Abstract
Tissue engineering potentially offers new treatments for disorders of the temporomandibular joint which frequently afflict patients. Damage or disease in this area adversely affects masticatory function and speaking, reducing patients’ quality of life. Effective treatment options for patients suffering from severe temporomandibular joint disorders are in high demand because surgical options are restricted to removal of damaged tissue or complete replacement of the joint with prosthetics. Tissue engineering approaches for the temporomandibular joint are a promising alternative to the limited clinical treatment options. However, tissue engineering is still a developing field and only in its formative years for the temporomandibular joint. This review outlines the anatomical and physiological characteristics of the temporomandibular joint, clinical management of temporomandibular joint disorder, and current perspectives in the tissue engineering approach for the temporomandibular joint disorder. The tissue engineering perspectives have been categorized according to the primary structures of the temporomandibular joint: the disc, the mandibular condyle, and the glenoid fossa. In each section, contemporary approaches in cellularization, growth factor selection, and scaffold fabrication strategies are reviewed in detail along with their achievements and challenges.
1. Introduction
Tissue engineering of the temporomandibular joint (TMJ) focuses on regenerative solutions when surgical management of temporomandibular joint disorder (TMD) is required. An epidemiological study of TMD revealed that 60–70% of adults experience symptoms relating to TMD[3]. Overall, the diagnosis and treatment of TMD costs four billion dollars per year in the United States, affecting an estimated 20 million adults in 2006, according to the NIH[3]. Tissue engineering aims to improve the outcomes of patients suffering TMD by providing an alternative to total joint replacement (TJR). A review of current approaches used to treat TMD, alongside strategies applied to similar anatomical structures, may ultimately guide researchers to develop consistent TMD treatments when surgical intervention is required.
1-1. Anatomical and physiological overview of the TMJ
The TMJ is a ginglymoarthrodial joint consisting of three primary structures listed inferior to superiorly: the mandibular condyle, the articular disc, and the articular eminence and glenoid fossa (Fig. 1)[4]. The function of the TMJ is to provide the pivot point for mandibular motion during movements such as chewing and speaking[5]. During maximal opening, the range of motion consists of condyle rotation in the glenoid fossa and anteroposterior translation over the articular eminence. The mandible can also be translated laterally and anterior-posteriorly such as in retrusion and protrusion during mastication. Connective tissue surrounds the joint creating a capsule that is lubricated by synovial fluid. The joint capsule is divided into two compartments by the anchor points of the articular disc. The articular surfaces of the TMJ are covered by fibrocartilage instead of the typical hyaline cartilage found on the articulating surfaces such as the knee and hip joints[6]. The primary nutrition source runs through the retrodiscal tissue termed the maxillary artery, but also, branches from blood vessels within a 3 cm radius contribute to the TMJ disc[7].
Figure 1.
Anatomic visualization of the TMJ.
1-2. Etiology and diagnosis of TMD
The primary symptom of TMD is the presence of pain in the TMJ area, and additional symptoms include popping, grinding, and locking in the joint[3, 8]. These problems can result in compromised joint function and reducing maximum mouth opening from 52 mm of a normal adult to less than 20 mm[9]. TMD includes disc dislocation, osteoarthritis, degenerative joint disease, and muscle pain[10]. Also, there have been multiple studies that focus on the link between TMD and depression, but whether mental disorders are a cause or a result of TMD is still debatable[11, 12]. To diagnose TMD, researchers recently revised the diagnosis criterion which consists of 81 questions which focus on the location of the pain, joint function, and psychological distress. Joint disease can be confirmed by computed tomography (CT) scans or magnetic resonance imaging (MRI), especially in the case of disc displacement[13, 14].
The etiology of TMD has been associated with gender, parafunction, malocclusion, trauma, and psychological factors, yet often the underlying cause is often unknown. Chisnoiu et al. recently published a review that detailed the etiology of TMD[15]. Gender is the most prominent risk factor for TMD with symptoms occurring four times as often in females as compared to males. However, the reason for the discrepancy has not been linked to hormonal or behavioral factors. It is worth noting in a rat model, elevated levels of testosterone do decrease pain in the TMJ after formalin induction[16]. A heavily debated topic is the correlation between TMD and malocclusion. Many publications have concluded malocclusion is not an underlying cause of TMD, but actually may result from TMD[17–19]. Parafunctions such as bruxism and excessive gum chewing have also been linked to increasing the risk of TMD[20, 21]. This correlation is likely due to the increased loading of the TMJ as evident by finite element analysis[22]. Trauma due to fracture or whiplash has also been evaluated as a contributing factor for TMD, and both of these injuries are correlated with an increased risk of TMD[21, 23, 24].
1-3. Conservative treatment
TMD is often treated primarily with conservative options as the symptoms often spontaneously disappear. Exercise consisting of stretching and manual movement of the TMJ has been demonstrated to improve maximal mouth opening and reduce pain; however, these activities have not been shown to restore the morphology of the TMJ[25]. Splints are used to reduce muscle strain and temporarily correct mandible malalignment and come in a variety of materials and styles[26, 27]. The use of stabilization splints has had inconsistent results in treating TMD. There is controversy over patient whether the splints reduce pain, and finite element analysis suggests these splints do not reduce pressure on TMJ components[28, 29]. In contrast, anterior repositioning splints consistently provide relief to patients suffering from disc displacement and general TMD symptoms[29, 30]. Another treatment is the use of pharmacological agents such as NSAIDs, muscle relaxers, corticosteroids, and antidepressants to reduce TMD pain[31]. Even though clinical studies of medications to treat TMD are rare, most evidence suggests pharmaceuticals are effective in lowering TMD symptoms but are often associated with side effects such nausea and dizziness[31,32].
1-4. Minimally invasive treatment
If conservative treatments are ineffective, there are minor procedures that can be employed to improve TMD symptoms such as arthrocentesis, arthroplasty, and hyaluronic acid injections. Arthrocentesis is an office visit procedure performed by lavaging the joint capsule with a solution that may contain steroids. A systematic review suggested arthrocentesis improved symptoms in over 83% of TMD cases making arthrocentesis a viable treatment option[33]. Another common treatment option is arthroscopy, which involves the practitioner inserting a small camera into the joint along with other tools to remove debris, lavage, and reposition the articular disc. Arthroscopy is considered a safe procedure and is generally as effective in treating TMD as arthrocentesis with the added advantage of visualization of the joint for more accurate diagnosis[34, 35]. Hyaluronic acid injections are also being considered for use in treating TMD, but have remained outside of routine clinical use. A recent study compared hyaluronic acid injections to stabilization splints to address TMJ disc displacement with reduction and found both groups decreased pain significantly, and the hyaluronic acid injections were significantly more effective than the stabilization splints[37].
1-5. Major surgery
When more conservative treatments fail, or the symptoms are too severe, open surgery may be required. Surgical procedures for TMD include discectomy, condylectomy, and in extreme cases, TJR may be necessary. Discectomy, or the removal of the articular disc, has consistently been demonstrated to reduce pain and improve joint function over at least five years[38]. To further mitigate crepitus and degradation of the condyle, surgeons have used a host of materials to cushion the joint after disc removal albeit with limited success[39, 40]. Condylectomy is implemented to repair damage to the mandibular condyle including bony erosion, and joint immobility, also called ankylosis [41]. The procedure often consists of resecting the upper portion of the condyle and replacing it with a costochondral autograft that has been tissue harvested from a rib of the patient. Overall, publications suggest condylectomy treats TMD in over 80% of cases when the patient presents with joint ankylosis or with failure of conservative treatment[42, 43]. TJR devices have been used with reasonable outcomes with some achieving over 90% success. Patients reported decreased pain and an increase in maximal opening as compared to pre-surgery immediately, as well as 3, 5, and 20 years post-surgery[44, 45].
1-6. The role of tissue engineering
Disc replacement materials, structural degradation, and alternatives to TJR are all areas where tissue engineering may provide improved solutions. Concerning disc replacements, the infamous Teflon-protoplastic implants of the 1960s provided patients with immediate relief from the symptoms associated with TMD[46]. However, the implants ultimately degraded leading to implant failure, osseous degeneration, foreign body granulomas, and pain[47]. Use of adipose tissue to cushion the joint is also hindered by the rapid reduction in the volume of the graft. A tissue engineering approach may overcome these issues of limited longevity by generating viable tissue capable of self-renewal with normal function. For bone regeneration, tissue engineering may improve the restoration of complex structures such as the condyle and fossa through anatomically accurate and osteoinductive scaffolds. Eventually, tissue engineering devices may even reduce the need for TJR devices by giving surgeons the tools to regenerate the damaged structures of the TMJ completely. Challenges for this approach include an optimal selection of cells, scaffold materials, and growth factors that work together. The purpose of this review is to provide a comprehensive description of current strategies used in tissue engineering for each component of the TMJ and to provide insight into which approaches show the most promise.
2. Articular Disc
2-1. Anatomy
The primary function of the articular disc is to provide a cushion during locomotion because the condyle and fossa are incongruent which would otherwise produce points of high stress[48]. The disc, housed in the joint capsule, is attached mediolaterally to the condylar head through the collateral ligaments, anteriorly to the joint capsule and the lateral pterygoid, and posteriorly to the glenoid fossa[49]. The posterior attachment is referred to as the retrodiscal tissue. This region is where the disc blends into highly vascularized and innervated ligaments inserting into the condyle and the tympanic plate[50]. The TMJ disc is concave in the inferior portion in which the condyle rests and concavo-convex or saddle-like in the superior portion for ease of movement across the articular eminence and glenoid fossa.
The disc can be divided into three sections: the anterior band, the intermediate region, and the posterior band[49]. The medial portion of the disc is the thinnest portion, yet it contains the highest density of collagen fibers allowing it to handle high stress during loading[51]. The collagen fibers consist of both thick and thin strands that are orientated anteroposterior in the center of the disc as seen in Fig. 2–A. In the distal portions of the disc, the fibers run parallel to the outer edge resulting in a ring formation following the periphery of the disc[51–53].
Figure 2.
Anatomy and tissue engineering strategies for the articular disc. Anatomy of the articular disc (A), and attempted tissue engineering strategies specific for the disc (B).
Collagen is the primary material of the disc comprising 37% of the weight of the hydrated disc. Collagen I, II, and III are present in the disc with type I being the predominant [54]. Embedded within the collagen, elastin, and glycosaminoglycans (GAGs) make up 3–7% and 1–10% of the dry weight of the disc, respectively[55]. The elastin fibers are generally oriented parallel to the collagen and are thought to aid in restoring the shape of the disc after loading[51]. The GAGs are located primarily in the intermediate zone and are believed to improve the compressive strength of the disc since GAGs perform this role in hyaline cartilage[55]. The overall structure gives the human TMJ disc Young’s modulus of 11–16 MPa in the mediolateral direction and 9–15 MPa in the anteroposterior direction[56].
The cells responsible for forming the structure of the disc, based on a porcine model, are chondrocytes and fibroblasts with a ratio of 30% chondrocytes and 70% fibroblasts[57]. The cell density of TMJ disc is 681 ± 197 cells/mm2, and slightly higher concentration of cells can be found in medial-lateral portions of the disc. Of note, the native chondrocytes are sometimes referred to as fibrochondrocytes as these cells do not exhibit the pericellular matrix capsule similar to articular chondrocytes[58]. Also, there are sporadic blood vessels throughout the disc, and perforation induces neovascularization in the disc[59]. The following sections describe tissue engineering strategies that have been applied to the articular disc and a short summary can be viewed in Fig 1–B.
2-2. Cells
Many cell types can be utilized for seeding scaffolds for articular disc replacement or partial regeneration including differentiated chondrocytes, stem cells, and induced pluripotent stem cells (iPSCs). Seeding constructs with autologous chondrocytes improves tissue regeneration rates while avoiding rejection concerns associated with allografting cells. However, spontaneous dedifferentiation during expansion is a challenge associated with using differentiated cells [60]. Mesenchymal Stem cells (MSCs) are an attractive alternative because they can be stably stored and differentiation into desired cell types can be controlled with growth factor induction [61]. Utilizing iPSCs in TMJ disc tissue engineering has yet to occur, however, this is a promising approach for regeneration of the TMJ disc because iPSCs can be readily generated and differentiated into cell types that form the TMJ such as rare fibrochondrocytes that are present in the disc [62].
2-2-1. Stem cells
MSCs can be harvested from adult and embryonic tissues, and offer low immunogenicity while retaining the ability to differentiate[63]. MSCs have been collected from a multitude of sites for TMJ disc bioengineering including adipose tissue[56], bone tissue[64], and synovial fluid[65]. Bone marrow-derived stem cells (BMSCs) have been used to regenerate the disc, but the harvesting procedure entails significant donor-site morbidity[66]. In a rabbit TMJ disc perforation model, autologous BMSCs were collected from the femur and were seeded into collagen scaffolds[67]. After eight weeks, the rabbits implanted with the seeded scaffolds exhibited dense connective tissue at the site of the initial perforation while the rabbits implanted with empty scaffolds merely demonstrated reduced perforation diameters.
Adipose tissue provides a more readily available source of stem cells, however adipose-derived MSCs (ADMSCs) require more growth factors than BMSCs for chondrocyte differentiation[68]. Gene expression of ADMSCs was assessed in vitro after culturing in differentiation media supplemented with transforming growth factor beta-1 (TGF-β1); The ADMSCs expressed similar levels of collagen I, however collagen II, collagen X, and aggrecan were significantly lower compared to the TMJ disc cells[69]. For an in vivo assessment, differentiated ADMSCs were embedded in a polymeric scaffold and sutured to the zygomatic arch post-excision of the TMJ disc in a rabbit model[70]. The condyle head treated with the differentiated cells scaffold retained a more native cartilage surface as compared to the control, but the displacement of the scaffold may have compromised the outcome.
Less frequently studied stem cells that are suitable for disc regeneration are synovium-derived stem cells (SDSCs) and dental pulp stem cells (DPSCs). SDSCs, harvested from the knee of a mouse, were injected into a meniscus defect and demonstrated improved regeneration of cartilaginous tissue[71]. Also, Shirakawa et al. compared BMSCs to SDSCs cultured as cell pellets in chondrogenic media and found similar proliferation rates, and the SDSCs produced more cartilage in the pellets[72]. When TMJ-SDSC-seeded scaffolds were implanted subcutaneously in a murine model, the seeded scaffold produced measurable levels of GAG and collagen compared to the minimal levels provided by the scaffold control[65]. DPSCs were investigated as a potential stem cell source for the TMJ disc because they are readily harvested and show promise for chondrogenic differentiation[73]. The DPSCs were seeded on 3D construct and culture in chondrogenic media for upwards of 8 weeks. Real-time polymerase chain reaction (qPCR) and histology demonstrated the DPSCs had upregulated expression of chondrogenic markers and were capable of depositing a cartilaginous extracellular matrix (ECM) (Fig. 3).
Figure 3.
Histology and qPCR data for DPSCs in chondrogenic media. Imaging for cartilage deposition in the ECM was performed using hematoxylin and eosin stain, alcian blue, and immunohistochemistry for aggrecan. DPSCs cultured in chondrogenic media for 14 days were assess using qPCR for chondrogenic markers Sex determining region Y-box 9 [Sox9], Collagen I [COL I], Collagen II [COL X], Aggrecan [ACAN], Cartilage Oligomeric Matrix Protein [COMP] and compared to the baseline of day 7 gene expression. Scale bars are 50 μm; error bars represent SD. Modified from “Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration” by Bousnaki et al. with permission from Springer Nature, 2018[73].
Other potential sources of stem cells include dermal derived stem cells (DDSCs) and iPCSs. Due to their novelty, DDSCs have yet to be used in TMJ disc engineering. They are active producers of cartilage when induced with aggrecan surfaces, bone morphogenetic protein 2 (BMP-2), TGF-β1, or hypoxic conditions[74, 75]. Also, TGF-β1 can substantially increase the mechanical properties of DDSC-seed scaffolds, which is often valuable in scaffolds for the TMJ disc[75]. iPSCs are a renewable cell type with the potential to be used as personalized cell therapy[76]. While iPSCs have not been used to regenerate fibrocartilage directly, an effort to use iPSCs to regenerate articular cartilage has resulted in the formation of fibrocartilage[77]. Also, a new protocol for rapid chondrocyte induction has been developed thereby increasing the attractiveness of using iPSCs in clinical work[78, 79].
2-2-2. Somatic Cells
Autologous chondrocytes can be harvested from the patient, expanded, and reintroduced in association with the scaffold to the site of the defect. However, donor site morbidity, dedifferentiation, and expansion all pose challenges for this cell source. Cells from the native disc of the TMJ, dermal fibroblasts, and costal chondrocytes were compared for collagen deposition and cell proliferation, and it was found that the costal chondrocytes outperformed the other cell types[80]. To address the de-differentiation issue of these cells, Johns et al. compared costal chondrocytes at passage number five to freshly harvested cells, and the passaged cells demonstrated equivalent capabilities of depositing collagen and GAGs[80]. A direct comparison of costal to hyaline chondrocytes in 3D agarose constructs was also performed, and costal chondrocytes produced more GAGs but failed to produce more collagen[81]. Furthermore, the collagen produced by the costal chondrocytes contained a high concentration of type II collagen. Of note, passaged costal chondrocytes produced more collagen and a more robust ECM pellet than the initial harvest of chondrocytes suggesting more cells can be obtained through multiple passages without loss of function. Recently, costal chondrocytes were used to develop a cartilage sheet using an aggregate redifferentiation method to repair a TMJ disc perforation in a minipig model[82, 83]. Once the self-assembled sheet demonstrated similar mechanical properties to the native tissue, the sheet was implanted for eight weeks. The repaired discs showed improved outcomes as determined by histology, percent closure, mechanical testing, and osteoarthritis scoring (Fig. 4).
Figure 4.
Self-assembled cartilage constructs implanted in a minipig TMJ disc perforation model assessed after eight weeks. Histology (A), defect perimeter closure (B), mechanical testing (C), and osteoarthritis [OA] score (D) all indicate the tissue engineer [TE] implant group improved wound healing. Scale bar is 2 mm; error bars represent SD. Reproduced from “Tissue engineering toward temporomandibular joint disc regeneration” by Vapniarsky et al. with permission from AAAS, 2018[83].
2-3. Growth factors
To supplement the few studies that have reported the impact of growth factors directly on TMJ articular disc cells, also included here are studies that examine the effects of growth factors on chondrocytes for fibrocartilage production. The prominent growth factors for the TMJ disc are fibroblast growth factor 2 (FGF-2), TGF-β1, and insulin growth factor (IGF); others include platelet-derived growth factor (PDGF), epidermal growth factor (EGF), interleukin 1 (IL-1), high mobility group 1 protein, and tumor necrosis factor alpha (TNF-α)[57, 84–89]. Based on these publications, many of the proposed growth factors increased parameters relevant to the TMJ disc such as proliferation, collagen production, and GAG production. However, only TGF-β1 was demonstrated to enhance the mechanical properties of the cell-embedded scaffold[86].
To improve TMJ constructs, researchers have used growth factors in combinational applications involving concomitant delivery, sequential delivery, or spatial delivery. Controlled delivery of multiple growth factors can improve the healing process because natural healing requires more than one growth factor to be upregulated, and often involves concentrations of growth factors varying in a time-dependent manner[90]. Also of note, these studies have only delivered growth factor proteins, thus gene-based growth factors remain mostly uninvestigated in TMJ disc cells[91].
In many of the experiments described in table 1, growth factors were incubated in the media to allow for interaction with the cells. Addition of growth factors to a scaffold has been achieved by embedding the TGF-β1 protein in poly-(lactic-co-glycolic acid) (PLGA) microparticles and adding the particles to a polycaprolactone (PCL) powder for fused deposition modeling fabrication[93]. PLGA undergoes bulk erosion which facilitates extended drug release. In addition, PLGA is a thermal insulator, so PLGA can protect the protein during the hot-melt extrusion process required by fused deposition modeling[94]. Fluorescent PLGA particles were embedded in the PCL scaffold, and confocal images demonstrated spatial control of the particles was achieved[2]. This concept was also utilized to 3D print an entire TMJ disc where microparticles loaded with connective tissue growth factor protein were incorporated throughout the scaffold and TGF-β3-microparticles were distributed in the center of the scaffold. TGF- β3 induces aggrecan deposition, so by design, the aggrecan deposition would mimic the native tissue of the disc.
Table 1.
List of growth factors that have been used in TMJ disc engineering.
| Growth Factors | Amount; Time | Structure | Result | Cite |
|---|---|---|---|---|
| FGF-2 | 10 ng/mL, 100 ng/mL; 2 weeks | Porcine TMJ disc cells, cell culture wells | Greatly increased cell proliferation and increased GAG and collagen production | [84] |
| 10 ng/mL, 100 ng/mL; 3, 6 weeks | Porcine TMJ disc cells, PGA mesh | No increase in mechanical strength, high collagen production with 10 ng | [92] | |
| 10 ng/mL; 3, 6 weeks | Costal Chondrocytes, Agarose Gel | Increased cell proliferation, Decrease in GAG, collagen, and mechanical strength | [85] | |
| 3 ng/mL; 5, 20, 60, 120 minutes | Bovine TMJ disc cells, cell culture wells | 4 and 8 fold increase in Erk1/Erk2 and p38 phosphorylation | [88] | |
| TGF-β1 | 5/ 30 ng/mL; 3, 6 weeks | Porcine TMJ disc cells, PGA mesh | No increase in mechanical strength, increase in collagen and GAG | [92] |
| 1 ng/mL; 3, 6 weeks | Costal Chondrocytes, Agarose Gel | Decrease in GAG, collagen, and mechanical strength | [85] | |
| 1 ng/mL; 5, 20, 60, 120 minutes | Bovine TMJ disc cells, cell culture wells | No increase in Erk1/Erk2 or p38 phosphorylation | [88] | |
| 0.00–3.00 ng/mL; 24 hours | Bovine TMJ disc cells, cell culture wells | Increase in cell proliferation | [87] | |
| 10 ng/ml; 4 weeks | Bovine chondrocytes and fibrochondrocytes, agarose gels | Increase in collagen production and mechanical strength, but no increase in GAG production | [86] | |
| IGF | 10 ng/mL, 100 ng/mL; 2 weeks | Porcine TMJ disc cells, cell culture wells | Increased cell proliferation, increased collagen production | [84] |
| 10 ng/mL, 100 ng/mL; 3, 6 weeks | Porcine TMJ disc cells, PGA mesh | No increase in mechanical strength, increased collagen production | [92] | |
| 100 ng/mL; 3, 6 weeks | Costal Chondrocytes, Agarose Gel | Increased cell proliferation | [85] | |
| 5 ng/ml; 4 weeks | Bovine chondrocytes and fibrochondrocytes, agarose gels | No increase in mechanical strength, collagen production, or GAG production | [86] | |
| PDGF | 10 ng/mL, 100 ng/mL; 2 weeks | Porcine TMJ disc cells, cell culture wells | Increased cell proliferation, increased GAG production | [84] |
| 10 ng/mL; 3, 6 weeks | Costal Chondrocytes, Agarose Gel | Decrease in GAG and collagen production, and mechanical strength | [85] | |
| 20 ng/ml; 5, 20, 60, 120 minutes | Bovine TMJ disc cells, cell culture wells | 6- and 4- fold increase in Erk1/Erk2 and p38 phosphorylation at 20 minutes | [88] | |
| IL-1 | 5 ng/ml; 5, 20, 60, 120 minutes | Bovine TMJ disc cells, cell culture wells | 2 fold increase in p38 phosphorylation | [88] |
| TNF-a | 30 ng/ml; 5, 20, 60, 120 minutes | Bovine TMJ disc cells, cell culture wells | 6- and 4- fold increase in Erk1/Erk2 and p38 phosphorylation | [88] |
| EGF | 30 ng/ml; 3, 6 weeks | Costal Chondrocytes, Agarose Gel | Decrease in GAG, collagen, and mechanical strength, increase in cell proliferation | [85] |
Multiple growth factors have been shown to independently increase cell proliferation, collagen production, and GAG synthesis, but rarely an increase in mechanical strength was observed. For future work, gene-based delivery and spatiotemporal parameters could be investigated to improve further the efficacy of growth factor treatment for regenerating the TMJ disc.
2.4. Scaffolds
Scaffolding material for articular disc regeneration requires adequate mechanical strength, biocompatibility, and long-term stability to ensure the new tissue can properly form. The TMJ disc is under high amounts of stress, often in motion, and is mostly avascular as previously mentioned. These factors contribute to making a long-term replacement of the disc problematic in clinical applications[95]. The first disc replacements were made from Teflon bonded to carbon Proplast I in 1973[96], however, the material proved to be an unsatisfactory replacement due to fibrosis, large cell body reactions, and morphology changes of the condyle[97, 98]. Furthermore, the failed implants warranted investigation into treatment options for patients that received a failed Teflon implant[99]. Thus, development of a scaffold suited to long-term replacement of the TMJ is vital for an effective treatment of TMD due to currently available disc replacement issues. Herein the types of materials used for disc replacement will be divided into two categories; natural and synthetic.
2-4-1. Natural Materials
Natural materials for TMJ disc scaffolds include collagen, fibrin, chitosan, and decellularized ECM sheets. Collagen is one of the main components of native disc. Collagen naturally creates a porous structure for cell infiltration and GAGs are readily deposited on its surface[100]. Generally, collagen is a weak, flexible material but can be thermally crosslinked for more robust mechanical properties. When seeded with BMSCs, a collagen scaffold successfully closed a perforation in the TMJ disc of a Japanese rabbit model[100]. Fibrin gel also has been used to regenerate soft tissues[101]. However, issues with fibrin gels include poor mechanical strength, rapid degradation and shrinkage volume during formation[102]. A composite scaffold of fibrin gel and lyophilized chitosan forms a stable structure with enhanced cell proliferation and disc ECM deposition (Fig. 5)[65]. Derived from crustacean shells, chitosan is a biodegradable material that forms a gel that can be modified based on pH. Although these techniques have yet to be applied to the TMJ, the chitosan gel properties may be improved by the incorporation of small molecules for local controlled release, and the β-glycerophosphate concentration in the chitosan can be altered to control the gelation temperature[103, 104]. Decellularized ECM scaffolds are often derived from either porcine bladder or decellularized TMJ discs. The advantages of using pre-formed tissues are they possess mechanical stability and are biocompatible. The porcine bladder based scaffold was constructed by sandwiching powdered porcine bladder between two hydrated sheets of the bladder creating a pillow-like structure[105]. After 24 weeks, the implanted scaffold resembled the native disc based on morphological findings. A follow-up study found the post-implant scaffold also contained GAG and collagen in concentrations similar to the native disc, along with possessing comparable mechanical properties[106]. However, these scaffolds were anchored to the temporal fossa. Thus, the natural motion of the TMJ would not be possible using this method. Porcine TMJ discs were decellularized and subsequently made porous by laser-ablation to increase hydraulic conductivity[107, 108]. The goal of the microporation was to improve cell adhesion to the scaffold and increase cell density throughout the core of the scaffold. The enhanced porosity facilitated elevated levels of cell populations in the center of the core, likely due to increased diffusivity and cellular adherence; however, this technique was limited to an in vitro study.
Figure 5.
TMJ-SDSCs seeded on fibrin/chitosan scaffolds implanted in a murine model for four weeks. Immunohistochemical staining for collagen I (Col I) and collagen II (Col II) was performed demonstrating more collagen was deposited in the fibrin-coated scaffold (A). Additionally, cell viability testing (B) and qPCR for collage I and collagen II (C) were performed. Error bars represent SD and asterisks indicate P < 0.05. Reproduced from “The Pilot Study of Fibrin with Temporomandibular Joint Derived Synovial Stem Cells in Repairing TMJ Disc Perforation” by Wu et al. under the CC BY 2.0, 2014[65].
2-4-2. Synthetic materials
In contrast to natural products, synthetic materials lack inherent differentiation properties but provide enhanced control over mechanical properties while remaining biocompatible. Polymers that have been investigated for use in the articular disc include polytetrafluoroethylene (PTFE), polyglycolic acid (PGA), polylactic acid (PLA), PLGA and, more recently, PCL. As previously stated, Teflon was among the first materials used for TMJ disc prosthetics because of its durability and non-porous nature[109]. However, long-term use resulted in degradation of the prosthetic and condyle resulting in continual pain experienced by the patients[97, 110]. After the failure of Teflon as a prosthetic for the TMJ, research has been more focused on an integrative approach where the body will replace the scaffold over time.
Biodegradable polymers offer high mechanical properties initially, and over time they are designed to degrade at the same rate the new tissue is formed. PGA scaffolds have been used to demonstrate the feasibility of seeding TMJ disc cells upon a polymeric scaffold to regenerate native tissues[111]. Woven PGA scaffolds were placed in spinner flasks, and over the course of six weeks, the seeded TMJ disc cells continually deposited collagen[112]. However, the tissue requires at least six weeks to form organized tissue constructs; therefore, PLA was investigated because of its slower degradation rate[113, 114]. Biphasic PLA discs were fabricated with one side as a non-woven, porous mat for cell seeding and the other as a solid PLA layer as articulating surface. The PLA scaffold was still visible after a 12 month period; however, dislocation and osteoarthritis present in the joint suggested the scaffold did not adequately protect the TMJ[114]. Scaffolds were fabricated from PCL embedded with PLGA microspheres to improve the generation of fibrocartilage. Due to the substantial difference in melting temperatures between PCL and PLGA, PLGA can remain stable in melted PCL, protecting the growth factor proteins encapsulated in the PLGA[2]. The resulting microsphere embedded scaffolds demonstrated enhanced collagen production, increased presence of deposited GAGs, and enhanced mechanical properties after six weeks incubation in vitro compared to the scaffold alone[2]. A relatively unique polymer, poly (glycerol sebacate) (PGS), demonstrated increased cellularity over initial seeding suggesting high cellular adherence and compatibility. The scaffolds also showed minimal decomposition over the course of four weeks[115]. In contrast to polymers, titanium oxide has also been investigated as a surface for the growth of fibrochondrocytes. When titanium oxide was deposited as a surface coating, cell viability and protein deposition were significantly increased over the control surface of hydrophilic glass[116].
In addition to selecting the appropriate material, the structure of the scaffold must also be optimized to maximize cell and nutrient infiltration while retaining the mechanical properties necessary to facilitate the function of the TMJ. Important considerations to make during scaffold design are pore size, porosity, overall shape, mechanical strength, flexibility, and region-specific variations. To achieve a scaffold optimized for these parameters, investigators have tried a wide variety of approaches ranging from conventional methods to 3-dimensional (3D) printing. Based on the available literature, the ideal properties for the TMJ disc scaffold will be described followed by a review of current fabrication techniques applied to TMJ disc scaffolds.
The natural TMJ disc is a biconcave fibrocartilage disc that contains both chondrocytes and fibrochondrocytes. Since, the regenerative scaffold needs to support nutrient and cellular infiltration while maintaining adequate mechanical strength. There is generally a compromise between porosity and mechanical integrity as an increase in porosity results in decreased mechanical strength[117]. Also, pore interconnectivity should be maximized to allow for uninterrupted diffusion of cells, nutrients, and waste[117].
Scaffold pore size also impacts cellular function specific for cartilage regeneration. Unlike bone tissue regeneration where 300 μm or greater is required for integration with the native tissue, seeded chondrocytes appear to have increased rates of proliferation and ECM production when pore sizes are below 100 μm. The ranges of pore sizes examined using collagen[118, 119], synthetic polymers[120], gelatin[121], titanium[122], and silk[123] were 20–500 μm, 200–1650 μm, 50–150 μm, 13–68 μm, and 90–425 μm, respectively. In all of these studies, barring the silk study, the smallest pore size resulted in the highest amount of GAG deposited and greatest cellular density. It is worth noting that the smaller pore size also resulted in decreased diffusion[124], however, by combining macropores (> 400 μm) with the micropores (< 50 μm), the best of both systems could be obtained[121].
Fabrication methods for TMJ disc scaffolds include decellularized ECM lamination, hydrogels, mold casting, and 3D printing. Lamination of decellularized porcine bladder sheets was able to form a pillow-like structure when packed with powdered ECM[105]. The lamination was performed by compressing two sheets of ECM in a mold with a void for the addition of filling material. This technique allows for the formation of a biocompatible anchor system that can be used to fix material into the TMJ disc space. Hydrogels containing cells can be used to inject into a porous preformed scaffold for seeding and improvement of the biocompatibility of the scaffold surface properties. This was achieved using a fibrin gel loaded with SDSCs, and upon implantation, the scaffold demonstrated enhanced cellularity compared to the scaffold control[65]. 3D printing is a promising new technique for articular disc scaffold fabrication because it enables rapid-prototyping and incorporation of biomolecules in a spatially controlled manner. PLGA microspheres containing TGF, BMP-2, or CTGF were incorporated into distinct regions of PCL scaffolds by blending the particles in PCL powder that was printed by fuse-deposition modeling. The resulting scaffolds released the growth factors in a sustained fashion for up to 42 days and were able to differentiate SDSCs into chondrogenic, fibrogenic, and osteogenic cells in vitro (Fig. 6)[2]. Furthermore, the seeded scaffolds were able to form fibrocartilaginous tissues with region-specific tissue phenotypes and tensile properties, mimicking the native tissue[2, 93]. Another group reported 3D printed PCL scaffolds coated with poly(ethylene glycol) diacrylate (PEGDA) hydrogels better mimic the mechanical properties of native articular discs as compared to PCL disc alone[125].
Figure 6.
3D printed PCL scaffold embedded with protein-loaded microspheres for TMJ disc regeneration. Laser scan and 3D print of the TMJ disc (A, B). The tensile modulus of the 3D printed scaffolds compared to the native TMJ disc (C). Fluorescently labeled particles embedded in a scaffold to demonstrate spatiocontrol (D). The release of the growth factors from the scaffold (E). Reproduced from “Engineering human TMJ discs with protein-releasing 3D-printed scaffolds” by Legemate et al. with permission from SAGE Publications, 2016[2].
3. Mandibular Condyle
3-1. Anatomy
Originating from the ramus of the mandible, the mandibular condyle widens into the articulating surface of the TMJ. During mandibular movement, the condyle rotates in the glenoid fossa and then transverses over the articular eminence as the jaw is maximally opened. Also, the condyle provides anchoring points for the articular disc, the capsule, and the lateral pterygoid[126]. The overall size of the condylar head is 690 ± 50 mm3 with a surface area of 400 ± 60 mm2, and in the mediolateral direction, the adult condyle is 19.0 ± 3.0 mm, while in the anteroposterior direction it is 8.7 ± 1.7 mm[127, 128]. The typical shape of the condyle is convex with bilateral symmetry[129]. Following is a review of the structure of the bone and articular cartilage which constitute the mandibular condyle.
The condyle neck consists of periosteal and endosteal cortical bone and trabecular bone. The two bone regions have been investigated through both nanoindentation and micro-CT, and the mechanical properties can be seen in table 2[130–132]. Based on these results, the cortical bone provides stiffness whereas the trabecular bone provides energy dissipation. Also, the cortical bone is in mediolateral aligned in the superior region and superior-inferior aligned in the neck of the condyle, suggesting that each area of the condyle is under differing stresses during movement[130].
Table 2.
Properties of cortical and trabecular bone of the mandibular condyle.
| Characteristic | Cortical Bone | Trabecular Bone |
|---|---|---|
| Thickness (mm) | 1.49 ± 0.14 | - |
| Porosity (%) | 3.53 ± 1.19 | 79.3 ± 5.1 |
| Mineralization (mg HA/mL) | 1045 ± 57 | 857 ± 41 |
| Elastic modulus and Plastic hardness (E/H) (GPa) | 7.5 ± 3.0 / 0.3 ± 0.2 | 4.2 ± 3 / 0.14 ± 0.12 |
| Viscosity (GPa * S) | 16,000 ± 16000 | 7500 ± 7500 |
On top of the condylar neck, articular cartilage coats the condyle. Unlike the majority of joints where hyaline cartilage is present, the condyle articular cartilage is made up of fibrocartilage. The regions of the condyle fibrocartilage are commonly divided into four zones: fibrous, proliferative, mature, and the hypertrophic zone listed from superior to inferior[133]. The fibrous zone contains fibroblasts and organized collagen I primarily; the proliferative zone houses the MSCs responsible for repopulating fibroblasts and chondrocytes; and the mature and hypertrophic zones contain mature chondrocytes embedded in loosely organized collagen II. The collagen network orientation is debated among researchers, but the majority concur that the fibers are arranged in the anteroposterior direction with some fibrils running parallel to the subchondral bone in a radial orientation[133–135]. This contributes to the collagen network providing the tensile and shear strength. For compression resistance, the proteoglycans can bind the interstitial fluid to create a pressurized osmotic system to reduce this force[133, 134].
The bone and the articular cartilage, which constitute the mandibular condyle, each contain specialized components to handle stresses applied during regular motion. Tissue engineering strategies must account for these localized variations within a single part to ensure adequate regeneration of the functioning tissue. Exploring current attempts at regenerating the condyle and the bone-cartilage interface by utilizing cells, growth factors, and scaffolds will provide insight for future research.
3-2. Cells
Cellularized scaffolds have been implemented to improve the efficacy of tissue regeneration strategies for the mandibular condyle[136]. Cells examined for mandibular condyle scaffolds include stem cells and somatic cells. In particular, the osteochondral interface poses a unique challenge because multiple cells types are required to form this tissue interface. Common obstacles to using stem cells include harvesting, expansion, differentiation and uniform seeding[137–139]. Somatic cells are already differentiated to a functioning cell but are unable to undergo extensive subculturing due to dedifferentiation concerns. Here we will introduce the types of cells used in mandibular condyle engineering and discuss the advantages and disadvantages of each.
3-2-1. Stem cells
The source of stem cells can either be from adult or embryonic tissue; however, due to ethical concerns, most research has focused on adult stem cells[140]. MSCs are of particular interest in tissue engineering because they are readily extracted from a multitude of sites such as adipose tissue, bone marrow, and cartilage. Also, these cells can be differentiated into various lineages which makes them attractive for regeneration because one source can be used to regenerate multiple types of tissues[141]. Pluripotency is especially useful in the mandibular cartilage because of the osteochondral interface[141]. However, forming a continuous transition from bone tissue to cartilage remains difficult[142].
BMSCs have been used in both cartilage and bone tissue engineering for the mandibular condyle. An in vitro study demonstrated the feasibility of regenerating bony tissue by seeding BMSCs in decellularized trabecular bone[143]. After five weeks of culture in a bioreactor, increased mineral density and osteoid formation were present based on micro-CT analysis and histological findings. In a separate investigation, BMSCs were differentiated in osteogenic media before implantation and were demonstrated to promote bone and cartilage formation throughout the pores of scaffolds when implanted in the dorsal side of nude mice[144]. For the osteochondral interface, bladder-derived laminate structures were embedded with differentiated BMSCs fixed to the heads of excised rabbit condyles[145]. The scaffold supported the growth of both bone and cartilage as determined by micro-CT and histology results.
In the bony tissue of the condyle, ADMSCs have been demonstrated to improve healing outcomes significantly. Harvested ADMSCs autogenously implanted into mandibular fractures showed a 36% increase in ossification rate compared to the control after 12 weeks[146]. The implantation of ADMSCs in a mandibular bony defect leads to increased bone formation through secretion of paracrine factors; the researchers concluded paracrine factors are responsible because the original cells are no longer present after only 12 days[147]. ADMSCs have yet to be used for the osteochondral interface in the mandibular condyle but have been implanted in both animals and humans with successful outcomes[148, 149].
Although embryonic stem cells (ESCs) are capable of producing unlimited cells without losing their pluripotency, legal and moral issues prevent widespread use in tissue therapies and currently no research has investigated using ESCs in TMJ tissue engineering. However, these cells do hold promise in osteochondral defects as their chondrogenic, and osteogenic potential is significant[150, 151]. In contrast to ESCs, umbilical cord MSCs (UCMSCs) are not restricted by ethical issues and still offer multipotency. Furthermore, UCMSCs have been directly compared to TMJ condyle chondrocytes in vitro and significantly outperform the differentiated chondrocytes in GAG and collagen sythesis and proliferation when seeded on a PGA scaffold[152]. Bone regeneration at the osteochondral interface are primary targets for UCMSCs due to their regenerative capacity; however, these cells have yet to be used in scaffolds implanted into a TMJ condyle[153,154].
Researchers identified a subset of stem cells that reside in the superficial layers of the TMJ condyle: fibrocartilage stem cells (FCSCs). These cells were assessed through qPCR, flow cytometry, and growth curves and compared to mandibular chondrocytes and BMSCs. The FCSCs expressed less osteogenic markers such as osteocalcin and were more proliferative than the condylar chondrocytes suggesting this was a unique cell population. It was also demonstrated FCSCs were capable of cartilage and bone formation in a murine model (Fig. 8)[141]. Induction of FCSC homing using various chemoattractants without cell transplantation is an attractive alternative for condyle cartilage regeneration.
Figure 8.
Single FCSC expanded, seeded on a collagen sponge, and implanted in the dorsum of nude mice (A). After three weeks H&E staining and immunohistochemistry staining for aggrecan (ACAN) revealed cartilage formation (B). Six weeks post-implantation, H&E staining and immunohistochemistry staining for osteocalcin (OCN) revealed bone formation (C) Scale bars are 50 μm. Reproduced from “Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury” by Embree et al. under CC BY 4.0, 2016[141].
3-2-2. Somatic cells
Somatic cells such as chondrocytes, osteoblasts, and fibrochondrocytes are all of use in tissue engineering of the mandibular condyle. Autologous cells reduce the risk of rejection when transplanted and can be cultured to increase cell number albeit by a finite amount[155]. So far hyaline chondrocytes, mandibular chondrocytes, costal chondrocytes, and osteoblasts have been investigated for tissue engineering the mandibular condyle. However, low availability and donor site morbidity limit the usefulness of somatic cells.
Chondrocytes extracted from the mandibular condyle have been reseeded into both PGA scaffolds and self-assembled agarose scaffolds. In both scaffolds, GAGs and collagen production were minimal[156]. Another study compared hyaline chondrocytes harvested from the ankle to TMJ chondrocytes. The results indicated the hyaline chondrocytes produced significantly more collagen and GAGs, but similar to the costal chondrocytes, type II collagen made up a large portion of the deposited collagen[157]. A polymeric scaffold was shaped to resemble the condyle[158]. The articulating surface was coated with hyaline chondrocytes, and the core of the scaffold was seeded with osteoblasts. The scaffolds were implanted in the dorsum of nude mice and allowed to generate tissue for twelve weeks. The histology analysis revealed a continuous transition from cartilage to bone occurred within the constructs suggesting seeding with osteoblast and chondrocytes is a viable option for engineering the interface.
3-3. Growth factors
Osteoinductivity is a major weakness of synthetic scaffolds because the synthetic materials alone do not promote endogenous cells to differentiate. Even natural materials may require additional growth factors to promote the differentiation of stem cells to the correct phenotype [159]. Thus, chemoattractants and growth factors are necessary to facilitate the influx of stem cells to the injury site and the subsequent differentiation into functioning adult cells. For the mandibular condyle, BMP-2, vascular endothelial growth factors (VEGF), TGF-β1[136, 160], IGF[161] and FGF[161] have been utilized to repair defects with some success.
For bony tissue regeneration of the condyle, the primary growth factor investigated has been BMP-2 as it is well-established in literature and approved by the FDA[162].A 15 mm segmental defect in a monkey mandible model was bridged with a polymeric scaffold loaded with BMP-2 and BMSCs. Although complete regeneration of bone was not present in any of the specimens, the BMP-2 group performed significantly better than the controls in mechanical testing and bone formation[163]. When BMP-2 was added to an osteochondral scaffold in a rabbit condyle defect, the presence of new bone was apparent[164]. However, the results were not significantly different from the control as the model used was likely not rigorous enough to produce a significant difference. The effects of FGF and VEGF on mandibular condyle growth have also been investigated[1]. Plasmid DNA (pDNA) encoding FGF was complexed with a lipopolymer. The complexes were injected into the condyle of adult rats, and after 30 days, the condyle was harvested for micro-CT and histological analysis. The induction of FGF significantly enhanced both bone formation, and the proliferative layer cell counts in the condyle[165]. For VEGF treatment, an adenovirus was used to deliver the pDNA encoding VEGF locally to the condyle and glenoid fossa of 35-day-old rats. When exposed to VEGF, the proliferative layer of the condyle stained more intensively for proliferating cell nuclear antigen suggesting overall proliferation was increased. Also, significantly higher levels of osteocalcin and alkaline phosphatase expression were observed in the VEGF treated group, albeit only at the 28-day time point, (Fig. 9)[1]. However, injections of protein-based VEGF into the TMJ of a mouse model has been demonstrated to induce osteoarthritis, so regimented dosing may be necessary when using VEGF in tissue engineering applications[166]. Overall, these results suggest that FGF and VEGF are vital to increasing proliferation rates of the mandibular condyle stem cells, whereas BMP-2 increases bone formation. A combination of these growth factors may produce a synergistic effect on condyle growth, as this has been observed elsewhere in bone tissue engineering[167].
Figure 9.
Injection of VEGF adenovirus gene therapy into the TMJ. Protein expression for alkaline phosphatase (A) and osteocalcin (B) were upregulated in the treated group at 28 days Asterisks represent *P < 0.05 and **P < 001 Reproduced from “Recombinant AAV-mediated VEGF gene therapy induces mandibular condylar growth” by Rabie et al. with permission from Springer Nature and Copyright Clearance Center, 2007[1].
To promote differentiation of cells into chondrocytes in the condylar cartilage, common growth factors used include TGF-β1, FGF, IGF, and BMP-2. Wang et al. independently examined the effects of FGF, TGF-β1, and IGF growth factors on TMJ chondrocytes in vitro, and found a concentration of 10 ng/mL of IGF-1 significantly improved the deposition of collagen and increased proliferation of TMJ chondrocytes. FGF at 100 ng/mL increased proliferation of mandibular condylar cartilage cells, but neither FGF nor TGF increased collagen or GAG production[161]. As previously discussed, TMJ condyle chondrocytes were compared to UCMSCs for collagen production, GAG synthesis, and cell proliferation using TMJ chondrocytes that were placed in media containing TGF or control media. Similar to the study of Wang et al., TGF-β1 did not enhance proliferation nor the synthesis of biomolecules[152]. However, TGF-β1 did significantly improve the histological scores when loaded into PLGA microspheres as part of the osteochondral graft used in a rabbit condyle model[164]. In addition to BMP-2 being used for osteogenesis, BMP-2 has also been used to support the formation of articular cartilage in vivo. Sponges fabricated from BMP-2 mixed with collagen were placed into a 2 mm defect in rabbit condyle cartilage and allowed to heal for three weeks. Afterward, the condyles were extracted for histology demonstrating new cartilage formation in the BMP-2 groups and only soft fibrous tissue formation in the controls[168]. Overall, these studies indicate that BMP-2 and IGF are the most promising growth factors for promoting cartilage synthesis in the condyle while results with TGF-β1 were conflicting.
3-4. Scaffolds
In the pursuit of developing a successful tissue engineering approach to repair or replace the mandibular condyle, both natural and synthetic materials have been investigated. The ideal scaffold mimics the structural integrity of the native tissue and supports growth and proliferation of cells ultimately resulting in the replacement of the scaffold with healthy tissue. An evaluation of current research describing condyle scaffolds will help direct future research towards the more promising approaches. Scaffold materials for each section of the condyle, cartilaginous and bone, will be described followed by techniques to form the osteochondral interface and scaffold fabrication methods.
Concerning the bony tissue of the condyle, synthetic scaffolds offer many advantages such as high mechanical integrity, porosity, and the capacity for the incorporation of growth factors. Materials used for bioengineered condyles include polymers such as PLGA[164], PGA[169], PCL[174] PLA[169] and mineral based scaffolds such as hydroxyapatite (HA)[172]. In general, polymeric structures are easy to mold, flexible, potentially bioabsorbable, and can be integrated and coated with other materials, whereas, mineral-based scaffolds provide high mechanical strength and are structurally similar to native bone. To develop the scaffold model, a CT scanner took a series of images of the beagle condyle and was used to generate a positive mold via 3D printing. The template was impressed into gypsum to make the negative mold which was filled with PGA fibers and a PLA solution. The scaffold was seeded with BMSCs, and after seven days in culture the BMSCs were adhering to the scaffold, and ECM deposition was detected[169]. A pure HA scaffold was constructed by sintering foamed, aqueous HA scaffolds in molds at 1250 degrees celcius for 3 hours. With a total porosity of 70%, the HA scaffolds maintained an adequate compressive strength of 5.6 ± 1.5 MPa. After four months of implantation in a rabbit model, the scaffold contained both organized cartilage at the superior portion and new bone, and more impressively, the TMJ disc adhered to the scaffold via dense connective tissue[172].
In comparison to synthetic materials, natural materials offer the distinct advantage of being naturally osteoinductive. Natural materials that have been explored for condylar bone replacement include coral[144], chitosan[174], and collagen[173]. Natural coral (porosity of 150–220 μm) was sculpted to resemble a condyle with a dental bur, and BMSCs were seeded at 20 million cells per construct. Dorsal implantation in nude mice for 8 weeks demonstrated endochondral bone formation had occurred in 6 of the 6 seeded scaffolds, but in the empty scaffold, no osteogenesis occurred[144]. In a separate study, a block of HA mixed with collagen was prepared to fit the mandibular condyle using a bur and was then implanted in seven patients presenting with TMJ ankylosis. A collagen sponge soaked with bone marrow aspirate from the iliac crest was placed between the resected condyle and the scaffold. Additionally, The temporalis fascia muscle was moved between the graft and the glenoid fossa likely to further increase cushion in the joint[175]. At the one-year follow up the average mouth opening increased from 4.14±2.3 mm to 34.57±3.8 mm, however, one patient did experience an infection that required removal surgery.
Scaffold material for the fibrocartilage of the condyle must support chondrogenesis and protect the underlying osseous tissue. Studies investigating materials for regeneration of only the articular cartilage are limited; thus both synthetic and natural materials will be described here. A study seeded TMJ chondrocytes into a PEG hydrogel and tested the scaffolds under dynamic loading[171]. While the chondrocytes were viable when residing in the scaffold, the dynamic loading significantly reduced the collagen I and II and aggrecan expression based on qPCR results. To better mimic the ECM and improve integrity, fibers can be embedded within the hydrogel[176]. Electrospun PCL fibers were mixed with PEG hydrogel to form the biomimetic scaffold, and BMSCs seeded on the scaffold had improved viability and GAG deposition as compared to the hydrogel alone.
For regeneration of the osteochondral interface, researchers have developed biphasic scaffolds that support the growth of bone and cartilage in distinct sections. Schek et al. created a ceramic-polymer based scaffold using HA and fibroblasts to promote bone growth and PLA sponge and hyaline chondrocytes for cartilage regeneration. After four weeks of dorsal implantation in a murine model, both novel bone and cartilage tissue were present within the scaffold; however, this study was limited by the lack of characterization of the collagen and implantation into a non-load bearing site[177]. A gradient growth factor-based scaffold was synthesized by encapsulating protein growth factors, BMP-2 and TGF-β1, into separate PLGA microspheres. The microspheres were loaded into a cylindrical mold where the inferior portion contained only BMP-2 loaded microspheres and then gradually transitioned to only TGF-β encapsulating microspheres in the superior portion. When loaded into rabbits, the scaffold group did show recovery of the cartilage and bony tissue, but the differences were not significantly different from the sham group[164]. A more recent study investigated combining a PCL/HA ceramic phase with either a PGA/PLA mesh or a cartilage cell sheet (Fig. 10). To cellularize the scaffold, BMSCs were implanted into the ceramic phase, and auricular chondrocytes were seeded into the PGA/PLA mesh. Twelve weeks after dorsal implantation in nude mice, both constructs exhibited novel bone and cartilage formation with minimal irregularity, shrinkage of the scaffold, and cellular attachment[178].
Figure 10.
Biphasic scaffold for osteochondral integration. The PLG/PLA cartilage scaffold (A) was sutured to the superior surface of the 3D printed HA/PCL scaffold (B) to form the biphasic scaffold (C). After 12 weeks of implantation, gross morphology (D), H&E staining (E), and safranin staining (F) were performed. The cartilaginous area [C] and the cartilage-bone interface [i] are indicated in panel E&F. Reproduced from “Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice” by Wang et al. with permission from Elsevier and Copyright Clearance Center, 2017[178].
The mandibular condyle exhibits large variations in size and morphology between patients; however, customized scaffolds produced by 3D printing in conjunction with CT scanning are capable of matching the original structure of the patient’s condyle. In one approach decellularized bovine trabecular bone blocks were milled with a 4-axis CNC milling machine into the shape of anatomically correct TMJ condyles[179]. The resulting scaffold was seeded with ADMSCs and cultured for six weeks in a bioreactor[179]. At the end of the cultivation period, the ADMSCs had differentiated, depositing new mineralized tissue. Another group seeded ADMSCs in fibrin gel onto 3D printed PCL scaffolds and examined differentiation in vitro[179]. Histological examination revealed the presence of vascularization or mineralization depending on the differentiation media the cells were exposed to[179]. In vivo implantation of the scaffolds resulted in vascular infiltration after seven days; the ability to print porous PCL scaffolds representing the full mandible (including the mandibular condyle) was also demonstrated[179]. A biphasic condyle scaffold was created by suturing cultured cartilage cell sheets onto a 3D-printed HA-PCL scaffold to regenerate the articular cartilage and bone respectively[178]. The scaffold was seeded with chondrocytes and implanted in the dorsum of a mouse for 12 weeks; the surface of the sheet was covered with a cartilage-like tissue, but there was minimal bone formation in the HA-PCL section of the scaffolds[178]. Interestingly, there is a case report describing a human mandibular condyle being wholly replaced with a 3D-printed prosthetic made from nanoscale HA-polyamide rather than an autologous graft[180]. Despite the implant being partly made from osteoconductive HA, the purpose of the case report was to describe the use of a 3D printed implant in place of an autologous graft in a human and so long-term bone regeneration in and around the implant was not reported here[180]. Additionally, 3D printed negative molds have been used during the fabrication of condyle scaffolds to generate patient-specific scaffolds using more traditional techniques. A CT scan of a beagle mandibular condyle was used as a template to 3D print a model for negative mold fabrication. PGA and PLA fibers were cast into the mold to create a porous scaffold capable of facilitating ECM deposition in vitro[169].
4. Glenoid Fossa/Articular Eminence
Although the glenoid fossa and the articular eminence are rarely studied, treatment options have been studied. A possible reason for the lack of investigation is the low incident rate of fossa fractures, making up only 1.4% of total condylar fractures[181]. Also, in most of cases treatment through conservative means provides acceptable functionality. However, when these treatments fail in cases such as bony erosion, significant trauma, and unsuccessful discectomy, procedures involving surgical intervention may be required in the fossa region[182]. The most accepted surgical treatment is a prosthetic replacement. The first implementations were all metal cups inserted into the glenoid fossa, but poor adaptability and metal-on-metal grinding, in the case of TJR, resulted in poor fit and fibrotic tissue formation[182]. To improve the compatibility and longevity, a prosthetic consisting of titanium shell coated with ultra-high-molecular-weight polyethylene on the articulating surface is now reported to have a 94% success rate and is FDA approved[183, 184].
In addition to prosthetics, autografts offer an alternative to replacing the damaged tissue of the glenoid fossa[185]. In a case study, cranial bone was harvested and fixed in the place of the glenoid fossa using a combination of wire and silk sutures[186]. Postoperative results showed no significant deterioration of function and the patient had no complaints of pain at the four-year follow-up[186]. In another case study, the native fossa was removed due to a giant cell tumor. The surgeon harvested a section of parietal bone, contoured the bone to replace the glenoid fossa, and it was fixed with two mini plates. After ten months, the patient did have minor deflection to the defect side with a maximal opening of 33.1 mm[187].
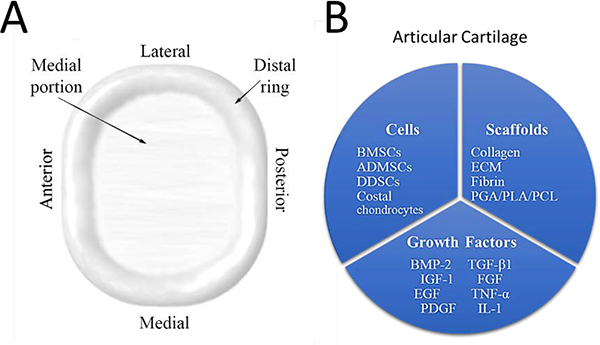
For tissue engineering of the articular eminence and glenoid fossa, morphology and the bone-cartilage interface pose the most significant challenges to overcome. Furthermore, no attempt at tissue engineering of these structures has been made[188]. The scaffold must be able to retain its shape during loading of the TMJ, otherwise undesirable flattening of the articular eminence may occur. Adequate regeneration of the bone-cartilage interface has been a long-standing issue in tissue engineering as the cartilage is highly avascular and the transition is difficult to integrate[189]. The following sections will include anatomy and recent studies relevant to the tissue engineering of glenoid fossa and articular eminence including discussions of cells, growth factors and scaffolding materials (Fig. 11–B).
Figure 11.
Anatomy and potential tissue engineering strategies for the glenoid fossa and articular eminence. Anatomy of the glenoid fossa and articular eminence (A), and attempted tissue engineering strategies specific for each tissue type present in the glenoid fossa and articular eminence (B).
4-1. Anatomy
The glenoid fossa is located on the inferior most edge of the temporal bone. The fossa is a concave structure in which the disc and condyle rotate during minimal opening of the jaw. As the jaw continues to open, the articular disc and condyle slide down and over the anterior portion of the fossa, the articular eminence. The fossa is bound posteriorly by the petrotympanic fissure which houses nerves and blood vessel[190]. The fossa measures 15.05 ± 1.79 mm in the anterior-posterior direction, and 22.03 ± 2.08 mm medial-laterally in the average adult and the fossa surrounds a 2,000 ± 900 mm3 space[191]. The roof thickness of the glenoid fossa is on average 0.9 ± 0.4 mm based on cone beam computed tomography imaging. These measurements appear to be independent of age or gender[192].
The fossa is made up of bony tissue covered on the articulating surface by a thin layer of articular cartilage (Fig. 11–A). The dense fibrocartilage of a porcine model was analyzed by nanoindentation, and it was found that the aggregate modulus of the fossa was 41.9 ± 16.8 kPa[52]. The authors compared this value to the stiffness of the human hip and knee joint and found the aggregate modulus to be 1/30 and 1/15, respectively. Because of the low modular values, they postulated that the condyle fossa is a low weight bearing joint. Underneath the articular cartilage are a few layers of flattened stem cells that appear to be pre-osteoblasts[193]. These cells have been known to proliferate and begin forming new bone in response to forward mandible positioning without formation of a callus as seen in long bone wound fractures. This is possible because the bony tissue of the fossa is formed through intramembranous ossification instead of endochondral ossification[193, 194]. The bone structure is trabecular bone covered with a thin layer of cortical bone; however, at the thinnest points of the fossa, the bone is primarily cortical.
In contrast to the fossa, the articular eminence is load bearing during translation of the mandible and varies with gender[195]. The shape of the eminence can be classified into four categories: box, sigmoid, flattened, and deformed and this categorization is based on how pronounced the eminence appears[196]. Shallow articular eminences are associated more with internal derangement without reduction than the more pronounced eminence morphologies. Using rhesus monkeys as a model, the eminence was also found to be covered with a thick layer of fibrocartilage consisting of three zones[197]. The first is a thin layer of collagen and elastic fibers sparsely seeded with rounded cells suspected of providing lubrication for the joint. The second layer contains a high cell density with randomly oriented collagen fibrils, and the third zone is the bone-cartilage interface where the dense cartilage is potentially replaced by bone as the chondrocytes undergoing pyknosis are visible. This is further reinforced by the presence of chondroid bone during mandibular advancement[198].
4-2. Cells
Since the glenoid fossa and articular eminence are bony tissue covered by a fibrocartilage layer: chondrocytes, osteoblasts, BMSCs, ADMSCs, and other stem cells are relevant cell types for regenerating this tissue[199]. The most suitable cell type for articular cartilage regeneration are BMSCs due to their ability to migrate to the damage site, secrete chemotactic factors, and differentiate into both chondrocytes and osteoblasts[200]. A calcium phosphate cement scaffold loaded with platelet-rich plasma (PRP) and BMSCs was packed into 8 mm femoral defects in a minipig model[201]. The BMSC-PRP scaffold more than doubled the amount of new bone regeneration and facilitated significantly more angiogenesis throughout the defect site.
iPSCs are another source of multipotent cells that are of particular interest for tissue engineering because readily available fibroblasts can be used to create a large pool of patient-matched chondrocytes[202]. One research group produced iPSCs and differentiated them into cells that were very similar to adult chondrocytes and were capable of generating cartilage both in vivo and in vitro without detectable tumorigenesis[203]. Another study converted iPSCs to neural crest cells as a source of MSCs. In the presence of differentiating factors in vitro the neural crest cells stained positive for collagen II and collagen I, but when implanted into an osteochondral defect, there was no significant improvement over the untreated control in regards to defect regeneration[204]. iPSCs have the potential to be used in the TMJ because high cell counts can be achieved with minimal harvesting.
4-3. Growth factors
Although tissue engineering strategies have not focused on the glenoid fossa and articular eminence, some researchers have investigated growth factors upregulated during bone formation due to forward mandibular position[198, 205, 206]. These studies have given some insight into which growth factors are responsible for natural bone formation in the glenoid fossa. VEGF and bone formation were found to be upregulated in the glenoid fossa when rats were fitted with bite-jumping appliances[205]. A similar study found that SOX9 and type II collagen were also increased in the fossa during forward mandible positioning[198]. This reverse engineering approach is a useful tool for understanding which growth factors are essential for osteogenesis in the fossa.
Extracellular vesicles (EVs) are another avenue to influence cell-to-cell communication and improve tissue regeneration[207–209]. EVs are categorized by their size and can be loaded with different paracrine signaling agents including amino acids, lipids, metabolites, DNAs, mRNAs, miRNAs, and long non-coding RNAs[210–213]. Previous studies have shown the therapeutic potential of the exosomes in wound and fracture healing, cancer therapy, and intervertebral disc regeneration[214–217]. Recent studies have shown that MSC- and ESC-derived exosomes induced osteogenic and chondrogenic differentiation in the knee joint and calvarial defect models[213, 218]. Exosome concentrations proportionally increased chondrocyte migration and proliferation in a dose and time-dependent manner, and the mRNA level of TGF-β1 and cartilage matrix protein were also similarly increased. Likewise, significant bone regeneration was observed in rat calvarial defects when osteogenic miRNA enriched BMSCs-derived EVs were delivered from a hydrogel. Regarding the mandibular fossa, it has not been extensively studied, but some recent studies imply stem cell-derived exosomes induce progenitor cell migration, cartilage and bone restoration, and pain attenuation[219, 220]. Therefore, exosomes may be a potential, novel strategy for osteochondral repair of the glenoid fossa and the articular eminence.
4-4. Scaffolds
Since there have not been any tissue engineering investigations of either the glenoid fossa or the articular eminence, this section will focus on scaffolds that have been used recently in similar fibrocartilage-bone applications. The goal is to provide insights into which materials and fabrication techniques have shown promise in restoring the cartilage-bone interface. Since the articular eminence is a non-load bearing joint and the articular cartilage is fibrocartilage, the mechanical properties do not have to be as robust as joints such as the knee. Also, an undesirable outcome of many tissue engineering attempts has been the production of fibrocartilage. Thus, tissue engineering of the fossa may be easier to achieve than typical hyaline cartilage covered joints. Many of the scaffolds already discussed for the condyle will be applicable to the glenoid fossa; however, this section will focus on recent publications of tissue engineering in fibrocartilage and osteochondral defects.
The shape of the glenoid fossa and the articular eminence are unique and are located along the inferior edge of the skull; thus, the scaffold must mimic the anatomical shape and retain its structure throughout the regeneration process. Materials such as collagen do not offer the mechanical strength, nor the longevity required to facilitate guided regeneration. Instead, materials such and calcium phosphates (CaP) and PCL are often used to develop anatomically similar scaffolds[221]. CaP have robust mechanical properties and possess inherent osteoinductive properties, and the mechanical properties can be tailored based on the mineral structure of the CaP[222–225]. Additionally, CaP can be modified through ion replacement or incorporation of growth factors within the lattice structure. Replacement of the some of the calcium ions with strontium can increase osteoinduction, whereas magnesium and silicon can induce angiogenesis. Also, growth factors can be embedded within the CaP by co-precipitation using simulated body fluid[226]. PCL, on the other hand, is more malleable; however, it does not possess effective osteoconductive properties and residence times that are associated with CaP. Blended materials offer the most promise, for example, HA mechanical properties such as brittleness can be improved with a wide range of synthetic and natural polymers[227]. These blends can be 3D printed to achieve customized structures based on CT scans.
To regenerate the fibrocartilage, materials such as alginate[229], PLA[230] and PCL[231] have been used successfully, and Lowe et al. have summarized recent publications focused on this area [232]. Lee et al. developed a growth factor embedded PCL scaffold to produce fibrocartilage in a sheep meniscus model[231]. The scaffold consisted of 3D printed 300 μm PCL strands arranged following the natural collagen alignment and embedded with CTGF and TGF-β3 loaded microspheres. The empty PCL scaffold demonstrated similar mechanical properties to the native meniscus tissue after 12 weeks, and when combined with the growth factors, the scaffold was not significantly different from the native tissue. Also, 3D printed PCL scaffolds were enhanced by the addition of BMSCs to the scaffold before implantation (Fig. 12). In New Zealand White rabbits, a menisectomy corrected with the scaffolds was compared to a sham surgery[228]. After 24 weeks the seeded scaffolds contained comparable levels of both collagen I and II to that of the sham group and reduced inflammatory cytokines, whereas the empty scaffold followed a similar trend but to a lesser extent.
Figure 12.
3D printed PCL scaffolds seeded with BMSCs for knee meniscus repair in a rabbit model. The scaffolds were implanted for 24 weeks, and gross morphology (upper row; scale bar is 10 mm) and toluidine blue staining (low row; scale bar is 100 μm) were performed (A). The native tissue and the implanted scaffolds are indicated as [N] and [S] respectively. Immunohistochemical staining was performed and quantified demonstrating collagen I (Col I) (B) was upregulated at 24 weeks whereas collagen II (Col II) (C) was significantly upregulated at 12 and 24 weeks in the cell-seeded scaffolds. Error bars represent SD; asterisks represent *** P < 0.001. Reproduced from “3D-printed poly(epsilon-caprolactone) scaffold augmented with mesenchymal stem cells for total meniscal substitution: a 12- and 24-week animal study in a rabbit model” by Zhang et al. with permission from SAGE Publications, 2017[228].
Another challenge, in the case of degradation of the bone tissue, is integrating the bony tissue to the fibrocartilage, as a continuous transition between bone and cartilage without delamination is difficult to achieve[233, 234]. The majority of efforts in this area have been attempts at reconnecting ligaments. However, the strategies applied are useful for creating a combinational scaffold. A review of polyphasic scaffolds demonstrated the success of both triphasic and biphasic scaffolds in restoring osteochondral defects. In triphasic scaffolds, there is a cartilage layer, a calcified cartilage layer, and a bony layer, while the biphasic scaffolds are limited to cartilage and bone in separate layers[235]. Triphasic scaffolds were prepared with a collagen I/HA bone layer, a collagen I and II/HA interconnecting layer, and a collagen I and II/hyaluronic acid cartilage layer. The scaffold, intended to repair hyaline cartilage on a rabbit femoral head, produced significantly more bone than the empty defect and fibrocartilage formation was present on the surface[236]. A similarly composed scaffold was tested in condyles of horses[237]. Histology revealed defects were completely covered with cartilage with no gaps between the native and newly formed cartilage. A pilot study investigated a spatio-gene activated chitosan-PLGA scaffold for cartilage and bone integration[238]. Two months after implantation, the scaffold reformed both tissues, however, the extent of the regeneration was not assessed quantitatively.
Direct and indirect 3D printing fabrication methods are both able to create scaffolds for the articular eminence and glenoid fossa with anatomical accuracy. Direct 3D printing encompasses a broad range of techniques such as stereolithography, fused deposition modeling, and bioplotting, and these techniques are described in detail by Do et al. in a review of 3D printing for tissue regeneration[239]. To directly print HA, the standard technique is to blend the HA particles in a polymeric material[240]. A blend of PCL and HA was printed into a femoral condyle shape using fused deposition modeling based upon CT images of the femur[241]. Another study combined a HA/alginate blotted scaffold with a layer of methacrylated gelatin to promote tissue regeneration in osteochondral defects[242]. The photocrosslinking of the gelatin improved the mechanical properties and sustained cellular proliferation and ECM deposition over 28 days[243]. Due to further developments in 3D printing, coaxial heads are available to incorporate more than one material which may be necessary for the cartilage-bone interface. Negative mold fabrication is also capable of forming the complicated structure of the articular eminence and glenoid fossa with added versatility during the scaffold fabrication process[244].
In conclusion, 3D printing HA composite scaffolds that are capped with a polymeric or alginate layer may be suitable for regenerating the articular eminence and glenoid fossa. HA composites provide robust mechanical properties, and the polymeric layer provides a suitable medium for fibrocartilage deposition. The scaffold could also be improved by the addition of growth factors and vitamins. Current publications offer excellent insight into scaffold materials and fabrication processes that may overcome the challenges of tissue engineering the articular eminence and glenoid fossa.
5. Animal Models for TMJ Tissue Engineering
Animal models for TMD have included a range of different mammals, and two recent reviews that focus on preclinical animal models can be found here[245, 246]. Characterization data of the TMJ has been published for rabbits[247], canines[248], sheep[249] and swine[250]. Rodents have been the primary model for studying TMD progression through chemical and physical induction[251]. However, the limited joint space of the rat TMJ restricts in vivo studies to distal implant sites such as subcutaneous pockets. The most common animal model for in vivo studies in the TMJ are rabbits, but large animal models such as dogs and goats have also been utilized[245]. Rabbits have an advantage in TMD modeling because of their low cost, ease of handling and anatomical similarities to the human TMJ. The drawback of using a rabbit model is their TMJ loading patterns do not represent a human’s likely due to their diet. Large animal models generally translate into clinical practice more readily as the joints’ tissue and loading more closely resemble the human TMJ. Limited work has been performed in minipigs, yet based an anatomical analysis, swine would be an ideal tissue engineering model for the TMJ. The paucity of data is most likely due to the high cost and difficulty in performing surgery on these animals[246]. A short list of anatomical similarity and motion of the joint can be found in the table 3.
Table 3.
Scaffolds and fabrication techniques used for TMJ condyle regeneration.
| Scaffold Material | Fabrication technique | Experimental model | Result(s) | Cite | |
|---|---|---|---|---|---|
| Synthetic materials | PLA, PGA, PLGA | PGA fibers coated with PLA solution, Negative mold | In vitro, BMSCs | Demonstrated cellular compatibility | [169] |
| EtOH sintered PLGA particles, Freeze dried | Rabbit condyle defect, 6 weeks | Slight increase in cartilage formed over empty defect | [164] | ||
| PCL | PCL surface treated with NaOH, 3D printed | Dorsum of rats, 7 days | Supported blood vessel formation; printed entire mandible | [170] | |
| PEG | PEG hydrogel, Photocrosslinked | In vitro, TMJ chondrocytes | Mechanical strain reduced collagen I and II | [171] | |
| HA | Gas foamed HA sintered at 1200°C, Milled | Sheep condyle replacement, 16 weeks | Supported cartilage and bone formation; attachment of the TMJ disc | [172] | |
| Natural materials | Coral | Coral cleaned with NaClO, Milled | Dorsum of mice, 8 weeks | Cell seeding resulted in new hard tissue and osteocytes | [144] |
| Collagen | HA-collagen composite and collagen sponge, Milled | Clinical trial, 1 year | Greatly increased mandible range of motion and patient quality of life | [173] | |
| Chitosan | PCL-HA-chitosan composite, Freeze dried | Mechanical and chemical assessment | Mechanical properties were similar to the native condyle | [174] |
Even though the animal models for TMD have been reviewed, a widely accepted defect model for the glenoid fossa and articular eminence has not been established[263]. An overview of the similarities of TMJs from different animals to the human TMJ will aid in the selection of an appropriate model. Anatomical analysis of the TMJ has been performed in rats[264], canines[265], rabbits[266], goats[267], and minipigs[267, 268]. Both canines and rats do not have an articular eminence as the jaw only rotates in the TMJ[264, 265]. There is some debate about the anatomy of rabbit as to whether or not the glenoid fossa is present or if it is only a slit between the zygomatic root and temporal bone[265]. Goats have both the articular eminence and glenoid fossa and have been used as a model for TMJ ankylosis. However, macroscopic analysis found that the glenoid fossa was concave-convex instead of just concave as seen in humans [267]. As herbivores, there are some slight differences in loading throughout the TMJ of goats. The pig model contains highly similar features to that of a human for loading, but similar to the goat, the fossa is concave-convex[267]. Based on these finding, goats and minipigs offer the most similar glenoid fossa and articular eminence loading and structure for an animal model.
6. Conclusion
Tissue engineering of the TMJ is, and will continue to be, an area of interest due to the prevalence of TMD. Tissue engineering is a rapidly evolving field with the ongoing development in scaffold fabrication, cellularization strategies, and growth factor delivery; and many of these techniques have been applied to the TMJ. Based on this literature review, there has been notable progress in fabricating scaffolds in the correct anatomical shape, and the materials utilized have been shown to increase tissue regeneration in models for TMD. However, there are still challenging problems that remained unsolved. Remaining barriers in tissue engineering of the TMJ include restoration and incorporation of the fibrocartilage on the articulating surfaces, displacement of the implant material, and evaluation of long-term outcomes from the use of regenerative approaches. Additionally, tissue engineering strategies have yet to be applied directly to the glenoid fossa and articular eminence. Further studies will elucidate a future when TMJ pathologies can be treated effectively and thus improve patient outcomes.
Figure 7.
Anatomy and tissue engineering strategies for the mandibular condyle. Anatomy of the mandibular condyle (A), and attempted tissue engineering strategies specific for each tissue type present in the mandibular condyle (B).
Table 4.
Overview of animal models used in TMJ tissue engineering.
| Species | Cost* | TMJ motion | Models | Pros | Cons |
|---|---|---|---|---|---|
| Rodent | $ | Rotation | Disease and subcutaneous implants | Cost-effective Genetic control | Limited surgical site |
| Rabbit | $$ | Translation | Preclinical, disc and condyle | Cost-effective | Spontaneous healing, clinical translation |
| Minipig | $$$$$ | Rotation and translation | Preclinical, disc | Human-like model, easy to handle | Expensive |
| Dog | $$$$ | Rotation | Preclinical, disc | - | Ethical concerns |
| Goat/Sheep | $$$ | Translation | Preclinical, disc and condyle | Cost effective, obtainability | Non-human TMJ movement |
| Pig | $$$$ | Rotation and translation | None published | Human-like model | Rearing time, housing requirements |
| Monkey (Rhesus) | $$$$$ | Rotation and translation | Preclinical, disc | Human-like model | Expensive, ethical concerns |
Acknowledgments
This work was financially supported by the Martin “Bud” Schulman Postdoctoral Fellowship Award from the American Association of Orthodontists Foundation, 2017, NIH R21 grant (1R21DE024206-01A1), and the Lyle and Sharon Bighley Professorship.
References
- 1.Rabie ABM, Dai J, and Xu R, Recombinant AAV-mediated VEGF gene therapy induces mandibular condylar growth. Gene Therapy, 2007. 14: p. 972. [DOI] [PubMed] [Google Scholar]
- 2.Legemate K, et al. , Engineering Human TMJ Discs with Protein-Releasing 3D-Printed Scaffolds. Journal of Dental Research, 2016. 95(7): p. 800–807. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, et al. , Etiological factors of temporomandibular joint disorders. Natl J Maxillofac Surg, 2011. 2(2): p. 116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorland WAN, et al. , Dorland’s illustrated medical dictionary. 1957, Philadelphia; London: W. B. Saunders Co. [Google Scholar]
- 5.Helland MM, Anatomy and function of the temporomandibular joint. J Orthop Sports Phys Ther, 1980. 1(3): p. 145–52. [DOI] [PubMed] [Google Scholar]
- 6.Temenoff JS and Mikos AG, Review: tissue engineering for regeneration of articular cartilage. Biomaterials, 2000. 21(5): p. 431–440. [DOI] [PubMed] [Google Scholar]
- 7.Cuccia AM, et al. , The arterial blood supply of the temporomandibular joint: an anatomical study and clinical implications. Imaging Sci Dent, 2013. 43(1): p. 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oral K, et al. , Etiology of temporomandibular disorder pain. Agri, 2009. 21(3): p. 89–94. [PubMed] [Google Scholar]
- 9.Li X-Y, Jia C, and Zhang Z-C, The normal range of maximum mouth opening and its correlation with height or weight in the young adult Chinese population. Journal of Dental Sciences, 2017. 12(1): p. 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman E, et al. , Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network() and Orofacial Pain Special Interest Group(). J Oral Facial Pain Headache, 2014. 28(1): p. 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter S, et al. , Comorbidity between depression and anxiety in patients with temporomandibular disorders according to the research diagnostic criteria for temporomandibular disorders. J Oral Facial Pain Headache, 2015. 29(2): p. 135–43. [DOI] [PubMed] [Google Scholar]
- 12.Kindler S, et al. , Depressive and Anxiety Symptoms as Risk Factors for Temporomandibular Joint Pain: A Prospective Cohort Study in the General Population. The Journal of Pain, 2012. 13(12): p. 1188–1197. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad M, et al. , Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD): Development of Image Analysis Criteria and Examiner Reliability for Image Analysis. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics, 2009. 107(6): p. 844–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiffman E, et al. , Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network() and Orofacial Pain Special Interest Group(). Journal of oral & facial pain and headache, 2014. 28(1): p. 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chisnoiu AM, et al. , Factors involved in the etiology of temporomandibular disorders - a literature review. Clujul Medical, 2015. 88(4): p. 473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer L, Clemente JT, and Tambeli CH, The protective role of testosterone in the development of temporomandibular joint pain. J Pain, 2007. 8(5): p. 437–42. [DOI] [PubMed] [Google Scholar]
- 17.Barrera-Mora JM, et al. , The relationship between malocclusion, benign joint hypermobility syndrome, condylar position and TMD symptoms. Cranio, 2012. 30(2): p. 121–30. [DOI] [PubMed] [Google Scholar]
- 18.Mohlin B, et al. , TMD in relation to malocclusion and orthodontic treatment. Angle Orthod, 2007. 77(3): p. 542–8. [DOI] [PubMed] [Google Scholar]
- 19.Caldas W, et al. , Occlusal changes secondary to temporomandibular joint conditions: a critical review and implications for clinical practice. Journal of Applied Oral Science, 2016. 24(4): p. 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandwani B, et al. , Incidence of bruxism in TMD population. N Y State Dent J, 2011. 77(5): p. 54–7. [PubMed] [Google Scholar]
- 21.Tabrizi R, et al. , Does gum chewing increase the prevalence of temporomandibular disorders in individuals with gum chewing habits? J Craniofac Surg, 2014. 25(5): p. 1818–21. [DOI] [PubMed] [Google Scholar]
- 22.Commisso MS, Martínez-Reina J, and Mayo J, A study of the temporomandibular joint during bruxism. International Journal of Oral Science, 2014. 6(2): p. 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salé H, Bryndahl F, and Isberg A, A 15-year follow-up of temporomandibular joint symptoms and magnetic resonance imaging findings in whiplash patients: a prospective, controlled study. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 2014. 117(4): p. 522–532. [DOI] [PubMed] [Google Scholar]
- 24.Haggman-Henrikson B, et al. , Pain and Disability in the Jaw and Neck Region following Whiplash Trauma. J Dent Res, 2016. 95(10): p. 1155–60. [DOI] [PubMed] [Google Scholar]
- 25.Miernik M and Wieckiewicz W, The Basic Conservative Treatment of Temporomandibular Joint Anterior Disc Displacement Without Reduction--Review. Adv Clin Exp Med, 2015. 24(4): p. 731–5. [DOI] [PubMed] [Google Scholar]
- 26.Luther F, Layton S, and McDonald F, Orthodontics for treating temporomandibular joint (TMJ) disorders. Cochrane Database Syst Rev, 2010(7): p. Cd006541. [DOI] [PubMed] [Google Scholar]
- 27.Alqutaibi A and Aboalrejal A, Types of Occlusal Splint in Management of Temporomandibular Disorders (TMD). J Arthritis, 2015. 4(176): p. 2. [Google Scholar]
- 28.Ferreira FM, et al. , Effect of Occlusal Splints on the Stress Distribution on the Temporomandibular Joint Disc. Brazilian Dental Journal, 2017. 28: p. 324–329. [DOI] [PubMed] [Google Scholar]
- 29.Amin A, Meshramkar R, and Lekha K, Comparative evaluation of clinical performance of different kind of occlusal splint in management of myofascial pain. The Journal of the Indian Prosthodontic Society, 2016. 16(2): p. 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti PCR, et al. , Partial time use of anterior repositioning splints in the management of TMJ pain and dysfunction: a one-year controlled study. Journal of Applied Oral Science, 2005. 13: p. 345–350. [DOI] [PubMed] [Google Scholar]
- 31.Ouanounou A, Goldberg M, and Haas DA, Pharmacotherapy in Temporomandibular Disorders: A Review. J Can Dent Assoc, 2017. 83: p. h7. [PubMed] [Google Scholar]
- 32.Yuasa H and Kurita K, Randomized clinical trial of primary treatment for temporomandibular joint disk displacement without reduction and without osseous changes: A combination of NSAIDs and mouth-opening exercise versus no treatment. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 2001. 91(6): p. 671–675. [DOI] [PubMed] [Google Scholar]
- 33.Al-Belasy FA and Dolwick MF, Arthrocentesis for the treatment of temporomandibular joint closed lock: a review article. International Journal of Oral and Maxillofacial Surgery, 2007. 36(9): p. 773–782. [DOI] [PubMed] [Google Scholar]
- 34.Sidebottom AJ, Current thinking in temporomandibular joint management. Br J Oral Maxillofac Surg, 2009. 47(2): p. 91–4. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed N, et al. , Prospective outcome assessment of the therapeutic benefits of arthroscopy and arthrocentesis of the temporomandibular joint. British Journal of Oral and Maxillofacial Surgery, 2012. 50(8): p. 745–748. [DOI] [PubMed] [Google Scholar]
- 36.Manfredini D, Piccotti F, and Guarda-Nardini L, Hyaluronic acid in the treatment of TMJ disorders: a systematic review of the literature. Cranio, 2010. 28(3): p. 166–76. [DOI] [PubMed] [Google Scholar]
- 37.Korkmaz YT, et al. , Is Hyaluronic Acid Injection Effective for the Treatment of Temporomandibular Joint Disc Displacement With Reduction? Journal of Oral and Maxillofacial Surgery, 2016. 74(9): p. 1728–1740. [DOI] [PubMed] [Google Scholar]
- 38.Dimitroulis G, McCullough M, and Morrison W, Quality-of-Life Survey Comparing Patients Before and After Discectomy of the Temporomandibular Joint. Journal of Oral and Maxillofacial Surgery, 2010. 68(1): p. 101–106. [DOI] [PubMed] [Google Scholar]
- 39.Dimitroulis G, The use of dermis grafts after discectomy for internal derangement of the temporomandibular joint. Journal of Oral and Maxillofacial Surgery, 2005. 63(2): p. 173–178. [DOI] [PubMed] [Google Scholar]
- 40.Dimitroulis G, A critical review of interpositional grafts following temporomandibular joint discectomy with an overview of the dermis-fat graft. International Journal of Oral and Maxillofacial Surgery, 2011. 40(6): p. 561–568. [DOI] [PubMed] [Google Scholar]
- 41.Sharma H, et al. , Costochondral Graft as Interpositional material for TMJ Ankylosis in Children: A Clinical Study. Journal of maxillofacial and oral surgery, 2015. 14(3): p. 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Sayed KM, Temporomandibular joint reconstruction with costochondral graft using modified approach. Int J Oral Maxillofac Surg, 2008. 37(10): p. 897–902. [DOI] [PubMed] [Google Scholar]
- 43.Guarda-Nardini L, Manfredini D, and Ferronato G, Temporomandibular joint total replacement prosthesis: current knowledge and considerations for the future. Int J Oral Maxillofac Surg, 2008. 37(2): p. 103–10. [DOI] [PubMed] [Google Scholar]
- 44.Wolford LM, et al. , Twenty-Year Follow-up Study on a Patient-Fitted Temporomandibular Joint Prosthesis: The Techmedica/TMJ Concepts Device. Journal of Oral and Maxillofacial Surgery, 2015. 73(5): p. 952–960. [DOI] [PubMed] [Google Scholar]
- 45.Gruber EA, McCullough J, and Sidebottom AJ, Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg, 2015. 53(5): p. 412–5. [DOI] [PubMed] [Google Scholar]
- 46.Henry CH and Wolford LM, Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J Oral Maxillofac Surg, 1993. 51(4): p. 352–8; discussion 359–60. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira JNAR, et al. , Evaluation of Surgically Retrieved Temporomandibular Joint Alloplastic Implants - Pilot Study. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons, 2008. 66(6): p. 1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuroda S, et al. , Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis Cartilage, 2009. 17(11): p. 1408–15. [DOI] [PubMed] [Google Scholar]
- 49.Alomar X, et al. , Anatomy of the temporomandibular joint. Semin Ultrasound CT MR, 2007. 28(3): p. 170–83. [DOI] [PubMed] [Google Scholar]
- 50.Miloro M, et al. , Peterson’s principles of oral and maxillofacial surgery. 2nd ed. 2004, Hamilton, Ont.; London: B C Decker. [Google Scholar]
- 51.Detamore MS and Athanasiou KA, Structure and function of the temporomandibular joint disc: implications for tissue engineering. J Oral Maxillofac Surg, 2003. 61(4): p. 494–506. [DOI] [PubMed] [Google Scholar]
- 52.Kim KW, et al. , Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng, 2003. 31(8): p. 924–30. [DOI] [PubMed] [Google Scholar]
- 53.Minarelli AM and Liberti EA, A microscopic survey of the human temporomandibular joint disc. J Oral Rehabil, 1997. 24(11): p. 835–40. [DOI] [PubMed] [Google Scholar]
- 54.Scapino RP, Obrez A, and Greising D, Organization and function of the collagen fiber system in the human temporomandibular joint disk and its attachments. Cells Tissues Organs, 2006. 182(3–4): p. 201–25. [DOI] [PubMed] [Google Scholar]
- 55.Detamore MS, et al. , Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol, 2005. 24(1): p. 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahtiainen K, et al. , Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. J R Soc Interface, 2013. 10(85): p. 20130287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Detamore MS, et al. , Cell Type and Distribution in the Porcine Temporomandibular Joint Disc. J Oral Maxillofac Surg, 2006. 64(2): p. 243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Almarza AJ and Athanasiou KA, Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng, 2004. 32(1): p. 2–17. [DOI] [PubMed] [Google Scholar]
- 59.Helmy ES, Bays RA, and Sharawy MM, Histopathological study of human TMJ perforated discs with emphasis on synovial membrane response. J Oral Maxillofac Surg, 1989. 47(10): p. 1048–52. [DOI] [PubMed] [Google Scholar]
- 60.Caron MMJ, et al. , Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis and Cartilage, 2012. 20(10): p. 1170–1178. [DOI] [PubMed] [Google Scholar]
- 61.Ng F, et al. , PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood, 2008. 112: p. 295–307. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi K, et al. , Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861–72. [DOI] [PubMed] [Google Scholar]
- 63.Jin Yap U, A. and Toh WS, Repair and Regeneration of Temporomandibular Joint: The Future of Stem Cell-Based Therapies. 2016. 47–75. [Google Scholar]
- 64.Chen K, et al. , Effect of in vitro chondrogenic differentiation of autologous mesenchymal stem cells on cartilage and subchondral cancellous bone repair in osteoarthritis of temporomandibular joint. Int J Oral Maxillofac Surg, 2013. 42(2): p. 240–8. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, et al. , The Pilot Study of Fibrin with Temporomandibular Joint Derived Synovial Stem Cells in Repairing TMJ Disc Perforation. Biomed Res Int, 2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fraser JK, et al. , Fat tissue: an underappreciated source of stem cells for biotechnology. Trends in Biotechnology, 2006. 24(4): p. 150–154. [DOI] [PubMed] [Google Scholar]
- 67.Kobayashi E, et al. , Experimental Study on In Situ Tissue Engineering of the Temporomandibular Joint Disc using Autologous Bone Marrow and Collagen Sponge Scaffold. Journal of Hard Tissue Biology, 2015. 24(2): p. 211–218. [Google Scholar]
- 68.Hennig T, et al. , Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol, 2007. 211(3): p. 682–91. [DOI] [PubMed] [Google Scholar]
- 69.Mäenpää K, et al. , Use of adipose stem cells and polylactide discs for tissue engineering of the temporomandibular joint disc. Journal of The Royal Society Interface, 2009. 7(42): p. 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahtiainen K, et al. , Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. Journal of The Royal Society Interface, 2013. 10(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mak J, et al. , Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci Rep, 2016. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shirasawa S, et al. , In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem, 2006. 97(1): p. 84–97. [DOI] [PubMed] [Google Scholar]
- 73.Bousnaki M, et al. , Fibro/chondrogenic differentiation of dental stem cells into chitosan/alginate scaffolds towards temporomandibular joint disc regeneration. Journal of Materials Science: Materials in Medicine, 2018. 29(7): p. 97. [DOI] [PubMed] [Google Scholar]
- 74.Kalpakci KN, et al. , Cartilage Tissue Engineering Using Dermis Isolated Adult Stem Cells: The Use of Hypoxia during Expansion versus Chondrogenic Differentiation. PLOS ONE, 2014. 9(5): p. e98570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sanchez-Adams J and Athanasiou KA, Dermis isolated adult stem cells for cartilage tissue engineering. Biomaterials, 2012. 33(1): p. 109–19. [DOI] [PubMed] [Google Scholar]
- 76.Wu SM and Hochedlinger K, Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature cell biology, 2011. 13(5): p. 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu J, et al. , The Effect of 3D Nanofibrous Scaffolds on the Chondrogenesis of Induced Pluripotent Stem Cells and Their Application in Restoration of Cartilage Defects. PLOS ONE, 2014. 9(11): p. e111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nejadnik H, et al. , Improved Approach for Chondrogenic Differentiation of Human Induced Pluripotent Stem Cells. Stem Cell Reviews and Reports, 2015. 11(2): p. 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guzzo M, R. and O’Sullivan MB, Human Pluripotent Stem Cells: Advances in Chondrogenic Differentiation and Articular Cartilage Regeneration. 2016. [Google Scholar]
- 80.Johns D and Athanasiou K, Passaged costal chondrocytes provide a viable cell source for temporomandibular joint tissue engineering. Ann Biomed Eng, 2008. 36(12): p. 1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johns D and Athanasiou K, A comparison of primary and passaged chondrocytes for use in engineering the temporomandibular joint. Arch Oral Biol, 2009. 54(2): p. 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy MK, et al. , Engineering a fibrocartilage spectrum through modulation of aggregate redifferentiation. Cell Transplant, 2015. 24(2): p. 235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vapniarsky N, et al. , Tissue engineering toward temporomandibular joint disc regeneration. Science Translational Medicine, 2018. 10(446). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Detamore MS and Athanasiou KA, Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol, 2004. 49(7): p. 577–83. [DOI] [PubMed] [Google Scholar]
- 85.Johns DE and Athanasiou KA, Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell and tissue research, 2008. 333(3): p. 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalpakci KN, Kim EJ, and Athanasiou KA, Assessment of Growth Factor Treatment on Fibrochondrocyte and Chondrocyte Co-Cultures for TMJ Fibrocartilage Engineering. Acta biomaterialia, 2011. 7(4): p. 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Landesberg R, Takeuchi E, and Puzas JE, Cellular, biochemical and molecular characterization of the bovine temporomandibular joint disc. Archives of Oral Biology, 1996. 41(8): p. 761–767. [DOI] [PubMed] [Google Scholar]
- 88.Landesberg R, Takeuchi E, and Puzas JE, Differential activation by cytokines of mitogen-activated protein kinases in bovine temporomandibular-joint disc cells. Archives of Oral Biology, 1999. 44(1): p. 41–48. [DOI] [PubMed] [Google Scholar]
- 89.Feng Y, et al. , HMGB1-induced angiogenesis in perforated disc cells of human temporomandibular joint. J Cell Mol Med, 2018. 22(2): p. 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ai-Aql ZS, et al. , Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res, 2008. 87(2): p. 107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang F, et al. , Effect of Concentrated Growth Factors on the Repair of the Goat Temporomandibular Joint. J Oral Maxillofac Surg, 2017. 75(3): p. 498–507. [DOI] [PubMed] [Google Scholar]
- 92.Detamore MS and Athanasiou KA, Evaluation of Three Growth Factors for TMJ Disc Tissue Engineering. Annals of biomedical engineering, 2005. 33(3): p. 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tarafder S, et al. , Micro-precise spatiotemporal delivery system embedded in 3D printing for complex tissue regeneration. Biofabrication, 2016. 8(2): p. 025003. [DOI] [PubMed] [Google Scholar]
- 94.Makadia HK and Siegel SJ, Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers, 2011. 3(3): p. 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morouço P, et al. , Tissue engineering for temporomandibular joint disc repair and regeneration: a methodological perspective. Advances in Cellular and Molecular Otolaryngology, 2016. 4(1): p. 33709. [Google Scholar]
- 96.Kent JN, et al. , Temporomandibular joint condylar prosthesis: A ten-year report. Journal of Oral and Maxillofacial Surgery, 1983. 41(4): p. 245–254. [DOI] [PubMed] [Google Scholar]
- 97.Heffez L, et al. , CT evaluation of TMJ disc replacement with a proplast-teflon laminate. Journal of Oral and Maxillofacial Surgery, 1987. 45(8): p. 657–665. [DOI] [PubMed] [Google Scholar]
- 98.Trumpy IG, Roald B, and Lyberg T, Morphologic and immunohistochemical observation of explanted Proplast-Teflon temporomandibular joint interpositional implants. Journal of Oral and Maxillofacial Surgery, 1996. 54(1): p. 63–68. [DOI] [PubMed] [Google Scholar]
- 99.Milam SB, A protocol for the management of failed alloplastic temporomandibular joint disc implants. Journal of Oral and Maxillofacial Surgery, 1995. 53(11): p. 1248–1249. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi E, et al. , Experimental Study on In Situ Tissue Engineering of the Temporomandibular Joint Disc using Autologous Bone Marrow and Collagen Sponge Scaffold. Vol. 24 2015. 211–218. [Google Scholar]
- 101.Moreno-Arotzena O, et al. , Characterization of fibrin and collagen gels for engineering wound healing models. Materials, 2015. 8(4): p. 1636–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y, et al. , Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. The Scientific World Journal, 2015. 2015: p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giri TK, et al. , Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications. Acta Pharmaceutica Sinica B, 2012. 2(5): p. 439–449. [Google Scholar]
- 104.Zhou HY, et al. , Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydrate Polymers, 2015. 117: p. 524–536. [DOI] [PubMed] [Google Scholar]
- 105.Brown BN, et al. , Extracellular Matrix as an Inductive Template for Temporomandibular Joint Meniscus Reconstruction: A Pilot Study. Journal of Oral and Maxillofacial Surgery, 2011. 69(12): p. e488–e505. [DOI] [PubMed] [Google Scholar]
- 106.Brown BN, et al. , Inductive, Scaffold-Based, Regenerative Medicine Approach to Reconstruction of the Temporomandibular Joint Disk. Journal of Oral and Maxillofacial Surgery, 2012. 70(11): p. 2656–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matuska AM and McFetridge PS, Laser micro-ablation of fibrocartilage tissue: Effects of tissue processing on porosity modification and mechanics. J Biomed Mater Res B Appl Biomater, 2018. 106(5): p. 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Juran CM, Dolwick MF, and McFetridge PS, Engineered Microporosity: Enhancing the Early Regenerative Potential of Decellularized Temporomandibular Joint Discs. Tissue Engineering. Part A, 2015. 21(3–4): p. 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Homsy CA, Recommended use of proplast. Journal of Oral and Maxillofacial Surgery, 1990. 48(3): p. 328–329. [DOI] [PubMed] [Google Scholar]
- 110.Fontenot MG and Kent JN, In vitro wear performance of proplast TMJ disc implants. Journal of Oral and Maxillofacial Surgery, 1992. 50(2): p. 133–139. [DOI] [PubMed] [Google Scholar]
- 111.Puelacher WC, et al. , Temporomandibular joint disc replacement made by tissue-engineered growth of cartilage. Journal of Oral and Maxillofacial Surgery, 1994. 52(11): p. 1172–1177. [DOI] [PubMed] [Google Scholar]
- 112.J Almarza A and Athanasiou K, Effects of Initial Cell Seeding Density for the Tissue Engineering of the Temporomandibular Joint Disc. Annals of Biomedical Engineering, 2005. 33(7): p. 943–950. [DOI] [PubMed] [Google Scholar]
- 113.Springer I, et al. , Culture of cells gained from temporomandibular joint cartilage on non-absorbable scaffolds. Biomaterials, 2001. 22(18): p. 2569–77. [DOI] [PubMed] [Google Scholar]
- 114.Ahtiainen K, et al. , Autologous adipose stem cells and polylactide discs in the replacement of the rabbit temporomandibular joint disc. Journal of the Royal Society Interface, 2013. 10(85): p. 20130287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.K Hagandora C, et al. , Poly (Glycerol Sebacate): A Novel Scaffold Material for Temporomandibular Joint Disc Engineering. Tissue Engineering Part A, 2012. 19(5–6): p. 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ronald S and Mills KD, Fibrochondrocyte Growth and Functionality on TiO2 Nanothin Films. Journal of Functional Biomaterials, 2016. 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hutmacher D, et al. , Scaffold Design and Fabrication. Tissue Engineering. 2008. [Google Scholar]
- 118.Nehrer S, et al. , Matrix collagen type and pore size influence behaviour of seeded canine chondrocytes. Biomaterials, 1997. 18(11): p. 769–76. [DOI] [PubMed] [Google Scholar]
- 119.Zhang Q, et al. , Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater, 2014. 10(5): p. 2005–13. [DOI] [PubMed] [Google Scholar]
- 120.Woodfield T, et al. , Polymer Scaffolds Fabricated with Pore-Size Gradients as a Model for Studying the Zonal Organization within Tissue-Engineered Cartilage Constructs. Vol. 11 2005. 1297–311. [DOI] [PubMed] [Google Scholar]
- 121.Chen S, et al. , Gelatin Scaffolds with Controlled Pore Structure and Mechanical Property for Cartilage Tissue Engineering. Tissue Engineering Part C: Methods, 2015. 22(3): p. 189–98. [DOI] [PubMed] [Google Scholar]
- 122.Bhardwaj T, et al. , Effect of material geometry on cartilagenous tissue formation in vitro. Vol. 57 2001. 190–9. [DOI] [PubMed] [Google Scholar]
- 123.Han K-S, et al. , Effect of Pore Sizes of Silk Scaffolds for Cartilage Tissue Engineering. Macromolecular Research, 2016. 23(12): p. 1091–1097. [Google Scholar]
- 124.Hamngren Blomqvist C, et al. , Pore size effects on convective flow and diffusion through nanoporous silica gels. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015. 484: p. 288–296. [Google Scholar]
- 125.Francisco L, et al. , Poly(ε-caprolactone) and Polyethylene Glycol Diacrylate-based Scaffolds for TMJ Bioengineered Disc Implants. Procedia Manufacturing, 2017. 12: p. 291–297. [Google Scholar]
- 126.Sakaguchi-Kuma T, et al. , An anatomic study of the attachments on the condylar process of the mandible: muscle bundles from the temporalis. Surg Radiol Anat, 2015. 38(4): p. 461–7. [DOI] [PubMed] [Google Scholar]
- 127.Kurita H, et al. , Alteration of the horizontal mandibular condyle size associated with temporomandibular joint internal derangement in adult females. Dentomaxillofacial Radiology, 2002. 31(6): p. 373–8. [DOI] [PubMed] [Google Scholar]
- 128.Tecco S, et al. , Condylar volume and surface in Caucasian young adult subjects. BMC Medical Imaging, 2010. 10(1): p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hegde S, Bn P, and Shetty S, Morphological and Radiological Variations of Mandibular Condyles in Health and Diseases: A Systematic Review. Vol. 3 2013. [Google Scholar]
- 130.Renders GAP, et al. , Porosity of human mandibular condylar bone. Journal of Anatomy, 2007. 210(3): p. 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim D-G, et al. , Regional variation of bone tissue properties at the human mandibular condyle. Bone, 2015. 77: p. 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sugisaki M, et al. , Three-Dimensional Analysis of the Internal Structure of the Mandibular Condyle in Dentulous and Edentulous Jaws Using Micro-CT. Cranio, 2009. 27(2): p. 78–87. [DOI] [PubMed] [Google Scholar]
- 133.Singh M and Detamore MS, Biomechanical properties of the mandibular condylar cartilage and their relevance to the TMJ disc. Journal of Biomechanics, 2009. 42(4): p. 405–417. [DOI] [PubMed] [Google Scholar]
- 134.Kuroda S, et al. , Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis and Cartilage, 2009. 17(11): p. 1408–1415. [DOI] [PubMed] [Google Scholar]
- 135.Mizoguchi I, et al. , An immunohistochemical study of regional differences in the distribution of type I and II collagens in rat mandibular condylar cartilage. Arch Oral Biol, 1996. 41(8–9): p. 863–9. [DOI] [PubMed] [Google Scholar]
- 136.Alhadlaq A and Mao JJ, Tissue-engineered Neogenesis of Human-shaped Mandibular Condyle from Rat Mesenchymal Stem Cells. J Dent Res, 2004. 82(12): p. 951–6. [DOI] [PubMed] [Google Scholar]
- 137.Ikada Y, Challenges in tissue engineering. Journal of the Royal Society Interface, 2006. 3(10): p. 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Samsudin R, Stem Cell and Tissue Engineering – The Challenge of Imitating Nature. The Malaysian Journal of Medical Sciences : MJMS, 2003. 10(2): p. 1–3. [PMC free article] [PubMed] [Google Scholar]
- 139.Amini AR, Laurencin CT, and Nukavarapu SP, Bone Tissue Engineering: Recent Advances and Challenges. Critical reviews in biomedical engineering, 2012. 40(5): p. 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yousefi A, et al. , Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cell International, 2016: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Embree MC, et al. , Exploiting endogenous fibrocartilage stem cells to regenerate cartilage and repair joint injury. Nature Communications, 2016. 7: p. 13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nukavarapu SP and Dorcemus DL, Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv, 2013. 31(5): p. 706–21. [DOI] [PubMed] [Google Scholar]
- 143.Grayson WL, et al. , Engineering anatomically shaped human bone grafts. Proceedings of the National Academy of Sciences, 2010. 107(8): p. 3299–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen F, et al. , Bone graft in the shape of human mandibular condyle reconstruction via seeding marrow-derived osteoblasts into porous coral in a nude mice model. Journal of Oral and Maxillofacial Surgery. 60(10): p. 1155–1159. [DOI] [PubMed] [Google Scholar]
- 145.El-Bialy T, et al. , In Vivo Ultrasound-Assisted Tissue-Engineered Mandibular Condyle: A Pilot Study in Rabbits. Tissue Engineering Part C: Methods, 2010. 16(6): p. 1315–23. [DOI] [PubMed] [Google Scholar]
- 146.Castillo-Cardiel G, et al. , Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent Traumatol, 2016. 33(1): p. 38–44. [DOI] [PubMed] [Google Scholar]
- 147.Linero I and Chaparro O, Paracrine Effect of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Bone Regeneration. PLOS ONE, 2014. 9(9): p. e107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Freitag J, et al. , The effect of autologous adipose derived mesenchymal stem cell therapy in the treatment of a large osteochondral defect of the knee following unsuccessful surgical intervention of osteochondritis dissecans – a case study. BMC Musculoskeletal Disorders, 2017. 18: p. 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jurgens WJFM, et al. , One-Step Surgical Procedure for the Treatment of Osteochondral Defects with Adipose-Derived Stem Cells in a Caprine Knee Defect: A Pilot Study . BioResearch Open Access, 2013. 2(4): p. 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hwang NS, et al. , In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(52): p. 20641–20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.T Kuhn L, et al. , Developmental-Like Bone Regeneration by Human Embryonic Stem Cell-Derived Mesenchymal Cells. Tissue Engineering Part A, 2013. 20(1–2): p. 365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bailey M, et al. , A Comparison of Human Umbilical Cord Matrix Stem Cells and Temporomandibular Joint Condylar Chondrocytes for Tissue Engineering Temporomandibular Joint Condylar Cartilage. Tissue Eng, 2007. 13(8): p. 2003–10. [DOI] [PubMed] [Google Scholar]
- 153.Zhao L, Weir MD, and Xu HHK, Human umbilical cord stem cell encapsulation in calcium phosphate scaffolds for bone engineering. Biomaterials, 2010. 31(14): p. 3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park Y-B, et al. , Restoration of a large osteochondral defect of the knee using a composite of umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel: a case report with a 5-year follow-up. BMC Musculoskeletal Disorders, 2017. 18: p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heath CA, Cells for tissue engineering. Trends in Biotechnology. 18(1): p. 17–19. [DOI] [PubMed] [Google Scholar]
- 156.Johns DE and Athanasiou KA, Passaged costal chondrocytes provide a viable cell source for temporomandibular joint tissue engineering. Annals of biomedical engineering, 2008. 36(12): p. 1992–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L, Lazebnik M, and Detamore MS, Hyaline cartilage cells outperform mandibular condylar cartilage cells in a TMJ fibrocartilage tissue engineering application. Osteoarthritis and Cartilage, 2009. 17(3): p. 346–353. [DOI] [PubMed] [Google Scholar]
- 158.Weng Y, et al. , Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. Journal of Oral and Maxillofacial Surgery, 2001. 59(2): p. 185–190. [DOI] [PubMed] [Google Scholar]
- 159.Martino MM, et al. , Extracellular matrix-inspired growth factor delivery systems for bone regeneration. Advanced Drug Delivery Reviews, 2015. 94: p. 41–52. [DOI] [PubMed] [Google Scholar]
- 160.Alhadlaq A and J Mao J, Tissue-Engineered Osteochondral Constructs in the Shape of an Articular Condyle. J Bone Joint Surg Am, 2005. 87(5): p. 936–44. [DOI] [PubMed] [Google Scholar]
- 161.Wang L and Detamore MS, Effects of growth factors and glucosamine on porcine mandibular condylar cartilage cells and hyaline cartilage cells for tissue engineering applications. Archives of Oral Biology, 2009. 54(1): p. 1–5. [DOI] [PubMed] [Google Scholar]
- 162.James AW, et al. , A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Engineering. Part B, Reviews, 2016. 22(4): p. 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chanchareonsook N, et al. , Segmental mandibular bone reconstruction with a carbonate-substituted hydroxyapatite-coated modular endoprosthetic poly(ε-caprolactone) scaffold in Macaca fascicularis. J Biomed Mater Res B Appl Biomater., 2014. 102(5): p. 962–76. [DOI] [PubMed] [Google Scholar]
- 164.Dormer NH, et al. , Osteochondral interface regeneration of rabbit mandibular condyle with bioactive signal gradients. Journal of oral and maxillofacial surgery, 2011. 69(6): p. e50–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kaur H, Uludağ H, and El-Bialy T, Effect of Nonviral Plasmid Delivered Basic Fibroblast Growth Factor and Low Intensity Pulsed Ultrasound on Mandibular Condylar Growth: A Preliminary Study. BioMed Research International, 2014. 2014: p. 426710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shen P, et al. , Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Scientific Reports, 2015. 5: p. 16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee K, Silva EA, and Mooney DJ, Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of the Royal Society Interface, 2011. 8(55): p. 153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Suzuki T, et al. , Regeneration of defects in the articular cartilage in rabbit temporomandibular joints by bone morphogenetic protein-2. British Journal of Oral and Maxillofacial Surgery, 2002. 40(3): p. 201–206. [DOI] [PubMed] [Google Scholar]
- 169.Xu H, et al. , Rapid prototyped PGA/PLA scaffolds in the reconstruction of mandibular condyle bone defects. Int J Med Robot, 2010. 6(1): p. 66–72. [DOI] [PubMed] [Google Scholar]
- 170.Temple JP, et al. , Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. Journal of Biomedical Materials Research Part A, 2014. 102(12): p. 4317–4325. [DOI] [PubMed] [Google Scholar]
- 171.Nicodemus GD, Villanueva I, and Bryant SJ, Mechanical stimulation of TMJ condylar chondrocytes encapsulated in PEG hydrogels. Journal of Biomedical Materials Research Part A, 2007. 83A(2): p. 323–331. [DOI] [PubMed] [Google Scholar]
- 172.Ciocca L, et al. , CAD-CAM-generated hydroxyapatite scaffold to replace the mandibular condyle in sheep: Preliminary results. J Biomater Appl, 2012. 28(2): p. 207–18. [DOI] [PubMed] [Google Scholar]
- 173.Howlader D, et al. , Hydroxyapatite collagen scaffold with autologous bone marrow aspirate for mandibular condylar reconstruction. Journal of Cranio-Maxillofacial Surgery, 2017. 45(9): p. 1566–1572. [DOI] [PubMed] [Google Scholar]
- 174.Wang Z, et al. , Preparation and Characterization of Integrated Condylar Biomimetic Scaffolds: A Pilot Study. Journal of Hard Tissue Biology, 2016. 25(1): p. 89–94. [Google Scholar]
- 175.Rajurkar SG, et al. , Use of Temporalis Fascia Flap in the Treatment of Temporomandibular Joint Ankylosis: A Clinical Audit of 5 Years. Contemporary Clinical Dentistry, 2017. 8(3): p. 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Coburn J, et al. , Biomimetics of the Extracellular Matrix: An Integrated Three-Dimensional Fiber-Hydrogel Composite for Cartilage Tissue Engineering. Smart structures and systems, 2011. 7(3): p. 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schek R, et al. , Tissue engineering osteochondral implants for temporomandibular joint repair. Vol. 8 2005. 313–9. [DOI] [PubMed] [Google Scholar]
- 178.Wang F, et al. , Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice. Journal of Cranio-Maxillofacial Surgery, 2017. 45(6): p. 855–861. [DOI] [PubMed] [Google Scholar]
- 179.Temple JP, et al. , Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A, 2014. 102(12): p. 4317–25. [DOI] [PubMed] [Google Scholar]
- 180.Li J, et al. , Computer-aided design and manufacturing and rapid prototyped nanoscale hydroxyapatite/polyamide (n-HA/PA) construction for condylar defect caused by mandibular angle ostectomy. Aesthetic Plast Surg, 2011. 35(4): p. 636–40. [DOI] [PubMed] [Google Scholar]
- 181.Ogura I, Sasaki Y, and Kaneda T, Analysis of mandibular condylar and glenoid fossa fractures with computed tomography. Eur Radiol, 2013. 24(4): p. 902–6. [DOI] [PubMed] [Google Scholar]
- 182.Kent JN, et al. , Experience with a polymer glenoid fossa prosthesis for partial or total temporomandibular joint reconstruction. Journal of Oral and Maxillofacial Surgery, 1986. 44(7): p. 520–533. [DOI] [PubMed] [Google Scholar]
- 183.Wolford LM and Mehra P, Custom-made total joint prostheses for temporomandibular joint reconstruction. Proceedings (Baylor University. Medical Center), 2000. 13(2): p. 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mercuri LG and Anspach WE 3rd, Principles for the revision of total alloplastic TMJ prostheses. Int J Oral Maxillofac Surg, 2003. 32(4): p. 353–9. [DOI] [PubMed] [Google Scholar]
- 185.Vega LG, Gonzalez-Garcia R, and Louis PJ, Reconstruction of acquired temporomandibular joint defects. Oral Maxillofac Surg Clin North Am, 2013. 25(2): p. 251–69. [DOI] [PubMed] [Google Scholar]
- 186.Lee JJ and Worthington P, Reconstruction of the temporomandibular joint using calvarial bone after a failed teflon-proplast implant. Journal of Oral and Maxillofacial Surgery, 1999. 57(4): p. 457–461. [DOI] [PubMed] [Google Scholar]
- 187.Jang HW, et al. , Mandibular condyle and infratemporal fossa reconstruction using vascularized costochondral and calvarial bone grafts. Journal of the Korean Association of Oral and Maxillofacial Surgeons, 2014. 40(2): p. 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aryaei A, et al. , Recent Tissue Engineering Advances for the Treatment of Temporomandibular Joint Disorders. Curr Osteoporos Rep, 2016. 14(6): p. 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Groen WM, et al. , From intricate to integrated: Biofabrication of articulating joints. Journal of Orthopaedic Research, 2017. 35(10): p. 2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eckerdal O, The Petrotympanic Fissure: A Link Connecting the Tympanic Cavity and the Temporomandibular Joint. Cranio, 1991. 9(1): p. 15–22. [DOI] [PubMed] [Google Scholar]
- 191.Ejima K, et al. , Relationship between the thickness of the roof of glenoid fossa, condyle morphology and remaining teeth in asymptomatic European patients based on cone beam CT data sets. Dento maxillo facial radiology, 2013. 42(3): p. 90929410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kijima N, et al. , Relationship between patient characteristics, mandibular head morphology and thickness of the roof of the glenoid fossa in symptomatic temporomandibular joints. Dentomaxillofacial Radiology, 2007. 36(5): p. 277–281. [DOI] [PubMed] [Google Scholar]
- 193.Rabie ABM, Wong L, and Tsai M, Replicating mesenchymal cells in the condyle and the glenoid fossa during mandibular forward positioning. American Journal of Orthodontics and Dentofacial Orthopedics, 2003. 123(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 194.Bruder SP, Fink DJ, and Caplan AI, Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem, 1994. 56(3): p. 283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ilgüy D, et al. , Articular Eminence Inclination, Height, and Condyle Morphology on Cone Beam Computed Tomography. The Scientific World Journal, 2014. 2014: p. 761714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Henrique Hirata F, et al. , Evaluation of TMJ articular eminence morphology and disc patterns in patients with disc displacement in MRI. Braz Oral Res, 2007. 21(3): p. 265–71. [DOI] [PubMed] [Google Scholar]
- 197.Furseth Klinge R, The structure of the fibrous tissue on the articular surface of the temporal bone in the monkey (Macaca mulatta). Micron, 2001. 32(6): p. 551–557. [DOI] [PubMed] [Google Scholar]
- 198.Rabie AB, She TT, and Harley VR, Forward mandibular positioning up-regulates SOX9 and type II collagen expression in the glenoid fossa. J Dent Res, 2003. 82(9): p. 725–30. [DOI] [PubMed] [Google Scholar]
- 199.Baug, et al. , Use of Adult Stem Cells for Cartilage Tissue Engineering: Current Status and Future Developments. Stem Cells International, 2015. 2015: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Makris EA, et al. , Repair and tissue engineering techniques for articular cartilage. Nature reviews. Rheumatology, 2015. 11(1): p. 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qiu G, et al. , Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J Tissue Eng Regen Med, 2018. 12(2): p. e937–e948. [DOI] [PubMed] [Google Scholar]
- 202.Guzzo RM and O’Sullivan MB, Human Pluripotent Stem Cells: Advances in Chondrogenic Differentiation and Articular Cartilage Regeneration. Current Molecular Biology Reports, 2016. 2(3): p. 113–122. [Google Scholar]
- 203.Lee J, et al. , Human iPSC-derived chondrocytes mimic juvenile chondrocyte function for the dual advantage of increased proliferation and resistance to IL-1β. Stem Cell Research & Therapy, 2017. 8(1): p. 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chijimatsu R, et al. , Characterization of Mesenchymal Stem Cell-Like Cells Derived From Human iPSCs via Neural Crest Development and Their Application for Osteochondral Repair. Stem Cells International, 2017. 2017: p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rabie ABM, Shum L, and Chayanupatkul A, VEGF and bone formation in the glenoid fossa during forward mandibular positioning. American Journal of Orthodontics and Dentofacial Orthopedics, 2002. 122(2): p. 202–209. [DOI] [PubMed] [Google Scholar]
- 206.Shum L, Rabie AB, and Hagg U, Vascular endothelial growth factor expression and bone formation in posterior glenoid fossa during stepwise mandibular advancement. Am J Orthod Dentofacial Orthop, 2004. 125(2): p. 185–90. [DOI] [PubMed] [Google Scholar]
- 207.Ruetze M and Richter W, Adipose-derived stromal cells for osteoarticular repair: trophic function versus stem cell activity. Expert Rev Mol Med, 2014. 16: p. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Vonk LA, et al. , Autologous, allogeneic, induced pluripotent stem cell or a combination stem cell therapy? Where are we headed in cartilage repair and why: a concise review. Stem Cell Res Ther, 2015. 6: p. 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Toh WS, et al. , Advances in mesenchymal stem cell-based strategies for cartilage repair and regeneration. Stem Cell Rev, 2014. 10(5): p. 686–96. [DOI] [PubMed] [Google Scholar]
- 210.Zhang H, et al. , Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol, 2018. 20(3): p. 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hewson C and Morris KV, Form and Function of Exosome-Associated Long Non-coding RNAs in Cancer, in Long Non-coding RNAs in Human Disease, Morris KV, Editor. 2016, Springer International Publishing: Cham: p. 41–56. [DOI] [PubMed] [Google Scholar]
- 212.Zaborowski MP, et al. , Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience, 2015. 65(8): p. 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sun Z, et al. , Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol Cancer, 2018. 17(1): p. 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hu L, et al. , Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Scientific Reports, 2016. 6: p. 32993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Furuta T, et al. , Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med, 2016. 5(12): p. 1620–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Johnsen KB, et al. , A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta, 2014. 1846(1): p. 75–87. [DOI] [PubMed] [Google Scholar]
- 217.Lu K, et al. , Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther, 2017. 8(1): p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qin Y, et al. , Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep, 2016. 6: p. 21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Toh W, et al. , 25 - MSC exosomes alleviate pain and degeneration in A rat model of temporomandibular joint osteoarthritis. Cytotherapy, 2018. 20(5, Supplement): p. S16. [Google Scholar]
- 220.Song I, 3039 - Chemotactic Effect of Bone Marrow Stromal Cell-derived Microvesicles on Progenitor cell homing in Injured Temporomandibular Joint Cartilage in Exosomes/Microvesicles: Heterogeneity, Biogenesis, Function and Therapeutic Developments. 2018: Breckenridge, Colorado USA. [Google Scholar]
- 221.Elgali I, et al. , Guided bone regeneration: materials and biological mechanisms revisited. European Journal of Oral Sciences, 2017. 125(5): p. 315–x337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Le Huec JC, et al. , Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials, 1995. 16(2): p. 113–118. [DOI] [PubMed] [Google Scholar]
- 223.Woodard JR, et al. , The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials, 2007. 28(1): p. 45–54. [DOI] [PubMed] [Google Scholar]
- 224.Trombetta R, et al. , 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann Biomed Eng, 2017. 45(1): p. 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tang Z, et al. , Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics. Journal of Biomedical Materials Research Part A, 2014. 103(3): p. 1001–1010. [DOI] [PubMed] [Google Scholar]
- 226.Shin K, et al. , Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine(). Tissue Eng Part A, 2017. 23(19–20): p. 1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hajiali F, Tajbakhsh S, and Shojaei A, Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polymer Reviews, 2018. 58(1): p. 164–207. [Google Scholar]
- 228.Zhang ZZ, et al. , 3D-Printed Poly(epsilon-caprolactone) Scaffold Augmented With Mesenchymal Stem Cells for Total Meniscal Substitution: A 12- and 24-Week Animal Study in a Rabbit Model. Am J Sports Med, 2017. 45(7): p. 1497–1511. [DOI] [PubMed] [Google Scholar]
- 229.Almeida HV, et al. , Anisotropic Shape-Memory Alginate Scaffolds Functionalized with Either Type I or Type II Collagen for Cartilage Tissue Engineering. Tissue Eng Part A, 2017. 23(1–2): p. 55–68. [DOI] [PubMed] [Google Scholar]
- 230.Baek J, et al. , Meniscus tissue engineering using a novel combination of electrospun scaffolds and human meniscus cells embedded within an extracellular matrix hydrogel. Journal of Orthopaedic Research, 2015. 33(4): p. 572–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lee CH, et al. , Protein-releasing polymeric scaffolds induce fibrochondrocytic differentiation of endogenous cells for knee meniscus regeneration in sheep. Science Translational Medicine, 2014. 6(266): p. 266ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lowe J and Almarza AJ, A review of in-vitro fibrocartilage tissue engineered therapies with a focus on the temporomandibular joint. Arch Oral Biol, 2017. 83: p. 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Brown WE, et al. , Functional self-assembled neocartilage as part of a biphasic osteochondral construct. PLOS ONE, 2018. 13(4): p. e01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lu HH, et al. , Tissue Engineering Strategies for the Regeneration of Orthopaedic Interfaces: Interface Tissue Engineering Strategies. Annals of biomedical engineering, 2010. 38(6): p. 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Atesok K, et al. , Multilayer scaffolds in orthopaedic tissue engineering. Knee Surg Sports Traumatol Arthrosc, 2016. 24(7): p. 2365–73. [DOI] [PubMed] [Google Scholar]
- 236.Levingstone TJ, et al. , Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater, 2016. 32: p. 149–160. [DOI] [PubMed] [Google Scholar]
- 237.Kon E, et al. , Novel nanostructured scaffold for osteochondral regeneration: pilot study in horses. J Tissue Eng Regen Med, 2010. 4(4): p. 300–8. [DOI] [PubMed] [Google Scholar]
- 238.Han F, et al. , A pilot study of conically graded chitosan-gelatin hydrogel/PLGA scaffold with dual-delivery of TGF-beta1 and BMP-2 for regeneration of cartilage-bone interface. J Biomed Mater Res B Appl Biomater, 2015. 103(7): p. 1344–53. [DOI] [PubMed] [Google Scholar]
- 239.Do A-V, et al. , 3D Printing of Scaffolds for Tissue Regeneration Applications. Advanced healthcare materials, 2015. 4(12): p. 1742–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kuswanto D, Composite of [HA/PMMA] for 3D-Printer Material Application. 2015. [Google Scholar]
- 241.Yao Q, et al. , Design, construction and mechanical testing of digital 3D anatomical data-based PCL-HA bone tissue engineering scaffold. J Mater Sci Mater Med, 2015. 26(1): p. 5360. [DOI] [PubMed] [Google Scholar]
- 242.Bartnikowski M, et al. , A Hydrogel Model Incorporating 3D-Plotted Hydroxyapatite for Osteochondral Tissue Engineering. Materials (Basel), 2016. 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rowland CR, et al. , The Effects of Crosslinking of Scaffolds Engineered from Cartilage ECM on the Chondrogenic Differentiation of MSCs. Biomaterials, 2013. 34(23): p. 5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Du D, et al. , Microstereolithography-based fabrication of anatomically shaped beta-tricalcium phosphate scaffolds for bone tissue engineering. BioMed research international, 2015. 2015: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Helgeland E, et al. , Scaffold-Based Temporomandibular Joint Tissue Regeneration in Experimental Animal Models: A Systematic Review. Tissue Eng Part B Rev, 2018. 24(4): p. 300–316. [DOI] [PubMed] [Google Scholar]
- 246.A.A. J, et al. , Preclinical Animal Models for Temporomandibular Joint Tissue Engineering. Tissue Engineering Part B: Reviews, 2018. 24(3): p. 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Weijs WA and Dantuma R, Functional Anatomy of the Masticatory Apparatus in the Rabbit (Oryctolagus Cuniculus L.). Netherlands Journal of Zoology, 1980. 31(1): p. 99–147. [Google Scholar]
- 248.Lin AW, et al. , The Temporomandibular Joint of the Domestic Dog (Canis lupus familiaris) in Health and Disease. Journal of Comparative Pathology, 2018. 161: p. 55–67. [DOI] [PubMed] [Google Scholar]
- 249.Angelo DF, et al. , Choosing sheep (Ovis aries) as animal model for temporomandibular joint research: Morphological, histological and biomechanical characterization of the joint disc. Morphologie, 2016. 100(331): p. 223–233. [DOI] [PubMed] [Google Scholar]
- 250.Natalia V, et al. , The Yucatan Minipig Temporomandibular Joint Disc Structure–Function Relationships Support Its Suitability for Human Comparative Studies. Tissue Engineering Part C: Methods, 2017. 23(11): p. 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Almarza AJ, Hagandora CK, and Henderson SE, Animal Models of Temporomandibular Joint Disorders: Implications for Tissue Engineering Approaches. Annals of Biomedical Engineering, 2011. 39(10): p. 2479. [DOI] [PubMed] [Google Scholar]
- 252.Ellegaard, Gottingen Minipigs Price List 2018 2018. Available from: https://minipigs.dk/.
- 253.River C, 2018 Catalog, 2018. Available from: www.criver.com.
- 254.University, A., Animal Facility Per Diem and Service Rates, 2018. Available from: www.augusta.edu.
- 255.Montana, T.U.o., Laboratory Animal Resources Projected Animal Per Diem Rates FY 17–21, 2018. Available from: www.umt.edu.
- 256.University, B., Per Diem Housing Rates (animal rates). 2018.
- 257.University, P., Animal Per Diem Rates. 2018.
- 258.Pittsburgh, U.o., Animal husbandry Per Diem Rate Schedule. 2018.
- 259.Minnesota, U.o., FT19 Animal per Diem Rates. 2018.
- 260.Michigan, U.o., Animal Care & Use Program: Rates. 2018.
- 261.University of California, L.A., Monkey Deficit Crimps Laboratories As Scientists Scramble for Alternatives. 2002.
- 262.Society, N.A.-V., Dogs Used in Research. 2018.
- 263.Herring SW, TMJ anatomy and animal models. Journal of musculoskeletal & neuronal interactions, 2003. 3(4): p. 391. [PMC free article] [PubMed] [Google Scholar]
- 264.Porto GG, et al. , Comparison between human and rat TMJ: anatomic and histopathologic features. Acta Cirurgica Brasileira, 2010. 25: p. 290–293. [DOI] [PubMed] [Google Scholar]
- 265.Rashed F, A Comparative Study of the Dentition and Temporomandibular Joint Anatomy and Histology Adult Dogs. Biol syst Open Access, 2015. 4(147): p. 2. [Google Scholar]
- 266.Poikela A, Pirttiniemi P, and Kantomaa T, Location of the glenoid fossa after a period of unilateral masticatory function in young rabbits. The European Journal of Orthodontics, 2000. 22(2): p. 105–112. [DOI] [PubMed] [Google Scholar]
- 267.Cheung LK, Shi X.-j., and Zheng L.-w., Surgical induction of temporomandibular joint ankylosis: an animal model. Journal of oral and maxillofacial surgery, 2007. 65(5): p. 993–1004. [DOI] [PubMed] [Google Scholar]
- 268.Kim K-W, et al. , Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Annals of biomedical engineering, 2003. 31(8): p. 924–930. [DOI] [PubMed] [Google Scholar]