Abstract
The genus Neocosmospora (Fusarium solani species complex) contains saprobes, plant endophytes and pathogens of major economic significance as well as opportunistic animal pathogens. Advances in biological and phylogenetic species recognition revealed a rich species diversity which has largely remained understudied. Most of the currently recognised species lack formal descriptions and Latin names, while the taxonomic utility of old names is hampered by the lack of nomenclatural type specimens. Therefore, to stabilise the taxonomy and nomenclature of these important taxa, we examined type specimens and representative cultures of several old names by means of morphology and phylogenetic analyses based on rDNA (ITS and LSU), rpb2 and tef1 sequences. Sixty-eight species are accepted in Neocosmospora, 29 of them described herein as new; while 13 new combinations are made. Eleven additional phylogenetic species are recognized, but remain as yet undescribed. Lectotypes are proposed for eight species, seven species are epitypified and two species are neotypified. Notes on an additional 17 doubtful or excluded taxa are provided.
Keywords: Fusarium, new taxa, systematics, taxonomy
INTRODUCTION
The genus Neocosmospora (Hypocreales, Nectriaceae) includes ubiquitous, widely distributed fungi that are commonly found in soil, plant debris, living plant material, air and water. Previously assigned to the Fusarium solani species complex (FSSC; O’Donnell 2000) and before that, to sect. Martiella (Wollenweber 1913), this genus encompasses one of the most important groups of plant pathogenic fungi. The included species and varieties have been recorded from nearly 500 different plant hosts, spanning more than 100 families (Farr & Rossman cont. updated). Common plant diseases attributed to these taxa include: dry and jelly-end potato rot (Carpenter 1915); head blight of wheat (Triticum aestivum) (Balmas et al. 2015); root rot of Citrus spp. (Menge 1988, Polizzi et al. 1992, Sandoval-Denis et al. 2018), common bean (Phaseolus vulgaris; Roy 1997), pea (Pisum sativum; Porter et al. 2015), peanut (Arachis hypogaea; Rojo et al. 2007), sweet potato (Ipomoea batatas; McClure 1951) and wheat (Nirenberg 1981); root and fruit rot of cucurbits (Hawthorne et al. 1992), and tomato (Solanum lycopersicum); stem and fruit rot of sweet peppers (Capsicum annuum; Fletcher 1994, Jarvis et al. 1994), and mango (Mangifera indica); fruit malformation (Liew et al. 2016); stem canker of cottonwood (Populus spp.; Toole 1963), avocado (Persea americana; Guarnaccia et al. 2018), red oak (Quercus rubra; Vujanovic et al. 1999), and walnut (Juglans spp.; Tisserat 1987, Chen & Swart 2000); and sudden death syndrome (SDS) of soybean (Glycine max; Achenbach et al. 1996, Aoki et al. 2005). Neocosmospora also includes economically important tree-pathogenic mutualists of the shot-hole borer beetle (Euwallaceae spp.) originally associated with dieback of avocado and tea (Camelia sinensis; Freeman et al. 2013). This mutualistic association, however, is now known from various other woody hosts that include castor bean (Ricinus communis), fig (Ficus carica), kaki persimmon (Diospyros kaki), ashleaf maple/ Manitoba maple/ box elder (Acer negundo), oak (Quercus spp.), oriental plane (Platanus orientalis), and the tree of heaven (Ailanthus altissima; Freeman et al. 2013, O’Donnell et al. 2016), as well as several native host species in South Africa (Paap et al. 2018).
Given their importance as plant pathogens, species of Neocosmospora have been used as model organisms in molecular plant pathology (VanEtten et al. 1989, Crowhurst et al. 1992, O’Donnell 2000 and additional references therein) and for the study of fungal cell biology (Wu et al. 1998, Aist 2002, Coleman 2016). They are also known as producers of bioactive natural products including antibacterial agents (Bacon et al. 1996, Merlin et al 2013); cytotoxic compounds like the immunosuppressive agents cyclosporine A and C, and naphthoquinones (Nakajima et al. 1989, Sugiura et al. 1999, Lee et al. 2014, Takemoto et al. 2014, Rathna et al. 2016, Chowdhury et al. 2017). They are sources of diverse enzymes with industrial applications including chitosanases (Liu & Bao 2009), cutinases (Mannesse 1997, Longhi et al. 2005), hydrolases (Jallouli et al. 2015), laccases (Wu & Nian 2014), and lyases (Longhi et al. 2005); and for the biosynthesis of nanoparticles (Fathima & Balakrishnan 2014).
Neocosmospora species have also sporadically been associated with human and animal mycotoxicoses (Ishii et al. 1971, Pitt & Hocking 2009, Antonissen et al. 2014), being producers of a wide range of toxins displaying activities against plants and animals, cell cultures and diverse microorganisms. The list of known toxic metabolites includes furanoterpenoids, ipomeanols and ipomeanine (Nelson et al. 1983, 1994, Mawhinney et al. 2008, Pitt & Hocking 2009), naphthazarins (Achor et al. 1993, Van Rensburg et al. 2001), while the alleged production of the trichothecenes scirpentriol, NT-2, T-1, T-2 toxins and neosolaniol are most likely based on misidentified isolates (Ishii et al. 1971, Ueno et al. 1975, Chełkowski 1989).
Despite their relative rarity compared to infections caused by other fungal pathogens such as Aspergillus and Candida spp., human and animal infections caused by Neocosmospora spp. are on the rise (Anaissie et al. 1986, Sutton & Brandt 2011). This increase is driven by a multitude of causes, mostly related to the increased incidence of specific host predisposing factors and immunocompromising conditions that include corticosteroid therapy, grafts, haematological malignancies, HIV infection, prolonged neutropenia, prosthetic devices and transplantations. Additionally, the development of new diagnostic tools and the currently improved awareness of medical personnel on the importance of fungal infections have greatly increased accurate identification of the causal agent (Guarro 2013, Slavin et al. 2015). Although nearly 50 % of fusarial infections have been attributed to the traditional concept of N. solani, recent phylogenetic studies have shown that infections due to Neocosmospora spp. are not limited to N. solani s.str. (O’Donnell et al. 2008, Guarro 2013, Sandoval-Denis & Crous 2018). Other Neocosmospora spp. that include N. petroliphila, N. falciformis, and in particular N. keratoplastica are now also known to be more frequently associated with cutaneous, subcutaneous or deep seated, commonly devastating infections of highly immunocompromised patients (Guarro 2013, Short et al. 2013).
Species concepts in Neocosmospora, the asexual-morph dilemma
Members of Neocosmospora (as the FSSC) have been traditionally classified in the asexual genus Fusarium, and characterised based on morphological features of the asexual morph, pathogenicity on specific hosts, and sexual or asexual compatibility (Matuo & Snyder 1973, Chitrampalam & Nelson 2016). However, the many different taxonomic schemes applied to this fungal group have led to a confused application of names as well as obscure synonymies.
In the taxonomic scheme proposed by Wollenweber (1913), morphological sections were erected to accommodate fusaria sharing similar cultural and macroconidial features. Three of these sections comprised taxa that, at some point, have been included in what it is now considered the genus Neocosmospora. Section Martiella was originally erected to accommodate species producing thick-walled and thick-septate macroconidia with inequilaterally tapered and rounded apices, and somewhat apiculate basal cells (Wollenweber 1913, 1918). Typified by Fusarium solani, the section included two additional species, F. caeruleum and F. martii. A fourth species, Fusarium eumartii, was included in a subsequent amendment of sect. Martiella by Carpenter (1915). The sister monotypic sections Pseudomartiella and Ventricosum included F. javanicum and F. argillaceum (F. ventricosum), respectively (Wollenweber 1918). In subsequent revisions by Reinking & Wollenweber (1927), Wollenweber (1931, 1943) and Wollenweber & Reinking (1935a, b), sect. Pseudomartiella was synonymised under sect. Martiella, revised to include four species: F. caeruleum, F. caucasicum, F. javanicum and F. solani, 10 varieties (F. eumartii and F. martii were reduced to varieties under F. solani), and three forms, roughly distinguished by cultural and micromorphological characters. These works formed the foundation from which most subsequent taxonomic treatments of ‘Fusarium’ solani were derived (Snyder & Hansen 1941, Toussoun & Nelson 1975, Joffe 1974, Gerlach 1981, Gerlach & Nirenberg 1982, Burgess et al. 1994). Snyder & Hansen (1941), however, considered Wollenweber & Reinking’s system to be impractical. According to their interpretations, the morphological features used for distinguishing the many species and varieties overlapped when a large number of monosporic isolates and nutritional conditions were studied. Therefore, all of Wollenweber & Reinking’s taxa in sections Martiella and Ventricosum were reduced to a single species, F. solani, with five formae, based on pathogenicity, now referred to as formae speciales (ff. spp.). This widely comprehensive concept was largely followed and is still in use, particularly by plant pathologists, given its simplicity and practicality (Nelson et al. 1983, Leslie & Summerell 2006). Nevertheless, several authors rejected Snyder & Hansen’s system in favour of narrower species circumscriptions. Raillo (1950) acknowledged four species, F. caeruleum, F. javanicum, F. martii and F. solani, plus numerous morphological varieties and formae, and also added new combinations including eight new formae and two subspecies. Bilai (1955) emended sect. Martiella to include taxa from sections Eupionnotes and Ventricosum, though she recognised only three species: F. javanicum, F. merismoides and F. solani. Fusarium caeruleum was reduced to being a variety of F. solani. Booth (1971) partially followed both Wollenweber & Reinking and Snyder & Hansen’s systems, and combined sections Martiella and Ventricosum. He accepted four species, including F. illudens, F. solani with 18 ff. spp., F. tumidum and F. ventricosum. He agreed with Bilai on the varietal status of F. caeruleum. Similarly, Joffe & Palti (1972) and Joffe (1974) accepted only two species: F. solani with its varieties caeruleum and ventricosum, and F. javanicum. More recently, Gerlach & Nirenberg (1982) accepted six species, F. caeruleum, F. caucasicum, F. eumartii, F. illudens, F. javanicum with two varieties and F. solani with four varieties. Fusarium ventricosum was re-classified as a distinct species in sect. Ventricosum, and was later transferred to the genus Rectifusarium by Lombard et al. (2015). Matuo & Snyder (1973), however, were the first to prove that F. solani constitutes a species complex rather than a discrete taxon. In that study, they were able to demonstrate through mating experiments, the existence of at least seven biologically isolated lineages termed mating populations (MP) I to VII of Nectria (Nec.) haematococca. These biological lineages are now recognised to agree, based on phylogeny, with the previously nominated formae speciales ff. spp. of F. solani (O’Donnell 2000, Schroers et al. 2016).
Phylogenetic studies have shown the existence of numerous discrete and cryptic phylogenetic species within F. solani, providing further evidence of the monophyly of most of its ff. spp. (O’Donnell 2000, O’Donnell et al. 2008). Although several ff. spp. have been proven to be monophyletic (O’Donnell 2000, O’Donnell et al. 2008), host specificity is not always a reliable character for taxonomic classification in Neocosmospora. For example, ‘F.’ solani f. sp. pisi (also known as ‘F.’ martii var. pisi or Nec. haematococca MPVI), a recognised pathogen of green peas (Pisum sativum), can also be pathogenic to a vast range of hosts that include alfalfa (Medicago sativa), chickpea (Cicer arietinum), cottonwood (Populus spp.), potato (Solanum tuberosum), sainfoin (Onobrychis viciifolia) and tulip tree (Liriodendron tulipifera) (VanEtten 1978, VanEtten & Kistler 1988, O’Donnell 2000). Similarly, ‘F.’ solani f. sp. eumartii, traditionally considered an agent of potato wilt, is also pathogenic to eggplant (Solanum melongena), tomato and pepper (Piper nigrum; Romberg & Davis 2007, Coleman 2016). Moreover, host specificity observed in this genus might not always have a single evolutionary origin. For instance, ‘F.’ solani f. sp. glycines, the commonly cited causal agent of sudden death syndrome (SDS) of soybean is a polyphyletic group, as is ‘F.’ solani f. sp. cucurbitae, a pathogen of melon (Cucumis melo) (Matuo & Snyder 1973, Aoki et al. 2003, Short et al. 2013). Furthermore, early genome comparisons of Nec. haematococca MPVI and Neocosmospora boninensis showed that the latter species, although not naturally occurring on peas, possesses the genomic traits needed to become pathogenic to that host (Temporini & VanEtten 2004). This pathogen was demonstrated to cause disease on peas in a laboratory setting (Temporini & VanEtten 2004). Despite the limitations of the ff. spp. classification system, it is still in use among plant pathologists, with new names still being assigned under this informal, sub-specific taxonomic rank (Chung et al. 2011, Bueno et al. 2014). Presently, 29 ff. spp. have been designated in Neocosmospora (as Fusarium solani).
Given the difficulties in assigning Latin binomials for commonly isolated cryptic species in Neocosmospora, Zhang et al. (2006) and O’Donnell et al. (2008), developed a multilocus haplotype classification specially directed for use among clinical microbiologists and clinicians in order to facilitate the management of epidemiological data. Following O’Donnell (2000), Zhang et al. (2006) and O’Donnell et al. (2008), it was clear that Neocosmospora (as FSSC) was composed of numerous phylogenetic species, distributed within three main clades with some degree of biogeographic differentiation: clades 1 and 2 restricted to plant-associated species from New Zealand and South America, respectively; and clade 3 comprising the highest phylogenetic and ecological diversity, including saprobic, plant pathogenic and all veterinary and human clinically relevant species. Summerbell & Schroers (2002) estimated more than 50 phylogenetic species in FSSC, and more than 60 phylogenetic species have to date been allocated in this species complex (Schroers et al. 2016). Recently, Schroers et al. (2016) assigned N. solani s.str. to phylogenetic species 5 within FSSC clade 3, fixing the use of the name by epitypification. Presently, only 23 phylogenetic species in FSSC clade 3 are unambiguously and validly connected to Latin binomials, namely ‘F.’ euwallaceae, ‘F.’ oligoseptatum, ‘F.’ riograndense, ‘F.’ stercicola, ‘F.’ witzenhousenense, Neocosmospora ambrosia, N. catenata, N. croci, N. cyanescens, N. falciformis, N. gamsii, N. haematococca, N. keratoplastica, N. lichenicola, N. macrospora, N. metavorans, N. perseae, N. petroliphila, N. pseudensiformis, N. solani, N. suttoniana, N. tonkinensis and N. vasinfecta.
The quest for the sexual-morph name and the one fungus-one name initiative
In the past, sexual morphs of FSSC have been assigned to several generic names, i.e., Cucurbitaria, Dialonectria, Haematonectria and Hypomyces, all derived from Nec. haematococca, a lignicolous fungus originally isolated in Ceylon (Sri Lanka; Berkeley & Broome 1873) and traditionally considered the sexual morph of ‘Fusarium’ solani. Wollenweber & Reinking (1935a, b) erroneously allocated the sexual morphs of sect. Martiella to the genus Hypomyces, with two species, Hyp. haematococcus and Hyp. ipomoeae, including two and one varieties, respectively. Snyder & Hansen (1941), based on their single-species system, considered Hypomyces solani (syn Nec. haematococca) as the sexual morph of ‘F.’ solani, reducing Hyp. ipomoeae to synonymy under Hyp. solani f. cucurbitae (sexual morph of ‘F.’ solani f. cucurbitae). This latter system was also followed by Nelson et al. (1983).
Rossman et al. (1999) introduced the genus Haematonectria to accommodate the sexual morphs of Fusarium sect. Martiella, based on Nec. haematococca as lectotypified by Samuels (1976). Members of Haematonectria could not be retained in Nectria as circumscribed by Rossman (1989) since they differed ecologically, genetically and morphologically from the type species (Nectria cinnabarina) of Nectria as well as from other genera with fusarium-like asexual morphs, i.e., Albonectria, Cosmospora and Gibberella (Samuels 1976, Guadet et al. 1989, Rossman 1989, O’Donnell 1993). However, Haematonectria (1999) is predated by the genus Neocosmospora (Smith 1899). Despite their minor morphological differences, Neocosmospora has been shown to be congeneric with Nec. haematococca, being nested deeply within the ‘F.’ solani species complex (Guadet et al. 1989, O’Donnell 1993, Spatafora & Blackwell 1994, Rehner & Samuels 1995). Although both Haematonectria and Neocosmospora are characterised by a two-layered perithecial wall, and ornamented, yellow-brown ascospores (Rossman et al. 1999, O’Donnell 2000), Rossman et al. (1999) argued that the morphological differences in both the sexual morphs (warted perithecial walls and 1-septate ascospores vs smooth perithecial walls and aseptate, only occasionally 1-septate ascospores in Neocosmospora) and the asexual morphs (fusarium-like vs acremonium-like in Neocosmospora) prevented the genera from being synonymised. O’Donnell (2000) hypothesised that the Fusarium asexual morphs of Neocosmospora might have lost the ability to produce sporodochia and macroconidia, resembling Acremonium spp. Similarly, Summerbell & Schroers (2002) considered the archetypal ‘F.’ solani conidia a symplesiomorphy from which several phenotypes have evolved. These include the highly divergent, asymmetrical clavate macroconidia of ‘F.’ ambrosium, ‘F.’ euwallaceae, and ‘F.’ oligoseptatum (Gadd & Loos 1947, Freeman et al. 2013, O’Donnell et al. 2008, 2016, Aoki et al. 2018), the cylindrical conidia of N. tonkinensis (Bugnicourt 1939, Sandoval-Denis et al. 2018), and reduced forms like the acremonium-like microconidial morphology commonly observed in N. falciformis (syn. Acremonium falciforme; Gams 1971, Summerbell & Schroers 2002), much similar to the acremonioid asexual morphs of Neocosmospora.
Because Neocosmospora and Haematonectria showed a clear common evolutionary origin, Nalim et al. (2011) stabilised the application of the name Nec. haematococca by epitypification, recombining this taxon as Neocosmospora haematococca (asexual morph ‘F.’ haematococcum), finally breaking the link between ‘F.’ solani and its previously assumed sexual morph, which is currently unknown (Schroers et al. 2016). Based on phylogeny and morphology of the sexual morphs, Gräfenhan et al. (2011) and Schroers et al. (2011) provided evidence supporting the segregation of taxa with Albonectria, Cyanonectria, Geejayesia and Neocosmospora sexual morphs from the main core of Fusarium, characterised by Gibberella sexual morphs. As a response, a petition to conserve the long-standing use of the name Fusarium was proposed and backed by numerous researchers (Geiser et al. 2013). Additional evidence, however, showed that this proposal needed to be reconsidered. Lombard et al. (2015) confirmed the original observations by Gräfenhan et al. (2011) and Schroers et al. (2011) using a 10-gene phylogeny spanning the complete Nectriaceae family. Considering the new phylogenetic evidence, which corresponded with morphological and ecological data, Lombard et al. (2015) segregated the Martiella fusaria (FSSC) from Fusarium, confining these taxa to the genus Neocosmospora, providing one new species and 12 new combinations, including the most commonly recognised species of the genus, Neocosmospora solani. Since taxonomy is not static and must progress with accumulated knowledge, we disagree with the proposal of Geiser et al. (2013) which necessarily implies a denial of the current phenotypic and phylogenetic evidence. On taxonomic grounds, the genus Neocosmospora (FSSC) is recognised here as a distinct genus from Fusarium as stated in Lombard et al. (2015).
Fusarium and Neocosmospora are clearly distinguished phylogenetically and morphologically based on both sexual and asexual morphs. Neocosmospora is characterised by forming orange to red-brown, smooth-walled to coarsely warted perithecia, producing globose to ellipsoidal, 0–1-septate, distinctly ornamented (striate, cerebriform to spinulose), yellow golden-brown ascospores; while asexual morphs produce distinctive very long and narrow, acremonium-like aerial monophialides. By contrast, Fusarium (Gibberella) species have dark-blue to purple, black with reflecting light, warted perithecia; and ellipsoidal to fusoidal, straight to curved, (0–)1–3-septate, smooth-walled, pale brown ascospores; their asexual morphs produce relatively short, mono- or polyphialides, while holoblastic conidiogenous cells producing solitary conidia can be also present.
Because of recent taxonomic changes related to the generic circumscription of the genus ‘Fusarium’, significant confusion has been created, especially among clinical microbiologists and phytopathologists, a situation exacerbated by the increasing number of recognised phylogenetic species known only from DNA, but lacking formal descriptions and Latin binomials. Conversely, a large number of taxa in Neocosmospora, including relatively recently described species, lack available DNA sequence data, while for many established species, original material has not been traced, resulting in ambiguous application of names, and their exclusion from modern studies.
The present study therefore aims to explore the phylogenetic breadth of Neocosmospora by updating and expanding currently available DNA sequence datasets (O’Donnell 2000, O’Donnell et al. 2008), and assigning names to presently undescribed taxa based on thorough morphological observations. Old species circumscriptions are revisited and their taxonomy is reconsidered based on a re-examination of original material and DNA sequence data.
MATERIAL AND METHODS
Fungal isolates and fungarium specimens
Strains included in this study were obtained from diverse culture collections, namely the Belgian Coordinated Collections of Microorganisms / Agro-food & Environmental Fungal Collection (MUCL), the Centre for Agriculture and Bioscience International (CABI) collection (IMI), the U.S. Agricultural Research Service culture collection (NRRL), the Westerdijk Fungal Biodiversity Institute (WI) collection (CBS) and the personal collection of P.W. Crous (CPC) held at WI. Strains obtained as metabolically inactive cultures (lyophilised) were revived in 2 mL malt/peptone broth (1 : 1) and transferred to oatmeal agar (OA; Crous et al. 2019). Strains retrieved from liquid nitrogen storage and as slant cultures, were directly plated onto OA. Additionally, fungarium specimens were obtained from the U.S. National Fungus Collections (BPI) and the WI (CBS; Table 1).
Table 1.
Cultures, specimens and DNA accesion numbers included in this study.
| Species name1 | Culture/specimen2 | Substrate | Country | GenBank/ENA accession number3 | |||
|---|---|---|---|---|---|---|---|
| tef1 | ITS | LSU | rpb2 | ||||
| F. caeruleum | CBS 113.23 = NRRL 20843 | Solanum tuberosum | Germany | LR583588 | LR583695 | LR583695 | – |
| CBS 133.73 = NRRL 22286 = ATCC 24389 = IMI 163397 | Solanum tuberosum | Sweden | LR583589 | LR583696 | LR583906 | – | |
| CBS 836.85 = NRRL 20434 = BBA 64413 | Solanum tuberosum | Germany | LR583590 | LR583697 | LR583907 | – | |
| F. caucasicum | CBS 179.35 = NRRL 13954 = IFO5979 | Unknown | USSR | LR583591 | LR583698 | LR583698 | – |
| F. javanicum | BPI 452276 | Unknown | Honduras | – | – | – | – |
| Fusarium sp. | BPI 452275 = ATCC 22403 | Unknown | Unknown | LR583592 | LR583699 | LR583699 | – |
| Geejayessia atrofusca | NRRL 22316 | Staphylea trifolia | USA | AF178361 | AF178423 | AF178392 | EU329502 |
| Geejayessia cicatricum | CBS 125552 | Dead twig | Slovenia | HM626644 | HQ728145 | – | HQ728153 |
| N. acutispora | CBS 145461T = NRRL 22574 = BBA 62213 | Coffea arabica | Guatemala | LR583593 | LR583700 | LR583908 | LR583814 |
| N. ambrosia | CBS 571.94ET = NRRL 22346 = BBA 65390 = MAFF 246287 (ex-epitype of Monacrosporium ambrosium) | Euwallacea fornicatus on Camellia sinensis | India | FJ240350 | EU329669 | EU329669 | EU329503 |
| NRRL 20438 = IMI 296597 = MAFF 246291 | Euwallacea fornicatus on Camellia sinensis | India | AF178332 | AF178397 | DQ236357 | JX171584 | |
| N. ampla | CBS 202.32T = BBA 4170 | Coffea sp. | German East Africa | LR583594 | LR583701 | LR583909 | LR583815 |
| N. batatas | CBS 144397 = NRRL 22400 = BBA 64683 | Ipomoea batatas | USA | AF178343 | AF178407 | AF178376 | EU329509 |
| CBS 144398T = NRRL 22402 = BBA 64954 = FRC S-0567 | Ipomoea batatas | USA | AF178344 | AF178408 | AF178377 | FJ240381 | |
| N. borneensis | CBS 145462 = NRRL 22579 = BBA 65095 = GJS 85-197 | Bark or recently dead tree | Indonesia | AF178352 | AF178415 | AF178384 | EU329515 |
| N. bostrycoides | CBS 239.39 = NRRL 22656 | Atta sp. fungus garden | Unknown | LR583595 | LR583702 | LR583910 | LR583816 |
| CBS 102824 | Leaf litter | Colombia | LR583596 | LR583703 | LR583911 | LR583817 | |
| CBS 130328 = NRRL 31169 | Human oral wound | USA | DQ246923 | DQ094396 | DQ236438 | EU329564 | |
| CBS 130391 = NRRL 46707 = FMR 8030 | Human eye | Brazil | HM347127 | EU329716 | EU329716 | EU329665 | |
| CBS 144.25NT (ex-neotype of Fusarium bostrycoides) | Soil | Honduras | LR583597 | LR583704 | LR583912 | LR583818 | |
| CBS 392.66 = NRRL 25325 = BBA 69595 | Bertholletia excelsa | Unknown | LR583598 | LR583705 | LR583913 | LR583819 | |
| NRRL 52701 = ARSEF 6602 | Hypothenemus hampei | Colombia | JF740784 | JF740906 | JF740906 | JF741110 | |
| N. brevicona | CBS 203.31 = NRRL 22234 = BBA 2019 | Twig | Philippines | LR583599 | LR583706 | LR583914 | LR583820 |
| CBS 204.31ET = NRRL 22659 = BBA 2123 (ex-type of Hypomyces haematococcus var. breviconus) | Gladiolus sp. | Indonesia | LR583600 | LR583707 | LR583915 | LR583821 | |
| N. brevis | F86 | Unknown | Unknown | * | * | * | * |
| F93 | Unknown | Unknown | * | * | * | * | |
| CBS 130326 = NRRL 28009 = CDC B-5543 | Human eye | USA | DQ246869 | DQ094351 | DQ236393 | EF470136 | |
| CBS 144387T = MUCL 16108 | Polluted soilwater | Belgium | LR583601 | LR583708 | LR583916 | LR583822 | |
| CPC 27190 | Citrus sinensis | Italy | LT746199 | LT746247 | LT746247 | LT746312 | |
| CPC 27191 | Citrus sinensis | Italy | LT746200 | LT746248 | LT746248 | LT746313 | |
| NRRL 32792 = FRC S-1143 | Human subcutaneous nodules | Japan | DQ247101 | DQ094561 | DQ236603 | EU329621 | |
| N. catenata | CBS 143228 = NRRL 54992 = UTHSC 09-1008 | Stegostoma fasciatum multiple tissues | USA | KC808213 | KC808255 | KC808255 | KC808354 |
| CBS 143229T = NRRL 54993 = UTHSC 09-1009 | Stegostoma fasciatum multiple tissues | USA | KC808214 | KC808256 | KC808256 | KC808355 | |
| N. crassa | CBS 144386T = MUCL 11420 | Unknown | France | LR583604 | LR583709 | LR583917 | LR583823 |
| NRRL 46596 | Human toenail | Italy | GU170627 | GU170647 | GU170647 | GU170592 | |
| NRRL 46703 = FMR 8281 | Nematode egg | Spain | HM347126 | EU329712 | EU329712 | EU329661 | |
| NRRL 54203 | Musa acuminata cv. Cavendish | Guatemala | * | * | * | * | |
| N. cryptoseptata | CBS 145463T = NRRL 22412 = BBA 65024 | Bark | French Guiana | AF178351 | AF178414 | AF178383 | EU329510 |
| N. cucurbitae | CBS 410.62 = NRRL 22658 = CECT 2864 | Cucurbita viciifolia | Netherlands | DQ247640 | LR583710 | LR583918 | LR583824 |
| CBS 616.66T = NRRL 22399 = BBA 64411 | Cucurbita viciifolia | Netherlands | DQ247592 | LR583711 | LR583919 | LR583825 | |
| NRRL 22098 = GJS 92-25 | Cucurbit | USA | AF178327 | DQ094301 | DQ236343 | EU329489 | |
| NRRL 22153 = ATCC 18099 | Cucurbit | USA | AF178346 | DQ094302 | DQ236344 | EU329492 | |
| N. cyanescens | CBS 518.82T | Human foot | Netherlands | LR583605 | AB190389 | LR583920 | LR583826 |
| CBS 637.82 | Human foot | Netherlands | LR583606 | LR583712 | LR583921 | LR583827 | |
| N. diminuta | CBS 144390 = MUCL 18798T | Coelocaryon preusii treated wood | Unknown | LR583607 | LR583713 | LR583922 | LR583828 |
| FRC S-S2432 | Bathroom sink drain | USA | * | * | * | * | |
| N. elegans | CBS 144395 = NRRL 22163 = MAFF 238540 = ATCC 18690 | Xanthoxylum piperitum branch | Japan | AF178328 | AF178394 | AF178363 | EU329496 |
| CBS 144396ET = NRRL 22277 = MAFF 238541 = ATCC 42366 (ex-epitype of Nectria elegans) | Xanthoxylum piperitum trunk | Japan | AF178336 | AF178401 | AF178370 | FJ240380 | |
| N. euwallaceae | CBS 135854T = NRRL 54722 (ex-type of Fusarium euwallaceae) | Euwallacea sp. on Persea americana | Israel | JQ038007 | JQ038014 | JQ038014 | JQ038028 |
| NRRL 62626 | Euwallacea sp. on Persea americana | USA | KC691532 | KC691560 | KC691560 | KU171702 | |
| N. falciformis | CBS 475.67T = IMI 268681 | Human mycetoma | Puerto Rico | LT906669 | MG189935 | MG189915 | LT960558 |
| CBS 121450 | Declined grape vine | Syria | JX435161 | JX435211 | JX435211 | JX435261 | |
| CBS 124627 | Human nail | France | JX435134 | JX435184 | JX435184 | JX435234 | |
| CBS 141593T = CML 1830 (ex-type of F. paranaense) | Glycine max | Brazil | KF597797 | MG787463 | MG787463 | KF680011 | |
| CBS 141594PT = CML 860 (ex-paratype of F. paranaense) | Glycine max | Brazil | KF597814 | MG787462 | MG787463 | KF680005 | |
| NRRL 22781 = IMI 215769 | Human cornea | Venezuela | DQ246849 | DQ094334 | DQ236376 | EU329527 | |
| NRRL 25746 = ATCC 62877 | Human skin | USA | * | KF030979 | KF030979 | * | |
| NRRL 28562 = UTHSC 97-946 | Human bonee | USA | DQ246903 | DQ094376 | DQ236418 | EU329553 | |
| NRRL 28563 = UTHSC 97-1127 | Clinical isolate | USA | DQ246904 | DQ094377 | DQ236419 | EU329554 | |
| NRRL 32307 = UTHSC 00-1855 | Human sputum | Unknown | DQ246935 | DQ094405 | DQ236447 | EU329569 | |
| NRRL 32313 = UTHSC 00-920 | Human corneal ulcer | Unknown | DQ246941 | EU329678 | EU329678 | EU329573 | |
| NRRL 32331 = UTHSC 98-1911 | Human leg wound | Unknown | DQ246959 | DQ094428 | DQ236470 | EU329577 | |
| NRRL 32339 = UTHSC 01-2314 | Human | Unknown | DQ246967 | DQ094436 | DQ236478 | EU329578 | |
| NRRL 32540 = CDC 1437-02 | Human eye | India | DQ247006 | DQ094471 | DQ236513 | EU329589 | |
| NRRL 32544 = CDC 1705-02 | Human eye | India | DQ247010 | DQ094475 | DQ236517 | EU329591 | |
| NRRL 32547 = CDC 1792-02 | Human eye | India | DQ247012 | EU329680 | EU329680 | EU329593 | |
| NRRL 32714 = FRC S-0406 | Human eye | USA | DQ247034 | DQ094496 | DQ236538 | EU329598 | |
| NRRL 32718 = FRC S-0410 | Human eye | USA | DQ247038 | DQ094500 | DQ236542 | EU329599 | |
| NRRL 32729 = FRC S-0421 | Human eye | USA | DQ247049 | DQ094510 | DQ236552 | EU329604 | |
| NRRL 32738 = FRC S-0431 | Human eye | USA | DQ247058 | DQ094519 | DQ236561 | EU329607 | |
| NRRL 32754 = FRC S-0449 | Turtle nare lesion | USA | DQ247072 | DQ094533 | DQ236575 | EU329612 | |
| NRRL 32778 = FRC S-0802 | Equine corneal ulcer | USA | DQ247088 | DQ094549 | DQ236591 | EU329616 | |
| NRRL 32798 = FRC S-1158 | Human | USA | DQ247107 | DQ094567 | DQ236609 | EU329623 | |
| NRRL 43441 = CDC 43441 | Human cornea | USA | DQ790478 | DQ790522 | DQ790522 | DQ790566 | |
| NRRL 43536 = CDC 2006743582 | Human cornea | USA | EF452966 | EF453118 | EF453118 | EF470005 | |
| NRRL 43537 = CDC 2006743583 | Human cornea | USA | DQ790506 | DQ790550 | DQ790550 | DQ790594 | |
| NRRL 46683 = FMR 7988 | Human eye | Brazil | * | EU329692 | EU329692 | EU329641 | |
| NRRL 46687 = FMR 7994 | Human eye | Unknown | * | EU329696 | EU329696 | EU329645 | |
| NRRL 46692 = FMR 7242 | Human skin | Spain | * | EU329701 | EU329701 | EU329650 | |
| NRRL 52832 | Human toenail | Italy | GU170631 | GU170651 | GU170651 | GU170596 | |
| NRRL 54219 | Human vertebral mass | USA | HQ401721 | * | * | HQ401723 | |
| NRRL 54966 = UTHSC 02-687 | Equine eye | USA | KC808193 | KC808233 | KC808233 | KC808331 | |
| NRRL 54983 = UTHSC 07-2890 | Equine eye | USA | KC808206 | KC808248 | KC808248 | KC808346 | |
| N. ferruginea | CBS 109028T = NRRL 32437 | Human subcutaneous nodule | Switzerland | DQ246979 | DQ094446 | DQ236488 | EU329581 |
| CPC 28194 | Citrus sinensis | Italy | LR583602 | LT746276 | LT746276 | LT746341 | |
| CPC 28195 | Citrus sinensis | Italy | LR583603 | LT746277 | LT746277 | LT746342 | |
| NRRL 52705 = ARSEF 6587 | Unknown | Unknown | JF740787 | * | * | JF741113 | |
| N. gamsii | CBS 217.53 = NRRL 22655 | Plywood | Nigeria | DQ247637 | MG189936 | MG189916 | LT960559 |
| CBS 700.86 = NRRL 22236 | Unknown | Brazil | DQ247624 | DQ094763 | MG189917 | LT960560 | |
| CBS 130181 = NRRL 43502 = CDC 2006743469 | Human cornea | USA | DQ790488 | DQ790532 | DQ790532 | DQ790576 | |
| CBS 143207T = NRRL 32323 = UTHSC 99-205 | Human bronchoalveolar lavage fluid | USA | DQ246951 | DQ094420 | DQ236462 | EU329576 | |
| CBS 143209 = NRRL 32770 = FRC S-0524 | Human eye | USA | DQ247083 | DQ094544 | DQ236586 | EU329615 | |
| CBS 143211 = NRRL 32794 = FRC S-1152 | Humidifier coolant | USA | DQ247103 | DQ094563 | DQ236605 | EU329622 | |
| N. guarapiensis† | CBS 131752 = GJS 93-44 | Bark | China | LR583608 | LR583714 | LR583714 | LR583829 |
| N. haematococca | CBS 119600ET = FRC S-1832 (ex-epitype of Nectria haematococca) | Dying tree | Sri Lanka | DQ247510 | KM231797 | KM231664 | LT960561 |
| N. henyangensis | HMAS 254518T | Twigs of unknown host | China | KY829448 | KY829446 | – | – |
| N. hypothenemi | CBS 145464T = NRRL 52782 = ARSEF 5878 | Hypothenemus hampei | Benin | JF740850 | LR583715 | LR583923 | JF741176 |
| CBS 145466 = NRRL 52783 = ARSEF 5879 | Hypothenemus hampei | Uganda | JF740851 | LR597067 | LR597068 | JF741177 | |
| N. illudens | NRRL 22090 = BBA 67606 = GJS 82-98 | Beilschmiedia tawa | New Zealand | AF178326 | AF178393 | AF178362 | JX171601 |
| N. ipomoeae | BPI 453044 | Unknown | Unknown | – | – | – | – |
| CBS 225.58 = NRRL 22235 = BBA 64431 | Cotton duck | Panama | LR583609 | LR583716 | LR583924 | LR583830 | |
| CBS 353.87 = NRRL 22657 | Capsicum annuum | Netherlands | DQ247639 | LR583717 | LR583925 | LR583831 | |
| CBS 354.87 = NRRL 22238 | Gerbera sp. | Netherlands | LR583610 | LR583718 | LR583926 | LR583832 | |
| CBS 833.97 | Rosa sp. dead parts | Netherlands | LR583611 | LR583719 | LR583927 | LR583833 | |
| NRRL 22101 | Cotton cloth | Panama | AF178333 | AF178398 | AF178367 | MG282399 | |
| NRRL 52699 = ARSEF 6461 | Mahanarva andigena adult | Colombia | JF740782 | JF740905 | JF740905 | JF741108 | |
| N. keleraja | CBS 125720PT = FRC S-1837 = GJS 02-114 | On branch of unidentified tree | Sri Lanka | LR583612 | LR583720 | LR583928 | LR583834 |
| CBS 125722PT = FRC S-1836 = GJS 02-114 | On branch of unidentified tree | Sri Lanka | DQ247515 | JF433039 | JF433039 | LR583835 | |
| FRC S-1839T = GJS 02-122 | Trunk of Yakuda marang | Sri Lanka | DQ247518 | JF433041 | JF433041 | – | |
| N. keratoplastica | CBS 490.63T (ex-type of Cephalosporium keratoplasticum) | Human | Japan | LT906670 | LR583721 | LR583929 | LT960562 |
| CBS 144389 = MUCL 18301 | Greenhouse humic soil | Belgium | LR583613 | LR583722 | LR583930 | LR583836 | |
| FRC S-2477T (ex-type of F. keratoplasticum) | Indoor plumbing | USA | JN235712 | NR130690 | JN235282 | JN235897 | |
| NRRL 22640 = FRC S-1486 = ATCC 32793 | Human cornea | Argentina | DQ246842 | DQ094327 | DQ236369 | EU329520 | |
| NRRL 22791 = FRC S-2194 = IMI 095994 | Iguana tail | Unknown | DQ246853 | DQ094337 | DQ236379 | EU329530 | |
| NRRL 28014 = CDC B-5754 | Human | USA | DQ246872 | DQ094354 | DQ236396 | EF470139 | |
| NRRL 28561 = UTHSC 97-1423 | Human wound | USA | DQ246902 | DQ094375 | DQ236417 | EU329552 | |
| NRRL 32707 = FRC S-0399 = FRC S-2226 | Human eye | USA | DQ247027 | DQ094490 | DQ236532 | EU329595 | |
| NRRL 32710 = FRC S-0402 = FRC S-2227 | Human eye | USA | DQ247030 | DQ094492 | DQ236534 | EU329596 | |
| NRRL 32780 = FRC S-0906 = FRC S-2239 | Sea turtle | USA | DQ247090 | DQ094551 | DQ236593 | EU329617 | |
| NRRL 32838 = FRC S-1268 | Sea turtle | USA | DQ247144 | EU329681 | EU329681 | EU329627 | |
| NRRL 32959 = UTHSC 97-425 | Manatee skin | USA | DQ247178 | DQ094632 | DQ236674 | EU329634 | |
| NRRL 43490 = CDC 2006743457 | Human eye | USA | DQ790485 | DQ790529 | DQ790529 | DQ790573 | |
| NRRL 43649 = CDC 2006743509 | Human eye | USA | EF452980 | EF453132 | EF453132 | EU329639 | |
| NRRL 46437 | Human toenail | Italy | GU170623 | GU170643 | GU170643 | GU170588 | |
| NRRL 46438 | Human toenail | Italy | GU170624 | GU170644 | GU170644 | GU170589 | |
| NRRL 46696 = FMR 7989 | Human eye | Brazil | AM397219 | EU329705 | EU329705 | EU329654 | |
| NRRL 46697 = FMR 8482 | Human tissue | Qatar | AM397224 | EU329706 | EU329706 | EU329655 | |
| NRRL 52704 = ARSEF 6572 | Tetranychus urticae | USA | JF740786 | JF740908 | JF740908 | JF741112 | |
| NRRL 54524 | Unknown | Unknown | * | * | * | * | |
| N. kuroshio | CBS 142642T | Euwallacea sp. on Platanus racemosa | USA | KX262216 | LR583723 | LR583931 | LR583837 |
| HFEW-16-IV-019 | Euwallacea sp. | Mexico | PRJNA387548 | PRJNA387548 | PRJNA387548 | PRJNA387548 | |
| NRRL 62945 | Euwallacea sp. on Platanus racemosa | USA | KM406629 | KM406636 | KM406636 | KM406649 | |
| NRRL 62946 | Euwallacea sp. on Platanus racemosa | USA | KM406630 | KM406637 | KM406637 | KM406650 | |
| N. kurunegalenis | CBS 119599T = GJS 02-94 | Recently cut tree | Sri Lanka | DQ247511 | JF433036 | JF433036 | LR583838 |
| N. lichenicola | CBS 166.67T = IMUR 1797 (ex-type of Moeszia pernambucensis) | Soil | Brazil | LR583614 | LR583724 | LR583932 | LR583839 |
| CBS 279.34T (ex-type of Monacrosporium tedeschii) | Human | Somalia | LR583615 | LR583725 | LR583933 | LR583840 | |
| CBS 279.59T = ATCC 13427 (type of Mastigosporium heterosporium) | Soil | Tahiti | LR583616 | LR583726 | LR583934 | LR583841 | |
| CBS 483.96 = IFO 30561 = NBRC 30561 | Human eye | Japan | LR583617 | LR583727 | LR583935 | LR583842 | |
| CBS 509.63T = MUCL 8050 = IMUR 410 (ex-type of Hyaloflorea ramosa) | Air | Brazil | LR583618 | LR583728 | LR583936 | LR583843 | |
| CBS 522.63T = MUCL 8049 = IMUR 320 (ex-type of Euricoa dominguesii) | Air | Brazil | LR583619 | LR583729 | LR583937 | LR583844 | |
| CBS 623.92ET (ex-epitype of Fusarium lichenicola) | Human necrotic wound | Germany | LR583620 | LR583730 | LR583938 | LR583845 | |
| NRRL 28030 | Human | Thailand | DQ246877 | DQ094355 | DQ236397 | KR674002 | |
| NRRL 34123 | Human eye | India | DQ247192 | DQ094645 | DQ236687 | EU329635 | |
| N. liriodendri | CBS 117481T = NRRL 22389 = BBA 67587 = GJS 91-148 | Liriodendron tulipifera | USA | AF178340 | AF178404 | AF178373 | EU329506 |
| N. longissima | CBS 126407T = GJS 85-72 | Tree bark | New Zealand | LR583621 | LR583731 | LR583939 | LR583846 |
| KOD614 | Unknown | Unknown | * | * | * | * | |
| N. macrospora | CBS 142424T = CPC 28191 | Citrus sinensis | Italy | LT746218 | LT746266 | LT746281 | LT746331 |
| CPC 28192 | Citrus sinensis | Italy | LT746219 | LT746267 | LT746282 | LT746332 | |
| CPC 28193 | Citrus sinensis | Italy | LT746220 | LT746268 | LT746283 | LT746333 | |
| N. mahasenii | CBS 119594T | Dead branch on live tree | Sri Lanka | DQ247513 | JF433045 | JF433045 | LT960563 |
| FRC S-1840 = GJS 02-124 | Rotting wood | Sri Lanka | DQ247520 | JF433042 | JF433042 | – | |
| N. martii | BPI 452379 | Solanum tuberosum | Netherlands | LR583622 | LR583732 | LR583940 | – |
| BPI 452383 | Pisum sativum | Netherlands | LR583623 | LR583733 | LR583941 | – | |
| BPI 452384 | Solanum tuberosum | Germany | LR583624 | LR583734 | LR583942 | – | |
| BPI 452385LT (lectotype of Fusarium martii) | Solanum tuberosum | Germany | LR583625 | LR583735 | LR583943 | – | |
| CBS 115659ET = FRC S-0679 = MRC 2198 (ex-epitype of Fusarium martii) | Solanum tuberosum var. Maritta | Germany | JX435156 | JX435206 | JX435206 | JX435256 | |
| CBS 127135 = RMF7653 | Soil | USA | LR583626 | LR583736 | LR583944 | LR583847 | |
| CBS 142423T (ex-type of Neocosmospora croci) | Citrus sinensis | Italy | LT746216 | LT746264 | LT746264 | LT746329 | |
| CPC 27187 | Citrus sinensis | Italy | LT746217 | LT746265 | LT746265 | LT746330 | |
| N. metavorans | CBS 233.36 = NRRL 22654 | Malus sylvestris | Italy | DQ247636 | LR583737 | LR583945 | LR583848 |
| CBS 135789T | Human pleural effusion | Greece | LR583627 | LR583738 | LR583946 | LR583849 | |
| CBS 130400 = NRRL 43489 = CDC 2006743456 | Human eye | USA | DQ790484 | DQ790528 | DQ790528 | DQ790572 | |
| CBS 143194 = NRRL 22782 = IMI 226114 | Human corneal ulcer | Spain | DQ246850 | EU329670 | EU329670 | EU329528 | |
| CBS 143195 = NRRL 22792 | Human eye | USA | DQ246854 | EU329671 | EU329671 | EU329531 | |
| CBS 143198 = NRRL 28016 = CDC B-5779 | Human | USA | DQ246873 | EU329673 | EU329673 | EF470140 | |
| CBS 143199 = NRRL 28017 = CDC B-5780 | Human | USA | LR583628 | LR583739 | LR583947 | LR583850 | |
| CBS 143200 = NRRL 28018 = CDC B-5781 | Human | USA | DQ246875 | LR583740 | FJ240360 | EF470142 | |
| CBS 143201 = NRRL 28019 = CDC B-5782 | Human | USA | DQ246876 | LR583741 | FJ240361 | EF470143 | |
| CBS 143202 = NRRL 28542 = UTHSC 98-1246 | Human bone | USA | DQ246883 | EU329675 | EU329675 | EU329543 | |
| CBS 143210 = NRRL 32785 = FRC S-1123 | Human toenail | USA | DQ247094 | LR583742 | FJ240371 | EU329618 | |
| CBS 143213 = NRRL 32849 = FRC S-1355 = UTHSC 95-2552 | Human eye | USA | DQ247155 | EU329682 | EU329682 | EU329628 | |
| CBS 143215 = NRRL 37640 = UTHSC R-3564 | Human | Turkey | FJ240355 | EU329685 | EU329685 | EU329638 | |
| CBS 143216 = NRRL 43717 | Human | USA | FJ240356 | EU329688 | EU329688 | EF470233 | |
| CBS 143218 = NRRL 46237 | Human | USA | FJ240357 | LR583743 | FJ240378 | FJ240411 | |
| CBS 143219 = NRRL 46708 = FMR 8634 | Human foot | Spain | LR583629 | LR583744 | LR583948 | LR583851 | |
| NRRL 28553 = UTHSC 97-2574 | Human foot | USA | DQ246894 | EU329676 | EU329676 | EU329548 | |
| NRRL 44892 | Human face | Italy | GU170618 | GU170638 | GU170638 | GU170583 | |
| NRRL 44904 | Human toenail | Italy | GU170621 | GU170641 | GU170641 | GU170586 | |
| NRRL 52746 = ARSEF 8279 | Ceresa bubalus | India | JF740822 | JF740921 | JF740921 | JF740994 | |
| N. mori | CBS 145467T = NRRL 22230 = MAFF 238539 | Morus alba branch | Japan | AF178358 | DQ094305 | DQ236347 | EU329499 |
| CBS 145468 = NRRL 22157 = MAFF 238538 | Morus alba branch | Japan | AF178359 | DQ094306 | DQ236348 | EU329493 | |
| JS-169 | Morus alba | South Korea | NGZQ01000005 | NGZQ01000004 | NGZQ01000004 | NGZQ01000006 | |
| N. nirenbergiana | CBS 145469T = NRRL 22387 = BBA 65023 = GJS 87-127 | Bark | French Guiana | AF178339 | AF178403 | AF178372 | EU329505 |
| N. noneumartii | CBS 115658T = FRC S-0661 | Solanum tuberosum | Israel | LR583630 | LR583745 | LR583949 | LR583852 |
| F97 | Unknown | Unknown | * | * | * | * | |
| F113 | Unknown | Unknown | * | * | * | * | |
| Fs112 | Solanum lycopersicum | USA | DQ164848 | DQ164844 | – | – | |
| Fs306 | Solanum tuberosum | USA | DQ164847 | DQ164843 | – | – | |
| N. oblonga | CBS 130325T = NRRL 28008 = CDC B-4701 | Human eye | USA | LR583631 | LR583746 | LR583950 | LR583853 |
| KOD253 | Unknown | Unknown | * | * | * | * | |
| N. oligoseptata | CBS 143241T = NRRL 62579 = FRC S-2581 = MAFF 246283 (ex-type of Fusarium oligoseptatum) | Euwallacea validus on Ailanthus altissima | USA | KC691538 | KC691566 | KC691566 | LR583854 |
| NRRL 62578 = FRC S-2576 | Euwallacea validus on Ailanthus altissima | USA | KC691537 | KC691565 | KC691565 | KC691626 | |
| NRRL 62606 | Euwallacea interjectus on Acer negundo | USA | KC691533 | KC691561 | KC691561 | KC691622 | |
| N. paraeumartii | CBS 487.76T = NRRL 13997 = BBA 62215 | Solanum tuberosum | Argentina | DQ247549 | LR583747 | LR583951 | LR583855 |
| N. parceramosa | CBS 115695T = CPC 1246 = STE-U 1246 | Soil | South Africa | JX435149 | JX435199 | JX435199 | JX435249 |
| NRRL 31158 | Human wound | USA | DQ246916 | DQ094389 | DQ236431 | EU329559 | |
| NRRL 32301 = UTHSC 01-595 | Human eye | USA | DQ246929 | EU329677 | EU329677 | EU329567 | |
| N. parva† | CBS 466.70IT = ATCC 28343 | Silty loam soil | Ecuador | LR583632 | LR583748 | LR583952 | LR583856 |
| N. perseae | CBS 144142T = CPC 26829 | Persea americana | Italy | LT991902 | LT991940 | LT991947 | LT991909 |
| CBS 144143 = CPC 26830 | Persea americana | Italy | LT991903 | LT991941 | LT991948 | LT991910 | |
| CBS 144144 = CPC 26831 | Persea americana | Italy | LT991904 | LT991942 | LT991949 | LT991911 | |
| CBS 144145 = CPC 26832 | Persea americana | Italy | LT991905 | LT991943 | LT991950 | LT991912 | |
| CBS 144146 = CPC 26833 | Persea americana | Italy | LT991906 | LT991944 | LT991951 | LT991913 | |
| N. petroliphila | CBS 203.32 = NRRL 13952 | Pelargonium sp. root | South Africa | DQ246835 | DQ094320 | DQ236362 | LR583857 |
| CBS 224.34 = NRRL 28579 | Human toenail | Cuba | DQ246910 | DQ094383 | DQ236425 | LR583858 | |
| CBS 398.66 | Saccharum officinarum | Brazil | LR583633 | LR583749 | LR583953 | LR583859 | |
| NRRL 22141 = MAFF 238536 | Cucurbita sp. | New Zealand | AF178329 | DQ094307 | DQ236349 | EU329491 | |
| NRRL 22142 = MAFF 238537 = FRC S-220 | Cucurbita sp. | USA | AF178347 | AF178411 | DQ236350 | FJ240379 | |
| NRRL 22610 | Human | USA | DQ246840 | DQ094325 | DQ236367 | * | |
| NRRL 32177 = FRC S-2206 | Unknown | Unknown | DQ246925 | DQ094397 | DQ236439 | * | |
| NRRL 32304 = UTHSC 00-2181 | Human nail | USA | DQ246932 | DQ094402 | DQ236444 | EU329568 | |
| NRRL 32315 = UTHSC 00-332 | Human groin ulcer | USA | DQ246943 | DQ094412 | DQ236454 | * | |
| NRRL 32856 = FRC S-1380 | Plaster from ceiling | USA | DQ247161 | EU329683 | EU329683 | EU329629 | |
| NRRL 43812 = CDC 2006743705 | Contact lens solution | Unknown | EF453054 | EF453205 | EF453205 | EF470093 | |
| NRRL 46706 = FMR 8340 | Human blood | Qatar | AM412594 | EU329715 | EU329715 | EU329664 | |
| NRRL 53401 = CDC 2008732272 | Human thigh tissue | Unknown | * | * | * | * | |
| N. phaseoli | BPI 452391 | Citrus aurantifolia | Honduras | – | – | – | – |
| CBS 265.50 | Phaseolus sp. | USA | FJ919464 | LR583750 | LR583954 | KJ511278 | |
| NRRL 22276 = ATCC 38466 | Phaseolus vulgaris | USA | AY220186 | EU329668 | EU329668 | JX171608 | |
| NRRL 22743 = BBA 68441 = MAFF 239041 | Glycine max | Brazil | AY320145 | EF408512 | AY320127 | EU329525 | |
| NRRL 22825 | Glycine max | USA | GU170635 | AF178419 | AF178388 | EU329533 | |
| NRRL 31041T = MAFF 238553 (ex-type of F. virguliforme) | Glycine max | USA | AY220193 | AY220239 | AY220169 | JX171643 | |
| NRRL 31096T = MAFF 238418 (ex-type of F. tucumaniae) | Glycine max | Argentina | AY220181 | AY220231 | AY220161 | GU170600 | |
| NRRL 31104 = MAFF 305607 | Phaseolus vulgaris | Japan | AY320159 | EF408518 | AY320141 | EU329558 | |
| NRRL 31156 = MAFF 239050 = FRC S-1550 | Phaseolus vulgaris | USA | AY220187 | EF408521 | AY220166 | KJ511281 | |
| NRRL 31157T = MAFF 239038 = FRC S-1551 (ex-type of F. cuneirostrum) | Phaseolus vulgaris | USA | MAEA01003816 | EF408519 | MAEA01000003 | KJ511282 | |
| NRRL 31757T = MAFF 239050 (ex-type of F. brasiliense) | Glycine max | Brazil | AY320148 | EF408514 | AY320130 | EU329565 | |
| NRRL 31949 = MAFF 239052 | Glycine max | Brazil | AY320161 | AY320197 | AY320143 | EU329566 | |
| NRRL 36877T = MAFF 239757 (ex-type of F. crassistipitatum) | Glycine max | Argentina | FJ240351 | FJ240376 | FJ240376 | FJ240405 | |
| NRRL 54364T = MAFF 242371 (ex-type of F. azukicola) | Vigna angularis | Japan | JQ670137 | MAEG01000005 | MAEG01000005 | KJ511287 | |
| N. piperis | CBS 145470T = NRRL 22570 = GJS 89-14 = CML 1888 | Piper nigrum | Brazil | AF178360 | AF178422 | AF178391 | EU329513 |
| N. pisi | CBS 178.47 | Pisum sativum | Netherlands | LR583634 | LR583751 | LR583955 | LR583860 |
| CBS 181.29 | Solanum tuberosum | Germany | JX435151 | JX435201 | JX435201 | JX435251 | |
| CBS 188.34 | Soil | Netherlands | LR583635 | LR583752 | LR583956 | LR583861 | |
| CBS 231.31 | Quercus garyana | USA | JX435160 | JX435210 | JX435210 | JX435260 | |
| CBS 123669ET = NRRL 45880 = ATCC MYA-4622 = Vanetten 77-13-4 (ex-epitype of F. martii var. pisi) | Progeny of parentals from Pisum sativum and soil | USA | LR583636 | LR583753 | LR583957 | LR583862 | |
| CBS 124896 = IHEM 15469 | Human skin | France | JX435130 | JX435180 | JX435180 | JX435230 | |
| CBS 127118 | Soil | USA | LR583637 | LR583754 | LR583958 | LR583863 | |
| CBS 142372 | Trifolium subterraneum | Germany | KY556454 | LR583755 | LR583959 | LR583864 | |
| CBS 144391 = MUCL 20258 | Greenhouse soil | Belgium | LR583638 | LR583756 | LR583960 | LR583865 | |
| CBS 144392 = MUCL 20260 | Greenhouse soil | Belgium | LR583639 | LR583757 | LR583961 | LR583866 | |
| NRRL 22278 | Pisum sativum | USA | AF178337 | DQ094309 | DQ236351 | EU329501 | |
| NRRL 22820 | Glycine max | USA | AF178355 | DQ094310 | DQ236352 | EU329532 | |
| NRRL 52721 = ARSEF 6403 | Eurygaster sp. | Turkey | JF740803 | * | * | JF741129 | |
| NRRL 52790 = ARSEF 6397 | Eurygaster sp. | Turkey | JF740858 | * | * | JF741184 | |
| NRRL 53339 | Unknown | Unknown | * | * | * | * | |
| N. plagianthi | BPI 801937IT (isotype of Nectria plagianthi) | Plagianthus betulinus | New Zealand | – | – | – | – |
| NRRL 22632 = GJS 83-146 | Hoheria glabrata | New Zealand | AF178354 | AF178417 | AF178386 | JX171614 | |
| N. protoensiformis | CBS 145471T = NRRL 22178 = GJS 90-168 | Dicot tree | Venezuela | AF178334 | AF178399 | AF178368 | EU329498 |
| N. pseudensiformis | CBS 130.78 = NRRL 22575 = NRRL 22653 | Cocos nucifera | Indonesia | DQ247635 | LR583759 | LR583963 | LR583868 |
| CBS 241.93 | Human mycetoma | Suriname | JX435148 | JX435198 | JX435198 | JX435248 | |
| CBS 125729T = NRRL 46517 = GJS 02-95 = GJS 9318 = FRC S-1834 | Dead tree | Sri Lanka | DQ247512 | KC691584 | KC691584 | KC691645 | |
| NRRL 22354 = BBA 65035 | Bark | French Guiana | AF178338 | AF178402 | DQ236358 | EU329504 | |
| N. pseudoradicicola | CBS 145472T = NRRL 25137 = ARSEF 2313 | Diseased cocoa pods | Papua New Guinea | JF740757 | JF740899 | JF740899 | JF741084 |
| NRRL 25138 = ARSEF 2314 | Diseased cocoa pods | Papua New Guinea | JF740758 | JF740900 | JF740900 | JF741085 | |
| N. pseudotonkinensis | CBS 143038 | Human cornea | Netherlands | LR583640 | LR583758 | LR583962 | LR583867 |
| N. quercicola | CBS 141.90T = NRRL 22652 | Quercus cerris | Italy | DQ247634 | LR583760 | LR583964 | LR583869 |
| CBS 334.92 = NRRL 25726 | Human toenail | Germany | DQ246863 | DQ094345 | DQ236387 | EU329539 | |
| CBS 130177 = NRRL 22611 = UTHSC 93-2524 | Human cornea | USA | DQ246841 | DQ094326 | DQ236368 | EU329518 | |
| NRRL 32705 = FRC S-0390 | Human skin lesions | USA | DQ247025 | DQ094488 | DQ236530 | EU329594 | |
| NRRL 32736 = FRC S-0429 | Human eye | USA | DQ247056 | DQ094517 | DQ236559 | EU329605 | |
| N. rectiphora | CBS 119603 = GJS 02-129 = FRC S-1843 | Dead tree branch | Sri Lanka | JF433027 | JF433044 | JF433044 | – |
| CBS 124755 = GJS 04-179 | Tree | Sri Lanka | LR583641 | LR583761 | LR583965 | LR583870 | |
| CBS 125726 = FRC S-1842 | Dead tree | Sri Lanka | JF433026 | JF433043 | JF433043 | – | |
| CBS 125727T = GJS 02-89 = FRC S-1831 | Dead tree | Sri Lanka | DQ247509 | JF433034 | JF433034 | LR583871 | |
| HMAS 254519T (ex-type of N. bomiensis) | Twigs of unknown host | China | KY829449 | KY829447 | – | – | |
| NRRL 22396 = BBA 65075 | Bark | French Guiana | AF178342 | AF178406 | AF178375 | EU329508 | |
| N. regularis | CBS 190.35 | Phaseolus sp. | USA | LR583642 | LR583762 | LR583966 | LR583872 |
| CBS 230.34T | Pisum sativum | Netherlands | LR583643 | LR583763 | LR583967 | LR583873 | |
| N. riograndensis | UFMG CM F12570T | Human nasal cavity | Brazil | KX534002 | KT186366 | KX534001 | KX534003 |
| N. robusta | CBS 145473T = NRRL 22395 = BBA 65682 | Bark | Venezuela | AF178341 | AF178405 | LR583968 | EU329507 |
| N. samuelsii | CBS 114067T = GJS 89-70 | Bark | Guyana | LR583644 | LR583764 | LR583969 | LR583874 |
| N. silvicola | CBS 119601 = GJS 98-135 | Populus nigra | France | LR583645 | LR583765 | LR583970 | LR583875 |
| CBS 123846T = GJS 04-147 | Liriodendron tulipifera | USA | LR583646 | LR583766 | LR583971 | LR583876 | |
| NRRL 22161 = ATCC 18692 | Robinia pseudoacacia | Japan | AF178330 | DQ094311 | DQ236353 | EU329494 | |
| NRRL 22162 = ATCC 18693 = MATUO 577 | Robinia pseudoacacia | Japan | DQ247561 | EU329667 | EU329667 | EU329495 | |
| NRRL 22586 | Robinia pseudoacacia | USA | AF178353 | DQ094312 | DQ236354 | EU329516 | |
| NRRL 53333 | Unknown | Unknown | * | * | * | * | |
| N. solani | BPI 451321 (lectotype of F. aduncisporum) | Meliotus alba stems | USA | LR583647 | LR583767 | LR583972 | – |
| BPI 452104 | Solanum tuberosum | USA | LR583648 | LR583768 | LR583973 | – | |
| BPI 452278 | Theobroma cacao fruit | Cameroon | LR583649 | – | – | – | |
| BPI 453134 | Unknown | Honduras | – | – | – | – | |
| CBS 165.87 | Solanum tuberosum | Denmark | JX435157 | JX435207 | JX435207 | JX435257 | |
| CBS 166.87 | Soil under Castanea sp. | USA | JX435157 | JX435207 | JX435207 | JX435257 | |
| CBS 208.29 | Hyacinthus orientalis | Germany | LR583650 | LR583769 | LR583974 | LR583877 | |
| CBS 101018T (ex-type of N. rubicola) | Raspberry | Italy | LR583651 | LR583770 | LR583975 | LR583878 | |
| CBS 111722 | Soil on wheat field | Japan | LR583652 | LR583771 | LR583976 | LR583879 | |
| CBS 112101 | Human vocal prosthesis | Belgium | LR583653 | LR583772 | LR583977 | LR583880 | |
| CBS 117149 | Mixture of cheese and soil | Austria | LR583654 | LR583773 | LR583978 | LR583881 | |
| CBS 119996 | Daucus carota | Netherlands | JX435152 | JX435202 | JX435202 | JX435252 | |
| CBS 124893 | Human nail | France | JX435141 | JX435191 | JX435191 | JX435241 | |
| CBS 140079ET = NRRL 66304 = GJS 09-1466 = FRC S-2364 (ex-epitype of Fusisporium solani) | Solanum tuberosum | Slovenia | KT313611 | KT313633 | KT313633 | KT313623 | |
| CBS 144393 = MUCL 34689 | Timber on tropical greenhouse | Belgium | LR583655 | LR583774 | LR583979 | LR583882 | |
| NRRL 22779 = IMI 307740 | Human toenail | New Zealand | DQ246848 | DQ094333 | DQ236375 | EU329526 | |
| NRRL 28679 | Unknown | Unknown | DQ246912 | DQ094385 | DQ236427 | EU329556 | |
| NRRL 31168 | Human toe | USA | DQ246922 | DQ094395 | DQ236437 | EU329563 | |
| N. solani (cont.) | NRRL 32484 = FRC S-1242 | Human | USA | DQ246982 | DQ094449 | DQ236491 | EU329583 |
| NRRL 32492 = FRC S-1327 | Human | USA | DQ246990 | EU329679 | EU329679 | EU329584 | |
| NRRL 32737 = FRC S-0430 | Human eye | USA | DQ247057 | DQ094518 | DQ236560 | EU329606 | |
| NRRL 32791 = FRC S-1142 | Unknown | USA | DQ247100 | DQ094560 | DQ236602 | EU329620 | |
| NRRL 32810 = FRC S-1198 | Human corneal ulcer | USA | DQ247118 | DQ094577 | DQ236619 | EU329624 | |
| NRRL 43468 = CDC 2006743431 | Human eye | USA | EF452941 | EF453093 | EF453093 | EF469980 | |
| NRRL 43474 | Human eye | USA | EF452945 | EF453097 | EF453097 | EF469984 | |
| NRRL 44896 | Human toenail | Italy | GU170619 | GU170639 | GU170639 | GU170584 | |
| NRRL 44924 | Unknown | Unknown | GU250537 | GU250660 | GU250660 | GU250722 | |
| NRRL 46598 | Human toenail | Italy | GU170628 | GU170648 | GU170648 | GU170593 | |
| NRRL 46643 | Unknown | Unknown | GU250544 | GU250667 | GU250667 | GU250729 | |
| NRRL 46702 = FMR 8673 | Nematode | Spain | AM397216 | AM412603 | EU329711 | EU329660 | |
| NRRL 52798 = ARSEF 7382 | Tetanops myopaeformis pupa | USA | JF740866 | JF740936 | JF740936 | JF741191 | |
| NRRL 53511 | Unknown | Unknown | * | * | * | * | |
| N. spathulata | CBS 145474T = NRRL 28541 = UTHSC 98-1305 | Human synovial fluid | USA | DQ246882 | EU329674 | EU329674 | EU329542 |
| N. stercicola | CBS 187.35 = BBA 2318 | Unknown | Unknown | LR583656 | LR583775 | LR583980 | LR583883 |
| CBS 260.54 | Unknown | Unknown | LR583657 | LR583776 | LR583981 | LR583884 | |
| CBS 618.76 = NRRL 22239 | Nematode egg | Germany | DQ247562 | LR583777 | LR583982 | LR583885 | |
| CBS 142480T (ex-type of F. witzenhousenense) | Hibiscus sp. branch | Germany | KY556525 | LR583778 | LR583983 | LR583886 | |
| CBS 142481T = DSM 106211 (ex-type of F. stercicola) | Compost yard debris | Germany | LR583658 | LR583779 | LR583984 | LR583887 | |
| CBS 144388 = MUCL 18299 | Greenhouse humic soil | Belgium | LR583659 | LR583780 | LR583985 | LR583888 | |
| FRC S-2570 = GJS 09-1458 | Solanum tuberosum | Slovenia | KT313605 | KT313627 | KT313627 | KT313616 | |
| GJS 09-1459 | Solanum tuberosum | Slovenia | KT313617 | KT313628 | KT313628 | KT313606 | |
| N. striata† | CBS 105.77T = NRRL 22427 = NRRL 22443 = ATCC 34720 = IFM 4528 = IMI 210879 = NHL 2745 | Soil | Japan | DQ247604 | LR583781 | LR583781 | LR583889 |
| N. suttoniana | CBS 124892 | Human nail | Gabon | JX435139 | JX435189 | JX435189 | JX435239 |
| CBS 130178 = NRRL 22608 = UTHSC 93-1547 | Human | USA | DQ246838 | DQ236365 | DQ094323 | EU329517 | |
| CBS 143197 = NRRL 28000 | Human blood | USA | DQ246865 | DQ094347 | DQ236389 | EF470128 | |
| CBS 143204 = NRRL 32316 | Human cornea | USA | DQ246944 | DQ094413 | DQ236455 | EU329574 | |
| CBS 143214T = NRRL 32858 | Human wound | USA | DQ247163 | DQ094617 | DQ236659 | EU329630 | |
| CBS 143224 = NRRL 54972 | Equine eye | USA | KC808197 | MG189940 | MG189925 | KC808336 | |
| NRRL 28001 = CDC B-4572 | Human | USA | DQ246866 | DQ094348 | DQ236390 | EF470129 | |
| N. tenuicristata† | IMI 277708IT = NHL 2911 | Marine sludge | Japan | – | LR583782 | LR583986 | – |
| N. theobromae | BPI 453072NT | Theobroma cacao fruits and seeds | Cameroon | LR583660 | – | LR583987 | – |
| N. tonkinensis | CBS 115.40T | Musa sapientum | Vietnam | LT906672 | MG189941 | MG189926 | LT960564 |
| CBS 222.49 | Euphorbia fulgens | Netherlands | LR583661 | LR583783 | LR583988 | LR583890 | |
| CBS 118931 | Solanum lycopersicum | UK | LR583662 | LR583784 | LR583989 | LR583891 | |
| CBS 143208 = NRRL 32755 = FRC S-0452 | Turtle head lesion | USA | DQ247073 | DQ094534 | DQ236576 | EU329613 | |
| CBS 143217 = NRRL 43811 | Human cornea | USA | EF453053 | EF453204 | EF453204 | EF470092 | |
| NRRL 46615 | Unknown | Unknown | GU250543 | GU250666 | GU250666 | GU250728 | |
| NRRL 46676 | Unknown | Unknown | GU250546 | GU250669 | GU250669 | GU250731 | |
| N. vasinfecta | CBS 237.55 = BBA 4647 = DSM 62822 = IMI 302624 = MUCL 9814 | Medicago sativa | South Africa | LR583663 | LR583785 | LR583990 | LR583892 |
| CBS 325.54IT = IMI 251386 = ATCC 162388 = IFO 7591 (ex-isotype of N. africana) | Soil | South Africa | LR583664 | KM231803 | KM231670 | KM232370 | |
| CBS 332.52 = IMI 302623 = LCP 58.1542 = LCP 812 | Soil on coffee plantation | Ivory Coast | LR583665 | LR583786 | LR583991 | LR583893 | |
| CBS 362.84 | Donkey dung | Venezuela | LR583666 | LR583787 | LR583992 | LR583894 | |
| CBS 398.67 | Soil in cotton plantation | Argentina | LR583667 | LR583788 | LR583993 | LR583895 | |
| CBS 405.82 | Cow dung | Venezuela | LR583668 | LR583789 | LR583994 | LR583896 | |
| CBS 406.82 | Donkey dung | Venezuela | LR583669 | LR583790 | LR583995 | LR583897 | |
| CBS 446.93T = IMI 316967 = NHL 2919 (ex-type of N. boninensis) | Soil | Japan | LR583670 | LR583791 | LR583996 | LR583898 | |
| CBS 533.65 = IMI 302625 | Unknown | India | LR583671 | LR583792 | LR583997 | LR583899 | |
| CBS 554.94 = ATCC 76350 = IMI 346678 = TRTC 51334 = UAMH 6870 | Soil | Australia | LR583672 | LR583793 | LR583998 | LR583900 | |
| CBS 562.70T = ATCC 32363 = IMI 251387 (ex-type of N. ornamentata) | Arachis hypogaea nut | Guinea-Bissau | DQ247606 | AF178413 | AF178382 | LR583901 | |
| CBS 602.67IT = ATCC 32362 = IMI 251388 = VKM F-1139 (ex-isotype of N. vasinfecta f. conidiifera) | Soil | Russia | LR583673 | LR583794 | LR583999 | LR583902 | |
| CBS 709.96 = FMR 5546 | Soil | India | LR583674 | LR583795 | LR584000 | LR583903 | |
| CBS 882.85 | Soil | Turkey | LR583675 | LR583796 | LR584001 | LR583904 | |
| CBS 101957 | Human blood, sputum and wound | Germany | LR583676 | LR583797 | LR584002 | LR583905 | |
| CBS 130182 = NRRL 43467 = CDC 2006743430 | Human eye | USA | EF452940 | EF453092 | EF453092 | EF469979 | |
| NRRL 22166 = ATCC 62199 | Heterodera glycines | USA | AF178350 | DQ094319 | DQ236361 | EU329497 | |
| NRRL 34174 = UTHSC 03-1457 | Human | USA | * | * | * | EU329636 | |
| Neocosmospora sp. | IBFS07 | Passiflora edulis f. flavicarpa | Brazil | JX524768 | FJ200220 | – | – |
| IBFS08 | Passiflora edulis f. flavicarpa | Brazil | JX524769 | FJ200221 | – | – | |
| IBFS09 | Passiflora edulis f. flavicarpa | Brazil | JX524770 | FJ200222 | – | – | |
| IBFS11 | Passiflora edulis f. flavicarpa | Brazil | JX524771 | FJ200223 | – | – | |
| IBFS12 | Passiflora edulis f. flavicarpa | Brazil | JX524772 | FJ200224 | – | – | |
| IBFS18 | Passiflora edulis f. flavicarpa | Brazil | JX524773 | FJ200226 | – | – | |
| IBFSE | Passiflora edulis f. flavicarpa | Brazil | JX524774 | FJ200227 | – | – | |
| IBFSRJ | Passiflora edulis f. flavicarpa | Brazil | JX524775 | FJ200228 | – | – | |
| Neocosmospora sp. (AF-1) | NRRL 22231 | Beetle on Hevea brasiliensis | Malaysia | KC691542 | KC691570 | KC691570 | KC691631 |
| NRRL 46518 | Beetle on Hevea brasiliensis | Malaysia | KC691543 | KC691571 | KC691571 | KC691632 | |
| NRRL 46519 | Beetle on Hevea brasiliensis | Malaysia | KC691544 | KC691572 | KC691572 | KC691633 | |
| Neocosmospora sp. (AF-3) | NRRL 62629 | Euwallacea interjectus on Acer negundo | USA | KC691536 | KC691564 | KC691564 | KC691625 |
| Neocosmospora sp. (AF-6) | NRRL 62590 | Euwallacea fornicatus on Persea americana | USA | KC691546 | KC691574 | KC691574 | KC691635 |
| NRRL 62591 | Euwallacea fornicatus on Persea americana | USA | KC691545 | KC691573 | KC691573 | KC691634 | |
| Neocosmospora sp. (AF-7) | NRRL 62610 | Euwallacea sp. on Persea americana | Australia | KC691547 | KC691575 | KC691575 | KC691636 |
| NRRL 62611 | Euwallacea sp. on Persea americana | Australia | KC691548 | KC691576 | KC691576 | KC691637 | |
| Neocosmospora sp. (AF-8) | NRRL 62584 | Euwallacea fornicatus on Persea americana | USA | KC691554 | KC691582 | KC691582 | KC691643 |
| NRRL 62585 | Euwallacea fornicatus on Persea americana | USA | KC691549 | KC691577 | KC691577 | KC691638 | |
| Neocosmospora sp. (AF-9) | NRRL 22643 = ATCC 44215 | Xyleborus ferrugineus | Costa Rica | DQ247628 | KC691583 | KC691583 | KC691644 |
| NRRL 66088 | Beetle on Delonix regia | USA | KM406625 | KM406632 | KM406632 | KM406646 | |
| Neocosmospora sp. (AF-10) | NRRL 62941 = IMI 351954 | Unknown | Singapore | KM406626 | KM406633 | KM406633 | KM406647 |
| Neocosmospora sp. (AF-11) | NRRL 62944 | Euwallacea sp. on Camellia sinensis | Sri Lanka | KM406627 | KM406634 | KM406634 | KM406648 |
| Neocosmospora sp. (FSSC 12) | CBS 143203 = NRRL 32309 = UTHSC 00-1608 | Sea turtle | USA | DQ246937 | DQ094407 | DQ236449 | EU329571 |
| CBS 143206 = NRRL 32317 = UTHSC 99-1886 | Treefish | USA | DQ246945 | DQ094414 | DQ236456 | EU329575 | |
| CBS 143212 = NRRL 32821 = FRC S-1230 | Turtle egg | USA | DQ247128 | DQ094587 | DQ236629 | EU329625 | |
| CBS 143220 = NRRL 54720 = UTHSC 10-3125 | Lined sea horse aquarium water | USA | JQ743207 | JQ743209 | JQ743209 | JQ743211 | |
| CBS 143221 = NRRL 54968 | Bonnet head shark | USA | LT906671 | KC808234 | KC808234 | KC808332 | |
| CBS 143222 = NRRL 54970 = UTHSC 05-175 | Antler crab | USA | KC808195 | MG189938 | MG189919 | KC808334 | |
| CBS 143223 = NRRL 54971 = UTHSC 05-2774 | Reptile bronchus | USA | KC808196 | KC808237 | MG189920 | KC808335 | |
| CBS 143225 = NRRL 54974 = UTHSC 06-1538 | Honeycomb fish | USA | KC808198 | KC808239 | MG189921 | KC808337 | |
| CBS 143226 = NRRL 54979 = UTHSC 06-3660 | Kemps Ridley turtle | USA | KC808202 | KC808244 | MG189922 | KC808342 | |
| CBS 143227 = NRRL 54982 = UTHSC 07-1869 | Kemps Ridley turtle | USA | KC808205 | MG189939 | MG189923 | KC808345 | |
| CBS 143230 = NRRL 62549 = UTHSC 08-1422 | Horseshoe crab | USA | KC808220 | KC808264 | MG189924 | KC808352 | |
| Neocosmospora sp. (FSSC 12) | NRRL 22642 = ATCC 38341 | Penaceous japonicus gill | Japan | DQ246844 | DQ094329 | DQ236371 | EU329522 |
| NRRL 22834 | Lobster | Australia | DQ247663 | * | * | FJ240382 | |
| NRRL 25392 = ATCC 32752 | American lobster | USA | DQ246861 | EU329672 | EU329672 | EU329537 | |
| NRRL 46704 = FMR 7140 | Aquarium sand | Spain | * | EU329713 | EU329713 | EU329662 | |
| NRRL 46705 = FMR 7141 | Aquarium sand | Spain | * | EU329714 | EU329714 | EU329663 | |
| Neocosmospora sp. (FSSC 40) | F1285 | Unknown | Unknown | * | * | * | * |
| Neocosmospora sp. (FSSC 41) | F57 | Unknown | Unknown | * | * | * | * |
| F59 | Unknown | Unknown | * | * | * | * | |
1† Excluded species. Codes between parentheses indicate phylogenetic species according to the nomenclature proposed by O’Donnell et al. (2008, 2016).
2ARSEF: Collection of entomopathogenic fungal cultures, US Department of Agriculture (USDA), Agricultural Research Service (ARS), Ithaca, NY, USA; ATCC: American Type Culture Collection, Manassas, VA, USA; BBA: Biologische Bundesanstalt für Land- und Forstwirtschaft, Institut für Mikrobiologie, Berlin, Germany; BPI: U.S. National Fungus Collections, USDA-ARS, Beltsville, MD, USA; CBS: Westerdijk Fungal Biodiverity Institute (WI), Utrecht, The Netherlands; CDC: Centers for Disease Control and Prevention, Atlanta, GA, USA; CECT: Spanish Type Culture Collection, Universidad de Valencia, Burjassot, Spain; CML: Coleção Micológica de Lavras, Universidade Federal de Lavras, Minas Gerais, Brazil; CPC: Collection of P.W. Crous, held at WI; DSM: DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany; F: As in O’Donnell et al. (2008) DNA sequence dataset; FMR: Facultat de Medicina i Ciències de la Salut, Reus, Spain; FRC: Fusarium Research Center, Pennsylvannia State University, PA, USA; Fs: As in Romberg & Davis (2007); GJS: Collection of G.J. Samuels, USDA-ARS, USA; HFEW: As in Ibarra-Laclette et al. (2017); HMAS: Herbarium Mycologicum Academiae Sinicae, Chinese Academy of Sciences, Beijing, China; IBFS: As in Bueno et al (2014); IFM: Medical Mycology Research Center, Chiba University, Chiba, Japan; IFO: Institute for Fermentation, Osaka, Yodogawa-ku, Osaka, Japan; IMI: CABI Bioscience, Eggham, UK; IMUR: Institute of Mycology, University of Recife, Recife, Brazil; JS: As in Kim et al. (2017); KOD: Collection of K. O’Donnell, USDA-ARS, Peoria, IL, USA; LCP: Laboratory of Cryptogamy, National Museum of Natural History, Paris, France; MAFF: Ministry of Agriculture, Forestry and Fisheries, Tsukuba, Ibaraki, Japan; MRC: National Research Institute for Nutritional Diseases, Tygerberg, South Africa; MUCL: Mycothèque de l′Université Catholique de Louvain, Louvain-la-Neuve, Belgium; NHL: National Institute of Hygienic Sciences, Tokyo, Japan; NRRL: Agricultural Research Service Culture Collection, National Center for Agricultural Utilization Research, USDA, Peoria, IL, USA; RMF: Rocky Mountain Herbarium, Fungi, University of Wyoming, Laramie, WY, USA; STE-U: Stellenbosch University Botanical Garden, Stellenbosch, South Africa; TRTC: Royal Ontario Museum Fungarium, Ontario, Canada; UAMH: University of Alberta Microfungus Collection and Herbarium, Canada; UFMG:Coleção de Micro-organismos, DNA e Células da Universidade Federal de Minas Gerais, Belo Horizonte, Brazil; UTHSC: Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center, San Antonio, USA; Vanetten: Collection of H.D. VanEtten, Department of Plant Pathology, University of Arizona, Tucson, AZ, USA; VKM: All-Russian Collection of Microorganisms, Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Moscow Region, Russia. ET: Ex-epitype, IT: Ex-isotype, LT: Lectotype, NT: Ex-neotype, PT: Ex-paratype, T: Ex-type.
3ENA: European Nucleotide Archive; ITS: internal transcribed spacer region of the rDNA; LSU: large subunit of the rDNA; rpb2: RNA polymerase’s second largest subunit; tef1: translation elongation factor 1-alpha; Sequences generated in this study are shown in bold;
*Sequences not available at GenBank/ENA, obtained from K. O’Donnell’s alignment datasets.
Morphological observations
The species concepts in this work essentially follow those of Wollenweber (1931), Wollenweber & Reinking (1935a) and Gerlach & Nirenberg (1982), based on standard culture media and conditions as indicated in Leslie & Summerell (2006). Cultural growth and micromorphological observations were assessed as described previously (Aoki et al. 2003, 2005, 2013, Sandoval-Denis et al. 2018). Colony morphology, presence or absence of pigments and odours where documented on potato dextrose agar (PDA; Crous et al. 2019) and OA after incubation for 7 and 14 d at 24 °C in darkness, under continuous fluorescent light and using a 12/12 h cool fluorescent light/dark cycle. For growth rate determination, cultures were inoculated on PDA by depositing overgrown 5 × 5 mm agar blocks, obtained from 7-d-old cultures growing on synthetic nutrient poor agar (SNA; Nirenberg 1976). Plates were incubated in darkness at 25 °C and growth rates were recorded after 3 and 7 d of incubation by measuring radial colonial growth in at least four different directions. Unless otherwise noted, micromorphological observations and photographs were performed using water as mounting medium from fungal structures grown on carnation leaf agar (CLA; Fisher et al. 1982), incubated at room temperature (22–24 °C) under a 12/12 h near UV light/dark cycle (Fisher et al. 1982, Leslie & Summerell 2006). To study features of the sexual morph, crosses were carried out pairwise in all possible combinations using carrot agar (CA; Gams et al. 1999), but also on OA and SNA. Fungal materials from fungarium specimens were rehydrated in 3 % aqueous KOH for a few minutes, and then rinsed by replacing the KOH solution with distilled water (Samuels 1976). A Nikon Eclipse 80i microscope with Differential Interference Contrast (DIC) optics and a Nikon AZ100 dissecting microscope, both equipped with a Nikon DS-Ri2 high definition colour digital camera, and a Nikon SMZ1000 dissecting microscope equipped with a Nikon DS-Fi1 colour digital camera were employed. Digital imaging and measurements were done using the Nikon software NIS-elements D software v. 4.50. The dimensions of no less than 30 randomly selected elements were recorded for every fungal structure; and average, standard deviation and maximum–minimum values were determined for elements with five or more individual measurements.
PCR and sequencing
Total genomic DNA was extracted from isolates grown on malt extract agar (MEA; Crous et al. 2019), incubated at room temperature for 7–10 d. Mycelium was scraped from the colony surface and genomic DNA isolated using the Wizard® Genomic DNA purification Kit (Promega Corporation, Madison, WI, USA) following the manufacturer’s instructions. For fungarium specimens, genomic DNA was obtained from fragments of sporodochia and conidia using the EZNA Forensic DNA reagent set (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s standard protocol with an added initial homogenization step using a TissueLyser II (Qiagen, Germantown, MD, USA) apparatus.
Four gene fragments were amplified according to protocols described previously (Sandoval-Denis et al. 2018), using the following primer pairs: ITS4/ITS5 (White et al. 1990) for the internal transcribed spacer region of the rDNA (ITS), LR0R/LR5 (Vilgalys & Hester 1990, Vilgalys & Sun 1994), for a partial fragment of the 28S large subunit of the rDNA (LSU), 5f2/7cr and 7cf/11ar (Liu et al. 1999, Sung et al. 2007) for two, non-contiguous fragments of the RNA polymerase’s second largest subunit (rpb2); and EF1/EF2 (O’Donnell et al. 2008) for a portion of the translation elongation factor 1-alpha (tef1).
Sequencing was done in both directions using the same primer pairs used for amplification. For DNA derived from fungarium specimens, the additional sequencing primers LR3/LSU2Rd (Hopple & Vilgalys 1994, Crous et al. 2009) and ITS1Fd (Crous et al. 2009), were included to ensure high sequencing quality for LSU and ITS, respectively. Sequencing was carried out using an Applied Biosystems, Hitachi 3730xl DNA analyser (Applied Biosystems Inc., Foster City, California, USA). Consensus sequences were assembled using Seqman Pro v. 10.0.1 (DNASTAR, Madison, WI, USA). All newly generated sequences were lodged in GenBank and the European Nucleotide Archive (ENA; Table 1).
Phylogenetic analyses
Sequence alignments for each gene region were generated using MAFFT v. 7 (Katoh & Standley 2013) under the European Bioinformatics Institute (EMBL-EBI, https://www.ebi.ac.uk) platform (Li et al. 2015), and manually checked and corrected if needed using MEGA v. 7 (Kumar et al. 2016). Phylogenetic analyses based on the Maximum Likelihood (ML) and Bayesian inference (BI) algorithms were used for both the individual gene partitions as well as the combined four-gene dataset, all executed on the CIPRES Science Gateway portal (https://www.phylo.org; Miller et al. 2012). The best evolutionary model for each gene partition was calculated using MrModeltest v. 2.3 (Nylander 2004). For ML analyses, RAxML v. 8.2.10 (randomised accelerated (sic) maximum likelihood for high performance computing; Stamatakis 2014) was used, employing the default parameters. Bayesian analyses employed MrBayes v. 3.2.6 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003), and included four parallel runs of 90 M generations starting from a random tree topology, and a sampling frequency of every 1 000 generations. The 50 % majority rule consensus tree and posterior probability (PP) values were calculated after discarding the initial 25 % of saved trees as the ‘burn-in’ phase. Individual gene phylogenies were checked for conflicts between significantly supported clades (ML-BS ≥ 70 %, BI-PP ≥ 0.95), after which the four gene datasets were concatenated (Mason-Gamer & Kellogg 1996, Wiens 1998).
Genealogical concordance phylogenetic species recognition (GCPSR)
The pairwise homoplasy index (PHI; Bruen et al. 2006) was determined using SplitsTree v. 4.14.4 (Huson & Bryant 2006) using the four-gene alignment dataset as described by Quaedvlieg et al. (2014). A PHI value below 0.05 (Φw < 0.05) indicated the presence of significant recombination in the dataset. In addition, split graphs were constructed to ease visualisation of the relationship between closely related species.
RESULTS
Phylogeny
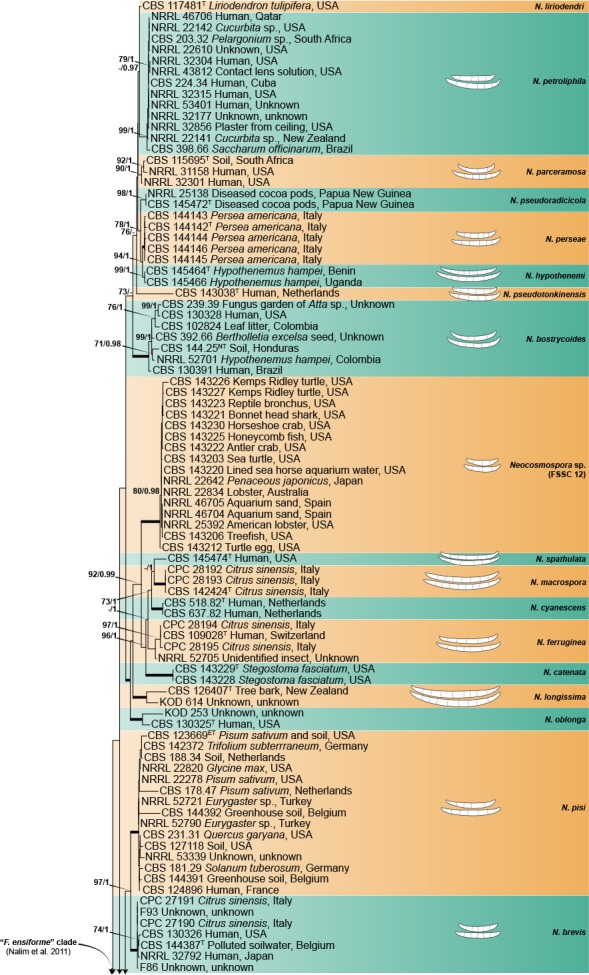
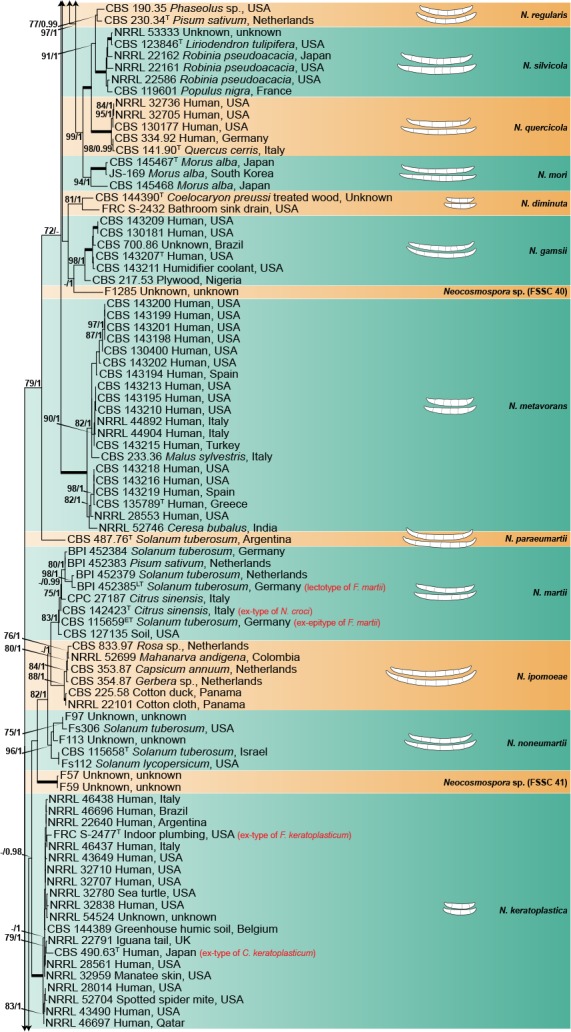
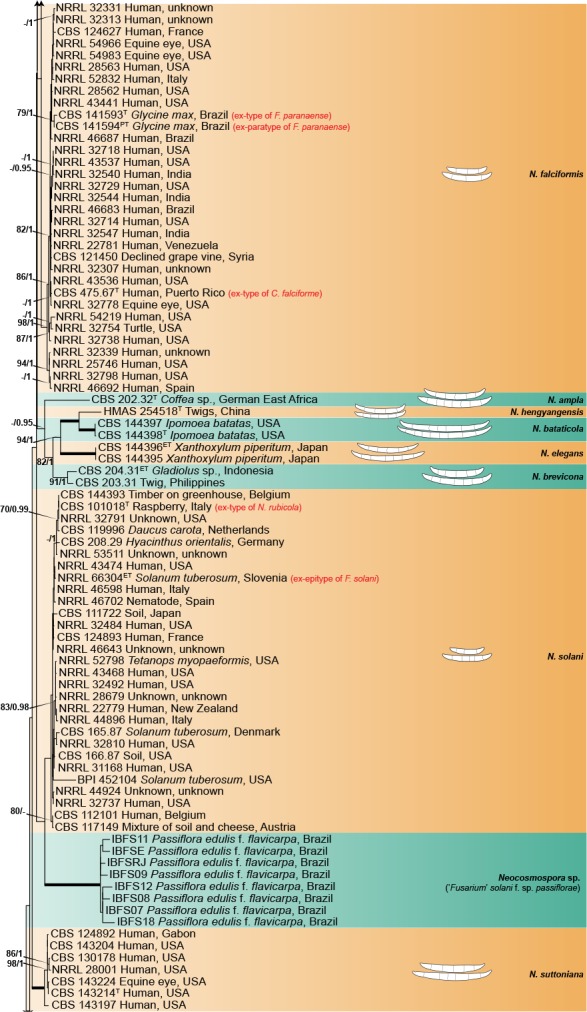
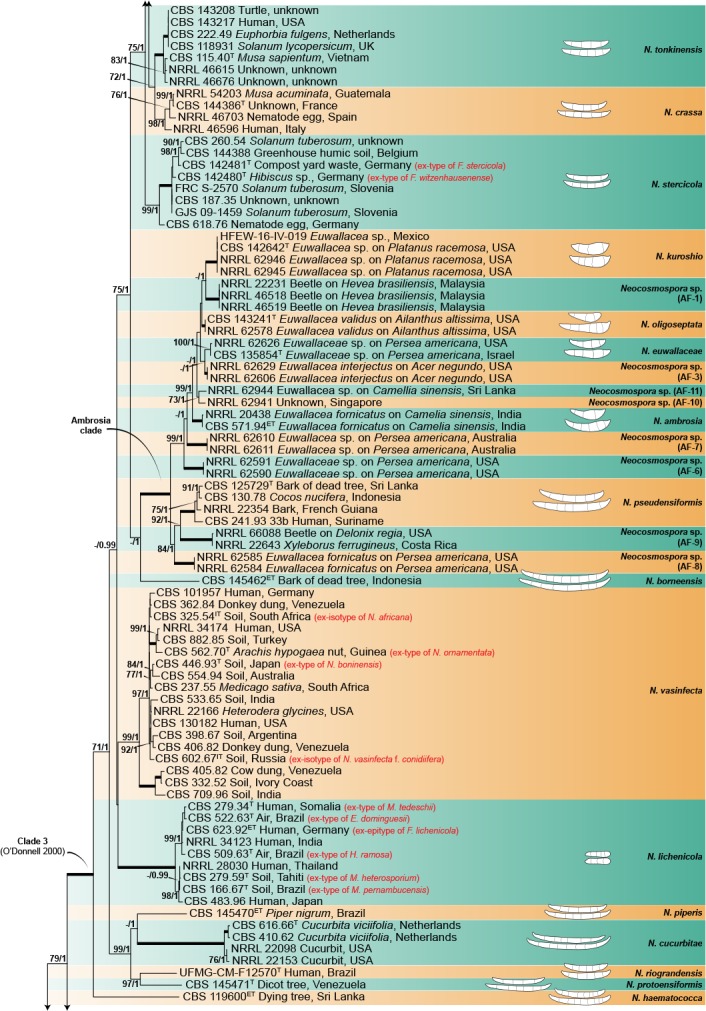
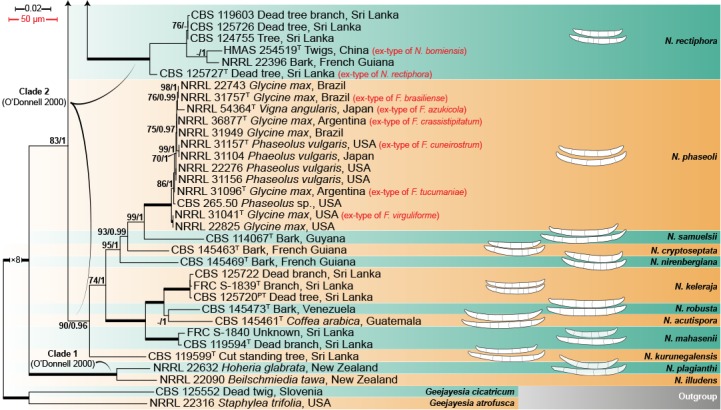
The combined four gene dataset included sequences from 378 isolates, including the two outgroup taxa, Geejayesia atrofusca (NRRL 22316) and G. cicatricum (CBS 125552). The multilocus dataset included 3 281 characters (tef1 692, ITS 489, LSU 485 & rpb2 1 615), including alignment gaps. Of these, 2 061 were conserved (tef1 341, ITS 269, LSU 414 & rpb2 1 037), 1 192 were variable (tef1 337, ITS 209, LSU 68 & rpb2 578), 938 were phylogenetically informative (tef1 284, ITS 148, LSU 37 & rpb2 469), and included 1 515 Bayesian unique site patterns (tef1 433, ITS 278, LSU 97 & rpb2 707). The best-fit models of evolution selected according to the Akaike criterion were GTR+I+G for the tef1, ITS and LSU and SYM+I+G for rpb2. For BI, the 50 % majority rule tree and posterior probabilities were calculated from a total of 135 002 trees, with 22 500 trees discarded as the ‘burn-in’ phase. The topologies observed for both the ML and BI analyses where congruent.
The multilocus analyses revealed a total of 77 species-level clades within the ingroup taxa, distributed among four main clades (Fig. 1), three of which largely corresponded to Clades 1–3 resolved by O’Donnell (2000). The most basal and fully-supported (ML-BS 100 %, BI-PP 1) Clade 1 includes N. illudens and N. plagianthi, both species known from New Zealand. Clade 2 is paraphyletic, and is divided into a fully-supported monotypic lineage, here assigned to N. rectiphora and a species-rich, well-supported (ML-BS 90 %, BI-PP 0.96) main clade including mostly tropical and neo-tropical species. The more speciose, fully-supported Clade 3 resolved into 65 species clades, not showing discernible biogeographic patterns. The fully-supported Ambrosia clade (Kasson et al. 2013) is contained within Clade 1, and includes 13 species, mostly obligated symbionts of the Euwallacea fornicatus species complex (Coleoptera, Xyleborini).
Fig. 1.
Maximum-Likelihood tree (ML) obtained from ITS, LSU, tef1 and rpb2 sequences of 376 strains from Neocosmospora species. Branch lengths are proportional to distance. Numbers on the nodes are ML bootstrap values (BS) ≥ 70 %, followed by Bayesian posterior probability values (BI-PP) ≥ 0.95. Full supported branches (ML-BS = 100 / BI-PP = 1) are indicated in bold. Line drawings representing the mean size (up) and maximum size (down) of macroconidia were plotted for each respective species clade with known morphology. Ex-type, ex-epitype, ex-isotype, ex-neotype and ex-paratype strains are indicated with T, ET, IT, NT and PT, respectively. The tree was rooted to Geejayesia atrofusca (NRRL 22316) and G. cicatricum (CBS 125552).
The PHI test was executed to predict genetic recombination between new species proposed here and phylogenetically related species, as well as among species with complex internal phylogenetic structures (Fig. 2). Statistically significant evidence for the existence of recombination events (Φw < 0.05) was found between the phylogenetic species FSSC 25 and FSSC 35, F. stercicola and F. witzenhousenense, as well as within the phylogenetic clades encompassing N. metavorans, N. phaseoli and N. vasinfecta. In contrast, no evidence of recombination was found between the closely related lineages corresponding to N. bataticola, N. brevicona and N. elegans. Additional data are given in the respective species notes below.
Fig. 2.
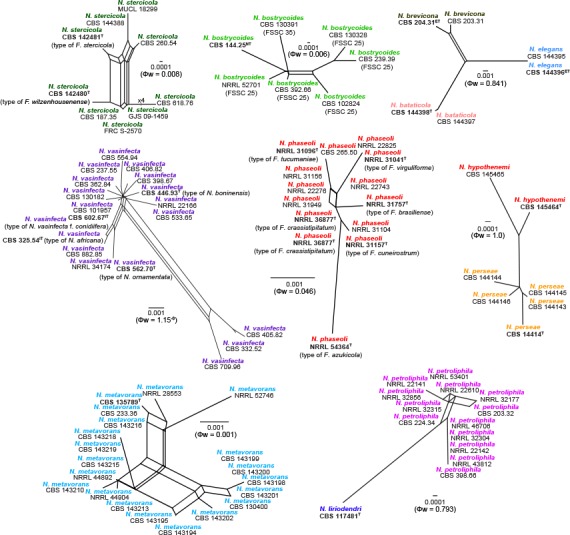
Split graphs showing the results of the pairwise homoplasy index (PHI) tests of closely related taxa using LogDet transformation and splits decomposition. PHI test results (Φw) ≤ 0.05 indicate significant recombination within the dataset. Ex-type, ex-epitype and ex-neotype strains are in bold and indicated with T, ET and NT, respectively.
TAXONOMY
Neocosmospora E.F. Sm., Bull. U.S.D.A. 17: 45. 1899
Synonyms. Euricoa Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 151. 1955.
Hyaloflorea Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 154. 1955.
Haematonectria Samuels & Nirenberg, Stud. Mycol. 42: 134. 1999.
Type species. Neocosmospora vasinfecta E.F. Sm.
Descriptions & Illustrations — Cannon & Hawksworth (1984), Samuels & Brayford (1994), Rossman et al. (1999), Samuels et al. (2006), Nalim et al. (2011), Guarro et al. (2012), Lombard et al. (2015).
Ascomata perithecial, orange-brown to bright red, darkening in 3 % KOH, globose to pyriform, papillate or with short ostiolar neck; superficial, solitary or gregarious, non-stromatic or with a reduced basal stroma, commonly warted, rarely smooth-walled; peridium of textura angularis; asci saccate, clavate to cylindrical, unitunicate, apex simple, rounded or flattened, 8-spored; ascospores uniseriate to irregularly biseriate, globose to ellipsoid, with or without slightly truncate ends, 0–1-septate, hyaline when young becoming yellow golden-brown at maturity, thick-walled, longitudinally striate, cerebriform or spinulose. Conidiophores simple, sparingly to highly branched, mononematous (aerial conidiophores) or grouped on sporodochia. Conidiogenous cells monophialidic; aerial phialides elongate subulate to subcylindrical; sporodochial phialides doliiform, short subcylindrical to subulate. Aerial conidia subglobose, ellipsoid to clavate, 0–2(–4)-septate or falcate and multiseptate, grouped on false heads at tips of phialides. Sporodochial conidia falcate and multiseptate, thick-walled, with well-developed foot-cells.
Notes — The generic name Lachnidium (Giard 1891), has been listed as a competing synonym (Summerbell & Schroers 2002, Lombard et al. 2015) of Neocosmospora. The taxonomic history of the generic type, L. acridiorum, based on Botrytis acridiorum, a locust ectoparasite isolated in Algeria (Trabut 1891), is complex and confusing (Madelin 1966, Kendrick 1974, Lombard et al. 2015). Following Brogniart’s transfer of this locust-associated fungus to Fusarium (as F. acridiorum, Brogniart 1891), Wollenweber & Reinking (1935a) synonymized this taxon under F. solani, a broadly accepted synonymy although in conflict with the basionym’s morphological features (Madelin 1966, Summerbell & Schroers 2002, Seifert et al. 2011). Madelin (1966) concluded that the circumscription of L. acridiorum was most likely based on more than one fungus, and after studying fresh isolations of the locust parasite, transferred B. acridiorum to Trichothecium, which is accepted here. The name Neocosmospora is therefore retained for this genus as stated in Nalim et al. (2011) and Lombard et al. (2015).
The type species of the asexual genera Euricoa, E. dominguesii (ex-type culture CBS 522.63) and Hyaloflorea, H. ramosa (ex-type culture CBS 509.63) belong to Neocosmospora (Summerbell & Schroers 2002, Gräfenhan et al. 2011, Seifert et al. 2011, Lombard et al. 2015); both taxa had been previously considered synonyms of Neonectria (= Cylindrocarpon, Carmichael et al. 1980, Kirk et al. 2008), but, current phylogenetic evidence placed them as synonyms of Neocosmospora lichenicola (Sandoval-Denis & Crous 2018).
The most important morphological features that differentiate Neocosmospora from its closest relatives in Fusarium are the orange-brown to bright red perithecia producing 0–1-septate, yellow-brown, ornamented ascospores. The asexual morphs of Neocosmospora can also easily be recognised by producing long and narrow, subcylindrical aerial monophialides (acremonium-like), and their large, multiseptate, thick-walled falcate conidia with well-developed foot cells; these morphological features have been largely recognised as unique for sect. Martiella (Wollenweber 1913, 1918).
Neocosmospora acutispora Sand.-Den. & Crous, sp. nov. — MycoBank MB831170; Fig. 3
Fig. 3.
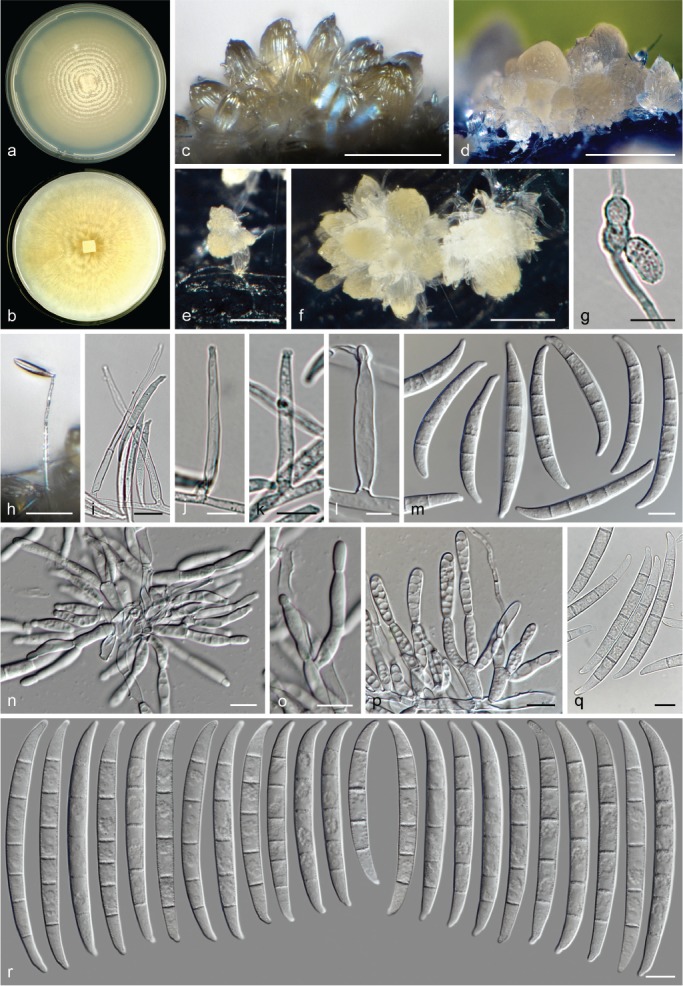
Neocosmospora acutispora (ex-type culture CBS 145461). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g. chlamydospores; h–l. aerial conidiophores and phialides; m. aerial conidia; n–p. sporodochial conidiophores and phialides; q–r. sporodochial conidia. — Scale bars: c–f = 100 μm; h = 50 μm; i = 20 μm; l = 5 μm; all others = 10 μm.
Etymology. From Latin acuō, meaning ‘sharpen, make sharp’; referring to the somewhat pointed apical cells of the sporodochial macroconidia.
Typus. Guatemala, from Coffea arabica, unknown date and collector (holotype CBS H-23969 designated here, culture ex-type CBS 145461 = NRRL 22574 = BBA 62213).
Conidiophores erect from substrate mycelium and produced laterally on aerial mycelium in abundance, straight, smooth- and thin-walled, simple, sometimes reduced to a single phialide borne laterally on aerial hyphae, more rarely sparingly branched, bearing terminal, single monophialides; phialides long-ampulliform, subulate to subcylindrical, (29.5–)32.5–43(–48.5) × (3–)3.5–4.5(–5) μm (av. 38 × 4 μm, n = 40), smooth- and thin-walled, periclinal thickening inconspicuous or absent, short-, non-flared collarette often present; aerial conidia falcate to wedge-shaped and multiseptate, markedly curved apically and tapering distinctly toward both ends, basal cell moderately to distinctly notched, (3–)4–5-septate, smooth- and thick-walled; 3-septate conidia: (46–)47–52(–53) × (4.5–)5–6.5 μm (av. 49.4 × 5.6 μm, n = 10); 4-septate conidia: (48.5–)49.5–55.5(–57) × 5–6.5 μm (av. 52.5 × 5.7 μm, n = 32); 5-septate conidia: (51.5–)56–66.5(–72) × (5–)5.5–6.5(–7) μm (av. 61.2 × 6 μm, n = 58); overall: (46–)49.5–63.5(–72) × (4.5–)5.5–6.5(–7) μm (av. 57.2 × 5.9 μm, n = 100). Sporodochia straw, pale luteous to pale sienna. Sporodochial conidiophores densely packed and sparingly or densely branched, verticillately, or sympodially; sporodochial phialides subcylindrical, subulate to somewhat lageniform, (11–)14–19.5(–23) × (3.5–)4–5(–5.5) μm (av. 16.8 × 4.6 μm, n = 46), smooth- and thin-walled, periclinal thickening and collarettes inconspicuous or absent. Sporodochial conidia, falcate to somewhat straight, narrowing gently toward both ends; apical cell often longer than adjacent cell, conical with a moderately curved, rounded to somewhat pointy and slightly elongated apex; basal cell barely to distinctly notched, (4–)5–6(–7)-septate, hyaline, smooth- and thick-walled; 4-septate conidia: 53.5–57 × 5.5–6.5 μm (n = 2); 5-septate conidia: (51–)67–80(–87.5) × (5.5–)6–7 μm (av. 73.5 × 6.4 μm, n = 102); 6-septate conidia: (67–)72.5–84.5(–87.5) × 6–7 μm (av. 78.4 × 6.5 μm, n = 40); 7-septate conidia 88.5 × 6.8 μm (n = 1); overall: (51–)67–82(–88.5) × (5.5–)6–7 μm (av. 74.5 × 6.4 μm, n = 145). Chlamydospores abundant, globose to subglobose, smooth- or rough- and thick-walled, (5.5–)6–6.5(–7) μm diam, terminal or intercalary, solitary, in chains or clusters. Colonies on PDA and OA growing in the dark with an average radial growth rate of 3–4 mm/d at 24 °C, reaching 45–50 mm diam in 7 d at 24 °C; surface sulphur yellow to pale luteous, flat, velvety to felty becoming densely granular at centre, with abundant, thin concentric rings of dense aerial mycelium, then quickly becoming pionnotal; margin entire. Reverse straw, pale luteous to saffron. On OA straw, sulphur yellow to amber, flat, membranous with irregular radial patches giving a feathery appearance, velvety to somewhat granulose at periphery, quickly becoming pionnotal; margin entire. Reverse straw to pale luteous.
Notes — Neocosmopora acutispora is one of the few species strictly producing macroconidia in sporodochial and aerial conidiophores, a feature common in Clade 2 of Neocosmospora, where it nests closely related to N. keleraja, N. mahasenii and N. robusta. These last three species also lack microconidia. Neocosmospora acutispora differs from N. robusta by its predominantly 5-septate sporodochial conidia with typical elongated and somewhat constricted apical and basal cells, contrasting with the mostly 6-septate sporodochial conidia with short and rounded apical cells of the latter species. Neocosmospora keleraja and N. mahasenii are known from Asia inhabiting living and dead tree bark, and rotting wood (Nalim et al. 2011). The ex-type of N. acutispora (CBS 145461) was isolated from Coffea arabica in Guatemala, while cultural and micromorphological characteristics, including colony and sporodochia colour and especially the size of the macroconidia and its apical cells, resemble those of N. cucurbitae and N. silvicola, two members of Clade 1 of Neocosmospora. However, macroconidia in N. acutispora are more robust and less septate than in N. cucurbitae, while the macroconidia in the latter species tend to be slightly more cylindrical, especially those produced in the aerial conidiophores, which also differ significantly in shape and size from their sporodochial counterparts. The macroconidia of N. silvicola are similar in overall size, shape and degree of septation. However, macroconidia of N. acutispora have distinctly longer, not commonly hooked apical cells and less developed basal cells. In addition, aerial microconidia are abundantly formed in both N. cucurbitae and N. silvicola. Neocosmospora acutispora also differs from closely related species by its rather short, somewhat ampulliform aerial phialides.
Neocosmospora ambrosia (Gadd & Loos) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015
Basionym. Monacrosporium ambrosium Gadd & Loos, Trans. Brit. Mycol. Soc. 31: 17. 1947.
Synonyms. Fusarium ambrosium (Gadd & Loos) Agnihothr. & Nirenberg, Stud. Mycol. 32: 98. 1990.
Dactylella ambrosia (Gadd & Loos) K.Q. Zhang, Xing Z. Liu & L. Cao, Mycosystema 7: 112. 1995.
Fusarium bugnicourtii Brayford, Trans. Brit. Mycol. Soc. 89: 350. 1987.
Typus. India, Upari Tea Institute, gallery of Euwallacea fornicatus infesting Camellia sinensis, 9 May 1990, V. Agnihothrudu (epitype of Monacrosporium ambrosium BPI 910524, designated by Aoki et al. (2018)). – Sri Lanka, an illustration of the fungus from a gallery of E. fornicatus on C. sinensis, C.H. Gadd & C.A. Loos (Trans. Brit. Mycol. Soc. 31: 16, f. 5 (1987) (lectotype of M. ambrosium designated by Aoki et al. (2018)).
Descriptions & Illustrations — Gadd & Loos (1947), Brayford (1987), Aoki et al. (2018).
Additional material examined. India, Upara Tea Institute, gallery of E. fornicatus in C. sinensis, 9 May 1990, V. Agnihothrudu, CBS 571.94 = BBA 65390 = MAFF 246287 = NRRL 22346 (culture ex-epitype of M. ambrosium).
Notes — Initially erroneously assigned to Monacrosporium given its oddly shaped conidia (Gadd & Loos 1947), N. ambrosia was recently described as the first representative of a single lineage, the Ambrosia clade, now belonging to Neocosmospora (O’Donnell et al. 2008). This clade includes species that share a symbiotic lifestyle and that have co-evolved with their respective hosts, members of the shot hole borer beetle genus Euwallacea (Ambrosia beetles: Coleoptera, Xyleborini; Mendel et al. 2012, Freeman et al. 2013, Kasson et al. 2013, Aoki et al. 2018). At least 12 species and one interspecific hybrid have been recognised in the Ambrosia clade of Neocosmospora. However, only four phylogenetic species have been formally described and assigned Latin binomials (Aoki et al. 2018).
The most significant morphological distinctive feature of N. ambrosia is the presence of irregularly clavate and swollen conidia, regarded as an evolutionary adaptation for its symbiotic lifestyle (Freeman et al. 2013, Kasson et al. 2013). This feature is shared, although not universally, among other members of the Ambrosia clade of Neocosmospora, particularly in N. euwallaceae and N. oligoseptata, its closest morphological allies. Neocosmospora ambrosia can be distinguished by its olive to olive-brown conidial pigmentation unlike the blue-green colouration in N. euwallaceae and always hyaline in N. oligoseptata (Freeman et al. 2013, Kasson et al. 2013, Aoki et al. 2018).
Neocosmospora ampla Sand.-Den. & Crous, sp. nov. — MycoBank MB831171; Fig. 4
Fig. 4.
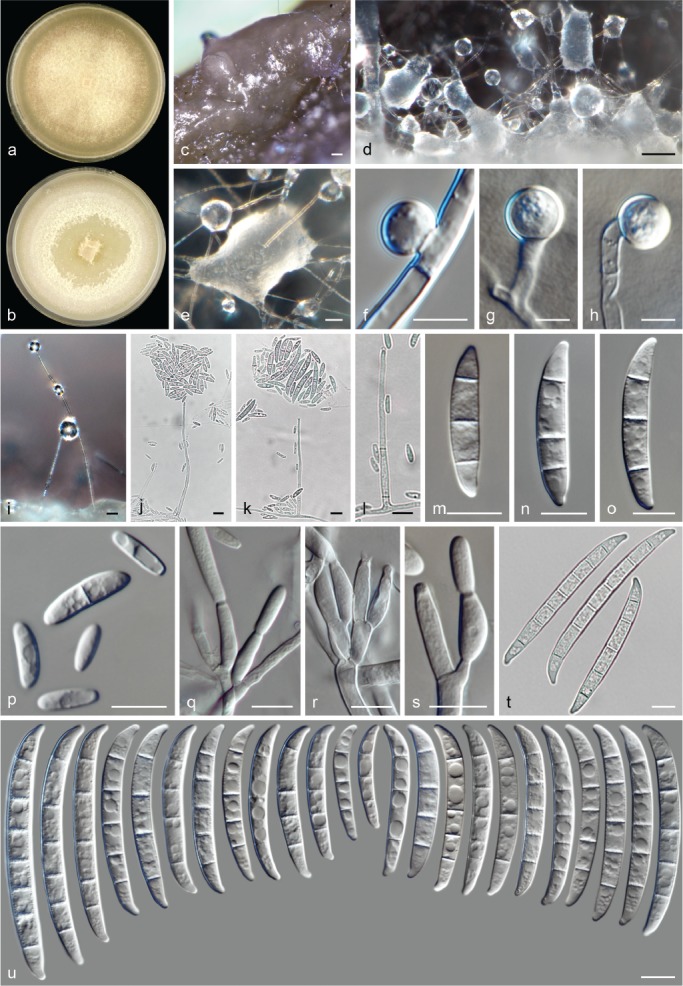
Neocosmospora ampla (ex-type culture CBS 202.32). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–h. chlamydospores; i–l. aerial conidiophores; m–o. aerial macroconidia; p. microconidia; q–s. sporodochial conidiophores and phialides; t–u. sporodochial conidia. — Scale bars: c = 200 μm; d = 100 μm; e, i = 50 μm; g–h = 5 μm; all others = 10 μm.
Etymology. From Latin amplus, meaning ‘large, wide, ample’, referring to its wide sporodochial conidia.
Typus. German East Africa, from Coffea sp., c. 1930, H.W. Wollenweber (holotype CBS H-23970 designated here, culture ex-type CBS 202.32 = BBA 4170).
Conidiophores erect from vegetative mycelium, highly abundant on aerial mycelium, straight, smooth- and thin-walled, often simple or sparingly irregularly or sympodially branched, bearing terminal and lateral monophialides; phialides subulate to subcylindrical, (24–)40.5–60(–75) × (1.5–)2–3.5(–4) μm (av. 50.4 × 2.8 μm, n = 107), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared, or less commonly flared collarette; aerial conidia of two types: microconidia oval, ellipsoidal, clavate to subcylindrical, straight or gently curved, 0–1(–2)-septate, hyaline, smooth- and thin-walled, (4–)7–14.5(–23.5) × (2–)2.5–4.5(–6) μm (av. 10.8 × 3.5 μm, n = 196), clustering in rather voluminous false heads on tips of monophialides; macroconidia fusiform to falcate, straight or gently dorsiventrally curved, with an indistinct papillate to notched basal cell, (1–)3(–5)-septate, smooth- and thick-walled; 1-septate conidia: (16.5–)17–30.5(–31) × 4–5.5 μm (av. 23.6 × 4.6 μm, n = 8); 3-septate conidia: (20.5–)25–37(–42.5) × (4–)4.5–6(–7.5) μm (av. 31 × 5.3 μm, n = 88); 4-septate conidia: 39 × 7 μm (n = 2); 5-septate conidia: 44.5 × 6 μm (n = 2); overall: (17–)24.5–37.5(–44.5) × (4–)4.5–6(–7.5) μm (av. 30.9 × 5.3 μm, n = 100). Sporodochia cream, light green to olivaceous, quickly developing into pionnotes. Sporodochial conidiophores densely verticillately or sympodially branched; sporodochial phialides subcylindrical, subulate to doliiform, (11.5–)13.5–22(–32.5) × (2.5–)3.5–5(–6.5) μm (av. 17.8 × 4.2 μm, n = 106), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, non-flared to rarely flared collarette. Sporodochial conidia almost straight to moderately dorsiventrally curved and wedge-shaped with nearly parallel lines, tapering toward base; apical cell mostly of equal length or shorter than adjacent cell, blunt with curved apex; basal cell barely notched, (1–)3–7-septate, hyaline, smooth- and thick-walled; 1-septate conidia: 26.5 × 4.5 μm (n = 2); 3-septate conidia: (29.5–)34–47(–51.5) × (4–)4.5–6(–6.5) μm (av. 40.5 × 5.2 μm, n = 42); 4-septate conidia: (38.5–)40.5–50(–55) × 5–6.5 μm (av. 45.1 × 5.8 μm, n = 44); 5-septate conidia: (42.5–)51.5–64.5(–71) × (5–)5.5–7(–7.5) μm (av. 58 × 6.4 μm, n = 100); 6-septate conidia: (59.5–)60.5–73(–85) × (6–)6.5–7.5 μm (av. 66.6 × 6.9 μm, n = 26); 7-septate conidia: (65–)66.5–75(–76.5) × 6.5–7.5 μm (av. 70.8 × 7 μm, n = 22); overall: (26.5–)43–67.5(–85) × (4–)5.5–7(–7.5) μm (av. 55.3 × 6.3 μm, n = 236). Chlamydospores abundant, globose to subglobose, smooth- and thick-walled, (3.5–)5.5–9(–10.5) μm diam, terminal or intercalary in hyphae and conidia, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 3.7–4.7 mm/d at 24 °C, reaching 52–66 mm diam in 7 d at 24 °C; white, straw to pale luteous, flat with slight central elevation, velvety, felty or floccose, white to straw aerial mycelium abundant; margin entire; reverse white to pale straw. On OA incubated in dark reaching 72–86 mm diam in 7 d at 24 °C; white, cream to buff, flat, velvety to felty, commonly wit concentric rings of white to straw aerial mycelium; margin entire. Reverse white, straw to luteous.
Notes — Neocosmospora ampla is introduced here for a monotypic lineage that includes a strain originally identified by H.W. Wollenweber as F. javanicum, and later assigned to F. eumartii. However, this strain did not satisfy the morphological characteristics of either of these taxa. Contrasting with the original concept of F. javanicum (Koorders 1907, Wollenweber 1931), the original description of F. eumartii (Carpenter 1915) or the long-standing modern concept of the latter species (Gerlach & Nirenberg 1982), sporodochial conidia of N. ampla are much wider, markedly tapered and more distinctly and regularly curved, especially on its dorsal side. In addition, N. ampla exhibits somewhat shorter and blunter apical cells while foot cells are barely notched and not as pronounced as described for F. eumartii.
Another distinguishing morphological feature of N. ampla is the additional formation of aerial macroconidia, clearly differentiated from the sporodochial conidia by being slightly wider toward the base (vs wedge-shaped in the sporodochial conidia). Other species showing similarly sized and septate sporodochial conidia are N. elegans and N. quercicola. Sporodochial conidia of N. elegans are similarly tapered at both ends, although narrower (up to 6.5 μm wide and average L/W ratio of 11.8 vs up to 7.5 μm and average L/W ratio of 8.7 in N. ampla). The sporodochial conidia of N. quercicola are typically almost straight, with well-developed, somewhat protuberant foot cells. Furthermore, N. elegans and N. quercicola lack aerial macroconidia.
Neocosmospora bataticola Sand.-Den. & Crous, sp. nov. — MycoBank MB831172; Fig. 5
Fig. 5.
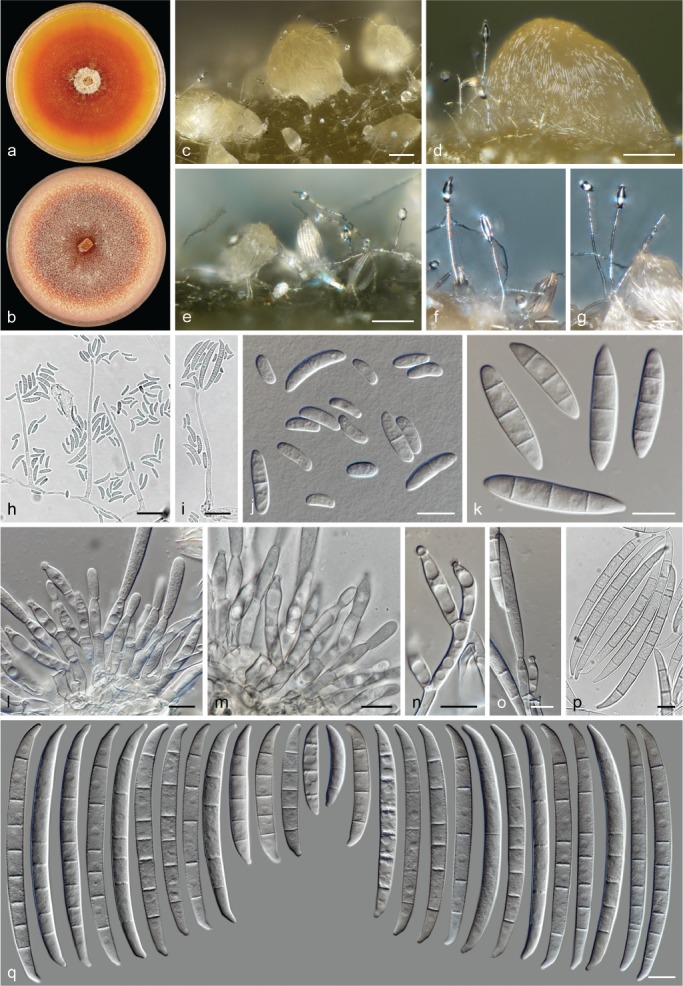
Neocosmospora bataticola (ex-type culture CBS 144398). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–i. aerial conidiophores; j. aerial microconidia; k. aerial macroconidia; l–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–e = 50 μm; f–i = 20 μm; all others = 10 μm.
Synonym. (Fusarium solani f. batatas T.T. McClure, Phytopathology 41: 75. 1951. Nom. inval., Art. 39.1).
Etymology. Name refers to the plant species Ipomoea batatas from which this fungus was isolated.
Typus. USA, North Carolina, from Ipomoea batatas, unknown date and collector (holotype CBS H-23971 designated here, culture ex-type CBS 144398 = NRRL 22402 = BBA 64954 = FRC S-0567).
Conidiophores abundant on substrate and aerial mycelium, straight, smooth- and thin-walled, simple or branched several times, mostly verticillately, more rarely irregularly or sympodially, bearing terminal and lateral, single monophialides; phialides subcylindrical, subulate to acicular, (27–)32.5–45.5(–57.5) × (1.5–)2–3(–3.5) μm (av. 39 × 2.5 μm, n = 68), smooth- and thin-walled, commonly proliferating, conidiogenous loci with rather inconspicuous periclinal thickening and collarettes; aerial conidia of two types: microconidia obovate, ellipsoidal to reniform, 0–1-septate, hyaline, smooth- and thin-walled, (6.5–)7–14(–19.5) × 2–4.5(–6) μm (av. 10.3 × 3.2 μm, n = 164), clustering in false heads at tip of monophialides; macroconidia fusiform to falcate, multiseptate, straight or dorsiventrally curved, base somewhat flattened to barely notched, 1–3(–5)-septate, smooth- and thick-walled; 1-septate conidia: (13.5–)15–20.5(–23) × (2.5–)3.5–6 μm (av. 17.8 × 4.5 μm, n = 32); 2-septate conidia: 24 × 5 μm (n = 2); 3-septate conidia: (21–)24–35(–41) × (3.5–)4–6.5(–7.5) μm (av. 29.4 × 5.3 μm, n = 78); 4-septate conidia: 40–43 × 5–5.5 μm (av. 41.6 × 5.1 μm, n = 16); 5-septate conidia: 43.5 × 5 μm (n = 4); overall: (13.5–)21–37(–43.5) × (2.5–)4–6.5(–7.5) μm (av. 29 × 5.2 μm, n = 130). Sporodochia cream, light green, olivaceous buff to olivaceous. Sporodochial conidiophores sparingly verticillately or laterally branched, bearing single lateral monophialides or terminating in whorls of 2–3 or single monophialides; sporodochial phialides in sporodochia subcylindrical, subulate, ampulliform or doliiform, (10–)14–19.5(–26.5) × (3–)4–5.5(–6) μm (av. 16.9 × 4.7 μm, n = 82), smooth- and thin-walled, conidiogenous loci with inconspicuous or absent periclinal thickening and a minute, non-flared collarette. Sporodochial conidia moderately dorsiventrally curved to nearly straight, tapering gently toward both ends; apical cell of equal length or slightly longer than adjacent cell, blunt to conical with an abruptly curved to somewhat hooked, rounded apex; basal cell notched and mostly slightly extended, (3–)5–7(–8)-septate, hyaline, smooth- and thick-walled; 3-septate conidia: 30.5–41.5 × 6–6.5 μm (n = 3); 4-septate conidia: 46–46.5 × 5.5–6.5 μm (n = 3); 5-septate conidia: (47–)59.5–85(–91) × 5.5–6.5(–7) μm (av. 72.4 × 6.2 μm, n = 72); 6-septate conidia: (62.5–)73–91(–96) × (5.5–)6–6.5(–7) μm (av. 82 × 6.2 μm, n = 90); 7-septate conidia: (72–)84–96(–101.5) × (5.5–)6–6.5 μm (av. 90.1 × 6.2 μm, n = 36); 8-septate conidia: (88.5–)90.5–96.5(–97) × (5.5–)6–6.5 μm (av. 93.6 × 6.2 μm, n = 9); overall: (30.5–)65–94(–101.5) × (5.5–)6–6.5(–7) μm (av. 79.5 × 6.2 μm, n = 213). Chlamydospores globose to subglobose, smooth-walled to finely granulate and thick-walled, 7–11.5 μm diam, terminal or intercalary, solitary, in pairs or short chains.
Colonies on PDA growing in dark with an average radial growth rate of 2.6–3 mm/d at 24 °C, reaching 35–42 mm diam in 7 d at 24 °C; straw, pale luteous, sienna, saffron, orange to red, flat, velvety, felty or lanose, commonly with short aerial mycelium arranged in concentric rings and a faint radiated pattern; margin entire. Reverse white to pale straw, an amber to pure yellow diffusible pigment can be produced. On OA incubated in dark covering an entire 9 mm Petri dish in 7 d at 24 °C; white, cream, scarlet to rust, flat, velvety, felty to granulose; margin entire. Reverse ochreous to red.
Additional material examined. USA, North Carolina, from Ipomoea batatas, unknown date and collector, CBS 144397 = NRRL 22400 = BBA 64683.
Notes — Isolates treated here are representatives of the genetically and biologically well-characterised F. solani f. batatas (McClure 1951), F. solani f. sp. batatas (Matuo & Snyder 1973, O’Donnell 2000, O’Donnell et al. 2008), also known as Nec. haematococca MPII or phylogenetic species FSSC 23. Nevertheless, F. solani f. batatas was not validly published since a Latin description or diagnosis was not included (Art. 39.1). In its formae speciales nomenclature, and largely relying on its host association with Ipomoea batatas, this group was considered a synonym of Hyp. ipomoeae (now Neocosmospora ipomoeae; Matuo & Snyder 1973). This synonymy is not supported here, given their clearly distinct evolutionary origins. Moreover, other taxa have also been recovered from the same host, e.g., F. javanicum, N. brevicona (as F. solani var. minus) and N. solani (Wollenweber 1931).
The clade representing N. bataticola in this study clusters within a marginally supported clade containing N. brevicona and N. hengyangensis, both species with known sexual-morphs (Wollenweber 1930, Zeng & Zhuang 2017). However, their distinctive morphology and lack of evidence of sexual recombination (Φw > 0.6, Fig. 2) between the different clades support their recognition as distinct species.
The most noticeable macroscopic characteristic of N. bataticola is the production of intense orange to red coloured diffusible pigments on PDA and OA. This feature is particularly evident for the ex-type culture of this species. Additionally, N. bataticola also produces distinct large multiseptate, straight to moderately curved sporodochial conidia with well-developed foot cells. The sporodochial conidia are reminiscent of N. ipomoeae, as the latter species also produces similar aerial micro- and macroconidia, and both species are hardly differentiable by their asexual morphs. The aerial macroconidia of N. bataticola are typically fusiform and somewhat wider at the base in comparison to the fusiform to almost cylindrical and commonly flattened at the base, aerial macroconidia of N. ipomoeae.
Other comparable species with similar sized sporodochial conidia are N. borneensis and N. longissima, also showing similar conidial septation. However, conidia in N. bataticola are less curved and less robust, while aerial macroconidia are much smaller than those in N. borneensis or N. longissima. In contrast, microconidia are never produced in N. longissima.
Neocosmospora borneensis (Petr.) Sand.-Den. & Crous, comb. nov. — MycoBank MB831173; Fig. 6
Fig. 6.
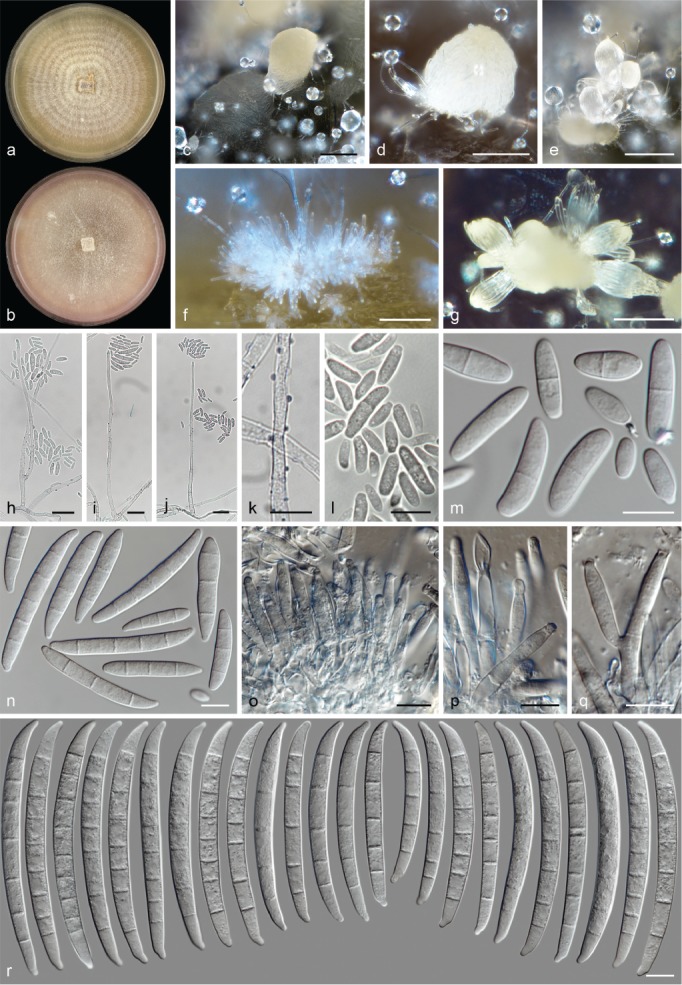
Neocosmospora borneensis (ex-epitype culture CBS 145462). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–g. sporodochia formed on the surface of carnation leaves; h–k. aerial conidiophores; l–m. aerial microconidia; n. aerial macroconidia; o–q. sporodochial conidiophores and phialides; r. sporodochial conidia. — Scale bars: c–g = 100 μm; h–k = 20 μm; all others = 10 μm.
Basionym. Nectria borneensis Petr., Sydowia 8: 20. 1954.
Typus. British North Borneo (Malaysia), Sabah, Tenompok, Mount Kinabalu, Jungle lodge, 25 Apr. 1933, Clemens (holotype K(M) 252860). – Indonesia, North Sulawesi, Bogani Nani Wartabone National Park (formerly the Dumoga Bone National Park), from bark of a recently dead unidentified tree, 26 Oct. 1985, G.J. Samuels (epitype of Nec. borneensis CBS H-23972 designated here, MBT387201, culture ex-epitype CBS 145462 = NRRL 22579 = BBA 65095 = G.J.S. 85-197).
Descriptions & Illustrations of the sexual morph — Samuels et al. (1990), Samuels & Brayford (1994).
Conidiophores abundant, erect or prostrate on substrate mycelium and laterally on the aerial mycelium, straight or flexuous, smooth- and thin-walled, often sparingly verruculose at basal and middle portions, simple or sparingly dichotomously branched, bearing terminal, single monophialides; phialides subulate to subcylindrical, (57–)60.5–71.5(–78.5) × (2.5–)3–4 μm (av. 66.1 × 3.3 μm, n = 52), smooth- and thin-walled, conidiogenous loci with visible periclinal thickening and a short, not-flared collarette; aerial conidia of two types: microconidia ellipsoidal to subcylindrical, straight or with a slight dorsal curvature, 0–1(–2)-septate, hyaline, smooth- and thin-walled, (6.5–)8.5–20(–29.5) × (2.5–)3.5–6(–7.5) μm (av. 14.3 × 4.6 μm, n = 95), clustering in false heads on tip of monophialides; macroconidia navicular to falcate and multiseptate, straight to gently dorsiventrally curved, base flattened, barely to moderately notched, (2–)3–5-septate, smooth- and thick-walled; 2-septate conidia: (22–)23–27(–28) × (5.5–)6–7.5 μm (av. 24.8 × 6.5 μm, n = 12); 3-septate conidia: (25.5–)30.5–44.5(–48) × (4.5–)5.5–7(–7.5) μm (av. 37.6 × 6.2 μm, n = 80); 4-septate conidia: (41–)43–50.5(–53) × (5.5–)6–7.5 μm (av. 46.8 × 6.5 μm, n = 24); 5-septate conidia: (47.5–)49.5–56.5(–59) × (5.5–)6–7 μm (av. 52.9 × 6.4 μm, n = 22); overall: (22–)31–50(–59) × (4.5–)5.5–7(–7.5) μm (av. 40.5 × 6.3 μm, n = 138). Sporodochia pale luteous, primrose to dull-green. Sporodochial conidiophores sparingly dichotomously branched or simple, densely packed; sporodochial phialides subcylindrical to cylindrical, (13.5–)18–25(–32) × (2.5–)3–4.5(–5) μm (av. 21.3 × 3.9 μm, n = 101), smooth- and thin-walled, conidiogenous loci with visible periclinal thickening and a minute, not-flared collarette. Sporodochial conidia falcate with nearly parallel lines, sometimes almost straight, narrowing gently toward base; apical of equal length to adjacent cell, conical with rounded, somewhat papillate apex; basal cell distinctly notched, 5–8(–9)-septate, hyaline, smooth- and thick-walled; 5-septate conidia: (59–)65.5–78(–79.5) × (5.5–)6–7 μm (av. 71.8 × 6.3 μm, n = 36); 6-septate conidia: (71–)73–80.5(–81.5) × 6–7 μm (av. 76.7 × 6.5 μm, n = 16); 7-septate conidia: (75–)80.5–90(–93) × 6.5–7.5(–8) μm (av. 85.2 × 7.1 μm, n = 84); 8-septate conidia: (82.5–)83–87.5(–88.5) × (6.5–)7–7.5 μm (av. 85 × 7.1 μm, n = 24); 9-septate conidia: (86.5–)88–99(–104) × 6.5–7.5 μm (av. 92.7 × 7.2 μm, n = 20); overall: (59–)74.5–91(–104) × (5.5–)6–7.5(–8) μm (av. 82.5 × 6.9 μm, n = 180). Chlamydospores not observed.
Colonies on PDA growing in dark with an average radial growth rate of 4.6–5 mm/d at 24 °C, reaching 64–70 mm diam in 7 d at 24 °C; pale luteous to ochreous, flat, felty to cottony, with conspicuous concentric rings of white aerial mycelium, becoming somewhat granulose with production of abundant pale luteous sporodochia; margin entire. Reverse pale luteous to ochreous, with pale umber patches. On OA incubated in dark reaching 70–74 mm diam in 7 d at 24 °C; flesh to pale salmon, flat, velvety; margin entire. Reverse pale luteous with peach to pale rose periphery.
Notes — The description of the asexual morph, the most common morphology observed in culture, is included to supplement the observations originally made by Samuels et al. (1990) and Samuels & Brayford (1994) of the same isolate studied here. Additional information was also partially taken from unpublished morphological notes and illustrations obtained from G.J. Samuels. The former studies also described and illustrated the sexual morph, which could not be induced on artificial media here.
Although originally described from a fungus collected on the bark of rotting branches of an unidentified plant in the Malaysian state of Sabah, the culture studied here has slightly smaller ascospores than the type collection (G.J. Samuels, pers. comm.). This culture also commonly exhibited a sparingly verruculose surface on its conidiophores, a feature not reported previously for this species but regularly observed on all the culture media studied here. Nevertheless, the studied strain was collected in North Sulawesi, Indonesia, a zone with similar environmental conditions as the holotype collection site (Sabah), while it has been regarded as a representative isolate of Nec. borneensis (Samuels et al. 1990, Samuels & Brayford 1994, O’Donnell 2000). Therefore, a specimen derived from CBS 145462 is here designated as epitype to fix the use of the species name.
According to Samuels & Brayford (1994), the sexual morph of N. borneensis cannot be differentiated morphologically from those of N. illudens or Nec. subsequens. The morphology of the asexual morph is quite unique for N. borneensis, producing much longer and more robust, up to 9-septate sporodochial conidia. Other species with similar sexual morphologies are Nec. balansae and Nec. pulcherrima (Samuels & Brayford 1994). Nectria balansae has a pycnidial asexual morph (Samuels & Brayford 1994), and it is phylogenetically related to Nectria s.str. (Chaverri et al. 2011). In contrast, Nec. pulcherrima has been morphologically connected to the genus Rugonectria (Chaverri et al. 2011).
Other species producing conidia of similar size and shape and known sexual morphs include N. ipomoeae and N. bataticola. Neocosmospora borneensis and N. ipomoeae both produce mostly 7–8-septate sporodochial conidia. Those of N. borneensis are slightly shorter and wider, often with a less pronounced curvature. Neocosmospora bataticola produces mostly 5–6-septate sporodochial conidia, which are longer, slender, less curved and more robust than those of N. borneensis. In addition, verruculose aerial conidiophores have not been observed for either N. bataticola or N. ipomoeae.
Neocosmospora bostrycoides (Wollenw. & Reinking) Sand.-Den., L. Lombard & Crous, comb. nov. — MycoBank MB831174; Fig. 7
Fig. 7.
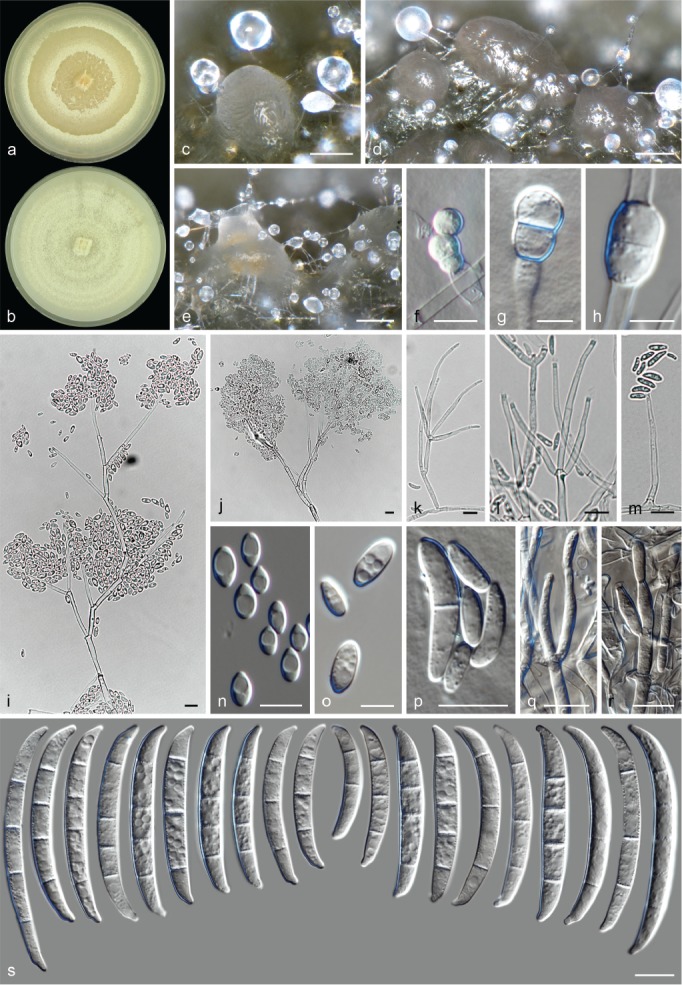
Neocosmospora bostrycoides (ex-neotype culture CBS 144.25). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–h. chlamydospores; i–m. aerial conidiophores and phialides; n–p aerial conidia; q–r. sporodochial conidiophores; s. sporodochial conidia. — Scale bars: c–e = 100 μm; g–h, o = 5 μm; all others = 10 μm.
Basionym. Fusarium bostrycoides Wollenw. & Reinking, Phytopathology 15: 166. 1925.
Typus. Honduras, Tela, from soil, unknown date and collector (neotype of F. bostrycoides CBS H-23973 designated here, MBT387203, culture ex-neotype CBS 144.25).
Conidiophores abundant on aerial and substrate mycelium, erect and simple or reduced to conidiogenous cells, mostly densely branched laterally and verticillately, straight or flexuous, smooth- and thin-walled, bearing terminal or lateral, single monophialides; phialides subcylindrical to subulate, (27.5–)40–57(–66) × (2–)2.5–3.5(–4) μm (av. 48.5 × 2.8 μm, n = 106), smooth- and thin-walled, often proliferating, conidiogenous loci with periclinal thickening and a short-, non-flared collarette; aerial conidia obovoidal, broadly ellipsoidal to ellipsoidal, straight or rarely curved, base narrow and flattened, slightly protruding, 0(–1)-septate, hyaline, smooth- and thin-walled, 5–11.5(–21) × (2–)2.5–4.5(–6) μm (av. 8.4 × 3.4 μm, n = 91), clustering abundantly in false heads at tip of monophialides. Sporodochia cream, pale green, hazel to pale greyish sepia. Sporodochial conidiophores sparingly verticillately branched, bearing terminal whorls of 2–3-monophialides; sporodochial phialides flask-shaped, subcylindrical to short subulate, (13–)15–23.5(–29) × (2–)2.5–4(–4.5) μm (av. 19.3 × 3.4 μm, n = 48), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and collarette. Sporodochial conidia moderately to distinctly dorsiventrally curved narrowing gently toward base, dorsal line usually almost straight; apical cell often equal in length to adjacent cell, blunt and rounded with curved apex; basal cell often distinctly notched, 3–5-septate, hyaline, smooth- and thick-walled; 3-septate conidia: (29–)39.5–49.5(–54) × (4–)4.5–5.5(–6) μm (av. 44.4 × 4.9 μm, n = 124); 4-septate conidia: (41.5–)43–53(–63) × 5–6.5(–7) μm (av. 48 × 5.7 μm, n = 48); 5-septate conidia: (45–)45.5–56.5(–62) × (4.5–)5–6.5(–7) μm (av. 51 × 5.6 μm, n = 24); overall: (29–)40.5–51.5(–63) × (4–)4.5–6(–7) μm (av. 46.1 × 5.2 μm, n = 196). Chlamydospores abundantly formed, globose to subglobose, smooth- to verruculose and thick-walled, (5–)5.5–7.5(–8) μm diam, terminal or intercalary in hyphae or conidia, solitary, in chains or in clusters.
Colonies on PDA growing in dark with an average radial growth rate of 2–2.8 mm/d at 24 °C, reaching 36–40 mm diam in 7 d at 24 °C; straw, buff to pale luteous, flat, membranous, velvety to granulose, floccose at centre, with or without concentric rings of aerial mycelium; margin entire. Reverse straw to pale luteous. On OA incubated in dark covering a 9 mm diam Petri Dish in 7 d at 24 °C; white to pale straw, flat, velvety, cottony to granular with entire margin. Reverse pale straw to buff.
Additional materials examined. Brazil, Sao Paulo, human eye lesion, 22 Feb. 2000, P. Godoy, CBS 130391 = NRRL 46707 = FMR 8030. – Colombia, Araracuaro, leaf litter, Feb. 2000, C. López-Quintero, CBS 102824. – USA, from human oral wound of leukaemia patient, unknown date and collector, CBS 130328 = NRRL 31169. – Unknown, from fungus garden of Atta sp., unknown date and collector, CBS 239.39 = NRRL 22656; from seed of Bertholletia excelsa, Nov. 1965, W. Gams, CBS 392.66 = BBA 69595 = NRRL 25325.
Notes — Significant evidence links one of the strains examined here (CBS 144.25) to authentic material of F. bostrycoides, also matching with the collection data and morphological features depicted in Wollenweber & Reinking (1925), Reinking & Wollenweber (1927) and ‘Fusaria Autographice Delineata No. 978’ (Wollenweber 1930). This particular culture was received at the WI in 1925, sent by the head pathologist of the Bureau of Plant Industry, US Department of Agriculture, where the original cultures of all the new species and varieties described in Wollenweber & Reinking (1925) were kept. However, since the type status of CBS 144.25 could not be unambiguously established and no type was selected nor illustrations were made in the protologue of F. bostrycoides, a specimen derived from CBS 144.25 is here selected as neotype, in order to fix the use of the name. The morphological characteristics of this clade are strikingly similar to those described for F. bostrycoides, easily recognisable by its relatively short, mostly 3-septate macroconidia, which resemble those of F. oxysporum. This feature partially explains its former allocation in the morphological sect. Elegans (subsection Orthoceras), resulting in its posterior synonymy with F. oxysporum s.lat. (Wollenweber & Reinking 1925, Reinking & Wollenweber 1927, Domsch et al. 2007). One of the most important characteristics of the species, its highly branched conidiophores bearing terminal, long and slender monophialides, allocate this species in sect. Martiella. This is further confirmed here by DNA sequence data, demonstrating that the former circumscription of this taxon was incorrect. Furthermore, the morphology of additional strains examined here demonstrated that N. bostrycoides can also produce typical 3–5-septate, robust sporodochial conidia, more characteristic of sect. Martiella, hence the name is resurrected and recombined as N. bostrycoides.
Neocosmospora bostrycoides (as the phylogenetic species FSSC 25 and 35) has also been isolated from clinical samples (human and animal; O’Donnell et al. 2008, 2012, Herkert et al. 2019) and is, therefore, considered a putative agent of opportunistic infections. The current circumscription also includes strains obtained from human clinical specimens. However, the pathogenicity of this species is doubtful.
The clade representing N. bostrycoides includes two previously determined phylogenetic species FSSC 25 and FSSC 35 (O’Donnell et al. 2008). Based on morphological and phylogenetic data, and as significant evidence of sexual recombination was found between the two subclades (Φw = 0.006, Fig. 2), these are here reduced to a single species.
Neocosmospora brevicona (Wollenw.) Sand.-Den. & Crous, comb. & stat. nov. — MycoBank MB831175; Fig. 8
Fig. 8.
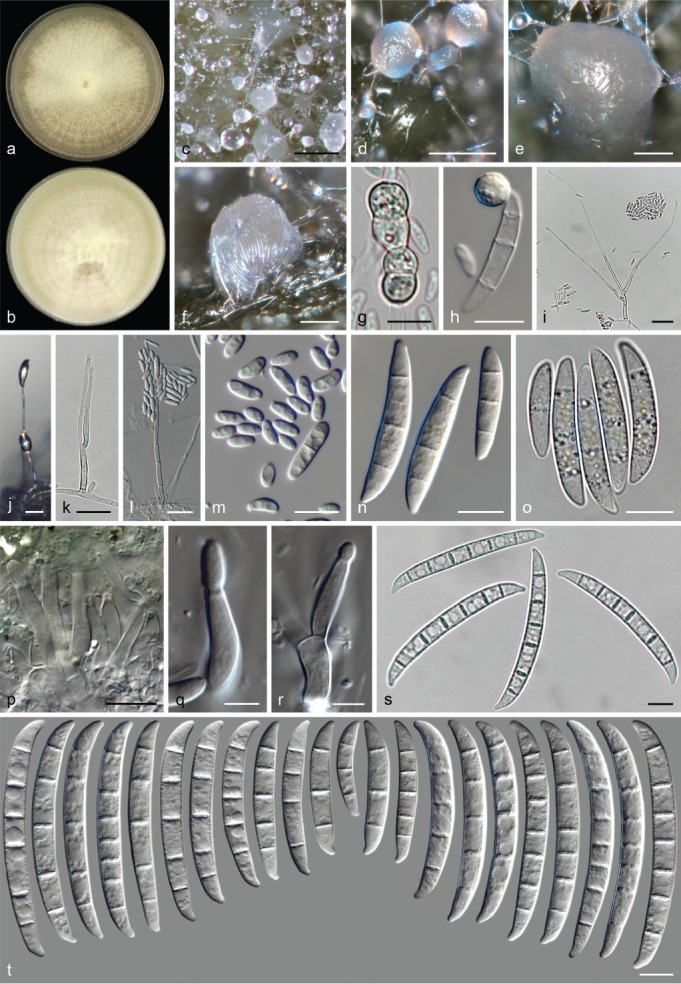
Neocosmospora brevicona (ex-epitype culture CBS 204.31). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g–h. chlamydospores; i–l. aerial conidiophores; m. aerial microconidia; n–o. aerial macroconidia; p–r. sporodochial conidiophores and phialides; s–t. sporodochial conidia. — Scale bars: c–e = 100 μm; f = 50 μm; i, k–l = 20 μm; q–r = 5 μm, all others = 10 μm.
Basionym. Hypomyces haematococcus var. breviconus Wollenw., Fusaria Autographice Delineata 3: 828. 1930.
Synonyms. Nectria haematococca var. brevicona (Wollenw.) Gerlach, Fusarium: Diseases, Biology, and Taxonomy (Philadelphia): 422. 1981.
Fusarium solani var. minus Wollenw., Die Fusarien: 134. 1935.
Typus. Indonesia, Java, Buitenzorg, from rotten bulb of Gladiolus sp., Jan. 1926, unknown collector (lectotype of Hyp. haematococcus var. breviconus designated here: illustration f. 828 in Wollenweber HW (1930). Fusaria Autographice Delineata 3: no. 828, MBT387205); from Gladiolus sp., 1926, unknown collector (epitype of Hyp. haematococcus var. breviconus CBS H-23974 designated here, MBT387206, culture ex-epitype CBS 204.31 = BBA 2123 = NRRL 22659).
Conidiophores erect and prostrate on substrate mycelium, or most commonly borne laterally on aerial mycelium, straight, smooth- and thin-walled, simple, sparingly or branched multiple times verticillately or sympodially, bearing terminal and lateral, single monophialides; phialides subulate to subcylindrical, (26.5–)40–53.5(–59.5) × 2–3.5(–4) μm (av. 46.6 × 2.8 μm, n = 74), smooth- and thin-walled, conidiogenous loci with noticeable periclinal thickening and a short, non- to somewhat flared collarette; aerial conidia of two types: microconidia obovoidal, ellipsoidal, clavate to somewhat reniform, straight or gently curved, 0(–1)-septate, hyaline, smooth- and thin-walled, (3.5–)5–11.5(–20.5) × (2–)3–4(–5) μm (av. 8.4 × 3.4 μm, n = 159), clustering in false heads at tip of monophialides; macroconidia fusiform to falcate and multiseptate, straight to gently dorsiventrally curved, base blunt to inconspicuously notched, 1–3(–4)-septate, smooth- and thick-walled; 1-septate conidia: (16–)17.5–22 × (3–)4–5.5 μm (av. 19.6 × 4.5 μm, n = 16); 2-septate conidia: (18–)19–28.5(–30.5) × 3.5–5.5 μm (av. 23.6 × 4.3 μm, n = 14); 3-septate conidia: (21–)25–37.5(–43) × (4–)4.5–6(–6.5) μm (av. 31.3 × 5.1 μm, n = 64); 4-septate conidia: 44 × 5.5 μm (n = 2); overall: (16–)21–36.5(–44) × (3–)4–5.5(–6.5) μm (av. 28.8 × 4.9 μm, n = 96). Sporodochia cream, pale rosy buff to pale green. Sporodochial conidiophores simple or sparingly verticillately branched, bearing terminal monophialides, single or in whorls of 2–3 phialides; sporodochial phialides subcylindrical to subulate, 14.5–16.5 × (3–)4–5.5(–6.5) μm (av. 15.5 × 3.7 μm, n = 30), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, a short, not-flared collarette may be present. Sporodochial conidia, falcate and moderately curved; apical cell of equal length than adjacent cell, blunt with rounded and discreetly curved apex; basal cell notched, protruding slightly, (3–)4–7-septate, hyaline, smooth- and thick-walled; 3-septate conidia: (29–)32–41.5(–43.5) × 5–6.5 μm (av. 37.7 × 5.8 μm, n = 16); 4-septate conidia: (38–)38.5–43.5(–47) × 5.5–6.5 μm (av. 40.7 × 6 μm, n = 28); 5-septate conidia: (42.5–)51.5–64.5(–69.5) × (5.5–)6–7(–7.5) μm (av. 58 × 6.4 μm, n = 120); 6-septate conidia: (59.5–)62–68.5(–71) × (5.5–)6.5–7.5 μm (av. 65.1 × 6.7 μm, n = 48); 7-septate conidia: (64.5–)66–73(–74.5) × 6.5–7.5 μm (av. 69.5 × 6.9 μm, n = 36); overall: (29–)47–68.5(–74.5) × (5–)6–7(–7.5) μm (av. 57.8 × 6.5 μm, n = 248). Chlamydospores globose to subglobose, smooth- or slightly verrucose and thick-walled, (5.5–)6.5–8.5(–9.5) μm diam, abundantly formed terminally or intercalary on hyphae or conidia, solitary, in chains or clusters. Colonies on PDA growing in dark with an average radial growth rate of 22–25 mm/d at 24 °C, reaching 61–70 mm diam in 7 d at 24 °C; white, pale luteous to primrose, flat, velvety to cottony, with or without white to straw concentric rings; margin entire. Reverse white to pale straw. On OA incubated in dark reaching 75–82 mm diam in 7 d at 24 °C; white, buff to primrose, flat with more or less defined concentric rings of white mycelium and buff sporodochia, velvety to floccose; margin entire. Reverse white, straw to buff.
Additional material examined. Honduras, Ulna District, Tela, on Musa × sapientum, 17 Feb. 1923, O.A. Reinking, BPI 453021. – Philippines, Los Baños, from twig, 1925, H.W. Wollenweber, CBS 203.31 = NRRL 22234 = BBA 2019.
Notes — Isolate CBS 204.31 was deposited in WI and identified by H.W. Wollenweber as Fusarium solani var. minus. It matches the original collection site, date and host reported in the protologue of its sexual morph Hyp. haematococcus var. breviconus, presumably representing authentic material of the latter taxon (Wollenweber 1930). Nevertheless, no type was designated and it is not possible to unequivocally assign CBS 204.31 as the ex-type of the latter variety. The designation N. brevicona is coined here based on Hyp. haematococcus var. breviconus. To preserve its taxonomic concept, the name is fixed by lectotypification based on the original illustration which is reproduced here (Fig. 9). Furthermore, CBS 204.31 is apparently a subculture from the authentic collection of this species, and is here designated as epitype.
Fig. 9.
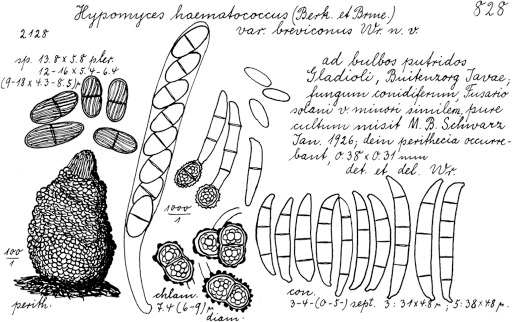
Neocosmospora brevicona (lectotype of Hypomyces haematococcus var. breviconus) as illustrated in the protologue by Wollenweber (1930).
The morphological features observed in this clade are similar to Wollenweber’s concept for F. solani var. minus and Hyp. haematococcus var. breviconus, especially regarding the morphological features of the aerial macroconidia. The original description of Hyp. haematococcus var. breviconus is clearly based on a mixture of aerial and sporodochial conidia, as depicted in Wollenweber (1930, No 828). No sexual morph developed in culture; the selected epitype is probably derived from the original collection where the sexual morph was present.
Previous descriptions of this taxon presumably included morphological characters from other taxa, especially N. solani s.str., which is also supported by the extensive list of host species commonly cited (Wollenweber 1931, Wollenweber & Reinking 1935a, b). Wollenweber (1931) synonymises F. solani var. minus under F. carneolum Sacc., an obscure, not effectively published species, and homonym of the later validly published F. carneolum (Karsten 1888). The latter species is morphologically unrelated to F. solani var. minus. This synonymy was also followed by Hering (1997). However, their concept of the species was polyphyletic according to available DNA sequence data of strains identified as such at that time. Fusarium caucasicum has also been treated as a synonym (Hering 1997). The ex-type culture of this species was examined here and found to represent a Fusarium species significantly different from the protologue or most modern observations (Gerlach & Nirenberg 1982; see notes under F. caucasicum).
Other taxa have also been cited as synonymous with Hyp. haematococcus var. breviconus, namely Fusarium roseum var. cucurbitacearum and F. uredinicola Petch (Wollenweber 1931) for which some authors have given nomenclatural priority (Hering 1997, Schütt 2001). However, these names were either not effectively published or are illegitimate, hence are not considered here, and the varietal name is raised to species level.
Neocosmospora brevis Sand.-Den. & Crous, sp. nov. — MycoBank MB831176; Fig. 10
Fig. 10.
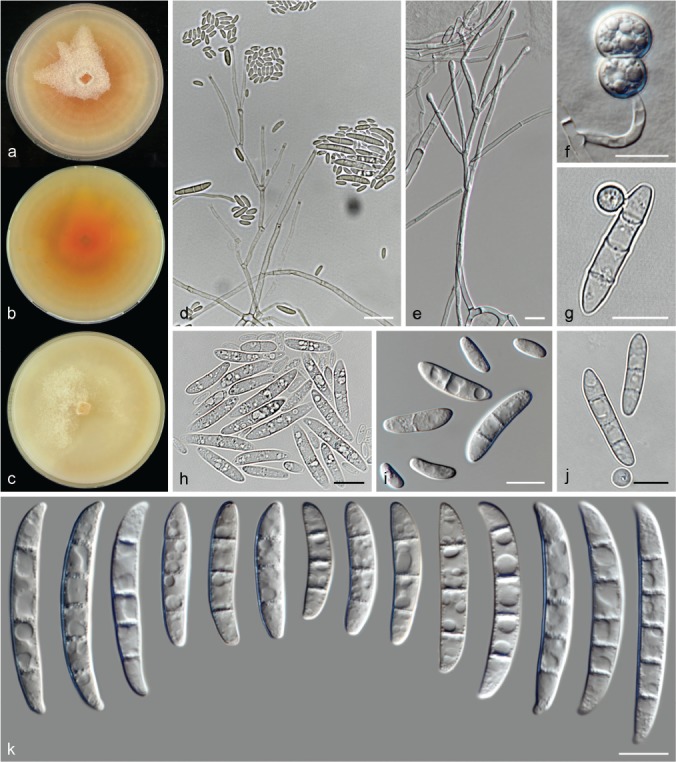
Neocosmospora brevis (ex-type culture CBS 144387). a–c. Colonies (a–b. PDA obverse and reverse, respectively; c. OA) after 14 d at 24 °C in the dark; d–e. aerial conidiophores; f–g. chlamydospores; h–j. aerial conidia; k. sporodochial conidia. — Scale bars: d = 20 μm; all others = 10 μm.
Etymology. From Latin brevis, meaning ‘short, small’; referring to its small sized macroconidia.
Typus. Belgium, Heverlee, soil-water polluted with diethyleneglycerol and ethylenglycerol, unknown date, G.L. Hennebert (holotype CBS H-23975 designated here, culture ex-type CBS 144387 = MUCL 16108).
Conidiophores scarce and scattered, erect and prostrate on substrate mycelium or on aerial mycelium, straight, smooth- and thin-walled, branched several times verticillately or sympodially or unbranched bearing terminal monophialides; phialides subulate to subcylindrical, (30–)36.5–59(–75.5) × 2–3(–3.5) μm (av. 47.6 × 2.6 μm, n = 88), smooth- and thin-walled, conidiogenous loci with rather conspicuous periclinal thickening and a non-flared collarette; aerial conidia of two types: microconidia oval, ellipsoidal to subclavate, often with somewhat flattened basal and apical ends, straight or slightly curved, 0–1(–2)-septate, hyaline, smooth- and thin-walled, (5.5–)7–17.5(–27) × (2–)3–5.5 μm (av. 12.4 × 3.9 μm, n = 214), clustering in false heads at tip of monophialides; macroconidia falcate, multiseptate, slightly dorsiventrally, curved, curvature slightly more pronounced toward base, apical cell blunt and rounded, basal cell without a well-developed foot shape to barely notched, 3–5-septate, smooth- and thick-walled; 3-septate conidia: (20–)24.5–37(–43.5) × (4–)5–6(–6.5) μm (av. 30.8 × 5.5 μm, n = 128); 4-septate conidia: 39.5–49(–52.5) × (5–)5.5–6 μm (av. 44.2 × 5.7 μm, n = 82); 5-septate conidia: 42.5–48.5(–49.5) × (5.5–)6–6.5 μm (av. 45.5 × 6.2 μm, n = 40); overall: (20–)28.5–46.5(–52.5) × (4–)5–6.5 μm (av. 37.5 × 5.7 μm, n = 250); Sporodochia not observed. Chlamydospores abundant, globose to subglobose, smooth- and thick-walled, (5–)6.5–9.5(–11) μm diam, terminal or intercalary on hyphae or conidia, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 2.4–2.8 mm/d at 24 °C, reaching 33–40 mm diam in 7 d at 24 °C; orange to saffron with straw to pale luteous periphery, flat, velvety with cottony to felty patches of white aerial mycelia and fine, short mycelia forming multiple concentric rings; margin entire. Reverse orange, luteous to amber, with scarce straw to bright orange diffusible pigment. On OA incubated in dark reaching 28–40 mm diam in 7 d at 24 °C; white, straw to buff, flat, dusty, velvety to floccose, margin entire. Reverse buff.
Additional materials examined. Italy, Catania, from Citrus sinensis, 2015, V. Guarnaccia, CPC 27190, CPC 27191. – USA, Texas, from human eye, unknown collector and date, CBS 130326 = NRRL 28009 = CDC B-5543.
Notes — Microconidia were the dominant conidial type observed for N. brevis, although not abundant. Aerial macroconidia were rarely observed, and true sporodochia were not observed. Abortive sporodochium-like structures forming irregularly shaped, rounded multiseptate conidia were only rarely present in the ex-type culture, formed on the agar surface on PDA and SNA; however, they were not considered in the species description.
Neocosmospora brevis is morphologically similar to but phylogenetically distant from N. diminuta. Both species are characterised by the absence of true sporodochia and presence of short falcate, multiseptate conidia produced from aerial conidiophores, where macro- and microconidia are produced in an apparent mixture. However, true macroconidia with well-developed foot cells are rare. Neocosmospora brevis differs from N. diminuta by having larger, more elongated microconidia (av. 12.4 × 3.9 μm vs 6.6 × 2.8 μm in N. diminuta) and longer, more regularly septate macroconidia (up to 52.5 μm long and 3–5-septate vs up to 38 μm long and 1–3-septate in N. diminuta). In addition, N. diminuta is homothallic and therefore develops the sexual morph readily, whereas a sexual morph is not known for N. brevis.
Currently, N. brevis includes isolates of clinical origin; whether this species encompasses true infectious agents or merely contaminants is not known.
Neocosmospora catenata Sand.-Den. & Crous, Persoonia 41: 115. 2018
Typus. USA, Georgia, from multiple tissues of Stegostoma fasciatum, unknown date and collector (holotype CBS H-23225, culture ex-type CBS 143229 = NRRL 54993 = UTHSC 09-1009).
Description & Illustration — Sandoval-Denis & Crous (2018).
Additional material examined. USA, Georgia, S. fasciatum multiple tissues, unknown date and collector, CBS 143228 = NRRL 54992 = UTHSC 09-1008.
Notes — This species was recently described from clinical animal specimens. Its circumscription encompasses the phylogenetic species previously known as FSSC 43 (O’Donnell et al. 2016, Sandoval-Denis & Crous 2018).
Neocosmospora crassa Sand.-Den. & Crous, sp. nov. — MycoBank MB831177; Fig. 11
Fig. 11.
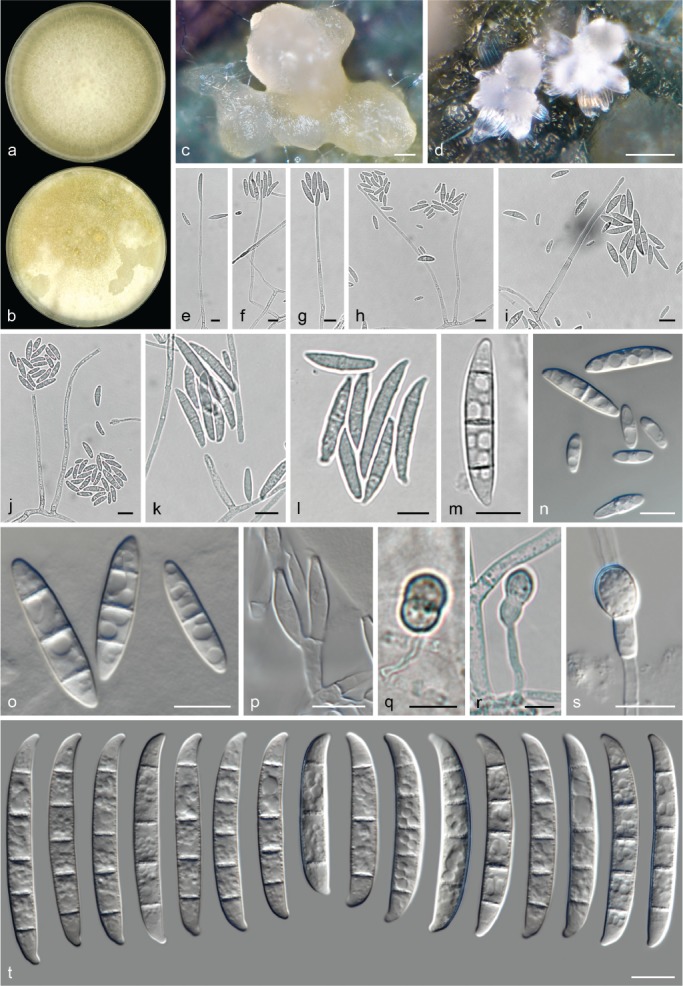
Neocosmospora crassa (ex-type culture CBS 144386). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochia formed on the surface of carnation leaves; e–k. aerial conidiophores and phialides; l–o. aerial conidia; p. sporodochial conidiophore and phialides; q–s. chlamy-dospores; t. sporodochial conidia. — Scale bars: c–d = 50 μm; all others = 10 μm.
Etymology. From Latin crassus meaning ‘thick, fat’; referring to its wide sporodochial conidia.
Typus. France, Paris, unknown substrate and date, J. Brun (holotype CBS H-23976 designated here, culture ex-type CBS 144386 = MUCL 11420).
Conidiophores on aerial mycelium straight, smooth- and thin-walled, mostly simple or sparingly irregularly branched, bearing terminal, single monophialides; phialides subcylindrical, subulate to somewhat acicular, (24.5–)49–69.5(–81.5) × (1.5–)2.5–4 μm (av. 59.4 × 3.2 μm, n = 154), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, collarettes short and non-flared; aerial conidia of two types: microconidia oval, ellipsoidal to subcylindrical, straight, often with a flattened base, straight to slightly curved, 0–1-septate, hyaline, smooth- and thin-walled, (8–)11.5–21.5(–25) × 4–6(–6.5) μm (av. 16.4 × 5 μm, n = 66), clustering in false heads at tip of monophialides; macroconidia fusiform to falcate, with a small flattened base or barely notched, straight to slightly curved, (1–)2–3-septate, hyaline, smooth- and thin-walled; 1-septate conidia: (13.5–)16–23.5(–25) × (4.5–)5–6(–6.5) μm (av. 19.7 × 5.4 μm, n = 36); 2-septate conidia: 24–25 × 5–6 μm (n = 4); 3-septate conidia: (25–)26.5–34.5(–39) × (5.5–)6–7 μm (av. 29.8 × 6.5 μm, n = 12); overall: (13.5–)16.5–28(–39) × (4.5–)5–6.5(–7) μm (av. 22.4 × 5.7 μm, n = 52). Sporodochia white, cream, light yellow-brown to green coloured. Sporodochial conidiophores densely irregularly to verticillately branched; sporodochial phialides subulate to doliiform, (10.5–)12–17(–18.5) × (3–)3.5–4.5(–5) μm (av. 14.4 × 4 μm, n = 92), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a minute, non-flared collarette. Sporodochial conidia straight to slightly dorsiventrally curved with nearly parallel lines, often gently widening at centre or basal half and tapering gently toward both ends; apical cell blunt and often shorter than adjacent cell; basal cell barely to distinctly notched and prominent, 3–5-septate, hyaline, smooth- and thick-walled; 3-septate conidia: (26.5–)32–41.5 × (5–)6–7 μm (av. 36.9 × 6.4 μm, n = 24); 4-septate conidia: (41.5–)43.5–50(–51) × 5.5–7(–7.5) μm (av. 46.5 × 6.2 μm, n = 80); 5-septate conidia: (44.5–)47–52(–54.5) × (5.5–)6–7(–7.5) μm (av. 49.5 × 6.3 μm, n = 64); overall: (26.5–)41–51.5(–54.5) × (5–)6–7(–7.5) μm (av. 46.3 × 6.3 μm, n = 168). Chlamydospores abundant, globose to subglobose, smooth- and thick-walled, (5.5–)6.5–9(–10) μm diam, terminal or intercalary in hyphae, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 5–5.6 mm/d at 24 °C, reaching 70–80 mm diam in 7 d at 24 °C; white, straw to buff, flat, felty to floccose; margin entire. Reverse white to pale straw. On OA incubated in dark covering an entire 90 mm diam Petri dish in 7 d at 24 °C; white, quickly becoming cream, pale luteous to buff; flat, velvety to felty, margin entire. Reverse white to straw.
Notes — Informally known as phylogenetic species FSSC 34 (O’Donnell et al. 2008), this clade is characterised by isolates originating from a vast array of hosts, including human clinical samples from superficial infections (Migheli et al. 2010).
Neocosmospora crassa is morphologically similar to N. pseudoradicicola and N. pseudotonkinensis, based on size and septation of the sporodochial conidia. However, N. crassa differs from N. pseudoradicicola by having much wider sporodochial conidia (av. width 6.3 μm vs 4.7 μm in N. pseudoradicicola), straighter and with less hooked apical cells, and an absence of microcyclic conidiation. In addition, the latter species exhibits only 0–1-septate microconidia. The morphological differentiation between N. crassa and N. pseudotonkinensis is less straightforward. Both species are clearly delimited and are distant phylogenetically, although they share remarkably similar cultural and microscopic features. Nonetheless, N. crassa shows slight differences in its conidial shape, being typically wider in the lower half, with less protuberant foot cells. In contrast, those of N. pseudotonkinensis are typically more tapered and wedge-shaped.
Neocosmospora cryptoseptata Sand.-Den. & Crous, sp. nov. — MycoBank MB831178; Fig. 12
Fig. 12.
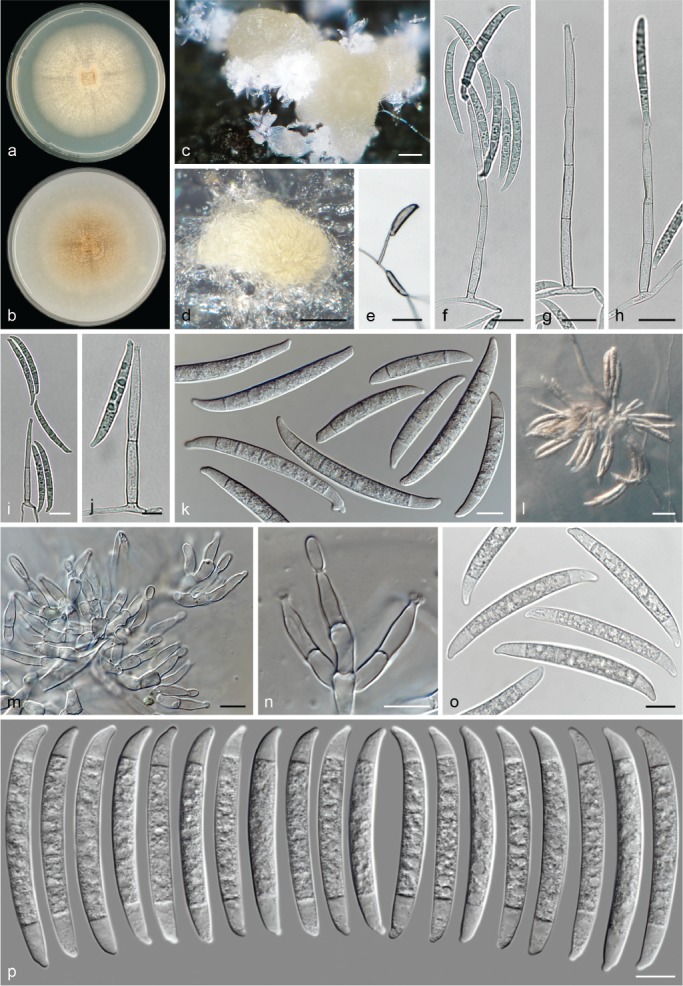
Neocosmospora cryptoseptata (ex-type culture CBS 145463). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochial formed on the surface of carnation leaves; e–j. aerial conidiophores; k. aerial conidia; l. sporodochia formed on the agar surface; m–n. sporodochial conidiophores and phialides; o–p. sporodochial conidia. — Scale bars: c–d = 100 μm; e = 50 μm; f–i, l = 20 μm all others = 10 μm.
Etymology. From Latin crypto meaning ‘secret or covert’, and sēptum meaning ‘enclosure, fence’; referring to the indistinct septation of the conidia.
Typus. French Guiana, from bark, unknown date and collector (holotype CBS H-23977 designated here, culture ex-type CBS 145463 = NRRL 22412 = BBA 65024).
Conidiophores abundant, formed laterally on aerial mycelium or erect on substrate mycelium, smooth- and thick-walled, often simple and straight, rarely sparingly laterally branched, proliferating sympodially and laterally from below conidiogenous loci, bearing terminal, single monophialides; phialides subcylindrical, subulate to somewhat lageniform, (39–)42.5–55(–62.5) × (3.5–)4–6(–6.5) μm (av. 48.9 × 5 μm, n = 72), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and non-flared, minute collarettes; aerial conidia falcate to wedge-shaped and multiseptate, base somewhat flattened, with an inconspicuous foot cell or distinctly and irregularly notched, 3–5(–6)-septate, smooth- and thick-walled; 3-septate conidia: (39–)42–50(–51) × (5.5–)6–7 μm (av. 45.9 × 6.4 μm, n = 36); 4-septate conidia: (49.5–)53–61(–62) × 6–7.5 μm (av. 57.1 × 6.6 μm, n = 28); 5-septate conidia: (42–)54–70.5(–71) × (5.5–)6–7 μm (av. 62.3 × 6.6 μm, n = 68); 6-septate conidia: 65–67.5 × 6–6.5 μm (av. 66.4 × 6.5 μm, n = 12); overall: (39–)48–67(–71) × (5.5–)6–7(–7.5) μm (av. 57.5 × 6.6 μm, n = 144). Sporodochia cream, straw, pale luteous to pale citrine coloured. Sporodochial conidiophores verticillately or laterally branched, bearing terminal, single monophialides or terminal whorls of up to three monophialides; sporodochial phialides subulate to lageniform, (10.5–)11.5–16.5(–20.5) × (3–)3.5–4.5(–5.5) μm (av. 14.1 × 4.1 μm, n = 56), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short or absent, non-flared collarette. Sporodochial conidia, falcate, rarely straight with curved ends, tapering toward base, septation often indistinct or with thin transverse septa; apical cell shorter than adjacent cell, blunt to somewhat conical with slight to markedly curved and rounded apex; basal cell inconspicuously to moderately notched to somewhat papillate, (3–)4–5-septate, hyaline, smooth- and thick-walled; 3-septate conidia: 53 × 7 μm (n = 4); 4-septate conidia: (52.5–)53.5–57.5(–59.5) × 5.5–6.5(–7) μm (av. 55.5 × 6.1 μm, n = 144); 5-septate conidia: (52.5–)55–61(–62.5) × (5.5–)6–6.5(–7) μm (av. 58 × 6.3 μm, n = 112); overall: (52.5–)53.5–59.5(–62.5) × (5.5–)6–6.5(–7) μm (av. 56.5 × 6.2 μm, n = 260). Chlamydospores not observed.Colonies on PDA growing in dark with an average radial growth rate of 2.5–3 mm/d at 24 °C, reaching 40–43 mm diam in 7 d at 24 °C; straw, pale luteous to pale ochreous, flat or inconspicuously radially folded, velvety to felty, with concentric rings of short aerial mycelium; margin filiform. Reverse white, pale straw to pale luteous. On OA incubated in dark reaching 38–41 mm diam in 7 d at 24 °C; pale luteous to pale sienna with white margin, flat, velvety and quickly becoming pionnotal; margin entire. Reverse pale luteous with umber to pale sienna centre.
Notes — Neocosmospora cryptoseptata is one of the few species in the genus lacking microconidia. Except for N. phaseoli, a lack of microconidia is a common feature for species within Clade 2 of Neocosmospora sensu O’Donnell (2000). The sporodochial conidia produced by this species, and to a lesser extent, the aerial conidia often appear to have indistinct septation, commonly with thin septal walls, often masked by fine but dense granular cell content. This feature has been reported for F. caeruleum, a species which is not related to Neocosmospora (see notes under F. caeruleum in ‘Doubtful and Excluded Taxa’).
In terms of sporodochial conidial size, N. cryptoseptata is related to N. regularis and N. bostrycoides. The latter two species differ in several aspects including conidial shape. Neocosmospora cryptoseptata produces less curved conidia, with shorter and rounder apical cells, and less pronounced foot cells. The absence of microconidia in N. cryptoseptata (present in the other two species), branching of the aerial conidiophores (profusely branched in N. bostrycoides), and the presence of microcyclic conidiation in N. regularis clearly distinguish these species from one another.
Similarly shaped sporodochial conidia are produced by N. robusta, a species also lacking microconidia and phylogenetically related to N. cryptoseptata. In the latter species the conidia are significantly shorter and less septate (av. 56.5 μm long, 3–5-septate, mostly 3-septate vs av. 69.1 μm long, up to 7-septate, mostly 6-septate in N. robusta).
Neocosmospora cucurbitae Sand.-Den., L. Lombard & Crous, sp. nov. — MycoBank MB831179; Fig. 13
Fig. 13.
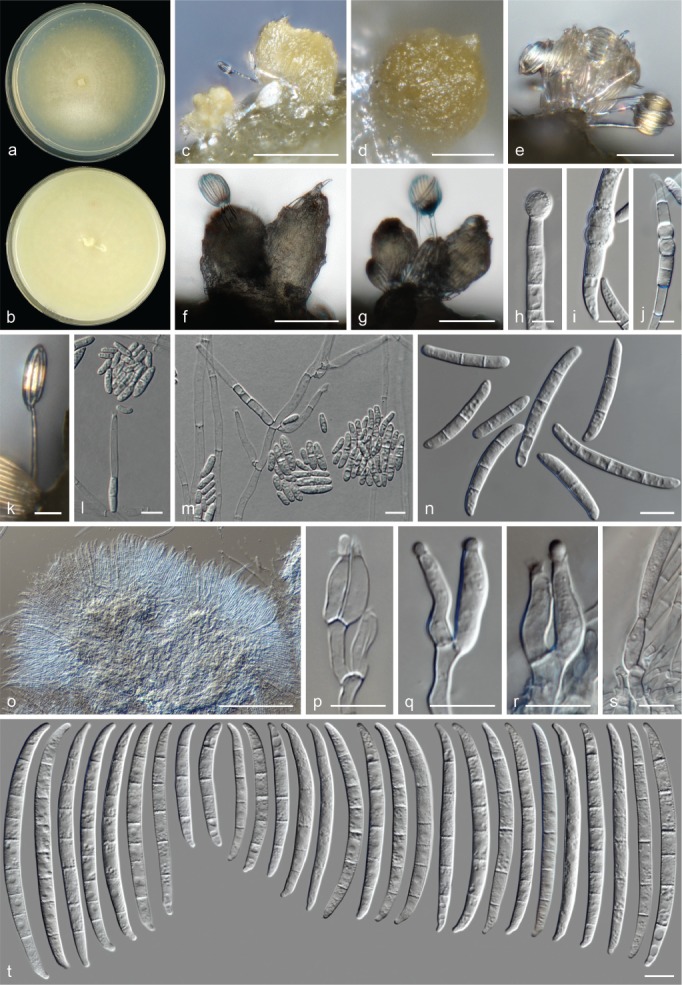
Neocosmospora cucurbitae (ex-type culture CBS 616.66). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–g. sporodochia formed on the surface of carnation leaves; h–j. chlamydospores; k–m. aerial conidiophores; n. aerial conidia; o–s. sporodochial conidiophores and phialides; t. sporodochial conidia. — Scale bars: c, e, o = 100 μm; d, f–g = 50 μm; h–j = 5 μm; k = 20 μm; all others = 10 μm.
Synonyms. Fusarium solani f. cucurbitae W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium solani f. sp. cucurbitae W.C. Snyder & H.N. Hansen, Root rots caused by Phycomycetes 28: 740. 1941.
Hypomyces solani f. cucurbitae W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 741. 1941.
Nectria haematococca var. cucurbitae (W.C. Snyder & H.N. Hansen) Dingley, New Zealand J. Agric. Res. 4: 337. 1961.
Nectria solani f. cucurbitae (W.C. Snyder & H.N. Hansen) G.R.W. Arnold, Z. Pilzk. 37: 193. 1972.
Etymology. Name refers to the plant genus Cucurbita from which this fungus was isolated.
Typus. Netherlands, from Cucurbita viciifolia, unknown date and collector (holotype CBS H-23978 designated here, culture ex-type CBS 616.66 = NRRL 22399 = BBA 64411).
Conidiophores mostly erect and simple, rarely sparingly branched, borne on substrate and aerial mycelium, straight, smooth- and thin-walled, bearing terminal, single monophialides; phialides subcylindrical to cylindrical, (11.5–)17.5–35(–52) × (2–)3–4.5(–6) μm (av. 26.3 × 3.6 μm, n = 55), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, non-flared collarette; aerial conidia of two types: microconidia ellipsoidal to clavate, straight, 0–2(–3)-septate, hyaline, smooth- and thin-walled, (4–)6.5–17.5(–42) × (2.5–)3–5 μm (av. 12 × 3.5 μm, n = 155), clustering in false heads at tip of monophialides; macroconidia falcate to cylindrical and multiseptate, moderately dorsiventrally curved, base rounded or barely notched, 2–3(–4)-septate, smooth- and thick-walled; 2-septate conidia: (19–)22–31.5(–36.5) × (3–)4–5(–6) μm (av. 26.5 × 4.3 μm, n = 48); 3-septate conidia: (17–)26–41.5(–51.5) × (3–)4–5(–6) μm (av. 33.6 × 4.4 μm, n = 44); 4-septate conidia: (36.5–)38.5–50.5(–51) × 4–5.5(–6) μm (av. 44.4 × 4.7 μm, n = 12); overall: (16.5–)22.5–40(–51.5) × (3–)3.5–5(–6) μm (av. 31.1 × 4.4 μm, n = 106). Sporodochia cream, light green to pale luteous. Sporodochial conidiophores sparingly verticillately branched, densely packed; sporodochial phialides subcylindrical, subulate to ampulliform, (11.5–)12–18.5(–27) × (3–)3.5–4.5(–5) μm (av. 15.3 × 4 μm, n = 52), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a minute, non-flared collarette. Sporodochial conidia moderately curved to wedge-shaped, narrowing gently toward base; apical cell of about equal length to the adjacent cell, blunt with rounded, somewhat papillate curved apex; basal cell distinctly notched, (4–)5–8(–9)-septate, hyaline, smooth- and thick-walled; 4-septate conidia: 42.7–57.5(–65.5) × 4–5.5 μm (av. 50.1 × 4.8 μm, n = 18); 5-septate conidia: (47–)55–74.5(–88) × (4–)4.5–5.5(–6) μm (av. 64.8 × 5 μm, n = 44); 6-septate conidia: (67.5–)70–81(–91) × (4.5–)5–6.5 μm (av. 75.6 × 5.4 μm, n = 39); 7-septate conidia: 73.5–74.5(–86) × 4.5–6 μm (av. 79.1 × 5.3 μm, n = 30); 8-septate conidia: (78–)81–91 × 5.5–6 μm (av. 86 × 5.7 μm, n = 8); 9-septate conidia: (87.5–)90–102 × 5.5–6.5 μm (av. 96.2 × 6.1 μm, n = 5); overall: (42.5–)59–83(–102) × (4–)4.5–6(–6.5) μm (av. 71.1 × 5.2 μm, n = 144). Chlamydospores globose to subglobose, smooth- or rough- and thick-walled, (4.5–)5.5–8(–9) μm diam, terminal or intercalary in hyphae or conidia, solitary or in chains. Colonies on PDA growing in dark with an average radial growth rate of 2.4–4.2 mm/d at 24 °C, reaching 34–59 mm diam in 7 d at 24 °C; straw to pale luteous, flat, velvety to felty with concentric floccose rings of white to straw aerial mycelium; margin entire. Reverse white to pale straw. On OA incubated in dark filling an entire 90 mm diam Petri dish in 7 d at 24 °C; white to straw, flat, membranous, velvety to granular; margin entire. Reverse straw to buff.
Additional material examined. Netherlands, from Cucurbita viciifolia, unknown date, Lab. W.C. Scholten, CBS 410.62 = NRRL 22658 = CECT 2864.
Notes — This clade is informally known as phylogenetic species FSSC 10, F. solani f. sp. cucurbitae race 1 and Nec. haematococca MPI. This phylogenetic species represents one of the two known biologically isolated populations to induce fruit rot of cucurbits (Mehl & Epstein 2007a). The other, F. solani f. sp. cucurbitae race 2 (Nec. haematococca MPV) falls within the circumscription of N. petroliphila, an important pathogen of plant and animal species, including humans (Mehl & Epstein 2007b, Short et al. 2013, Sandoval-Denis & Crous 2018).
Strains CBS 410.62 and CBS 616.66 studied here were previously identified as F. ensiforme. They do not morphologically match with the features of that species, nor cluster within the ‘ensiforme-clade’ sensu Nalim et al. (2011). In contrast, several studies link this clade with Nec. haematococca MPI (O’Donnell 2000, O’Donnell et al. 2008). Records indicate that strain CBS 616.66 is able to produce perithecia when crossed with isolates of F. solani f. cucurbitae race 1 from the collection of W.C. Snyder, while isolate NRRL 22153 (Table 1) is derived from a white perithecial strain reported by W.C. Snyder for this biological species (Snyder et al. 1975).
Morphologically, N. cucurbitae can easily be identified by its long, rather narrow sporodochial conidia with elongated apical and foot cells, and for its cylindrical, commonly flat-based aerial macroconidia. Similarly sized sporodochial conidia, with comparable septation can also be seen in N. mori. In the latter species, however, the sporodochial conidia are almost straight and markedly tapered, while aerial macroconidia are absent.
Neocosmospora cyanescens (G.A. de Vries et al.) Summerb. et al., Biology of Microfungi (Cham): 183. 2016
Basionym. Phialophora cyanescens G.A. de Vries et al., Antonie van Leeuwenhoek 50: 150. 1984.
Synonym. Cylindrocarpon cyanescens (G.A. de Vries et al.) Sigler, J. Clin. Microbiol. 29: 1858. 1991.
Typus. Netherlands, Groningen, subcutaneous tissue of the right foot of a male human patient, unknown date, Acad. Ziekenhuis Groningen, No. 689b (holotype CBS 518.82, maintained as a permanently metabolically inactive culture).
Descriptions & Illustrations — De Vries et al. (1984), Zoutman & Sigler (1991).
Additional material examined. Netherlands, Groningen, subcutaneous tissues of the right foot of a male human patient, unknown date, Acad. Ziekenhuis Groningen No. 689b, CBS 637.82.
Notes — Neocosmospora cyanescens, representing phylogenetic species FSSC 27, is an extremely rare and pleomorphic species. Its morphological characters render it hard to place in Neocosmospora, being characterised by forming aggregates of chlamydospore-like cells and sparse ellipsoidal conidia formed on long phialides. This species was originally isolated from a white-grain mycetoma in a man, initially described as a new species in Phialophora (De Vries et al. 1984), and then recombined in Cylindrocarpon, although multiseptate conidia are not produced (Zoutman & Sigler 1991). Based on DNA sequence data, this species was recently reallocated to Neocosmospora with the odd morphological features being attributed to years enduring extreme conditions in the host (Summerbell & Scott 2016). Neocosmospora cyanescens is only known from a single collection, the additional isolate CBS 637.82 being a white mutant from the original collection.
Neocosmospora diminuta Sand.-Den. & Crous, sp. nov. — MycoBank MB831180; Fig. 14
Fig. 14.
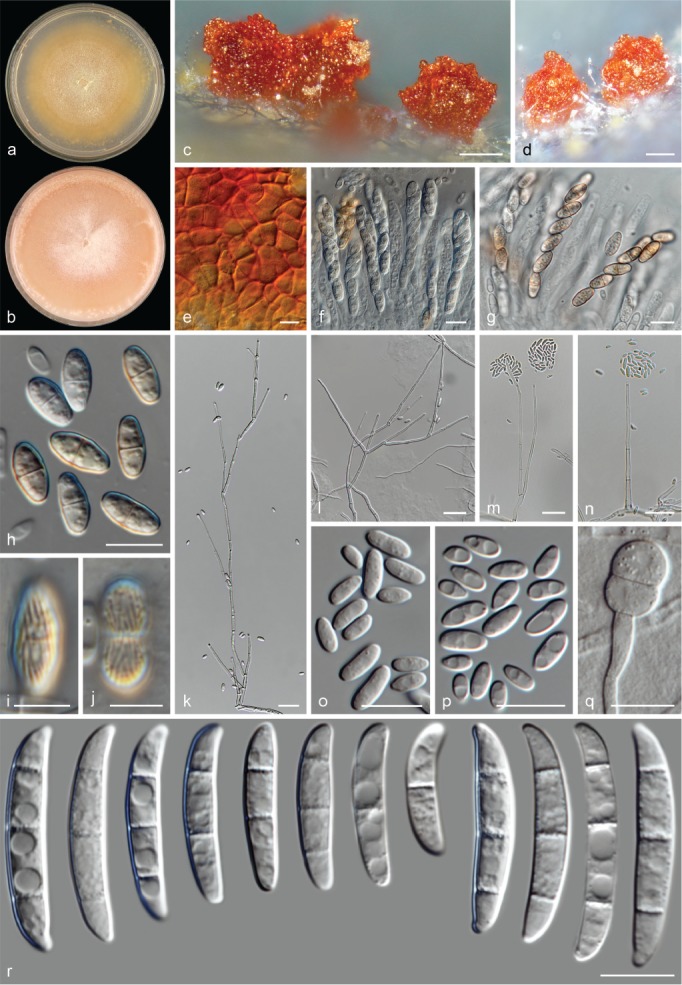
Neocosmospora diminuta (ex-type culture CBS 144390). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. perithecia; e. detail of the peridium cells; f–g. asci; h–j. ascospores; k–n. aerial conidiophores; o–p. aerial microconidia; q. chlamydospores; r. aerial macroconidia. — Scale bars: c–d = 100 μm; i–j = 5 μm; k–n = 20 μm; all others = 10 μm.
Etymology. From Latin diminuto meaning ‘minute, tiny’; refers to its small conidia.
Typus. Unknown (presumptively Ivory Coast), from Coelocaryon preussii treated wood, before 1972, Centre Technique Forestier Tropical (holotype CBS H-23979 designated here, culture ex-type CBS 144390 = MUCL 18798).
Perithecia orange to dark-red, globose to pyriform, papillate, superficial, solitary or gregarious, coarsely warted, glabrous; peridial wall composed of thick-walled cells of textura angularis. Asci subcylindrical, clavate to saccate, unitunicate, apex rounded and simple, (59.5–)62.5–74.5(–82.5) × (7.5–)8–11(–11.5) μm (av. 68.6 × 9.5 μm, n = 48). Ascospores obliquely uniseriate or irregularly biseriate at apex of asci, ellipsoidal to subfusiform, 1-septate, (9–)10.5–12(–14.5) × (4–)4.5–5.5(–6) μm (av. 11.2 × 5.1 μm, n = 99), yellow-brown to golden yellow, thick-walled, longitudinally finely striated.
Conidiophores abundant on aerial mycelium or erect from substrate mycelium, straight or flexuous, smooth- and thin-walled, simple or branched several times verticillately or sympodially, bearing terminal monophialides; phialides subulate to subcylindrical, (8.5–)36.5–56.5(–69) × (1.5–)2–3 μm (av. 46.4 × 2.4 μm, n = 72), smooth- and thin-walled, proliferating frequently, conidiogenous loci with periclinal thickening and a short, non-flared collarette; aerial conidia of two types: microconidia oval to ellipsoidal, straight or slightly curved, 0(–1)-septate, hyaline, smooth- and thin-walled, (4–)5–8.5(–14.5) × 2–3.5(–4.5) μm (av. 6.6 × 2.8 μm, n = 108), clustering in false heads at tip of monophialides; macroconidia short falcate and multiseptate, gently dorsiventrally curved with almost straight ventral line, base rounded, inconspicuously or distinctly notched, 1–3-septate, smooth- and thick-walled; 1-septate conidia: (14.5–)17.5–22.5 × (3.5–)4–5 μm (av. 19.9 × 4.4 μm, n = 29); 2-septate conidia: 21–24(–24.5) × 3.5–4.5 μm (av. 22.4 × 4.1 μm, n = 5); 3-septate conidia: (22.5–)27–35(–38) × 4–5(–5.5) μm (av. 31.1 × 4.7 μm, n = 52); overall: (14.5–)20.5–33.5(–38) × (3.5–)4–5(–5.5) μm (av. 26.8 × 4.5 μm, n = 86). Sporodochia not observed. Chlamydospores subglobose to globose, smooth- and thick-walled, (6–)7–10.5(–11) μm diam, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 4–4.8 mm/d at 24 °C, reaching 56–70 mm diam in 7 d at 24 °C; luteous to ochreous, flat, velvety to felty, with or without concentric rings of short aerial mycelium; margin entire. Reverse pale luteous to orange. On OA incubated in dark reaching 50–57 mm diam in 7 d at 24 °C; salmon to flesh with abundant white mycelium at centre, flat, velvety to floccose; margin entire. Reverse peach, salmon to flesh.
Notes — Previously known as phylogenetic species FSSC 39, this clade was recognised as a putative novel species during a screening for human pathogenic fusaria in plumbing drains, but not formally described based on its cryptic morphology (Short et al. 2011). Neocosmospora diminuta produces the smallest falcate multiseptate conidia in the genus. These macroconidia are not produced on sporodochia, but on tall aerial conidiophores, mixed with microconidia.
Morphologically, N. diminuta is comparable to N. brevis, with both species lacking sporodochia and producing short, multiseptate, aerial falcate conidia. Conidia of N. diminuta, are considerably smaller and less septate (up to 14.5 μm long, av. size 11.2 × 5.1 μm and 1–3-septate, vs up to 52.5 μm long, av. size 37.5 × 5.7 μm and 3–5-septate in N. brevis). In addition, N. diminuta has a conspicuous sexual morph, while an asexual morph is not known for N. brevis.
Other species with similarly sized macroconidia are N. keratoplastica and the unnamed phylogenetic species FSSC 12 (not yet formally introduced, D. Geiser pers. comm.). In the latter species macroconidia are produced on well-developed sporodochia.
Neocosmospora elegans (Y. Yamam. & Maeda) Sand.-Den. & Crous, comb. nov. — MycoBank MB831226; Fig. 15
Fig. 15.
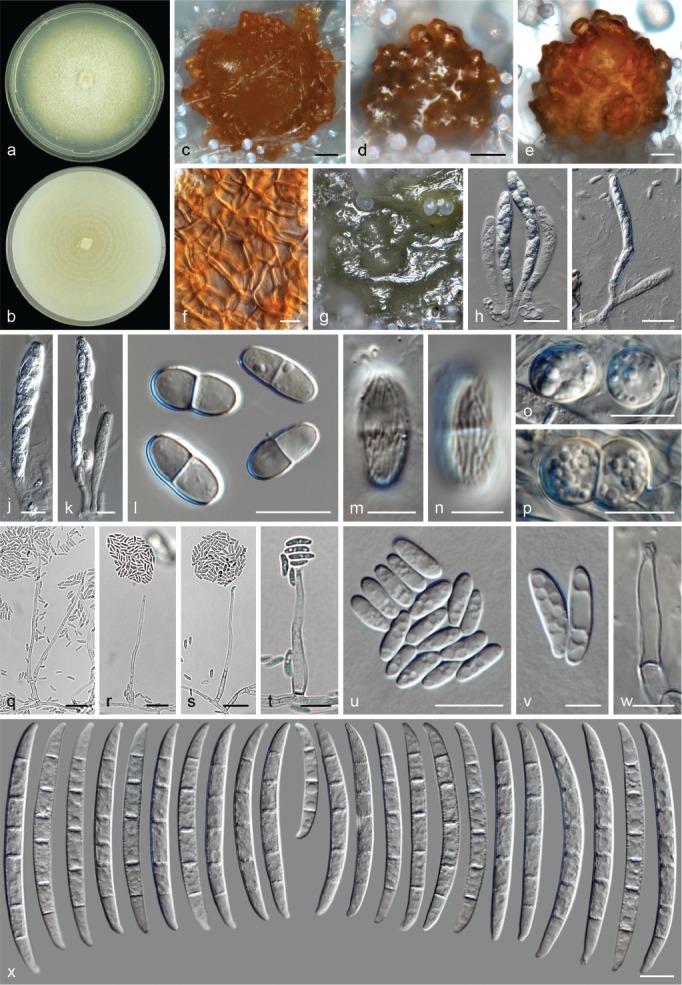
Neocosmospora elegans (ex-epitype culture CBS 144396). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. perithecia; f. detail of peridium cells; g. sporodochia formed on the surface of carnation leaves; h–k. asci; l–n. ascospores; o–p. chlamydospores; q–t. aerial conidiophores and phialides; u–v. aerial conidia; w. sporodochial phialide; x. sporodochial conidia. — Scale bars: c–e, g = 100 μm; h–i, q–s = 20 μm; m–n, v–w = 5 μm; all others = 10 μm.
Basionym. Nectria elegans Y. Yamam. & Maeda, Sci. Rep. Hyogo Univ. Agric. 3: 15. 1957.
Synonyms. (Fusarium solani f. xanthoxyli Y. Sakurai & Matuo, Ann. Phytopathol. Soc. Japan 26: 117. 1961 (Nom. inval., Art. 39.1.)).
(Hypomyces solani f. xanthoxyli Y. Sakurai & Matuo, Ann. Phytopathol. Soc. Japan 26: 117. 1961 (Nom. inval., Art. 39.1.)).
Typus. Japan, Prov. Tamba, Nishiki-mura, on twigs and trunks of Xanthoxylum piperitum (lectotype of Nectria elegans, designated here: illustration f. 1–9, p. 16, in Yamamoto et al. (1957), Science Reports of the Hyogo University of Agriculture. 3: 15–18, MBT387213); Hyōgo, from trunk of X. piperitum, 1959, Y. Sakurai (epitype of N. elegans CBS H-23980 designated here, MBT387214; culture ex-epitype CBS 144396 = NRRL 22277 = MAFF 238541 = ATCC 42366 = SUF XV-1).
Perithecia dark-orange to dark-red, globose, subglobose or pyriform, superficial or partially embedded in substrata, solitary or gregarious, coarsely warted, glabrous; peridial wall composed of thick-walled cells of textura angularis. Asci subcylindrical to clavate, unitunicate, apex flat to rarely rounded and simple, (68.5–)70–85.5(–94) × (7.5–)8–10 μm (av. 76.8 × 9.1 μm, n = 48). Ascospores obliquely uniseriate or irregularly biseriate at apex of asci; broadly ellipsoidal to ellipsoidal, 1-septate, (9–)10.5–12.5(–14) × 4–5.5(–6.5) μm (av. 11.6 × 4.9 μm, n = 132), pale yellow-brown to golden yellow, thick-walled, longitudinally finely to coarsely striated, often rounded at both ends and constricted at septum.
Conidiophores erect and prostrate on substrate mycelium, abundantly produced on aerial mycelium, straight, smooth- and thin-walled, often short and simple, reduced to conidiogenous cells borne laterally on hyphae, or sparingly branched irregularly, verticillately or sympodially, bearing terminal and lateral, single monophialides; phialides subulate, subcylindrical to acicular, (28.5–)36–53(–63) × 2–3.5(–5) μm (av. 44.5 × 2.8 μm, n = 104), smooth- and thin-walled, walls sometimes slightly thickened at base, conidiogenous loci with rather conspicuous periclinal thickening, collarettes short- and non-flared or absent; aerial conidia ellipsoidal to clavate, straight, rarely slightly curved, 0(–1)-septate, hyaline, smooth- and thin-walled, (5–)6–7(–16) × (1.5–)2–3.5(–4.5) μm (av. 8 × 2.9 μm, n = 129). Sporodochia cream to olivaceous. Sporodochial conidiophores sparingly irregularly or verticillately branched, bearing terminal, single monophialides; sporodochial phialides subcylindrical to subulate, (12–)13.5–17.5(–18) × 2–3.5 μm (av. 15.3 × 2.9 μm, n = 80), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and collarettes. Sporodochial conidia discreetly dorsiventrally curved, often more pronouncedly curved in bottom half of the conidia; broad in middle portion or just above it and narrowing gradually toward both ends; apical cell often longer than the adjacent cell, blunt to conical with a discreetly curved, rounded apex; basal cell blunt and straight or notched, (3–)5–6-septate, hyaline, smooth- and thick-walled; 3-septate conidia: 35.5 × 4 μm (n = 2); 5-septate conidia: (50.5–)57–66.5(–70) × (4.5–)5–6(–6.5) μm (av. 61.8 × 5.4 μm, n = 76); 6-septate conidia: (63–)65.5–75(–78.5) × 5–6 μm (av. 70.1 × 5.5 μm, n = 34); overall: (34.5–)56.5–71(–78.5) × (4–)5–6(–6.5) μm (av. 63.8 × 5.4 μm, n = 112). Chlamydospores abundant, globose to subglobose, smooth- and thick-walled, 8.5–11(–12.5) μm diam, turning pale golden yellow when mature; terminal or intercalary, solitary, in chains or clusters.
Colonies on PDA growing in dark with an average radial growth rate of 2.3–3 mm/d at 24 °C, reaching 31–40 mm diam in 7 d at 24 °C; straw to pale luteous coloured, flat, velvety to felty, with abundant white aerial mycelium; margin entire. Reverse straw to pure yellow. On OA incubated in dark reaching 34–42 mm diam in 7 d at 24 °C; cream, straw to pale buff coloured, flat, velvety to granular, with concentric rings of white to cream aerial mycelium; margin entire. Reverse straw to buff.
Additional material examined. Japan, Hyōgo, from a branch of Xanthoxylum piperitum, 1959, Y. Sakurai, CBS 144395 = NRRL 22163 = MAFF 238540 = ATCC 18690 = MATUO XV-23.
Notes — This species was initially described as the causal agent of trunk-blight of Xanthoxylum piperitum in Japan (Yamamoto et al. 1957), with the sexual morph placed in Nectria. The asexual morph was assigned to Fusarium sect. Elegans (F. oxysporum s.lat.), but was later re-identified by Sakurai & Matuo (1961) as F. solani, and reduced to a formae speciales of the latter taxon (as F. solani f. xanthoxyli), and the sexual morph recombined into the genus Hypomyces (as Hyp. solani f. sp. xanthoxyli). However, sexual incompatibility (Matuo & Snyder 1973), phylogenetic inference (O’Donnell 2000, O’Donnell et al. 2008), and RAPD and ITS-RFLP banding patterns (Hering 1997) demonstrated that this taxon is not conspecific with N. solani. Therefore, it has been informally assigned to F. solani f. sp. xanthoxyli and later to phylogenetic species FSSC 22 (O’Donnell 2000). Accordingly, the basionym is resurrected here and recombined as Neocosmospora elegans. The two heterothallic strains studied here formed part of the mating studies by Sakurai & Matuo (1961) and are able to reproduce sexually in vitro.
Type material of N. elegans could not be traced; the current location of the type specimen cited in the protologue (Japan, Prov. Tamba, Nishiki-mura, on twigs and trunks of X. piperitum, 16 Sept. 1955, W. Yamamoto) is unknown and is therefore presumed lost (C. Nakashima & Y. Hirooka, pers. comm.). A living strain identified by W. Yamamoto is housed in NBRC (NBRC 7187 = IFO 7187), however, not as type. In order to fix the use of the name, a lectotype is designated from the original illustration, which is reproduced here (Fig. 16) and an epitype is chosen from material obtained from the same geographical region (prefecture) where the holotype was originally collected.
Fig. 16.
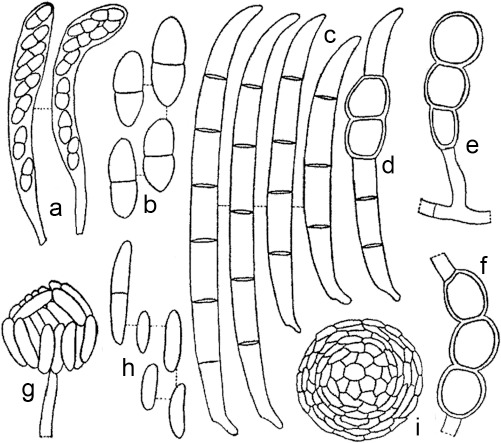
Neocosmospora elegans (lectotype of Nectria elegans) adapted from the original drawings by Yamamoto et al. (1957). a. Asci; b. ascospores; c. sporodochial conidia; d–f. chlamydospores; g–h. aerial conidia; i. sclerotium.
An unusual characteristic of this species is the presence, although rare, of short rather ampulliform aerial phialides born laterally on the hyphae, which somehow resemble those of F. oxysporum s.lat. Nevertheless, the most commonly formed conidiogenous apparatus corresponds to the archetypal F. solani/Neocosmospora type.
Species showing similar dimensions of sporodochial macroconidia include N. hypothenemi and N. silvicola. Neocosmospora elegans can be distinguished by its regularly sparingly branched conidiophores, the luteous coloration of its colonies and its narrow (av. width 2.9 μm) microconidia. In contrast, N. hypothenemi and N. silvicola form highly branched, tall conidiophores; orange to scarlet colonies in the former and saffron in the latter, and wider microconidia (av. width of 3.9 μm and 3.7 μm for N. hypothenemi and N. silvicola, respectively). In addition, N. hypothenemi lacks a known sexual morph.
Neocosmospora euwallaceae (S. Freeman et al.) Sand.-Den., L. Lombard & Crous, comb. nov. — MycoBank MB831181
Basionym. Fusarium euwallaceae S. Freeman, Z. Mendel, T. Aoki & O’Donnell, Mycologia 105: 1599. 2013.
Typus. Israel, Kibbutz Glil Yam central coastal region, Euwallacea sp. beetle infecting Persea americana (cultivar Hass), 17 Feb. 2010, S. Freeman & Z. Mendel (Freeman 1) (holotype BPI 884203; culture ex-type CBS 135854 = NRRL 54722).
Description & Illustration — Freeman et al. (2013).
Notes — A member of the Ambrosia clade of Neocosmospora, it is also referred to as Clade AF2 (Freeman et al. 2013) and is a serious threat to avocado production in Israel and California (Mendel et al. 2012, Eskalen et al. 2013, O’Donnell et al. 2016). It was recently introduced into South Africa where c. 80 tree host species have been identified, including native and exotic trees (Paap et al. 2018, retrieved from www.fabinet.up.ac.za/index.php/pshb on April 2019). Neocosmospora euwallaceae is clearly recognisable by its blue-brown, irregularly clavate sporodochial conidia (see notes under N. ambrosia).
A draft genome sequence was recently published of a strain regarded as N. euwallaceae (as Fusarium euwallaceae strain HFEW-16-IV-019; Ibarra-Laclette et al. 2017), and, rDNA, tef1 and rpb2 sequences were retrieved from the genome scaffolds available in GenBank and included in this study (Table 1, Fig. 1). The results indicate that the reported genome sequences belong to a different, although closely related species, N. kuroshio (see below). This is also supported by the host insect association reported for that strain (Kuroshio shot hole borer beetle, Euwallacea sp. nr. fornicatus #5, KSHB; O’Donnell et al. 2015, Stouthamer et al. 2017, Na et al. 2018).
Neocosmospora falciformis (Carrión) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015
Basionym. Cephalosporium falciforme Carrión, Mycologia 43: 523. 1951.
Synonyms. Acremonium falciforme (Carrión) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 139. 1971.
Fusarium falciforme (Carrión) Summerb. & Schroers, J. Clin. Microbiol. 40: 2872. 2002.
Fusarium paranaense Costa, Matos & Pfenning, Fungal Biol. 120: 55. 2017.
Typus. Puerto Rico, from a human mycetoma, 1967, J.E. MacKinnon (holotype CBS 475.67, permanently preserved as metabolically inactive culture, culture ex-type CBS 475.67 = IHM 939 = IMI 268681).
Descriptions & Illustrations — Carrión (1951), Gams (1971), Costa et al. (2016), Sandoval-Denis & Crous (2018).
Additional materials examined. Brazil, Distrito Federal, Brasília, from Glycine max, unknown date and collector, CBS 141594 = CML 860 (culture ex-paratype of F. paranaense); Goiás, Cristalina, from basal stem of Glycine max, Feb. 2000, collector unknown, CBS 141593 = CML 1830 = MES 24 (culture ex-type of F. paranaense). – France, Nancy, human nail, unknown date, N. Contet-Audonneau, CBS 124627. – Syria, declined grapevine, unknown date, K.A. Halim, CBS 121450.
Notes — Also known as phylogenetic species FSSC 3+4 (O’Donnell et al. 2008), N. falciformis is one of the most prevalent clinically relevant species of the genus along with N. keratoplastica, N. metavorans, N. petroliphila and N. solani (Short et al. 2013, Sandoval-Denis & Crous 2018). These species are opportunistic taxa, capable of inducing disease in humans and other animals (O’Donnell et al. 2008, 2016, Sarmiento-Ramírez et al. 2014, Sandoval-Denis & Crous 2018), with some also found as weak plant pathogens (Short et al. 2011, 2013, Scheel et al. 2013). It is one of the most frequently encountered species from natural substrates.
The ex-type and ex-paratype cultures of Fusarium paranaense (CBS 141593 and CBS 141594, respectively), a recently published species responsible for root rot of Glycine max in Brazil (Costa et al. 2016), cluster within the main clade of N. falciformis, and this species is thus here reduced to synonymy under N. falciformis. Interestingly, F. paranaense was described with a sexual morph, a feature previously not known for N. falciformis, and that indicates the existence of a heterothallic sexual cycle in the latter taxon.
The presence of sporodochial macroconidia is largely strain dependant in N. falciforme. The ex-type strain, CBS 475.67, is known to exhibit a microconidial morphology, lacking typical falcate multiseptate conidia, which explains its original allocation in Cephalosporium and later in Acremonium (Carrión 1951, Gams 1971). When present, sporodochial conidia are rather short and bulky, similar in size and appearance to those of N. metavorans and N. solani. In N. falciformis, sporodochial conidia are less septate, shorter and narrower, while aerial conidia are larger than those of N. metavorans (Sandoval-Denis & Crous 2018). In contrast, the sporodochial conidia of N. metavorans and N. solani tend to be more clearly tapered.
Neocosmospora ferruginea Sand.-Den. & Crous, sp. nov. — MycoBank MB831182; Fig. 17
Fig. 17.
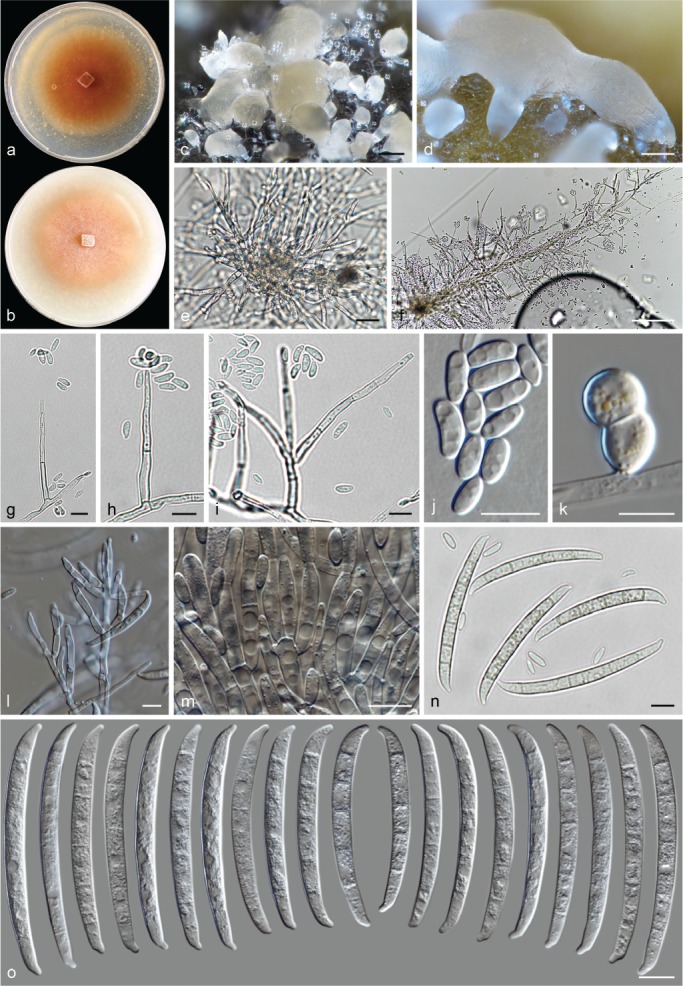
Neocosmospora ferruginea (ex-type culture CBS 109028). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d = sporodochia formed on the surface of carnation leaves; e. pseudostromatic structures; f–i. aerial conidiophores; j, aerial conidia; k. chlamydospores; l–m. sporodochial conidiophores and phialides; n–o. sporodochial conidia. — Scale bars: c–d = 100 μm; e = 20 μm; all others = 10 μm.
Etymology. From Latin ferrugineus (‘rust colour’). Referring to the colony colour on artificial media.
Typus. Switzerland, from human subcutaneous nodule, unknown date, G. Schär (holotype CBS H-23981 designated here, culture ex-type CBS 109028 = NRRL 32437).
Conidiophores abundant on aerial mycelium, commonly borne from dense mycelial tufts or cords, or emerging on agar surface from pseudostromatic structures composed of pale brown, irregularly shaped, smooth- and thick-walled cells; straight, smooth- and thin-walled, simple or sparingly branched verticillately or irregularly, bearing terminal, single monophialides; phialides subulate, subcylindrical to acicular, (29.5–)31–40(–45) × 2.5–3.5 μm (av. 35.5 × 3 μm, n = 96), hyaline to subhyaline when mature, smooth- and thick-walled, conidiogenous loci with inconspicuous periclinal thickening, collarettes absent or minute; aerial conidia ellipsoidal to allantoid, 0–1(–2)-septate, hyaline, smooth- and thin-walled, (4–)4.5–11(–20) × (1.5–)2–4(–6) μm (av. 7.6 × 3 μm, n = 92), clustering in false heads at tip of monophialides. Sporodochia white to buff. Sporodochial conidiophores irregularly and verticillately branched and densely packed; sporodochial phialides subcylindrical to doliiform, 16.5–22(–24.5) × 3.5–4.5(–5) μm (av. 19.4 × 4.1 μm, n = 42), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, without collarette or with a short, non-flared collarette. Sporodochial conidia moderately curved, gently tapered and straighten toward base; apical cell of equal length than adjacent cell, blunt to conical, with a somewhat constricted, barely hooked and rounded apex; basal cell distinctly notched, often protuberant, (4–)5–6(–7)-septate, hyaline, smooth- and thick-walled; 4-septate conidia: 53.5–57.5 × 5–5.5 μm (av. 55.6 × 5.3 μm, n = 6); 5-septate conidia: (55.5–)58.5–67(–70) × (5–)5.5–6(–6.5) μm (av. 62.4 × 5.7 μm, n = 72); 6-septate conidia: (56.5–)61.5–69.5(–71.5) × 5–6.5 μm (av. 65.4 × 5.7 μm, n = 33); 7-septate conidia: 69.5 × 6 μm (n = 3); overall: (54–)58–69(–71.5) × 5–6.5 μm (av. 63.1 × 5.7 μm, n = 114). Chlamydospores globose to subglobose, smooth- and thick-walled, 8–10 μm diam, terminal or intercalary, solitary or in short chains.
Colonies on PDA growing in dark with an average radial growth rate of 2–2.5 mm/d at 24 °C, reaching 27–30 mm diam in 7 d at 24 °C; sienna, rust to brick, flat, velvety, margin entire. Reverse apricot to rust. Colonies on OA incubated in dark reaching 23–28 mm diam in 7 d at 24 °C; peach to rosy buff, flat, velvety to dusty, margin entire. Reverse saffron to rosy buff.
Additional materials examined. Italy, from Citrus sinensis, 3 Mar. 2015, V. Guarnaccia, CPC 28194, CPC 28195.
Notes — Previously known as phylogenetic species FSSC 28, Neocosmospora ferruginea was assigned to the ‘F. ensiforme clade’ by Nalim et al. (2011). This group encompass an array of phylogenetic species with morphologies comparable to that of F. ensiforme (F. javanicum var. ensiforme). The latter species is characterised by producing long, somewhat straight and acute sporodochial conidia with protuberant foot cells (Wollenweber & Reinking 1925, 1935a, Gerlach & Nirenberg 1982). Although largely resembling the historical concept of F. ensiforme, N. ferruginea differs in various aspects that include conidial dimensions, forming shorter, rarely 7-septate sporodochial conidia with rounded and hooked apices, and wider microconidia (up to 6 μm vs up to 4 μm wide in F. ensiforme). In addition, N. ferruginea forms rust-coloured colonies on PDA, contrary to the white to pink colonies of F. ensiforme. The presence of pseudostromatic structures in N. ferruginea could be considered a differential character. However, this feature was observed only in the ex-type isolate.
Several species share similar morphological features with N. ferruginea, particularly those previously assigned to the ‘F. ensiforme clade’ (Nalim et al. 2011), i.e., N. hypothenemi, and N. spathulata, but also genetically unrelated species like N. piperis and N. protoensiformis. These taxa produce similarly shaped and relatively large, up to 6- or rarely 7-septate sporodochial conidia. Nevertheless, N. ferruginea differs from N. piperis by the absence of aerial macroconidia and sexual morph in the former species. Neocosmospora hypothenemi, N. protoensiformis and N. spathulata differ by exhibiting sporodochial conidia with tapered, somewhat acute and straight apical cells, and protuberant foot cells vs the rounded and hooked apical cells and less developed foot cells of N. ferruginea.
Neocosmospora gamsii Sand.-Den. & Crous, Persoonia 41: 116. 2018
Typus. USA, Pennsylvania, from human bronchoalveolar lavage fluid, unknown date, D.A. Sutton (holotype CBS H-23226, culture ex-type CBS 143207 = NRRL 32323 = UTHSC 99-205).
Description & Illustration — Sandoval-Denis & Crous (2018).
Additional materials examined. Brazil, unknown substrate, date and collector, CBS 700.86 = NRRL 22236. – Nigeria, from plywood, unknown date and collector, CBS 217.53 = NRRL 22655. – USA, Illinois, Urbana, human eye, unknown date and collector, CBS 143209 = NRRL 32770 = FRC S-0524; New York, coolant fluid from humidifier, unknown date and collector, CBS 143211 = NRRL 32794 = FRC S-1152; Tennessee, human cornea, unknown date and collector, CBS 130181 = NRRL 43502 = CDC 2006743469.
Notes — A name recently proposed for the previously informally known phylogenetic species FSSC 7 (O’Donnell et al. 2008, Sandoval-Denis & Crous 2018), N. gamsii is a pathogenic species known mostly from human clinical samples. This species is isolated with lesser frequency than the more prevalent pathogenic species N. keratoplastica, N. metavorans, N. petroliphila and N. solani. Among the clinically relevant species of the genus, N. gamsii is recognisable by having long and narrow sporodochial conidia with elongated, curved and rounded apical cells and a conspicuous homothallic sexual morph (Sandoval-Denis & Crous 2018).
Morphologically, it is a close ally to N. ampla and N. elegans. The latter species are presently only known from plant hosts. Nonetheless, a number of features distinguish N. gamsii, including its narrow sporodochial conidia with elongated, hooked and rounded apical cells, smaller microconidia (up to 11 μm long vs up to 16 and 23 μm long, respectively for N. elegans and N. ampla), sporodochia being yellow-blue-green at maturity (vs cream to olivaceous in N. ampla and N. elegans), the presence of a sexual morph (absent in N. ampla) and the absence of aerial macroconidia (present in N. ampla; Sandoval-Denis & Crous 2018).
Neocosmospora haematococca (Berk. & Broome) Samuels et al., Mycologia 103: 1322. 2011
Basionym. Nectria haematococca Berk. & Broome, J. Linn. Soc., Bot. 14: 116. 1875.
Synonyms. Dialonectria haematococca (Berk. & Broome) Cooke, Grevillea 12: 110. 1884.
Cucurbitaria haematococca (Berk. & Broome) Kuntze, Revis. Gen. Pl. 3: 461. 1898.
Hypomyces haematococcus (Berk. & Broome) Wollenw., Angew. Bot. 8: 191. 1926.
Haematonectria haematococca (Berk. & Broome) Samuels & Nirenberg, Stud. Mycol. 42: 135. 1999.
Fusarium haematococcum Nalim et al., Mycologia 103: 1322. 2011.
?Nectria lanata Pat., Bull. Soc. Mycol. France 8: 52. 1892 (fide Samuels 1976).
?Nectria aurantiella Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 6: 287. 1898 (fide Samuels 1976).
?Nectria episphaerioides Penz. & Sacc., Malpighia 11: 511. 1898 (fide Samuels 1976).
?Nectria cinnabarina var. jaraguensis Höhn., Denkschr. Kaiserl. Akad. Wiss., Wien. Math.-Naturwiss. Kl. 83: 18. 1907 (fide Rossman et al. 1999).
?Nectria victoriae Henn., Ann. Mycol. 5: 81. 1907 (fide Samuels 1976).
?Nectria calonectricola Henn., Hedwigia 48: 105. 1908 (fide Rossman et al. 1999).
?Nectria citri Henn., Hedwigia 48: 104. 1908 (fide Samuels 1976).
?Nectria luteococcinea Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1. 118: 299. 1909 (fide Rossman et al. 1999).
?Nectria bainii var. hypoleuca Sacc., Nuovo Giorn. Bot. Ital. 23: 205. 1916 (fide Samuels 1976).
?[Nectria confluens Seaver, Sci. Surv. Porto Rico & Virgin Islands 8: 44. 1926 (Nom. illegit., Art. 53.1) (fide Rossman et al. 1999)].
?Nectria bogoriensis C. Bernard, Bull. Dépt. Agric. Indes Néerl. 11: 45. 1907 (fide Samuels 1976, Rossman et al. 1999).
Typus. Sri Lanka, Ceylon, Central Province, on bark, 1868, G.H.K. Thwaites no. 1104, (lectotype of Nectria haematococca K(M) 252877, designated by Samuels (1976)); Sabaragamua, Singaharaj Man and Biosphere Reserve, Morningside, vicinity Bungalow in forested slope, on dying tree, 10 Dec. 2002, G.J. Samuels, A. Nalim, N. Dayawansa, K. Poldmaa (epitype of Nectria haematococca BPI 871363, designated by Nalim et al. (2011), culture ex-epitype CBS 119600 = FRC S-1832).
Descriptions & Illustrations — Samuels (1976), Rossman et al. (1999), Samuels et al. (2006), Nalim et al. (2011).
Notes — Many of the synonyms listed in Samuels (1976) and Rossman et al. (1999) are here listed as doubtful. Neocosmospora haematococca was recently epitypified and confined to a discrete phylogenetic lineage (Nalim et al. 2011). Therefore, the list of synonyms certainly needs to be re-evaluated according to the wide genetic diversity derived from recent phylogenetic evidence, contrasting with the morphologically broad and polyphyletic traditional concept of Nec. haematococca.
Neocosmospora hengyangensis Z.Q. Zeng & W.Y. Zhuang, Phytotaxa 319: 179. 2017
Typus. China, Hunan, Hengyang, Gouloufeng, on twigs, 24 Oct. 2015, Z.Q. Zeng, X.C. Wang, K. Chen, Y.B. Zhang (holotype HMAS 254518, culture ex-type HMAS 254518).
Description & Illustration — Zeng & Zhuang (2017).
Notes — A recently described taxon and one of the five Neocosmospora sexual species so far known from China (Zeng & Zhuang 2017). Only DNA sequences were available to us for this species. Molecular data indicate that this is a well-resolved species, nested within a discrete lineage which includes closely related taxa with known sexual morphs, i.e., N. bataticola, N. brevicona, N. elegans and N. hengyangensis. Morphological features of the asexual morph, as described in the protologue of N. hengyangensis, clearly differentiate this species from its closest phylogenetic relative N. bataticola, the former species lacking aerial macroconidia (present in N. bataticola) and possessing markedly smaller sporodochial conidia (4–6-septate, up to 60 μm long vs up to 8-septate and 101.5 μm long in N. bataticola).
Neocosmospora hypothenemi Sand.-Den. & Crous, sp. nov. — MycoBank MB831183; Fig. 18
Fig. 18.
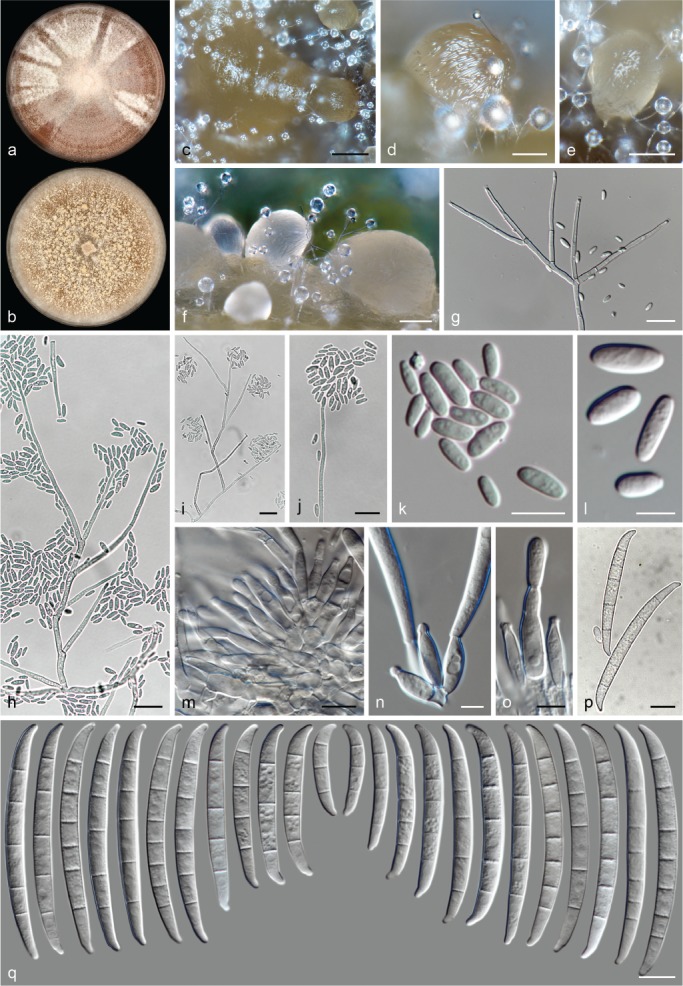
Neocosmospora hypothenemi (ex-type culture CBS 145464). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g–j = aerial conidiophores; k–l. aerial conidia; m–o. sporodochial conidia and phialides; p–q. sporodochial conidia. — Scale bars: c = 100 μm; d–f = 50 μm; g–i = 20 μm; l–m = 5 μm; all others = 10 μm.
Etymology. Name refers to the host genus Hypothenemus (Coleoptera: Scolytidae) from which this fungus was isolated.
Typus. Benin, Niaouli, from adult Hypothenemus hampei, unknown date and collector (holotype CBS H-23982 designated here, culture ex-type CBS 145464 = NRRL 52782 = ARSEF 5878).
Conidiophores erect on substrate mycelium, highly abundant on aerial mycelium, straight or flexuous, smooth-walled or finely verruculose and thin-walled, rarely simple, often copiously branched sympodially and verticillately, bearing terminal, single monophialides; phialides subulate to subcylindrical, (35–)45.5–55.5(–60) × 2–3.5(–4) μm (av. 50.6 × 2.8 μm, n = 102), smooth- and thin-walled, conidiogenous loci with periclinal thickening and a short-, non-flared collarette; aerial conidia ellipsoidal to cylindrical, with or without a slightly protruding base, straight or discreetly curved, 0-septate, hyaline, smooth- and thin-walled, (5.5–)6.5–10(–13.5) × (2.5–)3–4.5(–5) μm (av. 8.2 × 3.6 μm, n = 96), clustering in false heads at tip of monophialides. Sporodochia cream, ochreous to pale citrine. Sporodochial conidiophores densely verticillately branched and packed, bearing terminal, single monophialides or whorls of up to four phialides; sporodochial phialides subcylindrical to ampulliform, (8.5–)11.5–16.5(–21) × (2.5–)3.5–4.5(–5.5) μm (av. 14.1 × 3.9 μm, n = 110), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and collarettes. Conidia in sporodochia, moderately dorsiventrally curved to almost straight, somewhat strongly curved at both ends and tapering toward base; apical cell elongated, of equal length to the adjacent cell, blunt with a rounded and curved apex; basal cell elongated and distinctly notched, often protuberant, (2–)3–6(–7)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 24 × 4.5 μm (n = 3); 3-septate conidia: (29–)33–44.5(–46) × 4.5–5 μm (av. 39.5 × 4.8 μm, n = 12); 4-septate conidia: (40–)43.5–53(–56.5) × (4–)4.5–5.5(–6) μm (av. 48.3 × 4.9 μm, n = 75); 5-septate conidia: (47.5–)55–63 (–66.5) × (4.5–)5–5.5 μm (av. 59 × 5.1 μm, n = 273); 6-septate conidia: (60–)61–64(–65) × (4.5–)5.5–6 μm (av. 62.6 × 5.2 μm, n = 33); overall: (24–)49–64(–66.5) × (4–)4.5–5.5(–6) μm (av. 56.4 × 5.1 μm, n = 399). Chlamydospores globose to subglobose, smooth- and thick-walled, 5.5–10 μm diam, terminal or intercalary, solitary or in short chains.
Colonies on PDA growing in dark with an average radial growth rate of 3.2–4.2 mm/d at 24 °C, reaching 45–63 mm diam in 7 d at 24 °C; white, pale luteous to saffron, flat, felty to granulose, with abundant white aerial mycelium in a radiate and concentric arrangement; margin entire. Reverse saffron, orange to rust, often with abundant scarlet to rust diffusible pigment. On OA incubated in dark covering an entire 90 mm Petri dish in 7 d at 24 °C; white to pale ochreous, flat, velvety to granulose; margin entire. Reverse pale luteous, buff to pale rust.
Additional material examined. Uganda, Chesogok, from adult H. hampei, unknown date and collector, CBS 145466 = NRRL 52783 = ARSEF 5879.
Notes — Previously known as phylogenetic species FSSC 38, N. hypothenemi is described associated with the coffee borer beetle or coffee berry borer (Hypothenemus hampei: Curculionidae: Scolytinae). It is phylogenetically distant and unrelated to the main cluster of arthropod symbiont species of the Ambrosia clade. Neocosmospora hypothenemi also retains the typically shaped falcate sporodochial conidia of Neocosmospora, contrasting with the irregular, asymmetrical-clavate conidia of the most important beetle-associated pathogens in the Ambrosia clade (Aoki et al. 2018). This is most likely a reflection of the non-symbiont nature of this species.
Morphologically, N. hypothenemi is different from its closest phylogenetic relatives, N. perseae and N. pseudoradicicola. Both N. hypothenemi and N. perseae exhibit red coloured colonies on PDA. However, in the former species these are saffron coloured instead of bright red as observed in N. perseae. In addition, N. hypothenemi produces highly branched aerial conidiophores and its sporodochial conidia are slender, relatively long (up to 66.5 μm long) with elongated apical and basal cells, contrasting with the simpler conidiophores of N. perseae and the latter’s shorter (up to 55.5 μm long) and more robust sporodochial conidia. Neocosmospora pseudoradicicola is morphologically differentiated by its short, less septate, curved and apically hooked conidia and the presence of a microcyclic conidiogenous cycle. The morphologically close, although genetically distinct species N. spathulata, is almost indistinguishable from N. hypothenemi (see notes under N. spathulata).
Neocosmospora illudens (Berk.) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015
Basionym. Nectria illudens Berk., Bot. Antarct. Voy. II (Fl. Nov.-Zeal.): 203. 1855.
Synonyms. Cucurbitaria illudens (Berk.) Kuntze, Revis. Gen. Pl. 3: 461. 1898.
Haematonectria illudens (Berk.) Samuels & Nirenberg, Stud. Mycol. 42: 136. 1999.
Fusarium illudens C. Booth, The Genus Fusarium: 54. 1971.
Typus. New Zealand, North Island, Bay Islands, on bark, J. Hooker (fide Samuels & Brayford 1994, not located).
Descriptions & Illustrations — Booth (1971), Gerlach & Nirenberg (1982), Samuels & Brayford (1994), Rossman et al. (1999).
Material examined. New Zealand, from Beilschmiedia tawa, unknown date or collector, NRRL 22090 = BBA 67606 = G.J.S 82-98.
Notes — This species is here represented by DNA sequences of a single isolate only, matching morphologically and geographically with the original description of the species. It is regarded as representative of N. illudens (Samuels & Brayford 1994, O’Donnell 2000, O’Donnell et al. 2008), although we could not confirm the existence of a type collection. Numerous specimens are housed at K marked as putative types; however, none match the collection data of the protologue. Similarly, Samuels & Brayford (1994) cited the holotype details but seemed unable to locate type material. Several specimens from New Zealand later collected by J.M. Dingley and G.J. Samuels are available in BPI and PDD, likely suitable to fix the application of the name if the holotype is confirmed lost.
The closely related species, N. plagianthi, clusters as a sister lineage to N. illudens and are the only known members of Neocosmospora Clade 1 (O’Donnell 2000). Both species are recognisable by several features of their sexual morphs and the absence of an asexual morph in N. plagianthi (Samuels & Brayford 1994, but see notes under N. plagianthi).
Descriptions of the asexual morph of N. illudens (as Fusarium illudens) by Booth (1971) and Samuels & Brayford (1994) indicate sporodochial conidia in the size and septation range of N. robusta and N. silvicola. Both these phylogenetically unrelated species nest within Clades 2 and 3 of Neocosmospora (sensu O’Donnell 2000), and can be distinguished by the absence of microconidia in N. robusta, while the aerial conidia in N. silvicola are much longer (up to 23.5 μm long vs up to 8 μm long in N. illudens; Booth 1971, Gerlach & Nirenberg 1982, Samuels & Brayford 1994).
Neocosmospora ipomoeae (Halst.) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015. — Fig. 19
Fig. 19.
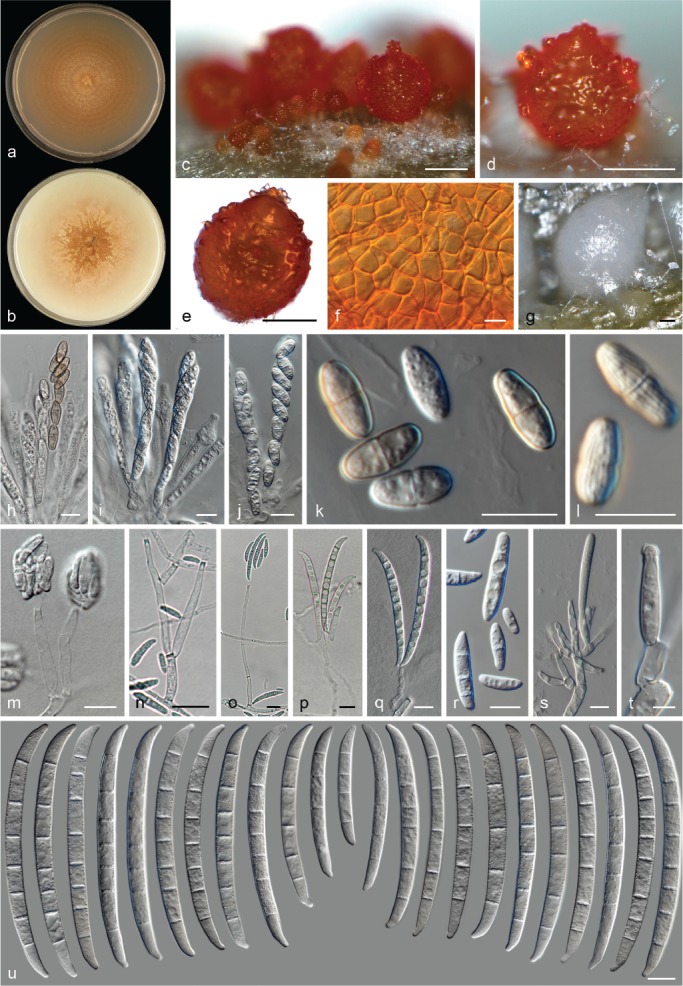
Neocosmospora ipomoeae. a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. perithecia; f. detail of peridium cells; g. sporodochia formed on the surface of carnation leaves; h–j. asci; k–l. ascospores; m–q. aerial conidiophores; r. aerial conidia; s–t. sporodochial conidiophores and phialides; u. sporodochial conidia. — Scale bars: c–e, g = 100 μm; all others = 10 μm.
Basionym. Nectria ipomoeae Halst., Rep. (Annual) New Jersey Agric. Exp. Sta. 12: 281. 1891.
Synonyms. Cucurbitaria ipomoeae (Halst.) Kuntze, Revis. Gen. Pl. 3: 461. 1898.
Creonectria ipomoeae (Halst.) Seaver, N. Amer. Fl. 3: 22. 1910.
Hypomyces ipomoeae (Halst.) Wollenw., Phytopathology 3: 34. 1913.
Haematonectria ipomoeae (Halst.) Samuels & Nirenberg, Stud. Mycol. 42: 136. 1999.
Nectria ipomoeae f. ipomoeae Halst., Rep. (Annual) New Jersey Agric. Exp. Sta. 12: 281. 1891.
Nectria ipomoeae var. ipomoeae Halst., Rep. (Annual) New Jersey Agric. Exp. Sta. 12: 281. 1891.
Hypomyces ipomoeae var. ipomoeae (Halst.) Wollenw., Phytopathology 3: 34. 1913.
Hypomyces ipomoeae var. major Wollenw., Fusaria Autographice Delineata 3: 826. 1930.
?Fusarium striatum Sherb., Cornell Univ. Agric. Exp. Sta. Mem. 6: 255. 1915 (fide Nirenberg & Brielmaier-Liebetanz 1996).
?Fusarium solani var. striatum (Sherb.) Wollenw., Z. Parasitenk. (Berlin) 3: 451. 1931 (fide Nirenberg & Brielmaier-Liebetanz 1996).
Typus. USA, New Jersey, Mickelton, on Solanum melongena, 8 July 1891, B.D. Halsted (holotype BPI 552416).
Perithecia orange to dark-red, globose to pyriform, superficial, solitary or gregarious, coarsely warted, glabrous; peridial wall composed of thick-walled cells of textura angularis. Asci subcylindrical to clavate, unitunicate, apex flat to rarely rounded, (63–)67.5–80.5(–89) × (8.5–)9–12(–14.5) μm (av. 74 × 4 μm, n = 142). Ascospores obliquely uniseriate or irregularly biseriate at apex of the asci, broadly ellipsoidal to ellipsoidal, 1-septate, (10–)11–13.5(–15) × 4.5–5.5(–6) μm (av. 12.2 × 5.3 μm, n = 202), pale yellow-brown to golden yellow, thick-walled, longitudinally finely striated, often constricted at septum.
Conidiophores erect or prostate from substrate and abundant on aerial mycelium straight or flexuous, smooth- and thin-walled, simple or branched several times irregularly or sympodially, bearing terminal and lateral, single monophialides; phialides subulate to subcylindrical, (16–)17.5–43.5(–67.5) × (2–)3–5 μm (av. 31.5 × 3.6 μm, n = 126), smooth- and thin-walled, conidiogenous loci with rather conspicuous periclinal thickening and a short, flared collarette; aerial conidia of two types: microconidia ellipsoidal, clavate to subcylindrical, straight or more often gently curved, 0–1(–2)-septate, hyaline, smooth- and thin-walled, (5.5–)9–16.9(–23.5) × (2.5–)3.5–5(–6) μm (av. 13.1 × 4 μm, n = 142), clustering in false heads at tip of monophialides; macroconidia falcate and multiseptate, gently dorsiventrally curved, base flattened, inconspicuously or distinctly notched, when notched almost indistinguishable in shape from sporodochial conidia, (1–)3–6-septate, smooth- and thick-walled; 1-septate conidia: (16.5–)19–23(–24.5) × 4–4.5 μm (av. 21.7 × 4.3 μm, n = 14); 2-septate conidia: (23–)24–28.5(–29.5) × 4.5–6 μm (av. 25.8 × 5.2 μm, n = 8); 3-septate conidia: (24.5–)28.5–40(–41.5) × 4–5.5(–6) μm (av. 34 × 4.7 μm, n = 50); 4-septate conidia: (39.5–)45–57.5 × 4.5–5.5(–6) μm (av. 51.3 × 5.1 μm, n = 60); 5-septate conidia: (59–)60–75.5(–80) × 5–6(–6.5) μm (av. 67.6 × 5.6 μm, n = 80); 6-septate conidia: (69.5–)70–77.5(–79) × (4.5–)5–5.5(–6) μm (av. 73.6 × 5.3 μm, n = 50); overall: (16.5–)36.5–73(–80) × (4–)4.5–6(–6.5) μm (av. 54.9 × 5.2 μm, n = 262). Sporodochia cream, pale green to olive-green. Sporodochial conidiophores sparingly branched, verticillately or irregularly; sporodochial phialides subcylindrical, subulate to somewhat doliiform, often slightly swollen near base, (13.5–)15–20(–22.5) × (3.5–)4–5.5 μm (av. 17.6 × 4.6 μm, n = 96), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared collarette. Sporodochial conidia moderately dorsiventrally curved with nearly parallel lines, narrowing gently toward base; apical cell often about equal length than adjacent cell, blunt to somewhat conical with curved apex; basal cell barely to distinctly notched, (2–)5–8(–9)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 28 × 4.5 μm (n = 2); 3-septate conidia: 49.5 × 5.5 μm (n = 2); 4-septate conidia: 43.5–50.5(–51.5) × 4.5–5.5 μm (av. 47.1 × 5.1 μm, n = 12); 5-septate conidia: (51.5–)56.5–77.5(–82.5) × (4.5–)5–6(–6.5) μm (av. 67 × 5.5 μm, n = 44); 6-septate conidia: (74–)76–86(–87.5) × (5.5–)6–7(–7.5) μm (av. 81.1 × 6.3 μm, n = 40); 7-septate conidia: (77–)81–90(–95.5) × (5.5–)6–7 μm (av. 85.5 × 6.3 μm, n = 128); 8-septate conidia: (80.5–)84.5–92.5(–94) × (5.5–)6–7 μm (av. 88.6 × 6.4 μm, n = 48); 9-septate conidia: 90.5 × 7 μm (n = 2); overall: (28–)66–93.5(–95.5) × (4.5–)5.5–7(–7.5) μm (av. 79.6 × 6.1 μm, n = 278). Chlamydospores abundant, globose to subglobose, smooth- or rough- and thick-walled, 5.5–10 μm diam, terminal or intercalary in hyphae or conidia, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 1.3–3.3 mm/d at 24 °C, reaching 20–46 mm diam in 7 d at 24 °C; white, pale luteous, saffron to sienna, flat, regular or irregular in shape, velvety to felty, with or without concentric rings of aerial mycelium; margin entire. Reverse white to pale straw. On OA incubated in dark reaching 66–78 mm diam in 7 d at 24 °C; white, cream to buff, flat, at first membranous turning velvety to floccose; margin entire. Reverse white, cream to brick or umber.
Additional materials examined. Netherlands, Ouderkerk aan de Amstel, glasshouse, from Rosa sp. dead parts, May 1997, H.A. v. Kesteren, CBS 833.97; unknown location, from Capsicum annuum, 25 May 1987, J.W. Veenbaas-Rijks, CBS 353.87 = NRRL 22657 = PD 87/236; from stem spot in Gerbera sp. besides Rhizoctonia solani, unknown date and collector, CBS 354.87 = NRRL 22238 = PD 87/238. – Panama, Panama Canal Zone, Fort Sherman, from unsterilized dewaxed cotton duck, 4 Aug. 1945, A.S. Barghoorn, CBS 225.58 = NRRL 22235 = BBA 64431 = QM 679. – Unknown, unknown substrate and date, C.D. Sherbakoff (BPI 453044).
Notes — This homothallic species, also known as phylogenetic species FSSC 21, produces abundant perithecia on diverse artificial media, irrespective of culture conditions. As frequently found with old species descriptions, the protologue of this species does not differentiate between aerial or sporodochial macroconidia. The original description measurements are close to those observed for the aerial macroconidia here. These include mostly 3–4-septate (predominantly 4-septate) conidia with an average size of 34 × 4.7 μm (34.7 × 4.6 μm in the protologue) for the 3-septate conidia.
Neocosmopora ipomoeae (as Hyp. ipomoeae) was historically connected to the asexual morph F. javanicum. However, F. javanicum was later thought to be not conspecific with N. ipomoeae on the basis of its pathogenicity to cucumbers (Nirenberg & Brielmaier-Liebetanz 1996). The same authors connected Hyp. ipomoeae to the asexual morph F. striatum, based on pathogenicity and morphological features. In this study, the morphology of the asexual morph of N. ipomoeae disagrees with the original concept of F. striatum. A photograph deposited by C.D. Sherbakoff illustrating conidia of F. striatum, is available at BPI (BPI 453044) although without a clear indication of the specimen’s original host. The structures depicted (sporodochial conidia and what seems to be a single microconidium) are largely reminiscent of Wollenweber’s illustration of authentic material of F. striatum (Wollenweber 1916, No 406; Fig. 20) though they deviate in size, shape and morphology of the apical and basal cell compared to the modern concepts of either F. javanicum or F. striatum (Gerlach & Nirenberg 1982, Nirenberg & Brielmaier-Liebetanz 1996), showing shorter and fewer-septate conidia. Therefore, the doubtful synonymy of F. striatum under H. ipomoeae is retained here.
Fig. 20.
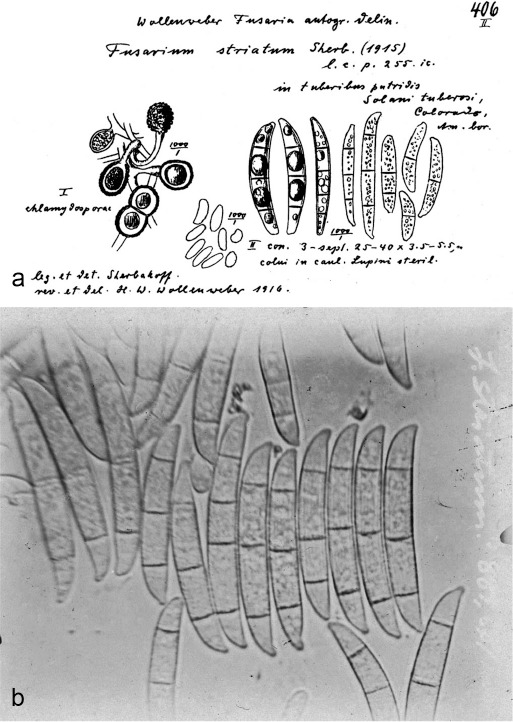
Fusarium striatum. a. Drawing of an authentic specimen by Wollenweber (1916); b. photograph by C.D. Sherbakoff depicting conidia (BPI 453044).
No authentic living biological material appears to exist for either F. javanicum or F. striatum. Three specimens identified as F. javanicum by C.D. Sherbakoff, W.H. Wollenweber and O.A. Reinking were retrieved from BPI (BPI 452275–452278) and DNA extraction was attempted. These specimens proved to be an assembly of different taxa. Specimen BPI 452275 (ATCC 22403), a dried culture on PDA from an undetermined substrate, exhibits discordant morphological features, while rDNA and tef1 sequences relate this fungus with Fusarium poae, hence not Neocosmospora (data not shown). Only tef1 sequences were obtained for BPI 452278, collected from dead fruits of Theobroma cacao in Cameroon. This single locus indicated that this fungus clusters within N. solani s.str., which agrees to some degree with its micromorphology. The third specimen, BPI 452276, from an undetermined substrate, although not in good condition, could represent F. javanicum, although it is morphologically different to the asexual morph of N. ipomoeae, having much shorter 2–5-septate conidia. However, genomic DNA extraction was not attempted from this specimen.
Neocosmospora keleraja Samuels et al., Mycologia 103: 1326. 2011
Synonym. Fusarium kelerajum Samuels et al., Mycologia 103: 1326. 2011.
Typus. Sri Lanka, North Central Province, Minneriya National Forest, c. 2 km from forest headquarters, mixed evergreen dry forest, on trunk of Yakuda marang, fallen but still alive, 14 Dec. 2002, G.J. Samuels, A. Nalim, N. Dayawansa & R. Wiejesendera (holotype BPI 871413, culture ex-type FRC S-1839 = G.J.S. 02-122).
Description & Illustration — Nalim et al. (2011).
Additional materials examined. Sri Lanka, North Central Province, vic. Giritale, Giritale Forest Training Centre, in low mixed evergreen forest, semi-arid, on small branch of standing dead small tree, 13 Dec. 2002, G.J. Samuels, A. Nalim, N. Dayawansa, K. Poldmaa, CBS 125720 = FRC S-1837 = G.J.S. 02-114 (culture ex-paratype), CBS 125722 = FRC S-1836 = G.J.S. 02-113 (culture ex-paratype).
Notes — Neocosmospora keleraja is similar to N. mahasenii but can be distinguished by shorter 4-septate conidia and longer ascospores. The former species is also characterised by tall aerial conidiophores, often coronated by a branched apical penicillus of long phialides (Nalim et al. 2011). This species is also similar to N. acutispora and N. robusta. However, N. keleraja can be distinguished by the frequent septation of its sporodochial conidia (up to 9-septate vs up to 7-septate in N. acustispora and N. robusta), which are somewhat wider, with relatively short and hooked apical cells, than the longer and tapered apical cells of N. acutispora and the blunt apical cells of N. robusta. The last two species also differ from N. keleraja in their biogeography and host associations: N. acutispora is known from Coffea arabica in Guatemala and N. robusta from bark in Venezuela.
Neocosmospora keratoplastica (Geiser et al.) Sand.-Den. & Crous, Persoonia 41: 120. 2017 (2018)
Basionym. Fusarium keratoplasticum Geiser et al., Fungal Genet. Biol. 53: 68. 2013.
Synonyms. (Cephalosporium keratoplasticum T. Morik., Mycopathologia 2: 66. 1939 (Nom. inval., Art. 39.1)).
(Hyalopus keratoplasticum T. Morik. ex M.A.J. Barbosa, Subsidios Para o Estudo Parasitologico do Genero Hyalopus Corda, 1838: 19. 1941 (Nom. inval., Art. 39.1)).
Typus. USA, Virginia, Winchester, from indoor plumbing, June 2009, unknown collector (holotype FRC S-2477, culture ex-type FRC S-2477).
Descriptions & Illustrations — Short et al. (2013), Sandoval-Denis & Crous (2018).
Additional materials examined. Belgium, Heverlee, greenhouse humic soil, B.G. Desai, CBS 144389 = MUCL 18301. – Japan, Nagao Institute, from human eye, Harada, CBS 490.63 = NRRL 22661 (culture ex-type of C. keratoplasticum).
Notes — Neocosmospora keratoplastica is one of the most important human pathogenic species in the genus. It is often associated with human corneal infections, but also known to infect other superficial locations and to cause deep-seated infections. This species is also known to attack various animal hosts, particularly in aquatic environments (O’Donnell et al. 2008, 2016, Short et al. 2013, Sarmiento-Ramírez et al. 2014, 2017, Fernando et al. 2015).
Known as phylogenetic species FSSC 2, this taxon was recently recombined in Neocosmospora with an erroneous type citation for the basionym (Sandoval-Denis & Crous 2018), which is corrected here.
Morphologically, N. keratoplastica is distinguishable from other clinically relevant species by the production of small-sized sporodochial conidia, overlapping in conidial size and septation with the non-human-pathogenic species N. brevis, N. diminuta and the unnamed phylogenetic species FSSC 12. However, N. keratoplatica can be distinguished from N. brevis and N. diminuta based on the falcate multiseptate conidia formed on the aerial conidiophores only, whereas N. keratoplastica produces true sporodochia and sporodochial conidia. Neocosmospora keratoplastica can be distinguished from FSSC 12 based on the production of more cylindrical sporodochial conidia, with rounded apical cells and barely notched foot cells, contrasting with the more wedge-shaped sporodochial conidia of FSSC 12 that have more or less pyramidal apical cells and commonly protruding foot cells (Sandoval-Denis & Crous 2018).
Neocosmospora kuroshio F. Na et al. ex Sand.-Den. & Crous, sp. nov. — MycoBank MB831184
Synonym. (Fusarium kuroshium F. Na et al., Plant Disease 102: 1159. 2018 (Nom. inval., Art. 40.7)).
Etymology. Derived from the common name of the ambrosia beetle vector, Kuroshio Shot Hole Borer (from Na et al. 2018).
Typus. USA, California, San Diego County, El Cajon, surface of Euwallacea sp. galleries in infested Platanus racemosa, 14 Dec. 2013, A. Eskalen (holotype BPI 910340 designated here, culture ex-type CBS 142642, isotype UCR 3641).
Description & Illustration — Na et al. (2018).
Notes — Fusarium kuroshium was invalidly published as the causal agent responsible for dieback of numerous tree species (Na et al. 2018). The name was invalid since two specimens on two different collections were cited as holotype (Art. 40.7). The phylogenetic boundaries of the clade representing N. kuroshio (as phylogenetic species AF-12) have been well-studied and delimited (O’Donnell et al. 2015, 2016); while morphological and ecological information attributed to this taxon clearly indicate that it is a true species. Therefore, the name is validated here based on the original published data and its ex-type culture CBS 142642 (Na et al. 2018) as a new species in Neocosmospora.
Neocosmospora kurunegalensis Samuels et al., Mycologia 103: 1324. 2011
Synonym. Fusarium kurunegalense Samuels et al., Mycologia 103: 1323. 2011.
Typus. Sri Lanka, Wagamba Province, Kurunegala. private garden, on recently cut tree, 12 Dec. 2002, G.J. Samuels, A. Nalim, K. Poldmaa (holotype BPI 871391, culture ex-type CBS 119599 = G.J.S. 02-94).
Description & Illustration — Nalim et al. (2011).
Additional material examined. Germany, Göttingen, from Phaseolus vulgaris, 30 Aug. 1983, H. Nirenberg, CBS 835.85 = NRRL 22662 = BBA 64384.
Notes — Nalim et al. (2011) indicated that this species is recognisable by the formation of ‘intercalary phialides’ on the aerial conidiophores, recorded as proliferating conidiophores on which a new axis develops just below the previous conidiogenous locus. Thus, giving the appearance of intercalary phialides, contrasting with the more common type of proliferating conidiophores on which a new axis develops through the previous conidiogenous opening. This seems to be a rather uncommon feature, also observed here, on the closely related species N. cryptoseptata, N. nirenbergiana, and more rarely in N. samuelsii. These species all belong to Clade 2 of Neocosmospora (O’Donnell 2000). An exception to this clade is N. diminuta, a member of Clade 3, for which this proliferating pattern was also observed.
Typification details on the original publication cite BPI 872931 as holotype, which is incorrect. The correct holotype specimen is BPI 871391 (as stated in BPI: ‘typographic error in protologue cites BPI 872931’). Strain CBS 835.85 is tentatively included in N. kurunegalensis based on phylogenetic data, although with a markedly different host and geographic origin.
Neocosmospora lichenicola (C. Massal.) Sand.-Den. & Crous, Persoonia 41: 120. 2017 (2018) — Fig. 21
Fig. 21.
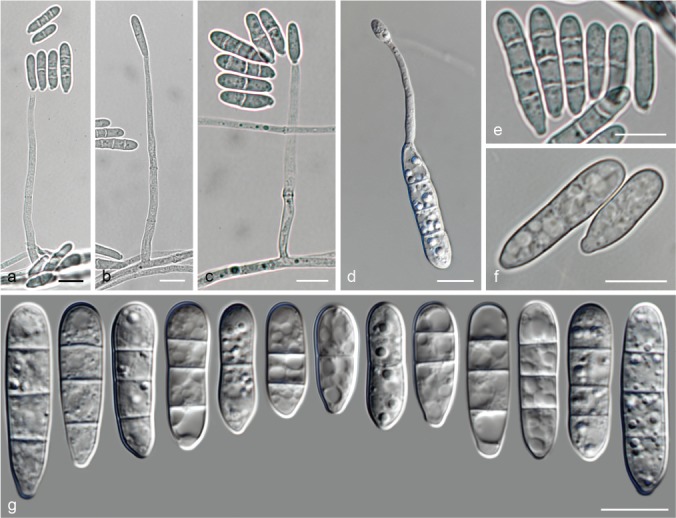
Neocosmospora lichenicola (ex-epitype culture CBS 623.92). a–c. conidiophores; d. microcyclic conidiogenesis; e–g. conidia. — Scale bars = 10 μm.
Basionym. Fusarium lichenicola C. Massal., Ann. Mycol. 1: 223. 1903.
Synonyms. Bactridium lichenicolum (C. Massal.) Wollenw., Fusaria Autographice Delineata 1: 456. 1916.
Cylindrocarpon lichenicola (C. Massal.) D. Hawksw., Bull. Brit. Mus. (Nat. Hist.), Bot. 6: 273. 1979.
Monacrosporium tedeschii A. Agostini, Atti Ist. Bot. Lab. Crittog. Univ. Pavia 4: 195. 1933.
Euricoa dominguiesii Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 152. 1955.
Hyaloflorea ramosa Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 155. 1955.
Neocosmospora ramosa (Bat. & H. Maia) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015.
Mastigosporium heterosporum R.H. Petersen, Mycologia 51: 729. 1959.
Typus. Italy, Verona, Tregnago, on Polycauliona candelaria (as Candelaria concolor), Nov. 1902, C. Massalongo (holotype PAD). – Germany, Göttingen, necrotic wounds of patient under chemotherapy, unknown date, Staatliches Medizinaluntersuchungsamt Göttingen (epitype of Fusarium lichenicola CBS H-23983 designated here, MBT387219, culture ex-epitype CBS 623.92).
Descriptions & Illustrations — Wollenweber (1916), Petersen (1959), Hawksworth (1979), Summerbell & Schroers (2002).
Additional materials examined. Brazil, Bahía, from air, 2 Feb. 1955, E.A.F. da Matta, CBS 522.63 = MUCL 8049 = IMUR 320 (culture ex-type of E. dominguesii); Pernambuco, Recife, Dois Irmãos, Universidade Rural, from soil, 16 Mar. 1961, unknown collector, CBS 166.67 = IMUR 1797 (culture ex-type of M. pernambucensis); Salvador, from air, 2 Apr. 1955, E.A.F. de Matta, CBS 509.63 = MUCL 8050 = IMUR 410 (culture ex-type of H. ramosa). – Japan, from human eye, unknown date and collector, CBS 483.96 = IFO 30561 = NBRC 30561. – Somalia, Mogadishu, human scabies-like skin condition, unknown date, A. Agostini, CBS 279.34 (culture ex-type of M. tedeschii). – Tahiti, French Polynesia, soil from damp river bed, unknown date and collector CBS 279.59 = ATCC 13427 (culture ex-type of M. heterosporum).
Notes — Also known as phylogenetic species FSSC 16, this taxon was recently recombined in Neocosmospora, based on F. lichenicola, an infrequent human pathogen. Although reported from superficial and invasive human infections, this fungus is also able to cause disease in a diverse group of plant hosts (Summerbell & Schroers 2002, Sandoval-Denis et al. 2018).
Morphologically, this species is unique in the genus Neocosmospora based on the ellipsoidal to cylindrical, 0–3-septate aerial conidia with the basal cells displaying a short, truncate base instead of the typical foot-shaped cell common among members of this genus. However, a phylogenetic study that included several strains of C. lichenicola indicated that this species belonged to Neocosmospora (as the F. solani species complex; Summerbell & Schroers 2002).
An epitype is designated here to stabilise the species and fix the application of the name to this phylogenetic clade, largely known to represent N. lichenicola. Although CBS 623.92 is not from the same host or country, but from human clinical origin, N. lichenicola is a recognised human pathogenic species. The selected epitype is in excellent morphological condition and represents the morphological concept of the species.
Neocosmospora liriodendri Sand.-Den. & Crous, sp. nov. — MycoBank MB831185; Fig. 22
Fig. 22.
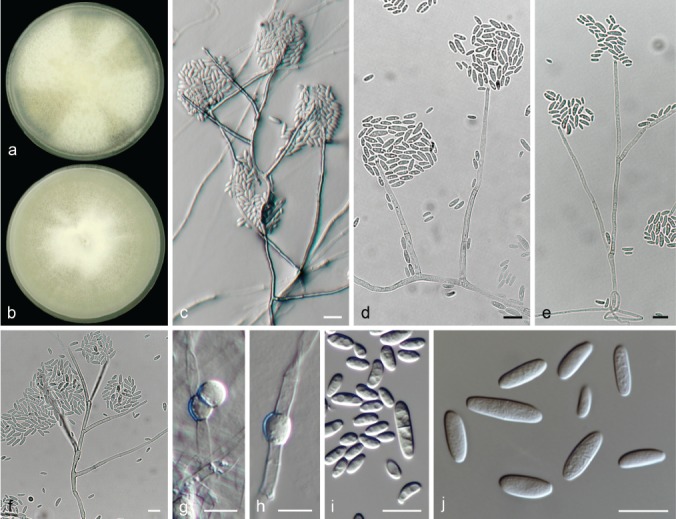
Neocosmospora liriodendri (ex-type culture CBS 117481). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. conidiophores; g–h. chlamydospores; i–j. conidia. — Scale bars = 10 μm.
Etymology. Name refers to the host genus Liriodendron from which this fungus was isolated.
Typus. USA, Maryland, from Liriodendron tulipifera, unknown date and collector (holotype CBS H-23984 designated here, culture ex-type CBS 117481 = NRRL 22389 = BBA 67587 = G.J.S 91-148).
Conidiophores prostrate or erect, abundant on substrate and aerial mycelium, straight, smooth- and thin-walled, simple or sparingly verticillately and laterally branched, bearing terminal, single monophialides; phialides subulate to subcylindrical, (40–)48–66.5(–71.5) × (2.5–)3–4(–5) μm (av. 57.3 × 3.4 μm, n = 48), smooth- and thin-walled, conidiogenous loci with or without inconspicuous periclinal thickening and a short, somewhat flared collarette; conidia obovoidal, subcylindrical to clavate, straight or gently curved 0(–1)-septate, hyaline, smooth- and thin-walled, (4.5–)5.5–11(–16) × (2–)2.5–4(–5) μm (av. 8.2 × 3.2 μm, n = 82), crowding in false heads at tip of monophialides. Sporodochia not observed. Chlamydospores globose to subglobose, smooth-walled, (4.5–)6–10.5 μm diam; terminal or intercalary, solitary or in short chains.
Colonies on PDA growing in dark with an average radial growth rate of 2.9–3.2 mm/d at 24 °C, reaching 40–45 mm diam in 7 d at 24 °C; white, straw to primrose; with thick, white radial patches of dense, white aerial mycelium; flat to slightly raised, velvety to cottony; margin entire. Reverse straw, buff to honey. On OA incubated in dark reaching 37–43 mm diam in 7 d at 24 °C; white, flat, velvety to granular with inconspicuous concentric rings of aerial mycelium; margin entire. Reverse straw to buff.
Notes — Neocosmospora liriodendri is a well-defined phylogenetic species, previously known as FSSC 24 (O’Donnell et al. 2008). It is only known from a single haplotype. Morphologically, it resembles N. parceramosa, both species producing much similar conidia formed only from relatively long phialides on simple aerial conidiophores, and showing comparable macroscopic features. Nevertheless, N. liriodendri differs by commonly forming sparingly branched conidiophores, a reduced growth rate on artificial media (2.9–3.2 vs 3.2–3.5 mm/d in N. parceramosa), and larger, smooth chlamydospores (up to 10.5 vs up to 8.5 μm diam, smooth to tuberculate in N. parceramosa).
Neocosmospora longissima Sand.-Den. & Crous, sp. nov. — MycoBank MB831186; Fig. 23
Fig. 23.
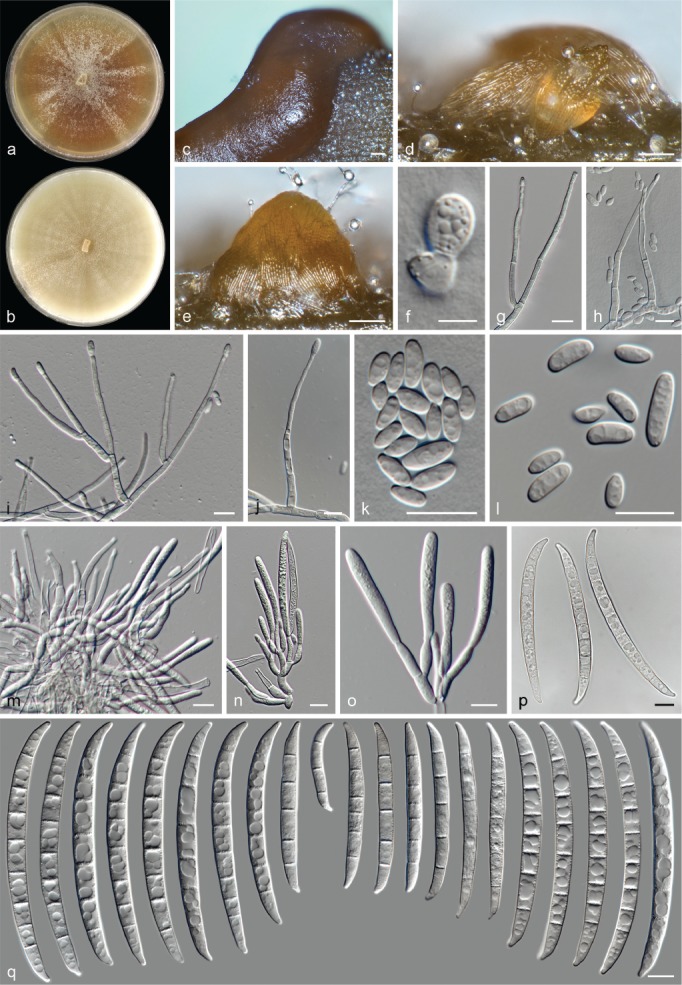
Neocosmospora longissima (ex-type culture CBS 126407). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f. chlamydospore; g–j. aerial conidiophores; k–l. aerial conidia; m–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–e = 100 μm; f = 5 μm; all others = 10 μm.
Etymology. From Latin longissimus meaning ‘longest, very long’; referring to its large sporodochial conidia.
Typus. New Zealand, Russell State Forest, Ngaiotonga Scenic Reserve, from tree bark, unknown date, G.J. Samuels, L.M. Kohn, D. Brown & E. Brown (holotype CBS H-23985 designated here, culture ex-type CBS 126407 = G.J.S. 85-72).
Conidiophores erect or prostrate on substrate, abundant on aerial mycelium, straight, smooth- and thin-walled, simple or commonly irregularly or verticillately branched several times, bearing terminal, single monophialides; phialides subulate to subcylindrical, (32–)37–53.5(–69) × (2–)2.5–3.5(–4) μm (av. 45.2 × 3 μm, n = 82), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, collarettes absent; aerial conidia obovate, ellipsoidal or reniform, 0-septate, hyaline, smooth- and thin-walled, (4.5–)5–9(–13) × (2–)2.5–4(–5.5) μm (av. 7.1 × 3.2 μm, n = 108), clustering in false heads at tip of monophialides. Sporodochia at first cream, becoming golden brown and quickly clustering into pionnotes. Sporodochial conidiophores densely verticillately branched; sporodochial phialides subcylindrical, ampulliform to doliiform, (9–)12–16.5(–21) × 3–4.5(–5.5) μm (av. 14.2 × 3.9 μm, n = 112), smooth- and thin-walled, conidiogenous loci lacking periclinal thickening or collarettes. Sporodochial conidia mostly wedge-shaped with marked dorsal curvature, rarely almost straight, tapering toward both ends and abruptly toward apex; apical cell often about equal length or longer than adjacent cell, conical with curved, somewhat elongate and rounded apex; basal cell barely to distinctly notched, straight, (3–)5–8-septate, hyaline, smooth- and thick-walled; 3-septate conidia: (34–)46–86.5(–79) × (4.5–)5–6 μm (av. 66.4 × 5.5 μm, n = 16); 5-septate conidia: (50.5–)56–71(–76) × 5–6(–6.5) μm (av. 63.5 × 5.7 μm, n = 60); 6-septate conidia: (61–)63.5–79.5(–91) × 5–6.5(–7) μm (av. 70 × 5.9 μm, n = 28); 7-septate conidia: (77–)80.5–95.5(–98) × (5–)6–8 μm (av. 88 × 6.9 μm, n = 36); 8-septate conidia: (86.5–)89.5–98.5(–99) × (6–)6.5–8 μm (av. 94.1 × 7.2 μm, n = 48); overall: (34–)61.5–93(–99) × (4.5–)5.5–7.5(–8) μm (av. 77.2 × 6.3 μm, n = 188). Chlamydospores abundant, globose to obpyriform, smooth- and thick-walled, 6.5–8.5(–9) μm diam, terminal, intercalary or lateral in hyphae, solitary or in groups.
Colonies on PDA growing in dark with an average radial growth rate of 6–8 mm/d at 24 °C, filling a 90 mm diam Petri dish in 7 d at 24 °C; sienna, umber to rust, with radial patches of white to cream aerial mycelia, flat, velvety to felty; margin entire. Reverse ochreous to umber. On OA incubated in dark filling a 90 mm diam Petri dish in 7 d at 24 °C; buff with radial patches of short, white aerial mycelium, flat, velvety to granulose; margin entire. Reverse buff.
Notes — Neocosmospora longissima is rather unique in the genus Neocosmospora based on the remarkably long, typically acute and tapered, almost pointy sporodochial conidia produced. Morphologically, the most similar species to N. longissima include N. bataticola and N. macrospora. However, N. longissima is clearly distinct based on conidial shape and other micromorphological and cultural characteristics. Macroscopically, N. longissima exhibits sienna to rust coloured colonies, in contrast to the intense orange or red coloured colonies of N. bataticola. Furthermore, N. longissima lacks aerial macroconidia, which N. bataticola readily produces in culture. Neocosmospora macrospora, in comparison with N. longissima, produces up to 9-septate sporodochial conidia, which are slightly shorter and slenderer (up to 90 μm long and 6.7 μm wide vs up to 99 μm long and 8 μm wide in N. longissima), with inconspicuously tapered ends.
Neocosmospora macrospora Sand.-Den. et al., Persoonia 40: 21. (2017) 2018
Typus. Italy, Sicily, Catania, Guardia, from Citrus sinensis crown, 9 Mar. 2015, V. Guarnaccia (holotype CBS H-23023, culture ex-type CBS 142424 = CPC 28191).
Description & Illustration — Sandoval-Denis et al. (2018).
Additional materials examined. Italy, Sicily, Catania, Guardia, from Citrus sinensis crown, 9 Mar. 2015, V. Guarnaccia, CPC 28192, CPC 28193.
Notes — This species was recently described from Citrus trees in Sicily, Italy, that were showing symptoms of dry root rot. The isolates were not proven to be the etiologic agent of the rot (Sandoval-Denis et al. 2018). The study authors provided a brief discussion on the similarity between N. macrospora and the traditional morphological concepts of F. eumartii and F. ensiforme. However, these discordant species concepts are shown here to be erroneous. Neocosmospora macrospora is morphologically close to N. longissima in terms of conidial size, although the two species are phylogenetically unrelated and morphologically distinct (see notes under N. longissima).
Neocosmospora mahasenii Samuels et al., Mycologia 103: 1325. 2011
Synonym. Fusarium mahasenii Samuels et al., Mycologia 103: 1325. 2011.
Typus. Sri Lanka, North Central Province, vic. Giritale. Giritale Forest Training Center, on small branch of live tree, 13 Dec. 2002, G.J. Samuels, A. Nalim, N. Dayawansa & K. Poldmaa (holotype BPI 871393, culture ex-type CBS 119594 = FRC S-1845 = G.J.S. 02-105).
Description & Illustration — Nalim et al. (2011).
Notes — Neocosmospora mahasenii is known from living and dead wood in Sri Lanka, and it clusters in Clade 2 of Neocosmospora (O’Donnell 2000, Nalim et al. 2011). According to Nalim et al. (2011), N. mahasenii is morphologically similar to N. rectiphora, another species known from dead plant material in South Asia. However, N. rectiphora is phylogenetically distant and clusters in a monotypic lineage, in the paraphyletic Clade 2 (O’Donnell 2000). Neocosmospora mahasenii is distinct from N. rectiphora in the production of a blue pigment in mature sporodochia, slower growth rates on PDA and shorter ascospores (Nalim et al. 2011).
Neocosmospora martii (Appel & Wollenw.) Sand.-Den. & Crous, comb. nov. — MycoBank MB831187; Fig. 24
Fig. 24.

Neocosmospora martii (lectotype of Fusarium martii BPI 452385). a. Original illustration by Wollenweber (1916); b–c. herbarium specimen; d–f. sporodochia, g. aerial microconidia; h. aerial macroconidia; i. sporodochial conidiophore; j–k. sporodochial conidia. — Scale bars: c = 1 cm; d = 2 mm; e–f = 100 μm; all others = 10 μm.
Basionym. Fusarium martii Appel & Wollenw., Arbeiten Kaiserl. Biol. Anst. Ld.- u. Forstw. 8: 83. 1910.
Synonyms. Fusarium solani var. martii (Appel & Wollenw.) Wollenw., Fusaria Autographice Delineata 3: 1034. 1930.
Neocosmospora croci Guarnaccia et al., Persoonia 40: 17 (2017) 2018.
Typus. Germany, Berlin, on rotten tuber of Solanum tuberosum, Sept. 1909, unknown collector (lectotype of Fusarium martii BPI 452385 here designated, MBT387222); from S. tuberosum cultivar Maritta, unknown date and collector (epitype of F. martii CBS H-23986 designated here, MBT387223; culture ex-epitype CBS 115659 = FRC S-0679 = MRC 2198).
Descriptions & Illustrations — Appel & Wollenweber (1910), Reinking & Wollenweber (1927), Wollenweber (1931, 1935), Sandoval-Denis et al. (2018, as N. croci).
Additional materials examined. Germany, Berlin, dried cultivated isolate from Solanum tuberosum, 1909 (on culture 1909/1910), H.W. Wollenweber, BPI 452384. – Italy, Sicily, Catania, Paternó, Citrus sinensis crown, 9 Mar. 2015, V. Guarnaccia, CBS 142423 = CPC 27186 (culture ex-type of N. croci); Sicily, Catania, Paternó, C. sinensis crown, 9 Mar. 2015, V. Guarnaccia, CPC 27187. – Netherlands, Amsterdam, North Holland, from Pisum sativum stem, 1910, W.C. Scholten (presumptive collector), BPI 452383; unknown location, from S. tuberosum, 8 Mar. 1911, J. Westerdijk (presumptive collector), BPI 452379. – USA, Nebraska, Buffalo County, Kearney landfill, soil, unknown date and collector, CBS 127135 = RMF 7653.
Notes — Material of Fusarium martii as well as later collections of the same fungus from different hosts were received from BPI, all authenticated by H.W. Wollenweber. Significant uncertainty exists regarding the nomenclatural status of the original material from that author, who often proposed new taxa without designating type collections (Stevenson 1971). Although unambiguously linked to the original publication, the specimens treated here are considered as authentic material rather than as types. To stabilize the use of the name, a lectotype is selected here for F. martii, while an epitype is also designated in order to assure access to DNA data for future studies. The selected lectotype (BPI 452385) undoubtedly represents original material for the species, matching with the specimen illustrated as specimen originale in ‘Fusaria Autographice Delineata No 413’, reproduced in Fig. 24. All BPI specimens examined were in excellent condition; micro-morphological characteristics are clearly recognisable and match the original concept of the species as well as the morphological characteristics of modern isolates included in this clade. Moreover, ITS, LSU and tef1 DNA barcodes were obtained for four specimens (BPI 452379, 452383–452385) and analyses of these barcodes demonstrated that these collections all belong to the same taxon.
Neocosmospora croci, a species recently described from Citrus in Sicily, Italy (Sandoval-Denis et al. 2018) is here reduced to synonymy under N. martii. The morphological and ecological characteristic of N. croci match with those reported for F. martii (Appel & Wollenweber 1910, Sherbakoff 1915, Wollenweber 1931); however, the uncertain application of the latter taxon’s name prevented the two species from being properly compared.
Fusarium martii was originally described as having a blue colouration (Appel & Wollenweber 1910, Sherbakoff 1915), which was not observed here on synthetic media. However, a feature quite evident from the original specimens of F. martii is the red pigmentation of the fungal material. This character also matched with our morphological observations and with the characteristics described for N. croci (Sandoval-Denis et al. 2018).
Neocosmospora metavorans (Al-Hatmi et al.) Sand.-Den. & Crous, Persoonia 41: 121. 2018
Basionym. Fusarium metavorans Al-Hatmi et al., Med. Mycol. 56: S147. 2018.
Typus. Greece, Athens, from human pleural effusion, 2013, M. Drogari (holotype CBS 135789, maintained as metabolically inactive culture, culture ex-type CBS 135789).
Descriptions & Illustrations — Al-Hatmi et al. (2018), Sandoval-Denis et al. (2018).
Additional materials examined. Italy, from Malus sylvestris, unknown date and collector, CBS 233.36 = NRRL 22654. – Spain, Reus, human foot, unknown date, F. Ballester, CBS 143219 = NRRL 46708 = FMR 8634; unknown location, human corneal ulcer, unknown date and collector, CBS 143194 = IMI 226114 = NRRL 22782. – Turkey, from human, unknown date and collector, CBS 143215 = NRRL 37640 = UTHSC R-3564. – USA, Illinois, from human, unknown date, P. Kammeyer, CBS 143218 = NRRL 46237; Maryland, from human eye, unknown date and collector, CBS 130400 = NRRL 43489 = CDC 2006743456; human toenail cancer, unknown date and collector, CBS 143210 = NRRL 32785 = FRC S-1123; Michigan, from human, unknown date, M. Brandt, CBS 143216 = NRRL 43717; New England, from human bone, unknown date, A. Fothergill, CBS 143202 = NRRL 28542 = UTHSC 98-1246; San Francisco, human eye, unknown date and collector, CBS 143195 = NRRL 22792 = IMI 153617; Texas, human eye, unknown date and collector, CBS 143213 = NRRL 32849 = FRC S-1355 = UTHSC 95-2552; human foot, unknown date and collector, NRRL 28553 = UTHSC 97-2574; unknown location, from human, unknown date and collector, CBS 143198 = NRRL 28016 = CDC B-5779; CBS 143199 = NRRL 28017 = CDC B-5780; CBS 143200 = NRRL 28018 = CDC B-5781; CBS 143201 = NRRL 28019 = CDC B-5782.
Notes — Neocosmospora metavorans is a species mostly known from human clinical samples, but can also be found on other animals and less frequently on plant hosts (O’Donnell et al. 2008, Al-Hatmi et al. 2018, Sandoval-Denis & Crous 2018). Previously known as phylogenetic species FSSC 6, N. metavorans is one of the most relevant species in medical mycology, associated with infections ranging from being superficial to disseminated (O’Donnell et al. 2008). The clade representing N. metavorans displays significant genetic variation, with a complex internal phylogenetic structure, supported by significant evidence of sexual recombination (Φw = 0.001, Fig. 2).
Among the clinically relevant species of Neocosmospora, N. metavorans is morphologically reminiscent of N. solani and N. suttoniana, but distinct based on the wider sporodochial conidia with conspicuous foot cells (Sandoval-Denis & Crous 2018). Sporodochial conidia of N. metavorans are similar in size and septation to those of N. petroliphila and N. tonkinensis. However, the former taxon can be distinguished by its conidial shape, with blunt, short apical cells and protuberant foot-cells, vs the elongated apical cells and barely notched foot cells of N. petroliphila, and by short ellipsoidal aerial conidia, vs the elongate clavate to ellipsoidal, multiseptate aerial conidia of N. tonkinensis.
Neocosmospora mori Sand.-Den. & Crous, sp. nov. — MycoBank MB831188; Fig. 25
Fig. 25.
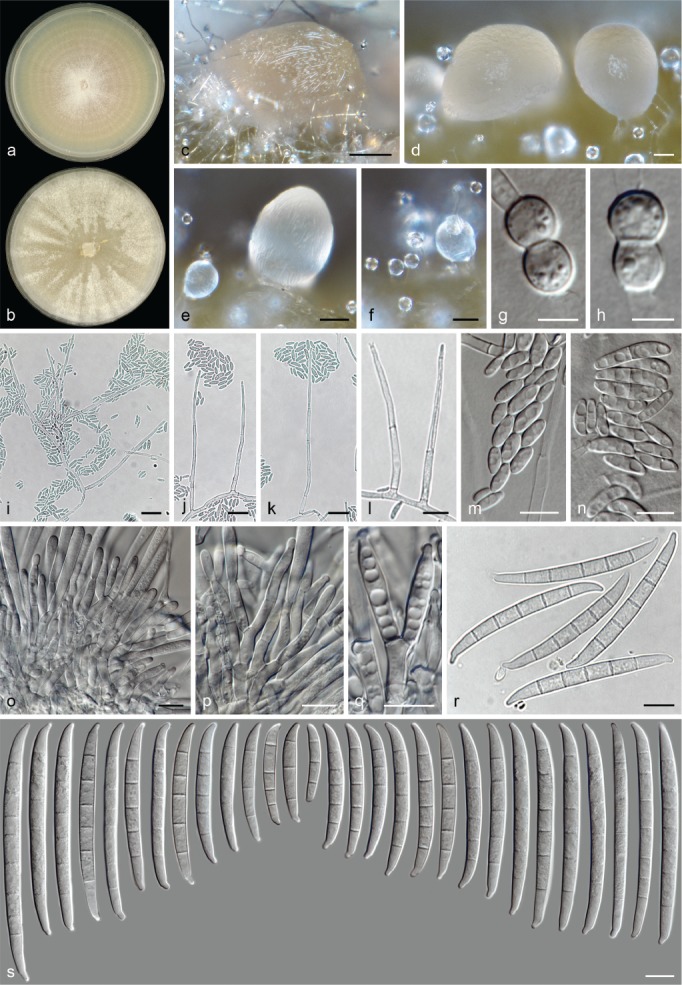
Neocosmospora mori (ex-type culture CBS 145467). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g–h. chlamydospores; i–l. aerial conidiophores; m–n. aerial conidia; o–q. sporodochial conidiophores and phialides; r–s. sporodochial conidiophores. — Scale bars: c–f = 100 μm; g–h = 5 μm; i–k = 20 μm; all others = 10 μm.
Synonyms. (Fusarium solani f. mori Sawada, Special Publication College of Agriculture, National Taiwan University 8: 222. 1959 (Nom. inval., Art. 39.1)).
(Hypomyces solani f. mori Y. Sakurai & Matuo, Ann. Phytophathol. Soc. Japan 24: 222. 1959 (Nom. inval., Art. 39.1)).
(Fusarium solani f. mori Y. Sakurai & Matuo, Ann. Phytopathol. Soc. Japan 24: 222. 1959 (Nom. inval., Art. 39.1)).
Etymology. Name refers to the host genus Morus from which this fungus was isolated.
Typus. Japan, Miyazaki, from Morus alba twig, unknown date and collector (holotype CBS H-23987 designated here, culture ex-type CBS 145467 = NRRL 22230 = MAFF 238539 = ATCC 44934).
Conidiophores erect on substrate mycelium or produced laterally on aerial hyphae, straight, smooth- and thin-walled, commonly unbranched or sparingly irregularly or dichotomously branched, bearing terminal, single monophialides; phialides subulate to acicular, (33–)38–54.5(–65) × 2–3.5 μm (av. 46.4 × 2.7 μm, n = 74), smooth- and thin-walled, conidiogenous loci with rather conspicuous periclinal thickening and a short, non-flared collarette; aerial conidia obovoidal, ellipsoidal to clavate, straight or more often slightly curved, 0(–1–2)-septate, hyaline, smooth- and thin-walled, (5–)6.5–15(–24) × (2–)3–4.5(–6) μm (av. 10.6 × 3.7 μm, n = 117), clustering in false heads at tip of monophialides. Sporodochia cream, pale ochreous to pale olivaceous. Sporodochial conidiophores densely branched, dichotomously, verticillately or irregularly and tightly packed, bearing terminal solitary monophialides or whorls of 2–3 phialides; sporodochial phialides subulate to subcylindrical, (13–)15–21.5(–25) × (3–)4–4.5(–5.5) μm (av. 18.4 × 4 μm, n = 49), smooth- and thin-walled, with inconspicuous periclinal thickening and a short, non-flared collarette. Sporodochial conidia straight or moderately curved at basal and apical cells, widest just above the mid portion and distinctly tapering toward base; apical cell of equal length or slightly longer than adjacent cell, blunt to slightly hooked with rounded apex; basal cell distinctly notched and somewhat bulging, (2–)3–7(–8)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 26–33 × 4–4.5 μm (n = 2); 3-septate conidia: (28–)34–47(–51) × (3.5–)4–5.5 μm (av. 40.5 × 4.6 μm, n = 28); 4-septate conidia: (37–)40–51.5(–55) × 4–5.5 μm (av. 45.8 × 4.7 μm, n = 20); 5-septate conidia: (43–)50.5–60.5(–65.5) × (4–)5–5.5 μm (av. 55.6 × 5 μm, n = 146); 6-septate conidia: (67–)68.5–76(–79) × 5–6(–6.5) μm (av. 72.2 × 5.5 μm, n = 44); 7-septate conidia (66.5–)72–81(–82.5) × (4.5–)5–6 μm (av. 76.6 × 5.5 μm, n = 9); 8-septate conidia: 91.5 × 6 μm (n = 1); overall: (26–)45–69.5(–91.5) × (3.5–)4.5–5.5(–6.5) μm (av. 57.3 × 5 μm, n = 250). Chlamydospores abundant, globose to subglobose, smooth-walled to verruculose, thick-walled, 6–7(–8) μm diam, terminal or intercalary in hyphae or conidia, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 4.3–5 mm/d at 24 °C, reaching 60–65 mm diam in 7 d at 24 °C; white to pale luteous, flat, velvety to felty, with concentric rings of short buff to rosy buff aerial mycelium; margin entire. Reverse pale orange to rosy buff. On OA incubated in dark reaching 40–50 mm diam in 7 d at 24 °C; white, becoming ochreous after the formation of numerous sporodochia, flat, velvety to floccose; margin entire. Reverse pale luteous, pale saffron at centre.
Additional material examined. Japan, Nagano, from Morus alba twig, c. 1956, unknown collector, CBS 145468 = NRRL 22157 = MAFF 238538.
Notes — This clade encompasses strains previously assigned to F. solani f. sp. mori, also known as Nec. haematococca MPIII and phylogenetic species FSSC 17. Members of this clade are known to induce stem blight on mulberry (Morus alba) trees (Sawada 1959, Sakurai & Matuo 1959, Matuo & Snyder 1973). Additional sequences from South Korea (M. alba, JS-169), were also included in this study, originating from the whole genome sequence of a non-pathogenic endophytic strain published in Kim et al. (2017; Table 1).
Neocosmospora mori produces sporodochial conidia intermediate in size to those of N. cucurbitae and N. samuelsii. However, the sporodochial conidia of N. mori are typically almost straight with notably tapered ends, while only microconidia are produced from aerial conidiophores. In contrast, the sporodochial conidia in N. cucurbitae are somewhat more cylindrical and curved. Neocosmospora cucurbitae also produces additional cylindrical, multiseptate aerial macroconidia. Neocosmospora samuelsii has thick-walled, robust and relatively strongly curved sporodochial conidia, with elongated and hooked apical cells, and it lacks microconidia, forming instead only typical flat-based and pointy aerial macroconidia.
Neocosmospora nirenbergiana Sand.-Den. & Crous, sp. nov. — MycoBank MB831189; Fig. 26
Fig. 26.
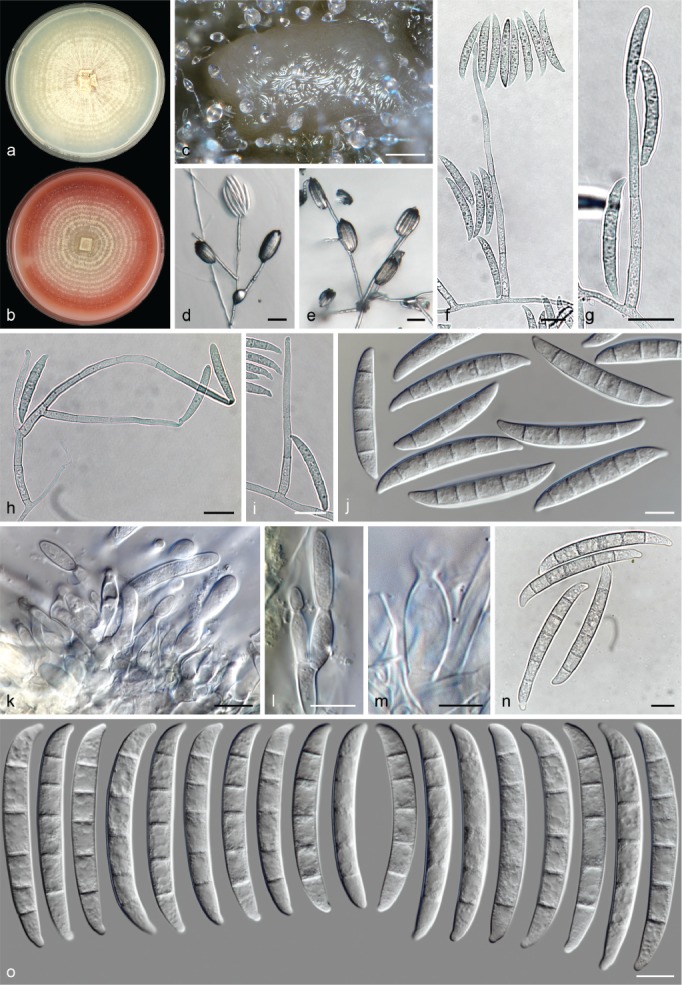
Neocosmospora nirenbergiana (ex-type culture CBS 145469). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c. sporodochium formed on the surface of carnation leave; d–i. aerial conidiophores; j. aerial conidia; k–m. sporodochial conidiophores and phialides; n–o. sporodochial conidia. — Scale bars: c = 100 μm; d–i = 20 μm; m = 5 μm; all others = 10 μm.
Etymology. In honour of Dr Helgard Nirenberg, for her significant contributions to the taxonomy of Fusarium and related genera.
Typus. French Guiana, on bark of unidentified tree, unknown date and collector (holotype CBS H-23988 designated here, culture ex-type CBS 145469 = NRRL 22387 = BBA 65023 = G.J.S. 87-127).
Conidiophores abundant on substrate mycelium, erect and prostrate, and laterally borne on aerial mycelium, straight, smooth- and thin-walled, often simple or sparingly dichotomously branched, irregular or sympodial, often proliferating through conidiogenous opening, or laterally just below previous conidiogenous locus; bearing terminal and lateral, single monophialides; phialides subulate to subcylindrical, (43–)46–56.5(–61) × 3.5–5 μm (av. 51.1 × 4.2 μm, n = 64), smooth- and thin-walled, with rather inconspicuous periclinal thickening and collarettes; aerial conidia falcate and multiseptate, often with an almost straight ventral line, indistinctly notched to papillate, 4–5-septate, smooth- and thick-walled; 4-septate conidia: (37.5–)40–49(–52) × (6.5–)7–7.5 μm (av. 44.6 × 7.2 μm, n = 28); 5-septate conidia: (42.5–)46.5–54.5(–59.5) × (6.5–)7–8(–8.5) μm (av. 50.5 × 7.3 μm, n = 124); overall: (37.5–)45–54(–59.5) × (6.5–)7–8(–8.5) μm (av. 49.4 × 7.3 μm, n = 152). Sporodochia cream, pale luteous, pale sienna to pale citrine. Sporodochial conidiophores sparingly branched, verticillately or irregularly; sporodochial phialides subcylindrical, subulate to somewhat doliiform, (9–)10.5–15(–18) × (3–)3.5–5(–5.5) μm (av. 12.7 × 4.3 μm, n = 74), smooth- and thin-walled, periclinal thickening at conidiogenous loci inconspicuous or visible, collarettes often wide and somewhat flared. Sporodochial conidia falcate, rarely straight, curvature often more pronounced toward basal end, narrowing toward base; apical cell of equal length to adjacent cell, blunt to somewhat conical with curved and rounded apex; basal cell indistinctly to moderately notched, (2–)5(–6)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 51–52.5 × 6–6.5 μm (n = 2); 5-septate conidia: (49.5–)53.5–62.5(–73.5) × (5.5–)6–7(–7.5) μm (av. 58 × 6.6 μm, n = 134); 6-septate conidia: 60 × 7 (n = 1); overall: (49.5–)53.5–62.5(–73.5) × (5.5–)6–7(–7.5) μm (av. 57.9 × 6.6 μm, n = 137). Chlamydospores not observed.
Colonies on PDA growing in dark with an average radial growth rate of 3.5–4.1 mm/d at 24 °C, reaching 54–60 mm diam in 7 d at 24 °C; straw, sulphur yellow to pale luteous, flat, velvety to felty, with concentric rings and radial patches of dense and short aerial mycelium; margin entire and filiform with abundant submerged mycelium. Reverse pale luteous, luteous to pale orange at centre. On OA incubated in dark reaching 46–52 mm diam in 7 d at 24 °C; straw, sulphur yellow to pale luteous, becoming scarlet, red to rust at the periphery, flat, velvety to felty, aerial mycelium dense and short arranged in concentric rings and radial patches; margin entire. Reverse pale luteous at centre, with abundant production of a scarlet to red diffusible pigment.
Notes — Neocosmospora nirenbergiana has long been recognised as a distinct species by means of RAPD and ITS-RFLP patterns (Hering 1997), and phylogenies based on the rDNA (Schütt 2001) and rDNA plus tef1 sequences (O’Donnell 2000, Nalim et al. 2011) but has heretofore not formally named. This species clusters within Clade 2 of Neocosmospora and is similar to most members of this clade based on the absence of aerial microconidia. Instead, several members of this clade are characterised by the formation of relatively short and robust aerial conidiophores from which only falcate, multiseptate conidia are produced. In addition, aerial conidiophores tend to proliferate laterally from below the previous conidiogenous locus, a feature also observed for the closest phylogenetic relatives N. cryptoseptata, N. kurunegalensis and N. samuelsii, but not seen for the remaining species known in Clade 2. Neocosmospora nirenbergiana is easily distinguished from N. samuelsii and N. kurunegalense by the production of much shorter sporodochial conidia which are similar in size to those of N. cryptoseptata. However, the sporodochial conidia of N. nirenbergiana are slender, with somewhat longer and hooked apical cells. In addition, N. nirenbergiana produces a scarlet pigment on OA, but not on PDA.
This lineage is only known from the ex-type culture (CBS 145469), which has been recorded to form a sexual morph (O’Donnell 2000, and personal notes from G.J. Samuels). We were, however, unable to induce the sexual morph in this study.
Neocosmospora noneumartii Sand.-Den. & Crous, sp. nov. — MycoBank MB831190; Fig. 27
Fig. 27.
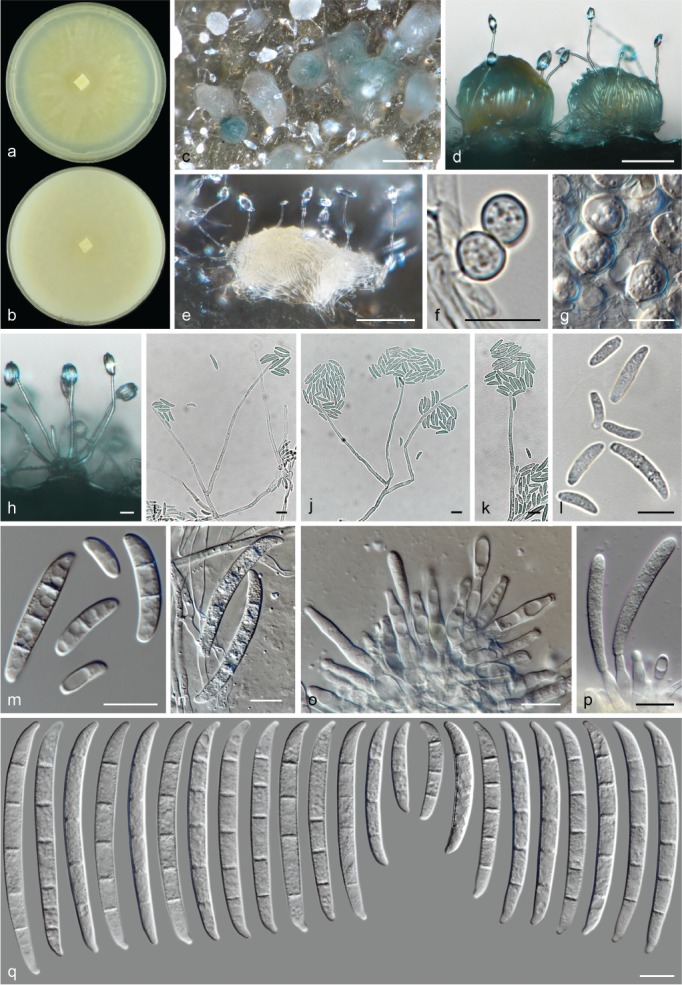
Neocosmospora noneumartii (ex-type culture CBS 115658). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–g. chlamydospores; h–k. aerial conidiophores; l–n. aerial microconidia and macroconidia; o–p. sporodochial conidiophores and phialides; q. sporodochial conidia. — Scale bars: c = 100 μm; d–e = 50 μm; all others = 10 μm.
Etymology. Named after the species similarity with the modern morphological and ecological concept of Fusarium eumartii, but being phylogenetically and morphologically distinct.
Typus. Israel, Palestine, from Solanum tuberosum, unknown date and collector (holotype CBS H-23989 designated here, culture ex-type CBS 115658 = FRC S-0661).
Conidiophores abundant on the aerial mycelium or erect and prostrate on vegetative mycelium, smooth- and thin-walled, at first simple, at maturity sparingly irregularly, verticillately or sympodially branched, bearing terminal monophialides; phialides subulate to subcylindrical, (19–)33.5–64.5(–75) × (2–)2.5–4 (–4.5) μm (av. 49.2 × 3.2 μm, n = 134), smooth- and thin-walled, rarely proliferating, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared collarette; aerial conidia of two types: microconidia ellipsoidal to subcylindrical, straight to slightly curved, 0–1-septate, hyaline, smooth- and thin-walled, (4.5–)9–15.5(–18.5) × (2–)2.5–4(–4.5) μm (av. 12.2 × 3.2 μm, n = 146), grouped in false heads at tip of monophialides; macroconidia short-falcate and multiseptate, slightly dorsiventrally curved or almost straight, basal cell inconspicuously notched, 1–4-septate, smooth- and thin-walled; 1-septate conidia: 13.5–22.5(–24) × (3.5–)4–5(–5.5) μm (av. 17.6 × 4.4 μm, n = 26); 2-septate conidia: (21–)21.5–24(–25) × (4.5–)5–5.5 μm (av. 22.6 × 5 μm, n = 18); 3-septate conidia: (25.5–)31.5–46(–48.5) × (3.5–)4–5(–6) μm (av. 38.8 × 4.4 μm, n = 70); 4-septate conidia: 44.5–46 × 5–5.5 μm (av. 45.3 × 5.4 μm, n = 24); overall: (13.5–)22–45.5(–48.5) × 4–5.5(–6) μm (av. 33.8 × 4.7 μm, n = 138). Sporodochia cream, ochreous, pale green to grey-green. Sporodochial conidiophores unbranched to densely branched, verticillately or irregularly and tightly packed; sporodochial phialides ampulliform, subcylindrical to subulate, (12–)13.5–18.5(–21) × (3–)3.5–5(–5.5) μm (av. 15.8 × 4.2 μm, n = 74), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared collarette. Sporodochial conidia straight or discreetly dorsiventrally curved, slightly wider on the upper third and tapering toward base; apical cell of equal length than adjacent cell, apex blunt to somewhat papillate; basal cell distinctly notched, (1–)3–6-septate, hyaline, smooth- and thick-walled; 1-septate conidia: 24 × 5 (n = 1); 2-septate conidia: 29.5–30 × 4–5 μm (av. 29.9 × 4.4 μm, n = 8); 3-septate conidia: (26.5–)32.5–53.5(–64.5) × (4–)4.5–6 μm (av. 43.2 × 5.1 μm, n = 116); 4-septate conidia: (34.5–)45–65.5(–67.5) × (4–)4.5–6 μm (av. 55.1 × 5.1 μm, n = 72); 5-septate conidia: (51.5–)59–67.5(–73.5) × 5–6(–6.5) μm (av. 63 × 5.5 μm, n = 140); 6-septate conidia: 68.5–69 × 5.5–6 μm (av. 68.7 × 5.6 μm, n = 8); overall: (24–)40.5–66.5(–73.5) × (4–)4.5–6(–6.5) μm (av. 53.7 × 5.3 μm, n = 345). Chlamydospores abundant, subglobose to globose, smooth- and thick-walled, (7.5–)8.5–11.5(–12) μm diam, terminal or intercalary in hyphae or conidia, solitary, in chains or clusters.
Colonies on PDA growing in dark with an average radial growth rate of 4.7–8 mm/d at 24 °C, reaching 86–90 mm diam in 7 d at 24 °C; white, pale luteous, buff, ochreous to olivaceous, often flat with regular shape, membranous, velvety or felty with sparse, often white, aerial mycelium; margin entire. Reverse white to pale straw. On OA incubated in dark covering an entire 90 mm diam Petri dish in 7 d at 24 °C; white, luteous, buff, flat, at first membranous turning velvety to floccose; margin entire. Reverse white, cream to brick or umber.
Notes — Neocosmospora noneumartii was proposed to represent the important potato storage rot pathogen Fusarium eumartii (as Fusarium solani f. sp. eumartii) by means of host pathogenicity and DNA sequence analyses (Romberg & Davis 2007). DNA barcodes derived from that study showed it corresponds to phylogenetic species FSSC 42. The same authors demonstrated that this lineage also encompasses agents of potato wilt and foot rot of tomato (Solanum lycopersicum). However, the modern concept of Fusarium eumartii is largely polyphyletic, while its morphological circumscription has been subjected to multiple interpretations, all of which diverge significantly from the original description (Gerlach & Nirenberg 1982, Hering 1997, Romberg & Davis 2007, Sandoval-Denis et al. 2018). Morphologically, N. noneumartii fits the modern concepts of ‘Fusarium’ eumartii sensu Wollenweber (1943) and sensu Gerlach & Nirenberg (1982) by forming large, multiseptate (up to 6-septate) sporodochial conidia. Nevertheless, a re-examination of the original material of F. eumartii showed that the species differs sharply from the previously mentioned modern concept, and this name is here reduced to synonymy under N. solani. As stated in earlier circumscriptions of the two species (Sherbakoff 1915, Wollenweber & Reinking 1935a), this synonymy is also supported by our phylogenetic analyses of the original material of F. eumartii. Therefore, N. noneumartii is proposed here as a novel species.
The sporodochial conidia of N. noneumartii are similar in size and septation to those of N. ampla and N. quercicola. These three species include strains that were, historically, identified as F. eumartii based on the broad modern concept of this species. However, N. noneumartii can be distinguished from N. quercicola by the latter’s absence of aerial falcate, multiseptate conidia. Neocosmospora quercicola produces only microconidia on its aerial conidiophores and also produces sporodochial conidia with somewhat elongated foot cells. The cultural characteristics of N. noneumartii and N. ampla appear to be similar, as is the range of conidia produced by both species, although the sporodochial conidia of N. noneumartii are usually slender, with somewhat longer apical cells.
Neocosmospora oblonga Sand.-Den. & Crous, sp. nov. — MycoBank MB831191; Fig. 28
Fig. 28.
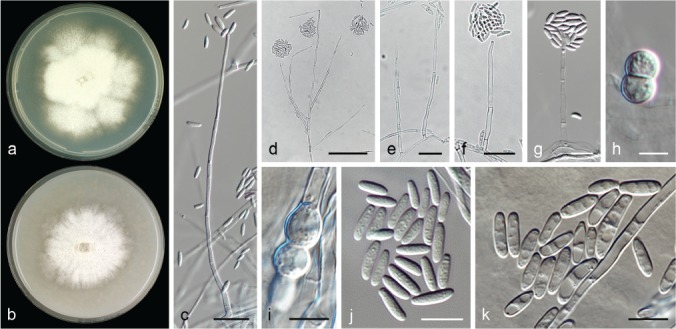
Neocosmospora oblonga (ex-type culture CBS 130325). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–g. conidiophores; h–i. chlamydospores; j–k. conidia. — Scale bars: c–d = 20 μm; h–i = 5 μm; all others = 10 μm.
Etymology. From Latin oblongum meaning ‘prolate spheroid’; referring to its conidial shape.
Typus. USA, from human eye, unknown date and collector (holotype CBS H-23990 designated here, culture ex-type CBS 130325 = NRRL 28008 = CDC B-4701).
Conidiophores abundant on aerial and substrate mycelium, straight, smooth- and thin-walled, commonly simple or moderately dichotomously, verticillately, or irregularly branched, often proliferating, bearing terminal, single monophialides; phialides subulate, subcylindrical to acicular, (3.5–)13.3–26.5(–33.5) × (0.5–)1–1.5 μm (av. 19.9 × 1.2 μm, n = 76), hyaline, smooth- and thick-walled, conidiogenous loci with visible periclinal thickening and a short, flared or non-flared collarette; aerial conidia ellipsoidal, clavate or bullet shaped, rarely short falcate, 0(–1)-septate, hyaline, smooth- and thin-walled, (5–)8–14(–22) × (2–)3–4(–5.5) μm (av. 10.9 × 3.3 μm, n = 178), clustering in false heads at tip of monophialides. Sporodochia not seen. Chlamydospores abundant, globose to subglobose, smooth- to finely verruculose and thick-walled, (5.5–)6–7.5(–8) μm diam, terminal or intercalary in hyphae or conidia, solitary or in chains. Colonies on PDA growing in dark with an average radial growth rate of 2.3–3 mm/d at 24 °C, reaching 16–21 mm diam in 7 d at 24 °C; white to pale straw at margin, flat, velvety to finely dusty or granular; margin lobate and highly irregular. Reverse pale straw to pale luteous. On OA incubated in dark reaching 16–20 mm diam in 7 d at 24 °C; white, flat, velvety to dusty; margin entire. Reverse saffron to rosy buff.
Notes — Neocosmospora oblonga is introduced here for the clade informally known as phylogenetic species FSSC 29 (O’Donnell et al. 2008). The isolate studied here (CBS 130325) produces microconidia only, while no sporodochia were observed. This lineage was included in the ‘F. ensiforme clade’ by Nalim et al. (2011). However, despite its simple morphology, cultural and micromorphological features this novel species is distinct from any plausible concept of F. ensiforme, an obscure name of uncertain application. Conidia of N. oblonga are typically ellipsoidal to clavate and straight, although short, falcate, 1-septate conidia can also rarely be observed on the aerial phialides. In contrast, F. ensiforme is described as forming mostly unicellular, ovoid and slightly curved microconidia and long, up to 7-septate sporodochial conidia. Additionally, the colonies of F. ensiforme grown on OA were reported to develop a thick and dense pink to buff-coloured mycelium, contrasting with the white and short aerial mycelium seen in N. oblonga (Wollenweber & Reinking 1925, Reinking & Wollenweber 1927). For additional comparisons, see notes under N. parceramosa.
Neocosmospora oligoseptata (T. Aoki et al.) Sand.-Den. & Crous, comb. nov. — MycoBank MB831192
Basionym. Fusarium oligoseptatum T. Aoki et al., Fungal Systematics and Evolution 1: 29. 2018.
Typus. USA, Pennsylvania, Dauphin Co., from a live female ambrosia beetle (Euwallacea validus), extracted from a gallery in a tree-of-heaven (Ailanthus altissima), 30 Jan. 2010, M.T. Kasson (holotype BPI 910525, culture ex-type CBS 143241 = NRRL 62579 = FRC S-2581 = MAFF 246283).
Description & Illustration — Aoki et al. (2018).
Notes — Neocosmospora oligoseptata is here coined for the recently described F. oligoseptatum. This species is a well-recognised and characterised phylogenetic species (as AF-1) within the Ambrosia clade of Neocosmospora. This clade is known to include symbionts of Euwallacea validus (Curculionidae: Xyleborini), an ambrosia beetle known from Asia and North America, and commonly associated with the transmission of Verticillium nonalfalfae, the agent of Verticillium wilt on the invasive tree-of-heaven (Ailanthus altissima; Cognato et al. 2015, Kasson et al. 2015).
According to Aoki et al. (2018), N. oligoseptata forms irregularly falcate to clavate, apically swollen sporodochial conidia similar to those of N. ambrosia and N. euwallaceae. However, N. oligoseptata can be distinguished by frequently producing 0–2-septate sporodochial conidia instead of the more regularly 3–5-septate sporodochial conidia of other species in this clade.
Neocosmospora paraeumartii Sand.-Den. & Crous, sp. nov. — MycoBank MB831193; Fig. 29
Fig. 29.
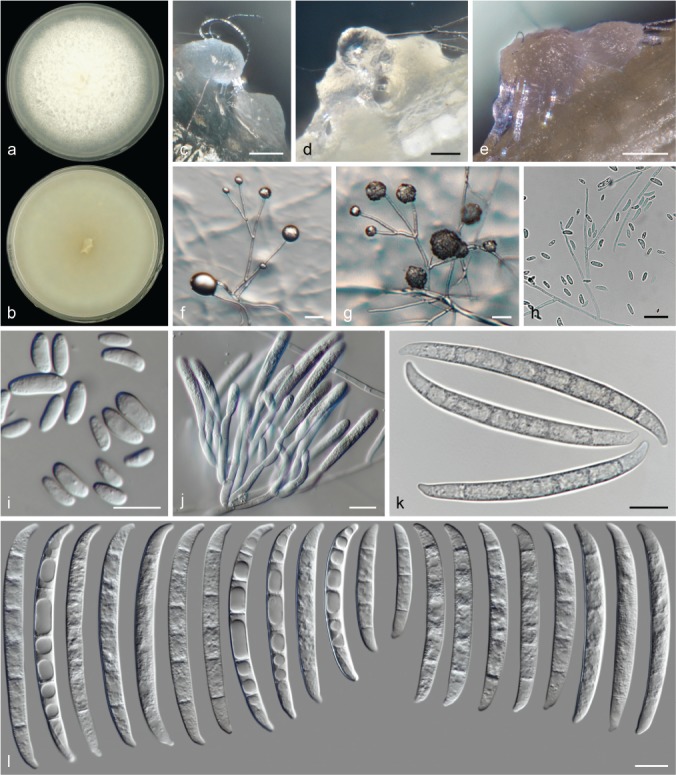
Neocosmospora paraeumartii (ex-type culture CBS 487.76). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–h. aerial conidiophores; i. aerial conidia; j. sporodochial conidiophore; k–l. sporodochial conidia. — Scale bars: c–e = 100 μm; f–h = 20 μm; all others = 10 μm.
Etymology. From Greek  (pará), meaning ‘against, contrary to’. According to the modern morphological concept of F. eumartii, the ex-type culture N. paraeumartii was erroneously thought to represent this historic species.
(pará), meaning ‘against, contrary to’. According to the modern morphological concept of F. eumartii, the ex-type culture N. paraeumartii was erroneously thought to represent this historic species.
Typus. Argentina, from decaying stem base of Solanum tuberosum, unknown date or collector (holotype CBS H-23991 designated here, culture ex-type CBS 487.76 = NRRL 13997 = BBA 62215).
Conidiophores abundant on the aerial mycelium, erect or prostrate on vegetative mycelium, smooth- and thin-walled, at first simple, later sparingly and irregularly branched, bearing terminal monophialides; phialides subulate to subcylindrical, (16–)35.5–60(–67) × 2–3(–3.5) μm (av. 48 × 2.7 μm, n = 28), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared collarette; aerial conidia ellipsoidal to subcylindrical, often dorsiventrally curved, 0(–1)-septate, hyaline, smooth- and thin-walled, (5–)6.5–11.5(–16.5) × (2.5–)3–4.5(–5.5) μm (av. 9 × 3.7 μm, n = 91), grouped in false heads on tip of monophialides. Sporodochia cream, ochreous to pale green. Sporodochial conidiophores densely and irregularly branched; sporodochial phialides ampulliform, subcylindrical to subulate, (12–)15–21(–22) × 3.5–5(–5.5) μm (av. 18 × 4.3 μm, n = 34), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a minute, non-flared collarette. Sporodochial conidia almost straight, discreetly to moderately dorsiventrally curved, wedge-shaped; apical cell of more or less equal length than adjacent cell, apex blunt and often distinctly hooked; basal cell notched and moderately protuberant (1–)4–6-septate, hyaline, smooth- and thick-walled; 1-septate conidia: 22.5 × 4 μm (n = 2); 3-septate conidia: (34–)35.5–48(–50) × 4.5–5.5 μm (av. 41.7 × 5 μm, n = 6); 4-septate conidia: (39.5–)49–64(–65) × (5–)5.5–6(–7) μm (av. 56.5 × 5.9 μm, n = 40); 5-septate conidia: (52.5–)56–66(–73) × 5–6(–6.5) μm (av. 61 × 5.8 μm, n = 88); 6-septate conidia: (57.5–)60.5–73(–75.5) × 5.5–6.5 μm (av. 66.6 × 5.9 μm, n = 44); overall: (22.5–)48.5–69(–75.5) × (4–)5–6.5(–7) μm (av. 58.9 × 5.8 μm, n = 180). Chlamydospores subglobose to globose, smooth- or rough- and thick-walled, 5–12.5 μm diam, terminal or intercalary.
Colonies on PDA growing in dark with an average radial growth rate of 2.8–3.1 mm/d at 24 °C, reaching 38–42 mm diam in 7 d at 24 °C; white, pale luteous to luteous at centre, flat to slightly raised, felty to cottony, with abundant irregularly distributed aerial mycelium; colony margin filiform. Reverse pale straw to pale luteous. On OA incubated in dark reaching 36–45 mm diam in 7 d at 24 °C; straw to buff, flat, membranous with scant white aerial mycelium; margin entire. Reverse buff.
Notes — This species is based on a single isolate that is genetically and morphologically well-delimited. The ex-type of N. paraeumartii was illustrated as representative of F. eumartii by Gerlach & Nirenberg (1982). The latter taxon is synonymised here under N. solani based on morphological and phylogenetic analyses of a specimen derived from the type material of F. eumartii (see notes under N. solani).
Morphologically, N. paraeumartii is comparable to, i.e., N. ampla, N. nirenbergiana, N. noneumartii and N. quercicola. All these species display morphologies concordant with the modern concept of F. eumartii, characterised by robust, predominantly 5–7-septate sporodochial conidia. However, these morphological features are highly polyphyletic in Neocosmospora.
Neocosmospora paraeumartii is phylogenetically distant and unrelated to all of the above-mentioned species. In addition, N. paraumartii differs from N. ampla, N. nirenbergiana and N. noneumartii by the absence of aerial macroconidia. The sporodochial conidia in N. ampla and N. nirenbergiana are much wider (av. 6.3 and 6.6 μm wide, respectively) than those of N. paraeumartii, while N. nirenbergiana clusters in Clade 2. The distinction between N. paraeumartii and N. quercicola is virtually impossible based on morphology alone. The sporodochial conidia of N. paraeumartii display a slightly more pronounced curvature than those of N. quercicola. Although both species are easily distinguished genetically, morphological measurements largely overlap.
Neocosmospora parceramosa Sand.-Den. & Crous, sp. nov. — MycoBank MB831194; Fig. 30
Fig. 30.
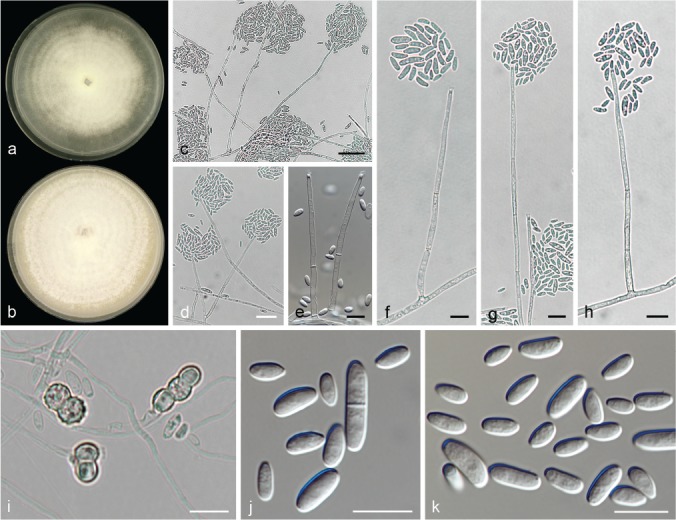
Neocosmospora parceramosa (ex-type culture CBS 115695). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–h. conidiophores; i. chlamydospores; j–k. conidia. — Scale bars: c–d = 20 μm; all others = 10 μm.
Etymology. From Latin parce, meaning ‘sparingly, moderately’; and rāmus, meaning ‘branch’. After its sparingly branched conidiophores.
Typus. South Africa, from soil, 1995, unknown collector (holotype CBS H-23992 designated here, culture ex-type CBS 115695 = CPC 1246).
Conidiophores on the substrate and aerial mycelium straight or flexuous, smooth- and thin-walled, mostly simple or sparingly branched, bearing terminal, single monophialides; phialides subulate to subcylindrical, (35–)47.5–63.5(–74) × (2–)2.5–3.5(–4) μm (av. 55.5 × 2.9 μm, n = 84), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, somewhat flared collarette; aerial conidia ellipsoidal, subcylindrical to clavate, straight, gently curved or rarely apically hooked, 0(–1)-septate, hyaline, smooth- and thin-walled, (4–)5.5–10(–16) × (2.5–)3–4(–5) μm (av. 7.9 × 3.4 μm, n = 169), clustering in false heads on tip of monophialides. Sporodochia not observed. Chlamydospores globose to subglobose, smooth-walled, warted or tuberculate and thick-walled, (5.5–)6–7.5(–8.5) μm diam, terminal, intercalary or more often borne on short lateral hyphal pegs, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 3.2–3.5 mm/d at 24 °C, reaching 44–50 mm diam in 7 d at 24 °C; white to pale straw, flat, velvety, cottony to felty, with concentric rings of white to pale straw aerial mycelium; margin entire. Reverse straw to honey. On OA incubated in dark reaching 63–78 mm diam in 7 d at 24 °C; white, flat, velvety to granular with concentric rings of dense, white aerial mycelium; margin entire. Reverse straw to buff.
Notes — Neocosmospora parceramosa, previously known as phylogenetic species FSSC 18, has been mostly isolated as a putative rare human pathogen (O’Donnell 2000) which requires further investigation. Neocosmospora parceramosa is also known to inhabit plumbing systems (Short et al. 2011), while the type was obtained from an environmental soil sample in South Africa. The ex-type strain, an isolate producing only microconidia from aerial conidiophores was originally identified and deposited as a Verticillium sp., which may indicate that the lack of sporodochia is not a result of strain deterioration after long-term storage, but a distinctive feature of the species.
Identifying species that only produce simple aerial conidiophores and lacking sporodochial conidia, i.e., N. catenata, N. diminuta, N. liriodendri, N. oblonga, N. parceramosa and N. vasinfecta, can be problematic. Neocosmospora diminuta differs conspicuously by the noticeable production of a sexual morph, an attribute shared with N. vasinfecta, distinguished by producing aseptate ascospores contained in smooth-walled, bright red perithecia. Distinctively, N. diminuta can produce falcate, multiseptate aerial conidia. Neocosmospora catenata commonly exhibits conspicuous chains of pale brown chlamydospores and rather dramatically flared phialidic collarettes. Neocosmospora parceramosa and N. oblonga are phylogenetically well-defined, even though the two species exhibit similar macroscopic features, in addition to having overlapping conidial and chlamydospore features. The main distinguishing morphological features of N. parceramosa include a fast growth rate on OA at 24 °C (63–78 vs 16–20 mm diam at 7 d in N. oblonga) and the very long phialides up to 74 μm vs up to 33.5 μm long in N. oblonga. Likewise, N. parceramosa can be distinguished from N. liriodendri by its faster growth rate on artificial media; longer aerial phialides (up to 71.5 μm long in N. liriodendri); and shorter, smooth to tuberculate chlamydospores (up to 8.5 vs up to 10.5 μm diam, and smooth-walled in N. liriodendri).
Neocosmospora perseae Sand.-Den. & Guarnaccia, Fungal Systematics and Evolution 1: 136. 2018
Typus. Italy, Catania, San Leonardello, from trunk canker lesions on Persea americana, 25 Mar. 2015, G. Polizzi (holotype CBS H-23433, culture ex-type CBS 144142 = CPC 26829).
Description & Illustration — Guarnaccia et al. (2018).
Additional material examined. Italy, Catania, San Leonardello, from trunk canker lesions on Persea americana, 25 Mar. 2015, G. Polizzi, CBS 144143 = CPC 26830, CBS 144144 = CPC 26831, CBS 144145 = CPC 26832, CBS 144146 = CPC 26833.
Notes — Recently introduced as a novel pathogen of Persea americana in Italy, N. perseae is discussed in detail by Guarnaccia et al. (2018). The phylogenetically unrelated species N. pseudotonkinensis, described here, produces similar sporodochial conidia. However, N. pseudotonkinensis forms cylindrical aerial macroconidia, while only microconidia are formed on aerial conidiophores in N. perseae. In addition, both species produce a similar luteous colouration in young cultures, which is retained in colonies of N. pseudotonkinensis. In contrast, those of N. persea become distinctly bright red after long-term incubation (Guarnaccia et al. 2018).
Neocosmospora petroliphila (Q.T. Chen & X.H. Fu) Sand.-Den. & Crous, Persoonia 41: 121. 2018
Basionym. Fusarium solani var. petroliphilum Q.T. Chen & X.H. Fu, Acta Mycol. Sin., Suppl. 1: 330. 1987.
Synonyms. Fusarium solani f. sp. cucurbitae (Race 2) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium petroliphilum (Q.T. Chen & X.H. Fu) Geiser et al., Fungal Genet. Biol. 53: 69. 2013.
Typus. China, Beijing, from deteriorated petroleum (holotype of F. solani var. petroliphilum HMAS 43748; culture ex-type NF4475 = NRRL 22268 = FRC S-2176).
Descriptions & Illustrations — Short et al. (2013), Sandoval-Denis & Crous (2018).
Additional materials examined. Brazil, Recife, Saccharum officinarum, unknown date, A.C. Batista, CBS 398.66. – Cuba, from human toenail, unknown date and collector, CBS 224.34 = NRRL 28579. – South Africa, Pretoria, from root of Pelargonium sp., unknown date, H.W. Wollenweber, CBS 203.32 = NRRL 13952.
Notes — Neocosmospora petroliphila is one of the most prevalent species associated with human infections and one of the most common species isolated from human-made environments (Short et al. 2011, Sandoval-Denis & Crous 2018). Several subspecific and informal names were applied to this taxon prior to formal acceptance at species level by Short et al. (2013), i.e., F. solani f. sp. cucurbitae Race 2, N. haematococca MP V, F. solani var. petroliphilum, and phylogenetic species FSSC 1.
Among the most prevalent clinically relevant species of Neocosmospora, N. petroliphila has sporodochial conidia larger than those of N. keratoplastica, N. metavorans and N. solani, and also has longer and more curved apical cells. These characteristic apical cells and the absence of cylindrical aerial conidia distinguish N. petroliphila from N. tonkinensis, which forms similar-sized sporodochial conidia. The sporodochial conidia of N. petroliphila are similar in shape to those of N. gamsii and N. suttoniana, but those of N. gamsii are considerably smaller (Short et al. 2013, Sandoval-Denis & Crous 2018).
Neocosmospora phaseoli (Burkh.) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015
Basionym. Fusarium martii f. phaseoli Burkh., Cornell Univ. Agric. Exp. Sta. Mem. 26: 1007. 1919.
Synonyms. Fusarium solani f. phaseoli (Burkh.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium phaseoli (Burkh.) T. Aoki & O’Donnell, Mycologia 95: 671. 2003.
Fusarium martii var. minus Sherb., Cornell Univ. Agric. Exp. Sta. Mem. 6: 249. 1915.
Fusarium solani var. martii Appel & Wollenw. f. 3 Snyder, Zentralbl. Bakteriol., 2. Abt. 91: 179. 1934.
Fusarium solani f. sp. glycines K. Roy, Plant Disease 81: 264. 1997.
Fusarium tucumaniae T. Aoki et al., Mycologia 95: 664. 2003.
Neocosmospora tucumaniae (T. Aoki et al.) L. Lombard & Crous, Stud. Mycol. 80: 228. 2015.
Fusarium virguliforme O’Donnell & T. Aoki, Mycologia 95: 667. 2003.
Neocosmospora virguliformis (O’Donnell & T. Aoki) L. Lombard & Crous, Stud. Mycol. 80: 228. 2015.
Fusarium brasiliense T. Aoki & O’Donnell, Mycoscience 46: 166. 2005.
Fusarium cuneirostrum O’Donnell & T. Aoki, Mycoscience 46: 170. 2005.
Fusarium crassistipitatum Scandiani et al., Mycoscience 53: 171. 2011.
Fusarium azukiicola T. Aoki et al., Mycologia 104: 1075. 2012.
Typus. USA, New York, on roots of Phaseolus vulgaris, unknown date and collector (fide Index Fungorum, not located).
Descriptions & Illustrations — Burkholder (1919), Aoki et al. (2003, 2005, 2012a, b).
Materials examined. Honduras, Puerto Arturo, Tela, on Citrus aurantifolia, 1 Mar. 1923, O.A. Reinking, BPI 452391. – USA, California, from Phaseolus sp., unknown date, W.C. Snyder, CBS 265.50.
Notes — Diverse studies suggested that the different taxa here reduced to synonymy under N. phaseoli differ by biogeographical patterns, pathogenicity and morphology (Aoki et al. 2003, 2005, 2012a, b, O’Donnell et al. 2010). These variations are not mirrored in genus-wide phylogenetic studies using markers traditionally employed for this genus (Nalim et al. 2011, O’Donnell et al. 2013, Chitrampalam & Nelson 2016, Costa et al. 2016, Dallé Rosa et al. 2018). The phylogenetic results here showed that an internal phylogenetic structure does exist within the N. phaseoli clade (Fig. 1). However, the marginal genetic variation between the included ex-types (Table 1) does not warrant their recognition at species level, a situation parallel to that described here for N. vasinfecta and its synonyms. Previously noted morphological and ecological differences are here regarded as subspecific populations of N. phaseoli, but we refrain from recognising these populations at a varietal level.
Neocosmospora phaseoli differs mostly from its closest relatives in Clade 2 (O’Donnell 2000) based on host preference and biogeography. Most species in Clade 2 are known to inhabit bark and wood, and have chiefly been isolated from dead trees, particularly in South Asia, Central America and the Caribbean region of South America. In contrast, N. phaseoli is a devastating pathogen of plant hosts in the Fabaceae, and is well documented from temperate and subtropical regions of North and South America, as well as Japan (Aoki et al. 2003, 2005, 2012a, b, O’Donnell et al. 2010).
Neocosmospora piperis (F.C. Albuq.) Sand.-Den. & Crous, comb. & stat. nov. — MycoBank MB831195; Fig. 31
Fig. 31.
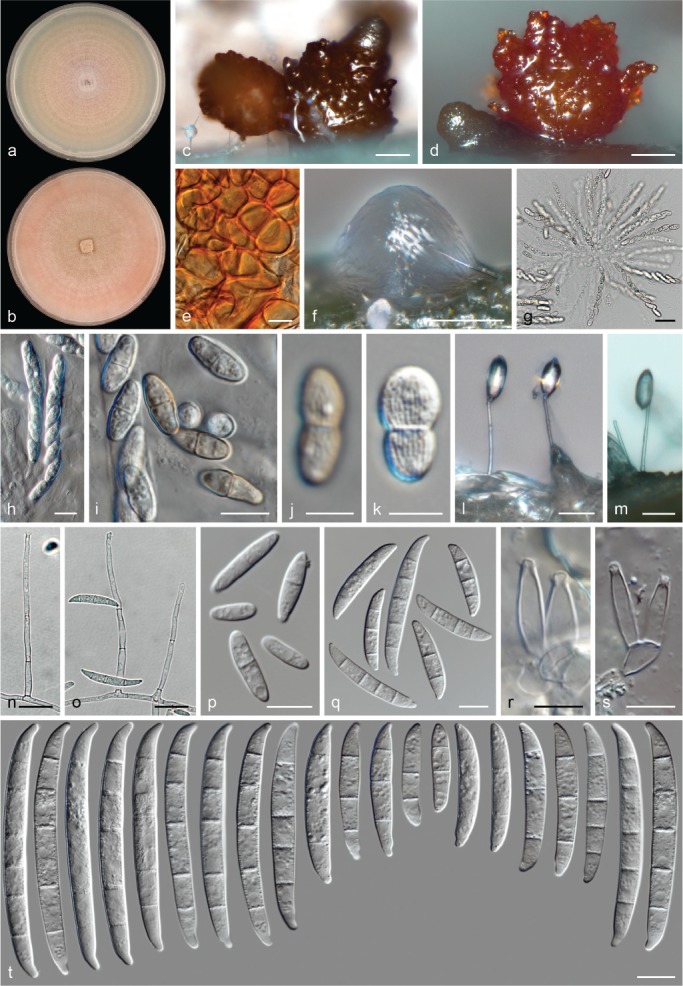
Neocosmospora piperis (ex-epitype culture CBS 145470). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. perithecia; e. detail of peridial cells; f. sporodochium formed on the surface of carnation leave; g–h. asci; i–k. ascospores; l–o. aerial conidiophores; p. aerial microconidia; q. aerial macroconidia; r–s. sporodochial conidiophores and phialides; t. sporodochial conidia. — Scale bars: c–d, f = 100 μm; g, l–o = 20 μm; j–k = 5 μm; all others = 10 μm.
Basionym. Fusarium solani f. piperis F.C. Albuq., Circular do Instituto Agronómico do Norte 5: 19. 1961.
Synonym. Nectria haematococca f. sp. piperis F.C. Albuq. & Ferraz, Experientia (Basel) 22: 136. 1976.
Typus. Brazil, Pará, Santa Izabel, on roots and stem of Piper nigrum, 21 Dec. 1960, F.C. Albuquerque (holotype IAN 825, held at the herbarium of Embrapa Amazônia Oriental); unknown location, from Piper nigrum, unknown date, G.J. Samuels (epitype of Fusarium solani f. piperis CBS H-23993 designated here, MBT387232, culture ex-epitype CBS 145470 = NRRL 22570 = G.J.S. 89-14 = CML 1888).
Perithecia dark orange to dark brown-red, globose to pyriform, often with a minute papilla, superficial, solitary or gregarious, coarsely irregularly warted, glabrous; peridial wall composed of thick-walled cells of textura angularis to globulosa. Asci subcylindrical to clavate, unitunicate, apex flat to rounded and simple, (57–)67–85(–96) × (6.5–)7.5–11(–12.5) μm (av. 75.8 × 9.1 μm, n = 40). Ascospores obliquely uniseriate, biseriate at apical third of asci, broadly ellipsoidal to broadly fusiform, 1-septate, (10–)11–13(–14.5) × (4–)4.5–5(–6) μm (av. 12 × 4.9 μm, n = 141), pale yellow-brown to golden yellow at maturity, thick-walled, longitudinally finely striated, often conspicuously constricted at the septum.
Conidiophores mostly erect or prostrate on substrate mycelium, less abundantly produced laterally on aerial mycelium, straight to somewhat flexuous, smooth- and thin-walled, mostly simple, rarely sparingly branched, bearing terminal, single monophialides; phialides subulate to subcylindrical, (30.5–)43.5–57(–64.5) × 2.5–3.5(–4.5) μm (av. 50.3 × 3.2 μm, n = 46), smooth- and thin-walled, periclinal thickening at conidiogenous loci inconspicuous or absent, collarette short and some-what flared; aerial conidia of two types: microconidia ellipsoidal, clavate to cylindrical, straight or gently curved, 0–1-septate, hyaline, smooth- and thin-walled, (5.5–)9–16(–20) × (2–)2.5–4.5(–6) μm (av. 12.4 × 3.5 μm, n = 65), clustering in false heads at tip of monophialides; macroconidia falcate and multiseptate, gently dorsiventrally curved, base inconspicuously to distinctly notched, often irregularly shaped, sometimes hardly distinguishable from sporodochial conidia, 1–4(–5)-septate, smooth- and thick-walled; 1-septate conidia: (14–)14.5–20.5(–22.5) × 3–5 μm (av. 17.5 × 3.7 μm, n = 28); 2-septate conidia: (20–)20.5–27.5(–31.5) × 4–6 μm (av. 24 × 4.9 μm, n = 40); 3-septate conidia: (26–)28–38(–44) × 5–6.5 μm (av. 33.1 × 5.7 μm, n = 48); 4-septate conidia: (36–)37–44(–46) × 5.5–6 μm (av. 40.2 × 5.6 μm, n = 20); 5-septate conidia: (40.5–)41.5–6 × 5.5–6 μm (av. 46.1 × 5.8 μm, n = 12); overall: (14–)20–39.5(–50) × (3–)4–6(–6.5) μm (av. 29.7 × 5.1 μm, n = 148). Sporodochia cream, pale flesh to pale olivaceous. Sporodochial conidiophores sparingly verticillately or irregularly branched, bearing single or paired terminal phialides; sporodochial phialides subcylindrical to subulate, (9.5–)11.5–15.5(–17) × (3–)4–5(–5.5) μm (av. 29.7 × 5.1 μm, n = 74), smooth- and thin-walled, conidiogenous loci with visible periclinal thickening and a minute, non-flared collarette. Sporodochial conidia almost straight to wedge-shaped; apical cell somewhat longer than adjacent cell, blunt to conical with a curved and slightly extended apex; basal cell barely to distinctly notched, occasionally indistinct from apical cell (2–)3–5(–6)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 27 × 4.8 μm (n = 2); 3-septate conidia: (33–)34.5–41.5(–43) × 5–6.5(–7) μm (av. 37.9 × 5.8 μm, n = 36); 4-septate conidia: (42.5–)43–59.5(–63.5) × (5–)5.5–7 μm (av. 51.2 × 5.8 μm, n = 28); 5-septate conidia: (47–)54.5–65(–67.5) × (5–)5.5–7 μm (av. 60 × 6.2 μm, n = 96); 6-septate conidia: (63–)63.5–67 × (5–)5.5–7 μm (av. 65.2 × 6.1 μm, n = 16); overall: (27–)42.5–65.5(–67.5) × (4.5–)5.5–6.5(–7) μm (av. 53.9 × 6 μm, n = 178). Chlamydospores not observed.
Colonies on PDA growing in dark with an average radial growth rate of 4–5 mm/d at 24 °C, reaching 56–60 mm diam in 7 d at 24 °C; saffron to pale rose, pale luteous to ochreous at periphery, flat, velvety to felty, with abundant concentric rings of pale rose aerial mycelium; margin entire with abundant submerged mycelium. Reverse a gradation of pale scarlet at centre to pale luteous on periphery. On OA incubated in dark reaching 52–65 mm diam in 7 d at 24 °C; peach, pale rose to pale scarlet, flat, velvety with abundant, dense aerial mycelium; margin entire. Reverse peach to pale rose.
Notes — The circumscription of Neocosmospora piperis includes the phylogenetic species FSSC 31, also known as F. solani f. sp. piperis or Nec. haematococca f. sp. piperis, responsible for Nectria blight (‘Mariquita disease’ or Fusarium disease) of black pepper (Piper nigrum; Hamada et al. 1988). The epitype designated here originates from a culture previously subjected to study and identified as belonging to the aforementioned special form, for which it had served as reference strain (O’Donnell 2000, Costa et al. 2017).
This species forms robust and markedly tapering sporodochial conidia, similar in size to those of N. nirenbergiana and N. protoensiformis. However, the sporodochial conidia of N. piperis are often slender, less curved and thinner-walled than those of N. nirenbergiana. The sporodochial conidia of N. protoensiformis show a high resemblance to those of N. piperis, but the latter commonly exhibit much more slender basal cells, with a protuberant, extended and somewhat acute foot.
Neocosmospora pisi (F.R. Jones) Sand.-Den. & Crous, comb. & stat. nov. — MycoBank MB831196
Basionym. Fusarium martii var. pisi F.R. Jones, J. Agric. Res. 26: 459. 1923.
Synonyms. Fusarium solani f. pisi (F.R. Jones) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
(Fusarium pisi (F.R. Jones) A. Šišić et al., Sci. Rep. 8: 2. 2018 (Nom. inval., Art. 42.1)).
Fusarium solani var. martii f 2 Wollenw., Z. Parasitenk. (Berlin) 3: 290. 1931.
Hypomyces solani f. sp. pisi Reichle, W.C. Snyder & Matuo, Nature 203: 664. 1964.
Typus. USA, on Pisum sativum (lectotype of F. martii var. pisi, designated here: illustration f. 1, p. 463, in Jones (1923). Journal of Agricultural Research 26: 459–476, MBT387234); from the sexual cross of parents from P. sativum and soil from a potato field, unknown date and collector (epitype of F. martii var. pisi CBS H-23994, designated here, MBT387235; culture ex-epitype CBS 123669 = NRRL 45880 = ATCC MYA-4622 = Vanetten 77-13-4).
Description & Illustrations — Jones (1923), Reichle et al. (1964), Šišić et al. (2018b).
Additional materials examined. Belgium, Heverlee, from greenhouse soil, unknown date, V. van Assche, CBS 144391 = MUCL 20258, CBS 144392 = MUCL 20260. – France, Paris, from human skin, unknown date and collector, CBS 124896 = IHEM 15469. – Germany, Hessen, Witzenhausen, Neu Eichenberg, root system of Trifolium subterraneum, unknown date, A. Šišić, CBS 142372 = FS21; unknown location, from Solanum tuberosum, unknown date, H.W. Wollenweber, CBS 181.29. – Netherlands, Wageningen, from Pisum sativum, unknown date and collector, CBS 178.47; Zuid Beveland, near Kloetinge, from soil, unknown date, J.C. Went, CBS 188.34. – USA, Oregon, from Quercus garyana, 1922, H.W. Wollenweber, CBS 231.31; Iowa, Cherokee County, Steele Prairie, from soil, unknown date and collector, CBS 127118 = RMF 7536.
Notes — The phylogenetic clade now representing N. pisi has also been informally referred to as F. solani f. sp. pisi, phylogenetic species FSSC 11 and Nec. haematococca MPVI (O’Donnell 2000, O’Donnell et al. 2008). This taxon was recently recognised at the species level by Šišić et al. (2018b) as Fusarium pisi. However, it was invalidly published because an identifier from a recognised repository of fungal names was not included. The authors also discussed the pathogenicity of N. pisi and found this fungus to be an aggressive root pathogen of Pisum sativum, but not being exclusive to that particular host.
No type material was located for this species and it is presumed lost. An illustration from the protologue depicting sporodochial conidia and chlamydospores is here selected to serve as lectotype in order to stabilise the name (Fig. 32). A supporting epitype is also designated here from a living culture (CBS 123669), in order to ensure accessibility to culturable and genetic material. The selected ex-epitype culture is a single-ascospore isolate of the third-generation progeny from a laboratory cross between two parental strains from the USA: one from P. sativum (strain T2) and the second one from soil (strain T129; Coleman et al. 2009). Although only one parent matches the type host (P. sativum), the epitype corresponds well with the pathogenicity, morphological and geographic width of the species. The ex-epitype culture has been extensively studied for its pathogenicity towards peas (Temporini & VanEtten 2002, Enkerli et al. 2007, Rodriguez-Carres et al. 2008, Coleman 2016) and has been commonly used as a reference strain for the phylogenetic clade referred to a F. solani f. sp. pisi (O’Donnell et al. 2008, Guarnaccia et al. 2018, Papizadeh et al. 2018, Sandoval-Denis et al. 2018). Additionally, the complete genome of this isolate (as Nec. haematococca MPVI 77-13-4) has been made available at the Joint Genome Institute (https://genome.jgi.doe.gov/Necha2/Necha2.home.html) and studied in depth (Coleman et al. 2009, Coleman 2016).
Fig. 32.
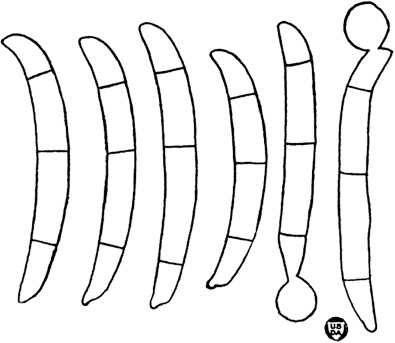
Neocosmospora pisi (lectotype of Fusarium martii var. pisi). Original illustration depicting conidia and chlamydospores by Jones (1923).
Morphologically, sporodochial conidia of N. pisi are similar in size to those of N. bostrycoides, N. cryptoseptata and N. protoensiformis. However, N. pisi differs from N. bostrycoides and N. protoensiformis by its slightly curved sporodochial conidia with barely notched foot cells and the presence of aerial, falcate, multiseptate conidia compared to the strongly curved conidia with well-developed, protuberant foot cells, and absence of aerial macroconidia in the last two species. Neocosmospora pisi differs from N. cryptoseptata in its slender sporodochial conidia with thick septa, while both micro- and macroconidia are present on aerial phialides. Microconidia are not produced in N. cryptoseptata.
Neocosmospora plagianthi (Dingley) L. Lombard & Crous, Stud. Mycol. 80: 227. 2015 — Fig. 33
Fig. 33.

Neocosmospora plagianthi (isotype of Nectria plagianthi BPI 801937). a–b. Herbarium specimen; c. detail of perithecia; d–e. detail of peridium cells (d. mounted on water; e. mounted on lactic acid); f–g. ascospores; h. conidia. — Scale bars: b–c = 1 cm; all others = 10 μm.
Basionym. Nectria plagianthi Dingley, Trans. Roy. Soc. New Zealand 79: 196. 1951.
Synonyms. Fusarium plagianthi (Dingley) O’Donnell & Geiser, Phytopathology 103: 404. 2013.
?Nectria pulverulenta Dingley, Trans. Roy. Soc. New Zealand 83: 657. 1956 (fide Samuels & Brayford 1994).
Typus. New Zealand, Fiordland, Hollyford Valley, on Plagianthus betulinus, 30 Dec. 1949, J.M. Dingley (holotype PDD 10916).
Descriptions & Illustrations — Dingley (1951), Samuels & Brayford (1994).
Additional material examined. New Zealand, Fiordland, Hollyford Valley, on Plagianthus betulinus, 30 Dec. 1949, J.M. Dingley, BPI 801937 (isotype of Nec. plagianthi); from Hoheria glabrata, G.J. Samuels, NRRL 22632 = G.J.S. 83-146.
Notes — Neocosmospora plagianthi and N. illudens comprise Clade 1 of Neocosmospora (O’Donnell 2000). Despite their genetic similarity, the two species exhibit clear morphological differences, particularly in their perithecial anatomy. This has resulted in the classification of the two species in different subgroups of Nectria in the past (Samuels & Brayford 1994). Both species are characterised by forming rather large striate ascospores, which distinguish them from most species with known sexual morphs in Clades 2 and 3. Neocosmospora plagianthi is unique in the genus since the ornamentation of its ascospores originates from a collapsed sheath that covers the young ascospores while they are still inside the asci. In contrast, the irregular striate ascospore pattern observed for other Neocosmospora spp., including N. illudens, is an integral part of the spore surface (Samuels & Brayford 1994). Thus far, no asexual morph is known for N. plagianthi. However, examination of the isotype revealed the presence of abundant 3–4-septate, (31–)34–44(–46.5) × 5–6 μm conidia (Fig. 33), similar to aerial macroconidia of known asexual morphs in Neocosmospora. Fresh collections of the fungus are needed in order to test these observations.
Neocosmospora protoensiformis Sand.-Den. & Crous, sp. nov. — MycoBank MB831197; Fig. 34
Fig. 34.
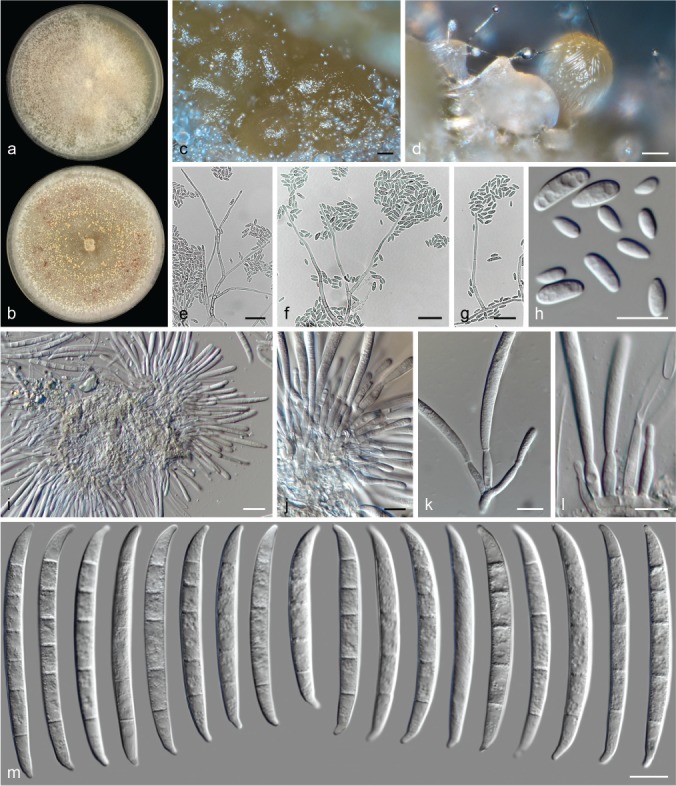
Neocosmospora protoensiformis (ex-type culture CBS 145471). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochia formed on the surface of carnation leaves; e–g. aerial conidiophores; h. aerial conidia; i–l. sporodochial conidiophores and phialides; m. sporodochial conidia. — Scale bars: c–d = 100 μm; e–g, i = 20 μm; all others = 10 μm.
Etymology. From Greek 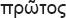 (prôtos), meaning ‘first’, and ensiforme; in reference to the species early evolutionary origin and its morphological similarity to Fusarium ensiforme.
(prôtos), meaning ‘first’, and ensiforme; in reference to the species early evolutionary origin and its morphological similarity to Fusarium ensiforme.
Typus. Venezuela, bark of dicot tree, unknown date, G.J. Samuels (holotype CBS H-23995 designated here, culture ex-type CBS 145471 = NRRL 22178 = G.J.S. 90-168).
Conidiophores abundant on substrate and aerial mycelia, erect, straight, smooth- and thin-walled, simple or branched several times irregularly, dichotomously or sympodially, bearing terminal, single monophialides; phialides subulate, subcylindrical to acicular, (26–)36–51(–58) × 2–3(–3.5) μm (av. 46.6 × 2.6 μm, n = 53), smooth- and thin-walled, rarely proliferating, conidiogenous loci without noticeable periclinal thickening or collarettes; aerial conidia obovate, short clavate to ellipsoidal, straight, rarely gently curved, 0–1-septate, hyaline, smooth- and thin-walled, (5–)6–9.5(–15) × (2.5–)3–4.5(–5.5) μm (av. 7.6 × 3.6 μm, n = 132), clustering in densely crowded false heads at tip of monophialides. Sporodochia cream, pale luteous, pale sienna to honey, turning brick to dark brick with age. Sporodochial conidiophores densely verticillately branched and packed, bearing terminal pairs of monophialides; sporodochial phialides subcylindrical to subulate, (10–)12.5–17(–19.5) × 3–4.5(–5) μm (av. 14.7 × 3.8 μm, n = 53), smooth- and thin-walled, conidiogenous loci without periclinal thickening or collarettes. Sporodochial conidia straight with curved ends to falcate, noticeably wider in upper third and tapering toward base; apical cell often longer than adjacent cell, conical with slightly curved and rounded apex; basal cell distinctly notched, basal tip sometimes slightly elongated, 4–6(–9)-septate, hyaline, smooth- and thick-walled; 4-septate conidia: (45–)49.5–60.5(–64) × 4.5–5.5(–6) μm (av. 55 × 5.2 μm, n = 40); 5-septate conidia: (49–)55–64.5(–69.5) × (4–)5–6 μm (av. 59.9 × 5.3 μm, n = 68); 6-septate conidia: 65.5–67.5 × 4.5–5 μm (av. 66.5 × 4.9 μm, n = 8); 9-septate conidia: 74.5 × 6 μm (n = 2); overall: (45–)53–65.5(–74.5) × (4–)4.5–6 μm (av. 59.2 × 5.3 μm, n = 116). Chlamydospores not observed.
Colonies on PDA growing in dark with an average radial growth rate of 4.2–5.5 mm/d at 24 °C, reaching 59–73 mm diam in 7 d at 24 °C; pale luteous to pale brick, flat, velvety, soon covered by patches of abundant, floccose, white to pale buff aerial mycelium forming inconspicuous concentric rings; margin entire. Reverse white to pale luteous with saffron patches. On OA incubated in dark reaching 75–81 mm diam in 7 d at 24 °C; white, pale buff at centre, flat, velvety to cottony with floccose periphery and abundant aerial mycelium, pale luteous to luteous sporodochia abundantly formed from centre of the colony, darkening when old; margin entire. Reverse pale luteous to ochreous, pale brick toward periphery.
Notes — The morphological and ecological characteristics of N. protoensiformis are similar to those reported for F. ensiforme, an old name of uncertain application that was characterised by isolates with large, up-to-7-septate sporodochial conidia, somewhat constricted at the apex and originally isolated from decaying Ficus sp. fruits in Honduras (Wollenweber & Reinking 1925). Nalim et al. (2011) ascribed these morphological features to a diverse arrangement of phylogenetic species, termed the ‘F. ensiforme clade’. Neocosmospora protoensiformis, however, did not cluster within the aforementioned clade, but clustered as one of the most basal lineages in Clade 3 in this study.
Notes by G.J. Samuels (pers. comm.) on the same isolate studied here, indicate that N. protoensiformis is a homothallic species, conspicuously producing the sexual morph, bearing the following characters: ascospores 9.7–14 × 4.7–7.3 μm (av. ± SD: 11.6 ± 1.2 × 6.2 ± 0.7 μm, n = 30), asci 53–105 × 8–13.8 μm (av. ± SD: 79 ± 13 × 10.6 ± 1.4 μm, n = 34) and perithecia 214–484 × 215–353 μm (av. ± SD: 310 ± 61 × 283 ± 35 μm, n = 21). We were unable to induce the sexual morph in culture, which indicates possible strain degeneration after long-term storage. However, these sexual features further distinguish N. protoensiformis from the described sexual morph of F. ensiforme (Hypomyces ipomoeae var. major), stated to produce smaller perithecia and asci (330 × 250 and 60–80 × 7–11 μm, respectively), but somewhat larger ascospores (13.6 × 5 μm; Wollenweber 1916, 1931), data supporting recognizing N. protoensiformis as a distinct species.
Neocosmospora pseudensiformis Samuels et al., Mycologia 103: 1323. 2011
Synonym. Fusarium pseudensiforme Samuels et al., Mycologia 103: 1323. 2011.
Typus. Sri Lanka, Wagamba Province, vic. Kurunegala, Arangakele, bark of tree, 12 Dec. 2002, G.J. Samuels, A. Nalim & K. Poldmaa (holotype BPI 871390, culture ex-type CBS 125729 = NRRL 46517 = G.J.S 02-95 = G.J.S 9318 = FRC S-1834).
Description & Illustration — Nalim et al. (2011).
Additional materials examined. Indonesia, Java, Bogor, on leaf of Cocos nucifera, unknown date, N. Hasnam, CBS 130.78 = NRRL 22575 = NRRL 22653. – Suriname, from human mycetoma, unknown date, A. Buiting, CBS 241.93.
Notes — Originally described from bark collected in Sri Lanka, N. pseudensiformis is now known to inhabit not only Southern Asia, but also other tropical regions around the globe including Indonesia and north eastern regions of South America (French Guiana and Suriname). Neocosmospora pseudensiformis is mostly known as a saprobic species but is also acknowledged as a human pathogen, recovered from a single case of mycetoma (De Hoog et al. 1993, Al-Hatmi et al. 2016).
As stated in the protologue of the species (Nalim et al. 2011), N. pseudensiformis shares morphological features with the concept of F. ensiforme, but clusters within the Ambrosia clade. Members of this clade often differ considerably from other species in Neocosmospora, not only in their morphology but also in their symbiotic ecologies. These species form an essential part of the life cycle of diverse shot-hole borer beetle species (Euwallaceae spp.; Freeman et al. 2013, O’Donnell et al. 2016, Aoki et al. 2018). Neocosmospora pseudensiformis differs from the other ambrosia-associated species in the production of typical wedge-shaped conidia instead of the irregularly clavate conidia commonly observed in the Ambrosia fusaria. In addition, the sporodochial conidia of N. pseudensiformis differ considerably from those attributed to F. ensiforme by exhibiting rounded and hooked apical cells and barely notched basal cells, differing from the rather acute, non-hooked conidia with elongated foot cells described for the latter species (Wollenweber & Reinking 1925).
Neocosmospora pseudoradicicola Sand.-Den. & Crous, sp. nov. — MycoBank MB831198; Fig. 35
Fig. 35.
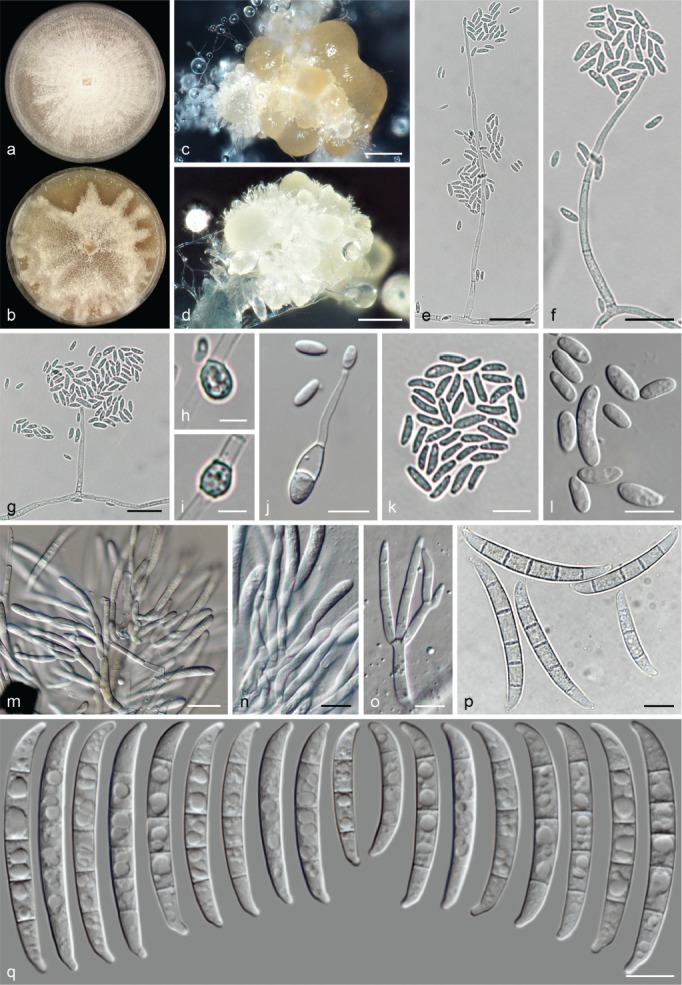
Neocosmospora pseudoradicicola (ex-type culture CBS 145472). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochia formed on the surface of carnation leaves; e–g. aerial conidiophores; h–i. chlamydospores; j. microcyclic conidiogenesis on aerial conidia; k–l. aerial conidia; m–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–d = 100 μm; e–g, m = 20 μm; h–i = 5 μm; all others = 10 μm.
Etymology. Name refers to its morphological approximation of the concept of Fusarium radicicola (syn. N. solani), notwithstanding slight differences.
Typus. Papua New Guinea, East New Britain, Keravat, Lowlands Agricultural Experiment Station, from diseased cocoa pods, unknown date and collector (holotype CBS H-23996, designated here, culture ex-type CBS 145472 = NRRL 25137 = ARSEF 2313).
Conidiophores abundant on substrate and aerial mycelium, straight, smooth- and thin-walled, rarely minutely verruculose at base, mostly simple or sparingly dichotomously branched, bearing terminal, single monophialides; phialides subulate, (39.5–)41.5–55.5(–78) × (2–)2.5–4(–4.5) μm (av. 48.7 × 3.1 μm, n = 54), smooth- and thin-walled, often proliferating, conidiogenous loci with conspicuous periclinal thickening and a minute, non-flared collarette; aerial conidia obovoidal, ellipsoidal, clavate or allantoid, base discreetly flattened, 0(–1)-septate, hyaline, smooth- and thin-walled, (5.5–)6.5–11.5(–18.5) × (2.5–)3–4.5(–6.5) μm (av. 8.9 × 3.7 μm, n = 84), clustering in false heads on tip of monophialides, microcyclic conidiation commonly observed. Sporodochia cream, pale straw to luteous. Sporodochial conidiophores dense, verticillately or irregularly branched; sporodochial phialides subcylindrical to somewhat doliiform, (12.5–)14–20(–26) × (3.5–)4–5 μm (av. 17.1 × 4.2 μm, n = 39), smooth- and thin-walled, periclinal thickening and collarettes inconspicuous or absent. Sporodochial conidia moderately curved, wedge-shaped, apical cell usually shorter than adjacent cell, blunt to slightly hooked and constricted; basal cell distinctly notched and protuberant, (2–)3–5-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 24.5 × 4 (n = 2); 3-septate conidia: (24.5–)29.5–40(–45) × (3.5–)4–5(–5.5) μm (av. 34.9 × 4.5 μm, n = 66); 4-septate conidia: (39–)41.5–50.5(–53.5) × (4–)4.5–5.5 μm (av. 45.9 × 4.9 μm, n = 38); 5-septate conidia: (47–)48–53.5 × (4.5–)5–6 μm (av. 50.9 × 5.4 μm, n = 8); overall: (24.5–)31.5–47.5(–53.5) × (3.5–)4–5(–6) μm (av. 39.5 × 4.7 μm, n = 114). Chlamydospores globose to subglobose, smooth-walled to verruculose and thick-walled, 5–6.5(–7) μm diam, terminal or intercalary, solitary or in short chains.
Colonies on PDA growing in dark with an average radial growth rate of 3.9–5 mm/d at 24 °C, reaching 55–70 mm diam in 7 d at 24 °C; white, flat, velvety, felty to granulose, with abundant white mycelium forming concentric rings and radial patches; margin entire. Reverse pale luteous, luteous to ochreous. On OA incubated in dark reaching 65–74 mm diam in 7 d at 24 °C; white, pale luteous to pale brick, flat, velvety to granulose, with abundant white aerial mycelium in irregular patches; margin entire. Reverse pale luteous, pale orange or peach.
Notes — Morphologically, N. pseudoradicicola is similar to Wollenweber’s concept of F. radicicola (syn. N. solani = F. javanicum var. radicicola), an old species originally recovered from I. batatas and S. tuberosum and characterised by slightly curved, pedicellate sporodochial conidia with distinctly constricted apices (Wollenweber 1914, 1931, Sherbakoff 1915). The latter species, however, here reduced to synonymy with N. solani s.str. (Schroers et al. 2016) The distinct host association (Malvales in N. pseudoradicicola vs Solanales in F. radicicola) and biogeography support the proposal of N. pseudoradicicola as a new, distinct species.
The sporodochial conidia of N. pseudoradicicola match in overall size and septation with those of N. crassa, N. stercicola and N. solani, but the conidia of the last three species are more robust and less tapered, with rounded apices, and their basal cells are less developed and not as protuberant as those of N. pseudoradicicola. In addition, N. pseudoradicicola lacks aerial, multiseptate, falcate conidia, but exhibits microcyclic conidiation in vitro.
Neocosmospora pseudotonkinensis Sand.-Den. & Crous, sp. nov. — MycoBank MB831199; Fig. 36
Fig. 36.
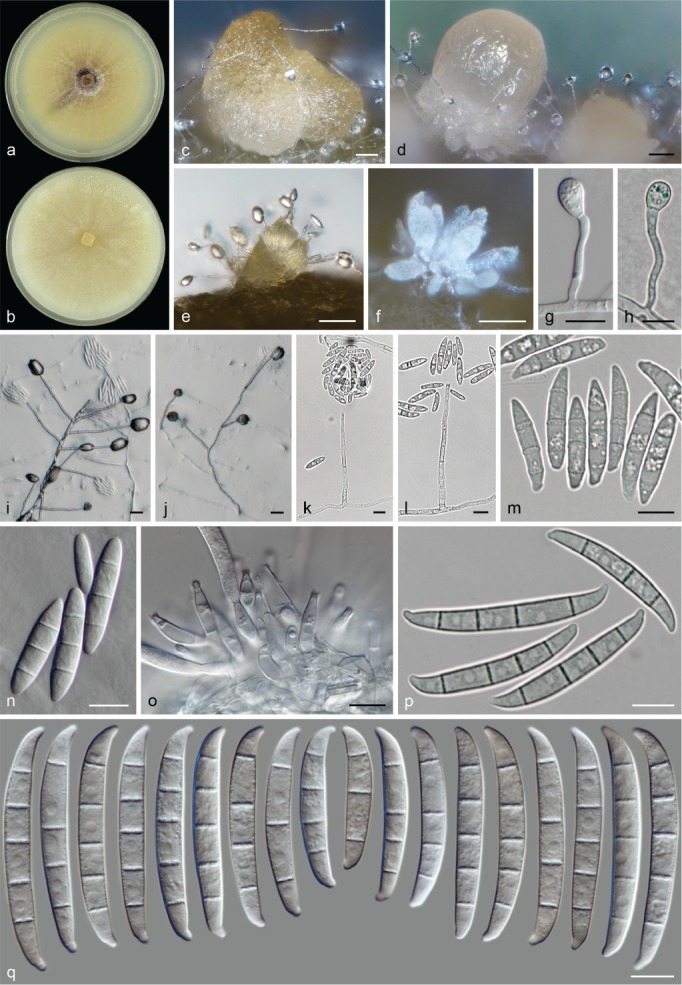
Neocosmospora pseudotonkinensis (ex-type culture CBS 143038). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g–h. chlamydospores; i–l. aerial conidiophores; m–n. aerial conidia; o. sporodochial conidiophores; p–q. sporodochial conidia. — Scale bars: c–e = 50 μm; f, i–j = 20 μm; all others = 10 μm.
Etymology. Name refers to its morphological affinity, although this species differs to some extent from N. tonkinensis.
Typus. Netherlands, Leiden, from human cornea, unknown date, M.T. van der Beek (holotype CBS H-23997 designated here, culture ex-type CBS 143038).
Conidiophores abundant on substrate mycelium, erect or prostrate, or lateral on aerial mycelium, straight, smooth- and thin-walled, mostly simple, rarely sparingly branched irregularly or verticillately, bearing terminal and lateral, single monophialides; phialides subulate, subcylindrical to acicular, (47.5–)50.5–66(–74) × (2–)2.5–4(–4.5) μm (av. 58.3 × 3.3 μm, n = 50), smooth- and thin-walled, often proliferating percurrently, conidiogenous loci with periclinal thickening and an short, flared collarette; aerial conidia of two types: microconidia oval, ellipsoidal to clavate, straight, 0–1-septate, hyaline, smooth- and thin-walled, (9.5–)14.5–22(–25) × (3.5–)4.5–6(–6.5) μm (av. 18.3 × 5.1 μm, n = 92), clustering in false heads on tip of monophialides; macroconidia fusiform, falcate to navicular and multiseptate, gently dorsiventrally curved, base barely notched, (2–)3-septate, smooth- and thick-walled; 2-septate conidia: 24–26.5(–27.5) × 5–6.5 μm (av. 25.4 × 5.5 μm, n = 14); 3-septate conidia: (20–)24.5–32(–39) × (4.5–)5–6(–7) μm (av. 28.1 × 5.5 μm, n = 90); overall: (20–)24–31.5(–39) × (4.5–)5–6(–7) μm (av. 27.6 × 5.5 μm, n = 104). Sporodochia white, cream, light green to olive-green. Sporodochial conidiophores simple or sparingly branched, verticillately or irregularly; sporodochial phialides subcylindrical, subulate to ampulliform, (13.5–)15.5–19.5(–22.5) × (3.5–)4–4.5(–5.5) μm (av. 17.3 × 4.2 μm, n = 96), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and a short, non-flared collarette. Sporodochial conidia moderately dorsiventrally curved with nearly parallel lines, slightly widened in middle portion or the upper third and narrowing toward base; apical cell often of equal length or shorter than adjacent cell, blunt, sometimes somewhat conical with a curved and rounded apex; basal cell distinctly notched, prominent, with a broad, rounded end, 3–5-septate, hyaline, smooth- and thick-walled; 3-septate conidia: (31.5–)35.5–44(–44.5) × (5–)5.5–6.5 μm (av. 39.7 × 5.8 μm, n = 26); 4-septate conidia: (37.5–)42.5–50(–54.5) × (5–)5.5–6.5(–7) μm (av. 46.3 × 5.9 μm, n = 192); 5-septate conidia: (45–)46.5–52.5(–57) × (5–)5.5–6.5 μm (av. 49.5 × 6 μm, n = 66); overall: (31.5–)42–51(–57) × (5–)5.5–6.5(–7) μm (av. 46.5 × 5.9 μm, n = 284). Chlamydospores abundant, globose to subglobose, smooth- and thick-walled, (6–)6.5–10(–13) μm diam, terminal or intercalary in hyphae or conidia, mostly borne on stalk-like, subcylindrical lateral hyphal projections up to 50 μm long, solitary or in short chains.
Colonies on PDA growing in dark with an average radial growth rate of 2.5–3 mm/d at 24 °C, reaching 34–40 mm diam in 7 d at 24 °C; pale luteous to ochreous, occasionally olivaceous to fuscous black at centre, flat, velvety to cottony, concentric rings of sparse aerial mycelium may be formed; margin entire with abundant submerged mycelium. Reverse pale luteous, sienna to olivaceous. On OA incubated in dark reaching 40–45 mm diam in 7 d at 24 °C; white, cream to pale buff, flat, velvety to granulose; margin entire. Reverse cream to buff.
Notes — Neocosmospora pseudotonkinensis mirrors the cultural and microscopic characteristics of N. tonkinensis. Despite this, the two species are clearly genetically distinct. They are difficult to distinguish from each other based on morphology, as they both exhibit typical 3-septate, fusiform to navicular aerial conidia, produced on slender, elongated aerial phialides. The only features that can be used to distinguish them are the sporodochial conidia, which are larger in N. pseudotonkinensis (31.5–57 μm vs 27.5–50.5 μm long in N. tonkinensis), and have slightly elongated and more strongly curved apical cells. Another species with similar conidial dimensions is N. crassa, but it differs from N. pseudotonkinensis by the production of almost straight sporodochial conidia, commonly widened in the basal third.
Neocosmospora quercicola Sand.-Den. & Crous, sp. nov. — MycoBank MB831200; Fig. 37
Fig. 37.
Neocosmospora quercicola (ex-type culture CBS 141.90). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–l. aerial conidiophores; m. aerial conidia; n–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–f = 100 μm; g–i = 20 μm; o = 5 μm; all others = 10 μm.
Etymology. Name refers to the plant host genus Quercus, from which the ex-type strain was isolated in association with tree decline.
Typus. Italy, from Quercus cerris wood, declined tree, 25 m high on the trunk, unknown date, A. Ragazzi (holotype CBS H-23998 designated here, culture ex-type CBS 141.90 = NRRL 22652).
Conidiophores erect or prostrate on substrate mycelium, abundant on aerial mycelium, straight, smooth- and thin-walled, simple or sparingly verticillately branched, bearing terminal monophialides; phialides subcylindrical to subulate, (27–)40–56.5(–62.5) × 2–3(–3.5) μm (av. 48.1 × 2.6 μm, n = 68), smooth- and thin-walled, rarely proliferating, conidiogenous loci with inconspicuous or absent periclinal thickening, collarettes if present, relatively short and non-flared; aerial conidia oval, ellipsoidal to clavate, straight or gently curved, 0(–1)-septate, hyaline, smooth- and thin-walled, (4–)5–12(–22) × (2–)2.5–4.5(–5.5) μm (av. 8.5 × 3.4 μm, n = 98), clustering in false heads on tip of monophialides. Sporodochia pale luteous, light green to rosy-buff. Sporodochial conidiophores unbranched or sparingly verticillately branched; sporodochial phialides lageniform, subulate to subcylindrical, (11.5–)14.5–21.5(–23.5) × (2.5–)3–4 μm (av. 18 × 3.3 μm, n = 76), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, non-flared collarette. Sporodochial conidia barely dorsiventrally curved, almost straight in its ventral portion, tapering toward base; apical cell of equal or shorter length than adjacent cell, blunt and slightly hooked; basal cell often distinctly notched, (1–)3–6(–7)-septate, hyaline, smooth- and thick-walled; 1-septate conidia: 24 × 4 μm (n = 1); 3-septate conidia: (32–)35–45(–49) × (3.5–)4–6(–6.5) μm (av. 40 × 4.9 μm, n = 18); 4-septate conidia: (43–)47.5–61.5(–67.5) × 4.5–5.5(–6) μm (av. 54.7 × 5.1 μm, n = 22); 5-septate conidia: (51–)61–71.5(–75.5) × (4.5–)5–6(–7) μm (av. 66.3 × 5.6 μm, n = 94); 6-septate conidia: (62–)65–82(–96.5) × 5–6.5 μm (av. 73.7 × 5.7 μm, n = 24); overall: (24–)50–75(–96.5) × (3.5–)4.5–6(–7) μm (av. 62.5 × 5.4 μm, n = 159). Chlamydospores abundant, globose to subglobose, smooth-walled, rarely slightly verruculose and thick-walled, 4–6 μm diam, terminal or intercalary, solitary, in chains or clusters. Colonies on PDA growing in dark with an average radial growth rate of 3.8–4.6 mm/d at 24 °C, reaching 52–64 mm diam in 7 d at 24 °C; straw, sulphur yellow to pale luteous, flat, velvety, felty to granular, commonly with concentric rings and irregular patches of straw aerial mycelium; margin entire. Reverse pale luteous, saffron to ochreous. On OA incubated in dark reaching 26–31 mm diam in 7 d at 24 °C; white, salmon to pale saffron, flat, velvety, floccose or granular; margin entire. Reverse pale luteous to luteous.
Additional materials examined. Germany, from human toenail, unknown date, E. Döllefeld, CBS 334.92 = NRRL 25726. – USA, Michigan, from human eye, 1977, M. Bianchedi, CBS 130177 = NRRL 22611 = UTHSC 93-2524.
Notes — The circumscription of N. quercicola includes a fungus suspected of being responsible for the decline of forests of Quercus spp. in Cornuda and Muzzana del Turgnano, north-eastern Italy, during the late 1980s (Ragazzi et al. 1993). The selected holotype of N. quercicola was originally isolated from woody tissues of declining Quercus cerris, and was then assigned to F. eumartii. The identification, however, was based on the wide, polyphyletic morphological concept of the species (Gerlach & Nirenberg 1982). Morphologically, this species resembles F. eumartii sensu Wollenweber (1931) and Gerlach & Nirenberg (1982) by its relatively large, up to 7-septate sporodochial conidia. However, the sporodochial conidia show little resemblance to structures described in the original circumscription of F. eumartii (Carpenter 1915), now reduced to synonymy under N. solani.
Sporodochial conidia of N. quercicola are markedly slender and tapered toward the base, differing from the robust conidia of N. brevicona and N. suttoniana, the closest morphological relatives. Neocosmospora paraeumartii, a phylogenetically distant species, is morphologically almost indistinguishable from N. quercicola (see notes under N. paraeumartii).
Neocosmospora rectiphora Samuels et al., Mycologia 103: 1324. 2011
Synonyms. Fusarium rectiphorum Samuels et al., Mycologia 103: 1324. 2011.
Neocosmospora bomiensis Z.Q. Zeng & W.Y. Zhuang, Phytotaxa 319: 177. 2017.
Typus. Sri Lanka, Wagamba Province, vic. Kurunegala, Arangakele, on recently dead tree, 12 Dec. 2002, G.J. Samuels, A. Nalim & K. Poldmaa, (holotype BPI 871364, culture ex-type CBS 125727 = G.J.S. 02-89 = FRC S-1831).
Description & Illustration — Nalim et al. (2011).
Additional materials examined. Sri Lanka, Southern Province, Yala National Park, Block 1, c. 10 km NE of park headquarters in low thorn scrub forest interspersed with grassland, Akasa chawtya rock area, from a dead branch of small Kora gaha, 18 Dec. 2002, G.J. Samuels, A. Nalim & N. Dayawansa, CBS 119603 = G.J.S. 02-129 = FRC S-1843; from a tree, unknown date, G.J. Samuels, CBS 124755 = G.J.S. 04-179; Block 3, along a newly cut road through more or less tall forest to bungalow from army post, from a recently dead tree, 19 Dec. 2002, G.J. Samuels & N. Dayawansa, CBS 125726 = G.J.S. 02-127 = FRC S-1842.
Notes — According to Nalim et al. (2011), N. rectiphora, a member of Clade 2 (O’Donnell 2000), is characterised by its short, erect aerial conidiophores. It is morphologically similar to N. mahasenii from which it differs by having faster growth rates and by producing somewhat longer ascospores. It has shorter sporodochial conidia than most members of Clade 2, including N. acutispora, N. cryptoseptata, N. kurunegalensis, N. nirenbergiana, N. robusta and N. samuelsii, although larger than those of N. keleraja.
Based on DNA barcodes, Neocosmospora bomiensis, a recently introduced species from an unknown host in Tibet (Zeng & Zhuang 2017), clusters within the wide phylogenetic span known for N. rectiphora. The ex-type strain of N. bomiensis formed a long branch together with strain NRRL 22396, from French Guiana, mostly due to missing sequence data (LSU and rpb2 sequences). Unfortunately, these strains were not available to us. Therefore, N. bomiensis is here reduced to synonymy under N. rectiphora with some reservations given the discordant biogeography of HMAS 254519 and NRRL 22396.
Neocosmospora regularis Sand.-Den. & Crous, sp. nov. — MycoBank MB831201; Fig. 38
Fig. 38.
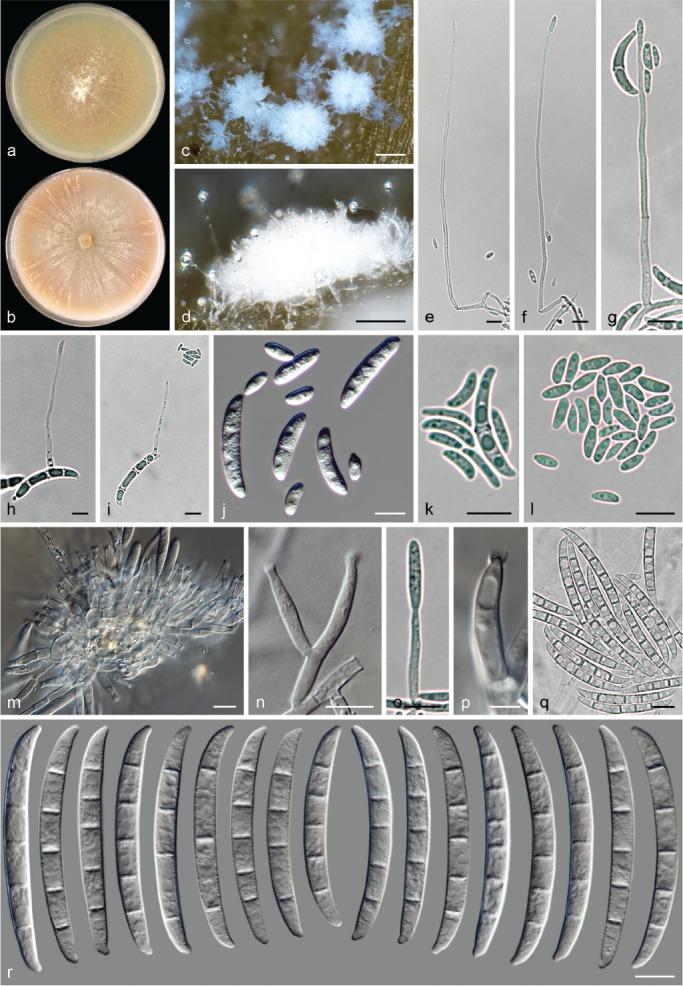
Neocosmospora regularis (ex-type culture CBS 230.34). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochia formed on the surface of carnation leaves; e–g. aerial conidiophores; h–i. microcyclic conidiogenesis on aerial conidia; j–l. aerial conidia; m–p. sporodochial conidiophores and phialides; q–r. sporodochial conidia. — Scale bars: c–d = 100 μm; p = 5 μm; all others = 10 μm.
Etymology. From Latin rēgulāris, meaning regular, having a constant pattern, and showing evenness of form or appearance; referring to the continuous and regular curvature of sporodochial macroconidia.
Typus. Netherlands, Zuid Beveland, near Kloetinge, from Pisum sativum, unknown date, J.C. Went (holotype CBS H-23999 designated here, culture ex-type CBS 230.34).
Conidiophores on substrate and aerial mycelium straight, smooth- and thin-walled, mostly simple, rarely sparingly verticillately or sympodially branched, bearing terminal, single monophialides; phialides elongate subulate to subcylindrical, (44.5–)51.5–67(–77) × (1.5–)2–3.5(–4) μm (av. 59.2 × 2.9 μm, n = 92), smooth- and thin-walled, conidiogenous loci with periclinal thickening and a short non-flared collarette; aerial conidia of two types: microconidia ovoid, ellipsoidal to subcylindrical, straight to slightly reniform, 0–1-septate, hyaline, smooth- and thin-walled, (6–)7.5–13.5(–20.5) × (2.5–)3–4.5(–5) μm (av. 10.6 × 3.6 μm, n = 95), clustering in discrete false heads on tip of monophialides; macroconidia short falcate and multiseptate, gently dorsiventrally curved, base often rounded, rarely barely notched, 3–4-septate, smooth- and thick-walled, produced rarely and clustering as false heads on tip of monophialides; 3-septate conidia: (23–)23.5–26.5 × 4–5.5 μm (av. 25.1 × 4.8 μm, n = 50); 4-septate conidia: 23–29.5(–30) × 3.5–5.5 μm (av. 26.3 × 4.7 μm, n = 36); overall: 23–28(–29.5) × (3.5–)4–5.5 μm (av. 25.5 × 4.7 μm, n = 86), microcyclic conidiation often observed on short falcate aerial conidia. Sporodochia white to pale luteous, often dry in appearance. Sporodochial conidiophores densely irregularly or verticillately branched; sporodochial phialides subcylindrical, subulate to doliiform, (10.5–)14.5–19.5(–22) × (2.5–)3–4.5(–5.5) μm (av. 17.1 × 3.8 μm, n = 43), smooth- and thin-walled, conidiogenous loci with inconspicuous or lacking periclinal thickening and collarette. Sporodochial conidia moderately to distinctly curved, regularly arcuate with nearly parallel lines, gently tapering toward both ends; apical cell often about equal length than adjacent cell, blunt, non-hooked; basal cell barely to distinctly notched, (3–)4–5(–6)-septate, hyaline, smooth- and thick-walled; 3-septate conidia: 43.5–54.5(–57) × 4.5–5.5(–6) μm (av. 49.1 × 5.1 μm, n = 10); 4-septate conidia: (46.5–)49.5–56.5(–60.5) × 4.5–6 μm (av. 52.9 × 5.3 μm, n = 58); 5-septate conidia: (49–)54.5–62(–69) × (4.5–)5.5–6(–7) μm (av. 58.2 × 5.7 μm, n = 170); 6-septate conidia: 58–59.5 × 5.5–7 μm (av. 58.7 × 6.2 μm, n = 5); overall: (43.5–)52–61(–69) × (4.5–)5–6(–7) μm (av. 56.5 × 5.6 μm, n = 243). Chlamydospores not seen.
Colonies on PDA growing in dark with an average radial growth rate of 3.6–4 mm/d at 24 °C, reaching 50–56 mm diam in 7 d at 24 °C; straw, luteous, sienna, pale grey-green to olivaceous buff, flat, velvety to floccose, radially striated and with irregular patches of white aerial mycelium; margin entire. Reverse pale luteous, ochreous to olivaceous buff. On OA incubated in dark covering an entire 90 mm diam Petri dish in 7 d at 24 °C; pale saffron to brick or olivaceous buff to green olivaceous, flat, at first membranous turning granular to velvety and then pionnotal after strong sporodochia formation; margin entire. Reverse pale brick, cinnamon to grey-yellow-green.
Additional material examined. USA, California, from Phaseolus sp., unknown date, W.C. Snyder, CBS 190.35.
Notes — This clade includes strains previously assigned to the special forms phaseoli and pisi. These two special forms are currently restricted to isolates of N. phaseoli and N. pisi. The last two species and N. regularis all produce at least two types of aerial conidia (microconidia and short falcate, multiseptate conidia) from long and thin monophialides, plus falcate sporodochial conidia. In N. regularis, short falcate conidia are rare, and are commonly formed intermixed with microconidia on the same aerial monophialide, often lacking a clearly defined fusarium-like shape and foot cells. Sporodochial conidia are highly similar in general shape and size for all three taxa. However, in N. regularis, they are distinguished by septation (up to 6-septate vs up to 5-septate in N. phaseoli and N. pisi), and by having conical, barely curved apical cells in contrast to the acute cells seen in N. phaseoli. Additionally, the sporodochial conidia of N. regularis are slightly larger than those of N. pisi, and have a much more pronounced central curvature and a less protuberant basal cell. Similarly, aerial falcate conidia are shorter but more frequently septate (3–4-septate) than those of N. phaseoli (commonly 3-septate, rarely 4-septate) and N. pisi (1–3-septate), while N. regularis exhibits microcyclic conidiation, a feature uncommonly observed in the genus Neocosmospora.
Neocosmospora riograndensis (Dallé Rosa et al.) Sand.-Den. & Crous, comb. nov. — MycoBank MB831202
Basionym. Fusarium riograndense Dallé Rosa et al., J. Mycol. Med. 28: 33. 2018.
Typus. Brazil, Rio Grande do Sul, Porto Alegre, Hospital de Clínicas de Porto Alegre, from human nasal cavity, 2014, unknown collector (holotype UFMG-CM F12570, culture ex-type UFMG-CM F12570).
Description & Illustration — Dallé Rosa et al. (2018).
Notes — This monotypic, human pathogenic species was recently introduced for an isolate described as the cause of invasive rhinosinusitis in a leukemic patient in Brazil (Dallé Rosa et al. 2018). It was erroneously assigned to phylogenetic species FSSC 36, a clade designation already occupied by the ambrosia fungus N. euwallaceae (FSSC 36 = AF-2). Neocosmospora riograndensis is phylogenetically closely related to N. cucurbitae and N. protoensiformis, but can be distinguished by the production of much shorter and bulkier sporodochial conidia.
Neocosmospora robusta Sand.-Den. & Crous, sp. nov. — MycoBank MB831203; Fig. 39
Fig. 39.
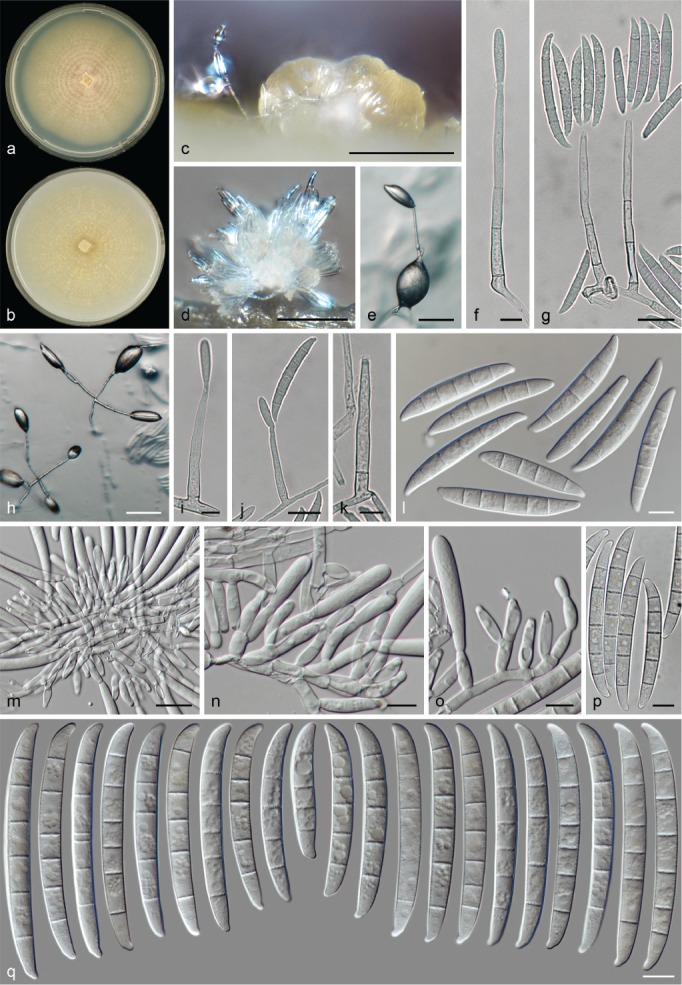
Neocosmospora robusta (ex-type culture CBS 145473). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–d. sporodochia formed on the surface of carnation leaves; e–k. aerial conidiophores and phialides; l. aerial conidia; m–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–d = 100 μm; e, h = 50 μm; g, j, m = 20 μm; all others = 10 μm.
Etymology. From Latin rōbustus, meaning ‘robust’; referring to its wide, thick-walled sporodochial conidia.
Typus. Venezuela, from bark, unknown date and collector (holotype CBS H-24000 designated here, culture ex-type CBS 145473 = NRRL 22395 = BBA 65682).
Conidiophores erect or prostrate on substrate mycelium and abundantly produced laterally on aerial mycelium, smooth- and thin-walled, often simple and straight, rarely sparingly verticillately or sympodially branched, bearing terminal, single monophialides; phialides subulate to acicular, (31–)38.5–52(–62.5) × (3–)3.5–5.5(–6) μm (av. 45.4 × 4.4 μm, n = 64), smooth- and thin-walled, conidiogenous loci with visible periclinal thickening and a minute, non-flared collarette; aerial conidia navicular to falcate and multiseptate, straight or gently curved, base flattened or barely to distinctly notched, 3–5-septate, smooth- and thick-walled; 3-septate conidia: (31–)33.5–42.5(–45.5) × 6–7.5 μm (av. 38.1 × 6.8 μm, n = 40); 4-septate conidia: (43–)46–56.5(–59) × (5.5–)6–7.5(–8) μm (av. 51.4 × 6.8 μm, n = 60); 5-septate conidia: (41.5–)45.5–57.5(–58.5) × (6–)6.5–8(–8.5) μm (av. 51.5 × 7.1 μm, n = 44); overall: (31–)39.5–56(–59) × (5.5–)6–7.5(–8.5) μm (av. 47.7 × 6.9 μm, n = 144). Sporodochia cream to pale luteous. Sporodochial conidiophores densely verticillately or irregularly branched; sporodochial phialides subcylindrical to doliiform, (11.5–)13.5–18.5(–22) × (3.5–)4–5(–5.5) μm (av. 15.9 × 4.5 μm, n = 104), smooth- and thin-walled, periclinal thickening and collarettes inconspicuous or absent. Sporodochial conidia falcate to almost straight; apical cell slightly larger than adjacent cell, blunt and moderately curved with rounded apex; basal cell notched, often prominent, (3–)5–6(–7)-septate, hyaline, smooth- and thick-walled; 3-septate conidia: 37–48 × 6–7 μm (n = 4); 4-septate conidia: 39.5–43 × 66.5–68.5 μm (n = 3); 5-septate conidia: (48.5–)60.5–71.5(–74) × (6–)6.5–7.5 μm (av. 66 × 6.9 μm, n = 82); 6-septate conidia: (64.5–)68.5–76(–82) × (6–)6.5–7.5 μm (av. 72.4 × 7 μm, n = 122); 7-septate conidia: (69.5–)70–79(–85.5) × 6.5–7.5 μm (av. 74.6 × 7 μm, n = 32); overall: (37–)61–77.5(–85.5) × (6–)6.5–7.5 μm (av. 69.1 × 6.9 μm, n = 243). Chlamydospores not observed.
Colonies on PDA growing in dark with an average radial growth rate of 3–4 mm/d at 24 °C, reaching 41–51 mm diam in 7 d at 24 °C; straw to pale luteous, pale sienna at centre, flat to slightly folded radially, velvety to felty, finely granular at the periphery, with central concentric rings of short and dense aerial mycelium; margin entire. Reverse straw, pale luteous to pale orange. On OA incubated in dark reaching 28–47 mm diam in 7 d at 24 °C; straw to sulphur yellow, flat, velvety becoming granulose, with scant aerial mycelium arranged in radial patches and concentric rings, soon becoming pionnotal; margin entire. Reverse pale luteous.
Notes — Known from a single collection from South America, N. robusta clusters within Clade 2, sharing the typical morphological features of members of this clade, i.e., producing large, multiseptate, falcate conidia from sporodochia as well as from relatively short and robust mononematous aerial conidiophores.
Neocosmospora robusta is distinguished from the closest phylogenetic relative, N. acutispora, by the production of mostly 5-septate sporodochial conidia with elongated apical and basal cells, contrasting with the predominantly 6-septate conidia with short and rounded apical cells of N. robusta.
The phylogenetically distant species, N. silvicola, can also produce sporodochial conidia of similar size and septation, but differs in having narrower (av. 5.9 μm wide) and predominantly 5-septate sporodochial conidia. Additionally, N. silvicola produces microconidia on its aerial conidiophores, while aerial macroconidia are absent.
Neocosmospora samuelsii Sand.-Den. & Crous, sp. nov. — MycoBank MB831204; Fig. 40
Fig. 40.
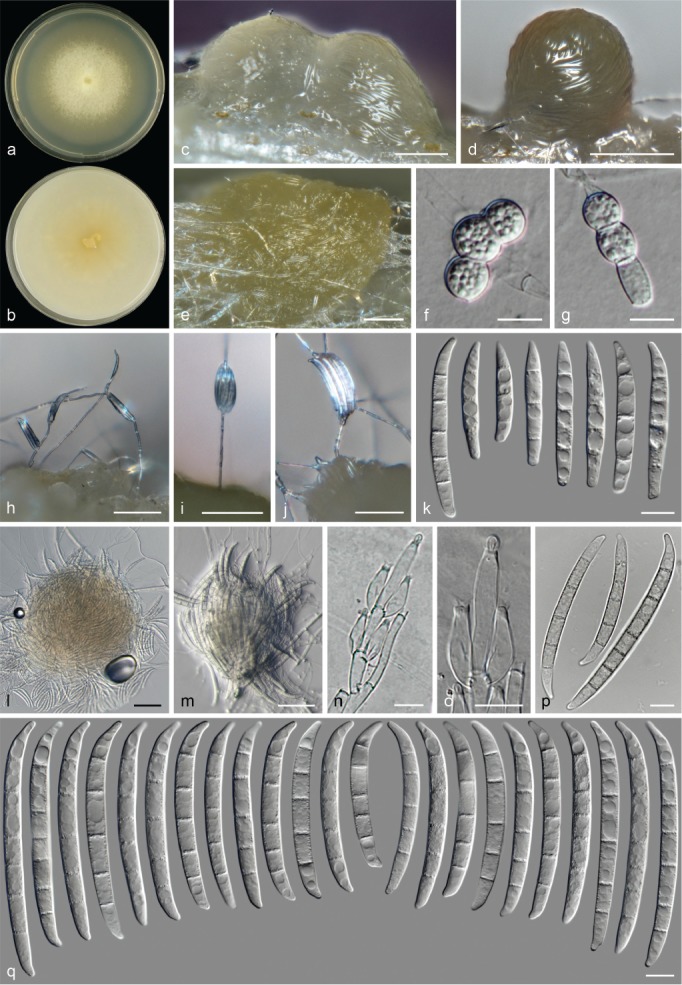
Neocosmospora samuelsii (ex-type culture CBS 114067). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. sporodochia formed on the surface of carnation leaves; f–g. chlamydospores; h–j. aerial conidiophores; k. aerial conidia; l–m. sporodochia formed on the agar surface; n–o. sporodochial conidiophores and phialides; p–q. sporodochial conidia. — Scale bars: c–e = 100 μm; j, l–m = 50 μm; all others = 10 μm.
Etymology. In honour of Dr Gary J. Samuels, for his contributions to the taxonomy of hypocrealean fungi, and collector of many of the isolates included in this study.
Typus. Guyana, Mt Wokomung, on ridge leading NW toward summit, 0.5–1 h walk from Base Camp, on bark, 1 July 1989, G.J. Samuels, B.M. Boom & G. Bacchus (holotype CBS H-24001 designated here, culture ex-type CBS 114067 = G.J.S. 89-70).
Conidiophores commonly erect, borne on substrate mycelium or more rarely formed laterally on aerial mycelium, straight, smooth- and thin-walled, simple or irregularly or sympodially branched, rarely proliferating laterally to previous conidiogenous loci, bearing terminal and lateral, single monophialides; phialides subulate to subcylindrical, (35–)45.5–56(–60) × 2–3.5(–4) μm (av. 50.7 × 2.8 μm, n = 60), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening and collarettes; aerial conidia navicular, fusiform to falcate and multiseptate, straight or curved, base commonly flattened to barely notched, 3(–4–5)-septate, smooth- and thick-walled; 3-septate conidia: (31–)33.5–43(–47) × 4.5–5.5(–6) μm (av. 38.4 × 5.1 μm, n = 44); 4-septate conidia: 44.5–49 × 5–6 μm (av. 46.8 × 5.5 μm, n = 8); 5-septate conidia: 47.5–51.5 × 5.5–6.5 μm (av. 49.4 × 5.9 μm, n = 8); overall: (31–)35–47(–51) × 4.5–6(–6.5) μm (av. 41 × 5.3 μm, n = 60). Sporodochia cream, light green to olive-green. Sporodochial conidiophores verticillately branched; sporodochial phialides subcylindrical to doliiform, 13.5–21.5(–26.5) × (3.5–)4.5–6(–6.5) μm (av. 17.4 × 5.1 μm, n = 60), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, non- to slightly flared collarette. Sporodochial conidia moderately curved, wedge-shaped, often tapering and straighten toward base; apical cell of equal length or larger than adjacent cell, blunt to conical, with a somewhat extended, hooked and rounded apex; basal cell barely to distinctly notched and straight, (4–)5–6(–7)-septate, hyaline, smooth- and thick-walled; 4-septate conidia: 57 × 6.5 μm (n = 2); 5-septate conidia: (52–)58.5–74.5(–82.5) × (5–)5.5–7 μm (av. 66.5 × 6.4 μm, n = 100); 6-septate conidia: (73–)75.5–88(–91.5) × (5.5–)6–7 μm (av. 81.9 × 6.5 μm, n = 40); 7-septate conidia: 85 × 6.5 μm (n = 2); overall: (52–)60–81.5(–91.5) × (5–)6–7 μm (av. 70.9 × 6.4 μm, n = 144). Chlamydospores globose to subglobose, smooth- and thick-walled, rarely granulate to verruculose, 5.5–8(–10) μm diam, terminal or intercalary, solitary, in chains or clusters. Colonies on PDA growing in dark with an average radial growth rate of 2.5–3 mm/d at 24 °C, reaching 34–41 mm diam in 7 d at 24 °C; straw to pale luteous, flat, velvety to felty, with abundant peripheral submerged mycelium; margin entire. Reverse white to luteous. On OA incubated in dark reaching 18–21 mm diam in 7 d at 24 °C; ochreous to buff, pale luteous to luteous at centre, flat, membranous becoming velvety; margin entire. Reverse cream to pale luteous.
Notes — The ex-type strain of N. samuelsii was originally identified as belonging to H. haematococca, indicating that a sexual morph was present. However, we were not able to induce this morph in culture during this study. This species produces the longest sporodochial conidia of all species included in Clade 2. However, sporodochial conidia of similar size are known for members of Clade 1, namely, N. borneensis and N. mori. Neocosmospora samuelsii is distinguished from both these Clade 1 species by lacking microconidia, while also showing distinct wedge-shaped sporodochial conidia. This contrasts with the continuous and regular curvature of sporodochial conidia in N. borneensis and the almost straight sporodochial conidia of N. mori.
Neocosmospora silvicola Sand.-Den. & Crous, sp. nov. — MycoBank MB831205; Fig. 41
Fig. 41.
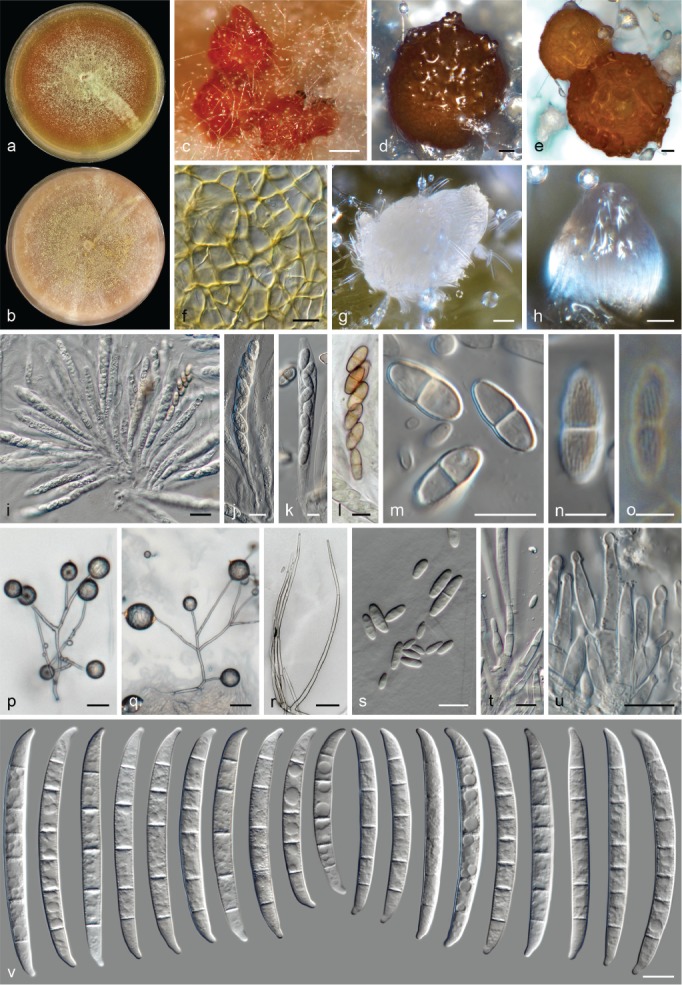
Neocosmospora silvicola (ex-type culture CBS 123846). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–e. perithecia; f. detail of peridium cells (mounted on lactic acid); g–h. sporodochia formed on the surface of carnation leaves; i–l. asci; m–o. ascospores; p–r. aerial conidiophores; s. aerial conidia; t–u. sporodochial conidiophores; v. sporodochial conidia. — Scale bars: c = 100 μm; d–e, g–i, p–r = 20 μm; k–l, n–o = 5 μm; all others = 10 μm.
Synonyms. Fusarium solani f. robiniae Matuo & Y. Sakurai, Ann. Phytophathol. Soc. Japan 30: 35. 1965.
Hypomyces solani f. robiniae Matuo & Y. Sakurai, Ann. Phytophathol. Soc. Japan 30: 35. 1965.
Nectria solani f. robiniae (Matuo & Y. Sakurai) G.R.W. Arnold, Z. Pilzk. 37: 193. 1972.
Etymology. From Latin silva (‘woods’, ‘forest’) and -cola (‘inhabitor’). Referring to the diverse woody plant hosts from which this species was collected.
Typus. USA, Tennessee, Great Smoky Mountains National Park, vic. Cosby, Snake Den Rock Trail, from Liriodendron tulipifera fallen trunk, 14 July 2004, G.J. Samuels (holotype CBS H-24002 here designated, culture ex-type CBS 123846 = G.J.S. 04-147).
Perithecia dark-orange to dark-red, globose to pyriform, superficial, solitary or gregarious, coarsely warted, glabrous; peridial wall composed of thick-walled cells of textura angularis. Asci clavate, unitunicate, apex flattened and simple (74.5–)82.5–98(–102) × (7–)7.5–10.5(–11.5) μm (av. 90.2 × 9.1 μm, n = 60). Ascospores uniseriate, irregularly biseriate at apex of asci, ellipsoidal with slightly truncate ends, 1-septate, (9.5–)11–13.5(–15.5) × (3.5–)4.5–6 μm (av. 12.4 × 5.2 μm, n = 156), pale yellow to golden brown, thick-walled, longitudinally finely striated, often constricted at septum.
Conidiophores erect or prostrate on substrate, abundant in aerial mycelium, straight or flexuous, smooth- and thin-walled, branched several times irregularly, verticillately or sympodially, bearing terminal or lateral monophialides, rarely unbranched; phialides subulate to subcylindrical, (32.5–)44.5–62.5(–74.5) × (2–)2.5–3.5 μm (av. 53.4 × 3 μm, n = 84), smooth- and thin-walled, conidiogenous loci with rather conspicuous periclinal thickening and a short-flared collarette; aerial conidia oval, obovoidal, ellipsoidal to somewhat reniform, 0–1-septate, hyaline, smooth- and thin-walled, (4.5–)6–14.5(–23.5) × (2–)2.5–4.5(–5.5) μm (av. 10.2 × 3.7 μm, n = 91), clustering in discrete false heads on tip of monophialides. Sporodochia pale luteous, ochreous or pale citrine, quickly developing into pionnotes. Sporodochial conidiophores densely verticillately or irregularly branched; sporodochial phialides ampulliform, subulate to subcylindrical, rarely proliferating, (10.5–)14–20(–23) × (2.5–)3.5–5(–5.5) μm (av. 17 × 4.2 μm, n = 116), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, collarettes often absent or inconspicuous and non-flared. Sporodochial conidia subtly dorsiventrally curved or almost straight, gradually tapering toward base; apical cell about equal length than adjacent cell, conical and blunt to slightly papillate, often gently hooked apically; basal cell distinctly notched and protuberant, (1–)4–6(–7)-septate, hyaline, smooth- and thick-walled; 1-septate conidia: 25 × 5 μm (n = 1); 4-septate conidia: (38–)39.5–56.5(–59) × 4–6 μm (av. 48 × 5.2 μm, n = 8); 5-septate conidia: (50–)57.5–72(–85.5) × 5–6.5(–7.5) μm (av. 64.8 × 6 μm, n = 154); 6-septate conidia: (60.5–)66–76.5(–80.5) × 5–7(–7.5) μm (av. 71.4 × 6 μm, n = 52); 7-septate conidia: (71.5–)72–78.5(–80) × 5.5–7 μm (av. 75.1 × 6.2 μm, n = 8); overall: (25–)55–74.5(–85.5) × (4–)5–6.5(–7.5) μm (av. 64.8 × 5.9 μm, n = 223). Chlamydospores abundant, globose to subglobose, smooth to finely roughened and thick-walled, 8–11(–12) μm diam, terminal or intercalary in hyphae or conidia, solitary or in chains.
Colonies on PDA growing in dark with an average radial growth rate of 3.5–3.9 mm/d at 24 °C, reaching 48–56 mm diam in 7 d at 24 °C; white, quickly turning into diverse shades of luteous, orange to scarlet or ochreous, blue-green to pale citrine, flat or slightly elevated at centre, felty to floccose, with or without concentric rings of white aerial mycelium; margin entire. Reverse scarlet, ochreous to citrine, with or without orange to scarlet diffusible pigments. Colonies on OA incubated in dark covering an entire 90 mm Petri dish in 7 d at 24 °C; pale orange, pale scarlet to pale citrine, flat, at first velvety to floccose with radial patches of white mycelium, quickly covering with abundant luteous, ochreous to citrine sporodochia, converging into pionnotes; margin entire. Reverse pale scarlet to citrine with scarce production of scarlet or citrine diffusible pigments.
Additional material examined. France, Pyrenees Atlantiques, Isle de Sauveterre de Bearn, from Populus nigra, 25 Oct. 1998, G.J. Samuels & F. Candoussau, CBS 119601 = G.J.S. 98-135.
Notes — Previously known as phylogenetic species FSSC 13, F. solani f. sp. robiniae or N. haematococca MP VII (Matuo & Snyder 1973, O’Donnell et al. 2008), this clade encompasses mostly homothallic strains, with the sexual morph abundantly formed on the surface of carnation leaves, getting quickly covered by aerial mycelium. DNA sequence data from two authentic strains (NRRL 22161, 22162) studied in the protologue of F. solani f. robiniae are included here (Matuo & Sakurai 1965). However, in the current circumscription, the species includes not only isolates from Robinia (Fabaceae) but also from plant hosts in the Salicaceae and Magnoliaceae. Therefore, the name N. silvicola is coined here.
Hering et al. (1997) established a connection between one strain studied here (NRRL 22586) and F. epimyces, a fungicolous species known from the UK, and one of the many taxa listed as synonyms of F. solani var. martii (Wollenweber 1931, Wollenweber & Reinking 1935a, b). Nonetheless, the latter taxon proved to belong to a different lineage (see notes under N.? martii). Neocosmospora silvicola exhibits distinct morphological features that separate it from the concept of F. epimyces, which was characterised by forming 3-septate sporodochial conidia measuring on average 50–60 × 4 μm in contrast to the 4–7-septate conidia that extend to 80 μm long in N. silvicola; Wollenweber 1931).
Similar-sized sporodochial conidia can be formed by N. acustispora and N. robusta, but the sporodochial conidia of N. silvicola are markedly less tapered than those of either species, with apical cells shorter and rounded than those of N. acustispora, and more curved and apically rounded than those of N. robusta.
Neocosmospora solani (Mart.) L. Lombard & Crous, Stud. Mycol. 80: 228. 2015
Basionym. Fusisporium solani Mart., Die Kartoffel-Epidemie der letzten Jahre oder die Stockfäule und Räude der Kartoffeln: 20. 1842.
Synonyms. Pionnotes viridis Lechmere, Compt. Rend. Hebd. Séances Acad. Sci. 155: 178. 1912.
Fusarium radicicola Wollenw., J. Agric. Res. 2: 257. 1914.
Fusarium javanicum var. radicicola Wollenw., Z. Parasitenk. (Berlin) 3: 286. 1931.
Fusarium solani f. radicicola (Wollenw.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium viride (Lechmere) Wollenw., Fusaria Autographice Delineata 1: 418. 1916.
Fusarium eumartii C.W. Carp., J. Agric. Res. 5: 204. 1915.
Fusarium solani var. eumartii (C.W. Carp.) Wollenw., Z. Parasitenk. (Berlin) 3: 452. 1931.
Fusarium solani f. eumartii (C.W. Carp.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium solani f. sp. eumartii (C.W. Carp.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
Fusarium aduncisporum Weimer & Harter, J. Agric. Res. 32: 312. 1926.
Fusarium solani var. aduncisporum (Weimer & Harter) Wollenw., Fusaria Autographice Delineata 3: 1035. 1930.
Neocosmospora rubicola L. Lombard & Crous, Stud. Mycol. 80: 227. 2015.
For additional synonyms see Index Fungorum and MycoBank.
Typus. Germany, from dry-rotten potato, von Martius (1842), taf. III, f. 29, illustration of a macroconidium (lectotype of Fusisporium solani, designated by Schroers et al. 2016). – Slovenia, Doljenska, Radohova vas (Dinaric valley systems and corrosion plains), from tuber of Solanum tuberosum in field following harvest, 18 Aug. 2009, H.-J. Schroers (epitype of Fusisporium solani CBS H-22335, designated by Schroers et al. (2016), culture ex-epitype CBS 140079 = NRRL 66304 = G.J.S 09-1466 = FRC S-2364).
Description & Illustration — Schroers et al. (2016)
Additional materials examined. Austria, Graz, from mixture of soil and cheese used as food for mites, unknown date, E. Ebermann, CBS 117149. – Belgium, Gent, from an artificial vocal prosthesis on human, unknown date and collector, CBS 112101; Meise, Jardin Botanique, from timber on tropical greenhouse, unknown date, J. Rammeloo, CBS 144393 = MUCL 34689. – Cameroon, Victoria, from dead fruit of Theobroma cacao, 1905, W. Busse, BPI 452278. – Denmark, from Solanum tuberosum, unknown date and collector, CBS 165.87 = IAM 14681. – France, Limoges, from human nail, unknown date and collector, CBS 124893. – Germany, former West-Germany, from Hyacinthus orientalis, unknown date, H.W. Wollenweber, CBS 208.29. – Honduras, Tela, unknown host and date, O.A. Reinking 170, BPI 453134. – Italy, from raspberry, unknown date and collector, CBS 101018 (culture ex-type of N. rubicola). – Japan, Nagasaki Prefecture, Isahaya, from soil on wheat field, unknown date, Y. Sugiura, CBS 111722. – Netherlands, from Daucus carota, unknown date and collector, CBS 119996. – USA, California, Ventura, a culture on stems of Meliotus alba, 24 June 1924, unknown collector, (lectotype of Fusarium aduncisporum designated here BPI 451321, MBT387246); Pennsylvania, from rotten tuber of Solanum tuberosum, unknown date, Carpenter, BPI 452104; from soil under Castanea sp., unknown date and collector, CBS 166.87; unknown location, from Solanum tuberosum, unknown date and collector (lectotype of Fusarium eumartii designated here: illustration plate XIV, number 4, in Carpenter CW. (1915) J. Agric. Res. 5: 183–209, MBT387247); Potomac Flats, near Washington, D.C., from tuber of Solanum tuberosum, 1912, H.W. Wollenweber (lectotype of Fusarium radicicola designated here: illustration plate XVI, f. K, in Wollenweber HW. (1914) J. Agric. Res. 2: 251–285, MBT388026).
Notes — Certainly the most recognised species of the genus, N. solani was recently epitypified and assigned to phylogenetic species FSSC 5 (O’Donnell et al. 2008) by Schroers et al. (2016). The numerous synonyms listed in various fungal databases must be carefully scrutinised, since they date back to pure morphological studies and many of these taxa are obscure and lack original material. Therefore, only synonyms linked to N. solani based on existent collections are listed. Most infraspecific taxa currently synonymised under N. solani are defined mostly based on cultural differences. For example, these include F. solani var. cyanum, F. solani var. suffuscum and F. solani var. medium, which are probably conspecific, although in need of further investigation.
Although not from the original host (Phaseolus vulgaris), the studied specimen of F. aduncisporum (BPI 451321) was deposited as an ‘ex-type culture’ of the species. This material, however, represents instead a subculture of the original collection on Meliotus alba stems, which formed part of the original material on which the species diagnosis was based (Weimer & Harter 1926). Since this is the only original material left for the species and a holotype was not designated on the protologue of F. aduncisporum, BPI 451321 is here selected as lectotype. Despite the strikingly discordant morphological features of the species, which are still clearly recognisable in BPI 451321 (Fig. 42), DNA barcodes generated from this herbarium specimen confirmed its synonymy under N. solani.
Fig. 42.
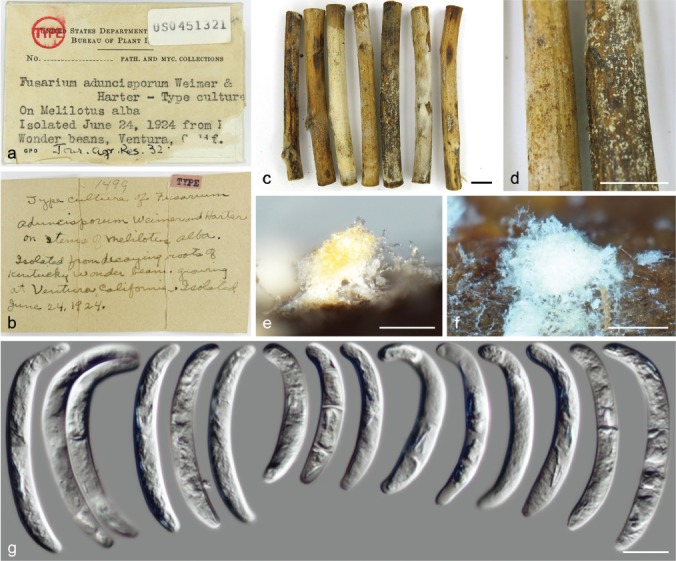
Neocosmospora solani (lectotype of Fusarium aduncisporum BPI 451321). a–c = Holotype specimen; d–f. sporodochia; g. sporodochial conidia. — Scale bars: c–d = 1 cm; e–f = 50 μm; g = 10 μm.
The specimen of F. eumartii (BPI 452104) included here seems to be the only available material for the species linked to the original publication (Carpenter 1915). This particular collection was subcultured by H.W. Wollenweber (Fusaria culta No 90) from original material obtained from C.W. Carpenter and is depicted in ‘Fusaria Autographice Delineata no 422’ (reproduced in Fig. 43c). Stevenson (1971) also cited this among the type collections studied by H.W. Wollenweber. However, since no type was designated for F. eumartii, original material from its protologue (an illustration) is here designated as lectotype to stabilise the application of the name (reproduced in Fig. 43a). Barcodes (rDNA and tef1) were successfully generated from BPI 452104, confirming its synonymy with N. solani. Although the mentioned specimen could serve as epitype for the species, we refrain to do so, pending the recollection of fresh living material. As seen from the morphological observations from BPI 452104 and DNA sequence data obtained from this specimen, significant differences exist between the original description of F. eumartii and the diverse morphological and pathological concepts applied to this taxon over the last century (Wollenweber 1916, 1931, Wollenweber & Reinking 1935a, Ragazzi et al. 1993, Gerlach & Nirenberg 1982, Romberg & Davis 2007 and references therein). Of the species recognised here, at least nine, i.e., N. ampla, N. gamsii, N. ipomoeae, N. metavorans, N. nirenbergiana, N. noneumartii, N. paraeumartii, N. quercicola and N. robusta exhibit sporodochial conidia compatible with the modern circumscription of F. eumartii (Gerlach & Nirenberg 1982), or include isolates previously identified as such based largely on morphology and pathogenicity data. The widely used concept of F. eumartii, however, most likely represents a range of different taxa, which is the result of the ambiguous use of the formae and special form nomenclature in literature. In view of the recent phylogenetic knowledge of the species, the host range and biogeography of N. solani needs to be reassessed.
Fig. 43.
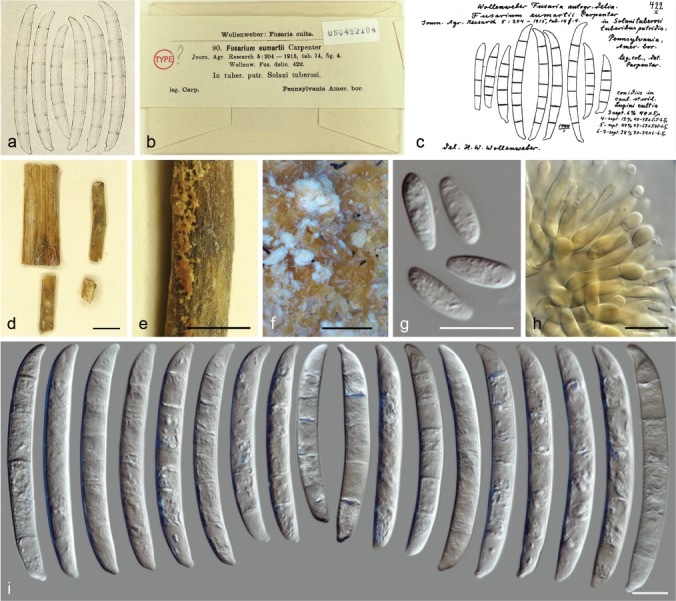
Neocosmospora solani (a. lectotype of Fusarium eumartii; b, d–i. herbarium material of Fusarium eumartii BPI 452104). a. Reproduction of original illustration depicting conidia by Carpenter (1915); b, d. herbarium specimen; c. original drawing by Wollenweber (1916); e–f. sporodochia; g. aerial conidia; h. sporodochial conidiophores; i. sporodochial conidia. — Scale bars: d = 1 cm; e = 5 mm; f = 50 μm; all others = 10 μm.
Fusarium radicicola, initially isolated from decaying tubers and roots of S. tuberosum and I. batatas is here synonymized under N. solani and a lectotype is designated from the original published material (digitally enhanced and reproduced in Fig. 44). The original concept of F. radicicola sensu Wollenweber (1914) exhibit the same range of morphological characters and host preference known for N. solani as epitypified by Schroers et al. (2016). This concept differs from that of other species common on sweet potato that includes F. javanicum, N. bataticola (syn. F. solani f. batatas) and N. brevicona; but also F. solani f. radicicola (syn. F. solani f. sp. radicicola, Snyder & Hansen 1941, Matuo & Snyder 1973), which is a polyphyletic assemblage based on host preference. Suga et al. (2000) found that strains of F. solani f. sp. radicicola belong to two distinct and distantly related genotypes, later termed genotypes 1 and 2 (Honraet et al. 2005). Genotype 1 contains mostly isolates from solanaceous hosts; genotype 2 includes isolates from diverse plant hosts from different plant families. Both genotypes also include opportunistic human pathogens (Honraet et al. 2005). Analyses of DNA barcodes from representatives of both genotypes (data not shown) indicated that genotype 1 falls within the current phylogenetic circumscription of N. solani s.str., while genotype 2 clusters within N. falciformis.
Fig. 44.
Neocosmospora solani (lectotype of Fusarium radicicola). a. Reproduction of original illustration depicting conidia by Wollenweber (1914). — Scale bar = 10 μm.
In terms of sporodochial conidial size and septation, N. solani is closely related to N. falciformis, N. metavorans and N. pseudoradicicola, but is clearly differentiated phylogenetically. Neocosmospora solani differs from N. falciformis and N. metavorans mainly by narrower sporodochial conidia (av. 5.1 μm wide vs 6 μm and 6.2 μm wide for N. falciformis and N. metavorans, respectively), which are also more regularly septate than those of N. falciformis (up to 5-septate vs up to 4-septate for N. falciformis), and with more rounded apices than those of N. metavorans. Contrasting with N. pseudoradicicola, N. solani exhibits more robust, less curved and commonly apically rounded sporodochial conidia, with less developed basal cells. It also differs by lacking microcyclic conidiation.
Neocosmospora spathulata Sand.-Den. & Crous, sp. nov. — MycoBank MB831206; Fig. 45
Fig. 45.
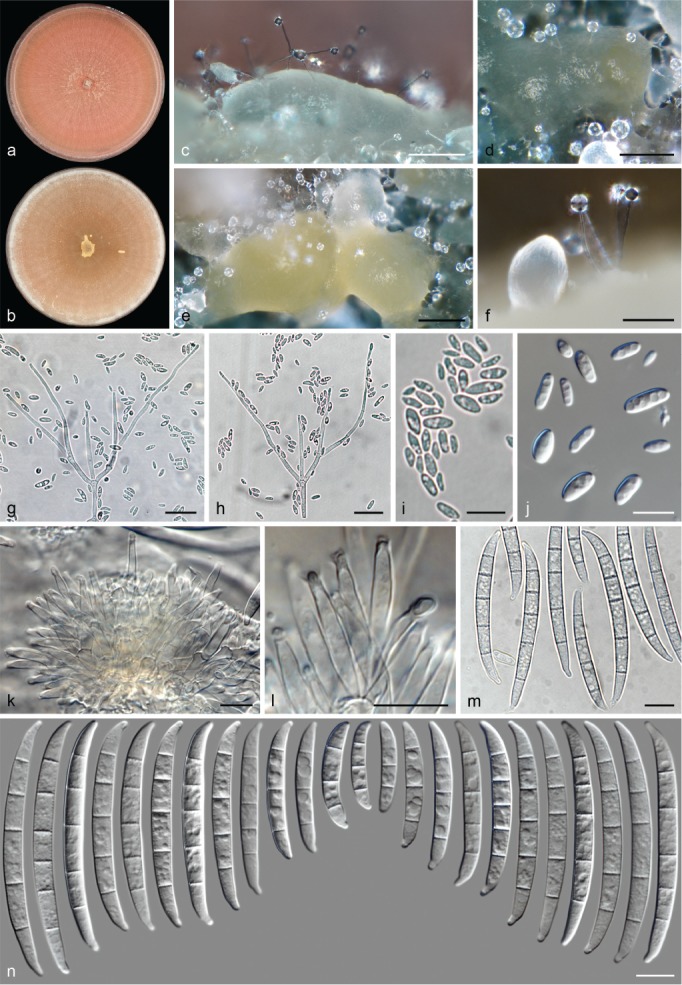
Neocosmospora spathulata (ex-type culture CBS 145474). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c–f. sporodochia formed on the surface of carnation leaves; g–h. aerial conidiophores; i–j. aerial conidia; k–l. sporodochial conidiophores and phialides; m–n. sporodochial conidia. — Scale bars: c–f = 100 μm; g–h = 20 μm; all others = 10 μm.
Etymology. From Latin spatula (‘spatula, a flat piece’). Named after the shape of the sporodochial conidial apex, resembling a spatula.
Typus. USA, New England, from human synovial fluid, unknown collector and date (holotype CBS H-24003 designated here, culture ex-type CBS 145474 = NRRL 28541 = UTHSC 98-1305).
Conidiophores abundant on substrate and aerial mycelium, erect or lateral, straight, smooth- and thin-walled, simple or two to three times verticillately, dichotomously or more uncommonly irregularly or sympodially branched, bearing terminal, single monophialides; phialides subulate, subcylindrical to acicular, (31–)39.5–51(–58.5) × 2–3 μm (av. 45.3 × 2.5 μm, n = 58), smooth- and thin-walled, conidiogenous loci with inconspicuous periclinal thickening, collarettes minute and non-flared or absent; aerial conidia, short clavate, ellipsoidal or cylindrical, straight, 0(–1)-septate, hyaline, smooth- and thin-walled, (4.5–)6–11(–16.5) × (2–)2.5–4(–5) μm (av. 8.5 × 3.2 μm, n = 93), clustering in false heads on tip of monophialides. Sporodochia cream, pale luteous, pale to dark blue-green. Sporodochial conidiophores sparingly verticillately branched; sporodochial phialides subcylindrical to subulate, (9.5–)11.5–16.5(–20) × (2.5–)3–4(–4.5) μm (av. 14.1 × 3.3 μm, n = 60), smooth- and thin-walled, conidiogenous loci with conspicuous periclinal thickening and a short, non-flared or flared collarette. Sporodochial conidia, moderately curved with nearly parallel ventral and dorsal lines; apical cell of variable length in relation to the adjacent cell, blunt to somewhat conical with slightly curved, rounded to barely papillate apex; basal cell distinctly notched with rather protuberant foot, (2–)3–5(–6)-septate, hyaline, smooth- and thick-walled; 2-septate conidia: 21–24 × 3.5–4.5 μm (n = 2); 3-septate conidia: (23.5–)27–38.5(–40) × 4–5 μm (av. 32.8 × 4.4 μm, n = 20); 4-septate conidia: (40–)42.5–55(–59) × (4–)4.5–5.5 μm (av. 48.9 × 4.9 μm, n = 20); 5-septate conidia: (44.5–)51.5–63.5(–69) × 4–5.5 μm (av. 57.5 × 5 μm, n = 88); 6-septate conidia: 66–73 × 5.3 μm (n = 2); overall: (21.5–)40–64(–73) × (3.5–)4.5–5.5 μm (av. 51.9 × 4.9 μm, n = 132). Chlamydospores not observed.
Colonies on PDA and OA growing in dark with an average radial growth rate of 6–7 mm/d at 24 °C, filling an entire 90 mm diam Petri dish in 7 d at 24 °C. On PDA peach, scarlet to coral, flat and regular, velvety to felty, floccose at centre, with concentric rings and radial patches of thin and short aerial mycelium and central saffron to coral area with floccose aerial mycelium; margin regular with abundant submerged mycelium. Reverse scarlet to red. On OA, salmon, apricot to umber, flat, at first membranous, quickly becoming velvety with cottony margin, later becoming pionnotal with abundant, confluent, pale luteous to ochreous sporodochia; margin entire. Reverse saffron, salmon to pale rust.
Notes — Originally known as phylogenetic species FSSC 29, N. spathulata forms part of the ‘F. ensiforme clade’ sensu Nalim et al. (2011), a cluster of phylogenetic species with morphologies comparable to that described for F. ensiforme, one of the oldest names among the Martiella fusaria, although of uncertain application. Neocosmospora spathulata differs from the morphological concept of F. ensiforme by its peach to red colonies, and less septate, rarely 6-septate sporodochial conidia (up to 7-septate in F. ensiforme) with elongated and spatulate apical cells. Other morphologically similar species include N. hypothenemi and N. noneumartii, although, the last two species significantly differ in the presence of aerial macroconidia, which are absent in N. hypothenemi and N. spathulata. The distinction between N. spathulata and N. hypothenemi is less straightforward and can only be done by employing phylogenetic markers. Although not genetically related, both species produce similar microconidia and sporodochial conidia with protuberant foot cells, and also greatly overlap in the size of aerial and sporodochial phialides. Marginal differences also exist regarding sporodochial conidial septation (rarely 6-septate in N. spathulata vs up to 7-septate in N. hypothenemi) and the shape of the apical cells. Additionally, the microconidia are slightly longer in N. spathulata (up to 16.5 μm vs up to 13.5 μm in N. hypothenemi).
Neocosmospora sphaerospora (Q.T. Chen & X.H. Fu) Sand.-Den. & Crous, comb. nov. — MycoBank MB832096
Basionym. Fusarium sphaerosporum Q.T. Chen & X.H. Fu, Acta Mycol. Sin., Suppl. 1: 331. 1987.
Typus. China, Guangtung, Maoming, from water from underground pipes of oil field, unknown date and collector (holotype HMAS 43749, culture ex-type NF 5840).
Description & Illustrations — Chen et al. (1987).
Notes — This species was isolated from polluted water and allocated in sect. Martiella by Chen et al. (1987). Described as producing typical elongated phialides borne on simple to sparingly branched conidiophores, it differs from other known Neocosmospora spp. by its subglobose to pyriform microconidia.
Neocosmospora spinulosa Pfenning, Sydowia 47: 66. 1995 — Fig. 46
Fig. 46.
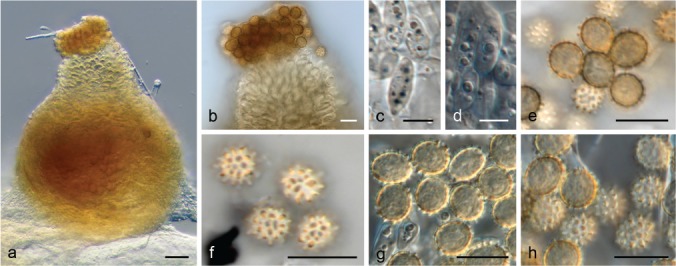
Neocosmospora spinulosa (CBS H-5443). a. Perithecia; b. detail of perithecium ostiole extruding ascospores; c–d. conidia; e–h. ascospores. — Scale bars: a = 20 μm; c–d = 5 μm; all others = 10 μm.
Typus. Brazil, State de Pará, Capitão Poço, from soil under Theobroma cacao, 1994, L. Pfenning (holotype CBS H-5452a).
Descriptions & Illustrations — Pfenning (1995), Guarro et al. (2012).
Additional material examined. Brazil, State de Pará, Capitão Poço, from soil under Theobroma cacao, 1994, L. Pfenning, CBS H-5443.
Notes — The species protologue reads ‘Holotypus siccus (et cultura viva) CBS 321.93’, but this living culture was never preserved in WI, and no copies or other living cultures exist for this species (L. Pfenning, pers. comm.). However, two specimens are conserved in WI, CBS H-5452a, the holotype of the species derived from CBS 321.93; and CBS H-5443 which was later labelled as ‘typus of Neocosmospora spinulosa Pfenning’. However, this specimen was labelled with a different and incorrect culture number association (CBS 393.93). This is most likely a typographic error judging from the micromorphology of the specimen. The two specimens are in excellent conserved condition, showing identical morphological features, also matching with the original description of the species (Pfenning 1995). Genomic DNA extraction was successfully done from specimen CBS H-5443. However, the DNA barcodes were not included in the final analyses due to significant topological alterations attributed to missing sequence data. Partial rDNA and tef1 barcodes demonstrated the genealogical exclusivity of this species (data not shown), which is also supported by its unique morphological features. Neocosmospora spinulosa, known from soil under Brachiaria humidicola and T. cacao, is the only species of the genus producing unicellular, spiny ascospores (Pfenning 1995).
Neocosmospora stercicola (Šišić et al.) Sand.-Den. & Crous, comb. nov. — MycoBank MB831207
Basionym. Fusarium stercicola Šišić et al., Antonie van Leeuwenhoek 111: 1793. 2018.
Synonyms. Fusarium martii var. viride Sherb., Cornell Univ. Agric. Exp. Sta. Mem. 6: 247. 1915.
Fusarium solani var. martii f. 1 Wollenw., Z. Parasitenk. (Berlin) 3: 290. 1931.
Fusarium witzenhousenense Šišić et al., Antonie van Leeuwenhoek 111: 1795. 2018.
Typus. Germany, Hessen, Witzenhausen, Neu Eichenberg, compost yard waste plant debris, unknown date, A. Šišić (holotype CBS H-23352, culture ex-type CBS 142481 = DSM 106211 = FS 89).
Description & Illustrations — Wollenweber (1931, as F. solani var. martii f. 1), Šišić et al. (2018a).
Additional materials examined. Belgium, Heverlee, greenhouse humic soil, unknown date, B.G. Desai, CBS 144388 = MUCL 18299. – Germany, Münster, from nematode egg, unknown date, Goswami, CBS 618.76 = NRRL 22239; Hessen, Witzenhausen, Neu Eichenberg, branch of Hibiscus sp., unknown date, A. Šišić, CBS 142480 = FS 90 (culture ex-type of F. witzenhausenense). – Unknown, unknown collection data, H.W. Wollenweber, CBS 187.35 = BBA 2318; unknown collection data, Y. van Koot, CBS 260.54.
Notes — Wollenweber (1931, 1935) illustrated Sherbakoff’s F. martii var. minus (icones 415) and F. martii var. viride (icones 417), obtained from potatoes in USA, recombining both taxa as a variety of F. solani (as F. solani var. martii f. 1; Sherbakoff 1915, Wollenweber 1931). While F. martii var. minus is currently synonymised under N. phaseoli (Aoki et al. 2003), original specimens could not be located and are most likely non-existent. However, strain CBS 187.35 was deposited in WI by H.W. Wollenweber as a representative of F. solani var. martii f. 1, and fits well with the morphological circumscription (Wollenweber & Reinking 1935a, b). The ex-type cultures of the recently introduced species, F. stercicola and F. witzenhousenense, also cluster within this clade (Šišić et al. 2018a). In addition to their genetic similarity, these two species lack clear distinctive morphological traits and are therefore reduced to synonymy, which is also supported by the significant evidence of recombination within this clade according to the PHI test (Φw = 0.008, Fig. 2).
Neocosmospora suttoniana Sand.-Den. & Crous, Persoonia 41: 123. 2018
Typus. USA, Louisiana, from human wound, unknown date and collector (holotype CBS H-23224, culture ex-type CBS 143214 = NRRL 32858).
Description & Illustration — Sandoval-Denis et al. (2018).
Additional materials examined. Gabon, from human nail, unknown date and collector, CBS 124892. – USA, Florida, from human corneal ulcer, unknown date, D.A. Sutton, CBS 143204 = NRRL 32316 = UTHSC 00-264; from equine eye, unknown date and collection, CBS 143224 = NRRL 54972 = UTSCH 05-2900; Georgia, from human blood, unknown date and collector, CBS 143197 = NRRL 28000; Massachusetts, Burlington, from human, unknown date and collector, CBS 130178 = NRRL 22608 = UTHSC 93-1547.
Notes — Known as phylogenetic species FSSC 20, N. suttoniana is an important human and veterinary pathogen (O’Donnell et al. 2008, 2016, Sandoval-Denis & Crous 2018). Although this species is not related to the ‘F. ensiforme clade’ sensu Nalim et al. (2011), its sporodochial conidia resemble those described for F. ensiforme, but are more robust, with a typically hooked apex (Sandoval-Denis & Crous 2018). Furthermore, the sporodochial conidia of N. suttoniana have the same dimensions as those of N. quercicola and N. robusta, but differ based on septation and width (up to 6-septate and av. 7 μm wide vs up to 7-septate and av. 5.4 μm wide in N. quercicola). Neocosmospora suttoniana can also be distinguished from N. robusta by its gently tapering sporodochial conidia with elongated and distinctly hooked apical cells compared to the short and blunt apical cells of N. robusta. In addition, N. robusta lacks aerial microconidia, producing only falcate, multiseptate aerial conidia.
Neocosmospora theobromae (Appel & Strunk) Sand.-Den. & Crous, comb. nov. — MycoBank MB831208; Fig. 47
Fig. 47.

Neocosmospora theobromae (neotype of Fusarium theobromae BPI 453072). a–c. Herbarium specimen; d. drawing of BPI 453072 by Wollenweber (1916); e–f. sporodochia; g. sporodochial conidiophores and chlamydospores (arrows); h, aerial conidia; i. sporodochial conidia. — Scale bars: c = 5 mm; e–f = 200 μm; all others = 10 μm.
Basionym. Fusarium theobromae Appel & Strunk, Centralbl. Bakteriol. Parasitenk., 1. Abth. 13: 685. 1904.
Typus. Cameroon, Victoria, from fruits and seeds of Theobroma cacao, 1902, Strunk (neotype of Fusarium theobromae BPI 453072, designated here, MBT387250).
Descriptions & Illustrations — Appel & Strunk (1904), Wollenweber (1917).
Notes — Neocosmospora theobromae was originally described growing as a secondary invader on wounded cacao fruits in Cameroon (Appel & Strunk 1904). However, no type specimen was designated, and the original collections could not be located. An illustration was included in the protologue, but is not eligible to be an iconotype since it only shows the macroscopic effects of fungal growth on cocoa fruits, with no fungal elements depicted. A specimen housed at BPI matches with the original collection details (BPI 453072). This specimen represents a subculture made by H.W. Wollenweber of the original collection of F. theobromae, which is depicted in ‘Fusaria Autographice Delineata No 428’ (reproduced in Fig. 47). Since original material could not be located and is presumed lost, BPI 453072 is here designated as neotype in order to fix the application of the name. Partial DNA barcodes were successfully obtained from the neotype specimen and analyses of these barcodes showed that this taxon forms a single lineage in Neocosmospora (data not shown), further supported by its sporodochial conidial morphology, typically dorsiventrally curved, with tapered and almost acute apical cells, and barely notched foot cells. These features are somewhat similar to those observed in N. pseudoradicicola and also to the conidia reported for F. radicicola (Sherbakoff 1915, Wollenweber 1916, 1931, Reinking & Wollenweber 1927). Despite overlapping conidial dimensions, N. theobromae differs from N. pseudoradicicola in the production of larger sporodochial conidia (av. 44.8 × 5.1 μm in the neotype, up to 75 μm long in the protologue of F. theobromae vs 39.5 × 4.7 μm in N. pseudoradicicola) which are predominantly 4-septate (5-septate in N. pseudoradicicola), as well as in producing slightly longer microconidia (av. 12.4 vs 8.9 μm long in N. pseudoradicicola).
The combination of F. theobromae as a variety of F. javanicum as proposed by Wollenweber (1931) is rejected here based on the examination of BPI 453072. The morphological characters differ greatly from those of F. javanicum.
An additional specimen of F. theobromae from T. cacao is housed at BPI (BPI 453071), collected by O.A. Reinking in 1918 in the Philippines. However, this specimen is not in good condition. The micromorphology displays characters of a different and unknown species, with larger sporodochial conidia showing longer, stretched foot cells, similar to those reported for F. ensiforme. Unfortunately, no usable DNA could be extracted from this specimen.
Neocosmospora tonkinensis (Bugnic.) Sand.-Den. & Crous, Persoonia 41: 126. 2017 (2018)
Basionym. Cylindrocarpon tonkinense Bugnic., Encycl. Mycol. 11: 181. 1939.
Synonym. Fusarium ershadii Papizadeh et al., Eur. J Plant Pathol. 151: 693. 2018 (Nom. illegit., Art 52.1).
Typus. Vietnam, Tonkin, from Musa sapientum, 1936, F. Bugnicourt No 498 (holotype IMI 113868).
Descriptions & Illustrations — Bugnicourt (1939), Booth (1966), Papizadeh et al. (2018), Sandoval-Denis & Crous (2018).
Additional materials examined. Netherlands, from Euphorbia fulgens, unknown date and collector, CBS 222.49. – UK, from Solanum lycopersicum, unknown date and collector, CBS 118931. – USA, Florida, from turtle head lesion, unknown date and collector, CBS 143208 = NRRL 32755 = FRC S-0452; Ohio, from human cornea, unknown date, M. Brandt, CBS 143217 = NRRL 43811. – Vietnam, Tonkin, from Musa sapientum, 1936, F. Bugnicourt, CBS 115.40 = IMI 113868 (culture ex-type of C. tonkinense). – Unknown, unknown collection data, NRRL 46615, NRRL 46676.
Notes — Also known as phylogenetic species FSSC 9, N. tonkinensis has been recorded from soil and various plant hosts (Booth 1966), as well as human-made environments (Short et al. 2011). This species also represents a recognised human and animal pathogen (O’Donnell et al. 2008, 2016, Sandoval-Denis & Crous 2018).
Morphologically, the sporodochial conidia of N. tonkinensis are similar in size to those of N. metavorans, N. pseudotonkinensis and N. theobromae. However, these species are not phylogenetically closely related. Neocosmospora tonkinensis can be distinguished from N. pseudotonkinensis by the slightly shorter sporodochial conidia, with shorter and straighter apical cells. It is distinguished from N. metavorans by the elongate clavate to ellipsoidal and multiseptate aerial conidia, and from N. theobromae by the straighter and less apically tapering sporodochial conidia. See additional comments in the notes under the respective species.
Neocosmospora vasinfecta E.F. Sm., Bull. U.S.D.A. 17: 45. 1899 — Fig. 48
Fig. 48.
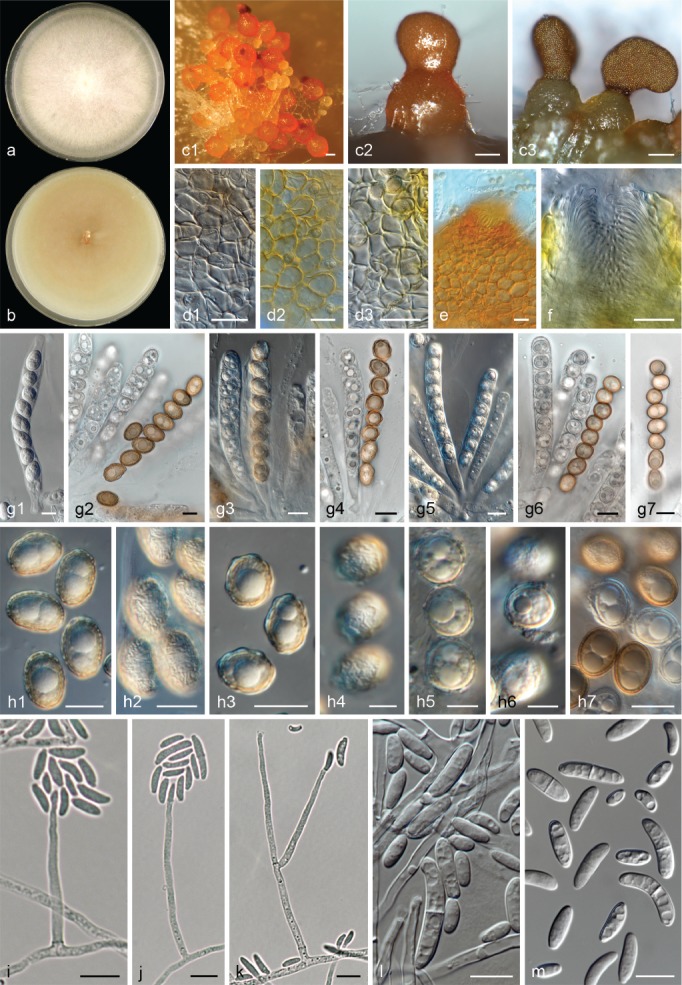
Neocosmospora vasinfecta (c1, d1, g1, g2, h1, h2. N. boninensis ex-type CBS 446.93; c2, d2, e, f, g3, g4, h3, h4. N. ornamentata ex-type CBS 562.72; a, b, c3, d3, g5, g6, h5, h6, i–m. N. vasinfecta CBS 101957; g7, h7. N. africana CBS 863.70). a–b. Colonies on PDA and OA, respectively, after 14 d at 24 °C in the dark; c. perithecia; d. detail of peridial cells (d2, d3. on lactic acid); e–f. detail of ostiolar opening (f. on lactic acid); g. asci; h. ascospores; i–k. conidiophores; l–m. conidia. — Scale bars: c1–c3 = 200 μm; d1–e = 20 μm; h4–h6 = 5 μm; all others = 10 μm.
Synonyms. (Nectriella tracheiphila E.F. Sm., AAAS Bull. 44: 190. 1896 (Nom. inval. fide Cannon & Hawksworth 1984)).
Neocosmospora vasinfecta var. nivea E.F. Sm., Bull. U.S.D.A. 17: 45. 1899.
Neocosmospora vasinfecta var. tracheiphila E.F. Sm., Bull. U.S.D.A. 17: 45. 1899.
Fusarium vasinfectum var. pisi C.J.J. Hall, Ber. Deutsch. Bot. Ges. 21: 4. 1903.
Neocosmospora vasinfecta var. pisi (C.J.J. Hall) Sacc., Syll. Fung. 20: 192. 1911.
Neocosmospora africana Arx, Antonie van Leeuwenhoek 21: 161. 1955.
Neocosmospora vasinfecta var. africana (Arx) P.F. Cannon & D. Hawksw., Trans. Brit. Mycol. Soc. 82: 676. 1984.
?Pseudonectria ornata Bat. & Maia, Anais Soc. Biol. Pernambuco 13: 74. 1955 (fide Cannon & Hawksworth 1984).
Neocosmospora vasinfecta var. major P. Rama Rao, Mycopathol. Mycol. Appl. 21: 218. 1963.
Neocosmospora ornamentata M.A.F. Barbosa, Garcia de Orta, Ser. Bot. 13: 17. 1965.
Neocosmospora vasinfecta f. conidiifera Kamyschko, Novosti Sist. Nizsh. Rast. 1965: 115. 1965.
Neocosmospora boninensis Udagawa, Y. Horie & P.F. Cannon, Sydowia 41: 350. 1989.
Fusarium neocosmosporiellum O’Donnell & Geiser, Phytopathology 103: 405. 2013.
Typus. USA, South Carolina, Williamsburg County, Salters, Salters Depot, on Gossypium hirsutum, 8 Oct. 1895, unknown collector (lectotype of N. vasinfecta designated here: illustration f. 1 and 2 on plate V in Smith EF. (1899). Bull. U.S.D.A. 17: 1–72, MBT387252).
Descriptions & Illustrations — Cannon & Hawksworth (1984), Rossman et al. (1999), Samuels et al. (2006), Guarro et al. (2012).
Additional materials examined. Argentina, near Buenos Aires, from soil in cotton plantation, unknown date, J.E. Wright, CBS 398.67. – Australia, Queensland, Cape York Peninsula, Iron Range National Park, soil of dry woodland under Eucalyptus, 20 Feb. 1990, N.G. Dangler, CBS 554.94 = ATCC 76350 = IMI 346678 = TRTC 51334 = UAMH 6870. – Germany, Berlin, Robert Koch Institute, blood culture, sputum and wound smear of an African patient, unknown date, K. Tintelnot, CBS 101957. – Guinea-Bissau, from stored nut of Arachis hypogaea, unknown date, M.A. de Freitas Barbosa, CBS 562.70 = ATCC 32363 = IMI 251387 (culture ex-type of N. ornamentata). – India, Agra, from soil, Nov. 1995, J. Gené, CBS 709.96 = FMR 5546; unknown collection data, CBS 533.65 = IMI 302625. – Ivory Coast, from soil on coffee plantation, unknown date and collector, CBS 332.52 = IMI 302623 = LCP 58.1542 = LCP 812. – Japan, Tokyo, Ogasawara-mura, from forest soil, 15 June 1983, T. Sato, CBS 446.93 = IMI 316967 = NHL 2919 (culture ex-type of N. boninensis). – Russia, St. Petersburg, from soil, unknown date and collector, CBS 602.67 = ATCC 32362 = IMI 251388 = VKM F-1139 (culture ex-isotype of N. vasinfecta f. conidiifera). – South Africa, Johannesburg, Frankenwald, from soil, W.J. Lutjeharms, CBS 325.54 = IMI 251386 = ATCC 162388 = IFO 7591 (culture ex-isotype of N. africana); Pretoria, Medicago sativa rotting stem base, 1932, unknown collector, CBS 237.55 = BBA 4647 = DSM 62822 = IMI 302624 = MUCL 9814. – Turkey, from soil, unknown date, G. Turhan, CBS 882.85. – USA, Louisiana, from human eye, unknown date and collector, CBS 130182 = NRRL 43467 = CDC 2006743430. – Venezuela, Anzoategui State, just north of Cantaura on road between Barcelona and Cd. Bolivar, from dung of donkey, J.C. Krug, CBS 406.82; Monagas State, El Zamura, dung of donkey, 1972, unknown collector, CBS 362.84; Monagas State, c. 12 km W of La Toscana on road to Jusepin, from dung of cow, 16 July 1972, K.P. Dumont, R.F. Cain, G.J. Samuels & G. Morillo, CBS 405.82.
Notes — As stated by Cannon & Hawksworth (1984), N. vasinfecta was originally published without a proper typification and initially erroneously proposed as a new combination based on its assumed asexual morph F. vasinfectum (F. oxysporum s.lat.), now known not to be congeneric. Therefore, a neotype (BPI 630336) was designated from the original host and geographic region, which was also followed by Rossman et al. (1999). However, according to Art. 9.19 (Shenzhen Code) a neotype designation is superseded if any of the original materials are found to exist. Detailed illustrations of N. vasinfecta growing on various hosts were included in the description by Smith (1899), hence original material according to Art. 9.4 (Shenzhen Code) exists; one of these illustrations is here designated as lectotype (on Gossypium hirsutum, adapted and reproduced in Fig. 49).
Fig. 49.
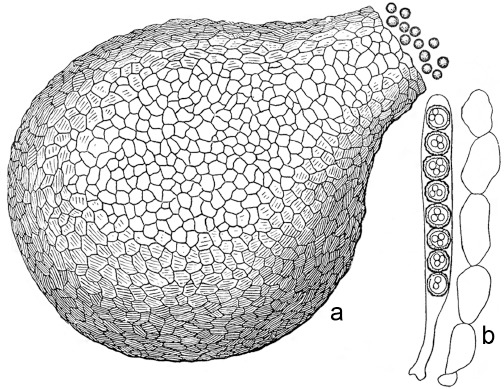
Neocosmospora vasinfecta (lectotype). Adapted from the original illustration by Smith (1899). a. Perithecium and ascospores; b. ripe ascus and paraphysis.
The ex-types cultures of N. africana, N. boninensis, N. ornamentata and N. vasinfecta f. conidiifera, often considered distinct at specific or subspecific levels based on ascospore ornamentation, all cluster within the same clade and are here reduced to synonymy under N. vasinfecta, as has been suggested in the past (Van Warmelo 1976, Mahoney 1976). Based on phylogenetic inference, there is no evidence to support the varietal status of N. vasinfecta var. africana. Isolates of this variety are morphologically compatible with the type concept of this taxon and are scattered within the N. vasinfecta clade. Additionally, a wide genetic diversity has been observed for this clade. Interestingly, two highly supported internal clades were consistently resolved, including a radiating one containing all the type material available, and a second one which included CBS 332.52, CBS 405.82 and CBS 709.96. There are no clear morphological and ecological differences observed for these isolates, and significant evidence of recombination events was found between these two internal clades (Φw = 1.15−6; Fig 2).
Neocosmospora vasinfecta is morphologically unique in producing smooth-walled perithecia and mostly 0-septate, rarely 1-septate ascospores with rugose to cerebriform ornamentation. Additionally, it lacks falcate, multiseptate conidia. The asexual morph was traditionally classified as a member of the genus Acremonium (Cannon & Hawksworth 1984, Rossman et al. 1999), but is analogous to the microconidial asexual morphs commonly observed in Neocosmospora.
DOUBTFUL AND EXCLUDED TAXA
Several additional Neocosmospora species have already been excluded from the genus based on the morphology of the asexual morphs (Rossman et al. 1999) and, more recently, on phylogenetic inference (Lombard et al. 2015). However, additional species have been found to belong to distant, unrelated lineages. As the status of some old names thought to belong to sect. Martiella could not be traced, the application of these names remains obscure.
Fusarium caeruleum Lib. ex Sacc., Syll. Fung. 4: 705. 1886
Synonyms. (Fusarium solani var. caeruleum (Lib. ex Sacc.) Bilaĭ, Fuzarii: 287. 1955 (Nom. inval., Art. 41.5)).
Fusarium solani var. caeruleum (Lib. ex Sacc.) C. Booth, The Genus Fusarium: 51. 1971.
(Selenosporium caeruleum Lib., 1834. (in herb.) (Nom. inval., Art. 32.1a)).
(Fusarium violaceum Fuckel, Jahrb. Nassauischen Vereins Naturk. 23–24: 369. 1870 (Nom. illegit., Art. 53.1)).
Fusarium caeruleum var. cellulosae Sartory, R. Sartory, J. Mey. & Bamuli, Papier 38: 43. 1935.
?Hypomyces asclepiadis Zerova, Zhurn. Inst. Bot. Vseukrajins’k. Akad. Nauk 11: 103. 1937 (fide Zerova (1937)).
Materials examined. Germany, former West-Germany, from Solanum tuberosum, unknown date, H.W. Wollenweber, CBS 113.23 = NRRL 20843; Berlin, from Solanum tuberosum ‘Hansa’, unknown date and collector, CBS 836.85 = NRRL 20434 = BBA 64413. – Sweden, from Solanum tuberosum, unknown date, B. Nedstam, CBS 133.73 = NRRL 22286 = ATCC 24389 = IMI 163397.
Notes — Often spelled as ‘F. coeruleum’, F. caeruleum is an important causal agent of potato storage rot, particularly in temperate zones, and one of the most commonly found agents associated with dry rot of potato in Europe and Canada. Although isolates matching this concept are also documented from other plant hosts worldwide, these records must be carefully scrutinised, as they provide conflicting circumscriptions of this taxon, and commonly treat this fungus as a variety of F. solani as suggested by Nelson et al. (1983), without a proper morphological assessment. The morphology of F. caeruleum is quite distinct, differing from species in Neocosmospora by the production of apedicellate conidia, distinctive conidiophores and phialides and the absence of a distinct microconidial state. However, F. caeruleum was retained in sect. Martiella since the overall conidial shape matched the shapes originally described for this section (Wollenweber 1931, Wollenweber & Reinking 1935a, b, Gerlach & Nirenberg 1982 and references therein). DNA barcodes obtained from three reference strains of F. caeruleum (CBS 113.23, 133.73 and 836.85), showed that they all belong to the genus Fusarium and not Neocosmospora. However, additional pathogenicity and morphological studies are required to fix the classification of F. caeruleum. The connection between F. caeruleum and the presumed sexual morph, H. asclepiadis, is still doubtful and requires further investigation (Zerova 1937).
Fusarium caucasicum Letov, Mater. Mikol. Fitopatol. 8: 225. 1929
Material examined. USSR, from cotton, 1928, A.S. Letov, CBS 179.35 = IFO 5979 = NRRL 13954 (ex-type culture).
Notes — The morphology of this species resembles that of F. caeruleum, particularly in the morphology of the conidiophores and in the apedicellate conidia, differing only by the absence of microconidia in the latter species. However, F. caeruleum belongs to Fusarium and not to Neocosmospora. The type culture of F. caucasicum was examined by Gerlach & Nirenberg (1982) who then retained the species in sect. Martiella, but re-examination of several batches of the ex-type culture held at WI (CBS 179.35) showed that the morphology corresponds to a completely different Fusarium species, having distinctly falcate and pedicellate sporodochial conidia. This could indicate a strain transposition or contamination by another Fusarium species.
Fusarium ensiforme Wollenw. & Reinking, Phytopathology 15: 169. 1925
Notes — The last assessments of this species by Hering (1997) and Schütt (2001), presented a highly polyphyletic circumscription judging from the available DNA barcodes of isolates included in those studies. Based on these, phylogenetic inference indicated that this species encompasses several phylogenetic species later included in the ‘F. ensiforme clade’ sensu Nalim et al. (2011). These species include N. ferruginea and N. oblonga, as well as two related single lineages, all distinct from N. petroliphila and the unnamed FSSC 12. Most of these species are morphologically comparable to the concept of F. ensiforme. However, other species outside of the ‘F. ensiforme clade’ also exhibit morphological similarities with the traditional concept of the species. No original material could be traced and communications from the WI archive indicate that a number of strains that included representative cultures of all the novelties from Wollenweber & Reinking (1925), was received in 1925, among them a culture of F. ensiforme. This culture matched the original host and geographic origin, and could have represented the ex-type of the species. Unfortunately, this strain is lost.
Fusarium javanicum Koord., Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., sect. 2, 13: 247. 1907
Synonyms. Fusarium javanicum var. chrysanthemi-leucanthemi Batikyan, Biol. Zhurn. Armenii 22: 86. 1969 (Nom. inval., Art. 39.1).
Fusarium javanicum var. sclerotii Batikyan, Biol. Zhurn. Armenii 22: 85. 1969 (Nom. inval., Art. 39.1).
Material examined. Cameroon, Victoria, on dead fruit of Theobroma cacao, 1905, W. Busse, BPI 452278 = Wollenweber: Fusaria culta 91. — Honduras, Tela, from unknown substrate, date unknown (before 1924), O.A. Reinking 161, BPI 452276. — Unknown, from unknown substrate, 10 May 1971, unknown collector, BPI 452275 = ATCC 22403.
Notes — Fusarium javanicum was assigned to the asexual morph of H. ipomoeae (Wollenweber 1930, Wollenweber & Reinking 1935a). However, the two taxa were later found not to be conspecific based on morphology, sexual behaviour and pathogenicity (Nirenberg & Brielmaier-Liebetanz 1996). This difference is further confirmed by morphological observations in the present study on materials assigned to F. javanicum by C.D. Sherbakoff and H.W. Wollenweber: these materials differ significantly from those of the asexual morph of N. ipomoeae. Fusarium javanicum produces shorter and more curved, 2–5-septate sporodochial conidia (Fig. 50; see additional notes under N. ipomoeae). The type specimen of F. javanicum could not be located, and therefore, it is not possible to presently assign F. javanicum to any of the phylogenetic clades resolved here.
Fig. 50.

Fusarium javanicum (a–d, f. BPI 452278; e BPI 452276). a–b. Herbarium specimen; c. drawing by Wollenweber (1916); d. sporodochia; e. photograph by C.D. Sherbakoff depicting conidia of specimen R161; f. conidia. — Scale bars: b = 1 cm; d = 200 μm; f = 10 μm.
Fusarium pestis Sorauer, Öst. Landw. Wochenbl.: 32. 1888
Notes — This obscure species was reduced to synonymy under F. solani var. martii f. 1 by Wollenweber (1931), re-named here as N. stercicola. The conclusions of Wollenweber (1931) were later accepted by Hering (1997). However, since no type material of this species could be located, the synonymy remains doubtful.
Fusarium solani f. sp. passiflorae C.J. Bueno, et al., Pl. Pathol. 63: 388. 2014
Notes — This special form was introduced as affecting yellow passion fruit (Passiflora edulis f. flavicarpa) in Brazil (Bueno et al. 2014). It most likely represents a distinct species based on available DNA barcodes derived from the original study. It is closely related to, but distinct from, N. solani. However, DNA barcodes were not available for all the loci included in this study, and the inclusion of additional gene data could significantly alter the phylogenetic results. The incomplete barcode dataset and the lack of morphological information, made it impossible to further assess the status of this taxon.
Fusarium solani f. sp. phalaenopsis W.C. Chung et al., Pl. Pathol. 60: 251. 2011
Notes — Described as the causal agent of orchid leaf yellowing (Phalaenopsis and Cymbidium spp.) in Taiwan (Chung et al. 2011), this special form was also recently identified in Australia (Laurence et al. 2016). As with F. solani f. sp. passiflorae, this informally designated group seems to refer to a genetically distinct population in Neocosmospora. Unfortunately, collections of F. solani f. sp. phalaenopsis were unavailable for study.
Nectria bolbophylli Henn., Hedwigia 44: 171. 1905
Synonym. Hypomyces ipomoeae f. 1 (Henn.) Wollenw., Fusaria Autographice Delineata 3: 824. 1930.
Notes — Nectria bolbophylli was considered as the sexual morph of F. radicicola by Wollenweber (1930). The original description mentions the lack of an asexual morph, but according to Wollenweber (1930), multiseptate, falcate conidia similar to those reported for F. radicicola were present on the original specimen of Nec. bolbophylli. The morphology of the holomorph certainly resembles that of a typical Neocosmospora species. Several strains considered as representative of this species were studied by Hering (1997). Based on analyses of the tef1 DNA barcodes derived from two of these strains (NRRL 22148 and NRRL 22733), they form a distinct, genetically exclusive group (data not shown) that merits further investigation.
Nectria subsequens Rehm, Hedwigia 37: 191. 1898
Notes — Samuels & Brayford (1994) assigned Nec. subsequens to the Nec. haematococca group based on the anatomy of the ascomata. The absence of an asexual morph and lack of DNA barcodes for the species prevent us from recombining it in Neocosmospora.
Neocosmospora guarapiensis (Speg.) Hirooka et al., Stud. Mycol. 71: 185. 2012
Basionym. Nectria guarapiensis Speg., Anales Soc. Ci. Argent. 19: 37. 1885.
Synonym. Cucurbitaria guarapiensis (Speg.) Kuntze, Rev. Gen. Pl. 3: 461. 1898.
Material examined. China, on bark, 3 Oct. 1993, Y. Doi, CBS 131752 = G.J.S 93-44.
Notes — Samuels & Brayford (1994) indicated perithecial features similar to those of the Nectria cinnabarina group (Rossman 1989), but this species also shares anatomical characters and ascospore morphologies with the Nec. haematococca group (Gerlach & Nirenberg 1982, Samuels et al. 1990). Although the species was described as lacking an asexual morph, Hirooka et al. (2012) reported the presence of ‘Fusarium cf. solani’ on the holotype specimen, as well as on other collections, and consequently recombined this taxon in Neocosmospora. Analyses of DNA barcodes from one of the cultures included in Hirooka et al. (2012; CBS 131752) indicated that this strain is more closely related to the Bionectriaceae than to the Nectriaceae. However, we were unable to study the type material.
Neocosmospora indica Wadhwani, Indian Bot. Reporter 2: 158. 1984
Notes — Accepted in Neocosmospora by Cannon & Hawksworth (1984) and Rossman et al. (1999), this species differs from other Neocosmospora species by having ascomatal walls of textura intricata, and by the production of irregular, verrucose ascospores, similar to those reported for N. parva (Mahoney 1976, Cannon & Hawksworth 1984). Presently, this species is excluded from Neocosmospora. Further studies of the type material are needed to confirm the taxonomic placement of this fungus.
Neocosmospora lushanensis (J. Luo & W.Y. Zhuang) Z.Q. Zeng & W.Y. Zhuang, Mycosystema 36: 280. 2017
Basionym. Haematonectria lushanensis J. Luo & W.Y. Zhuang, Sci. China, Life Sci. 53: 911. 2010.
Notes — Although the protologue appears to describe a good species, N. lushanensis is characterised as having 1-septate, spiny ascospores rather than the typical striate spores of Neocosmospora. Unfortunately, this important diagnostic phenotype was not clearly illustrated by the authors. Neocosmospora lushanensis has a well-developed asexual morph characterised by long and narrowly falcate, multiseptate conidia, which appears to be distinct from the asexual morphs known in Neocosmospora. No molecular data are presently available.
Neocosmospora parva Mahoney, Mycologia 68: 1111. 1976
Material examined. Ecuador, from silty loam soil, May 1969, D.P. Mahoney, CBS 466.70 = ATCC 28343 (ex-isotype culture).
Notes — Described from soil collected from the Galapagos Island and only known from the type collection (Rossman et al. 1999), N. parva differs greatly from other Neocosmospora species. This species is characterised by ascomatal walls of textura intricata, much smaller asci and ellipsoidal, finely warted ascospores (Mahoney 1976). DNA barcodes of the ex-isotype culture CBS 466.70 indicates that N. parva does fit within the Nectriaceae, but not in the genera Neocosmospora or Fusarium. Phylogenetic inference showed that this species is distantly related to members of the genus Chaetopsina (data not shown).
Neocosmospora rehmiana (Kirschst.) Hirooka et al., Stud. Mycol. 71: 186. 2012
Basionym. Calonectria rehmiana Kirschst., Verh. Bot. Vereins Prov. Brandenburg 48: 59. 1906.
Synonym. Nectria rehmiana (Kirschst.) Rossman, Mycol. Pap. 150: 24. 1983.
Notes — Lectotypified by Rossman (1983), this species was recombined into Neocosmospora by Hirooka et al. (2012) based on the shape, colour and coarsely warted walls of the ascomata. However, the presence of up to 3-septate, hyaline and smooth ascospores, perithecia formed on a well-developed stroma and a dark purple reaction of the ascomatal walls to 3 % KOH, indicates this taxon does not belong in the genus Neocosmospora.
Neocosmospora striata Udagawa & Y. Horie, Trans. Mycol. Soc. Japan 16: 340. 1975
Material examined. Japan, Nagano Pref., from soil, 20 Sept. 1974, Y. Horie, CBS 105.77 = NRRL 22427 = NRRL 22443 = ATCC 34720 = IFM 4528 = IMI 210879 = NHL 2745 (ex-type culture).
Notes — Cannon & Hawksworth (1984) tentatively retained N. striata in Neocosmospora, but highlighted the fact that this taxon greatly differs morphologically from any other species in Neocosmospora based on the brown, hairy ascomata, asci with apical rings, ascospore shape, and transversely striate ascospore ornamentation. A tef1 barcode derived from the ex-type is available (DQ247604; Zhang et al. 2006) and links this species to Neocosmospora. However, the ex-type culture (CBS 105.77) was re-sequenced here and rDNA and rpb2 barcodes resolved this species as more closely related to Dothideomycetes. It is therefore excluded from Neocosmospora.
Neocosmospora tenuicristata S. Ueda & Udagawa, Mycotaxon 16: 387. 1983
Synonym. Acremonium tenuicristatum S. Ueda & Udagawa, Mycotaxon 16: 387. 1983.
Material examined. Japan, Nagasaki, Higashisonogi-gun, Oomura Bay, from marine sludge, 26 Jan. 1981, S. Ueda, IMI 277708 = NHL 2911 (culture ex-isotype).
Notes — This species is known only from the type collection (Rossman et al. 1999). The ex-isotype culture, maintained as metabolically inactive, is not available anymore (H. Stewart, pers. comm.). However, perithecial structures and ascospores were still recognisable after rehydration of the freeze-dried ex-isotype isolate (IMI 277708; Fig. 51). As stated by Cannon & Hawksworth (1984), the ascospores of N. tenuicristata have transverse striations rather than the typical ornamentation observed in Neocosmospora spp. Based on rDNA barcodes obtained from the preserved non-living structures of the ex-isotype, N. tenuicristata does not belong in Neocosmospora, but rather is closely related to the genus Fusicolla. However, the presence of an acremonium-like asexual morph in N. tenuicristata distinguishes it from the latter genus.
Fig. 51.
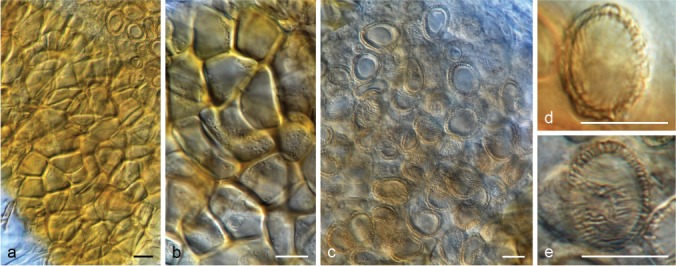
Neocosmospora tenuicristata (ex-isotype IMI 277708). a–b. Detail of ascomatal peridium cells (on lactic acid); c–e. ascospores. — Scale bars = 10 μm.
Neocosmospora termitum (Höhn.) L. Lombard & Crous, Stud. Mycol. 80: 228. 2015
Basionym. Neoskofitzia termitum Höhn., Sitzungsber. Heidelberger Akad. Wiss., Math.-Naturwiss. Kl. 117: 998. 1908.
Synonym. Haematonectria termitum (Höhn.) Samuels & Rossman, Stud. Mycol. 42: 137. 1999.
Notes — Neoskofitzia termitum was examined and lectotypified by Rossman et al. (1999), and transferred to Haematonectria because it showed a similar ascomatal morphology. This combination was followed by Lombard et al. (2015) who then renamed the species in Neocosmospora. However, the absence of an asexual morph, and above all, the morphological features of ascospores being ‘1-septate, disarticulating into sixteen part-ascospores, part ascospores broadly ellipsoid, 3–4(–4.5) × 3–3.5 μm, translucent yellow-brown, becoming densely spinulose’ (Rossman et al. 1999), indicate that this taxon does not belong to Neocosmospora.
Acknowledgements
We want to thank the curators and managers of the following fungaria and culture collections for sending specimens and important information regarding original material: S. Dominick (BPI), D. Pfister and G.E. Tocci (FH), T. Merkx, A. van Iperen and G. Verkley (CBS); H.J.R. Souza (IAN), H. Stewart (IMI), B. Aguirre-Hudson (K), C. Decock (MUCL), T.W. Adkins, J. Swezey and T. Ward (NRRL) and R. Marcucci (PAD). Kerry O’Donnell is thanked for providing sequence datasets. Gary J. Samuels and K.A. Seifert are thanked for giving access to collection data and unpublished notes. Vladimiro Guarnaccia and A. Akulov are thanked for providing literature. Yuuri Hirooka and C. Nakashima for their help in locating specimens. Paul Cannon, K. Bensch, U. Braun and S. Pennycook are thanked for their help on nomenclatural issues.
REFERENCES
- Achenbach LA, Patrick J, Gray L. 1996. Use of RAPD markers as a diagnostic tool for the identification of Fusarium solani isolates that cause soybean sudden death syndrome. Plant Disease 80: 1228–1232. [Google Scholar]
- Achor DS, Nemec S, Baker RA. 1993. Effects of Fusarium solani naphthazarin toxins on the cytology and ultrastructure of rough lemon seedlings. Mycopathologia 123: 117–126. [Google Scholar]
- Aist JR. 2002. Mitosis and motor proteins in the filamentous ascomycete, Nectria haematococca, and some related fungi. International Review of Cytology 2012: 239–263. [DOI] [PubMed] [Google Scholar]
- Al-Hatmi AMS, Ahmed SA, Van Diepeningen AD, et al. 2018. Fusarium metavorans sp. nov.: the frequent opportunist ‘FSSC6’. Medical Mycology 56: S144–S152. [DOI] [PubMed] [Google Scholar]
- Al-Hatmi AMS, Bonifaz A, Tirado-Sánchez A, et al. 2016. Fusarium species causing eumycetoma: Report of two cases and comprehensive review of the literature. Mycoses 60: 204–212. [DOI] [PubMed] [Google Scholar]
- Anaissie E, Kantarjian H, Jones P, et al. 1986. Fusarium. A newly recognized fungal pathogen in immunosuppressed patients. Cancer 57: 2141–2145. [DOI] [PubMed] [Google Scholar]
- Antonissen G, Martel A, Pasmans F, et al. 2014. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel) 6: 430–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Kasson MT, Berger MC, et al. 2018. Fusarium oligoseptatum sp. nov., a mycosymbiont of the ambrosia beetle Euwallacea validus in the Eastern U.S. and typification of F. ambrosium. Fungal Systematics and Evolution 1: 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, O’Donnell K, Homma Y, et al. 2003. Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex – F. virguliforme in North America and F. tucumaniae in South America. Mycologia 95: 660–684. [PubMed] [Google Scholar]
- Aoki T, O’Donnell K, Scandiani MM. 2005. Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliense sp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme. Mycoscience 46: 162–183. [Google Scholar]
- Aoki T, Scandiani MM, O’Donnell K. 2012a. Phenotypic, molecular phylogenetic, and pathogenetic characterization of Fusarium crassistipitatum sp. nov., a novel soybean sudden death syndrome pathogen from Argentina and Brazil. Mycoscience 53: 167–186. [Google Scholar]
- Aoki T, Smith JA, Mount LL, et al. 2013. Fusarium torreyae sp. nov., a pathogen causing canker disease of Florida torreya (Torreya taxifolia), a critically endangered conifer restricted to northern Florida and southwestern Georgia. Mycologia 105: 312–319. [DOI] [PubMed] [Google Scholar]
- Aoki T, Tanaka F, Suga H, et al. 2012b. Fusarium azukicola sp. nov., an exotic azuki bean root-rot pathogen in Hokkaido, Japan. Mycologia 104: 1068–1084. [DOI] [PubMed] [Google Scholar]
- Appel O, Strunk HF. 1904. Ueber einige in Kamerun auf Theobroma cacao beobachtete pilze. Zentralblatt für Bakteriologie und Parasitenkunde, Abteilung 2, 11: 551–557. [Google Scholar]
- Appel O, Wollenweber HW. 1910. Grundlagen einer monographie der gattung Fusarium (Link). Kaiserlichen Biologischen Anstalt für Land- und Forstwirtschaft 8: 1–207. [Google Scholar]
- Bacon CW, Porter JK, Norred WP, et al. 1996. Production of fusaric acid by Fusarium species. Applied and Environmental Microbiology 62: 4039–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmas V, Scherm B, Marcello A, et al. 2015. Fusarium species and chemotypes associated with fusarium head blight and fusarium root rot on wheat in Sardinia. Plant Pathology 64: 972–979. [Google Scholar]
- Berkeley MJ, Broome CE. 1873. Enumeration of the fungi of Ceylon. Part II, containing the remainder of the Hymenomycetes, with the remaining established tribes of Fungi (continued). Journal of the Linnean Society of London, Botany 14: 65–140. [Google Scholar]
- Bilai VI. 1955. The fusaria (biology and systematics). Akademia Naukwe Ukrainskii, SSR, Kiev. [Google Scholar]
- Booth C. 1966. The genus Cylindrocarpon. Mycological Papers 104: 1–56. [Google Scholar]
- Booth C. 1971. The genus Fusarium. Commonwealth Mycology Institute, Kew, England. [Google Scholar]
- Brayford D. 1987. Fusarium bugnicourtii sp. nov., and its relationship to F. tumidum and F. tumidum var. coeruleum. Transactions of the British Mycological Society 89: 347–351. [Google Scholar]
- Brogniart C. 1891. Les champignons parasites observés sur les criquets pèlerins en Algérie (Acridium peregrinum Oliv.). Bulletin de la Société Nationale d’Agriculture de France 51: 627–636. [Google Scholar]
- Bruen TC, Philippe H, Bryant D. 2006. A simple and robust statistical test for detecting the presence of recombination. Genetics 172: 2665–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno CJ, Fischer IH, Rosa DD, et al. 2014. Fusarium solani f. sp. passiflorae: a new forma specialis causing collar rot in yellow passion fruit. Plant Pathology 63: 382–389. [Google Scholar]
- Bugnicourt F. 1939. Les Fusarium et Cylindrocarpon de l’Indochine. Encyclopédie Mycologique 11: 1–206. [Google Scholar]
- Burgess LW, Summerell BA, Bullock S, et al. 1994. Laboratory manual for Fusarium research. Fusarium Research Laboratory, The University of Sydney, Australia. [Google Scholar]
- Burkholder WH. 1919. The dry root rot of the bean. Memoirs of the Cornell University Agricultural Experimental Station 26: 1003–1033. [Google Scholar]
- Cannon PF, Hawksworth DL. 1984. A revision of the genus Neocosmospora (Hypocreales). Transactions of the British Mycological Society 82: 673–688. [Google Scholar]
- Carmichael JW, Kendrick WB, Conners IL, et al. 1980. Genera of Hyphomycetes. University of Alberta Press, Edmonton, Canada. [Google Scholar]
- Carpenter CW. 1915. Some potato tuber-rots caused by species of Fusarium. Journal of Agricultural Research 5: 183–209. [Google Scholar]
- Carrión AL. 1951. Cephalosporium falciforme sp. nov., a new etiologic agent of maduromycosis. Mycologia 43: 522–523. [Google Scholar]
- Chaverri P, Salgado C, Hirooka Y, et al. 2011. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology 68: 57–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chełkowski J. 1989. Fusarium: mycotoxins, taxonomy and pathogenicity, vol. 2. Topics in Secondary Metabolism Series. Elsevier Science & Technology Books. University of Wisconsin – Madison. [Google Scholar]
- Chen QT, Fu XH, Chen YD. 1987. Two petroliphilous taxa of Fusarium. Acta Mycologica Sinica, Supplement 1: 328–333. [Google Scholar]
- Chen W, Swart WJ. 2000. First report of stem canker of English walnut caused by Fusarium solani in South Africa. Plant Disease 84: 592. [DOI] [PubMed] [Google Scholar]
- Chitrampalam P, Nelson B., Jr 2016. Multilocus phylogeny reveals an association of agriculturally important Fusarium solani species complex (FSSC) 11, and clinically important FSSC 5 and FSSC 3 + 4 with soybean roots in the north central United States. Antonie Van Leeuwenhoek 109: 335–347. [DOI] [PubMed] [Google Scholar]
- Chowdhury NS, Sohrab MH, Rana MS, et al. 2017. Cytotoxic naphthoquinone and azaanthraquinone derivatives from an endophytic Fusarium solani. Journal of Natural Products 80: 1173–1177. [DOI] [PubMed] [Google Scholar]
- Chung WC, Chen LW, Huang JH, et al. 2011. A new ‘forma specialis’ of Fusarium solani causing leaf yellowing of Phalaenopsis. Plant Pathology 60: 244–252. [Google Scholar]
- Cognato AI, Hoebeke ER, Kajimura H, et al. 2015. History of the exotic Ambrosia beetles Euwallacea interjectus and Euwallacea validus (Coleoptera: Curculionidae: Xyleborini) in the United States. Journal of Economic Entomology 108: 1129–1135. [DOI] [PubMed] [Google Scholar]
- Coleman JJ. 2016. The Fusarium solani species complex: ubiquitous pathogens of agricultural importance. Molecular Plant Pathology 17: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JJ, Rounsley SD, Rodriguez-Carres M, et al. 2009. The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genetics 5: e1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa SS, Matos KS, Tessmann DJ, et al. 2016. Fusarium paranaense sp. nov., a member of the Fusarium solani species complex causes root rot on soybean in Brazil. Fungal Biology 120: 51–60. [DOI] [PubMed] [Google Scholar]
- Costa SS, Moreira GM, Pfenning LH. 2017. Development of a PCR protocol for the identification and detection of Fusarium solani f. sp. piperis from soil and roots of black pepper (Piper nigrum). Tropical Plant Pathology 42: 55–59. [Google Scholar]
- Crous PW, Schoch CL, Hyde KD, et al. 2009. Phylogenetic lineages in the Capnodiales. Studies in Mycology 64: 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. 2019. Westerdijk Laboratory Manual Series 1: Fungal Biodiversity. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- Crowhurst RN, Rees-George J, Rikkerink EHA, et al. 1992. High efficiency transformation of Fusarium solani f. sp. cucurbitae race 2 (mating population V). Current Genetics 21: 463–469. [DOI] [PubMed] [Google Scholar]
- Dallé Rosa P, Ramirez-Castrillon M, Valente P, et al. 2018. Fusarium riograndense sp. nov., a new species in the Fusarium solani species complex causing fungal rhinosinusitis. Journal de Mycologie Médicale 28: 29–35. [DOI] [PubMed] [Google Scholar]
- De Hoog GS, Buiting A, Tan CS, et al. 1993. Diagnostic problems with imported cases of mycetoma in The Netherlands. Mycoses 36: 81–87. [DOI] [PubMed] [Google Scholar]
- De Vries GA, De Hoog GS, De Bruyn HP. 1984. Phialophora cyanescens sp. nov. with Phaeosclera-like synanamorph, causing white-grain mycetoma in man. Antonie van Leeuwenhoek 50: 149–153. [DOI] [PubMed] [Google Scholar]
- Dingley JM. 1951. The Hypocreales of New Zealand II. The genus Nectria. Transactions of the Royal Society of New Zealand 79: 177–202. [Google Scholar]
- Domsch KH, Gams W, Anderson TH. 2007. Compendium of soil fungi, ed 2. IHW Verlag, Eching. [Google Scholar]
- Enkerli J, Bhatt G, Covert SF. 2007. Maackiain detoxification contributes to the virulence of Nectria haematococca MP VI on chickpea. Molecular Plant-Microbe Interactions 11: 317–326. [Google Scholar]
- Eskalen A, Stouthamer R, Lynch SC, et al. 2013. Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Disease 97: 938–951. [DOI] [PubMed] [Google Scholar]
- Farr DF, Rossman AY. Continuously updated. Fungal databases, U.S. National Fungus Collections, ARS, USDA; Retrieved 13 March 2017, from https://nt.ars-grin.gov/fungaldatabases/. [Google Scholar]
- Fathima BS, Balakrishnan RM. 2014. Biosynthesis and optimization of silver nanoparticles by endophytic fungus Fusarium solani. Material Letters 132: 428–431. [Google Scholar]
- Fernando N, Hui SW, Tsang CC, et al. 2015. Fatal Fusarium solani species complex infections in elasmobranchs: the first case report for black spotted stingray (Taeniura melanopsila) and a literature review. Mycoses 58: 422–431. [DOI] [PubMed] [Google Scholar]
- Fisher NL, Burguess LW, Toussoun TA, et al. 1982. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 72: 151–153. [Google Scholar]
- Fletcher JT. 1994. Fusarium stem and fruit rot of sweet peppers in the glasshouse. Plant Pathology 43: 225–227. [Google Scholar]
- Freeman S, Sharon M, Maymon M, et al. 2013. Fusarium euwallaceae sp. nov. – a symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 105: 1595–1606. [DOI] [PubMed] [Google Scholar]
- Gadd CH, Loos CA. 1947. The ambrosia fungus of Xyleborus fornicatus Eich. Transactions of the British Mycological Society 31: 13–19. [Google Scholar]
- Gams W. 1971. Cephalosporium-artige Schimmelpilze. Fischer, Stuttgart, Germany. [Google Scholar]
- Gams W, Klamer M, O’Donnell K. 1999. Fusarium miscanthi sp. nov. from Miscanthus litter. Mycologia 91: 263–268. [Google Scholar]
- Geiser DM, Aoki T, Bacon CW, et al. 2013. One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology 103: 400–408. [DOI] [PubMed] [Google Scholar]
- Gerlach W. 1981. The present concept of Fusarium classification. In: Nelson PE, Toussoun TA, Cook RJ. (eds), Fusarium diseases, biology and taxonomy: 413–426. Pennsylvania State University Press, USA. [Google Scholar]
- Gerlach W, Nirenberg HI. 1982. The genus Fusarium – a pictorial atlas. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 209: 1–406. [Google Scholar]
- Giard MA. 1891. Sur les cladosporiées entomophytes, nouveau groupe de champignons parasites des insectes. Comptes Rendus Hebdomadaires des Séances de l’Academie des Sciences. Paris 112: 1518–1521. [Google Scholar]
- Gräfenhan T, Schroers HJ, Nirenberg HI, et al. 2011. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology 68: 79–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadet J, Julien J, Lafay JF, et al. 1989. Phylogeny of some Fusarium species, as determined by large-subunit rRNA sequence comparison. Molecular Biology and Evolution 6: 227–242. [DOI] [PubMed] [Google Scholar]
- Guarnaccia V, Sandoval-Denis M, Aiello D, et al. 2018. Neocosmospora perseae sp. nov., causing trunk cankers on avocado in Italy. Fungal Systematics and Evolution 1: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J. 2013. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. European Journal of Clinical Microbiology & Infectious Diseases 32: 1491–1500. [DOI] [PubMed] [Google Scholar]
- Guarro J, Gené J, Stchigel AM, et al. 2012. Atlas of soil Ascomycetes. CBS Biodiversity Series 10. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Hamada M, Uchida T, Tsuda M. 1988. Ascospore dispersion of the causal agent of Nectria blight of Piper nigrum. Annals of the Phytopathological Society of Japan 54: 303–308. [Google Scholar]
- Hawksworth DL. 1979. The lichenicolous hyphomycetes. Bulletin of the British Museum (Natural History) 6: 183–300. [Google Scholar]
- Hawthorne BT, Rees-George J, Broadhurst PG. 1992. Mating behaviour and pathogenicity of New Zealand isolates of Nectria haematococca (Fusarium solani). New Zealand Journal of Crop and Horticultural Science 20: 51–57. [Google Scholar]
- Hering O. 1997. Charakterisierung und Differenzierung bei Fusarium Link mittels RAPD und ITS-RFLP. Biologische Bundesanstalt für Land- und Forstwirtschaft, Institut für Mikrobiologie, Berlin-Dahlem. [Google Scholar]
- Herkert PF, Al-Hatmi AMS, De Oliveira Salvador GL, et al. 2019. Molecular characterization and antifungal susceptibility of clinical Fusarium species from Brazil. Frontiers in Microbiology 10: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Rossman AY, Samuels GJ, et al. 2012. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology 71: 1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honraet K, De Vos MM, Summerbell RC, et al. 2005. Recurrent colonization of successively implanted tracheoesophageal vocal prostheses by a member of the Fusarium solani species complex. Journal of Clinical Microbiology 43: 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopple JS, Vilgalys R. 1994. Phylogenetic relationships among coprinoid taxa and allies based on data from restriction site mapping of nuclear rDNA. Mycologia 86: 96–107. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. [DOI] [PubMed] [Google Scholar]
- Ibarra-Laclette E, Sánchez-Rangel D, Hernández-Domínguez E, et al. 2017. Draft genome sequence of the phytopathogenic fungus Fusarium euwallaceae, the causal agent of fusarium dieback. Genome Announcements 5: e00881–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Sakai K, Ueno Y, et al. 1971. Solaniol, a toxic metabolite of Fusarium solani. Applied Microbiology 22: 718–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallouli R, Othman H, Amara S, et al. 2015. The galactolipase activity of Fusarium solani (phospho)lipase. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1851: 282–289. [DOI] [PubMed] [Google Scholar]
- Jarvis WR, Khosla SK, Barrie SD. 1994. Fusarium stem and fruit rot of sweet pepper in Ontario greenhouses. Canadian Plant Disease Survey 74: 131–134. [Google Scholar]
- Joffe AZ. 1974. A modern system of Fusarium taxonomy. Mycopathologia et Mycologia Applicata 53: 201–228. [DOI] [PubMed] [Google Scholar]
- Joffe AZ, Palti J. 1972. Fusarium species of the Martiella section in Israel. Journal of Phytopathology 73: 123–148. [Google Scholar]
- Jones FR. 1923. Stem and root rot of peas in the United States caused by species of Fusarium. Journal of Agricultural Research 26: 459–476. [Google Scholar]
- Karsten PA. 1888. Symbolae ad mycologiam Fennicam. XXVII. Meddelanden af Societas pro Fauna et Flora Fennica 16: 33–36. [Google Scholar]
- Kasson MT, O’Donnell K, Rooney AP, et al. 2013. An inordinate fondness for Fusarium: Phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genetics and Biology 56: 147–157. [DOI] [PubMed] [Google Scholar]
- Kasson MT, O’Neal ES, Davis DD. 2015. Expanded host range testing for Verticillium nonalfalfae: potential biocontrol agent against the invasive Ailanthus altissima. Plant Disease 99: 823–835. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software v. 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick B. 1974. The generic iceberg. Taxon 23: 747–753. [Google Scholar]
- Kim JA, Jeon J, Park SY, et al. 2017. Genome sequence of an endophytic fungus, Fusarum solani JS-169, which has antifungal activity. Genome Announcements 5: e01071–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter R, et al. 2008. Ainsworth and Bisby’s dictionary of fungi. CABI Publishing, UK. [Google Scholar]
- Koorders SH. 1907. Botanische Untersuchungen. Verhandelingen Koninklijke Nederlandse Akademie van Wetenschappen Afdeling Natuurkunde 13: 1–264. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence MH, Howard C, Summerell BA, et al. 2016. Identification of Fusarium solani f.sp. phalaenopsis in Australia. Australasian Plant Disease Notes 11: 15. [Google Scholar]
- Lee HS, Phat C, Nam WS, et al. 2014. Optimization of culture conditions of Fusarium solani for the production of neoN-methylsansalvamide. Bioscience, Biotechnology, and Biochemistry 78: 1421–1427. [DOI] [PubMed] [Google Scholar]
- Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames. [Google Scholar]
- Li W, Cowley A, Uludag M, et al. 2015. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Research 43: W580–W584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew ECY, Laurence MH, Pearce CA, et al. 2016. Review of Fusarium species isolated in association with mango malformation in Australia. Australasian Plant Pathology 45: 547–559. [Google Scholar]
- Liu H, Bao X. 2009. Characterization of a chitosanase from Fusarium solani and its expression in an industrial strain of Saccharomyces cerevisiae. Wei Sheng Wu Xue Bao 49: 1607–1612. [Article in Chinese.] [PubMed] [Google Scholar]
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2015. Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhi S, Nicolas A, Jelsch C, et al. 2005. Structure-function studies on cutinase, a small lipolytic enzyme with a water accessible active site. In: Alberghina L. (ed), Protein engineering in industrial biotechnology: 75–114. Harwood Academic Publishers, Amsterdam, The Netherlands. [Google Scholar]
- Madelin MF. 1966. Trichothecium acridiorum (Trabut) comb. nov. on red locust. Transactions of the British Mycological Society 49: 275–288. [Google Scholar]
- Mahoney DP. 1976. A new Neocosmospora from Galapagos Island soil. Mycologia 68: 1111–1116. [Google Scholar]
- Mannesse MLM. 1997. Enantioselectivity of cutinase from Fusarium solani. PhD Thesis, University of Utrecht, The Netherlands. [Google Scholar]
- Martius CFP. 1842. Die Kartoffel-Epidemie der letzten Jahre oder die Stockfäule und Räude der Kartoffeln. Verlag der königlich bayerischen Akademie der Wissenschaften, München. [Google Scholar]
- Mason-Gamer R, Kellogg E. 1996. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 45: 524–545. [Google Scholar]
- Matuo T, Sakurai Y. 1965. Fusarium solani f. robiniae n. f., one of the causal fusaria of the twig blight of Robinia pseudoacacia. Annals of the Phytopathological Society of Japan 30: 31–36. [Google Scholar]
- Matuo T, Snyder WC. 1973. Use of morphology and mating populations in the identification of formae speciales in Fusarium solani. Phytopathology 63: 562–565. [Google Scholar]
- Mawhinney I, Woodger N, Trickey S, et al. 2008. Suspected sweet potato poisoning in cattle in the UK. Veterinary Record 162: 62–63. [DOI] [PubMed] [Google Scholar]
- McClure TT. 1951. Fusarium foot rot of sweet-potato sprouts. Phytopathology 41: 72–77. [Google Scholar]
- Mehl HL, Epstein L. 2007a. Identification of Fusarium solani f. sp. cucurbitae race 1 and race 2 with PCR and production of disease-free pumpkin seeds. Plant Disease 91: 1288–1292. [DOI] [PubMed] [Google Scholar]
- Mehl HL, Epstein L. 2007b. Fusarium solani species complex isolates conspecific with Fusarium solani f. sp. cucurbitae race 2 from naturally infected human and plant tissue and environmental sources are equally virulent on plants, grow at 37°C and are interfertile. Environmental Microbiology 9: 2189–2199. [DOI] [PubMed] [Google Scholar]
- Mendel Z, Protasov A, Sharon M, et al. 2012. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 40: 235–238. [Google Scholar]
- Menge JA. 1988. Dry root rot. In: Whiteside JO, Garnsey SM, Timmer LW. (eds), Compendium of citrus diseases: 14–15. APS Press, USA. [Google Scholar]
- Merlin JN, Christudas IVSN, Kumar PP, et al. 2013. Optimization of growth and bioactive metabolite production: Fusarium solani. Asian Journal of Pharmaceutical and Clinical Research 6: 98–103. [Google Scholar]
- Migheli Q, Balmas V, Harak H, et al. 2010. Molecular phylogenetic diversity of dermatologic and other human pathogenic fusarial isolates from hospitals in northern and central Italy. Journal of Clinical Microbiology 45: 1076–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2012. The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st Conference of the Extreme Science and Engineering Discovery Environment: Bridging from the extreme to the campus and beyond: 1–8. Association for Computing Machinery, USA. [Google Scholar]
- Na F, Carrillo JD, Mayorquin JS, et al. 2018. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio shot hole borer (Euwallaceae sp. nr. fornicatus) cause fusarium dieback on woody host species in California. Plant Disease 102: 1154–1164. [DOI] [PubMed] [Google Scholar]
- Nakajima H, Hamasaki T, Tanaka K, et al. 1989. Production of cyclosporin by fungi belonging to the genus Neocosmospora. Agricultural and Biological Chemistry 53: 2291–2292. [Google Scholar]
- Nalim FA, Samuels GJ, Wijesundera RL, et al. 2011. New species from the Fusarium solani species complex derived from perithecia and soil in the old World tropics. Mycologia 103: 1302–1330. [DOI] [PubMed] [Google Scholar]
- Nelson PE, Dignani MC, Anaissie EJ. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clinical Microbiology Reviews 7: 479–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PE, Toussoun TA, Marasas WFO. 1983. Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, University Park, USA. [Google Scholar]
- Nirenberg HI. 1976. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Nirenberg HI. 1981. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany 59: 1599–1609. [Google Scholar]
- Nirenberg H, Brielmaier-Liebetanz U. 1996. Nectria ipomoeae Halst., anamorph: Fusarium striatum Sherb. an Passiflora edulis Sims. Nachrichten Blatt des Deutschen Pflanzenschutzdienstes 48: 270–275. [Google Scholar]
- Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K. 1993. Fusarium and its near relatives. In: Reynolds DR, Taylor JW. (eds), The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics: 225–236 CAB International, UK [Google Scholar]
- O’Donnell K. 2000. Molecular phylogeny of the Nectria haematococca–Fusarium solani species complex. Mycologia 92: 919–938. [Google Scholar]
- O’Donnell K, Humber RA, Geiser DM, et al. 2012. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia 104: 427–445. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Rooney AP, Proctor RH, et al. 2013. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genetics and Biology 52: 20–31. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Sink S, Libeskind-Hadas R, et al. 2015. Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Genetics and Biology 82: 277–290. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Sink S, Scandiani MM, et al. 2010. Soybean sudden death syndrome species diversity within North and South America revealed by multilocus genotyping. Phytopathology 100: 58–71. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Sutton DA, Fothergill A, et al. 2008. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. Journal of Clinical Microbiology 46: 2477–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K, Sutton DA, Wiederhold N, et al. 2016. Veterinary fusarioses within the United States. Journal of Clinical Microbiology 54: 2813–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paap T, De Beer ZW, Migliorini D, et al. 2018. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: a new invasion in South Africa. Australasian Plant Pathology 47: 231–237. [Google Scholar]
- Papizadeh M, Van Diepeningen AD, Zamanizadeh HR, et al. 2018. Fusarium ershadii sp. nov., a pathogen on Asparagus officinalis and Musa acuminata. European Journal of Plant Pathology 151: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RH. 1959. A new species of Mastigosporium from tropical soil. Mycologia 51: 729–733. [Google Scholar]
- Pfenning L. 1995. A new species of Neocosmospora from Brazil. Sydowia 47: 65–69. [Google Scholar]
- Pitt JI, Hocking AD. 2009. Fungi and food spoilage ed. 3. Springer Science & Business Media. [Google Scholar]
- Polizzi G, Magnano di San Lio G, Catara A. 1992. Dry root rot of citranges in Italy. Proceedings of the International Society of Citriculture. VII International Citrus Congress, Acireale 1992: 890–893. [Google Scholar]
- Porter LD, Pasche JS, Chen W, et al. 2015. Isolation, identification, storage, pathogenicity tests, hosts, and geographic range of Fusarium solani f. sp. pisi causing Fusarium root rot of pea. Plant Health Progress 16: 136–145. [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragazzi A, Mugnai L, Moricca S, et al. 1993. Requirements and biological aspects of Fusarium eumartii and its possible role in oak decline in North-eastern Italian oak forests. European Journal of Forest Pathology 23: 171–177. [Google Scholar]
- Raillo A. 1950. Fungi of the genus Fusarium. State Publishing House of Agricultural Literature, Moscow, USSR. [Google Scholar]
- Rathna J, Yazhini B, Kennedy AAA, et al. 2016. Production of napthoquinones and phenolics by a novel isolate Fusarium solani PSC-R of Palk Bay and their industrial applications. Bioresource Technology 213: 289–298. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. 1995. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their ananmorphs. Canadian Journal of Botany 73: S816–S823. [Google Scholar]
- Reichle RE, Snyder WC, Matuo T. 1964. Hypomyces stage of Fusarium solani f. pisi. Nature 203: 664–665. [Google Scholar]
- Reinking OA, Wollenweber HW. 1927. Tropical fusaria. The Phillipine Journal of Science 32: 103–253. [Google Scholar]
- Rodriguez-Carres M, White G, Tsuchiya D, et al. 2008. The supernumerary chromosome of Nectria haematococca that carries pea-pathogenicity-related genes also carries a trait for pea rhizosphere competitiveness. Applied and Environmental Microbiology 74: 3849–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo FG, Reynoso MM, Ferez M, et al. 2007. Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Protection 26: 549–555. [Google Scholar]
- Romberg MK, Davis RM. 2007. Host range and phylogeny of Fusarium solani f. sp. eumartii from potato and tomato in California. Plant Disease 91: 585–592. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Rossman AY. 1983. The phragmosporous species of Nectria and related genera. Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY. 1989. A synopsis of the Nectria cinnabarina-group. Memoirs of the New York Botanical Garden 49: 253–265. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Roy KW. 1997. Fusarium solani on soybean roots: Nomenclature of the causal agent of sudden death syndrome and identity and relevance of F. solani form B. Plant Disease 81: 259–266. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Matuo T. 1959. On the form name and race of Hypomyces solani (Rke. et Berth.) Snyd. et Hans. which is pathogenic to the mulberry trees. Annals of the Phytopathological Society of Japan 24: 219–223. [Google Scholar]
- Sakurai Y, Matuo T. 1961. Taxonomy of the causal fungus of trunk-blight of Xanthoxylum piperitum and heterothallism in this fungus. Annals of the Phytopathological Society of Japan 26: 112–117. [Google Scholar]
- Samuels GJ. 1976. A revision of the fungi formerly classified in Nectria subg. Hyphonectria. Memoires of the New York Botanical Garden 26: 1–126. [Google Scholar]
- Samuels GJ, Brayford D. 1994. Species of Nectria (sensu lato) with red perithecia and striate ascospores. Sydowia 46: 75–161. [Google Scholar]
- Samuels GJ, Doi Y, Rogerson CT. 1990. Hypocreales. Memoires of the New York Botanical Garden 59: 6–108. [Google Scholar]
- Samuels GJ, Rossman AY, Chaverri P, et al. 2006. Hypocreales of the southeastern United States: An identification guide. CBS Biodiversity Series 4. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Sandoval-Denis M, Crous PW. 2018. Removing chaos from confusion: assigning names to common human and animal pathogens in Neocosmospora. Persoonia 41: 109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Denis M, Guarnaccia V, Polizzi G, et al. 2018. Symptomatic Citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia 40: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento-Ramírez JM, Abella-Pérez E, Phillott AD, et al. 2014. Global distribution of two fungal pathogens threatening endangered sea turtles. PLoS ONE 9: e85853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiento-Ramírez JM, Sim J, Van West P, et al. 2017. Isolation of fungal pathogens from eggs of the endangered sea turtle species Chelonia mydas in Ascension Island. Journal of the Marine Biological Association of the United Kingdom 97: 661–667. [Google Scholar]
- Sawada K. 1959. Descriptive catalogue of Taiwan (Formosan) fungi. Part XI. Special Publication College of Agriculture National Taiwan University 8: 1–268. [Google Scholar]
- Scheel CM, Hurst SF, Barreiros G, et al. 2013. Molecular analyses of Fusarium isolates recovered from a cluster of invasive mold infections in a Brazilian hospital. BMC Infectious Diseases 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroers HJ, Gräfenhan T, Nirenberg HI, et al. 2011. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Studies in Mycology 68: 115–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroers HJ, Samuels GJ, Zhang N, et al. 2016. Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia 108: 806–819. [DOI] [PubMed] [Google Scholar]
- Schütt F. 2001. Moniliforminbildung von Fusarium-Arten unter definierten Bedingungen [thesis dissertation]. Fakultät für Prozesswissenschaften, Technischen Universität Berlin. [Google Scholar]
- Seifert KA, Morgan-Jones GA, Gams W, et al. (eds). 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9, CBS Fungal Diversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Sherbakoff CD. 1915. Fusaria of potatoes. Memoirs of the Cornell University Agricultural Experimental Station 6: 87–270. [Google Scholar]
- Short DP, O’Donnell K, Thrane U, et al. 2013. Phylogenetic relationships among members of the Fusarium solani species complex in human infections and the descriptions of F. keratoplasticum sp. nov. and F. petroliphilum stat. nov. Fungal Genetics and Biology 53: 59–70. [DOI] [PubMed] [Google Scholar]
- Short DP, O’Donnell K, Zhang N, et al. 2011. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. Journal of Clinical Microbiology 49: 4264–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šišić A, Al-Hatmi AMS, Baćanović-Šišić J, et al. 2018a. Two new species of the Fusarium solani species complex isolated from compost and hibiscus (Hibiscus sp.). Antonie van Leeuwenhoek 111: 1785–1805. [DOI] [PubMed] [Google Scholar]
- Šišić A, Baćanović-Šišić J, Al-Hatmi AMS, et al. 2018b. The ‘forma specialis’ issue in Fusarium: A case study in Fusarium solani f. sp. pisi. Scientific Reports 8: 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin M, Van Hal S, Sorrell TC, et al. 2015. Invasive infections due to filamentous fungi other than Aspergillus: epidemiology and determinants of mortality. Clinical Microbiology and Infection 21: 490.e1–10. [DOI] [PubMed] [Google Scholar]
- Smith EF. 1899. Wilt disease of cotton, watermelon and cowpea (Neocosmospora nov. gen.). Bulletin, Division of Vegetable Physiology and Pathology, U S Department of Agriculture. Washington, DC. 17: 1–73. [Google Scholar]
- Snyder WC, Georgopoulos SG, Webster RK, et al. 1975. Sexuality and genetic behavior in the fungus Hypomyces (Fusarium) solani f. sp. cucurbitae. Hilgardia 43: 161–185. [Google Scholar]
- Snyder WC, Hansen HN. 1941. The species concept in Fusarium with reference to section Martiella. American Journal of Botany 28: 738–742. [Google Scholar]
- Spatafora JW, Blackwell M. 1994. The polyphyletic origins of ophiostomatoid fungi. Mycological Research 98: 1–9. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JA. 1971. An account of fungus exsiccati, containing material from the Americas. Beihefte zur Nova Hedwigia 36: 1–563. [Google Scholar]
- Stouthamer R, Rugman-Jones P, Thu PQ, et al. 2017. Tracing the origin of a cryptic invader: phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agricultural and Forest Entomology 19: 366–375. [Google Scholar]
- Suga H, Hasegawa T, Mitsui H, et al. 2000. Phylogenetic analysis of the phytopathogenic fungus Fusarium solani based on the rDNA-ITS region. Mycological Research 10: 1175–1183. [Google Scholar]
- Sugiura Y, Barr JR, Barr DB, et al. 1999. Physiological characteristics and mycotoxins of human clinical isolates of Fusarium species. Mycological Research 103: 1462–1468. [Google Scholar]
- Summerbell RC, Schroers HJ. 2002. Analysis of phylogenetic relationship of Cylindrocarpon lichenicola and Acremonium falciforme to the Fusarium solani species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. Journal of Clinical Microbiology 40: 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC, Scott JA. 2016. Conidiogenesis: its evolutionary aspects in the context of a philosophy of opportunity (lectics). In: Li DW. (ed), Biology of Microfungi: 169–196. Springer, Switzerland. [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. 2007. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Sutton DA, Brandt MB. 2011. Fusarium and other opportunistic hyaline fungi. In: Versalovic J, Carroll K, Funke G, et al. (eds), Manual of clinical microbiology, 10th edn: 1853–1879. ASM Press, Washington, USA. [Google Scholar]
- Takemoto K, Kamisuki S, Chia PT, et al. 2014. Bioactive dihydronaphthoquinone derivatives from Fusarium solani. Journal of Natural Products 77: 1992–6. [DOI] [PubMed] [Google Scholar]
- Temporini ED, VanEtten HD. 2002. Distribution of the pea pathogenicity (PEP) genes in the fungus Nectria haematococca mating population VI. Current Genetics 41: 107–114. [DOI] [PubMed] [Google Scholar]
- Temporini ED, VanEtten HD. 2004. An analysis of the phylogenetic distribution of the pea pathogenicity genes of Nectria haematococca MPVI supports the hypothesis of their origin by horizontal transfer and uncovers a potentially new pathogen of garden pea: Neocosmospora boniensis. Current Genetics 46: 29–36. [DOI] [PubMed] [Google Scholar]
- Tisserat N. 1987. Stem canker of black-walnut caused by Fusarium solani in Kansas. Plant Disease 71: 557. [Google Scholar]
- Toole ER. 1963. Cottonwood canker caused by Fusarium solani. Plant Reproduction 47: 1032–1036. [Google Scholar]
- Toussoun TA, Nelson PE. 1975. Variation and speciation in the fusaria. Annual Review of Phytopathology 13: 71–82. [Google Scholar]
- Trabut L. 1891. Sur une maladie cryptogamique du criquet pèlerin (Acridium peregrinum). Comptes rendus hebdomadaires des séances de l’Académie des sciences 112: 1383–1384. [Google Scholar]
- Ueno Y, Sawano M, Ishii K. 1975. Production of trichothecene mycotoxins by Fusarium species in shake culture. Journal of Applied Microbiology 30: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rensburg JCJ, Labuschagne N, Nemec S. 2001. Occurrence of Fusarium-produced naphthazarins in citrus trees and sensitivity of rootstocks to isomarticin in relation to citrus blight. Plant Pathology 50: 258–265. [Google Scholar]
- Van Warmelo KT. 1976. Scanning electron microscopy of Neocosmospora ascospores. Mycologia 68: 1181–1187. [Google Scholar]
- VanEtten HD. 1978. Identification of additional habitats of Nectria haematococca mating population VI. Phytopathology 68: 1552–1556. [Google Scholar]
- VanEtten HD, Kistler HC. 1988. Nectria haematococca, mating population I and VI. Advances in Plant Pathology 6: 189–206. [Google Scholar]
- VanEtten HD, Matthews DE, Matthews PS. 1989. Phytoalexin detoxification: importance for pathogenicity and practical implications. Annual Review of Phytopathology 27: 143–164. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Sun BL. 1994. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences of the United States of America 91: 4599–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujanovic V, Cogliastro A, St-Arnaud M, et al. 1999. First report of Fusarium solani canker and wilt symptoms on red oak (Quercus rubra) in Quebec. Canadian Plant Disease Survey 83: 78. [DOI] [PubMed] [Google Scholar]
- Weimer JL, Harter LL. 1926. Root rot of the bean in California caused by Fusarium martii phaseoli Burk. and F. aduncisporum n. sp. Journal of Agricultural Research 4: 311–319. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innes MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: a guide to methods and applications: 315–322. Academic Press, USA. [Google Scholar]
- Wiens JJ. 1998. Testing phylogenetic methods with tree congruence: phylogenetic analysis of polymorphic morphological characters in phrynosomatid lizards. Systematic Biology 47: 427–444. [Google Scholar]
- Wollenweber HW. 1913. Studies on the Fusarium problem. Phytopathology 3: 24–50. [Google Scholar]
- Wollenweber HW. 1914. Identification of species of Fusarium occurring on the sweet potato, Ipomoea batatas. Journal of Agricultural Research 2: 251–285. [Google Scholar]
- Wollenweber HW. 1916. Fusaria Autographice Delineata 1: 1–509. Berlin. [Google Scholar]
- Wollenweber HW. 1917. Fusaria Autographice Delineata. Annales Mycologici 15: 1–56. [Google Scholar]
- Wollenweber HW. 1918. Conspectus analyticus Fusariorum. Berichte der Deutschen Botanischen Gesellschaft 35: 732–742. [Google Scholar]
- Wollenweber HW. 1930. Fusaria Autographice Delineata 3: 660–1100. Berlin. [Google Scholar]
- Wollenweber HW. 1931. Fusarium – Monographie. Fungi parasitici et saprophytici. Zeitschrift für Parasitenkunde 3: 260–516. [Google Scholar]
- Wollenweber HW. 1935. Fusaria Autographice Delineata 4: 1101–1200. Berlin. [Google Scholar]
- Wollenweber HW. 1943. Fusarium – Monographie II. Fungi parasitici et saprophytici. Zentralblatt für Bakteriologie und Parasitenkunde Abteilung 2, 106: 171–202. [Google Scholar]
- Wollenweber HW, Reinking OA. 1925. Aliquot fusaria tropicalia nova vel revisa. Phytopathology 15: 155–169. [Google Scholar]
- Wollenweber HW, Reinking OA. 1935a. Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung. Parey, Berlin. [Google Scholar]
- Wollenweber HW, Reinking OA. 1935b. Die Verbreitung der Fusarien in der Natur. Friedländer & Sohn, Berlin. [Google Scholar]
- Wu Q, Sandrock TM, Turgeon BG, et al. 1998. A fungal kinesin required for organelle motility, hyphal growth, and morphogenesis. Molecular Biology of the Cell 9: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YR, Nian DL. 2014. Production optimization and molecular structure characterization of a newly isolated novel laccase from Fusarium solani MAS2, an anthracene-degrading fungus. International Biodeterioration & Biodegradation, vol. 86: 382–389. [Google Scholar]
- Yamamoto W, Maeda M, Oyasu N. 1957. Some Nectriaceae and Elsinoe species from Japan. Science Reports of the Hyogo University of Agriculture 3: 15–18. [Google Scholar]
- Zeng ZQ, Zhuang WY. 2017. Two new species of Neocosmospora from China. Phytotaxa 319: 175–183. [Google Scholar]
- Zerova M. 1937. About the ascigerous stage of Fusarium coeruleum (Lib.) Sacc. Zhurnal Instytuto botaniiky Vseukrajins’koji 11: 101–104. [Google Scholar]
- Zhang N, O’Donnell K, Sutton DA, et al. 2006. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. Journal of Clinical Microbiology 44: 2186–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoutman DE, Sigler L. 1991. Mycetoma on the foot caused by Cylindrocarpon destructans. Journal of Clinical Microbiology 29: 1855–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]


