Abstract
Background:
Psychosocial adversity escalates medical risk for poor outcomes in infants born <30 weeks’ gestation. Neonatal neurobehavior and maternal psychological and socioenvironmental assessments may identify the earliest specific intervention needs. We hypothesized that maternal prenatal anxiety, depression, and adverse medical and socioenvironmental conditions would be associated with less optimal neonatal neurobehavior at NICU discharge.
Methods:
We studied 665 infants at 9 university NICUs. Risk indices of socioenvironmental, maternal, and neonatal medical factors were obtained from standardized, structured maternal interviews and medical record reviews. Brain injuries were classified by consensus ultrasonogram readings. NICU Network Neurobehavioral Scale (NNNS) exams were conducted at NICU discharge.
Results:
On the NNNS, generalized estimating equations indicated infants of mothers with prenatal anxiety had less optimal attention, and those born to mothers with prenatal depression had increased lethargy. Maternal medical complications predicted suboptimal reflexes. Socioenvironmental risk predicted lower self-regulation and movement quality. Infants with more severe neonatal medical complications had lower attention, increased lethargy, and suboptimal reflexes.
Conclusions:
Combined information from the observed associations among adverse prenatal maternal medical and psychosocial conditions, and neonatal complications may assist in the early identification of infants at elevated neurobehavioral risk.
Introduction
Adverse psychosocial and environmental conditions in pregnancy compromise the intrauterine environment, are associated with very premature birth, and escalate medical risks, leading to poor outcomes for infants born before 30 weeks post-menstrual age (PMA) (1, 2). These same risk factors can disrupt brain development (3, 4), and are associated with atypical newborn neurobehavior, as well as adverse neurodevelopmental and psychological outcomes throughout childhood (5–7). Similarly, very premature birth also presents challenges to maternal affective warmth and responsive interactions that support the infant’s postnatal development and the developing mother-infant relationship (8). Risk is further elevated in the presence of prenatal maternal depression and anxiety (9, 10), and for mothers with economic disadvantage, less than a high school education, or inadequate social support (11, 12). These collective findings suggest that measuring multiple maternal, neonatal, and environmental factors may be the most effective approach to identifying infants and mothers at greatest risk for persistent problems (1, 3).
In a recent validation of the NICU Network Neurobehavioral Scale (NNNS) risk profiles in a sample of very premature infants, Everson et al. (13) studied associations between NNNS profiles at NICU discharge with methylation levels of specific genes in buccal cells collected concurrently. This study found that an atypical, poorly regulated NNNS profile was associated with methylation levels in a subset of genes related to neurological structure and function, and to neurodevelopmental disorders.
In the present study, we expand on Everson et al.’s above work with this cohort (13) to examine the influences of adverse prenatal and perinatal medical and socioenvironmental conditions, including prenatal maternal depression and anxiety, on NNNS summary scores. NNNS summary scores are validated, cohesive composites of individual items combined to reflect varied aspects of neurobehavioral functioning (14), and are used to calculate the NNNS risk profiles (13, 15).
Our primary goal was to detect maternal and infant characteristics that may be amenable to targeted interventions beginning during pregnancy, and continuing from the NICU onward. In light of findings about the risk profiles, which represent discrete categories of performance patterns across the summary scores, we hypothesized that (a) NNNS summary scores would indicate decreased neurobehavioral regulation in infants with elevated scores on prenatal and neonatal risk indices, and (b) neurobehavior would be less regulated among infants whose mothers reported diagnoses of prenatal anxiety or depression. To date, this is the first large multisite study of infants born before 30 weeks PMA to describe the relative influences of psychosocial and medical risk on individual neurobehavioral constructs at NICU discharge.
Methods
We studied 665 infants and used standardized procedures to collect information about maternal depression and anxiety histories, as well as socioenvironmental and medical risk factors in order to examine associations with individual NNNS summary scores. This section summarizes published methods detailed previously (13).
Sample
The Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) Study enrolled infants from nine NICUs affiliated with six universities from April, 2014 through June, 2016. Eligibility inclusion criteria were as follows: 1) birth <30 weeks PMA (16); 2) maternal ability to read and speak English, Spanish, Japanese or Chinese; and 3) residence within 3 hours of the NICU and follow-up clinic. Infants were excluded for major congenital anomalies (17), and anomalies that would have required altering NNNS administration.
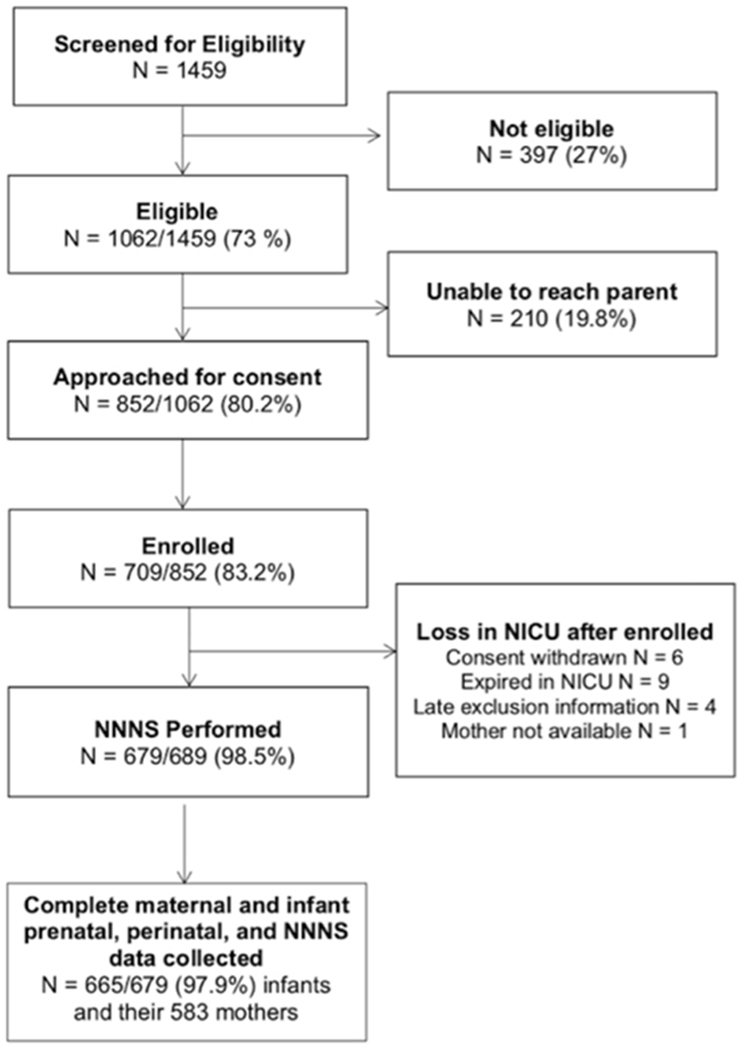
Parents were invited to participate when their infants reached 31-32 weeks PMA, or when their survival to discharge was determined to be likely by the attending neonatologist. Study details were explained, and informed consent obtained in accordance with each institution’s review board. We enrolled 709 infants (83.2% consent rate), and this study sample included 665 infants and their 583 mothers with complete maternal and infant assessments (Figure 1).
Figure 1:
Study Flow and Eligibility
Procedures
Maternal Data Collection:
Prior to data collection, research staff participated in multisite training sessions and individual reliably assessments with the trainer (SDG) to standardize the reliable administration of the structured maternal interview conducted at enrollment. Multisite reliability criteria for medical record reviews were established by consensus agreement among study neonatologists. During data collection, neonatologists at each study site provided oversight for trained research personnel who reviewed infant medical records to collect prenatal and intrapartum variables, prescribed medications, and the use of alcohol, tobacco, marijuana, and other psychoactive substances. Socioenvironmental variables, including race/ethnicity, partner and cohabitation status, insurance type, education, occupation, and self-reported substance use were obtained during standardized maternal enrollment interviews. Information about prior diagnoses of anxiety, depression, and the receipt of counseling and/or prescribed medication for these conditions was obtained from neonatal medical records and maternal interviews.
Maternal Risk Indices (Table 1).
Table 1:
Maternal and Neonatal Characteristics and Risk Indices
| Maternal Psychosocial and Medical Characteristics (n=583) | N (%) or Mean (SD) |
|---|---|
| Socioenvironmental Risk Index1 | 1.1 (0.91) |
| Minority race or ethnicity | 333 (57.1) |
| Maternal age > 35 at childbirth | 104 (17.8) |
| No prenatal care | 16 (2.7) |
| No partner | 145 (24.9) |
| Low socioeconomic status (Hollingshead category 5) | 55 (9.4) |
| Maternal Medical Complications Index | 0.94 (0.87) |
| Diabetes mellitus or gestational diabetes | 36 (6.2) |
| Pregnancy infection (vaginal, cervical, urinary, or kidney) | 60 (10.3) |
| Hypertension (chronic or pregnancy induced) | 159 (27.3) |
| HIV/AIDS, other sexually transmitted infections | 38 (6.5) |
| Pre-pregnancy obesity, BMI>30 | 198 (34.0) |
| Pre-pregnancy underweight, BMI<18.5 | 29 (5.0) |
| Congenital anomaly (not affecting NNNS administration) | 33 (5.7) |
| Substance Use Index | 0.30 (0.68) |
| Tobacco | 84 (14.4) |
| Alcohol | 19 (3.3) |
| Marijuana | 57 (9.8) |
| Other recreational substance use | 26 (4.5) |
| Opiates | 14 (2.4) |
| Stimulants | 13 (2.2) |
| Benzodiazepines/Barbiturates | 5 (0.97) |
| Hallucinogens | 2 (0.3) |
| Maternal Psychological History and Treatment | |
| Prenatal depression | 35 (6.0) |
| Prescribed antidepressants during pregnancy | 30 (5.1) |
| Depression counseling during pregnancy | 25 (4.3) |
| Prenatal anxiety | 42 (7.2) |
| Prescribed anxiolytics during pregnancy | 31 (5.3) |
| Anxiety counseling during pregnancy | 31 (5.3) |
| Neonatal Medical Characteristics (N=665) | N (%) or Mean(SD) |
| Female | 291 (43.8) |
| Cesarean Delivery | 471 (70.8) |
| Multiple Gestation | 176 (26.5) |
| PMA at birth (weeks) | 27.0 (1.9) |
| ≤25 | 140 (21.1) |
| >25 to ≤28 | 299 (45.0) |
| >28 to <30 | 226 (34.0) |
| Birthweight (grams) | 951.6(278.6) |
| Head Circumference | 24.5 (2.4) |
| Intrauterine Growth Restriction (birthweight <10%ile for PMA) | 48 (7.2) |
| PMA at NNNS exam (range 32.1-51.3 weeks) | 39.3 (3.4) |
| <34 weeks | 6 (0.9) |
| ≥34 to <36 weeks | 98 (14.7) |
| ≥36 to <38 weeks | 167 (25.1) |
| ≥38 to <42 weeks | 259(38.9) |
| ≥42 to <46 weeks | 96 (14.4) |
| ≥ 46 weeks | 39(5.9) |
| PMA at NICU Discharge | 40.4 (5.1) |
| Length of NICU Stay (Days) | 93.3 (42.4) |
| Out-born | 128 (19.2) |
| Neonatal Medical Complications Index: | 0.87(0.87) |
| Severe Retinopathy of Prematurity | 40 (6.0) |
| Sepsis | 87 (13.1) |
| Necrotizing enterocolitis | 44 (6.6) |
| Chronic Lung Disease | 336 (50.5) |
| Severe Brain Injuries (parenchymal echodensity, periventricular leukomalacia, ventricular dilatation) | 146 (22.0) |
| Brain Injuries | |
| Parenchymal echodensity on any scan* | 52 (7.8) |
| Periventricular leukomalacia on any scan* | 43 (6.5) |
| Periventricular leukomalacia on late scan | 26 (3.9) |
| Germinal matrix hemorrhage on any scan | 149 (22.4) |
| Intraventricular hemorrhage on any scan | 119 (17.9) |
| Intraventricular hemorrhage on late scan | 69 (10.4) |
| Ventricular dilatation on any scan* | 51 (7.7) |
| Ventricular dilatation on late scan | 40 (6.0) |
| Intracerebral hemorrhage on any scan | 3 (0.5) |
| Cerebellar hemorrhage on any scan1 | 12 (1.8) |
Note: NNNS, NICU Network Neurobehavioral Scale (NNNS);
Brain injuries included in the Neonatal Medical Complications Index;
Lower than expected rate in this <30 week PMA sample may be due to the absence of mastoid CUS views
Hollingshead socioeconomic status (SES) was calculated based on maternal education and occupation (18), with one risk point assigned for the lowest SES category. Prenatal maternal socioenvironmental, substance use, and medical complications included in the risk indices were calculated as the respective sums of the sets of binary indicators of risk factors associated with prematurity (3), and validated in previous studies of neurodevelopmental outcomes (11).
Neonatal Data Collection:
Using Vermont-Oxford Network (VON) definitions and criteria (19), neonatal medical records were reviewed to collect information about infections, grades of retinopathy of prematurity (ROP), respiratory, renal, neurologic, cardiac, genetic, hematologic, and gastrointestinal characteristics.
Neonatal Medical Complications Index.
A neonatal medical complications index was constructed based on a previously validated composite (20), including one point each for the following conditions: (a) brain injury, defined per methods below as PVL, moderate-severe ventricular dilatation (VDIL) with or without IVH, or parenchymal echodensity (PED); (b) any culture-positive sepsis; (c) severe ROP (stage 4-5 or surgery); (d) necrotizing enterocolitis (NEC; ≥Bell’s stage 2); and (e) chronic lung disease (CLD) defined as oxygen requirement at 36 weeks PMA.
For descriptive purposes, and separate from the index, infants with CLD were further classified as having mild CLD if treated at 36 weeks PMA with supplemental oxygen, or with high flow nasal cannula with FiO2=.21. They were classified as having moderate CLD if treated at 36 weeks PMA with either supplemental oxygen via high flow nasal canula, or nasal continuous positive airway pressure, or nasal intermittent positive pressure ventilation. Severe CLD was defined as treatment at 36 weeks PMA with mechanical ventilation through an endotracheal tube (21).
Brain Injury Diagnoses.
Brain injury diagnoses were based on routine cranial ultrasonograms performed at each site, and readings were categorized as early (day 7-10) or late (between 36 weeks PMA and discharge). At all sites, cranial sonograms were performed using high-frequency transducers, and included six standard quasi-coronal views and five para-sagittal views, using the anterior fontanel as the sonographic window. The mastoid window was used at two of the nine study sites.
Each sonogram was read initially as part of site-specific routine clinical care. For the current study, a subsequent reading was randomly assigned to and conducted by one of six NOVI Study neuroradiologists, identified as central reader-radiologists. NOVI Study neuroradiologists were masked to medical characteristics, and classified their observations according to criteria established in the Extremely Low Gestational Age Newborn Study (ELGAN) (22). A third reading, by a different NOVI Study neuroradiologist, was performed if the initial and second readings disagreed about the presence of one or more of the following observations: IVH, parenchymal echodensity, parenchymal echolucency, or moderate-to-severe ventricular enlargement. Abnormalities were classified as present if identified by at least two readers.
Neonatal Neurobehavioral Assessments.
The NICU Network Neurobehavioral Scale (NNNS) is a 20 to 30 minute standardized, well validated exam that assesses active and passive muscle tone, primitive reflexes, movement, social behaviors (e.g., cuddling and soothability), attention to visual and auditory stimuli, and a checklist of stress signs organized by organ systems (14).
The NNNS was administered during the week of NICU discharge ± 3 days, by certified examiners trained to reliability using standardized procedures and criteria (14). Examiners were masked to chronological age, PMA at exam, and medical history; the exception was infants requiring visible nasal cannula for respiratory support. Exams were conducted prior to a scheduled feeding or routine care to maximize the likelihood of alertness, and to avoid disrupting sleep and NICU routines. Validated algorithms were used to compute 13 NNNS summary scores (14), where higher scores reflect more of the construct measured.
Statistical Analyses
Generalized estimating equations (GEE) accounted for correlations for women with multiple births by nesting infants within families. These analyses examined the influences of prenatal maternal depression and anxiety, and maternal and neonatal medical and socioenvironmental adversity on individual NNNS summary scores. Regression models tested the influences of prenatal anxiety and depression diagnoses separately, as well as the influences of the socioenvironmental adversity index, maternal medical complications index, substance use index, neonatal medical complications index, and PMA at exam date, after adjusting for site differences. Data were analyzed using SPSS 24.0 (Chicago: SPSS, Inc.), with continuous covariates centered and scaled to z-scores, and the Bonferroni correction applied to account for testing of the multiple NNNS summary score outcome models. In the results presented below and in Table 3 for multivariable GEE models, the regression coefficients are estimates of the increment in NNNS scores that would be expected for a one unit increment in each of the significant risk factors, after controlling for the influences of all other variables in the model.
Table 3:
Multivariable GEE Significance Tests
| Prenatal Anxiety1 | Prenatal Depression1 | |||||
|---|---|---|---|---|---|---|
| NNNS Summary Score and Predictors | B | SE | Adjusted Means Anxiety ; No Anxiety | B | SE | Adjusted Means Depression; No Depression |
| Attention (625) | −0.43* | 0.18 | 4.88 ; 5.31 | −0.45 | 0.29 | 4.86 ; 5.31 |
| Neonatal Medical Complications | −0.36** | 0.07 | −0.36** | 0.07 | ||
| PMA at Exam | 0.31** | 0.08 | 0.31** | 0.08 | ||
| Handling (652) | −0.02 | 0.04 | 0.39 ; 0.41 | −0.06 | 0.04 | 0.35 ; 0.41 |
| PMA at Exam | −0.03* | 0.01 | −0.03* | 0.01 | ||
| Quality of Movement (657) | −0.02 | 0.12 | 4.57 ; 4.59 | −0.18 | 0.13 | 4.41 ; 4.60 |
| Socioenvironmental Risk | −0.08** | 0.03 | −0.07** | 0.03 | ||
| Self-Regulation (654) | −0.10 | 0.13 | 5.53 ; 5.62 | −0.29 | 0.16 | 5.34 ; 5.63 |
| Socioenvironmental Risk | −0.07* | 0.03 | −0.07* | 0.03 | ||
| Excitability (657) | −0.06 | 0.35 | 2.44 ; 2.50 | 0.34 | 0.43 | 2.81 ; 2.48 |
| PMA at Exam | 0.25* | 0.11 | 0.26* | 0.11 | ||
| Lethargy (657) | 0.50 | 0.28 | 5.01 ; 4.51 | 1.01* | 0.41 | 5.49 ; 4.49 |
| Neonatal Medical Complications | 0.36** | 0.10 | 0.35** | 0.11 | ||
| PMA at Exam | −0.30** | 0.11 | −0.30** | 0.11 | ||
| Arousal (657) | −0.04 | 0.09 | 3.71 ; 3.75 | −0.08 | 0.13 | 3.67 ; 3.75 |
| PMA at Exam | 0.08* | 0.03 | 0.08* | 0.03 | ||
| Stress-Abstinence (657) | −0.01 | 0.01 | 0.13 ; 0.14 | −0.01 | 0.01 | 0.13 ; 0.14 |
| Substance Use Index | −0.01** | 0.003 | −0.01** | 0.003 | ||
| Hypertonicity (657) | 0.14 | 0.14 | 0.50 ; 0.36 | 0.15 | 0.15 | 0.51 ; 0.36 |
| PMA at Exam | 0.11** | 0.04 | 0.11** | 0.04 | ||
| Hypotonicity (657) | -*** | - | −0.02 | 0.07 | 0.21 ; 0.23 | |
| Substance Use Index | - | - | −0.03** | 0.01 | ||
| Non-optimal Reflexes (657) | 0.19 | 0.28 | 5.55 ; 5.36 | −0.11 | 0.35 | 5.27 ; 5.38 |
| Neonatal Medical Complications | 0.33** | 0.10 | 0.33** | 0.10 | ||
| Maternal Medical Risk | 0.20* | 0.09 | 0.20* | 0.09 | ||
| Asymmetrical Reflexes (657) | 0.04 | 0.19 | 1.01 ; 0.97 | −0.08 | 0.19 | 0.90 ; 0.98 |
Notes: Significant predictors are tabled for *p<.05, **p<.01 with Bonferroni correction for multiple tests. Separate models testing anxiety and depression examined associations with all of the following: neonatal medical risk, maternal medical risk, socioenvironmental risk, and PMA at NNNS exam, after adjusting for site.
Model did not converge.
There were no significant associations between NNNS scores and prenatal use of prescribed antidepressant or anxiolytic medication.
Results
Maternal and Newborn Characteristics (Table 1)
At enrollment, mean maternal age was 29 years (standard deviation = 6.4), and 25% of mothers were not involved with a partner. Fifty seven percent of mothers self-identified as minority ethnicity (Native American, Black, Hispanic, Native Hawaiian and Pacific Islander, Asian, other). Thirteen percent of mothers did not complete high school, 29% were high school graduates, 35% attended some college, and 23% graduated from college. In addition to the 9% in the lowest Hollingshead socioeconomic category, 64% of mothers reported receiving income-based public assistance or health insurance.
The most prevalent prenatal complications were chronic or pregnancy-induced hypertension (27%), genitourinary infections (10%), pre-pregnancy obesity (34%), and fetal exposure to tobacco (14%) or marijuana (10%). Among women who reported a prenatal diagnosis of anxiety (7%) or depression (6%), 71-86% reported receiving counseling and/or pharmacological treatment.
With respect to PMA groups at birth, 21% were born before 25 weeks, 45% were born 25-27 weeks, and 34% were born at 28-29 weeks PMA. The sample included 7% of infants with NEC, 13% with early- or late-onset sepsis, 50% with CLD (24% mild, 26% moderate/severe), and 22% with severe brain injuries (PED, PVL, or VDIL); the most prevalent brain injuries were germinal matrix hemorrhage (22%) and IVH (18%). In addition, 23% of infants had 2 or more severe conditions included in the neonatal medical complications index, whereas 40% of the sample had none of these severe conditions.
Mean PMA at NNNS exam during the NICU discharge week was 39 weeks (range= 32 to 51). This range reflects earlier exams for back-transported infants, with later exams for extended hospitalization due to surgeries, illness, feeding problems, and growth problems.
Prediction of NNNS Summary Scores at NICU Discharge
Descriptive statistics for NNNS Summary Scores are presented in Table 2, and the results of multivariable GEE models are discussed below, with significant predictors presented in Table 3. There were no significant associations between any exposures and the summary score for asymmetrical reflexes.
Table 2:
Sample Descriptive Statistics for NNNS Summary Scores1
| NNNS Summary Score (N) | Mean | SD | Min-Max Range | Percentiles | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | ||||
| Attention (634) | 5.29 | 1.50 | 1.00-9.00 | 3.00 | 3.43 | 4.14 | 5.14 | 6.50 | 7.43 | 7.71 |
| Handling (660) | 0.41 | 0.27 | 0.00-1.00 | 0 | 0 | 0.25 | 0.38 | 0.63 | 0.75 | 0.88 |
| Quality of Movement (665) | 4.59 | 0.69 | 2.00-6.40 | 3.40 | 3.67 | 4.17 | 4.67 | 5.00 | 5.40 | 5.60 |
| Self-Regulation (662) | 5.62 | 0.80 | 2.64-7.82 | 4.27 | 4.60 | 5.14 | 5.62 | 6.08 | 6.63 | 6.92 |
| Excitability (665) | 2.48 | 2.07 | 0.00-10.0 | 0 | 0 | 1.00 | 2.00 | 3.00 | 5.00 | 7.00 |
| Lethargy (665) | 4.55 | 2.11 | 0.00-15.0 | 2.00 | 2.00 | 3.00 | 4.00 | 6.00 | 7.00 | 9.00 |
| Arousal (665) | 3.74 | 0.65 | 1.71-5.86 | 2.71 | 3.00 | 3.29 | 3.71 | 4.14 | 4.57 | 4.86 |
| Stress-Abstinence (665) | 0.14 | 0.07 | 0.00-0.39 | 0.02 | 0.04 | 0.08 | 0.12 | 0.18 | 0.24 | 0.27 |
| Hypertonicity (665) | 0.38 | 0.74 | 0.00-5.00 | 0 | 0 | 0 | 0 | 1.00 | 1.00 | 2.00 |
| Hypotonicity (665) | 0.22 | 0.51 | 0.00-4.00 | 0 | 0 | 0 | 0 | 0 | 1.00 | 1.00 |
| Asymmetric Reflexes (665) | 0.97 | 1.29 | 0.00-6.00 | 0 | 0 | 0 | 0 | 2.00 | 3.00 | 4.00 |
| Non-optimal Reflexes (665) | 5.36 | 2.06 | 0.00-11.0 | 2.00 | 3.00 | 4.00 | 5.00 | 7.00 | 8.00 | 9.00 |
Habituation was not able to be administered for infants initially in awake states, and was not included in analyses.
Maternal Psychological and Socioenvironmental Risks.
In unadjusted analyses, infants of mothers with prenatal anxiety had less optimal attention (poor alertness, visual, and auditory responses) and were more lethargic (subdued responsivity). Infants whose mothers reported a prenatal depression diagnosis were more lethargic and had lower scores on attention and selfregulation (unmodulated physiology, movement, and arousal). After adjustment for covariates, prenatal anxiety was associated with lower attention, and prenatal depression was associated with increased lethargy (Table 3 and Figure 2). No association was found between NNNS scores and maternal use of prescribed antidepressant or anxiolytic medication.
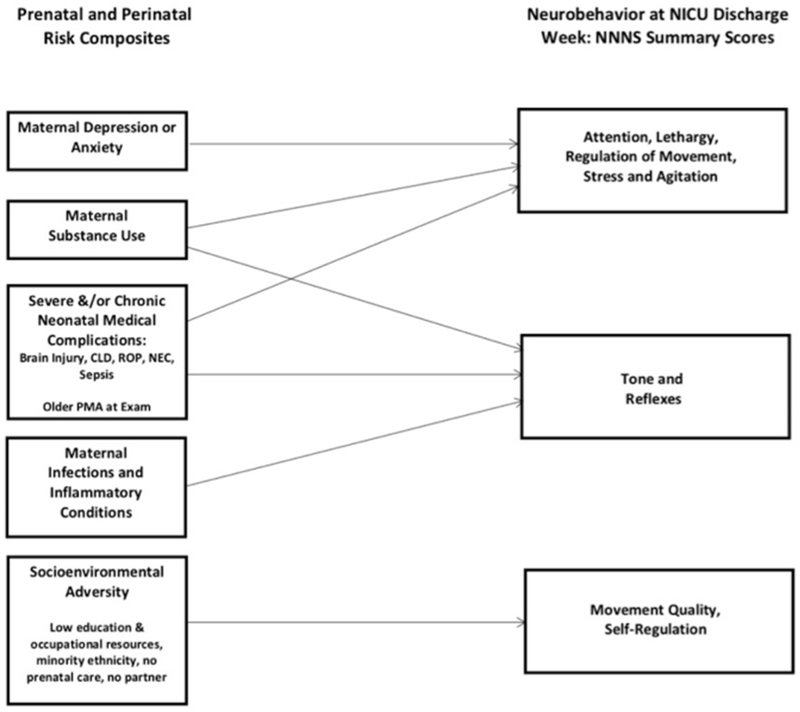
Figure 2:
Conceptual Model of Influences on Neurobehavioral Constructs at NICU Discharge
In both anxiety and depression models, socioenvironmental risk was associated with lower scores for self-regulation and movement quality (activity, coordination, range of motion). Maternal medical risk was associated with more suboptimal reflexes, and substance use was associated with fewer stress-abstinence signs. In the depression model, substance use was associated with less hypotonicity.
Neonatal Medical Complications Index.
In both anxiety and depression models, infants with higher neonatal medical complications scores had lower attention, increased lethargy, and more suboptimal reflexes. Infants whose PMA was older at exam had higher attention scores, less lethargy, and required fewer handling adaptations to maintain relaxed alertness. However, these infants also had increased hypertonicity, arousal, and excitability (agitation and distress).
Discussion
Our primary aim was to identify indicators of risk for varied neurobehavioral regulation characteristics on the NNNS that could inform the design of targeted interventions antenatally, and from NICU admission onward. Towards this goal, we have delineated the distinct contributions of prenatal maternal anxiety and depression, adverse socioenvironmental conditions, and maternal and neonatal medical complications to NNNS performance at NICU discharge. The present study expands on recent NOVI Study findings about atypical patterns of behavior reflected in risk profiles (13) by identifying perinatal characteristics that detect risk for multiple specific aspects of neurobehavioral function.
Overall, NOVI sample characteristics on NNNS summary scores are comparable to other studies of very premature infants tested at term (23), and show gestational age-expected differences compared to infants born moderate and late preterm (24). In comparison with reports of healthy full term infants tested at one month (25), our very preterm sample, as expected, had increased hypertonicity, lethargy, suboptimal reflexes, and stress signs. However, attention, arousal, quality of movement, and self-regulation scores were similar to those of term infants.
Compared to previous reports of maternal characteristics in other multicenter studies of very premature infants in the United States (26, 27), the NOVI sample included higher rates of mothers who reported minority ethnicity, were living without a partner, and were receiving low income-based public resources. In contrast, the NOVI sample included higher rates of mothers who completed more years of college, and had a lower rate classified in the lowest Hollingshead SES category. It is important to point out here that, to date, no multisite studies of infants in the United States born <30 weeks have examined neonatal neurobehavior. However, the combined pattern of maternal characteristics we found may be a reflection of the post-2008 loss of economic and employment resources for educated young adults included in the NOVI sample of parents (28).
Multivariable results indicated that maternal depression and anxiety were associated with attention, lethargy, and self-regulation scores. Notably, both maternal psychological conditions and the NNNS summaries that they predict in part reflect hypothalamic-pituitary-adrenal regulation (2). These adaptations are required to modulate responses to internal and external multisensory input along a continuum from dampened or subdued to extremely agitated or distressed. Attention scores for infants of mothers with anxiety were comparable to those in the atypical NNNS risk profile 3, and infants of mothers with depression had scores for lethargy and regulation similar to those averages for the atypical NNNS risk profile 5 (13). Our findings are in concert with Salisbury et al., who reported lower scores on regulation associated with depression (10), and with Conradt et al., who reported lower regulation, with increased lethargy and hypotonicity in infants born to mothers who reported prenatal anxiety or depression, findings that were also associated with epigenetic alterations of stress-related genes (9).
It is noteworthy that the extent of neonatal complications was associated with lower attention, increased lethargy, and more suboptimal reflexes, even after controlling for PMA at exam date. Further, associations between older PMA at exam and increased hypertonicity, arousal, and excitability may be capturing a pattern of difficulty in regulating distress and agitation. These are comparable to findings by Pineda et al. (23), who described less regulated performance compared to full term infants on scores for stress signs, lethargy, excitability, suboptimal reflexes, hypotonicity, and hypertonicity. The collective stress-reactivity features may be common in infants with more severe CLD, feeding problems, and growth delays that resulted in lengthier hospitalization, and hence, older ages at discharge exams.
The observed pattern of difficulty with stress regulation captured in various NNNS summaries is one that has been demonstrated in multiple studies of very preterm infants, alone and in combination with significant prognostic information about inflammatory conditions and structural brain abnormalities on MRI (7, 23, 24). Everson et al.’s findings (13) further suggest that intrauterine and extrauterine inflammatory and stress exposures may alter epigenetic processes and patterns of associations with neurobehavior, as well as influencing mother-infant interactions and early relationship characteristics. Similar associations have been described in full term infants (9), and would be useful to incorporate in future longitudinal studies.
Importantly, previous single-site studies demonstrated associations between maternal-infant relationship features and NNNS assessments that may underlie the infant’s regulation of attention and autonomic processes. In a study of single family room NICU outcomes, Lester et al. (29) found that “maternal involvement”, a composite of maternal presence, care, feeding, and skin-to-skin contact, was associated with better attention and fewer stress signs on the NNNS. Reynolds et al. (30) also found that parental presence and holding were related to NNNS summary scores for quality of movement and arousal regulation. In addition, focused attention and distress/agitation during the NNNS-comparable orientation component of the Assessment of Preterm Infant Behavior at 35 weeks PMA were correlated with concurrent respiratory sinus arrhythmia, a measure of parasympathetic regulation of heart rate patterns, as well as with infant attention and distress/agitation during mother-infant interaction at term (31). The present study adds to the collective earlier findings and, where feasible, future research could examine more proximal mother-infant interaction measures to provide information about influences on autonomic and epigenetic processes important for maternal and infant well-being.
Identifying these early prenatal and perinatal risks for poorly regulated neurobehavior is particularly relevant in light of the body of evidence demonstrating prediction from the NNNS to subsequent neurodevelopmental impairments throughout childhood. For example, in the Maternal Lifestyles Study, a sample of preterm and full term infants with varied substance exposures, NNNS scores for stress, handling, regulation, lethargy, hypotonicity, hypertonicity, and suboptimal reflexes predicted poor neurodevelopmental and behavioral outcomes at age 1 through ages 4.5 (15) and 7 years (32).
Findings from preterm samples indicate that poor Bayley outcomes at 18 months adjusted age were associated with NNNS scores for poor regulation, suboptimal reflexes, hypertonicity, and increased handling needs (5). Bayley scores at two years were associated with lethargy and increased excitability (33), and lethargy and low movement quality also predicted cerebral palsy (34). An NNNS “dysregulation” aggregate score predicted more internalizing behavior problems at 18 months (7). In addition, increased suboptimal reflexes and stress signs predicted later sensory processing disorders at four to six years (35).
It is important to note that because of the observational design of the study, no causal inferences can be made with respect to our findings. Further, despite encouraging consistency with prior work, and the added generalizability of including out-born infants, a limitation of our study arose from the need to rely on infant histories and admission records for maternal medical information. Postnatal recruitment also resulted in maternal interviews that included retrospective questions about the mother’s prior diagnosis of prenatal depression and anxiety, as well as her use of counseling, and/or prescribed antidepressants or anxiolytics. Prescribed medication and other psychoactive substance use were also obtained by review of the infant medical record and from maternal self-reports on the interview. In the absence of toxicology screens to document specific exposures, and the requisite use of retrospective interview questions, it is likely that substance use, depression, and anxiety were underreported in this sample.
Our data about cranial ultrasound abnormalities is limited by the reliance, at a majority of study sites, on the anterior fontanelle acoustic window (rather than the mastoid window) to ascertain cerebellar injury. We expect that the magnitude of the bias resulting from under-ascertainment of cerebellar injury was small because at the two sites where mastoid views were routinely used, the prevalence of cerebellar injury was only 1%. Under-ascertainment of cerebellar injury would not have biased results of analyses of the relationship of prenatal maternal factors and neurobehavior.
Strengths of this study include the large multisite sample size, and the integration of detailed measures of maternal, infant, and environmental characteristics. The rigorous training and protocol standardization procedures for recruitment and data collection resulted in high rates of complete data collection. Other strengths include the use of neonatal variables defined by the NICHD Neonatal Research Network and VON, centralized cranial sonogram readings using ELGAN Study training and diagnostic criteria, and standardized NNNS protocols and examiner training and certification procedures. This level of standardization and reliability has been essential for comparing findings, and facilitates generalizability across measures, examiners, and babies in studies cited.
In summary, the combined psychosocial and medical predictors of NNNS outcomes here provide a more complete picture of the influences on specific aspects of newborn neurobehavioral regulation at NICU discharge. It will be important to determine in future studies if, in addition to routinely documented medical risks, the NNNS summaries themselves may be useful in detecting more subtle but salient characteristics during the NICU stay that could inform individualized supportive pre- and post-discharge interventions. We know from earlier studies that the NNNS contributes additive information for predicting subsequent outcomes after controlling for adverse medical and socioenvironmental conditions (5, 15, 34, 35). As such, important next steps would be to determine if intervention targeted to specific early NNNS findings is associated with improved neurobehavior at NICU discharge, and if targeted interventions are also correlated with long term outcomes. This ability to detect neurobehavioral risk is important in order to prevent infants from “slipping through the services cracks,” particularly for those who were not previously identified by illness acuity or severity, nor by adverse environmental characteristics.
Here we have identified infants who demonstrate difficulty regulating and adapting tone, movement, and stress-coping processes that are the prerequisites of well-regulated attention, behavior, and later cognitive processes. These are also the building blocks of essential strengths needed for long-term adaptation and well-being - outcomes that have been consistently found to be associated with NNNS performance (7, 15).
Our findings provide information about potential intervention targets that are identifiable early, and could address the combined challenges of maternal psychological vulnerability and the very premature newborn’s poor neurobehavioral regulation. There is consistent evidence that these combined threats have long-term consequences for maternal and child psychopathology (1, 4, 7).
With respect to preventive interventions that could begin during routine prenatal care, updated best practice guidelines (36) with demonstrated effectiveness (37, 38) include providing assessment and support services for pregnant and postpartum women to address psychological distress and threatening or stressful living conditions. In the context of antenatal consultation and in the NICU, supportive surveillance and collaborative care teams and community providers could facilitate continuous and more comprehensive and individualized care planning. Care components could incorporate additional resources for support during vulnerable maternal transitions, such as at birth, NICU admission and family care adjustments, and at the time of discharge to home.
To target individualized interventions to meet specific infant needs in the NICU and continue post-discharge, multidisciplinary team consultation can integrate supportive services based on each infant’s identified problems and strengths to build on. In addition to routine NICU assessments, individual needs can be identified using the NNNS, which measures varied aspects of newborn functioning across multiple developmental domains. Critical domains measured using the NNNS include autonomic and central nervous system integration with the regulation of biobehavioral responses reflected in movement and muscle tone, attention, as well as arousal in the context of responses during multisensory exchanges and experiences with the examiner and with the inanimate environment.
Integrating findings from the NNNS and other routine infant assessments, along with those that identify the needs of the parents can result in more comprehensive family-centered care practices. Towards this goal, several randomized clinical trials have demonstrated effectiveness in concurrently facilitating both the infants’ neurobehavioral adaptation and the parents’ sense of well-being and their ability to interpret infant cues and provide comfort and support, in addition to reinforcing parents’ importance as key members of the NICU care team (39, 40).
Conclusion
This study demonstrates that sufficient information is available at admission to the NICU to identify significant prenatal risks for specific neurobehavioral outcomes on the NNNS, as well as infants with additional postnatal complications who may benefit from targeted interventions. Earlier research has documented prediction from specific early neurobehavioral deficits identified using the NNNS (5, 15, 34) to subsequently impaired outcomes in multiple developmental domains. In combination with research that has demonstrated individualized interventions result in improved infant outcomes, we speculate that multifactorial prescriptive assessments of mothers and infants may identify specific needs for individualizing targeted and preventive NICU care practices. This unified approach has the potential to ameliorate the threat of complex exposures, and improve outcomes for vulnerable infants and their families.
Acknowledgements
We greatly appreciate the individuals whose expertise has so enriched the NOVI Study: We thank colleagues at the University of North Carolina at Chapel Hill, Samantha Meltzer-Brody, MD, and Alison Stuebe, MD, for consultation regarding maternal assessments and well-being; Victoria Childers, RN, Nanette Coulon, Wayne Price, MD, Carl Seashore, MD, Karen Wood, MD, for assistance with multisite NNNS training, Carl Bose, MD, and Martin McCaffrey, MD, for consultation regarding neonatal medical data collection; Brown University faculty Linda LaGasse, PhD, for early leadership with the NOVI Data Center, and Lynne Andreozzi Fontaine, PhD, for consultation on NNNS training; and ELGAN Study investigators (NIH grant 1U01 NS 40069, Alan Leviton, PI), generously provided us with materials for training and diagnostic criteria regarding brain abnormalities.
Our immense gratitude goes to our NOVI families, whose participation has made our study successful, and to the data collection site teams of Co-PIs, coordinators, NNNS examiners and neuroradiologists listed below, for their commitment to our families and study goals.
In memory of Zack Boukydis, PhD, whose caring ways with vulnerable babies and their parents continue to inspire us.
Brown Alpert Medical School and Women and Infants Hospital, Providence, RI: Amy Salisbury, PhD, Elizabeth Danella MOT, OTR/L, Lynne Andreozzi, PhD, Erica Oliveira, BA, Brenda Rosario Perez, BA, BS.
Children’s Mercy Hospital, Kansas City, MO: Howard Kilbride, MD, Anne Holmes, RN, MSN, CCRC, Allison Knutson, RNC-NIC, Denise Smith, RN, MSN, RNC, NNP.
Harbor-UCLA Medical Center, Torrance, CA: Lucinda Santos, MHA, Jennifer Huynh, RN.
Miller Children’s and Women’s Hospital Long Beach, Long Beach, CA: Lucinda Santos, MHA, Aimee Burdick, PT, DPT, PCS, Alison Yamaguchi, PT.
Spectrum Health-Helen DeVos Hospital, Grand Rapids, MI: Edgar J. Beaumont, MD, Virginia DeWitt, BSN, RN, BS, Stephanie Fagerman, MS, MB, Kathy Nystrom, BSN, RN, Emily Gleason, BSN, RN, Karen Pawloski, RN, Rebecca McCurdy, PNP-PC, Jason Powell, PT.
University of Hawaii John A. Burns School of Medicine, Honolulu, HI: Venkataraman Balaraman MBBS, Mari Uehara, MD, Joann Cheung, MA, CCRC, Micah Tong, CCRP, Pattaraporn Chun, MD, Eydie Nakasone, Jayna Lee.
Wake Forest School of Medicine, Winston Salem, NC: Jennifer Check, MD, MS, Shannon Green Hanson, PhD, MPH, April Stewart, Heather Vye, PT, MPT, PCS, Kerry Dudziak MS, OTR/L, Kristi Lanier, RN, BSN, Nancy Peters, RN, Caroline Ludwig, BS, Melissa Tuttle.
Ultrasound Neuroradiology Consultants: Steve Bezinque, DO, Heather Borders, MD, Joseph Junewick, MD, Brad Betz, MD, Spectrum Health-Helen Devos Hospital; and Barbara Specter, MD, Wake Forest School of Medicine.
Financial Support: Funded by National Institutes of Health (NIH)/ Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) grant R01HD072267.
Footnotes
Disclosure Statement: The authors have no financial relationships or conflicts of interest to disclose relevant to this article.
References:
- 1.Field T 2017. Prenatal Depression Risk Factors, Developmental Effects and Interventions: A Review. Journal of pregnancy and child health 4:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadhwa PD, Entringer S, Buss C, et al. 2011. The contribution of maternal stress to preterm birth: issues and considerations. Clinics in perinatology 38:351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vohr BR, Poggi Davis E, Wanke CA, et al. 2017. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 139:S38–S49. [DOI] [PubMed] [Google Scholar]
- 4.Montagna A, Nosarti C 2016. Socio-Emotional Development Following Very Preterm Birth: Pathways to Psychopathology. Frontiers in psychology 7:80–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Dib M, Massaro AN, Glass P, et al. 2012. Neurobehavioral assessment as a predictor of neurodevelopmental outcome in preterm infants. J Perinatol 32:299–303. [DOI] [PubMed] [Google Scholar]
- 6.Bangma JT, Kwiatkowski E, Psioda M, et al. 2019. Early life antecedents of positive child health among 10-year-old children born extremely preterm. Pediatric Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montirosso R, Giusti L, De Carli P, et al. 2018. Developmental care, neonatal behavior and postnatal maternal depressive symptomatology predict internalizing problems at 18 months for very preterm children. J Perinatol 38:191–195. [DOI] [PubMed] [Google Scholar]
- 8.Hane AA, LaCoursiere JN, Mitsuyama M, et al. 2019. The Welch Emotional Connection Screen: validation of a brief mother-infant relational health screen. Acta Paediatrica 108:615–625. [DOI] [PubMed] [Google Scholar]
- 9.Conradt E, Lester BM, Appleton AA, et al. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 8:1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salisbury AL, Wisner KL, Pearlstein T, et al. 2011. Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 28:1008–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans GW, Li D, Whipple SS 2013. Cumulative risk and child development. Psychol Bull 139:1342–1396. [DOI] [PubMed] [Google Scholar]
- 12.Joseph RM, O’Shea TM, Allred EN, et al. 2017. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatric Research 83:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everson TM, Marsit CJ, Michael O’Shea T, et al. 2019. Epigenome-wide Analysis Identifies Genes and Pathways Linked to Neurobehavioral Variation in Preterm Infants. Scientific Reports 9:6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester B, Tronick E 2004. The Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 113 (Suppl. 3 Pt. 2):631–695, [PubMed] [Google Scholar]
- 15.Liu J, Bann C, Lester B, Tronick E, Das A, et al. 2010. Neonatal Neurobehavior Predicts Medical and Behavioral Outcome. Pediatrics 125:e90–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Shea TM, Allred EN, Dammann O, et al. 2009. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev 85:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walden RV, Taylor SC, Hansen NI, et al. 2007. Major congenital anomalies place extremely low birth weight infants at higher risk for poor growth and developmental outcomes. Pediatrics 120:e1512–1519. [DOI] [PubMed] [Google Scholar]
- 18.Hollingshead AB 1975. Four Factor Index of Social Status. In University Y (ed), New Haven, CT. [Google Scholar]
- 19.Vermont-Oxford Network 2013 Manual of Operations (Part 2) http://www.vtoxford.org/tools/manualofoperationspart2.pdf.
- 20.Bassler D, Stoll BJ, Schmidt B, et al. 2009. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 123:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen EA, Dysart K, Gantz MG, et al. 2019. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants: An Evidence-Based Approach. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuban K, Adler I, Allred EN, et al. 2007. Observer variability assessing US scans of the preterm brain: the ELGAN study. Pediatr Radiol 37:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pineda RG, Tjoeng TH, Vavasseur C, et al. 2013. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. J Pediatr 162:470–476.e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eeles AL, Walsh JM, Olsen JE, et al. 2017. Continuum of neurobehaviour and its associations with brain MRI in infants born preterm. BMJ Paediatrics Open 1:e000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzi L, Olson K, Giusti L, et al. 2018. NICU Network Neurobehavioral Scale: 1-month normative data and variation from birth to 1 month. Pediatr Res 83:1104–1109. [DOI] [PubMed] [Google Scholar]
- 26.McElrath TF, Hecht JL, Dammann O, et al. 2008. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 168:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoll BJ, Hansen NI, Bell EF, et al. 2010. Neonatal Outcomes of Extremely Preterm Infants From the NICHD Neonatal Research Network. Pediatrics 126:443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klass P 2016. Saving Tiny Tim — Pediatrics and Childhood Poverty in the United States. New England Journal of Medicine 374:2201–2205. [DOI] [PubMed] [Google Scholar]
- 29.Lester BM, Hawes K, Abar B, et al. 2014. Single-Family Room Care and Neurobehavioral and Medical Outcomes in Preterm Infants. Pediatrics 134:754–760. [DOI] [PubMed] [Google Scholar]
- 30.Reynolds LC, Duncan MM, Smith GC, et al. 2013. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J Perinatol 33:636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofheimer JA, Wood BR, Porges SW, et al. 1995. Respiratory sinus arrhythmia and social interaction patterns in preterm newborns. Infant Behavior and Development 18:233–245. [Google Scholar]
- 32.Lester BM, Bagner DM, Liu J, et al. 2009. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics 124:1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spittle AJ, Walsh JM, Potter C,et al. 2017. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Dev Med Child Neurol 59:207–215. [DOI] [PubMed] [Google Scholar]
- 34.Stephens B, Liu J, Lester B, et al. 2010. Neurobehavioral assessment predicts motor outcome in preterm infants. J Pediatr 156:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryckman J, Hilton C, Rogers C, et al. 2017. Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior. Early Human Development 113:18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinney J, Keyser L, Clinton S, et al. 2018. ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstetrics & Gynecology 132:784–785. [DOI] [PubMed] [Google Scholar]
- 37.Milgrom J, Schembri C, Ericksen J, et al. 2011. Towards parenthood: An antenatal intervention to reduce depression, anxiety and parenting difficulties. Journal of Affective Disorders 130:385–394. [DOI] [PubMed] [Google Scholar]
- 38.Melnyk BM, Crean HF, Feinstein NF, et al. 2008. Maternal anxiety and depression after a premature infant’s discharge from the neonatal intensive care unit: explanatory effects of the creating opportunities for parent empowerment program. Nurs Res 57:383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milgrom J, Newnham C, Anderson PJ, et al. 2010. Early Sensitivity Training for Parents of Preterm Infants: Impact on the Developing Brain. Pediatr Res 67:330–335. [DOI] [PubMed] [Google Scholar]
- 40.Welch MG, Firestein MR, Austin J, et al. 2015. Family Nurture Intervention in the Neonatal Intensive Care Unit improves social-relatedness, attention, and neurodevelopment of preterm infants at 18 months in a randomized controlled trial. J Child Psychol Psychiatry 56:1202–1211. [DOI] [PubMed] [Google Scholar]