Abstract
Prostaglandins (PGs) are a family of lipid compounds that are derived from arachidonic acid via the cyclooxygenase pathway, and consist of PGD2, PGI2, PGE2, PGF2, and thromboxane B2. PGs signal through G-protein coupled receptors, and individual PGs affect allergic inflammation through different mechanisms according to the receptors with which they are associated. In this review article, we have focused on the metabolism of the cyclooxygenase pathway, and the distinct biological effect of each PG type on various cell types involved in allergic airway diseases, including asthma, allergic rhinitis, nasal polyposis, and aspirin-exacerbated respiratory disease.
Keywords: prostaglandins, allergy, asthma, allergic rhinitis, AERD, PGD2, PGE2
1. Introduction
Prostaglandins (PGs) are lipid mediators, generated from arachidonic acid (AA) metabolism via cyclooxygenases (COX). They were discovered in the 1930s as regulators of blood pressure and smooth muscle contraction [1]. The distribution of synthases and receptors for each PG is different in various cell types, and PGs are activated via either paracrine or autocrine signaling on the surface of each cell type [2]. PGs bridge the interactions between various immune-modulating cells, and are considered key players in regulating pro-inflammatory and anti-inflammatory responses [3]. The role of PGs in allergic disease was first introduced in an article published in 1969, which reported the release of PGE2 and PGF2α during anaphylaxis in an animal model [4]. Since then, numerous studies have reported on the role of PGs in allergic diseases, and technologies have been developed to produce stable analogs and generate genetically engineered animal models [5].
Allergic reactions are characterized as immunoglobulin E (IgE)-dependent mechanisms, orchestrated by various cellular components and resulting in antigen-induced type 2 inflammation. Allergic diseases in the upper and lower airways include asthma, allergic rhinitis, and nasal polyposis. In airway allergic reactions, antigens that have penetrated epithelial layers are engulfed by dendritic cells (DCs), inducing them to mature and migrate to the lymph nodes, where they activate naïve T cells into effecter T helper 2 (Th2) cells [6]. Cytokines such as interleukin-4 (IL-4), IL-5, and IL-13 are released from Th2 cells or type 2 innate lymphoid (ILC2) cells. These cytokines, as well as epithelial-derived cytokines such as thymic stromal lymphopoietin (TSLP) and IL-33, activate the mast cells, eosinophils, and other structural cells, including smooth muscle cells and fibroblasts. These cells can also interact with each other to induce hyperreactivity [7]. Although aspirin-exacerbated respiratory disease (AERD) is not induced by the IgE-mediated hypersensitivity reaction, it is characterized by asthma, eosinophilic nasal polyposis, and nonsteroidal anti-inflammatory drug (NSAID) sensitivity, and it is a serious airway disease that affects both the upper and the lower airways [8]. AERD is dominantly regulated by lipid mediators such as PGs and leukotrienes (LTs) because the disease pathogenesis is characterized by alteration of AA metabolism [9,10].
Although PGs and their role in allergic reactions have been known about for a long time, the development of novel drugs targeting their signaling and metabolism is currently in progress, and clinical studies applying these to allergic airway diseases are being undertaken. In this review, we will discuss the potential of PGs to serve as therapeutic targets in allergic airway inflammation of the upper and lower airways. Based on an introduction of the pathways of PG generation, and the mechanisms of individual PGs in important cellular allergic reactions, we will present the regulation of PGs in clinical settings and the effects of pharmacological agents that modulate PG activation in various airway diseases such as asthma, allergic rhinitis, AERD, and nasal polyposis.
2. Biosynthesis of Prostaglandins
2.1. Phospholipase A2 and Arachidonic Acid
All PGs and LTs derived from AA are called “eicosanoids,” following the Greek word “eikosi,” meaning “20,” referring to the number of carbon atoms in AA. Free fatty acids, including AA, are obtained from the hydrolysis of fatty acids by phospholipase A2 (PLA2) at the sn-2 position of membrane phospholipids [11]. PLA2 enzymes are classified into six major groups: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2), Ca2+ dependent PLA2 (iPLA2), platelet-activating factor acetylhydrolases (PAF-AH), lysosomal PLA2, and adipose-specific PLA2 [12]. In addition, based on their catalytic mechanisms, functions, and structures, they are further categorized into 16 groups. Among them, groups IIA/V/X of the sPLA2 group, group IVA of cPLA2, and group VI of iPLA2 lead to AA production by hydrolyzing membrane phospholipids, which are then metabolized sequentially to produce PGs and LTs [13].
2.2. The Cyclooxygenase Pathway
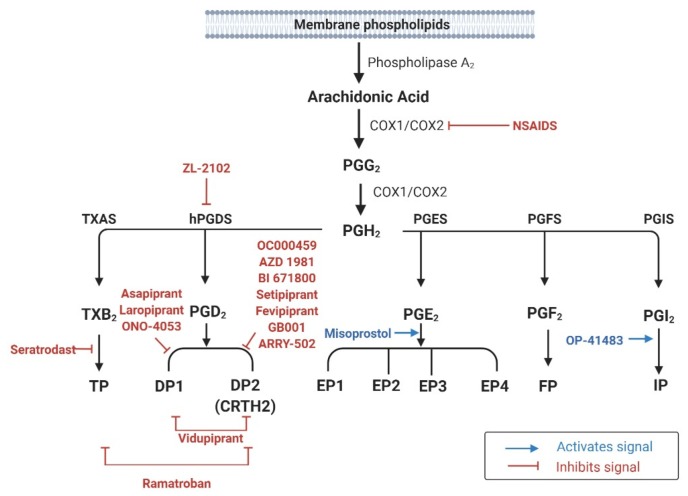
PGs are derived from the COX pathway [14]. COX catalyzes the initial reaction in the generation of PGG2 by inserting two oxygen molecules into AA (Figure 1). Thereafter, sequential peroxidase reactions transform PGG2 into PGH2, the precursor for PGD2, PGE2, PGF2α, PGI2, and thromboxane A2 (TXA2) [14].
Figure 1.
Prostaglandin biosynthesis pathways and pharmacologic agents used in clinical trials for human respiratory allergic diseases.
In humans, COX-1 and COX-2 comprise the functional COX enzymes, whereas COX-3, which is encoded by COX-1, does not have known function yet. The two homologous COX enzymes have distinct genetic locations and roles in inflammatory reactions. In humans, COX-1 and COX-2 are located on chromosomes 9 and 1, respectively [14]. The expression of COX-1 is constitutive in most tissues, where it participates in the synthesis of homeostatic PGs [15]. However, COX-2 expression is usually transient, and induced by cellular stress or inflammatory reactions, for example after stimulation by lipopolysaccharide (LPS) or the secretion of cytokines, such as interleukin (IL)-1, IL-2, and tumor necrosis factor (TNF)-α. COX-2 has been proposed to have a central role in the production of a wide range of PGs during the inflammatory response [15].
2.3. Generation of Individual PGs and Their Receptors
PGH2 is believed to be converted immediately to its downstream product rather than accumulating in cells. Except for PGF2α, PGH2 metabolites such as PGD2, PGE2, PGI2, and TXA2 require specific synthases (Figure 1) [14]. All PGs elicit their biological effects by activating cell surface G protein-coupled receptors (GPCRs)—seven transmembrane spanning receptors [16]. PG receptors are classified into nine subfamilies (DP1, DP2, EP1–4, FP, IP, and TP) according to their affinities for individual PGs (Table 1) [17].
Table 1.
Prostaglandins and their specific receptors, downstream signaling, and region of expression.
| Ligands | Production | Receptor | Downstream Signaling |
Receptor Expression |
|---|---|---|---|---|
| PGD2 | mast cells, eosinophils, T cells, dendritic cells, macrophages, endothelial cells, platelets, lung parenchyma | DP1 | ↑cAMP | mucus-secreting goblet cells, nasal serous glands, vascular endothelial cells, T cells, dendritic cells, eosinophils |
| DP2 (CRTH2) |
↓cAMP, ↑Ca2+ | T cells, basophils, eosinophils, ILC2 | ||
| PGE2 | epithelial cells, fibroblasts, macrophage, smooth muscle cells, platelets | EP1 | ↑Ca2+ | T cells, dendritic cells, B cells, smooth muscle cells |
| EP2 | ↑cAMP | T cells, dendritic cells, B cells, ILC2, mast cells, basophils, smooth muscle cells | ||
| EP3 | ↓cAMP | T cells, B cells, dendritic cells, smooth muscle cells | ||
| EP4 | ↑cAMP | T cells, B cells, dendritic cells, smooth muscle cells | ||
| PGF2α | lung parenchyma, vascular smooth muscle cells, peripheral blood lymphocytes | FP | ↑IP3/DAG/Ca2+ | none |
| PGI2 | endothelial cells, vascular smooth muscle cells, lung parenchyma | IP | ↑cAMP | T cells, dendritic cells, B cells, ILC2, endothelial cells, platelets |
| TXA2 | platelets, vascular smooth muscle cells, macrophages | TP | ↑IP3/DAG/Ca2+, ↑↓cAMP |
megakaryocytes, monocytes |
PG—prostaglandin; cAMP—cyclic adenosine 3′,5′-monophosphate; ILC—innate lymphoid cell; IP—Inositol trisphosphate; DAG—diacylglycerol. An upward arrow (↑) indicates an increased intracellular signaling pathway, and a downward arrow (↓) denotes a decrease in an intracellular signaling pathway.
Two groups of synthases are involved in PGD2 production—hematopoietic-PGD2 synthases (H-PGDS) and lipocalin-PGD2 synthases (L-PGDS) [18]—of which only H-PGDS are responsible for PGD2 production by mast cells or other hematopoietic cells, such as eosinophils [19,20]. In humans, H-PGDS are abundantly expressed in the lungs, adipose tissue, placenta (at the tissue level), mast cells, T cells, dendritic cells, and megakaryocytes (at the cellular level) [18]. PGD2 signals through two receptors: DP1 and DP2 (also known as CRTH2). In the airways, DP1 is expressed in the nasal mucous-secreting goblet cells, nasal serous glands, vascular endothelium, DCs, T cells, basophils, and eosinophils [21]. DP2 expression has been observed in both hematopoietic cells such as T cells, ILC2s, mast cells, basophils, and eosinophils, as well as in structural cells such as epithelial cells and smooth muscle cells [22]. Stimulation of DP1 increases the intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) level, which is believed to inhibit target cell functions and suppress innate immune functions, for example the generation of inflammatory mediators. Conversely, PGD2 signaling through DP2 decreases the cAMP level [14,23]. In addition to these functions PGD2 can activate the thromboxane receptor (TP), even at low concentrations [24].
Synthesis of PGE2 involves three distinct PGH2-metabolizing enzymes: microsomal PGE synthase (mPGES)-1 and -2, and cytosolic PGE synthase (cPGES) [14]. mPGES-1 is glutathione-dependent, with an inducible expression, and it preferentially catalyzes PGH2 produced by COX-2. In contrast, mPGES-2 utilizes PGE2 derived from either COX-1 or COX-2, and it shows constitutive expression in various cell types, including human embryonic kidney cells (HEK293), human colon adenocarcinoma cells (HCA-7) and human lung epithelial cells (BEAS-2B) [25]. cPGES is also expressed constitutively, and it generates PGE2 using COX-1, rather than COX-2. Four distinct receptors, termed EP receptors 1–4, are activated by PGE2, and are present in various organs associated with allergic reactions, including the lungs [26]. Stimulation of the EP1 receptor causes increase in inositol triphosphate (IP3) and diacylglycerol, followed by increase in intracellular calcium concentrations. Signaling through the EP2 and EP4 receptors is known to increase the intracellular cAMP level, whereas EP3 activation results in decreased cAMP synthesis in cells [14].
The formation of PGF2α is catalyzed by PGF synthase (PGFS) via two pathways, utilizing NADPH and PGD2, respectively [27]. PGFS has been identified in lung and peripheral blood lymphocytes, suggesting their role in inducing airway allergic reactions [27]. A single receptor, termed FP, mediates PGF2α signaling. FP has high affinity for PGF2, and its stimulation results in an increase in intracellular calcium concentrations [16].
PGI2 is generated from PGH2 by PGI synthase, which is highly expressed in the lungs, smooth muscle, heart, kidneys, and ovaries [28]. PGI2 activates adenylate cyclase and increases intracellular cAMP levels by stimulating its receptor, known as IP [16], which is strongly expressed in the thymus, lungs, heart, spleen, and neurons [28].
TXA2 accounts for the majority of AA metabolites, but it is unstable in nature, with a short half-life of 30 s [26]. TXA2 is hydrolyzed to thromboxane B2 (TXB2) in the absence of an enzyme. The synthase that catalyzes the formation of TXA2 from PHG2 is named thromboxane synthase, which shows strong expression in the lungs, kidneys, liver, monocytes, and megakaryocytes [29]. TXA2 is predominantly produced by cells such as platelets, neutrophils, monocytes, macrophages, and lung parenchyma cells [30]. TXA2 signals through its receptor TP, which has two isoforms, TPα and TPβ. Although both of these isoforms lead to phospholipase C activation, calcium release, and protein kinase C activation, they have opposing functions when coupled to a Gq protein: TPα activates adenylate cyclase, followed by increased cAMP levels and the induction of cAMP-dependent intracellular signaling pathways, while TPβ inhibits them [31].
3. The Role of Prostaglandins in Various Cell Types Involved in Allergic Reactions
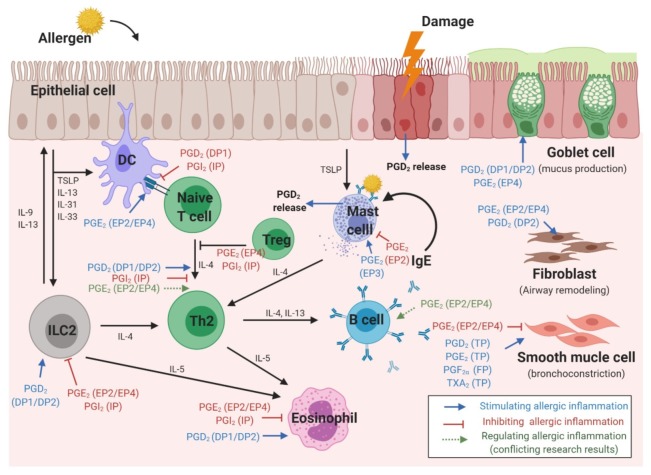
Allergic respiratory reactions occur because of interactions between various cells and cytokines [32]. When an allergen reaches the respiratory mucosa, epithelial cells and ILC2 secrete cytokines, while DCs present allergens, resulting in Th2 differentiation of naïve T cells and IgE production from B cells [33,34]. Eosinophilic inflammation caused by intercellular reactions leads to epithelial mucus hypersecretion, bronchoconstriction, and airway remodeling. PGs affect all stages of this reaction [35] (Figure 2).
Figure 2.
Stimulatory and inhibitory effects of prostaglandins, and their receptors, in different cell types involved in the pathophysiology of respiratory allergies.
3.1. Epithelial Cells
In human nasal polyps, stronger expression of H-PGDS and PGD2 was observed than in normal nasal mucosa. Moreover, PGD2 in airway epithelial cells induced increased MUC5B expression and mucin hypersecretion by the DP1 receptor [36,37]. A recent study using primary bronchial epithelial cell reported that PGD2 promoted epithelial cell migration as well as goblet cell hyperplasia, which were attenuated by DP2-selective antagonist [38].
Human bronchial epithelial cells treated with IL-13 showed increased airway mucin production, with increased expression of the mucin gene MUC5AC after PGE2 stimulation via the EP4 receptor [39]. Moreover, epithelial cells derived from the nasal polyps of patients with aspirin sensitivity showed dysregulated AA metabolism, which abnormally reduced the spontaneous production of PGE2, suggesting a mechanism of chronic inflammation in these patients [40]. PGE2 derived from epithelial cells also affected cells such as DCs and smooth muscle cells. Activation of the EP4 receptor by PGE2 released from airway epithelial cells limits DC activation and reduces the pro-inflammatory properties of DCs [41]. In addition, epithelia-derived PGE2 generated in response to bradykinin stimulation reduces airway smooth muscle contraction [42].
3.2. Dendritic Cells
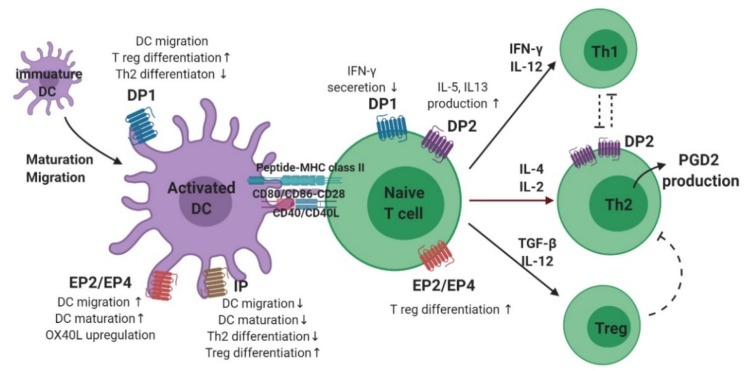
The maturation and migration of DCs is affected by PGs (Figure 3) [2]. In the presence of PGE2, CCL7 ligands triggered DC migration through the activation of signaling pathways, including cAMP-dependent PKA and rho kinase [43]. Interestingly, the presence of PGE2 was found to be effective only at initiation, and not termination, of DC maturation. In addition, modulation of DC migration by PGE2 reportedly requires a signal in the form of stimulation of either EP2, EP4, or both [44,45]. In contrast, PGD2 and PGI2 reduce DC maturation and migration. The inhibitory effect of PGD2 administration was observed in Balb/c mice exposed to fluorescein isothiocyanate (FITC)-OVA inhalation as a decreased migration of FITC+ DC to draining lymph nodes. This effect was also mediated by a DP1 agonist, but not by a DP2 agonist, suggesting that DP1 is responsible for modulating DC migration [46]. Another study showed that DP1 activation in DCs induced FOXP3 (+), CD4 (+), and regulatory T (Treg) cells, but DP1 depletion in DCs enhanced Th2 response in the airways, supporting this hypothesis [47]. In a murine model of asthma, inhalation of a stable PGI2 analog, iloprost, reduced the maturation and migration of lung DCs, thereby reducing the allergen-specific Th2 response in mediastinal lymph nodes [48]. In addition, iloprost treatment of DCs decreased differentiation of naïve T cells into effector Th2 cells [49].
Figure 3.
The role of prostaglandins and their receptors in dendritic cell–T cell interaction and Th2 differentiation.
PGE2 also has an important role in DC–T cell interactions as well as T cell differentiation (Figure 3). For Th1/Th2 balance, the ratio between PGE2 and IL-12 from DCs is important, suggesting that PGE2 induces Th2-skewing responses by inhibiting IL-12 production [50,51,52]. Reduction of IL-12 by PGE2 also inhibits interferon (IFN)-γ release from T cells and natural killer cells [51,53]. A recent study revealed a new mechanism for activation of Th2 immune response mediated by DCs, wherein allergen-induced PGE2 secretion by DCs resulted in autocrine OX40 ligand upregulation [54]. In a study on murine bone marrow-derived DCs (BMDCs), PGE2 inhibited TNF-α and IL-6 secretion by inducing IL-10 [55,56,57]. However, in the presence of TNF-α, PGE2 stimulation of human BMDCs increased IL-12 production, inducing a Th1 response [58,59]. In addition, BMDCs stimulated by PGE2 promoted IL-23 production, and induced Th17 differentiation [60]. In a recently published study of an OVA-induced asthma model, treatment of DCs with a PGI2 analogue, iloprost, induced differentiation of naïve T cells to Treg cells, leading to immune tolerance [61].
3.3. T Cells
PGD2 production was found to occur preferentially in antigen-stimulated human Th2 cells, but not in Th1 cells (Figure 3) [26]. PGD2 inhibits IFN-γ secretion by activating DP1, and stimulates Th2 cytokine expression through DP2 (CRTH2) activation [62]. A more recent study showed that the IL-5 positive effector T cell population expressing the CRTH2 receptor, was the pro-inflammatory Th2 cell population and induced allergic inflammation [63]. Moreover, mast cell-derived PGD2 activated type-2 CD8 (+) T cells via the CRTH2 receptor, inducing migration and cytokine (IL-5 and IL-13) production in severe eosinophilic asthma [64]. Studies supporting these findings have shown that a larger population of CRTH2 (+) T cells was identified from the peripheral blood of patients with severe asthma, and allergen-specific Th2 cells could be identified by stable co-expression of CRTH2 [65,66,67].
PGE2 has been proposed to induce Th2 responses by attenuating the production of IL-2 and IFN-γ by Th1 cells without enhancing the release of IL-4 by Th2 cells [26,68]. In an in vitro study, PGE2 stimulation of CD4 (+) T cells downregulated not only Th1 cytokines such as IFN-γ and TNF-α, but also IL-4. Nevertheless, differentiation skewed toward Th2, because of a much stronger inhibition of Th1 cytokine production [69]. However, studies regarding individual PGE2 receptors have shown conflicting results. Increased cAMP signaling via PGE2–EP2/EP4 in naïve T cells promoted the upregulation of IL-12Rβ2 and IFN-γ R1, thereby inducing Th1 differentiation [70]. The endogenous PGE2–EP2 axis was shown to have a role in controlling allergen sensitization and reducing theTh2 immune response in a murine model of asthma [71]. Furthermore, recent studies have shown that endogenous PGE2 coupled with the EP2–EP4 receptor induces the activation of pathogenic Th17 cells in both murine and human T cells [72,73]. Another study showed that PGE2 stimulation of T cells from the peripheral blood of patients with allergic rhinitis caused them to differentiate into Treg cells via EP4 receptor activation [74]. Although conflicting results have been reported on whether PGE2 induces differentiation of naïve T cells to Th1 or Th2, there is a consensus on the effect of PGE2 in Treg cell-induced inhibition of effector T cells [75].
3.4. B Cells
Most studies on the role of PGs in B cells have investigated the effect of PGE2 on the development of B cells and their production of IgE. However, studies on the role of PGE2 on IgE production have shown conflicting results. In the most recent study, B cells from EP2-deficient mice showed impaired IgE production and airway inflammation after an OVA challenge, suggesting the role of PGE2–EP2 signaling in promoting IgE production in allergic inflammation [76]. In support of this finding, PGE2 was shown to promote immunoglobulin class switching to IgE in B cells in the presence of IL-4 and LPS [77]. In contrast, PGE2 was shown to reduce MHC-II expression in B cells and suppress IgE production, which was mediated by either EP2 or EP4 [78]. Another study on B cell responses in an OVA-induced asthma murine model also showed that EP2 deficiency resulted in elevation of serum IgE level [71]. Taken together, although the exact effect of PGE2 is unclear, these studies emphasize the role of PGE2 in modulating IgE production.
3.5. Type 2 Innate Lymphoid Cells
The relationship between ILC2 cell function and PGs in allergic disease was first reported in a study showing that PGD2 released from mast cells induced IL-13 production by ILC2 cells, via DP1 or DP2 receptors (Figure 4) [79]. ILC2 cells obtained from the peripheral blood of patients with allergic rhinitis induced IL-5 production after stimulation by PGD2 [80]. Furthermore, activation of DP2 receptor on human ILC2 cells induced their migration, production of type 2 cytokines, and increased expression of receptors for IL-33 and IL-25 [81]. ILC2 accumulation, induced by IL-33, was not observed in a DP2-deficient murine model [82]. In the sputum of patients with asthma, a DP2-positive ILC2 population, in addition to IL-5- or IL-13–positive ILC2s, was observed to be significantly higher after allergen challenge than in the controls [83]. In addition, the number of ILC2s after allergen challenge was elevated in the bronchoalveolar lavage (BAL) fluid and reduced in the blood. PGD2 levels in the BAL fluid showed a significant correlation with a decrease in ILC2 in the blood, indicating that PGD2 is essential for ILC2 migration [84]. In patients with AERD, recruitment of ILC2 to the nasal mucosa was observed upon treatment with a COX-1 inhibitor, with a corresponding decrease in ILC2 numbers in the blood, supporting the correlation with enhanced PGD2 production [85]. Further supporting these findings, ILC2s isolated from human blood that were treated with the PGD2 antagonist, fevipiprant, showed reduced aggregation and diminished cytokine production [86]. Moreover, human ILC2 cells treated with the H-PGDS enzyme inhibitor, KMN698, showed a diminished release of IL-5 and IL-13 [87].
Figure 4.
The role of prostaglandins and their receptors in type 2 innate lymphoid cells.
Among four receptors for PGE2, only the EP2 and EP4 receptors showed strong expression in single-cell transcriptome sequencing of human tonsillar ILC2 cells [88]. In the same study, PGE2 attenuated ILC2 function by inhibiting GATA-3 expression, and decreasing the release of Th2 cytokines, including IL-5 and IL-13. Another study revealed that PGE2–EP4–cAMP signaling inhibited IL-33-induced Th2 cytokine production in pulmonary ILC2 cells by suppressing GATA3 and ST2 expression [89]. Similarly, PGI2 inhibited the expression of IL-5 and IL-13 in IL-33-stimulated ILC2 cells from the bone marrow in a mouse model challenged intranasally with Alternaria alternata [90].
3.6. Eosinophils
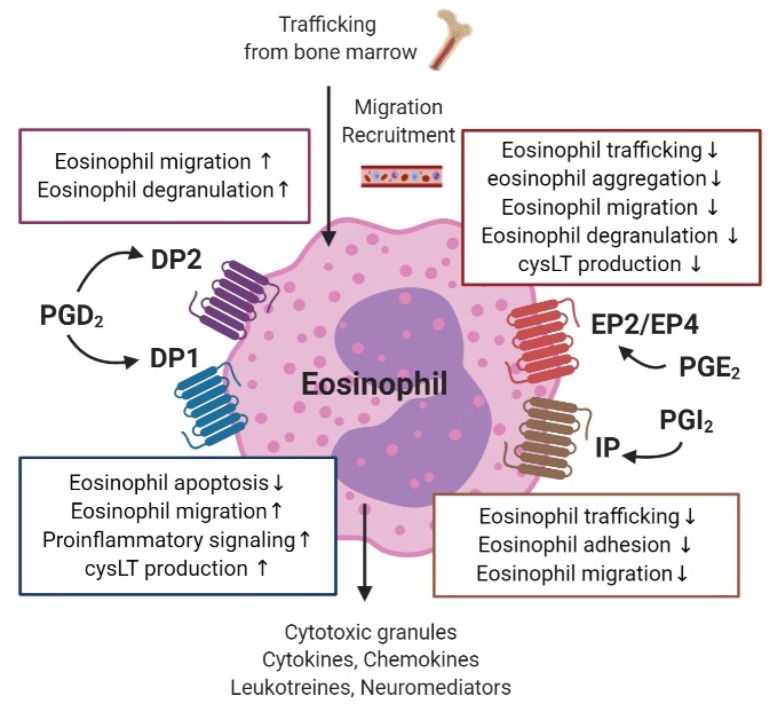
PG receptors expressed on human eosinophils include DP1 and DP2 for PGD2, EP2 and EP4 for PGE2, and IP for PGI2 (Figure 5) [91].
Figure 5.
The role of prostaglandins and their receptors in eosinophil function, including bone marrow trafficking, migration, and degranulation.
Both DP1 and DP2 are present on the surface of eosinophils [92]. In addition to PGD2-induced stimulation of these receptors, the major metabolite of PGD2, 15-deoxy-Δ12,14-PGJ2, activates the peroxisome proliferator-activated receptor-γ on eosinophils [93]. PGD2 is known to modulate eosinophil migration via the DP2 receptor [94,95]. PGD2-DP2 signaling also enhances Ca2+ morphologic changes and degranulation of eosinophils in allergic inflammatory responses [94]. A recent study revealed that blocking the PGE2-DP2 pathway inhibits migration of eosinophils toward mast cells—the initial stage in an allergic reaction [96]. In addition, by mediating its effect through DP2, PGD2 also enhances the function of other chemoattractants, such as eotaxin, complement factor C5a, or 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) on eosinophils [97,98]. In contrast, eotaxin and 5-oxo-ETE diminished eosinophil migration toward PGD2, and their effect was decreased in the presence of blood or plasma, while no effect was identified for PGD2, suggesting that it acts as the initial chemoattractant, while eotaxin is the endpoint chemoattractant [98]. The DP1 receptor on eosinophils inhibits eosinophil apoptosis and cooperates with DP2 receptors to induce LTC4 synthesis, eosinophil mobilization, and pro-inflammatory signaling [92,94,99,100]. In eosinophils obtained from the peripheral blood of patients with AERD, high expression of H-PGDS was identified, and inhibitory action of the H-PGDS enzyme suppressed the release of PGD2 [101]. This suggests that eosinophil activation could occur via autocrine signaling in patients with AERD and allergic inflammation [20].
In eosinophils, PGE2 decreases the production of the eosinophil cationic protein and the aggregation of eosinophils [102,103]. EP2 receptor activation in eosinophils attenuates eosinophil trafficking and PGE2–EP4 signaling, and decreases migration and degranulation of eosinophils [104,105]. PGE2 also inhibits the spontaneous apoptosis of eosinophils in human peripheral blood and suppresses eosinophil–endothelial cell interactions, including adhesion and transmigration, by altering β2 integrin and L-selectin function [106,107]. Eosinophils obtained from human peripheral blood showed reduced production of LTB4 and cysteinyl LTs (cysLTs) when treated with a low dose of PGE2 after lysine aspirin stimulation, and this effect was mediated by EP2 receptor activation [108].
PGI2 has similar properties to PGE2, as both act as immune suppressors in eosinophils through the IP receptor, and both modulate intracellular cAMP [91]. In guinea pigs, both PGI2 and a PGI2 analogue, iloprost, inhibit bone marrow eosinophil trafficking [109]. In addition, endothelial-derived PGI2 negatively modulates eosinophil–endothelial interactions by inhibiting eosinophil adhesion and transendothelial migration [110].
3.7. Mast Cells
Mast cells are an important source of endogenous eicosanoids, and PGD2 is the predominantly released mediator, with cysLTs. Production of PGD2 from mast cells isolated from human lungs depends on COX-1 and H-PGDS, but not L-PGDS [111]. In the nasal mucosa of patients with allergic rhinitis, the number of mast cells with H-PGDS expression was high, while that with L-PGDS expression was low [21]. In humans, there was a linear correlation between PGD2 generation and histamine secretion after IgE-dependent activation of human mast cells [19]. A recent study, using nasal polyp tissue from patients with chronic rhinosinusitis and AERD, showed that TSLP induces the production of PGD2 by mast cells [112]. Another recent study showed intracellular expression of DP2 receptors in mast cells isolated from nasal polyps [113]. However, the majority of mast cells (either cell-line-derived human mast cells or cells harvested from the nasal cavity) did not express the PGD2 receptors, and did not respond to a DP2 agonist or antagonist [114].
Human mast cells also express EP2, EP3, and EP4 receptors for PGE2 signaling, and PGE2 stimulation coupled with EP2 activation inhibited mast cell degranulation, whereas EP3 receptor activation enhanced mast cell mediator release [115,116,117].
3.8. Smooth Muscle Cells
The role of PGs in modulating contraction of airway smooth muscles is widely accepted [39]. Four distinct receptors (PGE2, EP2, EP3, and EP4) have been detected on human airway smooth muscle cells [118]. Inhalation of PGE2 diminished bronchoconstriction in a methacholine airway test after an allergen challenge, and decreased bronchoconstriction induced by exercise or aspirin [119]. In humans, the EP4 receptor appears to mediate this effect, as a selective EP4 agonist could reverse the histamine-induced contraction of airway smooth muscle cells [120]. In other species, including mice, guinea pigs, and monkeys, PGE2-mediated regulation of smooth muscle contraction was regulated by EP2 [121]. Conversely, the activation of PGE2 is known to induce bronchial contraction, emphasizing the importance of receptor selectivity. In a recent study, bronchoconstriction caused by PGE2 and other PGs involved TP receptor activation pathways, and the powerful bronchoprotective effect of PGE2 was generated through mast cell-mediated bronchoconstriction via activation of the EP2 receptor, while EP4 stimulation had less efficacy than a long-acting β-receptor agonist [121]. In addition to muscle tone regulation in airway cultured smooth muscle cells, PGE2 limits the release of the granulocyte macrophage-colony stimulating factor, an important mediator of allergic inflammation and eosinophil survival [122]. Moreover, PGE2-induced stimulation of the EP2 or EP4 receptors inhibits migration of smooth muscle cells of the airways through the cAMP/PKA pathway, suggesting a potential pharmacological role of PGE2 in preventing airway remodeling in asthma [123].
In contrast to PGE2, PGD2 acts as a bronchoconstrictor and has a potent effect on muscle contraction, mediated via the TP receptor [24]. In humans, the increase in nasal resistance induced by PGD2 nasal instillation was greater than that of histamine (10-fold) or bradykinin (100-fold) [24]. A recent in vitro study showed that inhibition of the PGD2-DP2 pathway reduced the migration of airway smooth muscle cells, suggesting a role for DP2 antagonists in airway remodeling [124].
PGF2α inhalation in healthy and asthmatic subjects caused bronchoconstriction in a dose-dependent manner; however, the sensitivity was approximately 150 times greater in asthmatic patients than in healthy controls [125]. In healthy controls, the responsive dose varied widely across individuals, but females generally had a smaller contractile response to PGF2α than males [125].
TXA2 is also reported to be a potent bronchoconstrictor. In asthmatic patients, bronchoconstriction induced by antigen inhalation was shown to involve high concentrations of TXB2, which is a stable metabolite of TXA2, suggesting the possible role of TXA2 in airway remodeling or hyper-responsiveness [126]. This effect was mediated through c-Jun N-terminal kinase–mitogen-activated protein kinase signaling, and increased the extracellular calcium influx after stimulation of the TP receptor [127].
3.9. Fibroblasts (in Nasal Polyps)
The role of PGs in fibroblasts has also been studied, as formation of eosinophilic nasal polyps is a typical phenotype of patients with dysregulated AA metabolism.
Nasal polyp-derived fibroblasts showed increased production of vascular endothelial growth factor after PGE2 stimulation via EP4 receptor activation, indicating that PGE2 has a role in increased micro-vascular permeability during tissue remodeling in nasal polyps [128]. In a study using cultured fibroblasts from healthy nasal mucosa and nasal polyps from patients with aspirin-tolerant and -sensitive asthma, PGE2 concentration was lower in patients with both aspirin-tolerant and -sensitive nasal polyps after IL-1β stimulation, than in the controls [129]. In addition, increased expression of the EP2 receptor in fibroblasts after IL-1β stimulation was identified only in normal mucosa, but not in nasal polyps from either aspirin-tolerant or -sensitive patients [129]. Another study suggested that decreased expression of the EP2 receptor in fibroblasts from nasal polyps of patients with AERD resulted in resistance to the anti-proliferative effects of PGE2 [130].
PGD2 stimulation also induced the release of vascular endothelial growth factor, but this release occurred via the DP1 receptor, not the DP2 receptor [131].
4. Clinical Studies of Prostaglandins in Allergic Airway Diseases
The expression and role of PGs in allergic airway diseases have been studied for a long time. Recently, clinical phase II and III studies were conducted to evaluate the therapeutic effects of PGs, especially for asthma and allergic rhinitis (Table 2). This section briefly introduces the role of PGs in each disease (asthma, allergic rhinitis, AERD, and nasal polyps) in clinical settings, and provides an overview of the clinical studies that have used pharmacologic agents targeting PGs.
Table 2.
Summary of clinical studies on pharmacologic agents targeting PGs in asthma and allergic rhinitis.
| Drug and Dose | Indication (Sample Size) |
Key Results | Ref. |
|---|---|---|---|
| DP2 antagonist | |||
| Fevipiprant (QAW039), oral administration, 500 mg daily for 28 days | Mild to moderate uncontrolled allergic asthma (n = 170) |
Improvement of lung function in patients with FEV1 <70% | [132] |
| Fevipiprant (QAW039), oral administration, 1–450 mg daily or 2–150 mg twice daily, with inhaled budesonide 200 µg twice a day, for 12 weeks | Allergic asthma uncontrolled by a low-dose inhaled corticosteroid (n = 2598) |
Total daily dose of 150 mg (150 mg once or 75 mg twice per a day) showed an improvement in forced expiratory volume | [133] |
| Fevipiprant (QAW039), oral administration, 225 mg twice daily for 12 weeks | Moderate to severe asthma with serum eosinophil ≥ 2% (n = 61) |
Decreased sputum eosinophil count | [134] |
| ARRY-502, oral administration, 200 mg twice daily for four weeks | Mild allergic asthma (n = 184) |
Reduction of FeNO level and decreased serum markers of Th2 inflammation | [135] |
| AZD1981, oral administration, 100 mg twice daily | Stable asthma withdrawn from inhaled corticosteroid (n = 209) |
No efficacy on morning peak expiratory flow | [136] |
| AZD1981, oral administration, 50–1000 mg twice daily, for four weeks, with an inhaled corticosteroid | Asthma uncontrolled by inhaled corticosteroid (n = 510) |
400 mg group showed improved FEV1, significant improvement in questionnaire score and FEV1 in atopic subgroup | [136] |
| OC000549, oral administration, 25 mg daily/200 mg daily/100 mg twice daily, for 12 weeks, with use of short-acting β2 agonist | Mild to moderate asthma (n = 460) |
Improved FEV1 (prominent in eosinophilic subjects), lower incidence of symptom exacerbation and respiratory infection | [137] |
| OC000549, oral administration, 200 mg twice daily for eight days | Seasonal allergic rhinitis (n = 35) |
Reduced grass-pollen induced nasal and ocular symptoms | [138] |
| GB001, oral administration, 30 mg daily for 28 days, with use of low dose inhaled fluticasone propionate | Mild to moderate atopic asthma (n = 36) |
Improved FEV1 (prominent in patients with high FeNO or high blood eosinophil) | [139] |
| BI671800, oral administration, 50/200/400 mg twice daily for six weeks | Mild to moderate asthma (n = 389) |
Greater improvement in FEV1 compared to moderate doses of fluticasone | [140] |
| BI671800, oral administration, 400 mg twice daily with inhaled fluticasone (88 µg) | Mild to moderate asthma with inhaled corticosteroid (n = 243) | Improvement in FEV1 compared to placebo; however, not significantly improved over montelukast | [140] |
| Setipiprant (ACT-129968), oral administration, 1000 mg twice daily for five days, washout period of three weeks | Stable allergic asthma (n = 15) |
Reduction in both allergen-induced late asthmatic responses and airway hyper-responsiveness | [141] |
| Setipiprant (ACT-129968), oral administration, 100/500/1000 mg twice daily or 1000 mg daily for two weeks | Seasonal allergic rhinitis (n = 557) |
Dose-related improvements in both nasal and ocular symptom scores | [142] |
| Setipiprant (ACT-129968), oral administration, 1000 mg twice daily for two weeks | Seasonal allergic rhinitis (n = 604) |
No significant effect on either nasal or ocular symptom scores | [142] |
| Dual antagonist for DP2 and TP | |||
| Ramatroban (BYAu3405), oral administration, 150 mg twice daily for four weeks | Perianal allergic rhinitis (n = 10) |
Inhibitory effect on allergen challenge-induced nasal mucosal swelling | [143] |
| Ramatroban (BYAu3405), oral administration, 150 mg twice daily for four weeks | Perianal allergic rhinitis (n = 11) |
Inhibitory effect on histamine-induced nasal reactivity, decreased eosinophil counts in nasal lavage fluid | [144] |
| TP antagonist | |||
| Seratrodast, oral administration, 80 mg daily for four weeks | Asthma (n = 14) |
Decreased airway hyper-responsiveness, no definite effect on exhaled nitric oxide and sputum eosinophils | [145] |
| AA02414, oral administration, 80 mg daily for four months | Asthma (n = 31) |
Improved symptom score, peak expiratory flow, and bronchial responsiveness to metacholine, decreased activated eosinophil infiltration | [146] |
| DP1 antagonist | |||
| ONO-4053, oral administration, 300 mg daily for two weeks | Seasonal allergic rhinitis (Japanese cedar pollen) (n = 200) |
Greater improvement in nasal symptoms compared to either placebo or pranlukast | [147] |
| PGI2 analogue | |||
| OP-41483, oral administration, 200 µg 4 times daily for four weeks | Stable asthma (n = 8) |
No direct effect on bronchial responsiveness | [148] |
FEV1—one second-forced expiratory volume; FeNO—fractional exhaled nitric oxide.
4.1. Asthma
Numerous studies have focused on the clinical relevance of prostaglandins in asthma. In a study that examined concentrations of PG mediators from BAL fluid, both PGD2 and PGF2α showed 12- and 20-fold higher levels in asthmatic patients than nonallergic controls, respectively [149]. Another study reported that concentrations of PGD2, TXB2, and PGI2 metabolites was much higher (up to 208-fold) in BAL fluid 5 min after an allergen challenge in allergic asthmatic patients compared to the controls [150]. In addition, epithelial cells obtained from severely asthmatic patients showed higher levels of H-PGDS compared to controls, correlating with PGD2 levels. Furthermore, BAL fluid collected from severely asthmatic patients showed stronger expression of DP2 receptors than that from mild asthmatics or heathy controls; however, there was no difference in DP1 receptor expression among groups [151]. As for PGE2 levels, studies regarding their correlation with eosinophils reported that a lower sputum PGE2 level is associated with eosinophilia in asthmatic patients [152].
To date, numerous clinical trials have shown that DP2 antagonists are effective for patients with asthma (Figure 1) [153,154,155]. Fevipiprant (QAW039), an oral DP2 antagonist, improved pulmonary function in asthmatic patients with severely impaired lung functions and patients with uncontrolled asthma using an inhaled corticosteroid (ICS) [132,133,156]. In a single-center randomized controlled trial, decreased sputum eosinophil count was observed in patients with moderate to severe asthma after treatment with fevipiprant [134]. Indeed, to date, fevipiprant is recognized as the most potent pharmacologic agent targeting PG receptors [157,158]. All phase II studies have agreed on the safety and tolerability of fevipiprant [159]. Several phase III trials are ongoing to determine its potential to prevent asthma exacerbation (LUSTER1 & LUSTER2), and its safety in moderate uncontrolled asthma in ICS-treated asthmatic patients (ZEAL1 & ZEAL2) [160,161,162,163,164]. Another DP2 antagonist, ARRY-502, improved one second-forced expiratory volume (FEV1) results as well as symptom-related quality of life of patients with asthma relative to control patients. This effect was greater in patients with elevated Th2-associated biomarkers [135]. Treatment with AZD1981, a DP2 receptor antagonist, showed improvements in the asthma control questionnaire score as well as FEV1 in patients with allergic asthma after four weeks [136]. OC000459 was also found to be safe and effective in inhibiting the DP2 receptor, showing significant improvements in pulmonary function in patients with eosinophil-dominant allergic asthma [137]. Other DP2 selective antagonists that were effective from phase II trials include GB001, BI671800, and setipiprant (ACT-129968) [139,140,141,165].
Recently, a phase I clinical trial for evaluating the efficacy of ZL-2010, an H-PGDS inhibitor, in asthma treatment has been completed, but the results are not yet published (Figure 1) [166].
In patients with asthma, PGE2 levels in sputum were negatively correlated with the eosinophil count, and inhaled PGE2 was effective in preventing pulmonary hyper-reactivity after an allergen challenge [167,168], but inhaled PGE2 did not alter baseline pulmonary functions without this challenge [168]. For investigating the effects of oral PGE2 administration, a synthetic PGE2 analog named misoprostol was used, because of the short half-life of PGE2 (Figure 1) [169]. However, no protective effect of misoprostol on pulmonary function, asthma symptom scores, or serum eosinophil count was identified in patients with aspirin-sensitive asthma, suggesting that oral administration could not deliver a sufficient concentration to the pulmonary system for pharmacologic activation [169].
Although PGF2α has not been studied as much as PGD2 or PGE2, studies of its effect on allergic reactions have shown that PGF2α inhalation decreases specific airway conductance in a dose-related manner, and induces bronchoconstriction, which can shorten the recovery by PGE2 [125,170]. In addition, inhaled PGF2α decreased the exhaled nitric oxide (NO) level, although the clinical implications of this finding have not yet been identified [171].
The effects of allergic response treatment with PGI2 have been extensively studied; however, to our knowledge the most recent article was published in 1991 [172]. This might be because of the unstable nature of PGI2 that catalyzes within 3 to 5 min, and the absence of a stable PGI2 analog. Previous studies have shown that PGI2 inhalation did not exert a protective effect against bronchoconstriction after an allergen challenge, with no change in airway conductance [148,173]. Moreover, another study using an oral analog of PGI2 (OP-41483), showed no effect on FEV1 and airway responsiveness in patients with asthma (Figure 1) [172]. However, the converse result has also been reported—that PGI2 inhalation causes a dose-dependent decrease in FEV1 with no effect on airway conductance, suggesting narrowing of the airway due to mucosal blood engorgement via the vasodilatory effects of PGI2 [174].
As for TXA2, treatment of patients with asthma using a TP antagonist, seratrodast (AA-2414), resulted in improvement of symptom scores, bronchial responsiveness, and peak expiratory flow, and a reduction of inflammatory biomarkers in the epithelium of patients after four weeks [145,146].
4.2. Allergic Rhinitis
Clinical studies of allergic rhinitis have also suggested the importance of PGs in the disease. In nasal lavage fluid (NALF), the PGD2 level was higher in patients with allergic rhinitis compared to those with nonallergic rhinitis [175]. Nasal mucosa samples obtained from the turbinates of patients with allergic rhinitis revealed stronger DP2 expression in eosinophils, mast cells, T lymphocytes, and epithelial cells compared to the nonallergic nasal mucosa [176]. In patients with seasonal allergic rhinitis and sensitization to grass pollen, the PGE2 level in NALF decreased significantly during the pollen-exposure season, which was negatively correlated with nasal eosinophilia [177].
Although fewer than for asthma, several clinical trials of PG-targeting pharmacologic agents have been conducted for allergic rhinitis (Table 2). In a randomized, double-blind, placebo-controlled study, a DP2 antagonist (OC000459) was effective in reducing nasal and ocular symptoms in patients with allergic rhinitis caused by grass pollen [138]. Clinical trials of the dual DP2/TP receptor antagonist, ramatroban (BAY u3405), in patients with rhinitis showed conflicting results. In a study of patients with atrophic rhinitis, PGD2-induced nasal congestion was not relieved by the administration of a single 20-mg dose of ramatroban [178]. However, when administrated twice a day at a higher dose (150 mg) for four weeks, it effectively decreased eosinophil levels in the nasal lavage fluid and reduced mucosal swelling after house dust challenges in patients with perennial allergic rhinitis [143,144]. In a phase II clinical study, setipiprant showed a significant improvement in nasal symptoms, during the day and night, in patients with seasonal allergic rhinitis; however, in the phase III trial it did not show efficacy at the endpoint [142]. The DP1 antagonist, ONO-4053, also reduced nasal symptoms including rhinorrhea, sneezing, and nasal itching in patients with seasonal allergic rhinitis, without causing safety issues, in a phase II trial [147].
Although previous studies have shown that selective inhibition of H-PGDS (TFC-007 and TAS-204) is effective in reducing nasal blockage and eosinophil infiltration in animal models of allergic rhinitis, no studies have yet been conducted in humans to determine the role of H-PGDS inhibitors in allergic rhinitis [179,180].
4.3. Aspirin-Exacerbated Respiratory Disease
AERD is a nonallergic disease caused by dysregulation of eicosanoids and characterized by eosinophilia, Th2 dominant cytokines, and a marked increase of CysLTs [181]. In AERD patients, COX-2 expression and PGE2 level are significantly reduced, because the mPGES-1 gene is functionally coupled to COX-2. However, there was no change in the expression of COX-1. Conversely, the 5-lipoxygenase (5-LO) pathway, which metabolizes AA into LTs, is markedly upregulated in these patients, resulting in an extremely high level of CysLTs, which activate mast cells and eosinophils [10,182]. Addition of nonsteroidal anti-inflammatory drugs, especially COX-1 inhibitors, forces AA metabolism towards LT synthesis and further decreases PGE2 production, resulting in the uncontrolled release of LTC4, LTD4, LTE4, and PGD2 by countering the suppressive effect of PGE2 on 5-LO [183,184,185].
Studies of the nasal mucosa or nasal polyps of patients with AERD have shown lower expression levels of the EP2 receptor than in healthy patients, or patients with aspirin tolerance, suggesting that the decreased expression of EP2 in AERD might have a synergistic effect on the anti-inflammatory and bronchoprotective capacity of the PGE2–EP2 axis [186,187]. Interference with PGE2 production by oral administration of the PGE2 analog misoprostol did not protect against the symptoms induced by NSAIDs in patients with AERD [188].
The importance of PGD2 in AERD pathogenesis has also been studied [189]. In patients with AERD, an aspirin challenge did not reduce the PGD2 concentration in BAL fluid, unlike in patients with asthma [190,191]. Moreover, frequent aspirin challenges increased PGD2 metabolites in the urine and serum of patients with AERD, possibly owing to the abnormal activation of mast cells [192]. A study evaluating the role of PGD2 in AERD showed that severe extra-pulmonary reactions are associated with PGD2 production, and high-dose aspirin therapy could be used to inhibit PGD2 generation and suppress inflammatory cell recruitment [189]. In patients with AERD, intravenous injection of PGI2 had no effect in preventing decreased airflow after aspirin stimulation [193].
4.4. Nasal Polyps
Few studies have investigated PGs in nasal polyps, whereas comparatively more have assessed their roles in asthma or allergic rhinitis. However, the clinical relevance of PGs has been well demonstrated, even disregarding their association with aspirin sensitivity [194].
PGE2 concentrations and COX-2 mRNA expression were lower in nasal polyp tissues than in nasal mucosas, either from healthy controls or from patients with chronic rhinosinusitis without nasal polyps. Higher production of LT metabolites, such as LTC4, LTD4, and LTE4 was correlated with lower PGE2 concentrations [195]. Another study revealed a decreased level of PGD2 and its metabolites in the serum of patients with chronic rhinosinusitis [196]. Supporting this finding, the mRNA expression of PGDS and PGES was higher and lower, respectively, in nasal polyps than in the normal uncinate process, and a negative correlation was observed between the PGDS and PGES levels [197]. Moreover, the high PGDS and low PGES levels were correlated with increased eosinophil infiltration, as well as disease severity, suggesting that PGs might have a role in eosinophilic inflammation in nasal polyps [197].
Regarding the expression of individual PGE2 receptors in nasal polyps, the EP2 receptor showed a lower expression in immunohistochemistry of nasal biopsies from patients with both aspirin-sensitive and -tolerant chronic rhinosinusitis than controls. However, the expression was lowest in aspirin-sensitive patients [198,199]. Moreover, increased EP1 receptor expression was associated with the eosinophilic phenotype in patients with nasal polyps without aspirin intolerance [194]. In a recent study, exposure to cigarette smoking was related with diminished expression of EP2 and EP4 receptors in nasal polyps of patients with chronic rhinosinusitis, which might explain the severe inflammation caused by smoking [200]. In a study of PGD2 receptors in nasal polyp tissue, higher DP1 and lower DP2 receptor expression was identified relative to normal nasal mucosa, and both these findings correlated with increased levels of PGDS and eotaxin [201].
Despite the promising importance of PGs in nasal polyps reported by previous studies, to our knowledge, clinical trials for nasal polyps with pharmacologic agents modulating PGs have not yet been conducted, and future studies are required to provide clinical evidence.
5. Conclusions
In this review, we described the biosynthesis of PGs and their roles in various cell types relating to allergic airway inflammation. We summarized up-to-date information about the clinical relevance of PGs and clinical trials regulating their activation in each allergic airway disease. Owing to their short half-life, researchers have faced difficulties in conducting in vivo studies and clinical trials of PGs. However, with the development of stable PG analogs, their promising role in regulating allergic reactions, and the possibility for future therapeutic agents based on their biochemistry has been demonstrated by both animal and human studies. Nevertheless, little is known about their role in modulating the pathogenesis of nasal polyps, or the effect of PG synthase inhibitors on allergic inflammation. Future studies should focus on methods for maintaining the biological activity of PGs, and the development of pharmacological agents targeting PG synthase to assist in modulating allergic airway diseases, such as nasal polyps.
Abbreviations
| AA | arachidonic acid |
| AERD | aspirin exacerbated respiratory disease |
| cAMP | cyclic adenosine 3′,5′-monophosphate |
| BAL | bronchoalveolar lavage |
| COX | cyclooxygenase |
| cPGES | cytosolic prostaglandin E synthase |
| cysLT | Cysteinyl leukotriene |
| DC | dendritic cell |
| FEV1 | forced expiratory volume 1 |
| H-PGDS | hematopoietic-prostaglandin D synthase |
| IgE | immunoglobulin E |
| IL | interleukin |
| ILC | innate lymphoid cell |
| INF-γ | interferon γ |
| L-PGDS | lipocalin-prostaglandin D synthase |
| LPS | lipopolysaccharide |
| LT | leukotriene |
| LTC4 | leukotrieneC4 |
| LTD4 | leukotrieneD4 |
| LTE4 | leukotrieneE4 |
| mPGES | microsomal prostaglandin E synthase |
| NALF | nasal lavage fluid |
| NSAID | nonsteroidal anti-inflammatory drug |
| PG | prostaglandin |
| PGD2 | prostaglandin D2 |
| PGE2 | prostaglandin E2 |
| PGF2α | prostaglandin F2α |
| PGFS | prostaglandin F synthase |
| PGG2 | prostaglandin G2 |
| PGH2 | prostaglandin H2 |
| PGI2 | prostaglandin I2 |
| PLA2 | phospholipase A2 |
| TNF-α | tumor necrosis factor α |
| Treg | regulatory T cell |
| TSLP | thymic stromal lymphopoietin |
| TXA2 | thromboxane A2 |
| TXB2 | thromboxane B2 |
Author Contributions
All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Basic Science Research Program, National Research Foundation of Korea, funded by the Ministry of Science and Technology and the Ministry of Science, ICT & Future Planning (2017R1A2B2003575), and the Korea Health Technology R&D Project (HI17C0387), Korea Health Industry Development Institute (KHIDI), and the Ministry of Health & Welfare. This research was also supported by a Korea University grant, and a grant from Korea University Medical Center as well as by Anam Hospital, Seoul, Republic of Korea (O1905011).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Korbecki J., Baranowska-Bosiacka I., Gutowska I., Chlubek D. Cyclooxygenase pathways. Acta Biochim. Pol. 2014;61:639–649. doi: 10.18388/abp.2014_1825. [DOI] [PubMed] [Google Scholar]
- 2.Debeuf N., Lambrecht B.N. Eicosanoid Control Over Antigen Presenting Cells in Asthma. Front. Immunol. 2018;9:2006. doi: 10.3389/fimmu.2018.02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arter. Thromb Vasc. Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piper P.J., Vane J.R. Release of additional factors in anaphylaxis and its antagonism by anti-inflammatory drugs. Nature. 1969;223:29–35. doi: 10.1038/223029a0. [DOI] [PubMed] [Google Scholar]
- 5.Claar D., Hartert T.V., Peebles R.S., Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 2015;9:55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakayama T. Introduction to “allergic inflammation”. Immunol. Rev. 2017;278:5–7. doi: 10.1111/imr.12566. [DOI] [PubMed] [Google Scholar]
- 7.Elieh Ali Komi D., Bjermer L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin. Rev. Allergy Immunol. 2019;56:234–247. doi: 10.1007/s12016-018-8720-1. [DOI] [PubMed] [Google Scholar]
- 8.White A.A., Stevenson D.D. Aspirin-Exacerbated Respiratory Disease. N. Engl. J. Med. 2018;379:1060–1070. doi: 10.1056/NEJMra1712125. [DOI] [PubMed] [Google Scholar]
- 9.Sanak M. Eicosanoid Mediators in the Airway Inflammation of Asthmatic Patients: What is New? Allergy Asthma Immunol. Res. 2016;8:481–490. doi: 10.4168/aair.2016.8.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laidlaw T.M. Pathogenesis of NSAID-induced reactions in aspirin-exacerbated respiratory disease. World J. Otorhinolaryngol. Head Neck Surg. 2018;4:162–168. doi: 10.1016/j.wjorl.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis E.A., Cao J., Hsu Y.H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M., Kudo I. Phospholipase A2. J. Biochem. 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- 13.Hallstrand T.S., Lai Y., Ni Z., Oslund R.C., Henderson W.R., Jr., Gelb M.H., Wenzel S.E. Relationship between levels of secreted phospholipase A(2) groups IIA and X in the airways and asthma severity. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2011;41:801–810. doi: 10.1111/j.1365-2222.2010.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith W.L., Urade Y., Jakobsson P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011;111:5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang Y.J., Mbonye U.R., DeLong C.J., Wada M., Smith W.L. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog. Lipid Res. 2007;46:108–125. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata A.N., Breyer R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004;103:147–166. doi: 10.1016/j.pharmthera.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Narumiya S., FitzGerald G.A. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Investig. 2001;108:25–30. doi: 10.1172/JCI200113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo M.J., Oh D.K. Prostaglandin synthases: Molecular characterization and involvement in prostaglandin biosynthesis. Prog. Lipid Res. 2017;66:50–68. doi: 10.1016/j.plipres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lewis R.A., Soter N.A., Diamond P.T., Austen K.F., Oates J.A., Roberts L.J., 2nd Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J. Immunol. 1982;129:1627–1631. [PubMed] [Google Scholar]
- 20.Luna-Gomes T., Magalhaes K.G., Mesquita-Santos F.P., Bakker-Abreu I., Samico R.F., Molinaro R., Calheiros A.S., Diaz B.L., Bozza P.T., Weller P.F., et al. Eosinophils as a novel cell source of prostaglandin D2: Autocrine role in allergic inflammation. J. Immunol. 2011;187:6518–6526. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okano M., Fujiwara T., Sugata Y., Gotoh D., Masaoka Y., Sogo M., Tanimoto W., Yamamoto M., Matsumoto R., Eguchi N., et al. Presence and characterization of prostaglandin D2-related molecules in nasal mucosa of patients with allergic rhinitis. Am. J. Rhinol. 2006;20:342–348. doi: 10.2500/ajr.2006.20.2865. [DOI] [PubMed] [Google Scholar]
- 22.Brightling C.E., Brusselle G., Altman P. The impact of the prostaglandin D2 receptor 2 and its downstream effects on the pathophysiology of asthma. Allergy. 2019 doi: 10.1111/all.14001. [DOI] [PubMed] [Google Scholar]
- 23.Serezani C.H., Ballinger M.N., Aronoff D.M., Peters-Golden M. Cyclic AMP: Master regulator of innate immune cell function. Am. J. Respir. Cell Mol. Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston S.L., Freezer N.J., Ritter W., O’Toole S., Howarth P.H. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. Eur. Respir. J. 1995;8:411–415. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- 25.Murakami M., Nakashima K., Kamei D., Masuda S., Ishikawa Y., Ishii T., Ohmiya Y., Watanabe K., Kudo I. Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J. Biol. Chem. 2003;278:37937–37947. doi: 10.1074/jbc.M305108200. [DOI] [PubMed] [Google Scholar]
- 26.Betz M., Fox B.S. Prostaglandin E2 inhibits production of Th1 lymphokines but not of Th2 lymphokines. J. Immunol. 1991;146:108–113. [PubMed] [Google Scholar]
- 27.Komoto J., Yamada T., Watanabe K., Woodward D.F., Takusagawa F. Prostaglandin F2alpha formation from prostaglandin H2 by prostaglandin F synthase (PGFS): Crystal structure of PGFS containing bimatoprost. Biochemistry. 2006;45:1987–1996. doi: 10.1021/bi051861t. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T. Prostacyclin analogues: Prevention of cardiovascular diseases. Cardiovasc. Hematol. Agents Med. Chem. 2006;4:351–359. doi: 10.2174/187152506784111463. [DOI] [PubMed] [Google Scholar]
- 29.Miyata A., Yokoyama C., Ihara H., Bandoh S., Takeda O., Takahashi E., Tanabe T. Characterization of the human gene (TBXAS1) encoding thromboxane synthase. Eur. J. Biochem. 1994;224:273–279. doi: 10.1111/j.1432-1033.1994.00273.x. [DOI] [PubMed] [Google Scholar]
- 30.Ruan K.H. Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway. Mini Rev. Med. Chem. 2004;4:639–647. doi: 10.2174/1389557043403710. [DOI] [PubMed] [Google Scholar]
- 31.Hirata T., Ushikubi F., Kakizuka A., Okuma M., Narumiya S. Two thromboxane A2 receptor isoforms in human platelets. Opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J. Clin. Investig. 1996;97:949–956. doi: 10.1172/JCI118518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenzel S.E. Emergence of Biomolecular Pathways to Define Novel Asthma Phenotypes. Type-2 Immunity and Beyond. Am. J. Respir. Cell Mol. Biol. 2016;55:1–4. doi: 10.1165/rcmb.2016-0141PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parulekar A.D., Kao C.C., Diamant Z., Hanania N.A. Targeting the interleukin-4 and interleukin-13 pathways in severe asthma: Current knowledge and future needs. Curr. Opin. Pulm. Med. 2018;24:50–55. doi: 10.1097/MCP.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 34.Karta M.R., Broide D.H., Doherty T.A. Insights into Group 2 Innate Lymphoid Cells in Human Airway Disease. Curr. Allergy Asthma Rep. 2016;16:8. doi: 10.1007/s11882-015-0581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuchiwal S.K., Boyce J.A. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J. Allergy Clin. Immunol. 2018;141:1182–1190. doi: 10.1016/j.jaci.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y.H., Lee S.N., Aoyagi H., Yamasaki Y., Yoo J.Y., Park B., Shin D.M., Yoon H.G., Yoon J.H. The extracellular signal-regulated kinase mitogen-activated protein kinase/ribosomal S6 protein kinase 1 cascade phosphorylates cAMP response element-binding protein to induce MUC5B gene expression via D-prostanoid receptor signaling. J. Biol. Chem. 2011;286:34199–34214. doi: 10.1074/jbc.M111.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright D.H., Ford-Hutchinson A.W., Chadee K., Metters K.M. The human prostanoid DP receptor stimulates mucin secretion in LS174T cells. Br. J. Pharm. 2000;131:1537–1545. doi: 10.1038/sj.bjp.0703688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sally E.S., Yassine A., Christopher E.B. D prostanoid receptor 2 (chemoattractant receptor-homologous molecule expressed on TH2 cells) protein expression in asthmatic patients and its effects on bronchial epithelial cells. J. Allergy Clin. Immunol. 2015;135:395–406. doi: 10.1016/j.jaci.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akaba T., Komiya K., Suzaki I., Kozaki Y., Tamaoki J., Rubin B.K. Activating prostaglandin E2 receptor subtype EP4 increases secreted mucin from airway goblet cells. Pulm. Pharmacol. Ther. 2018;48:117–123. doi: 10.1016/j.pupt.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Kowalski M.L., Pawliczak R., Wozniak J., Siuda K., Poniatowska M., Iwaszkiewicz J., Kornatowski T., Kaliner M.A. Differential metabolism of arachidonic acid in nasal polyp epithelial cells cultured from aspirin-sensitive and aspirin-tolerant patients. Am. J. Respir. Crit. Care Med. 2000;161:391–398. doi: 10.1164/ajrccm.161.2.9902034. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt L.M., Belvisi M.G., Bode K.A., Bauer J., Schmidt C., Suchy M.T., Tsikas D., Scheuerer J., Lasitschka F., Grone H.J., et al. Bronchial epithelial cell-derived prostaglandin E2 dampens the reactivity of dendritic cells. J. Immunol. 2011;186:2095–2105. doi: 10.4049/jimmunol.1002414. [DOI] [PubMed] [Google Scholar]
- 42.Barnett K., Jacoby D.B., Nadel J.A., Lazarus S.C. The effects of epithelial cell supernatant on contractions of isolated canine tracheal smooth muscle. Am. Rev. Respir. Dis. 1988;138:780–783. doi: 10.1164/ajrccm/138.4.780. [DOI] [PubMed] [Google Scholar]
- 43.Scandella E., Men Y., Legler D.F., Gillessen S., Prikler L., Ludewig B., Groettrup M. CCL19/CCL21-triggered signal transduction and migration of dendritic cells requires prostaglandin E2. Blood. 2004;103:1595–1601. doi: 10.1182/blood-2003-05-1643. [DOI] [PubMed] [Google Scholar]
- 44.Harizi H., Grosset C., Gualde N. Prostaglandin E2 modulates dendritic cell function via EP2 and EP4 receptor subtypes. J. Leukoc. Biol. 2003;73:756–763. doi: 10.1189/jlb.1002483. [DOI] [PubMed] [Google Scholar]
- 45.Legler D.F., Krause P., Scandella E., Singer E., Groettrup M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006;176:966–973. doi: 10.4049/jimmunol.176.2.966. [DOI] [PubMed] [Google Scholar]
- 46.Hammad H., de Heer H.J., Soullie T., Hoogsteden H.C., Trottein F., Lambrecht B.N. Prostaglandin D2 inhibits airway dendritic cell migration and function in steady state conditions by selective activation of the D prostanoid receptor 1. J. Immunol. 2003;171:3936–3940. doi: 10.4049/jimmunol.171.8.3936. [DOI] [PubMed] [Google Scholar]
- 47.Hammad H., Kool M., Soullie T., Narumiya S., Trottein F., Hoogsteden H.C., Lambrecht B.N. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. J. Exp. Med. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Idzko M., Hammad H., van Nimwegen M., Kool M., Vos N., Hoogsteden H.C., Lambrecht B.N. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J. Clin. Investig. 2007;117:464–472. doi: 10.1172/JCI28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou W., Hashimoto K., Goleniewska K., O’Neal J.F., Ji S., Blackwell T.S., Fitzgerald G.A., Egan K.M., Geraci M.W., Peebles R.S., Jr. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J. Immunol. 2007;178:702–710. doi: 10.4049/jimmunol.178.2.702. [DOI] [PubMed] [Google Scholar]
- 50.Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv. Exp. Med. Biol. 1997;417:363–367. doi: 10.1007/978-1-4757-9966-8_59. [DOI] [PubMed] [Google Scholar]
- 51.Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 52.Kalinski P., Schuitemaker J.H., Hilkens C.M., Kapsenberg M.L. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: The levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J. Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 53.Walker W., Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12- and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaisar M.M.M., Ritter M., Del Fresno C., Jonasdottir H.S., van der Ham A.J., Pelgrom L.R., Schramm G., Layland L.E., Sancho D., Prazeres da Costa C., et al. Dectin-1/2-induced autocrine PGE2 signaling licenses dendritic cells to prime Th2 responses. PloS Biol. 2018;16:e2005504. doi: 10.1371/journal.pbio.2005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harizi H., Norbert G. Inhibition of IL-6, TNF-alpha, and cyclooxygenase-2 protein expression by prostaglandin E2-induced IL-10 in bone marrow-derived dendritic cells. Cell Immunol. 2004;228:99–109. doi: 10.1016/j.cellimm.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Vassiliou E., Jing H., Ganea D. Prostaglandin E2 inhibits TNF production in murine bone marrow-derived dendritic cells. Cell Immunol. 2003;223:120–132. doi: 10.1016/S0008-8749(03)00158-8. [DOI] [PubMed] [Google Scholar]
- 57.Harizi H., Juzan M., Pitard V., Moreau J.F., Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J. Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 58.Rieser C., Papesh C., Herold M., Bock G., Ramoner R., Klocker H., Bartsch G., Thurnher M. Differential deactivation of human dendritic cells by endotoxin desensitization: Role of tumor necrosis factor-alpha and prostaglandin E2. Blood. 1998;91:3112–3117. doi: 10.1182/blood.V91.9.3112. [DOI] [PubMed] [Google Scholar]
- 59.Rieser C., Bock G., Klocker H., Bartsch G., Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: Synergistic activation of interleukin 12 production. J. Exp. Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chizzolini C., Chicheportiche R., Alvarez M., de Rham C., Roux-Lombard P., Ferrari-Lacraz S., Dayer J.M. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong T.H., Gau R.J., Chen Y.F., Shen H.H., Lin C.T., Chen S.L., Suen J.L. Dendritic cells treated with a prostaglandin I2 analog, iloprost, promote antigen-specific regulatory T cell differentiation in mice. Int. Immunopharmacol. 2019;79:106106. doi: 10.1016/j.intimp.2019.106106. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka K., Hirai H., Takano S., Nakamura M., Nagata K. Effects of prostaglandin D2 on helper T cell functions. Biochem. Biophys. Res. Commun. 2004;316:1009–1014. doi: 10.1016/j.bbrc.2004.02.151. [DOI] [PubMed] [Google Scholar]
- 63.Mitson-Salazar A., Yin Y., Wansley D.L., Young M., Bolan H., Arceo S., Ho N., Koh C., Milner J.D., Stone K.D., et al. Hematopoietic prostaglandin D synthase defines a proeosinophilic pathogenic effector human T(H)2 cell subpopulation with enhanced function. J. Allergy Clin. Immunol. 2016;137:907–918. doi: 10.1016/j.jaci.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 64.Hilvering B., Hinks T.S.C., Stoger L., Marchi E., Salimi M., Shrimanker R., Liu W., Chen W., Luo J., Go S., et al. Synergistic activation of pro-inflammatory type-2 CD8(+) T lymphocytes by lipid mediators in severe eosinophilic asthma. Mucosal Immunol. 2018;11:1408–1419. doi: 10.1038/s41385-018-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wambre E., Bajzik V., DeLong J.H., O’Brien K., Nguyen Q.A., Speake C., Gersuk V.H., DeBerg H.A., Whalen E., Ni C., et al. A phenotypically and functionally distinct human TH2 cell subpopulation is associated with allergic disorders. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aam9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palikhe N.S., Laratta C., Nahirney D., Vethanayagam D., Bhutani M., Vliagoftis H., Cameron L. Elevated levels of circulating CD4(+) CRTh2(+) T cells characterize severe asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2016;46:825–836. doi: 10.1111/cea.12741. [DOI] [PubMed] [Google Scholar]
- 67.Shrestha Palikhe N., Bosonea A.M., Laratta C., Gandhi V.D., Nahirney D., Hillaby A., Bowen M., Bhutani M., Mayers I., Cameron L., et al. Stability of peripheral blood immune markers in patients with asthma. Allergy Asthma Clin. Immunol. 2019;15:30. doi: 10.1186/s13223-019-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snijdewint F.G., Kalinski P., Wierenga E.A., Bos J.D., Kapsenberg M.L. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J. Immunol. 1993;150:5321–5329. [PubMed] [Google Scholar]
- 69.Bao Y.S., Zhang P., Xie R.J., Wang M., Wang Z.Y., Zhou Z., Zhai W.J., Feng S.Z., Han M.Z. The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2. Int. Immunopharmacol. 2011;11:1599–1605. doi: 10.1016/j.intimp.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 70.Yao C., Hirata T., Soontrapa K., Ma X., Takemori H., Narumiya S. Prostaglandin E(2) promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat. Commun. 2013;4:1685. doi: 10.1038/ncomms2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaslona Z., Okunishi K., Bourdonnay E., Domingo-Gonzalez R., Moore B.B., Lukacs N.W., Aronoff D.M., Peters-Golden M. Prostaglandin E(2) suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J. Allergy Clin. Immunol. 2014;133:379–387. doi: 10.1016/j.jaci.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao C., Sakata D., Esaki Y., Li Y., Matsuoka T., Kuroiwa K., Sugimoto Y., Narumiya S. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat. Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
- 73.Lee J., Aoki T., Thumkeo D., Siriwach R., Yao C., Narumiya S. T cell-intrinsic prostaglandin E2-EP2/EP4 signaling is critical in pathogenic TH17 cell-driven inflammation. J. Allergy Clin. Immunol. 2019;143:631–643. doi: 10.1016/j.jaci.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li L., Guan K., Zhou Y., Wu J., Wang Y., Wang W. Prostaglandin E2 signal inhibits T regulatory cell differentiation during allergic rhinitis inflammation through EP4 receptor. World Allergy Organ. J. 2019;12:100090. doi: 10.1016/j.waojou.2019.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawahara K., Hohjoh H., Inazumi T., Tsuchiya S., Sugimoto Y. Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim. Biophys. Acta. 2015;1851:414–421. doi: 10.1016/j.bbalip.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Gao Y., Zhao C., Wang W., Jin R., Li Q., Ge Q., Guan Y., Zhang Y. Prostaglandins E2 signal mediated by receptor subtype EP2 promotes IgE production in vivo and contributes to asthma development. Sci. Rep. 2016;6:20505. doi: 10.1038/srep20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roper R.L., Conrad D.H., Brown D.M., Warner G.L., Phipps R.P. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J. Immunol. 1990;145:2644–2651. [PubMed] [Google Scholar]
- 78.Fedyk E.R., Phipps R.P. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc. Natl. Acad. Sci. USA. 1996;93:10978–10983. doi: 10.1073/pnas.93.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barnig C., Cernadas M., Dutile S., Liu X., Perrella M.A., Kazani S., Wechsler M.E., Israel E., Levy B.D. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tojima I., Matsumoto K., Kikuoka H., Hara S., Yamamoto S., Shimizu S., Kouzaki H., Shimizu T. Evidence for the induction of Th2 inflammation by group 2 innate lymphoid cells in response to prostaglandin D2 and cysteinyl leukotrienes in allergic rhinitis. Allergy. 2019;74:2417–2426. doi: 10.1111/all.13974. [DOI] [PubMed] [Google Scholar]
- 81.Xue L., Salimi M., Panse I., Mjosberg J.M., McKenzie A.N., Spits H., Klenerman P., Ogg G. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 2014;133:1184–1194. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oyesola O.O., Duque C., Huang L.C., Larson E.M., Fruh S.P., Webb L.M., Peng S.A., Tait Wojno E.D. The Prostaglandin D2 Receptor CRTH2 Promotes IL-33-Induced ILC2 Accumulation in the Lung. J. Immunol. 2020 doi: 10.4049/jimmunol.1900745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen R., Smith S.G., Salter B., El-Gammal A., Oliveria J.P., Obminski C., Watson R., O’Byrne P.M., Gauvreau G.M., Sehmi R. Allergen-induced Increases in Sputum Levels of Group 2 Innate Lymphoid Cells in Subjects with Asthma. Am. J. Respir. Crit. Care Med. 2017;196:700–712. doi: 10.1164/rccm.201612-2427OC. [DOI] [PubMed] [Google Scholar]
- 84.Winkler C., Hochdorfer T., Israelsson E., Hasselberg A., Cavallin A., Thorn K., Muthas D., Shojaee S., Luer K., Muller M., et al. Activation of group 2 innate lymphoid cells after allergen challenge in asthmatic patients. J. Allergy Clin. Immunol. 2019;144:61–69. doi: 10.1016/j.jaci.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 85.Eastman J.J., Cavagnero K.J., Deconde A.S., Kim A.S., Karta M.R., Broide D.H., Zuraw B.L., White A.A., Christiansen S.C., Doherty T.A. Group 2 innate lymphoid cells are recruited to the nasal mucosa in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2017;140:101–108. doi: 10.1016/j.jaci.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hardman C., Chen W., Luo J., Batty P., Chen Y.L., Nahler J., Wu Y., Pavord I.D., Erpenbeck V.J., Sandham D.A., et al. Fevipiprant, a selective prostaglandin D2 receptor 2 antagonist, inhibits human group 2 innate lymphoid cell aggregation and function. J. Allergy Clin. Immunol. 2019;143:2329–2333. doi: 10.1016/j.jaci.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 87.Maric J., Ravindran A., Mazzurana L., Van Acker A., Rao A., Kokkinou E., Ekoff M., Thomas D., Fauland A., Nilsson G., et al. Cytokine-induced endogenous production of prostaglandin D2 is essential for human group 2 innate lymphoid cell activation. J. Allergy Clin. Immunol. 2019;143:2202–2214. doi: 10.1016/j.jaci.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 88.Maric J., Ravindran A., Mazzurana L., Bjorklund A.K., Van Acker A., Rao A., Friberg D., Dahlen S.E., Heinemann A., Konya V., et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J. Allergy Clin. Immunol. 2018;141:1761–1773. doi: 10.1016/j.jaci.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou Y., Wang W., Zhao C., Wang Y., Wu H., Sun X., Guan Y., Zhang Y. Prostaglandin E2 Inhibits Group 2 Innate Lymphoid Cell Activation and Allergic Airway Inflammation Through E-Prostanoid 4-Cyclic Adenosine Monophosphate Signaling. Front. Immunol. 2018;9:501. doi: 10.3389/fimmu.2018.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou W., Toki S., Zhang J., Goleniewksa K., Newcomb D.C., Cephus J.Y., Dulek D.E., Bloodworth M.H., Stier M.T., Polosuhkin V., et al. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am. J. Respir. Crit. Care Med. 2016;193:31–42. doi: 10.1164/rccm.201410-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peinhaupt M., Sturm E.M., Heinemann A. Prostaglandins and Their Receptors in Eosinophil Function and As Therapeutic Targets. Front. Med. (Lausanne) 2017;4:104. doi: 10.3389/fmed.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sedej M., Schroder R., Bell K., Platzer W., Vukoja A., Kostenis E., Heinemann A., Waldhoer M. D-type prostanoid receptor enhances the signaling of chemoattractant receptor-homologous molecule expressed on T(H)2 cells. J. Allergy Clin. Immunol. 2012;129:492–500. doi: 10.1016/j.jaci.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 93.Ueki S., Adachi T., Bourdeaux J., Oyamada H., Yamada Y., Hamada K., Kanda A., Kayaba H., Chihara J. Expression of PPARgamma in eosinophils and its functional role in survival and chemotaxis. Immunol. Lett. 2003;86:183–189. doi: 10.1016/S0165-2478(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 94.Gervais F.G., Cruz R.P., Chateauneuf A., Gale S., Sawyer N., Nantel F., Metters K.M., O’Neill G P. Selective modulation of chemokinesis, degranulation, and apoptosis in eosinophils through the PGD2 receptors CRTH2 and DP. J. Allergy Clin. Immunol. 2001;108:982–988. doi: 10.1067/mai.2001.119919. [DOI] [PubMed] [Google Scholar]
- 95.Monneret G., Gravel S., Diamond M., Rokach J., Powell W.S. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–1948. doi: 10.1182/blood.V98.6.1942. [DOI] [PubMed] [Google Scholar]
- 96.Shamri R., Dubois G., Erpenbeck V.J., Mankuta D., Sandham D.A., Levi-Schaffer F. Fevipiprant, a DP2 receptor antagonist, inhibits eosinophil migration towards mast cells. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019;49:255–257. doi: 10.1111/cea.13304. [DOI] [PubMed] [Google Scholar]
- 97.Heinemann A., Schuligoi R., Sabroe I., Hartnell A., Peskar B.A. Delta 12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J. Immunol. 2003;170:4752–4758. doi: 10.4049/jimmunol.170.9.4752. [DOI] [PubMed] [Google Scholar]
- 98.Schratl P., Sturm E.M., Royer J.F., Sturm G.J., Lippe I.T., Peskar B.A., Heinemann A. Hierarchy of eosinophil chemoattractants: Role of p38 mitogen-activated protein kinase. Eur J. Immunol. 2006;36:2401–2409. doi: 10.1002/eji.200535672. [DOI] [PubMed] [Google Scholar]
- 99.Schratl P., Royer J.F., Kostenis E., Ulven T., Sturm E.M., Waldhoer M., Hoefler G., Schuligoi R., Lippe I.T., Peskar B.A., et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J. Immunol. 2007;179:4792–4799. doi: 10.4049/jimmunol.179.7.4792. [DOI] [PubMed] [Google Scholar]
- 100.Mesquita-Santos F.P., Bakker-Abreu I., Luna-Gomes T., Bozza P.T., Diaz B.L., Bandeira-Melo C. Co-operative signalling through DP(1) and DP(2) prostanoid receptors is required to enhance leukotriene C(4) synthesis induced by prostaglandin D(2) in eosinophils. Br. J. Pharm. 2011;162:1674–1685. doi: 10.1111/j.1476-5381.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feng X., Ramsden M.K., Negri J., Baker M.G., Payne S.C., Borish L., Steinke J.W. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2016;138:1089–1097. doi: 10.1016/j.jaci.2016.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teixeira M.M., al-Rashed S., Rossi A.G., Hellewell P.G. Characterization of the prostanoid receptors mediating inhibition of PAF-induced aggregation of guinea-pig eosinophils. Br. J. Pharm. 1997;121:77–82. doi: 10.1038/sj.bjp.0701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butchers P.R., Vardey C.J. The effect of prostanoids on the function of human eosinophils. Agents Actions Suppl. 1990;31:103–112. doi: 10.1007/978-3-0348-7379-6_12. [DOI] [PubMed] [Google Scholar]
- 104.Luschnig-Schratl P., Sturm E.M., Konya V., Philipose S., Marsche G., Frohlich E., Samberger C., Lang-Loidolt D., Gattenlohner S., Lippe I.T., et al. EP4 receptor stimulation down-regulates human eosinophil function. Cell Mol. Life Sci. 2011;68:3573–3587. doi: 10.1007/s00018-011-0642-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sturm E.M., Schratl P., Schuligoi R., Konya V., Sturm G.J., Lippe I.T., Peskar B.A., Heinemann A. Prostaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptors. J. Immunol. 2008;181:7273–7283. doi: 10.4049/jimmunol.181.10.7273. [DOI] [PubMed] [Google Scholar]
- 106.Peacock C.D., Misso N.L., Watkins D.N., Thompson P.J. PGE 2 and dibutyryl cyclic adenosine monophosphate prolong eosinophil survival in vitro. J. Allergy Clin. Immunol. 1999;104:153–162. doi: 10.1016/S0091-6749(99)70127-2. [DOI] [PubMed] [Google Scholar]
- 107.Konya V., Philipose S., Balint Z., Olschewski A., Marsche G., Sturm E.M., Schicho R., Peskar B.A., Schuligoi R., Heinemann A. Interaction of eosinophils with endothelial cells is modulated by prostaglandin EP4 receptors. Eur J. Immunol. 2011;41:2379–2389. doi: 10.1002/eji.201141460. [DOI] [PubMed] [Google Scholar]
- 108.Pal K., Ramsden M., Shim Y.M., Borish L., Payne S.C., Steinke J.W. Suppression of aspirin-mediated eosinophil activation by prostaglandin E2: Relevance to aspirin and nonsteroidal anti-inflammatory drug hypersensitivity. Ann. Allergy Asthma Immunol. 2019;123:503–506. doi: 10.1016/j.anai.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sturm E.M., Schuligoi R., Konya V., Sturm G.J., Heinemann A. Inhibitory effect of prostaglandin I2 on bone marrow kinetics of eosinophils in the guinea pig. J. Leukoc. Biol. 2011;90:285–291. doi: 10.1189/jlb.0211087. [DOI] [PubMed] [Google Scholar]
- 110.Konya V., Sturm E.M., Schratl P., Beubler E., Marsche G., Schuligoi R., Lippe I.T., Peskar B.A., Heinemann A. Endothelium-derived prostaglandin I(2) controls the migration of eosinophils. J. Allergy Clin. Immunol. 2010;125:1105–1113. doi: 10.1016/j.jaci.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Baothman B.K., Smith J., Kay L.J., Suvarna S.K., Peachell P.T. Prostaglandin D2 generation from human lung mast cells is catalysed exclusively by cyclooxygenase-1. Eur. J. Pharm. 2018;819:225–232. doi: 10.1016/j.ejphar.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 112.Buchheit K.M., Cahill K.N., Katz H.R., Murphy K.C., Feng C., Lee-Sarwar K., Lai J., Bhattacharyya N., Israel E., Boyce J.A., et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2016;137:1566–1576. doi: 10.1016/j.jaci.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Moon T.C., Campos-Alberto E., Yoshimura T., Bredo G., Rieger A.M., Puttagunta L., Barreda D.R., Befus A.D., Cameron L. Expression of DP2 (CRTh2), a prostaglandin D(2) receptor, in human mast cells. PLoS ONE. 2014;9:e108595. doi: 10.1371/journal.pone.0108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xia J., Abdu S., Maguire T.J.A., Hopkins C., Till S.J., Woszczek G. Prostaglandin D2 receptors in human mast cells. Allergy. 2019 doi: 10.1111/all.14161. [DOI] [PubMed] [Google Scholar]
- 115.Feng C., Beller E.M., Bagga S., Boyce J.A. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kay L.J., Yeo W.W., Peachell P.T. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharm. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Torres R., Picado C., de Mora F. The PGE2-EP2-mast cell axis: An antiasthma mechanism. Mol. Immunol. 2015;63:61–68. doi: 10.1016/j.molimm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 118.Bradbury D., Clarke D., Seedhouse C., Corbett L., Stocks J., Knox A. Vascular endothelial growth factor induction by prostaglandin E2 in human airway smooth muscle cells is mediated by E prostanoid EP2/EP4 receptors and SP-1 transcription factor binding sites. J. Biol. Chem. 2005;280:29993–30000. doi: 10.1074/jbc.M414530200. [DOI] [PubMed] [Google Scholar]
- 119.Pavord I.D., Wong C.S., Williams J., Tattersfield A.E. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am. Rev. Respir. Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 120.Benyahia C., Gomez I., Kanyinda L., Boukais K., Danel C., Leseche G., Longrois D., Norel X. PGE(2) receptor (EP(4)) agonists: Potent dilators of human bronchi and future asthma therapy? Pulm. Pharmacol. Ther. 2012;25:115–118. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 121.Buckley J., Birrell M.A., Maher S.A., Nials A.T., Clarke D.L., Belvisi M.G. EP4 receptor as a new target for bronchodilator therapy. Thorax. 2011;66:1029–1035. doi: 10.1136/thx.2010.158568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lazzeri N., Belvisi M.G., Patel H.J., Yacoub M.H., Chung K.F., Mitchell J.A. Effects of prostaglandin E2 and cAMP elevating drugs on GM-CSF release by cultured human airway smooth muscle cells. Relevance to asthma therapy. Am. J. Respir. Cell Mol. Biol. 2001;24:44–48. doi: 10.1165/ajrcmb.24.1.4027. [DOI] [PubMed] [Google Scholar]
- 123.Aso H., Ito S., Mori A., Suganuma N., Morioka M., Takahara N., Kondo M., Hasegawa Y. Differential regulation of airway smooth muscle cell migration by E-prostanoid receptor subtypes. Am. J. Respir. Cell Mol. Biol. 2013;48:322–329. doi: 10.1165/rcmb.2012-0158OC. [DOI] [PubMed] [Google Scholar]
- 124.Saunders R., Kaul H., Berair R., Gonem S., Singapuri A., Sutcliffe A.J., Chachi L., Biddle M.S., Kaur D., Bourne M., et al. DP2 antagonism reduces airway smooth muscle mass in asthma by decreasing eosinophilia and myofibroblast recruitment. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aao6451. [DOI] [PubMed] [Google Scholar]
- 125.Smith A.P., Cuthbert M.F., Dunlop L.S. Effects of inhaled prostaglandins E1, E2, and F2alpha on the airway resistance of healthy and asthmatic man. Clin. Sci. Mol. Med. 1975;48:421–430. doi: 10.1042/cs0480421. [DOI] [PubMed] [Google Scholar]
- 126.Davi G., Basili S., Vieri M., Cipollone F., Santarone S., Alessandri C., Gazzaniga P., Cordova C., Violi F. Enhanced thromboxane biosynthesis in patients with chronic obstructive pulmonary disease. The Chronic Obstructive Bronchitis and Haemostasis Study Group. Am. J. Respir. Crit. Care Med. 1997;156:1794–1799. doi: 10.1164/ajrccm.156.6.9706026. [DOI] [PubMed] [Google Scholar]
- 127.Lei Y., Cao Y., Zhang Y., Edvinsson L., Xu C.B. Enhanced airway smooth muscle cell thromboxane receptor signaling via activation of JNK MAPK and extracellular calcium influx. Eur J. Pharm. 2011;650:629–638. doi: 10.1016/j.ejphar.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 128.Han D.Y., Cho J.S., Moon Y.M., Lee H.R., Lee H.M., Lee B.D., Baek B.J. Effect of prostaglandin e2 on vascular endothelial growth factor production in nasal polyp fibroblasts. Allergy Asthma Immunol. Res. 2013;5:224–231. doi: 10.4168/aair.2013.5.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roca-Ferrer J., Garcia-Garcia F.J., Pereda J., Perez-Gonzalez M., Pujols L., Alobid I., Mullol J., Picado C. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J. Allergy Clin. Immunol. 2011;128:66–72. doi: 10.1016/j.jaci.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 130.Cahill K.N., Raby B.A., Zhou X., Guo F., Thibault D., Baccarelli A., Byun H.M., Bhattacharyya N., Steinke J.W., Boyce J.A., et al. Impaired E Prostanoid2 Expression and Resistance to Prostaglandin E2 in Nasal Polyp Fibroblasts from Subjects with Aspirin-Exacerbated Respiratory Disease. Am. J. Respir. Cell Mol. Biol. 2016;54:34–40. doi: 10.1165/rcmb.2014-0486OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanai K., Okano M., Fujiwara T., Kariya S., Haruna T., Omichi R., Makihara S.I., Hirata Y., Nishizaki K. Effect of prostaglandin D2 on VEGF release by nasal polyp fibroblasts. Allergol. Int. 2016;65:414–419. doi: 10.1016/j.alit.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Erpenbeck V.J., Popov T.A., Miller D., Weinstein S.F., Spector S., Magnusson B., Osuntokun W., Goldsmith P., Weiss M., Beier J. The oral CRTh2 antagonist QAW039 (fevipiprant): A phase II study in uncontrolled allergic asthma. Pulm. Pharmacol. Ther. 2016;39:54–63. doi: 10.1016/j.pupt.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 133.Bateman E.D., Guerreros A.G., Brockhaus F., Holzhauer B., Pethe A., Kay R.A., Townley R.G. Fevipiprant, an oral prostaglandin DP2 receptor (CRTh2) antagonist, in allergic asthma uncontrolled on low-dose inhaled corticosteroids. Eur. Respir. J. 2017;50 doi: 10.1183/13993003.00670-2017. [DOI] [PubMed] [Google Scholar]
- 134.Gonem S., Berair R., Singapuri A., Hartley R., Laurencin M.F.M., Bacher G., Holzhauer B., Bourne M., Mistry V., Pavord I.D., et al. Fevipiprant, a prostaglandin D2 receptor 2 antagonist, in patients with persistent eosinophilic asthma: A single-centre, randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir. Med. 2016;4:699–707. doi: 10.1016/S2213-2600(16)30179-5. [DOI] [PubMed] [Google Scholar]
- 135.Wenzel S., Chantry D., Eberhardt C., Hopkins R., Saunders M., Anderson L., Aitchinson R., Bell S., Izuhara K., Ono J., et al. ARRY-502, a potent, selective, oral CRTh2 antagonist reduces Th2 mediators in patients with mild to moderate Th2-driven asthma. Eur. Respir. J. 2014;44:4836. [Google Scholar]
- 136.Kuna P., Bjermer L., Tornling G. Two Phase II randomized trials on the CRTh2 antagonist AZD1981 in adults with asthma. Drug Des. c. 2016;10:2759–2770. doi: 10.2147/DDDT.S105142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pettipher R., Hunter M.G., Perkins C.M., Collins L.P., Lewis T., Baillet M., Steiner J., Bell J., Payton M.A. Heightened response of eosinophilic asthmatic patients to the CRTH2 antagonist OC000459. Allergy. 2014;69:1223–1232. doi: 10.1111/all.12451. [DOI] [PubMed] [Google Scholar]
- 138.Horak F., Zieglmayer P., Zieglmayer R., Lemell P., Collins L.P., Hunter M.G., Steiner J., Lewis T., Payton M.A., Perkins C.M., et al. The CRTH2 antagonist OC000459 reduces nasal and ocular symptoms in allergic subjects exposed to grass pollen, a randomised, placebo-controlled, double-blind trial. Allergy. 2012;67:1572–1579. doi: 10.1111/all.12042. [DOI] [PubMed] [Google Scholar]
- 139.Ortega H., Fitzgerald M., Raghupathi K., Tompkins C.A., Shen J., Dittrich K., Pattwell C., Singh D. A phase 2 study to evaluate the safety, efficacy and pharmacokinetics of DP2 antagonist GB001 and to explore biomarkers of airway inflammation in mild-to-moderate asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2019 doi: 10.1111/cea.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hall I.P., Fowler A.V., Gupta A., Tetzlaff K., Nivens M.C., Sarno M., Finnigan H.A., Bateman E.D., Rand Sutherland E. Efficacy of BI 671800, an oral CRTH2 antagonist, in poorly controlled asthma as sole controller and in the presence of inhaled corticosteroid treatment. Pulm. Pharmacol. Ther. 2015;32:37–44. doi: 10.1016/j.pupt.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 141.Diamant Z., Sidharta P.N., Singh D., O’Connor B.J., Zuiker R., Leaker B.R., Silkey M., Dingemanse J. Setipiprant, a selective CRTH2 antagonist, reduces allergen-induced airway responses in allergic asthmatics. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014;44:1044–1052. doi: 10.1111/cea.12357. [DOI] [PubMed] [Google Scholar]
- 142.Ratner P., Andrews C.P., Hampel F.C., Martin B., Mohar D.E., Bourrelly D., Danaietash P., Mangialaio S., Dingemanse J., Hmissi A., et al. Efficacy and safety of setipiprant in seasonal allergic rhinitis: Results from Phase 2 and Phase 3 randomized, double-blind, placebo- and active-referenced studies. Allergy Asthma Clin. Immunol. 2017;13:18. doi: 10.1186/s13223-017-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Terada N., Yamakoshi T., Hasegawa M., Tanikawa H., Maesako K., Ishikawa K., Konno A. The effect of ramatroban (BAY u 3405), a thromboxane A2 receptor antagonist, on nasal cavity volume and minimum cross-sectional area and nasal mucosal hemodynamics after nasal mucosal allergen challenge in patients with perennial allergic rhinitis. Acta Otolaryngol. Suppl. 1998;537:32–37. doi: 10.1080/00016489850182323. [DOI] [PubMed] [Google Scholar]
- 144.Terada N., Yamakoshi T., Hasegawa M., Tanikawa H., Nagata H., Maesako K.-i., Konno A. Effect of a thromboxane A2 receptor antagonist ramatroban (BAY u 3405), on inflammatory cells, chemical mediators and non-specific nasal hyperreactivity after allergen challenge in patients with perennial allergic rhinitis. Allergol. Int. 1998;47:59–67. doi: 10.2332/allergolint.47.59. [DOI] [Google Scholar]
- 145.Aizawa H., Inoue H., Nakano H., Matsumoto K., Yoshida M., Fukuyama S., Koto H., Hara N. Effects of thromboxane A2 antagonist on airway hyperresponsiveness, exhaled nitric oxide, and induced sputum eosinophils in asthmatics. Prostaglandins Leukot Essent Fat. Acids. 1998;59:185–190. doi: 10.1016/S0952-3278(98)90061-8. [DOI] [PubMed] [Google Scholar]
- 146.Hoshino M., Sim J., Shimizu K., Nakayama H., Koya A. Effect of AA-2414, a thromboxane A2 receptor antagonist, on airway inflammation in subjects with asthma. J. Allergy Clin. Immunol. 1999;103:1054–1061. doi: 10.1016/S0091-6749(99)70179-X. [DOI] [PubMed] [Google Scholar]
- 147.Okubo K., Hashiguchi K., Takeda T., Baba K., Kitagoh H., Miho H., Tomomatsu H., Yamaguchi S., Odani M., Yamamotoya H. A randomized controlled phase II clinical trial comparing ONO-4053, a novel DP1 antagonist, with a leukotriene receptor antagonist pranlukast in patients with seasonal allergic rhinitis. Allergy. 2017;72:1565–1575. doi: 10.1111/all.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bianco S., Robuschi M., Grugni A., Ceserani R., Gandolfi C. Effect of prostacyclin on antigen induced immediate bronchoconstriction in asthmatic patients. Prostaglandins Med. 1979;3:39–45. doi: 10.1016/0161-4630(79)90014-4. [DOI] [PubMed] [Google Scholar]
- 149.Liu M.C., Bleecker E.R., Lichtenstein L.M., Kagey-Sobotka A., Niv Y., McLemore T.L., Permutt S., Proud D., Hubbard W.C. Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am. Rev. Respir. Dis. 1990;142:126–132. doi: 10.1164/ajrccm/142.1.126. [DOI] [PubMed] [Google Scholar]
- 150.Liu M.C., Hubbard W.C., Proud D., Stealey B.A., Galli S.J., Kagey-Sobotka A., Bleecker E.R., Lichtenstein L.M. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am. Rev. Respir. Dis. 1991;144:51–58. doi: 10.1164/ajrccm/144.1.51. [DOI] [PubMed] [Google Scholar]
- 151.Fajt M.L., Gelhaus S.L., Freeman B., Uvalle C.E., Trudeau J.B., Holguin F., Wenzel S.E. Prostaglandin D(2) pathway upregulation: Relation to asthma severity, control, and TH2 inflammation. J. Allergy Clin. Immunol. 2013;131:1504–1512. doi: 10.1016/j.jaci.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pavord I.D., Ward R., Woltmann G., Wardlaw A.J., Sheller J.R., Dworski R. Induced sputum eicosanoid concentrations in asthma. Am. J. Respir. Crit. Care Med. 1999;160:1905–1909. doi: 10.1164/ajrccm.160.6.9903114. [DOI] [PubMed] [Google Scholar]
- 153.Marone G., Galdiero M.R., Pecoraro A., Pucino V., Criscuolo G., Triassi M., Varricchi G. Prostaglandin D2 receptor antagonists in allergic disorders: Safety, efficacy, and future perspectives. Expert Opin. Investig. Drugs. 2019;28:73–84. doi: 10.1080/13543784.2019.1555237. [DOI] [PubMed] [Google Scholar]
- 154.Wendell S.G., Fan H., Zhang C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020;72:1–49. doi: 10.1124/pr.118.016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu W., Min J., Jiang H., Mao B. Chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) antagonists in asthma: A systematic review and meta-analysis protocol. Bmj Open. 2018;8:e020882. doi: 10.1136/bmjopen-2017-020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Murillo J.C., Dimov V., Gonzalez-Estrada A. An evaluation of fevipiprant for the treatment of asthma: A promising new therapy? Expert Opin. Pharmacother. 2018;19:2087–2093. doi: 10.1080/14656566.2018.1540589. [DOI] [PubMed] [Google Scholar]
- 157.White C., Wright A., Brightling C. Fevipiprant in the treatment of asthma. Expert Opin. Investig. Drugs. 2018;27:199–207. doi: 10.1080/13543784.2018.1432592. [DOI] [PubMed] [Google Scholar]
- 158.Erpenbeck V.J., Popov T.A., Miller D., Weinstein S.F., Spector S., Magnusson B., Osuntokun W., Goldsmith P., Weiss M., Beier J. Data on the oral CRTh2 antagonist QAW039 (fevipiprant) in patients with uncontrolled allergic asthma. Data Brief. 2016;9:199–205. doi: 10.1016/j.dib.2016.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kao C.C., Parulekar A.D. Spotlight on fevipiprant and its potential in the treatment of asthma: Evidence to date. J. Asthma Allergy. 2019;12:1–5. doi: 10.2147/JAA.S167973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pelaia C., Crimi C., Vatrella A., Busceti M.T., Gaudio A., Garofalo E., Bruni A., Terracciano R., Pelaia G. New treatments for asthma: From the pathogenic role of prostaglandin d2 to the therapeutic effects of fevipiprant. Pharmacol. Res. 2019;155:104490. doi: 10.1016/j.phrs.2019.104490. [DOI] [PubMed] [Google Scholar]
- 161.Study of Efficacy and Safety of QAW039 in Patients with Severe Asthma Inadequately Controlled with Standard of Care Asthma Treatment. [(accessed on 8 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02555683.
- 162.Study of Efficacy and Safety of QAW039 in Patients with Severe Asthma Inadequately Controlled with Standard of Care Asthma Treatment. [(accessed on 8 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02563067.
- 163.Study of Efficacy and Safety of QAW039 When Added to Standard-of-Care Asthma Therapy in Patients with Uncontrolled Asthma. [(accessed on 8 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03215758.
- 164.Study of Efficacy and Safety of QAW039 When Added to Standard-of-Care Asthma Therapy in Patients with Uncontrolled Asthma. [(accessed on 8 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03226392.
- 165.Asano K., Sagara H., Ichinose M., Hirata M., Nakajima A., Ortega H., Tohda Y. A Phase 2a Study of DP2 antagonist GB001 for Asthma. J. Allergy Clin. Immunol. Pract. 2019 doi: 10.1016/j.jaip.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 166.Langfang Xinghe Industry Co., Ltd. Study of the Tolerability and Pharmacokinetic of ZL-2102 with an Investigation of Food Effect in Healthy Male Subjects. [(accessed on 8 January 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02397005.
- 167.Aggarwal S., Moodley Y.P., Thompson P.J., Misso N.L. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: Association with asthma severity and eosinophilic inflammation. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010;40:85–93. doi: 10.1111/j.1365-2222.2009.03386.x. [DOI] [PubMed] [Google Scholar]
- 168.Gauvreau G.M., Watson R.M., O’Byrne P.M. Protective effects of inhaled PGE2 on allergen-induced airway responses and airway inflammation. Am. J. Respir. Crit. Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- 169.Wasiak W., Szmidt M. A six week double blind, placebo controlled, crossover study of the effect of misoprostol in the treatment of aspirin sensitive asthma. Thorax. 1999;54:900–904. doi: 10.1136/thx.54.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mathe A.A., Hedqvist P., Holmgren A., Svanborg N. Bronchial hyperreactivity to prostaglandin F 2 and histamine in patients with asthma. Br. Med. J. 1973;1:193–196. doi: 10.1136/bmj.1.5847.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kharitonov S.A., Sapienza M.A., Barnes P.J., Chung K.F. Prostaglandins E2 and F2alpha reduce exhaled nitric oxide in normal and asthmatic subjects irrespective of airway caliber changes. Am. J. Respir. Crit. Care Med. 1998;158:1374–1378. doi: 10.1164/ajrccm.158.5.9707076. [DOI] [PubMed] [Google Scholar]
- 172.Fujimura M., Ozawa S., Matsuda T. Effect of oral administration of a prostacyclin analog (OP-41483) on pulmonary function and bronchial responsiveness in stable asthmatic subjects. J. Asthma. 1991;28:419–424. doi: 10.3109/02770909109110624. [DOI] [PubMed] [Google Scholar]
- 173.Bianco S., Robuschi M., Ceserani R., Gandolfi C. Effects of prostacyclin on aspecifically and specifically induced bronchoconstriction in asthmatic patients. Eur J. Respir. Dis. Suppl. 1980;106:81–87. [PubMed] [Google Scholar]
- 174.Hardy C.C., Bradding P., Robinson C., Holgate S.T. Bronchoconstrictor and antibronchoconstrictor properties of inhaled prostacyclin in asthma. J. Appl. Physiol. (1985) 1988;64:1567–1574. doi: 10.1152/jappl.1988.64.4.1567. [DOI] [PubMed] [Google Scholar]
- 175.Naclerio R.M., Creticos P.S., Norman P.S., Lichtenstein L.M. Mediator release after nasal airway challenge with allergen. Am. Rev. Respir. Dis. 1986;134:1102. doi: 10.1164/arrd.1986.134.5.1102. [DOI] [PubMed] [Google Scholar]
- 176.Shirasaki H., Kikuchi M., Kanaizumi E., Himi T. Accumulation of CRTH2-positive leukocytes in human allergic nasal mucosa. Ann. Allergy Asthma Immunol. 2009;102:110–115. doi: 10.1016/S1081-1206(10)60239-6. [DOI] [PubMed] [Google Scholar]
- 177.Ciebiada M., Gorski P., Antczak A. Evaluation of eicosanoids in nasal lavage as biomarkers of inflammation in patients with allergic rhinitis. Arch. Med. Sci. 2014;10:1123–1128. doi: 10.5114/aoms.2015.47655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnston S.L., Smith S., Harrison J., Ritter W., Howarth P.H. The effect of BAY u 3405, a thromboxane receptor antagonist, on prostaglandin D2-induced nasal blockage. J. Allergy Clin. Immunol. 1993;91:903–909. doi: 10.1016/0091-6749(93)90348-J. [DOI] [PubMed] [Google Scholar]
- 179.Kajiwara D., Aoyagi H., Shigeno K., Togawa M., Tanaka K., Inagaki N., Miyoshi K. Role of hematopoietic prostaglandin D synthase in biphasic nasal obstruction in guinea pig model of experimental allergic rhinitis. Eur. J. Pharm. 2011;667:389–395. doi: 10.1016/j.ejphar.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 180.Nabe T., Kuriyama Y., Mizutani N., Shibayama S., Hiromoto A., Fujii M., Tanaka K., Kohno S. Inhibition of hematopoietic prostaglandin D synthase improves allergic nasal blockage in guinea pigs. Prostaglandins Other Lipid Mediat. 2011;95:27–34. doi: 10.1016/j.prostaglandins.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 181.Steinke J.W., Wilson J.M. Aspirin-exacerbated respiratory disease: Pathophysiological insights and clinical advances. J. Asthma Allergy. 2016;9:37–43. doi: 10.2147/JAA.S88739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li K.L., Lee A.Y., Abuzeid W.M. Aspirin Exacerbated Respiratory Disease: Epidemiology, Pathophysiology, and Management. Med. Sci. 2019;7:45. doi: 10.3390/medsci7030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steinke J.W., Borish L. Factors driving the aspirin exacerbated respiratory disease phenotype. Am. J. Rhinol. Allergy. 2015;29:35–40. doi: 10.2500/ajra.2015.29.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Parker A.R., Ayars A.G., Altman M.C., Henderson W.R., Jr. Lipid Mediators in Aspirin-Exacerbated Respiratory Disease. Immunol. Allergy Clin. North. Am. 2016;36:749–763. doi: 10.1016/j.iac.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 185.Mastalerz L., Tyrak K.E., Ignacak M., Konduracka E., Mejza F., Cmiel A., Buczek M., Kot A., Oles K., Sanak M. Prostaglandin E2 decrease in induced sputum of hypersensitive asthmatics during oral challenge with aspirin. Allergy. 2019;74:922–932. doi: 10.1111/all.13671. [DOI] [PubMed] [Google Scholar]
- 186.Adamusiak A.M., Stasikowska-Kanicka O., Lewandowska-Polak A., Danilewicz M., Wagrowska-Danilewicz M., Jankowski A., Kowalski M.L., Pawliczak R. Expression of arachidonate metabolism enzymes and receptors in nasal polyps of aspirin-hypersensitive asthmatics. Int. Arch. Allergy Immunol. 2012;157:354–362. doi: 10.1159/000329744. [DOI] [PubMed] [Google Scholar]
- 187.Machado-Carvalho L., Torres R., Perez-Gonzalez M., Alobid I., Mullol J., Pujols L., Roca-Ferrer J., Picado C. Altered expression and signalling of EP2 receptor in nasal polyps of AERD patients: Role in inflammation and remodelling. Rhinology. 2016;54:254–265. doi: 10.4193/Rhin15.207. [DOI] [PubMed] [Google Scholar]
- 188.Walters K.M., Simon R.A., Woessner K.M., Wineinger N.E., White A.A. Effect of misoprostol on patients with aspirin-exacerbated respiratory disease undergoing aspirin challenge and desensitization. Ann. Allergy Asthma Immunol. 2017;119:71–76. doi: 10.1016/j.anai.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 189.Cahill K.N., Bensko J.C., Boyce J.A., Laidlaw T.M. Prostaglandin D(2): A dominant mediator of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2015;135:245–252. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Szczeklik A., Sladek K., Dworski R., Nizankowska E., Soja J., Sheller J., Oates J. Bronchial aspirin challenge causes specific eicosanoid response in aspirin-sensitive asthmatics. Am. J. Respir. Crit. Care Med. 1996;154:1608–1614. doi: 10.1164/ajrccm.154.6.8970343. [DOI] [PubMed] [Google Scholar]
- 191.Rusznak M., Peebles R.S., Jr. Prostaglandin E2 in NSAID-exacerbated respiratory disease: Protection against cysteinyl leukotrienes and group 2 innate lymphoid cells. Curr. Opin. Allergy Clin. Immunol. 2019;19:38–45. doi: 10.1097/ACI.0000000000000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bochenek G., Nagraba K., Nizankowska E., Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J. Allergy Clin. Immunol. 2003;111:743–749. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 193.Nizankowska E., Czerniawska-Mysik G., Szczeklik A. Lack of effect of i.v. prostacyclin on aspirin-induced asthma. Eur. J. Respir. Dis. 1986;69:363–368. [PubMed] [Google Scholar]
- 194.Xie L., Liu A.G., Cui Y.H., Zhang Y.P., Liao B., Li N.N., Wang X.S. Expression profiles of prostaglandin E2 receptor subtypes in aspirin tolerant adult Chinese with chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2015;29:322–328. doi: 10.2500/ajra.2015.29.4205. [DOI] [PubMed] [Google Scholar]
- 195.Perez-Novo C.A., Watelet J.B., Claeys C., Van Cauwenberge P., Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J. Allergy Clin. Immunol. 2005;115:1189–1196. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 196.Kim J.H., Choi G.E., Lee B.J., Kwon S.W., Lee S.H., Kim H.S., Jang Y.J. Natural killer cells regulate eosinophilic inflammation in chronic rhinosinusitis. Sci. Rep. 2016;6:27615. doi: 10.1038/srep27615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Okano M., Fujiwara T., Yamamoto M., Sugata Y., Matsumoto R., Fukushima K., Yoshino T., Shimizu K., Eguchi N., Kiniwa M., et al. Role of prostaglandin D2 and E2 terminal synthases in chronic rhinosinusitis. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2006;36:1028–1038. doi: 10.1111/j.1365-2222.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 198.Ying S., Meng Q., Scadding G., Parikh A., Corrigan C.J., Lee T.H. Aspirin-sensitive rhinosinusitis is associated with reduced E-prostanoid 2 receptor expression on nasal mucosal inflammatory cells. J. Allergy Clin. Immunol. 2006;117:312–318. doi: 10.1016/j.jaci.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 199.Machado-Carvalho L., Roca-Ferrer J., Picado C. Prostaglandin E2 receptors in asthma and in chronic rhinosinusitis/nasal polyps with and without aspirin hypersensitivity. Respir. Res. 2014;15:100. doi: 10.1186/s12931-014-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xie L., Liu A.G., Peng L.Y., Wang S.J., Zhang Y.P., Wang X.S. Expression of E-prostanoid receptors in nasal polyp tissues of smoking and nonsmoking patients with chronic rhinosinusitis. PLoS ONE. 2018;13:e0200989. doi: 10.1371/journal.pone.0200989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yamamoto M., Okano M., Fujiwara T., Kariya S., Higaki T., Nagatsuka H., Tsujigiwa H., Yamada M., Yoshino T., Urade Y., et al. Expression and characterization of PGD2 receptors in chronic rhinosinusitis: Modulation of DP and CRTH2 by PGD2. Int. Arch. Allergy Immunol. 2009;148:127–136. doi: 10.1159/000155743. [DOI] [PubMed] [Google Scholar]