Abstract
Salt affected soil inhibits plant growth, development and productivity, especially in case of rice crop. Ion homeostasis is a candidate defense mechanism in the salt tolerant plants or halophyte species, where the salt toxic ions are stored in the vacuoles. The aim of this investigation was to determine the OsNHX1 (a vacuolar Na+/H+ exchanger) and OsHKT2;1 (Na+/K+ transporter) regulation by salt stress (200 mM NaCl) in two rice cultivars, i.e. Pokkali (salt tolerant) and IR29 (salt susceptible), the accumulation of Na+ in the root and leaf tissues using CoroNa Green® staining dye and the associated physiological changes in test plants. Na+ content was largely increased in the root tissues of rice seedlings cv. Pokkali (15 min after salt stress) due to the higher expression of OsHKT2;1 gene (by 2.5 folds) in the root tissues. The expression of OsNHX1 gene in the leaf tissues was evidently increased in salt stressed seedlings of Pokkali, whereas it was unchanged in salt stressed seedlings of IR29. Na+ in the root tissues of both Pokkali and IR29 was enriched, when subjected to 200 mM NaCl for 12 h and easily detected in the leaf tissues of salt stressed plants exposed for 24 h, especially in cv. Pokkali. Moreover, the overexpression of OsNHX1 gene regulated the translocation of Na+ from root to leaf tissues, and compartmentation of Na+ into vacuoles, thereby maintaining the photosynthetic abilities in cv. Pokkali. Overall growth performance, maximum quantum yield (Fv/Fm), photon yield of PSII (ΦPSII) and net photosynthetic rate (Pn) was improved in salt stressed leaves of Pokkali than those in salt stressed IR29.
Electronic supplementary material
The online version of this article (10.1007/s12298-020-00769-3) contains supplementary material, which is available to authorized users.
Keywords: Gene expression, Growth performance, Net photosynthetic rate, OsNHX1, OsHKT2;1, Photosynthetic ability
Introduction
Global climate change may induce the expansion of soil salinization throughout the world, resulting in low quality of irrigation practices (salt-contaminated fresh water), rise in sea level and expansion of the arid and semiarid lands (Jesus et al. 2015). Saline soil is currently spread over a large area in 100 countries (~ 10 billion ha of salt affected soil), and it is estimated to increase at a rate of 10–16% per annum (Qadir and Oster 2002). Irrigated land is very sensitive and can get affected by the salt concentrations present in irrigated water, resulting in crop yield reduction (Pitman and Läuchli 2004; Rengasamy 2010; Krishnamurthy et al. 2017). Soil salinity causes an economic loss of 11 billion USD every year (Hussain et al. 2017). Rice is an important carbohydrate source cultivated in 156 million ha land in the world (with 650 million tons of grain yield), providing the world population > 50% of staple food as food security, especially in Asia (Ghosh et al. 2016; Hussain et al. 2018). The major areas of rice cultivation (> 90%) are located in Asia, including China, India, Pakistan, Thailand, Indonesia, Myanmar and Japan, which are facing concerns pertaining to soil salinization, especially in the irrigated zones (Wang et al. 2003). Rice has been identified as a salt sensitive glycophytic species, especially during the germination, seedling and reproductive stages (Grattan et al. 2002). Previously, studies have screened the salt tolerant genotypes from rice germplasm, including weedy rice (Zhang et al. 2018) and widely cultivated genotypes (Rahman et al. 2016; Bertazzini et al. 2018); and salt tolerant cultivars, Pokkali (Yasmin et al. 2015), CSR10 (Singh et al. 2018), Panvel-3 (Khare et al. 2015) are generally used as positive check.
In general, a plethora of defense mechanisms, including ion homeostasis, osmoregulation, antioxidant regulation and hormonal regulation are activated in plants in response to ionic and osmotic stresses derived from saline soil (Roy et al. 2014; Han et al. 2015; Parihar et al. 2015). Na+ homeostasis is a very important salt defense mechanism for improving salt tolerance by controlling Na+ uptake in the root tissues (Kumar and Mosa 2015), Na+ loading in the vascular system (Shabala et al. 2010; Zhu et al. 2017), and Na+ translocation to leaves (Ochiai and Matoh 2002; Plett and Møller 2010; Wu 2018). Na+ influx from the soil solution into the root cells via antiporter channels (i.e. HKTs) is the first trigger for perception, signaling and regulation of salt stress in higher plants grown under saline soil (Julkowska and Testerink 2015). In rice, HKT transporters of both class I (OsHKT1;x) and class II (OsHKT2;1, OsHKT2;2, OsHKT2;2/1, OsHKT2;3 and OsHKT2;4) have been identified (Almeida et al. 2013). It has been suggested that Na+ and K+ transport using OsHKT2;1 (a class II transporter) is largely dependent on the external concentration of these ions, as confirmed by earlier studies in yeast cells and Xenopus oocytes (Jabnoune et al. 2009). Na+ toxicity is reduced by vacuole secreting system (i.e. NHXs), together with the H+ generating from activities of V-PPase (vacuolar H+- pyrophosphatse) and V-H+-ATPase on the vacuolar membrane, especially in the old leaves (Maathuis 2014; Shabala et al. 2015). Recently, we have reported that carbohydrate-related gene(s) function as osmoregulators in Pokkali cultivar of salt tolerant rice under salt stress conditions (Theerawitaya et al. 2015). However, studies pertaining to the early regulation (within 6 h NaCl treatment) of Na+ in different rice genotypes are still lacking. In the present study, we hypothesized that the ion homeostasis-related genes (OsNHXs, OsHKT2;1 and OsAVP1), Na+ enrichment in the root and leaf tissues, and physiological and morphological changes are activated as counter response mechanisms under salt stress in Pokkali (salt tolerant) and IR29 (salt sensitive) rice genotypes.
Materials and methods
Plant materials and salt treatment
Rice cvs. Pokkali (salt-tolerant) and IR29 (salt-sensitive) obtained from Rice Germplasm Bank, Rice Department, Ministry of Agriculture and Cooperative, Thailand, were hand-dehusked, sterilized once in 5% (v/v) Clorox® [0.05% (w/v) sodium hypochlorite, active ingredient] for 12 h, once in 25% (v/v) Clorox® [0.25% (w/v) ai] for 30 min, and then rinsed thrice with sterile distilled water. Surface-sterilized seeds were cultivated on solidified MS medium (Murashige and Skoog 1962) using 0.25% Phytagel® supplemented with 3% (w/v) sucrose in a glass vessel. The pH of MS medium was adjusted to 5.7 using 1 N HCl before autoclaving. Culture vessel containing rice seedlings were incubated under 60 ± 5% relative humidity (RH), 25 ± 2 °C ambient temperature, and 60 ± 5 μmol m−2 s−1 photosynthetic photon flux (PPF) intensity provided by fluorescent lamps with a 16 h d−1 photoperiod. Fourteen-day-old germinated seedlings were directly transferred to new MS-liquid sugar-free medium and vermiculite was used as supporting material for 2 weeks. Air ventilation rate inside and outside culture vessel was adjusted to 2.32 μmol CO2 h−1 by punching a hole in the plastic cap (ø = 1 cm) and covering the hole with gas-permeable microporous polypropylene film (0.22 μm pore size, Nihon Millipore Ltd., Japan). After acclimatizing the seedlings for a week, sodium chloride (NaCl) was added to the culture medium at 0 (control) or 200 mM (salt stress) (Fig. S1).
Total RNA extraction and cDNA preparation
Root and leaf tissues of rice seedlings in each treatment were harvested at 0, 15, 30, 45, 60, 180, and 360 min after NaCl treatment and immediately frozen at −80 °C, prior to the extraction of total RNA. Total RNA in the roots and leaves was extracted from rice seedlings using guanidine hydrochloride following the details of Sambrook et al. (1989). In brief, roots and leaves of rice seedlings were ground and homogenized in a solution containing guanidinium thiocyanate (0.75 M Na-citrate at pH 7.0, 10% sarcosyl and 2 M 2-β-mercaptoethanol), Na-acetate (pH 4.0) and phenol–chloroform. After chilling the homogenate on ice, it was centrifuged at 10,000g for 20 min under 4 °C. The supernatant so obtained was again subjected to centrifugation as previously described after adding 1× vol isopropanol and the aqueous phase was collected and stored at − 20 °C for 1 h. The pellet was quickly dissolved in 0.3 mL of guanidinium thiocyanate solution and precipitated by absolute ethanol. RQ1 RNase-free DNase (Promega) was used as decontaminant DNA in the RNA preparations and total RNA was purified by phenol–chloroform extraction. First-strand DNA was synthesized with 3 μg total RNA per sample, using ImPromp-II™ Reverse Transcriptase (Promega) and oligo-dT15 primer.
Semi-quantitative PCR
A Veriti® Thermal Cycler (Applied Biosystems, CA, USA) was used to perform the PCR reaction. Following primer sequences were used: NHX1, (F) 3′-tacacgacctccgact acgc-5′ and (R) 5′-ctcctacatccagcgttcc-3′, NHX3 (F) 3′-cagcttggggactatcttgc-5′ and (R) 5′-tgtccagtgcatccatcc-3′, NHX5 (F) 3′-gcagcagctggggagaac-5′ and (R) 5′-gccatggcccctctaagc-3′, HKT2, (F) 3′-ctcattctcggtcaccgtct-5′ and (R) 5′-gatgagtgagcagtcgatgg-3′, AVP1 (F) 3′-ctgctgatgttggtgctgac-5´ and (R) 5′-ggaggcctaaccaactgaca-3′, 18SrRNA (F) 3′-gtgcaacaaaccccgact-5′ and (R) 5′-gctgctggcaccagactt-3′ (Table S1). The PCR reaction was performed with 70–100 ng synthesized cDNA, 10 pM primer and EmeraldAmp® GT PCR Master Mix (Takara, Japan) using the conditions: 94 °C for 3 min, 18–37 cycles of 94 °C for 30 s, 56–67 °C for 30 s, 72 °C for 30 s and 72 °C for 5 min. The conditions and cycle numbers were determined in order to avoid saturation of DNA amplification (Table S1). In addition, OsEF1α, a housekeeping gene was tested (Fig. S2). The DNA obtained was subjected to agarose gel electrophoresis and stained with ethidium bromide. The size of target genes was validated (Fig. S3). The signal intensity of stained bands was photographed with a Gel Doc image analysis system (Bio-Rad, Hercules) and the intensity data were analyzed using GeneTools™ (Syngene, Cambridge, UK) analysis software.
Na+ and K+ assay
Na+ and K+ were analyzed according to the modified method of Tanaka et al. (1999) and Hossain et al. (2006). In brief, root and leaf tissues of rice seedlings exposed to salt stress were collected. Then, the samples were washed by deionized water to avoid surface Na+ contamination, and dried at 60 °C. The dry tissues were ground into a powder using liquid nitrogen, extracted with boiling distilled water, and centrifuged at 10,000×g for 10 min. The extracted solution was filtered through a 0.45 μm membrane filter (VertiPure, Vertical Chromatography), and then 20 μL solution was injected to HPLC (Waters Associates, Milford, MA, USA). Na+ and K+ concentrations in each organ were determined using HPLC connected with 432 Conductivity Detector and WATER IC-PACK™ ion exclusion column. Mobile phase containing 26.7 mg of Na-EDTA and 189 μL of nitric acid L−1 was used at a flow rate of 0.8 mL min−1. Na+ and K+ concentrations were calculated from the calibration curves by directly injecting different concentrations of Na+ and K+ standards (Sigma) into the HPLC.
CoroNa-Green® Na+ localization assay
CoroNa Green® staining to reveal Na+ content in the target organs was performed as described by Oh et al. (2009). Fresh leaves and root tips of in vitro seedlings were sliced and incubated in chilled medium A (0.5 M Sorbitol, 1 mM CaCl2, 0.20% Poly-vinyl-pyrrolidone and 5 mM Tris-MES pH 5.5) containing 10 μM CoroNa Green® AM (Molecular Probes, Eugene, OR, USA) and 0.02% Pluromic F-127 for 1 h. After removing excess CoroNa Green® from the medium by washing twice with deionized water, the explants were immediately observed under Confocal laser scanning microscope (FV 1000-D, Olympus, Japan) at excitation and emission wavelengths of 488 nm and 516 nm, respectively, and photographed.
Photosynthetic abilities
Chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll (TC) concentrations were determined following the method of Shabala et al. (1998) and total carotenoids (Cx+c) concentration was assayed according to Lichtenthaler (1987). Briefly, hundred milligrams of leaf tissue were placed in a 25 mL glass vial (Opticlear® KIMBLE, Vineland, New Jersey, USA) and 10 mL of 95.5% acetone was supplied and then fragmented with a homogenizer (T25 basic ULTRA-TURRAX®, IKA, Kuala Lumpur, Malaysia). The sealed glass vials using Parafilm® to prevent evaporation were processed and then, stored at 4 °C for 48 h. Chl a, Chl b and Cx+c concentrations were estimated using a UV–visible spectrophotometer (DR/4000, HACH, Loveland, Colorado, USA). A solution of 95.5% acetone was used as blank.
Chlorophyll fluorescence emission in the leaves of rice seedlings was measured using a fluorescence monitoring system (model FMS 2; Hansatech Instruments Ltd., Norfolk, UK) in the pulse amplitude modulation mode (Loggini et al. 1999). In brief, the tissue was kept in dark for 30 min using a leaf clip and the modulated beam of far-red light (LED source) with typical peak at a wavelength of 735 nm was measured. Original (F0) and maximum (Fm) fluorescence yields were collected under weak modulated red light (< 85 μmol m−2 s−1) with 1.6 s pulses of saturating light (> 1500 μmol m−2 s−1 PPFD) and calculated using FMS software for Windows®. The variable fluorescence yield (Fv) was auto-calculated following the equation: Fv = Fm–F0. The ratio of variable to maximum fluorescence (Fv/Fm) was represented as the maximum quantum yield of PSII photochemistry. The photon yield of PSII (ΦPSII) in light was calculated using equation: ΦPSII = (Fm′ − F)/Fm′ after 45 s of illumination, when steady state was achieved (Maxwell and Johnson 2000).
Net photosynthetic rate (Pn; μmol m–2 s–1) in the leaf tissues was calculated following the method of Fujiwara et al. (1987). CO2 concentration inside and outside of culture vessels containing rice seedlings at steady state were measured by Gas Chromatography (GC; Model GC-17A, Shimadzu Co. Ltd., Tokyo, Japan).
Growth characters
Shoot height (SH), root length (RL), fresh weight (FW), dry weight (DW), and leaf area of rice seedlings were collected, as described by Cha-um et al. (2006). For the dry weight, rice seedlings were dried at 80 °C in a hot air oven for 48 h, and then kept in desiccator before the measurements of dry weight. The leaf area of rice seedlings in each treatment was measured using a Root/Leaf Area Meter DT-scan (Delta-Scan Version 2.03, Delta-T Devices, Ltd, Cambridge, UK).
Experimental design and statistical analysis
The experiment was arranged as 2 × 2 factorial (two rice genotypes and two salt stress treatments) in a Completely Randomized Design (CRD) with six replicates (n = 6) for biochemical, physiological and morphological parameters and 3 biological replications (n = 3) for relative expression in each gene. Analysis of variance (ANOVA) and the mean values obtained were analyzed and compared using Tukey’s HSD at p ≤ 0.01 using SPSS software.
Results
Expression level of ion homeostasis-related genes
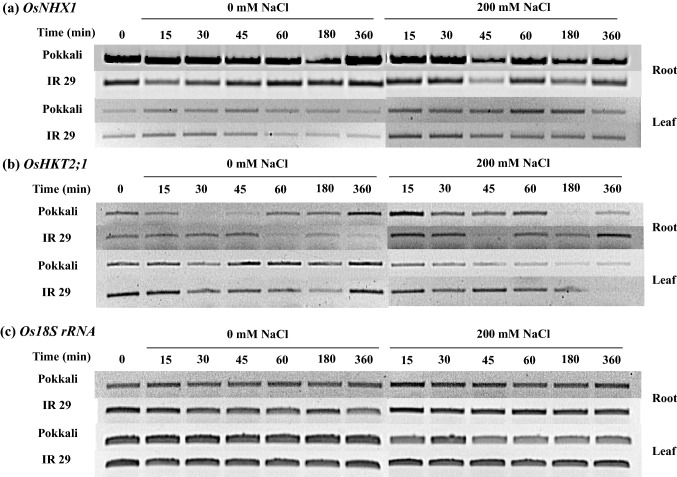
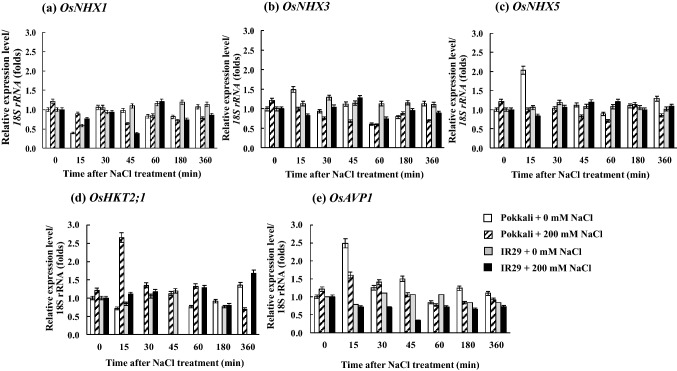
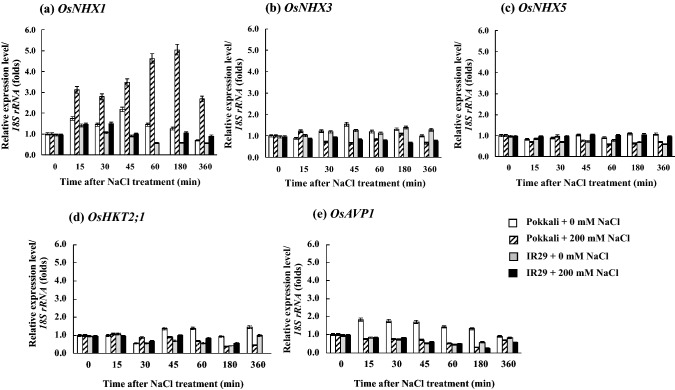
OsNHX1 mRNA expression level in the root tissues of rice cvs. Pokkali (salt tolerant) and IR29 (salt sensitive) was higher than in the leaf tissues (Fig. 1a). OsNHX1 expression in the leaves of rice seedlings cvs. Pokkali and IR29 under 200 mM NaCl (salt stress) was upregulated, whereas it was unchanged in the root tissues (Fig. 1a). Transcriptional expression level of OsHKT2;1 gene in the root tissues of both Pokkali and IR29 under control condition (0 mM NaCl) was fluctuated; however, it was down-regulated by salt stress treatments, especially in cv. Pokkali (after 180–360 min of salt stress treatment). Likewise, OsHKT2;1 expression level in the leaf tissues of both Pokkali and IR29 was down-regulated, when subjected to 200 mM NaCl (Fig. 1b). In addition, the housekeeping gene, Os18S rRNA retained a high expression level in both the cultivars and under the salt stress treatment (Fig. 1c). In the root tissues, expression levels of OsNHX1, OsNHX3, OsNHX5, OsHKT2;1 and OsAVP1 were fluctuated during early salt stress treatment (≤ 60 min), and then adjusted to stable contour (Fig. 2). OsNHX1, OsNHX3, OsNHX5 and OsHKT2;1 mRNA expression was lowest in cv. Pokkali under 200 mM NaCl for 6 h (Fig. 2a–d), whereas the expression of OsAVP1 gene was unchanged (Fig. 2e). The expression of OsAVP1 in the root tissues of IR29 under salt stress was down-regulated during early salt exposure period (≤ 45 min) compared to Os18S rRNA housekeeping gene. Interestingly, OsNHX1 expression level in the leaf tissues of salt tolerant rice cv. Pokkali was immediately increased by 3 times over the control after 200 mM NaCl treatment for 15 min and maintained at high levels during prolonged salt stress (360 min), whereas it was unchanged in IR29 (salt sensitive) (Fig. 3a). Relative expression of OsNHX3, OsNHX5, OsHKT2;1 and OsAPV1 in rice crop was down-regulated by salt treatment (Fig. 3b–e).
Fig. 1.
Semi-quantitative RT-PCR results of vacuolar Na+/H+ antiporter (OsNHX1) of root and leaf tissues in Pokkali and IR29 (a), plasma membrane Na+/H+ antiporter (OsHKT2;1) of root and leaf tissues in Pokkali and IR29 (b), and Os18S rRNA of root and leaf tissues in Pokkali and IR29 rice seedlings (c), when exposed to control (0 mM NaCl) and salt stress (200 mM) for 0, 15, 30, 45, 60,180, and 360 min
Fig. 2.
Expression profile of vacuolar Na+/H+ antiporter isoform 1 (OsNHX1) (a), isoform 3 (OsNHX3) (b), isoform 5 (OsNHX5) (c) plasma membrane Na+/H+ antiporter isoform 2 (OsHKT2;1) (d), and vacuolar pyrophosphatase (OsAPV1) (e) in root tissues of Pokkali and IR29, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 0, 15, 30, 45, 60, 180, and 360 min. Error bars represent standard error (± SE)
Fig. 3.
Expression profile of vacuolar Na+/H+ antiporter isoform 1 (OsNHX1) (a), isoform 3 (OsNHX3) (b), isoform 5 (OsNHX5) (c), plasma membrane Na+/H+ antiporter isoform 2 (OsHKT2;1) (d), and vacuolar pyrophosphatase (OsAPV1) (e) in leaf tissues of Pokkali and IR29, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 0, 15, 30, 45, 60,180, and 360 min. Error bars represent standard error (± SE)
Na+, Na+/K+ ratio and localization in the root and leaf tissues
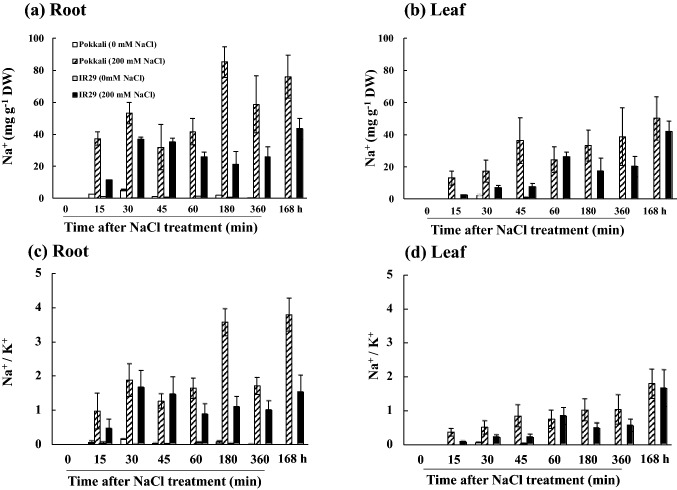
Na+ in the root and leaf tissues of salt stressed rice cvs. Pokkali and IR29 was increased in relation to the salt exposure time (Fig. 4a, b). Accumulation of Na+ in salt stressed seedlings of cv. Pokkali was greater than cv. IR29, especially in the root tissues as indicated by Na+/K+ ratio (Fig. 4c). In the root tissues of cv. Pokkali, the maximum accumulation of Na+ was found to be 40–85 mg g−1 DW, whereas in cv. IR29, it was found to be 10–50 mg g−1 DW (Fig. 4a). Na+/K+ ratio in the leaf tissues of cvs. Pokkali and IR29 was increased instantly on NaCl exposure (Fig. 4d). In the root tissues, green fluorescence of Na+ CoroNa Green® in salt stressed seedlings was clearly observed, especially at the root primordial zone (Fig. 5). In addition, green color staining was evidently found in apoplastic route, and a faint staining was detected inside the root cells of salt stressed seedlings of cv. IR29, especially in the elongation zone (Fig. 5). In the leaf tissues, green color staining was clearly demonstrated in the vascular bundles of salt stressed seedlings of cvs. Pokkali and IR29. Number of stained cells in the leaf tissues of cv. Pokkali was more than those in cv. IR29 (Fig. 5).
Fig. 4.
Na+ in root (a) and leaf tissues (b) and Na+/K+ ratio in root (c) and leaf tissues (d) of Pokkali and IR29, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 0, 15, 30, 45, 60, 180, 360 min and 168 h. Error bars represent standard error (± SE)
Fig. 5.
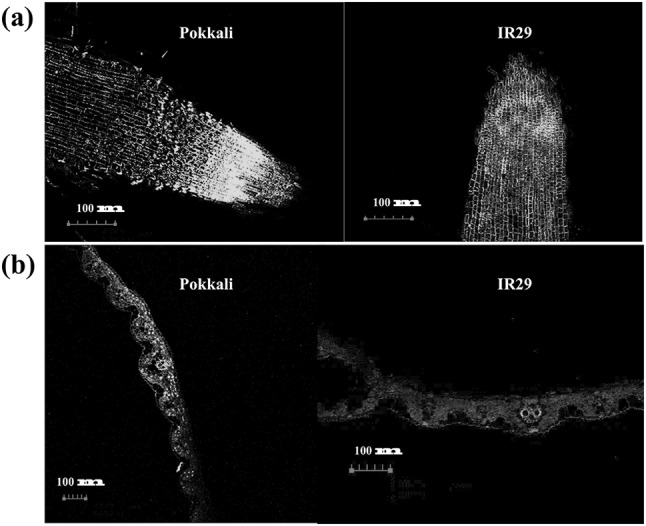
Na+ localization traced by CoroNa Green® in root (a) and leaf (b) of Pokkali and IR29 treated with 200 mM NaCl for 24 h. Green represents Na+, whereas red depicts chlorophyll fluorescence
Photosynthetic abilities and growth performances
Chl a, Chl b, TC and Cx+c in the leaf tissues of salt stressed seedlings of cv. Pokkali were significantly degraded by 10.83%, 12.15%, 11.36% and 11.19%, respectively, whereas those in cv. IR29 were sharply declined by 67.34%, 72.15%, 69.16% and 71.58%, respectively (Table 1). Fv/Fm and ΦPSII in salt stressed seedlings of cv. Pokkali were maintained (only 1.83% and 11.08% diminution over the control), whereas those in cv. IR29 were sharply declined by 41.12% and 70.31%, respectively, leading to Pn reduction (73.49% over the control) (Table 2). Pn was significantly dropped in seedlings of both cvs. Pokkali and IR29 exposed to 200 mM NaCl, resulting in reduced dry weight. Shoot height, root length, fresh weight and leaf area in salt stressed seedlings of cv. Pokkali were retained (only 0.61%, 5.44%, 3.74% and 5.02% reduction to that of control, respectively), while those of cv. IR29 were significantly decreased by 21.96%, 37.42%, 16.90% and 22.96%, respectively (Table 3). In addition, plant dry weight in salt stressed seedlings of cvs. Pokkali (11.42% reduction to that of control) and IR29 (27.19% reduction to that of control) was significantly repressed.
Table 1.
Chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (TC), and total carotenoid (Cx+c) concentrations in Pokkali and IR 29 rice seedlings, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 7 days
| Varieties | NaCl (mM) |
Chl a (μg g−1 FW) |
Chl b (μg g−1 FW) |
TC (μg g−1 FW) |
Cx+c (μg g−1 FW) |
|---|---|---|---|---|---|
| Pokkali | 0 | 80.23 ± 1.49a | 55.46 ± 0.20a | 135.67 ± 1.38a | 5.63 ± 0.04b |
| 200 |
71.54 ± 2.63b (10.83%) |
48.72 ± 1.13b (12.15%) |
120.26 ± 1.56b (11.36%) |
5.00 ± 0.09b (11.19%) |
|
| IR29 | 0 | 88.53 ± 1.48a | 53.96 ± 1.07a | 142.49 ± 1.97a | 6.51 ± 0.23a |
| 200 |
28.91 ± 0.18c (67.34%) |
18.30 ± 0.42c (72.15%) |
43.94 ± 0.32c (69.16%) |
1.85 ± 0.04c (71.58%) |
Figures in parentheses represent percent degradation in photosynthetic pigments of salt stressed seedlings in each cultivar. Data presented as mean ± standard error
Different letters in each column represent significant difference at P ≤ 0.01 according to Tukey’s HSD
Table 2.
Maximum quantum yield of PSII (Fv/Fm), photon yield of PSII (ΦPSII), and net photosynthetic rate (Pn) in Pokkali and IR 29 rice seedlings, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 7 days
| Varieties | NaCl (mM) |
Fv/Fm | ΦPSII | Pn (μmol m−2 s−1) |
|---|---|---|---|---|
| Pokkali | 0 | 0.875 ± 0.013a | 0.749 ± 0.008a | 6.01 ± 0.16b |
| 200 |
0.859 ± 0.008a (1.83%) |
0.666 ± 0.011b (11.08%) |
3.29 ± 0.23c (42.26%) |
|
| IR29 | 0 | 0.852 ± 0.010a | 0.704 ± 0.013ab | 8.30 ± 0.42a |
| 200 |
0.493 ± 0.015b (42.14%) |
0.209 ± 0.005c (70.31%) |
2.20 ± 0.26c (73.49%) |
Figures in parentheses represent percent diminution in photosynthetic abilities of salt stressed seedlings in each cultivar. Data presented as mean ± standard error
Different letters in each column represent significant difference at P ≤ 0.01 according to Tukey’s HSD
Table 3.
Shoot height (SH), root length (RL), fresh weight (FW), dry weight (DW), and leaf area (LA) in Pokkali and IR 29 rice seedlings, when exposed to control (0 mM NaCl) and salt stress (200 mM NaCl) for 7 days
| Varieties | NaCl (mM) |
SH (cm) |
RL (cm) |
FW (mg) |
DW (mg) |
LA (cm2) |
|---|---|---|---|---|---|---|
| Pokkali | 0 | 28.00 ± 0.83a | 6.98 ± 0.06a | 277.67 ± 8.25a | 68.67 ± 0.93a | 17.92 ± 0.17a |
| 200 |
27.83 ± 0.080a (0.61%) |
6.60 ± 0.14ab (5.44%) |
267.30 ± 3.10a (3.74%) |
60.83 ± 0.74b (11.42%) |
17.02 ± 0.07a (5.02%) |
|
| IR29 | 0 | 27.32 ± 0.68a | 6.44 ± 0.06b | 260.33 ± 5.113a | 68.30 ± 1.05a | 13.85 ± 0.41b |
| 200 |
21.32 ± 0.50b (21.96%) |
4.03 ± o.02c (37.42%) |
216.33 ± 4.50b (16.90%) |
49.73 ± 1.09c (27.19%) |
10.67 ± 0.18c (22.96%) |
Figures in parentheses represent percent reduction in growth parameters of salt stressed seedlings in each cultivar. Data presented as mean ± standard error
Different letters in each column represent significant difference at P ≤ 0.01 according to Tukey’s HSD
Discussion
Expression level of OsNHX1 gene in rice crop cvs. Pokkali and IR29 under NaCl stress was upregulated in plant tissues (root > leaf), especially during the early period of salt exposure (15–30 min after salt treatment). In general, Na+ secretion into the vacuole via Na+/H+ antiporter (NHX1) present in the vacuolar membrane is one of the most important salt defense mechanism in rice crop to cope with salt stress conditions (Plett and Møller 2010; Horie et al. 2012; Reddy et al. 2017). Expression level of OsNHX1 in FR13A (salt tolerant) was up-regulated by 150 mM NaCl for 1 h, whereas it was delayed in BRRI dhan29 (salt susceptible) (Hossain et al. 2017). As per the previous reports, relative expression of OsNHX1 in the root of rice crop cvs. 9311, JYGY-1 exposed to 150 mM NaCl for 24 h was found to be the maximum and greater than in the shoot tissues. After the prolonged NaCl exposure (72 h), OsNHX1 expression level in salt stressed seedlings was declined (Zhang et al. 2018). In another study, relative expression of OsNHX1 in the shoot of cv. Nipponbare exposed to 100 mM NaCl for 4–7 d (Fu et al. 2018) and in the leaves of cv. Sakha 102 exposed to 50 mM NaCl for 14 d (Mekawy et al. 2015) was upregulated over the control. It is possible that excess Na+ was compartmented in the old leaves, which contained large-sized vacuoles (Wang et al. 2012). OsNHX1 protein functions via H+ derived from the activities of OsAVP1 (H+-pyrophosphatase) (Shen et al. 2015) and/or OsVHA (H+-ATPase) located on vacuolar membrane (Kader et al. 2006). On the other hand, expression level of OsHKT2;1 gene was down-regulated, especially in the root tissues of cv. Pokkali, when subjected to 200 mM NaCl for 180–360 min. Previously, OsHKT2;1 gene has been reported as key transporter for Na+ and K+ influx in the root organ of rice crop (Huang et al. 2008). OsHKT2 proteins are located on the plasma membrane (in the root organ), controlling the Na+ influx from the medium to plant cells (Horie et al. 2007; Plett and Møller 2010; Wu 2018). Down regulation of OsHKT2;1 in four rice cultivars, Nipponbare, 9311, JYGY-1 and JYFN-4, exposed to 125 mM NaCl for 24 and 72 h was demonstrated by Zhang et al. (2018). In addition, transcriptional level of OsHKT2;1 in two rice cultivars, Sakha 102 and Egyptian Yasamine, under salt stress was the lowest in the root tissues, when compared to leaf sheath and leaf blade (Mekawy et al. 2015) and a down-regulation was observed in the root tissues of Pokkali (Kader et al. 2006). In general, relative expression level of OsHKT2;1 in the leaves and roots of two rice cultivars, Tampha and MSE9, exposed to 120 mM NaCl for 96 h was very low as detected from semi-quantitative PCR using OsActin normalization (Omisun et al. 2018). Therefore, Na+ was enriched in the root tissues of rice cv. Pokkali and it was quickly translocated from root to leaf tissues. In addition, down-regulation of OsHKT2;1 transporter in both the root and leaf tissues limited Na+ influx and Na+ unloading from xylem to leaf organ. K+ deficiency plays an important role in reducing the expression level of OsHKT2;1, which is largely regulated by presence of K+ ions, when plants are exposed to high NaCl stress (Jabnoune et al. 2009; Wu et al. 2009). Under K+-deficiency, OsHKT2;1 expression level in 11 rice cultivars was closely related to Na-uptake within 24 h (Miyamoto et al. 2015). Light dependent circadian clock is another factor that is directly regulated by the expression level of target genes in rice seedlings (cvs. Pokkali, IR29 and Nonabokra) under salt stress (200 mM NaCl) (Basu and Roychoudhury 2014). Moreover, the functional analysis of OsHKT2;1 in both gene-knock down (reducing Na+ compartmentation in the root tissues) (Horie et al. 2007) and over-expression in rice cv. Nipponbare (promoting Na+ enrichment in transgenic lines) (Yao et al. 2010) was validated. In the present study, other isoforms (OsNHX3 and OsNHX5), OsAVP1, and H+ generation that served as energy to the Na+/H+ exchangers present in the vacuolar membrane of both the root and leaf tissues of cvs. Pokkali and IR29 were fluctuated. Absolute gene expression in several NHX isoforms including OsNHX2, OsNHX3, OsNHX4, OsNHX5 and OsNHX6, in salt stressed seedlings of rice cvs. XZ26 and Nipponbare was found to show different patterns, depending on the salt exposure period, genetic variation and their interactions (Fu et al. 2018). Interestingly, relative mRNA expression of OsNHX2, OsNHX3, OsNHX4, OsNHX5 in the salt stressed roots of cv. JYGY-1 was found to be high compared to other cultivars (Zhang et al. 2018).
Na+ and Na+/K+ ratio in the roots was rapidly increased, when compared with those in the leaf tissues. Na+ uptake via influx flow channels from the external to internal roots cells has been well established in several plant species including rice crop (Garciadeblás et al. 2003; Rahman et al. 2016; Hamam et al. 2018). Na+ enrichment and Na+/K+ ratio in the root tissues of salt stressed seedlings of rice cvs. IR64 and Pokkali under 200 mM NaCl for 2 h were greater than those in the leaf tissues (Lakra et al. 2019). Likewise, Na+ content and Na+/K+ ratio in the roots of rice cvs. CSR10 and MI48 under 150 mM NaCl for 3d and 6d were greater to that of the leaves (Singh et al. 2018). However, Na+ in the leaves of rice cultivars was greater than in the roots after prolonged NaCl exposure period (7–14 d) (Mekawy et al. 2015; Hazman et al. 2016; Fu et al. 2018; Zhang et al. 2018). In rice crop, Na+, Na+:K+ ratio in old leaves of salt stressed plants were enriched over young leaves due to down-regulation of OsHKT2;1 (reduced root Na uptake) and up-regulation of OsNHX1, preventing the young tissues from Na+ toxicity (Wang et al. 2012). Alkalinity (alkali + salinity) is another factor that is tightly regulated by OsNHX1 (within 24 h) and leads to higher accumulation of Na+ in shoots than roots of rice cv. Sakha 102 (Hazman et al. 2016). In addition, Na-labeling using green fluorescence staining was well established to chase the Na localization and xylem loading/transport as a probe and to estimate the enlargement of vacuole in the protoplast of rice crop under salt stress conditions (Kader and Lindberg 2005). In the present study, Na+ enrichment in salt tolerant Pokkali (root and leaf tissues) was evidently observed. It was dependent on the high salt concentration (200 mM NaCl) in MS medium, in vitro seedling test and early salt exposure period (within 6 h). An enrichment of Na+ in the leaf tissues significantly damaged the chlorophyll pigments, especially in the salt susceptible cultivar, MI48 (Singh et al. 2018) and IR64 (Basu et al. 2017), leading to Pn reduction (Fu et al. 2018), cell death (Khare et al. 2015) and growth inhibition (Theerawitaya et al. 2015; Basu et al. 2017; Singh et al. 2018). Chl b in rice cvs. FL478, IR72, IR83142-B-61-B and IR88611-B-5 under salt stressed seedlings was found to be dependent on the degree of salt concentration (Shakeela et al. 2016). In addition, degradation of chlorophyll pigments was positively related with Pn reduction and shoot/root growth inhibition (Basu et al. 2017; Fu et al. 2018; Singh et al. 2018).
Conclusion
In conclusion, down regulation of OsHKT2;1 in the root tissues and upregulation of OsNHX1 in the leaf tissues of Pokkali rice cultivar under salt stress was largely responsible for the reduction in Na+ induced toxicity leading to pigment stabilization, chlorophyll fluorescence retention and stable Pn, thereby resulting in overall growth maintenance.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
The funding was provided by BIOTEC (Grant number P1750079).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almeida P, Katschnig D, de Boer A. HKT transporters—state of the art. Int J Mol Sci. 2013;14:20359–20385. doi: 10.3390/ijms141020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Roychoudhury A (2014) Expression profiling of abiotic stress-inducible genes in response to multiple stresses in rice (Oryza sativa L.) varieties with contrasting level of stress tolerance. BioMed Res Int. Article ID 706890 [DOI] [PMC free article] [PubMed]
- Basu S, Giri RK, Benazir I, Kumar S, Rajwanshi R, Dwivedi SK, Kumar G. Comprehensive physiological analyses and reactive oxygen species profiling in drought tolerant rice genotypes under salinity stress. Physiol Mol Biol Plants. 2017;23:837–850. doi: 10.1007/s12298-017-0477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzini M, Sacchi GA, Forlani G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes dose not rely on Na+ exclusion from shoots. J Plant Physiol. 2018;226:145–153. doi: 10.1016/j.jplph.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Cha-um S, Supaibulwatana K, Kirdmanee C. Water relation, photosynthetic ability and growth of Thai jasmine rice (Oryza sativa L. ssp. indica cv. KDML 105) to salt stress by application of exogenous glycinebetaine and choline. J Agron Crop Sci. 2006;192:25–36. doi: 10.1111/j.1439-037X.2006.00186.x. [DOI] [Google Scholar]
- Fu L, Shen Q, Kuang L, Yu J, Wu D, Zhang G. Metabolite profiling and gene expression of Na/K transporter analyses reveal mechanisms of the difference in salt tolerance between barley and rice. Plant Physiol Biochem. 2018;130:248–257. doi: 10.1016/j.plaphy.2018.07.013. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Kozai T, Watanabe L. Fundamental studies on environment in plant tissue culture vessel. (3) Measurement of carbon dioxide gas concentration in closed vessels containing tissue cultured plantlets and estimates of net photosynthetic rates of the plantlets. J Agric Methodol. 1987;43:21–30. doi: 10.2480/agrmet.43.21. [DOI] [Google Scholar]
- Garciadeblás B, Senn ME, Bañuelos MA, Rodríguez-Navarro A. Sodium transport and HKT transporters: the rice model. Plant J. 2003;34:788–801. doi: 10.1046/j.1365-313X.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- Ghosh B, Ali NM, Gantait S. Response of rice under salinity stress: a review update. J Res Rice. 2016;4:2. doi: 10.4172/2375-4338.1000167. [DOI] [Google Scholar]
- Grattan S, Zeng L, Shannon M, Roberts S. Rice is more sensitive to salinity than previously thought. Calif Agric. 2002;56:189–198. doi: 10.3733/ca.v056n06p189. [DOI] [Google Scholar]
- Hamam AM, Coskun D, Britto DT, Plett D, Kronzucker HJ. Plasma-membrane electrical responses to salt and osmotic gradients contradict radiotracer kinetics, and reveal Na+-transport dynamics in rice (Oryza sativa L.) Planta. 2018 doi: 10.1007/s00425-018-3059-7. [DOI] [PubMed] [Google Scholar]
- Han Y, Yin S, Huang L. Towards plant salinity tolerance-implications from ion transporters and biochemical regulation. Plant Growth Regul. 2015;76:13–23. doi: 10.1007/s10725-014-9997-6. [DOI] [Google Scholar]
- Hazman M, Hause B, Eiche E, Riemann M, Nick P. Different forms of osmotic stress evoke qualitatively different responses in rice. J Plant Physiol. 2016;202:45–56. doi: 10.1016/j.jplph.2016.05.027. [DOI] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung HY, Miyao A, Hirochika H, An G, Schroeder JI. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Karahara I, Katsuhara M. Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice. 2012;5:11. doi: 10.1186/1939-8433-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain GS, Waditee R, Hibino T, Tanaka Y, Takabe T. Root specific expression of Na+/H+ antiporter gene from Synechocystis sp. PCC6803 confers salt tolerance of tobacco plant. Plant Biotechnol. 2006;23:275–281. doi: 10.5511/plantbiotechnology.23.275. [DOI] [Google Scholar]
- Hossain MM, Imran S, Islam MA, Kader MA, Uddin MI. Expressional analysis of OsNHX1, OsNHX2, OsSOS1 and OsDREB transporters in salt tolerant (FR13A) and salt sensitive rice (BRRI dhan29) induced by salinity stress. IOSR J Agric Veter. 2017;10:64–70. [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, Munns R. Comparative mapping of HKT genes in wheat, barley, and rice, key determinants of Na+ transport, and salt tolerance. J Exp Bot. 2008;59:927–937. doi: 10.1093/jxb/ern033. [DOI] [PubMed] [Google Scholar]
- Hussain S, Zhang JH, Zhong C, Zhu LF, Cao XC, Yu SM, James AB, Hu JJ, Jin QY. Effects of salt stress on rice growth, development characteristics, and the regulating ways: a review. J Integr Agric. 2017;16:2357–2374. doi: 10.1016/S2095-3119(16)61608-8. [DOI] [Google Scholar]
- Hussain M, Ahmad S, Hussain S, Lal R, Ul-Allah S, Nawaz A. Rice in saline soils: physiology, biochemistry, genetics, and management. Adv Agron. 2018;148:231–287. doi: 10.1016/bs.agron.2017.11.002. [DOI] [Google Scholar]
- Jabnoune M, Espeout S, Mieulet D, Fizames C, Verdeil JL, Conéjéro G, Rodríguez-Navarro A, Sentenac H, Guiderdoni E, Abdelly C, Véry AA. Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol. 2009;150:1955–1971. doi: 10.1104/pp.109.138008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesus JM, Danko AS, Fiúza A, Borges MT. Phytoremediation of salt-affected soils: a review of processes, applicability, and the impact of climate change. Environ Sci Pollut Res. 2015;22:6511–6525. doi: 10.1007/s11356-015-4205-4. [DOI] [PubMed] [Google Scholar]
- Julkowska MM, Testerink C. Tuning plant signaling and growth to survive salt. Trend Plant Sci. 2015;20:586–594. doi: 10.1016/j.tplants.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Kader MA, Lindberg S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. determined by the fluorescent dye SBFI. J Exp Bot. 2005;56:3149–3158. doi: 10.1093/jxb/eri312. [DOI] [PubMed] [Google Scholar]
- Kader MA, Seidel T, Golldack D, Lindberg S. Expression of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J Exp Bot. 2006;57:4257–4268. doi: 10.1093/jxb/erl199. [DOI] [PubMed] [Google Scholar]
- Khare T, Kumar V, Kishor PBK. Na+ and Cl− ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma. 2015;252:1149–1165. doi: 10.1007/s00709-014-0749-2. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy SL, Sharma PC, Sharma DK, Ravikiran KT, Singh YP, Mishra VK, Burman D, Maji B, Mandal S, Sarangi SK, Gautam RK, Singh PK, Manohara KK, Marandi BC, Padmavathi G, Vanve PB, Patil KD, Thirumeni S, Verma OP, Khan AH, Tiwari S, Geetha S, Shakila M, Gill R, Yadav VK, Roy SKB, Prakash M, Bonifacio J, Ismail A, Gregorio GB, Singh RK. Identification of mega-environments and rice genotypes for general and specific adaptation to saline and alkaline stresses in India. Sci Rep. 2017;7:7968. doi: 10.1038/s41598-017-08532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Mosa KA. Ion transporters: a decisive component of salt stress tolerance in plants. In: Wani SH, Anwar M, editors. Managing salt tolerance in plants: molecular and genomic perspectives. Boca Raton: CRC Press, Taylor & Francis Group; 2015. pp. 373–390. [Google Scholar]
- Lakra N, Kaur C, Singla-Pareek SL, Pareek A. Mapping the ‘early salinity response’ triggered proteome adaptation in contrasting rice genotypes using iTRAQ approach. Rice. 2019;12:3. doi: 10.1186/s12284-018-0259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol. 1987;148:350–380. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F. Antioxidant defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol. 1999;119:1091–1099. doi: 10.1104/pp.119.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM. Sodium in plants: perception, signaling, and regulation of sodium fluxes. J Exp Bot. 2014;65:849–858. doi: 10.1093/jxb/ert326. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence-a practical guide. J Exp Bot. 2000;51:659–668. doi: 10.1093/jexbot/51.345.659. [DOI] [PubMed] [Google Scholar]
- Mekawy AMM, Assaha DVM, Yahaki H, Tada Y, Ueda A, Seneoka H. Growth, physiological adaptation, and gene expression analysis of two Egyptian rice cultivars under salt stress. Plant Physiol Biochem. 2015;87:17–25. doi: 10.1016/j.plaphy.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Ochiai K, Nonoue Y, Matsubara K, Yano M, Matoh T. Expression level of the sodium transporter gene OsHKT2;1 determines sodium accumulation of rice cultivars under potassium-deficient conditions. Soil Sci Plant Nutr. 2015;61:481–492. doi: 10.1080/00380768.2015.1005539. [DOI] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Ochiai K, Matoh T. Characterization of the Na+ delivery from roots to shoots in rice under saline stress: excessive salt enhances apoplastic transport in rice plants. Soil Sci Plant Nutr. 2002;48:371–378. doi: 10.1080/00380768.2002.10409214. [DOI] [Google Scholar]
- Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, Jiang X, D’Urzo MP, Lee SY, Zhao Y, Bahk JD, Bressan RA, Yun DJ, Pardo JM, Bohnert HJ. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009;151:210–222. doi: 10.1104/pp.109.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omisun T, Sahoo S, Saha B, Panda SK. Relative salinity tolerance of rice cultivars native to North East India: a physiological, biochemical and molecular perspective. Protoplasma. 2018;255:193–202. doi: 10.1007/s00709-017-1142-8. [DOI] [PubMed] [Google Scholar]
- Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plans and its tolerance strategies: a review. Environ Sci Pollut Res. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- Pitman M, Läuchli A. Global impact of salinity and agricultural ecosystems. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht: Springer; 2004. pp. 3–20. [Google Scholar]
- Plett DC, Møller IS. Na+ transport in glycophytic plants: what we know and would like to know. Plant Cell Environ. 2010;33:612–626. doi: 10.1111/j.1365-3040.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Qadir M, Oster J. Vegetative bioremediation of calcareous sodic soils: history, mechanisms, and evaluation. Irrig Sci. 2002;21:91–101. doi: 10.1007/s00271-001-0055-6. [DOI] [Google Scholar]
- Rahman MA, Thomson MJ, Shah-E-Alam M, de Ocampo M, Egdane J, Ismail AM. Exploring novel genetic sources of salinity tolerance in rice through molecular and physiological characterization. Ann Bot. 2016;117:1083–1097. doi: 10.1093/aob/mcw030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy INBL, Kim BK, Yoon IS, Kim KH, Kwon TR. Salt tolerance in rice: focus on mechanisms and approaches. Rice Sci. 2017;24:123–144. doi: 10.1016/j.rsci.2016.09.004. [DOI] [Google Scholar]
- Rengasamy P. Soil processes affecting crop production in salt-affected soil. Funct Plant Biol. 2010;37:613–620. doi: 10.1071/FP09249. [DOI] [Google Scholar]
- Roy SJ, Negrão S, Tester M. Salt resistant crop plants. Curr Opin Biotechnol. 2014;26:115–124. doi: 10.1016/j.copbio.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Shabala SN, Shabala SI, Martynenko AI, Babourina O, Newman IA. Salinity effect on bioelectric activity, growth, Na+ accumulation and chlorophyll fluorescence of maize leaves: a comparative survey and prospects for screening. Aust J Plant Physiol. 1998;25:609–616. [Google Scholar]
- Shabala S, Shabala S, Cuin TA, Pang J, Percey W, Chen Z, Conn S, Eing C, Wegner LH. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010;62:839–853. doi: 10.1111/j.1365-313X.2009.04110.x. [DOI] [PubMed] [Google Scholar]
- Shabala S, Wu H, Bose J. Salt stress sensing and early signalling events in plant roots: current knowledge and hypothesis. Plant Sci. 2015;241:109–119. doi: 10.1016/j.plantsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Shakeela BS, Chachar QI, Chachar SD, Soloangi AB, Solangi JA. Effect of salinity (NaCl) stress on physiological characteristics of rice (Oryza sativa L.) at early seedling stage. Int J Agric Technol. 2016;12:263–279. [Google Scholar]
- Shen G, Wei J, Qiu X, Hu R, Kuppu S, Auld D, Blumwald E, Gaxiola R, Payton P, Zhang H. Co-overexpression of AVP1 and AtNHX1 in cotton further improves drought and salt tolerance in transgenic cotton plants. Plant Mol Biol Rep. 2015;33:167–177. doi: 10.1007/s11105-014-0739-8. [DOI] [Google Scholar]
- Singh V, Singh AP, Bhadoria J, Giri J, Singh J, Vineeth TV, Sharma PC. Differential expression of salt-tolerant and salt sensitive rice (Oryza sativa L.) at seedling stage. Protoplasma. 2018;255:1667–1681. doi: 10.1007/s00709-018-1257-6. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ohta K, Haddad PR, Fritz JS, Lee KP, Hasebe K, Ieuji A, Miyanaga A. Acid-rain monitoring in East Asia with a portable-type ion-exclusion-cation-exchange chromatographic analyzer. J Chromatogr. 1999;850:311–317. doi: 10.1016/S0021-9673(99)00286-1. [DOI] [PubMed] [Google Scholar]
- Theerawitaya C, Yamada N, Samphumphuang T, Cha-um S, Kirdmanee C, Takabe T. Evaluation of Na+ enrichment and expression of some carbohydrate related genes in indica rice seedlings under salt stress. Plant Omic J. 2015;8:130–140. [Google Scholar]
- Wang W, Vinocur B, Altman A. Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang MS, Guo R, Shi DC, Liu B, Lin XY, Yang CW (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol 12:194 [DOI] [PMC free article] [PubMed]
- Wu H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018;6:215–225. doi: 10.1016/j.cj.2018.01.003. [DOI] [Google Scholar]
- Wu Y, Hu Y, Xu G. Interactive effects of potassium and sodium on root growth and expression of K/Na transporter genes in rice. Plant Growth Regul. 2009;57:271–280. doi: 10.1007/s10725-008-9345-9. [DOI] [Google Scholar]
- Yao X, Horie T, Xue S, Leung HY, Katsuhara M, Brodsky DE, Wu Y, Schroeder JI. Differential sodium and potassium transport selectivities of the rice OsHKT2;1 and OsHKT2;2 transporters in plant cells. Plant Physiol. 2010;152:341–355. doi: 10.1104/pp.109.145722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmin F, Biswas S, Nurnabi GM, Jewel NA, Elias SM, Seraj ZI. Constitutive overexpression of the plasma membrane Na+/H+ antiporter for conferring salinity tolerance in rice. Plant Tiss Cult Biotechnol. 2015;25:257–272. doi: 10.3329/ptcb.v25i2.26259. [DOI] [Google Scholar]
- Zhang Y, Fang J, Wu X, Dong L. Na+/K+ balance and transport regulatory mechanisms in weedy and cultivated rice (Oryza sativa L.) under salt stress. BMC Plant Biol. 2018;18:375. doi: 10.1186/s12870-018-1586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Zhou M, Shabala L, Shabala S. Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context salinity stress tolerance. Plant Cell Environ. 2017;40:1009–1020. doi: 10.1111/pce.12727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.