Abstract
Some cancer survivors experience marked cognitive impairment, referred to as cancer-related cognitive impairment (CRCI). CRCI has been linked to the genetic factor APOE4, the strongest genetic risk factor for Alzheimer’s disease (AD). We used APOE knock-in mice to test whether the relationship between APOE4 and CRCI can be demonstrated in a mouse model, to identify associations of chemotherapy with behavioural and structural correlates of cognition, and to test whether chemotherapy affects markers of AD. Twelve-month old C57BL/6J female APOE3 (n=30) and APOE4 (n=31) knock-in mice were randomized to treatment with either doxorubicin (10 mg/kg) or saline. Behavioural assays at 2–21 weeks-post exposure included open field maze, elevated zero maze, pre-pulse inhibition, Barnes maze, and fear conditioning. Ex-vivo magnetic resonance imaging was used to determine regional volume differences at 31–35 weeks-post exposure, and tissue sections were analyzed for markers of AD pathogenesis. Minimal toxicities were observed in the aged mice after doxorubicin exposure. In the Barnes maze assay, APOE3 mice did not exhibit impairment in spatial learning after doxorubicin treatment, but APOE4 mice demonstrated significant impairments in both the initial identification of the escape hole and the latency to full escape at 6 weeks post-exposure. Both APOE3 and APOE4 mice treated with doxorubicin showed impairment of spatial memory. Grey matter volume in the frontal cortex decreased in APOE4 mice treated with doxorubicin vs. APOE3 mice. This study demonstrates cognitive impairments in aged APOE4 knock-in mice after doxorubicin treatment and establishes this system as a novel and powerful model of CRCI.
Keywords: cancer, chemotherapy, Apolipoprotein E, Alzheimer’s Disease, cognitive impairment, mouse behaviour
1. Introduction
Cognitive impairment has been reported by 13–70% of cancer survivors, greatly affecting their quality of life [1, 2]. Cancer-related cognitive impairment (CRCI) occurs in several domains, including processing speed, visual memory, verbal memory, and executive function, and can persist over years [3, 4]. The etiology of CRCI is unknown, but may be influenced by age, treatment type, cognitive reserve, and genetic factors [5].
The apolipoprotein E (APOE) gene exists in humans in three common alleles (APOE2, APOE3, and APOE4). APOE4 constitutes the strongest genetic risk factor for late-onset Alzheimer’s Disease (AD) [6], with an earlier effect on AD risk in females [7]. Interestingly, the APOE4 allele is also the most reproducible genetic risk factor for CRCI, supported in five independent studies of cancer survivors [8–11]. Aging is a risk factor for cognitive decline in many contexts (such as AD); older age also contributes to cognitive declines after chemotherapy in breast cancer survivors [5]. In the largest study of cognitive impairment in older breast cancer survivors, APOE4-positive survivors who received chemotherapy exhibited greater CRCI over two years post treatment than survivors with other APOE alleles [8].
APOE targeted-replacement mice [12] are a preclinical model for investigating CRCI in a genetically susceptible context [13]. In the APOE targeted replacement, or knock-in mice, the human APOE3 or APOE4 alleles are under the control of the endogenous murine APOE promoter, generating mice homozygous for APOE3 or APOE4 [12]. In these mice, APOE protein levels are similar to human APOE levels in hippocampus, frontal cortex, and cerebellum [14]. Importantly, while APOE targeted-replacement mice do not develop overt AD brain pathology, the APOE4 mice do show distinct cognitive and brain structure differences compared to APOE3 mice [13, 15–17].
In the current study, we use aged female APOE mice to recapitulate the older breast cancer survivor population, in order to understand how APOE genotype affects cognition and non-cognitive behaviours in the context of female sex and aging. The earlier experiments in this model found deficiencies in spatial learning and memory in five-month old female APOE4 mice exposed to doxorubicin [13]; the current work examines more clinically relevant aged mice and tests other cognitive domains. This mouse model also allows testing of the mechanisms underlying this complex genetic-environmental interaction, particularly whether it is related to AD pathological changes commonly associated with APOE genotype.
2. Materials and Methods
2.1. Ethics Statement
This study was conducted in accordance with ethical standards and relevant national and international guidelines for animal welfare, including the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures and handling of the animals were performed according to protocols approved by the Georgetown University Medical Center Institutional Animal Care and Use Committee.
2.2. Animal Population
The human APOE3 (n = 30) and APOE4 (n = 31) targeted-replacement C57BL/6J mice, which have either the human APOE3 or APOE4 allele in the mouse APOE gene locus thereby maintaining the normal expression of the APOE gene, were bred and maintained at Georgetown University as previously described [13]. All mice were bred as homozygous for their respective alleles and the APOE genotype was confirmed by DNA sequencing. Female mice were used for the study and all mice were housed under the same conditions and entered into the experiment at 12–13 months of age. APOE3 mice were chosen as appropriate controls for APOE4 mice as both mice have the same targeted replacement of the murine APOE gene, allowing comparison between behaviour of the human alleles in a mouse model. Research staff were not blinded to APOE genotype of mice in this study.
2.3. Chemotherapeutic drug exposure
Doxorubicin hydrochloride (Sigma) was prepared as a 10 mM solution in ultrapure DMSO and frozen at 4°C for a maximum of one month before use. Animals were treated with two intra-peritoneal (IP) injections of either 5 mg/kg doxorubicin (total dose 10 mg/kg) or DMSO vehicle, given in in 470 uL sterile PBS, spaced one week apart. There were a total of four groups: APOE3 saline control, APOE3 doxorubicin-treated, APOE4 saline control, and APOE4 doxorubicin-treated mice (n = 15–16/group). The total dose and dosing schedule were chosen based on similar in vivo experiments [18–20]. Our experiments verified that this dose produced limited whole-body toxicity (≤ 10% total body weight loss followed by full weight recovery) and limited temporary reductions in locomotive behaviour (data not shown). Mice were weighed prior to the initial injection and weekly thereafter.
2.4. Behavioural Assessments
Mice were behaviourally tested using a series of assessments. For each procedure, each mouse was habituated to the testing room for 30 minutes in its home cage and, when appropriate, allowed to explore the apparatus freely for five minutes. Each apparatus was thoroughly cleaned and wiped down with 70% ethanol prior to each test to eliminate the potential influence of olfactory cues. Procedures for video recording and software or blinded analyses of behaviours are noted for these tasks.
2.4.1. Open Field Task
The Open Field task was used as an assessment of locomotion, anxiety, and exploratory behaviour. The open field apparatus was a plain plexiglass field of 43 cm square. Laser detectors tracked mouse movement and location on the apparatus and data was directly collected by MedAssociates activity monitor. The outermost 8 cm of the field were considered the “outer zone” and the remaining area considered the “inner zone”. Time spent in the inner zone was used as a measure of anxiety. Total distance traveled was used to assess locomotion. Mice were assessed one week prior to initial doxorubicin or saline injection, three weeks following completion of treatment, and fifteen weeks following completion of treatment.
2.4.2. Elevated Zero Maze.
The Elevated Zero Maze consists of a ring-shaped platform 600 mm in diameter, with two sections protected by walls 6” high (“closed zone”) and two sections lacking walls (“open zone”). Mice were placed at the edge of a closed zone and movement recorded by overhead camcorder and analyzed using the AnyMaze software suite. Percent time spent in the open zone was used as a measure of exploratory behaviour and as a negative measure of anxiety. Mice were assessed two weeks prior to initial doxorubicin or saline injection, two weeks following completion of treatment, and fifteen weeks following completion of treatment.
2.4.3. Pre-Pulse Inhibition (PPI).
PPI was used to assess sensorimotor gating at one week prior to initial treatment and three weeks after treatment was completed. Animals were exposed to consistent background noise (70 dB) alone for five minutes; the background noise was continuous throughout the session. Afterwards, five startle-inducing pulses (30 ms) of 120 dB broadband noise generated stable baseline startle responses. Mice were exposed to trials either of the pulse alone or the pulse preceded by a 30 ms “pre-pulse” of noise 3, 6, 9 and 12 dB above background. In the prepulse+pulse trials, the onset of the prepulse and the onset of the pulse were separated by 130 ms. Animals were tested on a total of 50 trials (10 Pulse-Alone trials, 10 of each of the prepulse trials) in a pseudorandom order of series of pulses with or without pre-pulses of 3, 6, 9, or 12 dB above background white noise (PP3-PP12). An average of 15 seconds (range 5–25 seconds) separated the trials. The testing parameters employed produce robust PPI [21, 22].
2.4.4. Barnes Maze.
The Barnes maze (San Diego Instruments Inc., San Diego, CA) was used to assess spatial learning and memory six weeks following the second doxorubicin or saline injection [23]. The maze was in a space containing extra-maze cues, illumination of 150 lux and 75 dB white noise; it had 19 shallow decoy containers and one escape hole around the edge of the disk. For each trial, the mouse was allowed to explore the maze freely for 180 seconds or until entering the escape hole. If the mouse did not attain the escape hole, the experimenter gently guided it there. The apparatus was captured by overhead video and analyzed using AnyMaze software. One day prior to the start of training, a habituation task consisted of a single trial with the escape hole placed in a location that would not be used for the remainder of the protocol. On the day following habituation, the training protocol was performed with the escape hole in the target location, which remained constant during the full training protocol. On each of four consecutive training days, the mice were put through four trials separated by an interval of 15 minutes. Seventy-two hours following the final training trial, each mouse underwent a single probe trial. Three APOE3 mice (two control, one doxorubicin-treated) were excluded from Barnes Maze testing due to barbering between cage-mates and related stress behaviour observed in the home cage within a week prior to scheduled testing on the apparatus. One APOE3 control mouse was excluded from Barnes Maze testing due to a cataract observed prior to study start.
2.4.5. Fear Conditioning.
Fear conditioning was used to assess amygdala-related learning and memory. The conditioning chamber was 12” by 9” in the presence of 60 dB of white noise. Video footage was captured using an LG Phoenix 3 smartphone running Android software using the default camera application. After habituation, each mouse was exposed to a 4000 kHz cue tone of 78 dB for 30 seconds. Coinciding with the end of the tone, the mouse received a foot shock of 0.7 mA for one second through the floor grid (“delayed conditioning”). After 120 seconds, the mouse was returned to its home cage. The shock conditioning protocol was repeated four hours later. The following day, each mouse was placed in a novel black foam chamber of 10” by 6” in a different room. After 180 seconds, the cue tone was played for 30 seconds without the conditioning shock. After 120 more seconds, the mouse was removed to its home cage. Four hours after the cued fear response test, each mouse was placed in the conditioning chamber for five minutes as the context fear response. Videos were reviewed by a blinded observer who noted duration of freezing behaviour throughout. Percent time freezing was defined as no motion of the mouse outside of breathing.
2.5. Tissue Collection and Immunohistochemistry
At 31 to 35 weeks following treatment (20–21 months of age), the mice were euthanized by CO2 inhalation. Any variation in time of euthanasia was due to choosing an early euthanasia time for some mice due to spontaneous age-related mortality observed among some of the mice (in a manner unrelated to APOE genotype or doxorubicin treatment); tissue was only used from mice that were healthy at the time of euthanasia. Blood was collected via cardiac puncture and centrifuged at 4°C at 2000g for 15 minutes to isolate plasma, which was stored at −80°C. The mice were perfused with 5–10 mL of either ice-cold 10% neutral buffered formalin or ice-cold PBS, depending on the intended tissue usage. For formalin-perfused mice, the organ tissue (brain, heart, lung, liver, kidney, and spleen and pancreas) was placed in 10% neutral buffered formalin. For PBS-perfused mice, heart, liver, kidney, and spleen and pancreas were collected and snap-frozen. PBS-perfused brains were hemisected, with one hemisphere dissected into cortex, cerebellum, and hippocampus, and snap-frozen; the other hemisphere was placed in a 4% formalin/10% sucrose solution. Fixed brain hemispheres were transferred through a sucrose gradient, then washed and soaked in PBS and 70% ethanol prior to paraffin-embedding and slicing. Five-micron thick slices were processed for immunohistochemistry and stained with antibodies against Iba-1 (Ionized calcium binding adaptor molecule 1, Wako) for microglia, GFAP (Cell Signaling Technology) for astrocytes, AT8 (Phospho-Tau: Ser199, Ser202) (Invitrogen), Aβ42 (Millipore), and activated caspase 3 (Biocare), and imaged via brightfield microscopy.
2.6. Magnetic Resonance Imaging
Formalin perfused whole brains were harvested and embedded in 3% agar in PBS for ex vivo MRI. High-resolution three-dimensional anatomical imaging was performed in order to analyze the differences in regional brain anatomy using voxel-based morphometry (VBM), as described previously [13]. Briefly, MRI was performed on a 7-T Bruker spectrometer (Bruker Biospin, Ettlingen, Germany) run by Paravision 5.1 software, with a 32-mm mouse brain volume coil. The imaging sequence was a 3-D T2-weighted rapid acquisition with relaxation enhancement (RARE) with the following parameters: repetition time = 2500 ms, effective echo time = 36 ms, RARE factor = 12, flip angle = 90 degrees, field of view = 17.5 × 17.5 × 20 mm, matrix size = 190 × 190 × 170, and spatial resolution of 92 × 92 × 118 mm.
To perform voxel-based morphometry, we extracted the raw Bruker 2dseq image datasets as DICOM files, and converted them to NIfTI (Neuroimaging Informatics Technology Initiative (NIH)) format files using MRIcron software. Images were then skull-stripped using MIPAV 7.1.1 (Medical Image Processing, Analysis, and Visualization) software and aligned to the SPMMouse mouse brain template (spmmouse.org) via affine transformation with 12 parameters (translations, shears, rotation, and scale factors in three dimensions) using FSL (FMRIB Software Library, Oxford, UK). Based on the unified segmentation algorithm, each image was segmented into gray matter (GM) and white matter using SPMMouse. The warped modulated GM maps were smoothed with a 0.5-mm FWHM (full width at the half maximum) Gaussian kernel. For the ROI-VMB analysis, regions of interest were manually drawn in the areas that had been identified through the whole brain VBM. Specifically, ROIs were generated in the frontal cortex of the gray matter template, followed by VBM as described above.
2.7. Statistical Analysis
All statistical analysis was performed in GraphPadPrism 8. For the Barnes Maze, we used 3-way ANOVA with Tukey’s multiple comparisons test comparing genotype, treatment status, and test day to assess differences in learning over time. For the Open Field and Elevated Zero assessments, we used three-way ANOVA with Tukey’s multiple comparisons test to assess differences between time points followed by two-way ANOVA with Sidak’s multiple comparisons test to assess interactions of time point and genotype. For Fear Conditioning, we used a mixed-effects analysis with Tukey’s multiple comparisons test to evaluate freezing behaviour across tasks. For freezing in different portions of the cue test, we used a three-way ANOVA with Tukey’s multiple comparisons test to assess differences between time points followed by two-way ANOVA with Sidak’s multiple comparisons test to assess genotype effects. For MRI, data were used to test for group-wise differences in the GM maps across the whole brain with SPMMouse toolbox by performing a voxel-by-voxel two-sample t test. Whole brain analysis was performed with a significance level of p < 0.05, uncorrected for multiple comparisons.
3. Results
3.1. Doxorubicin exposure
At the beginning of the experiment (with mice at 12 months of age), the two APOE genotype groups were similar in weight. Doxorubicin caused an average maximum weight loss of 6.5% in APOE3 mice and 5.2% in APOE4 mice, with no significant difference between genotypes. The greatest extent of weight loss was observed at three weeks post-treatment, after which weight recovered in both APOE genotypes.
3.2. Exploration and anxiety
Prior to treatment, APOE3 mice spent 50% more time in the exposed Inner Zone of the Open Field and 50% more time on the Open Zone of the Elevated Zero Maze than APOE4 mice, indicating slightly lower baseline anxiety-like behaviour than APOE4 mice (ANOVA, F(2,110) = 7.041, p = 0.02 and F(2,104) = 8.858, p = 0.004 respectively) (Figure 1). Two weeks post-treatment, control and doxorubicin-treated mice of both APOE genotypes traveled the same distance in the Open Field Assessment and Elevated Zero Maze (ANOVA, F(2,110) = 0.1123, p = 0.05 and F(2,100) = 0.04967, p = 0.08 respectively) (Figure 1). The distance traveled was reduced in all groups post-treatment by 35%. After 15 weeks, control and doxorubicin-treated mice of both APOE genotypes also traveled the same distance in both apparatus (ANOVA, F(1,53) = 0.1292, p = 0.72). Time spent in the exposed zones of the Elevated Zero Maze was reduced at both post-treatment timepoints in all groups.
Figure 1: Doxorubicin treatment does not alter locomotive and anxiety behaviours.

A. Timeline of doxorubicin or control exposure, behavioural assessments, and euthanasia. B-E, Results of Open Field and Elevated Zero behavioural assessments; “pre-treatment” time point two weeks prior to doxorubicin or control exposure, “post 1” two weeks following completion of treatment, “post 2” 15 weeks following completion of treatment. B. Distance traveled in a 5 minute Open Field exploration task. C. Time spent in the Inner Zone of the Open Field. Distance traveled in a 5 minute Elevated Zero exploration task. D. Distance traveled in a 5 minute Elevated Zero exploration task. E. Time spent in the Open Zone of the Elevated Zero apparatus. Three-way ANOVA with Tukey’s multiple comparisons test to assess differences between time points followed by two-way ANOVA with Sidak’s multiple comparisons test to assess interactions of time point and genotype, * p <.05, ** p<.01.
3.3. Sensorimotor gating
For pre-pulse inhibition (PPI), the pre-pulse of 3 dB above background caused about 25% of startle inhibition (Figure 2A) and was chosen for analyses of the effects of APOE genotype and doxorubicin exposure. APOE3 and APOE4 groups did not differ in their degree of PPI with the 3 dB pre-pulse at baseline (t-test, t(59) = 1.647, p = .10) (Figure 2A). Doxorubicin treatment had no significant effect on PPI at any of the post-treatment time points (ANOVA, F(3,54) = 0.5794, p = 0.63) (Figure 2B).
Figure 2: Doxorubicin treatment does not alter Pre-Pulse Inhibition (PPI).

A. Prior to treatment, percent inhibition of startle response to a short tone following a pre-pulse of 3, 6, 9, or 12 decibels (dB) above background noise (PP3 – PP12) compared to startle with no pre-pulse. B. Following treatment, percent inhibition of startle response to a tone following a pre-pulse 3 decibels above background noise compared to startle with no pre-pulse. One way ANOVA with Tukey’s multiple comparisons test **** p<.0001
3.4. Hippocampus-related Spatial Learning and Memory
Treated and untreated APOE3 and APOE4 mice explored the Barnes maze during habituation at approximately the same speed (.046 − .067 meters/second), although the control APOE4 subgroup was significantly faster than doxorubicin-treated APOE3 subgroup (ANOVA, F (3, 53) = 4.495, p = 0.004) (Figure 3A). Control APOE4 mice took longer to escape than control APOE3 mice, particularly on training days 1 (146 vs. 74 seconds mean latency to escape) and 2 (118 vs. 54 seconds) (Figure 3B, ANOVA, F(1,896) = 221.6 p <.0001). Over training days 3 and 4, APOE4 control mice exhibited reduced total latency until Day 4, when total latency became comparable to APOE3 mice (F(1,896) = 6.117, p =0.97). Doxorubicin treatment had no effect on the response of APOE3 mice across the four training days (F(1,896) = 6.117, p > .99). In contrast, APOE4 mice treated with doxorubicin had significantly higher total latency to escape than the three other groups. Compared to APOE4 control mice, APOE4 mice treated with doxorubicin exhibited significantly higher total latency on training days 3 and 4 (mean of 120 vs. 73 seconds, ANOVA, F(1,896) = 6.117, p = .0007 and 106 vs. 51 seconds, p = .0012 respectively). This deficit was also reflected in the fact that APOE4 mice treated with doxorubicin had high rates of failure to full escape in the 180 second task.
Figure 3: Doxorubicin treatment impairs spatial learning in APOE4 but not APOE3 mice.
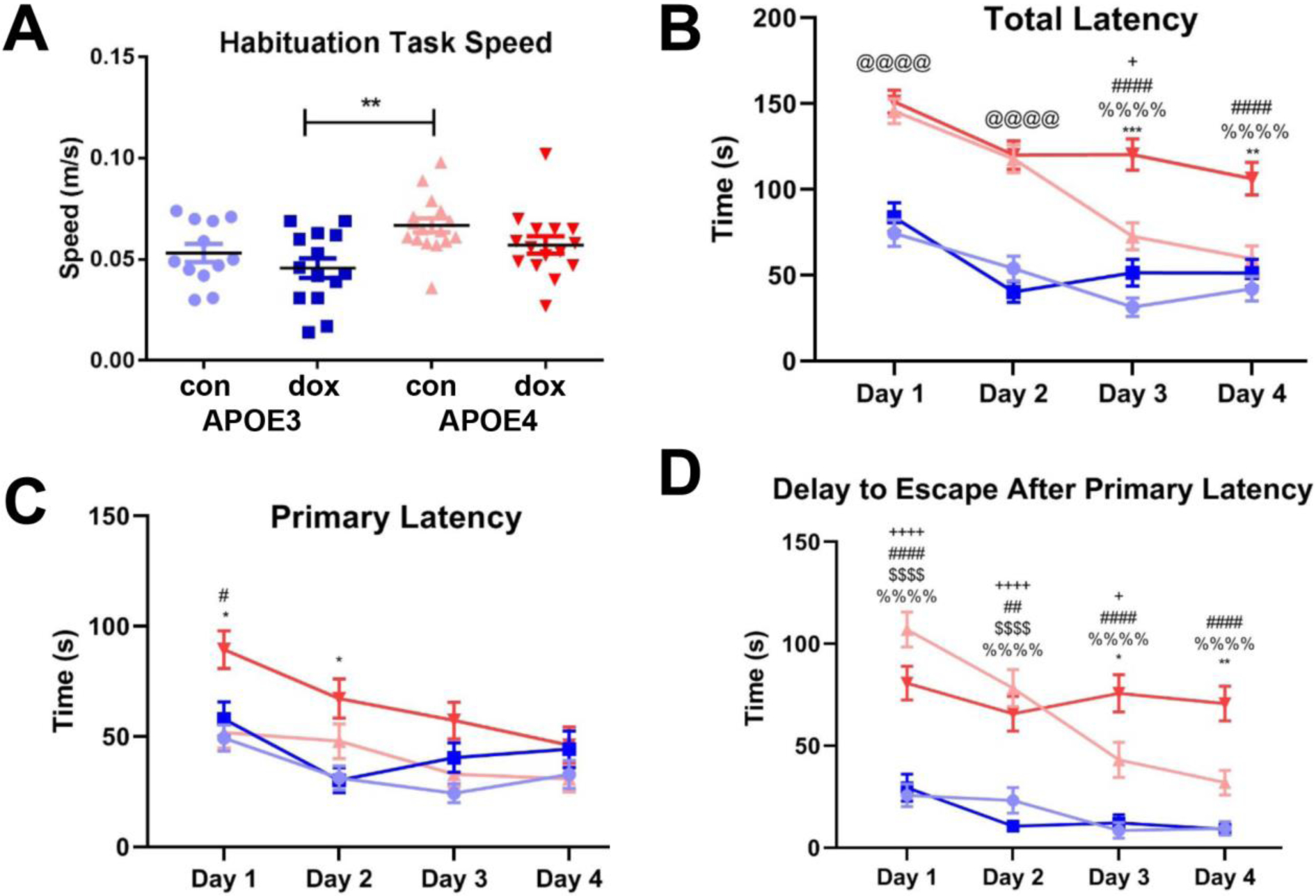
A. Average speed of locomotion on apparatus during Barnes Maze habituation task. B. Latency to full entry to escape hole by day, average of four trials per day for each of four consecutive days. C. Latency to first approach to escape hole by day, average of four trials per day for each of four consecutive days. D. Delay to complete escape after first approach to escape hole, average of four trials per day for each of four consecutive days (excludes all mice that did not make an approach to the escape hole during the trial). Three-way ANOVA with Tukey’s multiple comparisons test: &, APOE3 Ctrl vs. APOE3 Doxo; +, APOE3 Ctrl vs. APOE4 Ctrl; #, APOE3 Ctrl vs. APOE4 Doxo; $, APOE3 Doxo vs. APOE4 Ctrl; %, APOE3 Doxo vs. APOE4 Doxo; *, APOE4 Ctrl vs. APOE4 Doxo; @, All APOE3 vs. all APOE4. * p<.05, ** p<.01, ***p<.001, ****p<.0001.
Next, we assessed primary latency, defined as the time for the mouse to make a first nose poke into the escape hole (Figure 3C). A three-way ANOVA corrected for multiple comparisons by Tukey’s test identified a genotype-by-treatment effect across the four training days (F(1,896) = 4.465). In same-day comparisons, APOE4 doxorubicin-treated mice had significantly higher primary latency compared to the untreated APOE3 and untreated APOE4 mice on training day 1 (p = .015 and p = .012 respectively) and compared to the treated APOE3 mice on training day 2 (p = .025). By training day 4, APOE4 doxorubicin-treated mice performed similarly to all other groups (p = .97).
We also examined the delay to full escape following the first approach to the hole. We found that APOE4 mice, regardless of treatment, had a longer delay to escape after the first correct nose poke during training days 1 and 2 compared to treated and untreated APOE3 mice (Figure 3D) (mean 95 vs. 28 seconds on day1 and 72 vs. 16 seconds on day 2, ANOVA, F(3,794) = 5.062, p < .0001). This delay to escape decreased in APOE4 control mice on training days 3 and 4 but remained significantly higher in APOE4 doxorubicin-treated mice (mean 76 vs. 43 seconds day 3, ANOVA, F(3,794) = 5.062p = .047 and mean 70 vs. 32 seconds day 4, p = .003). The results of doxorubicin treatment failing to cause an observable deficit in spatial learning by the APOE3 mice coincides with prior experiments in wild-type female C57BL/6J mice: mice treated with 10 mg/kg doxorubicin performed the same as saline-injected control mice in the same Barnes Maze paradigm (data not shown).
Three days after training, mice were again exposed to the Barnes maze in a probe trial (Figure 4). Doxorubicin treatment was associated with a significant increase in the time to enter the target hole, in both APOE3 and APOE4 mice (F(1,49) = 15.59, p = .0003). Interestingly, two of the 14 doxorubicin-treated APOE3 mice (14.3%) and seven of the 15 (46.7%) doxorubicin-treated APOE4 mice did not enter the target hole at all by the end of the three minute trial, indicative of strong memory deficits (Figure 4).
Figure 4: Doxorubicin impairs spatial memory.
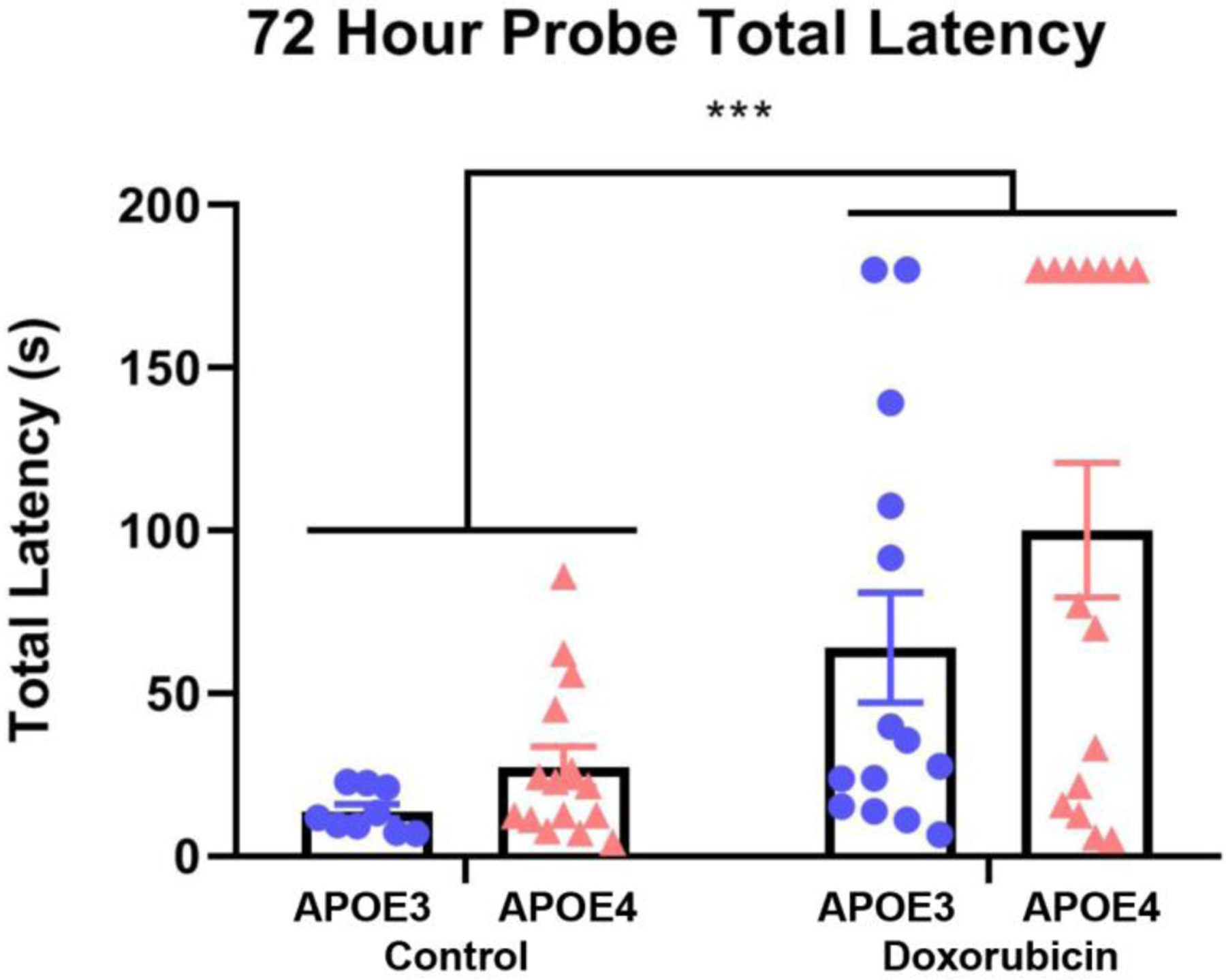
A. Time until total escape from Barnes Maze, single trial conducted 72 hours following the final training trial. ROUT removal of outliers Q = 1% followed by two-way ANOVA. *** p<.001.
3.5. Amygdala-related Learning and Memory
To analyze amygdala-related learning alongside hippocampal learning, we used cued and contextual fear conditioning tasks 21 weeks after treatments (Figure 5). Mice in all groups showed similar high rates of freezing behaviour in response to the second of two conditioning shocks compared to the initial unconditioned shock (ANOVA, F(2.763,151.0) = 75.92, p < .0001) (Figure 5A). In the cued fear conditioning assessment, mice in all groups showed similar elevated freezing, both in response to the tone cue presented in the novel (non-conditioned) environment and during the time-matched portion of the contextual fear response assessment (in the absence of the tone cue) (ANOVA, F (2.763, 151.0) = 75.92, p > .99) (Figure 5A). Examining the response to the cue tone in more detail, we confirmed mice in all groups froze in response to the tone cue presented in the novel environment but not to the initial presentation of the novel environment (F(1.533,84.33) = 229.7, p < .0001) (Figure 5B). We also found that APOE4 mice in both treatment group showed elevated freezing behaviour after the tone cue was complete compared to APOE3 mice F(2,114) = 5.295, p = .001) (Figure 5B).
Figure 5: Doxorubicin does not impair conditioned or cued memory in APOE3 or APOE4 mice.
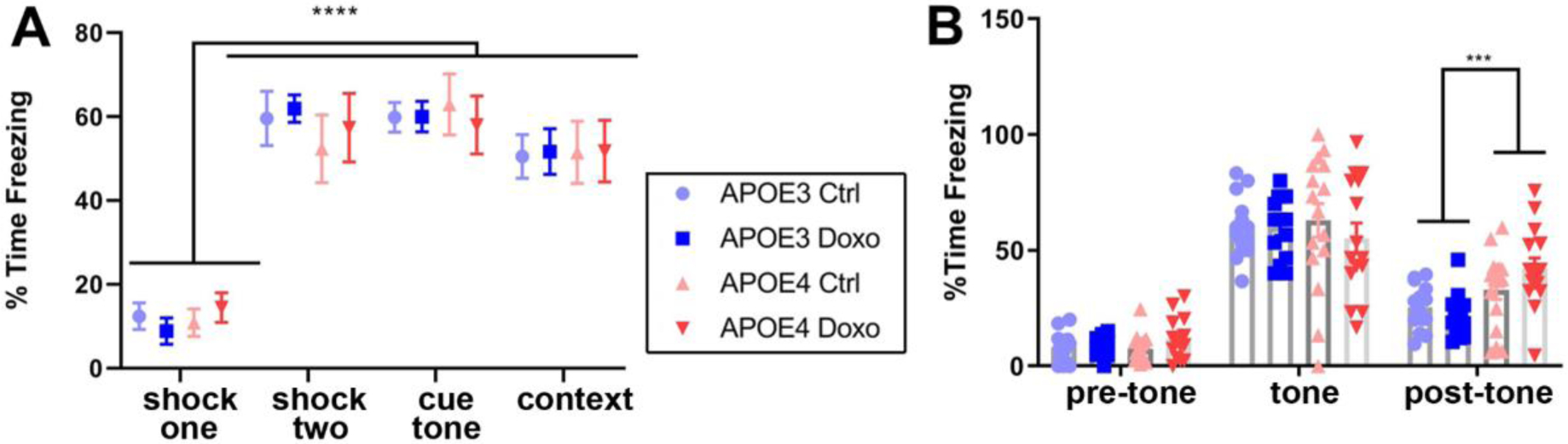
A. Percent time spent freezing during the second of two training shocks, during tone cue in cue test, and in the conditioned environment. B. Percent time spent freezing during the five minute cue test, comparison of pre-tone, tone, and post-tone periods. Mixed-effects analysis with Tukey’s multiple comparisons test to evaluate freezing behaviour in different tasks. Three-way ANOVA with Tukey’s multiple comparisons test to assess differences between time points followed by two-way ANOVA with Sidak’s multiple comparisons test to assess genotype effects in cue test. ** p<.01, **** p<.0001.
3.6. Structural Changes in the Brain
In order to assess the long-term effects of doxorubicin on brain structure, we performed VBM on three-dimensional anatomical MR images of ex-vivo brains. VBM analysis did not detect any persistent, long-term cerebral anatomical alterations associated with doxorubicin treatment in the APOE3 mice (data not shown). However, long-term effects were observed in the APOE4 model. Specifically, the doxorubicin-treated APOE4 mice exhibited subtle atrophy primarily in the grey matter of the frontal cortex, versus control APOE4 mice (Figure 6A, B). In order to assess whether these genotype-based differences in brain anatomy persisted in older age, we compared all APOE3 (both untreated and treated) with all APOE4 mice (Figure 6C, D). Compared to APOE3 mice, APOE4 mice showed lower grey matter volumes in the cerebral cortex and the striatum, regardless of treatment. No differences were observed between groups in hippocampal volume. When corrected for multiple comparisons using FWE (family-wise error) correction, the above described differences are no longer statistically significant, suggesting that the FWE correction was too stringent for the small sample size (3–4 mouse brain/group). In order to minimize the risk of type 1 errors, we performed a region of interest-VBM (ROI-VBM) analysis to substantially decrease the number of voxels tested. The ROIs were placed on the areas of the frontal cortex that had shown differences by whole brain VBM. The ROI-VBM results were consistent with those generated in the whole brain analysis (Supplemental Figure 1); specifically, APOE4 mice exhibited a slight long-term decrease in gray matter areas of the frontal cortex in response to doxorubicin treatment and, when comparing all APOE4 mice versus all APOE3 mice, the anatomical effect of APOE genotype was evident.
Figure 6: Long-term effects of doxorubicin on the brain structure of APOE mice are modest.
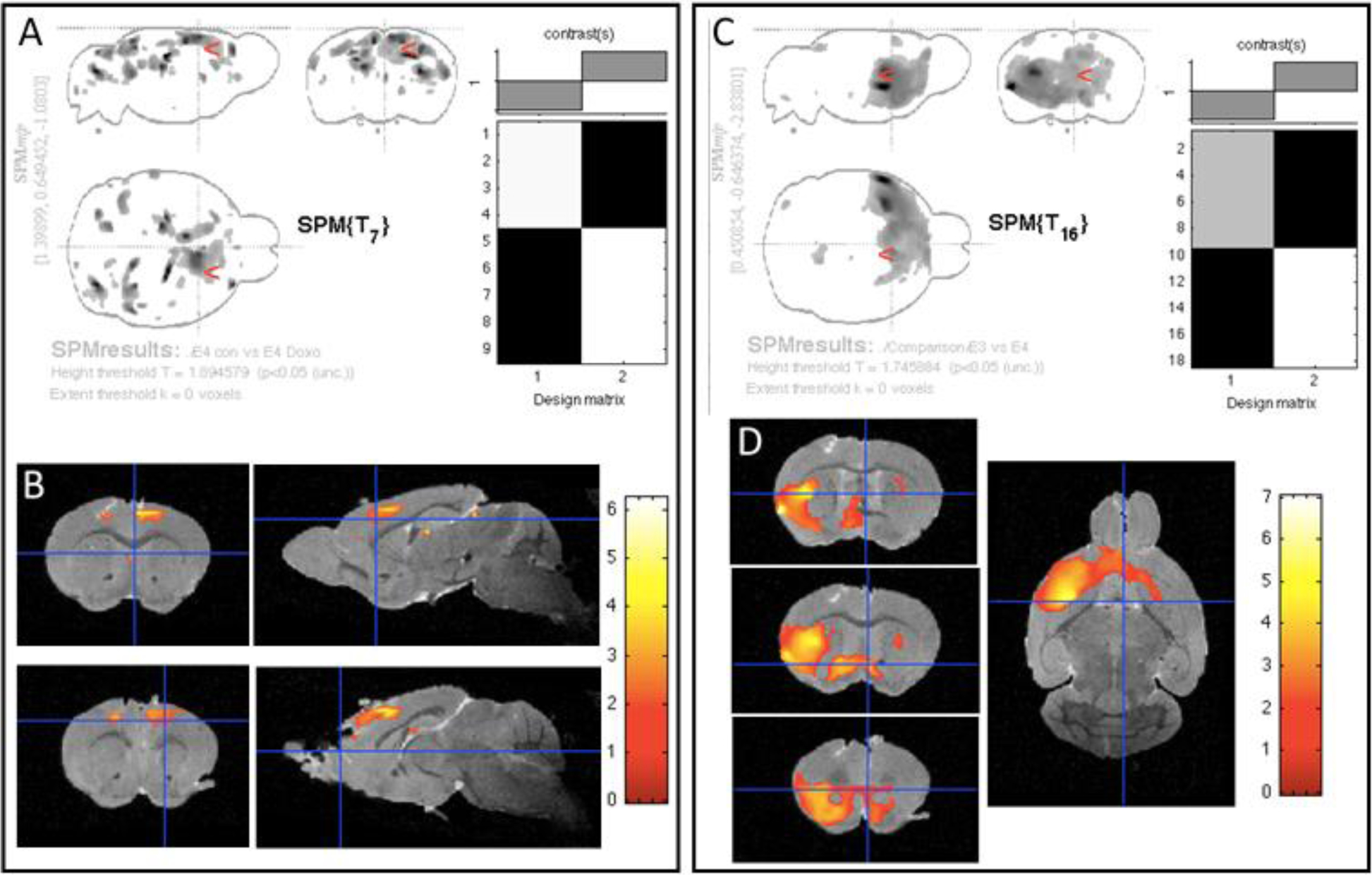
Nine months after treatment, 3-D MRI and VMB were performed on control and doxorubicin-treated APOE3 and APOE4 mice. A and C show maximum intensity projections (MIP) and the design of the matrix for the study. B and D depict color overlays of the t-test values on the co-registered template image and the location of significant clusters in this comparison. The color bar measures t test values of the statistical analyses. A & B. VBM analysis comparing the grey matter of untreated versus doxorubicin-treated APOE4 mice revealed a mild atrophy predominantly in the frontal cortex (colored areas). APOE3 mice did not show any long-term brain regional differences in response to doxorubicin (data not shown). C & D. The comparison of all (both treated and untreated) APOE3 mice versus all APOE4 mice, detected regions of grey matter that were significantly larger in APOE3 mice than in APOE4 mice, regardless of the treatment.
3.7. Immunohistochemistry for AD Markers and Markers of Neurodegeneration
Postmortem tissue was immunostained for markers of Alzheimer’s disease pathogenesis: Aβ42, phospho-tau, glial activation (microglia: Iba-1; astrocytes: GFAP), and apoptosis (Figure 7). We did not detect accumulation of Aβ42 (data not shown), which was expected given that APOE mice are not models of amyloid accumulation. No differences in staining intensity of localization of Iba-1, GFAP or phospho-tau were observed based either on APOE genotypes or by doxorubicin treatment (Figure 7). Isolated cells showing strong staining for activated caspase 3, a marker of apoptosis, were observed across cortex, hippocampus, and cerebellum. No differences in frequency of caspase 3 positive cells were observed across groups (data not shown).
Figure 7: Immunohistochemical staining for pathogenic markers of AD.
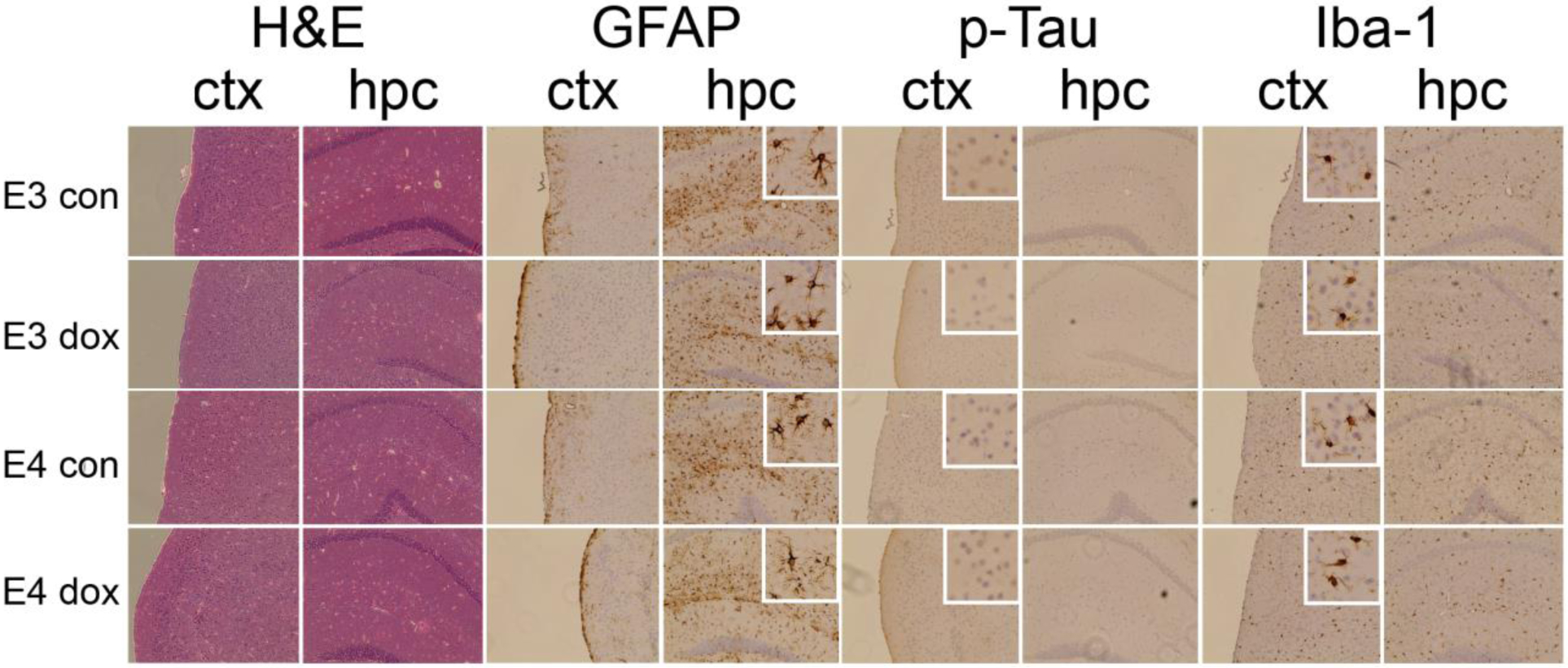
Coronal sections across the anterior/posterior brain regions were stained from 3–5 brains of APOE3 and APOE4 mice treated with control (con) or doxorubicin (dox). Images show staining of frontal cortex (ctx) and hippocampus (hip): Hematoxylin and eosin staining (H&E), IBA-1, GFAP, phosphorylated tau. Images were taken using brightfield microscopy at 20× magnification.
4. Discussion
To our knowledge, this is the first pre-clinical, controlled study that examines cancer-related cognitive impairment in older, genetically susceptible (APOE4) mice. We found that older mice could tolerate chemotherapy and we observed short-term cognitive deficits in spatial learning and memory in APOE4 mice after exposure to chemotherapy, which were not seen in exposed APOE3 mice. Despite these short-term cognitive deficits, anatomical brain assessment of long term effects (nine months post-treatment) showed only mild decrements in the frontal cortex volume, and no differences in hippocampal volume between the APOE4 doxorubicin-exposed mice versus the other groups. These impairments were observed in the context of no alterations in anxiety-like and locomotive behaviour between groups, as well as no deficits in sensory gating or fear-conditioned memory, establishing neurological processes and cognitive domains that are spared by doxorubicin in this model. Finally, in this APOE knock-in model, there were no changes in markers associated with Alzheimer’s disease after doxorubicin treatment as measured by immunohistochemical staining.
Cognitive impairment has been reported among older breast cancer survivors, especially those with an APOE4 allele [8, 10, 11]. We now add a critical preclinical finding to support these clinical data: in older female mice, APOE4 increases susceptibility to deficits in spatial learning after chemotherapy. Since cognitive impairment is associated with older age, and chemotherapy is thought to accelerate aging processes, we chose to use older mice for our experiments in this report. The older mice tolerated chemotherapy, showing only a small, transient weight loss, and successfully completed a panel of behavioural tasks. This demonstrates the feasibility of using older mice to study long-term effects of chemotherapy. This is particularly important since 75% of breast cancer survivors are ages 60 and above at the time of their diagnosis [24].
CRCI seen in chemotherapy-treated breast cancer survivors has most commonly affected processing speed, attention, and executive function [8, 10, 11]; among APOE4 carriers, pronounced differences are seen in these domains. We observed cognitive behavioural impairment in our pre-clinical model of older APOE4 mice exposed to chemotherapy in spatial learning and memory functions. We found impairment in the time to learn the location of the target hole of the Barnes Maze, and increased time to enter the hole after finding it. This impairment occurred in the absence of differences in locomotive and exploratory behaviour between groups, ruling out treatment-related fatigue as a major factor in primary latency and latency to escape. These findings show specific deficits of APOE4 mice after chemotherapy in the domain of spatial learning but also in another domain, perhaps related to attention or motivation needed to enter the target hole. Importantly, attention and executive function are among the cognitive domains most often found to be impaired in CRCI [4, 8, 25], while learning and memory impairments, including in spatial domains, have also been reported (although visuospatial outcomes among cancer survivors are less frequently assessed [8, 25–28]). The finding of connections between APOE4 and CRCI in both humans and mice provides a compelling justification for determining APOE genotype in cancer patients considering treatment options.
This work, consistent with our work with young (5 month old) APOE mice [13], identified spatial learning behaviours that were affected by doxorubicin chemotherapy. In our current work, we tested an expanded panel of behavioural assays for other deficits: we found these assays were unaffected by chemotherapy, including locomotive and anxiety behaviours, PPI response, and cued or contextual fear conditioning. These observations establish that the impairments in spatial learning identified in APOE mice treated with doxorubicin occur in a context where multiple basic features of normal behaviour and sensory processing, as well as some forms of non-navigational memory, are unimpaired.
In terms of brain structure, we only observed mild brain atrophy at 21 months of age among APOE4 mice compared to APOE3 mice after doxorubicin exposure at 12 months of age. Previously, VBM revealed significant changes in cortical grey matter and hippocampal volume in younger APOE3 and APOE4 mice analyzed two months after exposure to doxorubicin [13]. Our current observation of mild alterations in frontal cortex volumes in APOE4 mice in the aged cohort suggests that the acute effects of doxorubicin, at least partially, resolve over time, albeit with some persistent residual damage in the APOE4 model. ROI analyses within the brain areas identified in our initial analyses were consistent with the whole brain VBM analyses.
In addition to increasing the risk of CRCI in older survivors, APOE4 is also the strongest genetic risk factor for Alzheimer’s disease [29]. This overlap suggests that cognitive impairment in CRCI and AD might share some mechanistic features. We examined whether long-term survival after chemotherapy resulted in classical histopathological signs of AD pathogenesis, namely the accumulation of plaques, tangles, and gliosis, and signs of neuronal loss such as apoptosis and hippocampal atrophy. We did not observe differences in cellular hallmarks of AD or other signs of neurodegeneration related to APOE genotype or treatment condition. Since the APOE targeted-replacement model does not develop AD pathology [12, 30, 31], possible effects of APOE4 genotype and doxorubicin on brain histopathology can be pursued in a model that includes signs of Aβ accumulation and neurodegeneration. The chemotherapy may induce important contributors to AD-related cognitive impairment outside these prominent neuropathological changes, such as specific inflammatory pathways [32], loss of blood brain barrier integrity [33], decreased neurogenesis [34] or increased glial senescence [35]. These processes are mechanistically related to APOE genotype [36], and could be exacerbated by exposure to cytotoxic agents.
The mechanisms by which an APOE genotype impacts the risk of AD are complex, with evidence for pathways dependent on Aβ accumulation and pathways independent of Aβ accumulation [37]. A role for APOE in risk of CRCI supports an Aβ independent pathway for effects on cognitive impairment. In humans, APOE4 increases the risk of impairment following traumatic brain injury [38] and stroke [39]. In preclinical models, APOE4 increases susceptibility to several conditions of inflammation, including traumatic brain injury [40], lipopolysaccharide exposure [37, 41] and stroke [42]. Genome-wide association studies have identified many inflammation genes as risk factors for AD [43], further supporting the importance of inflammation in cognitive impairment in AD [44]. Several types of cancer chemotherapy induce peripheral inflammation in clinical studies [45, 46] and CNS inflammation in preclinical studies [47]. Inflammation is also one of the central pillars of aging [48, 49]. The connection of APOE4 genotype to an increased predisposition to inflammation may underlie some of its effects on increased risk of CRCI in older mice rather than through mechanisms involving only Aβ accumulation.
While we used a rigorous, controlled experiment, there are several caveats that should be considered in understanding the scope of our results. First, several cognitive tests were limited to single time points due to practice effects; doxorubicin treatment may cause behavioural impairments at other time points. Second, we studied female mice, given that the preponderance of data linking APOE4 to CRCI is in breast cancer survivors [8, 50]; links may also exist between APOE4 and CRCI in males [10, 51]. Third, we focused our experiments on two APOE genotypes: mice homozygous for APOE3 or APOE4. We did not assess effects in APOE3/4 heterozygotes, or investigate the possible effects of the APOE2 allele, which is associated with up to 50% decreased risk of AD [52] and present in 12% of the US population [29, 53]. Fourth, research staff was not blinded to APOE genotype of mice in this study, though this was mitigated by use of automated tracking software or scoring by a blinded observer to analyze all behavioural data collected. Finally, we chose to initially study the effects of doxorubicin, one of the most commonly used chemotherapeutics [54, 55]. Doxorubicin is generally excluded by the blood-brain barrier, suggesting that at least some of the effects of CRCI are mediated by systemic insults [56, 57]. Different chemotherapies may have different risks of CRCI or effects on different cognitive domains; these questions can be addressed systematically in this APOE mouse model.
The establishment of this preclinical model of genetically influenced CRCI in older mice has the potential to accelerate discovery. First, our model fulfills many of the priority criteria laid out by the International Cognition and Cancer Task Force for preclinical research in CRCI [1]. It is ideally suited for multilateral investigations into multiple potential mechanisms of CNS insult by chemotherapy, including neuroinflammation, oxidative stress, and blood-brain barrier challenge, in the context of a genetically vulnerable population. Second, our model allows analysis of a complex condition in ways that are not possible in human studies [1, 58]. For example, there are significant impediments to comprehensive clinical studies of CRCI, with the most significant being the limited numbers of patients, the heterogeneity of patient populations and treatments, and the lack of availability of tissue specimens (e.g. brain biopsies) for direct study [59, 60]. Third, this model can be used to test the separate and combined effects of different chemotherapy and hormonal therapy regimens on CRCI in diverse age groups.
In summary, we have established a reliable and tractable preclinical model of the specific vulnerability of behavioural and anatomical measures related to CRCI in older APOE4 carriers following doxorubicin treatment. Our model system is useful for studying the underlying molecular mechanisms of cognitive insult by chemotherapeutic agents in cancer survivors and enables the study of potential interventions and preventative approaches for CRCI.
Supplementary Material
Figure 1: Region of Interest (ROI) – VBM analysis. Analyses were performed on ROIs localized in the frontal cortex, the area positive for regional differences in the whole brain VBM analysis. Panels A and C show maximum intensity projections (MIP) and the design of the matrix for the study. Panels B and D depict color overlays of the t-test values on the co-registered template image and the location of significant clusters in this comparison. A, B) ROI-VBM analysis comparing the grey matter of untreated versus doxorubicin-treated APOE4 mice. Only mild atrophy, predominantly in the frontal cortex, was detected (colored areas); APOE3 mice do not show any long-term brain regional differences in response to doxorubicin (data not shown). C, D) Comparisons of all (both treated and untreated) APOE3 mice versus all APOE4 mice. Regions of grey matter that were significantly larger in APOE3 mice than in APOE4 mice, regardless of the treatment, were observed (colored areas). The color bar defines the t test values of the statistical analyses. E) A representative region of interest used for ROI-VBM analysis is depicted in red.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 NS100704 (to GWR); R35 CA197289 and R01 CA129769 (to JM); T32 CA009686 and TL1 TR001431 (to TD); and P30 CA51008), and from the Georgetown Lombardi Comprehensive Cancer Center Women & Wine group.
Abbreviations:
- AD
Alzheimer’s Disease
- APOE
Apolipoprotein E
- CRCI
cancer-related cognitive impairment
- CNS
central nervous system
- GFAP
Glial fibrillary acidic protein
- Iba-1
Ionized calcium binding adaptor molecule
- MRI
magnetic resonance imaging
- PPI
pre-pulse inhibition
- RARE
rapid acquisition with relaxation enhancement
- ROI
region of interest
- VBM
voxel-based morphometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Winocur G, Johnston I, Castel H, Chemotherapy and cognition: International cognition and cancer task force recommendations for harmonising preclinical research, Cancer Treatment Reviews 69 (2018) 72–83. [DOI] [PubMed] [Google Scholar]
- [2].Vardy J, Tannock I, Cognitive function after chemotherapy in adults with solid tumours, Critical Reviews in Oncology/Hematology 63(3) (2007) 183–202. [DOI] [PubMed] [Google Scholar]
- [3].Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function, Cancer 104(10) (2005) 2222–2233. [DOI] [PubMed] [Google Scholar]
- [4].Ahles TA, Root JC, Ryan EL, Cancer-and cancer treatment-associated cognitive change: an update on the state of the science, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30(30) (2012) 3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA, Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve, Journal of Clinical Oncology 28(29) (2010) 4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM, Prevalence of Apolipoprotein E4 Genotype and Homozygotes (APOE e4/4) among Patients Diagnosed with Alzheimer’s Disease: A Systematic Review and Meta-Analysis, Neuroepidemiology 38(1) (2012) 1–17. [DOI] [PubMed] [Google Scholar]
- [7].Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P, Wang L-S, Romero K, Arneric SP, Redolfi A, Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis, JAMA neurology 74(10) (2017) 1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC, Graham D, Zhou X, Clapp J, Zhai W, Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study, Journal of Clinical Oncology 36(32) (2018) 3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Buskbjerg CDR, Amidi A, Demontis D, Nissen ER, Zachariae R, Genetic risk factors for cancer-related cognitive impairment: a systematic review, Acta Oncologica 58(5) (2019) 537–547. [DOI] [PubMed] [Google Scholar]
- [10].Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA, The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy, Psycho-Oncology 12(6) (2003) 612–619. [DOI] [PubMed] [Google Scholar]
- [11].Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ, Moore JH, Saykin AJ, Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking, Psycho-Oncology 23(12) (2014) 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N, Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis, J Biol Chem 272(29) (1997) 17972–80. [DOI] [PubMed] [Google Scholar]
- [13].Speidell AP, Demby T, Lee Y, Rodriguez O, Albanese C, Mandelblatt J, Rebeck GWJNR, Development of a Human APOE Knock-in Mouse Model for Study of Cognitive Function After Cancer Chemotherapy, 35(2) (2018) 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sullivan PM, Mace BE, Maeda N, Schmechel DE, Marked regional differences of brain human apolipoprotein e expression in targeted replacement mice, Neuroscience 124(4) (2004) 725–733. [DOI] [PubMed] [Google Scholar]
- [15].Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart J-C, Bales K, Mathis C, Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior, Behavioural Brain Research 159(1) (2005) 1–14. [DOI] [PubMed] [Google Scholar]
- [16].Bour A, Grootendorst J, Vogel E, Kelche C, Dodart J-C, Bales K, Moreau P-H, Sullivan PM, Mathis C, Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks, Behavioural Brain Research 193(2) (2008) 174–182. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW, Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex, Learn Mem 20(5) (2013) 256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Salas-Ramirez KY, Bagnall C, Frias L, Abdali SA, Ahles TA, Hubbard K, Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways, Behavioural Brain Research 292 (2015) 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fremouw T, Fessler CL, Ferguson RJ, Burguete Y, Preserved learning and memory in mice following chemotherapy: 5-Fluorouracil and doxorubicin single agent treatment, doxorubicin–cyclophosphamide combination treatment, Behavioural Brain Research 226(1) (2012) 154–162. [DOI] [PubMed] [Google Scholar]
- [20].Seigers R, Loos M, Van Tellingen O, Boogerd W, Smit AB, Schagen SB, Cognitive impact of cytotoxic agents in mice, Psychopharmacology 232(1) (2015) 17–37. [DOI] [PubMed] [Google Scholar]
- [21].Geyer MA, Wilkinson LS, Humby T, Robbins TW, Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia, Biological Psychiatry 34(6) (1993) 361–372. [DOI] [PubMed] [Google Scholar]
- [22].Mansbach RS, Geyer MA, Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine, Psychopharmacology 105(2) (1991) 162–168. [DOI] [PubMed] [Google Scholar]
- [23].Rosenfeld CS, Ferguson SA, Barnes maze testing strategies with small and large rodent models, JoVE (Journal of Visualized Experiments) (84) (2014) e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bluethmann SM, Mariotto AB, Rowland JH, Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States, Cancer Epidemiology Biomarkers & Prevention 25(7) (2016) 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bernstein LJ, McCreath GA, Komeylian Z, Rich JB, Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis, Neuroscience & Biobehavioral Reviews 83 (2017) 417–428. [DOI] [PubMed] [Google Scholar]
- [26].Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM, Cognitive impairment associated with adjuvant therapy in breast cancer, Psycho‐Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 15(5) (2006) 422–430. [DOI] [PubMed] [Google Scholar]
- [27].Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, Witmer M, Van Gorp WG, Fornier M, D’Andrea G, Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study, Journal of the American Geriatrics Society 54(6) (2006) 925–931. [DOI] [PubMed] [Google Scholar]
- [28].Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G, Predictors of cognitive decline after chemotherapy in breast cancer patients, Journal of the International Neuropsychological Society 15(6) (2009) 951–962. [DOI] [PubMed] [Google Scholar]
- [29].Raber J, Huang Y, Ashford JW, ApoE genotype accounts for the vast majority of AD risk and AD pathology, Neurobiology of Aging 25(5) (2004) 641–650. [DOI] [PubMed] [Google Scholar]
- [30].Kitamura HW, Hamanaka H, Watanabe M, Wada K, Yamazaki C, Fujita SC, Manabe T, Nukina N, Age-dependent enhancement of hippocampal long-term potentiation in knock-in mice expressing human apolipoprotein E4 instead of mouse apolipoprotein E, Neurosci Lett 369(3) (2004) 173–8. [DOI] [PubMed] [Google Scholar]
- [31].Korwek KM, Trotter JH, Ladu MJ, Sullivan PM, Weeber EJ, ApoE isoform-dependent changes in hippocampal synaptic function, Molecular neurodegeneration 4 (2009) 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barroeta-Espar I, Weinstock LD, Perez-Nievas BG, Meltzer AC, Siao Tick Chong M, Amaral AC, Murray ME, Moulder KL, Morris JC, Cairns NJ, Parisi JE, Lowe VJ, Petersen RC, Kofler J, Ikonomovic MD, Lopez O, Klunk WE, Mayeux RP, Frosch MP, Wood LB, Gomez-Isla T, Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology, Neurobiol Dis 121 (2019) 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV, Apolipoprotein E controls cerebrovascular integrity via cyclophilin A, Nature 485 (2012) 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zetterberg H, Schott JM, Biomarkers for Alzheimer’s disease beyond amyloid and tau, Nat Med 25(2) (2019) 201–203. [DOI] [PubMed] [Google Scholar]
- [35].Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A, Astrocytes in physiological aging and Alzheimer’s disease, Neuroscience 323 (2016) 170–82. [DOI] [PubMed] [Google Scholar]
- [36].Flowers SA, Rebeck GW, APOE in the normal brain, Neurobiol Dis (2020) 104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kanekiyo T, Xu H, Bu G, ApoE and Aβ in Alzheimer’s Disease: Accidental Encounters or Partners?, Neuron 81(4) (2014) 740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Friedman G, Froom P, Sazbon L, Grinblatt I, Shochina M, Tsenter J, Babaey S, Yehuda AB, Groswasser Z, Apolipoprotein E-ε4 genotype predicts a poor outcome in survivors of traumatic brain injury, Neurology 52(2) (1999) 244–244. [DOI] [PubMed] [Google Scholar]
- [39].Wagle J, Farner L, Flekkøy K, Wyller TB, Sandvik L, Eiklid KL, Fure B, Stensrød B, Engedal K, Association between ApoE ε4 and Cognitive Impairment after Stroke, Dementia and Geriatric Cognitive Disorders 27(6) (2009) 525–533. [DOI] [PubMed] [Google Scholar]
- [40].Main BS, Villapol S, Sloley SS, Barton DJ, Parsadanian M, Agbaegbu C, Stefos K, McCann MS, Washington PM, Rodriguez OC, Burns MP, Apolipoprotein E4 impairs spontaneous blood brain barrier repair following traumatic brain injury, Molecular Neurodegeneration 13(1) (2018) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT, APOE Genotype and an ApoE-mimetic Peptide Modify the Systemic and Central Nervous System Inflammatory Response, Journal of Biological Chemistry 278(49) (2003) 48529–48533. [DOI] [PubMed] [Google Scholar]
- [42].Sheng H, Laskowitz DT, Bennett E, Schmechel DE, Bart RD, Saunders AM, Pearlstein RD, Roses AD, Warner DS, Apolipoprotein E Isoform-Specific Differences in Outcome from Focal Ischemia in Transgenic Mice, Journal of Cerebral Blood Flow & Metabolism 18(4) (1998) 361–366. [DOI] [PubMed] [Google Scholar]
- [43].Malik M, Parikh I, Vasquez JB, Smith C, Tai L, Bu G, LaDu MJ, Fardo DW, Rebeck GW, Estus S, Genetics ignite focus on microglial inflammation in Alzheimer’s disease, Molecular Neurodegeneration 10(1) (2015) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McGeer PL, McGeer EG, Polymorphisms in Inflammatory Genes and the Risk of Alzheimer Disease, JAMA Neurology 58(11) (2001) 1790–1792. [DOI] [PubMed] [Google Scholar]
- [45].Vyas D, Laput G, Vyas AK, Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis, OncoTargets and therapy 7 (2014) 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang L, Chen Q, Qi H, Wang C, Wang C, Zhang J, Dong L, Doxorubicin-Induced Systemic Inflammation Is Driven by Upregulation of Toll-Like Receptor TLR4 and Endotoxin Leakage, Cancer Research 76(22) (2016) 6631–6642. [DOI] [PubMed] [Google Scholar]
- [47].Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, St. Clair W, Ratanachaiyavong S, St. Clair DK, Butterfield DA, Adriamycin-induced, TNF-α-mediated central nervous system toxicity, Neurobiology of Disease 23(1) (2006) 127–139. [DOI] [PubMed] [Google Scholar]
- [48].Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C, Molecular inflammation: Underpinnings of aging and age-related diseases, Ageing Research Reviews 8(1) (2009) 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sarkar D, Fisher PB, Molecular mechanisms of aging-associated inflammation, Cancer Letters 236(1) (2006) 13–23. [DOI] [PubMed] [Google Scholar]
- [50].Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, Hussin MG, Jacobsen PB, Small BJ, Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30(29) (2012) 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Amidi A, Agerbæk M, Wu LM, Pedersen AD, Mehlsen M, Clausen CR, Demontis D, Børglum AD, Harbøll A, Zachariae R, Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment, Brain Imaging and Behavior 11(3) (2017) 769–783. [DOI] [PubMed] [Google Scholar]
- [52].West HL, William Rebeck G, Hyman BT, Frequency of the apolipoprotein E ε2 allele is diminished in sporadic Alzheimer disease, Neuroscience Letters 175(1) (1994) 46–48. [DOI] [PubMed] [Google Scholar]
- [53].Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM, Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease: A Meta-analysis, JAMA 278(16) (1997) 1349–1356. [PubMed] [Google Scholar]
- [54].Hortobágyi GN, Anthracyclines in the Treatment of Cancer, Drugs 54(4) (1997) 1–7. [DOI] [PubMed] [Google Scholar]
- [55].Turner N, Biganzoli L, Di Leo A, Continued value of adjuvant anthracyclines as treatment for early breast cancer, The Lancet Oncology 16(7) (2015) e362–e369. [DOI] [PubMed] [Google Scholar]
- [56].Bigotte L, Arvidson B, Olsson Y, Cytofluorescence localization of adriamycin in the nervous system, Acta Neuropathologica 57(2) (1982) 121–129. [DOI] [PubMed] [Google Scholar]
- [57].Bigotte L, Olsson Y, Cytofluorescence localization of adriamycin in the nervous system, Acta Neuropathologica 58(3) (1982) 193–202. [DOI] [PubMed] [Google Scholar]
- [58].Seigers R, Schagen SB, Van Tellingen O, Dietrich J, Chemotherapy-related cognitive dysfunction: current animal studies and future directions, Brain Imaging and Behavior 7(4) (2013) 453–459. [DOI] [PubMed] [Google Scholar]
- [59].Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, Shi B, Breast cancer intrinsic subtype classification, clinical use and future trends, American journal of cancer research 5(10) (2015) 2929–2943. [PMC free article] [PubMed] [Google Scholar]
- [60].G. Early Breast Cancer Trialists’ Collaborative, Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials, The Lancet 379(9814) (2012) 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Region of Interest (ROI) – VBM analysis. Analyses were performed on ROIs localized in the frontal cortex, the area positive for regional differences in the whole brain VBM analysis. Panels A and C show maximum intensity projections (MIP) and the design of the matrix for the study. Panels B and D depict color overlays of the t-test values on the co-registered template image and the location of significant clusters in this comparison. A, B) ROI-VBM analysis comparing the grey matter of untreated versus doxorubicin-treated APOE4 mice. Only mild atrophy, predominantly in the frontal cortex, was detected (colored areas); APOE3 mice do not show any long-term brain regional differences in response to doxorubicin (data not shown). C, D) Comparisons of all (both treated and untreated) APOE3 mice versus all APOE4 mice. Regions of grey matter that were significantly larger in APOE3 mice than in APOE4 mice, regardless of the treatment, were observed (colored areas). The color bar defines the t test values of the statistical analyses. E) A representative region of interest used for ROI-VBM analysis is depicted in red.


