Bone adaptation to persistent overloading can be counteracted by superimposed inflammatory and loading-induced damage that can tip the balance from bone accrual to loss.
Supplemental digital content is available in the text.
Key Words: repetitive overuse injury, repetitive motion injury, bone, anabolism, catabolism, work related
Abstract
It is commonly assumed that beneficial adaptations in bone occur with vigorous exercise, yet any adaptive re/modeling in bone undergoing persistent overloading can be counteracted by superimposed inflammatory, compressive, and tensile loading–induced damage responses above thresholds of tissue fatigue failure and repair. This leads to a tenuous balance between achieving bone accrual and loss.
Key Points
Responses in bone are complex and multifactorial. Yet unclear are load exposure thresholds and mechanisms that lead to pathological bone responses with persistent muscle contraction overloading of bone.
It is commonly assumed that bone mass increases with all types of vigorous physical activity, yet persistent excessive loading with either exercise or occupational tasks can lead to diminishing returns in bone mass or quality and even induce microcracks or stress fractures.
Repetitive overloading induces systemic and local inflammatory responses that can stimulate bone adaptation, repair, resorption, or further injury, based on superimposed processes in all involved tissues.
Applied loads typically suppress sclerostin production, which releases the break on Wnt signaling and allows for bone formation; however, loading-induced matrix microdamage in which osteocyte apoptosis occurs enhances the release of both receptor activator of nuclear factor kappa-B ligand (RANKL) and sclerostin, changes that promote bone catabolism.
INTRODUCTION
In general, physical activity is considered to be beneficial for bone health because these activities can increase bone mass or help prevent bone loss typically seen with aging or estrogen deficiency. However, there is a point at which prolonged high-demand activities can tip the balance and induce adverse bone outcomes. It is well accepted that osteocytes function as the primary mechanosensing cells in bone and can respond to mechanical stimuli through molecular signals that can regulate osteoblastic bone formation and osteoclastic bone resorption (1). It is the balance between the opposing functions of osteoblasts and osteoclasts, which also are capable of regulating one another, that determines whether the net effect of mechanical loading results in bone accrual (anabolic effect), bone loss (catabolic effect), or maintenance of preexisting bone mass.
A key question to ask is, “What factors influence the point at which the interaction of repetition, force, and duration shifts from driving a net response of bone formation to bone resorption during muscle contraction–induced loading of bone” (Fig. 1). There are a multitude of factors that can influence the response of bone to mechanical loading, including, but not limited to, sex, age, genetic and epigenetic traits, hormonal status, serum mineral balance, nutritional status/diet, smoking status, emotional status (e.g., stress/anxiety), and the presence of comorbidities that can affect bone health (e.g., hypertension, obesity, hypercholesterolemia, diabetes) (Table) (2–4). Even when some factors are controlled, it is difficult to predict which factor or group of factors will prevail given their interwoven complexity. Furthermore, the contributions of various stimuli generated by muscle on bones are still under investigation (5,6).
Figure 1.

Schematic demonstrating that interactions of force, repetition, and duration of bone loading can elicit a pro-anabolic (bone accrual) response up to a point at which the combination of these factors exceeds a threshold (the transition region between the green and red zones), resulting in a net catabolic response due to tissue fatigue failure and damage (microcracks, osteocyte apoptosis). The point at which the threshold is crossed varies from one individual to another, and the factors that influence its location (e.g., age, sex, genetic background) should be considered in future studies. The figure was created in consultation with Susan Fecho, M.F.A., Barton College.
TABLE.
Factors that favor bone anabolic versus catabolic responses

Because the responses of bone to exercise-related physical activity have been reviewed extensively (3,7–12), the focus of this contribution will primarily be on the less studied responses of bone to occupational-related physical activity. Many scientific questions remain to be answered to determine limits of high-demand occupational-related physical activity that would avoid bone catabolism and that would inform treatments and preventative approaches.
Physical Activities: Exercise Versus Occupational Tasks
Exercise is the deliberate performance of physical activity with the intent to improve performance/health/fitness. It is a subcomponent of physical activity. Because muscle and bone are biomechanically linked, most types of physical activities are considered beneficial to bone mass or quality, whether sports, planned exercise, or household work (6,12–14). It is commonly assumed that bone mass and quality increase with vigorous exercise and decrease as a result of unloading or reduced activity. Several studies show evidence of site-specific correlations between muscle activity in the form of grip strength and bone mineral density (BMD) in the radius of both nonathletes and athletes (15,16). Yet, there also are studies showing that increasing weight-bearing loads and muscle loading exercise to excessive levels is associated with diminishing returns in bone mass and quality and can even lead to increased stress fractures (7,9,17).
Unlike exercise, occupational-related physical activities usually provide no cardiovascular benefits (18) and may even promote myocardial infarctions in men with low levels of leisure time physical activity (19). Occupational-related physical activity involves repeated or sustained exertions of the body while often in biomechanically awkward postures. They include occupational computer work, hand tool use, and intense practice and playing of muscle instruments, in which there is prolonged positioning of the neck and shoulders in one position (20–23) or prolonged and sustained gripping and redundant movement of the thumb and digits (21,23–26). Occupational-related physical activity also includes manual material handling tasks that require lifting/lowering, pushing/pulling, and holding/carrying loads (27,28). Work-related musculoskeletal disorders (MSDs) encompass rotator cuff tears, tendinitis, arthritis, carpal tunnel syndrome, and more (25,29–31). These injuries are precipitated or aggravated by high repetitiveness, prolonged muscular exertions (particularly forceful exertions), chronic exposure to vibration of high intensity, or sustained awkward postures (25,32,33). Risk factors also include duration of exposure, female sex, aging, genetic traits (e.g., interleukin-1 [IL-1] gene polymorphisms), smoking status, low serum 25-OH vitamin D levels, and diabetes (4,22,29,34). The interaction of cumulative exposure to multiple mechanical factors also needs to be considered as an additional risk factor (Fig. 1), as do other aspects of a particular occupation (32,35,36).
Neck and Shoulder Responses to Occupational Related Physical Activity
MSDs involving the shoulder accounted for 14.9% of all work-related MSDs in the United States in 2016, with heavy tractor-trailer truck drivers and laborers/material movers having a greater proportion of injuries affecting the shoulder than other occupations (37). Risk factors included frequent manual handling of loads, high-force highly repetitive work, working above shoulder level or other awkward postures and vibration (35,38), task duration (22), and female sex (39). Continuous low-intensity muscle contractions also increase the prevalence of neck-shoulder complaints and syndromes, including acromioclavicular syndrome (20,33,40). Miners that use vibration tools show increased frequency of radiologically detected shoulder lesions (40.7% of 152 miners) and lesions that included degenerative bone changes (34.5%), primarily in the acromioclavicular joint (17.8%) (41).
Forelimb and Hand Responses to Occupational Related Physical Activity
Although MSDs involving the arm and hand account for only 5.1% of all work-related MSDs (37), disorders of the hand and wrist constitute 40% and 13%, respectively, of such cases (42). Risk factors for wrist and hand MSDs include repetitive pushing, hand force, combined exposure to both force and repetition, sustained gripping (e.g., computer or hand tool use), repetitive redundant movement of the thumb and digits (e.g., typing or texting), sustained or repeated static loading of the weight of an instrument or tool, and use of vibrating tools (24–26,41,43). Continuous movement of a joint into end of range, for example, with repeated hyperextension of metacarpalphalageal joints, may be another causative factor due to enhanced inflammation in joint and tendon tissues (43,44). Individuals with prolonged heavy or one-sided hand workloads, or increased high-impact “jolting” of the hand show increased incidence of hand osteoarthritis, with higher incidence in females (29,30,45–47).
Carpal tunnel syndrome is a condition of median nerve compression that frequently presents in working aged adults, particularly in association with prolonged and highly repetitive flexion and extension of the wrist, especially when combined with forceful gripping (48). Patients with this syndrome show decreased areal BMD in distal forearm bones (i.e., radius and ulna) and reduced bone in hand phalanges, assayed using quantitative ultrasound measurements (amplitude-dependent speed of sound, m s−1) (49,50).
A Clinically Relevant Animal Model of Occupational Work
To explore underlying mechanisms of bone changes occurring with occupational tasks, we developed an operant and clinically relevant rat model of work-related MSDs. Rats are taught a reaching and lever bar grasping and pulling task (36,51–53). For this, they reach forward using their whole forearm into a shoulder-height portal to pull isometrically on a lever bar located outside the chamber at learned and defined reach rates ranging from two to four reaches min−1 and target forces of 15% to 55% of the rats' maximum voluntary pulling force (Fig. 2; see Video, Supplemental Digital Content 1 for demonstration, http://links.lww.com/ESSR/A52).
Figure 2.
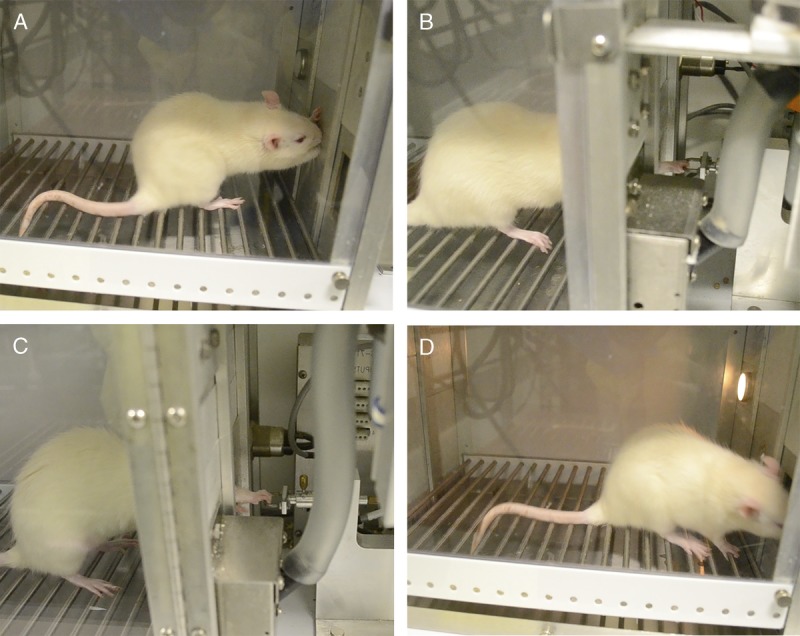
Image of rat performing the upper extremity reaching and grasping lever pulling task. A. Rat in an operant chamber with one limb extended into a portal located at shoulder height. B. Rat shown pulling on the lever bar, with digits four and five positioned in an ulnar position, indicative of ulnar deviation while pulling. C. Rat shown pulling in a neutral position. D. Rat retrieving a food reward.
In this model, different osteogenic responses were seen with different force loads and repetition rates. Young adult rats performing the lever bar pulling task at the lowest level of demand, that is, a low-force low-repetition task for 12 wk (an LFLR task; defined as 15% maximum voluntary pulling force, two reaches min−1, i.e., 240 cycles d−1, for 3 d wk−1, which for 12 wk equals 8640 cycles total), show no changes in trabecular bone volume and no significant osteoblast or osteoclast responses compared with control rats (36). Yet, rats performing a low-force high-repetition task for 12 to 18 wk (an LFHR task; again 15% maximum voluntary force, yet now at 4 reaches min−1, i.e., 480 cycles d−1, for 3 d wk−1, which is 17,280 to 25,920 cycles total for 12 and 18 wk, respectively) show net adaptive bone changes, including greater increases in osteoblasts than osteoclasts and increased trabecular bone volume and formation in distal radial metaphyses (Figs. 3A, B) (36,51). The latter results are consistent with human and animal studies reporting bone anabolism in response to high-impact exercise and moderate loading levels (8,10–12,17).
Figure 3.
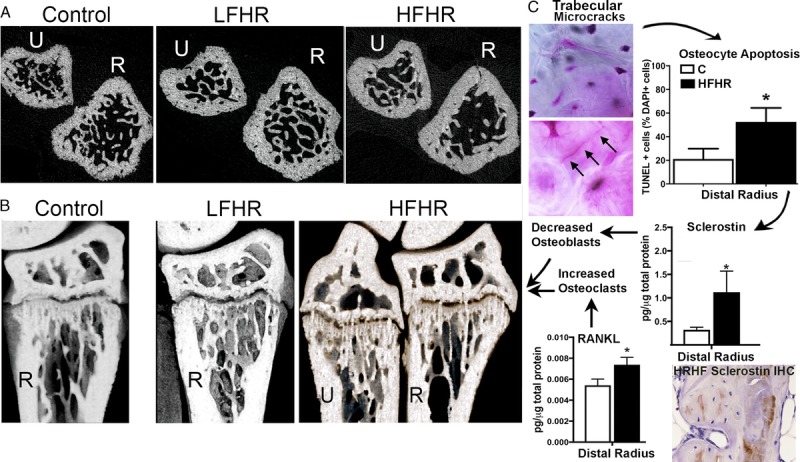
A and B. MicroCT images of ulnar (U) and radius (R) of control, 18-wk LFHR task, and 18-wk HFHR task rats. A. Transaxial images of the distal metaphyseal ulna and radius. Note the reduced numbers of trabeculae in HFHR rat radius. B. Sagittal images. C. Microcracks in the radial trabeculae (after basic fuchsin staining) of 18-wk HFHR rats lead to increased osteocyte apoptosis, sclerostin, and RANKL, which decreased osteoblast and increased osteoclasts numbers, respectively, compared with controls (C). (Adapted from (51). Copyright © 2019 Elsevier. Used with permission.)
In contrast, rats performing a high-force high-repetition task for 12 and 18 wk (an HFHR task; now at 55% maximum voluntary force, yet still 4 reaches min−1, for 3 d wk−1, which is 17,280 to 25,920 cycles total for 12 and 18 wk, respectively) show net bone catabolism, with significant increases in osteoclast numbers, significant increases in several inflammatory cytokines (including tumor necrosis factor-α [TNFα]), trabecular bone volume loss, and cortical bone thinning in primarily the distal radius (Figs. 3A, B) (36,51,52). The radiocarpal cartilage of the wrist joint of HFHR rats also shows the onset of osteoarthritis (54), consistent with the earlier-mentioned human findings of increased incidence of hand osteoarthritis with hand-intensive tasks. The HFHR task responses are more consistent with human and animal studies showing net bone catabolism when the bone is not provided sufficient time to recover from mechanically induced microdamage that has accumulated with prolonged static or cyclic overloading (55,56).
Lumbar Vertebral Responses to Occupational Related Physical Activity
MSDs involving the back account for 38.5% of work-related MSDs in the United States (37). Occupational physical activities, such as carrying, lifting heavy weight while inclined, and awkward postures (e.g., bending, twisting, squatting, and kneeling), are associated with a higher prevalence of recurrent low back pain (57,58). In professional drivers, whole-body vibration exposures contribute heavily to this risk (59), as do awkward postures while driving (60). Regarding bone, professional tractor drivers, urban taxi drivers, and helicopter pilots have increased exposure-dependent degenerative changes in the spinal column, e.g., spondylolisthesis (61–64). The degenerative changes are thought to be due to enhanced mechanical overloading and shocks to the spinal column that increase internal lumbar vertebral load (compressive and shear peak forces) (59). Individual differences likely also alter ultimate stress values to the same load before rapid damage accumulation occurs in involved tissues, for example, differences in muscle mass, strength, kinematic strategies, and experience-driven differences in muscle recruitment patterns (27,65).
Lower Extremity Responses to Occupational Related Physical Activity
Although evidence is scarce that work-related physical activity can be beneficial to bone health, three studies have reported positive associations between occupational activity and areal hip/femoral BMD (66–68). Female nurses aged 47–48 yr had higher femoral neck BMD than female clerks, findings related to duration of standing at work, indicating that prolonged working in a sitting position may lower hip BMD (68), similar to findings of higher hip BMD in female postmenopausal agricultural workers than female office workers or housewives (66). Femoral neck and total hip BMDs were also found to be higher in men engaged in moderate work and travel-related physical activity, than inactive men (67). Similar relationships were not seen in work-active versus inactive women (67). These findings contrast with others showing no evidence that occupational physical activity is associated with higher hip BMD in either sex (69,70), and positive associations only with standing at 30 yr of age (70).
Yet, there is clear evidence that physical work activities can increase risk of knee osteoarthritis (45,71). Specifically, work-related and repetitive kneeling, bending, lifting, climbing, jolting of the legs, and whole-body vibration can cause or aggravate knee osteoarthritis (45,46,71). Obesity adds an additional risk of knee osteoarthritis, as well as subchondral tibial bone degeneration, in both workers and in general (45,72).
Potential Adaptive Re/Modeling May Be Countered by Other Superimposed Responses
Factors other than muscle size account for 12%–16% of the variations in differences in bone mass (73). Several studies have shown increased systemic and local inflammatory responses in humans engaged in occupational physical activity or frequent cell phone and keypad texting (43,74–76). Inflammatory responses can stimulate tissue adaptation, repair, resorption, or further injury based on superimposed processes in involved tissues. In bone, they may contribute to overuse-induced trabecular bone loss because increased inflammatory cytokines promote osteoclast activity and bone resorption (77). Involvement of inflammatory cytokines in repetitive overuse-induced bone loss has been confirmed with findings that anti-inflammatory treatments (ibuprofen and anti-TNFα) provided at peak points of inflammation improved trabecular bone volume and reduced osteoclast numbers and activity in rats performing the HFHR reaching and grasping task for 12 wk (52,78). Three-phase bone scintigraphy has been used to examine upper extremity bones of patients with occupational repetitive strain injury and demonstrated increased blood flow and pooling in wrist bones of affected limbs (79,80), suggestive of similar increases in inflammatory processes that might enhance osteoclastogenesis.
Mechanostat Theory Versus the Damage-Repair Theory
The mechanostat theory proposes that bones adapt their strength to keep the strain caused by physiological loads close to a set point (81) and, consequently, that physical activity typically increases bone adaptation in response to loading (81,82). Another theory, the damage-repair theory, was developed to explain catabolic changes in cortical bone and postulates that damage accumulates in the bone if the loading is so high that self-repair mechanisms cannot keep pace with the level of damage or overload-induced resorption (83). This damage-repair theory is relevant to trabecular changes seen in the rat model of repetitive reaching and grasping, in rats that performed the HFHR task for 18 wk (Fig. 3C) (51). These animals showed several catabolic indices in their distal radial metaphyses, including decreased trabecular bone volume, increased woven bone, microcracks, and osteocyte apoptosis, compared with control rats. The microcracks were likely the result of repeated high-force muscle compressive forces above fatigue failure thresholds for trabecular bone (51,82). The loss of trabecular bone volume has been shown to enhance brittleness and to increase fracture risk (84). Increased disorganization of trabecular patterning was also observed, a change that would further increase stress in trabeculae (47). Such responses are consistent with the damage-repair theory suggested previously for cortical bone (83) and now extended to trabecular bone. Such catabolic bone changes with high-demand tasks also are consistent with the fatigue-failure theory for MSD injuries (85).
Roles of RANKL and Sclerostin in Responsiveness to Bone Loading and Unloading
Osteocytes show metabolic responsiveness to bone loading and unloading (86) and have been implicated as the primary cells in bone that transduce mechanical signals into molecular signals capable of regulating bone formation and resorption (1). RANKL (Receptor activator of nuclear factor kappa-B ligand) is released by apoptotic osteocytes after loading-induced microdamage (87), thereby promoting increased osteoclast activity (88). This response is believed to encourage removal of damaged matrix by osteoclasts (87). It has been suggested that production of RANKL and osteoprotegerin (OPG), its receptor decoy, is dictated by the severity of damage disrupting the osteocytic network (89). Although osteocyte apoptosis and RANKL release are essential for bone re/modeling, a prolonged shift in the RANKL and OPG ratio toward more RANKL enhances osteoclastogenesis and net bone resorption (87).
Sclerostin also is expressed by osteocytes (90) and is a negative regulator of osteoblast differentiation, making it a potent inhibitor of bone formation (91). Sclerostin production typically decreases with physiological bone loading and increases with bone unloading (92,93). Applied mechanical loads usually suppress sclerostin production, a change that releases the inhibition of Wnt signaling and, thus, increases bone formation (86). On the contrary, loading-induced matrix microdamage in which osteocyte apoptosis occurs results in enhanced release of sclerostin into the bone matrix (91), changes that promote bone catabolism. Recombinant sclerostin upregulates RANKL production and increases osteoclast formation (88), changes that enhance bone resorption. In the rat model of repetitive reaching and grasping, when bones were loaded at high-force levels for 18 wk, loading-induced microdamage develops in the trabecular bone matrix (Fig. 3C) (51), through which the osteocytes extend their canaliculi (94). Subsequent osteocyte apoptosis triggered increased sclerostin and RANKL release into the bone matrix (Fig. 3C) (51), matching other studies demonstrating enhanced release of these factors after osteocyte apoptosis after microcrack damage (91). Thus, microdamage, sclerostin, and RANKL tip the balance from net bone formation (accrual of bone) to net bone resorption (bone loss) that occurs under extreme loading conditions.
Role of Nerves in Bone
As mentioned earlier, carpal tunnel syndrome is a condition of median nerve compression that frequently presents in workers. Patients with this syndrome show decreased areal BMD in distal forearm bones (49,50) that usually improves with surgical release of median nerves (50,95). Although yet unknown, median nerve compression may reduce neural growth factors provided to distal forearm bones, as found after spinal cord injury or chronic constriction injury of the sciatic nerve (96,97).
Studies have established that neurons communicate with cells in the bone microenvironment and regulate bone homeostasis (98). Both the periosteum and trabecular bone compartments contain a dense network of TrkA (the high-affinity receptor for nerve growth factor)–positive sensory fibers that are responsive to mechanical stimuli (99). These sensory neurons sense and respond to mechanical stimuli. A study in mice investigating the role of sensory neurons during forelimb axial loading demonstrated their importance in potentiating the anabolic response to mechanical stimuli to achieve maximal load-induced bone formation (100). It is generally believed that the somatosensory system plays a role in mediating the anabolic response of bone to physiological mechanical loading via the Wnt/β-catenin pathway.
In contrast, activation of the sympathetic nervous system (SNS) elicits a catabolic response in bone through an increase in bone resorption and a decrease in bone formation (101). Increased SNS activity stimulates the production of bone-resorptive cytokines, for example, RANKL and IL-6 (102,103). Although nerve fibers that are immunoreactive for various sympathetic markers, for example, vasoactive intestinal peptide, tyrosine hydroxylase, and neuropeptide Y, have been identified in bone (104), it appears that only a limited number of bone cells are in direct contact with sympathetic nerve terminals (105,106). Furthermore, beta-adrenergic receptors (βARs) (predominantly type beta 2 [β2]) are found on osteocytes, osteoblast, and osteoclasts (98). Studies have shown that βAR agonists trigger a bone catabolic response (increased bone resorption and decreased bone formation), whereas βAR blockade using antagonists has an anticatabolic effect, particularly in conditions when bone re/modeling is high, such as in young mice and estrogen-deficient bone loss (106,107).
Although somatosensory nerves are believed to play an important role in mediating the anabolic response of bone to physiological mechanical loading, the role of the SNS appears minimal under these circumstances (98,108). However, in cases in which the loading exceeds the beneficial threshold and results in tissue damage (e.g., bone microcracks), hyperactivation of the SNS may tip the balance in favor of bone catabolism by overriding the positive effects of mechanical loading on bone. The advantageous effects of exercise on bone mass in rats can be suppressed by β2 agonists, thereby suggesting that the positive effects of physical activity can be overridden by activation of the SNS (107).
This is interesting in light of evidence of reduced skin temperatures in hands of individuals with advanced upper extremity MSDs, changes thought to reflect underlying dysfunction in peripheral sympathetic nerves (109,110). Although more work is needed to confirm this possible contribution, hyperactivation of sympathetic nerves may tip the balance toward bone loss, thereby negating the positive effects of mechanical loading on bone.
Key Questions That Need to Be Addressed (Future Perspectives)
The effects of repetitive high-demand activities on bones in human populations are not as well defined as in animal models. Longitudinal studies should be performed in young adult, mature, and aged humans to capture the effects of changing repair mechanisms, hormone levels, changes in metabolism, and inflamm-aging (the increase in inflammation occurring with aging) (111). We have shown that aged rats performing the same LFHR task for 12 wk as young adult rats develop greater levels of trabecular and cortical bone degradation, likely due to observed increases in systemic and tissue inflammatory cytokine responses occurring as a consequence of both aging and continued loading (53). Similar studies should be repeated in human populations, particularly in light of extended retirement times for workers (112).
Repair of biological tissues can be accomplished through the processes of inflammation and re/modeling, as long as the damage does not exceed the ability of tissues to repair.
Thresholds for damage need to be defined, as do rest and recovery allowances that would enhance tissue recovery and bone adaptation (i.e., accrual). Current equations for repetitive occupational task thresholds were developed from 69 short-term psychophysical data tasks (each <4 wk in duration) and using physiological data from six studies demonstrating muscle fatigue after only 1 h (113). Duty cycle is the frequency of effort duration an individual worker is engaged in a repetitive task. Lowering duty cycles may increase the allowable maximum acceptable forces and enhance tissue recovery (113). Increasing periods of complete relaxation between work cycles also reduces health risks (114), supporting a need for further exploration of rest periods in loading models.
Future experiments also should consider the sexual dimorphism of bone (e.g., structural or hormonal response differences) in their study design and data interpretation. Such studies would elucidate if male-female differences are from sex-linked biological factors versus gendered social dynamics, which might include differences in dress, occupation, physical activity, sun exposure, vitamin D synthesis, and weight-bearing activities in which the different sexes are typically engaged.
Supplementary Material
Acknowledgments
Professor Susan Fecho, M.F.A., at Barton College, NC, enhanced the 3D effect of the microCT images in Figure 3 and created the Supplemental movie pdf. The research reported in this study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR056019 to M.F.B.
Footnotes
Editor: Monica J. Hubal, Ph.D., FACSM.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-essr.org).
References
- 1.Bonewald LF. The amazing osteocyte. J. Bone Miner. Res. 2011; 26(2):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos L, Elliott-Sale KJ, Sale C. Exercise and bone health across the lifespan. Biogerontology. 2017; 18(6):931–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wohl GR, Boyd SK, Judex S, Zernicke RF. Functional adaptation of bone to exercise and injury. J. Sci. Med. Sport. 2000; 3(3):313–24. [DOI] [PubMed] [Google Scholar]
- 4.Romano A, Vigna L, Belluigi V, et al. Shift work and serum 25-OH vitamin D status among factory workers in northern Italy: cross-sectional study. Chronobiol. Int. 2015; 32(6):842–7. [DOI] [PubMed] [Google Scholar]
- 5.Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle-bone interaction. Curr. Osteoporos. Rep. 2015; 13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone. 2015; 80:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunier J, Chapurlat R. Stress fracture in athletes. Joint Bone Spine. 2018; 85(3):307–10. [DOI] [PubMed] [Google Scholar]
- 8.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc. Sport Sci. Rev. 2003; 31(1):45–50. [DOI] [PubMed] [Google Scholar]
- 9.Bourrin S, Genty C, Palle S, Gharib C, Alexandre C. Adverse effects of strenuous exercise: a densitometric and histomorphometric study in the rat. J. Appl. Physiol. 1994; 76(5):1999–2005. [DOI] [PubMed] [Google Scholar]
- 10.Daly RM. Exercise and nutritional approaches to prevent frail bones, falls and fractures: an update. Climacteric. 2017; 20(2):119–24. [DOI] [PubMed] [Google Scholar]
- 11.Sanudo B, de Hoyo M, Del Pozo-Cruz J, et al. A systematic review of the exercise effect on bone health: the importance of assessing mechanical loading in perimenopausal and postmenopausal women. Menopause. 2017; 24(10):1208–16. [DOI] [PubMed] [Google Scholar]
- 12.Willems HME, van den Heuvel E, Schoemaker RJW, Klein-Nulend J, Bakker AD. Diet and exercise: a match made in bone. Curr. Osteoporos. Rep. 2017; 15(6):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blair SN, Kohl HW, Gordon NF, Paffenbarger RS., Jr How much physical activity is good for health? Annu. Rev. Public Health. 1992; 13:99–126. [DOI] [PubMed] [Google Scholar]
- 14.Clark EA, Goodship AE, Lanyon LE. Locomotor bone strain as the stimulus for bone's mechanical adaptability. J. Physiol. 1975; 245(2):57P. [PubMed] [Google Scholar]
- 15.Aydin G, Atalar E, Keles I, et al. Predictive value of grip strength for bone mineral density in males: site specific or systemic? Rheumatol. Int. 2006; 27(2):125–9. [DOI] [PubMed] [Google Scholar]
- 16.Chan DC, Lee WT, Lo DH, Leung JC, Kwok AW, Leung PC. Relationship between grip strength and bone mineral density in healthy Hong Kong adolescents. Osteoporos. Int. 2008; 19(10):1485–95. [DOI] [PubMed] [Google Scholar]
- 17.Umemura Y, Ishiko T, Yamauchi T, Kurono M, Mashiko S. Five jumps per day increase bone mass and breaking force in rats. J. Bone Miner. Res. 1997; 12(9):1480–5. [DOI] [PubMed] [Google Scholar]
- 18.Kivimaki M, Jokela M, Nyberg ST, et al. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet. 2015; 386(10005):1739–46. [DOI] [PubMed] [Google Scholar]
- 19.Holtermann A, Marott JL, Gyntelberg F, et al. Occupational and leisure time physical activity: risk of all-cause mortality and myocardial infarction in the Copenhagen City Heart Study. A prospective cohort study. BMJ Open. 2012; 2(1):e000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huysmans MA, Blatter BM, van der Beek AJ. Perceived muscular tension predicts future neck-shoulder and arm-wrist-hand symptoms. Occup. Environ. Med. 2012; 69(4):261–7. [DOI] [PubMed] [Google Scholar]
- 21.IJmker S, Blatter BM, van der Beek AJ, van Mechelen W, Bongers PM. Prospective Research On Musculoskeletal disorders in Office workers (PROMO): study protocol. BMC Musculoskelet. Disord. 2006; 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punnett L, Bergqvist U. Musculoskeletal disorders in visual display unit work: gender and work demands. Occup. Med. 1999; 14(1):113–24, iv. [PubMed] [Google Scholar]
- 23.Cohn L, Lowry RM, Hart S. Overuse syndromes of the upper extremity in interpreters for the deaf. Orthopedics. 1990; 13(2):207–9. [DOI] [PubMed] [Google Scholar]
- 24.Gupta AD, Mahalanabis D. Study of hand function in a group of shoe factory workers engaged in repetitive work. J. Occup. Rehabil. 2006; 16(4):675–84. [DOI] [PubMed] [Google Scholar]
- 25.Barr AE, Barbe MF, Clark BD. Work-related musculoskeletal disorders of the hand and wrist: epidemiology, pathophysiology, and sensorimotor changes. J. Orthop. Sports Phys. Ther. 2004; 34(10):610–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry HJ. Overuse syndrome of the upper limb in musicians. Med. J. Aust. 1986; 144(4):182–3, 185. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher S, Heberger JR. The effects of operator position, pallet orientation, and palletizing condition on low back loads in manual bag palletizing operations. Int. J. Ind. Ergon. 2015; 47:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nimbarte AD, Sun Y, Jaridi M, Hsiao H. Biomechanical loading of the shoulder complex and lumbosacral joints during dynamic cart pushing task. Appl. Ergon. 2013; 44(5):841–9. [DOI] [PubMed] [Google Scholar]
- 29.Blumenfeld O, Williams FM, Valdes A, et al. Association of interleukin-6 gene polymorphisms with hand osteoarthritis and hand osteoporosis. Cytokine. 2014; 69(1):94–101. [DOI] [PubMed] [Google Scholar]
- 30.Rossignol M, Leclerc A, Allaert FA, et al. Primary osteoarthritis of hip, knee, and hand in relation to occupational exposure. Occup. Environ. Med. 2005; 62(11):772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold JE, Hallman DM, Hellstrom F, et al. Systematic review of quantitative imaging biomarkers for neck and shoulder musculoskeletal disorders. BMC Musculoskelet. Disord. 2017; 18(1):395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher S, Heberger JR. Examining the interaction of force and repetition on musculoskeletal disorder risk: a systematic literature review. Hum. Factors. 2013; 55(1):108–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visser B, van Dieen JH. Pathophysiology of upper extremity muscle disorders. J. Electromyogr. Kinesiol. 2006; 16(1):1–16. [DOI] [PubMed] [Google Scholar]
- 34.Bouffard J, Martinez R, Plamondon A, Côté JN, Begon M. Sex differences in glenohumeral muscle activation and coactivation during a box lifting task. Ergonomics. 2019; 62:1327–38. [DOI] [PubMed] [Google Scholar]
- 35.Linaker CH, Walker-Bone K. Shoulder disorders and occupation. Best Pract. Res. Clin. Rheumatol. 2015; 29(3):405–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbe MF, Gallagher S, Massicotte VS, Tytell M, Popoff SN, Barr-Gillespie AE. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2013; 14(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bureau of Labor Statistics Back injuries prominent in work-related musculoskeletal disorder cases in 2016. 2018. Available from: https://www.bls.gov/opub/ted/2018/back-injuries-prominent-in-work-related-musculoskeletal-disorder-cases-in-2016.htm.
- 38.van Rijn RM, Huisstede BM, Koes BW, Burdorf A. Associations between work-related factors and specific disorders of the shoulder—a systematic review of the literature. Scand. J. Work Environ. Health. 2010; 36(3):189–201. [DOI] [PubMed] [Google Scholar]
- 39.Juul-Kristensen B, Sogaard K, Stroyer J, Jensen C. Computer users' risk factors for developing shoulder, elbow and back symptoms. Scand. J. Work Environ. Health. 2004; 30(5):390–8. [DOI] [PubMed] [Google Scholar]
- 40.Balogh I, Arvidsson I, Bjork J, et al. Work-related neck and upper limb disorders—quantitative exposure-response relationships adjusted for personal characteristics and psychosocial conditions. BMC Musculoskelet. Disord. 2019; 20(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakosy T, Nemeth L, Kiss G, Laszloffy M, Kardos K. Clinical features of the hand-arm vibration syndrome in miners. Orv. Hetil. 2006; 147(18):833–9. [PubMed] [Google Scholar]
- 42.Bureau of Labor Statistics Type of injury or illness and body parts affected by nonfatal injuries and illnesses in 2014. 2015. Available from: https://www.bls.gov/opub/ted/2015/type-of-injury-or-illness-and-body-parts-affected-by-nonfatal-injuries-and-illnesses-in-2014.htm.
- 43.Gold JE, Mohamed FB, Ali S, Barbe MF. Serum and MRI biomarkers in mobile device texting: a pilot study. Hum. Factors. 2014; 56(5):864–72. [DOI] [PubMed] [Google Scholar]
- 44.Walsh T, Delahunt E, McCarthy Persson U. Effects of taping on thumb alignment and force application during PA mobilisations. Man. Ther. 2011; 16(3):264–9. [DOI] [PubMed] [Google Scholar]
- 45.Palmer KT. Occupational activities and osteoarthritis of the knee. Br. Med. Bull. 2012; 102:147–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernard TE, Wilder FV, Aluoch M, Leaverton PE. Job-related osteoarthritis of the knee, foot, hand, and cervical spine. J. Occup. Environ. Med. 2010; 52(1):33–8. [DOI] [PubMed] [Google Scholar]
- 47.Ding M, Odgaard A, Hvid I. Changes in the three-dimensional microstructure of human tibial cancellous bone in early osteoarthritis. J. Bone Joint Surg. 2003; 85(6):906–12. [PubMed] [Google Scholar]
- 48.Palmer KT. Carpal tunnel syndrome: the role of occupational factors. Best Pract. Res. Clin. Rheumatol. 2011; 25(1):15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erselcan T, Topalkara K, Nacitarhan V, Akyuz A, Dogan D. Carpal tunnel syndrome leads to significant bone loss in metacarpal bones. J. Bone Miner. Metab. 2001; 19(5):317–20. [DOI] [PubMed] [Google Scholar]
- 50.Kisala A, Pluskiewicz W, Adamczyk P. Skeletal status in women with carpal tunnel syndrome—A 1-yr prospective study. J. Clin. Densitom. 2019; 22(3):305–10. [DOI] [PubMed] [Google Scholar]
- 51.Barbe MF, Massicotte VS, Assari S, et al. Prolonged high force high repetition pulling induces osteocyte apoptosis and trabecular bone loss in distal radius, while low force high repetition pulling induces bone anabolism. Bone. 2018; 110:267–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jain NX, Barr-Gillespie AE, Clark BD, et al. Bone loss from high repetitive high force loading is prevented by ibuprofen treatment. J. Musculoskelet. Neuronal Interact. 2014; 14(1):78–94. [PMC free article] [PubMed] [Google Scholar]
- 53.Massicotte VS, Frara N, Harris MY, et al. Prolonged performance of a high repetition low force task induces bone adaptation in young adult rats, but loss in mature rats. Exp. Gerontol. 2015; 72:204–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driban JB, Barr AE, Amin M, Sitler MR, Barbe MF. Joint inflammation and early degeneration induced by high-force reaching are attenuated by ibuprofen in an animal model of work-related musculoskeletal disorder. J. Biomed. Biotechnol. 2011; 2011:691412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamamoto E, Paul Crawford R, Chan DD, Keaveny TM. Development of residual strains in human vertebral trabecular bone after prolonged static and cyclic loading at low load levels. J. Biomech. 2006; 39(10):1812–8. [DOI] [PubMed] [Google Scholar]
- 56.Chapurlat RD, Delmas PD. Bone microdamage: a clinical perspective. Osteoporos. Int. 2009; 20(8):1299–308. [DOI] [PubMed] [Google Scholar]
- 57.Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. Physical load during work and leisure time as risk factors for back pain. Scand. J. Work Environ. Health. 1999; 25(5):387–403. [DOI] [PubMed] [Google Scholar]
- 58.B Amorim A, Simic M, Pappas E, et al. Is occupational or leisure physical activity associated with low back pain? Insights from a cross-sectional study of 1059 participants. Braz. J. Phys. Ther. 2019; 23(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bovenzi M, Schust M, Mauro M. An overview of low back pain and occupational exposures to whole-body vibration and mechanical shocks. Med. Lav. 2017; 108(6):419–33. [DOI] [PubMed] [Google Scholar]
- 60.Bovenzi M, Schust M, Menzel G, Prodi A, Mauro M. Relationships of low back outcomes to internal spinal load: a prospective cohort study of professional drivers. Int. Arch. Occup. Environ. Health. 2015; 88(4):487–99. [DOI] [PubMed] [Google Scholar]
- 61.Chen JC, Chan WP, Katz JN, Chang WP, Christiani DC. Occupational and personal factors associated with acquired lumbar spondylolisthesis of urban taxi drivers. Occup. Environ. Med. 2004; 61(12):992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christ W, Dupuis H. Studies on the possibility of physical damage in the spinal region of tractor operators. I. Report on a 2d mass screening of 137 young farmers. Med. Welt. 1968; 36:1919–20 contd. [PubMed] [Google Scholar]
- 63.Byeon JH, Kim JW, Jeong HJ, et al. Degenerative changes of spine in helicopter pilots. Ann. Rehabil. Med. 2013; 37(5):706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froom P, Froom J, Van Dyk D, et al. Lytic spondylolisthesis in helicopter pilots. Aviat. Space Environ. Med. 1984; 55(6):556–7. [PubMed] [Google Scholar]
- 65.Marras WS, Parakkat J, Chany AM, Yang G, Burr D, Lavender SA. Spine loading as a function of lift frequency, exposure duration, and work experience. Clin. Biomech. (Bristol, Avon). 2006; 21(4):345–52. [DOI] [PubMed] [Google Scholar]
- 66.Damilakis J, Perisinakis K, Kontakis G, Vagios E, Gourtsoyiannis N. Effect of lifetime occupational physical activity on indices of bone mineral status in healthy postmenopausal women. Calcif. Tissue Int. 1999; 64(2):112–6. [DOI] [PubMed] [Google Scholar]
- 67.Sritara C, Thakkinstian A, Ongphiphadhanakul B, et al. Work- and travel-related physical activity and alcohol consumption: relationship with bone mineral density and calcaneal quantitative ultrasonometry. J. Clin. Densitom. 2015; 18(1):37–43. [DOI] [PubMed] [Google Scholar]
- 68.Weiss M, Yogev R, Dolev E. Occupational sitting and low hip mineral density. Calcif. Tissue Int. 1998; 62(1):47–50. [DOI] [PubMed] [Google Scholar]
- 69.Walker-Bone K, D'Angelo S, Syddall HE, et al. Exposure to heavy physical occupational activities during working life and bone mineral density at the hip at retirement age. Occup. Environ. Med. 2014; 71(5):329–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coupland CA, Grainge MJ, Cliffe SJ, Hosking DJ, Chilvers CE. Occupational activity and bone mineral density in postmenopausal women in England. Osteoporos. Int. 2000; 11(4):310–5. [DOI] [PubMed] [Google Scholar]
- 71.Yucesoy B, Charles LE, Baker B, Burchfiel CM. Occupational and genetic risk factors for osteoarthritis: a review. Work. 2015; 50(2):261–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Driban JB, Eaton CB, Lo GH, et al. Overweight older adults, particularly after an injury, are at high risk for accelerated knee osteoarthritis: data from the osteoarthritis initiative. Clin. Rheumatol. 2016; 35(4):1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daly RM, Saxon L, Turner CH, Robling AG, Bass SL. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone. 2004; 34(2):281–7. [DOI] [PubMed] [Google Scholar]
- 74.Gold JE, Hallman DM, Hellstrom F, et al. Systematic review of biochemical biomarkers for neck and upper-extremity musculoskeletal disorders. Scand. J. Work Environ. Health. 2016; 42(2):103–24. [DOI] [PubMed] [Google Scholar]
- 75.Matute Wilander A, Karedal M, Axmon A, Nordander C. Inflammatory biomarkers in serum in subjects with and without work related neck/shoulder complaints. BMC Musculoskelet. Disord. 2014; 15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carp SJ, Barbe MF, Winter KA, Amin M, Barr AE. Inflammatory biomarkers increase with severity of upper-extremity overuse disorders. Clin. Sci. (Lond.). 2007; 112(5):305–14. [DOI] [PubMed] [Google Scholar]
- 77.Gowen M, Wood DD, Ihrie EJ, McGuire MK, Russell RG. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983; 306(5941):378–80. [DOI] [PubMed] [Google Scholar]
- 78.Rani S, Barbe MF, Barr AE, Litivn J. Role of TNF alpha and PLF in bone remodeling in a rat model of repetitive reaching and grasping. J. Cell. Physiol. 2010; 225(1):152–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.al-Nahhas AM, Jawad AS, Norman A, McCready VR. 99Tcm-MDP blood-pool phase in the assessment of repetitive strain injury. Nucl. Med. Commun. 1997; 18(10):927–31. [DOI] [PubMed] [Google Scholar]
- 80.Amorim BJ, Etchebehere EC, Dalla Torre G, et al. Low sensitivity of three-phase bone scintigraphy for the diagnosis of repetitive strain injury. Sao Paulo Med. J. 2006; 124(3):145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frost HM. Wolff's law and bone's structural adaptations to mechanical usage: an overview for clinicians. Angle Orthod. 1994; 64(3):175–88. [DOI] [PubMed] [Google Scholar]
- 82.Laurent MR, Dubois V, Claessens F, et al. Muscle-bone interactions: from experimental models to the clinic? A critical update. Mol. Cell. Endocrinol. 2016; 432:14–36. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Li J, Zou Z, Fok AS. Fracture simulation of restored teeth using a continuum damage mechanics failure model. Dent. Mater. 2011; 27(7):e125–33. [DOI] [PubMed] [Google Scholar]
- 84.Turner CH. Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos. Int. 2002; 13(2):97–104. [DOI] [PubMed] [Google Scholar]
- 85.Gallagher S, Schall MC., Jr Musculoskeletal disorders as a fatigue failure process: evidence, implications and research needs. Ergonomics. 2017; 60(2):255–69. [DOI] [PubMed] [Google Scholar]
- 86.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2008; 283(9):5866–75. [DOI] [PubMed] [Google Scholar]
- 87.Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone. 2014; 64:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PloS one. 2011; 6(10):e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mulcahy LE, Taylor D, Lee TC, Duffy GP. RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone. 2011; 48(2):182–8. [DOI] [PubMed] [Google Scholar]
- 90.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011; 17(10):1231–4. [DOI] [PubMed] [Google Scholar]
- 91.Mabilleau G, Mieczkowska A, Edmonds ME. Thiazolidinediones induce osteocyte apoptosis and increase sclerostin expression. Diabet Med. 2010; 27(8):925–32. [DOI] [PubMed] [Google Scholar]
- 92.Nguyen J, Tang SY, Nguyen D, Alliston T. Load regulates bone formation and sclerostin expression through a TGFβ-dependent mechanism. PloS One. 2013; 8(1):e53813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tu X, Rhee Y, Condon KW, et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone. 2012; 50(1):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012; 50(5):1115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schorn D, Hoskinson J, Dickson RA. Bone density and the carpal tunnel syndrome. Hand. 1978; 10(2):184–6. [DOI] [PubMed] [Google Scholar]
- 96.Voor MJ, Brown EH, Xu Q, et al. Bone loss following spinal cord injury in a rat model. J. Neurotrauma. 2012; 29(8):1676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suyama H, Moriwaki K, Niida S, Maehara Y, Kawamoto M, Yuge O. Osteoporosis following chronic constriction injury of sciatic nerve in rats. J. Bone Miner. Metab. 2002; 20(2):91–7. [DOI] [PubMed] [Google Scholar]
- 98.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018; 98(3):1083–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takegahara N, Takamatsu H, Toyofuku T, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 2006; 8(6):615–22. [DOI] [PubMed] [Google Scholar]
- 100.Tomlinson RE, Li Z, Li Z, et al. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl. Acad. Sci. U. S. A. 2017; 114(18):E3632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qiao Y, Wang Y, Zhou Y, et al. The role of nervous system in adaptive response of bone to mechanical loading. J. Cell. Physiol. 2019; 234(6):7771–80. [DOI] [PubMed] [Google Scholar]
- 102.Kondo A, Mogi M, Koshihara Y, Togari A. Signal transduction system for interleukin-6 and interleukin-11 synthesis stimulated by epinephrine in human osteoblasts and human osteogenic sarcoma cells. Biochem. Pharmacol. 2001; 61(3):319–26. [DOI] [PubMed] [Google Scholar]
- 103.Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem. Pharmacol. 2001; 61(5):579–86. [DOI] [PubMed] [Google Scholar]
- 104.Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J. Auton. Nerv. Syst. 1988; 25(2-3):119–25. [DOI] [PubMed] [Google Scholar]
- 105.Denes A, Boldogkoi Z, Uhereczky G, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005; 134(3):947–63. [DOI] [PubMed] [Google Scholar]
- 106.Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002; 111(3):305–17. [DOI] [PubMed] [Google Scholar]
- 107.Bonnet N, Beaupied H, Vico L, et al. Combined effects of exercise and propranolol on bone tissue in ovariectomized rats. J. Bone Miner. Res. 2007; 22(4):578–88. [DOI] [PubMed] [Google Scholar]
- 108.de Souza RL, Pitsillides AA, Lanyon LE, Skerry TM, Chenu C. Sympathetic nervous system does not mediate the load-induced cortical new bone formation. J. Bone Miner. Res. 2005; 20(12):2159–68. [DOI] [PubMed] [Google Scholar]
- 109.Gold JE, Cherniack M, Hanlon A, Dennerlein JT, Dropkin J. Skin temperature in the dorsal hand of office workers and severity of upper extremity musculoskeletal disorders. Int. Arch. Occup. Environ. Health. 2009; 82(10):1281–92. [DOI] [PubMed] [Google Scholar]
- 110.Nakamoto M. Responses of sympathetic nervous system to cold exposure in vibration syndrome subjects and age-matched healthy controls. Int. Arch. Occup. Environ. Health. 1990; 62(2):177–81. [DOI] [PubMed] [Google Scholar]
- 111.Salvioli S, Capri M, Valensin S, et al. Inflamm-aging, cytokines and aging: state of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 2006; 12(24):3161–71. [DOI] [PubMed] [Google Scholar]
- 112.Welsh J, Strazdins L, Charlesworth S, Kulik CT, Butterworth P. Health or harm? A cohort study of the importance of job quality in extended workforce participation by older adults. BMC Public Health. 2016; 16:885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Potvin JR. Predicting maximum acceptable efforts for repetitive tasks: an equation based on duty cycle. Hum. Factors. 2012; 54(2):175–88. [DOI] [PubMed] [Google Scholar]
- 114.Sjøgaard G, Lundberg U, Kadefors R. The role of muscle activity and mental load in the development of pain and degenerative processes at the muscle cell level during computer work. Eur. J. Appl. Physiol. 2000; 83(2-3):99–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


