Abstract
Introduction:
Periodontitis is a chronic inflammatory condition initiated by microorganisms and is positively linked to systemic conditions such as cancer, cardiovascular disease, and diabetes mellitus.
Objectives:
To prospectively investigate associations between empirically derived clusters of IgG antibodies against 19 selected periodontal microorganisms and cancer mortality in a representative sample of the US population.
Methods:
We evaluated 6,491 participants aged ≥40 y from the Third National Health and Nutrition Examination Survey (1988 to 1994), who had complete data on IgG antibody titers against 19 selected periodontal microorganisms and were free of cardiovascular disease and cancer. In a prior study, antibodies were categorized into 4 mutually exclusive groups via cluster analysis: red-green, orange-red, yellow-orange, and orange-blue. Cluster scores were estimated by summing z scores of the antibody titers making up each cluster. Participants were followed up to death until December 31, 2011. Cox proportional hazard models were applied to estimate hazard ratios (HRs) and 95% CIs for all-cancer mortality by tertiles of cluster scores.
Results:
During follow-up for a median of 15.9 y, there were 2,702 deaths (31.3%), including 631 cancer-related deaths (8.1%). After adjusting for multiple confounders, the orange-blue cluster was inversely associated with cancer mortality (tertile 2 vs. tertile 1: HR = 0.67, 95% CI = 0.54 to 0.84; tertile 3 vs tertile 1: HR = 0.62, 95% CI = 0.46 to 0.84). The association between the yellow-orange cluster and all-cancer mortality was also inverse but not significant, and the orange-red cluster and the red-green cluster were not associated with all-cancer mortality.
Conclusions:
Antibodies against Eubacterium nodatum and Actinomyces naeslundii may be novel predictors of cancer mortality. If further studies establish a causal relationship between these antibodies and cancer mortality, they could be targets to prevent possible systemic effects of periodontal disease with potential interventions to raise their levels.
Knowledge Transfer Statement:
Periodontal antibodies against Eubacterium nodatum and Actinomyces naeslundii were inversely associated with cancer mortality among adults followed up for an average of 16 y. Periodontal antibodies may predict cancer mortality.
Keywords: bacterial antibodies, periodontal diseases, neoplasms, mortality rate, cluster analysis, oral health
Introduction
Inflammation is a key mediator of overall cancer risk (Heikkila et al. 2018). As periodontal disease is a low-grade chronic infection of supporting tooth structures leading to systemic inflammation and affecting almost half of all US adults, it is plausible that periodontal disease could play a role in cancer pathology (Eke et al. 2012). Emerging evidence from epidemiologic studies has linked periodontal disease with various types of cancer, including cancer of the oral cavity and other sites (Fitzpatrick and Katz 2010; Ahn, Segers, and Hayes 2012; Michaud et al. 2013; Zeng et al. 2013; Heikkila et al. 2018). Higher oral cancer risk was observed for individuals with periodontitis as compared with those with gingivitis, further suggesting that periodontal infection may affect cancer risk (Wen et al. 2014). Individuals with periodontal disease receiving treatment had lower cancer risk versus those not receiving treatment in a large population-based cohort in Taiwan (Hwang et al. 2014).
The observed associations between periodontal disease and cancer are suspected to have a microbial basis. Individuals with oral cancer show altered composition of oral microorganisms (Mager et al. 2005), which are suspected to initiate the carcinogenesis. A recent study reported that carriage of periodontal pathogens such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were related to increased risk of pancreatic cancer (Fan et al. 2016). Serum IgG antibodies, which are induced by periodontal microorganisms, can remain elevated for up to 15 y (Papapanou et al. 2004) and may thus provide long-lasting protection against subsequent periodontal diseases (Papapanou et al. 2004; Rams et al. 2006) and be a surrogate marker for clinical periodontal status in epidemiologic studies (Papapanou et al. 2001). A twofold increased risk of pancreatic cancer was detected in individuals with greater serum P. gingivalis IgG in a large European prospective cohort study (Michaud et al. 2013). Similarly, high levels of antibody against P. gingivalis tended to be associated with higher orodigestive cancer mortality (Ahn, Segers, and Hayes 2012). Although there is evidence linking periodontal disease with cancer risk, the underlying mechanisms are uncertain. As the mouth harbors approximately 700 microorganisms, identifying relevant serum IgG antibodies that can serve as markers is challenging (Schenkein et al. 1993).
The Centers for Disease Control and Prevention released data for antibodies against 19 periodontal microorganisms using stored blood samples from participants of the Third National Health and Nutrition Examination Survey (NHANES III) in 2008 (Vlachojannis et al. 2010). In a prior analysis, we categorized these antibodies into 4 groups via cluster analysis, an empirical approach giving mutually exclusive groups reflecting the way the antibodies grouped together in vivo (Merchant et al. 2014). Specific groups of antibodies defined this way were positively or negatively associated with hyperglycemia, metabolic syndrome, and cardiovascular risk factors (Merchant et al. 2014). These clusters were found to modify the associations between periodontal disease and alcohol intake and physical activity (Merchant et al. 2016; Anderson et al. 2018). The advantages of this approach to examine the relation between periodontal antibodies and systemic outcomes were that type 1 error was minimized and the groups reflected the way that the antibodies clustered in vivo. The aim of this study was to prospectively investigate the underlying associations between these serum IgG antibody clusters and cancer mortality with the nationally representative NHANES III data.
Methods
Data Source
The NHANES III was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention from 1988 to 1994 among a representative sample of the noninstitutionalized civilian US population. Data were collected via household interview, medical and dental examination, and laboratory tests in a standardized way with strict quality control procedures (Centers for Disease Control and Prevention 1992; National Center for Health Statistics 1994).
Population Description
The CDC used stored serum from NHANES III participants ≥40 y old at the time of examination to test for IgG antibodies (gravimetric units) against 19 oral bacterial species related to periodontal disease via the “checkerboard” immunoassay technique at the Columbia University College of Dental Medicine. Details are described in NHANES III documentation (Centers for Disease Control and Prevention 2008). Complete IgG data were available for 8,153 individuals. After exclusion of individuals with a self-reported history of CVD or cancer, 6,491 participants were left in the final sample. All participants were followed up for mortality status until December 31, 2011 (National Center for Health Statistics 2013).
Exposure Measures
Cluster Formation and Naming the Clusters
Data from NHANES III participants with data on IgG antibodies were used to derive antibody clusters in a prior study (Merchant et al. 2014). Briefly, serum IgG antibody titers against periodontal microorganisms were grouped into 4 mutually distinct groups via cluster analysis. To name these clusters, we adapted the nomenclature used by Socransky and Haffajee to describe periodontal microorganism groups related to clinical periodontal conditions. They classified the microorganisms into complexes, which they named using the following color scheme: organisms related to periodontal disease (red and orange complexes), to a healthy periodontal state (yellow and purple complexes), to healthy and diseased states (blue complex), and weakly to periodontal disease (green complex; Socransky and Haffajee 2002, 2005).
Antibody clusters were named in the following way. Cluster 1 contained antibodies against 4 organisms, of which 3 were from the orange complex and 1 from the red complex, and was named the orange-red cluster (Prevotella melaninogenica, Prevotella intermedia, Prevotella nigrescens, P. gingivalis). The red-green (Tannerella forsythia, Treponema denticola, A. actinomycetemcomitans, Eikenella corrodens, Selenomonas noxia, Veillonella parvula, Campylobacter rectus), yellow-orange (Streptococcus intermedius, Streptococcus oralis, Streptococcus mutans, Fusobacterium nucleatum, Parvimonas micra, Capnocytophaga ochracea), and orange-blue (Eubacterium nodatum, Actinomyces naeslundii) clusters were similarly named.
Calculating Cluster Score
In the present analyses, we computed z scores for all 19 antibody titers for each individual. Next, we summed z scores for the antibody titers making up each cluster to get a cluster score for each participant. For example, the orange-blue cluster consisted of antibody titers against E. nodatum and A. naeslundii; z scores of these 2 antibody titers were summed to obtain a score for that cluster, and so on (Merchant et al. 2014).
Outcome Measures
Data on total cancer mortality were abstracted from the NHANES III Linked Mortality File, which contains mortality data for all eligible NHANES III participants. Follow-up started from the date of survey participation and lasted through December 31, 2011, or the date of death, whichever occurred first; the average length of follow-up period in our study was 15.9 y. Underlying cause of death was obtained from the death certificate. According to the National Center for Health Statistics, 96.1% of decedents and 99.4% of living participants were correctly classified with the probabilistic matching algorithm (Brown et al. 2017). Details can be found elsewhere (National Center for Health Statistics 2013).
Covariates
The following covariables were adjusted for in this study to eliminate confounding influences: age, sex, race, educational level, income, smoking status, drinking status, body mass index, diabetes, hypertension, and annual dentist visits. Age at baseline was self-reported and categorized into 2 groups, representing middle-aged people (40 to 64 y) and elderly people (≥65 y). Data on sex (male and female) and race (black, white, and other) were based on self-report. Poverty income ratio and education level, as indicators of socioeconomic status, were divided into 3 groups (≤1.3, >1.3 to ≤3.5, >3.5) and 2 groups (<12 y, ≥12 y completed education), respectively. Smoking status (current, former, never smoker) and drinking status (drinker and nondrinker) were also self-reported. Body mass index was calculated as weight divided by height squared and categorized into normal (≤24.9 kg/m2), overweight (25 to ≤29.9 kg/m2), and obese (≥30 kg/m2). History of diabetes and hypertension were captured according to whether the participant reported being diagnosed with these conditions by a doctor. Annual visit to a dentist was a binary variable. Oral health measures were obtained from the oral examination component of the NHANES III (Centers for Disease Control and Prevention 1992).
Statistical Analysis
SAS 9.4 (SAS Institute) was used to manage and analyze the data. SAS survey procedures used mobile examination center 6-y weights, with weights recommended for strata and primary sampling unit for the NHANES III, to account for the multistage clustered sampling design, unequal probability of selection, missing data, and poststratification to the US population and to get accurate variance estimates. Failure time was defined as the time from examination at the mobile examination center to death from any type of cancer, or December 31, 2011, whichever occurred first; survival time was the duration from the start of follow-up to the failure time. The level of significance was set as 0.05.
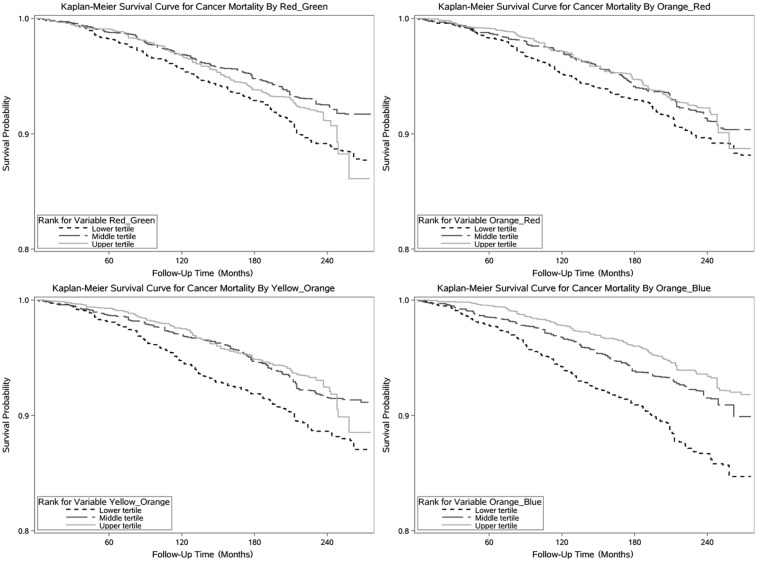
Basic descriptive statistics were calculated for covariates across extreme tertiles of cluster scores. Specifically, for categorical variables, total original number and weighted percentage across categories were presented; for continuous variables, the means and standard errors were calculated. Kaplan-Meier survival curves, the standard method to describe and compare graphically the overall survival, were plotted by 3 tertiles of different cluster scores.
Cox proportional hazard models were run to estimate the hazard ratio and 95% CI, with survival time to cancer mortality as the outcome and with tertiles of scores for orange-red, red-green, yellow-orange, and orange-blue clusters as the exposures. First, we modeled the crude model with only 4 variables representing the tertiles of 4 clusters; then, we adjusted for the sociodemographic variables, including sex, race, and age group; third, we fully adjusted with education level, income (poverty income ratio), smoking, drinking, body mass index, diabetes status, history of hypertension, and annual dentist visits as other covariates. Variables to control for confounding were chosen per prior studies. During model building, estimates were examined for evidence of collinearity while adding variables. To further evaluate potential confounding that could affect the association between periodontal disease and cancer, we conducted subgroup analyses by sex, age group, race, smoking status, and periodontal status. As males and older individuals have a higher cancer risk, we evaluated the associations in joint subgroups of age (40 to 64 vs. ≥65 y) by sex. The proportional hazards assumption was tested by Schoenfeld residuals to ensure appropriate model fit. The study was exempted from full Institutional Review Board review. The manuscript is in compliance with the STROBE statement.
Results
The cohort of 6,491 individuals was followed up to 23 y (mean, 19.0 y), during which there were 2,702 deaths (31.3%), including 631 cancer-related deaths (8.1%). At the start of follow-up, the individuals were aged 40 to 90 y (median, 52.15 y); 45% were male; and 86% and 10% were White and Black, respectively.
Table 1 describes the demographic and clinical characteristics of the participants by antibody score tertiles. Individuals in the top tertiles of the red-green and orange-red clusters were more likely to be Black, male, and never smokers as compared with those in the bottom tertiles. People in the top tertiles of the yellow-orange and orange-blue clusters were more likely to be younger, nonsmokers, and with higher education and income levels as compared with those in lower tertiles. The unadjusted Kaplan-Meier survival curves for all-cancer mortality by tertiles of each cluster showed that the overall survival probability was >80% over 15.9 y (Fig. 1). Table 2 shows the hazards ratios and 95% CIs from Cox proportional hazard models. The orange-red cluster and the red-green cluster were not associated with all-cancer mortality. The orange-blue cluster was consistently inversely associated with all-cancer mortality. The associations persisted in fully adjusted models; the hazards ratios (95% CIs) were as follows: 0.67 (0.54 to 0.84), tertile 2 versus tertile 1; 0.62 (0.46 to 0.84), tertile 3 versus tertile 1. The association between the yellow-orange cluster and all-cancer mortality was also inverse, but the 95% CIs included 1.
Table 1.
Descriptive Characteristics (Weighted) of Study Population.
| Red-Green |
Orange-Red |
||||
|---|---|---|---|---|---|
| Participants, na | Tertile 1 | Tertile 3 | Tertile 1 | Tertile 3 | |
| Cluster score range | 6,491 | −36.09 to −0.38 | 2.34 to 13.21 | −27.28 to −0.65 | 1.27 to 10.76 |
| Cancer mortality | 631 | 9.70 | 7.72b | 9.02 | 7.39 |
| Age, y | |||||
| Middle-aged, 40 to 64 | 4,441 | 77.52 | 78.03 | 77.63 | 78.96 |
| Elderly, ≥65 | 2,050 | 22.48 | 21.97 | 22.37 | 21.04 |
| Race | |||||
| White | 4,568 | 90.40 | 81.83b | 91.62 | 77.82b |
| Black | 1,719 | 7.85 | 12.55 | 6.43 | 15.56 |
| Other | 204 | 1.75 | 5.63 | 1.96 | 6.62 |
| Sex | |||||
| Male | 2,942 | 41.30 | 49.25b | 39.98 | 49.07b |
| Female | 3,549 | 58.70 | 50.75 | 60.02 | 50.93 |
| Educational level, y | |||||
| <12 | 2,952 | 28.95 | 26.56 | 27.13 | 30.24 |
| ≥12 | 3,493 | 71.05 | 73.44 | 72.87 | 69.76 |
| Income (poverty income) | |||||
| Low | 1,717 | 14.25 | 12.51 | 13.59 | 14.57 |
| Middle | 3,185 | 49.41 | 45.66 | 44.71 | 47.26 |
| High | 1,589 | 36.34 | 41.82 | 41.70 | 38.17 |
| Smoking status | |||||
| Current | 1,518 | 29.21 | 17.19 b | 28.63 | 18.43 b |
| Ever smoker | 1,945 | 32.43 | 34.56 | 30.52 | 32.97 |
| Never smoker | 3,028 | 38.36 | 48.24 | 40.85 | 48.60 |
| Drinking status | |||||
| Drinker | 2,789 | 50.67 | 48.24 | 50.95 | 50.79 |
| Nondrinker | 3,701 | 49.33 | 51.76 | 49.05 | 49.21 |
| Body mass index, kg/m2 | |||||
| Normal, <25 | 2,095 | 38.59 | 33.08 | 37.88 | 32.89 |
| Overweight, 25 to 29.9 | 2,481 | 35.81 | 38.17 | 38.23 | 36.90 |
| Obesity, ≥30 | 1,903 | 25.60 | 28.76 | 23.89 | 30.21 |
| Diabetes status | |||||
| Absent | 5,797 | 93.32 | 94.09 | 94.48 | 92.85 |
| Present | 687 | 6.68 | 5.91 | 5.52 | 7.15 |
| History of hypertension | |||||
| Absent | 4,211 | 68.13 | 68.63 | 69.25 | 66.14 b |
| Present | 2,251 | 31.87 | 31.37 | 30.75 | 33.86 |
| Annual dentist visits | |||||
| No | 3,806 | 49.54 | 48.71 | 47.24 | 47.99 |
| Yes | 2,476 | 50.46 | 51.29 | 52.76 | 52.01 |
| Yellow-Orange |
Orange-Blue |
||||
| Participants, na | Tertile 1 | Tertile 3 | Tertile 1 | Tertile 3 | |
| Cluster score range | 6,491 | −30.20 to −0.26 | 1.92 to 11.08 | −11.72 to −0.45 | 0.80 to 4.89 |
| Cancer mortality | 631 | 9.91 | 6.90b | 11.43 | 6.05b |
| Age, y | |||||
| Middle-aged, 40 to 64 | 4,441 | 74.51 | 79.79b | 73.09 | 83.45b |
| Elderly, ≥65 | 2,050 | 25.49 | 20.21 | 26.91 | 16.55 |
| Race | |||||
| White | 4,568 | 89.00 | 81.53 b | 84.63 | 87.58 |
| Black | 1,719 | 8.51 | 12.82 | 11.55 | 9.20 |
| Other | 204 | 2.49 | 5.65 | 3.82 | 3.22 |
| Sex | |||||
| Male | 2,942 | 42.44 | 44.81b | 46.16 | 45.83 |
| Female | 3,549 | 57.56 | 55.19 | 53.84 | 54.17 |
| Educational level, y | |||||
| <12 | 2,952 | 30.80 | 27.33b | 31.65 | 21.42b |
| ≥12 | 3,493 | 69.20 | 72.67 | 68.35 | 78.58 |
| Income (poverty income) | |||||
| Low | 1,717 | 16.44 | 13.00b | 17.78 | 10.17b |
| Middle | 3,185 | 47.28 | 47.45 | 49.01 | 43.28 |
| High | 1,589 | 36.28 | 39.55 | 33.21 | 46.56 |
| Smoking status | |||||
| Current | 1,518 | 29.15 | 18.33 b | 30.06 | 17.65 b |
| Ever smoker | 1,945 | 31.39 | 33.26 | 30.99 | 33.69 |
| Never smoker | 3,028 | 39.46 | 48.41 | 38.95 | 48.65 |
| Drinking status | |||||
| Drinker | 2,789 | 49.76 | 49.27 | 46.74 | 53.40 b |
| Nondrinker | 3,701 | 50.24 | 50.73 | 53.26 | 46.60 |
| Body mass index, kg/m2 | |||||
| Normal, <25 | 2,095 | 39.84 | 34.01 | 37.62 | 34.12 |
| Overweight, 25 to 29.9 | 2,481 | 35.35 | 37.74 | 37.46 | 38.23 |
| Obesity, ≥30 | 1,903 | 24.81 | 28.25 | 24.92 | 27.65 |
| Diabetes status | |||||
| Absent | 5,797 | 92.66 | 93.62 | 91.81 | 95.41 |
| Present | 687 | 7.34 | 6.38 | 8.19 | 4.59 |
| History of hypertension | |||||
| Absent | 4,211 | 68.67 | 69.05 | 65.94 | 72.18b |
| Present | 2,251 | 31.33 | 30.95 | 34.06 | 27.82 |
| Annual dentist visits | |||||
| No | 3,806 | 50.56 | 45.45b | 52.04 | 44.12b |
| Yes | 2,476 | 49.44 | 54.55 | 47.96 | 55.88 |
Unless indicated otherwise, all values are expressed as weighted percentages.
Values are unweighted; total numbers may be different as result of missing data.
P < 0.05, indicating differences across tertiles for each cluster.
Figure 1.
Kaplan-Meier survival curves for cancer mortality by tertiles of 4 cluster scores.
Table 2.
Cancer Mortality in Relation to 4 Cluster Scores of Serum IgG Antibody Titers against 19 Periodontal Microorganisms.
| Cancer Mortality: HR (95% CI)a |
|||
|---|---|---|---|
| Cluster: Levelb | Model 1 | Model 2 | Model 3 |
| Red-green | |||
| Middle | 0.95 (0.65 to 1.40) | 0.90 (0.62 to 1.31) | 0.93 (0.65 to 1.33) |
| High | 1.47 (0.88 to 2.44) | 1.32 (0.81 to 2.15) | 1.41 (0.86 to 2.29) |
| Orange-red | |||
| Middle | 0.91 (0.68 to 1.22) | 0.85 (0.63 to 1.14) | 0.83 (0.62 to 1.12) |
| High | 0.94 (0.58 to 1.51) | 0.82 (0.51 to 1.32) | 0.86 (0.54 to 1.37) |
| Yellow-orange | |||
| Middle | 0.78 (0.52 to 1.18) | 0.87 (0.58 to 1.30) | 0.86 (0.58 to 1.26) |
| High | 0.70 (0.49 to 1.00)c | 0.80 (0.55 to 1.18) | 0.80 (0.56 to 1.13) |
| Orange-blue | |||
| Middle | 0.62 (0.50 to 0.77)c | 0.65 (0.53 to 0.81)c | 0.67 (0.54 to 0.84)c |
| High | 0.48 (0.36 to 0.65)c | 0.54 (0.40 to 0.72)c | 0.62 (0.46 to 0.84)c |
HR, hazard ratio.
Model 1: crude model. Model 2: adjusted for age group, sex, and race. Model 3: adjusted further for education level, income, smoking status, drinking status, body mass index, hypertension, diabetes, and annual dentist visits.
The lowest tertile was used as the reference group; the middle level corresponds to the second tertile; and the high level corresponds to the highest tertile.
P < 0.05, vs. reference group.
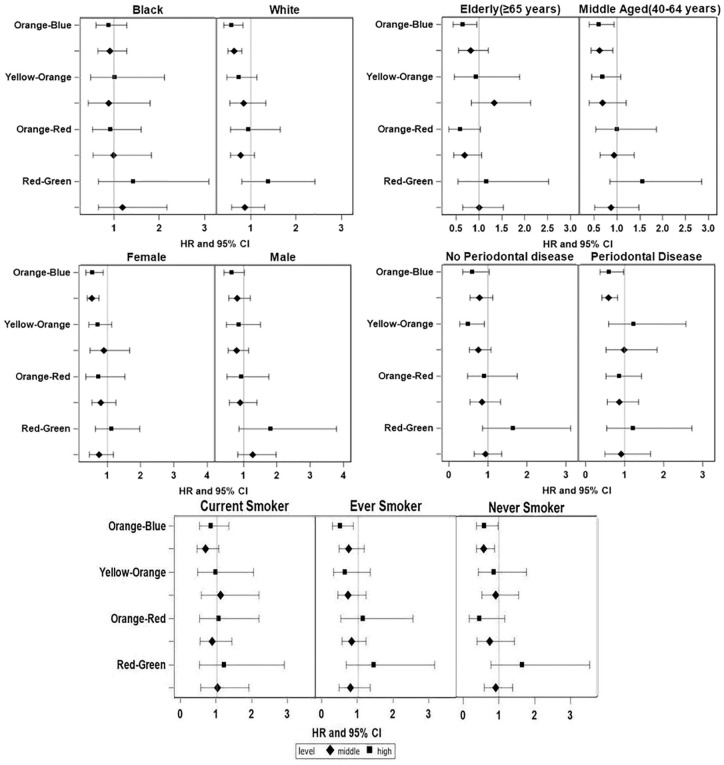
Subgroup analyses (Fig. 2) show results for cancer mortality with forest plots of hazards ratios and 95% CIs from fully adjusted models for each cluster level (lowest tertile reference). Though the trend of the results in the subgroups were similar to the overall findings, associations appeared stronger for middle-aged individuals, those with periodontal disease, Whites, ever and never smokers, and females. In the joint strata of age (40 to 64 vs. ≥65 y) by sex, the associations were qualitatively similar but stronger among the females and the younger age group (Appendix Fig.).
Figure 2.
Adjusted hazard ratios (95% CIs) of cancer mortality according to tertiles of 4 cluster scores by subgroups of age, race, sex, periodontal status, and smoking status. HR, hazard ratio.
Discussion
In the present study, we related empirically derived IgG antibody clusters against specific periodontal microorganisms with overall cancer mortality. In multivariable models, individuals with higher levels of orange-blue cluster scores were associated with lower cancer mortality, while the other 3 clusters (yellow-orange, red-green, and orange-red) were not related with cancer mortality. Consistent results were also observed for middle-aged people, Whites, females, and noncurrent smokers. The qualitatively similar but stronger associations among females and younger individuals in the joint strata of age and sex were likely driven by higher sample sizes in these groups.
The orange-blue cluster consisted of antibodies against E. nodatum and A. naeslundii. A. naeslundii has been linked to good oral health (Dewhirst et al. 2010; Desvarieux et al. 2013), with higher counts of this species being found in individuals without periodontal disease as compared with those with periodontitis (Papapanou et al. 2000). Although higher levels of the E. nodatum microorganism are detected in individuals with periodontal disease (Haffajee et al. 2006), higher antibody titers against E. nodatum have been found in individuals without periodontal disease (Socransky et al. 1998; Papapanou et al. 2000). Although this is a novel finding, the orange-blue cluster defined in the same way has been inversely related with other systemic conditions, such as hyperglycemia and elevated fasting glucose (Merchant et al. 2014; Shrestha et al. 2015), suggesting that the orange-blue cluster may represent a protective factor in the development of subsequent systemic diseases. The causal role of the orange-blue antibodies in the prevention of periodontal disease and its possible systemic effects needs to be established in future studies, and if the relation is determined to be causal, these antibodies could be a potentially novel target for intervention to improve oral and systemic health.
A few studies have related periodontal microorganisms to risks of developing cancer, indicating a microbial basis of the association between periodontal disease and cancer. Although we did not observe a significant association between the orange-red cluster and cancer mortality, elevated serum IgG antibody against P. gingivalis, a widely recognized periodontal pathogen belonging to this cluster, has been associated with higher risk of orodigestive cancer independent of periodontal disease (Ahn, Segers, and Hayes 2012). Similarly, results from a large European nested case-control study indicated that individuals with higher antibody titers against P. gingivalis had a twofold increased risk of developing pancreatic cancer, while higher antibodies to commensal oral bacteria were linked to a 45% lower risk of pancreatic cancer (Michaud et al. 2013), which is similar to our finding. Heavier loads of oral pathogens P. gingivalis and A. actinomycetemcomitans were related with an increased risk of pancreatic cancer in prospective cohort studies, pointing to a potential role of periodontal microorganisms in carcinogenesis, while an inverse association was observed between specific commensal microorganisms (phylum Fusobacteria and its genus Leptotrichia) and a decreased pancreatic cancer risk (Fan et al. 2016). Data derived from the same study also indicated that a greater abundance of genera Corynebacterium and Kingella, both of which are commensal microorganisms in the oral cavity, was associated with a decreased risk of head and neck squamous cell cancer (Hayes et al. 2018). It is thus plausible that individuals with oral microbial stability, periodontal health, and a strong immune response toward microorganisms may be less likely to develop cancer.
Although certain microorganisms and antibodies have been linked to periodontal disease and various subsequent systemic conditions, the role of periodontal microorganisms in carcinogenesis is not completely understood. However, several plausible hypotheses have been proposed to explain the association. Immune response is a plausible mediator in the causal pathway. It is estimated that infection-driven inflammations are involved in the pathogenesis of 15% of human tumors (Heikkila et al. 2018), while a great many studies report that periodontal disease leads to elevated levels of inflammatory markers, such as CRP and TNF-alpha, which were associated with various systemic conditions, including carcinogenesis (Meyer et al. 2008; Demmer et al. 2012). In addition, microorganisms associated with periodontal disease have been identified at distant sites, including plaque in the intimal wall of blood vessels and pancreas duct (Kozarov et al. 2005; Swidsinski et al. 2005). In a review of randomized controlled trials, periodontal treatment tended to reduce oral and systemic inflammation and counts of periodontal microorganisms (Merchant and Virani 2017). However, in poor oral health states, specific periodontal bacteria species have the capacity to produce nitrosamines and acetaldehyde; both chemicals are recognized human carcinogens that have been linked to oral cancer and certain upper gastrointestinal cancer types. Thus, increased exposure to these 2 carcinogenic metabolic by-products can result in a higher risk of developing cancer (Fitzpatrick and Katz 2010; Ahn, Chen, and Hayes 2012).
The study had several strengths. It was conducted in a representative US population; data collection was rigorous; and physical examinations and laboratory tests strictly adhered to standardized protocols to minimize measurement errors. In addition, it had a prospective design with a median follow-up of 15.91 y. Serum samples were collected before cancer diagnosis, making it possible to observe the temporal association between exposure variables and subsequent cancer risk; outcome ascertainment was valid and almost complete. Another advantage was the application of cluster analysis. The oral microbiome is complex, consisting of hundreds of microorganisms (Schenkein et al. 1993), which, if evaluated one at a time, would increase the chances of type 1 error because of multiple testing. Antibodies against 19 oral microorganisms that were well studied in relation to periodontal disease were assayed in NHANES III. The chances of false-positive findings were further reduced by grouping these antibodies with cluster analysis to define the groups as captured via their natural grouping in vivo. Finally, the association persisted in subgroups, including among never smokers.
However, our study has some limitations. First, antibodies against just 19 periodontal microorganisms were assessed in NHANES III. It is possible that the unmeasured antibodies were causal and were correlated with the 19 measured antibodies. Second, small numbers of cases limited our ability to evaluate the risk for specific cancers. Given strong associations between periodontitis and cancer type, particularly oral and pancreatic cancer, further studies are required to investigate these associations. The observed association may be underestimated because the outcome was cancer mortality rather than incidence. Increased screening and emerging treatments have increased survival from common cancers, such as those of the breast and colon, but similar trends have not been reported for pancreatic and oral cancers, which have been related to periodontal disease (Torre et al. 2016; Siegel et al. 2017). Third, we did not explicitly characterize the relation among antibodies against periodontal microorganisms, periodontal disease, and mortality. It is also possible that antibodies making up the groups had opposing actions, which could have attenuated the associations. However, in subgroup analyses, the associations between antibody clusters and mortality were similar among those with and without periodontal disease. Fourth, although several sociodemographic, anthropometric, and behavioral factors were adjusted for, several of which were self-reported, residual confounding may still exist.
In summary, we grouped IgG antibodies against 19 periodontal microorganisms using empirical methods and found that the orange-blue cluster, comprising E. nodatum and A. naeslundii, was inversely associated with overall cancer mortality. If further studies establish that higher antibodies against these microorganisms are protective against periodontal disease and its possible systemic effects and that this relationship is causal, then interventions to raise these antibodies may be possible.
Author Contributions
Z. Zhong, Q. Jin, contributed to data analysis and interpretation, drafted the manuscript; J. Zhang, contributed to design, data acquisition, and analysis, critically revised the manuscript; Y.M. Park, contributed to design, data analysis, and interpretation, critically revised the manuscript; D. Shrestha, contributed to design, data acquisition, and interpretation, critically revised the manuscript; J. Bai, contributed to data interpretation, critically revised the manuscript; A.T. Merchant, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_2380084419859484 for Serum IgG Antibodies against Periodontal Microbes and Cancer Mortality by Z. Zhong, Q. Jin, J. Zhang, Y.M. Park, D. Shrestha, J. Bai and A.T. Merchant in JDR Clinical & Translational Research
Footnotes
The authors received funding from the American Diabetes Association (1-13-MUl-08). This research was supported in part by the Intramural Program in the National Institutes of Health, National Institute of Environmental Health Sciences.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
ORCID iD: A.T. Merchant  https://orcid.org/0000-0003-1045-5079
https://orcid.org/0000-0003-1045-5079
References
- Ahn J, Chen CY, Hayes RB. 2012. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 23(3):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Segers S, Hayes RB. 2012. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 33(5):1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AP, Park YM, Shrestha D, Zhang J, Liu J, Merchant AT. 2018. Cross-sectional association of physical activity and periodontal antibodies. J Periodontol. 89(12):1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Harhay MO, Harhay MN. 2017. Anthropometrically-predicted visceral adipose tissue and mortality among men and women in the Third National Health and Nutrition Examination Survey (NHANES III). Am J Hum Biol [epub ahead of print 17 Jul 2016]. 29(1). doi: 10.1002/ajhb.22898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 1992. NHANES III: oral examination component; [accessed 2019 Jun]. https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/dental.pdf.
- Centers for Disease Control and Prevention. 2008. NHANES III: antibodies to periodontal pathogens; [accessed 2019 Jun]. https://wwwn.cdc.gov/nchs/data/nhanes3/30a/spsdeppx.pdf.
- Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, Desvarieux M. 2012. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 35(11):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. 2013. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J Am Heart Assoc. 2(6):e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance Workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G. 2016. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 67(1):120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SG, Katz J. 2010. The association between periodontal disease and cancer: a review of the literature. J Dent. 38(2):83–95. [DOI] [PubMed] [Google Scholar]
- Haffajee A, Teles R, Socransky S. 2006. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Mol Oral Microbiol. 21(5):269–282. [DOI] [PubMed] [Google Scholar]
- Hayes RB, Ahn J, Fan X, Peters BA, Ma Y, Yang L, Agalliu I, Burk RD, Ganly I, Purdue MP. 2018. Association of oral microbiome with risk for incident head and neck squamous cell cancer. JAMA Oncol. 4(3):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila P, But A, Sorsa T, Haukka J. 2018. Periodontitis and cancer mortality: register-based cohort study of 68,273 adults in 10-year follow-up. Int J Cancer. 142(11):2244–2253. [DOI] [PubMed] [Google Scholar]
- Hwang IM, Sun LM, Lin CL, Lee CF, Kao CH. 2014. Periodontal disease with treatment reduces subsequent cancer risks. QJM. 107(10):805–812. [DOI] [PubMed] [Google Scholar]
- Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Progulske-Fox A. 2005. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 25(3):e17–e18. [DOI] [PubMed] [Google Scholar]
- Mager DL, Haffajee AD, Devlin PM, Norris CM, Posner MR, Goodson JM. 2005. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med. 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AT, Park YM, Dodhia S, Shrestha D, Choi YH, Pitiphat W. 2016. Cross-sectional analysis of alcohol intake and serum antibodies to oral microorganisms. JDR Clin Trans Res. 2(2):168–178. [DOI] [PubMed] [Google Scholar]
- Merchant AT, Shrestha D, Chaisson C, Choi YH, Hazlett LJ, Zhang J. 2014. Association between serum antibodies to oral microorganisms and hyperglycemia in adults. J Dent Res. 93(8):752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant AT, Virani SS. 2017. Evaluating periodontal treatment to prevent cardiovascular disease: challenges and possible solutions. Curr Atheroscler Rep. 19(1):4. [DOI] [PubMed] [Google Scholar]
- Meyer MS, Joshipura K, Giovannucci E, Michaud DS. 2008. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 19(9):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjonneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, et al. 2013. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 62(12):1764–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. 1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat. 32:1–407. [PubMed] [Google Scholar]
- National Center for Health Statistics. 2013. Analytic guidelines for NCHS 2011 linked mortality files. 2013. Hyattsville (MD): Centers for Disease Control and Prevention; [accessed 2019 June]. https://www.cdc.gov/nchs/data/datalinkage/2011_linked_mortality_analytic_guidelines.pdf.
- Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlen G. 2004. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin Periodontol. 31(11):985–990. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Papadimitriou A, Sandros J, Dahlen G. 2000. “Checkerboard” assessments of periodontal microbiota and serum antibody responses: a case-control study. J Periodontol. 71(6):885–897. [DOI] [PubMed] [Google Scholar]
- Papapanou PN, Neiderud AM, Sandros J, Dahlén G. 2001. Checkerboard assessments of serum antibodies to oral microbiota as surrogate markers of clinical periodontal status. J Clin Periodontol. 28(1):103–106. [DOI] [PubMed] [Google Scholar]
- Rams TE, Listgarten MA, Slots J. 2006. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J Periodontal Res. 41(3):228–234. [DOI] [PubMed] [Google Scholar]
- Schenkein H, Burmeister J, Koertge T, Brooks C, Best A, Moore L, Moore W. 1993. The influence of race and gender on periodontal microflora. J Clin Periodontol. 64(4):292–296. [DOI] [PubMed] [Google Scholar]
- Shrestha D, Choi Y-H, Zhang J, Hazlett LJ, Merchant AT. 2015. Relationship between serologic markers of periodontal bacteria and metabolic syndrome and its components. J Periodontol. 86(3):418–430. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2017. Cancer statistics, 2017. CA Cancer J Clin. 67(1):7–30. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. 2002. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 28:12–55. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. 2005. Periodontal microbial ecology. Periodontol 2000. 38:135–187. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol. 25(2):134–144. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Bärlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U. 2005. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 54(3):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Siegel RL, Ward EM, Jemal A. 2016. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev. 25(1):16–27. [DOI] [PubMed] [Google Scholar]
- Vlachojannis C, Dye BA, Herrera-Abreu M, Pikdoken L, Lerche-Sehm J, Pretzl B, Celenti R, Papapanou PN. 2010. Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J Clin Periodontol. 37(8):685–696. [DOI] [PubMed] [Google Scholar]
- Wen BW, Tsai CS, Lin CL, Chang YJ, Lee CF, Hsu CH, Kao CH. 2014. Cancer risk among gingivitis and periodontitis patients: a nationwide cohort study. QJM. 107(4):283–290. [DOI] [PubMed] [Google Scholar]
- Zeng XT, Deng AP, Li C, Xia LY, Niu YM, Leng WD. 2013. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PLoS One. 8(10):e79017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_2380084419859484 for Serum IgG Antibodies against Periodontal Microbes and Cancer Mortality by Z. Zhong, Q. Jin, J. Zhang, Y.M. Park, D. Shrestha, J. Bai and A.T. Merchant in JDR Clinical & Translational Research