Abstract
Rumen fermentation parameters and microbiota were evaluated in 3 in vitro rumen fermentation experiments after addition of chestnut tannins (CWT) or an extract from Stevia rebaudiana Bertoni (SB) to substrates. A control (CTR) substrate was fermented alone or added with 1.5% of CWT or SB extracts in a batch culture system (Exp. 1, fermentation in 500 mL for 24 h) and in a subsequent continuous culture system (Exp. 2, fermentation in 2 L bottles for 9 d). Experiment 3 used the fermentation system of Exp. 1 and tested 7 doses of each extract added to CTR (additions of 0.2%, 0.4%, 0.6%, 0.8%, 1.0%, 1.2% and 1.4% for 48 h). The addition of CWT lowered (P < 0.01) the in vitro rumen ammonia concentration in all experiments and reduced the protozoa counts in Exp. 1 (P < 0.05). In contrast, the SB extract did not modify the ammonia concentrations, but significantly lowered the protozoa counts in all 3 experiments (reduction of 47% and 20% in Exp. 1 and 2, P < 0.05; and a quadratic reduction in Exp. 3, R2 = 0.63, P < 0.01). Neither extract affected the fermentation in terms of gas production (Exp. 1 and 3) nor volatile fatty acids (VFA) yield (Exp. 1 and 2), if we exclude a reduction at the highest CWT concentration in Exp. 3. Changes in VFA profile were induced by CWT and were limited to reductions in the iso-valerate (P < 0.01, in Exp. 2) and iso-butyrate levels (P < 0.01, Exp. 2). The CWT increased the abundance of Prevotella ruminicola and Selenomonas ruminantium and decreased that of Ruminobacter amylophilus (P < 0.01, P < 0.05 and P < 0.05, respectively). The SB extract increased the relative abundance of Treponema saccarophylum (P < 0.05). Both of the studied substances had an impact on rumen metabolism, with SB reducing protozoa counts and CWT lowering the rumen ammonia concentration. The effects of both extracts on the rumen were appreciable at low dietary doses, and the negative impacts on fermentation were limited to the reduction in protein degradation with the addition of CWT.
Keywords: Rumen, Ammonia, Protozoa, Chestnut tannin, Stevia rebaudiana Bertoni
1. Introduction
Natural substances extracted from plants can be used as safe dietary additives to favorably modify rumen fermentation and microbiota in terms of pollution mitigation (e.g. methane and ammonia) and improve feed efficiency.
Tannins are known to be bioactive in the rumen, mainly for their capacity to form complexes with proteins and/or to inhibit the enzymatic activity of rumen protease and urease (Patra and Saxena, 2011, Patra and Aschenbach, 2018). However, tannins are a complex family of compounds (e.g. hydrolysable and condensed) with different chemical structures depending on their botanical origin. Moreover, the availability of literature for condensed tannins is extensive, whereas hydrolysable tannins have been less studied in animal nutrition. Chestnut tannins (extracted from chestnut wood, Castanea sativa L.; CWT) are hydrolysable tannins, that have been studied alone (Hassanat and Benchaar, 2013, Liu et al., 2011, Witzig et al., 2018) or in association with condensed tannins (Aboagye et al., 2018) with positive effects in terms of mitigation of rumen N degradation. Moreover, reductions in methane were measured in vitro at high dietary concentrations of CWT (Hassanat and Benchaar, 2013, Witzig et al., 2018), and negative impacts on protozoa were found in vivo in sheep (Liu et al., 2011).
Stevia (Stevia rebaudiana Bertoni, SB) is a perennial shrubby plant belonging to the Asteraceae family known for its steviol glycosides content (mainly, stevioside and rebaudioside A) which have high-intensity sweetness. In addition, Stevia glycosides are thought to have antimicrobial effects and were used to reduce unfavorable bacteria in the gut of young pigs to prevent diarrhea (Munro et al., 2000, Wang et al., 2014). Knowledge on the effects of stevia on the rumen is limited to a recent work (Ramos-Morales et al., 2017) that tested the effects of addition of Stevia leaf extract on rumen fermenters and found a depressive effect on the rumen protozoal population and ammonia and shifts in the bacterial community.
The present research has the aim to study the effects on in vitro rumen fermentation of dietary addition of these 2 natural substances at low doses, typical of additive supplements. We performed a series of in vitro rumen fermentations to evaluate changes in fermentability and the rumen microbiota and to uncover possible dose-dependent effects of the 2 extracts. In our experiments we used a maximum dose of pure tannin (1.1% dry matter [DM] of tannic acid equivalent) much lower than doses (2.0% DM) assumed to limit intake in ruminants (Jayanegara et al., 2012) and compatible with a practical dose of additive supplements in ruminant diets. We adopted the same doses used for CWT for SB despite the higher doses already tested in vitro (Ramos Morales et al., 2017).
2. Materials and methods
2.1. Diets and plant extracts
SaviotaN Feed supplement, made of hydrolyzable tannins from C. sativa Miller, was provided by Gruppo Mauro Saviola s.r.l. (Viadana, MN, Italy). SaviotaN Feed supplement was extracted from chestnut wood and used in feed formulations as a source of CWT (750 g of tannic acid equivalent/kg of DM). The chemical composition and gas chromatographic profile of the CWT is available in the study of Campo et al. (2012).
Stevia leaves were obtained by Bio Mondo (Porcia, PN, Italy). Dried and milled leaves were maintained in ethanol solution for 6 h (Ramos-Morales et al., 2017) and then filtered, and the liquid extract was dried by a Univapo 100 ECH vacuum concentrator centrifuge (UniEquip, Planegg, Germany).
The extracts were added to ground corn meal in Exp. 1 or to a mixture of feeds, which simulated a total mixed ratio for high producing ruminants in Exp. 2 and 3, in amounts ranging from zero to 1.5% of DM. The feed ingredients used as a substrate for the 3 experiments were ground through a 1.0-mm screen (Ciclotec Tecator) and were analyzed (AOAC, 2000) for DM, crude protein (CP) and neutral detergent fiber (NDF, Van Soest et al., 1991) using an the Ankom 200 Fiber Analyzer (ANKOM Technology, Macedon, NY).
2.2. In vitro experiments
This study employed 3 different in vitro rumen fermentation experiments: a batch system was used to evaluate the fermentation parameters, gas production and protozoa population (Exp. 1), a rumen continuous fermenter to determine the effects of the additives on the microbiome (Exp. 2) and a batch system to study the dose–response effect of the 2 additives (Exp. 3). The rumen fluid for all the fermentation runs of each experiment was collected in the same slaughterhouse in controlled conditions (e.g. from 4 culled dairy cows for each rumen collection, animals fed productive total mixed rations based on corn silage, not slaughtered in emergency, in good health status, transported from farms located near the slaughterhouse, rumen fluid sampled within 20 min of slaughter) and was delivered to the laboratory within 0.5 h in airtight glass-bottles refluxed with carbon dioxide and maintained at 39 °C.
2.2.1. Rumen fermentation in a batch system (Exp. 1)
Rumen fermentation was conducted in 3 runs using a gas production fermentation system consisting of six 500-mL bottles, closed by a stirring device and connected to a MilliGascounter (measuring range: from 1 mL/h to 1 L/h; measuring accuracy: 3%; Dr. Ing. Ritter Apparatebau GmbH & Co. KG). The incubation medium contained 125 mL of the rumen fluid and a double strength buffer (375 mL of bicarbonate-mineral-distilled water mixture, 2:1:3, vol:vol:vol) according to Blümmel and Becker (1997). This adaptation of the original buffer of Menke et al. (1979) permits an increase in the amount of the sample for fermentation because the added bicarbonate buffer neutralizes a higher amount of volatile fatty acids (VFA). A larger amount of feed substrate allows to minimize the error in the additives weighing phase. Corn meal (5.5 g of DM) was introduced in each fermentation bottle and was incubated alone as a control and with the addition of one of the 2 extracts, SB or CWT (1.5% of corn DM); each treatment was incubated in duplicate bottles per fermentation run. Fermentation bottles were maintained at 39 °C for 24 h, and samples for protozoa counts were collected at 4 and 24 h, and samples for ammonia, VFA and pH were collected at 24 h. Gas data were automatically recorded in a computer connected with the gas counters and equipped with a specific software (Rigamo v3.1, 2012 Dr. Ing. Ritter Apparatebau GmbH & Co. KG) until the end of the fermentation. Fermentation fluid samples at 24 h were taken by completely opening the fermenters at the end of fermentation, the 4-h samples were collected by a syringe through the gas outflow hole. The gas losses during the sample collection were negligible given the small hole with respect to the fermentation bottle volume (10 mm diameter vs. 500 mL), the short sampling duration (approximated 10 s) and the system of gas recording, which is based on a continuous measurement at ultralow flow rates that does not require accumulation of gas under pressure within the bottle.
2.2.2. Rumen fermentation in a continuous system (Exp. 2)
Two different fermentation runs were performed using six 2-L single-flow continuous fermenters, as described in Mason et al. (2015). A standard diet composed on a DM basis of 15% hay, 35% corn silage and 50% concentrate for dairy cows was used as a control (88% DM, 14.3% CP and 42.6% NDF). The 2 treatments consisted of the addition of 1.5% DM of SB or CWT. Diets were given to each fermenter (2 bottles per treatment) in 2 equal doses (at 09:00 and 17:00) for a total of 18 g/d of DM. Each run lasted 9 d, with 6 d of adaptation and 3 d of sampling, and artificial saliva (McDougall, 1948) was continuously infused by a peristaltic pump at 1.3 mL/min. Each day, samples of fermentation fluid were collected for protozoa counts. During the last 3 d before the morning feeding samples for ammonia, pH, and bacterial DNA analysis were collected and stored at –20 °C. For VFA analysis, the samples were acidified with 0.05 mol/L H2SO4 and stored at –20 °C. Ammonia samples were collected just before the morning meal and every hour thereafter for 3 h.
2.2.3. Dose-effect studied by a rumen fermentation batch system (Exp. 3)
An independent fermentation experiment was conducted for each extract. Each experiment used 8 fermentation bottles with the same apparatus used in Exp. 1 during 3 subsequent fermentation runs. The mixed diet of Exp. 2 was incubated in each bottle alone (5.5 g DM/bottle) or with the following doses of the extracts: 0.2%, 0.4%, 0.6%, 0.8%, 1.0%, 1.2% and 1.4% of incubated DM. Samples of fermentation fluid were collected at 24 h for protozoa counts (SB addition) and ammonia determination (CWT addition) as previously described for Exp. 1, and gas production was automatically recorded continuously until 48 h by Rigamo v3.1 software (2012 Dr. Ing. Ritter Apparatebau GmbH & Co. KG).
2.3. Sample analysis
Protozoa were counted as described by Dehority (2003), and pH was immediately measured after sample collection (GLP 22, Crison Instruments, S.A. Barcelona, Spain).
Samples of fermentation liquid for ammonia analysis were stored at –20 °C. Before the analysis, samples were thawed at room temperature and measured by an ammonia electrode (Ammonia Gas Sensing Combination Electrode, Hach Company, 2001). Then the samples were thawed at room temperature and centrifuged at 20,000 × g for 30 min at 20 °C and the supernatant was filtered using 0.45 μm polypore filters (Agilent Technologies, Milano, Italy). The filtrate was injected into a high-performance liquid chromatography instrument (Perkin–Elmer, Norwalk, CN, USA) set to 220 nm according to the method described by Martillotti and Puppo (1985).
2.4. DNA extraction and quantitative PCR
Before DNA extraction fermenter fluid samples from the 3 sampling days were thawed on ice and pooled per bottle. Total DNA was extracted from approximated 800 μL of fermentation fluid using the PowerSoil DNA extraction kit (MoBio Laboratories Inc., Carlsbad, CA, USA) with some modifications. The DNA elution volume was 50 μL, and the isolated DNA concentration was determined by a NanoDrop One (Thermo Fisher Scientific Inc., Wilmington, USA).
Quantitative PCR (qPCR) was performed using the CFX96 Real Time System (Bio-Rad Technologies Inc, Hercules, California, USA). The total reaction volume was 20 μL and consisted of 0.3 μL of each forward and reverse primers (0.3 μmol/L), 10 μL of iQ SYBR Green Supermix (Bio-Rad Technologies Inc., Hercules, California), 8.4 μL of sterile water and 1 μL of gDNA (Appendix Table 1). The amplification program included polymerase activation and denaturation at 98 °C for 3 min, 40 cycles of 98 °C for 15 s, annealing at 60 °C for 30 s and elongation at 72 °C for 30 s. A melting curve was established to determine the specificity of the amplification. The amplification efficiency (E) was calculated using the formula: E = 10−1/slope. The relative abundance of the target bacterial and archaeal groups or species was expressed in proportion to the total bacterial 16S rRNA gene and archaeal 16S rRNA gene, respectively. The relative abundance was calculated using the following formula:
Relative abundance = 2−ΔCT.
where CT is cycle threshold, and ΔCT = CT (calibrator) – CT (sample).
2.5. Statistical analyses
In all experiments, the fermentation runs were performed in different periods (weeks) and replicates between runs were the statistical replicates in Exp. 1 and 3. In Exp. 2, continuous fermenters (2 per treatment within each fermentation run) were considered experimental units.
The data from Exp. 1 and 2 (except for protozoa counts of Exp. 1 and NH3 of Exp. 2) were statistically analyzed with a factorial randomized complete block (fermentation run) design:
| Yijk = μ + αi + βj + εijk, |
where yijk, is the experimental data; μ is the overall mean, αi is the random effect (block) of the fermentation trial (i = 1, 3 in Exp. 1 and i = 1, 2 in Exp. 2); βj is the fixed effect of the dietary treatment ( j = 1, 3); and εijk is the random error (k = 1, 2 in Exp. 2).
The protozoa composition of Exp. 1 and 2 and ammonia data of Exp. 2 were analyzed according to a repeated measurement design:
| Yijkl = μ + αi + βj + γk + (βγ)jk + δl + εijkl, |
where y, μ, α, β and ε are as described for the previous model; γk is the fixed effect of sampling time (for protozoa cell count: 4 and 24 h in Exp. 1 and d 1 to 9 in Exp. 2, k = 1, 2 and 1, 9, respectively; for ammonia of Exp. 2: k = 1, 2); and δl is the random effect of the fermentation bottle (l = 1, 6).
Volumes of gas for each fermentation bottle were recorded continuously until 48 h in Exp. 3, and the cumulative gas values at 2 h intervals were fitted with the following exponential model without a lag phase:
| y = B × (1 – exp–kt), |
where y is the cumulative gas volume (mL) produced at time t (h); B is the asymptotic gas volume (mL) and k is a constant rate (mL/h).
The asymptotic gas volume and the constant rate of gas production of Exp. 3 were statistically analyzed with a factorial randomized complete block (fermentation run) design:
| Yij = μ + αi + βj + εij |
where yij is the experimental data; μ is the overall mean; αi is the random effect (block) of the fermentation trial (i = 1, 3); βj is the fixed effect of the dose of each extract (j = 1, 8); and εij is the random error.
The ammonia and protozoal counts measured in fermentation fluids in Exp. 3 were regressed on increasing doses of CWT and SB according to the following second-order polynomial mixed model:
| Yij = β0 + β1xij + β2x2ij + si + eij, |
where β0 is the overall intercept across fermentation runs (fixed effect); β1 and β2 are the overall regression coefficients for the linear and quadratic effect of x (fixed effects) across fermentation runs; xij is the dependent variable for the ith extract doses of the jth fermentation run (i = 1, nj; j = 1, 8); si is the random effect of fermentation run i, approximately normal (0, σ2s); and eij is the residual error, approximately normal (0, σ2e). Adjusted values for the fermentation run effect, calculated according to St-Pierre (2001), were used to generate 2 dimensional graphs.
For all statistical analyses, the probability significance levels (P) were 0.05 and 0.01 (P < 0.05 and <0.01, respectively).
3. Results
3.1. Experiment one
The addition of CWT or SB to corn meal had no effect on total gas production and total VFA and ammonia was decreased by CWT (P < 0.05, Table 1). The protozoa count (Table 2) was significantly affected by treatment (P < 0.05) with the lowest value be observed with SB in comparison with that of CWT and CTR and there was a significant reduction from 4 to 24 h (P < 0.05). The dietary treatment did not modify the proportion of protozoan subfamilies, except for a reduction (P < 0.05) of Ophryscolecinae due to SB addition compared with CWT. Finally, Ophryscolecinae increased their relative abundance from 4 to 24 h (P < 0.01).
Table 1.
Effect of addition of chestnut tannin (CWT) or Stevia rebaudiana Bertoni (SB) extracts to a control (CTR) substrate (corn meal) on the pH, ammonia and VFA of fermentation fluid and gas yield measured at 24 h (Exp. 1).
| Item | Treatment |
SEM | ||
|---|---|---|---|---|
| CTR | CWT | SB | ||
| 24-h gas yield, mL | 1,621 | 1,584 | 1,648 | 23.9 |
| pH | 7.26 | 7.25 | 7.32 | 0.027 |
| Ammonia, mg/dL | 27.9a | 24.8b | 27.5a | 0.58 |
| Total VFA, mmol/L | 115.4 | 117.7 | 121.4 | 1.49 |
| VFA, mol/100 mL | ||||
| Acetate | 55.46 | 54.87 | 54.75 | 0.299 |
| Propionate | 26.16 | 27.05 | 27.55 | 0.427 |
| Iso-butyrate | 1.34 | 1.24 | 1.26 | 0.138 |
| Butyrate | 12.98 | 12.89 | 12.46 | 0.259 |
| Iso-valerate | 2.23 | 2.08 | 2.34 | 0.055 |
| Valerate | 1.85 | 1.88 | 1.64 | 0.240 |
| Acetate:Propionate ratio | 2.17 | 2.06 | 2.01 | 0.049 |
SEM = standard error of the means.
a,b Within rows, means without a common superscript differ (P < 0.05).
Table 2.
Effect of addition of chestnut tannin (CWT) or Stevia rebaudiana Bertoni (SB) extracts to a control (CTR) substrate (corn meal) on protozoa population in the fermentation fluid measured at 4 and 24 h (Exp. 1).
| Item | Treatment1 |
SEM | Sampling time, h |
SEM | |||
|---|---|---|---|---|---|---|---|
| CTR | CWT | SB | 4 | 24 | |||
| Protozoa, × 103 cells/mL | 479a | 316b | 255b | 56.6 | 442a | 258b | 46.2 |
| Protozoan subfamilies, % of total protozoa | |||||||
| Entodiniinae | 68.4 | 64.6 | 71.0 | 2.25 | 68.5 | 67.4 | 1.84 |
| Diplodiniinae | 16.0 | 17.3 | 16.3 | 1.52 | 18.0 | 15.0 | 1.24 |
| Ophryoscolecinae | 11.5ab | 13.7a | 8.5b | 1.08 | 8.4B | 14.1A | 0.88 |
| Isotricha | 2.2 | 2.0 | 1.6 | 0.43 | 2.1 | 1.8 | 0.35 |
| Dasytricha | 1.9 | 2.4 | 2.6 | 0.70 | 3.0 | 1.7 | 0.57 |
SEM = standard error of the means.
a, bWithin rows, means without a common superscript differ (P < 0.05).
A, BWithin rows, means without a common superscript differ (P < 0.01).
Interaction “treatment × sampling time” not significant.
3.2. Experiment two
The average ammonia concentration of fermented liquid in the rumen continuous culture (CC) fermenter was lower with CWT addition than with the other treatments (P < 0.01, Table 3). Dietary treatments did not modify the total VFA yield and the CWT inclusion decreased the proportions of iso-butyrate and iso-valerate (P < 0.01) and increased the valerate proportion (P < 0.01) compared with the other treatments. The following rumen microbiota changes were detected: Prevotella ruminicola and Selenomonas ruminantium were increased by the CWT treatment whereas Ruminobacter amylophilus decreased (P < 0.05, P < 0.01 and P < 0.01, respectively). SB positively affected the relative abundance of Treponema saccarophylum (P < 0.01) compared with CTR and CWT.
Table 3.
Effect of addition of chestnut tannin (CWT) or Stevia rebaudiana Bertoni (SB) extracts to a control (CTR) substrate (dry total mixed ration) on the pH, ammonia, VFA and bacterial composition of fermentation fluid in a continuous rumen fermenter (Exp. 2).
| Item | Treatment |
SEM | ||
|---|---|---|---|---|
| CTR | CWT | SB | ||
| pH | 6.05 | 6.03 | 6.10 | 0.065 |
| Ammonia, mg/dL1 | 20.3A | 10.5B | 19.2A | 1.08 |
| Total VFA, mmol/L | 47.3 | 46.1 | 48.5 | 3.68 |
| VFA, mol/100 mL | ||||
| Acetate | 50.3 | 44.9 | 47.2 | 1.55 |
| Propionate | 30.2 | 33.0 | 32.3 | 2.24 |
| Iso-butyrate | 0.63A | 0.25B | 0.50A | 0.071 |
| Butyrate | 9.01 | 10.3 | 9.65 | 0.682 |
| Iso-valerate | 4.11A | 3.48B | 3.93A | 0.123 |
| Valerate | 5.78B | 8.09A | 6.45B | 0.403 |
| Acetate:Propionate ratio | 1.71 | 1.42 | 1.48 | 0.175 |
| Relative abundance, % of total bacteria | ||||
| Genus Prevotella | 35.8 | 41.8 | 40.8 | 2.91 |
| Prevotella ruminicola | 4.66b | 5.28a | 4.39b | 0.928 |
| Fibrobacter succinogenes | 0.928 | 1.02 | 0.520 | 0.2090 |
| Lactobacillus | 0.145 | 0.103 | 0.075 | 0.0240 |
| Ruminococcus albus | 0.085 | 0.075 | 0.035 | 0.0286 |
| Megasphaera elsdenii | 0.318 | 0.410 | 0.418 | 0.1333 |
| Selenomonas ruminantium | 0.280B | 0.390A | 0.243B | 0.0151 |
| Ruminobacter amylophilus | 2.19A | 0.08B | 2.09A | 0.274 |
| Treponema saccarophylum | 0.618B | 0.552B | 1.943A | 0.2816 |
| Relative abundance, % of Archea | ||||
| Methanobrevibacter spp. | 79.2 | 85.4 | 82.1 | 4.20 |
| Methanosphaera spp. | 1.75 | 2.42 | 1.81 | 0.281 |
SEM = standard error of the means.
A−C Within rows, means without a common superscript differ (P < 0.01).
a–c Within rows, means without a common superscript differ (P < 0.05).
Samples for ammonia analysis were collected just before substrate addition to fermenter in the morning and 3 h after. The data were analyzed considering time effect (not significant) and a repeated measure model (see material and method section).
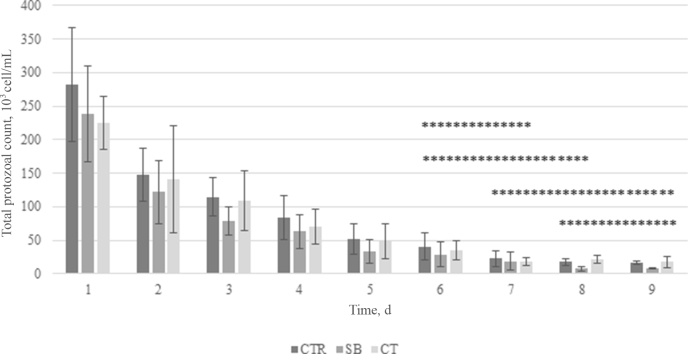
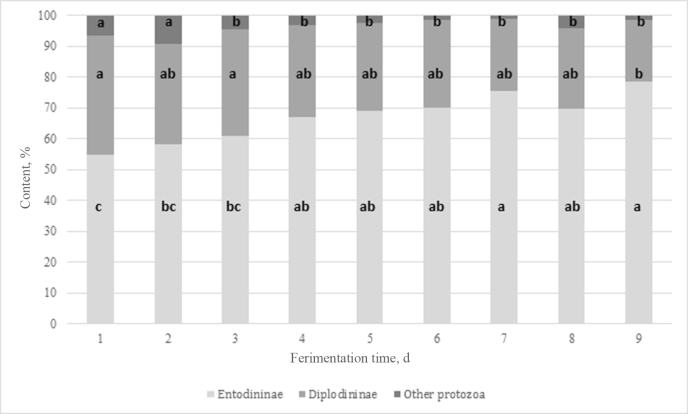
For protozoa counts the interaction between the sampling day (time) and the dietary treatment was not significant (Fig. 1). Average dietary treatment means of CTR and CWT (86.3 × 103 and 76.2 × 103 cell/mL, respectively) differed (P < 0.05) from the mean of SB (66.7 × 103 cell/mL). Protozoa counts decreased progressively (P < 0.05) with the day of sampling during the first 4 d, and in the following days (from 5 to 9 d) the decrease was less intense (Fig. 1). The percentage of Entodinium among the protozoal counts (Fig. 2) increased during the 9 d of sampling and the proportion measured on d 1 was lower (P < 0.05) than that on d 9 (45% vs. 78% of total). In contrast, the relative abundance of Diplodiniinae decreased during this period, with an average lowest value on d 9 (P < 0.05).
Fig. 1.
Effect of addition of Chestnut tannin (CWT) or Stevia rebaudiana Bertoni (SB) extracts to a control (CTR) substrate (dry total mixed ration) on total protozoal counts during the days of fermentation in a continuous rumen fermenter (Exp. 2). (Treatment means of CTR and CT (86.3 × 103 and 76.2 × 103 cell/mL, respectively) differ (P < 0.05) from SB mean (66.7 × 103 cell/mL, standard error of the treatment means is 4.65 × 103 cell/mL); interaction effect of fermentation day × treatment was not significant; means of days under the horizontal asterisk lines are not significantly different; standard error of the fermentation day means: 8.06 × 103 cell/mL).
Fig. 2.
Percentage of Entodiniinae, Diplodiniinae and remaining other protozoa on total counts over dietary treatments during the days of fermentation in a continuous rumen fermenter (experiment 2). (different letters in the bars denote difference between day averages (P < 0.05); the effects of dietary treatment and interaction fermentation day × dietary treatment were not significant; standard error of the fermentation day means is 3.12%, 3.15% and 0.95% for the percentage of Entodiniinae, Diplodiniinae and remaining other protozoa, respectively).
3.3. Experiment three
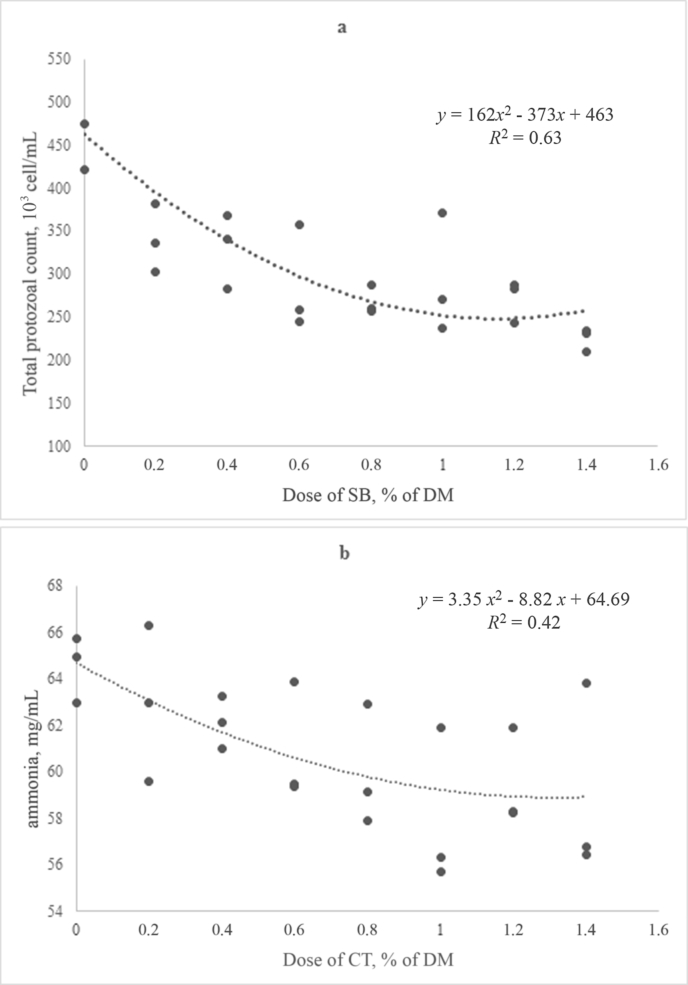
The simple exponential model used to interpolate the gas yields demonstrated satisfactory fitting properties to the experimental data with an average residual mean square errors of ±8.7 and ±6.8 mL, respectively for CWT and SB datasets. The calculated kinetic values from the fitting production curve as a result of increasing the dose of CWT and SB are shown in Table 4. The maximum cumulative volume of gas production did not differ based on the doses for either additive, and the rate of gas production decreased significantly with increasing dose of CWT (P < 0.05). Protozoa counts and ammonia concentrations decreased according to a curvilinear trend (P < 0.05) with increasing doses of CWT and SB, respectively (Fig. 3A and B).
Table 4.
Effect of addition of increasing doses of chestnut tannin (CWT) or Stevia rebaudiana Bertoni (SB) extracts to a control (CTR) substrate (dry total mixed ration) on kinetic parameters (maximum volume and rate of gas production, V (mL) and k (%/h) measured for 48 h (Exp. 3).
| Item | Treatment |
|||
|---|---|---|---|---|
| CWT |
SB |
|||
| V | k | V | k | |
| Dose, % of DM | ||||
| 0 | 1640 | 9.07a | 1522 | 8.62 |
| 0.2 | 1579 | 8.95a | 1494 | 8.45 |
| 0.4 | 1619 | 8.93a | 1518 | 8.58 |
| 0.6 | 1594 | 8.87ab | 1517 | 8.55 |
| 0.8 | 1551 | 8.86ab | 1474 | 8.71 |
| 1.0 | 1551 | 8.74ab | 1497 | 8.89 |
| 1.2 | 1676 | 8.71ab | 1504 | 8.68 |
| 1.4 | 1629 | 8.52b | 1539 | 8.41 |
| Significance | ns | <0.05 | ns | ns |
| SEM | 26.2 | 0.087 | 30.5 | 0.135 |
SEM = standard error of the means; ns = not significant.
a–c Within columns, means without a common superscript differ (P < 0.05).
Fig. 3.
Polynomial (second-order) regression between total protozoal count and increasing doses of stevia extract (SB, A), or between ammonia and increasing doses of chestnut tannin (CWT, B), measured after 24 h of fermentation and adjusted for the fermentation run effect (Exp. 3).
4. Discussion
In all the experiments of this research we used as inoculum the rumen fluid collected from cows immediately after slaughter. The collection at slaughter is one of the methods of sampling rumen fluid for microbiota studies (Rumen Microbial Genomics Network, 2019) and is an accepted alternative to sampling through rumen cannula (Yáñez-Ruiz et al., 2016). In the present experiments possible differences between rumen fluids collected in different sampling sessions were attenuated by selecting the animal donors (culled dairy cows in good health and fed productive total mixed rations based on corn silage) and by mixing individual rumen fluid of 4 cows for each sampling.
The in vitro tests in the present research utilized a rumen CC fermenter designed and tested in our department (Mason et al., 2015) and a batch culture system suitable to evaluate fermentation in terms of continuous gas yield. An initial experiment (Exp. 1) utilized a highly fermentable substrate (e.g. corn meal) to create the conditions for intense bacterial growth (and protein degradation) and to stimulate maximal protozoal growth. In such an environment, we hoped to more effectively demonstrate any effect of the additives (CWT and SB) than in less favorable fermentation conditions. Given the quite clear results obtained in Exp. 1, we moved to the CC trial with the objective of evaluating any possible adaptation of the microbiota to the additives and to replicate the effects with a more fibrous substrate, such as a total mixed ration. Finally, in the last experiment, lower doses than those used in previous experiments were studied for each extract in dose–response tests.
4.1. Effects of stevia extract and chestnut tannins on rumen fermentation intensity
The extracts studied in this research did not affect the fermentation intensity measured in terms of gas yield (Exp. 1 and 3) and VFA yield (Exp. 1 and 2), if we exclude a modest reduction in the rate of gas production at the highest CWT concentration in Exp. 3 (e.g., 1.4% dietary addition). While there is no comparable information in the literature for SB, tannins are known to exert negative effects on fermentation, although with variable effects according to the family (condensed or hydrolysable), plant origin (Patra and Saxena, 2011) and molecular structure (Mueller-Harvey et al., 2019). However, Hassanat and Benchaar (2013) examined increasing doses of CWT in vitro and obtained a reduction in gas and VFA yield only with additions (e.g. 5%) much higher than the dose levels used in the present trial. Low CWT doses (0.15% and 1.5%) were tested in vivo on growing young steers (Aboagye et al., 2018) and did not modify growth performance or rumen VFA concentrations.
4.2. Effect of chestnut tannins on rumen fermentation and microbiota
The in vitro results showed that the addition of CWT reduced the yield of ammonia. This result confirms the impact of CWT on the overall reduction of proteolytic activity in the rumen, which is well known to be the result of the formation of a reversible complex with proteins and/or inhibition of the enzymatic activity of rumen protease (Patra and Saxena, 2011). The effect was more intense in the CC experiment than in the previous batch fermentation, which could be due to the higher CP content of the substrate used in that experiment. The decrease in rumen ammonia as a consequence of to the CWT addition was associated with an increase of concentration of noncellulolytic bacteria, such as P. ruminicola and S. ruminantium, which are known to use ammonia for amino acid synthesis (Atasoglu et al., 1998), and the concentration of Ruminococus amilophylus was reduced. We were not able to detect a variation in the number of methanogens due to CWT addition whereas Witzig et al. (2018) recently measured such a reduction following the addition of a high dose (approximated 10%) of CWT.
In both experiments the only significant modification of the VFA profile of the fermentation fluids induced by the CWT extract regarded iso-valerate, which was present at levels lower than that in control diet. The iso-valerate comes from the degradation of leucine and its reduction due to CWT addition is a well-known effect, demonstrated by the meta-analysis of Jayanegara et al. (2012) and in vivo by the work of Liu et al. (2011) and recently by Aboagye et al. (2018). The effect of CWT on the protozoa population was different between trials: in the first experiment, there was a reduction in protozoa counts in comparison with the control substrate; in the second experiment this was not confirmed. The effect of CWT on the protozoa population is quite controversial in the literature (Patra and Saxena, 2011): Liu et al. (2011) registered a reduction with CWT inclusions of 1% and 3% whereas Aboagye et al. (2018) did not find reductions with CWT doses of 0.15% and 1.5%. It is known that protozoa growth is favored by diets rich in concentrates (Franzolin and Dehority, 1996, Hook et al., 2011). Therefore, it could be speculated that a depressive effect of CWT on protozoa was detected in vitro only in the first experiment, where a starchy substrate was used and not in those case in which a fibrous substrate was used (Exp. 2).
4.3. Effect of stevia extract on rumen microbiota
In contrast with CWT, the SB extract lowered the protozoa counts significantly, and this effect was reproduced in all 3 experiments. In the batch fermentation system (Exp. 1), we measured a reduction in 47% of protozoa (the average between the 4 and 24 h reductions), which is comparable with that reported by Ramos-Morales et al. (2017) after 24 h of incubation (−56%). In the short sampling interval of Exp. 1 (4 and 24 h) the Ophryoscolecinae increased their relative abundance, probably because these organisms have a long generation time (up to 3 d, Sylvester et al., 2009) and could survive more than faster reproducing protozoa strains.
During fermentation in the second experiment, we observed a progressive reduction in the protozoa populations, which are known to be very sensitive to in vitro conditions (Cabeza-Luna et al., 2018, Muetzel et al., 2009). The protozoa decline was not attenuated by SB addition and therefore, from the second experiment, we conclude that protozoa were not able to adapt to the SB dietary addition with respect to the control diet. However, the decrease of protozoa induced by SB was lower (approximated −20%, −30%) than that observed in the first experiment and this was probably due to the different conditions of the CC system, which utilizes a continuous flow of liquids and a wash out of the additives.
Additionally, in the third experiment, SB reduced the protozoa counts in decrements proportional to the dose added and Ramos-Morales et al. (2017) speculated that the reduction in the protozoa number was determined by the iminosugar content of SB, even if other unknown phytochemicals may contributing to the effect. Protozoa decrement is generally considered positive in terms of reducing rumen polluting compounds (e.g. ammonia and methane, Newbold et al., 2015), but despite the decrease in the protozoa number, no effects on rumen fermentation parameters were observed in present work. In contrast with the results of Ramos-Morales et al. (2017), SB addition did not result in any reduction in ammonia or any appreciable variation in the patterns of fermentation (total yield and composition of VFA). Moreover, despite the antimicrobial activity claimed for SB extracts (Munro et al., 2000, Wang et al., 2014), we did not observe variation in the relative abundance of bacteria strains, with the only exception of the tripled concentration of T. saccarophylum. Therefore, the unaffected composition of the Archaea community and the VFA profile would suggest a nonsignificant variation in methane production, but the gas measure is requested to confirm this indirect suggestion.
5. Conclusion
Both natural substances considered in the present paper had an impact on rumen metabolism, but in different ways. For the CWT, the lowering rumen ammonia effect was confirmed, however, the assumed action of CWT against rumen protozoa was less clear from the present results. The SB extract had a relevant negative effect on the rumen protozoa population and no appreciable effects on rumen ammonia levels. While the implication that CWT reduce pollution is clear, for SB extract, it will be necessary to better identify the role of protozoa in contributing to the increase in rumen of ammonia and/or methane. However, for both extracts, their effects on the rumen were appreciable at low dietary doses, and the negative impacts on fermentation were limited to the reduction in protein degradation with the addition of CWT.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2019.11.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aboagye I.A., Oba M., Castillo A.R., Koenig K.M., Iwaasa A.D., Beauchemin K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J Anim Sci. 2018;96:5276–5286. doi: 10.1093/jas/sky352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 17th ed. Association of Official Analytical Chemists; 2000. Official methods of analysis of AOAC international. [Google Scholar]
- Atasoglu C., Valdés C., Walker N.D., Newbold C.J., Wallace R.J. De novo synthesis of amino acids by the ruminal bacteria Prevotella bryantii B14, Selenomonas ruminantium HD4, and Streptococcus bovis ES1. Appl Environ Microbiol. 1998;64:2836–2843. doi: 10.1128/aem.64.8.2836-2843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümmel M., Becker K. The degradability characteristics of fifty- four roughages and roughage neutral detergent fiber as described by in vitro gas production and their relationship to voluntary feed intake. Br J Nutr. 1997;77:757–786. doi: 10.1079/bjn19970073. [DOI] [PubMed] [Google Scholar]
- Cabeza-Luna I., Carro M.D., Fernández-Yepes J., Molina-Alcaide E. Effects of modifications to retain protozoa in continuous-culture fermenters on ruminal fermentation, microbial populations, and microbial biomass assessed by two different methods. Anim Feed Sci Technol. 2018;240:117–127. [Google Scholar]
- Campo M., Pinelli P., Romani A. Proceedings of the XXVI international conference on polyphenols. 2012. HPLC/DAD/MS characterization and antioxidant activity of sweet chestnut (Castanea sativa Mill.) fractions; pp. 135–136. Florence, Italy. [Google Scholar]
- Dehority B.A. Nottingham University Press; Nottingham: 2003. Rumen microbiology. [Google Scholar]
- Franzolin R., Dehority B.A. Effect of prolonged high concentrate feeding on ruminal protozoa concentrations. J Anim Sci. 1996;74:2803–2809. doi: 10.2527/1996.74112803x. [DOI] [PubMed] [Google Scholar]
- Hassanat F., Benchaar C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J Sci Food Agric. 2013;93:332–339. doi: 10.1002/jsfa.5763. [DOI] [PubMed] [Google Scholar]
- Hook S.E., Steele M.A., Northwood K.S., Wright A.D.G., McBride B.W. Impact of high-concentrate feeding and low ruminal pH on methanogens and Protozoa in the rumen of dairy cows. Microb Ecol. 2011;62:94–105. doi: 10.1007/s00248-011-9881-0. [DOI] [PubMed] [Google Scholar]
- Jayanegara A., Leiber F., Kreuzer M. Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro experiments. J Anim Physiol Anim Nutr. 2012;96:365–375. doi: 10.1111/j.1439-0396.2011.01172.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Vaddella V., Zhou D. Effects of chestnut tannins and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J Dairy Sci. 2011;94:6069–6077. doi: 10.3168/jds.2011-4508. [DOI] [PubMed] [Google Scholar]
- Martillotti F., Puppo S. Liquid chromatographic determination of organic acids in silages and rumen fluids. Ann dell’Istituto Sper Zootec. 1985;18:1–10. [Google Scholar]
- Mason F., Zanfi C., Spanghero M. Testing a stratified continuous rumen fermenter system. Anim Feed Sci Technol. 2015;201:104–109. [Google Scholar]
- McDougall E.I. Studies on ruminant saliva. I: the composition and output of sheep's saliva. Biochem J. 1948;43:99–109. [PMC free article] [PubMed] [Google Scholar]
- Menke K.H., Raab L., Salewski A., Steingass H., Fritz D., Schneider W. The estimation of the digestibility and metabolizable energy content of ruminant feedstuffs from the gas production when they are incubated with rumen liquor. J Agric Sci. 1979;92:499–503. [Google Scholar]
- Mueller-Harvey I Bee, Dohme-Meier G., Hoste F., Karonen H., Kölliker M., Lüscher R., Niderkorn A., Pellikaan V. Benefits of condensed tannins in forage legumes fed to ruminants: importance of structure, concentration, and diet composition. Crop Sci. 2019;59:1–25. [Google Scholar]
- Muetzel S., Lawrence P., Hoffmann E.M., Becker K. Evaluation of a stratified continuous rumen incubation system. Anim Feed Sci Technol. 2009;151:32–43. [Google Scholar]
- Munro P.J., Lirette A., Anderson D.M., Ju H.Y. Effects of a new sweetener, Stevia, on performance of newly weaned pigs. Can J Anim Sci. 2000;80:529–531. [Google Scholar]
- Newbold C.J., de la Fuente G., Belanche A., Ramos-Morales E., McEwan N.R. The role of ciliate Protozoa in the rumen. Front Microbiol. 2015;6:1313. doi: 10.3389/fmicb.2015.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A.K., Aschenbach J.R. Ureases in the gastrointestinal tracts of ruminant and monogastric animals and their implication in urea-N/ammonia metabolism: a review. J Adv Res. 2018;13:39–50. doi: 10.1016/j.jare.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A.K., Saxena J. Exploitation of dietary tannin to improve rumen metabolism and ruminant nutrition. J Sci Food Agric. 2011;91:24–37. doi: 10.1002/jsfa.4152. [DOI] [PubMed] [Google Scholar]
- Ramos-Morales E., de la Fuente G., Nash R.J., Braganca R., Duval S., Bouillon M.E., Lahmann M., Newbold C.J. Improving the antiprotozoal effect of saponins in the rumen by combination with glycosidase inhibiting iminosugars or by modification of their chemical structure. PLoS One. 2017;12:e0184517. doi: 10.1371/journal.pone.0184517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumen Microbial Genomics Network . 2019. A report in support of the Rumen Microbial Genomics (RMG) Network describing standard guidelines and protocols for data acquisition, analysis and storage.http://www.rmgnetwork.org/user/file/37/pdf [Google Scholar]
- St-Pierre N.R. Invited review: integrating quantitative findings from multiple studies using mixed model methodology. J Dairy Sci. 2001;84:741–755. doi: 10.3168/jds.S0022-0302(01)74530-4. [DOI] [PubMed] [Google Scholar]
- Sylvester J.T., Karnati S.K.R., Dehority B.A., Morrison M., Smith G.L., St-Pierre N.R., Firkins J.L. Rumen ciliated protozoa decrease generation time and adjust 18S ribosomal DNA copies to adapt to decreased transfer interval, starvation, and monensin. J Dairy Sci. 2009;92:256–269. doi: 10.3168/jds.2008-1417. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang L.S., Shi Z., Shi B.M., Shan A.S. Effects of dietary stevioside/rebaudioside A on the growth performance and diarrhea incidence of weaned piglets. Anim Feed Sci Technol. 2014;187:104–109. [Google Scholar]
- Witzig M., Zeder M., Rodehutscord M. Effect of the ionophore monensin and tannin extracts supplemented to grass silage on populations of ruminal cellulolytics and methanogens in vitro. Anaerobe. 2018;50:44–54. doi: 10.1016/j.anaerobe.2018.01.012. [DOI] [PubMed] [Google Scholar]
- Yáñez-Ruiz D.R., Bannink A., Dijkstra J., Kebreab E., Morgavi D.P., O'Kiely P., Reynolds C.K., Schwarm A., Shingfield K.J., Yu Z., Hristov A.N. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants - a review. Anim Feed Sci Technol. 2016;216:1–18. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.