Abstract
Introduction:
Approximately 5–8% of children report severe chronic pain and disability. Although evidence supports pain-self management as effective for reducing pain and disability, most youth do not have access to psychological intervention. Our prior studies demonstrate that an existing internet-delivered pain self-management program (WebMAP) can reduce barriers to care, is feasible, acceptable, and is effective in reducing pain-related disability in youth with chronic pain. The current trial seeks to: 1) determine the effectiveness of a mobile app version of WebMAP for improving patient pain-related outcomes, and 2) evaluate a novel implementation strategy to maximize the public health impact of the intervention including the determination of adoption, reach, and sustainability of WebMAP in specialty clinics serving children with chronic pain in the United States.
Methods:
This hybrid effectiveness-implementation cluster randomized controlled trial employs a stepped wedge design in which the WebMAP mobile intervention is sequentially implemented in 8 specialty pain clinics following a usual care period. This trial aims to enroll a minimum of 120 youth (ages 10–17) who have chronic pain. Outcome analyses will determine effectiveness of treatment on adolescent pain-related outcomes as well as public health impact via reach, adoption, implementation, and maintenance.
Conclusions:
This trial examines an innovative approach to evaluate a technology-delivered pain self-management program for youth with chronic pain. Findings are expected to yield a strategic approach for delivering a digital pain management program for youth with chronic pain that can be sustained in clinical settings.
Clinical Trial Registration #:
Keywords: Chronic pain, Adolescence, Psychological intervention, Cluster randomized controlled trial, Technology, Mobile application
1. Introduction
Approximately 20% of youth report chronic pain, with around 5–8% experiencing significant associated disability [1]. Psychological treatments for chronic pain that teach pain self-management skills are recommended to address pain-related disability, and have been shown effective for children and adolescents [2]. However, major barriers exist, including geographical distance, limited scheduling, and long waiting lists, preventing most youth from receiving psychological treatment for chronic pain [3]. In the United States, there are only about 45 interdisciplinary pediatric chronic pain programs to serve these patients [4], creating a challenge as the demand for pain services is greater than the supply of services available. Innovations are needed to improve access, and to identify cost-effective and sustainable delivery mechanisms such as through use of technology.
Digital health interventions delivered via technologies such as smartphones, web sites, and text messaging are an ideal medium to provide evidence-based, low-cost pain self-management interventions. Access to Internet and smartphone technologies among American adolescents and parents is high (> 92%), even among those of low income [5]. A Cochrane systematic review of the pediatric chronic pain literature identified eight randomized controlled trials (RCTs), which all delivered cognitive-behavioral therapy (CBT) interventions using technology, and promising emerging data indicate improvements in pain and disability [6].
Our research group developed the WebMAP program, an Internet-delivered CBT pain intervention for children with chronic pain showing excellent patient engagement, feasibility across numerous clinics, and effectiveness in improving relevant pain-related outcomes [7–9]. In our recent multicenter RCT with 273 adolescents with chronic abdominal, headache, or musculoskeletal pain [8] we reported improvements in daily physical functioning, depressive symptoms, and parent-perceived impact of pain in families receiving the 8-week Internet pain self-management program, compared to an Internet pain education control group. Treatments delivered remotely overcome many barriers of access to health care professionals and require a fraction of the therapist time, which is particularly relevant in pediatric chronic pain, where few resources exist to provide interdisciplinary pain care for children. However, digital health interventions for pediatric chronic pain have not been implemented or sustained in routine care settings that serve children with chronic pain.
The first aim of this hybrid effectiveness-implementation trial is to determine effectiveness of a smartphone application version of this program (WebMAP mobile) on individual pain-related outcomes when delivered in real-world settings. The primary outcome is pain-related disability, and secondary outcomes include pain symptoms, anxiety and depressive symptoms, insomnia, and self-efficacy for managing pain. Our secondary aim is to evaluate an implementation and dissemination strategy for adoption and sustainability of WebMAP mobile in specialty care settings treating youth with chronic pain in the United States. To reach this aim, we will use the Reach, Effectiveness, Adoption, Implementation, Maintenance (RE-AIM) public health impact framework [10]. Given the high prevalence and impact of pediatric chronic pain and the promise of digital technologies to extend access to care, results of this trial are expected to yield important data on a strategic approach to dissemination of digital health interventions in clinical settings for youth with chronic pain.
2. Methods
2.1. Participants and setting
The sample will include a minimum of 120 youth ages 10–17 years with chronic pain and one participating parent recruited from 8 specialty clinics at 5 children’s hospitals across the United States: Seattle Children’s Hospital (gastroenterology clinic, pain clinic), Children’s Mercy Medical Center (abdominal pain clinic, pain clinic), C.S. Mott Children’s Hospital (pain clinic), Nationwide Children’s Hospital (pain clinic), and Connecticut Children’s Medical Center (gastroenterology clinic, pain clinic). Study sites were selected to obtain a range of clinic sizes and regions of the United States.
Criteria for patient inclusion are purposefully broad to study real-world effectiveness: 1) child age 10–17 years, 2) has chronic pain de-fined as pain present for at least 3 months, and 3) has access to a smartphone. Exclusion criteria include: 1) non-English speaking, 2) presently in a psychiatric crisis (e.g., recent inpatient admission, suicide attempt), 3) does not have access to a smartphone, and 4) is unable to read at the 5th grade level. This study has been approved by the Institutional Review Board at the primary site (Seattle Children’s Research Institute) and all the Institutional Review Boards of the referring study sites.
2.2. Recruitment
Providers at referring centers will determine interest in the study, provide potential participants with a flyer about the study and ask whether they are willing to be contacted by phone by study staff to undergo additional screening. Providers will then securely transfer referral information to study staff via a dedicated study website, email, or fax. Potential participants can also contact study staff directly by calling a toll-free number provided on the study flyer. Study staff not involved in intervention assignment will screen potential participants via phone and if eligible will obtain verbal parent consent and child assent for study participation.
2.3. Study design
This is a hybrid effectiveness-implementation trial employing a stepped wedge cluster randomized design with clinic as the unit of randomization (see Figure 1). This design includes an initial control period during which none of the clinics are exposed to the intervention and participants receive care as usual at the respective clinic (“un-exposed period”). Subsequently, each of the 8 clinics are randomized to cross from the control period (“non-exposure period”) to the intervention period (“exposure period”) at four randomized waves (2 clinics per wave). During exposure periods, patients in these clinics will receive access to the WebMAP interventions (mobile app for children and web site for parents) to learn pain self-management skills. The intervention phase for individual patients will last 8 weeks. In total, the study will be conducted over a 24-month period: Eighteen months will be devoted to study enrollment, randomization, and completion of the intervention. An additional 6 months will be dedicated to completing T2 and T3 follow-up assessments and examining implementation outcomes.
Fig. 1.
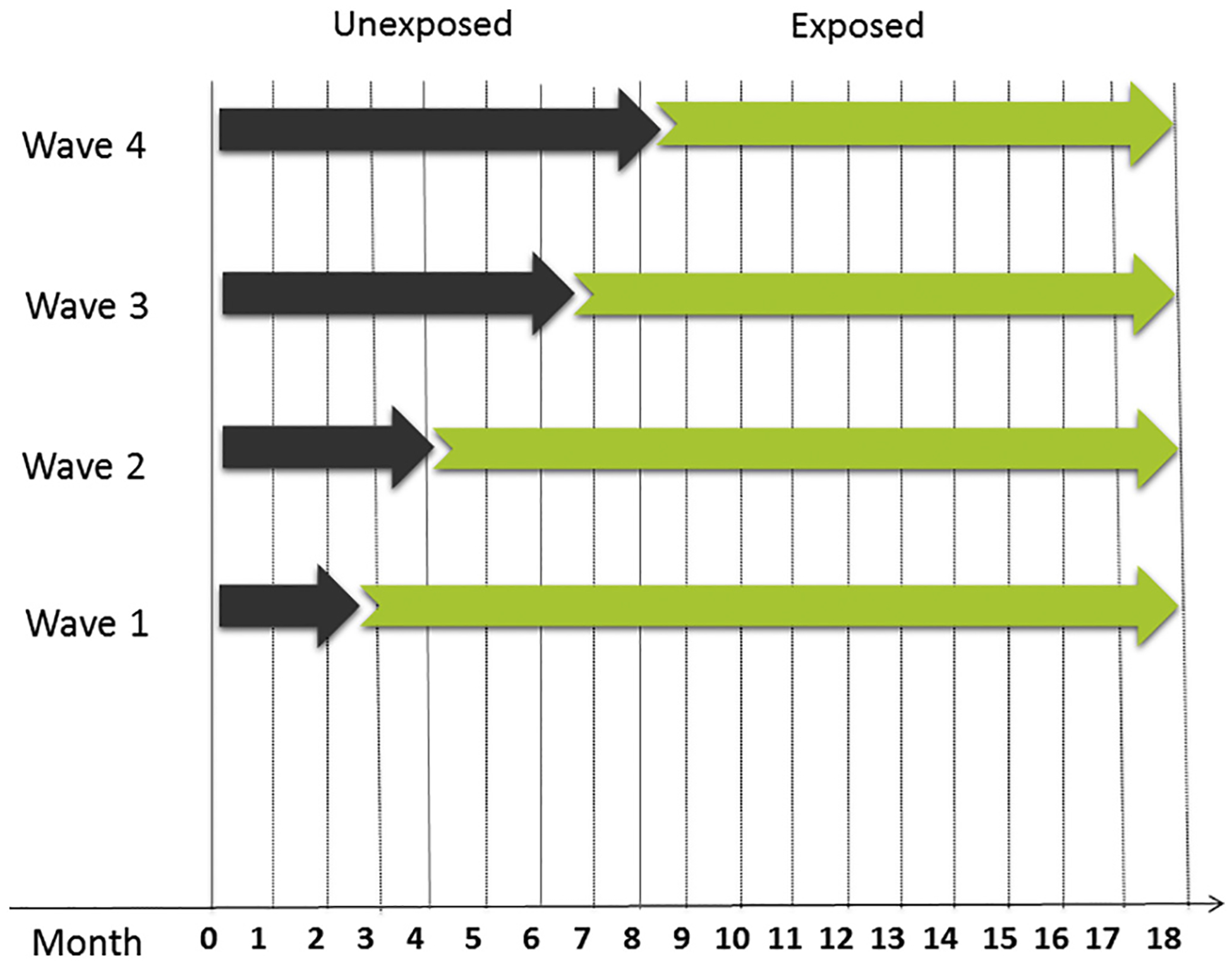
Stepped wedge study design.Note. The 8 clinics are randomized to cross from the “unexposed period” to the “exposed” period (intervention period) at four randomized waves with 2 clinics per wave.
2.4. Rationale for study design
We chose to use a stepped wedge cluster randomized controlled trial with clinic as the unit of randomization (rather than individual patients) because we are testing both effectiveness and implementation. The stepped wedge design is recommended in hybrid effectiveness-implementation trials. This design promotes external validity and allows a blended ability to test both intervention effectiveness and effectiveness of implementation strategies [11]. We are testing the clinical effectiveness in real world clinic settings with limited inclusion/exclusion criteria in order to study outcomes in the typical patients presenting to these clinics. The design includes random and sequential crossover of clusters from control to intervention until all clusters are exposed. Data collection continues throughout the study, so that each cluster contributes observations under both control (“non-exposed”) and intervention (“exposed”) observation periods. Each clinic ends in the exposed period with the intervention in place so that implementation outcomes can be studied. This design is preferred when evidence exists in support of the intervention (as is the case with our WebMAP intervention) in order to offer known effective treatment to a clinic.
2.5. Randomization
Randomization will be implemented using a computer-generated randomization schedule to derive a random assignment for each of the 8 clinics to the four exposure intervals in the stepped wedge design. The randomization schedule will be created by the study biostatistician who is not involved in participant recruitment or intervention assignment. The randomization schedule will be stored in a password-protected document accessible only to study staff involved in intervention assignment. To ensure that study staff who perform the intervention assignment are blinded to the randomized allocation sequences, each participant’s group assignment will be revealed only after the participant completes the pre-treatment assessment. Study staff involved in participant recruitment will not be involved in intervention assignment or outcome assessment procedures. Because we are employing a usual care unexposed period, it will not be possible for participants to be blinded to their intervention group assignment.
2.6. Intervention procedures
As part of their routine care, all enrolled participants will receive an initial evaluation for chronic pain at their referring specialty clinic and subsequently may receive recommendations for treatment (e.g., for physical therapy, psychology, and/or medication management). Usual care as recommended by each participant’s specialty clinic team will not be altered for this clinical trial. All study-related interventions will be adjunctive to care provided in the specialty clinics.
2.6.1. Usual care control condition (non-exposure period)
All clinics will have a non-exposure period during which patients will receive usual care for their chronic pain from the specialty clinic. The 8 clinics will be randomized in 4 waves (i.e., 2 clinics per wave) following non-exposure periods of 2 to 9 months.
2.6.2. WebMAP mobile intervention condition (exposure period)
In addition to usual care, during the exposure period, youth recruited into the study will receive access to the mobile app version of the program, called WebMAP Mobile, available on Android and iPhone operating systems. Their parents will receive access to our WebMAP parent internet program.
2.6.2.1. Teen Intervention: Web-MAP mobile.
The program design and treatment content of WebMAP follow cognitive-behavioral, social learning, and family systems frameworks. The WebMAP Mobile program is an interactive, self-guided intervention including six core treatment modules and two supplementary treatment modules. Core treatment modules include: 1) pain education, 2) stress, emotions, and thoughts (e.g., pleasant activity scheduling, thought stopping), 3) relaxation and imagery, 4) lifestyle and school interventions (e.g., sleep habits, school plan), 5) staying active (e.g., activity pacing, graded exposure), and 6) maintenance and relapse prevention. Within the app, youth complete screening questions to set up their personal profile and to evaluate the need for modules targeting negative mood and insomnia symptoms. The Patient Health Questionnaire 2 [12] screens for depressive symptoms, with scores of 3 or higher targeting release of a supplementary module to address negative mood. Difficulties with falling or staying asleep are screened using two items from the Adolescent Sleep Wake Scale [13] with endorsement of “quite often” or greater for either symptom triggering release of a supplementary module targeting insomnia symptoms. Treatment duration is 6 to 8 weeks depending on the number of supplementary modules assigned, and youth can continue using the app to monitor and practice skills as long as they desire.
The main adaptations required to transform the web-based program into an app consisted of trimming text redundancies and adding new features that are unique to mobile technologies including text notifications for positive reinforcement and encouragement to continue the program. The main functional components of the app include the following (see Figure 2):
Fig. 2.
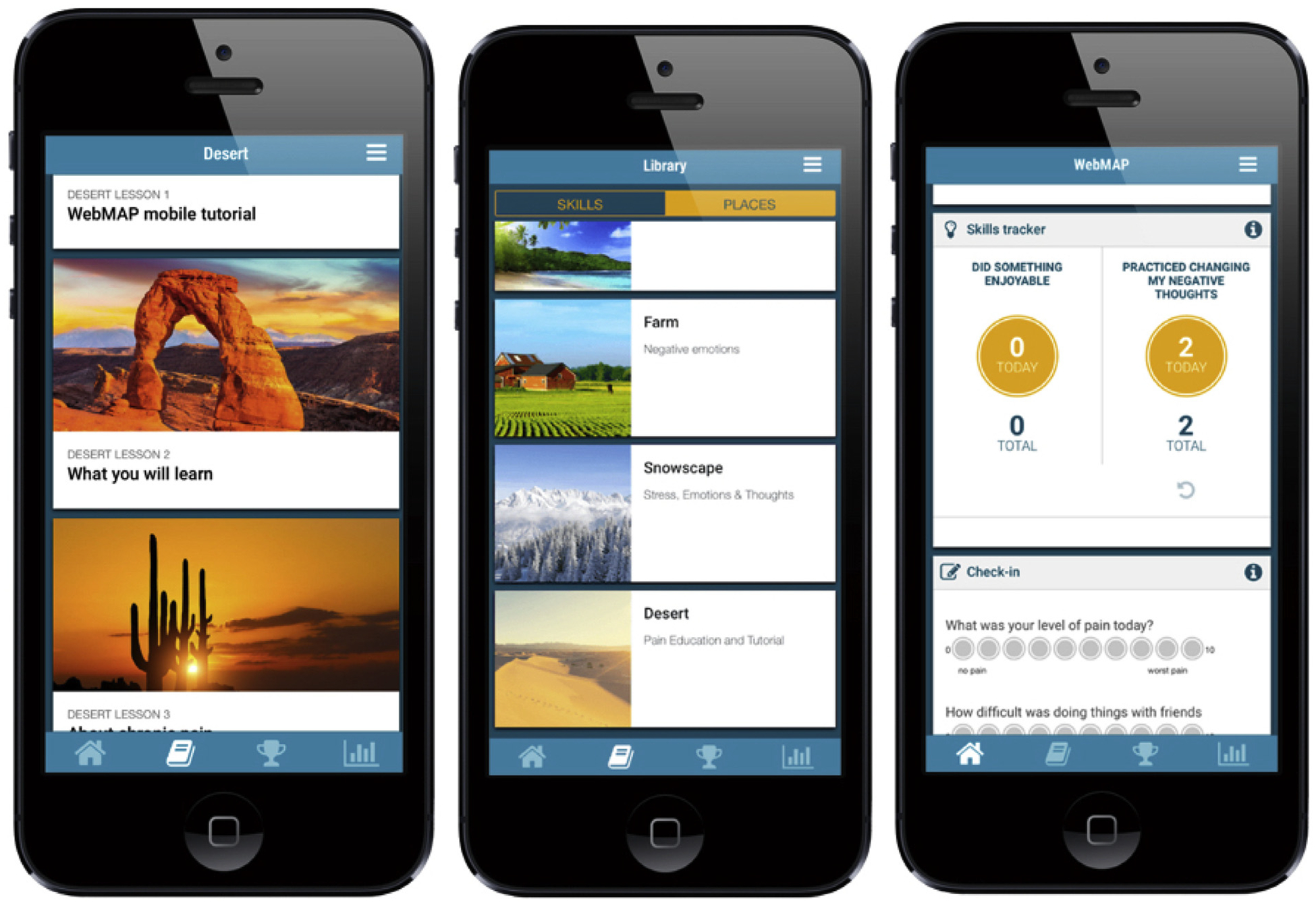
Screen shots from the WebMAP Mobile Program.
Places and Lessons. The program has a travel theme and youth will be able to visit 6–8 “Places” (e.g., desert) corresponding with the core treatment modules (above). Each place has 3–5 lessons that provide education and teach pain management skills. Once participants finish the lessons, they review their knowledge with brief quizzes and complete an assignment corresponding with skills taught in the modules (e.g., daily relaxation practice using audio files provided on the app). Youth are asked to practice skills in each assignment for at least 5 days. Upon completion of assignments, youth receive a reward (i.e., badge) in the app and are allowed access to the next module.
Library. The Library contains information pertaining to each skill learned in the program delivered via audio clips, videos of peers, and infographics. The library is intended to assist with skills practice and increase interactivity.
Check in. The app allows youth to track their pain level, pain impact, sleep, and mood symptoms on 0 to 10 scales through a Daily Check in. These scores generate a customized graph that is updated daily to allow users to track their symptoms and progress.
Skills Tracker. In the Skills Tracker, youth will record practice of specific skills (e.g., improving sleep, relaxation, etc). As youth complete modules, new skills learned appear in the Skills Tracker.
2.6.2.2. Parent Intervention: Web-MAP Web Site.
During the intervention period, parents of children will receive access to the WebMAP parent web site, which has already been developed and evaluated by our team and is described in detail elsewhere [8]. We did not develop a mobile app version for parents but instead chose to provide them with access to the existing internet version of WebMAP. This decision was based on qualitative data collected from families, which specifically revealed that youth desired a mobile app version. Parents are taught a range of strategies including operant strategies, the importance of modeling, supporting independence, and enhancing communication with their adolescent. The eight parent modules are: 1) education about chronic pain, 2) recognizing stress and negative emotions, 3) operant strategies I (using attention and praise to increase positive coping), 4) operant strategies II (using rewards to increase positive coping; strategies to support school goals), 5) modeling, 6) sleep hygiene and lifestyle, 7) communication, and 8) relapse prevention. The WebMAP parent program includes weekly behavioral assignments to facilitate skills practice in 6 of the 8 modules, as well as vignettes, videos of peers, illustrations, and reinforcing quizzes. Parents will be asked to complete 1 module per week over the 8-week intervention period.
2.7. Effectiveness outcomes
Clinical effectiveness outcomes will be evaluated via patient and parent surveys, which will be completed online through the secure, web-based application REDCap (Research Electronic Data Capture) [14]. All participants will complete an initial baseline assessment including completion of daily diaries for 7-days prior to randomization. The intervention period is 8 weeks. At eight weeks, participants will repeat assessments including the 7-day post-treatment diary assessment, and similarly at three months they will complete outcome measures including the 7-day follow-up diary assessment. Patient surveys will be completed independently by children and their parents. See Table 1 for a list of the measures administered at each assessment point.
Table 1.
Study measures.
| Measure | Description of measure | Respondent | Time point administered | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Screening and background measures | |||||
| Pain History Form | Includes questions about spatial distribution of pain, health services and medications used during treatment period, pain diagnose(s), and pain duration. | Youth, Caregiver | ✓ | ✓ | ✓ |
| Caregiver Information Form | Report on sociodemographic information including age, ethnicity, parent martial status, education, and family income. | Caregiver | ✓ | ||
| Patient Health Questionnaire (PHQ-2) | 2-item measure that screens for frequency of depressive symptoms in the past 2 weeks. Used to screen for delivering the optional app module addressing negative mood. | Youth | ✓ | ||
| Adolescent Sleep Wake Scale (ASWS) - Insomnia | 2 items from the ASWS screen for frequency of insomnia symptoms over the past month. Used to screen for delivering the optional app module addressing insomnia. | Youth | ✓ | ||
| Primary outcome measure | |||||
| Activity Limitations (CALI-9) | Completed on the online diary to rate perceived difficulty in completing 9 daily activities. | Youth | ✓ | ✓ | ✓ |
| Secondary outcome measures | |||||
| Pain intensity and frequency (Daily Diary) | Assess daily pain intensity (NRS-11), location, activity limitations, and mood. Completed daily for 7 days. | Youth | ✓ | ✓ | ✓ |
| PROMIS Pain Interference - Short Form | 8-item measure used to assess extent to which pain hinders engagement in social, cognitive, emotional, physical, and recreational activities. | Youth | ✓ | ✓ | ✓ |
| PROMIS Pediatric Emotional Distress | Includes 8-item scale of anxiety that assesses fear, anxious misery, and hyperarousal and an 8-item scale of depressive symptoms. | Youth | ✓ | ✓ | ✓ |
| Chronic Pain Self-Efficacy Scale-4 | 4-item measure that assesses beliefs in carrying out activities when in pain. | Youth | ✓ | ✓ | ✓ |
| Insomnia Severity Index (ISI) | 7-item measure used to rate severity and impact of insomnia symptoms. | Youth | ✓ | ✓ | ✓ |
| Patient’s Global Impression of Change Scale | Assess perception of improvement since beginning the intervention. | Youth | ✓ | ✓ | |
| Adult Responses to Child Symptoms (ARCS) | Assess caregiver response to youth pain behavior including protectiveness, minimizing, and encouraging. | Caregiver, Youth | ✓ | ✓ | ✓ |
| PROMIS anxiety and depression | Assess parent depression and anxiety symptoms; includes 4-item scale of anxiety and 4-item scale of depression. | Caregiver | ✓ | ✓ | ✓ |
| Treatment Evaluation Inventory | Assess perceptions and acceptability of the intervention. | Youth, Caregiver | ✓ | ||
2.7.1. Primary outcome
Child-reported pain-related disability is the primary outcome to examine clinical effectiveness of the WebMAP mobile program and will be measured by the Child Activity Limitations Interview (CALI-9) in daily diaries over 7-day periods at each assessment point. The CALI-9 is a validated instrument to prospectively collect ratings of difficulty in performing usual daily physical, social, and recreational activities (e.g., running, walking 1 or 2 blocks, doing school work, doing things with friends) [15]. The items are scored on a 5-point scale, and total scores are the average of the 7-days, with higher scores indicating greater pain-related disability.
2.7.2. Secondary outcomes
2.7.2.1. Pain.
Child-reported pain intensity will be rated in daily diaries using an 11-point numerical rating scale (NRS) (0 = no pain, 10 = worst pain ever) over 7-day periods at each assessment point. Total pain intensity scores will be the average of the 7-days. Pain frequency will be assessed as number of pain-days occurring during each 7-day measurement period [16].
2.7.2.2. Pain interference.
Child report on the PROMIS Pain Interference short form will be used to assess pain interference [17]. This measure includes 8-items that assess the extent to which pain hinders the child’s engagement in social, cognitive, emotional, physical, and recreational activities. Items are scored on a 5-point scale with higher scores indicating greater pain-related interference.
2.7.2.3. Anxiety and depression.
Child-report on the PROMIS Pediatric Emotional Distress scales will be used to assess anxiety and depressive symptoms [18] This includes an 8-item scale of anxiety that assesses fear, anxious misery, and hyperarousal and an 8-item scale of depressive symptoms. Raw scores on both scales are converted to T-scores for analyses. The T-score distribution has a mean of 50 (SD ± 10), and scores of more than one standard deviation above the mean indicate clinically meaningful symptoms.
2.7.2.4. Pain self-efficacy.
To assess pain self-efficacy, the 4-item Chronic Pain Self-Efficacy Scale-4 [19] will be completed by youth to assess their beliefs in their ability to carry out activities when in pain. Items are scored on a 7-point scale, with higher scores indicating greater pain self-efficacy.
2.7.2.5. Insomnia.
Child-reported insomnia severity will be assessed using the Insomnia Severity Index [20,21], a 7-item measure used to rate severity and impact of insomnia symptoms. Items are scored on a 5-point Likert scale, with scores ranging from 0 to 28 and higher scores indicating greater insomnia symptoms.
2.7.2.6. Patient global impression of change.
will be assessed using child-report at post-treatment and 3-month follow-up on a single item: “Since the start of the study my overall status is… (1 = very much improved to 7 = very much worse”).
2.7.2.7. Parent protective behaviors.
The Adult Responses to Child Symptoms (ARCS [22]) will be completed by children and parents to assess parent protective behaviors. The ARCS is a 29-item scale with subscale scores for three factors, Protect, Minimize, Distract and Monitor. Each subscale is scored by computing the mean of the item responses ranging from 0 to 4, with higher scores indicating more maladaptive parenting behaviors.
2.7.2.8. Parent anxiety and depression.
Parent will complete the PROMIS Adult Emotional Distress scale [23] to assess their anxiety and depressive symptoms. This includes a 4-item scale of anxiety and a 4-item scale of depression. Scores range from 4 to 20 and are converted to T-scores for analyses. The T-score distribution has a mean of 50 (SD ± 10), and scores of more than one standard deviation above the mean indicate clinically meaningful symptoms.
2.8. Covariates
Patient demographic information (age, sex, race, income) and pain history (spatial distribution of pain, health services used during the treatment period, pain diagnosis, duration) will be collected.
2.8.1. Implementation outcomes
Implementation outcomes will be assessed at the end of the study via clinic records, WebMAP administrative tracking, and online provider surveys completed securely via REDCap. Implementation will be evaluated via standardized metrics suggested by the RE-AIM work-group.
2.8.2. Adoption
Adoption at the clinic level will be calculated as the proportion of children referred to WebMAP mobile at each clinic/Number seen at each clinic. Clinic record data on the number of patients seen at each clinic during the study period will be collected.
2.8.3. Reach
Reach will be calculated at the individual level as the proportion of children who agree to participate in WebMAP mobile /Number of eligible participants referred for intervention.
2.8.4. Implementation
Implementation at the clinic level will be assessed via a provider survey completed online regarding the feasibility of implementing WebMAP mobile and attitudes toward adoption. Items include relevance of the program (“The modules had helpful skills for my patients”), barriers (“The program takes too long”), global appraisal (“My patients benefited from the WebMAP mobile program), and sustainability (“I would implement the program in my clinic, even if I were not enrolled in the study”). Likert scale ratings will be used: 0 = strongly disagree to 5 = strongly agree. The survey will also collect provider and clinic characteristics including the provider professional background (e.g., physicians, psychologists), practice size, and location. Implementation at the patient level will be assessed by collecting app analytics on usage of the program.
2.8.5. Maintenance
Maintenance at the clinic level will be computed as the proportion of clinics agreeing to continue using WebMAP mobile /Number of clinics in the trial. Maintenance at the individual level will be evaluated by pain-related outcomes at the 3-month follow up period.
2.9. Data analysis plan
To test clinical effectiveness, mixed effects regression models will be used, which allows for the modeling of longitudinal data on patients, nested within clinic sites. This approach has the ability to use partial data on those subjects with missing data, which can ameliorate selection bias due to drop out. Mixed models naturally structure patient and clinic site heterogeneity specifically allowing for random effects such as individual intercepts and slopes over time. Longitudinal regression models also allow the use of baseline covariates (e.g., patient sex) that may be prognostic. All primary statistical analyses will be conducted using intent-to-treat methods. Because the stepped wedge design randomizes clinic sites to sequentially initiate the intervention, we will compare within site pre- and post-intervention, as well as between sites during unexposed vs exposed periods.
To evaluate implementation, standardized metrics suggested by the RE-AIM workgroup (www.re-aim.org) include Individual Level Impact which is computed as Sum across target behaviors of (Reach X Average of Individual Change at Long-Term Follow-up) and Organizational Level Impact, which is computed as Adoption X Implementation. We will also assess individual clinic performance (e.g., the reach for each clinic) as well as tracking variation by time, by implementation strategy, and by clinic and patient characteristics. We will calculate the variability in reach using a generalized linear random effect model including a random practice effect. We will use a logit link and binomial distribution. In addition, to assess the association between reach and implementation strategy, clinic characteristics, and patient demographics, logistic regression will be used.
2.10. Sample size and power analysis
Sample size estimates were adjusted for the clustering of patients within clinic sites, using appropriate intraclass correlations (ICCs) derived from the study team’s prior multisite investigations, ICC = 0.02 for this estimate. Minimal attrition is expected in the study sample based on prior studies by the research group that have consistently achieved follow-up completion rates > 90% at 6–12 months follow-up with similar populations. With 8 clinic sites recruiting patients into the study, using an ICC of 0.02, and assuming a two tailed alpha of 0.05, the study has 80% power to detect an effect size of 0.25 on the primary outcome of pain-related disability in mixed linear models for examining differential changes between the treatment conditions. This is the same effect size for pain-related disability that we have found in prior trials of WebMAP.
3. Discussion
One promising solution for improving access to evidence-based psychological treatment for chronic pain is through the use of technology-delivered interventions. Our trial addresses a critical national need for low-cost, accessible pain self-management for children and adolescents with chronic pain. WebMAP is an internet-delivered pain self-management intervention for youth that has proven efficacy in several clinical trials [7–9]. We now focus on scaling up this effective Internet pain self-management program through evaluating a dissemination approach through implementing a mobile app version (WebMAP mobile) with children receiving care for chronic pain in specialty clinics. The goal of this hybrid effectiveness-implementation RCT is therefore to evaluate the efficacy, reach, adoption, implementation, and maintenance of the WebMAP mobile intervention to facilitate large-scale dissemination.
Understanding of strategies to best disseminate and implement technology-delivered interventions represents an important gap in knowledge. Ideally, Internet and smartphone interventions could be globally implemented to all youth living with chronic pain. However, at present, interventions developed for research studies are not readily available for public access. And, even when available, it is unknown how to best deliver these resources to children who might benefit from them. Results of this trial are expected to provide information on the effectiveness and public health impact of a mobile app version of a pain self-management program that can be readily disseminated in clinical settings for youth with chronic pain.
Funding
This research was supported by the American Pain Society and Pfizer Independent Grants for Learning and Change (Grant ID #: 27971161, PI: Palermo). The study sponsor and funders had no role in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
References
- [1].Huguet A, Miro J, The severity of chronic pediatric pain: an epidemiological study, J. Pain 9 (3) (2008) 226–236. [DOI] [PubMed] [Google Scholar]
- [2].Fisher E, et al. , Systematic review and meta-analysis of psychological therapies for children with chronic pain, J. Pediatr. Psychol 39 (8) (2014) 763–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Peng P, et al. , Dedicated multidisciplinary pain management centres for children in Canada: the current status, Can. J. Anaesth 54 (12) (2007) 985. [DOI] [PubMed] [Google Scholar]
- [4].Cipher DJ, Fernandez E, Clifford A, Cost-effectiveness and health care utilization in a multidisciplinary pain center: comparison of three treatment groups, J. Clin. Psychol. Med. Settings 8 (4) (2001) 237–244. [Google Scholar]
- [5].Croft P, Lewis M, Hannaford P, Is all chronic pain the same? A 25-year follow-up study, Pain 105 (1–2) (2003) 309–317. [DOI] [PubMed] [Google Scholar]
- [6].Fisher E, et al. , Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents, Cochrane Database Syst. Rev 3 (2015) p. CD011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Law EF, et al. , Pilot randomized controlled trial of internet-delivered cognitive-behavioral treatment for pediatric headache, Headache 55 (10) (2015) 1410–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Palermo TM, et al. , Internet-delivered cognitive-behavioral treatment for adolescents with chronic pain and their parents: A randomized controlled multicenter trial, Pain 157 (1) (2016) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Palermo TM, et al. , Randomized controlled trial of an Internet-delivered family cognitive–behavioral therapy intervention for children and adolescents with chronic pain, Pain 146 (1–2) (2009) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dzewaltowski DA, et al. , RE-AIM: evidence-based standards and a Web resource to improve translation of research into practice, Ann. Behav. Med 28 (2) (2004) 75. [DOI] [PubMed] [Google Scholar]
- [11].Curran GM, et al. , Effectiveness-implementation Hybrid Designs Combining Elements of Clinical Effectiveness and Implementation Research to Enhance Public Health Impact, Med. Care 50 (3) (2012) 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Arroll B, et al. , Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population, Ann. Fam. Med 8 (4) (2010) 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Essner B, et al. , Examination of the Factor Structure of the Adolescent Sleep-Wake Scale (ASWS), Behav. Sleep Med 13 (4) (2015) 296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris PA, et al. , Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Holley AL, et al. , The CALI-9: A brief measure for assessing activity limitations in children and adolescents with chronic pain, Pain 159 (1) (2018) 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].von Baeyer CL, et al. , Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity, Pain 143 (3) (2009) 223–227. [DOI] [PubMed] [Google Scholar]
- [17].Amtmann D, et al. , Development of a PROMIS item bank to measure pain interference, Pain 150 (1) (2010) 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Irwin DE, et al. , An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales, Qual. Life Res 19 (4) (2010) 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McWilliams LA, Kowal J, Wilson KG, Development and evaluation of short forms of the Pain Catastrophizing Scale and the Pain Self-efficacy Questionnaire, Eur. J. Pain 19 (9) (2015) 1342–1349. [DOI] [PubMed] [Google Scholar]
- [20].Bastien CH, Vallières A, Morin CM, Validation of the Insomnia Severity Index as an outcome measure for insomnia research, Sleep Med. 2 (4) (2001) 297–307. [DOI] [PubMed] [Google Scholar]
- [21].Chung K-F, Kan KK-K, Yeung W-F, Assessing insomnia in adolescents: comparison of insomnia severity index, athens insomnia scale and sleep quality index, Sleep Med. 12 (5) (2011) 463–470. [DOI] [PubMed] [Google Scholar]
- [22].Claar RL, et al. , Factor structure of the Adult Responses to Children’s Symptoms: validation in children and adolescents with diverse chronic pain conditions, Clin. J. Pain 26 (5) (2010) 410–417. [DOI] [PubMed] [Google Scholar]
- [23].Cella D, et al. , The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years, Med. Care 45 (5 Suppl 1) (2007) S3. [DOI] [PMC free article] [PubMed] [Google Scholar]


