Abstract
The molecular mechanisms underlying the ‘seed and soil’ theory are unknown. S100A8/A9 (a heterodimer complex of S100A8 and S100A9 proteins that exhibits a ‘soil signal’) is a ligand for Toll-like receptor 4, causing distant melanoma cells to approach the lung as a ‘seeding’ site. Unknown soil sensors for S100A8/A9 may exist, e.g., extracellular matrix metalloproteinase inducer, neuroplastin, activated leukocyte cell adhesion molecule, and melanoma cell adhesion molecule. We call these receptor proteins ‘novel S100 soil sensor receptors (novel SSSRs).’ Here we review and summarize a crucial role of the S100A8/A9-novel SSSRs' axis in cancer metastasis. The binding of S100A8/A9 to individual SSSRs is important in cancer metastasis via upregulations of the epithelial-mesenchymal transition, cellular motility, and cancer cell invasiveness, plus the formation of an inflammatory immune suppressive environment in metastatic organ(s). These metastatic cellular events are caused by the SSSR-featured signal transductions we identified that provide cancer cells a driving force for metastasis. To deprive cancer cells of these metastatic forces, we developed novel biologics that prevent the interaction of S100A8/A9 with SSSRs, followed by the efficient suppression of S100A8/A9-mediated lung-tropic metastasis in vivo.
Introduction
We have been investigating cancer metastasis mechanisms at the molecular level, based on the ‘seed and soil’ theory that was first proposed by Paget et al. in 1989 [1]. The term ‘seed’ represents the dissemination of cancer cells, and ‘soil’ represents a favorable environment for the cancer cells to grow within a given organ. Several research groups have examined the lung-secreted ligand S100A8/A9 (a heterodimer complex of S100A8 and S100A9 proteins), which attracts cancer cells originating from distant organs [3,2]. Lung S100A8/A9 acts as a strong ‘soil signal’ to its receptors in cancer cells, which act as ‘S100A8/A9-soil signal sensors.’ In addition to the two known S100A8/A9 receptors, i.e., toll-like receptor 4 (TLR4) [3,2] and receptor for advanced glycosylation end products (RAGE) [4], we have identified the following novel S100A8/A9 receptors: extracellular matrix metalloproteinase inducer (EMMPRIN) [5], neuroplastin (NPTN) α and β (NPTNβ compensates for NPTNα) [7,6], activated leukocyte cell adhesion molecule (ALCAM), and melanoma cell adhesion molecule (MCAM) [[8], [9], [10]]. We named this group of receptors the ‘novel S100 soil sensor receptors (SSSRs)’ [12,11].
As do the two previously identified receptors, the newly identified receptors play a crucial role in cancer metastasis in response to S100A8/A9. This implies that these receptors may be useful candidate molecular targets to prevent cancer metastasis [12,11]. In this mini-review, based mainly on our research history, we first describe how these receptors are identified, and we then discuss their roles in cancer and signal transduction, and we consider the therapeutic possibilities when these molecules are targeted.
Identification of SSSRs and Their Functions in Cancer Metastasis
Identification of EMMPRIN and its Paralogs
S100A8/A9 is a heterodimer complex of S100A8 and S100A9 proteins [13] that correspond to 20 of the S100 family proteins (S100A1–A16, β, G, P and Z) in humans [14]. Among the S100 family proteins, S100A8/A9 has an interesting trait; it shows markedly high production and secretion in the lung even when solid cancer is present at a site that is remote from the lung. Due to a neoplastic feature of cancers as a foreign substance, the lung senses the presence of cancer, and cancer-mediated inflammation develops at that site as a protection mechanism against cancer since the lung is one of the sensitive tissues that generally recognizes many types of foreign substances in the air. A high expression of S100A8/A9 can be induced by the inflammation, since several inflammatory cytokines and chemokines stimulate both the production and the secretion of S100A8/A9, and secreted S100A8/A9 further stimulates these inflammatory soluble factors in a process that leads to the formation of a feed-forward loop between inflammatory soluble factors and S100A8/A9, resulting in S100A8/A9-integrated and persistent inflammation [15].
We observed that S100A8/A9 secretion in the lung is also stimulated by cancer-derived exosomes [7]. The lung-secreted S100A8/A9 attracts remote cancer cells to the lung. S100A8/A9 requires its receptor(s) in order to act on cancer cells. TLR4 and RAGE are representative receptors for S100A8/A9 [[2], [3], [4]], but S100A8/A9-mediated cancer metastasis is not explained by only TLR4 and RAGE since many sorts of metastatic cancer cell lines show poor expressions of these receptors at both the mRNA and protein levels (unpubl. data).
Our previous research indicated the potential presence of unidentified receptors for S100A8/A9, since we observed that S100A8/A9-dependent signals in cells never faded out even in the setting of a functional inhibition of both TLR4 and RAGE [16]. To identify novel receptors, we performed a pull-down assay using S100A8/A9 recombinant protein as a bait to pick up its receptor candidate(s) from protein extracts. In our subsequent liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis of the precipitated proteins with S100A8/A9, we fortunately succeeded in identifying EMMPRIN as a putative S100A8/A9 receptor [5].
EMMPRIN, also known as basigin or CD147, is a glycoprotein that is a member of the immunoglobulin superfamily (as is RAGE), and EMMPRIN plays a crucial role in cancer progression via several functions, including the following: a facilitated secretion of matrix metalloproteinases (MMPs) [17], enhanced tumor angiogenesis via the induction of vascular endothelial growth factor (VEGF) [19,18], adhesion with extracellular matrixes [20], and cancer-associated anaerobic glycolysis by regulating the activity of the membrane monocarboxylate transporters-1 (MCT-1) and MCT-4, which transport lactic acid [21]. It was demonstrated that cyclophilinA (CyPA) functions as an EMMPRIN ligand; the interaction of CyPA and EMMPRIN stimulates cancer proliferation [17]. We revealed that S100A8/A9 also functions as a ligand of EMMPRIN; their engagement plays a crucial role in the lung-tropic metastasis of melanoma [5], as S100A8/A9 was highly induced in the lungs of melanoma-bearing mice.
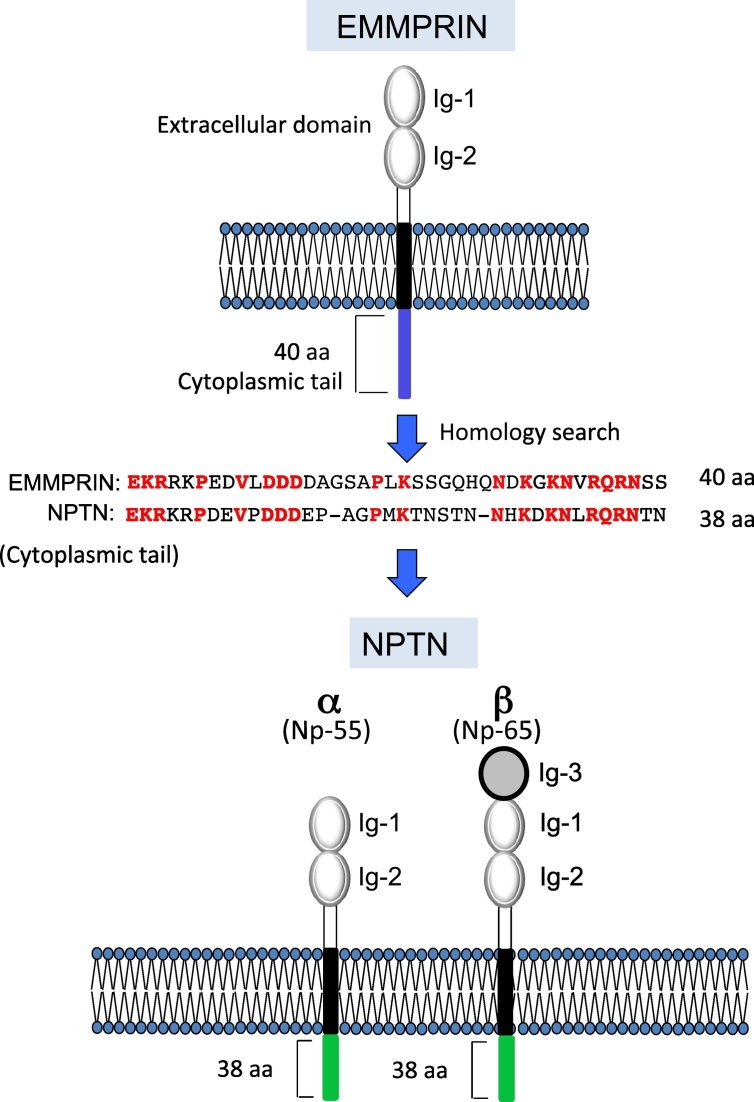
To force melanoma cells to metastasize by EMMPRIN triggered by S100A8/A9 or S100A9 binding, tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2)-mediated NFκB activation is required; this is because the cytoplasmic tail of EMMPRIN recruits TRAF2 adaptor protein in response to S100A8/A9-EMMPRIN interaction [5]. In light of the interaction of EMMPRIN with S100A8/A9 via its S100A9 side, we hypothesized that another receptor that can couple with EMMPRIN and bind with the S100A8 side of the heterodimer might be present. Such a receptor may be structurally similar to EMMPRIN, and we thus conducted a homology search on the basis of the amino acid sequence of the full length of EMMPRIN. However, no hitting of any appreciable candidates was observed. Faced with this result, we modified the strategy to conduct an individual domain-based search to identify a greater number of hitting sequences. The results revealed the presence of NPTN, whose cytoplasmic domain sequence is very similar to that of EMMPRIN [6] (Figure 1).
Figure 1.
The domain structures for EMMPRIN, NPTNα, and NPTNβ. EMMPRIN and NPTN belong to the Ig superfamily consisting of extracellular Ig domains, one transmembrane domain, and a short cytoplasmic domain. NPTN has alternative splicing variants called NPTNα (Np-55) and NPTNβ (Np-65). A homology search showed very similar amino acids sequences between EMMPRIN and NPTN.
NPTN is a member of the immunoglobulin (Ig) superfamily. It has two isoforms, NPTNα (Np-55) and NPTNβ (Np-65) that contain two and three extracellular immunoglobulin domains, respectively. NPTNα and NPTNβ have been reported to play important roles in a number of key neuronal and synaptic functions because of their abundant expression in the brain [23,22]. They also exhibited an unusual role in cancer progression: we revealed this role in the context of S100A8/A9-related metastasis [7]. We observed that both the NPTNα and NPTNβ isoforms are able to bind with S100A9. However, due to its additional N-terminal Ig domain, NPTNβ can interact with S100A8. We also observed that NPTNβ is able to undergo dimerization with its own NPTNβ, its α-isoform, and EMMPRIN [6].
In light of the dominant expression of NPTNβ but not NPTNα in cases of melanoma, it should be noted that either the NPTNβ/NPTNβ homodimer or the EMMPRIN/NPTNβ heterodimer presents on the surface of melanoma and functions as an S100A8/A9 receptor, leading melanoma cells to lung-tropic metastasis. The NPTNβ/NPTNβ homodimer may be dominant in lung cancer, since NPTNβ expression is markedly upregulated in lung cancer species in a consistent manner compared to the expression profiling of EMMPRIN and NPTNα. In the lung cancer context, we reported that the S100A8/A9-NPTNβ axis plays a critical role in disseminating the progression of lung cancer in vitro and in vivo. What downstream signal(s) does the S100A8/A9-NPTNβ axis use as a driving force for cancer progression in the lung? We observed a key transcription factor, nuclear factor I (NFI)A/NFIB, which is positively regulated mostly by TRAF2, but the growth factor receptor-bound protein 2 (GRB2)-renin-angiotensin system (RAS) pathway also contributes to the activation of NFIA/NFIB in an orchestrated manner with TRAF2.
In the case of RAS, we also observed the importance of ERAS and RASL11A, which are different from KRAS. The well-known KRAS gene (which is frequently mutated as an active form in lung cancer) was not involved in the identified pathway. The activation of NFIA/NFIB eventually led to the induction of many cancer-related genes. One of the most interesting genes was SPDEF (SAM pointed-domain containing ETS transcription factor). When we artificially regulated the expression of the SPDEF gene, we observed that SPDEF greatly contributed to lung cancer progression with enhanced anchorage-independent growth and disseminative activities. Taken together, our data support the notion that the newly identified NPTNβ signaling pathway that is initiated by cancer cells surrounding extracellular S100A8/A9 worsens lung cancers toward their disseminating progression [7].
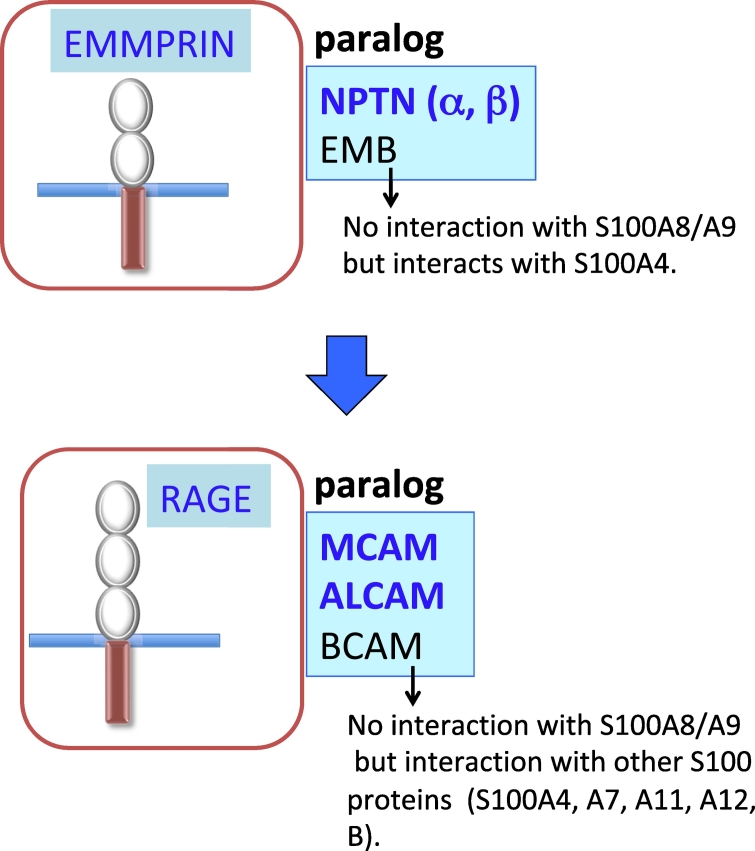
We used the structural similarity between EMMPRIN and NPTN and observed that these molecules are in paralog relation. We also identified another candidate, embigin (EMB) [24] (Figure 2). EMB is a member of the Ig superfamily that was first observed in embryonic carcinoma cells. EMB is highly expressed in pancreatic ductal adenocarcinoma (PDAC) cells and contributes to the development of PDAC. Jung et al. reported that the overexpression of EMB in PDAC induces upregulations of the proliferation, migration, and invasion of PDAC cells [25]. The EMB-mediated enhanced cell motility is associated with the tumor growth factor beta (TGFβ) pathway-relevant epithelial-mesenchymal transition (EMT) [25]. In that study, we first expected that like EMMPRIN and NPTN, EMB must also work as an S100A8/A9 receptor and would contribute to the progression of PDAC. However, contrary to our expectation, EMB could not bind with S100A8/A9, but it was able to bind with another cancer-relevant S100 protein, S100A4 (by another name, metastasis). We therefore eliminated EMB from the group of S100A8/A9-associated SSSRs.
Figure 2.
The presence of paralogs for EMMPRIN (top) and RAGE (bottom).
Regarding the S100A4-EMB axis, we focused on prostate cancer since EMB showed the highest expression in prostate cancer cells among the cancer cell lines that we examined, including PDAC cells. An unusual role of the S100A4-EMB axis in the progression of prostate cancer was observed, and we identified a key signal pathway that is regulated by EMB upon S100A4 binding: an identified AMPK/mTORC1/NFκB/MMP9 pathway is used as a vital oncogenic molecular cascade in prostate cancer cells for their progression. Our RNA-seq analysis data further showed that EMB is associated mostly with cancer-related genes [24].
RAGE Paralogs
According to the idea that paralog(s) should also exist for RAGE, we searched for the presence of RAGE paralogs and identified basal cell adhesion molecule (BCAM), ALCAM, and MCAM [8] (Figure 2). These are cell adhesion molecules that belong to the Ig superfamily, like RAGE. Because there was no binding of BCAM to S100A8/A9, we removed BCAM from the group of SSSRs and decided to focus on ALCAM and MCAM, which interact with S100A8/A9. We observed that a functional inhibition of the intrinsic ALCAM (but not of MCAM) in melanoma cells had no effect on the lung-tropic metastasis of melanoma cells in a mouse model, and we therefore paid more attention to MCAM as a key regulator for melanoma metastasis in response to S100A8/A9.
MCAM, which was originally identified as a tumor biomarker of melanoma [27,26], has long been known to be highly involved in the metastasis of not only melanoma but many other types of cancer through the regulation of cell migration, invasion, angiogenesis, and immune response [[28], [29], [30]]. It is not yet known how MCAM provides the driving force for metastasis upon S100A8/A9 binding to cancer cells, and we therefore investigated an unidentified MCAM downstream signal. Our findings demonstrated that the cytoplasmic tail of MCAM recruits a specific mitogen-activated protein kinase kinase kinase (MAPKKK) named tumor progression locus 2 (TPL2), resulting in the activation of ERK1/2 at a significant level. The activated ERK1/2 was observed to lead to an active form of ETS variant transcription factor 4 (ETV4) and to the subsequent induction of matrix metallopeptidase 25 (MMP25) by the active ETV4, eventually leading to the skin dissemination of melanoma cells (an event that is required as a prerequisite for remote metastasis) [9].
Interestingly, the ETV4-target molecule is changed in different cancer cells. We revealed that the S100A8/A9-MCAM-TPL2-ETV4 axis leads to an EMT-mediated disseminative progression of breast cancer cells via a marked induction of zinc finger E-box binding homeobox 1 (ZEB1) transcription factor [10]. That study was the first report of the unveiled S100A8/A9-MCAM-mediated signal pathways that lead to aggressive metastasis of melanoma and breast cancer cells [10,9].
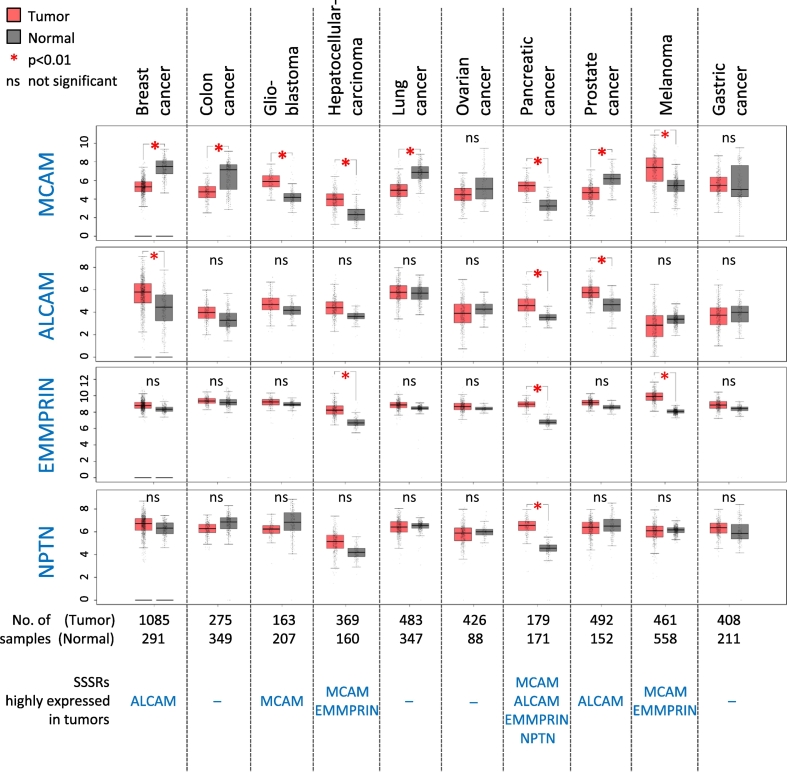
We summarized how these novel SSSRs are related to various primary cancers with their tissue (cell) specificity, by using publicly available data (Figure 3) (GEPIA: http://gepia.cancer-pku.cn/) and the available scientific reports. They are presented in Table 1: MCAM [[31], [32], [33], [34], [35],9,8], ALCAM [[36], [37], [38], [39]], and EMMPRIN [[40], [41], [42], [43],5]). We first examined the expression levels of the novel SSSRs in several types of primary cancers through the website database (Figure 3), and then the data obtained about relevancy between the elevated expressions of individual novel SSSRs and their corresponding primary cancer species were further checked through the scientific reports (Table 1). By reviewing the combined results from Figure 3 and Table 1, we found that MCAM is upregulated in glioblastoma, hepatocellular carcinoma, and melanoma; ALCAM is upregulated in breast cancer, pancreatic cancer and prostate cancer; EMMPRIN is upregulated in hepatocellular carcinoma, pancreatic cancer, and melanoma. In light of these data, our findings from recent melanoma studies (MCAM [35,9,8], EMMPRIN [5]) were consistent with the summarized results (Table 1). In the case of NPTN, no report in pancreatic cancers was identified, but we observed that NPTN (especially in its isoform β) is commonly upregulated in lung cancer cells and is associated with an increased potential for the cells' metastasis [7].
Figure 3.
Gene expression plots of the indicated genes (SSSRs) from cancer specimens were obtained from a publicly available website (http://gepia.cancer-pku.cn/). Data are mean ± SD. * P < .01. ns: not significant.
Table 1.
The elevated expressions and potentially metastatic implications of SSSRs in human cancers.
| Novel SSSR |
Cancer species that highly express SSSRs | References |
|---|---|---|
| MCAM | Glioblastoma | [31] Yawata et al., J. Neurooncol. (2019) |
| Hepatocellular carcinoma |
[32] Wang et al., Oncogene. (2015) | |
| [33] Jiang et al., J. Exp. Clin. Cancer Res. (2016) | ||
| Pancreatic cancer | (−) | |
| Melanoma | [34] Pearl et al., J. Plast. Reconstr. Aesthet. Surg. (2008) | |
| [8] Ruma et al., Clin. Exp. Metastasis. (2016) | ||
| [35] Sumardika et al., Oncol. Res. (2018) | ||
| [9] Chen et al., Cancer Lett. (2019) | ||
| ALCAM | Breast cancer | [36] Ihnen et al., Breast Cancer Res. Treat. (2008) |
| Pancreatic cancer | [37] Kahlert et al., Br. J. Cancer. (2009) | |
| Prostate cancer | [38] Kristiansen et al., Prostate. (2003) | |
| [39] Hansen et al., Cancer Res. (2014) | ||
| EMMPRIN | Hepatocellular carcinoma |
[40] Li et al., World J. Gastroenterol. (2005) |
| Pancreatic cancer | [41] Zhang et al., Int. J. Exp. Pathol. (2016) | |
| Melanoma | [42] Kanekura et al., Int. J. Cancer. (2002) | |
| [5] Hibino et al., Cancer Res. (2013) | ||
| [43] Caudron et al., Exp. Dermatol. (2016) | ||
| NPTN | Pancreatic cancer | (−) |
The expression of the novel SSSRs will elevate further in accord with the cancer malignancy. We examined two types of melanoma cell lines (WM-115 and WM-266-4) that were obtained from the same patient: the WM-115 cells were from the primary tumor in the skin, and the WM-266-4 cells were from a metastasized area [44]. Our analyses revealed that MCAM was highly upregulated in the WM-266-4 cells compared to the WM-115 cells [9]. This observation was also made in breast cancer cells [10]. The expression of MCAM is also proportionally elevated with the progression of malignant stages of breast cancer [10]. Collectively, these results suggest that the expression levels of individual SSSRs are closely associated with the primary cancer species, and their expressions can be altered through their malignant conversion.
Novel Insights Into the Functions of SSSRs in Metastasis
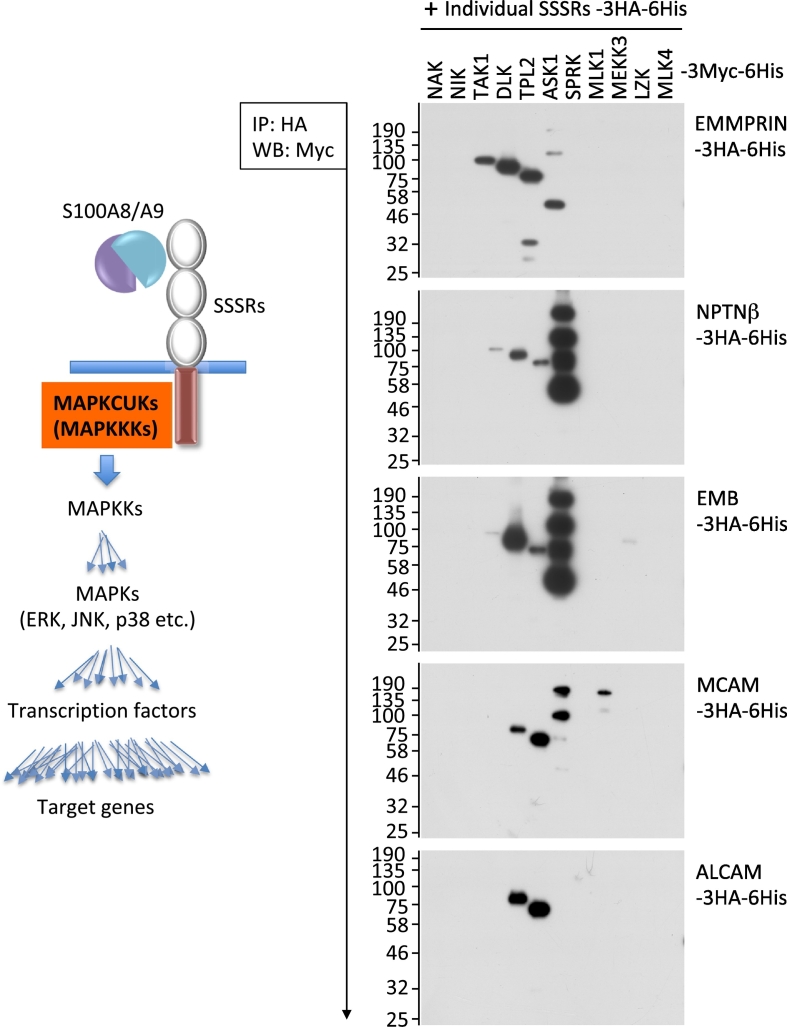
A discussion of potential common pathway(s) among SSSRs is necessary, since the functions of all of the SSSRs are very similar to one another in the context of metastasis. The presence of similar pathway(s) is very important to mutually compensate and synergistically enhance individual signal pathway(s) from SSSRs. Figure 4 (unpubl. data) illustrates our novel findings regarding which types of MAPK cascade upstream kinases (MAPKCUKs) interact with each SSSR. Because the signal cascade ‘snowballs’ (Figure 4, left panel), analyses of the signal onset are important to determine the signal dimensions. For MAPKCUKs, the MAPKKKs are located mostly at the very top of the receptor signal cascades. We thus investigated the potential recruitment of MAPKKKs to SSSRs.
Figure 4.
The interaction of the MAPK cascade upstream kinases (MAPKCUKs) with SSSRs. To examine certain interaction(s) between individual SSSRs and MAPKCUKs, we constructed a series of expression vectors that express foreign genes of interest at much higher levels. We inserted the cDNAs of interest into pIDT-SMART (C-TSC) vector [81]. The prepared cDNAs were as follows: human cDNAs encoding SSSRs (EMMPRIN, NPTNβ, RAGE, MCAM and ALCAM) and MAPKCUKs (NAK, NIK, TAK1, DLK, TPL2, ASK1, SPRK, MLK1, MEKK3, LZK and MLK4), which are designed for expression as C-terminal 3xHA-6His-tagged forms for SSSRs and as C-terminal 3xMyc-6His-tagged forms for MAPKCUKs [9]. HEK292T cells were transiently transfected with the indicated combinations of the plasmid vectors using FuGENE-HD (Promega, Madison, WI). The co-immunoprecipitation and Western blotting were performed as follows. Monoclonal anti-HA tag (clone HA-7) agarose (Sigma-Aldrich, St. Louis, MO) was used for the co-immunoprecipitation experiments. The tag-agarose beads were mixed with various cell extracts expressing an excess amount of foreign kinases (MAPKCUKs) and incubated for 3 h at 4 °C. After the samples were incubated, bound proteins were pulled down by centrifugation and the precipitates were subjected to SDS-PAGE followed by Western blotting with mouse anti-Myc tag antibody (Cell Signaling Technology, Beverly, MA). Left panel: Schematic of the receptor-mediated signal ‘snowball.’ Right: The results of the interactions examined.
A similar interaction pattern of MAPKKKs with individual SSSRs was detected. Among the EMMPRIN paralogs (EMMPRIN, NPTNβ and EMB), DLK, TPL2 and ASK1 were commonly bound to them, and TAK1 was strongly recruited to EMMPRIN. In contrast, RAGE paralogs (MCAM and ALCAM) recruited DLK and TPL2 in a consistent manner, whereas ASK1 and MLK1 were additionally associated with MCAM (Figure 4, right panel). Our previous work showed that RAGE has two MAPKKKs, TPL2 and ASK1, for its key signal onset [45].
Taking these findings together, we observed that TPL2 is the single MAPKKK that is commonly located at the signal onset of the SSSRs. TPL2 may therefore have functions that are similar among all SSSRs. In the context of cancer, TPL2 may give cancer cells increased abilities of cellular motility and survival, in light of the unusual role of the kinase in melanoma and breast cancers' aggressiveness [10,9]. Regarding the SSSRs' signals upon S100A8/A9 binding, on the basis of the central role of TPL2, another binding MAPKKK may modify the downstream signals, and this may be followed by altered cellular behaviors even in the same metastatic event. Thus, the new finding of the potential usage of MAPKKKs in SSSRs may help establish the details of the complex mechanisms of S100A8/A9-SSSR-related cancer metastasis.
In addition to the above signal transductions of SSSRs that supply a metastatic driving force to cancer cells, certain proteins interacting with SSSRs together on the cancer plasma membrane may also affect cancer metastasis. Tasdogan et al. recently reported a very interesting outcome that indicates a critical role of lactate in cancer metastasis [46]. They demonstrated that an enhanced lactate uptake through the major lactate transporter MCT1 in melanoma cells promotes the cells' survival and metastasis in vivo. One of the SSSRs, EMMPRIN, can bind to lactate transporters such as MCT1 and MCT4 [47] and regulate their channel activity of lactate [48,49]. For example, EMMPRIN modulates lactate uptake via an interactive regulation of MCT1 and MCT4 in many types of cancer cells that promotes cancer survival, drug resistance, dissemination and metastasis [[49], [50], [51], [52], [53]]. The disruption of the EMMPRIN-MCT1 interaction by immunomodulatory drugs exerted efficient antitumor activity [54].
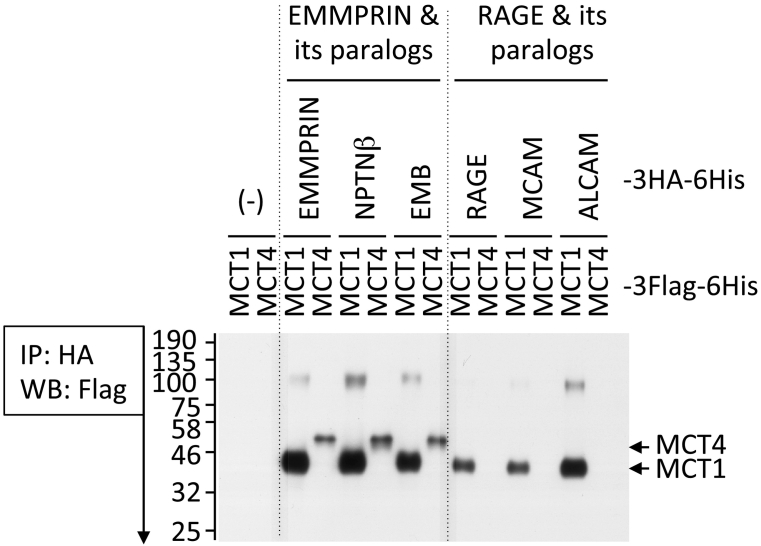
We thus hypothesized that one or more other SSSRs would also bind with MCT(s) and regulate their activity, which may contribute to cancer metastasis. To test this hypothesis, we examined the potential interaction between individual SSSRs and MCT1 or MCT4. Surprisingly, our data (Figure 5, unpubl. data) showed that EMMPRIN paralogs (NPTNα and NPTNβ) bind with both MCT1 and MCT4, as does EMMPRIN. On the other hand, the RAGE and RAGE paralogs ALCAM and MCAM can both bind with the single MCT1 in a similar manner. These results suggest that all SSSRs can control the intracellular lactate level via MCT, by which they contribute in part to cancer metastasis. These data are part of our ongoing research, and we expect that further investigations will uncover the complex cross-talk pathways with mutual signal transductions that lead to cancer metastasis.
Figure 5.
The interaction of SSSRs with MCT1 (gene name: SLC16A1) or MCT4 (gene name: SLC16A4). We inserted the cDNAs of MCT1 and MCT4 into pIDT-SMART (C-TSC) vector [81]; the cDNAs were designed for expression as C-terminal 3xFlag-6His-tagged forms. HEK292T cells were transiently transfected with the indicated combinations of the plasmid vectors (individual SSSRs-3HA-6His plasmids and MCT1-3Flag-6His or MCT4-3Flag-6His plasmid) using FuGENE-HD. The co-immunoprecipitation and Western blotting were performed by a method similar to that described in Figure 3 except for the use of mouse anti-Flag tag antibody for Western blotting.
Therapeutic Approaches to Cancer Metastasis Based on the S100A8/A9-SSSRs
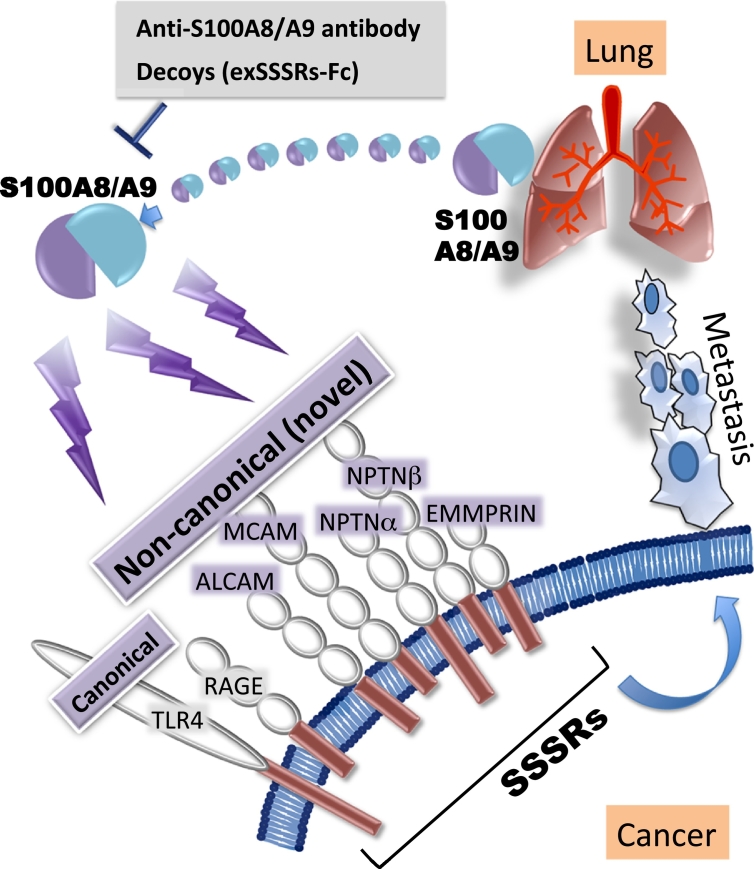
Strategies that prevent the signaling between S100A8/A9 and these receptors as described above may be useful for the suppression of ‘soil and seed’-based lung-directed cancer metastasis. This idea spurred us to develop a decoy and neutralizing antibody against extracellular S100A8/A9 (Figure 6). We then developed a therapeutic decoy [11] and S100A8/A9 antibody [12] and evaluated the suppressive effect of the antibody on lung-directed cancer metastasis.
Figure 6.
A strategy for preventing melanoma lung-tropic metastasis in response to S100A8/A9.
We prepared extracellular regions of these novel receptors and fused them to human IgG2-Fc to extend the half-life expectancy [55] (novel receptor-based decoys: exEMMPRIN-Fc, exNPTNβ-Fc, exMCAM-Fc and exALCAM-Fc; classical receptor decoy: exRAGE-Fc). Because of the much lower affinity of the IgG2 to Fc receptor compared to IgG1 [56], we used IgG2 to avoid unexpected inflammatory events that might be caused by the IgG-Fc receptor binding. Among the prepared decoys, exMCAM-Fc showed the highest activity in suppressing metastasis. The exMCAM-Fc-induced suppressive effect may be explained by the down-regulation of a series of metastatic soil-educating genes trigged by S100A8/A9 in the lungs.
To determine whether cancer-mediated gene alterations in the lungs are affected by exMCAM-Fc, we performed an RNA-seq-based comprehensive analysis of gene expressions, using mouse lung. The results showed that quite high numbers of genes were differentially expressed among the several decoys compared to the control phosphate-buffered saline (PBS). We therefore decided to focus on soluble factors such as cytokines, chemokines, growth factors and secretory enzymes, since a metastatic soil feature is critically affected by certain inflammation-related soluble factors. Notably, the genes that encoded soluble factors in reduction with exMCAM-Fc included secretory proteinases, i.e., Mmp12, Mmp13 and Pla2g2d; an interleukin, Il33; chemokines, Ccl19, Ccl1, Ccl2, Ccl5, Ccl8, Ccl12, Ccl21, Cxcl12 and Cxcl5/6; and complement factor C5. Mmp12 and Mmp13 are secretory matrix metalloproteinases that contribute to cancer invasion via a matrix collapse surrounding cancer cells, leading to cancer invasion [58,57]. In addition to its proteinase activity, Mmp13 contributes to an increase in the maturation of immune-suppressing Treg cells via the induction of TGFβ [59].
Il33 acts as a positive regulator of Treg cells at the functional side [60]. The group IID-secreted phospholipase A2 (Pla2g2d) is an immunosuppressive phospholipase A2 that is secreted via the production of free fatty acids from membrane lipids that are associated with chronic inflammation and immune suppression [61]. An immune-suppressive environment triggered by Mmp13, Il33 and Pla2g2d may thus be involved in raising the metastatic soil formation in the lungs. We also observed that the expressions of many chemokines that are highly induced by chronic inflammation were decreased by exMCAM-Fc treatment. All of the identified chemokines may play an important role in a distant cancer's attraction to the lung area in an additive or a synergistic manner. In particular, Ccl19, Ccl2, Ccl21 and Cxcl12 attracted our attention.
The expression of the C-C chemokine receptor 7 (CCR7) that binds with both Ccl19 and Ccl21 mediated the metastasis of breast cancer to the lymph nodes in mice [62]. The Cxcl12 receptor C-X-C chemokine receptor 4 (CXCR4) promotes breast cancer cell metastasis to the lung [63]. Kitamura et al. reported that a CCL2-induced chemokine cascade promotes the pulmonary metastasis of breast cancer cells [64]. Complement C5 functions as a strong chemokine to attract not only inflammatory cells but also cancer cells [65].
Regarding the chemokines mentioned above, Eisenblaetter et al. revealed an unusual role of the Ccl2 protein in the event of S100A8/A9-mediated breast cancer metastasis toward the lung [66]. They succeeded in the in vivo imaging of S100A8/A9 by using its antibody-based single photon emission computed tomography (SPECT). By using this advanced technique, Eisenblaetter et al. demonstrated that Ccl2 mediates the tumor metastatic niche formation in the lung before breast cancer travels there. Namely, breast cancer located at the mammary fat pad stimulates both the induction of Ccl2 in the remote lung and an increase in the CCR2highCX3CR1low monocyte population at the same site, where Ccl2 readily binds to its receptor CCR2 in the CCR2highCX3CR1low monocytes, resulting in a significant level of S100A8/A9 secretion from the monocytes. Interestingly, this process is associated with the increased presence of immunosuppressive Treg cells in mouse lung.
In the Eisenblaetter study, the anti-Ccl2 neutralizing antibody markedly reduced the number of CCR2highCX3CR1low monocytes, S100A8/A9 secretion, and lung-tropic breast cancer metastasis, indicating the presence of a cancer-mediated positive feed-forward circuit of Ccl2-CCR2-S100A8/A9 in the lung for creating a pre-metastatic niche there. These results may provide one reason explaining the higher suppressive effect of the exMCAM-Fc decoy on lung metastasis in the case of breast cancer cells, since exMCAM-Fc remarkably reduced the Ccl2 expression in breast cancer-bearing mouse lung. Taking these findings together, we speculate that the identified soluble factors may all play a critical role in metastatic soil formation in the lung by modulating inflammation, immune activity, and cancer chemotactic attraction with invasiveness. In addition, a series of simultaneous downregulations of all of the identified genes may reinforce the highest effect of exMCAM-Fc on the suppression of the lung-tropic metastasis of breast cancer cells.
For the production of an optimal S100A8/A9 antibody, the preparation of an optimal antigen is the most important step. To establish an inhibitory antibody to S100A8/A9, we used our original S100A8/A9 recombinant protein, which has been maintained as a 100% heterodimer with high purity, normal folding, and strong biological activity. After a multi-step screening of antibodies for the desired inhibition, we identified one antibody (clone #45) that significantly suppresses lung-directed cancer metastasis in melanoma as well as breast cancer cells. The humanized antibody of clone #45, abbreviated as chimeric-45, showed similar activity in vivo.
Questions About the Blockade Biologics Against the S100A8/A9-SSSRs Axis (SSSR blockers)
An initial question is: what is the advantage of these SSSRs' blockers in comparison to anti-CCL2 antibody in the suppressive function of lung-tropic cancer metastasis? Bonapace et al. reported that it was difficult to use an anti-CCL2 antibody as a single agent in cancer therapy [67]. Of course, the anti-CCL2 antibody highly reduced cancer metastasis in mice, but an unexpected cessation of the treatment led to enhanced metastasis and accelerated the death of the mice, since the interruption of the treatment induced an influx of monocytes into the pre-metastatic lung and an increase in IL-6 levels there, leading to a local enhancement of angiogenesis in the lung [67]. Interestingly, this series of events was overcome by using anti-IL-6 antibody in combination with the anti-CCL2 antibody.
On the other hand, we have shown that S100A8/A9 effectively induces not only CCL2 [11] but also IL-6 [12]. The use of these blockers therefore may be inhibitory to both CCL2 and IL-6 at the same time. Our ongoing research includes functional comparisons of anti-CCL2 antibody and SSSRs' blockers (especially anti-S100A8/A9 antibody and the exMCAM-Fc decoy) and evaluations of their therapeutic cessation for the prevention of metastasis.
A logical next question is whether our SSSRs' blockers own suppressive function is also exerted in other organ-tropic metastasis. To answer this question, we need to learn more about the functional importance of S100A8/A9 in organs such as the liver and brain (which are also major metastatic destinations of cancers) for their metastasis profiles. We plan to focus first on liver metastasis, since patients with melanoma also frequently suffer from liver metastasis [68,69]. In our metastatic mouse models, i.e., an experimental forced model (melanoma injection by way of the tail vein) and an autonomous model (making solid melanoma in the skin), B16-BL6 mouse melanoma cells showed mainly lung-tropic metastasis [11,12] but showed liver metastasis too, at a lower level than the lung metastasis (unpubl. data). In this setting, we observed that S100A8/A9 was also induced in the liver (unpubl. data).
In contrast, Saito et al. clearly revealed an interesting mechanism of liver-tropic metastasis [70]. They used pancreatic cancer cells in which complement component 5 alpha (C5a) — which is induced and secreted from liver hepatocytes when pancreatic cancers appear in the pancreas in a human (or in one or more organs other than the liver such as the spleen in their model mouse of liver metastasis) — attracts pancreatic cancer cells to the liver. Because C5a receptor (C5aR), an inflammatory relevant chemokine receptor, is highly stabilized on the cell membrane in podocalyxin-like 1 (PODXL1)-positive pancreatic cancer cells, the liver-mediated C5a readily stimulates remote PODXL1-positive pancreatic cancer cells through a C5a-C5aR interaction, leading to their liver-tropic cancer metastasis [70]. Interestingly, our RNA-seq data revealed that C5 mRNA-encoded C5a is highly induced by S100A8/A9 [11].
Lee et al. proposed that in patients with pancreatic or colorectal cancer, liver-mediated serum amyloid A1 (SAA1) and A2 (SAA2) play crucial roles in the formation of a pro-metastatic niche in the liver that recruits cancer cells to the liver [71]. In their report, in addition to SAA1 and 2, S100A8/A9 was also markedly elevated in the liver of mice bearing cancers that exhibited liver-tropic metastasis. Those findings suggested that S100A8/A9 contributes in part to liver-tropic cancer metastasis. S100A8/A9 is also likely to be involved in brain-tropic cancer metastasis. Liu et al. reported that S100A8/A9 was highly induced in the CD11b+Gr1+ myeloid cells that accumulated in the brain of mice bearing mouse breast cancer (4 T1) cells, and S100A8/A9 thereby actively attracted 4 T1 cells [72].
Lastly, what are the precise clinical settings of cancer metastasis for which the developed biologics are applicable? Our SSSRs' blockers exhibited a great suppressive effect on the S100A8/A9-mediated lung-tropic cancer metastasis when we used them before cancer metastasis [12]. We thus speculate that the developed biologics will be useful for cancer patients as an effective anti-metastasis precaution after the surgical resection of solid cancer, in combination with chemotherapeutic compounds. Further studies may reveal additional uses of the developed biologics metastatic patients with cancers at advanced stages.
We expect that the newly developed antibody and decoys will eventually be of great help to the creation of antidotes to life-threatening cancer metastasis — not only at the lung but also organs such as the liver and brain — when combined with surgery and chemotherapeutic anticancer drugs.
Conclusion
Researchers continue to observe that in the complex cancer metastatic processes, novel receptors appear to act in cooperation with each other with individual featured signal cascades at many specific stages, not only on the cancer side (i.e., the EMT, adhesion, invasion, migration and colonization with re-proliferation) but also on the metastatic organ side (the inflammatory immune-suppressive environment). The blockade biologics against the S100A8/A9-SSSRs axis have shown significant preventive effects on cancer metastasis. In addition, S100A8/A9 heterodimer-mediated diseases are not restricted to cancer metastasis; the S100A8/A9 protein contributes to many types of inflammation-associated diseases (e.g., autoimmune diseases including atopic dermatitis and arthritis, diabetes, and neurodegenerative diseases) [6,[73], [74], [75], [76], [77], [78], [79], [80]]. We thus believe that our prior and ongoing studies will help us gain a better understanding of the complexities of cancer metastasis and other inflammatory diseases at the molecular level, and our findings will contribute to the establishment of innovative methods for preventing cancer metastasis and other inflammatory diseases.
Acknowledgments
Acknowledgements
This research was supported in part by a grant from the JSPS KAKENHI (no. 17H03577 to M.S.) and by funds to M.S. from the Smoking Research Foundation, the Terumo Life Science Foundation, and the Takeda Science Foundation.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Contributor Information
Nahoko Tomonobu, Email: pl5i0zd8@s.okayama-u.ac.jp.
Rie Kinoshita, Email: rie-k@okayama-u.ac.jp.
Masakiyo Sakaguchi, Email: masa-s@md.okayama-u.ac.jp.
References
- 1.S. Paget. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 8 (1989) 98–101. [PubMed]
- 2.Hiratsuka S., Watanabe A., Aburatani H., Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 3.Hiratsuka S., Watanabe A., Sakurai Y., Akashi-Takamura S., Ishibashi S., Miyake K., Shibuya M., Akira S., Aburatani H., Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 4.Saha A., Lee Y.C., Zhang Z., Chandra G., Su S.B., Mukherjee A.B. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J. Biol. Chem. 2010;285:10822–10831. doi: 10.1074/jbc.M109.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hibino T., Sakaguchi M., Miyamoto S., Yamamoto M., Motoyama A., Hosoi J., Shimokata T., Ito T., Tsuboi R. NH. Huh. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2013;73:172–183. doi: 10.1158/0008-5472.CAN-11-3843. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi M., Yamamoto M., Miyai M., Maeda T., Hiruma J., Murata H., Kinoshita R., Ruma I.M.W., Putranto E.W., Inoue Y., Morizane S., Huh N.H., Tsuboi R., Hibino T. Identification of an S100A8 receptor neuroplastin-β and its heterodimer formation with EMMPRIN. J. Invest. Dermatol. 2016;136:2240–2250. doi: 10.1016/j.jid.2016.06.617. [DOI] [PubMed] [Google Scholar]
- 7.Sumardika I.W., Chen Y., Tomonobu N., Kinoshita R., Ruma I.M.W., Sato H., Kondo E., Inoue Y., Yamauchi A., Murata H., Yamamoto K.I., Tomida S., Shien K., Yamamoto H., Soh J., Futami J., Putranto E.W., Hibino T., Nishibori M., Toyooka S., Sakaguchi M. Neuroplastin-β mediates S100A8/A9-induced lung cancer disseminative progression. Mol. Carcinog. 2019;58:980–995. doi: 10.1002/mc.22987. [DOI] [PubMed] [Google Scholar]
- 8.Ruma I.M.W., Putranto E.W., Kondo E., Murata H., Watanabe M., Huang P., Kinoshita R., Futami J., Inoue Y., Yamauchi A., Sumardika I.W., Youyi C., Yamamoto K., Nasu Y., Nishibori M., Hibino T., Sakaguchi M. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-κB and ROS formation upon ligand binding. Clin. Exp. Metastasis. 2016;33:609–627. doi: 10.1007/s10585-016-9801-2. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Sumardika I.W., Tomonobu N., Ruma I.M.W., Kinoshita R., Kondo E., Inoue Y., Sato H., Yamauchi A., Murata H., Yamamoto K.I., Tomida S., Shien K., Yamamoto H., Soh J., Liu M., Futami J., Sasai K., Katayama H., Kubo M., Putranto E.W., Hibino T., Sun B., Nishibori M., Toyooka S., Sakaguchi M. Melanoma cell adhesion molecule is the driving force behind the dissemination of melanoma upon S100A8/A9 binding in the original skin lesion. Cancer Lett. 2019;452:178–190. doi: 10.1016/j.canlet.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Sumardika I.W., Tomonobu N., Kinoshita R., Inoue Y., Iioka H., Mitsui Y., Saito K., Ruma I.M.W., Sato H., Yamauchi A., Murata H., Yamamoto K.I., Tomida S., Shien K., Yamamoto H., Soh J., Futami J., Kubo M., Putranto E.W., Murakami T., Liu M., Hibino T., Nishibori M., Kondo E., Toyooka S., Sakaguchi M. Critical role of the MCAM-ETV4 axis triggered by extracellular S100A8/A9 in breast cancer aggressiveness. Neoplasia. 2019;21:627–640. doi: 10.1016/j.neo.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinoshita R., Sato H., Yamauchi A., Takahashi Y., Inoue Y., Sumardika I.W., Chen Y., Tomonobu N., Araki K., Shien K., Tomida S., Torigoe H., Namba K., Kurihara E., Ogoshi Y., Murata H., Yamamoto K.I., Futami J., Putranto E.W., Ruma I.M.W., Yamamoto H., Soh J., Hibino T., Nishibori M., Kondo E., Toyooka S., Sakaguchi M. exSSSRs (extracellular S100 soil sensor receptors)-Fc fusion proteins work as prominent decoys to S100A8/A9-induced lung tropic cancer metastasis. Int. J. Cancer. 2019;144:3138–3145. doi: 10.1002/ijc.31945. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita R., Sato H., Yamauchi A., Takahashi Y., Inoue Y., Sumardika I.W., Chen Y., Tomonobu N., Araki K., Shien K., Tomida S., Torigoe H., Namba K., Kurihara E., Ogoshi Y., Murata H., Yamamoto K.I., Futami J., Putranto E.W., Ruma I.M.W., Yamamoto H., Soh J., Hibino T., Nishibori M., Kondo E., Toyooka S., Sakaguchi M. Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int. J. Cancer. 2019;145:569–575. doi: 10.1002/ijc.31982. [DOI] [PubMed] [Google Scholar]
- 13.Vogl T., Stratis A., Wixler V., Völler T., Thurainayagam S., Jorch S.K., Zenker S., Dreiling A., Chakraborty D., Fröhling M., Paruzel P., Wehmeyer C., Hermann S., Papantonopoulou O., Geyer C., Loser K., Schäfers M., Ludwig S., Stoll M., Leanderson T., Schultze J.L., König S., Pap T., Roth J. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Invest. 2018;128:1852–1866. doi: 10.1172/JCI89867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi M., Huh N.H. S100A11, a dual growth regulator of epidermal keratinocytes. Amino Acids. 2011;41:797–807. doi: 10.1007/s00726-010-0747-4. [DOI] [PubMed] [Google Scholar]
- 15.Nukui T., Ehama R., Sakaguchi M., Sonegawa H., Katagiri C., Hibino T., Huh N.H. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J. Cell. Biochem. 2008;104:453–464. doi: 10.1002/jcb.21639. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi M., Murata H., Yamamoto K., Ono T., Sakaguchi Y., Motoyama A., Hibino T., Kataoka K., Huh N.H. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long T., Su J., Tang W., Luo Z., Liu S., Liu Z., Zhou H., Qi M., Zeng W., Zhang J., Chen X. A novel interaction between calcium-modulating cyclophilin ligand and basigin regulates calcium signaling and matrix metalloproteinase activities in human melanoma cells. Cancer Lett. 2013;339:93–101. doi: 10.1016/j.canlet.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Su J., Chen X., Kanekura T. A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett. 2009;273:140–147. doi: 10.1016/j.canlet.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Bougatef F., Quemener C., Kellouche S., Naïmi B., Podgorniak M.P., Millot G., Gabison E.E., Calvo F., Dosquet C., Lebbé C., Menashi S., Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009;114:5547–5556. doi: 10.1182/blood-2009-04-217380. [DOI] [PubMed] [Google Scholar]
- 20.Delyon J., Khayati F., Djaafri I., Podgorniak M.P., Sadoux A., Setterblad N., Boutalbi Z., Maouche K., Maskos U., Menashi S., Lebbé C., Mourah S. EMMPRIN regulates β1 integrin-mediated adhesion through Kindlin-3 in human melanoma cells. Exp. Dermatol. 2015;24:443–448. doi: 10.1111/exd.12693. [DOI] [PubMed] [Google Scholar]
- 21.Le Floch R., Chiche J., Marchiq I., Naiken T., Ilc K., Murray C.M., Critchlow S.E., Roux D., Simon M.P., Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beesley P.W., Herrera-Molina R., Smalla K.H., Seidenbecher C. The neuroplastin adhesion molecules: key regulators of neuronal plasticity and synaptic function. J. Neurochem. 2014;131:268–283. doi: 10.1111/jnc.12816. [DOI] [PubMed] [Google Scholar]
- 23.Herrera-Molina R., Mlinac-Jerkovic K., Ilic K., Stöber F., Vemula S.K., Sandoval M., Milosevic N.J., Simic G., Smalla K.H., Goldschmidt J., Bognar S.K., Montag D. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regulation: Implication for cognitive deterioration. Sci. Rep. 2017;7:7273. doi: 10.1038/s41598-017-07839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruma I.M.W., Kinoshita R., Tomonobu N., Inoue Y., Kondo E., Yamauchi A., Sato H., Sumardika I.W., Chen Y., Yamamoto K.I., Murata H., Toyooka S., Nishibori M., Sakaguchi M. Embigin promotes prostate cancer progression by S100A4-dependent and-independent mechanisms. Cancers (Basel) 2018;10 doi: 10.3390/cancers10070239. pii: E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung D.E., Kim J.M., Kim C., Song S.Y. Embigin is overexpressed in pancreatic ductal adenocarcinoma and regulates cell motility through epithelial to mesenchymal transition via the TGF-β pathway. Mol. Carcinog. 2016;55:633–645. doi: 10.1002/mc.22309. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann J.M., Riethmüller G., Johnson J.P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc. Natl. Acad. Sci. U. S. A. 1989;86:9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapanotti M.C., Bianchi L., Ricozzi I., Campione E., Pierantozzi A., Orlandi A., Chimenti S., Federici G., Bernardini S. Melanoma-associated markers expression in blood: MUC-18 is associated with advanced stages in melanoma patients. Br. J. Dermatol. 2009;160:338–344. doi: 10.1111/j.1365-2133.2008.08929.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnson J.P. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1999;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- 29.McGary E.C., Lev D.C., Bar-Eli M. Cellular adhesion pathways and metastatic potential of human melanoma. Cancer Biol. Ther. 2002;1:459–465. doi: 10.4161/cbt.1.5.158. [DOI] [PubMed] [Google Scholar]
- 30.Melnikova V.O., Bar-Eli M. Bioimmunotherapy for melanoma using fully human antibodies targeting MCAM/MUC18 and IL-8. Pigment Cell Res. 2006;19:395–405. doi: 10.1111/j.1600-0749.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- 31.Yawata T., Higashi Y., Kawanishi Y., Nakajo T., Fukui N., Fukuda H., Ueba T. CD146 is highly expressed in glioma stem cells and acts as a cell cycle regulator. J. Neuro-Oncol. 2019;144:21–32. doi: 10.1007/s11060-019-03200-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Tang X., Weng W., Qiao Y., Lin J., Liu W., Liu R., Ma L., Yu W., Yu Y., Pan Q., Sun F. The membrane protein melanoma cell adhesion molecule (MCAM) is a novel tumor marker that stimulates tumorigenesis in hepatocellular carcinoma. Oncogene. 2015;34:5781–5795. doi: 10.1038/onc.2015.36. [DOI] [PubMed] [Google Scholar]
- 33.Jiang G., Zhang L., Zhu Q., Bai D., Zhang C., Wang X. CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2016;35:38. doi: 10.1186/s13046-016-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearl R.A., Pacifico M.D., Richman P.I., Wilson G.D., Grover R. Stratification of patients by melanoma cell adhesion molecule (MCAM) expression on the basis of risk: Implications for sentinel lymph node biopsy. J. Plast. Reconstr. Aesthet. Surg. 2008;61:265–271. doi: 10.1016/j.bjps.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Sumardika I.W., Youyi C., Kondo E., Inoue Y., Ruma I.M.W., Murata H., Kinoshita R., Yamamoto K.I., Tomida S., Shien K., Sato H., Yamauchi A., Futami J., Putranto E.W., Hibino T., Toyooka S., Nishibori M., Sakaguchi M. β-1,3-Galactosyl-O-glycosyl-glycoprotein β-1,6-N-acetylglucosaminyltransferase 3 increases MCAM stability, which enhances S100A8/A9-mediated cancer motility. Oncol. Res. 2018;26:431–444. doi: 10.3727/096504017X15031557924123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ihnen M., Müller V., Wirtz R.M., Schröder C., Krenkel S., Witzel I., Lisboa B.W., Jänicke F., Milde-Langosch K. Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res. Treat. 2008;112:419–427. doi: 10.1007/s10549-007-9879-y. [DOI] [PubMed] [Google Scholar]
- 37.Kahlert C., Weber H., Mogler C., Bergmann F., Schirmacher P., Kenngott H.G., Matterne U., Mollberg N., Rahbari N.N., Hinz U., Koch M., Aigner M., Weitz J. Increased expression of ALCAM/CD166 in pancreatic cancer is an independent prognostic marker for poor survival and early tumour relapse. Br. J. Cancer. 2009;101:457–464. doi: 10.1038/sj.bjc.6605136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kristiansen G., Pilarsky C., Wissmann C., Stephan C., Weissbach L., Loy V., Loening S., Dietel M., Rosenthal A. ALCAM/CD166 is up-regulated in low-grade prostate cancer and progressively lost in high-grade lesions. Prostate. 2003;54:34–43. doi: 10.1002/pros.10161. [DOI] [PubMed] [Google Scholar]
- 39.Hansen A.G., Arnold S.A., Jiang M., Palmer T.D., Ketova T., Merkel A., Pickup M., Samaras S., Shyr Y., Moses H.L., Hayward S.W., Sterling J.A., Zijlstra A. ALCAM/CD166 is a TGFβ responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res. 2014;74:1404–1415. doi: 10.1158/0008-5472.CAN-13-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H.G., Xie D.R., Shen X.M., Li H.H., Zeng H., Zeng Y.J. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J. Gastroenterol. 2005;11:1445–1451. doi: 10.3748/wjg.v11.i10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z., Zhang Y., Chen R., Luo D., Chen Z.N. Clinical impact and prognostic value of CD147 and MMP-7 expression in patients with pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2016;9:9175–9183. [Google Scholar]
- 42.Kanekura T., Chen X., Kanazaki T. BASIGIN (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int. J. Cancer. 2002;99:520–528. doi: 10.1002/ijc.10390. [DOI] [PubMed] [Google Scholar]
- 43.Caudron A., Battistella M., Feugeas J.P., Pages C., Basset-Seguin N., Dorval S.M., Brentano E.F., Sadoux A., Podgorniak M.P., Menashi S., Janin A., Lebbe C., Mourah S. EMMPRIN/CD147 is an independent prognostic biomarker in cutaneous melanoma. Exp. Dermatol. 2016;25:618–622. doi: 10.1111/exd.13022. [DOI] [PubMed] [Google Scholar]
- 44.Saltari Y.A., Truzzi F., Quadri M., Lotti R., Palazzo E., Grisendi G., Tiso N., Marconi A., Pincelli C. CD271 down-regulation promotes melanoma progression and invasion in three-dimensional models and in zebrafish. J. Invest. Dermatol. 2016;136:2049–2058. doi: 10.1016/j.jid.2016.05.116. [DOI] [PubMed] [Google Scholar]
- 45.Mitsui Y., Tomonobu N., Watanabe M., Kinoshita R., Sumardika I.W., Youyi C., Murata H., Yamamoto K.I., Sadahira T., Rodrigo A.G.H., Takamatsu H., Araki K., Yamauchi A., Yamamura M., Fujiwara H., Inoue Y., Futami J., Saito K., Iioka H., Kondo E., Nishibori M., Toyooka S., Yamamoto Y., Nasu Y., Sakaguchi M. Upregulation of mobility in pancreatic cancer cells by secreted S100A11 through activation of surrounding fibroblasts. Oncol. Res. 2019;27:945–956. doi: 10.3727/096504019X15555408784978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tasdogan A., Faubert B., Ramesh V., Ubellacker J.M., Shen B., Solmonson A., Murphy M.M., Gu Z., Gu W., Martin M., Kasitinon S.Y., Vandergriff T., Mathews T.P., Zhao Z., Schadendorf D., DeBerardinis R.J., Morrison S.J. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature. 2020;577:115–120. doi: 10.1038/s41586-019-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirk P., Wilson M.C., Heddle C., Brown M.H., Barclay A.N., Halestrap A.P. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–3904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slomiany M.G., Grass G.D., Robertson A.D., Yang X.Y., Maria B.L., Beeson C., Toole B.P. Hyaluronan, CD44, and EMMPRIN regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009;69:1293–1301. doi: 10.1158/0008-5472.CAN-08-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneiderhan W., Scheler M., Holzmann K.H., Marx M., Gschwend J.E., Bucholz M., Gress T.M., Seufferlein T., Adler G., Oswald F. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58:1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- 50.Chen H., Wang L., Beretov J., Hao J., Xiao W., Li Y. Co-expression of CD147/EMMPRIN with monocarboxylate transporters and multiple drug resistance proteins is associated with epithelial ovarian cancer progression. Clin. Exp. Metastasis. 2010;27:557–569. doi: 10.1007/s10585-010-9345-9. [DOI] [PubMed] [Google Scholar]
- 51.Le Floch R., Chiche J., Marchiq I., Naiken T., Ilc K., Murray C.M., Critchlow S.E., Roux D., Simon M.P., Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Afonso J., Santos L.L., Miranda-Gonçalves V., Morais A., Amaro T., Longatto-Filho A., Baltazar F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol. Carcinog. 2015;54:1451–1466. doi: 10.1002/mc.22222. [DOI] [PubMed] [Google Scholar]
- 53.Marchiq I., Albrengues J., Granja S., Gaggioli C., Pouysségur J., Simon M.P. Knock out of the BASIGIN/CD147 chaperone of lactate/H+ symporters disproves its pro-tumour action via extracellular matrix metalloproteases (MMPs) induction. Oncotarget. 2015;6:24636–24648. doi: 10.18632/oncotarget.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eichner R., Heider M., Fernández-Sáiz V., van Bebber F., Garz A.K., Lemeer S., Rudelius M., Targosz B.S., Jacobs L., Knorn A.M., Slawska J., Platzbecker U., Germing U., Langer C., Knop S., Einsele H., Peschel C., Haass C., Keller U., Schmid B., Götze K.S., Kuster B., Bassermann F. Immunomodulatory drugs disrupt the cereblon-CD147-MCT1 axis to exert antitumor activity and teratogenicity. Nat. Med. 2016;22:735–743. doi: 10.1038/nm.4128. [DOI] [PubMed] [Google Scholar]
- 55.Vidarsson U.G., Dekkers G., Rispens T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang T.X.R., Song A., Bergelson S., Arroll T., Parekh B., May K., Chung S., Strouse R., Mire-Sluis A., Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011;10:101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 57.Lv F.Z., Wang J.L., Wu Y., Chen H.F., Shen X.Y. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int. J. Immunopathol. Pharmacol. 2015;28:77–84. doi: 10.1177/0394632015572557. [DOI] [PubMed] [Google Scholar]
- 58.Datar I., Feng J., Qiu X., Lewandowski J., Yeung M., Ren G., Aras S., Al-Mulla F., Cui H., Trumbly R., Arudra S.K., De Las Casas L.E., de la Serna I., Bitar M.S., Yeung K.C. RKIP inhibits local breast cancer invasion by antagonizing the transcriptional activation of MMP13. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu M., Feng L., Gu J., Xia Z., Zhang H., Zheng S., Duan Z., Hu R., Wang J., Shi W., Ji C., Shen Y., Chen G., Zheng S.G., Han Y.P. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-β is critical for immune homeostasis following acute liver injury. J. Mol. Cell Biol. 2013;5:369–379. doi: 10.1093/jmcb/mjt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schiering C., Krausgruber T., Chomka A., Fröhlich A., Adelmann K., Wohlfert E.A., Pott J., Griseri T., Bollrath J., Hegazy A.N., Harrison O.J., Owens B.M.J., Löhning M., Belkaid Y., Fallon P.G., Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miki Y., Kidoguchi Y., Sato M., Taketomi Y., Taya C., Muramatsu K., Gelb M.H., Yamamoto K., Murakami M. Dual roles of Group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 2016;291:15588–15601. doi: 10.1074/jbc.M116.734624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cunningham H.D., Shannon L.A., Calloway P.A., Fassold B.C., Dunwiddie I., Vielhauer G., Zhang M., Vines C.M. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl. Oncol. 2010;3:354–361. doi: 10.1593/tlo.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee D., Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am. J. Cancer Res. 2013;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- 64.Kitamura T., Qian B.Z., Soong D., Cassetta L., Noy R., Sugano G., Kato Y., Li J., Pollard J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015;212:1043–1059. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Darling V.R., Hauke R.J., Tarantolo S., Agrawal D.K. Immunological effects and therapeutic role of C5a in cancer. Expert. Rev. Clin. Immunol. 2015;11:255–263. doi: 10.1586/1744666X.2015.983081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisenblaetter M., Flores-Borja F., Lee J.J., Wefers C., Smith H., Hueting R., Cooper M.S., Blower P.J., Patel D., Rodriguez-Justo M., Milewicz H., Vogl T., Roth J., Tutt A., Schaeffter T., Ng T. Visualization of tumor-immune interaction – Target-specific imaging of S100A8/A9 reveals pre-metastatic niche establishment. Theranostics. 2017;7:2392–2401. doi: 10.7150/thno.17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonapace L., Coissieux M.M., Wyckoff J., Mertz K.D., Varga Z., Junt T., Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 68.J.K. Patel, M.S. Didolkar, J.W.P ickren, R.H. Moore. Metastatic pattern of malignant melanoma: A study of 216 autopsy cases. Am. J. Surg. 135 (1978) 807–810. [DOI] [PubMed]
- 69.S. C.M. Balch, S.J Soong, T.M. Murad, J.W. Smith, W.A. Maddox, J.R. Durant. A multifactorial analysis of melanoma. IV. Prognostic factors in 200 melanoma patients with distant metastases (stage III). J. Clin. Oncol. 1 (1983) 126–134. [DOI] [PubMed]
- 70.Saito K., Iioka H., Maruyama S., Sumardika I.W., Sakaguchi M., Kondo E. PODXL1 promotes metastasis of the pancreatic ductal adenocarcinoma by activating the C5aR/C5a axis from the tumor microenvironment. Neoplasia. 2019;21:1121–1132. doi: 10.1016/j.neo.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee V.J.W., Stone M.L., Porrett P.M., Thomas S.K., Komar C.A., Li J.H., Delman D., Graham K., Gladney W.L., Hua X., Black T.A., Chien A.L., Majmundar K.S., Thompson J.C., Yee S.S., O'Hara M.H., Aggarwal C., Xin D., Shaked A., Gao M., Liu D., Borad M.J., Ramanathan R.K., Carpenter E.L., Ji A., de Beer M.C., de Beer F.C., Webb N.R., Beatty G.L. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature. 2019;567:249–252. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu W.Y., Kosaka A., Ikeura M., Kohanbash G., Fellows-Mayle W., Snyder L.A., Okada H. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro-Oncology. 2013;15:891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogl T., Stratis A., Wixler V., Völler T., Thurainayagam S., Jorch S.K., Zenker S., Dreiling A., Chakraborty D., Fröhling M., Paruzel P., Wehmeyer C., Hermann S., Papantonopoulou O., Geyer C., Loser K., Schäfers M., Ludwig S., Stoll M., Leanderson T., Schultze J.L., König S., Pap T., Roth J. S100 proteins in rheumatic diseases. J. Clin. Invest. 2018;128:1852–1866. doi: 10.1172/JCI89867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Austermann J., Spiekermann C., Roth J. S100 proteins in rheumatic diseases. Nat. Rev. Rheumatol. 2018;14:528–541. doi: 10.1038/s41584-018-0058-9. [DOI] [PubMed] [Google Scholar]
- 75.Marinković G., Grauen Larsen H., Yndigegn T., Szabo I.A., Mares R.G., de Camp L., Weiland M., Tomas L., Goncalves I., Nilsson J., Jovinge S., Schiopu A. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur. Heart J. 2019;40:2713–2723. doi: 10.1093/eurheartj/ehz461. [DOI] [PubMed] [Google Scholar]
- 76.Frangogiannis N.G. S100A8/A9 as a therapeutic target in myocardial infarction: Cellular mechanisms, molecular interactions, and translational challenges. Eur. Heart J. 2019;40:2724–2726. doi: 10.1093/eurheartj/ehz524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.A. Tammaro, S. Florquin, M. Brok, N. Claessen, L.M. Butter, G.J.D. Teske, T. de Boer OJ, Vogl, J.C. Leemans, M.C. Dessing. S100A8/A9 promotes parenchymal damage and renal fibrosis in obstructive nephropathy. Clin. Exp. Immunol. 193 (2018) 361–375. [DOI] [PMC free article] [PubMed]
- 78.Kraakman M.J., Lee M.K., Al-Sharea A., Dragoljevic D., Barrett T.J., Montenont E., Basu D., Heywood S., Kammoun H.L., Flynn M., Whillas A., Hanssen N.M., Febbraio M.A., Westein E., Fisher E.A., Chin-Dusting J., Cooper M.E., Berger J.S., Goldberg I.J., Nagareddy P.R., Murphy A.J. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J. Clin. Invest. 2017;127:2133–2147. doi: 10.1172/JCI92450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vogl T., Gharibyan A.L., Morozova-Roche L.A. Pro-inflammatory S100A8 and S100A9 proteins: Self-assembly into multifunctional native and amyloid complexes. Int. J. Mol. Sci. 2012;13:2893–2917. doi: 10.3390/ijms13032893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hagmeyer S., Romão M.A., Cristóvão J.S., Vilella A., Zoli M., Gomes C.M., Grabrucker A.M. Distribution and relative abundance of S100 proteins in the brain of the APP23 Alzheimer's disease model mice. Front. Neurosci. 2012;13:2893–2917. doi: 10.3389/fnins.2019.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sakaguchi M., Watanabe M., Kinoshita R., Kaku H., Ueki H., Futami J., Murata H., Inoue Y., Li S.A., Huang P., Putranto E.W., Ruma I.M.W., Nasu Y., Kumon H., Huh N.H. Dramatic increase in expression of a transgene by insertion of promoters downstream of the cargo gene. Mol. Biotechnol. 2014;56:621–630. doi: 10.1007/s12033-014-9738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]