Abstract
Background
Recruiting participants to trials can be extremely difficult. Identifying strategies that improve trial recruitment would benefit both trialists and health research.
Objectives
To quantify the effects of strategies for improving recruitment of participants to randomised trials. A secondary objective is to assess the evidence for the effect of the research setting (e.g. primary care versus secondary care) on recruitment.
Search methods
We searched the Cochrane Methodology Review Group Specialised Register (CMR) in the Cochrane Library (July 2012, searched 11 February 2015); MEDLINE and MEDLINE In Process (OVID) (1946 to 10 February 2015); Embase (OVID) (1996 to 2015 Week 06); Science Citation Index & Social Science Citation Index (ISI) (2009 to 11 February 2015) and ERIC (EBSCO) (2009 to 11 February 2015).
Selection criteria
Randomised and quasi‐randomised trials of methods to increase recruitment to randomised trials. This includes non‐healthcare studies and studies recruiting to hypothetical trials. We excluded studies aiming to increase response rates to questionnaires or trial retention and those evaluating incentives and disincentives for clinicians to recruit participants.
Data collection and analysis
We extracted data on: the method evaluated; country in which the study was carried out; nature of the population; nature of the study setting; nature of the study to be recruited into; randomisation or quasi‐randomisation method; and numbers and proportions in each intervention group. We used a risk difference to estimate the absolute improvement and the 95% confidence interval (CI) to describe the effect in individual trials. We assessed heterogeneity between trial results. We used GRADE to judge the certainty we had in the evidence coming from each comparison.
Main results
We identified 68 eligible trials (24 new to this update) with more than 74,000 participants. There were 63 studies involving interventions aimed directly at trial participants, while five evaluated interventions aimed at people recruiting participants. All studies were in health care.
We found 72 comparisons, but just three are supported by high‐certainty evidence according to GRADE.
1. Open trials rather than blinded, placebo trials. The absolute improvement was 10% (95% CI 7% to 13%).
2. Telephone reminders to people who do not respond to a postal invitation. The absolute improvement was 6% (95% CI 3% to 9%). This result applies to trials that have low underlying recruitment. We are less certain for trials that start out with moderately good recruitment (i.e. over 10%).
3. Using a particular, bespoke, user‐testing approach to develop participant information leaflets. This method involved spending a lot of time working with the target population for recruitment to decide on the content, format and appearance of the participant information leaflet. This made little or no difference to recruitment: absolute improvement was 1% (95% CI −1% to 3%).
We had moderate‐certainty evidence for eight other comparisons; our confidence was reduced for most of these because the results came from a single study. Three of the methods were changes to trial management, three were changes to how potential participants received information, one was aimed at recruiters, and the last was a test of financial incentives. All of these comparisons would benefit from other researchers replicating the evaluation. There were no evaluations in paediatric trials.
We had much less confidence in the other 61 comparisons because the studies had design flaws, were single studies, had very uncertain results or were hypothetical (mock) trials rather than real ones.
Authors' conclusions
The literature on interventions to improve recruitment to trials has plenty of variety but little depth. Only 3 of 72 comparisons are supported by high‐certainty evidence according to GRADE: having an open trial and using telephone reminders to non‐responders to postal interventions both increase recruitment; a specialised way of developing participant information leaflets had little or no effect. The methodology research community should improve the evidence base by replicating evaluations of existing strategies, rather than developing and testing new ones.
Plain language summary
What improves trial recruitment?
Key messages
We had high‐certainty evidence for three methods to improve recruitment, two of which are effective:
1. Telling people what they are receiving in the trial rather than not telling them improves recruitment.
2. Phoning people who do not respond to a postal invitation is also effective (although we are not certain this works as well in all trials).
3. Using a tailored, user‐testing approach to develop participant information leaflets makes little or no difference to recruitment.
Of the 72 strategies tested, only 7 involved more than one study. We need more studies to understand whether they work or not.
Our question
We reviewed the evidence about the effect of things trial teams do to try and improve recruitment to their trials. We found 68 studies involving more than 74,000 people.
Background
Finding participants for trials can be difficult, and trial teams try many things to improve recruitment. It is important to know whether these actually work. Our review looked for studies that examined this question using chance to allocate people to different recruitment strategies because this is the fairest way of seeing if one approach is better than another.
Key results
We found 68 studies including 72 comparisons. We have high certainty in what we found for only three of these.
1. Telling people what they are receiving in the trial rather than not telling them improves recruitment. Our best estimate is that if 100 people were told what they were receiving in a randomised trial, and 100 people were not, 10 more would take part n the group who knew. There is some uncertainty though: it could be as few as 7 more per hundred, or as many as 13 more.
2. Phoning people who do not respond to a postal invitation to take part is also effective. Our best estimate is that if investigators called 100 people who did not respond to a postal invitation, and did not call 100 others, 6 more would take part in the trial among the group who received a call. However, this number could be as few as 3 more per hundred, or as many as 9 more.
3. Using a tailored, user‐testing approach to develop participant information leaflets did not make much difference. The researchers who tested this method spent a lot of time working with people like those to be recruited to decide what should be in the participant information leaflet and what it should look like. Our best estimate is that if 100 people got the new leaflet, 1 more would take part in the trial compared to 100 who got the old leaflet. However, there is some uncertainty, and it could be 1 fewer (i.e. worse than the old leaflet) per hundred, or as many as 3 more.
We had moderate certainty in what we found for eight other comparisons; our confidence was reduced for most of these because the method had been tested in only one study. We had much less confidence in the other 61 comparisons because the studies had design flaws, were the only studies to look at a particular method, had a very uncertain result or were mock trials rather than real ones.
Study characteristics
The 68 included studies covered a very wide range of disease areas, including antenatal care, cancer, home safety, hypertension, podiatry, smoking cessation and surgery. Primary, secondary and community care were included. The size of the studies ranged from 15 to 14,467 participants. Studies came from 12 countries; there was also one multinational study involving 19 countries. The USA and UK dominated with 25 and 22 studies, respectively. The next largest contribution came from Australia with eight studies.
The small print
Our search updated our 2010 review and is current to February 2015. We also identified six studies published after 2015 outside the search. The review includes 24 mock trials where the researchers asked people about whether they would take part in an imaginary trial. We have not presented or discussed their results because it is hard to see how the findings relate to real trial decisions.
Summary of findings
Summary of findings for the main comparison. Open trial versus blinded trial.
| Open RCT versus blinded RCT | |||||
| Patient or population: individuals eligible for a trial Settings: any Intervention: open trial Comparison: blinded, placebo trial | |||||
| Outcomes | Illustrative effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with blinded trial | Effect with open trial | ||||
| Number recruited | As measureda | RR 1.25 (1.18 to 1.34) | 4833 (2 studies) | ⊕⊕⊕⊕ High | |
| 41 per 100 | 50 per 100 (51 to 55) | ||||
| Lowb | |||||
| 10 per 100 | 13 per 100 (12 to 13) | ||||
| Moderateb | |||||
| 30 per 100 | 38 per 100 (35 to 40) | ||||
| Highb | |||||
| 50 per 100 | 63 per 100 (59 to 67) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect for the open trial (and its 95% confidence interval) is based on the assumed risk in the the comparison group (blinded trial) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment.
Summary of findings 2. Telephone reminder versus no telephone reminder.
| Telephone reminder versus no telephone reminder | ||||||
| Patient or population: individuals eligible for a trial Settings: any Intervention: telephone reminder Comparison: no telephone reminder | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Effect with no telephone reminder | Effect with telephone reminder | |||||
| Number recruited | As measureda | RR 1.90 (1.35 to 2.67) | 978 (2 studies) | ⊕⊕⊕⊕ Highc | Both included studies had very low baseline recruitment of < 10%. | |
| 6 per 100 | 11 per 100 (8 to 16) |
|||||
| Lowb | ||||||
| 10 per 100 | 19 per 100 (14 to 27) | |||||
| Moderateb | ||||||
| 30 per 100 | 57 per 100 (41 to 80) | |||||
| Highb | ||||||
| 50 per 100 | 95 per 100 (68 to 100) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with the telephone reminder (and its 95% confidence interval) is based on the assumed risk in the comparison group (no reminder) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment.. cThe evidence for this intervention comes entirely from trials with low (< 10%) underlying recruitment. When applied to trials with higher recruitment we would downgrade the assessment of certainty to moderate due to indirectness.
Summary of findings 3. Bespoke, user‐tested participant information leaflet (PIL) vs usual PIL.
| Bespoke user‐tested participant information leaflet (PIL) vs usual PIL | |||||
| Patient or population: individuals eligible for trial Settings: any Intervention: bespoke, user‐tested PIL Comparison: usual PIL | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with usual PIL | Effect with bespoke user‐tested PIL | ||||
| Willingness to participate/number recruited | As measureda | RR 1.15 (0.92 to 1.44) | 6634 (3 studies) | ⊕⊕⊕⊕ High | |
| 5 per 100 |
6 per 100 (5 to 7) |
||||
| Lowb | |||||
| 10 per 100 | 12 per 100 (9 to 14) | ||||
| Moderateb | |||||
| 30 per 100 | 35 per 100 (28 to 43) | ||||
| Highb | |||||
| 50 per 100 | 58 per 100 (46 to 72) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with the bespoke user‐tested PIL (and its 95% confidence interval) is based on the assumed risk in the comparison group (usual PIL) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment..
Summary of findings 4. Brief participant information leaflet (PIL) vs usual PIL.
| Brief participant information leaflet (PIL) vs usual PIL | |||||
| Patient or population: individuals eligible for a trial Settings: any Intervention: brief PIL Comparison: usual PIL | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with usual PIL | Effect with brief PIL | ||||
| Number recruited | As measureda | RR 1.00 (0.93 to 1.07) | 4633 (2 studies) | ⊕⊕⊕⊝ Moderatec | |
| 33 per 100 |
33 per 100 (31 to 35) |
||||
| Lowb | |||||
| 10 per 100 | 10 per 100 (9 to 11) | ||||
| Moderateb | |||||
| 30 per 100 | 30 per 100 (28 to 32) | ||||
| Highb | |||||
| 50 per 100 | 50 per 100 (47 to 54) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with the brief PIL (and its 95% confidence interval) is based on the assumed risk in the comparison group (usual PIL) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment. cWe downgraded the certainty by 1 level because of indirectness: Chen 2011 actually measures entry to pre‐randomisation phase, not recruitment.
Summary of findings 5. Participant information leaflet (PIL) developed with feedback from users vs usual PIL.
| Participant information leaflet (PIL) developed with feedback from users vs usual PIL | |||||
| Patient or population: individuals eligible for a trial Settings: any Intervention: PIL developed with feedback from users Comparison: usual PIL | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with usual PIL | Effect with PIL developed with feedback from users | ||||
| Number recruited | As measureda | RR 1.09 (0.96 to 1.25) | 16763 (2 studies) | ⊕⊕⊕⊝ Moderatec | |
| 5 per 100 |
5 per 100 (5 to 6) |
||||
| Lowb | |||||
| 10 per 100 | 11 per 100 (10 to 13) | ||||
| Moderateb | |||||
| 30 per 100 | 33 per 100 (29 to 38) | ||||
| Highb | |||||
| 50 per 100 | 55 per 100 (48 to 63) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with a PIL developed with feedback from users (and its 95% confidence interval) is based on the assumed risk in the comparison group (usual PIL) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment. cWe downgraded evidence by 1 level because of indirectness: Chen 2011 actually measures entry to pre‐randomisation phase, not recruitment.
Summary of findings 6. Providing information by video versus by standard means alone.
| Video information versus standard information alone | |||||
| Patient or population: individuals eligible for trial Settings: any Intervention: video information Comparison: standard information (mixed but not including video) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with standard information | Effect with video information | ||||
| Number recruited | As measureda | RR 1.08 (0.89 to 1.31) | 4695 (3 studies) | ⊕⊝⊝⊝ Very lowc, d, e | |
| 33 per 100 |
36 per 100 (29 to 43) |
||||
| Lowb | |||||
| 10 per 100 | 11 per 100 (9 to 13) | ||||
| Moderateb | |||||
| 30 per 100 | 32 per 100 (27 to 39) | ||||
| Highb | |||||
| 50 per 100 | 54 per 100 (45 to 66) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with the video information (and its 95% confidence interval) is based on the assumed risk in the comparison group (standard information) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. bWe selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment. cWe downgraded by 1 level because of study limitations: both Du 2008 and Du 2009 were at unclear risk of bias. dWe downgraded 1 level because of inconsistency. All 3 studies suggest little or no difference in recruitment due to the intervention but the Hutchison 2007 point estimate was in favour of control, while that of Du 2008 and Du 2009 studies was in favour of the intervention. eWe downgraded 1 level because of imprecision and wide CIs.
Summary of findings 7. Financial incentive vs no incentive.
| Financial incentive vs no incentive | |||||
| Patient or population: individuals eligible for a trial Settings: any Intervention: financial incentive Comparison: no incentive | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Effect with no incentive | Effect with financial incentive | ||||
| Number recruited | As measureda | RR 1.48 (0.85 to 2.58) | 1506 (6 studies) | ⊕⊕⊕⊝ Moderatec | |
| 9 per 100 |
13 per 100 (8 to 23) |
||||
| Lowb | |||||
| 10 per 100 | 15 per 100 (9 to 26) | ||||
| Moderateb | |||||
| 30 per 100 | 44 per 100 (26 to 77) | ||||
| Highb | |||||
| 50 per 100 | 74 per 100 (43 to 100) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The effect with a financial incentive (and its 95% confidence interval) is based on the assumed risk in the comparison group (no incentive) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aThis is the baseline recruitment measured in the studies presented in the 'Summary of findings' table. b We selected the low, moderate and high illustrative recruitment levels of 10%, 30% and 50% based on our prior experience with trial recruitment. cWe downgraded 1 level for inconsistency. There was substantial heterogeneity, I2 = 65%.
Background
All randomised trials need to recruit participants, but this is often a challenge. Poor recruitment can lead to an underpowered study, which may report clinically relevant effects as statistically non‐significant. A non‐significant finding increases the risk that an effective intervention will be abandoned before its true value is established, or that there will be a delay in demonstrating this value while more trials or meta‐analyses are done. Underpowered trials also raise an ethical problem: trialists have exposed participants to an intervention with uncertain benefit but may still be unable to determine whether the intervention does more good than harm on completion. Poor recruitment can also lead to the extension of the trial, increasing costs.
Although investigations differ in their estimates of how many studies achieve their recruitment targets, the proportion is likely to be less than half (Charlson 1984; Foy 2003; Haidich 2001; McDonald 2006; Sully 2013). For example, McDonald 2006 found that only 38 (31%) of 114 trials achieved their original recruitment target, and 65 (53%) were extended. More recent replications of this work by Sully 2013 and Walters 2017 found that the number of trials meeting recruitment targets had increased to around 50%. In Sully 2013, the overall start to recruitment was delayed in 47 (41%) trials and early recruitment problems occurred in 77 (63%). The costs of poor recruitment can be huge (Kitterman 2011).
Trialists use many interventions to improve recruitment (see for example Caldwell 2010, Watson 2006 and Prescott 1999), but it is generally difficult to predict their effect.
This review updates our previous reviews (Treweek 2010; Treweek 2013). In addition to updating the search, we have made some important changes that affect how studies are selected for presentation in the Results and Discussion sections; essentially we neither present nor discuss studies that we consider are at high risk of bias unless it was possible to include them in a meta‐analysis.
Objectives
To quantify the effects of strategies for improving recruitment of participants to randomised trials. A secondary objective is to assess the evidence for the effect of the research setting (e.g. primary care versus secondary care) on recruitment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials of interventions to improve recruitment of participants to randomised trials.
Types of data
Randomised and quasi‐randomised trials of recruitment strategies set in the context of trials but not limited to health care; interventions that work in other fields (e.g. education, housing) could be applicable to healthcare settings. Strategies both within real settings and in hypothetical trials (studies that ask potential participants whether they would take part in a trial if it was run but the trial does not actually exist) are eligible for this version of the review.
However, in future versions of this review we will exclude hypothetical trials since we consider their design to confer a high risk of bias because the recruitment decision is not a real one; many also have other methodological problems. The three main reasons for excluding these trials in future versions of the review are as follows.
The relevance of the results of hypothetical trials will always be in doubt because of uncertainty as to how people would have reacted had the decision to take part in a trial been real rather than hypothetical.
It is possible to study recruitment interventions in real trials, avoiding the above problem.
Now that the number of evaluations in real trials has increased, we do not think the trade‐off between value added and work involved to include hypothetical trials is worthwhile for future versions of this review.
We excluded research into ways to improve questionnaire response and research looking at incentives and disincentives for clinicians to recruit participants to trials, as complementary Cochrane Methodology Reviews address these issues (Edwards 2009; Rendell 2007; Preston 2016). We also excluded studies of retention strategies, as a Cochrane Methodology Review on strategies to reduce attrition from trials already exists (Brueton 2013).
Types of methods
Any intervention that aimed to improve recruitment of participants to a randomised trial. The interventions being studied could be directed at potential participants (e.g. patients being randomised to a trial), collaborators (e.g. clinicians recruiting patients for a trial), or others (e.g. research ethics committees). Examples of such interventions are signed letters introducing the trial from influential people, alternative methods of providing information about the trial to potential participants, presenting ethics committees with (and getting approval for) a ranked list of recruitment strategies that might be used depending how recruitment goes so as to avoid delays before trials teams can implement additional recruitment strategies, additional training for collaborators, financial incentives for participants, telephone follow‐up of expressions of interest and modifications to the design of the trial (e.g. using a preference design).
Types of outcome measures
Primary outcomes
Proportion of eligible individuals or centres recruited.
Secondary outcomes
None.
Note: the lack of any secondary outcomes is a change from the previous version of the review, which gave 'Rate at which participants were recruited' as a secondary outcome. We have removed this because rate is rarely reported. We will continue to report rate of recruitment if the primary outcome is not available but will no longer consider it as a secondary outcome. We will reconsider this decision in future versions of this review.
Search methods for identification of studies
We searched the following electronic databases without language restriction for eligible studies.
The Cochrane Methodology Review Group Specialised Register (CMR) in the Cochrane Library (July 2012; searched 11 February 2015).
MEDLINE and MEDLINE In Process (OVID) (1946 to 10 February 2015).
Embase (OVID) (1996 to 2015 Week 06).
Science Citation Index & Social Science Citation Index (ISI) (2009 to 11 February 2015)
ERIC (EBSCO) (2009 to 11 February 2015).
Appendix 1 details the full search strategies for all databases. We downloaded the search results to Endnote reference management software and de‐duplicated them.
Data collection and analysis
We prepared a revised protocol for this updated review, including it as Appendix 2 to make it available alongside this review in the Cochrane Library.
Selection of studies
Two review authors independently screened the titles and abstracts of all references identified by the search strategy. We obtained the full versions of papers not definitely excluded at that stage for detailed review. Two review authors independently assessed all potentially eligible studies to determine if they met the inclusion criteria. We discussed differences of opinion and when necessary, a third review author read the full papers.
Data extraction and management
Two review authors independently carried out data extraction for each included record (using a proforma specifically designed for the purpose). We resolved differences in data extraction by discussion. We extracted data on the method evaluated; country where the study took place; nature of the population; nature of the study setting; nature of the study to be recruited into; randomisation or quasi‐randomisation method; and numbers and proportions of participants in the intervention and comparator groups of the study comparing recruitment strategies.
Assessment of risk of bias in included studies
We assessed the risk of bias using the Cochrane 'Risk of bias' tool (Cochrane Risk of Bias tool), including reassessing all 44 of the included studies from the previous version of this review carried forward into the update. We used GRADE on all studies where relevant data were available (Guyatt 2008). Where we have done a meta‐analysis, we provide the details of the GRADE assessment in the relevant 'Summary of findings' table. Where we used GRADE on a single study, we used the following rules for assigning a GRADE rating of high, moderate, low or very low certainty.
Baseline rating: all studies start at high.
Study limitations: downgrade all studies at high risk of bias by two levels; downgrade all studies at uncertain risk of bias by one level.
Inconsistency: assume no serious inconsistency.
Indirectness: downgrade all hypothetical studies by two levels.
Imprecision: downgrade all single studies by one level because of the sparsity of data; downgrade by a further level if the confidence interval is wide and includes a risk difference of 0.
Reporting bias: assume no serious reporting bias.
At least two reviewers performed all GRADE assessments. We generated 'Summary of findings' tables using only studies with real recruitment (i.e. not data for hypothetical studies). We present information on risk of bias for all included studies in Characteristics of included studies.
Although we did not exclude studies because of a high of risk of bias, we do not mention them in the text of the Results or Discussion because of the low confidence we have in the data they present, except in cases where we could include them in a meta‐analysis and interpret the datatogether with data from other studies.
Studies at high risk of bias do appear in Data and analyses, but we suggest that readers use these data only to make decisions as to whether they would like to evaluate the intervention themselves in a more rigorous way. We do not believe the data support judgements about effect.
Data for hypothetical studies are included in Data and analyses for this version of the review. We will exclude these studies from future versions of this review.
Assessment of heterogeneity
We sought statistical evidence of heterogeneity of results of trials using the Chi2 test for heterogeneity, and we quantified the degree of heterogeneity observed in the results using the I2 statistic (Higgins 2003). Where we detected substantial heterogeneity, we informally investigated possible explanations and summarised the data using a random‐effects analysis if appropriate. We planned to explore the following factors in subgroup analyses, assuming enough studies were identified, as we believed that these were plausible explanations for heterogeneity.
Type of design used to evaluate recruitment strategies (randomised versus quasi‐randomised) and allocation concealment (adequate versus inadequate or unclear).
Setting of the study recruiting participants (e.g. primary versus secondary care; healthcare versus non‐healthcare settings).
Disease area in which the evaluation was done (e.g. cancer versus lifestyle change).
Design of the study recruiting participants (e.g. open versus blinded studies, trials with placebo arms versus those without).
Target group (e.g. ethics committees, clinicians, patients).
Recruitment to hypothetical versus real trials (future versions of this review, which will exclude hypothetical trials, will not include this subgroup).
Assessment of reporting biases
We investigated reporting (publication) bias for the primary outcomes using a funnel plot where 10 or more studies were available.
Data synthesis
We grouped trials according to the type of intervention based on the categorisation used in the Online Resource for Recruitment research in Clinical triAls (ORRCA) project. We split one ORRCA category (Recruitment Information Needs) into two so as to separate out interventions aimed at the consent process from those aimed at more general participant information. This classification results in seven categories.
Design (category A). This includes changes to the general design of the trial specifically done to increase recruitment.
Pre‐trial planning (category B). This includes work done before the trial starts (possibly in a separate study) to explicitly make it more likely that recruitment will be successful.
Trial conduct changes (category C). This includes initiatives implemented once the trial has started such as better ways of identifying participants, changes to how data are collected, changes to the type of data collected and tailoring recruitment to different types of participant.
Modifications to the consent process (category D). This includes changes to the staff member helping with consent, when consent is taken, what sort of consent information is presented and how it is presented.
Modification to the information given to potential participants about the trial (category E). This includes who provides it, when, where what sort of information is presented, how the information is presented.
Interventions aimed at the recruiter or recruitment site (category F). This includes anything that is aimed at the recruiter or recruitment site staff rather than the person being recruited, such as changes to training.
Incentives (category G). Financial and other incentives for participants (but not staff, which is covered by a separate review).
We present results as risk differences (RD) with the associated 95% confidence intervals (CIs) where sufficient data were available. We only included cluster‐randomised trials in the meta‐analysis if sufficient data were reported to allow inclusion of analyses that adjusted for clustering; an odds ratio (OR) was used as the summary effect in the meta‐analysis result if risk difference or risk ratio clustering adjusted analyses were not possible with available data. Where two or more studies could be included in a meta‐analyses, we used a fixed‐effect approach to produce a pooled estimate in the absence of substantial heterogeneity.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
We screened 25,432 titles and abstracts (9098 in this update) and sought the full text of 377 records (76 in this update) to confirm inclusion or clarify uncertainties regarding eligibility, generally due to the lack of an abstract. We were able to obtain the full text of 374 of these articles; the remaining three records were not retrievable because the title or abstract reference was incomplete or incorrect.
Additionally, we retrieved the full text of six articles identified outside the search. A colleague identified Fleissig 2001 as missed in the previous version of the review; our search strategy had picked up the article, but we had rejected it in error during abstract checking. Man 2015a and Man 2015b (a single study describing two embedded recruitment trials), Jennings 2015a, Jennings 2015b, Jennings 2015c, Jennings 2015d, Jennings 2015e (a single study describing five embedded recruitment trials), Foss 2016, Lee 2017 and Cockayne 2017 are more recent studies that we identified while updating the review. We excluded one study that we had included in the previous version of the review, Harris 2008, because it was not recruiting to a trial and was therefore ineligible.
A total of 68 studies were eligible for inclusion. Studies came from 12 countries; there was also one multinational study involving 19 countries. The USA and UK dominated, with 25 and 22 studies, respectively. The next largest was Australia with eight studies. The full breakdown is given in Table 8.
1. Countries where the included studies took place.
| Country | Number of studies |
| Australia | 8 |
| Austria | 1 |
| Canada | 4 |
| Denmark | 1 |
| Estonia | 1 |
| France | 1 |
| Italy | 1 |
| Multinational | 1 (involved 19 countries) |
| Norway | 1 |
| Sweden | 1 |
| Tanzania | 1 |
| UK | 22 |
| USA | 25 |
There were 63 studies involving interventions aimed directly at trial participants, and five evaluated interventions aimed at those recruiting participants. At least 74,519 individuals were involved in the 68 studies; it was not clear how many participants were recruited in two studies. The figure of 74,519 includes both individuals who were recruited as well as those who were approached about recruitment but declined. A breakdown of participant numbers is given in Appendix 3.
There were too few studies evaluating the same or similar interventions to allow us to do any of our planned subgroup analyses.
Risk of bias in included studies
See Characteristics of included studies; Figure 1; Figure 2.
1.
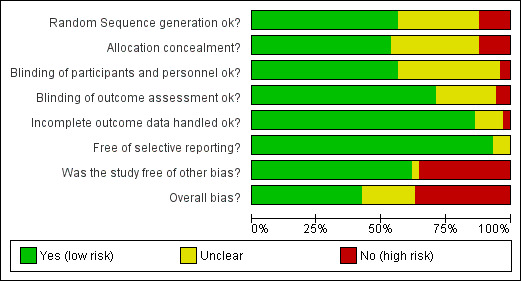
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
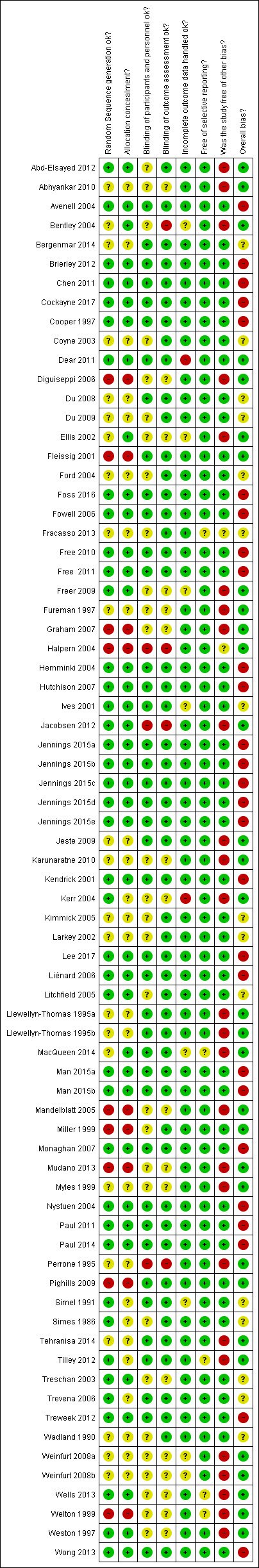
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Trialists described all their studies as either randomised (62 studies) or quasi‐randomised (6 studies). We considered the overall assessment of the risk of bias as low for 22 studies, unclear for 14 studies and high for 32 studies.
There were 26 studies involving hypothetical trials, and we judged 24 of these to be at high risk of bias because the participation decision was not a real one (there may also have been other weaknesses). We judged Treschan 2003 to be at unclear risk of bias because although participants were not told the trial was hypothetical initially, it was not clear if this remained the case throughout. Simel 1991 also involved a hypothetical trial, but participants were unaware of this; the use of a hypothetical trial did not therefore affect our risk of bias assessment for this study, and we judged it to be at unclear risk of bias.
Effect of methods
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Table 9 shows the list of included studies in each of our seven categories. The divisions between categories were not always clear, and we placed studies according to the original study authors' stated focus.
2. Intervention categories.
| Study | Host trial intervention | Type of participants |
| A–Design. This includes changes to the general design of the trial specifically done to increase recruitment. | ||
| Avenell 2004 | Drug: vitamin D tablet | Patients (adults): attending a fracture clinic or orthopaedic ward |
| Cooper 1997 | Drug/surgery: medical management or transcervical resection of the endometrium | Patients (adults): first‐time attendees at a gynaecological clinic |
| Fowell 2006 | Drug: anti‐emetics only if symptomatic | Patients (adults): cancer inpatients receiving palliative care |
| Hemminki 2004 | Drug: HRT | Patients (adults): postmenopausal women considering HRT |
| Litchfield 2005 | Device: alternative delivery systems (NovoPen and Innovo) for insulin | Patients (probably adults): people with type 1 diabetes |
| Paul 2011 | Drug: adjuvant treatment | Patients (probably adults): with colorectal cancer |
| Tehranisa 2014a | Hypothetical drug: acute stroke trial | Patients (adults): people attending emergency department |
| Welton 1999a | Hypothetical drug: HRT | Healthy volunteers (adults): women who had not had a hysterectomy |
| B–Pre‐trial planning. This includes work done before the trial starts (possibly in a separate study) that explicitly aims to increase recruitment success. | ||
| None | ||
| C–Trial conduct changes. This includes initiatives implemented once the trial has started, such as better ways of identifying participants, changes to how data are collected, changes to the type of data collected and tailored recruitment to different types of participant. | ||
| Diguiseppi 2006a | Hypothetical behavioural trial | Patients (adults): attending hospital with acute injury |
| Free 2010 | Behaviour: mobile phone‐based smoking cessation | Healthy volunteers (adults): smokers |
| Free 2011 | Behaviour: mobile phone‐based smoking cessation | Healthy volunteers (adults): smokers |
| Graham 2007a | Hypothetical lifestyle trial | Patients (adults): attending hospital with acute injury |
| Miller 1999 | Drug or therapy: psychotherapy, antidepressant medication, or both | Patients (adults): eligible for 1 of the 2 trials being run through the unit: 18‐75 years old and DSM‐IV dysthymic disorder, double depression (major depression superimposed on antecedent dysthymia), or chronic major depression |
| Mudano 2013 | Hypothetical drug: osteoporosis | Healthy volunteers (adults): women 65 years or over with no reported use of osteoporosis medication in last year |
| Nystuen 2004 | Therapy: psychologist intervention for issues linked to psychological problems or musculoskeletal pain | Patients (adults): on sick leave receiving benefits |
| Treweek 2012 | Drug: antibiotic prescribing | Health professionals (adults): family doctors |
| Wong 2013 | Screening: colorectal cancer screening | Healthy volunteers (adults): eligible for colorectal cancer screening |
| D–Modification to the consent form or process. This includes changes to the staff member helping with consent, when consent is taken, what sort of consent information is presented and how it is presented. | ||
| Abd‐Elsayed 2012 | Drug or blood storage trials | Patients (adults): eligible for 1 of 3 trials, all of whom had substantial illness requiring major surgery (cardiac) |
| Abhyankar 2010a | Hypothetical drug or surgery | Healthy volunteers (adults): women and students on university mailing list |
| Coyne 2003 | Drug: various | Patients (adults): eligible for cancer trial |
| MacQueen 2014a | Hypothetical drug: HIV treatment | Healthy volunteers (adults): sexually active women |
| Myles 1999a | Hypothetical drug: various | Patients (adults): eligible for surgery |
| Perrone 1995a | Hypothetical drug: various | Healthy volunteers (adults): attending a public event |
| Trevena 2006 | Screening: colorectal cancer | Healthy volunteers (adults): eligible for colorectal screening |
| Wadland 1990 | Lifestyle: smoking cessation | Healthy volunteers (adults): smokers |
| E–Modification to the information given to potential participants about the trial. This includes who provides it, when, where what sort of information is presented, how the information is presented. | ||
| Bergenmar 2014 | Drug: various | Patients (probably adults): eligible for cancer trials |
| Brierley 2012 | Therapy: cognitive behavioural therapy | Patients (adults): depression |
| Chen 2011 | Unclear | Patients (probably adults): unclear what type |
| Cockayne 2017 | Device: orthosis | Patients (adults): podiatry |
| Dear 2011 | Information: access to cancer trials site | Patients (adults): have cancer |
| Du 2008 | Cancer trials (unspecified) | Patients (adults): lung cancer |
| Du 2009 | Cancer trials (unspecified) | Patients (adults): women with breast cancer |
| Ellis 2002a | Hypothetical cancer trials (unspecified) | Patients (adults): women with breast cancer |
| Ford 2004 | Screening: prostate, lung and colorectal cancer screening | Healthy volunteers (adults): men eligible for prostate, lung and colorectal cancer screening |
| Foss 2016 | Vaccination | Healthy volunteers (adults): pregnant women |
| Fracasso 2013 | Cancer trials (unspecified) | Patients (adults): cancer (various) |
| Freer 2009a | Hypothetical intensive care (unspecified) | Healthy volunteers (adults): parents of infants admitted to hospital |
| Fureman 1997a | Hypothetical vaccine trial: HIV | Healthy volunteers (adults): drug users |
| Hutchison 2007 | Cancer trials (unspecified) | Patients (probably adults): cancer (various) |
| Ives 2001 | Unclear but probably drug | Patients (adults): people with HIV |
| Jacobsen 2012a | Hypothetical cancer trial | Patients (adults): cancer (various) |
| Jeste 2009a | Hypothetical drug trial | Patients (adults): schizophrenia |
| Karunaratne 2010a | Hypothetical device trial | Patients (adults): diabetes |
| Kendrick 2001 | Injury prevention trial | Healthy volunteers (adults and children): families |
| Kerr 2004a | Hypothetical drug trial | Healthy volunteers (adults): attending college |
| Kimmick 2005 | Cancer trials (various) | Patients (adults): cancer (various) |
| Larkey 2002 | Various targeting cardiovascular disease, cancer and osteoporosis | Healthy volunteers: (adults) women |
| Llewellyn‐Thomas 1995aa | Hypothetical drug trial | Patients (adults): colorectal cancer |
| Llewellyn‐Thomas 1995ba | Hypothetical drug trial | Patients (adults): cancer |
| Man 2015ab | Therapy: telephone support and self‐management | Patients (adults): cardiovascular |
| Man 2015bb | Therapy: telephone support and self‐management | Patients (adults): cardiovascular |
| Mandelblatt 2005a,c | Hypothetical drug trial | Healthy volunteers (adults): cancer prevention |
| Paul 2014 | Screening: colorectal cancer | Healthy volunteers (adults): colorectal cancer screening |
| Pighills 2009 | Therapy: falls prevention | Healthy volunteers (adults): older people at risk of falling |
| Simel 1991a,c | Hypothetical drug trial (participants were not told it was hypothetical) | Patients (adults): people attending ambulatory care clinic |
| Simes 1986 | Unclear: cancer | Patients (adults): cancer |
| Treschan 2003a,c | Hypothetical surgery trial (participants were not told it was hypothetical) | Patients (adults): people undergoing minor surgery with general anaesthetic |
| Weinfurt 2008aa | Hypothetical drug trial | Patients (adults): coronary heart disease |
| Weinfurt 2008ba | Hypothetical drug trial | Patients (adults): coronary heart disease |
| Wells 2013a | Hypothetical: unclear what type, probably drug | Patients (adults): cancer |
| Weston 1997a | Hypothetical surgery trial | Healthy volunteers (adults): women attending antenatal clinics. |
| F–Interventions aimed at the recruiter or recruitment site. This includes anything that is aimed at the recruiter or recruitment site staff rather than the person being recruited such as changes to training | ||
| Fleissig 2001 | Diverse: cancer | Patients (adults): cancer |
| Lee 2017 | Therapy: pain education | Staff at primary care clinics (sites are target, not patients) |
| Liénard 2006 | Drug: breast cancer treatment | Staff at breast cancer treatment centres (sites are target, not patients) |
| Monaghan 2007 | Unclear: diabetes management | Staff at clinical sites recruiting to a diabetes and vascular disease treatment trial (sites are target, not patients) |
| Tilley 2012 | Drug: Parkinson's disease | Neurologists, primary care doctors and internists (adults) |
| G–Incentives. Financial and other incentives for participants | ||
| Bentley 2004a | Hypothetical drug trial | Healthy volunteers (adults): students |
| Free 2010 | Lifestyle: mobile phone‐based smoking cessation | Healthy volunteers (adults): smokers |
| Halpern 2004a,c | Hypothetical drug study | Patients (probably adults): mild hypertension |
| Jennings 2015ad | Drug: NSAID | Patients (adults): arthritis |
| Jennings 2015bd | Drug: hyperuricaemia | Patients (adults): symptomatic hyperuricaemia |
| Jennings 2015cd | Drug: hypertension | Patients (adults): hypertension |
| Jennings 2015dd | Drug: hypertension | Patients (adults): hypertension |
| Jennings 2015ed | Drug: diuretic therapy | Patients (adults): metabolic syndrome |
DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; HRT: hormone replacement therapy; NSAID: non‐steroidal anti‐inflammatory drugs. aStudies were recruiting to hypothetical trials or asking questions about intention to participate rather than asking people to make a real decision about participation. bMan 2015a and Man 2015b are actually a single study that describes 2 embedded recruitment trials. cSimel 1991, Treschan 2003 and Halpern 2004 used hypothetical trials but did not tell participants until after they had made their decisions; Mandelblatt 2005 involved a real trial but asked about intention to take part, not actual taking part. dJennings 2015a, Jennings 2015b, Jennings 2015c, Jennings 2015d and Jennings 2015e are actually a single study that describes 5 embedded recruitment trials.
We report the results of studies rated as being at low or uncertain risk of bias here. The full list of 72 comparisons tested, irrespective of risk of bias, is given in Appendix 4.
We produced 'Summary of findings' tables for all interventions where more than one study done in a real trial was available, giving seven in total (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7).
Design ‐ category A
Eight studies focused on trial design as a way to improve recruitment; we judged two (25%) of these to be at high risk of bias and do not present them here. The remaining six studies involved 5637 participants; one study also targeted general practices and recruited 28 centres.
We summarise the results for the six studies as follows.
An open design compared to a blinded, placebo‐controlled design increases recruitment: RD = 10% (95% CI 7% to 13%); GRADE: high; Analysis 1.1; Table 1. This is based on two studies: Avenell 2004 (fracture prevention); RoB: low; Hemminki 2004 (postmenopausal hormone therapy) RoB: low.
A patient preference design increased total participation but made little or no difference to recruitment to the randomised trial: RD = ‐4% (reduced recruitment) (95% CI ‐15% to 7%); GRADE: low (‐2 levels: imprecision– single study; wide CI crossing RD=0); Analysis 2.1. This is based on one study: Cooper 1997 (management strategies for heavy menstrual bleeding) RoB: low.
Internet‐based, electronic data collection compared to paper‐based may reduce recruitment: RD = ‐13% (reduced recruitment) (95% CI ‐24% to ‐3%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 3.1. This is based on one study: Litchfield 2005 (delivery systems for insulin) RoB: unclear.
Cluster‐randomised design compared to Zelen design. The study had only two sites (clusters) with few participants: 6 out of 24 potential participants were recruited in the cluster arm, compared to 0 out of 29 in the Zelen arm; RoB: low. This is based on one study: Fowell 2006 (palliative care) RoB: low.
Two‐stage randomisation to choose duration of treatment. Data on numbers recruited not available for one arm but up‐front randomisation to 3 or 6 months treatment gave a recruitment rate of 5.21 per year per centre compared to 4.09 for delayed randomisation to decide whether second 3 month treatment given. This is based on one study: Paul 2011 (adjuvant treatment for colorectal cancer) RoB: low.
1.1. Analysis.
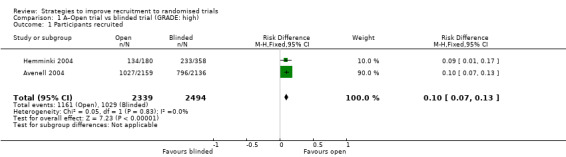
Comparison 1 A–Open trial vs blinded trial (GRADE: high), Outcome 1 Participants recruited.
2.1. Analysis.
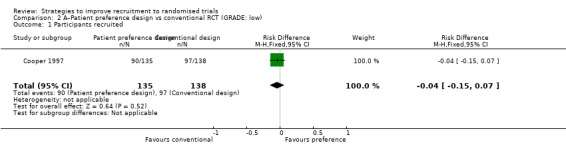
Comparison 2 A–Patient preference design vs conventional RCT (GRADE: low), Outcome 1 Participants recruited.
3.1. Analysis.
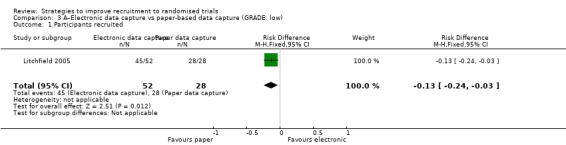
Comparison 3 A–Electronic data capture vs paper‐based data capture (GRADE: low), Outcome 1 Participants recruited.
Pre‐trial planning ‐ category B
There were no studies in this category.
Trial conduct changes ‐ category C
Nine studies assessed changes in trial conduct to improve recruitment. We judged four (44%) to be at high risk of bias and do not present them here. The remaining five studies involved 4531 participants.
Using a telephone reminder to contact non‐responders to a postal invitation increases recruitment. RD = 6% (95% CI 3% to 9%); GRADE: high; Analysis 6.1; Table 2. This is based on two studies: Nystuen 2004 (getting people to return to work); RoB: low; Wong 2013 (colorectal cancer) RoB: low. NOTE: the evidence for this intervention comes entirely from trials with low (<10%) underlying recruitment. When applied to trials with higher recruitment we would downgrade the GRADE assessment because of Indirectness to moderate.
Mentioning scarcity of trial places in SMS messages probably increased recruitment. RD = 3% (95% CI = 1% to 6%); GRADE: moderate (‐1 level: imprecision–single study); Analysis 7.1. This is based on one study: Free 2011 (smoking cessation) RoB: low..
Giving quotes from previous participants in SMS messages probably increased recruitment. RD = 4% (95% CI = 2% to 6%); GRADE: moderate (‐1 level: imprecision–single study); Analysis 8.1. This is based on one study: Free 2010 (smoking cessation) RoB: low.
Using email invitations made little or no difference to recruitment compared to postal invitations. RD = 1% (95% CI = ‐3% to 4%); GRADE: moderate (‐1 level: imprecision–single study); Analysis 9.1. This is based on one study: Treweek 2012 (antibiotic prescribing by GPs) RoB: low.
6.1. Analysis.
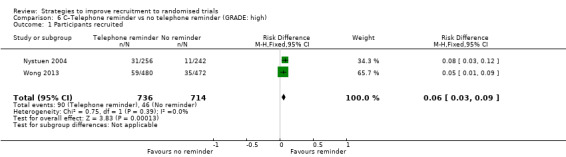
Comparison 6 C–Telephone reminder vs no telephone reminder (GRADE: high), Outcome 1 Participants recruited.
7.1. Analysis.
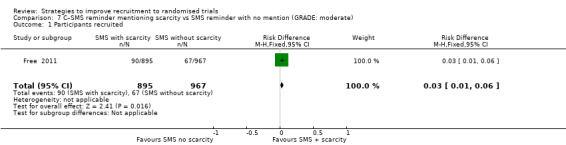
Comparison 7 C–SMS reminder mentioning scarcity vs SMS reminder with no mention (GRADE: moderate), Outcome 1 Participants recruited.
8.1. Analysis.
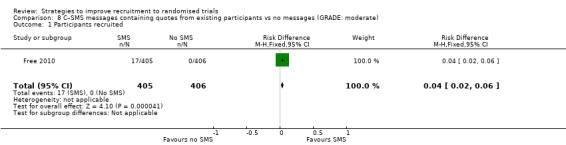
Comparison 8 C–SMS messages containing quotes from existing participants vs no messages (GRADE: moderate), Outcome 1 Participants recruited.
9.1. Analysis.
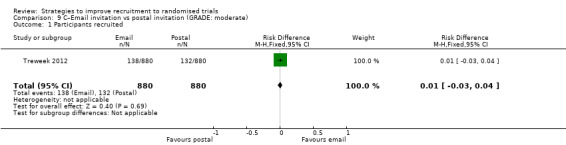
Comparison 9 C–Email invitation vs postal invitation (GRADE: moderate), Outcome 1 Participants recruited.
Modification to the consent process ‐ category D
Eight studies assessed the effect of modifying the consent process on trial recruitment. Of the five (63%) we judged to be at high risk of bias, we could have combined two (Myles 1999; Perrone 1995): however, both were hypothetical, and we do not present them here. The three studies presented here involved 482 participants.
Opt‐out consent may improve recruitment. RD = 19% (95% CI = 3% to 35%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 15.1. This is based on one study: Trevena 2006 (colorectal cancer) RoB: unclear.
It is very uncertain whether a researcher reading out the consent details affects recruitment. RD = 6% (95% CI = ‐13% to 25%); GRADE: very low (‐1 level: study limitations–unclear RoB; ‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 18.1. This is based on one study: Wadland 1990 (smoking cessation) RoB: unclear.
Easy to read consent form. Although the authors of this cluster trial did not present centre‐level recruitment data, or provide an intracluster correlation coefficient, they did consider intracluster correlation in their analysis and found that recruitment did not differ significantly between the two trial groups (RD=3; P = 0.32). This is based on one study: Coyne 2003 (cancer) RoB: unclear.
15.1. Analysis.
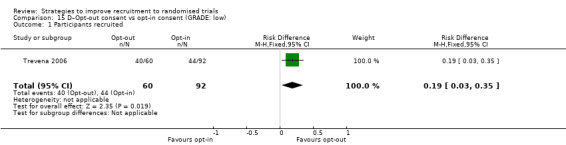
Comparison 15 D–Opt‐out consent vs opt‐in consent (GRADE: low), Outcome 1 Participants recruited.
18.1. Analysis.
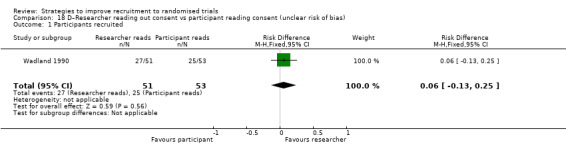
Comparison 18 D–Researcher reading out consent vs participant reading consent (unclear risk of bias), Outcome 1 Participants recruited.
Modification to the information given to potential participants about the trial ‐ category E
Thirty‐five studies assessed the effects of modifying the information given to potential participants about the trial for trial recruitment. We judged 17 (49%) to be at high risk of bias and do not present them here. The remaining 17 studies involved 42,826 participants.
Optimising the participant information leaflet (PIL) through a particular, bespoke process involving formal user‐testing makes little or no difference to recruitment. RD = 1% (95% CI = ‐1% to 3%); GRADE: high; Analysis 25.1; Table 3. This is based on three studies: Man 2015a (depression) RoB: low; Man 2015b (cardiovascular disease) RoB: low; Cockayne 2017 (falls prevention) RoB: low.
Using a brief patient information leaflet (PIL) makes little or no difference to recruitment compared to a full PIL. RD = 0% (95% CI = ‐2% to 2%); GRADE: moderate (‐1 level: indirectness, Chen 2011 actually measures entry to pre‐randomisation phase); Analysis 26.1; Table 4. This is based on two studies: Chen 2011 (unclear) RoB: low; Brierley 2012 (depression) RoB: low.
Enclosing a questionnaire covering issues relevant to trial with the invitation probably increases recruitment. RD = 18% (95% CI = 16% to 20%); GRADE: moderate (‐1 level: imprecision–single study); Analysis 27.1 This is based on one study: Kendrick 2001 (injury prevention, recruiting family units) RoB: low.
Optimising the PIL through using user feedback probably makes little or no difference in recruitment. RD = 0% (95% CI = 0% to 1%); GRADE: moderate (‐1 level: indirectness, Chen 2011 actually measures entry to pre‐randomisation phase); Analysis 28.1; Table 5 This is based on two studies: Chen 2011 (unclear) RoB: low; Cockayne 2017 (falls prevention) RoB: low.
Sending a recruitment primer letter may have little or no effect on recruitment. RD = 0% (95% CI = ‐6% to 6%); GRADE: low (‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 29.1 This is based on one study: Paul 2014 (colorectal cancer) RoB: low.
Providing information over the telephone may have little or no effect on recruitment. RD = ‐7% (reduced recruitment) (95% CI = ‐18% to 5%); GRADE: low (‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 30.1 This is based on one study: Foss 2016 (vaccination) RoB: low.
Recruitment at a church and other enhancements may improve recruitment. RD = 1% (95% CI = 0% to 2%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 31.1 This is based on one study: Ford 2004 (cancer) RoB: unclear.
An enhanced recruitment package including more contact may make little or no difference in recruitment. RD = 0% (95% CI = ‐1% to 0%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 32.1 This is based on one study: Ford 2004 (cancer) RoB: unclear.
An enhanced recruitment package including more contact by telephone may make little or no difference in recruitment. RD = 0% (95% CI = ‐1% to 1%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 33.1 This is based on one study: Ford 2004 (cancer) RoB: unclear.
Emphasising risk in information may make little or no difference to recruitment. RD = 0% (95% CI = ‐1% to 1%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 34.1 This is based on one study: Treschan 2003 (unclear) RoB: unclear.
Writing treatment effect as 'twice as fast' rather than 'half as fast' may improve recruitment. RD = 26% (95% CI = 7% to 45%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 35.1 This is based on one study: Simel 1991 (pain relief) RoB: unclear.
Emphasising pain in information may reduce recruitment. RD = ‐29% (reduced recruitment) (95% CI = ‐48% to ‐10%); GRADE: low (‐1 level: study limitations–unclear RoB; ‐1 level: imprecision–single study); Analysis 36.1 Thsi is based on one study: Treschan 2003 (unclear) RoB: unclear.
It is very uncertain whether providing trial information by video affects recruitment. RD = 3% (95% CI = ‐3% to 9%); GRADE: very low (‐1 level: study limitations–unclear RoB; ‐1 level: inconsistency; ‐1 level: imprecision–wide CI crossing RD=0); Analysis 37.1; Table 6 This is based on three studies: Hutchison 2007 (cancer) RoB: low; Du 2008 (lung cancer) RoB: unclear; Du 2009 (breast cancer) RoB: unclear.
It is very uncertain whether providing an audio record of the discussion about the trial affects recruitment. RD = ‐3% (reduced recruitment) (95% CI = ‐19% to 13%); GRADE: very low (‐1 level: study limitations–unclear RoB; ‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 38.1 This is based on one study: Bergenmar 2014 (cancer) RoB: unclear.
It is very uncertain whether providing a clinical trial booklet together with standard information affects recruitment. RD = 20% (95% CI = ‐5% to 46%); GRADE: very low (‐1 level: study limitations–unclear RoB; ‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 39.1 This is based on one study: Ives 2001 (HIV) RoB: unclear.
It is very uncertain whether providing total information disclosure rather than leaving it to recruiters as to what to reveal affects recruitment. RD = 11% (95% CI = ‐6% to 28%); GRADE: very low (‐1 level: study limitations–unclear RoB; ‐2 levels: imprecision–single study; wide CI crossing RD=0); Analysis 40.1 This is based on one study: Simes 1986 (cancer) RoB: unclear.
Educational material to provide additional information about a trial. Although the authors of this cluster trial did not present centre‐level recruitment data, or provide an intracluster correlation coefficient, they did consider intracluster correlation in their analysis. An educational package did not significantly increase recruitment compared to standard information alone (31% of participants aged over 65 in both intervention and control groups in year 2, P = 0.83). This is based on one study: Kimmick 2005 (cancer) RoB: unclear.
Trained recruiters from a similar ethnic background to study population already taking part in a trial as lay advocates. The authors of this cluster trial did not report an analysis that corrected for the clustering or provide an intracluster correlation coefficient. Data at the recruiter aggregate level were reported on whether a recruiter did or did not recruit anyone to the trial. Eight of the 28 trained Hispanic recruiters recruited one or more women to the trial whereas none of the 26 untrained Hispanic women recruited anyone the trial. Two of the 42 untrained Anglo control group recruited two women. This is based on one study: Larkey 2002 (unclear) RoB: low.
25.1. Analysis.
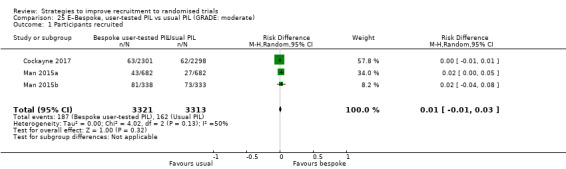
Comparison 25 E–Bespoke, user‐tested PIL vs usual PIL (GRADE: moderate), Outcome 1 Participants recruited.
26.1. Analysis.
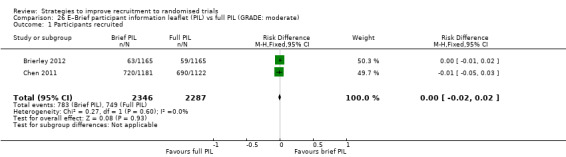
Comparison 26 E–Brief participant information leaflet (PIL) vs full PIL (GRADE: moderate), Outcome 1 Participants recruited.
27.1. Analysis.
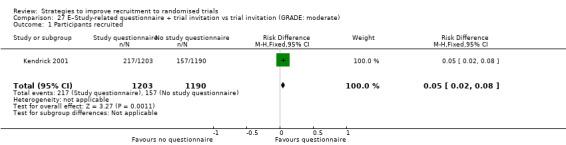
Comparison 27 E–Study‐related questionnaire + trial invitation vs trial invitation (GRADE: moderate), Outcome 1 Participants recruited.
28.1. Analysis.
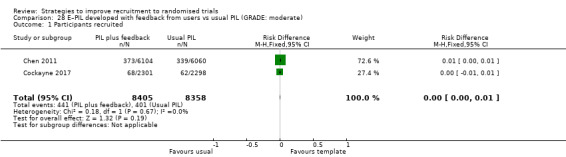
Comparison 28 E–PIL developed with feedback from users vs usual PIL (GRADE: moderate), Outcome 1 Participants recruited.
29.1. Analysis.
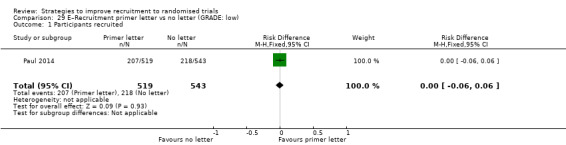
Comparison 29 E–Recruitment primer letter vs no letter (GRADE: low), Outcome 1 Participants recruited.
30.1. Analysis.
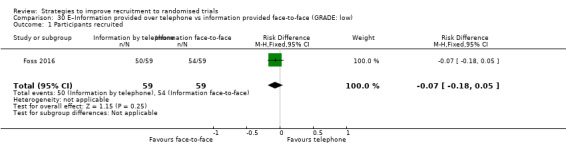
Comparison 30 E–Information provided over telephone vs information provided face‐to‐face (GRADE: low), Outcome 1 Participants recruited.
31.1. Analysis.
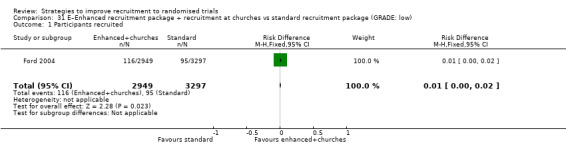
Comparison 31 E–Enhanced recruitment package + recruitment at churches vs standard recruitment package (GRADE: low), Outcome 1 Participants recruited.
32.1. Analysis.
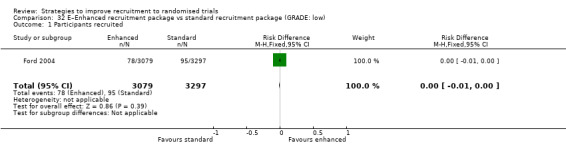
Comparison 32 E–Enhanced recruitment package vs standard recruitment package (GRADE: low), Outcome 1 Participants recruited.
33.1. Analysis.
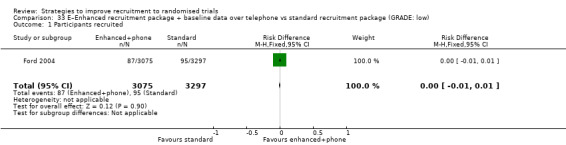
Comparison 33 E–Enhanced recruitment package + baseline data over telephone vs standard recruitment package (GRADE: low), Outcome 1 Participants recruited.
34.1. Analysis.
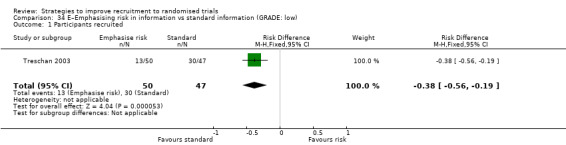
Comparison 34 E–Emphasising risk in information vs standard information (GRADE: low), Outcome 1 Participants recruited.
35.1. Analysis.
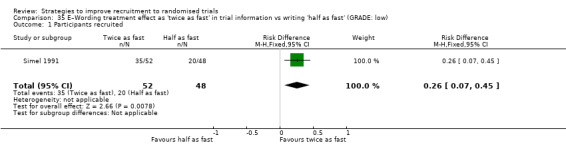
Comparison 35 E–Wording treatment effect as 'twice as fast' in trial information vs writing 'half as fast' (GRADE: low), Outcome 1 Participants recruited.
36.1. Analysis.
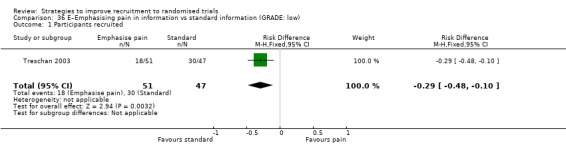
Comparison 36 E–Emphasising pain in information vs standard information (GRADE: low), Outcome 1 Participants recruited.
37.1. Analysis.
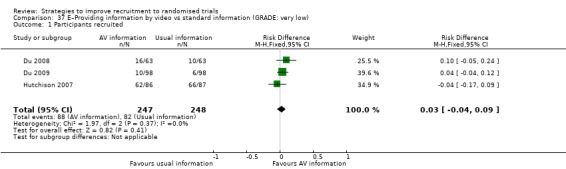
Comparison 37 E–Providing information by video vs standard information (GRADE: very low), Outcome 1 Participants recruited.
38.1. Analysis.
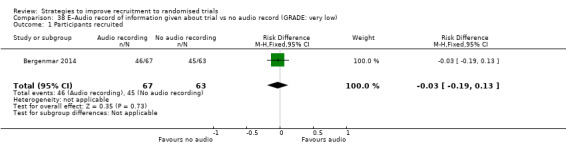
Comparison 38 E–Audio record of information given about trial vs no audio record (GRADE: very low), Outcome 1 Participants recruited.
39.1. Analysis.
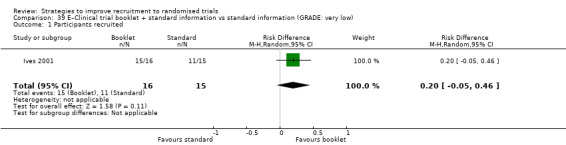
Comparison 39 E–Clinical trial booklet + standard information vs standard information (GRADE: very low), Outcome 1 Participants recruited.
40.1. Analysis.
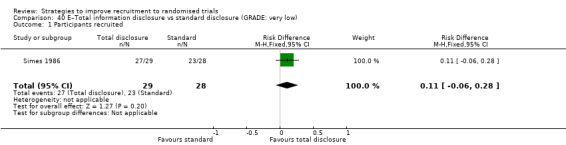
Comparison 40 E–Total information disclosure vs standard disclosure (GRADE: very low), Outcome 1 Participants recruited.
Interventions aimed at the recruiter or recruitment site ‐ category F
Five studies assessed interventions aimed at the recruiter or recruitment site. We judged two (40%) of these to be at high risk of bias and do not present them here. The remaining three studies involved at least 602 participants; it was not clear how many participants were involved in one study, although 167 recruitment sites were involved.
Using a postcard teaser campaign made little or no difference to recruitment. RD = 0% (95% CI = ‐4% to 5%); GRADE: moderate (‐1 level: imprecision–single study); Analysis 55.1 This is based on one study: Lee 2017 (recruiting GP practices to low back pain trial) RoB: low.
Onsite initiation visits. The authors did not present the proportion of eligible participants recruited, only the number recruited: visited sites recruited 302 participants while those not receiving visits recruited 271. This is based on one study: Liénard 2006 (breast cancer) RoB: low.
Additional communication strategies such as tailored feedback on recruitment. The median total number of participants in the additional communication group was 37.5, compared to 37.0 in the standard communication group. Intervention centres achieved half their recruitment targets in 4.4 months, compared to 5.8 months for control centres. This is based on one study: Monaghan 2007 (diabetes) RoB: low.
55.1. Analysis.
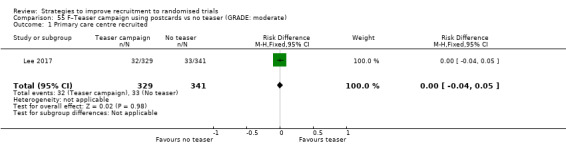
Comparison 55 F–Teaser campaign using postcards vs no teaser (GRADE: moderate), Outcome 1 Primary care centre recruited.
Incentives ‐ category G
Four studies assessed incentives for recruitment, but we judged two (50%) to be at high risk of bias and do not present them here. The remaining two studies included one that involved five trials of the same intervention and together both studies involved a total of 1,506 participants.
1. Financial incentives offered to potential participants probably improve recruitment. RD = 4% (95% CI = ‐1% to 8%); GRADE: moderate (‐1 level: inconsistency); Analysis 57.1; Table 7 This is based on six studies, one including five trials within a single published study: Free 2010 (smoking cessation) RoB: low; Jennings 2015a; Jennings 2015b; Jennings 2015c; Jennings 2015d; Jennings 2015e (primary care, older people, mainly hypertension) RoB: low.
57.1. Analysis.
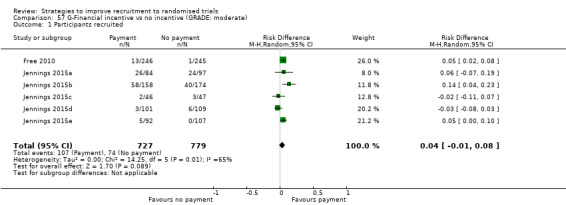
Comparison 57 G‐Financial incentive vs no incentive (GRADE: moderate), Outcome 1 Participants recruited.
Discussion
Principal findings
Trialists looking to the literature to select components of an evidence‐informed trial recruitment strategy will be disappointed to find that the literature has plenty of variety but little depth, and therefore much uncertainty. There are three findings that carry a GRADE high certainty of the evidence.
An open design compared to a blinded, placebo‐controlled design increases recruitment (RD 10%, 95% CI 7% to 13%; Analysis 1.1; Table 1; intervention category A).
Using a telephone reminder to contact non‐responders to a postal invitation increases recruitment (RD 6%, 95% CI 3% to 9%; Analysis 6.1; Table 2); intervention category C; see note below).
Optimising the participant information leaflet (PIL) through bespoke development plus formal user‐testing makes little or no difference to recruitment (RD 1%, 95% CI −1% to 3%; Analysis 25.1; Table 3; intervention category E).
Findings 2 and 3 could in principle be considered for many trials. Finding 1 is unlikely to be widely attractive because of the internal validity problem that open trial designs present. Moreover, the evidence for finding 2 comes entirely from trials with low (< 10%) underlying recruitment. When seeking to apply this to trials with higher recruitment, we would downgrade the GRADE assessment to moderate certainty due to indirectness.
There are eight findings that carry a moderate GRADE certainty of the evidence, mostly from single, well‐conducted studies (three in intervention category C, three in category E, one in category F and one in Category G). We rated the GRADE certainty of the evidence for all other findings as low or very low, or as being at high risk of bias if insufficient data were available to do a GRADE assessment. There are no evaluations of an intervention used pre‐trial to support recruitment (category B) and no evaluations of a consent‐related intervention (category D) with a GRADE certainty of the evidence better than low.
Of the 68 included studies, none addresses recruitment to paediatric trials (see Table 9), meaning trialists lack any evidence to inform decisions around participation in these trials. Therefore, identifying effective interventions to support recruitment to paediatric trials is also a priority. Researchers may be wary of adding research methods evaluations to paediatric trials because of, among other challenges, additional ethical requirements. However, because the challenges of recruitment to paediatric trials are likely to be different from those of other trials, extrapolating from trials in adults is unlikely to be sufficient. Moreover, one of the key ethical requirements for research with children – that it is not possible to do the work with adults – is met. For some trials it is likely that the target of the recruitment intervention will be parents rather than children despite being a paediatric trial, so the ethical requirements may in fact be similar to those for trials in adults. Finally, recruitment to paediatric trials will remain less efficient than it could be without work evaluating alternative approaches to recruitment.
While new studies were added to the review, the overall picture with regard to interventions to improve recruitment to trials remains similar to our 2010 version (Treweek 2010), which was in turn largely unchanged from the 2007 version before it (Mapstone 2007). In other words, a decade of research into the effect of interventions to improve trial recruitment has not substantively reduced our uncertainty with regards to which interventions make recruitment more likely. The chief reasons for this are a preference for methodology researchers to evaluate new interventions rather than to replicate evaluations of existing interventions. Poor reporting also leads to uncertain risk of bias assessments.
There is some good news, though. While the intervention type of the studies added to this update is the same as in the 2010 update (Category E, modification to the information given to participants dominates both updates), the methodological quality of studies seems to be improving. Of the 18 studies new to the 2010 update, 12 were at high risk of bias (66%), compared to 11 out of 24 (46%) added in 2017. We judged all 5 of the included studies published in the last three years (2015 to 2017) and all 10 of the recruitment evaluations they describe, to be at low risk of bias (Cockayne 2017; Foss 2016; Jennings 2015a; Jennings 2015b; Jennings 2015c; Jennings 2015d; Jennings 2015e; Lee 2017; Man 2015a; Man 2015b). Equally important, initiatives such as START (research.bmh.manchester.ac.uk/mrcstart) are leading to coordinated evaluation of recruitment interventions in many trials, participant information leaflets and video information in the case of START. The three studies in the bespoke, user‐tested participant information leaflet analysis (Analysis 25.1; Table 3) came via START over a three‐year period (2015 to 2017). By contrast, the two studies in the telephone reminder analysis (Analysis 6.1; Table 2) are nine years apart (2004 to 2013). START will provide more studies for the next update of this review. Timely reduction in uncertainty around interventions needs focus, coordination and replication.
Nevertheless, we judged around half of the 68 included studies to be at high risk of bias, meaning that we have so little confidence in their findings that we chose to neither present nor discuss their results. We will continue to make this choice in future versions of this review. Encouragingly, more recent studies are better reported and much more likely to be judged to be at low risk of bias. A recent reporting standard for embedded recruitment studies may improve things further (Madurasinghe 2016).
We will exclude 24 hypothetical studies from future versions of this review because their findings are not based on real decisions and provide only indirect evidence. It is clearly possible to do studies in real trials, and these will be our focus inthe future.
Finally, we would welcome feedback about studies that we have missed or newly published studies that we should include in future versions of the review.
Authors' conclusions
Implication for methodological research.
The methodological literature with regard to recruitment needs more depth. The current approach of uncoordinated evaluation has led to the usable information content of this review remaining largely unchanged for more than a decade despite the addition of 41 studies. The implications for methodological research are clear.
The research community should establish a process for prioritising which recruitment interventions are most in need of evaluation. While an ongoing, formal process is developed, we suggest that trialists focus on the evaluations highlighted below and the comparisons in this review with moderate‐certainty evidence, especially where there is still only a single study. The PRioRiTy project, which ran a James Lind Alliance prioritisation process for recruitment methods research, is due to publish in 2018 and will provide an excellent list of prioritised areas in need of recruitment intervention work.
The development and evaluation of recruitment interventions for use in paediatric trials is a priority.
We need much more replication and perhaps a little less innovation. This review of 72 comparisons has a total of only seven meta‐analyses. The remainder of the comparisons are single study evaluations of a new intervention.
Trialists evaluating recruitment interventions should do so through Studies Within A Trial (SWATs), using a registered protocol for replication or developing one for new evaluations (Clarke 2015). The SWAT Repository (go.qub.ac.uk/SWAT‐SWAR) supports this at no cost.
Trialists should consider notifying Trial Forge (www.trialforge.org) about their planned recruitment (and other trial process) evaluations to favour better coordination and wider dissemination of evaluation efforts.
Trialists should aim to include evaluations of recruitment strategies in their trials, preferably using a SWAT for a prioritised intervention. Funders should support this to avoid another decade with little progress regarding which interventions are effective in improving trial recruitment.
Based on the results of this review we suggest prioritising evaluations in three SWATs.
Although telephone reminders seem effective and have a high certainty of the evidence rating (Analysis 6.1, Table 2), both included studies had underlying recruitment of less than 10%. Beyond trials with low underlying recruitment, the GRADE certainty in the evidence is moderate due to indirectness. Evaluations in trials expected to have higher underlying recruitment are needed, especially given the potentially substantial workload and cost of involving a telephone reminder component to a recruitment strategy. The SWAT‐61 protocol is available through the Northern Ireland Network for Trials Methodology Research.
Use of a financial incentive probably improves recruitment (Analysis 57.1, Table 7), but the GRADE certainty of the evidence is currently moderate because of inconsistency between included study results. Moreover, financial incentives are widely used but at more modest levels than the GBP 100 used in Jennings 2015a, Jennings 2015b, Jennings 2015c, Jennings 2015d and Jennings 2015e. Use of incentives, including financial ones, also matches Priority no. 17 from the PRioRiTy top 20. More evaluations of financial incentives would therefore be welcome. The SWAT‐59 protocol is available through the Northern Ireland Network for Trials Methodology Research.
There are two text message‐based interventions in the review (Analysis 7.1; Analysis 8.1), both of which suggest small but potentially useful improvements in recruitment. We rated both as having moderate‐certainty evidence because the comparisons are based only on single evaluations. Text messaging is cheap, can be easily scaled up and could be widely applicable given the high usage of mobile telephones. The content of messages needs further work, though, including replications with regard to scarcity and quotes from participants, which are the two interventions evaluated in this review. Use of text messaging also matches priorities no. 2, 4 and 10 in the PRioRiTy top 10. We have developed the SWAT‐60 protocol for the intervention used in Analysis 7.1 on scarcity as a template for such evaluations, and it is available through the Northern Ireland Network for Trials Methodology Research.
Feedback
Michaels, 2 March 2010
Summary
I suggest that the next iteration of this report take into account, assuming it does exist in the literature, researcher relationships with the community. I am not only referring to Community Based Participatory Research (CBPR) in relation to clinical research (see www.communitiespartners.org), but also to researcher relationships with referring physicians and community based organizations. These relationships are critical to the success of clinical research, especially in the community setting.
The review also needs to take into account disease states in terms of recruitment. The patient with controllable diabetes vs the patient needing cancer treatment have very different information needs when it comes to clinical trial participation.
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
(Feedback submitted by Margo Micheals March 2010)
Reply
Many thanks for this suggestion, which we would like to build into our review. In terms of managing this, we think the best way to incorporate this comment would be to create a new category of intervention where researchers have specifically evaluated the impact on recruitment of building close collaborative relationships with potential participants, be they patients, healthy volunteers, or health professionals. Here we would be looking to studies that compared such an intervention against what might be called traditional recruitment strategies. We will also add disease as a potential subgroup analysis. We agree that it is highly plausible that disease (especially chronic versus acute) plays a role in recruitment.
As you mention, we may not find primary studies that allow us to act on these suggestions straight away. We did not identify studies that evaluated the kind of interventions mentioned above in our initial search though this may change as the review is updated.
Thanks again for your interest in our review.
Update to the 2010 feedback
We have added disease to our subgroup analysis list although we did not find enough studies to do this analysis, which is what we found for all of our proposed subgroup analyses. We think the new category of intervention we mentioned is nicely covered by Category F (Interventions aimed at the recruiter or recruitment site) as these would include the type of relationship‐building interventions mentioned in the feedback. This category also has the advantage of coming from the ORCCA process so matches the categories used elsewhere within the field of trial recruitment.
Contributors
Reply received from the review team, April 2010.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2018 | New citation required and conclusions have changed | Review updated |
| 9 June 2017 | New search has been performed | Review updated: search extended to February 2015; 24 additional included studies, including 6 recent studies identified outside the search (two from 2017) and 1 study missed in earlier searches. One previously included study excluded (it was included in error). Changes to protocol for next update introduced, chiefly linked to hypothetical trials, which will be excluded in future updates. While we added new studies to the review, the overall picture with regard to interventions for improving recruitment to trials remains similar to the previous version of the review. We have updated the 'Implications for methodological research' section to suggest interventions that methodological researchers should prioritise for enhanced evaluation, along with protocols for Studies Within A Trial (SWATs) to support these areas. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 10 June 2011 | New search has been performed | Review updated: search extended to April 2010, 18 additional included studies. While new studies were added to the review, the overall picture with regard to interventions to improve recruitment to trials remains similar to the previous version of the review. |
| 16 April 2010 | Feedback has been incorporated | Feedback from Margo Michaels added with reply from authors. |
| 10 November 2009 | New search has been performed | New search conducted September 2007. Twelve new studies identified. |
| 10 November 2009 | New citation required but conclusions have not changed | The title of this review has changed, as have the authors. |
| 27 December 2007 | Amended | Converted to new review format. |
| 20 February 2007 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We dedicate this update to our good friend and colleague, Monica Kjeldstrøm, who was an author on the protocol and previous versions of this review but died too young in 2014.
We would like to thank Marit Johansen, Ritu Jones, Pauline Lockhart and Sue Wilson for their contributions as authors to previous versions of this review, along with Hatim Osman, who screened abstracts for previous versions and Marian Pandal and Gail Morrison, who helped to obtain full‐text articles for previous versions. We would like thank Adel El Feky for his help with 'Risk of bias' assessment in this version of the review.
The Health Services Research Unit, University of Aberdeen receives core funding from the Chief Scientist Office of the Scottish Government Health Directorates. The views expressed are those of the authors and do not necessarily reflect those of the funders.
Many thanks to Valerie Ann Jenkins for identifying a study that we had missed in the previous version of this review. We would also like to thank Arthur Wong and colleagues for pointing out in their article, Wong 2013, that we should not have included Harris 2008 in our review because it was not recruiting to a trial. We agree with their determination and have now excluded Harris 2008 from the review.
Appendices
Appendix 1. Search strategies
Searches undertaken 11 February 2015
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to February Week1 2015>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Patient Selection/ (50436)
2 ((participat$ or recruit$ or enrol$) adj4 trial?).tw. (16427)
3 1 or 2 (65322)
4 Informed Consent/ (31549)
5 informed consent.tw. (24225)
6 4 or 5 (47497)
7 exp Clinical Trials as Topic/ (283986)
8 Research Subjects/ (5055)
9 (trial? or study or studies or research).tw. (7218575)
10 7 or 8 or 9 (7314164)
11 3 or (6 and 10) (86896)
12 (research support nih extramural or research support nih intramural or research support non us govt or research support us govt non phs or research support us govt phs).pt. (7410137)
13 recruitment.ab. /freq=2 (18332)
14 participation.ab. /freq=2 (16979)
15 12 or 13 or 14 (7422665)
16 11 and 15 (27568)
17 randomized controlled trial.pt. (383951)
18 controlled clinical trial.pt. (88580)
19 random$.ab. (724307)
20 17 or 18 or 19 (914167)
21 16 and 20 (9907)
22 exp animals/ not humans/ (3982927)
23 21 not 22 (9883)
24 23 not (comment or editorial).pt. (9860)
25 24 and ("2009" or "2010" or "2011" or "2012" or "2013" or "2014" or "2015").yr. (4913)
26 25 not 2009$.ed (4453)
***************************
Database: Ovid Embase <1996 to 2015 Week 06>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 ((participat$ or recruit$ or enrol$ or enter$ or entry) and (trial? or study)).ti. (9063)
2 (select$ adj3 (participants or patients or controls)).tw. (102178)
3 recruit$.ab. /freq=2 (46720)
4 participat$.ab. /freq=2 (55568)
5 research.tw. (987167)
6 2 and (3 or 4 or 5) (7329)
7 Informed Consent/ (55296)
8 (informed consent or consent process$ or consent procedure?).tw. (40057)
9 exp "controlled clinical trial (topic)"/ (67171) term
10 (trial? or study or studies or research).tw. (6952871)
11 (7 or 8) and (9 or 10) (40723)
12 1 or 6 or 11 (56375)
13 Randomized Controlled Trial/ (313117)
14 Cross‐over Procedure/ (37035)
15 random$.tw. (807376)
16 (factorial or crossover or cross‐over or assign$ or allocat$).tw. (345538)
17 13 or 14 or 15 or 16 (1062995)
18 nonhuman/ (3059129)
19 editorial.pt. (373977)
20 conference abstract.pt. (1746506)
21 17 not (18 or 19 or 20) (749148)
22 12 and 21 (8476)
23 limit 22 to yr="2009 ‐Current" (3953)
24 23 not 2009$.dd (3534)
The Cochrane Library Cochrane Methodology Register : Issue 3 of 4, July 2012
#1 "accrual and sample size" or "attitudes to trials" or "informed consent":kw (Word variations have been searched) 3040
#2 (participat* or recruit* or enrol* or select*) near/8 (trial* or research or study):ti (Word variations have been searched) 3910
#3 (participat* or recruit* or enrol* or select*) near/8 (trial* or research or study):ab (Word variations have been searched) 59388
#4#1 or #2 or #3 515
Publication Year from 2009 to 2012, in Methods Studies
SCI & SSCI (ISI)
# 5 #4 OR #3 OR #2 OR #1 629
# 4 (TS=(recruitment NEAR/8 "controlled trial")) AND DOCUMENT TYPES: (Article) 175
# 3 (TS=(recruitment NEAR/8 "controlled trials")) AND DOCUMENT TYPES: (Article) 54
# 2 (TS=(recruitment NEAR/8 "clinical trials")) AND DOCUMENT TYPES: (Article) 306
# 1 ((TS=(recruitment NEAR/8 "clinical trial"))) AND DOCUMENT TYPES: (Article) 187
Indexes=SCI‐EXPANDED, SSCI Timespan=2009‐2015
ERIC (EBSCO)
S4 (S1 AND S2) Limiters ‐ Date Published: 20090101‐20141231 521
S3 (S1 AND S2) 884
S2 clinical trial* OR controlled trial* OR randomi* 4379
S1 (recruit* or participat*) 152,558
Appendix 2. Protocol
Cover sheet
Title
Strategies to improve recruitment to randomised trials
Reviewers
Treweek S, Pitkethly M, Cook J, Mitchell E, Sullivan F,Fraser C, Jackson C, Johansen M, Taskila T, Wilson S, Jones R, Lockhart P, Gardner H.
Contact reviewer
Prof Shaun Treweek Health Services Research Unit University of Aberdeen 3rd Floor, Health Sciences Building Foresterhill Aberdeen AB25 2ZD UK Telephone: +44 1224 438145 Mobile:+44 777 901 6955 E‐mail: streweek@mac.com Secondary contact person's name: Heidi Gardner; heidi.gardner.10@aberdeen.ac.uk.
Contribution of reviewers
All authors contributed to the writing of the protocol.
Internal sources of support
Scottish Higher Education Funding Council, Scotland
External sources of support
None
Background
Essentially all trials need to recruit participants but this is often a challenge. Poor recruitment can lead to an underpowered study, which may report clinically relevant effects to be statistically non‐significant. A non‐significant finding increases the risk that an effective intervention will be abandoned before its true value is established, or that there will be a delay in demonstrating this value while more trials or meta‐analyses are done. Underpowered trials also raise an ethical problem: trialists have exposed participants to an intervention with uncertain benefit but may still be unable to determine whether the intervention does more good than harm on completion of the trial. Poor recruitment can also lead to the trial being extended, increasing costs.
Although investigations of recruitment differ in their estimates of the proportion of studies that achieve their recruitment targets, it is likely that less than 50% meet their target (Charlson 1984; Foy 2003; Haidich 2001; McDonald 2006; Sully 2013). For example, McDonald and colleagues found that only 38 (31%) of 114 trials achieved their original recruitment target and 65 (53%) were extended (McDonald 2006). More recent replications of this work by Sully and colleagues and by Walters and colleagues found that the the number of trials meeting recruitment targets had increased to around 50% (Sully 2013; Walters 2017). The overall start to recruitment was delayed in 47 (41%) trials and early recruitment problems were identified in 77 (63%) trials (Sully 2013). The costs of poor recruitment can be huge (Kitterman 2011).
Trialists use many interventions to improve recruitment (see for example Caldwell 2010, Watson 2006 and Prescott 1999) but it is generally difficult to predict the effect of these interventions.
This review updates the Treweek 2010 review.
Objectives
The primary objective is to quantify the effects of strategies to improve recruitment of participants to randomised controlled trials. A secondary objective is to assess the evidence for the effect of the research setting (e.g. primary care versus secondary care) on recruitment.
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised trials of interventions to improve recruitment to randomised trials.
Types of participants
Randomised and quasi‐randomised trials of recruitment strategies set in the context of trials but not limited to health care; interventions that work in other fields (e.g. education, housing) could be applicable to healthcare settings. Strategies both within real settings and in hypothetical trials (studies that ask potential participants whether they would take part in a trial if it was run but the trial does not actually exist) are eligible for this version of the review.
Note: future versions of this review will exclude hypothetical trials since these are all considered to be at high risk of bias because the recruitment decision is not a real one; many also have other methodological problems. There are three reasons for deciding to exclude them in future versions:
The relevance of the results of hypothetical trials will always be in doubt because of uncertainty as to how people would have reacted had the decision to take part in a trial been a real one not a hypothetical one.
It clearly is possible to study recruitment interventions in real trials, avoiding the above problem.
Now that the number of evaluations in real trials has increased, we do not think the trade‐off between value‐added and work involved to include hypothetical trials comes down in favour of including hypothetical trials in future versions of this review.
We excluded research into ways to improve questionnaire response and research looking at incentives and disincentives for clinicians to recruit patients to trials as these issues are addressed by complementary Cochrane Methodology Reviews (Edwards 2009; Rendell 2007). Studies of retention strategies were also excluded as a Cochrane Methodology Review on strategies to reduce attrition from trials is already exists (Brueton 2013).
Types of interventions
Any intervention that aimed to improve recruitment of participants to a randomised trial. The interventions being studied could be directed at potential participants (e.g. patients being randomised to a trial), collaborators (e.g. clinicians recruiting patients for a trial), or others (e.g. research ethics committees). Examples of such interventions are letters introducing the trial being signed by influential people, alternative methods of providing information about the trial to potential participants, additional training for collaborators, financial incentives for participants, telephone follow‐up of expressions of interest and modifications to the design of the trial (e.g. using a preference design).
Types of outcome measures
Primary
Proportion of eligible individuals or centres recruited.
Secondary
None.
Search strategy for identification of studies
We will search the following electronic databases without language restriction for eligible studies:
The Cochrane Methodology Review Group Specialised Register (CMR)
MEDLINE and MEDLINE In Process (OVID)
EMBASE (OVID)
Science Citation Index & Social Science Citation Index (ISI)
ERIC (EBSCO)
The search results will be downloaded to Endnote reference management software and de‐duplicated.
The following MEDLINE search strategy will be adjusted according to the above listed databases.
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Patient Selection/ (50436)
2 ((participat$ or recruit$ or enrol$) adj4 trial?).tw. (16427)
3 1 or 2 (65322)
4 Informed Consent/ (31549)
5 informed consent.tw. (24225)
6 4 or 5 (47497)
7 exp Clinical Trials as Topic/ (283986)
8 Research Subjects/ (5055)
9 (trial? or study or studies or research).tw. (7218575)
10 7 or 8 or 9 (7314164)
11 3 or (6 and 10) (86896)
12 (research support nih extramural or research support nih intramural or research support non us govt or research support us govt non phs or research support us govt phs).pt. (7410137)
13 recruitment.ab. /freq=2 (18332)
14 participation.ab. /freq=2 (16979)
15 12 or 13 or 14 (7422665)
16 11 and 15 (27568)
17 randomized controlled trial.pt. (383951)
18 controlled clinical trial.pt. (88580)
19 random$.ab. (724307)
20 17 or 18 or 19 (914167)
21 16 and 20 (9907)
22 exp animals/ not humans/ (3982927)
23 21 not 22 (9883)
24 23 not (comment or editorial).pt. (9860)
25 24 and ("2009" or "2010" or "2011" or "2012" or "2013" or "2014" or "2015").yr. (4913)
26 25 not 2009$.ed (4453)
***************************
Methods of the review
Identifying trials
Two authors will independently screen the titles and abstracts of all records retrieved from the searches of the electronic bibliographic databases. Any disagreements will be resolved through discussion and, if necessary, the involvement of a third author. The full text will be obtained for studies that appear to meet the inclusion criteria. All potentially eligible studies will be independently assessed by two authors to determine if they meet the inclusion criteria. Any disagreements will be resolved through discussion or the involvement of a third author.
Assessment of methodological quality
We will use the Cochrane Risk of Bias tool (Cochrane Risk of Bias tool) to assess risk of bias. We will use GRADE (Guyatt 2008) on all studies where relevant data are available. Where we do a meta‐analysis, the details of the GRADE assessment will be given in the relevant Summary of Findings table. Where we use GRADE on a single study, we will use the following rules for assigning a GRADE rating of High, Moderate, Low or Very low:
All studies start at High
Study limitations: downgrade all high RoB studies by two levels; downgrade all uncertain RoB studies by one level.
Inconsistency: assume no serious inconsistency.
Indirectness: downgrade all hypothetical studies by two levels.
Imprecision: downgrade all single studies by one level because of the sparseness of data; downgrade by a further one level if the confidence interval is wide and crosses the line where risk difference = 0.
Reporting bias: assume no serious reporting bias.
Data on methodological quality will be presented in an additional table for all included studies.
Although we will not exclude studies because of a high of risk of bias, the low confidence we have in the data they present means that these studies will not be mentioned in the text of the Results or Discussion, except where it has been possible to include them in a meta‐analysis and the data can be interpreted together with data from other studies.
High risk of bias studies will appear in Data and analyses but we suggest that readers use these data only to make decisions as to whether they would like to evaluate the intervention themselves in a more rigorous way. We do not believe they should be used to make judgements about effect.
Data for hypothetical studies will be included in Data and analyses for this version of the review. All of these studies will be excluded from future versions of this review.
Data extraction
Two review authors independently carried out data extraction of each included article (using a proforma specifically designed for the purpose). Differences in data extraction were resolved by discussion. We extracted data on the method evaluated; country in which the study was carried out; nature of the population; nature of the study setting; nature of the study to be recruited into; randomisation or quasi‐randomisation method; and numbers and proportions of participants in the intervention and comparator groups of the study comparing recruitment strategies.
Data analysis
Trials will be grouped according to the type of intervention based on the categorisation used in the Online Resource for Recruitment research in Clinical triAls (ORRCA) project. We split one ORRCA category (Recruitment Information Needs) into two so as to separate out interventions aimed at the consent process from those aimed at more general participant information. Our seven categories are therefore:
Design (Category A). This includes changes to the general design of the trial specifically done to increase recruitment.
Pre‐trial planning (Category B). This includes work done before the trial starts (possibly in a separate study) to explicitly make it more likely that recruitment will be successful.
Trial conduct changes (Category C). This includes initiatives implemented once the trial has started such as better ways of identifying participants, changes to how data are collected, changes to the type of data collected, tailor recruitment to different types of participant.
Modifications to the consent process (Category D). This includes changes to the staff member helping with consent, when consent is taken, what sort of consent information is presented and how it is presented.
Modification to the information given to potential participants about the trial (Category E). This includes who provides it, when, where what sort of information is presented, how the information is presented.
Interventions aimed at the recruiter or recruitment site (Category F). This includes anything that is aimed at the recruiter or recruitment site staff rather than the person being recruited such as changes to training.
Incentives (Category G). Financial and other incentives for participants (but not staff, which is covered by a separate review).
We will present results as risk difference (RD) with the associated 95% confidence intervals (CIs) where sufficient data are available. We will only include cluster‐randomised trials in the meta‐analysis if sufficient data were reported to allow inclusion of analyses that adjusted for clustering; an odds ratio (OR) wil be used as the summary effect in the meta‐analysis result if risk difference or risk ratio clustering adjusted anlayses were not possible with available data. Where two or more studies could be included in a meta‐analyses we will use a fixed effect approach to produce a pooled estimate in the absence of susbtantial heterogeneity.
Publication bias will be investigated for the primary outcomes using a funnel plot where 10 or more studies are available.
Potential conflict of interest
None known.
Additional references
None. All are listed in main review reference list.
Contributions to the protocol
Updated May 2017 by Treweek S, Pitkethly M, Cook J, Mitchell E, Sullivan F, Fraser C, Jackson C, Gardner H.
Contributing authors (October 2007): Treweek S, Sullivan F, Pitkethly M, Jackson C, Wilson S, Kjeldstrøm M, Johansen M, Jones R, Cook J. Comments on drafts (October 2007): Treweek S, Sullivan F, Pitkethly M, Jackson C, Wilson S, Kjeldstrøm M, Johansen M, Jones R, Cook J.
Glossary of selected terms
See the GET IT Glossary (http://getitglossary.org) for plain language definitions of a wide range of terms relevant to fair tests of treatments.
Appendix 3. Participant numbers per study
| Category A ‐ Design | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
| Avenell 2004 Cooper 1997 Fowell 2006 Hemminki 2004 Litchfield 2005 Paul 2011 | 538 273 53 4295 80 398 | 28 | Tehranisa 2014 Welton 1999 | 418 436 | — |
| Total | 5637 | 28 | Total | 854 | — |
| Category B ‐ pre‐trial planning | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
| None | |||||
| Total | 0 | — | Total | 0 | — |
| Category C ‐ Trial conduct changes | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
| Free 2010a Free 2011 Nystuen 2004 Treweek 2012 Wong 2013 | 811 1862 498 880 480 | — | Diguiseppi 2006 Graham 2007 Miller 1999 Mudano 2013 | 469 370 347 155 | — |
| Total | 4531 | Total | 1341 | ||
| Category D ‐ Modification to the consent process | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
| Coyne 2003 Trevena 2006 Wadland 1990 | 226 152 104 | — | Abhyankar 2010 Abd‐Elsayed 2012 MacQueen 2014 Myles 1999 Perrone 1995 | 30 499 80 769 3217 | — |
| Total | 482 | — | Total | 4595 | — |
| Category E ‐ Modification to the information given to potential participants about the trial | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
|
Bergenmar 2014
Brierley 2012
Chen 2011
Cockayne 2017
Du 2008
Du 2009
Ford 2004
Foss 2016
Hutchison 2007
Ives 2001
Kendrick 2001
Kimmick 2005
Larkey 2002 Man 2015ab Man 2015bb Paul 2014 Simel 1991 Simes 1986 Treschan 2003 |
130
2330
14,467
6,900
126
196
12,400
118
173
50
2393
126
15 1364 671 1062 100 57 148 |
— | Dear 2011 Ellis 2002 Freer 2009 Fracasso 2013 Fureman 1997 Jacobsen 2012 Jeste 2009 Karunaratne 2010 Kerr 2004 Llewellyn‐Thomas 1995a Llewellyn‐Thomas 1995b Mandelblatt 2005 Pighills 2009 Weinfurt 2008a Weinfurt 2008b Wells 2013 Weston 1997 | 340 60 41 69 186 462 188 60 130 90 100 450 4488 3623 470 31 90 | — |
| Total | 42,826 | — | Total | 10,878 | — |
| Category F ‐ Interventions aimed at the recruiter or recruitment site | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
|
Monaghan 2007
Liénard 2006 Lee 2017 |
573 29 |
167 744 |
Fleissig 2001 Tilley 2012 | 265 606 | 32 |
| Total | 602 | 1046 | Total | 871 | 32 |
| Category G ‐ Incentives | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| Study | N participants | N clusters | Study | N participants | N clusters |
|
Free 2010c Jennings 2015ad Jennings 2015bd Jennings 2015cd Jennings 2015dd Jennings 2015ed |
491 181 332 93 210 199 |
— | Bentley 2004 Halpern 2004 | 270 126 | — |
| Total | 1506 | — | Total | 396 | — |
| Overall totals | |||||
| Low and uncertain risk of bias | High risk of bias | ||||
| N studies | N participants | N clusters | N studies | N participants | N clusters |
| 36 | 55,584 | 1343 | 32 | 18,935 | 32 |
| All risk of bias | |||||
| N studies | N participants | N clusters | |||
| 66 | 74,519 | 1405 | |||
aContained two interventions (see Category G). bBoth included in same article. cIncluded two interventions (see Category C). dAll included in same article.
Appendix 4. Full list of interventions
-
Design (Category A)
Open RCT versus blinded RCT (GRADE: high; Analysis 1.1)
Patient preference design versus conventional RCT design (GRADE: low; Analysis 2.1)
Electronic data capture versus paper‐based data capture (GRADE: low; Analysis 3.1)
Cluster randomisation versus Zelen design (risk of bias: low Analysis 4.1)
Two‐stage randomisation to choose duration of treatment versus single randomisation (low risk of bias; Paul 2011)
Placebo versus other comparator (high risk of bias; Analysis 4.1)
Video describing response‐adaptive design vs video describing standard design (high risk of bias; Analysis 5.1)
-
Pre‐trial planning (Category B)
None
-
Trial conduct changes (Category C)
Telephone reminder versus no telephone reminder (GRADE: high; Analysis 6.1)
SMS reminder mentioning scarcity vs SMS reminder with no mention (GRADE: moderate; Analysis 7.1)
SMS messages containing quotes from existing participants vs no messages (GRADE: moderate; Analysis 8.1)
Email invitation versus postal invitation (GRADE: moderate; Analysis 9.1)
Telephone screening versus face‐to‐face screening (high risk of bias; Analysis 10.1)
Screening by senior investigator versus screening by research assistant (high risk of bias; Analysis 11.1)
Tablet computer to support screening vs voice response system to support screening (high risk of bias; Analysis 12.1)
Electronic completion of screening questionnaire versus standard paper completion (high risk of bias; Analysis 13.1)
Oral completion of screening questionnaire versus standard paper completion (high risk of bias; Analysis 14.1)
-
Modifications to the consent process (Category D)
Opt‐out consent versus opt‐in consent (GRADE: low; Analysis 15.1)
Consent to experimental care versus usual consent (GRADE: very low; Analysis 16.1)
Consent to standard care versus usual consent (GRADE: very low; Analysis 17.1)
Researcher reading our consent versus participant reading consent (GRADE: very low; Analysis 18.1)
Easy to read consent versus standard consent (unclear risk of bias; Coyne 2003)
Information printed on heavyweight paper and blue folio vs standard (high risk of bias; Analysis 19.1)
Refusers choose treatment versus usual consent (high risk of bias; Analysis 20.1)
Physician‐modified consent versus usual consent (high risk of bias; Analysis 21.1)
Participant‐modified consent versus usual consent (high risk of bias; Analysis 22.1)
Implicit participant values clarification task vs standard (high risk of bias; Analysis 23.1)
Explict participant values clarification task vs standard (high risk of bias; Analysis 24.1)
Open ended assessment of comprehension versus closed‐ended assessment (high risk of bias; MacQueen 2014)
-
Modification to the information given to potential participants about the trial (Category E)
Bespoke user‐tested PIL vs usual PIL (GRADE: high; Analysis 25.1)
Brief participant information leaflet (PIL) vs full PIL (GRADE: moderate; Analysis 26.1)
Study‐related questionnaire + trial invitation versus trial invitation (GRADE: moderate; Analysis 27.1)
PIL developed with feedback from users vs usual PIL (GRADE: moderate; Analysis 28.1)
Recruitment primer letter vs no letter (GRADE: low; Analysis 29.1)
Information provided over telephone vs information provided face‐to‐face (GRADE: low; Analysis 30.1)
Enhanced recruitment package + recruitment at churches versus standard recruitment package (GRADE: low; Analysis 31.1)
Enhanced recruitment package versus standard recruitment package (GRADE: low; Analysis 32.1)
Enhanced recruitment package + baseline data over telephone versus standard recruitment package (GRADE: low; Analysis 33.1)
Emphasising risk in information versus standard information (GRADE: low; Analysis 34.1)
Wording treatment effect is 'twice as fast' in trial information versus writing 'half as fast' (GRADE: low; Analysis 35.1)
Emphasising pain in information versus standard information (GRADE: low; Analysis 36.1)
Providing information by video versus standard information (GRADE: very low; Analysis 37.1)
Audio record of information given about trial vs no audio record (GRADE: very low; Analysis 38.1)
Clinical trial booklet + standard information versus standard information (GRADE: very low; Analysis 39.1)
Total information disclosure versus standard disclosure (GRADE: very low; Analysis 40.1)
Standard information about trial plus symposium + other educational material versus standard information (unclear risk of bias; Kimmick 2005)
Newspaper article + study information versus study information only (high risk of bias; Analysis 41.1)
Interactive computer presentation of trial information versus standard paper presentation (high risk of bias; Analysis 42.1)
Access to cancer trials website vs no access (high risk of bias; Analysis 43.1)
More favourable newspaper article + study information versus less favourable article + study information (high risk of bias; Analysis 44.1)
Clinical trial booklet + standard information versus standard information (high risk of bias; Analysis 45.1)
Educational audiovisual information + help versus standard information + general audiovisual information + help (high risk of bias; Analysis 46.1)
Educational audiovisual information with written information versus written information (high risk of bias; Analysis 47.1)
Negative framing of side effects versus neutral framing (high risk of bias; Analysis 48.1)Positive framing of side effects versus neutral framing (high risk of bias; Analysis 49.1)
Less detailed presentation of risk and other information versus more detailed presentation (high risk of bias; Analysis 50.1)
Information leaflet with explanation versus information leaflet without explanation (high risk of bias; Analysis 51.1)
Brief counselling + print materials versus print materials (high risk of bias; Analysis 52.1)
Interactive computer presentation of trial information versus audio‐taped presentation (high risk of bias; Analysis 53.1)
One new versus both standard (description of intervention) (high risk of bias; Analysis 54.1)
Coach to support recruitment of minority participants versus no coach (high risk of bias; Fracasso 2013)
Financial disclosure saying drug company pays investigator versus no disclosure (high risk of bias; Weinfurt 2008a)
Presenting increasing amounts of financial disclosure information about investigator (high risk of bias; Weinfurt 2008b)
Video + pamphlet describing the trial versus pamphlet only (high risk of bias; Fureman 1997)
Multimedia psychoeducational DVD and written information providing trial information versus written information only (high risk of bias; Jacobsen 2012)
Spanish‐language multimedia information versus Spanish‐language written information (high risk of bias; Wells 2013)
Use of Hispanic lay advocates versus no advocates (unclear risk of bias; Larkey 2002)
Interventions aimed at the recruiter or recruitment site (Category F)
Teaser campaign using postcards vs no teaser (GRADE: moderate; Analysis 55.1)
Additional communication from central trial coordinator to sites versus standard communication (low risk of bias; Monaghan 2007)
Site initiation visit versus no initiation visit (low risk of bias; Liénard 2006)
Recruitment coordinator plus training vs usual recruitment (high risk of bias; Analysis 56.1)
Doctor knows patient preferences about participation vs standard (high risk of bias; Analysis 56.1)
-
Incentives (Category G)
Financial incentive vs no incentive (GRADE: moderate; Analysis 57.1)
Variation in information provided about adverse events, participants receiving placebo and payments to participants (high risk of bias; Halpern 2004)
Variation in hourly payment plus risk‐based bonuses (high risk of bias; Bentley 2004)
4.1. Analysis.
Comparison 4 A–Placebo vs other comparator (high risk of bias; hypothetical), Outcome 1 Participants recruited.
5.1. Analysis.
Comparison 5 A–Video describing response‐adaptive design vs video describing standard design (high risk of bias; hypothetical), Outcome 1 Participants recruited.
10.1. Analysis.
Comparison 10 C–Telephone screening vs face‐to‐face screening (high risk of bias), Outcome 1 Participants recruited.
11.1. Analysis.
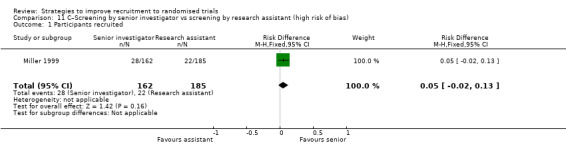
Comparison 11 C–Screening by senior investigator vs screening by research assistant (high risk of bias), Outcome 1 Participants recruited.
12.1. Analysis.
Comparison 12 C–Tablet computer to support screening vs voice response system to support screening (high risk of bias), Outcome 1 Willingness to take part if eligible.
13.1. Analysis.
Comparison 13 C–Electronic completion of screening questionnaire vs standard paper completion (high risk of bias; hypothetical), Outcome 1 Participants recruited.
14.1. Analysis.
Comparison 14 C–Oral completion of screening questionnaire vs standard paper completion (high risk of bias; hypothetical), Outcome 1 Participants recruited.
16.1. Analysis.
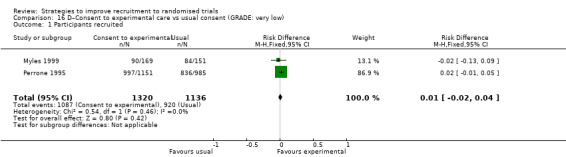
Comparison 16 D–Consent to experimental care vs usual consent (GRADE: very low), Outcome 1 Participants recruited.
17.1. Analysis.
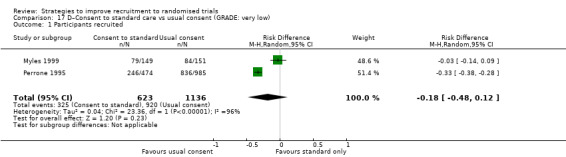
Comparison 17 D–Consent to standard care vs usual consent (GRADE: very low), Outcome 1 Participants recruited.
19.1. Analysis.
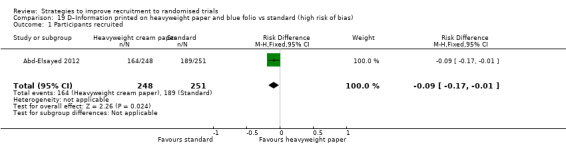
Comparison 19 D–Information printed on heavyweight paper and blue folio vs standard (high risk of bias), Outcome 1 Participants recruited.
20.1. Analysis.
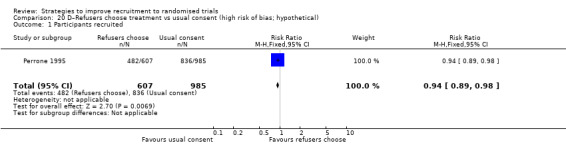
Comparison 20 D–Refusers choose treatment vs usual consent (high risk of bias; hypothetical), Outcome 1 Participants recruited.
21.1. Analysis.
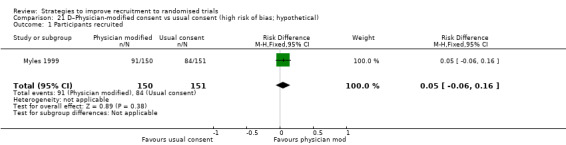
Comparison 21 D–Physician‐modified consent vs usual consent (high risk of bias; hypothetical), Outcome 1 Participants recruited.
22.1. Analysis.
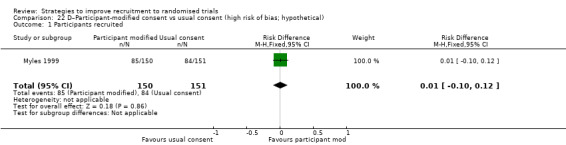
Comparison 22 D–Participant‐modified consent vs usual consent (high risk of bias; hypothetical), Outcome 1 Participants recruited.
23.1. Analysis.
Comparison 23 D–Implicit participant values clarification task vs standard consent procedure (high risk of bias; hypothetical), Outcome 1 Participants recruited.
24.1. Analysis.
Comparison 24 D–Explicit participant values clarification task vs standard (high risk of bias; hypothetical), Outcome 1 Participants recruited.
41.1. Analysis.
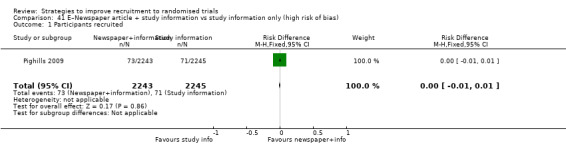
Comparison 41 E–Newspaper article + study information vs study information only (high risk of bias), Outcome 1 Participants recruited.
42.1. Analysis.
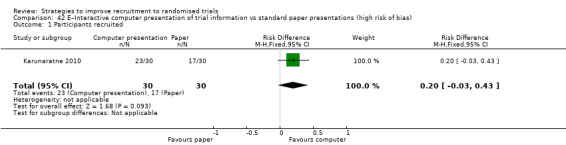
Comparison 42 E–Interactive computer presentation of trial information vs standard paper presentations (high risk of bias), Outcome 1 Participants recruited.
43.1. Analysis.
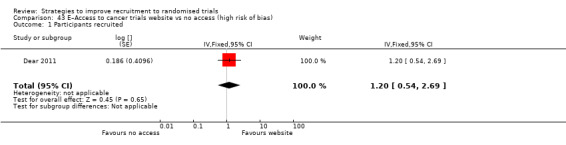
Comparison 43 E–Access to cancer trials website vs no access (high risk of bias), Outcome 1 Participants recruited.
44.1. Analysis.
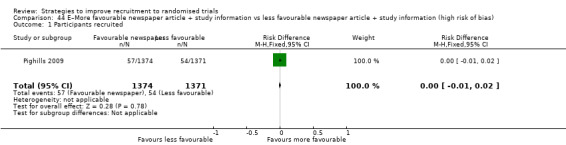
Comparison 44 E–More favourable newspaper article + study information vs less favourable newspaper article + study information (high risk of bias), Outcome 1 Participants recruited.
45.1. Analysis.
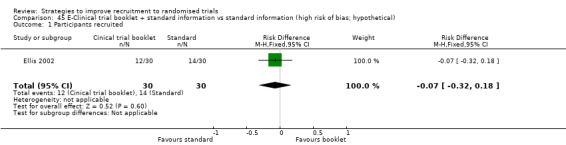
Comparison 45 E‐Clinical trial booklet + standard information vs standard information (high risk of bias; hypothetical), Outcome 1 Participants recruited.
46.1. Analysis.
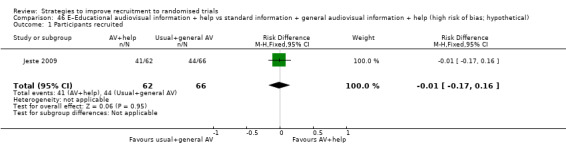
Comparison 46 E–Educational audiovisual information + help vs standard information + general audiovisual information + help (high risk of bias; hypothetical), Outcome 1 Participants recruited.
47.1. Analysis.
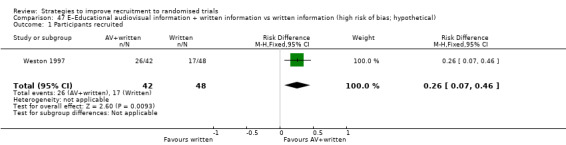
Comparison 47 E–Educational audiovisual information + written information vs written information (high risk of bias; hypothetical), Outcome 1 Participants recruited.
48.1. Analysis.
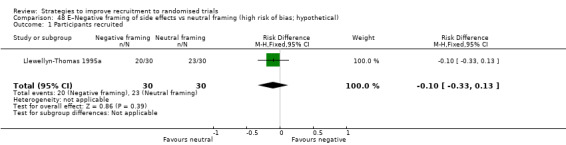
Comparison 48 E–Negative framing of side effects vs neutral framing (high risk of bias; hypothetical), Outcome 1 Participants recruited.
49.1. Analysis.
Comparison 49 E–Positive framing of side effects vs neutral framing (high risk of bias; hypothetical), Outcome 1 Participants recruited.
50.1. Analysis.
Comparison 50 E–Less detailed presentation of risk and other information vs more detailed presentation (high risk of bias; hypothetical), Outcome 1 Participants recruited.
51.1. Analysis.
Comparison 51 E–Information leaflet with explanation vs information leaflet without explanation (high risk of bias; hypothetical), Outcome 1 Participants recruited.
52.1. Analysis.
Comparison 52 E–Brief counselling + print materials vs print alone (high risk of bias; hypothetical), Outcome 1 Participants recruited.
53.1. Analysis.
Comparison 53 E–Interactive computer presentation of trial information vs audio‐taped presentation (high risk of bias; hypothetical), Outcome 1 Participants recruited.
54.1. Analysis.
Comparison 54 E–One new vs both standard (intervention description) (high risk of bias; hypothetical), Outcome 1 Participants recruited.
56.1. Analysis.
Comparison 56 F–Doctor knows patient preferences about participation vs standard (high risk of bias), Outcome 1 Participants recruited.
Data and analyses
Comparison 1. A–Open trial vs blinded trial (GRADE: high).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 4833 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [0.07, 0.13] |
Comparison 2. A–Patient preference design vs conventional RCT (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 273 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.15, 0.07] |
Comparison 3. A–Electronic data capture vs paper‐based data capture (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 80 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.13 [‐0.24, ‐0.03] |
Comparison 4. A–Placebo vs other comparator (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 436 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.18, ‐0.00] |
Comparison 5. A–Video describing response‐adaptive design vs video describing standard design (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 418 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.22] |
Comparison 6. C–Telephone reminder vs no telephone reminder (GRADE: high).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 1450 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [0.03, 0.09] |
Comparison 7. C–SMS reminder mentioning scarcity vs SMS reminder with no mention (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 1862 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.06] |
Comparison 8. C–SMS messages containing quotes from existing participants vs no messages (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 811 | Risk Difference (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.06] |
Comparison 9. C–Email invitation vs postal invitation (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 1760 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.04] |
Comparison 10. C–Telephone screening vs face‐to‐face screening (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 469 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.24] |
Comparison 11. C–Screening by senior investigator vs screening by research assistant (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 347 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.02, 0.13] |
Comparison 12. C–Tablet computer to support screening vs voice response system to support screening (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Willingness to take part if eligible | 1 | 155 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [0.01, 0.29] |
Comparison 13. C–Electronic completion of screening questionnaire vs standard paper completion (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 292 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.20, 0.03] |
Comparison 14. C–Oral completion of screening questionnaire vs standard paper completion (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 219 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.14, 0.14] |
Comparison 15. D–Opt‐out consent vs opt‐in consent (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 152 | Risk Difference (M‐H, Fixed, 95% CI) | 0.19 [0.03, 0.35] |
Comparison 16. D–Consent to experimental care vs usual consent (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 2456 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.04] |
Comparison 17. D–Consent to standard care vs usual consent (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 1759 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.48, 0.12] |
Comparison 18. D–Researcher reading out consent vs participant reading consent (unclear risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 104 | Risk Difference (M‐H, Fixed, 95% CI) | 0.06 [‐0.13, 0.25] |
Comparison 19. D–Information printed on heavyweight paper and blue folio vs standard (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 499 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.17, ‐0.01] |
Comparison 20. D–Refusers choose treatment vs usual consent (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 1592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.89, 0.98] |
Comparison 21. D–Physician‐modified consent vs usual consent (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 301 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
Comparison 22. D–Participant‐modified consent vs usual consent (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 301 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.10, 0.12] |
Comparison 23. D–Implicit participant values clarification task vs standard consent procedure (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 20 | Risk Difference (M‐H, Fixed, 95% CI) | 0.15 [‐0.23, 0.53] |
Comparison 24. D–Explicit participant values clarification task vs standard (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 19 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.50, 0.37] |
Comparison 25. E–Bespoke, user‐tested PIL vs usual PIL (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 3 | 6634 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
Comparison 26. E–Brief participant information leaflet (PIL) vs full PIL (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 4633 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.02] |
Comparison 27. E–Study‐related questionnaire + trial invitation vs trial invitation (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 2393 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [0.02, 0.08] |
Comparison 28. E–PIL developed with feedback from users vs usual PIL (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 2 | 16763 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.00, 0.01] |
Comparison 29. E–Recruitment primer letter vs no letter (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 1062 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.06, 0.06] |
Comparison 30. E–Information provided over telephone vs information provided face‐to‐face (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 118 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.18, 0.05] |
Comparison 31. E–Enhanced recruitment package + recruitment at churches vs standard recruitment package (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 6246 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.02] |
Comparison 32. E–Enhanced recruitment package vs standard recruitment package (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 6376 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.00] |
Comparison 33. E–Enhanced recruitment package + baseline data over telephone vs standard recruitment package (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 6372 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.01, 0.01] |
Comparison 34. E–Emphasising risk in information vs standard information (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 97 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.38 [‐0.56, ‐0.19] |
Comparison 35. E–Wording treatment effect as 'twice as fast' in trial information vs writing 'half as fast' (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.45] |
Comparison 36. E–Emphasising pain in information vs standard information (GRADE: low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 98 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.29 [‐0.48, ‐0.10] |
Comparison 37. E–Providing information by video vs standard information (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 3 | 495 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.04, 0.09] |
Comparison 38. E–Audio record of information given about trial vs no audio record (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 130 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.03 [‐0.19, 0.13] |
Comparison 39. E–Clinical trial booklet + standard information vs standard information (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 31 | Risk Difference (M‐H, Random, 95% CI) | 0.20 [‐0.05, 0.46] |
Comparison 40. E–Total information disclosure vs standard disclosure (GRADE: very low).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 57 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [‐0.06, 0.28] |
Comparison 41. E–Newspaper article + study information vs study information only (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 4488 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
Comparison 42. E–Interactive computer presentation of trial information vs standard paper presentations (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | 0.20 [‐0.03, 0.43] |
Comparison 43. E–Access to cancer trials website vs no access (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | (Fixed, 95% CI) | 1.20 [0.54, 2.69] |
Comparison 44. E–More favourable newspaper article + study information vs less favourable newspaper article + study information (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 2745 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
Comparison 45. E‐Clinical trial booklet + standard information vs standard information (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.32, 0.18] |
Comparison 46. E–Educational audiovisual information + help vs standard information + general audiovisual information + help (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 128 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.01 [‐0.17, 0.16] |
Comparison 47. E–Educational audiovisual information + written information vs written information (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 90 | Risk Difference (M‐H, Fixed, 95% CI) | 0.26 [0.07, 0.46] |
Comparison 48. E–Negative framing of side effects vs neutral framing (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.10 [‐0.33, 0.13] |
Comparison 49. E–Positive framing of side effects vs neutral framing (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 60 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.17 [‐0.40, 0.06] |
Comparison 50. E–Less detailed presentation of risk and other information vs more detailed presentation (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 19 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.37, 0.50] |
Comparison 51. E–Information leaflet with explanation vs information leaflet without explanation (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 37 | Risk Difference (M‐H, Fixed, 95% CI) | 0.19 [‐0.13, 0.50] |
Comparison 52. E–Brief counselling + print materials vs print alone (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 450 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.18] |
Comparison 53. E–Interactive computer presentation of trial information vs audio‐taped presentation (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 100 | Risk Difference (M‐H, Fixed, 95% CI) | 0.2 [0.01, 0.39] |
Comparison 54. E–One new vs both standard (intervention description) (high risk of bias; hypothetical).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 124 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.16 [‐0.31, ‐0.01] |
Comparison 55. F–Teaser campaign using postcards vs no teaser (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary care centre recruited | 1 | 670 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.04, 0.05] |
Comparison 56. F–Doctor knows patient preferences about participation vs standard (high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 1 | 265 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
Comparison 57. G‐Financial incentive vs no incentive (GRADE: moderate).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants recruited | 6 | 1506 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd‐Elsayed 2012.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care in USA. 499 participants were eligible for 1 of 3 trials; all had substantial illness requiring major surgery (cardiac) at least 24 hours after being asked about consent | |
| Comparisons | Investigated the use of different consent form presentations Intervention A: consent documents on heavy weight cream‐coloured paper (20‐pound) and a blue folder Comparator: consent documents as photocopies stapled together. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Unclear | Participants did not know there was a study. Personnel knew, and there was possibility that this could influence consent conversation, but there was substantial training so the effect is less clear. |
| Blinding of outcome assessment ok? | Yes | Participants were blind and data entered by someone who was blinded |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Review only interested in recruitment, which is reported |
| Was the study free of other bias? | No | Trial stopped early because of host trials stopping early and consent responsibility for the third trial site moving to a different department |
| Overall bias? | Yes | High risk of bias |
Abhyankar 2010.
| Methods | Randomised controlled trial | |
| Data | Setting: university, UK. 30 participants were women students and staff aged over 18 years on the university email list | |
| Comparisons | Investigated the use of trial information with clarification of values Intervention A: study information plus implicit values clarification task (look at info) Intervention B: study information plus implicit and explicit values clarification task (look at info and engage with it by making ratings of what is important to you) Comparator: routine information |
|
| Outcomes | Willingness to take part in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Insufficient detail in paper to be sure what was done |
| Allocation concealment? | Unclear | Uncertain if the random numbers list was open and so investigators could in principle influence allocation |
| Blinding of participants and personnel ok? | Unclear | Linked to qualitative work; possible that investigators could influence quantitative work through qualitative work and they know allocation by this stage (if not before). |
| Blinding of outcome assessment ok? | Unclear | Willingness to take part is self‐report; not clear what participants were told beforehand, which could influence what they report |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported, and this is the only outcome needed for review. |
| Was the study free of other bias? | No | Trial is hypothetical so outcome is just a proxy for real decision |
| Overall bias? | Yes | High risk of bias |
Avenell 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, UK. 538 participants aged 70 years or over, attending a fracture clinic or orthopaedic ward | |
| Comparisons | Investigated the effect of different trial designs Open trial design comparing vitamin D versus calcium versus vitamin D plus calcium versus no tablets. Compared to conventional trial comparing vitamin D versus calcium versus vitamin D plus calcium versus placebo. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Pre‐programmed laptop computer‐generated sequence |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | Not all participants were blinded, but this was the point of the evaluation so the trial has not been penalised on this risk of bias item. Those in comparison group were blinded. Tablets were sent out centrally by trial staff, not handed out by clinical staff. |
| Blinding of outcome assessment ok? | Yes | Objective outcome recorded by trial team |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Bentley 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: university, USA. 270 pharmacy student participants | |
| Comparisons | Investigated the effect of financial incentives and trial risk 9‐arm trial looking at the effect of financial incentives and bonus based on the level of risk (high, medium or low) associated with the intervention drug Interventions A‐C: information on high‐risk trial for a drug not yet tested on humans, paying USD 1800, USD 800 or USD 350 Interventions D‐F: information on medium‐risk study for a generic drug already on the market, paying USD 1800, USD 800 or USD 350 Intervention G‐I: information on low‐risk study measuring salivary levels of stress hormones, paying USD 1800, USD 800 or USD 350 |
|
| Outcomes | Willingness to take part in hypothetical studies | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Text just says 'randomly distributed' but does not say how the randomisation was done. |
| Allocation concealment? | Yes | Not entirely clear, but trial team handed packs to course instructors to distribute, and it is unlikely that instructors of students receiving packs could foresee allocation. |
| Blinding of participants and personnel ok? | Unclear | Participants potentially able to discuss, though people handing out envelopes (course instructors) were blinded |
| Blinding of outcome assessment ok? | No | Participants gave self‐reported 'willingness to participate' response, which could potentially have been influenced by ability to discuss allocation with other participants |
| Incomplete outcome data handled ok? | Unclear | Some responses were discarded because of missing data, unclear why |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Bergenmar 2014.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Sweden. Participants were 130 patients eligible for a phase II or III cancer drug trial involving 1 of 13 oncologists consenting to be recorded during study period | |
| Comparisons | Investigated use of audio recording to improve communication about the trial Intervention: an audio recording (CD), using a portable voice recorder, of the information given at the medical consultation in which the patients were informed about a clinical drug trial Comparator: no CD |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Nurse did randomisation but does not say how |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Yes | Adequate |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Brierley 2012.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. 2330 participants were people eligible for a trial about computerised CBT in depression | |
| Comparisons | Investigated effect of length of the participant information leaflet on recruitment. Intervention: short participant information leaflet (not clear how short) as initial info about trial Comparator: full length participant information leaflet (8‐pages) as initial info about trial |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | People sending out packs blind, as well as potential participants |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review. |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Chen 2011.
| Methods | Randomised controlled trial | |
| Data | Setting: unclear but probably secondary, UK. Participants were eligible for 3 host trials but unclear what the trials were. 2 comparisons against original PIL: 2302 participants in analysis for first, 12,164 participants in analysis for second | |
| Comparisons | Investigated different version of the participant information leaflet (PIL) Intervention 1: invitation letter with brief summary of PIL Intervention 2: PIL modified after focus group discussions; enclosed with letter Comparator: invitation letter with full original PIL |
|
| Outcomes | Proportion recruited to pre‐randomisation phase of trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Conference abstract and limited details. Additional information from co‐author R Haynes: randomisation by computer (Haynes 2016). |
| Allocation concealment? | Yes | As above. R Haynes provided datasets from hospitals with typically thousands of potentially eligible participants and (under section 251 support) we mailed these patients from Cancer Trials Support Unit. The invitations were generated by a computer programme with an incorporated randomisation element (so the different invitations were produced automatically according to the random allocation); this is how allocation was kept concealed so the investigator had no way of knowing what their patients were going to receive. |
| Blinding of participants and personnel ok? | Yes | Participants definitely blinded. Staff blinding unclear but effect of knowing on recruitment probably minimal |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported, and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Cockayne 2017.
| Methods | Randomised controlled trial | |
| Data | Setting: community NHS clinics, UK. 6900 patients eligible for the REFORM study (over 64 years, routine podiatry appointment in past 6 months) and offered an appointment at NHS podiatry clinics across 5 centres. Ineligible if report neuropathy, dementia or other neurological condition, unable to walk unaided, lower limb amputation, unwilling to attend local podiatry clinic. 3‐arm trial of a bespoke user‐tested PIL and a template‐developed PIL against the usual PIL | |
| Comparisons | Investigated different version of the participant information leaflet (PIL) Intervention 1: bespoke, user‐tested PIL and letter, with graphic design input Intervention 2: template developed PIL and original study letter with public and patient involvement (PPI) feedback but no user‐testing or design input Comparator: PIL developed for REFORM trial using NRES (ethics) template with study invitation letter |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Generated electronically, stratified by centre |
| Allocation concealment? | Yes | Independent data manager, IDs used, invitation packs sent centrally |
| Blinding of participants and personnel ok? | Yes | Participants and research staff blinded; not admin staff but unlikely to have affected the allocation |
| Blinding of outcome assessment ok? | Yes | Objective assessment |
| Incomplete outcome data handled ok? | Yes | No missing data |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent. Sensitivity analysis showed negligible effect of newsletter in pack. May be underpowered |
| Overall bias? | No | Low risk of bias |
Cooper 1997.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, UK. 273 first‐time attendees at a gynaecological clinic | |
| Comparisons | Investigated the effect of different trial designs Partially randomised patient preference design allocating to medical management or transcervical resection of the endometrium or preferred option. Comparator was a conventional trial design allocating to medical management or transcervical resection of the endometrium. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated list |
| Allocation concealment? | Yes | Series of sealed, opaque envelopes |
| Blinding of participants and personnel ok? | Yes | Participants were blinded but not investigators. All participants (intervention and control) were seen by the same trial investigator. Impossible not to unblind investigator since he/she had to know allocation to deliver information to participant |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Coyne 2003.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: secondary care, USA. 226 patients eligible for participation in a cancer treatment trial | |
| Comparisons | Investigated the effect of different consent methods Easy to read consent statements (altered text style, layout, font size, vocabulary; reading level 7th to 8th grade) were compared to standard consent statements |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Definitely randomised but unclear how this was done |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Unclear | Nurse clearly knew that the participant had intervention or control consent statement; not clear how much participant was told about the intervention. Not clear if telephone interviewers knew the allocation |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Dear 2011.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: secondary care, Australia. 340 participants with cancer who had Internet access | |
| Comparisons | Investigated whether information provided through a website improved recruitment Intervention: access to a consumer‐friendly cancer clinical trials site, which enables people to search for trials Comparator: usual care (no access to site) |
|
| Outcomes | Self‐reported (by participant) recruitment to a trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | Participants were blind to purpose of study. Doctors knew purpose but only intervention group got link to website. |
| Blinding of outcome assessment ok? | Yes | Assessors were blinded |
| Incomplete outcome data handled ok? | No | More than double amount of missing data in intervention group because consultations not recorded and participants not completing follow‐up questionnaires. |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Yes | High risk of bias |
Diguiseppi 2006.
| Methods | Quasi‐randomised controlled trial | |
| Data | Setting: health maintenance organisation, USA. Participants were 469 patients aged 18 or over attending the HMO with an acute injury | |
| Comparisons | Investigated the effect of different methods of pre‐screening participants Telephone administered questionnaire on hazardous drinking and willingness to participate in lifestyle intervention. This was compared to face‐to‐face administered questionnaire on hazardous drinking and willingness to participate in behavioural intervention |
|
| Outcomes | Proportion recruited to hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | By week |
| Allocation concealment? | No | As above |
| Blinding of participants and personnel ok? | Unclear | Potential participants were probably blind but researchers and practice staff were not blind. |
| Blinding of outcome assessment ok? | Unclear | Not clear what impact researcher and practice staff being unblinded may have on discussions with participants. Outcome not objective (willingness to participate not actual participation) |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Du 2008.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. 126 patients aged 21 to 80 attending multidisciplinary lung clinic at a cancer centre | |
| Comparisons | Investigated the effect of different methods of providing information about the trial 18‐minute educational video giving an overview of clinical trials and the importance of cancer clinical research to society. This was compared to standard care (i.e. normal first visit to oncologist). |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomised but no more details |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Yes | Oncologist was blinded but the participant was not (not clear if they were told that intervention was a video versus standard care). Outcome objective so probably not a problem |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Du 2009.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. 196 women scheduled for treatment evaluation by medical oncology specialist at Karmanos Cancer Institute (KCI) breast clinic. Aged 21 to 80, new female patient at clinic, with diagnosis of histologically confirmed invasive breast cancer, and self‐determined as white or African American. Plus: the ability to read and understand English at least at the 6th grade level, the capability to make their own treatment decisions, not having previously participated in a cancer clinical trial, and performance status (PS) B 2 (Southwest Oncology Group (SWOG) scale) | |
| Comparisons | Intervention: 18‐minute video. The video presents an overview of phase I, II and III clinical trials and the importance of cancer clinical research to society. The video addresses common concerns regarding clinical trials and cancer treatment from the patient's perspective such as side effects, expected risks and benefits, eligibility criteria, the enrolment process, and treatment costs. Comparator: usual practice ‐ return to waiting room but not clear what 'standard care' actually is | |
| Outcomes | Enrolment in therapeutic trials | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomised but no more details |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Unclear | Not clear if staff were blinded, and for participants it depended on what they had been told about study. Participants completed questionnaires themselves so may not have been influenced by staff if staff were unblinded. |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Ellis 2002.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Australia. 60 women undergoing definitive surgical operation for early stage breast cancer | |
| Comparisons | Intervention: booklet explaining trials, how treatment is selected in RCT, discussion of treatment options, examples of trials, where to get more info, advantages and disadvantages of participating + usual information from clinician, discussion of treatment which may include discussion of RCT, no standardisation of what is discussed Comparator: usual information from clinician, discussion of treatment which may include discussion of RCT, no standardisation of what is discussed | |
| Outcomes | Willingness to take part in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomised but no more details |
| Allocation concealment? | Yes | Text says 'randomised centrally' but doesn't say how |
| Blinding of participants and personnel ok? | Unclear | Not clear what participants were told. Not clear if clinicians providing general advice knew allocation. |
| Blinding of outcome assessment ok? | Unclear | Outcome not objective and not clear what influence lack of blinding might have had on this. |
| Incomplete outcome data handled ok? | Unclear | 84 were randomised but only had baseline data for 79 and outcome data for 60. No difference across groups in number of questionnaires not returned. |
| Free of selective reporting? | Yes | Willingness to take part was outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Fleissig 2001.
| Methods | Quasi‐randomised trial (used order in which people turned up for consultations) | |
| Data | Setting: secondary care, UK. 265 participants were cancer patients 16 or older eligible for 1 of 40 local trials. 23 trials were offered to both control and intervention groups | |
| Comparisons | Investigated improving communication between recruiter and potential participant Intervention: doctor presented with patient preferences on trial participation prior to discussion about trial participation Comparator: doctor does normal trial discussion without knowing patient preferences |
|
| Outcomes | Proprortion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Consultation sequence is part of allocation, so it is possible to predict who will get control and who gets intervention |
| Allocation concealment? | No | As above |
| Blinding of participants and personnel ok? | Yes | Participants blinded but not doctors, but hard to avoid this |
| Blinding of outcome assessment ok? | Yes | Main outcome for review is recruitment, which is objective. Also some independent assessment though probably not necessary for recruitment |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Yes | High risk of bias |
Ford 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: community, USA. 12,400 African American men aged 55 to 74 eligible for a prostate, lung and colorectal cancer screening trial | |
| Comparisons | Investigated the effect of different trial information and consent methods Intervention A: enhanced recruitment letter, telephone call by African American interviewer, baseline information by mail, reminder calls/mailings for baseline information/consent Intervention B: enhanced recruitment letter, telephone call by African American interviewer, baseline information over telephone, reminder calls/mailings for consent form Intervention C: enhanced recruitment letter, telephone call by African American interviewer, church session, baseline information at church session Compared to standard recruitment letter, telephone assessment by African American or white interviewer, baseline information by mail, reminder calls/mailings for baseline information/consent |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomised but no more details |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Unclear | Potential participants were blinded but the researchers probably were not blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Foss 2016.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Denmark. 118 women giving birth at 1 of 3 hospitals and eligible for the Danish Calmette Study | |
| Comparisons | Investigated the effect of different trial information and consent methods | |
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Central, web‐based block‐randomisation with variable block sizes of 2, 4, and 6 in random order |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Yes | Participants blinded although staff giving information were not , though they followed an SOP regarding what to say. Probably didn't affect outcome |
| Blinding of outcome assessment ok? | Yes | Outcome objective |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Fowell 2006.
| Methods | Cluster‐randomised cross‐over trial | |
| Data | Setting: secondary care, UK. 53 Cancer inpatients receiving palliative care and starting on a syringe driver | |
| Comparisons | Investigated the effect of different trial designs Cluster‐randomisation compared to Zelen's design (in which only those randomised to the intervention group were asked for consent) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Coin‐tossing for initial allocation to cluster or Zelen (2 sites only) |
| Allocation concealment? | Yes | Only 2 sites and allocation to intervention (Zelen or cluster) by coin toss almost certainly done centrally |
| Blinding of participants and personnel ok? | Yes | Blinding only partial, but looking at the effect of open study design was the purpose of the study |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Fracasso 2013.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. Participants were 60 patients with cancer recruited through the Siteman Cancer Center (SCC). Patients were identified by their medical, radiation, or surgical oncologist at the time of evaluation for treatment. Patients were ≥ 18 years of age; English speaking; self‐reported as a member of a racial or ethnic minority; diagnosed with advanced breast, colorectal, lung, or prostate carcinoma with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2 | |
| Comparisons | Investigated coaching as a way of improving recruitment Intervention: African American coach providing individualised, flexible education and support to create context of trust promoting trial enrollment Comparator: no coach (usual care) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Says randomly allocated but nothing more |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Unclear | Not clear what participants knew about the intervention prior to being randomised; all provided consent so they were told something |
| Blinding of outcome assessment ok? | Yes | Objective outcome (recruitment) |
| Incomplete outcome data handled ok? | Yes | 6 died or were lost to follow‐up, but not clear which groups they were in. But unlikely due to intervention. |
| Free of selective reporting? | Unclear | Recruitment reported, and this is only outcome needed for review |
| Was the study free of other bias? | Unclear | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Free 2011.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 1592 smokers eligible for a smoking cessation trial | |
| Comparisons | Investigated effect of mentioning scarcity on recruitment Intervention: SMS reminder message including scarcity message 'only 300 places left' Comparator: SMS reminder without mention of scarcity |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | Adequate |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Free 2010.
| Methods | Randomised controlled trial | |
| Data | Setting: community, UK. Participants were 1302 daily smokers, 16 or over, wanting to stop smoking in next month | |
| Comparisons | Investigated whether including GBP 5 with invitation or sending SMS messages to potential participants increased recruitment Intervention A: GBP 5 with participant info sheet and consent form Intervention B: series of 4 text messages with quotes from existing participants Comparator: normal trial procedures ‐ letter with participant information sheet and consent form |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | For the 2 trials covered in this review the data manager placed registration ID numbers of participants in ascending numerical order and alternate participants were allocated systematically to the intervention or control group. The ID numbers were not linked to any names or other personally identifying information, so allocation was concealed. Additional information from the study author: all the data manager had was a list of numbers with no other linked information. The order of numbers were generated by the timing of recruitment to the txt2stop randomisation. The allocation could be checked, i.e. there was no way of manipulating it. |
| Allocation concealment? | Yes | Central (web‐based)/data manager |
| Blinding of participants and personnel ok? | Yes | Participants blind but not research staff, unlikely to affect outcome measurement (assessment was blinded) |
| Blinding of outcome assessment ok? | Yes | Objective outcome and assessors were blind |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Registration to trial outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Freer 2009.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, UK. Participants were 41 parents of immature infant(s) were admitted to a large tertiary NICU but who did not require intensive care (i.e. not requiring mechanical ventilation or continuous observation) | |
| Comparisons | Intervention A: US trial leaflet with explanation Intervention B: US trial leaflet alone Intervention C: UK trial leaflet with explanation Intervention D: UK trial leaflet alone | |
| Outcomes | Willingness to take part in a hypothetical study | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Randomisation done by independent person using sequential, sealed opaque envelopes |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Unclear | Depends what researchers providing standard statements knew and what participants were told about the study. |
| Blinding of outcome assessment ok? | Unclear | Outcome not objective and not clear what influence lack of blinding might have had on this. |
| Incomplete outcome data handled ok? | Unclear | 54 were randomised but 41 provided questionnaires. Reasons for non‐completion are not given per group. No real difference in the number of questionnaires returned per group. |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs. |
| Was the study free of other bias? | No | Hypothetical trial. |
| Overall bias? | Yes | High risk of bias |
Fureman 1997.
| Methods | Randomised controlled trial | |
| Data | Setting: university, USA. 188 participants in the Risk Assessment Project (injection drug users) | |
| Comparisons | Investigated the effect of different trial information methods Enhanced video on an HIV vaccine trial plus 1‐hour pamphlet presentation (5 minutes pre‐test, 26 minutes of video, 10 minutes to review pamphlet, research assistant initiated question and answer session, post‐test questionnaire, survey at 1 month. This was compared to standard half‐hour pamphlet‐only presentation (5 minutes pre‐test, 10 minutes to review trial information pamphlet; research assistant initiated question and answer session, post‐test questionnaire, survey at 1 month |
|
| Outcomes | Willingness to take part in hypothetical trial (expressed as a score on a willingness scale) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no details |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Not clear how much participants were told before the study, not clear what the research assistant running sessions knew about randomisation; probably knew that video was the intervention. Assistant could in principle influence post‐test questionnaire responses of participants because these were done during the session |
| Blinding of outcome assessment ok? | Unclear | Outcome not objective and not clear what influence lack of blinding might have had on this |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Graham 2007.
| Methods | Quasi‐randomised controlled trial | |
| Data | Setting: health maintenance organisation, USA. 370 participants were patients aged 18 or over attending the HMO with an acute injury | |
| Comparisons | Investigated the effect of different methods of pre‐screening participants Intervention A: electronic questionnaire on hazardous drinking and willingness to participate in lifestyle intervention Intervention B: oral questionnaire read aloud to patients in the clinic, potential answers printed on cards and patients asked to point Compared to standard self‐completed paper questionnaire |
|
| Outcomes | Willingness to take part in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Allocated by week |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Unclear | Potential participants probably blind but not researchers or practice staff |
| Blinding of outcome assessment ok? | Unclear | Outcome not objective and not clear what influence lack of blinding might have had on this |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Halpern 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. 126 participants who had mild to moderate hypertension and who met standard entry criteria (unclear what these are) for phase II and III trials at the clinic), attending clinic on selected interview days. Exclusion criteria were unable/unwilling to give oral informed consent and any exclusion criteria for the current phase III trials at the clinic (it was unclear what these were) | |
| Comparisons | Intervention A: the variables altered were information regarding the percentage of previous patients who experienced adverse effects from the study drug (10%, 20% and 30%) and the payment participants would receive (USD 100, USD 1000, and USD 2000). Intervention B: the variables altered were the percentage of patients who would be assigned to placebo (10%, 30% and 50%) and the payment level |
|
| Outcomes | Willingness to participate in a hypothetical trial (patients were told the trial was real but then told trial was not after decision) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Allocated by alternate day of week |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | No | Participants blind but not investigator, who could, in principle, influence their responses because data collection was via interview |
| Blinding of outcome assessment ok? | No | Outcome not objective and not clear what influence unblinded investigator might have had on this. |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | Unclear | Hypothetical study, though participants were initially told it was real; yet each was told about 9 scenarios "after patients had indicated their [willingness to participate] in all 9 trials …" Not clear if participant considered these real or not. |
| Overall bias? | Yes | High risk of bias |
Hemminki 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: 'local clinics', Estonia. 4295 postmenopausal women aged 50 to 64 | |
| Comparisons | Investigated the effect of different design methods Non‐blinded allocation comparing active HRT treatment versus no treatment. This was compared to traditional blinded allocation comparing active HRT treatment versus placebo. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐based random number sequence |
| Allocation concealment? | Yes | Sealed opaque envelope with ID on it |
| Blinding of participants and personnel ok? | Yes | Blinding only partial but looking at the effect of open study design was the purpose of the study |
| Blinding of outcome assessment ok? | Yes | Partial (see above) but objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Hutchison 2007.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, UK. 173 patients with colorectal, breast, lung cancer and clinically eligible to enter 1 of centre's trials; access to a video recorder, CD‐ROM or DVD player; can understand English | |
| Comparisons | Intervention: video covering general trial info, randomisation, pictures of patients receiving care + voiceover discussing uncertainty + standard practice (clinician discussing treatment options and possibility of taking part in a trial) + standard practice Comparator: standard practice (clinician discussing treatment options and possibility of taking part in a trial) | |
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Minimisation in Oracle database done by clinical trials unit |
| Allocation concealment? | Yes | Centrally by CTU |
| Blinding of participants and personnel ok? | Yes | Not clear if patients know about video versus normal info when consenting. Staff may also be unblinded although materials are sent to them at home and all participants receive standard care so probably small chance of introducing bias. |
| Blinding of outcome assessment ok? | Yes | Partial (see above) but objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Ives 2001.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, UK. 50 patients attending an HIV hospital clinic | |
| Comparisons | Investigated the effect of different trial information methods Standard trial information plus booklet entitled, 'Clinical Trials in HIV and AIDS: Information for people who are thinking about joining a trial'. This was compared to standard trial information (information sheet specific to proposed trial, plus discussion with trial doctor and research nurse) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Randomisation done sequence of numbered envelopes |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Yes | Patients and investigators not blinded. Not clear if interviewers were the investigators and therefore blind or unblinded. Unlikely to have affected outcome |
| Blinding of outcome assessment ok? | Yes | Partial (see above) but objective outcome |
| Incomplete outcome data handled ok? | Unclear | 50 were randomised but outcome data available for only 31, most of whom had joined a trial. There were some difference between those who provide only baseline data and those who provided follow‐up data. Not clear if there were differences between groups |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Jacobsen 2012.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary and university‐based cancer centre, community‐based oncology centres, USA. Participants were 462 people 18 or over diagnosed with cancer who were scheduled for a visit with an oncologist and who had not been in a trial before. Could speak and read English | |
| Comparisons | Investigated of multimedia provision of trial information. Intervention: multimedia (DVD) psychoeducation giving general info and addressing misperceptions and concerns about trials Comparator: written information about trials |
|
| Outcomes | Willingness to participate in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | No | Unclear what participants knew beforehand but outcome was self‐reported. Staff were not blinded. |
| Blinding of outcome assessment ok? | No | Willingness to take part is self‐report, and it's not clear what participants were told beforehand, which could influence what they report. Staff were not blinded but not clear if central person doing outcome assessments was also blinded. |
| Incomplete outcome data handled ok? | Yes | Only an 'as treated'/'per protocol' analysis was done and there was more deviation from the intended treatment in the intervention group. |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | No | Hypothetical trial so not a real decision about trial recruitment |
| Overall bias? | Yes | High risk of bias |
Jennings 2015a.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 181 people who were over 60 taking long‐term NSAIDS for arthritis. | |
| Comparisons | Investigated effect of financial incentive on recruitment Intervention: offer of GBP 100 Comparison: no offer |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Done centrally using a computer algorithm. There was a slight imbalance in favour of control because of algorithm used but allocation still random |
| Allocation concealment? | Yes | Done centrally |
| Blinding of participants and personnel ok? | Yes | Research nurses and staff not blinded but interventions sent out to patients on GP list so staff could not influence response. Patients blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment data reported, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Jennings 2015b.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 332 people who were aged over 60 with symptomatic hyperuricaemia | |
| Comparisons | Investigated effect of financial incentive on recruitment Intervention: offer of GBP 100 Comparison: no offer |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Done centrally using the computer algorithm. There was a slight imbalance in favour of control because of algorithm used but allocation still random |
| Allocation concealment? | Yes | Done centrally |
| Blinding of participants and personnel ok? | Yes | Research nurses and staff not blinded but invitations sent out to patients on GP list so staff could not influence response. Participants blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment data reported, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Jennings 2015c.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 93 people who were aged 18 to 79 years comparing monotherapy with dual therapy as initial hypertension treatment. | |
| Comparisons | Investigated effect of financial incentive on recruitment. Intervention: offer of GBP 100 Comparison: no offer |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Done centrally using computer algorithm. There was a slight imbalance in favour of control because of algorithm used but allocation still random |
| Allocation concealment? | Yes | Done centrally |
| Blinding of participants and personnel ok? | Yes | Research nurses and staff not blinded but invitations sent out to patients on GP list so staff could not influence response. Participants blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment data reported, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Jennings 2015d.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 210 people who were aged 18 to 79 years with uncontrolled blood pressure on 3 antihypertensive agents | |
| Comparisons | Investigated effect of financial incentive on recruitment Intervention: offer of GBP 100 Comparison: no offer |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Done centrally using computer algorithm. There was a slight imbalance in favour of control because of algorithm used but allocation still random |
| Allocation concealment? | Yes | Done centrally |
| Blinding of participants and personnel ok? | Yes | Research nurses and staff not blinded but invitations sent out to patients on GP list so staff could not influence response. Participants blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment data reported, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Jennings 2015e.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 199 people who were 18 to 80 years with at least 1 component of the metabolic syndrome | |
| Comparisons | Investigated effect of financial incentive on recruitment Intervention: offer of GBP 100 Comparison: no offer |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Done centrally using computer algorithm. There was a slight imbalance in favour of control because of algorithm used but allocation still random |
| Allocation concealment? | Yes | Done centrally |
| Blinding of participants and personnel ok? | Yes | Research nurses and staff not blinded but invitations sent out to patients on GP list so staff can not influence response. Participants blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment data reported, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Jeste 2009.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. The 128 participants were > 40 years, with schizophrenia, fluency in English and an absence of a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV), 34 diagnosis of current substance use disorder, dementia or other known conditions likely to influence decisional capacity independent of the effects of schizophrenia and/or by verbal report from the patients' treating clinicians. | |
| Comparisons | Intervention: DVD presenting key information from consent form plus a narrator explaining consent relevant info, video and slides as well. A research assistant was also there to answer questions. Comparator: printed consent information plus a 10‐minute control DVD giving general info about research. A research assistant was also there to answer questions. | |
| Outcomes | Willingness to participate in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but doesn't say more |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Yes | Researchers were blind but not clear how much participants knew about aim of study. They were probably blind. |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Karunaratne 2010.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Australia. Participants were English speaking, computer‐literate 60 patients with diabetes aged 18 to 70, able to travel to hospital. | |
| Comparisons | Intervention: computer‐based presentation of information on leaflet but with interactive explanatory features, e.g. text linked to keywords, video clips Comparator: paper‐based information | |
| Outcomes | Willingness to take part in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but doesn't say more |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Unclear if participants knew nature of the intervention when consenting. Not clear if staff doing 1‐to‐1 interviews were blinded. |
| Blinding of outcome assessment ok? | Unclear | See above and not objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Kendrick 2001.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: primary care, UK. Families with children aged under 5 years, living in deprived areas; 2393 participants | |
| Comparisons | Investigated the effect of different trial information methods Mailed invitation to participate in an injury prevention trial, including a home safety questionnaire. This was compared to mailed invitation to participate excluding the home safety questionnaire. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Randomised using ACCESS software by neutral researcher |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Yes | Participants blinded, but researchers know (probably). However, because questionnaire was mailed, there was no way researchers could influence result. |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Kerr 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: further Education colleges, UK. 130 participants were aged 18 or over and enrolled on further education and leisure courses | |
| Comparisons | Investigated the effect of describing trial treatments as new or standard for 2 disease areas, arthritis and back pain Intervention A: arthritis: treatment A described as standard, treatment B described as standard Intervention B: arthritis: treatment A described as new, treatment B described as standard Intervention C: arthritis: treatment A described as new, treatment B described as new Intervention D: back pain: treatment A described as standard, treatment B described as standard Intervention E: back pain: treatment A described as new, treatment B described as standard Intervention F: back pain: treatment A described as new, treatment B described as new |
|
| Outcomes | Willingness to participate in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Random number tables |
| Allocation concealment? | Unclear | The starting point was selected randomly, from then on there is no concealment because the scenarios were ordered consecutively from a starting point. Materials handed to students where they chose to sit. Not clear if materials were in an envelope or open to staff. |
| Blinding of participants and personnel ok? | Unclear | Students were probably blind but not clear about staff |
| Blinding of outcome assessment ok? | Unclear | Partial blinding (see above) and not objective outcome |
| Incomplete outcome data handled ok? | No | Willingness to participate responses only given for 113/130 |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Kimmick 2005.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: secondary care and academic institutions, USA. Practitioners and researchers from 126 Cancer and Leukaemia Group B (CALGB) institutions | |
| Comparisons | Investigated the effect of different trial information methods Educational intervention of standard information plus an educational symposium, geriatric oncology educational materials, monthly mailings and emails for 1 year, lists of available protocols for use on patient charts, case discussion seminar. This was compared to standard information of periodic notification of all existing CALGB trials by the CALGB Central Office, and CALGB website access. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | Clustering was accounted for in the analysis. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no more details |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Unclear | Not clear what details were given to the participants about the study before it started |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Larkey 2002.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: various existing trial sites, USA. 96 participants in the Women's Health Initiative trial | |
| Comparisons | Investigated the effect of different methods of training lay advocates for trials Intervention A: Hispanic lay advocates; attended 6 hour‐long training sessions, 5 quarterly meetings and received brochures with interest cards to distribute to other women Intervention B: Hispanic women controls, received quarterly telephone calls and brochures with interest cards to distribute to other women Compared to Anglo women controls, received quarterly telephone calls and brochures with interest cards to distribute to other women |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no more details |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Not clear if the participants were blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Lee 2017.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: primary care, Australia. 744 primary care clinics (372 general practice and 372 physiotherapy clinics) in the Sydney metropolitan area. Recruiting clinics for a trial of an intervention to reduce low back pain | |
| Comparisons | Investigated the use of a teaser campaign to increase recruitment of clinical centres Mailed 3 postcards out as a part of a staged teaser campaign to raise awareness of trial prior to invitation letter. This was compared to no teaser postcards. |
|
| Outcomes | Proportion of clinics recruited | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | An investigator not involved in outcome assessment generated a 1:1 randomisation schedule using a random number generator and assigned clinics to the groups. |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Yes | The clinicians and support staff were blind to the different recruitment strategies that were being tested in this study. |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome available, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Litchfield 2005.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were general practices participating in a trial of 2 delivery systems for insulin, NovoPen and Innovo. 28 practices were involved and 73 participants recruited | |
| Comparisons | Intervention: electronic data capture Comparator: paper data capture |
|
| Outcomes | Number of participants recruited to the trial. Improving recruitment was not the main aim (improving efficiency was the main aim) of the study though this information is provided. | |
| Notes | Clustering was not accounted for in analysis. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated randomisation code in compliance with FDA and EU regulations |
| Allocation concealment? | Yes | Done centrally (inferred rather than explicit but seems reasonable to assume for this cluster trial) |
| Blinding of participants and personnel ok? | Unclear | Investigators knew that both paper and electronic data collection were to be used so study was not blinded. Unlikely that patient decisions to join study would be affected by this. Not clear how much influence knowledge of data collection method might have had on practices. |
| Blinding of outcome assessment ok? | Yes | Objective outcome. Improving recruitment was not the main aim (improving efficiency was the main aim) of the study, though this information is provided |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Liénard 2006.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: secondary care, France. Centres recruiting to a randomised controlled trial for breast cancer; 573 participants | |
| Comparisons | Investigated the effect of organising visits by the trial co‐ordination team to centres participating in a multicentre trial Site visits including an initiation visit to review trial protocol, inclusion/exclusion criteria, safety, randomisation etc. plus ongoing review visits. This was compared to no site visits (unless requested). |
|
| Outcomes | Proportion recruited to trial | |
| Notes | Clustering was not accounted for in the analysis. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Minimisation |
| Allocation concealment? | Yes | Done centrally by the coordinating office |
| Blinding of participants and personnel ok? | Yes | Centres blind. Somewhat unclear if monitors were blind but probably were not |
| Blinding of outcome assessment ok? | Yes | Partial (see above) but objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Llewellyn‐Thomas 1995a.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Canada. 90 colorectal cancer patients attending cancer hospital as outpatients | |
| Comparisons | Investigated the effect of different trial information methods Intervention A: booklet with negatively‐framed intervention about treatment side effects and survival Intervention B: booklet with positively‐framed intervention about treatment side effects and survival Compared to booklet with neutrally framed intervention about treatment side effects and survival |
|
| Outcomes | Proportion recruited to hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Mentions randomisation but no further details. |
| Allocation concealment? | Unclear | Used sealed envelopes although doesn't mention numbering |
| Blinding of participants and personnel ok? | Yes | Interviewer was blinded, but unclear about participants |
| Blinding of outcome assessment ok? | Yes | Partial (see above) but subjective outcome but probably not influenced by partial blinding (interviewer was blind, probably tricky for participant to figure out what was being tested). |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Llewellyn‐Thomas 1995b.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Canada. 100 patients attending the outpatient department of a cancer hospital | |
| Comparisons | Investigated the effect of different trial information methods Searchable computerised information on a hypothetical trial, including purpose, description of treatment group and randomisation, possible benefits, side effects and patients' rights. This was compared to tape‐recorded information on a hypothetical trial, including purpose, description of treatment arm and randomisation, possible benefits, side effects and patients' rights |
|
| Outcomes | Proportion recruited to hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Just says framing was randomly determined |
| Allocation concealment? | Unclear | Used sealed envelopes although doesn't mention numbering |
| Blinding of participants and personnel ok? | Yes | Unclear if the interviewer or the participants were blinded. It depends on what the participants were told. Interviewer did not seem to do more than help with equipment, so perhaps limited room for bias |
| Blinding of outcome assessment ok? | Yes | Somewhat unclear (see above), subjective outcome but probably did not affect outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
MacQueen 2014.
| Methods | Randomised controlled trial | |
| Data | Setting: community care, Tanzania. Participants were women aged 18 to 35 living in particular districts, had had sex in last 14 days, or had more than 1 sexual partner in last 30 days. Women who had been in trial before excluded | |
| Comparisons | Investigated alternative ways of assessing informed consent (comprehension) Intervention: open‐ended (verbal description of each of 7 components) comprehension assessment of informed consent information prior to deciding whether to take part Comparator: closed‐ended (true or false rating of statements read out by interviewer of each of 7 components) comprehension assessment of informed consent information prior to deciding whether to take part |
|
| Outcomes | Willingness to take part in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | No mention of method |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | Participants were blinded, staff weren't but probably given outcome of willingness to take part in trial |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Unclear | Doesn't specify how many women responded to willingness question |
| Free of selective reporting? | Unclear | Recruitment data are presented but not clear if they are all presented |
| Was the study free of other bias? | No | Trial was hypothetical |
| Overall bias? | Yes | High risk of bias |
Man 2015a.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. 1364 participants who were identified as potentially eligible for the Healthlines CVD study | |
| Comparisons | Investigated the alternative was of presenting patient information materials Intervention: participant information that developed in collaboration with patients together with a graphic designer Comparator: standard participant information materials |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated random numbers to split those to be invited |
| Allocation concealment? | Yes | Use of IDs, sorted by random number |
| Blinding of participants and personnel ok? | Yes | Patients unaware of recruitment study. Researchers blind to patient allocation |
| Blinding of outcome assessment ok? | Yes | Objective outcomes |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Man 2015b.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. 671 participants who were identified as potentially eligible for the Healthlines CVD study | |
| Comparisons | Investigated the alternative ways of presenting patient information materials Intervention: participant information that developed in collaboration with patients together with a graphic designer Comparator: standard participant information materials |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated random numbers to split those to be invited |
| Allocation concealment? | Yes | Use of IDs, sorted by random number |
| Blinding of participants and personnel ok? | Yes | Patients unaware of recruitment study. Researchers blind to patient allocation |
| Blinding of outcome assessment ok? | Yes | Objective outcomes |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Mandelblatt 2005.
| Methods | Randomised controlled trial | |
| Data | Setting: community cancer clinics, USA. 450 participants who were eligible for cancer prevention trial (high risk of breast cancer but low risk of side effects) | |
| Comparisons | Intervention: 5, 10‐minute educational sessions about STAR cancer prevention trial following short interview about prior knowledge, risk perceptions and background. Education emphasised benefits of participation, lack of financial burden and need for minority participation in trials. Also given a brochure. Comparator: brochure plus short background interview | |
| Outcomes | Intention/likelihood of taking part in STAR cancer prevention trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Based on clinic day |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Unclear | Not clear how much info participants given about intervention during consent process, or whether staff doing interviews were blind |
| Blinding of outcome assessment ok? | Unclear | See above. Outcome was intention to participate so possible to introduce bias depending on what information participants were given |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Intention to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Intention to participate, not actual participation |
| Overall bias? | Yes | High risk of bias |
Miller 1999.
| Methods | Quasi‐randomised controlled trial | |
| Data | Setting: USA, secondary care, 347 participants. Participants were eligible for 1 of the 2 trials being run through the unit: 18 to 75 years old and DSM‐IV dysthymic disorder, double depression (major depression superimposed on antecedent dysthymia), or chronic major depression. Exclusion criteria were history of psychosis, mania or hypomania; comorbid substance abuse; severe medical illness; failed 3 adequate trials of antidepressants from 2 different classes of antidepressants in the past 3 years; and failed study medication or study psychotherapy | |
| Comparisons | Investigated whether screening by research assistants was more cost‐effective than by senior investigators Intervention: screening by senior investigator Comparator: screening by research assistant |
|
| Outcomes | Proportion recruited to trials | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Alternating screening calls were given to senior investigator |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Unclear | Investigator and research assistants knew allocation, and they were the people interviewing potential participants (who would be blind) |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Yes | High risk of bias |
Monaghan 2007.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: existing, multicentre, international trial. 167 clinical sites in 19 countries recruiting to a diabetes and vascular disease treatment trial | |
| Comparisons | Investigated the effect of different levels of communication between the trial co‐ordination team and participating sites Additional communication – usual plus frequent emails, regular personalised mail‐outs of league tables/graphs of performance against other sites, certificates of achievement for recruitment/other study items (1 per month). This was compared to usual communication (provided via the regional centre) plus occasional direct communications from the co‐ordinating centre in the form of generic newsletters, emails and faxes. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | Clustering was not accounted for in analysis. | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated randomisation |
| Allocation concealment? | Yes | Central randomisation |
| Blinding of participants and personnel ok? | Yes | Centres were blinded, but the central office was not blind |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome (per site) presented, which is what review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Mudano 2013.
| Methods | Quasi‐randomised trial (used date of birth) | |
| Data | Setting: primary care, USA. Participants were 155 women ≥ 65 years with Medicare drug coverage and no reported use of osteoporosis medication in last year. Also bone fracture since 50, or osteo diagnosis by healthcare professional (based on self‐report) | |
| Comparisons | Investigated effect of systems to support eligibility screening Intervention: tablet computer to support eligibility screening Comparator: integrated voice response system (IVRS) to support eligibility screening |
|
| Outcomes | Willingness to participate in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Used day of birth, even date allocated to tablet |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Unclear | Unclear how much participants knew; study staff not blinded |
| Blinding of outcome assessment ok? | Unclear | Outcome was willingness to take part, and participants possibly knew that they were in study and therefore that there was another arm to which they could have been allocated. Could influence this subjective outcome. |
| Incomplete outcome data handled ok? | Yes | 160 participants, all 93 in tablet arm completed, only 46 of 67 in IVRS arm completed screening. Does seem that most provided willingness to participate data though |
| Free of selective reporting? | Yes | Willingness to take part is reported, and this is only outcome needed for review. |
| Was the study free of other bias? | No | Trial was hypothetical. Almost a third more people in intervention arm than in control. |
| Overall bias? | Yes | High risk of bias |
Myles 1999.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Australia. 769 inpatients aged 18 or over, scheduled for elective surgery | |
| Comparisons | Investigated the effect of different consent methods Intervention A: pre‐randomised to experimental drug and asked to provide consent; if no consent, standard treatment given Intervention B: pre‐randomised to standard drug and asked to provide consent; if no consent, experimental treatment given Intervention C: told that the physician thinks experimental drug superior, if consent given, has 70% chance of receiving this; if no consent, standard treatment given Intervention D: allowed to increase or decrease their chance of receiving the experimental drug if consent given, and if no preference, 50% chance of receiving it; if no consent, standard treatment given Compared to standard randomisation method (equal chance of experimental or standard drug) |
|
| Outcomes | Proportion recruited to hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Mentions randomisation but no details given |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Patient is blinded (they are not told the exact details of the study in the patient information). Researchers (probably) knew the allocation. |
| Blinding of outcome assessment ok? | Unclear | Outcome was subjective and unclear what potential researchers had to influence this while participants answered questions about intentions |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Nystuen 2004.
| Methods | Randomised controlled trial | |
| Data | Setting: community, Norway. 498 sick‐listed employees attending a participating social security office | |
| Comparisons | Investigated the effect of different telephone reminders Written invitation to participate in a community‐based trial followed by a telephone reminder if no response within 2 weeks; guide used for discussion. This was compared to written invitation to participate in a community‐based trial followed by no reminder if no response within 2 weeks. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated list |
| Allocation concealment? | Yes | Central allocation |
| Blinding of participants and personnel ok? | Yes | Participants were blinded but not the research team who makes the phone calls. The team do not contact the control group. |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Paul 2011.
| Methods | Randomised controlled trial | |
| Data | Setting: secondaty care, UK. Participants were patients with colorectal cancer receiving adjuvant treatment. 215 were allocated to the comparator; it was unclear how many received the intervention. | |
| Comparisons | Investigated the effect of the randomisation time point Intervention: randomise prior to treatment to get 3 or 6 months treatment Comparator: randomise after 3 months of treatment to see if participant gets another 3 months of treatment |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Received additional information from Jim Paul by email (Paul 2016). Minimisation programmed in PL/SQL in Oracle |
| Allocation concealment? | Yes | Central allocation |
| Blinding of participants and personnel ok? | Yes | Participants blinded |
| Blinding of outcome assessment ok? | Yes | Objective outcome (recruitment) |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome available, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Paul 2014.
| Methods | Randomised controlled trial | |
| Data | Setting: community (via cancer registry), Australia. 1062 participants were 18 years or older, primary colorectal cancer diagnosis and within 3 months of diagnosis and on registry | |
| Comparisons | Investigated pre‐recruitment primer letter Intervention: pre‐recruitment primer letter designed to encourage participation Comparison: no primer letter |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Done centrally from register |
| Blinding of participants and personnel ok? | Yes | Adequate |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported ,and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Perrone 1995.
| Methods | Randomised controlled trial | |
| Data | Setting: community, Italy. 3573 members of the general public aged under 80 years, attending a scientific exhibition | |
| Comparisons | Intervention A: 1‐sided informed consent (participants refusing were given standard treatment) Intervention B: 2‐sided informed consent (participants refusing could choose between experimental and standard treatment) Intervention C: randomised to experimental (participants refusing were given standard treatment) Intervention D: randomised to standard (participants refusing were given experimental treatment) | |
| Outcomes | Willingness to participate in a hypothetical trial | |
| Notes | This is same trial as Gallo 1995 but Perrone 1995 includes participants under 20 | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no details given |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | No | Not clear what participants were told. Researchers unblinded and since researcher asked participants for his/her views at end of test, there is the potential for bias |
| Blinding of outcome assessment ok? | No | See above |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Pighills 2009.
| Methods | Quasi‐randomised controlled trial | |
| Data | Setting: community, UK. 4488 participants were over 70 and on a participating GP's listarticipants. | |
| Comparisons | Intervention A: newspaper article about the trial Intervention B: more favourable newspaper article about the trial Intervention C: the original newspaper article Comparator: no article (i.e. usual recruitment materials) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | Control and intervention were stacked alternately in packs given to GP practice |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Yes | Recipients and practice staff blinded |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Yes | High risk of bias |
Simel 1991.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. 100 patients attending an ambulatory care clinic | |
| Comparisons | Investigated the effect of different consent methods Consent form including a statement that the new treatment may work twice as fast as usual treatment. This was compared to a consent form including a statement that the new treatment may work half as fast as usual treatment |
|
| Outcomes | Number consenting (inferred from data rather than being an outcome presented by authors) | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Randomisation using a computer‐generated scheme |
| Allocation concealment? | Unclear | Single centre and unclear whether the randomisation list was open or not |
| Blinding of participants and personnel ok? | Yes | Participants probably were blind but the investigators were not. Investigators got an independent reviewer to look at a portion of interviews, and he/she thought they were fair. They also used a script so less room for investigator initiative. |
| Blinding of outcome assessment ok? | Yes | See above |
| Incomplete outcome data handled ok? | Unclear | Adequate |
| Free of selective reporting? | Yes | Number consenting not presented as an outcome but inferred from data, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent. Trial was hypothetical but participants were not told this so they thought decision was real |
| Overall bias? | Unclear | Unclear risk of bias |
Simes 1986.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Australia. 57 patients attending an oncology unit | |
| Comparisons | Investigated the effect of different consent methods Individual approach to consent – patients given information about aims, expected results, potential toxicities of treatment; details of treatment left to discretion of consultant; patients given opportunity to ask questions, verbal consent obtained. This was compared to total disclosure approach – participants were fully informed about all trial aspects by consultant, with opportunity to ask questions and a consent form outlining the information; this was kept overnight, and written consent was obtained the following day. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Sealed envelopes using balanced randomisation |
| Allocation concealment? | Unclear | Unclear if envelopes were sequentially numbered |
| Blinding of participants and personnel ok? | Unclear | Participants were probably blinded. Clinicians were probably not blinded. It is not clear if it is the same clinicians provided information in to both groups. |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Tehranisa 2014.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. Participants were 418 non‐critically ill emergency department adult (18 or older) patients without without presenting symptoms consistent with stroke, altered mental status, or alcohol intoxication. | |
| Comparisons | Investigated the use of response‐adaptive designs Intervention: video describing a hypothetical trial that uses a response‐adaptive design Comparator: video describing a hypothetical trial that uses a standard design |
|
| Outcomes | Willingness to take part in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Mentions block size and randomisation in protocol |
| Allocation concealment? | Unclear | As above |
| Blinding of participants and personnel ok? | Yes | Participants were blind but not investigators. Outcome (willingness to take part in hypothetical trial) unlikely to be influenced by investigators because intervention is watching a video alone |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to take part in trial reported and this is only outcome needed for review |
| Was the study free of other bias? | No | Trial was hypothetical |
| Overall bias? | Yes | High risk of bias |
Tilley 2012.
| Methods | Cluster‐randomised controlled trial | |
| Data | Setting: primary care, USA. Participants were neurologists, primary care docs and internists within 30 miles of trial site. Intention was that this would increase proportion of non‐white, non‐Hispanic participants into the trial. Participants being enrolled had Parkinson's. 606 participants in analysis | |
| Comparisons | Investigated effect of a recruitment coordinator Intervention: recruitment coordinator plus package of training, materials and events, some carrying CME points. Comparator: whatever recruitment procedures sites wanted to use |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Unclear | No details given |
| Blinding of participants and personnel ok? | Yes | Possible that intervention sites mentioned what they were doing to control sites but controls did not have the coordinator and funding for events so unlikely to really influence outcome, which was anyway objective (recruitment) |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Unclear | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | No | Stopped early because of a formal stopping rule |
| Overall bias? | Yes | High risk of bias |
Treschan 2003.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Austria. Participants were 150 patients undergoing minor surgery with general anaesthetic, 19 to 80 years old. Exclusion criteria were pain, cancer, unable to give unformed consent, could not speak German | |
| Comparisons | Investigated the effect of mentioning risk or discomfort on recruitment Intervention A: said no risk but emphasised the painful nature of tests. etc. Intervention B: said no pain but emphasised risk Comparator: said extra oxygen is harmless and the wound evaluations are painless. This study thus poses essentially no risk and will not produce any significant pain |
|
| Outcomes | Willingness to participate in a hypothetical trial ‐ participants were not told the trial was hypothetical until after decision to take part | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated randomisation code |
| Allocation concealment? | Yes | Randomisation assignment held in sealed, opaque envelopes opened just before presentation |
| Blinding of participants and personnel ok? | Unclear | Participants were blinded (just given general statement that study was about pain and risk) but not clear if interviewers were. They were, however, told not to give personal comments to influence the decision‐making process. |
| Blinding of outcome assessment ok? | Unclear | Subjective outcome and interviewers could potentially influence, depending on whether they were blind or not. |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | Hypothetical trial but patients were not told the trial was hypothetical until after decision to take part |
| Overall bias? | Unclear | Unclear risk of bias |
Trevena 2006.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, Australia. 152 participants aged 50 to 74 eligible for a colorectal cancer screening trial | |
| Comparisons | Investigated the effect of different trial information methods Opt‐in recruitment; letter from doctor advising that the practice is taking part in screening trial; would only be contacted if contact details returned. This was compared to opt‐out recruitment; letter from doctor advising that the practice is taking part in screening trial; would be contacted unless the practice was advised to withhold contact details The distribution of participants between intervention and comparison groups is uneven: 60 versus 92, respectively. This was due to a change in legislation in Australia, which meant that the trialists could no longer continue with the opt‐out procedure and had to change to opt‐in to keep their ethical approval. |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Computer‐generated randomisation |
| Allocation concealment? | Unclear | Unclear if randomisation list was open |
| Blinding of participants and personnel ok? | Yes | Participants not told about different recruitment methods. Not clear if clinicians were blinded but they were not involved in recruitment, which was done by letter and then contact with research team. |
| Blinding of outcome assessment ok? | Yes | See above |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Treweek 2012.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, UK. Participants were 1760 GPs | |
| Comparisons | Investigated use of different modes of invitation to take part in trial Intervention: email invitation (email plus link to info sheet ‐ text the same as with intervention) Comparator: postal invitation (letter plus 2‐page information sheet) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Centrally generated by statistician using computer |
| Allocation concealment? | Yes | 3rd party used to send out invitations |
| Blinding of participants and personnel ok? | Yes | Research team blind. Participants did not know study was ongoing so also blind |
| Blinding of outcome assessment ok? | Yes | Adequate |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
Wadland 1990.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, USA. Participants were 104 smokers > 18 years old | |
| Comparisons | Intervention: consent form read out by researcher Comparator: consent form read by patient |
|
| Outcomes | Proportion recruited to trial | |
| Notes | Only site 2 in the study ran a randomised evaluation so only its data are included | |
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no more details |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Both actively involved but not clear if the participants were told about how consent might be varied |
| Blinding of outcome assessment ok? | Yes | Objective outcome |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment outcome presented, which is all the review needs |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | Unclear | Unclear risk of bias |
Weinfurt 2008a.
| Methods | Randomised controlled trial | |
| Data | Setting: community, USA. 3623 participants aged 18 or over and diagnosed with coronary artery disease | |
| Comparisons | Intervention A: drug company pays investigator running costs plus general statement saying ethics committee did not think this would affect patient safety Intervention B: drug company pays investigator money for things outside the study plus general statement saying ethics committee did not think this would affect patient safety Intervention C: Investigator owns part of drug company plus general statement saying ethics committee did not think this would affect patient safety. Intervention D: Institution owns part of drug company plus general statement saying ethics committee did not think this would affect patient safety Comparator: generic financial disclosure: general statement about investigator possibly gaining financially plus general statement saying ethics committee did not think this would affect patient safety |
|
| Outcomes | Willingness to take part in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no more details |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Not clear what participants were told about the purpose of the study although there were 5 disclosure statements so everyone got a statement (i.e. hard to tell which group they were in). Participants completed a questionnaire (probably) so research team unable to influence |
| Blinding of outcome assessment ok? | Unclear | See above |
| Incomplete outcome data handled ok? | Unclear | Only P values presented, not absolute numbers |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Weinfurt 2008b.
| Methods | Randomised controlled trial | |
| Data | Setting: community but recruited through outpatient dept, USA. The 470 participants were 18 or over and diagnosed with coronary artery disease. articipants. | |
| Comparisons | Intervention A: financial disclosure saying that the drug company pays hospital Intervention B: financial disclosure saying that the drug company pays the investigator Comparator: no financial disclosure |
|
| Outcomes | Willingness to take part in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Unclear | Randomisation mentioned but no more details |
| Allocation concealment? | Unclear | See above |
| Blinding of participants and personnel ok? | Unclear | Not clear what participants were told about disclosure study; not clear if interviewers knew allocation |
| Blinding of outcome assessment ok? | Unclear | See above |
| Incomplete outcome data handled ok? | Unclear | Only a mean score presented, not absolute numbers so hard to know |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Wells 2013.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, USA. Participants were Hispanic cancer 31 patients, scheduled for consultation with medical oncologist, never asked about cancer trial, Spanish as preferred language | |
| Comparisons | Investigated multimedia presentation of information Intervention: Spanish‐language multimedia information about clinical trials Comparator: Spanish‐language written information about clinical trials |
|
| Outcomes | Willingness to participate in a hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Unclear | Given that trial was hypothetical, not clear whether being unblinded might influence stated willingness to take part in a future trial, especially if it was the same research assistant who was there when participants watched video/read booklet, and phoned them to do outcome assessment |
| Blinding of outcome assessment ok? | Unclear | As above |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Unclear | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | No | Trial was hypothetical |
| Overall bias? | Yes | High risk of bias |
Welton 1999.
| Methods | Quasi‐randomised controlled trial | |
| Data | Setting: primary care, UK. 436 women aged 45 to 64 who had not had a hysterectomy | |
| Comparisons | Investigated the effect of different trial information methods Verbal information about a trial of HRT, comparing oestrogen only versus combined oestrogen and progestogen. This was compared to verbal information about a trial of HRT, comparing oestrogen only, versus oestrogen plus progestogen versus placebo |
|
| Outcomes | Willingness to take part in hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | No | By week |
| Allocation concealment? | No | See above |
| Blinding of participants and personnel ok? | Unclear | Participants were blinded but the nurses were not |
| Blinding of outcome assessment ok? | Unclear | Subjective outcome and not clear what influence nurses might have |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Unclear | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Weston 1997.
| Methods | Randomised controlled trial | |
| Data | Setting: secondary care, Canada. 90 women attending for antenatal visits | |
| Comparisons | Investigated the effect of different trial information methods Written study information followed by viewing of Term Prelabour Rupture of the Membranes (Term PROM) video. This was compared to written study information only. |
|
| Outcomes | Proportion recruited to hypothetical trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Randomisation used random numbers table held centrally |
| Allocation concealment? | Yes | See above |
| Blinding of participants and personnel ok? | Unclear | Depends if the women were told they might watch a video ‐ they were probably told. Women completed a questionnaire so they were probably not influenced by the study nurse. |
| Blinding of outcome assessment ok? | Unclear | See above |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Willingness to participate outcome presented, which is all the review needs |
| Was the study free of other bias? | No | Hypothetical trial |
| Overall bias? | Yes | High risk of bias |
Wong 2013.
| Methods | Randomised controlled trial | |
| Data | Setting: primary care, Canada. Participants were 952 people aged 50‐70 years who had not responded to initial invitation by 4 weeks. People were being recruited to a colorectal cancer screening trial not had recent colorectal cancer screening | |
| Comparisons | Investigated use of telephone reminders to non‐responders Intervention: up to 3 telephone reminders to those not responding to initial posted invitation Comparison: no telephone reminders (but did get a 2nd invitation) |
|
| Outcomes | Proportion recruited to trial | |
| Notes | ||
| Risk of bias | ||
| Item | Authors' judgement | Description |
| Random Sequence generation ok? | Yes | Adequate |
| Allocation concealment? | Yes | Adequate |
| Blinding of participants and personnel ok? | Yes | Participants blinded, study nurse making calls clearly not but outcome objective |
| Blinding of outcome assessment ok? | Yes | Recruitment objective (this was study's secondary outcome, primary was attendance at eligibility screening) |
| Incomplete outcome data handled ok? | Yes | Adequate |
| Free of selective reporting? | Yes | Recruitment reported and this is only outcome needed for review |
| Was the study free of other bias? | Yes | No other biases apparent |
| Overall bias? | No | Low risk of bias |
CBT: cognitive behavioural therapy; CME: continuing medical education; CVD: cardiovascular disease; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GP: general practitioner; HRT: hormone replacement therapy; NICU: neonatal intensive care unit; NSAIDs: non‐steroidal anti‐inflammatory drugs; PIL: participant information leaflet; PL/SQL: procedural language extension to Structured Query Language; RCT: randomised controlled trial; SMS: short message service; SOP: standard operating protocol.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aalborg 2012 | Engagement not recruitment |
| Aaronson 1996 | Not studying a recruitment intervention |
| Agoritsas 2010 | Not studying recruitment intervention |
| Alexander 2008 | Not recruiting to a trial |
| Andrew 1993 | Used Zelen design but its use was not part of a randomised evaluation of the design to increase recruitment |
| Barnard 2010 | Systematic review |
| Berman 2005 | Allocation not randomised |
| Brach 2013 | Allocation not randomised |
| Brealey 2007 | Allocation not randomised |
| Breland‐Noble 2012 | Engagement not recruitment |
| Brocklehurst 2007 | The study never started (personal communication from member of study team, 6 April 2017) Farrell 2017 |
| Brown 2012 | Response not recruitment |
| Burns 2008 | Not studying a recruitment intervention |
| Caldwell 2002 | An earlier version of work later published in a systematic review (Caldwell 2010), the references of which we checked for this Cochrane Review |
| Calimlim 1977 | Not studying a recruitment intervention |
| Carney 2014 | Not recruiting to a trial |
| Celentano 1995 | Recruiting to a survey |
| Chin Feman 2008 | Allocation not randomised |
| Chlebowski 2010 | Allocation not randomised |
| Clagett 2013 | Not recruiting to a trial |
| Cook 2010 | Allocation not randomised |
| Coronado 2012 | Allocation not randomised |
| Dal‐Ré 1991 | Not recruiting to a randomised controlled trial (simulated trial was a non‐randomised phase I study) |
| Davis 1998 | Allocation not randomised |
| Donovan 2009 | Allocation not randomised |
| Donovan 2010 | Allocation not randomised |
| Eckardt 2011 | Not recruiting to a trial |
| Embi 2012 | Allocation not randomised |
| Enama 2012 | Not a recruitment study. Participants already had decided to take part; this study was just to see if different consent forms would have different levels of comprehension and satisfaction. |
| Feman 2008 | Allocation not randomised |
| Foradori 2012 | Not studying a recruitment intervention |
| Gallo 1995 | This study presents a subset of the data given in Perrone 1995, which is included in this review |
| Gillan 2009 | Not recruiting to a trial |
| Gilligan 2014 | Not recruiting to a trial |
| Gillon 2009 | Not studying a recruitment intervention |
| Ginexi 2003 | Allocation not randomised |
| Gitanjali 2003 | Allocation not randomised |
| Goldstein 2010 | Allocation not randomised |
| Gomez 1998 | Letter |
| Graham 2011 | Allocation not randomised |
| Grubbs 2009 | Not studying a recruitment intervention |
| Halpern 2002 | Allocation not randomised |
| Harris 2008 | Not recruiting to a trial |
| Harron 2012 | Allocation not randomised |
| Heiney 2010 | Allocation not randomised |
| Henkel 2010 | Not studying recruitment intervention |
| Hillsdon 2011 | This conference abstract only presents time to recruit first patient; it isn't studying actual rate of recruitment into the trial. |
| Hoffner 2011 | Not studying a recruitment intervention |
| Homish 2009 | Not recruiting to a trial |
| Jaffee 2009 | Allocation not randomised |
| Jay 2007 | Not studying a recruitment intervention |
| Jenkins 2013 | No recruitment outcome, just number of patients approached |
| Ji 2008 | Allocation not randomised |
| Junghans 2005 | Not recruiting to a trial but to an observational study of patients with angina |
| Juraskova 2014 | Not studying recruitment |
| Karlawish 2008 | Allocation not randomised |
| Keedy 2009 | Allocation not randomised |
| Kelechi 2010 | Allocation not randomised |
| Kernan 2009 | Hospitals not randomised to intervention |
| Kiernan 2000 | Studying response to an advertisement not actual recruitment |
| Kirkby 2013 | Allocation not randomised |
| Korde 2009 | Allocation not randomised |
| Kruse 2000 | Looking at impact on knowledge, not recruitment |
| Labrique 2011 | Not studying recruitment intervention |
| Lancet 2001 | Editorial |
| Lang 1991 | Not studying a recruitment intervention |
| Larkey 2009 | Allocation not randomised |
| Leader 1978 | Allocation not randomised |
| Lee 2011 | Allocation not randomised |
| Lichter 1991 | Editorial |
| Lloyd‐Williams 2002 | Not studying a recruitment intervention |
| Macias 2005 | Not studying a recruitment intervention |
| Marco 2008 | Not recruiting to a trial |
| Masood 2006 | Not recruiting to a trial |
| May 2007 | Not studying a recruitment intervention |
| McGuire 2011 | Not recruiting to a trial |
| Menoyo 2006 | Not studying a recruitment intervention |
| Monane 1991 | Not studying a recruitment intervention |
| Murphy 2011 | Allocation not randomised |
| O'Lonergan 2011 | Does not present recruitment data; about understanding |
| Olver 2009 | Not recruiting to a trial |
| Paskett 2002 | Allocation not randomised |
| Perri 2006 | Allocation not randomised |
| Porucznik 2010 | Allocation not randomised |
| Quinaux 2003 | An earlier version of Liénard 2006, which is included in this review |
| Rogers 1998 | Studying recall, understanding and satisfaction rather than effect on recruitment |
| Rowbotham 2013 | Not studying recruitment |
| Ruffin 2011 | Allocation not randomised |
| Santoyo‐Olsson 2011 | Allocation not randomised |
| Saul 2002 | News item |
| Scholes 2007 | Not recruiting to a trial |
| Schrott 1982 | Not studying a recruitment intervention |
| Schroy 2009 | Allocation not randomised |
| Sherman 2009 | Allocation not randomised |
| Swain 2011 | Allocation not randomised |
| Tenorio 2014 | Allocation not randomised |
| Ubel 1997 | Allocation not randomised |
| Unger 2006 | Not studying a recruitment intervention |
| Unger 2010 | Allocation not randomised |
| Vaidya 2010 | Not studying recruitment intervention |
| Wang 2014 | Allocation not randomised |
| Woodford 2011 | Allocation not randomised |
| Wragg 2000 | Allocation not randomised |
| Yates 2009 | Allocation not randomised |
| Zhou 2013 | Allocation not randomised |
Most studies that we considered in detail but excluded arose from records that we had retrieved because the database reference gave no abstract and it was not possible to exclude them on the basis of the title. We excluded most of the records falling into this category as soon as we checked the full text, with the most common reason being that the study did not evaluate a recruitment intervention.
The two exceptions are Aaronson 1996 and Kiernan 2000, which we excluded at the data extraction stage for the reasons given in the table.
Characteristics of studies awaiting assessment [ordered by study ID]
Cramer 1993.
| Methods | — |
| Data | — |
| Comparisons | — |
| Outcomes | — |
| Notes | Full text to be obtained |
Glen 1980.
| Methods | — |
| Data | — |
| Comparisons | — |
| Outcomes | — |
| Notes | Full text to be obtained |
Greenlee 2003.
| Methods | — |
| Data | — |
| Comparisons | — |
| Outcomes | — |
| Notes | Full text to be obtained |
Differences between protocol and review
Below we describe the key differences between the protocol used in our previous review and this version. An updated version of the protocol is available describesing the methods used in this version of the review (Appendix 2).
Although we did not exclude studies at high of risk of bias, the low confidence we have in the data they present means that we no longer mention these studies in the text of the Results or Discussion, except where it was possible to include them in a meta‐analysis.
Studies at high risk of bias do appear in Data and analyses, but we recommend readers use these data only to make decisions as to whether they would like to evaluate the intervention themselves in a more rigorous way. We do not believe these studies can support judgements about the effects of the tested interventions.
We include data for hypothetical studies in Data and analyses for this version of the review, but we will exclude them from future versions of this review, because:
the relevance of the results of hypothetical trials will always be in doubt due to uncertainty as to how people would have reacted had the decision to take part in a trial been a real one, not a hypothetical one;
it is possible to study recruitment interventions in real trials, avoiding the above problem;
now that the number of evaluations in real trials has increased, we do not think the trade‐off between value added and work involved to include hypothetical trials is worthwhile.
Contributions of authors
For this update, Shaun Treweek, Jonathan Cook, Heidi Gardner, Catherine Jackson, Elizabeth Mitchell, Marie Pitkethly and Frank Sullivan contributed to study design, record screening, full‐text review of retrieved records and drafting of the report. Shaun Treweek, Marie Pitkethly and Heidi Gardner extracted the data. Jonathan Cook and Shaun Treweek analysed them. Cynthia Fraser developed and ran the electronic searches. Tyna Taskila contributed to the final report. All authors approved the final version of the review.
Sources of support
Internal sources
Scottish Funding Council, UK.
Rigshospitalet, Denmark.
External sources
Department of Health, Cochrane Review Incentive Scheme 2008, UK.
Department of Health, Cochrane Review Incentive Scheme 2011, UK.
-
Medical Research Council, UK.
Jonathan Cook holds a Medical Research Council UK personal fellowship (G0601938).
Declarations of interest
Shaun Treweek and Frank Sullivan are coauthors of Treweek 2012; they were not involved in data extraction or risk of bias assessment for this study for this review. Although Shaun Treweek was not involved in Cockayne 2017, he was involved in the wider START study in which Cockayne 2017 was nested; he was not involved in data extraction or risk of bias assessment for this study for this review. Shaun Treweek was a reviewer for Jennings 2015a; Jennings 2015b; Jennings 2015c; Jennings 2015d; Jennings 2015e (all included in a single article). Shaun Treweek and Frank Sullivan declare no further conflict of interest.
Marie Pitkethly: none known.
Jonathan Cook: none known.
Cynthia Fraser: none known.
Elizabeth Mitchell: none known.
Catherine Jackson: none known.
Tyna Taskila: none known.
Heidi Gardner: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abd‐Elsayed 2012 {published data only}
- Abd‐Elsayed AA, Sessler DI, Mendoza‐Cuartas M, Dalton JE, Said T, Meinert J, et al. A randomized controlled study to assess patients' understanding of and consenting for clinical trials using two different consent form presentations. Minerva Anestesiologica 2012;78:564‐73. [PubMed] [Google Scholar]
Abhyankar 2010 {published data only}
- Abhyankar P, Bekker HL, Summers BA, Velikova G. Why values elicitation techniques enable people to make informed decisions about cancer trial participation. Health Expectations 2010;14(Suppl 1):20‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avenell 2004 {published data only}
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA, the RECORD Trial Management Group. The effects of an open design on trial participant recruitment, compliance and retention ‐ a randomized controlled trial comparison with a blinded, placebo‐controlled design. Clinical Trials 2004;1:490‐8. [DOI] [PubMed] [Google Scholar]
Bentley 2004 {published data only}
- Bentley JP, Thacker PG. The influences of risk and monetary payment on the research participation decision making process. Journal of Medical Ethics 2004;30:293‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergenmar 2014 {published data only}
- Bergenmar M, Johansson H, Wilking N, Hatschek T, Brandberg Y. Audio‐recorded information to patients considering participation in cancer clinical trials – a randomized study. Acta Oncologica 2014;53:1197‐204. [DOI: 10.3109/0284186X.2014.921726] [DOI] [PubMed] [Google Scholar]
Brierley 2012 {published data only}
- Brierley G, Richardson R, Torgerson DJ. Using short information leaflets as recruitment tools did not improve recruitment: a randomized controlled trial. Journal of Clinical Epidemiology 2012;65:147‐54. [DOI: 10.1016/j.jclinepi.2011.06.005] [DOI] [PubMed] [Google Scholar]
Chen 2011 {published and unpublished data}
- Chen F, Rahimi K, Haynes R, Naessens K, Taylor‐Clarke M, Murray C, et al. Investigating strategies to improve attendance at screening visits in a randomized trial. Trials 2011;12(Suppl 1):A111. [DOI: 10.1186/1745-6215-12-S1-A111] [DOI] [Google Scholar]
Cockayne 2017 {published data only}
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18:144. [DOI: 10.1186/s13063-017-1797-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1997 {published data only}
- Cooper KG, Grant AM, Garratt AM. The impact of using a partially randomised patient preference design when evaluating alternative managements for heavy menstrual bleeding. Journal of Obstetrics and Gynaecology 1997;104:1367‐73. [DOI] [PubMed] [Google Scholar]
Coyne 2003 {published data only}
- Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, et al. Randomized, controlled trial of an easy‐to‐read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2003;21(5):836‐42. [DOI] [PubMed] [Google Scholar]
Dear 2011 {published data only}
- Dear RF, Barratt AL, Askie LM, Butow PN, McGeechan K, Crossing S, et al. Impact of a cancer clinical trials web site on discussions about trial participation: a cluster randomized trial. Annals of Oncology 2012;23:1912‐8. [DOI: 10.1093/annonc/mdr585] [DOI] [PubMed] [Google Scholar]
Diguiseppi 2006 {published data only}
- Diguiseppi C, Goss C, Xu S, Magid D, Graham A. Telephone screening for hazardous drinking among injured patients seen in acute care clinics: feasibility study. Alcohol & Alcoholism 2006;41(4):438‐45. [DOI] [PubMed] [Google Scholar]
Du 2008 {published data only}
- Du W, Mood D, Gadgeel S, Simon MS. An educational video to increase clinical trials enrolment among lung cancer patients. Journal of Thoracic Oncology 2008;3(1):23‐9. [DOI] [PubMed] [Google Scholar]
Du 2009 {published data only}
- Du W, Mood M, Gadgeel S, Simon MS. An educational video to increase clinical trials enrollment among breast cancer patients. Breast Cancer Research and Treatment 2009;117:339‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 2002 {published data only}
- Ellis PM, Butow PN, Tattersall MH. Informing breast cancer patients about clinical trials: a randomized clinical trial of an educational booklet. Annals of Oncology 2002;13:1414‐23. [DOI] [PubMed] [Google Scholar]
Fleissig 2001 {published data only}
- Fleissig A, Jenkins V, Fallowfield L. Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Euorpean Journal of Cancer 2001;37:322‐31. [DOI] [PubMed] [Google Scholar]
Ford 2004 {published data only}
- Ford ME, Havstad SL, Davis SD. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Clinical Trials 2004;1:343‐51. [DOI] [PubMed] [Google Scholar]
Foss 2016 {published and unpublished data}
- Foss KT, Kjærgaard J, Stensballe LG, Greisen G. Recruiting to clinical trials on the telephone – a randomized controlled trial. Trials 2016;17:552. [DOI: 10.1186/s13063-016-1680-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fowell 2006 {published data only}
- Fowell A, Johnstone R, Finlay IG, Russell D, Russell IT. Design of trials with dying patients: a feasibility study of cluster randomisation versus randomised consent. Palliative Medicine 2006;20:799‐804. [DOI] [PubMed] [Google Scholar]
Fracasso 2013 {published data only}
- Fracasso PM, Goodner SA, Creekmore AN, Morgan HP, Foster DM, Hardmon AA, et al. Coaching intervention as a strategy for minority recruitment to cancer clinical trials. Journal of Oncology Practice 2013;9:294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Free 2010 {published and unpublished data}
- Free C, Hoile E, Robertson S, Knight R. Three controlled trials of interventions to increase recruitment to a randomized controlled trial of mobile phone based smoking cessation support. Clinical Trials 2010;7:265‐73. [DOI] [PubMed] [Google Scholar]
Free 2011 {published data only}
- Free CJ, Hoile E, Knight R, Robertson S, Devries KM. Do messages of scarcity increase trial recruitment?. Contemporary Clinical Trials 2011;32:36‐9. [DOI: 10.1016/j.cct.2010.09.002] [DOI] [PubMed] [Google Scholar]
Freer 2009 {published and unpublished data}
- Freer Y, McIntosh N, Teunisse S, Kanwaljeet JSA, Boyle EM. More information, less understanding: a randomized study on consent issues in neonatal research. Pediatrics 2009;123:1301‐5. [DOI] [PubMed] [Google Scholar]
Fureman 1997 {published data only}
- Fureman I, Meyers K, McLellan AT, Metzger D, Woody G. Evaluation of a video‐supplement to informed consent: injection drug users and preventive HIV vaccine efficacy trials. AIDS Education and Prevention 1997;9(4):330‐41. [PubMed] [Google Scholar]
Graham 2007 {published data only}
- Graham A, Goss C, Xu S, Magid D, Diguiseppi C. Effect of using different modes to administer the AUDIT‐C on identification of hazardous drinking and acquiescence to trial participation among injured patients. Alcohol & Alcoholism 2007;42(5):423‐9. [DOI] [PubMed] [Google Scholar]
Halpern 2004 {published data only}
- Halpern SD, Karlawish JHT, Casarett, D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Annals of Internal Medicine 2004;164:801‐3. [DOI] [PubMed] [Google Scholar]
Hemminki 2004 {published data only}
- Hemminki E, Hovi SL, Veerus P, Sevon T, Tuimala R, Rahu M, et al. Blinding decreased recruitment in a prevention trial of postmenopausal hormone therapy. Journal of Clinical Epidemiology 2004;57:1237‐43. [DOI] [PubMed] [Google Scholar]
Hutchison 2007 {published data only}
- Hutchison C, Cowan C, McMahon T, Paul J. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. British Journal of Cancer 2007;97:705‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ives 2001 {published data only}
- Ives NJ, Troop M, Waters A, Davies S, Higgs C, Easterbrook PJ. Does an HIV clinical trial information booklet improve patient knowledge and understanding of HIV clinical trials?. HIV Medicine 2001;2:241‐9. [DOI] [PubMed] [Google Scholar]
Jacobsen 2012 {published data only}
- Jacobsen PB, Wells KJ, Meade CD, Quinn GP, Lee JH, Fulp WJ, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. Journal of Clinical Oncology 2012;30:2516‐21. [DOI: 10.1200/JCO.2011.39.5186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015a {published data only}
- Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants?. Trials 2015;16:80. [DOI: 10.1186/s13063-015-0582-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015b {published data only}
- Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants?. Trials 2015;16:80. [DOI: 10.1186/s13063-015-0582-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015c {published data only}
- Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants?. Trials 2015;16:80. [DOI: 10.1186/s13063-015-0582-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015d {published data only}
- Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants?. Trials 2015;16:80. [DOI: 10.1186/s13063-015-0582-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jennings 2015e {published data only}
- Jennings CG, MacDonald TM, Wei L, Brown MJ, McConnachie L, Mackenzie IS. Does offering an incentive payment improve recruitment to clinical trials and increase the proportion of socially deprived and elderly participants?. Trials 2015;16:80. [DOI: 10.1186/s13063-015-0582-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeste 2009 {published data only}
- Jeste DV, Palmer BW, Golshan S, Eyler LT, Dunn LB, Meeks T, et al. Multimedia consent for research in people with schizophrenia and normal subjects: a randomized controlled trial. Schizophrenia Bulletin 2009;35:719‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karunaratne 2010 {published data only}
- Karunaratne A, Koremann SG, Thomas SL, Myles PS, Komeraroff PA. Improving communication when seeking informed consent: a randomised controlled study of a computer‐based method for providing information to prospective clinical trial participants. Medical Journal of Australia 2010;192:388‐92. [DOI] [PubMed] [Google Scholar]
Kendrick 2001 {published data only}
- Kendrick D, Watson M, Dewey M, Woods AJ. Does sending a home safety questionnaire increase recruitment to an injury prevention trial? A randomised controlled trial. Journal of Epidemiology and Community Health 2001;55:845‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerr 2004 {published and unpublished data}
- Kerr CEP, Robinson EJ, Lilford RL, Edwards SLJ, Braunholtz DA, Stevens AJ. The impact of describing clinical trial treatments as new or standard. Patient Education and Counseling 2004;53:107‐13. [DOI] [PubMed] [Google Scholar]
Kimmick 2005 {published data only}
- Kimmick GG, Peterson BL, Kornblith AB, Mandelblatt J, Johnson JL, Wheeler J, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. Journal of Clinical Oncology 2005;23(10):2201‐7. [DOI] [PubMed] [Google Scholar]
Larkey 2002 {published data only}
- Larkey LK, Staten LK, Ritenbaugh C, Hall RA, Buller DB, Bassford T, et al. Recruitment of Hispanic women to the Women's Health Initiative: the case of Embajadoras in Arizona. Controlled Clinical Trials 2002;23:289‐98. [DOI] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee H, Hubscher M, Moseley GL, Kamper SJ, Traeger AC, Skinner IW, et al. An embedded randomised controlled trial of a teaser campaign to optimise recruitment in primary care. Clinical Trials 2017;14:162‐9. [DOI: 10.1177/1740774516683921] [DOI] [PubMed] [Google Scholar]
Liénard 2006 {published data only}
- Liénard J‐L, Quinax E, Fabre‐Guillevin E, Piedbois P, Jouhaud A, Decoster G, et al. Impact of on‐site initiation visits on patient recruitment and data quality in a randomized trial of adjuvant chemotherapy for breast cancer. Clinical Trials 2006;3:486‐92. [DOI] [PubMed] [Google Scholar]
Litchfield 2005 {published data only}
- Litchfield J, Freeman J, Schou H, Elsley M, Fuller R, Chubb B. Is the future for clinical trials internet‐based? A cluster randomized clinical trial. Clinical Trials 2005;2:72‐9. [DOI] [PubMed] [Google Scholar]
Llewellyn‐Thomas 1995a {published data only}
- Llewellyn‐Thomas HA, McGreal MJ, Thiel EC. Cancer patients decision making and trial‐entry preferences: the effects of framing information about short‐term toxicity and long‐term survival. Medical Decision Making 1995;15:4‐12. [DOI] [PubMed] [Google Scholar]
Llewellyn‐Thomas 1995b {published data only}
- Llewellyn‐Thomas HA, Thiel EC, Sem FWC, Woermke DEH. Presenting clinical trial information: a comparison of methods. Patient Education and Counseling 1995;25:97‐107. [DOI] [PubMed] [Google Scholar]
MacQueen 2014 {published data only}
- MacQueen KM, Chen M, Ramirez C, Nnko SEA, Earp KM. Comparison of closed‐ended, open‐ended, and perceived informed consent comprehension measures for a mock HIV prevention trial among women in Tanzania. PLOS One 2013;9:e105720. [DOI: 10.1371/journal.pone.0105720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Man 2015a {published data only}
- Man MS, on behalf of the Healthlines Study Group, Rick J, Bower P, on behalf of the MRC‐START Group. Improving recruitment to a study of telehealth management for long‐term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16:309. [DOI: 10.1186/s13063-015-0820-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Man 2015b {published data only}
- Man MS, on behalf of the Healthlines Study Group, Rick J, Bower P, on behalf of the MRC‐START Group. Improving recruitment to a study of telehealth management for long‐term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16:309. [DOI: 10.1186/s13063-015-0820-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandelblatt 2005 {published data only}
- Mandelblatt J, Kaufmann E, Sheppard VB, Pomeroy J, Kavanaugh J, Canar J, et al. Breast cancer prevention in community clinics: will low‐income Latina patients participate in clinical trials?. Preventive Medicine 2005;40:611‐8. [DOI] [PubMed] [Google Scholar]
Miller 1999 {published data only}
- Miller NL, Markowitz JC, Kocsis JH, Leon AC, Brisco ST, Garno JL. Cost effectiveness of screening for clinical trials by research assistants versus senior investigators. Journal of Psychiatric Research 1999;33:81‐5. [DOI] [PubMed] [Google Scholar]
Monaghan 2007 {published data only}
- Monaghan H, Richens A, Colman S, Currie R, Girgis S, Jayne K, et al. A randomised trial of the effects of an additional communication strategy on recruitment into a large‐scale, multi‐centre trial. Contemporary Clinical Trials 2007;28:1‐5. [DOI] [PubMed] [Google Scholar]
Mudano 2013 {published data only}
- Mudano AS, Gary LC, Oliveira A, Wright MM, Curtis J, Delzell E, et al. Using tablet computers compared to interactive voice response to improve subject recruitment in osteoporosis pragmatic clinical trials: feasibility, satisfaction, and sample size. Patient Preference and Adherence 2013;7:517‐23. [DOI: 10.2147/PPA.S44551] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myles 1999 {published data only}
- Myles PS, Fletcher HE, Cairo S, Madder H, McRae R, Cooper J, et al. Randomized trial of informed consent and recruitment for clinical trials in the immediate preoperative period. Anesthesiology 1999;91:969‐78. [DOI] [PubMed] [Google Scholar]
Nystuen 2004 {published data only}
- Nystuen P, Hagen KB. Telephone reminders are effective in recruiting nonresponding patients to randomized controlled trials. Journal of Clinical Epidemiology 2004;57:773‐6. [DOI] [PubMed] [Google Scholar]
Paul 2011 {published data only}
- Paul J, Iveson T, Midgely R, Harkin A, Masterton M, Alexander L, Cassidy J. Choice of randomisation time‐point in non‐inferiority studies of reduced treatment duration: experience from the SCOT study. Trials 2011;12:A30. [Google Scholar]
Paul 2014 {published data only}
- Paul C, Courtney R, Sanson‐Fisher R, Carey M, Hill D, Simmons J, Rose S. A randomized controlled trial of the effectiveness of a pre‐recruitment primer letter to increase participation in a study of colorectal screening and surveillance. BMC Medical Research Methodology 2014;14:44. [DOI: 10.1186/1471-2288-14-44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perrone 1995 {published data only}
- Perrone F, Placido S, Giusti C, Gallo C. Looking for consent in RCTs: a randomised trial with surrogate patients [La richiesta del consenso nella ricerca clinica: uno studio randomizzato in soggetti sani]. Epidemiologia e Prevenzione 1995;19:282‐90. [PubMed] [Google Scholar]
Pighills 2009 {published data only}
- Pighills A, Torgerson DJ, Sheldon T. Publicity does not increase recruitment to falls prevention trials: the results of two quasi‐randomized trials. Journal of Clinical Epidemiology 2009;62:1332‐5. [DOI] [PubMed] [Google Scholar]
Simel 1991 {published data only}
- Simel DL, Feussner JR. A randomized controlled trial comparing quantitative informed consent formats. Journal of Clinical Epidemiology 1991;44(8):771‐7. [DOI] [PubMed] [Google Scholar]
Simes 1986 {published data only}
- Simes RJ, Tattersall MHN, Coates AS, Raghavan D, Solomon HJ. Randomised comparison of procedures for obtaining informed consent in clinical trials of treatment for cancer. BMJ 1986;293:1065‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tehranisa 2014 {published data only}
- Tehranisa JS, Meurer WJ. Can response‐adaptive randomization increase participation in acute stroke trials?. Stroke 2014;45:2131‐3. [DOI: 10.1161/STROKEAHA.114.005418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tilley 2012 {published data only}
- Tilley BC, Mainous AG, Elm JJ, Pickelsimer E, Soderstrom LH, Ford ME, et al. A randomized recruitment intervention trial in Parkinson's disease to increase participant diversity: early stopping for lack of efficacy. Clinical Trials 2012;9:188‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treschan 2003 {published data only}
- Treschan TA, Scheck T, Kober A, Fleischmann E, Birkenberg B, Petschnigg B, et al. The influence of protocol pain and risk on patients' willingness to consent for clinical studies: a randomized trial. Economics, Education, and Health Systems Research 2003;96:498‐506. [DOI] [PubMed] [Google Scholar]
Trevena 2006 {published data only}
- Trevena L, Irwig L, Barratt A. Impact of privacy legislation on the number and characteristics of people who are recruited for research: a randomised controlled trial. Journal of Medical Ethics 2006;32:473‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treweek 2012 {published data only}
- Treweek S, Barnett K, Maclennan G, Bonetti D, Eccles MP, Francis JJ, et al. E‐mail invitations to general practitioners were as effective as postal invitations and were more efficient. Journal of Clinical Epidemiology 2012;65:793‐7. [DOI: 10.1016/j.jclinepi.2011.11.010] [DOI] [PubMed] [Google Scholar]
Wadland 1990 {published data only}
- Wadland WC, Hughes JR, Secker‐Walker RH, Bronson DL, Fenwick J. Recruitment in a primary care trial on smoking cessation. Family Medicine 1990;22:201‐4. [PubMed] [Google Scholar]
Weinfurt 2008a {published data only}
- Weinfurt KP, Hall MA, Friedman JY, Hardy NC, Fortune‐Greeley AK, Lawlor JS, et al. Effects of disclosing financial interests on participation in medical research: a randomized vignette trial. Journal of General Internal Medicine 2008;23:860‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinfurt 2008b {published data only}
- Weinfurt KP, Hall MA, Friedman JY, Hardy NC, Fortune‐Greeley AK, Lawlor JS, et al. Effects of disclosing financial interests on participation in medical research: a randomized vignette trial. American Heart Journal 2008;156:689‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2013 {published data only}
- Wells KJ, McIntyre J, Gonzalez LE, Lee JH, Fisher KJ, Jacobsen PB. Feasibility trial of a Spanish‐language multimedia educational intervention. Clinical Trials 2013;10:767‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welton 1999 {published data only}
- Welton AJ, Vickers MR, Cooper JA, Meade TW, Marteau TM. Is recruitment more difficult with a placebo arm in randomised controlled trials? A quasi randomised, interview based study. BMJ 1991;318:1114‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weston 1997 {published data only}
- Weston J, Hannah M, Downes J. Evaluating the benefits of a patient information video during the informed consent process. Patient Education and Counseling 1997;30:239‐45. [DOI] [PubMed] [Google Scholar]
Wong 2013 {published data only}
- Wong AD, Kirby J, Guyatt GH, Moayyedi P, Vora P, You JJ. Randomized controlled trial comparing telephone and mail follow‐up for recruitment of participants into a clinical trial of colorectal cancer screening. Trials 2013;14:40. [DOI: 10.1186/1745-6215-14-40] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aalborg 2012 {published data only}
- Aalborg AE, Miller BA, Husson G, Byrnes HF, Bauman KE, Spoth RL. Implementation of adolescent family‐based substance use prevention programs in health care settings: comparisons across conditions and programs. Health Education Journal 2012;71:53‐61. [DOI: 10.1177/0017896910386209] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aaronson 1996 {published data only}
- Aaronson NK, Visser‐Pol E, Gerleen HMW, Muller MJ, Schot AC, Dam FS, et al. Telephone‐based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. Journal of Clinical Oncology 1996;14:984‐96. [DOI] [PubMed] [Google Scholar]
Agoritsas 2010 {published data only}
- Agoritsas T, Deom M, Perneger TV. Study design attributes influenced patients' willingness to participate in clinical research: a randomized vignette‐based study. Journal of Clinical Epidemiology 2011;64:107‐15. [DOI: ] [DOI] [PubMed] [Google Scholar]
Alexander 2008 {published data only}
- Alexander GL, Divine GW, Couper MP, McClure JB, Stopponi MA, Fortman KK, et al. Effect of incentives and mailing features on online health program enrollment. American Journal of Preventive Medicine 2008;34:382‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrew 1993 {published data only}
- Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. Journal of Pediatrics 2003;123:285‐91. [DOI] [PubMed] [Google Scholar]
Barnard 2010 {published data only}
- Barnard KD, Dent L, Cook A. A systematic review of models to predict recruitment to multicentre clinical trials. BMC Medical Research Methodology 2011;10:63. [DOI: 10.1186/1471-2288-10-63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 2005 {published data only}
- Berman SR, Friedman EC, Callan JO, Fasiczka AL, McLean M, Hunter DM, et al. Recruitment and screening strategies for bipolar clinical trials. Bipolar Disorders 2005;7(Suppl 2):27‐117. [Google Scholar]
Brach 2013 {published data only}
- Brach M, Moschny A, Bücker B, Klaaßen‐Mielke R, Trampisch M, Wilm S, et al. Recruiting hard‐to‐reach subjects for exercise interventions: a multi‐centre and multi‐stage approach targeting general practitioners and their community‐dwelling and mobility‐limited patients. International Journal of Environmental Research and Public Health 2013;10:6611‐29. [DOI: 10.3390/ijerph10126611] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brealey 2007 {published data only}
- Brealey SD, Atwell C, Bryan S, Coulton S, Cox H, Cross B, et al. Using postal randomization to replace telephone randomization had no significant effect on recruitment of patients. Journal of Clinical Epidemiology 2006;60:1046‐51. [DOI] [PubMed] [Google Scholar]
Breland‐Noble 2012 {published data only}
- Breland‐Noble AM, AAKOMA Project Adult Advisory Board. Community and treatment engagement for depressed African American youth: the AAKOMA FLOA pilot. Journal of Clinical Psychology in Medical Settings 2012;19:41‐8. [DOI: 10.1007/s10880-011-9281-0] [DOI] [PubMed] [Google Scholar]
Brocklehurst 2007 {published data only}
- Brocklehurst P, Tarnow‐Mordi W, Farrell B, Quigley M. INLET trial ‐ cluster trial of newsletters and educational supplements to centres participating in INIS trial. Oxford: National Perinatal Epidemiology Unit; 2007. Annual Report 2006.
Brown 2012 {published data only}
- Brown SD, Lee K, Schoffman DE, King AC, Crawley LM, Kiernan M, et al. Minority recruitment into clinical trials: experimental findings and practical implications. Contempary Clinical Trials 2012;33:620‐3. [DOI: 10.1016/j.cct.2012.03.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2008 {published data only}
- Burns D, Soward AC, Skelly AH, Leeman J, Carlson J. Effective recruitment and retention strategies for older members of rural minorities. Diabetes Educator 2008;34:1045‐52. [DOI] [PubMed] [Google Scholar]
Caldwell 2002 {published data only}
- Caldwell P, Craig J, Hamilton S. [Strategies for recruitment to RCTs: a systematic review of controlled trials and observational studies]. International Clinical Trials Symposium: improving health care in the new millennium, 2002 October 21‐23; Sydney. 2002:34‐5.
Calimlim 1977 {published data only}
- Calimlim J, Wardell WM, Lasagna L. Selection, attrition, and consent in recruitment of patients for a clinical trial. Clinical Pharmacology & Therapeutics 1977;21:100. [Google Scholar]
Carney 2014 {published data only}
- Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. Journal of Cancer Education 2014;29(1):181‐7. [DOI: 10.1007/s13187-013-0567-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celentano 1995 {published data only}
- Celentano DD, Beyrer C, Natpratan C, Eiumtrakul S, Sussman L, Renzullo PO, et al. Willingness to participate in AIDS vaccine trials among high‐risk populations in northern Thailand. AIDS 1995;9:1079‐83. [DOI] [PubMed] [Google Scholar]
Chin Feman 2008 {published data only}
- Chin Feman SP, Nguyen LT, Quilty MT, Kerr CE, Nam BH, Conboy LA, et al. Effectiveness of recruitment in clinical trials: an analysis of methods used in a trial for irritable bowel syndrome patients. Contemporary Clinical Trials 2008;29:241‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlebowski 2010 {published data only}
- Chlebowski RT, Menon R, Chaisanguanthum RM, Jackson DM. Prospective evaluation of two recruitment strategies for a randomized controlled cancer prevention trial. Clinical Trials 2010;7:744‐8. [DOI: 10.1177/1740774510383886] [DOI] [PubMed] [Google Scholar]
Clagett 2013 {published data only}
- Clagett B, Nathanson KL, Ciosek SL, McDermoth M, Vaughn DJ, Mitra N, et al. Comparison of address‐based sampling and random‐digit dialing methods for recruiting young men as controls in a case‐control study of testicular cancer susceptibility. American Journal of Epidemiology 2013;178(11):1638‐47. [DOI: 10.1093/aje/kwt164] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 2010 {published data only}
- Cook ED, Arnold KB, Hermos JA, McCaskill‐Stevens W, Moody‐Thomas S, Probstfield JL, et al. Impact of supplemental site grants to increase African American accrual for the Selenium and Vitamin E Cancer Prevention Trial. Clinical Trials 2010;7(1):90‐9. [DOI: 10.1177/1740774509357227] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coronado 2012 {published data only}
- Coronado GD, Ondelacy S, Schwarz Y, Duggan C, Lampe JW, Neuhouser ML. Recruiting underrepresented groups into the Carbohydrates and Related Biomarkers (CARB) cancer prevention feeding study. Contemporary Clinical Trials 2012;33(4):641‐6. [DOI: 10.1016/j.cct.2012.03.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dal‐Ré 1991 {published data only}
- Dal‐Ré R. Clinical research with drugs: a study of the influence of information about adverse reactions on obtaining informed consent [Investigación clínica con fármacos: estudio de la influencia de la informacíon sobre reacciones adversas en la obtención del consentimiento informado]. Medicina Clínica (Barcelona) 1991;96:566‐9. [PubMed] [Google Scholar]
Davis 1998 {published data only}
- Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. Journal of the National Cancer Institute 1998;90:668‐74. [DOI] [PubMed] [Google Scholar]
Donovan 2009 {published data only}
- Donovan J, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. Journal of Clinical Epidemiology 2009;62:29‐36. [DOI] [PubMed] [Google Scholar]
Donovan 2010 {published data only}
- Donovan J, Lane A, Mills N, Neal D, Hamdy F, the ProtecT Study Group. Development and application of a complex intervention to improve recruitment to randomised controlled trials. Clinical Trials 2010;7:469. [Google Scholar]
Eckardt 2011 {published data only}
- Eckardt JR, DeMaggio AW, Peracha O, Levonyak M, Ku N. Impact of direct physician‐to‐physician contact on accelerating oncology clinical trials accrual. Journal of Clinical Oncology 2011;29(15 Suppl). [Google Scholar]
Embi 2012 {published data only}
- Embi PJ, Leonard AC. Evaluating alert fatigue over time to EHR‐based clinical trial alerts: findings from a randomized controlled study. Journal of the American Medical Informatics Association: JAMIA 2012;19:145‐8. [DOI: 10.1136/amiajnl-2011-000743] [DOI] [PMC free article] [PubMed] [Google Scholar]
Enama 2012 {published data only}
- Enama ME, Hu Z, Gordon I, Costner P, Ledgerwood JE, Grady C, VRC 306 and 307 Consent Study Teams. Randomization to standard and concise informed consent forms: development of evidence‐based consent practices. Contemporary Clinical Trials 2012;33(5):895‐902. [DOI: 10.1016/j.cct.2012.04.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feman 2008 {published data only}
- Feman SPC, Nguyen LI, Quilty MT, Kerr CE, Nam BH, Conboy LA, et al. Effectiveness of recruitment in clinical trials: an analysis of methods used in a trial for irritable bowel syndrome patients. Contemporary Clinical Trials 2008;29:241‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foradori 2012 {published data only}
- Foradori MA, Nolan MT. Effect of a study map intended to support informed consent in transplant research. Progress in Transplantation 2012;22(1):56‐61. [DOI: 10.7182/pit2012553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallo 1995 {published data only}
- Gallo C, Perrone F, Placido S, Giusti C. Informed versus randomised consent to clinical trials. Lancet 1995;346:1060‐4. [DOI] [PubMed] [Google Scholar]
Gillan 2009 {published data only}
- Gillan MG, Gilbert FJ, Flight H, Cooper J, Wallis MG, James JJ, et al. Increasing participant recruitment into large‐scale screening trials: experience from the CADET II study. Journal of Medical Screening 2009;16(4):180‐5. [DOI: 10.1258/jms.2009.009023] [DOI] [PubMed] [Google Scholar]
Gilligan 2014 {published data only}
- Gilligan C, Kypri K. Recruiting by registered versus standard mail. Epidemiology 2014;25(2):317. [DOI: 10.1097/EDE.0000000000000065] [DOI] [PubMed] [Google Scholar]
Gillon 2009 {published data only}
- Gillan MG, Gilbert FJ, Flight H, Cooper J, Wallis MG, James JJ, et al. Increasing participant recruitment into large‐scale screening trials: experience from the CADET II study. Journal of Medical Screening 2009;16:180‐5. [DOI] [PubMed] [Google Scholar]
Ginexi 2003 {published data only}
- Ginexi EM, Crosse SB, Caudill BD. Alcohol abuse prevention programming for fraternity members: are we reaching the heaviest drinkers?. 11th Annual Meeting of the Society for Research Prevention. 2003.
Gitanjali 2003 {published data only}
- Gitanjali B, Raveendran R, Pandian DG, Sujindra S. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference?. Stroke 2003;34:e109‐37. [PubMed] [Google Scholar]
Goldstein 2010 {published data only}
- Goldstein JN, Delaney KE, Pelletier AJ, Fisher J, Blanc PG, Halsey M, et al. A brief educational intervention may increase public acceptance of emergency research without consent. Journal of Emergency Medicine 2010;39(4):419‐35. [DOI: 10.1016/j.jemermed.2007.12.033] [DOI] [PubMed] [Google Scholar]
Gomez 1998 {published data only}
- Gómez Arnáu JI. Preoperative information and informed consent from surgical patients. Revista Española de Anestesiologia y Reanimacion 1998;45(9):401. [PubMed] [Google Scholar]
Graham 2011 {published data only}
- Graham AL, Lopez‐Class M, Mueller NT, Mota G, Mandelblatt J. Efficiency and cost‐effectiveness of recruitment methods for male Latino smokers. Health Education & Behavior 2011;38(3):293‐300. [DOI: 10.1177/1090198110372879] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grubbs 2009 {published data only}
- Grubbs SS, Gonzalez M, Krasna M, Siegel R, Bryant D, Tschetter L, et al. Tracking clinical trial accrual strategies and barriers via a Web‐based screening tool. Journal of Clinical Oncology 2009;27(15 Suppl). [Google Scholar]
Halpern 2002 {published data only}
- Halpern S. Challenges to improving the impact of worksite cancer prevention programs: comparing reach, enrollment, and attrition using active versus passive recruitment strategies. Controlled Clinical Trials 2002;24:274‐88. [DOI] [PubMed] [Google Scholar]
Harris 2008 {published data only}
- Harris TJ, Carey IM, Victor CR, Adams R, Cook DG. Optimising recruitment into a study of physical activity in older people: a randomised controlled trial of different approaches. Age and Ageing 2008;37:659‐65. [DOI] [PubMed] [Google Scholar]
Harron 2012 {published data only}
- Harron K, Lee T, Ball T, Mok Q, Gamble C, Macrae D, et al. CATCH team. Making co‐enrolment feasible for randomised controlled trials in paediatric intensive care. PLOS ONE 2012;7(8):e41791. [DOI: 10.1371/journal.pone.0041791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiney 2010 {published data only}
- Heiney SP, Arp Adams S, Drake BF, Bryant LH, Bridges L, Hebert JR. Successful subject recruitment for a prostate cancer behavioral intervention trial. Clinical Trials 2010;7(4):411‐7. [DOI: 10.1177/1740774510373491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henkel 2010 {published data only}
- Henkel V, Mergl R, Allgaier AK, Hautzinger M, Kohnen R, Coyne JC, et al. Treatment of atypical depression: post‐hoc analysis of a randomized controlled study testing the efficacy of sertraline and cognitive behavioural therapy in mildly depressed outpatients. European Psychiatry 2010;25(8):491‐8. [DOI: 10.1016/j.eurpsy.2010.01.010] [DOI] [PubMed] [Google Scholar]
Hillsdon 2011 {published data only}
- Hillsdon M. Rates of recruitment from systematic and opportunistic methods: preliminary results from the DDELPHI study. Trials 2011;12(Suppl 1):A112. [Google Scholar]
Hoffner 2011 {published data only}
- Hoffner B, Bauer‐Wu S, Hitchcock‐Bryan S, Powell M, Wolanski A, Joffe S. "Entering a Clinical Trial: Is it Right for You?": a randomized study of the clinical trials video and its impact on the informed consent process. Cancer 2012;118(7):1877‐83. [DOI: 10.1002/cncr.26438] [DOI] [PMC free article] [PubMed] [Google Scholar]
Homish 2009 {published data only}
- Homish GG, Leonard KE. Testing methodologies to recruit adult drug‐using couples. Addictive Behaviors 2009;34(1):96‐9. [DOI: 10.1016/j.addbeh.2008.08.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaffee 2009 {published data only}
- Jaffee WB, Bailey GL, Lohman M, Riggs P, McDonald L, Weiss RD. Methods of recruiting adolescents with psychiatric and substance use disorders for a clinical trial. American Journal of Drug and Alcohol Abuse 2009;35(5):381‐4. [DOI: 10.1080/00952990903150860] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jay 2007 {published data only}
- Jay F, Chantler T, Lees A, Pollard AJ. Children's participation in vaccine research: parents' views. Paediatric Nursing 2007;19:14‐8. [DOI] [PubMed] [Google Scholar]
Jenkins 2013 {published data only}
- Jenkins VA, Farewell D, Farewell V, Batt L, Wagstaff J, Langridge C, et al. Teams Talking Trials: results of an RCT to improve the communication of cancer teams about treatment trials. Contemporary Clinical Trials 2013;35(1):43‐51. [DOI: 10.1016/j.cct.2013.02.003] [DOI] [PubMed] [Google Scholar]
Ji 2008 {published data only}
- Ji P, DuBois DL, Flay BR, Brechling V. "Congratulations, you have been randomized into the control group!(?)": issues to consider when recruiting schools for matched‐pair randomized control trials of prevention programs. Journal of School Health 2008;78:131‐9. [DOI] [PubMed] [Google Scholar]
Junghans 2005 {published data only}
- Junghans C, Feder G, Hemingway H, Timmis A, Jones M. Recruiting patients to medical research: double blind randomised trial of "opt‐in" versus "opt‐out" strategies. BMJ 2005;331:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juraskova 2014 {published data only}
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AB, Seccombe M, et al. Improving decision making about clinical trial participation ‐ a randomised controlled trial of a decision aid for women considering participation in the IBIS‐II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1‐7. [DOI: 10.1038/bjc.2014.144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlawish 2008 {published data only}
- Karlawish J, Cary MS, Rubright J, Tenhave T. How redesigning AD clinical trials might increase study partners willingness to participate. Neurology 2008;71:1883‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keedy 2009 {published data only}
- Keedy VL, Horn L, Hayes A, Spencer B, Garcia G, Campbell N, et al. Enrollment of lung cancer patients on clinical trials at an NCI comprehensive cancer center. Journal of Clinical Oncology 2009;27(Suppl 15):355s. [Google Scholar]
Kelechi 2010 {published data only}
- Kelechi T, Watts A, Wiseman J. Recruitment strategy effectiveness for a cryotherapy intervention for a venous leg ulcer prevention study. Journal of Wound, Ostomy and Continence Nursing 2010;37:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kernan 2009 {published data only}
- Kernan WN, Viscoli CM, Demarco D, Mendes B, Shrauger K, Schindler JL, et al. Boosting enrollment in neurology trials with Local Identification and Outreach Networks (LIONs). Neurology 2009;72:1345‐51. [DOI: 10.1212/WNL.0b013e3181a0fda3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiernan 2000 {published data only}
- Kiernan M, Phillips K, Fair J, King AC. Using direct mail to recruit Hispanic adults into a dietary intervention: an experimental study. Annals of Behavioral Medicine 2000;22(1):89‐93. [DOI] [PubMed] [Google Scholar]
Kirkby 2013 {published data only}
- Kirkby HM, Calvert M, McManus RJ, Draper H. Informing potential participants about research: observational study with an embedded randomized controlled trial. PLOS ONE 2013;8(10):e76435. [DOI: 10.1371/journal.pone.0076435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Korde 2009 {published data only}
- Korde LA, Micheli A, Smith AW, Venzon D, Prindiville SA, Drinkard B, et al. Recruitment to a physical activity intervention study in women at increased risk of breast cancer. BMC Medical Research Methodology 2009;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruse 2000 {published data only}
- Kruse AY, Kjaergard LL, Krogsgaard K, Gluud C, Mortensen EL, Gottschau A, et al. A randomized trial assessing the impact of written information on outpatients' knowledge about and attitude toward randomized clinical trials. The INFO trial group. Controlled Clinical Trials 2000;21:223‐40. [DOI] [PubMed] [Google Scholar]
Labrique 2011 {published data only}
- Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie Aet al. A cluster‐randomized, placebo‐controlled, maternal vitamin a or beta‐carotene supplementation trial in Bangladesh: design and methods. Trials 2011;12:102. [DOI: 10.1186/1745-6215-12-102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lancet 2001 {published data only}
- Recruitment of women to clinical trials. Lancet 2001;358:853. [PubMed] [Google Scholar]
Lang 1991 {published data only}
- Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run‐in on the power of the Physicians' Health Study. Statistics in Medicine 1991;10:1585‐93. [DOI] [PubMed] [Google Scholar]
Larkey 2009 {published data only}
- Larkey LK, Gonzalez JA, Mar LE, Glantz N. Latina recruitment for cancer prevention education via Community Based Participatory Research strategies. Contemporary Clinical Trials 2009;30:47‐54. [DOI] [PubMed] [Google Scholar]
Leader 1978 {published data only}
- Leader MA, Neuwirth E. Clinical research and the noninstitutional elderly: a model for subject recruitment. Journal of the American Geriatrics Society 1978;26:27‐31. [DOI] [PubMed] [Google Scholar]
Lee 2011 {published data only}
- Lee JY, Foster HE Jr, McVary KT, Meleth S, Stavris K, Downey J, Kusek JW. Recruitment of participants to a clinical trial of botanical therapy for benign prostatic hyperplasia. Journal of Alternative and Complementary Medicine (New York) 2011;17(5):469‐72. [DOI: 10.1089/acm.2010.0300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichter 1991 {published data only}
- Lichter PR. Patient recruitment for clinical trials. Ophthalmology 1991;98:1489‐90. [DOI] [PubMed] [Google Scholar]
Lloyd‐Williams 2002 {published data only}
- Lloyd‐Williams F, Beaton S, Mair FS, Shiels C, Goldstein P, Hanratty B, et al. Recruiting heart failure patients to clinical trials: the experience of a randomised controlled trial in primary care. Society for Social Medicine 46th Annual Scientific Meeting. 2002.
Macias 2005 {published data only}
- Macias C, Barreira P, Hargreaves W, Bickman L, Fisher W, Aronson E. Impact of referral source and study applicants' preference for randomly assigned service on research enrollment, service engagement, and evaluative outcomes. American Journal of Psychiatry 2005;162:781‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marco 2008 {published data only}
- Marco CA. Impact of detailed informed consent on research subjects' participation: a prospective, randomized trial. Journal of Emergency Medicine 2008;34:269‐75. [DOI] [PubMed] [Google Scholar]
Masood 2006 {published data only}
- Masood J, Hafeez A, Wiseman O, Hill JT. Informed consent: are we deluding ourselves? A randomized controlled study. BJU International 2006;99:4‐5. [DOI] [PubMed] [Google Scholar]
May 2007 {published data only}
- May DE, Hallin MJ, Kratochvil CJ, Puumala SE, Smith LS, Reinecke MA, et al. Factors associated with recruitment and screening in the Treatment for Adolescents With Depression Study (TADS). Journal of the American Academy of Child and Adolescent Psychiatry 2007;46:801‐10. [DOI] [PubMed] [Google Scholar]
McGuire 2011 {published data only}
- McGuire AL, Oliver JM, Slashinski MJ, Graves JL, Wang T, Kelly PA, et al. To share or not to share: a randomized trial of consent for data sharing in genome research. Genetics in Medicine 2011;13(11):948‐55. [DOI: 10.1097/GIM.0b013e3182227589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menoyo 2006 {published data only}
- Menoyo E, Perez M, Torre R, Farré M. Telephone screening to improve recruitment of healthy volunteers in neuropsychopharmacology clinical trials: role of nurses. Proceedings of the 20th Congress of the Spanish Clinical Pharmacology Society. 2006.
Monane 1991 {published data only}
- Monane M, et al. The randomized controlled trial in the long‐term care setting ‐ recruitment and enrollment. Journal of the American Geriatrics Society 1991;39:A51. [Google Scholar]
Murphy 2011 {published data only}
- Murphy M, Merenstein D. Grassroots campaign trail methods to recruit for clinical trials: recruitment lessons learned from trail to trial. Clinical Medicine Insights: Pediatrics 2011;5:1‐7. [DOI: 10.4137/CMPed.S6488] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Lonergan 2011 {published data only}
- O'Lonergan TA, Forster‐Harwood JE. Novel approach to parental permission and child assent for research: improving comprehension. Pediatrics 2011;127(5):917‐24. [DOI: 10.1542/peds.2010-3283] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olver 2009 {published data only}
- Olver IN, Whitford HS, Denson LA, Peterson MJ, Olver SI. Improving informed consent to chemotherapy: a randomized controlled trial of written information versus an interactive multimedia CD‐ROM. Patient Education and Counseling 2009;74:197‐204. [DOI] [PubMed] [Google Scholar]
Paskett 2002 {published data only}
- Paskett ED, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell T, et al. Clinical trial enrollment of rural patients with cancer. Cancer Practice 2002;10:28‐35. [DOI] [PubMed] [Google Scholar]
Perri 2006 {published data only}
- Perri R, Wollin S, Drolet N, Mai S, Awad M, Feine J. Monitoring recruitment success and cost in a randomized clinical trial. European Journal of Prosthodontics & Restorative Dentistry 2006;14:126‐30. [PubMed] [Google Scholar]
Porucznik 2010 {published data only}
- Porucznik CA, Schliep KC, Stanford JB. Participant recruitment in a virtually interconnected world: comparative effectiveness and considerations of bias. American Journal of Epidemiology 2010;171(Suppl):s147. [Google Scholar]
Quinaux 2003 {published data only}
- Quinaux E, Liénard JL, Slimani Z, Jouhaud A, Piedbois P, Buyse M. Impact of monitoring visits on patient recruitment and data quality: case study of a phase IV trial in oncology. Controlled Clinical Trials 2003;24:99S. [Google Scholar]
Rogers 1998 {published data only}
- Rogers CG, Tyson JE, Kennedy KA, Broyles RS, Hickman JF. Conventional consent with opting in versus simplified consent with opting out: an exploratory trial for studies that do not increase patient risk. Journal of Pediatrics 1998;132:606‐11. [DOI] [PubMed] [Google Scholar]
Rowbotham 2013 {published data only}
- Rowbotham MC, Astin J, Greene K, Cumming SR. Interactive informed consent: randomized comparison with paper consents. PLOS ONE 2013;8(3):e58603. [DOI: 10.1371/journal.pone.0058603] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruffin 2011 {published data only}
- Ruffin MT, Nease DE. Using patient monetary incentives and electronically derived patient lists to recruit patients to a clinical trial. Journal of the American Board of Family Medicine 2011;24:569‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santoyo‐Olsson 2011 {published data only}
- Santoyo‐Olsson J, Cabrera J, Freyre R, Grossman M, Alvarez N, Mathur D, et al. An innovative multiphased strategy to recruit underserved adults into a randomized trial of a community‐based diabetes risk reduction program. Gerontologist 2011;51(Suppl 1):S82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saul 2002 {published data only}
- Saul H. Influences on recruitment in clinical trials. European Journal of Cancer 2002;38:2334. [Google Scholar]
Scholes 2007 {published data only}
- Scholes D, Heidrich FE, Yarbro P, Lindenbaum JE, Marrazzo JM. Population‐based outreach for Chlamydia screening in men: results from a randomized trial. Sexually Transmitted Diseases 2007;34:837‐9. [DOI] [PubMed] [Google Scholar]
Schrott 1982 {published data only}
- Schrott HG, Merideth N. Recruitment by screening entire communities. The Iowa Lipid Research Clinic Experience. Circulation 1982;66(6 Pt 2):IV23‐6. [PUBMED: 7127714] [PubMed] [Google Scholar]
Schroy 2009 {published data only}
- Schroy PC 3rd, Glick JT, Robinson P, Lydotes MA, Heeren TC, Prout M, et al. A cost‐effectiveness analysis of subject recruitment strategies in the HIPAA era: results from a colorectal cancer screening adherence trial. Clinical Trials 2009;6(6):597‐609. [DOI: 10.1177/1740774509346703] [DOI] [PubMed] [Google Scholar]
Sherman 2009 {published data only}
- Sherman KJ, Hawkes RJ, Ichikawa L, Cherkin DC, Deyo RA, Avins AL, Khalsa PS. Comparing recruitment strategies in a study of acupuncture for chronic back pain. BMC Medical Research Methodology 2009;9:69. [DOI: 10.1186/1471-2288-9-69] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swain 2011 {published data only}
- Swain J, Parish SL, Luken K, Atkins L. Recruitment and consent of women with intellectual disabilities in a randomised control trial of a health promotion intervention. Journal of Intellectual Disability Research 2011;55(5):474‐83. [DOI: 10.1111/j.1365-2788.2011.01399.x] [DOI] [PubMed] [Google Scholar]
Tenorio 2014 {published data only}
- Tenorio SL, O'Donnell CI, Hernandez J, Rozjabek HM, Lynch D, Marcus PM. Culturally sensitive approaches to recruitment and retention of Hispanics in the national lung screening trial. Journal of Immigrant and Minority Health/Center for Minority Public Health 2014;16(4):761‐4. [DOI: 10.1007/s10903-013-9862-0] [DOI] [PubMed] [Google Scholar]
Ubel 1997 {published data only}
- Ubel PA, Merz JF, Shea J, Asch DA. How preliminary data affect people's stated willingness to enter a hypothetical randomized controlled trial. Journal of Investigative Medicine 1997;45:561‐6. [PubMed] [Google Scholar]
Unger 2006 {published data only}
- Unger JM, Coltman CA Jr, Crowley JJ, Hutchins LF, Martino S, Livingston RB, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. Journal of Clinical Oncology 2006;24:141‐4. [DOI] [PubMed] [Google Scholar]
Unger 2010 {published data only}
- Unger S, Wylie L, Fallah S, Heinrich L, O'Brien K. Motivated by money? The impact of financial incentive for the research team on study recruitment. IRB: Ethics & Human Research 2010;32(1):16‐9. [PUBMED: 20184220] [PubMed] [Google Scholar]
Vaidya 2010 {published data only}
- Vaidya VS. Pragmatism in the TARGIT trial encouraged wider participation of centres yet yielded an unexpected homogeneous patient profile. EJC Supplements 2010;8(3):131. [Google Scholar]
Wang 2014 {published data only}
- Wang JH, Sheppard VB, Liang W, Ma GX, Maxwell AE. Recruiting Chinese Americans into cancer screening intervention trials: strategies and outcomes. Clinical Trials 2014;11(2):167‐77. [DOI: 10.1177/1740774513518849] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodford 2011 {published data only}
- Woodford J, Farrand P, Bessant M, Williams C. Recruitment into a guided internet based CBT (iCBT) intervention for depression: lesson learnt from the failure of a prevalence recruitment strategy. Contemporary Clinical Trials 2011;32(5):641‐48. [DOI: 10.1016/j.cct.2011.04.013] [DOI] [PubMed] [Google Scholar]
Wragg 2000 {published data only}
- Wragg JA, Robinson EJ, Lilford RJ. Information presentation and decisions to enter clinical trials: a hypothetical trial of hormone replacement therapy. Social Science and Medicine 2000;51:453‐62. [DOI] [PubMed] [Google Scholar]
Yates 2009 {published data only}
- Yates BC, Dodendorf D, Lane J, LaFramboise L, Pozehl B, Duncan K, et al. Testing an alternate informed consent process. Nursing Research 2009;58:135‐9. [DOI] [PubMed] [Google Scholar]
Zhou 2013 {published data only}
- Zhou ES, Dunsiger SI, Pinto BM. Proactive versus reactive recruitment to a physical activity intervention for breast cancer survivors: does it matter?. Clinical Trials 2013;10(4):587‐92. [DOI: 10.1177/1740774513480004] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cramer 1993 {published data only}
- Cramer JA. Patient recruitment and compliance issues in clinical trials. Epilepsy Research. Supplement 1993;10:211‐22. [PubMed] [Google Scholar]
Glen 1980 {published data only}
- Glen VH. Patient involvement utilizing video cassettes. Ontario Dentist 1980;57:20‐1. [PubMed] [Google Scholar]
Greenlee 2003 {published data only}
- Greenlee H, Gonzalez AJ, Lampe JW. Recruitment feasibility for a pilot randomized controlled trial on the effect of naturopathic therapies on estrogen metabolism. Cancer Epidemiology Biomarkers & Prevention 2003;12:1302s. [Google Scholar]
Additional references
Brueton 2013
- Brueton VC, Rait G, Tierney J, Meredith S, Darbyshire J, Harding S, et al. Strategies to improve retention in randomised trials. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.MR000032.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2010
- Caldwell PH, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLOS Medicine 2010;7:e1000368. doi:10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charlson 1984
- Charlson ME, Horwitz RI. Applying results of randomised trials to clinical practice: impact of losses before randomisation. BMJ 1984;289:1281‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2015
- Clarke M, Savage G, Maguire L, McAneney H. The SWAT (study within a trial) programme; embedding trials to improve the methodological design and conduct of future research. Trials 2015;16(Suppl 2):P209. [DOI: 10.1186/1745-6215-16-S2-P209] [DOI] [Google Scholar]
Edwards 2009
- Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response rates to postal questionnaires. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.MR000008.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 2017
- Farrell B. Status of our study. Email to S Treweek 6/4/2017.
Foy 2003
- Foy R, Parry J, Duggan A, Delaney B, Wilson S, Lewin‐van den Broek NTh, et al. How evidence‐based are recruitment strategies for randomized controlled trials in primary care? Experience from seven studies. Family Practice 2003;20:83‐92. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haidich 2001
- Haidich AB, Ioannidis JPA. Patterns of patient enrolment in randomized controlled trials. Journal of Clinical Epidemiology 2001;54:877‐83. [DOI] [PubMed] [Google Scholar]
Haynes 2016
- Haynes R. Randomisation used on our study. Email to S Treweek 23/11/2016.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitterman 2011
- Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low‐enrolling clinical studies at an academic medical center. Academic Medicine 2011;11:1360‐6. [DOI: 10.1097/ACM.0b013e3182306440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Madurasinghe 2016
- Madurasinghe VW, Eldridge S, on behalf of MRC START Group, Forbes G, on behalf of the START Expert Consensus Group. Guidelines for reporting embedded recruitment trials. Trials 2016;17:27. [DOI: 10.1186/s13063-015-1126-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2006
- McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2016
- Paul J. Ramdomisation used in our study. Email to S Treweek 21/12/2016.
Prescott 1999
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality,number and progress of randomised controlled trials. Health Technology Assessment 1999;3(20):1‐143. [PubMed] [Google Scholar]
Preston 2016
- Preston NJ, Farquhar MC, Walshe CE, Stevinson C, Ewing G, Calman LA, et al. Strategies designed to help healthcare professionals to recruit participants to research studies. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.MR000036.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rendell 2007
- Rendell JM, Merritt RK, Geddes JR. Incentives and disincentives to participation by clinicians in randomised controlled trials. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000021.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sully 2013
- Sully BGO, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013;14:166. [DOI: 10.1186/1745-6215-14-166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2017
- Walters SJ, Bonacho dos Anjos Henriques‐Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:e015276. [10.1136/bmjopen‐2016‐ 015276] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2006
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Medical Research Methodology 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mapstone 2007
- Mapstone J, Elbourne D, Roberts IG. Strategies to improve recruitment to research studies. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000013.pub3] [DOI] [PubMed] [Google Scholar]
Treweek 2010
- Treweek S, Pitkethly M, Cook J, Kjeldstrøm M, Taskila T, Johansen M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.MR000013.pub5] [DOI] [PubMed] [Google Scholar]
Treweek 2013
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrøm M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta‐analysis. BMJ Open 2013;3(2):e002360. [DOI: 10.1136/bmjopen-2012-002360] [DOI] [PMC free article] [PubMed] [Google Scholar]


