Abstract
Hepatic steatosis, the excess storage of intrahepatic lipids, is a rampant clinical problem associated with the obesity epidemic. Hepatic steatosis is linked to increased risk for insulin resistance, type 2 diabetes, and cardiovascular and advanced liver disease. Accumulating evidence shows that physical activity, exercise, and aerobic capacity have profound effects on regulating intrahepatic lipids and mediating susceptibility for hepatic steatosis. Moreover, exercise can effectively reduce hepatic steatosis independent of changes in body mass. In this perspective, we highlight 1) the relationship between obesity and metabolic pathways putatively driving hepatic steatosis compared with changes induced by exercise; 2) the impact of physical activity, exercise, and aerobic capacity compared with caloric restriction on regulating intrahepatic lipids and steatosis risk; 3) the effects of exercise training (modalities, volume, intensity) for treatment of hepatic steatosis, and 4) evidence for a sustained protection against steatosis induced by exercise. Overall, evidence clearly indicates that exercise powerfully regulates intrahepatic storage of fat and risk for steatosis.
Introduction
Excessive storage of intrahepatic fat, i.e., hepatic steatosis, is recognized as a significant metabolic and hepatic pathology associated with the ongoing obesity epidemic. Nonalcoholic fatty liver disease (NAFLD) is a spectrum of liver pathologies ranging from hepatic steatosis to steatohepatitis (NASH) (hepatocellular inflammation), fibrosis, and cirrhosis (liver failure). NAFLD is the most common chronic liver disease in the U.S., estimated to impact ∼25% of the general population and >75% of individuals with obesity and morbid obesity (1). Rates of NAFLD in other continents are on par with those of the U.S. or higher, except for much lower rates in Africa (1). NAFLD also is intimately linked with insulin resistance, type 2 diabetes, cardiovascular disease mortality, and risk for hepatocellular carcinoma. Currently, there are no proven pharmacological therapies for the effective treatment of NAFLD, leading health organizations to advocate lifestyle modification (exercise and diet) as the cornerstone for treatment. Accumulating clinical and epidemiological evidence shows that physical activity levels, exercise behavior, and aerobic capacity can independently impact risk for hepatic steatosis. Moreover, exercise training is a potent treatment for hepatic steatosis.
In this perspective, we 1) highlight the relationship between obesity and metabolic pathways putatively driving hepatic steatosis, 2) highlight the impact of physical activity, exercise, and aerobic capacity on hepatic steatosis; 3) highlight the impact of exercise training in the treatment of hepatic steatosis, and 4) discuss the recidivism of hepatic steatosis after cessation of caloric restriction versus exercise. Finally, we explore known and hypothesized mechanisms by which exercise mediates susceptibility and effectively treats hepatic steatosis.
Obesity and Hepatic Steatosis Paradox
Hepatic steatosis is linked to a constant positive energy balance associated with obesity, expansion of adiposity, and ectopic storage of fat in nonadipose tissues. Although greater obesity undoubtedly correlates with intrahepatic fat storage in examination of large numbers of patients, the degree of intrahepatic fat between subjects at the same BMI varies widely (2). Certain genetic polymorphisms (for instance, PNPLA3), ethnicity, and sex also powerfully modulate the associations between BMI status and steatosis (1). There are also normal weight patients who develop hepatic steatosis (3). Moreover, greater intrahepatic fat storage does not always correlate with pathological outcomes like insulin resistance, further highlighting the heterogeneity of this condition (4). In addition, the composition and the lipid species present in the liver beyond total triglyceride (TG) accumulation are another very importance factor to consider. Here, we posit that the paradox in these findings may be driven by individual differences in physical activity, exercise, and aerobic capacity.
Systemic and Hepatic Drivers of Steatosis
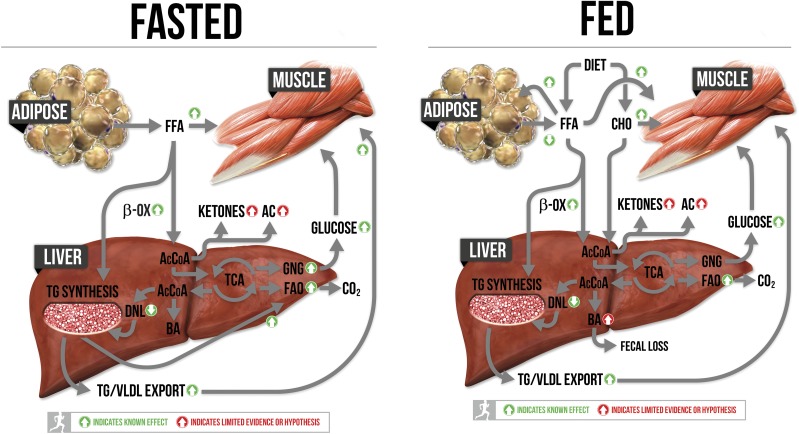
Hepatic steatosis is likely caused by multiple levels of metabolic insults. In fact, ectopic storage of fat in liver is not necessarily pathological but may have been designed as a transitory condition that can occur in conditions in which free fatty acids (FFAs) increase dramatically. Increases in intrahepatic lipid storage may not be pathological if combined with higher oxidative capacity or increased turnover rates much like the athlete paradox of elevated levels of intramuscular TG in endurance athletes (5), but we are unaware of data comparing liver TG levels in endurance trained versus untrained subjects who are normal weight. It is also possible that exercise promotes different intrahepatic lipid storage patterns (size, location, fatty acid saturation status, and changes in lipid droplet proteins controlling access to lipases). One human study showed that a short, 7-day exercise training paradigm increased the liver polyunsaturated lipid index, showing that even small doses of exercise can likely alter hepatic lipid composition (6). We are not aware of further studies examining transitory changes in liver lipids or of studies delineating physiological versus pathological levels of steatosis in human subjects, leaving these important questions largely unanswered. Finally, as shown in Fig. 1, current data suggest that metabolic pathways in both fed (postprandial) and fasted (postabsorptive) states are implicated in the development of hepatic steatosis.
Figure 1.
Metabolic pathways implicated in the development of hepatic steatosis in both the fed and fasted condition are highlighted. The effects of exercise and/or aerobic capacity on pathways are highlighted with green and red arrows. AcCoA, acetyl-coA; BA, bile acids; β-Ox, β-oxidation; CHO, carbohydrates; TCA, tricarboxylic acid cycle.
Metabolic Pathways Involved in Steatosis
Systemic insulin resistance contributes to steatosis through impaired suppression of adipose tissue lipolysis resulting in elevated portal and systemic delivery of FFAs to the liver both during fasting and postabsorptive conditions (7). Increased delivery of FFAs challenges the liver to enhance esterification of TG, increase oxidation, or both. Tracer approaches in human patients with hepatic steatosis (measured in postabsorptive state) revealed that ∼60% of intrahepatic lipids were derived from circulating FFAs emerging from adipose depots via lipolysis (8). Systemic insulin resistance is also associated with hyperinsulinemia, which drives increased hepatic TG synthesis and enhanced de novo lipogenesis (DNL). DNL is transcriptionally driven by insulin and fueled by elevated glucose, which are both elevated in individuals with systemic insulin resistance (8). In the same previously mentioned tracer studies, it was revealed that DNL accounted for ∼26% of intrahepatic lipid stores (8); however, these studies were done in postabsorptive conditions when DNL is largely suppressed. We are unaware of studies that have quantified DNL contribution to hepatic fat stores in humans during fed conditions, but it is likely to be further elevated.
While excess energy balance, hormonal, and substrate-driven systemic factors push susceptibility for hepatic steatosis, hepatic-specific cellular mechanisms also likely impact risk. The degree to which hepatic fatty acid oxidation (FAO) can increase in response to elevations in lipids may play a primary role. Current evidence in rodents and humans using both in vivo and in vitro tools shows that obesity and high-fat diets, factors that drive steatosis, lead to compensatory increases in measures of mitochondrial oxidative capacity including tricarboxylic acid cycle flux, FAO, and mitochondrial respiratory capacity (9–11), while transitioning to advanced liver disease (NASH) is associated with a loss of these compensatory responses (11).
The capacity to rid the liver of excess substrates is also compromised in hepatic steatosis. The liver readily packages and exports TG systemically into VLDLs, and multiple lines of evidence show that TG export is compromised with hepatic steatosis such that VLDL-TG export becomes saturated despite increasing intrahepatic lipids (reviewed in ref. 12). Impaired hepatic ketogenesis may also play a role in hepatic steatosis. Ketogenesis uses excess hepatic acetyl-CoA derived from β-oxidation to synthesize β-hydroxybutarate and acetoacetate, which is exported and catabolized by extrahepatic tissues (muscle, heart, brain, etc.). Typically ketogenesis is upregulated with fasting when lipolysis and hepatic β-oxidation are increased, but rodent findings have shown that ketogenesis is also increased initially in high-fat diet–induced hepatic steatosis (10,13) and in human subjects with steatosis (14). However, this diet-induced upregulation in ketogenesis transitions and becomes suppressed with prolonged high-fat feeding (10,13). Recent human data show that patients with greater intrahepatic lipids have lower hepatic ketogenic capacity (14), linking ketogenic insufficiency to worsening hepatic steatosis and metabolic dysfunction (13–15). The mechanisms for suppressed ketogenesis with steatosis are not completely understood but could be linked to altered hormonal actions (hyperinsulinemia and glucagon) and an increased usage of acetyl-CoA toward tricarboxylic acid cycle and gluconeogenesis (GNG) rather than ketone synthesis.
Other mechanisms for exporting excess hepatic substrates may also contribute to hepatic steatosis. Intracellular intermediate substrates acetyl-CoA and acyl-CoA pools are tightly regulated through conversion to acetylcarnitine and acylcarnitine (via carnitine acyltransferase enzymes) allowing them to be exported into systemic circulation. The capacity for acylcarnitine export is downregulated in obesity (16), and hepatic acylcarnitines are increased with steatosis and NASH (17). Finally, the liver’s role in synthesizing and exporting cholesterol and bile acids may also be implicated. Excess hepatic acetyl-CoA is also used to synthesize cholesterol and then bile acids. Upregulating bile acid synthesis and fecal loss through bile acid sequestrants lowers intrahepatic fat storage in rodents (18), but this has not been tested in human subjects to our knowledge. Each of these factors is highlighted in Fig. 1.
Relationship of Physical Activity and Aerobic Capacity With Incidence of Hepatic Steatosis
Physical activity is the physical movement (steps, distance, etc.) achieved through daily living activities outside of “programmed” exercise. Exercise is defined as programmed time devoted to multiple modalities of movement (continuous aerobic, high-intensity interval training, resistance exercise, etc.). Adjusting the intensity of these modalities differentially impacts physiological outcomes related to kilocalories expended, metabolic demand, and substrate utilization. Aerobic capacity, also termed cardiorespiratory or aerobic fitness, represents the maximal capacity to utilize oxygen at the whole-body level (VO2peak) during maximal effort (graded exercise tests to failure). Aerobic capacity is driven by genetics, age, and exercise/activity behavior (19). Individuals who regularly exercise or have high levels of vigorous daily activity, particularly in early life, have a significantly higher lifetime apex and higher long-term maintenance of aerobic capacity than inactive individuals, despite both groups experiencing declines with aging. Thus, exercise, activity, and aerobic capacity are interrelated variables and aerobic capacity can be used as a surrogate measure of vigorous physical activity behavior or exercise habits.
Both higher physically activity levels and higher aerobic capacity are associated with reduction in mortality (19). It is largely underappreciated that low aerobic capacity, independent of physical activity levels, BMI, or other risk factors, is the best overall predictor of early mortality and is also tightly linked to the development of cardiovascular disease, type 2 diabetes, and other numerous disease conditions (19). In addition, multiple reports have recently shown an important connection between low aerobic capacity and risk for NAFLD. Church et al. (20) first reported that aerobic capacity was linked to susceptibility for NAFLD in a cohort of middle age men independent of obesity. Subsequently, additional reports have confirmed an inverse association between aerobic capacity and prevalence of NAFLD (21–23). Importantly, even in young obese subjects, higher aerobic capacity dramatically reduces NAFLD prevalence, with the biggest effect seen in moving from the lowest to the second-lowest quartile of aerobic capacity (24). Furthermore, higher aerobic capacity at baseline predicts a greater effectiveness for lifestyle modifications to reduce hepatic steatosis in patients with NAFLD (22), and hepatic fat content is higher in healthy monozygotic twins with a lower measured cardiorespiratory fitness than their sibling due to differences in exercise behavior (25). Aerobic capacity levels of at least ∼9 METS or ∼30 mL/kg−1/min−1 appear to be a cutoff where risk for NAFLD is significantly decreased (20,24). The links between aerobic capacity and NAFLD have been complemented by our findings in a rat model selectively bred over several generations to be divergent in intrinsically high versus low running capacity (HCR and LCR rats). The HCR rats display ∼40% higher intrinsic aerobic capacity than the LCR rats while being maintained in a sedentary condition. We have shown that the HCR rats do not develop hepatic steatosis with acute or chronic high- fat/sucrose diet exposure and that the LCR rats are extremely susceptible (26,27).
Physical inactivity, which occurs in >75% of U.S. adults, is also a significant mediator of obesity-associated metabolic disorders. Cross-sectional observations indicate that decreased levels of daily physical activity are also independently associated with increased incidence of NAFLD (28) and higher intrahepatic lipid content (29). Another related outcome is sedentary behavior: the quantity of time individuals spend sitting or lying down versus standing and/or moving. Sedentary behavior has also been linked to poor metabolic outcomes but has not been examined as extensively in relation to steatosis. In one recent report, sedentary time was associated with greater intrahepatic fat, such that for every 60 min/day spent in sedentary behavior, intrahepatic fat was increased by 1.9% (30). In addition, very recent evidence suggests that aerobic capacity is more important for NAFLD prevalence than time spent sedentary (31).
Exercise Reduces Intrahepatic Lipids With and Without Weight Loss
Aerobic exercise and caloric restriction to induce weight loss are the most commonly prescribed therapies for NAFLD (32). Currently, there is no recognized dietary strategy that appears to be superior in the treatment of NAFLD; rather, overall caloric restriction to achieve weight loss should be the goal. Minimal weight loss (∼3% of body weight) by caloric restriction with or without aerobic exercise training effectively lowers hepatic steatosis (32). Twelve months of intensive lifestyle intervention (combination of caloric restriction and increased physical activity with a goal of 175 min/week) has been shown to reduce intrahepatic lipids, with greater reductions in those with greater weight loss (33). Combining aerobic exercise training and caloric restriction reduces intrahepatic lipids in both normal weight individuals and individuals with obesity, and greater weight loss results in larger resolution of NAFLD in both groups (34). Existing literature suggests that weight loss of >7% may be necessary to see improvement in histological inflammation and/or fibrosis (reviewed in ref. 32). However, no studies to date have examined whether exercise training, independent of weight loss, can effectively reduce histological hepatocellular inflammation or fibrosis. Moreover, we are unaware of studies examining whether exercise interacts uniquely with factors that dramatically impact risk for steatosis including genetic polymorphisms, ethnicity, and sex influence.
Importantly, studies combining both exercise and caloric restriction make it difficult to ascertain whether exercise modifies hepatic fat through altering energy balance or through systemic or hepatic-specific metabolic mechanisms. However, emerging evidence clearly demonstrates that exercise training, independent of reductions in body mass, can effectively reduce intrahepatic lipids. Interestingly, this appears to be the case for both resistance training and aerobic exercise training, which have been shown to reduce intrahepatic lipids in adults with type 2 diabetes (35) and adolescent boys with obesity (36) with minimal changes in total body mass. In addition, the majority of studies performed in participants with clinically defined hepatic steatosis (>5.5%, quantified by MRI/MRS) have shown that aerobic and resistance exercise training without weight loss is effective in reducing intrahepatic lipids by 20–40% (see excellent reviews in refs. 37–39). Collectively, there does not appear to be a relationship between reduced intrahepatic lipids and exercise intervention duration (2–26 weeks), sessions of exercise per week (range of 3–6 sessions/week), or total weekly exercise volume (range 60–300 min/week) based on these published work, although this has not been examined systematically. However, higher exercise intensity may be more effective, although the findings may not be universal across all study populations. We have recently shown that both 4 weeks of moderate-intensity continuous training and high-intensity interval training were effective in reducing intrahepatic lipids independent of changes in abdominal adiposity or body mass (40). High-intensity exercise (80% VO2peak) lowered intrahepatic lipids by 37% compared with only a 20% reduction with moderate-intensity exercise (55% VO2peak). Exercise intensity and aerobic capacity may also be related to prevalence of advanced NAFLD, including NASH and fibrosis. Cross-sectional data support this notion, where meeting vigorous physical activity guidelines (>75 min/week) reduced the odds of hepatic steatosis progressing to NASH and exceeding the vigorous activity guidelines (>150 min/week) decreased odds of having fibrosis (41), whereas meeting or exceeding the recommendations for moderate-intensity activity (>150 min/week) was not associated with a reduced incidence of NASH or fibrosis. Again, these data are complemented by findings in a rodent model of high and low aerobic capacity (HCR/LCR) challenged with a NASH-inducing Western diet (high fat, high sucrose, high cholesterol). The Western diet induced significant steatosis in both groups, but the high–aerobic capacity strain (HCR rats) was largely protected against NASH-related outcomes, including inflammation, Kupffer cell activation, and fibrosis, that were induced within the low–aerobic capacity strain (LCR rats) (42).
These data collectively highlight that exercise training in the absence of weight loss can effectively reduce intrahepatic lipids and point to the clinical potential of high-intensity exercise training in the management of NAFLD, but randomized clinical trials are clearly necessary. Currently, there is no clear-cut threshold of physical activity (steps or MET/h) per day that is known to provide protection against excess intrahepatic lipid accumulation. Thus, global recommendations of accumulation of >150 min/week of moderate-intensity aerobic exercise or >75 min/week of high-intensity exercise training should be the target goals for patients. As stated previously, cross-sectional studies suggest that aerobic capacity is critical for preventing NAFLD, but it is unknown whether vigorous exercise is more effective at treating NAFLD/NASH and whether this possible benefit is contingent on increases in aerobic capacity. In addition, it is currently unknown whether combining aerobic with resistance training would be more effective in the reduction in intrahepatic lipids. Moreover, the capacity for regular exercise and aerobic capacity to modulate intrahepatic fat without modifying body weight suggests that underlying mechanisms are not solely driven by increased energy expenditure.
How Does Exercise Influence Intrahepatic Fat Storage?
Figure 1 shows pathways implicated in steatosis development during fasting and feeding conditions combined with the known and hypothesized/limited evidence effects of exercise on these pathways. First and foremost, exercise is a powerful regulator of systemic insulin sensitivity that exerts its effects on hepatic metabolism at multiple levels. Regular exercise increases the sensitivity of insulin to 1) increase glucose transport into muscle (fed), 2) inhibit lipolysis in adipose (fed), and 3) and inhibit hepatic glucose output (fed). Exercise also reduces fasting and fed insulin levels due to enhanced systemic sensitivity, thus lowering the capacity of insulin to promote lipid synthesis pathways in the liver. Together, these systemic effects work to reduce intrahepatic fat by reducing hepatic exposure to substrates used to synthesize lipids and reducing transcriptional upregulation of machinery to synthesize the lipids. Furthermore, one of the most consistent effects of aerobic exercise in our hands and others’ is to promote the downregulation of genes and proteins in the DNL pathway as already stated (43). It is unknown whether these effects are solely due to insulin or due to exercise-specific signaling within the liver.
In addition, exercise uniquely prepares the liver for excess delivery of FFAs. We have shown repeatedly that exercise and elevations in intrinsic aerobic capacity lead to increased hepatic FAO, improved mitochondrial respiration, and increases in other associated mitochondrial outcomes (citrate synthase activity, β-HAD activity, cytochrome c content, etc.) (44–46). These increases in markers of hepatic oxidative capacity are retained chronically in the liver and are even programmed by breeding for high and low intrinsic aerobic capacity, suggesting that they are critical for divergence in intrinsic aerobic capacity (46). Hepatic mitochondrial adaptations to exercise are critical because higher hepatic FAO is needed to provide ATP for the energy costly process of GNG (26,47), which is increased during prolonged exercise. Robust upregulation of GNG is needed to provide glucose to working skeletal muscles and to maintain euglycemia. Extensive work has shown that the onset of exercise immediately increases hepatic glucose output as ATP demands in muscle increase (48). Moreover, exercise training increases gluconeogenic capacity (48). Breakdown and release of hepatic glycogen provide the initial glucose output from the liver, but as exercise duration continues, GNG contributes to a greater extent (47). The liver also likely increases the breakdown of intrahepatic lipids to fuel FAO during exercise, and it is easy to speculate that regular exercise increases turnover and storage patterns of hepatic lipid pools. Thus, both exercise and aerobic capacity upregulate hepatic oxidative pathways due to the energy demands of exercise pulling on hepatic metabolism, responses that are associated with improved metabolic health. In contrast, the compensatory increases in hepatic oxidative metabolism found with pushing energy through the liver (high-fat feeding, obesity, steatosis) exert negative outcomes (oxidative stress, hyperglycemia, hepatocellular injury) (9). Exercise and high-fat diet/obese conditions all likely expose the liver to elevated lipids and boost hepatic oxidative capacity but clearly lead to divergent outcomes. As shown in Fig. 1, the increased capacity for skeletal muscle glucose disposal induced by chronic exercise may play an important role in the protective adaptations in liver associated with exercise. Enhanced glucose disposal in muscle would lead to reduced insulin levels and would also reduce oxidative stress that has been found with steatosis and hyperglycemia in conditions of excess energy consumption.
There is also limited evidence that exercise and aerobic capacity can increase the export of substrates out of the liver through ketogenesis, acylcarnitines, cholesterol, and bile acids. In each of these areas, there are data that exercise upregulates these processes, but they have not been tied to the treatment of hepatic steatosis. Both acute and chronic exercise upregulate the capacity for ketogenesis, particularly in a fasted condition (49). In fact, exercise after an overnight fast increase ketogenic flux to levels in humans subjects similar to those measured after a 4-day fast (49). Therefore, exercise-induced upregulation of ketogenic capacity may play an important role during the fasted state, but the impact in a fed condition is also possible, particularly when aerobic exercise is employed as a treatment for already existing steatosis when ketogenic capacity is suppressed (14,15).
Current evidence using the collection of blood across the hepatic/splanchnic bed in humans suggests that skeletal muscle releases a great deal of long-chain acylcarnitines during exercise that are taken by the liver, chain shortened, and then released as C2 and C3 carnitines into circulation (50). Thus, there is evidence of increased acylcarnitine efflux capacity during exercise, but we are unaware of data testing whether acylcarnitine efflux aids exercise in mitigating excess hepatic lipids during nonexercise conditions. More recently, it has been shown in humans that a number of other metabolites are released by muscle and taken up by the liver during exercise, including malate, succinate, and several short-chain fatty acids in addition to established lactate (51). Finally, there is evidence that exercise in rodents can increase cholesterol and bile acid synthesis, turnover, and fecal loss. During fed conditions, the oxidation of glucose and fatty acids to acetyl-CoA far outpaces mitochondrial demand, resulting in the accumulation of citrate. Citrate is then exported out of hepatic mitochondria where it is converted back to acetyl-CoA and becomes substrate for DNL or is trafficked to cholesterol synthesis. Cholesterol is then the substrate for bile acid synthesis. Both cholesterol and bile acid synthesis are energy costly processes requiring large quantities of ATP for production. Thus, bile acids are largely recycled back to the liver via the portal vein after resorption in the intestines, with only 5% being lost in feces. Long-term exercise in rodents was shown to increase biliary bile acid and cholesterol secretion and fecal bile acid loss, likely stimulating increased bile acid synthesis to maintain the overall bile acid pool size (52). If exercise can increase fecal cholesterol and bile acid loss and increase overall turnover, it could preferentially traffic acetyl-CoA away from DNL and provide a siphon to dump excess energy from the liver, much like bile acid sequestrants (18) or CYP7a1 overexpression studies (53). Unfortunately, we are unaware of studies exploring exercise on the effects of bile acid metabolism in the context of steatosis.
Exercise Versus Caloric Restriction for NAFLD—Fixing Versus Bandaging
Both exercise training and caloric restriction reduce intrahepatic lipids. However, the residual benefit of these diverse lifestyle modifications is another important consideration in the long-term treatment/management of NAFLD, given the difficulty in sustaining healthy dietary and physical activity habits indefinitely. It currently is unknown how long an individual is protected against NAFLD after ceasing diet or exercise regimens. To our knowledge, studies of this nature have not been conducted in humans but are definitely warranted, as rates of recidivism for weight regain are rampant.
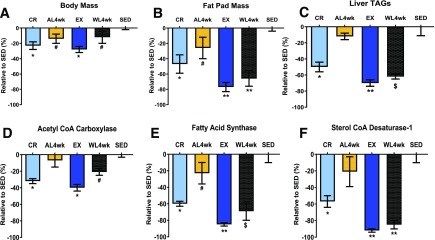
In an attempt to better understand alterations in hepatic lipid metabolism and systemic factors linked to NAFLD risk caused by physical inactivity or overnutrition, our research group has utilized the Otsuka Long-Evans Tokushima Fatty (OLETF) rat. OLETF rats lack the cholecystokinin-1 receptor and are hyperphagic, leading to progressively worsening obesity, insulin resistance, type 2 diabetes, and NAFLD (54). However, these pathologies are entirely prevented when OLETF rats are either given daily access to voluntary running wheels or energy restricted to a normophagic control level (44,55,56). We conducted a series of studies where OLETF rats either were allowed to exercise on voluntary running wheels or were calorically restricted (30% reduction from ad libitum fed rats) for 12 weeks in order to prevent NAFLD development (57,58). The animals were then transitioned to a physically inactive state (running wheels locked) or overnutrition state (allowed ad libitum access to low-fat rodent chow) for 4 weeks. By design, both exercise and caloric restriction resulted in similar reductions in body mass compared with that in ad libitum fed sedentary controls (Fig. 2A); however, exercise further suppressed fat pad mass, liver TG, and hepatic DNL markers fatty acid synthase (FAS) and stearoyl-CoA desaturase 1 (SCD-1) compared with restricted diet alone (57,58) (Fig. 2B, C, E, and F). In addition, while there was a slight return in the DNL markers acetyl-CoA carboxylase (ACC) and FAS, much of the benefits of prior exercise was maintained 4 weeks after exercise cessation (58) including only minimal increases in liver TG (58) (Fig. 2C). This protection against a return of steatosis is in agreement with similar findings after only 7 days of physical inactivity (55). Interestingly, activity-induced reductions in hepatic FAS and SCD-1 were almost completely maintained following 4 weeks of physical inactivity and hyperphagia. In comparison, these findings differ dramatically from those observed in the previously calorically restricted animals, where a 4-week return to ad libitum feeding resulted in a greater degree of fat mass gain and also a return in hepatic ACC, FAS, and SCD-1 to levels approaching those in the chronically sedentary, hyperphagic animals (57) (Fig. 2). It should be noted that hepatic mitochondrial adaptations witnessed with either exercise or caloric restriction were largely lost with either transition (57,58), highlighting the acute nature by which these adaptations are regulated and the importance of DNL in rodent models of NAFLD.
Figure 2.
Residual benefits of prior caloric restriction vs. exercise. Data are summarized and adapted from previously published articles (57,58) and presented as % difference (±SE) from ad libitum fed sedentary OLETF rats (SED). Percent change relative to ad libitum fed sedentary OLETF rats for body mass (A), fat pad mass (B), liver triglycerides (TAGs) (C), hepatic ACC (D), FAS (E), and sterol CoA desaturase-1 (F). AL4wk, ad libitum fed for 4 weeks following 12 weeks of caloric restriction (CR); EX, voluntary wheel-running exercise for 12 weeks; WL4wk, wheel lock for 4 weeks to induce physical inactivity following 12 weeks of voluntary wheel-running exercise. *P < 0.01, #P < 0.05 vs. ad libitum fed sedentary OLETF rats; **P < 0.05 vs. 12 weeks of caloric restriction, ad libitum feeding for 4 weeks following 12 weeks of caloric restriction, and ad libitum fed sedentary OLETF rats; $P < 0.05 vs. ad libitum feeding for 4 weeks following 12 weeks of caloric restriction, voluntary wheel-running exercise for 12 weeks, and ad libitum fed sedentary OLETF rats.
These findings may have important clinical implications, as the residual benefits of 12 weeks of exercise appear to offer more persistent protection against susceptibility to NAFLD due in part to a continued suppression of hepatic DNL, whereas the benefits of caloric restriction–induced prevention of hepatic steatosis are rapidly lost. Whether this exercise-induced protection translates to other model systems or in a human clinical population needs rigorous investigation. It is doubtful that the benefits of prior exercise would persist indefinitely.
Conclusion
Exercise and aerobic capacity have profound effects on regulating intrahepatic lipids and the protection/treatment of hepatic steatosis, revealed through multiple lines of evidence (rodent studies, clinical results, and epidemiological findings). Almost every known system believed to contribute to the pathology of steatosis is countered or reversed with exercise or high levels of aerobic capacity (Fig. 1). The effects of exercise are multifactorial, with robust systemic (extrahepatic) and hepatic-specific mechanisms being altered. Future work should explore whether exercise is required to work through each of these mechanisms to influence hepatic steatosis outcomes. In addition, future studies should consider the potential role of hepatokine secretion from liver during exercise affecting systemic metabolism, as well as myokines being releasing from working muscle during exercise, which could have a direct impact on hepatic metabolism. Furthermore, exercise training may offer longer residual benefits for hepatic steatosis compared with just caloric restriction alone. We posit that it will be difficult or near impossible to find another therapeutic agent that can effectively protect/treat steatosis through systemic and hepatic-specific mechanisms like exercise. Moreover, the existing evidence clearly shows that programmed exercise with or without weight loss should be employed as “medical rehab” for patients who are at risk or who have NAFLD.
Article Information
Acknowledgments. The authors thank Gaige Larson (GaigeLarsonDesigns) for designing Fig. 1.
Funding. R.S.R. is supported by Veterans Affairs (VA) Merit Grant I01BX003271 and National Institutes of Health (NIH) grant R01 DK113701. J.P.T. is supported by VA Merit Grant 1I01BX002567 and NIH grants R01 KD121497 and R01 AR071263.
References
- 1.Younossi Z, Anstee QM, Marietti M, et al. . Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20 [DOI] [PubMed] [Google Scholar]
- 2.Portillo-Sanchez P, Bril F, Maximos M, et al. . High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanWagner LB, Armstrong MJ. Lean NAFLD: a not so benign condition? Hepatol Commun 2018;2:5–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev 2008;29:939–960 [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 [DOI] [PubMed] [Google Scholar]
- 6.Haus JM, Solomon TP, Kelly KR, et al. . Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2013;98:E1181–E1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 2005;42:987–1000 [DOI] [PubMed] [Google Scholar]
- 8.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satapati S, Kucejova B, Duarte JA, et al. . Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest 2015;125:4447–4462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satapati S, Sunny NE, Kucejova B, et al. . Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 2012;53:1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koliaki C, Szendroedi J, Kaul K, et al. . Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–746 [DOI] [PubMed] [Google Scholar]
- 12.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol 2008;14:185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter DG, Ercal B, Huang X, et al. . Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest 2014;124:5175–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher JA, Deja S, Satapati S, Fu X, Burgess SC, Browning JD. Impaired ketogenesis and increased acetyl-CoA oxidation promote hyperglycemia in human fatty liver. JCI Insight 2019;4:e127737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017;25:262–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noland RC, Koves TR, Seiler SE, et al. . Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 2009;284:22840–22852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng KY, Watt MJ, Rensen S, et al. . Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J Lipid Res 2018;59:1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe M, Morimoto K, Houten SM, et al. . Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS One 2012;7:e38286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc 2015;90:1533–1540 [DOI] [PubMed] [Google Scholar]
- 20.Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology 2006;130:2023–2030 [DOI] [PubMed] [Google Scholar]
- 21.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 2003;284:E1065–E1071 [DOI] [PubMed] [Google Scholar]
- 22.Kantartzis K, Thamer C, Peter A, et al. . High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009;58:1281–1288 [DOI] [PubMed] [Google Scholar]
- 23.Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2008;47:1158–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pälve KS, Pahkala K, Suomela E, et al. . Cardiorespiratory fitness and risk of fatty liver: the Young Finns Study. Med Sci Sports Exerc 2017;49:1834–1841 [DOI] [PubMed] [Google Scholar]
- 25.Hannukainen JC, Borra R, Lindeborg K, et al. . Liver and pancreatic fat content and metabolism in healthy monozygotic twins with discordant physical activity. J Hepatol 2011;54:545–552 [DOI] [PubMed] [Google Scholar]
- 26.Morris EM, Jackman MR, Johnson GC, et al. . Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 2014;307:E355–E364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris EM, Meers GM, Koch LG, et al. . Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab 2016;311:E749–E760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, et al. . Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 2008;48:1791–1798 [DOI] [PubMed] [Google Scholar]
- 29.Perseghin G, Lattuada G, De Cobelli F, et al. . Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care 2007;30:683–688 [DOI] [PubMed] [Google Scholar]
- 30.Henson J, Edwardson CL, Morgan B, et al. . Sedentary time and MRI-derived measures of adiposity in active versus inactive individuals. Obesity (Silver Spring) 2018;26:29–36 [DOI] [PubMed] [Google Scholar]
- 31.Croci I, Coombes JS, Bucher Sandbakk S, et al. Non-alcoholic fatty liver disease: prevalence and all-cause mortality according to sedentary behaviour and cardiorespiratory fitness . The HUNT Study. Prog Cardiovasc Dis 2019;62:127–134 [DOI] [PubMed] [Google Scholar]
- 32.Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005–2023 [DOI] [PubMed] [Google Scholar]
- 33.Lazo M, Solga SF, Horska A, et al.; Fatty Liver Subgroup of the Look AHEAD Research Group . Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong VW, Wong GL, Chan RS, et al. . Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349–1356 [DOI] [PubMed] [Google Scholar]
- 35.Bacchi E, Negri C, Targher G, et al. . Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013;58:1287–1295 [DOI] [PubMed] [Google Scholar]
- 36.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 2012;61:2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brouwers B, Hesselink MK, Schrauwen P, Schrauwen-Hinderling VB. Effects of exercise training on intrahepatic lipid content in humans. Diabetologia 2016;59:2068–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashida R, Kawaguchi T, Bekki M, et al. . Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol 2017;66:142–152 [DOI] [PubMed] [Google Scholar]
- 39.Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829–846 [DOI] [PubMed] [Google Scholar]
- 40.Winn NC, Liu Y, Rector RS, Parks EJ, Ibdah JA, Kanaley JA. Energy-matched moderate and high intensity exercise training improves nonalcoholic fatty liver disease risk independent of changes in body mass or abdominal adiposity - a randomized trial. Metabolism 2018;78:128–140 [DOI] [PubMed] [Google Scholar]
- 41.Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB; NASH CRN Research Group . Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol 2011;106:460–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris EM, McCoin CS, Allen JA, et al. . Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J Physiol 2017;595:4909–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol (1985) 2011;111:1828–1835 [DOI] [PubMed] [Google Scholar]
- 44.Rector RS, Thyfault JP, Morris RT, et al. . Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 2008;294:G619–G626 [DOI] [PubMed] [Google Scholar]
- 45.Linden MA, Fletcher JA, Morris EM, et al. . Treating NAFLD in OLETF rats with vigorous-intensity interval exercise training. Med Sci Sports Exerc 2015;47:556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thyfault JP, Rector RS, Uptergrove GM, et al. . Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 2009;587:1805–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wasserman DH, Cherrington AD. Hepatic fuel metabolism during muscular work: role and regulation. Am J Physiol 1991;260:E811–E824 [DOI] [PubMed] [Google Scholar]
- 48.Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl Sci 2015;135:203–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Féry F, Balasse EO. Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am J Physiol 1983;245:E318–E325 [DOI] [PubMed] [Google Scholar]
- 50.Xu G, Hansen JS, Zhao XJ, et al. . Liver and muscle contribute differently to the plasma acylcarnitine pool during fasting and exercise in humans. J Clin Endocrinol Metab 2016;101:5044–5052 [DOI] [PubMed] [Google Scholar]
- 51.Hu C, Hoene M, Plomgaard P, et al. . Muscle-liver substrate fluxes in exercising humans and potential effects on hepatic metabolism. J Clin Endocrinol Metab. 11 December 2019 [Epub ahead of print]. DOI: 10.1210/clinem/dgz266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK. Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 2011;218:323–329 [DOI] [PubMed] [Google Scholar]
- 53.Li T, Owsley E, Matozel M, Hsu P, Novak CM, Chiang JY. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 2010;52:678–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rector RS, Thyfault JP, Uptergrove GM, et al. . Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 2010;52:727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rector RS, Thyfault JP, Laye MJ, et al. . Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 2008;586:4241–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rector RS, Uptergrove GM, Morris EM, et al. . Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 2011;300:G874–G883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linden MA, Fletcher JA, Meers GM, Thyfault JP, Laughlin MH, Rector RS. A return to ad libitum feeding following caloric restriction promotes hepatic steatosis in hyperphagic OLETF rats. Am J Physiol Gastrointest Liver Physiol 2016;311:G387–G395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linden MA, Meers GM, Ruebel ML, et al. . Hepatic steatosis development with four weeks of physical inactivity in previously active, hyperphagic OLETF rats. Am J Physiol Regul Integr Comp Physiol 2013;304:R763–R771 [DOI] [PMC free article] [PubMed] [Google Scholar]