Abstract
The C-N cross coupling reaction has always been a fundamental task in organic synthesis. However, the direct use of N-H group of aryl amines to generate N-centered radicals which would couple with alkyl radicals to construct C-N bonds is still rare. Here we report a visible light-promoted C-N radical cross coupling for regioselective amination of remote C(sp3)-H bonds. Under visible light irradiation, the N-H groups of aryl amines are converted to N-centered radicals, and are then trapped by alkyl radicals, which are generated from Hofmann-Löffler-Freytag (HLF) type 1,5-hydrogen atom transfer (1,5-HAT). With the same strategy, the regioselective C(sp3)-C(sp3) cross coupling is also realized by using alkyl Hantzsch esters (or nitrile) as radical alkylation reagents. Notably, the α-C(sp3)-H of tertiary amines can be directly alkylated to form the C(sp3)-C(sp3) bonds via C(sp3)-H − C(sp3)-H cross coupling through the same photoredox pathway.
Subject terms: Reaction mechanisms, Synthetic chemistry methodology, Photocatalysis
C-N bond forming is an established strategy to form amines, which are quintessential in chemical synthesis and in nature. Here, the authors report three classes of photoredox reactions, involving C(sp3)-N coupling between N-centered radicals and alkyl radicals and C(sp3)- C(sp3) coupling via C(sp3)-H alkylation.
Introduction
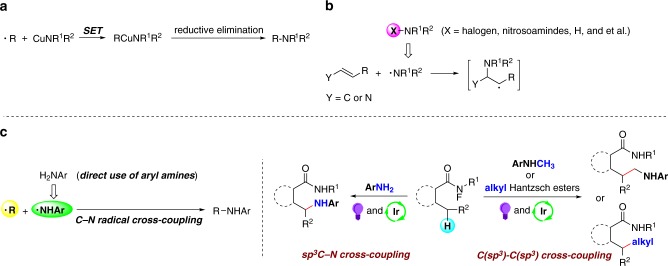
Amines are quintessential moieties in pharmaceuticals, nature products, and organic materials1,2. In the past few decades, transition-metal-catalyzed sp2 C–N couplings of aryl halides (and pseudo halides) with amine nucleophiles have been well developed, such as Buchwald–Hartwig reaction3, Ullmann coupling4, and Chan–Lam amination5. However, the alkylation of amines using alkyl electrophiles is largely underdeveloped due to the β-hydrogen elimination from the metal-alkyl intermediate6–8. Recently, significant progress has been made in transition-metal-catalyzed radical sp3 C–N bond formations. Fu and coworkers recently disclosed the photoinduced, Cu-catalyzed intermolecular and intramolecular alkylation of amides9,10. Very recently, Macmillan11 and Hu12 reported a series of Cu-catalyzed, photoinduced decarboxylative sp3 C–N coupling reactions, respectively. In these approaches, the trapping of alkyl radicals by Cu-amine species and the reductive eliminations of Cu intermediates were key steps for the cross-couplings (Fig. 1a).
Fig. 1. sp3 C–N bond formations with N-center radicals.
a Cu-catalyzed radical sp3 C–N cross-couplings6–8. b Addition reactions between N-center radicals and alkenes (enamines)9. c This work: sp3 C–N cross-coupling between alkyl radical and N-center radical. X = halogen, nitrosoamides, H, and so on. Y = C or N.
In the past few years, the addition reactions of N-center radical to alkenes (or enamine intermediates) have been developed (Fig. 1b)13–20. However, the direct cross-coupling between alkyl- and N-based radicals in the absence of stabilization by transition-metal complex has been rarely explored. Moreover, in the reactions, the amine (or amide) compounds need to be converted to the corresponding N-radical precursors (e.g., N-halogens and N-nitrosoamides) by separated steps. The direct use of the N–H group of aryl amines to generate Naryl-center radicals and couple with alkyl radicals is still rare17.
The regioselective C–H functionalization is one of the most fundamental reactions in organic synthetic chemistry. In recent years, the functionalization of C(sp3)–H bonds has become an important and intensive task to the organic synthetic community. In the past decade, great progress has been achieved in C(sp3)–H functionalization at unactivated sites, which allows streamlined synthesis of target compounds and late-stage modification of complex structures. Recently, the application of Hofmann–Löffler–Freytag (HLF)-type 1,5-hydrogen atom transfer (1,5-HAT) in C(sp3)–H functionalization reactions received much attention due to their unique regioselectivities21,22. Although the amidyl radical formation and its subsequent 1,5-HAT process have been well established, the followed transformations of the C-center radical are still limited, and the reactions mainly focused on cyclization23–29, atom transfer (halogenation)30–33, Giese reaction34–37, azidation38,39, cyanation40,41, trifluoromethylation42, and arylation)43,44. So far, using the HLF-type C-center radical for sp3 C–N or C(sp3)–C(sp3) couplings is still very rare45, and the direct cross-coupling between C(sp3)–H and N–H is not realized. We here report an example of sp3 C–N cross-coupling reaction between N-center- and alkyl radicals. Notably, the aryl amines are directly converted to N-center radicals under visible-light irradiation (Fig. 1c). By using the same photoredox catalytic 1,5-HAT strategy, the regioselective C(sp3)–H alkylation can also be realized when Hantzsch esters are used as alkylation reagents. The primary, secondary, and tertiary alkylation are all compatible under standard conditions. It is worth noting that the α-C(sp3)–H of tertiary amines can be directly alkylated to form C(sp3)–C(sp3) bonds without pre-functionalization.
Results
Investigation of the sp3 C–N coupling reaction conditions
The investigation was initiated by using N-(tert-butyl)-N-fluoro-2-methylbenzamide (1a) and aniline (2a) as model substrates. A series of photocatalysts (Ir and Ru complexes, or organic photocatalysts), light sources, solvents, additives, and the substrate ratios were tested (see the Supplementary Information for details). The results indicated the optimal reaction conditions (condition A): under 24-W violet LED (390–410 nm) irradiation, 1a and 2a (3 equiv) were dissolved in DMF (0.1 M), Ir(ppy)2(dtbpy)PF6 (1 mol %) was used as the photocatalyst, K2CO3 (3.0 equiv) was used as basic additive, and the reaction was stirred at room temperature for 12 h. Under these reaction conditions, the desired sp3 C–N coupling product (3a) was isolated in 73% yield. Notably, to achieve this transformation, a suitable photocatalyst with well-balanced redox potential was required. The organic photocatalysts A–D have relatively strong oxidative properties, whereas the reductive activities were moderate. In contrast, the Ir- and Ru-based photocatalysts have good redox abilities, which were compatible for the reaction (Table 1).
Table 1.
Optimization of reaction conditionsa.
| Entry | Change to “condition A” | Yield (%)b |
|---|---|---|
| 1 | Condition A | 73 |
| 2 | No light | 0 |
| 3 | No photocatalyst | 0 |
| 4 | Without K2CO3 | 0 |
| 5 | A,B,C,D instead of G | 0 |
| 6 | E, F, H instead of G | 20–64 |
| 7 | Other bases instead of K2CO3 | 0–49 |
| 8 | Other solvents instead of DMF | 0–55 |
aUnless noted, the reactions were carried out using 1a (0.1 mmol), 2a (3 equiv), photocatalyst (1 mol %), base (3.0 equiv), and DMF (1 ml), under Ar, and stirred at rt for 12 h under 24-W violet LED irradiation.
bIsolated yields.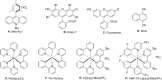
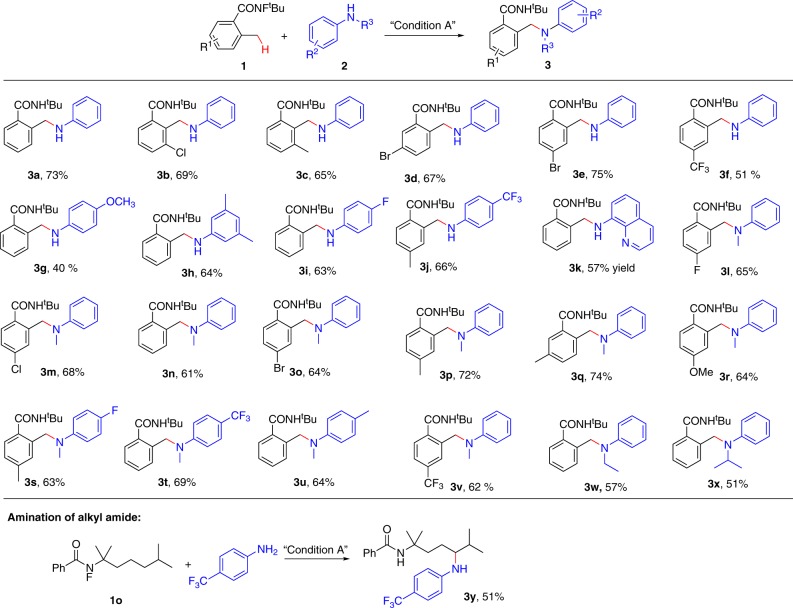
Scope of sp3 C–N coupling reactions
With the optimal reaction conditions in hand, the substrate scope of carboxylamides and anilines was examined, and the results are summarized in Fig. 2. To our delight, the carboxamides and anilines bearing electron-donating and electron-withdrawing groups at o-, m-, or p-position of the aryl ring were compatible with moderate-to-good yields (Fig. 2, 3a–3x). A range of functional groups, such as –CH3, –OCH3, –CF3, and halides (–F, –Cl, and –Br) were all tolerated. The bulkier amines, such as EtNHPh and i-PrNHPh, were successfully converted to the corresponding products 3w and 3x with satisfactory yields, respectively. Notably, the amination of alkyl amide was also achieved under standard conditions (3y). However, the alkyl-substituted amines, such as CyNH2 and n-Bu2NH, failed to give the corresponding amination products.
Fig. 2. Substrate scope of sp3 C–N coupling reactions.
All reactions were conducted in 0.2 mmol scale. Yields referred to isolated yields.
Alkylation of tertiary amine α-C(sp3)–H bonds
Tertiary amine motifs are widely represented in many pharmaceuticals and advanced materials1,2. Direct functionalization of tertiary amine provides an efficient pathway to synthesize structurally diversified tertiary amines. In 2006, Li and coworkers reported a cross-dehydrogenative-coupling reaction, which could directly couple the α-C(sp)3–H of tertiary amines with nucleophiles under oxidative conditions46–49. Very recently, the photoredox-induced α-C(sp)3–H functionalization of tertiary amines was achieved, which could functionalize the α-C(sp)3–H under mild and external oxidant-free conditions50,51. Despite these achievements, the direct C(sp3)–C(sp3) cross-coupling reactions between tertiary amines α-C(sp3)–H and unactivated C(sp3)–H were still not realized.
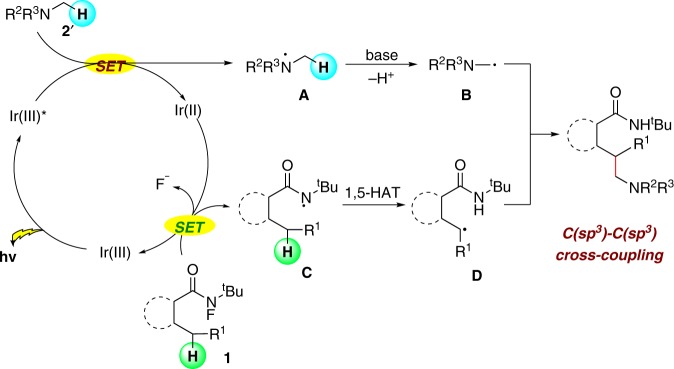
Encouraged by the success of photoredox sp3 C–N coupling, we decided to explore an alternate route to realize regioselective C(sp3)–C(sp3) coupling between tertiary amines α-C(sp3)–H and unactivated C(sp3)–H using the photoredox 1,5-HAT strategy. In our initial hypothesis, upon irradiation, the high valent photocatalyst could accept an electron from amine and simultaneously generate an amino radical cation A through single-electron transfer (SET) process (Fig. 3). The amino radical cation would then form α-amino alkyl radical B by deprotonation. The intermediate B could be captured by C-center radical D that was generated through 1,5-HAT, and furnished the C(sp3)–C(sp3) coupling.
Fig. 3. Design plan for alkylation of tertiary amine α-C(sp3)–H bonds.
Hypothesis of the mechanism for photoinduced C(sp3)–C(sp3) coupling reactions.
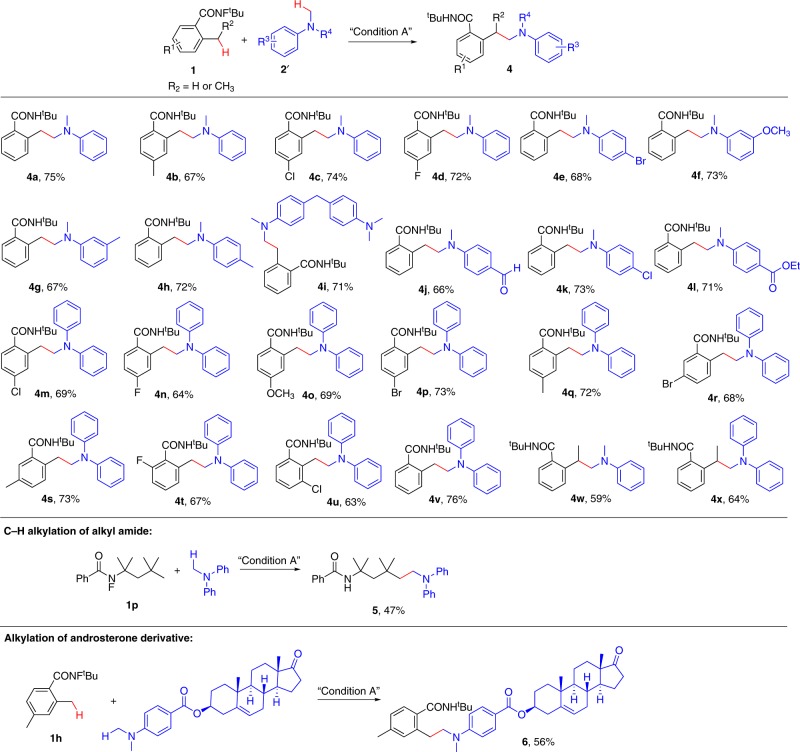
Scope of alkylation of tertiary amine α-C(sp3)–H bonds
With the above hypothesis in mind, we began our study by using 1a and N,N-dimethylaniline (2a’) as model substrates to optimize the reaction conditions (see the Supplementary Information for details). Under the optimal conditions (condition A), the desired C(sp3)–C(sp3) cross-coupling product (4a) was obtained in 75% yield. The generality of the reaction was examined by using a variety of tertiary amines and carboxamides (Fig. 4, 4a–4o). To our delight, uniformly good results were obtained with various substrates bearing sensitive functional groups. The C–H functionalization of alkyl amide was also realized with good yield (5). Furthermore, the late-stage modification of androsterone-derived amine was achieved in 56% yield with the ester group untouched 6.
Fig. 4. Substrate scope of alkylation of tertiary amine α-C(sp3)–H bonds.
All reactions were conducted in 0.2 mmol scale. Yields referred to isolated yields.
C(sp3)–C(sp3) coupling reaction using alkyl Hantzsch ester
In the past few years, the HLF-type radical cross-coupling reactions were intensively studied21–44. However, its application in the construction of C(sp3)–C(sp3) was still rare45. Hantzsch esters were first synthesized by A. R. Hantzsch in 1881, and widely used in pharmaceutical chemistry. With the rapid development of radical chemistry, various alkylation reactions using 4-substituted Hantzsch esters as alkylation reagent have been developed52–55. However, the cross-coupling between alkyl Hantzsch esters and C(sp3)–H was still not realized.
In the above successful C(sp3)–H alkylation reactions (Fig. 4), the alkyl radicals were generated through 1,2-SET of N-center radical, which restricted the scope of alkyl substrates. Alkyl Hantzsch ester has the ability to serve both as a single-electron reductant and alkyl radical precursor. We envisioned that alkyl Hantzsch esters could be used instead of tertiary amine as the alkylation reagents for the direct C(sp3)–C(sp3) cross-coupling.
Scope of C(sp3)–C(sp3) coupling
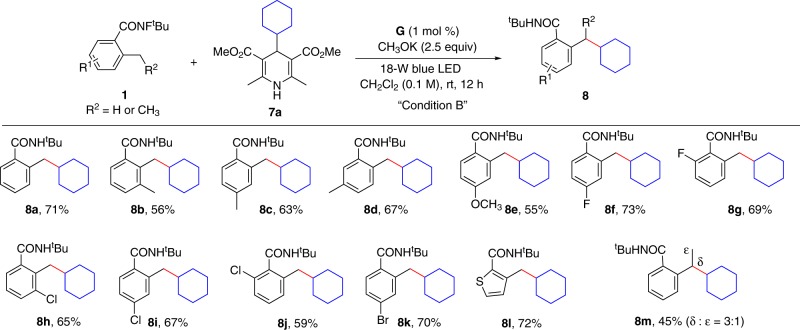
Initially, cyclohexyl Hantzsch ester (7a) was used as model substrate to optimize the reaction conditions. After the screening of reaction parameters, the desired C(sp3)–C(sp3) cross-coupling product (8a) could be obtained in 71% yield (condition B, see the Supplementary Information for details). Then, the substrate scope of carboxylamides was examined (Fig. 5). To our delight, the carboxamides bearing electron-donating and electron-withdrawing groups at o-, m-, or p-position of the aryl ring were compatible with moderate-to-good yields (8a–8l). A range of functional groups, such as –CH3, –OCH3, and halides (–F, –Cl, and –Br), were all tolerated (8a–8k). The thiophene-derived substrate delivered the desired product with 72% yield (8l). The regioisomers were found in the case of 8m, which might be attributed to the competing 1,6-HAT pathway32,56.
Fig. 5. Substrate scope of carboxylamides with cyclohexyl Hantzsch ester.
All reactions were conducted in 0.2 mmol scale. Yields referred to isolated yields.
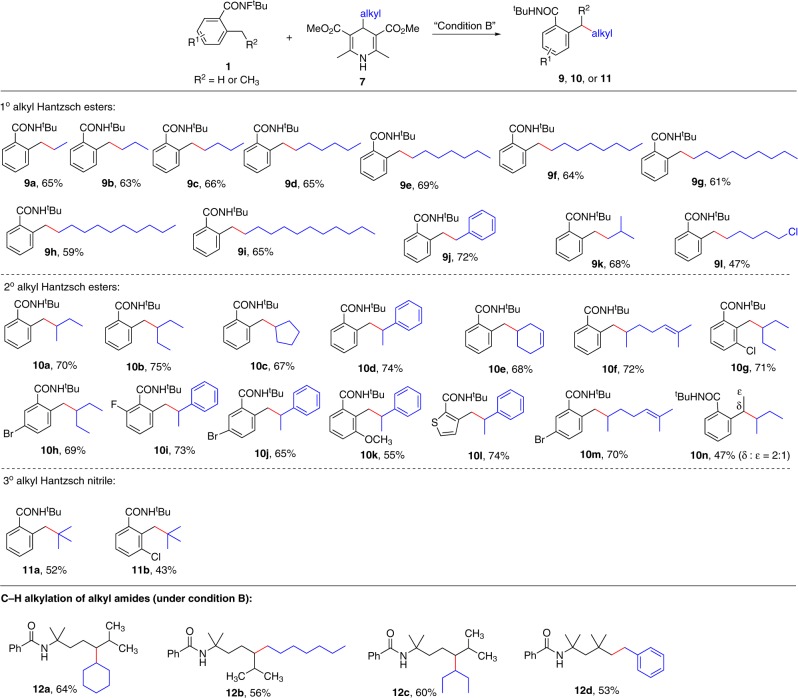
To further explore the substrate scope, a variety of alkyl Hantzsch esters were examined (Fig. 6). To our delight, the primary and the secondary alkyl Hantzsch esters, as well as the tertiary alkyl Hantzsch nitrile, all proceeded smoothly in satisfactory results (9a–11b) with the sensitive functional groups (halogens and alkenes) untouched. The results indicated the general ability of our strategy for the construction of C(sp3)–C(sp3) bonds in the synthetic chemistry. Notably, the aryl Hantzsch esters failed to give any desired products under our standard conditions. It should be noted that our method was suitable not only for o-methylbenzamide, but also alkyl amide. As shown in Fig. 6, under the standard reaction conditions, 1o and 1p were smoothly coupled with alkyl Hantzsch esters in satisfactory yields (12a–12d).
Fig. 6. Substrate scope of alkyl Hantzsch esters and nitrile.
All reactions were conducted in 0.2 mmol scale. Yields referred to isolated yields.
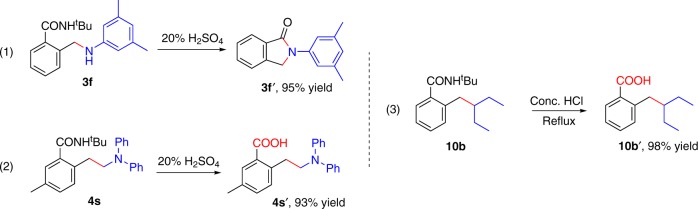
Synthetic applications
To demonstrate the synthetic application of our method, the amination and alkylation products were readily converted to the corresponding lactam (3f’) and acid (4s’ and 10b’) through simple operations with excellent yields, respectively (Fig. 7).
Fig. 7. Synthetic applications.
(1) Synthesis of lactam 3f’. (2) and (3) Converted amides to acids.
Mechanistic investigations
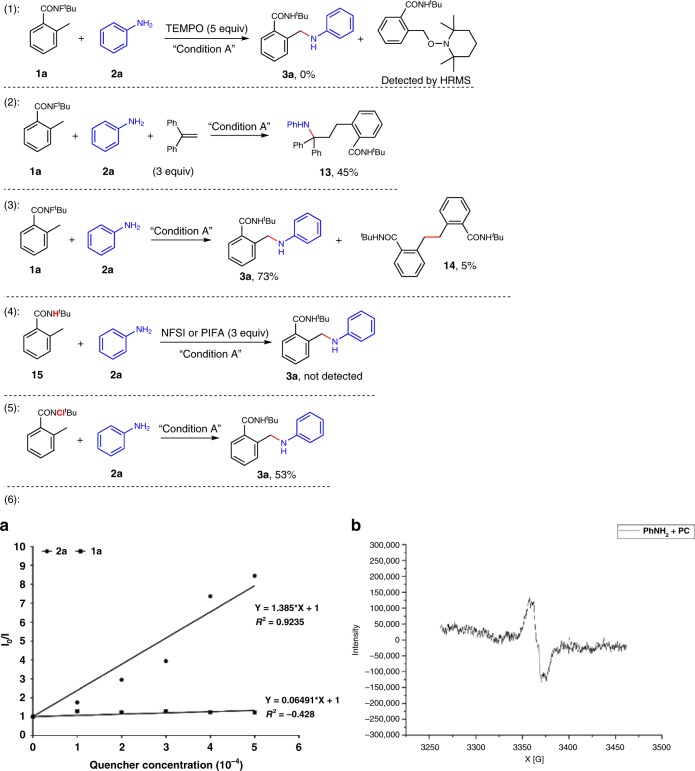
In order to gain some mechanistic insight of this sp3 C–N coupling reaction, several control experiments were carried out (Fig. 8). The reaction was completely shut down by 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO, Equation (1)). Furthermore, when the radical scavenger ethane-1,1-diyldibenzene was added to the reaction, the corresponding three-component-type product 13 was obtained in 45% yield (Equation (2)). When 1a and aniline (2a) were used as substrates, the desired product 3a was isolated in 73% yield. Notably, the homo-coupling product 14 was also obtained in the reaction system with 5% yield (Equation (3)). These results suggested that a) the radical pathway might be involved in the reaction; b) the HLF-type 1,5-HAT proceeded in the system and formed the C-center radical; c) aryl amine possibly converted to the corresponding N-center radical under standard conditions; d) the radical–radical coupling route might be responsible for this sp3 C–N bond formation reaction. We also tried this reaction under oxidative conditions. In the presence of N-fluorobenzenesulfonimide (NFSI, 3 equiv)43,44 or [bis(trifluoroacetoxy)iodo]benzene (PIFA, 3 equiv)57, the un-fluoride amide substrate 15 failed to produce the amination product under standard conditions (Equation (4)). In addition, the N-chloroamide could also give the desired product with modest yield (Equation (5)). These results indicated that the pre-activation of the substrates is crucial to this coupling reaction. In our initial hypothesis, the step that aryl amine converts to the corresponding N-center radical was crucial for this transformation. To verify this hypothesis, emission quenching and electron paramagnetic resonance experiments have been conducted, and the results indicated that the radical species was generated in the system (Equation (6)), see the Supplementary Information for details). The Stern−Volmer plot showed strong quenching of Ir(ppy)2(dtbpy)PF6 (E1/2*III/II = +0.66 V vs. SCE) by PhNH2 (2a) (E1/2red = +0.94 V vs. SCE), favoring a reductive quenching cycle. These evidences indicated that the excited-state Ir(ppy)2(dtbpy)PF6 might undergo a SET process that furnished the formation of Naryl-center radical.
Fig. 8. Mechanistic studies.
Equation (1) Radical trapping reaction with TEMPO. (2) Radical trapping reaction with ethane-1,1-diyldibenzene. (3) Homo-coupling product. (4) The results under oxidative conditions. (5) The result with N-chloroamide substrate. (6) (a) Fluorescence quenching of Ir(ppy)2(dtbpy)PF6 by 2a and 1a. (b) EPR experiment result of PhNH2 (2a, PhNH2 (0.1 mmol) and Ir(ppy)2(dtbpy)PF6 (5 mol%) in hexafluoroisopropanol (1 ml), stirred at room temperature for 1 h under 400-nm irradiation, and directly used for EPR experiments).
Proposed mechanism
Based on our investigations and previous reports, a plausible mechanism is proposed in Fig. 9 (see the Supplementary Information for details). The reaction starts with the oxidation of 2 by the excited-state Ir(III)* in the presence of a base, yielding amine radical A and Ir(II). Then, the Ir(II) (E1/2III/II = −1.51 V vs. SCE)58,59 species facilitated the second SET process of substrate 1 (Ep0/–1(1a) = –0.84 V vs. SCE in MeCN) to generate the amidyl radical B. The subsequent 1,5-HAT formed the radical intermediate C along with the oxidation of Ir(II) to Ir(III) to close the catalytic cycle. Finally, the radical–radical cross-coupling between N-center radical A and C-center radical intermediate C was proposed to provide the sp3 C–N cross-coupling product 3.
Fig. 9. Proposed mechanism.
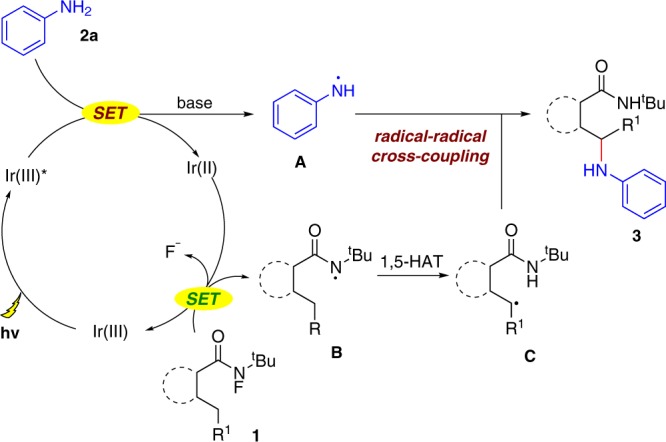
Proposed mechanism for photoinduced C(sp3)–N coupling reactions.
Discussion
In conclusion, we disclosed a visible-light-promoted C–N-radical cross-coupling to realize the regioselective amination of remote C(sp3)–H bonds. In the reactions, the N-center radicals were directly generated from aryl amines under visible-light irradiation. Using the photoinduced HLF-type 1,5-HAT strategy, the regioselective C(sp3)–C(sp3) cross-coupling was also achieved by using alkyl Hantzsch esters (or nitrile) as alkylation reagents. Notably, the α-C(sp3)–H of tertiary amines was directly alkylated to form the C(sp3)–C(sp3) bonds via C(sp3)–H–C(sp3)–H cross-coupling. All the reactions proceeded at room temperature without the assistance of external oxidants.
Methods
General procedure for condition A
In a dry 10-ml glass test tube, substrate N-fluoroamides (0.2 mmol), amine (0.6 mmol, 3 equiv), Ir(ppy)2(dtbpy)PF6 (1 mol%), and K2CO3 (0.6 mmol, 3 equiv) were dissolved in DMF (2.0 mL) under Ar atmosphere. The glass test tube was then transferred to a 24-W violet-light photoreactor, where it was irradiated for 12 h. The residue was added water (10 mL) and extracted with ethyl acetate (5 mL × 3). The combined organic phase was dried over Na2SO4. The resulting crude residue was purified via column chromatography on silica gel to afford the desired products.
General procedure for condition B
In a dry 10-ml glass test tube, substrate N-fluoroamides (0.2 mmol), Hantzsch esters or Hantzsch nitrile (0.6 mmol, 3 equiv), Ir(ppy)2(dtbpy)PF6 (1 mol%), and MeOK (0.5 mmol, 2.5 equiv) were dissolved in DCM (2.0 mL) under Ar atmosphere. The glass test tube was then transferred to a 18-W blue LED photoreactor, where it was irradiated for 12 h. The residue was added water (10 mL) and extracted with DCM (5 mL × 3). The combined organic phase was dried over Na2SO4. The resulting crude residue was purified via column chromatography on silica gel to afford the desired products.
Supplementary information
Acknowledgements
We are grateful for the grants from the NSFC (21971098) and the Fundamental Research Funds for the Central Universities (lzujbky-2018-k9). The authors also thank Prof. Rui Wang, Prof. Yawen Wang, Prof. Pinxian Xi, and Prof. Qiang Liu at Lanzhou University for helpful discussion and technical assistance.
Author contributions
Z.X. conceived and designed the research. Q.G., Q.P., H.C., Y.H., and S.W. performed the research. Q.G. and Z.X. cowrote the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information. Data are also available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-15167-2.
References
- 1.Ricci A. Amino Group Chemistry: From Synthesis to the Life Sciences. Weinheim, Germany: Wiley-VCH; 2008. [Google Scholar]
- 2.Lawrence SA. Amines: Synthesis, Properties and Applications. Cambridge: Cambridge University Press; 2004. [Google Scholar]
- 3.Ruiz-Castillo P, Buchwald SL. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016;116:12564–12649. doi: 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sambiagio C, Marsden SP, Blacker AJ, McGowan PC. Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 2014;43:3525–3550. doi: 10.1039/C3CS60289C. [DOI] [PubMed] [Google Scholar]
- 5.Qiao JX, Lam PYS. Copper-promoted carbon-heteroatom bond cross-coupling with boronic acids and derivatives. Synthesis. 2011;6:829–856. doi: 10.1055/s-0030-1258379. [DOI] [Google Scholar]
- 6.Choi J, Fu GC. Transition metal-catalyzed alkyl-alkyl bond formation: another dimension in cross-coupling chemistry. Science. 2017;356:152–160. doi: 10.1126/science.aaf7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu X. Nickel-catalyzed cross coupling of non-activated alkyl halides: a mechanistic perspective. Chem. Sci. 2011;2:1867–1886. doi: 10.1039/c1sc00368b. [DOI] [Google Scholar]
- 8.Tasker SZ, Standley EA, Jamison TF. Recent advances in homogeneous nickel catalysis. Nature. 2014;509:299–309. doi: 10.1038/nature13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do H-Q, Bachman S, Bissember AC, Peters JC, Fu GC. Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature. J. Am. Chem. Soc. 2014;136:2162–2167. doi: 10.1021/ja4126609. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Wurz RP, Peters JC, Fu GC. Photoinduced, copper-catalyzed decarboxylative C-N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc. 2017;139:12153–12156. doi: 10.1021/jacs.7b07546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran EB, et al. Aryl amination using ligand-free Ni(II) salts and photoredox catalysis. Science. 2016;353:279–283. doi: 10.1126/science.aag0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mao R, Frey A, Balon J, Hu X. Decarboxylative C(sp3)–N cross-coupling via synergetic photoredox and copper catalysis. Nat. Catal. 2018;1:120–126. doi: 10.1038/s41929-017-0023-z. [DOI] [Google Scholar]
- 13.Kemper J, Studer A. Stable reagents for the generation of N-centered radicals: hydroamination of norbornene. Angew. Chem. Int. Ed. 2005;44:4914–4917. doi: 10.1002/anie.200463032. [DOI] [PubMed] [Google Scholar]
- 14.Guin J, Mück-Lichtenfeld C, Grimme S, Studer A. Radical transfer hydroamination with aminated cyclohexadienes using polarity reversal catalysis: scope and limitations. J. Am. Chem. Soc. 2007;129:4498–4503. doi: 10.1021/ja0692581. [DOI] [PubMed] [Google Scholar]
- 15.Guin J, Frohlich R, Studer A. Thiol-catalyzed stereoselective transfer hydroamination of olefins with N-aminated dihydropyridines. Angew. Chem. Int. Ed. 2008;47:779–782. doi: 10.1002/anie.200703902. [DOI] [PubMed] [Google Scholar]
- 16.Cecere G, König CM, Alleva JL, MacMillan DWC. Enantioselective direct α-amination of aldehydes via a photoredox mechanism: a strategy for asymmetric amine fragment coupling. J. Am. Chem. Soc. 2013;135:11521–11524. doi: 10.1021/ja406181e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu X-Q, et al. Photocatalytic generation of N-centered hydrazonyl radicals: a strategy for hydroamination of β,γ-unsaturated hydrazones. Angew. Chem. Int. Ed. 2014;53:12163–12167. doi: 10.1002/anie.201406491. [DOI] [PubMed] [Google Scholar]
- 18.Wappes EA, Nakafuku KM, Nagib DA. Directed β C-H amination of alcohols via radical relay chaperones. J. Am. Chem. Soc. 2017;139:10204–10207. doi: 10.1021/jacs.7b05214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stateman LM, Wappes EA, Nakafuku KM, Edwards KM, Nagib DA. Catalytic β C–H amination via an imidate radical relay. Chem. Sci. 2019;10:2693–2699. doi: 10.1039/C8SC05685D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakafuku KM, Fosu SC, Nagib DA. Catalytic alkene difunctionalization via imidate radicals. J. Am. Chem. Soc. 2018;140:11202–11205. doi: 10.1021/jacs.8b07578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofman AW. Ueber die einwirkung des broms in alkalischer Lösung auf die amine. Ber. Dtsch. Chem. Ges. 1883;16:558–560. doi: 10.1002/cber.188301601120. [DOI] [Google Scholar]
- 22.Löffler K, Freytag C. Über das ω-Oxy-α-propyl-piperidin und eine neue synthese des piperolidins (δ-coniceins) Ber. Dtsch. Chem. Ges. 1909;42:3427–3431. doi: 10.1002/cber.19090420377. [DOI] [Google Scholar]
- 23.Hlrnández R, Rivera A, Salazar JA, Suárez E. Nitroamine radicals as intermediates in the functionalization of non-activated carbon atoms. J. Chem. Soc. Chem. Commun. 1980;20:958–959. doi: 10.1039/C39800000958. [DOI] [Google Scholar]
- 24.Betancor C, Concepción JI, Hernández R, Salazar JA, Suárez E. Intramolecular functionalization of phosphoramidate radicals. Synthesis of 1,4-Epimine compounds. J. Org. Chem. 1983;48:4430–4432. doi: 10.1021/jo00171a066. [DOI] [Google Scholar]
- 25.DeArmas P, et al. Synthesis of 1,4-epimine compounds. Iodosobenzene diacetate, an efficient for neutral nitrogen radical generation. Tetrahedron Lett. 1985;26:2493–2496. doi: 10.1016/S0040-4039(00)94862-7. [DOI] [Google Scholar]
- 26.Paz NR, et al. Chemoselective intramolecular functionalization of methyl groups in nonconstrained molecules promoted by N‑iodosulfonamides. Org. Lett. 2015;17:2370–2373. doi: 10.1021/acs.orglett.5b00866. [DOI] [PubMed] [Google Scholar]
- 27.Richers J, Heilmann M, Drees M, Tiefenbacher K. Synthesis of lactones via C–H functionalization of nonactivated C(sp3)–H bonds. Org. Lett. 2016;18:6472–6475. doi: 10.1021/acs.orglett.6b03371. [DOI] [PubMed] [Google Scholar]
- 28.Martínez C, Muñiz K. An iodine-catalyzed Hofmann–Löffler reaction. Angew. Chem. Int. Ed. 2015;54:8287–8291. doi: 10.1002/anie.201501122. [DOI] [PubMed] [Google Scholar]
- 29.Wappes EA, Fosu SC, Chopko TC, Nagib DA. Triiodide-mediated d-amination of secondary C-H bonds. Angew. Chem. Int. Ed. 2016;55:9974–9978. doi: 10.1002/anie.201604704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy ER, Reddy BVS, Corry EG. Efficient method for selective introduction of substituents as C(5) of isoleucine and other α-amino acids. Org. Lett. 2006;8:2819–2821. doi: 10.1021/ol060952v. [DOI] [PubMed] [Google Scholar]
- 31.Richers J, Heilmann M, Drees M, Tiefenbacher K. Synthesis of lactones via C−H functionalization of nonactivated C(sp3)−H bonds. Org. Lett. 2016;18:6472–6475. doi: 10.1021/acs.orglett.6b03371. [DOI] [PubMed] [Google Scholar]
- 32.Liu T, Myers MC, Yu J-Q. Copper-catalyzed bromination of C(sp3)-H Bonds distal to functional groups. Angew. Chem. Int. Ed. 2017;56:306–309. doi: 10.1002/anie.201608210. [DOI] [PubMed] [Google Scholar]
- 33.Groendyke BJ, AbuSalim DI, Cook SP. Iron-catalyzed, fluoroamide-directed C–H fluorination. J. Am. Chem. Soc. 2016;138:12771–12774. doi: 10.1021/jacs.6b08171. [DOI] [PubMed] [Google Scholar]
- 34.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu JCK, Rovis T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp3 C–H bonds. Nature. 2016;539:272–275. doi: 10.1038/nature19810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu W, Genoux A, Li Z, Nevado C. γ-Functionalizations of amines through visible-light-mediated, redox-neutral C−C bond cleavage. Angew. Chem. Int. Ed. 2017;56:10521–10524. doi: 10.1002/anie.201704068. [DOI] [PubMed] [Google Scholar]
- 37.Wu K, Wang L, Colón-Rodríguez S, Flechsig G-U, Wang T. Amidyl radical directed remote allylation of unactivated sp3 C-H bonds by organic photoredox catalysis. Angew. Chem. Int. Ed. 2019;58:1774–1778. doi: 10.1002/anie.201811004. [DOI] [PubMed] [Google Scholar]
- 38.Bao X, Wang Q, Zhu J. Copper-catalyzed remote C(sp3)-H azidation and oxidative trifluoromethylation of benzohydrazides. Nat. Commun. 2019;10:1–7. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia Y, Wang L, Studer A. Site-selective remote radical C-H functionalization of unactivated C-H bonds in amides using sulfone reagents. Angew. Chem. Int. Ed. 2018;57:12940–12944. doi: 10.1002/anie.201807455. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Zhou Y, Tian P, Jiang C. Copper-catalyzed amide radical-directed cyanation of unactivated Csp3-H bonds. Org. Lett. 2019;21:1921–1925. doi: 10.1021/acs.orglett.9b00553. [DOI] [PubMed] [Google Scholar]
- 41.Morcillo SP, et al. Photoinduced remote functionalization of amides and amines using electrophilic nitrogen radicals. Angew. Chem. Int. Ed. 2018;57:12945–12949. doi: 10.1002/anie.201807941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, et al. Copper-catalyzed remote C(sp3)-H trifluoromethylation of carboxamides and sulfonamides. Angew. Chem. Int. Ed. 2019;58:2510–2513. doi: 10.1002/anie.201813425. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Wang Q, Zhu J. Copper-catalyzed arylation of remote C(sp3)-H bonds in carboxamides and sulfonamides. Angew. Chem. Int. Ed. 2018;57:13288–13292. doi: 10.1002/anie.201807623. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Stateman LM, Nagib DA. δ C–H (hetero)arylation via Cu-catalyzed radical relay. Chem. Sci. 2019;10:1207–1211. doi: 10.1039/C8SC04366C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thullen SM, Treacy SM, Rovis T. Regioselective alkylative cross-coupling of remote unactivated C(sp3)-H bonds. J. Am. Chem. Soc. 2019;141:14062–14067. doi: 10.1021/jacs.9b07014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoo W-J, Li C-J. Highly efficient oxidative amidation of aldehydes with amine hydrochloride salts. J. Am. Chem. Soc. 2006;128:13064–13065. doi: 10.1021/ja064315b. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Li C-J. CuBr-catalyzed direct indolation of tetrahydroisoquinolines via cross-dehydrogenative coupling between sp3 C−H and sp2 C−H Bonds. J. Am. Chem. Soc. 2005;127:6968–6969. doi: 10.1021/ja0516054. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Li C-J. Highly efficient copper-catalyzed nitro-Mannich type reaction: cross-dehydrogenative-coupling between sp3 C−H bond and sp3 C−H bond. J. Am. Chem. Soc. 2005;127:3672–3673. doi: 10.1021/ja050058j. [DOI] [PubMed] [Google Scholar]
- 49.Li Z, Li C-J. CuBr-catalyzed efficient alkynylation of sp3 C−H bonds adjacent to a nitrogen atom. J. Am. Chem. Soc. 2004;126:11810–11811. doi: 10.1021/ja0460763. [DOI] [PubMed] [Google Scholar]
- 50.Le C, Liang Y, Evans RW, Li X, MacMillan DWC. Selective sp3 C-H alkylation via polarity-match-based cross-coupling. Nature. 2017;547:79–83. doi: 10.1038/nature22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou W-J, et al. Visible-light-driven palladium-catalyzed radical alkylation of C-H bonds with unactivated alkyl bromides. Angew. Chem. Int. Ed. 2017;56:15683–15687. doi: 10.1002/anie.201704513. [DOI] [PubMed] [Google Scholar]
- 52.Li G, et al. Alkyl transfer from C-C cleavage. Angew. Chem. Int. Ed. 2013;52:8432–8436. doi: 10.1002/anie.201303696. [DOI] [PubMed] [Google Scholar]
- 53.Leeuwen TV, Buzzetti L, Perego LA, Melchiorre P. A redox-active nickel complex that acts as an electron mediator in photochemical Giese reactions. Angew. Chem. Int. Ed. 2019;58:4953–4957. doi: 10.1002/anie.201814497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen W, et al. Building congested ketone: substituted Hantzsch ester and nitrile as alkylation reagents in photoredox catalysis. J. Am. Chem. Soc. 2016;138:12312–12315. doi: 10.1021/jacs.6b06379. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima K, Nojima S, Nishibayashi Y. Nickel- and photoredox-catalyzed cross-coupling reactions of aryl halides with 4-alkyl-1,4-dihydropyridines as formal nucleophilic alkylation reagents. Angew. Chem. Int. Ed. 2016;55:14106–14110. doi: 10.1002/anie.201606513. [DOI] [PubMed] [Google Scholar]
- 56.Nechab M, Mondal S, Bertrand MP. 1,n-Hydrogen-atom transfer (HAT) reactions in which n ≠ 5: an updated inventory. Chem. Eur. J. 2014;20:16034–16059. doi: 10.1002/chem.201403951. [DOI] [PubMed] [Google Scholar]
- 57.Tang N, Wu X, Zhu C. Practical, metal-free remote heteroarylation of amides via unactivated C(sp3)-H bond functionalization. Chem. Sci. 2019;10:6915–6919. doi: 10.1039/C9SC02564B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowry MS, et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 2005;17:5712–5719. doi: 10.1021/cm051312+. [DOI] [Google Scholar]
- 59.Slinker JD, et al. Efficient yellow electroluminescence from a single layer of a cyclometalated iridium complex. J. Am. Chem. Soc. 2004;126:2763–2767. doi: 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information. Data are also available from the corresponding author on request.