Abstract
Infection during pregnancy increases the risk of neurodevelopmental disorders, such as autism, in offspring. Mouse studies now reveal a link between gut bacteria and atypical brain-circuit connections.
What do pregnancy, viral infection, gut bacteria, the immune response and the function of a neuronal circuit in the brain have in common? More than you might expect, according to a pair of studies, by Kim et al.1 and Yim et al.2, published online in Nature
Animal studies and epidemiological analysis in humans have shown that if a mother is infected by certain viruses during pregnancy, there is a risk that her offspring will develop autism or other neurodevelopmental disorders3,4. This phenomenon is often studied using a mouse model in which viral infection is mimicked by exposing pregnant animals to a synthetic molecule called poly(I:C) that is structurally similar to double-stranded RNA, a common hallmark of viral infection (Fig. 1). This exposure triggers an immune response in the mother that is termed maternal immune activation (MIA), which can lead to atypical social and repetitive behaviours in her offspring5. However, the molecular and cellular basis for this phenomenon has remained poorly understood until now.
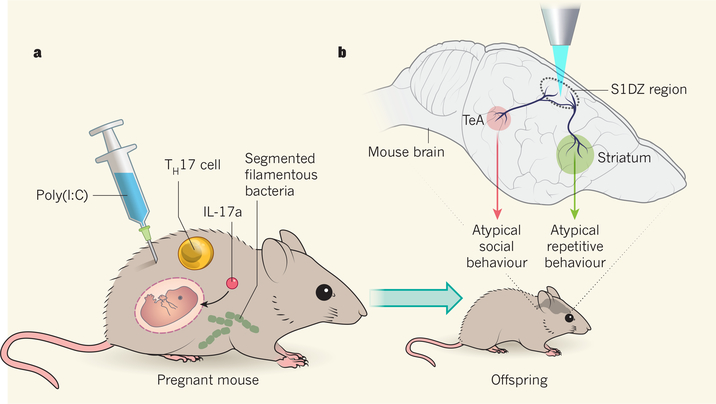
Figure 1 |. Maternal immune response and the brain circuits underlying atypical offspring behaviour.
Viral exposure during pregnancy can sometimes result in offspring that have neurodevelopmental disorders3,4. This phenomenon is linked to a maternal immune response5 associated with TH17 cells and the signalling molecule IL-17a. a, Kim et al.1 and Yim et al.2 investigated this process using a model in which pregnant mice are injected with a molecule called poly(I:C) that is structurally similar to the double-stranded RNA characteristic of viral infection. The authors demonstrated that the presence of a type of gut microbe called segmented filamentous bacteria is required for the animals’ offspring to show atypical behaviours. b, Applying a technique known as optogenetics, in which light of a certain wavelength (shown here in blue) is used to activate or inhibit neurons genetically engineered to express light-sensitive ion channels, the authors reveal that activation of the S1DZ region of the cortex is associated with atypical offspring behaviour. They found that neurons in the S1DZ connect to neurons in a brain region called the temporal association area (TeA) that influence social behaviours, and to neurons in the striatum that affect repetitive behaviours.
In a previous study5, Kim, Yim and their colleagues used this mouse model of MIA to show that interleukin 17a (IL-17a), a protein secreted into the pregnant mother’s blood-stream, is required for the behavioural abnormalities to develop in offspring. The authors also found that IL-17a is secreted by maternal immune cells called T helper 17 (TH17) cells.
Kim et al. now reveal key missing links in the process that activates this TH17-cell immune response in mothers. First, they found that IL-17a secretion did not occur in non-pregnant female mice that had undergone MIA, indicating that pregnancy is an important factor. The authors also showed that specific bacteria must be present in the mother’s intestine for the TH17 immune cells to respond to the mimicked state of viral infection. Pretreatment with antibiotics decreased this maternal immune response. Moreover, after antibiotic pretreatment, offspring did not show abnormal brain development or atypical behaviours, revealing a link between microbes present in the pregnant mother and the risk of autism-like features developing in her offspring.
The authors investigated which bacteria targeted by the antibiotic might be responsible for the changes observed. They tested a type of bacterium commonly resident in the gut called segmented filamentous bacteria, which are associated with higher levels of TH17 cells than are found in mice without these bacteria6. Using microscopy and analysis of DNA in faecal samples, they confirmed that these microbes were the antibiotic target. When mice lacking the bacteria underwent MIA, their offspring did not show atypical brain development or behaviour. However, if the mice were exposed to segmented filamentous bacteria, either through direct introduction of the microbes or by coming into contact with mice that harboured them, the animals’ offspring showed both of these abnormal features.
Yim and colleagues delved further into the underlying mechanism by trying to determine exactly where in the brain the MIA-associated abnormalities occur. Microscope analysis of brain regions revealed that abnormalities in the dysgranular zone of the primary somatosensory cortex (S1DZ) were most closely correlated with the atypical behaviour. Not satisfied with correlation alone, the authors carried out a remarkable series of manipulations of this tiny brain region to examine how it affects behaviour.
A technique called optogenetics, in which animals are genetically engineered to express light-responsive versions of ion-channel proteins, enabled the authors to use specific wavelengths of light to control neuronal activation. When they engineered animals to express such proteins in the S1DZ, they found that activating this brain region caused atypical social and repetitive behaviours in the offspring of wild-type mice that had not undergone MIA. Crucially, when the authors used a light-responsive channel to inhibit neuronal activity in the S1DZ in the offspring of mothers that had undergone MIA, the change in neuronal activity in this region alone was sufficient to stop the atypical behaviour that these animals had shown. Indeed, the authors obtained similar effects on behaviour by using optogenetics either to specifically activate just the excitatory neurons or to inhibit the inhibitory neurons in this brain region. Such specific recapitulations of behaviour by the manipulation of a subset of neurons in a precise cortical region is a state-of-the-art experimental demonstration of the link between an individual brain region and distinct behaviours.
In a final tour de force, using a viral-based technique to determine cellular connections, Yim and colleagues identified a region of the cortex called the temporal association area (TeA), together with the striatum, as the brain areas to which cells in the S1DZ project. When the authors expressed light-sensitive ion channels in the subset of S1DZ neurons projecting to the TeA, stimulation of these neurons in offspring of wild-type mice that had not undergone MIA resulted in atypical social behaviours, but not in repetitive behaviours. Inhibition of these neurons in offspring of mothers that had undergone MIA restored normal social behaviours, but not repetitive behaviours. Similar experiments on S1DZ neurons projecting to the striatum showed effects on repetitive behaviours but not on social behaviours. The authors could therefore implicate a subregion of the brain as a key circuit hub for atypical behaviours that resemble features of autism in humans. They also discovered that the two behaviours, social and repetitive, were linked to different brain regions, with both regions being modulated, to some degree, by the S1DZ hub.
Do cortical abnormalities due to maternal viral infections have a role in the development of autism in humans? This link has not been clearly established, but infections during the first and second trimesters of pregnancy are probably risk factors3, and areas of abnormally developed cortex have been identified in individuals with autism7. Additional experimental work and analyses of post-mortem brain samples will be required to answer this question. If this connection were confirmed, one might consider strategies to reduce the risk of harm from maternal infections during pregnancy through something as straightforward as manipulation of the mother’s intestinal bacterial population, whether by simple dietary modification or by other means.
Even if the mechanisms described by Kim et al. and Yim et al. are not involved in autism in humans, these papers still provide valuable insights by revealing the complexity of the interactions between gut bacteria, the immune system and brain development. Furthermore, the authors have identified a specific brain circuit in mice that has a role in modulating social and repetitive behaviours, offering a major advance in our understanding of the circuits responsible for such atypical characteristics.
References
- 1.Kim S et al. Nature 10.1038/nature23910 (2017). [DOI] [Google Scholar]
- 2.Yim YS et al. Nature 10.1038/nature23909 (2017). [DOI] [Google Scholar]
- 3.Patterson PH Trends Mol. Med 17, 389–394 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estes ML & McAllister AK Science 353, 772–777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi GB et al. Science 351, 933–939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov II et al. Cell 139, 485–498 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoner R et al. N. Engl. J. Med 370, 1209–1219 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]