Summary
The cost effectiveness of reusable vs. single‐use flexible bronchoscopy in the peri‐operative setting has yet to be determined. We therefore aimed to determine this and hypothesised that single‐use flexible bronchoscopes are cost effective compared with reusable flexible bronchoscopes. We conducted a systematic review of the literature, seeking all reports of cross‐contamination or infection following reusable bronchoscope use in any clinical setting. We calculated the incidence of these outcomes and then determined the cost per patient of treating clinical consequences of bronchoscope‐induced infection. We also performed a micro‐costing analysis to quantify the economics of reusable flexible bronchoscopes in the peri‐operative setting from a high‐throughput tertiary centre. This produced an accurate estimate of the cost per use of reusable flexible bronchoscopes. We then performed a cost effectiveness analysis, combining the data obtained from the systematic review and micro‐costing analysis. We included 16 studies, with a reported incidence of cross‐contamination or infection of 2.8%. In the micro‐costing analysis, the total cost per use of a reusable flexible bronchoscope was calculated to be £249 sterling. The cost per use of a single‐use flexible bronchoscope was £220 sterling. The cost effectiveness analysis demonstrated that reusable flexible bronchoscopes have a cost per patient use of £511 sterling due to the costs of treatment of infection. The findings from this study suggest benefits from the use of single‐use flexible bronchoscopes in terms of cost effectiveness, cross‐contamination and resource utilisation.
Keywords: airway management, bronchoscope, economics, infection, intubation
Introduction
Flexible bronchoscopes allow visualisation of the nasopharynx, oropharynx, larynx, trachea and its subsequent divisions for diagnostic and therapeutic purposes. It is estimated that 500,000 bronchoscopic procedures are performed annually in the USA alone 1. The risk of transmission of infection following bronchoscopy with reusable flexible bronchoscopes is often under‐considered, even when they are reprocessed according to infection control guidelines and recommendations 2, 3, 4, 5. However, the transmission of pathogenic organisms via contaminated reusable flexible bronchoscopes remains an evident risk 6, 7, 8, even if appropriate decontamination procedures are adhered to 9. There are unquantifiable risks of cross‐contamination and infection from reusable flexible bronchoscopes, along with uncertainty regarding their cost effectiveness. Reusable flexible bronchoscopes are often used by anaesthetists to place tracheal tubes, either awake or asleep, and to check adequate positioning of double‐lumen tubes. Therefore, cross‐contamination risk will also apply to these patients.
Single‐use flexible bronchoscopes are delivered sterile and thus should minimise the risk of infection transmission and cross‐contamination compared with reusable flexible bronchoscopes. A previously reported cost effectiveness study of single‐use flexible bronchoscopes in a typical intensive care unit (ICU) setting in the USA demonstrated that subsequent implementation is cost effective when looking at cross‐contamination and potential subsequent infection, and it is associated with increased patient safety 10. There are several reports of cross‐contamination of reusable flexible bronchoscopes due to inappropriate cleaning, disinfection or lack of leak testing and drying 11, 12. These reports do not provide a quantifiable risk for cross‐contamination and subsequent infection, but it is accepted that there is a risk and cases are under‐reported 8, 13, 14, 15; consequently, the literature lacks a quantified risk of cross‐contamination and subsequent infection due to flexible bronchoscopy 8, 16, 17. Moreover, several micro‐costing studies of reusable flexible bronchoscopes do not include costs of infections, which is why there is some uncertainty regarding these estimates 18, 19, 20.
We therefore aimed to determine the cost per use and cross‐contamination risk of reusable flexible bronchoscopes and to ascertain the cost effectiveness of single‐use flexible bronchoscopes compared with reusable flexible bronchoscopes in various clinical settings. To achieve this we conducted a micro‐costing analysis of flexible bronchoscope utilisation from a high‐throughput tertiary centre 21 and a systematic review of the literature. Our primary hypothesis was that single‐use flexible bronchoscopes are equally or more cost effective than reusable flexible bronchoscopes.
Methods
The preferred reporting items for systematic reviews and meta‐analyses (PRISMA) guidance was adhered to in the conduct of the systematic review 22. Given an evident risk of patient cross‐contamination and infection with reusable flexible bronchoscopes 6, 8 and no risk using single‐use flexible bronchoscope due to its single‐use modality, the effect measure in this cost effectiveness analysis was defined as the avoided risk of infection using single‐use compared with reusable flexible bronchoscopes. A comprehensive search strategy was conducted of the PubMed, MEDLINE and Embase databases to identify relevant literature for the risk of cross‐contamination and infection associated with reusable flexible bronchoscopes. The following search terms or combinations were employed: ‘bronchoscopy’; ‘bronchoscope’ determine ‘infection’ determine ‘cross‐infection’ determine ‘pseudo‐outbreak’ determine ‘outbreak’ determine ‘device contamination’ determine and ‘hospital infection’, including word variations and assorted permutations. English‐language studies on humans were included from 1982 to 2018. Retrospective and prospective observational studies were included when studying the risk of cross‐contamination and infection post‐bronchoscopy. Reference lists of review articles were hand‐searched to locate studies that may have been missed in our initial search. Eligibility criteria of studies were met if cross‐contamination of patients who underwent bronchoscopic procedures was detected by traditional typing systems based on phenotypes or more recent methods that examine the relatedness of isolates at a molecular level, such as polymerase chain reaction or pulse‐field gel electrophoresis 23. To quantify the risk of infection, we needed the number of: bronchoscopic procedures; patients who underwent bronchoscopy; contaminated patients; and infected patients. Studies were included in the quantitative analysis if at least three of these four variables were reported. Studies were excluded if they had a non‐quantifiable risk. A study with a non‐quantifiable risk was defined as one in which less than three of the above‐mentioned parameters were reported. Other exclusion criteria were other endoscopic procedures (e.g. gastroscopy), if bronchoscope contamination was not detected or reported by recognised typing methods, or if the setting of the studies was not clinically relevant for answering our hypothesis. The risk of infection was used as the measurement of effectiveness. In the event of incomplete data on the number of bronchoscopic procedures and number of patients included, a simple regression method was applied to predict missing data in this large heterogeneous group of patients eligible for bronchoscopy. The relationship was used to predict missing data points within these two variables. Once regression methods were applied, all studies were included for quantitative synthesis. The risk of cross‐contamination and infection was determined by a weighted average using a fixed‐effects model to reflect a more precise estimate. There are currently no reported cases of cross‐contamination using single‐use flexible bronchoscopes, which is why the risk is expected to be 0%.
The cost effectiveness of single‐use vs. reusable flexible bronchoscopes was estimated by using a literature review to obtain the best available evidence of effects. The effect measure was the risk of infection. The time horizon of the cost‐effectiveness analysis was within 1 year. The micro‐costing analysis was conducted at Guy's and St. Thomas’ NHS Foundation Trust Department of Anaesthesia. The total cost per use of reusable flexible bronchoscopes for tracheal intubation and double‐lumen tube position verification was estimated, and the cost per use of single‐use flexible bronchoscopes (including the monitor) was determined. All costs identified were adjusted to pounds sterling (£) in 2017. To determine the present value of capital expenditures, a discount rate of 3.5% was used. Capital acquisition costs were annualised across a 5‐year period for bronchoscopes and related equipment, and a 30‐year period for buildings.
A decision tree was constructed using Tree Age (2016 version, TreeAge Software, MA, USA), which enabled the comparison of the cost effectiveness of single‐use flexible bronchoscopes to reusable flexible bronchoscopes (Fig. 1). The modelling approach was based on principles of good practice for decision analytic modelling in healthcare analyses 24.
Figure 1.

Decision tree model used in this cost effectiveness analysis.
The cost perspective used in this analysis was a UK government third‐party payer, and the clinical setting was an anaesthetics department where tracheal intubations and double‐lumen tube position verification were frequently carried out. Multiple bronchoscopies for either tracheal intubation or double‐lumen tube position verification were examined to determine the resources and costs associated with the procedures. The procedures were monitored in detail from start to finish.
Data obtained retrospectively from various fiscal years (2000–2017) were used to quantify costs for capital acquisitions, repairs, consumables, disposables and service agreements. Additionally, labour time associated with the reprocessing cycles of the reusable flexible bronchoscopes was meticulously measured. The department has 19 reusable flexible bronchoscopes. Of these, 12 are used for tracheal intubation (either awake or asleep), whereas seven are reserved for double‐lumen tube position verification. The activities of performing bronchoscopy are dispersed across various operating theatres on one floor, whereas the reprocessing is divided on two different floors. The reusable flexible bronchoscopes undergo a precleaning cycle on the same floor as the operating theatre using equipment including detergents and brushes, followed by a second‐stage manual clean performed in a central cleaning facility on another floor. Costs were estimated using the mean annual number of bronchoscopic procedures and reprocessing volumes done by the automated endoscope reprocessors in this tertiary hospital.
In the Supporting Information (Data S1), all collected cost data from the micro‐costing analysis are presented in detail. This includes: (1) capital and repair costs of reusable flexible bronchoscopes and rack systems; (2) capital and repair costs of reprocessing capital and additional equipment; (3) time measurements of the specific reprocessing steps; (4) average cost of labour‐related reprocessing; (5) reprocessing equipment and costs incurred; and (6) the allocation keys and reason for usage 25.
The costs of the clinical outcome were determined by identifying treatment costs related to the clinical manifestations of a post‐bronchoscopic contamination or infection. The incidence of postoperative pulmonary complications (including pneumonia and sepsis) is estimated to be up to 23% in an unselected group of patients having general anaesthesia 26. This figure is higher in patients undergoing thoracic or head and neck surgery 27, which are the cohort of patients in whom flexible bronchoscopes are most commonly used. However, there are no data directly demonstrating bronchoscope‐induced cross‐contamination or infection in these patients, as infection is often assumed to be multifactorial. We were therefore only able to use published data of infection and cross‐contamination from the ICU or elective endoscopy or bronchoscopy population. The cost of the clinical outcome was estimated from the studies that were included according to the clinical manifestations that were reported in included studies, such as respiratory tract infection prophylaxis and therapy 28, 29, 30, 31, 32, 33, 34, 35, 36 and sepsis 33, 35. In the cohort of patients of interest for this study, we considered ventilator‐associated pneumonia, sepsis and community‐acquired pneumonia as suitable outcomes. The average cost of ventilator‐associated pneumonia was identified from a review of 28 US community hospitals to be £25,426 sterling per patient 37. From a systematic review of hospital‐related cost of sepsis, the treatment‐related costs were identified as £27,123 sterling per patient 38. The costs of inpatient and outpatient community‐acquired pneumonia were estimated from > 28,000 community‐acquired pneumonia episodes from a large US database study at £13,151 and £1948, respectively, per patient 39. The weighted average was defined as the treatment‐related costs per patient infected. This value was imputed in the cost‐effectiveness analysis. A summary of all costs and effect inputs for the cost‐effectiveness analysis is presented in Table 1.
Table 1.
Inputs for the cost effectiveness model. Base‐case value, the standard error (SE) and the distribution are provided
| Parameters | Base‐case value (SE) | Distribution |
|---|---|---|
| Effects | ||
| Risk of patient contamination using a reusable FB | 0.153 (0.009) | Beta |
| Risk of subsequent infection using a reusable FB | 0.181 (0.018) | Beta |
| Risk of patient contamination using a single‐use FB | 0.0 (0.001) | Beta |
| Risk of subsequent infection using a single‐use FB | 0.0 (0.001) | Beta |
| Costs | ||
| Capital cost per use of a reusable FB (reusable FB, stack systems, reprocessing capital) | £116.4a (29.10) | Gamma |
| Repair cost per use of a reusable FB (reusable FB, stack systems, reprocessing capital) | £92.9a (23.20) | Gamma |
| Reprocessing cost per use a reusable FB (labour time and equipment) | £39.9a (10.00) | Gamma |
| Cost of per clinical outcome 37, 38, 39 (per patient infection) | £9,454 (£1,158) | Gamma |
| Cost per use of a single‐use FB, including monitor | £220 (21.80) | Gamma |
SE was not estimated. However, a conservative approach was taken by varying the parameter with a SE of 25%. FB, flexible bronchoscope.
Using the results from the literature review and the micro‐costing analysis to compute the cost‐effectiveness analysis, a base‐case result was generated. Sensitivity analyses were undertaken to capture uncertainty within parameters and to provide sufficient insight to decision‐makers. Uncertainty is captured through deterministic and probabilistic sensitivity analyses. One‐way (univariate) sensitivity analyses were applied to all parameters in the model to test its robustness by examining the impact on the incremental cost effectiveness ratio. All cost parameters were varied by ± 50%. Considering the average period of an 8‐month investigation across the 16 studies, the effect parameters were varied from a low value of 0% risk to a high value of 20% risk. A scenario analysis was conducted from a previous Delphi approach to the general risk of patient cross‐contamination and infection 10. Furthermore, the impact of the incremental cost‐effectiveness ratio was observed by varying the amortisation period of capital investments related to bronchoscopy materials to 10 years from the previous 5 years.
The probabilistic sensitivity analysis quantifies the overall uncertainty within parameters using pre‐specified distributions (Table 1). The modality of the probabilistic sensitivity analysis was a second‐order Monte Carlo simulation with 10,000 iterations of the mean incremental cost‐effectiveness ratios. These 10,000 iterations were drawn up in a cost effectiveness scatterplot to represent the expected avoidance of infection risk using a single‐use compared with reusable flexible bonchoscopes.
According to International Guidelines in Health Economics, the mean is used as it is the only relevant measure for economic decision making 40. In economic calculations, we aim to capture the uncertainty of the sample mean, that is, parameter uncertainty, rather than variability or heterogeneity, that is, stochastic variability. The uncertainty in the expected mean is the standard error (SE) 40. Consequently, we report all cost data as mean (SE).
Results
We identified 890 citations, of which 12 were duplicates. Seven additional studies were considered through hand‐searching of two review articles 7, 8. Across numerous studies a non‐quantifiable risk was identified 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, thus not fulfilling the inclusion criteria for the quantitative analysis. After screening based on title and abstract and full‐text review, we identified 16 studies for quantitative analysis of the cross‐contamination and infection risk (Fig. 2) 12, 28, 29, 30, 31, 32, 33, 34, 35, 36, 63, 64, 65, 66, 67, 68. The setting of these studies were patients who underwent bronchoscopy in a hospital intensive care or respiratory unit setting, or during bronchoscopy or endoscopy in the UK, USA, France, Spain, Australia or Taiwan (Table 2). The study designs were prospective observational and retrospective studies, and the period of contamination and infection investigation of patients undertaken was from one to 23 months 30, 33. A total of 2351 patients underwent 3120 various bronchoscopic procedures. Of these procedures, 476 cases of patient contamination were detected. Eighty‐six of these patients were reported to have a bronchoscope‐linked manifestation of infection, including pneumonia or other respiratory tract infection. Seven of the included studies contained a missing data‐point relating to the number of bronchoscopic procedures (five studies) and the number of patients who underwent a bronchoscopy (two studies) 12, 30, 31, 35, 63, 64, 68. These missing data‐points were predicted using a linear regression method. There was a strong correlation between the number of patients who underwent bronchoscopy and the number of bronchoscopic procedures (r = 0.92) (see Supporting Information, Data S2). From this, 118 and 299 patients were predicted, respectively, for two of the studies with a missing data point 30, 68. A strong correlation was also demonstrated between the number of bronchoscopic procedures carried out and the number of patients who underwent bronchoscopy (r = 0.93). Five studies contained a missing data‐point in terms of number of bronchoscopic procedures, which were therefore calculated as 163, 429, 27, 76 and 63, respectively 12, 31, 35, 63, 64.
Figure 2.

Study flow chart.
Table 2.
Sixteen studies identified for quantitative analysis of the cross‐contamination and infection risk. Values are number or mean (SD)
| Source | Study design | Period of investigation (months) | Country | Setting | Patient age (years) | Procedures | Patients | Cases of contamination | Cases of infection | Detection of contamination | Typing system used | Infection(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blanc et al. 68 | Observational | 6 | USA | NA | NA | 410 | 299a | 35 | 0 | Yes | Ribotyping | No infections |
| Botana‐Rial et al. 30 | Observational | 1 | Spain | BU | NA | 154 | 118a | 39 | 21 | Yes | REP‐PCR | Treatment to prevent the development of pneumonia |
| Chroneou et al. 63 | Observational | 2 | USA | NA | 71 (49–86) | 76a | 57 | 9 | 0 | Yes | REP‐PCR | No infections |
| DiazGranados et al. 35 | Observational | 2 | USA | BU | NA | 27a | 20 | 12 | 2 | Yes | PFGE | Pneumonia and sepsis |
| Waite et al. 64 | Retrospective | 12 | UK | ICU | NA | 63a | 47 | 18 | 0 | Yes | PFGE | No infections |
| Wang et al. 31 | Retrospective | 4 | Taiwan | NA | 60 (45–79) | 163a | 123 | 18 | 8 | Yes | AFB | Patients were treated as mycobacterial infected |
| Silva et al. 12 | Retrospective | 22 | USA | EU | NA | 429a | 324 | 41 | 0 | Yes | Ribotyping | No infections |
| Nye et al. 65 | Retrospective | 6 | UK | EU | 61 (40–80) | 58 | 7 | 7 | 0 | Yes | Culturing and lipid analysis | No infections |
| Guy et al. 32 | Retrospective | 7 | France | ICU | 62 (49–73) | 216 | 157 | 10 | 8 | Yes | PFGE | Treatment to prevent the development of pneumonia |
| Sammartino et al. 36 | Retrospective | 3 | USA | NA | 56 (36–76) | 19 | 19 | 11 | 1 | Yes | Serotyping | Pneumonia |
| Campagnaro et al. 66 | Retrospective | 5 | Australia | NA | NA | 65 | 65 | 12 | 0 | Yes | DNA probing | No infections |
| Corne et al. 28 | Retrospective | 8 | France | ICU | NA | 61 | 36 | 16 | 4 | Yes | PFGE | Pneumonia |
| Kirschke et al. 34 | Retrospective | 4 | USA | NA | 59 (24–88) | 66 | 60 | 20 | 1 | Yes | PFGE | Pneumonia |
| Srinivasan et al. 33 | Retrospective | 23 | USA | EU | NA | 665 | 414 | 39 | 39 | Yes | PFGE | Pneumonias, sepsis, respiratory tract infection |
| Pappas et al. 29 | Retrospective | 11 | USA | NA | NA | 195 | 187 | 72 | 2 | Yes | Culturing | No data |
| Cêtre et al. 67 | Observational/retrospective | 8 | France | EU | NA | 453 | 418 | 117 | 0 | Yes | PFGE | No infections |
| Total | 3120 | 2351 | 476 | 86 |
AFB, acid‐fast bacillus testing; BU, bronchoscopic unit; DNA, deoxyribonucleic acid; EU, endoscopic unit; ICU, intensive care unit; NA, not available; PFGE, pulsed‐field gel electrophoresis; REP‐PCR, repetitive extragenic palindromic‐polymerase chain reaction.
Predicted data‐point.
From the included studies, the differentiation between a pre‐existing infection and a flexible bronchoscope‐related infection was determined using traditional bacterial recognition or more recent methods that examine the association of isolates at a molecular level (Table 2).
One thousand annual bronchoscopic procedures are performed by the Department of Anaesthesia at Guy's Hospital. The reprocessing volumes by the automated endoscope reprocessor were 10,075 cycles per year. The results from the micro‐costing analysis revealed a mean (SE) capital cost per use of a reusable flexible bronchoscope at £116.40 (£29.10), whereas the repair and reprocessing cost per use of a reusable flexible bronchoscope was estimated at £92.90 (£23.20) and £39.90 (£10.00), respectively. This equates to a total cost per use of a reusable flexible bronchoscope at £249.20 sterling. The mean (SE) cost per use of a single‐use flexible bronchoscope were provided by Ambu (Ambu® aScope™ 4, Copenhagen, Denmark) at £220.00 sterling (£21.80), including the monitor.
In the Supporting Information (Data S1), a detailed overview and description are provided of all costs incurred, and the allocation keys that were employed to more accurately reflect reality.
In the cost‐effectiveness analysis, we found reusable flexible bronchoscopes to have a mean (SE) cost per patient of £511.00 sterling (£59.60), with an associated risk of infection of 2.8%. The mean (SE) cost per patient with single‐use flexible bronchoscopes are estimated at £220.00 (£21.80) and a 0% risk of infection. Base‐case results indicate a net saving of £291.00 to hospitals and an avoided risk of infection of patients undergoing bronchoscopy at 2.8% with single‐use flexible bronchoscopes compared with reusable flexible bronchoscopes. The base‐case incremental cost‐effectiveness ratio is −£10,505, which is interpreted as the cost of avoided patient infection.
Varying cost inputs by ± 50% did not have a significant impact on the expected value of the incremental cost‐effectiveness ratio, which varied from −£15,232 to −£5,778. From Fig. 3, the cost parameter with the greatest impact on cost effectiveness is the cost of clinical outcome, whereas the parameter with the lowest impact is the reprocessing cost per use of a reusable flexible bronchoscope.
Figure 3.
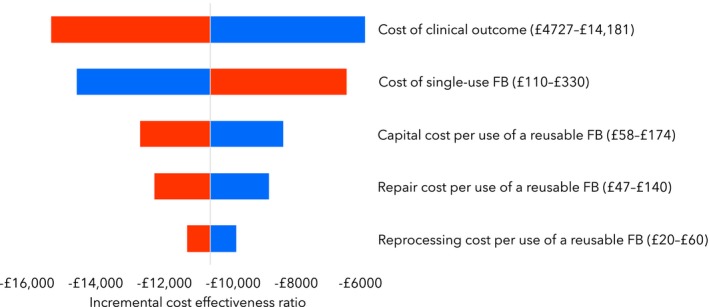
Tornado chart showing multiple one‐way (univariate) sensitivity analyses of cost input parameters varied by ± 50%. The incremental cost‐effectiveness ratio (ICER) midpoint is −£10,505 sterling and is equal to the base‐case result from the cost‐effectiveness analysis. Low values (blue) for cost of clinical outcome, capital, repair and reprocessing cost per use of a reusable flexible bronchoscope increase the ICER, whereas high values (red) reduce the ICER. Low values (blue) for cost of a single‐use bronchoscope reduce the ICER, whereas high values (red) increase the ICER. FB, flexible bronchoscope.
Considering the average of 8 months of investigation across the 16 studies of patient contamination and infection, one‐way sensitivity results from the CEA and a scenario analysis is presented in Table 3.
Table 3.
Base‐case result and one‐way sensitivity analyses of effect parameters
| Description | Difference in cost | Difference in effects | ICER (cost per avoided patient infection) |
|---|---|---|---|
| Base‐case | −£291 | 2.8% | Dominant |
| One‐way sensitivity of effects | |||
| Cross‐contamination risk of 0% | −£29 | No difference | Dominant |
| Cross‐contamination risk of 20% | −£371 | 3.6% | Dominant |
| Infection risk of 0% | −£29 | No difference | Dominant |
| Infection risk of 20% | −£318 | 3.1% | Dominant |
| One‐way sensitivity of amortisation of capital investments | |||
| Capital investments amortised across 10 years | −£239 | 2.8% | Dominant |
| Scenario analysis using estimates of cross‐contamination and infection risk obtained from a Delphi approach10 | |||
| Cross‐contamination risk of 3.38% and infection risk of 21.3% | −£97 | 0.7% | Dominant |
ICER, incremental cost effectiveness ratio.
The probabilistic sensitivity analysis was indicative of a potential net savings to hospitals ranging from £34 to 577 sterling per use and eliminating the risk of infection of approximately 1.71–4.07% using single‐use flexible bronchoscopes compared with reusable flexible bronchoscopes (Fig. 4).
Figure 4.
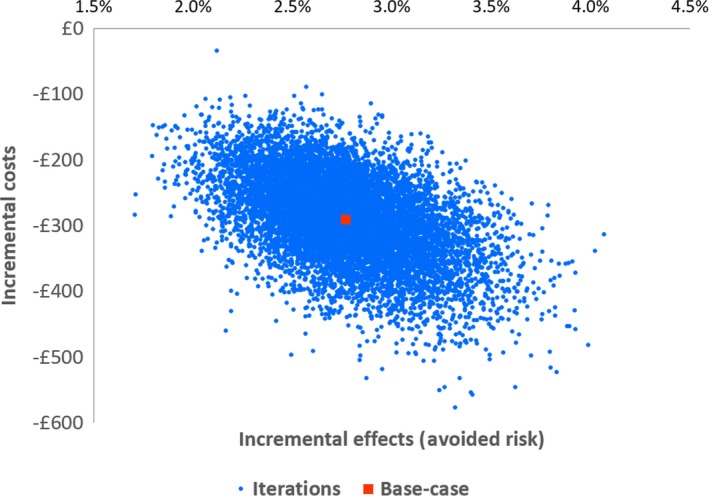
Probabilistic sensitivity analysis with 10,000 iterations (blue) and the base‐case value (red).
Discussion
This systematic review and cost‐effectiveness analysis found that single‐use flexible bronchoscopes are cost effective and associated with a lower risk of infection compared with reusable flexible bronchoscopes. Sensitivity analyses support these findings. Our systematic review demonstrated that the risk of patient infection post‐bronchoscopy was 2.8%, with a cost per use of a reusable flexible bronchoscope of £249 sterling and of a single‐use flexible bronchoscope of £220 sterling. Our cost‐effectiveness analysis demonstrated that reusable flexible bronchoscopes have a cost per patient use of £511 sterling due to the potential costs of treatment of infection.
The risk for patient contamination (15%) and infection (18%) resulted in a 2.8% risk of patient infection post‐bronchoscopy. In a previous study, a Delphi approach was used to estimate the general risk of patient contamination (3.4%) and infection (21%) in critical care settings 10. This generated a risk of post‐bronchoscopy infection of 0.7%. The estimate from this present study (2.8%) is of high accuracy because patient contamination and infection were linked to bronchoscopes and the data were sourced from international, multicentre settings with more than 2300 patients undergoing approximately 3100 various procedures.
The cost per use of reusable flexible bronchoscopes for tracheal intubation in a UK hospital carrying out 141 flexible bronchoscope‐assisted tracheal intubations annually was previously estimated as £340 sterling 69. In this present study, the total cost per use of reusable flexible bronchoscopes in an institution performing 1000 annual procedures was estimated to be £249 sterling. The cost per use for capital, reprocessing and repairs are highly dependent on local and clinical setting. The reprocessing costs are dependent on the length of non‐usage before reusable flexible bronchoscopes need reprocessing again. This is a variable time, but can be as low as 12 h 5. Staffing costs and use of productive working hours are also factors that may have an economic impact. Furthermore, repair costs per use of reusable flexible bronchoscopes are also highly dependent on the local setting. McCahon and Whynes conducted an analysis at a teaching hospital with repair cost per use of £146 sterling 69, which contrasts to the repair cost per use at Guy's Hospital of £93 sterling. This could be due to service agreements to cover repairs of all capital equipment in some institutions but not others. Finally, capital costs per use are dependent on economies of scale advantages, that is, the volume of annual bronchoscopies. Comparing McCahon and Whynes data to that in Guy's Hospital, the capital cost per use was £141 and £114, respectively. Other costing analyses have been conducted with total cost per use ranging from £111 to £540 sterling 18, 19, 20. From these studies, the clinical setting in terms of capital and repair expenditures play an important role when determining the capital and repair cost per use, whereas the reprocessing costs were similar between settings.
There are two main strengths of this study, the first being the micro‐costing analysis as the cost comparison between reusable and single‐use equipment is complex. Numerous overhead cost elements must be considered, and this study captures more than previous studies 18, 19, 20. The other main strength is the fact that this is the first study to identify risk of patient contamination and infection from the published literature.
In general, cost analyses lack precision in terms of including all relevant overhead costs 18, 19, 20. This is difficult to manage because numerous elements contribute to the overall cost per use estimate, such as water consumption, electricity, training of new personnel in cleaning techniques, maintaining updated and compliant guidelines on reprocessing, handling of automated endoscope reprocessor cycle failures, among others. Moreover, the cost of the tracking systems is often left out 18, 19, 20. If all relevant indirect and overhead costs were identified and included in those analyses, evidently it would add to the cost per use. To advance the precision of the cost per use estimate in this present micro‐costing analysis, some of the overhead costs mentioned above could have been included. If done so, it would have added to the cost effectiveness of single‐use flexible bronchoscopes. Findings from sensitivity analyses of cost per use of single‐use flexible bronchoscopes and reusable flexible bronchoscopes support the cost effectiveness of single‐use flexible bronchoscopes, even when varying cost parameters ± 50%.
In the institution examined in this study, the availability of reusable flexible bronchoscopes remains limited due to the unplanned requirement for flexible bronchoscopes, the additional time required for reprocessing and the ongoing requirement for bronchoscopes to be repaired. Availability was still an issue, thus investment in a subset of single‐use flexible bronchoscope for emergencies was undertaken. The availability of single‐use flexible bronchoscopes is constant as long as stocks are replenished, but the availability of reusable flexible bronchoscopes are hampered by the need for reprocessing.
The environmental impact of clinical care is also an element to consider. The disposable equipment, chemical detergents, water, electricity, and other resources used during the reprocessing cycles have an environmental impact, whereas single‐use flexible bronchoscopes are disposed of after each use. From research comparing carbon dioxide (CO2) emissions and resource consumption from a single‐use flexible bronchoscope (Ambu aScope™ 4) to a reusable flexible bronchoscope, results show that the materials used for reprocessing are substantial when comparing the two types of bronchoscopes 70. The use of cleaning materials and personal protective equipment contributes to a comparable or potentially higher material and energy consumption as well as emissions of CO2 equivalents and value of resource consumption for reusable compared with single‐use flexible bronchoscopes 70.
This study has several limitations. Our cost‐effectiveness analysis show that, ceteris paribus, the single‐use technology is superior in terms of costs and patient safety, and a mix of single‐use and reusable equipment may be the only realistic alternative. But not all overhead costs related to reusable equipment will be possible to eliminate, and the predicted savings may be smaller under such circumstances. Further research should be conducted to investigate the cost effectiveness of a mixed usage strategy for single‐use and reusable bronchoscopes. Another potential limitation is that we were unable to perform a formal risk of bias assessment of the included studies as no appropriate, validated tool was suitable. An additional limitation was that we included data from patients presenting for outpatient bronchoscopy, who may sometimes have systemic pathology, could be immunocompromised and are, therefore, prone to infection 6, 7, 8. However, the cohort of patients sought in the micro‐costing analysis also included patients undergoing thoracic surgical procedures and patients undergoing major head and neck surgery, both of which may be associated with high risk of infection transmission and immunocompromise. Moreover, the risk of transmission of infection remains, regardless of patient baseline characteristics. Furthermore, our cost calculations are primarily relevant to the peri‐operative setting, but the published data used come from outpatient bronchoscopic and critical care settings, and therefore there may be some discrepancy that is unaccounted for due to this assumption. There was heterogeneity in the lengths of investigations (1–23 months), patient contamination rates (4.6–58%) and infection risk (0–100%) among included studies. The variation was partly accounted for by utilising a fixed‐effects model. When varying risks of patient cross‐contamination and infection in the sensitivity analysis, single‐use flexible bronchoscopes remained financially superior to reusable flexible bronchoscopes. In addition, one of the assumptions that were made was that the risk of infection of single‐use flexible bronchoscopes was 0%. However, there always remain unaccounted‐for risks of infection transmission, even with single‐use flexible bronchoscopes, due to unsterile operators and surrounding equipment or re‐use in the same patient 71. Finally, there remains a previously unreported risk of nosocomial infection from tracheal intubation in elective patients who are not critically unwell and who are intubated for a limited duration of time, which might account for some of the incidence of infection after bronchoscopy. However, given that there are virtually no data reporting nosocomial infection in these settings, this may have a limited impact on our data. Moreover, the data we used on the published incidence of infection could be secondary to tracheal intubation rather than the use of a flexible bronchoscope. However, given that only 44 of the included patients with evidence of infection were in critical care settings, it is unlikely that the incidence of infection could be primarily attributed to non‐bronchoscopic sources.
In conclusion, our systematic review has demonstrated that the risk of patient infection following bronchoscopy with reusable flexible bronchoscopes is significant, warranting a need for guidelines on reprocessing to be stricter to ensure greater patient safety 8, 9, 72. The total cost per use of a reusable flexible bronchoscope was calculated to be £249. The cost per use of a single‐use flexible bronchoscope was £220 sterling. When considering the risk of infection in the cost analysis, reusable flexible bronchoscopes have a mean cost per patient of £511 sterling and an associated risk of infection at 2.8%. The findings from this study suggest benefits of single‐use flexible bronchoscopes in terms of cost effectiveness, cross‐contamination and resource utilisation.
Supporting information
Data S1. Micro‐costing analysis.
Data S2. Data prediction.
Acknowledgements
This review was registered on PROSPERO (CRD42019134573). KE is an Editor of Anaesthesia. IA and KE have previously received honoraria for consulting for Ambu, but there was no involvement of any industry in any aspect of this study, nor has anyone bar the authors had sight of the results of this study before submission for publication. No external funding or competing interests declared for JM, LE and JK.
References
- 1. Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures. Chest 2003; 123: 1693–4. [DOI] [PubMed] [Google Scholar]
- 2. Van Wicklin SA, Conner R, Spry C. Guideline for processing flexible endoscopes In: Connor R, ed. 2016 Guidelines for Perioperative Practice. Denver, CO: Association of Peri‐operative Registered Nurses, 2016. 675–758. [Google Scholar]
- 3. Society of Gastroenterology Nurses and Associates . Standard of infection prevention in the gastroenterology setting. 2015. https://www.sgna.org/Portals/0/Standard of Infection Prevention_FINAL_2.22.pdf?ver = 2016‐02‐22‐153052‐487 (accessed 06/05/2019).
- 4. Society of Gastroenterology Nurses and Associates . Standards of infection prevention in reprocessing flexible gastrointestinal endoscopes. 2015. https://www.sgna.org/Portals/0/Education/PDF/Standards-Guidelines/sgna_stand_of_infection_control_0812_FINAL.pdf (accessed 06/05/2019).
- 5. Advancing Safety in Healthcare Technology . American National Standard ‐ Flexible and semi‐rigid endoscope processing in health care facilities. 2015. https://my.aami.org/aamiresources/previewfiles/ST91_1504_preview.pdf (accessed 06/05/2019).
- 6. Kovaleva J, Meessen N, Peters F, et al. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy 2009; 41: 913–16. [DOI] [PubMed] [Google Scholar]
- 7. Culver DA, Gordon SM, Mehta AC. Infection control in the bronchoscopy suite: a review of outbreaks and guidelines for prevention. American Journal of Respiratory and Critical Care Medicine 2003; 167: 1050–6. [DOI] [PubMed] [Google Scholar]
- 8. Kovaleva J, Peters FTM, van der Mei HC, Degener JE. Transmission of infection by flexible gastrointestinal endoscopy and bronchoscopy. Clinical Microbiology Reviews 2013; 26: 231–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ofstead CL, Quick MR, Wetzler HP, et al. Effectiveness of reprocessing for flexible bronchoscopes and endobronchial ultrasound bronchoscopes. Chest 2018; 154: 1024–34. [DOI] [PubMed] [Google Scholar]
- 10. Terjesen CL, Kovaleva J, Ehlers L. Early assessment of the likely cost effectiveness of single‐use flexible video bronchoscopes. PharmacoEconomics – Open 2017; 1: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bou R, Aguilar A, Perpiñán J, et al. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. Journal of Hospital Infection 2006; 64: 129–35. [DOI] [PubMed] [Google Scholar]
- 12. Silva CV, Magalhães VD, Pereira CR, Kawagoe JY, Ikura C, Ganc AJ. Pseudo‐outbreak of Pseudomonas aeruginosa and Serratia marcescens related to bronchoscopes. Infection Control and Hospital Epidemiology 2003; 24: 195–7. [DOI] [PubMed] [Google Scholar]
- 13. Mehta AC, Prakash UBS, Garland R, et al. American College of Chest Physicians and American Association for Bronchoscopy Consensus Statement: prevention of flexible bronchoscopy‐associated infection. Chest 2005; 128: 1742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan A. Epidemiology and prevention of infections related to endoscopy. Current Infectious Disease Reports 2003; 5: 467–72. [DOI] [PubMed] [Google Scholar]
- 15. Mughal MM, Minai OA, Culver DA, Mehta AC. Reprocessing the bronchoscope: the challenges. Seminars in Respiratory and Critical Care Medicine 2004; 25: 443–9. [DOI] [PubMed] [Google Scholar]
- 16. Ramsey AH, Oemig TV, Davis JP, Massey JP, Török TJ. An outbreak of bronchoscopy‐related Mycobacterium tuberculosis infections due to lack of bronchoscope leak testing. Chest 2002; 121: 976–81. [DOI] [PubMed] [Google Scholar]
- 17. Larson JL, Lambert L, Stricof RL, Driscoll J, McGarry MA, Ridzon R. Potential nosocomial exposure to Mycobacterium tuberculosis from a bronchoscope. Infection Control and Hospital Epidemiology 2006; 24: 825–30. [DOI] [PubMed] [Google Scholar]
- 18. Tvede MF, Kristensen MS, Nyhus‐Andreasen M. A cost analysis of reusable and disposable flexible optical scopes for intubation. Acta Anaesthesiologica Scandinavica 2012; 56: 577–84. [DOI] [PubMed] [Google Scholar]
- 19. Perbet S, Blanquet M, Mourgues C, et al. Cost analysis of single‐use (Ambu® aScopeTM) and reusable bronchoscopes in the ICU. Annals of Intensive Care 2017; 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gupta D, Wang H. Cost‐effectiveness analysis of flexible optical scopes for tracheal intubation: a descriptive comparative study of reusable and single‐use scopes. Journal of Clinical Anesthesia 2011; 23: 632–5. [DOI] [PubMed] [Google Scholar]
- 21. El‐Boghdadly K, Onwochei DN, Cuddihy J, et al. A prospective cohort study of awake fibreoptic intubation practice at a tertiary centre. Anaesthesia 2017; 72: 694–703. [DOI] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, John PA. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. British Medical Journal 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabat AJ, Budimir A, Nashev D, et al. Overview of molecular typing methods for outbreak detection and epidemiological surveillance. European Communicable Disease Bulletin 2013; 18: 20380. [DOI] [PubMed] [Google Scholar]
- 24. Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health‐care evaluation: report of the ISPOR Task Force on Good Research Practices‐Modeling Studies. Value in Health 2003; 6: 9–17. [DOI] [PubMed] [Google Scholar]
- 25. Ofstead CL, Quick MR, Eiland JE, Adams SJ. A glimpse at the true cost of reprocessing endoscopes. 2017. https://www.bostonscientific.com/content/dam/bostonscientific/uro-wh/portfolio-group/LithoVue/pdfs/Sterilization-Resource-Handout.pdf (accessed 19/09/2019).
- 26. Miskovic A, Lumb AB. Postoperative pulmonary complications. British Journal of Anaesthesia 2017; 118: 317–34. [DOI] [PubMed] [Google Scholar]
- 27. Brueckmann B, Villa‐Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013; 118: 1276–85. [DOI] [PubMed] [Google Scholar]
- 28. Corne P, Godreuil S, Jean‐Pierre H, et al. Unusual implication of biopsy forceps in outbreaks of Pseudomonas aeruginosa infections and pseudo‐infections related to bronchoscopy. Journal of Hospital Infection 2005; 61: 20–6. [DOI] [PubMed] [Google Scholar]
- 29. Pappas SA, Schaaff DM, DiCostanzo MB, King FW, Sharp JT. Contamination of flexible fiberoptic bronchoscopes. American Review of Respiratory Disease 1983; 127: 391–2. [DOI] [PubMed] [Google Scholar]
- 30. Botana‐Rial M, Leiro‐Fernández V, Núñez‐Delgado M, et al. A pseudo‐outbreak of Pseudomonas putida and Stenotrophomonas maltophilia in a bronchoscopy unit. Respiration 2016; 92: 274–8. [DOI] [PubMed] [Google Scholar]
- 31. Wang HC, Liaw YS, Yang PC, Kuo SH, Luh KT. A pseudoepidemic of Mycobacterium chelonae infection caused by contamination of a fibreoptic bronchoscope suction channel. European Respiratory Journal 1995; 8: 1259–62. [DOI] [PubMed] [Google Scholar]
- 32. Guy M, Vanhems P, Dananché C, et al. Outbreak of pulmonary Pseudomonas aeruginosa and Stenotrophomonas maltophilia infections related to contaminated bronchoscope suction valves, Lyon, France, 2014. Eurosurveillance 2016; 21: 30286. [DOI] [PubMed] [Google Scholar]
- 33. Srinivasan A, Wolfenden LL, Song X, et al. An outbreak of Pseudomonas aeruginosa Infections associated with flexible bronchoscopes. New England Journal of Medicine 2003; 348: 221–7. [DOI] [PubMed] [Google Scholar]
- 34. Kirschke DL, Jones TF, Craig AS, et al. Pseudomonas aeruginosa and Serratia marcescens contamination associated with a manufacturing defect in bronchoscopes. New England Journal of Medicine 2003; 348: 214–20. [DOI] [PubMed] [Google Scholar]
- 35. DiazGranados Ca, Jones MY, Kongphet‐Tran T, et al. Outbreak of Pseudomonas aeruginosa infection associated with contamination of a flexible bronchoscope. Infection Control and Hospital Epidemiology 2009; 30: 550–5. [DOI] [PubMed] [Google Scholar]
- 36. Sammartino MT, Israel RH, Magnussen CR. Pseudomonas aeruginosa contamination of fiberoptic bronchoscopes. Journal of Hospital Infection 1982; 3: 65–71. [DOI] [PubMed] [Google Scholar]
- 37. Anderson DJ, Kirkland KB, Kaye KS, et al. Underresourced hospital infection control and prevention programs: penny wise, pound foolish? Infection Control and Hospital Epidemiology 2007; 28: 767–73. [DOI] [PubMed] [Google Scholar]
- 38. Arefian H, Heublein S, Scherag A, et al. Hospital‐related cost of sepsis: a systematic review. Journal of Infection 2017; 74: 107–17. [DOI] [PubMed] [Google Scholar]
- 39. Sato R, Gomez Rey G, Nelson S, Pinsky B. Community‐acquired pneumonia episode costs by age and risk in commercially insured US adults aged ≥ 50 years. Applied Health Economics and Health Policy 2013; 11: 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty analysis: a report of the ISPOR‐SMDM modeling good research practices task force working group‐6. Medical Decision Making 2012; 32: 722–32. [DOI] [PubMed] [Google Scholar]
- 41. Anon . Bronchoscopy‐related infections and pseudoinfections – New York, 1996 and 1998. Morbidity and Mortality Weekly Report 1999; 48: 557–60. [PubMed] [Google Scholar]
- 42. Schelenz S, French G. An outbreak of multidrug‐resistant Pseudomonas aeruginosa infection associated with contamination of bronchoscopes and an endoscope washer‐disinfector. Journal of Hospital Infection 2000; 46: 23–30. [DOI] [PubMed] [Google Scholar]
- 43. Kressel A, Kidd F. Pseudo‐outbreak of Mycobacterium chelonae and Methylobacterium mesophilicum caused by contamination of an automated endoscopy washer. Infection Control and Hospital Epidemiology 2000; 21: 394–7. [DOI] [PubMed] [Google Scholar]
- 44. Bennett SN, Peterson DE, Johnson DR, Hall WN, Robinson‐Dunn B, Dietrich S. Bronchoscopy‐associated Mycobacterium xenopi pseudoinfections. American Journal of Respiratory and Critical Care Medicine 1994; 150: 245–50. [DOI] [PubMed] [Google Scholar]
- 45. Webb SF, Vall Spinosa A. Outbreak of Serratia marcescens associated with the flexible fiberbronchoscope. Chest 1975; 68: 703–8. [Google Scholar]
- 46. Siegman‐igra Y, Inbar G, Campus A. An, “outbreak” of pulmonary pseudoinfection by Serratia marcescens. Journal of Hospital Infection 1985; 6: 218–20. [DOI] [PubMed] [Google Scholar]
- 47. Vandenbroucke‐Grauls CMJE, Baars ACM, Visser MR, Hulstaert PF, Verhoef J. An outbreak of Serratia marcescens traced to a contaminated bronchoscope. Journal of Hospital Infection 1993; 23: 263–70. [DOI] [PubMed] [Google Scholar]
- 48. Goldstein B, Abrutyn E. Pseudo‐outbreak of Bacillus species: related to fibreoptic bronchoscopy. Journal of Hospital Infection 1985; 6: 194–200. [PubMed] [Google Scholar]
- 49. Hagan ME, Klotz SA, Bartholomew W, Potter L, Nelson M. A pseudoepidemic of Rhodotorula rubra: a marker for microbial contamination of the bronchoscope. Infection Control and Hospital Epidemiology 1995; 16: 727–8. [DOI] [PubMed] [Google Scholar]
- 50. Machida H, Seki M, Yoshioka N, et al. Correlation between outbreaks of multidrug‐resistant Pseudomonas aeruginosa infection and use of bronchoscopes suggested by epidemiological analysis. Biological and Pharmaceutical Bulletin 2014; 37: 26–30. [DOI] [PubMed] [Google Scholar]
- 51. Peaper DR, Havill NL, Aniskiewicz M, et al. Pseudo‐outbreak of Actinomyces graevenitzii associated with bronchoscopy. Journal of Clinical Microbiology 2015; 53: 113–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guimarães T, Chimara E, do Prado GVB, et al. Pseudooutbreak of rapidly growing mycobacteria due to Mycobacterium abscessus subsp bolletii in a digestive and respiratory endoscopy unit caused by the same clone as that of a countrywide outbreak. American Journal of Infection Control 2016; 44: e221–6. [DOI] [PubMed] [Google Scholar]
- 53. Sorin M, Segal‐Maurer S, Mariano N, Urban C, Combest A, Rahal JJ. Nosocomial transmission of imipenem‐resistant pseudomonas aeruginosa following bronchoscopy associated with improper connection to the steris system I processor. Infection Control and Hospital Epidemiology 2001; 22: 409–13. [DOI] [PubMed] [Google Scholar]
- 54. Peltroche‐Llacsahuanga H, Lütticken R, Haase G. Temporally overlapping nosocomial outbreaks of Serratia marcescens infections: an unexpected result revealed by pulsed‐field gel electrophoresis. Infection Control and Hospital Epidemiology 1999; 20: 387–8. [DOI] [PubMed] [Google Scholar]
- 55. Barton E, Borman A, Johnson E, Sherlock J, Giles A. Pseudo‐outbreak of Fusarium oxysporum associated with bronchoscopy. Journal of Hospital Infection 2016; 94: 197–8. [DOI] [PubMed] [Google Scholar]
- 56. Raz DJ, Nelson RA, Grannis FW, Kim JY. Natural history of typical pulmonary carcinoid tumors: a comparison of nonsurgical and surgical treatment. Chest 2015; 147: 1111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nelson KE, Larson PA, Schraufnagel DE, Jackson J. Transmission of tuberculosis by flexible fiberbronchoscopes. American Review of Respiratory Disease 1983; 127: 97–100. [DOI] [PubMed] [Google Scholar]
- 58. Wheeler PW, Lancaster D, Kaiser AB. Bronchopulmonary cross‐colonization and infection related to mycobacterial contamination of suction valves of bronchoscopes. Journal of Infectious Diseases 1989; 159: 954–8. [DOI] [PubMed] [Google Scholar]
- 59. Agerton T, Valway S, Gore B, et al. Transmission of a highly drug‐resistant strain (strain W1) of Mycobacterium tuberculosis. Community outbreak and nosocomial transmission via a contaminated bronchoscope. Journal of the American Medical Association 1997; 278: 1073–7. [PubMed] [Google Scholar]
- 60. Prigogine T, Glupczynski Y, Van Molle P, Schmerber J. Mycobacterial cross‐contamination of bronchoscopy specimens. Journal of Hospital Infection 1988; 11: 93–5. [DOI] [PubMed] [Google Scholar]
- 61. Uttley AH, Honeywell KM, Fitch LE, Yates MD, Collins CH, Simpson RA. Cross contamination of bronchial washings. British Medical Journal 1990; 301: 1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Takigawa K, Fujita J, Negayama K, et al. Eradication of contaminating Mycobacterium chelonae from bronchofibrescopes and an automated bronchoscope disinfection machine. Respiratory Medicine 1995; 89: 423–7. [DOI] [PubMed] [Google Scholar]
- 63. Chroneou A, Zimmerman SK, Cook S, et al. Molecular typing of mycobacterium chelonae isolates from a pseudo‐outbreak involving an automated bronchoscope washer. Infection Control and Hospital Epidemiology 2008; 29: 1088–90. [DOI] [PubMed] [Google Scholar]
- 64. Waite TDD, Georgiou A, Abrishami M, Beck CRR. Pseudo‐outbreaks of Stenotrophomonas maltophilia on an intensive care unit in England. Journal of Hospital Infection 2016; 92: 392–6. [DOI] [PubMed] [Google Scholar]
- 65. Nye K, Chadha DK, Hodgkin P, Bradley C, Hancox J, Wise R. Mycobacterium chelonei isolation from broncho‐alveolar lavage fluid and its practical implications. Journal of Hospital Infection 1990; 16: 257–61. [DOI] [PubMed] [Google Scholar]
- 66. Campagnaro RL, Teichtahl H, Dwyer B. A pseudoepidemic of Mycobacterium chelonae: contamination of a bronchoscope and autocleaner. Australian and New Zealand Journal of Medicine 1994; 24: 693–5. [DOI] [PubMed] [Google Scholar]
- 67. Cêtre JC, Nicolle M‐CC, Salord H, et al. Outbreaks of contaminated broncho‐alveolar lavage related to intrinsically defective bronchoscopes. Journal of Hospital Infection 2005; 61: 39–45. [DOI] [PubMed] [Google Scholar]
- 68. Blanc DS, Parret T, Janin B, Raselli P, Francioli P. Nosocomial infections and pseudoinfections from contaminated bronchoscopes: two‐year follow up using molecular markers. Infection Control and Hospital Epidemiology 1997; 18: 134–6. [DOI] [PubMed] [Google Scholar]
- 69. McCahon RA, Whynes DK. Cost comparison of re‐usable and single‐use fibrescopes in a large English teaching hospital. Anaesthesia 2015; 70: 699–706. [DOI] [PubMed] [Google Scholar]
- 70. Lilholt Sørensen B, Grüttner H. Comparative study on environmental impacts of reusable and single‐use bronchoscopes. American Journal of Environmental Protection 2019; 7: 55. [Google Scholar]
- 71. McGrath BA, Ruane S, McKenna J, Thomas S. Contamination of single‐use bronchoscopes in critically ill patients. Anaesthesia 2017; 72: 36–41. [DOI] [PubMed] [Google Scholar]
- 72. Rutala WA, Weber DJ. Gastrointestinal endoscopes a need to shift from disinfection to sterilization? Journal of the American Medical Association 2014; 312: 1405–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Micro‐costing analysis.
Data S2. Data prediction.


