Short abstract
Endometriosis affects up to 10% of women of childbearing age, causing symptoms that can include chronic pelvic pain and reduced fertility. The symptoms are not specific to the disease and can be confused with other gynecological conditions or normal menstruation. Currently, the disease can be only definitively diagnosed by laparoscopy, as no clinically accepted biomarker exists. Biomarker discovery can either follow a hypothesis-driven approach selecting targets to be tested based on current knowledge of the disease, or take an unbiased high-throughput screening “omics” approach, such as transcriptomics or proteomics, to identify markers that are unique or elevated in accessible bodily fluids of patients with the disease. Numerous studies have been conducted using these approaches to try and identify endometriosis biomarkers, but variabilities in study design, cohort selection, and analysis, together with the fact that most studies were small-scale, have made independent validation of biomarker candidates difficult. Therefore, efforts are underway to standardize cohort selection, patient data, and sample collection to allow better cross-study comparisons. Large scale multi-center studies using this standardized approach are necessary to validate existing endometriosis biomarker candidates and uncover potential new markers. Given the complexity and heterogeneity of the disease, it is likely that a panel of biomarkers will be necessary to diagnose and categorize endometriosis.
Impact statement
Endometriosis is a common disease affecting reproductive age women, which is associated with chronic pain and reduced fertility reducing the quality of life of many women. Definitive diagnosis requires invasive laparoscopic surgery creating a high barrier to diagnosis that can delay the onset of treatment significantly. Clinically approved biomarkers of endometriosis are currently lacking, making the discovery and validation of biomarkers that would lead to earlier diagnosis a priority for improving treatment of the disease.
Keywords: Endometriosis, fertility, dysmenorrhea, dyspareunia, biomarkers, endometrium
The challenging nature of endometriosis
Endometriosis is a common and burdensome chronic disease that affects up to 10% of women of reproductive age.1 The severity and type of symptoms are variable and not specific to endometriosis, but can include dysmenorrhea, dyspareunia, chronic pelvic pain, and infertility.2 Currently, the disease can only be definitely diagnosed by laparoscopic surgery. Therefore, the non-specific nature of the symptoms and the need for invasive surgery for diagnosis often lead to a significant delay in diagnosis and onset of treatment.1 Endometriosis is typified by abnormalities in the hormone response and production in both the ectopic endometrium found in lesions, and the eutopic endometrium in the uterus.3–5 The interplay between the impact of the local cellular and signaling environment in the lesions and eutopic endometrium and the effect of altered sensitivity to circulating levels of steroid hormones adds to the complexity of the disease.
Although twin studies indicate there can be a genetic component in endometriosis,6 genome-wide-association (GWAS) studies have detected only a minor effect at multiple loci,2 indicating that susceptibility to the disease is multigenic with a large environmental component. A number of risk factors have been reported to increase the risk of developing endometriosis, such as low birth weight, in utero exposure to chemicals such as the non-steroidal estrogen diethylstilbestrol, early age of menarche, shorter menstrual cycles, and a low body mass index (BMI), while giving birth and breast feeding have been associated with a protective effect.7 Lifestyle factors such as diet and exercise have also been reported to affect the risk of developing the disease.2 However, in each case, the effect was small, indicating that the predictive value of individual environmental and lifestyle factors to develop endometriosis is limited. Therefore, given that susceptibility to develop endometriosis cannot be accurately predicted from genetic and environmental information, and that diagnosis is often delayed by the non-specific symptoms and the need for invasive laparoscopic surgery for diagnosis, there is an urgent need to develop a reliable non-invasive diagnostic test for the disease.
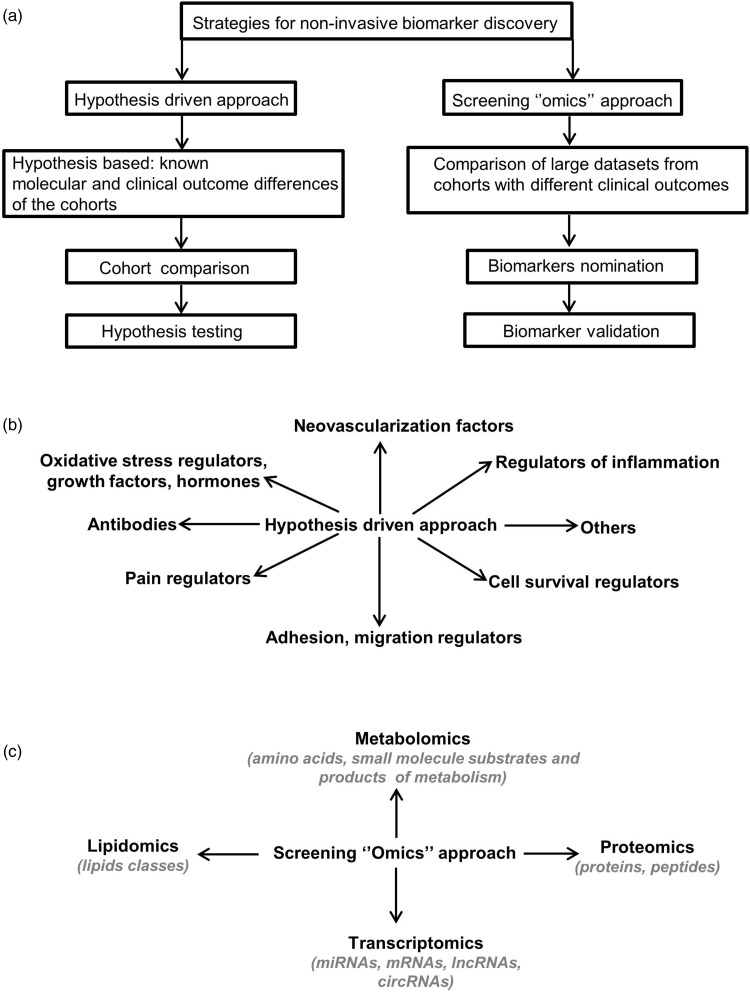
In this mini-review, we provide a concise view of current knowledge of the pathogenesis, diagnosis, and treatment of endometriosis. We outline alternative strategies and challenges to uncover and validate diagnostic biomarkers of the disease to allow earlier diagnosis and onset of treatment. Specifically, we focus on the two main alternative approaches for biomarker discovery: hypothesis-driven research and the high-through put “omics” approaches (Figure 1), highlighting the advantages and disadvantages of each approach using examples from our work and from others.
Figure 1.
Strategies for non-invasive biomarker discovery. (a) Strategies for non-invasive biomarker discovery. Biomarkers can be uncovered and validated using two different methodologies: a hypothesis-driven or a hypothesis-free “omics” approach. (b) Hypothesis-driven approach. Using existing knowledge from different sources illustrated here, candidate biomarkers are hypothesized then subject to testing. (c) Screening “omics’’ approach. Diverse high-throughput technologies, such as those indicated here, are used to uncover candidate endometriosis biomarkers by comparing patient tissue to controls.
Pathogenesis
In order for endometriosis lesions to develop, (1) the cells that initiate the lesion must be present at the ectopic site, and (2) these cells must be able to implant, differentiate into endometrium-like tissue, and evade the immune system. The cells, proteins, and other molecules associated with each stage of pathogenesis are potential biomarkers if they can be detected in accessible body fluids such as blood, urine, saliva, and cervicovaginal fluid (CVF). In contrast to the blood, urine, and saliva, as the bodily fluid directly exposed to the uterine environment, CVF may provide more sensitive and specific biomarkers of endometriosis.8 A number of hypotheses that are not necessarily mutually exclusive have been developed to explain the first part of lesion development: the source of the cells. The most widely accepted theory is the retrograde menstruation hypothesis, whereby cells are introduced into the peritoneal cavity via the fallopian tubes.1,9 A second hypothesis proposes that endometrial cells may be transported to the site of the lesion via the lymphatic or blood vessels, a hypothesis that may explain the uncommon occurrence of lesions outside the peritoneal cavity at other sites.10 A third hypothesis poses that tissues may trans-differentiate into endometrium-like tissue at the site of the lesion, a hypothesis that could explain the rare reports of endometriosis-like lesions in men.2 These hypotheses and others provide plausible explanations for the origin of endometrial-like cells at ectopic sites, but they do not explain how the lesions become established or why the eutopic endometrium of patients may also be defective compared to controls.
Once at the site of the lesion, cells must adhere, implant, and differentiate while avoiding the immune system. Endometriosis patients display abnormal levels of the adhesion molecules VCAM-1 and ICAM-1 in their serum,11 along with elevated levels of the metalloproteinases such as MMP-2 and MMP-9, which are thought to be required for remodeling the extracellular matrix at the site of implantation.12
It is currently unclear whether lesions arise from a single cell or a group of cells. The clonality of the epithelial layer indicates that they may arise from a single cell,13 although the stromal layer has not yet been investigated. Endometriosis is also associated with an acquired invasive mesenchymal phenotype due to an increased level of epithelial to mesenchymal transition (EMT) that is often facilitated by a loss of E-cadherin and a gain of N-cadherin, or the presence of EMT-promoting factors such as TGFβ1.2 After a lesion has been established, it may become infiltrated by neural, endothelial, and immune cells. The neurovascularization process is associated with the expression of the endothelial marker VEGF and neuronal markers such as NGF, which have been detected at higher levels in the peritoneal fluid of endometriosis patients.14,15 Neurovascularization of ectopic lesions in endometriosis patients is thought to contribute to the chronic pain associated with the disease,16 with distortion and infiltration of pelvic organs also thought to play a role.2
A hallmark of endometriosis pathogenesis is the dysregulation of steroid hormone production and receptors in the eutopic endometrium and ectopic lesions. Studies in the baboon model have shown that endometriosis is characterized by estrogen dependence and progesterone resistance, with aberrant angiogenesis and chronic inflammation.3,4 This is in agreement with the altered expression of estrogen and progesterone receptors in ectopic lesions in human patients, and also changed expression of enzymes involved in estrogen metabolism in the eutopic endometrium.5 The estrogen-dependent nature of endometriosis is indicated by the usual regression of the disease following menopause or ovariectomy.5 Evidence from genetic and epigenetic studies strongly support the notion that the eutopic endometrium of women with endometriosis responds differently to circulating progesterone, compared to women without the disease.17,18 In vivo studies in the baboon endometriosis model revealed that progesterone resistance is an event that occurs late in disease development after the early dominance of an estrogenic phenotype lacking steroid hormone receptor abnormalities.19 This study indicated that the molecular changes in the eutopic endometrium are directly influenced by endometriotic lesions, although the mechanism how this occurs is unclear. Despite altered levels of steroid hormone receptor expression in eutopic and ectopic endometrial tissue reported in these studies and others, these tissues are not available for a non-invasive diagnostic test. Although lesions produce additional estrogen, there are no reports that elevated circulating estrogen levels is diagnostic of endometriosis, which may be due to intra- and inter-individual variability obscuring any effect. Endometriosis lesions can be broadly characterized based on their localization as peritoneal, ovarian, or deep infiltrating endometriosis, although they can also rarely occur at other sites outside of the peritoneum cavity.2 Adenomyosis results from growth of endometrium into the myometrium of the uterus and is often categorized as a sub-type of endometriosis because it results from abnormal endometrial growth, causes similar symptoms, and frequently co-exists with other forms of endometriosis. However, there is no consensus in the literature, with adenomyosis and endometriosis often being considered separate disease entities.20
Endometriosis patients show both an altered local and systemic immune response thought to be a complex combination of a defective immune state that allows establishment of the disease, as well as the inflammatory response induced by the lesions.2 The local immune environment that is most relevant for endometriosis is the peritoneal fluid, as most lesions develop at sites in the peritoneum. Endometriosis has been associated with a defective natural killer (NK) cell response, and activation of macrophage, T cell and B cells.2 In endometriosis patients, peritoneal macrophages are polarized from a M1 to a M2 response,21 which is associated with a change in the T helper cell populations with an increase in the Th2, Th17, and Treg populations.22,23 Associated with these cellular changes in the immune system in endometriosis are changes in the chemokines and cytokines that can be detected in the peritoneal fluid and serum. In particular, the CC-chemokines CCL5, CCL2, and CCL11 and CXC-chemokines CXCL1, CXCL8, CXCL5, and CXCL12 have been reported to be elevated in endometriosis.24 Increases in the cytokines IL-1, IL-6, IL-8, IL-12 and growth factors IGFI, PDGF, VEGF, and TNF are also associated with endometriosis.2 Dissecting the complex relationships between the immune cells and the chemokines and cytokines that they release is difficult, but it is clear that an altered immune state contributes to the disease, and the factors involved are potential biomarkers of the disease.
Comorbidities
A number of comorbidities can be associated with endometriosis such as reduced fertility, cancer, autoimmune, and cardiovascular diseases that may have connected underlying causes. As mentioned above, adenomyosis, which is sometimes considered a sub-type of endometriosis, often co-occurs with other endometriosis forms. Fertility problems occur in 30–50% of women with endometriosis, but the underlying mechanisms remain unclear.2 Distortion of the pelvic anatomy by endometriotic lesions may reduce the chance of pregnancy, while women with endometriosis may have a reduced quality of oocytes that may result from damage to the ovary by infiltrating endometriosis lesions or by the surgery to remove them.2 It has been shown that decidualization of stromal cells in the eutopic endometrium is compromised, indicating that this may be a contributing factor to causing increased infertility in endometriosis.25 However, data on the receptiveness of the uterus to implantation are unclear, with one prospective study comparing the success of in vitro fertilization with healthy donor oocytes finding no difference,26 and a more recent study showing that women with endometriosis had a reduced chance of getting pregnant.27
Endometriosis has also been associated with an increased risk of some neoplastic diseases, in particular with clear cell and endometrioid ovarian cancers.28 There is also an increased risk of autoimmune diseases such as systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. Finally, endometriosis has also been associated with an increased risk for cardiovascular disease, as well as hypertension and hypercholesterolemia.2 It is unclear whether endometriosis is predisposing women to these diseases, or vice versa, or if a more general pro-inflammatory disorder can lead to both diseases.
Diagnosis
Taking an exact medical history and performing a palpatory examination may indicate symptoms consistent with endometriosis that warrant further investigation. A series of non-invasive imaging techniques are then available to determine if any further evidence of endometriosis can be detected. First in line is a transvaginal ultrasound (TVS), which has advantages such as ready availability and lack of radiation. However, the specificity and sensitivity of TVS are strongly dependent on the experience of the examiner. In cases where deep-infiltrating endometriosis is suspected, further imaging techniques such as an MRI may be helpful to assess the degree of infiltration into organs like the rectum and bladder and to aid in surgery planning.29 These techniques can be helpful, but as mentioned previously, the gold standard for diagnosis of endometriosis remains laparoscopy with histological verification.29 Through visualization during surgery, the disease can be staged according to the revised American Society for Reproductive Medicine (rASRM) score, from minimal (stage I)—mild (II)—moderate (III) to severe (IV).30 Deep-infiltrating lesions are further classified using the ENZIAN score, depending on localization and the degree of infiltration.30 Some reported biomarkers have been associated with the stage or severity of the disease (Table 1), although there are currently no clinically accepted biomarkers that can aid in classifying the stage of the disease without surgery. Validation of biomarkers that indicate the endometriosis stage would be a useful tool in helping to decide whether surgery is necessary or not.
Table 1.
Selected candidate biomarkers for endometriosis chosen based on the quality of evidence, reproducibility, and to illustrate different potential types of biomarkers and samples.
| Biomarker or combination of markers | Sample type | Diagnostic clinical relevance | Current gaps | References |
|---|---|---|---|---|
| Soluble BDNF | Plasma, serum | Minimal and mild EM, pain | Validation | 33,39,40 |
| lncRNA UCA1 | Serum | Ovarian EM, disease stage | Function, validation | 69 |
| Annexin V+VEGF+CA-125+ I-CAM | Serum | EM | Validation | 37,70 |
| sVCAM-1/sICAM-1 ratio | Serum | EM | Validation | 11 |
| mtDNA deletions in EM | Blood | EM | Function, validation | 9 |
| miR-145+miR-923+miR141 | Plasma | EM | Function, validation | 71 |
| mir122-5p | Serum | EM | Function, validation | 52 |
| miR-199-5p | Serum | EM | Function, validation | 52 |
| let7d | Serum | Severe EM, treatment of EM | Validation | 72 |
| KLK13 | Cervicovaginal fluid | EM-associated infertility | Validation | 65 |
| sFLT-1 | Urine | Minimal and mild EM | Validation | 73 |
| Leptin | Peritoneal fluid | Minimal and mild EM, pain | Validation | 74 |
| AOPP | Peritoneal fluid | EM, disease type (DIE, PE), disease stage | Validation | 75 |
| Nitrates/nitrites | Peritoneal fluid | EM, DIE, severe EM | Validation | 75 |
| miR-122+miR-145+miR-199a+miR-542-3p | Serum | EM | Function, validation | 54 |
EM: endometriosis; DIE: deep infiltrating endometriosis; PE: peritoneal endometriosis.
Strategies for non-invasive biomarker discovery
Studies seeking to uncover non-invasive biomarkers for endometriosis have employed two main experimental strategies: a hypothesis-driven approach or a high-throughput screening “omics” approach (Figure 1(a)).31 The hypothesis-driven approach uses current knowledge of endometriosis pathogenesis derived from animal models, in vitro experimental data, and in vivo data from patients to propose potential biomarkers for the disease that can then be tested in experimental and clinical studies. The potential biomarker, or combination of biomarkers, are then usually tested experimentally by comparing a cohort of patients and controls using small-scale multiplex experimental techniques like ELISA and reverse transcription quantitative PCR (RT-qPCR). In contrast, the “omics” screening approach uses high-throughput technologies such as RNA-seq, proteomics, and lipidomics to compare cohorts of control and endometriosis patients to identify candidate biomarkers in an unbiased fashion.
Hypothesis-driven biomarker discovery
The hypothesis-driven approach has been taken to try and identify endometriosis biomarkers by examining factors known to be associated with various aspects of the disease including the regulation of pain, inflammation, oxidative stress, cellular proliferation, migration, adhesion, survival, and immune responses. Many studies have examined candidate biomarkers in accessible body fluids (primarily blood) based on current knowledge of the pathogenesis of endometriosis, as has been well summarized by previous reviews (Figure 1(b)).12,31,32 We used this strategy to test the applicability of the differences in the levels of secretion of the adhesion molecules VCAM-1 and ICAM-1,11 pain regulatory protein BDNF,33 and the regulators of inflammation (soluble CD40 Ligand and CXCL1)34 in the serum of women with and without endometriosis. However, only one of the three studies validated our initial hypothesis. We could show that the soluble VCAM-1/ICAM-1 ratio is a promising non-invasive biomarker for the diagnosis of endometriosis.11 Comparing serum protein levels in endometriosis patients, compared to controls, we found that women with endometriosis had lower serum levels of sICAM-1 (P = 0.042) and higher levels of sVCAM-1 (P < 0.001). Our analysis revealed that the serum levels of sVCAM-1 was not affected by lesion type, menstrual cycle phase, or disease severity. A receiver operating characteristics curve (ROC), calculated to determine whether preoperative serum sVCAM-1 concentration can be used to predict endometriosis, found an AUC of 0.868 with 80% specificity and 84% sensitivity at a cutoff value of 370 pg/ml. This predictive performance was further improved by calculating the sVCAM-1/sICAM-1 ratio, leading to an AUC of 0.929 with 86.7% specificity and 90.3% sensitivity at a cutoff ratio value of 1.55. This study indicates that using a combination of targets could provide better specificity and sensitivity than single target studies in its ability to diagnose women with endometriosis. Similarly, it was shown that sensitivity and specificity for predicting endometriosis were improved by combining the measurements of CCR1 mRNA with the levels of MCP1 and CA-125 proteins in the blood of women compared to the use of the individual factors,35 or by assaying six inflammatory cytokines where a combination of increased levels of IL-6, IL-8 and CA-125 was the most predictive of disease.36 One of the more large-scale studies of this type examined the levels of 28 of inflammatory and non-inflammatory proteins including growth factors and adhesion molecules in serum from 121 controls and 232 endometriosis patients.37 Using univariate and multivariate statistical analyses, they found that two alternative combinations of the levels of four factors gave the best predictive value for endometriosis: (1) annexin V, VEGF, CA-125 and glycodelin or (2) annexin V, VEGF, CA-125, and ICAM-1. In contrast, a comprehensive review of the literature recommended that VEGF should not be used as an endometriosis biomarker,38 although it could be that it is a useful marker in combination with other markers as this study indicated.37
Pain is a common symptom of endometriosis, so neural molecules that can be detected in the blood are potential biomarkers of endometriosis. Therefore, several studies have examined the expression of brain-derived neurotrophic factor (BDNF) in endometriosis and have reported increased levels of soluble total BDNF protein in plasma of patients with endometriosis compared to women without the disease.39,40 BDNF was also shown to be upregulated in women with minimal and mild endometriosis compared to women without the disease, where it showed an AUC of 0.75 with a sensitivity of 91.7% and specificity of 69.4% at a cut-off of 1000 pg/ml.39 Additionally, the plasma levels of total BDNF have been correlated with pain and could discriminate between women with ovarian endometriosis and other benign gynecological disorders with an AUC of 0.72.39 Our study showed that the mature form of the secreted BDNF protein (mBDNF), which is part of the total secreted BDNF, is elevated in serum of women with endometriosis compared to women without endometriosis.33 This association was only with minimal and mild endometriosis and not the more severe forms of the disease, was independent of menstrual cycle phase, and was not associated with self-reported pelvic pain intensity VAS scores of either overall or menstruation-related pain. However, ROC curve analysis showed that despite this correlation, mBDNF is not a strong endometriosis biomarker candidate (AUC 0.6242, P = 0.02). Soluble BDNF protein is present in two forms as pro-BDNF and mBDNF, which might have a different impact on certain pathological conditions.41 This example illustrates the importance of assaying different forms of the same protein separately when they can have a different predictive value for diagnosing the disease.
Other aspects of endometriosis have also been explored as a potential source of biomarkers. Endometriosis fulfils most of the criteria for an autoimmune disorder,42 making autoantibodies promising candidates for diagnostic biomarkers. For example, the evaluation of the combination of the anti-IMP1 and ant-cylinB1 autoantibodies in serum showed a sensitivity of 83.9% and specificity of 72.7% to distinguish women with ovarian endometriosis from controls.43In contrast, some other autoantibodies, such as anti-syntaxin5, have shown lower diagnostic potential.44
Elevated levels of circulating cell-free DNA can reflect increased levels of cell death and is associated with inflammatory diseases such as rheumatoid arthritis.45 Therefore, it has been proposed that endometriosis patients may also show elevated levels of cell-free DNA in plasma and serum.46Circulating cell-free DNA can be of nuclear (nDNA) or mitochondrial (mtDNA) origin. A study by Zachariah et al. comparing 19 patients with 15 controls found that nDNA was significantly elevated in the plasma of endometriosis patients, but not in the serum, while no significant increase was seen for mtRNA in either fluid. A larger more recent study by Creed et al. looked for mutations of mitochondrial DNA in a cohort of 182 women.9 They found that the deletions of 1.2 and 3.7 kB can differentiate between endometriosis and controls in 9 out of 10 women, providing a potential biomarker for endometriosis that can be detected in the blood of patients.
The published data from others and our own experience have shown that a disadvantage of the hypothesis-driven approach is that it normally focuses on a narrow aspect of the disease, which can often lead to a negative outcome.34,38 For example, in the Cochrane Review by Gupta et al.38 from 54 studies included in the systematic analysis, 31 studies evaluating 77 different biomarkers for endometriosis have shown negative outcome of initial hypothesis and could not distinguish women with and without endometriosis. There is statistical evidence suggesting a publication bias against negative study outcomes,47 so this is likely an underestimate. In our opinion, it is important to report the results of well-designed studies with a negative outcome in order to avoid unnecessary replication and to better understand the complexity of the disease.
Contrarily, while the hypothesis-driven approach may miss some non-obvious opportunities for discovering biomarkers for the disease, by building a large body of established knowledge, this approach still has the potential for identifying new biomarkers that may not be obvious from hypothesis-free large-scale approaches.
High-throughput “omics” biomarker discovery
In recent years, an alternative approach for non-invasive biomarker discovery in endometriosis using high-throughput screening approaches has emerged and continues to evolve with the development of new technologies. These studies use screening technologies to detect multiple differences between studied cohorts and are therefore not restricted to a specific set of markers or to known or suspected regulators of disease pathogenesis. This unbiased approach may help to bring to light new regulators of disease pathogenesis that may not otherwise be uncovered with a hypothesis-based approach. The most commonly used screening techniques are focused on evaluating the differences in transcriptome, metabolome, proteome, and lipidome and include microarrays, next generation sequencing, mass spectrometry, 2 D–3D electrophoresis, and large-scale qPCR chips (Figure 1(c)). These techniques produce large and complex datasets that are challenging to analyze, requiring specialized software and skilled bioinformatic personal.48 The proper design and selection of the approach that best addresses the study question is important to generate relevant data that can be mined for potential biomarkers.
Transcriptome analysis based on microarray or RNA sequencing data evaluates the expression levels and variants present in four main groups of transcripts: messenger (m), micro (mi), long non-coding (lnc), and circular (circ) RNAs. Analysis of mRNAs often acts as a proxy for protein levels, although differences in mRNA expression levels do not necessarily translate to differences in protein levels, and obviously no information on post-translation modifications can be detected. However, such studies can still be valuable, as they do not require as much material as proteomics studies, are cheaper to conduct, and may detect potential endometriosis biomarkers at the mRNA level that could be assayed by PCR. MiRNAs are the best studied class of non-coding stable RNAs that can be detected in the circulation.49 These small (22 nucleotides (nt) in length), single-stranded RNA molecules act at the post-transcriptional level to either repress transcription or degrade their target mRNA.49 In our lab, we have used large-scale RT-qPCR arrays to identify differences in the levels of expression/secretion of miRNAs in plasma of women with and without endometriosis.50 We showed that a specific plasma miRNA signature is associated with endometriosis, with miR-154-5p alone (AUC = 0.72, P = 0.001) or in combination with miR-196b, miR-378-3p, and miR-33a-5p and the clinical parameters of body mass index and age (AUC = 0.72. P = 0.0002), showing diagnostic potential for the disease. To date, around 15 studies including our own have evaluated the potential of circulating miRNAs as diagnostic marker for endometriosis, with several single or panels of miRNAs showing promising diagnostic properties, as detailed in a series of reviews.50–53 For example, Wang et al.54 reported that a panel of four miRNAs (miR-122 + miR-145 + miR-199a + miR-542-3p) has 93.2% specificity and 96% sensitivity (AUC 0.994) in detecting women with endometriosis. Using RT-qPCR, Suryawanshi et al.55 examined 1113 miRNAs and identified 23 miRNAs showing significant differences in the plasma of women with endometriosis compared to controls. In the validation phase of the study, they found three miRNAs (miR-16, miR-191, and miR-195) that could distinguish between women with and without endometriosis with a sensitivity of 88% and specificity of 60%. Vanhie et al.53 used genome-wide miRNA expression profiling by small RNA sequencing to detect differences in the plasma levels of miRNAs in women with and without endometriosis. They found three miRNAs (miR-125-5p, miR-28-5p, and miR-29a-3p) that had a diagnostic power above chance in distinguishing women with minimal and mild endometriosis from women without the disease (AUC = 60%), with a sensitivity of 78%, but poor specificity (37%). Although these data support the potential of miRNAs as diagnostic marker, to date, there is no significant overlap between the targets identified in the different studies. This is likely due to a lack of consensus for data normalization methodology within the same or different experimental platforms and the high heterogeneity of study designs and biological samples analyzed.
Long non-coding RNAs (lncRNAs) are a class of RNAs greater than 200 nucleotides in length that show similar RNA biology features to mRNAs except that they do not code for a protein, but may be involved in gene regulation or other biological functions in the cell.56 They can be involved directly in the regulation of gene expression, or indirectly as regulators of miRNAs by acting as competing endogenous RNAs (ceRNAs), which titrate miRNAs away from natural mRNA targets.57 A growing number of lncRNAs have been shown to play important roles in development and disease,58 but to date, only one study has evaluated the potential of the circulating lncRNAs for the diagnosis of the endometriosis. Wang et al.59 showed that a combination of five serum lncRNAs can differentiate women with endometriosis from disease-free women with 89.7% sensitivity and 73.2% specificity. Recently, another class of non-coding RNAs called circular RNAs (circRNAs) has been detected in extracellular vesicles called exosomes, with their relative stability and abundance in bodily fluids making them attractive for non-invasive biomarker research.60 A study by Khalaj et al. on extracellular vesicles including exosomes in tissues and plasma from endometriosis patients and controls found that endometriosis patients had a distinctive miRNA and lncRNA expression signature, although circRNAs were not investigated in this study.61 These studies indicate that in addition to miRNAs, lncRNAs and perhaps circRNAs have potential as biomarkers for endometriosis.
Proteomics research focuses on the identification of proteins, their expression, localization, post-transcriptional modifications, and protein interactions on a large scale mostly using mass spectrometry-based technologies. The most commonly applied technologies in proteomic analysis for endometriosis biomarkers are surfaced enhanced laser desorption isolation (SELDI)-time of life (TOF)-mass spectrometry (MS), matrix-assisted laser desorption isolation (MALDI)-TOF-MS, and two-dimensional difference gel electrophoresis (2D-DGEF). These methods have been used to detect differentially expressed proteins and peptides in the peripheral blood of endometriosis patients, some of which have been proposed as potential endometriosis biomarkers, as has been previously summarized.62 For example, Liu et al.63 used SELDI-TOF-MS to assay plasma protein expression in patients with endometriosis compared to disease-free controls. They found 20 differential protein peaks with a sensitivity of 87.5% and specificity of 80% to distinguish between the analyzed groups. A proteome fingerprint model based on a cohort of 126 patients with endometriosis and 120 healthy controls was able to distinguish women with and without endometriosis with a sensitivity of 91.4% and specificity of 95.0%.64 The strength of this study over others is that the model based on three diagnosis mass-to-charge (m/z) ratios was validated on independent serum samples based on blind testing (sensitivity 89.3%, specificity 90.0%). Although biomarkers with high sensitivity and specificity for diagnosis of all stages of endometriosis were found using SELDI-TOF-MS and MALDI-TOF-MS, a disadvantage remains in the inability of these techniques to identify the underlying proteins, making validation of the targets and the development of diagnostic tests difficult.
Using LC-MS/MS assay, Grande et al.65 have found 30 differentially expressed protein in vaginal fluid of women with endometriosis, compared to controls and including proteins involved in regulation of immunity (SLURP1, PGLYRP1) and fertility (KLK13). As the latest are not expressed in women with the disease, they can be considered as candidates with high specificity and sensitivity to discriminate women with and without endometriosis. Several metabolites have also shown diagnostic potential as non-invasive biomarkers of endometriosis, as has been reviewed previously.66. For example, recently, Dutta et al.67 identified altered tissue metabolites for minimal and mild endometriosis, which they further explored in serum of the same patients to assess their value as diagnostic markers. For minimal endometriosis, alanine was found to have 90% sensitivity and 58% specificity, and for mild endometriosis, a panel of five amino acids (alanine, leucine, lysine, proline, and phenylalanine) showed a sensitivity of 100% and specificity of 83%. The lipidomic profile was also considered in the search for endometriosis biomarkers in endometrial fluid,68 but studies in blood are still lacking.
In summary, by characterizing body fluids of women with endometriosis compared to controls with diverse high-throughput “omics” methods potential biomarkers of the disease have been identified that have been missed by hypothesis-driven approaches. However, as with candidate biomarkers identified in hypothesis-driven studies, validation by independent studies is often lacking. A disadvantage can be that these studies lack the focus of hypothesis-driven studies, and that they can be overwhelmed by data, so appropriate analysis is required to identify the most promising biomarkers.
Concluding remarks
Despite numerous studies that have taken a diverse array of experimental approaches to uncover multiple candidate non-invasive biomarkers for endometriosis (Table 1), no clinically accepted biomarker is available. There are a number of explanations for this failure. Endometriosis is a common disease with diverse symptoms, and there is no consensus control group. Although healthy controls would be the ideal control group, for many studies, this is not possible, and patients that have been laparoscopically proven not to have endometriosis, but who may have other gynecological conditions, are often used as controls. The criteria by which patients are including in the control group may vary, adding extra heterogeneity to the existing studies. In order to assess the quality of endometriosis biomarkers studies, May et al.32 and Gupta et al.38 adopted the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) standards. They found that a lack of consensus in study design, data normalization, patient selection for cohort building, and clinical data characteristics often compromise the quality of studies and hinder combined systematic analysis of existing datasets. In addition, they observed that most current knowledge in endometriosis biomarker research has been acquired from relatively small study cohorts that often have not been validated in prospective studies.
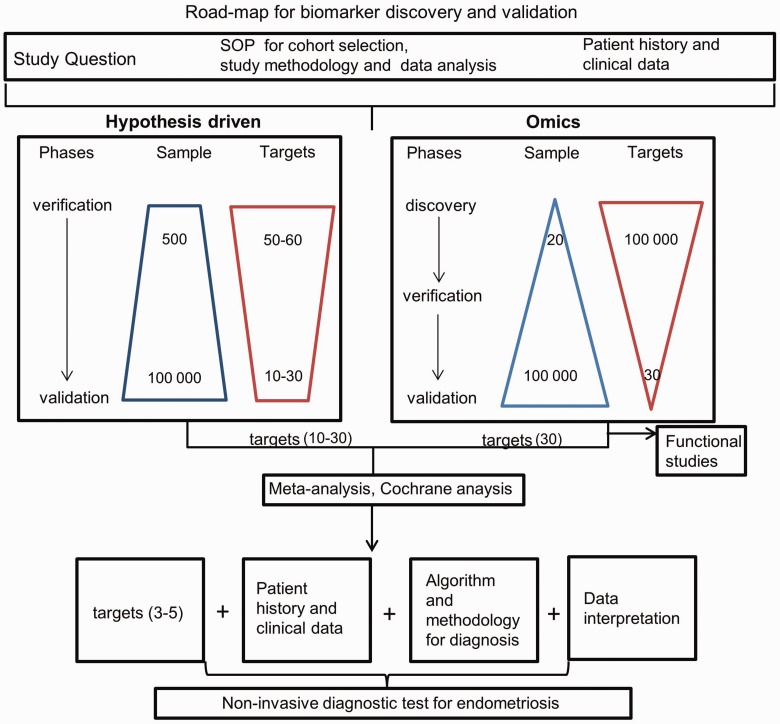
To overcome these limitations, there is a need to perform large-scale studies with the participation of multiple centers with agreed standard operating procedures (SOPs) for patient and control selection, patient data, and sample collection. For this purpose, the World Endometriosis Research foundation has initiated the Endometriosis Phenome and Bio-banking harmonization project (http://endometriosisfoundation.org/ephect/)76 to establish SOPs for sample collection, storage, and data collection. Despite the shortcomings of some previous endometriosis biomarker studies, there have been a number of candidates that have been detected in multiple studies as mentioned above. With this in mind, a multi-center endometriosis biomarker validation study (https://clinicaltrials.gov/; study ID: NCT03376451) has been initiated within the framework of the Horizon 2020 Scientific Initiative funded by the European Commission. The aim of the study is to validate a cluster of endometrial and blood biomarkers identified in previous studies for disease diagnosis and prognosis. Patient material and associated data, acquired using the SOPs described above, will be collected in 15 clinical centers located in Europe, USA, Canada, and Dubai. There may also be value in conducting a large-scale “omics” studies based on such a well-controlled cohort both as an unbiased method to validate existing biomarker candidates and to potentially uncover novel markers. For such a complex and heterogeneous disease like endometriosis, a biomarker panel will most likely be more accurate than a single marker, both for diagnosing the disease and perhaps also as an aid for classifying sub-types of the disease. By taking these approaches, the prospects for developing a non-invasive biomarker assay for endometriosis may finally be within reach, opening the opportunity for earlier diagnosis and onset of treatment that would help many women with this condition worldwide. Here, we propose a logistical road-map (Figure 2) for non-invasive biomarker discovery and validation that we believe would be useful for guiding future investigations.
Figure 2.
Road-map for biomarker discovery and validation. The pipeline for biomarker discovery and validation taking a hypothesis-driven approach (left) or “omics” approach (right). Meta-analysis of candidates emerging from these two approaches leads to the selection of the most promising candidates for validation studies. Candidates that pass this final validation step can then be introduced to the clinic. (A color version of this figure is available in the online journal.)
Authors’ contributions
QJH and IY wrote the original draft and prepared the table. IY prepared the figures. AP and RW reviewed and edited the article.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Iveta Yotova https://orcid.org/0000-0003-1810-4951
References
- 1.Giudice LC. Clinical practice. Endometriosis. N Engl J Med 2010; 362:2389–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Vigano P. Endometriosis. Nat Rev Dis Primers 2018; 4:1–25 [DOI] [PubMed] [Google Scholar]
- 3.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci USA 1999; 96:2543–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol 2006; 4: 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol 2002; 83:149–55 [DOI] [PubMed] [Google Scholar]
- 6.Lee SH, Sapkota Y, Fung J, Montgomery GW. Genetic biomarkers for endometriosis In: T D'Hooghe. (ed.) Biomarkers for endometriosis: state of the art. Cham: Springer International Publishing, 2017, pp.83–93 [Google Scholar]
- 7.Farland LV, Shah DK, Kvaskoff M, Zondervan KT, Missmer SA. Epidemiological and clinical risk factors for endometriosis In: T D'Hooghe. (ed.) Biomarkers for endometriosis: state of the art. Cham: Springer International Publishing, 2017, pp.95–121 [Google Scholar]
- 8.Schmidt A, Aebersold R. High-accuracy proteome maps of human body fluids. Genome Biol 2006; 7: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creed J, Maggrah A, Reguly B, Harbottle A. Mitochondrial DNA deletions accurately detect endometriosis in symptomatic females of child-bearing age. Biomark Med 2019; 13:291–306 [DOI] [PubMed] [Google Scholar]
- 10.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012; 98:511–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuessel L, Wenzl R, Proestling K, Balendran S, Pateisky P, Yotova S, Yerlikaya G, Streubel B, Husslein H. Soluble VCAM-1/soluble ICAM-1 ratio is a promising biomarker for diagnosing endometriosis. Hum Reprod 2017; 32:770–9 [DOI] [PubMed] [Google Scholar]
- 12.Fassbender A, Dorien O, Becker CM, D’Hooghe T. Peripheral blood biomarkers for endometriosis In: T D'Hooghe. (ed.) Biomarkers for endometriosis: state of the art. Cham: Springer International Publishing, 2017, pp.123–39 [Google Scholar]
- 13.Wu Y, Basir Z, Kajdacsy-Balla A, Strawn E, Macias V, Montgomery K, Guo SW. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil Steril 2003; 79:710–7 [DOI] [PubMed] [Google Scholar]
- 14.Barcena de Arellano ML, Arnold J, Vercellino F, Chiantera V, Schneider A, Mechsner S. Overexpression of nerve growth factor in peritoneal fluid from women with endometriosis may promote neurite outgrowth in endometriotic lesions. Fertil Steril 2011; 95:1123–6 [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod 1998; 13:1686–90 [DOI] [PubMed] [Google Scholar]
- 16.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am J Physiol Regul Integr Comp Physiol 2008; 294:R162–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol 2012; 358:208–15 [DOI] [PubMed] [Google Scholar]
- 18.Barragan F, Irwin JC, Balayan S, Erikson DW, Chen JC, Houshdaran S, Piltonen TT, Spitzer TL, George A, Rabban JT, Nezhat C, Giudice LC. Human endometrial fibroblasts derived from mesenchymal progenitors inherit progesterone resistance and acquire an inflammatory phenotype in the endometrial niche in endometriosis. Biol Reprod 2016; 94: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afshar Y, Hastings J, Roqueiro D, Jeong JW, Giudice LC, Fazleabas AT. Changes in eutopic endometrial gene expression during the progression of experimental endometriosis in the baboon, Papio anubis. Biol Reprod 2013; 88: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res 2019; 8: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol 2009; 175:547–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olkowska-Truchanowicz J, Bocian K, Maksym RB, Bialoszewska A, Wlodarczyk D, Baranowski W, Zabek J, Korczak-Kowalska G, Malejczyk J. CD4(+) CD25(+) FOXP3(+) regulatory T cells in peripheral blood and peritoneal fluid of patients with endometriosis. Hum Reprod 2013; 28:119–24 [DOI] [PubMed] [Google Scholar]
- 23.Izumi G, Koga K, Takamura M, Makabe T, Satake E, Takeuchi A, Taguchi A, Urata Y, Fujii T, Osuga Y. Involvement of immune cells in the pathogenesis of endometriosis. J Obstet Gynaecol Res 2018; 44:191–8 [DOI] [PubMed] [Google Scholar]
- 24.Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update 2013; 19:406–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab 2012; 97:E35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diaz I, Navarro J, Blasco L, Simon C, Pellicer A, Remohi J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: matched case-control study. Fertil Steril 2000; 74:31–4 [DOI] [PubMed] [Google Scholar]
- 27.Prapas Y, Goudakou M, Matalliotakis I, Kalogeraki A, Matalliotaki C, Panagiotidis Y, Ravanos K, Prapas N. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod Biomed Online 2012; 25:543–8 [DOI] [PubMed] [Google Scholar]
- 28.Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS. Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 2018; 12: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuldeore M, Yang H, Du EX, Soliman AM, Wu EQ, Winkel C. Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: a longitudinal analysis of claims databases. Fertil Steril 2015; 103:163–71 [DOI] [PubMed] [Google Scholar]
- 30.Haas D, Shebl O, Shamiyeh A, Oppelt P. The rASRM score and the Enzian classification for endometriosis: their strengths and weaknesses. Acta Obstet Gynecol Scand 2013; 92:3–7 [DOI] [PubMed] [Google Scholar]
- 31.Dorien FO, Flores I, Waelkens E, D'Hooghe T. Noninvasive diagnosis of endometriosis: review of current peripheral blood and endometrial biomarkers. Best Pract Res Clin Obstet Gynaecol 2018; 50:72–83 [DOI] [PubMed] [Google Scholar]
- 32.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 2010; 16:651–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perricos A, Ashjaei K, Husslein H, Proestling K, Kuessel L, Obwegeser R, Wenzl R, Yotova I. Increased serum levels of mBDNF in women with minimal and mild endometriosis have no predictive power for the disease. Exp Biol Med (Maywood) 2018; 243:50–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pateisky P, Pils D, Kuessel L, Szabo L, Walch K, Obwegeser R, Wenzl R, Yotova I. The serum levels of the soluble factors sCD40L and CXCL1 are not indicative of endometriosis. Biomed Res Int 2016; 2016: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agic A, Djalali S, Wolfler MM, Halis G, Diedrich K, Hornung D. Combination of CCR1 mRNA, MCP1, and CA125 measurements in peripheral blood as a diagnostic test for endometriosis. Reprod Sci 2008; 15:906–11 [DOI] [PubMed] [Google Scholar]
- 36.Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De Smet F, De Moor B, Meuleman C, Billen J, Blanckaert N, Vodolazkaia A, Fulop V, D'Hooghe TM. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod 2010; 25:654–64 [DOI] [PubMed] [Google Scholar]
- 37.Vodolazkaia A, El-Aalamat Y, Popovic D, Mihalyi A, Bossuyt X, Kyama CM, Fassbender A, Bokor A, Schols D, Huskens D, Meuleman C, Peeraer K, Tomassetti C, Gevaert O, Waelkens E, Kasran A, De Moor B, D'Hooghe TM. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod 2012; 27:2698–711 [DOI] [PubMed] [Google Scholar]
- 38.Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PM, Johnson N, Nisenblat V. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016; 4: 1–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels JM, Kay VR, Leyland NA, Agarwal SK, Foster WG. Assessing brain-derived neurotrophic factor as a novel clinical marker of endometriosis. Fertil Steril 2016; 105:119–28 e1-5 [DOI] [PubMed] [Google Scholar]
- 40.Rocha AL, Vieira EL, Ferreira MC, Maia LM, Teixeira AL, Reis FM. Plasma brain-derived neurotrophic factor in women with pelvic pain: a potential biomarker for endometriosis? Biomark Med 2017; 11:313–7 [DOI] [PubMed] [Google Scholar]
- 41.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci 2005; 6:603–14 [DOI] [PubMed] [Google Scholar]
- 42.Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, Missmer SA, Rahmioglu N, Zondervan KT, Becker CM. The association between endometriosis and autoimmune diseases: a systematic review and Meta-analysis. Hum Reprod Update 2019; 25:486–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi YC, Wang SC, Chao CC, Su CL, Lee YL, Chen LY. Evaluation of serum autoantibody levels in the diagnosis of ovarian endometrioma. J Clin Lab Anal 2010; 24:357–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabeta M, Abe Y, Takaoka Y, Kusanagi Y, Ito M. Identification of anti-syntaxin 5 autoantibody as a novel serum marker of endometriosis. J Reprod Immunol 2011; 91:48–55 [DOI] [PubMed] [Google Scholar]
- 45.Zhong XY, von Muhlenen I, Li Y, Kang A, Gupta AK, Tyndall A, Holzgreve W, Hahn S, Hasler P. Increased concentrations of antibody-bound circulatory cell-free DNA in rheumatoid arthritis. Clin Chem 2007; 53:1609–14 [DOI] [PubMed] [Google Scholar]
- 46.Zachariah R, Schmid S, Radpour R, Buerki N, Fan AX, Hahn S, Holzgreve W, Zhong XY. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod Biomed Online 2009; 18:407–11 [DOI] [PubMed] [Google Scholar]
- 47.Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, Decullier E, Easterbrook PJ, Von Elm E, Gamble C, Ghersi D, Ioannidis JP, Simes J, Williamson PR. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One 2008; 3:e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misra BB, Langefeld C, Olivier M, Cox LA. Integrated omics: tools, advances and future approaches. J Mol Endocrinol 2019; 62:R21–R45 [DOI] [PubMed] [Google Scholar]
- 49.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105:10513–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pateisky P, Pils D, Szabo L, Kuessel L, Husslein H, Schmitz A, Wenzl R, Yotova I. Hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod Biomed Online 2018; 37:449–66 [DOI] [PubMed] [Google Scholar]
- 51.Agrawal S, Tapmeier T, Rahmioglu N, Kirtley S, Zondervan K, Becker C. The miRNA mirage: how close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. Int J Mol Sci 2018; 19: 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maged AM, Deeb WS, El Amir A, Zaki SS, El Sawah H, Al Mohamady M, Metwally AA, Katta MA. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int J Gynaecol Obstet 2018; 141:14–9 [DOI] [PubMed] [Google Scholar]
- 53.Vanhie ADorien OPeterse D Beckers A Cuellar A Fassbender A Meuleman C Mestdagh P D'Hooghe T.. Plasma miRNAs as biomarkers for endometriosis. Hum Reprod 2019; 34:1650–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab 2013; 98:281–9 [DOI] [PubMed] [Google Scholar]
- 55.Suryawanshi S, Vlad AM, Lin HM, Mantia-Smaldone G, Laskey R, Lee M, Lin Y, Donnellan N, Klein-Patel M, Lee T, Mansuria S, Elishaev E, Budiu R, Edwards RP, Huang X. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res 2013; 19:1213–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uszczynska-Ratajczak B, Lagarde J, Frankish A, Guigo R, Johnson R. Towards a complete map of the human long non-coding RNA transcriptome. Nat Rev Genet 2018; 19:535–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Hou J, He D, Sun M, Zhang P, Yu Y, Chen Y. The emerging function and mechanism of ceRNAs in cancer. Trends Genet 2016; 32:211–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Pathogenesis of endometriosis: the genetic/epigenetic theory. Fertil Steril 2019; 111:327–40 [DOI] [PubMed] [Google Scholar]
- 59.Wang WT, Sun YM, Huang W, He B, Zhao YN, Chen YQ. Genome-wide long non-coding RNA analysis identified circulating LncRNAs as novel non-invasive diagnostic biomarkers for gynecological disease. Sci Rep 2016; 6 (23343):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in exosomes. Adv Exp Med Biol 2018; 1087:109–17 [DOI] [PubMed] [Google Scholar]
- 61.Khalaj K, Miller JE, Lingegowda H, Fazleabas AT, Young SL, Lessey BA, Koti M, Tayade C. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019; 4: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fassbender A Hubert A Waelkens EDorien OD’Hooghe T.. Proteomic biomarkers for endometriosis In: T D'Hooghe. (ed.) Biomarkers for endometriosis: State of the art. Cham: Springer International Publishing, 2017, pp.185–98 [Google Scholar]
- 63.Liu H, Lang J, Zhou Q, Shan D, Li Q. Detection of endometriosis with the use of plasma protein profiling by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry. Fertil Steril 2007; 87:988–90 [DOI] [PubMed] [Google Scholar]
- 64.Zheng N, Pan C, Liu W. New serum biomarkers for detection of endometriosis using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Int Med Res 2011; 39:1184–92 [DOI] [PubMed] [Google Scholar]
- 65.Grande G, Vincenzoni F, Milardi D, Pompa G, Ricciardi D, Fruscella E, Mancini F, Pontecorvi A, Castagnola M, Marana R. Cervical mucus proteome in endometriosis. Clin Proteomics 2017; 14: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizner TL. Noninvasive biomarkers of endometriosis: myth or reality? Expert Rev Mol Diagn 2014; 14:365–85 [DOI] [PubMed] [Google Scholar]
- 67.Dutta M, Singh B, Joshi M, Das D, Subramani E, Maan M, Jana SK, Sharma U, Das S, Dasgupta S, Ray CD, Chakravarty B, Chaudhury K. Metabolomics reveals perturbations in endometrium and serum of minimal and mild endometriosis. Sci Rep 2018; 8: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dominguez F, Ferrando M, Diaz-Gimeno P, Quintana F, Fernandez G, Castells I, Simon C. Lipidomic profiling of endometrial fluid in women with ovarian endometriosisdagger. Biol Reprod 2017; 96:772–9 [DOI] [PubMed] [Google Scholar]
- 69.Huang H, Zhu Z, Song Y. Downregulation of lncRNA UCA1 as a diagnostic and prognostic biomarker for ovarian endometriosis. Rev Assoc Med Bras (1992) 2019; 65:336–41 [DOI] [PubMed] [Google Scholar]
- 70.Dorien FO, Fassbender A, Van Bree R, Laenen A, Peterse DP, Vanhie A, Waelkens E, D'Hooghe TM. Technical verification and assessment of independent validation of biomarker models for endometriosis. Biomed Res Int 2019; 2019:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nisenblat V, Sharkey DJ, Wang Z, Evans SF, Healey M, Ohlsson Teague EMC, Print CG, Robertson SA, Hull ML. Plasma miRNAs display limited potential as diagnostic tools for endometriosis. J Clin Endocrinol Metab 2019; 104:1999–2022 [DOI] [PubMed] [Google Scholar]
- 72.Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril 2015; 103:1252–60 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho SH, Oh YJ, Nam A, Kim HY, Park JH, Kim JH, Park KH, Cho DJ, Lee BS. Evaluation of serum and urinary angiogenic factors in patients with endometriosis. Am J Reprod Immunol 2007; 58:497–504 [DOI] [PubMed] [Google Scholar]
- 74.Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A. Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Hum Reprod 2003; 18:1205–9 [DOI] [PubMed] [Google Scholar]
- 75.Santulli P, Chouzenoux S, Fiorese M, Marcellin L, Lemarechal H, Millischer AE, Batteux F, Borderie D, Chapron C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum Reprod 2015; 30:49–60 [DOI] [PubMed] [Google Scholar]
- 76.Rahmioglu N, Fassbender A, Vitonis AF, Tworoger SS, Hummelshoj L, D'Hooghe TM, Adamson GD, Giudice LC, Becker CM, Zondervan KT, Missmer SA. World endometriosis research foundation endometriosis phenome and biobanking harmonization project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil Steril 2014; 102:1233–43 [DOI] [PMC free article] [PubMed] [Google Scholar]