Abstract
Objectives
To develop computer algorithms that can recognize physiologic patterns in traumatic brain injury patients that occur in advance of intracranial pressure and partial brain tissue oxygenation crises. The automated early detection of crisis precursors can provide clinicians with time to intervene in order to prevent or mitigate secondary brain injury.
Design
A retrospective study was conducted from prospectively collected physiologic data. Intracranial pressure, and partial brain tissue oxygenation crisis events were defined as intracranial pressure greater than or equal to 20 mm Hg lasting at least 15 minutes and partial brain tissue oxygenation value less than 10 mm Hg for at least 10 minutes, respectively. The physiologic data preceding each crisis event were used to identify precursors associated with crisis onset. Multivariate classification models were applied to recorded data in 30-minute epochs of time to predict crises between 15 and 360 minutes in the future.
Setting
The neurosurgical unit of Ben Taub Hospital (Houston, TX).
Subjects
Our cohort consisted of 817 subjects with severe traumatic brain injury.
Measurements and Main Results
Our algorithm can predict the onset of intracranial pressure crises with 30-minute advance warning with an area under the receiver operating characteristic curve of 0.86 using only intracranial pressure measurements and time since last crisis. An analogous algorithm can predict the start of partial brain tissue oxygenation crises with 30-minute advanced warning with an area under the receiver operating characteristic curve of 0.91.
Conclusions
Our algorithms provide accurate and timely predictions of intracranial hypertension and tissue hypoxia crises in patients with severe traumatic brain injury. Almost all of the information needed to predict the onset of these events is contained within the signal of interest and the time since last crisis.
Keywords: brain tissue hypoxia, forecasting, data mining, intracranial pressure, neuromonitoring, prediction algorithm, traumatic brain injury
Two central physiologic targets in the management of severe traumatic brain injury (TBI) are the intracranial pressure (ICP) and the partial brain tissue oxygen tension (PbtO2). Despite the lack of high-level evidence on impacting clinical outcome via control of these variables, most ICUs treating TBI incorporate ICP and PbtO2 into step-tiered clinical protocols. This approach is recommended by the Brain Trauma Foundation (BTF) guidelines and by more recent international multidisciplinary consensus conferences (1–4).
Although the initial injury incurred by TBI patients is usually irreversible, the original trauma often starts a series of physiologic changes that may cause long-term damage. These “secondary injuries” can be mitigated and even reversed, to some extent, by careful management, particularly of ICP hypertension. The current BTF guidelines offer thresholds at which treatment is recommended in order to prevent or mitigate secondary brain injury. However, by the time treatment is provided, it may be too late. The ability to predict the onset of these “crisis” events would provide clinicians with valuable time to attempt aborting the episode and/or to appropriately manage it. Combining statistical machine learning with physiologic and clinical insight allows the construction of robust quantitative models to predict future events such as elevated ICP or low PbtO2 Such models provide targets for evidence-based individualized treatment in real time (5, 6).
We seek to predict episodes of intracranial hypertension and brain tissue hypoxia 30 minutes to several hours in advance of overt deterioration using intuitive and simple models whose inputs are the recent ICP or PbtO2 values, the time since the last crisis and the fraction of the time spent with high/low ICP or oxygen levels. We investigate how model performance changes as the prediction time increases, providing an estimate of the clinical timescale of crisis precursors. We also investigate the importance of these crises with regards to clinical outcomes by computing correlations between both the total time and fraction of time patients spend in each type of crisis and their 1-, 3-, and 6-month Glasgow Outcome Scale (GOS) (7).
To the best of our knowledge, this article represents the first attempt to build a predictive model for brain tissue hypoxia in TBI patients. Although previous work has been published on predicting ICP crises, these approaches use more than 100 independent variables (8). In contrast, we propose that it is possible to achieve good predictive performance using five extracted features, forming intuitive models that are perhaps less prone to overfitting and more likely to generalize to clinical settings.
MATERIALS AND METHODS
Database
We constructed a database of 874 subjects who experienced severe TBI. Patient data were collected between 1989–2000 and 2006–2013. All subjects were admitted to the neurosurgical ICU of Ben Taub General Hospital located in Houston, TX. ICP, mean arterial pressure (MAP), end-tidal CO2 (ETco2), oxygen saturation, and PbtO2 measurements were recorded every 36 seconds from the patient monitors for each subject over the course of their ICU admission. ICP measurements were made using either a extraventricular drain or an intraparenchymal fiberoptic probe. PbtO2 measurements were made using the Licox (Integra Life Sciences, Plainsboro, NJ) device. ICP recordings were present in 817 subjects for subsequent analysis of intracranial hypertension. PbtO2 recordings were present in 242 subjects for analysis of brain tissue hypoxia. The Institutional Review Board of Baylor College of Medicine approved the use of this dataset for retrospective analysis (protocol H-34134).
Prediction Task
Our goal was to discover algorithms that could provide early warning of impending crises in TBI patients. We developed and evaluated a set of statistical models to predict periods of elevated ICP, as well as a second set of models to predict depressed PbtO2. ICP crises were defined as time periods where ICP was greater than or equal to 20 mm Hg for at least 15 minutes. PbtO2 crises were defined as time periods where PbtO2 was less than 10 mm Hg for at least 10 minutes. In both cases, a 30-minute window of physiologic data and the time since last crises were used to predict impending crisis events between 15 and 360 minutes in the future.
Evaluation Process
The database was separated into three cohorts. Data collected from subjects between 1989 and 1996 formed the study cohort; data between 1996 and 2000 comprised the model selection cohort; whereas data between 2006 and 2013 defined the validation cohort. The study cohort was used to develop candidate predictive models, while the model selection cohort was used to evaluate their performance. The best performing models were subsequently validated on the independent validation cohort. This strict separation of model development, selection, and validation makes it highly unlikely for the model to be over-fit to the training data.
Because data were collected over a period of 24 years, changes in patient care practices could impact model applicability. Our groups are well matched in terms of age, gender, and admission Glasgow Coma Scale. No attempt was made to create strictly matched groups because our primary aim was event prediction, not outcome comparisons between cohorts. Differences in mortality rates may be due to trauma causes and advancement of care practices. Models were constructed and tuned using the oldest of the recorded data and evaluated on temporally distinct and more recent data. If such models work well across this wide time range, it provides strong evidence that the models are robust to changes in care management, which may make them widely applicable.
Different types of statistical models were developed using the study cohort. The top 6 models were evaluated using the model selection cohort. We used the area under the receiver operating characteristic curve (AUC) to evaluate discrimination performance. We tested the two best performing models on the independent validation cohort.
Two tests were performed using the validation cohort. The first used 10-fold cross validation to calculate an average AUC. This test measures the ideal-case performance of the models.
In the second test, model parameters optimized using the study cohort are directly applied to the validation cohort, without modification. This more pessimistic evaluation measures how the models perform when parameters are fit to data that are distinct from the dataset used to construct the model.
The process for developing predictive models of PbtO2 crises was similar, but because PbtO2 crises are more rare than ICP crisis events, the epochs prior to PbtO2 crises were replicated 100 times in the data used for model development to increase their prevalence, a standard method for dealing with rare classes (9).
Models Evaluated
Three types of classification algorithms were studied: Gaussian processes (GP), logistic regression, and the Autoregressive Ordinal-Regression (AR-OR) model described by Myers et al (10).
Gaussian process models were evaluated by duplicating the steps used by Güiza et al (8). Their selection method generated a set of features for each epoch, after which a GP model evaluated the predictive ability of each feature individually to predict crisis states. This process generated 145 features in our dataset. We used the top 5% of these features in combination to predict crises 30 minutes in the future.
Logistic regression models were evaluated based on the 145 features identified by Güiza et al (8). A univariate analysis was performed to measure the association between each individual feature with the onset of crisis events. This feature set was truncated to include only those features with the strongest associations.
The AR-OR model utilizes a hidden Markov model to learn a set of latent time series states. The fraction of time spent in each state was used as an input to a logistic/probit regression (along with other features) to predict the value of a dependent variable. The AR-OR model has demonstrated success in classifying vital sign time series (9).
Several combinations of these model generation methods were applied to the study cohort. Additional physiologic signals (CPP, MAP, and Petco2) were included in the model construction. A piecewise-linear approximation of the time since last crisis was also included in the models.
Additional information related to the methods of this article can be found in the online supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/B869).
RESULTS
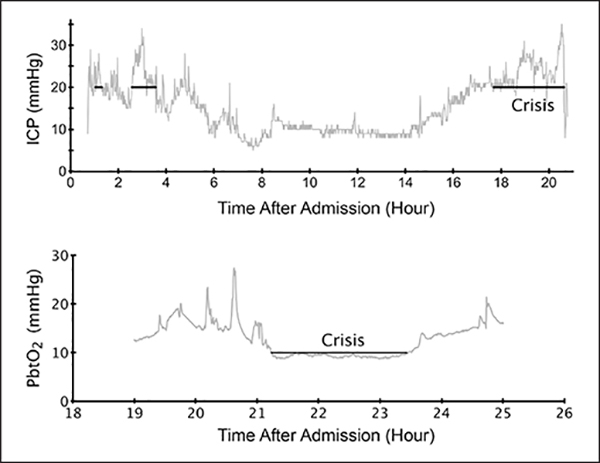
A breakdown of the study cohorts by demographics and types of trauma can be seen in Tables 1 and 2. Typical ICP and PbtO2 recordings can be seen in Figure 1. The time since the last crisis was found to be strongly predictive of when another crisis would occur. Figure S1 (Supplemental Digital Content 1, http://links.lww.com/CCM/B869) shows the probability of a second crisis occurring as a function of the amount of time elapsed since the last observed crisis event. The probability of having a crisis is higher if the subject had recently experienced a crisis.
TABLE 1.
Cohort Characteristics for the Intracranial Pressure Crisis Prediction Study
| ICP Study | Study Cohort (n = 368) | Model Selection Cohort (n = 188) | Validation Cohort (n = 261) | All Patients (n = 817) |
|---|---|---|---|---|
| ICU LOS (d), median (IQR) | 16.4 (8.6–27) | 17.7 (8.6–28.1) | 16.3 (10.5–23.5) | 16.8 (9.2–25.5) |
| Hospital LOS (d), median (IQR) | 26.0 (11.8–43) | 23.5 (11–41.8) | 23 (15–42) | 24 (13–42) |
| Age (yr), median (IQR) | 29 (21–40) | 32 (23–45) | 30 (23–46) | 30 (22–42) |
| Gender (% male) | 87 | 83.2 | 85.1 | 85 |
| Trauma (%) | ||||
| Assault | 33.2 | 22.9 | 11.9 | 24 |
| Transportation | 53 | 50 | 70.5 | 57.9 |
| Explosion | 0.3 | 0 | 0 | 0.1 |
| Fall | 8.4 | 14.9 | 13.4 | 11.5 |
| Falling object | 0.3 | 1.6 | 0.4 | 0.6 |
| Work | 0.3 | 1.1 | 0.4 | 0.5 |
| Sports | 0.5 | 0.5 | 0 | 0.4 |
| Unknown | 4.1 | 9 | 3.4 | 5 |
| GCS eye, median (IQR) | 7 (4–9) | 7 (4–9) | 7 (4–8) | 7 (4–8) |
| GCS motor, median (IQR) | 5 (2–5) | 4 (2–5) | 5 (2–5) | 5 (2–5) |
| 1-mo GOS (%) | 24/18/36/17/4 | 21/20/38/8/4 | 10/23/46/5/2 | 19/20/40/11/3 |
| Missing, % | 1 | 8 | 14 | 7 |
| 3-mo GOS (%) | 26/9/23/23/10 | 22/10/32/12/9 | 13/10/39/13/8 | 21/10/30/18/9 |
| Missing, % | 8 | 15 | 16 | 12 |
| 6-mo GOS (%) | 28/5/15/18/17 | 24/5/23/13/13 | 14/5/30/17/14 | 23/5/22/17/15 |
| Missing, % | 17 | 21 | 19 | 18 |
| Elevated ICP episodes per patient, median (IQR) | 13(2–44.5) | 19 (3–66.5) | 11 (1–40) | 14 (2–46) |
| Duration of ICP episodes (min), median (IQR) | 34.2 (22–65.2) | 34.2 (22–69.2) | 30.7 (21.4–54.3) | 33.7 (21.8–62.7) |
ICP = intracranial pressure, LOS = length of stay, IQR = interquartile range, GCS = Glasgow Coma Scale.
TABLE 2.
Cohort Characteristics for the Brain Hypoxia Prediction Study
| PbtO2 Study | Study Cohort (n = 73) | Model Selection Cohort (n = 37) | Validation Cohort (n = 132) | All Patients (n = 242) |
|---|---|---|---|---|
| ICU LOS (d), median (IQR) | 18.7 (10.8–28.4) | 17.1 (8.8–272) | 18.1 (11.4–24.3) | 18.3 (10.6–26.3) |
| Hospital LOS (d), median (IQR) | 24.5 (13–42.3) | 23 (11.5–38.5) | 26 (16.5–46) | 26 (15–43) |
| Age (yr), median (IQR) | 27 (20–44) | 33 (24–40) | 31 (23–46) | 30 (23–44) |
| Gender (% male) | 79.5 | 81.1 | 88.6 | 85.4 |
| Missing, % | 0 | 2.6 | 0.8 | 0.4 |
| Trauma, % | ||||
| Assault | 21.9 | 27 | 15.9 | 19.4 |
| Transportation | 60.3 | 45.9 | 72 | 64.5 |
| Fall | 12.3 | 13.5 | 10.6 | 11.6 |
| Falling object | 1.4 | 2.7 | 0 | 0.8 |
| Work | 0 | 0 | 0.8 | 0.4 |
| Unknown | 4.1 | 10.8 | 0.8 | 3.3 |
| GCS eye, median (IQR) | 6 (4–8) | 6.5 (3.8–9.3) | 7 (3–8) | 7 (4–8) |
| GCS motor, median (IQR) | 4 (2–5) | 4 (1.8–5) | 5 (2–5) | 4 (2–5) |
| 1-mo GOS (%) | 19/18/48/5/4 | 19/16/38/5/14 | 10/29/45/5/4 | 14/24/45/5/5 |
| Missing, % | 5 | 8 | 7 | 6 |
| 3-mo GOS (%) | 19/8/36/22/7 | 22/5/30/16/16 | 14/9/45/10/10 | 17/8/40/14/10 |
| Missing, % | 8 | 11 | 12 | 11 |
| 6-mo GOS (%) | 22/4/23/16/15 | 22/5/19/19/22 | 15/5/38/18/10 | 18/5/31/18/13 |
| Missing, % | 19 | 14 | 14 | 16 |
| Depressed PbtO2 episodes per patient, median (IQR) | 2 (0–5) | 1 (0–6) | 3 (1–9) | 2 (0–6.8) |
| Duration of PbtO2 episodes, median (IQR) | 43 (18.9–93.2) | 48.6 (19.1–192.1) | 46.3 (21.7–100.9) | 44.9 (20.6–103.8) |
PbtO2 = partial brain tissue oxygen tension, LOS = length of stay, IQR = interquartile range, GCS = Glasgow Coma Scale.
Figure 1.
Typical intracranial pressure (ICP) and partial brain tissue oxygenation (PbtO2) recordings. Crisis periods are annotated with lines.
Prediction of ICP Crises
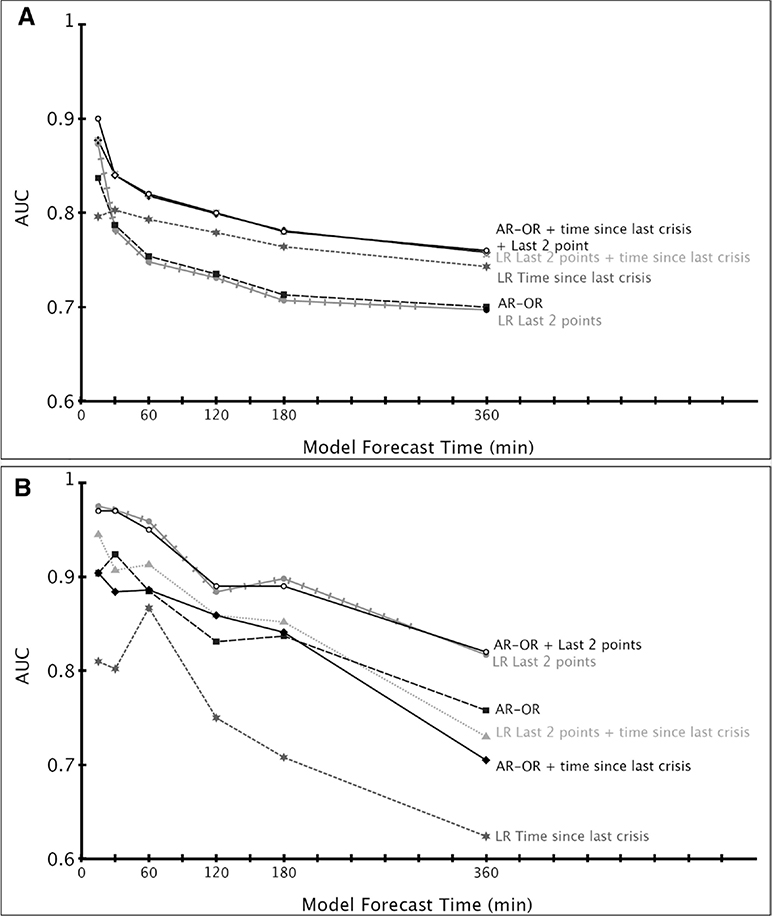
The physiologic signals found to be most associated with precursors to intracranial hypertension were ICP and the change in ICP (AUC = 0.85 at 30 min from event) (Fig. 2A). We found that CPP, ETco2, and MAP, as well as changes in these signals, had little predictive power when used in isolation (AUC = 0.49, 0.57, 0.58, respectively, at 30 min from event). The time since last crisis performed better than any individual physiologic signal at prediction times greater than 30 minutes. We found that the majority of the information associated with the precursors of ICP crisis was contained within the last two ICP values of the 30-minute epoch, no matter how far in advance this epoch is from the crisis event. The best model for predicting ICP crisis events 30 minutes in advance uses two states in the AR-OR model to obtain two fractions, along with the time since last crisis and the last two ICP values. Because this is a rare class problem, the F2-score was used to compare the model with a null hypothesis where all epochs are classified as no crisis (11–14). We obtain a maximal F2-score of 0.79 for the ICP predictive model and 0.37 using the null hypothesis.
Figure 2.
A, Performance of several classes of predictive models of Intracranial pressure (ICP) crises as a function of model forecast time, based on data from the model selection cohort. B, Performance of several classes of predictive models of partial brain tissue oxygenation (PbtO2) crises as a function of model forecast time, based on data from the model selection cohort. AR-OR = Autoregressive Ordinal-Regression, AUC = area under the receiver operating characteristic curve.
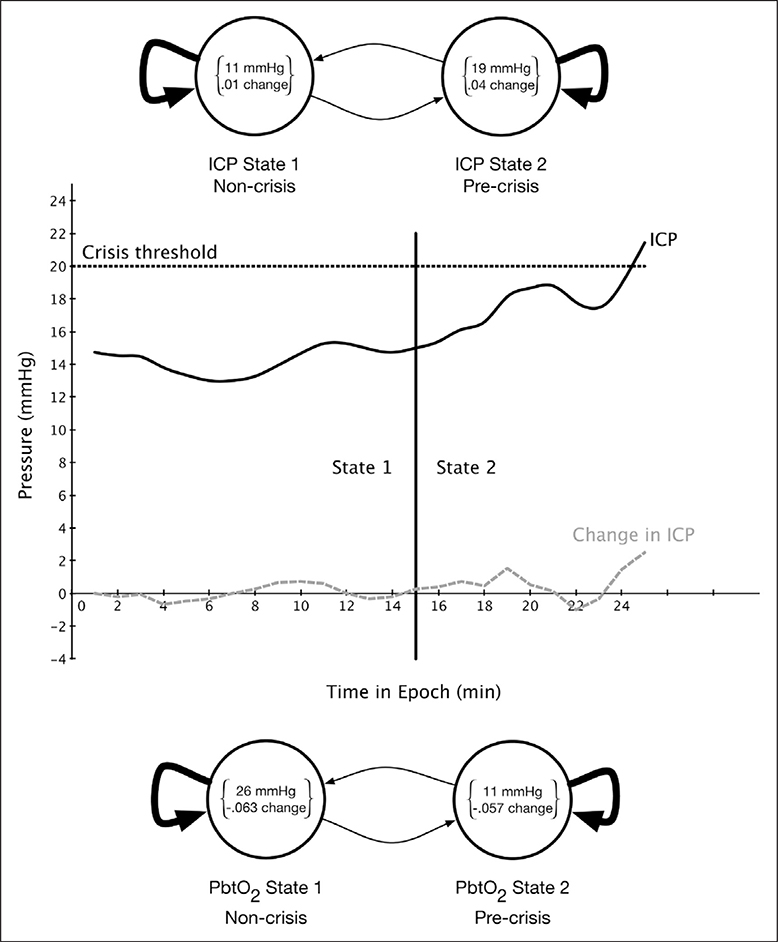
In Figure 3, we show the two-state hidden Markov model state diagram learned to predict ICP crises. The two states correspond to a “precrisis” state, with mean value of 19 mm Hg for the ICP and a small positive change in ICP and a “noncrisis” state with mean value of 11 mm Hg and little change in ICP. The amount of time spent in the precrisis state contributes to the likelihood that a patient will have an ICP crisis in 30 minutes, whereas time spent in the noncrisis state reduces this likelihood.
Figure 3.
State diagram of the hidden Markov model for predicting intracranial pressure (ICP) crisis (top), state labels for sample precrisis epoch (middle), and state diagram of the hidden Markov model for predicting partial brain tissue oxygen tension (PbtO2) crisis (bottom).
Prediction of Brain Tissue Oxygen Crises
The physiologic signals found to be most associated with precursors to PbtO2 crisis were PbtO2 and the change in PbtO2 (AUC = 0.91 at 30 min from event in the model selection cohort) (Fig. 2B). We found that arterial oxygen saturation and CPP, as well as changes in these signals, had little predictive power (AUC = 0.53 and 0.58). In the case of PbtO2 crises, the time since last crisis had the lowest performance compared to the individual physiologic signals. We obtained an F2-score of 0.43 using the predictive model for PbtO2 and 0.07 for the null hypothesis. For a given signal, we found that the majority of the information associated with the onset of PbtO2 crisis was contained in the last two points of the 30-minute epoch, no matter how far in advance this epoch is from the crisis event. The best model for predicting PbtO2 crisis events used the two-state AR-OR model, the time since last crisis and the last two PbtO2 values.
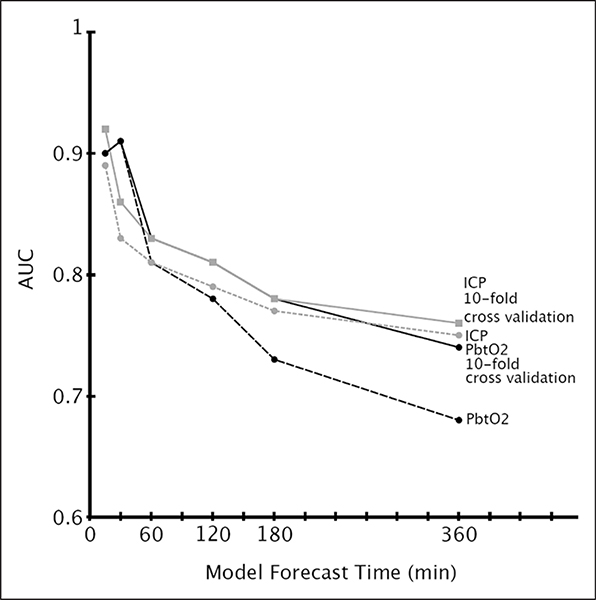
The prediction performance of both the ICP and PbtO2 algorithms as a function of forecasting time can be seen in Figure 4. Additional performance measures can be found in the supplemental material (Supplemental Digital Content 1, http://links.lww.com/CCM/B869).
Figure 4.
Best predictive model performance as a function of model forecast time, based on data from the validation cohort. AUC = area under the receiver operating characteristic curve, ICP = intracranial pressure, PbtO2 = partial brain tissue oxygen tension.
Patient Outcomes
We correlated the fraction of time patients spend in crises and their 1-, 3-, and 6-month GOS. Because the values of the fraction of time in crises are non-Gaussian, standard tests such as a two-sample t test are not applicable. A nonparametric bootstrap test (15) was used to compare the median cumulative time in crisis or fraction of time in crisis for patients with GOS of 1–3 against the median values for patients with GOS of 4–5 (Table S11, Supplemental Digital Content 1, http://links.lww.com/CCM/B869). Statistically significant correlations were found.
DISCUSSION
Our goal was to discover models that could be incorporated into bedside monitors in order to provide clinicians early warning of ICP and PbtO2 crises in TBI patients. Research related to forecasting ICP measurements has been ongoing over the past 30 years. In 1983, R. Allen discussed the importance of automated ICP data collection and suggested that short-term predictions may be more useful than long-term predictions due to changes in patient conditions. This observation supports the use of short epochs of time for making clinical predictions (16) and was the basis for choosing a 30-minute epoch for this study.
Several works have been published attempting to either predict the onset of ICP crisis events or to forecast future ICP values themselves with limited success (17–19). The closest work to this study is that of Güiza et al (8). They used a 4-hour epoch of minute-byminute measurements of ICP and MAP in conjunction with patient length of stay and other time series features, to predict ICP crises in the next 30 minutes. The authors were able to predict ICP crises (defined as ICP ≥ 30 mm Hg for at least 10 min) with an AUC of 0.87 (8). This work represents the best prediction to date.
However, despite the high AUC they achieved, Güiza et al (8) used a very large number of features, testing an initial set of 1,000 potential features and utilizing approximately 100. The large number of features increases the potential of overfitting. In contrast, the best approach we considered uses five features (time spent in each of two states, the last two points in the primary signal epoch, and the time since last crisis). Güiza et al (8) reported that the 10 most important predictors in their model include the most recently observed ICP value, which is consistent with our results. However, this set also includes the 68th and 143rd most recent observations. It is difficult to posit a clinical justification for why these particular observations are important, suggesting the possibility of overfitting. Indeed, we were unable to replicate the performance of GP-based predictive models of Güiza et al (8) on our own data sets.
In our study, we found that the most accurate predictive model for ICP crises was a five-feature logistic regression using the AR-OR output along with the last two measurements of ICP and the time since last crisis (AUC of 0.83 with direct validation; 0.86 using 10-fold cross validation).
For predicting PbtO2 crises, we found that the last two observed PbtO2 measurements alone are an extremely useful predictor of an upcoming crisis. Using the last two measurements along with the two AR-OR features achieved an AUC of 0.97 for predicting PbtO2 crisis events 30 minutes in the future on the model selection cohort, and achieved an AUC of 0.91 on the validation cohort (0.91 using 10-fold cross-validation). For predicting ICP and PbtO2 crises, we found that none of the other recorded physiologic signals increased the model’s predictive ability. This implies that much of the information necessary to predict the onset of crisis events is contained in the ICP or PbtO2 signals. These results are consistent with observations from Güiza et al (8).
Given that our models can forecast ICP and PbtO2 crises, it is natural to ask whether preventing such crises in TBI patients would be clinically beneficial. Although providing a definitive answer to this question is difficult, our results indicate that time spend in crisis state seems to be correlated with outcome (Table S11, Supplemental Digital Content 1, http://links.lww.com/CCM/B869). Although correlation does not imply causation, these results suggest that an outcome benefit may be achieved by reducing the cumulative amount of time that a patient spends in a crisis state.
Limitations
Although the data were prospectively collected, the analysis is retrospective. Also, because this is a treated cohort of subjects, it is likely that the clinical team detected subjects that were heading towards crisis states and intervened. However, it is unlikely that this occurred for every patient, and it is unlikely that physicians could consistently detect the onset of such crisis events 30 minutes beforehand. Because we do not have records of interventions performed, it was not possible to distinguish between a precrisis epoch that was successfully averted by intervention and a similar looking epoch where the patient recovered without intervention.
Although our definitions for ICP and PbtO2 crises may not be universally acceptable, they follow current BTF guidelines and are consistent with contemporary clinical intuition. More importantly, our methodology is not dependent on the particular crisis definition and can be applied to variable thresholds as deemed necessary.
CONCLUSION
We have presented a simple, robust, and validated model for predicting ICP and PbtO2 crises in patients with severe TBI within an adequate time window for clinical intervention. Using ICP measurements and the time from last crisis, our model can predict ICP crises from 15 minutes to 6 hours in the future with AUCs ranging from 0.76 to 0.92. Using measurements of PbtO2, our model can predict PbtO2 crises in the same time span with AUCs ranging from 0.74 to 0.91. Both models were validated using independent validation cohorts. Our approach uses a minimum set of features that are easily understood and requires only a 30-minute lead-in period. We found that adding additional physiologic signals to the prediction model did not improve results, implying that these signals either do not contain additional information or that changes in these signals are already incorporated in the main input features. Finally, we demonstrate that the 1-, 3-, and 6-month GOS scores are correlated with time spent in these crises states. This finding may indicate that the ability to predict the onset of crisis events would be valuable, as it would provide time for early intervention, reducing the time a patient spends in crisis, which may improve patient outcomes.
Supplementary Material
Acknowledgments
Supported, in part, by a training fellowship from the Keck Center of the Gulf Coast Consortia, on Rice University’s NLM Training Program in Biomedical Informatics (grant number T15LM007093) and by the NSF under grant number 0964526.
This work was performed at Baylor College of Medicine, Houston, TX.
Dr. Myers received support for article research from the National Institutes of Health (NIH) and the National Science Foundation. Her institution received funding from the NIH, National Library of Medicine, and from the National Science Foundation. She received supported, in part, by the Keck Center of the Gulf Coast Consortia, on Rice University’s NLM Training Program in Biomedical Informatics (grant T15LM007093) and by the NSF (grant 0964526). Dr. Jermaine received support for article research from the US National Science Foundation. His institution received funding from the US National Science Foundation. Dr. Rusin is a co-founder of Medical Informatics; however, MIC has no interests in this work. All other authors report no conflicts of interest. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. : Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007; 24(Suppl 1):S55–58 [DOI] [PubMed] [Google Scholar]
- 2.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. : Guidelines for the management of severe traumatic brain injury. X. Brain Oxygen Monitoring and Thresholds. J Neurotrauma. 2007; 24(Suppl 1):S65–70 [DOI] [PubMed] [Google Scholar]
- 3.Le Roux P, Menon DK, Citerio G, et al. : The International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A list of recommendations and additional conclusions: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Neurocrit Care 2014; 21(Suppl 2):S282–S296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stocchetti N, Picetti E, Berardino M, et al. : Clinical applications of intracranial pressure monitoring in traumatic brain injury: Report of the Milan consensus conference. Acta Neurochir (Wien) 2014; 156:1615–1622 [DOI] [PubMed] [Google Scholar]
- 5.Buchman TG: The digital patient: Predicting physiologic dynamics with mathematical models. Crit Care Med 2009; 37:1167–1168 [DOI] [PubMed] [Google Scholar]
- 6.Hemphill JC, Andrews P, De Georgia M: Multimodal monitoring and neurocritical care bioinformatics. Nat Rev Neurol 2011; 7:451–460 [DOI] [PubMed] [Google Scholar]
- 7.Jennett B, Bond M: Assessment of outcome after severe brain damage. Lancet 1975; 1:480–484 [DOI] [PubMed] [Google Scholar]
- 8.Güiza F, Depreitere B, Piper I, et al. : Novel methods to predict increased intracranial pressure during intensive care and long-term neurologic outcome after traumatic brain injury: Development and validation in a multicenter dataset. Crit Care Med 2013; 41:554–564 [DOI] [PubMed] [Google Scholar]
- 9.Weiss GM: Mining with rarity: A unifying framework. New York, ACM; 2004; 6(1):7–19. [Google Scholar]
- 10.Myers RB, Frenzel JC, Ruiz JR, Jermaine CM: Correlating Surgical Vital Sign Quality with 30-Day Outcomes using Regression on Time Series Segment Features, Society of Industrial and Applied Mathematics (SIAM) International Conference on Data Mining, Vancouver, BC, Canada, 2015 [Google Scholar]
- 11.van Rijsbergen CJ: Information Retrieval. Second Edition. London, Butterworths, 1979 [Google Scholar]
- 12.Lewis DD: Evaluating and optimizing autonomous text classification systems. Proceedings of the 18th Annual International ACM SIGIR Conference on Research and Development in Information Retrieval ACM, 1995 [Google Scholar]
- 13.Mladenic D, Grobelnik M: Feature selection for unbalanced class distribution and naive Bayes. ICML ‘99 Proceedings of the Sixteenth International Conference on Machine Learning, Morgan Kaufmann Publishers, San Francisco, CA, 1999 [Google Scholar]
- 14.Köknar-Tezel S, Latecki LJ: Improving SVM Classification on Imbalanced Data Sets in Distance Spaces. Data Mining, 2009. ICDM’09. Ninth IEEE International Conference on IEEE, 2009, pp 59–267 [Google Scholar]
- 15.Efron B, Tibshirani RJ: An Introduction to the Bootstrap. Boca Raton, FL, CRC Press, 1994 [Google Scholar]
- 16.Allen R. Time series methods in the monitoring of intracranial pressure. Part 1: Problems, suggestions for a monitoring scheme and review of appropriate techniques. J Biomed Eng. 1983;5(1):5–18. [DOI] [PubMed] [Google Scholar]
- 17.Swiercz M, Mariak Z, Krejza J, et al. : Intracranial pressure processing with artificial neural networks: Prediction of ICP trends. Acta Neurochir (Wien) 2000; 142:401–406 [DOI] [PubMed] [Google Scholar]
- 18.Narotam PK, Morrison JF, Schmidt MD, et al. : Physiological complexity of acute traumatic brain injury in patients treated with a brain oxygen protocol: Utility of symbolic regression in predictive modeling of a dynamical system. J Neurotrauma 2014; 31:630–641 [DOI] [PubMed] [Google Scholar]
- 19.Wakeland W, Agbeko R, Vinecore K, et al. : Assessing the prediction potential of an in silico computer model of intracranial pressure dynamics. Crit Care Med 2009; 37:1079–1089 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.