Abstract
Objective
To determine whether β-amyloidopathy correlates with apathy rating scores independently of mood changes and other neurodegenerative processes in Parkinson disease (PD).
Methods
In this cross-sectional study, patients with PD (n = 64, 48 male and 16 female, mean age 69.2 ± 6.7 years, Hoehn & Yahr stage 2.7 ± 0.5, Montreal Cognitive Assessment score 25.3 ± 3.0) underwent [11C]Pittsburgh compound B β-amyloid, [11C]dihydrotetrabenazine vesicular monoamine transporter type 2 (VMAT2), and [11C]methyl 4 piperidinyl propionate acetylcholinesterase brain PET imaging and clinical assessments, including the Marin Apathy Evaluation Scale, Clinician Version. Patients were recruited on the basis of having at least 1 risk factor for PD dementia, but they were excluded if they had dementia.
Results
Mean apathy rating score was 25.4 ± 6.4, reflecting predominantly subclinical apathy. Apathy rating scale scores correlated with amyloid binding, cognitive, depressive, and anxiety scores but not significantly with age, duration of disease, striatal VMAT2, or cholinergic binding. Multiple regression analysis model (p < 0.0001) showed significant regressor effects for global β-amyloid burden (p = 0.0038) with significant covariate effects for global cognitive z scores (p = 0.028) and for anxiety (p = 0.038) but not with depressive scores. Voxel-based analysis showed robust correlation between apathy rating scale scores and β-amyloid binding in bilateral nuclei accumbens, inferior frontal, and cingulate cortices (family-wise error rate–corrected p < 0.005).
Conclusion
Apathy is independently associated with β-amyloidopathy in patients with PD at risk of dementia. Regional brain findings are most robust for β-amyloidopathy in the nuclei accumbens, inferior frontal, and cingulate regions. Findings may provide an explanation for the often treatment-refractory nature of apathy in advancing PD despite optimized dopaminergic and antidepressant pharmacotherapy.
ClinicalTrials.gov identifier:
Apathy, or lack of motivation in completing goal-oriented activities, commonly occurs among patients with Parkinson disease (PD), with reported prevalence rates ranging from 23% to 70%.1 Apathy negatively affects quality of life and, in turn, increases caregiver burden and adds to overall disability.1,2 Apathy is distinct from depression and anhedonia and is thought to be due in large part to mesolimbic dopaminergic denervation and reduced integrity of associated prefrontal cortex network connections in PD.2,3
A recent meta-analysis showed that apathy is associated with older age, lower mean cognitive score, increased risk of comorbid depression, higher parkinsonian motor score, and more severe disability in PD.1,3,4 Although there is a clear dopaminergic contribution to the apathy syndrome of PD, there is also evidence that other pathophysiologies may play a role. Apathy is common not only in PD but also in other neurodegenerative disorders such as Alzheimer disease (AD) that generally lack nigrostriatal degeneration.5,6 This observation suggests that Alzheimer-type neuropathology such as amyloidopathy may also be a determinant of apathy.2 This notion is supported by in vivo PET imaging studies showing that patients with AD and apathy have higher cortical β-amyloid levels compared to those without apathy.7 Similar findings of increased cortical amyloidopathy and apathy are reported in patients with mild cognitive impairment.8 Amyloidopathy may also occur in PD and has been associated with worsening of cognitive impairment and dementia.9 It is possible that more severe amyloidopathy in PD may be associated with apathy in PD, at least in the subset of patients at higher risk of dementia. The objective of this study was to test the hypothesis that amyloidopathy is associated with apathy independently of mood disturbances, cognition, and other neurodegenerative pathologies in PD. We also assessed whether such hypothesized association may localize to amyloid plaque deposition in specific brain regions.
Methods
Patients
This was a cross-sectional study conducted from 2007 to 2013 in an academic movement disorder clinic setting. Patients of either sex were invited to enroll and provide written informed consent if they met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.10 Study inclusion was also based on the presence of at least 1 of the following risk factors for PD dementia: older age, subjective cognitive complaints, mild cognitive impairment, and imbalance.11–13 Patients were excluded if they showed no signs of nigrostriatal denervation on vesicular monoamine transporter type 2 (VMAT2) PET imaging or if they were on anticholinergic or cholinesterase inhibitor drugs. Other exclusion criteria included evidence of a stroke in a clinically relevant area (i.e., cerebral cortex, basal ganglia, thalamus) or mass lesion on MRI, contraindication for MRI, severe claustrophobia, previous participation in research procedures involving ionizing radiation, or positive pregnancy test or breastfeeding in women.
The Movement Disorder Society–revised Unified Parkinson Disease Rating Scale was assessed in the practically defined “off” state (i.e., the examination was performed in the morning after overnight withholding of dopaminergic medication).14
The Apathy Evaluation Scale clinician version was used to assess apathy severity.15 The Apathy Evaluation Scale has been validated in PD.16 The Beck Depression Inventory II was used to rate depressive symptoms,17 and the Spielberger18 State Trait Anxiety Inventory was used to rate trait anxiety.
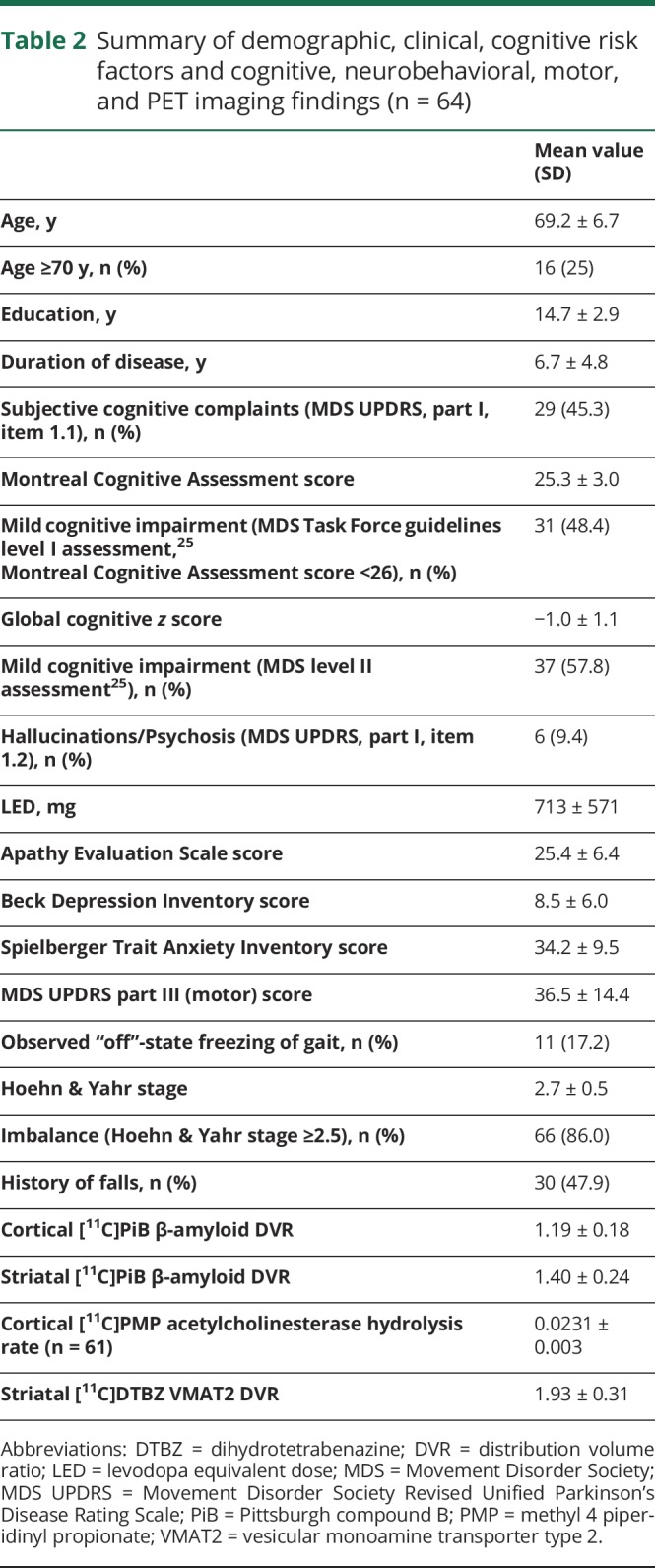
Each participant underwent a detailed cognitive examination (while on dopaminergic medications) following the approach previously reported for cognitive impairment in PD.19 These tests included measures of verbal memory with the California Verbal Learning Test20; executive/reasoning functions with the Wechsler Adult Intelligence Scale III Picture Arrangement test21 and Delis Kaplan Executive Function System Sorting Test22; attention/psychomotor speed as absolute time on the Stroop naming test23; and visuospatial function with the Benton Judgment of Line Orientation test.24 A z score for every individual on every test was calculated on the basis of a dataset of a healthy elderly control group of similar age, sex, and educational level distribution. A z score for each cognitive domain was obtained by averaging all z scores on the tests or subtasks. A global cognitive Z score was computed as the average of all different domains. Details of the cognitive test scores in the patients and control persons can be found in table 1. After completion of detailed neuropsychological testing and assessment of functional abilities, 4 patients met criteria for mild dementia on the basis of abnormal cognitive test scores and impaired instrumental activities of daily living.25–27
Table 1.
Demographic and cognitive characteristics of normal control vs participants with PD
Cognitive and motor correlates of β-amyloidopathy of subsets of the patients from the present study have been reported previously.28–31
Standard protocol approvals, registrations, and patient consents
This study (ClinicalTrials.gov identifier: NCT01565473) was approved by and study procedures were followed in accordance with the ethical standards of the Institutional Review Board of the University of Michigan. Written informed consent was obtained before any study procedures from all patients.
Imaging techniques
MRI was performed on a 3T Philips Achieva system (Philips, Best, the Netherlands), and PET imaging was performed in 3D imaging mode with an ECAT Exact HR+ tomograph (Siemens Molecular Imaging, Inc, Knoxville, TN), as previously reported.32
[11C]Pittsburgh compound B (PiB) β-amyloid, [11C]dihydrotetrabenazine (DTBZ) VMAT2, and [11C]methyl 4 piperidinyl propionate (PMP) acetylcholinesterase were prepared as described previously.33,34 Bolus/infusion protocols were used for [11C]DTBZ (15 mCi) in 60 minutes and for [11C]PiB (18 mCi) in 70 minutes.35 Dynamic PET scanning was performed for 70 minutes with a bolus dose of 15 mCi [11C]PMP. [11C]PMP PET scans were not available in 2 patients.
Image analysis
Imaging registration and volume-of-interest definition were performed as previously reported.32 MRI-based partial volume correction was performed.36 Cortical acetylcholinesterase [11C]PMP hydrolysis rates (k3) were estimated with the striatal volume as the tissue reference.37
[11C]PiB and [11C]DTBZ distribution volume ratios (DVRs) were estimated with the Logan plot graphical analysis method using cerebellar cortical and supratentorial cortical reference tissues, respectively.38 Striatal [11C]DTBZ, striatal [11C]PiB, and global cortical [11C]PiB DVRs were calculated. We computed percentage scores of cortical and striatal PiB DVRs using the mean PiB DVR from healthy middle-aged controls and summed the cortical and striatal PiB DVR percentage scores to derive a global brain PiB DVR measure, as previously reported.31
Data analysis: Global β-amyloid burden analysis
Bivariate Pearson correlation coefficients were computed for the relationship between apathy rating scores and the clinical, cognitive, neurobehavioral, motor, and global β-amyloid burden and other PET imaging variables. Multivariable linear regression analysis was then used to evaluate the association between global brain β-amyloidopathy and apathy while accounting for confounder variables that were significantly associated with apathy rating scale score in the bivariate correlation analysis.
Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC).
Regional brain voxel-based β-amyloid analysis
Voxel-based [11C]PiB PET analyses were performed to explore regional cerebral β-amyloid binding correlates of apathy rating scale scores. With the use of statistical parametric mapping software (SPM12, Wellcome Department of Cognitive Neurology, London, UK), whole-brain voxel-based correlation was analyzed to assess the relationship between DVRs and apathy rating scores. The same confounder variables as in the volume of interest–based multiple regression model were used for the voxel-based model. The results were considered statistical significant at the peak level threshold p < 0.001 and corrected for multiple comparisons on the voxel level (family-wise error–corrected p < 0.005), in conjunction with a cluster threshold of 20 voxels. The significant clusters were overlaid with MRIcroGL (mccauslandcenter.sc.edu/mricrogl/) onto the PiB PET template derived as a mean of images of all patients.
Data availability
Anonymized data will be shared by request from any qualified investigator.
Results
This study involved 64 patients with PD (48 male, 16 female) with a mean age of 69.2 ± 6.7 years and mean duration of motor disease of 6.7 ± 4.8 years. Patients with PD met the UK Parkinson's Disease Society Brain Bank clinical diagnostic criteria.10 All but 1 of the patients were on dopaminergic drugs; none were on anticholinergic or cholinergic drugs. Twenty-four patients were taking a combination of dopamine agonist and carbidopa levodopa medications; 29 were using carbidopa levodopa alone; and 10 were taking a dopamine agonist alone. Mean levodopa equivalent dose was 713 ± 571 mg.39 Most patients had moderate severity of disease: 2 patients were in modified Hoehn and Yahr stage 1.5, 7 were in stage 2, 27 were in stage 2.5, 25 were in stage 3, and 3 were in stage 4.40 The mean Movement Disorder Society–revised Unified Parkinson Disease Rating Scale part III motor score was 36.5 ± 14.4. The mean Montreal Cognitive Assessment test score was 25.3 ± 3.0.41
Table 2 provides a summary of the clinical, cognitive, neurobehavioral, and PET imaging findings. Mean apathy rating score was 25.4 ± 6.4, reflecting predominantly subclinical apathy in the study population. Table 2 also lists the frequency of risk factors for PD dementia, including presence of subjective cognitive complaints, presence of mild cognitive impairment, older age, and presence of postural instability and gait difficulties motor features. All participants had at least 1 of these risk factors.
Table 2.
Summary of demographic, clinical, cognitive risk factors and cognitive, neurobehavioral, motor, and PET imaging findings (n = 64)
Clinical and imaging correlates of apathy rating scale scores
Bivariate correlation coefficients between apathy rating scores and the cognitive, neurobehavioral, motor, and PET imaging variables are shown in table 3. Higher ratings of apathy scores correlated with greater cognitive deficits, higher depression scores, anxiety ratings, and more severe global β-amyloid burden.
Table 3.
Bivariate correlation coefficients between apathy rating scores and cognitive, neurobehavioral, motor, and PET imaging variables (n = 64)
Multivariate confounder analysis of main voxel of interest–based cortical β-amyloid PET analysis
Multiple regression analysis using the apathy rating scale score as the outcome parameter was then used to evaluate the specificity of the association between apathy rating scores (dependent variable) and global brain β-amyloidopathy burden while accounting for confounder variables (independent variables). The significant confounder variables that were used simultaneously for the model were global cognitive z score, Beck Depression Inventory score, and Spielberger Trait Anxiety score, as shown in table 3. Regression diagnostics, including tests of homogeneity of variance, multicollinearity testing, and normality of residuals, were performed and model assumptions were met. The analysis showed a significant model (F4,59 = 13.0, p < 0.0001) with significant regressor effects for global β-amyloid burden (standardized β = 0.30, 95% confidence interval [CI] 0.05–0.23, t = 3.0, p = 0.0038) with significant covariate effects for global cognitive z score (β = 0.23, 95% CI 2.6–0.2, t = 2.3, p = 0.028), and anxiety ratings (β = 0.31, 95% CI 0.01–0.41, t = 2.1, p = 0.038).
Regional brain voxel-based β-amyloid PET analysis
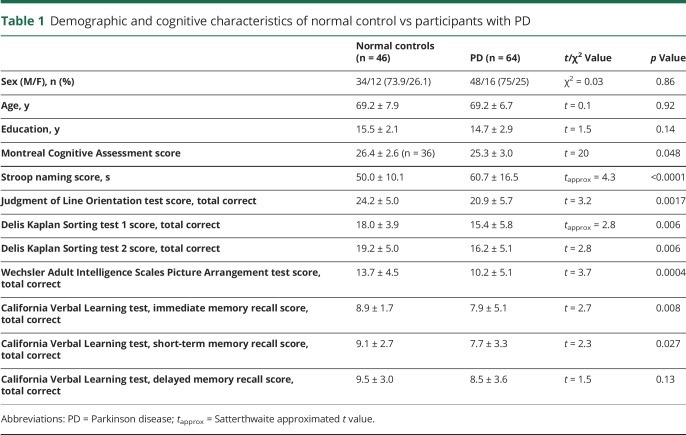
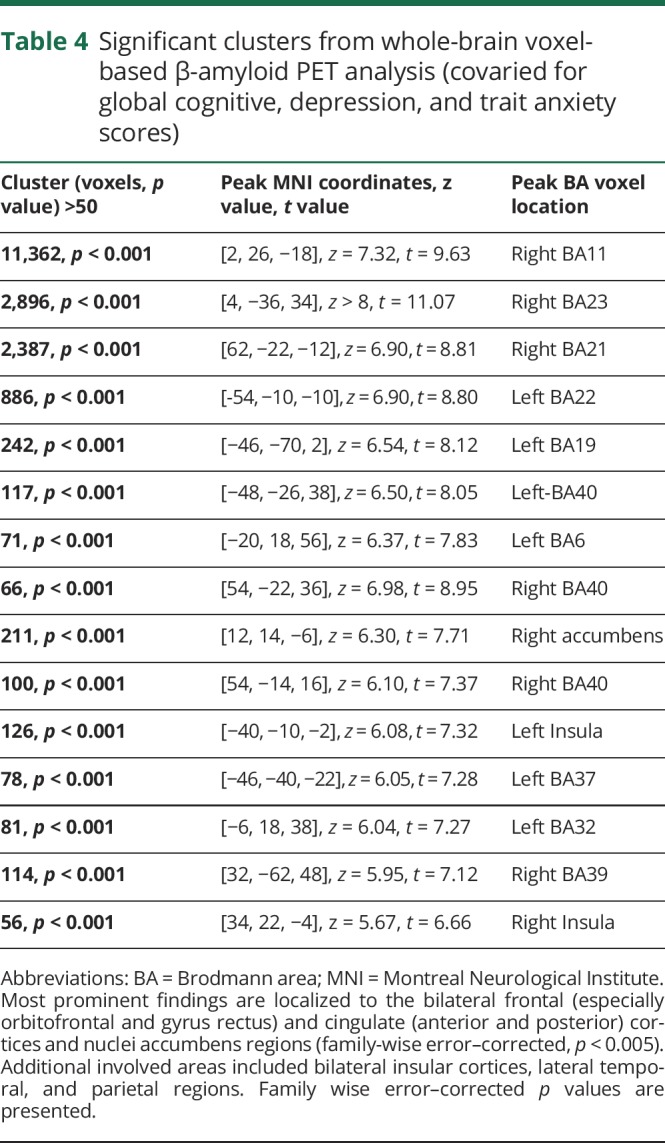
Voxel-based regression analysis was performed with apathy rating scale scores as the dependent parameter and β-amyloid binding as the independent parameter while adjusting for the same confounding variables used in the voxel of interest–based model (i.e., global cognitive z score, Beck Depression Inventory score, and Spielberger Trait Anxiety score). The voxel-based analysis showed regionally significant correlations between the apathy rating scale scores and amyloid binding in the bilateral frontal (especially the orbitofrontal cortices and gyri rectus), cingulate (anterior and posterior) cortices, and nuclei accumbens regions (family-wise error–corrected p < 0.005). Additional involved areas included bilateral insular cortices and lateral temporal and parietal regions (figure and table 4).
Figure. Significant clusters of positive correlation between regional β-amyloid deposition and apathy rating scores (covaried for cognitive, depression, and trait anxiety rating) depicted in fire color.
The most prominent findings localized to the bilateral frontal (especially orbitofrontal and gyrus rectus) and cingulate (anterior and posterior) cortices and nuclei accumbens regions (family-wise error–corrected p < 0.005). Additional involved areas included bilateral insular cortices and lateral temporal regions.
Table 4.
Significant clusters from whole-brain voxel-based β-amyloid PET analysis (covaried for global cognitive, depression, and trait anxiety scores)
Discussion
Our analyses suggest that cerebral amyloidopathy, notably in limbostriatal, limbofrontal, and cingulate regions, is a strong correlate of apathy ratings in PD. Because dopaminergic losses are already prominent in early PD, progressive apathy with worsening disease may be driven by nondopaminergic mechanisms in PD. These mechanisms may also contribute to cognitive decline. Our multivariate analysis shows that amyloidopathy is independently associated with apathy ratings even after accounting for confounders, including cognitive, depression, and anxiety rating scores. These observations support the hypothesis that apathy, cognitive, and mood disturbances are partially distinct disorders.42 Our findings showed an independent regressor effect for cognitive functions in the statistical model. Although apathy can occur independently of cognitive impairment, overlap is common.43 The partial overlap between apathy and cognition may relate to our observation that apathy ratings are associated with posterior cingulum amyloidopathy. Similarly, a voxel-based MRI morphometry study found evidence of atrophy in the posterior cingulum in patients with PD with apathy.44 We also observed independent regressor effects between the anxiety trait rating and apathy rating scale scores. The instruction for the trait anxiety scale was based on a more general assessment of how somebody feels, and this may likely reflect a component of more recent state-dependent anxiety ratings. Future research is needed to disentangle the complex interrelationships among apathy and cognitive and mood disturbances in PD in appropriately powered study samples.
We did not observe an association between striatal VMAT2 changes and apathy in our study. Our PD population was selected on the basis of an increased risk of dementia, i.e., older age, imbalance, and cognitive complaints. The relatively more advanced stage of PD in our study population may result in a dopaminergic denervation “floor” effect, thereby attenuating possible statistical associations. In contrast, other studies in drug-naive patients with early-stage PD have shown cumulative evidence for anteroventral striatal and extrastriatal dopaminergic changes associated with apathy.2 This correlates to findings in MPTP-lesioned monkeys in which postlesioning apathetic behavior was associated with extrastriatal dopaminergic changes in the ventral tegmental area insular cortical pathways.45
Using PiB PET and voxel-based whole-brain analysis, we performed an exploratory analysis on the regional brain correlation between β-amyloid deposition and the severity of apathy in PD. The results showed that β-amyloid deposition had significant positive correlation with apathy scores in the predominant limbic striatal and limbofrontal regions. It has been suggested that dysfunction of reward-related circuits that connect the orbitofrontal cortex, ventromedial prefrontal cortex, anterior cingulum, and nucleus accumbens may contribute to the presence of apathy in PD.2 A relationship between apathy and β-amyloid deposition in regions of bilateral frontal, temporal, and right anterior cingulum has also been reported in patients with AD.7 This cortical deposition pattern is consistent with our findings, implicating a possible common neural pathway for apathy in both PD and AD, at least in advancing disease stages. In addition to cortical regions, we found an association of amyloidopathy with apathy in the bilateral nuclei accumbens. The results of this granular analysis are consistent with prior work associating anteroventral striatal changes with apathy. The ventral striatum is a key node in motivated behavior, and there are several possible explanations for the effects of β-amyloid deposition in this region. Using [11C]DASB PET to image serotonin transporters, a recent study showed that apathetic patients with PD had greater serotonergic terminal reductions in the ventral striatum.46 Our previous research showed evidence of an inverse relationship between amyloidopathy and loss of serotonin nerve terminals in PD, especially in the striatum.47 Therefore, some β-amyloid deposition effects on apathy might be mediated by local serotoninergic deficits.
In addition to the frontostriatal circuits commonly associated with apathy, our study found significant β-amyloidopathy in other regions, including the temporal and insular cortices. Similar cortical changes extending beyond the frontostriatal circuits have been observed in structural whole-brain MRI morphometric studies.44,48
Coincident AD pathology is regionally associated with increased Lewy body pathology in PD, indicating an interactive effect between these 2 proteinopathies.49 A possible interactive vs common driver effect is also supported by a postmortem study showing that the APOE ε4 genotype is independently associated with a greater severity of Lewy body pathology independently of Alzheimer pathology in patients with Lewy body disorders.50 It is possible that the increased PiB PET binding signal in our study may preferentially reflect sites of parallel occurrence of regional proteinopathies, including Lewy body pathology, rather than exclusively representing amyloid plaques per se.
A limitation of our study was that it did not include patients with more severe apathy. This is likely due to selection bias because patients with significant dementia were not eligible for the study. Another selection bias may be that patients with more severe apathy may be less motivated to volunteer for a multimodal imaging research study. Therefore, the average apathy rating scale scores in this study reflect a PD population with subclinical or borderline apathy ratings.
Our findings may explain the relatively greater severity of apathy in patients with dementia with Lewy bodies, who tend to have greater deposition of β-amyloid plaques compared to patients with PD or PD with dementia.9 Our study, however, was limited by the exclusion of patients with dementia with Lewy bodies to confirm this hypothesized correlation. Nevertheless, these data reveal an association between amyloidopathy and apathy ratings that is independent of cognition, depression, and anxiety. This has implications for amyloid-based therapeutic strategies in alleviating not only cognitive but also apathetic aspects of PD. These results may also provide an explanation for the often treatment-refractory nature of apathy in advancing PD despite optimized dopaminergic or antidepressant pharmacotherapy.
Glossary
- AD
Alzheimer disease
- CI
confidence interval
- DTBZ
dihydrotetrabenazine
- DVR
distribution volume ratio
- FWE
family-wise error rate
- PD
Parkinson disease
- PiB
Pittsburgh compound B
- PMP
methyl 4 piperidinyl propionate
- VMAT2
vesicular monoamine transporter type 2
Appendix. Authors
Study funding
This work was supported by the Department of Veterans Affairs (I01 RX000317), the Michael J. Fox Foundation, and the NIH (grants P01 NS015655, P50 NS091856, and R01 NS070856).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E. Apathy in Parkinson's disease: a systematic review and meta analysis. Mov Disord 2015;30:759–769. [DOI] [PubMed] [Google Scholar]
- 2.Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson's disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol 2015;14:518–531. [DOI] [PubMed] [Google Scholar]
- 3.Sierra M, Carnicella S, Strafella AP, et al. Apathy and impulse control disorders: yin & yang of dopamine dependent behaviors. J Parkinsons Dis 2015;5:625–636. [DOI] [PubMed] [Google Scholar]
- 4.Lee WJ, Tsai CF, Gauthier S, Wang SJ, Fuh JL. The association between cognitive impairment and neuropsychiatric symptoms in patients with Parkinson's disease dementia. Int Psychogeriatr 2012;24:1980–1987. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki M, Desmond TJ, Albin RL, Frey KA. Striatal monoaminergic terminals in Lewy body and Alzheimer's dementias. Ann Neurol 2002;51:767–771. [DOI] [PubMed] [Google Scholar]
- 6.Tokuchi R, Hishikawa N, Sato K, et al. Differences between the behavioral and psychological symptoms of Alzheimer's disease and Parkinson's disease. J Neurol Sci 2016;369:278–282. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Shimada H, Shinotoh H, et al. Apathy correlates with prefrontal amyloid beta deposition in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2014;85:449–455. [DOI] [PubMed] [Google Scholar]
- 8.Marshall GA, Donovan NJ, Lorius N, et al. Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci 2013;25:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey KA, Petrou M. Imaging amyloidopathy in Parkinson disease and parkinsonian dementia syndromes. Clin Transl Imaging 2015;3:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves G, Larsen JP, Emre M, Wentzel Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson's disease. Mov Disord 2006;21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 12.Anang JB, Gagnon JF, Bertrand JA, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology 2014;83:1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogland J, Boel JA, de Bie RMA, et al. Risk of Parkinson's disease dementia related to level I MDS PD MCI. Mov Disord 2019;34:430–435. [DOI] [PubMed] [Google Scholar]
- 14.Goetz CG, Fahn S, Martinez Martin P, et al. Movement Disorder Society sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 15.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 16.Santangelo G, Barone P, Cuoco S, et al. Apathy in untreated, de novo patients with Parkinson's disease: validation study of Apathy Evaluation Scale. J Neurol 2014;261:2319–2328. [DOI] [PubMed] [Google Scholar]
- 17.Beck AT, Brown G, Steer RA. Beck Depression Inventory II Manual. San Antonio: Psychological Corp.; 1996. [Google Scholar]
- 18.Spielberger CD. The measurement of state and trait anxiety: conceptual and methodological issues. In: Levi L, editor. Emotions: Their Parameters and Measurement. New York: Raven Press; 1975:713–725. [Google Scholar]
- 19.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009;72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 20.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual, Adult Version. 2nd ed. San Antonio: Psychological Corp; 2000. [Google Scholar]
- 21.Wechsler D. WAIS III Technical Manual. San Antonio: Psychological Corp; 1997. [Google Scholar]
- 22.Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System (D KEFS): Examiner's Manual. San Antonio: Psychological Corp; 2001. [Google Scholar]
- 23.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662. [Google Scholar]
- 24.Benton AL, Varney NR, Hamsher K. Judgment of Line Orientation, Form V. Iowa City: University of Iowa Hospitals; 1975. [Google Scholar]
- 25.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 27.Lawton MP, Brody EM. Assessment of older people: self maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 28.Petrou M, Bohnen NI, Muller ML, Koeppe RA, Albin RL, Frey KA. Abeta amyloid deposition in patients with Parkinson disease at risk for development of dementia. Neurology 2012;79:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller ML, Frey KA, Petrou M, et al. β Amyloid and postural instability and gait difficulty in Parkinson's disease at risk for dementia. Mov Disord 2013;28:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohnen NI, Frey KA, Studenski S, et al. Extra nigral pathological conditions are common in Parkinson's disease with freezing of gait: an in vivo positron emission tomography study. Mov Disord 2014;29:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah N, Frey KA, Muller MLTM, et al. Striatal and cortical beta amyloidopathy and cognition in Parkinson's disease. Mov Disord 2016;31:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bohnen NI, Muller MLTM, Kotagal V, et al. Heterogeneity of cholinergic denervation in Parkinson's disease without dementia. J Cereb Blood Flow Metab 2012;32:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao X, Hoareau R, Hockley BG, et al. Highlighting the versatility of the Tracerlab synthesis modules, part 1: fully automated production of [18F]labelled radiopharmaceuticals using a Tracerlab FXFN. J Labelled Comp Radiopharm 2011;54:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klunk WE, Engler E, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease using the novel positron emission tomography tracer, Pittsburgh compound B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 35.Koeppe RA, Frey KA, Kume A, Albin R, Kilbourn MR, Kuhl DE. Equilibrium versus compartmental analysis for assessment of the vesicular monoamine transporter using (+) alpha [11C]dihydrotetrabenazine (DTBZ) and positron emission tomography. J Cereb Blood flow Metab 1997;17:919–931. [DOI] [PubMed] [Google Scholar]
- 36.Muller Gartner H, Links J, Prince J, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI based correction of partial volume effects. J Cereb Blood flow Metab 1992;12:571–583. [DOI] [PubMed] [Google Scholar]
- 37.Nagatsuka S, Fukushi K, Shinotoh H, et al. Kinetic analysis of [(11)C]MP4A using a high radioactivity brain region that represents an integrated input function for measurement of cerebral acetylcholinesterase activity without arterial blood sampling. J Cereb Blood flow Metab 2001;21:1354–1366. [DOI] [PubMed] [Google Scholar]
- 38.Logan J, Fowler JS, Volkow ND, Ding YS, Wang GJ, Alexoff DL. A strategy for removing the bias in the graphical analysis method. J Cereb Blood flow Metab 2001;21:307–320. [DOI] [PubMed] [Google Scholar]
- 39.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 40.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 2004;19:1020–1028. [DOI] [PubMed] [Google Scholar]
- 41.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 42.Kirsch Darrow L, Fernandez HF, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology 2006;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weintraub D, Mamikonyan E. The neuropsychiatry of Parkinson disease: a perfect storm. Am J Geriatr Psychiatry 2019;27:998–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF. Neuroanatomical correlates of apathy in Parkinson's disease: a magnetic resonance imaging study using voxel based morphometry. Mov Disord 2010;25:2318–2325. [DOI] [PubMed] [Google Scholar]
- 45.Tian L, Xia Y, Flores HP, Campbell MC, Moerlein SM, Perlmutter JS. Neuroimaging analysis of the dopamine basis for apathetic behaviors in an MPTP lesioned primate model. PLoS One 2015;10:e0132064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maillet A, Krack P, Lhommee E, et al. The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson's disease. Brain 2016;139:2486–2502. [DOI] [PubMed] [Google Scholar]
- 47.Kotagal V, Bohnen NI, Müller MLTM, Koeppe RA, Frey KA, Albin RL. Cerebral amyloid deposition correlates inversely with serotoninergic innervation in Parkinson disease. Arch Neurol 2012;69:1628–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alzahrani H, Antonini A, Venneri A. Apathy in mild Parkinson's disease: neuropsychological and neuroimaging evidence. J Parkinsons Dis 2016;6:821–832. [DOI] [PubMed] [Google Scholar]
- 49.Toledo JB, Gopal P, Raible K, et al. Pathological alpha synuclein distribution in subjects with coincident Alzheimer's and Lewy body pathology. Acta Neuropathol 2016;131:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dickson DW, Heckman MG, Murray ME, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018;91:e1182–e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.