Abstract
Background
von Willebrand factor (VWF) and factor VIII (FVIII) circulate in the plasma as a non-covalent complex, and the majority of FVIII is likely cleared by VWF-dependent pathways. Clearance of VWF-free FVIII is rapid and underlies the pathological basis of some quantitative FVIII deficiencies. The receptor pathways that regulate the clearance of VWF-bound and VWF-free FVIII are incompletely uncharacterized. The human liver expressed endothelial lectin CLEC4M has been previously characterized as a clearance receptor for VWF, although its influence on FVIII is unknown.
Objectives
The interaction between FVIII and CLEC4M was characterized in the presence or absence of VWF.
Methods
FVIII interactions with CLEC4M were evaluated by an in vitro cell based and solid phase binding assays. Interactions between FVIII and CLEC4M or liver sinusoidal endothelial cells were evaluated in vivo by immunohistochemistry.
Results
CLEC4M-expressing HEK 293 cells bound and internalized recombinant and plasma-derived FVIII through VWF-dependent and -independent mechanisms. CLEC4M-binding to recombinant FVIII was dependent on mannose-exposed N-linked glycans. CLEC4M mediated FVIII internalization via a clathrin-coated pit-dependent mechanism, resulting in transport of FVIII from early and late endosomes for catabolism by lysosomes. In vivo hepatic expression of CLEC4M after hydrodynamic liver transfer was associated with a decrease in plasma levels of endogenous murine FVIII:C in normal mice, while infused recombinant human FVIII associated with sinusoidal endothelial cells in the presence or absence of VWF.
Conclusions
These findings suggest that CLEC4M is a novel clearance receptor that interacts with mannose-exposed glycans on FVIII in the presence or absence of VWF.
Introduction
Plasma levels of the glycoprotein coagulation factor VIII (FVIII) are highly variable in the normal population (50–200%). Low levels of FVIII associate with the inherited bleeding disorders hemophilia A and von Willebrand disease (VWD) (<1 – 50%), while epidemiological studies and animal models have linked elevated plasma FVIII levels to an increased risk for venous and arterial thrombosis (>150%) [1–3]. Plasma FVIII levels are influenced by the rate at which FVIII is synthesized and secreted, its rate of clearance from the plasma, and its interaction with the multimeric glycoprotein von Willebrand Factor (VWF). Approximately 95–97% of plasma FVIII circulates in the plasma in a dynamic equilibrium with VWF [4–6]. VWF protects FVIII from proteolysis [7], as well as from accelerated clearance from the plasma [8] and thus the concentration of circulating VWF, and the binding affinity between VWF and FVIII regulate plasma FVIII levels. The majority of circulating FVIII is thus likely cleared through VWF-dependent receptor-ligand interactions.
However, VWF-independent clearance pathways for FVIII have both physiologic and pathophysiologic relevance. Although the amount of VWF-free FVIII in the circulation is relatively low, it has a 6–8-fold faster clearance rate than VWF-bound FVIII, suggesting that the proportion of FVIII cleared in a VWF-independent manner is thus substantial. Moreover, inherited bleeding disorders involving quantitative FVIII deficiency can result from accelerated clearance of VWF-free FVIII. Type 2N VWD is characterized by pathogenic variants in the D’D3 FVIII-binding region of the VWF gene that result in impaired binding of VWF to FVIII, resulting in isolated FVIII deficiency [9]. Conversely some mild/moderate forms of hemophilia A are the result of F8 gene variants that impair FVIII binding to VWF [10]. In both cases, the pathways that underlie this pathological enhanced clearance of VWF-free FVIII are largely unknown. Furthermore, as elevated plasma FVIII is a risk factor for thrombosis, the rapid clearance of VWF-free FVIII in normal individuals may be crucially important in maintaining physiological FVIII levels, and dysregulation of these clearance pathways could contribute to elevated plasma FVIII levels and an increased risk for thrombosis.
Variants in the VWF gene and the VWF-modifying ABO blood group locus account for approximately 50% of the variability in plasma FVIII levels [11]. As every 1% change in plasma VWF levels is associated with a ~0.54% change in plasma FVIII:C [12], it is thought that the majority of quantitative trait loci that modify plasma VWF also modify FVIII but with a decreased magnitude of influence and statistical association. GWAS analyses have identified variants in genes involved in biosynthesis and secretion and receptor-mediated clearance as associating with both plasma levels of VWF and FVIII [13–15]. Interestingly, VWF but not FVIII plasma levels associated with a common variant within the CLEC4M gene (rs868875), which encodes a transmembrane calcium-dependent lectin receptor (encoding CLEC4M (C-type lectin member 4 family M, also termed L-SIGN or DC-SIGNR) expressed on the sinusoidal endothelial cells of the liver and lymphoid tissues [16]. CLEC4M had been previously described as an adhesive receptor for pathogens such as HIV, capable of mediating infection in trans in an ICAM-3-dependent manner [17]. Importantly while no association between CLEC4M gene variants and plasma FVIII levels was reported, this may be related to the genome-wide significance cut-off threshold for reporting this association, rather than an absence of a biological interaction between FVIII and CLEC4M.
We and others have previously found that variants within the CLEC4M gene, including the GWAS-identified SNV rs868875, or a variable number of tandem repeat (VNTR) polymorphism in the neck region of CLEC4M which is in linkage disequilibrium with the rs868875 SNV, can modify the phenotype of type 1 VWD [18,19]. We have also demonstrated that CLEC4M can bind VWF through interactions with its N-linked glycans, and mediate the internalization of VWF to early endosomes [18]. However, the ability of CLEC4M to interact with FVIII in the presence or absence of VWF has not been characterized. Additionally, the mechanistic basis by which CLEC4M is able to facilitate internalization of its glycoprotein ligands has not been demonstrated. In these studies, we investigate the ability of CLEC4M to act as an endocytic receptor for FVIII in the presence and absence of VWF. We further characterize the mechanism by which CLEC4M binds and internalizes FVIII and describe the subsequent endocytic pathway of this ligand.
Methods and Materials
FVIII Products
Plasma-derived VWF-FVIII (2.4:1, Humate-P) was from CSL Behring (King of Prussia, USA), and (1:1, Wilate) was from Octapharma, Lachen, Switzerland. Human recombinant FVIII (rFVIII) products included Advate (Baxter, Deerfield, USA), Kogenate-FS and Kovaltry (Bayer, Leverkusen, Germany), Xyntha (Pfizer, New York, USA), and Nuwiq (Octapharma). Plasma-derived FVIII (pdFVIII) (Haemocetin) (Biotest, Dreieich, Germay) had been purified to contain 1% VWF. All experiments unless otherwise specified were performed with Advate (rFVIII) or Humate P (pdVWF-FVIII).
Imaging Studies
Cell Culture
A HEK 293 stable cell line expressing CLEC4M possessing the 7 copy VNTR polymorphic allele and control (pCIneo stable cells) were generated and maintained as described [18].
Antibodies
Antibodies used included: sheep anti-F8 (Affinity Biologicals), rabbit anti-VWF (DAKO), mouse anti-CLEC4M (R&D), rabbit anti-CLEC4M (Novus, Littleton, USA), rabbit anti-EEA1 (Cell Signalling Technology, Beverly, MA, USA), rabbit anti-Rab9 (Cell Signaling Technology), mouse anti-LAMP1 (Abcam), and rat anti-murine CD31 (Dianova, Hamburg, Germany).
Immunocytochemistry
CLEC4M-expressing cells were exposed to rFVIII or pdVWF-FVIII in binding buffer buffer (10 mM HEPES pH 7.4, 135 mM NaCl, 10 mM KCl, 5 mM CaCl2, 2 mM MgSO4) for 15 – 120 minutes. Cells were washed and prepared as previously described. Slides were imaged with a Quorum Wave FX Spinning Disc confocal microscope and Hamamastsu Orca high resolution camera. Images were analyzed using ImageJ software (NIH). For some experiments, cells were preincubated with DMSO (0.2%, 30 minutes), methyl-β-cyclodextrin (5 mM, 60 minutes) (Sigma Aldrich, St. Lousi, MO. USA), pitstop-2 (20 μM, 10 minutes) (Abcam, Cambridge, England), or dynasore hydrate (80 μM, 30 minutes) (Sigma Aldrich). Quantification of immunofluorescent images was performed using ImagePro Plus Software (Media Cybernetics, Rockville, USA).
Immunohistochemistry
FVIII KO or VWF/FVIII DKO mice received tail vein injections of human rFVIII in accordance with previous studies [20]. 30 minutes post-infusion, tissues were prepared by saline and formaldehyde perfusion. IHC analysis was performed on formalin fixed paraffin-embedded 7 μM tissue sections as described [20]. Imaging was performed using a Leica SP8 laser scanning confocal microscope using 63X oil immersion objective. Images were analyzed using ImageJ or FIJI software (NIH, Bethesda, USA).
Enhanced sensitivity human FVIII:Ag ELISA
FVIII ELISA (Affinity Biologicals, Ancaster, Canada) was performed according to manufacturer’s protocols using a biotinylated sheep anti-Factor VIII (Affinity Biologicals) antibody followed by incubation with streptavidin poly-HRP (Pierce/Thermo Scientific, Rockford, IL USA) and developed with o-Phenylenediamine (Sigma Aldrich). To quantify FVIII:Ag in cell lysates, FVIII levels were normalized to total protein concentration (BioRad, Hercules, USA). For some experiments, cells were preincubated with rFVIII for 5 hours followed by incubation with chloroquine disulfide (250 μM) (Sigma Aldrich), MG132 (50 μM in DMSO) (Sigma Aldrich), NH4Cl (20 mM) (Sigma Aldrich), or DMSO (0.2%) vehicle control for 2 hours prior to lysis.
Solid phase immunosorbent assay
The interaction between FVIII and recombinant CLEC4M-Fc chimera (R&D Minneapolis, USA) was measured as described [18] with several modifications. FVIII was coated on Maxisorp plates (Nunc, Rochester, USA) in 50 mM Na2HCO3 and Fc-chimera binding was detected with an HRP-conjugated goat anti-human Fc antibody (AbCam). Alternatively, CLEC4M-Fc was coated in carbonate buffer and FVIII binding was detected with an anti-FVIII-HRP antibody (Affinity Biologicals) or a monoclonal anti-FVIII A1 antibody 8002 (Green Mountain Antibodies, Burlington, USA) with a goat-anti Mouse Ig-HRP (Southern Biotech, Birmingham, USA). For all experiments, CLEC4M-Fc or FVIII binding was compared with background binding to a BSA-coated negative control. For some experiments, FVIII and/or -Fc chimera proteins were preincubated with 1 mg/mL mannan or anti-VWF (DAKO), or anti-FVIII polyclonal (Affinity Biologicals) or monoclonal antibodies GMA-8002, −8016, −8011, or −8008 (Green Mountain Antibodies, Burlington, USA) (100 μg/mL) for 30 minutes at room temperature. Mannan, α-mannosidase used for some experiments were from Sigma Aldrich.
Animal models
Hepatic expression of CLEC4M in mice was induced via hydrodynamic gene transfer of the CLEC4M cDNA in the pLIVE liver expression vector [18]. FVIII:C was measured using the Chromogenix Coatest assay (Diapharma, West Chester, USA) according to the manufacturer’s directions against a normal C56Bl/6 mouse plasma pool. Recombinant human FVIII was administered by tail vein infusion (100 U/mouse) and tissues were perfused with saline and formalin after 30 minutes. VWF/FVIII DKO mice were generated on a C57BL/6 background [21,22]. To induce liver sinusoidal endothelial cell (LSEC) cytotoxicity, mice received an IP injection of 200 mg/kg cyclophosphamide (Sigma Aldrich) 24 hours prior to study [23]. Human rFVIII was administered at 200 IU/kg by tail vein injection and blood was collected by retro-orbital plexus into 10% buffered citrate. Samples were centrifuged at 10,000xg for 10 minutes and maintained at −80°C until FVIII:Ag ELISA.
Statistical analysis
T-tests or one-way ANOVA was performed on experiments with n≥4 using GraphPad InStat software version 3.06 (La Jolla, CA, USA) or SPSS version 16 (IBM, Armonk, NY, USA). Values are expressed as mean ± standard error. Figures denote P<0.05 with * and P <.001 with **.
Results
FVIII and VWF-FVIII complex is internalized by CLEC4M-expressing cells in vitro and/or in vivo
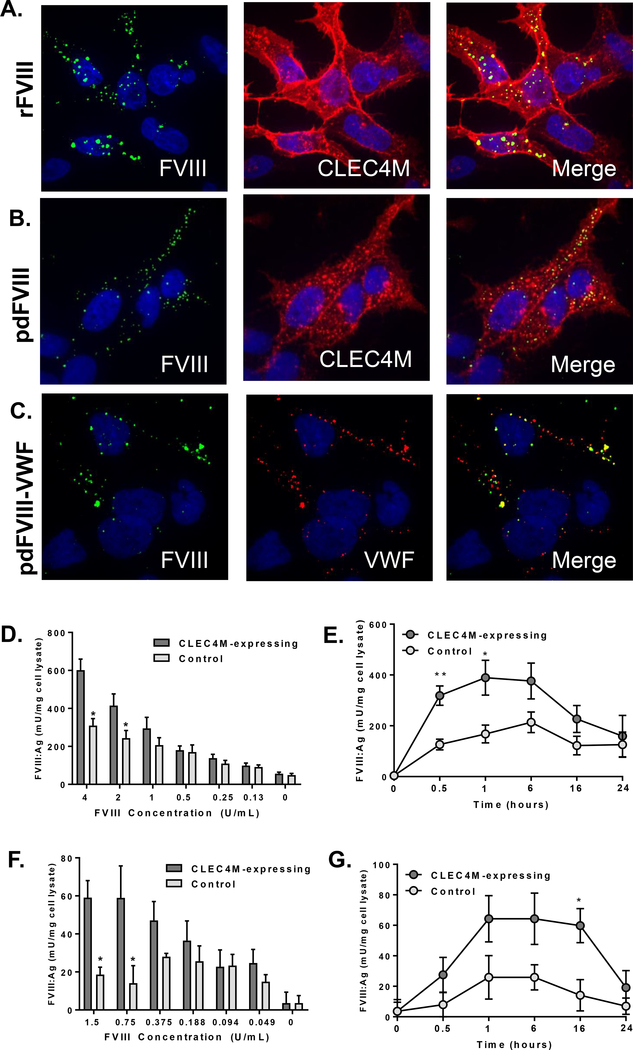
CLEC4M is expressed on the sinusoidal endothelial cells of the liver and lymphoid tissues [16] which rapidly lose their phenotype in culture [24] and commercially available primary liver or lymphatic sinusoidal endothelial cells do not express CLEC4M (unpublished observations). We therefore generated a stable CLEC4M-expressing HEK 293 cell line that was >90% CLEC4M-positive [18]. CLEC4M-expressing and pCIneo (stable vector backbone) control cells were exposed to FVIII products at physiological concentrations [25]. Immunofluorescence demonstrated that rFVIII, pdFVIII, and pdVWF-FVIII complex were internalized by CLEC4M-expressing cells after 1 hour (Figure 1A–C), and not by control cells that did not express CLEC4M (Supplement Figure 1D and 1E). FVIII co-localized with CLEC4M in cells exposed to rFVIII (Figure 1A), pdFVIII (Figure 1B) or the VWF-FVIII complex (overlay not shown). Additionally, we observed that on CLEC4M-expressing cells exposed to the VWF-FVIII complex, VWF and FVIII partially colocalized (Figure 1C), suggesting that CLEC4M can also bind and internalize VWF and FVIII as a complex. For all imaging conditions, CLEC4M-negative and isotype controls were performed (Supplemental Figure 1).
Figure 1. Internalization of FVIII by CLEC4M-expressing HEK 293 cells.
CLEC4M-expressing cells were exposed to recombinant (r) or plasma-derived (pd) FVIII and internalization was measured using immunofluorescence or ELISA. (A) Internalization of rFVIII, (B) pdFVIII by CLEC4M expressing cells using immunofluoresence (2 U/mL FVIII, 1 hour incubation); CLEC4M (red), FVIII (green), DAPI (blue), colocalization (yellow). (C) Internalization of VWF-FVIII complex by CLEC4M-expressing cells; VWF (red), FVIII (green), DAPI (blue), colocalization (yellow) (2 U/mL VWF, 0.83 U/mL FVIII, 1 hour incubation). Images are representative of 5–6 independent experiments. Quantification of rFVIII internalization by CLEC4M-expressing cells by FVIII:Ag ELISA; dose response (1 hour) (D), time course (2 U/mL FVIII) (E). Quantification of pd-VWF-FVIII internalization by CLEC4M-expressing cells by FVIII:Ag ELISA; dose response (1 hour) (F), time course (1.5 U/mL FVIII) (G). For all ELISA conditions, n=3–5 independent experiments. ± SEM, *p<0.05, **p<0.001.
The binding and internalization of rFVIII (Figures 1D and 1E) or pdVWF-FVIII (Figures 1F and 1G) by CLEC4M-expressing cells was quantified. Cells were exposed to physiological or therapeutic FVIII concentrations as described, and cells were washed, lysed, and FVIII:Ag in cell lysates was measured using an enhanced sensitivity ELISA. Cells expressing CLEC4M had approximately two-fold more FVIII detected in cell lysates than cells that did not express CLEC4M, suggesting that CLEC4M is able to bind and/or internalize FVIII alone or as part of a complex with VWF. FVIII observed in the lysate of cells that did not express CLEC4M may be bound to the HEK 293 cell surface via FVIII C2 domain interactions with the phospholipid membranes, or by heparan sulfate proteoglycans. It is currently unknown if these interactions enhance the capture and endocytosis of FVIII by endocytic receptors such as CLEC4M, as previous reports have also indicated that FVIII interactions with heparan sulfate proteoglycans on the cell surface can enhance the internalization by LRP-1 [26].
We observed a difference in the total amount of FVIII internalized between the two products. As the ratio of VWF to FVIII in the plasma-derived complex is 2.4:1, FVIII only accounts for a fraction of the total material internalized, and the relative size of the VWF-FVIII complex is much larger than rFVIII alone which may alter the rate of internalization. Additionally, rFVIII (VWF-free) was used as a standard for both assays, as internalized FVIII is rapidly denatured and degraded, and thus may lose its VWF-binding ability. However, the presence of some VWF-bound FVIII in these lysates may decrease the apparent amount of FVIII detected by the polyclonal anti-FVIII antibody (Supplemental Figure 5).
CLEC4M binds mannose exposed glycans on FVIII
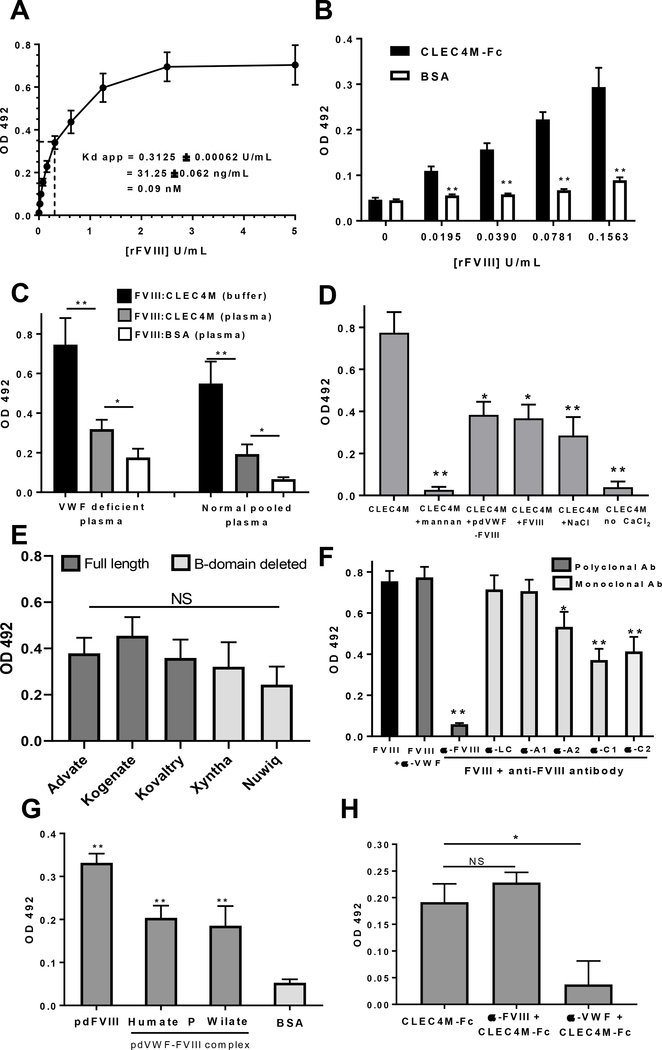
We next confirmed that CLEC4M interacts with FVIII using a solid phase binding assay with recombinant CLEC4M-Fc fusion protein in dose-dependent manner; assuming the specific activity of this product is 1 IU = 100 ng/mL [27], this interaction has an apparent Kd of <0.1 nM (Figures 2A and 2B). We further demonstrated that binding to rFVIII occurred in a plasma environment ±VWF (Figure 2C), was calcium-dependent, reversible, and could be partially blocked by pre-incubation with the mannose polymer mannan or with soluble pdVWF-FVIII, or rFVIII (Figure 2D). Immobilized CLEC4M-Fc bound to 2nd and 3rd generation rFVIII products (Figure 2E), and pdFVIII (<1% VWF) and pdVWF-FVIII complex (Figure 2G). Of note, differences in the interaction between FVIII products may be related to glycan content or specific activity of each product, and the variable recognition of FVIII (±VWF) products by the polyclonal anti-FVIII detection antibody (Supplemental Figure 5). Preincubation of immobilized FVIII with monoclonal antibodies to the A2, C1, and C2 domains (Figure 2E) could partially attenuate binding to CLEC4M-Fc. Pre-incubation of Humate P with a polyclonal anti-VWF antibody (Affinity Biologicals) attenuate binding to CLEC4M-Fc, while an anti-FVIII antibody did not (Figure 2H).
Figure 2. Binding of FVIII to recombinant CLEC4M-Fc.
The binding of recombinant CLEC4M-Fc fusion protein to FVIII was characterized using a solid phase immunosorbent assay as described in Methods. (A) Soluble rFVIII binds to immobilized CLEC4M-Fc (10 μg/mL) in a dose-dependent manner with an apparent Kd < 0.1 nM. (B) Soluble rFVIII binds to immobilized CLEC4M-Fc (10 μg/mL) at low concentrations (< 0.2 U/mL) when compared with binding to a BSA (10 μg/mL) control. (C) Soluble rFVIII (5 U/mL) binds to immobilized CLEC4M-Fc (10 μg/mL) in defibrinated normal and VWF-deficient pooled plasma. (D) The interaction between soluble CLEC4M-Fc (10 μg/mL) and immobilized FVIII (30 U/mL) was blocked with mannan (1 mg/mL), calcium-dependent, partially reversible with a 500 mM NaCl wash, and competed with soluble plasma-derived VWF-FVIII complex (10 U/mL) and recombinant FVIII (100 U/mL). (E) Various soluble rFVIII products (full-length and B-domain deleted) (2 U/mL) bound to immobilized CLEC4M-Fc (5 μg/mL). FVIII binding was detected using a monoclonal anti-FVIII A1 antibody as described in Methods. (F) Preincubation of immobilized rFVIII (30 U/mL) with domain specific antibodies (100 μg/mL) attenuated the interaction between FVIII and soluble CLEC4M-Fc (10 μg/mL). (G) Immobilized CLEC4M-Fc (5 μg/mL) bound to plasma-derived FVIII (1 U/mL) containing (<1% VWF), and pdVWF-FVIII complex (1 U/mL) (2.4 VWF: 1 FVIII, or 1 VWF:1 FVIII). (H) Pre-incubation of immobilized 2.4:1 VWF-FVIII complex (1 U/mL) with a polyclonal anti-VWF antibody (100 μg/mL, Affinity Biologicals) attenuate binding to CLEC4M-Fc, while an anti-FVIII antibody (100 μg/mL, Affinity Biologicals) did not (Figure 2H). For all assays, n=3–5 independent experiments, ± SEM, *p<0.05, **p<0.001.
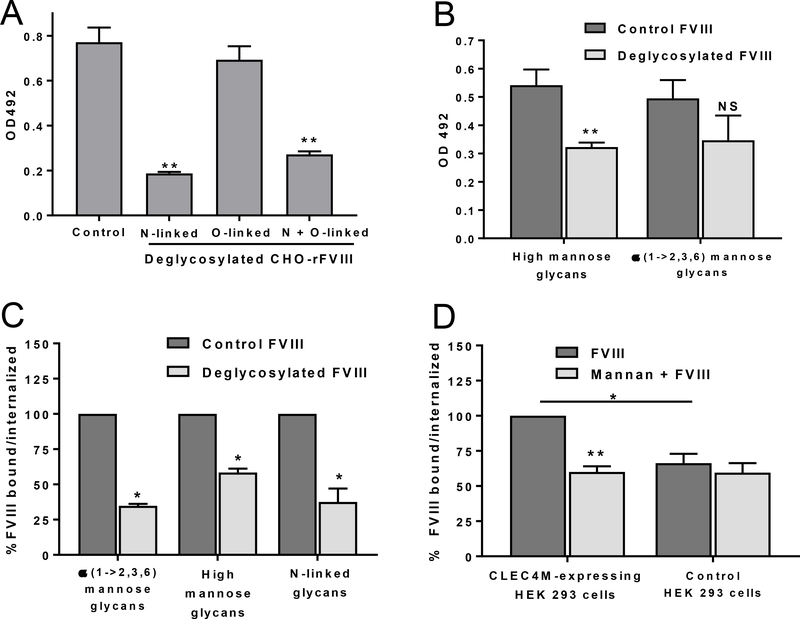
We have previously demonstrated that CLEC4M-Fc interacts with N-linked glycans on VWF [18]. Interestingly, while CLEC4M has been described as a mannose-binding lectin, glycomic analysis of human plasma-derived VWF identified predominantly complex-type N-linked glycans with only trace evidence of high mannose structure [28]. While the majority of N-linked glycans on FVIII are also of complex type, the A1 (Asn-258) and C1 (Asn-2137) domains each contain a single high mannose glycan [27,29,30]. Here, the ability of CLEC4M to interact with N- and O-linked glycans on rFVIII was characterized. Removal of N-linked, O-linked, N -and O-linked, high mannose glycans, and α(1→2,3,6)-linked mannose sugars was performed using endoglycosidase digestion as directed by the manufacturer (Sigma Aldrich or New England Biolabs, Ipswich, USA) and confirmed by Galanthus nivalis lectin binding (Vector Labs, Burlingame, USA) (Supplemental Figure 2). Removal of the rFVIII N- but not O-linked glycans attenuated binding to CLEC4M-Fc (Figure 3A). Removal of high mannose glycans greatly and α(1–2,3,6) linked mannose sugars modestly decreased the interaction between rFVIII and CLEC4M-Fc respectively (Figure 3B). CLEC4M-expressing cells were incubated with deglycosylated or control rFVIII for 2 hours and FVIII:Ag in cell lysates was measured with ELISA. Removal of N-linked glycans, high mannose glycans, or α(1–2,3,6) linked mannose sugars decreased internalization of rFVIII relative to controls (Figure 3C). Additionally, preincubation of CLEC4M-expressing cells with the mannose polymer mannan (100 μg/mL) attenuated the binding/internalization of FVIII (Figure 3D).
Figure 3. Glycan-dependent binding/internalization of FVIII by CLEC4M.
(A) Soluble CLEC4M-Fc (10 μg/mL) binds to immobilized deglycosylated FVIII (30 U/mL). (B) Soluble CLEC4M-Fc binds to immobilized demannosylated FVIII (30 U/mL). (C) Internalization of deglycosylated/demannosylated FVIII (2 U/mL, 1-hour incubation) by CLEC4M-expressing cells. (D) Internalization of rFVIII (2 U/mL,1-hour incubation) by CLEC4M-expressing cells preincubated with mannan (100 μg/mL). For all assays, n=3–5 independent experiments, ± SEM, *p<0.05, **p<0.001.
CLEC4M internalizes VWF and FVIII through clathrin-coated pits
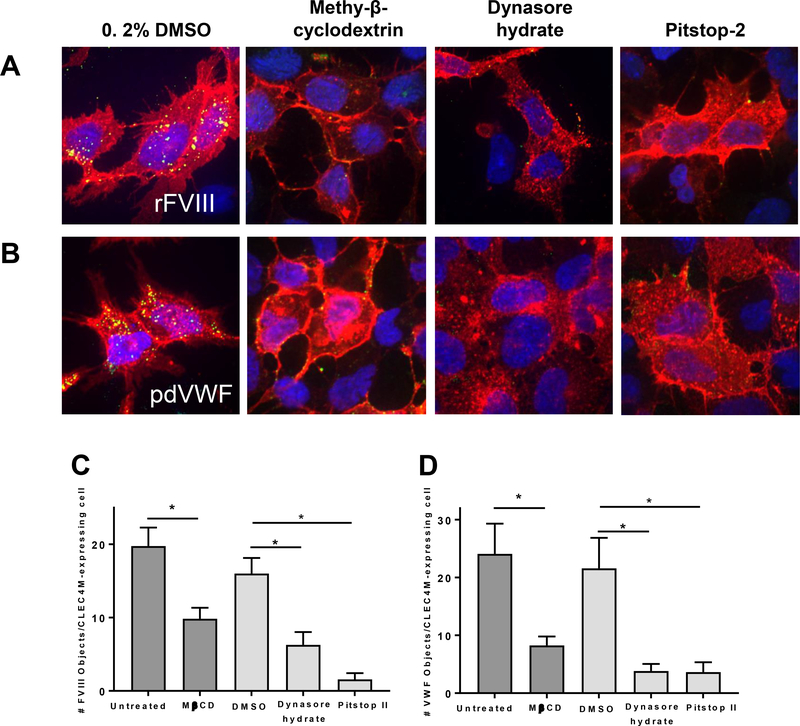
We have previously observed that VWF internalized by CLEC4M-expressing HEK 293 cells is transported to early endosomes [18]. To better characterize this endocytic pathway, CLEC4M-expressing cells were pretreated with methyl-β-cyclodextrin to deplete cell membrane cholesterol and disrupt lipid rafts, dynasore hydrate to inhibit dynamin GTPase activity, and pitstop-2 that binds the terminal domain of clathrin and prevents internalization of clathrin-coated pits. Cells were then incubated with human rFVIII or pdVWF-FVIII for 1 hour and internalization was visualized by immunofluorescence (Figure 4A and 4B). Internalized rFVIII or VWF was quantified by ImagePro analysis and demonstrated that binding/internalization of rFVIII or pdVWF by CLEC4M-expressing cells was reduced by each of the three reagents used to disrupt the clathrin-coated pit-mediated endocytic pathway - methyl-β-cyclodextrin, dynasore hydrate, and pitstop-2 (Figure 4A–D).
Figure 4. Internalization of FVIII by CLEC4M by a clathrin-coated pit-dependent pathway.
CLEC4M-expressing cells were preincubated with endocytosis inhibitors for 1 hour as described in Methods. (A and B) Association of rFVIII (2 U/mL) or pdVWF (2 U/mL VWF, 2.4:1 VWF-FVIII complex) with CLEC4M-expressing cells (1 hour) pre-treated with inhibitors of endocytic pathways, methyl-β-cyclodextrin (MBCD), pitstop-2 (PS), dynasore hydrate (DH) as described in Methods. Images are representative from 4–5 independent experiments. Cells were imaged with immunofluorescence; CLEC4M (red), FVIII (green), DAPI (blue), colocalization (yellow). (C and D) Images from 4–5 independent experiments were quantified using ImagePro software and cell-bound VWF or FVIII was normalized to the number of CLEC4M expressing cells per field of view. ± SEM, p<0.05 = *, p<0.001 = **.
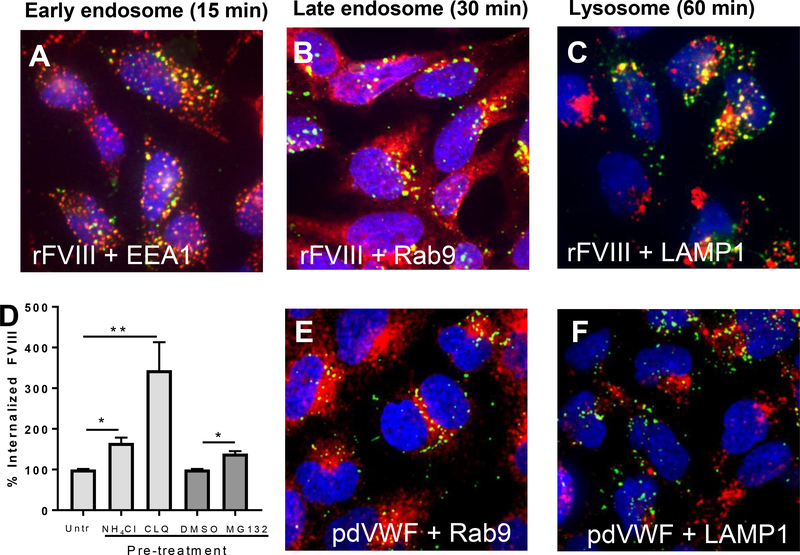
To visualize the endocytic pathway of FVIII, CLEC4M-expressing HEK 293 cells were incubated with rFVIII for 15, 30, or 60 minutes [30]. When CLEC4M-expressing cells were exposed to rFVIII for 15 minutes, we observed colocalization between FVIII and early endosomal antigen 1 (EEA1), confirming that upon internalization by CLEC4M, FVIII is trafficked to early endosomes (Figure 5A). When CLEC4M-expressing cells were incubated with rFVIII for 30 minutes, we observed colocalization with Rab-9, a marker for late endosomes (Figure 5B). When CLEC4M-expressing cells were incubated with rFVIII for 1 hour, we observed colocalization of FVIII with LAMP1, a lysosomal marker, suggesting that the internalization of FVIII by CLEC4M leads to lysosomal-mediated catabolism of the FVIII protein in this HEK 293-based model (Figure 5C). To confirm the role of lysosomes and proteasomes in the catabolism of FVIII, CLEC4M-expressing HEK 293 cells were treated with rFVIII for 5 hours, cells were washed, and then exposed to the lysosome inhibitors chloroquine disulfide (CLQ) and NH4Cl, and the proteasome inhibitor MG132 for 2 hours. FVIII in cell lysates was measured by ELISA. Exposure of the cells to both the lysosomal inhibitors (CLQ and NH4Cl), and the proteasome inhibitor MG132 significantly increased detectable FVIII in the lysates of CLEC4M-expressing cells (Figure 5D).
Figure 5. Intracellular trafficking and catabolism of FVIII and VWF by CLEC4M-expressing cells.
CLEC4M-expressing HEK 293 cells were incubated with rFVIII (2 U/mL) or pdVWF (2 U/mL VWF, 2.4:1 VWF-FVIII complex) for 15, 30, and 60 minutes and imaged with immunofluorescence. (A) Colocalization with early endosomes. Early endosomal antigen 1 (EEA1) (red), FVIII (green), DAPI (blue), colocalization (yellow). (B) Colocalization with late endosomes. Late endosomal marker Rab-9 (red), FVIII (green), DAPI (blue), colocalization (yellow). (C) Colocalization with lysosomes. Lysosomal marker LAMP-1 (red), FVIII (green), DAPI (blue), colocalization (yellow). Images are representative of 5–6 independent experiments. (D) Catabolism of FVIII by lysosomes/proteasomes. CLEC4M-expressing cells were preincubated with 2 U/mL rFVIII for 5 hours, washed, and incubated with two lysosomal inhibitors (chloroquine disulfide (CLQ), and NH4Cl), and a proteasome inhibitor (MG132) for 2 hours. FVIII levels were then quantified by ELISA, n=3–5 independent experiments. ± SEM, *p<0.05, **p<0.001. (E) Colocalization with late endosomes. Late endosomal marker Rab-9 (red), VWF (green), DAPI (blue), colocalization (yellow). (F) Colocalization with lysosomes. Lysosomal marker LAMP-1 (red), VWF (green), DAPI (blue), colocalization (yellow). Images are representative of 5–6 independent experiments.
We have previously demonstrated that VWF colocalizes with EEA1 post-internalization by CLEC4M-expressing cells [18]. Here we demonstrate that when CLEC4M-expressing cells were incubated with pdVWF-FVIII for 30 minutes, VWF co-localized with Rab-9 (Figure 5E). However, when CLEC4M-expressing cells were incubated with pdVWF-FVIII for 1 hour, and in contrast to what we had seen with FVIII, we observed little colocalization of VWF with LAMP1 (Figure 5F).
Association of FVIII with CLEC4M-expressing cells in vivo
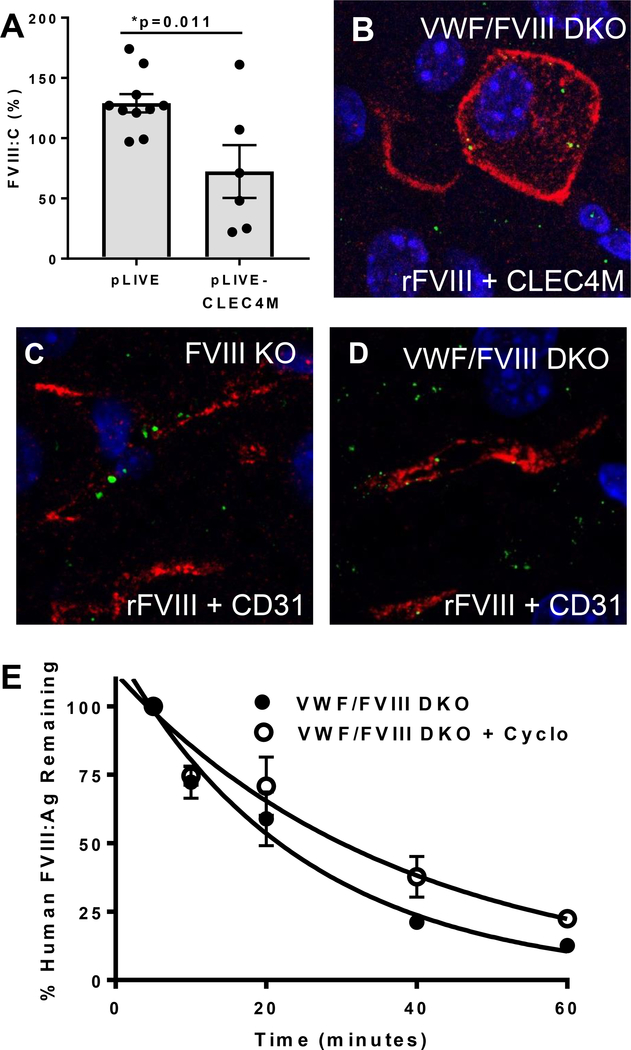
As mice do not express a CLEC4M ortholog, we have previously induced hepatic CLEC4M expression using hydrodynamic gene transfer [18]. Four days post-injection, mice with effective gene transfer had a 46% decrease in plasma levels of VWF:Ag. Plasma samples were then further characterized to examine endogenous levels of FVIII:C. In mice where 10–30% of hepatocytes expressed CLEC4M (as characterized by immunohistochemistry), plasma levels of FVIII:C were decreased compared with control mice that received hydrodynamic injections of the empty vector backbone (Figure 6A). See Supplemental Figure 3 for control experiments.
Figure 6. Association between CLEC4M-expressing cells and FVIII in vivo.
(A) Plasma FVIII:C levels in mice expressing CLEC4M on >10% of hepatocytes as compared with mice receiving pLIVE vector backbone alone (n=16). ± SEM, p<0.05 = *. (B) Association of infused human rFVIII (100 U/mouse) with a CLEC4M-expressing hepatocyte from a VWF-FVIII DKO mouse. (C) Association of infused human rFVIII (100 U/mouse) with a CD31-expressing LSEC in a FVIII KO mouse. (D) Association of human rFVIII with a CD31-expressing LSEC in a VWF/FVIII DKO mouse. (E) Half-life of human rFVIII (200 U/kg) in VWF/FVIII DKO mice compared with VWF/FVIII DKO treated with cyclophosphamide.
We were unable to observe a change in endogenous plasma FVIII:C in VWF KO mice that received hydrodynamic gene transfer of the CLEC4M cDNA compared with controls (data not shown). To demonstrate that CLEC4M-expressing cells can interact with human FVIII in vivo, we infused human rFVIII (10 μg) into VWF/FVIII DKO mice that had received a hydrodynamic gene transfer of the CLEC4M cDNA four days previously. 30 minutes post-FVIII injection, the tissues were prepared for IHC and imaged using anti-human FVIII and anti-human CLEC4M antibodies. We observed that rFVIII was internalized by cells expressing CLEC4M as well as cells that did no express CLEC4M (Figure 6B). Finally, we demonstrate that LSECs, even in the absence of CLEC4M expression, are capable of endocytosing FVIII. FVIII KO or VWF-FVIII DKO mice were infused with human rFVIII as described, and FVIII localization with CD31-expressing LSECs was observed 30 minutes post-injection (Figures 6C and 6D). VWF/FVIII DKO mice were pre-treated with cyclophosphamide to induce LSEC cytotoxicity. We observed an extended half-life of infused human rFVIII in the context of LSEC cytotoxicity (VWF/FVIII DKO mice t1/2 = 17.03 min; cyclophosphamide-treated VWF/FVIII DKO mice = 25.79 min; p=0.0075) (Figure 6E).
Discussion
VWF-bound FVIII has a prolonged half-life compared with VWF-free FVIII, and it is thought that the majority of VWF-bound FVIII is cleared by receptors that bind only to VWF, or to both VWF and FVIII such as LRP1, stabilin-2, or SIGLEC-5 [31–34]. However, VWF-free FVIII has a short in vivo half life, and up to ~24% of plasma FVIII may be cleared by receptors capable of binding VWF-free FVIII such as LRP1, SIGLEC5, and the asialoglycoprotein receptor (Supplemental Figure 4) [35–39]. While we have previously demonstrated that CLEC4M is a clearance receptor for VWF, here we extended this work to show that CLEC4M can regulate the binding, internalization and plasma levels of FVIII in both the absence and presence of VWF [18].
The N-linked glycans of FVIII have been shown to mediate interactions with the lectin receptors SIGLEC-5, the asialoglycoprotein receptor and the CLEC4M homolog DC-SIGN [37,39,40], suggesting that glycans expressed on FVIII are important determinants of its in vivo half-life. In the absence of VWF, CLEC4M interacts with both the high mannose and complex type N-linked glycans that are expressed throughout the FVIII molecule (Figure 3). Importantly, while glycosylation differences exist between plasma derived and rFVIII preparations, including ABO blood group determinants, sialic acid content, and occupancy of glycan sites, expression of high mannose glycans is relatively conserved and CLEC4M was able to interact with all FVIII products [27,41]. To date, CLEC4M is the first mannose-binding lectin clearance receptor for FVIII, although high mannose glycans are involved in the recognition of FVIII by the mannose receptor (CD206) on dendritic cells and macrophages in vitro [42].
VWF and FVIII are among the first identified endogenous glycoprotein ligands for CLEC4M. Previous studies have characterized CLEC4M as an adhesive receptor capable of mediating viral infection in trans in an ICAM-3-dependent manner [17] and CLEC4M has not been previously shown to endocytose other proteins expressing high mannose glycans [43]. Thus, the mechanism by which CLEC4M internalizes VWF and FVIII, and the subsequent fate of these ligands, is uncharacterized. We observed that depletion of membrane cholesterol, and inhibition of clathrin and dynamin GTPase activity impaired the endocytic activity of CLEC4M (Figure 4). This suggests that the CLEC4M receptor mediates endocytosis of its ligands through a clathrin-coated pit-dependent mechanism that involves lipid rafts. Consistent with these findings, microdomains of the CLEC4M-homolog DC-SIGN exist within lipid rafts, and depletion of membrane cholesterol attenuated binding and internalization of viral particles by DC-SIGN [44].
Mice do not express a CLEC4M ortholog, and we have previously demonstrated that the CLEC4M murine homologue SIGNR1 (~55% amino acid identity) which is expressed on LSECs, does not modify VWF-FVIII plasma levels or half-life [34]. In this study we have demonstrated using a solid phase assay that CLEC4M is capable of binding to human rFVIII in the presence of defibrinated, recalcified normal human plasma (containing VWF) when compared to a BSA control (Figure 2B) suggesting that FVIII and CLEC4M interact in vivo. We have previously demonstrated that transgenic expression of CLEC4M by hepatocytes using hydrodynamic injection of the CLEC4M cDNA was associated with decreased plasma levels of VWF [18]. Here, we observed a decrease in endogenous FVIII in mice expressing CLEC4M on 10–30% of hepatocytes relative to mice that received the empty pLIVE vector via hydrodynamic injection (Figure 6A), suggesting that hepatic expression of CLEC4M can facilitate clearance of the endogenous murine VWF-FVIII complex. We further demonstrated that infused human rFVIII can associate with CD31-expressing LSECs in the liver of FVIII KO mice where circulating murine VWF is present (Figure 6C). This suggests that even in the absence of CLEC4M expression, the pattern of blood flow and anatomical structure of the liver promote the association between FVIII and LSECs and is consistent with our previous observation that treatment of mice with cyclophosphamide, which is cytotoxic to sinusoidal endothelial cells, can increase plasma levels of endogenous FVIII in normal mice and prolong the half-life of human rFVIII in FVIII KO mice [34].
As CLEC4M can also directly interact with FVIII in the absence of VWF, we confirmed that this interaction can occur in recalcified, defibrinated VWF-deficient plasma when compared with a BSA control (Figure 2B). However, the quantitative influence of CLEC4M on FVIII plasma levels in vivo was more challenging to demonstrate in the absence of VWF, likely due to the short in vivo half-life of VWF-free FVIII and the variable and artificial expression of hepatic CLEC4M by hydrodynamic expression. We observed that infused rFVIII associated with CLEC4M-expressing hepatocytes in our hydrodynamic gene transfer model (Figure 6B). However, as FVIII was also taken up by other cells in the liver, and the hepatocyte asialoglycoprotein receptor has been reported to bind VWF-free FVIII [39,45,46], it is not possible to definitively state that hepatocyte uptake of FVIII in this model was mediated by CLEC4M. We did confirm, however, using VWF/FVIII DKO mice that human rFVIII can be taken up by CD31-expressing LSECs, suggesting that these cells can contribute to the clearance of FVIII in the presence or absence of VWF. While supra-physiological levels of rFVIII were utilized to facilitate FVIII detection in these imaging studies, these doses are similar to those employed in previous publications and are in line with the range of physiological antigen concentrations of other large plasma proteins such as VWF and fibrinogen [20,47,48]. Moreover, we demonstrate that infusion of therapeutic levels of human rFVIII (200 U/kg) into VWF/FVIII DKO mice treated with the LSEC cytotoxic agent cyclophosphamide prolongs the half-life of FVIII in the absence of VWF (Figure 6E) confirming that LSEC-expressed endocytic pathways contribute to the clearance of VWF-free FVIII even in the absence of CLEC4M expression.
Together, this data suggests that CLEC4M, a previously characterized clearance receptor for VWF, can also bind and internalize human FVIII in both a VWF-dependent and independent manner. Elucidation of the mechanistic basis of VWF-independent FVIII clearance provides new information concerning quantitative trail loci influencing FVIII plasma levels and contributes insights into novel strategies for improving the half-life of FVIII replacement products. LSECs are recognized to be the cellular source for a substantial proportion of FVIII production [49–51], as well a location of VWF-FVIII clearance and catabolism, and are thus likely to be important regulators of plasma FVIII levels. Future studies on the interactions between VWF, FVIII and the sinusoidal endothelial cell secretory and clearance pathways, including those involving CLEC4M, will likely yield novel insights into the FVIII life cycle.
Supplementary Material
Essentials:
CLEC4M is an endocytic receptor for factor FVIII
CLEC4M interacts with FVIII in a VWF-dependent and -independent manner
CLEC4M binds to mannose-containing glycans on FVIII
CLEC4M internalization of FVIII involves clathrin coated pits
Acknowledgements
This research was supported by grants from the Canadian Institute of Health Research (FDN 154285), the Heart and Stroke Foundation of Canada (NA 6881), and the National Institutes of Health Zimmerman Program for the Molecular and Clinical Biology of VWD (HL081588). L. Swystun was supported by a Heart and Stroke Fellowship award, a Queen’s University Senate Advisory Research Council fellowship, and a Canadian Hemophilia Society fellowship grant. D. Lillicrap is the recipient of a Canadian Research Chair in Molecular Hemostasis. The authors acknowledge M. Gordon and J. Mewburn from Queen’s University for technical assistance, and S. Kistner and C. Ungerer from Biotest for providing plasma-derived FVIII.
Conflict-of-interest disclosure: D.L. receives grant support from Bayer, Bioverativ, CSL, and Octapharma. P.D.J. receives research funding from CSL Behring and honoraria from CSL Behring, Bayer, and Baxter for educational presentations. The remaining authors declare no competing financial interests.
References
- 1.Zimmerman B, Valentino L. Hemophilia: in review. Pediatr Rev 2013; 34: 289–95. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins PV, Rawley O, Smith OP, O’Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol 2012; 157: 653–63. [DOI] [PubMed] [Google Scholar]
- 3.Golder M, Mewburn J, Lillicrap D. In vitro and in vivo evaluation of the effect of elevated factor VIII on the thrombogenic process. Thromb Haemost 2013; 109: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leyte A, Verbeet MP, Brodniewicz-Proba T, Van Mourik JA, Mertens K. The interaction between human blood-coagulation factor VIII and von Willebrand factor. Biochem J 1989; 257: 679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer BE, Kramer G, Mitterer A, Grillberger L, Reiter M, Mundt W, Dorner F, Eibl J. Effect of multimerization of human and recombinant von Willebrand factor on platelet aggregation, binding to collagen and binding of coagulation factor VIII. Thromb Res 1996; 84: 55–66. [DOI] [PubMed] [Google Scholar]
- 6.Noe D A mathematical model of coagulation factor VIII kinetics. Haemostasis 1996; 26: 289–303. [DOI] [PubMed] [Google Scholar]
- 7.Fay PJ, Coumans JV, Walker FJ. von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation. J Biol Chem 1991; 266: 2172–7. [PubMed] [Google Scholar]
- 8.Brinkhous KM, Sandberg H, Garris JB, Mattsson C, Palm M, Griggs T, Read MS. Purified human factor VIII procoagulant protein: comparative hemostatic response after infusions into hemophilic and von Willebrand disease dogs. Proc Natl Acad Sci 1985; 82: 8752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazurier C, Goudemand J, Hilbert L, Caron C, Fressinaud E, Meyer D. Type 2N von Willebrand disease: clinical manifestations, pathophysiology, laboratory diagnosis and molecular biology. Clin Haematol 2001; 14: 337–47. [DOI] [PubMed] [Google Scholar]
- 10.Jacquemin M, Lavend’homme R, Benhida A, Vanzieleghem B, D’Oiron R, Lavergne JM, Brackmann HH, Schwaab R, VandenDriessche T, Chuah MK, Hoylaerts M, Gilles JG, Peerlinck K, Vermylen J, Saint-Remy JM. A novel cause of mild/moderate hemophilia A: mutations scattered in the factor VIII C1 domain reduce factor VIII binding to von Willebrand factor. Blood 2000; 96: 958–65. [PubMed] [Google Scholar]
- 11.Morange PE, Tregouet DA, Frere C, Saut N, Pellegrina L, Alessi MC, Visvikis S, Tiret L, Juhan-Vague I. Biological and genetic factors influencing plasma factor VIII levels in a healthy family population: Results from the Stanislas cohort. Br J Haematol 2005; 128: 91–9. [DOI] [PubMed] [Google Scholar]
- 12.Song J, Chen F, Campos M, Bolgiano D, Houck K, Chambless LE, Wu KK, Folsom AR, Couper D, Boerwinkle E, Dong JF. Quantitative influence of ABO blood groups on factor VIII and its ratio to von willebrand factor, novel observations from an ARIC study of 11,673 subjects. PLoS One 2015; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith NL, Chen M-H, Dehghan A, Strachan DP, Basu S, Soranzo N, Hayward C, Rudan I, Sabater-Lleal M, Bis JC, de Maat MPM, Rumley A, Kong X, Yang Q, Williams FMK, Vitart V, Campbell H, Mälarstig A, Wiggins KL, Van Duijn CM, et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation 2010; 121: 1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antoni G, Oudot-Mellakh T, Dimitromanolakis A, Germain M, Cohen W, Wells P, Lathrop M, Gagnon F, Morange P-E, Tregouet D-A. Combined analysis of three genome-wide association studies on vWF and FVIII plasma levels. BMC Med Genet 2011; 12: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman JE, De Vries PS, Morrison AC, Sabater-lleal M, Kacprowski T, Auer PL, Brody JA, Chasman DI, Chen M, Guo X, Lin L, Marioni RE, Martina M, Cushman M, Wiggins KL, Qi L, Sennblad B, Harris SE, Polasek O, Riess H, et al. Rare and low-frequency variants and their association with plasma levels. Blood 2015; 126: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoo U-S, Chan KYK, Chan VSF, Lin CLS. DC-SIGN and L-SIGN: the SIGNs for infection. J Mol Med 2008; 86: 861–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bashirova AA, Geijtenbeek TBH, Van Duijnhoven GCF, Van Vliet SJ, Eilering JBG, Martin MP, Wu L, Martin TD, Viebig N, Knolle PA, Kewalramani VN, Van Kooyk Y. A Dendritic Cell – specific Intercellular Adhesion Molecule 3 – grabbing Nonintegrin ( DC-SIGN )– related Protein Is Highly Expressed on Human Liver Sinusoidal Endothelial Cells and Promotes HIV-1 Infection. J Exp Med 2001; 193: 671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rydz N, Swystun LL, Notley C, Paterson AD, Riches JJ, Sponagle K, Boonyawat B, Montgomery RR, James PD, Lillicrap D. The C-type lectin receptor CLEC4M binds, internalizes, and clears von Willebrand factor and contributes to the variation in plasma von Willebrand factor levels. Blood 2013; 121: 5228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanders YV, van der Bom JG, Isaacs A, Cnossen MH, de Maat MPM, Laros-van Gorkom BAP, Fijnvandraat K, Meijer K, van Duijn CM, Mauser-Bunschoten EP, Eikenboom J, Leebeek FWG. CLEC4M and STXBP5 gene variations contribute to von Willebrand factor level variation in von Willebrand disease. J Thromb Haemost 2015; 13: 956–66. [DOI] [PubMed] [Google Scholar]
- 20.Lai JD, Cartier D, Hartholt RB, Swystun LL, van Velzen AS, den Haan JMM, Hough C, Voorberg J, Lillicrap D. Early cellular interactions and immune transcriptome profiles in human factor VIII-exposed hemophilia A mice. J Thromb Haemost 2018; 16: 533–45. [DOI] [PubMed] [Google Scholar]
- 21.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet 1995; 10: 196–201. [DOI] [PubMed] [Google Scholar]
- 22.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Culleré M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci 1998; 95: 9524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Til NP, Markusic DM, van der Rijt R, Kunne C, Hiralall JK, Vreeling H, Frederiks WM, Oude-Elferink RPJ, Seppen J. Kupffer cells and not liver sinusoidal endothelial cells prevent lentiviral transduction of hepatocytes. Mol Ther 2005; 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 24.Elvevold K, Smedsrød B, Martinez I. The liver sinusoidal endothelial cell: a cell type of controversial and confusing identity. Am J Physiol 2008; 294: G391–400. [DOI] [PubMed] [Google Scholar]
- 25.Pipe SW, Montgomery RR, Pratt KP, Lenting PJ, Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood 2016; : 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarafanov G, Ananyeva NM, Shima M, Saenko EL. Cell surface heparan sulfate proteoglycans participate in factor VIII catabolism mediated by low density lipoprotein receptor-related protein. J Bol Chem 2001; 276: 11970–9. [DOI] [PubMed] [Google Scholar]
- 27.Lai J, Swystun L, Cartier D, Nesbitt K, Zhang C, Hough C, Dennis J, Lillicrap D. N-linked glycosylation modulates the immunogenicity of recombinant human factor VIII in hemophilia A mice. Haematologica 2018; 103: 1925–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canis K, McKinnon TAJ, Nowak A, Haslam SMM, Panico M, Morris HRR, Laffan MAA, Dell A. Mapping the N-glycome of human von Willebrand factor. Biochem J 2012; 447: 217–28. [DOI] [PubMed] [Google Scholar]
- 29.Lenting P, Pegon J, Christophe O, Denis C. Factor VIII and von Willebrand factor--too sweet for their own good. Haemophilia 2010; 16: 194–9. [DOI] [PubMed] [Google Scholar]
- 30.Canis K, Anzengruber J, Garenaux E, Feichtinger M, Benamara K, Scheiflinger F, Savoy LA, Reipert BM, Malisauskas M. In-depth comparison of N-glycosylation of human plasma-derived factor VIII and different recombinant products: from structure to clinical implications. J Thromb Haemost 2018; 16: 1592–603. [DOI] [PubMed] [Google Scholar]
- 31.Rastegarlari G, Pegon JN, Casari C, Odouard S, Navarrete A-M, Saint-Lu N, van Vlijmen BJ, Legendre P, Christophe OD, Denis CV, Lenting PJ. Macrophage LRP1 contributes to the clearance of von Willebrand factor. Blood 2012; 119: 2126–34. [DOI] [PubMed] [Google Scholar]
- 32.Saenko EL, Yakhyaev A, Mikhailenko I, Strickland D, Sarafanov A. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem 1999; 274: 37685–92. [DOI] [PubMed] [Google Scholar]
- 33.Pegon JN, Kurdi M, Casari C, Odouard S, Denis CV, Christophe OD, Lenting PJ. Factor VIII and von Willebrand factor are ligands for the carbohydrate-receptor Siglec-5. Haematologica 2012; 97: 1855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swystun LL, Lai JD, Notley C, Georgescu I, Paine AS, Mewburn J, Nesbitt K, Schledzewski K, Géraud C, Kzhyshkowska J, Goerdt S, Hopman W, Montgomery R, James PD, Lillicrap D. The endothelial cell receptor stabilin-2 regulates VWF-FVIII complex half-life and immunogenicity. J Clin Investig 2018; 128: 4057–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss HJ, Sussman II, Hoyer LW. Stabilization of factor VIII in plasma by the von Willebrand factor. J Clin Invest 1977; 60: 390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenting PJ, Neels JG, van den Berg BM, Clijsters PP, Meijerman DW, Pannekoek H, van Mourik J a, Mertens K, van Zonneveld a J. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem 1999; 274: 23734–9. [DOI] [PubMed] [Google Scholar]
- 37.Pegon JN, Kurdi M, Casari C, Odouard S, Denis C V, Christophe OD, Lenting PJ. Factor VIII and von Willebrand factor are ligands for the carbohydrate-receptor Siglec-5. Haematologica 2012; 97: 1855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarafanov AG, Ananyeva NM, Shima M, Saenko EL. Cell surface heparan sulfate proteoglycans participate in factor VIII catabolism mediated by low density lipoprotein receptor-related protein. J Biol Chem 2001; 276: 11970–9. [DOI] [PubMed] [Google Scholar]
- 39.Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJM, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J Thromb Haemost 2005; 3: 1257–65. [DOI] [PubMed] [Google Scholar]
- 40.Herczenik E, van Haren SD, Wroblewska A, Kaijen P, van den Biggelaar M, Meijer AB, Martinez-Pomares L, ten Brinke A, Voorberg J. Uptake of blood coagulation factor VIII by dendritic cells is mediated via its C1 domain. J Allergy Clin Immunol 2012; 129: 501–9, 509.e1–5. [DOI] [PubMed] [Google Scholar]
- 41.Hironaka T, Furukawa K, Esmon P, Fournel M, Sawada S, Kato M, Minaga T, Kobata A. Comparative study of the sugar chains of factor VIII purified from human plasma and from the culture media of recombinant baby hamster kidney cells. J Biol Chem 1992; 267: 8012–20. [PubMed] [Google Scholar]
- 42.Dasgupta S, Navarrete A, Bayry J, Delignat S, Wootla B, Andre S, Christophe O, Nascimbeni M, Jacquemin M, Martinez-Pomares L, Geijtenbeek T, Moris A, Saint-Remy J, Kazatchkine M, Kaveri S, Lacroix-Desmazes S. A role for exposed mannosylations in presentation of human therapeutic self-proteins to CD4+ T lymphocytes. Proc Natl Acad Sci 2007; 104: 8965–8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Y, Feinberg H, Conroy E, Mitchell D a, Alvarez R, Blixt O, Taylor ME, Weis WI, Drickamer K. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat Struct Mol Biol 2004; 11: 591–8. [DOI] [PubMed] [Google Scholar]
- 44.Cambi A, De Lange F, Van Maarseveen NM, Nijhuis M, Joosten B, Van Dijk EMHP, De Bakker BI, Fransen JAM, Bovee-geurts PHM, Van Leeuwen FN, Van Hulst NF, Figdor CG. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J Cell Biol 2004; 164: 145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saenko EL. Regulation of Factor VIII Life-Cycle by Receptors from LDL Receptor Superfamily. 36th Hemophilia Symposium Hamburg 2005 2007. p. 23–33. [Google Scholar]
- 46.Pipe SW, Montgomery RR, Pratt KP, Lenting PJ, Lillicrap D. Life in the shadow of a dominant partner: the FVIII-VWF association and its clinical implications for hemophilia A. Blood 2016; 128: 2007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Navarrete A, Dasgupta S, Delignat S, Caligiuri C, Christophe O, Bayry J, Nicoletti A, Kaveri S, Lacroix-Desmazes S. Splenic marginal zone antigen-presenting cells are critical for the primary allo-immune response to therapeutic factor VIII in hemophilia A. J Thromb Haemost 2009; 7: 1816–23. [DOI] [PubMed] [Google Scholar]
- 48.Van Der Flier A, Liu Z, Tan S, Chen K, Drager D, Liu T, Patarroyo-White S, Jiang H, Light DR. FcRn rescues recombinant factor VIII Fc fusion protein from a VWF independent FVIII clearance pathway in mouse hepatocytes. PLoS One 2015; 10: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Do H, Healey J, Waller E, Lollar P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J Biol Chem 1999; 274: 19587–93. [DOI] [PubMed] [Google Scholar]
- 50.Fahs SA, Hille MT, Shi Q, Weiler H, Montgomery RR. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood 2014; 123: 3706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Everett L, Cleuren A, Khoriaty R, Ginsburg D. Coagulation factor VIII is synthesized in endothelial cells. Blood 2014; 123: 3697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.