Abstract
Objective
As diabetes develops, marked reductions of insulin secretion are associated with very modest elevations of glucose. We wondered if these glucose changes disrupt beta cell differentiation enough to account for the altered function.
Methods
Rats were subjected to 90% partial pancreatectomies and those with only mild glucose elevations 4 weeks or 10 weeks after surgery had major alterations of gene expression in their islets as determined by RNAseq.
Results
Changes associated with glucose toxicity demonstrated that many of the critical genes responsible for insulin secretion were downregulated while the expression of normally suppressed genes increased. Also, there were marked changes in genes associated with replication, aging, senescence, stress, inflammation, and increased expression of genes controlling both class I and II MHC antigens.
Conclusions
These findings suggest that mild glucose elevations in the early stages of diabetes lead to phenotypic changes that adversely affect beta cell function, growth, and vulnerability.
Keywords: Diabetes, Pancreatic beta cell, Insulin secretion, Glucose toxicity, RNAseq
Highlights
-
•
Exposure of beta cells to mild hyperglycemia leads to major changes in gene expression.
-
•
Beta cells show evidence of growth arrest and DNA damage.
-
•
Expression of disallowed genes is increased.
-
•
There was increased expression of genes controlling both class I and II MHC antigens.
1. Introduction
Modest dysglycemia in type 1 diabetes (T1D) [1] and type 2 diabetes (T2D) [2] is associated with a marked loss of glucose-induced insulin secretion (GSIS), more notably with early secretion known as the first phase. It is becoming accepted that this change of function results from beta cells undergoing phenotypic change due to chronic exposure to higher than normal glucose levels, which is a reversible process commonly called “glucose toxicity” [3]. This is referred to as dedifferentiation, but it is more often considered a change in identity or phenotype rather than assuming that the beta cells move to particular types of precursor cells such as multipotent pancreatic duct cells or pluripotent embryonic stem cells [4].
It is important to understand the phenotypic changes in beta cells that occur during the early progression to diabetes not only because of their impact upon secretory function but also because these changes may lead to increased vulnerability to autoimmunity in T1D. Additionally, in T2D, these changes could contribute to beta cell death resulting from undefined mechanisms.
While there are many interventions that can produce changes in beta cell phenotype, it is important to determine the differences between changes due to hyperglycemia and those caused by other disruptions, such as the genetic disruption of specific transcription and other factors including Pdx1 [5], Pax4 [6], Mafa [7], Tshz1 [8], Foxo1 [9], Pax6 [10], Neurod1 [11], NKX6-1 [12], Nkx2-2 [13], Abcc [14], and Rfx6 [15]. Glucose toxicity leads to the downregulation of multiple transcription factors that are important for beta cell identity. It is unlikely that the resultant change in phenotype from glucotoxicity would be similar to that caused by knocking down just one important transcription factor. Interpretation can be complicated with such genetic studies; when individual transcription factors are genetically disrupted, it becomes difficult to determine how much of the changed function and phenotype was caused by the genetic intervention and how much was caused by the resultant glucose toxicity. Added complexity comes from the recently renewed acceptance of the heterogeneity of pancreatic beta cells [[16], [17], [18]].
While it is difficult to expect that the perturbation of any one transcription factor would reproduce what occurs with glucose toxicity, other genetic manipulations can create hyperglycemia and are more likely to create a true glucotoxicity phenotype. One valuable model relies on shutting down insulin secretion by mutations in Kir6.2 leading to gain of function of the KATP channel. Other studies have exploited this approach [[19], [20], [21]] and their data suggest that the changes in beta cell phenotype are very similar to those induced by chronic hyperglycemia.
To extend our understanding of the phenotypic changes in beta cells that occur in the presence of varying diabetes severity, we revisited our 90% partial pancreatectomy (PX) model, which has a well-characterized defect in glucose-induced insulin secretion and a marked change in beta cell identity due to chronic, albeit mild, hyperglycemia [[22], [23], [24], [25], [26], [27], [28]]. We revisited this model to measure gene expression as determined by RNAseq in islets in mild to modest hyperglycemia. Islets were obtained either 4 or 10 weeks after PX surgery. These data provide new information about changes in beta cell identity due to diabetes and insights into beta cell function, growth, and vulnerability.
2. Materials and methods
2.1. Experimental groups and sample collection
2.1.1. Four-week PX group
Male inbred Lewis rats (4–5 weeks old for recipients or 8 weeks old for donor islets) were obtained from Envigo (South Easton, MA, USA). Male rats were used to allow a comparison with our earlier work with this model. There were four experimental groups with islet transplants followed by 90% partial pancreatectomy or corresponding sham surgeries: Sham TX-PX, Sham TX-Sham PX, TX-PX, and TX-Sham PX. Anesthesia (ketamine and xylazine) was used for all of the surgical procedures. The Animal Care Committee of the Joslin Diabetes Center approved all of the animal procedures.
Islet transplants (TX) consisted of 1200–1500 islets obtained from the 8-week-old Lewis rats. They were isolated with collagenase digestion followed by density gradient and hand picking as previously described [29] and then cultured overnight in RPMI 1640 medium with 10% fetal calf serum and antibiotics (Life Technologies, Carlsbad, CA, USA). The islets were pelleted into a PE 50 tube attached to a threaded Hamilton syringe and gently injected under the kidney capsule of the left kidney as previously described [29]. A similar procedure was used for the Sham TX.
Seven days after TX or Sham TX, the rats underwent a second surgery to remove 90% of their pancreas (PX) or a Sham PX as previously described [22,23]. Under anesthesia, pancreatic tissue was removed by gentle abrasion with cotton-tipped applicators, leaving a small remnant from the common bile duct and extending to the first loop of the duodenum. Flunixin was administered as an analgesic for 3 days.
Morning random fed blood glucose levels were measured in samples obtained by tail snipping using a Bayer Contour Glucose Meter (Bayer, Whippany, NJ, USA). Blood glucose and body weights were determined on surgery days and then weekly until sacrifice.
Four weeks after the PX or Sham PX, islets were isolated from the remaining pancreas, handpicked, and then immediately extracted for RNA. Total RNA from the islets was extracted using miRNeasy Mini kits (Qiagen, Germantown, MD, USA). Then two aliquots were taken, one for RNA quality control analysis and the other for sequencing to avoid any freezing/thawing. The kidney grafts were examined to ensure that they were intact in the TX groups.
2.1.2. Ten-week PX group
Approximately one year after the 4-week group was completed, the 10-week group was started using the same techniques for PX and sham surgeries on inbred Lewis rats. No islet transplants were conducted in this set of experiments.
2.2. RNA quality control and library preparation
RNA samples were extracted using miRNeasy (Qiagen, Germantown, MD, USA) and assessed for quantity and quality by NanoDrop 3000 and BioAnalyzer 2100 (Agilent). In the 4-week cohort, one rat in the PX group was excluded due very low RNA quantity. A total of 42 samples from individual rats were used for the 4-week cohort library and 15 samples from individual rats were used for the 10-week cohort library preparation, sequencing, and data analysis.
For the 4-week library preparation, the samples were enriched using a RiboZero Gold rRNA Removal kit (Illumina, San Diego, CA, USA) to remove the abundant rRNA while maintaining lncRNAs and premature mRNAs in our samples. The lncRNA data in the 4-week group was not informative. For the 10-week library preparation, the RNA samples were enriched using a TrueSeq Poly-A mRNA Isolation kit (Illumina, San Diego, CA, USA) selecting for mature mRNA molecules.
Directional WaferGen RNASeq library preparation (WaferGen Biosystems, Fremont, CA, USA) was done by the Biopolymers Facility at Harvard Medical School. To assess the integrity of the libraries, 2200 TapeStation (Agilent Technologies, Santa Clara, CA, USA) and qPCR assays were used to test the library concentrations and size distribution. Furthermore, in the 4-week groups, a MiSeq Standard-V3 75 bp long (Illumina) was run on the prepared libraries to ensure that the libraries and indices were correct and equally presented. The MiSeq reads were then added to their corresponding samples and used in the analysis.
2.3. Next-generation RNA sequencing
The 4-week group was sequenced by Illumina HiSeq 2500, and for the 10-week group, NextSeq 500 High Output flow cells were used, both 100 cycles, paired-end, run by the BioPolymers Facility at Harvard Medical School. The mean library size was 21.6 (1.3) million reads, and the libraries then were transferred to the shared high-performance computing cluster O2 of Harvard Medical School.
2.4. Data analysis
A reference genome index was prepared using Subread [30] software and Ensembl [31] Rattus norvegicus reference genome sequence and annotation release 93, and the libraries were aligned to this index. The gene quantifications were done with Subread and featureCounts. Low abundant genes were excluded from differentially expressed genes per Bioconductor manual recommended thresholds. Differentially expressed genes were calculated using the Limma-Voom pipeline. The Benjamini-Hochberg procedure was used to calculate adjusted p-values and false discovery rate (FDR) [32]. The gene set enrichment analysis was conducted using the CAMERA method to account for inter-gene correlations in gene sets [33]. The Molecular Signatures Database (v.5.2) of the Broad Institute was used for pathway analysis.
2.5. Immunostaining and quantification
In parallel to those for islet isolation, additional animals were sacrificed under anesthesia and the pancreas excised for histology (4-week PX: 6 PX and 5 Sham at 4-week PX; at 10-week PX: 4 PX and 3 Sham). The excised pancreas was spread flat and fixed for 2 h in 4% paraformaldehyde for paraffin embedding. Additional sections from previous studies of 4-week PX (9) and Sham (6) pancreas were also used. Paraffin sections of rat pancreas with primary Ab (Table S6) overnight at 4 °C followed by incubation with anti-insulin Ab for 2 h at RT. Fluorochrome-conjugated secondary antibodies were from Jackson ImmunoResearch. For each antibody, at least 3 animals for each group were studied. Images were obtained in the confocal mode using a Zeiss LSM710 microscope with the same settings in a systematic manner.
To assess replication, sections from 4-week PX were triple immunostained for replication marker Ki67, duct marker pan-cytokeratin, and insulin. Biotin amplification was used for Ki67. A total of 4228 insulin-positive cells in 46 islets from 9 PX animals and 2207 insulin-positive cells in 30 islets from 6 Sham animals (a range of 153–492 cells per animal) were systematically photographed and counted. Ki67 and high expression of pan-cytokeratin insulin-positive cells were quantified as a percentage of the total insulin-positive cells.
2.6. Supplemental information
The Supplemental Information includes 5 supplemental tables and 4 supplemental figures that can be found online at https://doi.org/10.1016/j.molmet.2020.02.002. These include. xlsx files: Table S2 Cell identity genes, Table S3 Regulation genes, Table S4 Cell cycle genes, and Table S5 Inflammation and stress genes. The RNAseq data can be found as GSE134963 for 4 weeks and GSE134966 for 10 weeks.
3. Results
3.1. Blood glucose levels following PX and TX
Lewis rats aged 4–5 weeks underwent transplants of 1200–1500 islets (TX) or sham transplants (Sham TX). One week later, they underwent either a 90% partial pancreatectomy (PX) or a Sham PX. Initially, there were four experimental groups (Sham TX-PX, Sham TX-Sham PX, TX-PX, and TX-Sham PX). The rationale for including the islet transplants (TX) was the prediction that these transplants would prevent the hyperglycemia expected following PX and thus help determine if islet changes after PX were independent of dysglycemia. The TX interventions failed to normalize glucose levels, perhaps because of insufficient islet numbers, thus making the data difficult to interpret, so these rats were not included in the analysis. Table S1 presents the glucose values and body weights of the different groups.
The PX (originally called Sham TX-PX) group consisting of 14 rats of which 11 were sacrificed 4 weeks after PX had only mild hyperglycemia (ranging from 88 to 132 mg/dl, 4-week mean 108 ± 3.9), yet higher than the control rats (Sham, originally called Sham TX-Sham PX: n = 10, range 85–107 mg/dl, 4-week mean 97 ± 2.1; p = 0.026). The group of 11 marginally hyperglycemic PX rats (Figure 1A) was analyzed separately to understand what happens to beta cells in a very early stage of progression to diabetes. The other 3 rats in the PX group were clearly hyperglycemic with average values of 317 at 2 weeks, 423 at 3 weeks, and 336 at sacrifice; these were not included. Although the group of 11 that we analyzed had glucose values in the range of 170 at 2 weeks and 150 at 3 weeks, we think it reasonable to consider the group to have very mild hyperglycemia.
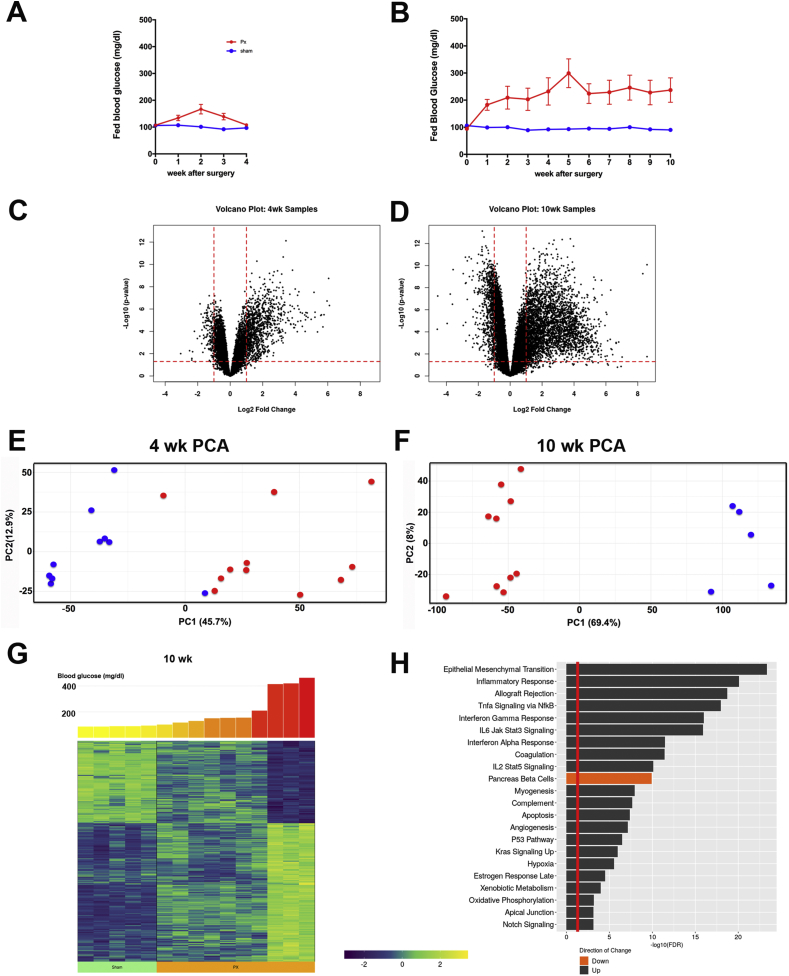
Figure 1.
Gene expression changes in islets after partial pancreatectomy (PX). A. Blood glucose levels after PX for the 4-week experiment (PX = 11; Sham = 10). B. Blood glucose levels after PX for the 10-week experiment (PX = 10, Sham = 5). C. Volcano plot of results from the 4-week experiment. D. Volcano plot from the 10-week experiment. E. Principle component analysis (PCA) of the 4-week data. F. PCA of the 10-week data. G. Heat map of the 428 most differentially expressed genes 10 weeks after PX linked to their ascending blood glucose levels. H. Hallmark pathway analysis of expression 10 weeks after PX: red line represents significance at the 0.05 level, black bars represent upregulated pathways, and the red bar depicts downregulation of the pancreatic beta cell pathway.
To control for possible time-related changes related to the regeneration, post-surgical inflammation, or some other variable in the 4-week group, we also examined islets in another cohort of animals 10 weeks post-surgery with only the PX and Sham groups. Blood sugar levels 10 weeks after surgery before sacrifice for islet isolation were higher than the previous cohort, for the PX group were 238 ± 45 (range 99–467, n = 10) mg/dl and for the Sham group were 90 ± 2 (range 83–98, n = 5), Figure 1B. Table S1 presents the glucose values and body weights.
3.2. Changes in gene expression correlate with diabetes severity
The prediction was that very modest increases in blood glucose after PX would be associated with important alterations in beta cell phenotype and that higher glucose levels would lead to more differential expression. Indeed, for the 4-week PX vs Sham comparison, which only included the 11 rats with slightly elevated blood glucose levels, 2313 of 13,971 genes (17%) were differentially expressed with adjusted p values of <0.01. In contrast, at 10 weeks, the rats with PX, which had modestly higher glucose levels compared with the Sham rats, 7844 of 15,207 genes (52%) were differentially expressed. The differences could also be shown with volcano plots (Figure 1C,D), which clearly demonstrate the greater differential expression in the more hyperglycemic 10-week group and that considerably more genes were upregulated than downregulated at both time points. Using principle component analysis (PCA), the PX and Sham were separated as groups at both time points (Figure 1E,F). The correlation of changes in gene expression with glucose levels at 10 weeks is best shown with a heat map in which the 428 most differentially expressed genes are depicted (Figure 1G), while the correlations for these same 428 genes were less obvious at 4 weeks (Fig. S1). The correlations with glucose occurred with genes that were both up- and downregulated after PX, but after PX, many more genes were upregulated. The differentiation process may depend more on shutting off the expression of genes to obtain specificity than turning on expression.
Using a hallmark pathways analysis of the 10-week samples, 40 pathways were expressed with a false discovery rate of less than 0.05; the top 22 are depicted in Figure 1H. Pathways associated with inflammation were conspicuously evident but the following were also noteworthy: epithelial–mesenchymal transition, apoptosis, hypoxia, oxidative phosphorylation, and notch signaling. Unsurprisingly, a collection of genes associated with beta cell identity was downregulated.
3.3. Changes in beta cell identity
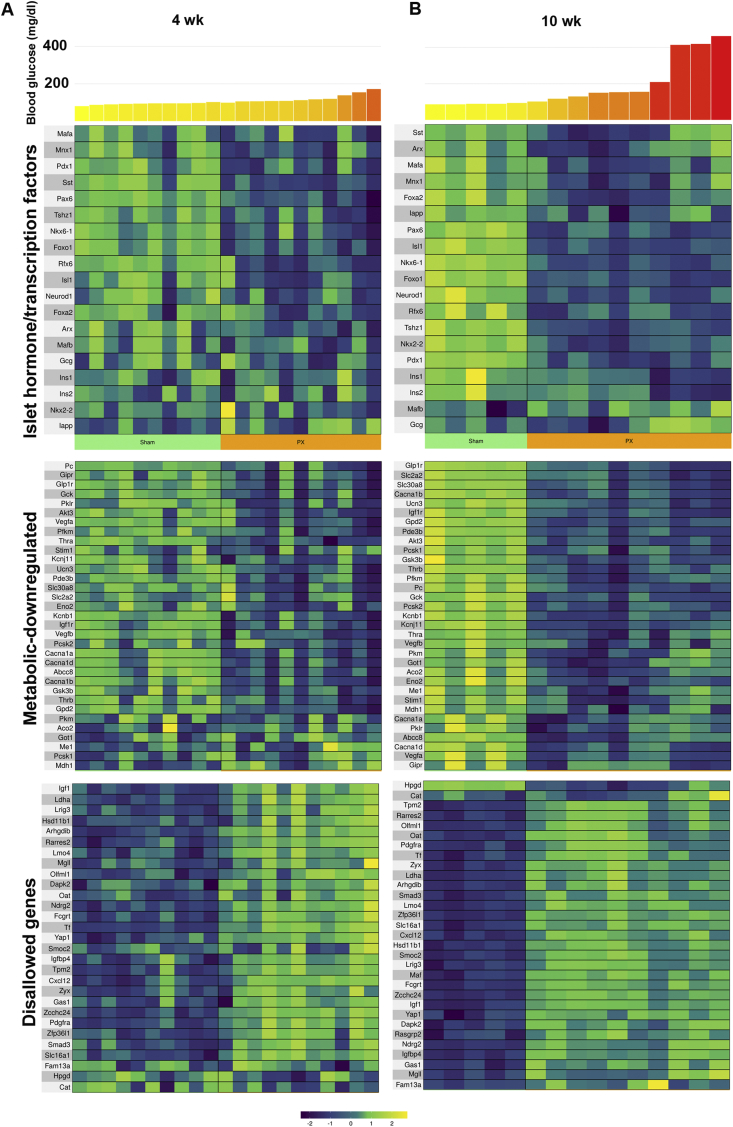
Even with only mildly increased glucose levels in the 4-week PX group, there were marked changes in the beta cell identity genes (Figure 2). Many important beta cell transcription factors were downregulated (Figure 2A and Table 1), including pancreas and duodenal homeobox 1 (Pdx1), NKX6 homeobox 1 (Nkx6-1), forkhead box protein O1 (Foxo1), insulin gene enhancer protein (Isl), paired box protein 6 (Pax6), regulatory factor X6 (Rfx6), and t-shirt zinc finger homeobox 1 (Tshz1). We previously used qPCR to show that some of the same beta cell transcription factors were downregulated 4 weeks after PX [23,25,34]. At 10 weeks, when hyperglycemia was slightly more severe, the differential downregulation of these genes was more marked (Figure 2B and Table 1) with additional significant changes including Foxa2, Mafa, motor neurons, and pancreas homeobox 1 (Mnx1), and there was even a modest increase in Mafb expression. Surprisingly, Mafa and Mnx1 had higher expression in the three animals with the highest glucose levels in the 10-week PX group than those with lower glucose levels. We have no explanation for this.
Figure 2.
Gene expression for key genes in beta cell identity and function changed with PX. Heat maps showing differential expression of gene expression as they relate to blood glucose levels for the last two weeks before sacrifice in islets at 4 weeks (A) and 10 weeks (B) after PX for islet transcription factors and hormones, downregulation of β-cell metabolic genes, and upregulation of disallowed genes. Data used to construct these heat maps are shown in Tables 1 and S2.
Table 1.
Islet cell transcription factors and islet hormones.
| Gene symbol | 4 weeks |
10 weeks |
Gene description | ||
|---|---|---|---|---|---|
| Fold change | Adj. p value | Fold change | Adj. p value | ||
| Arx | 0.5 | 0.044 | 0.7 | 0.04 | Aristaless-related homeobox |
| Foxa2 | 0.9 | 0.2 | 0.5 | 0.00018 | Forkhead box A2 |
| Foxo1 | 0.6 | 0.00064 | 0.4 | 3.3e-08 | Forkhead box O1 |
| Gcg | 0.8 | 0.39 | 1 | 0.93 | Glucagon |
| Iapp | 1.4 | 0.071 | 0.7 | 0.017 | Islet amyloid polypeptide |
| Ins1 | 0.9 | 0.59 | 0.5 | 0.00083 | Insulin 1 |
| Ins2 | 1.0 | 0.96 | 0.5 | 0.0085 | Insulin 2 |
| Isl1 | 0.7 | 0.024 | 0.5 | 3.2e-08 | ISL LIM homeobox 1 |
| Mafa | 0.8 | 0.12 | 0.5 | 0.0041 | MAF bZIP transcription factor a |
| Mafb | 0.6 | 0.092 | 1.6 | 0.036 | MAF bZIP transcription factor a |
| Mnx1 | 0.8 | 0.064 | 0.5 | 0.015 | Motor neuron and pancreas homeobox 1 |
| Neurod1 | 0.8 | 0.088 | 0.6 | 2.7e-06 | Neuronal differentiation 1 |
| Nkx2-2 | 1.0 | 0.8 | 0.4 | 1.3e-06 | NK2 homeobox 2 |
| Nkx6-1 | 0.6 | 0.0048 | 0.3 | 1.6e-08 | NK6 homeobox 1 |
| Pax6 | 0.6 | 0.00095 | 0.5 | 5.9e-07 | Paired box 6 |
| Pdx1 | 0.7 | 0.01 | 0.5 | 2.2e-06 | Pancreatic and duodenal homeobox 1 |
| Rfx6 | 0.6 | 0.00087 | 0.4 | 7e-05 | Regulatory factor X, 6 |
| Sst | 0.4 | 3.9e-05 | 0.5 | 0.041 | Somatostatin |
| Tshz1 | 0.7 | 0.0057 | 0.5 | 1.4e-07 | T-shirt zinc finger family member 1 |
Regarding islet hormone expression, there was decreased somatostatin (Sst) mRNA expression at both 4 and 10 weeks, while decreased insulin 1 and 2 (Ins1 and Ins2) and islet-associated polypeptide (Iapp) mRNA were found only at 10 weeks.
Additionally, a number of genes well known to be critical for beta cell function and metabolism had decreased expression after PX, with this again being more evident at 10 weeks than 4 weeks (Figure 2C,D and Table S2). These included the ATP-dependent potassium channel Kcnj11, the sulfonylurea receptor (Abcc8), the zinc transporter (Slc30a8), glucokinase (Gck), and various subunits of the voltage-dependent calcium channel (Cacna1a, Cacna1b, and Cacna1d). Kcnb1 (Kv2.1), which influences exocytosis, was also downregulated, as was found in T2D [35]. For glucose metabolism, there was a reduction in Glut2 (Slc2a2) and a few genes associated with glycolysis: enolase 2 (Eno2), muscle phosphofructokinase (Pfkm), pyruvate kinase (Pkm), the Krebs cycle pyruvate carboxylase (Pc), and aconitase (Aco2). Notable reductions were also found for mitochondrial shuttle components glycerol-3-phosphate dehydrogenase 2 (Gpd2) and malate dehydrogenase 1 (Mdh1). Similarly reduced expression of genes associated with glucose metabolism were previously found in immature beta cells from newborn rats [36]. Another downregulated gene was the ER calcium sensor (Stim1), which has been demonstrated to be reduced in islets from T2D subjects and may contribute to impaired insulin secretion and ER stress [37]. The expected decrease in the maturity marker urocortin 3 (Unc3) was also found. It is of particular interest that there was a marked reduction in the expression of sentrin/SUMO-specific protease-1 (SenP1), which is thought to play an essential role in the amplification of insulin exocytosis by glucose [38].
Already at 4-weeks post-PX, there was a markedly increased expression of genes that were disallowed or downregulated in beta cells. These can be considered in two categories, first those that are considered disallowed, meaning that their expression in normal β cells is severely reduced. The second category can be thought of as genes that become less active when beta cells are fully mature. There is increasing interest in these disallowed genes [39,40]. Rutter's group recently examined RNAseq data from purified mouse beta cells and listed the top 20 genes with markedly reduced expression compared with other tissues [41]. We examined these and additional genes recently described by Schuit's group [40]. Of these genes, expression was determined for 31 in our data sets, of which 23 were upregulated in the islets of the PX rats at 4 weeks and 30 at 10 weeks (Figure 2 and Table S2). Interestingly, methyltransferase Dnmt3a contributed to the repression of disallowed genes [40,42] and its expression was reduced (Table S3). Other disallowed genes were upregulated (Table S2) as expected based on studies of islets of other diabetic models [20,43] and our own PX research [23,44]. These include lactate dehydrogenase A (LdhA) and monocarboxylic acid transporter (Slc16a1), which are considered disallowed genes.
Mature beta cells are known to have low expression of antioxidant defenses [45], so it was not surprising to find upregulation of glutathione peroxidases (Gpx 1 and 2); however, catalase and superoxide dismutase (Sod2) expression were unchanged. Other upregulated genes that normally play little role in normal beta cell function include hexokinases 1, 2, and 3 (Hk1, Hk2, and Hk3), and the glucose transporter glut3 (Slc2a3). Also of potential interest was the increased expression of aldolase B (Aldob), which has been found to be upregulated in T2D [46]. Calcium-binding proteins S100a4 and S100a6, which were recently described to be upregulated in mice lacking Abcc8 [14] and in db/db mice [47], were also upregulated. Increased expression of tryptophan hydroxylase (Tph1) can lead to increased serotonin that could enhance insulin secretion [48] and beta cell proliferation [49].
3.4. Downregulation of genes associated with secretory machinery
There was a striking reduction in the expression of many genes related to distal secretory mechanisms, those responsible for trafficking of granules from the ER to Golgi and docking (Table S2). The hallmark pathway protein secretion was downregulated with an FDR of 0.023. This suggests that there was a general reduction in the activity of the machinery responsible for moving insulin to its final release. Special note should be made of the marked decreased expression of neuronatin (Nnat), which may be related to insulin signal peptide cleavage by binding to the signal peptidase complex [50]. Major changes were also found with marked downregulation of the beta site amyloid cleaving enzymes 1 (Bace1), while Bace2 was markedly upregulated. Deficiency of this plasma membrane protease Bace2 is thought to enhance beta cell function and proliferation [51]. One of the targets of these proteases was found to be Igf2 receptor.
3.5. Mitochondrial changes
Of the 37 genes transcribed by mitochondrial DNA, the expression of 13 was determined, and of these, 10 were significantly downregulated (Table S2). None were upregulated. This striking finding raises a variety of questions about the structure and function of mitochondria in the diabetic state. Despite these changes, there was no evidence of the general downregulation of genes that comprise electron chain complexes 1–4. However, mitochondrial GTP has been postulated to have an important influence on GSIS [52], and its production relative to ATP can be determined by the ratio of two succinyl-CoA synthetase isoforms, one generating ATP, the other generating GTP. In the PX model, the expression of SCS-ATP (Sucla2) was upregulated (Table S2), while the expression of SCS-GTP isoforms (Suclg1 and Suclg2) were unchanged. This shift in the ratio could lead to less production of mitochondrial GTP and contribute to the reduced GSIS found in hyperglycemia.
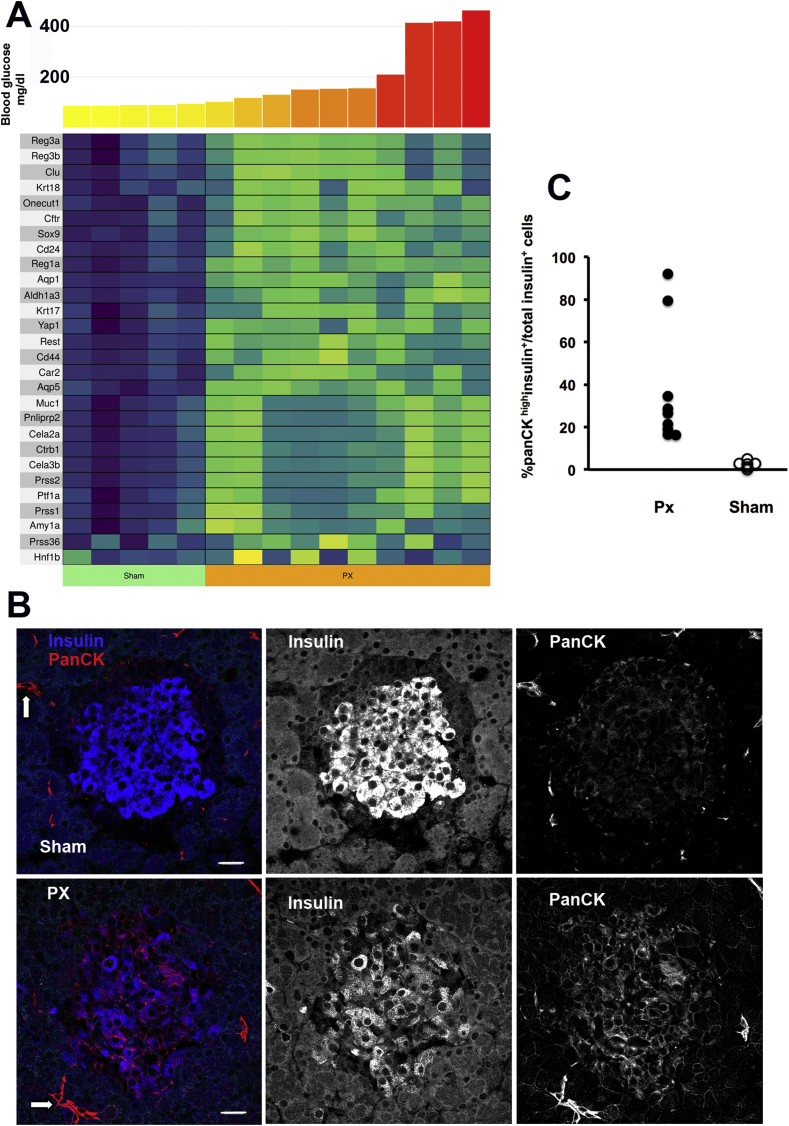
3.6. Increased expression of genes associated with pancreatic ducts and acinar cells
Many genes that are normally found in duct and acinar cells were expressed in the islets after PX at both 4 and 10 weeks (Figure 4A and Table S2). These had not been reported in other hyperglycemia models although some exocrine markers have recently been found in beta cells in human T2D [53]. Because beta cells are derived from duct cells, this reversion to an earlier state of differentiation may not be surprising. Some of the upregulated duct genes included carbonic anhydrase (Car2), aquaporin (Aqp5), and cytokeratins 17 and 18 (Krt17 and Krt18). Interestingly, the major exocrine pancreas-specific transcription factors Ptf1a, HNF6 (Onecut1), and Sox9 expression were markedly increased, but Hnf1b was not. Ptf1a is vital for the development of the endocrine pancreas [54]. We found expression of Rest and Yap1 in ducts in the past (unpublished), and these were markedly elevated in the islets after PX. We also found increased expression of genes associated with acinar cells after PX, including amylase (Amy1a), lipase (Pnliprp2), chymotrypsin (Ctrb1), elastase (Cela3b), and trypsin (Prss1).
Figure 4.
Upregulation of exocrine gene expression 10 weeks after PX and increased protein expression of pan-cytokeratin (panCK) in beta cells 4 weeks after PX. A. Heat map of upregulated exocrine genes 10 weeks after PX. Data for expression at both 4 and 10 weeks are shown in Table S2. B. Immunostaining for pan-cytokeratin (red) and insulin (blue) of Sham and PX shows strong expression in the pancreatic ducts as expected (arrows) but also in many of the insulin-expressing beta cells in the PX animals. The first panel shows the merged image with adjacent panels showing separate channels for insulin and panCK. Mag bar = 20 μm. C. Quantification of the percentage of insulin-positive cells that had high cytokeratin expression in the 4-week PX (PX: 2210 insulin + cells, 46 islets from 9 animals; Sham: 1329 insulin + cells, 30 islets from 6 animals). Each symbol is the mean of one animal; PX = 9, Sham = 6.
In addition, there were marked increases in the expression of Reg genes that are associated with regeneration but remain poorly understood [43,[55], [56], [57], [58]]. Large increases were found at 4 weeks but even more so at 10 weeks: Reg1a (53-fold increase), Reg3a (46-fold), and Reg 3b (48-fold) (Table S2).
To address whether these exocrine genes had increased expression in islets or were from contaminating exocrine cells, we immunostained pancreatic sections for pan-cytokeratin, which was abundant in the beta cells of the 4-week PX rats compared to its minimal presence in the beta cells of the Sham rats (Figure 4B,C). Pan-cytokeratin was expressed in 20–90% of the insulin-positive beta cells in the PX rats, which was far higher than could be accounted for by beta cells newly generated by neogenesis with a residual expression of duct markers. Carboxypeptidase B and SPP1 (Figure S2) were both strongly stained in the pancreatic ducts and occasional insulin-positive cells. Since a few islets in the PX were noticeably irregular in shape with small ducts interspersed, we cannot rule out minor ductal contamination; nonetheless, each of 3 antigens we tested had some beta cell staining. To further address the possibility of contamination, we examined the variability among the samples. Thus, if there was contamination in the PX preps, it would likely be variable such that a contaminated sample from one PX rat would have consistently higher expression of multiple exocrine genes than values from another PX rat. We thoroughly examined the individual preps for evidence of such linked contamination and could not find support for that hypothesis. Another way to test this was to determine the coefficient of variation (COV) for the exocrine genes that were highly expressed in the islets. In the 10-week group, the islets from the 10 PX rats had large fold increases in a number of exocrine genes (Table S2) and the COVs of some of these determined from the Voom normalization were as follows: Prss1 5%, Krt17 28%, CD44 11%, and Cela3b 6%. These low values show the impressive consistency of the differential expression.
3.7. Beta cell regulatory mechanisms
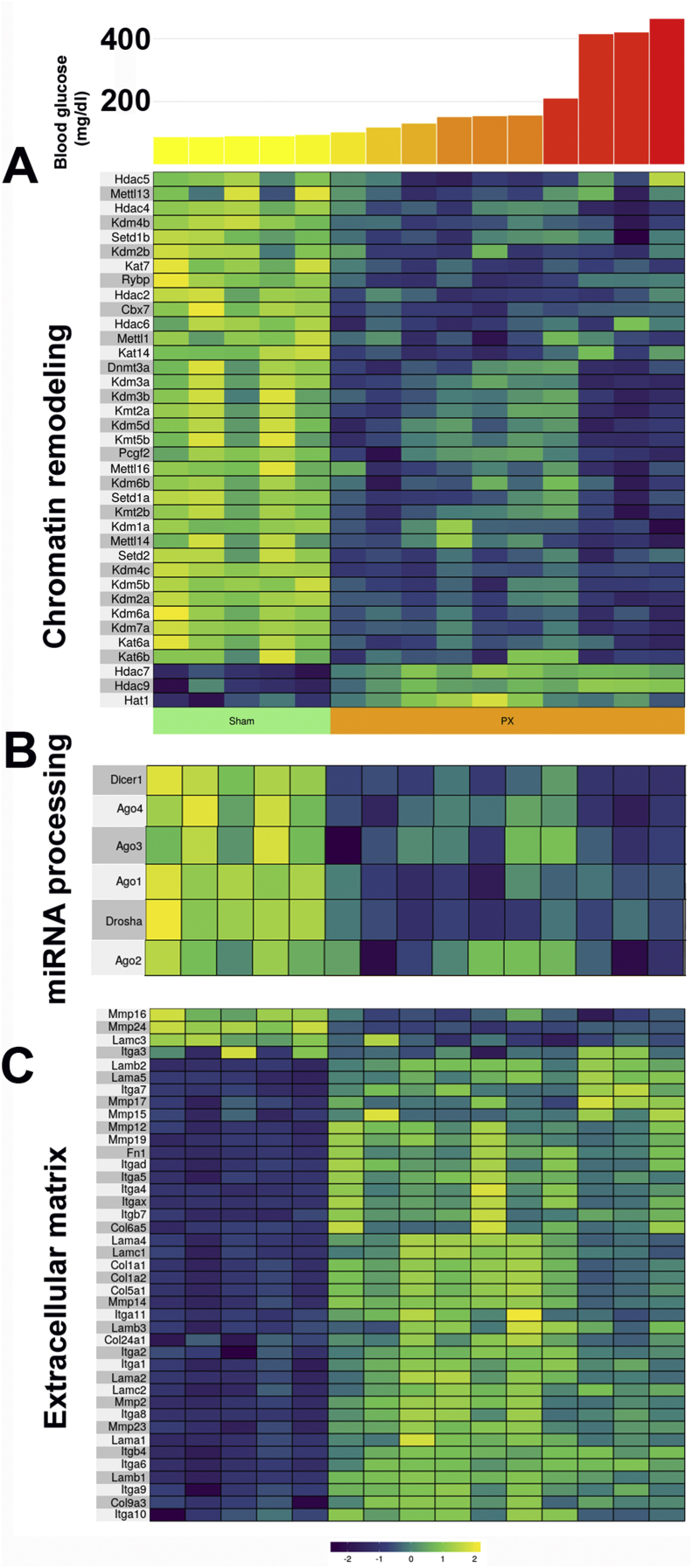
3.7.1. Additional layers of the regulation of gene expression
In addition to the previously described transcription factors changes, several other mechanisms of the regulation of gene expression had meaningful changes. Perhaps the most striking was the reduction in the enzymes related to epigenetics, with many decreased at 4 weeks and even lower at 10 weeks (Figure 3A and Table S3), including numerous Hdac genes (histone deacetylase), Kat genes (lysine acetyltransferase), and Kdm genes (lysine demethylase), although there was upregulation of Hat1 (histone acetyltransferase). As previously noted, methyltransferase Dnmt3a contributes to the repression of disallowed genes [40,42] and its expression was reduced.
Figure 3.
Gene expression of other layers of regulation after PX. Heat maps showing differential expression of gene expression 10 weeks after PX as they relate to blood glucose levels for the last two weeks before sacrifice. A. Genes associated with chromatin modification. B. Genes associated with miRNA processing. C. Genes associated with extracellular matrix. Data for these heat maps are shown in Table S3.
A second altered layer of regulation was a marked reduction in the genes whose products are involved in the processing of miRNAs (Figure 3B and Table S3). These included the ribonucleases Dicer1 and Drosha and Argonaute (Ago1, 2, 3, and 4). These were downregulated at 4 weeks and 10 weeks.
The third level was a surprising increase in the expression of major components of the extracellular matrix, most notably collagens, integrins, and laminins, along with an increase in fibronectin (Fn1) (Figure 3C and Table S3). This suggests some remodeling of the islets after PX, which could have been related either to the beta cell hypertrophy we observed [23] or to an increase in senescent beta cells that secrete senescent-associated secretory products (SASP) including a variety of cytokines, chemokines, and components of the ECM [18,59].
Still, other layers could have been the result of downregulation of many circadian rhythm genes at 10 weeks (Table S3) and notable changes in the expression of receptor genes (Table S3). Downregulated receptors included the adenosine A1 receptor (Adora1), glucagon-like peptide 1 receptor (Glp1r), insulin-like growth factor 1 receptor (Igf1r), growth hormone receptor (Ghr), prolactin receptor (Prlr), somatostatin receptor 5 (Sstr5), vitamin D receptor (Vdr), and thyroid receptors alpha and beta (Thra and Thrb). Upregulated receptor genes included the secretin receptor (Sctr), toll-like receptors 2, 3, 4, 6, 7, 8, and13 (Tlr), and interferon gamma receptor 1 (Ifngr1).
3.8. Genes associated with development
There were a variety of changes in the expression of genes related to development (Table S3). Perhaps it is not surprising that cells undergoing a major change in phenotype would have changes in genes that influence development. Some of those upregulated included Jag1, Jag2, Notch2, Wnt3, Wnt5a, Smo, Fzd2, and Hat1; the downregulated genes included Bmp3, Ptch1, Ptch2, Dvl2, Dvl3, Glis3, Phc1, Phc3, and Prox1.
Fizzled 2 (Fzd2) was upregulated, which is of interest because we previously found its expression to be higher in neonatal islets than adult islets [60]. Another upregulated gene myocyte-specific enhancer factor 2c (Mef2c) was found to be upregulated in mice lacking Abcc8, a model of beta cell dedifferentiation [61]. Also of note, glycoprotein 2 (GP2), which has been found to be expressed on progenitor cells [62], had markedly increased expression after PX.
Upregulated genes related to beta cell vulnerability and death that are likely to be important are Il1beta, the Il1 decoy receptor (Il1r2), and the Il1beta receptor antagonist (Il1rn). Others involved in cytokine signaling include Tnfrsf1a, Tnfrsf1b, fas (TNF receptor), Cd40 (TNF receptor superfamily), Myd88 and the interferon gamma receptor 1 (Ifngr1). An increase in Tnfaip3, also known as A20, was found in our earlier studies using the PX model [26]. Upregulated factors influencing the NFkB pathways include Nfkbia, Nfkbiz, Nfkb1, and Nfkb2. There are also a variety of upregulated cytokines that can regulate the immune system: Il-16, Il-18, Il-33, Ccl2 (Mcp1), Ccl-6, Ccl-7, Cx3cr1, Cxcl11, Cxcr4, and Cxcr6. Jun (c-Jun) expression was also upregulated.
3.9. Beta cell growth, replication, cell cycle arrest, and DNA damage
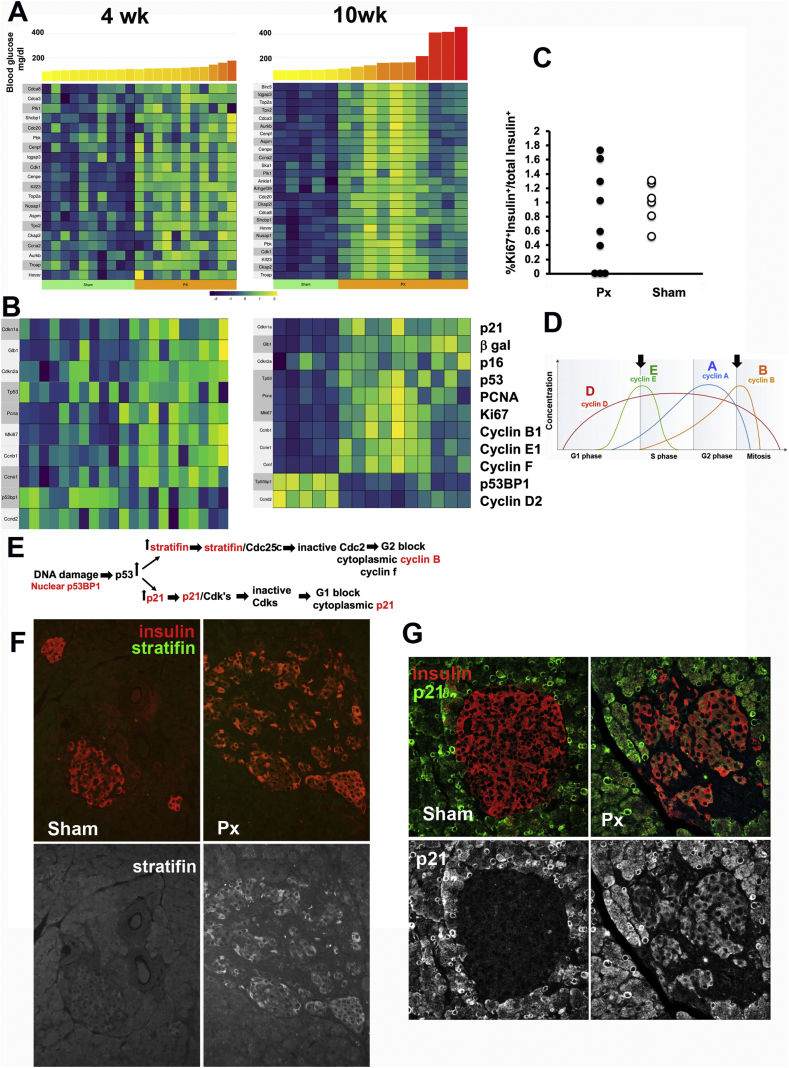
The ability of glucose to acutely stimulate beta cell replication is well known, but the complex effects of chronic hyperglycemia raise questions about growth stimulation, cell cycle arrest, DNA damage, aging, and senescence. A recent publication studied the transcriptome of replicating beta cells from transgenic mice accumulating GFP (from the deconstruction box of cyclin B1) in cells reaching the late (S-G2-M) cell cycle stages [63]. Of the 30 top upregulated genes in that study, 25 had increased expression in islets 10 weeks after PX (Figure 5A and Table S4). In the PX islets, mki67 and Pcna mRNA, whose products are indicative of cells within the replicative cycle, were upregulated (Figure 5B and Table S4), yet the proportion of Ki67+ immunostained beta cells at 4 weeks PX did not differ from that of the Sham group (Figure 5C). Previously we reported beta cell hypertrophy with a 1.8-fold increase in volume 4 weeks and 12 weeks after PX [23,25]. Cellular hypertrophy has been reported in numerous tissues as an early compensatory mechanism caused by the expression of p27 and/or p21 resulting in G1 growth arrest [64]. Together these data suggest that following PX, beta cells enter the cell cycle but become arrested in G1 or G2.
Figure 5.
Beta cells after PX became arrested in cell cycle. A. Heat maps of the genes identified by Klochendler et al. [63] in cells that are in replication as they are expressed in the islets from the 4-week and 10-week PX animals. Of the top 30 genes they identified, 25 were found upregulated in 10-week PX islets. B. Key genes of replication, growth arrest, and senescence also had changes as shown in the heat maps for 4- and 10-week PX. C. Although the expression of Pcna and ki67 mRNA was significantly elevated in the PX islets, there was no increase in the Ki67+ nuclei in the beta cells of the 4-week animals by immunostaining (same images as in Figure 4C). Each symbol is the mean of one animal; PX = 9, Sham = 6. D. Schematic from Wikipedia of the patterns of expression of key cyclins across the cell cycle, with the arrows indicating stages for G1 and G2 arrest. E. Schematic modified by Hermeking et al. [81] of the molecular basis of G1 and G2 arrest. The proteins in red were shown by immunostaining to be increased in beta cells of the 10-week PX. F. YWHAH (stratifin) has increased expression in some insulin + cells after PX compared to Sham (image from 10-week PX with 156 mg/dl mean blood glucose value; 97 mg/dl Sham). G. Cytoplasmic p21expression was observed variably in the beta cells after PX with little expression in Sham (image from 10-week PX with 156 mg/dl mean glucose value; Sham with 89 mg/dl). Data for these heat maps and growth arrest genes are shown in Table S4, respectively.
Following PX, there were several changes indicating growth arrest of beta cells. Growth arrest in G2 is thought to allow for DNA repair, whereas senescence, a permanent growth arrest, prevents the replication of cells with unrepaired DNA damage. DNA damage in beta cells was reported previously in both T1D and T2D [18,65]. Senescent cells have been shown to be arrested either in G1 or G2, yet not all arrested cells are senescent [66,67]. We wondered whether stress on beta cells exerted by hyperglycemia could increase the expression of genes associated with aging and senescence; we recently reported enhanced expression of such genes with the induction of insulin resistance [18,59]. After PX, there were mixed changes in the expression of markers of aging and senescence (Figure 5B and Table S4). The classic markers of senescence p16ink4a, p21cis, and acid β-galactosidase had increased expression both 4 and 10 weeks after PX.
In addition, increased nuclear p53BP1 protein (Figure S3A), a sign of DNA damage, was found along with increased p53 gene expression (Figure 5B). Arrest at the G1-S checkpoint (Figure 5D) was suggested by reduced expression of cyclin D2 but increased cyclin E and p21 and cytoplasmic P21 in the beta cells of some PX islets (Figure 5G). Arrest at the G2-M checkpoint was suggested by increased stratifin protein in some islets after PX (Figure 5E,F) and its (Sfn) increased gene expression (Figure S3C) but little to no expression in the Sham islets. Increased expression of Rprm and Rprml, both P53-dependent G2 arrest mediators, and Gtse1 and Cdc25c associated with G2-M checkpoint arrest was observed. DNA damage was also suggested by increased expression of Cxcl13, Cxcl14, and Cxcl11 (Table S5).
From these data, we conclude that following PX, some beta cells became growth arrested with DNA damage and some progressed even to the permanent growth arrest of senescence.
3.10. Inflammation, stress, and beta cell vulnerability
There were a wide variety of changes in expression in the genes associated with beta cell health and vulnerability. Noteworthy gene expression changes can be found in the supplementary Table S5 for the following categories: inflammation, cytokines and chemokines, SASP, interleukins, TNF and toll receptors, NFkB, ER stress, and apoptosis.
As previously mentioned, there is a link between DNA damage and inflammatory response characterized by activation of p53BP1 and an upregulation of various chemokines [65]. Senescent cells are known to secrete a tissue-specific panel of cytokines, chemokines, and interleukins called senescence-associated secretory (SASP) factors [68]. We recently identified a SASP signature for beta cells [69] that overlap with the cytokines identified by [65]. Many of these as well as other inflammatory genes were elevated after PX.
In the face of the increased expression of these genes was a balancing of enhanced protective genes such as glutathione peroxidases, heme oxygenase 1, and A20/Tnfaip3, as demonstrated in this study as well as previously [26]. Additionally, two of the pro-survival pathways activated in senescent cells [70] (here the MdM2-P53-p21-serpine and the HSP90 pathways) were upregulated in the 10-week PX. At present, it is difficult to explain these changes because despite the upregulation of so many genes that should have adverse effects, some will have protective effects. The NFkB pathway is a particularly good example as its activation can lead to the expression of both pro- and anti-apoptotic genes as well as pro- and anti-inflammatory genes.
There has been considerable interest in ER stress in beta cells because of the unfolded protein response (UPR) and its potential contribution to apoptosis. There were many changes in the markers of ER stress, but the downregulation of Atf6, Hyou1, Pdia4, and Sec24d, along with similar reductions in several chaperone genes, raises questions as to whether the impaired insulin production of glucose toxicity leads to reduced UPR [71].
Because of the increased expression of genes associated with inflammation, we searched for inflammatory cells in the islets. Macrophages were of particular interest because the macrophage marker CD68 had a 22-fold increased expression in the PX islets at 10 weeks (Table S5). Using immunofluorescent immunostaining, CD68+ macrophages were observed at the periphery of an occasional islet in both the Sham and PX groups. There were also rare islets in the PX with strongly stained CD68+ insulin + cells (Figure S4). This finding is consistent with our published data [69] that CD68, a recognized SASP factor, had increased expression (4.7-fold) in FACS purified senescent (βgal +) beta cells compared to non-senescent (βGal-) beta cells from the same mice. In addition, we wondered whether there was evidence of contamination, which might have been reflected by the variable expression in the islets from the 10 PX rats at 10 weeks. The Voom normalized expression level of CD68 in the islets from these 10 rats was 4.78 ± 0.78 (SD), with a very low coefficient of variation of 16%, which argues against variable contamination. We also looked for lymphocytes using CD3 immunostaining, but they were only rarely observed in the PX and Sham islets, which was probably attributable to their presence in circulating blood (data not shown).
Among the last set of genes that may have changed the vulnerability of the beta cells was the impressive increase in the expression of β2 microglobulin (B2m), a component of the MHC class I complex and genes associated with activation of the MHC class II complex [72], in particular Ciita (Class II MHC transactivator), Irf8 (interferon regulatory factor 8), Spi1 (transcription factor PU.1), Cd74 (HLA class II histocompatibility antigen gamma chain), and Ctss (cathepsin S). Increased class I MHC antigen is seen on beta cells as T1D develops and is thought to make beta cells more vulnerable to killing by CD8 effector T cells. This increase is thought to be caused by inflammation induced by interferon gamma. Recent studies made a strong case for beta cells with class 1 antigen being generally associated with lymphocytic infiltration [73,74]. Nonetheless, it is possible that the increasing glucose levels in T1D contribute to these increases and accelerate the presentation of beta cell antigens to the immune system.
The importance of the changing beta cell phenotype occurring as glucose levels rise as T1D develops is that it may alter their susceptibility to autoimmunity [75,76]. This possibility fits with recent data suggesting there is an intensification of beta cell killing shortly before the diagnosis [77]. Also, it is entirely possible that beta cells in T2D are more vulnerable to death as the beta cell phenotype changes.
4. Discussion
Our study provides new information about the changes in the identity of beta cells that are presumably driven by elevated glucose levels, which is considered glucose toxicity. It may seem surprising that so many genes were differentially expressed. Part of the reason is that we used a large number of animals and benefitted from the precision of RNAseq measurements, which meant that we could detect highly significant differences that were only 20% or less. This is an important concept because such seemingly small changes, as might occur with an enzyme or transport system, may result in major biological effects. Thus, the “tightness” of our data provides a much more complete picture of how beta cell identity is altered in the diabetic state.
One of our major findings is that important changes in gene expression could be found when glucose levels were only slightly elevated, being only 11 mg/dl higher than the Sham controls. Only 4 weeks after a 90% PX, the beta cell phenotype was markedly altered as demonstrated by a surprising number of changes in gene expression. These beta cell changes provide insights into the pathophysiology of the early stages of the progression of both types 1 and 2 diabetes. In both cases, GSIS is already impaired [1,2] even when dysglycemia is not severe enough to be defined as diabetes. These changes in beta cells also raise questions about whether beta cells in the early stages of T1D become more or less susceptible to autoimmune killing [26,76]. Similar questions can be asked about whether beta cell death is accelerated in the early stages of T2D because it has been found that beta cell mass is already reduced by approximately 40% when subjects have impaired fasting glucose levels [78]. This may fit with recent findings in mice that the induction of insulin resistance in mice can push beta cells to show markers of aging [18,59], suggesting that they are more vulnerable.
A complexity of islet studies is that they are a heterogeneous collection of cells but it is likely that most of the changes in gene expression found in this study reflected events in beta cells, which comprise approximately 80% of the cell volume of islets as measured in both the PX and control animals at 10 weeks [22]. In addition, we know from studies of parameters other than gene expression such as protein measurements that beta cell phenotype is more altered by glucose changes and the diabetic state than is the case for other islet cell types. Another issue is contamination by other cell types such as duct or acinar exocrine cells or inflammatory cells. Some contamination is inevitable and this would be reflected as increases in the expression of genes such as exocrine enzymes or inflammatory cytokines. One should be more suspicious about contamination when increased expression is present; for example, a trivial number of exocrine cells may contain high expression of genes that have much lower expression in beta cells. However, when gene expression in islets is greatly reduced, as found with many genes, one can be more confident that the changes are occurring in beta cells because they greatly outnumber other cell types. At some point, single cell RNAseq will be used to study the islet cells of this PX model, but this has its own set of drawbacks, including the limited number of genes that can be analyzed.
The question must be addressed as to whether these many changes in gene expression can be explained entirely by hyperglycemia. In particular, it might be argued that the upregulation of genes associated with pancreatic acinar and duct cells, as well as those associated with beta cell proliferation, might represent the regeneration of beta cells that occurred in the pancreatic remnant. Both replication and neogenesis occur shortly after PX but settle down by 4 weeks [79] and certainly by 14 weeks as we studied previously [25] and by 10 weeks in the present study. Beta cells developing from duct cells by neogenesis were a minority population compared with beta cells that either have or have not replicated. With regard to recently replicated beta cells, Klochendler et al. found that as expected a variety of genes associated with the cell cycle machinery are upregulated, but these changes are transient, and most beta cell identity genes had normal expression shortly after division [63]. This means that beta cells a few days after replication must have very different gene expression profiles than those originating from multipotent duct cells, which presumably undergo a more extended maturation period. The genes associated with proliferation are of particular interest because we previously found hypertrophy of beta cells in the PX model [23,25] and yet there was no increase in Ki67+ beta cells even at 4-week PX. It thus seems likely that elevations of glucose are pushing beta cells into the cell cycle, with most beta cells instead of dividing ending up in growth arrest and a hypertrophic state.
In line with our earlier assessment of gene expression using multiplex PCR in islets obtained 4 weeks after PX [[23], [24], [25], [26]], we found reduced expression of many key beta cell transcription factors identified before as well as a number of others due to the breadth of the RNAseq approach (Table 1). As expected, there was the downregulation of multiple metabolic genes (Table S2) that are important for beta cell function including Kcnj11, Abcc8, Slc30a8, and Vdcc subunits and Gck (HK4), but we also found decreased expression of the GLP-1 and GIP receptors. Other noteworthy changes included downregulation of the critical shuttle gene Gpd2 (mitochondrial glycerol phosphate dehydrogenase) and PC (pyruvate carboxylase).
The number of upregulated genes related to beta cell metabolism was also impressive (Table S2). The concept of disallowed genes is receiving attention now that RNAseq and other tools allow better analyses [40,41]. These are genes with a very low or negligible expression in beta cells compared to other tissues. As the list of disallowed genes continues to grow, it seems interesting that so many of them have increased expression in the PX model of glucose toxicity. Why did beta cells evolve to have such low expression of these genes and what might they have to do with beta cell function or survival? Perhaps optimal oxidative phosphorylation is dependent upon suppression of some of these genes. The expression of Ldha and Slc16a1 would allow the loss of carbons as lactate that would reduce efficiency. Likewise, increased expression of Hk1, Hk2, and Hk3 (hexokinases 1, 2, and 3) should interfere with the key glucose-sensing role of Hk4 (glucokinase). Also noteworthy is the upregulation of antioxidant genes that are normally reduced in beta cells [80] including Gpx 1 and 2. These increases along with the increase in Hmox1 could provide some protection against injury but may also have a negative effect on secretion.
The increase in beta 2 microglobulin (B2m) and other MHC associated genes in islets after PX with only mild dysglycemia merits attention. There is considerable interest as to whether its increased expression in beta cells in T1D makes it more of a target for autoimmune killing. While inflammation may be the dominant force responsible for the increase [73,74], it would be of great interest if the development of dysglycemia also made an important contribution. In addition to the increase in B2m, there was also a marked increase in the expression of genes related to activation of class II MHC, which raises questions about whether increased antigen presentation by beta cells leads to increased cell death as glucose levels rise.
There was a notable increase in the expression of genes associated with stress, inflammation, and cell death that are difficult to explain, in part because some of the changes could promote damage to beta cells while others could be protective. However, the changes are striking enough to draw attention to the possibility, if not the likelihood, that beta cells become a changing target for autoimmunity as glucose levels rise [76].
On the background of the beta cell hypertrophy previously demonstrated after PX [23], we now find evidence for entry into the cell cycle, but with both G1 and G2 arrest, as well as DNA damage. We also find increased expression of genes associated with senescence, SASP, and aging. These changes vary from cell to cell, which demonstrates what must be studied in the future to elucidate the pathogenesis of diabetes. We must find ways to identify populations of cells to understand their vulnerability and even their potential for regeneration. What can we learn from markers of aging and senescence? Can their expression be reversed? Can we find ways to identify beta cells that are close to death? Ongoing progress with the characterization of single cells should provide answers to these fundamental questions.
Author contributions
A.G.E., G.C.W., S.B-W., and J.H-L. designed the experiments, interpreted the results, and wrote the manuscript. A.G.E, S.B-W., J.H-L., B.A.S., and R.T. conducted the experiments.
Acknowledgments
This study was supported by grants from the NIH (R01 DK110390 [S.B-W.] and P30 DK036836), the Joslin Diabetes Research Center (DRC, PODK036836), the Diabetes Research and Wellness Foundation, and an important group of private donors. We thank Drs. Jonathan M. Dreyfuss and Hui Pan of the Bioinformatics and Biostatistics Core of the DRC for helpful advice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2020.02.002.
Conflict of interest
G.C.W. is on the Scientific Advisory Board of Beta O2 Biotechnology Ltd.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Vardi P., Crisa L., Jackson R.A. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):93–102. doi: 10.1007/BF00500379. [DOI] [PubMed] [Google Scholar]
- 2.Brunzell J.D., Robertson R.P., Lerner R.L., Hazzard W.R., Ensinck J.W., Bierman E.L. Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. Journal of Clinical Endocrinology & Metabolism. 1976;42:222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 3.Weir G.C., Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Annals of the New York Academy of Sciences. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir G.C., Aguayo-Mazzucato C., Bonner-Weir S. Beta cell dedifferentiation in diabetes is important, but what is it? Islets. 2013;5(5):233–237. doi: 10.4161/isl.27494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metabolism. 2014;19(2):259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prado C.L., Pugh-Bernard A.E., Elghazi L., Sosa-Pineda B., Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proceedings of the National Academy of Sciences of the U S A. 2004;101(9):2924–2929. doi: 10.1073/pnas.0308604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang C., Moriguchi T., Kajihara M., Esaki R., Harada A., Shimohata H. MafA is a key regulator of glucose-stimulated insulin secretion. Molecular and Cellular Biology. 2005;25(12):4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raum J.C., Soleimanpour S.A., Groff D.N., Core N., Fasano L., Garratt A.N. Tshz1 regulates pancreatic beta cell maturation. Diabetes. 2015;64(8):2905–2914. doi: 10.2337/db14-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talchai C., Xuan S., Lin H.V., Sussel L., Accili D. Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell. 2012;150(6):1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swisa A., Avrahami D., Eden N., Zhang J., Feleke E., Dahan T. PAX6 maintains beta cell identity by repressing genes of alternative islet cell types. Journal of Clinical Investigation. 2017;127(1):230–243. doi: 10.1172/JCI88015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu C., Stein G.H., Pan N., Goebbels S., Hornberg H., Nave K.A. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metabolism. 2010;11(4):298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor B.L., Liu F.F., Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Reports. 2013;4(6):1262–1275. doi: 10.1016/j.celrep.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez G.D., Bender A.S., Cirulli V., Mastracci T.L., Kelly S.M., Tsirigos A. Pancreatic beta cell identity requires continual repression of non-beta cell programs. Journal of Clinical Investigation. 2017;127(1):244–259. doi: 10.1172/JCI88017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stancill J.S., Cartailler J.P., Clayton H.W., O'Connor J.T., Dickerson M.T., Dadi P.K. Diabetes; 2017. Chronic beta cell depolarization impairs beta cell identity by disrupting a network of Ca2+-regulated genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piccand J., Strasser P., Hodson D.J., Meunier A., Ye T., Keime C. Rfx6 maintains the functional identity of adult pancreatic beta cells. Cell Reports. 2014;9(6):2219–2232. doi: 10.1016/j.celrep.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T. Human islets contain four distinct subtypes of beta cells. Nature Communications. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bader E., Migliorini A., Gegg M., Moruzzi N., Gerdes J., Roscioni S.S. Identification of proliferative and mature beta cells in the islets of Langerhans. Nature. 2016;535(7612):430–434. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- 18.Aguayo-Mazzucato C., van Haaren M., Mruk M., Lee T.B., Jr., Crawford C., Hollister-Lock J. Beta cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metabolism. 2017;25(4):898–910. doi: 10.1016/j.cmet.2017.03.015. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z., York N.W., Nichols C.G., Remedi M.S. Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metabolism. 2014;19(5):872–882. doi: 10.1016/j.cmet.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brereton M.F., Rohm M., Shimomura K., Holland C., Tornovsky-Babeay S., Dadon D. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic beta cells. Nature Communications. 2016;7:13496. doi: 10.1038/ncomms13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haythorne E., Rohm M., van de Bunt M., Brereton M.F., Tarasov A.I., Blacker T.S. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic beta cells. Nature Communications. 2019;10(1):2474. doi: 10.1038/s41467-019-10189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonner-Weir S., Trent D.F., Weir G.C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. Journal of Clinical Investigation. 1983;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jonas J.C., Sharma A., Hasenkamp W., Ilkova H., Patane G., Laybutt R. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. Journal of Biological Chemistry. 1999;274(20):14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- 24.Laybutt D.R., Sharma A., Sgroi D.C., Gaudet J., Bonner-Weir S., Weir G.C. Genetic regulation of metabolic pathways in beta cells disrupted by hyperglycemia. Journal of Biological Chemistry. 2002;277(13):10912–10921. doi: 10.1074/jbc.M111751200. [DOI] [PubMed] [Google Scholar]
- 25.Laybutt D.R., Glandt M., Xu G., Ahn Y.B., Trivedi N., Bonner-Weir S. Critical reduction in beta cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. Journal of Biological Chemistry. 2003;278(5):2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- 26.Laybutt D.R., Kaneto H., Hasenkamp W., Grey S., Jonas J.C., Sgroi D.C. Increased expression of antioxidant and antiapoptotic genes in islets that may contribute to beta cell survival during chronic hyperglycemia. Diabetes. 2002;51(2):413–423. doi: 10.2337/diabetes.51.2.413. [DOI] [PubMed] [Google Scholar]
- 27.Leahy J.L., Bumbalo L.M., Chen C. Diazoxide causes recovery of beta cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes. 1994;43:173–179. doi: 10.2337/diab.43.2.173. [DOI] [PubMed] [Google Scholar]
- 28.Li W.C., Rukstalis J.M., Nishimura W., Tchipashvili V., Habener J.F., Sharma A. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. Journal of Cell Science. 2010;123(Pt 16):2792–2802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montana E., Bonner-Weir S., Weir G.C. Beta cell mass and growth after syngeneic islet cell transplantation in normal and streptozocin diabetic C57BL/6 mice. Journal of Clinical Investigation. 1993;91(3):780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao Y., Smyth G.K., Shi W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Research. 2013;41(10):e108. doi: 10.1093/nar/gkt214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aken B.L., Ayling S., Barrell D., Clarke L., Curwen V., Fairley S. Database; Oxford): 2016. The Ensembl gene annotation system. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B. 1995;57(1):289–300. [Google Scholar]
- 33.Wu D., Smyth G.K. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Research. 2012;40(17):e133. doi: 10.1093/nar/gks461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kondo T., El Khattabi I., Nishimura W., Laybutt D.R., Geraldes P., Shah S. p38 MAPK is a major regulator of MafA protein stability under oxidative stress. Molecular Endocrinology. 2009;23(8):1281–1290. doi: 10.1210/me.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu J., Dai X., Plummer G., Suzuki K., Bautista A., Githaka J.M. Kv2.1 clustering contributes to insulin exocytosis and rescues human beta cell dysfunction. Diabetes. 2017 doi: 10.2337/db16-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jermendy A., Toschi E., Aye T., Koh A., Aguayo-Mazzucato C., Sharma A. Rat neonatal beta cells lack the specialised metabolic phenotype of mature beta cells. Diabetologia. 2011;54(3):594–604. doi: 10.1007/s00125-010-2036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kono T., Tong X., Taleb S., Bone R.N., Iida H., Lee C.C. Impaired store-operated calcium entry and STIM1 loss lead to reduced insulin secretion and increased endoplasmic reticulum stress in the diabetic beta cell. Diabetes. 2018 doi: 10.2337/db17-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferdaoussi M., Dai X., Jensen M.V., Wang R., Peterson B.S., Huang C. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional beta cells. Journal of Clinical Investigation. 2015;125(10):3847–3860. doi: 10.1172/JCI82498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorrez L., Laudadio I., Van Deun K., Quintens R., Hendrickx N., Granvik M. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Research. 2011;21(1):95–105. doi: 10.1101/gr.109173.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemaire K., Granvik M., Schraenen A., Goyvaerts L., Van Lommel L., Gomez-Ruiz A. How stable is repression of disallowed genes in pancreatic islets in response to metabolic stress? PloS One. 2017;12(8) doi: 10.1371/journal.pone.0181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pullen T.J., Huising M.O., Rutter G.A. Analysis of purified pancreatic islet beta and alpha cell transcriptomes reveals 11beta-hydroxysteroid dehydrogenase (Hsd11b1) as a novel disallowed gene. Frontiers in Genetics. 2017;8:41. doi: 10.3389/fgene.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhawan S., Tschen S.I., Zeng C., Guo T., Hebrok M., Matveyenko A. DNA methylation directs functional maturation of pancreatic beta cells. Journal of Clinical Investigation. 2015;125(7):2851–2860. doi: 10.1172/JCI79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Churchill A.J., Gutierrez G.D., Singer R.A., Lorberbaum D.S., Fischer K.A., Sussel L. Genetic evidence that Nkx2.2 acts primarily downstream of Neurog3 in pancreatic endocrine lineage development. Elife. 2017;6 doi: 10.7554/eLife.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laybutt R., Hasenkamp W., Groff A., Grey S., Jonas J.C., Kaneto H. Beta cell adaptation to hyperglycemia. Diabetes. 2001;50(Suppl 1):S180–S181. doi: 10.2337/diabetes.50.2007.s180. [DOI] [PubMed] [Google Scholar]
- 45.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared to with various other mouse tissues. Free Radical Biology and Medicine. 1996;320:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 46.Gerst F., Jaghutriz B.A., Staiger H., Schulte A.M., Lorza-Gil E., Kaiser G. TRThe expression of aldolase B in islets is negatively associated with insulin secretion in humans. Journal of Clinical Endocrinology & Metabolism. 2018 doi: 10.1210/jc.2018-00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue H., Shirakawa J., Togashi Y., Tajima K., Okuyama T., Kyohara M. Signaling between pancreatic beta cells and macrophages via S100 calcium-binding protein A8 exacerbates beta cell apoptosis and islet inflammation. Journal of Biological Chemistry. 2018;293(16):5934–5946. doi: 10.1074/jbc.M117.809228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Deng R., Yang X., Xu W., Liu Y., Li F. Glucose potentiates beta cell function by inducing Tph1 expression in rat islets. The FASEB Journal. 2017;31(12):5342–5355. doi: 10.1096/fj.201700351R. [DOI] [PubMed] [Google Scholar]
- 49.Kim H., Toyofuku Y., Lynn F.C., Chak E., Uchida T., Mizukami H. Serotonin regulates pancreatic beta cell mass during pregnancy. Nature Medicine. 2010;16(7):804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millership S.J., Da Silva Xavier G., Choudhury A.I., Bertazzo S., Chabosseau P., Pedroni S.M. Neuronatin regulates pancreatic beta cell insulin content and secretion. Journal of Clinical Investigation. 2018;128(8):3369–3381. doi: 10.1172/JCI120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stutzer I., Selevsek N., Esterhazy D., Schmidt A., Aebersold R., Stoffel M. Systematic proteomic analysis identifies beta site amyloid precursor protein cleaving enzyme 2 and 1 (BACE2 and BACE1) substrates in pancreatic beta cells. Journal of Biological Chemistry. 2013;288(15):10536–10547. doi: 10.1074/jbc.M112.444703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kibbey R.G., Pongratz R.L., Romanelli A.J., Wollheim C.B., Cline G.W., Shulman G.I. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metabolism. 2007;5(4):253–264. doi: 10.1016/j.cmet.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masini M., Marselli L., Himpe E., Martino L., Bugliani M., Suleiman M. Co-localization of acinar markers and insulin in pancreatic cells of subjects with type 2 diabetes. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0179398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan F.C., Wright C. Pancreas organogenesis: from bud to plexus to gland. Developmental Dynamics. 2011;240(3):530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 55.Terazono K., Yamamoto H., Takasawa S., Shiga K., Yonemura Y., Tochino Y. A novel gene activated in regenerating islets. Journal of Biological Chemistry. 1988;263(5.5):2111–2114. [PubMed] [Google Scholar]
- 56.Okamoto H., Takasawa S. Recent advances in the Okamoto model: the CD38-cyclic ADP-ribose signal system and the regenerating gene protein (Reg)-Reg receptor system in beta cells. Diabetes. 2002;51(Suppl 3):S462–S473. doi: 10.2337/diabetes.51.2007.s462. [DOI] [PubMed] [Google Scholar]
- 57.Unno M., Nata K., Noguchi N., Narushima Y., Akiyama T., Ikeda T. Production and characterization of Reg knockout mice: reduced proliferation of pancreatic beta cells in Reg knockout mice. Diabetes. 2002;51(Suppl 3):S478–S483. doi: 10.2337/diabetes.51.2007.s478. S478-483. [DOI] [PubMed] [Google Scholar]
- 58.Planas R., Alba A., Carrillo J., Puertas M.C., Ampudia R., Pastor X. Reg (regenerating) gene overexpression in islets from non-obese diabetic mice with accelerated diabetes: role of IFNbeta. Diabetologia. 2006;49(10):2379–2387. doi: 10.1007/s00125-006-0365-6. [DOI] [PubMed] [Google Scholar]
- 59.Aguayo-Mazzucato C., Andle J., Lee T.B., Jr., Midha A., Talemal L., Chipashvili V. Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019 doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aye T., Toschi E., Sharma A., Sgroi D., Bonner-Weir S. Identification of markers for newly formed beta cells in the perinatal period: a time of recognized beta cell immaturity. Journal of Histochemistry and Cytochemistry. 2010;58(4):369–376. doi: 10.1369/jhc.2009.954909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stancill J.S., Cartailler J.P., Clayton H.W., O'Connor J.T., Dickerson M.T., Dadi P.K. Chronic beta cell depolarization impairs beta cell identity by disrupting a network of Ca(2+)-regulated genes. Diabetes. 2017;66(8):2175–2187. doi: 10.2337/db16-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ameri J., Borup R., Prawiro C., Ramond C., Schachter K.A., Scharfmann R. Efficient generation of glucose-responsive beta cells from isolated GP2+ human pancreatic progenitors. Cell Reports. 2017;19(1):36–49. doi: 10.1016/j.celrep.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 63.Klochendler A., Caspi I., Corem N., Moran M., Friedlich O., Elgavish S. The genetic program of pancreatic beta cell replication in vivo. Diabetes. 2016;65(7):2081–2093. doi: 10.2337/db16-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf G. Molecular mechanisms of renal hypertrophy: role of p27Kip1. Kidney International. 1999;56:1262–1265. doi: 10.1046/j.1523-1755.1999.00695.x. [DOI] [PubMed] [Google Scholar]
- 65.Horwitz E., Krogvold L., Zhitomirsky S., Swisa A., Fischman M., Lax T. Beta cell DNA damage response promotes islet inflammation in type 1 diabetes. Diabetes. 2018;67(11):2305–2318. doi: 10.2337/db17-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blagosklonny M.V. Cell cycle arrest is not senescence. Aging. 2011;3(2):94–101. doi: 10.18632/aging.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan T.A., Hwang P.M., Hermeking H., Kinzler K.W., Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes & Development. 2000;14(13):1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 68.Campisi J., d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nature Reviews Molecular Cell Biology. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 69.Aguayo-Mazzucato C., Andle J., Lee T.B., Jr., Midha A., Talemal L., Chipashvili V. Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metabolism. 2019;30(1):129–142.e4. doi: 10.1016/j.cmet.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kirkland J.L., Tchkonia T., Zhu Y., Niedernhofer L.J., Robbins P.D. The clinical potential of senolytic drugs. Journal of the American Geriatrics Society. 2017;65(10):2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma R.B., O'Donnell A.C., Stamateris R.E., Ha B., McCloskey K.M., Reynolds P.R. Insulin demand regulates beta cell number via the unfolded protein response. Journal of Clinical Investigation. 2015;125(10):3831–3846. doi: 10.1172/JCI79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russell M.A., Redick S.D., Blodgett D.M., Richardson S.J., Leete P., Krogvold L. HLA class II antigen processing and presentation pathway components demonstrated by transcriptome and protein analyses of islet beta cells from donors with type 1 diabetes. Diabetes. 2019;68(5):988–1001. doi: 10.2337/db18-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez-Calvo T., Suwandi J.S., Amirian N., Zapardiel-Gonzalo J., Anquetil F., Sabouri S. Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase. Journal of Histochemistry and Cytochemistry. 2015;63(8):626–636. doi: 10.1369/0022155415576543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson S.J., Rodriguez-Calvo T., Gerling I.C., Mathews C.E., Kaddis J.S., Russell M.A. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59(11):2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skowera A., Ellis R.J., Varela-Calvino R., Arif S., Huang G.C., Van-Krinks C. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. Journal of Clinical Investigation. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weir G.C., Bonner-Weir S. Glucose driven changes in beta cell identity are important for function and possibly autoimmune vulnerability during the progression of type 1 diabetes. Frontiers in Genetics. 2017;8:2. doi: 10.3389/fgene.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herold K.C., Usmani-Brown S., Ghazi T., Lebastchi J., Beam C.A., Bellin M.D. Beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. Journal of Clinical Investigation. 2015;125(3):1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta cell deficit and increased beta cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 79.Brockenbrough J.S., Weir G.C., Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes. 1988;37(2):232–236. doi: 10.2337/diab.37.2.232. [DOI] [PubMed] [Google Scholar]
- 80.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 81.Hermeking H., Lengauer C., Polyak K., He T.C., Zhang L., Thiagalingam S. 14-3-3sigma is a p53-regulated inhibitor of G2/M progression. Molecular Cell. 1997;1(1):3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.