Abstract
This systematic review and meta-analysis examined predictors of successful antipsychotic dose reduction in schizophrenia. Prospective clinical trials and randomized controlled trials (RCTs) investigating antipsychotic dose reduction in schizophrenia were selected for systematic review and meta-analysis, respectively. In total, 37 trials were identified. Only 8 studies focused on second-generation antipsychotics (SGAs); no studies investigated long-acting injectable SGAs. Of 24 studies evaluating relapse or symptom changes, 20 (83.3%) met the criteria for successful dose reduction. Factors associated with successful dose reduction were study duration < 1 year, age > 40 years, duration of illness > 10 years, and post-reduction chlorpromazine equivalent (CPZE) dose > 200 mg/day. Clinical deterioration was mostly re-stabilized by increasing the dose to the baseline level (N = 7/8, 87.5%). A meta-analysis of 18 RCTs revealed that relapse rate was significantly higher in the reduction group than the maintenance group (risk ratio [RR] = 1.96; 95% confidence interval [CI], 1.23–3.12), whereas neurocognition was significantly improved (standardized mean difference = 0.69; 95% CI, 0.25–1.12). A subgroup analysis indicated that only a post-reduction CPZE dose ≤ 200 mg/day was associated with an increased risk of relapse (RR = 2.79; 95% CI, 1.29–6.03). Thus, when reducing antipsychotic doses, clinicians should consider the long-term risk of relapse in younger patients with a relatively short illness duration and keep the final doses higher than CPZE 200 mg/day. Further studies, particularly those involving SGAs, are warranted to determine the optimal strategies for successful antipsychotic dose reduction in schizophrenia.
Subject terms: Outcomes research, Psychiatric disorders
Introduction
Maintenance treatment with antipsychotics is critical to prevent negative outcomes in patients with schizophrenia [1, 2]. A meta-analysis of 65 randomized controlled trials (RCTs) provided compelling evidence that antipsychotic maintenance treatment is superior to placebo in reducing the risk of relapse in stable schizophrenia [1]. Another meta-analysis showed that total symptom scores remained almost unchanged over 1 year in patients continuing antipsychotics, whereas symptoms continuously worsened over time in those who switched to placebo [3]. However, antipsychotics are associated with various undesirable adverse effects, such as extrapyramidal symptoms (EPSs) [4], neurocognitive impairment [5–7], and sudden cardiac death [8], at least partly in a dose-dependent manner.
Accordingly, it is clinically relevant to minimize long-term antipsychotic exposure. However, the consensus as to whether, to what extent, and how to reduce antipsychotics has not been fully established [9, 10]. As a result, patients with schizophrenia may be maintained on higher doses of antipsychotics than those needed for relapse prevention. It would thus be helpful to characterize patients who are unlikely to relapse after antipsychotic dose reduction during maintenance treatment of schizophrenia. To address this clinically important issue, we conducted a systematic review of prospective clinical trials and a meta-analysis of RCTs to explore the predictors of successful antipsychotic dose reduction.
Materials and methods
First, we conducted qualitative analyses of prospective antipsychotic dose reduction trials, including RCTs. These trials were identified from a systematic literature review to explore factors associated with successful dose reduction based on pre-defined criteria. Second, we performed quantitative analyses (i.e., meta-analysis) of RCTs exclusively to identify factors associated with successful dose reduction using the cut-off suggested in the qualitative analyses. Then, we considered the factors replicated in both the qualitative and quantitative analyses as robust predictors of successful antipsychotic dose reduction in schizophrenia.
Literature search and study selection
Systematic literature search for prospective trials
We conducted a systematic literature search for studies examining antipsychotic dose reduction in schizophrenia on 31 March 2019, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [11], using the MEDLINE and Embase databases with the following search terms: ((antipsychotic or neuroleptic or tranquiliz*) AND (dose or dosage) AND (reduce or reduction or low*-dose or minim* or decrease) AND schizophreni* AND adult) with limitations of human subjects and English language. We also performed cross-referencing and hand searches. We selected studies that met the following criteria: (1) original prospective clinical trials that examined antipsychotic dose reduction; (2) ≥70% of participants with a diagnosis of schizophrenia or schizoaffective disorder; and (3) ≥5 participants included in the study. We excluded studies that aimed to alter the antipsychotic formulation (e.g., oral to long-acting injectable antipsychotics [LAI-APs]) or simplify antipsychotic polypharmacy (e.g., switching 2 concurrent antipsychotics to monotherapy) because these were beyond the scope of the present study. Two authors (H.T. and S.T.) independently identified the relevant studies. Any discrepancies in study selection were resolved by consensus with the senior corresponding author (H.T.).
Selection and evaluation of RCTs
We selected RCTs comparing antipsychotic dose reduction with dose maintenance from the included studies to conduct a meta-analysis. Risk of bias for each included study was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions (available at http://handbook.cochrane.org). Two authors (H.T. and S.T.) independently identified the relevant studies. Any discrepancies in study selection and evaluation were resolved by consensus with the senior corresponding author (H.T.).
Data extraction
For qualitative analysis of prospective trials
We extracted the following data: (1) study information (i.e., publication year, study design, study duration, inclusion criteria, and definition of relapse); (2) patients’ demographic and clinical characteristics (i.e., age, sex, treatment setting, duration of illness, and duration of treatment); (3) clinical outcomes (i.e., pre- and post-intervention symptom severity, relapse and hospitalization, treatment strategies when symptoms deteriorated and their clinical consequences, study discontinuation, and adverse effects); and (4) antipsychotic use (i.e., antipsychotic types and formulations, target doses after reduction, actual pre- and post-intervention doses of antipsychotics, and procedure, duration, and speed of reduction). The presence or absence of statistically significant differences was also extracted for rates of relapse or hospitalization, and score changes in symptoms or adverse effects between pre- and post-dose reduction in single-arm prospective trials and between the dose reduction and maintenance groups in RCTs. Two authors (H.T. and S.T.) independently extracted the data. Any discrepancies in data extraction were resolved by consensus with the senior corresponding author (H.T.).
For meta-analysis of RCTs
We extracted the following clinical outcome data for both dose reduction and maintenance groups from the included RCTs: (1) number of patients who relapsed (primary outcome) and were hospitalized, (2) number of patients who discontinued the study due to all causes, inefficacy, and intolerability, (3) mean ± standard deviation (SD) changes from baseline to endpoint in psychopathology scores (total, and positive and negative symptom subscale scores) [12–15], and (4) mean ± SD changes in the scores for adverse effects (EPSs [15–18], body weight, neurocognition [19, 20], and quality of life (QOL) [21–23]). The detailed scales included in this meta-analysis are described in Supplementary Figure S1A. Two authors (H.T. and S.T.) independently extracted the data. Any discrepancies in data extraction were resolved by consensus with the senior corresponding author (H.T.). If the articles did not provide sufficient data, we contacted the corresponding authors to request additional data.
Data analysis
Qualitative analysis of prospective trials
We conducted qualitative analyses of the identified prospective clinical trials. A successful dose reduction was defined as (1) any significant improvement or no significant change in symptom severity between pre- and post-reduction along with any significant improvement or no significant change in adverse effects after dose reduction, or (2) any significant superiority or no significant difference in relapse rates or changes in symptom severity (if relapse rates were not available) between the dose reduction and maintenance groups along with any significant superiority or no significant difference in adverse effects. We counted the number of successful and unsuccessful studies for each item of the study information and the factors related to patients’ demographic and clinical characteristics, antipsychotic use, and dose reduction procedures. The cut-off was set to obtain the highest sensitivity and specificity to differentiate the factor in terms of the proportion of successful studies (Supplementary Fig. S1B). A factor was considered a predictor of successful dose reduction if it satisfied both of the following criteria: (1) all included studies identified a particular factor as a predictor of successful dose reduction and (2) more than half of the unsuccessful studies did not identify that specific factor as a predictor of unsuccessful dose reduction (i.e., less than half of the unsuccessful studies were grouped as “not available”) to increase the certainty of the findings (Supplementary Fig. S1C).
Meta-analysis of RCTs
We performed meta-analyses of RCTs using Review Manager (RevMan) version 5.3. We combined and compared the outcome data between the dose reduction and maintenance groups for each extracted outcome. Pooled estimates of risk ratios (RRs) for dichotomous outcomes and standardized mean differences (SMDs) for continuous variables were calculated with 2-sided 95% confidence intervals (CIs) using a random-effects model. Antipsychotic doses reported in the included studies were converted to chlorpromazine equivalent (CPZE) doses [24].
In addition, we conducted subgroup analyses with the following factors using the cut-off identified as described above: publication year, study duration, illness stability, age, treatment setting, duration of illness and treatment, baseline symptom severity, antipsychotic type and formulation, pre- and post-reduction doses of antipsychotics, reduction rates, and duration and speed of reduction. When some factors were found to be significantly associated with an increased risk of relapse, we conducted further subgroup analyses classified by factors related to antipsychotic dose to see if there were other variables independent of antipsychotic dose (Supplementary Fig. S1D). Sensitivity analyses were performed if there were studies that included a factor just right on the threshold dividing the subgroups (Supplementary Fig. S1E). All effect sizes with P < 0.05 were considered statistically significant. Study heterogeneities were quantified by using the I2 statistic with I2 ≥ 50% indicating significant heterogeneity. Publication bias was assessed by visual inspection of funnel plots.
Results
A total of 37 prospective clinical trials involving 2,080 subjects that met our eligibility criteria were identified for the systematic review [25–61]. The characteristics of all the included studies are shown in Table 1. The PRISMA flow diagram of the literature search is shown in Supplementary Fig. S2. Of these studies, 18 RCTs involving 1,385 subjects were included in the meta-analysis. The risk of bias for the RCTs is shown in Supplementary Fig. S3A.
Table 1.
Characteristics of included studies.
| Author (year) | Outcome: overall | Outcome: symptoms | Outcome: adverse effects | n | Study design | Study duration | Age, year | DOI, year | Baseline symptoms | APs | Baseline CPZE dose, mg/dayd | CPZE dose after reduction, mg/dayd | Reduction duration | Relapse rate in reduction group (that in non-reduction group) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. [25] | Success | No change | Improved | 75 | SBRCT | 52 w | 44.3 | NA | PANSS | 66.2 |
RIS OLZ |
510 585 |
330 234 |
16 w | 10.8% (15.8%) |
| Yamanouchi et al. [26] | Success | No change | No change | 163 | OLRCT | RP + 3 m | 60 | 32 | MS | 12.7 | FGAs/ SGAs | 1012 | 763 | 12–24 w | NA |
| Takeuchi et al. [27] | Success | No change | Improved | 61 | OLRCT | 28 w | 40.9 | 15.5 | PANSS | 56.4 |
RIS OLZ |
370 414 |
210 213 |
4 w | 3.2% (3.3%) |
| Wang et al. [28] | Failure | Deteriorated | Improved | 264 | OLRCT | 1 y | 32.7 | 6.7 | PANSS | 39.6 | RIS | 420 | 200 | ≤8 wg | 15.8% (7.8%) |
| Rouillon et al. [29] | Success | No change | No change | 97 | OLRCT | 6 m | 39.5 | NA | PANSS | 61.3 | OLZ | 528 | 399 | 6 m | 8.2% (6.3%) |
| Uchida et al. [30] | Success | No change | Improved | 34 | OLRCT | 36 w | 40.1 | 15.8 | PANSS | 55.0 | NA | 703 | 413 | 12 w | 0% (0%) |
| Kinion et al. [31] | NA | NA | No change | 27 | SBRCT | RP + 6 m | 73 | 35a | NA | NA | FGAs | 370 | 173 | ≤6 m | NA |
| Volavka et al. [32] | Success | No change | No change | 23 | DBRCT | 28 w | 40.1 | 20.4a | PANSS | 82.7 | HPD | 1974 | 1164 | 12 w | NA |
| Hirschowitz et al. [33] | Success | No change | No change | 24 | DBRCT | RP+1 y | 43.1 | 19.9a | PANSS | 82.1 | HPD | 1200 | 666 | 1 w | NA |
| Schooler et al. [34] | Success | No change | NA | 212 | DBRCT | 2 y | 29.6 | 8.5 | BPRS | 29.4 | FPZ-LAI | 300–1200 | 215 | 0 | 25%h (25%) |
| Hogarty et al. [35] | NA | NA | No change | 79 | DBRCT | 12 w | NA | NA | NA | NA | FPZ-LAI | 446.4e 396.0f |
256.8 242.4 |
12 w | NA |
| Inderbitzin et al. [36] | Success | No change | Improved | 37 | DBRCT | 1 y | 41.2 | 18.2a | BPRS | 32.9 | FPZ-LAI | 545 | 276 | 5 m | 25.0% (23.5%) |
| Newcomer et al. [37] | NA | NA | Improved | 24 | DBRCT | 4 w | 39 | 15.8 | BPRS | 35.7 | HPD | 1704 | NA | 4 w | 21.4% (NA) |
| Faraone et al. [38] | Success | No change | NA | 29 | DBRCT | 6 m | 37–74 | NA | NA | NA | NA | NA | NA | 2 w/8 w | 36.4% (0%) |
| Hogarty et al. [39] | Success | No change | No change | 70 | DBRCT | 2 y | 28.3 | 7 | BPRS | NA | FPZ-LAI | 516 | 91 | 2 y | 24.3% (18.2%) |
| Cookson et al. [40] | NA | NA | NA | 18 | DBRCT | 44 w | 46 | 14 | BPRS | 20.2 | FUL-LAI | 3540 | 1770 | 0 | 33.3% (11.1%) |
| Johnson et al. [41] | Failure | Deteriorated | No change | 59 | DBRCT | 1 y | 40 | 10 | BPRS | 1.0 | FUL-LAI | NA | 90 | 1 y | 32.1% (9.7%) |
| Kane et al. [42] | Failure | Deteriorated | Improved | 126 | DBRCT | 1 y | 28.9 | 6.1a | BPRS | NA | FPZ-LAI | 300–1200 | 30–120 | 0 | 56.0% (7.0%) |
| Bogers et al. [43] | Success | No change | No change | 24 | Prospective | RP + 1 y | 53 | NA | PANSS | 104 | FGAs | 1086 | 300 | 0–24 w | 20.8% |
| Graff-Guerrero et al. [44] | Success | Improved | Improved | 35 | Prospective | RP + 5.9 w | 60.1 | 33.1 | PANSS | 61.3 |
RIS OLZ |
440 624 |
300 405 |
2.0 w | NA |
| Uchida et al. [45] | NA | NA | NA | 9 | Prospective | 3 m | 58 | 34 | PANSS | 43.4 | RIS | 340 | NA | NA | NA |
| Kawai et al. [46] | Success | No change | Improved | 23 | Prospective | 21.3 w | 42.4 | 23.8 | PANSS | 92.4 | FGAs | 2253 | 1315 | 21.3 w | NA |
| Tsuruta et al. [47] | Failure | Deteriorated | No change | 170 | Prospective | 2 y | NA | NA | BPRS | NA | NA | NA | NA | ≤5 m | 59.5% (12.5%) |
| Gefvert et al. [48] | NA | NA | NA | 5 | Prospective | 35 w | 33.4 | NA | BPRS | 52.0 | QTP | 600/360 | 360/240 | 1 w | NA |
| Nyberg et al. [49] | NA | NA | Improved | 8 | Prospective | 2 w | 30.9 | 5.5 | PANSS | 85.6 | RIS | 600 | 300 | 0 | NA |
| Harris et al. [50] | Success | No change | Improved | 49 | Prospective | 11 m | 61.8 | 26.2 | PANSS | 59.5 | NA | 190 | 108 | NA | 0%h (0%) |
| Canuso et al. [51] | NA | NA | No change | 5 | Prospective | 12.2 w | 39.4 | 17.0 | NA | NA | NA | 1980 | 631 | 12.2 w | 100% |
| Dale et al. [52] | NA | NA | NA | 22 | Prospective | 1 y | 33.4 | 9.9 | NA | NA | FGA-LAIs | 318 | NA | 6 m | 54.5% |
| Leblenc et al. [53] | Success | Improved | Deteriorated | 32 | Prospective | 1 y | 37 | 11.7b | BPRS | 38.1 | FGA-LAIs | 3702 | 1800 | 5 m | 18.8% |
| Smith et al. [54] | Success | No change | NA | 16 | Prospective | 10.3 m | 46.5 | 11.5c | BPRS | NA | NA | 1290 | 437 | 10.3 m | NA |
| Solgaard et al. [55] | Success | No change | NA | 23 | Prospective | NA | 45.7 | 18.8 | BPRS | 9.8 | ZUC-LAI | 656 | 599 | NA | 4.3% |
| Heresco-Levy et al. [56] | NA | NA | NA | 41 | Prospective | 2 y |
41.6 39.6 |
19.3 16.7 |
BPRS |
31.4 33.5 |
FPZ-LAI FPZ-LAI |
258 665 |
120 420 |
2 m 2 m |
36.0% 78.0% |
| Van Putten et al. [57] | Success | Improved | Improved | 13 | Prospective | 1 y | 32.6 | NA | BPRS | NA | HP | 3786 | 1410 | 32 w | NA |
| Hirschowitz et al. [58] | NA | NA | No change | 16 | Prospective | NA | 53 | 22 | SAPS | NA | HPD | 1680 | NA | NA | NA |
| Kistrup et al. [59] | NA | NA | NA | 44 |
Prospective Prospective |
5–14 m 3–9 m |
45.8 44.1 |
16.8 16.6 |
BPRS BPRS |
10.0 9.5 |
PPZ-LAI FUL-LAI |
700 975 |
596 900 |
8.0 m 5.1 m |
NA NA |
| Faraone et al. [60] | NA | NA | NA | 29 | Prospective | 1 y | 48 | 23 | BPRS | 32.6 | FGAs | NA | NA | 2 w | 44.8% |
| Lehman et al. [61] | Success | No change | NA | 94 | Prospective | 48 w | 45 | NA | BPRS |
25.9 32.6 |
NA |
266 475 |
50 100 |
1 m | 25.8–32.3% (21.9%) |
APs antipsychotics, BPRS Brief Psychiatric Rating Scale, CGI-S Clinical Global Impressions – Severity scale, CPZE chlorpromazine equivalent, DBRCT double-blind randomized controlled trial, DOI duration of illness, FPZ fluphenazine, FGAs first-generation antipsychotics, FUL flupentixol, HPD haloperidol, LAI long-acting injection, MS Manchester Scale, n number of subjects, NA not available, m months, OLRCT open-label randomized controlled trial, OLZ olanzapine, PANSS Positive and Negative Syndrome Scale, PPZ perphenazine, RIS risperidone, RP reduction period, SAPS Scale for the Assessment of Positive Symptoms, SBRCT single-blind randomized controlled trial, SGAs second-generation antipsychotics, w weeks, y years, ZUC zuclopenthixol
aDuration from the first hospitalization to study participation
bDuration of antipsychotic treatment
cDuration of current hospitalization
dCPZE dose was calculated according to Gardner et al.24
eDistressed group
fDefect group
gDose was maintained for 26 weeks before reduction
hHospitalization rate
Six studies assessed a change in symptom severity after dose reduction and 18 RCTs compared relapse rates or changes in symptom severity between the dose reduction and maintenance groups. Of these 24 studies, 20 (83.3%) showed a significant improvement or no significant difference in symptom severity or relapse rates between pre- and post-dose reduction or between the dose reduction and maintenance groups, along with no worsening or no inferiority of adverse effects, which was considered to indicate a successful dose reduction by our definition. Nine studies reported a significant improvement in adverse effects after dose reduction, including EPSs and neurocognitive impairment.
Factors related to successful dose reduction identified by qualitative analysis of prospective trials
Table 2 shows the number of studies classified by outcome for each of the factors related to study design, patient characteristics, and antipsychotic dose reduction strategy. Relapse definition varied among the studies and included exceeding a certain threshold score on a scale (N = 9), an increase in antipsychotic dose (N = 4), hospitalization (N = 2), or a combination of them (i.e., an increase in antipsychotic doses or hospitalization) (N = 4), whereas relapse was not clearly defined in 18 studies. In addition, 21 (56.8%) and 11 (29.7%) studies targeted first-generation antipsychotics (FGAs) and LAI-APs only, respectively. Only 8 studies (21.6%; 4 RCTs and 4 non-RCTs) focused on second-generation antipsychotics (SGAs) and no studies examined dose reduction of LAI-SGAs. Sixteen studies (43.2%) included patients receiving a mean CPZE ≥ 600 mg/day at baseline. In 7 out of 8 studies (87.5%), patients who experienced clinical deterioration were re-stabilized by increasing the doses back to the pre-reduction baseline.
Table 2.
Number of studies classified by outcome in each factor.
| Number of studies | |||||
|---|---|---|---|---|---|
| Total | By outcome classification | ||||
| Success (%) | Failure (%) | NA | |||
| Factors related to study design | |||||
| Publication year |
2003– –2002 |
10 | 8 (89) | 1 (11) | 1 |
| 27 | 12 (80) | 3 (20) | 12 | ||
| Study design | Randomized controlled trial | 18 | 11 (79) | 3 (21) | 4 |
| Blind | 13 | 7 | 2 | 4 | |
| Open label | 5 | 4 | 1 | 0 | |
| Prospective trial | 19 | 9 (90) | 1 (10) | 9 | |
| Study duration | ≥1 year | 14 | 7 (64) | 4 (36) | 3 |
| ≥6 months and <1 year | 14 | 10 (100) | 0 (0) | 4 | |
| <6 months | 7 | 2 (100) | 0 (0) | 5 | |
| NA | 2 | 1 | 0 | 1 | |
| Inclusion criteria: illness stability | Stable condition | 19 | 9 (69) | 4 (31) | 6 |
| Less than a certain score on a scale | 4 | 2 | 2 | 0 | |
| Longer than a certain period | 5 | 2 | 1 | 2 | |
| ≥3 months | 4 | 1 | 1 | 2 | |
| <3 months | 1 | 1 | 0 | 0 | |
| Mixed (less than a certain score and longer than a certain period) | 4 | 2 | 1 | 1 | |
| Unstable condition | 5 | 4 (100) | 0 (0) | 1 | |
| NA | 13 | 7 | 0 | 6 | |
| Inclusion criteria: antipsychotic dose | Stable dose for a certain period | 7 | 2 (100) | 0 (0) | 5 |
| More than a certain dose | 3 | 0 (0) | 2 (100) | 1 | |
| More than a certain dose and period | 15 | 12 (92) | 1 (8) | 2 | |
| NA | 12 | 6 | 1 | 5 | |
| Relapse definition | More than a certain score on a scale | 9 | 5 (57) | 3 (43) | 1 |
| Increase in antipsychotic dose | 4 | 2 (67) | 1 (33) | 1 | |
| Hospitalization | 2 | 2 (100) | 0 (0) | 0 | |
| Mixed (hospitalization or increase in antipsychotic dose) | 4 | 2 (100) | 0 (0) | 2 | |
| NA | 18 | 9 | 0 | 9 | |
| Factors related to patients’ demographic and clinical characteristics | |||||
| Mean age | >40 years | 21 | 14 (100) | 0 (0) | 7 |
| ≤40 years | 13 | 5 (63) | 3 (38) | 5 | |
| NA | 3 | 1 | 1 | 1 | |
| Treatment setting | Outpatients only | 15 | 9 (82) | 2 (18) | 4 |
| Inpatients only | 10 | 5 (100) | 0 (0) | 5 | |
| Mixed (outpatients and inpatients) | 7 | 6 (100) | 0 (0) | 1 | |
| NA | 5 | 0 | 2 | 3 | |
| Mean illness durationa | >10 years | 17 | 8 (100) | 0 (0) | 9 |
| ≤10 years | 7 | 2 (40) | 3 (60) | 2 | |
| NA | 13 | 10 | 1 | 2 | |
| Mean treatment duration | >10 years | 11 | 8 (100) | 0 (0) | 3 |
| ≤10 years | 2 | 0 (0) | 2 (100) | 0 | |
| NA | 24 | 12 | 2 | 10 | |
| Antipsychotics: type | FGAs only | 21 | 10 (83) | 2 (17) | 9 |
| SGAs only | 8 | 4 (80) | 1 (20) | 3 | |
| Mixed (FGAs and SGAs) | 1 | 1 (100) | 0 (0) | 0 | |
| NA | 7 | 5 | 1 | 1 | |
| Antipsychotics: formulation | Oral | 14 | 6 (86) | 1 (14) | 7 |
| LAI | 11 | 4 (67) | 2 (33) | 5 | |
| Mixed (oral and LAI) | 3 | 2 (100) | 0 (0) | 1 | |
| NA | 9 | 8 | 1 | 0 | |
| Antipsychotics: mean CPZE dose | ≥430 mg/day | 22 | 16 (100) | 0 (0) | 6 |
| >600 mg/day | 15 | 9 | 0 | 6 | |
| <430 mg/day | 9 | 2 (67) | 1 (33) | 6 | |
| NA | 6 | 2 | 3 | 1 | |
| Mean symptom severity | >Mild | 14 | 10 (100) | 0 (0) | 4 |
| PANSS total >58 | 9 | 8 | 0 | 1 | |
| BPRS total >31 | 5 | 2 | 0 | 3 | |
| CGI-S >3 | 0 | 0 | 0 | 0 | |
| ≤Mild | 10 | 5 (71) | 2 (29) | 3 | |
| PANSS total ≤58 | 4 | 2 | 1 | 1 | |
| BPRS total ≤31 | 6 | 3 | 1 | 2 | |
| CGI-S ≤3 | 0 | 0 | 0 | 0 | |
| NA | 13 | 5 | 2 | 6 | |
| Factors related to antipsychotic dose reduction strategy | |||||
| Goal of reduction | % reduction | 17 | 9 (75) | 3 (25) | 5 |
| 50% reduction | 11 | 6 | 2 | 3 | |
| To target dose | 7 | 4 (100) | 0 (0) | 3 | |
| Mixed (% reduction and to target dose) | 1 | 1 (100) | 0 (0) | 0 | |
| To MED for each patient | 6 | 3 (100) | 0 (0) | 3 | |
| NA | 5 | 3 | 0 | 2 | |
| Actual proportion of reduction | >52% | 11 | 6 (75) | 2 (25) | 3 |
| >80% | 3 | 2 | 1 | 0 | |
| ≤52% | 17 | 12 (100) | 0 (0) | 5 | |
| NA | 9 | 2 | 2 | 5 | |
| Antipsychotic dose after reduction (CPZE) | >600 mg/day | 8 | 6 (100) | 0 (0) | 2 |
| >200 mg/day and ≤600 mg/day | 14 | 10 (100) | 0 (0) | 4 | |
| ≤200 mg/day | 8 | 3 (50) | 3 (50) | 2 | |
| NA | 7 | 1 | 1 | 5 | |
| Duration of reduction | >2 month | 21 | 12 (86) | 2 (14) | 7 |
| ≤2 month | 9 | 4 (67) | 2 (33) | 3 | |
| NA | 7 | 4 | 0 | 3 | |
| Speed of reduction | <6.5%/week | 16 | 11 (100) | 0 (0) | 5 |
| ≥6.5%/week | 8 | 4 (67) | 2 (33) | 2 | |
| NA | 13 | 5 | 2 | 6 | |
BPRS Brief Psychiatric Rating Scale, CGI-S Clinical Global Impressions – Severity scale, CPZE chlorpromazine equivalent, FGAs first-generation antipsychotics, LAI long-acting injectable, MED minimum effective dose, NA not available, PANSS Positive and Negative Syndrome Scale, SGAs second-generation antipsychotics
aDuration of illness or since first hospitalization
Factors that satisfied the criteria for predicting successful dose reduction were study duration < 1 year, age > 40 years, duration of illness > 10 years, and a post-reduction antipsychotic CPZE dose > 200 mg/day. Other factors including illness stability or antipsychotic dose at baseline did not fulfill the criteria for predicting successful dose reduction.
Factors related to an increased risk of relapse after dose reduction identified by meta-analysis of RCTs
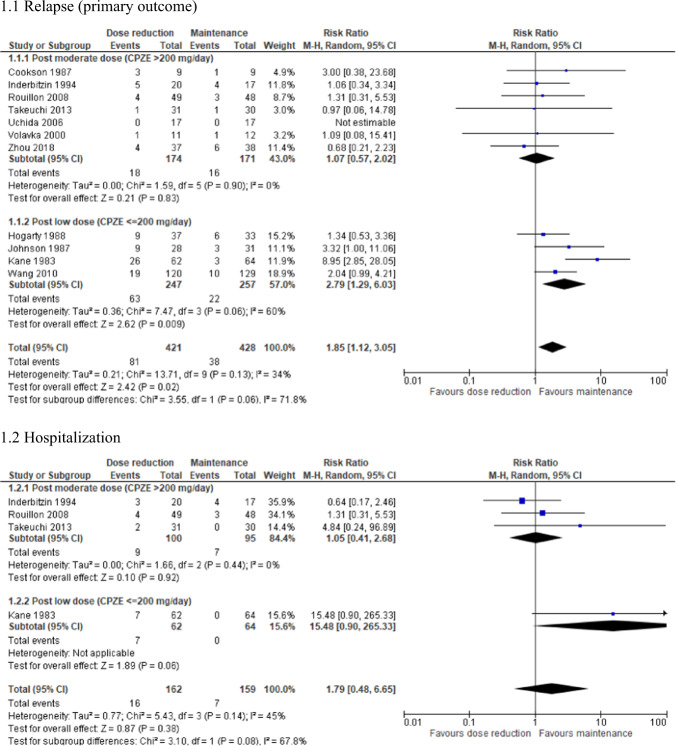
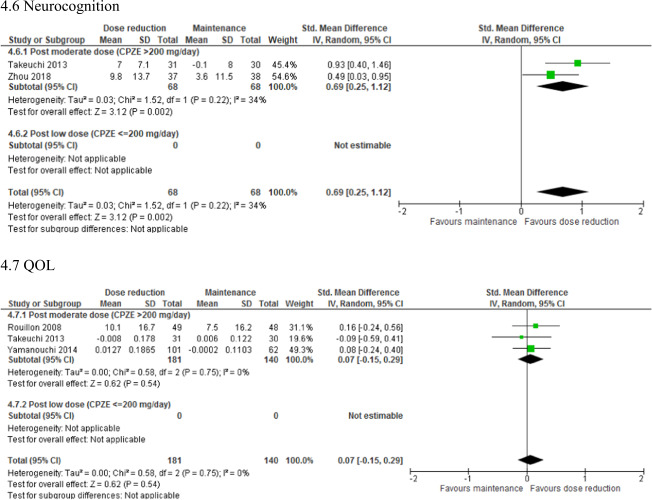
The quantitative results of the meta-analyses are summarized in Table 3 and the corresponding forest plots are shown in Supplementary Fig. S4. Relapse rate was significantly higher in the dose reduction group than in the maintenance group (N = 13; n = 902; RR = 1.96; 95% CI, 1.23–3.12; P = 0.005; I2 = 27%). In contrast, a significantly greater improvement in neurocognition was found in the dose reduction group compared with the maintenance group (N = 2; n = 136; SMD = 0.69; 95% CI, 0.25–1.12; P = 0.002; I2 = 34%). There were no significant differences in hospitalization, study discontinuation, psychopathology, EPSs, body weight, or QOL between the 2 groups.
Table 3.
Effect estimate of each outcome.
| Studies | Participants | Statistics | Effect estimate | Overall effect | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| [95% CI] | P-value | I2 (%) | P-value | ||||
| Relapse/Hospitalization | |||||||
| Relapse (primary outcome) | 13 | 902 | RR | 1.96 [1.23–3.12] | 0.005* | 27 | 0.18 |
| Hospitalization | 5 | 350 | RR | 1.79 [0.60–5.30] | 0.30 | 30 | 0.22 |
| Study discontinuation | |||||||
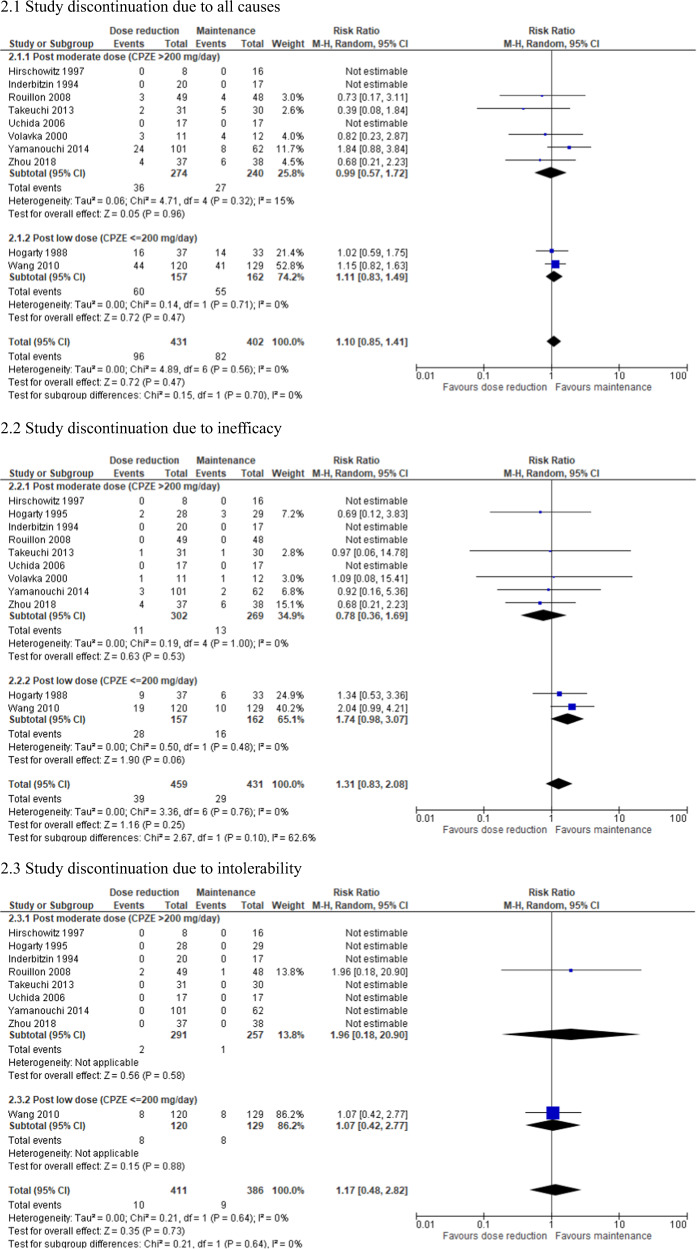
| due to all causes | 11 | 857 | RR | 1.11 [0.86–1.42] | 0.42 | 0 | 0.54 |
| due to inefficacy | 12 | 914 | RR | 1.36 [0.86–2.14] | 0.19 | 0 | 0.75 |
| due to intolerability | 9 | 797 | RR | 1.17 [0.48–2.82] | 0.73 | 0 | 0.64 |
| Psychopathology | |||||||
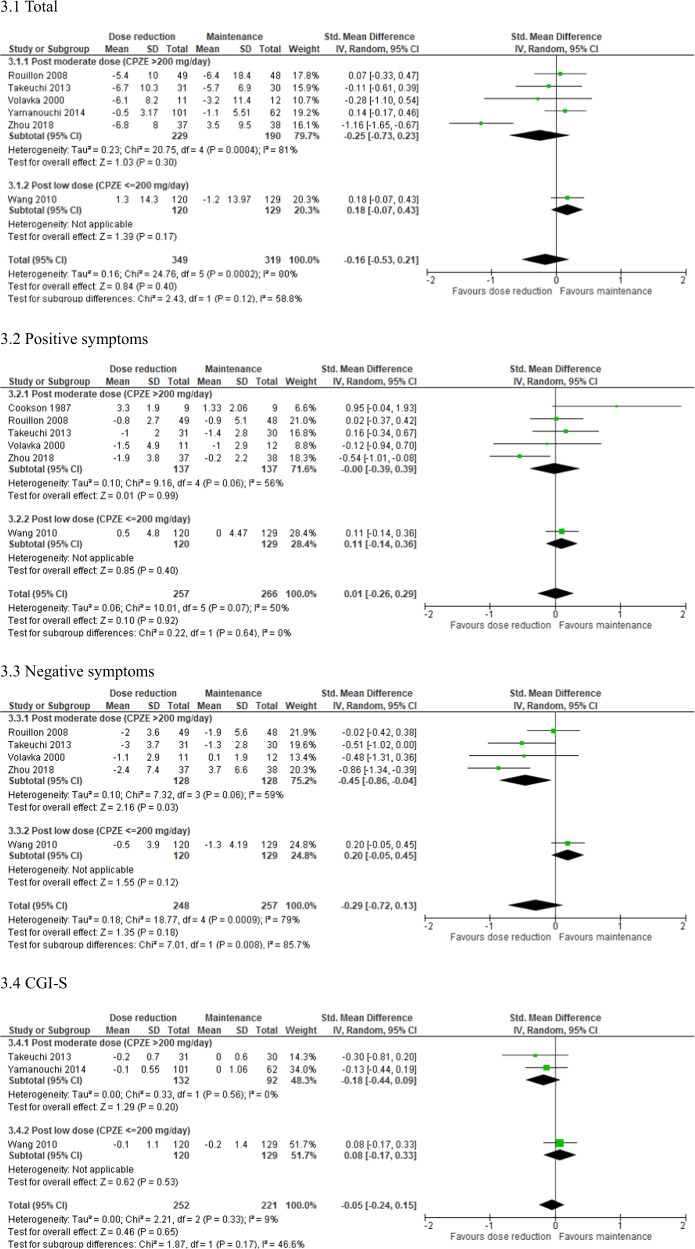
| Total | 6 | 668 | SMD | −0.16 [−0.53–0.21] | 0.40 | 80 | 0.0002 |
| Positive symptoms | 6 | 523 | SMD | 0.01 [−0.26–0.29] | 0.92 | 50 | 0.07 |
| Negative symptoms | 5 | 505 | SMD | −0.29 [−0.72–0.13] | 0.18 | 79 | 0.0009 |
| CGI-S | 3 | 473 | SMD | −0.05 [−0.24–0.15] | 0.65 | 9 | 0.33 |
| Adverse effects | |||||||
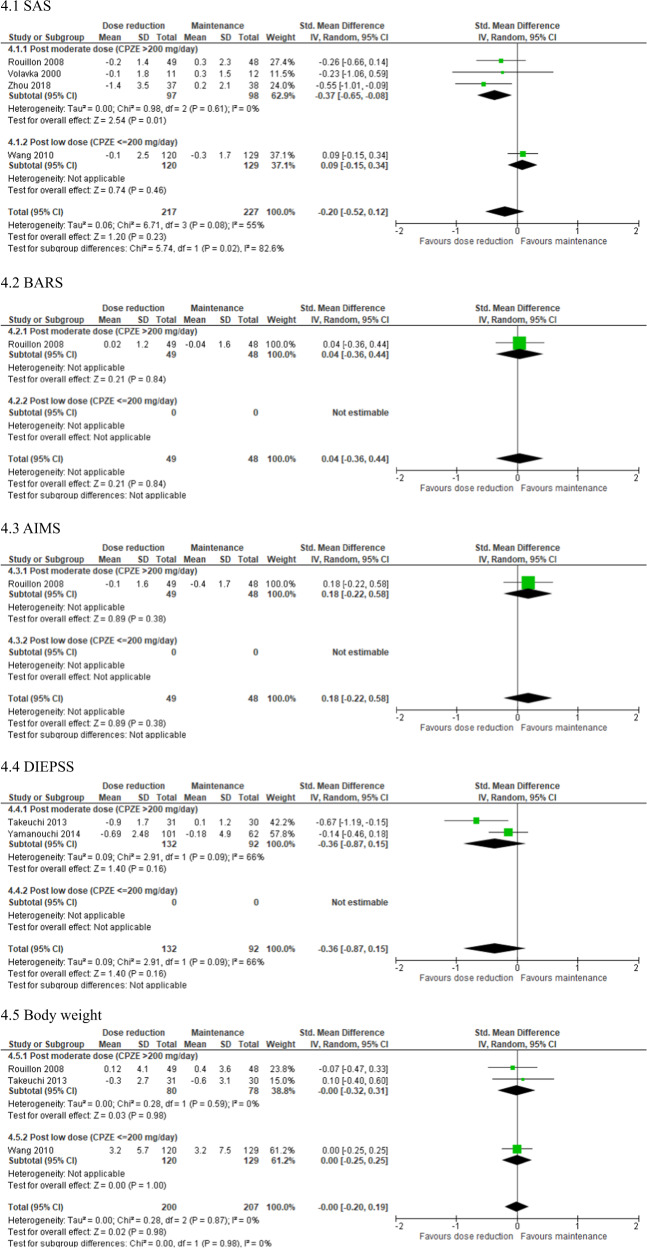
| SAS | 4 | 444 | SMD | −0.20 [−0.52–0.12] | 0.23 | 55 | 0.08 |
| BARS | 1 | 97 | SMD | 0.04 [−0.36–0.44] | 0.84 | NA | NA |
| AIMS | 1 | 97 | SMD | 0.18 [−0.22–0.58] | 0.38 | NA | NA |
| DIEPSS | 2 | 224 | SMD | −0.36 [−0.87–0.15] | 0.16 | 66 | 0.09 |
| Body weight | 3 | 407 | SMD | −0.00 [−0.20–0.19] | 0.98 | 0 | 0.87 |
| Neurocognition | 2 | 136 | SMD | 0.69 [0.25–1.12] | 0.002* | 34 | 0.22 |
| QOL | 3 | 321 | SMD | 0.07 [−0.15–0.29] | 0.54 | 0 | 0.75 |
AIMS Abnormal Involuntary Movement Scale, BARS Barnes Akathisia Rating Scale, CGI-S Clinical Global Impression – Severity scale, DIEPSS Drug-Induced Extrapyramidal Symptoms Scale, NA not applicable, QOL quality of life, RR risk ratio, SAS Simpson-Angus Scale, SMD standardized mean difference
*P < 0.05 overall effect
The subgroup analyses of relapse rate are summarized in Table 4 and the corresponding forest plots are shown in Figs. 1–4 and Supplementary Figs. S5–7. The following factors were associated with an increased risk of relapse: publication before 2002, study duration ≥ 1 year, stable illness at enrollment, mean age ≤ 40 years, outpatient setting, mean illness duration ≤ 10 years, mean treatment duration ≤ 10 years, use of FGAs, use of LAI-APs, mild or lower symptom severity, post-reduction CPZE dose ≤ 200 mg/day, and duration of reduction ≤ 2 months. However, after the sensitivity analyses, study duration and duration of illness were no longer significant (Supplementary Table S1). Moreover, when the further subgroup analyses of studies with a post-reduction CPZE dose > 200 mg/day were conducted, no factors remained significant (Supplementary Table S2).
Fig. 2. Forest plot: study discontinuation, subgroup analysis by antipsychotic dose after reduction.
CPZE chlorpromazine equivalent.
Fig. 3. Forest plot: psychopathology, subgroup analysis by antipsychotic dose after reduction.
CGI-S Clinical Global Impressions – Severity scale, CPZE chlorpromazine equivalent.
Table 4.
Subgroup analysis of effect estimate in relapse rate.
| Factors | Subgroup | Studies | Participants | Effect estimate | Overall effect | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| [95% CI] | P-value | I2 (%) | P-value | ||||
| Overall | 13 | 902 | 1.96 [1.23–3.12] | 0.005* | 27 | 0.18 | |
| Publication year |
2003– –2002 |
5 | 516 | 1.45 [0.83–2.54] | 0.19 | 0 | 0.47 |
| 8 | 386 | 2.59 [1.33–5.05] | 0.005* | 35 | 0.15 | ||
| Study duration | ≥1 year | 6 | 616 | 1.96 [1.00–3.84] | 0.05* | 61 | 0.02 |
| <1 year | 7 | 286 | 1.95 [0.80–4.77] | 0.14 | 0 | 0.84 | |
| Illness stability | Stable | 8 | 761 | 2.06 [1.13–3.74] | 0.02* | 45 | 0.08 |
| Unstable | 3 | 70 | 2.70 [0.67–10.95] | 0.16 | 0 | 0.66 | |
| Mean age | >40 years | 6 | 248 | 1.02 [0.50–2.07] | 0.96 | 0 | 0.83 |
| ≤40 years | 6 | 625 | 2.56 [1.38–4.75] | 0.003* | 41 | 0.13 | |
| Treatment setting | Outpatient only | 6 | 418 | 2.37 [1.10–5.11] | 0.03* | 53 | 0.06 |
| Inpatient only | 1 | 23 | 1.09 [0.08–15.41] | 0.95 | NA | NA | |
| Mean illness duration | >10 years | 6 | 197 | 1.44 [0.62–3.36] | 0.40 | 0 | 0.79 |
| ≤10 years | 4 | 504 | 2.79 [1.29–6.03] | 0.009* | 60 | 0.06 | |
| Mean treatment duration | >10 years | 2 | 60 | 1.07 [0.37–3.05] | 0.90 | 0 | 0.99 |
| ≤10 years | 2 | 185 | 5.55 [2.06–14.94] | 0.0007* | 30 | 0.23 | |
| Antipsychotics: type | FGAs | 7 | 357 | 2.48 [1.21–5.07] | 0.01* | 41 | 0.12 |
| SGAs | 4 | 482 | 1.45 [0.83–2.54] | 0.19 | 0 | 0.47 | |
| Antipsychotics: formulation | Oral | 6 | 529 | 1.50 [0.88–2.56] | 0.14 | 0 | 0.65 |
| LAI | 5 | 310 | 2.53 [1.09–5.90] | 0.03* | 58 | 0.05 | |
| Antipsychotics: mean dose | ≥430 mg/day | 8 | 378 | 1.22 [0.72–2.06] | 0.46 | 0 | 0.84 |
| <430 mg/day | 2 | 310 | 1.94 [0.97–3.92] | 0.06 | 0 | 0.60 | |
| Mean symptom severity | >Mild | 5 | 256 | 1.05 [0.54–2.06] | 0.88 | 0 | 0.77 |
| ≤Mild | 5 | 421 | 2.28 [1.28–4.07] | 0.005* | 0 | 0.82 | |
| Actual proportion of reduction | ≥52% | 3 | 445 | 2.73 [0.99–7.51] | 0.05 | 73 | 0.03 |
| <52% | 6 | 270 | 1.28 [0.60–2.73] | 0.52 | 0 | 0.94 | |
| Antipsychotic dose after reduction | >200 mg/day | 7 | 345 | 1.07 [0.57–2.02] | 0.83 | 0 | 0.90 |
| ≤200 mg/day | 4 | 504 | 2.79 [1.29–6.03] | 0.009* | 60 | 0.06 | |
| Duration of reduction | >2 months | 7 | 395 | 1.32 [0.80–2.17] | 0.28 | 0 | 0.61 |
| ≤2 months | 4 | 460 | 3.39 [1.22–9.41] | 0.02* | 47 | 0.13 | |
| Speed of reduction | <6.5%/week | 6 | 336 | 1.08 [0.62–1.88] | 0.78 | 0 | 0.93 |
| ≥6.5%/week | 3 | 436 | 3.20 [0.93–11.00] | 0.07 | 64 | 0.06 | |
FGAs first-generation antipsychotics, LAI long-acting injectable, NA not applicable, SGAs second-generation antipsychotics
*P < 0.05 for overall effect
Fig. 1. Forest plot: relapse/hospitalization, subgroup analysis by antipsychotic dose after reduction.
CPZE chlorpromazine equivalent.
Fig. 4. Forest plot: adverse effects, subgroup analysis by antipsychotic dose after reduction.
AIMS Abnormal Involuntary Movement Scale, BARS Barnes Akathisia Rating Scale, CPZE chlorpromazine equivalent, DIEPSS Drug-Induced Extrapyramidal Symptoms Scale, QOL quality of life, SAS Simpson-Angus Scale.
The results of the subgroup analyses of studies with a post-reduction CPZE dose > 200 mg/day are shown in Figs. 1–4. Negative symptoms (N = 4; n = 256; SMD = −0.45; 95% CI, −0.86 to −0.04; P = 0.03; I2 = 59%), EPSs assessed with the Simpson-Angus Scale (N = 2; n = 195; SMD = −0.37; 95% CI, −0.65 to −0.08; P = 0.01; I2 = 0%), and neurocognition (N = 2; n = 136; SMD = 0.69; 95% CI, 0.25–1.12; P = 0.002; I2 = 34%) were improved to a significantly greater extent in the dose reduction group than in the maintenance group. Funnel plots showed no significant publication bias in these findings (Supplementary Fig. S3B, C).
Discussion
Our qualitative analyses of prospective trials suggested that study duration < 1 year, age > 40 years, duration of illness > 10 years, and post-reduction CPZE dose > 200 mg/day were associated with a successful antipsychotic dose reduction in patients with schizophrenia. Our quantitative results from a meta-analysis of RCTs showed that dose reduction increased the risk of relapse but improved neurocognitive function. A subgroup analysis confirmed that an antipsychotic reduction that remained above the minimum effective dose (i.e., CPZE 200 mg/day) did not increase the risk of relapse compared with a maintained dose.
Factors related to a successful dose reduction identified by qualitative analysis of prospective trials
Although studies with a shorter duration were associated with greater success of antipsychotic dose reduction, these studies may underestimate the risk of relapse because it increases over time [1, 62]. Older age was another factor associated with a successful dose reduction, which suggests that elderly patients may need lower antipsychotics doses, given that an age-related decline in the dopaminergic system has been consistently reported; for example, dopamine receptor availability decreases by about 10% per decade [63, 64]. Moreover, a longer duration of illness was another factor associated with a successful dose reduction, which may indicate that chronic patients are a good candidate for dose reduction. Because functional deterioration as an index of illness severity is supposed to plateau 10 years after the onset of illness [65–67], patients with chronic schizophrenia may be treated with lower doses of antipsychotics once their illness has stabilized. Alternatively, in younger patients with a shorter illness duration, antipsychotic dose reduction should be more carefully implemented with close monitoring.
A moderate dose reduction seems to be a reasonable treatment option in the maintenance phase of schizophrenia. This approach is consistent with the results of a previous meta-analysis examining the effectiveness of antipsychotic low-dose vs. standard-dose treatment [68], although there are substantial differences in study design between low-dose treatment trials and dose reduction trials. This meta-analysis found no significant difference in relapse between low-dose treatment (i.e., 0.5–1.0 defined daily dose [DDD]) and standard-dose treatment (i.e., >1.0 DDD) but significant inferiority of very low-dose treatment (i.e., <0.5 DDD) vs. standard-dose treatment. This is in line with the concept of minimum effective dose, which is defined as the lowest dose that shows superiority over placebo [1, 69, 70]. Moreover, it is reassuring that, even if symptoms deteriorate, the patient’s condition can generally be re-stabilized by simply increasing the doses back to those at baseline.
Antipsychotic discontinuation is the most straightforward approach to nullify the exposure to antipsychotics. A systematic review suggested that older age, maintenance on a lower antipsychotic dose before discontinuation, shorter duration of untreated psychosis, older age at the onset of illness, lower severity of positive symptoms at baseline, better social functioning, and fewer previous relapses were associated with a lower risk of relapse after antipsychotic discontinuation [71]. These factors, except for older age, were not identified as risk factors in antipsychotic dose reduction in our results. Another review focusing on first-episode psychosis found no replicated predictive factors for continuing remission after discontinuation of antipsychotics [72]. These findings highlight the difference between antipsychotic discontinuation and dose reduction.
Factors related to an increased risk of relapse after dose reduction identified by meta-analysis of RCTs
The subgroup meta-analyses found that the following factors were associated with an increased risk of relapse: publication before 1999, study duration of ≥1 year, stable illness, age ≤ 40 years, outpatient setting, illness or treatment duration ≤ 10 years, use of FGAs or LAI-APs, mild or lower symptom severity, reduction rate ≥ 50%, post-reduction CPZE dose ≤ 200 mg/day, and reduction duration ≤ 2 months. Because older studies often used FGAs or LAI-APs and reduced the doses aggressively (e.g., by 80–90%), these factors seem to be closely related to each other. An inpatient setting would be advantageous for clinicians to closely monitor patients and more precisely detect signs of imminent worsening, which can avert a full-blown relapse. Stable or less severe illness was also associated with an increased risk of relapse, which means that patients with more severe illness are more likely to experience successful dose reduction. This seemingly paradoxical finding may indicate that the antipsychotic drugs used for these patients did not work in the first place and therefore dose reduction did not result in any symptom change. It may also be possible that clinicians were less likely to recognize relapse when a patient was already symptomatic. A shorter duration of dose reduction was related to an increased risk of relapse, suggesting that gradual dose reduction should be recommended to ensure stabilization before the next step of dose reduction and to avoid withdrawal/rebound symptoms. The speed of the reduction was not statistically significant but did show a similar trend in which a gradual dose reduction may be more favorable. This finding is supported by a meta-analysis indicating that gradual withdrawal for at least 3 weeks was associated with a lower risk of relapse [73], although the result was not consistent with a recent meta-analysis [1]. Further research is warranted to elucidate the relationship between the speed of reduction and risk of relapse. Reduction in the number of antipsychotics (e.g., switching from antipsychotic polypharmacy to monotherapy) is another clinically important issue because antipsychotic dose is associated with the number of antipsychotics. A recent meta-analysis showed that conversion of polypharmacy to monotherapy was related to an increased risk of study discontinuation [74], which should be taken into consideration when clinicians simultaneously reduce the dose and the number of antipsychotics.
Given that the sensitivity analyses did not find any significance for study duration, illness duration, and reduction rate, they do not seem to be robust factors for successful dose reduction. Moreover, antipsychotic dose before reduction was not associated with the risk of relapse, even when the cut-off was set beyond the upper end of the therapeutic dose range (i.e., CPZE dose > 600 mg/day). Furthermore, in the further subgroup analyses of studies with the predictive factor identified by the aforementioned subgroup analyses (i.e., a post-reduction CPZE dose > 200 mg/day), no other factors remained significant, suggesting that post-reduction CPZE dose is a robust factor for successful dose reduction.
Other outcomes of the meta-analysis
Our meta-analysis found that antipsychotic dose reduction significantly improved neurocognitive function, which aligns with the recent evidence indicating that neurocognitive impairment is a dose-dependent adverse effect of antipsychotics [5, 6]. Both studies included in the meta-analysis conducted a 50% dose reduction of risperidone and olanzapine, indicating that dose reduction of SGAs is still beneficial, even though they are considered to have fewer risks of neurocognitive dysfunction than FGAs [75, 76]. Impaired neurocognition is related to disturbances in the dopaminergic and cholinergic systems in the brain. All antagonist antipsychotic drugs are believed to exert their effects by blocking mesolimbic dopamine D2 receptors; however, they also block dopamine receptors in the prefrontal cortex, which can lead to impairment in cognitive control and working memory [77]. In addition, some antipsychotics, in particular olanzapine and clozapine, have marked anticholinergic effects, which can be associated with impairment in attention and memory [78]. An excessive blockade of dopaminergic and cholinergic transmission with antipsychotics can be relieved by antipsychotic dose reduction, which can ameliorate neurocognitive adverse effects. However, this finding in our meta-analysis was from only 2 RCTs and further studies are clearly needed to identify a strategy to counteract this problematic adverse effect of antipsychotics.
The subgroup analyses found that an antipsychotic dose reduction that did not exceed the minimum effective dose (i.e., CPZE 200 mg/day) was not associated with an increased risk of relapse. Moreover, such a reduction improved not only neurocognitive function but also EPSs and negative symptoms. EPSs are another dose-dependent adverse effect of antipsychotics [79]. Negative symptoms are one of the core features of schizophrenia, although some of them are represented by secondary negative symptoms due to excessive exposure to antipsychotics. Furthermore, given that (1) EPSs are correlated with negative symptoms [80], (2) negative symptoms are associated with neurocognitive impairment [81], and (3) poor neurocognitive performance is linked to severe EPSs [82], there is a close relationship among EPSs, neurocognitive impairment, and negative symptoms. Antipsychotic dose reduction may be a viable strategy for these domains, given that currently available treatment options for negative symptoms and neurocognitive impairment are limited both in quality and quantity [83, 84].
It should be emphasized that 2 studies revealed worsened clinical symptoms but improved adverse effects after dose reduction [28, 42], whereas 1 study showed a paradoxical finding in which symptoms improved but adverse effects worsened [53]; tardive adverse effects may worsen upon antipsychotic dose reduction. Physicians should consider this clinical dilemma during the therapeutic decision-making process. Although we also investigated QOL as an index of functioning, there was no difference between the dose reduction and maintenance groups.
Limitations
This systematic review should be interpreted in light of some limitations. First, the heterogeneity of the study design made comparisons among the studies difficult; however, the aim of this study was to identify factors associated with successful dose reduction rather than to simply synthesize relapse rates in dose reduction studies. For this purpose, we sorted the studies by study design, participants’ demographic and clinical characteristics, and dose reduction procedures that were potentially related to successful dose reduction. Second, the definition of relapse was not consistent across the studies; a temporary deterioration was managed by an increase in antipsychotics in some studies, whereas other studies recognized it as a relapse. Moreover, the results of 3 possible causes of study discontinuation could overlap with relapse and may not be independently interpreted because 10 studies included relapse in the criteria of withdrawal from the study. Third, an arbitrary definition of successful dose reduction was adopted in the qualitative analysis. Fourth, half of the included RCTs were conducted with an open-label design that is susceptible to biases such as patient and rater expectations. Fifth, the small number of studies included in the subgroup analysis is prone to a potential type II error for predictors of successful dose reduction. It should be emphasized that only 4 studies used SGAs and that no study examined dose reduction of LAI-SGAs. Further trials with a double-blind design examining antipsychotic dose reduction with oral SGAs or LAI-SGAs are certainly warranted to support the findings in this meta-analysis.
Conclusions
Antipsychotic dose reduction increased the risk of relapse but improved neurocognitive function. In most studies, patients were re-stabilized by increasing the doses back to the baseline level, even when their symptoms worsened. A subgroup analysis indicated that modest dose reduction not exceeding the minimum effective dose (i.e., CPZE 200 mg/day) was the only robust predictor of successful dose reduction; modest dose reduction was associated with improvements in EPSs and negative symptoms in the maintenance treatment of schizophrenia. Given a lack of RCTs of SGAs, clinicians are advised to closely monitor patients when reducing the doses of these antipsychotics. Considering substantial heterogeneity in study designs and insufficient quality of the data, optimal antipsychotic dose reduction strategies should currently be guided by individual patient characteristics.
Funding and disclosure
H.T. has received a research grant from Eli Lilly, a fellowship grant from the Japanese Society of Clinical Neuropsychopharmacology, and manuscript or speaker’s fees from Otsuka, Sumitomo Dainippon Pharma, Wiley, and Yoshitomiyakuhin. S.T. has no competing interests to disclose. H.U. has received research grants from Eisai, Meiji Seika Pharma, Otsuka, Sumitomo Dainippon Pharma, speaker’s fees from Eli Lilly, Meiji Seika Pharma, MSD, Otsuka, Pfizer, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin, and advisory panel fees from Sumitomo Dainippon Pharma. T.S. has received research grants from Eisai, Meiji Seika Pharma, and Mochida, and manuscript or speaker’s fees from Astellas Pharma, Eli Lilly, Elsevier, Janssen, Kyowa, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Novartis Pharma, Otsuka, Shionogi, Sumitomo Dainippon Pharma, Tsumura, Wiley, and Yoshitomiyakuhin. M.M. has received research grants from Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Pfizer, Shionogi, Takeda, and Tsumura, and speaker’s fees from Daiichi Sankyo, Eisai, Eli Lilly, Fujifilm RI Pharma, Janssen, Mochida, MSD, Nippon Chemipher, Novartis Pharma, Ono, Otsuka, Pfizer, Sumitomo Dainippon Pharma, Takeda, Tsumura, and Yoshitomiyakuhin. H.T. has received fellowship grants from Astellas Foundation for Research on Metabolic Disorders, the Canadian Institutes of Health Research (CIHR), Centre for Addiction and Mental Health (CAMH) Foundation, and the Japanese Society of Clinical Neuropsychopharmacology, speaker’s fees from Meiji Seika Pharma, Mochida, Otsuka, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin, and manuscript fees from Sumitomo Dainippon Pharma. We declare no financial relationships to disclose related to this study.
Supplementary information
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/23/2020
A Correction to this paper has been published: 10.1038/s41386-019-0598-y
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0573-7).
References
- 1.Leucht S, Tardy M, Komossa K, Heres S, Kissling W, Salanti G, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–71. doi: 10.1016/S0140-6736(12)60239-6. [DOI] [PubMed] [Google Scholar]
- 2.Goff DC, Falkai P, Fleischhacker WW, Girgis RR, Kahn RM, Uchida H, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174:840–9. doi: 10.1176/appi.ajp.2017.16091016. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi H, Kantor N, Sanches M, Fervaha G, Agid O, Remington G. One-year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: meta-analysis. Br J Psychiatry. 2017;211:137–43. doi: 10.1192/bjp.bp.116.186007. [DOI] [PubMed] [Google Scholar]
- 4.Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol. 1997;17:194–201. doi: 10.1097/00004714-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai H, Bies RR, Stroup ST, Keefe RS, Rajji TK, Suzuki T, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39:564–74. doi: 10.1093/schbul/sbr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori H, Yoshimura R, Katsuki A, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, et al. Several prescription patterns of antipsychotic drugs influence cognitive functions in Japanese chronic schizophrenia patients. Int J Psychiatry Clin Pract. 2012;16:138–42. doi: 10.3109/13651501.2011.631018. [DOI] [PubMed] [Google Scholar]
- 7.Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–35. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 8.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–35. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi H, Suzuki T, Uchida H, Watanabe K, Mimura M. Antipsychotic treatment for schizophrenia in the maintenance phase: a systematic review of the guidelines and algorithms. Schizophr Res. 2012;134:219–25. doi: 10.1016/j.schres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Shimomura Y, Kikuchi Y, Suzuki T, Uchida H, Mimura M, Takeuchi H. Treatment in the maintenance phase of schizophrenia: an updated systematic review of the guidelines and algorithms. Schizophr. Res. In press. 10.1016/j.schres.2019.09.013. [DOI] [PubMed]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 14.Manchanda R, Saupe R, Hirsch SR. Comparison between the Brief Psychiatric Rating Scale and the Manchester Scale for the rating of schizophrenic symptoms. Acta Psychiatr Scand. 1986;74:563–8. doi: 10.1111/j.1600-0447.1986.tb06285.x. [DOI] [PubMed] [Google Scholar]
- 15.Guy W. ECDEU Assessment manual for psychopharmacology-revised. DHEW Publication No. ADM 76-338. Rockville, MD: US Dept of Health, Education, andWelfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. p. 218–22.
- 16.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 17.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 18.Inada T. Evaluation and Diagnosis of Drug-Induced Extrapyramidal Symptoms [commentary on the DIEPSS and guide to its usage] Tokyo: Seiwa Shoten; 1996. [Google Scholar]
- 19.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–9. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 20.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–13. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 21.Auquier P, Simeoni MC, Sapin C, Reine G, Aghababian V, Cramer J, et al. Development and validation of a patient-based health-related quality of life questionnaire in schizophrenia: the S-QoL. Schizophr Res. 2003;63:137–49. doi: 10.1016/s0920-9964(02)00355-9. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchiya A, Ikeda S, Ikegami N, Nishimura S, Sakai I, Fukuda T, et al. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–53. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Li G, Li D, Cui H, Ning Y. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32:524–32. doi: 10.1177/0269881118756062. [DOI] [PubMed] [Google Scholar]
- 26.Yamanouchi Y, Sukegawa T, Inagaki A, Inada T, Yoshio T, Yoshimura R, et al. Evaluation of the individual safe correction of antipsychotic agent polypharmacy in Japanese patients with chronic schizophrenia: validation of safe corrections for antipsychotic polypharmacy and the high-dose method. Int J Neuropsychopharmacol. 2014;18:pyu016. [DOI] [PMC free article] [PubMed]
- 27.Takeuchi H, Suzuki T, Remington G, Bies RR, Abe T, Graff-Guerrero A, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39:993–8. doi: 10.1093/schbul/sbt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CY, Xiang YT, Cai ZJ, Weng YZ, Bo QJ, Zhao JP, et al. Risperidone maintenance treatment in schizophrenia: a randomized, controlled trial. Am J Psychiatry. 2010;167:676–85. doi: 10.1176/appi.ajp.2009.09030358. [DOI] [PubMed] [Google Scholar]
- 29.Rouillon F, Chartier F, Gasquet I. Strategies of treatment with olanzapine in schizophrenic patients during stable phase: results of a pilot study. Eur Neuropsychopharmacol. 2008;18:646–52. doi: 10.1016/j.euroneuro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Uchida H, Suzuki T, Yamazawa R, Tomita M, Nemoto T, Kimura Y, et al. Reducing the dose of antipsychotic agents ameliorates visual hypersensitivity attack: an ideal treatment option in terms of the adverse effect. J Clin Psychopharmacol. 2006;26:50–5. doi: 10.1097/01.jcp.0000195384.04008.25. [DOI] [PubMed] [Google Scholar]
- 31.Kinion ES, Campbell JM, Linc LG, Paradise N. Decreasing neuroleptic doses in older adults with schizophrenia. J Clin Geropsychol. 2000;6:15–23. [Google Scholar]
- 32.Volavka J, Cooper TB, Czobor P, Lindenmayer JP, Citrome LL, Mohr P, et al. High-dose treatment with haloperidol: the effect of dose reduction. J Clin Psychopharmacol. 2000;20:252–6. doi: 10.1097/00004714-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Hirschowitz J, Hitzemann R, Piscani K, Burr G, Frecska E, Culliton D, et al. The Dose Reduction in Schizophrenia (DORIS) Study: a final report. Schizophr Res. 1997;23:31–43. doi: 10.1016/S0920-9964(96)00074-6. [DOI] [PubMed] [Google Scholar]
- 34.Schooler NR, Keith SJ, Severe JB, Matthews SM, Bellack AS, Glick ID, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54:453–63. doi: 10.1001/archpsyc.1997.01830170079011. [DOI] [PubMed] [Google Scholar]
- 35.Hogarty GE, McEvoy JP, Ulrich RF, DiBarry AL, Bartone P, Cooley S, et al. Pharmacotherapy of impaired affect in recovering schizophrenic patients. Arch Gen Psychiatry. 1995;52:29. doi: 10.1001/archpsyc.1995.03950130029004. [DOI] [PubMed] [Google Scholar]
- 36.Inderbitzin LB, Lewine RR, Scheller-Gilkey G, Swofford CD, Egan GJ, Gloersen BA, et al. A double-blind dose-reduction trial of fluphenazine decanoate for chronic, unstable schizophrenic patients. Am J Psychiatry. 1994;151:1753–9. doi: 10.1176/ajp.151.12.1753. [DOI] [PubMed] [Google Scholar]
- 37.Newcomer JW, Riney SJ, Vinogradov S, Csernansky JG. Plasma prolactin and homovanillic acid as markers for psychopathology and abnormal movements after neuroleptic dose decrease. Psychopharmacol Bull. 1992;28:101–7. [PubMed] [Google Scholar]
- 38.Faraone SV, Green AI, Brown W, Yin P, Tsuang MT. Neuroleptic dose reduction in persistently psychotic patients. Hosp Community Psychiatry. 1989;40:1193–5. doi: 10.1176/ps.40.11.1193. [DOI] [PubMed] [Google Scholar]
- 39.Hogarty GE, McEvoy JP, Munetz M, DiBarry AL, Bartone P, Cather R, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry. 1988;45:797–805. doi: 10.1001/archpsyc.1988.01800330021002. [DOI] [PubMed] [Google Scholar]
- 40.Cookson IB. The effects of a 50% reduction of cis(z)-flupenthixol decanoate in chronic schizophrenic patients maintained on a high dose regime. Int Clin Psychopharmacol. 1987;2:141–9. doi: 10.1097/00004850-198704000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Johnson DA, Ludlow JM, Street K, Taylor RD. Double-blind comparison of half-dose and standard-dose flupenthixol decanoate in the maintenance treatment of stabilised out-patients with schizophrenia. Br J Psychiatry. 1987;151:634–8. doi: 10.1192/bjp.151.5.634. [DOI] [PubMed] [Google Scholar]
- 42.Kane JM, Rifkin A, Woerner M, Reardon G, Sarantakos S, Schiebel D, et al. Low-dose neuroleptic treatment of outpatient schizophrenics. I. Preliminary results for relapse rates. Arch Gen Psychiatry. 1983;40:893–6. doi: 10.1001/archpsyc.1983.01790070083010. [DOI] [PubMed] [Google Scholar]
- 43.Bogers J, Schulte PFJ, Broekman TG, Moleman P, de Haan L. Dose reduction of high-dose first-generation antipsychotics or switch to ziprasidone in long-stay patients with schizophrenia: A 1-year double-blind randomized clinical trial. Eur Neuropsychopharmacol. 2018;28:1024–34. doi: 10.1016/j.euroneuro.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 receptor occupancy study. JAMA Psychiatry. 2015;72:927–34. doi: 10.1001/jamapsychiatry.2015.0891. [DOI] [PubMed] [Google Scholar]
- 45.Uchida H, Suzuki T, Graff-Guerrero A, Mulsant BH, Pollock BG, Arenovich T, et al. Therapeutic window for striatal dopamine D(2/3) receptor occupancy in older patients with schizophrenia: a pilot PET study. Am J Geriatr Psychiatry. 2014;22:1007–16. doi: 10.1016/j.jagp.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Kawai N, Yamakawa Y, Baba A, Nemoto K, Tachikawa H, Hori T, et al. High-dose of multiple antipsychotics and cognitive function in schizophrenia: the effect of dose-reduction. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1009–14. doi: 10.1016/j.pnpbp.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Tsuruta S, Nomura S, Yoshino A. Neuroleptic dose reduction in stable chronic schizophrenia. Schizophr Res. 2003;59:95–6. doi: 10.1016/s0920-9964(01)00374-7. [DOI] [PubMed] [Google Scholar]
- 48.Gefvert O, Lundberg T, Wieselgren IM, Bergstrom M, Langstrom B, Wiesel F, et al. D(2) and 5HT(2A) receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. Eur Neuropsychopharmacol. 2001;11:105–10. doi: 10.1016/s0924-977x(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 49.Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156:869–75. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 50.Harris MJ, Heaton RK, Schalz A, Bailey A, Patterson TL. Neuroleptic dose reduction in older psychotic patients. Schizophr Res. 1997;27:241–8. doi: 10.1016/S0920-9964(97)00083-2. [DOI] [PubMed] [Google Scholar]
- 51.Canuso CM, Goldman MB. Does minimizing neuroleptic dosage influence hyponatremia? Psychiatry Res. 1996;63:227–9. doi: 10.1016/0165-1781(96)02793-x. [DOI] [PubMed] [Google Scholar]
- 52.Dale R, Longdon M, Seeman MV. Reducing the dose of depot neuroleptics in stable schizophrenia. J Psychiatry Neurosci. 1994;19:278–81. [PMC free article] [PubMed] [Google Scholar]
- 53.Leblanc G, Cormier H, Gagne MA, Vaillancourt S. Effects of neuroleptic reduction in schizophrenic outpatients receiving high doses. Can J Psychiatry. 1994;39:223–9. doi: 10.1177/070674379403900406. [DOI] [PubMed] [Google Scholar]
- 54.Smith RC. Lower-dose therapy with traditional neuroleptics in chronically hospitalized schizophrenic patients. Arch Gen Psychiatry. 1994;51:427–9. doi: 10.1001/archpsyc.1994.03950050087011. [DOI] [PubMed] [Google Scholar]
- 55.Solgaard T, Kistrup K, Aaes-Jorgensen T, Gerlach J. Zuclopenthixol decanoate in maintenance treatment of schizophrenic outpatients. Minimum effective dose and corresponding serum levels. Pharmacopsychiatry. 1994;27:119–23. doi: 10.1055/s-2007-1014290. [DOI] [PubMed] [Google Scholar]
- 56.Heresco-Levy U, Greenberg D, Lerer B, Dasberg H, Brown WA. Trial of maintenance neuroleptic dose reduction in schizophrenic outpatients: two-year outcome. J Clin Psychiatry. 1993;54:59–62. [PubMed] [Google Scholar]
- 57.Van Putten T, Marshall BD, Liberman R, Mintz J, Kuehnel TG, Bowen L, et al. Systematic dosage reduction in treatment-resistant schizophrenic patients. Psychopharmacol Bull. 1993;29:315–20. [PubMed] [Google Scholar]
- 58.Hirschowitz J, Hitzemann R, Burr G, Schwartz A. A new approach to dose reduction in chronic schizophrenia. Neuropsychopharmacology. 1991;5:103–13. [PubMed] [Google Scholar]
- 59.Kistrup K, Gerlach J, Aaes-Jorgensen T, Larsen NE. Perphenazine decanoate and cis(z)-flupentixol decanoate in maintenance treatment of schizophrenic outpatients. Serum levels at the minimum effective dose. Psychopharmacology. 1991;105:42–8. doi: 10.1007/BF02316862. [DOI] [PubMed] [Google Scholar]
- 60.Faraone SV, Curran JP, Laughren T, Faltus F, Johnston R, Brown WA. Neuroleptic bioavailability, psychosocial factors, and clinical status: a 1-year study of schizophrenic outpatients after dose reduction. Psychiatry Res. 1986;19:311–22. doi: 10.1016/0165-1781(86)90124-1. [DOI] [PubMed] [Google Scholar]
- 61.Lehmann HE, Wilson WH, Deutsch M. Minimal maintenance medication: effects of three dose schedules on relapse rates and symptoms in chronic schizophrenic outpatients. Compr Psychiatry. 1983;24:293–303. doi: 10.1016/0010-440x(83)90057-3. [DOI] [PubMed] [Google Scholar]
- 62.Kishi T, Ikuta T, Matsui Y, Inada K, Matsuda Y, Mishima K, et al. Effect of discontinuation v. maintenance of antipsychotic medication on relapse rates in patients with remitted/stable first-episode psychosis: a meta-analysis. Psychol Med. 2018:49;1–8. [DOI] [PubMed]
- 63.Matuskey D, Worhunksy P, Correa E, Pittman B, Gallezot JD, Nabulsi N, et al. Age-related changes in binding of the D2/3 receptor radioligand [(11)C](+)PHNO in healthy volunteers. Neuroimage. 2016;130:241–7. doi: 10.1016/j.neuroimage.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida H, Mamo DC, Mulsant BH, Pollock BG, Kapur S. Increased antipsychotic sensitivity in elderly patients: evidence and mechanisms. J Clin Psychiatry. 2009;70:397–405. doi: 10.4088/jcp.08r04171. [DOI] [PubMed] [Google Scholar]
- 65.Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–97. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- 66.Belitsky R, McGlashan TH. The manifestations of schizophrenia in late life: a dearth of data. Schizophr Bull. 1993;19:683–5. doi: 10.1093/schbul/19.4.683. [DOI] [PubMed] [Google Scholar]
- 67.McGlashan TH. A selective review of recent North American long-term followup studies of schizophrenia. Schizophr Bull. 1988;14:515–42. doi: 10.1093/schbul/14.4.515. [DOI] [PubMed] [Google Scholar]
- 68.Uchida H, Suzuki T, Takeuchi H, Arenovich T, Mamo DC. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr Bull. 2011;37:788–99. doi: 10.1093/schbul/sbp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40:314–26. doi: 10.1093/schbul/sbu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 71.Tani H, Suzuki T, Wolfgang Fleischhacker W, Tomita M, Mimura M, Uchida H. Clinical characteristics of patients with schizophrenia who successfully discontinued antipsychotics: a literature review. J Clin Psychopharmacol. 2018;38:582–9. doi: 10.1097/JCP.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 72.Bowtell M, Ratheesh A, McGorry P, Killackey E, O'Donoghue B. Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. A systematic review. Schizophr Res. 2018;197:9–18. doi: 10.1016/j.schres.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Viguera AC, Baldessarini RJ, Hegarty JD, van Kammen DP, Tohen M. Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54:49–55. doi: 10.1001/archpsyc.1997.01830130055011. [DOI] [PubMed] [Google Scholar]
- 74.Matsui K, Tokumasu T, Takekita Y, Inada K, Kanazawa T, Kishimoto T, et al. Switching to antipsychotic monotherapy vs. staying on antipsychotic polypharmacy in schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2019;209:50–7. doi: 10.1016/j.schres.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 75.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–72. [DOI] [PubMed]
- 76.Désaméricq G, Schurhoff F, Meary A, Szöke A, Macquin-Mavier I, Bachoud-Lévi AC, Maison P. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol. 2014;70:127–34. [DOI] [PubMed]
- 77.Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–25. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–24. [DOI] [PubMed]
- 79.Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31:497–502. doi: 10.1097/JCP.0b013e3182214aad. [DOI] [PubMed] [Google Scholar]
- 80.Farreny A, Savill M, Priebe S. Correspondence between negative symptoms and potential sources of secondary negative symptoms over time. Eur Arch Psychiatry Clin Neurosci. 2018;268:603–09. doi: 10.1007/s00406-017-0813-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dominguez Mde G, Viechtbauer W, Simons CJ, van Os J, Krabbendam L. Are psychotic psychopathology and neurocognition orthogonal? A systematic review of their associations. Psychol Bull. 2009;135:157–71. doi: 10.1037/a0014415. [DOI] [PubMed] [Google Scholar]
- 82.Fervaha G, Agid O, Takeuchi H, Lee J, Foussias G, Zakzanis KK, et al. Extrapyramidal symptoms and cognitive test performance in patients with schizophrenia. Schizophr Res. 2015;161:351–6. doi: 10.1016/j.schres.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 83.Iwata Y, Nakajima S, Suzuki T, Keefe RS, Plitman E, Chung JK, et al. Effects of glutamate positive modulators on cognitive deficits in schizophrenia: a systematic review and meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2015;20:1151–60. doi: 10.1038/mp.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang AC, Tsai SJ. New targets for schizophrenia treatment beyond the dopamine hypothesis. Int J Mol Sci. 2017;18:E1689. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.