Abstract
The 6′-fluorinated aristeromycins were designed as dual-target antiviral compounds aimed at inhibiting both the viral RNA-dependent RNA polymerase (RdRp) and the host cell S-adenosyl-l-homocysteine (SAH) hydrolase, which would indirectly target capping of viral RNA. The introduction of a fluorine at the 6′-position enhanced the inhibition of SAH hydrolase and the activity against RNA viruses. The adenosine and N6-methyladenosine analogues 2a–e showed potent inhibition against SAH hydrolase, while only the adenosine derivatives 2a–c exhibited potent antiviral activity against all tested RNA viruses such as Middle East respiratory syndrome-coronavirus (MERS-CoV), severe acute respiratory syndrome-coronavirus, chikungunya virus, and/or Zika virus. 6′,6′-Difluoroaristeromycin (2c) showed the strongest antiviral effect for MERS-CoV, with a ∼2.5 log reduction in infectious progeny titer in viral load reduction assay. The phosphoramidate prodrug 3a also demonstrated potent broad-spectrum antiviral activity, possibly by inhibiting the viral RdRp. This study shows that 6′-fluorinated aristeromycins can serve as starting points for the development of broad-spectrum antiviral agents that target RNA viruses.
Introduction
Over the past 15 years, outbreaks of a number of emerging positive-stranded RNA (+RNA) viruses,1 such as the severe acute respiratory syndrome coronavirus (SARS-CoV),2 Middle East respiratory syndrome coronavirus (MERS-CoV),3 chikungunya virus (CHIKV),4 and Zika virus (ZIKV)5 have seriously threatened human health and have had a substantial socio-economic impact. SARS-CoV and MERS-CoV cause serious respiratory diseases6 that can be fatal in approximately 10 and 35% of cases, respectively. CHIKV is transmitted by mosquitoes and causes a painful arthritis that can persist for months.7 ZIKV is also transmitted by mosquitoes,8 although sexual transmission8 occurs as well. This virus usually causes mild disease, but can cause neurological complications in adults and fetal death or severe complications, including microcephaly in infants when women are infected during pregnancy.9 CHIKV and ZIKV have caused massive outbreaks, totaling millions of infections over the past decade. Currently, there are no effective chemotherapeutic agents or vaccines that can prevent or cure infections of any of these four serious pathogens.
The aforementioned viruses belong to the +RNA virus group (Baltimore class IV),1 which indicates that their genomic RNA has the same polarity as mRNA and can be directly translated by host ribosomes upon release into the cytoplasm of a host cell. After infection, the genomes of these viruses are translated into polyproteins that are subsequently cleaved into individual proteins by viral and/or host proteases. The nonstructural proteins (nsps) of these viruses harbor a variety of enzymatic activities that are required for the replication of the viral RNA and invariably include a RNA-dependent RNA polymerase (RdRp),10 an enzyme which is not present in uninfected cells. The RdRp transcribes the genomic RNA into a complementary negative-stranded RNA that subsequently serves as the template for the synthesis of new positive-stranded RNA.
Many +RNA viruses (including coronaviruses, CHIKV, and ZIKV) also encode methyltransferases (MTases)11 that are required for methylations of viral mRNA cap structures.12 Because this capping is crucial for stability and translation of the viral RNA, and evasion of the host innate immune response, the viral MTases are considered promising targets for the development of antiviral therapy.12 Inhibition of MTases can be indirectly achieved by the inhibition of S-adenosyl-l-homocysteine (SAH) hydrolase.13 The SAH hydrolase catalyzes the interconversion of SAH into adenosine and l-homocysteine. Inhibition of this enzyme leads to the accumulation of SAH in the cell, which in turn inhibits S-adenosyl-l-methionine (SAM)-dependent transmethylase reactions by feedback inhibition.13,14 Most of the viral MTases are dependent on SAM as the only methyl donor. Compounds that target cellular proteins might exhibit a broader spectrum of activity, are less likely to lead to drug-resistance, but have a higher likelihood of toxicity. Compounds that are specifically aimed at viral proteins are expected to be less cytotoxic, but might have a narrower spectrum of antiviral activity and might have a lower barrier antiviral drug-resistance14 Thus, the approach of targeting cellular proteins such as SAH hydrolase can be considered as a promising strategy for the development of broad-spectrum antiviral agents.14 A number of compounds have been reported to act as SAH hydrolase inhibitors.14 Type I inhibitors act through inactivation of the NAD+ cofactor, and their inhibitory effect on the catalytic activity of the enzyme can be reversed by the addition of excess NAD+.14 Type II inhibitors are irreversible inhibitors of the SAH hydrolase that form covalent bonds with amino acid residues in the active site of the enzyme. This irreversible inhibition cannot be reversed by the addition of NAD+ or adenosine or by dialysis.14
Because both the viral RdRp and host SAH hydrolase are critical for virus replication, we aimed to design broad-spectrum nucleoside analogue inhibitors that could directly target RdRp activity and/or indirectly inhibit the methylation of viral RNA through their effect on the host SAH hydrolase. Modified nucleosides are usually taken up by the cell via nucleoside transporters and can be successively converted into mono-, di-, and triphosphates by cellular kinases.15 Then, these modified nucleoside triphosphates (NTPs) can compete with natural NTPs during RNA synthesis or can be incorporated into the nascent viral RNA, leading to chain termination or detrimental mutations.15
(−)-Aristeromycin (1) is a naturally occurring carbocyclic nucleoside that was originally identified as a metabolite of Streptomyces citricolor in 1967.16a The first synthesis of 1 as racemate was reported by Clayton and his co-worker,16b−16d and its asymmetric syntheses have since been reported.16e−16h It is a type I SAH hydrolase inhibitor and exhibits potent antiviral activity against many viruses.14a However, it could not be further advanced into clinical development because of its cytotoxicity.17 Compound 1 was found to be toxic at low concentrations in both adenosine kinase-positive (AK+) and AK– cells. AK+ cells were presumably killed by the 5′-phosphorylated form of 1, while the toxicity in AK– cells was caused by 1 itself.17 However, this compound is also metabolized into a triphosphate form and has been observed to exert a variety of metabolic effects.17 We aimed to use 1 as a prototype for the design of dual-target compounds intended at directly inhibiting the viral RdRp and indirectly inhibiting the capping process through targeting of cellular SAH hydrolase.
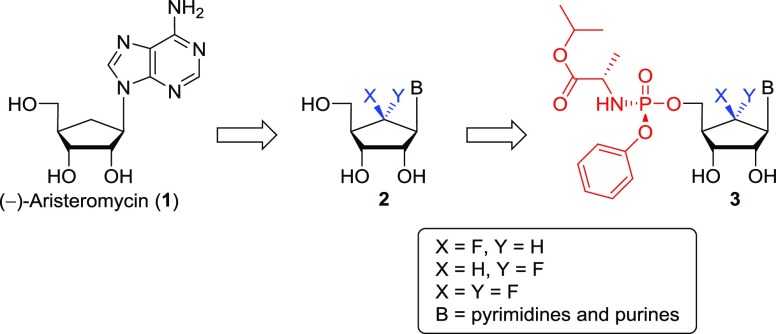
Since the introduction of a fluorine at the 6′-position of carbocyclic nucleosides has been known to affect biological activities to a significant extent,18 we aimed to synthesize the 6′-fluorinated-aristeromycin analogues 2 by introducing fluorine at the 6′-position of 1 (Figure 1). Prisbe and his co-workers18a have reported the synthesis of (±)-6′-α- and (±)-6′-β-fluorinated aristeromycins and their inhibitory activity on SAH hydrolase, but the synthesis and biological activity of (±)-6′,6′-difluoroaristeromycin was not reported, despite the fact that the structure was claimed in the patent.18b Thus, we set out to synthesize the 6′-fluorinated-aristeromycin analogues 2 in the optically pure d-forms because biological activity can generally be attributed to one enantiomer, the d-isomer. Yin and co-workers18c reported the elegant synthesis of optically pure (−)-6′-β-fluoro-aristeromycin, but its biological activity was not reported. Their synthetic route involved the 6-β-fluoroazide as the key intermediate, which was synthesized by employing SN2 fluorination of the 6-α-triflic azide with tris(dimethylamino)sulfur(trimethylsilyl)difluoride, whereas our current approach19 included the stereoselective electrophilic fluorination of silyl enol ether with Selectfluor as the fluorine source. In addition to the adenosine analogues, aimed at inhibiting SAH hydrolase and/or RdRp, we have also synthesized 6′-fluorinated purine and pyrimidine nucleosides (changes in B of the structures in Figure 1), which could interfere with viral RNA synthesis by targeting the viral RdRp after their phosphorylation by cellular kinases.15 To bypass the first and rate-limiting 5′-monophosphorylation step, we have also synthesized a phosphoramidate prodrug 3 of nucleoside 2, using the McGuigan ProTides.20 Herein, we report the synthesis of the 6′-fluoro-aristeromycin analogues 2 and 3 and a preliminary characterization of their effect on several +RNA viruses, which provided insight into structure–activity relationships (SARs).
Figure 1.
Rationale for the design of the target nucleosides 2 and 3.
Results and Discussion
Chemistry
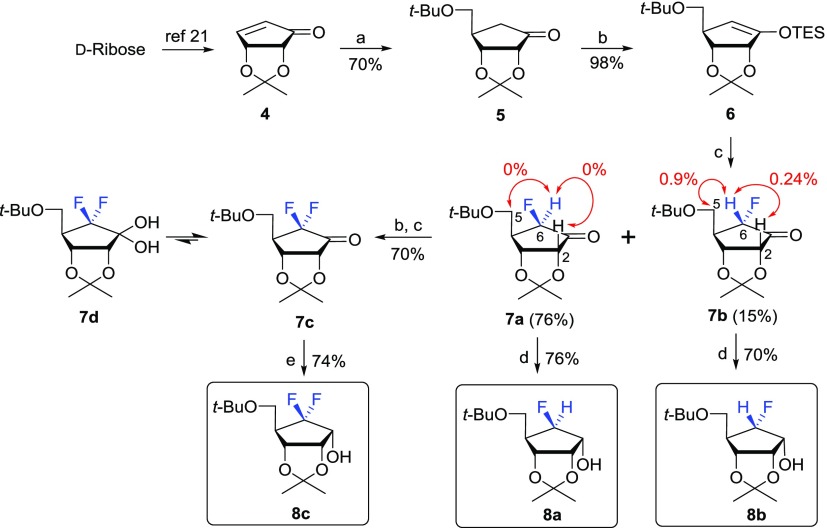
For the synthesis of the target nucleosides 2, the key fluorosugars 8a–c were synthesized from d-ribose via electrophilic fluorination, as shown in Scheme 1.
Scheme 1. Synthesis of 6-β-Fluoro-, 6-α-Fluoro-, and 6-Difluorosugar 8a–c.
Reagents and conditions: (a) LiCu(CH2Ot-Bu)2; (b) TESCl, LiHMDS, THF, −78 °C, 10 min; (c) Selectfluor, DMF, 0 °C, 12 h; (d) NaBH4, MeOH, 0 °C, 30 min. (e) LiBH4, MeOH, 0 °C, 30 min.
d-Ribose was converted to d-cyclopentenone 4 according to our previously published procedure.21 The 1,4-conjugated addition of 4 with Gilman reagent yielded the d-cyclopentanone derivative 5.19,22 Treatment of 5 with lithium hexamethyldisilazide (LiHMDS) followed by trapping with triethylsilyl chloride (TESCl) gave silylenol ether 6, which was treated with (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate): Selectfluor) in dimethylformamide (DMF) at 0 °C to yield a 5:1 ratio of 6-β-fluorosugar 7a to 6-α-fluorosugar 7b.19 The stereochemistry of the fluorine in 7a and 7b was confirmed by 1H NOE experiments. Irradiation of 6-H of 7b gave NOE effects on its 2-H and 5-H, indicating the 6-α-fluoro configuration, but no NOE effects were observed on the same experiment in the case of 7a, confirming the 6-β-fluoro configuration. The configuration of the fluorine in 7b was further confirmed by the X-ray crystal structure obtained after it was converted to the final uracil derivative 2g (Scheme 5). Further electrophilic fluorination of 6-β-fluorosugar 7a or 6-α-fluorosugar 7b under the same conditions yielded the 6,6-difluorosugar 7c, which was equilibrated to form a geminal diol because of the presence of electronegative fluorine atoms. Electrophilic fluorinations with other electrophilic fluorines such as N-fluorobenzenesulfonimide (NFSI) or N-fluoro-O-benzenedisulfonimide (NFOBS) were problematic, resulting in low yields with many side spots. The reduction of 7a–c with sodium borohydride (NaBH4) or lithium borohydride (LiBH4) in MeOH resulted in the production of the 1-hydroxyl derivatives 8a–c.
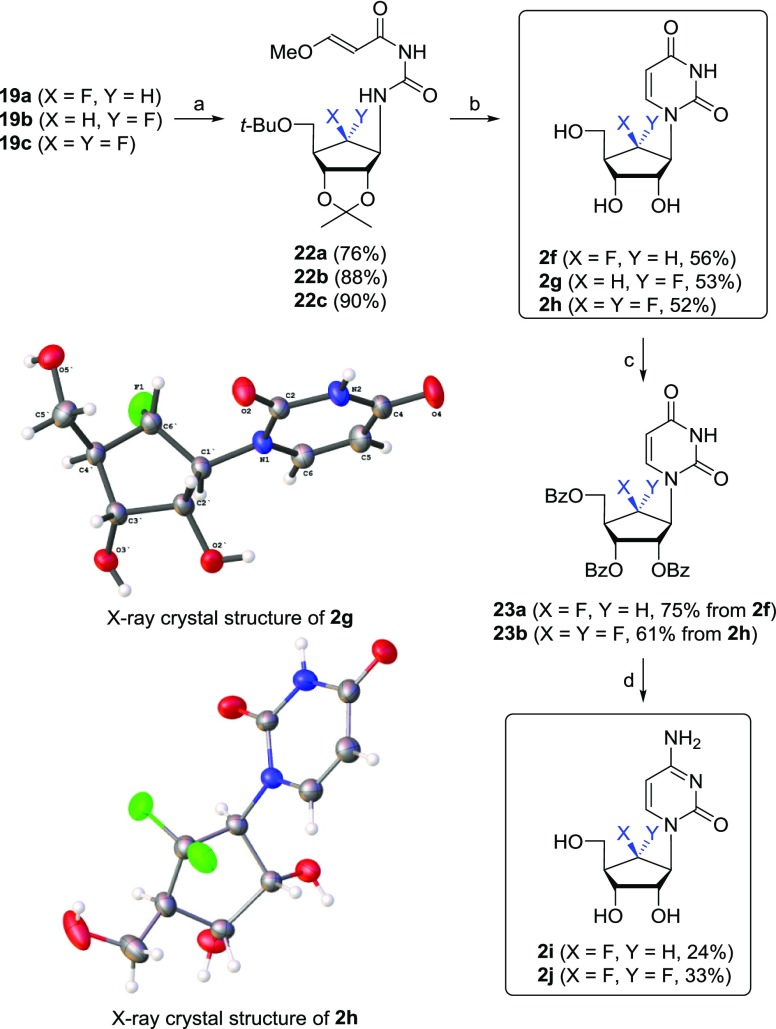
Scheme 5. Synthesis of Fluorinated Pyrimidine Nucleoside Analogues 2f–j.
Reagents and conditions: (a) (E)-3-methoxy-2-propenoyl isocyanate, benzene, 4 Å-MS, DMF, −20 °C to rt, 15 h; (b) 2 M H2SO4, dioxane, reflux, 1.5 h; (c) BzCl, pyridine, CH2Cl2, rt, 15 h; (d) (i) 1,2,4-triazole, POCl3, Et3N, CH3CN, rt, 15 h. (ii) NH4OH, dioxane, rt, 15 h. (iii) NH3/MeOH, rt, 15 h.
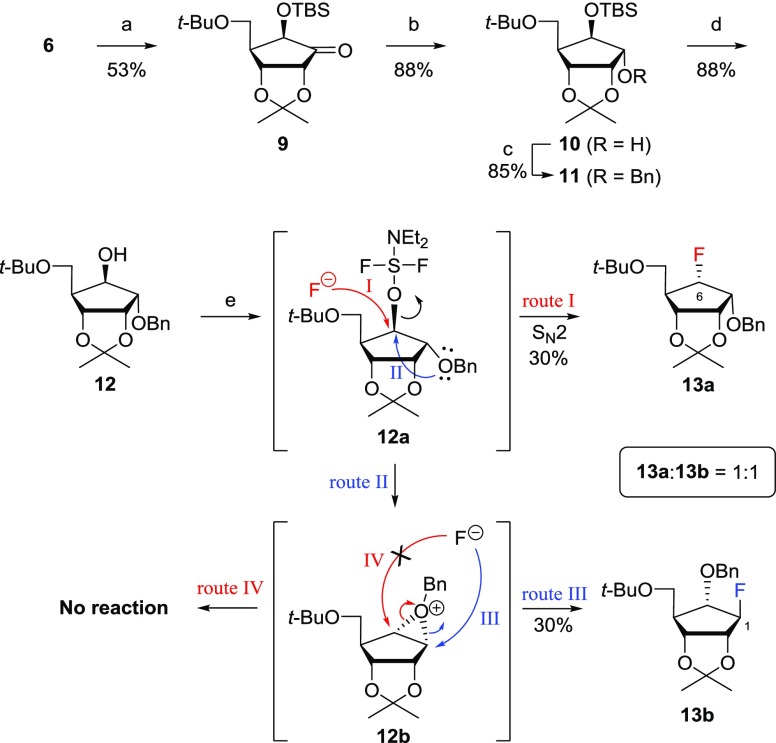
As the α-fluoro derivative 8b was obtained as the minor isomer, as shown in Scheme 1, we wanted to improve the stereoselective synthesis of 8b, by using Rubottom23 oxidation as the key step, as illustrated in Scheme 2. Rubottom oxidation of silylenol ether 6 with osmium tetroxide (OsO4) and N-methylmorpholine-N-oxide (NMO) followed by trapping with t-butyldimethylsilyl chloride (TBSCl) produced 6-β-alkoxyketone 9 as a single stereoisomer in 53% yield. The reduction of ketone 9 with NaBH4 gave alcohol 10, which was protected with a benzyl group to give 11. Removal of the t-butyldimethylsilyl (TBS) group in 11 with tetra-n-butylammonium fluoride (TBAF) yielded the 6-β-alcohol 12. To our disappointment, the treatment of 12 with N,N-diethylaminosulfur trifluoride (DAST) gave the desired product, 6-α-fluoride 13a, but also the undesired product 1-β-fluoride 13b at a 1:1 ratio. The formation of 13a (route I) resulted from the direct SN2 reaction of 12a with fluoride, while 12a was readily converted into the oxonium ion 12b (route II) via its participation of the neighboring benzyl group, which was attacked exclusively by the fluoride at the less sterically hindered 1-position to yield the undesired product 13b (route III). However, the product via route IV was not formed because of the steric effect of the t-butyloxymethyl substituent.
Scheme 2. Synthetic Approach to 6-α-Fluorosugar 8b via Rubottom Oxidation.
Reagents and conditions: (a) (i) OsO4, NMO·H2O, THF, rt, 1 h, then NaHCO3, MeOH, rt, 3 h; (ii) TBSCl, imidazole, DMF, rt, 3 h; (b) NaBH4, MeOH, rt, 1 h; (c) BnBr, NaH, DMF, 0 °C to rt, 12 h; (d) TBAF, THF, rt, 12 h; (e) DAST, toluene, 0 °C to rt, 2 h.
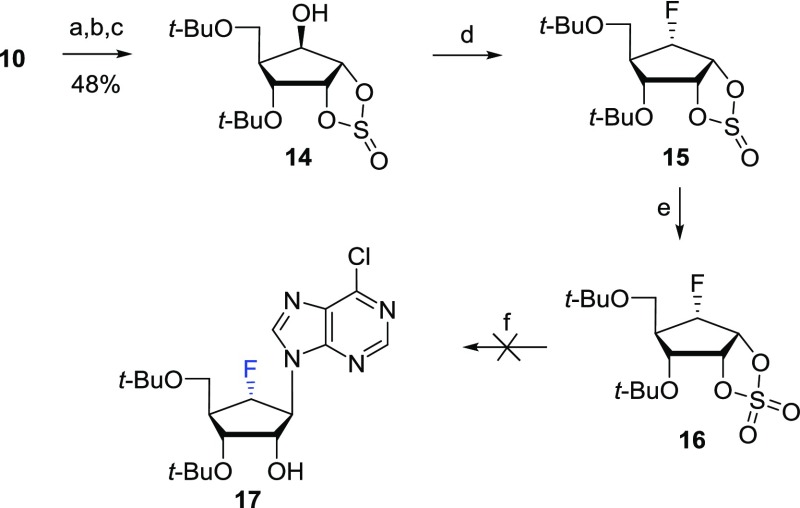
To avoid the participation of the neighboring group, we considered using a cyclic sulfate substrate with electron-withdrawing property and conformational restraint to be the best choice. Furthermore, cyclic sulfate has the advantage that it can be utilized as a surrogate for epoxide during nucleobase condensation, as shown in Scheme 3. The regioselective cleavage of the 2,3-acetonide in 10 with trimethylaluminum (AlMe3) followed by treatment of the resulting diol with thionyl chloride (SOCl2) yielded the 6-β-hydroxyl cyclic sulfite 14 after the removal of the TBS group. The treatment of 14 with DAST yielded the desired 6-α-fluoro cyclic sulfite 15 as a single stereoisomer. The cyclic sulfite 15 was oxidized to form cyclic sulfate 16, which was subsequently condensed with 6-chloropurine anions; however, this resulted in decomposition.19 Thus, we decided to synthesize the 6-α-fluoro derivative 8b according to Scheme 1.
Scheme 3. Synthetic Approach to 2b via Cyclic Sulfate.
Reagents and conditions: (a) AlMe3, CH2Cl2, −78 °C to rt, 12 h; (b) SOCl2, Et3N, CH2Cl2, 0 °C, 10 min; (c) TBAF, AcOH, THF, rt, 12 h; (d) DAST, CH2Cl2, 0 °C to rt, 4 h; (e) RuCl3, NaIO4, CCl4/CH3CN/H2O (1/1/1.5), rt, 20 min; (f) (i) 6-chloropurine, 18-crown-6, NaH, THF, 65 °C, 15 h; (ii) 20% H2SO4, rt, 1 h.
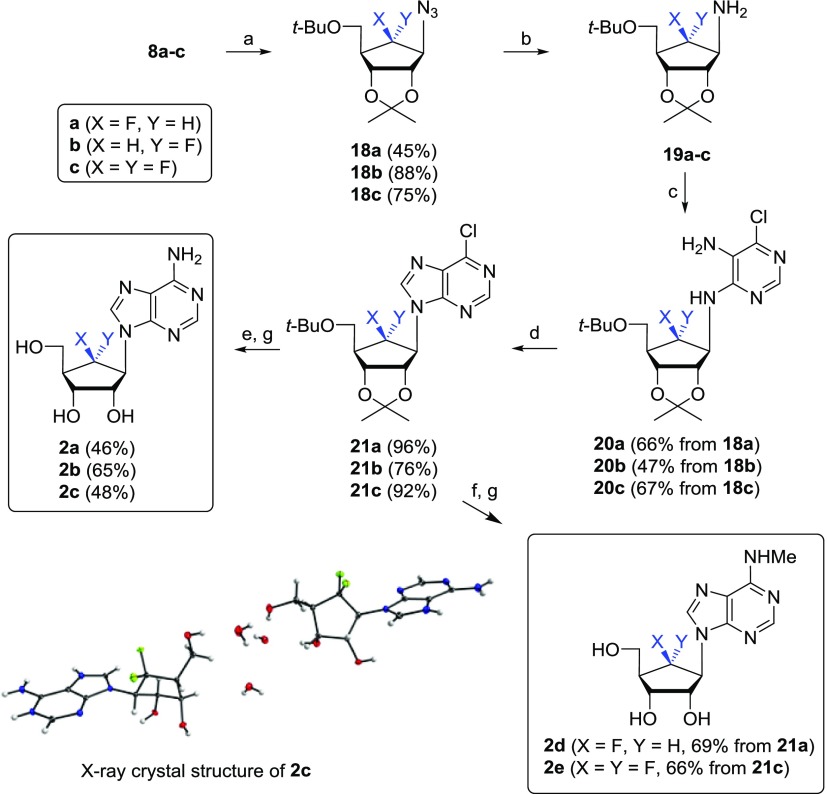
Scheme 4 depicts the synthesis of the aristeromycin analogues 2a–e from the 6-β-fluoro-, 6-α-fluoro-, and 6,6-difluorosugars 8a–c.19 Compounds 8a–c were treated with triflic anhydride (Tf2O) followed by treatment with sodium azide to give azido derivatives 18a–c. The catalytic hydrogenation of 18a–c yielded the amino derivatives 19a–c, respectively, which are starting compounds for the base-building process. The treatment of 19a–c with 5-amino-4,6-dichloropyrimidine18a−18c,24 in the presence of N,N-diisopropylethylamine (DIPEA) under microwave radiation conditions yielded 20a–c, which were cyclized with diethoxymethyl acetate18a−18c,24 in the presence of microwave radiation to produce the 6-chloropurine derivatives 21a–c. The treatment of 21a–c with t-butanolic ammonia followed by the removal of protective groups under acidic conditions yielded the 6′-β-fluoro-, 6′-α-fluoro-, and 6′,6′-difluoroaristeromycins 2a–c, respectively. The structure of compound 2c was confirmed by a single-crystal X-ray analysis (see the Supporting Information).25 The treatment of 21a and 21c with 40% aqueous methylamine followed by aqueous trifluoroacetic acid (TFA) resulted in N6-methyl-aristeromycin analogues 2d and 2e, respectively.
Scheme 4. Synthesis of β-Fluoro-, α-Fluoro-, and Difluoro-Aristeromycin Analogues 2a–e.
Reagents and conditions: (a) (i) Tf2O, pyridine, 0 °C, 30 min; (ii) NaN3, DMF, 60–100 °C, 4–15 h; (b) Pd/C, H2, MeOH, rt, 18 h; (c) 5-amino-4,6-dichloropyrimidine, DIPEA, n-BuOH, 170–200 °C, 4–7 h, MW; (d) CH3C(O)OCH(OEt)2, 140 °C, 3 h, MW; (e) NH3/t-BuOH, 120 °C, 15 h; (f) NH2Me/H2O, (40 wt %), EtOH, 30 °C, 2 h; (g) 67% aq TFA, 50 °C, 15 h.
The amino derivatives 19a–c were also converted into the pyrimidine nucleoside derivatives 2f–j, as shown in Scheme 5. Treatment of 19a–c with (E)-3-methoxy-2-propenoyl isocyanate, which was prepared by reacting 3-methoxyacryloyl chloride with silver cyanate,26 in benzene produced 22a–c, respectively, which were cyclized with 2 M H2SO4 to yield the uridine derivatives 2f–h, respectively. The structures of 2g and 2h were confirmed by the X-ray crystallography (see the Supporting Information) (Scheme 5).27 To synthesize the cytidine derivatives 2i and 2j, compounds 2f and 2h were benzoylated to give 23a and 23b, respectively, which were converted to the cytidine derivatives 2i and 2j using conventional three-step procedures.28
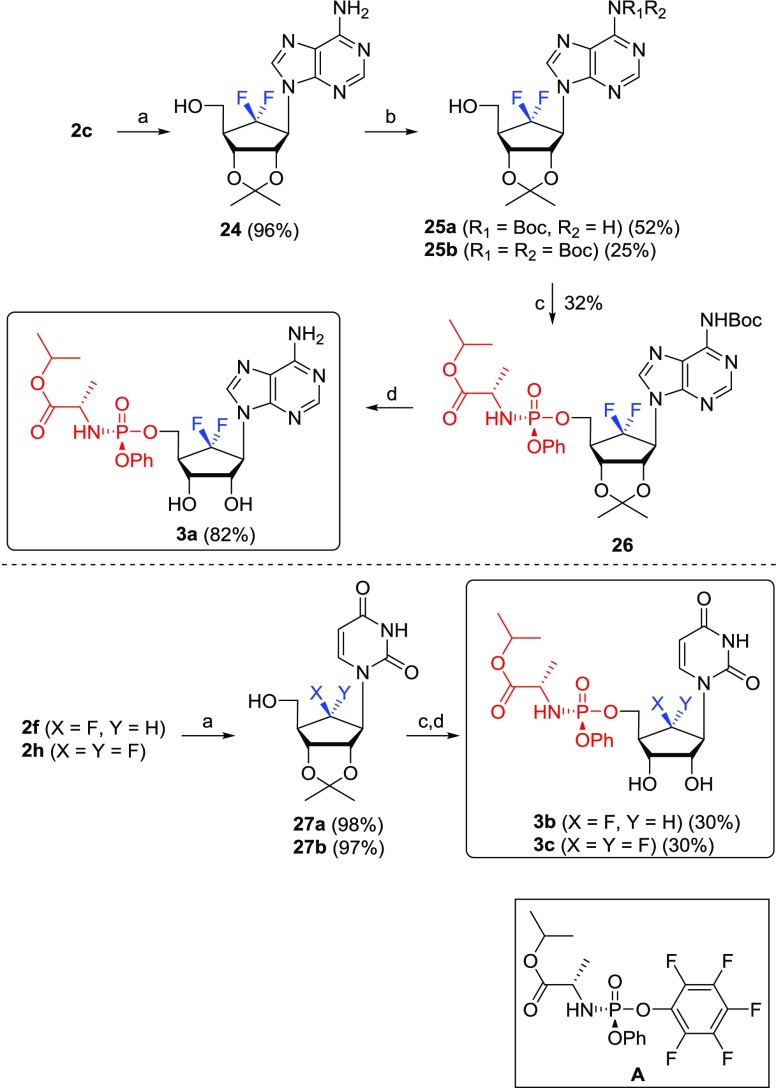
The uracil phosphoramidate analogue Sofosbuvir20 is used in the clinic as a powerful anti-hepatitis C virus agent. Therefore, we have also synthesized the uracil phosphoramidate prodrugs 3b–c and the adenine phosphoramidate prodrug 3a derived from the purine and pyrimidine nucleoside analogues 2a–j by using McGuigan’s ProTide prodrug methodology,20 as shown in Scheme 6. 6′,6′-Difluoro-aristeromycin (2c) was treated with acetone under acidic conditions to give 2,3-acetonide 24. The treatment of 24 with di-tert-butyl dicarbonate (Boc2O) yielded a mixture of 25a and 25b in a 2:1 ratio, which was converted to the phosphoramidate prodrug 26 by treating with phosphoramiditing reagent (A)29 in the presence of t-butylmagnesium chloride. The treatment of 26 with 50% formic acid produced the final product, prodrug 3a. The monofluoro- and difluoropyrimidine derivatives 2f and 2h were similarly converted to the final prodrugs 3b and 3c.
Scheme 6. Synthesis of Phosphoramidate Prodrugs 3a–c.
Reagents and conditions: (a) cH2SO4, acetone, rt, 4 h; (b) (i) TMSOTf, DMAP, HMDS, 75 °C, 2 h; (ii) Boc2O, THF, rt, 4 h; (iii) MeOH/Et3N (5:1), 55 °C, 16 h; (c) A, t-BuMgCl, 4 Å-MS, THF, 0 °C to rt, 36 h; (d) 50% HCOOH, rt, 8 h.
Inhibition of SAH Hydrolase
All compounds 1, 2a–j, and 3a–c were assayed for their ability to inhibit recombinant human SAH hydrolase protein, expressed in Escherichia coli JM109, using a 5,5′-dithiobis-2-nitrobenzoate (DTNB) coupled assay as described by Lozada-Ramírez et al.30 As expected, all adenosine derivatives 2a–e potently inhibited SAH hydrolase, but none of the pyrimidine analogues 2f–j showed any inhibitory activity at concentrations up to 100 μM. None of the prodrugs 3a–c exhibited inhibitory activity at concentrations up to 100 μM. This result is not surprising because adenosine is the substrate for SAH hydrolase. Among the adenosine analogues, 6′-β-fluoroaristeromycin (2a) exhibited the most potent inhibitory activity (IC50 = 0.37 μM), which was 3.6-fold more potent than the control 1 (IC50 = 1.32 μM). However, 6′-α-fluoroaristeromycin (2b, IC50 = 9.70 μM) was 26-fold less potent than the corresponding 6′-β-fluoro analogue 2a and 7.4-fold less active than the 6′-unsubstituted compound 1. This indicates that the stereochemistry at the 6′-position is important for inhibitory activity. Interestingly, the introduction of two fluorines at the 6′-position resulted in 2c (IC50 = 1.06 μM), which was slightly more potent than the control 1. The inhibitory activity of the 6′-fluoro-aristeromycin series can be ranked in the following order: 6′-β-F > 6′,6′-F,F > 6′-H > 6′-α-F. The introduction of a methyl group at the N6-amino group of 2a, resulting in 2d, decreased the inhibitory activity (IC50 = 4.39 μM) by 11.9-fold, while the addition of a methyl group to the N6-amino group of 2c, resulting in 2e, increased the inhibitory activity (IC50 = 0.76 μM) by 1.7-fold. These results demonstrate that the N6-methyladenine and the adenine moieties do not lead to a decrease in inhibitory activity.
Antiviral Activity
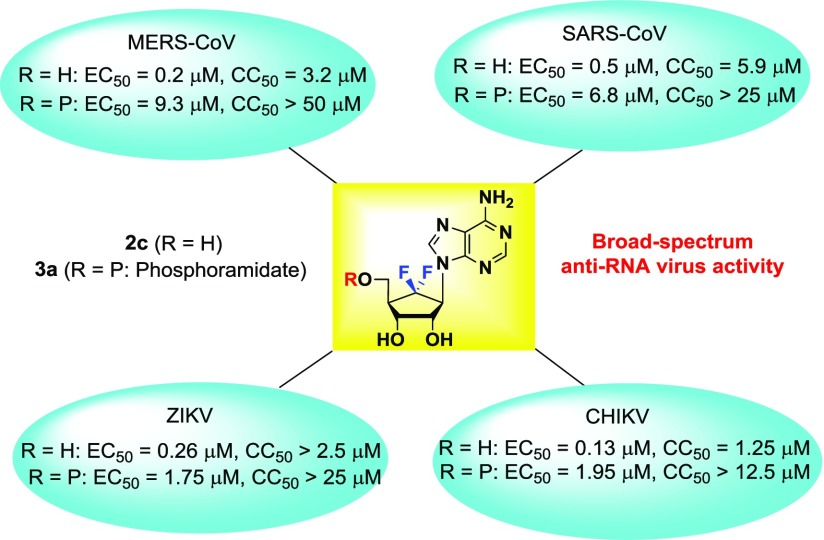
The novel 6′-fluoro-aristeromycin analogues 2a–j and 3a–c were screened for antiviral activity against a variety of +RNA viruses. The compounds were tested for antiviral activity in cytopathic effect (CPE) reduction assays at 4 concentrations, that is, 150, 50, 16.7, and 5.6 μM by preparing 3-fold serial dilutions. Compounds that demonstrated antiviral activity in this primary screen were further tested more extensively in dose response experiments at 8 different concentrations to determine the EC50. Cytotoxicity (CC50) was determined in parallel in uninfected cells (Table 1).
Table 1. Inhibition of SAH Hydrolase and the Replication of Several +RNA Viruses by All Final Nucleoside Analogues 2a–j and 3a–ca,b,c,d.
| MERS-CoV |
SARS-CoV |
ZIKV |
CHIKV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compound no. | SAH hydrolase IC50 (μM) | EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI | EC50 (μM) | CC50 (μM) | SI |
| 1 | 1.32 | >50 | 2 | >50 | >5 | 0.64 | 2.4 | 3.8 | 0.8 | 6.3 | 7.9 | ||
| 2a | 0.37 | 0.20 | 0.60 | 3 | ND | ND | ND | ND | >100 | >100 | |||
| 2b | 9.70 | ND | ND | ND | ND | 2.54 | 3.97 | 1.56 | 0.53 | 1.32 | 2.49 | ||
| 2c | 1.06 | 0.2 | 3.2 | 16 | 0.5 | 5.9 | 11.8 | 0.26 | >2.5 | >9.6 | 0.13 | >1.25 | >9.6 |
| 2d | 4.39 | >50 | >50 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2e | 0.76 | >50 | 12.5 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2f | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2g | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2h | >100 | >50 | >50 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2i | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 2j | >100 | >50 | >50 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 3a | >100 | 9.3 | >50 | 6.8 | >25 | >3.7 | 1.75 | >25 | >14.3 | 1.95 | >12.5 | >6.4 | |
| 3b | >100 | >50 | >50 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
| 3c | >100 | >50 | >50 | >100 | >100 | >100 | >100 | >100 | >100 | ||||
ND: not determined; SI = CC50/EC50.
EC50: effective concentration to inhibit the replication of the virus by 50%.
CC50: cytotoxic concentration to inhibit the replication of normal cells by 50%.
EC50 > 100 indicates that no antiviral activity was observed at the highest concentration tested because either there was no protection or the compound was toxic.
As shown in Table 1, only the adenosine derivatives 2a–c exhibited potent antiviral activities against +RNA viruses, while the other purine N6-methyladenine derivatives 2d and 2e and pyrimidine derivatives 2f–j did not show significant antiviral activities, not even at 100 μM. This result suggests that the antiviral activity might be due to an (indirect) effect on viral MTase activity through the inhibition of host SAH hydrolase. Inhibition of the viral RdRp appears not to be important. The mechanism of action of these compounds has been studied in more detail and results will be published elsewhere.
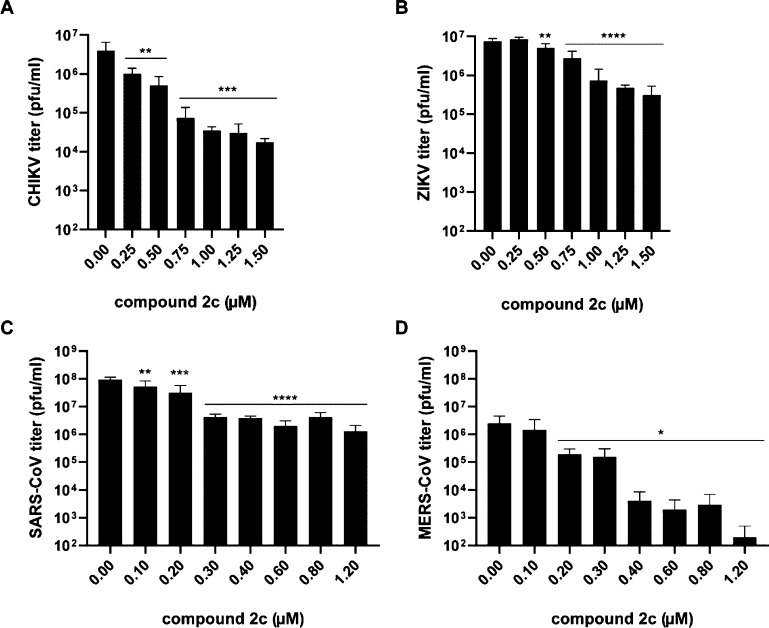
Compound 2a inhibited MERS-CoV replication with an EC50 of 0.20 μM; however, it was also rather cytotoxic, resulting in a selectivity index (SI) of 3. Replacement of the remaining 6′-H in 2a with F resulted in compound 2c, which exhibited a > 5-fold reduction in cytotoxicity, while its antiviral activity remained unchanged, with an EC50 of ∼0.20 μM and an SI of 15 for MERS-CoV. This compound was also active against SARS-CoV with an SI of 12.5, suggesting that it may be a broad-spectrum coronavirus inhibitor. In addition, it also inhibited ZIKV replication with an EC50 of 0.26 μM (SI > 10) and was active against CHIKV with an EC50 of 0.13 μM. Compound 2b showed some inhibitory effects on CHIKV and ZIKV replication, but this was likely due to pleiotropic cytotoxic effects, as the SI was <3. Among the phosphoramidate prodrugs 3a–c, only the adenosine prodrug 3a exhibited significant broad-spectrum antiviral activities, demonstrating that it may inhibit the RdRp of RNA viruses after conversion into the triphosphate form, although it remains to be determined in biochemical assays whether the triphosphate form affects RdRp activity.20 Compound 3a had an EC50 of 9.3 μM for MERS-CoV and 6.8 μM for SARS-CoV, but it also had an SI < 10, and it was therefore not considered a potent inhibitor of coronavirus replication. However, for CHIKV and ZIKV, 3a had EC50 values of 1.95 and 1.75 μM, respectively, with good selectivity indices. Interestingly, the prodrug 3a was less potent, but also much less cytotoxic than the parent compound 2c, which is unusual as regularly the phosphoramidate is more potent than the parent drug.20 The phosphoramidate 3a might be slowly hydrolyzed to the 5′-monophosphate by metabolic enzymes, or to the parent drug 2c by a phosphatase, which could inhibit SAH hydrolase, explaining the observed antiviral effect. Viral load reduction assays were performed with compound 2c by infecting cells with CHIKV, ZIKV, SARS-CoV, and MERS-CoV, followed by treatment with different concentrations of 2c. At 30 hpi (CHIKV) or 48 hpi (ZIKV, SARS- and MERS-CoV), infectious progeny titers in the medium were determined by plaque assay (Figure 2). Treatment with concentrations higher than 1 μM of 2c reduced infectious CHIKV titers by more than 2 log. The effect on ZIKV infectious progeny titers was limited and showed a ∼1 log reduction. For SARS-CoV, the reduction in infectious progeny titer was ∼1.5 log at 2c concentrations above 0.3 μM. The strongest antiviral effect was observed for MERS-CoV, with a ∼2.5 log reduction in infectious progeny titers when infected cells were treated with 2c concentrations above 0.3 μM. Follow-up studies to gain more insights into the mode of action of 2c and 3a and related compounds are currently ongoing, and results will be published elsewhere.
Figure 2.
Effect of 2c on the infectious progeny of CHIKV, ZIKV, SARS-CoV, and MERS-CoV. Cells were infected with the virus indicated on the y-axis of the graph in medium with various concentrations of 2c. Infectious progeny titers were determined by plaque assay (n = 4) and viability of noninfected cells was monitored using the CellTiter 96AQueous Non-Radioactive Cell Proliferation Assay (Promega). Significant differences are indicated by *: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Finally, we measured the log P of the most active compound 2c by the pH-metric method, using a T3 Sirius instrument, because the lipophilicity is a major determinant for compound absorption, distribution in the body, penetration across biological barriers, metabolism, and excretion. The measured log P was 0.02, indicating that it is almost equally partitioned between the lipid and aqueous phases. The relatively low log P of 2c is expected to be overcome by converting it to the phosphoramidate 3a.
Conclusions
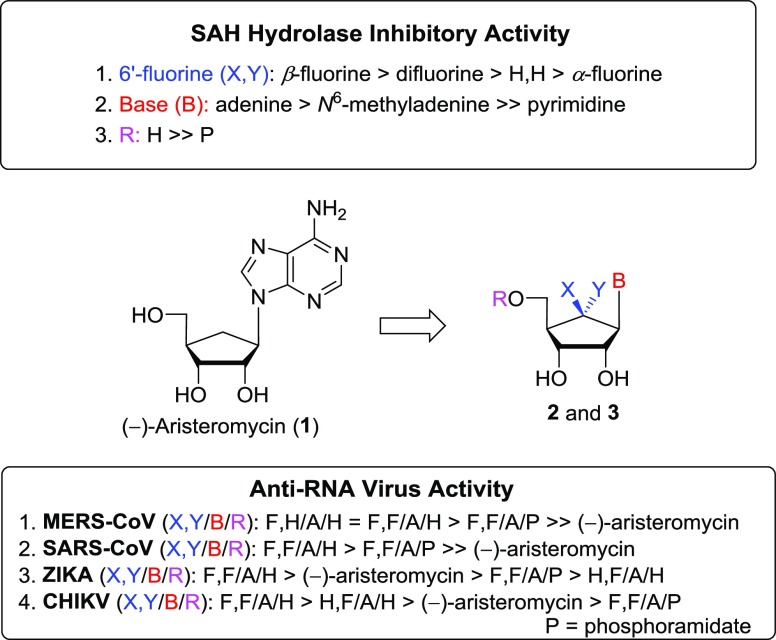
We have synthesized the 6′-fluorinated aristeromycin analogues 2a–j, which were designed as dual-target antiviral compounds aimed at inhibiting both the viral RdRp and the host SAH hydrolase. The electrophilic fluorination of silyl enol ether with Selectfluor was the key step in the synthesis. We have also synthesized the phosphoramidate prodrugs 3a–c to determine whether these would inhibit virus replication through an effect on the viral RNA polymerase. Figure 3 depicts the summarized SAR of the synthesized 6′-fluorinated final nucleoside analogues 2a–j and 3a–c concerning the inhibition of human SAH hydrolase and the inhibition of the replication of various +RNA viruses with capped genomes. It was discovered that the introduction of fluorine at the 6′-position increases the inhibitory activity on SAH hydrolase and the replication of selected +RNA viruses. Compared to the 6′-unsubstituted compound 1, the 6′-fluorinated aristeromycin analogues 2a and 2c more potently inhibited SAH hydrolase activity and the replication of MERS-CoV, SARS-CoV, ZIKV, and CHIKV. Among these compounds, 6′-β-fluoroaristeromycin (2a) was the most potent with an IC50 of 0.37 μM for SAH hydrolase activity and an EC50 of 0.20 μM for MERS-CoV replication. There was a correlation between the inhibition of SAH hydrolase and the antiviral activity of the compounds, suggesting that the latter was mainly due to indirect targeting of viral methylation reactions. The SAR studies and a lack of antiviral effects of several purine and pyrimidine analogues suggest that the antiviral effect of 1, 2a, and 2c is unlikely due to targeting of the viral RdRp. Compound 2c appears to be an interesting compound for further development and evaluation as a broad-spectrum antiviral agent, as it inhibited several coronaviruses, CHIKV, and ZIKV. More detailed biological studies on the efficacy of these compounds in virus-infected cells and into their mode of action are currently ongoing and will be published elsewhere.
Figure 3.
Summarized SAR of 6′-fluorinated aristeromycin analogues 2 and 3.
Experimental Section
Chemical Synthesis
General Methods
Proton (1H) and carbon (13C) NMR spectra were obtained on a Bruker AV 400 (400/100 MHz), Bruker AMX 500 (500/125 MHz), JEOL JNM-ECA600 (600/150 MHz), or Bruker AVANCE III 800 (800/200 MHz) spectrometer. Chemical shifts are reported as parts per million (δ) relative to the solvent peak. Coupling constants (J) are reported in hertz. Mass spectra were recorded on a Thermo LCQ XP instrument. Optical rotations were determined on Jasco III in appropriate solvent. UV spectra were recorded on U-3000 made by Hitachi in methanol or water. Infrared spectra were recorded on FT-IR (FTS-135) made by Bio-Rad. Melting points were determined on a Buchan B-540 instrument and are uncorrected. The crude compounds were purified by column chromatography on a silica gel (Kieselgel 60, 70–230 mesh, Merck). Elemental analyses (C, H, and N) were used to determine the purity of all synthesized compounds, and the results were within ±0.4% of the calculated values, confirming ≥95% purity.
(((3aR,6R,6aR)-6-(tert-Butoxymethyl)-2,2-dimethyl-6,6a-dihydro-3aH-cyclopenta[d][1,3]dioxol-4-yl)oxy)triethylsilane (6)
To a cooled (−78 °C) solution of 5 (1568.0 mg, 6.470 mmol) in anhydrous tetrahydrofuran (THF; 32.0 mL, 0.2 M) was dropwise added chlorotriethylsilane (5.4 mL, 32.355 mmol), followed by addition of LiHMDS (19.0 mL, 1.0 M solution in THF, 19.0 mmol) under N2. After being stirred at the same temperature for 10 min, the reaction mixture was quenched with saturated aqueous NH4Cl (80 mL). The layers were separated, and the aqueous layer was extracted with ethyl acetate (EtOAc; 150 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 100/1 to 30/1) to give 6 (2267.0 mg, 98%) as colorless oil: [α]D20 = +36.48 (c 1.23, CHCl3); 1H NMR (400 MHz, CDCl3): δ 4.73 (dd, J = 1.1, 6.0 Hz, 1H), 4.58 (d, J = 2.1 Hz, 1H), 4.36 (d, J = 6.1 Hz, 1H), 3.27 (dd, J = 5.6, 8.6 Hz, 1H), 3.15 (dd, J = 6.6, 8.6 Hz, 1H), 2.72 (dd, J = 5.9, 5.9 Hz, 1H), 1.42 (s, 3H), 1.32 (s, 3H), 1.12 (s, 9H), 0.96 (t, J = 8.0 Hz, 9H), 0.66–0.72 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 154.1, 110.3, 104.4, 82.8, 79.7, 72.5, 63.9, 47.9, 27.4 (3 × CH3-tert-butyl), 27.3, 25.8, 6.5 (3 × triethylsilyl), 4.6 (3 × triethylsilyl); IR (neat): 2973, 1648, 1363, 1262, 1204, 1056, 851, 748 cm–1; HRMS (FAB): found, 356.2388 [calcd for C19H36O4Si+ (M + H)+, 356.2383].
(3aR,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one (7a) and (3aR,5S,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one (7b)
To a cooled (0 °C) solution of silyl enol ether 6 (8.75 g, 24.548 mmol) in anhydrous DMF (123.0 mL, 0.20 M) was added 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (13.04 g, 36.824 mmol, Selectfluor) in one portion under N2. After being stirred at the same temperature for 12 h, the reaction mixture was quenched with saturated aqueous NH4Cl (130 mL), diluted with EtOAc (130 mL). The layers were separated and the aqueous layer was extracted with EtOAc (2 × 100 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 40/1 to 20/1) to give 7a and 7b (5.80 g, 91%, total yield, 7a/7b = 5.2:1 by 1H NMR analysis).
Compound 7a
It was obtained as a white solid; [α]D25 = −156.69 (c 0.735, CHCl3); 1H NMR (400 MHz, CDCl3): δ 5.29 (dd, J = 8.2, 49.5 Hz, 1H), 4.70 (t, J = 5.7 Hz, 1H), 4.20 (dd, J = 2.4, 6.1 Hz, 1H), 3.61 (dd, J = 1.6, 8.6 Hz, 1H) 3.38–3.41 (m, 1H), 2.75 (d, J = 8.2 Hz, 1H), 1.41 (s, 3H), 1.30 (s, 3H), 1.06 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 203.0 (d, J = 12.9 Hz), 111.4, 88.5 (d, J = 201.5 Hz), 78.2 (d, J = 6.9 Hz), 75.0 (d, J = 3.1 Hz), 74.3, 56.6 (d, J = 6.6 Hz), 40.5 (d, J = 15.5 Hz), 26.8 (3 × CH3-tert-butyl), 26.2, 23.6; 19F NMR (376 MHz, CDCl3): δ −220.60 to –221.14 (m); LRMS (ESI+): found, 283.13 [calcd for C13H21FO4Na+ (M + Na)+, 283.1322]; Anal. Calcd for C13H21FO4: C, 59.98; H, 8.13. Found: C, 59.99; H, 8.53.
Compound 7b
It was obtained as a white solid; [α]D25 = −83.72 (c 0.495, CHCl3); 1H NMR (600 MHz, CDCl3): δ 5.21–5.36 (ddd, J = 1.3, 4.5, 50.8 Hz, 1H), 4.55 (d, J = 5.9 Hz, 1H), 4.50 (d, J = 5.9 Hz, 1H), 3.63 (d, J = 2.2 Hz, 2H), 2.52–2.58 (m, 1H), 1.41 (s, 3H), 1.33 (s, 3H), 1.13 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 207.8 (d, J = 12.9 Hz), 112.2, 91.9 (d, J = 192.4 Hz), 78.78 (d, J = 3.5 Hz), 78.74, 73.6, 60.5 (d, J = 4.3 Hz), 45.0 (d, J = 17.9 Hz), 27.2 (3 × CH3-tert-butyl), 26.8, 25.2; 19F NMR (376 MHz, CDCl3): δ −196.0 to –196.2 (m); HRMS (FAB): found, 262.1679 [calcd for C13H22FO4+ (M + H)+, 261.1505]; Anal. Calcd for C13H21FO4: C, 59.98; H, 8.13. Found: C, 59.77; H, 8.45.
(3aR,6R,6aR)-6-(tert-Butoxymethyl)-5,5-difluoro-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one (7c)
It was obtained in 70% yield (mixture of 7c and 7d) as a white solid; [α]D25 = −4.34 (c 0.21, MeOH); 1H NMR (7c and 7d mixture, 400 MHz, CDCl3; 7c and 7d mixture): δ 4.82 (s, 1H), 4.72 (t, J = 6.1 Hz, 1H), 4.52–4.57 (m, 1H), 4.35–4.41 (m, 1H), 4.25 (dd, J = 8.0, 4.0 Hz, 1H), 3.74 (s, 1H), 3.69 (d, J = 8.0 Hz, 1H), 3.67–3.60 (m, 1H), 3.54–3.59 (m, 1H), 3.46 (d, J = 8.3 Hz, 1H), 2.68 (d, J = 17.4 Hz, 1H), 2.53–2.62 (m, 1H), 1.48 (s, 3H), 1.44 (s, 3H), 1.34 (s, 3H), 1.32 (s, 3H), 1.21 (s, 9H), 1.06 (s, 9H).
General Procedure for the Synthesis of 8a–c
To a cooled (0 °C) solution of 7a–c (1 equiv) in MeOH (0.18 M), sodium borohydride or lithium borohydride was added in a single portion in a N2 atmosphere. After stirring for 30 min at the same temperature, the reaction mixture was neutralized with acetic acid (2 mL) and evaporated. The residue was diluted with saturated aqueous NH4Cl, and the aqueous layer was extracted with EtOAc (2 × 100 mL). The combined organic layers were dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 20/1) to give 8a–c.
(3aS,4R,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol (8a)
It was obtained in 71% yield as a colorless syrup; [α]D25 = −47.46 (c 0.395, CHCl3); 1H NMR (400 MHz, CDCl3): δ 4.91 (td, J = 6.6, 52.5 Hz, 1H), 4.51–4.52 (m, 1H), 4.47 (ddd, J = 1.6, 6.3, 7.8 Hz, 1H), 4.26–4.34 (m, 1H), 3.52 (dd, J = 3.3, 8.8 Hz, 1H), 3.36–3.39 (m, 1H), 2.67 (d, J = 7.9 Hz, 1H), 2.46 (br s, 1H), 1.45 (s, 3H), 1.32 (s, 3H), 1.14 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 111.1, 99.5 (d, J = 185.9 Hz), 81.2 (d, J = 4.4 Hz), 76.3 (d, J = 9.0 Hz), 74.0 (d, J = 23.4 Hz), 73.0, 56.8 (d, J = 8.2 Hz), 44.6 (d, J = 18.1 Hz), 27.3 (3 × CH3-tert-butyl), 26.1, 24.1; 19F NMR (376 MHz, CDCl3) −211.0 to −211.21 (m); HRMS (FAB): found, 263.1662 [calcd for C13H24FO4+ (M + H)+, 263.1659]; Anal. Calcd for C13H23FO4: C, 59.52; H, 8.84. Found: C, 59.32; H, 9.15.
(3aS,4R,5S,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol (8b)
It was obtained in 67% yield as a colorless syrup; [α]D25 = −40.42 (c 0.22, MeOH); 1H NMR (500 MHz, CDCl3): δ 4.68 (dd, J = 4.1, 52.4 Hz, 1H), 4.46–4.53 (m, 2H), 4.13–4.24 (m, 1H), 3.33–3.40 (m, 1H), 2.81 (d, J = 11.4 Hz, 1H), 2.50 (dt, J = 2.9, 22.9 Hz, 1H), 1.46 (s, 3H), 1.30 (s, 3H), 1.08 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 111.4, 98.4 (d, J = 181.5 Hz), 82.8, 79.3, 73.8 (d, J = 16.3 Hz), 73.0, 60.6 (d, J = 12.1 Hz), 49.2 (d, J = 18.3 Hz), 27.1 (3 × CH3-tert-butyl), 26.2, 24.2; HRMS (ESI+): found, 285.1480 [calcd for C13H23FNaO4+ (M + Na)+, 285.1478]; Anal. Calcd for C13H23FO4: C, 55.70; H, 7.91. Found: C, 55.40; H, 7.75.
(3aS,4R,6R,6aR)-6-(tert-Butoxymethyl)-5,5-difluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol (8c)
It was obtained in 74% yield as a colorless syrup; [α]D25 = 22.37 (c 0.28, MeOH); 1H NMR (500 MHz, CDCl3): δ 4.53 (t, J = 5.7 Hz, 1H), 4.44 (ddd, J = 2.6, 6.4, 8.9 Hz, 1H), 4.20–4.29 (m, 1H), 3.55 (d, J = 8.7 Hz, 1H), 3.39 (d, J = 8.8 Hz, 1H), 2.76 (d, J = 11.5 Hz, 1H), 2.43 (d, J = 17.2 Hz, 1H), 1.46 (s, 3H), 1.31 (s, 3H), 1.12 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 126.9 (dd, J = 252.3, 260.3 Hz), 110.9, 79.6 (d, J = 5.9 Hz), 75.5 (d, J = 11.3 Hz), 73.7 (dd, J = 18.5, 25.8 Hz), 73.4, 57.6 (dd, J = 4.6, 8.5 Hz), 48.7 (t, J = 20.8 Hz), 27.2 (3 × CH3-tert-butyl), 25.9, 24.2; HRMS (ESI+): found, 298.1834 [calcd for C13H26F2NO4+ (M + NH4)+, 298.1830 ]; Anal. Calcd for C13H22F2O4: C, 55.70; H, 7.91. Found: C, 55.45; H, 7.56.
(3aR,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-((tert-butyldimethylsilyl)oxy)-2,2-dimethyldihydro-3aH-cyclopenta[d][1,3]dioxol-4(5H)-one (9)
To a cooled (0 °C) solution of 6 (1275 mg, 3.57 mmol) in anhydrous THF (12 mL, 0.3 M) were added 4-methylmorpholine N-oxide monohydrate (967 mg, 7.15 mmol, 2 equiv) and osmium tetroxide (1000 mg, 3.93 mmol, 1.1 equiv) under a N2 atmosphere. After stirring for 30 min, to the reaction mixture were added sodium thiosulfate pentahydrate (300 mg), sodium sulfite (300 mg), and acetone (30 mL) and stirred for additional 1 h at the same temperature. The layers were separated, and the aqueous layer was extracted with EtOAc (100 mL). The combined organic layers were washed with H2O followed by saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was used for the next step without further purification. To a solution of above generated intermediate in anhydrous DMF (18 mL, 0.19 M) were added TBSCl (1614 mg, 10.71 mmol) and imidazole (729 mg, 10.71 mmol) under a N2 atmosphere. After stirring for 3 h at room temperature, the reaction mixture was quenched with saturated aqueous NH4Cl (50 mL) and diluted with EtOAc (50 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (2 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 40/1 to 20/1) to give 9 (705 mg, 53%) as a colorless syrup: [α]D25 = −103.19 (c 0.30, MeOH); 1H NMR (400 MHz, CDCl3): δ 4.65 (d, J = 6.4 Hz, 1H), 4.53 (d, J = 8.0 Hz, 1H), 4.11 (d, J = 6.3 Hz, 1H), 3.61 (dd, J = 1.6, 8.0 Hz, 1H), 3.30 (dd, J = 2.4, 8.1 Hz, 1H), 2.41–2.46 (m, 1H), 1.42 (s, 3H), 1.30 (s, 3H), 1.03 (s, 9H), 0.88 (s, 9H), 0.13 (s, 3H), 0.05 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 207.2, 110.9, 78.1, 75.8, 73.7, 71.3, 56.9, 42.3, 27.0 (3 × CH3-tert-butyl), 26.4, 25.7 (3 × CH3-tert-butyl), 23.8, 18.3, −4.4, −5.6; HRMS (FAB+) (m/z): found, 373.2398 [calcd for C19H37O5Si+ (M + H)+, 373.2410]; Anal. Calcd for C19H36O5Si: C, 61.25; H, 9.74. Found: C, 61.26; H, 9.75.
(3aS,4R,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-((tert-butyldimethylsilyl)oxy)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-ol (10)
To a cooled (0 °C) solution of 9 (471 mg, 1.26 mmol) in methanol (6.3 mL, 0.2 M) was added sodium borohydride (144 mg, 3.79 mmol, 3 equiv) under a N2 atmosphere. After being stirred at the same temperature for 1 h, the reaction mixture was diluted with H2O (20 mL) and EtOAc (20 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 30/1 to 20/1) to give 10 (415 mg, 88%) as a colorless syrup: [α]D25 = −40.39 (c 0.32, MeOH); 1H NMR (500 MHz, CDCl3): δ 4.49 (d, J = 6.1 Hz, 1H), 4.41 (t, J = 6.2 Hz, 1H), 4.07 (t, J = 6.9 Hz, 1H), 3.95 (dd, J = 6.8, 14.7 Hz, 1H), 3.48 (dd, J = 3.9, 8.5 Hz, 1H), 3.32 (dd, J = 4.6, 8.5 Hz, 1H), 2.43 (d, J = 8.4 Hz, 1H), 2.12–2.18 (m, 1H), 1.45 (s, 3H), 1.32 (s, 3H), 1.12 (s, 9H), 0.87 (s, 9H), 0.09 (s, 3H), 0.05 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 110.4, 81.0, 78.8, 77.0, 76.1, 72.6, 57.3, 46.0, 27.4 (3 × CH3-tert-butyl), 26.2, 25.8 (3 × CH3-tert-butyl), 24.0, 18.1, −4.5, −5.1; HRMS (FAB+) (m/z): found, 375.2584 [calcd for C19H39O5Si+ (M + H)+, 375.2567]; Anal. Calcd for C19H38O5Si: C, 60.92; H, 10.23. Found: C, 60.91; H, 10.25.
(((3aR,4R,5R,6R,6aR)-4-(Benzyloxy)-6-(tert-butoxymethyl)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-5-yl)oxy)(tert-butyl)dimethylsilane (11)
To a cooled (0 °C) solution of 10 (193 mg, 0.515 mmol) in DMF (5.2 mL, 0.1 M) was added benzyl chloride (0.12 mL, 1.030 mmol, 2.0 equiv) and sodium hydride (41 mg, 1.030 mmol, 2.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 12 h, the reaction mixture was diluted with H2O (20 mL) and EtOAc (20 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 50/1) to give 11 (204 mg, 85%) as a colorless syrup: [α]D25 = −46.64 (c 0.66, MeOH); 1H NMR (400 MHz, CDCl3): δ 7.22–7.39 (m, 5H), 4.76 (d, J = 12.4 Hz, 1H), 4.59 (d, J = 12.4 Hz, 1H), 4.45 (d, J = 6.0 Hz, 1H), 4.33–4.37 (m, 2H), 3.83 (dd, J = 5.6, 8.8 Hz, 1H), 3.39 (dd, J = 4.4, 8.8 Hz, 1H), 3.32 (dd, J = 4.0, 8.4 Hz, 1H), 2.05–2.11 (m, 1H), 1.48 (s, 3H), 1.29 (s, 3H), 1.03 (s, 9H), 0.88 (s, 9H), 0.09 (s, 3H), 0.05 (s, 3H); 13C NMR (200 MHz, CDCl3): δ 138.9, 128.4, 128.1, 127.9, 127.7, 127.2, 110.0, 82.1, 80.2, 76.0, 75.6, 72.4, 71.7, 57.5, 45.7, 27.3 (3 × CH3-tert-butyl), 26.4, 25.8 (3 × CH3-tert-butyl), 24.2, −4.7, −4.9; HRMS (FAB+) (m/z): found, 465.3001 [calcd for C26H45O5Si+ (M + H)+, 465.3029]; Anal. Calcd for C26H44O5Si: C, 67.20; H, 9.54. Found: C, 67.22; H, 9.55.
(3aR,4S,5R,6S,6aR)-4-(Benzyloxy)-6-(tert-butoxymethyl)-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-5-ol (12)
To a cooled (0 °C) solution of 11 (179 mg, 0.385 mmol) in anhydrous THF (3.8 mL, 0.1 M) was added TBAF solution (1.2 mL, 1.0 M solution in THF, 1.2 mmol, 3.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 12 h, the reaction mixture was diluted with H2O (30 mL) and EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 8/1) to give 12 (129 mg, 88%) as a colorless syrup: [α]D25 = −49.04 (c 0.28, MeOH); 1H NMR (400 MHz, CDCl3): δ 7.39 (d, J = 7.2 Hz, 2H), 7.29–7.35 (m, 2H), 7.23–7.28 (m, 1H), 4.85 (d, J = 12.4 Hz, 1H), 4.62 (d, J = 12.4 Hz, 1H), 4.51 (t, J = 6.0 Hz, 1H), 4.40–4.45 (m, 2H), 3.81 (dd, J = 4.8, 7.2 Hz, 1H), 3.58 (dd, J = 3.6, 8.8 Hz, 1H), 3.44 (dd, J = 4.4, 8.8 Hz, 1H), 2.70 (br s, 1H), 2.26–2.32 (m, 1H), 1.48 (s, 3H), 1.31 (s, 3H), 1.08 (s, 9H); 13C NMR (200 MHz, CDCl3): δ 138.5, 128.3 (2 × CH-benzene), 128.0 (2 × CH-benzene), 127.5, 111.1, 82.7, 80.6, 77.2, 76.7, 73.4, 71.9, 59.3, 45.4, 27.2 (3 × CH3-tert-butyl), 26.5, 24.6; Anal. Calcd for C20H30O5: C, 68.54; H, 8.63. Found: C, 68.52; H, 8.64.
(3aR,4R,5S,6R,6aR)-4-(Benzyloxy)-6-(tert-butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxole (13a)
To a cooled (0 °C) solution of 12 (20 mg, 0.052 mmol) in anhydrous toluene (2.0 mL, 0.026 M) was dropwise added diethylaminosulfur trifluoride (30 μL, 0.210 mmol, 4.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 2 h, the reaction mixture was quenched with saturated aqueous NH4Cl (30 mL) and EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 30/1) to give 13a (5.6 mg, 30%) and 13b (5.6 mg, 30%) as a colorless syrup.
Compound 13a
[α]D25 = −26.59 (c 0.22, MeOH); 1H NMR (500 MHz, CDCl3): δ 7.25–7.34 (m, 5H), 4.96 (ddd, J = 2.6, 6.8, 52.7 Hz, 1H), 4.72 (dd, J = 0.8, 11.6 Hz, 1H), 4.54 (d, J = 11.6 Hz, 1H), 4.44–4.52 (m, 2H), 4.02–4.09 (m, 1H), 3.41–3.47 (m, 2H), 2.15–2.18 (m, 1H), 1.47 (s, 3H), 1.28 (s, 3H), 1.12 (s, 9H); 13C NMR (200 MHz, CDCl3): δ 137.8, 128.3 (2 × CH-benzyl), 128.1 (2 × CH-benzyl), 127.8, 111.8, 96.0 (d, J = 187.1 Hz), 81.6, 79.3, 78.2 (d, J = 15.7 Hz), 72.6, 71.8, 60.6 (d, J = 11.0 Hz), 50.2 (d, J = 18.7 Hz), 27.0 (3 × CH3-tert-butyl), 26.6, 24.4; HRMS (FAB+) (m/z): found, 353.2121 [calcd for C20H30FO4+ (M + H)+, 353.2128]; Anal. Calcd for C20H29FO4: C, 68.16; H, 8.29. Found: C, 68.13; H, 8.27.
Compound 13b
[α]D25 = −61.72 (c 0.42, MeOH); 1H NMR (500 MHz, CDCl3): δ 7.38 (t, J = 7.3 Hz, 2H), 7.31 (t, J = 7.2 Hz, 2H), 7.25 (d, J = 7.2 Hz, 1H), 5.18 (dt, J = 7.8, 53.7 Hz, 1H), 4.76 (d, J = 12.2 Hz, 1H), 4.66 (d, J = 12.2 Hz, 1H), 4.45–4.49 (m, 1H), 4.41–4.44 (m, 1H), 4.19 (ddd, J = 5.9, 7.7, 16.5 Hz, 1H), 3.45 (dd, J = 3.0, 8.8 Hz, 1H), 3.31–3.34 (m, 1H), 2.37–2.43 (m, 1H), 1.47 (s, 3H), 1.28 (s, 3H), 1.01 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 138.0, 128.3, 127.9 (2 × CH-benzyl), 127.7 (2 × CH-benzyl), 112.2, 103.5, 102.1, 81.5 (d, J = 27.5 Hz), 81.1 (d, J = 20.0 Hz), 72.6, 72.4, 57.6, 48.8 (d, J = 6.2 Hz), 27.4 (3 × CH3-tert-butyl), 27.1, 25.0; HRMS (FAB+) (m/z): found, 353.2131 [calcd for C20H30FO4+ (M + H)+, 353.2128]; Anal. Calcd for C20H29FO4: C, 68.16; H, 8.29. Found: C, 68.13; H, 8.27.
(3aR,4R,5S,6R,6aS)-4-(tert-Butoxy)-5-(tert-butoxymethyl)-6-hydroxytetrahydro-3aH-cyclopenta[d][1,3,2]dioxathiole 2-Oxide (14)
To perform regioselective cleavage, to a cooled (−78 °C) solution of 10 (420 mg, 1.121 mmol) in anhydrous CH2Cl2 (5.6 mL, 0.2 M) was dropwise added trimethylaluminum (3.4 mL, 2.0 M solution in hexane, 6.727 mmol, 6.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 12 h, the reaction mixture was quenched with saturated aqueous NH4Cl (30 mL) and EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 10/1) to give diol intermediate (245 mg, 56%) 10a as a colorless syrup. For the introduction of cyclic sulfite, to a cooled (0 °C) solution of diol intermediate 10a (250 mg, 0.639 mmol) in anhydrous CH2Cl2 (6.4 mL, 0.1 M) was dropwise added triethylamine (0.3 mL, 2.239 mmol, 3.5 equiv) followed by thionyl chloride (70 μL, 0.959 mmol) under a N2 atmosphere. After being stirred at room temperature for 30 min, the reaction mixture was quenched with saturated aqueous NH4Cl (30 mL) and diluted with EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by flash column chromatography (silica gel, hexanes/EtOAc, 10/1) to give cyclic sulfite intermediate 10b (249 mg, 89%) as a colorless syrup. For TBS deprotection, to a cooled (0 °C) solution of 10b (286 mg, 0.654 mmol) in anhydrous THF (6.5 mL, 0.1 M) was added acetic acid (0.13 mL, 0.131 mmol, 0.2 equiv) followed by TBAF solution (2.6 mL, 1.0 M solution in THF, 2.6 mmol, 4.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 12 h, the reaction mixture was quenched with H2O (30 mL) and diluted with EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 6/1) to give 14 (202 mg, 96%, two diastereomers A and B were generated from sulfoxide stereogenic center) as a colorless syrup: for A: 1H NMR (400 MHz, CDCl3): δ 5.27 (t, J = 5.4 Hz, 1H), 5.02 (d, J = 5.9 Hz, 1H), 4.79 (s, 1H), 4.44 (dd, J = 4.8, 11.4 Hz, 1H), 4.19 (d, J = 3.9 Hz, 1H), 3.80 (dd, J = 2.6, 9.3 Hz, 1H), 1.90–1.94 (m, 1H), 1.27 (s, 9H), 1.21 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 86.9, 82.6, 74.9, 74.5, 74.1, 69.4, 58.2, 43.6, 28.3 (3 × CH3-tert-butyl), 27.2 (3 × CH3-tert-butyl); HRMS (FAB+) (m/z): found, 323.1530 [calcd for C14H27O6S+ (M + H)+, 323.1528]; for B: 1H NMR (500 MHz, CDCl3): δ 4.98–5.07 (m, 2H), 4.79 (d, J = 6.4 Hz, 1H), 4.36 (dd, J = 4.6, 11.5 Hz, 1H), 4.31 (d, J = 4.1 Hz, 1H), 3.84 (d, J = 9.2 Hz, 1H), 3.77 (d, J = 9.3 Hz, 1H), 2.65 (d, J = 10.1 Hz, 1H), 1.25 (s, 9H), 1.21 (s, 9H).
(3aR,4R,5R,6S,6aR)-4-(tert-Butoxy)-5-(tert-butoxymethyl)-6-fluorotetrahydro-3aH-cyclopenta[d][1,3,2]dioxathiole 2-Oxide (15)
To a cooled (0 °C) solution of 14 (33 mg, 0.102 mmol) in anhydrous CH2Cl2 (1.5 mL, 0.068 M) was dropwise added diethylaminosulfur trifluoride (60 μL, 0.434 mmol, 4.0 equiv) under a N2 atmosphere. After being stirred at room temperature for 4 h, the reaction mixture was quenched with saturated aqueous NH4Cl (30 mL) and diluted with EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by flash column chromatography (silica gel, hexanes/EtOAc, 15/1) to give 15 (12 mg, 37%) as a colorless syrup: 1H NMR (600 MHz, CDCl3): δ 5.17 (ddd, J = 4.6, 7.8, 52.7 Hz, 1H), 5.03 (t, J = 8.2 Hz, 1H), 4.92 (ddd, J = 5.0, 8.7, 17.8 Hz, 1H), 4.06 (ddd, J = 7.8, 11.0, 16.5 Hz, 1H), 3.53 (ddd, J = 2.7, 2.7, 6.8 Hz, 1H), 3.44 (dd, J = 2.2, 9.1 Hz, 1H), 2.54–2.58 (m, 1H), 1.17 (s, 18H); 13C NMR (125 MHz, CDCl3): δ 102.1 (d, J = 191.2 Hz), 87.2 (d, J = 28.2 Hz), 81.9 (d, J = 5.8 Hz), 74.5, 72.8, 72.4 (d, J = 19.2 Hz), 55.5, 50.4 (d, J = 6.5 Hz), 28.6 (3 × CH3-tert-butyl), 27.5 (3 × CH3-tert-butyl).
(3aR,4R,5R,6S,6aR)-4-(tert-Butoxy)-5-(tert-butoxymethyl)-6-fluorotetrahydro-3aH-cyclopenta[d][1,3,2]dioxathiole 2,2-Dioxide (16)
To a solution of cyclic sulfite 15 (13 mg, 0.040 mmol) in CCl4/CH3CN/H2O (1:1:1.5, total 1.75 mL, 0.14 M) was added in one portion sodium periodate (26 mg, 0.120 mmol), followed by ruthenium(III) chloride trihydrate (2 mg, 0.008 mmol) at room temperature under a N2 atmosphere. After being stirred at the same temperature for 20 min, the reaction mixture was quenched with H2O (20 mL) and diluted with CH2Cl2 (20 mL). The layers were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 50 mL). The combined organic layers were washed successively with H2O and saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The crude product 16 was used for the next step without further purification.
General Procedure for the Synthesis of 18a–c
Triflation
To a cooled (0 °C) solution of 8a–c (1 equiv) in anhydrous pyridine (0.32 M), trifluoromethanesulfonic anhydride (2 equiv) was added dropwise in a N2 atmosphere. After stirring at the same temperature for 30 min, the reaction mixture was quenched with H2O (50 mL) and diluted with EtOAc (30 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (2 × 30 mL). The combined organic layers were washed with saturated aqueous CuSO4 followed by water, dried over anhydrous MgSO4, filtered, and evaporated. The residue was used for the next step without further purification.
Azidation
To a solution of triflate intermediate (1 equiv) in anhydrous DMF (0.19 M), sodium azide (3 equiv) was added in a single portion at room temperature. After being heated to 60–100 °C and stirred for 4–15 h, the reaction mixture was cooled to room temperature, quenched with H2O (50 mL), and diluted with EtOAc (50 mL). The layers were separated, and the aqueous layer was extracted with EtOAc (2 × 50 mL). The combined organic layers were washed with H2O followed by saturated brine, dried over anhydrous MgSO4, filtered, and evaporated. The residue was purified by column chromatography (silica gel, hexanes/EtOAc, 10/1) to give 18a–c.
(3aS,4S,5R,6R,6aR)-4-Azido-6-(tert-butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxole (18a)
It was obtained in 45% yield as a colorless syrup; [α]D25 = −24.42 (c 0.016, CH2Cl2); 1H NMR (500 MHz, CDCl3): δ 5.16 (td, J = 52.4, 3.1 Hz, 1H), 4.66 (t, J = 6.0 Hz, 1H), 4.41 (t, J = 6.5 Hz, 1H), 3.62–3.69 (m, 1H), 3.54 (s, 1H), 3.50 (s, 1H), 2.27–2.36 (m, 1H), 1.47 (s, 3H), 1.29 (s, 3H), 1.16 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 114.1, 96.9 (d, J = 182.6 Hz), 82.0, 80.2, 73.1, 67.9 (d, J = 15.7 Hz), 57.8 (d, J = 7.2 Hz), 49.4 (d, J = 17.6 Hz), 27.3 (3 × CH3-tert-butyl), 27.1, 24.6; 19F NMR (376 MHz, CDCl3) −206.9 to –207.2 (m); IR (neat): 2108 cm–1; LR-MS (ESI+): 310.15 [calcd for C13H22FN2NaO3+ (M + Na)+, 310.1543]; Anal. Calcd for C13H22FN3O3: C, 54.34; H, 7.72; N, 14.62. Found: C, 54.35; H, 7.45; N, 14.23.
(3aS,4S,5S,6R,6aR)-4-Azido-6-(tert-butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxole (18b)
It was obtained in 88% yield as a colorless syrup; [α]D25 = 9.66 (c 0.51, MeOH); 1H NMR (500 MHz, CDCl3): δ 4.75 (dt, J = 7.7, 53.0 Hz, 1H), 4.41 (dd, J = 4.5, 6.7 Hz, 1H), 4.22 (t, J = 5.7 Hz, 1H), 4.00 (ddd, J = 5.5, 7.4, 16.6 Hz, 1H), 3.43–3.50 (m, 2H), 2.33–2.44 (m, 1H), 1.50 (s, 3H), 1.27 (s, 3H), 1.15 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 112.7, 95.8 (d, J = 188.9 Hz), 81.0 (d, J = 8.6 Hz), 77.8 (d, J = 7.2 Hz), 73.0, 70.9 (d, J = 20.1 Hz), 57.9, 49.1 (d, J = 18.7 Hz), 27.3 (3 × CH3-tert-butyl), 27.2, 25.0; IR (neat): 2111 cm–1; Anal. Calcd for C13H22FN3O3: C, 54.34; H, 7.72; N, 14.62. Found: C, 54.12; H, 7.94; N, 14.33.
(3aS,4S,6R,6aR)-4-Azido-6-(tert-butoxymethyl)-5,5-difluoro-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxole (18c)
It was obtained in 75% yield as a colorless syrup; [α]D25 = −43.39 (c 0.36, MeOH); 1H NMR (500 MHz, CDCl3): δ 4.40–4.44 (m, 1H), 4.34–4.39 (m, 1H), 3.87–3.95 (m, 1H), 3.61 (dd, J = 6.5, 9.3 Hz, 1H), 3.48 (t, J = 7.6 Hz, 1H), 2.54–2.66 (m, 1H), 1.49 (s, 3H), 1.28 (s, 3H), 1.17 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 127.1 (dd, J = 255.9, 260.9 Hz), 113.0, 80.0 (d, J = 5.9 Hz), 78.4 (d, J = 5.6 Hz), 73.4, 69.1 (dd, J = 18.8, 25.1 Hz), 57.2 (d, J = 6.4 Hz), 50.8 (t, J = 20.0 Hz), 27.3 (3 × CH3-tert-butyl), 26.9, 24.7; IR (neat): 2116 cm–1; Anal. Calcd for C13H21F2N3O3: C, 51.14; H, 6.93; N, 13.76. Found: C, 51.45; H, 7.21; N, 14.10.
General Procedure for the Synthesis of 19a–c
To a suspension of 18a–c (1 equiv) in methanol (0.2 M), 10% palladium on activated carbon (0.03 equiv) was added and stirred overnight at room temperature in a H2 atmosphere. After filtration, the solvent was removed, and the residue was used for the next step without further purification.
General Procedure for the Synthesis of 20a–c
To a solution of 19a–c (1 equiv) in n-butanol (0.38 M), 5-amino-4,6-dichloro pyrimidine (3–10 equiv) and diisopropylamine (10 equiv) were added. The reaction mixture was placed under microwave irradiation at 170–200 °C for 4–7 h. The solvent was co-evaporated with MeOH, and the residue was purified with column chromatography (silica gel, hexane/EtOAc, 4/1) to give 20a–c, respectively.
N4-((3aS,4S,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloropyrimidine-4,5-diamine (20a)
It was obtained in 66% yield from 18a; yellow foam; [α]D25 = −53.8 (c 0.10, CH2Cl2); 1H NMR (500 MHz, CDCl3): δ 8.08 (s, 1H), 5.27–5.33 (br s, 1H), 5.24 (td, J = 3.5, 52.9 Hz, 1H), 4.71–4.81 (m, 1H), 4.57 (t, J = 6.1 Hz, 1H), 4.44 (t, J = 6.3 Hz, 1H), 3.58–3.63 (m, 1H), 3.53 (t, J = 9.2 Hz, 1H), 3.39 (br s, 2H), 2.42–2.55 (m, 1H), 1.52 (s, 3H), 1.30 (s, 3H), 1.18 (s, 9H); 13C NMR (200 MHz, CDCl3): δ 154.4, 149.0, 122.4, 113.8, 95.9 (d, J = 178.7 Hz), 84.2, 80.1, 77.1, 73.3, 59.8 (d, J = 15.9 Hz), 58.0 (d, J = 7.0 Hz), 49.4 (d, J = 17.6 Hz), 27.4 (3 × CH3-tert-butyl), 27.2, 24.8; 19F NMR (376 MHz, CDCl3) −212.8 to –213.1 (m); UV (CH2Cl2): λmax 287 nm; LRMS (ESI+): found, 388.17 [calcd for C17H27ClFN4O3+ (M + H)+, 389.1756]; Anal. Calcd for C17H26ClFN4O3: C, 52.51; H, 6.50; N, 14.45. Found: C, 52.45; H, 6.13; N, 14.15.
N4-((3aS,4S,5S,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloropyrimidine-4,5-diamine (20b)
It was obtained in 47% yield from 18b; yellow foam; [α]D25 = −11.79 (c 0.36, MeOH); 1H NMR (500 MHz, CDCl3): δ 8.10 (s, 1H), 5.56 (d, J = 9.2 Hz, 1H), 4.89 (dt, J = 3.1, 51.0 Hz, 1H), 4.77 (dd, J = 9.1, 21.2 Hz, 1H), 4.61 (dd, J = 2.5, 5.0 Hz, 1H), 4.51 (dd, J = 2.4, 6.0 Hz, 1H), 3.60 (dd, J = 2.6, 9.2 Hz, 1H), 3.55 (dd, J = 2.5, 9.3 Hz, 1H), 3.39 (br s, 2H), 2.60 (d, J = 23.5 Hz, 1H), 1.54 (s, 3H), 1.29 (s, 3H), 1.21 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 154.2, 149.6, 143.4, 122.4, 111.7, 101.3 (d, J = 185.1 Hz), 85.5 (d, J = 3.3 Hz), 82.0 (d, J = 2.6 Hz), 74.0, 63.7 (d, J = 26.6 Hz), 60.6 (d, J = 7.1 Hz), 51.3 (d, J = 20.5 Hz), 27.5 (3 × CH3-tert-butyl), 27.1, 24.9; UV (MeOH): λmax 297.60, 265.07 nm; HRMS (ESI+): found, 389.1762 [calcd for C17H27ClFN4O3+ (M + H)+, 389.1756]; Anal. Calcd for C17H26lFN4O3: C, 52.51; H, 6.50; N, 14.45. Found: C, 52.56; H, 6.51; N, 14.43.
N4-((3aS,4S,6R,6aR)-6-(tert-Butoxymethyl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloropyrimidine-4,5-diamine (20c)
It was obtained in 67% yield from 18c; yellow foam; [α]D25 = −61.76 (c 0.23, MeOH); 1H NMR (500 MHz, CDCl3): δ 8.11 (s, 1H), 5.71 (d, J = 10.1 Hz, 1H), 5.03 (t, J = 12.7 Hz, 1H), 4.56 (t, J = 4.6 Hz, 1H), 4.40–4.45 (m, 1H), 3.69 (dd, J = 2.6, 9.5 Hz, 1H), 3.57 (dd, J = 4.4, 9.4 Hz, 1H), 3.38 (br s, 2H), 2.72 (d, J = 14.7 Hz, 1H), 1.53 (s, 3H), 1.44 (s, 3H), 1.25 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 154.5, 149.6, 143.9, 128.0 (dd, J = 257.3, 260.0 Hz), 122.3, 111.7, 84.5, 79.7 (d, J = 4.1 Hz), 74.5, 61.7 (dd, J = 18.1, 31.9 Hz), 58.3 (t, J = 5.8 Hz), 51.6 (t, J = 22.6 Hz), 27.5 (3 × CH3-tert-butyl), 26.7, 24.6; UV (MeOH): λmax 297.39, 263.29 nm; HRMS (ESI+): found, 407.1658 [calcd for C17H26ClF2N4O3+ (M + H)+, 407.1661]; Anal. Calcd for C17H25ClF2N4O3: C, 50.19; H, 6.19; N, 13.77. Found: C, 50.11; H, 6.23; N, 13.65.
General Procedure for the Synthesis of 21a–c
A solution of 20a–c in diethoxymethyl acetate (0.15 M) was placed under microwave irradiation at 140 °C for 3 h. The mixture was then co-evaporated with MeOH three times, and the resulting residue was purified with column chromatography (silica gel, hexane/EtOAc, 7/1) to give 21a–c.
9-((3aS,4S,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloro-9H-purine (21a)
It was obtained in 96% yield as yellow foam; [α]D25 = −29.2 (c 0.17, CH2Cl2); 1H NMR (400 MHz, CDCl3): δ 8.74 (s, 1H), 8.34 (d, J = 2.4 Hz, 1H), 5.28–5.43 (td, J = 2.8, 52.8 Hz, 1H), 5.12–5.23 (m, 2H), 4.61 (t, J = 5.0 Hz, 1H), 3.65–3.69 (m, 1H), 3.61 (t, J = 9.2 Hz, 1H), 2.56–2.71 (m, 1H), 1.56 (s, 3H), 1.32 (s, 3H), 1.17 (s, 9H); 13C NMR (100 MHz, CDCl3): δ 152.3, 151.4, 144.2, 144.1, 131.4, 115.4, 97.7–95.9 (d, J = 181.2 Hz), 82.9, 80.1, 73.5, 63.1 (d, J = 16.1 Hz), 58.0 (d, J = 7.4 Hz), 50.0 (d, J = 17.5 Hz), 27.6 (3 × CH3-tert-butyl), 27.5, 25.1; 19F NMR (376 MHz, CDCl3) −202.6–202.9 (m); UV (CH2Cl2): λmax 271 nm; LRMS (ESI+): found, 399.16 [calcd for C18H25ClFN4O3+ (M + H)+, 399.1599]; Anal. Calcd for C18H24ClFN4O3: C, 54.20; H, 6.06; N, 14.05. Found: C, 54.12; H, 6.34; N, 14.23.
9-((3aS,4S,5S,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloro-9H-purine (21b)
It was obtained in 76% yield as yellow foam; [α]D25 = −31.54 (c 0.54, MeOH); 1H NMR (500 MHz, CDCl3): δ 8.67 (s, 1H), 8.15 (s, 1H), 5.55 (dt, J = 8.4, 53.6 Hz, 1H), 5.02 (t, J = 6.4 Hz, 1H), 4.84–4.94 (m, 1H), 4.65 (t, J = 5.1 Hz, 1H), 3.53–3.63 (m, 2H), 2.47–2.57 (m, 1H), 1.54 (s, 3H), 1.25 (s, 3H), 1.17 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 151.7, 151.5, 151.3, 144.8, 132.3, 113.1, 93.9 (d, J = 191.0 Hz), 79.1 (d, J = 7.9 Hz), 77.6 (d, J = 7.9 Hz), 73.1, 67.8 (d, J = 20.8 Hz), 58.1, 48.7 (d, J = 18.7 Hz) 27.5 (3 × CH3-tert-butyl), 27.3, 25.0; UV (MeOH): λmax 264.36 nm; HRMS (ESI+): found, 399.1589 [calcd for C18H25ClFN4O3+ (M + H)+, 399.1599]; Anal. Calcd for C18H24ClFN4O3: C, 54.20; H, 6.06; N, 14.05. Found: C, 54.34; H, 6.46; N, 13.99.
9-((3aS,4S,6R,6aR)-6-(tert-Butoxymethyl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-6-chloro-9H-purine (21c)
It was obtained in 92% yield as yellow foam; [α]D25 = −46.05 (c 0.43, MeOH); 1H NMR (500 MHz, CDCl3): δ 8.73 (s, 1H), 8.28 (d, J = 2.1 Hz, 1H), 5.30 (dt, J = 6.9, 20.1 Hz, 1H), 5.10 (t, J = 6.7 Hz, 1H), 4.57–4.62 (m, 1H), 3.63–3.73 (m, 2H), 2.81–2.93 (m, 1H), 1.56 (s, 3H), 1.30 (s, 3H), 1.18 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 152.4, 152.4, 151.3, 143.9 (d, J = 4.0 Hz), 131.2, 125.6 (dd, J = 253.4, 264.6 Hz), 114.0, 79.5 (d, J = 7.7 Hz), 77.9 (d, J = 7.5 Hz), 73.7, 64.6 (dd, J = 19.3, 24.3 Hz), 57.1 (d, J = 7.1 Hz), 50.3 (t, J = 19.8 Hz), 27.3 (3 × CH3-tert-butyl), 27.2, 25.0; UV (MeOH): λmax 263.74 nm; HRMS (ESI+): found, 417.1500 [calcd for C18H24ClF2N4O3+ (M + H)+, 417.1505]; Anal. Calcd for C18H23ClF2N4O3: C, 51.86; H, 5.56; N, 13.44. Found: C, 51.56; H, 5.96; N, 13.13.
General Procedure for the Synthesis of 2a–c
To a solution of 21a–c in tert-butanol (2 mL, 0.27 M) contained in a stainless steel bomb reactor, saturated ammonia in tert-butanol (15 mL) was added and the reactor was locked. After being heated to 120 °C with stirring for 15 h, the mixture was cooled to room temperature and co-evaporated with MeOH. Without purification, the residue was added to a TFA/H2O solution (2:1, v/v, total 15 mL) and heated to 50 °C with stirring for 15 h. After the reaction mixture was evaporated, the residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 9/1) to give 2a–c.
(1R,2S,3S,4R,5R)-3-(6-Amino-9H-purin-9-yl)-4-fluoro-5-(hydroxymethyl)cyclopentane-1,2-diol (2a)
It was obtained in 43% yield as a white solid; mp 172–177 °C; [α]D25 = −64.49 (c 0.22, MeOH); 1H NMR (800 MHz, CD3OD-d6): δ 8.26 (d, J = 2.0 Hz, 1H), 8.21 (s, 1H), 5.21 (dt, J = 4.0, 54.6, 1H), 4.99 (ddd, J = 3.4, 10.8, 29.5 Hz, 1H), 4.75 (dd, J = 6.7, 9.4 Hz, 1H), 4.02 (dd, J = 4.8, 6.4 Hz, 1H), 3.79–3.85 (m, 2H), 2.42–2.51 (m, 1H); 13C NMR (200 MHz, CD3OD): δ 158.1, 154.6, 152.2, 142.4 (d, J = 3.3 Hz), 120.5, 92.8 (d, J = 180.7 Hz), 74.3, 71.8, 64.0 (d, J = 17.0 Hz), 60.6 (d, J = 10.7 Hz), 54.3 (d, J = 17.9 Hz); 19F NMR (376 MHz, CD3OD): δ −204.7 to −205.4 (m); UV (MeOH): λmax 259.90 nm; HRMS (ESI+): found, 284.1161 [calcd for C11H15FN5O3+ (M + H)+, 284.1159]; Anal. Calcd for C11H14FN5O3: C, 46.64; H, 4.98; N, 24.72. Found: C, 46.65; H, 5.38; N, 25.10.
(1R,2S,3S,4S,5R)-3-(6-Amino-9H-purin-9-yl)-4-fluoro-5-(hydroxymethyl)cyclopentane-1,2-diol (2b)
It was obtained in 71% yield as a white solid; mp 182–186 °C; [α]D25 = −11.85 (c 0.26, MeOH); 1H NMR (500 MHz, CD3OD): δ 8.19 (s, 1H), 8.18 (s, 1H), 5.40 (ddd, J = 5.2, 7.3, 54.4 Hz, 1H), 5.03 (ddd, J = 7.5, 9.8, 20.7 Hz, 1H), 4.60 (dd, J = 5.1, 9.9 Hz, 1H), 4.05–4.09 (m, 1H), 3.80 (d, J = 5.8 Hz, 2H), 2.28–2.40 (m, 1H); 13C NMR (125 MHz, CD3OD): δ 158.0, 154.3, 151.9, 143.4, 121.6, 95.8 (d, J = 186.4 Hz), 74.2 (d, J = 7.4 Hz), 73.2 (d, J = 3.3 Hz), 68.6 (d, J = 21.1 Hz), 62.6, 54.6 (d, J = 19.0 Hz); 19F NMR (378 MHz, CD3OD): δ −185.244 (dt, J = 23.8, 53.7 Hz); UV (MeOH): λmax 260.88 nm; HRMS (ESI+): found, 284.1155 [calcd for C11H15FN5O3+ (M + H)+, 284.1159]; Anal. Calcd for C11H14FN5O3: C, 46.64; H, 4.98; N, 24.72. Found: C, 46.38; H, 5.12; N, 24.33.
(1R,2S,3S,5R)-3-(6-Amino-9H-purin-9-yl)-4,4-difluoro-5-(hydroxymethyl)cyclopentane-1,2-diol (2c)
It was obtained in 61% yield as a white solid; mp 180–185 °C; [α]D25 = −56.51 (c 0.30, MeOH); 1H NMR (500 MHz, CD3OD): δ 8.26 (d, J = 19.5 Hz, 1H), 8.20 (s, 1H), 5.33 (dt, J = 10.0, 17.0 Hz, 1H), 4.79 (dd, J = 5.2, 10.6 Hz, 1H, merged with solvent peak), 4.13–4.17 (m, 1H), 3.79–3.91 (m, 2H), 2.60–2.71 (m, 1H); 13C NMR (200 MHz, CD3OD): δ 158.2, 154.8, 152.6, 142.7 (d, J = 2.4 Hz), 125.9 (dd, J = 252.3, 258.4 Hz), 120.6, 73.7 (d, J = 7.3 Hz), 71.8 (d, J = 3.3 Hz), 64.8 (dd, J = 19.4, 23.8 Hz), 59.6 (d, J = 10.8 Hz), 56.4 (t, J = 19.9 Hz); 19F NMR (378 MHz, CD3OD): δ −97.5 (d, J = 238.5 Hz), −115.4 (dt, J = 15.9, 238.9 Hz); UV (MeOH): λmax 259.92 nm; HRMS (ESI+): found, 302.1066 [calcd for C11H14F2N5O3+ (M + H)+, 302.1065]; Anal. Calcd for C11H13F2N5O3: C, 43.86; H, 4.35; N, 23.25. Found: C, 44.17; H, 4.14; N, 23.05.
General Procedure for the Synthesis of 2d and 2e
To a solution of 21a and 21c (0.283 mmol) in EtOH (1.5 mL, 0.19 M) in a sealed glass tube, methylamine (40 wt % in H2O, 10 mL) was added. After being stirred at room temperature for 2 h, the mixture was concentrated and added to a TFA/H2O solution (2:1, v/v, total 15 mL) without purification. After being heated to 50 °C with stirring for 15 h, the reaction mixture was evaporated. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 9/1) to give 2d and 2e.
(1S,2R,3R,4R,5S)-4-Fluoro-3-(hydroxymethyl)-5-(6-(methylamino)-9H-purin-9-yl)cyclopentane-1,2-diol (2d)
It was obtained in 67% yield as a white solid; mp 197–201 °C; [α]D25 = −61.46 (c 0.40, MeOH); 1H NMR (800 MHz, CD3OD): δ 8.27 (s, 1H), 8.20 (d, J = 18.4 Hz, 1H), 5.21 (dt, J = 4.0, 54.6 Hz, 1H), 4.98 (ddd, J = 3.4, 10.0, 29.6 Hz, 1H), 4.74 (dd, J = 6.7, 9.4 Hz, 1H), 4.01 (dd, J = 4.9, 6.4 Hz, 1H), 3.79–3.85 (m, 2H), 3.11 (br s, 3H), 2.42–2.51 (m, 1H); 13C NMR (200 MHz, CD3OD): δ 157.5, 154.6, 151.1, 141.8 (d, J = 3.7 Hz), 121.1, 92.9 (d, J = 180.8 Hz), 74.3, 71.8, 64.0 (d, J = 17.0 Hz), 60.6 (d, J = 10.5 Hz), 54.3 (d, J = 18.0 Hz), 28.5; 19F NMR (376 MHz, CD3OD): δ −206.3 (dt, J = 29.7, 53.4 Hz); UV (MeOH): λmax 266.89 nm; HRMS (ESI+): found, 298.1317 [calcd for C12H17FN5O3+ (M + H)+, 298.1315]; Anal. Calcd for C12H16FN5O3: C, 48.48; H, 5.42; N, 23.56. Found: C, 48.50; H, 5.22; N, 23.93.
(1S,2R,3R,5S)-4,4-Difluoro-3-(hydroxymethyl)-5-(6-(methylamino)-9H-purin-9-yl)cyclopentane-1,2-diol (2e)
It was obtained in 76% yield as a white solid; mp 125–129 °C; [α]D25 = −48.62 (c 0.25, MeOH); 1H NMR (500 MHz, CD3OD): δ 8.24 (s, 1H), 8.20 (s, 1H), 5.33 (dt, J = 9.9, 18.4 Hz, 1H), 4.79 (dd, J = 10.3, 10.2 Hz, 1H), 4.17 (s, 1H), 3.81–3.90 (m, 2H), 3.10 (br s, 3H), 2.67 (m, 1H); 13C NMR (125 MHz, CD3OD): δ 157.5, 154.7, 151.5, 142.1, 125.9 (dd, J = 252.4, 258.1 Hz), 121.1, 73.7 (d, J = 7.25 Hz), 71.9 (d, J = 3.1 Hz), 64.7 (dd, J = 20.0, 24.3 Hz), 59.6 (d, J = 10.8 Hz), 56.4 (t, J = 19.9 Hz), 28.6; 19F NMR (378 MHz, CD3OD): δ −97.4 (d, J = 238.5 Hz), −115.3 (d, J = 238.9 Hz); UV (MeOH): λmax 263.72 nm; HRMS (ESI+): found, 316.1227 [calcd for C12H16F2N5O3+ (M + H)+, 316.1221]; Anal. Calcd for C12H15F2N5O3: C, 45.71; H, 4.80; N, 22.21. Found: C, 45.99; H, 4.47; N, 22.02.
General Procedure for the Synthesis of 22a–c
To a cooled (−20 °C) solution of 19a–c (1 equiv) in DMF (0.2 M), 3-methoxyacryloyl isocyanate (2 equiv) in benzene was added dropwise in a N2 atmosphere. After the reaction mixture was slowly warmed to room temperature for 15 h with stirring, the reaction mixture was filtered with CH2Cl2 and co-evaporated with toluene and ethanol. The residue was purified by column chromatography (silica gel, hexane/EtOAc, 1.5/1) to give 22a–c.
(E)-N-(((3aS,4S,5R,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)carbamoyl)-3-methoxyacrylamide (22a)
It was obtained in 76% yield as a colorless syrup; [α]D25 = −19.41 (c 0.37, MeOH); 1H NMR (600 MHz, CDCl3): δ 10.24 (s, 1H), 9.16 (d, J = 8.2 Hz, 1H), 7.61 (d, J = 12.4 Hz, 1H), 5.35 (d, J = 12.4 Hz, 1H), 5.06 (dt, J = 3.2, 52.7 Hz, 1H), 4.51 (t, J = 6.6 Hz, 1H), 4.29–4.38 (m, 2H), 3.64 (s, 3H), 3.45–3.52 (m, 2H), 2.21–2.31 (m, 1H), 1.41 (s, 3H), 1.21 (s, 3H), 1.10 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 168.0, 163.3, 155.4, 113.7, 97.5, 96.7 (d, J = 178.8 Hz), 84.4, 80.1, 72.9, 58.6 (d, J = 15.8 Hz), 57.8 (d, J = 6.5 Hz), 57.4, 49.8 (d, J = 17.2 Hz), 27.2 (3 × CH3-tert-butyl), 27.1, 24.6; UV (MeOH): λmax 243.14 nm; HRMS (ESI+): found, 389.2088 [calcd for C18H30FN2O6+ (M + H)+, 389.2088].
(E)-N-(((3aS,4S,5S,6R,6aR)-6-(tert-Butoxymethyl)-5-fluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)carbamoyl)-3-methoxyacrylamide (22b)
It was obtained in 88% yield as a colorless syrup; [α]D25 = −20.47 (c 0.34, MeOH); 1H NMR (500 MHz, CDCl3): δ 10.33 (s, 1H), 8.96 (d, J = 7.4 Hz, 1H), 7.63 (d, J = 12.3 Hz, 1H), 5.39 (d, J = 12.3 Hz, 1H), 4.80 (dt, J = 6.4, 52.5 Hz, 1H), 4.44 (t, J = 5.5 Hz, 1H), 4.33–4.41 (m, 2H), 3.67 (s, 3H), 3.46 (d, J = 32.5 Hz, 2H), 2.33–2.42 (m, 1H), 1.46 (s, 3H), 1.24 (s, 3H), 1.13 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 168.1, 163.2, 155.5, 111.9, 97.9 (d, J = 187.4 Hz), 97.5, 83.3 (d, J = 7.2 Hz), 79.0 (d, J = 6.5 Hz), 73.1, 61.9 (d, J = 23.7 Hz), 58.6 (d, J = 2.1 Hz), 57.4, 49.9 (d, J = 19.4 Hz), 27.3 (3 × CH3-tert-butyl), 27.2, 25.0; UV (MeOH): λmax 242.93 nm; HRMS (ESI+): found, 389.2098 [calcd for C18H30FN2O6+ (M + H)+, 389.2088].
(E)-N-(((3aS,4S,6R,6aR)-6-(tert-Butoxymethyl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)carbamoyl)-3-methoxyacrylamide (22c)
It was obtained in 90% yield as a colorless syrup; [α]D25 = −40.41 (c 0.52, MeOH); 1H NMR (500 MHz, CDCl3): δ 10.26 (s, 1 Η), 9.11 (d, J = 8.7 Hz, 1H), 7.65 (d, J = 12.3 Hz, 1H), 5.37 (d, J = 12.4 Hz, 1H), 4.52–4.62 (m, 1H), 4.39 (s, 2H), 3.67 (s, 3H), 3.53–3.60 (m, 2H), 2.57–2.68 (m, 1H), 1.47 (s, 3H), 1.27 (s, 3H), 1.16 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 167.9, 163.4, 155.7, 126.9 (dd, J = 252.9, 261.3 Hz), 112.4, 97.4, 82.5 (d, J = 6.9 Hz), 78.6 (d, J = 4.9 Hz), 73.5, 60.6 (dd, J = 19.4, 29.2 Hz), 57.4 (d, J = 6.1 Hz), 57.3, 50.8 (t, J = 20.8 Hz), 27.1 (3 × CH3-tert-butyl), 27.0, 24.9; UV (MeOH): λmax 242.22 nm; HRMS (ESI+): found, 407.1991 [calcd for C18H29F2N2O6+ (M + H)+, 407.1994].
General Procedure for the Synthesis of 2f–h
To a stirred solution of 22a–c in 1,4-dioxane (3 mL, 2.5 M), 2 M sulfuric acid (0.3 mL) was dropwise added. After refluxing with stirring for 1 h, the reaction mixture was cooled to room temperature and neutralized with DOWEX 66 ion-exchange resin. The mixture was filtered and evaporated. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 9/1) to give 2f–h.
1-((1S,2R,3R,4R,5S)-2-Fluoro-4,5-dihydroxy-3-(hydroxymethyl)cyclopentyl)pyrimidine-2,4(1H,3H)-dione (2f)
It was obtained in 56% yield as a white solid; mp 112–118 °C; [α]D25 = −77.11 (c 0.20, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.70 (dd, J = 1.1, 8.1 Hz, 1H), 5.69 (d, J = 8.0 Hz, 1H), 5.10 (dt, J = 4.1, 55.3 Hz, 1H), 4.91 (dd, J = 3.4, 10.2 Hz, 1H, merged with solvent peak), 4.46 (dd, J = 6.6, 10.1 Hz, 1H), 3.93 (t, J = 4.8 Hz, 1H), 3.70–3.80 (m, 2H), 3.69 (s, 1H), 2.29–2.41 (m, 1H); 13C NMR (125 MHz, CD3OD): δ 166.9, 154.2, 145.5 (d, J = 3.8 Hz), 102.7, 93.0 (d, J = 180.1 Hz), 72.4, 71.7, 64.4 (d, J = 16.6 Hz), 60.6 (d, J = 11.4 Hz), 53.8 (d, J = 17.9 Hz); 19F NMR (378 MHz, CD3OD): δ −208.9 (dt, J = 29.9, 59.7 Hz); UV (MeOH): λmax 264.11 nm; HRMS (ESI+): found, 261.0886 [calcd for C10H14FN2O5+ (M + H)+, 261.0887]; Anal. Calcd for C10H13FN2O5: C, 46.16; H, 5.04; N, 10.77. Found: C, 45.98; H, 5.44; N, 10.98.
1-((1S,2S,3R,4R,5S)-2-Fluoro-4,5-dihydroxy-3-(hydroxymethyl)cyclopentyl)pyrimidine-2,4(1H,3H)-dione (2g)
It was obtained in 53% yield as a white solid; mp 195–200 °C; [α]D25 = −16.89 (c 0.35, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.60 (d, J = 7.9 Hz, 1H), 5.69 (d, J = 7.9 Hz, 1H), 5.07–5.21 (ddd, J = 5.10, 6.8, 55.2 Hz, 1H), 4.61–4.69 (ddd, J = 7.3, 8.7, 22.6 Hz, 1H), 4.32 (dd, J = 5.25, 9.0 Hz, 1H), 3.98 (t, J = 3.7 Hz, 1H), 3.70 (m, 2H), 2.24 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 167.1, 153.6, 147.4, 103.5, 94.8 (d, J = 183.9 Hz), 73.4 (d, J = 7.3 Hz), 73.1 (d, J = 22.0 Hz), 72.7 (d, J = 3.5 Hz), 62.3 (d, J = 1.8 Hz), 54.1 (d, J = 18.9 Hz); 19F NMR (378 MHz, CD3OD): δ −184.3 (dt, J = 23.8, 53.7 Hz); UV (MeOH): λmax 265.33 nm; HRMS (ESI+): found, 261.0894 [calcd for C10H14FN2O5+ (M + H)+, 261.0887]; Anal. Calcd for C10H13FN2O5: C, 46.16; H, 5.04; N, 10.77. Found: C, 46.24; H, 5.23; N, 10.78.
1-((1S,3R,4R,5S)-2,2-Difluoro-4,5-dihydroxy-3-(hydroxymethyl)cyclopentyl)pyrimidine-2,4(1H,3H)-dione (2h)
It was obtained in 52% yield as a white solid; mp 164–169 °C; [α]D25 = −31.06 (c 0.30, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.67 (dd, J = 2.3, 8.1 Hz, 1H) 5.71 (d, J = 8.0 Hz, 1H), 5.36 (dt, J = 10.3, 17.7 Hz, 1H), 4.41 (dd, J = 5.15, 10.7 Hz, 1H), 4.07 (m, 1H), 3.73–3.82 (m, 2H), 2.53 (m, 1H); 13C NMR (150 MHz, CD3OD): δ 166.6, 154.1, 145.3 (d, J = 4.3 Hz), 126.8 (dd, J = 252.8, 258.5 Hz), 103.4, 72.5 (d, J = 7.9 Hz), 71.8 (d, J = 2.9 Hz), 64.4 (dd, J = 18.7, 25.1 Hz), 59.5 (d, J = 11.5 Hz), 56.3 (t, J = 20.1 Hz); 19F NMR (378 MHz, CD3OD): δ −96.6 (d, J = 238.9 Hz), −116.9 (dt, J = 15.1, 238.5 Hz); UV (MeOH): λmax 262.41 nm; HRMS (ESI+): found, 279.0801 [calcd for C10H13F2N2O5+ (M + H)+, 279.0793]; Anal. Calcd for C10H12F2N2O5: C, 43.17; H, 4.35; N, 10.07. Found: C, 43.34; H, 4.67; N, 9.94.
General Procedure for the Synthesis of 2i and 2j
Benzoylation
To a cooled (0 °C) solution of 2f or 2h (1 equiv) in CH2Cl2 (0.07 M), benzoyl chloride (6 equiv) and pyridine (6.7 equiv) were added in a N2 atmosphere. After being stirred for 15 h at room temperature, the reaction mixture was quenched with H2O and extracted with CH2Cl2. The organic layers were combined and washed with H2O followed by brine, dried over MgSO4, filtered, and evaporated. The residue was purified with column chromatography (silica gel, hexane/EtOAc, 1/1) to give the benzoylated intermediate.
Introduction of Triazole
To a cooled (0 °C) suspension of 1,2,4-triazole (10 equiv) in anhydrous MeCN (0.6 M), phosphoryl chloride (10 equiv) was added dropwise in a N2 atmosphere. After stirring, the benzoylated intermediate (1 equiv) in MeCN (0.14 M) followed by trimethylamine (10 equiv) were added to the reaction mixture. After additional stirring at room temperature for 15 h, the reaction mixture was evaporated. The reaction mixture was diluted with CH2Cl2 and H2O. The layers were separated, and the organic layers were washed with H2O, dried over MgSO4, filtered, and evaporated.
Amination
In the sealed glass tube, the above-generated intermediate in 1,4-dioxane (0.06 M) was added to excess saturated aqueous ammonia at room temperature. After being stirred at the same temperature for 2 h, the reaction mixture was evaporated and purified with flash chromatography (silica gel, CH2Cl2/MeOH, 7/1) to give the benzoyl protected cytosine intermediate.
Benzoyl Deprotection
In a sealed glass tube, the above-generated benzoyl-protected cytosine intermediate in MeOH (0.2 M) was added to saturated ammonia in MeOH (0.2 M). After being stirred at the same temperature for 2 d, the reaction mixture was evaporated and diluted with H2O and CH2Cl2. The layers were separated, and the H2O layers were washed with CH2Cl2 10 times and evaporated to give 2i and 2j, respectively.
4-Amino-1-((1S,2R,3R,4R,5S)-2-fluoro-4,5-dihydroxy-3-(hydroxymethyl)cyclopentyl)pyrimidin-2(1H)-one (2i)
It was obtained in 17% yield as a white solid; mp 230–233 °C; [α]D25 = −84.26 (c 0.20, MeOH); 1H NMR (800 MHz, CD3OD): δ 7.67 (dd, J = 1.3, 7.5 Hz, 1H), 5.88 (d, J = 7.4 Hz, 1H), 5.23 (dt, J = 3.7, 55.4 Hz, 1H), 4.93 (ddd, J = 3.4, 10.3, 30.4 Hz, 1H), 4.44 (dd, J = 6.6, 10.3 Hz, 1H), 3.92 (dd, J = 4.5, 6.3 Hz, 1H), 3.71–3.78 (m, 2H), 2.31–2.40 (m, 1H); 13C NMR (200 MHz, CDCl3): δ 168.3, 160.3, 145.7 (d, J = 3.1 Hz), 96.2, 93.0 (d, J = 179.9 Hz), 72.5, 71.8, 65.3 (d, J = 16.6 Hz), 60.7 (d, J = 11.3 Hz), 53.9 (d, J = 17.9 Hz); 19F NMR (376 MHz, CD3OD): δ −209.4 (dt, J = 29.3, 53.4 Hz); UV (MeOH): λmax 274.67 nm; HRMS (ESI+): found, 260.1041 [calcd for C10H15FN3O4+ (M + H)+, 260.1047]; Anal. Calcd for C10H14FN3O4: C, 46.33; H, 5.44; N, 16.21. Found: C, 46.71; H, 5.12; N, 15.99.
4-Amino-1-((1S,3R,4R,5S)-2,2-difluoro-4,5-dihydroxy-3 (hydroxymethyl)cyclopentyl)pyrimidin-2(1H)-one (2j)
It was obtained in 20% yield as a white solid; mp 242–245 °C; [α]D25 = −39.85 (c 0.30, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.62 (dd, J = 7.45, 2.35 Hz, 1H), 5.90 (d, J = 7.40 Hz, 1H), 5.51 (dt, J = 18.2, 10.0 Hz, 1H), 4.37 (dd, J = 10.6, 5.25 Hz, 1H), 4.06 (m, 1H), 3.73–3.83 (m, 2H), 2.54 (m, 1H); 13C NMR (150 MHz, CD3OD): δ 168.2, 160.1, 145.7 (d, J = 3.6 Hz), 126.9 (dd, J = 252.1, 259.2 Hz), 96.8, 72.9 (d, J = 8.6 Hz), 71.7 (d, J = 3.6 Hz), 65.1 (dd, J = 18.7, 23.0 Hz), 59.6 (d, J = 10.8 Hz), 56.3 (t, J = 20.1 Hz); 19F NMR (378 MHz, CD3OD): δ −97.4 (d, J = 235.9 Hz), −117.4 (dt, J = 14.7, 238.9 Hz); UV (MeOH): λmax 272.27, 237.93 nm; HRMS (ESI+): found, 278.0954 [calcd for C10H14F2N3O4+ (M + H)+, 278.0952]; Anal. Calcd for C10H13F2N3O4: C, 43.32; H, 4.73; N, 15.16. Found: C, 43.56; H, 4.56; N, 15.44.
General Procedure for the Synthesis of 24, 27a and 27b
To a cooled (0 °C) suspension of 2c, 2f, and 2h (1 equiv) in acetone (0.005 M) were added 1–2 drops of cH2SO4 in N2 (g). After being stirred at room temperature for 4 h, the reaction mixture was neutralized with solid NaHCO3, filtered, and evaporated under reduced pressure. The residue was further purified by silica gel column chromatography to give 24, 27a, and 27b, respectively.
((3aR,4R,6S,6aS)-6-(6-Amino-9H-purin-9-yl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)methanol (24)
It was obtained in 96% yield as a colorless syrup; 1H NMR (500 MHz, CD3OD): δ 8.31 (s, 1H), 8.21 (s, 1H), 5.30–5.40 (m, 2H), 4.70 (br s, 1H), 3.94 (dd, J = 6.8, 11.4 Hz, 1H), 3.86 (dd, J = 6.8, 11.4 Hz, 1H), 2.81–2.90 (m, 1H), 1.58 (s, 3H), 1.35 (s, 3H); 13C NMR (125 MHz, CD3OD): δ 163.5 (dd, J = 33.1, 69.2 Hz), 156.5, 152.2, 152.0, 143.4, 128.0 (dd, J = 251.7, 263.6 Hz), 116.0, 80.7 (d, J = 7.3 Hz), 79.6 (d, J = 8.3 Hz), 66.1 (dd, J = 19.2, 22.8 Hz), 59.2 (d, J = 8.0 Hz), 53.8 (t, J = 19.4 Hz), 28.2, 25.9; HRMS (ESI+) (m/z): found, 342.1370 [calcd for C14H18F2N5O3+ (M + H)+, 342.1372]; Anal. Calcd for C14H17F2N5O3: C, 49.27; H, 5.02; N, 20.52. Found: C, 49.28; H, 4.98; N, 20.91.
1-((3aS,4S,5R,6R,6aR)-5-Fluoro-6-(hydroxymethyl)-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)pyrimidine-2,4(1H,3H)-dione (27a)
It was obtained in 98% yield as a colorless syrup; 1H NMR (500 MHz, CD3OD): δ 7.75 (dd, J = 1.4, 8.1 Hz, 1H), 5.70 (d, J = 8.1 Hz, 1H), 5.20 (dt, J = 3.1, 54.1 Hz, 1H), 5.01–5.13 (m, 2H), 4.58 (d, J = 6.3 Hz, 1H), 3.73–3.83 (m, 2H), 2.42–2.56 (m, 1H), 1.50 (s, 3H), 1.32 (s, 3H); 13C NMR (125 MHz, CD3OD): δ 166.7, 153.7, 145.3 (d, J = 5.9 Hz), 116.5, 103.2, 99.2 (d, J = 180.2 Hz), 82.2, 82.0, 65.0 (d, J = 15.7 Hz), 60.3 (d, J = 8.7 Hz), 53.2 (d, J = 17.7 Hz), 28.4, 25.9; HRMS (ESI+) (m/z): found, 301.1185 [calcd for C13H18FN2O5+ (M + H)+, 301.1194]; Anal. Calcd for C13H17FN2O5: C, 52.00; H, 5.71; N, 9.33. Found: C, 52.15; H, 5.47; N, 9.15.
1-((3aS,4S,6R,6aR)-5,5-Difluoro-6-(hydroxymethyl)-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)pyrimidine-2,4(1H,3H)-dione (27b)
It was obtained in 97% yield; 1H NMR (500 MHz, CD3OD): δ 7.71 (dd, J = 2.0, 8.1 Hz, 1H), 5.73 (d, J = 8.1 Hz, 1H), 5.33 (dt, J = 6.8, 21.3 Hz, 1H), 4.94 (d, J = 6.8 Hz, 1H), 4.57–4.63 (m, 1H), 3.88 (dd, J = 6.7, 11.4 Hz, 1H), 3.81 (dd, J = 6.7, 11.4 Hz, 1H), 2.68–2.79 (m, 1H), 1.54 (s, 3H), 1.34 (s, 3H); HRMS (ESI+) (m/z): found, 319.1104 [calcd for C13H17F2N2O5+ (M + H)+, 319.1100]; Anal. Calcd for C13H16F2N2O5: C, 49.06; H, 5.07; N, 8.80. Found: C, 49.43; H, 5.47; N, 8.43.
Synthesis of tert-Butyl-(9-((3aS,4S,6R,6aR)-5,5-difluoro-6-(hydroxymethyl)-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)-9H-purin-6-yl)carbamate (25a) and Its N6-Di-Boc derivative (25b)
To a suspension of 24 (20 mg, 0.058 mmol) and 4-dimethylaminopyridine (1 mg, 0.0058 mmol) in hexamethyldisilazane (3 mL), trimethylsilyl trifluoromethanesulfonate (TMSOTf; 5 μL) was added dropwise at room temperature in a N2 atmosphere (g). After being heated to 75 °C with stirring for 2 h, the reaction mixture was evaporated, and anhydrous THF (7 mL) was added. To a cooled (0 °C) reaction mixture, di-t-butyl dicarbonate (63 mg, 0.29 mmol) was added. After stirring for 4 h at room temperature, the reaction mixture was evaporated, and the residue was added to MeOH/trimethylamine (6 mL, 5:1 (v/v)). After heating to 55 °C with stirring for 16 h, the reaction mixture was evaporated, and the residue was purified with column chromatography (silica gel, CH2Cl2/MeOH, 50/1) to give 25a (13 mg, 52%) and 25b (8 mg, 25%) as a colorless syrup.
Compound 25a
1H NMR (500 MHz, CD3OD): δ 8.59 (s, 1H), 8.49 (s, 1H), 5.36–5.50 (m, 2H), 4.72 (d, J = 5.6 Hz, 1H), 3.95 (dd, J = 6.8, 11.4 Hz, 1H), 3.87 (dd, J = 6.8, 11.4 Hz, 1H), 2.83–2.95 (m, 1H), 1.57 (s, 12H), 1.34 (s, 3H); HRMS (ESI+) (m/z): found, 442.1899 [calcd for C19H26F2N5O5+ (M + H)+, 442.1897].
Compound 25b
1H NMR (500 MHz, CD3OD): δ 8.87 (s, 1H), 8.73 (d, J = 1.8 Hz, 1H), 5.46–5.57 (m, 2H), 4.75 (d, J = 5.4 Hz, 1H), 3.95 (dd, J = 6.8, 11.4 Hz, 1H), 3.88 (dd, J = 6.8, 11.4 Hz, 1H), 2.84–2.95 (m, 1H), 1.59 (s, 3H), 1.37 (s, 21H); 13C NMR (125 MHz, CD3OD): δ 156.2, 154.2, 152.2, 152.1 (2 × C(O)-Boc protection group), 147.8 (d, J = 2.4 Hz), 130.6, 128.1 (dd, J = 251.8, 263.3 Hz), 116.0, 86.1, 80.4 (d, J = 7.4 Hz), 79.7 (d, J = 8.2 Hz), 72.7, 66.5 (dd, J = 19.1, 23.1 Hz), 59.2 (d, J = 8.0 Hz), 53.8 (t, J = 19.2 Hz), 28.7 (6 × CH3-tert-butyl), 28.3, 25.9; HRMS (ESI+) (m/z): found, 542.2411 [calcd for C24H34F2N5O7+ (M + H)+, 542.2421].
Iso-propyl ((S)-(((3aR,4R,6S,6aS)-6-(6-((tert-Butoxycarbonyl)amino)-9H-purin-9-yl)-5,5-difluoro-2,2-dimethyltetrahydro-4H-cyclopenta[d][1,3]dioxol-4-yl)methoxy)(phenoxy)phosphoryl)-l-alaninate (26)
To a stirred suspension of 25a (16 mg, 0.036 mmol), 25b (7 mg, 0.012 mmol), and powdered molecular sieves (4 Å, 62 mg) in anhydrous THF (20 mL), tert-butylmagnesium chloride solution (0.26 mL, 1.0 M in THF, 0.26 mmol) was added at 0 °C in a nitrogen atmosphere. After 10 min, a solution of pentafluorophosphoramidate reagent A (47 mg, 0.10 mmol) in THF (12 mL) was slowly added, and the reaction mixture was stirred at room temperature for 36 h. Then, it was quenched by the dropwise addition of methanol (10 mL), filtered, and evaporated. The residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 9/1) to give the phosphoramidate 26 as a colorless liquid (12 mg, 33%): 1H NMR (500 MHz, CD3OD): δ 8.59 (s, 1H), 8.45 (s, 1H), 7.37 (d, J = 7.8 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 7.19 (d, J = 7.5 Hz, 1H), 5.50 (dt, J = 5.9, 22.3 Hz, 1H), 5.40–5.45 (m, 1H), 4.92–4.99 (m, 1H), 4.73–4.80 (m, 1H), 4.36–4.50 (m, 2H), 3.86–3.98 (m, 1H), 3.07–3.19 (m, 1H), 1.58 (s, 12H), 1.34 (s, 6H), 1.21 (d, J = 6.2 Hz, 3H), 1.17 (d, J = 6.2 Hz, 3H); HRMS (ESI+) (m/z): found, 711.2716 [calcd for C31H42F2N6O9P+ (M + H)+, 711.2713].
Iso-propyl ((S)-(((1R,3S,4S,5R)-3-(6-Amino-9H-purin-9-yl)-2,2-difluoro-4,5-dihydroxycyclopentyl)methoxy)(phenoxy)phosphoryl)-l-alaninate (3a)
A solution of 26 (15 mg, 0.021 mmol) in 10 mL of formic acid/H2O (1:1, v/v) was stirred at room temperature for 8 h. After evaporation, the crude product was purified by column chromatography (silica gel, CH2Cl2/MeOH, 6/1) to give 3a (9.9 mg, 82%) as a colorless solid: mp 95–100 °C; UV (MeOH): λmax 259.6 nm; [α]D25 = −38.06 (c 0.1, MeOH); 1H NMR (400 MHz, CD3OD): δ 8.18 (s, 1H), 8.17 (d, J = 1.6 Hz, 1H), 7.35 (d, J = 8.4 Hz, 2H), 7.23 (d, J = 8.6 Hz, 2H), 7.18 (d, J = 8.0 Hz, 1H), 5.26–5.38 (m, 1H), 4.81–4.98 (m, merged with H2O peak, 1H), 4.74 (dd, J = 4.8, 10.0 Hz, 1H), 4.29–4.43 (m, 2H), 7.17 (br s, 1H), 3.82–3.93 (m, 1H), 2.79–2.94 (m, 1H), 1.32 (d, J = 6.8 Hz, 3H), 1.19 (d, J = 6.2 Hz, 3H), 1.14 (d, J = 6.2 Hz, 3H); 13C NMR (150 MHz, CD3OD): δ 175.2 (d, J = 5.7 Hz), 158.1, 154.7, 153.0, 152.9, 152.6, 142.6, 131.6 (2 × CH-phenyl), 127.0, 124.4 (dd, J = 253.5, 260.6 Hz), 122.2 (d, J = 4.3 Hz), 120.6 (2 × CH-phenyl), 73.3 (d, J = 7.1 Hz), 71.2 (d, J = 5.0 Hz), 70.9, 64.6, 64.1 (dd, J = 5.0, 10.7 Hz), 52.4, 22.6 (d, J = 2.9 Hz, 2 × CH3), 21.2 (d, J = 6.5 Hz); 19F NMR (376 MHz, CD3OD): δ −98.71 (d, J = 238.4 Hz), −115.13 (dt, J = 14.9, 236.4 Hz); HRMS (ESI+) (m/z): found, 571.1889 [calcd for C23H30F2N6O7P+ (M + H)+, 571.1876]; Anal. Calcd for C23H29F2N6O7P: C, 48.42; H, 5.12; N, 14.73. Found: C, 48.74; H, 4.98; N, 14.54.
Iso-propyl ((S)-(((1R,2R,3S,4S,5R)-3-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2-fluoro-4,5-dihydroxycyclopentyl)methoxy)(phenoxy)phosphoryl)-l-alaninate (3b)
Introduction of Phosphoramidate
To a cooled (0 °C) suspension of 27a (21 mg, 0.069 mmol) and molecular sieves (4 Å, 35 mg) in anhydrous THF (15 mL, 0.005 M), tert-butylmagnesium chloride solution (0.34 mL, 1.0 M in THF, 0.34 mmol) was added dropwise in a N2 atmosphere (g). After being stirred for 5 min, a solution of the phosphoramidate reagent A (31 mg, 0.069 mmol) in anhydrous THF (7 mL) was added dropwise, and the reaction mixture was stirred at room temperature for 36 h, quenched with MeOH (5 mL), filtered, and evaporated, and the residue was purified by column chromatograph (silica gel, CH2Cl2/MeOH, 24/1) to give phosphoramidate as a colorless liquid (13 mg, 33%): 1H NMR (500 MHz, CD3OD): δ 7.73 (dd, J = 1.3, 8.1 Hz, 1H), 7.36 (d, J = 7.8 Hz, 2H), 7.24 (d, J = 7.8 Hz, 2H), 7.19 (d, J = 7.4 Hz, 1H), 5.70 (d, J = 8.1 Hz, 1H), 5.02–5.22 (m, 3H), 4.93–5.01 (m, 1H), 4.66 (d, J = 6.3 Hz, 1H), 4.29 (d, J = 7.6 Hz, 2H), 3.87–3.95 (m, 1H), 2.62–2.73 (m, 1H), 1.51 (s, 3H), 1.34 (d, J = 7.7 Hz, 3H), 1.32 (s, 3H), 1.22 (d, J = 6.2 Hz, 6H); HRMS (ESI+) (m/z): found, 570.2003 [calcd for C25H34FN3O9P+ (M + H)+, 570.2011].
Hydrolysis
A solution of phosphoramidate (13 mg, 0.022 mmol) in a formic acid/H2O solution (1:1, v/v, 7 mL total) was stirred at room temperature for 8 h. The reaction mixture was evaporated and the residue was purified by column chromatography (silica gel, CH2Cl2/MeOH, 7/1) to give the phosphoramidate prodrug 3b (10.8 mg, 90%) as a white solid: mp 107–110 °C; UV (MeOH): λmax 262.8 nm; [α]D25 = −59.40 (c 0.1, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.64 (d, J = 8.1 Hz, 1H), 7.36 (d, J = 7.9 Hz, 2H), 7.23 (d, J = 7.9 Hz, 2H), 7.19 (d, J = 7.4 Hz, 1H), 5.68 (d, J = 8.1 Hz, 1H), 5.04 (dt, J = 4.1, 55.4 Hz, 1H), 4.87–4.98 (m, merged with H2O peak, 2H), 4.45 (dd, J = 6.6, 9.7 Hz, 1H), 4.26 (d, J = 7.1 Hz, 2H), 3.99 (d, J = 5.4 Hz, 1H), 3.85–3.93 (m, 1H), 2.49–2.60 (m, 1H), 1.33 (d, J = 7.0 Hz, 3H), 1.21 (d, J = 6.1 Hz, 6H); 13C NMR (125 MHz, CD3OD): δ 175.2, 166.9, 154.0, 151.1, 145.4, 131.5 (2 × CH-phenyl), 126.9 (2 × CH-phenyl), 122.2 (d, J = 4.6 Hz), 102.8, 93.3 (d, J = 184.5 Hz), 80.3, 79.9 (d, J = 32.5 Hz), 72.3, 71.2, 70.9, 64.2 (d, J = 16.0 Hz), 52.4, 22.7 (d, J = 9.2 Hz, 2 × CH3), 21.2 (d, J = 6.8 Hz); 19F NMR (376 MHz, CD3OD): δ −208.27 (dt, J = 29.7, 59.4 Hz); HRMS (ESI+) (m/z): found, 530.1685 [calcd for C22H30FN3O9P+ (M + H)+, 530.1698]; Anal. Calcd for C22H29FN3O9P: C, 49.91; H, 5.52; N, 7.94. Found: C, 50.03; H, 5.32; N, 7.54.
Iso-propyl ((S)-(((1R,3S,4S,5R)-3-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2,2-difluoro-4,5-dihydroxycyclopentyl)methoxy)(phenoxy)phosphoryl)-l-alaninate (3c)
Compound 3c was synthesized according the same procedure used in the preparation of 3b: yield = 30%; white solid; mp 174 °C (decomp); UV (MeOH): λmax 262.8 nm; [α]D25 = −19.40 (c 0.1, MeOH); 1H NMR (500 MHz, CD3OD): δ 7.53 (dd, J = 2.1, 8.1 Hz, 1H), 7.36 (d, J = 7.8 Hz, 2H), 7.25 (d, J = 7.8 Hz, 2H), 7.20 (d, J = 7.6 Hz, 1H), 5.70 (d, J = 8.1 Hz, 1H), 5.29–5.39 (m, 1H), 4.93–5.02 (m, 1H), 4.30–4.39 (m, 2H), 4.23–4.29 (m, 1H), 4.08 (br s, 1H), 3.84–3.92 (m, 1H), 2.69–2.80 (m, 1H), 1.33 (d, J = 7.1 Hz, 3H), 1.22 (d, J = 6.2 Hz, 6H); 19F NMR (376 MHz, CD3OD): δ −98.47 (d, J = 237.2 Hz), −116.91 (dt, J = 17.6, 237.2 Hz); HRMS (ESI+) (m/z): found, 548.1619 [calcd for C22H29F2N3O9P+ (M + H)+, 548.1604]; Anal. Calcd for C22H28F2N3O9P: C, 48.27; H, 5.16; N, 7.68. Found: C, 48.12; H, 4.98; 8.01.
SAH Hydrolase Assay18e−18g,30
The gene encoding human placental SAH hydrolase was cloned into expression plasmid pPROKcd20. Recombinant SAH hydrolase protein was produced in E. coli JM109 in 50 mM Tris-HCl (pH 7.5) containing 2 mM ethylenediaminetetraacetic acid and was purified by DEAE-cellulose column (2.8 cm × 6 cm), ammonium sulfate fractionation (35–60%), Sephacryl S-300HR (1.0 cm × 105 cm), and DEAE cellulose (2.8 cm × 24 cm). The protein homogeneity was confirmed by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The protein concentration was determined by using the Bradford method. Bovine serum albumin was a standard material for protein assay. Enzyme activity was determined in reaction mixtures (250 μL) that contain 50 mM sodium phosphate (pH 8.0), 2 μM SAH hydrolase (0.5 μM tetrameric form), and varying concentrations of compounds. The reaction mixtures were first preincubated with the compounds for 10 min at 37 °C, after which the reaction was initiated by adding 100 μM SAH. The reaction was allowed to proceed for 20 min, followed by the addition of 5,5′-dithiobis-2-nitrobenzoate (DNTB) to a final concentration of 200 μM. The absorbance of the product 5-thio-2-nitrobenzoic acid (TNB) was measured at 412 nm using a spectrophotometer (Varian, Cary 100). The molar extinction coefficient for TNB (ε412 = 13 700 M–1 cm–1) was used in calculations to quantify TNB formation.
Cells, Viruses, and Compounds
Vero E6 and Vero CCL81 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Lonza), supplemented with 8% fetal calf serum (FCS; PAA), 2 mM l-glutamine, 100 IU/mL of penicillin and 100 μg/mL of streptomycin, and were grown at 37 °C in a humidified incubator with 5% CO2. Vero cells were maintained in Eagle’s minimum essential medium (EMEM; Lonza), supplemented with 8% FCS (FCS; PAA), 100 IU/mL of penicillin and 100 μg/mL of streptomycin, and were grown at 37 °C in a humidified incubator with 5% CO2. Infections were performed in EMEM with 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Lonza) supplemented with 2% FCS, l-glutamine, and antibiotics. Infectious clone-derived CHIKV(CHIKV-LS3) was generated as described by Scholte et al.31 The ZIKV strain SL0612 was isolated from an infected traveler returning from Suriname as described by van Boheemen et al.32 The Sindbis virus (SINV) strain HR and Semliki forest virus (SFV) strain SFV4 are part of the LUMC virus collection. The MERS-CoV strain EMC/2012 was isolated from patient material in the Dr. Soliman Fakeeh Hospital, Jeddah, Saudi Arabia, and was obtained from Erasmus Medical Center, Rotterdam.33 The SARS-CoV strain Frankfurt 1 was provided by H. F. Rabenau and H. W. Doerr (Johann Wolfgang Goethe-Universität, Frankfurt am Main, Germany).34 The compounds were dissolved in dimethylsulfoxide to obtain 20 mM stock solutions. All work with infectious CHIKV, MERS-CoV, SARS-CoV, and ZIKV was performed inside biosafety cabinets in the BSL-3 facilities of the Leiden University Medical Center.
Antiviral CPE-Reduction Assays
VeroE6 cells were seeded at a density of 5000 cells/well (CHIKV) and 10 000 cells/well (SARS-CoV, SFV and SINV) in a total volume of 100 μL per well in 96-well plates. Vero cells were seeded at a density of 20 000 cells/well when used for MERS-CoV infections, and Vero CCL81 cells were seeded at a density of 5000 cells/well for ZIKA infections under the same conditions as described for Vero E6. The following day, compound dilutions with concentrations of 150, 50, 16.7, and 5.6 μM were prepared in the infection medium by 3-fold serial dilution of the 150 μM solution. After replacing the culture medium with the respective dilutions of the compound, the cells were infected with CHIKV (MOI 0.005), SFV (MOI 0.025), SINV (MOI 0.025), ZIKV (MOI 0.05), MERS-CoV (MOI 0.005), or SARS-CoV (MOI 0.01). Viability assays were conducted in parallel. Each compound was tested at each concentration in quadruplicate (4 biological replicates per concentration). An MTS colorimetric assay was conducted 40 hpi for SFV, 76 hpi for SINV, 72 h hpi for MERS- and SARS-CoV, and 96 hpi for CHIKV and ZIKV by adding 20 μL/well of the CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) reagent (Promega). The assay was stopped after 2–2.5 h by fixing the cells with 37% formaldehyde. The absorbance was measured at 495 nm in a Berthold Mithras LB 940 plate reader, and the values were expressed relative to uninfected (infection) or untreated (viability) samples. The results represent the average of quadruplicate samples expressed as the mean ± SD. Compounds that were found to be protective were further evaluated in CPE reduction assays by testing 8 different concentrations to determine the EC50 as previously described.31,34 The cytotoxicity (CC50) of the compounds was determined in parallel, and all experiments were performed in quadruplicate. Graph-Pad Prism 8.0.1 was used for EC50 and CC50 determination by nonlinear regression.
Viral Load Reduction Assays
VeroE6 (CHIKV, ZIKV) cells were seeded at a density of 7.5 × 104 cells/well in 0.5 mL DMEM/8%FCS in 24-well cell culture plates and allowed to adhere overnight. For MERS-CoV and SARS-CoV, a cell density of or 6.0 × 104 cells/well of Vero E6 and Vero cells was used, respectively, under the same conditions as described above. The next day, compound dilutions (0–1.5 μM) were prepared in EMEM/2%FCS to which virus was added to yield inocula for infecting the cells with an MOI of 0.1 for CHIKV, MOI of 1 for ZIKV, and MOI of 0.01 for SARS- and MERS-CoV. Cells were incubated at 37 °C with 250 μL/well of the inoculum for 1 h (CHIKV and SARS- and MERS-CoV) or 2 h (ZIKV). After the infection, the cells were washed twice with 1 mL/well warm phosphate-buffered saline and 0.5 mL/well fresh EMEM/2%FCS with different concentrations of compound 2c (0–1.5 μM) was added. The cells were incubated for 30 h (CHIKV) or 48 h (ZIKV, SARS- and MERS-CoV) at 37 °C, after which supernatants were harvested and stored at −80 °C for determination of the infectious virus titer by plaque assay. Viability assays were conducted in parallel as described in the previous paragraph. Plaque assays with CHIKV and SARS-CoV on VeroE6 cells, MERS-CoV on Vero cells, and ZIKV on Vero CCL81 cells were performed as described previously.31,34a,35 Compound 2c was tested at each concentration in duplicate in two independent experiments (n = 4). Graph-Pad Prism 8.0.1 was used for statistical analysis with a one-way ANOVA multiple comparison test.
Acknowledgments
This research was supported by grants from Mid-career Research Program (2016R1A2B3010164) and the Ministry of Science, ICT & Future Planning (2017M3A9A8032086) of the National Research Foundation (NRF), Korea. The work in Leiden (N.S.O. and K.K.) was supported by the EU Marie Skłodowska-Curie ETN “ANTIVIRALS” (grant agreement no. 642434).
Glossary
Abbreviations
- RdRp
RNA-dependent RNA polymerase
- SAH
S-adenosyl-l-homocysteine
- SARS-CoV
severe acute respiratory syndrome coronavirus
- CHIKV
chikungunya virus
- ZIKV
Zika virus
- nsps
nonstructural proteins
- MTase
methyltransferase
- NTP
nucleoside triphosphate
- SAM
S-adenosyl-l-methionine
- AK
adenosine kinase
- LiHMDS
lithium hexamethyldisilazide
- TESCl
triethylsilyl chloride
- NFSI
N-fluorobenzenesulfonimide
- NFOBS
N-fluoro-O-benzenedisulfonimide
- NaBH4
sodium borohydride
- LiBH4
lithium borohydride
- NMO
N-methylmorpholine-N-oxide
- TBS
t-butyldimethylsilyl
- TBAF
tetra-n-butylammonium fluoride
- DAST
N,N-diethylaminosulfur trifluoride
- AlMe3
trimethylaluminum
- SOCl2
thionyl chloride
- DIPEA
N,N-diisopropylethylamine
- TFA
trifluoroacetic acid
- Boc2O
di-tert-butyl dicarbonate
- DNTB
5,5′-dithiobis-2-nitrobenzoate
- CPE
cytopathic effect
- TMSOTf
trimethylsilyl trifluoromethanesulfonate
- DMEM
Dulbecco’s modified Eagle’s medium
- FCS
fetal calf serum
- NEAA
non-essential amino acid
- EMEM
Eagle’s minimum essential medium
- SINV
Sindbis virus
- SFV
Semliki forest virus
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.9b00781.
Author Contributions
J.-s.Y. and G.K. contributed equally to this work. All authors have contributed to the manuscript and given approval to the final version of the manuscript.
The authors declare no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
Supplementary Material
References
- a Baltimore D. Expression of animal virus genomes. Bacteriol. Rev. 1971, 35, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Modrow S.; Falke D.; Truyen U.; Schätzl H.. Viruses with Single-Stranded, Positive-Sense RNA Genomes. Molecular Virology; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 185–349. [Google Scholar]
- Thiel V.Coronaviruses: Molecular and Cellular Biology, 1st ed.; Caister Academic Press: Poole, UK, 2007. [Google Scholar]
- Zumla A.; Hui D. S.; Perlman S. Middle East Respiratory Syndrome. Lancet 2015, 386, 995–1007. 10.1016/s0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglioti C.; Lalle E.; Castilletti C.; Carletti F.; Capobianchi M. R.; Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013, 36, 211–227. [PubMed] [Google Scholar]
- a Musso D.; Gubler D. J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524. 10.1128/cmr.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Agumadu V. C.; Ramphul K. Zika Virus: A Review of Literature. Cureus 2018, 10, e3025. 10.7759/cureus.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Forgie S.; Marrie T. Healthcare-Associated Atypical Pneumonia. Semin. Respir. Crit. Care Med. 2009, 30, 067–085. 10.1055/s-0028-1119811. [DOI] [PubMed] [Google Scholar]; b Chan-Yeung M.; Xu R.-H. SARS: epidemiology. Respirology 2003, 8, S9–S14. 10.1046/j.1440-1843.2003.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Hui D. S.; Azhar E. I.; Kim Y.-J.; Memish Z. A.; Oh M.-d.; Zumla A. Middle East Respiratory Syndrome Coronavirus: Risk Factors and Determinants of Primary, Household, and Nosocomial Transmission. Lancet Infect. Dis. 2018, 18, e217–e227. 10.1016/s1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Schwartz O.; Albert M. L. Biology and Pathogenesis of Chikungunya Virus. Nat. Rev. Microbiol. 2010, 8, 491–500. 10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]; b Presti A. L.; Lai A.; Cella E.; Zehender G.; Ciccozzi M. Chikungunya Virus, Epidermiology, Clinics, and Pathogenesis: A Review. Asian Pac. J. Trop. Med. 2014, 7, 925–932. 10.1016/s1995-7645(14)60164-4. [DOI] [PubMed] [Google Scholar]; c Ng L. F. P. Immunopathology of Chikungunya Virus Infection: Lessons Learned from Patients and Animal Models. Ann. Rev. Virol. 2017, 4, 413–427. 10.1146/annurev-virology-101416-041808. [DOI] [PubMed] [Google Scholar]
- Abushouk A. I.; Negida A.; Ahmed H. An Updated Review of Zika Virus. J. Clin. Virol. 2016, 84, 53–58. 10.1016/j.jcv.2016.09.012. [DOI] [PubMed] [Google Scholar]
- a Musso D.; Roche C.; Robin E.; Nhan T.; Teissier A.; Cao-Lormeau V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Oster A. M.; Russell K.; Stryker J. E.; Friedman A.; Kachur R. E.; Petersen E. E.; Jamieson D. J.; Cohn A. C.; Brooks J. T. Update: Interim Guidance for Prevention of Sexual Transmission of Zika Virus. Morb. Mortal.Wkly Rep. 2016, 65, 323–325. 10.15585/mmwr.mm6512e3. [DOI] [PubMed] [Google Scholar]
- Ahlquist P. RNA-Dependent RNA Polymerases, Viruses, and RNA Silencing. Science 2002, 296, 1270–1273. 10.1126/science.1069132. [DOI] [PubMed] [Google Scholar]
- a Coutard B.; Barral K.; Lichière J.; Selisko B.; Martin B.; Aouadi W.; Lombardia M. O.; Debart F.; Vasseur J.-J.; Guillemot J. C.; Canard B.; Decroly E. Zika Virus Methyltransferase: Structure and Functions for Drug Design Perspectives. J. Virol. 2017, 91, e02202–e02216. 10.1128/jvi.02202-16. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Case J. B.; Ashbrook A. W.; Dermody T. S.; Denison M. R. Mutagenesis ofS-Adenosyl-l-Methionine-Binding Residues in Coronavirus nsp14 N7-Methyltransferase Demonstrates Differing Requirements for Genome Translation and Resistance to Innate Immunity. J. Virol. 2016, 90, 7248–7256. 10.1128/jvi.00542-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Decroly E.; Ferron F.; Lescar J.; Canard B. Conventional and Unconventional Mechanisms for Capping Viral mRNA. Nat. Rev. Microbiol. 2011, 10, 51–65. 10.1038/nrmicro2675. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cougot N.; Van Dijk E.; Babajko S.; Séraphin B. Cap-tabolism. Trends Biochem. Sci. 2004, 29, 436–444. 10.1016/j.tibs.2004.06.008. [DOI] [PubMed] [Google Scholar]; c Ferron F.; Decroly E.; Selisko B.; Canard B. The Viral RNA Capping Machinery as a Target for Antiviral Drugs. Antiviral Res. 2012, 96, 21–31. 10.1016/j.antiviral.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Turner M. A.; Yang X.; Yin D.; Kuczera K.; Borchardt R. T.; Howell P. L. Structure and Function of S-Adenosylhomocysteine Hydrolase. Cell Biochem. Biophys. 2000, 33, 101–125. 10.1385/cbb:33:2:101. [DOI] [PubMed] [Google Scholar]; b Cantoni G. L.The Centrality of S-Adenosylhomocysteinase in the Regulation of the Biological Utilization of S-Adenosylmethionine. In Biological Methylation and Drug Design; Borchardt R. T., Creveling C. R., Ueland P. M., Eds.; Humana Press: Clifton, NJ, 1986; pp 227–238. [Google Scholar]
- a Wolfe M. S.; Borchardt R. T. S-Adenosyl-L-Homocysteine Hydrolase as a Target for Antiviral Chemotherapy. J. Med. Chem. 1991, 34, 1521–1530. 10.1021/jm00109a001. [DOI] [PubMed] [Google Scholar]; b De Clercq E. Strategies in the Design of Antiviral Drugs. Nat. Rev. Drug Discovery 2002, 1, 13–25. 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- Jordheim L. P.; Durantel D.; Zoulim F.; Dumontet C. Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases. Nat. Rev. Drug Discovery 2013, 12, 447–464. 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- a Kusaka T.; Yamamoto H.; Shibata M.; Muroi M.; Kishi T.; Mizuno K. Streptomyces Citricolor Nov. Sp. and a New Antibiotic, Aristeromycin. J. Antibiot. 1968, 21, 255–263. 10.7164/antibiotics.21.255. [DOI] [PubMed] [Google Scholar]; b Shealy Y. F.; Clayton J. D. 9-[β-DL-2α,3α-Dihydroxy-4β-(hydroxymethyl)- cyclopentyl]adenine, the Carbocyclic Analog of Adenosine1,2. J. Am. Chem. Soc. 1966, 88, 3885–3887. 10.1021/ja00968a055. [DOI] [Google Scholar]; c Shealy Y. F.; Clayton J. D. Synthesis of Carbocyclic Analogs of Purine Ribonucleosides. J. Am. Chem. Soc. 1969, 91, 3075–3083. 10.1021/ja01039a042. [DOI] [Google Scholar]; d Shealy F. Y.; Thorpe M. C.; Coburn W. C. J. Jr.; Clayton J. D. Identity of the Synthetic Carbocylic Analog of Adenosine and Aristeromycin. Chem. Pharm. Bull. 1980, 28, 3114–3117. 10.1248/cpb.28.3114. [DOI] [Google Scholar]; e Arita M.; Adachi K.; Ito Y.; Sawai H.; Ohno M. Enantioselective synthesis of the carbocyclic nucleosides (-)-aristeromycin and (-)-neplanocin A by a chemicoenzymatic approach. J. Am. Chem. Soc. 1983, 105, 4049–4055. 10.1021/ja00350a050. [DOI] [Google Scholar]; f Yoshikawa M.; Okaichi Y.; Cheon Cha B.; Kitagawa I. Synthesis of (-)-Aristeromycin from D-glucose. Tetrahedron 1990, 46, 7459–7470. 10.1016/s0040-4020(01)89060-8. [DOI] [Google Scholar]; g Wolfe M. S.; Lee Y.; Bartlett W. J.; Borcherding D. R.; Borchardt R. T. 4′-Modified Analogs of Aristeromycin and Neplanocin A: Synthesis and Inhibitory Activity Toward S-Adenosyl-L-Homocysteine Hydrolase. J. Med. Chem. 1992, 35, 1782–1791. 10.1021/jm00088a013. [DOI] [PubMed] [Google Scholar]; h Madhavan G. V. B.; Martin J. C. A Novel and Stereospecific Synthesis of (±)- and (−)-Aristeromycin. J. Org. Chem. 1986, 51, 1287–1293. 10.1021/jo00358a023. [DOI] [Google Scholar]
- a Bennett L. L. Jr.; Allan P. W.; Rose L. M.; Comber R. N.; Secrist J. A. III Differences in the Metabolism and Metabolic Effects of the Carbocyclic Adenosine Analogs, Neplanocin A and Aristeromycin. Mol. Pharmacol. 1986, 29, 383–390. [PubMed] [Google Scholar]; b Bennett L. L.; Bowdon B. J.; Allan P. W.; Rose L. M. Evidence that the Carbocyclic Analog of Adenosine Has Different Mechanisms of Cytotoxicity to Cells with Adenosine Kinase Activity and to Cells Lacking this Enzyme. Biochem. Pharmacol. 1986, 35, 4106–4109. 10.1016/0006-2952(86)90036-5. [DOI] [PubMed] [Google Scholar]
- a Madhavan G. V. B.; McGee D. P. C.; Rydzewski R. M.; Boehme R.; Martin J. C.; Prisbe E. J. Synthesis and Antiviral Evaluation of 6′-Substituted Aristeromycins: Potential Mechanism-Based Inhibitors of S-Adenosylhomocysteine Hydrolase. J. Med. Chem. 1988, 31, 1798–1804. 10.1021/jm00117a021. [DOI] [PubMed] [Google Scholar]; b Verheyden J. P.; Martin J. C.; Madhaven B.; McGee D. P. C.; Prisbe E. J.. Purinyl or Pyrimidinyl Substituted Hydroxycyclopentane Compounds Useful as Antivirals. U.S. Patent 4,605,659, Aug 12, 1986.; c Yin X.-q.; Schneller S. W. Chiral syntheses of 6′-β-fluoroaristeromycin, 6′-β-fluoro-5′-noraristeromycin and aristeromycin. Tetrahedron Lett. 2005, 46, 7535–7538. 10.1016/j.tetlet.2005.08.163. [DOI] [Google Scholar]; d Jeong L. S.; Moon H. R.; Park J. G.; Shin D. H.; Choi W. J.; Lee K. M.; Kim H. O.; Chun M. W.; Kim H.-D.; Kim J. H. Synthesis and Biological Evaluation of Halo-neplanocin A as Novel Mechanism-Based Inhibitors ofS-Adenosylhomocysteine Hydrolase. Nucleotides Nucleic Acids 2003, 22, 589–592. 10.1081/ncn-120021961. [DOI] [PubMed] [Google Scholar]; e Jeong L. S.; Yoo S. J.; Lee K. M.; Koo M. J.; Choi W. J.; Kim H. O.; Moon H. R.; Lee M. Y.; Park J. G.; Lee S. K.; Chun M. W. Design, Synthesis, and Biological Evaluation of Fluoroneplanocin A as the Novel Mechanism-Based Inhibitor ofS-Adenosylhomocysteine Hydrolase. J. Med. Chem. 2003, 46, 201–203. 10.1021/jm025557z. [DOI] [PubMed] [Google Scholar]; f Lee K. M.; Choi W. J.; Lee Y.; Lee H. J.; Zhao L. X.; Lee H. W.; Park J. G.; Kim H. O.; Hwang K. Y.; Heo Y.-S.; Choi S.; Jeong L. S. X-ray Crystal Structure and Binding Mode Analysis of HumanS-Adenosylhomocysteine Hydrolase Complexed with Novel Mechanism-Based Inhibitors, Haloneplanocin A Analogues. J. Med. Chem. 2011, 54, 930–938. 10.1021/jm1010836. [DOI] [PubMed] [Google Scholar]; g Chandra G.; Moon Y. W.; Lee Y.; Jang J. Y.; Song J.; Nayak A.; Oh K.; Mulamoottil V. A.; Sahu P. K.; Kim G.; Chang T.-S.; Noh M.; Lee S. K.; Choi S.; Jeong L. S. Structure-Activity Relationships of Neplanocin A Analogues as S-Adenosylhomocysteine Hydrolase Inhibitors and Their Antiviral and Antitumor Activities. J. Med. Chem. 2015, 58, 5108–5120. 10.1021/acs.jmedchem.5b00553. [DOI] [PubMed] [Google Scholar]
- Kim G.; Yoon J.-S.; Jarhad D. B.; Shin Y. S.; Majik M. S.; Mulamoottil V. A.; Hou X.; Qu S.; Park J.; Baik M.-H.; Jeong L. S. Asymmetric Synthesis of (−)-6′-β-Fluoro-aristeromycin via Stereoselective Electrophilic Fluorination. Org. Lett. 2017, 19, 5732–5735. 10.1021/acs.orglett.7b02470. [DOI] [PubMed] [Google Scholar]
- a Siddiqui A. Q.; Ballatore C.; McGuigan C.; De Clercq E.; Balzarini J.; De Clercq E. The Presence of Substituents on the Aryl Moiety of the Aryl Phosphoramidate Derivative of d4T Enhances Anti-HIV Efficacy in Cell Culture: A Structure–Activity Relationship. J. Med. Chem. 1999, 42, 393–399. 10.1021/jm9803931. [DOI] [PubMed] [Google Scholar]; b Mehellou Y.; Rattan H. S.; Balzarini J. The ProTide Prodrug Technology: From the Concept to the Clinic. J. Med. Chem. 2018, 61, 2211–2226. 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mehellou Y.; Balzarini J.; McGuigan C. Aryloxy Phosphoramidate Triesters: A Technology for Delivering Monophosphorylated Nucleosides and Sugars into Cells. Chem. Med. Chem. 2009, 4, 1779–1791. 10.1002/cmdc.200900289. [DOI] [PubMed] [Google Scholar]; d McGuigan C.; Pathirana R. N.; Balzarini J.; De Clercq E. Intracellular Delivery of Bioactive AZT Nucleotides by Aryl Phosphate Derivatives of AZT. J. Med. Chem. 1993, 36, 1048–1052. 10.1021/jm00060a013. [DOI] [PubMed] [Google Scholar]; e McGuigan C.; Harris S. A.; Daluge S. M.; Gudmundsson K. S.; McLean E. W.; Burnette T. C.; Marr H.; Hazen R.; Condreay L. D.; Johnson L.; De Clercq E.; Balzarini J. Application of Phosphoramidate Pronucleotide Technology to Abacavir Leads to a Significant Enhancement of Antiviral Potency. J. Med. Chem. 2005, 48, 3504–3515. 10.1021/jm0491400. [DOI] [PubMed] [Google Scholar]; f McGuigan C.; Hassan-Abdallah A.; Srinivasan S.; Wang Y.; Siddiqui A.; Daluge S. M.; Gudmundsson K. S.; Zhou H.; McLean E. W.; Peckham J. P.; Burnette T. C.; Marr H.; Hazen R.; Condreay L. D.; Johnson L.; Balzarini J. Application of Phosphoramidate ProTide Technology Significantly Improves Antiviral Potency of Carbocyclic Adenosine Derivatives. J. Med. Chem. 2006, 49, 7215–7226. 10.1021/jm060776w. [DOI] [PubMed] [Google Scholar]; g Sofia M. J.; Bao D.; Chang W.; Du J.; Nagarathnam D.; Rachakonda S.; Reddy P. G.; Ross B. S.; Wang P.; Zhang H.-R.; Bansal S.; Espiritu C.; Keilman M.; Lam A. M.; Steuer H. M. M.; Niu C.; Otto M. J.; Furman P. A. Discovery of a β-d-2′-Deoxy-2′-α-fluoro-2′-β-C-methyluridine Nucleotide Prodrug (PSI-7977) for the Treatment of Hepatitis C Virus. J. Med. Chem. 2010, 53, 7202–7218. 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]; h Slusarczyk M.; Lopez M. H.; Balzarini J.; Mason M.; Jiang W. G.; Blagden S.; Thompson E.; Ghazaly E.; McGuigan C. Application of ProTide Technology to Gemcitabine: A Successful Approach to Overcome the Key Cancer Resistance Mechanisms Leads to a New Agent (NUC-1031) in Clinical Development. J. Med. Chem. 2014, 57, 1531–1542. 10.1021/jm401853a. [DOI] [PubMed] [Google Scholar]
- a Choi W. J.; Park J. G.; Yoo S. J.; Kim H. O.; Moon H. R.; Chun M. W.; Jung Y. H.; Jeong L. S. Syntheses ofd- andl-Cyclopentenone Derivatives Using Ring-Closing Metathesis: Versatile Intermediates for the Synthesis ofd- andl-Carbocyclic Nucleosides. J. Org. Chem. 2001, 66, 6490–6494. 10.1021/jo015733w. [DOI] [PubMed] [Google Scholar]; b Moon H. R.; Choi W. J.; Kim H. O.; Jeong L. S. Improved and Alternative Synthesis of d- and l-Cyclopentenone Derivatives, the Versatile Intermediates for the Synthesis of Carbocyclic Nucleosides. Tetrahedron:Asymmetry 2002, 13, 1189–1193. 10.1016/s0957-4166(02)00316-6. [DOI] [Google Scholar]; c Mulamoottil V. A.; Nayak A.; Jeong L. S. Recent Advances in the Synthesis of Carbocyclic Nucleosides via Ring-Closing Metathesis. Asian J. Org. Chem. 2014, 3, 748–761. 10.1002/ajoc.201402032. [DOI] [Google Scholar]
- a Gilman H.; Jones R. G.; Woods L. A. The Preparation of Methylcopper and Some Observations on the Decomposition of Organocopper Compounds. J. Org. Chem. 1952, 17, 1630–1634. 10.1021/jo50012a009. [DOI] [Google Scholar]; b Song G. Y.; Paul V.; Choo H.; Morrey J.; Sidwell R. W.; Schinazi R. F.; Chu C. K. Enantiomeric Synthesis ofd- andl-Cyclopentenyl Nucleosides and Their Antiviral Activity Against HIV and West Nile Virus. J. Med. Chem. 2001, 44, 3985–3993. 10.1021/jm010256v. [DOI] [PubMed] [Google Scholar]
- Rubottom G. M.; Gruber J. M.; Boeckman R. K. Jr; Ramaiah M.; Medwid J. B. Clarification of the Mechanism of Rearrangement of Enol Silyl Ether Epoxides. Tetrahedron Lett. 1978, 19, 4603–4606. 10.1016/s0040-4039(01)85682-3. [DOI] [Google Scholar]
- Montgomery J. A.; Temple C. Jr. Synthesis of Potential Anticancer Agents. IX. 9-Ethyl-6-substituted-purines2. J. Am. Chem. Soc. 1957, 79, 5238–5242. 10.1021/ja01576a046. [DOI] [Google Scholar]
- The X-ray CIF File for 2c Has Been Deposited at the Cambridge Crystallographic Data Centre (Deposition Number: CCDC 1914672).
- a Shaw G.; Warrener R. N. Purines, pyrimidines, and glyoxalines. Part VIII. New syntheses of uracils and thymines. J. Chem. Soc. 1958, 157–159. 10.1039/jr9580000157. [DOI] [Google Scholar]; b Jeong L. S.; Buenger G.; McCormack J. J.; Cooney D. A.; Hao Z.; Marquez V. E. Carbocyclic Analogues of the Potent Cytidine Deaminase Inhibitor 1-(β-d-Ribofuranosyl)-1,2-dihydropyrimidin-2-one (Zebularine). J. Med. Chem. 1998, 41, 2572–2578. 10.1021/jm980111x. [DOI] [PubMed] [Google Scholar]
- The X-ray CIF Files for 2g and 2h Have Been Deposited at the Cambridge Crystallographic Data Centre (Deposition Numbers: CCDC 1871331 for 2g and CCDC 1871332 for 2h).
- Divakar K. J.; Reese C. B. 4-(1,2,4-Triazol-1-yl)- and 4-(3-nitro-1,2,4-triazol-1-yl)-1-(β-D-2,3,5-tri-O-acetylarabinofuranosyl)pyrimidin-2(1H)-ones. Valuable Intermediates in the Synthesis of Derivatives of 1-β-D-Arabinofuranosyl)cytosine (Ara-C). J. Chem. Soc., Perkin Trans. 1 1982, 1171–1176. 10.1039/p19820001171. [DOI] [Google Scholar]
- Ross B. S.; Ganapati Reddy P.; Zhang H.-R.; Rachakonda S.; Sofia M. J. Synthesis of Diastereomerically Pure Nucleotide Phosphoramidates. J. Org. Chem. 2011, 76, 8311–8319. 10.1021/jo201492m. [DOI] [PubMed] [Google Scholar]
- Lozada-Ramírez J. D.; Martínez-Martínez I.; Sánchez-Ferrer A.; García-Carmona F. A Colorimetric Assay for S-Adenosylhomocysteine Hydrolase. J. Biochem. Biophys. Methods 2006, 67, 131–140. 10.1016/j.jbbm.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Scholte F. E. M.; Tas A.; Martina B. E. E.; Cordioli P.; Narayanan K.; Makino S.; Snijder E. J.; van Hemert M. J. Characterization of Synthetic Chikungunya Viruses Based on the Consensus Sequence of Recent E1-226V Isolates. PLoS One 2013, 8, e71047. 10.1371/journal.pone.0071047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boheemen S.; Tas A.; Anvar S. Y.; van Grootveld R.; Albulescu I. C.; Bauer M. P.; Feltkamp M. C.; Bredenbeek P. J.; van Hemert M. J. Quasispecies Composition and Evolution of a Typical Zika Virus Clinical Isolate from Suriname. Sci. Rep. 2017, 7, 2368. 10.1038/s41598-017-02652-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boheemen S.; de Graaf M.; Lauber C.; Bestebroer T. M.; Raj V. S.; Zaki A. M.; Osterhaus A. D. M. E.; Haagmans B. L.; Gorbalenya A. E.; Snijder E. J.; Fouchier R. A. M. Genomic Characterization of a Newly Discovered Coronavirus Associated with Acute Respiratory Distress Syndrome in Humans. mBio 2012, 3, e00473-12. 10.1128/mbio.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Albulescu I. C.; Kovacikova K.; Tas A.; Snijder E. J.; van Hemert M. J. Suramin Inhibits Zika Virus Replication by Interfering with Virus Attachment and Release of Infectious Particles. Antiviral Res. 2017, 143, 230–236. 10.1016/j.antiviral.2017.04.016. [DOI] [PubMed] [Google Scholar]; b de Wilde A. H.; Jochmans D.; Posthuma C. C.; Zevenhoven-Dobbe J. C.; van Nieuwkoop S.; Bestebroer T. M.; van den Hoogen B. G.; Neyts J.; Snijder E. J. Screening of an FDA-Approved Compound Library Identifies Four Small-Molecule Inhibitors of Middle East Respiratory Syndrome Coronavirus Replication in Cell Culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. 10.1128/aac.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Worm S. H. E.; Eriksson K. K.; Zevenhoven J. C.; Weber F.; Züst R.; Kuri T.; Dijkman R.; Chang G.; Siddell S. G.; Snijder E. J.; Thiel V.; Davidson A. D. Reverse Genetics of SARS-Related Coronavirus Using Vaccinia Virus-Based Recombination. PLoS One 2012, 7, e32857. 10.1371/journal.pone.0032857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.