Abstract
Background.
Pediatric Zika remains an understudied topic. The World Health Organization (WHO) and Pan American Health Organization (PAHO) Zika case definitions have not been assessed in children. We aimed to characterize clinical profiles and evaluate the diagnostic performance of the WHO and PAHO case definitions in a large cohort of pediatric Zika cases.
Methods.
We prospectively followed a cohort of ~3,700 healthy children aged 2–14 years old in Managua, Nicaragua, from January 2016 to February 2017, encompassing the major 2016 Zika epidemic. We characterized acute clinical findings (signs, symptoms, and complete blood counts) and tested participants with a broad range of clinical profiles suspected of Zika using molecular and serological assays.
Findings.
We analyzed 556 laboratory-confirmed Zika and 548 non-Zika cases. The WHO and PAHO case definitions captured 176 and 109 confirmed Zika cases, respectively, who presented with the most clinical findings and a dengue-like clinical profile. The remaining sixty percent of Zika cases, principally characterized by undifferentiated fever or afebrile rash, were missed. Among Zika cases, rash (n=440) – particularly generalized erythematous rash (n=334), fever (n=333), leukopenia (n=217), and headache (n=203) were most common and peaked within three days of illness onset. The most common Zika presentation over the first week of illness was rash only (n=80). The sensitivity of Zika case definitions increased across pediatric age (from 11% to 56% for the WHO case definition and from 6% to 37% for the PAHO case definition), as the prevalence of most clinical findings (particularly arthralgia) increased with age, irrespective of prior dengue virus infection.
Interpretation.
We provide the most thorough description of pediatric Zika to date. Most pediatric Zika cases go undetected under the WHO and PAHO case definitions, suggesting current standards for Zika case ascertainment require revision. Zika manifests with mild but differing clinical profiles across pediatric age, presenting major challenges to diagnosis, surveillance, and efforts to control future Zika epidemics.
Keywords: Zika, symptoms, age, case definition, sensitivity
INTRODUCTION
Zika virus (ZIKV), a member of the Flavivirus genus,1 was believed to cause a self-limiting febrile illness without severe complications for decades after its discovery in 1947.2 However, Zika became a global public health concern when, during explosive epidemics throughout the Pacific Islands and the Americas from 2013 to 2016,1 ZIKV infection was responsible for severe congenital complications such as microcephaly and neurological disabilities.3 Over 828,000 Zika cases have been reported to the Pan American Health Organization (PAHO), the Regional Office for the Americas of the World Health Organization (WHO), since the Zika pandemic started in 2015.4 ZIKV is usually transmitted by Aedes mosquitoes, although other transmission routes exist.1
ZIKV infection is primarily concerning in pregnant women and their fetuses, as Zika in adulthood, except for rare neurological complications like Guillain-Barré Syndrome, presents with mild fever, maculopapular rash, conjunctivitis, and arthralgia,1 generally resolving within a week. The clinical profile of Zika patients is thought to resemble mild cases of dengue, which is caused by the closely related dengue flavivirus (DENV).1 Immunological cross-reactivity between ZIKV and DENV results from the high degree of nucleotide and antigenic homology.1 Heterologous, secondary DENV infections can induce a life-threatening condition due to immunological cross-reactivity to different DENV serotypes,5 a phenomenon termed antibody-dependent enhancement. It is unknown whether prior DENV infection modulates the spectrum of Zika severity.
Most of what is known about Zika derives from adult studies. Clinical studies of pediatric Zika are relatively scarce and feature modest sample sizes,6–10 precluding a full understanding of the disease in children. Using the largest clinical dataset of laboratory-confirmed pediatric Zika cases to date,11 this study characterizes the acute clinical profile of Zika and assesses the diagnostic performance of the WHO12 and PAHO13 case definitions in children.
METHODS
Ethics statement
Institutional review boards of the University of California, Berkeley, the University of Michigan, and the Nicaraguan Ministry of Health approved the Pediatric Dengue Cohort Study (PDCS) protocol. Participants’ parents or legal guardians provided written informed consent. Subjects ≥6 years old provided verbal assent.
Study design and eligibility criteria
The PDCS is an ongoing prospective cohort study of 2–14-year-olds based in Managua, Nicaragua, originally established to study DENV infections and later expanded to include ZIKV; its full study protocol has been described (appendix).14,15 PDCS participants, healthy at enrollment, were recruited before this study’s initiation (appendix). Approximately 3,700 PDCS participants were followed from January 2016 to February 2017, spanning the initiation and cessation of Managua’s first Zika epidemic.11,16 Participants were encouraged to visit the study health center at first indication of any illness. During the study period, 1,100 PDCS cases exhibited any of four broad clinical profiles suspected of Zika and were thus eligible for inclusion; there were no exclusion criteria. These clinical profiles were: 1) fever and at least two of the following: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, and leukopenia (1997 WHO dengue case definition17); 2) fever and at least two of the following: (nausea or vomiting), rash, (aches and pains), positive tourniquet test, leukopenia, and any dengue warning sign (2009 WHO dengue case definition18); 3) undifferentiated fever without evident cause, with or without any other clinical finding; and 4) afebrile rash with or without any other clinical finding. For analysis purposes, Zika cases meeting the 1997 and/or 2009 WHO dengue case definitions were collapsed into a clinical profile, termed WHO dengue case definition. Acute-phase serum and/or urine samples of eligible participants were tested for ZIKV infection by real-time RT-PCR (rRT-PCR). Paired acute-phase and convalescent-phase serum samples were tested using an algorithm based on five serological assays (appendix).11 Based on such testing, cases were categorized as either Zika cases or non-Zika cases. The latter group contained all non-Zika illnesses that met one of the clinical profiles during the study period. We collected and analyzed clinical findings (a collective term for signs, symptoms, and complete blood count [CBC] findings) through the initial seven days of illness (appendix). Conjunctival involvement collectively refers to conjunctivitis and/or conjunctival injection. Rash without qualifications refers to any type of rash (appendix).
Study participants
Of the 1,100 PDCS cases, 560 were laboratory confirmed by either rRT-PCR or serology as Zika cases and the remaining 550 as non-Zika cases. Four Zika cases (three co-infections with DENV, one missing CBC data) and two non-Zika cases (missing CBC data) were dropped from the analysis.
Statistical analyses
The WHO case definition for Zika is: (rash and/or fever) and (at least one of: arthralgia, arthritis, conjunctivitis).12 The PAHO case definition for Zika is: (rash) and (at least two of: fever, conjunctivitis, arthralgia, myalgia, peri-articular edema).13 Using the Zika and non-Zika cases, we assessed the sensitivity, specificity, and predictive values of these case definitions (appendix). Sensitivity was modeled as a function of age using generalized additive models (GAMs),19 which capture nonlinear trends (appendix). We calculated the percentage of laboratory-confirmed Zika cases that would be missed by standard (WHO and PAHO) case definitions if cases had to meet both definitions to be captured, and, as a robustness check (sensitivity analysis), if cases had to meet only one definition to be captured (appendix).
The age and sex distributions of the non-Zika cases, Zika cases, and Zika cases stratified by clinical profile were examined (appendix). The prevalence of each clinical finding for Zika and non-Zika cases was calculated. Clustering of clinical findings among Zika cases was visualized through a co-occurrence dendrogram (appendix). We used GAMs to characterize the prevalence of each clinical finding across age. We created a severity score for each Zika case by summing the number of clinical findings (appendix) and modeled this severity score with zero-truncated models as functions of age, sex, number of days from illness onset to the last medical consult, and number of medical consults (appendix). The severity score and models were stratified by DENV infection history. Data were analyzed using STATA v14, SAS v9·4, and R v3·5·1.
Role of the funding source
The study funder had no role in design, recruitment, data collection, analysis, and interpretation, or writing phase of the study. The corresponding author had full data access and had final responsibility for submitting this report for publication.
RESULTS
Participant characteristics
The study contained 548 non-Zika cases (Figure S1) and 556 Zika cases; of the latter, 370 (66·5%) were rRT-PCR-confirmed and 186 (33·4%) were rRT-PCR-negative but confirmed by serology. Zika cases were older and more frequently female than non-Zika cases (Table S1, Figure S2). Both groups had similar numbers of medical consults, but non-Zika cases had a higher hospitalization rate (5·1%) than Zika cases (0·9%). No case experienced Guillain-Barré Syndrome. Fifty-nine percent of the Zika cases presented with undifferentiated fever (107/556, 19·2%) or afebrile rash (223/556, 40·1%) (Table 1). Only 226 (40·6%) Zika cases met the WHO dengue case definition.
Table 1.
Cross-tabulation of the overall study group by identification method (rRT-PCR vs. serology) and clinical profile (WHO dengue case definition vs. undifferentiated fever vs. afebrile rash).
| rRT-PCR N Row proportion (R%) Column proportion (C%) | Serology N Row proportion (R%) Column proportion (C%) | Row total | |
|---|---|---|---|
| WHO dengue case definition | 169 | 57 | 226 |
| R%: 74·8 | R%: 25·2 | ||
| C%: 45·7 | C%: 30·6 | ||
| Undifferentiated fever | 59 | 48 | 107 |
| R%: 55·1 | R%: 44·9 | ||
| C%: 15·9 | C%: 25·8 | ||
| Afebrile rash | 142 | 81 | 223 |
| R%: 63·7 | R%: 36·3 | ||
| C%: 38·4 | C%: 43·5 | ||
| Column total | 370 | 186 | 556 |
Acronyms: rRT-PCR, real-time reverse transcription polymerase chain reaction; WHO, World Health Organization
Pediatric Zika is often missed by standard case definitions
Overall, 176 (31·7%) and 109 (19·6%) laboratory-confirmed Zika cases met the WHO and PAHO Zika case definitions, respectively (Table 2, Table S2). Only 100 of Zika cases were captured by both case definitions (sensitivity=18·3%; 95% CI:15·0%, 21·4%), and 185 of Zika cases were captured by either case definition (sensitivity=33·3%; 95% CI:29·5%, 37·3%) (Figure 1A).
Table 2.
Sensitivity, specificity, and predictive values for the WHO case definition and the PAHO case definition for the 556 Zika and 548 non-Zika PDCS cases reported January 2016 – February 2017.a
| Case definition | Elements of the case definition | Sensitivity (95% CI) | Specificity (95% CI)b | Positive predictive value (95% CI)b | Negative predictive value (95% CI) b |
|---|---|---|---|---|---|
| WHO | Rash and/or fever AND At least 1 of the following: • Arthralgia • Arthritisc • Conjunctivitisd |
31·7% (27·9%, 35·6%) |
74·3% (70·4%, 77·8%) |
55·5% (50·0%, 60·9%) |
51·7% (48·2%, 55·2%) |
| PAHO | Rash AND At least 2 of the following: •Fever •Conjunctivitisd •Arthralgia •Myalgia •Peri-articular edema |
19·6 % (16·5%, 23·1%) |
98·2% (96·6%, 99·1%) |
91·6% (85·1%, 95·5%) |
54·6% (51·5%, 57·7%) |
Acronyms: CI, confidence interval; PAHO, Pan American Health Organization; PDCS, Pediatric Dengue Cohort Study; WHO, World Health Organization
An expanded version of this table, with the raw data and additional diagnostic indices, is provided in Table S2.
During the Zika outbreak that occurred during the study period, January 2016-February 2017, there were uncharacteristically few cases of dengue in the study population. Thus, the specificity and predictive values are higher than they would be if the non-Zika cases included typical levels of dengue and/or chikungunya.
Arthritis is not a recorded variable for medical assessments of our pediatric population. Instead, doctors record proximal and distal arthralgias. The estimated indices are lower for the WHO case definition than they would be if arthritis was a recorded variable.
The four indices of interest are calculated with conjunctival involvement (conjunctivitis and/or conjunctival injection). The estimated indices are slightly higher than if they were estimated using conjunctivitis alone.
Figure 1. Distribution of the severity score in Zika cases by case definition and clinical profile.
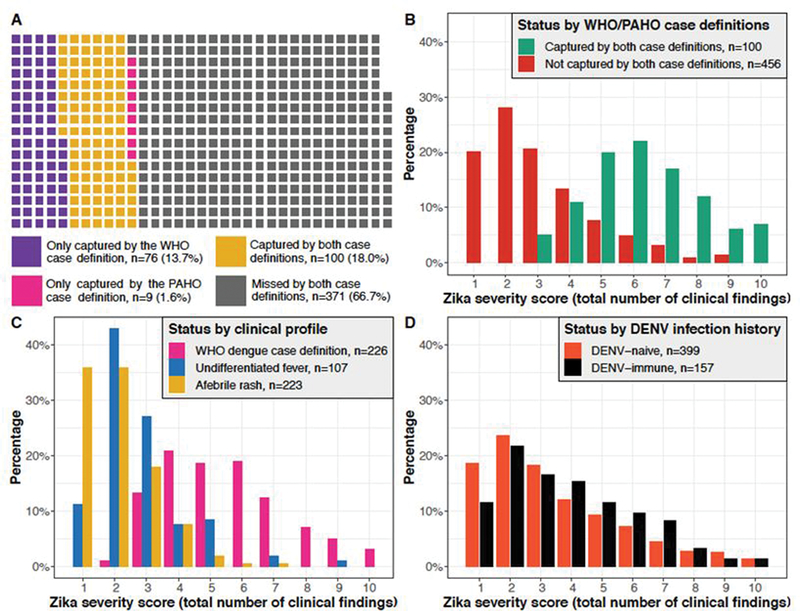
(A) Waffle chart showing the degree of overlap between Zika cases captured by the WHO and PAHO case definitions. Each square represents one Zika case in the study. (B) The distribution of the severity score for the 556 confirmed Zika cases, stratified by whether the cases were captured by both the WHO and PAHO case definitions (yellow squares in Figure 1A) or not (purple, pink, and gray squares in Figure 1A). (C) The distribution of the severity score for the 556 confirmed Zika cases, stratified by clinical profile. (D) The distribution of the severity score for the 556 confirmed Zika cases, stratified by DENV infection history. DENV, dengue virus; PAHO, Pan American Health Organization; WHO, World Health Organization.
To understand clinical differences between captured and missed cases, we compared the 100 Zika cases that were captured by both case definitions to the remaining 456 cases. Fourteen of the 22 clinical findings we assessed were significantly more prevalent in the group that met both case definitions, and no clinical findings were significantly more prevalent in the missed group. Thus, missed cases displayed milder manifestations than captured cases (Figure S3). The overall distribution of the severity score (Figure S4) demonstrated that Zika cases mostly experienced few clinical findings, with the most common severity score (128/556, 23·0%) involving only two clinical findings (usually rash and leukopenia, 47/128, 36·7%). Such children would not be captured by the WHO or PAHO case definition for Zika, as leukopenia (Figure S5) does not appear in either. Additionally, 92 (16·5%) Zika cases experienced one clinical finding (usually rash, 80/92, 80·7%), too few to meet either case definition. Only rash and only rash with leukopenia were the first and second most common pediatric Zika manifestations, respectively, when we considered combinations of the four most common clinical findings (i.e., rash, fever, headache, and leukopenia) (Figure S6). Among all 556 Zika cases, twelve (2·2%) experienced only fever.
A bimodal distribution was apparent when the overall severity score distribution was stratified by whether cases were captured by both standard case definitions (Figure 1B). Missed cases experienced a median of three clinical findings, whereas captured cases experienced a median of six clinical findings. A similar bimodal distribution was recapitulated when stratifying by clinical profile (Figure 1C). Zika cases meeting the WHO dengue case definition experienced a median of five clinical findings. Both cases with undifferentiated fever and cases with afebrile rash experienced a median of two clinical findings. Thus, the standard case definitions for Zika chiefly captured cases meeting the WHO dengue case definition (Table S3). As a robustness check (sensitivity analysis), we repeated the above analyses by comparing the 185 cases that were captured by either standard case definition and the remaining 371 cases, with equivalent conclusions (Figures S7-S8; Table S4).
Pediatric Zika manifests with mild and non-specific acute clinical findings
Among Zika cases, rash (440/556, 79·1%), fever (333/556, 59·9%), leukopenia (217/556, 39·0%), and headache (203/556, 36·5%) were the four most common clinical findings (Table S5). While both case definitions include conjunctivitis, only 15 (2·7%) Zika cases experienced conjunctivitis, and only two (0·4%) Zika cases experienced peri-articular edema, a clinical finding appearing in the PAHO case definition. Respiratory and gastrointestinal conditions were rare among the Zika cases. Zika cases predominantly presented with generalized rash (404/556, 72·7%) and erythematous rash (359/556, 64·6%) (Figure S9); hence, generalized erythematous rash was common (334/556, 60·1%). Maculopapular (105/556, 18·9%) and other rash subtypes were less common.
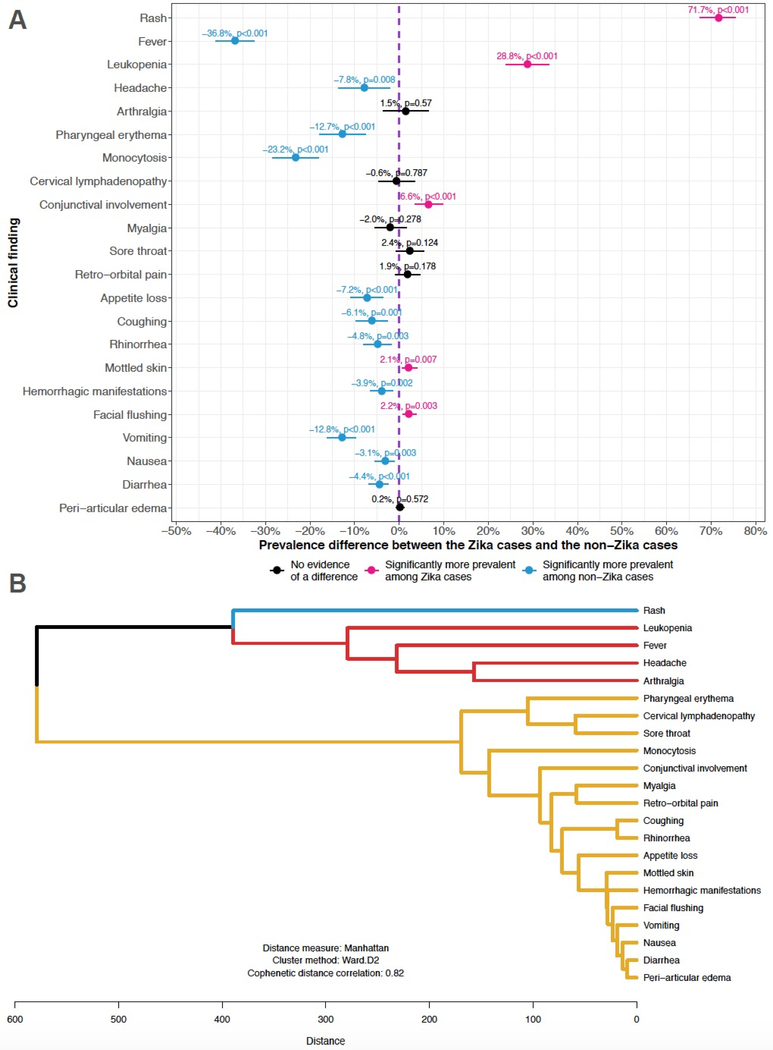
Rash and leukopenia were significantly more prevalent among Zika cases (Figure 2A). Pediatric Zika presents non-specifically, as the majority of clinical findings were more prevalent among non-Zika cases. There was no evidence of a difference in prevalence between Zika and non-Zika cases regarding arthralgia (in both case definitions) and myalgia and peri-articular edema (in the PAHO case definition).
Figure 2. Analyses of clinical findings.
(A) Prevalence differences for each clinical finding comparing the 556 Zika cases to the 548 non-Zika cases. The prevalence difference expresses the excess prevalence in the former group relative to the prevalence in the latter group. The point estimates along with associated with 95% confidence intervals and p-values are graphed on a forest plot. The dashed purple line at 0% represents the null value for prevalence differences; 95% confidence intervals including the dashed purple line correspond to non-significant prevalence differences. The prevalence differences are ordered by the prevalence of each clinical finding in the Zika group, as per Table S5. (B) Hierarchical clustering dendrogram showing co-occurrence of clinical findings among the 556 Zika cases. Clinical findings closer together along branches of the tree structure (vertical axis) are more likely to either co-occur or jointly not occur in the same person. The high cophenetic distance correlation coefficient indicates that the dendrogram is reproducing an underlying hierarchical structure found in the original Manhattan distances used to construct the dendrogram. Clusters of clinical findings are color-coded. Rash, in the first cluster (blue), was the most common clinical finding among the Zika cases (Table S5). This clustering of rash by itself indicates that many (n=80) Zika cases had rash and no other clinical finding. Leukopenia, fever, headache, and arthralgia, in the second cluster (red), were the third, second, fourth, and fifth most common clinical finding, respectively. This clustering of leukopenia, fever, headache, and arthralgia implies that whenever one of these clinical findings occurs in a pediatric Zika case, the others are very likely to co-occur in the same case. Conversely, when one of them is missing in a particular Zika case, the others are also likely to be absent in the same case. All other clinical findings are in the third cluster (yellow). PAHO, Pan American Health Organization; WHO, World Health Organization
Among the Zika cases, the average number of signs/symptoms per medical visit remained relatively constant over the first three days post-illness onset and subsequently declined, as expected (Figure S10). The prevalence of the more common clinical findings exhibited a similar pattern (Figure S11). Leukopenia was the exception; its prevalence was more stable over seven days post-illness onset.
Standard case definitions do not reflect observed patterns of pediatric Zika clinical findings
Rash formed its own co-occurrence cluster (Figure 2B) among the Zika cases. Leukopenia, fever, headache, and arthralgia formed second cluster, while all other clinical findings constituted a third cluster. These co-occurrence patterns of clinical findings were discordant with the clinical findings in the WHO and PAHO case definitions. Neither standard case definition captured Zika cases with rash only. Myalgia, conjunctival involvement, and peri-articular edema clustered together but not with the other clinical findings in the PAHO case definition.
Age drives the prevalence of clinical findings, the clinical profile, the severity score, and the sensitivity of Zika case definitions
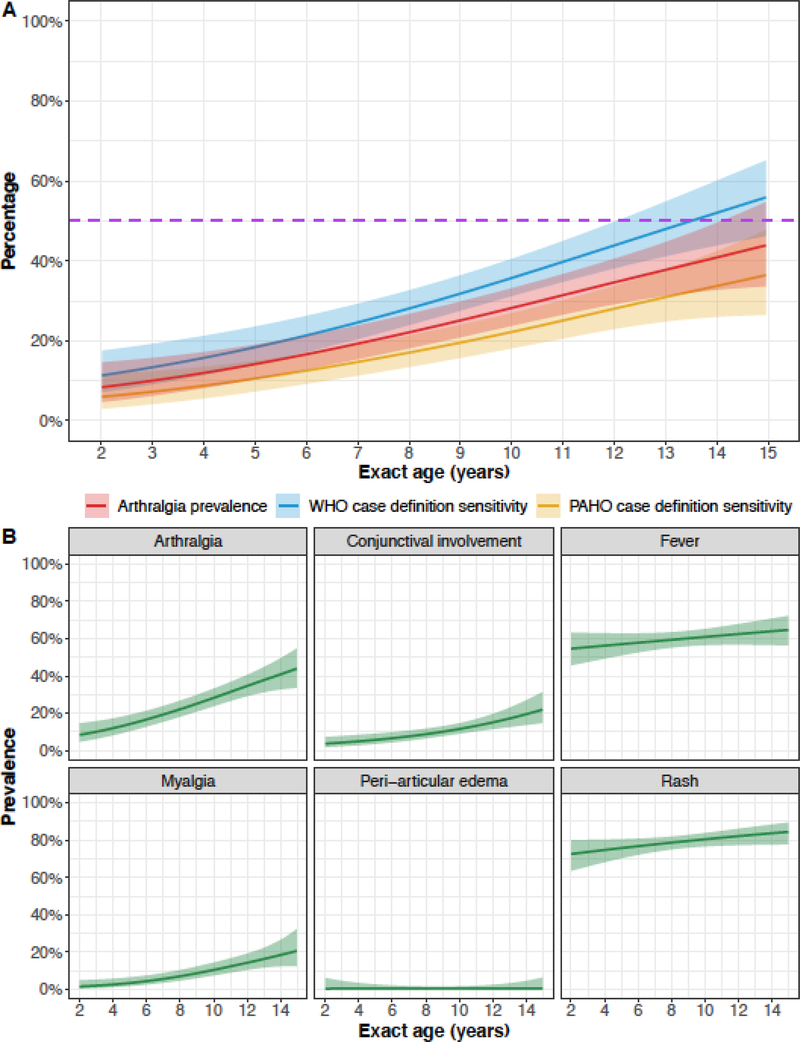
We examined the clinical profile and severity score of the laboratory-confirmed Zika cases for an explanation underlying all prior observations. Among Zika cases, there was no evidence that clinical profile varied by sex; however, Zika cases with a dengue-like clinical profile were significantly older than Zika cases with other clinical profiles (Figure S12). Similarly, there was no evidence that the severity score was associated with sex (Figure S13). However, the adjusted risk ratio per additional year of age was 1·04 (95% CI: 1·02, 1·05), indicating that the number of Zika manifestations significantly increased with age. While the expected change in severity score per year of age is small, the model implied that Zika cases that aged out of the PDCS at age 15 had, on average, 1·6 (95% CI: 1·4, 2·0) times the number of clinical findings than two-year-old Zika cases, after accounting for sex, number of medical consults, and number of days from illness onset to the last medical consult. We further observed that the sensitivity of the WHO case definition ranged from 11·3% (95% CI: 7·1%, 17·5%) at age two to 56·1% (95% CI: 46·3%, 65·4%) at age 15 (Figure 3A). Similarly, the sensitivity of the PAHO case definition ranged from 6·0% (95% CI: 3·0%, 11·6%) at age two to 36·6% (95% CI: 26·5%, 48·0%) at age 15.
Figure 3. Model predictions from logistic generalized additive models.
For all models, exact age is based on the date of birth and the date of the first medical consult for a Zika-associated illness. All models were run with the sample of Zika cases (n=556). (A) The predicted sensitivity of the WHO case definition and the PAHO case definition across pediatric age is shown, with pointwise 95% confidence bands. The dashed purple line indicates a sensitivity of 50%, the expected sensitivity if true Zika cases were randomly categorized as Zika-positive or Zika-negative. The predicted prevalence of arthralgia (from panel B) is also shown. The near parallelism exhibited by the three curves implies that the main determining factor for whether the case definitions classify a true case as Zika-positive is the presence of arthralgia. (B) The predicted prevalence for each clinical finding in the PAHO case definition across pediatric age is shown. Pointwise 95% confidence bands are provided. PAHO, Pan American Health Organization; WHO, World Health Organization.
Among Zika cases, the prevalence of all measured clinical findings in the standard case definitions also varied by age, except for peri-articular edema, which was rare (Figure 3B). Rash and fever were common across age since children with either sign were tested for ZIKV infection. However, the prevalence of both signs increased ~10–15 percentage points across pediatric age. The positive age-prevalence trend for rash reflected similar trends for generalized erythematous rashes (Figure S14); the prevalence of maculopapular rashes decreased across age. The prevalence of arthralgia sharply increased from 8·4% (95% CI: 4·7%, 14·6%) at age two to 44·0% (95% CI: 33·7%, 55·0%) at age 15, an average increase of 2·7 percentage points per year of age. The age-prevalence curve for arthralgia displayed near parallelism to the age-sensitivity curves for the WHO and PAHO case definitions (Figure 3A), implying that the presence of arthralgia principally determined whether standard case definitions correctly classified laboratory-confirmed Zika cases as Zika-positive. The prevalence of several other clinical findings also varied across age, including leukopenia, which increased in prevalence ~40 percentage points across pediatric age (Figure S15).
The proportion of Zika cases meeting the WHO dengue case definition increased ~32 percentage points over pediatric age (Figure S16A). Twenty-four of the 32 percentage points were offset by commensurate decreases in the proportion of undifferentiated fever, with the rest explained by the decreasing proportion of afebrile rash. Thus, the clinical spectrum of Zika changed with age. Stratifying the severity score by DENV infection history did not recapitulate a bimodal distribution (Figure 1D). Similarly, stratifying the models of 1) prevalence of clinical findings by DENV infection history (Figure S17) and 2) prevalence of clinical profiles by DENV infection history (Figure S16B) did not produce different results. Thus, our major findings were not explained by the positive association between existing humoral immunity to DENV and age (Figure S18).
DISCUSSION
We describe the clinical presentation of 556 Zika cases aged 2–14 years in a prospective cohort in Managua, Nicaragua, using an extensive molecular and serological laboratory workup on a wide range of clinical profiles. Zika cases experienced mild and non-specific clinical findings, but age was positively associated with additional clinical findings (including signs such as fever, generalized erythematous rash, cervical lymphadenopathy, and leukopenia) and hence the spectrum of severity, regardless of prior DENV infection. Pediatric Zika cases infrequently exhibited maculopapular rash. Our analyses indicate that the WHO and PAHO Zika case definitions anticipate a more severe Zika presentation than was typically found in pediatric cases. Accordingly, these case definitions missed most (68–80%) laboratory-confirmed cases in an age-dependent manner and largely captured older children who met the WHO dengue case definition. Most Zika cases that presented with undifferentiated fever and afebrile rash were not captured. To our knowledge, this study is the most extensive analysis of pediatric Zika to date.
A mild and self-limited illness is conventionally of little medical concern. However, the mild and nonspecific presentation of pediatric Zika presents numerous clinical and public health challenges. In our study, 40% of Zika cases presented with at most two clinical findings and were only captured because of our expansive testing scheme. As such cases do not meet standard case definitions, physicians may miss many, particularly younger, Zika cases. Consequently, persons living with ZIKV-infected children – especially women of child-bearing age – may be exposed to ZIKV without their knowledge.
Future Zika case definitions should prioritize sensitivity over specificity, as a high degree of false negatives can hinder time-sensitive outbreak control. A score-based case definition in a general population with dengue virus and chikungunya virus transmission has high levels of sensitivity and specificity.20 However, the study’s sole reliance on RT-PCR to confirm ZIKV infection suggests that its published sensitivity is overestimated because RT-PCR-negative, serology-positive cases were misclassified as non-Zika cases. The description of rash in Zika case definitions should be reexamined. We find little evidence that pediatric Zika presents with maculopapular rash, the typical type of rash reported in adults with Zika. In our pediatric study, Zika-associated rash was predominantly characterized as generalized and erythematous without macules or papules. Only a minority of pediatric Zika cases exhibited maculopapular rash. Future Zika case definitions should capture Zika cases presenting with rash only and rash and leukopenia, as 23% of our large sample exhibited only these clinical findings.
That children with Zika tended to present with mild and non-specific clinical findings complicates the physician’s task of differential diagnosis, especially in areas with co-circulating dengue and chikungunya viruses. The use of broad or data-driven20 case definitions, combined with extensive and expensive laboratory testing to overcome immunological cross-reactivity, will be necessary to capture and monitor pediatric Zika cases, especially in dengue-endemic areas. These issues will only become more urgent over time, as climate change over the next century is projected to expand the niche of Aedes mosquitoes poleward. As a result, nearly a billion people, primarily in Europe and high-elevation tropical and subtropical areas, will be newly exposed to Aedes-associated arboviruses, including ZIKV.21
The low sensitivity of the standard case definitions in children suggests that the size of Zika epidemics may have been systematically underestimated in an age-dependent manner wherever these and similar case definitions have been used. Consequently, transmission models for Zika, mostly estimated from adult Zika data,22,23 may also be systematically biased. The large pediatric population of Latin American countries implies the potential for a sizable degree of bias in the epidemiological literature.24 The proportion of asymptomatic ZIKV infections varies considerably across populations (range: 0%–100%);25 our results suggest that different case definitions and the different age structures of studied populations contribute to this heterogeneity. To resolve this, future epidemiological studies should employ an extensive ZIKV testing scheme and use consistent case definitions that capture both pediatric and adult Zika cases. Future meta-analyses of the proportion of asymptomatic ZIKV infection should examine individual-level data from these epidemiological studies for age effects.
Arthralgia was the key (though not the only) age-varying factor determining whether the standard case definitions correctly classified a laboratory-confirmed Zika case, despite arthralgia not being considered a major aspect of Zika. The observed prevalence of arthralgia reflects both its true occurrence and the ability of a child to articulate his/her pain. If the prevalence of arthralgia were fully dependent on the latter, its age-prevalence curve would have plateaued during late childhood. Regardless of the degree to which the observed prevalence of arthralgia reflects occurrence or reporting, many Zika cases did not meet standard case definitions because study physicians, quite experienced with pediatric arthralgic diseases (i.e., dengue, chikungunya, and Zika), concluded that young children did not experience much Zika-associated arthralgia. The increasing prevalence of arthralgia, rash, and fever across pediatric age has been previously noted.8,9
The WHO and PAHO case definitions are likely based on adult data, as mostly adult data from Yap Island26 and French Polynesia27 were available when the case definitions were created1 and updated.12,13 Because Zika manifests differentially across childhood, and standard case definitions include clinical findings rarely observed in our large pediatric population, symptomatic ZIKV infection may manifest differently across the lifespan. This hypothesis is supported by the age-varying prevalence of various Zika clinical findings – observed both in younger children compared to older children, as in our study and others,8,9 and in children compared to adults.9 A comparison of Zika clinical findings in pediatric and general population studies (Table S6) provides further evidence for this hypothesis. Specifically, general-population Zika studies report higher prevalence of arthralgia, myalgia, conjunctival involvement, headache, peri-articular edema, and gastrointestinal/respiratory manifestations than studies of pediatric Zika. Our hypothesis that Zika manifests differently by age should be tested empirically with a broad case ascertainment strategy, rigorous laboratory testing, and appropriate methods for continuous data. Importantly, there was no evidence that Zika manifested differently by prior DENV infection. Thus, a mild form of antibody-dependent enhancement does not, epidemiologically, explain the age trends we documented.
We used rRT-PCR and a serological algorithm based on five assays to confirm ZIKV infection. Had we relied solely on rRT-PCR as others have done6,27,28 and only included cases with a dengue-like clinical profile, as is common,6, 27,29 the sample size would have been reduced by 75%, artefactually making the sample more concordant with Zika’s typical presentation in adults. Thus, commonly used approaches to ascertain and confirm pediatric Zika cases miss many true cases and substantially mischaracterize pediatric Zika. Better diagnostic and surveillance tools are needed to understand the 2015–2016 Zika pandemic and prepare for future Zika epidemics.21
Our study has several limitations. First, the number and schedule of medical consults varied by participant, potentially leading to an incomplete record of clinical findings. However, the severity model adjusted for these factors and PDCS participants are encouraged to visit the health center at the first indication of any illness, especially during outbreaks. Second, there were few dengue and chikungunya cases (compared to historical data30,31) in the non-Zika group; standard Zika case definitions would have lower specificity with contemporaneous arboviral transmission. Third, symptoms are subjective and therefore prone to age-dependent misreporting. Despite study physicians’ extensive attempts to diagnose symptoms like arthralgia (appendix), some degree of symptom misclassification is possible.
Overall, our results demonstrate that pediatric Zika manifestations are mild, non-specific, and vary by age, explaining why pediatric Zika cases are often missed by the WHO and PAHO case definitions. As our observations concern how pediatric Zika manifests, our results are likely to be generalizable to other settings, increasing the need for more accurate Zika case definitions.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before the study
We conducted a review of the published studies documenting the signs and symptoms of Zika in children. We searched the PubMed database for articles published between January 1, 2013, and April 23, 2019, using the following search strategy: (Zika) AND (Children). No language restrictions were used in the search. We selected articles describing the clinical features of symptomatic children who were postnatally infected with Zika virus (ZIKV). Articles describing Zika in adults, including pregnant women, and in children infected with ZIKV in utero were excluded. There are only a handful of studies in the published literature describing pediatric Zika, and most are small case reports. The few modestly sized studies describing Zika in children have either selected participants predominantly using a febrile surveillance system or been based on travelers to areas with ZIKV transmission. These previous studies of children with Zika have not included a comparison group of non-Zika cases, evaluated the temporality of Zika signs and symptoms, investigated age trends with statistical methods appropriate for continuous data, or assessed the diagnostic accuracy of standard Zika case definitions.
Added value of the study
This study used a wide case ascertainment strategy to identify potential cases and employed a serology-based algorithm to supplement the traditional confirmation of cases by real-time RT-PCR (rRT-PCR). The study expands our understanding of Zika by showing that the disease primarily manifests with undifferentiated fever or afebrile rash throughout childhood, with pediatric Zika tending to manifests like traditional dengue in late childhood and early adolescence. As a result of its mild presentation in childhood, Zika in children is often missed by current global case definitions.
Implications of all available evidence
Our study and evidence from the literature support the hypothesis that age is a key determinant of the clinical manifestations of Zika, with older cases presenting more signs and symptoms, and thus presenting more often with a dengue-like clinical profile, than younger cases. Current case definitions may require revision to capture the full clinical spectrum of Zika. Clinicians may need to use molecular and serological methods to confirm ZIKV infection, especially in children, as Zika’s mild and non-specific presentation complicates differential diagnosis.
ACKNOWLEDGMENTS
We are deeply grateful to our study team at the Centro de Salud Sócrates Flores Vivas and the Laboratorio Nacional de Virología at the Centro Nacional de Diagnóstico y Referencia, Nicaraguan Ministry of Health, as well as the Sustainable Sciences Institute in Nicaragua. We would also like to thank Arthur Reingold, Jennifer Ahern, Holly Elser, and other members of UC Berkeley’s Epidemiology Doctoral Seminar course for thoughtful feedback. Finally, and most importantly, we thank the study participants and their families. This study was supported by grants R01 AI099631 (AB), P01 AI106695 (EH), and U19 AI118610 (EH) from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Funding. US National Institutes of Health
Footnotes
DECLARATION OF INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Musso D, Gubler DJ Zika Virus. Clin Microbiol Rev [Internet]. 2016. July 30 [cited 2019 Jan 5];29(3):487–524. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27029595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick GWA, Kitchen SF, Haddow AJ Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg [Internet]. 1952. September [cited 2018 Apr 3];46(5):509–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12995440 [DOI] [PubMed] [Google Scholar]
- 3.Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB da, et al. Characterizing the Pattern of Anomalies in Congenital Zika Syndrome for Pediatric Clinicians. JAMA Pediatr [Internet]. 2017. March 1 [cited 2017 Mar 20];171(3):288 Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan American Health Organization. Cumulative Cases: Cases of Zika Virus Disease [Internet]. 2019. Available from: http://www.paho.org/data/index.php/en/?option=com_content&view=article&id=524:zika-weekly-en&Itemid=352
- 5.Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, Gordon A, et al. Antibody-dependent enhancement of severe dengue disease in humans. Science (80- ) [Internet]. 2017. November 17 [cited 2019 Jan 20];358(6365):929–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29097492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Chong CY, Tan NW, Yung CF, Tee NW, Thoon KC Characteristics of Zika Virus Disease in Children: Clinical, Hematological, and Virological Findings from an Outbreak in Singapore. Clin Infect Dis [Internet]. 2017. May 15 [cited 2019 Mar 29];64(10):1445–8. Available from: https://academic.oup.com/cid/article-lookup/doi/10.1093/cid/cix137 [DOI] [PubMed] [Google Scholar]
- 7.Waggoner JJ, Gresh L, Vargas MJ, Ballesteros G, Tellez Y, Soda KJ, et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected With Zika Virus, Chikungunya Virus, and Dengue Virus. Clin Infect Dis [Internet]. 2016. December 15 [cited 2017 Mar 17];63(12):1584–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27578819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read JS, Torres-Velasquez B, Lorenzi O, Rivera Sanchez A, Torres-Torres S, Rivera L V, et al. Symptomatic Zika Virus Infection in Infants, Children, and Adolescents Living in Puerto Rico. JAMA Pediatr [Internet]. 2018. July 1 [cited 2019 Jan 5];172(7):686 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29813148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsey NP, Porse CC, Potts E, Hyun J, Sandhu K, Schiffman E, et al. Postnatally acquired Zika virus disease among children, United States, 2016–2017. Clin Infect Dis [Internet]. 2019. March 11 [cited 2019 Mar 21]; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciz195/5373555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman AB, Dziuban EJ, Powell K, Bitsko RH, Langley G, Lindsey N, et al. Characteristics of Children Aged <18 Years with Zika Virus Disease Acquired Postnatally — U.S. States, January 2015–July 2016. MMWR Morb Mortal Wkly Rep [Internet]. 2016. October 7 [cited 2019 Jan 5];65(39):1082–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27711041 [DOI] [PubMed] [Google Scholar]
- 11.Gordon A, Gresh L, Ojeda S, Katzelnick LC, Sanchez N, Mercado JC, et al. Prior dengue virus infection and risk of Zika: A pediatric cohort in Nicaragua. von Seidlein L, editor. PLOS Med [Internet]. 2019. January 22 [cited 2019 Jan 22];16(1):e1002726. Available from: http://dx.plos.org/10.1371/journal.pmed.1002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Zika virus disease | Interim case definition [Internet]. World Health Organization; 2016. [cited 2019 Feb 3]. Available from: https://www.who.int/csr/disease/zika/case-definition/en/ [Google Scholar]
- 13.PAHO/WHO. Zika Resources: Case Definitions [Internet]. 2016. [cited 2018 Oct 7]. Available from: https://www.paho.org/hq/index.php?option=com_content&view=article&id=11117:zika-resources-case-definitions&Itemid=41532&lang=en
- 14.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, et al. The Nicaraguan Pediatric Dengue Cohort Study: Study Design, Methods, Use of Information Technology, and Extension to Other Infectious Diseases. Am J Epidemiol [Internet]. 2009. July 1 [cited 2017 Mar 13];170(1):120–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19435864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balmaseda A, Standish K, Mercado JCC, Matute JCC, Tellez Y, Saborío S, et al. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis [Internet]. 2010. January 1 [cited 2017 Mar 13];201(1):5–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19929380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zambrana JV, Bustos Carrillo F, Burger-Calderon R, Collado D, Sanchez N, Ojeda S, et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc Natl Acad Sci [Internet]. 2018. September 11 [cited 2019 Jan 19];115(37):9294–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30150394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Dengue haemorrhagic fever: Diagnosis, treatment, prevention, and control, 2nd ed Geneva; 1997. [Google Scholar]
- 18.World Health Organization. Dengue : Guidelines for Diagnosis Treatment Prevention and Control (New Edition 2009). World Health Organization; 2009. 158 p. [PubMed] [Google Scholar]
- 19.Hastie T, Tibshirani R. Generalized Additive Models. Stat Sci. 1986;1(3):297–318. [DOI] [PubMed] [Google Scholar]
- 20.Braga JU, Bressan C, Dalvi APR, Calvet GA, Daumas RP, Rodrigues N, et al. Accuracy of Zika virus disease case definition during simultaneous Dengue and Chikungunya epidemics. Ng LFP, editor. PLoS One [Internet]. 2017. June 26 [cited 2019 Aug 6];12(6):e0179725. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28650987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. Han BA, editor. PLoS Negl Trop Dis [Internet]. 2019. March 28 [cited 2019 Mar 31];13(3):e0007213. Available from: http://dx.plos.org/10.1371/journal.pntd.0007213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao D, Lou Y, He D, Porco TC, Kuang Y, Chowell G, et al. Prevention and Control of Zika as a Mosquito-Borne and Sexually Transmitted Disease: A Mathematical Modeling Analysis. Sci Rep [Internet]. 2016. September 17 [cited 2019 Feb 17];6(1):28070. Available from: http://www.nature.com/articles/srep28070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiura H, Kinoshita R, Mizumoto K, Yasuda Y, Nah K. Transmission potential of Zika virus infection in the South Pacific. Int J Infect Dis [Internet]. 2016. April 1 [cited 2019 Feb 17];45:95–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26923081 [DOI] [PubMed] [Google Scholar]
- 24.US Census Bureau. International Data Base [Internet]. 2018. Available from: https://www.census.gov/data-tools/demo/idb/region.php?N=Results&T=12&A=separate&RT=0&Y=2019&R=143&C=
- 25.Haby MM, Pinart M, Elias V, Reveiz L. Prevalence of asymptomatic Zika virus infection: a systematic review. Bull World Health Organ [Internet]. 2018. June 1 [cited 2018 Nov 3];96(6):402–413D. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29904223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N Engl J Med [Internet]. 2009. June 11 [cited 2017 Dec 14];360(24):2536–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19516034 [DOI] [PubMed] [Google Scholar]
- 27.Mallet H-P, Vial A-L, Musso D. Bilan de l’épidémie a virus ZIKA en Polynésie Français, 2013–2014 (in French). Bull d’information Sanit Epidemiol Stat. 2015;May(13):1–5. [Google Scholar]
- 28.Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika Virus Outbreak in Rio de Janeiro, Brazil: Clinical Characterization, Epidemiological and Virological Aspects. Powers AM, editor. PLoS Negl Trop Dis [Internet]. 2016. April 12 [cited 2019 Feb 1];10(4):e0004636. Available from: https://dx.plos.org/10.1371/journal.pntd.0004636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez Corona ME, De la Garza Barroso AL, Rodriguez Martínez JC, Luna Guzmán NI, Ruiz Matus C, Díaz Quiñonez JA, et al. Clinical and Epidemiological Characterization of Laboratory-Confirmed Authoctonous Cases of Zika Virus Disease in Mexico. PLoS Curr [Internet]. 2016. April 15 [cited 2019 Feb 3];8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27158557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzelnick LC, Montoya M, Gresh L, Balmaseda A, Harris E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc Natl Acad Sci [Internet]. 2016. January 19 [cited 2019 Mar 29];113(3):728–33. Available from: https://www.pnas.org/content/113/3/728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon A, Gresh L, Ojeda S, Chowell-Puente G, Gonzalez K, Sanchez N, et al. Differences in Transmission and Disease Severity between Two Successive Waves of Chikungunya. Clin Infect Dis [Internet]. 2018. April 25 [cited 2018 May 11]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29697796 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.