.cell.2015.08.055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shiuchi T et al. Induction of glucose uptake in skeletal muscle by central leptin is mediated by muscle beta2-adrenergic receptor but not by AMPK. Scientific reports 7, 15141, doi: 10.1038/s41598-017-15548-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waxman SG From neuroscience to neurology : neuroscience, molecular medicine, and the therapeutic transformation of neurology. (Elsevier Academic Press, 2005). [Google Scholar]
- 58.Korsching S The neurotrophic factor concept: a reexamination. J Neurosci 13, 2739–2748 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christian M Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte 4, 50–54, doi: 10.4161/adip.29853 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosell M et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. American journal of physiology. Endocrinology and metabolism 306, E945–964, doi: 10.1152/ajpendo.00473.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakagomi A et al. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NPJ aging and mechanisms of disease 1, 15009, doi: 10.1038/npjamd.2015.9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Y, Wang H & Zeng W Whole-tissue 3D imaging reveals intra-adipose sympathetic plasticity regulated by NGF-TrkA signal in cold-induced beiging. Protein & cell, doi: 10.1007/s13238-018-0528-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang GX et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20, 1436–1443, doi: 10.1038/nm.3713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min SY et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat Med 22, 312–318, doi: 10.1038/nm.4031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pearson RJ Jr. & Carroll SL ErbB transmembrane tyrosine kinase receptors are expressed by sensory and motor neurons projecting into sciatic nerve. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 52, 1299–1311, doi: 10.1177/002215540405201006 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Vetter I, Pujic Z & Goodhill GJ The response of dorsal root ganglion axons to nerve growth factor gradients depends on spinal level. J Neurotrauma 27, 1379–1386, doi: 10.1089/neu.2010.1279 (2010). [DOI] [PubMed] [Google Scholar]
- 67.Moy JK, Khoutorsky A, Asiedu MN, Dussor G & Price TJ eIF4E Phosphorylation Influences Bdnf mRNA Translation in Mouse Dorsal Root Ganglion Neurons. Front Cell Neurosci 12, 29, doi: 10.3389/fncel.2018.00029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cannon B et al. ‘Neuropeptide tyrosine’ (NPY) is co-stored with noradrenaline in vascular but not in parenchymal sympathetic nerves of brown adipose tissue. Experimental cell research 164, 546–550 (1986). [DOI] [PubMed] [Google Scholar]
- 69.Slavin BG & Ballard KW Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec 191, 377–389, doi: 10.1002/ar.1091910310 (1978). [DOI] [PubMed] [Google Scholar]
- 70.Bartness TJ & Bamshad M Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. The American journal of physiology 275, R1399–1411 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Carmeliet P & Tessier-Lavigne M Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200, doi: 10.1038/nature03875 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Shvartsman D et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther 22, 1243–1253, doi: 10.1038/mt.2014.76 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackenzie F & Ruhrberg C Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380, doi: 10.1242/dev.072348 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Guaiquil VH et al. VEGF-B selectively regenerates injured peripheral neurons and restores sensory and trophic functions. Proc Natl Acad Sci U S A 111, 17272–17277, doi: 10.1073/pnas.1407227111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park J et al. VEGF-A-Expressing Adipose Tissue Shows Rapid Beiging and Enhanced Survival After Transplantation and Confers IL-4-Independent Metabolic Improvements. Diabetes 66, 1479–1490, doi: 10.2337/db16-1081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun K et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A 109, 5874–5879, doi: 10.1073/pnas.1200447109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhondt J et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J 25, 1461–1473, doi: 10.1096/fj.10-170944 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedersen DJ et al. A major role of insulin in promoting obesity-associated adipose tissue inflammation. Molecular metabolism 4, 507–518, doi: 10.1016/j.molmet.2015.04.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardy OT et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis 7, 60–67, doi: 10.1016/j.soard.2010.05.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanos TP et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci U S A 115, E4843–E4852, doi: 10.1073/pnas.1719083115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osamura N et al. Induction of interleukin-6 in dorsal root ganglion neurons after gradual elongation of rat sciatic nerve. Exp Neurol 195, 61–70, doi: 10.1016/j.expneurol.2005.03.019 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Miller RJ, Jung H, Bhangoo SK & White FA Cytokine and chemokine regulation of sensory neuron function. Handb Exp Pharmacol, 417–449, doi: 10.1007/978-3-540-79090-7_12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nunez Ruiz A et al. Diminished levels of regulatory T cell subsets (CD8+Foxp3, CD4+Foxp3 and CD4+CD39+Foxp3) but increased Foxp3 expression in adipose tissue from overweight subjects. Nutrition (Burbank, Los Angeles County, Calif.) 32, 943–954, doi: 10.1016/j.nut.2016.02.006 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Cortez-Espinosa N et al. CD39 expression on Treg and Th17 cells is associated with metabolic factors in patients with type 2 diabetes. Hum Immunol 76, 622–630, doi: 10.1016/j.humimm.2015.09.007 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Herbert MK & Holzer P [Neurogenic inflammation. I. Basic mechanisms, physiology and pharmacology]. Anasthesiol Intensivmed Notfallmed Schmerzther 37, 314–325, doi: 10.1055/s-2002-32233 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Russell FA, King R, Smillie SJ, Kodji X & Brain SD Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94, 1099–1142, doi: 10.1152/physrev.00034.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brain SD & Grant AD Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84, 903–934, doi: 10.1152/physrev.00037.2003 (2004). [DOI] [PubMed] [Google Scholar]
- 88.Sung CP et al. CGRP stimulates the adhesion of leukocytes to vascular endothelial cells. Peptides 13, 429–434 (1992). [DOI] [PubMed] [Google Scholar]
- 89.Liu T et al. Endogenous Calcitonin Gene-Related Peptide Regulates Lipid Metabolism and Energy Homeostasis in Male Mice. Endocrinology 158, 1194–1206, doi: 10.1210/en.2016-1510 (2017). [DOI] [PubMed] [Google Scholar]
- 90.Walker CS et al. Mice lacking the neuropeptide alpha-calcitonin gene-related peptide are protected against diet-induced obesity. Endocrinology 151, 4257–4269, doi: 10.1210/en.2010-0284 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Johnson MB, Young AD & Marriott I The Therapeutic Potential of Targeting Substance P/NK-1R Interactions in Inflammatory CNS Disorders. Front Cell Neurosci 10, 296, doi: 10.3389/fncel.2016.00296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Recio S & Gascon P Biological and Pharmacological Aspects of the NK1-Receptor. Biomed Res Int 2015, 495704, doi: 10.1155/2015/495704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Connor TM et al. The role of substance P in inflammatory disease. J Cell Physiol 201, 167–180, doi: 10.1002/jcp.20061 (2004). [DOI] [PubMed] [Google Scholar]
- 94.Reilly SM & Saltiel AR Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol 13, 633–643, doi: 10.1038/nrendo.2017.90 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Ellis A & Bennett DL Neuroinflammation and the generation of neuropathic pain. Br J Anaesth 111, 26–37, doi: 10.1093/bja/aet128 (2013). [DOI] [PubMed] [Google Scholar]
- 96.Zanos TP et al. Identification of cytokine-specific sensory neural signals by decoding murine vagus nerve activity. Proc Natl Acad Sci U S A, doi: 10.1073/pnas.1719083115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yadav RL et al. Somatic neural alterations in non-diabetic obesity: a cross-sectional study. BMC Obes 3, 50, doi: 10.1186/s40608-016-0131-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richardson JD & Vasko MR Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 302, 839–845, doi: 10.1124/jpet.102.032797 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Xanthos DN & Sandkuhler J Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15, 43–53, doi: 10.1038/nrn3617 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Schaper NC, Huijberts M & Pickwell K Neurovascular control and neurogenic inflammation in diabetes. Diabetes Metab Res Rev 24 Suppl 1, S40–44, doi: 10.1002/dmrr.862 (2008). [DOI] [PubMed] [Google Scholar]
- 101.Farkas GJ & Gater DR Neurogenic obesity and systemic inflammation following spinal cord injury: a review. J Spinal Cord Med, 1–10, doi: 10.1080/10790268.2017.1357104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morrison SF, Madden CJ & Tupone D Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell metabolism 19, 741–756, doi: 10.1016/j.cmet.2014.02.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morrison SF Central neural control of thermoregulation and brown adipose tissue. Autonomic neuroscience : basic & clinical 196, 14–24, doi: 10.1016/j.autneu.2016.02.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams KW & Elmquist JK From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nature neuroscience 15, 1350–1355, doi: 10.1038/nn.3217 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Konner AC, Klockener T & Bruning JC Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol Behav 97, 632–638, doi: 10.1016/j.physbeh.2009.03.027 (2009). [DOI] [PubMed] [Google Scholar]
- 106.Timper K & Bruning JC Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech 10, 679–689, doi: 10.1242/dmm.026609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hollenberg AN The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid 18, 131–139, doi: 10.1089/thy.2007.0251 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Ryu V, Watts AG, Xue B & Bartness TJ Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. American journal of physiology. Regulatory, integrative and comparative physiology 312, R324–r337, doi: 10.1152/ajpregu.00456.2015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen NL, Randall J, Banfield BW & Bartness TJ Central sympathetic innervations to visceral and subcutaneous white adipose tissue. American journal of physiology. Regulatory, integrative and comparative physiology 306, R375–386, doi: 10.1152/ajpregu.00552.2013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bamshad M, Aoki VT, Adkison MG, Warren WS & Bartness TJ Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. The American journal of physiology 275, R291–299 (1998). [DOI] [PubMed] [Google Scholar]
- 111.Morton GJ & Schwartz MW Leptin and the central nervous system control of glucose metabolism. Physiological reviews 91, 389–411, doi: 10.1152/physrev.00007.2010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Contreras C, Nogueiras R, Dieguez C, Rahmouni K & Lopez M Traveling from the hypothalamus to the adipose tissue: The thermogenic pathway. Redox biology 12, 854–863, doi: 10.1016/j.redox.2017.04.019 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Myers MG Jr. & Olson DP Central nervous system control of metabolism. Nature 491, 357–363, doi: 10.1038/nature11705 (2012). [DOI] [PubMed] [Google Scholar]
- 114.Mahu I & Domingos AI The sympathetic neuro-adipose connection and the control of body weight. Experimental cell research 360, 27–30, doi: 10.1016/j.yexcr.2017.03.047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dodd GT & Tiganis T Insulin action in the brain: Roles in energy and glucose homeostasis. J Neuroendocrinol 29, doi: 10.1111/jne.12513 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Roh E, Song DK & Kim MS Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 48, e216, doi: 10.1038/emm.2016.4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benoit SC et al. The catabolic action of insulin in the brain is mediated by melanocortins. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 9048–9052 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choudhury AI et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. The Journal of clinical investigation 115, 940–950, doi: 10.1172/JCI24445 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dodd GT et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104, doi: 10.1016/j.cell.2014.12.022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dodd GT et al. A Hypothalamic Phosphatase Switch Coordinates Energy Expenditure with Feeding. Cell metabolism 26, 375–393 e377, doi: 10.1016/j.cmet.2017.07.013 (2017). [DOI] [PubMed] [Google Scholar]
- 121.Shin AC et al. Insulin Receptor Signaling in POMC, but Not AgRP, Neurons Controls Adipose Tissue Insulin Action. Diabetes 66, 1560–1571, doi: 10.2337/db16-1238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harris RB, Kelso EW, Flatt WP, Bartness TJ & Grill HJ Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147, 1365–1376, doi: 10.1210/en.2005-1156 (2006). [DOI] [PubMed] [Google Scholar]
- 123.Thaler JP et al. Obesity is associated with hypothalamic injury in rodents and humans. The Journal of clinical investigation 122, 153–162, doi: 10.1172/JCI59660 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kleinridders A et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell metabolism 10, 249–259, doi: 10.1016/j.cmet.2009.08.013 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thaler JP & Schwartz MW Minireview: Inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology 151, 4109–4115, doi: 10.1210/en.2010-0336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao Y et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25, doi: 10.1002/glia.22580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia-Caceres C, Yi CX & Tschop MH Hypothalamic astrocytes in obesity. Endocrinol Metab Clin North Am 42, 57–66, doi: 10.1016/j.ecl.2012.11.003 (2013). [DOI] [PubMed] [Google Scholar]
- 128.Baufeld C, Osterloh A, Prokop S, Miller KR & Heppner FL High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol 132, 361–375, doi: 10.1007/s00401-016-1595-4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schur EA et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 23, 2142–2148, doi: 10.1002/oby.21248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Valdearcos M et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell metabolism 26, 185–197 e183, doi: 10.1016/j.cmet.2017.05.015 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giordano A et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. American journal of physiology. Regulatory, integrative and comparative physiology 291, R1243–1255, doi: 10.1152/ajpregu.00679.2005 (2006). [DOI] [PubMed] [Google Scholar]
- 132.Bartness TJ, Liu Y, Shrestha YB & Ryu V Neural innervation of white adipose tissue and the control of lipolysis. Frontiers in neuroendocrinology 35, 473–493, doi: 10.1016/j.yfrne.2014.04.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scherer T et al. Brain insulin controls adipose tissue lipolysis and lipogenesis. Cell metabolism 13, 183–194, doi: 10.1016/j.cmet.2011.01.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vitali A et al. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. Journal of lipid research 53, 619–629, doi: 10.1194/jlr.M018846 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang H, Ding X, Cao Y, Wang H & Zeng W Dense Intra-adipose Sympathetic Arborizations Are Essential for Cold-Induced Beiging of Mouse White Adipose Tissue. Cell Metab 26, 686–692 e683, doi: 10.1016/j.cmet.2017.08.016 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Schulz TJ et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383, doi: 10.1038/nature11943 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu Y et al. Connexin 43 Mediates White Adipose Tissue Beiging by Facilitating the Propagation of Sympathetic Neuronal Signals. Cell Metab 24, 420–433, doi: 10.1016/j.cmet.2016.08.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burnstock G & Sneddon P Evidence for ATP and noradrenaline as cotransmitters in sympathetic nerves. Clin Sci (Lond) 68 Suppl 10, 89s–92s (1985). [DOI] [PubMed] [Google Scholar]
- 139.Pablo Huidobro-Toro J & Veronica Donoso M Sympathetic co-transmission: the coordinated action of ATP and noradrenaline and their modulation by neuropeptide Y in human vascular neuroeffector junctions. Eur J Pharmacol 500, 27–35, doi: 10.1016/j.ejphar.2004.07.008 (2004). [DOI] [PubMed] [Google Scholar]
- 140.Ralevic V & Dunn WR Purinergic transmission in blood vessels. Autonomic neuroscience : basic & clinical 191, 48–66, doi: 10.1016/j.autneu.2015.04.007 (2015). [DOI] [PubMed] [Google Scholar]
- 141.Razzoli M et al. Stress-induced activation of brown adipose tissue prevents obesity in conditions of low adaptive thermogenesis. Molecular metabolism 5, 19–33, doi: 10.1016/j.molmet.2015.10.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xie TR, Liu CF & Kang JS Sympathetic transmitters control thermogenic efficacy of brown adipocytes by modulating mitochondrial complex V. Signal Transduct Target Ther 2, 17060, doi: 10.1038/sigtrans.2017.60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Collins S beta-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Front Endocrinol (Lausanne) 2, 102, doi: 10.3389/fendo.2011.00102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zechner R, Madeo F & Kratky D Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18, 671–684, doi: 10.1038/nrm.2017.76 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Granneman JG, Li P, Zhu Z & Lu Y Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. American journal of physiology. Endocrinology and metabolism 289, E608–616, doi: 10.1152/ajpendo.00009.2005 (2005). [DOI] [PubMed] [Google Scholar]
- 146.Ramseyer VD & Granneman JG Adrenergic regulation of cellular plasticity in brown, beige/brite and white adipose tissues. Adipocyte 5, 119–129, doi: 10.1080/21623945.2016.1145846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bachman ES et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297, 843–845, doi: 10.1126/science.1073160 (2002). [DOI] [PubMed] [Google Scholar]
- 148.Douris N et al. Beta-adrenergic receptors are critical for weight loss but not for other metabolic adaptations to the consumption of a ketogenic diet in male mice. Molecular metabolism 6, 854–862, doi: 10.1016/j.molmet.2017.05.017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nisoli E, Tonello C, Briscini L & Carruba MO Inducible nitric oxide synthase in rat brown adipocytes: implications for blood flow to brown adipose tissue. Endocrinology 138, 676–682, doi: 10.1210/endo.138.2.4956 (1997). [DOI] [PubMed] [Google Scholar]
- 150.Petrovic V et al. NO modulates the molecular basis of rat interscapular brown adipose tissue thermogenesis. Comp Biochem Physiol C Toxicol Pharmacol 152, 147–159, doi: 10.1016/j.cbpc.2010.03.008 (2010). [DOI] [PubMed] [Google Scholar]
- 151.Takahashi H et al. Beta-3 adrenergic agonist, BRL-26830A, and alpha/beta blocker, arotinolol, markedly increase regional blood flow in the brown adipose tissue in anesthetized rats. Jpn Circ J 56, 936–942 (1992). [DOI] [PubMed] [Google Scholar]
- 152.Giordano A et al. Evidence for a functional nitric oxide synthase system in brown adipocyte nucleus. FEBS Lett 514, 135–140 (2002). [DOI] [PubMed] [Google Scholar]
- 153.Sipe LM et al. Differential sympathetic outflow to adipose depots is required for visceral fat loss in response to calorie restriction. Nutr Diabetes 7, e260, doi: 10.1038/nutd.2017.13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang W, Cline MA & Gilbert ER Hypothalamus-adipose tissue crosstalk: neuropeptide Y and the regulation of energy metabolism. Nutrition & metabolism 11, 27, doi: 10.1186/1743-7075-11-27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Turtzo LC, Marx R & Lane MD Cross-talk between sympathetic neurons and adipocytes in coculture. Proc Natl Acad Sci U S A 98, 12385–12390, doi: 10.1073/pnas.231478898 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang K, Guan H, Arany E, Hill DJ & Cao X Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22, 2452–2464, doi: 10.1096/fj.07-100735 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Kuo LE et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13, 803–811, doi: 10.1038/nm1611 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Burnstock G Purinergic cotransmission. Experimental physiology 94, 20–24, doi: 10.1113/expphysiol.2008.043620 (2009). [DOI] [PubMed] [Google Scholar]
- 159.Burnstock G & Gentile D The involvement of purinergic signalling in obesity. Purinergic Signal 14, 97–108, doi: 10.1007/s11302-018-9605-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tozzi M & Novak I Purinergic Receptors in Adipose Tissue As Potential Targets in Metabolic Disorders. Frontiers in pharmacology 8, 878, doi: 10.3389/fphar.2017.00878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ussar S et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci Transl Med 6, 247ra103, doi: 10.1126/scitranslmed.3008490 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bernhard F et al. Functional relevance of genes implicated by obesity genome-wide association study signals for human adipocyte biology. Diabetologia 56, 311–322, doi: 10.1007/s00125-012-2773-0 (2013). [DOI] [PubMed] [Google Scholar]
- 163.Guilherme A et al. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol Metab 6, 781–796, doi: 10.1016/j.molmet.2017.05.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Migrenne S, C. C-G, Kang L, Wang R, Rouch C, Lefèvre AL, Ktorza A, Routh VH, Levin BE, Magnan C. Fatty Acid Signaling in the Hypothalamus and the Neural Control of Insulin Secretion. Diabetes 55, 5 (2006). [Google Scholar]
- 165.Bazinet RP & Laye S Polyunsaturated fatty acids and their metabolites in brain function and disease. Nature reviews. Neuroscience 15, 771–785, doi: 10.1038/nrn3820 (2014). [DOI] [PubMed] [Google Scholar]
- 166.Matias I et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 91, 3171–3180, doi: 10.1210/jc.2005-2679 (2006). [DOI] [PubMed] [Google Scholar]
- 167.Gonthier MP et al. Identification of endocannabinoids and related compounds in human fat cells. Obesity (Silver Spring) 15, 837–845, doi: 10.1038/oby.2007.581 (2007). [DOI] [PubMed] [Google Scholar]
- 168.Matias I et al. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol 152, 676–690, doi: 10.1038/sj.bjp.0707424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ruiz de Azua I et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. The Journal of clinical investigation 127, 4148–4162, doi: 10.1172/JCI83626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cote M et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. International journal of obesity (2005) 31, 692–699, doi: 10.1038/sj.ijo.0803539 (2007). [DOI] [PubMed] [Google Scholar]
- 171.Di Marzo V et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 52, 213–217, doi: 10.1007/s00125-008-1178-6 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Wagner IV, Perwitz N, Drenckhan M, Lehnert H & Klein J Cannabinoid type 1 receptor mediates depot-specific effects on differentiation, inflammation and oxidative metabolism in inguinal and epididymal white adipocytes. Nutr Diabetes 1, e16, doi: 10.1038/nutd.2011.12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quarta C et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell metabolism 11, 273–285, doi: 10.1016/j.cmet.2010.02.015 (2010). [DOI] [PubMed] [Google Scholar]
- 174.Bajzer M et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 54, 3121–3131, doi: 10.1007/s00125-011-2302-6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pakdeechote P, Dunn WR & Ralevic V Cannabinoids inhibit noradrenergic and purinergic sympathetic cotransmission in the rat isolated mesenteric arterial bed. Br J Pharmacol 152, 725–733, doi: 10.1038/sj.bjp.0707397 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O’Keefe L, Simcocks AC, Hryciw DH, Mathai ML & McAinch AJ The cannabinoid receptor 1 and its role in influencing peripheral metabolism. Diabetes Obes Metab 16, 294–304, doi: 10.1111/dom.12144 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Pongratz G & Straub RH The sympathetic nervous response in inflammation. Arthritis Res Ther 16, 504 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xiong Y et al. Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Sci Transl Med 9, doi: 10.1126/scitranslmed.aan8732 (2017). [DOI] [PubMed] [Google Scholar]
- 179.Rines AK, Verdeguer F & Puigserver P Adenosine activates thermogenic adipocytes. Cell Res 25, 155–156, doi: 10.1038/cr.2014.157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bootcov MR et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A 94, 11514–11519 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tsai VW, Lin S, Brown DA, Salis A & Breit SN Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. International journal of obesity (2005) 40, 193–197, doi: 10.1038/ijo.2015.242 (2016). [DOI] [PubMed] [Google Scholar]
- 182.Tsai VW et al. Treatment with the TGF-b superfamily cytokine MIC-1/GDF15 reduces the adiposity and corrects the metabolic dysfunction of mice with diet-induced obesity. International journal of obesity (2005) 42, 561–571, doi: 10.1038/ijo.2017.258 (2018). [DOI] [PubMed] [Google Scholar]
- 183.O’Rahilly S GDF15-From Biomarker to Allostatic Hormone. Cell metabolism 26, 807–808, doi: 10.1016/j.cmet.2017.10.017 (2017). [DOI] [PubMed] [Google Scholar]
- 184.Strelau J, Schober A, Sullivan A, Schilling L & Unsicker K Growth/differentiation factor-15 (GDF-15), a novel member of the TGF-beta superfamily, promotes survival of lesioned mesencephalic dopaminergic neurons in vitro and in vivo and is induced in neurons following cortical lesioning. J Neural Transm Suppl, 197–203 (2003). [DOI] [PubMed] [Google Scholar]
- 185.Gnad T et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399, doi: 10.1038/nature13816 (2014). [DOI] [PubMed] [Google Scholar]
- 186.Dobson JG Jr. Mechanism of adenosine inhibition of catecholamine-induced responses in heart. Circ Res 52, 151–160 (1983). [DOI] [PubMed] [Google Scholar]
- 187.Rongen GA et al. Presynaptic inhibition of norepinephrine release from sympathetic nerve endings by endogenous adenosine. Hypertension 27, 933–938 (1996). [DOI] [PubMed] [Google Scholar]
- 188.Thorp AA & Schlaich MP Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res 2015, 341583, doi: 10.1155/2015/341583 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bougneres P et al. In vivo resistance of lipolysis to epinephrine. A new feature of childhood onset obesity. The Journal of clinical investigation 99, 2568–2573, doi: 10.1172/JCI119444 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Horowitz JF & Klein S Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. American journal of physiology. Endocrinology and metabolism 278, E1144–1152, doi: 10.1152/ajpendo.2000.278.6.E1144 (2000). [DOI] [PubMed] [Google Scholar]
- 191.Heinonen S et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 64, 3135–3145, doi: 10.2337/db14-1937 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Guo T et al. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245, doi: 10.7554/eLife.03245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Buettner C Is hyperinsulinemia required to develop overeating-induced obesity? Cell metabolism 16, 691–692, doi: 10.1016/j.cmet.2012.11.009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mehran AE et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell metabolism 16, 723–737, doi: 10.1016/j.cmet.2012.10.019 (2012). [DOI] [PubMed] [Google Scholar]
- 195.Collins S, Daniel KW & Rohlfs EM Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int J Obes Relat Metab Disord 23, 669–677 (1999). [DOI] [PubMed] [Google Scholar]
- 196.Surwit RS, Dixon TM, Petro AE, Daniel KW & Collins S Diazoxide restores beta3-adrenergic receptor function in diet-induced obesity and diabetes. Endocrinology 141, 3630–3637, doi: 10.1210/endo.141.10.7726 (2000). [DOI] [PubMed] [Google Scholar]
- 197.Rahmouni K et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. The Journal of clinical investigation 114, 652–658, doi: 10.1172/JCI21737 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Muntzel MS, Morgan DA, Mark AL & Johnson AK Intracerebroventricular insulin produces nonuniform regional increases in sympathetic nerve activity. The American journal of physiology 267, R1350–1355, doi: 10.1152/ajpregu.1994.267.5.R1350 (1994). [DOI] [PubMed] [Google Scholar]
- 199.Pirzgalska RM et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med 23, 1309–1318, doi: 10.1038/nm.4422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Komohara Y, Fujiwara Y, Ohnishi K, Shiraishi D & Takeya M Contribution of Macrophage Polarization to Metabolic Diseases. J Atheroscler Thromb 23, 10–17, doi: 10.5551/jat.32359 (2016). [DOI] [PubMed] [Google Scholar]
- 201.Olefsky JM & Glass CK Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219–246, doi: 10.1146/annurev-physiol-021909-135846 (2010). [DOI] [PubMed] [Google Scholar]
- 202.Lumeng CN, Bodzin JL & Saltiel AR Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation 117, 175–184, doi: 10.1172/JCI29881 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aron-Wisnewsky J et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 94, 4619–4623, doi: 10.1210/jc.2009-0925 (2009). [DOI] [PubMed] [Google Scholar]
- 204.Patsouris D et al. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism 8, 301–309, doi: 10.1016/j.cmet.2008.08.015 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Oh DY et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698, doi: 10.1016/j.cell.2010.07.041 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS & Ward PA Induction of M2 regulatory macrophages through the beta2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6, 607–618, doi: 10.1159/000358524 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tang L et al. Sympathetic Nerve Activity Maintains an Anti-Inflammatory State in Adipose Tissue in Male Mice by Inhibiting TNF-alpha Gene Expression in Macrophages. Endocrinology 156, 3680–3694, doi: 10.1210/EN.2015-1096 (2015). [DOI] [PubMed] [Google Scholar]
- 208.Camell CD et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123, doi: 10.1038/nature24022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Czech MP Macrophages dispose of catecholamines in adipose tissue. Nat Med 23, 1255–1257, doi: 10.1038/nm.4440 (2017). [DOI] [PubMed] [Google Scholar]
- 210.Cao Y Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell metabolism 18, 478–489, doi: 10.1016/j.cmet.2013.08.008 (2013). [DOI] [PubMed] [Google Scholar]
- 211.Fredriksson JM, Lindquist JM, Bronnikov GE & Nedergaard J Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta -adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. The Journal of biological chemistry 275, 13802–13811 (2000). [DOI] [PubMed] [Google Scholar]
- 212.Marko SB & Damon DH VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol 294, H2646–2652, doi: 10.1152/ajpheart.00291.2008 (2008). [DOI] [PubMed] [Google Scholar]
- 213.Long JB, Jay SM, Segal SS & Madri JA VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol 334, 119–132, doi: 10.1016/j.ydbio.2009.07.023 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Robciuc MR et al. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell metabolism 23, 712–724, doi: 10.1016/j.cmet.2016.03.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.During MJ et al. Adipose VEGF Links the White-to-Brown Fat Switch With Environmental, Genetic, and Pharmacological Stimuli in Male Mice. Endocrinology 156, 2059–2073, doi: 10.1210/en.2014-1905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim KH et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res 27, 1309–1326, doi: 10.1038/cr.2017.126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xue Y et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell metabolism 9, 99–109, doi: 10.1016/j.cmet.2008.11.009 (2009). [DOI] [PubMed] [Google Scholar]
- 218.Sun K et al. Brown adipose tissue derived VEGF-A modulates cold tolerance and energy expenditure. Molecular metabolism 3, 474–483, doi: 10.1016/j.molmet.2014.03.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Zhao Y, e. a. Transient Overexpression of VEGF-A in Adipose Tissue Promotes Energy Expenditure via Activation of the Sympathetic Nervous System. bioRxiv, doi:doi.org/10.1101/323659 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jiang Y, Berry DC & Graff JM Distinct cellular and molecular mechanisms for beta3 adrenergic receptor-induced beige adipocyte formation. Elife 6, doi: 10.7554/eLife.30329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ueta CB et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. The Journal of endocrinology 214, 359–365, doi: 10.1530/JOE-12-0155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shah SH et al. Neuropeptide Y gene polymorphisms confer risk of early-onset atherosclerosis. PLoS Genet 5, e1000318, doi: 10.1371/journal.pgen.1000318 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sung CP, Arleth AJ & Feuerstein GZ Neuropeptide Y upregulates the adhesiveness of human endothelial cells for leukocytes. Circ Res 68, 314–318 (1991). [DOI] [PubMed] [Google Scholar]
- 224.Claxson A et al. The anti-inflammatory effects of D-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Actions 29, 68–70 (1990). [DOI] [PubMed] [Google Scholar]
- 225.Singer K et al. Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation. PloS one 8, e57929, doi: 10.1371/journal.pone.0057929 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pandolfi J et al. Purinergic signaling modulates human visceral adipose inflammatory responses: implications in metabolically unhealthy obesity. J Leukoc Biol 97, 941–949, doi: 10.1189/jlb.3A1214-626R (2015). [DOI] [PubMed] [Google Scholar]
- 227.Eltzschig HK, Sitkovsky MV & Robson SC Purinergic signaling during inflammation. N Engl J Med 367, 2322–2333, doi: 10.1056/NEJMra1205750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jo EK, Kim JK, Shin DM & Sasakawa C Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol 13, 148–159, doi: 10.1038/cmi.2015.95 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Enjyoji K et al. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes 57, 2311–2320, doi: 10.2337/db07-1265 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen Z et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Molecular metabolism 6, 863–872, doi: 10.1016/j.molmet.2017.03.016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jeong JH, Chang JS & Jo YH Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Scientific reports 8, 6672, doi: 10.1038/s41598-018-25265-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Akhmedov D et al. Gs-DREADD Knock-In Mice for Tissue-Specific, Temporal Stimulation of Cyclic AMP Signaling. Mol Cell Biol 37, doi: 10.1128/MCB.00584-16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rojas JM & Schwartz MW Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab 16 Suppl 1, 33–40, doi: 10.1111/dom.12332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cantu RC & Goodman HM Effects of denervation and fasting on white adipose tissue. The American journal of physiology 212, 207–212, doi: 10.1152/ajplegacy.1967.212.1.207 (1967). [DOI] [PubMed] [Google Scholar]
- 235.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ & Song CK Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Molecular and cellular endocrinology 318, 34–43, doi: 10.1016/j.mce.2009.08.031 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]


