Abstract
Improving the nutritional quality of rice grains through modulation of bioactive compounds and micronutrients represents an efficient means of addressing nutritional security in societies which depend heavily on rice as a staple food. White rice makes a major contribution to the calorific intake of Asian and African populations, but its nutritional quality is poor compared to that of pigmented (black, purple, red orange, or brown) variants. The compounds responsible for these color variations are the flavonoids anthocyanin and proanthocyanidin, which are known to have nutritional value. The rapid progress made in the technologies underlying genome sequencing, the analysis of gene expression and the acquisition of global ‘omics data, genetics of grain pigmentation has created novel opportunities for applying molecular breeding to improve the nutritional value and productivity of pigmented rice. This review provides an update on the nutritional value and health benefits of pigmented rice grain, taking advantage of both indigenous and modern knowledge, while also describing the current approaches taken to deciphering the genetic basis of pigmentation.
Keywords: pigmented rice grain, nutrition, flavonoids, metabolites, genetics
Introduction
Rice is a staple food for over half of the world’s population (World Rice Production, 2019). Meeting the demand of future rice supply for the growing population, which has been predicted to reach 9.7 billion by 20501, is central for ensuring food and nutritional security. In addition to its critical importance to Asian populations as a source of food, rice also features in a range of social, cultural, economic, and religious activities (Ahuja et al., 2007; Hedge et al., 2013; Sathya, 2013). In sub-Saharan Africa the consumption of rice is projected to grow from its current level of 27–28 Mt per year to around 36 Mt by the end of 2026 (Terungwa and Yuguda, 2014; Nigatu et al., 2017), replacing some of the current demand for cassava, yam, maize, millet, and sorghum.
Most of the nutrients found in rice grain accumulate in the outer aleurone layer and embryo, the endosperm being composed primarily of starch. The process of dehulling and milling discards most micronutrients, fatty acids, anti-oxidants, and fiber. As a result, diets over-reliant on white rice risk deficiencies for several nutritional factors (Verma and Shukla, 2011; Sharma et al., 2013; Saneei et al., 2016; Sarma et al., 2018). The focus of rice breeding has long been concentrated on improving the crop’s productivity, although some emphasis has been given to improving the size, shape, and amylose content of the grain (Breseghello, 2013; Rao et al., 2014). The nutritional quality of the grain produced by certain traditional landraces has been shown to be higher than that of the grain produced by conventional, modern rice varieties, largely due to their more effective accumulation of bioactive compounds (Bhat and Riar, 2015; Berni et al., 2018). A growing consumer interest in health-promoting food products is generating a substantial market for more nutritionally valuable rice, creating health benefits for the large number of people for whom rice is a staple, while simultaneously generating economic benefits for the producers (Terungwa and Yuguda, 2014). As a result, the focus of a number of major rice research programs is turning to the issue of nutritional quality, encompassing an improved micronutrient and anti-oxidant content, along with a reduction in the grains’ glycemic index.
This review explores the nutritional and health attributes of pigmented rice grain, based on both indigenous knowledge and current research, and discusses the potential of pigmented rice grain to address nutritional food security. In addition, it explores the genetic basis of grain pigmentation, and suggests the potential contribution which ‘omics technologies can make to address the challenge of the double burden of malnutrition.
Indigenous Knowledge, Complemented With Corroborated Scientific Evidence, Informs on the Potential of Pigmented Rice Grain to Improve Nutrition and Health
Indigenous diets have developed to meet the needs of local communities over a long period of time, and the knowledge associated with these should be viewed as a resource to inform the discussion concerning the place of rice in the modern diet (Berni et al., 2018; Khatri, 2018). The value of such landraces in the context of both human nutrition and health (Rahman et al., 2006; Chunta et al., 2014) can be exemplified by the proven advantages of consuming pigmented grain (Figure 1; Rahman et al., 2006; Umadevi et al., 2012; Sathya, 2013). In particular, pigmented rice has been associated with anti-inflammatory and diuretic activity (Umadevi et al., 2012). Based on native indigenous knowledge, it has also been recommended for the treatment of diarrhea, vomiting, fever, hemorrhaging, chest pain, wounds, burns, and gastrointestinal problems, as well as addressing various liver and kidney disorders (Hedge et al., 2013; Sathya, 2013). Certain pigmented rice varieties are still used to treat skin diseases, blood pressure, fever, paralysis, rheumatism, and leucorrhea, and even as the basis of a general health tonic (Ahuja et al., 2007). In the Philippines, “tiki tiki,” derived from rice bran, has been used to cure thiamine deficiency (Umadevi et al., 2012). In India, the grain of pigmented rice landraces is offered to lactating mothers, and is used to both treat jaundice and cure paralysis. The rice variety “Laicha” was given its name because of its ability to prevent an eponymous skin disease (Das and Oudhia, 2000). For more than 2,000 years, grain of the South Indian landrace “Kavuni” has been reported to exhibit anti-oxidant, anti-arthritic, and anti-diabetic properties, and has been used to cure gastritis and peptic ulcers, as well as to enhance blood circulation (Valarmathi et al., 2014; Hemamalini et al., 2018).
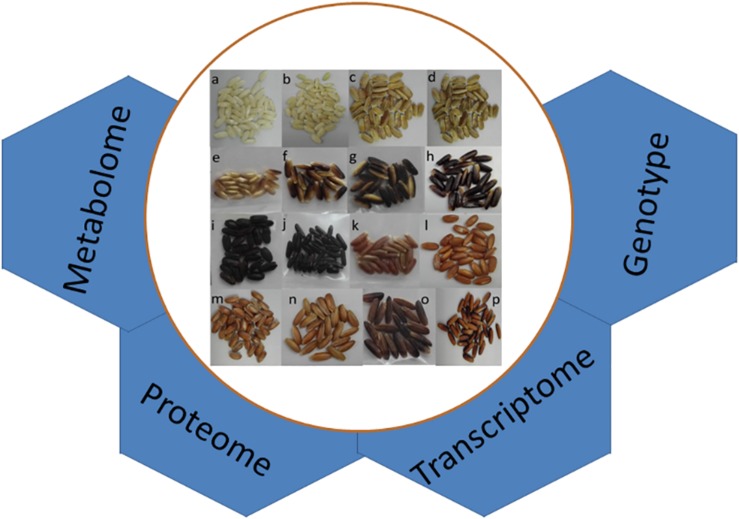
FIGURE 1.
Genetic variation for grain pigmentation in rice. Grains featuring (a,b) white, (c,d) brown, (e–h) purple, (i,j) dark purple or black, (k–n) red, and (o,p) mixed colored pericarp. The application of various genomic approaches to understand the genetic pathway of grain pigmentation is outlined.
A number of scientifically based studies have provided evidence to support the hypothesis that pigmented rice grain possesses anti-oxidant, anti-diabetic, anti-hyperlipidemic, and anti-cancer activity (Baek et al., 2015; Boue et al., 2016), which is reviewed below.
Anti-oxidant Activity
Dietary anti-oxidants represent an effective means of combating the accumulation of harmful reactive oxygen species and of balancing the redox status of the body (Krishnanunni et al., 2014). Analysis of extracts made from pigmented rice grain has shown that the phenolic compounds tocopherol and anthocyanin are efficient neutralizers of reactive oxygen species (Zhang et al., 2015; Ghasemzadeh et al., 2018), while animal tests have proven that these compounds are bioavailable (Tantipaiboonwong et al., 2017). Several studies have shown that the elevated anti-oxidation activity exhibited by pigmented rice grains (most markedly by black rice) can be used to mitigate the inflammatory response (Chakuton et al., 2012; Petroni et al., 2017).
Anti-diabetic Activity
The grain of some traditional pigmented rice varieties have proven to be effective in supporting glucose homeostasis, and are thus useful for the management of diabetes mellitus (Hemamalini et al., 2018). Unlike white rice grain consumption, which raises blood glucose levels, consuming pigmented grain can reduce blood glucose levels. Extracts of pigmented rice grain and bran have been shown to effectively inhibit the activity of endogenous α-amylase and α-glucosidase, thereby inhibiting the conversion of starch to glucose in the small intestine, which acts as a source of resistant starch to be utilized by gut microbiota in the colon (Boue et al., 2016; Chiou et al., 2018). While extracts made from both red and purple grain have been reported to inhibit α-glucosidase activity, only the former was effective in also inhibiting α-amylase activity (Valarmathi et al., 2014; Boue et al., 2016). The anthocyanins found in the whole grain of black rice acted as a potent inhibitor of β-glucosidase, thus delaying the absorption of carbohydrates (Chandramouli et al., 2018). Extracts of black rice bran have also been shown to induce the repair and regeneration of pancreatic beta cells (Wahyuni et al., 2016). Overall, the anti-diabetic effects of pigmented rice seem to arise from a synergistic effect of anthocyanin, proanthocyanidin, vitamin E, γ-oryzanol, and various flavonoids (Tantipaiboonwong et al., 2017). Black rice extracts reduced blood glucose levels more quickly than did extracts from red rice, a difference which was attributed to the presence of cyanidin 3-glucoside, a compound which activates insulin sensitivity, glucose uptake, and adiponectin secretion (Matsukawa et al., 2016; Tantipaiboonwong et al., 2017). However, many of the black rice are low in its amylose content and upon milling most of the anthocyanins accumulated in aleurone will be lost, thus not necessarily would possess low GI property when consumed in the form of milled rice (Kang et al., 2011).
Anti-cancer Activity
A considerable body of evidence suggests that consumption of pigmented rice has a protective effect against certain cancers. Ghasemzadeh et al. (2018) demonstrated that extracts of both black and red rice inhibit the proliferation of breast cancer cells. The phenolic acids, flavonoids, anthocyanins, and phytic acid present in extracts of purple rice bran have been shown to act as anti-mutagens and potential suppressors of cancer. It has been proposed that these phytochemicals act by either blocking the carcinogenetic cytochromes P450 CYP1A1 and CYP1B1 and/or by effectively scavenging free radicals (Insuan et al., 2017). Bioactive compounds of pigmented grains can reduce the viability of cancer cells and even induce their apoptosis. The mechanistic basis of this effect has been found to be variety-dependent, reflecting differences in the spectrum of bioactive compounds present in each rice variety (Baek et al., 2015). The high anthocyanin content of purple rice has been associated with an inhibitory effect on the growth of human hepatocellular carcinoma cells (Banjerdpongchai et al., 2013), while extracts of purple rice bran were able to block the first stage of aflatoxin B1-initiated hepatocarcinogenesis by inhibiting key metabolic activating enzymes (Suwannakul et al., 2015). Extracts of red rice have been shown to limit the invasiveness of cancer cells in a dose-dependent manner (Pintha et al., 2014). The phytosterols 24-methylenecycloartanol, β-sitosterol, gramisterol, campesterol, stigmasterol, cycloeucalenol, 24-methylene-ergosta-5-en-3β-ol, and 24-methylene-ergosta-7-en-3β-ol, all of which are present in extracts of black rice bran, have also been reported to be effective as agents restricting the proliferation of murine leukemic cells (Suttiarporn et al., 2015). Consequently, one of the long-term strategies proposed by Luo et al. (2014) to prevent breast cancer metastasis relies on the inclusion of pigmented rice in the human diet.
The Biochemical Properties of Pigmented Rice Grain
Phytosterols, Carotenoids, Vitamins, and Micronutrients in Pigmented Rice Grain
Phytosterols
Rice grains contain a wide range of secondary metabolites (Table 1). Pigmented grain appears to accumulate a higher level of γ-oryzanol than does non-pigmented grain (Chakuton et al., 2012). The grain accumulates the active anti-oxidant γ-oryzanol, which comprises a mixture of several phytosteryl ferulates (Chakuton et al., 2012), in particular 24-methylenecycloartanyl ferulate, cycloartenyl ferulate, campesteryl ferulate, and β-sitosteryl ferulate (Zubair et al., 2012; Pereira-Caro et al., 2013). The most important nutritional benefit of the phytosterols is their ability to both inhibit the absorption of cholesterol and to control the blood’s content of undesirable lipoproteins (Jesch and Carr, 2017). The predominant phystosterols detected in commercial rice varieties are β-sitosterol, followed by campesterol, Δ5-avenasterol, and stigmasterol (Zubair et al., 2012). The bran of the black rice variety “Riceberry” also harbors three additional sterols, namely 24-methylene-ergosta-5-en-3β-ol, 24-methylene-ergosta-7-en-3β-ol, and fucosterol (Suttiarporn et al., 2015).
TABLE 1.
Bioactive and nutritional compounds identified in pigmented rice.
| Compound | PubChem CID | Compound class | Figure 2a | References |
| Cyanine 3-glucoside | 197081 | Anthocyanin | ✓ | Pereira-Caro et al., 2013; |
| Peonidin-3-glucoside | 443654 | Anthocyanin | ✓ | Zhang et al., 2015; |
| Cyanidin | 128861 | Anthocyanin | Tantipaiboonwong et al., 2017; | |
| Cyanidin-3,5-diglucoside | 44256718 | Anthocyanin | ✓ | Shao et al., 2018 |
| Cyanidin-3-O-(6”-O-p-coumaroyl)glucoside | Anthocyanin | ✓ | ||
| Pelargonidin-3-O-glucoside | 443648 | Anthocyanin | ||
| Peonidin-3-O-(6”-O-p-coumaroyl)glucoside | Anthocyanin | ✓ | ||
| Cyanidin-3-O-arabidoside | Anthocyanin | ✓ | ||
| Flavone | 10680 | Flavone | ||
| Luteolin-6/8-C-pentoside-6/8-C-hexoside (2 isomers) | Flavone | Pereira-Caro et al., 2013; | ||
| Apigenin-6/8-C-pentoside-8/6-C-hexoside (three isomers) | Flavone glycoside | Kim et al., 2014; | ||
| Apigenin-6-C-glucosyl-8-C-arabinoside | Flavone | Ghasemzadeh et al., 2018; | ||
| Tricin-O-rhamnoside-O-hexoside | Flavone | Poulev et al., 2019 | ||
| Tricin | 5281702 | Flavone | ✓ | |
| Chrysoeriol | 5280666 | Flavone | ✓ | |
| Luteolin | 5280445 | Flavone | ✓ | |
| Apigenin | 5280443 | Flavone | ✓ | |
| Caffeic acid | 689043 | Hydrocinnamic acid | ✓ | Gunaratne et al., 2013; |
| p-Coumaric acid | 637542 | Hydrocinnamic acid | ✓ | Zhang et al., 2015; |
| Ferulic acid | 445858 | Hydrocinnamic acid | ✓ | Irakli et al., 2016; |
| Sinapic acid | 637775 | Hydrocinnamic acid | ✓ | Tantipaiboonwong et al., 2017; |
| Isoferulic acid | 736186 | Hydrocinnamic acid | Chiou et al., 2018; | |
| Chlorogenic acid | 1794427 | Hydrocinnamic acid | Ghasemzadeh et al., 2018; Shao et al., 2018 | |
| 2,5-Dihydroxybenzoic acid | 3469 | Hydroxybenzoic acid | ✓ | Kim et al., 2014; |
| p-Hydroxybenzoic acid | 135 | Hydroxybenzoic acid | ✓ | Valarmathi et al., 2014; |
| Gallic acid | 370 | Hydroxybenzoic acid | ✓ | Suwannakul et al., 2015; |
| Vanillic acid | 8468 | Hydroxybenzoic acid | ✓ | Huang and Lai, 2016; |
| Syringic acid | 10742 | Hydroxybenzoic acid | ✓ | Irakli et al., 2016; |
| Protocatechuic acid | 72 | Hydroxybenzoic acid | ✓ | Tantipaiboonwong et al., 2017; |
| Salicylic acid | 338 | Hydroxybenzoic acid | ✓ | Ghasemzadeh et al., 2018; |
| β-Resorcylic acid | 1491 | Hydroxybenzoic acid | Shao et al., 2018 | |
| Protocatechualdehyde | 8768 | Phenolic aldehyde | Huang and Lai, 2016 | |
| 8-5′-Coupled diferulic acid | Phenolic dehydrodimer | Zhang et al., 2015 | ||
| 5-5′-Coupled diferulic acid | Phenolic dehydrodimer | |||
| 8-8′-Coupled diferulic acid benzofuran form | Phenolic dehydrodimer | |||
| Proanthocyanidin dimer | Proanthocyanin | ✓ | Gunaratne et al., 2013 | |
| Proanthocyanidin trimer | Proanthocyanin | ✓ | ||
| Catechin | 73160 | Flavanonol | ✓ | Tantipaiboonwong et al., 2017; |
| Epicatechin | 72276 | Flavanonol | ✓ | Ghasemzadeh et al., 2018 |
| Quercetin | 5280343 | Flavonol | Pereira-Caro et al., 2013; | |
| Quercetin-3-O-glucoside | Flavonol | Valarmathi et al., 2014; | ||
| Quercetin-3-O-rutinoside | Flavonol | Chiou et al., 2018; | ||
| Isorhamnetin-3-O-glucoside | 5318645 | Flavonol | Ghasemzadeh et al., 2018; | |
| Myricetin | 5281672 | Flavonol | Poulev et al., 2019 | |
| Rutin | 5280805 | Flavonol | ||
| Kaempferol | 5280863 | Flavonol | ||
| Kaempferide | 5281666 | Flavonol | ||
| Naringenin | 932 | Flavanone | ✓ | Chiou et al., 2018 |
| Cycloartenol ferulate | 134695320 | γ-Oryzanol | Chakuton et al., 2012; | |
| 24-Methylenecycloartenol ferulate | 9920169 | γ-Oryzanol | Gunaratne et al., 2013; | |
| Campesteryl ferulate | 15056832 | γ-Oryzanol | Pereira-Caro et al., 2013 | |
| β-Sitosteryl ferulate | 9938436 | γ-Oryzanol | ||
| Δ7-Campesteryl ferulate | γ-Oryzanol | |||
| Campestanyl ferulate | 13786591 | γ-Oryzanol | ||
| Sitostanyl ferulate | 11227138 | γ-Oryzanol | ||
| Phytic acid | 890 | Phytic acid | Chakuton et al., 2012; Insuan et al., 2017 | |
| Tocotrienols (α-, β-, γ-, δ-forms) | 9929901 | Vitamin E | Zubair et al., 2012; | |
| Tocopherols (α-, β-, γ-, δ-forms) | 14986 | Vitamin E | Gunaratne et al., 2013; Irakli et al., 2016 | |
| Riboflavin | 493570 | Vitamin B2 | Valarmathi et al., 2014 | |
| Nicotinic acid | 938 | Vitamin B3 | Kim et al., 2014 | |
| Lutein | 5281243 | Carotenoid | Pereira-Caro et al., 2013; | |
| Zeaxanthin | 5280899 | Carotenoid | Valarmathi et al., 2014; | |
| β-Carotene | 5280489 | Carotenoid | Irakli et al., 2016; | |
| Lycopene | 446925 | Carotenoid | Melini and Acquistucci, 2017 | |
| β-Carotene-4,4′-dione | Carotenoid | |||
| all-trans-3,3’,4,4’-Tetrahydrospirilloxanthin | 5366411 | Carotenoid | ||
| 10′-Apo-β-carotenoic acid | Carotenoid | |||
| 24-Methylene-ergosta-5-en-3β-ol | Phytosterol | Suttiarporn et al., 2015 | ||
| 24-Methylene-ergosta-7-en-3β-ol | Phytosterol | |||
| Fucosterol | 5281326 | Phytosterol | ||
| Gramisterol | 5283640 | Phytosterol | ||
| Campesterol | 173183 | Phytosterol | ||
| Stigmasterol | 5280794 | Phytosterol | ||
| β-Sitosterol | 222284 | Phytosterol | ||
| Cycloeucalenol | 101690 | Triterpenoid | Suttiarporn et al., 2015 | |
| Lupenone | 92158 | Triterpenoid | ||
| Lupeol | 259846 | Triterpenoid | ||
| 24-Methylenecycloartanol | 94204 | Triterpenoid | ||
| LysoPC 14:0 | 460604 | Phospholipid | Kim et al., 2014 | |
| LysoPC 18:2 | 11005824 | Phospholipid | ||
| LysoPC 16:0 | 460602 | Phospholipid | ||
| LysoPC 18:1 | 53480465 | Phospholipid | ||
| Histidine | 6274 | Essential amino acid | Kim et al., 2014; | |
| Threonine | 6288 | Essential amino acid | Thomas et al., 2015 | |
| Valine | 6287 | Essential amino acid | ||
| Methionine | 6137 | Essential amino acid | ||
| Lysine | 5962 | Essential amino acid | ||
| Isoleucine | 6306 | Essential amino acid | ||
| Leucine | 6106 | Essential amino acid | ||
| Phenylalanine | 6140 | Essential amino acid | ✓ | |
| L-Aspartate | 5460294 | Non-essential amino acid | Kim et al., 2014; | |
| Serine | 5951 | Non-essential amino acid | Valarmathi et al., 2014; | |
| Glutamine | 5961 | Non-essential amino acid | Thomas et al., 2015 | |
| Glycine | 750 | Non-essential amino acid | ||
| Arginine | 6322 | Non-essential amino acid | ||
| Alanine | 602 | Non-essential amino acid | ||
| Proline | 614 | Non-essential amino acid | ||
| Tyrosine | 6057 | Non-essential amino acid | ||
| α-Aminobutyric acid (AABA) | 6657 | Non-essential amino acid | ||
| Potassium (K) | 5462222 | Mineral | Valarmathi et al., 2014; | |
| Calcium (Ca) | 5460341 | Mineral | Thomas et al., 2015; | |
| Magnesium (Mg) | 5462224 | Mineral | Raghuvanshi et al., 2017; | |
| Sodium (Na) | 5360545 | Mineral | Shozib et al., 2017; | |
| Chromium (Cr) | 23976 | Mineral | Hurtada et al., 2018 | |
| Iron (Fe) | 23925 | Mineral | ||
| Manganese (Mn) | 23930 | Mineral | ||
| Zinc (Zn) | 23994 | Mineral | ||
| Copper (Cu) | 23978 | Mineral | ||
| Phosphorus (P) | 5462309 | Mineral | ||
| Caproic acid | 8892 | Fatty acid | Valarmathi et al., 2014; | |
| Caprylic acid | 379 | Fatty acid | Thomas et al., 2015 | |
| Capric acid | 2969 | Fatty acid | ||
| Lauric acid | 3893 | Fatty acid | ||
| Tridecanoic acid | 12530 | Fatty acid | ||
| Myristic acid | 11005 | Fatty acid | ||
| Pentadecanoic acid | 13849 | Fatty acid | ||
| Palmitic acid | 985 | Fatty acid | ||
| Stearic acid | 5281 | Fatty acid | ||
| Arachidic acid | 10467 | Fatty acid | ||
| 9-Octadecanoic acid | 965 | Fatty acid | ||
| Undecanoic acid | 8180 | Fatty acid | ||
| Oleanolic acid | 10494 | |||
| Myristoleic acid | 5281119 | Mono-unsaturated fatty acid | Valarmathi et al., 2014; | |
| cis-10-Pentadecenoic acid | 5312411 | Mono-unsaturated fatty acid | Thomas et al., 2015 | |
| Oleic acid | 445639 | Mono-unsaturated fatty acid | ||
| cis-Vaccenic acid | 5282761 | Mono-unsaturated fatty acid | ||
| Erucic acid | 5281116 | Mono-unsaturated fatty acid | ||
| Hexadecadienoic acid | Polyunsaturated fatty acid | Valarmathi et al., 2014; | ||
| Hexadecatrienoic acid | 6506600 | Polyunsaturated fatty acid | Thomas et al., 2015 | |
| Linoleic acid | 5280450 | Polyunsaturated fatty acid | ||
| Octadecatetraenoic acid | 11778225 | Polyunsaturated fatty acid | ||
| cis-11,14,17-Eicosatrienoic acid | 5312529 | Polyunsaturated fatty acid | ||
| cis-5,8,11,14-Eicosatetraenoate acid | Polyunsaturated fatty acid | |||
| Eicosatetraenoic acid | 21863049 | Polyunsaturated fatty acid | ||
| Pinellic acid | 9858729 | Oxylipin | Kim et al., 2014 | |
| Succinic acid | 1110 | Carboxylic acid | ||
| Maleic acid | 444266 | Carboxylic acid | ||
| Malonic acid | 867 | Carboxylic acid | ||
| Citric acid | 311 | Carboxylic acid | ||
| Cinnamic acid | 444539 | Carboxylic acid | ✓ | |
| D-Xylose | 135191 | Sugar | Kim et al., 2014; | |
| D-Fructose | 2723872 | Sugar | Valarmathi et al., 2014 | |
| D-Glucose | 5793 | Sugar | ||
| Maltose | 439341 | Sugar | ||
| myo-Inositol | 892 | Sugar | ||
aCompounds present in Figure 2.
Carotenoids
Carotenoids represent another class of nutritionally beneficial compounds (Roberts and Dennison, 2015). Lutein and zeaxanthin represent together >90% of the carotenoids produced by rice, with carotenes, lycopenes, and β-carotene present in trace amounts (Pereira-Caro et al., 2013; Melini and Acquistucci, 2017). Most of this class of compound is present in the bran, with little or no carotenoids being found in milled rice (Petroni et al., 2017). Grain carotenoid content is a genetically variable trait, and is strongly correlated with grain pigmentation (Ashraf et al., 2017). Red and black rice accumulate a particularly high carotenoid content, while white rice accumulates very little (Ashraf et al., 2017; Petroni et al., 2017).
Vitamins
Rice grain represents a good source of vitamin E, including both the tocopherols and the tocotrienols (Zubair et al., 2012). The β- and γ-tocotrienols are the most abundant forms present in rice (Irakli et al., 2016). According to Gunaratne et al. (2013), red rice grains harbor higher levels of total tocopherol and tocotrienol than do the grains of modern white rice varieties. Note, however, that dehulling and milling strongly reduce the tocopherol content of the grain (Zubair et al., 2012).
Micronutrients
Rice grain contains traces of a number of essential micronutrients, namely zinc, magnesium, iron, copper, potassium, manganese, and calcium (Table 1; Raghuvanshi et al., 2017; Shozib et al., 2017; Shao et al., 2018). Some genetic variation in mineral content has been reported; but in general, pigmented rice grain accumulates higher amounts than does white grain rice (Shozib et al., 2017). Other studies have suggested that pigmented rice contains higher levels of zinc, iron, and manganese than does white grain, but a lower level of phosphorus (Raghuvanshi et al., 2017; Hurtada et al., 2018; Shao et al., 2018). Brown rice can provide as much as 75% of the recommended daily intake of zinc, copper, and iron, but this falls to just 37% for white rice (Hashmi and Tianlin, 2016).
Flavonoid Metabolism in Pigmented Rice Grain
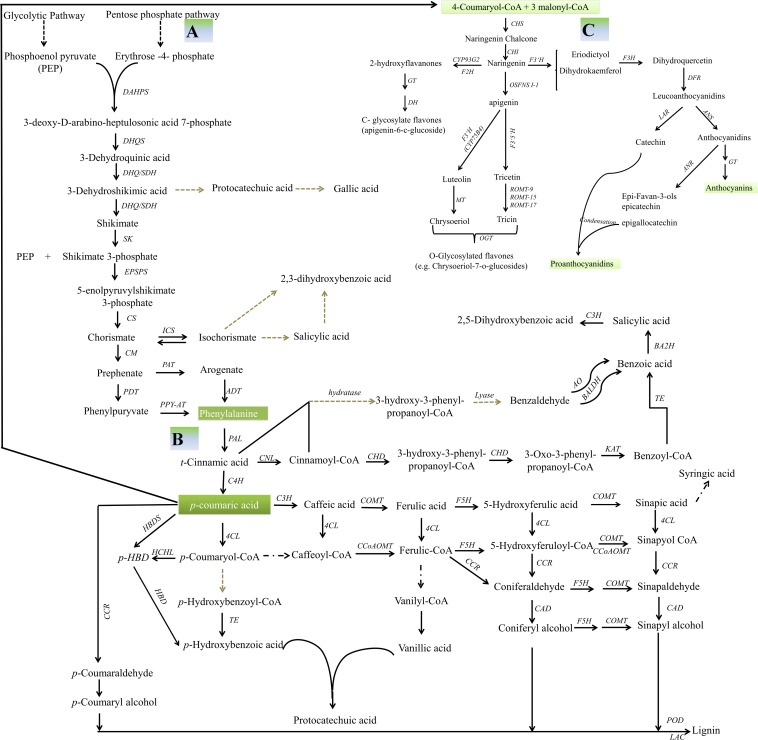
The major flavonoids present in pigmented rice grain are proanthocyanidins and anthocyanins (Table 1). The synthesis of the flavonoids is initiated by the deamination of phenylalanine to form cinnamic acid, a reaction catalyzed by phenylalanine ammonia lyase. Cinnamate 4-hydroxylase catalyses the oxidation of cinnamic acid to 4-coumaric acid, which is in turn converted to 4-coumaroyl-CoA through the action of 4-coumaroyl-CoA ligase (Cheng et al., 2014). The rate limiting step is the conversion of cinnamic acid to p-coumaroyl-CoA, which affects the synthesis of phenolic acids, flavanones, proanthocyanidins, and anthocyanidins (Figure 2).
FIGURE 2.
Secondary metabolism in rice. (A) A schematic representation of the shikimic acid pathway. DAHP, 3-deoxy-D-arabino-heptulosonic acid 7-phosphate; DAHPS, 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase; DHQ/SDH, 3-dehydroquinate dehydratase/shikimate 5 dehydrogenase; DHQS, 3-dehydroquinate synthetase; DHS, 3-dehydroshikimic acid; SDH, shikimate dehydrogenase; SK, shikimate kinase; S3P, shikimic acid 3-phosphate; EPSPS, 5-enolpyruvylshikimate 3-phosphate synthase; EPSP, 5-enolpyruvylshikimate 3-phosphate; CS, chorismate synthase; CM, chorismate mutase; PAT, prephenate aminotransferase; ADT, arogenate dehydratase; PDT, prephenate dehydratase; PPY-AT, phenylpyruvate aminotransferase (Tzin and Galili, 2010; Widhalm and Dudareva, 2015; Santos-Sánchez et al., 2019). (B) Possible routes to the production of benzoic acid, benzoic acid-derived compounds and lignin. CNL, cinnamate-CoA ligase; CHD, cinnamoyl-CoA-dehydrogenase/hydratase; KAT1, 3-ketoacyl-CoA thiolase; TE, CoA thioesterase; BA2H, benzoic acid 2-hydroxylase; BALDH, benzaldehyde dehydrogenase; AO, aldehyde oxidase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:CoA ligase; ICS, isochorismate synthase; CCR, cinnamoyl-CoA reductase; CCoAOMT, caffeoyl-CoA O-methyltransferase; F5H, ferulate 5-hydroxylase; CSE, caffeoyl shikimate esterase; COMT, caffeic acid O-methyltransferase; CAD, cinnamyl alcohol dehydrogenase; LAC, laccase; POD, peroxidase; p-HBD, p-hydroxybenzaldehyde; HBDS, 4-hydroxybenzaldehyde synthase; HCHL, 4-hydroxycinnamoyl-CoA hydratase/lyase; HBD, 4-hydroxybenzaldehyde dehydrogenase (Qualley et al., 2012; Gallage and Møller, 2015; Widhalm and Dudareva, 2015; Liu et al., 2018). (C) Flavonoid metabolism. PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; CHS, chalcone synthetase; CHI, chalcone isomerase; F3′H, flavone 3-hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanin synthase; ANR, anthocyanin reductase; GT, glucosyltransferase; LAR, leucoanthocyanidin reductase; MT, O-methyltransferase; F2H, flavanone 2-hydroxylase (Chen et al., 2013; Galland et al., 2014). The square dot arrows indicates steps which have not yet been fully elucidated, while the black arrows indicate steps supported by genetic evidence.
Phenolic Acids
Compared to white grain, pigmented grain contains a higher level of phenolic acids (Gunaratne et al., 2013; Zhang et al., 2015). Cinnamic acid serves as a precursor for the synthesis of various phenolic acids, including p-coumaric acid, ferulic acid, sinapic acid, isoferulic acid, and 2,5-dihydroxybenzoic acid (Zhang et al., 2015; Shao et al., 2018). The predominant phenolic acids present in white rice are p-coumaric acid and ferulic acid; these are largely utilized as building blocks for lignin synthesis (Figure 2). In an alternative pathway, particularly active in black rice, cinnamic acid is converted to vanillic acid and protocatechuic acid (Zhang et al., 2015; Shao et al., 2018). In red rice, caffeic acid has been identified as a minor phenolic acid, while this compound is not detectable in brown rice (Gunaratne et al., 2013; Zhang et al., 2015; Irakli et al., 2016; Shao et al., 2018). Additional phenolic acids identified include syringic acid in the extract of brown, red, and black rice (Ghasemzadeh et al., 2018; Shao et al., 2018), pinellic acids in red and white rice (Kim et al., 2014), hydroxybenzoic acid in black rice extracts (Tantipaiboonwong et al., 2017), and gallic acid in the extracts of the red rice mutant AM-425 (Chiou et al., 2018). Four diferulic acids (phenolic acid dehydrodimers) are present in the insoluble-bound (Table 1; Zhang et al., 2015).
Flavanones
The condensation and subsequent intramolecular cyclization of three molecules of malonyl CoA and one of 4-coumaroyl-CoA is then catalyzed by chalcone synthetase to produce naringenin chalcone. Naringenin chalcone is isomerized into naringenin by the action of chalcone isomerase to form the flavones (Figure 2). Small quantities of flavones and flavanol glycosides have been detected in the grain, notably luteolin-6/8-C-pentoside-6/8-C-hexoside and certain derivatives of apigenin (Table 1). In the tricin pathway, a flavone synthase II enzyme converts naringenin to apigenin, which is then converted first to luteonin by flavonoid 3′-hydroxylase, and then to tricin by O-methyltransferase and chrysoeriol 5′-hydroxylase (Park et al., 2016; Figure 2). Apigenin, luteolin, tricetin, tricin, quercetin, and myricetin have all been detected in extracts of red and brown rice bran (Table 1; Galland et al., 2014; Ghasemzadeh et al., 2018). The synthesis of C-glycosylated flavanones begins with the conversion of naringenin to 2-hydroxyflavanone by flavanone 2-hydroxylase, which is then C-glycosylated by C-glucosyltransferase and finally is dehydrated by an as yet unknown enzyme (Figure 2; Du et al., 2010; Galland et al., 2014; Sasaki et al., 2014; Park et al., 2016; Poulev et al., 2019). Other flavonoid-like compounds identified in rice include quercetin-3-O-glucoside, quercetin-3-O-rutinoside, methoxy-flavanol-3-O-glucoside, and isorhamnetin-3-O-glucoside (Pereira-Caro et al., 2013; Kim et al., 2014); tricin-O-rhamnoside-O-hexoside and apigenin-6-C-glucosyl-8-C-arabinoside are particularly predominant in white rice grains (Kim et al., 2014).
Proanthocyanidins
Proanthocyanidins are oligomers and polymers of flavan-3-ols (Gunaratne et al., 2013). Naringenin, the universal substrate for their synthesis, is 3′-hydroxylated by flavonoid 3′-hydroxylase, producing eriodictyol, which is then converted to dihydroquercetin by the action of flavone 3-hydroxylase (Figure 2). Dihydroflavonol 4-reductase catalyses the conversion of dihydroquercetin into leucoanthocyanidins. Leucocyanidin is converted into the flavan-3-ol catechin by leucoanthocyanidin reductase, while catechin monomers are polymerized by a yet unknown pathway to form proanthocyanidin (Figure 2; Zhao et al., 2010; Galland et al., 2014). Proanthocyanidins and catechins make up the bulk of the phenolic compounds found in red rice, being responsible for the red pigmentation of the pericarp (Pereira-Caro et al., 2013; Kim et al., 2014). No proanthocyanidins have been detected in white rice accessions (Gunaratne et al., 2013), while some black rice varieties have been reported to contain them (Vichit and Saewan, 2015).
Anthocyanidins
The oxidization of leucoanthocyanidin to form cyanidin, pelargonidin, and delphinidin is catalyzed by anthocyanin synthase (Figure 2; Cheng et al., 2014; Galland et al., 2014). Anthocyanins, which are responsible for purple to blue pigmentation, represent the bulk of the flavonoids present in black and purple rice (Pereira-Caro et al., 2013; Zhang et al., 2015). The compounds cyanidin-3-O-glucoside and peonidin-3-O-glucoside are the most prominent, but also represented are cyanidin-3,5-diglucoside, cyanidin-3-O-(6″-O-p-coumaroyl)glucoside, pelargonidin-3-O-glucoside, peonidin-3-O-(6″-O-p-coumaroyl)glucoside, and cyanidin-3-O-arabidoside. Red and white rice grains have been classified as lacking anthocyanin (Gunaratne et al., 2013; Xiongsiyee et al., 2018), but both Boue et al. (2016) and Ghasemzadeh et al. (2018) have been able to detect a low level in both red and brown rice accessions. Unstable anthocyanidins can be converted into the colorless flavan-3-ols epiafzelechin, epicatechin, and epigallocatechin through the action of anthocyanin reductase, and when glycosylated, a wide array of distinct molecules are generated (Ko et al., 2006; Sasaki et al., 2014; Kim et al., 2015; Figure 2). Although the major enzymes operating in the flavonoid pathway are well known and their encoding genes have been identified (Table 2), many aspects underlying the synthesis of these pigments in rice have yet to be fully elucidated.
TABLE 2.
Regulatory and structural genes shown to be involved in the biosynthesis of anthocyanin and proanthocyanidin in rice.
| Locus | Allelic locus | Gene name | Locus ID |
CHRXa | Pericarp | References | |
| Rice Annotation Project (RAP) | Rice Genome Annotation Project (MSU) | ||||||
| Regulatory genes | |||||||
| Kala1 | Rd | OsDFR | Os01g0633500 | LOC_Os01g44260 | 1 | Red and black | Furukawa et al., 2006; Shih et al., 2008; Maeda et al., 2014; Sun et al., 2018 |
| Pp | 1 | Red and black | Caixia and Qingyao, 2007 | ||||
| Kala3 | OsMYB3 | Os03t0410000 | LOC_Os03g29614 | 3 | Black | Maeda et al., 2014 | |
| Kala4 | Plw | OSB1/Pb/Ra | Os04g0557800 | LOC_Os04g47080 | 4 | Hu et al., 1996; Sakamoto et al., 2001; Caixia and Qingyao, 2007; Sakulsingharoj et al., 2016 | |
| OSB2 | Os04g0557500 | LOC_Os04g47059 | 4 | Sakamoto et al., 2001; Sakulsingharoj et al., 2014; Oikawa et al., 2015 | |||
| Rc | Rc-s | bHLH | Os07g0211500 | LOC_Os07g11020 | 7 | Red | Sweeney et al., 2006 |
| Rc | 7 | Red | Furukawa et al., 2006 | ||||
| rc | 7 | White | Furukawa et al., 2006; Sweeney et al., 2006 | ||||
| Rc-g | 7 | Red | Brooks et al., 2008 | ||||
| Rcr | 7 | Red | Ferrari et al., 2015 | ||||
| Rc-gl | 7 | White | Gross et al., 2010 | ||||
| Chromogen | OsC1 | Os06g0205100 | LOC_Os06g10350 | 6 | Black | Saitoh et al., 2004; Rachasima et al., 2017; Sun et al., 2018 | |
| Structural genes | |||||||
| Chalcone synthetase (CHS) | OsCHS1 | Os11g0530600 | LOC_Os11g32650 | 11 | Common intermediate | Shih et al., 2008 | |
| OsCHS2 | Os07g0214900 | LOC_Os07g11440 | 7 | Common intermediate | |||
| Chalcone isomerase (CHI) | OsCHI | Os03g0819600 | LOC_Os03g60509 | 3 | Common intermediate | Shih et al., 2008 | |
| Flavanone 3-hydroxylase (F3H) | OsF3H-1 | Os04g0662600 | LOC_Os04g56700 | 4 | Common intermediate | Kim et al., 2008 | |
| OsF3H-2 | Os10g0536400 | LOC_Os10g39140 | 10 | ||||
| OsF3H-3 | Os04g0667200 | LOC_Os04g57160 | 4 | ||||
| Flavanone 3′-hydroxylase (F3′H) | OsF3′H | Os10g0320100 | LOC_Os10g17260 | 10 | Common intermediate | Shih et al., 2008 | |
| Leucoanthocyanidin reductase (LAR) | OsLAR | Os03g0259400 | LOC_Os03g15360 | 3 | Black rice | Kim et al., 2015 | |
| Anthocyanidin synthase (ANS) | OsANS1 | Os01g0372500 | LOC_Os01g27490 | 1 | Black rice | Shih et al., 2008 | |
| OsANS2 | Os06g0626700 | LOC_Os06g42130 | 6 | ||||
| UDP-glycosyltransferase (UF3GT) | OsUGT | Os06g0192100 | LOC_Os06g09240 | 6 | Black rice | Yoshimura et al., 2012 | |
| Os07g0148200 | LOC_Os07g05420 | 7 | Black rice | ||||
| Anthocyanin reductase (ANR) | OsANR | Os04g0630800 | LOC_Os04g53850 | 4 | Black rice | Kim et al., 2015 | |
aCHRX: chromosome.
The Genetic Basis of Rice Grain Pigmentation
The rice genome harbors at least two genes encoding chalcone synthetase: CHS1 on chromosome 11 and CHS2 on chromosome 7 (Shih et al., 2008; Han et al., 2009; Cheng et al., 2014), each contributing to flavanone biosynthesis. For the production of proanthocyanidins, three flavone 3-hydroxylase are relevant: namely F3H-1 (chromosome 4), F3H-2 (chromosome 10), and F3H-3 (chromosome 4; Kim et al., 2008; Park et al., 2016). Two anthocyanin synthases are critical for the synthesis of anthocyanins, namely ANS1 (chromosome 1) and ANS2 (chromosome 6) (Shih et al., 2008; Table 2).
Rc Role in Red Pericarp in Ancestral Rice
White grained rice was selected during rice’s domestication. The two complementary genes Rc (on chromosome 7), which encodes a basic helix-loop-helix (bHLH) transcription factor, and Rd (chromosome 1) encoding a form of dihydroflavonol 4-reductase, an enzyme which enhances the accumulation of proanthocyanidin, are together responsible for the red pericarp color. Rc is closely associated with shattering and grain dormancy (Sweeney et al., 2006), so therefore was selected against during domestication. Rc-Rd genotypes produce red grain, while Rc-rd genotypes produce brown grain (Furukawa et al., 2006). The three common Rc alleles are the wild type Rc, and mutant alleles Rc-s and rc. Rc-s differs from Rc due to the presence of a premature stop codon, while rc lacks a 14 bp stretch of the wild type sequence (Furukawa et al., 2006; Sweeney et al., 2006). Carriers of rc produce a colorless pericarp, while those of Rc-s produce a range of pericarp pigmentation (Sweeney et al., 2007). A number of variants have been identified as restoring the wild type (red) pericarp pigmentation: Rc-g carries a 1 bp deletion 20 bp upstream of the 14 bp rc deletion (Brooks et al., 2008), while Rcr features a 44 bp deletion upstream of the 14 bp segment, which restores the wild type reading frame (Ferrari et al., 2015). Most varieties of African domesticated rice (Oryza glaberrima) produce a red pericarp, and white variants harbor a loss-of-function mutation in Rc. An exception is the O. glaberrima specific mutation rc-gl, which carries a premature stop codon 146 bp upstream of the site of the Rc-s point mutation (Gross et al., 2010).
Regulatory Cascade Influencing Purple Rice Color
Anthocyanins are responsible for the black-purple pigmentation in rice grain. The variation seen in pigmentation intensity has been taken to imply that the trait is under polygenic control, involving as yet unidentified genes (Ham et al., 2015). A number of publications report the identification of rice genes that regulate anthocyanin production, each adopting a different gene coding system, which only adds to the confusion. According to Hu et al. (1996), two classes of regulatory gene (R/B and C1/Pl) govern both the accumulation of anthocyanin and the regulation of its deposition. Two R genes have been characterized: Ra maps to chromosome 4 and Rb to chromosome 1. The former gene is thought to be a homolog of the maize R/B gene. Three alleles of Pl (chromosome 4) have been identified, namely Plw, Pli, and Plj, and each is responsible for a distinctive pattern of pigmentation. Plw activates anthocyanin synthesis in most of the aerial parts of the rice plant (although not in either the stem or the internode). The Pl locus harbors the two genes, OSB1 and OSB2, each of which encodes a bHLH transcription factor (Table 2; Sakamoto et al., 2001). Other studies found the purple pericarp trait to be genetically determined by the dominant complementary genes Pb (synonym Prp-b) and Pp (synonym Prp-a), mapping to chromosomes 4 and 1, respectively (Table 2; Rahman et al., 2013; Ham et al., 2015). While the product of Pb appears to be responsible for the accumulation of pigment in the pericarp of brown grain, that of Pp increases the amount of the pigment, giving rise to purple grain. The number of copies of the Pp gene present is correlated with the intensity of the purple pigmentation (Rahman et al., 2013). In the absence of Pp, plants harboring Pb produce grain with a brown pericarp, while the pericarp of Pp carriers, lacking Pb, are white (Rahman et al., 2013). The Pb locus comprises of two genes, a myc transcription factor (Ra), along with bHLH16. The bHLH16 has been shown to be involved in proanthocyanidin synthesis, while Ra is involved in anthocyanin synthesis. Ra and OSB1 are believed to have synonymous functions (Hu et al., 1996; Sakamoto et al., 2001; Caixia and Qingyao, 2007). A 2 bp (GT) insertion in exon 7 of Ra abolishes purple pigmentation (Caixia and Qingyao, 2007; Lim and Ha, 2013; Rahman et al., 2013). Similarly, Sakulsingharoj et al. (2016) have found that a 2 bp (GT) insertion in exon 7 of OSB1, which along with a 1 bp deletion of a guanine nucleotide in exon 8, results in a threonine for methionine substitution at position 64, resulting in a white grain phenotype. Carriers of the three loci Kala1 (chromosome 1), Kala3 (chromosome 3), and Kala4 (chromosome 4) express a black pericarp trait (Maeda et al., 2014). It has been suggested that Kala4 is synonymous with Pb, and Kala1 with Pp. Kala4 encodes a bHLH transcription factor and corresponds to OSB2 (Table 2). OSB2 regulates a number of genes encoding enzymes involved in anthocyanin synthesis, including F3H, DFR, and ANS (Sakulsingharoj et al., 2014). The chromosomal region harboring Kala1 includes Rd (dihydroflavonol 4-reductase). Kala3 is likely be a synonym of MYB3 (Maeda et al., 2014). The black grain phenotype occurring in tropical japonica germplasm has been attributed to structural variants in the Kala4 promoter sequence. Oikawa et al. (2015) have proposed that Kala4 has been introgressed several times from japonica to indica germplasm.
The R2R3-MYB transcription factor Os06g0205100 has been proposed as a candidate for the C gene, functioning as a possible activator of DFR and ANS (Saitoh et al., 2004; Rachasima et al., 2017; Sun et al., 2018). Os01g0633500 (A1) is a dihydroflavonol reductase gene involved in anthocyanin synthesis (Table 2). Thus, A1 and C1 determine the purple color of grain. The S1 gene (Os04g0557500) encodes a bHLH protein, and contributes to hull-specific pigmentation. The presence of a functional copy of both C1 and S1 has been shown to be required for hull pigmentation, while the product of A1 acts as a catalyst for the development of purple hulls (Sun et al., 2018). The pattern of anthocyanin pigmentation is determined by the allelic status of A1, C1, and S1 (Sun et al., 2018). Several authors have attempted to correlate sequence variants of a number of regulatory genes, e.g., C1 and OSB2, with phenotypic variation in rice grain pigmentation (Sakulsingharoj et al., 2016; Rachasima et al., 2017; Sun et al., 2018). Lachagari et al. (2019) conducted comparative genomics in 108 rice lines and identified novel allelic variants in a number of genes belonging to the flavonoid pathway, cytokinins glucoside, and betanidin degradation biosynthesis that were associated with purple pigmentation. Although a number of genes responsible for grain pigmentation have already been identified (Table 2), there is still a possibility that additional genes and variants thereof, remain to be discovered.
The Genetic Basis of Grain Pigmentation Inferred From Quantitative Trait Loci Analysis or Genome Wide Association Studies
A number of attempts have been made to exploit the quantitative trait loci (QTL) mapping approach as a means of inferring the genetic basis of grain pigmentation (Table 3). Tan et al. (2001) identified nine QTL in an analysis of flour pigmentation in a recombinant inbred line (RIL) population. Three QTL reflected variation in the CIE 1976 color parameter L∗ (lightness), two in a∗ (red-green), and four in b∗ (yellow-blue). In a backcross RIL population, made from a cross between the rice varieties “Kasalath” (red pericarp) and “Koshihikari” (white pericarp), Dong et al. (2008) identified four QTL underlying variation in red pigmentation, with the two largest effect QTL co-locating with Rc and Rd, the two minor effect QTL being novel. An analysis carried out by Matsuda et al. (2012) suggested that flavonoid content was governed by genetic factors which control flavone glycosylation. In a recent study 21 QTL, responsible for variation in the content and composition of anthocyanin and proanthocyanidin, were identified (Xu et al., 2017). While some mapped to locations occupied by already known genes, others mapped to genomic regions not previously identified as harboring genes involved in rice grain pigmentation.
TABLE 3.
Quantitative trait loci identified for colored related traits, anthocyanin and proanthocyanidin.
| No. | Population | Size | Markers | Trait category | QTLs/QTNs | Closest structural and/or regulatory genes | Chromosome | Effect (%) | References |
| 1 | RILs | 238 | 162 RFLP and 48 SSRs | Flour color | 9 | 1,3,4,5,6,7,8 | 4.3–25.4 | Tan et al., 2001 | |
| L* (3) | 5,6,8 | 4.5–15.7 | |||||||
| a* (2) | 4,7 | 6.9–10.5 | |||||||
| b* (4) | 1, 3,6,8 | 4.3–25.4 | |||||||
| 2 | BRILs | 182 | 162 RFLP | Degree of red coloration | 4 | 1,7,9,11 | 2.1–83.7 | Dong et al., 2008 | |
| qDRC-1* | Rd | 1 | 3.6–3.7 | ||||||
| qDRC-7* | Rc | 7 | 75.9 -83.7 | ||||||
| qDRC-9 | 9 | 2.1–3.2 | |||||||
| qDRC-11 | 11 | 3.3–3.4 | |||||||
| 3 | RILs | 182 | 126 SSRs | Anthocyanin and proanthocyanidin | 21 | 1,2,3,7,8,10,12 | 3.8–34.8 | Xu et al., 2017 | |
| ANC (8) | 1,2,3,7,10 | 8.8–34.8 | |||||||
| PAC (13) | 1,2,7,8,10,12 | 3.8–17.0 | |||||||
| 4 | Diversity panel | 416 | 100 SSRs and 10 gene markers | Grain color | 25 | 1,4,6,7,8,9,10,11,12 | 1.39–86.68 | Shao et al., 2011 | |
| L* (3) | Ra, Rc | 4,7,10 | 4.96–31.23 | ||||||
| a* (8) | Ra, Rc | 1,4,7,8,9,11,12 | 1.51–19.65 | ||||||
| b* (6) | Ra | 4,6,8,9,10 | 4.38–49.82 | ||||||
| c (3) | Ra | 4,8,10 | 1.39–3.99 | ||||||
| H° (5) | Ra | 4,6,8,9 | 5.4–86.68 | ||||||
| Phenolic and flavonoid content | 10 | 4,7,8,9,10 | 2.64–39.67 | ||||||
| PC (4) | Ra, Rc | 4,7,8,9 | 5.87–39.67 | ||||||
| FC (6) | Ra, Rc | 4,7,8,9,10 | 2.64–35.35 | ||||||
| 5 | Diversity panel | 203 | sequencing data | Pericarp color (PC) | 4 | 7,10 | Wang et al., 2016 | ||
| Rc-s | Rc-s | 7 | |||||||
| qPc10 | F3H | 10 | |||||||
| 6 | Diversity panel | 244 | 122,785 SNPs | Red seed color | snp_07_6067391 | bHLH | Butardo et al., 2017 | ||
| 7 | Diversity panel | 419 | 208,993 SNPs | Pericarp color_whole panel | 763 | 1,3,4,7,8,10,11 | Yang et al., 2018 | ||
| Rd | 1 | ||||||||
| MYB family transcription factors | 10,11 | ||||||||
| WD domain, G-beta repeat domain containing protein | 8 | ||||||||
| Pericarp color_indica | 99 | ||||||||
| MYB family transcription factors | 10,11 | ||||||||
| OsCHI | 3 | ||||||||
| Kala4 | 4 | ||||||||
| Rc | 7 | ||||||||
| WD domain, G-beta repeat domain containing protein | 8 | ||||||||
| OsCHI | 3 | ||||||||
| Kala4 | 4 | ||||||||
| Rc | 7 |
RILs, recombination inbred lines; BRILs, backcross-recombinant inbred lines.
The genome wide association study (GWAS) approach, which has certain advantages over QTL mapping (Korte and Farlow, 2013), has been applied in a few cases to determine the genetic basis of grain pigmentation (Table 3). Shao et al. (2011) used GWAS to identify 25 marker–trait associations for grain pigmentation: some related to pigment intensity, others to hue angle, L∗, a∗, or b∗. Their analysis confirmed the importance of Ra and Rc. Butardo et al. (2017) used GWAS to uncover a number of single nucleotide polymorphism loci (SNPs) linked to Rc. The 763 SNPs associated with pericarp pigmentation uncovered by Yang et al. (2018) mapped to 6 of the 12 rice chromosomes (chromosomes 1, 3, 4, 8, 10, and 11); some of the most significantly associated SNPs lying close to previously identified structural or regulatory genes, but others map to regions not previously associated with variation in rice grain pigmentation.
‘Omics Approaches Taken to Unraveling the Mechanistic Basis of Grain Pigmentation
High throughput genomics, including transcriptomics, proteomics, and metabolomics, have contributed to the unraveling of biochemical pathways underlying target traits. By combining genetics with systems biology tools the target genomic regions were narrowed down to identify candidate genes and proteins influencing key nutritional traits of interest in rice (Butardo et al., 2017; Anacleto et al., 2019).
Differential transcriptomic analyses between pigmented and non-pigmented rice grains identified regulators and downstream targets of flavonoid pathway genes (Oh et al., 2018). The high anthocyanin content of black rice was associated with enhanced transcription of genes encoding anthocyanidin synthase, while high proanthocyanin content, characteristic of red rice, was accompanied by a notable abundance of transcript for a gene encoding leucoanthocyanidin reductase (Chen et al., 2013). Transcript abundance of genes encoding chalcone synthetase, chalcone isomerase, flavanone 3-hydroxylase, dihydroflavonol 4-reductase, and anthocyanin synthetase was compared in white, black, and red rice grain by Lim and Ha (2013). Four genes were markedly up-regulated in pigmented grain varieties, while the gene encoding chalcone isomerase displayed a similar level of transcription in both white and pigmented varieties. The enhanced abundance of transcripts of chalcone synthetase, flavanone 3-hydroxylase, and anthocyanin synthetase seen in some black varieties implied a strong correlation between transcription and pigment content. Sun et al. (2018) found that flavonoid pathway genes were regulated by ternary MYB-bHLH-WD40 transcriptional complexes (Xu et al., 2013; Zhang et al., 2018). A microarray-based comparison of black and white rice identified nearly 1,300 differentially transcribed genes, of which 137 were predicted to encode transcription factors belonging to 1 of 10 different classes (Kim et al., 2011). When Kim et al. (2018) applied RNA-seq to analyze differential transcription, it was concluded that the B-box protein encoded by BBX14 was a key regulator of the anthocyanin synthesis pathway. Anthocyanin production in pigmented grain appeared to be induced and fine-tuned by BBX14 in conjunction with the basic leucine zipper transcription factor HY5. Both irradiation at a high light intensity and the plant’s sugar content can influence anthocyanin and proanthocyanin synthesis (Xu et al., 2013; Ma et al., 2018; Zhang et al., 2018). Therefore it would be of value to search for linkages between photoreceptor and light signal transduction elements associated with anthocyanin/proanthocyanidin synthesis in pigmented rice (Teng et al., 2005). While transcriptomic analyses have succeeded in shedding some light on the transcriptional regulation of secondary metabolites, unraveling post-transcriptional and post-translation processes may well provide further insights into the identity of the rate-limiting steps of grain pigmentation (Merchante et al., 2017; Spoel, 2018).
The abundance of a given transcript and its translation product are not always linearly related, due to post-transcriptional regulation, translation and post-translational processing, and peptide modification (Chen et al., 2017; Zhang et al., 2017). Thus, a proteomic analysis can give a more nuanced picture of the differences between pigmented and non-pigmented grains, than is possible from a transcriptomic analysis. A total of 230 differentially abundant proteins, involved in various metabolic processes, were identified by Chen et al. (2016) from a comparison between black and white grains sampled at five stages of grain development. A number of proteins involved in the synthesis of flavonoids and sugars were found to be more abundant in the black grain, while proteins associated with signal transduction, redox homeostasis, photosynthesis, nitrogen metabolism, and tocopherol synthesis were less abundant. In particular, chalcone synthetase was pinpointed as a key component required for the synthesis of anthocyanin.
Metabolomic analyses have also been successfully used to characterize the cellular composition of rice (Table 1). Comparative metabolome studies of black, red, and non-colored rice revealed that various anthocyanins, tocopherol, fatty acid methyl esters, free sugars, and fatty acids were found to be significantly different (Frank et al., 2012). de Guzman et al. (2017) were able to monitor more than 1,000 metabolites in a screen of several rice varieties differing with respect to their nutritional quality and glycemic response (de Guzman et al., 2017). Comparisons of the grain metabolomes of diverse rice accessions have revealed that a substantial degree of variation exists at this level (Gong et al., 2013; Pereira-Caro et al., 2013). However, the grain metabolome is highly dynamic, responding strongly to the plant’s external environment, so this variation is as a consequence of genetic and environmental variation and the interaction there-of. Correlation analyses carried out between individual metabolites have nevertheless revealed the regulation of the grain metabolome, with clusters of co-accumulated metabolites appearing to be under the control of shared genetic factors (Matsuda et al., 2012, 2015).
The application of ‘omics-based platforms have begun to reveal the genetic and biochemical basis of the grain pigment parameters a∗, b∗, L∗, hue, and chroma. While transcriptomic and metabolomic analyses have identified certain important structural and regulatory genes influencing core components of flavonoid synthesis (Lee et al., 2015; Oh et al., 2018), what is still lacking is a comprehensive understanding of the molecular machinery underlying key metabolic processes such as the polymerization and transport of tannins. A more integrated approach, focusing on identifying linkages between regulatory networks is needed (Figure 2). Combining diverse datasets facilitates the reconstruction of regulatory networks and the identification of key modulators (Butardo et al., 2017; Wambugu et al., 2018). As an example, an exploration of the key genetic influences affecting grain amylose–amylopectin composition has implicated two genomic regions, one on chromosome 6 and the other on chromosome 7 (Butardo et al., 2017). The genetic region on chromosome 7 is in the vicinity of Rc and includes a haplotype associated with increased amylose and reduced accumulation of short chain amylopectin. The bHLH transcription factor encoded by Rc activates the gene encoding dihydroflavonol 4-reductase, thereby influencing the formation of red pigmentation. The same transcription factor has also been proposed to act as a regulator of starch structure, as it engages within the network regulating granule-bound starch synthase activity.
Conserving and Utilizing Pigmented Rice Landraces for Future Breeding
Although rice landraces with pigmented grain represent an important genetic reservoir for rice improvement, these populations are rapidly being lost as a result of the introduction of more productive, modern white rice varieties. Some of these materials have been safeguarded in ex situ gene banks, such as the major gene bank curated by the International Rice Research Institute2. Few of these landraces have been systematically characterized in terms of their grain end-use quality, their nutritional features and potential health benefits. Therefore, there is an urgent need to validate the traditional knowledge associated with these materials with scientific-based analyses. While the productivity of the landrace materials is undoubtedly lower than that of modern white rice varieties, their market value is potentially quite high, given the growing consumer preference for nutritious foods (Islam et al., 2018). Looking forward, there is a major opportunity for breeding programs to develop productive pigmented varieties (Voss-Fels et al., 2019).
Understanding the mode of inheritance of grain pigmentation, identifying beneficial alleles of the key genes underlying these traits, and developing trait-specific markers, will contribute to accelerating efforts to breed high yielding pigmented rice varieties. Advanced generation breeding lines of pigmented lines have been developed (Bhuiyan et al., 2011; Arbelaez et al., 2015). A black rice line has been developed in the genetic background of a leading Japanese white rice variety (Koshihikari); which has eating quality superior to that of the widely cultivated black rice variety “Okunomurasaki” (Maeda et al., 2014). Crosses have been initiated between pigmented and non-pigmented varieties to develop pigmented varieties adapted to the growing conditions in Kazakhstan (Rysbekova et al., 2017). The Thai aromatic, deep purple indica-type rice variety “Riceberry” has developed a reputation for its health-promoting properties. Riceberry combines the desirable features of two prominent rice varieties, one a local, non-glutinous purple rice and the other an aromatic white jasmine rice (Waiyawuththanapoom et al., 2015; Gene Discovery Rice and Rice Science Center, 2017). Two improved pigmented varieties (the red rice “Rubi” and the black rice “Onix”) have been released in Brazil (Wickert et al., 2014).
Conclusion
Pigmented rice varieties are gaining popularity among consumers, and demand is only expected to rise. The seed supply chain of pigmented rice is weak and thus rice value chain opportunities have to evolve to meet the current nutritional demand. Production of pigmented rice using landraces is unable to meet market demand, emphasizing the need to genetically improve these landrace materials. Systematic nutritional characterization of the 130,657 accessions curated by International Rice Research Institute’s gene bank and Africa Rice3, which including pigmented entries, will create new avenues for nutritional diversification that reaches lower income target countries. These, as well as other, national ex situ collections, represent a valuable source of genetic variation for the improvement of pigmented rice, providing materials to elucidate the genetic basis of grain pigmentation and associated nutrition-related traits. The process of identifying as yet unknown genes influencing flavonoid metabolism and grain pigmentation could be accelerated by whole genome re-sequencing, allowing novel allelic variants to be harnessed for use as markers. Fine mapped genetic regions associated with proanthocyanidins and anthocyanin needs to be undertaken to develop quality markers to support marker-assisted-selection breeding of these nutritional traits into high yielding rice backgrounds. A systems approach to study implication of diet based health benefits would require holistic understanding of the molecular basis of human health benefits of consuming grain pigmentation, enabling the identification of the modulators involved to overcome the prevailing double burden malnutrition and communicable diseases in the target communities. While several health benefits were shown to possess to consume pigmented rice, its texture and palatability is found to be poor and thus its acceptance rate is lower. To address this limitation, we need to explore the genetic variation for the retention of flavonoids in the milled endosperm.
Author Contributions
EM and NS drafted the manuscript. TK, HJ, NE, CB, and LB edited the part of the sections.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. The authors thank the UK Biotechnology and Biological Sciences Research Council’s Newton Fund Sustainable Rice Research Initiative (Project BB/N013603/1) for its financial support.
References
- Ahuja U., Ahuja S., Chaudhary N., Thakrar R. (2007). Red rices - past, present, and future. Asian Agri Hist. 11 291–304. [Google Scholar]
- Anacleto R., Badoni S., Parween S., Butardo V. M., Misra G., Cuevas R. P., et al. (2019). Integrating a genome wide association study with a large scale transcriptome analysis to predict genetic regions influencing the glycaemic index and texture in rice. Plant Biotechnol. J. 17 1261–1275. 10.1111/pbi.13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbelaez J. D., Moreno L. T., Singh N., Tung C. W., Maron L. G., Ospina Y., et al. (2015). Development and GBS-genotyping of introgression lines (ILs) using two wild species of rice. O. meridionalis and O. rufipogon, in a common recurrent parent, O. sativa cv. Curinga. Mol. Breed. 35 81. 10.1007/s11032-015-0276-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf H., Murtaza I., Nazir N., Wani A. B., Naqash S., Husaini A. M. (2017). Nutritional profiling of pigmented and scented rice genotypes of Kashmir Himalayas. J. Pharmacogn. Phytochem. 6 910–916. [Google Scholar]
- Baek J. A., Chung N. J., Choi K. C., Hwang J. M., Lee J. C. (2015). Hull extracts from pigmented rice exert antioxidant effects associated with total flavonoid contents and induce apoptosis in human cancer cells. Food Sci. Biotechnol. 24 241–247. 10.1007/s10068-015-0032-0 [DOI] [Google Scholar]
- Banjerdpongchai R., Wudtiwai B., Sringarm K. (2013). Cytotoxic and apoptotic-inducing effects of purple rice extracts and chemotherapeutic drugs on human cancer cell lines. Asian Pacific J. Cancer Prev. 14 6541–6548. 10.7314/apjcp.2013.14.11.6541 [DOI] [PubMed] [Google Scholar]
- Berni R., Id C. C., Romi M., Hausman J., Guerriero G., Cai G. (2018). Agrobiotechnology goes wild?: ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 19:E2248. 10.3390/ijms19082248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat F., Riar C. (2015). Health benefits of traditional rice varieties of temperate regions. Med. Aromat. Plants 4 3–5. 10.4172/2167-0412.1000198 [DOI] [Google Scholar]
- Bhuiyan M. A. R., Narimah M. K., Rahim H. A., Abdullah M. Z., Wickneswari R. (2011). Transgressive variants for red pericarp grain with high yield potential derived from Oryza rufipogon×Oryza sativa: field evaluation, screening for blast disease, QTL validation and background marker analysis for agronomic traits. Field Crops Res. 121 232–239. 10.1016/j.fcr.2010.12.012 [DOI] [Google Scholar]
- Boue S. M., Daigle K. W., Chen M.-H., Cao H., Heiman M. L. (2016). Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J. Agric. Food Chem. 64 5345–5353. 10.1021/acs.jafc.6b01909 [DOI] [PubMed] [Google Scholar]
- Breseghello F. (2013). Traditional and modern plant breeding methods with examples in rice (Oryza sativa L.). J. Agric. Food Chem. 61 8277–8286. 10.1021/jf305531j [DOI] [PubMed] [Google Scholar]
- Brooks S. A., Yan W., Jackson A. K., Deren C. W. (2008). A natural mutation in rc reverts white-rice-pericarp to red and results in a new, dominant, wild-type allele: Rc-g. Theor. Appl. Genet. 117 575–580. 10.1007/s00122-008-0801-8 [DOI] [PubMed] [Google Scholar]
- Butardo V. M., Jr., Anacleto R., Parween S., Samson I., Guzman K., De, et al. (2017). Systems genetics identifies a novel regulatory domain of amylose synthesis. Plant Physiol. 173 887–906. 10.1104/pp.16.01248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caixia W., Qingyao S. (2007). Fine mapping and candidate gene analysis of purple pericarp gene Pb in rice (Oryza sativa L.). Chinese Sci. Bull. 52 3097–3104. 10.1007/s11434-007-0472-x [DOI] [Google Scholar]
- Chakuton K., Puangpronpitag D., Nakornriab M. (2012). Phytochemichal content and antioxidant activity of colored and non-colored Thai rice cultivars. Asian J. Plant Sci. 6 285–293. 10.3923/ajps.2012.285.293 [DOI] [Google Scholar]
- Chandramouli B., Latha M. M., Narendra K., Mallikarjuna K. (2018). Phytochemical and antimicrobial investigations of methanolic extract & ethyl acetate extract of rice husk (Oryza sativa) mentioned in an ancient palm leaf manuscript (Talapatra). World J. Pharm. Res. 7 598–616. 10.20959/wjpr20183-10797 [DOI] [Google Scholar]
- Chen L., Huang Y., Xu M., Cheng Z., Zhang D., Zheng J. (2016). iTRAQ-based quantitative proteomics analysis of black rice grain development reveals metabolic pathways associated with anthocyanin biosynthesis. PLoS One 11:e0159238. 10.1371/journal.pone.0159238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang Y., Xu M., Cheng Z., Zheng J. (2017). Proteomic analysis reveals coordinated regulation of anthocyanin biosynthesis through signal transduction and sugar metabolism in black rice leaf. Int. J. Mol. Sci. 18:E2722. 10.3390/ijms18122722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Itani T., Wu X., Chikawa Y., Irifune K. (2013). Physiological factors affecting transcription of genes involved in the flavonoid biosynthetic pathway in different rice varieties. Plant Signal. Behav. 8:e27555. 10.4161/psb.27555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Han X., Wu Y., Lou H. (2014). The Function and catalysis of 2-oxoglutarate-dependent oxygenases involved in plant flavonoid biosynthesis. Int. J. Mol. Sci. 15 1080–1095. 10.3390/ijms15011080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou S. Y., Lai J.-Y., Liao J.-A., Sung J.-M., Lin S.-D. (2018). In vitro inhibition of lipase, α-amylase, α-glucosidase, and angiotensin-converting enzyme by defatted rice bran extracts of red-pericarp rice mutant. Cereal Chem. 95 167–176. 10.1002/cche.10025 [DOI] [Google Scholar]
- Chunta S., Prathepha P., Jongdee B. (2014). Nuances of traditional knowledge in utilization of rice landraces by a farming community in North-Eastern Thailand. Indian J. Tradit. Knowl. 13 473–483. [Google Scholar]
- Das D. K., Oudhia P. (2000). Rice as a medicinal plant in Chhattisgarh, India. NBPGR Newsl. 122:46. [Google Scholar]
- de Guzman M. K., Parween S., Butardo V. M., Alhambra C. M., Anacleto R., Seiler C., et al. (2017). Investigating glycemic potential of rice by unraveling compositional variations in mature grain and starch mobilization patterns during seed germination. Sci. Rep. 7:5854. 10.1038/s41598-017-06026-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Xu J., Xiao K., Zhang Y., Zhang J., Luo L., et al. (2008). Genomic regions associated with the degree of red coloration in pericarp of rice (Oryza sativa L.). J. Cereal Sci. 48 556–560. 10.1016/j.jcs.2007.11.011 [DOI] [Google Scholar]
- Du Y., Chu H., Chu I. K., Lo C. (2010). CYP93G2 Is a flavanone 2-hydroxylase required for c -glycosylflavone biosynthesis in rice. Plant Physiol. 154 324–333. 10.1104/pp.110.161042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari B., Gianinetti A., Finocchiaro F., Terzi V. (2015). Rc gene sequence and expression evaluation in a red-kernel rice. J. Rice Res. 3:145. 10.4172/2375-4338.1000145 22140118 [DOI] [Google Scholar]
- Frank T., Reichardt B., Shu Q., Engel K.-H. (2012). Metabolite profiling of colored rice (Oryza sativa L.) grains. J. Cereal Sci. 55 112–119. 10.1016/j.jcs.2011.09.009 [DOI] [Google Scholar]
- Furukawa T., Maekawa M., Oki T., Suda I., Iida S., Shimada H., et al. (2006). The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 49 91–102. 10.1111/j.1365-313X.2006.02958.x [DOI] [PubMed] [Google Scholar]
- Gallage N. J., Møller B. L. (2015). Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 8 40–57. 10.1016/j.molp.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Galland M., Boutet-Mercey S., Lounifi I., Balzergue S., Grandjean O., Morin H., et al. (2014). Compartmentation and Dynamics of flavone metabolism in dry and germinated rice seeds. Plant Cell Physiol. 55 1646–1659. 10.1093/pcp/pcu095 [DOI] [PubMed] [Google Scholar]
- Gene Discovery Rice and Rice Science Center (2017). Riceberry. Available online at: http://dna.kps.ku.ac.th/index.php/news-articles-rice-rsc-rgdu-knowledge/rice-breeding-lab/riceberry-variety (accessed May 13, 2019) [Google Scholar]
- Ghasemzadeh A., Karbalaii M. T., Jaafar H. Z. E., Rahmat A. (2018). Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem. Cent. J. 12:17. 10.1186/s13065-018-0382-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Chen W., Gao Y., Liu X., Zhang H., Xu C., et al. (2013). Genetic analysis of the metabolome exemplified using a rice population. Proc. Natl. Acad. Sci. U.S.A. 110 20320–20325. 10.1073/pnas.1319681110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross B. L., Steffen F. T., Olsen K. M. (2010). The molecular basis of white pericarps in African domesticated rice: novel mutations at the Rc gene. J. Evol. Biol. 23 2747–2753. 10.1109/TMI.2012.2196707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne A., Wu K., Li D., Bentota A., Corke H., Cai Y. (2013). Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 138 1153–1161. 10.1016/j.foodchem.2012.11.129 [DOI] [PubMed] [Google Scholar]
- Ham T., Kwon S. W., Ryu S., Koh H. (2015). Correlation analysis between grain color and cyanidin-3-glucoside content of rice grain in segregate population. Plant Breed. Biotechnol. 3 160–166. 10.9787/pbb.2015.3.2.160 [DOI] [Google Scholar]
- Han Y. Y., Wang J. W., Han N., Liu Q. J., Liu T. M., Guan F. M., et al. (2009). Duplication and sequence divergence of rice chalcone synthase genes. Russ. J. Plant Physiol. 56 417–422. 10.1134/s1021443709030169 [DOI] [Google Scholar]
- Hashmi M. I., Tianlin J. S. (2016). Minerals contents of some indigenous rice varieties of Sabah Malaysia. Int. J. Agric. For. Plant. 2 31–34. [Google Scholar]
- Hedge S., Yenagi N. B., Kasturiba B. (2013). Indigenous knowledge of the traditional and qualified ayurveda practitioners on the nutritional significance and use of red rice in medications. Indian J. Tradit. Knowl. 12 506–511. [Google Scholar]
- Hemamalini S., Umamaheswari D. S., Lavanya D. R., Umamaheswara R. D. C. (2018). exploring the therapeutic potential and nutritional properties of ‘Karuppu Kavuni’ variety rice of Tamil Nadu. Int. J. Pharma Bio Sci. 9 88–96. [Google Scholar]
- Hu J., Anderson B., Wessler S. R. (1996). Isolation and characterization of rice R genes: evidence for distinct evolutionary paths in rice and maize. Genetics 142 1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. P., Lai H. M. (2016). Bioactive compounds and antioxidative activity of colored rice bran. J. Food Drug Anal. 24 564–574. 10.1016/j.jfda.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtada W. A., Barrion A. S. A., Nguyen-Orca M. F. R. (2018). Mineral content of dehulled and well-milled pigmented and non-pigmented rice varieties in the Philippines. Int. Food Res. J. 25 2063–2067. [Google Scholar]
- Insuan O., Chariyakornkul A., Rungrote Y., Wongpoomchai R. (2017). Antimutagenic and antioxidant activities of Thai rice brans. J. Cancer Prev. 22 89–97. 10.15430/jcp.2017.22.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irakli M. N., Samanidou V. F., Katsantonis D. N., Billiaderis C. G., Papadoyannis I. N. (2016). Phytochemical profiles and antioxidant capacity of pigmented and non-pigmented genotypes of rice (Oryza sativa L.). Cereal Res. Commun. 44 98–110. 10.1556/0806.43.2015.033 [DOI] [Google Scholar]
- Islam M. Z., Khalequzzaman M., Prince M. F. R. K., Siddique M. A., Rashid E. S. M. H., Ahmed M. S. U., et al. (2018). Diversity and population structure of red rice germplasm in Bangladesh. PLoS One 13:e0196096. 10.1371/journal.pone.0196096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesch E. D., Carr T. P. (2017). Food ingredients that inhibit cholesterol absorption. Prev. Nutr. Food Sci. 22 67–80. 10.3746/pnf.2017.22.2.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.-Y., Kim J.-H., Rico C. W., Nam S.-H. (2011). A comparative Study on the physicochemical characteristics of black rice varieties. Int. J. Food Property 14 1241–1254. 10.1080/10942911003637350 [DOI] [Google Scholar]
- Khatri (2018). The Protection of Indigenous Knowledge for Peoples Health Rose. Available online at: https://www.ghwatch.org/sites/www.ghwatch.org/files/indig_kph.pdf (accessed May 13, 2019). [Google Scholar]
- Kim C., Cho M., Choi Y., Kim J. (2011). Identification and characterization of seed-specific transcription factors regulating anthocyanin biosynthesis in black rice. J. Appl. Genet. 52 161–169. 10.1007/s13353-011-0027-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. K., Seol Y. J., Shin Y., Lim H. M., Lee G. S., Kim A. R., et al. (2015). Whole-genome resequencing and transcriptomic analysis to identify genes involved in leaf-color diversity in ornamental rice plants. PLoS One 10:e0124071. 10.1371/journal.pone.0124071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Park S., Lee J. Y., Ha S. H., Lee J. G., Lim S. H. (2018). A rice B-Box protein, OsBBX14, finely regulates anthocyanin biosynthesis in rice. Int. J. Mol. Sci. 9:E2190. 10.3390/ijms19082190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G. R., Jung E. S., Lee S., Lim S., Ha S., Lee C. H. (2014). Combined mass spectrometry-based metabolite profiling of different pigmented rice (Oryza sativa L.) seeds and correlation with antioxidant activities. Molecules 19 15673–15686. 10.3390/molecules191015673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Lee Y. J., Kim B. G., Lim Y., Ahn J.-H. (2008). Flavanone 3ß-hydroxylases from rice: key enzymes for favonol and anthocyanin biosynthesis. Mol. Cells 25 312–316. [PubMed] [Google Scholar]
- Ko J. H., Kim B. G., Hur H. G., Lim Y., Ahn J. H. (2006). Molecular cloning, expression and characterization of a glycosyltransferase from rice. Plant Cell Rep. 25 741–746. 10.1007/s00299-006-0119-4 [DOI] [PubMed] [Google Scholar]
- Korte A., Farlow A. (2013). The advantages and limitations of trait analysis with GWAS?: a review. Plant Methods 9:29. 10.1186/1746-4811-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnanunni K., Ramaiah S., Anbarasu A. (2014). Total phenolic content and “in-vitro” antioxidant assay of two medicinal rice varieties - Karungkavuni and Kuzhiadichan. Int. J. Pharma Bio Sci. 5 540–548. 10.1007/s13197-014-1292-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachagari V. B. R., Gupta R., Lekkala S. P., Mahadevan L., Kuriakose B., Chakravartty N., et al. (2019). Whole genome sequencing and comparative genomic analysis reveal allelic variations unique to a purple colored rice landrace (Oryza sativa ssp. Indica cv. Purpleputtu). Front. Plant Sci. 10:513. 10.3389/fpls.2019.00513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Seol Y., Hahn J., Won S., Won Y., Kim Y., et al. (2015). Transcriptomics analyses of genes regulating anthocyanin production in black rice. BioChip J. 9 59–66. 10.1007/s13206-014-9108-9 [DOI] [Google Scholar]
- Lim S. H., Ha S. H. (2013). Marker development for the identification of rice seed color. Plant Biotechnol. Rep. 7 391–398. 10.1007/s11816-013-0276-1 [DOI] [Google Scholar]
- Liu Q., Luo L., Zheng L. (2018). Lignins: biosynthesis and biological functions in plants. Int. J. Mol. Sci. 19:E335. 10.3390/ijms19020335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. P., Han B., Yu X. P., Chen X. Y., Zhou J., Chen W., et al. (2014). Anti-metastasis activity of black rice anthocyanins against breast cancer: analyses using an ErbB2 positive breast cancer cell line and tumoral xenograft model. Asian Pacific J. Cancer Prev. 15 6219–6225. 10.7314/APJCP.2014.15.15.6219 [DOI] [PubMed] [Google Scholar]
- Ma Y., Id H. D., Zhang F., Li Y., Yang H., Tian F.-P., et al. (2018). Transcriptomic analysis of Lycium ruthenicum Murr. during fruit ripening provides insight into structural and regulatory genes in the anthocyanin biosynthetic pathway. PLoS One 13:e0208627. 10.1371/journal.pone.0208627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Yamaguchi T., Omoteno M., Takarada T., Fujita K., Murata K., et al. (2014). Genetic dissection of black grain rice by the development of a near isogenic line. Breed. Sci. 64 134–141. 10.1270/jsbbs.64.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Nakabayashi R., Yang Z., Okazaki Y., Yonemaru J. I., Ebana K., et al. (2015). Metabolome-genome-wide association study dissects genetic architecture for generating natural variation in rice secondary metabolism. Plant J. 81 13–23. 10.1111/tpj.12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda F., Okazaki Y., Oikawa A., Kusano M., Nakabayashi R., Kikuchi J., et al. (2012). Dissection of genotype – phenotype associations in rice grains using metabolome quantitative trait loci analysis. Plant J. 70 624–636. 10.1111/j.1365-313X.2012.04903.x [DOI] [PubMed] [Google Scholar]
- Matsukawa T., Villareal M. O., Isoda H. (2016). The Type 2 diabetes-preventive effect of cyanidin-3-glucoside on adipocytes. J. Dev. Sustain. Agric. 11 31–35. [Google Scholar]
- Melini V., Acquistucci R. (2017). Health-promoting compounds in pigmented Thai and wild rice. Foods 6:E9. 10.3390/foods6010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchante C., Stepanova A. N., Alonso J. M. (2017). Translation regulation in plants?: an interesting past, an exciting present and a promising future. Plant J. 90 628–653. 10.1111/tpj.13520 [DOI] [PubMed] [Google Scholar]
- Nigatu G., Hansen J., Childs N., Seeley R. (2017). Sub-Saharan Africa is Projected to be the Leader in Global Rice Imports Highlights. Available online at: https://ideas.repec.org/a/ags/uersaw/266026.html (accessed May 13, 2019). [Google Scholar]
- Oh H. J., Lee Y.-J., Byeon E.-J., Kang B.-C., Kyeong D.-S., Kim C.-K. (2018). Whole - genome resequencing and transcriptomic analysis of genes regulating anthocyanin biosynthesis in black rice plants. 3 Biotech 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa T., Maeda H., Oguchi T., Yamaguchi T., Tanabe N., Ebana K., et al. (2015). The birth of a black rice gene and its local spread by introgression. Plant Cell 27 2401–2414. 10.1105/tpc.15.00310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Choi M. J., Lee J. Y., Kim J. K., Ha S. H., Lim S. H. (2016). Molecular and biochemical analysis of two rice flavonoid 3’-hydroxylase to evaluate their roles in flavonoid biosynthesis in rice grain. Int. J. Mol. Sci. 17 1549–1562. 10.3390/ijms17091549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira-Caro G., Watanabe S., Crozier A., Fujimura T., Yokota T. (2013). Phytochemical profile of a Japanese black – purple rice. Food Chem. 141 2821–2827. 10.1016/j.foodchem.2013.05.100 [DOI] [PubMed] [Google Scholar]
- Petroni K., Landoni M., Tomay F., Calvenzani V. (2017). Proximate composition, polyphenol content and anti-inflammatory properties of white and pigmented Italian rice varieties. Univ. J. Agric. Res. 5 312–321. 10.13189/ujar.2017.050509 [DOI] [Google Scholar]
- Pintha K., Yodkeeree S., Pitchakarn P., Limtrakul P. (2014). Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pacific J. Cancer Prev. 15 4601–4607. 10.7314/apjcp.2014.15.11.4601 [DOI] [PubMed] [Google Scholar]
- Poulev A., Heckman J. R., Raskin I., Belanger F. C. (2019). Tricin levels and expression of flavonoid biosynthetic genes in developing grains of purple and brown pericarp rice. PeerJ 7:e6477. 10.7717/peerj.6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualley A. V., Widhalm J. R., Adebesin F., Kish C. M., Dudareva N. (2012). Completion of the core-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. U.S.A. 109 16383–16388. 10.1073/pnas.1211001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachasima L. N., Sukkasem R., Pongjaroenkit S., Sangtong V., Chowpongpang S., Sakulsingharoj C. (2017). Expression analysis and nucleotide variation of OsC1 gene associated with anthocyanin pigmentation in Thai rice cultivars. Genomics Genet. 10 46–53. [Google Scholar]
- Raghuvanshi R. S., Dutta A., Tewari G., Suri S. (2017). Qualitative characteristics of red rice and white rice procured from local market of Uttarakhand?: a comparative study. J. Rice Res. 10 49–53. [Google Scholar]
- Rahman M. M., Lee K. E., Lee E. S., Matin M. N., Lee D. S., Yun J. S., et al. (2013). The genetic constitutions of complementary genes Pp and Pb determine the purple color variation in pericarps with cyanidin-3-O-glucoside depositions in black rice. J. Plant Biol. 56 24–31. 10.1007/s12374-012-0043-9 [DOI] [Google Scholar]
- Rahman S., Sharma M. P., Sahai S. (2006). Nutritional and medicinal values of some indigenous rice varieties. Indian J. Tradit. Knowl. 5 454–458. [Google Scholar]
- Rao Y., Li Y., Qian Q. (2014). Recent progress on molecular breeding of rice in China. Plant Cell Rep. 33 551–564. 10.1007/s00299-013-1551-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. E., Dennison J. (2015). The photobiology of lutein and zeaxanthin in the eye. J. Ophtalmol. 2015:687173. 10.1155/2015/687173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysbekova A. B., Kazkeyev D. T., Usenbekov B. N., Mukhina Z. M., Zhanbyrbaev E. A., Sartbaeva I. A., et al. (2017). Prebreeding selection of rice with colored pericarp based on genotyping Rc and Pb genes. Russ. J. Genet. 53 49–58. 10.1134/s1022795416110119 [DOI] [PubMed] [Google Scholar]
- Saitoh K., Onishi K., Mikami I., Thidar K., Sano Y. (2004). Allelic diversification at the C (OsC1) locus of wild and cultivated rice: nucleotide changes associated with phenotypes. Genetics 168 997–1007. 10.1534/genetics.103.018390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto W., Ohmori T., Kageyama K., Miyazaki C., Saito A., Noda K., et al. (2001). The purple leaf (Pl) Locus of Rice?: the Plw allele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis. Plant Cell Physiol. 42 982–991. 10.1093/pcp/pce128 [DOI] [PubMed] [Google Scholar]
- Sakulsingharoj C., Inta P., Sukkasem R., Pongjaroenkit S., Chowpongpang S., Sangtong V. (2014). Overexpression of OSB2 gene in transgenic rice up-regulated expression of structural genes in anthocyanin biosynthesis pathway. Thai J. Genet. 7 173–182. 10.14456/tjg.2014.24 [DOI] [Google Scholar]
- Sakulsingharoj C., Inta P., Sukkasem R., Pongjaroenkit S., Chowpongpang S., Sangtong V. (2016). Cloning and characterization of OSB1 gene controlling anthocyanin biosynthesis from Thai black rice. Genomics Genet. 9 7–18. 10.14456/gag.2016.2 [DOI] [Google Scholar]
- Saneei P., Larijani B., Esmaillzadeh A. (2016). Rice consumption, incidence of chronic diseases and risk of mortality?: meta-analysis of cohort studies. Public Health Nutr. 20 233–244. 10.1017/S1368980016002172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sánchez N. F., Salas-Coronado R., Hernández-Carlos B., Villanueva-Cañongo C. (2019). “Shikimic Acid pathway in biosynthesis of phenolic compounds,” in Plant Physiological Aspects of Phenolic Compounds, eds Soto-Hernández M., García-Mateos R., Palma-Tenango M. (London: IntechOpen; ), 10.5772/intechopen.83815 [DOI] [Google Scholar]
- Sarma R., Swamy H. V. V. K., Shashidhar H. E. (2018). Dealing with zinc and iron deficiency in rice?: combine Strategies to fight hidden hunger in developing countries. Int. J. Curr. Microbiol. Appl. Sci. 7 1887–1895. 10.20546/ijcmas.2018.703.224 [DOI] [Google Scholar]
- Sasaki N., Nishizaki Y., Ozeki Y., Miyahara T. (2014). The role of acyl-glucose in anthocyanin modifications. Molecules 19 18747–18766. 10.3390/molecules191118747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathya A. (2013). Are the Indian rice landraces a heritage of biodiversity to reminisce their past or to reinvent for future? Asian Agrihist. 17 221–232. [Google Scholar]
- Shao Y., Hu Z., Yu Y., Mou R., Zhu Z., Beta T. (2018). Phenolic acids, anthocyanis, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 239 733–741. 10.1016/j.foodchem.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Shao Y., Jin L., Zhang G., Lu Y., Shen Y., Bao J. (2011). Association mapping of grain color, phenolic content, flavonoid content and antioxidant capacity in dehulled rice. Theor. Appl. Genet. 122 1005–1016. 10.1007/s00122-010-1505-4 [DOI] [PubMed] [Google Scholar]
- Sharma A., Patni B., Shankhdhar D., Shankhdhar S. C. (2013). Zinc – an indispensable micronutrient. Physiol. Mol. Biol. Plants 19 11–20. 10.1007/s12298-012-0139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. H., Chu H., Tang L. K., Sakamoto W., Maekawa M., Chu I. K., et al. (2008). Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 228 1043–1054. 10.1007/s00425-008-0806-1 [DOI] [PubMed] [Google Scholar]
- Shozib H. B., Jahan S., Das S. C., Alam S., Amin R. B., Hasan M., et al. (2017). Mineral profiling of HYV rice in Bangladesh. Vitam. Miner. 6:164 10.4172/2376-1318.1000164 [DOI] [Google Scholar]
- Spoel S. H. (2018). Orchestrating the proteome with post-translational modifications. J. Exp. Bot. 69 4499–4503. 10.1093/jxb/ery295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhang Z., Chen C., Wu W., Ren N., Jiang C., et al. (2018). The C–S–A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J. Exp. Bot. 69 1485–1498. 10.1093/jxb/ery001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttiarporn P., Chumpolsri W., Mahatheeranont S., Luangkamin S., Teepsawang S., Leardkamolkarn V. (2015). Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 7 1672–1687. 10.3390/nu7031672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwannakul N., Punvittayagul C., Jarukamjorn K., Wongpoomchai R. (2015). Purple rice bran extract attenuates the aflatoxin B1-induced initiation stage of hepatocarcinogenesis by alteration of xenobiotic metabolizing enzymes. Asian Pacific J. Cancer Prev. 16 3371–3376. 10.7314/APJCP.2015.16.8.3371 [DOI] [PubMed] [Google Scholar]
- Sweeney M. T., Thomson M. J., Cho Y. G., Park Y. J., Williamson S. H., Bustamante C. D., et al. (2007). Global dissemination of a single mutation conferring white pericarp in rice. PLoS Genet. 3:e133. 10.1371/journal.pgen.0030133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M. T., Thomson M. J., Pfeil B. E., Mccouch S. (2006). Caught red-handed?: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 18 283–294. 10.1105/tpc.105.038430.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. F., Sun M., Xing Y. Z., Hua J. P., Sun X. L., Zhang Q. F., et al. (2001). Mapping quantitative trait loci for milling quality, protein content and color characteristics of rice using a recombinant inbred line population derived from an elite rice hybrid. Theor. Appl. Genet. 103 1037–1045. 10.1007/s001220100665 [DOI] [Google Scholar]
- Tantipaiboonwong P., Pintha K., Chaiwangyen W., Chewonarin T., Pangjit K., Chumphukam O., et al. (2017). Anti-hyperglycaemic and anti-hyperlipidaemic effects of black and red rice in streptozotocin-induced diabetic rats. ScienceAsia 43 281–288. [Google Scholar]
- Teng S., Keurentjes J., Bentsink L., Koornneef M., Smeekens S. (2005). Sucrose-specific induction of anthocyanin biosynthesis in arabidopsis requires the MYB75 / PAP1 Gene. Plant Physiol. 139 1840–1852. 10.1104/pp.105.066688.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terungwa T. I., Yuguda M. A. (2014). The impact of rice production, consumption and importation in Nigeria?: the political economy perspectives. Int. J. Sustain. Dev. World Policy 3 90–99. [Google Scholar]
- Thomas R., Bhat R., Kuang Y. T. (2015). Composition of amino acids, fatty acids, minerals and dietary fiber in some of the local and import rice varieties of Malaysia. Int. Food Res. J. 22 1148–1155. [Google Scholar]
- Tzin V., Galili G. (2010). New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 3 956–972. 10.1093/mp/ssq048 [DOI] [PubMed] [Google Scholar]
- Umadevi M., Pushpa R., Sampathkumar K. P., Bhowmik D. (2012). Rice-traditional medicinal plant in India. J. Pharmacogn. Phytochem. 1 6–12. [Google Scholar]
- Valarmathi R., Raveendran M., Senthil N. (2014). Unraveling the nutritional and therapeutic properties of ‘Kavuni’ a traditional rice variety of Tamil Nadu. J. Plant Biochem. Biotechnol. 24 305–315. 10.1007/s13562-014-0274-6 [DOI] [Google Scholar]
- Verma D. K., Shukla K. (2011). Nutritional value of rice and their importance. Indian Farmers Dig. 44 21–35. [Google Scholar]
- Vichit W., Saewan N. (2015). antioxidant activities and cytotoxicity of Thai pigmented rice. Int. J. Pharm. Pharm. Sci. 7 329–334. 25518304 [Google Scholar]
- Voss-Fels K. P., Stahl A., Hickey L. T. (2019). Q&A: modern crop breeding for future food security. BMC Biol. 17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahyuni A. S., Munawaroh R., Da’i M. (2016). Antidiabetic mechanism of ethanol extract of black rice bran on diabetic rats. Natl. J. Physiol. Pharm. Pharmacol. 6 106–110. 10.5455/njppp.2015.5.1111201590 [DOI] [Google Scholar]
- Waiyawuththanapoom P., Waiyawuththanapoom W., Tirastittam P. (2015). Social media as a channel for Thailand’s Rice Berry Product. Int. J. Econ. Manag. Eng. 9 904–907. [Google Scholar]
- Wambugu P., Ndjiondjop M., Furtado A., Henry R. (2018). Sequencing of bulks of segregants allows dissection of genetic control of amylose content in rice. Plant Biotechnol. J. 16 100–110. 10.1111/pbi.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xu X., Vieira F. G., Xiao Y., Li Z., Wang J., et al. (2016). The power of inbreeding: NGS-based GWAS of rice reveals convergent evolution during rice domestication. Mol. Plant 9 975–985. 10.1016/j.molp.2016.04.018 [DOI] [PubMed] [Google Scholar]
- Wickert E., Schiocchet M. A., Noldin J. A., Raimondi J. V., De Andrade A., Scheuermann K. K., et al. (2014). Exploring variability?: New Brazilian varieties SCS119 Rubi and SCS120 Onix for the specialty rices market. Open J. Genet. 4 157–165. 10.4236/ojgen.2014.42016 [DOI] [Google Scholar]
- Widhalm J. R., Dudareva N. (2015). A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 8 83–97. 10.1016/j.molp.2014.12.001 [DOI] [PubMed] [Google Scholar]
- World Rice Production (2019). World Rice Production 2019/2020. Available online at: http://www.worldagriculturalproduction.com/crops/rice.aspx/ (accessed May 17, 2019). [Google Scholar]
- Xiongsiyee V., Rerkasem B., Veeradittakit J., Saenchai C., Lordkaew S., Prom-U-Thai C. T. (2018). Variation in grain quality of upland rice from Luang Prabang province, Lao PDR. Rice Sci. 25 94–102. 10.1016/j.rsci.2018.02.002 [DOI] [Google Scholar]
- Xu T. Y., Sun J., Chang H. L., Zheng H. L., Wang J. G., Liu H. L., et al. (2017). QTL mapping for anthocyanin and proanthocyanidin content in red rice. Euphytica 213:243. [Google Scholar]
- Xu W., Grain D., Bobet S., Le Gourrierec J., Thevenin J., Kelemen Z., et al. (2013). Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB – bHLH – WDR complexes and their targets in Arabidopsis seed. New Phytol. 202 132–144. 10.1111/nph.12620 [DOI] [PubMed] [Google Scholar]
- Yang X., Xia X., Zeng Y., Nong B., Zhang Z., Wu Y., et al. (2018). Identification of candidate genes for gelatinization temperature, gel consistency and pericarp color by GWAS in rice based on SLAF-sequencing. PLoS Genet. 13:e0196690. 10.1371/journal.pone.0196690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y., Zaima N., Moriyama T., Kawamura Y. (2012). Different localization patterns of anthocyanin species in the pericarp of black rice revealed by imaging mass spectrometry. PLoS One 7:e31285. 10.1371/journal.pone.0031285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Shao Y., Bao J., Beta T. (2015). Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 172 630–639. 10.1016/j.foodchem.2014.09.118 [DOI] [PubMed] [Google Scholar]
- Zhang L., Dong Y., Wang Q., Du C., Xiong W., Li X., et al. (2017). iTRAQ-based proteomics analysis and network integration for kernel tissue development in maize. Mol. Sci. 18:1840. 10.3390/ijms18091840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Xu S., Cheng Y., Peng Z. (2018). Transcriptome profiling of anthocyanin- related genes reveals effects of light intensity on anthocyanin biosynthesis in red leaf lettuce. PeerJ 6:e4607. 10.7717/peerj.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Pang Y., Dixon R. A. (2010). The mysteries of proanthocyanidin transport and polymerization. Plant Physiol. 153 437–443. 10.1104/pp.110.155432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M., Anwar F., Ashraf M., Uddin K. (2012). Characterization of high-value bioactives in some selected varieties of Pakistani rice (Oryza sativa L.). Int. J. Mol. Sci. 13 4608–4622. 10.3390/ijms13044608 [DOI] [PMC free article] [PubMed] [Google Scholar]