Abstract
Introduction:
Placental viral infections are associated with fetal inflammation and adverse pregnancy outcomes. However, there have been limited studies on how placental macrophages in the villous and adjacent fetal umbilical endothelial cells respond to a viral insult. This study aimed to evaluate the communication between Hofbauer cells (HBCs) and human umbilical vein endothelial cells (HUVECs) during a viral infection.
Methods:
HBCs were either uninfected or infected with the γ-herpesvirus, MHV-68, and the conditioned medium (CM) collected. HUVECs were exposed to HBC CM and the levels of the pro-neutrophilic response markers: IL-8; E-selectin; intercellular adhesion molecule 1 (ICAM-1); and vascular adhesion molecule 1 (VCAM-1) measured by ELISA and qPCR. The role of HBC-derived IL-1β was investigated using an IL-1β blocking antibody (Ab) or IL-1 receptor antagonist (IL-1Ra).
Results:
MHV-68 infection of HBCs induced a significant increase in IL-1β secretion. CM from infected HBCs induced HUVEC expression of IL-8, E-selectin, VCAM-1, ICAM-1 mRNA, and secretion of IL-8. The HUVEC response to the CM of MHV-infected HBCs was inhibited by a neutralizing IL-1β Ab and by IL-1Ra.
Discussion:
Virally-induced HBC IL-1β activates HUVECs to generate a pro-neutrophilic response. This novel cell-cell communication pathway may play an important role in the genesis of fetal inflammation associated with placental viral infection.
Keywords: Placenta, Hofbauer cells, Macrophages, Herpesvirus, IL-1β, FIRS
1. Introduction
Hofbauer cells (HBCs) are macrophages of fetal origin that are found in the placenta [1]. They can be found as early as 18 days of gestation, prior to the development of a fetal circulation, and are present until delivery [2, 3]. It is suggested that they originate from villous mesenchymal stem cells early in pregnancy, and later from the differentiation of recruited fetal monocytes [4]. Hofbauer cells are located below the syncytiotrophoblast in the villous mesenchyme and are adjacent to fetal capillaries [1]. Thus, HBCs are uniquely positioned to react to pathogens crossing the syncytiotrophoblast, directly affecting inflammatory responses in the fetus.
Hofbauer cells, like other macrophages, show plasticity in their function [4–8]. At baseline they function as the classically defined M2 or anti-inflammatory macrophages. It is suggested that M2 HBC functions include maturation and support of the placental stroma [5–7, 9]. There is evidence to suggest their involvement with angiogenesis, stromal fluid homeostasis, and development of the placental villous tree [5–7, 9]. However, in the presence of pathogen, or under inflammatory conditions, they may manifest a M1 or pro-inflammatory function [6, 8]. HBCs have a role in the inflammatory processes in response to infection, but most of the published data in this regard focuses on bacterial infections [10]. HBCs in preterm placentas with chorioamnionitis express increased Toll-like receptor 4 (TLR4), suggesting HBCs are an important part of the innate defenses of the fetus to bacterial insult [11]. Toti et al. reported a MCP-1-mediated mechanism by which fibroblasts recruit HBCs in response to pathogens [12], and Matsubara et al. noted an increase in activated HBCs in placentas from infected miscarriages compared to normal placentas [13]. Inflammation in the placenta is known to be associated with fetal inflammatory response syndrome (FIRS) and its subsequent neonatal morbidity and mortality [14, 15]. FIRS is an often subclinical activation of the fetal immune system eliciting an inflammatory response. Funisitis (inflammation of the umbilical cord) is the pathologic hallmark of the syndrome; and is associated with poor neonatal neurological outcomes including periventricular white matter lesions and cerebral palsy [16–18].
There have also been associations made between viral infections and poor obstetrical outcomes, including chorioamnionitis and preterm labor [19, 20]. Most of the published data regarding HBCs cells and viral infection are focused on HIV, for which they are susceptible due to their CD4 receptor expression [21, 22]. Recent data also indicates that Zika virus can replicate to high levels in HBCs [23–26], suggesting an important role in adverse fetal/neonatal outcomes seen in these pregnancies. Overall, the HBC response to viral insult is largely understudied, and the down-stream effects of this response are even less well studied.
Interleukin 1β (IL-1β) is a pro-inflammatory cytokine that is secreted by activated macrophages [27, 28]. It is known that endothelial cells are a major target of IL-1β - up-regulating levels of other inflammatory cytokines (e.g. IL-8), that can initiate neutrophilic responses, and promote leukocyte recruitment by endothelial cells [29].
In the current study we focused on the interactions between HBCs and human umbilical vein endothelial cells (HUVECs) in the presence of a viral infection, a physiologically relevant and well-studied model of umbilical vessel response. We also employed human endometrial endothelial cells (HEECs) to determine whether our observed effects were specific to HUVECs. In our model, isolated HBCs were infected with the murine γ-herpesvirus, MHV-68, and the collected conditioned media (CM) was then added to cultured HUVECs or HEECs. MHV-68 has been previously shown to infect human cells and tissues [30–32], and to promote preterm birth and adverse fetal outcomes in a mouse model [30]. Thus, we used this as a general model of a live viral infection of HBCs during pregnancy. We used endothelial cell markers of inflammation to measure endothelial cell activation. This included the levels mRNA expression and secreted protein of the neutrophil chemokine, interleukin-8 (IL-8); and the mRNA levels of cellular adhesion molecules involved with leukocyte binding and recruitment including E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular adhesion molecule 1 (VCAM-1) [29]. Herein we report that following infection with MHV-68, HBC-derived IL-1β induces a pro-neutrophilic response in HUVECs.
2. Methods
2.1. Collection of Placentas
Placentas (n=7) were collected from normal uncomplicated term singleton pregnancies delivered by cesarean section prior to labor. Infection was excluded on the basis of standard clinical criteria (absence of fever, uterine tenderness, maternal/fetal tachycardia, and foul vaginal discharge). Processing for placental cell culture was started within 30 min of surgery. Each placenta was processed separately. Approval for this study was granted our institution’s Human Investigation Committee (HIC# 1208010742).
2.2. Isolation of Hofbauer Cells (HBCs)
Isolation of HBCs was carried out using protocols that we have previously described.[33] Briefly, trypsin and collagenase digested tissue fragments were washed and loaded onto a discontinuous Percoll gradient (35%/30%/25%/20%) and centrifuged. Cells from 20%/25% to 30%/35% interfaces were combined and were immunopurified by negative selection using sequential treatment with anti-EGFR (clone #528, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then anti-CD10 (clone #HI10a, Biolegend, San Diego, CA, USA) antibodies conjugated to magnetic beads. Cells from the final supernatant were counted using a hemocytometer and then plated in RPMI medium, and floating and weakly attached cells were washed off after 1 h. The remaining adherent cells were treated as described below.
2.3. HBC culture and treatment
Prior to experiments, HBCs were maintained in FBSM (medium containing a 1:1 mixture of F12:DME and 10% fetal bovine serum). For experiments, HBCs were maintained for the indicated time in either FBSM or serum-free medium (SFM, DMEM/F12 with 50 μg/ml ascorbic acid and ITS+ Premix, a culture supplement which yields a final concentration of insulin of 6.25 mg/ml; transferrin, 6.25 mg/ml; selenous acid, 6.25 ng/ml; bovine serum albumin, 1.25 mg/ml; and linoleic acid, 5.35 μg/ml). HBCs were cultured in a six-well plate and infected with 1 ml of 10,000 PFU/ml MHV-68 for 1 h.[30, 31] The culture media was replaced with fresh SFM for 48 h and then collected.
2.4. Treatment of endothelial cell cultures with HBC conditioned medium
Human umbilical vein endothelial cells (HUVECs) were obtained from the Yale Institutional Tissue Core Laboratory and were maintained in endothelial growth medium (EGM)-2 (Lonza, Walkersville, MD) as previously described.[34] Human endometrium endothelial cells (HEECs) were obtained and immortalized as previously described.[35] HUVECs and HEECs were cultured in EGM-2 containing 24% SFM, and 6% HBC conditioned media (CM). EGM-2 and 30% SFM without CM was employed as a negative control. In some experiments, HBC CM was pretreated for 2 h with a mouse anti-human IL-1β antibody (Ab) (Cat# MAB201, R&D systems, Minneapolis, MN) or a mouse IgG1 isotype control (Cat# MAB002, R&D systems), each diluted in PBS to achieve a final concentration of 10 μg/ml. Protein G Separose (10μl/ml) diluted in PBS was then added for 1 h, washed and the process repeated after centrifugation. The supernatant was collected and diluted for treatment of HUVECs and HEECs as described above. Note, that the values presented in Figures 2, 5, and 6 for IL-8 secretion by HUVECs and HEECs in the presence of HBC CM were obtained by subtracting the levels of HBC secretion from the total levels of IL-8 detected in culture media. In another set of experiments, HUVECs were pretreated with for 1 h with 1μg/ml of IL-1 receptor antagonist (IL-1Ra, Peprotech, Rocky Hill, NJ) prior to exposure to HBC media and controls. For all experiments, culture media from HUVECS and HEECs were collected for analysis and HUVECs were washed with PBS and 1 ml of Trizol (Life Technologies, Grand Island, NY) was added for RNA extraction. Alternatively, to study the dose-dependent effects of IL-1β on HUVEC gene expression, cells were treated for 24 h with 0 - 1000pg/ml recombinant IL-1β (Cat# 201-LB-010/CF, R&D systems) for 24 h and RNA was extracted using Trizol.
Figure 2. Secretion of IL-8 by HUVECs following treatment with HBC CM.
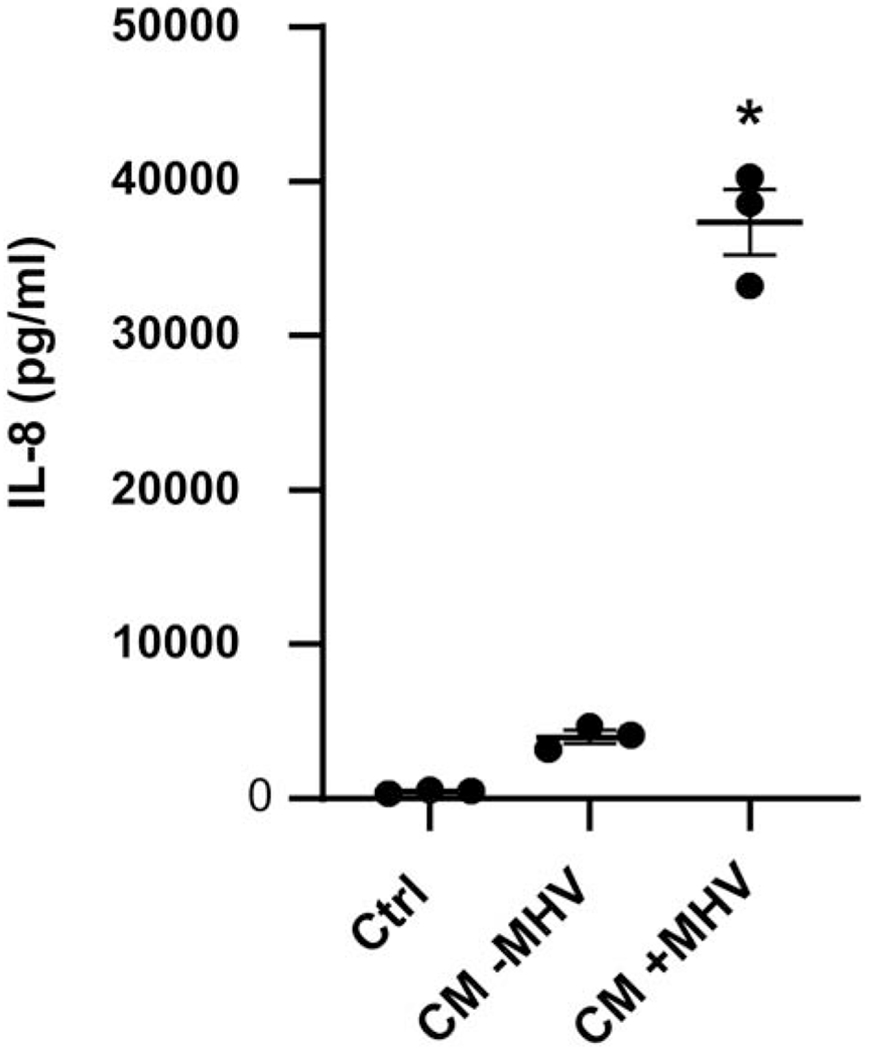
HUVECs were incubated for 24 h with CM from uninfected (CM − MHV) or MHV infected (CM + MHV) HBCs. Unconditioned serum-free medium (SFM) was used as control (CTRL). Levels of IL-8 in three independent experiments were determined by ELISA. Results are presented as a mean ± S.E.M and as individual data points. *p<0.05 vs uninfected Ctrl and CM-MHV groups.
Figure 5. Effect of IL-1 receptor antagonist (IL-1rA) on HUVEC IL-8 secretion in response to CM from MHV-infected HBCs.
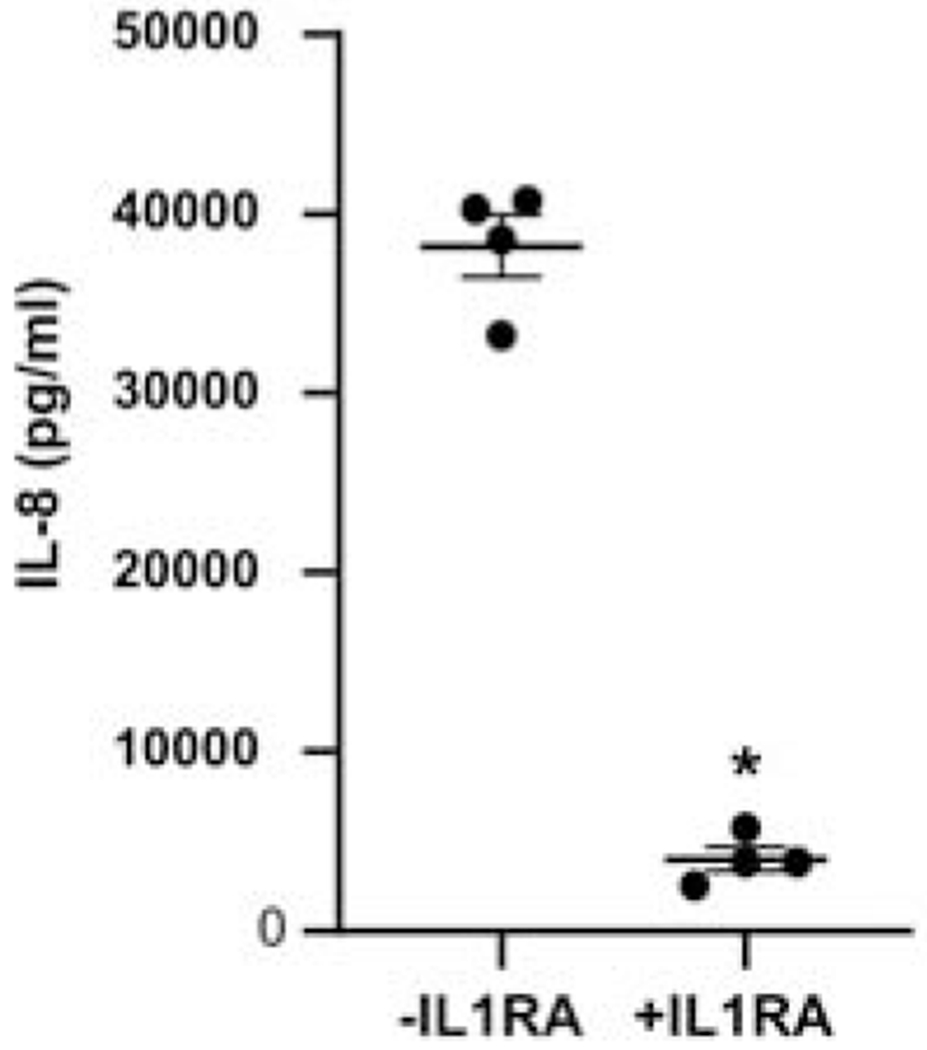
HUVECs were treated for 1 h with (+IL1rA) or without (− IL-1rA) and then incubated for an additional 24 h with CM from MHV-infected HBCs. Levels of IL-8 from four independent experiments were determined by ELISA and are expressed as a mean ± S.E.M and as individual data points. *p<0.05 vs −IL1rA.
Figure 6. Effect of IL-1β blocking antibody on HEEC IL-8 secretion in response to CM from MHV-infected HBCs.
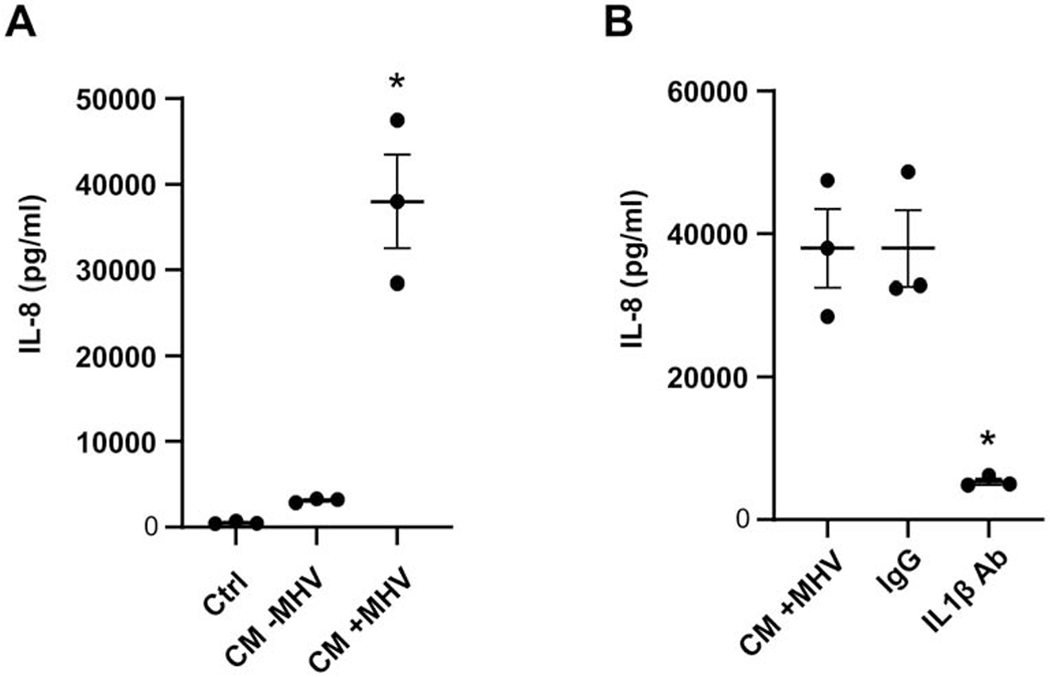
(A) HEECs were incubated for 24 h with CM from uninfected (CM − MHV) or infected (CM + MHV) HBCs, and unconditioned SFM was used as control (CTRL). Levels of IL-8 in three independent experiments were determined by ELISA and are expressed as a mean ± S.E.M. *p<0.05 versus CTRL and CM-MHV groups. (B) HEECs were incubated with CM from MHV-infected HBCs that were pretreated with either an IL-1β blocking antibody (IL-1β Ab), an isotype-matched control antibody (IgG), or no pretreatment (CM +MHV). Results are expressed as a mean ± S.E.M. from three independent experiments and as individual data points. *p<0.05 versus CM+MHV and IgG control groups.
2.5. ELISA
Levels of secreted IL-1β and IL-8 were measured using R&D Systems DuoSet ELISAs (Cat# DY201 and DY208, respectively). Levels of IL-1α were also measured by ELISA (Cat# 900-TM11, Peprotech).
2.6. qPCR
Levels of human mRNA were determined with an ABI 7500 RealTime PCR System (Applied Biosystems, Foster City, CA, USA) using TaqMan Gene Expression Assays for IL-1β (Cat# Hs01555410_m1), IL-8 (Cat# Hs99999034_m1), E-selectin (Cat# Hs00950401_m1), VCAM-1 (Cat# Hs01003372_m1), ICAM-1 (Cat# Hs00164932_m1) and 18S RNA (Cat# Hs99999901_s1). PCR reactions were performed in duplicate in a 20 μL volume of TaqMan Universal PCR Master Mix containing 1 μL of reverse transcribed cDNA and 1 μL of assay primer–probe mix. Mean CT values were analyzed using the ABI 7500 System SDS software 1.3.1. Gene expression was normalized to the 18S RNA housekeeping gene 18S using the formula 2−ΔΔCT Results are expressed as relative expression compared to an endogenous control in each experiment.
To detect of MHV-68 replication in HBCs and HUVECs cells were infected by MHV68 virus at an MOI of 0.1 for 1 hr and then maintained in SFM and EGM2 media respectively for 24 hours. Genomic DNA (gDNA) and viral DNA (vDNA) were isolated and purified using a Monarch Genomic DNA Purification Kit (NEB). qPCR was carried out using 100ng of gDNA+vDNA. The following primer sets were employed : MHV-68 ORF53 (Forward: 5’-AAAGACACCACTAGACACTG-3’, Reverse: 5’-CCAAGAAACCACACCTACTC-3’) and 18S (Forward: 5’-CGGACAGGATTGACAGATTGA-3’, Reverse: 5’-CCAGAGTCTCGTTCGTTATCG-3’). A Luna Universal qPCR Master Mix (NEB) was used to amplify targeted sequences and products were analyzed using a Bio-Rad iCycler iQ Real Time PCR Detection System. Data was collected and analyzed using iCycler program version 3.1 software and relative levels of MHV-68 DNA were calculated using the formula 2−ΔΔCt.
2.7. Statistics
Student’s t-test and one-way analysis of variance (ANOVA) were used to compare results that were normally distributed and are presented as a mean ± standard error of the mean (S.E.M). Mann-Whitney Rank Sum Test and Kruskal-Wallis ANOVA were carried out for data that were not normally distributed and are presented as a median with quartiles. SIGMASTAT software (San Jose, CA, USA) was used for statistical analyses. A p<0.05 was considered significant.
3. Results
3.1. HBCs secrete high levels of IL-1β in response to MHV-68
MHV-68 infection of HBCs at a MOI of 0.1 elicited a significant 64-fold increase in secreted IL-1β levels compared to uninfected controls (Fig. 1). A MOI of 0.1 was chosen based on the optimal enhancement of levels of lL-1β secretion by HBCs (Supplemental Figure 1). Successful infection of HBCs by MHV-68 was confirmed by measuring the early-late lytic gene, ORF-53 by qPCR [31] (data not shown). Levels of secreted IL-1α were also increased 10-fold compared to uninfected controls, although levels of secreted IL-1β were 8-fold that of IL-1α in infected HBCs (Supplemental Fig. 2). This indicated that IL-1β is the major IL-1 cytokine secreted by HBCs in response to MHV-68 infection. Of note, HBCs also secreted elevated levels of IL-8, IL-6, TNFα and MCP-1 following infection with MHV-68 (data not shown).
Figure 1. Secretion of IL-1β by HBCs following viral infection.
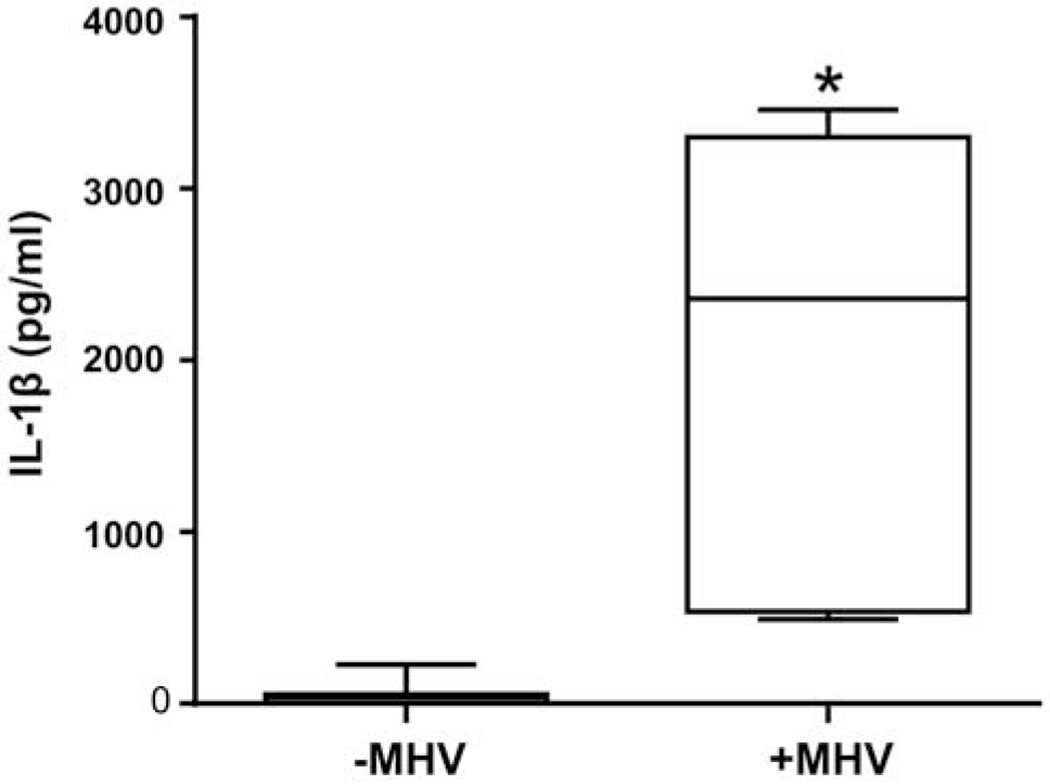
HBCs were infected with MHV-68 for 1 h, fresh media was added, and then HBCs were incubated for an additional 48 h in SFM. Uninfected HBC CM (−MHV) was used as control. Levels of secreted IL-1β, quantitated in seven independent experiments, were determined by ELISA. Results are presented as medians and percentiles; the lines inside the box indicate the median, the ends of the box describe the lower and upper quartiles, and the whiskers define the smallest and largest values. *p<0.001 versus uninfected control.
3.2. HUVECs secrete IL-8 in response to conditioned media (CM) from MHV-68-infected HBCs
We next sought to determine whether secreted factors in conditioned medium (CM) from MHV-68-infected HBCs induced HUVEC activation. As shown in Fig. 2, CM from MHV-68-infected HBCs promoted a significant 85-fold increase in IL-8 secretion by HUVECs compared to the negative control. A significant 9-fold increase in HUVEC IL-8 secretion was also noted compared to HUVECs treated with CM from uninfected HBCs (Fig. 2). Note, that the levels of IL-8 secretion by HUVECs were obtained by subtracting the levels of HBC IL-8 secretion from the total levels of IL-8 detected in culture media. Levels of IL-8 secreted by uninfected HBCs and infected HBCs were 10,845 ± 1466 pg/ml and 34,021 ± 2070 pg/ml (mean ± S.E.M.), respectively. This indicates that viral infection per se induces the secretion of HBC factors which in turn promote HUVEC expression of pro-neutrophilic markers.
Levels of IL-8, E-Selectin, ICAM-1, and VCAM-1 mRNA in HUVECs were measured by qPCR after treatment with CM from uninfected or MHV-68-infected HBCs. Expression of IL-8, E-selectin, ICAM-1, and VCAM-1 mRNAs, reflecting endothelial cell activation, was significantly increased in HUVECs (680, 4240-, 57-, and 191-fold, respectively) compared to the SFM control, and (56-, 49-, 6-, 13-fold, respectively) compared to the CM from uninfected HBCs (Fig. 3). To ensure that the HUVECs were responding to HBC-derived factors, rather than any residual virus in the HBC CM, we tested the direct effect of MHV-68 infection on HUVECs. Importantly, and despite HUVECs replicating MHV-68 to approximately a 6-fold greater degree (data not shown), we observed that there was no significant HUVEC response to MHV-68 infection in terms of the expression levels of IL-8, E-selectin, ICAM-1, and VCAM-1 mRNA when compared to uninfected controls (Supplemental Fig. 3).
Figure 3. Effect of HBC CM on levels of HUVEC IL-8, E-selectin, ICAM-1, and VCAM-1 mRNA.
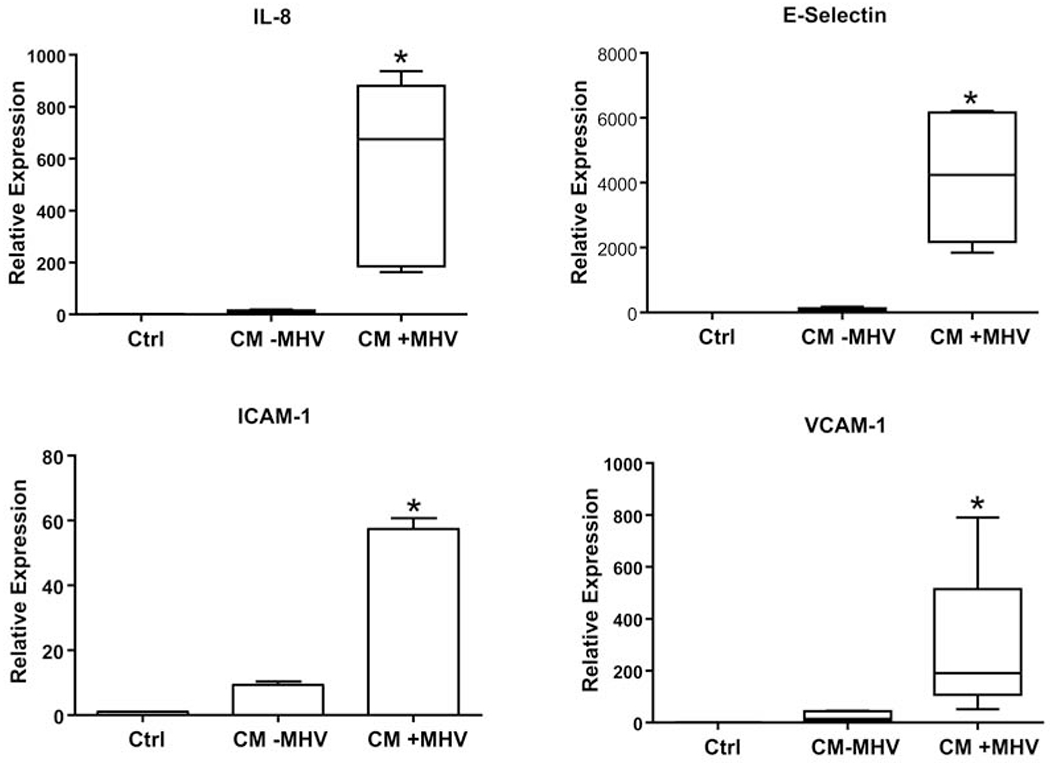
HUVECs were treated with HBC CM for 24 hours from MHV-infected HBCs (CM+MHV), uninfected HBC (CM−MHV), or unconditioned SFM control (Ctrl). Levels of mRNA were analyzed by qPCR and normalized to 18S RNA levels. Results are expressed as a mean + S.E.M. or as a median and percentiles from five independent experiments. *p<0.05 versus Ctrl and CM-MHV groups.
3.3. Enhanced pro-neutrophilic factor expression by HUVECs in response to CM from MHV-infected HBCs is mediated by IL-1β
Since HBCs secreted very high levels of IL-1β in response to MHV-68 infection and since IL-1β through the IL-1R can elicit many inflammatory responses, we sought to determine whether this HBC-derived factor was responsible for the pro-neutrophilic responses of HUVECs. Levels of IL-8 secreted by HUVECs in response to CM from MHV-68-infected HBCs were significantly reduced 77% by the presence of an IL-1β blocking Ab compared to the both untreated and IgG isotype-matched control Ab treated groups (Fig. 4A). Similarly, pretreatment of HBC CM with the IL-1β blocking antibody significantly reduced the CM-mediated increase in HUVEC IL-8; E-selectin; VCAM-1; and ICAM-1 mRNA levels by 90%; 92%; 58%; and 67%, respectively when compared to the isotype-matched control Ab (Fig. 4B).
Figure 4. Effect of IL-1β blocking antibody on HUVEC IL-8 secretion and levels of HUVEC IL-8, E-selectin, ICAM-1, and VCAM-1 mRNA in response to CM from MHV-infected HBCs.
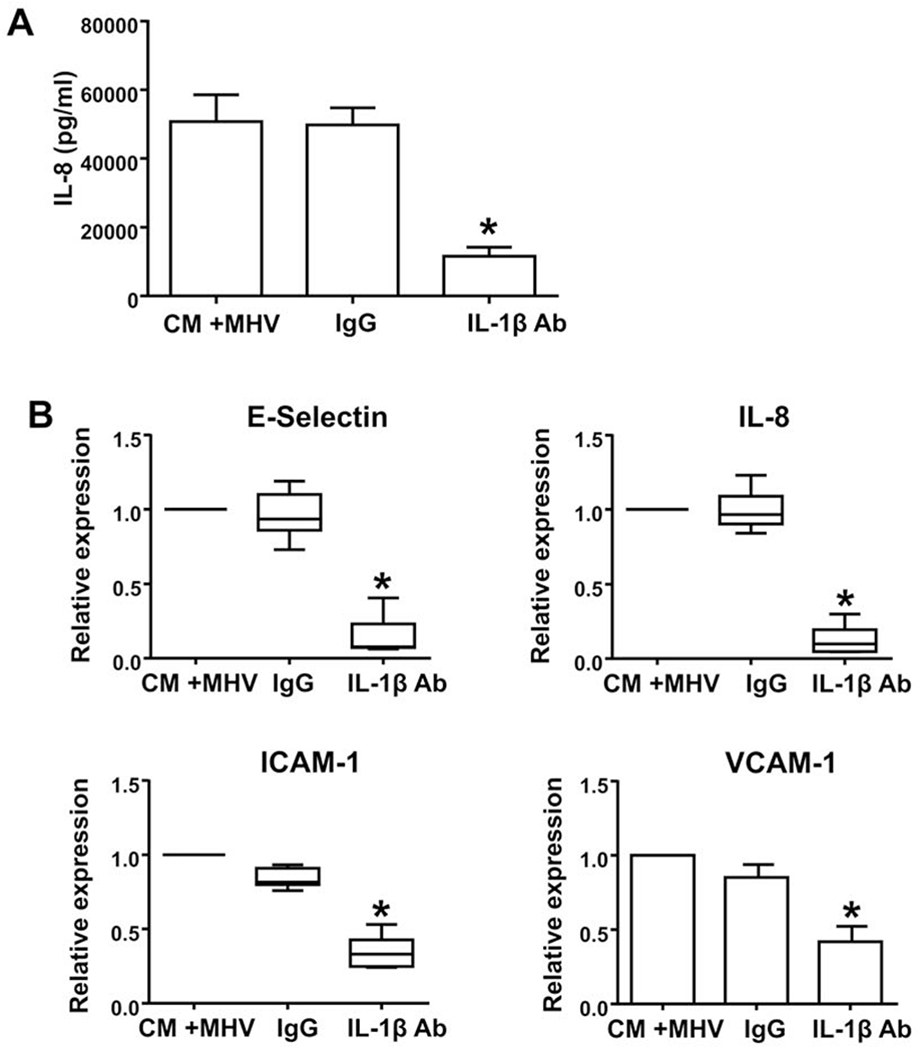
(A) HUVECs were incubated with CM from MHV-infected HBCs that were either pretreated with an IL-1β blocking antibody (IL1β Ab), an isotype-matched control antibody (IgG), or no pretreatment (CM +MHV) (Panel A). Results are expressed as a mean + S.E.M. from four independent experiments. *p<0.05 versus CM+MHV and IgG control groups. (B) HUVECs were treated as described above, and levels of mRNA were analyzed by qPCR and normalized to 18S RNA levels. Results are expressed as a mean + S.E.M. or as a median and percentiles from five independent experiments. *p< 0.05 versus CM+MHV and IgG control groups.
To confirm that HUVECs respond to IL-1β by generating a pro-inflammatory neutrophilic response, HUVECs were exposed to exogenous IL-1β. As shown in Supplemental Figure 4, HUVEC expression levels of IL-8, E-selectin, ICAM-1, and VCAM-1 mRNA were increased by recombinant IL-1β in a dose-dependent manner.
To provide further support of a direct role of HBC-derived IL-1β in promoting pro-neutrophilic responses by HUVECs, CM from MHV-68-infected HBCs was added to HUVECs pretreated with or without IL-1Ra. HUVEC secretion of IL-8 was significantly reduced by 90% in the presence of IL-1Ra (Fig. 5).
3.4. CM from MHV-infected HBCs promotes similar IL-β-dependent response in HEECs
HEECs, a second endothelial cell line, were studied to determine whether HBC CM uniquely promoted pro-inflammatory responses in HUVECs. Similar to observations made with HUVECs, the presence of CM from MHV-68-infected HBCs promoted a significant 76-fold increase in IL-8 secretion by HEECs compared to control (Fig. 6A); this level of induction was also significant and 12-fold greater than that observed in HEECs treated with CM from uninfected HBCs (Fig. 6A). Importantly, the stimulatory effect of CM from HBCs infected with MHV-68 on IL-8 secretion by HEECs was significantly inhibited 86% by the presence of IL-1β blocking antibody compared to control and treatment with isotype-matched control Ab groups (Fig. 6B). This indicates that, like responses noted in HUVECs, viral infection per se induces the secretion of HBC factors that promote a pro-inflammatory response in HEECs.
Taken together, these results indicate that IL-1β secreted by HBCs in response to viral infection promotes pro-inflammatory responses in endothelial cells.
4. Discussion
Inflammation in the human placental/fetal unit secondary to an infectious insult in pregnancy is associated with poor pregnancy outcomes, including the morbidity associated with both preterm delivery and fetal inflammatory response syndrome [14, 36, 37]. Placental inflammation is associated with FIRS, which is pathologically characterized by neutrophilic infiltration into the umbilical cord (funisitis) [38]. In the current study we examined whether interactions between HBCs and HUVECs in the presence of a viral infection could promote a pro-neutrophilic response. With respect to viral infection, the placental/fetal inflammatory response and sequela are less well described. Besides the known teratogenic viral outcomes included in the TORCH family (varicella-zoster, parvovirus B19, rubella, cytomegalovirus (CMV), and herpes simplex virus (HSV)), it is thought subclinical and unrecognized maternal viral infections are associated with poor prenatal outcomes [19, 20, 30]. Gervasi et al. evaluated midtrimester amniotic fluid for viral genetic material to determine the prevalence of subclinical infections and their associated outcomes. Out of 729 samples studied, 2.2 % or 16 samples had viral infections identified (7 cases of human herpes virus 6, 6 cases of CMV, 2 cases or parvovirus B19, and 1 case of Epstein-Barr virus).[19] Satosar et al. studied the placentas from pregnancies complicated by severe fetal or neonatal morbidity and mortality. They found viral rRNA in a large portion of the affected pregnancies (31/60) compared to normal controls (0/17). The infectious agents identified where largely enterovirus (23/60), specifically coxsackie virus. Other viruses identified included CMV (4/60), HSV (2/60), and parvovirus (2/60). With the exception of parvovirus, the virus was found in the placental macrophages (i.e. HBCs) [20].
The rationale for using MHV-68 in the current studies is based, in part, on a previous study that used a mouse model to demonstrate that prior infection of pregnant mothers with MHV-68 sensitized pregnancies to subsequent LPS-induced preterm delivery [30]. This led to the postulation of a two-hit hypothesis, suggesting that the presence of a non-clinical viral infection may predispose a group of women to bacterial-induced preterm delivery [30]. Of note, and of importance for our study, findings indicated that although maternal MHV-infection did not result in significant vertical transmission of virus from mother to the fetus, inflammatory cytokines derived from the placental and/or decidua were suggested to be responsible for neurological sequelae reminiscent of FIRS. We do acknowledge, however, that while the use of MHV-68 provided us with a model for studying host-pathogen interactions, it may differ from human pathogens important for pregnancy complications, such as HSV and CMV.
In the current report we noted that infection of HBCs with MHV-68 promoted robust levels of IL-1β secretion by this cell type. In contrast, infection with the MHV-68 alone had no significant effect on fetal membrane IL-1β secretion [31]. In addition, infection of human primary trophoblasts with MHV-68 only modestly increased IL-1β secretion [30]. This suggests the presence of cell-type specific response to virus within placenta and fetal membranes. In our current study, we also determined that CM from MHV-infected HBCs promoted a pro-inflammatory and pro-neutrophilic response in cultures of both HUVECs and HEECs as evidenced by increased IL-8 secretion and/or enhanced mRNA levels of markers of endothelial cell activation (IL-8, E-selectin, ICAM-1 and VCAM-1). Moreover, the HUVEC response to the CM of MHV-infected HBCs was inhibited by a neutralizing IL-1β Ab and by IL-1Ra, confirming that effects of HBC-derived IL-1β were mediated through the IL-1 receptor expressed by endothelial cells. However, since MHV-68 can induce HBC secretion of other inflammatory cytokines, these factors may also play a contributory role. Whether, the pro-neutrophil response generated by HUVECs after exposure to virally-infected HBCs has a functional impact on neutrophils is the focus of future studies. Based on a recent study by our group showing that in the presence of an infection fetal membranes recruit and activate neutrophils [39] which could lead to tissue injury, we speculate that this may also be the case in the umbilical cord with HUVECs recruiting neutrophils and promoting their activation.
Animal models of intrauterine inflammation following intrauterine or systemic administration of LPS have been used to mimic neurological disorders similar to those observed in exposed children [40–43]. Cytokine responses in these models include increased levels of in IL-1β in the fetal brain and placenta. In mice, blockade of IL-1 receptor protected against placental as well as neurodevelopmental and fetal cortical brain injury effects resulting from LPS treatment [41, 44]. These studies indicate that IL-1β plays a key role in inflammation-associated fetal brain injury. Thus, HBC-derived IL-1β in response to a viral infection may also pose a similar fetal risk.
With the knowledge that pregnant women represent a high risk population during viral pandemics, [45] a recent review stressed the important contributions of maternal, placental, as well as fetal viral infection on fetal development as well as maternal morbidity and pregnancy outcomes [46]. Their conclusion is that it is imperative that we gain a better understanding of how viruses infect and affect the placenta across gestation and how direct and indirect fetal infections differentially affect development.
Taken together, our results specifically indicate that a viral infection of HBCs promotes a pro-inflammatory/pro-neutrophilic response in endothelial cells through an IL-1β-dependent mechanism. This suggests that viral-induced-inflammatory responses in HBCs, which are topographically adjacent to fetal umbilical endothelial cells, could promote transplacental inflammation with or without vertical transmission of virus leading to adverse pregnancy outcomes. The importance of HBCs has been demonstrated in pregnancies complicated with infection by HIV [21, 22], and more recently, for those with Zika virus.[23–26] Thus, targeting microbial-driven inflammation in HBCs may have a significant impact in our efforts to reduce viral-associated adverse pregnancy outcomes.
Supplementary Material
Highlights.
Viral infection of Hofbauer cells induced a significant increase in IL-1β secretion.
Virally infected Hofbauer cells induced a HUVEC pro-neutrophilic response.
The HUVEC response to virally-infected Hofbauer cells was IL-1β mediated.
This cell-cell communication may play a role in the genesis of fetal inflammation.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health Grants RO1AI131613 (to SG and VMA) and P01HD054713 (to GM and SG)
Abbreviations:
- CM
conditioned medium
- FIRS
fetal inflammatory response syndrome
- HBCs
Hofbauer cells
- HEECs
human endometrial endothelial cells
- HUVECs
human umbilical vein endothelial cell
- ICAM
intercellular adhesion molecule
- IL-1Ra
IL-1 receptor antagonist
- MHV
murine herpes virus
- SFM
serum-free medium
- TLR
Toll-like receptor
- VCAM
vascular adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- [1].Castellucci M, Zaccheo D, Pescetto G, A three-dimensional study of the normal human placental villous core. I. The Hofbauer cells, Cell Tissue Res 210(2) (1980) 235–47. [DOI] [PubMed] [Google Scholar]
- [2].Fox H, The incidence and significance of Hofbauer cells in the mature human placenta, J Pathol Bacteriol 93(2) (1967) 710–7. [DOI] [PubMed] [Google Scholar]
- [3].Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS, Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease, J Immunol 182(6) (2009) 3919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benirshke K BG, Baergen RN, Basic structure of villous trees , in: Benirschke K BG, Baergen RN (Ed.), Pathology of the Human Placenta, Springer, New York, NY, 2012, pp. 55–100. [Google Scholar]
- [5].Anteby EY, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Haimov-Kochman R, Holzer H, Yagel S, Human placental Hofbauer cells express sprouty proteins: a possible modulating mechanism of villous branching, Placenta 26(6) (2005) 476–83. [DOI] [PubMed] [Google Scholar]
- [6].Joerink M, Rindsjo E, van Riel B, Alm J, Papadogiannakis N, Placental macrophage (Hofbauer cell) polarization is independent of maternal allergen-sensitization and presence of chorioamnionitis, Placenta 32(5) (2011) 380–5. [DOI] [PubMed] [Google Scholar]
- [7].Seval Y, Korgun ET, Demir R, Hofbauer cells in early human placenta: possible implications in vasculogenesis and angiogenesis, Placenta 28(8-9) (2007) 841–5. [DOI] [PubMed] [Google Scholar]
- [8].Young OM, Tang Z, Niven-Fairchild T, Tadesse S, Krikun G, Norwitz ER, Mor G, Abrahams VM, Guller S, Toll-like receptor-mediated responses by placental Hofbauer cells (HBCs): a potential pro-inflammatory role for fetal M2 macrophages, Am J Reprod Immunol 73(1) (2015) 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ingman K, Cookson VJ, Jones CJ, Aplin JD, Characterisation of Hofbauer cells in first and second trimester placenta: incidence, phenotype, survival in vitro and motility, Placenta 31(6) (2010) 535–44. [DOI] [PubMed] [Google Scholar]
- [10].Tang Z, Abrahams VM, Mor G, Guller S, Placental Hofbauer cells and complications of pregnancy, Ann N Y Acad Sci 1221 (2011) 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y, Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis, Hum Pathol 35(1) (2004) 47–54. [DOI] [PubMed] [Google Scholar]
- [12].Toti P, Arcuri F, Tang Z, Schatz F, Zambrano E, Mor G, Niven-Fairchild T, Abrahams VM, Krikun G, Lockwood CJ, Guller S, Focal increases of fetal macrophages in placentas from pregnancies with histological chorioamnionitis: potential role of fibroblast monocyte chemotactic protein-1, American journal of reproductive immunology 65(5) (2011) 470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matsubara S, Takayama T, Yamada T, Usui R, Izumi A, Watanabe T, Ohkuchi A, Shibahara H, Sato I, Suzuki M, Hofbauer cell activation and its increased glucose-6-phosphate dehydrogenase activity in second trimester-spontaneous abortion: an ultrastructural dual staining enzyme-cytochemical study, American journal of reproductive immunology 49(4) (2003) 202–9. [DOI] [PubMed] [Google Scholar]
- [14].Bashiri A, Burstein E, Mazor M, Cerebral palsy and fetal inflammatory response syndrome: a review, J Perinat Med 34(1) (2006) 5–12. [DOI] [PubMed] [Google Scholar]
- [15].Romero R, Chaiworapongsa T, Espinoza J, Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome, J Nutr 133(5 Suppl 2) (2003) 1668S–1673S. [DOI] [PubMed] [Google Scholar]
- [16].Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR, Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years, Am J Obstet Gynecol 182(3) (2000) 675–81. [DOI] [PubMed] [Google Scholar]
- [17].Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK, The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis, Am J Obstet Gynecol 183(5) (2000) 1124–9. [DOI] [PubMed] [Google Scholar]
- [18].Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC, Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia, Am J Obstet Gynecol 174(5) (1996) 1433–40. [DOI] [PubMed] [Google Scholar]
- [19].Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, Mor G, Barzon L, Franchin E, Militello V, Palu G, Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy, J Matern Fetal Neonatal Med 25(10) (2012) 2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ, Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn, Hum Pathol 35(5) (2004) 536–45. [DOI] [PubMed] [Google Scholar]
- [21].Boily-Larouche G, Milev MP, Zijenah LS, Labbe AC, Zannou DM, Humphrey JH, Ward BJ, Poudrier J, Mouland AJ, Cohen EA, Roger M, Naturally-occurring genetic variants in human DC-SIGN increase HIV-1 capture, cell-transfer and risk of mother-to-child transmission, PloS one 7(7) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Soilleux EJ, Morris LS, Lee B, Pohlmann S, Trowsdale J, Doms RW, Coleman N, Placental expression of DC-SIGN may mediate intrauterine vertical transmission of HIV, J Pathol 195(5) (2001) 586–92. [DOI] [PubMed] [Google Scholar]
- [23].Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, Fikrig E, Zika virus productively infects primary human placenta-specific macrophages, JCI Insight 1(13) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS, Zika Virus Infects Human Placental Macrophages, Cell Host Microbe 20(1) (2016) 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S, Zika virus infection of Hofbauer cells, American journal of reproductive immunology 77(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L, Zika Virus Targets Different Primary Human Placental Cells, Suggesting Two Routes for Vertical Transmission, Cell Host Microbe 20(2) (2016) 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A, Macrophage plasticity, polarization, and function in health and disease, J Cell Physiol 233(9) (2018) 6425–6440. [DOI] [PubMed] [Google Scholar]
- [28].Taniguchi T, Matsuzaki N, Kameda T, Shimoya K, Jo T, Saji F, Tanizawa O, The enhanced production of placental interleukin-1 during labor and intrauterine infection, Am J Obstet Gynecol 165(1) (1991) 131–7. [DOI] [PubMed] [Google Scholar]
- [29].Mantovani A, Bussolino F, Introna M, Cytokine regulation of endothelial cell function: from molecular level to the bedside, Immunol Today 18(5) (1997) 231–40. [DOI] [PubMed] [Google Scholar]
- [30].Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G, Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor, J Immunol 185(2) (2010) 1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cross SN, Potter JA, Aldo P, Kwon JY, Pitruzzello M, Tong M, Guller S, Rothlin CV, Mor G, Abrahams VM, Viral Infection Sensitizes Human Fetal Membranes to Bacterial Lipopolysaccharide by MERTK Inhibition and Inflammasome Activation, J Immunol 199(8) (2017) 2885–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Racicot K, Kwon JY, Aldo P, Abrahams V, El-Guindy A, Romero R, Mor G, Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth, Am J Reprod Immunol 75(4) (2016) 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tang Z, Tadesse S, Norwitz E, Mor G, Abrahams VM, Guller S, Isolation of hofbauer cells from human term placentas with high yield and purity, Am J Reprod Immunol 66(4) (2011) 336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma Y, Kadner SS, Guller S, Differential effects of lipopolysaccharide and thrombin on interleukin-8 expression in syncytiotrophoblasts and endothelial cells: implications for fetal survival, Ann N Y Acad Sci 1034 (2004) 236–44. [DOI] [PubMed] [Google Scholar]
- [35].Krikun G, Mor G, Huang J, Schatz F, Lockwood CJ, Metalloproteinase expression by control and telomerase immortalized human endometrial endothelial cells, Histol Histopathol 20(3) (2005) 719–24. [DOI] [PubMed] [Google Scholar]
- [36].Goncalves LF, Chaiworapongsa T, Romero R, Intrauterine infection and prematurity, Ment Retard Dev Disabil Res Rev 8(1) (2002) 3–13. [DOI] [PubMed] [Google Scholar]
- [37].Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M, The preterm parturition syndrome, Bjog 113 Suppl 3 (2006) 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM, Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance, Am J Obstet Gynecol 213(4 Suppl) (2015) S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tong M, Potter JA, Mor G, Abrahams VM, Lipopolysaccharide-Stimulated Human Fetal Membranes Induce Neutrophil Activation and Release of Vital Neutrophil Extracellular Traps, J Immunol 203(2) (2019) 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Burd I, Chai J, Gonzalez J, Ofori E, Monnerie H, Le Roux PD, Elovitz MA, Beyond white matter damage: fetal neuronal injury in a mouse model of preterm birth, Am J Obstet Gynecol 201(3) (2009) 279 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Girard S, Tremblay L, Lepage M, Sebire G, IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation, J Immunol 184(7) (2010) 3997–4005. [DOI] [PubMed] [Google Scholar]
- [42].Leviton A, Gressens P, Neuronal damage accompanies perinatal white-matter damage, Trends Neurosci 30(9) (2007) 473–8. [DOI] [PubMed] [Google Scholar]
- [43].Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D, Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy, Am J Obstet Gynecol 199(6) (2008) 651 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Leitner K, Al Shammary M, McLane M, Johnston MV, Elovitz MA, Burd I, IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury, Am J Reprod Immunol 71(5) (2014) 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G, Viral infections during pregnancy, Am J Reprod Immunol 73(3) (2015) 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Racicot K, Mor G, Risks associated with viral infections during pregnancy, J Clin Invest 127(5) (2017) 1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


