Abstract
BACKGROUD
Spinal cord injury (SCI) is damage that can cause a temporary or permanent change in spinal cord functions.
AIM:
This work evaluates clinical signs, mental signs, and quality of life (QoL) after autologous adipose-derived stem cells (ADSCs) transplantation to treat acute spinal cord injury (SCI).
METHODS:
In this study, 47 SCI patients were recruited and divided into two groups: intervention and control. ADSCs were isolated and cultured under the cell culture quality control procedure. All patients in both groups underwent neurosurgery with or without ADSC transplantation. The recovery regarding neurological muscle, QoL, neurogenic bladder, and mental improvement was assessed after transplantation.
RESULTS:
All patients had improved in terms of motor function, bladder function, and daily living. No patients reported any side effect. MRI imaging showed significant changes in the lesion length of the spinal canal and the thickening of the spinal cord. Mental improvement was highest at six months after transplantation and lowest at one month after transplantation. The proportion of patients whose quality of life improved after treatment was 100%, while 80% of patients were satisfied with treatment outcomes.
CONCLUSIONS
Thus, our data suggested that ADSCs transplantation was safe and effective for the treatment of SCI patients. Neurological muscle and neurogenic bladder were improved significantly after transplantation.
Keywords: Spinal cord injury, Adipose-derived stem cells, Safety, Feasibility, Quality of life
Introduction
Spinal cord injury (SCI) is damage that can cause a temporary or permanent change in spinal cord functions. SCI causes a variety of severe issues such as loss of bowel faculties, sensory and motor function impairment, and intractable pain. It can be categorized as primary and secondary injuries — both share a common mechanism that leads to the death of glial and neuronal cells, demyelination, axonal degeneration, and consequently manifests as severe impairment in neurological functions [1]. SCI can be treated by several methods such as surgical decompression and fixation, and physical rehabilitation; however, these treatment methods still have some limitations [2].
Stem cell therapy has emerged and been applied successfully in the treatment of many diseases. It has also shown potential as a treatment method for disorders in the refractory nervous system, including SCI. In the most recent cell therapy clinical trials for SCI, mesenchymal stem cells (MSCs) were specifically studied for their therapeutic potential in various treatment modalities such as modulation at the injury site, cell rescue, promotion of directed progenitor precursor cell migration, differentiation and re-myelination, angiogenesis, and neovascularization [3]. There are a number of MSC types applied in SCI treatment in which the stem cells are isolated from bone marrow (bone marrow stem cells – BMSCs), umbilical cord blood (umbilical cord blood cells – UCBCs), adipose tissues (adipose-derived stem cells – ADSCs) and placental MPCs [4].
It is also well-known that subcutaneous adipose depots are ubiquitous, thereby, providing a higher number of MSCs compared to bone marrow [5]. Adipose tissue contains multipotent cells that are suitable to develop stem cell therapies. It is evident that the number of stem and progenitor cells in stromal vascular fraction (SVF) from fat tissue can be 3%, which is 2500-fold higher compared to the population isolated from bone marrow [6].
Additionally, ADSCs are easily isolated, cultured, and widely used in basic and clinical research, and the stem cell extraction technique for ADSCs (e.g., liposuction aspiration) is less invasive compared to bone marrow aspiration. Liposuction surgery is safer, less invasive, and cheaper in stem cell isolation [7], [8]. Stem cell therapy based on ADSCs can take advantage of lipoaspirate, which is considered as medical waste, as a good source for stem cells.
The application of ADSCs therapy has been explored in various treatments for conditions such as myocardial infarction, heart failure, diabetes, and obesity [9], [10], [11], [12], [13], [14], [15]. A recent systematic review and meta-analysis indicated that treatment for SCI using stem cell transplantation is efficient and safe, with improved sensory and bladder functions [16]. Besides, hADSCs could be converted into electrophysiologically active motoneuron-like cells (hADSC-MNs). After transplantation, they survived, migrated, and integrated into the injured site and this led to partial functional recovery in SCI mice. These results suggest the use of ADSC for cell replacement therapy for spinal cord injury [17]. The safety and feasibility of autologous ADSCs in SCI treatment on humans have not been fully explored. Thus, this study evaluates the safety and feasibility of autologous ADSCs transplantation for treating acute SCI, in terms of clinical signs, mental signs, and quality of life.
Materials and Methods
Participants
Patients were eligible if they A) were aged from 18 to 60 years and B) had an acute complete SCI (e.g., ASIA-A, between T1 and T12 of traumatic etiology within 2 weeks). They were excluded if they A) were under mechanical ventilation; B) had diseases other than SCI (e.g., melanoma within 5 years); C) had an infectious diseases (i.e., HIV); body temperature higher than 38°C; D) had a predisposing disease (e.g., thrombocytopenia); E) received cytotoxic drug treatment; F) were participating in a clinical trial within 3 months; G) had current psychiatric illness; H) had traumatic brain injury related to SCI, or I) did not provide fully informed consent.
Based on the inclusion and exclusion criteria, 100 participants were screened. Then, the participants were randomly allocated into treatment and control group with the ratio 2: 1, respectively. Of the patients screened, 48 met the inclusion criteria and were allocated a group, with 32 in the treated group and 16 in the control group, which consisted of 18 males and 2 females aged 18 – 59 years old. One patient in the treatment group withdrew from the study for personal reasons. Finally, 47 patients were included in the analysis, with 31 in the treated group and 16 in the control group (Figure 1) (Table 1 and Table 2).
Figure 1.
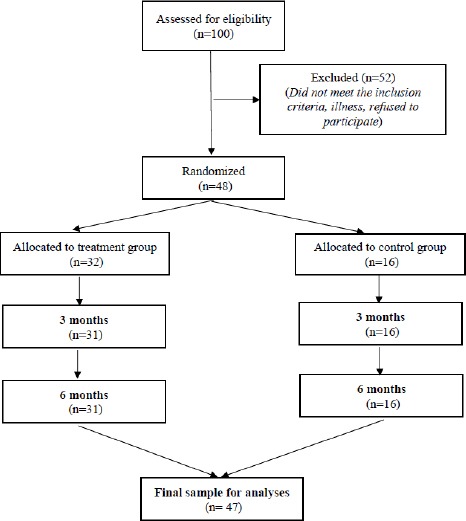
Flow chart of patients from recruitment to the end of 6-month randomized control trial; There were 100 patients assessed for eligibility if they A) were aged from 18 to 60 years and B) had an acute complete SCI; They were excluded, if they A) were under mechanical ventilation; B) had diseases other than SCI (e.g., melanoma within 5 years); C) had an infectious disease (i.e., HIV); body temperature higher than 38°C; D) had a predisposing disease (e.g., thrombocytopenia); E) received cytotoxic drug treatment; F) were participating in a clinical trial within 3 months; G) had current psychiatric illness; H) had a traumatic brain injury related to SCI, or I) who did not provide fully informed consent; Screening based on inclusion and exclusion criteria resulted in 100 participants. Then, the participants were randomly allocated into treatment and control group with the ratio 2: 1, respectively. Of the patients screened, 48 met the inclusion criteria and were allocated groups, with 32 in the treated group and 16 in the control group, which consisted of 18 males and 2 females aged 18-59 years old. One patient in the treatment group withdrew from the study for personal reasons. Finally, 47 patients were included in the analysis, with 31 in the treated group and 16 in the control group
Table 1.
Summary results
| Patients number (n) | |||||||
|---|---|---|---|---|---|---|---|
| Patients | Motor change | EMG/SSEP change | MRI change | Bladder function change | Improve in daily living | Side effect | Time observation |
| N = 31 | 2 | 3 | 31 | 3 | 31 | 0 | 3 months |
| N = 31 | 5 | 3 | 31 | 9 | 31 | 0 | 6 months |
| N = 31 | 5 | 3 | 31 | 11 | 31 | 0 | 12 months |
Table 2.
Change in ASIA between treatment and control group
| AIS | 12 months after injection | Total | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Control group | A | 15 | 1 | 0 | 0 | 0 | 16 |
| Treatment group | A | 16 | 10 | 1 | 2 | 0 | 31 |
| Total | 31 | 11 | 1 | 2 | 0 | 47 | |
AIS: American Spinal Injury Association Impairment Scale.
The ratio of patients in treatment/control group was 2: 1; patients in the treatment group received surgical decompression and spinal fix using the classic method and then they were transplanted with the stem cells, while the other group received only surgical decompression and spinal fix using the classic method. The assumption was that the real recovery rate was applied to AIS A after 7 days: 3% in control candidates (based on the data above) after one year for AIS C, and a 23% recovery effect for actually-treated candidates. At baseline, there are no differences between intervention and control group regarding basic characteristics such as mean age, proportion of male, and level of injury as well as AIS-A (Table 1).
The sample size was based on several factors such as important clinical differences and setting the sensory/motor score on the AIS scale. In addition, clinical experts in the field were asked for clinical outcomes that were needed to determine significant improvement in both AIS and success rates in-patient rehabilitation. In this study, the treatment was clinically significant if 20% or more cases appeared to improve AIS scores from A to B (Table 2).
Adipose-Derived Stem Cells Culture
Participants in the transplant group underwent stomach anesthesia for liposuction. Fat tissues was injected with 100 mL 0.9% sodium chloride mixed with 5 mL 1% lidocaine, 1 ml adrenaline (1mg/mL), and then 80 mL fat tissue was aspirated into the syringe. The fat syringes were placed immediately into a sterile cold container. The collected fat tissues were transported from the operation room (Viet Duc Hospital) to the isolation facility (Tri Phuoc Company).
Fat tissues were totally manipulated in sterile lamina to isolate ADSCs. The tissues were washed briefly in PBS plus antibiotics, digested by DMEM containing collagenase type I at 37°C in a water-bath shaker for 40 – 60 minutes, followed by centrifugation to remove undigested fat tissues. After centrifugation, the supernatant was then discarded, the stromal vascular fraction (SVF) was resuspended in a stromal medium. ADSCs were counted under a microscope, identified by surface marker expression of MSCs, and tested for sterility. Then, half of the SVF were diluted in 8 mL sodium chloride 0.9% and transferred to a syringe for the first injection. The cell-storing syringe was placed in a sterile cold container and was transferred back to Viet Duc Hospital.
The rest of the cells were seeded in a flask and incubated at 37°C with 5% CO2, the medium was changed every 48 hours [18]. Those cells were passaged when they were 80% confluent. After the second passage, ADSCs were divided into 3 parts, each corresponding to an injection. Each part was mixed with freezing protective agents at 80°C for 24 hours and stored in liquid nitrogen. Ten days prior to transplantation, the cells were quickly thawed at 37°C and sub-cultured for 1 – 2 next passage times. On transplantation day, the cells were harvested using trypsinization, then transferred into a sterile syringe at a concentration of 30 x 106 cells/8 mL 0.9% NaCl for the 2nd and 3rd injection, and a concentration of 100 x 106 cells/10 mL 0.9% NaCl for the 4th injection, and delivered to the operating hospital.
Cell Quality Control
Immunophenotyping characterization of the cell population was done by flow cytometric analysis. The freshly-isolated SVF and ADSCs were immunophenotyped before any transplantation. To identify the surface markers of MSCs, such as CD73, CD105, CD90, CD166, 1 x 106 cells dissolved in 1 mL PBS were used. The morphology of the cells was observed using a microscope. All materials and media in the procedure adhered to WHO-GMP, ISO 9001-2008, and ISO 15189 standards to ensure sterility.
First Transplantation
In the same day, the participants in each group underwent surgery in which the spinal cord received fix and decompression, liposuction, and first transplantation.
We adapted the procedure of transplantation by Féron et al., (2005) using adipose tissue instead of olfactory ensheathing cells [19]. All patients received general endotracheal anesthesia before starting transplantation. The incision line was infiltrated with a mixture of adrenaline: lidocaine with rate 1: 100000. Before performing the surgery, an x-ray was done to locate the lesion site and stem cell infusion site. Five main surgical steps were performed to deliver the stem cells to the injury site: 1) Midline incision at the vertebral column rostral and caudal to the site of the injury; 2) Dura bared at the site of injury through rostral and caudal multiple level laminectomies. Extreme caution should be taken so facet joints would not interfere; 3) Midline durotomy away from the injury site. To complete the opening of the durotomy, normal dura and sharp dissection was done after removing adhesions through the injury; 4) Dorsal adhesiolysis under the magnification of an operating microscope through sharp and blunt dissections in the injury site. This was done to restore the structure of the cord to as normal as possible; 5) Two mL SVF solutions were delivered to the subarachnoid space of the injury at above, center, and below of injury site. The remaining cell solution was injected into subarachnoid space before closing duration [16], [17], [18], [19].
Second and third ADSCs Transplantation
Second and third ADSCs transplantation took place on day 30, and day 45 respectively. At which point, 30 x 106 cells/8 mL 0.9% NaCl was delivered into the lumbar spine.
Fourth ADSCs Transplantation
Fourth ADSCs transplantation took place on day 75, when 100 x 106 cells/8 mL 0.9% NaCl was intravenously injected into the lumbar spine.
Recovery measurements
The patients were observed and assessed for neurological muscle recovery at 3 months and 6 months after the first injection through (1) somatosensory evoked potentials (SSEPs); (2) cord recovery at injury site using MRI; and (3) neurogenic bladder improvement using Exploration Urinary Kinetics (EUK). Mental improvements were also evaluated using (1) the Short Form 36 items (SF-36) index; (2) Oswestry; and (3) Barthel Index of Activities of Daily Living (BIADL). The quality of life (QoL) was evaluated by short-form 36 items (SF-36), and patient satisfaction with treatment results was measured.
Statistical analysis
SPSS (SPSS Inc., Chicago, III, v.20 for Windows) was used data analysis, and statistical analysis was done by t-Test. P-value of < 0.05 was employed to identify statistical significance unless otherwise indicated.
Results
Adipose-Derived Stem Cell Culture
The cultured cell presented characteristics of ADSC/MSC, which followed the criteria of the IFATS/ISCT [20]. The percentages of MSCs ranged from 86–99% (Figure 2). No cell was positive for CD14 (a marker for hematopoietic stem cells) (Figure 3). For fresh SVF, the percentages of CD73, CD90, CD105, and CD166-positive ranged from 61 to 68%; CD14-positive cells were at 8% of the total cell population.
Figure 2.
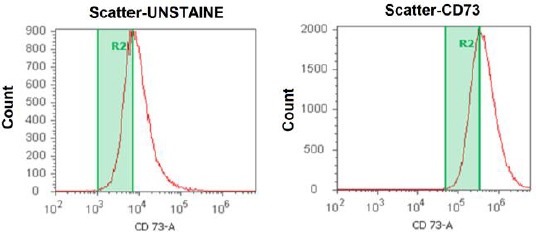
CD73 marker – MSC culture in Phase 2 with positive percent (97.5%). Adipose-derived stem cell cultures predominantly contain MSCs identified by immunostaining for CD73. The percentages of CD 73+ cell ranged 97.5%, which contained the majority of adipose-derived stem cells
Figure 3.
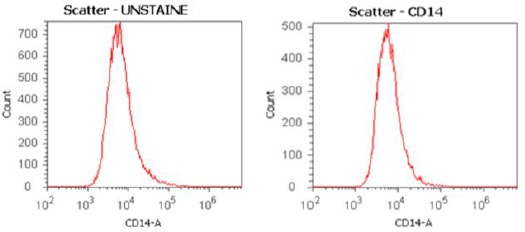
CD14 marker – MSC culture in Phase 2 with positive percent (0%). The adipose-derived stem cell cultures were checked with CD14 marker, a marker for hematopoietic stem cells. The result showed no cell was positive for CD14
Electrophysiological parameters
Changes in motor function were recorded based on complete paraplegia or quadriplegia. In the study, three out of five patients recovered motor functions (Table 1); they could stand and walk on their own legs without support or with a little assistance. Three patients had SSEP changes with the cortical response on tibia nerves and positive P37. Changes in electromyography (EMG) were recorded in three patients showing some motor units on the muscle electromechanical table (Figure 4).
Figure 4.
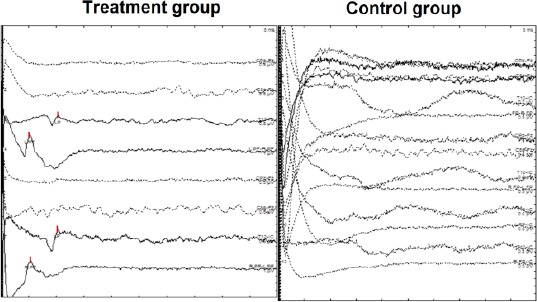
Changes in Electromyography (EMG) in the treatment versus the control group. The electromyography (EMG) was recorded in the treated group and compared to the untreated group. The results showed some motor units on the muscle electromechanical table
Radiology
Changes in the lesion length of the spinal canal, thickening of the spinal cord, and MCC and MSCC were statistically significant (p < 0.05) at time points of 3 months and 6 months compared to before treatment.
These changes were recorded using MRI (Table 3) (Figure 5).
Table 3.
MRI results of 31 patients, 3 months after the first injection
| L (mm) | R (mm) | MCC (%) | MSCC (%) | |
|---|---|---|---|---|
| Before treatment (n = 31) | 60.97 ± 3.934 | 6.18 ± 0.486 | 32.73 ± 2.950 | 36.95 ± 3.193 |
| 3 months after first treatment (n=31) | 48.548 ± 3.998 | 7.76 ± 0.320 | 20.38 ± 2.165 | 19. 23 ± 1.934 |
| P-value | P = 0.0179 | P = 0.0122 | P = 0.0013 | P < 0.01 |
L: lesion length, R: thickening of the cord, MCC: maximum spinal canal compromise, MSCC: maximum spinal cord compression, (p values were determined by t-test).
Figure 5.
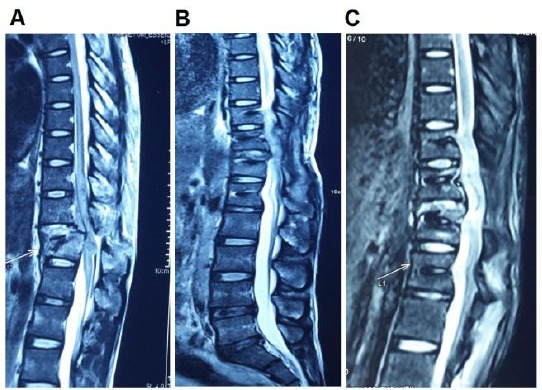
Comparison of a lesion in MRI; A) Before treatment; B) 3 months after the first treatment; and C) 6 months after the first treatment; The MRI image of the lesion region was taken before the treatment A); 3 months after the first treatment B); and 6 months after the first treatment C); B) and C) present the recovery of the injured spinal cord
Neurogenic bladder measurement
There were changes in neurogenic bladder recorded based on significant changes in the involuntary bladder contractions, maximum bladder pressure (Pdetmax), and maximum bladder volume (VH2Omax) (Table 4).
Table 4.
MRI results of 31 patients, 6 months after the first injection
| L (mm) | R (mm) | MCC (%) | MSCC (%) | |
|---|---|---|---|---|
| Before treatment (n = 31) | 51.34 ± 5.052 | 5.75 ± 0.559 | 27.135 ± 3.968 | 29.43 ± 3.674 |
| 6 months after first treatment (n = 31) | 36.94 ± 4.083 | 8.59 ± 0.448 | 13.59 ± 1.67 | 11.14 ± 1.599 |
| P-value | P = 0.0343 | P = 0.0004 | P = 0.0037 | P = 0.0001 |
L: lesion length; R: thickening of the cord; MCC: maximum spinal canal compromise; MSCC: maximum spinal cord compression; p values were determined by t-test.
However, this result does not reflect the effectiveness of treatment because it depends on the care of the bladder and rehabilitation after treatment (Table 5).
Table 5.
Result for changes in involuntary bladder contractions
| Months after the first treatment | n | Mean ± Stand | P |
|---|---|---|---|
| 3 | 18 | 2.5 ± 0.246 | P < 0.01 |
| 6 | 10 | 0.4 ± 0.221 |
p values were determined by t-test.
Mental improvement
SF-36 results showed that the general QoL of the patients improved from poor to good, while the back-pain rate, which affects patients’ daily activities, measured through the Oswestry questionnaire, changed from serious to mild. Barthel ADL increased to 3 points at 6 months after the first treatment (Table 6). No side effects were reported during 6 months after treatment.
Table 6.
Mental improvement of patients in different time points
| Index | Before treatment (n = 31) | 1 month after first treatment (n = 31) | 3 months after first treatment (n = 31) | 6 months after first treatment (n = 31) |
|---|---|---|---|---|
| SF-36 score | 23.50 ± 7.89 | 31.60 ± 11.18 | 41.25 ± 13.35 | 53.16 ± 14.47 |
| General quality of life | Bad | Bad | Average | Excellent |
| Oswestry | 79.48 ± 7.33 | 73.38 ± 8.07 | 65.41 ± 7.55 | 58.22 ± 7.66 |
| Back pain level | Serious | Serious | Mild | Mild |
| Barthel ADL | 3.35 ± 1.35 | 4.41 ± 1.78 | 5.51 ± 2.06 | 6.48 ± 2.14 |
| P-value | P < 0.05 | P > 0.05 | ||
p values were determined by t-test.
Quality of life
All patients rated their quality of life improved after treatment. Moreover, 80% of patients reported that they were satisfied and happy with the results of the treatment.
Discussion
SCI patients have shown significant improvement after autologous ADSCs therapy. Of the patients, 48.1% exhibited improved functions based on the AIS scale (A-B) after 6 months. Further follow-up with patients for the next 9 months showed 7.4% of patients at the B-C level. Clinical evaluation according to the AIS scale reflected the extent of anatomical lesions. In MRI imaging, hematoma and compression support clinicians regarding prognosis, recovery, and post-transplant evaluation through MCCs and MSCCs. We found that there were statistically significant differences between the study groups. Although MRI has limited discrepancies between white matter and gray matter, the use of MCCs and MSCCs combination with the AIS scale is a good way to predict marrow recovery.
Further, the result of our study was congruent with other published data. Cases in point, a study by Park et al., (2005) reported that the application of MSCs in 18-month spine trauma patients resulted in improvement of 6/20 (20%) patients with A – B changes [21]. Another US multi-center study of 1816 patients with 50 randomly selected stem cell transplant recipients scored 3/13 patients who improved on the AIS (AC) scale [16]. A study by Yoon et al., (2007) demonstrated that after one year of follow-up, 29.5% of patients who received stem cell transplant had neurological recovery (A – B) [22]. Ramesh Kumar et al., (2011), in a study of 24 patients, reported a 30% increase in the MCC and MSCC scores to assess changes in the structure of the spinal cord [17]. This was similar to our finding in this study, in which the index was 33.5%.
We used both direct and indirect injection at different times, and cells were cultured to obtain enough numbers per time point. The clinical efficacy of the transplant was evaluated. The combination of stem cells transplanted into the lumbar at L2 and the intravenous infusion was also a highlight in this study. Elevated efficacy through cerebrovascular drainage and acute myeloid lesions provide anti-inflammatory, directed, and attractive cytokines by the homing mechanism to the lesion site. In this study, we have not applied grafting techniques and tissue fixators.
There are some limitations to this study. The sample size was limited and we only conducted the study in one hospital. The follow-up duration was 6 months. Additionally, preoperative and postoperative imaging methods such as MEP and MRI were insufficient. Regarding cell quality, the survival rate and the number of cells differentiated were not investigated.
The study is still on-going in several centers that have a follow-up period of five years. We will conduct a further subgroup study in chronic patients or in patients’ ages possibly with many different cell sources such as bone marrow stem cells, induced pluripotent stem cells, and neural stem cells. Grafting can be used by utilizing robots to calculate specific doses and tissue fixation agents in combination with neuronal differentiation-oriented proteins.
In conclusion, all treated patients with motor and sensory deficits have shown improvement. Neurological muscle recovery and neurogenic bladder contraction improved significantly. The recovery rate was not high, although most of the treated patients had an improved quality of life and reported high satisfaction with the results of the treatment.
Acknowledgments
We would like to thank our American colleagues and Ms. Bui Nhat Le (Faculty of Clinical Pharmacy, University of Pharmacy, Hanoi, Vietnam) for checking and improving the English in the manuscript. Especially, the revised mauscript has undergone English language editing by MDPI. The text has been checked for correct use of grammar and common technical terms, and edited to a level suitable for reporting research in a scholarly journal.
Footnotes
Funding: This study was sponsored by Tri Phuoc Biotechnology Joint Stock Company
Competing Interests: The authors have declared that no competing interests exist
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research was approved by an ethical committee of the Vietnam Ministry of Health following the decision No 1456/QDBYT.
Informed Consent
Informed consent was obtained from patients included in the study before the procedure was done.
References
- 1.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury:An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Frontiers in Neurology. 2019;10(282) doi: 10.3389/fneur.2019.00282. https://doi.org/10.3389/fneur.2019.00282 PMid:3096783 PMCid:PMC6439316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cristante AF, et al. Therapeutic approaches for spinal cord injury. Clinics. 2012;67(10):1219–1224. doi: 10.6061/clinics/2012(10)16. https://doi.org/10.6061/clinics/2012(10)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qu J, Zhang H. Roles of Mesenchymal Stem Cells in Spinal Cord Injury. Stem Cells Int. 2017;2017:5251313. doi: 10.1155/2017/5251313. https://doi.org/10.1155/2017/5251313 PMid:2↶630 PMCid:PMC5467343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries:A review. World J Stem Cells. 2014;6(2):120–33. doi: 10.4252/wjsc.v6.i2.120. https://doi.org/10.4252/wjsc.v6ai2.120 PMid:24772239 PMCid:PMC3999770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells:tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693. doi: 10.1155/2012/812693. https://doi.org/10.1155/2012/81269 PMid:22577397 PMCid:PMC3345279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baer PC. Adipose-derived mesenchymal stromal/stem cells:An update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6(3):256–65. doi: 10.4252/wjsc.v6.i3.256. https://doi.org/10.4252/wjsc.v6ai3.256 PMid:25126376 PMCid:PMC4131268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider S, et al. Adipose-derived mesenchymal stem cells from liposuction and resected fat are feasible sources for regenerative medicine. Eur J Med Res. 2017;22(1):17. doi: 10.1186/s40001-017-0258-9. https://doi.org/10.1186/s40001-017-0258-9 PMid:28526089 PMCid:PMC5438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu M, et al. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;79:e50585. doi: 10.3791/50585. https://doi.org/10.3791/50585 PMCid:PMC3935774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panfilov IA, et al. Clinical study using adipose-derived mesenchymal-like stem cells in acute myocardial infarction and heart failure. Methods Mol Biol. 2013;1036:207–12. doi: 10.1007/978-1-62703-511-8_16. https://doi.org/10.1007/978-1-62703-511-8_16 PMid:23807797. [DOI] [PubMed] [Google Scholar]
- 10.Lindroos B, Suuronen R, Miettinen S. The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 2011;7(2):269–91. doi: 10.1007/s12015-010-9193-7. https://doi.org/10.1007/s12015-010-9193-7 PMid:20853072. [DOI] [PubMed] [Google Scholar]
- 11.Chu DT, Tao Y. Human thermogenic adipocytes:a reflection on types of adipocyte, developmental origin, and potential application. Journal of Physiology and Biochemistry. 2017;73(1):1–4. doi: 10.1007/s13105-016-0536-y. https://doi.org/10.1007/s13105-016-0536-y PMid:27826900. [DOI] [PubMed] [Google Scholar]
- 12.Chu DT, Tao Y. A homologous stem cell therapy for obesity and its related metabolic disorders. Medical Hypotheses. 2017;103:26–28. doi: 10.1016/j.mehy.2017.03.034. https://doi.org/10.1016/j.mehy.2017.03.034 PMid:28571802. [DOI] [PubMed] [Google Scholar]
- 13.Chu DT, et al. Cell source, differentiation, functional stimulation, and potential application of human thermogenic adipocytes in vitro. Journal of Physiology and Biochemistry. 2016;73(3):315–321. doi: 10.1007/s13105-017-0567-z. https://doi.org/10.1007/s13105-017-0567-z PMid:2↤196. [DOI] [PubMed] [Google Scholar]
- 14.Rogne M, Chu DT, Küntziger TM, Mylonakou MN, Collas P, Tasken K. OPA1-anchored PKA phosphorylates perilipin 1 on S522 and S497 in adipocytes differentiated from human adipose stem cells. Molecular biology of the cell. 2018;29(12):1487–501. doi: 10.1091/mbc.E17-09-0538. https://doi.org/10.1091/mbc.E17-09-0538 PMid:29688805 PMCid:PMC6014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu DT, et al. Adipose Tissue Stem Cells for Therapy:An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. Journal of Clinical Medicine. 2019;8(7):917. doi: 10.3390/jcm8070917. https://doi.org/10.3390/jcm8070917 PMid:31247996 PMCid:PMC6678927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, et al. Stem cell transplantation for spinal cord injury:a meta-analysis of treatment effectiveness and safety. Neural Regen Res. 2017;12(5):815–825. doi: 10.4103/1673-5374.206653. https://doi.org/10.4103/1673-5374.206653 PMid:28616040 PMCid:PMC5461621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S, et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death &Disease. 2019;10(8):597. doi: 10.1038/s41419-019-1772-1. https://doi.org/10.1038/s41419-019-1772-1 PMid:31395857 PMCid:PMC66≅1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bunnell BA, et al. Adipose-derived stem cells:isolation, expansion and differentiation. Methods. 2008;45(2):115–20. doi: 10.1016/j.ymeth.2008.03.006. https://doi.org/10.1016/j.ymeth.2008.03.006 PMid:1↑609 PMCid:PMC3668445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feron F, et al. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128(Pt 12):2951–60. doi: 10.1093/brain/awh657. https://doi.org/10.1093/brain/awh657 PMid:16219671. [DOI] [PubMed] [Google Scholar]
- 20.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells:a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–8. doi: 10.1016/j.jcyt.2013.02.006. https://doi.org/10.1016/j.jcyt.2013.02.006 PMid:23570660 PMCid:PMC3979435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park HC, et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11(5-6):913–22. doi: 10.1089/ten.2005.11.913. https://doi.org/10.1089/ten.2005.11.913 PMid:15998231. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor:Phase I/II clinical trial. Stem Cells. 2007;25(8):2066–73. doi: 10.1634/stemcells.2006-0807. https://doi.org/10.1634/stemcells.2006-0807 PMid:17464087. [DOI] [PubMed] [Google Scholar]


