Abstract
Amyotrophic lateral sclerosis (ALS) is a complex multi-system neurodegenerative disorder with currently limited diagnostic and no therapeutic options. Despite the intense efforts no clinically applicable biomarkers for ALS are yet established. Most current research is thus focused, in particular, in identifying potential non-invasive circulating biomarkers for more rapid and accurate diagnosis and monitoring of the disease. In this review, we have focused on messenger RNA (mRNA), non-coding RNAs (lncRNAs), micro RNAs (miRNAs) and circular RNA (circRNAs) as potential biomarkers for ALS in peripheral blood serum, plasma and cells. The most promising miRNAs include miR-206, miR-133b, miR-27a, mi-338-3p, miR-183, miR-451, let-7 and miR-125b. To test clinical potential of this miRNA panel, a useful approach may be to perform such analysis on larger multi-center scale using similar experimental design. However, other types of RNAs (lncRNAs, circRNAs and mRNAs) that, together with miRNAs, represent RNA networks, have not been yet extensively studied in blood samples of patients with ALS. Additional research has to be done in order to find robust circulating biomarkers and therapeutic targets that will distinguish key RNA interactions in specific ALS-types to facilitate diagnosis, predict progression and design therapy.
Keywords: amyotrophic lateral sclerosis, ALS, circulating biomarkers, circulating RNAs, blood, miRNA, lncRNA, circRNA, mRNA, ceRNET
1. Introduction
Amyotrophic lateral sclerosis (ALS) belongs to a group of complex multi-factorial neurodegenerative diseases. It is characterized by the selective loss of upper and lower motor neurons [1]. This neuronal degeneration leads to progressive skeletal muscle atrophy and death by respiration failure after 2–5 years from the onset of symptoms. The disease, presenting in middle age, has an incidence of 2 per 100,000 persons per year. The familial ALS forms represent only around 5% of cases, while a majority of cases are sporadic forms, which are mostly phenotypically indistinguishable from familial forms suggesting the existence of common pathways at the basis of neuronal death [2]. Mutations in ALS-causing genes are associated with the disease in approximately 70% of familial forms of ALS (FALS) and in 15% of sporadic forms of ALS (SALS) [3]. Several pathological mechanisms have been demonstrated to induce neuronal death in ALS, from oxidative stress and mitochondrial impairment to glutamate excitotoxicity, growth factor deficiency, neuro-inflammation, defective axonal transport [4], RNA metabolism [5,6,7,8,9] and impaired brain energy metabolism [10]. Since the current diagnosis is based on clinical examination and electrophysiological measurements, the establishment of diagnosis is often delayed due to the clinical symptoms overlapping with alike neurological conditions at the early stage [11]. Often patients receive an alternative diagnosis before the diagnosis of ALS [12,13], and especially for the majority of patients with no genetic mutation or familial background. To date, there is no cure for ALS, so early detection of the disease in order to improve diagnosis, to predict and slow down disease progression and to develop treatment is an urgent need [14]. Identification of a panel of biomarkers that accurately reflect ALS pathology is a priority. It has been suggested that, besides genetics, certain environmental factors may contribute to the disease pathology through epigenetic changes [15,16]. Studies that showed disease discordance in monozygotic twin pairs support the idea that epigenetic mechanisms are involved in ALS disease development [17,18,19]. Among the main epigenetic mechanisms are methylation of DNA, differential expression of long non-coding RNAs (lncRNAs), micro RNAs (miRNAs), circular RNA (circRNAs), other non-coding RNAs and histone modifications. The effects of these epigenetic mechanisms are closely related, since for example DNA methylation can control the expression of miRNA, and vice versa—miRNA can regulate the expression of methylation enzymes. Furthermore, there is also a complex temporal and spatial cross-talk or endogenous competition between long non-coding RNA (lncRNA), circular RNA (circRNA), micro (miRNA), messenger RNA (mRNA) and other RNAs, which form RNA networks and shape the disease phenotypic outcomes. Recent research has placed an increasing importance on discovering RNAs networks, for both, understanding molecular mechanisms and developing novel diagnostic and prognostic markers as well as therapeutic targets.
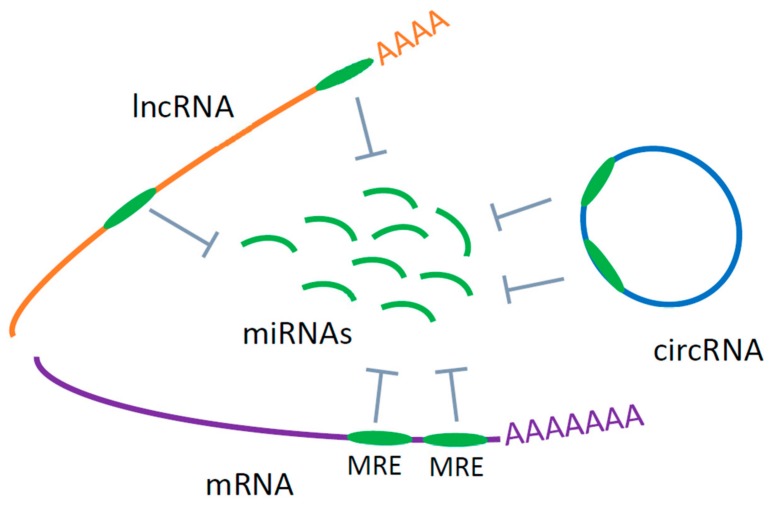
Despite the intense efforts, no clinically applicable biomarkers for ALS are yet established [12]. Development of biomarkers that are useful for early diagnosis and are selective for subgroups of disease as well as have prognostic potential and are indicators of treatment response are urgently needed. In addition, biomarkers should be minimally invasive and easily accessible from living patients. Most current research is thus focused, in particular, in identifying potential non-invasive RNA based circulating biomarkers for more rapid and accurate diagnosis and monitoring of the disease. Since ALS is a complex multi-factorial disease, no singular molecular species will be enough to enable the diagnosis or prognosis of ALS. Thus, an integrative approach based on several levels of RNA, including mRNA and non-coding RNAs, that form a potential biomarker signature is needed [20]. This approach has been already shown useful for other complex diseases, like cancers and neurodegenerative diseases [21,22]. In this review we have thus primarily concentrated on studies that have been foremost designed to find biomarkers for ALS based on mRNA, miRNA, lncRNA and circRNA in peripheral blood serum, plasma and cells, with little attempt to underlie the biology of the disease. We considered each RNA species separately as potential circulating biomarkers for ALS and in our conclusions we propose a potential integrated biomarker composed of the lncRNA/circRNA-miRNA-mRNA axis based on a competitive endogenous RNA network (ceRNET) hypothesis (see Figure 1 and Section 6) [22,23].
Figure 1.
Competitive endogenous RNA (ceRNA) hypothesis: MicroRNAs (miRNAs) are commonly considered as active negative regulators of gene expression, decreasing the stability of target mRNAs or limiting their translation. miRNAs act through specific complementary binding of its seed region (usually 6–8 nt long) to the 3′ untranslated region (UTR) of target mRNA, called miRNA response elements (MRE). However, recent studies have shown that the interaction with mRNA is not unidirectional, but that also other endogenous RNAs, like lncRNAs, and circRNAs possessing the same MRE sequence (shown in the figure) can compete for the same pool of miRNA thereby regulating miRNA activity [23]. (green oval represents miRNA response elements (MRE)).
Search Strategy and Criteria
We searched the PubMed and Google Scholar databases for articles in English. The main search terms included Amyotrophic Lateral Sclerosis, ALS, combined with RNA biomarkers and circulating RNA biomarkers. Since this is a relatively new research topic, no filter for the years was applied. We hand-searched the retrieved articles and selected the most relevant articles based on a subjective evaluation of their quality and relevance. Additional articles on specific topics were searched if needed.
2. mRNAs in ALS
The dysregulated RNA mechanism is present in the familial and sporadic form of ALS. Abnormal GGGGCC hexanucleotide repeat expansion in the Chromosome 9 open reading frame 72 (C9orf72) is associated with 30–40% of familial cases and 1%–10% of sporadic cases of ALS [24]. This alteration causes the formation of R-loops and thus alters the RNA binding activity of translated C9orf72 protein [25,26]. Mutated superoxide dismutase (SOD1) was detected in 10%–20% of familial cases and in 2%–7% of sporadic ALS cases [27]. Alterations in SOD1 produce increased intracellular toxicity and trigger the apoptosis of the neuronal cells [28]. It was shown also that mutated SOD1 affects expression of two other mRNAs involved in neurodegeneration, vascular endothelial growth factor-A (VEGFA) mRNA and neurofilament (NEFL) mRNA [26]. Fused in sarcoma (FUS) RNA binding protein is a nuclear protein involved in splicing of RNAs and in the initiation of transcription [29]. Its prevalence in familial cases is about 5–7% and approximately 1% in sporadic cases [30,31]. Transactive response DNA binding protein (TDP-43) is involved in mRNA stability, RNA splicing, microRNA processing, and transport and local translation in neurons. Mutations in TDP-43 are present in about 3%–5% familial cases and in 1%–2% sporadic cases [31]. Both, FUS and TDP-43 are predominantly found in nucleus, but their mutations produce an export to the cytoplasm, formation of stress granules and neurodegeneration [32,33,34]. It was shown recently that different ALS-linked FUS mutations potentially caused disparate pathogenic pathways, but strikingly, nuclear import receptor Karyopherin-beta2 recovered the mutant defects [35]. Archbold et al. showed that several overlapping mechanisms regulate TDP-43 nuclear export [36]. It was revealed that the proteostasis system and the macroautophagy system are both severely involved in TDP-43 aggregation [37,38]. Studying TDP-43 structure, François-Moutal et al. reviewed how different domains and post-translational modifications may influence aggregation and deleterious behavior of TDP-43 [39].
Recently, Maniatis et al. performed comprehensive gene expression analysis using RNA sequencing in different regions of the spinal cord of a mouse ALS model over the course of disease and of a postmortem human ALS to detect transcriptomic changes that contribute to motor neuron loss. They found regional differences between microglia and astrocyte populations at early time points and confirmed altered expression of several known ALS genes e.g., MATR3, KIF5A and PFN1 [40,41]. Furthermore, their data suggest that microglial dysfunction occurs well before onset of symptoms, precedes astroglial dysfunction in ALS and is proximal to motor neuron dysfunction. TREM2- and TYROBP-mediated signaling that modulates autophagy is an early step in the spatiotemporal ordering of disease-associated changes in microglial gene expression [41].
mRNAs as Candidate Circulating Biomarkers of ALS
Some comprehensive studies to detect differentially expressed mRNA in whole blood and peripheral blood mononuclear cells (PBMC) [42,43,44,45] and several studies on selected mRNAs potentially involved in ALS pathology [42,43,44,46,47,48,49] have been performed, however, no obvious candidate biomarkers have been yet identified. Vijayakumar et al. performed a systematic literature search and detected no reproducibility across different studies concerning the transcriptomic signature of ALS tissues [50]. The reasons for the lack of repeatability could be different study populations, different types of control subjects, different sample sources, different stages of the disease and the use of different methodological strategies [50]. The majority of detected differently expressed mRNAs have low or no ALS disease specificity. However, in the study of Kuzma-Kozakiewicz et al. using real-time qPCR and Western blotting PBMCs from 74 SALS patients with different clinical phenotypes, 65 blood donors (healthy control I) and 29 cases with other neurological diseases (disease control II) divided into subgroups IIA (atypical parkinsonism) and IIB (ALS-mimicking disorders) were investigated. KIF5C and KIFC3 expression were significantly lower and DCTN1 higher in SALS than in control I. KIF1B expression was significantly higher in SALS than in subgroup IIB, whereas DCTN1 and DCTN3 were higher in SALS than in subgroup IIA. Kinesin heavy chain isoform 5 (KIF5C) has critical roles in the developing brain, including organelle transport along microtubules [51]. Dynactin subunit 1 (DCTN1) is the largest subunit of the dynactin complex, an activator of the molecular motor protein complex dynein. Reduced levels of DCTN1 mRNA and protein have been detected in patients with sporadic amyotrophic lateral sclerosis, and mutations have been linked to disease [52]. KIF1B, Kinesin family member 1B is a motor protein that transports mitochondria and precursors of synaptic vesicle. Mutations in this gene cause Charcot–Marie–Tooth disease, type 2A1 [53]. Never the less, more studies are needed to find out whether the levels of KIF5C and DCTN1 may be useful in ALS diagnosis, and whether KIF1B expression may discriminate ALS from ALS-mimicking disorders [47]. Nachmany et al. detected a significant difference in mRNA expression levels of cytoplasmic FMR1 interacting protein 2 (CyFIP2) and RB binding protein 9 (RbBP9) genes in ALS compared to non-ALS peripheral blood leukocytes samples. CyFIP2 and RbBP9 proteins could be functionally related to cell cycle control and DNA damage mediated apoptosis [48]. CyFIP2 is expressed in the nervous system since it was found to directly interact with fragile X mental retardation protein (FMRP) and is present in mouse brain synaptosomal extracts [54]. We reported significant overexpression of apoptosis-associated tyrosine kinase, AAKT mRNA, a host gene of miR-338 and significant downregulation of the GTPase dynamin 2 (DNM2) gene in leukocytes of 84 SALS patients compared to healthy controls [55]. It has been shown in a neuroblastoma cell-line that the AATK gene produces neuronal differentiation [56]. In developing mouse brains, AATK was connected to cell death in mature neurons and to neurite extension in developing neurons [57]. DNM2 was also previously found mutated in Charcot–Marie–Tooth neuropathy type CMT2M, a motor and sensory neuropathy primarily affecting peripheral nerves [58] and in centronuclear myopathy (CNM), presenting with primary damage in skeletal muscles [59]. Loss of DNM2 in skeletal muscle initiates a chain of harmful parallel and serial events, involving dysregulation of lipid droplets and mitochondrial defects within altered muscle fibers, defective neuromuscular junctions and peripheral nerve degeneration [60]. Using deep sequencing of coding RNAs in monocytes of ALS patients compared to healthy controls a unique inflammation-related gene expression profile was detected, in which the most prominent mRNAs included IL1B, IL8, FOSB, CXCL1 and CXCL2 had higher expression levels in patients than in controls [61]. Gupta et al. reported that they found vascular endothelial growth factor-A (VEGF-A) and chemokine ligand (CCL2) genes upregulated in peripheral blood mononuclear cells in 50 Indian ALS patients compared with the same number of normal controls [46]. In a study that included 56 SALS patients and 20 healthy controls, Liguori et al. confirmed the importance of 12 genes whose mutations have been already identified as causative of SALS/FALS (PFN1, TUBA4A, PARK7, SQSTM1, DCTN1, C9orf72, TMEM106B, ALS2, TRPM7, MATR3, SPG11 and ATXN2) [3,9,62,63,64,65,66] by detecting their differential expression (mRNAs) in the peripheral blood samples of SALS patients compared to controls [67]. They also identified differential expression of other potential candidate genes like galectin 3 (LGALS3), which is implicated in neuroinflammation and protein kinase C delta (PRKCD), which is activated in mitochondrial-induced apoptosis [67]. Additional studies are needed to find out if any of these or other ALS associated mRNAs will be possible to use as circulating biomarkers for ALS.
3. Micro RNAs
MicroRNAs (miRNAs) are small 17–22 nucleotides (nt) long non-coding RNAs, which act as post-transcriptional regulators of gene expression either by causing the degradation of target mRNAs or the inhibition of their translation [68] through specific bounding of miRNA 6–8 nt long seed sequence to the 3′ untranslated region (UTR) of target mRNA [69]. Many miRNAs are evolutionarily conserved and have preferentially conserved interaction with most human mRNAs, which is indicative of their important biological functions [70]. Beside mRNA, miRNAs are also potent regulators of other miRNAs and non-coding RNAs, like lncRNAs and circRNAs. A single microRNA can regulate hundreds of RNA species, which makes miRNAs important regulators of cellular homeostasis [71]. MiRNAs are present in both intracellular and extracellular environments and in almost all biological fluids [72]. Extracellularly, miRNAs are detected within membrane vesicles and freely, forming complexes with other macromolecules. So far, more than 2500 miRNAs have been identified in the human genome (miRBase (www.mirbase.org)) [73] and for hundreds of them, the function in brain development and pathology have already been described [74].
3.1. MicroRNAs in Neurodegeneration and ALS
Several neurodegenerative diseases including ALS share numerous deregulated miRNAs, among them miR-9, miR-17, miR-21, miR-22, miR-25, miR-26, miR-29, miR-30, miR-34, miR-49, miR-98, miR-106, miR-107, miR-124, miR-125, miR-127, miR-128, miR-132, miR-132/212 cluster, miR-133b, miR-134, miR-136, miR-141, miR-146a, miR-153, miR-155, miR-181, miR-183, miR-183/96/182 cluster, miR-186, miR-193, miR-196, miR-200, miR-206, miR-210, miR-212,miR-221, miR-223, miR-320, miR-338, miR-365, miR-378, miR-494, miR-505, miR-512, miR-592, miR-let7 and miR-Let-7f-5p [75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109].
miRNAs have many characteristics to be promising biomarkers of human diseases. They have been shown to have high specificity, stability and recent studies have also indicated that miRNAs can be detected in biological fluids where they retain the expression profile of the original cells, including neurons [110].
3.2. miRNAs as Candidate Circulating Biomarkers of ALS
Numerous studies on miRNAs as potential circulating biomarkers for ALS have been published.
De Felice et al. detected several differentially expressed miRNAs in leukocytes from ALS patients including miR-149, miR-328, miR-338-3p, miR-451, miR-583, miR-638, miR-665 and miR-1275 [111].
CD14+CD16− monocytes isolated from ALS patients with SOD1 mutated familial form revealed a unique miRNA signature with miR-27a, miR-30b, miR-142-5p, miR-155, miR-223 and miR-532-3p highly overexpressed in an ALS patient comparing to healthy controls and patients with multiple sclerosis [109]. In addition, three of these miRNAs, miR-27b, miR-146a and miR-532-3p were upregulated also in sporadic ALS patients, and in monocytes and microglia from SOD1 mice [109].
In serum of sporadic ALS, Freischmidt et al. observed significant downregulation of TDP-43 binding miRNAs, miR-132-5p, miR-132-3p, miR-143-3p and miR-143-5p and let-7b [112].
Subsequently, miR-206 was reported elevated and as a potential biomarker in the serum of SALS patients [113].
Previously detected overexpression of miR-338-3p in blood leukocytes was confirmed in a larger cohort, and in addition, also in cerebrospinal fluid (CSF), serum and spinal cord obtained from SALS patients compared to healthy controls as well as to patients with other neurodegenerative disorders like Parkinson’s disease (PD), Alzheimer’s disease (AD) and Huntington disease (HD) [114].
Freischmidt et al. reported an miRNAs signature including miR-4745, miR-1915, miR-1825, miR-3613-3p, miR-3665, miR-3185, miR-4488, miR-3960, miR-4530, miR-1281, miR-4532, miR-4734, miR-477-5p, miR-4497, miR-3940-5p, miR-4466, miR-3196, miR-4270, miR-4507, miR-4505, miR-1469, miR-4741, miR-4787-5p, miR-371b-5p, miR-2861, miR-638, 149-3p, miR-4763-3p and miR-4516 that was significantly down-regulated in the serum of FALS and of presymptomatic mutation carriers compared to controls [115]. However, only two miRNAs, miR-1234-3p and miR-1825, were subsequently identified by the same group to be consistently downregulated in serum of sporadic ALS patients [116].
Takahashi et al. identified in the plasma of 48 SALS patients, compared to 47 healthy controls, miR-4649-5p up-regulated and miR-4299 down-regulated [117].
An miRNA panel of four under-expressed microRNAs, miR-183, miR-193b, miR-451 and miR-3935 was identified that distinguished among 83 SALS patients and 61 controls with high diagnostic accuracy of SALS (AUC 0.857). miR-183 also significantly discriminated between SALS patients and 24 Parkinson’s disease patients [118].
In plasma from 39 sporadic patients with spinal onset, miR-424 and miR-206 were found to be overexpressed compared to the same number of controls and their baseline expression correlated with clinical deterioration over time [119].
In 14 ALS patients (10 spinal, 4 bulbar) Tasca et al. measured the serum levels of muscle-specific miR-206, miR-1, miR-133a/b and miR-27a and found miR-206 and miR-133 significantly increased and miR-27a significantly reduced as compared to controls as well as between spinal vs. bulbar onset [120].
Thirty-seven brain-enriched miRNAs, inflammation-associated miRNAs, miRNAs highly enriched in muscle tissue and in cerebellum, and ubiquitous apoptosis-associated miRNAs were investigated in plasma of 250 patients with clinical diagnosis of either AD, frontotemporal dementia (FTD), PD or ALS. MicroRNA pairs and their combinations could differentiate all diseases from controls and from each other with high accuracy. Up-regulation of pairs miR-206/miR-338-3p, miR9*/miR-129-3p and miR-335-5p/miR-338-3p differentiated ALS from controls, while combinations miR-31/miR-206, miR-125b/miR-335-5p and miR-107/miR-491-5p differentiated ALS from AD, respectively [121].
miR-206 and miR-143-3p were increased and miR-374b-5p was decreased in the serum of SALS patients compared to controls and persisted during disease progression [122].
Serum miRNAs of 20 SALS and 3 FALS patients were compared to serum miRNAs of healthy controls, AD and multiple sclerosis patients. Seven microRNAs (miR-192-5p, miR-192-3p, miR-1, miR-133a-3p, miR-133b, miR-144-5p and miR-19a-3p) were identified as upregulated and six microRNAs (miR-320c, miR-320a, let-7d-3p, miR-425-5p, miR-320b and miR-139-5p) as downregulated in ALS patients in relation to healthy and ill controls [123].
We aimed to evaluate in leukocyte samples of 84 patients with sporadic ALS the differential expression of 10 miRNAs, for which some connection to ALS was shown previously in ALS culture cells, animal models or patients. We observed significant up-regulation across our patient cohort for miR-124a, miR-206, miR-9, let-7b, miR-638, miR-663a, miR-451, miR-132 and miR-338 [55].
Xu et al. isolated serum exosomes from ALS patients and healthy controls and compared the expression of miR-27a-3p. They found miR-27a-3p significantly down-regulated [124].
In serum from twenty sporadic ALS patients, a significant deregulation of miR-142-3p and miR-1249-3p was observed. miR-142-3p levels were found to negatively correlate with the ALS functional rating scale [125].
All the above described studies used as an miRNA detection approach combination of microarrays and qRT-PCR or qRT-PCR alone, however in three most recent studies, high-throughput next-generation RNA sequencing was applied [45,67,126].
In peripheral blood samples of SALS patients compared to healthy controls, results of analysis revealed that 38 miRNAs (let-7a-5p, let-7d-5p, let-7f-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-106b-3p, miR-128-3p, miR-130a-3p, miR-130b-3p, miR-144-5p, miR-148a-3p, miR-148b-3p, miR-15a-5p, miR-15b-5p, miR-151a-5p, miR-151b, miR-16-5p, miR-182-5p, miR-183-5p, miR-186-5p, miR-22-3p, miR-221-3p, miR-223-3p, miR-23a-3p, miR-26a-5p, miR-26b-5p, miR-27b-3p, miR-28-3p, miR-30b-5p, miR-30c-5p, miR-342-3p, miR-425-5p, miR-451a, miR-532-5p, miR-550a-3p, miR-584-5p and miR-93-5p) were significantly downregulated in patients. It was also found that different miRNAs profiles characterized the bulbar/spinal onset and the progression rate of disease [67].
When compared between the ALS blood and healthy control blood, De Felice et al. identified 696 known and 49 novel miRNAs differentially expressed in ALS tissues. Most upregulated miRNAs were miR-224-3p, miR-5684, miR-4695-3p, miR-1296-5p, miR-224-5p, miR-153-3p, miR-10b-5p, miR-1, miR-3194-3p, miR-877-3p, miR-326 and miR-338-3p, and the most downregulated were miR-144-5p, miR-190a-5p, miR-218-5p, hsa-miR-125a-3p miR-143-3p, miR-144-3p, miR-618, miR-338-5p, miR-4423-3p, miR-542-5p, miR-199b-5p and miR-193a-5p [45].
Differentially expressed miRNAs were detected in extracellular vesicles (EVs) from the plasma of persons living with ALS (PALS) and healthy controls using high-throughput sequencing and droplet digital PCR (ddPCR). Results revealed elevated levels of 5 miRNAs and reduced levels of 22 miRNAs. Deregulated miRNAs most relevant to ALS included miR-9-5p, miR-183-5p, miR-338-3p and miR-1246. miR-15a-5p was identified for its diagnostic potential and miR-193a-5p for disability progression [126].
3.3. The Most Promising Potential Circulating miRNA Biomarkers and Their Physiological Roles
Although numerous potential miRNAs were proposed in several studies mentioned above as biomarkers in peripheral blood from ALS patients, there is not much overlap between each study. Most clinically relevant diagnostic and prognostic biomarkers, as well as treatment targets, would be those which are unable to detect disease at most early stages of its molecular pathogenesis. Vijayakumar et al. performed a systematic literature search and identified only a relatively few number candidates that were consistently identified as potential biomarkers across multiple independent studies. These candidate biomarkers are predominantly involved in dysfunction of skeletal muscle and of motor neurons and in inflammatory process [50].
Before the disease is clinically detectable and during its progression, the skeletal muscle of ALS patients undergoes futile cycles of re-innervation and denervation, along with motor neuron degeneration [127]. Muscle fibers are classified into two main metabolic types, slow-twitch and fast-twitch. Slow-twitch fibers are innervated by small-caliber axons and fast-twitch fibers are innervated by large-caliber axons. Studies revealed that the number of large-caliber axons is significantly lower in the spinal cord of ALS patients than in controls, whereas the number of small-caliber axons is maintained [128,129]. In the ALS mouse model, muscles enriched in slow-twitch fibers undergo denervation at later stages, compared with fast-twitch fibers [130] what suggests that, during the early stages of ALS pathogenesis, the muscle fibers and the motor neurons innervating them closely collaborate to counteract the skeletal muscle atrophy [131]. The role of microRNAs in the control of these crucial mechanisms has been investigated, though not yet conclusively clarified. A set of microRNAs enriched and specifically expressed in the skeletal muscle have been identified including miR-1, miR-133a, miR-133b, miR-206 and miR-27a [131]. Interestingly, each of these miRNAs has been more frequently detected also as deregulated circulating miRNAs in ALS patients compared to controls [55,109,113,119,120,122,123,124]. These miRNAs are involved in the regulation of different processes during skeletal muscle development. miR-1 is acting on transcription factor histone deacetylase (HDAC4) and paired box 7 (PAX7) and is required for muscle differentiation, while miR-133 promotes proliferation [132]. Williams and colleagues [133] originally investigated microRNA expression in the skeletal muscle during ALS progression in muscle tissues of symptomatic G93A-SOD1 transgenic mice. In this model, the upregulation of miR-206 coincided with the onset of neurological symptoms, since the transcriptional activation of miR-206 was activated in response to skeletal muscle denervation before clinical symptoms. miR-206 mediated its effects by suppressing muscular histone deacetylase 4 (HDAC4) protein levels. HDAC4 inhibition, in turn, induced the expression of fibroblast growth factor binding protein 1 (FGFBP1), which promoted re-innervation and regeneration within the neuromuscular junction [133]. It seems that miR-206 is not involved in the ALS pathogenesis, but is rather a potential early circulating biomarker of ALS progression and promising target for testing new treatments based on physiologic re-innervation self-healing responses.
miR-338-3p is another potential circulating miRNA for ALS, since it was found consistently upregulated in several studies [45,50,111,114,121,126]. Its role as a functional miRNA in controlling different molecular pathways has been reported. miR-338-3p is involved in neuronal proliferation, maturation and neurite outgrowth and in organization in the dentate gyrus, while also acting as a tumor suppressor in vivo [134,135,136,137]. miR-338-3p is also expressed in spinal cord oligodendrocytes, where it positively regulates oligodendrocyte differentiation from precursors into mature oligodendrocytes by repressing Sox5 and Hes6, two maturation- and differentiation-inhibiting transcription factors [138,139]. Interestingly, miR-338-3p might also relate to the higher glutamate levels observed in ALS patients. Namely, one of putative targets of deregulated miR-338p is membrane-bound transporter protein SLC1A2/EAAT2, which clears the excitatory glutamate from the extracellular space at synapses in the CNS. Excitotoxicity thus caused by down-regulation of SLC1A2 /EAAT2 is thought to contribute to motor neuron death in ALS [140]. Li et al. recently showed that miR-338-3p binds glycogen phosphorylase (PYGB) and causes its diminished expression and regional accumulation of glycogen in the spinal cord of ALS mice. These findings provide new insights into the metabolic dysfunctions in the progression of ALS and into new potential therapeutic targets [141]. However, miR-338-3p deregulation was also observed in other neurodegenerative diseases including HD [142].
miR-183 was found dysregulated in peripheral blood samples of sporadic ALS patients in three different studies [67,118,126]. miR-183 family (including miR-183, miR-96 and miR-182) are highly conserved miRNAs with many revealed roles in different cancers, immunity and nervous system [143,144,145,146,147]. Jawaid et al. also showed that impaired biogenesis of miR-183/96/182 cluster influence age-related memory decline and that TDP-43 and FUS are involved in regulation of the biogenesis. In the postmortem brain of ALS patient’s miR-183/96/182 was decreased [148].
Maniatis et al. recently reported that microglial dysfunction occurs well before the onset of disease symptoms in an ALS mouse model [41]. Detection of circulating miRNAs biomarkers involved in neuroinflammation through microglial activation, dysregulation of immune-related genes and recruitment of monocytes to affected tissues represents important steps towards improving diagnosis and future treatment of ALS. Butovsky et al. already demonstrated that infiltration of peripheral inflammatory monocytes into the CNS played an important role in progression of ALS. Inflammation-related miRNAs including miRNA let-7a, let-7b, miR-27a, miR-146a, miR-451, miR-223, miR-142-5p, miR-532-3p and miR-155 were significantly upregulated in peripheral monocytes from SOD1 mice and ALS patients with familial and sporadic disease. In addition, MiR-27a was able to differentiate multiple sclerosis patients from patients with ALS [109]. Among miRNAs involved in neuroinflammation, miR-451 was revealed deregulated in blood samples of ALS patients in most studies [50,55,67,109,111,118].
miR-451 is involved in the regulation of various human physiological and pathological processes. It acts as a tumor suppressor gene in most cancer types. miR-451 can directly affect the biological functions of tumor cells but can also, upon secretion into the tumor microenvironment via exosomes, indirectly affect tumor cell invasion and metastasis [149]. It was shown that miR-451 is regulated in vivo by protein kinase AMP-activated catalytic subunit alpha 1 (AMPK) pathway and that AMPK/miR-451 loop has the ability to switch between proliferative and migratory pattern behavior of glioma cells [150,151]. It was also suggested that miR-451 might relieve chronic inflammatory pain by inhibiting microglia activation-mediated inflammation via targeting toll-like receptor 4 (TLR4) [152].
miRNAs let-7 were also found deregulated in ALS in several studies [55,67,109,112,123]. Let-7 family miRNAs are involved in multiple pathways contributing to cell survival and cell death and influence cancer risk and prognosis as well as neuroinflammation [153,154,155]. It was shown recently, that miR cluster MC-let-7a-1 ~ let-7d promotes glioma cell autophagy and apoptosis by repressing signal transducer and activator of transcription 3 (STAT3) [156].
Proinflammatory miR-125a/b has also been detected as circulating miRNA in ALS patients in more than one study [45,121]. Parisi et al. thoroughly investigated the role of miR-125b in the modulation of nuclear factor kappa B subunit (NF-κb) signaling in microglia. They identified a pathogenic mechanism in ALS microglia, in which miR-365 and miR-125b negatively regulated interleukin-6 (IL-6) and STAT3 pathway, respectively, causing an increase in tumor necrosis factor-alpha (TNFα) expression and switching microglia toward a detrimental phenotype [96,157].
In order to contribute to the diagnosis of ALS, it is important to detect and track miRNAs involved in early molecular manifestations of disease. No single miRNA of described deregulated miRNAs is specific for ALS. In addition, since ALS is a multi-systemic disease, a combination of several miRNAs will be needed to test simultaneously in order to reach higher specificity and accuracy of diagnosis. In some studies, combinations of several miRNAs have already shown a higher accuracy than single miRNAs in discriminating ALS from healthy controls or other neurological disorders [118,121,123]. Up-regulation of pairs miR-206/miR-338-3p, miR9*/miR-129-3p and miR-335-5p/miR-338-3p differentiated ALS from controls, while combinations miR-31/miR-206, miR-125b/miR-335-5p and miR-107/miR-491-5p differentiated ALS from AD, respectively [121].
miR-206, miR-133b, miR-27a, mi-338-3p, miR-183, miR-451, let-7 and miR-125b were most commonly deregulated in multiple studies reviewed above (Table 1). To test clinical potential of this miRNA panel, a useful approach may be to perform such analysis on larger multi-center scale using similar experimental design.
Table 1.
The most promising potential circulating miRNA biomarkers.
| miRNA | Sample | Level of miRNA | Reference |
|---|---|---|---|
| miR-206 | Leukocytes, plasma, serum | increased | [55,113,119,120,122] |
| miR-133b | serum | increased | [120,123] |
| miR-27a | CD14+CD16- monocytes, serum exosomes | increased, decreased | [109,120,124] |
| mi-338-3p | leukocytes, plasma, plasma extracellular vesicles | increased | [45,55,111,114,121,126] |
| miR-183 | leukocytes, plasma extracellular vesicles | decreased | [67,118,126] |
| miR-451 | leukocytes, peripheral monocytes | decreased, increased | [55,67,109,111,118] |
| let-7 | serum, leukocytes, peripheral monocytes | decreased, increased | [55,67,109,112,123] |
| miR-125 | leukocytes, plasma | decreased | [45,121] |
4. Long Non-Coding RNA
Long non-coding RNA (lncRNA) is another type of RNA molecule that could be investigated as circulating biomarkers in ALS. LncRNAs are transcripts greater than 200 bp in length with no or little translational potential. lncRNAs are classified according to the genomic position from which they are transcribed: intergenic RNAs (lincRNAs), intronic lncRNAs, antisense lncRNAs (aslncRNAs), bidirectional lncRNAs and enhancer RNAs (eRNAs). They can either silence or enhance the expression of a proximal gene at the level of epigenetics, transcription and post-transcription [158]. Lines of evidence revealed the role of dysregulated lncRNAs in cancer [159,160]. There is also increasing evidence of the importance of lncRNAs in development of brain, function, maintenance and differentiation of neurons, as well as in neurodegenerative diseases [161,162].
4.1. Long Non-Coding RNA in Neurodegeneration and ALS
In ALS, long non-coding transcripts have now also been found at the C9ORF72 locus. The C9ORF72 repeat expansion region can be transcribed bidirectional and both, sense and antisense C9ORF72 transcripts (C9ORF72-AS) are elevated in the brains of ALS patients where they form nuclear RNA foci. RNA foci were most abundant in the frontal cortex but also occurred in astrocytes, microglia and oligodendrocytes [163]. LncRNA, nuclear-enriched abundant transcript 1_2 (NEAT1_2T) contains a GC-rich sequence and is predominantly expressed in spinal motor neurons in an early phase of ALS [164]. A neurotoxic ataxin 2 antisense transcript ATXN2-AS with a CUG repeat expansion may also contribute to ALS pathogenesis [165].
4.2. LncRNAs as Candidate Circulating Biomarkers of ALS
To date, only one study reported differentially expressed lncRNAs in peripheral blood mononuclear cells (PBMCs) from ALS patients. Gagliardi et al. found in total of 293 dysregulated lncRNAs in sporadic ALS patients without detected mutation. The majority (184/293 transcripts) were antisense lncRNAs and mostly unknown. In patients with mutation in the FUS gene, 21 lncRNAs were identified, 11 of them were antisense. In TDP-43 mutated patients 7 antisense lncRNA was detected, and only one was already described, SNAP25-AS, antisense lncRNA of synaptic protein SNAP25. In ALS patients with SOD1 mutation, 2 novel antisense RNAs have been revealed, one of these is creatine kinase, mitochondrial 2 (CKMT2) antisense [44]. There is still a lot to be done in order to identify and understand the role of lncRNAs in ALS.
5. Circular RNAs
Circular RNAs (circRNAs) represent yet another class of important regulatory non-coding RNAs [166,167,168]. They arise from back-splicing events during precursor mRNA processing. CircRNAs are resistant to RNA exonucleases and are thus highly stable in cells. CircRNAs may function as miRNA sponges and may sequester RNA-binding protein (RBP), and thus influence gene regulation. Each circRNA competitively binds multiple miRNAs and reduces their mRNA silencing potential [169]. When circRNAs bind to RBP they can act as scaffolds for protein complexes [170].
5.1. Circular RNAs in Neurodegeneration and ALS
Precise temporal and spatial regulation of gene networks tailors the development, homeostasis and stress response of the central nervous system (CNS). Moreover, it has been revealed that circRNAs are spatiotemporally regulated and dynamically expressed during brain development and can thus significantly influence development of CNS and diseases [168]. circRNAs have been already implicated in several neurodegenerative diseases, such as AD [171] and PD [172]. RNA-binding protein FUS, has been recognized as an important modulator of circRNA expression. Errichelli et al. observed an overall downregulation of circRNA expression in FUS−/− mice and expression was dysregulated also in FUSR521C and FUSP525L human induced pluripotent stem cell-derived motor neurons. Since cognate linear transcripts showed no significant alteration in expression levels, circRNA deregulation can be attributed to altered splicing dynamics due to mutated or absent FUS. Whether this is the reason for altered circRNA expression also in human tissues, it remains to be determined [173]. These findings could have considerable implications for further research on circRNAs in ALS as mutations in FUS have been detected in ALS patients [3].
5.2. Circular RNA as Candidate Circulating Biomarkers of ALS
We investigated a microarray expression profile of circRNAs in leukocyte samples from SALS patients and age- and sex-matched healthy controls and identified 425 differentially expressed circRNAs. We selected 10 of them based on the function of the hosting gene for qPCR validation. The expression of 7/10 circRNAs was significant in a larger cohort of ALS patients. Four of them showed the highest significance as well as clinical relevance [174]. hsa_circ_0000567 is located in SETD3 gene, the product of which is histone methyltransferase that regulates muscle differentiation in mouse [175]. hsa_circ_0023919 is located in the PICALM gene that is involved in clathrin-mediated endocytosis at neuromuscular junctions [176]. hsa_circ_0023919 sequence contains two binding sites for hsa-miR-9 [177]. The upregulation of miR-9 was confirmed in both mouse model of ALS [178] and in human blood samples of ALS patients [55]. TNRC6B, a host gene of hsa_circ_0063411, guides Ago mediated gene silencing [179]. It contains one binding site for miR-647 [177]. Some connection between miR-647 and ALS was already detected in spinal cord samples from ALS patients where miR-647 was absent compared to controls [180]. hsa_circ_0088036 is located in sushi domain containing 1 (SUSD1) gene that is potentially associated with ALS [181]. In addition, hsa_circ_0023919, hsa_circ_0063411 and hsa_circ_0088036 also had AUC > 0.95, and sensitivity and specificity for the optimal threshold point >90%, showing their potential for using them as diagnostic biomarkers [174].
6. Conclusions and Future Perspectives
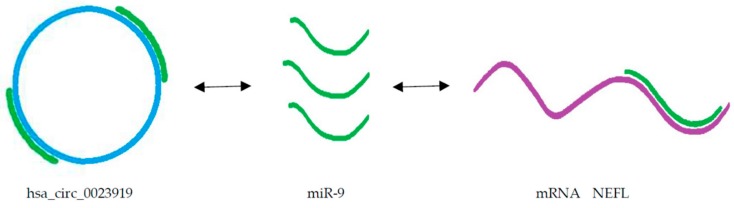
ALS is a complex multi-system neurodegenerative disorder with currently limited diagnostic and no therapeutic options. Early diagnosis of ALS is, therefore, most important for rapid identification and management of the disease. Most studies to date have investigated miRNAs as potential circulating biomarkers for ALS. From these studies, a panel of the most promising potential circulating miRNAs with physiological roles in ALS could be deduced (Table 1). However, other types of RNAs (lncRNAs, circRNAs and mRNAs) that together with miRNAs represent RNA networks, have not been yet extensively studied in blood samples of patients with ALS. The RNA network represents a large-scale regulatory network across the transcriptome that plays important roles in maintaining the homeostasis, while the disruption of this network leads to pathological conditions. Molecular biomarkers based on specific RNA networks would have more potential to successfully distinguish between different neurological conditions. Specific RNA networks based on cross-talk between lncRNA/circRNA, miRNA and mRNA have recently attracted more attention [182]. Salmena et al. proposed the hypothesis about competing endogenous RNAs (ceRNAs) (Figure 1). According to this hypothesis, different RNA molecules, including lncRNAs, circRNAs and mRNA act as miRNA sponges to inhibit miRNAs from binding to their target sites. RNA transcripts that share the same miRNA response element (MRE) can competitively inhibit the function of miRNA and then influence the expression of relevant mRNAs [23]. Based on the ceRNA hypothesis, a cross-talk is going on between RNA transcripts with identical MREs regulating their reciprocal expression levels. Because these interactions are interconnected an aberrant expression of any component of network could interrupt the complex regulatory circuitry, resulting in the development of disease. Since all these RNAs can be detected in blood, the lncRNA/circRNA–miRNA-mRNA axes may represent innovative circulating biomarkers. Such ceRNA axes and networks (ceRNETs) based on computational platforms and experimental approaches have already been proposed for several cancers and neurodegenerative diseases [21,22], but not yet for ALS. Extensive investigation of the role of circulating mRNAs, lncRNAs and circRNAs is therefore needed to detect RNA networks specific for disease type and progression. We have recently reported high diagnostic potential of circRNA hsa_circ_0023919 in blood samples of SALS patients [174]. hsa_circ_0023919 contains two binding sites for miR-9 [177]. Hawley et al. have shown that human neurofilament (NEFL) 3’UTR had two sites for miR-9 binding and that miR-9 was capable of reducing the expression of NEFL, and intermediate neurofilaments were observed in motor neurons of SALS patients [183]. Deduced from these results one potential example of biomarker based on circRNA-miRNA-mRNA axis for ALS could be: hsa_circ_0023919—miR-9—mNEFL, where circRNA hsa_circ_0023919 sponges miR-9, while miR-9 regulates metabolism of intermediate filaments (NEFL) observed in ALS motor neurons [174,183] (Figure 2). A lot still must be done in order to find robust circulating biomarkers and therapeutic targets that will distinguish key ceRNA interactions in specific ALS-types to facilitate diagnosis, predict progression and design therapy.
Figure 2.
Potential circRNA–miRNA–mRNA axis for amyotrophic lateral sclerosis (ALS). This axis represents endogenous competition between circRNA hsa_circ_0023919 and mRNA NEFL for binding of miR-9. circRNA hsa_circ_0023919 sponges miR-9, which regulates metabolism of neurofilament (NEFL) [174,183].
Author Contributions
M.R.-G. wrote the main text and produced figures. D.G. discussed and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovenian Research Agency (ARRS) under research program P3-0054.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tandan R., Bradley W.G. Amyotrophic lateral sclerosis: Part 1. Clinical features, pathology, and ethical issues in management. Ann. Neurol. 1985;18:271–280. doi: 10.1002/ana.410180302. [DOI] [PubMed] [Google Scholar]
- 2.Ajroud-Driss S., Siddique T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS) Biochim. Biophys. Acta. 2015;1852:679–684. doi: 10.1016/j.bbadis.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Chia R., Chio A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet. Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothstein J.D. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann. Neurol. 2009;65:S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 5.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y., et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 6.Neumann M., Bentmann E., Dormann D., Jawaid A., DeJesus-Hernandez M., Ansorge O., Roeber S., Kretzschmar H.A., Munoz D.G., Kusaka H., et al. FET proteins TAF15 and EWS are selective markers that distinguish FTLD with FUS pathology from amyotrophic lateral sclerosis with FUS mutations. Brain J. Neurol. 2011;134:2595–2609. doi: 10.1093/brain/awr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinchetti P., Rizzuti M., Faravelli I., Corti S. MicroRNA Metabolism and Dysregulation in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2018;55:2617–2630. doi: 10.1007/s12035-017-0537-z. [DOI] [PubMed] [Google Scholar]
- 9.Morgan S., Orrell R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016;119:87–98. doi: 10.1093/bmb/ldw026. [DOI] [PubMed] [Google Scholar]
- 10.Bordone M.P., Salman M.M., Titus H.E., Amini E., Andersen J.V., Chakraborti B., Diuba A.V., Dubouskaya T.G., Ehrke E., Espindola de Freitas A., et al. The energetic brain—A review from students to students. J. Neurochem. 2019;151:139–165. doi: 10.1111/jnc.14829. [DOI] [PubMed] [Google Scholar]
- 11.Andersen P.M., Abrahams S., Borasio G.D., de Carvalho M., Chio A., Van Damme P., Hardiman O., Kollewe K., Morrison K.E., Petri S., et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)--revised report of an EFNS task force. Eur. J. Neurol. 2012;19:360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 12.Benatar M., Boylan K., Jeromin A., Rutkove S.B., Berry J., Atassi N., Bruijn L. ALS biomarkers for therapy development: State of the field and future directions. Muscle Nerve. 2016;53:169–182. doi: 10.1002/mus.24979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paganoni S., Macklin E.A., Karam C., Yu H., Gonterman F., Fetterman K.A., Cudkowicz M., Berry J., Wills A.M. Vitamin D levels are associated with gross motor function in amyotrophic lateral sclerosis. Muscle Nerve. 2017;56:726–731. doi: 10.1002/mus.25555. [DOI] [PubMed] [Google Scholar]
- 14.Turner M.R., Bowser R., Bruijn L., Dupuis L., Ludolph A., McGrath M., Manfredi G., Maragakis N., Miller R.G., Pullman S.L., et al. Mechanisms, models and biomarkers in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013;14:19–32. doi: 10.3109/21678421.2013.778554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa-Romero C., Hur J., Bender D.E., Delaney C.E., Cataldo M.D., Smith A.L., Yung R., Ruden D.M., Callaghan B.C., Feldman E.L. Identification of epigenetically altered genes in sporadic amyotrophic lateral sclerosis. PLoS ONE. 2012;7:e52672. doi: 10.1371/journal.pone.0052672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Pacheco A., Franco J.M., Lopez S., Gomez-Zumaquero J.M., Magdalena Leal-Lasarte M., Caballero-Hernandez D.E., Cejudo-Guillen M., Pozo D. Epigenetic Mechanisms of Gene Regulation in Amyotrophic Lateral Sclerosis. Adv. Experimen. Med. Biol. 2017;978:255–275. doi: 10.1007/978-3-319-53889-1_14. [DOI] [PubMed] [Google Scholar]
- 17.Xi Z., Yunusova Y., van Blitterswijk M., Dib S., Ghani M., Moreno D., Sato C., Liang Y., Singleton A., Robertson J., et al. Identical twins with the C9orf72 repeat expansion are discordant for ALS. Neurology. 2014;83:1476–1478. doi: 10.1212/WNL.0000000000000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young P.E., Kum Jew S., Buckland M.E., Pamphlett R., Suter C.M. Epigenetic differences between monozygotic twins discordant for amyotrophic lateral sclerosis (ALS) provide clues to disease pathogenesis. PLoS ONE. 2017;12:e0182638. doi: 10.1371/journal.pone.0182638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M., Xi Z., Ghani M., Jia P., Pal M., Werynska K., Moreno D., Sato C., Liang Y., Robertson J., et al. Genetic and epigenetic study of ALS-discordant identical twins with double mutations in SOD1 and ARHGEF28. J. Neurol. Neurosurg. Psychiatry. 2016;87:1268–1270. doi: 10.1136/jnnp-2016-313592. [DOI] [PubMed] [Google Scholar]
- 20.Joilin G., Leigh P.N., Newbury S.F., Hafezparast M. An Overview of MicroRNAs as Biomarkers of ALS. Front. Neurol. 2019;10:186. doi: 10.3389/fneur.2019.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan J.J., Tay Y. Noncoding RNA:RNA Regulatory Networks in Cancer. Int. J. Mol. Sci. 2018;19:1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuzziello N., Liguori M. The MicroRNA Centrism in the Orchestration of Neuroinflammation in Neurodegenerative Diseases. Cells. 2019;8:1193. doi: 10.3390/cells8101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruts M., Gijselinck I., Van Langenhove T., van der Zee J., Van Broeckhoven C. Current insights into the C9orf72 repeat expansion diseases of the FTLD/ALS spectrum. Trends Neurosci. 2013;36:450–459. doi: 10.1016/j.tins.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Gibson S.B., Downie J.M., Tsetsou S., Feusier J.E., Figueroa K.P., Bromberg M.B., Jorde L.B., Pulst S.M. The evolving genetic risk for sporadic ALS. Neurology. 2017;89:226–233. doi: 10.1212/WNL.0000000000004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Recabarren-Leiva D., Alarcon M. New insights into the gene expression associated to amyotrophic lateral sclerosis. Life Sci. 2018;193:110–123. doi: 10.1016/j.lfs.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 27.Volk A.E., Weishaupt J.H., Andersen P.M., Ludolph A.C., Kubisch C. Current knowledge and recent insights into the genetic basis of amyotrophic lateral sclerosis. Med. Genet. 2018;30:252–258. doi: 10.1007/s11825-018-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 29.Yang L., Embree L.J., Hickstein D.D. TLS-ERG leukemia fusion protein inhibits RNA splicing mediated by serine-arginine proteins. Mol. Cell. Biol. 2000;20:3345–3354. doi: 10.1128/MCB.20.10.3345-3354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwiatkowski T.J., Jr., Bosco D.A., Leclerc A.L., Tamrazian E., Vanderburg C.R., Russ C., Davis A., Gilchrist J., Kasarskis E.J., Munsat T., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 31.Da Cruz S., Cleveland D.W. Understanding the role of TDP-43 and FUS/TLS in ALS and beyond. Curr. Opin. Neurobiol. 2011;21:904–919. doi: 10.1016/j.conb.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosco D.A., Lemay N., Ko H.K., Zhou H., Burke C., Kwiatkowski T.J., Jr., Sapp P., McKenna-Yasek D., Brown R.H., Jr., Hayward L.J. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 2010;19:4160–4175. doi: 10.1093/hmg/ddq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ling S.C., Albuquerque C.P., Han J.S., Lagier-Tourenne C., Tokunaga S., Zhou H., Cleveland D.W. ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS. Proc. Natl. Acad. Sci. USA. 2010;107:13318–13323. doi: 10.1073/pnas.1008227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolozin B. Regulated protein aggregation: Stress granules and neurodegeneration. Mol. Neurodegener. 2012;7:56. doi: 10.1186/1750-1326-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niaki A.G., Sarkar J., Cai X., Rhine K., Vidaurre V., Guy B., Hurst M., Lee J.C., Koh H.R., Guo L., et al. Loss of Dynamic RNA Interaction and Aberrant Phase Separation Induced by Two Distinct Types of ALS/FTD-Linked FUS Mutations. Mol. Cell. 2020;77:82–94. doi: 10.1016/j.molcel.2019.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Archbold H.C., Jackson K.L., Arora A., Weskamp K., Tank E.M., Li X., Miguez R., Dayton R.D., Tamir S., Klein R.L., et al. TDP43 nuclear export and neurodegeneration in models of amyotrophic lateral sclerosis and frontotemporal dementia. Sci. Rep. 2018;8:4606. doi: 10.1038/s41598-018-22858-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hergesheimer R.C., Chami A.A., de Assis D.R., Vourc’h P., Andres C.R., Corcia P., Lanznaster D., Blasco H. The debated toxic role of aggregated TDP-43 in amyotrophic lateral sclerosis: A resolution in sight? Brain J. Neurol. 2019;142:1176–1194. doi: 10.1093/brain/awz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cascella R., Fani G., Bigi A., Chiti F., Cecchi C. Partial Failure of Proteostasis Systems Counteracting TDP-43 Aggregates in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019;20:3685. doi: 10.3390/ijms20153685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francois-Moutal L., Perez-Miller S., Scott D.D., Miranda V.G., Mollasalehi N., Khanna M. Structural Insights Into TDP-43 and Effects of Post-translational Modifications. Front. Mol. Neurosci. 2019;12:301. doi: 10.3389/fnmol.2019.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan S., Duguez S., Duddy W. Personalized Medicine and Molecular Interaction Networks in Amyotrophic Lateral Sclerosis (ALS): Current Knowledge. J. Pers. Med. 2018;8:44. doi: 10.3390/jpm8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maniatis S., Aijo T., Vickovic S., Braine C., Kang K., Mollbrink A., Fagegaltier D., Andrusivova Z., Saarenpaa S., Saiz-Castro G., et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science (New York) 2019;364:89–93. doi: 10.1126/science.aav9776. [DOI] [PubMed] [Google Scholar]
- 42.Van Rheenen W., Diekstra F.P., Harschnitz O., Westeneng H.J., van Eijk K.R., Saris C.G.J., Groen E.J.N., van Es M.A., Blauw H.M., van Vught P.W.J., et al. Whole blood transcriptome analysis in amyotrophic lateral sclerosis: A biomarker study. PLoS ONE. 2018;13:e0198874. doi: 10.1371/journal.pone.0198874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saris C.G., Horvath S., van Vught P.W., van Es M.A., Blauw H.M., Fuller T.F., Langfelder P., DeYoung J., Wokke J.H., Veldink J.H., et al. Weighted gene co-expression network analysis of the peripheral blood from Amyotrophic Lateral Sclerosis patients. BMC Genom. 2009;10:405. doi: 10.1186/1471-2164-10-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gagliardi S., Zucca S., Pandini C., Diamanti L., Bordoni M., Sproviero D., Arigoni M., Olivero M., Pansarasa O., Ceroni M., et al. Long non-coding and coding RNAs characterization in Peripheral Blood Mononuclear Cells and Spinal Cord from Amyotrophic Lateral Sclerosis patients. Sci. Rep. 2018;8:2378. doi: 10.1038/s41598-018-20679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Felice B., Manfellotto F., Fiorentino G., Annunziata A., Biffali E., Pannone R., Federico A. Wide-Ranging Analysis of MicroRNA Profiles in Sporadic Amyotrophic Lateral Sclerosis Using Next-Generation Sequencing. Front. Genet. 2018;9:310. doi: 10.3389/fgene.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta P.K., Prabhakar S., Abburi C., Sharma N.K., Anand A. Vascular endothelial growth factor-A and chemokine ligand (CCL2) genes are upregulated in peripheral blood mononuclear cells in Indian amyotrophic lateral sclerosis patients. J. Neuroinflamm. 2011;8:114. doi: 10.1186/1742-2094-8-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuzma-Kozakiewicz M., Kazmierczak B., Chudy A., Gajewska B., Baranczyk-Kuzma A. Alteration of Motor Protein Expression Involved in Bidirectional Transport in Peripheral Blood Mononuclear Cells of Patients with Amyotrophic Lateral Sclerosis. Neuro-Degener. Dis. 2016;16:235–244. doi: 10.1159/000443664. [DOI] [PubMed] [Google Scholar]
- 48.Nachmany H., Wald S., Abekasis M., Bulvik S., Weil M. Two potential biomarkers identified in mesenchymal stem cells and leukocytes of patients with sporadic amyotrophic lateral sclerosis. Dis. Markers. 2012;32:211–220. doi: 10.1155/2012/824692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadanand A., Janardhanan A., Vanisree A.J., Pavai T. Neurotrophin Expression in Lymphocytes: A Powerful Indicator of Degeneration in Parkinson’s Disease, Amyotrophic Lateral Sclerosis and Ataxia. J. Mol. Neurosci. 2018;64:224–232. doi: 10.1007/s12031-017-1014-x. [DOI] [PubMed] [Google Scholar]
- 50.Vijayakumar U.G., Milla V., Cynthia Stafford M.Y., Bjourson A.J., Duddy W., Duguez S.M. A Systematic Review of Suggested Molecular Strata, Biomarkers and Their Tissue Sources in ALS. Front. Neurol. 2019;10:400. doi: 10.3389/fneur.2019.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirokawa N., Noda Y., Tanaka Y., Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 52.Bercier V., Hubbard J.M., Fidelin K., Duroure K., Auer T.O., Revenu C., Wyart C., Del Bene F. Dynactin1 depletion leads to neuromuscular synapse instability and functional abnormalities. Mol. Neurodegener. 2019;14:27. doi: 10.1186/s13024-019-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conforti L., Dell’Agnello C., Calvaresi N., Tortarolo M., Giorgini A., Coleman M.P., Bendotti C. Kif1Bbeta isoform is enriched in motor neurons but does not change in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. Res. 2003;71:732–739. doi: 10.1002/jnr.10517. [DOI] [PubMed] [Google Scholar]
- 54.Schenck A., Bardoni B., Moro A., Bagni C., Mandel J.L. A highly conserved protein family interacting with the fragile X mental retardation protein (FMRP) and displaying selective interactions with FMRP-related proteins FXR1P and FXR2P. Proc. Natl. Acad. Sci. USA. 2001;98:8844–8849. doi: 10.1073/pnas.151231598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrabec K., Bostjancic E., Koritnik B., Leonardis L., Dolenc Groselj L., Zidar J., Rogelj B., Glavac D., Ravnik-Glavac M. Differential Expression of Several miRNAs and the Host Genes AATK and DNM2 in Leukocytes of Sporadic ALS Patients. Front. Mol. Neurosci. 2018;11:106. doi: 10.3389/fnmol.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raghunath M., Patti R., Bannerman P., Lee C.M., Baker S., Sutton L.N., Phillips P.C., Damodar Reddy C. A novel kinase, AATYK induces and promotes neuronal differentiation in a human neuroblastoma (SH-SY5Y) cell line. Brain Res. Mol. Brain Res. 2000;77:151–162. doi: 10.1016/S0169-328X(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 57.Tomomura M., Hasegawa Y., Hashikawa T., Tomomura A., Yuzaki M., Furuichi T., Yano R. Differential expression and function of apoptosis-associated tyrosine kinase (AATYK) in the developing mouse brain. Brain Res. Mol. Brain Res. 2003;112:103–112. doi: 10.1016/S0169-328X(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 58.Zuchner S., Noureddine M., Kennerson M., Verhoeven K., Claeys K., De Jonghe P., Merory J., Oliveira S.A., Speer M.C., Stenger J.E., et al. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
- 59.Bitoun M., Maugenre S., Jeannet P.Y., Lacene E., Ferrer X., Laforet P., Martin J.J., Laporte J., Lochmuller H., Beggs A.H., et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 2005;37:1207–1209. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]
- 60.Tinelli E., Pereira J.A., Suter U. Muscle-specific function of the centronuclear myopathy and Charcot-Marie-Tooth neuropathy-associated dynamin 2 is required for proper lipid metabolism, mitochondria, muscle fibers, neuromuscular junctions and peripheral nerves. Hum. Mol. Genet. 2013;22:4417–4429. doi: 10.1093/hmg/ddt292. [DOI] [PubMed] [Google Scholar]
- 61.Zhao W., Beers D.R., Hooten K.G., Sieglaff D.H., Zhang A., Kalyana-Sundaram S., Traini C.M., Halsey W.S., Hughes A.M., Sathe G.M., et al. Characterization of Gene Expression Phenotype in Amyotrophic Lateral Sclerosis Monocytes. JAMA Neurol. 2017;74:677–685. doi: 10.1001/jamaneurol.2017.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renton A.E., Chio A., Traynor B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Chalabi A., van den Berg L.H., Veldink J. Gene discovery in amyotrophic lateral sclerosis: Implications for clinical management. Nat. Rev. Neurol. 2017;13:96–104. doi: 10.1038/nrneurol.2016.182. [DOI] [PubMed] [Google Scholar]
- 64.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 65.Zou Z.Y., Zhou Z.R., Che C.H., Liu C.Y., He R.L., Huang H.P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2017;88:540–549. doi: 10.1136/jnnp-2016-315018. [DOI] [PubMed] [Google Scholar]
- 66.Van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., van den Berg L.H. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 67.Liguori M., Nuzziello N., Introna A., Consiglio A., Licciulli F., D’Errico E., Scarafino A., Distaso E., Simone I.L. Dysregulation of MicroRNAs and Target Genes Networks in Peripheral Blood of Patients With Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2018;11:288. doi: 10.3389/fnmol.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pillai R.S., Bhattacharyya S.N., Filipowicz W. Repression of protein synthesis by miRNAs: How many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Cloutier F., Marrero A., O’Connell C., Morin P., Jr. MicroRNAs as potential circulating biomarkers for amyotrophic lateral sclerosis. J. Mol. Neurosci. 2015;56:102–112. doi: 10.1007/s12031-014-0471-8. [DOI] [PubMed] [Google Scholar]
- 70.Bartel D.P. Metazoan MicroRNAs. Cell. 2018;173:20–51. doi: 10.1016/j.cell.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eichhorn C.D., Kang M., Feigon J. Structure and function of preQ1 riboswitches. Biochim. Biophys. Acta. 2014;1839:939–950. doi: 10.1016/j.bbagrm.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber J.A., Baxter D.H., Zhang S., Huang D.Y., Huang K.H., Lee M.J., Galas D.J., Wang K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozomara A., Griffiths-Jones S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Godlewski J., Lenart J., Salinska E. MicroRNA in Brain pathology: Neurodegeneration the Other Side of the Brain Cancer. Non-Coding RNA. 2019;5:20. doi: 10.3390/ncrna5010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quinlan S., Kenny A., Medina M., Engel T., Jimenez-Mateos E.M. MicroRNAs in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2017;334:309–343. doi: 10.1016/bs.ircmb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 76.Ferrante M., Conti G.O. Environment and Neurodegenerative Diseases: An Update on miRNA Role. MicroRNA. 2017;6:157–165. doi: 10.2174/2211536606666170811151503. [DOI] [PubMed] [Google Scholar]
- 77.Majdi A., Mahmoudi J., Sadigh-Eteghad S., Farhoudi M., Shotorbani S.S. The interplay of microRNAs and post-ischemic glutamate excitotoxicity: An emergent research field in stroke medicine. Neurol. Sci. 2016;37:1765–1771. doi: 10.1007/s10072-016-2643-5. [DOI] [PubMed] [Google Scholar]
- 78.Schonrock N., Humphreys D.T., Preiss T., Gotz J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-beta. J. Mol. Neurosci. 2012;46:324–335. doi: 10.1007/s12031-011-9587-2. [DOI] [PubMed] [Google Scholar]
- 79.Banelli B., Forlani A., Allemanni G., Morabito A., Pistillo M.P., Romani M. MicroRNA in Glioblastoma: An Overview. Int. J. Genom. 2017;2017:7639084. doi: 10.1155/2017/7639084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao F., Qu Y., Zhu J., Zhang L., Huang L., Liu H., Li S., Mu D. miR-30d-5p Plays an Important Role in Autophagy and Apoptosis in Developing Rat Brains After Hypoxic-Ischemic Injury. J. Neuropathol. Experimen. Neurol. 2017;76:709–719. doi: 10.1093/jnen/nlx052. [DOI] [PubMed] [Google Scholar]
- 81.Han L., Zhou Y., Zhang R., Wu K., Lu Y., Li Y., Duan R., Yao Y., Zhu D., Jia Y. MicroRNA Let-7f-5p Promotes Bone Marrow Mesenchymal Stem Cells Survival by Targeting Caspase-3 in Alzheimer Disease Model. Front. Neurosci. 2018;12:333. doi: 10.3389/fnins.2018.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J., Yoon H., Chung D.E., Brown J.L., Belmonte K.C., Kim J. miR-186 is decreased in aged brain and suppresses BACE1 expression. J. Neurochem. 2016;137:436–445. doi: 10.1111/jnc.13507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oh S.E., Park H.J., He L., Skibiel C., Junn E., Mouradian M.M. The Parkinson’s disease gene product DJ-1 modulates miR-221 to promote neuronal survival against oxidative stress. Redox Biol. 2018;19:62–73. doi: 10.1016/j.redox.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salimian N., Peymani M., Ghaedi K., Nasr Esfahani M.H. Modulation in miR-200a/SIRT1axis is associated with apoptosis in MPP(+)-induced SH-SY5Y cells. Gene. 2018;674:25–30. doi: 10.1016/j.gene.2018.06.061. [DOI] [PubMed] [Google Scholar]
- 85.Li L., Xu J., Wu M., Hu J.M. Protective role of microRNA-221 in Parkinson’s disease. Bratisl. Lek. Listy. 2018;119:22–27. doi: 10.4149/BLL_2018_005. [DOI] [PubMed] [Google Scholar]
- 86.Je G., Kim Y.S. Mitochondrial ROS-mediated post-transcriptional regulation of alpha-synuclein through miR-7 and miR-153. Neurosci. Lett. 2017;661:132–136. doi: 10.1016/j.neulet.2017.09.065. [DOI] [PubMed] [Google Scholar]
- 87.Song S., Lin F., Zhu P., Wu C., Zhao S., Han Q., Li X. Extract of Spatholobus suberctus Dunn ameliorates ischemia-induced injury by targeting miR-494. PLoS ONE. 2017;12:e0184348. doi: 10.1371/journal.pone.0184348. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Jin R., Xu S., Lin X., Shen M. MiR-136 controls neurocytes apoptosis by regulating Tissue Inhibitor of Metalloproteinases-3 in spinal cord ischemic injury. Biomed. Pharm. 2017;94:47–54. doi: 10.1016/j.biopha.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 89.Salta E., De Strooper B. microRNA-132: A key noncoding RNA operating in the cellular phase of Alzheimer’s disease. FASEB J. 2017;31:424–433. doi: 10.1096/fj.201601308. [DOI] [PubMed] [Google Scholar]
- 90.Kunkanjanawan T., Carter R.L., Prucha M.S., Yang J., Parnpai R., Chan A.W. miR-196a Ameliorates Cytotoxicity and Cellular Phenotype in Transgenic Huntington’s Disease Monkey Neural Cells. PLoS ONE. 2016;11:e0162788. doi: 10.1371/journal.pone.0162788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fu M.H., Li C.L., Lin H.L., Tsai S.J., Lai Y.Y., Chang Y.F., Cheng P.H., Chen C.M., Yang S.H. The Potential Regulatory Mechanisms of miR-196a in Huntington’s Disease through Bioinformatic Analyses. PLoS ONE. 2015;10:e0137637. doi: 10.1371/journal.pone.0137637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hwang J.Y., Kaneko N., Noh K.M., Pontarelli F., Zukin R.S. The gene silencing transcription factor REST represses miR-132 expression in hippocampal neurons destined to die. J. Mol. Biol. 2014;426:3454–3466. doi: 10.1016/j.jmb.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang C., Ji B., Cheng B., Chen J., Bai B. Neuroprotection of microRNA in neurological disorders (Review) Biomed. Rep. 2014;2:611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heman-Ackah S.M., Hallegger M., Rao M.S., Wood M.J. RISC in PD: The impact of microRNAs in Parkinson’s disease cellular and molecular pathogenesis. Front. Mol. Neurosci. 2013;6:40. doi: 10.3389/fnmol.2013.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Junn E., Lee K.W., Jeong B.S., Chan T.W., Im J.Y., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parisi C., Arisi I., D’Ambrosi N., Storti A.E., Brandi R., D’Onofrio M., Volonte C. Dysregulated microRNAs in amyotrophic lateral sclerosis microglia modulate genes linked to neuroinflammation. Cell Death Dis. 2013;4:e959. doi: 10.1038/cddis.2013.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun L.Q., Guo G.L., Zhang S., Yang L.L. Effects of MicroRNA-592-5p on Hippocampal Neuron Injury Following Hypoxic-Ischemic Brain Damage in Neonatal Mice—Involvement of PGD2/DP and PTGDR. Cell. Physiol. Biochem. 2018;45:458–473. doi: 10.1159/000486923. [DOI] [PubMed] [Google Scholar]
- 98.Liang L., Wang J., Yuan Y., Zhang Y., Liu H., Wu C., Yan Y. MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed. Pharm. 2018;102:86–93. doi: 10.1016/j.biopha.2018.03.036. [DOI] [PubMed] [Google Scholar]
- 99.Harraz M.M., Eacker S.M., Wang X., Dawson T.M., Dawson V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA. 2012;109:18962–18967. doi: 10.1073/pnas.1121288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiong R., Wang Z., Zhao Z., Li H., Chen W., Zhang B., Wang L., Wu L., Li W., Ding J., et al. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiol. Aging. 2014;35:705–714. doi: 10.1016/j.neurobiolaging.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 101.Yang K., Yu B., Cheng C., Cheng T., Yuan B., Li K., Xiao J., Qiu Z., Zhou Y. Mir505-3p regulates axonal development via inhibiting the autophagy pathway by targeting Atg12. Autophagy. 2017;13:1679–1696. doi: 10.1080/15548627.2017.1353841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng S.J., Zhang X.Q., Li J.T., Dai X.M., Zhao F. miRNA-223 regulates ischemic neuronal injury by targeting the type 1 insulin-like growth factor receptor (IGF1R) Folia Neuropathol. 2018;56:49–57. doi: 10.5114/fn.2018.74659. [DOI] [PubMed] [Google Scholar]
- 103.Pichler S., Gu W., Hartl D., Gasparoni G., Leidinger P., Keller A., Meese E., Mayhaus M., Hampel H., Riemenschneider M. The miRNome of Alzheimer’s disease: Consistent downregulation of the miR-132/212 cluster. Neurobiol. Aging. 2017;50:167.e1–167.e10. doi: 10.1016/j.neurobiolaging.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Wang Q., Zhan Y., Ren N., Wang Z., Zhang Q., Wu S., Li H. Paraquat and MPTP alter microRNA expression profiles, and downregulated expression of miR-17-5p contributes to PQ-induced dopaminergic neurodegeneration. J. Appl. Toxicol. JAT. 2018;38:665–677. doi: 10.1002/jat.3571. [DOI] [PubMed] [Google Scholar]
- 105.Xu L.J., Ouyang Y.B., Xiong X., Stary C.M., Giffard R.G. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Experimen. Neurol. 2015;264:1–7. doi: 10.1016/j.expneurol.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mezache L., Mikhail M., Garofalo M., Nuovo G.J. Reduced miR-512 and the Elevated Expression of Its Targets cFLIP and MCL1 Localize to Neurons With Hyperphosphorylated Tau Protein in Alzheimer Disease. Appl. Immunohistochem. Mol. Morphol. 2015;23:615–623. doi: 10.1097/PAI.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 107.Yang Z.B., Zhang Z., Li T.B., Lou Z., Li S.Y., Yang H., Yang J., Luo X.J., Peng J. Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin. Sci. (Lond.) 2014;127:679–689. doi: 10.1042/CS20140084. [DOI] [PubMed] [Google Scholar]
- 108.Hoye M.L., Koval E.D., Wegener A.J., Hyman T.S., Yang C., O’Brien D.R., Miller R.L., Cole T., Schoch K.M., Shen T., et al. MicroRNA Profiling Reveals Marker of Motor Neuron Disease in ALS Models. J. Neurosci. 2017;37:5574–5586. doi: 10.1523/JNEUROSCI.3582-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Butovsky O., Siddiqui S., Gabriely G., Lanser A.J., Dake B., Murugaiyan G., Doykan C.E., Wu P.M., Gali R.R., Iyer L.K., et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Investig. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rao P., Benito E., Fischer A. MicroRNAs as biomarkers for CNS disease. Front. Mol. Neurosci. 2013;6:39. doi: 10.3389/fnmol.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Felice B., Guida M., Guida M., Coppola C., De Mieri G., Cotrufo R. A miRNA signature in leukocytes from sporadic amyotrophic lateral sclerosis. Gene. 2012;508:35–40. doi: 10.1016/j.gene.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 112.Freischmidt A., Muller K., Ludolph A.C., Weishaupt J.H. Systemic dysregulation of TDP-43 binding microRNAs in amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2013;1:42. doi: 10.1186/2051-5960-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toivonen J.M., Manzano R., Olivan S., Zaragoza P., Garcia-Redondo A., Osta R. MicroRNA-206: A potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS ONE. 2014;9:e89065. doi: 10.1371/journal.pone.0089065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Felice B., Annunziata A., Fiorentino G., Borra M., Biffali E., Coppola C., Cotrufo R., Brettschneider J., Giordana M.L., Dalmay T., et al. miR-338-3p is over-expressed in blood, CFS, serum and spinal cord from sporadic amyotrophic lateral sclerosis patients. Neurogenetics. 2014;15:243–253. doi: 10.1007/s10048-014-0420-2. [DOI] [PubMed] [Google Scholar]
- 115.Freischmidt A., Muller K., Zondler L., Weydt P., Volk A.E., Bozic A.L., Walter M., Bonin M., Mayer B., von Arnim C.A., et al. Serum microRNAs in patients with genetic amyotrophic lateral sclerosis and pre-manifest mutation carriers. Brain J. Neurol. 2014;137:2938–2950. doi: 10.1093/brain/awu249. [DOI] [PubMed] [Google Scholar]
- 116.Freischmidt A., Muller K., Zondler L., Weydt P., Mayer B., von Arnim C.A., Hubers A., Dorst J., Otto M., Holzmann K., et al. Serum microRNAs in sporadic amyotrophic lateral sclerosis. Neurobiol. Aging. 2015;36:2660.e15–2660.e20. doi: 10.1016/j.neurobiolaging.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi I., Hama Y., Matsushima M., Hirotani M., Kano T., Hohzen H., Yabe I., Utsumi J., Sasaki H. Identification of plasma microRNAs as a biomarker of sporadic Amyotrophic Lateral Sclerosis. Mol. Brain. 2015;8:67. doi: 10.1186/s13041-015-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen Y., Wei Q., Chen X., Li C., Cao B., Ou R., Hadano S., Shang H.F. Aberration of miRNAs Expression in Leukocytes from Sporadic Amyotrophic Lateral Sclerosis. Front. Mol. Neurosci. 2016;9:69. doi: 10.3389/fnmol.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Andrade H.M., de Albuquerque M., Avansini S.H., Rocha C.D.S., Dogini D.B., Nucci A., Carvalho B., Lopes-Cendes I., Franca M.C., Jr. MicroRNAs-424 and 206 are potential prognostic markers in spinal onset amyotrophic lateral sclerosis. J. Neurol. Sci. 2016;368:19–24. doi: 10.1016/j.jns.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 120.Tasca E., Pegoraro V., Merico A., Angelini C. Circulating microRNAs as biomarkers of muscle differentiation and atrophy in ALS. Clin. Neuropathol. 2016;35:22–30. doi: 10.5414/NP300889. [DOI] [PubMed] [Google Scholar]
- 121.Sheinerman K.S., Toledo J.B., Tsivinsky V.G., Irwin D., Grossman M., Weintraub D., Hurtig H.I., Chen-Plotkin A., Wolk D.A., McCluskey L.F., et al. Circulating brain-enriched microRNAs as novel biomarkers for detection and differentiation of neurodegenerative diseases. Alzheimer’s Res. 2017;9:89. doi: 10.1186/s13195-017-0316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Waller R., Goodall E.F., Milo M., Cooper-Knock J., Da Costa M., Hobson E., Kazoka M., Wollff H., Heath P.R., Shaw P.J., et al. Serum miRNAs miR-206, 143-3p and 374b-5p as potential biomarkers for amyotrophic lateral sclerosis (ALS) Neurobiol. Aging. 2017;55:123–131. doi: 10.1016/j.neurobiolaging.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Raheja R., Regev K., Healy B.C., Mazzola M.A., Beynon V., Von Glehn F., Paul A., Diaz-Cruz C., Gholipour T., Glanz B.I., et al. Correlating serum micrornas and clinical parameters in amyotrophic lateral sclerosis. Muscle Nerve. 2018;58:261–269. doi: 10.1002/mus.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu Q., Zhao Y., Zhou X., Luan J., Cui Y., Han J. Comparison of the extraction and determination of serum exosome and miRNA in serum and the detection of miR-27a-3p in serum exosome of ALS patients. Intractable Rare Dis. Res. 2018;7:13–18. doi: 10.5582/irdr.2017.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matamala J.M., Arias-Carrasco R., Sanchez C., Uhrig M., Bargsted L., Matus S., Maracaja-Coutinho V., Abarzua S., van Zundert B., Verdugo R., et al. Genome-wide circulating microRNA expression profiling reveals potential biomarkers for amyotrophic lateral sclerosis. Neurobiol. Aging. 2018;64:123–138. doi: 10.1016/j.neurobiolaging.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 126.Saucier D., Wajnberg G., Roy J., Beauregard A.P., Chacko S., Crapoulet N., Fournier S., Ghosh A., Lewis S.M., Marrero A., et al. Identification of a circulating miRNA signature in extracellular vesicles collected from amyotrophic lateral sclerosis patients. Brain Res. 2019;1708:100–108. doi: 10.1016/j.brainres.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 127.Loeffler J.P., Picchiarelli G., Dupuis L., Gonzalez De Aguilar J.L. The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis. Brain Pathol. 2016;26:227–236. doi: 10.1111/bpa.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bradley W.G., Good P., Rasool C.G., Adelman L.S. Morphometric and biochemical studies of peripheral nerves in amyotrophic lateral sclerosis. Ann. Neurol. 1983;14:267–277. doi: 10.1002/ana.410140304. [DOI] [PubMed] [Google Scholar]
- 129.Dyck P.J., Stevens J.C., Mulder D.W., Espinosa R.E. Frequency of nerve fiber degeneration of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. Morphometry of deep and superficial peroneal nerves. Neurology. 1975;25:781–785. doi: 10.1212/WNL.25.8.781. [DOI] [PubMed] [Google Scholar]
- 130.Schaefer A.M., Sanes J.R., Lichtman J.W. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J. Comp. Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- 131.Di Pietro L., Lattanzi W., Bernardini C. Skeletal Muscle MicroRNAs as Key Players in the Pathogenesis of Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2018;19:1534. doi: 10.3390/ijms19051534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nie M., Deng Z.L., Liu J., Wang D.Z. Noncoding RNAs, Emerging Regulators of Skeletal Muscle Development and Diseases. Biomed Res. Int. 2015;2015:676575. doi: 10.1155/2015/676575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Williams A.H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J.L., Bassel-Duby R., Sanes J.R., Olson E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science (New York) 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Song Y., Zhang F., Li L., Song B., Xu J., Wang G., Zheng Z. MiR-338-3p inhibits growth of glioblastoma through targeting MAP4K3. Minerva Med. 2019 doi: 10.23736/S0026-4806.19.06251-7. [DOI] [PubMed] [Google Scholar]
- 135.Zhang R., Shi H., Ren F., Feng W., Cao Y., Li G., Liu Z., Ji P., Zhang M. MicroRNA-338-3p suppresses ovarian cancer cells growth and metastasis: Implication of Wnt/catenin beta and MEK/ERK signaling pathways. J. Exp. Clin. Cancer Res. 2019;38:494. doi: 10.1186/s13046-019-1494-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Zou T., Duan J., Liang J., Shi H., Zhen T., Li H., Zhang F., Dong Y., Han A. miR-338-3p suppresses colorectal cancer proliferation and progression by inhibiting MACC1. Int. J. Clin. Exp. Pathol. 2018;11:2256–2267. [PMC free article] [PubMed] [Google Scholar]
- 137.Howe J.R., Li E.S., Streeter S.E., Rahme G.J., Chipumuro E., Russo G.B., Litzky J.F., Hills L.B., Rodgers K.R., Skelton P.D., et al. MiR-338-3p regulates neuronal maturation and suppresses glioblastoma proliferation. PLoS ONE. 2017;12:e0177661. doi: 10.1371/journal.pone.0177661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dugas J.C., Cuellar T.L., Scholze A., Ason B., Ibrahim A., Emery B., Zamanian J.L., Foo L.C., McManus M.T., Barres B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhao X., He X., Han X., Yu Y., Ye F., Chen Y., Hoang T., Xu X., Mi Q.S., Xin M., et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maragakis N.J., Dykes-Hoberg M., Rothstein J.D. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann. Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- 141.Li C., Wei Q., Gu X., Chen Y., Chen X., Cao B., Ou R., Shang H. Decreased Glycogenolysis by miR-338-3p Promotes Regional Glycogen Accumulation Within the Spinal Cord of Amyotrophic Lateral Sclerosis Mice. Front. Mol. Neurosci. 2019;12:114. doi: 10.3389/fnmol.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Diez-Planelles C., Sanchez-Lozano P., Crespo M.C., Gil-Zamorano J., Ribacoba R., Gonzalez N., Suarez E., Martinez-Descals A., Martinez-Camblor P., Alvarez V., et al. Circulating microRNAs in Huntington’s disease: Emerging mediators in metabolic impairment. Pharmacol. Res. 2016;108:102–110. doi: 10.1016/j.phrs.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 143.Ma Y., Liang A.J., Fan Y.P., Huang Y.R., Zhao X.M., Sun Y., Chen X.F. Dysregulation and functional roles of miR-183-96-182 cluster in cancer cell proliferation, invasion and metastasis. Oncotarget. 2016;7:42805–42825. doi: 10.18632/oncotarget.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang X.L., Pan S.H., Yan J.J., Xu G. The prognostic value of microRNA-183 in human cancers: A meta-analysis. Medicine. 2018;97:e11213. doi: 10.1097/MD.0000000000011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Song C.J., Chen H., Chen L.Z., Ru G.M., Guo J.J., Ding Q.N. The potential of microRNAs as human prostate cancer biomarkers: A meta-analysis of related studies. J. Cell. Biochem. 2018;119:2763–2786. doi: 10.1002/jcb.26445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ichiyama K., Dong C. The role of miR-183 cluster in immunity. Cancer Lett. 2019;443:108–114. doi: 10.1016/j.canlet.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 147.Banks S.A., Pierce M.L., Soukup G.A. Sensational MicroRNAs: Neurosensory Roles of the MicroRNA-183 Family. Mol. Neurobiol. 2020;57:358–371. doi: 10.1007/s12035-019-01717-3. [DOI] [PubMed] [Google Scholar]
- 148.Jawaid A., Woldemichael B.T., Kremer E.A., Laferriere F., Gaur N., Afroz T., Polymenidou M., Mansuy I.M. Memory Decline and Its Reversal in Aging and Neurodegeneration Involve miR-183/96/182 Biogenesis. Mol. Neurobiol. 2019;56:3451–3462. doi: 10.1007/s12035-018-1314-3. [DOI] [PubMed] [Google Scholar]
- 149.Bai H., Wu S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. OncoTargets Ther. 2019;12:11069–11082. doi: 10.2147/OTT.S230963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bronisz A., Chiocca E.A., Godlewski J. Response to energy depletion: miR-451/AMPK loop. Oncotarget. 2015;6:17851–17852. doi: 10.18632/oncotarget.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ogawa D., Ansari K., Nowicki M.O., Salinska E., Bronisz A., Godlewski J. MicroRNA-451 Inhibits Migration of Glioblastoma while Making It More Susceptible to Conventional Therapy. Non-Coding RNA. 2019;5:25. doi: 10.3390/ncrna5010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sun X., Zhang H. miR-451 elevation relieves inflammatory pain by suppressing microglial activation-evoked inflammatory response via targeting TLR4. Cell Tissue Res. 2018;374:487–495. doi: 10.1007/s00441-018-2898-7. [DOI] [PubMed] [Google Scholar]
- 153.Chirshev E., Oberg K.C., Ioffe Y.J., Unternaehrer J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019;8:24. doi: 10.1186/s40169-019-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Goh S.Y., Chao Y.X., Dheen S.T., Tan E.K., Tay S.S. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019;20:5649. doi: 10.3390/ijms20225649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Slota J.A., Booth S.A. MicroRNAs in Neuroinflammation: Implications in Disease Pathogenesis, Biomarker Discovery and Therapeutic Applications. Non-Coding RNA. 2019;5 doi: 10.3390/ncrna5020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang Z.Y., Wang Y., Liu Q., Wu M. microRNA cluster MC-let-7a-1~let-7d promotes autophagy and apoptosis of glioma cells by down-regulating STAT3. CNS Neurosci. Ther. 2019;00:1–13. doi: 10.1111/cns.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Parisi C., Napoli G., Amadio S., Spalloni A., Apolloni S., Longone P., Volonte C. MicroRNA-125b regulates microglia activation and motor neuron death in ALS. Cell Death Differ. 2016;23:531–541. doi: 10.1038/cdd.2015.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fernandes J.C.R., Acuna S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Non-Coding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dharap A., Nakka V.P., Vemuganti R. Effect of focal ischemia on long noncoding RNAs. Stroke. 2012;43:2800–2802. doi: 10.1161/STROKEAHA.112.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Riva P., Ratti A., Venturin M. The Long Non-Coding RNAs in Neurodegenerative Diseases: Novel Mechanisms of Pathogenesis. Curr. Alzheimer Res. 2016;13:1219–1231. doi: 10.2174/1567205013666160622112234. [DOI] [PubMed] [Google Scholar]
- 162.An H., Williams N.G., Shelkovnikova T.A. NEAT1 and paraspeckles in neurodegenerative diseases: A missing lnc found? Non-Coding RNA Res. 2018;3:243–252. doi: 10.1016/j.ncrna.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mizielinska S., Lashley T., Norona F.E., Clayton E.L., Ridler C.E., Fratta P., Isaacs A.M. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol. 2013;126:845–857. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nishimoto Y., Nakagawa S., Hirose T., Okano H.J., Takao M., Shibata S., Suyama S., Kuwako K., Imai T., Murayama S., et al. The long non-coding RNA nuclear-enriched abundant transcript 1_2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis. Mol. Brain. 2013;6:31. doi: 10.1186/1756-6606-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li P.P., Sun X., Xia G., Arbez N., Paul S., Zhu S., Peng H.B., Ross C.A., Koeppen A.H., Margolis R.L., et al. ATXN2-AS, a gene antisense to ATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis. Ann. Neurol. 2016;80:600–615. doi: 10.1002/ana.24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen L.L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 167.Rong D., Sun H., Li Z., Liu S., Dong C., Fu K., Tang W., Cao H. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget. 2017;8:73271–73281. doi: 10.18632/oncotarget.19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xie L., Mao M., Xiong K., Jiang B. Circular RNAs: A Novel Player in Development and Disease of the Central Nervous System. Front. Cell. Neurosci. 2017;11:354. doi: 10.3389/fncel.2017.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., Maier L., Mackowiak S.D., Gregersen L.H., Munschauer M., et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 170.Van Rossum D., Verheijen B.M., Pasterkamp R.J. Circular RNAs: Novel Regulators of Neuronal Development. Front. Mol. Neurosci. 2016;9:74. doi: 10.3389/fnmol.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao Y., Alexandrov P.N., Jaber V., Lukiw W.J. Deficiency in the Ubiquitin Conjugating Enzyme UBE2A in Alzheimer’s Disease (AD) is Linked to Deficits in a Natural Circular miRNA-7 Sponge (circRNA; ciRS-7) Genes. 2016;7:116. doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sang Q., Liu X., Wang L., Qi L., Sun W., Wang W., Sun Y., Zhang H. CircSNCA downregulation by pramipexole treatment mediates cell apoptosis and autophagy in Parkinson’s disease by targeting miR-7. Aging. 2018;10:1281–1293. doi: 10.18632/aging.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfo R., Peruzzi G., et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dolinar A., Koritnik B., Glavac D., Ravnik-Glavac M. Circular RNAs as Potential Blood Biomarkers in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019;56:8052–8062. doi: 10.1007/s12035-019-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eom G.H., Kim K.B., Kim J.H., Kim J.Y., Kim J.R., Kee H.J., Kim D.W., Choe N., Park H.J., Son H.J., et al. Histone methyltransferase SETD3 regulates muscle differentiation. J. Biol. Chem. 2011;286:34733–34742. doi: 10.1074/jbc.M110.203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tebar F., Bohlander S.K., Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: Localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Mol. Biol. Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dudekula D.B., Panda A.C., Grammatikakis I., De S., Abdelmohsen K., Gorospe M. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13:34–42. doi: 10.1080/15476286.2015.1128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhou F., Guan Y., Chen Y., Zhang C., Yu L., Gao H., Du H., Liu B., Wang X. miRNA-9 expression is upregulated in the spinal cord of G93A-SOD1 transgenic mice. Int. J. Clin. Exp. Pathol. 2013;6:1826–1838. [PMC free article] [PubMed] [Google Scholar]
- 179.Nishi K., Nishi A., Nagasawa T., Ui-Tei K. Human TNRC6A is an Argonaute-navigator protein for microRNA-mediated gene silencing in the nucleus. RNA. 2013;19:17–35. doi: 10.1261/rna.034769.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Campos-Melo D., Droppelmann C.A., He Z., Volkening K., Strong M.J. Altered microRNA expression profile in Amyotrophic Lateral Sclerosis: A role in the regulation of NFL mRNA levels. Mol. Brain. 2013;6:26. doi: 10.1186/1756-6606-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schymick J.C., Scholz S.W., Fung H.C., Britton A., Arepalli S., Gibbs J.R., Lombardo F., Matarin M., Kasperaviciute D., Hernandez D.G., et al. Genome-wide genotyping in amyotrophic lateral sclerosis and neurologically normal controls: First stage analysis and public release of data. Lancet Neurol. 2007;6:322–328. doi: 10.1016/S1474-4422(07)70037-6. [DOI] [PubMed] [Google Scholar]
- 182.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 183.Hawley Z.C.E., Campos-Melo D., Strong M.J. MiR-105 and miR-9 regulate the mRNA stability of neuronal intermediate filaments. Implications for the pathogenesis of amyotrophic lateral sclerosis (ALS) Brain Res. 2019;1706:93–100. doi: 10.1016/j.brainres.2018.10.032. [DOI] [PubMed] [Google Scholar]