Abstract
The host innate immune response either clears invading viruses or allows the adaptive immune system to establish an effective antiviral response. In this study, both pathogenic (passage 3, P3) and attenuated (P110) infectious bronchitis virus (IBV) strains were used to study the immune responses of chicken to IBV infection. Expression of avian β-defensins (AvBDs) and Toll-like receptors (TLRs) in 16 tissues of chicken were compared at 7 days PI. The results showed that P3 infection upregulated the expression of AvBDs, including AvBD2, 4, 5, 6, 9, and 12, while P110 infection downregulated the expression of AvBDs, including AvBD3, 4, 5, 6, and 9 in most tissues. Meanwhile, the expression level of several TLRs showed a general trend of upregulation in the tissues of P3-infected chickens, while they were downregulated in the tissues of P110-infected chickens. The result suggested that compared with the P110 strain, the P3 strain induced a more pronounced host innate immune response. Furthermore, we observed that recombinant AvBDs (including 2, 6, and 12) demonstrated obvious anti-viral activity against IBV in vitro. Our findings contribute to the proposal that IBV infection induces an increase in the messenger RNA (mRNA) expression of some AvBDs and TLRs, which suggests that AvBDs may play significant roles in the resistance of chickens to IBV replication.
Electronic supplementary material
The online version of this article (doi:10.1007/s00253-015-6786-8) contains supplementary material, which is available to authorized users.
Keywords: Chicken, Avian β-defensins, Toll-like receptors, Infectious bronchitis virus
Introduction
Respiratory pathogens, including infectious bronchitis virus (IBV), continue to have serious health and economic impacts on the poultry industry (Cavanagh 2003; Fang et al. 2007) despite the existence of vaccination programs. IBV is a coronavirus that causes an acute, highly contagious respiratory disease, and some strains can also cause nephritis. In addition, the reproductive tract of layer and breeder birds can be affected, causing decreased egg quality and production. As well as being an economically relevant pathogen in poultry, IBV also bears close resemblance to human coronaviruses, such as the pathogen severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus. Up to date, while much progress has been made in understanding the adaptive immune response to these respiratory pathogens (Ignjatovic and Galli 1995; Gelb et al. 2005), the innate immune response remains poorly characterized. The innate immune system is the first line of host defense against an invading viral pathogen, and the outcome of an infection is dependent on the ability of host cells to recognize the invading pathogen and activate appropriate signaling pathways (Liao et al. 2011). If the innate immune response fails, pathogens are susceptible to triggering what is referred to as a cytokine storm (Tisoncik et al 2012). The innate immune responses activated as a result of virus interaction with host cells play an essential role in the outcome of viral infection. The response is induced by recognition of specific components of the infecting pathogenic microorganisms known as pathogen-associated molecular patterns (PAMPs) by special receptors (Garcia et al 2007; Takeuchi and Akira 2009; Liao et al 2011). Toll-like receptors (TLRs) are the members of such receptors. To date, ten TLRs have been identified in chickens, namely TLR1-1 (TLR1A), TLR1-2 (TLR1B), TLR2-1 (TLR2A), TLR2-2 (TLR2B), TLR3, TLR4, TLR5, TLR7, TLR15, and TLR21. Interaction of TLRs with their specific ligands leads to an innate immune response, which leads to the expression and secretion of both pro-inflammatory cytokines and defensins as well (Abdel-Mageed et al. 2014).
Cytokines and chemokines are key mediators that initiate immune responses and ultimately shape the adaptive immune responses via chemoattraction of adaptive immune cells, activation of macrophages, differentiation of Th1 from T cells and control of intracellular pathogens, promoting protective adaptive immunity, upregulation of co-stimulatory molecules required for antigen presentation, and prevention of fatal immunopathology. Furthermore, they may be involved in both clearance of pathogens and pathological tissue damage (Carpenter and O’Neill 2007; Quintana et al. 2011). Defensins are small polypeptides that play an important role in the innate host defense against bacteria, fungi, and some viruses (Ganz and Lehrer 1999; Lehrer and Ganz 2002; Wang et al. 2010; Ma et al. 2011, 2012a, b, c, 2013, 2014; Barabas et al. 2013). In addition to their antimicrobial effect, defensins have been shown to activate monocyte-derived dendritic cells and modulate interferon (IFN)-γ production in antigen-presenting cells, suggesting that they may also play a role in the shaping of adaptive immunity (Presicce et al. 2009; Nijnik et al. 2012; Quintana et al. 2011). IBV infection of chickens causes an acute and highly contagious respiratory disease (Fang et al. 2007; Shen et al. 2004). It initially infects the upper respiratory tract, where it is restricted to the ciliated and mucus-secreting cells (Raj and Jones 1997). Following infection of chickens by IBV, IFN was detected in the trachea and lungs (Otsuki et al. 1987). Meanwhile, the production of pro-inflammatory cytokines IL-6 and IL-8 were also greatly increased during IBV infection of cultured cells (Liao et al. 2011). While the induction of cytokines has recently been observed in chickens infected with IBV (Pei et al. 2001; Liao et al. 2011), the regulation of defensins in the tissues of chicken, and their significance in the pathogenesis of IBV, remains poorly described. In this study, we demonstrate that prestimulation with a pathogenic IBV strain (passage 3 (P3)) results in a beneficial expression of several avian β-defensins (AvBDs) in the tissues of chicken compared with an attenuated strain (P110). Our results further provide evidence that this regulation of AvBD expression may be ascribed to their antiviral effect against IBV and seems to be mediated by TLRs.
Materials and methods
Viral infection and virus detection in kidney tissues of the infected chickens
One-day-old, specific pathogen-free (SPF) chickens were obtained from the Laboratory Animal Center, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Harbin, China).
The pathogenic IBV strain CK/CH/LHLJ/04 V (P3) and attenuated (P110) strain (Liu et al. 2009) were used in this study. The P3 and P110 viruses were propagated once in 9-day-old embryonated SPF chicken eggs, as described previously (Liu et al. 2013), to obtain titers of 106–108 50 % median embryo infectious doses (EID50) per 0.1 mL. Before use, viruses isolated from the allantoic fluids of inoculated eggs were confirmed by negative-contrast electron microscopy, real-time PCR (RT-PCR), and DNA sequencing (Liu et al. 2009).
Forty-five 1-day-old SPF White Leghorn chicks were housed in separate isolators and divided into three groups of 15 chicks each at 15 days of age. Chickens in groups 1 and 2 were inoculated either with P3 or with P110 respectively, by intraocular application, with a dose of 106 EID50 per chick. Birds in group 3, which served as the negative control, were mock-inoculated with sterile allantoic fluid. Five birds from each of the three groups were killed humanely at 7 days post-inoculation (dpi). The proventriculus, liver, spleen, lung, kidneys, duodenum, pancreas, small intestine, large intestine, cecum, cecal tonsil, rectum, bursa of Fabricius, trachea, Harderian gland, and thymus of each bird were collected, rinsed in cold sterile saline, snap-frozen in liquid nitrogen, and stored at −70 °C until further use. Serum samples were collected at 12 dpi from groups 1 and 2, as well as from control birds, and assayed in triplicate using a commercial enzyme-linked immunosorbent assay (ELISA) (IDEXX Laboratories, Inc., Westbrook, MA, USA) according to the manufacturer’s instructions. The remaining birds in groups 1 and 2 were killed humanely 15 dpi, and blood was collected for antibody tests, as described above. Serum-to-positive ratios were calculated as described previously (Liu et al. 2006). Individual serum titers were expressed as absorption values at an optical density (OD) at 650 nm according to the manufacturer’s instructions.
Real-time PCR
For RNA extraction, equal amounts of tissues (1 g) were excised in cold, RNase-free phosphate-buffered saline (PBS) as described previously (Ma et al. 2012a, b, c; Liu et al. 2013). Briefly, tissue samples were homogenized, ground, or cut into small pieces before being adjusted to a 10 % (w/v) suspension in PBS. Nucleic acid extractions were prepared from 50 μL of homogenized samples using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. RNA was air-dried for 2–10 min, redissolved in 40 μL of RNase-free water, and stored at −70 °C until use. To evaluate RNA quality, the ODs of RNA at 260 and 280 nm were examined. The OD260 to OD280 ratios were within 1.8 to 2.2 (data not shown). All of the processes were performed under RNase-free conditions.
One-step RT-PCR was performed using the One-step Real-time PrimeScript® RT-PCR kit (TaKaRa Biotechnology, Dalian Co., LTD.) on a LightCycler® 480 II RT-PCR system (Roche, Basel, Switzerland) according to previous studies (Ma et al. 2012a, b, c; Liu et al. 2013). Serial tenfold dilutions of plasmids containing chicken 18S ribosomal RNA (18S rRNA), AvBDs 1–14; TLRs 1–5, 7, 15, and 21; and inducible nitric oxide synthase (iNOS) were used as controls. Chicken TLRs 1–5 and 7 are the functional orthologs of mammalian TLRs 1–5 and 7, and chicken TLR15 and 21 have no orthologs in mammals (Keestra et al. 2013). Plasmids containing these genes were stored in our laboratory. 18S rRNA was selected as the most stable reference gene across all samples from a panel of potential genes, including 18S rRNA, β-actin, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), from chickens (data not shown). The initial DNA concentrations were quantified using a spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The optimal concentrations of forward and reverse primers were determined by titrating 1-, 2.5-, 5-, and 10-μmol concentrations (data not shown). As a result, the real-time RT-PCR reactions (25 μL) contained 1 μL (2.5 μmol) of each primer, 12.5 μL of 2× One Step SYBR RT-PCR Buffer 4, 1 μL of Primer Script 1 Step Enzyme Mix 2, 7.5 μL of nuclease-free water, and 2 μL of either a template RNA sample or a known concentration of standard plasmid. The primers are shown in Table 1.
Table 1.
Real-time PCR primer sequences used for profiling AvBDs, TLRs, iNOS, IBV strain P3, and 18S rRNA
| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) | Accession number | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| AvBD1 | GATCCTCCCAGGCTCTAGGAAG | GCCCCATATTCTTTTGC | NM_204993 | 137 | a |
| AvBD2 | GGTTGTCTTCGCCCCGGCGGGA | TTATGCATTCCAAGGCCATTTG | NM_204992 | 137 | a |
| AvBD3 | GAACTGCCACTCAGTGCAGAAT | ATGGGGGTTGTTTCCAGGAGC | NM_204650 | 182 | a |
| AvBD4 | TCATCGTGCTCCTCTTTGTG | AATACTTGGGACGGCATAGC | NM_001001610 | 153 | a |
| AvBD5 | GCTGTCCCTTGCTCGAGGATT | GGAATACCATCGGCTCCGGC | NM_001001608 | 139 | a |
| AvBD6 | GTCAGCCCTACTTTTCCAGC | GCCCACCTGTTCCTCACAC | NM_001001193 | 143 | a |
| AvBD7 | ACCTGCTGCTGTCTGTCCTC | TGCACAGCAAGAGCCTATTC | NM_001001194 | 173 | a |
| AvBD8 | TTCTCCTCACTGTGCTCCAA | AAGGCTCTGGTATGGAGGTG | NM_001001781 | 124 | a |
| AvBD9 | GCTTACAGCCAAGAAGACGCT | GGAGCTAGGTGCCCATTTGCA | NM_001001611 | 145 | a |
| AvBD10 | GGCTCAGCAGACCCACTTTTCC | CTGCGCCGGAATCTTGGCAC | NM_001001609 | 146 | a |
| AvBD11 | GGTCTCGGCTTGCCCAGAGAC | ATGGAAGTCTGATGTAGTGTC | AY621313 | 150 | a |
| AvBD12 | GGAACCTTTGTTTCGTGTTCA | GAGAATGACGGGTTCAAAGC | AY534898 | 155 | a |
| AvBD13 | GATCCTCCAGCTGCTCTTTG | AGTGGCCATGGTTGTTCCT | AY701473 | 104 | a |
| AvBD14 | CATATTCCTCCTGTTTCTTGTTCTC | GCCAGTCCATTGTAGCAGGT | AM402954 | 150 | a |
| TLR1 | AGTCCATCTTTGTGTTGTCGCC | ATTGGCTCCAGCAAGATCAGG | NM_001081709 | 127 | a |
| TLR2 | GATTGTGGACAACATCATTGACTC | AGAGCTGCTTTCAAGTTTTCCC | XM_001232192 | 294 | a |
| TR3 | TCAGTACATTTGTAACACCCCGCC | GGCGTCATAATCAAACACTCC | NM_001011691 | 256 | b |
| TLR4 | AGTCTGAAATTGCTGAGCTCAAAT | GCGACGTTAAGCCATGGAAG | NM_001030693.1 | 190 | a |
| TLR5 | CCTTGTGCTTTGAGGAACGAGA | CACCCATCTTTGAGAAACTGCC | NM_001024586 | 124 | a |
| TR7 | TTCTGGCCACAGATGTGACC | CCTTCAACTTGGCAGTGCAG | NM_001011688 | 219 | b |
| TLR15 | GTTCTCTCTCCCAGTTTTGTAAATAGC | GTGGTTCATTGGTTGTTTTTAGGAC | NM_001037835 | 262 | a |
| TLR21 | TGCCCCTCCCACTGCTGTCCACT | AAAGGTGCCTTGACATCCT | NM_001030558 | 112 | a |
| iNOS | GAACAGCCAGCTCATCCGATA | CCCAAGCTCAATGCACAACTT | GGU46504 | 103 | – |
| 18S rRNA | TCAGATACCGTCGTAGTTCC | TTCCGTCAATTCCTTTAAGTT | AF173612 | 154 | – |
| IBV | CTATCGCCAGGGAAATGTC | GCGTCCTAGTGCTGTACCC | FJ641062 | 174 | c |
The presence of IBV in tissue samples of the infected birds was confirmed by detection of the viral RNA using the One-step Real-time PrimeScript® RT-PCR kit (described above) according to the following steps: reverse transcription at 42 °C for 10 min, denaturation at 95 °C for 10 s, and 40 cycles at 95 °C for 5 s, 55 °C for 20 s, and 72 °C for 10 s, followed by a cooling step at 40 °C for 10 s (Cong et al. 2013).
Standard curves were obtained by plotting the crossing cycle number (the threshold or crossing point) as a function of the log plasmid DNA concentration for each target sequence. The concentration of target complementary DNA (cDNA) in a sample was deduced from the crossing point obtained and from the corresponding standard curve. The presence of IBV was expressed for each sample as the viral RNA copy number. The results for the other genes were expressed for each sample as the copy numbers of each target cDNA normalized to 109 times the copy number of the reference gene, 18S rRNA, using the following formula: (target gene cDNA copy number / 18S rRNA cDNA copy number) × 109. All real-time RT-PCR products were confirmed by electrophoresis on 2.0 % agarose gels, followed by ethidium bromide staining. Furthermore, the specificities of the reactions were checked by cloning and sequencing three independent PCR products. This experiment was performed in three independent experiments, with five replicates per experiment.
Protein expression and purification
The DNA fragments that encoded AvBD2, 6, and 12 were amplified by PCR from their respective plasmids (stored in our laboratory) using the primers (shown in Table 2). Amino acid sequences of these recombinant AvBDs are listed in Fig. 5a.
Table 2.
PCR primer sequences used for protein expression of the recombinant AvBDs (Ma et al. 2014)
| Gene name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| AvBD2 | GAATTCATGCCCCGGCGGGACATGCTG | CTCGAGTTATGCATTCCAAGGCCATTTG |
| AvBD6 | GAATTCATGCCCTACTTTTCCAGCCCTATTC | CTCGAGTCAGGCCCACCTGTTCCTCACAC |
| AvBD12 | GAATTCATGGACAGCTGTAACCACG | CTCGAGTCAGGTCTTGGTGGGAGTTG |
Fig. 5.
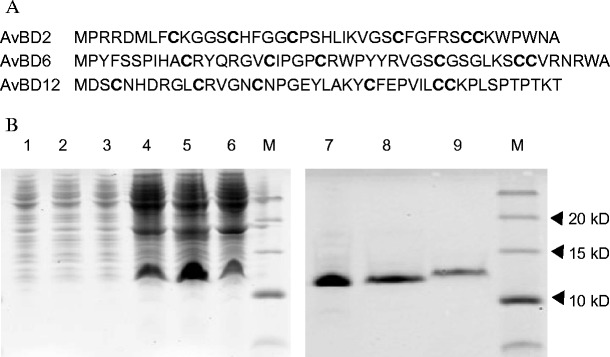
Amino acid sequences of the recombinant AvBDs and SDS-PAGE analysis of His-tagged recombinant AvBD (His-rAvBD) proteins expressed in E. coli BL21 (DE3) cells. a Amino acid sequences of the recombinant AvBDs. The conserved six cysteines (C) are in bold. b SDS-PAGE analysis of His-tagged recombinant AvBD (His-rAvBD) proteins expressed in E. coli BL21 (DE3) cells. Lanes: 1–3, total protein from BL21 containing AvBD2, 6, and 12 without IPTG induction; lanes 4–6, total protein from BL21 containing AvBD2, 6, and 12 with IPTG induction; lanes 7–9, purified protein of AvBD2, 6, 12, with IPTG induction; M, protein molecular weight marker. IPTG isopropyl-β-d-thiogalactoside
The PCR products, which contained the coding sequence of AvBD2, AvBD6, or AvBD12 flanked by EcoRI/XhoI sites, were inserted into the same sites of the pProEX-HTa expression vector (Invitrogen). The resultant plasmids were designated as recombinant AvBD2, 6, and 12, respectively, and were sequenced again. The constructs that were confirmed to contain these AvBDs were transformed into competent Escherichia coli BL21 (DE3) cells and induced subsequently with 0.6 mmol/L isopropyl-β-d-1-thiogalactopyranoside (IPTG). The proteins were purified and refolded using a purification and refolding kit (Novagen, Darmstadt, Germany), according to the manufacturer’s instructions. The proteins were quantified by the Bradford assay (Bradford 1976). The soluble fractions were recovered, analyzed on 15 % (w/v) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) at 80 V using the Mini-protean III system (Bio-Rad, Beijing, China), and stained with Coomassie brilliant blue R-250 (Schägger and von Jagow 1987).
Antiviral activity of recombinant His-tagged AvBDs against IBV in vitro
The antiviral activities against IBV strain CK/CH/LHLJ/04V P3 of a recombinant 6× His-tagged peptide and recombinant His-tagged AvBD2, 6, and 12 were determined according to methods described for neutralization assays (OIE 2004). A dose of 106 EID50 of IBV was serially diluted tenfold and treated with equal volumes of 6× His-tagged peptide, or His-tagged AvBDs (final concentration, 300 μg/mL in PBS), or PBS at 37 °C for 60 min. The mixture was inoculated into 11-day-old SPF chicken embryo (200 μL administered per egg). The inoculated chicken embryos were incubated at 37 °C. Fifty microliters of allantoic fluid were collected from each egg at 48 and 72 hpi, using a 26-g needle, through a small hole created in the shell near the air sac (Hewson et al. 2012). Viral RNA was extracted from the allantoic fluid and subjected to real-time RT-PCR as described above. The embryos were incubated at 37 °C for 7 days and then chilled at 4 °C and examined for characteristic IBV lesions such as the dwarfing, stunting, or curling of embryos. Embryo mortality recorded in the first 24 h post-inoculation was considered nonspecific. Samples were considered positive if the real-time RT-PCR was positive and embryos show lesions after three blind passages of 7-day duration. This experiment was performed in three independent experiments.
Statistics
Data are expressed as the mean ± SD. Statistical analyses, where appropriate, were performed using one-way analysis of variance (ANOVA) followed by Tukey’s test to identify differences between observations and groups using the generalized linear model (GLM) procedure of SAS software (SAS 1996). A P value <0.05 was considered to be statistically significant. Correlations between relative gene expressions of AvBDs and TLRs in the tissue samples of chickens in response to IBV infection were performed using Pearson’s tau of SAS software (SAS 1996). P value <0.01 was considered to be statistically significant.
Results
Clinical signs and viral detection
Similar to uninfected control chickens, no obvious clinical signs were observed in the CK/CH/LHLJ/04V P110-inoculated chickens during the experiment. In contrast, chicks inoculated with strain CK/CH/LHLJ/04V P3 showed obvious clinical signs, such as ruffled feathers and dark, shrunken combs, at 3–13 dpi. Two chicks died during the experiment, and nephritis was observed. The clinical signs of the survived birds tended to gradually disappear after 13 dpi. All chickens inoculated with strain CK/CH/LHLJ/04V P3 showed a positive serum antibody response at 12 and 15 dpi, respectively; however, four and five chickens inoculated with strain CK/CH/LHLJ/04V P110 showed a positive serum antibody response at 12 and 15 dpi, respectively, whereas those in the control group were negative for serum antibody response (Fig. 1a). To confirm the presence of IBV in the infected birds, viral loads in 16 tissue samples of chickens inoculated with the P3 and P110 strains were compared at 7 days PI. Viral RNA was not detected in the proventriculus, liver, spleen, lung, pancreas, or thymus of all five chickens inoculated with the P110 strain (Fig. 1b). In contrast, viral RNA was found in these same tissues in all five chickens inoculated with the P3 strain. Furthermore, the amount of P3 viral RNA was significantly greater than that of P110 viral RNA in the kidneys, duodenum, and large intestine. Similar to the P3 virus, the P110 virus showed an affinity for the bursa of Fabricius and gastrointestinal tract, especially the small intestine, cecal tonsil, and rectum, as reflected by high viral RNA copy numbers demonstrated by real-time RT-PCR. Collectively, these results confirmed the successful IBV infection of SPF chickens.
Fig. 1.
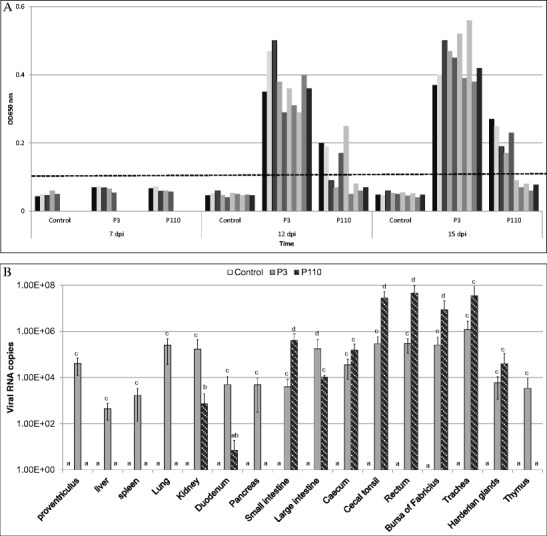
Humoral immune responses and viral RNA copies in the tissue samples of chickens inoculated with CK/CH/LHLJ/04V P3 or P110. a Humoral immune responses in SPF chickens inoculated with IBV CK/CH/LHLJ/04V passages evaluated by indirect ELISA. Five chickens were tested in each inoculated group on day 7 post-inoculation, and ten were tested at 12 and 15 days after inoculation. Dashes show the S/P ratios, calculated as described in “Materials and methods” section. Serum samples with S/P ratios equal or above the dashes were considered positive, and those below were considered negative. The serum sample S/P ratios of chickens in the negative control group were all below the dashes and are not indicated in the figures. b Viral RNA copy numbers in the tissue samples from five chickens in each group were measured by real-time RT-PCR on day 7 post-inoculation. All assays were performed in triplicate, with five replicates per experiment, and each bar is the mean ± SD. The statistical significance of differences between groups of the control, P3, and P110 was assessed using the generalized linear model (GLM) procedure of SAS software (SAS 1996). The values with different letters are significantly different (P < 0.05)
Virus-induced expression of AvBDs in tissues
AvBDs have been shown to play vital roles in the immune response of birds against viral pathogens. To analyze the expression of AvBDs 1–14 in the tissues of chicken in response to IBV infection, both highly pathogenic (P3) and attenuated (P110) IBV strains were used in this study. Sixteen tissues mentioned above were collected from chickens inoculated with the aforementioned viruses, as well as from control chickens. Compared with the control, both the P3 and P110 strains induced the expression of most of the AvBDs (Fig. 2). Compared with control chickens, the expression of AvBD2 in the large intestine, cecum, cecal tonsil, and thymus; AvBD4 in the trachea and thymus; AvBD5 in the small intestine and thymus; AvBD6 in the kidneys, duodenum, small intestine, large intestine, bursa of Fabricius, and thymus; AvBD9 in the small intestine and thymus; and AvBD12 in the pancreas, small intestine, bursa of Fabricius, and Harderian glands of P3-infected chickens was upregulated. Interestingly, in contrast to the upregulation of expression observed in the tissues of P3-infected chickens, for P110-infected chickens, the expression of nearly all of the AvBDs investigated in this study was downregulated in most of the tissues. In addition, compared with the high level of expression in most of the tissues in both control and P3-infected chickens, the expression of AvBD10 was undetectable in the lung, pancreas, small intestine, and cecum of P110-infected chickens. Similarly, little expression of AvBD11 was detected in the proventriculus, liver, pancreas, small intestine, and Harderian gland in P110-inoculated chickens. In addition, no significant differences in the expression of the other AvBDs were found in response to IBV infection. Because of their marked differences in expression in the tissues of chickens inoculated with IBV P3, as well as in P110-infected and control chickens, AvBD2, 6, and 12 were chosen for further analysis in the study.
Fig. 2.
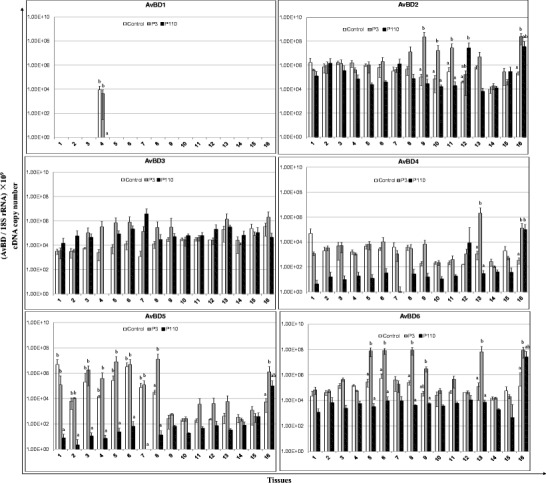
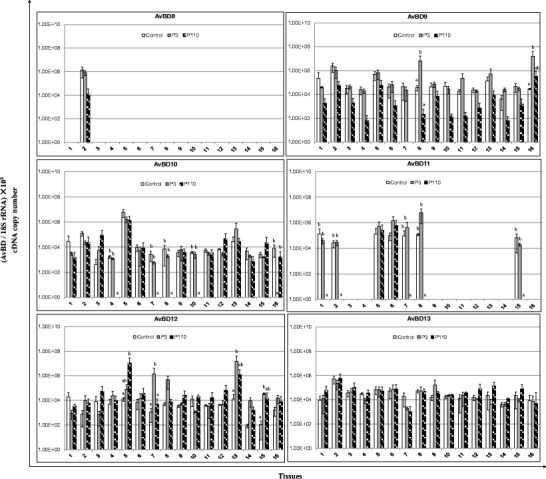
Relative mRNA expression of avian β-defensins (AvBDs) in the tissues of chickens in response to IBV infection. 1 Proventriculus, 2 liver, 3 spleen, 4 lung, 5 kidneys, 6 duodenum, 7 pancreas, 8 small intestine, 9 large intestine, 10 cecum, 11 cecal tonsil, 12 rectum, 13 bursa of Fabricius, 14 trachea, 15 Harderian gland, 16 thymus. cDNA copy numbers in the tissue samples from five chickens of each group were measured by real-time PCR on day 7 post-infection. Results are shown for each sample as the copy number of each target cDNA normalized to 109 times the copy number of the reference gene, 18S rRNA, using the following formula: (target gene cDNA copy number / 18S rRNA cDNA copy number) × 109. All assays were performed in triplicate, with five replicates per experiment, and each bar is the mean ± SD. The statistical significance of differences between groups of the control, P3, and P110 was assessed using the generalized linear model (GLM) procedure of SAS software (SAS 1996). The values with different letters are significantly different (P < 0.05)
Expressions of Toll-like receptors and inducible nitric oxide synthase
TLR gene expression data are illustrated in Fig. 3. The expression level of TLRs showed a general trend of upregulation in the tissues of P3-infected chickens and downregulation in the tissues of P110-infected chickens. Compared with control chickens, we observed an increase in the expression of TLR1 in the small and large intestines, TLR2 in the kidneys and small intestine, and TLR4 in the large intestine and bursa of Fabricius of P3-infected chickens. However, in P110-infected chickens, the expression of TLRs 1–3, 5–7, 15, and 21 was downregulated in most tissues, except for the rectum, when compared with both control and P3-infected chickens.
Fig. 3.
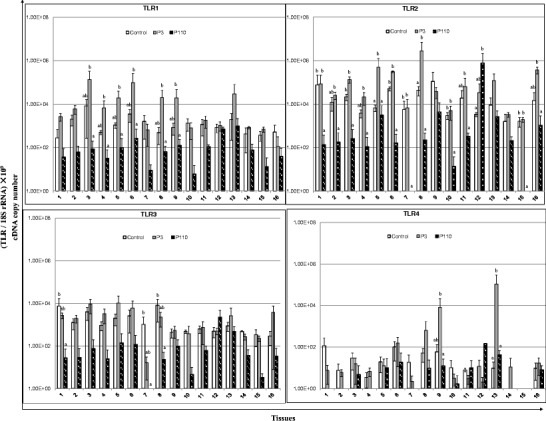
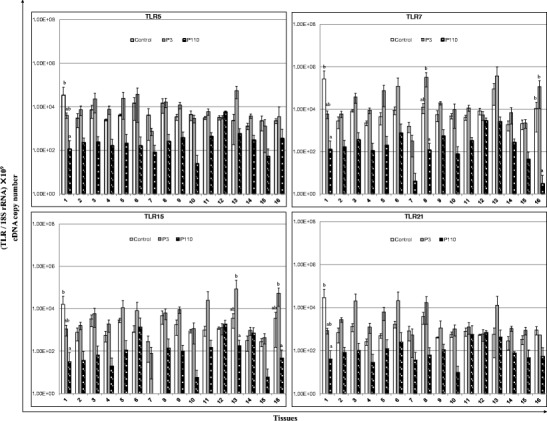
Relative mRNA expression of Toll-like receptors (TLRs) in the tissue samples of chickens in response to IBV infection. 1 Proventriculus, 2 liver, 3 spleen, 4 lung, 5 kidneys, 6 duodenum, 7 pancreas, 8 small intestine, 9 large intestine, 10 cecum, 11 cecal tonsil, 12 rectum, 13 bursa of Fabricius, 14 trachea, 15 Harderian gland, 16 thymus. cDNA copy numbers in the tissue samples from five chickens of each group were measured by real-time RT-PCR on day 7 post-infection. Results are shown for each sample as the copy number of each target cDNA normalized to 109 times the copy number of the reference gene, 18S rRNA, using the following formula: (target gene cDNA copy number / 18S rRNA cDNA copy number) × 109. All assays were performed in triplicate, with five replicates per experiment, and each bar is the mean ± SD. The statistical significance of differences between groups of the control, P3, and P110 was assessed using the generalized linear model (GLM) procedure of SAS software (SAS 1996). The values with different letters are significantly different (P < 0.05)
In contrast to the expression patterns of TLRs, no obvious changes in the expression of iNOS were observed in the tissues of P3-infected chickens, as compared with controls, whereas in P110-infected chicks, iNOS gene expression was downregulated in the proventriculus, pancreas, large intestine, and trachea (Fig. 4).
Fig. 4.
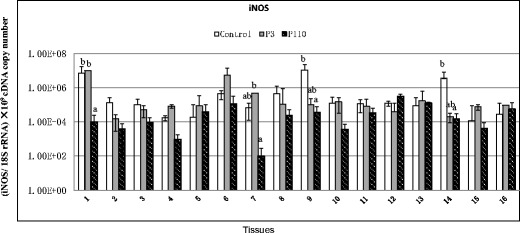
Relative mRNA expression of inducible nitric oxide synthase (iNOS) in the tissue samples of chickens in response to IBV infection. 1 Proventriculus, 2 liver, 3 spleen, 4 lung, 5 kidneys, 6 duodenum, 7 pancreas, 8 small intestine, 9 large intestine, 10 cecum, 11 cecal tonsil, 12 rectum, 13 bursa of Fabricius, 14 trachea, 15 Harderian gland, 16 thymus. cDNA copy numbers in the tissues from five chickens of each group were measured by real-time RT-PCR on day 7 post-infection. Results are shown finally for each sample as the copy number of each target cDNA normalized to 109 times the copy number of the reference gene, 18S rRNA, using the following formula: (target gene cDNA copy number / 18S rRNA cDNA copy number) × 109. All assays were performed in triplicate, with five replicates per experiment, and each bar is the mean ± SD. The statistical significance of differences between groups of the control, P3, and P110 was assessed using the generalized linear model (GLM) procedure of SAS software (SAS 1996). The values with different letters are significantly different (P < 0.05)
Relationship between gene expressions of AvBDs and TLRs
It was shown that gene expression of AvBD2 correlated positively with TLRs (1–5, 7, and 21) from P110-infected chicks (P < 0.01 or P < 0.001). For P3-infected chickens, significant correlations were observed between AvBD4 expression and expressions of both TLR4 and 5 (P < 0.001), expression of AvBD5 and expressions of both TLR2 and 7 (P < 0.01), both AvBD6 and 9 expression and TLR3 (P < 0.01), expressions of AvBD10 and TLR21 (P < 0.01), AvBD11 expression and expressions of both TLR1 and 7 (P < 0.01), and expressions of AvBD12 and TLR4 (P < 0.01) (Table S1).
Expression, purification, and antiviral activities of recombinant AvBD2, 6, and 12 against IBV in vitro
The cDNAs encoding AvBD2, 6, and 12 were produced as His-tagged AvBDs (molecular weights 10–15 kDa) in E. coli (Fig. 5b). The His-tagged AvBDs were purified and refolded by using a purification and refolding kit mentioned above.
Viral RNA loads in the allantoic fluids of P3-infected chicken embryos inoculated with 6× His peptide (which served as the control) or His-tagged AvBDs were investigated by real-time RT-PCR at 48 and 72 h post-infection (Fig. 6). Compared with the control, the viral load of AvBD2-inoculated embryos was significantly decreased at 48 h post-infection (P < 0.05). Furthermore, low viral loads were observed in the chicken embryos inoculated with AvBD6 or AvBD12 at 48 h post-infection (P < 0.01). However, no significant change was observed at 72 h post-infection for AvBD2. In contrast, only little viral RNA loads were detected for both AvBD6- and AvBD12-inoculated embryos at 72 h post-infection. The results showed that both AvBD6 and AvBD12 exhibit stronger antiviral activity against IBV (P3) than AvBD2 (P < 0.01).
Fig. 6.
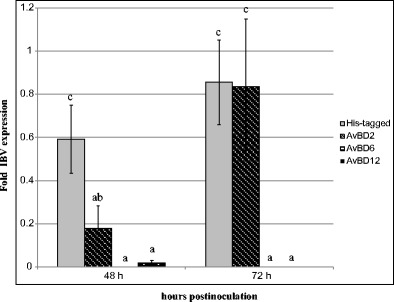
Antiviral activities of His-rAvBDs against IBV in chicken embryo. Values are indicated as the fold viral RNA copies compared with that of the control (PBS). All assays were performed in three independent experiments, with five replicates per experiment, and each bar is the mean ± SD. The statistical significance of differences between the His-tagged, AvBD2, AvBD6, and AvBD12 was assessed using the generalized linear model (GLM) procedure of SAS software (SAS 1996). The values with different letters are significantly different (P < 0.05)
Discussion
IBV exhibits mortality rates as high as 30 % in chickens less than 4 weeks old, and it causes losses in egg production (Kameka et al. 2014). Live attenuated viral vaccines are available to control IBV, and they have been very reliable (Cavanagh 2003, 2007; Kameka et al. 2014). However, the emergence of new IBV variants, which led to infectious bronchitis outbreaks in vaccinated flocks, resulted in significant production losses (Xu et al. 2007; Shimazaki et al. 2009; Kameka et al. 2014). Therefore, a novel approach that could be used as an alternative to, or in addition to, the existing means of control is urgently needed. One such approach may be the use of innate immune mediators to empower the innate immune system.
Generally, innate immunity induced by IBV occurs during the early phase of infection (e.g., 3–5 days after infection) (Wang et al. 2006). Our previous report showed that the immunogenicity of IBV strain CK/CH/LHLJ/04V decreased and that the immune response of chickens to a CK/CH/LHLJ/04V strain that was passaged 110 times in embryonated chicken eggs also delayed (Liu et al. 2009). None of the chickens showed seroconversion at 7 dpi with CK/CH/LHLJ/04V P110, and only 40 % of the P110-inoculated chickens showed seroconversion at 12 dpi (Liu et al. 2009). Hence, we investigated the differential modulation of AvBD and TLR expression in chickens infected with CK/CH/LHLJ/04V highly pathogenic P3 at 7 dpi in comparison with P110-infected and control chickens.
Our findings showed that chickens are likely to respond differently to various pathogenic IBV strains. We found that chickens infected with the P3 strain showed an increased production of AvBDs, including AvBD2, 4, 5, 6, 9, and 12, in comparison with the P110 strain, which downregulated the expression of AvBDs, including AvBD3, 4, 5, 6, and 9, in several tissues, including digestive and immune organs. The expression of AvBD2, 6, and 12 was particularly upregulated in P3-infected chickens. Consistent with the present study, two recent studies found that the expression of AvBDs in the livers of ducks was significantly upregulated in response to duck hepatitis virus (DHV) infection (Ma et al. 2011, 2012b). Similarly, increased levels of β-defensins in tissues or cells of other animal species, including lung homogenates of lambs and lungs of mice, and even human epithelial cells, have also been observed in vitro or in vivo following viral infections (Duits et al. 2003; Grubor et al. 2004; Proud et al. 2004; Chong et al. 2006, 2008). These results suggested that β-defensins, including AvBDs, play an essential role in the animal defense against viral infection. However, in contrast to the upregulation of expression that was observed in the tissues of P3-infected chickens, several AvBDs were downregulated in P110-infected chickens. It is likely that different signaling pathways of host were activated by the different IBVs with variant pathogenicity though the exact mechanism needed to be further investigated. Similar results were found that TLR7 gene expression was downregulated in variant infectious bursal disease virus (IBDV)-infected bursa; however, in classic IBDV-infected bursas, TLR7 gene expression was upregulated (Rauf et al. 2011).
Similar to the expression pattern of AvBDs, the expression level of TLRs showed a general trend toward upregulation in the tissues of P3-infected chickens, while they were downregulated in the tissues of P110-infected chickens. We observed a significant increase in TLR1 messenger RNA (mRNA) expression in both the small and large intestines, TLR2 mRNA expression in both the kidneys and small intestine, and TLR4 mRNA expression in both the large intestine and bursa of Fabricius in P3-infected chickens. These results paralleled the above findings that chickens infected with the P3 strain exhibited an increased production of AvBDs in digestive and immune organs. These observations are in accordance with earlier reports demonstrating that mouse β-defensin 2 activates immature dendritic cells through an interaction with TLR4 (Biragyn et al. 2001) and that human β-defensin 3 activates antigen-presenting cells via TLR1 and TLR2 in an nuclear factor kappa B (NF-κB)-dependent manner (Funderburg et al. 2007, 2011). Previous studies have also shown an increase in the mRNA expression of TLR2, TLR3, TLR6, and TLR7 following IBV immunization (Guo et al. 2008; Wang et al. 2006). The nature and the extent of the innate host responses elicited against IBV are not known. Surprisingly, we did not detect significant changes in TLR3, 5, 7, 15, and 21 expression following infection with the P3 strain. In contrast, a previous study detected a significant increase in both TLR3 and TLR7 mRNA expression in the tracheas of Massachusetts-type IBV-infected chickens (Kameka et al. 2014), which could be due to the use of different virulent vaccine strains in their study. However, because of the downregulation of mRNA expression that was observed for several AvBDs in most tissues in P110-infected chickens compared with P3-infected or control chickens, we were not surprised to see a similar decrease in the expression of TLRs in most of these tissues as well, though the reason was not known and needed to be further investigated. Furthermore, relationship between AvBD and TLR expression analysis showed that AvBDs (2, 4–6, 9–12) correlated positively with different TLRs (including 1–5, 7, and 21) from chickens infected by IBV.
Findings obtained in mammals showed that the microbial ligand-mediated induction of TLR signaling in cells, via activation of the transcription factor NF-κB, induces the expression and secretion not only of pro-inflammatory cytokines (Galvan-Moroyoqui et al. 2011), but also of defensins, such as HBD2 and HBD3, into the extracellular milieu (Abreu et al. 2002; Yoon et al. 2010). In a recent study, it is shown that the expression of AvBD10 can be downregulated by TLR ligands in the uterus of laying hens (Abdel-Mageed et al. 2014). The present results about correlation between TLR expression and AvBD expression in chickens infected with IBV further confirm these findings.
Of the 14 AvBDs detected in the present study, the mRNA expressions of AvBD2, 6, and 12 were found to be upregulated extensively in the tissues following infection with the IBV pathogenic P3 strain and were chosen for further discussion in this study. His-tagged AvBD2, 6, and 12 were produced and purified to further characterize their antiviral activities. In contrast to the extensive studies on the antimicrobial activities of AvBDs, reports on the antiviral activity of such peptides, especially AvBDs, are limited. In avian species, Apl-AvBDs exhibited significant antiviral activity against DHV in vitro (Ma et al. 2011, 2012b). Similarly, our experiment showed that AvBD2, 6, and 12 demonstrated obvious anti-viral activities against IBV in vitro. The results further confirm that AvBDs might play significant roles in the resistance of chickens to IBV replication.
It has been shown that viruses, including IBV, NDV, and AIV H9 subtype infection, can elicit inflammatory responses characterized by recruitment of myeloid or lymphoid cells. Consequently, the cellular types of tissues harvested from pathogenic and attenuated virus-inoculated chickens for gene expression analysis may be different due to immune cell infiltration. As a result, the changes revealed by gene expression analysis of some genes such as AvBDs and TLRs that could also be expressed in immune cells in the course of viral infection may be caused or partly caused by cellular heterogeneity occurring during the inflammatory response to viral infection (including infiltration by innate and adaptive immune cells). This needed to be further investigated.
In spite of the novel aspect our work on the evaluation of differential modulation of AvBD, TLR, and iNOS expression in response to IBV infection in vivo, analysis of expression of these molecules may not be sufficient to completely understand IBV immunologic mechanisms. In addition, the underlying mechanism of the natural host’s response to attenuated IBV strain (P110) could not be explained and remained unknown. Furthermore, whether known chicken TLR ligands can induce AvBD expression in vivo or in vitro in chickens needs to be studied further. Therefore, further analysis of the above mentioned concerns is warranted.
In conclusion, this study reports striking difference in the activation of host responses by a pathogenic IBV strain (P3) and an attenuated IBV strain (P110). Compared with the P110 strain, the P3 strain induced a more production of AvBDs and TLRs. The present study highlights some of the underlying mechanisms of induction of host responses by IBV in chickens. Further studies may employ these findings to select AvBDs for enhancing immune responses in chickens.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 355 kb)
Acknowledgments
The study was partly supported by Specialized Research Fund for the science and technological innovation talent of Harbin (2013RFXXJ019), Research Program for Applied Technology of Heilongjiang Province (PC13S02), Specialized Research Fund for the Doctoral Program of Higher Education (20122325110016), and grants from the China Agriculture Research Systerm (No. CARS-41-K12).
Ethics statement
The animals had received humane care throughout all the procedures in accordance with the Guide for the Care and Use of Laboratory Animals (http://www.nap.edu/openbook.php?record_id=5140&page=R1). All animal experimental procedures were approved by the Ethical and Animal Welfare Committee of Heilongjiang province, China (License no. SQ20130507).
Contributor Information
Deying Ma, Phone: (86)451-55190862, Email: mdy296@sohu.com.
Shengwang Liu, Phone: (86)451-51997169, Email: swliu@hvri.ac.cn.
References
- Abdel-Mageed AM, Isobe N, Yoshimura Y. Effect of different TLR ligands on the expression of proinflammatory cytokines and avian beta-defensins in the uterine and vaginal tissues of laying hens. Vet Immunol Immunopathol. 2014;162:132–141. doi: 10.1016/j.vetimm.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–20437. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- Barabas N, Röhrl J, Holler E, Hehlgans T. Beta-defensins activate macrophages and synergize in pro-inflammatory cytokine expression induced by TLR ligands. Immunobiology. 2013;218:1005–1011. doi: 10.1016/j.imbio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Biragyn A, Surenhu M, Yang D, Ruffini PA, Haines BA, Klyushnenkova E, Oppenheim JJ, Kwak LW. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. J Immunol. 2001;167:6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpenter S, O’Neill LA. How important are Toll-like receptors for antimicrobial responses? Cell Microbiol. 2007;9:1891–1901. doi: 10.1111/j.1462-5822.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chong KT, Xiang L, Wang X, Jun EL, Xi LF, Schweinfurth JM. High level expression of human epithelial β-defensins (hBD-1, 2 and 3) in papillomavirus induced lesions. Virol J. 2006;3:75. doi: 10.1186/1743-422X-3-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong KT, Thangavel RR, Tang X. Enhanced expression of murine β-defensins (MBD-1, -2, -3, and -4) in upper and lower airway mucosa of influenza virus infected mice. Virology. 2008;380:136–143. doi: 10.1016/j.virol.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Cong F, Liu X, Han Z, Shao Y, Kong X, Liu S. Transcriptome analysis of chicken kidney tissues following coronavirus avian infectious bronchitis virus infection. BMC Genomics. 2013;14:743. doi: 10.1186/1471-2164-14-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits LA, Nibbering PH, van Strijen E, Vos JB, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Rhinovirus increases human β-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol Med Microbiol. 2003;38:59–64. doi: 10.1016/S0928-8244(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Fang S, Chen B, Tay FP, Ng BS, Liu DX. An arginine-to-proline mutation in a domain with undefined functions within the helicase protein (Nsp13) is lethal to the coronavirus infectious bronchitis virus in cultured cells. Virology. 2007;358:136–147. doi: 10.1016/j.virol.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV, Weinberg A, Sieg SF. Human-defensin-3 activates professional antigen -presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci U S A. 2007;104:18631–18635. doi: 10.1073/pnas.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburg NT, Jadlowsky JK, Lederman MM, Feng Z, Weinberg A, Sieg SF. The Toll-like receptor 1/2 agonists Pam(3) CSK(4) and human β-defensin-3 differentially induce interleukin-10 and nuclear factor-κB signaling patterns in human monocytes. Immunology. 2011;134:151–160. doi: 10.1111/j.1365-2567.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Moroyoqui JM, Del Carmen Dominguez-Robles M, Franco E, Meza I. Pathogenic bacteria prime the induction of Toll-like receptor signalling in human colonic cells by the Gal/GalNAc lectin carbohydrate recognition domain of Entamoeba histolytica. Int J Parasotol. 2011;41:1101–1112. doi: 10.1016/j.ijpara.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Ganz T, Lehrer RI. Antibiotic peptides from higher eukaryotes: biology and applications. Mol Med Today. 1999;5:292–297. doi: 10.1016/S1357-4310(99)01490-2. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Gelb J, Jr, Weisman Y, Ladman BS, Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996-2000) Avian Pathol. 2005;34:194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- Grubor B, Gallup JM, Meyerholz DK, Crouch EC, Evans RB, Brogden KA, Lehmkuhl HD, Ackermann MR. Enhanced surfactant protein and defensin mRNA levels and reduced viral replication during parainfluenza virus type 3 pneumonia in neonatal lambs. Clin. Vaccine Immunol. 2004;11:599–607. doi: 10.1128/CDLI.11.3.599-607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Rosa AJ, Chen DG, Wang X. Molecular mechanisms of primary and secondary mucosal immunity using avian infectious bronchitis virus as a model system. Vet Immunol Immunopathol. 2008;121:332–343. doi: 10.1016/j.vetimm.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson KA, Scott PC, Devlin JM, Ignjatovic J, Noormohammadi AH. The presence of viral subpopulations in an infectious bronchitis virus vaccine with differing pathogenicity—a preliminary study. Vaccine. 2012;30:4190–4199. doi: 10.1016/j.vaccine.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J, Galli U. Immune responses to structural proteins of avian infectious bronchitis virus. Avian Pathol. 1995;24:313–332. doi: 10.1080/03079459508419072. [DOI] [PubMed] [Google Scholar]
- Kameka AM, Haddadi S, Kim DS, Cork SC, Abdul-Careem MF. Induction of innate immune response following infectious bronchitis coronavirus infection in the respiratory tract of chickens. Virology. 2014;450–451:114–121. doi: 10.1016/j.virol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra AM, de Zoete MR, Bouwman LI, Vaezirad MM, van Putten JPM. Unique features of chicken Toll-like receptors. Dev Comp Immunol. 2013;41:316–323. doi: 10.1016/j.dci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/S0952-7915(01)00303-X. [DOI] [PubMed] [Google Scholar]
- Liao Y, Wang X, Huang M, Tam JP, Liu DX. Regulation of the p38 mitogen-activated protein kinase and dual-specificity phosphatase 1 feedback loop modulates the induction of interleukin 6 and 8 in cells infected with coronavirus infectious bronchitis virus. Virology. 2011;420:106–116. doi: 10.1016/j.virol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang Q, Chen J, Han Z, Liu X, Feng L, Shao Y, Rong J, Kong X, Tong G. Genetic diversity of avian infectious bronchitis coronavirus strains isolated in China between 1995 and 2004. Arch Virol. 2006;151:1133–1148. doi: 10.1007/s00705-005-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhang X, Gong L, Yan B, Li C, Han Z, Shao Y, Li H, Kong X. Altered pathogenicity, immunogenicity, tissue tropism and 3′-7kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos. Vaccine. 2009;27:4630–4640. doi: 10.1016/j.vaccine.2009.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma H, Xu Q, Sun N, Han Z, Sun C, Guo H, Shao Y, Kong X, Liu S. Characterization of a recombinant coronavirus infectious bronchitis virus with distinct S1 subunits of spike and nucleocapsid genes and a 3′ untranslated region. Vet Microbiol. 2013;162:429–436. doi: 10.1016/j.vetmic.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DY, Lin LJ, Zhang KX, Han ZX, Shao YH, Liu XL, Liu SW. Three novel Anas platyrhynchos avian β-defensins, upregulated by duck hepatitis virus, with antibacterial and antiviral activities. Mol Immunol. 2011;49:84–96. doi: 10.1016/j.molimm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Ma DY, Lin LJ, Zhang KX, Han ZX, Shao YH, Wang RQ, Liu SW. Discovery and characterization of Coturnix chinensis avian β-defensin 10, with broad antibacterial activity. J Pept Sci. 2012;18:224–232. doi: 10.1002/psc.1437. [DOI] [PubMed] [Google Scholar]
- Ma DY, Zhang KX, Zhang MY, Liu XL, Han ZX, Shao YH, Liu SW (2012b) Identification, expression and activity analyses of five novel duck beta-defensins. PLoS ONE 7(10):e47743. doi:10.1371/journal.pone.0047743 [DOI] [PMC free article] [PubMed]
- Ma DY, Zhou CY, Zhang MY, Han ZX, Shao YH, Liu SW. Functional analysis and induction of four novel goose (Anser cygnoides) avian β-defensins in response to salmonella enteritidis infection. Comp Immunol Microbiol Infect Dis. 2012;35:197–207. doi: 10.1016/j.cimid.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Ma DY, Zhang MY, Zhang KX, Liu XL, Han ZX, Shao YH, Liu SW (2013) Identification of three novel avian beta-defensins from goose and their significance in the pathogenesis of Salmonella. Mol Immunol 56:521–529 [DOI] [PubMed]
- Ma DY, Xu Y, Li YY, Xu QQ, Zhang TT, Han ZX, Liu SW. Recombination expression and biological analysis of chicken β-defensin 12. J Northeast Agric Univ. 2014;10:49–56. [Google Scholar]
- Meade KG, Higgs B, Lloyd AT, Giles S, O’Farrelly C. Differential antimicrobial peptide gene expression patterns during early chicken embryological development. Dev Comp Immunol. 2009;33:516–524. doi: 10.1016/j.dci.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Nijnik A, Pistolic J, Filewod NC, Hancock RE. Signaling pathways mediating chemokine induction in keratinocytes by cathelicidin LL-37 and flagellin. J Innate Immun. 2012;4:377–386. doi: 10.1159/000335901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE (2004) Duck virus hepatitis. In: Manual of diagnostic tests and vaccines for terrestrial animals. Part 2. Section 2.7. Chapter 2.7.9. http://www.oie.int/eng/publicat/enstandards.htm
- Otsuki K, Nakamura T, Kubota N, Kawaoka Y, Tsubokura M. Comparison of two strains of avian infectious bronchitis virus for their interferon induction, viral growth and development of virus-neutralizing antibody in experimentally-infected chickens. Vet Microbio. 1987;15:31–40. doi: 10.1016/0378-1135(87)90126-X. [DOI] [PubMed] [Google Scholar]
- Pei J, Sekellick MJ, Marcus PI, Choi IS, Collisson EW. Chicken interferon type I inhibits infectious bronchitis virus replication and associated respiratory illness. J Interferon Cytokine Res. 2001;21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- Presicce P, Giannelli S, Taddeo A, Villa ML, Della Bella S. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J Leukoc Biol. 2009;86:941–948. doi: 10.1189/jlb.0708412. [DOI] [PubMed] [Google Scholar]
- Proud D, Sanders SP, Wiehler S. Human rhinovirus infection induces airway epithelial cell production of human β-defensin 2 both in vitro and in vivo. J Immunol. 2004;172:4637–4645. doi: 10.4049/jimmunol.172.7.4637. [DOI] [PubMed] [Google Scholar]
- Quintana AM, Landolt GA, Annis KM, Hussey GS. Immunological characterization of the equine airway epithelium and of a primary equine airway epithelial cell culture model. Vet Immunol Immunopathol. 2011;140:226–236. doi: 10.1016/j.vetimm.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Raj GD, Jones RC. Infectious bronchitis virus: immunopathogenesis of infection in the chicken. Avian Pathol. 1997;26:677–706. doi: 10.1080/03079459708419246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A, Khatri M, Murgia MV, Jung K, Saif YM. Differential modulation of cytokine, chemokine and Toll like receptor expression in chickens infected with classical and variant infectious bursal disease virus. Vet Res. 2011;42:85. doi: 10.1186/1297-9716-42-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS . Institute. SAS user’s guide: statistics. Cary: SAS Institute Inc.; 1996. [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shen S, Law YC, Liu DX. A single amino acid mutation in the spike protein of coronavirus infectious bronchitis virus hampers its maturation and incorporation into virions at the nonpermissive temperature. Virology. 2004;326:288–298. doi: 10.1016/j.virol.2004.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki Y, Watanable Y, Harada M, Seki Y, Kuroda Y, Fukuda M, Honda E, Suzuki S, Nakamura S. Genetic analysis of the S1 gene of 4/91 type infectious bronchitis virus isolated in Japan. J Vet Med Sci. 2009;71:583–588. doi: 10.1292/jvms.71.583. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva AI, Kulkarni RR, Sharif S. Synthetic double-stranded RNA oligonucleotides are immunostimulatory for chicken spleen cells. Dev Comp Immunol. 2011;35:28–34. doi: 10.1016/j.dci.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rosa AJ, Oliverira HN, Rosa GJ, Guo X, Travnicek M, Girshick T. Transcriptome of local innate and adaptive immunity during early phase of infectious bronchitis viral infection. Viral Immunol. 2006;19:768–774. doi: 10.1089/vim.2006.19.768. [DOI] [PubMed] [Google Scholar]
- Wang R, Ma D, Lin L, Zhou C, Han Z, Shao Y, Liao W, Liu S. Identification and characterization of an avian β-defensin orthologue, avian β-defensin 9, from quails. Appl Microbiol Biotechnol. 2010;87:1395–1405. doi: 10.1007/s00253-010-2591-6. [DOI] [PubMed] [Google Scholar]
- Xu C, Zhao J, Hu X, Zhang G. Isolation and identification of four infectious bronchitis virus strains in China and analyses of their S1 glycoprotein gene. Vet Microbiol. 2007;122:61–71. doi: 10.1016/j.vetmic.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Yoon YM, Lee JY, Yoo D, Sim YS, Kin YJ, Oh YK, Kang JS, Kim S, Kim JS, Kim JM. Bacteroides fragilis enterotoxin induces human beta-defensin-2 expression in intestinal epithelial cells via a mitogen-activated protein kinase/I kappaB kinase/NF-kappaB-dependent pathway. Infect Immun. 2010;78:2024–2033. doi: 10.1128/IAI.00118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 355 kb)


