Patients with a low platelet count (thrombocytopenia) have an increased risk of both spontaneous and post‐procedural bleeding.1, 2 Platelet transfusions are therefore recommended in various guidelines,3, 4, 5 either when the platelet count drops below a certain threshold or prior to invasive procedures. The clinical studies forming the basis of these guidelines are known to be of low quality,3, 4, 5, 6 essentially reducing the value of transfusion guidelines to the quality level of expert opinion.
Most studies designed to assess the optimal platelet transfusion trigger frequently include a clinical assessment of bleeding as outcome measure. A review of studies evaluating platelet transfusion triggers in patients with leukemia reported a spontaneous bleeding incidence that varied between 12 and 66%.7 The authors concluded that this wide variance was more likely a reflection of different methods of bleeding assessment than an actual difference in the occurrence of bleeding. A recent review on coagulopathy prior to central venous catheter (CVC) placement by our group, also found a large variance in the incidence of bleeding.1
Several bleeding scales have been developed to help clinicians and researchers assess bleeding. The most widely used of these is the World Health Organization (WHO) bleeding scale,8 which was created to standardize toxicity reporting in cancer treatment. The Society of Interventional Radiology (SIR) has developed standards for reporting post‐procedural complications that includes a bleeding scale.9 These bleeding scales are ordinal in nature.
An ordinal scale assigns grades to bleeding of increasing severity, whereas a singular definition gives criteria of bleeding to which the answer is either yes or no. In principle, an ordinal bleeding scale renders more details on bleeding complications than a singular definition, provided it is clear enough to allow unambiguous usage. The WHO bleeding scale in particular, is hampered by subjectivity and while none of the frequently used bleeding scales have ever been formally tested for reproducibility,10 a study on adjudication of the WHO scale revealed high inter‐observer variability.11
Another problem with designing adequate bleeding scales is their clinical relevance. Historically, many studies have used WHO Grade 2‐4 bleeding complications as an outcome, while Grade 2 bleeding (“mild blood loss”) is widely regarded as clinically irrelevant. Nonetheless, researchers often include grade 2 bleeding in order to capture enough endpoints. The incidence of grade 2 bleeding usually outweighs the incidence of grade 3‐4 bleeding. Therefore, while such studies pretend to report clinically relevant bleeding, they mostly report “mild blood loss”, in this case a surrogate outcome.12
In this systematic review, we expect to find different bleeding incidences depending on the assessment methods and bleeding definitions used, but also depending on the study design. Retrospective studies have been shown to be less accurate than prospective studies and heavily depend on chart review. Minor bleeding in particular is not regularly recorded in clinical practice, and may therefore be underreported.7, 13
The primary objective of our study was to systematically review the methods and definitions used to assess bleeding severity in clinical research on invasive procedures. The secondary objective was to investigate the role of the study design in the variability in bleeding incidence.
MATERIALS AND METHODS
Inclusion & exclusion criteria
We included clinical studies (randomized controlled trials [RCTs] and cohort studies), both prospective and retrospective, on the following invasive procedures: CVC placement, liver biopsy (LB), renal biopsy (RB), bone marrow biopsy (BMB), or lumbar puncture (LP). Included studies needed to have bleeding complications as their primary or secondary endpoint and had to include at least one thrombocytopenic (<150 × 109/L) patient. An overview of thrombocytopenia and coagulopathy in each included study can be found in Appendix 1. Animal studies and case reports or series were excluded. Additionally, we excluded studies that were unavailable in English or Dutch.
Search
We conducted a MEDLINE search in May 2019, for which we used the search strategy that was previously described by the AABB, for the development of platelet transfusion guidelines.3 The search was not limited in time. Two authors independently reviewed citations for eligibility (EvdW & FvB); if any disagreement occurred a third author adjudicated (BB). We manually checked platelet transfusion guidelines to identify missing articles.3, 4, 5 The complete MEDLINE search terms are described in Appendix 2.
Assessment of risk of bias in included studies
For RCTs, the Cochrane Collaboration tool for the assessment of the risk of bias was used.14 For observational studies, the Newcastle‐Ottawa Scale was used.15 Overall study quality was assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method.16 The quality assessment is provided in Appendix 3.
Statistical analysis
Continuous data was described as mean (SD) if normally distributed or as median (IQR) if not normally distributed. Categorical data was described as number (%). Non‐normally distributed data was analyzed with Mann–Whitney U‐tests, confidence intervals of bleeding incidences were calculated with the Wilson method17 and all statistical analyses were performed using R‐Studio (version 1.1.453).
RESULTS
Study selection and characteristics
Our MEDLINE search yielded a total of 2692 articles (1190 BMB, 211 CVC insertion, 1247 LB & RB and 44 LP), and the manual search of transfusion guidelines yielded another 472 articles. After removal of duplicates 3018 articles were left, of which 30 met the predefined inclusion and exclusion criteria (Fig. 1).
Figure 1.
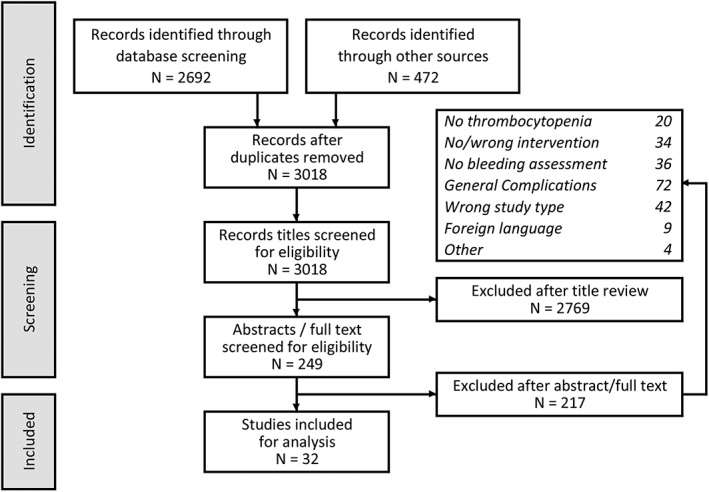
Study flow.
All studies were cohort studies, seven of which were prospective and 23 were retrospective. All studies had bleeding complications as their primary endpoint. There was reasonable variation in study types and populations studied (Table 1).
Table 1.
Study characteristics
| Number of participants, median (IQR) | 296 (108–1450) |
| Publication year, median (IQR) | 2012 (2000–2016) |
| Design | |
| RCT | 0 (0%) |
| Prospective cohort | 7 (23%) |
| Retrospective cohort* | 23 (77%) |
| Procedure type | |
| Central venous catheter* | 12 (40%) |
| Liver biopsy (LB) | 7 (23%) |
| Renal biopsy | 6 (20%) |
| Lumbar puncture (LP)* | 4 (13%) |
| Bone marrow biopsy (BMB) | 1 (3%) |
| Population | |
| General population | 12 (40%) |
| Advanced liver disease patients* | 5 (17%) |
| Hemato‐ / oncology* | 4 (13%) |
| Coagulopathic patients | 3 (10%) |
| TTP patients | 2 (7%) |
| Other | 4 (13%) |
Also includes studies in children.
BMB = bone marrow biopsy; IQR = interquartile range; RCT = randomized controlled trial; TTP = thrombotic thrombocytopenic purpura.
Differences in bleeding definitions
Overall, 11 studies used an ordinal bleeding scale, 13 used a singular bleeding definition and 6 reported no bleeding definition at all. Of the 24 studies with a bleeding definition, five used an existing ordinal bleeding scale (2) or incorporated elements of an existing ordinal bleeding scale in their singular definition (3). Nineteen studies used a bleeding definition (ordinal scale or singular definition) of the researchersʼ own design (Table 2). When investigators designed their own ordinal scale, it was always a two‐point scale (major and minor bleeding).
Table 2.
Use of bleeding definitions in studies of minimally invasive procedures
| Intervention | N | Categorical scale | Non‐categorical definition | No definition | ||
|---|---|---|---|---|---|---|
| Existing bleeding scale | Researchersʼ own design | Incorporating existing scale | Researchersʼ own design | |||
| Total | 30 | 2 (7%) | 9 (30%) | 3 (10%) | 10 (33%) | 6 (20%) |
| CVC placement | 12 | 1 (8%) | 5 (42%) | 1 (8%) | 3 (25%) | 2 (17%) |
| Liver biopsy | 7 | 1 (14%) | 1 (14%) | 0 (0%) | 3 (43%) | 2 (29%) |
| Renal biopsy | 6 | 0 (0%) | 3 (50%) | 1 (17%) | 2 (33%) | 0 (0%) |
| Lumbar puncture | 4 | 0 (0%) | 0 (0%) | 0 (0%) | 2 (50%) | 2 (50%) |
| BM biopsy | 1 | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
BM = bone barrow; CVC = central venous catheter.
The existing scales used in these studies included the SIR Technology Assessment Committee reporting standards9 and the National Cancer Instituteʼs Common Terminology Criteria for Adverse Events (CTCAE)18 (Table 3). A detailed overview of bleeding definitions for all included studies can be found in Table 4.
Table 3.
Bleeding scales used in studies of minimally invasive procedures
| Scale | Items |
|---|---|
| SIR |
A: no therapy, no consequence; B: requiring nominal therapy, no consequence, including overnight admission for observation; C: requiring therapy, minor hospitalization <48 hours; D: requiring major therapy, unplanned increase in level of care, prolonged hospitalization >48 hours; E: permanent adverse sequelae; F: death |
| CTCAE* |
1: mild symptoms not requiring invasive intervention; 2: mild symptoms requiring minimally invasive interventions or aspiration; 3: event indicating transfusion, radiological or surgical procedure; 4: life‐threatening consequences necessitating major urgent intervention; 5: death |
Zeidler et al used an adapted form of CTCAE that included prolonged compression as grade 2 bleeding.
SIR = Society of Interventional Radiology; CTCAE = Common Terminology Criteria for Adverse Events.
Table 4.
Bleeding definitions and assessment methods for all included studies
| Study | Year | Procedure | Bleeding definition* | Bleeding assessment / follow‐up | |
|---|---|---|---|---|---|
| (Minor) | (Major) | ||||
| Liu et al31 | 2017 | Bone marrow biopsy | ≥SIR grade C biopsy site bleeding, postprocedural imaging showing hematoma, >2g/dL Hb drop and requirement of vasopressors and/or inotropes | 1st hour vital signs monitoring every 15 minutes, then medical record review at 48 hours. | |
| Doerfler et al32 | 1996 | CVC (Landmark) | ‐ | Routine chest radiograph and nurses were instructed to report any evidence of bleeding or hematoma formation. | |
| Fisher et al33 | 1999 | CVC (Landmark) | Hemothorax or any other hemodynamically significant or life‐threatening hemorrhage | Superficial oozing >24 hours without hemodynamic consequence, or superficial hematoma (visible or palpable) | Routine chest radiograph and daily inspection until catheter removal. |
| Foster et al34 | 1992 | CVC (Landmark) | Insertion site bleeding: hemorrhage requiring removal of catheter or surgical intervention, including placement of suture ligatures, not including bleeding arrested with manual pressure; Hemothorax: pleural opacity on chest x‐ray, confirmed by aspiration of blood on thoracocentesis; Mediastinal hematoma: collection of blood in mediastinum clinically evident from serial hematocrit concentrations, confirmed by appropriate density on chest x‐ray or CT; Subcutaneous hematoma: subcutaneous bleeding at insertion site requiring surgical intervention to arrest bleeding or evacuate clot | ‐ | |
| Mumtaz et al35 | 2001 | CVC (Landmark) | Intervention necessary to stop hemorrhage, hematomas increasing in size, hemothorax, hemomediastinum | Bleeding arrested with digital manual pressure for approximately 20 minutes | Routine chest radiograph. Medical record review at undetermined time. |
| Pandey et al36 | 2017 | CVC (Landmark) | Requiring additional and non‐expected hemostatic measures (compression bandage >15 minutes; blood transfusions) and bleeding causing extension of hospital stay | Routine chest radiograph and observation for 6 hours. Blinded assessor. | |
| Zeidler et al37 | 2011 | CVC (Landmark) | CTCAE | Daily inspection by specialized nurses. | |
| Duffy et al38 | 2013 | CVC (Mixed US‐guided & landmark) | Requiring surgical intervention or causing significant morbidity/ mortality | Requiring minimal or no intervention | ‐ |
| Ong et al39 | 2012 | CVC (Mixed US‐guided & landmark) | ‐ | ‐ | |
| Vinson et al40 | 2014 | CVC (Mixed US‐guided & landmark) | 1) New postprocedural fluid collection or enlargement in the pleural cavity, mediastinum or neck <24 hours of CVC; 2) line‐related bleeding causing hemodynamic compromise requiring blood or fluid replacement, vasopressors or surgery | Oozing from a percutaneous puncture site or superficial hematoma <24 hours of CVC (includes use of manual pressure, no time‐limit given); Minor with procedural intervention: requiring line removal, suture placement or administration of blood products | Medical record review at 48 hours with complication assessment by two investigators and a third arbitrator from a pool of four trained abstractors. Additionally, 5% randomly selected for independent review by a second investigator (97.8%‐100% interrater agreement). |
| Haas et al41 | 2010 | CVC (US‐guided) | SIR, excluding minor oozing not requiring any intervention other than brief manual compression | ‐ | |
| Weigand et al42 | 2009 | CVC (US‐guided) | Drop in Hb >1,5g/dL within 24 to 36 hours | Routine chest radiograph and a laboratory test at least once within 24 to 36 hours. | |
| Olivieri et al43 | 2016 | CVC (Surgical) | Requiring surgical intervention or causing significant morbidity/ mortality | Requiring minimal or no intervention | Routine chest radiograph. Hemoglobin and platelet check within 24 hours. Medical record review at undetermined time. |
| McVay et al21 | 1990 | LB (blind percutaneous) | Hb decrease >2,0g/dL | Hb decrease <2,0g/dL, but RBC‐transfusion for hypovolemia given | Frequent monitoring of vital signs 1st 6 hours, routine hemoglobin check after 5 hours and often also the next day. |
| Sharma et al44 | 1982 | LB (blind percutaneous) | ‐ | 24 hours of bedrest. Frequent monitoring of vital functions for undetermined time. | |
| Sandrasegaran et al45 | 2016 | LB (Mixed blind & US‐guided percutaneous) | Acute hemoperitoneum; drop in hematocrit >2g/dL, requiring inotropic or blood transfusion support or need for embolization of hepatic artery branches | Review of medical records at 4 weeks | |
| Caturelli et al46 | 1993 | LB (US‐guided percutaneous) | ‐ | Frequent monitoring of vital signs, routine hematological studies, clinical and ultrasound examination of the abdomen within 6 hours. | |
| Kitchin et al20 | 2018 | LB (US‐guided percutaneous) | ≥CTCAE grade 2 | CTCAE grade 1 | Two to four hours monitoring in nursing unit, next day telephone call and medical record review at 1 month by a single investigator. |
| Kamphuisen et al47 | 2002 | LB (Plugged percutaneous) | Acute bleeding event requiring blood transfusion | Close monitoring and twice daily hemoglobin check until discharge (average 4 days). | |
| Ahmed et al48 | 2016 | LB (Transjugular) | Presence of an intraparenchymal liver hematoma, hemobilia, or subcapsular bleeding within 15 days following liver biopsy | Routine observation on nursing floor or interventional radiology recovery area for undetermined time. Review of records up to 15 days post‐procedure. | |
| Davis et al49 | 1995 | RB (US‐guided percutaneous) | Drop in hematocrit >6 within 6 hours of renal biopsy | Drop in hematocrit >4 or ultrasound evidence of new perirenal | Routine observation for 6 hours, with hematocrit check at 6 hours. |
| Islam et al50 | 2010 | RB (US‐guided percutaneous) | Hematuria, blood transfusion after biopsy or ultrasound‐detected hematoma formation | Routine ultrasound both post‐procedure and at discharge. | |
| Soares et al51 | 2008 | RB (US‐guided percutaneous) | Requiring one or more major interventions, such as blood transfusion, hospital admission, or interventional or surgical procedure. | All other procedure‐related bleeding not meeting the criteria for major bleeding | Routine ultrasound post‐procedure, observation at least 6 hours and review of clinical notes at 1 week. |
| Sun et al52 | 2018 | RB (US‐guided percutaneous) |
US or CT verified bleeding requiring blood transfusions, angiographic embolizations or surgical interventions. |
Not requiring intervention. | Routine admission for one night with 6 hours of sandbag compression and imaging when signs of bleeding occurred. |
| Xu et al53 | 2017 | RB (US‐guided percutaneous) | Requiring intervention, including blood transfusion or invasive procedure (radiological or surgical) due to bleeding, within 1 week post‐procedure | 24 hours of bedrest, regular measurement of vital functions, imaging only on indication. Medical record review at undetermined time. | |
| Monahan et al54 | 2019 | RB (US‐ & CT‐guided percutaneous) | ≥ CTCAE grade 3, within 3 months of biopsy | Routine imaging directly post‐procedure and when clinically indicated. A telephone call after 1, 2, or 3 days. Review of medical record at Days 1, 2, or 3 and after 3 months. | |
| Estepp et al55 | 2017 | Lumbar puncture | Objective confirmation on diagnostic imaging of a spinal hematoma, or a clinical suspicion leading to diagnostic imaging in a symptomatic patient | ‐ | |
| Foerster et al56 | 2015 | Lumbar puncture | ‐ | Chart review at undetermined time. | |
| Horlocker et al57 | 1995 | Lumbar puncture | ‐ | Observation until discharge and hospital record review at 6 months. | |
| Ning et al58 | 2016 | Lumbar puncture | Spinal, subdural, subarachnoid and epidural hematomas | Review of medical records at 1 week. | |
If two columns are used for bleeding definition, a distinction was made between minor and major bleeding. If one column was used, no such distinction was made.
CT = computed tomography; CTCAE = common terminology criteria for adverse events; CVC = central venous catheter; LB = liver biopsy; RB = renal biopsy; US = ultrasound.
Criteria used in bleeding definitions
The criteria used to define bleeding could be categorized into three distinct categories: symptoms, interventions, and laboratory results, which were all sometimes limited in time and/or size (Fig. 2). General symptoms included oozing, subcutaneous hematoma, and changes in hemodynamic function. Naturally, some symptoms differed between invasive procedures. Studies on CVC placement included hemothorax and mediastinal hematoma. Studies on LB included hemobilia, subcapsular liver bleeding, and hemoperitoneum. Studies on RB included (subcapsular) perirenal hematoma and hematuria. Studies on LP included spinal, subdural, subarachnoid, and epidural hematoma. The study on BMB did not include specific symptoms.
Figure 2.
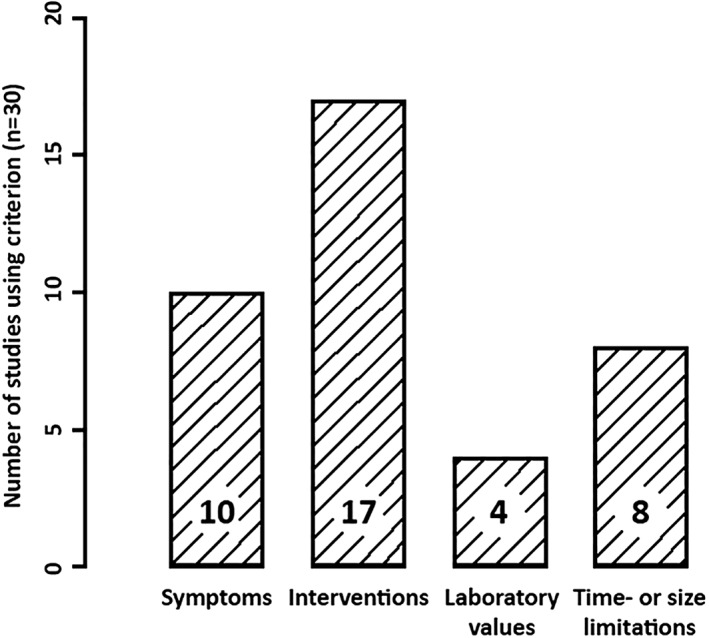
Criteria used in bleeding definitions.
Common interventional criteria included erythrocyte (RBC) transfusion, surgical and/or radiological intervention to stop bleeding, which, together, often determined major bleeding, if such a distinction was made. Others included need for vasopressor or fluid therapy, extension of hospital stay, placement of suture ligaments, compression bandage, or manual pressure. Studies on CVC placement also included catheter removal, while one of the RB studies explicitly included angiographic embolization as a rescue intervention. Laboratory results used to define bleeding were a decrease in either hemoglobin (Hb) or hematocrit (Ht).
Some studies put size‐ or time‐limitations on one or more of the prior criteria. Limitations in time were the most common, where the bleeding had to occur within a specified timeframe, varying between 24 hours and 3 months after the intervention. In other studies, symptoms and/or interventions needed a minimum duration, for instance manual compression for >15‐20 minutes or oozing of >24 hours. Pertaining to size, one study defined major bleeding as hematomas increasing in size.
Differences in bleeding assessment
In five studies there was no mention of routine clinical post‐procedural care. Routine care included post‐procedural imaging, laboratory and clinical examinations, (overnight) admission, or observation. In 14 of 30 studies at least some data on bleeding assessment were described, in varying details, including chart review without further details on the procedure. Only one study used blinded bleeding assessors, although no details on the blinding procedure were given. Only one study used multiple trained bleeding assessors with an independent arbitrator. No other studies used trained bleeding assessors and/or arbitrators.
Variability in bleeding incidence
Although we restricted our study to five predefined invasive procedures, there was little overlap between studies, due to different subtypes of procedures and different study populations. We could identify 23 different combinations of patient populations and procedures, of which only five were represented by at least two studies. Bleeding incidences varied widely between groups (Table 5), but even within groups we found non‐overlapping 95% confidence intervals (Fig. 3).
Table 5.
Bleeding incidence per procedure type
| Procedure | N | Bleeding incidence Median (IQR) |
|---|---|---|
| CVC | 12 | 5.4 (0.2‐13.5) |
| LB | 7 | 2.2 (0.4‐4.0) |
| RB | 6 | 9.3 (3.3‐24.1) |
| LP | 4 | 0 (0‐0) |
| BMB | 1 | 0 (0‐0) |
CVC = central venous catheter placement; LB = liver biopsy; RB = renal biopsy; LP = lumbar puncture; BMB = bone marrow biopsy; IQR = interquartile range.
Figure 3.
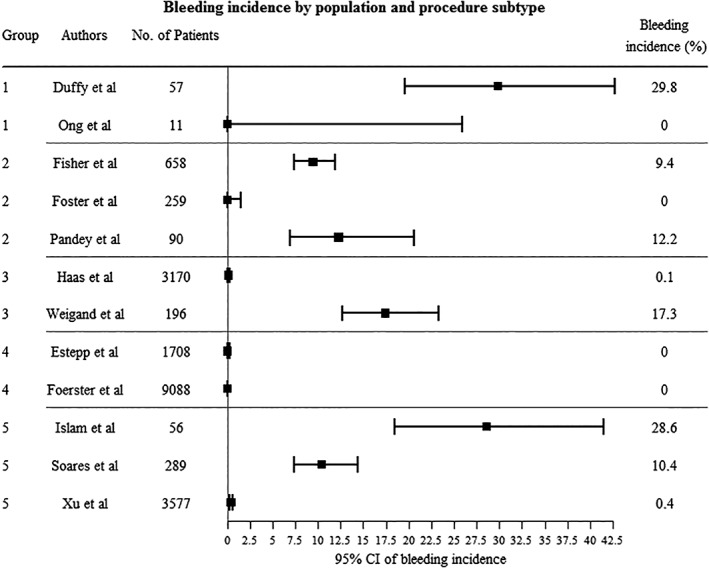
Bleeding incidence by population and procedure subtype. CI = Confidence Interval. 1 = CVC placement (ultrasound‐guided and landmark) in thrombotic thrombocytopenic purpura (TTP) patients, 2 = CVC placement (landmark) in advanced liver disease patients, 3 = CVC placement (ultrasound‐guided) in general population, 4 = Lumbar puncture (LP) in pediatric cancer patients, 5 = Renal biopsy (RB) (ultrasound‐guided percutaneous) in general population. Non‐overlapping 95% CI in Groups 2, 3, and 5 signify difference in bleeding incidence within groups.
A significant difference in median bleeding incidence was observed between prospective studies (12.2% [8.1%‐23.0%]) and retrospective studies (0.8% [0.0%‐4.3], p = 0.02). We performed a post‐hoc analysis on the ratio of major bleeding/minor bleeding for 10 studies that reported separate major and minor bleeding incidences. The median ratio was 0.1 (0.06‐0.14) in prospective studies (n = 2), meaning that for every major bleeding there were 10 minor bleeding episodes, and 0.4 (0.2‐1.2) in retrospective studies (n = 8), meaning five minor bleeding episodes for every two major episodes. This difference was not significant at p = 0.5.
DISCUSSION
In this study, we reviewed all studies on five frequently performed invasive procedures. We found a large variance in bleeding complications, even between studies assessing the same invasive procedure, mostly due to differences in the way clinical bleeding is assessed and defined, as suggested previously.10, 19
The large proportion (19/30) of studies using a bleeding definition of investigatorsʼ own design forms a major problem, the impact of which is illustrated in the following example: a LB complicated by subcapsular bleeding requiring embolization and causing a 1 g/dL drop in Hb. This would be classified as major bleeding in one study (Kitchin et al20), but would not even be classified as minor bleeding in another study (McVay et al21). This illustrates that the difference in bleeding definitions should be taken into account when interpreting these results. Moreover, in the six studies without bleeding definition it is impossible to interpret the results.
Five studies fully or partly used an existing bleeding scale, which seems to increase the validity of these studies. However, even these scales suffer from subjective criteria and have never been tested for inter‐observer variability. One of these bleeding scales was used in a different context than its intended use. The CTCAE scale was designed for toxicity reporting in cancer patients and it is therefore questionable to apply it in patients undergoing an invasive procedure. Moreover, the CTCAE scale has no predefined cut‐off between minor and major bleeding. Since researchers mostly report minor and major bleeding as separate entities, a clear distinction is needed.
Besides the two bleeding scales encountered in this review, many other bleeding scales have been published previously. Koreth et al22 have already analyzed the majority of these scales, all of which are used in settings other than invasive procedures. Interestingly, the HEME bleeding assessment by Arnold et al,23 which was specifically designed for critically ill patients, uses some objective criteria, like hemodynamic measures and specific bleeding sites, but retains subjectivity in defining major bleeding as bleeding requiring major therapeutic intervention. Another limitation of these interventional bleeding scales is the difference in the use of therapeutic interventions according to local clinical practice, as reported by Koreth et al.22
Methods of bleeding assessment varied also. Fourteen out of 30 reported their methods, which were mostly based on review of medical records, resulting in less accurate results than prospectively gathered data.13 The amount of studies mentioning bleeding assessors was especially low (2/30), and none scored full marks with multiple trained, blinded bleeding assessors using independent adjudication. A systematic review on blinded versus non‐blinded outcome assessors in RCTs showed that subjective binary endpoints suffer from bias when non‐blinded assessors are used.24 Furthermore, disagreement between two independent adjudicators using the WHO bleeding scale was as high as 31.2%.11
The necessity of adjudicating results has not been demonstrated in all situations. For instance, multicenter research seems to have more benefit than single center research, and vague, subjective endpoints need more adjudication than well‐defined, objective endpoints.25, 26, 27, 28 Not all measures allow for adjudication: a trial on thromboprophylaxis in intensive care patients showed that attribution of bleeding to anticoagulant use was too hard for an arbitrating committee, when so many different causes of bleeding co‐existed.29
Chart review is the predominant assessment method in retrospective studies. Our results show a significantly lower reported bleeding incidence in retrospective studies compared to prospective studies. This difference could be explained by the fact that in retrospective studies subtle positive outcomes (i.e., minor bleedings) are missed easily, since the assessment and documentation of minor bleeding is often not performed properly in general clinical practice.7, 13 The higher proportion of major bleeding that we found in retrospective studies further underlines this mechanism. However, due to the small number of prospective studies reporting minor and major bleeding, we were unable to demonstrate a statistically significant difference.
Our study is limited by heterogeneity of included studies (including the rate of thrombocytopenic patients), which is due to the broad range of patient populations undergoing different invasive procedures (as addressed in Fig. 3). Although this is a well‐known limitation in transfusion medicine research, current guidelines completely rely on these studies, so including them in this review is absolutely relevant.
Our results support the hypothesis that reported bleeding incidence depends more on methods of assessment and bleeding definition than on actual bleeding tendency. This is in line with earlier results concerning both SAE reporting and clinical bleeding.1, 7, 30 Also, we have shown that the way of reporting bleeding assessment is often limited. The lack of this essential information reduces the validity and hampers the reproducibility of these studies. A major concern is that these studies form the basis of both current clinical guidelines and sample size calculations for future studies. Clinicians and researchers should be aware of the importance of outcome assessment and bleeding definition.
Future research should focus on developing such a uniform, objective, and practical bleeding definition. Through detailing current practices and common criteria in bleeding definitions, the results of this study could form the basis of such a uniform definition. We suggest a definition that is specific to each intervention, proposed by specialists in each field, and perhaps with the help of patient‐advocates.12 A specific definition could entail specific symptoms without relying on interventions or on subjective words like “significant morbidity” and “minimal intervention.”
CONCLUSION
We demonstrate a high variability in definition and assessment of bleeding complications in studies on interventions in patients with thrombocytopenia. Hereby, interpretation and comparison of different study results is hampered. This has consequences for clinical practice (uncertainty about transfusion thresholds in guideline development) and clinical research (imprecise sample‐size calculations and hampered comparison of studies). There is a dire need of a consensus procedure‐related bleeding definition in the field of transfusion medicine, in patients undergoing invasive procedures.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Transfusion. This study is funded by ZonMW (Zorgonderzoek Medische Wetenschappen; part of the NWO [Nederlandse Organisatie voor Wetenschappelijk Onderzoek; the Dutch Organization for Scientific Research], Den Haag, The Netherlands), project number 843002625. The sponsors of this work were not involved in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the manuscript for publication.
| Study | N | Platelets | Prothrombin time/INR | Activated partial thromboplastin time | Other | |
|---|---|---|---|---|---|---|
| BMB | Liu et al31 | 981 |
<20: N = 33; 20‐50: N = 187; >50: N = 761 |
|||
| CVC | Doerfler et al32 | 104 |
Isolated <20: N = 11; 20‐50: N = 30; 50‐100: N = 22 |
Isolated 1.2‐1.5 × ULoN: N = 12 >1.5 × ULoN: N = 6 |
Isolated 1.2‐1.5 × ULoN: N = 4; >1.5 × ULoN: N=3 |
Combined PT & aPTT >1.5 × ULoN: N=3; PLT & coagulation abnormal: N = 13 |
| Fisher et al33 | 658 |
Median (IQR; range): Subclavian (n = 352) 81 (51‐133; 9‐1088) Internal Jugular (n = 306) 83 (53‐133; 10‐425) |
Median (IQR; range): Subclavian (n = 352) 2.4 (1.7‐3.9; 1‐16) Internal Jugular (n = 306) 2,7 (1.8‐4.7; 1‐17) |
|||
| Foster et al34 | 259 |
<80 (n = 122) Mean (range): 47 (8‐79) |
<40% (n = 122) Mean (range): 29% (39%‐10%) |
>77 (n = 3) Mean (range): 92 (78‐100) |
Normal coagulation: N = 57; 1) abnormal parameter: N = 160; 2) abnormal parameters: N = 40 3) abnormal parameters: N = 2 |
|
| Mumtaz et al35 | 2010 |
In 88 coagulopathic patients: Median (range): 95 (12‐330) |
In 88 coagulopathic patients: Median (range): 1.8 (1.2‐3.5) |
In 88 coagulopathic patients: Median (range): 54s (22‐100) |
1922 × normal (1680) or corrected (242) hemostasis | |
| Pandey et al36 | 90 | PLT <150 and/or INR >1.5: N = 86 | ||||
| Zeidler et al37 | 604 |
Mean: 48; <20: N = 14; 20‐29: N = 48; 30‐39: N = 56; 40‐49: N = 52; 50‐99: N = 140; >100: N = 272 |
||||
| Duffy et al38 | 57 |
Median (range): Overall (n = 57) 26 (3‐128) Transfused (n = 14) 50 (11‐100) Not transfused (n = 43) 25 (3‐128) |
||||
| Ong et al39 | 11 | Median (range): 28 (7‐129) | ||||
| Vinson et al40 | 936 |
<20: N = 16; 20‐50: N = 55; 50‐75: N = 100; 75‐100: N = 146 |
>3,0: N = 97; 2.0‐3.0: N = 139; 1.5‐2.0: N = 239; 1.3‐1.5: N = 293 |
>50: N = 17; 35‐50: N = 55 |
1) abnormal parameters: N = 732; 2) abnormal parameters: N = 187; 3) abnormal parameters: N = 17 |
|
| Haas et al41 | 3170 |
Isolated 3‐19: N = 14; 20‐24: N = 26; 25‐29: N = 45; 30‐34: N = 54; 34‐39: N = 65; 40‐44: N = 49; 45‐49: N = 47 |
Isolated 1.5‐1.6: N = 151; 1.7‐1.8: N = 67; 1.9‐2.0: N = 34; 2.1‐2.2: N = 20; 2.3‐3.8: N = 10 |
PLT < 50 & INR > 1,5: N = 44 | ||
| Weigand et al42 | 196 |
Isolated <50: N = 12 |
Isolated <50%: N = 32 |
Combined PLT < 50 & PT < 50%: N = 7 |
||
| Olivieri et al43 | 72 |
<50: N = 25 Mean (range): 251 (7‐834) |
Mean (range): 1.12 (0.96‐1.76) | Mean (range): 30.5 (20.1‐38.9) | All patients with PLT < 50 received PLT transfusion (1 unit/10kg). | |
| LB | McVay et al21 | 177 |
<50: N = 2; 50‐99: N = 18; ≥100: N = 157 |
13.6‐15.7: N = 11; 11.6‐13.5: N = 65; <11.5: N = 100 |
43.6: N = 14; 38.0‐43.5: N = 23; 34.1‐37.9: N = 37; <34: N = 103 |
|
| Sharma et al44 | 87 |
30‐60: N = 13; 60‐90: N = 16; 90‐120: N = 21; 120‐150: N = 13; 150‐180: N = 6; >180: N = 18 |
||||
| Sandrasegaran et al45 | 296 |
Mean: 205 In 7 transfused patients Range: 35‐96 |
Mean: 1.17 In 11 transfused patients Range: 1.25‐1.79 |
|||
| Caturelli et al46 | 85 |
Isolated <50: N = 36 Mean(range): 39.5 (18‐49) |
Isolated <50%: N =3 0 Mean(range): 44.3% 28%‐49%) |
PLT > 50 & PT < 50%: N = 19 Mean (range) PLT: 39.2 (22‐49) Mean (range) PT: 42.6% (29%‐49%) |
||
| Kitchin et al20 | 1846 |
<50: N = 21 50‐100: N = 110 >100: N = 1715 Mean (range): 219 (24‐751) |
>1.5: N = 40 1.0‐1.5: N=755 <1.0: N=1051 Mean (range): 1.08 (0.8‐2.7) |
|||
| Kamphuisen et al47 | 36 |
In 27 patients with coagulopathy Mean (range): 53 (19‐153) |
In 27 patients with coagulopathy Mean (range): 16.3s (11.4‐20.3) |
|||
| Ahmed et al48 | 1600 |
BMT group (n = 183) Mean (sd; range): 88 (71; 5‐336) Non‐BMT group (n = 1417) Mean (sd; range): 174 (107; 8‐1507) |
BMT group (n = 183) Mean (sd): 1.2 (0.5) Non‐BMT group (n=1417) Mean (sd): 1.2 (0.4) |
|||
| RB | Davis et al49 | 120 | <150: N = 3 | >13.6: N = 9 | >36: N = 2 | |
| Islam et al50 | 56 |
Mean (sd; range): 260 (85; 107‐442) |
Mean (sd; range): 11.1s (1.2; 9.3‐13.4) |
Mean (sd; range): 26.5 (3.2; 21.7‐37.1) |
||
| Soares et al51 | 289 |
Amyloidosis group (n = 101) Median (range): 282 (54‐824) Control group (n = 188) Median (range): 265 (35‐844) |
Amyloidosis group (n = 101) Median (range): 0.9 (0.8‐1.4) Control group (n = 188) Median (range): 0.9 (0.8‐1.4) |
Amyloidosis group (n = 101) Median (range): 26s (17‐54) Control group (n = 188) Median (range): 26s (20‐47) |
||
| Sun et al52 | 296 |
Mean: 248 <100: N = 6 (range: 75‐94); 100‐150: N = at least 5 |
Mean: 9.8 | Mean: 26.1 | ||
| Xu et al53 | 3577 |
Median (IQR): 226 (184‐273) |
Median (IQR): 10.1s (9.6s‐10.7s) |
Median (IQR): 31.3s (28.7‐33.8) |
||
| Monahan et al54 | 2204 |
Median (IQR): 236 (182‐297); <100: N=97; ≥100: N=1881 |
Median (IQR):1.0 (0.9‐1.1) | |||
| LP | Estepp et al55 | 1708 |
1‐25: N = 40; 26‐75: N = 236; 76‐99: N = 111; ≥100: N = 1321 |
|||
| Foerster et al56 | 9088 |
<10: N = 25; 10‐20: N = 67; 20‐30: N = 88; 30‐40: N = 92; 40‐50: N = 107; 50‐100: N = 729;>100: N = 7980 |
||||
| Horlocker et al57 | 1000 | Mean (sd; range) 277 (84; 94‐739) |
Mean (sd; range): Bleeding group (n = 223) 12 (0.7; 9.8‐13) Non‐bleeding group (n = 777) 12.0 (1.1; 8.9‐15.5) |
Mean (sd; range): Bleeding group (n = 223) 29 (2.9; 22‐37) Non‐bleeding group (n = 777) 31 (8.4; 22‐79) |
||
| Ning et al58 | 369 |
11‐20: N = 3; 21‐50: N = 17; 51‐100: N = 40; 101‐150: N = 52; >150: N = 242 |
All <1.5 | All <40s |
aPTT = activated partial thromboplastin time; BMB = bone marrow biopsy; CVC = central venous catheter placement; INR = international normalized ratio; IQR = interquartile range; LB = liver biopsy; LP = lumbar puncture; PT = prothrombin time; PLT = platelet; RB = renal biopsy; ULoN = upper limit of normal.
(“Platelet Count”[Mesh] OR “Platelet Count”[tiab] OR “Platelet Counts”[tiab] OR “Platelet Number”[tiab] OR “Platelet Numbers”[tiab] OR “Blood Platelet Disorders”[Mesh] OR “Blood Platelet Disorders”[tiab] OR “Blood Platelet Disorder”[tiab] OR “Thrombocytopenia”[tiab] OR “Platelet Storage Pool Deficiency”[tiab]) AND ((“Bone Marrow”[Mesh] AND “Biopsy”[Mesh]) OR “Bone Marrow Aspiration”[tiab] OR “Bone Marrow Biopsy”[tiab] OR “Bone Marrow Biopsies”[tiab])
(“Platelet Count”[Mesh] OR “Platelet Count”[tiab] OR “Platelet Counts”[tiab] OR “Platelet Number”[tiab] OR “Platelet Numbers”[tiab] OR “Blood Platelet Disorders”[Mesh] OR “Blood Platelet Disorders”[tiab] OR “Blood Platelet Disorder”[tiab] OR “Thrombocytopenia”[tiab] OR “Platelet Storage Pool Deficiency”[tiab])AND(“Catheterization, Central Venous”[Mesh] OR “Central Catheterization”[tiab] OR “Central Catheterizations”[tiab] OR “Central Venous Catheterization”[tiab] OR “Central Venous Catheterizations”[tiab] OR “CVC”[tiab] OR “CVL”[tiab] OR “CVCs”[tiab] OR “Central Vein Catheterization”[tiab] OR “Central Vein Catheterizations”[tiab])
(“Biopsy, Needle/adverse effects”[MAJR] OR “liver biopsy”[tiab] OR “renal biopsy”[tiab] OR “kidney biopsy” AND (“Platelet Count”[Mesh] OR “Platelet Count”[tiab] OR “Platelet Counts”[tiab] OR “Platelet Number”[tiab] OR “Platelet Numbers”[tiab] OR “Blood Platelet Disorders”[Mesh] OR “Blood Platelet Disorders”[tiab] OR “Blood Platelet Disorder”[tiab] OR Thrombocytopenia[tiab] OR “Platelet Storage Pool Deficiency”[tiab])
(“Platelet Count”[Mesh] OR “Platelet Count”[tiab] OR “Platelet Counts”[tiab] OR “Platelet Number”[tiab] OR “Platelet Numbers”[tiab] OR “Blood Platelet Disorders”[Mesh] OR “Blood Platelet Disorders”[tiab] OR “Blood Platelet Disorder”[tiab] OR Thrombocytopenia[tiab] OR “Platelet Storage Pool Deficiency”[tiab]) AND (“Puncture, Lumbar”[Mesh] OR “lumbar punct*”[tiab] OR “Spinal puncture”[Mesh])
| Study | Year | Risk of Bias* | Inconsistency* | Indirectness* | Imprecision* | Publication bias* | Large effect† | Dose response‡ | Residual confounding§ | Overall study quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al48 | 2016 | −1 | −1 | −1 | 0 | 0 | 0 | 0 | 0 | Very low |
| Caturelli et al46 | 1993 | −1 | 0 | −1 | −1 | 0 | 0 | 0 | 0 | Very low |
| Davis et al49 | 1995 | −1 | 0 | −1 | 0 | 0 | 0 | 0 | 0 | Very low |
| Doerfler et al32 | 1996 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Duffy et al38 | 2013 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Estepp et al55 | 2017 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Fisher et al33 | 1999 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Foerster et al56 | 2015 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Foster et al34 | 1992 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Haas et al41 | 2010 | −1 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Horlocker et al57 | 1995 | 0 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Islam et al50 | 2010 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Kamphuisen et al47 | 2002 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Kitchin et al20 | 2018 | −1 | 0 | 0 | −1 | 0 | 0 | 1 | 0 | Very low |
| Liu et al31 | 2017 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| McVay et al21 | 1990 | −1 | 0 | −1 | −1 | 0 | 0 | 0 | 0 | Very low |
| Monahan et al54 | 2019 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Low |
| Mumtaz et al35 | 2001 | −1 | −1 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Ning et al58 | 2016 | −1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | Low |
| Olivieri et al43 | 2016 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Ong et al39 | 2012 | −2 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Pandey et al36 | 2017 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Sandrasegaran et al45 | 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Low |
| Sharma et al44 | 1982 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Soares et al51 | 2008 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Sun et al52 | 2018 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
| Vinson et al40 | 2014 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Low |
| Weigand et al42 | 2009 | −1 | 0 | −1 | 0 | 0 | 0 | 0 | 0 | Very low |
| Xu et al53 | 2017 | −1 | 0 | 0 | −1 | 0 | 0 | 0 | 0 | Very low |
| Zeidler et al37 | 2011 | −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Very low |
Study quality can be “high,” “moderate,” “low,” or “very low.” Observational Studies start as low quality, there were no randomized controlled trials (RCTs) included. Each “‐1” or “+1” makes the study fall or rise a quality level.
*Serious = −1, very serious = −2.
†Large effect = +1, very large effect = +2.
‡Evidence of gradient = +1.
§All plausible residual confounding would reduce demonstrated effect or suggest spurious effect if no effect was observed = +1.
REFERENCES
- 1. van de Weerdt EK, Biemond BJ, Baake B, et al. Central venous catheter placement in coagulopathic patients: risk factors and incidence of bleeding complications. Transfusion 2017;57:2512‐25. [DOI] [PubMed] [Google Scholar]
- 2. Stanworth SJ, Estcourt LJ, Powter G, et al. A no‐prophylaxis platelet‐transfusion strategy for hematologic cancers. N Engl J Med 2013;368:1771‐80. [DOI] [PubMed] [Google Scholar]
- 3. Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 2015;162:205‐13. [DOI] [PubMed] [Google Scholar]
- 4. Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol 2017;176:365‐94. [DOI] [PubMed] [Google Scholar]
- 5. Haas FJLM, Van Rhenen DJ, De Vries RRP, Overbeeke MAM, Novotny VMJ, Henny CP. Richtlijn Bloedtransfusie 2011 [Guideline]. [cited 2011 Aug 1]. Available from: http://nvb-trip-symposium.nl/wp-content/uploads/2017/08/Richtlijnbloedtransfusie2011.pdf.
- 6. Kumar A, Mhaskar R, Grossman BJ, et al. Platelet transfusion: a systematic review of the clinical evidence. Transfusion 2015;55:1116‐27 quiz 5. [DOI] [PubMed] [Google Scholar]
- 7. Heddle NM, Cook RJ, Webert KE, et al. Methodologic issues in the use of bleeding as an outcome in transfusion medicine studies. Transfusion 2003;43:742‐52. [DOI] [PubMed] [Google Scholar]
- 8. Miller AB, Hoogstraten B, Staguet M, et al. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
- 9. Silberzweig JE, Sacks D, Khorsandi AS, et al. Reporting standards for central venous access. J Vasc Interv Radiol 2003;14(Suppl):S443‐52. [DOI] [PubMed] [Google Scholar]
- 10. Bercovitz RS, OʼBrien SH. Measuring bleeding as an outcome in clinical trials of prophylactic platelet transfusions. Hematology Am Soc Hematol Educ Program 2012;2012:157‐60. [DOI] [PubMed] [Google Scholar]
- 11. Heddle NM, Wu C, Vassallo R, et al. Adjudicating bleeding events in a platelet dosage study: impact on outcome results and challenges. Transfusion 2011;51:2304‐10. [DOI] [PubMed] [Google Scholar]
- 12. Heddle NM, Arnold DM, Webert KE. Time to rethink clinically important outcomes in platelet transfusion trials. Transfusion 2011;51:430‐4. [DOI] [PubMed] [Google Scholar]
- 13. Nagurney JT, Brown DFM, Sane S, et al. The accuracy and completeness of data collected by prospective and retrospective methods. Acad Emerg Med 2005;12:884‐95. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaborationʼs tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stang A. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol 2010;25:603‐5. [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209‐12. [Google Scholar]
- 18.Common terminology criteria for adverse events v3.0 (CTCAE). [cited 2006 Aug 9]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
- 19. Estcourt LJ, Heddle N, Kaufman R, et al. The challenges of measuring bleeding outcomes in clinical trials of platelet transfusions. Transfusion 2013;53:1531‐43. [DOI] [PubMed] [Google Scholar]
- 20. Kitchin DR, Munoz del Rio A, Woods M, et al. Percutaneous liver biopsy and revised coagulation guidelines: a 9‐year experience. Abdom Radiol (NY) 2018;43:1494‐501. [DOI] [PubMed] [Google Scholar]
- 21. McVay PA, Toy PTCY. Lack of increased bleeding after liver biopsy in patients with mild hemostatic abnormalities. Am J Clin Pathol 1990;94:747‐53. [DOI] [PubMed] [Google Scholar]
- 22. Koreth R, Weinert C, Weisdorf DJ, et al. Measurement of bleeding severity: a critical review. Transfusion 2004;44:605‐17. [DOI] [PubMed] [Google Scholar]
- 23. Arnold DM, Donahoe L, Clarke FJ, et al. Bleeding during critical illness: a prospective cohort study using a new measurement tool. Clin Invest Med 2007;30:E93‐102. [DOI] [PubMed] [Google Scholar]
- 24. Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
- 25. Eisenbud R, Assmann SF, Kalish LA, et al. Differences in difficulty adjudicating clinical events in patients with advanced HIV disease. J Acquir Immune Defic Syndr. 2001;28:43‐6. [DOI] [PubMed] [Google Scholar]
- 26. Pogue J, Walter SD, Yusuf S. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clin Trials 2009;6:239‐51. [DOI] [PubMed] [Google Scholar]
- 27. Mahaffey KW, Harrington RA, Akkerhuis M, et al. Disagreements between central clinical events committee and site investigator assessments of myocardial infarction end‐points in an international clinical trial: review of the PURSUIT study. Curr Control Trials Cardiovasc Med 2001;2:187‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Näslund U, Grip L, Fischer‐Hansen J, et al. The impact of an end‐point committee in a large multicenter, randomized, placebo‐controlled clinical trial. Eur Heart J 1999;20:771‐7. [DOI] [PubMed] [Google Scholar]
- 29. Cook D, Sinuff T, Zytaruk N, et al. Event adjudication and data monitoring in an intensive care unit observational study of thromboprophylaxis. J Crit Care 2009;24:168‐75. [DOI] [PubMed] [Google Scholar]
- 30. Kirwan BA, Lubsen J, De Brouwer S, et al. Diagnostic criteria and adjudication process both determine published event‐rates: the ACTION trial experience. Contemp Clin Trials 2007;28:720‐9. [DOI] [PubMed] [Google Scholar]
- 31. Liu B, Limback J, Kendall M, et al. Safety of CT‐guided bone marrow biopsy in thrombocytopenic patients: a retrospective review. J Vasc Interv Radiol 2017;28:1727‐31. [DOI] [PubMed] [Google Scholar]
- 32. Doerfler ME, Kaufman B, Goldenberg AS. Central venous catheter placement in patients with disorders of hemostasis. Chest 1996;110:185‐8. [DOI] [PubMed] [Google Scholar]
- 33. Fisher NC, Mutimer DJ. Central venous cannulation in patients with liver disease and coagulopathy – a prospective audit. Intensive Care Med 1999;25:481‐5. [DOI] [PubMed] [Google Scholar]
- 34. Foster PF, Moore LR, Sankary HN, et al. Central venous catheterization in patients with coagulopathy. Arch Surg 1992;127:273‐5. [DOI] [PubMed] [Google Scholar]
- 35. Mumtaz H, Williams V, Hauer‐Jensen M, et al. Central venous catheter placement in patients with disorders of hemostasis. Am J Surg 2001;180:503‐6. [DOI] [PubMed] [Google Scholar]
- 36. Pandey CK, Saluja V, Gaurav K, et al. K time & maximum amplitude of thromboelastogram predict post‐central venous cannulation bleeding in patients with cirrhosis: a pilot study. Indian J Med Res 2017;145:84‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeidler K, Arn K, Senn O, et al. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion 2011;51:2269‐76. [DOI] [PubMed] [Google Scholar]
- 38. Duffy SM, Coyle TE. Platelet transfusions and bleeding complications associated with plasma exchange catheter placement in patients with presumed thrombotic thrombocytopenic purpura. J Clin Apheresis 2013;28:356‐8. [DOI] [PubMed] [Google Scholar]
- 39. Ong M, Veillon D. Cotelingam J. Is platelet transfusion necessary prior to a central venous catheter placement in thrombocytopenic purpura patients? J La State Med Soc 2012;164:283‐4. [PubMed] [Google Scholar]
- 40. Vinson DR, Ballard DW, Hance LG, et al. Bleeding complications of central venous catheterization in septic patients with abnormal hemostasis. Am J Emerg Med 2014;32:737‐42. [DOI] [PubMed] [Google Scholar]
- 41. Haas B, Chittams JL, Trerotola SO. Large‐bore tunneled central venous catheter insertion in patients with coagulopathy. J Vasc Interv Radiol 2010;21:212‐7. [DOI] [PubMed] [Google Scholar]
- 42. Weigand K, Encke J, Meyer FJ, et al. Low levels of prothrombin time (INR) and platelets do not increase the risk of significant bleeding when placing central venous catheters. Med Klin 2009;104:331‐5. [DOI] [PubMed] [Google Scholar]
- 43. Olivieri C, Crocoli A, De Pasquale MD, et al. Central venous catheter placement in children with thrombocytopenia. Minerva Pediatr 2016;68:398‐403. [PubMed] [Google Scholar]
- 44. Sharma P, McDonald GB, Banaji M. The risk of bleeding after percutaneous liver biopsy: relation to platelet count. J Clin Gastroenterol 1982;4:451‐3. [DOI] [PubMed] [Google Scholar]
- 45. Sandrasegaran K, Thayalan N, Thavanesan R, et al. Risk factors for bleeding after liver biopsy. Abdom Radiol 2016;41:643‐9. [DOI] [PubMed] [Google Scholar]
- 46. Caturelli E, Squillante MM, Andriulli A, et al. Fine‐needle liver biopsy in patients with severely impaired coagulation. Liver 1993;13:270‐3. [DOI] [PubMed] [Google Scholar]
- 47. Kamphuisen PW, Wiersma TG, Mulder CJJ, et al. Plugged‐percutaneous liver biopsy in patients with impaired coagulation and ascites. Pathophysiol Haemost Thromb 2002;32:190‐3. [DOI] [PubMed] [Google Scholar]
- 48. Ahmed O, Ward TJ, Lungren MP, et al. Assessing the risk of hemorrhagic complication following transjugular liver biopsy in bone marrow transplantation recipients. J Vasc Interv Radiol 2016;27:551‐7. [DOI] [PubMed] [Google Scholar]
- 49. Davis CL, Chandler WL. Thromboelastography for the prediction of bleeding after transplant renal biopsy. J Am Soc Nephrol 1995;6:1250‐5. [DOI] [PubMed] [Google Scholar]
- 50. Islam N, Fulop T, Zsom L, et al. Do platelet function analyzer‐100 testing results correlate with bleeding events after percutaneous renal biopsy? Clin Nephrol 2010;73:229‐37. [DOI] [PubMed] [Google Scholar]
- 51. Soares SM, Fervenza FC, Lager DJ, et al. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single‐center experience in 101 patients. Am J Kidney Dis 2008;52:1079‐8. [DOI] [PubMed] [Google Scholar]
- 52. Sun YS, Sun IT, Wang HK, et al. Risk of complications of ultrasound‐guided renal biopsy for adult and pediatric patients with systemic lupus erythematosus. Lupus 2018;27:828‐36. [DOI] [PubMed] [Google Scholar]
- 53. Xu D, Chen M, Zhou F, et al. Risk factors for severe bleeding complications in percutaneous renal biopsy. Am J Med Sci 2017;353:230‐5. [DOI] [PubMed] [Google Scholar]
- 54. Monahan H, Gunderson T, Greene E, et al. Risk factors associated with significant bleeding events after ultrasound‐guided percutaneous native renal biopsies: a review of 2204 cases. Abdom Radiol 2019;44:2316‐22. [DOI] [PubMed] [Google Scholar]
- 55. Estepp JH, Smeltzer MP, Kang G, et al. Safe use of low‐molucular‐weight heparin in pediatric acute lymphoblastic leukemia and lymphoma around lumbar punctures. J Pediatr Hematol Oncol 2017;39:596‐601. [DOI] [PubMed] [Google Scholar]
- 56. Foerster MV. de Paula Ramos Pedrosa F, da Fonseca TCT, et al. Lumbar punctures in thrombocytopenic children with cancer. Pediatr Anesth 2015;25:206‐10. [DOI] [PubMed] [Google Scholar]
- 57. Horlocker TT, Wedel DJ, Schroeder DR, et al. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg 1995;80:303‐9. [DOI] [PubMed] [Google Scholar]
- 58. Ning S, Kerbel B, Callum J, et al. Safety of lumbar punctures in patients with thrombocytopenia. Vox Sang 2016;110:393‐400. [DOI] [PubMed] [Google Scholar]


