Abstract
The genus Minthostachys belonging to the Lamiaceae family, and is an important South American mint genus used commonly in folk medicine as an aroma in cooking. The phytochemical-rich samples of the aerial parts of Minthostachys diffusa Epling. were tested for pharmacological and health-promoting bioactivities using in vitro chemical and enzymatic assays. A range of radical scavenging activities of the samples against biological radicals such as nitric oxide and superoxide anion and against synthetic 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals, the ferric reducing antioxidant power and the lipid peroxidation inhibition were determined and ranked using the ‘relative antioxidant capacity index’ (RACI). The ethyl acetate fraction showed the highest RACI of +1.12. Analysis of the various fractions’ inhibitory ability against enzymes involved in diabetes (α-amylase and α-glucosidase), and against enzymes associated with Parkinson’s or Alzheimer’s diseases (acetylcholinesterase and butyrylcholinesterase) also suggested that the ethyl acetate fraction was the most active. Liquid chromatography–tandem mass spectrometry analysis of the ethyl acetate fraction showed more than 30 polyphenolic compounds, including triterpenes. The inhibitory cholinesterase effects of the triterpenes identified from M. diffusa were further analysed by in silico docking of these compounds into 3D-structures of acetylcholinesterase and butyrylcholinesterase. This is the first study on pharmacological activities and phytochemical profiling of the aerial parts of M. diffusa, showing that this plant, normally used as food in South America, is also rich in health-promoting phytochemicals.
Keywords: Minthostachys diffusa, Lamiaceae, DPPH, beta-carotene bleaching, relative antioxidant capacity index (RACI), polyphenols, terpenoids, liquid chromatography–mass spectrometry analysis (UHPLC-MS/MS), flavonoids
1. Introduction
Plant metabolism produces numerous secondary metabolites (phytochemicals) that are very specific to each plant family and do not participate directly in the growth and development of the plant and [1,2]. Phytochemicals are known to possess a wide range of properties including antioxidant, hypoglycaemic, anticholinesterase, hypolipidemic, antiviral, antibacterial, antifungal, anti-inflammatory and cytotoxic activities as comprehensively reviewed by Pinakin et al. 2020 and Tang et al. 2019 [3,4]. Although plants are considered an important natural source for therapeutic applications with well-known ethnomedical uses in literature, yet they have been poorly investigated from the phytochemical point of view as exemplified by a recent review on the genus Tragopogon of Asteraceae family [5]. The plants belonging to Minthostachys genus are also among the less studied. The local populations call them “peperina” in Argentina and “muña” in the area from central Peru to Bolivia.
From the early 16th century the folklore medicinal use of the Minthostachys genus has been reported for the treatment of several health-disorders such as headache, cold and flu, respiratory illnesses (asthma, bronchitis, cough), digestive disorders (indigestion, carminative, stomach-ache, diarrhea, colics), muscle spasms, rheumatism, impotence and amenorrhea [6,7]. Other traditional uses of Minthostachys include biopesticides (antimycotic and antiparasitic, against flea infestations) and for the protection of stored potato and oca tubers from aphids and pests [6,7]. In recent years, there have been numerous research studies on Minthostachys oils to provide scientific evidences on their medicinal properties [8,9,10,11]. For instance, the main components of Minthostachys verticillata (M. verticillata) essential oil, namely pulegone (63.4%), menthone (15.9%), and limonene (2.1%), have been linked immediate-type allergic reactions in vitro and in vivo [8]. Montironi et al. (2016) have shown the bactericidal efficacy of M. verticillata essential oil against Streptococcus uberis strains isolated from bovine mastitis [9], while the essential oil of Minthostachys mollis (M. mollis) that largely contained pulegone (55.2%) and trans-menthone (31.5%) showed significant efficacy against Gram-positive and Gram-negative bacteria, especially Bacillus subtilis and Salmonella typhi, at 4 μg/mL [10]. A natural product, (−)-(1S,2R,3R,4S)-1,2-epoxy-1-methyl-4-(1-methylethyl)-cyclohex-3-yl acetate, isolated from the volatile constituents of Minthostachys tomentosa exhibited significant insecticidal activity against Oncopeltus fasciatus, whereas its synthetic form was found inactive [11]. However, there is no report on the effects of Minthostachys on chronic degenerative diseases such as diabetes and Alzheimer’s diseases that are associated with oxidative stress. According to the World Health Organization, there are nearly 422 million people worldwide with diabetes, which is one of the major causes of death globally (https://www.who.int/health-topics/diabetes). An increasing number of studies have also linked diabetes with neurodegeneration, which, for example, is involved in the development of Alzheimer’s disease [12,13,14].
Although the Minthostachys genus has received growing attention from modern pharmacology and medicine, the interest in this genus has been concentrated only on few species, mainly M. verticillata, M. mollis, Minthostachys andina or Minthostachys glabrescens, whereas very few studies have been focused on Minthostachys diffusa (M. diffusa) Epling [6,7,8,9,10,11]. M. diffusa is also known as “tusuwaya”, an endemic species prevalent in Bolivia, where the local population uses its aerial part as tea and to treat digestive, spasms and carminative disorders [6], but its properties and phytochemical composition have not been explored yet.
In this study, the aerial parts of M. diffusa powders were subjected to sequential extraction using solvents of different polarities. All the samples were tested for their antioxidant activity with different in vitro methods. Furthermore, in vitro assays have been used to assess the sample inhibitory activities on enzymes involved in diabetes (i.e., α-amylase and α-glucosidase) and neurodegenerative diseases (acetylcholinesterase and butyrylcholinesterase). Spectrophotometric and liquid chromatography–tandem mass spectrometry (LC-MS/MS) methods were performed on the most active samples to characterize and quantify the secondary metabolites responsible for their biological activities. In silico docking analysis to confirm the inhibitory effects of the identified compounds against cholinesterase enzymes was also carried out. To the best of our knowledge, this is the first study on the potential pharmacological activity as antioxidant, antidiabetic, and anticholinesterase of M. diffusa, besides its phytochemical characterization by LC-MS/MS analysis.
2. Materials and Methods
2.1. Chemicals
Solvents such as chloroform, ethanol, ethyl acetate, glacial acetic acid, hydrochloric acid, methanol, n-butanol, n-hexane, and phosphoric acid were purchased from Carlo Erba (Milan, Italy). Acetonitrile, formic acid and Leucine-Enkephalin were purchased from Merck (Wicklow, Ireland). Folin-Ciocalteu reagent, sodium carbonate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, β-nicotinamide adenine dinucleotide reduced form (NADH), phenazine methosulfate (PMS), nitrotetrazolium blue chloride (NBT), sodium nitroprusside dehydrate (SNP), sulfanilamide, N-(1-naphthyl)ethylenediamine dihydrochloride, sodium acetate trihydrate, 2,4,6-tripyridyl-s-triazine, 3,5-dinitrosalicylic acid, 4-p-nitrophenyl-α-d-glucopyranoside, 5,5′-dithio-bis(2-nitrobenzoic acid), α-amylase from hog pancreas (CAS number: 9000-90-2), α-glucosidase from Saccharomyces cerevisiae (CAS number: 9001-42-7), acetylcholinesterase (AChE) from Electrophorus electricus (electric eel, type VI-s, lyophilized powder), acetylthiocholine iodide, β-carotene, bovine serum albumin, butyrylcholinesterase (BChE) from equine serum (lyophilized powder), iron (III) chloride (FeCl3.6H2O), linoleic acid, potassium phosphate monobasic, potassium sodium tartrate tetrahydrate, s-butyrylthiocholine chloride, sodium chloride, sodium hydroxide, sodium phosphate, starch, trizma hydrochloride, aluminium chloride, galantamine, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), acarbose, butylhydroxytoluene (BHT), gallic acid, quercetin, linalool and Tween 20 were purchased from Sigma-Aldrich (Milan, Italy). Polyphenol standards for LC-MS/MS were purchased either from Extrasynthese (Genay, France) or from Merck (Wicklow, Ireland). Milli-Q water was obtained from Mill-Q purification system (Millipore, Bedford, MA, USA).
2.2. Plant Material and Samples Preparation
The aerial parts of M. diffusa (Md) were collected near the Aymaya community (18°26′54″ S to 66°27′36″ W; 3750 msnm), Bustillo province, Potosí department, Bolivia, in 2014. A voucher specimen was stored at the National University Siglo XX, Llallagua, Potosí, Bolivia.
The aerial parts were dried at room temperature. Briefly, 95 g of dried plant material were crushed and subjected to exhaustive dynamic maceration in a shaker set at 25 °C with 96% ethanol (Md EtOH) for 24 h. Four extractions at a solid to solvent ratio of 1:15 (w/v) per extraction were performed. The four Md EtOH extracts from each plant material were combined and filtered through a Buchner funnel (0.45 µm) and dried. Then, a part of this extract (7 g in 100 mL of water) was subjected to liquid/liquid extraction in triplicate using n-hexane, chloroform, ethyl acetate and n-butanol in order to separate the compounds on the basis of their increasing solvent polarity [15,16,17]. All fractions (n-hexane (MdH), chloroform (MdC), ethyl acetate (MdEA), n-butanol (MdB) and water (MdW)) were dried and stored in darkness at room temperature until further experimental use. The extraction yield was determined as follows:
Extraction yield of Md EtOH (% w/w) = [total obtained dried extract (gram)/initial dried plant materials (gram)] × 100;
Extraction yields of fractions (% w/w) = (single obtained dried fraction (gram)/initial dried Md EtOH subjected to liquid/liquid extraction (gram)) × 100.
2.3. Total Phenolic, Flavonoid and Terpenoid Contents
The Folin–Ciocalteu reagent was used to determine the total phenolic content present in the analysed samples using a colorimetric assay by adapting the method of Singelton et al. [18]. A calibration curve with gallic acid as a standard was made and the results were expressed as ‘milligrams of gallic acid equivalents per gram’ (mg GAE/g) of dried sample.
Total flavonoid content was determined using aluminum chloride as the reactant reagent and quercetin to obtain the standard curve [19]. The results were expressed as ‘milligrams of Quercetin Equivalents per gram’ (mg QE/g) of dried sample. Monoterpene linalool was used as standard reagent for the determination of total terpenoids content as described previously [20]. The results were expressed as ‘milligrams of Linalool Equivalents per gram’ (mg LE/g) of dried sample.
2.4. Antioxidant Activity
2.4.1. Radical Scavenging Activity
All M. diffusa samples were tested for their radical scavenging activity by four different in vitro chemical assays targeted against the biological super oxide anion (O2−) and nitric oxide (NO) radicals, and the synthetic neutral DPPH and cationic ABTS+ radicals [21]. The ability of the various samples to scavenge the radicals was monitored spectrophotometrically and the results were expressed as the concentration (in mg/mL) inhibiting 25% of radicals (IC25) or quantified in ‘milligrams of Trolox Equivalents per gram’ (mg TE/g) of dried sample.
2.4.2. Ferric Reducing Antioxidant Power Assay (FRAP)
The ability of samples to reduce the Fe (III) in Fe (II) was monitored in FRAP assay at 593 nm [22]. The Trolox was used as a standard and FRAP values were expressed as mg TE/g.
2.4.3. β-carotene Bleaching Assay
The capacity of samples at final concentration of 0.05 mg/mL to inhibit the lipid peroxidation was evaluated in the β-carotene emulsion at 470 nm [14]. BHT was used as positive control and the results were expressed as percentage of β-carotene bleaching inhibition (% Antioxidant Activity (%AA)) [15].
2.4.4. Relative Antioxidant Capacity Index (RACI)
No single chemical test can define a complete antioxidant capacity of a sample. For this reason, it is necessary to perform more than one in vitro antioxidant assay. However, the measurement scale of antioxidant of each method is different, which makes difficult to define the antioxidant capacity of the sample. Here a statistical method RACI that integrates the results obtained from different in vitro antioxidant assays was used. RACI is an arbitrary index which allows to rank the antioxidant capacity derived from different antioxidant methods.
RACI is derived by comparing the mean and the standard deviation of the raw data of each antioxidant method. The standard score represents the distance between the raw data and the mean in units of the standard deviation, which is negative when the raw data are smaller than the mean and vice-versa. The final data of RACI were represented in a histogram similar to previously described publications [15,17].
2.5. Potential Antidiabetic Activity
2.5.1. α-amylase Inhibition
The α-amylase enzyme from hog pancreas was mixed with different concentrations of each sample and starch used as substrates [23]. Briefly, the aromatic yellow-orange 3,5-dinitrosalicylic acid reagent was added that reacts with reducing sugars released from starch hydrolysis and other reducing molecules to form 3-amino-5-nitrosalicylic acid, which was subsequently monitored at 540 nm. The clinical antidiabetic drug, acarbose, was used as a positive control and the results were expressed as ‘milligrams of acarbose equivalents per gram’ (mg AE/g) of dried sample or as the concentration (in mg/mL) of the sample required to inhibit the activity of the enzyme by 50% (IC50) calculated by non-linear regression analysis.
2.5.2. α-glucosidase Inhibition
The inhibition of α-glucosidase enzyme was performed as previously described [23]. The α-glucosidase enzyme, the substrate 4-p-nitrophenyl-α-d-glucopyranoside, and different concentrations of each sample were mixed, and the reaction was spectrophotometrically monitored at 405 nm by the release of yellow p-nitrophenol. In this assay also, acarbose was used as a positive control and the results were expressed as mg AE/g or IC50.
2.6. Anticholinesterase Activity
The two prevalent forms of cholinesterase in a healthy brain are acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Ellman’s reaction to analyse the AChE and BChE inhibition by the samples was used as described before [17]. The natural drug galantamine, commonly used in the treatment of Parkinson’s and Alzheimer’s diseases, was used as a positive control and the results were expressed as ‘milligrams of galantamine equivalents per gram’ (mg GE/g) of dried sample or IC50.
2.7. Liquid Chromatography Tandem Mass Spectrometry Analysis of Polyphenols
The selected samples based on the highest biological activities, in particular ethyl acetate and n-hexane fractions of M. diffusa, were chosen for structural characterization of polyphenols on a Q-Tof Premier mass spectrometer coupled to an Alliance 2695 HPLC system (Waters Corporation, Milford, MA, USA). The quantification of identified polyphenols was performed using a Waters Acquity ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS), as described previously [15,17].
2.8. Molecular Docking
Structural homology models of Electrophorus electricus AChE and equine BChE, which were used in the in vitro inhibition assays, were generated by Swiss-model based on the structures of Tetronarce californica AChE (PDBID: 1GQS, with 70% sequence identity with E. electricus AChE) and Homo sapiens BChE (PDBID: 5LKR, with 91% sequence identity with equine BChE), respectively [24]. The resulting models were further refined by YASARA energy minimization [25]. In silico molecular docking of conformationally flexible terpenes identified in M. diffusa into semi-rigid homology models of AChE and BChE was performed with AutoDock Vina [26].
2.9. Statistical Analysis
All analysis and assays were performed in triplicates and the data were expressed as mean ± standard deviation. The correlation among used assays was verified by the calculation of p value by one-way analysis of variance (ANOVA) using GraphPad Prism 5 Software (San Diego, CA, USA). Only the p ≤ 0.05 was considered significant.
3. Results and Discussion
3.1. Extraction Yield and Influence of Solvents on Total Polyphenolic, Flavonoid and Terpenoid Contents
The exhaustive extraction of aerial parts of M. diffusa in the 96% ethanol showed a yield of 12.30 ± 1.07%. Previous studies on other species of the Minthostachys genus have reported varying extraction yields. For example, extraction yield of ethanolic extracts of the aerial parts of M. verticillata was 3.60% [27], which is considerably lower than the values obtained in our study. On the other hand, the infusions of M. mollis and M. verticillata yielded a high extraction yield of 20.80% [28,29].
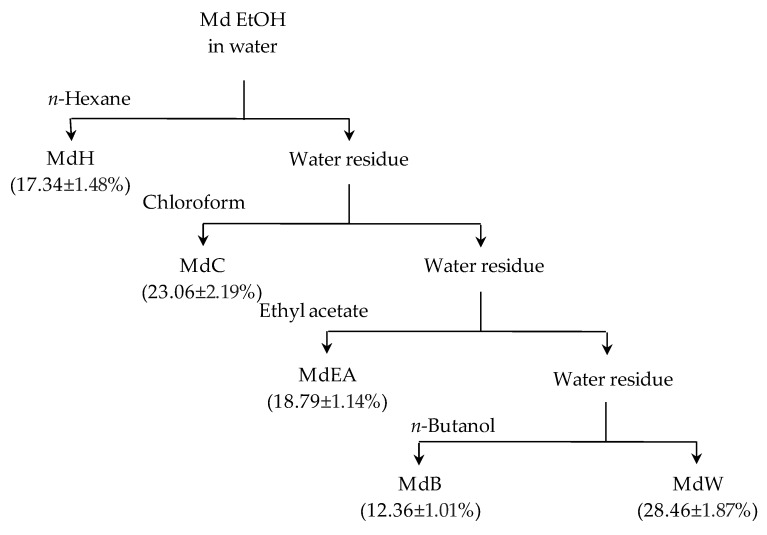
The extraction yields following the sequential liquid/liquid partitioning of ethanolic extract of M. diffusa (Md EtOH) in various solvents of different polarities are shown in Figure 1. The fractions that showed the highest extraction yields were water (MdW) and chloroform (MdC) fractions (28.46 ± 1.87% and 23.06 ± 2.19%, respectively). The butanol fraction (MdB) showed the lowest extraction yield (12.36 ± 1.01%).
Figure 1.
Extraction yields (%) of M. diffusa EtOH extract partitioned fractions with solvents of different polarities. Results are expressed as mean ± standard deviation of the triplicate experiments. Samples are crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA), n-butanol fraction (MdB) and water fraction (MdW).
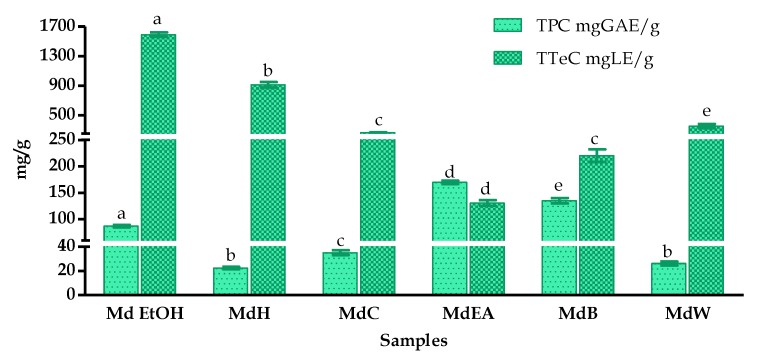
The samples from various partitioned fractions showed statistically significant differences in total phenolic content (TPC) and total terpenoid content (TTeC) as illustrated graphically in Figure 2. The mean TPC value of all fractions was 79.29 mg of GAE/g, where the MdEA and MdB fractions showed higher TPC values (i.e., 169.74 ± 3.10 and 135.11 ± 5.22 mg GAE/g, respectively) than other fractions. Similarly, the Md EtOH extract showed the highest TTeC (1590.31 ± 32.33 mg LE/g), which was significantly higher than the mean value of 577.80 mg LE/g. The total flavonoid content (TFC) assay was carried out only on M. diffusa fractions that presented a TPC value higher than the mean value, i.e., the Md EtOH, MdEA and MdB fractions. The Md EtOH fraction showed the highest TFC value (400.84 ± 26.94 mg QE/g) followed by MdEA and MdB (177.33 ± 14.05 and 114.23 ± 6.03 mg QE/g, respectively) (data not shown). Based on these findings, the phenolic, flavonoid, and terpenoid contents depend on the choice of solvents used for the extraction due to differences in the chemistry of these classes of compounds.
Figure 2.
Total Polyphenol Content (TPC) and Total Terpenoid Content (TTeC) of M. diffusa fractionated samples. Results are expressed as mean ± standard deviation of triplicate determinations in ‘mg of Gallic Acid Equivalents per gram’ (mg GAE/g) of dried sample and in ‘mg of Linalool Equivalents per gram’ (mg LE/g) of dried sample. In each test, the values with the same letter (a, b, c, d, e) are not significantly different at the 95% confidence limit according to one-way analysis of variance (ANOVA). Samples are crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA), n-butanol fraction (MdB) and water fraction (MdW).
3.2. Antioxidant Activity
Six complementary in vitro antioxidant assays were performed to determine the antioxidant activity of M. diffusa samples. The ability of samples to scavenge the biological superoxide anion (O2−) and nitric oxide (.NO) radicals was expressed as IC25 and results were compared with the ascorbic acid values.
All six samples caused a dose-dependent inhibition of superoxide anion. The butanol (MdB) fraction showed an IC25 of 0.26 ± 0.01 mg/mL that was very similar to that of ascorbic acid (IC25 of 0.26 ± 0.02 mg/mL). MdW and MdC fractions presented the lowest scavenging activity. This also corresponded with the biological nitric oxide scavenging assay, where the activity was detectable only in the MdB fraction (IC25 of 2.50 ± 0.15 mg/mL), which was better than the ascorbic acid (IC25 of 4.78 ± 0.09 mg/mL). On the contrary, the ethyl acetate (MdEA) fraction showed the highest radical scavenging-activity against synthetic radicals (Table 1) with 444.76 ± 28.24 mg TE/g and 281.64 ± 7.93 mg TE/g in ABTS and DPPH, respectively, followed by MdB (218.56 ± 9.38 and 122.30 ± 2.77 mg TE/g in ABTS and DPPH, respectively). The MdC and MdW fractions showed the lowest scavenging activity against the synthetic radicals. A similar trend was observed for the ferric reducing antioxidant power (574.86 ± 9.14 and 297.75 ± 5.71 mg TE/g for MdEA and MdB, respectively), while the MdC and MdH fractions were the least active. The inhibition of the lipid peroxidation determined by β-carotene bleaching (BCB) test showed the most active sample was the MdEA fraction (19.13 ± 0.93% AA), whereas the MdB and MdW fractions did not show any activity.
Table 1.
Results of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and Super Oxide anion (SO) scavenging activity, Ferric Reducing Antioxidant Power (FRAP) and β-Carotene Bleaching (BCB) of M. diffusa samples.
| Samples | ABTS (mgTE/g) |
DPPH (mgTE/g) |
SO IC25 (mg/mL) |
FRAP (mgTE/g) |
BCB %AA |
|---|---|---|---|---|---|
| Md EtOH | 146.07 ± 4.20 a | 113.05 ± 3.51 a | 0.92 ± 0.08 a | 224.67 ± 3.37 a | 10.12 ± 0.52 a |
| MdH | nc | nc | nc | 27.27 ± 0.99 b | 11.01 ± 0.33 a |
| MdC | 29.61 ± 0.74 b | 17.64 ± 0.03 b | 1.03 ± 0.08 a,b | 22.18 ± 0.90 b | 13.03 ± 0.49 b |
| MdEA | 444.76 ± 28.24 c | 281.64 ± 7.93 c | 0.62 ± 0.04 c | 574.86 ± 9.14 c | 19.13 ± 0.93 c |
| MdB | 218.56 ± 9.38 d | 122.30 ± 2.77 a | 0.26 ± 0.01 d | 297.75 ± 5.71 d | nc |
| MdW | 45.85 ± 0.42 b | 37.01 ± 1.63 d | 1.14 ± 0.09 b | 64.67 ± 1.63 e | nc |
Samples are crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA), n-butanol fraction (MdB) and water fraction (MdW). Data are expressed as means ± standard deviation from three experiments. Different superscripts in the same row (a, b, c, d, e) indicate significant difference (p < 0.05); nc = not calculable.
The correlation between the amount of polyphenols and the antioxidant activity of the fractions was evaluated by the Pearson correlation coefficient (Table 2). The highest correlation was observed between the total polyphenol content and radical-scavenging activity (rTPC/ABTS = 0.96 and rTPC/DPPH = 0.94) or ferric reducing power (rTPC/FRAP = 0.96). The ferric reducing power and the radical-scavenging activity against ABTS and DPPH radicals (rFRAP/ABTS = 1.00 and rFRAP/DPPH = 0.99) also displayed high correlation constants. There were also very good correlations between ABTS and DPPH assays (rABTS/DPPH = 0.99) and between NO and SO tests (rNO/SO = 0.91). The terpenoids were poorly correlated with the antioxidant activities (r < 0 for terpenoids against all assays except for BCB test rTTeC/BCB = 0.06).
Table 2.
Pearson correlation coefficient calculated among tested M. diffusa samples.
| TPC | TTeC | ABTS | DPPH | SO | NO | FRAP | BCB | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1.00 | |||||||
| TTeC | −0.26 | 1.00 | ||||||
| ABTS | 0.96 | −0.31 | 1.00 | |||||
| DPPH | 0.94 | −0.23 | 0.99 | 1.00 | ||||
| SO | 0.69 | −0.40 | 0.50 | 0.44 | 1.00 | |||
| NO | 0.44 | −0.31 | 0.21 | 0.13 | 0.91 | 1.00 | ||
| FRAP | 0.96 | −0.24 | 1.00 | 0.99 | 0.50 | 0.22 | 1.00 | |
| BCB | 0.26 | 0.06 | 0.40 | 0.43 | −0.40 | −0.58 | 0.38 | 1.00 |
Total Polyphenolic Content (TPC), Total Terpenoid Content (TTeC), ABTS assay, DPPH assay, Super Oxide anion scavenging activity (SO), Nitric Oxide radical scavenging activity (NO), Ferric Reducing Antioxidant Power assay (FRAP) and β-Carotene Bleaching assay (BCB).
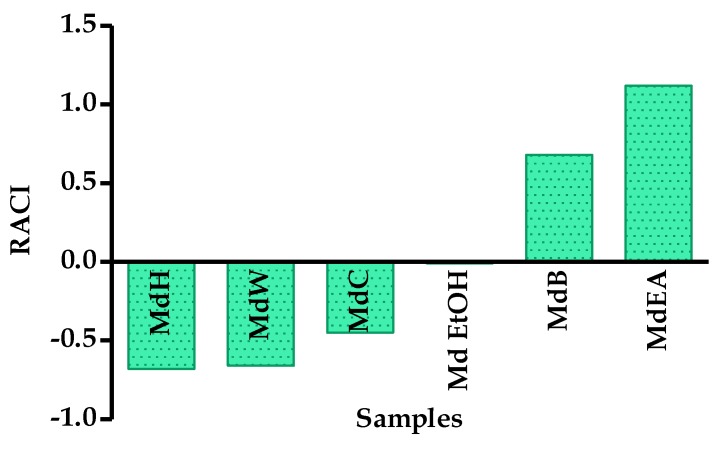
The integration of obtained results by the six different in vitro antioxidant assays was calculated through the relative antioxidant capacity index (RACI) in order to compare and rank the data (Figure 3). The MdEA fraction showed the highest RACI value (+1.12) followed by the MdB fraction (0.68). The MdW and MdH fractions presented the negative RACI (−0.66 and −0.68, respectively) implying a relative lack of antioxidant activity by these fractions.
Figure 3.
Relative Antioxidant Capacity Index (RACI) of different M. diffusa samples.
To the best of our knowledge, this is the first study of antioxidant activity of aerial parts of M. diffusa. However, studies on other species belonging to the Minthostachys genus are reported in the literature. The infusion extract of M. verticillata (2 g in 250 mL of boiling water) has been reported to have a low radical scavenging activity against the neutral DPPH due to its low phenolic content [28]. Interestingly, the essential oil obtained from leaves of M. spicata by hydrodistillation exhibited a higher DPPH radical scavenging efficacy (76.05 ± 2.40%) at 500 μg/mL and had an IC50 value of 82.19 ± 6.70 μg/mL, which may be compared to our results where the Md EtOH presented an IC50 value of 269.45 ± 8.24 μg/mL. The high activity in M. spicata was attributed to the major oil constituents (pulegone, isomenthone, and menthone) as well as oxygenated monoterpenes in general [29]. Nevertheless, the MdEA fraction, the most active sample of M. diffusa, showed an IC50 value of 108.14 ± 3.07 μg/mL after 30 min of incubation and IC50 of 90.04 ± 3.44 μg/mL after 90 min, which was close to that of essential oils from M. spicata leaves.
3.3. Potential Antidiabetic Activity
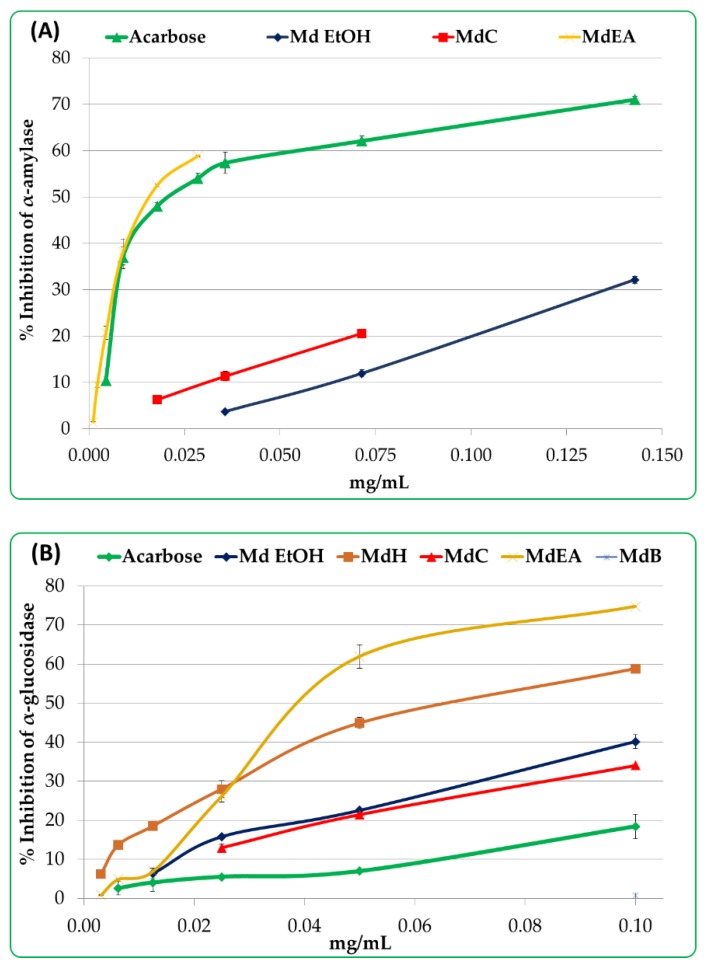
The capacity of the M. diffusa samples to inhibit enzymes, namely α-amylase and α-glucosidase that are involved in the pathogenesis of diabetes, were tested and the results show a concentration-dependent inhibition (Figure 4). The ethyl acetate fraction (MdEA) showed promising inhibition ability against α-amylase with an IC50 value of 16.40 ± 1.61 μg/mL. The MdH and MdB fractions did not inhibit α-amylase, while the MdW fraction had no effect in any of the two assays. In the α-glucosidase inhibition assay, IC50 for the MdB and MdW fractions was not reached in this concentration range.
Figure 4.
α-amylase (A) and α-glucosidase (B) inhibition activity of M. diffusa samples. Samples are acarbose, crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA) and n-butanol fraction (MdB).
To date, this is the first report on antidiabetic activity of the aerial parts of M. diffusa and of the Minthostachys genus as well. The results are very interesting and, in particular, the ethyl acetate fraction inhibitory activity against both the tested enzymes, and the n-hexane fraction against α-glucosidase activity.
3.4. Determination of Anticholinesterase Activity
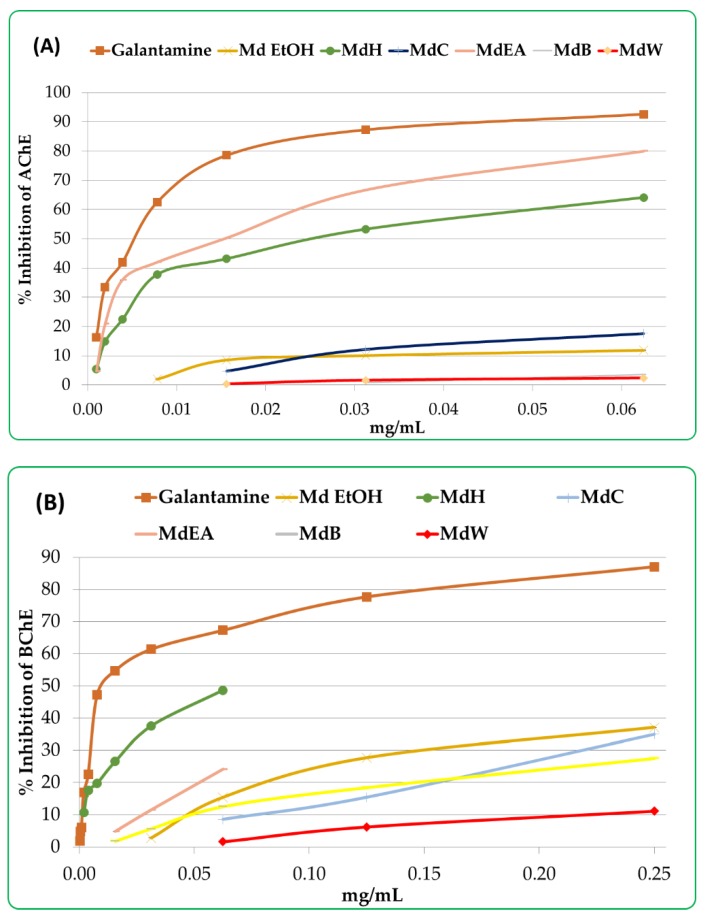
The inhibitory effects of the M. diffusa samples were also tested on acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE) activity and the enzymatic assays demonstrate a concentration-dependent activity (Figure 5).
Figure 5.
AChE (A) and BChE (B) inhibition activity of different M. diffusa samples. Samples are galantamine, crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA), n-butanol fraction (MdB) and water fraction (MdW).
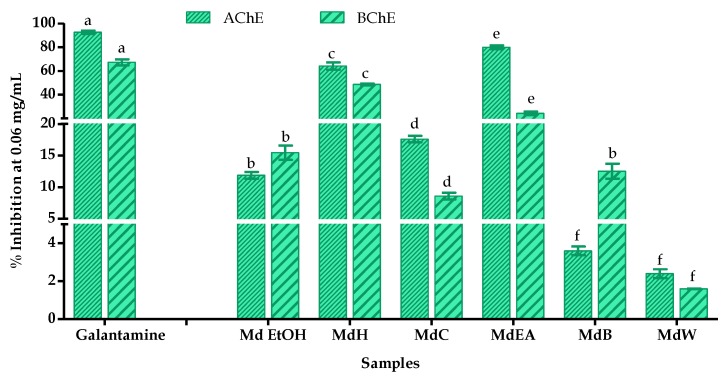
In order to assess the anticholinesterase activities, the results were expressed as percentage of inhibition at a normalized concentration of each sample and the positive control (galantamine) to 0.06 mg/mL (Figure 6). In doing so, the inhibition of AChE from all samples of M. diffusa was lower than that of galantamine (92.61 ± 1.41%). Amongst the samples, the MdEA and MdH fractions presented highest AChE inhibitions of 80.00 ± 1.49 and 64.11 ± 3.11%, respectively. A similar trend was observed in the BChE assays, where the MdH and MdEA fractions inhibited 48.65 ± 0.82 and 24.20 ± 1.57%, respectively that were lower compared to galantamine (67.26 ± 2.61%).
Figure 6.
AChE and BChE inhibition in % by M. diffusa samples and the positive control galantamine expressed as percentage of inhibition at 0.06 mg/mL. Samples are crude ethanol extract (Md EtOH), n-hexane fraction (MdH), chloroform fraction (MdC), ethyl acetate fraction (MdEA), n-butanol fraction (MdB) and water fraction (MdW). Data are expressed as mean ± standard deviation from three experiments. In each test, the values with the same letter (a, b, c, d, e, f) are not significant different at the p > 0.05 level, 95% confidence limit, according to one-way analysis of variance (ANOVA).
This is also the first report on anticholinesterase effects of M. diffusa extracts. To date, only the M. verticillata of Minthostachys genus have been tested for anticholinesterase potential [30]. In a similar approach to our experimental design, the ethanolic extract of M. verticillata was partitioned in CH2Cl2:H2O to obtain an organic and an aqueous fraction. Both showed poor AChE inhibitions (≤ 5% at 1 mg/mL). However, other species, such as the Salvia genus, belonging to the Lamiaceae family has been reported to possess good cholinesterase inhibition activities [31,32].
3.5. Identification and Quantification of Phytochemicals by Liquid Chromatography-Mass Spectrometry
The ethyl acetate fraction of M. diffusa (MdEA) was chosen for further LC-MS/MS characterization based on its highest RACI and, also, for its highest antidiabetic and anticholinesterase capacities. More than 30 compounds were detected and some of the polyphenols were (tentatively) identified, through accurate mass measurements, fragmentation pattern and aided by the existing literature, for the first time in M. diffusa and in general in the Minthostachys genus (Table 3). In the past, only the essential oil of other species belonging to Minthostachys genus had been profiled for its phytochemicals that showed mainly monoterpenes [33,34]. Fourteen compounds were identified in the MdEA fraction by comparing their retention times with those of the available commercial standards and subsequently quantified. M. diffusa predominantly contained flavonols (quercetin-3,4′-di-glucoside, rutin, quercetin-3-O-glucoside, quercetin-3-O-arabinoside, kaempferol-3-O-glucoside, and quercetin), flavones (apigenin-7-O-glycoside, luteolin-rutinoside), flavanones (hesperidin, and naringenin-7-O-glucoside), cinnamic acid derivatives (rosmarinic acid 4-coumaric acid and caffeic acid), and triterpenes (corosolic acid, betulinic acid, oleanolic acid and their derivatives).
Table 3.
Characterisation of phytochemicals present in ethyl acetate fraction of M. diffusa by liquid chromatography-tandem mass spectrometry. Quantities of the detected compounds were determined using commercial standards (in bolds); nq = not quantified.
| Pk. no. | RT (min) | [M-H]−m/z Observed | [M-H]−m/z Calculated | Predicted Molecular Formula |
MS/MS (m/z) |
Compound Identity | mg/g DW |
|---|---|---|---|---|---|---|---|
| 1 | 6.18 | 179.0367 | 179.0344 | C9H8O4 | 135, 79 | Caffeic acid | 2.49 ± 0.01 |
| 2 | 6.84 | 461.1769 | 461.1753 | C31H26O4 | 329, 301 | unknown | nq |
| 3 | 6.71 | 625.1441 | 625.1405 | C27H30O17 | 343, 301, 271, 255, 179, 151 | Quercetin-3,4′-di-glucoside | 0.03 ± 0.02 |
| 4 | 7.17 | 609.1473 | 609.1456 | C27H30O16 | 300, 285, 271, 255, 179, 151 | Rutin | 1.63 ± 0.07 |
| 5 | 7.37 | 463.0876 | 463.0877 | C21H20O12 | 300, 271, 255, 179, 151 | Quercetin-3-O-glucoside | 22.87 ± 0.25 |
| 6 | 7.67 | 505.0982 | 505.098 | C23H22O13 | 300, 271, 255, 179, 161, 151 | Quercetin derivative | Nq |
| 7 | 7.70 | 433.0742 | 433.0771 | C20H18O11 | 300, 271, 255, 179,151 | Quercetin-3-O-arabinoside | 1.92 ± 0.02 |
| 8 | 7.87 | 447.0921 | 447.0927 | C21H20O11 | 284, 255, 227 | Kaempferol-3-O-glucoside | 2.07± 0.15 |
| 9 | 8.00 | 609.1848 | 609.1819 | C28H34O15 | 325, 301, 286, 242, 199, 164, 125 | Hesperidin | 1.10 ± 0.09 |
| 10 | 431.0981 | 431.0978 | C21H20O10 | 269, 239, 224 | Apigenin-7-O-glycoside | 0.54 ± 0.05 | |
| 11 | 433.1125 | 433.1135 | C21H22O10 | 151, 107 | Naringenin-7-O-glucoside | 0.17 ± 0.01 | |
| 12 | 8.24 | 359.0766 | 359.0767 | C18H16O8 | 197, 179, 161, 135, 133, 123, 73 | Rosmarinic acid | 69.64 ± 1.53 |
| 13 | 8.73 | 609.1457 | 609.1456 | C27H30O16 | 463, 323, 300, 285, 271, 255, 179, 161, 151 | Quercetin-O-glucoside- rhamnoside | nq |
| 14 | 8.79 | 533.1882 | 533.1870 | C23H34O14 | 387, 374, 207, 163, 145, 119, 101 | Coumaric acid derivative | nq |
| 15 | 471.1216 | 471.1232 | C31H20O5 | 307, 205, 163, 145, 119, 101 | Coumaric acid derivative | nq | |
| 16 | 163.0392 | 163.0395 | C9H8O3 | 119, 93 | 4-Coumaric acid | 0.23 ± 0.02 | |
| 17 | 9.15 | 593.1697 | 593.1506 | C27H30O15 | 327, 309, 285, 270, 241, 164, 151 | Luteolin-rutinoside | nq |
| 18 | 285.0384 | 285.0399 | C15H10O6 | 151, 133 | Luteolin | 1.00 ± 0.00 | |
| 19 | 9.39 | 301.0362 | 301.0348 | C15H10O7 | 179, 151 | Quercetin | 2.07 ± 0.09 |
| 20 | 9.69 | 373.0908 | 373.0923 | C19H18O8 | 197, 179, 161, 135, 117, 107 | Rosmarinic acid methyl ester | nq |
| 21 | 11.34 | 487.3447 | 487.3424 | C30H48O5 | 469, 441, 405, 397,389, 85, 73 | Asiatic acid type | nq |
| 22 | 11.50 | 487.3447 | 487.3424 | C30H48O5 | 469, 441, 405, 397,389, 85, 73 | Asiatic acid type | nq |
| 23 | 12.03 | 829.4156 | 829.4163 | C48H62O12 | 811, 789, 667, 649, 553, 359, 179, 161, 135 | Rosmarinic acid derivative | nq |
| 24 | 12.82 | 471.3475 | 471.3474 | C30H48O4 | 427, 425, 409, 353, 337, 57 | Corosolic type triterpenoid | nq |
| 25 | 13.81 | 501.3549 | 501.3580 | C31H50O5 | 469, 421, 407, 389 | Asiatic acid methyl ester | nq |
| 26 | 14.18 | 813.4201 | 813.4214 | C48H62O11 | 651, 453, 359, 197, 179, 161, 135, 73 | Rosmarinic acid derivative | nq |
| 27 | 14.51 | 455.3527 | 455.3525 | C30H48O3 | 411, 393, 381, 351, 83, 71, 57 | Oleanolic type triterpenoid | nq |
| 28 | 15.22 | 469.3342 | 469.3318 | C30H46O4 | 451, 425, 421, 407, 391, 377, 353, 337, 137 | Corosolic type triterpenoid | nq |
| 29 | 15.67 | 471.3453 | 471.3474 | C30H48O4 | 453, 411, 353, 337, 121, 113, 97, 71, 57 | Corosolic type triterpenoid | nq |
| 30 | 16.38 | 469.3342 | 469.3318 | C30H46O4 | 451, 425, 421, 407, 391, 377, 353, 337, 137 | Corosolic type triterpenoid | nq |
| 31 | 17.04 | 469.3342 | 469.3318 | C30H46O4 | 451, 425, 421, 407, 391, 377, 353, 337, 137 | Corosolic type triterpenoid | nq |
| 32 | 17.41 | 471.3453 | 471.3474 | C30H48O4 | 453, 411, 353, 337, 121, 113, 97, 71, 57 | Corosolic acid | 4.06 ± 2.46 |
| 33 | 20.18 | 453.3460 | 453.3369 | C30H46O3 | 405, 391, 389, 371, 337, 97 | Oleanolic type triterpenoid | nq |
| 34 | 20.84 | 455.3521 | 455.3525 | C30H48O3 | 452, 407, 391, 389, 375, 373, 189, 183, 137 | Betulinic acid | 3.93 ± 0.63 |
| 35 | 21.33 | 455.3539 | 455.3525 | C30H48O3 | 407, 391, 389, 375, 373, 189, 183, 137, 97 | Oleanolic acid | 7.26 ± 1.56 |
The most abundant was rosmarinic acid (69.64 ± 1.53 mg/g DW) followed by the quercetin-3-O-glucoside (22.87 ± 0.25 mg/g DW). These phenolic compounds have been known for their antioxidant properties. Flavonoids such as luteolin and luteolin-glycosides have been reported to be extremely active with anti-inflammatory and antioxidant properties [35,36].
Triterpene and in particular, maslinic acid, betulinic acid, oleanolic acid as well ursolic acid have also been linked with several biological activities including antioxidant, cholinesterase and α-glucosidase inhibitions [37,38], which can explain the positive activity of the MdEA and MdH fractions. A vast majority of the tentatively identified compounds in Table 3 belonged to triterpenes, which were also present in the MdH fraction.
3.6. Potential Anticholinesterase Activity of Identified Triterpenes from MdEA and MdH Fractions
The phytochemical investigation of MdEA and MdH fractions led to the tentative identification of five triterpenes, in particular betulinic, corosolic and oleanolic acids in the MdEA fraction and betulinic, maslinic, oleanolic and ursolic acids in the MdH fraction.
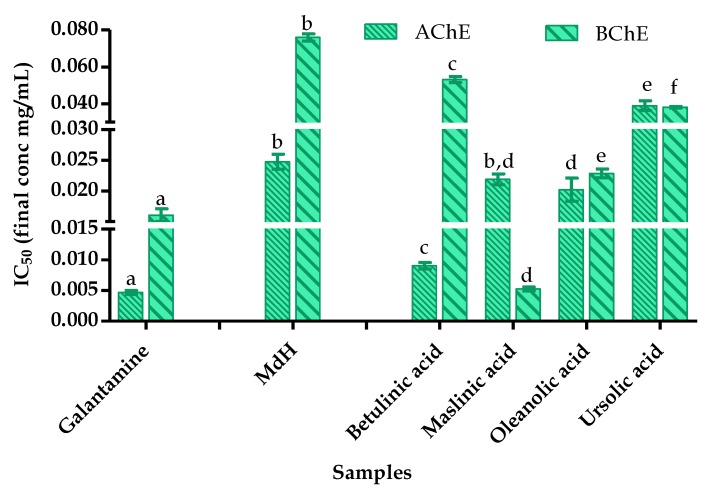
Terpenoids are reported as potential leads for the development of cholinesterase inhibitors [39,40,41]. For this reason, in vitro cholinesterase inhibition assays were also performed on the commercially available triterpenes betulinic, corosolic, maslinic, oleanolic and ursolic acids that were found in M. diffusa, in order to compare the activities of sample fractions containing these triterpenes. The results expressed as IC50 in mg/mL were compared with that of galantamine. All test compounds inhibited the AChE enzyme lesser than the galantamine, where the betulinic, maslinic and oleanolic acids showed IC50 of 0.009 ± 0.001 mg/mL, 0.022 ± 0.001 mg/mL and 0.020 ± 0.002 mg/mL, respectively. However, these triterpenes, on its own, were more active than the MdH fraction that contained all these triterpenes (IC50 of 0.025 ± 0.001 mg/mL, Figure 7). In BChE assay, only the maslinic acid (IC50 of 0.005 ± 0.001 mg/mL) inhibited the enzyme more effectively than the galantamine (IC50 of 0.016 ± 0.001 mg/mL). Nonetheless, all tested triterpenes inhibited the BChE enzyme more actively than the MdH fraction.
Figure 7.
AChE and BChE inhibition by galantamine and n-hexane fraction of M. diffusa and identified terpenes expressed as IC50 values in mg/mL. In each test, the values with the same letter (a, b, c, d, e, f) are not significant different at the p > 0.05 level, 95% confidence limit, according to one-way analysis of variance (ANOVA).
On the other hand, among all identified triterpenes from the MdEA fraction, only betulinic acid was more effective than the MdEA fraction (IC50 of 0.012 ± 0.001 mg/mL) in AChE inhibition. In the BChE assay, the MdEA fraction was not active and also the identified terpenes were less active than galantamine.
Triterpenoids, such as betulinic, corosolic, maslinic, oleanolic, and ursolic acids have been shown to have several biological activities including anticancer, cytotoxic, antitumor, antioxidant, anti-inflammatory, anti-HIV, anti-cholinesterase, α-glucosidase inhibition, antimicrobial, and hepatoprotective activities [37,38,39,40]. It has been demonstrated that betulinic acid also improves learning and memory of aged as well as scopolamine-induced amnesic rats. Furthermore, betulinic acid also decreased lipid peroxidation and nitrite level, and increased the levels of reduced glutathione and superoxide dismutase [40]. Ursolic acid isolated from Micromeria cilicica has been shown to inhibit AChE and BChE at IC50 of 93.80 and 41.10 μM, respectively [41]. In our assays, the IC50 values were similar: 85.35 ± 5.85 μM against AChE and IC50 of 83.50 ± 0.94 μM in BChE inhibition.
3.7. Molecular Docking of the Identified Terpenes into AChE and BChE
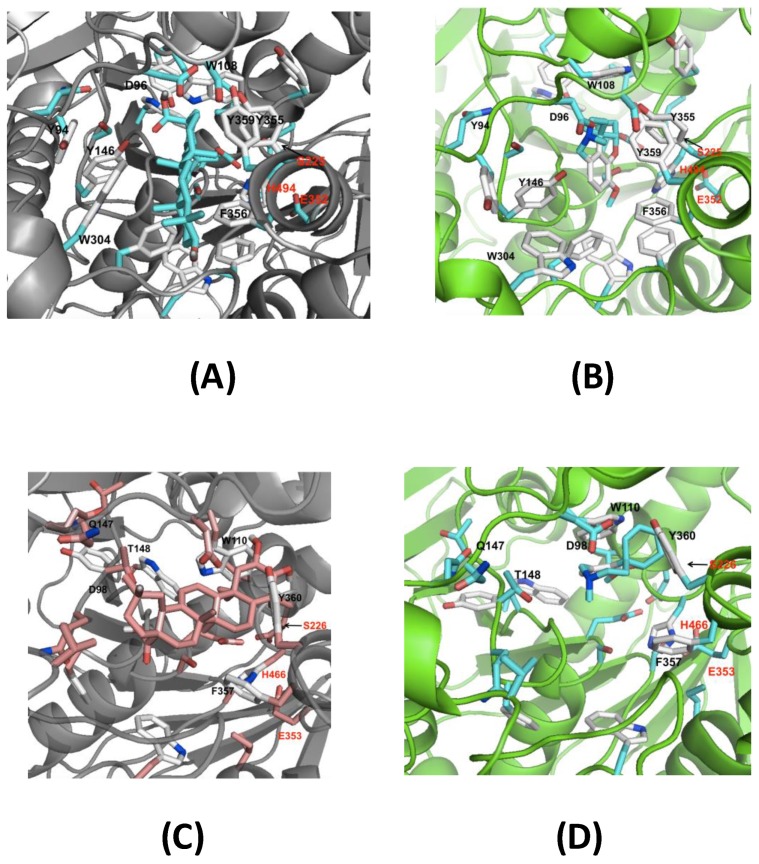
To get further insight into the inhibitory effects of the M. diffusa terpenes on AChE and BChE activity, structural homology models of these enzymes of the in vitro assays were made and used in docking studies. The docking procedure provides the treatment of ligand flexibility within the protein-binding site [42,43]. The results indicated that all terpenes tested had the ability to bind to the active sites of AChE and BChE with eminent affinities (estimated binding energies of −10 to −15 kcal/mol), which is in agreement with a previous study where some AChE-inhibiting compounds (corosolic, oleanolic and ursolic acids) were docked into the active site [42]. Galantamine binds close to the catalytic triad of AChE and BChE, whereas betulinic acid and maslinic acid, which were the most were the most active compounds in AChE and BChE assays, occupy almost the whole binding pocket surfaced with interacting aromatic residues in the corresponding enzyme (Figure 8). Thus, these results demonstrate that all of the terpenes have the potential of binding the active sites of both enzymes and provide valuable insights into the interactions of the inhibitors. The interesting inhibitory activity obtained from in vitro assays of terpenoids identified for the first time in M. diffusa suggests that follow-up studies of these compounds are warranted.
Figure 8.
Docking results of betulinic acid (A, central cyan moiety) and galantamine (B, central cyan/white moiety) in the homology model of AChE; maslinic acid (C, central salmon moiety) and galantamine (D, central cyan/white moiety) in the homology model of BChE. Residues interacting with the ligands are indicated in black and the residues of the catalytic triad in red.
4. Conclusions
The relative antioxidant capacity index (RACI) evidenced the ethyl acetate (MdEA) fraction of M. diffusa as the most active of the five different fractions and of the ethanol crude extract, which also showed the highest inhibition effects against α-amylase, α-glucosidase and cholinesterases. These health-promoting bioactivities may be attributed to the high content of polyphenols, in particular flavonoids and triterpenes. Molecular docking studies on individual authentic triterpenes have confirmed the anticholinesterase actions. The findings provide scientific explanation for the traditional uses of this specie and ascertain the health benefits of the infusions of M. diffusa commonly consumed by the local populations. These results also demonstrate that the M. diffusa represents a rich source of natural agents for nutraceuticals, food preservatives, functional foods, and cosmetics.
Author Contributions
Conceptualization, L.C., D.K.R., and L.M.; Methodology, I.F., D.R., A.C., E.F., L.C., M.M., D.K.R., and L.M.; Software, D.R., I.F., A.C., M.M.; Validation, L.C. D.K.R., and L.M.; Formal Analysis, D.R., I.F., A.C., E.F.; Investigation, D.R., I.F., A.C., E.F., and D.K.R.; Resources, L.C., D.K.R., and L.M.; Data Curation, L.C., M.M., D.K.R., and L.M.; Writing-Original Draft Preparation, D.R., I.F., D.K.R., E.F., M.M., and L.M.; Writing-Review & Editing, D.K.R., M.M. and L.M.; Supervision, L.M. and D.K.R.; Project Administration, L.M.; Funding Acquisition, L.C., D.K.R., and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Regione Basilicata; the Fondazione Enrico Mattei DGR n. 1490 del 4/12/2014, vs. rep. n. 163 n8; and the Regional Project ALIMINTEGRA, GO NUTRIBAS financed on 16.1 PSR Basilicata founding ex D.G.R. n° 312/17 CUP: C31G18000210002.
Conflicts of Interest
The authors declare no conflict of interests and they alone are responsible for the content and writing of the paper.
References
- 1.Buchanan B.B., Gruissem W., Jones R.L. Biochemistry & Molecular Biology of Plants. Volume 40 American Society of Plant Physiologists; Rockville, MD, USA: 2000. [Google Scholar]
- 2.Bourgaud F., Gravot A., Milesi S., Gontier E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001;161:839–851. doi: 10.1016/S0168-9452(01)00490-3. [DOI] [Google Scholar]
- 3.Pinakin D.J., Kumar V., Suri S., Sharma R., Kaushal M. Nutraceutical potential of tree flowers: A comprehensive review on biochemical profile, health benefits, and utilization. Food Res. Int. 2020;127:108724. doi: 10.1016/j.foodres.2019.108724. [DOI] [PubMed] [Google Scholar]
- 4.Tang G.-Y., Meng X., Gan R.-Y., Zhao C.-N., Liu Q., Feng Y.-B., Li S., Wei X.-L., Atanasov A.G., Corke H., et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019;20:6196. doi: 10.3390/ijms20246196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdalla M.A., Zidorn C. The genus Tragopogon (asteraceae): A review of its traditional uses, phytochemistry, and pharmacological properties. J. Ethnopharmacol. 2020;250:112466. doi: 10.1016/j.jep.2019.112466. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt-Lebuhn A.N. Ethnobotany, biochemistry and pharmacology of Minthostachys (Lamiaceae) J. Ethnopharmacol. 2008;118:343–353. doi: 10.1016/j.jep.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Lock O., Perez E., Villar M., Flores D., Rojas R. Bioactive compounds from plants used in Peruvian traditional medicine. Nat. Prod. Commun. 2016;11:315–337. [PubMed] [Google Scholar]
- 8.Cariddi L., Escobar F., Moser M., Panero A., Alaniz F., Zygadlo J., Sabini L., Maldonado A. Monoterpenes isolated from Minthostachys verticillata (Griseb.) Epling essential oil modulates immediate-type hypersensitivity responses in vitro and in vivo. Planta Med. 2011;77:1687–1694. doi: 10.1055/s-0030-1271090. [DOI] [PubMed] [Google Scholar]
- 9.Montironi I.D., Cariddi L.N., Reinoso E.B. Evaluation of the antimicrobial efficacy of Minthostachys verticillata essential oil and limonene against Streptococcus uberis strains isolated from bovine mastitis. Rev. Argent. Microbiol. 2016;48:210–216. doi: 10.1016/j.ram.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Mora F.D., Araque M., Rojas L.B., Ramirez R., Silva B., Usubillaga A. Chemical composition and in vitro antibacterial activity of the essential oil of Minthostachys mollis (Kunth) Griseb Vaught from the Venezuelan Andes. Nat. Prod. Commun. 2009;4:997–1000. doi: 10.1177/1934578X0900400726. [DOI] [PubMed] [Google Scholar]
- 11.Cantín Á., Lull C., Primo J., Miranda M.A., Primo-Yúfera E. Isolation, structural assignment and insecticidal activity of (−)-(1S,2R,3R,4S)-1,2-Epoxy-1-methyl-4-(1-methylethyl)-cyclohex-3-yl acetate, a natural product from Minthostachys tomentosa. Tetrahedron Asymmetry. 2001;12:677–683. [Google Scholar]
- 12.Roberts R.O., Knopman D.S., Przybelski S.A., Mielke M.M., Kantarci K., Preboske G.M., Senjem M.L., Pankratz V.S., Geda Y.E., Boeve B.F., et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132. doi: 10.1212/WNL.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre-Acevedo D.C., Lopera F., Henao E., Tirado V., Muñoz C., Giraldo M., Bangdiwala S.I., Reiman E.M., Tariot P.N., Langbaum J.B., et al. Cognitive decline in a colombian kindred with autosomal dominant Alzheimer disease: A retrospective cohort study. JAMA Neurol. 2016;73:431–438. doi: 10.1001/jamaneurol.2015.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redondo M.T., Beltrán-Brotóns J.L., Reales J.M., Ballesteros S. Executive functions in patients with Alzheimer’s disease, type 2 diabetes mellitus patients and cognitively healthy older adults. Exp. Gerontol. 2016;83:47–55. doi: 10.1016/j.exger.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Faraone I., Rai D., Chiummiento L., Fernandez E., Choudhary A., Prinzo F., Milella L. Antioxidant activity and phytochemical characterization of Senecio clivicolus Wedd. Molecules. 2018;23:2497. doi: 10.3390/molecules23102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamorte D., Faraone I., Laurenzana I., Milella L., Trino S., De Luca L., Del Vecchio L., Armentano M., Sinisgalli C., Chiummiento L. Future in the past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on Multiple Myeloma cells. Int. J. Mol. Sci. 2018;19:3348. doi: 10.3390/ijms19113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraone I., Rai D.K., Russo D., Chiummiento L., Fernandez E., Choudhary A., Milella L. Antioxidant, Aantidiabetic, and Aanticholinesterase Aactivities and Pphytochemical Pprofile of Azorella glabra Wedd. Plants. 2019;8:265. doi: 10.3390/plants8080265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 19.Lin J.-Y., Tang C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007;101:140–147. doi: 10.1016/j.foodchem.2006.01.014. [DOI] [Google Scholar]
- 20.Chakraborty S., Guchhait S., Saha S., Biswas S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoc. Exch. 2012;5:1038. [Google Scholar]
- 21.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 22.Stratil P., Klejdus B., Kubáň V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006;54:607–616. doi: 10.1021/jf052334j. [DOI] [PubMed] [Google Scholar]
- 23.Fidelis Q.C., Faraone I., Russo D., Aragão Catunda F.E., Jr., Vignola L., de Carvalho M.G., De Tommasi N., Milella L. Chemical and biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2019;33:1500–1503. doi: 10.1080/14786419.2017.1419227. [DOI] [PubMed] [Google Scholar]
- 24.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krieger E., Joo K., Lee J., Lee J., Raman S., Thompson J., Tyka M., Baker D., Karplus K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins Struct. Funct. Bioinform. 2009;77:114–122. doi: 10.1002/prot.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios S.M., del Corral S., Carpinella M.C., Ruiz G. Screening for natural inhibitors of germination and seedling growth in native plants from Central Argentina. Ind. Crops Prod. 2010;32:674–677. doi: 10.1016/j.indcrop.2010.05.004. [DOI] [Google Scholar]
- 28.Vaquero M.R., Serravalle L.T., De Nadra M.M., De Saad A.S. Antioxidant capacity and antibacterial activity of phenolic compounds from Argentinean herbs infusions. Food Control. 2010;21:779–785. doi: 10.1016/j.foodcont.2009.10.017. [DOI] [Google Scholar]
- 29.Solis-Quispe L., Tomaylla-Cruz C., Callo-Choquelvica Y., Solís-Quispe A., Rodeiro I., Hernández I., Fernández M.D., Pino J.A. Chemical composition, antioxidant and antiproliferative activities of essential oil from Schinus areira L. and Minthostachys spicata (Benth.) Epl. grown in Cuzco, Peru. J. Essent. Oil Res. 2016;28:234–240. doi: 10.1080/10412905.2015.1120691. [DOI] [Google Scholar]
- 30.Carpinella M.C., Andrione D.G., Ruiz G., Palacios S.M. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. 2010;24:259–263. doi: 10.1002/ptr.2923. [DOI] [PubMed] [Google Scholar]
- 31.Savelev S.U., Okello E.J., Perry E.K. Butyryl-and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004;18:315–324. doi: 10.1002/ptr.1451. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee P.K., Kumar V., Mal M., Houghton P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine. 2007;14:289–300. doi: 10.1016/j.phymed.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Alkire B.H., Tucker A.O., Maciarello M.J. Tipo, Minthostachys mollis (lamiaceae): An Ecuadorian mint. Econ. Bot. 1994;48:60–64. doi: 10.1007/BF02901380. [DOI] [Google Scholar]
- 34.Senatore F. Composition of the essential oil of Minthostachys spicata (Benth.) Epl. Flavour Fragr. J. 1995;10:43–45. doi: 10.1002/ffj.2730100107. [DOI] [Google Scholar]
- 35.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9:31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 36.Uma Devi P., Ganasoundari A., Vrinda B., Srinivasan K., Unnikrishnan M. Radiation protection by the Ocimum flavonoids orientin and vicenin: Mechanisms of action. Radiat. Res. 2000;154:455–460. doi: 10.1667/0033-7587(2000)154[0455:RPBTOF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Leal A.S.M. Ph.D. Thesis. Universidade de Coimbra; Coimbra, Portugal: 2012. Preparation and biological evaluation of new triterpene derivates of ursolic and oleanolic acid. [Google Scholar]
- 38.Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J. Enzym. Inhib. Med. Chem. 2011;26:616–642. doi: 10.3109/14756366.2010.546793. [DOI] [PubMed] [Google Scholar]
- 39.Jamila N., Khairuddean M., Yeong K.K., Osman H., urugaiyah V. Cholinesterase inhibitory triterpenoids from the bark of Garcinia hombroniana. J. Enzym. Inhib. Med. Chem. 2015;30:133–139. doi: 10.3109/14756366.2014.895720. [DOI] [PubMed] [Google Scholar]
- 40.Ruhal P., Dhingra D. Ameliorative effect of betulinic acid on ageing and scopolamine-induced learning and memory impairment in rats. Asian J. Pharm. Pharmacol. 2018;4:825–841. doi: 10.31024/ajpp.2018.4.6.17. [DOI] [Google Scholar]
- 41.Öztürk M., Kolak U., Topçu G., Öksüz S., Choudhary M.I. Antioxidant and anticholinesterase active constituents from Micromeria cilicica by radical-scavenging activity-guided fractionation. Food Chem. 2011;126:31–38. doi: 10.1016/j.foodchem.2010.10.050. [DOI] [Google Scholar]
- 42.Bahadori M.B., Dinparast L., Valizadeh H., Farimani M.M., Ebrahimi S.N. Bioactive constituents from roots of Salvia syriaca L.: Acetylcholinesterase inhibitory activity and molecular docking studies. South Afr. J. Bot. 2016;106:1–4. doi: 10.1016/j.sajb.2015.12.003. [DOI] [Google Scholar]
- 43.Geromichalos G.D., Lamari F.N., Papandreou M.A., Trafalis D.T., Margarity M., Papageorgiou A., Sinakos Z. Saffron as a source of novel acetylcholinesterase inhibitors: Molecular docking and in vitro enzymatic studies. J. Agric. Food Chem. 2012;60:6131–6138. doi: 10.1021/jf300589c. [DOI] [PubMed] [Google Scholar]