Abstract
The Arctic is subject to long-range atmospheric deposition of globally-distilled semi-volatile organic compounds (SVOCs) that bioaccumulate and biomagnify in lipid-rich food webs. In addition, locally contaminated sites may also contribute SVOCs to the arctic environment. Specifically, Alaska has hundreds of formerly used defense (FUD) sites, many of which are co-located with Alaska Native villages in remote parts of the state. The purpose of this study was to investigate the extent of SVOC contamination on Alaska’s St. Lawrence Island through the analysis of sentinel fish, the ninespine stickleback (Pungitius pungitius), collected from Troutman Lake located within the watershed of a FUD site and adjacent to the Yupik community of Gambell. We measured the concentrations of legacy and emerging SVOCs in 303 fish samples (81 composites), including polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), organophosphate esters (OPEs) and their diester metabolites, and per- and poly-fluoroalkyl substances (PFAS). PBDEs and PCBs were the most abundant SVOC groups found in stickleback with ΣPBDE and ΣPCB median concentrations of 25.8 and 10.9 ng/g ww, respectively, followed by PFAS (median ΣPFAS 7.22 ng/g ww). ΣOPE and ΣOPE metabolite concentrations were lower with median concentrations of 4.97 and 1.18 ng/g ww, respectively. Chemical patterns and distributions based on correlations and comparison with SVOC concentrations in stickleback from other parts of the island suggest strong local sources of PCBs, PBDEs, and PFAS on St. Lawrence Island.
Capsule
The purpose of this study was to investigate the extent of SVOC contamination on Alaska’s St. Lawrence Island through the analysis of sentinel fish, the ninespine stickleback (Pungitius pungitius), collected from Troutman Lake located within the watershed of a formerly used defense site and adjacent to the Yupik community of Gambell. We found elevated concentrations of PBDEs and PFAS in stickleback, suggesting local sources of contamination.
Graphical Abstract
INTRODUCTION
Semi-volatile organic compounds (SVOCs) are contaminants of global concern that adversely affect human health and the environment (Letcher et al., 2018; Nash, 2011). The Arctic is a hemispheric sink for certain SVOCs that reach the Arctic through long-range atmospheric transport from lower latitudes and bioaccumulate in lipid-rich food webs (Blais, 2005; Wania and Mackay, 1993, 1996). In addition, local sources of contamination also contribute SVOCs to the arctic environment (Brown et al., 2014). For example, in Alaska many formerly used defense (FUD) sites are located in or near Alaska Native villages in remote parts of the state (von Hippel et al., 2016). Many of these abandoned sites remain contaminated with persistent SVOCs, including polychlorinated biphenyls (PCBs), solvents, and other contaminants (Scrudato et al., 2012; von Hippel et al., 2018). Despite the large size of Alaska and high number of contaminated sites located throughout the state, pollution in the U.S. Arctic is poorly studied compared to the Canadian and European Arctic (Muir and de Wit, 2010; Riget et al., 2016). Alaska Native peoples are often exposed to disproportionately high levels of SVOCs through consumption of traditional subsistence foods that are lipid rich, often long-lived, and from high trophic levels. Because of the proximity of some Native villages to contaminated sites, chronic exposure to many SVOCs also occurs in local hot spots of pollution. This poses an important public health concern because chronic exposure to SVOCs is associated with endocrine, neurological, and reproductive disorders and cancers (Costa and Giordano, 2007; Hekster et al., 2003; Hou et al., 2016; Ulbrich and Stahlmann, 2004; Yu et al., 2016).
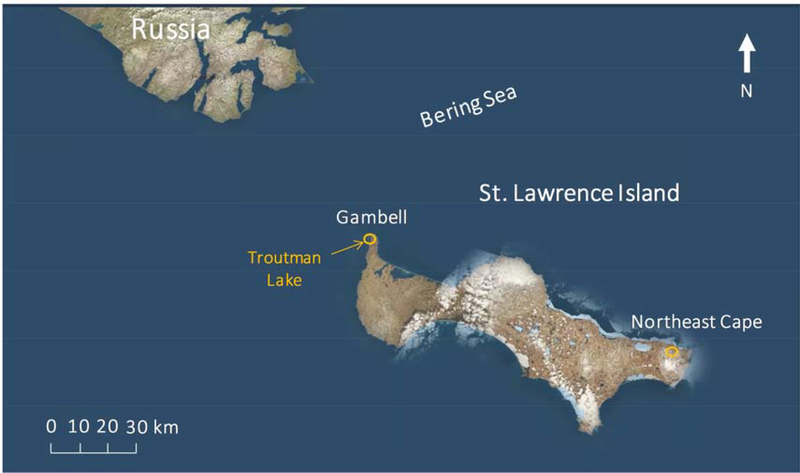
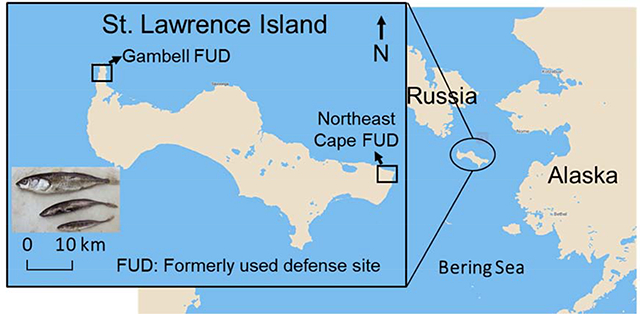
This study addresses SVOC contamination on Alaska’s St. Lawrence Island (the traditional Yupik name of the Island is Sivuqaq; hereafter, SLI), which is the largest island in the Bering Sea and located only 61 km from the Chukotka Peninsula of northern Russia. The island has a population of about 1600 Native Yupik residents living in two extant villages, Gambell and Savoonga. SLI contains two FUD sites that were established because of the island’s strategic location with respect to the former Soviet Union (Fig. 1). One of them is located at Northeast Cape, a former Yupik village that was acquired by the U.S. Air Force to establish a surveillance station in 1957 to provide early warning of enemy attacks during the Cold War (ADEC, 2019; USBLM, 2015). The Northeast Cape facility was abandoned in 1972, leaving a legacy of contamination which resulted in contaminated soil, surface and ground waters, and biota derived from fuel spills and releases of PCBs, pesticides, solvents, metals, and possibly other contaminants (ADEC, 2019; Stamatis et al., 2010). The other military base was located in the village of Gambell from 1948 to 1956. The operation of the military base in Gambell resulted in spilled fuels, oils, and other contaminants that have settled in course gravel and in a layer above and possibly sequestered within the permafrost. The Northeast Cape was extensively cleaned and remediated with an overall $125 million spent, making it the most expensive FUD site clean-up in Alaska to date (ADEC, 2019; USACE, 2012). The Northeast Cape remediation started in 1985 and included removal of structures, storage tanks and drums, contaminated wastes, soil and sediments (ADEC, 2019; Byrne et al., 2015; Scrudato, 2012). In 2014, The U.S. Army Corps of Engineers determined that sufficient cleanup occurred and all active remediation was ceased at Northeast Cape, while active remediation ceased at Gambell in 2008 (ADEC, 2019).
Figure 1.
Map of St. Lawrence Island, Alaska.
However, previous studies show that contamination on SLI remains. SLI residents have concentrations of PCBs in their blood that are several times higher than those in people who live in other U.S. states (Carpenter et al., 2005). Moreover, SLI residents who use Northeast Cape for subsistence activities have higher serum PCB levels than the residents who are not associated with this FUD site (Carpenter et al., 2005). These findings suggest that while bioaccumulation of PCBs in arctic subsistence foods results in elevated PCB levels in SLI residents, some residents experience added exposure from FUD sites (Carpenter et al., 2005). More recent research also demonstrated elevated levels of certain polybrominated diphenyl ethers (PBDEs) and per- and polyfluoroalkyl substances (PFAS) in blood serum of SLI residents, and associations with thyroid hormone disruption (Byrne et al., 2018a; Byrne et al., 2018b). Moreover, previous research showed elevated levels of several groups of SVOCs, including PCBs, in freshwater fish (ninespine stickleback, Pungitius pungitius; hereafter, “stickleback”, and Alaska blackfish, Dalliapectoralis) collected from the Suqitughneq (Suqi) River where it flows through the FUD site at Northeast Cape, suggesting that these contaminants originated in part from the local FUD site (von Hippel et al., 2018). Stickleback are effective sentinel species in part due to their circumpolar arctic distribution and presence at contaminated sites (von Hippel et al., 2016). In addition, stickleback are preyed upon by higher trophic level fish and thus may indirectly contribute to human SVOC exposure through the food web (Gallagher and Dick, 2011). Interestingly, SVOC patterns in sentinel fish reflected blood sera patterns of SLI residents and suggest that stickleback are an appropriate sentinel species for human exposure (Byrne et al., 2015; Byrne et al., 2017).
The current study is the follow-up of these prior studies (Carpenter et al., 2005; Byrne et al., 2015; Byrne et al., 2017; Byrne et al., 2018b; von Hippel et al., 2018) and aims to investigate environmental contamination on SLI through the analysis of stickleback collected from Troutman Lake, adjacent to the village of Gambell (Fig. 1). Our goals are two-fold: 1) to determine concentrations, patterns, and distributions of an expanded suite of legacy and emerging SVOCs in stickleback, including PCBs, PBDEs, organophosphate esters (OPEs) and their diester metabolites, and PFAS; and 2) to explore if remaining contamination is derived primarily from local sources or through atmospheric deposition.
MATERIALS AND METHODS
Sample collection
Stickleback were collected from two locations in Troutman Lake on SLI in July 2018. Samples were frozen, shipped to our laboratory at Indiana University, and stored at −20 °C until analysis. A total of 303 stickleback were analyzed in 81 composite samples.
Sample treatment
Whole-body fish samples were freeze-dried, ground and homogenized, and composited to include 3–5 individual fish per composite to achieve at least 1 g of sample (dry weight). Water content of each composite was determined based on the difference in sample mass before and after freeze drying. For PCB and PBDE analyses, the samples were extracted using a Dionex Accelerated Solvent Extraction (ASE) 350. Approximately 0.4 g of a dried fish composite was mixed with pre-cleaned diatomaceous earth, added to ASE 66 mL cells, and spiked with surrogate standards (BDE-77, −166 and 13C12-209 for PBDEs; PCB-14, −65 and −166 for PCBs). The samples were then extracted with hexane and acetone (1/1, v/v) at 90 °C and 1500 psi in three static cycles. The extract was reduced to 1 mL by rapid evaporation using nitrogen gas. The lipid content was determined gravimetrically using 10% of the extract. Samples were further cleaned on a multilayer silica column, packed from bottom to top with glass wool, 2 cm of neutral silica, 5 cm of acid silica, 2 cm of neutral silica, and 1 cm of sodium sulfate. The first fraction was eluted with 40 mL hexane and the second fraction was eluted with 40 mL hexane/dichloromethane (v/v, 1/1). PCBs and some PBDEs eluted in the first fraction; the remaining PBDEs eluted in the second fraction. Each fraction was then concentrated, solvent exchanged to hexane, blown down to 1 mL with nitrogen gas, and spiked with internal standards (BDE-118 and −181 for PBDEs; PCB-30 and −204 for PCBs).
For PFAS analysis, 0.1 g of a dried fish composite was spiked with surrogate standards (M3PFBA, M3PFBS, MPFHxA, MPFHxS, MPFOA, MPFOS, MPFUdA and M2PFTeDA; Table S1) and equilibrated overnight in a 15 mL polypropylene tube. The sample was then extracted with 4 mL acetonitrile in an ultrasonic environment for 1 h. After centrifugation (3000 rpm, 5 min), the supernatant was transferred to a new tube, and the sample was re-extracted twice. The combined extracts were concentrated to ~1 mL and diluted with 4 mL water. The resulting extract was further cleaned on an Oasis weak anion exchange (WAX) cartridge, which was pre-washed using 3 mL of 1% ammonium hydroxide in methanol, followed by 3 mL methanol and 3 mL water. The extract was loaded onto a WAX cartridge and rinsed with 3 mL water. Target analytes were eluted with 1 mL acetonitrile, followed by 3 mL 1% ammonium hydroxide in methanol. The collected fraction was then blown down with nitrogen to ~ 0.5 mL, filtered through a nylon syringe filter (0.2 μm), and spiked with internal standards (M3PFHxS, M7PFUdA, M8PFOA, M8PFOS and MPFBA; Table S1).
For OPE and OPE metabolite analyses, 0.2 g of a dried fish composite was spiked with surrogate standards [d12-tris(2-chloroethyl) phosphate (d12-TCEP) and C18-triphenyl phosphate (13C18-TPP) for OPEs; d8-bis(2-butoxyethyl) phosphate (d8-BBOEP), d8-bis(2-chloroethyl) phosphate (d8-BCEP), d12-bis(1-chloro-2- propyl) phosphate (d12-BCIPP), d10-bis(1,3-dichloro-2-propyl) phosphate (d10-BDCIPP), and d10-diphenyl phosphate (d10-DPHP) for OPE metabolites] and equilibrated overnight. The sample was sonicated in 4 mL acetonitrile for 1 h. After centrifugation (3000 rpm, 5 min), the supernatant was transferred to a pre-weighed glass vial, and the sample was re-extracted twice. The combined extracts were concentrated to dryness under nitrogen gas and solvent exchanged to 1 mL hexane. The extract was then further cleaned on a 1 g ISOLUTE® aminopropyl silica gel solid phase extraction (SPE) column. The SPE column was pre-conditioned with 15 mL 0.1 M ammonium acetate in methanol, 15 mL methanol, 15 mL dichloromethane, and 15 mL hexane. The sample was loaded on a cartridge and then conditioned with 4 mL hexane and 2 mL hexane in dichloromethane (4/1, v/v). OPEs were eluted with 4 mL hexane in dichloromethane (1/4, v/v) and 8 mL dichloromethane, and the two fractions were combined. OPE metabolites were eluted with 5 mL methanol and 5 mL methanol with 0.1 M ammonium acetate, and the- se two fractions were combined. The extracts were concentrated using nitrogen blow down, and then diluted with 4 mL acetone to remove ammonium acetate. The extracts were then blown down to dryness again using nitrogen gas, solvent exchanged to 1 mL methanol, filtered through nylon syringe filters (0.2 μm), and spiked with internal standards [d15-triethyl phosphate (d15-TEP), d21-tri-n-propyl phosphate (d21-TPRP), d15-tris(1,3-dichloro-2-propyl) phosphate (d15-TDCPP), d15-triphenyl phosphate (d15-TPHP), and d27-tri-n-butyl phosphate (d27-TNBP) for OPEs; 13C12-diphenyl phosphate (13C12-DPHP) for OPE metabolites].
Quality assurance and quality control (QA/QC)
One procedural blank and one matrix spike recovery sample were included in the extraction of every ten samples. The recoveries of matrix spike samples ranged from 70 to 134%. Each sample was spiked with surrogate standards, and the recoveries for the surrogate standards were 119 ± 23 (mean ± standard error), 103 ± 26, and 98 ± 17% for PCB-14, −65 and −166, respectively; 120 ± 27, 101 ± 18, and 76 ± 14% for BDE-77, −166 and 13C12–209, respectively; 72 ± 9 and 75 ± 14% for d12-TCEP and 13C18-TPP, respectively; 69 ± 18, 66 ± 13, 99 ± 16, 117 ± 12, and 125 ± 28% for dg-BCEP, d12-BCIPP, d10-DPHP, d10-BDCIPP, and d8-BBOEP, respectively; and 91 ± 7, 101 ± 8, 92 ± 7, 92 ± 7, 91 ±7, 89 ±8, 85 ±10, and 68 ± 12% for M3PFBA, M3PFBS, MPFHxA, MPFHxS, MPFOA, MPFOS, MPFUdA, and M2PFTeDA, respectively. Table S3 includes the data for matrix spike recoveries for all analytes.
Procedural blanks contained on average less than 1% of sample levels for all analytes. For compounds with detectable contamination in procedural blanks, the method detection limits (MDLs) were set at three times the standard deviation of the levels in blanks. For compounds not detected in blank samples, MDLs were based on a signal-to-noise ratio of three. The data for MDLs and procedural blanks are included in Table S4. All concentrations were corrected for blank levels by subtracting average blank amounts from those measured in samples.
Data analysis
All concentrations were normalized using either water or lipid content, and expressed as ng/g lipid weight or ng/g wet weight. Pearson’s correlation tests were used for correlations of logarithmically transformed concentrations with a critical α value of p < 0.05. The analysis of variance (ANOVA) was used to compare logarithmically transformed concentrations of target analytes. Statistical analysis, including heatmaps and hierarchical clustering, was performed using R 3.4.1, IBM SPSS Statistics 24, and Minitab 18. Descriptive statistics were calculated using Windows 10 Excel. Maps were created with ArcGIS 10.3.
RESULTS AND DISCUSSION
Concentrations
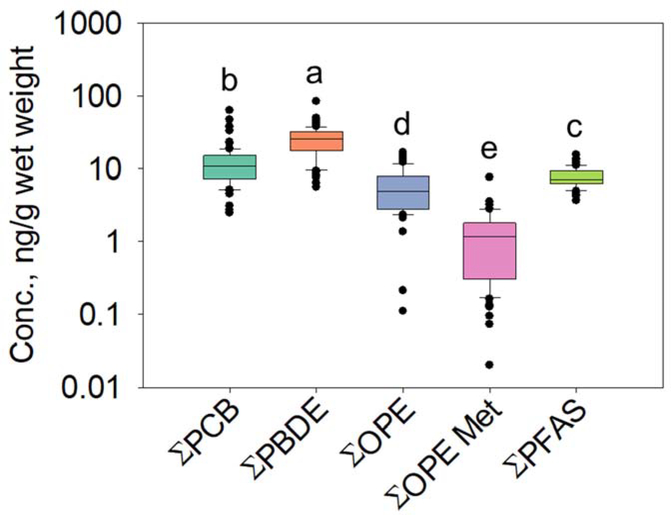
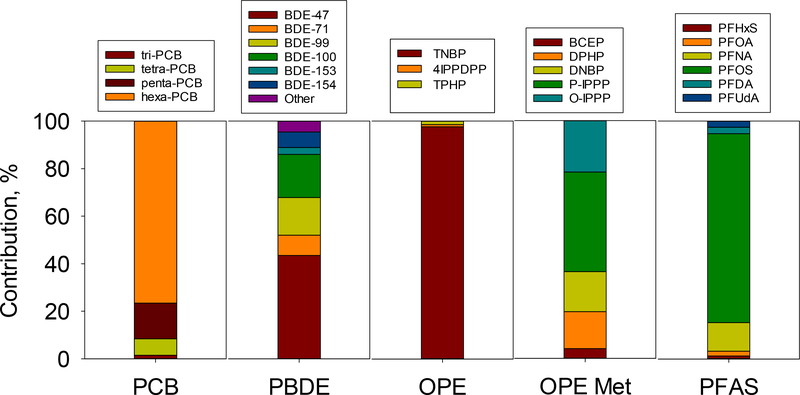
Table 1 shows the detection frequencies, minimum, maximum, median, and mean (with their standard errors) wet weight (ww)-based concentrations for PCBs, PBDEs, OPEs, OPE metabolites, and PFAS detected in more than 50% of the samples. Wet weight and lipid weight (lw) concentrations for all compounds measured in this study can be found in Tables S5 and S6, respectively. Figure 2 shows the concentrations for total PCBs (ΣPCBs, the sum of 70 PCBs), total PBDEs (ΣPBDEs, the sum of 36 PBDEs), total OPEs (ΣOPEs, the sum of 24 OPEs), total OPE metabolites (ΣOPE Met, the sum of 11 OPE diester metabolites), and total PFAS (ΣPFAS, the sum of 31 PFAS) as box plots. Figure 2 also includes the results of the analysis of variance (ANOVA); boxes sharing the same letter do not significantly differ at p < 0.05. Figure 3 shows median percent contributions of the most abundant chemicals in each analyzed chemical group to total concentrations.
Table 1.
Detection frequency (%), the minimum (Min), maximum (Max), median and mean (with standard error) wet weight concentrations of PCBs, PBDEs, PFAS, OPEs and OPE metabolites in stickleback (ng/g ww) collected from Troutman Lake, St. Lawrence Island, Alaska (n = 303 [81 composites]). <MDL: concentrations below the method detection limits.
| DF | Min | Max | Median | Mean ± std. err. | DF | Min | Max | Median | Mean ± std. err. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCBs | PBDEs | ||||||||||
| PCB22 | 56 | <MDL | 0.5 87 | 0.130 | 0.151 ± 0.014 | BDE17 | 53 | <MDL | 3.38 | 0.273 | 0.519 ± 0.117 |
| PCB42 | 79 | <MDL | 9.62 | 0.183 | 0.574 ± 0.189 | BDE28 | 96 | <MDL | 1.48 | 0.416 | 0.443 ± 0.028 |
| PCB52 | 67 | <MDL | 0.296 | 0.104 | 0.110 ± 0.007 | BDE47 | 100 | 0.858 | 40.8 | 10.2 | 10.4 ± 0.604 |
| PCB56+60 | 75 | <MDL | 0.483 | 0.156 | 0.186 ± 0.013 | BDE66 | 93 | <MDL | 3.36 | 0.241 | 0.318 ± 0.047 |
| PCB66 | 68 | <MDL | 0.846 | 0.056 | 0.092 ± 0.019 | BDE71 | 99 | <MDL | 12.0 | 1.98 | 2.26 ± 0.199 |
| PCB84+92 | 52 | <MDL | 0.775 | 0.188 | 0.217 ± 0.022 | BDE99 | 100 | 0.650 | 9.32 | 3.72 | 3.73 ± 0.187 |
| PCB87 | 93 | <MDL | 0.446 | 0.142 | 0.154 ± 0.009 | BDE100 | 98 | <MDL | 20.3 | 4.27 | 4.21 ± 0.273 |
| PCB95 | 95 | <MDL | 1.24 | 0.104 | 0.170 ± 0.022 | BDE-126 | 83 | <MDL | 0.146 | 0.071 | 0.072 ± 0.003 |
| PCB97 | 57 | <MDL | 0.170 | 0.059 | 0.060 ± 0.005 | BDE139 | 74 | <MDL | 1.24 | 0.085 | 0.196 ± 0.029 |
| PCB99 | 67 | <MDL | 0.503 | 0.210 | 0.214 ± 0.014 | BDE153 | 96 | <MDL | 1.80 | 0.679 | 0.677 ± 0.034 |
| PCB101 | 93 | <MDL | 0.701 | 0.353 | 0.366 ± 0.015 | BDE154 | 100 | 0.245 | 3.00 | 1.51 | 1.43 ± 0.061 |
| PCB105+132+153 | 100 | 0.381 | 6.66 | 2.06 | 2.36 ± 0.159 | ΣPBDEs | 5.59 | 84.7 | 25.8 | 25.5 ± 1.33 | |
| PCB110 | 88 | <MDL | 0.348 | 0.075 | 0.089 ± 0.007 | ||||||
| PCB118 | 94 | <MDL | 0.551 | 0.278 | 0.273 ± 0.013 | PFAS | |||||
| PCB123+149 | 84 | <MDL | 1.44 | 0.625 | 0.624 ± 0.028 | PFHxS | 65 | <MDL | 0.4 91 | 0.093 | 0.129 ± 0.015 |
| PCB128 | 64 | <MDL | 0.833 | 0.284 | 0.305 ± 0.031 | PFOA | 87 | <MDL | 0.359 | 0.145 | 0.157 ± 0.010 |
| PCB135+144 | 65 | <MDL | 0.303 | 0.089 | 0.118 ± 0.010 | PFNA | 100 | 0.449 | 2.28 | 0.867 | 0.965 ± 0.041 |
| PCB163+138 | 95 | <MDL | 1.06 | 0.566 | 0.553 ± 0.021 | PFOS | 100 | 2.73 | 11.9 | 5.76 | 6.16 ± 0.209 |
| PCB170+190 | 94 | <MDL | 4.51 | 0.384 | 0.528 ± 0.079 | PFDA | 98 | <MDL | 0.466 | 0.204 | 0.219 ± 0.010 |
| PCB174 | 74 | <MDL | 0.409 | 0.096 | 0.127 ± 0.012 | PFUdA | 100 | 0.01 | 0.623 | 0.183 | 0.184 ± 0.011 |
| PCB180 | 84 | <MDL | 0.564 | 0.241 | 0.237 ± 0.013 | ΣPFAS | 3.66 | 15.6 | 7.22 | 7.80 ± 0.263 | |
| PCB194 | 81 | <MDL | 0.416 | 0.159 | 0.159 ± 0.008 | ||||||
| PCB199 | 81 | <MDL | 2.60 | 0.832 | 0.853 ± 0.067 | OPE metabolites | |||||
| PCB201 | 93 | <MDL | 0.739 | 0.085 | 0.096 ± 0.010 | BCEP | 35 | <MDL | 0.260 | 0.071 | 0.081 ± 0.009 |
| PCB202+171 | 100 | 0.245 | 4.08 | 1.16 | 1.20 ± 0.063 | DNBP | 70 | <MDL | 2.35 | 0.290 | 0.436 ± 0.066 |
| PCB206 | 64 | <MDL | 0.756 | 0.084 | 0.151 ± 0.021 | P-IPPP | 91 | <MDL | 2.09 | 0.725 | 0.657 ± 0.058 |
| ΣPCBs | 2.46 | 63.5 | 10.9 | 12.8 ± 1.07 | DPHP | 14 | <MDL | 1.62 | 0.269 | 0.410 ± 0.143 | |
| O-IPPP | 88 | <MDL | 1.59 | 0.373 | 0.356 ± 0.037 | ||||||
| OPEs | ΣOPE Met | 0.020 | 7.61 | 1.18 | 1.31 ± 0.136 | ||||||
| TNBP | 88 | <MDL | 15.4 | 4.93 | 5.50 ± 0.362 | ||||||
| 4IPPDPP | 41 | <MDL | 0.157 | 0.048 | 0.053 ± 0.001 | ||||||
| TPHP | 49 | <MDL | 1.21 | 0.074 | 0.136 ± 0.032 | ||||||
| ΣOPEs | <MDL | 16.8 | 4.97 | 5.95 ± 0.430 | |||||||
Figure 2.
Concentrations of ΣPCBs, ΣPBDEs, ΣOPEs, ΣOPE metabolites (Met), and EPF AS in stickleback collected from Troutman Lake, St. Lawrence Island, Alaska (ng/g ww). Boxes represent the 25th-75th percentiles; whiskers represent the 10th and 90th percentiles; dots represent outliers. The black line represents the median. The letters represent the results of the analysis of variance (ANOVA); boxes not sharing the same letters are significantly different at p < 0.05.
Figure 3.
Percent contributions of individual compounds to concentrations of ΣPCBs, ΣPBDEs, ΣOPEs, ΣOPE metabolites (Met), and ΣPFAS in stickleback collected from Troutman Lake, St. Lawrence Island, Alaska.
PBDEs
PBDEs were the most abundant among all SVOC groups measured (Fig. 2). ΣPBDE concentrations ranged from 5.59 to 84.7 ng/g ww (median 25.8 ng/g ww; mean 25.5 ± 1.33 ng/g ww). These ΣPBDE concentrations were comparable with the levels measured in a previous study of stickleback from Troutman Lake (Byrne et al., 2017), but higher than those detected in several marine fish species from southern Greenland (Christensen et al., 2002), Hudson Bay, Canada (Kelly et al., 2008), and California coastal waters (Brown et al., 2006), as well as higher trophic level fish (e.g., lake trout (Salvelinus namaycush) and walleye (Sander vitreus)) from the Great Lakes (Guo et al., 2017). Tetra-BDE congeners were the predominant PBDEs in stickleback accounting for 53% of ΣPBDE concentrations, followed by penta- (34%) and hexa-BDEs (10%). BDE-47 was the most abundant PBDE in most of the samples (median 10.2 ng/g ww; mean 10.4 ± 0.604 ng/g ww) with an overall contribution of 43% to ΣPBDE concentrations. The other two most abundant PBDE congeners included BDE-99 (median 3.72 ng/g ww; mean 3.73 ± 0.187 ng/g ww) and −100 (median 4.27 ng/g ww; mean 4.21 ± 0.273 ng/g ww), with contributions of 16% and 18% to ΣPBDE concentrations, respectively. The high contribution of BDE-47 was consistent with other studies that measured PBDEs in fish and mammalian species (Byrne et al., 2017; Peng et al., 2007; Ramu et al., 2005; Wan et al., 2008; Zhang et al., 2010; Zhu et al., 2014). Highly brominated PBDEs (hexa-deca BDEs) comprised less than 3% of ΣPBDE concentrations. Higher brominated PBDEs have been reported to have a faster biotransformation rate in lake trout compared to less brominated PBDEs, suggesting that biotransformation of hexa- and other high molecular weight PBDEs could be responsible for lower occurrence of these congeners in stickleback (Roberts et al., 2011; Stapleton et al., 2004a; Stapleton et al., 2004b; Tomy et al., 2004; Zheng et al., 2016). Similar patterns of PBDEs were detected in several fish species from Bohai and Liaodong Bays in China (Wan et al., 2008; Zhang et al., 2010).
Along with PBDEs, several other brominated and chlorinated flame retardants were detected, including decabromodiphenylethane (DPDPE), pentabromoethylbenzene (PBEB) and Dechlorane Plus (DP). However, these chemicals were detected in less than half of the samples (9–40%, Table S5) and at lower concentrations than were the PBDEs.
PCBs
PCBs were the second most abundant class of chemicals in these samples (Fig. 2). ΣPCB concentrations ranged from 2.46 to 63.5 ng/g ww with the median and mean concentrations of 10.9 ng/g ww and 12.8 ± 1.07 ng/g ww, respectively. These PCB concentrations were comparable to previous studies on stickleback from SLI (5–50 ng/g ww) (von Hippel et al., 2018), but were generally lower than those detected in several fish species from other FUD sites located in Adak, Amchitka, Atka, and Kiska Islands in the Aleutian Archipelago of Alaska (104–285 ng/g ww) (Hardell et al., 2010). Interestingly, the PCB levels in stickleback in this study (59.3–1543 ng/g lw) were generally higher than concentrations found in higher trophic level marine fishes, such as polar cod (Boreogadus saida) (19–190 ng/g lw) and halibut (Reinhardtius hippoglossoides) from the Canadian Arctic (46–70 ng/g lw) (Kelly et al., 2008). Generally, persistent organic pollutants such as PCBs biomagnify through foodwebs and are elevated at higher trophic levels. High PCB concentrations in lower trophic level stickleback suggest local sources of PCBs on SLI, but these differences could also be due to different feeding patterns and growth rates between freshwater and marine fishes.
Hexa-chlorinated biphenyls were the predominant PCB congeners found in stickleback and constituted 76% of ΣPCB concentrations (Fig. 3). Specifically, PCB-153 (co-eluted with PCB-105 and −132) was the most abundant PCB congener (median 2.06 ng/g ww; mean 2.36 ± 0.159 ng/g ww), accounting for 24% of ΣPCBs. Penta- and tetra-chlorinated biphenyls constituted 15% and 7% of ΣPCB concentrations, respectively. These PCB patterns were consistent with a previous study of stickleback from the Suqitughneq (Suqi) and Tapisaggak (Tapi) Rivers on the Northeast Cape of SLI (von Hippel et al., 2018).
PFAS
ΣPFAS concentrations in stickleback ranged from 3.66 to 15.6 ng/g ww with the median and mean concentrations of 7.22 and 7.80 ± 0.263 ng/g ww, respectively, and were the third most abundant SVOC group found in stickleback. These concentrations were lower than those detected in a previous study on stickleback from Troutman Lake (13.7–21.7 ng/g ww) (Byrne et al., 2017). Comparable concentrations of PFAS have been reported in ringed seals (Pusa hispida) and polar bears (Ursus maritimus) from Alaska (5–8 ng/g ww) (Quakenbush and Citta, 2008; Ye et al., 2008). This is remarkable because stickleback are relatively short lived and are at a much lower trophic-level.
Perfluorooctanesulfonic acid (PFOS) was the predominant PFAS in nearly all fish samples (median 5.76 ng/g ww; mean 6.16 ± 0.209 ng/g ww), accounting for 79% of ΣPFAS concentrations (Fig. 3). Perfluoro-n-nonanoic acid (PFNA), perfluoro-n-decanoic acid (PFDA), perfluoro-n-undecanoic acid (PFUdA), perfluoro-n-octanoic acid (PFOA), and per-fluoro-1-hexanesulfonic acid (PFHxS) were also detected in 65–100% of the samples, but at lower levels, comprising 1–13% of ΣPFAS concentrations. A similar pattern was previously observed for PFAS concentrations in stickleback and human blood serum on SLI (Byrne et al., 2017), and for those in crucian carp (Carassius auratus) and mandarin fish (Siniperca scherzeri) in Korean rivers and lakes (Lam et al., 2014). Short-chain PFAS (C5-C7) were less abundant, and perfluorobutane sulfonate (PFBS) was not detected, suggesting lower bioaccumulation capability of short-chain PFAS in fish (Labadie and Chevreuil, 2011; Martin et al., 2003).
OPEs and their diester metabolites
Concentrations of ΣOPEs were significantly lower than those of PBDEs, PCBs, and PFAS (Fig. 2), and ranged from <MDL to 16.8 ng/g ww, with the median and mean concentrations of 4.97 and 5.95 ± 0.430 ng/g ww, respectively. Tri-n-butyl phosphate (TNBP) was the dominant OPE (median 4.93 ng/g ww; mean 5.50 ± 0.362 ng/g ww) found in stickleback, and was detected in 88% of samples. TNBP contributed more than 95% to ΣOPE concentrations. This pattern was consistent with that found in crucian carp from urban surface waters in Beijing, China (Hou et al., 2017). Interestingly, chlo- rinated-OPEs, which were frequently detected in several fish species from Taihu Lake (Zhao et al., 2018), and catfish (Clarias fuscus) and grass carp (Ctenopharyngodon idella) from the Pearl River, China (Ma et al., 2013), were rarely detected in our study. Possible explanations for this finding could include the small size of stickleback and / or different elimination rates of chlorinated OPEs in different fish species (Sasaki et al., 1981), or differences in the chemical makeup of the source pollution at Gambell.
Although OPEs have been detected in different environmental matrices (e.g., air, snow, seawater) in polar regions (Li et al., 2017; Salamova et al., 2014), there are few reports on the occurrence of OPEs in arctic biota. Low OPE levels were found in lake trout and walleye from Lake Athabasca, Canada (McGoldrick et al., 2014). ΣOPE concentrations in stickleback were lower than those in Atlantic cod (Gadus morhua) and polar cod from the Arctic Ocean and Greenland (36.6–102 ng/ww) (Evenset, 2009). OPE contamination in stickleback may be due to long-range atmospheric transport and/or from local sources of pollution (Li et al., 2017; Suhring et al., 2016).
Several studies suggest that OPEs are metabolized rapidly to diesters and other metabolites with short half-lives in biota (Hou et al., 2016; Kelly et al., 2007; Zheng et al., 2016). Thus, we also analyzed diester OPE metabolites in these samples. To our knowledge, this is the first report of the occurrence of OPE metabolites in arctic fish. ΣOPE Met concentrations ranged from 0.020 to 7.61 ng/g ww with the median and mean concentrations of 1.18 and 1.31 ± 0.136 ng/g ww, respectively. Bis(p-tert-butylphenyl) phenyl phosphate (P-IPPP), the metabolite of 4-isopropylphenyl diphenyl phosphate (4IPPDPP), was the predominant OPE metabolite found in stickleback, contributing 42% to ΣOPE Met concentrations, followed by bis(o-isopropylphenyl) phenyl phosphate (O-IPPP) (22%), which is the metabolite of 2-isopropylphenyl diphenyl phosphate (2IPPDPP).
We found a significant positive association between ΣOPE and ΣOPE Met levels (r = 0.59; p < 0.001; n = 72). In addition, TNBP and TPHP concentrations were strongly and positively correlated with the concentrations of their respective diester metabolites: DNBP (r = 0.62; p < 0.001; n = 50) and DPHP (r = 0.77; p < 0.001; n = 14), indicating that sources of OPE metabolites were the same as those of OPEs. No significant relationships were observed for the other metabolite - parent pairs due to low detections.
Figure S1 shows median relative abundances of OPEs and their respective metabolites in fish samples calculated as ratios of the concentrations of parent OPEs to the sum of the parent + metabolite concentrations (OPE / [OPE + OPE Met]). These metabolite/parent ratios provide useful information on the bioaccumulation potential of the metabolite. Among detected metabolites, DPHP, O-IPPP, and P-IPPP (the metabolites of TPHP, 2IPPDPP, and 4IPPDPP, respectively) had relative abundances of up to 90%, while DNBP (the metabolite of TNBP) and BCEP (the metabolite of TCEP) had much lower relative abundances of 6% and 42%, respectively. Consequently, the ratios of DNBP/TNBP and BCEP/TCEP were 0.06 and 0.72, respectively, with the parent compound representing 58–94% of the metabolite + parent concentrations. Although several in vitro and in vivo metabolism studies have shown that DNBP is the major metabolite of TNBP in fish (Sasaki et al., 1984), a recent study suggested that TNBP is metabolized through a different metabolic pathway to a hydroxylated metabolite, dibutyl-3-hydroxybutyl phosphate (3-OH-TNBP), instead of to DNBP (Hou et al., 2018). The ratios of DPHP/TPHP, O-IPPP/2IPPDPP and P-IPPP/4IPPDPP were 3.6, 1.8, and 15 for stickleback, respectively, indicating that TPHP, 2IPPDPP, and 4IPPDPP underwent rapid metabolic transformation in stickleback. Congener-specific metabolic processes may contribute to different metabolite/parent ratios observed in stickleback. Higher abundances of OPE metabolites raises the concern that little is known about bioaccumulation or toxicity of these compounds. For example, a recent in vitro study demonstrated that DPHP has stronger transcript alteration capability related to lipid and cholesterol metabolism genes in chicken embryonic liver cells than its parent compound TPHP (Su et al., 2014).
Concentration correlations
Pearson’s correlation analysis was performed using log-transformed concentrations of chemicals detected in more than half of the samples. Heat maps and cluster analyses (Fig. S2) demonstrated that the detected compounds were grouped into three distinctive clusters based on the Pearson’s correlation matrix. The largest cluster included PBDEs, hexa-PCBs, and penta-PCBs that were auto-correlated (r = 0.35–0.83; P < 0.001), followed by the PFAS cluster (r = 0.4–0.9; p < 0.001) and the OPE and OPE metabolite cluster (r = 02–06; P < 0.05). This clustering suggests that chemicals in each cluster have similar sources and physiochemical properties such as lipid solubility and binding affinity to proteins. For example, no relationships were observed between protein-bound PFAS and lipid soluble PBDEs and PCBs. A similar pattern was also reported for polar cod from the Barents Sea food web (Haukas et al., 2007) and for fish from the Czech Republic (Hlouskova et al., 2013). Interestingly, tri- and tetra-PCBs were not significantly correlated with hexa- and penta-PCBs and PBDEs, suggesting a different source for these less chlorinated PCBs. Long-range atmospheric transport has been shown to move a greater fraction of less chlorinated PCBs to higher latitudes, while heavier congeners, including hexa-PCBs, are more likely to move shorter distances via atmospheric processes and deposit closer to their emission sources (Cabrerizo et al., 2018; Mamontova et al., 2016; Shen et al., 2006). Consequently, PCB contamination that includes a higher fraction of highly chlorinated congeners indicates the presence of local sources of contamination near or in Troutman Lake.
Potential sources of contamination
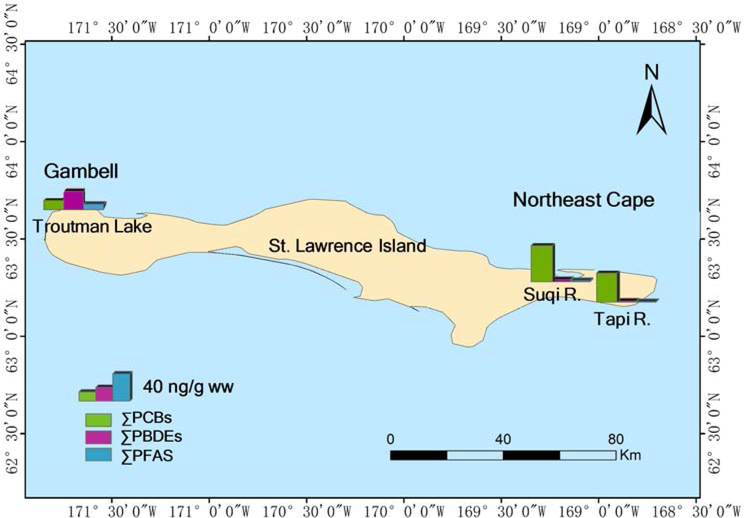
To further investigate potential sources of the most abundant chemical groups in stickleback samples (PBDEs, PCBs and PFAS), we compared PBDE, PCB and PFAS concentrations measured in this study with those found in stickleback collected from two other water bodies located on SLI, the Suqi and Tapi Rivers (Fig. 4). The Suqi River is located in the same drainage as the Northeast Cape FUD site, and the Tapi River is located ~5 km east of the Suqi River (Byrne et al., 2015). ΣPCB concentrations (n = 70 PCBs) in stickleback from Troutman Lake measured in this study (mean 12.8 ± 1.07 ng/g ww) were ~2 times lower than those in stickleback from the Suqi River (mean 24.1 ± 2.41 ng/g ww) and the Tapi River (mean 20.2 ± 6.42 ng/g ww) (von Hippel et al., 2018). The PCB profile at these three sites was dominated by hexa-PCBs (Fig. S3) and was similar to the composition of Aroclors 1254 and 1260 with the ratios of chlorine / biphenyl of 5.2–5.8. Moreover, the presence of Aroclors 1254 and 1260 was observed in the upper part of sediment cores from the Northeast Cape FUD site (Scrudato et al., 2012), further indicating possible local contamination with these Aroclor mixtures. In addition, PBDE (n = 11 PBDEs) and PFAS (n = 3 PFAS) concentrations in these stickleback samples (mean 25.5 ± 1.33 and 7.80 ± 0.263 ng/g ww, respectively) were up to ~9 times higher than those from the Suqi River (3.00 ± 0.425 ng/g ww and 1.45 ± 0.357 ng/g ww, respectively) and up to ~18 times higher than those from the Tapi River (1.40 ± 0.532 ng/g ww and 0.675 ± 0.477 ng/g ww, respectively) (Byrne et al., 2017). PBDE patterns in stickleback were similar among the three sites; however, the PFAS pattern in Troutman Lake was different from that in the Suqi and Tapi Rivers and was dominated by PFOS (Fig. S3). Elevated PCB, PBDE, and PFAS concentrations in stickleback from Troutman Lake suggest an unknown local contamination source and warrant further investigation.
Figure 4.
Concentrations of ΣPCBs, ΣPBDEs, and ΣPFAS in stickleback collected from Troutman Lake (this study) and the Suqitughneq (Suqi) and Tapisaggak (Tapi) Rivers (Byrne et al., 2017; von Hippel et al., 2018).
Supplementary Material
Highlights.
PCBs, PBDEs, PFAS, OPEs and their diester metabolites were measured in stickleback from St. Lawrence Island, Alaska
PBDEs and PCBs were the most abundant SVOC compounds
Chemical patterns and distributions in stickleback suggest strong local sources of PBDEs and PFAS
ACKNOWLEDGMENTS
This work was funded by the National Institute of Environmental Health Sciences (NIEHS 2R01ES019620). We would like to thank the people of Sivuqaq (St. Lawrence Island), the Native Village of Gambell, the Native Village of Savoonga, Sivuqaq Inc., Kukulget Corporation, the City of Savoonga, and the City of Gambell for their collaboration and support of this project. We also appreciate the on-going guidance and support of the St. Lawrence Island Working Group that informs this community-based participatory research project. In addition, we thank Alison Gardell, PhD of the University of Alaska Anchorage, Community Health Researchers Erika Apatiki and Bobby Ungwiluk, and intern Jing Si for field collection of the stickleback samples.
Footnotes
Supporting Information Available
Materials provided in the Supporting Information include: the complete list of compounds targeted in this study; the optimized MRM transitions for OPEs, OPE metabolites, and PFAS analytes; median and mean recovery (%) of each analyte in matrix spike samples; median and mean concentration for all analytes in procedural blanks, and method detection limits; the detection frequencies, minimum, maximum, median and mean (with standard error) wet weight and lipid weight concentration of all target analytes measured in stickleback; relative percent abundances of OPEs and their respective diester metabolites in stickleback.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alaska Department of Environmental Conservation (ADEC), 2019. Site report for Northeast Cape, St. Lawrence Island Alaska. Alaska Department of Environmental Conservation Division of Spill Prevention and Response Contaminated Sites Program. https://dec.alaska.gov/spar/csp/sites/st-lawrence/. Accessed on November 15, 2019.
- Blais JM, 2005. Biogeochemistry of persistent bioaccumulative toxicants: Processes affecting the transport of contaminants to remote areas. Can. J. Fish. Aquat. Sci. 62, 236–243. [Google Scholar]
- Brandsma SH, Leonards PEG, Leslie HA, de Boer J, 2015. Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web. Sci. Total Environ. 505, 22–31. [DOI] [PubMed] [Google Scholar]
- Brown FR, Winkler J, Visita P, Dhaliwal J, Petreas M, 2006. Levels of PBDEs, PCDDs, PCDFs, and coplanar PCBs in edible fish from California coastal waters. Chemosphere 64, 276–286. [DOI] [PubMed] [Google Scholar]
- Brown TM, Fisk AT, Helbing CC, Reimer KJ, 2014. Polychlorinated biphenyl profiles in ringed seals (Pusa Hispida) reveal historical contamination by a military radar station in Labrador, Canada. Environ. Toxicol. Chem. 33, 592–601. [DOI] [PubMed] [Google Scholar]
- Byrne S, Miller P, Waghiyi V, Buck CL, von Hippel FA, Carpenter DO, 2015. Persistent organochlorine pesticide exposure related to a formerly used defense site on St. Lawrence Island, Alaska: Data from sentinel fish and human sera. J. Toxicol. Env. Heal. A 78, 976–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne S, Seguinot-Medina S, Miller P, Waghiyi V, von Hippel FA, Buck CL, Carpenter DO, 2017. Exposure to polybrominated diphenyl ethers and perfluoroalkyl substances in a remote population of Alaska natives. Environ. Pollut. 231, 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SC, Miller P, Seguinot-Medina S, Waghiyi V, Buck CL, von Hippel FA, Carpenter DO, 2018a. Associations between serum polybrominated diphenyl ethers and thyroid hormones in a cross sectional study of a remote Alaska native population. Scientific Reports 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne SC, Miller P, Seguinot-Medina S, Waghiyi V, Buck CL, von Hippel FA, Carpenter DO, 2018b. Exposure to perfluoroalkyl substances and associations with serum thyroid hormones in a remote population of Alaska natives. Environ. Res. 166, 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO, DeCaprio AP, O’Hehir D, Akhtar F, Johnson G, Scrudato RJ, Apatiki L, Kava J, Gologergen J, Miller PK, Eckstein L, 2005. Polychlorinated biphenyls in serum of the Siberian Yupik people from St. Lawrence Island, Alaska. Int. J. Circumpol. Heal. 64, 322–335. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Glasius M, Pecseli M, Platz J, Pritzl G, 2002. Polybrominated diphenyl ethers (PBDEs) in marine fish and blue mussels from southern Greenland. Chemosphere 47, 631–638. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, 2007. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28, 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenset A, Leknes H, Christensen GN, Warner N, Remberger M, Gabrielsen GW, 2009. Screening of new contaminants in samples from the Norwegian arctic rapport. Akvaplanniva Report. Norwegian Pollution Control Agency. [Google Scholar]
- Guo JH, Venier M, Salamova A, Hites RA, 2017. Bioaccumulation of dechloranes, organophosphate esters, and other flame retardants in Great Lakes fish. Sci. Total Environ. 583, 1–9. [DOI] [PubMed] [Google Scholar]
- Hardell S, Tilander H, Welfinger-Smith G, Burger J, Carpenter DO, 2010. Levels of polychlorinated biphenyls (PCBs) and three organochlorine pesticides in fish from the Aleutian Islands of Alaska. PLoS ONE 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukas M, Berger U, Hop H, Gulliksen B, Gabrielsen GW, 2007. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 148, 360–371. [DOI] [PubMed] [Google Scholar]
- Hekster FM, Laane RWPM, de Voogt P, 2003. Environmental and toxicity effects of perfluoroalkylated substances. Rev. Environ. Contam. Toxicol. 179, 99–121. [DOI] [PubMed] [Google Scholar]
- Hlouskova V, Lankova D, Kalachova K, Hradkova P, Poustka J, Hajslova J, Pulkrabova J, 2013. Occurrence of brominated flame retardants and perfluoroalkyl substances in fish from the Czech aquatic ecosystem. Sci. Total Environ. 461, 88–98. [DOI] [PubMed] [Google Scholar]
- Hou R, Huang C, Rao KF, Xu YP, Wang ZJ, 2018. Characterized in vitro metabolism kinetics of alkyl organophosphate esters in fish liver and intestinal microsomes. Environ. Sci. Technol. 52, 3202–3210. [DOI] [PubMed] [Google Scholar]
- Hou R, Liu C, Gao XZ, Xu YP, Zha JM, Wang ZJ, 2017. Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ. Pollut. 229, 548–556. [DOI] [PubMed] [Google Scholar]
- Hou R, Xu YP, Wang ZJ, 2016. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 153, 78–90. [DOI] [PubMed] [Google Scholar]
- Iwata H, Tanabe S, Sakal N, Tatsukawa R, 1993. Distribution of persistent organochlorines in the oceanic air and surface seawater and the role of ocean on their global transport and fate. Environ. Sci. Technol. 27, 1080–1098. [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Gobas FAPC, 2008. Bioaccumulation behaviour of polybrominated diphenyl ethers (PBDEs) in a Canadian arctic marine food web. Sci. Total Environ. 401, 60–72. [DOI] [PubMed] [Google Scholar]
- Kelly BC, Ikonomou MG, Blair JD, Morin AE, Gobas FAPC, 2007. Food web-specific biomagnification of persistent organic pollutants. Science 317, 236–239. [DOI] [PubMed] [Google Scholar]
- Labadie P, Chevreuil M, 2011. Partitioning behaviour of perfluorinated alkyl contaminants between water, sediment and fish in the Orge River (nearby Paris, France). Environ. Pollut. 159, 1452–1453. [DOI] [PubMed] [Google Scholar]
- Lam NH, Cho CR, Lee JS, Soh HY, Lee BC, Lee JA, Tatarozako N, Sasaki K, Saito N, Iwabuchi K, Kannan K, Cho HS, 2014. Perfluorinated alkyl substances in water, sediment, plankton and fish from Korean rivers and lakes: A nationwide survey. Sci. Total Environ. 491, 154–162. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Morris AD, Dyck M, Sverko E, Reiner EJ, Blair DAD, Chu SG, Shen L, 2018. Legacy and new halogenated persistent organic pollutants in polar bears from a contamination hotspot in the arctic, Hudson Bay Canada. Sci. Total Environ. 610, 121–136. [DOI] [PubMed] [Google Scholar]
- Liu LY, Salamova A, Venier M, Hites RA, 2016. Trends in the levels of halogenated flame retardants in the Great Lakes atmosphere over the period 2005–2013. Environ. Int. 92-93, 442–449. [DOI] [PubMed] [Google Scholar]
- Ma YQ, Cui KY, Zeng F, Wen JX, Liu H, Zhu F, Ouyang GF, Luan TG, Zeng ZX, 2013. Microwave-assisted extraction combined with gel permeation chromatography and silica gel cleanup followed by gas chromatography-mass spectrometry for the determination of organophosphorus flame retardants and plasticizers in biological samples. Anal. Chim. Acta 786, 47–53. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DCG, 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus Mykiss). Environ. Toxicol. Chem. 22, 196–204. [PubMed] [Google Scholar]
- McGoldrick DJ, Letcher RJ, Barresi E, Keir MJ, Small J, Clark MG, Sverko E, Backus SM, 2014. Organophosphate flame retardants and organosiloxanes in predatory freshwater fish from locations across Canada. Environ. Pollut. 193, 254–261. [DOI] [PubMed] [Google Scholar]
- Nash SB, 2011. Persistent organic pollutants in Antarctica: Current and future research priorities. J. Environ. Monit. 13, 497–504. [DOI] [PubMed] [Google Scholar]
- Peng JH, Huang CW, Weng YM, Yak HK, 2007. Determination of polybrominated diphenyl ethers (PBDEs) in fish samples from rivers and estuaries in Taiwan. Chemosphere 66, 1990–1997. [DOI] [PubMed] [Google Scholar]
- Quakenbush LT, Citta JJ, 2008. Perfluorinated contaminants in ringed, bearded, spotted, and ribbon seals from the Alaskan Bering and Chukchi Seas. Mar. Pollut. Bull. 56, 1809–1814. [DOI] [PubMed] [Google Scholar]
- Ramu K, Kajiwara N, Tanabe S, Lam PKS, Jefferson TA, 2005. Polybrominated diphenyl ethers (PBDEs) and organochlorines in small cetaceans from Hong Kong waters: Levels, profiles and distribution. Mar. Pollut. Bull. 51, 669–676. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Suzuki T, Takeda M, Uchiyama M, 1984. Metabolism of phosphoric-acid triesters by rat-liver homogenate. Bull. Environ. Contam. Toxicol. 33, 281–288. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Takeda M, Uchiyama M, 1981. Toxicity, absorption and elimination of phosphoric-acid triesters by killifish and goldfish. Bull. Environ. Contam. Toxicol. 27, 775–782. [DOI] [PubMed] [Google Scholar]
- Scheringer M, 2009. Long-range transport of organic chemicals in the environment. Environ. Toxicol. Chem. 28, 677–690. [DOI] [PubMed] [Google Scholar]
- Scrudato RJ, Chiarenzelli J, Miller PK, Alexander CR, Arnason J, Zamzow K, Zweifel K, Gologergen J, Kava J, Waghiyi V, Carpenter DO, 2012. Contaminants at arctic formerly used defense sites. J. Local Glob. Health Sci. 2, 1–12. [Google Scholar]
- Stamatis N, Hela D, Konstantinou I, 2010. Occurrence and removal of fungicides in municipal sewage treatment plant. J. Hazard. Mater. 175, 829–835. [DOI] [PubMed] [Google Scholar]
- Stubbings WA, Guo JH, Simon K, Romanak K, Bowerman W, Venier M, 2018. Flame retardant metabolites in addled bald eagle eggs from the Great Lakes region. Environ. Sci. Technol. Lett. 5, 354–359. [Google Scholar]
- Su GY, Crump D, Letcher RJ, Kennedy SW, 2014. Rapid in vitro metabolism of the flame retardant triphenyl phosphate and effects on cytotoxicity and mRNA expression in chicken embryonic hepatocytes. Environ. Sci. Technol. 48, 13511–13519. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT, 2004. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus Namaycush). Environ. Sci. Technol. 38, 1496–1504. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, Stahlmann R, 2004. Developmental toxicity of polychlorinated biphenyls (PCBs): A systematic review of experimental data. Arch. Toxicol. 78, 483–487. [DOI] [PubMed] [Google Scholar]
- U.S. Army Corps of Engineers (USACE), 2012. Northeast Cape HTRW remedial actions report, Northeast Cape, St. Lawrence Island, Alaska. U.S: Army Corps of Engineers, contract number w911kb-12-c-003,. FUD Number F10AK0969–03. [Google Scholar]
- U.S. Bureau of Land Management (USBLM), 2015. Orders affecting public lands in Alaska. U.S. Bureau of Land Management.
- Venier M, Dove A, Romanak K, Backus S, Hites R, 2014. Flame retardants and legacy chemicals in Great Lakes’ water. Environ. Sci. Technol. 48, 9563–9572. [DOI] [PubMed] [Google Scholar]
- von Hippel FA, Miller PK, Carpenter DO, Dillon D, Smayda L, Katsiadaki I, Titus TA, Batzel P, Postlethwait JH, Buck CL, 2018. Endocrine disruption and differential gene expression in sentinel fish on St. Lawrence Island, Alaska: Health implications for indigenous residents. Environ. Pollut. 234, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hippel FA, Trammell EJ, Merila J, Sanders MB, Schwarz T, Postlethwait JH, Titus TA, Buck CL, Katsiadaki I, 2016. The ninespine stickleback as a model organism in arctic ecotoxicology. Evol. Ecol. Res. 17, 487–504. [Google Scholar]
- Wan Y, Hu JY, Zhang K, An LH, 2008. Trophodynamics of polybrominated diphenyl ethers in the marine food web of Bohai Bay, North China. Environ. Sci. Technol. 42, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Wania F, 2003. Assessing the potential of persistent organic chemicals for long-range transport and accumulation in polar regions. Environ. Sci. Technol. 37, 1344–1351. [Google Scholar]
- Wania F, Mackay D, 1993. Global fractionation and cold condensation of low volatility organochlorine compounds in polar-regions. Ambio 22, 10–18. [Google Scholar]
- Wania F, Mackay D, 1996. Tracking the distribution of persistent organic pollutants. Environ. Sci. Technol. 30, 390–396. [DOI] [PubMed] [Google Scholar]
- Ye XB, Strynar MJ, Nakayama SF, Varns J, Helfant L, Lazorchak J, Lindstrom AB, 2008. Perfluorinated compounds in whole fish homogenates from the Ohio, Missouri, and upper Mississippi rivers, USA. Environ. Pollut. 156, 1227–1232. [DOI] [PubMed] [Google Scholar]
- Yu G, Bu QW, Cao ZG, Du XM, Xia J, Wu M, Huang J, 2016. Brominated flame retardants (BFRs): A review on environmental contamination in China. Chemosphere 150, 479–490. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wan Y, An LH, Hu JY, 2010. Trophodynamics of polybrominated diphenyl ethers and methoxylated polybrominated diphenyl ethers in a marine food web. Environ. Toxicol. Chem. 29, 2792–2799. [DOI] [PubMed] [Google Scholar]
- Zhao HQ, Zhao FR, Liu JX, Zhang SY, Mu D, An LH, Wan Y, Hu JY, 2018. Trophic transfer of organophosphorus flame retardants in a lake food web. Environ. Pollut. 242, 1887–1893. [DOI] [PubMed] [Google Scholar]
- Zheng GM, Wan Y, Hu JY, 2016. Intrinsic clearance of xenobiotic chemicals by liver microsomes: Assessment of trophic magnification potentials. Environ. Sci. Technol. 50, 6343–6353. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Lai NLS, Wai TC, Chan LL, Lam JCW, Lam PKS, 2014. Changes of accumulation profiles from PBDEs to brominated and chlorinated alternatives in marine mammals from the South China Sea. Environ. Int. 66, 65–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.