Abstract
A key component of seed germination is the interplay of mechanical forces governing embryo growth and the surrounding restraining endosperm tissue. Endosperm cell separation is therefore thought to play a critical role in the control of this developmental transition. Here we demonstrate that in Arabidopsis thaliana seeds, endosperm cell expansion is a key component of germination. Endosperm cells expand to accommodate embryo growth prior to germination. We show that this is an actively regulated process supported by spatiotemporal control of the cell expansion gene EXPANSIN 2 (EXPA2). The NAC transcription factors NAC25 and NAC1L were identified as upstream regulators of EXPA2 expression, gibberellin-mediated endosperm expansion, and seed germination. The DELLA protein RGL2 repressed activation of the EXPA2 promoter by NAC25/NAC1L. Taken together, our findings uncover a key role of the GA/DELLA-NAC25/NAC1L-EXPA2 network in regulating endosperm cell expansion to control the seed-to-seedling transition.
Key words: cell expansion, cell-wall remodeling enzymes, endosperm, expansin, NAC transcription factors, seed germination
This study demonstrates that endosperm cell expansion is crucial for germination and that two NAC transcription factors play pivotal roles in this process. NAC25 and NAC1L act downstream of GAs and the RGL2 DELLA protein to upregulate cell-wall remodeling enzyme gene expression.
Introduction
In multicellular organisms, the regulation of growth requires precise coordination between cell division and cell expansion. In the case of plants, the ability to grow is largely influenced by the presence of a cell wall and the absence of cell movement (Coen et al., 2004). Therefore, plants require mechanisms to modify the physical properties of their cell walls according to tissue-specific growth rates and developmental stages as well as in response to environmental cues.
Seed germination in Arabidopsis thaliana (hereafter Arabidopsis) is a useful system to study growth regulatory mechanisms since the developmental transition from seed to seedling is driven exclusively by changes in cell-shape in the absence of cell division (Holdsworth et al., 2008, Sliwinska et al., 2009). The ability of seeds to germinate is thought to result from a balance between physical restrictions imposed by the embryo-surrounding tissues (testa and endosperm) and the ability of the embryo to grow and protrude (Finch-Savage and Leubner-Metzger, 2006, Holdsworth et al., 2008). The impact of the endosperm on plant survival and seed vigor has been well documented (Bassel, 2016, Finch-Savage and Bassel, 2016), and it was shown that the endosperm has the capacity to communicate bidirectionally with the embryo (Groot and Karssen, 1987, Bassel et al., 2006, Lee et al., 2010) and influence initial embryo growth (Lee et al., 2012b) or even inhibit germination altogether (Bethke et al., 2007, Lee et al., 2010). The decline in the mechanical resistance of the micropylar endosperm (the endosperm covering the radicle tip) leading to endosperm cell separation appears to be a general prerequisite for radicle (root tip) protrusion (germination sensu stricto) (Yan et al., 2014b, Steinbrecher and Leubner-Metzger, 2016).
In a number of plant species (including Arabidopsis) endosperm cell separation precedes germination, a process stimulated by gibberellins (GAs), and correlates with the GA-regulated coordinated expression of cell-wall remodeling enzymes (CWREs) (Groot and Karssen, 1987, Chen and Bradford, 2000, Dubreucq et al., 2000, Chen et al., 2002, Müller et al., 2006, Penfield et al., 2006, Morris et al., 2011, Endo et al., 2012, Lee et al., 2012a, Martínez-Andújar et al., 2012, Yan et al., 2014a, Scheler et al., 2015, Sechet et al., 2016). However, the molecular mechanisms linking GA action with CWRE expression and the effect of CWREs on endosperm functionality and germination remain poorly understood.
To better understand the relationship between embryo growth and endosperm functionality, we investigated the biophysical and molecular events that take place within cells of the endosperm during Arabidopsis seed imbibition prior to radicle protrusion. This analysis revealed the necessity of endosperm expansion for the completion of germination. Functional and genetic consequences underpinning these events were investigated, revealing a GA-mediated network of transcription factor (TF)-regulated CWREs.
Results
Endosperm Cells Expand during Seed Germination
We examined endosperm cell expansion dynamics during seed germination prior to radicle protrusion using confocal microscopy and quantitative imaging analysis. Specifically, changes in endosperm cell geometry before (6 h after imbibition [hai]) and after (27 hai) testa rupture were analyzed. Confocal z stacks of seeds with the testa removed were taken to study the underlying endosperm (Figure 1A). Using 2.5 D image analysis, the size of the endosperm cells was determined by cell segmentation (Figure 1B) and area analysis (Figure 1C) in MorphoGraphX (Barbier de Reuille et al., 2015). The endosperm was divided into four regions corresponding to the different anatomical parts of the underlying embryo it encapsulates (Figure 1D): radicle tip (micropylar endosperm [ME]; region 1); radicle and lower hypocotyl (adjacent ME; region 2); hypocotyl (peripheral endosperm [PE]; region 3); and cotyledons (cotyledon PE; region 4). Statistical analysis of the changes in cell surface area in these different regions before and after testa rupture identified significant differences in cell size for each region (Figure 1E). Endosperm cell growth was greatest in regions overlying the radicle and lower hypocotyl, which is consistent with these parts of the embryo being previously identified as the sites of cell expansion (Bassel et al., 2014).
Figure 1.
Endosperm Cell Expansion Is a Key Component of Seed Germination.
(A) Confocal image of a seed with the testa removed.
(B) Endosperm cell segmentation.
(C) Segmented cells colored based on surface area (μm2) as indicated by the scale bar.
(D) Cells clustered into four regions. Region 1, marked in green color, corresponds to the micropylar endosperm; region 2, colored in blue, is adjacent to the micropylar endosperm; region 3, tinted in yellow, is the peripheral endosperm; and region 4, in pink, is the area surrounding the cotyledons.
(E) Changes in endosperm cell surface area (average percentage + SE) per region. Cell surface area per region in samples at 27 hai was compared with that calculated in samples at 6 h after inhibition (hai). Five Arabidopsis Col-0 seeds for each time point were analyzed. Asterisks represent significant differences at **P < 0.01 and at *P < 0.05 using the Mann–Whitney test (two-sided).
To visualize changes in endosperm cells during Arabidopsis seed germination, we prepared microscopic sections of seeds before and after testa rupture. Concomitant formation of central vacuoles in the embryo and endosperm cells was seen at the time of testa rupture (Supplemental Figure 1A and 1B), reflecting reserve mobilization and resulting in the formation of protein storage vacuoles (Penfield et al., 2004, Bethke et al., 2007). When using a transgenic line with GFP targeted to the tonoplast (Cutler et al., 2000) we observed a high density of vacuoles at the early stages of germination representing the multiple protein storage vacuoles present at this stage (Supplemental Figure 1C). Progressive formation of a large central vacuole was observed during the later stages of germination, coincident with endosperm cell expansion (Supplemental Figure 1D). These results suggest that the observed subcellular events in the endosperm reflect those occurring in the embryo (Sliwinska et al., 2009) and are linked to endosperm cell expansion.
EXPA2 Is an Endosperm-Specific CWRE Marker of Cell Expansion
Previous work has shown that the expression of many CWREs, promoted by GAs, correlates with endosperm cell separation (Morris et al., 2011, Endo et al., 2012, Dekkers et al., 2013, Steinbrecher and Leubner-Metzger, 2016). Wherever cells are growing or modifying their cell walls, one or more expansin genes are usually involved (Cosgrove, 2015). EXPANSIN 2 (EXPA2, At5g05290) encodes an endosperm-specific α-expansin with a proven genetic role in enhancing germination that is induced by GAs, is not repressed by abscisic acid (ABA), and is under the control of DELLAs (Ogawa et al., 2003, Cao et al., 2006, Penfield et al., 2006, Morris et al., 2011, Endo et al., 2012). For these reasons, we decided to study the temporal and spatial expression kinetics of EXPA2 in the context of endosperm expansion prior to germination.
We generated transgenic seeds containing a 2-kb EXPA2 promoter (upstream of the ATG) fused to the β-glucuronidase gene (GUS) in the Columbia-0 (Col-0) background (ProEXPA2:GUS). Endosperms were separated from embryos at different times after seed imbibition (6, 12, 18, and 24 hai) and assayed for GUS activity to assess the temporal and spatial expression pattern of ProEXPA2 in the endosperm (no GUS staining was observed in the embryo as this gene is endosperm specific; Supplemental Figure 2). Initially, we analyzed expression driven by this promoter in the endosperm of intact seeds. As shown in Figure 2A, GUS expression was observed by 18 hai in the endosperm region overlying the radicle and lower hypocotyl, and subsequently extended along the region overlying the hypocotyl. This spatiotemporal activity of the EXPA2 promoter overlaps the regions of endosperm expansion prior to germination (Figure 1E). Histochemical staining of the ProEXPA2:GUS seeds in the presence or absence of ABA, paclobutrazol (PAC), and PAC + GAs confirmed that EXPA2 promoter activity in the endosperm depends on GAs and cannot be repressed by ABA (Figure 2B).
Figure 2.
EXPA2 Spatiotemporal Expression Pattern Is Tightly Associated with Endosperm Expansion and Depends on an Embryo Signal(s).
(A)ProEXPA2:GUS seeds were imbibed on half-strength Murashige and Skoog (½ MS) medium for the indicated times, and stained overnight to detect GUS activity after seed coats were removed. GUS staining was visualized after bleaching the testa.
(B)ProEXPA2:GUS seeds were imbibed on ½ MS medium in the absence or presence of ABA (10 μM), PAC (20 μM), or PAC (20 μM) + GA (10 μM) for 24 h, and stained overnight after seed coats were removed.
(C) (C) ProEXPA2:GUS seeds were imbibed on ½ MS medium and, at the indicated times (min), seed coats were separated from embryos, incubated on ½ MS for a total of 24 h and stained overnight.
The endosperm perceives environmental signals that affect germination control (Lee et al., 2010). Communication with the embryo is bidirectional and endosperm cell separation requires a signal from the embryo (Groot and Karssen, 1987, Morris et al., 2011, Bassel, 2016). To determine whether EXPA2 promoter activity depends on an embryo signal and its timing, we separated endosperms from embryos at different times during early seed imbibition (45, 60, 75, and 90 min after imbibition) and subsequently incubated them separately from the embryo for a total of 24 h to allow completion of downstream gene expression programs. This analysis shows that GUS expression, driven by the EXPA2 promoter, relies on an inductive signal that is initiated between 45 and 60 min after imbibition of the seed (Figure 2C).
The Promoter of EXPA2 Contains Conserved Sequences Required for Binding and Transactivation by Two NAC Transcription Factors
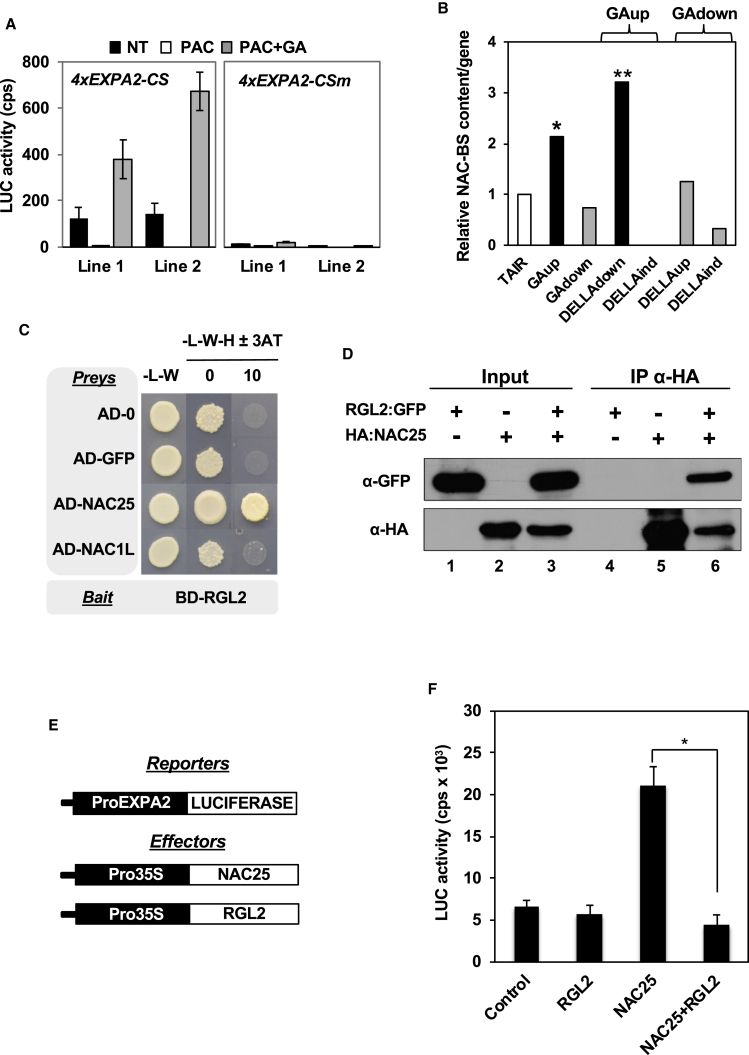
The above results prompted us to identify cis elements controlling EXPA2 expression and upstream transcriptional regulators. We used a phylogenetic shadowing approach (Castrillo et al., 2011) with orthologous EXPA2 gene promoters to define conserved cis elements. An 80-bp sequence was identified (EXPA2-CS) with almost 50% overall sequence identity among the eight species analyzed (Supplemental Figure 3). Transgenic seeds carrying a luciferase reporter gene under the control of four tandem copies of the EXPA2-CS and a minimal promoter (4xEXPA2-CS), or a minimal promoter alone (control), were used to quantify luciferase expression in vivo (Figure 3A). The 4xEXPA2-CS construct was able to increase luciferase expression 6-fold over the control construct at 24 hai, suggesting that the EXPA2-CS is bound by TFs that activate gene expression in seeds (Figure 3B).
Figure 3.
Two NAC TFs Bind to a Conserved Promoter Sequence to Activate EXPA2 Transcription.
(A) Construct used for functional analysis of the EXPA2 conserved sequence (EXPA2-CS; black box) and the control construct containing only the minimal promoter (Min; gray box).
(B) Seeds from two representative transgenic lines for each construct (Min or 4xEXPA2-CS) were imbibed for 24 h. Average luciferase levels and SE for at least 10 seeds for each line are shown.
(C) Sequence of the wild-type EXPA2-CS and a version with mutations in the putative NAC binding sites (EXPA2-CSm). Mutated bases are indicated in lowercase.
(D) Yeast strains containing one copy of the EXPA2-CS or EXPA2-CSm fused to the HIS3 reporter gene were mated to strains containing the AD-∅, AD-GFP, AD-NAC1L, or AD-NAC25 constructs. Diploid cells were grown on diploid (-L-W) and screening (-L-W-H) plates with increasing concentrations of 3-AT.
(E) Diagram of the EXPA2 promoter (reporters) and TF (effectors) constructs used for transient expression analyses in N. benthamiana leaves. N. benthamiana leaves were agroinfiltrated with the ProEXPA2 or the ProEXPA2m construct together with an effector construct overexpressing NAC25 or NAC1L.
(F) Control is the empty effector plasmid (pCX) co-expressed with each of the reporters. Average luciferase activities and SE from at least six replicates are shown, and similar results were obtained in an additional agroinfiltration experiment. Asterisks represent significant differences at **P < 0.01 and *P < 0.05 using the Mann–Whitney test (two-sided).
To identify TFs able to regulate EXPA2 expression through binding to the EXPA2-CS, we used the EXPA2-CS sequence as bait in a yeast one-hybrid screen of a library of Arabidopsis TFs (Castrillo et al., 2011, Sánchez-Montesino and Oñate-Sánchez, 2017, Sánchez-Montesino and Oñate-Sánchez, 2018). Only two yeast strains contained inserts able to activate the reporter gene (HIS3) under the control of the EXPA2-CS and grew on the screening medium (Supplemental Figure 4A). Isolation and sequencing of the corresponding GAL4AD-TF plasmids showed that they encoded two NAC TFs, NAC25 (At1g61110) and NAC1-like (NAC1L; At3g12977).
Two putative NAC binding sequences (NAC-BSs; 5′-ACG GAA TT-3') were identified in the EXPA2-CS, and a mutated version containing 2-bp changes in the core sequence (Puranik et al., 2012, Franco-Zorrilla et al., 2014) of each NAC-BS was generated (EXPA2-CSm; Figure 3C). Diploid cells containing the EXPA2-CS bait and AD-NAC25 or AD-NAC1L were able to grow in the presence of 3-amino-1,2,4-triazole (3-AT; a competitive inhibitor of the product of the HIS3 gene) at concentrations blocking the growth of cells harboring control plasmids (AD-∅ and AD-GFP; Figure 3D). No differences in the growth of control yeast cells were observed when the EXPA2-CSm or a GA-responsive sequence present in the promoters of genes expressed in the epidermis of the embryo (LIP1-CS; Rombolá-Caldentey et al., 2014) were used as bait (Figure 3D and Supplemental Figure 4B). In conclusion, our results indicate that both NAC TFs bind the EXPA2-CS in yeast and that integrity of the core NAC-BSs is required for binding.
To determine the relevance of the NAC-BSs for NAC transactivation of the EXPA2 promoter, we carried out transient expression analyses in planta. As a reporter, we fused the same 2-kb EXPA2 promoter included in the ProEXPA2:GUS reporter construct (see Figure 2) to the luciferase reporter gene (ProEXPA2). We also generated a version of the 2-kb EXPA2 promoter containing the same NAC-BS mutations shown in Figure 3C (ProEXPA2m). As effectors, we cloned the two NAC open reading frames (ORFs) under the control of the 35S promoter (Figure 3E). Nicotiana benthamiana leaves were then infiltrated with different combinations of Agrobacterium tumefaciens cultures carrying the reporters and effectors. Both NAC TFs were able to increase luciferase expression driven by ProEXPA2 but not that driven by the mutated version (ProEXPA2m), indicating that intact NAC-BSs are required for full transactivation mediated by the NAC TFs in planta (Figure 3F).
The NAC-BSs Mediate GA Responses and RGL2 Represses NAC-Mediated Transactivation
Endosperm cell separation and EXPA2 upregulation are both GA-mediated processes (Ogawa et al., 2003, Penfield et al., 2006, Holdsworth et al., 2008, Yan et al., 2014a, Yan et al., 2014b). To analyze the role of the NAC-BSs in the GA-mediated induction of EXPA2, we generated transgenic seeds containing either EXPA2-CS or EXPA2-CSm fused to luciferase. High levels of luciferase activity were detected in seeds carrying the EXPA2-CS reporter construct at 24 hai under control conditions, but levels were reduced by almost 8-fold in the presence of PAC, an inhibitor of GA biosynthesis (Figure 4A). In addition, luciferase levels in seeds imbibed with PAC but supplemented with GA were similar to those in the control. Seeds carrying the EXPA2-CSm reporter construct showed much lower basal luciferase activity and did not exhibit responsiveness to GA. These results indicate that transcription driven by the EXPA2-CS is modulated in response to GA and mediated by the NAC-BSs.
Figure 4.
The NAC-BSs Mediate GA Responses and RGL2 Represses NAC25 Transactivation via Physical Interaction.
(A) Seeds from transgenic lines with a 4xEXPA2-CS or a 4xEXPA2-CSm promoter fused to luciferase were imbibed for 48 h in the absence (NT) or presence of PAC (5 μM) or PAC (50 μM) + GA (5 μM). Average luciferase activities and SE from at least 10 seeds for each line are shown.
(B) NAC-BS content in the 500-bp promoters of genes upregulated (GAup) or downregulated (GAdown) in response to GA or upregulated (DELLAup) or downregulated (DELLAdown) in DELLA mutants as described by Cao et al. (2006). DELLAind, DELLA independent. The average NAC-BS occurrence in the Arabidopsis gene promoter dataset, Araport11 Loci Upstream Seq-500 bp, is taken arbitrarily as 1. Asterisks represent significant differences at **P < 0.01 or *P < 0.05, from the control (TAIR) using the Chi-squared (χ2)÷ test.
(C) Yeast strains containing the RGL2 CDS fused to the GAL4-BD (BD-RGL2; bait) were mated to strains containing the AD-Ø, AD-GFP, AD-NAC1L, or AD-NAC25 constructs. Diploid cells were grown on diploid (-L-W) and screening (-L-W-H) plates with or without 3-AT.
(D) Co-immunoprecipitation (Co-IP) assays with co-expressed HA-NAC25 and RGL2-GFP in leaves of N. benthamiana. Soluble protein extracts before (input) and after (IP α-HA) immunoprecipitation with an anti-HA antibody were subjected to immunoblotting with an anti-GFPHRP antibody or an anti-HAHRP antibody.
(E) Diagram of the EXPA2 promoter (reporter) and TF constructs (effectors) used for transient expression analyses in N. benthamiana leaves. N. benthamiana leaves were agroinfiltrated with the ProEXPA2 construct together with one or two effector constructs overexpressing NAC25 or RGL2.
(F) Luciferase activity in N. benthamiana leaves agroinfiltrated with the ProEXPA2:LUC construct and effector constructs overexpressing the indicated TFs. For infiltration of the control and single TF effector constructs, appropriate amounts of the empty effector plasmid (pCX) were co-infiltrated to equalize bacterial numbers between leaves. Average luciferase activities and SE from at least four replicates are shown, and similar results were obtained in an additional agroinfiltration experiment. Asterisks represent significant differences at *P < 0.05 using the Mann–Whitney test (two-sided).
To determine whether the NAC-BSs play a broader role in GA-regulated gene expression, we looked for enrichment of the NAC-BS (5′-ACG GAA TT-3') in the promoters of genes represented in the transcriptomes of germinating wild-type seeds compared with the GA-deficient ga1-3 mutant (Koornneef and van der Veen, 1980, Sun and Kamiya, 1994) and the ga1-3 gai-t6 rga-t2 rgl1-1 rgl2-1 mutant lacking four DELLA genes (Cao et al., 2006). The average occurrence of the NAC-BSs sequence was found to be significantly higher in GA-upregulated (GAup) and DELLA-downregulated (DELLAdown) gene promoters when compared with the control (Figure 4B). When the occurrence of a monocot GA-responsive cis element (Skriver et al., 1991, Gubler and Jacobsen, 1992) was analyzed in these gene promoters, no significant enrichment was found (Ogawa et al., 2003, Rombolá-Caldentey et al., 2014).
We analyzed the molecular mechanism mediating GA responsiveness of the EXPA2 promoter. Our results clearly indicate a role of the NAC-BSs in GA responses (Figure 4A and 4B). One possibility could be that DELLA proteins block NAC-BS-mediated gene expression by sequestering NACs through physical interaction. To test this possibility, we fused the coding sequence of the RGL2 protein, the predominant DELLA repressor of seed germination in Arabidopsis (Lee et al., 2002, Tyler et al., 2004, Cao et al., 2005), to the GAL4 DNA binding domain and used it as bait in yeast two-hybrid experiments. Yeast cells carrying BD-RGL2 and the AD-NAC25 construct, but not those harboring the control plasmids or the AD-NAC1L construct, were able to grow on the selection medium in the presence of 3-AT (Figure 4C), indicating that NAC25 is able to interact with the RGL2 protein. To validate this protein interaction in planta, we carried out co-immunoprecipitation (Co-IP) experiments using hemagglutinin (HA) and GFP translational fusions of NAC25 and RGL2, respectively. As shown in Figure 4D, the HA-NAC25 protein was detected by the anti-HAHRP antibody only in protein extracts from leaves that had been agroinfiltrated with the HA-NAC25construct (α-HA panels, lanes 2, 3, 5, and 6), and the HA epitope fused to the NAC25 protein was efficiently immunoprecipitated by the anti-HA antibody (α-HA panels, lanes 5 and 6). Likewise, the anti-GFPHRP antibody successfully detected the RGL2-GFP fusion protein (α-GFP panel, lanes 1 and 3). Since the RGL2-GFP protein was detected after HA-NAC25 was immunoprecipitated with the anti-HA antibody (α-GFP panel, lane 6), these results demonstrate that NAC25 interacts with RGL2 in planta.
To measure the effect of this interaction on the transactivation ability of NAC25, we carried out transient expression assays. As shown in Figure 4E and 4F, RGL2 is unable to regulate EXPA2 expression on its own, but efficiently blocks NAC25-mediated transactivation. It has been demonstrated that EXPA2 and RGL2 are expressed in the seed endosperm (Penfield et al., 2006, Lee et al., 2010, Yan et al., 2014a; this work). To check for expression of NAC genes in the endosperm, we isolated RNA from Arabidopsis embryos and endosperms dissected at different times after seed imbibition and quantified NAC mRNA expression levels by qRT–PCR. Our results (Supplemental Figure 5) show that NAC25 and NAC1L are expressed in both the embryo and endosperm at early time points but mainly in the endosperm at later time points.
NAC25 and NAC1L Are Positive Regulators of Seed Germination and CWRE Gene Expression
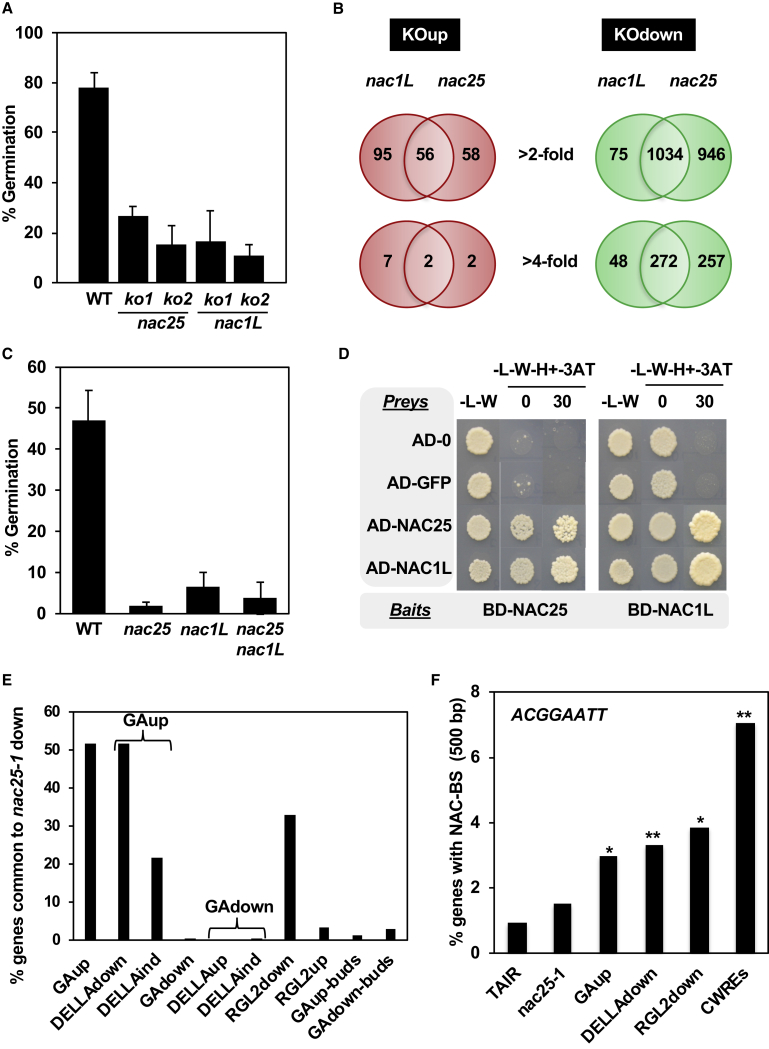
To analyze the role of these TFs in planta, we characterized the seed germination properties of two single loss-of-function mutants for the NAC25 and NAC1L genes (Supplemental Figure 6 and Supplemental Table 1). Mutation of either gene led to delayed germination when compared with the wild-type (Figure 5A), indicating that both NAC TFs are positive regulators of this process. To identify other genes regulated by the NAC TFs, we compared gene expression during seed germination in the nac25 and nac1L null mutants and wild-type controls by performing deep sequencing of RNA populations (RNA-seq). Analyses of RNA-seq results led to the identification of NAC25- and NAC1L-regulated genes (Supplemental Table 2). Using a 2-fold change in expression as a threshold for differential expression, we identified 1260 and 2094 genes as putative NAC1L- and NAC25-regulated genes, respectively (Figure 5B). In both null mutants most genes were downregulated—88% in nac1L-1 (1109 DOWN and 151 UP) and 94.5% in nac25-1 (1980 DOWN and 114 UP)—suggesting that both TFs are positive regulators of gene expression. The ratio of downregulated to upregulated genes in both transcriptomes increased when only genes differentially regulated above 4-fold were considered, further highlighting the role of both NAC proteins as activators of gene expression. Importantly, the overwhelming majority of nac1L-1 downregulated genes (93%) were commonly downregulated in nac25-1, while almost 48% of nac25-1 downregulated genes were specific to this mutant. These data suggest that NAC25 has a wider regulatory role than NAC1L and that NAC1L lies in the same regulatory pathway. In agreement with these results, a nac25-1 nac1L-1 double mutant produced a germination delay similar to that observed for each single mutant (Figure 5C). Thus, NAC25 and NAC1L may share regulatory roles by forming a protein complex through physical interaction. Our results from a yeast two-hybrid assay indicate that NAC25 and NAC1L are able to form homo- and heterodimers (Figure 5D). To validate these protein interactions in planta, we performed bimolecular fluorescence complementation (BiFC) experiments by agroinfiltration of N. benthamiana leaves. The NAC protein coding sequences (CDSs) were translationally fused to the N-terminal half of the yellow fluorescent protein (N-YFP) or the C-terminal half of the cyan fluorescent protein (C-CFP) as indicated in Supplemental Figure 7. A strong fluorescent signal was observed in nuclei of N. benthamiana cells in leaves co-infiltrated with pairs of NAC translational fusions but not with controls. These results indicate that NAC25 and NAC1L can interact in planta and support our genetic evidence indicating that they could be essential components of the same regulatory complex.
Figure 5.
NAC TFs Are Positive Regulators of Seed Germination and CWRE Gene Expression, and Mediate GA Responses.
(A) Germination Percentage of wild-type (WT; Col-0) and NAC knockout (ko) seeds at 48 hai. Results represent average values and SE for four biological replicates.
(B) Overlap in up- and downregulated genes in the nac25-1 and nac1L-1 mutants represented by Venn diagrams.
(C) Germination Percentage of wild-type (WT; Col-0) and NAC single (nac25-1 and nac1L-1) and double knockout seeds at 48 hai. Results represent average values and SE for four biological replicates.
(D) Yeast strains containing the NAC25 or NAC1L CDS fused to the GAL4-BD (BD-NAC; bait) were mated to strains containing the AD-Ø, AD-GFP, AD-NAC1L, or AD-NAC25 constructs. Diploid cells were grown on diploid (-L-W) and screening (-L-W-H) plates with or without 3-AT.
(E) Overlap between genes downregulated >4-fold in the nac25-1 transcriptome and genes deregulated >4-fold in GA-related transcriptomes.
(F) NAC-BS content in the 500-bp promoters of genes downregulated >4-fold in the nac25-1 mutant and those commonly deregulated in GA-related transcriptomes or belonging to the CWRE class. The average NAC-BS occurrence in the Arabidopsis gene promoter dataset, Araport11 Loci Upstream Seq-500 bp, is taken arbitrarily as 1 (TAIR). Asterisks represent significant differences, at **P < 0.01 or *P < 0.05, from the control (TAIR) using the Chi-squared (χ2)÷ test.
We carried out gene ontology analyses using genes downregulated at least 4-fold in nac25-1, whereby we observed over-representation of genes related to growth and cell-wall modification and response to GAs (Supplemental Table 3), two biological processes playing a central role in germination and endosperm function. qRT–PCR analyses for some of these genes confirmed their downregulation (Supplemental Figure 8). These analyses showed that EXPA2 is strongly downregulated in both the nac25-1 (almost 30-fold) and nac1L-1 (almost 20-fold) mutants, confirming our discovery of EXPA2 promoter activation by these TFs. We compared the nac25-1 downregulated transcriptome with transcriptomes related to GA responses during seed germination. Figure 5E shows that over 50% of the genes upregulated ≥4-fold by GAs (Cao et al., 2006) were also downregulated ≥4-fold in nac25-1. This percentage increased to almost 70% when genes with 2-fold changes in expression were compared (Supplemental Figure 9). In contrast, almost no overlap was observed with genes repressed by GAs. According to the results of Cao et al. (2006), almost 90% of GA-induced genes (≥4-fold) were downregulated by DELLAs (DELLAdown), and we observed that over 50% of the DELLAdown genes were also downregulated in nac25-1. Stamm et al. (2012) published a list of genes specifically downregulated by RGL2, the major DELLA repressor of seed germination, over 30% of which (≥4-fold) were also downregulated in nac25-1. No overlap was observed when GA-related transcriptomes from buds were compared (Cao et al., 2006), suggesting that NAC25 regulation is seed specific.
Eighty-five genes related to cell-wall modification were downregulated >2-fold in nac25-1, including expansins, xyloglucan endotransglucosylases, pectin methylesterases, and arabinogalactans, and most were inducible by GA under the control of DELLA (Supplemental Table 4) according to published transcriptomes (Ogawa et al., 2003, Cao et al., 2006, Stamm et al., 2012, De Giorgi et al., 2015). When the 500-bp promoters of the 85 CWRE genes (Supplemental Table 4) were analyzed, a significant enrichment of the NAC-BS was observed (Figure 5F). This enrichment was higher than that found in other transcriptomes, suggesting that many CWRE genes could be direct targets of NACs. However, no NAC-BS enrichment was observed in the nac25-1 downregulated genes, compared with all genes in The Arabidopsis Information Resource (TAIR), suggesting that other proteins might be involved in their regulation (Supplemental Table 5).
NAC25 and NAC1L Positively Regulate Endosperm Cell Expansion
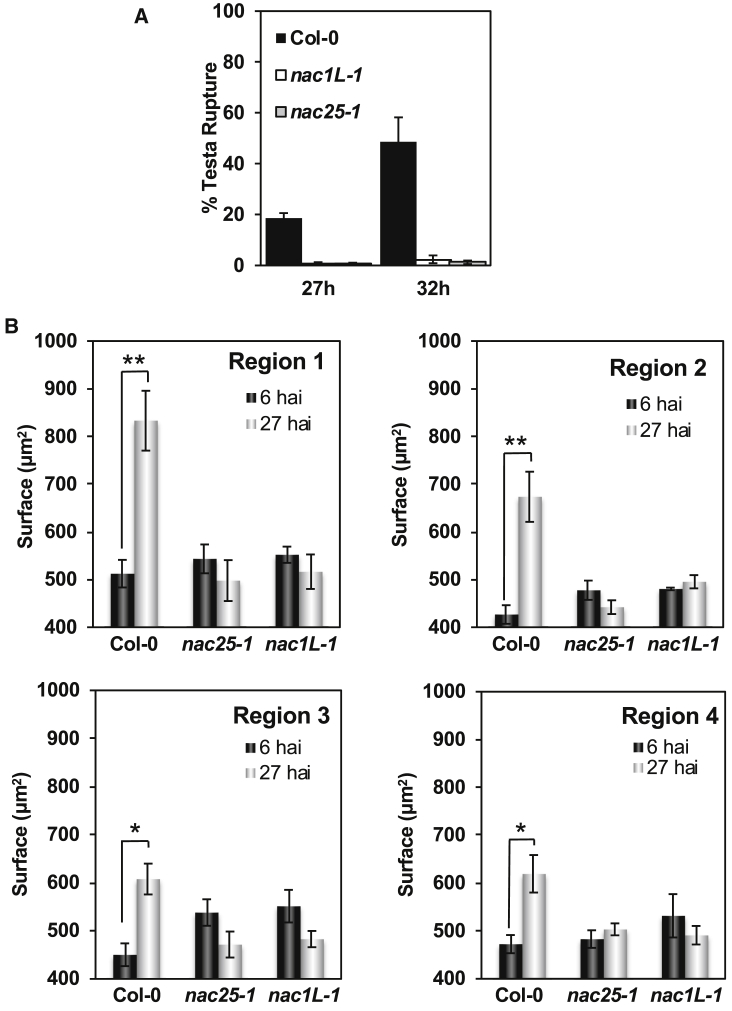
To assess the effect of NAC25 and NAC1L on endosperm physical properties, we measured endosperm cell expansion in imbibed wild-type, nac25-1, and nac1L-1 seeds. Since testa rupture is an early marker of germination, we determined the rupture kinetics in the genotypes under study. By 27 and 32 hai, testa rupture had occurred in 20%–50% of wild-type seeds, while the percentage of mutant seeds with testa rupture was almost negligible (Figure 6A). Next, we removed the testa of seeds at 6 and 27 hai and visualized them using confocal laser scanning microscopy and performed 3D reconstruction, segmentation, and clustering. Quantification of changes in cell surface area in the four endosperm regions was performed (Figure 6B). In wild-type seeds, the endosperm cell surface area increased in all regions examined, the highest increase being observed in regions 1 and 2 (70%) followed by regions 4 (44%) and 3 (26%). nac25-1 and nac1L-1 seeds, however, did not show any significant increase in cell surface area in any of the regions examined at the studied time points. These quantitative analyses of cell-shape changes indicate that NAC25 and NAC1L-1 regulate endosperm cell expansion.
Figure 6.
NAC25 and NAC1L TFs Are Positive Regulators of Testa Rupture and Endosperm Cell Expansion.
(A) Percentage of wild-type (Col-0) and NAC loss-of-function mutant seeds with testa rupture at 27 and 32 hai. Average percentages and SE for at least five biological replicates are shown.
(B) Cell surface area per region of every genotype at 27 hai was compared with that calculated at 6 hai in order to study cell elongation. Asterisks represent significant differences at **P < 0.01 and *P < 0.05 using the Mann–Whitney test (two-sided).
Discussion
This study provides new insights into the biophysical and molecular network underlying endosperm function in regulating Arabidopsis seed germination. While it has been clearly established that endosperm cell separation is important in the control of germination, our observation of endosperm expansion during imbibition represents a previously undescribed and necessary regulatory step in this developmental transition. This provides an important advance in our understanding of germination biology. In addition, we identified a gene network including previously undescribed genetic factors controlling endosperm expansion by modulating CWRE function.
By measuring changes in endosperm cell surface area using confocal imaging and 3D geometry reconstruction, we observed that all endosperm cells expand during imbibition but at different rates. The rate of expansion is greatest in the regions covering the radicle and lower hypocotyl, which coincide with the site of testa rupture and the highest expansion rates of the embryo axis (Stamm et al., 2017). These results highlight the different roles of the distinct regions of the endosperm.
Two NAC domain TFs (NAC25 and NAC1L) were identified as upstream regulatory factors controlling the expression of the EXPA2 gene and regulating endosperm expansion prior to germination. Both TFs bind to a conserved cis element within the EXPA2 promoter and activate transcription. The observed strong downregulation of EXPA2 in the nac25/1L mutant transcriptomes also confirms the importance of these TFs in regulating EXPA2 RNA accumulation in vivo. Neither NAC25 nor NAC1L are endosperm specific at the RNA level, suggesting that other factors are also important contributors to the endosperm-specific expression of EXPA2, perhaps through interaction with other cis elements in the EXPA2 promoter. Despite the fact that NACs are mainly expressed in endosperm after 6 hai, some of the downregulated CWRE genes shown in Supplemental Table 4 have been described as embryo specific (Dekkers et al., 2013), and therefore we cannot rule out the possibility that some of the phenotypes observed in the nac mutants could be partly due to reduced embryo growth. GA is a key positive regulator of endosperm function during germination, and DELLA regulators repress GA responses. We found that the major germination-associated DELLA, RGL2, interacts with NAC25 in vivo, and also inhibits the NAC25 activation of the EXPA2 promoter. This provides a simple mechanism for the regulation of EXPA2 transcription (Figure 7) where in low-GA conditions (e.g. dormant seeds) RGL2 would sequester NAC25, thereby inhibiting EXPA2 transcription. In high-GA conditions (e.g., leading to germination), GA-mediated destruction of RGL2 would allow NAC25 to interact with the EXPA2 promoter and thereby enhance EXPA2 expression. We found that the NAC25 binding element, NAC-BS, was significantly enriched in CWRE promoters. The gene expression module RGL2-NAC25-NAC-BS could therefore effectively modulate the expression of a cohort of CWREs, allowing expansion of the endosperm only in high-GA conditions and thus favoring subsequent germination. The endosperm cell expansion observed in wild-type seeds is blocked when NAC25 and NAC1L activity is removed, demonstrating their importance in controlling this process. De Giorgi et al. (2015) reported endosperm cell expansion in the ME and PE using seed histological sections and identified a role for cutin biosynthetic genes (BDG1, LACS2) in preventing cell expansion in imbibed seeds under low-GA conditions. However, we observed that in nac mutants BDG1 and LACS2 were downregulated (Supplemental Table 2) and that nac mutant transcriptomes and phenotypes resembled those of reduced GA signaling mutants. This suggests that the increased cell expansion observed in bdg1 and lacs2 depends on additional NAC-upregulated genes.
Figure 7.
Regulatory Model for GA-Mediated Endosperm Cell Expansion.
Our results suggest a regulatory model in which RGL2 blocks GA signaling in the endosperm by sequestering NACs. Upon imbibition, a signal from the embryo and/or GA biosynthesis destabilizes RGL2 and releases NACs to activate CWRE gene expression. CWREs then impact endosperm cell elongation, to accommodate embryo growth and facilitate radicle protrusion (that is germination sensu stricto).
Germination involves bidirectional interactions between the embryo and endosperm (Yan et al., 2014b). Endosperm cell separation requires the presence of the embryo, pointing to the existence of a diffusible signal from the embryo (Groot and Karssen, 1987, Groot et al., 1988, Müller et al., 2006). We showed that a signal requiring the embryo is perceived by the endosperm between 45 and 60 min after the initiation of seed imbibition, which activates the EXPA2 promoter. Comparison of the strength of GUS staining in endosperms from intact and separated seeds suggests that continued signaling is required for maximal EXPA2 promoter-driven expression. The nature of the initial signal is unknown and could be chemical, mechanical, or a combination of both. Genetic programs underpinning endosperm expansion, in addition to endosperm cell-wall separation, also therefore depend upon a mobile signal(s) requiring the embryo.
Previously, we described a molecular mechanism responsible for GA signaling in the embryo that involved two HD-ZIP TFs expressed in the epidermis (Rombolá-Caldentey et al., 2014). We have found that when GA signaling is blocked in the embryo epidermis during seed imbibition, EXPA2 expression is not affected (Supplemental Figure 10), suggesting that GA signaling in the endosperm does not require fully functional GA signaling in the embryo epidermis. These results are in line with previous findings pointing to different molecular mechanisms driving GA responses depending on the cell layer and developmental phase (Penfield et al., 2006, Ubeda-Tomas et al., 2008, Lee et al., 2010, Heo et al., 2011, Zhang et al., 2011, Duan et al., 2013, Geng et al., 2013, Lofke et al., 2013, Rombolá-Caldentey et al., 2014). This study identifies endosperm expansion during imbibition as a necessary key step in the regulation of germination and defines its control by an embryo-initiated gene network. Our findings provide important insight into the regulatory processes that underpin the seed-to-seedling transition in plants.
Methods
Plant Materials
Plants were germinated either on Petri dishes containing half-strength Murashige and Skoog (MS) medium buffered with 2 mM 2-(N-morpholino)ethanesulfonic acid (pH 5.7) and 0.7% (w/v) agar or in soil, and grown to maturity in 16 h light at 22°C/8 h dark at 20°C and 60% relative humidity. Seeds were harvested when plants had ceased flowering and siliques were starting to dehisce. Wild-type and mutant plants were in the Col-0 accession. NAC25 loss-of-function mutants were SM_3_16 875 (KO1) and SM_3_37 315 (KO2). NAC1L loss-of-function mutants were SALK_063384 (KO1) and SALK_049270 (KO2). Genotyping of the mutants was carried out by PCR amplification of genomic DNA (gDNA) using appropriate primer combinations (Supplemental Table 1). gDNA was isolated as described by Edwards et al. (1991). Appropriate constructs were introduced into A. thaliana (Col-0 accession) using the A. tumefaciens strain GV3101 and the floral-dip method (Clough and Bent, 1998).
Sources of Orthologous EXPA2 Gene Promoter Sequences from Several Brassicaceae Species, and Phylogenetic and Bioinformatic Analyses
Oligonucleotides to amplify EXPA2 orthologous promoter sequences from Descuriainia sophia, Carrichtera annua, Hornungia petraene, and Brassica oleracea were designed as described in Castrillo et al. (2011) and are listed in Supplemental Table 1 (EXPA2-rev and At5g05280-fw). The promoter sequence corresponding to the A. thaliana EXPA2 gene was downloaded from the TAIR webpage using the bulk data retrieval tool (https://www.arabidopsis.org/tools/bulk/sequences/index.jsp). Orthologous promoter sequences from Arabidopsis lyrata, Thelungiella halophila, and Capsella rubella were downloaded from the Phytozome webpage (https://phytozome.jgi.doe.gov/pz/portal.html). Alignment of promoter sequences and detection of conserved blocks were carried out as described in Castrillo et al. (2011). NAC-BS occurrence in promoters of genes differentially expressed in GA-related transcriptomes was calculated as described previously (Rombolá-Caldentey et al., 2014).
In Vivo Imaging of Bioluminescence
In vivo imaging and quantification of luciferase activity were carried out using a cooled CCD camera (NightOwl II LB 983 NC-100; Berthold Technologies) and the provided software. Seeds from representative lines were simultaneously sown and grown on 0.6% agarose or MS agar plates in the presence or absence of 5 μM PAC or 5 μM PAC plus 5 μM GA. Plates were directly transferred to a chamber at a constant temperature of 22°C and a photoperiod of 16 h light/8 h dark for 24 or 48 h. Plates were then sprayed with 100 μM luciferin and imaged after 40 min.
Generation of Constructs for Yeast and Plant Assays
A 2106-bp promoter fragment from the AtEXP2 gene (containing the first three EXPA2 ORF codons) was amplified by PCR from A. thaliana (Col-0 accession) gDNA using the LO1884 and LO1885 primers (Supplemental Table 1) and recombined with BP Clonase Enzyme Mix into the pDONR221 plasmid (Invitrogen). This construct was recombined with the LR Clonase Enzyme Mix into the pBGWL7 plasmid (VIB) containing the luciferase CDS to obtain the ProEXPA2:LUC construct or into the pKGWFS7 plasmid (Karimi et al., 2002) to obtain the ProEXPA2:GUS construct. To generate the mutated version of the EXPA2 promoter (ProEXPA2-mut:LUC), we used two different primer pairs (LO1 + LO1916 and LO1915 + LO604) to amplify two partially overlapping fragments containing the mutated nucleotides, using the wild-type version cloned in pBGWL7 as a template. Fragments were digested with AvrII and ligated after purification. The resulting fragment was used as a template to amplify the mutated 2106-bp fragment using the primers LO1 and LO604. The PCR product was recombined with BP Clonase Enzyme Mix into the pDONR221 plasmid and then recombined with the LR Clonase Enzyme Mix into the pBGWL7 plasmid.
To introduce four tandemly repeated EXPA2-CS into plants, we used a binary plasmid (pYRO) containing a luciferase (LUC+) CDS downstream of a minimal promoter sequence with BamHI and HindIII restriction sites at its 5′ end (min:LUC or -58F8-pYRO; Chen and Singh, 1999, Castrillo et al., 2011). The ProEXPA2 construct in the pDONR221 plasmid was used as template to amplify the EXPA2-CS using the primers LO839 and LO840, which contained BglII and BamHI/HindIII restriction sites, respectively (Supplemental Table 1). Then, the engineered BglII and HindIII sites in the EXPA2-CS product were used to clone it into the BamHI and HindIII sites of the min:LUC plasmid upstream of the minimal promoter and the LUC+ gene (1xEXPA2-CS:LUC). This process was repeated three more times to generate the 4xEXPA2-CS:LUC plasmid. To produce the 4xEXPA2-CSm:LUC construct, we synthesized and cloned the 4xEXPA2-CSm sequence (342 bp) into the BamHI/HindIII sites of the pUC57 plasmid (GeneScript, USA). The 4xEXPA2-CSm sequence was fused to the luciferase reporter gene by cloning into the BamHI/HindIII sites of the pYRO plasmid (4xEXPA2-CSm:LUC).
For yeast assays, the ProEXPA2 fragment in the pDONR221 plasmid was used as template for PCR amplification of the EXPA2-CS with the LO1201 and LO1202 primers (Supplemental Table 1). The amplified fragment was cloned into the XbaI and XmaI sites of the pTUY1H plasmid (Castrillo et al., 2011). To generate the EXPA2-CSm version, we used three different primer pairs (LO1040 + LO1914, LO1913 + LO1912, and LO1911 + 1041) to amplify three partially overlapping fragments containing the mutated nucleotides in the NAC-BS core sequence (5′-CGT[G/A]-3′ [Puranik et al., 2012, Franco-Zorrilla et al., 2014]), using the wild-type version cloned in pTUY1H as a template. The generated fragments were purified and combined to serve as templates for the amplification of a 180-bp DNA sequence using the primers LO1040 and LO1041. The 80 bp EXPA2-CSm was excised from the 180-bp fragment and cloned into the pTUY1H plasmid by using the XbaI and XmaI restriction enzymes. Full sequencing of the TFs isolated in the screening revealed that the first nine base pairs of the NAC25 ORF (coding for the first three amino acids of the protein) were missing. The full version of NAC25 was PCR-amplified using an SSP pUNI clone (U605068; Yamada et al., 2003) as a template and primers LO1344 and LO1345. The resulting 972 bp ORF was cloned into pDONR221 by a BP recombination reaction and translationally fused to the GAL4AD by an LR reaction between the pDONR221-NAC25 and the pDEST22 or the pGADT7(GW) plasmids, or to the GAL4BD by using pGBKT7(GW) as the destination vector. The NAC1L ORF contained in the library prey plasmid (pDEST22; GAL4AD-NAC1L) was cloned into pDONR221 by a BP recombination reaction and transferred to the pGBKT7(GW) or pGADT7(GW) plasmids by an LR reaction. All control constructs and constructs carrying GAL4BD-RGL2 (BD-RGL2) have been previously described (Rombolá-Caldentey et al., 2014).
For Co-IP assays, the NAC25-pDONR221 vector (entry vector) was digested with the MluI enzyme at 37°C for 2 h before the LR recombination reaction with pEarleyGate201 to obtain an N-terminal fusion to the HA epitope. The RGL2 ORF was obtained from the construct pDEST22-RGL2 isolated from the corresponding yeast strain in the RR library (Castrillo et al., 2011) and transferred to pDONR207 by a BP reaction. The RGL2 ORF was then transferred to the pMDC83 plasmid by an LR reaction to obtain a translational fusion to the complete sequence of the GFP.
For transient expression analyses, overexpression constructs for NAC25, NAC1L, and RGL2 were generated by transferring their ORFs to the PCX plasmid from ORF-pDONR221 in an LR reaction.
Transient Expression Analyses in N. benthamiana
The reporter constructs ProEXPA2:LUC and ProEXPA2m:LUC and the effector constructs (pCX plasmid) overexpressing NAC25, NAC1L, and RGL2 were introduced into A. tumefaciens C58 GV3101:pMP90, and the resulting strains were used for agroinfiltration of 4-week-old N. benthamiana leaves. The empty pCX plasmid was used as a negative control, and a pBIN61-35S:P19 plasmid was always co-infiltrated to avoid gene silencing (Voinnet et al., 2003). All Agrobacterium cultures were used at OD600 = 0.3. Three days after inoculation luciferase activity was measured in 0.6% agarose microtiter plates as described in Espinosa-Ruiz et al. (2017), using a cooled CCD camera (NightOwl II LB 983 NC-100).
Co-immunoprecipitation Assay
For the Co-IP assay, about 5 g of N. benthamiana leaves were ground in liquid nitrogen and homogenized in 5 ml of extraction buffer. Samples were centrifuged at 13 000 rpm at 4°C for 10 min, and the supernatants were filtered using Miracloth paper (Calbiochem 475855-1R) and transferred into a new tube. Also, as a positive control, 75 μl of each protein extract was reserved, mixed with 25 μl of 4× loading buffer 4, and 15 μl were loaded on a gel. Total protein concentration was determined using the Bio-Rad Bradford Protein Assay Kit, and 1 mg of total soluble proteins was used for further immunoprecipitation. Each protein extract was then incubated with 1 μl of Anti-HA antibody (1.9 μg/μl; kindly provided by Dr. Salomé Prat, Centro Nacional de Biotecnología, Madrid) for 2 h in a cold room. For protein immunoprecipitation, 20 μl of Dynabeads protein G (Life Technologies) was cleaned with 50 μl of extraction buffer and was then added into the protein solution, which had been previously incubated with the antibody. After 30 min of incubation at 4°C, beads were separated from the protein extract with a magnet and washed four times with 500 μl of extraction buffer. Finally, beads were collected and resuspended in 45 μl of extraction buffer, mixed with 15 μl of loading buffer, boiled at 95°C for 5 min, and loaded onto two separate gels. Interactions between fusion proteins HA:NAC25 and RGL2:GFP were detected by immunoblotting with 1:1000 diluted anti-GFPHRP (Miltenyi Biotec) and anti-HAHRP (Roche) antibodies.
Bimolecular Fluorescence Complementation Experiments
The NAC CDSs were translationally fused to N-YFP and C-CFP in the Gateway binary destination vectors pNXGW and pCXGW, respectively. All Agrobacterium cultures were used at OD600 = 0.3 and agroinfiltration of N. benthamiana leaves was carried out as previously described (Rombolá-Caldentey et al., 2014). BiFC images were taken three days after agroinfiltration with a Leica DM 2000 fluorescence microscope.
Germination and Testa Rupture Assays
Testa and endosperm rupture (germination) were scored at the indicated time points in germination assays carried out as described in Rombolá-Caldentey et al. (2014).
Yeast Transformation and One-Hybrid Screening
For a complete description of the methodology and yeast strains used, see Castrillo et al. (2011) and Sánchez-Montesino and Oñate-Sánchez, 2017, Sánchez-Montesino and Oñate-Sánchez, 2018).
RNA Isolation and qRT–PCR
RNA extraction was carried out as described previously (protocol 2 in Oñate-Sánchez and Vicente-Carbajosa, 2008) except that: in step 2, the mix containing the sample, phenol, and chloroform was applied to a Phase Lock (5-PRIME) Gel (Eppendorf) prior to centrifugation to prevent organic contamination of the aqueous phase; in step 4, the RNA pellet was dissolved in 88 μl of water, and 20 units of RNAse-free DNAse were used. First-strand cDNA synthesis and real-time qPCR has been previously described (Rombolá-Caldentey et al., 2014). For RNA sequencing, RNA isolation was followed by RNA cleanup using the RNeasy mini kit (Qiagen). Library construction and sequencing (mRNA-seq) on the Illumina HiSeq2500 was carried out at CNAG (National Center for Genomic Sequencing, Barcelona, Spain), resulting in 45–72 million 50-bp single reads per sample.
Sequence Processing
We analyzed the global change in gene expression in wild-type (Col-0) and one representative knockout (KO) line for each NAC gene by carrying out RNA-seq of seeds at 12 hai. Total RNA from three biological replicates for each genotype was isolated and used to prepare the corresponding libraries for mRNA sequencing (Illumina HiSeq 2500 with v4 chemistry). A minimum of 45 × 106 50-bp single reads were obtained for each sample (Supplemental Table 6), and more than the 99.3% of reads in each sample aligned against the Araport11 reference genome. Transcript data were downloaded in FastQ format from the sequencing facility and the quality was assessed using FastQC v0.11.5 (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc). To evaluate the expression levels of the aligned reads, we used the cuffdiff protocol under the local Galaxy interface v.2.2.1.0 (Afgan et al., 2016). The reads were aligned against the genome using TopHat v2.1.0 (Langmead et al., 2009) and then the expression levels were obtained with Cuffdiff v2.2.1.3 (Trapnell et al., 2010). The classic-fpkm normalization method, per-condition estimation, and bias correction were applied to the analysis. Only differentially expressed genes misregulated over 2-fold were considered for downstream analyses, and genes were referred to as being KO-upregulated or KO-downregulated only if the ratio of wild-type versus KO was greater than or equal to 2-fold, with a P-value cutoff at 0.05. Using these criteria, we identified 1260 and 2094 genes as putative KO1- and KO2-regulated genes, respectively.
Measuring Cell Expansion by Confocal Imaging and Geometry Reconstruction
Seeds of each wild-type and mutant line were sown on a filter paper placed onto 0.6% agarose plates and transferred to a chamber at a constant temperature of 22°C and a photoperiod of 16 h light/8 h dark. After 6 and 27 hai, testas from five seeds per line were carefully removed using two forceps, and the seeds were immediately transferred to a fixative solution (ethanol/acetic acid 3:1). After incubation in fixative solution for at least 24 h, the fixative was replaced with clearing solution (0.2 M NaOH, 1% SDS), and the sample was incubated for 1 week. After repeating the clearing step, seeds were left in water for several days. Cell walls were stained using the mPA-PI procedure as described previously (Bassel et al., 2014). Before imaging, seeds were placed in chloral hydrate clearing solution (4 g of chloral hydrate/1 ml of glycerol/2 ml of water), and z stacks were collected using a Zeiss LSM710 confocal microscope.
Image analysis was performed by using MorphoGraphX software (Barbier de Reuille et al., 2015). After applying a Gaussian Blur Stack, a surface (mask) surrounding the seed was generated. The surface was first fitted, then signal emerging from the cell walls was projected on the surface. Surfaces were then automatically segmented into cells and incorrectly segmented cells were manually discarded. Cell surface areas (μm2) were obtained using a heatmap tool. At least 180 cells from five different samples from every line and time after imbibition were used to determine differences in cell elongation. Student’s t-test was applied to the average data from 6-hai and 27-hai samples of every line, which were treated as independent lines. P values of less than 0.05 were considered indicative of a significant difference.
Funding
This work was supported with grants to L.O.-S by the Spanish Ministry of Economy and Competitiveness (BIO2013-46076-R and BIO2016-77840-R), by the Biotechnology and Biological Sciences Research Council (grant numbers BB/J017604/1, BB/L010232/1, BB/N009754/1, BB/G02488X/1) to M.J.H. and G.B., and the Leverhulme Trust grant RPG-2016-049 to G.B. together with S.D.-N.
Author Contributions
R.S.-M performed most of the experiments, L.B.-M. did the Co-IP experiments, and both were supervised by L.O.-S. Quantitative image analyses were carried out by R.S.-M and S.D.-N. under the supervision of G.B. GUS expression analyses were done by M.G. and J.M. under the supervision of M.J.H. L.G. helped with RNA-seq analyses and review of the manuscript. M.J.H., G.B., and L.O.-S. wrote the manuscript.
Acknowledgments
We wish to thank Dr. A. Muñoz for help with the alignment and evaluation of expression levels of the RNA-seq data, Dr. Javier Paz-Ares for providing some of the EXPA2 promoter sequences used in phylogenomic analyses, Dr. Mónica Pernas for critical reading of the manuscript, and Anne Medhurst for making the ProEXPA2:GUS construct. The NAC knockout mutant seeds were obtained from NASC (http://arabidopsis.info). No conflict of interest declared.
Published: November 8, 2018
Footnotes
Published by the Molecular Plant Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and IPPE, SIBS, CAS.
Supplemental Information is available at Molecular Plant Online.
Contributor Information
Michael J. Holdsworth, Email: michael.holdsworth@nottingham.ac.uk.
George Bassel, Email: g.w.bassel@bham.ac.uk.
Luis Oñate-Sánchez, Email: luis.onate@upm.es.
Supplemental Information
References
- Afgan E., Baker D., van den Beek M., Blankenberg D., Bouvier D., Cech M., Chilton J., Clements D., Coraor N., Eberhard C. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Res. 2016;44:W3–W10. doi: 10.1093/nar/gkw343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbier de Reuille P., Routier-Kierzkowska A.-L., Kierzkowski D., Bassel G.W., Schüpbach T., Tauriello G., Bajpai N., Strauss S., Weber A., Kiss A. MorphoGraphX: a platform for quantifying morphogenesis in 4D. Elife. 2015;4:e05864. doi: 10.7554/eLife.05864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel G.W. To grow or not to grow? Trends Plant Sci. 2016;21:498–505. doi: 10.1016/j.tplants.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Bassel G.W., Mullen R.T., Bewley J.D. ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J. Exp. Bot. 2006;57:1291–1297. doi: 10.1093/jxb/erj101. [DOI] [PubMed] [Google Scholar]
- Bassel G.W., Stamm P., Mosca G., Barbier de Reuille P., Gibbs D.J., Winter R., Janka A., Holdsworth M.J., Smith R.S. Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. Proc. Natl. Acad. Sci. U S A. 2014;111:8685–8690. doi: 10.1073/pnas.1404616111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke P.C., Libourel I.G., Aoyama N., Chung Y.Y., Still D.W., Jones R.L. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D., Hussain A., Cheng H., Peng J. Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta. 2005;223:105–113. doi: 10.1007/s00425-005-0057-3. [DOI] [PubMed] [Google Scholar]
- Cao D., Cheng H., Wu W., Soo H.M., Peng J. Gibberellin mobilizes distinct DELLA-dependent transcriptomes to regulate seed germination and floral development in Arabidopsis. Plant Physiol. 2006;142:509–525. doi: 10.1104/pp.106.082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo G., Turck F., Leveugle M., Lecharny A., Carbonero P., Coupland G., Paz-Ares J., Oñate-Sánchez L. Speeding cis-trans regulation discovery by phylogenomic analyses coupled with screenings of an arrayed library of Arabidopsis transcription factors. PLoS One. 2011;6:e21524. doi: 10.1371/journal.pone.0021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Bradford K.J. Expression of an expansin is associated with endosperm weakening during tomato seed germination. Plant Physiol. 2000;124:1265–1274. doi: 10.1104/pp.124.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Nonogaki H., Bradford K.J. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J. Exp. Bot. 2002;53:215–223. doi: 10.1093/jexbot/53.367.215. [DOI] [PubMed] [Google Scholar]
- Chen W., Singh K.B. The auxin, hydrogen peroxide and salicylic acid induced expression of the Arabidopsis GST6 promoter is mediated in part by an ocs element. Plant J. 1999;19:667–677. doi: 10.1046/j.1365-313x.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Coen E., Rolland-Lagan A.-G., Matthews M., Bangham J.A., Prusinkiewicz P. The genetics of geometry. Proc. Natl. Acad. Sci. U S A. 2004;101:4728. doi: 10.1073/pnas.0306308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. Plant expansins: diversity and interactions with plant cell walls. Curr. Opin. Plant Biol. 2015;25:162–172. doi: 10.1016/j.pbi.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Ehrhardt D.W., Griffitts J.S., Somerville C.R. Random GFP∷cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. U S A. 2000;97:3718. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi J., Piskurewicz U., Loubery S., Utz-Pugin A., Bailly C., Mene-Saffrane L., Lopez-Molina L. An endosperm-associated cuticle is required for Arabidopsis seed viability, dormancy and early control of germination. PLoS Genet. 2015;11:e1005708. doi: 10.1371/journal.pgen.1005708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers B.J., Pearce S., van Bolderen-Veldkamp R.P., Marshall A., Widera P., Gilbert J., Drost H.G., Bassel G.W., Muller K., King J.R. Transcriptional dynamics of two seed compartments with opposing roles in Arabidopsis seed germination. Plant Physiol. 2013;163:205–215. doi: 10.1104/pp.113.223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L., Dietrich D., Ng C.H., Chan P.M., Bhalerao R., Bennett M.J., Dinneny J.R. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell. 2013;25:324–341. doi: 10.1105/tpc.112.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq B., Berger N., Vincent E., Boisson M., Pelletier G., Caboche M., Lepiniec L. The Arabidopsis AtEPR1 extensin-like gene is specifically expressed in endosperm during seed germination. Plant J. 2000;23:643–652. doi: 10.1046/j.1365-313x.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- Edwards K., Johnstone C., Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res. 1991;19:1349. doi: 10.1093/nar/19.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Tatematsu K., Hanada K., Duermeyer L., Okamoto M., Yonekura-Sakakibara K., Saito K., Toyoda T., Kawakami N., Kamiya Y. Tissue-specific transcriptome analysis reveals cell wall metabolism, flavonol biosynthesis and defense responses are activated in the endosperm of germinating Arabidopsis thaliana seeds. Plant Cell Physiol. 2012;53:16–27. doi: 10.1093/pcp/pcr171. [DOI] [PubMed] [Google Scholar]
- Espinosa-Ruiz A., Martínez C., de Lucas M., Fábregas N., Bosch N., Caño-Delgado A.I., Prat S. TOPLESS mediates brassinosteroid control of shoot boundaries and root meristem development in Arabidopsis thaliana. Development. 2017;144:1619–1628. doi: 10.1242/dev.143214. [DOI] [PubMed] [Google Scholar]
- Finch-Savage W.E., Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Finch-Savage W.E., Bassel G.W. Seed vigour and crop establishment: extending performance beyond adaptation. J. Exp. Bot. 2016;67:567–591. doi: 10.1093/jxb/erv490. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla J.M., Lopez-Vidriero I., Carrasco J.L., Godoy M., Vera P., Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. U S A. 2014;111:2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Wu R., Wee C.W., Xie F., Wei X., Chan P.M., Tham C., Duan L., Dinneny J.R. A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell. 2013;25:2132–2154. doi: 10.1105/tpc.113.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot S.P., Karssen C.M. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta. 1987;171:525–531. doi: 10.1007/BF00392302. [DOI] [PubMed] [Google Scholar]
- Groot S.P.C., Kieliszewska-Rokicka B., Vermeer E., Karssen C.M. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta. 1988;174:500–504. doi: 10.1007/BF00634479. [DOI] [PubMed] [Google Scholar]
- Gubler F., Jacobsen J.V. Gibberellin-responsive elements in the promoter of a barley high-pI alpha-amylase gene. Plant Cell. 1992;4:1435–1441. doi: 10.1105/tpc.4.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.O., Chang K.S., Kim I.A., Lee M.H., Lee S.A., Song S.K., Lee M.M., Lim J. Funneling of gibberellin signaling by the GRAS transcription regulator scarecrow-like 3 in the Arabidopsis root. Proc. Natl. Acad. Sci. U S A. 2011;108:2166–2171. doi: 10.1073/pnas.1012215108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth M.J., Bentsink L., Soppe W.J. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Karimi M., Inzé D., Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- Koornneef M., van der Veen J.H. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) heynh. Theor. Appl. Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.J., Dekkers B.J., Steinbrecher T., Walsh C.T., Bacic A., Bentsink L., Leubner-Metzger G., Knox J.P. Distinct cell wall architectures in seed endosperms in representatives of the Brassicaceae and Solanaceae. Plant Physiol. 2012;160:1551–1566. doi: 10.1104/pp.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Piskurewicz U., Tureckova V., Strnad M., Lopez-Molina L. A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. U S A. 2010;107:19108–19113. doi: 10.1073/pnas.1012896107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.P., Piskurewicz U., Tureckova V., Carat S., Chappuis R., Strnad M., Fankhauser C., Lopez-Molina L. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev. 2012;26:1984–1996. doi: 10.1101/gad.194266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Cheng H., King K.E., Wang W., He Y., Hussain A., Lo J., Harberd N.P., Peng J. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002;16:646–658. doi: 10.1101/gad.969002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofke C., Zwiewka M., Heilmann I., Van Montagu M.C., Teichmann T., Friml J. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc. Natl. Acad. Sci. U S A. 2013;110:3627–3632. doi: 10.1073/pnas.1300107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Andújar C., Pluskota W.E., Bassel G.W., Asahina M., Pupel P., Nguyen T.T., Takeda-Kamiya N., Toubiana D., Bai B., Gorecki R.J. Mechanisms of hormonal regulation of endosperm cap-specific gene expression in tomato seeds. Plant J. 2012;71:575–586. doi: 10.1111/j.1365-313X.2012.05010.x. [DOI] [PubMed] [Google Scholar]
- Morris K., Linkies A., Muller K., Oracz K., Wang X., Lynn J.R., Leubner-Metzger G., Finch-Savage W.E. Regulation of seed germination in the close Arabidopsis relative Lepidium sativum: a global tissue-specific transcript analysis. Plant Physiol. 2011;155:1851–1870. doi: 10.1104/pp.110.169706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Tintelnot S., Leubner-Metzger G. Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006;47:864–877. doi: 10.1093/pcp/pcj059. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Hanada A., Yamauchi Y., Kuwahara A., Kamiya Y., Yamaguchi S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oñate-Sánchez L., Vicente-Carbajosa J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res. Notes. 2008;1:93. doi: 10.1186/1756-0500-1-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S., Li Y., Gilday A.D., Graham S., Graham I.A. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell. 2006;18:1887–1899. doi: 10.1105/tpc.106.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S., Rylott E.L., Gilday A.D., Graham S., Larson T.R., Graham I.A. Reserve mobilization in the Arabidopsis endosperm fuels hypocotyl elongation in the dark, is independent of abscisic acid, and requires PHOSPHOENOLPYRUVATE CARBOXYKINASE1. Plant Cell. 2004;16:2705–2718. doi: 10.1105/tpc.104.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S., Sahu P.P., Srivastava P.S., Prasad M. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17:369–381. doi: 10.1016/j.tplants.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Rombolá-Caldentey B., Rueda-Romero P., Iglesias-Fernández R., Carbonero P., Oñate-Sánchez L. Arabidopsis DELLA and two HD-ZIP transcription factors regulate GA signaling in the epidermis through the L1 box cis-element. Plant Cell. 2014;26:2905–2919. doi: 10.1105/tpc.114.127647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Montesino R., Oñate-Sánchez L. Yeast one- and two-hybrid high-throughput screenings using arrayed libraries. In: Kaufmann K., Mueller-Roeber B., editors. Plant Gene Regulatory Networks. vol. 1629. Humana Press; New York: 2017. pp. 47–65. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- Sánchez-Montesino R., Oñate-Sánchez L. Screening arrayed libraries with DNA and protein baits to identify interacting proteins. In: Oñate-Sánchez L., editor. Two-Hybrid Systems. vol. 1794. Humana Press; New York: 2018. pp. 131–149. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- Scheler C., Weitbrecht K., Pearce S., Hampstead A., Büttner-Mainik A., Lee K.J.D., Voegele A., Oracz K., Dekkers B.J.W., Wang X. Promotion of testa rupture during Lepidium sativum germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015;167:200–215. doi: 10.1104/pp.114.247429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechet J., Frey A., Effroy-Cuzzi D., Berger A., Perreau F., Cueff G., Charif D., Rajjou L., Mouille G., North H.M. Xyloglucan metabolism differentially impacts the cell wall characteristics of the endosperm and embryo during arabidopsis seed germination. Plant Physiol. 2016;170:1367–1380. doi: 10.1104/pp.15.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Olsen F.L., Rogers J.C., Mundy J. cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc. Natl. Acad. Sci. U S A. 1991;88:7266–7270. doi: 10.1073/pnas.88.16.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska E., Bassel G.W., Bewley J.D. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 2009;60:3587–3594. doi: 10.1093/jxb/erp203. [DOI] [PubMed] [Google Scholar]
- Stamm P., Ravindran P., Mohanty B., Tan E.L., Yu H., Kumar P.P. Insights into the molecular mechanism of RGL2-mediated inhibition of seed germination in Arabidopsis thaliana. BMC Plant Biol. 2012;12:179. doi: 10.1186/1471-2229-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm P., Topham A.T., Mukhtar N.K., Jackson M.D., Tome D.F., Beynon J.L., Bassel G.W. The transcription factor ATHB5 affects GA-mediated plasticity in hypocotyl cell growth during seed germination. Plant Physiol. 2017;173:907–917. doi: 10.1104/pp.16.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher T., Leubner-Metzger G. The biomechanics of seed germination. J. Exp. Bot. 2016;68:765–783. doi: 10.1093/jxb/erw428. [DOI] [PubMed] [Google Scholar]
- Sun T.P., Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L., Thomas S.G., Hu J., Dill A., Alonso J.M., Ecker J.R., Sun T.P. Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004;135:1008–1019. doi: 10.1104/pp.104.039578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Tomas S., Swarup R., Coates J., Swarup K., Laplaze L., Beemster G.T., Hedden P., Bhalerao R., Bennett M.J. Root growth in Arabidopsis requires gibberellin/DELLA signalling in the endodermis. Nat. Cell Biol. 2008;10:625–628. doi: 10.1038/ncb1726. [DOI] [PubMed] [Google Scholar]
- Voinnet O., Rivas S., Mestre P., Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- Yamada K., Lim J., Dale J.M., Chen H., Shinn P., Palm C.J., Southwick A.M., Wu H.C., Kim C., Nguyen M. Empirical analysis of transcriptional activity in the Arabidopsis genome. Science. 2003;302:842–846. doi: 10.1126/science.1088305. [DOI] [PubMed] [Google Scholar]
- Yan A., Wu M., Yan L., Hu R., Ali I., Gan Y. AtEXP2 is involved in seed germination and abiotic stress response in Arabidopsis. PLoS One. 2014;9:e85208. doi: 10.1371/journal.pone.0085208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Duermeyer L., Leoveanu C., Nambara E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014;55:1521–1533. doi: 10.1093/pcp/pcu089. [DOI] [PubMed] [Google Scholar]
- Zhang Z.L., Ogawa M., Fleet C.M., Zentella R., Hu J., Heo J.O., Lim J., Kamiya Y., Yamaguchi S., Sun T.P. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. U S A. 2011;108:2160–2165. doi: 10.1073/pnas.1012232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.