Abstract
Heterogeneity in symptom presentation, outcomes, and treatment response has long been a thorn in the side of researchers aiming to identify biological markers of schizophrenia or psychosis. However, there is increasing recognition that there may likely be no such general illness markers, consistent with the notion of a group of schizophrenia(s) that may have both shared and unique neurobiological pathways. Instead, strategies aiming to capitalize or leverage such heterogeneity may help uncover neurobiological pathways that can then be used to stratify groups of patients for prognostic purposes, or for therapeutic trials. A shift toward larger sample sizes with adequate statistical power to overcome small effect sizes and disentangle the shared variance among different brain imaging or behavioral variables has become a priority for the field. In addition, recognition that two individuals with the same clinical diagnosis may be more different from each other (at brain, genetic, behavioral-levels) than another in a different disorder or non-clinical control group, coupled with computational advances, has catapulted data-driven efforts forward. Emerging challenges for this new approach include longitudinal stability of new subgroups, demonstration of validity, and replicability. The ‘litmus test’ will be whether computational approaches that are successfully identifying groups of patients who share features in common more than current DSM diagnostic constructs, also provide better prognostic accuracy over time, and in addition lead to enhancements in treatment response and outcomes. These are the factors that matter most to patients, families, providers, and payers.
Keywords: Schizophrenia, Psychosis, Neuroimaging, Heterogeneity, Computation, Data-Driven, Multivariate, Brain-Behavior
Introduction
Schizophrenia and psychosis represent a disorder and syndrome respectively, in which heterogeneity is the rule rather than the exception. There is substantial overlap in clinical presentation with other illnesses, such as bipolar disorder or autism spectrum disorder, and among people with a diagnosis of schizophrenia, substantial heterogeneity in symptom presentation and functioning is present. Some people recover fully, while others remain institutionalized (1–3).
Such heterogeneity was typically ignored in early neuroimaging studies. These studies often used small sample sizes and a single brain measure (e.g. volume, or functional activation of a candidate region) to compare people with schizophrenia to people designated as ‘healthy’ or ‘normal’ controls (4). Recent analyses by the ENIGMA consortium have largely confirmed widespread volumetric reductions (and more recently cortical thickness reductions) in people with schizophrenia compared to people who are controls (5, 6). However, rather than large effect sizes (required for a significant result in studies with small sample sizes), smaller ones are present. Therefore, some patients with schizophrenia will have certain changes in brain structure or function, but many others will not -- and thus are no different on hypothesized biological measures -- compared to controls.
One of the most prominent post DSM-III attempts to dissect heterogeneity was through the definition of Type I and Type II schizophrenia (7). Similarly the conceptualization of ‘deficit/non-deficit’ schizophrenia was intended to capture a more clinically homogeneous subgroup of patients (deficit) with prominent negative symptoms and sustained poor functional outcomes to enhance biological discovery (8). Research studies using these categorical definitions provided some important insight into the neurobiology and neural circuitry of people with psychosis or schizophrenia who were more impaired, or clinically different than their counterparts with the same diagnosis. However, they ultimately suffer some of the same limitations as those using the current definition of schizophrenia in their reliance on group designation defined solely through behavioral criteria.
Conceptual and methodological advances are accelerating our ability to disentangle heterogeneity with a view toward prognostic and therapeutic impact. Among these, which will be the focus of this review, include the Research Domain Criteria (RDoC) framework supporting dimensional brain-behavior investigations; the emerging understanding and acceptance of antecedent childhood disorders as risk factors for psychosis and schizophrenia; clinical and neurobiological differences based on biological sex; and data-driven approaches that can combine and integrate information to redefine groups of patients beyond clinical criteria; and finally, how new definitions of patient subgroups can be used in the design of treatment studies. Implicit in all of the recent conceptual progress is a comfort and even embrace of the notion of heterogeneity, the use of large sample sizes, computational methods, and a greater acceptance of ‘following the data’.
Sources of Neurobiological Heterogeneity in Psychosis
Neurobiological Heterogeneity Related to Phenomenology, Cognition, Treatment Response, and other Clinical Factors
Among people with a diagnosis of schizophrenia, greater impairments in brain structure, demonstrated through some combination of lateral ventricle increases, smaller gray matter volumes, or impaired white matter microstructure appear to align with greater negative symptom burden and poorer outcome (9, 10). Network-based analyses of brain structure has also related right fronto-parietal and fronto-temporal circuitry to such clinical features (11). In contrast, in relation to cognitive impairments, patterns of ‘brain-wide’ impairment may be present (12, 13). Overall, replicability of structural brain-behavior associations has recently been seriously questioned (14). Part of the challenge may relate to the historical use of regional analyses, rather than structural covariance (15–17), but mainly small sample sizes, and studying a highly heterogeneous single-disorder group may be among the most important study design flaws. Functional magnetic resonance imaging (fMRI) studies have more recently moved to larger sample sizes. One of the largest studies to date showed that resting state functional networks were associated with cognitive performance (18); however, these associations were independent of diagnostic category; similarly, such associations have been shown with white matter microstructure (19) and gray matter microstructure (20), which may be a structural correlate of resting state functional networks (21). Aberrant frontostriatal connectivity has been associated with negative symptoms (22). Data from clinical high risk (CHR) patients shows an intrinsic “trait-like” abnormality in cerebello-thalamo-cortical circuitry that is associated with symptoms of disorganization, a pattern reliably detected in patients with schizophrenia (23). Fronto-striatal functional connectivity may be an important marker of treatment response to antipsychotic medications and change in psychosis symptoms, and thus in effect another source of heterogeneity (24). Comparison of clozapine responders vs. nonresponders suggests potential differences in white matter structure and glutamatergic magnetic resonance spectroscopy-related measures (25); however, while such treatment-refractory samples are admittedly very difficult to collect, the fact remains that sample sizes are small, and the findings have yet to be replicated. Larger sample sizes provide an opportunity to use multivariate approaches to determine relationships among brain and behavioral variables. In one study (26) substance use was associated with white matter integrity, negative symptoms with subcortical volume, and positive symptoms with cortical thickness. Many patients with schizophrenia are current or past substance users, and confounds related to alcohol, smoking, cannabis, cocaine and other substances are essential considerations given their own associations with different structural and functional brain features. The recent legalization of cannabis in a number of jurisdictions may lead to an increasing number of potential research participants with substance use histories, which would only increase the need to better understand its impact on brain structure and function.
Genetic variation has also been studied to attempt to dissect heterogeneity. For instance, genome-wide significant variants have shown associations with brain structure (27) and function (28). Also association has been shown with both age at onset and brain structure, where a risk variant was tied to earlier age at onset, smaller hippocampal volumes, larger ventricles, and lower white matter fractional anisotropy (FA) in people with schizophrenia (29). Others have explored the relationship between polygenic risk score and brain structure, function, or both. However, studies using an expression quantitative trait loci (eQTL) style approach to identify loci associated with structural or functional properties of brain regions or circuits, do not demonstrate that ‘top-hit’ variants associated with schizophrenia are the same as those with brain structure (30).
Neurobiological Heterogeneity Related to Sex
Sexual dimorphism in schizophrenia has been found in age of onset, premorbid deficits, symptom severity, cognitive functioning, course of illness, and treatment response (31). However, schizophrenia studies often include disproportionately fewer females. Findings from neuroanatomical studies have had mixed results, but men with schizophrenia tend to have more pronounced abnormalities including larger ventricles, smaller prefrontal and temporal cortical volumes, and greater asymmetry than women with schizophrenia (32). A large meta-analysis (33) found decreases in intracranial volumes were significantly more prominent in males, while findings from ENIGMA suggest that decreases in the accumbens and amygdala are more prominent (34). A recent review of diffusion tensor imaging-based white matter metrics in schizophrenia found that most studies showed similar patterns between sexes of decreases in FA in females, although not significant. A meta-analysis of the genu and splenium of the corpus callosum found significant differences between patient and controls in the genu, with greater effect sizes for females in both regions (35).
There is evidence that sex differences in schizophrenia may be related to estrogen, providing females with a neuroprotective effect tied to a later age of onset and reduced illness severity (36). Protective effects fluctuate with estrogen levels across the menstrual cycle and lifespan, and hormone modulating therapies have shown promise for symptom amelioration for women with schizophrenia (37). This protective effect in females may suggest a greater biological ‘hit’ is required to develop psychotic illness, as shown by increased white matter connectivity deficits and evidence that women have a higher genetic mutational burden than men (38).
Even less is known about sex differences in earlier stages of psychosis such as in high-risk groups, particularly during adolescence when brain change is highly dynamic. Premorbid adjustment and deterioration has been found to differ across development and between sexes (38). Males with CHR may have an increased risk of conversion, increased negative symptoms, and poorer functioning compared to increased affective symptoms in females (39). Studies have also found structural and functional brain abnormalities differ between the sexes at these earlier stages of psychosis risk, including smaller hippocampal volumes in males compared to females with CHR (40). Others have also shown increased anatomical abnormalities in males with CHR in total brain volume (41). Volume differences between CHR youth and controls were in opposing directions for sexes in cortical thickness. Opposing volume differences between sexes in youth with psychosis spectrum symptoms (PSS) from the Philadelphia Neurodevelopmental Cohort (PNC) (42) sample have also been observed in striatal and thalamic subdivision volumes, as well as differences in striato-cortical and thalamo-cortical connectivity (43).
More studies on earlier stages of psychosis and potential sex-dependencies of neurodevelopmental pathways for psychosis are needed. Recent large initiatives such as the PNC, Adolescent Brain Cognitive Development study (44) (https://abcdstudy.org/), and Healthy Brain Network (45) provide this opportunity. Datasets collected by the ENIGMA consortium also provide greater statistical power to detect diagnosis by sex interactions or differences in effect sizes.
Neurobiological Heterogeneity Related to Presence of Comorbid Neurodevelopmental Disorders or Dimensional Symptoms
Tracking atypical behavioral and brain development prior to the time of onset of secondary psychosis or schizophrenia spectrum disorder is an important, underutilized strategy to dissect heterogeneity across the illness spectrum, during the highest-risk time point for emerging psychotic illness, i.e. adolescence. With a new understanding of dimensional psychosis risk thanks in large part to the PNC study, coupled with a new understanding of antecedent childhood disorders as potential psychosis risk factors, an entirely new approach to early identification related to psychosis risk profiles is possible. At the population level, the prevalence of psychotic symptoms is 17% among children aged 9–12 years, and 7.5% among adolescents aged 13–18 years (46). The Dunedin Birth Cohort study demonstrated that a single psychotic symptom in children 11 years or younger was associated with adult diagnoses of schizophrenia, posttraumatic stress disorder, and suicide attempts, after controlling for gender, social class and childhood psychopathology (47). The PNC study of >7000 children and youth ranging from 8–21 years old demonstrated that nearly 20% of all youth in this community sample met criteria for threshold (delusions/hallucinations) or subthreshold (psychotic-like experiences) psychosis spectrum symptoms. Their PSS designation was linked to greater suicidal ideation, poorer neurocognitive performance, and poorer functioning (42). The presence of certain diagnosed childhood psychiatric disorders, in particular disruptive behavior disorders (DBDs: attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder), more than doubled the likelihood of a psychosis spectrum designation. Consistent with the PNC study, the ALSPAC (Avon, U.K.) birth cohort showed that any childhood disorder at age 8 predicted psychotic symptoms at age 13. In this study, the association was most notable in those with autism spectrum disorders (ASD) (48). A number of additional studies indicate a strong relationship between autistic spectrum and schizophrenia spectrum disorders (49). A Scandinavian registry study demonstrated that the presence of an ASD without intellectual disability was associated with a 5.6 fold increased risk for a nonaffective psychotic disorder (50). Higher co-occurrence of these disorders may stem from shared genetic risk, given that polygenic risk architecture in ASD features important overlap with that for schizophrenia (51). Interestingly risk overlap between ASD and schizophrenia (stemming from common genetic risk variants) may be particularly prominent in individuals with ASD that do not have intellectual disability (51).
Despite these data, few neuroimaging studies examining correlates of psychosis or schizophrenia account for childhood psychiatric disorders. A number of open questions remain, such as: what is the effect of different childhood disorders in conjunction with psychosis or schizophrenia on brain structure or function? How does complex premorbid psychopathology impact prognosis, potential illness trajectory, treatment response, and shape development of brain circuitry that might predict response? Even dimensional symptom impairment that may not reach diagnostic threshold could impact brain circuit structure or function. Therefore, one potential approach, given the high degree of comorbidity across childhood psychiatric disorders may be to focus more on dimensional brain-behavior relationships in youth (52) across the developmental stage where manifestations of illness symptoms most likely occur. Larger ‘n’ cross-sectional data are now emerging that demonstrate successful mapping of brain-behavioral relationships related to dimensions of psychopathology in adolescents (53, 54), but risk mechanisms for psychosis in longitudinal studies of children with psychopathology (other than in CHR cases, which account for a small minority of incident psychosis (55)) has yet to be addressed.
Longitudinal studies are necessary to understand how brain circuits implicated in ASD or other neurodevelopmental disorders such as ADHD create risk for psychosis, and how such circuit function interacts with environmental risk factors such as trauma or cannabis use. This longitudinal/developmental research approach may also yield identification of affected individuals presenting with secondary psychosis that feature qualitatively different genetic architecture and neurodevelopmental risk pathways than those exhibiting first illness in early adulthood, offering new opportunities to further parse heterogeneity.
Approaches to Resolving Neurobiological Heterogeneity in Psychosis: Multivariate and Data-Driven Techniques
Disentangling Shared Variance Through Conventional Multivariate Approaches for Identifying Brain-Behavior Relationships
Early studies typically used univariate modeling, e.g. Pearson correlations, aiming to relate the volume or function of a brain region with a specific type of psychopathology, or cognitive task. Many behavioral and neurocognitive domains are correlated, e.g. negative symptoms and social cognition, or neurocognition and social cognition. Conventional univariate strategies often employed do not permit an understanding of shared and unique variance that might contribute to the findings. Dozens of studies have related a structural variable (e.g. hippocampal volume) to negative symptom burden or neurocognitive performance, but often do not build a model addressing shared variance among these variables. Approaches such as structural equation modeling (SEM), canonical correlation, and partial least squares (PLS) can allow for incorporation of multimodal brain and behavior data acquired. While each approach has its advantages and limitations, they represent an important step forward in statistical modeling, and can illuminate relationships within correlated brain imaging variables or within correlated behavioral variables. SEM is largely hypothesis driven, while canonical correlation and PLS can be used as data-driven approaches. PLS has built-in reliability or bootstrapping assessments that provide some insight into the stability of the ascertained relationships (56, 57). In schizophrenia and psychosis research, these approaches are very important because different brain structural and behavioral measures are often correlated with each other (3). A limitation of canonical correlation or PLS is that these methods do not provide information regarding brain-behavior relationships among subsets of patients, unless those subsets are explicitly defined a priori. In addition, the need to leverage the statistical power of an entire dataset can make it challenging to explore multivariate relationships in a subset of patients. There is also no guarantee that the brain-behavior relationships in a ‘tipping-point’ subsample (e.g. the bottom quartile of functioning) might be the same as that across an entire sample. Given the increasing evidence of overlap across different patient groups and non-clinical controls on certain measures, the future of data-driven analyses is emerging as one that will need to test relationships among variables (and their potential prognostic and therapeutic ‘value’) in new groups of patients based on brain-behavior relationships.
Data-Driven Computational Approaches Toward Resolving Heterogeneity
Data-driven, computational approaches can be used to construct models of psychosis based on a combination of biological, clinical, and cognitive variables, independent from predefined clinical categories. New technologies, analytical approaches, and research initiatives that aim to sample both broadly (e.g large sample sizes) and deeply with multi-level phenotyping, have enabled this type of investigation of mental illnesses at increasingly larger scales and in multiple dimensions.
Multi-scale integration of data from the molecular to behavioral level can be used to capture multi-level profiles of individuals, rather than using clinical data alone (58). However, different methods used to reduce dimensionality of data and derive data-driven groupings pose their own set of advantages and challenges. Additionally, the emergence of large samples and meta-analyses can introduce forms of technical heterogeneity that need to be considered such as variation in scanners, study designs, data collected, participant inclusion criteria, and recruitment location.
Large consortia such as B-SNIP that include multimodal data across participants with schizophrenia, schizoaffective and bipolar disorder provide an optimal opportunity to study psychosis along a spectrum using data-driven techniques (59). In the B-SNIP sample, principal component analysis was used to reduce dimensionality of data types and a k-means algorithm was applied to integrate performance data from cognitive tasks and EEG variables to identify three novel subgroups with distinct clinical and neurobiological profiles that replicated in the relatives of participants. Another study identified biotypes of individuals with schizophrenia spectrum disorders that are normal (i.e. equivalent to controls) versus poor social cognitive and neurocognitive performers, by first using a canonical correlation analysis to find regions of fMRI brain connectivity related to cognitive scores. Then a support vector machine classifier based on neuroimaging data was used to predict cognitive scores with high accuracy in unseen data from other participants (60). A common challenge of machine learning methods is that they are often a ‘black box’ when interpreting how output relates to model input and what the importance of features contributing to classification decisions are. Additionally, reducing data dimensions and narrowing down chosen measures can lead to lost information or unintentionally bias results.
Recently, a relatively new approach known as Similarity Network Fusion (SNF) (61) was used to integrate data and identify social cognitive subgroups across schizophrenia spectrum disorders, autism spectrum disorder, and bipolar disorder using demographic, behavioral and different structural neuroimaging measures (62). SNF creates participant similarity matrices for each data type before iteratively fusing them so that participants can be clustered based on a spectrum of multivariate data. Identification of top contributing features to the similarity model is conducted using normalized mutual information between features and the fused similarity matrix. Additionally, this method handles noise and large amounts of measures well, reducing the number of a priori decisions needed.
These studies capture different aspects of heterogeneity within psychotic disorders, but also across disorders (i.e. bipolar and autism spectrum), to provide insight into brain circuitry that may be vulnerable in different patient groups with the same diagnosis, but also across patient groups. It is likely, however, that a continuum across newly identified groups is present. For instance, identification of subgroups based on fMRI task data using hierarchical clustering, support modelling social brain function in psychosis on a spectrum according to specific behavioral domains (neurocognitive and social cognitive performance) (63). Integration of cognitive and anatomical structural data from participants in the B-SNIP sample also identified a continuous set of relationships from affective to non-affective psychosis (64).
In terms of risk for psychosis, machine learning techniques have successfully been used with structural MRI data to predict longitudinal functional and clinical outcomes of CHR youth (65, 66). A combination of neuroimaging and clinical data were shown to be superior to clinical data alone in terms of prognosticating functional outcomes (67). These findings emphasize the importance of a multivariate approach to understanding, treating and identifying outcomes related to psychosis as a way to navigate heterogeneity.
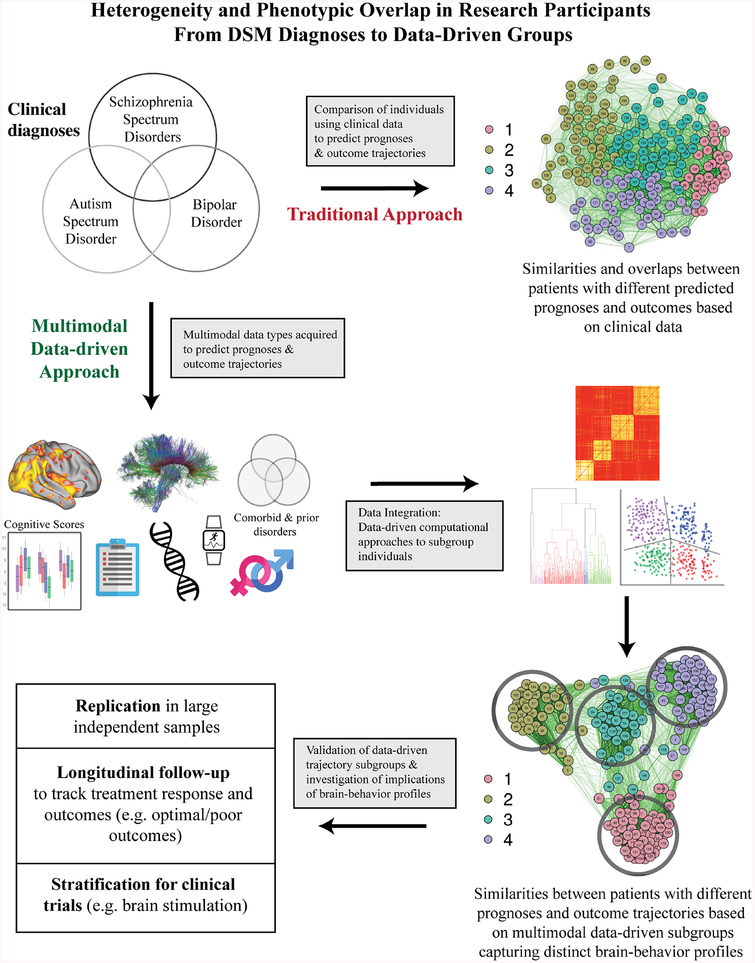
Validation of newly identified subgroups will depend on whether methods to parse heterogeneity within one domain are applicable across other relevant domains. For example, a ‘bottom-up’ approach as recently outlined (68) could be used to examine whether subgroups featuring distinct markers at one level (e.g., at the circuit-level) feature relevant distinctions at other levels (e.g., academic achievement). Replication is also critical, as one recent high profile study (69) failed to replicate (70). Overall, data-driven clustering approaches are promising for increasing our understanding of underlying mechanisms and biomarkers of psychosis (Figure 1), however, as clustering procedures will always produce clusters (even when data included is continuous), general caution for the field is needed in interpreting results. The next phase of discovery will need to minimize the potential to oversample from a specific subgroup that is not representative of the broader population. Measurement at different time points of progression of disease may contribute to inconsistent results across studies, further emphasizing the need to characterize how vulnerable circuits change over time, and their relationship with evolution of symptomatology and functioning. Sample size needs could potentially be reduced in subsequent studies targeting mechanistic underpinnings identified within a particular subgroup, where effect sizes for features differentiating a particular subgroup from others are large.
Figure 1.
Schematic comparing a more traditional approach with a multimodal data-driven approach reconsidering and capitalizing on heterogeneity in psychosis toward clinical impact. Such an approach as the latter can take into account multi-level phenotypic and biological data, then use data-driven techniques to reconfigure diagnostic groups into ones where individuals are similar to each other based on information within and between levels of data. These new groups must then be tested for longitudinal stability, reliability, and validity. Ultimately, whether these groups are more closely linked to prognostic and therapeutic outcomes compared to current DSM groups will determine their utility.
Design of Treatment Trials and Therapeutic Innovation
The data-driven clustering methods just discussed have shown that using multimodal data individuals can be sorted into new groups that feature more similar brain-behavior profiles than those with the same DSM diagnosis (62). The longitudinal utility of identified subgroups could be tracked through continual measurement over time or tracking downstream outcomes through medical records/events (e.g., later emergency room visits, need for repeated hospitalizations or different levels of care intensity). Identified subgroups can also facilitate treatment innovation by providing the opportunity for stratification in the context of clinical trials (71). A specific marker that differentiates a given subgroup from others at the biological level (and may be linked to an important clinical outcome) could be targeted to modulate circuitry and improve associated behaviors within participant subgroups (either using existing or novel approaches). Targeting high-impact biomarkers within more homogeneous participant subgroups could yield larger treatment effects than would be found in the broader population, where underlying biology contributes to attenuation of effect sizes. The translational impact of clustering individuals with psychiatric illnesses into more homogeneous data-driven subgroups will ultimately depend on clinical utility, including: stability and reliability of identified subgroups across different samples, predictive utility for different outcomes over time, and ultimately better treatment response or remission rates with existing or novel treatments compared to current rates based on DSM diagnosis.
The increasing interest and rapid progress in brain imaging analytic techniques related to individual variability in brain structure and function may also impact design of trials aimed at testing existing and novel therapeutics. The potential ability to ‘fingerprint’ an individual via their functional connectivity profile (72), along with new methods that take into account variability among individuals, moving away from ‘group’-based templates to accurately map functional organization at the individual level are important sources of progress (73). This progress is also making a ‘dent’ in our understanding of heterogeneity, whereby some of the findings based on group-based templates that may have characterized a diagnostic group, as recently shown in autism spectrum disorders, are no longer present (or reduced) after adjusting or optimizing for functional connectivity due to individual variability (74). These findings also have important implications for therapeutics in the brain stimulation armamentarium that can target brain regions. Such targeting can be optimized at the individual level based on any one person’s structural and functional connectivity profile (75). The natural assumption is that such approaches may enhance the likelihood of treatment response.
Conclusion
The timing of onset of psychosis or schizophrenia corresponds with the final years of adolescent development into early adulthood; a life-stage associated with higher educational attainment, formation of enduring relationships, and first employment. A negative transition to adulthood at this critical stage places an individual at risk for poor subsequent functioning (76). The brain is still quite plastic and dynamic during this crucial time. It is possible that an effective intervention during this high-risk period could alter illness trajectory (77). Given the substantial heterogeneity inherent to psychosis, overlap with other neurodevelopmental disorders (78), but also with unaffected controls (79), it is unlikely that a consistent neural signature of any particular domain of impairment is present across affected individuals. Nor is it probable that a ‘one size fits all’ treatment will have efficacy for all affected. Dimensional and data-driven approaches may help turn the challenge of heterogeneity into an opportunity (80). Reorienting investigations away from clinically based categories towards transdiagnostic domains of functioning with strong evidence of associated biological systems can advance research towards impactful understanding and treatments that have eluded us thus far (80).
Will prognostic accuracy in newly identified data-driven groups be superior what can be achieved using DSM definitions? Similarly, are such data-driven groups more likely to respond to select therapeutics than DSM defined groups? And how will decisions be made in terms of which therapeutics to test for which subgroups? These are all crucial questions that need to be answered. The future is bright, but circumspect decisions should be made by funding bodies and collaborative research groups regarding study design, scientific objectives, and deliverables. Clinicians, families, and patients continue to ask when neurobiological research will make an impact for people suffering from schizophrenia or psychosis.
Acknowledgements/Disclosures:
ANV currently receives funding from the National Institute of Mental Health (1/3R01MH102324 & 1/5R01MH114970), the former of which directly supports this work, Canadian Institutes of Health Research, Canada Foundation for Innovation, CAMH Foundation, and University of Toronto.
GRJ currently receives funding from an Ontario Graduate Scholarship and Ontario Student Opportunity Trust Fund.
SHA currently receives funding from the National Institute of Mental Health (R01MH114879), Canadian Institutes of Health Research, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, and CAMH Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors report any biomedical financial interests or potential conflicts of interest.
References
- 1.Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–36. [DOI] [PubMed] [Google Scholar]
- 3.Oliver LD, Haltigan JD, Gold JM, Foussias G, DeRosse P, Buchanan RW, et al. Lower- and Higher-Level Social Cognitive Factors Across Individuals With Schizophrenia Spectrum Disorders and Healthy Controls: Relationship With Neurocognition and Functional Outcome. Schizophr Bull. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49(1–2):1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. 2018;84(9):644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 2018;23(5):1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crow TJ. The two-syndrome concept: origins and current status. Schizophr Bull. 1985;11(3):471–86. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter WT Jr., The deficit syndrome. Am J Psychiatry. 1994;151(3):327–9. [DOI] [PubMed] [Google Scholar]
- 9.Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. 2009;34(6):1514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, et al. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry. 2013;70(5):472–80. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, et al. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry. 2015;72(5):446–55. [DOI] [PubMed] [Google Scholar]
- 12.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, et al. Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry. 2017;74(9):958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM, et al. Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex. 2013;23(9):2044–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, Genon S, Alzheimer’s Disease Neuroimaging I. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheeler AL, Chakravarty MM, Lerch JP, Pipitone J, Daskalakis ZJ, Rajji TK, et al. Disrupted prefrontal interhemispheric structural coupling in schizophrenia related to working memory performance. Schizophr Bull. 2014;40(4):914–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotiras A, Toledo JB, Gur RE, Gur RC, Satterthwaite TD, Davatzikos C. Patterns of coordinated cortical remodeling during adolescence and their associations with functional specialization and evolutionary expansion. Proc Natl Acad Sci U S A. 2017;114(13):3527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, et al. Evidence for Dissociable Linkage of Dimensions of Psychopathology to Brain Structure in Youths. Am J Psychiatry. 2019:appiajp201918070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, et al. Transdiagnostic Associations Between Functional Brain Network Integrity and Cognition. JAMA Psychiatry. 2017;74(6):605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahab S, Mulsant BH, Levesque ML, Calarco N, Nazeri A, Wheeler AL, et al. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology. 2019;44(5):898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nazeri A, Mulsant BH, Rajji TK, Levesque ML, Pipitone J, Stefanik L, et al. Gray Matter Neuritic Microstructure Deficits in Schizophrenia and Bipolar Disorder. Biol Psychiatry. 2017;82(10):726–36. [DOI] [PubMed] [Google Scholar]
- 21.Nazeri A, Chakravarty MM, Rotenberg DJ, Rajji TK, Rathi Y, Michailovich OV, et al. Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. J Neurosci. 2015;35(4):1753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant Frontostriatal Connectivity in Negative Symptoms of Schizophrenia. Schizophr Bull. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao H, Chen OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. 2018;9(1):3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata Y, Nakajima S, Plitman E, Caravaggio F, Kim J, Shah P, et al. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol Psychiatry. 2019;85(7):596–605. [DOI] [PubMed] [Google Scholar]
- 26.Moser DA, Doucet GE, Lee WH, Rasgon A, Krinsky H, Leibu E, et al. Multivariate Associations Among Behavioral, Clinical, and Multimodal Imaging Phenotypes in Patients With Psychosis. JAMA Psychiatry. 2018;75(4):386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, et al. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36(9):1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, et al. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324(5927):605. [DOI] [PubMed] [Google Scholar]
- 29.Lett TA, Chakavarty MM, Felsky D, Brandl EJ, Tiwari AK, Goncalves VF, et al. The genome-wide supported microRNA-137 variant predicts phenotypic heterogeneity within schizophrenia. Mol Psychiatry. 2013;18(4):443–50. [DOI] [PubMed] [Google Scholar]
- 30.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivieres S, Jahanshad N, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;2012:916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendrek A, Mancini-Marie A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 2016;67:57–78. [DOI] [PubMed] [Google Scholar]
- 33.Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahab S, Stefanik L, Foussias G, Lai MC, Anderson KK, Voineskos AN. Sex and Diffusion Tensor Imaging of White Matter in Schizophrenia: A Systematic Review Plus Meta-analysis of the Corpus Callosum. Schizophr Bull. 2018;44(1):203–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGregor C, Riordan A, Thornton J. Estrogens and the cognitive symptoms of schizophrenia: Possible neuroprotective mechanisms. Front Neuroendocrinol. 2017;47:19–33. [DOI] [PubMed] [Google Scholar]
- 37.Kulkarni J, Butler S, Riecher-Rossler A. Estrogens and SERMS as adjunctive treatments for schizophrenia. Front Neuroendocrinol. 2019. [DOI] [PubMed] [Google Scholar]
- 38.Jacquemont S, Coe BP, Hersch M, Duyzend MH, Krumm N, Bergmann S, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94(3):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barajas A, Ochoa S, Obiols JE, Lalucat-Jo L. Gender differences in individuals at high-risk of psychosis: a comprehensive literature review. ScientificWorldJournal. 2015;2015:430735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruessner M, Lepage M, Collins DL, Pruessner JC, Joober R, Malla AK. Reduced hippocampal volume and hypothalamus-pituitary-adrenal axis function in first episode psychosis: evidence for sex differences. Neuroimage Clin. 2015;7:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guma E, Devenyi GA, Malla A, Shah J, Chakravarty MM, Pruessner M. Neuroanatomical and Symptomatic Sex Differences in Individuals at Clinical High Risk for Psychosis. Front Psychiatry. 2017;8:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calkins ME, Moore TM, Merikangas KR, Burstein M, Satterthwaite TD, Bilker WB, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13(3):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobs GR, Ameis SH, Ji JL, Viviano JD, Dickie EW, Wheeler AL, et al. Developmentally divergent sexual dimorphism in the cortico-striatal-thalamic-cortical psychosis risk pathway. Neuropsychopharmacology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldstein Ewing SW, Bjork JM, Luciana M. Implications of the ABCD study for developmental neuroscience. Dev Cogn Neurosci. 2018;32:161–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexander LM, Escalera J, Ai L, Andreotti C, Febre K, Mangone A, et al. An open resource for transdiagnostic research in pediatric mental health and learning disorders. Sci Data. 2017;4:170181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42(9):1857–63. [DOI] [PubMed] [Google Scholar]
- 47.Fisher HL, Caspi A, Poulton R, Meier MH, Houts R, Harrington H, et al. Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychol Med. 2013;43(10):2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebald C, Khandaker GM, Zammit S, Lewis G, Jones PB. Association between childhood psychiatric disorders and psychotic experiences in adolescence: A population-based longitudinal study. Compr Psychiatry. 2016;69:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lugo Marin J, Alviani Rodriguez-Franco M, Mahtani Chugani V, Magan Maganto M, Diez Villoria E, Canal Bedia R. Prevalence of Schizophrenia Spectrum Disorders in Average-IQ Adults with Autism Spectrum Disorders: A Meta-analysis. J Autism Dev Disord. 2018;48(1):239–50. [DOI] [PubMed] [Google Scholar]
- 50.Selten JP, Lundberg M, Rai D, Magnusson C. Risks for nonaffective psychotic disorder and bipolar disorder in young people with autism spectrum disorder: a population-based study. JAMA Psychiatry. 2015;72(5):483–9. [DOI] [PubMed] [Google Scholar]
- 51.Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun. 2018;9(1):3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, et al. Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study. Am J Psychiatry. 2017;174(7):657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, et al. Common and Dissociable Mechanisms of Executive System Dysfunction Across Psychiatric Disorders in Youth. Am J Psychiatry. 2016;173(5):517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3(3 Pt 1):143–57. [DOI] [PubMed] [Google Scholar]
- 57.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23 Suppl 1:S250–63. [DOI] [PubMed] [Google Scholar]
- 58.Zitnik M, Nguyen F, Wang B, Leskovec J, Goldenberg A, Hoffman MM. Machine Learning for Integrating Data in Biology and Medicine: Principles, Practice, and Opportunities. Inf Fusion. 2019;50:71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016;173(4):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Viviano JD, Buchanan RW, Calarco N, Gold JM, Foussias G, Bhagwat N, et al. Resting-State Connectivity Biomarkers of Cognitive Performance and Social Function in Individuals With Schizophrenia Spectrum Disorder and Healthy Control Subjects. Biol Psychiatry. 2018;84(9):665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang B, Mezlini AM, Demir F, Fiume M, Tu Z, Brudno M, et al. Similarity network fusion for aggregating data types on a genomic scale. Nat Methods. 2014;11(3):333–7. [DOI] [PubMed] [Google Scholar]
- 62.Stefanik L, Erdman L, Ameis SH, Foussias G, Mulsant BH, Behdinan T, et al. Brain-Behavior Participant Similarity Networks Among Youth and Emerging Adults with Schizophrenia Spectrum, Autism Spectrum, or Bipolar Disorder and Matched Controls. Neuropsychopharmacology. 2018;43(5):1180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hawco C, Buchanan RW, Calarco N, Mulsant BH, Viviano JD, Dickie EW, et al. Separable and Replicable Neural Strategies During Social Brain Function in People With and Without Severe Mental Illness. Am J Psychiatry. 2019:appiajp201817091020. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigue AL, McDowell JE, Tandon N, Keshavan MS, Tamminga CA, Pearlson GD, et al. Multivariate Relationships Between Cognition and Brain Anatomy Across the Psychosis Spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(12):992–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Wit S, Ziermans TB, Nieuwenhuis M, Schothorst PF, van Engeland H, Kahn RS, et al. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: Applying machine learning techniques to brain imaging data. Hum Brain Mapp. 2017;38(2):704–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kambeitz-Ilankovic L, Meisenzahl EM, Cabral C, von Saldern S, Kambeitz J, Falkai P, et al. Prediction of outcome in the psychosis prodrome using neuroanatomical pattern classification. Schizophr Res. 2016;173(3):159–65. [DOI] [PubMed] [Google Scholar]
- 67.Koutsouleris N, Kambeitz-Ilankovic L, Ruhrmann S, Rosen M, Ruef A, Dwyer DB, et al. Individualized Prediction of Functional Outcomes in the Clinical High-Risk State for Psychosis and in Recent-Onset Depression: A Multi-Modal, Multi-Site Machine Learning Analysis. JAMA Psychiatry. 2018(In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lombardo MV, Lai MC, Baron-Cohen S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dinga R, Schmaal L, Penninx B, van Tol MJ, Veltman DJ, van Velzen L, et al. Evaluating the evidence for biotypes of depression: Methodological replication and extension of. Neuroimage Clin. 2019;22:101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science 2015;348(6234):499–500. [DOI] [PubMed] [Google Scholar]
- 72.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, et al. Parcellating cortical functional networks in individuals. Nat Neurosci. 2015;18(12):1853–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dickie EW, Ameis SH, Shahab S, Calarco N, Smith DE, Miranda D, et al. Personalized Intrinsic Network Topography Mapping and Functional Connectivity Deficits in Autism Spectrum Disorder. Biol Psychiatry. 2018;84(4):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage. 2013;66:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutter M Pathways from childhood to adult life. J Child Psychol Psychiatry. 1989;30(1):23–51. [DOI] [PubMed] [Google Scholar]
- 77.Kilford EJ, Garrett E, Blakemore SJ. The development of social cognition in adolescence: An integrated perspective. Neurosci Biobehav Rev. 2016;70:106–20. [DOI] [PubMed] [Google Scholar]
- 78.Chung YS, Barch D, Strube M. A meta-analysis of mentalizing impairments in adults with schizophrenia and autism spectrum disorder. Schizophr Bull. 2014;40(3):602–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okruszek L, Pilecka I. Biological motion processing in schizophrenia - Systematic review and meta-analysis. Schizophr Res. 2017;190:3–10. [DOI] [PubMed] [Google Scholar]
- 80.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]