Abstract
Context
Adenosine 5’-monophosphate-activated protein kinase-α (AMPKα) is a mediator of exercise-induced glucose uptake in skeletal muscle.
Objective
We evaluated whether AMPKα expression and phosphorylation are reduced in skeletal muscle and adipose tissue of patients with hypogonadotropic hypogonadism (HH), and whether testosterone replacement therapy results in restoration of the expression and phosphorylation of AMPKα.
Design
This is a secondary analysis of a previously completed trial that showed an insulin-sensitizing effect of testosterone therapy in men with type 2 diabetes and HH.
Setting
Clinical research center at university.
Patients
Thirty-two men with HH and 32 eugonadal men were compared at baseline.
Interventions
Men with HH were treated with intramuscular injections of testosterone or placebo every 2 weeks for 22 weeks. Quadriceps muscle biopsies and subcutaneous abdominal fat biopsies were obtained before and after 4-hour euglycemic hyperinsulinemic clamp, prior to and after testosterone or placebo therapy.
Outcome Measures and Results
mRNA expression of AMPKα in hypogonadal men was lower by 37% in adipose tissue and 29% in skeletal muscle, respectively, compared with levels in eugonadal men, while phosphorylated AMPKα was lower by 22% and 28%, respectively. Following testosterone replacement, the expression of AMPKα did not alter in the fasting state but increased markedly by 41% and 46% in adipose tissue and muscle, respectively, after the clamp. In contrast, phosphorylated AMPKα increased by 69% in muscle after testosterone therapy but did not change following the clamp.
Conclusions
Testosterone modulates the expression of AMPKα and phosphorylated AMPKα. These effects may contribute to the improved insulin sensitivity following testosterone therapy.
Keywords: testosterone, hypogonadism, AMPK, AMP kinase, adipose, muscle
Our previous work has shown that one-third of males with type 2 diabetes have hypogonadotropic hypogonadism (HH) (1). These men with HH are more insulin resistant, have increased fat mass and less lean mass as compared with eugonadal men. Testosterone replacement restores insulin sensitivity, as it lowers mediators of inflammation and insulin resistance and increases mediators of insulin signaling, namely insulin receptor, insulin receptor substrate (IRS)-1, AKT serine/threonine kinase 2 and glucose transporter type 4 (GLUT4) (2).
Adenosine 5′-monophosphate-activated protein kinase-α (AMPKα) induces the phosphorylation of AKT kinase, thus activating it (3). This, in turn, results in the transport of GLUT4 to the membrane to increase glucose transport. This mechanism, although independent of insulin action, can amplify insulin signal transduction, since insulin action also involves AKT kinase and GLUT4. AMPKα is important in mediating the action of exercise and in enhancing glucose transport. This pathway also partially mediates the insulin-sensitizing action of metformin in liver and of thiazolidinediones in skeletal muscle (4, 5).
Our recent work on the insulin-sensitizing effect of testosterone in patients with HH, along with the knowledge that muscular exercise induces AMPKα to increase glucose uptake and that testosterone administration increases muscle growth, led us to consider the possibility that AMPKα expression may be diminished in such patients and that testosterone replacement therapy may restore it. We hypothesized that (1) men with HH have a lower expression and phosphorylation of AMP kinase in muscle and adipose tissue, and (2) testosterone replacement restores cellular AMPKα expression and phosphorylation to normal.
Patients and Methods
We recruited men with type 2 diabetes aged 30 to 65 years, who had glycated hemoglobin A1c (HbA1c) ≤ 8% and stable diabetes regimen for 3 months, for a randomized placebo-controlled trial of testosterone replacement that was funded by the National Institutes of Health. HH was defined as subnormal free testosterone concentration (<5 ng/dL) on 2 occasions along with low or normal luteinizing hormone concentrations. Men with known organic causes of hypogonadism, such as panhypopituitarism, congenital HH, prolactinoma or severe head trauma, were excluded from the study. The study was designed to evaluate the metabolic aspects of testosterone replacement in men with obesity-associated HH. None of the study subjects had previously been diagnosed with hypogonadism. The details on study design, as well as the results on insulin sensitivity, inflammation and body composition from that trial, have previously been published (2). The results presented below are secondary analyses of observations from that trial. Briefly, 50 eugonadal men and 44 men with HH participated in the primary trial. They underwent euglycemic hyperinsulinemic clamp (EHC), subcutaneous fat biopsies, and quadriceps muscle biopsies. Hypogonadal men were randomized to receive intramuscular injections of testosterone (therapy initiated with 200 mg) or placebo (saline 1 mL) every 2 weeks for 22 weeks. EHC, muscle biopsies, and fat biopsies were performed 1 week after last injection (week 23). The dose of testosterone was adjusted to keep serum free testosterone concentrations in the mid-normal range. Two men in the testosterone group and 8 men in the placebo group dropped out and did not complete the study. The main cause of dropout was unwillingness to complete all required study procedures, which included sequential fat and muscle biopsies (2). There were no differences in baseline characteristics of study completers and dropouts. Some subjects completed the study visits but did not undergo fat and muscle biopsies. Fat biopsies were available on 32 eugonadal men and 32 hypogonadal men at baseline. Of the hypogonadal men, 17 men in the testosterone group and 11 men in the placebo group provided fat biopsies at 23 weeks. Twenty eugonadal men and 22 men with HH agreed to provide muscle biopsies at baseline (week 0). At the end of the study (week 23), 10 men in the testosterone arm and 4 men in the placebo arm agreed to provide muscle biopsies.
The protocol was approved by the Human Research Board of the State University of New York at Buffalo, informed consent was signed by all subjects, and the trial was registered with clinicaltrials.gov (NCT01127659) (2).
Hyperinsulinemic euglycemic clamp (EHC)
All subjects underwent 4-hour EHC at baseline and 23 weeks as previously described (2). Insulin clamp was started with a priming dose of short-acting human insulin (Humulin R; Eli Lilly & Co., Indianapolis, IN) given over 10 minutes and then an infusion at the rate of 80 mIU/m2/min. Infusion of 20% glucose was titrated to maintain arterialized blood glucose concentration at 100 mg/dL. Insulin sensitivity was calculated from the glucose infusion rates during the last 30 minutes of the 4-hour EHC (steady state) and expressed as glucose uptake per lean body mass (2). A biopsy from the vastus lateralis muscle was obtained at the start of the clamp, and then repeated on the contralateral vastus lateralis after 3.5 hours. Subcutaneous fat biopsy was obtained from abdominal area at the start of the clamp, and then repeated on the contralateral side of the abdomen after 3.5 hours. The EHC and biopsies were carried out at baseline and week 23.
The mean coefficient of variation of glucose during the clamps was 5.8%. The mean glucose concentrations during the last 30 minutes were 99.4 ± 4.9 mg/dL and 99.7 ± 3.4 mg/dL at 0 weeks in men with and without HH. The mean glucose concentration during steady state was 103.9 ± 15.8 mg/dL at 23 weeks in men with HH (P = 0.20 compared with 0 weeks). Mean insulin concentrations achieved during the clamps were 89 µIU/mL (75–110 µIU/mL) and were not different between baseline and end of the study clamps (2).
Fat aspiration procedure
Subcutaneous fat tissue aspiration was performed at baseline and at 23 weeks on abdomen at a 10-cm distance from umbilicus under sterile conditions and local anesthesia (6). A sample of 0.5 to 3 g was aspirated and cleared from blood and fluid contaminants by centrifugation. The adipose tissue was collected into a separate sterile tube, washed twice with cold sterile phosphate buffered saline (PBS) and centrifuged to remove the PBS. Total RNA and total cell lysates were prepared from the adipose tissue.
Percutaneous skeletal muscle biopsy
Muscle biopsies of vastus lateralis muscle were performed under local anesthesia (7). A 5-mm incision was made 10 to 15 cm above the patella and a biopsy needle connected to suction was inserted into the muscle. Approximately 200 mg of muscle tissue was obtained, washed with PBS, and then stored in liquid nitrogen (−80°C) until processing for specific assays.
Dual-energy x-ray absorptiometry(DEXA) scan
Total and regional (appendicular and trunk) lean body mass and fat mass were measured by DEXA at baseline and week 23. The within-center variability over time in measurement of lean mass is 0.9% and of fat mass is 1.2% (8, 9).
Laboratory assays
Total testosterone and estradiol concentrations were measured by liquid chromatography tandem mass spectrometry (Quest Diagnostics) (10). Tracer equilibrium dialysis was used to separate the free testosterone and free estradiol (Nichols Institute, Chantilly, VA and San Juan Capistrano, CA) (10, 11).
Quantification of mRNA Expression by reverse transcription-polymerase chain reaction (RT-PCR)
Expression of AMPKα1 was measured in adipose tissue and skeletal muscle by RT-PCR. Total RNA was isolated from adipose tissue and skeletal muscle using the commercially available RNAqueous-4PCR Kit (Ambion, Austin, TX). Real time RT-PCR was performed using Bio-Rad CFX-Connect (Hercules, CA), Sybergreen master mix (Qiagen, CA), and gene-specific primers (Life Technologies, MD). All values were normalized to the expression of a group of housekeeping genes including actin, ubiquitin C and cyclophilin A. The normalization factor used is calculated by geNorm software and is based on the values of all housekeeping genes used.
Western blotting
Activation of AMPKα subunit was assessed by measuring phosphorylation of residue T172 in total cell lysates prepared from adipose tissue and skeletal muscle samples. Polyclonal antibodies against pT172 AMPKα subunit (Abcam, Cambridge, MA) and actin (Santa Cruz Biotechnology, CA) were used and all values were corrected for loading to actin.
Statistical analysis
Group comparisons were performed by two-sided t tests, Mann-Whitney rank sum tests, and χ 2 tests as appropriate. Percentage change from baseline was calculated and statistical analysis for change from baseline was carried out using paired t test or one-way repeated measures analysis of variance (RMANOVA) followed by Holm-Sidak post hoc test. Data that were not normally distributed (Kolmogorov-Smirnov test) were log-transformed to perform the parametric statistical tests using SPSS software (SPSS Inc, Chicago, IL). Pearson correlation between variables of interest was performed. Data are presented as mean ± SD, except for expression and phosphorylation of AMPKα, which are shown in graphs as mean ± SE. Nonnormal data are presented as median (25th, 75th percentile). P < 0.05 was considered significant. Results for posttreatment group comparisons are presented as mean difference (95% confidence interval [CI]).
Results
Baseline demographics, laboratory tests, and medication use of participants for these analyses are shown in Table 1. These data are similar to the overall study population characteristics reported previously (2). Following testosterone treatment for 23 weeks, free testosterone concentration increased by 7.8 (4.2, 11.2) ng/dL (P < 0.001, placebo-subtracted difference, 95% CI). The mean free testosterone concentrations in the testosterone and placebo groups at study end were 12.0 ± 6.8 and 5.1 ± 1.7 ng/dL, respectively. The mean total testosterone concentrations in the testosterone and placebo groups at study end were 547 ± 234 and 280 ± 132 ng/dL, respectively. Total body subcutaneous fat mass decreased (−3.3 kg [−5.8, −0.9]; P = 0.009) and lean mass increased (2.9 kg [0.4, 5.4]; P = 0.03) after testosterone therapy. Glucose infusion rate increased after testosterone therapy (2.4 mg/kg/fat-free mass/min [0.2, 5.2]; P = 0.03). There was no change in HbA1c (0.2% [−0.6, 0.9]; P = 0.4).
Table 1.
Baseline Comparison Between Hypogonadal and Eugonadal Men
| Hypogonadal | Eugonadal | P | |
|---|---|---|---|
| Number of participants | 32 | 32 | |
| Age, years | 54.0 ± 7.2 | 51.9 ± 9.1 | 0.31 |
| BMI, kg/m2 | 39.8 ± 8.2 | 34.6 ± 6.2 | 0.005 |
| Duration of diabetes, years | 9.4 ± 8.6 | 10.9 ± 9.8 | 0.50 |
| Total testosterone, ng/dL | 248 ± 84 | 482 ± 202 | <0.001 |
| Free testosterone, ng/dL | 4.3 ± 1.2 | 7.5 ± 1.5 | <0.001 |
| Total estradiol, pg/mL | 27.4 ± 13.0 | 25.5 ± 12.4 | 0.65 |
| Free estradiol, pg/mL | 0.58 ± 0.26 | 0.60 ± 0.25 | 0.88 |
| HbA1c, % | 7.0% ± 1.1% | 7.1% ± 1.1% | 0.62 |
| Total body subcutaneous fat mass, kg | 44.3 ± 13.1 | 35.3 ± 13.2 | 0.009 |
| Total body lean mass, kg | 70.7 ± 10.9 | 65.5 ± 9.5 | 0.08 |
| GIR, mg/kg fat-free mass/min | 6.6 ± 3.7 | 9.6 ± 5.6 | 0.02 |
| Medications | |||
| Metformin | 91% | 78% | 0.19 |
| Insulin | 50% | 38% | 0.27 |
| Thiazolidinediones | 16% | 10% | 0.58 |
Abbreviations: BMI, body mass index; GIR, glucose infusion rate; HbA1c, glycated hemoglobin A1c
AMPKα expression and phosphorylation in hypogonadal and eugonadal men at 0 weeks
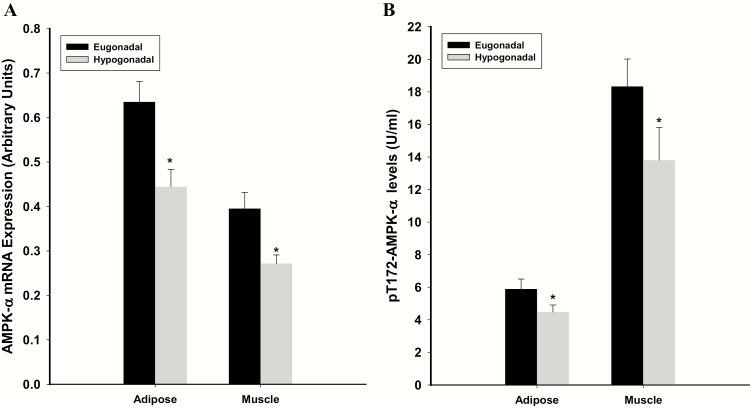
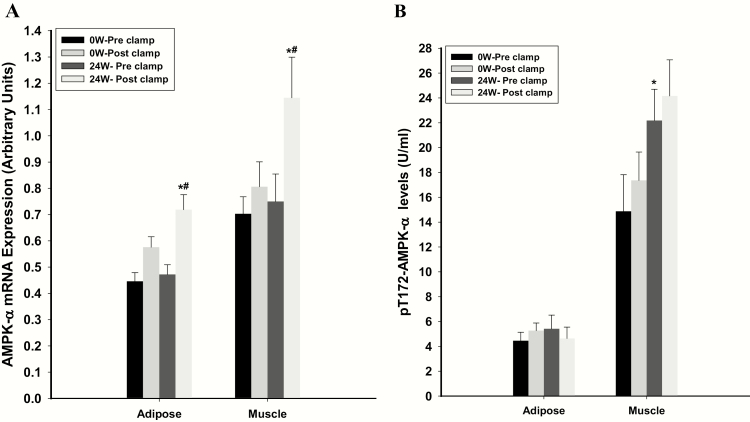
The mRNA expression of AMPKα in hypogonadal men was lower by 37% in adipose tissue and 29% in skeletal muscle, compared with eugonadal diabetic men (Fig. 1A). Phosphorylated AMPKα was lower in hypogonadal men by 22% in adipose tissue and 28% in skeletal muscle compared with eugonadal men (Fig. 1B). Insulin-glucose infusion during EHC did not alter AMPKα expression or phosphorylated AMPKα in muscle or adipose tissue at 0 week in hypogonadal men (Fig. 2). AMPKα expression in muscle increased by 38% (from 0.43 ± 0.06 to 0.59 ± 0.07 (relative units); P= 0.026) in eugonadal men following EHC, while phosphorylation did not change (P= 0.22). EHC did not induce a significant change in adipose tissue AMPK expression in eugonadal men (P= 0.46). We did not analyze AMPKα phosphorylation in adipose tissue in eugonadal men following EHC.
Figure 1.
AMPKα expression (A) and phosphorylation (B) in hypogonadal and eugonadal men at 0 weeks. *P < 0.05 between eugonadal and hypogonadal groups.
Figure 2.
AMPKα expression (A) and phosphorylation (B) in hypogonadal men pre- and post-EHC at 0 weeks and 24 weeks following testosterone therapy. *P < 0.05 for comparison with 0 weeks. #P < 0.05 for comparison with pre-clamp values.
AMPKα expression and phosphorylation after testosterone therapy
Following testosterone replacement, the expression of AMPKα did not alter in the fasting state but increased markedly by 41% ± 9% and 46% ± 11% in adipose tissue and muscle, respectively, after the infusion of insulin and glucose during the clamp procedure (Fig. 2A). In contrast, AMPKα phosphorylation markedly increased by 69% ± 31% in muscle after testosterone therapy but did not change following EHC (Fig. 2B). Change in insulin sensitivity (glucose infusion rate/lean body mass) was not related to the increase in AMPKα phosphorylation in muscle (r = 0.23; P= 0.48). There was no effect of testosterone therapy or EHC on AMPKα phosphorylation in adipose tissue (Fig. 2B). There were no changes in AMPKα expression or phosphorylation following placebo treatment (data not shown). The changes in expression or phosphorylation of AMPKα were similar in men using metformin for treatment of their diabetes (versus those who were not), thiazolidinediones (versus those who were not) or insulin (versus those who were not; data not shown).
Discussion
Our data show clearly that the expression of AMPKα, as well as its phosphorylation, is significantly reduced in the adipose tissue and skeletal muscle of patients with HH when compared with levels in eugonadal patients with type 2 diabetes. Following testosterone replacement, there was a significant increase in the phosphorylated AMPKα in skeletal muscle, but not in adipose tissue. These data link with our recent observations that patients with HH have 36% additional insulin resistance compared with eugonadal patients with type 2 diabetes, since the reduction in phosphorylated AMPKα would cause further diminishment of glucose uptake (2). The replacement of testosterone results in the restoration of insulin sensitivity and the increase in phosphorylated AMPKα. In this regard, the effects of testosterone are quite similar to well-known insulin sensitizers, the thiazolidinediones. Coletta et al showed that pioglitazone treatment for 6 months in patients with type 2 diabetes increases insulin sensitivity by 30% and AMPKα phosphorylation by 38% in skeletal muscle (5). It is believed that the effect of pioglitazone on AMPKα may be mediated via an increase in adiponectin (5). However, the increase in phosphorylated AMPKα following testosterone happened in the absence of change in adiponectin, which we have previously shown does not change following testosterone therapy (2).
It is of interest that the fasting levels of AMPKα expression did not alter after testosterone replacement. However, the expression of AMPKα increased following the infusion of insulin and glucose during EHC in both adipose and muscle tissues. This is also in contrast to a lack of change in the expression of AMPKα during the infusion of glucose and insulin prior to testosterone replacement. The increase in AMPKα expression following combined insulin and testosterone administration is perhaps a reflection of their synergistic anabolic effects on muscle protein synthesis and growth (7, 12, 13).
AMPKα is a ubiquitous enzyme known to regulate macronutrient intake and energy expenditure (14). It is present even in unicellular organisms performing similar energy related functions. Its synthesis and phosphorylation are dependent upon the nutritional status, such that caloric deprivation (fasting state) results in its activation/increase. Caloric deprivation results in a decrease in adenosine triphosphate/adenosine monophosphate ratio, triggering an increase in AMPKα. In contrast, caloric intake reduces phosphorylated AMPKα in the skeletal muscle of rats (15). Insulin and glucose infusion also reduce phosphorylated AMPKα in mouse skeletal muscle (16). In our study, we found that despite an increase AMPKα expression following EHC in both adipose tissue and skeletal muscle, there was no change in phosphorylated AMPKα. Thus, it may appear that AMPKα expression and phosphorylation status are not directly coupled. The AMPKα pathway may provide a non–insulin-mediated mechanism for glucose uptake in the fasting state following testosterone therapy. However, in the “fed state” of insulin-glucose infusion, the phosphorylation of AMPKα is not needed, or is relatively downregulated despite an increase in its expression. This may also explain the lack of correlation between the increase in phosphorylated AMPKα (pre-clamp) and the increase in glucose infusion rate during the clamp following testosterone therapy.
It has recently been shown that the deletion of AMPKα in myelogenous cells leads to accelerated atherogenicity in low-density lipoprotein receptor knock-out mice (17, 18). This implies that AMPKα exerts an antiatherogenic effect. Thus, it is possible that the decrease in AMPKα expression in HH patients may potentially contribute to greater atherogenicity. Indeed, the state of hypogonadism in the male results in an increase in cardiovascular events (19). The increase in AMPKα expression after testosterone replacement may help reduce atherogenesis and cardiovascular events in such patients. It is noteworthy that the pro-inflammatory cytokines secreted by macrophages which increase following AMPKα deletion are tumor necrosis factor-α (TNFα) and interleukin 1β (IL-1β) (17), which are also suppressed significantly after treatment of HH patients with testosterone (2). Thus, AMPKα provides a link between metabolic and inflammatory processes. In this context, it is important to note that while IL-1β is toxic to the insulin secreting β-cell in the pancreatic islet, TNFα is the major cytokine that interferes with insulin signal transduction (20, 21).
In conclusion, AMPKα expression and phosphorylation are reduced in the adipose tissue and the skeletal muscle of patients with HH. Following the replacement of testosterone, there is an increase in the expression of AMPKα in adipose tissue and skeletal muscle after EHC. There is also an increase in the phosphorylation of AMPKα in skeletal muscle after testosterone administration without EHC. Thus, testosterone and insulin modulate the expression and phosphorylation of AMPKα. These effects may contribute to the improved insulin sensitivity and glucose homeostasis after testosterone replacement.
Acknowledgments
The authors are grateful to Zahid Sayeed for assistance in formatting and submission of this manuscript.
Financial Support: Supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (RO1 grant to PD; R01DK075877).
Author contributions: P.D. put forth the hypothesis, planned and interpreted the study, and wrote the manuscript. H.G. and S.D. analyzed data and wrote the manuscript. H.G, S.A and K.G. analyzed samples. S.D., M.B., S.S., A.C. and A.M. executed the study. P.D., S.D. and H.G. are the guarantors of this work and, as such, have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Clinical Trial Information: Clinicaltrials.gov ID no. NCT01127659 (first posted May 21, 2010).
Glossary
Abbreviations
- AMPKα
adenosine 5’-monophosphate-activated protein kinase-α
- EHC
euglycemic hyperinsulinemic clamp
- GLUT4
glucose transporter type 4
- HbA1c
glycated hemoglobin A1c
- HH
hypogonadotropic hypogonadism
- PBS
phosphate buffered saline
Additional Information
H.G., M.B., K.G., N.K., A.M.: no conflicts of interest; S.D.: Bayer (consultant); A.C.: Eli Lilly, Sanofi-Aventis (speaker panel); PD: Research support from National Institutes of Health, JDRF, ADA, Novo Nordisk, Bristol Meyer Squibb, AbbVie Pharmaceuticals, Astra Zeneca, Boehringer Ingelheim Pharmaceuticals, and honorarium from Eli Lilly, Novartis, GlaxoSmithKline, Merck, Novo Nordisk, Takeda, and Sanofi-Aventis.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Dhindsa S, Ghanim H, Batra M, Dandona P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care. 2018;41(7):1516–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhindsa S, Ghanim H, Batra M, et al. . Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol Metab. 2017;28(8):545–560. [DOI] [PubMed] [Google Scholar]
- 4. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coletta DK, Sriwijitkamol A, Wajcberg E, et al. . Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52(4):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghanim H, Dhindsa S, Abuaysheh S, et al. . Diminished androgen and estrogen receptors and aromatase levels in hypogonadal diabetic men: reversal with testosterone. Eur J Endocrinol. 2018;178(3):277–283. [DOI] [PubMed] [Google Scholar]
- 7. Ghanim H, Dhindsa S, Batra M, et al. . Effect of testosterone on FGF2, MRF4, and myostatin in hypogonadotropic hypogonadism: relevance to muscle growth. J Clin Endocrinol Metab. 2019;104(6):2094–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Louis O, Verlinde S, Thomas M, De Schepper J. Between-centre variability versus variability over time in DXA whole body measurements evaluated using a whole body phantom. Eur J Radiol. 2006;58(3):431–434. [DOI] [PubMed] [Google Scholar]
- 9. Dhindsa S, Bhatia V, Dhindsa G, Chaudhuri A, Gollapudi GM, Dandona P. The effects of hypogonadism on body composition and bone mineral density in type 2 diabetic patients. Diabetes Care. 2007;30(7):1860–1861. [DOI] [PubMed] [Google Scholar]
- 10. Salameh WA, Redor-Goldman MM, Clarke NJ, Reitz RE, Caulfield MP. Validation of a total testosterone assay using high-turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75(2):169–175. [DOI] [PubMed] [Google Scholar]
- 11. Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. 2011;34(8):1854–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mandel JL, Pearson ML. Insulin stimulates myogenesis in a rat myoblast line. Nature. 1974;251(5476):618–620. [DOI] [PubMed] [Google Scholar]
- 13. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetologia. 2016;59(1):44–55. [DOI] [PubMed] [Google Scholar]
- 14. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson GJ, Layman DK, Moulton CJ, et al. . Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab. 2011;301(6):E1236–E1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee-Young RS, Bonner JS, Mayes WH, et al. . AMP-activated protein kinase (AMPK)α2 plays a role in determining the cellular fate of glucose in insulin-resistant mouse skeletal muscle. Diabetologia. 2013;56(3):608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao Q, Cui X, Wu R, et al. . Myeloid deletion of α1AMPK exacerbates atherosclerosis in LDL receptor knockout (LDLRKO) mice. Diabetes. 2016;65(6):1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaudhuri A, Ghanim H, Dandona P. Targeting AMP kinase in myeloid cells to reduce atherosclerosis. Diabetes. 2016;65(6):1493–1495. [DOI] [PubMed] [Google Scholar]
- 19. Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167(12): 1252–1260. [DOI] [PubMed] [Google Scholar]
- 20. Larsen CM, Faulenbach M, Vaag A, et al. . Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. [DOI] [PubMed] [Google Scholar]
- 21. Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91(11):4854–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]