Abstract
Background:
Alzheimer’s disease (AD) has a higher prevalence among African Americans. Targeting cardiovascular and metabolic risk factors may be potential mechanisms to modify AD risk and address racial/ethnic disparities in AD dementia.
Objective:
This study investigated relationships among cardiovascular and metabolic risk factors, APOE genotype, AD biomarkers, and intracranial arterial blood flow in Whites and African Americans enriched for AD risk.
Methods:
399 cognitively unimpaired adults from the Wisconsin Alzheimer’s Disease Research Center completed physical and neuroimaging examinations. A 4D Flow MRI sequence (phase-contrast vastly under sampled isotropic projection imaging) measured intracranial arterial flow in the Circle of Willis. Linear mixed-effects regression models estimated relationships between risk factors and intracranial arterial flow and tested interactions with racial group, APOE genotype, and AD biomarkers, with separate models per risk factor.
Results:
Higher fasting glucose was associated with lower intracranial arterial flow; no additional relationships between flow and risk factors were observed. Main effects of racial group were observed, without an interaction, indicating lower flow in African Americans compared to Whites. In race-stratified analyses, higher glucose and triglycerides were associated with lower flow for African Americans, but not for Whites. No main effects or interactions among risk factors, APOE or AD biomarkers, and flow were observed.
Conclusion:
Elevated fasting glucose and triglycerides were associated with lower intracranial arterial flow; these relationships were more prominent in African Americans. Targeting metabolic risk factors may impact intracranial arterial health. Additional research is needed to determine if this will impact disparities in dementia prevalence.
Keywords: Alzheimer Disease, cerebrovascular circulation, risk factors, African Americans, metabolic syndrome, glucose, Apolipoprotein E4, Aging, neuroimaging
INTRODUCTION
Alzheimer’s disease is the most common cause of dementia among older adults (≥60 years old), affecting more than 5.3 million people in the United States [1]. The prevalence of Alzheimer’s disease is estimated to triple over the next 30 years [2], with 106 million cases of Alzheimer’s disease expected worldwide. However, despite the urgency to identify potential Alzheimer’s disease modifying agents, there are still limited therapeutic options [2, 3]. There is growing evidence that the etiology of Alzheimer’s disease reflects complex interactions between age, genetic risk factors, health behavior (e.g. physical activity, diet, tobacco use), environmental factors, cardiovascular and metabolic risk factors [4]. Modifiable cardiovascular and metabolic risk factors (e.g., hypertension, dyslipidemia, obesity, diabetes mellitus, insulin resistance) present at middle age significantly increase risk for cognitive impairment, vascular dementia, and Alzheimer’s Disease [4, 5]. Therefore, the American Heart Association and American Stroke Association recommend that cardiovascular and metabolic risk factors be potential major targets for the prevention of dementia [6]. Worldwide, it is estimated that approximately 23 million cases of dementia due to Alzheimer’s disease could be avoided by the year 2050, if the onset of disease is delayed by 2 years with preventive interventions [6].
Although vascular risk factors may be associated with markers of Alzheimer’s disease pathophysiology (e.g., beta-amyloid [Aβ] burden) in midlife [7], the cerebrovascular mechanisms by which vascular disease increases risk for later-life Alzheimer’s disease dementia remain unknown. Changes in vasculature that become more common with advanced age, such as atherosclerosis and vessel stiffening, can disrupt delivery of essential nutrients to neurons via blood flow. Prior studies using a four-dimensional (4D) flow MRI technique to quantify intracranial arterial health have observed lower intracranial arterial blood flow in individuals with dementia and mild cognitive impairment [8–11]. Additionally, associations between lower intracranial arterial blood flow, elevated atrophy of brain tissue, and lower Aβ42 in the cerebrospinal fluid (CSF) were noted in a sample of cognitively healthy and cognitively impaired adults [12]. Our prior studies also showed that higher overall vascular risk burden was associated with lower blood flow in middle cerebral arteries (MCA) and internal carotid arteries (ICA) [13]; higher insulin resistance was also associated with lower ICA flow [14].
However, there is a growing body of evidence that African Americans have a greater risk of Alzheimer’s disease compared to the non-Hispanic white population [15–19]. The prevalence of cognitive impairment or Alzheimer’s disease is two to three times higher among older African Americans than in older non-Hispanic whites [16–20]. Per U.S. data, African Americans have a greater prevalence of vascular risk factors and cerebrovascular disease. Unfortunately, due to limited recruitment of African Americans within Alzheimer’s disease studies, there is minimal understanding of the pathways linking risk factors to Alzheimer’s disease across race/ethnicities [20].
Overall, prior results suggest that vascular risk factors may be associated with lower intracranial arterial blood flow in individuals at-risk for or diagnosed with cognitive impairment. However, more information is needed on the unique contributions of individual cardiovascular risk factors to intracranial arterial blood flow in adults at-risk for Alzheimer’s disease. Additional research is also needed to determine if genetic risk or presence of Alzheimer’s disease pathophysiology moderates the relationship between vascular risk factors and intracranial flow, and if relationships vary in populations with elevated vascular risk such as African Americans. Therefore, the aims of this study were to: 1) investigate the unique relationships between modifiable cardiovascular risk factors and intracranial arterial blood flow (as measured via 4D-Flow MRI), in a racially diverse cognitively unimpaired late middle-aged population, 2) examine if increased genetic risk for Alzheimer’s disease (Apolipoprotein E [APOE] ε4 carrier status) or biomarkers of Alzheimer’s disease (cerebrospinal fluid [CSF] beta-amyloid and tau, MRI hippocampal volume and atrophy) moderate the relationships between cardiovascular risk factors and intracranial arterial blood flow, and 3) determine if relationships between cardiovascular risk factors and intracranial arterial blood flow differ in African Americans compared to non-Hispanic Whites.
MATERIALS AND METHODS
Participants
This study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board and the Wisconsin Alzheimer’s Disease Research Center (ADRC). Participants included middle-aged and older adults enrolled in the Alzheimer’s Disease Research Center (ADRC) Clinical Core whose risk for Alzheimer’s disease was enriched due to a parental history of dementia. Participants were classified as cognitively unimpaired (e.g., no evidence of mild cognitive impairment or dementia) at their baseline study visit by consensus conference panel based on the National Institute on Aging – Alzheimer’s Association (NIA-AA) criteria [21,22]. Enrolled participants who self-identified as African American or non-Hispanic White as primary race/ethnicity and completed neuroimaging exams were included in this analysis.
Neuroimaging Data Acquisition
MRI scans were completed on a clinical 3T scanner (Discovery MR750, GE Healthcare, Waukesha, WI) using either a 32-channel (Nova Medical) or 8-channel head coil (Excite HD Brain Coil; GE Healthcare). All participants were instructed to abstain from food, tobacco, and caffeine at least four hours prior to the MRI scan. Phase-contrast vastly under sampled isotropic projection imaging (PC VIPR), a 4D flow MRI technique, which simultaneously captures vessel anatomy with dynamic velocity vector data allowing for detailed hemodynamic analysis, was used to acquire intracranial arterial blood flow [23–25]. Scan parameters for the PC VIPR acquisition were as detailed: venc = 80 cm/s, imaging volume = 22x22x10 cm3 , acquired isotropic spatial resolution = (0.7 mm)3 , TR (repetition time)/TE(echo time)=7.4/2.7ms, flip angle α=10°, bandwidth = 83.3KHz, 14,000 projection angles, scan time ~ 7 min, retrospective cardiac gating reconstructed into 20 cardiac phases with temporal interpolation [26]. A T1-weighted structural scan (BRAVO) was acquired axially using the following imaging parameters: 3D fast spoiled gradient echo sequence, inversion time = 450 ms; TR/TE = 8.1/3.2 ms; flip angle = 12°; acquisition matrix = 256 × 256; field of view (FOV) = 256x256 mm2; and slice thickness = 1.0 mm.
Intracranial arterial blood flow quantification
Intracranial arterial 4D-flow data were processed using an in-house Matlab (The Mathworks; Natick, MA) based application termed the Centerline Tool, which allows for semi-automated segmentation and quantification of the intracranial arteries and veins [27]. Compared to manual segmentation of the cerebrovasculature, which requires significant time and user input, analysis using the centerline algorithm is faster and more reproducible. The internal carotid arteries (ICA) were measured in two locations, one superior position (approximately 7 mm [10 centerline points] below the posterior genu) and one inferior position (approximately 7 mm [10 centerline points] below the straight horizontal segment). The middle cerebral arteries (MCA) were measured approximately 5 mm (7 centerline points) from the juncture of the ICA and the MCA. The basilar artery was measured 5 mm (7 centerline points) from its origin from the vertebral arteries. These measurement locations were determined in consultation with a senior neuroradiologist (PT). All measurement locations were identified by experienced raters and then reviewed by a second rater before use. Experienced raters had to demonstrate an inter-rater reliability (intra-class correlation, random effects, compared with an average of the two most experienced raters) at each location greater than 0.9 for their measurements to be considered and reviewed by a second rater.
Cardiovascular and metabolic risk factors
The selection of cardiovascular and metabolic risk factors was based upon prior research by the Wisconsin Alzheimer’s Disease Research Center [12, 14], U.S. guidelines and statements on cardiovascular and cerebrovascular risk, and prior observational and epidemiological studies on vascular risk, cognitive impairment, vascular dementia, and Alzheimer’s Disease [6, 28, 29, 30]. Risk factors included fasting total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), fasting glucose, waist circumference, body mass index (BMI), systolic and diastolic blood pressure, cigarette tobacco use (current/former smoker vs. never smoker), cigarette tobacco exposure (total years smoked multiplied by the range of cigarettes/packs smoked daily [1 cigarette – <1/2 pack, 1/2 pack – <1 pack, 1 pack – <1.5 packs, 1.5 packs – <2 packs, or ≥2 packs]), and a composite vascular risk score (the ACC/AHA atherosclerotic cardiovascular disease [ASCVD] 10-year risk score) [28]. Cardiovascular and metabolic risk factor data were acquired after a minimum 12-hour overnight fast, during the physical examination at the WADRC clinical core study visit, closest in time to the MRI scan date (mean ≈ 3 months difference between study visit and MRI scan).
DNA collection and APOE genotyping
DNA was extracted at the baseline study visit from whole blood samples using the 5 Prime PerfectPure™ DNA Blood Kit (Fisher Scientific Company, LLC, Pittsburgh, PA) or the Gentra Puregene Blood Kit (Qiagen N.V., Venlo, Netherlands). DNA concentrations were quantified using UV spectrophotometry (Synergy™ 2 Multi-Mode Microplate Reader, BioTek Instruments, Inc., Winooski, VT) or the Thermo Scientific™ NanoDrop™ One UV-Visible spectrophotometer. APOE genotyping was performed by LGC Genomics (Beverly, MA) using competitive allele-specific PCR based KASP™ genotyping assays.
Beta-amyloid, tau, and neurodegeneration biomarkers
Cerebrospinal fluid (CSF) data were available for n=183 participants in the sample, who had complete data in other covariates. CSF was collected in the morning after a minimum 12-hour fast. A Sprotte spinal needle was used to extract 22 mL of CSF from the L3-L4 or L4-L5 vertebral interspace via gentle extraction into polypropylene syringes. Within 30 minutes of collection, the CSF was combined, gently mixed, centrifuged to remove red blood cells or other debris, aliquoted into 0.5-mL polypropylene tubes, and stored at −80 degrees Celsius. Samples were analyzed at the Clinical Neurochemistry Laboratory at the Sahlgrenska Academy of the University of Gothenburg, Sweden, for Aβ1– 40 and Aβ1– 42 using commercially available enzyme-linked immunosorbent assay methods (INNOTEST assays; Fujirebio, Ghent, Belgium; Triplex assays, MSD Human Aβ peptide ultrasensitive kit; Meso Scale Discovery, Gaithersburg, MD). An Aβ42 value of ≤ 471.54 pg/mL or an Aβ42/Aβ40 ratio of ≤ 0.09 was used to define elevated beta-amyloid (Aβ). A p-tau value ≥ 59.5 or t-tau value ≥ 461.26 was used to define elevated tau based on prior receiver operating characteristic analyses showing these values best discriminated cognitively healthy adults from individuals with dementia [31]. Using these thresholds, a CSF biomarker group variable with 4 levels (Aβ−/Tau−, Aβ+/Tau−, Aβ−/Tau+, Aβ+/Tau+) was created.
The T1-weighted volume was segmented into tissue classes using the updated segmentation feature in Statistical Parametric Mapping version 12 (SPM12, www.fil.ion.ucl.ac.uk/spm). Total intracranial volume (ICV; mm3) was derived from the sum of the major tissue class volumes (gray and white matter and CSF volume). Global atrophy was quantified as CSF volume divided by gray matter volume plus white matter volume. Hippocampal volume (mm3) was calculated using the FMRIB Software Library FIRST tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST), a model-based segmentation/registration tool. Volumes for left and right hippocampus were summed to compute bilateral hippocampal volume. Bilateral hippocampal volume was divided by total ICV and this proportion value (hippocampal volume/ICV) was included in models [32].
Statistical analyses
Sample characteristics were compared across racial strata using Fisher’s exact test for categorical or binary covariates, and Mann-Whitney U test for continuous items.
Linear mixed effects models were used to examine adjusted relationships between the risk factors and intracranial arterial flow. All models included fixed effects of age at MRI scan, sex (male/female), years of education, genetic risk for late-onset Alzheimer’s disease (APOE ε4 carrier status), antihypertensive medication use, MR coil type (8- or 32-channel), vessel type (basilar, left and right MCA, left and right inferior and superior ICA), and two random intercepts: one for subject, and a second for vessel group nested within subject (ICA vs. non-ICA grouping). Diabetes mellitus was not included in all models due to collinearity with other risk factors (e.g., fasting glucose). The outcome variable of each model was the vessel specific intracranial arterial flow. Due to the concern of collinearity among the 13 risk factors of interests, separate models were run for each cardiovascular risk factor included as a covariate in the model, instead of including all 13 risk factors in the same model. The model described above, with a risk factor covariate included, was used to examine relationships between the risk factors and flow and represent the base models. There was reasonable concern that the effect of risk factor on flow might be vessel specific. Therefore, an interaction between the risk factor and vessel type was examined in these 13 models; however, Likelihood Ratio Tests did not indicate sufficient evidence to include these interactions in the base models.
In addition to the base models, several moderators of the risk factor / intracranial arterial flow were examined. Moderation by APOE ε4 carrier status was examined by including an interaction of APOE ε4 status and the risk factor to the base model. Moderation by Alzheimer’s disease biomarkers was examined by adding a main effect of the biomarker, and its interaction with the risk factor, to the base model. As with the risk factors, concern of multicolinearity was such that only one of the three Alzheimer’s disease biomarkers (CSF biomarker group, global atrophy, or hippocampal volume/ICV) was included in the model at a time. Due to the few participants who had both CSF and fasting glucose data available, no model for the CSF group moderation of fasting glucose could be performed. For most models, n=183 participants were included in analyses involving CSF and n=375 participants were included in analyses involving structural volume. Specific sample sizes for each of the models including the interaction of Alzheimer’s disease biomarker x vascular risk factor are presented in the Supplemental Table.
Moderation by racial group was examined in two ways. First, by inclusion of a main effect of race, and its interaction with the risk factor, to the base model. Models with only the race main effect (no interaction) were also examined, as it is well known that African Americans are typically at higher risk for many of the risk factors examined, compared to non-Hispanic Whites [33]. Second, by fitting race-specific models, as it is known that at least some covariates in the model (e.g. APOE ε4 carrier status) may not represent the same effect in both racial groups [34].
Asymptotic properties of fixed effects estimates were used to calculate p-values by Wald tests. Likelihood ratio tests were used to assess significant interactions with more than 1 degree of freedom (i.e., CSF group interactions), and for omnibus tests (e.g. race and its interaction with a risk factor both being in a model). The False Discovery Rate (FDR) correction was applied across the 13 different risk factor models using the Benjamini-Hochberg method at an FDR control of 5%. For items with the same interpretation across models (e.g. age main effect, sex main effect, race main effect, etc.) no FDR correction was performed. Statistical significance was assessed at α = 0.05 level.
All analyses were performed using R 3.5.1 [35]. Linear mixed effects were fitted using restricted maximum likelihood with ‘lmer()’ in the ‘lme4’ package [36]; for likelihood ratio tests, models were fit using maximum likelihood. Confidence intervals on figures were produced using the percentile method on parametric bootstrapping of the fitted model over 2000 iterations. Use of the vessel group random effect was necessary to alleviate a U-shaped trend in residuals, where halves of the U corresponded to the two vessel groups specified. With the vessel group random effect included, there were no major concerns for non-normal residuals, residuals trends, outliers, non-normal random effects, or correlation between random effects and residuals.
RESULTS
Sample characteristics
Summary characteristics are displayed in Table 1. On average, participants (n=399; n=332 Whites and n=67 African Americans) were late middle-aged (mean age = 62), highly educated (mean education = 16 years), and two-thirds were female (66%). There were no significant demographic differences between whites and African American participants. However, African Americans were generally more likely to have higher number of prevalent cardiovascular risk factors. African Americans had higher rates of diabetes mellitus. Of the 25 African Americans with diabetes mellitus, 24 had type 2 diabetes mellitus. Of the 31 White participants with diabetes mellitus, 25 had Type 2 diabetes mellitus.
TABLE 1.
Sample characteristics
| Total | White | African American | p | |
|---|---|---|---|---|
| N | 399 | 332 | 67 | |
| Age, years | 61.87 (8.65) | 61.60 (8.71) | 63.23 (8.28) | 0.123 |
| Female (%) | 262 (65.7) | 216 (65.1) | 46 ( 68.7) | 0.672 |
| APOE e4 carrier (%) | 137 (34.3) | 119 (35.8) | 18 ( 26.9) | 0.204 |
| Education, years | 16.14 (2.60) | 16.20 (2.61) | 15.88 (2.56) | 0.325 |
| Antihypertensive Medication Use (%) | 121 (30.5) | 81 (24.4) | 40 ( 61.5) | <0.001 |
| Diabetes mellitus (%) | 56 (14.0) | 31 ( 9.3) | 25 ( 37.3) | <0.001 |
| ASCVD 10-year risk score | 10.94 (12.92) | 9.65 (12.78) | 17.62 (11.61) | <0.001 |
| Systolic blood pressure, mmHg | 128.29 (17.47) | 125.93 (15.86) | 139.96 (20.31) | <0.001 |
| Diastolic blood pressure, mmHg | 76.74 (9.15) | 75.36 (8.68) | 83.61 (8.31) | <0.001 |
| Waist-to-hip ratio | 0.91 (0.09) | 0.90 (0.09) | 0.94 (0.08) | 0.001 |
| Waist circumference, cm | 96.93 (15.50) | 95.06 (14.92) | 106.20 (15.10) | <0.001 |
| Body Mass Index, kg/m2 | 29.22 (5.98) | 28.44 (5.62) | 33.09 (6.24) | <0.001 |
| LDL-c, mg/dL | 113.45 (33.29) | 114.61 (32.17) | 107.45 (38.27) | 0.225 |
| HDL-c, mg/dL | 62.09 (18.96) | 62.99 (18.54) | 57.51 (20.50) | 0.029 |
| Total Cholesterol , mg/dL | 196.48 (37.97) | 198.68 (37.16) | 185.29 (40.31) | 0.018 |
| Triglycerides, mg/dL | 111.80 (88.46) | 110.54 (90.12) | 118.18 (79.86) | 0.443 |
| Glucose, mg/dL | 100.47 (29.36) | 97.37 (22.30) | 113.65 (47.27) | 0.028 |
| Current or former tobacco use (%) | 145 (36.3) | 108 (32.5) | 37 ( 55.2) | 0.001 |
| Tobacco exposure, pack-years | 12.67 (26.05) | 10.74 (24.55) | 22.22 (30.94) | <0.001 |
| Hippocampal volume, mm3 | 7792.08 (891.03) | 7883.15 (852.49) | 7334.00 (944.93) | <0.001 |
| Intracranial volume (ICV), mm3 | 1473.95 (148.24) | 1481.63 (147.53) | 1435.89 (146.91) | 0.011 |
| Hippocampal volume/ICV | 0.53 (0.06) | 0.53 (0.06) | 0.51 (0.06) | 0.009 |
| Global atrophy | 3.36 (0.91) | 3.42 (0.88) | 3.08 (1.01) | 0.001 |
| Cerebrospinal Fluid Group (%) | 0.678 | |||
| Biomarker negative | 137 (71.0) | 133 (70.7) | 4 ( 80.0) | |
| Aβ+ and tau− | 22 (11.4) | 22 (11.7) | 0 ( 0.0) | |
| Tau+ and Aβ− | 16 ( 8.3) | 16 ( 8.5) | 0 ( 0.0) | |
| Aβ + and tau+ | 18 ( 9.3) | 17 ( 9.0) | 1 ( 20.0) | |
| MRI Head Coil Type (% 32-channel) | 129 (32.3) | 86 (25.9) | 43 ( 64.2) | <0.001 |
Values are given as mean (standard deviation) unless otherwise specified; LDL-c (low-density lipoprotein cholesterol); HDL-c (high-density lipoprotein cholesterol); Aβ (Beta-amyloid); ASCVD (atherosclerotic cardiovascular disease); Global atrophy was calculated as CSF volume (mm3) divided by gray matter volume (mm3) plus white matter volume (mm3); Diabetes mellitus included 7 participants with Type I diabetes (1 African American, 6 White) and 49 participants with Type II diabetes (24 African American and 25 White).
Relationships among vascular risk factors and intracranial arterial flow
Statistical parameters are displayed in Table 2. Covariates of age, sex, vessel, and head coil were significantly associated with intracranial arterial flow in most models. In contrast, covariates of education, antihypertensive medication use, and APOE genotype were non-significant in all models. When risk factor p-values were FDR-adjusted, a significant relationship was observed between higher fasting glucose and lower intracranial arterial flow (β=−0.004 mL/min per mg/dL, p<0.001); see Figure 1, top center panel. Without FDR-adjustment, a relationship was also observed between higher diastolic blood pressure and lower intracranial arterial flow (see Figure 1, top left panel). No relationships between the remaining vascular risk factors and intracranial arterial flow were observed when p-values were unadjusted (remaining p’s > .13) or FDR-adjusted (remaining p’s > .21).
Table 2.
Relationship between risk factor and intracranial arterial blood flow
| Model | Total sample | White sample | African American sample | ||||
|---|---|---|---|---|---|---|---|
| Risk Factor | N | Beta (SE) | N | Beta (SE) | N | Beta (SE) | |
| 1 | ASCVD 10-year risk score | 379 | −0.004 (.003) | 320 | −0.0002 (.004) | 59 | −0.010 (.006) |
| 2 | Systolic blood pressure | 381 | −0.002 (.002) | 321 | 0.0006 (.002) | 60 | −0.002 (.003) |
| 3 | Diastolic blood pressure | 381 | −0.006 (.003)* | 321 | −0.0032 (.003) | 60 | −0.004 (.006) |
| 4 | Waist circumference | 381 | −0.003 (.002) | 321 | 0.0002 (.002) | 60 | −0.006 (.003) |
| 5 | Waist-to-Hip Ratio | 381 | −0.356 (.355) | 321 | 0.0574 (.389) | 60 | −1.460 (.760) |
| 6 | Body Mass Index | 381 | −0.007 (.004) | 321 | 0.0006 (.005) | 60 | −0.015 (.008)* |
| 7 | LDL-c | 376 | 0.001 (.001) | 318 | −0.0001 (.001) | 58 | 0.002 (.001) |
| 8 | HDL-c | 379 | 0.001 (.002) | 320 | 0.00001 (.002) | 59 | 0.002 (.003) |
| 9 | Total cholesterol | 379 | 0.0004 (.001) | 320 | −0.0001 (.001) | 59 | 0.001 (.001) |
| 10 | Triglycerides | 378 | −0.0003 (.000) | 319 | 0.00002 (.000) | 59 | −0.002 (.001)*+ |
| 11 | Glucose | 277 | −0.004 (.001)*+ | 227 | −0.0042 (.002)* | 50 | −0.003 (.001)*+ |
| 12 | Current or former smoker | 381 | 0.013 (.055) | 321 | 0.0819 (.063) | 60 | −0.045 (.100) |
| 13 | Tobacco exposure | 135 | 0.001 (.001) | 102 | 0.0011 (.002) | 33 | 0.000 (.002) |
Each linear mixed-effects regression model included fixed effects of age, sex, education, antihypertensive medication use, vessel (Left ICA, Right ICA, Left SICA, Right SICA, Left MCA, Right MCA, Basilar), head coil type (8-channel, 32- channel), and intercept random effects for person and vessel group (non-ICA or ICA). For total sample analyses, covariates of age, sex, vessel type, and head coil type were significantly associated with intracranial arterial flow in most models, whereas covariates of education, antihypertensive medication use, and APOE genotype were non-significant in all models.
ASCVD (atherosclerotic cardiovascular disease); LDL-c (low-density lipoprotein cholesterol); HDL-c (high-density lipoprotein cholesterol);
p<.05 non-FDR-adjusted;
p<.05 FDR-adjusted
Figure 1 (color for online version).
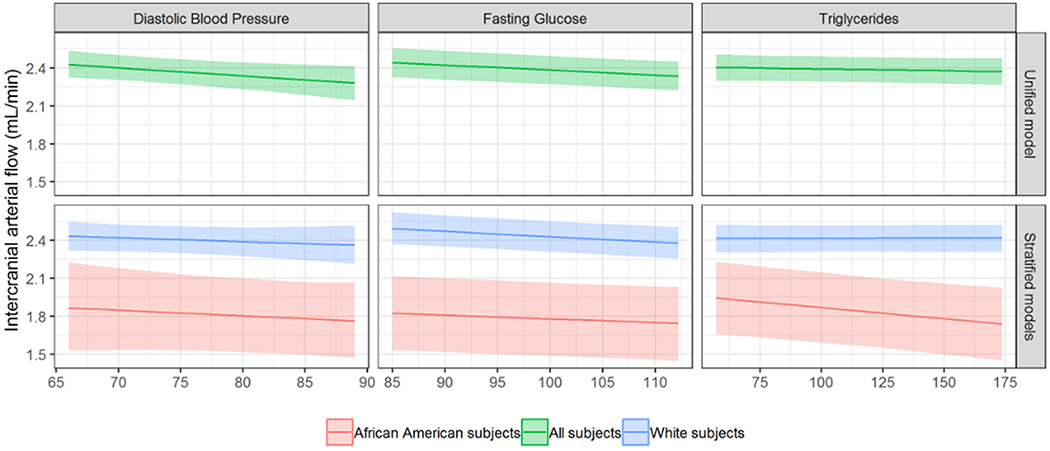
Estimated relationships among intracranial arterial blood flow and diastolic blood pressure (left), fasting glucose (center), and triglycerides (right) in the total sample of 399 participants (top row) and in separate models for African Americans (red; n=67) and Whites (blue; n=332) (bottom row). All non-risk factor covariates were set to their median value for continuous covariates, and their mode for binary or categorical covariates.
Effect of APOE genotype and Alzheimer’s disease biomarkers on risk factor-flow relationships
When interaction p-values were FDR-adjusted, no significant interactions between APOE genotype x vascular risk factors on intracranial arterial blood flow were observed. For the associated unadjusted p-values, interaction terms of APOE genotype x total cholesterol (p=.04), APOE genotype x HDL-c (p=.01), APOE genotype x LDL-c (p=.04), and APOE genotype x triglycerides (p=.006) were significantly associated with intracranial arterial flow. No significant interactions between vascular risk factors and Alzheimer’s disease biomarkers (CSF biomarker group, hippocampal volume/ICV, global atrophy) or main effects of Alzheimer’s disease biomarkers on intracranial arterial blood flow were observed when p-values were unadjusted or FDR-adjusted.
Effect of racial group on risk factor-flow relationships
No interactions between vascular risk factors and race were observed when p-values were unadjusted or FDR-adjusted. With these interactions removed from the models, significant main effects of race on intracranial arterial blood flow indicated lower flow among African American participants compared to Whites (average β=−0.308 mL/min per mg/dL, all p<0.001); see Table 3. In race-stratified models (see Table 2), there were no significant relationships between vascular risk factors and intracranial arterial flow in FDR-adjusted models for White participants. For African American participants, a significant relationship between higher glucose and triglycerides and lower intracranial arterial flow was observed in FDR-adjusted models (glucose β=−0.003 mL/min per mg/dL, p=0.006; triglycerides β=−0.002 mL/min per mg/dL, p=0.003; see Figure 1, bottom panel).
Table 3.
Relationship between racial group and intracranial arterial flow
| Interaction of risk factor x racial group | Main effect of racial group after interaction term removed from models | |||
|---|---|---|---|---|
| Model | Risk Factor | N | Beta (SE) | Beta (SE) |
| 1 | ASCVD 10-year risk score | 379 | 0.005 (0.006) | −0.289 (0.080)* |
| 2 | Systolic blood pressure | 381 | −0.001 (0.004) | −0.305 (0.081)* |
| 3 | Diastolic blood pressure | 381 | 0.000 (0.008) | −0.281 (0.081)* |
| 4 | Waist circumference | 381 | −0.006 (0.005) | −0.293 (0.079)* |
| 5 | Waist-to-Hip Ratio | 381 | −0.628 (0.900) | −0.3 (0.079)* |
| 6 | Body Mass Index | 381 | −0.015 (0.011) | −0.293 (0.080)* |
| 7 | LDL-c | 376 | 0.001 (0.002) | −0.287 (0.080)* |
| 8 | HDL-c | 379 | 0.001 (0.004) | −0.294 (0.079)* |
| 9 | Total cholesterol | 379 | 0.000 (0.002) | −0.295 (0.079)* |
| 10 | Triglycerides | 378 | −0.002 (0.001)* | −0.293 (0.079)* |
| 11 | Glucose | 277 | 0.002 (0.002) | −0.239 (0.096)* |
| 12 | Current or former smoker | 381 | −0.135 (0.143) | −0.319 (0.079)* |
| 13 | Tobacco exposure | 135 | 0.000 (0.003) | −0.51 (0.113)* |
Each linear mixed-effects regression model included fixed effects of age, sex, education, antihypertensive medication use, vessel (Left ICA, Right ICA, Left SICA, Right SICA, Left MCA, Right MCA, Basilar), head coil type (8-channel, 32- channel), and intercept random effects for person and vessel group (non-ICA or ICA).
ASCVD (atherosclerotic cardiovascular disease); LDL-c (low-density lipoprotein cholesterol); HDL-c (high-density lipoprotein cholesterol)
p<.05 non-FDR-adjusted
DISCUSSION
In this middle-aged and older adult sample of 399 cognitively healthy adults enriched for Alzheimer’s disease risk, we observed that elevated fasting glucose was associated with lower intracranial arterial blood flow. A weaker relationship (observed in individual model p-values >0.05 after FDR correction, but <0.05 before correction) was observed between elevated diastolic blood pressure and lower intracranial arterial flow; no significant relationships were observed between intracranial arterial blood flow and other cardiovascular or metabolic risk factors examined including systolic blood pressure, obesity (BMI, waist circumference, waist-to-hip ratio), lipids (total cholesterol, HDL-c, LDL-c, triglycerides), tobacco use, or composite vascular risk burden.
Interactions between racial group x risk factors on intracranial arterial blood flow were non-significant. When the interaction term was removed from all models, the main effect of race was significantly associated with intracranial arterial flow across all risk factor models. African American participants demonstrated lower intracranial arterial blood flow compared to Whites. When relationships between risk factors and flow were examined within racial groups separately, we observed significant relationships among elevated fasting glucose and triglycerides and lower intracranial arterial blood flow in the African Americans that survived FDR-correction, even with the relatively small sample size of the strata. These relationships did not remain statistically significant in the White participants after FDR-correction despite the larger sample size in that group compared to the African American strata. These findings suggest that the relationships observed among metabolic risk factors and intracranial arterial blood flow may be driven by the African American participants, who have on average higher rates of risk factors, and that these relationships may be race-specific.
Metabolic syndrome, defined as a clustering of at least three of the five conditions: elevated triglycerides, low high-density lipoprotein cholesterol (HDL-c), elevated blood pressure (hypertension), elevated fasting glucose, and abdominal obesity (elevated waist circumference) [37], has a higher prevalence among African Americans compared to non-Hispanic Whites and is an important risk factor for diabetes mellitus [38], cardiovascular disease [39] and cerebrovascular disease, including stroke and Alzheimer’s disease [40]; although the exact mechanisms are unknown. There is growing data that abnormalities in essential fatty acid metabolism, alterations in lipid metabolism, insulin resistance and abnormal leptin signaling may support the pathological association between metabolic syndrome and cerebrovascular disorders (“metabolic-cognitive syndrome”) [40]. In a small study of racially/ethnically diverse middle-aged U.S. adults (n=83, 40–60 years old) without cognitive impairment or prior cardiovascular or cerebrovascular events, a greater number of metabolic risk factors (per the metabolic syndrome score, MetS score) was associated with lower cerebrovascular conductance within the middle cerebral artery [41].
Furthermore, some prior studies report associations between elevated markers of cerebrovascular dysfunction, such as arterial stiffness and/or reduced cerebral blood flow, and elevated dementia-related pathology (e.g., beta-amyloid deposition [42, 43]); however, others have not observed direct relationships among cerebrovascular dysfunction and Alzheimer’s disease biomarkers, but have rather found interactions among them on cognitive decline [44]. In our prior work, we observed lower internal carotid artery flow in a small sample of older adults with mild cognitive impairment who had elevated brain markers of beta-amyloid. In contrast, in this larger racially diverse cognitively unimpaired sample, we did not observe relationships among markers of Alzheimer’s disease or dementia-related biomarkers (e.g., beta-amyloid, tau, hippocampal volume, global brain atrophy) and intracranial arterial blood flow; nor did we observe a moderation of these markers on the relationships between risk factors and flow. We also did not observe a relationship between genetic risk for late-onset Alzheimer’s disease (APOE ε4 carriage) and intracranial arterial blood flow; however, weak relationships (observed in individual model p-values that did not survive FDR-correction) between APOE genotype x total cholesterol, HDL-c, LDL-c, and triglycerides suggest possible differential relationships between lipids and intracranial blood flow in APOE ε4 vs. non-ε4 carriers. Prior literature suggests that genetic risk for late-onset Alzheimer’s disease may be associated with cerebrovascular dysfunction by reducing cerebral blood flow, modifying coupling between cerebral blood flow and neuronal signaling, and increasing blood-brain barrier leakiness, cerebral amyloid angiopathy, hemorrhages, and transport of nutrients and toxins [45].
There are several limitations to the current study that should be considered including generalizability of the study findings to those at elevated risk for Alzheimer’s disease, differences in vascular risk factors among African Americans and Whites and smaller sample of participants with CSF data available (particularly in the African American sample). MRI hardware changes (e.g., head coil) occurred during the course of the study and may increase noise in the outcome variable; however coil type was included in all models to minimize overall measurement variance. Additionally, although antihypertensive medication was included in the analysis, we were unable to evaluate the effect of diabetes medication on biomarker levels and vascular outcomes, and thus various levels of certain blood biomarkers might be a partial proxy for medication use as well. The complex nature of ASCVD scores along with use of covariates that are needed to calculate the score (age, sex, race, etc.) can make interpretation of the effect of ASCVD and its moderation/mediation difficult to interpret. Despite the limitations, this study suggests that elevated fasting glucose may be particularly deleterious for intracranial arterial vessel health; this may be a target for improving cerebral blood flow and reducing risk for cognitive decline. Our findings highlight the need to further examine metabolic risk factors, particularly in African Americans and other under-represented populations. Future directions include examination of additional markers of cerebrovascular health not included here such as white matter hyper intensities, arterial stiffness, and micro bleeds, as well as examining associations between cumulative vascular/metabolic risk factor burden, Alzheimer’s disease biomarkers, and cerebrovascular health in longitudinal models.
Supplementary Material
Figure 1 (grey-scale for print version).
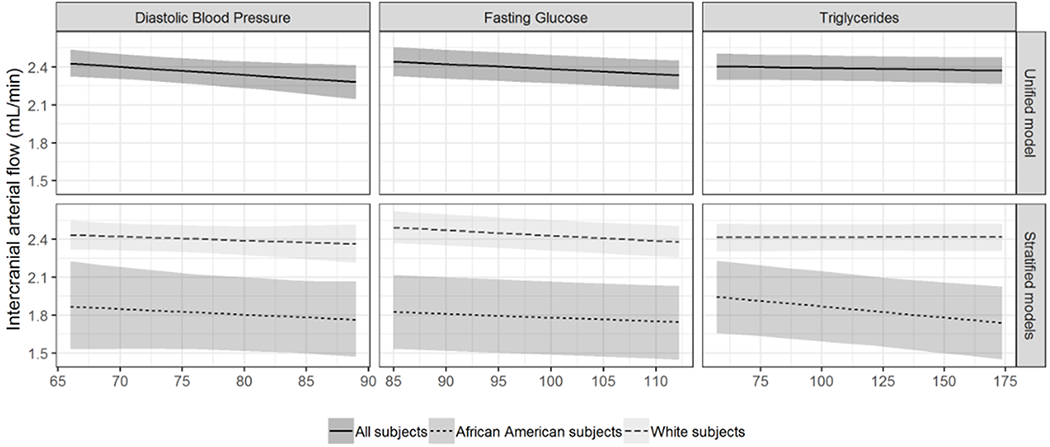
Relationships among intracranial arterial blood flow and diastolic blood pressure (left), fasting glucose (center), and triglycerides (right) in the total sample of 399 participants (top row) and in separate models for African Americans (darker grey; n=67) and Whites (n=332; light grey) (bottom row). All non-risk factor covariates were set to their median value for continuous covariates, and their mode for binary or categorical covariates.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of researchers and staff at the Wisconsin Alzheimer’s Disease Research Center for assistance in recruitment and data collection. We would like to acknowledge Fabu Carter, MS and Brieanna Harris, BA for their assistance in recruitment of under-represented populations into the study. We are grateful to Carson Hoffman for his contributions to improve the centerline tool for more streamlined and robust cranial processing. We would like to thank Heather Schoul, BS for assistance in data acquisition and database organization. We would like to thank Paul Cary and Natasha Ignatowski for their assistance in processing PC VIPR data for this study and Carson Hoffman for the implementation of improved tools for the semi-automated 4D flow analysis. Most importantly, we thank the dedicated Wisconsin ADRC participants for their continued support and participation in research. Research reported in this manuscript was supported by the National Institutes of Health, NIH-NIA grant number P50-AG033514, a Wisconsin Alzheimer’s Disease Research Center pilot award (HMJ, LRC), NIH-NIA R01 AG054059 (CEG), and the Veterans Affairs Advanced Fellowship in Women’s Health (MFW). The authors would like to acknowledge the clinical and neuropathology diagnostic support provided by the Wisconsin ADRC’s Clinical, Neuropathology and Biomarkers Cores, and biostatistical support provided by the Data Management and Biostatistics Core. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A portion of the findings described were presented by Lindsay Clark at the Alzheimer’s Association International Conference in Chicago on July 22, 2018.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- [1].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cummings J, Fox N (2017) Defining Disease Modifying Therapy for Alzheimer’s Disease. J Prev Alzheimers Dis 4, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Han J-Y, Han S-H (2014) Primary prevention of Alzheimer’s disease: is it an attainable goal? Journal of Korean medical science 29, 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de la Torre JC (2004) Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol 3, 184–190. [DOI] [PubMed] [Google Scholar]
- [6].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council CoE, Prevention CoCNCoCR, Intervention, Council on Cardiovascular S, Anesthesia (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Langbaum JB, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, Protas HD, Reeder SA, Bandy D, Yu M, Caselli RJ, Reiman EM (2012) Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging 33, 827e811–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rivera-Rivera LA, Turski P, Johnson KM, Hoffman C, Berman SE, Kilgas P, Rowley HA, Carlsson CM, Johnson SC, Wieben O (2016) 4D flow MRI for intracranial hemodynamics assessment in Alzheimer’s disease. J Cereb Blood Flow Metab 36, 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stefani A, Sancesario G, Pierantozzi M, Leone G, Galati S, Hainsworth AH, Diomedi M (2009) CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer’s and mixed dementia. J Neurol Sci 283, 109–115. [DOI] [PubMed] [Google Scholar]
- [10].Vicenzini E, Ricciardi MC, Altieri M, Puccinelli F, Bonaffini N, Di Piero V, Lenzi GL (2007) Cerebrovascular reactivity in degenerative and vascular dementia: a transcranial Doppler study. Eur Neurol 58, 84–89. [DOI] [PubMed] [Google Scholar]
- [11].Franceschi M, Alberoni M, Bressi S, Canal N, Comi G, Fazio F, Grassi F, Perani D, Volonté MA (1995) Dementia 6, 32–38. [DOI] [PubMed] [Google Scholar]
- [12].Berman SE, Rivera-Rivera LA, Clark LR, Racine AM, Keevil JG, Bratzke LC, Carlsson CM, Bendlin BB, Rowley HA, Blennow K, Zetterberg H, Asthana S, Turski P, Johnson SC, Wieben O (2015) Intracranial Arterial 4D-Flow is Associated with Metrics of Brain Health and Alzheimer’s Disease. Alzheimers Dement (Amst) 1, 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clark LR, Berman SE, Rivera-Rivera LA, Hoscheidt SM, Darst BF, Engelman CD, Rowley HA, Carlsson CM, Asthana S, Turski P, Wieben O, Johnson SC (2017) Macrovascular and microvascular cerebral blood flow in adults at risk for Alzheimer’s disease. Alzheimers Dement (Amst) 7, 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoscheidt SM, Kellawan JM, Berman SE, Rivera-Rivera LA, Krause RA, Oh JM, Beeri MS, Rowley HA, Wieben O, Carlsson CM, Asthana S, Johnson SC, Schrage WG, Bendlin BB (2017) Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J Cereb Blood Flow Metab 37, 2249–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].2019 Alzheimer’s disease facts and figures Alzheimers Dement 15, 3, 321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- [16].Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA (2016) Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 12, pp. 216–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, McGuire LC (2019) Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥ 65 years. Alzheimers Dement 15,17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rajan KB, Weuve J, Barnes LL, Wilson RS, Evans DA (2019) Prevalence and incidence of clinically diagnosed Alzheimer’s disease dementia from 1994 to 2012 in a population study. Alzheimers Dement 15, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeuz R (1999) Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry 14, 481–493 [PubMed] [Google Scholar]
- [20].Barnes LL, Bennett DA (2014) Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health affairs (Project Hope) 33, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O (2012) 4D flow MRI. J Magn Reson Imaging 36, 1015–1036. [DOI] [PubMed] [Google Scholar]
- [24].Johnson KM, Lum DP, Turski PA, Block WF, Mistretta CA, Wieben O (2008) Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 60, 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gu T, Korosec FR, Block WF, Fain SB, Turk Q, Lum D, Zhou Y, Grist TM, Haughton V, Mistretta CA (2005) PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR Am J Neuroradiol 26, 743–749. [PMC free article] [PubMed] [Google Scholar]
- [26].Liu J, Redmond MJ, Brodsky EK, Alexander AL, Lu A, Thornton FJ, Schulte MJ, Grist TM, Pipe JG, Block WF (2006) Generation and visualization of four-dimensional MR angiography data using an undersampled 3-D projection trajectory. IEEE Trans Med Imaging 25, 148–157. [DOI] [PubMed] [Google Scholar]
- [27].Schrauben E, Wahlin A, Ambarki K, Spaak E, Malm J, Wieben O, Eklund A (2015) Fast 4D flow MRI intracranial segmentation and quantification in tortuous arteries. J Magn Reson Imaging 42, 1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goff DC jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC jr, , Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC jr, , Tomaselli GF (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S49–73. [DOI] [PubMed] [Google Scholar]
- [29].Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, Mistur R, Pirraglia E, de Santi S, Li Y, Goldowsky A, de Leon MJ (2011) Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab 31, 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carlsson CM, Xu G, Wen Z, Barnet JH, Blazel HM, Chappell RJ, Stein JH, Asthana S, Sager MA, Alsop DC, Rowley HA, Fain SB, Johnson SC (2012) Effects of atorvastatin on cerebral blood flow in middle-aged adults at risk for Alzheimer’s disease: a pilot study. Curr Alzheimer Res 9, 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, Bendlin BB, Asthana S, Johnson SC, Zetterberg H, Carlsson CM (2018) Age-accelerated cognitive decline in asymptomatic adults with CSF β-amyloid. Neurology 90, e1306–e1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Voevodskaya O, Simmons A, Nordenskjöld R, Kullberg J, Ahlström H, Lind L, Wahlund LO, Larsson EM, Westman E, Alzheimer’s Disease Neuroimaging Initiative (2014) Front Aging Neurosci 6, 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bravata DM, Wells CK, Gulanski B, Kernan WN, Brass LM, Long J, Concato J (2005) Racial disparities in stroke risk factors: the impact of socioeconomic status. Stroke 36, 1507–1511. [DOI] [PubMed] [Google Scholar]
- [34].Barnes LL, Bennett DA (2014) Alzheimer’s disease in African Americans: risk factors and challenges for the future. Health Aff (Milwood) 33, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Team RC (2018) R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- [36].Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear Mixed-Effects Models Using lme4. 2015 67, 48. [Google Scholar]
- [37].Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645. [DOI] [PubMed] [Google Scholar]
- [38].Ginsberg HN, MacCallum PR (2009) The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. Journal of the cardiometabolic syndrome 4, 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Galassi A, Reynolds K, He J (2006) Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 119, 812–819. [DOI] [PubMed] [Google Scholar]
- [40].Farooqui AA, Farooqui T, Panza F, Frisardi V (2012) Metabolic syndrome as a risk factor for neurological disorders. Cellular and Molecular Life Sciences 69, 741–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pasha EP, Birdsill AC, Oleson S, Haley AP, Tanaka H (2017) Impacts of Metabolic Syndrome Scores on Cerebrovascular Conductance Are Mediated by Arterial Stiffening. American Journal of Hypertension 31, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, Wong D, Zhou Y, Knopman D, Mosley TH, Gottesman RF (2018) Arterial stiffness and dementia pathology: Atherosclerosis Risk in Communities (ARIC)-PET Study. Neurology 90, e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mattsson N, Tosun D, Insel PS, Simonson A, Jack CR jr, ., Beckett LA, Donohue M, Jagust W, Schuff N, Weiner MW (2014) Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain 137, 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bangen KJ, Clark AL, Edmonds EC, Evangelista ND, Werhane ML, Thomas KR, Locano LE, Tran M, Zlatar ZZ, Nation DA, Bondi MW, Delano-Wood L (2017) Cerebral Blood Flow and Amyloid-β Interact to Affect Memory Performance in Cognitively Normal Older Adults. Frontiers in aging neuroscience 9, 181–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tai LM, Thomas R, Marottoli FM, Koster KP, Kanekiyo T, Morris AW, Bu G (2016) The role of APOE in cerebrovascular dysfunction. Acta Neuropathol 131, 709–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


