Abstract
Objective
We conducted a nationwide case-control study in Sweden to test the hypothesis that specific clinical characteristics are associated with increased risk of sudden unexpected death in epilepsy (SUDEP).
Methods
The study included 255 SUDEP cases (definite and probable) and 1,148 matched controls. Clinical information was obtained from medical records and the National Patient Register. The association between SUDEP and potential risk factors was assessed by odds ratios (ORs) and 95% confidence intervals (CIs) and interaction assessed by attributable proportion due to interaction (AP).
Results
Experiencing generalized tonic-clonic seizures (GTCS) during the preceding year was associated with a 27-fold increased risk (OR 26.81, 95% CI 14.86–48.38), whereas no excess risk was seen in those with exclusively non-GTCS seizures (OR 1.15, 95% CI 0.54–48.38). The presence of nocturnal GTCS during the last year of observation was associated with a 15-fold risk (OR 15.31, 95% CI 9.57–24.47). Living alone was associated with a 5-fold increased risk of SUDEP (OR 5.01, 95% CI 2.93–8.57) and interaction analysis showed that the combination of not sharing a bedroom and having GTCS conferred an OR of 67.10 (95% CI 29.66–151.88), with AP estimated at 0.69 (CI 0.53–0.85). Among comorbid diseases, a previous diagnosis of substance abuse or alcohol dependence was associated with excess risk of SUDEP.
Conclusions
Individuals with GTCS who sleep alone have a dramatically increased SUDEP risk. Our results indicate that 69% of SUDEP cases in patients who have GTCS and live alone could be prevented if the patients were not unattended at night or were free from GTCS.
Sudden unexpected death in epilepsy (SUDEP) is the most important epilepsy-related cause of death, ranking second only to stroke among neurologic diseases in terms of potential years of life lost.1 Several case-control studies have attempted to identify risk factors for SUDEP2–5 to provide a basis for an individualized risk assessment. By pooling data from 4 such studies, frequency of generalized tonic-clonic seizures (GTCS) in particular, but also the duration of epilepsy, young age at epilepsy onset, and male sex, were identified as risk factors.6 However, a recent systematic review concluded that the frequency of GTCS was the only risk factor identified with a high level of confidence, whereas, e.g., lack of nighttime supervision and absence of nocturnal listening device were risk factors with moderate confidence.7 Other risk factors, including young age at epilepsy onset, long duration of epilepsy, focal epilepsy, and intellectual disability, have been proposed in individual studies,8 but the evidence was considered low in the systematic review.7 The uncertainty can be attributed to methodologic limitations such as small numbers and selected study populations affecting generalizability.2–5 Differences in definitions of potential risk factors have also hampered pooling of data.6,7 To guide patient counseling and for the development of effective SUDEP preventions, there is still need for large, high-quality studies to elucidate SUDEP risk factors.7 Therefore, we analyzed the risk of SUDEP in relation to a range of potential risk factors in a large, nationwide population-based case-control study in Sweden utilizing data from individual medical records and national registries.
Methods
SUDEP definition and classification
SUDEP is defined as sudden, unexpected, witnessed or unwitnessed, nontraumatic, and nondrowning death of patients with epilepsy with or without evidence of a seizure, excluding documented status epilepticus, and in whom postmortem examination does not reveal a structural or toxicologic cause for death.9 In the present study, we classified SUDEP cases according to Anneger10 criteria. This classification was selected to facilitate comparison since it has been used in most previous studies.2–5 SUDEP cases were divided into 3 subgroups based on the certainty of the diagnosis: (1) definite SUDEP when all clinical criteria are met and an autopsy is performed that reveals no alternative cause of death; (2) probable SUDEP when all clinical criteria are met but no autopsy is performed; and (3) possible SUDEP when SUDEP cannot be ruled out, but there is insufficient evidence regarding the circumstances of the death and no autopsy is performed.10
Study population
The Swedish National Patient Register (SNPR) contains all patients hospitalized (starting in 1968, with total national coverage from 1987) or managed in hospital-based ambulatory care (since 2001) in Sweden.11 Each individual's outpatient visit or hospital discharge diagnosis (ICD code) is linked with a unique personal identification number. We identified all persons who at some point during 1998–2005 were registered in the SNPR with an ICD-10 code for epilepsy (G40) (n = 78,424) and alive on June 30, 2006 (n = 60,952). This constituted our study population.
Cases
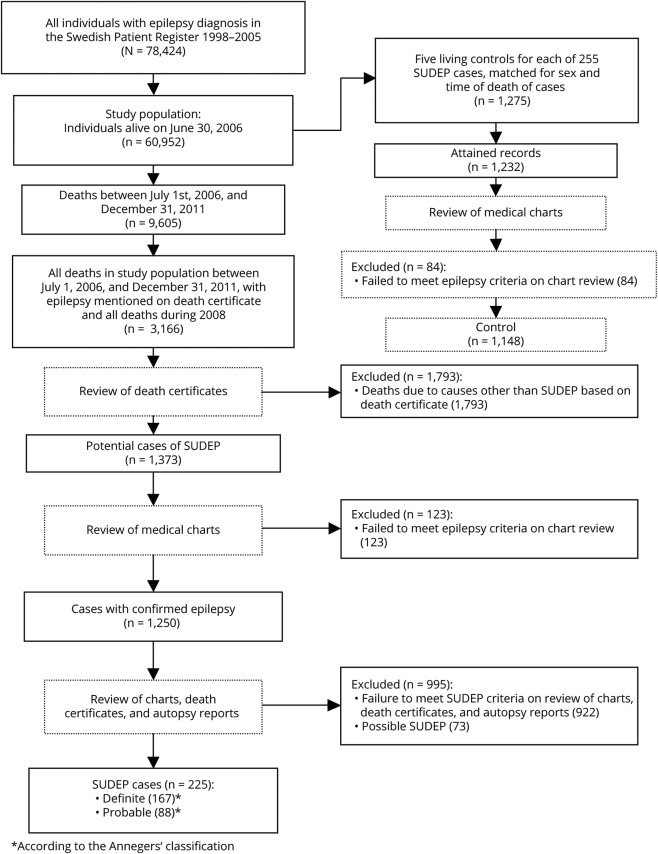
During follow-up from July 1, 2006, to December 31, 2011, 9,605 deaths were identified by linkage to the National Cause of Death Registry (ICD-10 classified since 1994).12 Eligible SUDEP cases were all deaths with epilepsy mentioned on the death certificate (n = 1,276), together will all individuals who died during 2008 (n = 1,890) (figure 1). We previously conducted a study of the incidence of SUDEP during 2008,13 which is why all deaths in the study population were reviewed that particular year. All death certificates were reviewed by one neurologist (O.S.). Obvious non-SUDEP deaths such as cancer, terminal illness, postmortem confirmed pneumonia, stroke, or myocardial infarction were excluded from further analysis based on the information in the death certificates (figure 1). This process considered all information on the death certificate, postmortem results, and whether the patient died in the hospital. For the remaining cases, where SUDEP could potentially be the cause of death (n = 1,373), patient records from family physicians, hospital records, nursing homes or other institutions, police records, and autopsy records were reviewed (O.S.) and all information was extracted using a standardized protocol. Emphasis was on attaining the doctor's or police report regarding circumstances surrounding the death, including documented interviews with eyewitnesses, caregivers, and relatives. All information was reviewed by 2 neurologists (O.S. and T.T.) and classification of the cases was made through consensus. From patient records, we determined if the patients met the criteria for a diagnosis of epilepsy according to the definition of the International League Against Epilepsy.14 In the end, 255 definite (n = 167) and probable (n = 88) cases according to the Anneger classification were found and served as cases for this study (figure 1). Possible SUDEP cases (n = 73) were not used in this study.
Figure 1. Flow chart describing the selection process.
SUDEP = sudden unexpected death in epilepsy.
Controls
From the study population, the National Board of Health and Welfare randomly selected 5 epilepsy controls (n = 1,275) for each person with SUDEP, of the same sex, who were alive at the case's time of death, which served as an index date for the controls. For these controls, we requested patient records from caregivers across the country and attained records for 1,232 (97%) individuals. Of these, 84 (6.8%) were judged not to have epilepsy. This left 1,148 individuals, who served as controls in the present study (figure 1).
Information from patient records
For all cases and controls, we used patient records to collect information on age, sex, and living condition (living alone or with others, including parents, partners, children, and siblings, and if sharing a bedroom). If cases or controls were married or had a partner, they were classified as sharing a bedroom, if not otherwise explicitly stated. Further information was collected on epilepsy onset, duration of epilepsy, type of epilepsy, etiology,15 history of tonic-clonic seizures (in this context including both generalized tonic-clonic seizures and focal to bilateral tonic-clonic seizures in accordance with most previous case-control studies of SUDEP),14 presence and frequency of tonic-clonic nocturnal seizures during the last year of observation, presence of other seizures during the last year of observation, history of nocturnal seizures, history of tonic-clonic nocturnal seizures, presence of tonic-clonic nocturnal seizures during the last year of observation, intellectual disability, antiepileptic drug (AED) treatment, and whether the patient had undergone epilepsy surgery or had ongoing treatment with vagus nerve stimulation (VNS).
Information from national registries
Information on psychiatric comorbidity, pulmonary disease, and cardiovascular disease was obtained from ICD codes in the national patient registry (from 1997 to death or index date). From the longitudinal integration database for health insurance and labor market studies (LISA), which holds annual registers since 1990 and includes all individuals 16–74 years of age, information on highest educational level was attained.16 In the LISA registry, this information is recorded as missing for individuals below 16 years and for those who did not attend regular school due to intellectual disability.
Statistics
Characteristics were expressed as mean (range) or proportion. The association between SUDEP and potential risk factors was estimated by odds ratios (ORs) with 95% confidence intervals (CIs) calculated by conditional logistic regression to account for matching by sex and calendar time. As the control participants were sampled with an incidence density method, the ORs can be interpreted as incidence rate ratios.17 In model 1, OR was adjusted for age and sex (matching variable). Model 2 included additional adjustments for GTCS frequency and model 3 included the same covariates as model 2 together with nocturnal GTCS last year of observation, living conditions, and AEDs. In the Results, all results are presented from model 3 unless stated otherwise. Interaction between GTCS during last year of observation (yes/no) and sharing a bedroom (yes/no), defined as departure from additivity of effects, was assessed with the proportion attributable to interaction (AP).18 The formula for AP is (OR11 − OR10 − OR01 + 1)/OR11, where OR11 indicates doubly exposed (having GTCS and sleeping alone) and OR01 or OR10 indicate either exposure (sleeping alone or having GTCS). The reference group is those with neither exposure and the ORs were adjusted for age and sex (matching variable). Statistical Analysis Software (SAS) 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Ethics Committee of Karolinska Institutet, which granted that individual informed consent was not needed.
Data availability
Anonymized data will be shared by request from qualified investigators.
Results
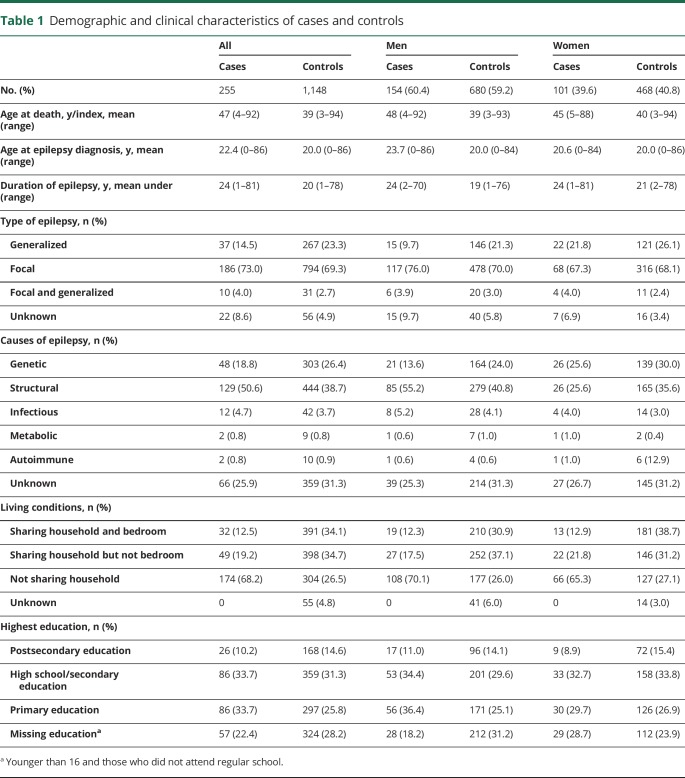
Characteristics of cases and controls are summarized in table 1. Among the 255 SUDEP decedents, 60.4% were men and due to matching, a similar male predominance was seen among controls. Mean age at diagnosis was 22.4 years for the SUDEP decedents and 20 years for controls and the decedents tended to have a slightly longer duration of epilepsy (24 vs 20 years). The majority of decedents had focal epilepsy (73.0%) and of structural origin (50.6%). Comparing cases and controls indicated small differences in the type and causes of epilepsy, but low education was slightly more common among cases (table 1). Decedents with SUDEP lived alone to a larger extent than controls, 68.2% vs 26.5%, and even if they shared their household, they were less likely than controls to share a bedroom. Generalized and genetic epilepsy was less common among men with SUDEP compared to women with SUDEP and male and female controls. In a similar fashion, men with SUDEP had a slightly higher age at epilepsy onset and more often had focal and structural epilepsy.
Table 1.
Demographic and clinical characteristics of cases and controls
Clinical characteristics, living conditions, education, and risk of SUDEP
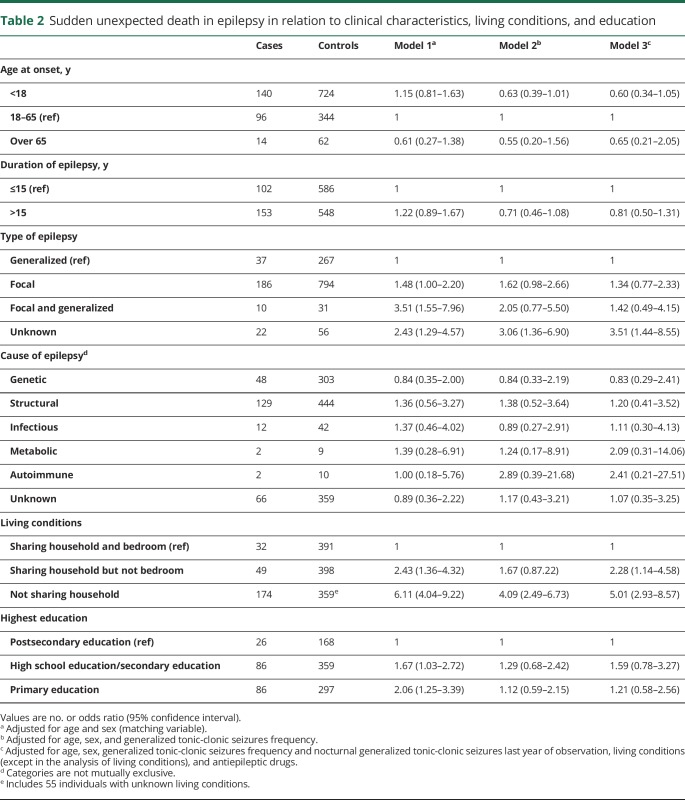
Previously proposed risk factors such as young age at epilepsy onset, longer duration of epilepsy, and structural etiology were not associated with SUDEP after adjustment for GTCS frequency (table 2). As for the type of epilepsy, no excess risk was seen in individuals with focal or focal and generalized epilepsy compared to generalized epilepsy after adjustment for GTCS frequency, but epilepsy of unknown type remained associated with SUDEP. Compared with sharing a bedroom, sharing household but not bedroom was associated with a twofold increased risk and living alone was associated with a fivefold increased risk of SUDEP (OR 5.01, 95% CI 2.93–8.57), even after adjustment for GTCS frequency and other covariates (table 2). No association between level of education and SUDEP was seen after adjustment for GTCS frequency.
Table 2.
Sudden unexpected death in epilepsy in relation to clinical characteristics, living conditions, and education
Seizures, treatment, and risk of SUDEP
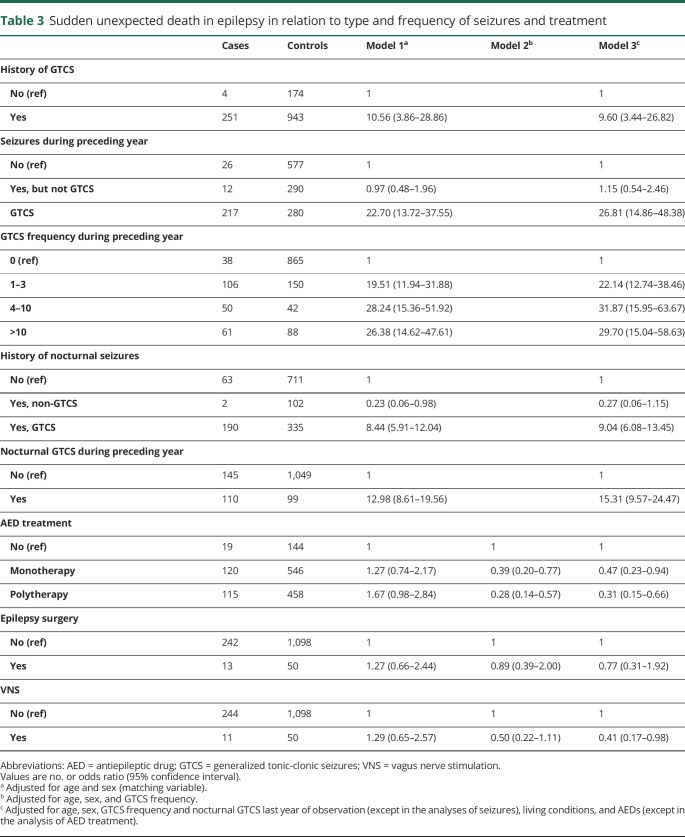
A history of GTCS was associated with a tenfold increased risk of SUDEP (OR 9.60, 95% CI 3.44–26.82) (table 3). Only 4 (1.6%) SUDEP cases did not have a history of GTCS compared to 15.1% among the controls. In those experiencing GTCS during the last year of observation, the risk was increased 27-fold (OR 26.81, 95% CI 14.86–48.38). Having 1–3 GTCS in the previous year was associated with a 22-fold risk (OR 22.14, 95% CI 12.74–38.46) and having 4–10 GTCS increased the risk to 32-fold (OR 31.87, 95% CI 15.95–63.67), while we did not see a further risk increase when the GTCS exceeded 10 during the preceding year.
Table 3.
Sudden unexpected death in epilepsy in relation to type and frequency of seizures and treatment
History of nocturnal GTCS was associated with a ninefold risk (OR 9.04, 95% CI 6.08–13.45) of SUDEP and the presence of nocturnal GTCS during last year of observation, with a 15-fold risk (OR 15.31, 95% CI 9.57–24.47). In individuals experiencing exclusively non-GTCS during the preceding year, no excess risk of SUDEP was seen (OR 1.15, 95% CI 0.54–2.46). Both monotherapy and polytherapy were associated with a reduced risk of SUDEP after adjusting for GTCS frequency and other covariates (table 3). Previous epilepsy surgery was not associated with SUDEP while vagus nerve stimulation was associated with a 59% reduced SUDEP risk after adjustment for covariates.
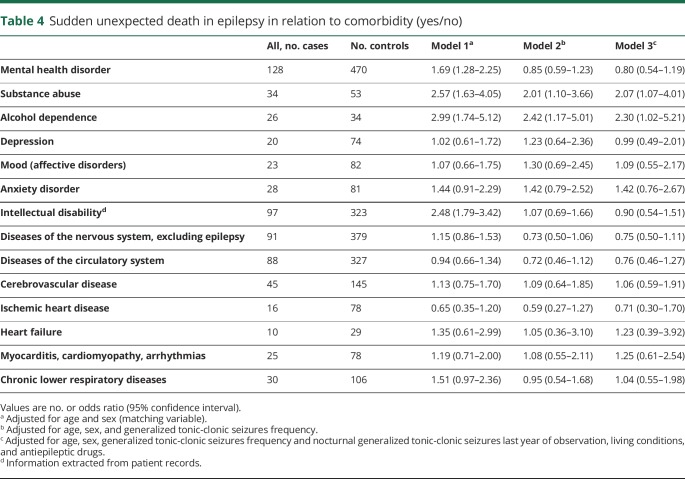
Comorbidity and risk of SUDEP
Among comorbid diseases, a twofold increased risk of SUDEP was seen in individuals with a previous diagnosis of substance abuse or alcohol dependence (table 4). Mental health disorders and intellectual disability was not associated with increased SUDEP risk once we adjusted for frequency of GTCS.
Table 4.
Sudden unexpected death in epilepsy in relation to comorbidity (yes/no)
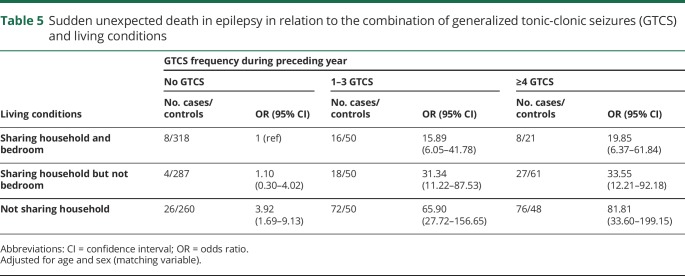
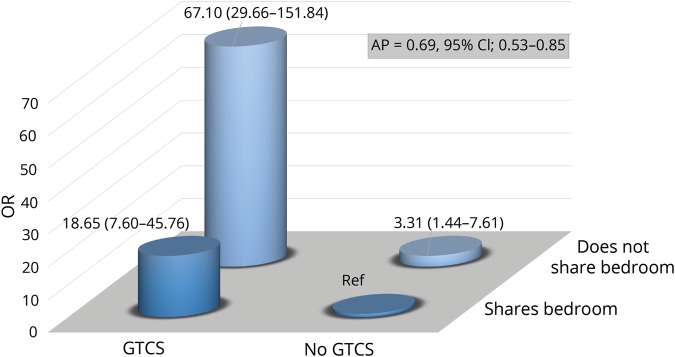
Interaction between living conditions and GTCS
Table 5 displays the risk of SUDEP in relation to the combination of living conditions and GTC seizure frequency. Individuals who experienced ≥4 GTCS had 20 times increased SUDEP risk if they shared a bedroom with someone, 34 times increased risk if they shared household but not bedroom, and an 82 times increased risk if they lived alone (table 5). Interaction analysis indicated that the combination of having at least one GTCS and not sharing a bedroom with someone conferred a 67-fold increased risk of SUDEP compared to not having GTCS and sharing a bedroom. AP was estimated at 0.69 (0.53–0.85) (figure 2).
Table 5.
Sudden unexpected death in epilepsy in relation to the combination of generalized tonic-clonic seizures (GTCS) and living conditions
Figure 2. Odds ratio (OR) (95% confidence interval [CI]) of sudden unexpected death in epilepsy by combinations of generalized tonic-clonic seizures (GTCS) and living conditions.
AP = attributable proportion due to interaction.
Discussion
Our results confirm the conclusion from previous case-control studies,2–6 and the recent systematic review,7 that the presence and frequency of GTCS is by far the most important risk factor for SUDEP. Importantly, we could demonstrate that having seizures other than GTCS, even at night, did not increase the risk for SUDEP. Living alone, especially not sharing a bedroom with anyone, was associated with a substantially increased risk of SUDEP and moreover, the combination of frequent GTCS and sleeping alone dramatically increased the risk of SUDEP. Taking AEDs as monotherapy or polytherapy and treatment with VNS was associated with significantly reduced risk of SUDEP whereas substance abuse and alcohol dependence appeared to increase the risk. A number of previously proposed risks were not associated with SUDEP, once we adjusted for GTCS frequency.
We saw an incremental risk increase from no seizures up to 4–10 GTCS (table 3), largely in line with the previous pooled analysis of case-control studies6 and the systematic review,7 although with somewhat higher risk estimates in our analysis. One explanation why having more than 10 GTCS per year did not increase the risk further could be that the recording of seizure counts in the medical records may be less precise in patients with a high frequency of seizures.
Interestingly, we did not observe an increased risk of SUDEP in patients with only non-GTCS. To our knowledge, this has not been specifically analyzed before.2–7 It was possible to extract this information from the extensive records we had on both cases and controls. This novel finding is important information when counseling the individual patient and in setting treatment goals. For example, improved treatment where GTCS are converted into non-GTCS could reduce the SUDEP risk for the individual patient. Even though there are a few reports of witnessed SUDEP without a preceding seizure or following a non-GTCS, this seems to be rare.19,20 In the MORTEMUS study of SUDEP during video-EEG monitoring, all cases followed in the aftermath of a GTCS.21
Nocturnal GTCS were associated with an increased risk of SUDEP. This fits with previous observations22 including a recent study on institutionalized individuals with epilepsy compared to controls living in the same institution.23 One novelty in our study was to analyze separately nocturnal non-GTCS demonstrating that such seizures were not associated with SUDEP.
As in previous studies,3–6 there was a trend towards increased risk in focal epilepsy which, however, disappeared after adjusting for other risk factors, especially frequency of GTCS. The group focal and generalized epilepsy was a risk factor before adjusting for GTCS, likely reflecting the severity of the epilepsy in this group. Interestingly, the unknown type of epilepsy remained a risk factor in all models. We have no clear explanation for this except that there could be similarities with this group and the focal and generalized group, where it is often difficult to classify the epilepsy due to its complex nature. It is also possible that failure to classify the type of epilepsy may be a reflection of suboptimal epilepsy management which in itself can contribute to an increased SUDEP risk.
We observed a substantial increase in SUDEP risk for those living alone, especially those not sharing a bedroom. Our observations are in line with a previous report of a protective effect of nighttime supervision, regular checks throughout the night, or use of listening devices to detect seizures.5 Furthermore, a recent study from 2 epilepsy residential care homes reported that SUDEP was more common in the center with less supervision at night.23 The greatest novelty in our findings, shown with interaction analysis, is the supra-additive increase in SUDEP risk for individuals having at least one GTCS during the last year of observation and sleeping alone. This demonstrates again that unattended GTCS are the most important risk factor in SUDEP.24 More than two-thirds of all cases exposed to both GTCS and not sharing a bedroom would be prevented by removal of one of these risk factors. This suggests that a patient with epilepsy with GTCS should share a room with someone else whenever possible. This can be difficult to organize but hopefully there will be an improvement in different types of seizure monitoring devices that could alert family members or caretakers when a seizure is detected. No prospective studies regarding the effectiveness of seizure monitoring devices in preventing SUDEP have been conducted.
Other risk factors could be hidden and sleeping alone could be a marker for fewer social connections/networks. We found substance abuse to be a risk factor that can be connected to a reduced social network. This field needs further research.
Early case-control studies identified polytherapy with AEDs as a risk factor for SUDEP.2,4,6 However, with pooled data from 4 case-control studies, polytherapy was no longer a risk factor after adjustment for GTCS frequency.25 We did find excess risk in individuals with polytherapy; however, once we adjusted for GTCS, both monotherapy and polytherapy was associated with a reduced risk of SUDEP. These observations are in line with the meta-analysis of placebo-controlled randomized add-on trials in refractory epilepsy, which showed a substantially lower SUDEP risk among those randomized to adjunctive active treatment compared with placebo.26 A major limitation of this meta-analysis, however, was that adjustment for GTCS frequency was not possible. Our findings indicate that AEDs may have a protective effect beyond the seizure-controlling properties. These potential mechanisms remain to be explored.
Several studies have observed a reduced SUDEP risk after successful epilepsy surgery.27,28 We could not confirm these findings, but our analyses were hampered by small numbers. Treatment with VNS was associated with a reduced risk of SUDEP. A possible protective effect of VNS has been discussed before,29 but our data should be interpreted with caution given the small numbers.
Comorbid mental health disorders have previously been associated with excess risk of SUDEP,13 but we did not observe an association once GTCS frequency was taken into account. In line with the pooled analysis6 of previous case-control studies, substance abuse, including alcohol abuse, was associated with an increased risk for SUDEP. This should be considered when counseling individual patients. We detected no increased risk associated with a medical history of ischemic heart disease, heart failure, myocarditis, cardiomyopathy, or arrhythmias. Neither was there an increased risk in individuals with a history of other neurologic disorders or those with a history of chronic lower respiratory diseases. It is conceivable that patients with epilepsy with comorbid cardiovascular and respiratory diseases are more likely to be classified as possible SUDEP, which was not included in our analysis.
The strengths of this study are its size, the population-based nationwide nature, and the fact that the controls came from the same population as the cases, and furthermore, that we were able to attain records for 97% of the 1,275 potential controls. In addition, the validity of the epilepsy diagnosis was ascertained with chart review, and those not meeting the epilepsy criteria were excluded. Among the weaknesses are that patient records have their inherent limitations, which can have an effect on, e.g., the possibility to classify epilepsy syndromes, even though we had extensive records for most cases and controls. In addition, the authors extracting information were not blinded to the outcome, and were aware of previous reports on SUDEP risk factors, which may introduce bias. The information was collected identically using a standardized protocol for both cases and controls. It is possible that information on living conditions was better documented among cases due to the more extensive records in connection with their death. However, information on living conditions was missing in only a small fraction of the controls (4.8%, n = 55), compared to in none of the SUDEP cases, and it is unlikely that this had a major effect on our results.
Having GTCS, nocturnal GTCS, and living alone are associated with markedly increased risk of SUDEP. Combining high frequency of GTCS and living alone is associated with a dramatically increased SUDEP risk, suggesting that unattended GTCS play a major role. The data suggest that better supervision is needed for high-risk patients with uncontrolled GTCS. However, such efforts to reduce SUDEP risks must be balanced against each patient's right to independence and integrity, which can only be done on an individual basis. Lately, there has been an increasing interest in the use of seizure detection devices, but it remains to be shown if these can reduce the SUDEP risk.30,31 The currently most important preventive method is to prescribe more effective treatments that reduce the occurrence of GTCS. Our data suggest that even a treatment that does not reduce the overall seizure frequency, but that prevents focal seizures from evolving to bilateral tonic-clonic seizures, may be beneficial. In a subsequent analysis, we intend to focus in more detail on the role of drug treatment utilizing data from the Swedish Drug Prescription Registry using the same study population.
Glossary
- AED
antiepileptic drug
- AP
proportion attributable to interaction
- CI
confidence interval
- GTCS
generalized tonic-clonic seizures
- ICD
International Classification of Diseases
- LISA
longitudinal integration database for health insurance and labor market studies
- OR
odds ratio
- SNPR
Swedish National Patient Register
- SUDEP
sudden unexpected death in epilepsy
- VNS
vagus nerve stimulation
Appendix. Authors
Footnotes
Patient page e436
CME Course: NPub.org/cmelist
Study funding
The study was supported by funding from Stockholm County Council, GlaxoSmithKline, and Citizens United for Research in Epilepsy. The sponsors had no influence on the conduct of the study, analysis, interpretation, writing of the manuscript, or the decision to publish the results.
Disclosure
O. Sveinsson has received grants from GSK, personal fees from Biogen, and honoraria to his institution from Biogen and UCB for lectures and advisory board, outside the submitted work. T. Andersson and S. Carlsson report no disclosures relevant to the manuscript. P. Mattsson received research support from the Uppsala County Council, Epilepsifonden, and Selander Foundation. T. Tomson is an employee of Karolinska Institutet, is associate editor of Epileptic Disorders, has received speaker's honoraria to his institution from Eisai, Sanofi, Sun Pharma, UCB, and Sandoz, and received research support from Stockholm County Council, EU, CURE, GSK, UCB, Eisai, and Bial. Go to Neurology.org/N for full disclosures.
References
- 1.Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia 2014;55:1479–1485. [DOI] [PubMed] [Google Scholar]
- 2.Walczak TS, Leppik IE, D'Amelio M, et al. Incidence and risk factors in sudden unexpected death in epilepsy: a prospective cohort study. Neurology 2001;56:519–525. [DOI] [PubMed] [Google Scholar]
- 3.Hitiris N, Suratman S, Kelly K, Stephen LJ, Sills GJ, Brodie MJ. Sudden unexpected death in epilepsy: a search for risk factors. Epilepsy Behav 2007;10:138–141. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: a case–control study. Lancet 1999;353:888–893. [DOI] [PubMed] [Google Scholar]
- 5.Langan Y, Nashef L, Sander JW. Case–control study of SUDEP. Neurology 2005;64:1131–1133. [DOI] [PubMed] [Google Scholar]
- 6.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–1159. [DOI] [PubMed] [Google Scholar]
- 7.Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2017;88:1674–1680. [DOI] [PubMed] [Google Scholar]
- 8.Tomson T, Surges R, Delamont R, Haywood S, Hesdorffer DC. Who to target in sudden unexpected death in epilepsy prevention and how? Risk factors, biomarkers, and intervention study designs. Epilepsia 2016;57(suppl 1):4–16. [DOI] [PubMed] [Google Scholar]
- 9.Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia 1997;38(suppl 11):6–8. [DOI] [PubMed] [Google Scholar]
- 10.Annegers IF. United States perspective on definitions and classifications. Epilepsia 1997;38(suppl 11):9–12. [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish National Inpatient Register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson LA, Björkenstam C, Westerling R. Unexplained differences between hospital and mortality data indicated mistakes in death certification: an investigation of 1,094 deaths in Sweden during 1995. J Clin Epidemiol 2009;62:1202–1209. [DOI] [PubMed] [Google Scholar]
- 13.Sveinsson O, Andersson T, Carlsson S, Tomson T. The incidence of SUDEP: a nationwide population-based cohort study. Neurology 2017;89:170–177. [DOI] [PubMed] [Google Scholar]
- 14.Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 15.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol 2019;34:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenbroucke JP, Pearce N. Case-control studies: basic concepts. Int J Epidemiol 2012;41:1480–1489. [DOI] [PubMed] [Google Scholar]
- 18.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–579. [DOI] [PubMed] [Google Scholar]
- 19.Sveinsson O, Andersson T, Carlsson S, Tomson T. Circumstances of SUDEP: a nationwide population-based case-series. Epilepsia 2018;59:1074–1082. [DOI] [PubMed] [Google Scholar]
- 20.Lhatoo SD, Nei M, Raghavan M, et al. Nonseizure SUDEP: sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 2016;57:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 22.Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 2012;53:253–257. [DOI] [PubMed] [Google Scholar]
- 23.van der Lende M, Hesdorffer DC, Sander JW, Thijs RD. Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology 2018;91:e1508–e1518. [DOI] [PubMed] [Google Scholar]
- 24.Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 2016;15:1075–1078. [DOI] [PubMed] [Google Scholar]
- 25.Hesdorffer DC, Tomson T, Benn E, et al. ; ILAE Commission on Epidemiology (Subcommission on Mortality). Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia 2012;53:249–252. [DOI] [PubMed] [Google Scholar]
- 26.Ryvlin P, Cucherat M, Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol 2011;10:961–968. [DOI] [PubMed] [Google Scholar]
- 27.Hennessy MJ, Langan Y, Elwes RD, et al. A study of mortality after temporal lobe epilepsy surgery. Neurology 1999;53:1276–1283. [DOI] [PubMed] [Google Scholar]
- 28.Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology 2016;86:1938–1944. [DOI] [PubMed] [Google Scholar]
- 29.Ryvlin P, So EL, Gordon CM, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 2018;59:562–572. [DOI] [PubMed] [Google Scholar]
- 30.Ryvlin P, Ciumas C, Wisniewski I, Beniczky S. Wearable devices for sudden unexpected death in epilepsy prevention. Epilepsia 2018;59(suppl 1):61–66. [DOI] [PubMed] [Google Scholar]
- 31.Rugg-Gunn F, Duncan J, Hjalgrim H, Seyal M, Bateman L. From unwitnessed fatality to witnessed rescue: nonpharmacologic interventions in sudden unexpected death in epilepsy. Epilepsia 2016;57(suppl 1):26–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from qualified investigators.