Summary
In situ measurement of cellular metabolites is still a challenge in biology. Conventional methods, such as mass spectrometry or fluorescence microscopy, would either destroy the sample or introduce strong perturbations to target molecules. Here, we present multiplex stimulated Raman scattering (SRS) imaging cytometry as a label-free single-cell analysis platform with chemical specificity and high-throughput capabilities. Using SRS imaging cytometry, we studied the metabolic responses of human pancreatic cancer cells under stress by starvation and chemotherapeutic drug treatments. We unveiled protrusions containing lipid droplets as a metabolic marker for stress-resistant cancer cells. Furthermore, by spectroscopic SRS mapping, we unveiled that triglyceride in lipid droplets are used for local energy production through lipolysis, autophagy, and β-oxidation. Our findings demonstrate the potential of targeting lipid metabolism for selective treatment of stress-resistant cancers. Collectively, these results highlight SRS imaging cytometry as a powerful label-free tool for biological discoveries with a high-throughput, high-content capacity.
Subject Areas: Optical Imaging, Metabolomics, Cancer
Graphical Abstract
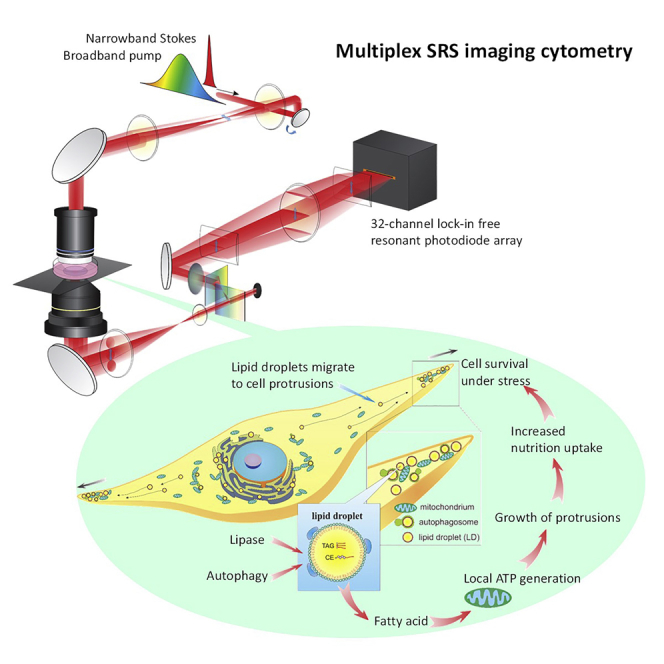
Highlights
-
•
Multiplex SRS imaging cytometry is developed for single-cell metabolic analysis
-
•
Lipid-rich protrusions are unveiled as a marker for stress-resistant cancer cells
-
•
Lipids in protrusions are used for local energy production via β-oxidation
-
•
Blockage of β-oxidation impairs survival of stress-resistant cancer cells
Optical Imaging; Metabolomics; Cancer
Introduction
Altered cell metabolism is recognized as one of the hallmarks of cancer (Hanahan and Weinberg, 2011). The reprogrammed metabolism, which is deployed by cancer cells to fulfill the demands of fast proliferation (Heiden et al., 2009, Warburg, 1956), offers opportunities for the diagnosis and treatment of cancer (Pelicano et al., 2006, Hamanaka and Chandel, 2012, Zhao et al., 2013). However, challenges remain in the quantification of cell metabolism, one of which is the inter- or intratumoral heterogeneous metabolic profiles of cancer cells. Although the ensemble measurement of large number of cancer cells identifies specific metabolic features under certain condition, individual cancer cells might show significantly different metabolic response (Altschuler and Wu, 2010). This cell heterogeneity is considered one of the major causes of incomplete tumor remission and relapse (Marusyk and Polyak, 2010, Meacham and Morrison, 2013). Such an inhomogeneous response of cells to environments and drugs cannot be addressed using conventional methods, such as biochemical assays or mass spectrometry, which are based on ensemble measurement of a cell population. High-efficiency imaging tools that can quantify metabolic features of individual cells with subcellular information for a large number of cells become essential.
Flow cytometry is one of the commonly used technologies for high-throughput single-cell analysis, which generates statistical information of a cell population (Hulett et al., 1969). However, conventional flow cytometry integrates the whole-cell signal to a single intensity measurement, lacking the information in spatial distributions of cellular compounds that are essential to understand cell heterogeneity in many cases. To take spatial information into account, one must switch to imaging cytometry or imaging flow cytometry (Blasi et al., 2016, Henriksen et al., 2011, McGrath et al., 2008, Nitta et al., 2018). Conventional imaging cytometry relies on fluorescence labeling, which is unsuitable to track endogenous metabolites in living cells. Photobleaching, non-specific binding, cytotoxicity, and molecular functional perturbation all raise issues for fluorescence-based measurement (Jensen, 2012, Zanetti-Domingues et al., 2013). In addition, high-specificity fluorescent probes do not exist for most small-molecule metabolites. To trace specific metabolic molecules in living cells, molecular analog with fluorescent conjugation might be used (Solanko et al., 2013, Yamada et al., 2007). However, the relatively large size of fluorescent probes, when tagged to small metabolic molecules, would significantly alter the biofunctions of these molecules (Lee et al., 2015, Wei et al., 2014).
Raman spectroscopy and microscopy have shown great potentials to solve problems encountered by fluorescence-based imaging. It circumvents the process of labeling and contains rich chemical information to characterize small molecules. The capacity of Raman spectroscopy/microscopy has been demonstrated to discover cholesteryl ester accumulation as a marker for aggressive cancer cells (Yue et al., 2014), visualize cytochrome c releases during apoptosis (Okada et al., 2012), and monitor the cellular stage such as macrophage activation (Pavillon et al., 2018). However, spontaneous Raman scattering is a very weak process, thus requiring hours to acquire a cellular image, which is impractical for live-cell imaging and imaging cytometry (Zhang et al., 2015a, Zhang et al., 2015b). The advent of coherent Raman scattering (CRS) techniques, including coherent anti-Stokes Raman scattering (CARS) and stimulated Raman scattering (SRS), enhanced the Raman efficiency by around seven orders of magnitude (Min et al., 2011, Cheng and Xie, 2016), and achieved imaging speed as fast as fluorescent microscopy (Evans et al., 2005, Ozeki et al., 2012, Saar et al., 2010). CRS microscopy has been used to study lipid metabolism (Fu et al., 2014, Yu et al., 2014, Yue et al., 2014, Li et al., 2017), protein metabolism (Wei et al., 2013, Wei et al., 2014), nucleic acid metabolism (Chen et al., 2014, Wei et al., 2014), retinoid metabolism (Chen et al., 2018, Liao et al., 2015a), cholesterol metabolism (García et al., 2015, Wang et al., 2013, Lee et al., 2015), and glucose metabolism (Li and Cheng, 2014, Hu et al., 2015, Zhang et al., 2019) and to monitor small molecular drug delivery (Gaschler et al., 2018, Tipping et al., 2016). To promote high-throughput analysis of single cells at a high speed, CARS and SRS flow cytometry have been demonstrated (Charles et al., 2011, Hiramatsu et al., 2019, Zhang et al., 2017). However, to generate enough signal, CRS usually requires tight laser focusing to a spot much smaller than a cell (Charles et al., 2011, Hiramatsu et al., 2019, Zhang et al., 2017). Therefore, CRS signals in flow cytometry might not represent the entire cell. To acquire spatial information from the cells and in flow cytometry settings, four-color SRS imaging flow cytometry was demonstrated recently to classify microalgal cells and cancer cells without the need for fluorescent labeling (Suzuki et al., 2019).
Here, we designed and constructed a prototype of high-content high-throughput imaging cytometer based on multiplex SRS. The multiplex SRS spectroscopy enabled acquisition of a Raman spectrum covering 200 wavenumbers at a speed of 5 μs in 32 spectral channels. We implemented a hybrid scanning scheme for high-speed hyperspectral cell imaging at a throughput of 30–50 cells per second at diffraction-limited spatial resolution. At a spectral resolution of 13.4 cm−1, we segregated the subcellular compartments based on their compositional difference. The high spatial and spectral resolution enables high-content single-cell analysis to address cellular heterogeneity by using our imaging cytometer. Through development of a quantitative analysis algorithm based on CellProfiler, we are able to distinguish 260 morphological and metabolic features in thousands of individual cells, which is not achievable with other technologies. Using our multiplex SRS imaging cytometer, we studied how human cancer cells reprogram their metabolism in response to stress conditions, including starvation and chemotherapy treatment. We found that nutrient starvation or chemotherapy treatment cause lipid droplet (LD) redistributions by forming LD-facilitated protrusions, which may promote cancer cell survival under stress by enhancing their nutrient uptake capacity. We also validated that LDs in protrusions are used for local energy production by SRS and two-photon fluorescence imaging on the same microscope. This finding not only opens opportunities of targeting the reprogrammed lipid metabolic pathway to treat stress-resistant cancer cells but also demonstrates the prowess of multiplex SRS imaging cytometry for discovering important metabolic markers of human diseases.
Results
Multiplex SRS-Based Label-free Chemical Imaging Cytometry
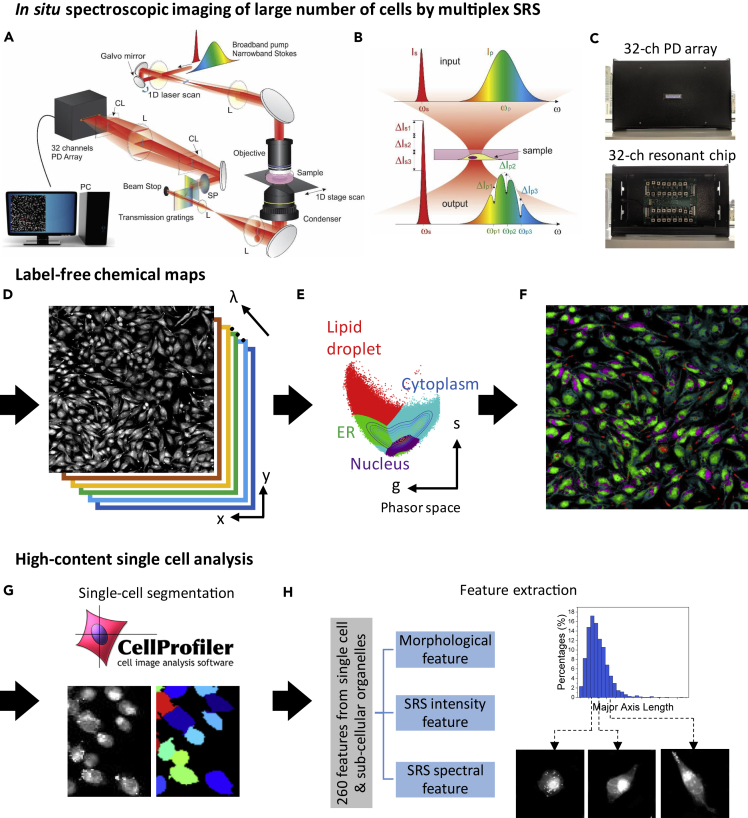
To quantify molecular information of a large numbers of cells at a high-throughput capacity, we developed a multiplex SRS imaging cytometer. The setup of our multiplex SRS imaging cytometer is shown in Figure 1A. A broadband pump and a narrowband Stokes laser beam simultaneously excite multiple Raman transition modes (Figure 1B). After the sample, the pump beam was dispersed by a grating pair and detected by a laboratory-built 32-channel lock-in free resonant photodiode array detector shown in Figure 1C. This parallel detection scheme allows high-speed, distortion-free, and sensitive Raman spectroscopy at 5 μs per spectrum. At such a speed, we are able to detect dimethyl sulfoxide (DMSO) as low as 0.1% (v/v) (see Figure S1A) covering a 200 cm−1 spectral window with a 13.4 cm−1 spectral resolution (see Figure S1B). Hybrid high-speed scanning was applied by combining one-dimensional laser scanning with a perpendicular one-dimensional translation of motorized stage (Figure 1A). With these implantations, our cytometric system achieved the throughput of mapping 30–50 cells per second with diffraction-limited spatial resolution.
Figure 1.
Workflow of the Single-Cell Analysis by Multiplex SRS Imaging Cytometry
(A–C) In situ spectroscopic imaging of a large number of cells by multiplex SRS. (A) Hybrid scanning was implemented by scanning a galvo mirror while moving the motorized stage. Multiple Raman modes are parallelly detected by a laboratory-built 32-channel lock-in free resonant photodiode array detector. (B) Multiple Raman shifts are excited by a broadband pump beam and a narrowband Stokes beam. (C) A photograph of our laboratory-built 32-channel lock-in free resonant photodiode (PD) array detector. Upper panel: the detector front side, showing a 32-channel PD array. Lower panel: the detector backside, showing 32-channel resonant circuit chips for lock-in free detection.
(D–F) Label-free chemical mapping. (D) x-y-λ, a three-dimensional dataset generated by the multiplex SRS imaging cytometer. (E) The SRS spectrum from each image pixel is projected onto a 2D phasor domain, followed by an unsupervised clustering algorithm to separate the ER, nuclei, cytosol, and LDs. (F) A chemical map is generated by remapping the clustered results in the phasor domain back to the SRS image.
(G and H) High-content single-cell analysis. (G) Single-cell segmentation by CellProfiler. (H) Left panel: a total of 260 features in each cell are extracted, which can be classified into morphological features, SRS intensity features, and SRS spectral features. Right: statistical analysis of each feature demonstrates cellular heterogeneity.
The Workflow of Single-Cell Analysis by Multiplex SRS Imaging Cytometry
We developed a workflow to achieve high-content single-cell analysis using the three-dimensional dataset generated by the high-speed multiplex SRS imaging cytometer. First, in situ spectroscopic imaging of a large number of cells is performed (Figures 1A–1D). To resolve weak chemical compositions, we apply a space-wavelength total variation denoizing algorithm to improve the signal-to-noise ratio and image quality (Liao et al., 2015b) (see Figures S2A and S2B). Second, chemical maps of subcellular compartments including ER LD, nucleus, and cytoplasm are generated (Figures 1D and 1E). Here, spectral phasor analysis is utilized to project the SRS spectrum from each pixel (Figure 1D) onto a two-dimensional phasor domain (Fu and Xie, 2014) (Figure 1E), followed by an unsupervised algorithm to cluster each cellular compartment based on its signature Raman spectrum (see Figures S2C–S2E, details can be found in Transparent Methods). Then, we remap the clustered pixels from the phasor domain back to the spatial domain to generate the chemical maps (Figure 1F). To validate the segmentation of cellular compartments, we compared our label-free imaging results with the images from fluorescent labeling. Our label-free chemical maps of ER, LD, and nucleus showed good agreement with the fluorescence images of ER, LD, and nucleus labeled by ER tracker, C12-BODIPY [C 12 (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid), and 4′,6-diamidino-2-phenylindole (DAPI), respectively (see Figure S3).
Third, to enable high-content single-cell analysis, we used CellProfiler for automatic single-cell segmentation (Figure 1G) and feature extraction (Figure 1H). A total of 260 features were extracted in each cell to describe cellular or subcellular characteristics. These features were categorized into the following three groups as shown in Figure 1H. (1) The morphological features, i.e., cell shape, size of ER, nuclear-cytoplasmic ratio, location of LD, etc. (2) SRS intensity features. The SRS signal is linearly proportional to the molecular concentration. Therefore, the average intensity reflects molecular concentration, the integrated intensity designates the total amount of chemicals, and the intensity distribution encloses texture information. (3) SRS spectral features. These features show chemical compositions of certain compartments in cells. Specifically, we measured and compared the ratio of triglyceride and cholesterol contents in LDs. For each feature, it can be displayed as a histogram (Figure 1H) to reflect the distribution of this feature in a cell population. As an example shown in Figure 1H, major axial length histogram illustrates the cellular morphology distribution. We confirm the consistency of quantified major axes value by validating the corresponding images of long, medium, and short major axes value.
Multiplex SRS Imaging Cytometry Identifies Lipid-Accumulated Protrusion as a Metabolic Signature for Cancer Cells under Stressed Conditions
We applied our system to study a fundamental yet elusive question: how human cancer cells reprogram their metabolism to cope with a stressed microenvironment. We chose pancreatic cancer as a test bed, because development of resistance to gemcitabine is a well-known challenge in pancreatic cancer treatment (Grasso et al., 2017). Owing to the dense extracellular matrix, lack of nutrition is another stress in pancreatic cancer (Derle et al., 2018). To systematically study the metabolic response to such stress, we imaged six groups of human pancreatic cancer cells under a variety of stress conditions, including a chemotherapy stress model: (1) MIA PaCa-2 pancreatic cancer cell treated with gemcitabine drug, (2) MIA PaCa-2 and MIA PaCa-2-derived gemcitabine-resistant cell line G3K, (3) G3K cells treated with gemcitabine drug, and a starvation stress model MIA PaCa-2 cells in glucose and serum-free medium for (4) 6 h, (5) 12 h, and (6) 24 h. A total of 260 features from three categories, including morphological features, SRS intensity features, SRS spectral features, are extracted (see Table S1).
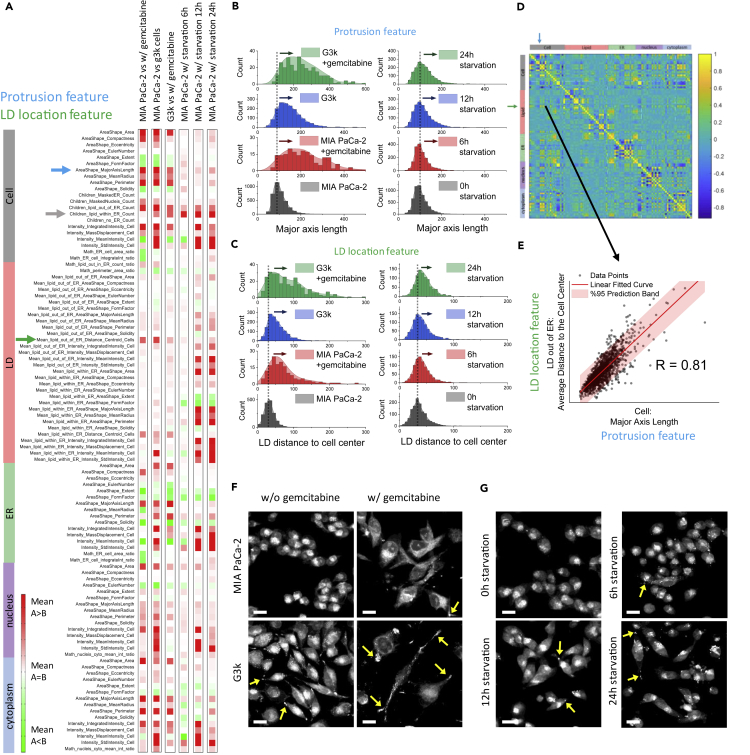
Figure 2A shows the extent of change of cellular features of MIA PaCa-2 or G3K cells under different conditions. From these feature heatmaps, we observed some common trends of morphological or metabolic change shared among gemcitabine-resistant G3K cells and gemcitabine-treated or starved cells. For example, gemcitabine-treated, gemcitabine-resistant, and starved cells all tend to have increased major axis length (blue arrow), increased LD counts within ER (gray arrow), and increased mean distances of LDs out of the ER to the center of the cell (green arrow). To better illustrate the distribution of these features in cell populations under different conditions, we elaborate individual features in histograms. Figure 2B demonstrates the distributions of major axis length (blue array in Figure 2A) of MIA PaCa-2 and G3K cells in different conditions. We found that gemcitabine treatment tends to broaden the distributions of both cell lines, with the maximum distribution shifting to a larger value; starvation of 12 h or longer also broadens the distribution, but with no obvious shifting of central distribution. These results indicate that under stress condition, a subpopulation of cancer cells tend to have spindle-like morphology, which is likely due to the formation of protrusion structures. Another example is the stress-altered distribution of LDs in cells. Figure 2C displays the mean distance of LDs out of the ER to the center of the cell (green array in Figure 2A), from which we find right shifts of the distribution in gemcitabine-treated or resistant cells and in cells under starvation. Such a change indicates a spatial redistribution of LDs toward the distal location of cancer cells in stress conditions.
Figure 2.
Multiplex SRS Imaging Cytometry Identifies Metabolic Signatures under Stressed Conditions
(A) Feature arrays of cells under chemotherapy stress model (columns 1–3) and starvation stress model (columns 4–6). Red or green color indicates a mean value higher or lower than the control group, respectively. From columns 1–6: MIA PaCa-2 cells treated with gemcitabine (n = 642) compared with MIA PaCa-2 cells (control, n = 1,150); G3K cells (n = 1,637) compared with MIA PaCa-2 cells (control, n = 1,150); G3K cells treated with gemcitabine (n = 313) compared with G3K cells (control, n = 1,637); MIA PaCa-2 cells starved for 6 h (n = 1,698) compared with MIA PaCa-2 cell without starvation (control, n = 5,259); MIA PaCa-2 cells starved for 12 h (n = 1,515) compared with MIA PaCa-2 cell without starvation (control, n = 5,259); MIA PaCa-2 cells starved for 24 h (n = 1,547) compared with MIA PaCa-2 cell without starvation (control, n = 5,259). Blue arrow indicates the celluar feature as the major axis length; Gray arrow indicates the feature of increased LD counts within ER; Green arrow indicates the feature of mean distances of LDs out of the ER to the center of the cell.
(B) Histograms of the cell “major axis length” for both the chemotherapy stress model (left panels) and the starvation stress model (right panels).
(C) Histograms of the “distance of lipid droplets to the cell center,” for both the chemotherapy stress model (left panel) and the starvation stress model (right panel).
(D) Correlation matrix of all the 260 features in gemcitabine-resistant G3K cells (n = 1,637). Yellow color indicates positive correlation coefficients with the highest value of 1. Blue color indicates negative correlation coefficients with the lowest value of −1.
(E) The “major axis length” (a protrusion feature) is positively correlated with “distance of lipid droplets to the cell center” (a lipid droplet feature), with a correlation coefficient of 0.81.
(F and G) Representative SRS images of cells from (F) the chemotherapy stress model and (G) the starvation stress model. Yellow arrows indicate the lipid-accumulated protrusion structures. Scale bar, 20 μm.
To better understand the relationships among the extracted 260 features, we generated a correlation matrix by calculating correlation coefficients from all features of the gemcitabine-resistant G3K cells (Figure 2D). From this table, we find some strong correlations between different morphological or metabolic features, most of which are features representing similar information. For example, we found that the protrusion features, such as major axis length, solidity, form factor, extent, compactness, and eccentricity strongly correlated with each other, presenting the morphological changes in multiple dimensions (see Figure S4). Surprisingly, we also found that the protrusion features correlate with the LD features, such as the LD location feature (Figure 2E). By plotting the major axis length of the cells to the average distance of LDs to the cell center, we found a strong positive correlation between them with a coefficient of 0.81. This suggests that in stress environments, the cells not only form protrusions but also have enriched LDs in the protrusions. This finding prompts us to perform a closer examination of the cells under SRS imaging. Consistent with our high-throughput analysis, we observed increased number of lipid-enriched protrusion structures in cells under a variety of stress conditions (Figures 2F and 2G), including gemcitabine treatment and starvation. Further experiments demonstrate that the number of LD-rich protrusions significantly increased in cells grown in either glucose-free or serum-free medium and increased even further when both glucose and serum were deprived (see Figure S5A). Quantitative analysis confirms a statistically significant increase of the percent of MIA PaCa-2 cells with LD-rich protrusions cultured in nutrition-deprived medium (see Figure S5B). Moreover, this phenomenon occurs not only in pancreatic cancer cells but also in cells of other types of cancer, including A549 lung cancer cells (see Figures S5C and S5D) and MDA-MB-231 breast cancer cells (see Figures S5E and S5F), suggesting LD protrusions as a general response for the cancer cells under stress. We also confirmed that the formation of LD protrusions is not dependent on cell density (see Figure S6). It is worth noting that time-lapse SRS imaging reveals LD transport from the perinuclear area of cells to the protrusions (see Figure S7 and Video S1), indicating that LD accumulation in protrusions is an active response of cancer cells under stress.
The LD trajectories are traced by color curves. Scale bar, 10 μm.
To summarize, by combining SRS imaging cytometry and high-throughput analysis of cellular features we identified both morphological and metabolic changes of cancer cells in stress conditions. Particularly, we found that the formation of lipid-rich protrusion structure is a response of cancer cells to stress environment and a potential marker for stress-resistant cancer cells. The following sections are devoted to further analysis of this metabolic marker and its potential role in cancer cells.
Multiplex SRS Imaging Cytometry Reveals a Location-Dependent Heterogeneous Composition of LDs within a Single Cell
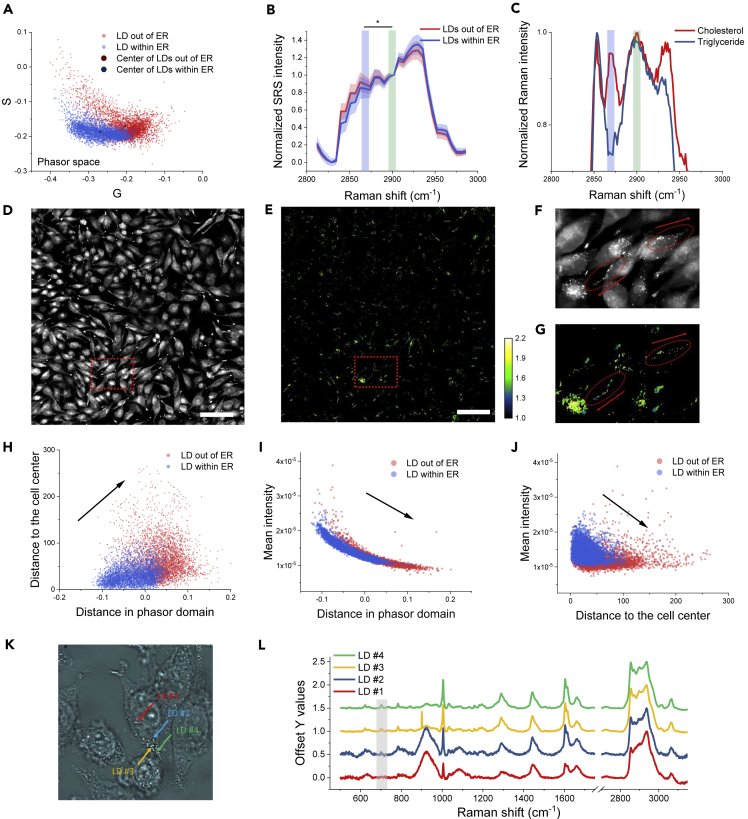
Besides providing 260 morphology or intensity-based feature analyses, an irreplaceable advantage of our SRS imaging cytometry is to provide spectrum-based feature analysis, such as to distinguish heterogeneity of chemical compositions in LDs in a single cell. Location and content of LDs are dynamically regulated through processes involving synthesis on ER and degradation by lipophagy or lipases in the cytoplasm (Walther and Farese, 2012). High-speed SRS imaging cytometry allowed us to acquire a hyperspectral image of 339 G3K cells, possessing 7,193 LDs, in 24 s. Among these LDs, we analyzed the compositional difference between LDs associated with ER (LDs within ER) and free LDs (LDs out of ER). The LDs out of ER showed different SRS spectrum compared with the LDs within ER, which can be separated in the spectral-phasor domain (Figure 3A). The spectral comparison highlighted a major difference at 2,870 cm−1 (Figure 3B). A cellular LD is mainly composed of triglycerides and cholesteryl esters. As triglyceride and cholesteryl ester have a major spectral difference at 2,870 cm−1 in the CH region (Figure 3C), we suspect that the spectral variation of LDs within ER and LDs out of ER at 2,870 cm−1 is due to the relative ratios between triglyceride and cholesteryl ester. To confirm such an LD compositional difference in a statistical manner, we performed SRS imaging of G3K cells (Figure 3D) and generated an LD composition map of triglyceride/cholesteryl ester by 2,900 cm−1 (asymmetry CH vibration) to 2,870 cm−1 (sterol ring CH vibration) SRS ratio image (Figure 3E). We found that LDs out of ER tend to have less triglyceride compositions but more cholesteryl ester contents (Figures 3F and 3G). Statistical analysis of all LDs in the image also showed a positive relationship between lower triglyceride content (higher cholesteryl ester content) and longer distance of LDs to the cell center (Figure 3H). In addition, the mean intensity of LDs is weaker with lower triglyceride content (Figure 3I) or farther away from the cell center (Figure 3J). These chemical content characteristics were also confirmed by the conventional spontaneous Raman spectroscopy measurement by analyzing the cholesterol signature peak around 704 cm−1 (Figures 3K and 3L). A quantitative analysis of spontaneous Raman signal at 704 cm−1 is shown in Figure S8.
Figure 3.
Multiplex SRS Imaging Cytometry Reveals the Heterogeneity of LDs in Single Cells
(A) A scatterplot of LDs outside the ER (red) and inside the ER (blue) boundaries in the phasor space.
(B) Averaged SRS spectra of LDs outside the ER (red) and inside the ER (blue) boundaries in the C-H region. Shaded area indicates the standard deviation of SRS spectral measurements from different LDs. SRS spectra of LDs outside the ER (red) and inside the ER are statistically different at 2,870 cm−1. ∗p < 0.05.
(C) Spontaneous Raman spectra of cholesteryl ester (red) and triglyceride (blue) in the C-H region.
(D) An SRS image of G3K cells.
(E) The molecular composition map of LDs in G3K cells by 2,900 cm−1/2,870 cm−1 SRS ratio image. Lower values indicate more cholesteryl ester contents, and higher values indicate more triglyceride contents. Scale bar, 100 μm.
(F and G) A zoom-in SRS image (F), and a zoom-in composition map (G) of the dashed rectangular region in (D) and (E), respectively. Red arrows indicate the directions of LDs away from the cell center.
(H–J) 2D scatterplots of two features from LDs outside the ER (red) and LDs inside the ER (blue) boundaries. The “distance in phasor domain” reflects “lipid compositions” of the LDs. The higher value of “distance in phasor domain” indicates more cholesteryl ester contents. The lower value of “distance in phasor domain” indicates more triglyceride contents. The features are (H) “lipid composition” versus “the distance of LD to the cell center,” (I) “lipid composition” versus “LD mean intensity,” and (J) “distance of LD to the cell center” versus “LD mean intensity.”
(K) A transmission image of G3K cells. Arrows indicate LDs analyzed by Raman spectroscopy.
(L) Spontaneous Raman spectra of selected LDs in (K). The gray region highlights the cholesteryl ester signature peak around 704 cm−1.
Collectively, the results indicate that even within a single cell, the contents of LDs are spatially different. The relatively higher triglyceride/cholesteryl ester ratio in the LDs within ER suggests the deposition of triglyceride during LD synthesis, whereas the relatively lower ratio of LDs out of ER is possibly due to the degradation of triglyceride in the cytosol, especially in the protrusions. Having shown that LDs are translocating from the perinuclear part of the cell to the protrusions (see Figure S7, Video S1), our results suggest that LDs are under active utilization to facilitate protrusion growth.
LDs in Protrusions Are Used for Local Energy Production
Next, we aim to understand how LDs in protrusions are being degraded and what is the function of these LDs. LD degradation happens through lipophagy or lipolysis (Kaushik and Cuervo, 2015, Thiam et al., 2013). In lipophagy, autophagosomes engulf the LDs and fuse with lysosomes to breakdown the LDs (Thiam et al., 2013). In lipolysis, lipases including adipose triglyceride lipase (ATGL), hormone-sensitive lipase, and monoglyceride lipase are involved in degrading triglyceride sequentially to release free fatty acids (Thiam et al., 2013).
We first examined the involvement of autophagosomes for LD degradation at the cell protrusions. To monitor autophagy, we labeled autophagosomes with monodansylcadaverine, a fluorescent dye that can be excited by the 800-nm femtosecond pulses through two-photon absorption. Then, we used SRS and two-photon excitation fluorescence (TPEF) to simultaneously image the LDs and the autophagosomes, respectively. From the images, we found the presence of both LDs and autophagosomes in proximity in the LD-rich protrusions in MIA PaCa-2 (Figure 4A), G3K (Figure 4A), and A549 (see Figure S9A) cells. Time course imaging shows that the LDs and autophagosomes are highly dynamic and closely interacting with each other in the protrusions (see Video S2), indicating that autophagy is likely involved in the degradation of protrusion LDs.
Figure 4.
LDs Are Degraded by Autophagy and Lipase and Used in Mitochondria for Energy
(A) SRS (left panel), TPEF (middle panel), and the composition (right panel) images from an MIA PaCa-2 cell (upper panel) and a G3K cell (lower panel). TPEF imaging detects the autophagosomes labeled by monodansylcadaverine. Zoom-in areas from the dashed yellow square are shown in the lower left corner of each panel. Scale bars, 10 μm.
(B) SRS (left panel), TPEF (middle panel), and the composition (right panel) images from an MIA PaCa-2 cell (upper panel) and a G3K cell (lower panel). TPEF imaging detects the mitochondria labeled by MitoTracker. Zoom-in areas from the dashed yellow square are shown in the lower left corner of each panel. Scale bar, 10 μm.
(C) Percentage of cells with LD-rich protrusions after treatment by atglistation in normal and starvation conditions (n = 5). The bars indicate means ± SEM.
(D) Percentage of cells with LD-rich protrusions after treatment by chloroquine sulfate in normal and starvation conditions (n = 5). The bars indicate means ± SEM.
(E) Percentage of cells with LD-rich protrusions after treatment by etomoxir in normal and starvation conditions (n = 5). The bars indicate means ± SEM.
(F and G) Histograms of one of the protrusion features “extent” for (F) G3K cells and (G) gemcitabine-treated G3K cells, treated with atglistatin (atglis), chloroquine sulfate (clq), and etomoxir (eto). The higher “extent” value indicates cells with less protrusion formation.
(H and I) Histograms of the “distance of LDs out of ER to the cell center” feature for (I) G3K cells and (J) gemcitabine-treated G3K cells, treated with atglistatin, chloroquine sulfate, and etomoxir. (J) Our hypothesis of LD degradation at the protrusion by autophagy and lipase, and the utilization of free fatty acids for energy production via β-oxidation in mitochondria. ∗∗p < 0.01, ∗∗∗p < 0.001.
Scale bar, 10 μm.
Excess free fatty acid released from LD degradation is toxic if not properly used. They can be used for a variety of biological processes including energy production or membrane synthesis or can be converted back to triglycerides. However, the enzymes related to membrane synthesis or triglyceride synthesis are majorly localized on the ER. Therefore, we hypothesize that the free fatty acids released at the cell protrusions would be used for energy production through mitochondria β-oxidation. We then studied the interaction of LDs in the cell protrusions with mitochondria. We labeled mitochondria with MitoTracker and imaged them by TPEF microscopy in parallel with the SRS imaging of the LDs. The dual-modality imaging reveals the co-presence of both organelles in proximity in the LD-rich protrusions in MIA PaCa-2 (Figure 4B), G3K (Figure 4B), and A549 cells (see Figure S9B). The evidence supports the local synthesis of ATP using LDs as an energy source.
The highly correlated LD accumulation/degradation and cell protrusions implies a critical role of LD function for cell protrusions. As we have shown that LDs are likely to be used as an energy source, it is reasonable to hypothesize that LD-supported local ATP production is a requisite for the formation of protrusions, and the inhibition of LD degradation would hamper the development of protrusions. To test this hypothesis, we treated cells with three inhibitors including chloroquine sulfate as an autophagy inhibitor, atglistatin as an ATGL inhibitor, and etomoxir as a β-oxidation (functions on carnitine palmitoyltransferase I, or CPT1) inhibitor in three protrusion-induced models including starvation stress model, drug-resistant model, and chemotherapy stress model.
In the starvation model, we imaged and counted the total number of cells and the number of cells with protrusions treated with the three inhibitors at various concentrations in both normal and 48-h starvation conditions. A quantitative analysis of the percentage of cells with protrusions shows that chloroquine sulfate (Figure 4C), atglistatin (Figure 4D), and etomoxir (Figure 4E) significantly reduced the formation of LD-rich protrusions in cells under starvation condition, but not under normal condition, for both MIA PaCa-2 (Figures 4C–4E) and A549 cells (see Figures S9C–S9E). These results support the fact that LD degradation by autophagosome and β-oxidation by mitochondria are critical for the formation of LD-rich protrusions under starvation when other energy sources are not present.
In the drug-resistant model, the protrusion structure is observed in G3K cells, a gemcitabine-resistant cell line (Figure 2B). We imaged G3K cells and G3K cells treated with chloroquine sulfate, atglistatin, and etomoxir. We found that each inhibitor effectively suppressed the formation of lipid-facilitated protrusions. The morphological feature of extent can be used to describe the cell protrusions. From the statistical analysis of the extent value of G3K cells, we found that although the cells show heterogeneous response to the inhibitors, the treatment tends to shift the extent distribution to larger values, indicating the decrease of the protrusions (Figure 4F). Other protrusion-related features such as higher solidity value and lower compactness value show similar trends (see Figures S10A and S10B). In addition, we observed a decrease of average distance to the cell center of the LDs through statistical analysis. For the G3K cells, 36% cells showed a distance of LD to the cell center above average. This percentage dropped to 20%, 3.2%, and 23% after treated with chloroquine sulfate, atglistatin, and etomoxir, respectively (Figure 4G). These results indicate that LD degradation and fatty acid oxidation are critical for the formation of LD-rich protrusions in the chemotherapy-drug-resistant cells.
In the chemotherapy stress model, we performed the same inhibitor treatment described previously and obtained similar results (Figures 4H, 4I, S10C, and S10D). We found that each inhibitor effectively suppressed the formation of LD-facilitated protrusions in gemcitabine-treated G3K cells. Collectively, our evidence shows that LDs are associated with energy production at the protrusions. Regulating the LD degradation and fatty acid oxidation processes impacts the formation of protrusions in cancer cells in stress conditions. An illustration of the role of LDs at the protrusions is shown in Figure 4J.
Blockage of Lipid Degradation or β-Oxidation Impairs the Survival of Stress-Resistant Cancer Cells
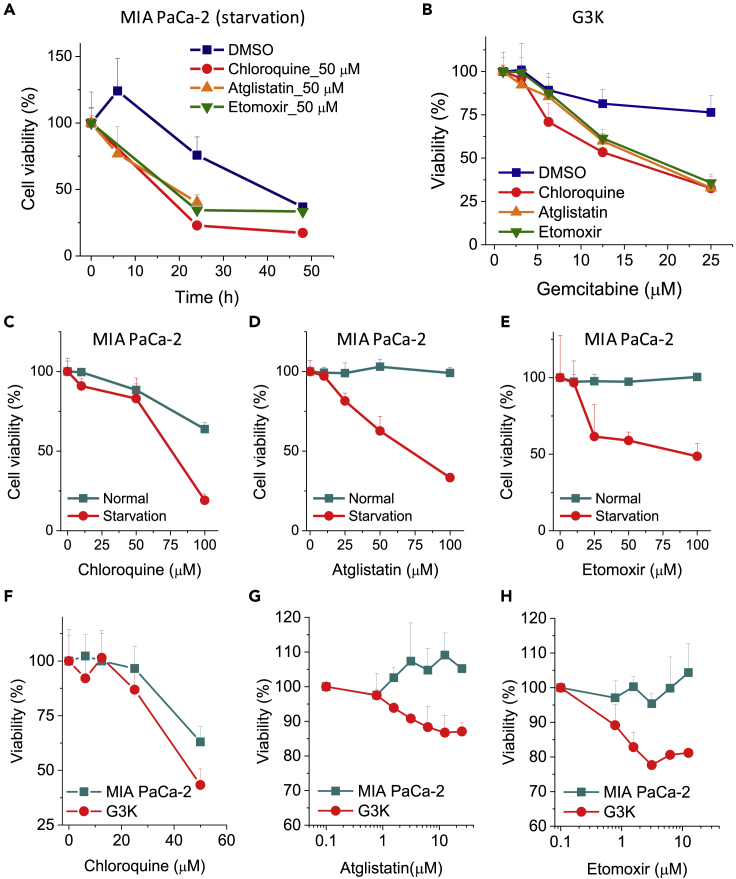
The observation that the residual cancer cells under stress have longer protrusions implies an important role of protrusion formation for the cells to survive in a stressed environment. We tested whether targeting the altered lipid metabolism can eliminate stress-resistant cancer cells. We measured the viability of MIA PaCa-2 cancer cells under starvation and G3K cells treated by the three previously mentioned inhibitors, which have been shown to reduce protrusion formation. For the starvation-resistant cancer cells, we first tested the cell survival under starvation with or without inhibitor treatment and found that 50 μM autophagy inhibitor chloroquine sulfate, 50 μM lipase inhibitor atglistatin, or 50 μM β-oxidation inhibitor etomoxir induce cell death much faster than vehicle treatments (Figure 5A). Then we examined whether the starved cancer cells are more susceptible than the non-starved ones in response to the inhibitor treatments. We found that the starved cells are more vulnerable to treatment of chloroquine sulfate (Figure 5C), atglistatin (Figure 5D), or etomoxir for 24 h (Figure 5E). Similarly, the treatment of inhibitors substantially diminished the survival of A549 cells under starvation condition (see Figure S11A), but not under normal conditions (see Figures S11B–S11D).
Figure 5.
Blockage of Lipid Metabolism Suppressed Cell Survival under Stress
(A) Time-dependent MIA PaCa-2 cell viability with and without chloroquine sulfate treatment, atglistatin treatment, and etomoxir treatment under starvation condition. The bars indicate means ± SEM.
(B) Gemcitabine concentration-dependent G3K cell viability with and without chloroquine sulfate treatment, atglistatin treatment, and etomoxir treatment for 72 h. The bars indicate means ± SEM.
(C–E) Concentration-dependent MIA PaCa-2 cell viability for cells treated by (C) chloroquine sulfate, (D) atglistatin, and (E) etomoxir under normal and starved conditions for 24 h. The bars indicate means ± SEM.
(F–H) Concentration-dependent viability for MIA PaCa-2 cells and G3K cells, treated by (F) chloroquine sulfate, (G) atglistatin, and (H) etomoxir, for 72 h, n = 3. The bars indicate means ± SEM.
We also tested whether drug-resistant cancer cells are more susceptible to synergistic treatment. The results show that synergistic treatment of gemcitabine drug with 50 μM autophagy inhibitor chloroquine sulfate, 50 μM lipase inhibitor atglistatin, or 50 μM β-oxidation inhibitor etomoxir induces a much faster cell death compared with vehicle treatments (Figure 5B). In addition, we found that the gemcitabine-resistant cancer cells are more vulnerable to chloroquine sulfate at 72 h (Figure 5F), atglistatin at 72 h (Figure 5G), and etomoxir at 72 h (Figure 5H).
These results collectively demonstrate that stress-resistant cancer cells rely on LD degradation and fatty acid oxidation processes for survival. As the inhibitors also reduce the formation of protrusions (Figure 4), our results indicate the important role of LD-rich protrusions in the survival mechanism of stress-resistant cancer cells.
LD-Rich Protrusions Enhance Glucose Uptake
The formation and growth of LD-rich protrusions can significantly alter the shape and size of the cells. As mentioned earlier, the elongated protrusions rendered the cells more polar shaped (Figures 2B–2D and S5). This phenotypic change is likely to extend the territory of the cell and potentially enhance the uptake of nutrition from the medium, consequently promoting cell survival. To test this supposition, we applied starvation model and measured glucose uptake in the starved cells for 24 h to stimulate the growth of LD-rich protrusions. Then, we fed the cells with a fluorescent glucose analog, 2-(n-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose (2-NBDG) for 1 h and imaged 2-NBDG uptake by TPEF microscopy in the cells. In both MIA PaCa2 (Figures 6A and 6B) and A549 cells (see Figure S12), starved cells with LD-rich protrusions showed much higher 2-NBDG uptake than cells without LD-rich protrusions or non-starved cells. Furthermore, we performed immunostaining and imaging of glucose transporter GLUT1 in normal and starved MIA PaCa2 cells (Figure 6C). Compared with normal cells, starved cells have a significantly higher expression level of GLUT1 (Figure 6D). Moreover, GLUT1 is highly expressed at the membranes of the protrusions in starved cells. In contrast, GLUT1 expression mostly localizes to cell body area in normal cells (Figure 6C). We also tested the supposition in the chemotherapy drug model and measured the glucose uptake of cells treated with gemcitabine to stimulate the growth of LD-rich protrusions (Figure 6E). In both MIA PaCa2 and G3K cells, gemcitabine-treated cells showed much higher 2-NBDG uptake than cells without gemcitabine treatment (Figure 6F). In addition, G3K cells, which are observed with more LD-rich protrusions, obtain much higher 2-NBDG uptake than MIA PaCa2 cells (Figure 6E). The evidence suggests that lipid-rich protrusions as a metabolic reprogramming signature may play an important role for cells to acquire glucose more efficiently. Although our preliminary data showed that the accumulation of LDs in protrusions is associated with the AMPK signaling (see Figure S13), the molecular mechanisms need to be further studied.
Figure 6.
Protrusions Increase Cellular Uptake of Glucose
(A) SRS (left panels) and TPEF (right panels) images from MIA PaCa-2 cells incubated with 2-NBDG for 1 h. The TPEF signal was from the 2-NBDG accumulated in the cells. The upper panels are the control group, whereas the lower panels are collected under the starvation condition. Scale bar, 10 μm.
(B) Quantitation of the 2-NBDG intensity for MIA PaCa-2 cells in normal (n = 5) and starvation conditions (with [n = 6] and without protrusions [n = 7]). The bars indicate means ± SEM.
(C) Representative TPEF images of MIA PaCa-2 cells immunostained with an antibody against GLUT1. Scale bar, 10 μm.
(D) Quantitation of fluorescent intensity from labeled GLUT1 in (C) (n = 6). The bars indicate means ± SEM.
(E) Transmission phase contrast (left panels) and TPEF (right panels) images of MIA PaCa-2 and G3K cells incubated with 2-NBDG for 2 h. Scale bar, 10 μm.
(F) Quantitation of the 2-NBDG intensity for MIA PaCa-2 and G3K cells without (MIA PaCa-2: n = 105; G3K: n = 67) and with gemcitabine treatment (MIA PaCa-2: n = 103; G3K: n = 68). The bars indicate means ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
Cancer cells are commonly exposed to stress environments. For example, due to the insufficient blood distribution, cancer cells at the center of a tumor often suffer from low oxygen and nutrition supply; chemotherapeutic drugs create significant challenges to cancer cells. However, a small portion of cancer cells manages to survive in the stress environments by reprogramming their metabolism, which may lead to incomplete tumor remission and eventually tumor relapse. Therefore, understanding the metabolic strategies utilized by cancer cells under stress conditions is critical to gain insights for developing more effective and targeted cancer therapy.
To address this challenge, a tool that can perform high-speed metabolic imaging and high-throughput analysis of cell metabolism is essential. We first achieve technical innovation by developing an in situ chemical imaging cytometer enabled by high-speed multiplex SRS imaging. This approach allows us to measure a large number of cells with immense information in cellular or subcellular contents. After separating different subcellular compartments, including ER, nucleus, LDs, and cytoplasm at high resolution, we were able to extract 260 morphological or metabolic features to understand and quantify cellular metabolic changes. Our method addresses challenges caused by cellular heterogeneity, including resolving cellular heterogeneity in spatial distribution and composition (for example, triglyceride and cholesterol contents), in a manner that lies beyond the scope of conventional fluorescence-based techniques. Taking advantage of our imaging cytometry, we discovered LD-enriched protrusions as a metabolic marker for cancer cells under stresses.
We note that the importance of LD in cancer cell survival has been well studied (Baenke et al., 2013, Itkonen et al., 2017, Petan et al., 2018, Shyu et al., 2018). In a recent review (Baenke et al., 2013), Baenke et al. summarized that the reprogramed lipid metabolism is involved in many aspects of cancer biology, including aberrant lipid synthesis, regulation of membrane structure, and participation of cellular signaling. In another review (Petan et al., 2018), Petan et al. proposed sequestration of toxic lipids, such as fatty acids, cholesterol and ceramides, thereby preventing lipotoxic cell damage. However, without spatial and spectral resolution, how cancer cells rewire their lipid metabolism reacting to stress is not fully understood. Here, we discovered that LDs actively transport from the cell body to protrusions. We further show that the LDs in protrusions serve as an alternative energy source to promote cell protruding out to enhance nutritional uptake. Such an adaptation strategy assists cancer cells to survive under the stress.
In our study, we showed LDs in protrusions are majorly used to produce energy in mitochondria to support the protrusion growth. However, serving as an energy source may not be the only possible function of lipids. We also tested the possible fate of lipids in LDs for membrane synthesis by using BODIPY-C12, a metabolizable fluorescent fatty acid analog and precursor for phospholipids and neutral lipids (Kolahi et al., 2016). In a previous report, BODIPY-C12 was shown to transport from LDs to organelles under starvation in fibroblast cells (Rambold et al., 2015). Here, using a similar approach, our results do not show the presence of BODIPY-C12 on plasma membrane after 12- or 24-h starvation (see Figure S14), suggesting that the fatty acids released from protrusion LDs are not used for membrane synthesis. On the other hand, inhibition of lipid β-oxidation pathway significantly suppresses protrusion formation, supporting lipids in protrusion LDs as an energy source. Transportation of LDs from cell body to protrusions is meaningful and necessary in the sense that co-localization of LDs, autophagosome/lipase, and mitochondria can maximize the efficiency of ATP production to maintain a favorable ATP concentration to support the fast expansion of the protrusions. However, the same strategy may not be applicable for efficient membrane construction. Synthesis of phospholipids is an energy-consuming process, which occurs in the ER. As a complex network structure, ER is primarily located in the cell body. Synthesis of phospholipids in the ER and their transport to the protrusion over long distance is a low-efficiency and energy-unfavorable process. In contrast, a more efficient way for membrane growth under starvation is probably through degradation and fusion of organelle membranes, which is consistent with the previous report that ER and other organelles are degraded under starvation condition (Nakatogawa, 2016). Future studies will be performed to fully understand the process of membrane construction under stress condition.
Although it is known that cancer cells under starvation or chemotherapy treatment become more vulnerable to other drugs, the inhibitors of autophagy/lipase/β-oxidation not only suppressed cell viability but also selectively reduced protrusion formation under stress, but not under normal condition. This correlation suggests these inhibitors act through a specific protrusion-associated mechanism, but not due to general chemical cytotoxicity. It is likely that the inhibitors will distribute through the entire cell instead of just localizing at the protrusions. However, after prolonged starvation (24 h or longer) or chemotherapy treatment, the majority of LDs have been transported from the cell body to the protrusions. It is, therefore, reasonable to consider that the inhibitors play their roles primarily at the protrusions.
Our current research explored the behaviors of cultured cancer cell-lines on a two-dimensional surface. The same methodology could be applied to three-dimensional cell culture or tissues for in situ analysis of metabolism at the single-cell level. A more delicate segmentation algorithm might be needed to segregate individual cells and distinguish different cell types in a complex tissue environment. In addition to starvation stress and chemotherapy stress, other metabolic stresses, such as hypoxia or oxidative stress, might also impose additional traits on the metabolism of cancer cells. For example, hypoxia condition may impair the capability of cancer cells to consume lipids through β-oxidation in mitochondria. In this case, alternative energy sources might be employed through different metabolic pathways. Future studies will be conducted to depict these metabolic adaptations in specific niches.
Our multiplex SRS imaging cytometry and pipelines not only apply for analyzing cultured cells as reported but also apply for in situ chemical mapping of larger biological objects, i.e., embryos, pathological tissue samples, and even whole organisms such as Drosophila, zebrafish, etc. Enabled by the hybrid scanning capacity, our multiplex SRS imaging cytometry can map the distributions of chemicals in individual cells throughout a bulk specimen, in addition to providing morphological information. With such information, we can examine the histopathological status of tissues from patients with diseases or discover potential biomarkers for disease identification. Moreover, assisted by deep learning we can potentially identify different cell types in complex tissue environments or in circulating systems.
Despite the power that multiplex SRS imaging cytometry has demonstrated in metabolic discovery, the capacity of SRS imaging cytometry can be further improved in the following aspects. One limitation of SRS imaging cytometry is sensitivity, which could be improved with the following methods. (1) Using a nonlinear spectral compression scheme (Liu et al., 2015), imaging sensitivity can be enhanced by improving the pulse shaping efficiency without sacrificing the spectral resolution. (2) Using pre-resonance SRS, electronic-resonant SRS, or plasmonic-enhanced method, the Raman cross-section of a molecule could be further enhanced for detection of molecules at sub-micromolar concentration (Shi et al., 2018, Wei et al., 2017, Zhang et al., 2014).
Segmentation of intracellular compartments, organelles, or even molecules could be improved with better chemical selectively, which could be another perspective for future development. (1) Using a laser system with a broader spectral width, the Raman spectral window can be widened (Figueroa et al., 2018, Hiramatsu et al., 2019). (2) Combining with Raman tags that have much higher Raman scattering cross-section than most inherent biomolecules, the multiplex SRS imaging cytometry would be able to measure more biomolecules such as amino acids, DNA/RNA molecules, glucose, small-molecule drugs, etc. (Zhao et al., 2017). (3) Shifting the current near-infrared laser excitation to the visible range is expected to improve the spatial resolution down to 130 nm to visualize fine structure such as dendritic spine (Bi et al., 2018). (4) It can be further extended to high-parameter cytometry by combining multiplex SRS cytometry and supermultiplex (24-color) Raman probes to overcome the color barrier limited by intrinsically broad fluorescence spectra (Wei et al., 2017).
Limitations of the Study
Owing to the focus spot of our system defined by the diffraction limit of the laser wavelength, SRS signal from LDs smaller than point spread function may be contributed from the surrounding protein. In future, higher spatial resolution of SRS imaging with visible wavelength excitation will improve the spatial resolution. Furthermore, to fully understand the mechanism behind the metabolic adaptations of protrusion formation for cancer cells under stress, the signaling pathway in the molecular level needs to be further studied.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Chien-Sheng Liao and Gregory Eakins for the help and the design of TAMP array. This work was funded by National Institutes of Health (NIH) grants GM118471 and CA223581 to J.-X.C.
Author Contributions
K.-C.H., J.L., and C.Z. designed the experiment. K.-C.H., J.L., and C.Z. performed SRS imaging. J.L. performed cell viability experiment. K.-C.H., J.L., and C.Z. analyzed the data. J.L. and Y.T. prepared the cell lines and the treatments. K.-C.H., J.L., and C.Z. wrote the manuscript. J.-X.C. supervised the project. All authors discussed the results and contributed to the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100953.
Data and Code Availability
The custom codes for image analysis and sort-decision making are available from the corresponding author upon request.
Supplemental Information
References
- Altschuler S.J., Wu L.F. Cellular heterogeneity: do differences make a difference? Cell. 2010;141:559–563. doi: 10.1016/j.cell.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenke F., Peck B., Miess H., Schulze A. Hooked on fat: the role of lipid synthesis in cancer metabolism and tumour development. Dis. Models Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y., Yang C., Chen Y., Yan S., Yang G., Wu Y., Zhang G., Wang P. Near-resonance enhanced label-free stimulated Raman scattering microscopy with spatial resolution near 130 nm. Light Sci. Appl. 2018;7:81. doi: 10.1038/s41377-018-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi T., Hennig H., Summers H.D., Theis F.J., Cerveira J., Patterson J.O., Davies D., Filby A., Carpenter A.E., Rees P. Label-free cell cycle analysis for high-throughput imaging flow cytometry. Nat. Commun. 2016;7:10256. doi: 10.1038/ncomms10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles H., Camp J., Yegnanarayanan S., Eftekhar A.A., Adibi A. Label-free flow cytometry using multiplex coherent anti-Stokes Raman scattering (MCARS) for the analysis of biological specimens. Opt. Lett. 2011;36:2309–2311. doi: 10.1364/OL.36.002309. [DOI] [PubMed] [Google Scholar]
- Chen A.J., Li J., Jannasch A., Mutlu A.S., Wang M.C., Cheng J.-X. Fingerprint stimulated Raman scattering imaging reveals retinoid coupling lipid metabolism and survival. ChemPhysChem. 2018;19:2500–2506. doi: 10.1002/cphc.201800545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Paley D.W., Wei L., Weisman A.L., Friesner R.A., Nuckolls C., Min W. Multicolor live-cell chemical imaging by isotopically edited alkyne vibrational palette. J. Am. Chem. Soc. 2014;136:8027–8033. doi: 10.1021/ja502706q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.-X., Xie X.S. CRC Press; 2016. Coherent Raman Scattering Microscopy. [Google Scholar]
- Derle A., Santis M.C.D., Gozzelino L., Ratto E., Martini M. The role of metabolic adaptation to nutrient stress in pancreatic cancer. Cell Stress. 2018;2:332–339. doi: 10.15698/cst2018.12.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.L., Potma E.O., Puoris’haag M., Côté D., Lin C.P., Xie X.S. Chemical imaging of tissue in vivo with video-rate coherent anti-Stokes Raman scattering microscopy. Proc. Natl. Acad. Sci. U S A. 2005;102:16807–16812. doi: 10.1073/pnas.0508282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa B., Fu W., Nguyen T., Shin K., Manifold B., Wise F., Fu D. Broadband hyperspectral stimulated Raman scattering microscopy with a parabolic fiber amplifier source. Biomed. Opt. Express. 2018;9:6116–6131. doi: 10.1364/BOE.9.006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D., Xie X.S. Reliable cell segmentation based on spectral phasor analysis of hyperspectral stimulated Raman scattering imaging data. Anal. Chem. 2014;86:4115–4119. doi: 10.1021/ac500014b. [DOI] [PubMed] [Google Scholar]
- Fu D., Yu Y., Folick A., Currie E., Farese R.V., Tsai T.-H., Xie X.S., Wang M.C. In vivo metabolic fingerprinting of neutral lipids with hyperspectral stimulated Raman scattering microscopy. J. Am. Chem. Soc. 2014;136:8820–8828. doi: 10.1021/ja504199s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A.A., Pfisterer S.G., Riezman H., Ikonen E., Potma E.O. D38-cholesterol as a Raman active probe for imaging intracellular cholesterol storage. J. Biomed Opt. 2015;21:061003. doi: 10.1117/1.JBO.21.6.061003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler M.M., Hu F., Feng H., Linkermann A., Min W., Stockwell B.R. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem. Biol. 2018;13:1013–1020. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso C., Jansen G., Giovannetti E. Drug resistance in pancreatic cancer: impact of altered energy metabolism. Crit. Rev. Oncol. Hematol. 2017;114:139–152. doi: 10.1016/j.critrevonc.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Hamanaka R.B., Chandel N.S. Targeting glucose metabolism for cancer therapy. J. Exp. Med. 2012;209:211–215. doi: 10.1084/jem.20120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heiden M.G.V., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen M., Miller B., Newmark J., Al-Kofahi Y., Holden E. Chapter 7 - laser scanning cytometry and its applications: a pioneering technology in the field of quantitative imaging cytometry. In: Darzynkiewicz Z., Holden E., Orfao A., Telford W., Wlodkowic D., editors. Methods in Cell Biology. Academic Press; 2011. pp. 159–205. [DOI] [PubMed] [Google Scholar]
- Hiramatsu K., Ideguchi T., Yonamine Y., Lee S., Luo Y., Hashimoto K., Ito T., Hase M., Park J.-W., Kasai Y. High-throughput label-free molecular fingerprinting flow cytometry. Sci. Adv. 2019;5:eaau0241. doi: 10.1126/sciadv.aau0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Chen Z., Zhang L., Shen Y., Wei L., Min W. Vibrational imaging of glucose uptake activity in live cells and tissues by stimulated Raman scattering. Angew. Chem. Int. Ed. 2015;54:9821–9825. doi: 10.1002/anie.201502543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett H.R., Bonner W.A., Barrett J., Herzenberg L.A. Cell sorting: automated separation of mammalian cells as a function of intracellular fluorescence. Science. 1969;166:747–749. doi: 10.1126/science.166.3906.747. [DOI] [PubMed] [Google Scholar]
- Itkonen H.M., Brown M., Urbanucci A., Tredwell G., Lau C.H., Barfeld S., Hart C., Guldvik I.J., Takhar M., Heemers H.V. Lipid degradation promotes prostate cancer cell survival. Oncotarget. 2017;8:38264–38275. doi: 10.18632/oncotarget.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen E.C. Use of fluorescent probes: their effect on cell biology and limitations. Anat. Rec. 2012;295:2031–2036. doi: 10.1002/ar.22602. [DOI] [PubMed] [Google Scholar]
- Kaushik S., Cuervo A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolahi K., Louey S., Varlamov O., Thornburg K. Real-time tracking of BODIPY-C12 long-chain fatty acid in human term placenta reveals unique lipid dynamics in cytotrophoblast cells. PLoS One. 2016;11:e0153522. doi: 10.1371/journal.pone.0153522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.J., Zhang W., Zhang D., Yang Y., Liu B., Barker E.L., Buhman K.K., Slipchenko L.V., Dai M., Cheng J.-X. Assessing cholesterol storage in live cells and C. elegans by stimulated Raman scattering imaging of phenyl-diyne cholesterol. Sci. Rep. 2015;5:7930. doi: 10.1038/srep07930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Cheng J.-X. Direct visualization of de novo lipogenesis in single living cells. Sci. Rep. 2014;4:6807. doi: 10.1038/srep06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.-S., Slipchenko M.N., Wang P., Li J., Lee S.-Y., Oglesbee R.A., Cheng J.-X. Microsecond scale vibrational spectroscopic imaging by multiplex stimulated Raman scattering microscopy. Light Sci. Appl. 2015;4:e265. doi: 10.1038/lsa.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Condello S., Thomes-Pepin J., Ma X., Xia Y., Hurley T.D., Matei D., Cheng J.X. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell. 2017;20:303–314.e5. doi: 10.1016/j.stem.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C.-S., Choi J.H., Zhang D., Chan S.H., Cheng J.-X. Denoising stimulated Raman spectroscopic images by total variation minimization. J. Phys. Chem. C. 2015;119:19397–19403. doi: 10.1021/acs.jpcc.5b06980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Wang P., Kim J.I., Zhang D., Xia Y., Chapple C., Cheng J.-X. Vibrational fingerprint mapping reveals spatial distribution of functional groups of lignin in plant cell wall. Anal. Chem. 2015;87:9436–9442. doi: 10.1021/acs.analchem.5b02434. [DOI] [PubMed] [Google Scholar]
- Marusyk A., Polyak K. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath K.E., Bushnell T.P., Palis J. Multispectral imaging of hematopoietic cells: where flow meets morphology. J. Immunol. Methods. 2008;336:91–97. doi: 10.1016/j.jim.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham C.E., Morrison S.J. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W., Freudiger C.W., Lu S., Xie X.S. Coherent nonlinear optical imaging: beyond fluorescence microscopy. Annu. Rev. Phys. Chem. 2011;62:507–530. doi: 10.1146/annurev.physchem.012809.103512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatogawa H. Eating the ER and the nucleus for survival under starvation conditions. Mol. Cell Oncol. 2016;3:e1073416. doi: 10.1080/23723556.2015.1073416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta N., Sugimura T., Isozaki A., Mikami H., Hiraki K., Sakuma S., Iino T., Arai F., Endo T., Fujiwaki Y. Intelligent image-activated cell sorting. Cell. 2018;175:266–276.e13. doi: 10.1016/j.cell.2018.08.028. [DOI] [PubMed] [Google Scholar]
- Okada M., Smith N.I., Palonpon A.F., Endo H., Kawata S., Sodeoka M., Fujita K. Label-free Raman observation of cytochrome c dynamics during apoptosis. Proc. Natl. Acad. Sci. U S A. 2012;109:28–32. doi: 10.1073/pnas.1107524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki Y., Umemura W., Otsuka Y., Satoh S., Hashimoto H., Sumimura K., Nishizawa N., Fukui K., Itoh K. High-speed molecular spectral imaging of tissue with stimulated Raman scattering. Nat. Photon. 2012;6:845–851. [Google Scholar]
- Pavillon N., Hobro A.J., Akira S., Smith N.I. Noninvasive detection of macrophage activation with single-cell resolution through machine learning. Proc. Natl. Acad. Sci. U S A. 2018;115:E2676–E2685. doi: 10.1073/pnas.1711872115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H., Martin D.S., Xu R.-H., Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- Petan T., Jarc E., Jusović M. Lipid droplets in cancer: guardians of fat in a stressful world. Molecules. 2018;23:1941. doi: 10.3390/molecules23081941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambold A.S., Cohen S., Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev. Cell. 2015;32:678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar B.G., Freudiger C.W., Reichman J., Stanley C.M., Holtom G.R., Xie X.S. Video-Rate molecular imaging in vivo with stimulated Raman scattering. Science. 2010;330:1368–1370. doi: 10.1126/science.1197236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Xiong H., Shen Y., Long R., Wei L., Min W. Electronic resonant stimulated Raman scattering micro-spectroscopy. J. Phys. Chem. B. 2018;122:9218–9224. doi: 10.1021/acs.jpcb.8b07037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu P., Wong X.F.A., Crasta K., Thibault G. Dropping in on lipid droplets: insights into cellular stress and cancer. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanko L.M., Honigmann A., Midtiby H.S., Lund F.W., Brewer J.R., Dekaris V., Bittman R., Eggeling C., Wüstner D. Membrane orientation and lateral diffusion of BODIPY-cholesterol as a function of probe structure. Biophys. J. 2013;105:2082–2092. doi: 10.1016/j.bpj.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Kobayashi K., Wakisaka Y., Deng D., Tanaka S., Huang C.-J., Lei C., Sun C.-W., Liu H., Fujiwaki Y. Label-free chemical imaging flow cytometry by high-speed multicolor stimulated Raman scattering. Proc. Natl. Acad. Sci. U S A. 2019;116:15842–15848. doi: 10.1073/pnas.1902322116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiam A.R., Farese R.V., Jr., Walther T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013;14:775–786. doi: 10.1038/nrm3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping W.J., Lee M., Serrels A., Brunton V.G., Hulme A.N. Stimulated Raman scattering microscopy: an emerging tool for drug discovery. Chem. Soc. Rev. 2016;45:2075–2089. doi: 10.1039/c5cs00693g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T.C., Farese R.V. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Li J., Wang P., Hu C.-R., Zhang D., Sturek M., Cheng J.-X. Label-free quantitative imaging of cholesterol in intact tissues by hyperspectral stimulated Raman scattering microscopy. Angew. Chem. Int. Ed. 2013;52:13042–13046. doi: 10.1002/anie.201306234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Wei L., Yu Y., Shen Y., Wang M.C., Min W. Vibrational imaging of newly synthesized proteins in live cells by stimulated Raman scattering microscopy. Proc. Natl. Acad. Sci. U S A. 2013;110:11226–11231. doi: 10.1073/pnas.1303768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Hu F., Shen Y., Chen Z., Yu Y., Lin C.-C., Wang M.C., Min W. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Methods. 2014;11:410–412. doi: 10.1038/nmeth.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Chen Z., Shi L., Long R., Anzalone A.V., Zhang L., Hu F., Yuste R., Cornish V.W., Min W. Super-multiplex vibrational imaging. Nature. 2017;544:465–470. doi: 10.1038/nature22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Saito M., Matsuoka H., Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat. Protoc. 2007;2:753–762. doi: 10.1038/nprot.2007.76. [DOI] [PubMed] [Google Scholar]
- Yu Y., Ramachandran P.V., Wang M.C. Shedding new light on lipid functions with CARS and SRS microscopy. Biochim. Biophys. Acta. 2014;1841:1120–1129. doi: 10.1016/j.bbalip.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S., Li J., Lee S.-Y., Lee H.J., Shao T., Song B., Cheng L., Masterson T.A., Liu X., Ratliff T.L. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti-Domingues L.C., Tynan C.J., Rolfe D.J., Clarke D.T., Martin-Fernandez M. Hydrophobic fluorescent probes introduce artifacts into single molecule tracking experiments due to non-specific binding. PLoS One. 2013;8:e74200. doi: 10.1371/journal.pone.0074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Huang K.-C., Rajwa B., Li J., Yang S., Lin H., Liao C., Eakins G., Kuang S., Patsekin V. Stimulated Raman scattering flow cytometry for label-free single-particle analysis. Optica. 2017;4:103–109. doi: 10.1364/optica.4.000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Shi L., Shen Y., Miao Y., Wei M., Qian N., Liu Y., Min W. Spectral tracing of deuterium for imaging glucose metabolism. Nat. Biomed. Eng. 2019;3:402–413. doi: 10.1038/s41551-019-0393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Ren L., Zhang X., Shan Y., Wang Y., Ji Y., Yin H., Huang W.E., Xu J., Ma B. Raman-activated cell sorting based on dielectrophoretic single-cell trap and release. Anal. Chem. 2015;87:2282–2289. doi: 10.1021/ac503974e. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang P., Gou H., Mou C., Huang W.E., Yang M., Xu J., Ma B. Towards high-throughput microfluidic Raman-activated cell sorting. Analyst. 2015;140:6163–6174. doi: 10.1039/c5an01074h. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhen Y.-R., Neumann O., Day J.K., Nordlander P., Halas N.J. Coherent anti-Stokes Raman scattering with single-molecule sensitivity using a plasmonic Fano resonance. Nat. Commun. 2014;5:4424. doi: 10.1038/ncomms5424. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Butler E.B., Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Shen Y., Hu F., Min W. Applications of vibrational tags in biological imaging by Raman microscopy. Analyst. 2017;142:4018–4029. doi: 10.1039/c7an01001j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The LD trajectories are traced by color curves. Scale bar, 10 μm.
Scale bar, 10 μm.
Data Availability Statement
The custom codes for image analysis and sort-decision making are available from the corresponding author upon request.